Patents
Literature
289results about How to "Good osteoinductivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
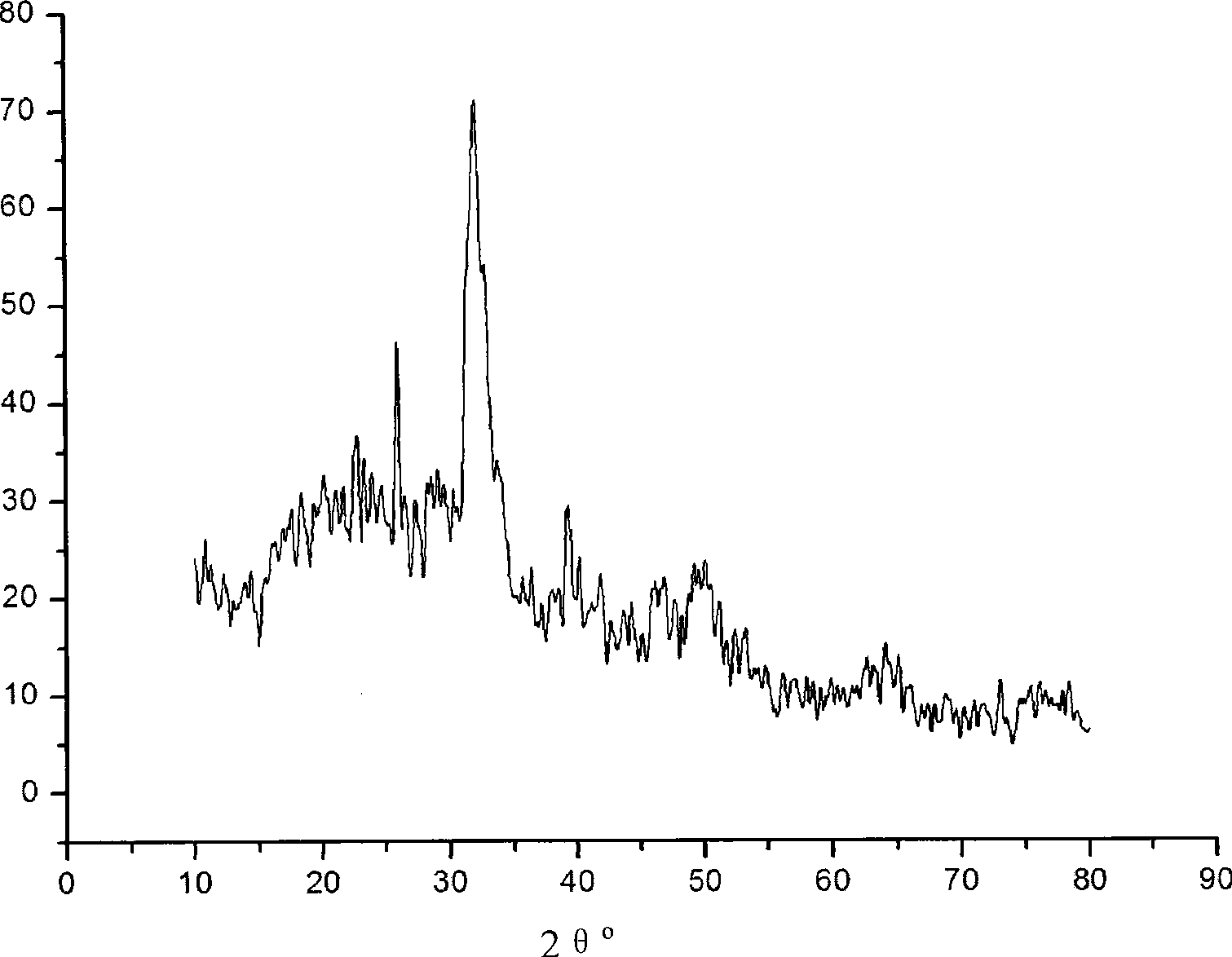
Bone Matrix Compositions and Methods
ActiveUS20070098756A1High activityEasy to addPeptide/protein ingredientsBone implantActive agentOSTEOINDUCTIVE FACTOR
An osteoinductive composition, corresponding osteoimplants, and methods for making the osteoinductive composition are disclosed. The osteoinductive composition comprises osteoinductive factors, such as may be extracted from demineralized bone, and a carrier. The osteoinductive composition is prepared by providing demineralized bone, extracting osteoinductive factors from the demineralized bone, and adding the extracted osteoinductive factors to a carrier. Further additives such as bioactive agents may be added to the osteoinductive composition. The carrier and osteoinductive factors may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, can induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. Also, in some embodiments, the osteoinductive composition can be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
Bone Matrix Compositions and Methods
ActiveUS20070110820A1High activityEasy to addPeptide/protein ingredientsImmunoglobulinsOSTEOINDUCTIVE FACTORBone implant
Owner:WARSAW ORTHOPEDIC INC
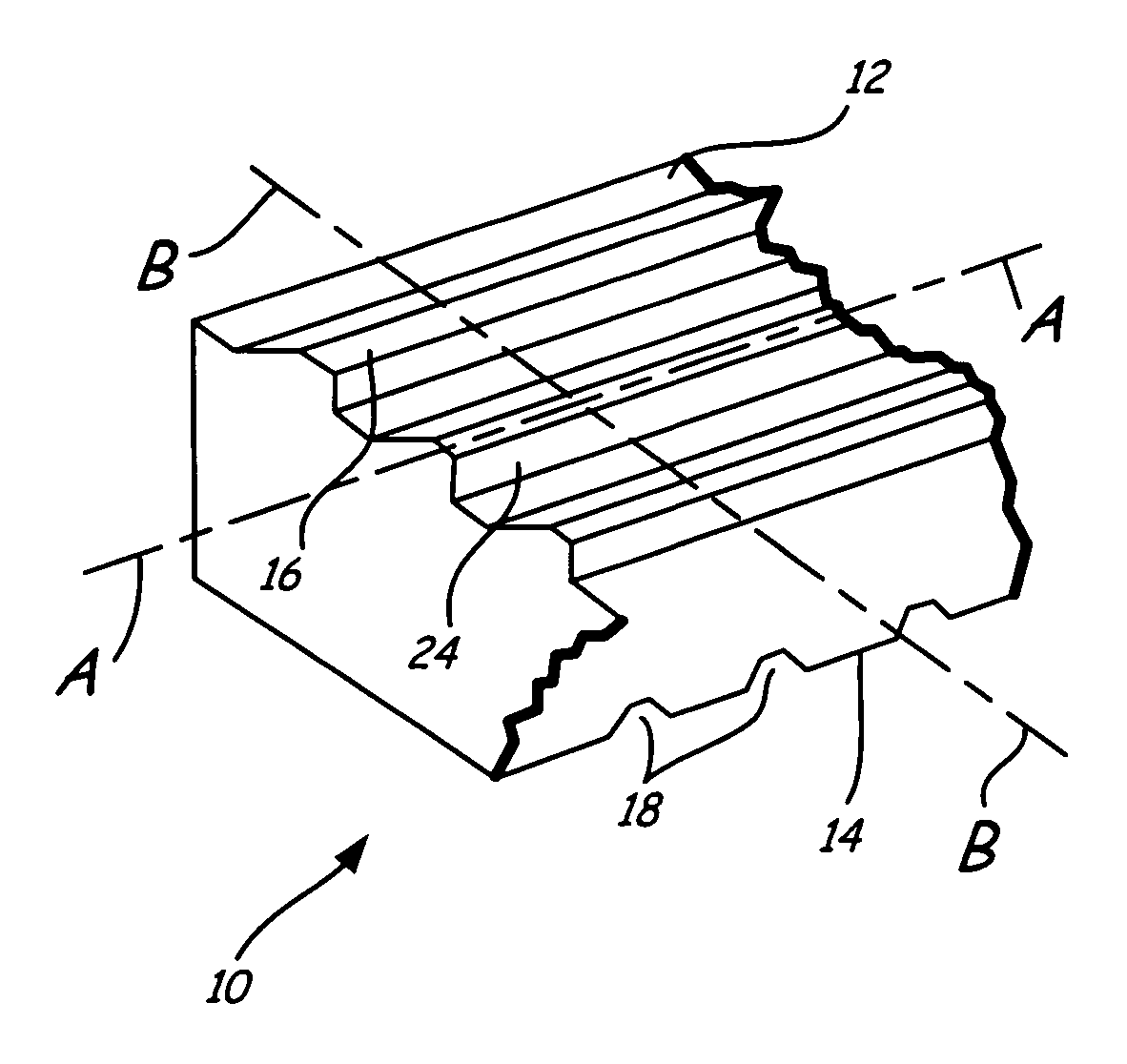
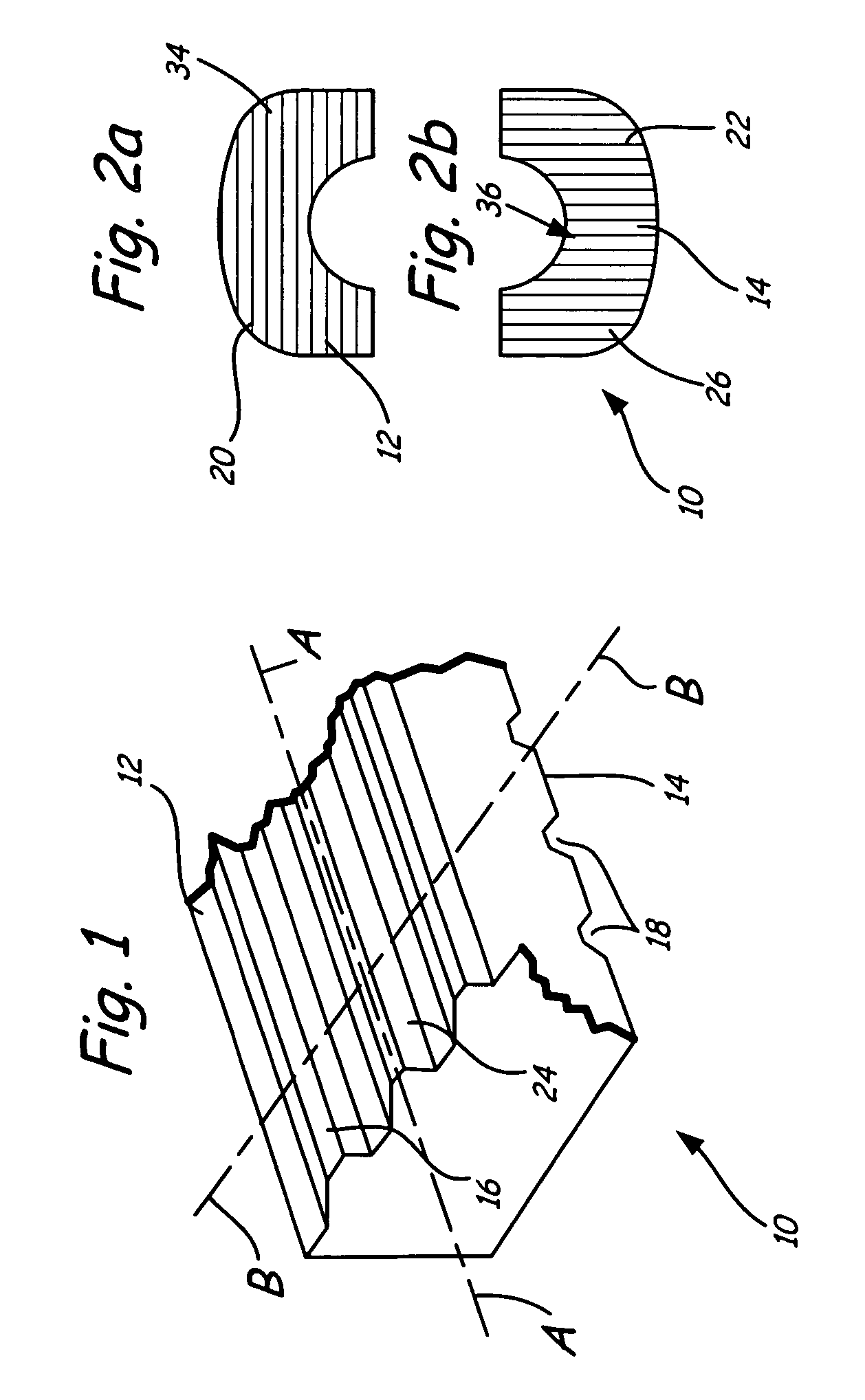
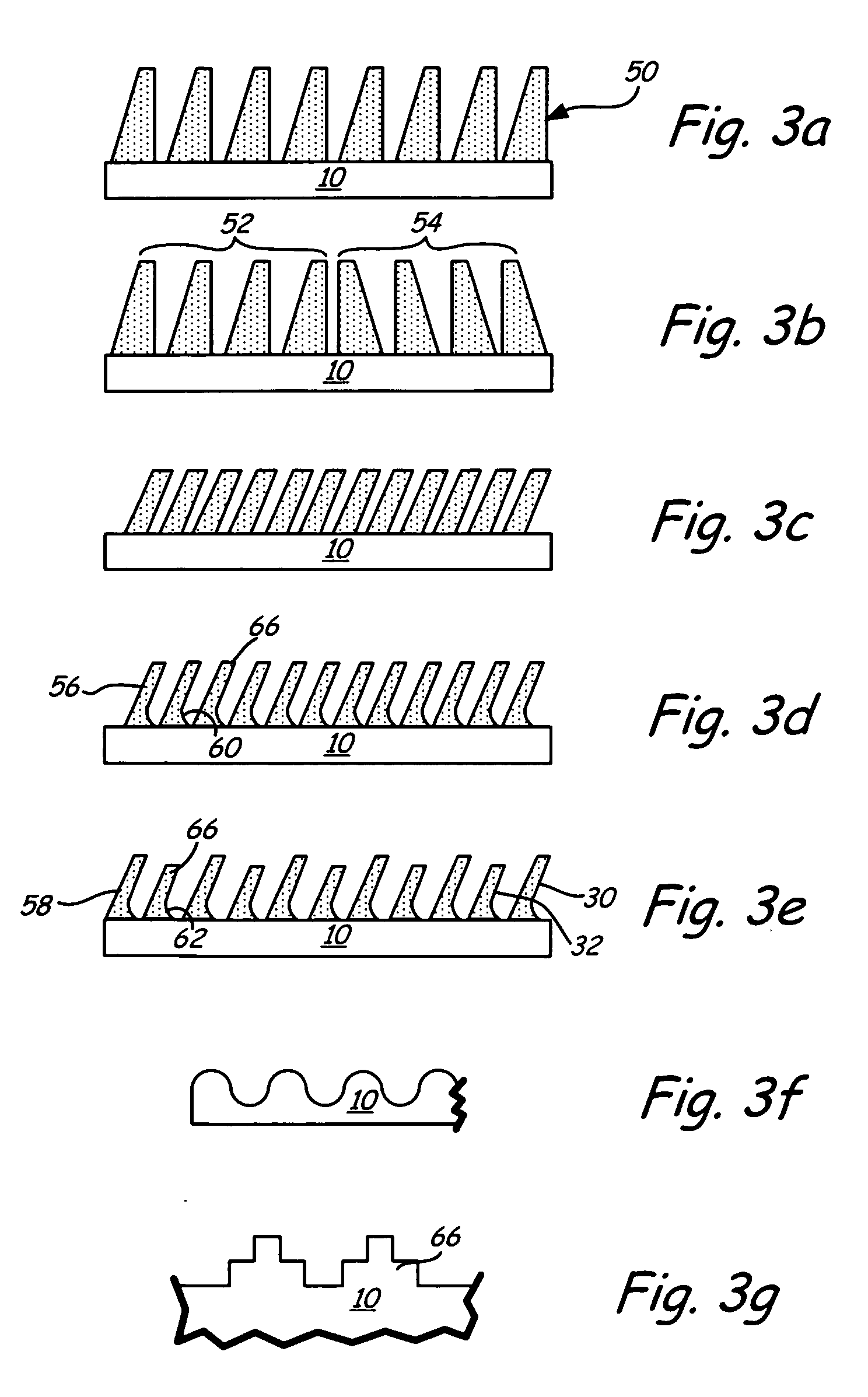
Bioimplant with nonuniformly configured protrusions on the load bearing surfaces thereof
ActiveUS20060149376A1Shorten the timeSaving in part set-upInternal osteosythesisBone implantEngineeringMechanical engineering
A bioimplant is configured with at least two load-bearing surfaces each having a plurality of protrusions oriented at an angle with respect to one another to resist translation in all directions when opposing load bearing surfaces are under normally applied compressive loads.
Owner:WARSAW ORTHOPEDIC INC
Stabilized bone graft
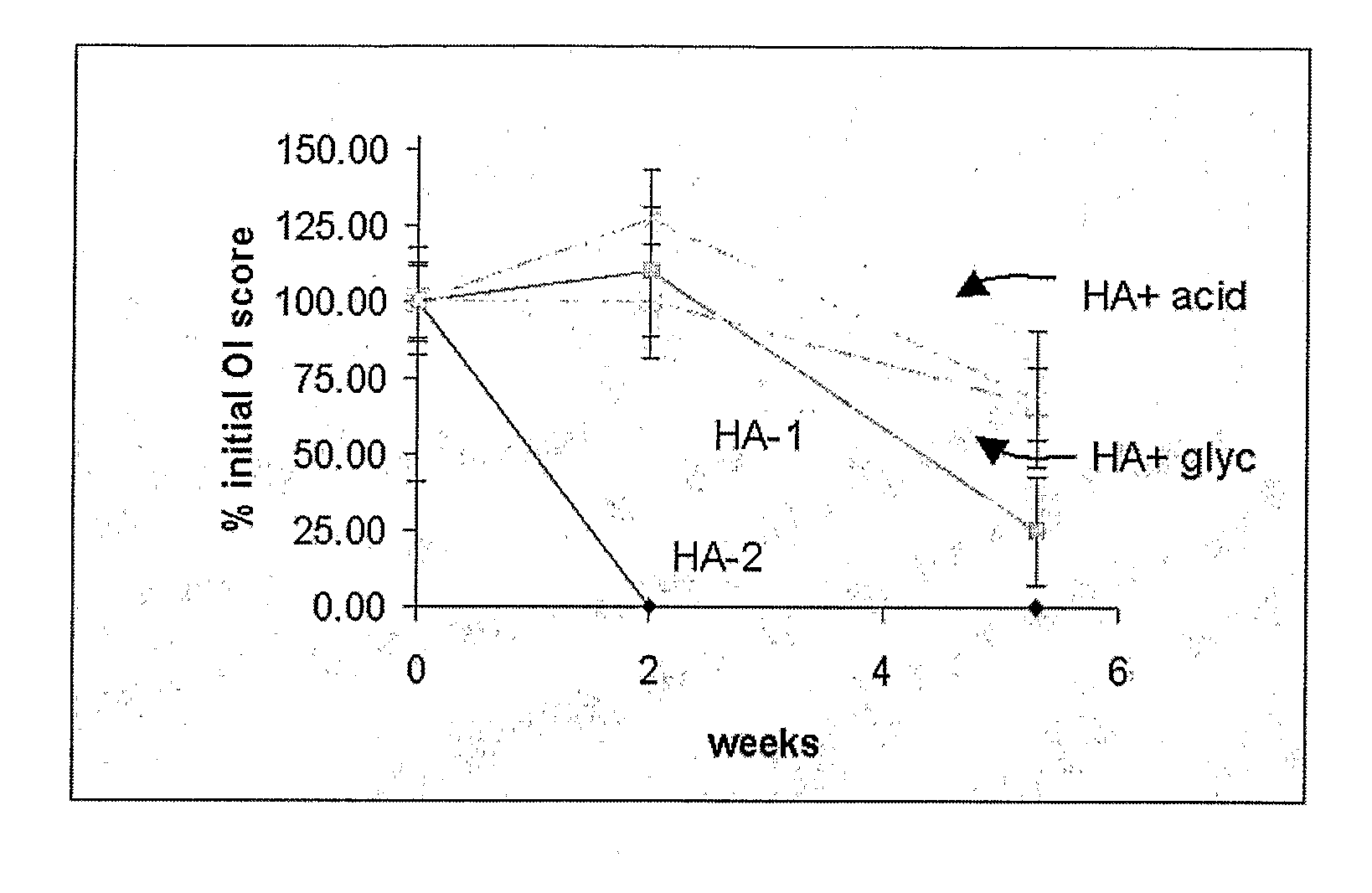
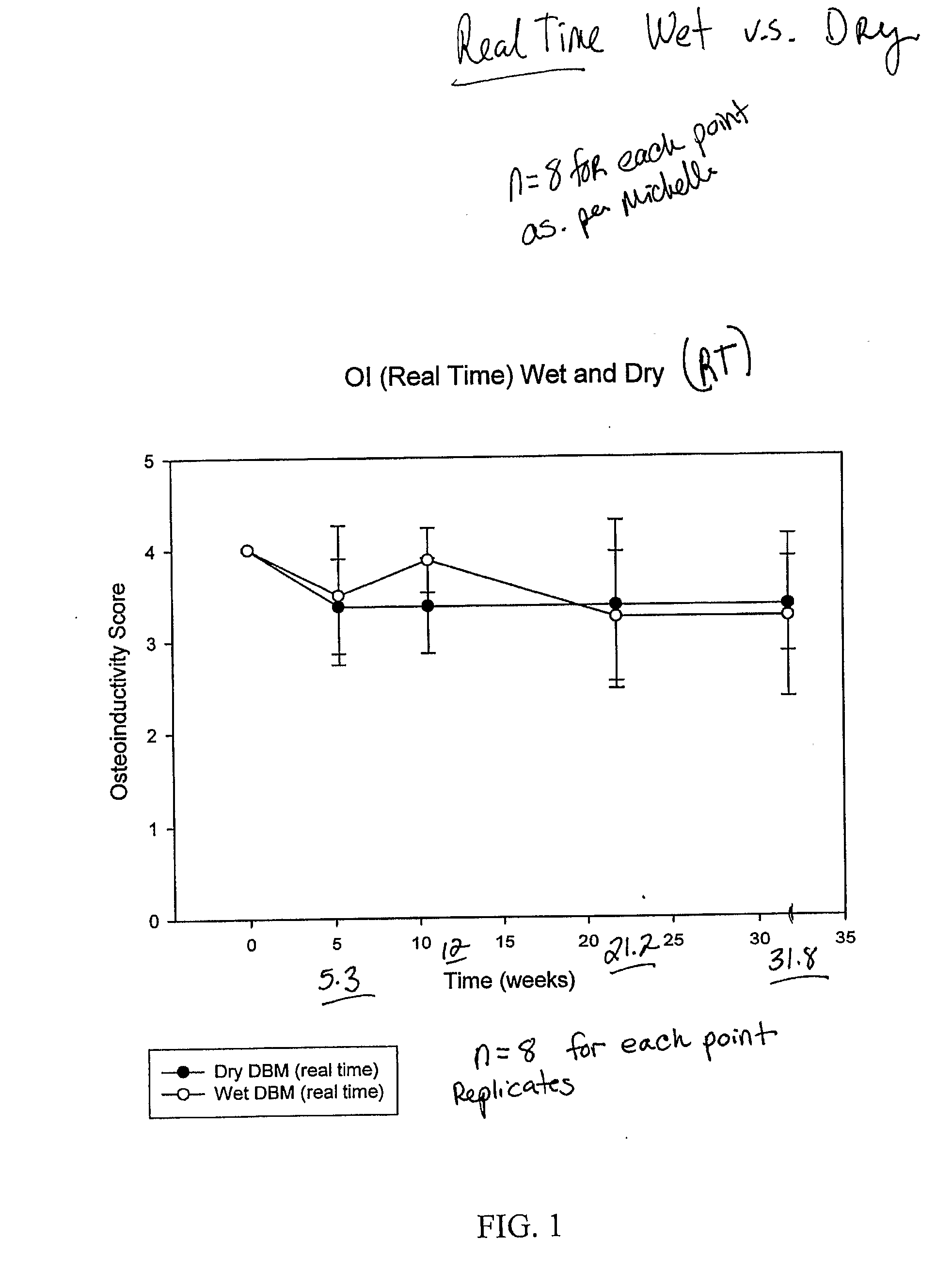
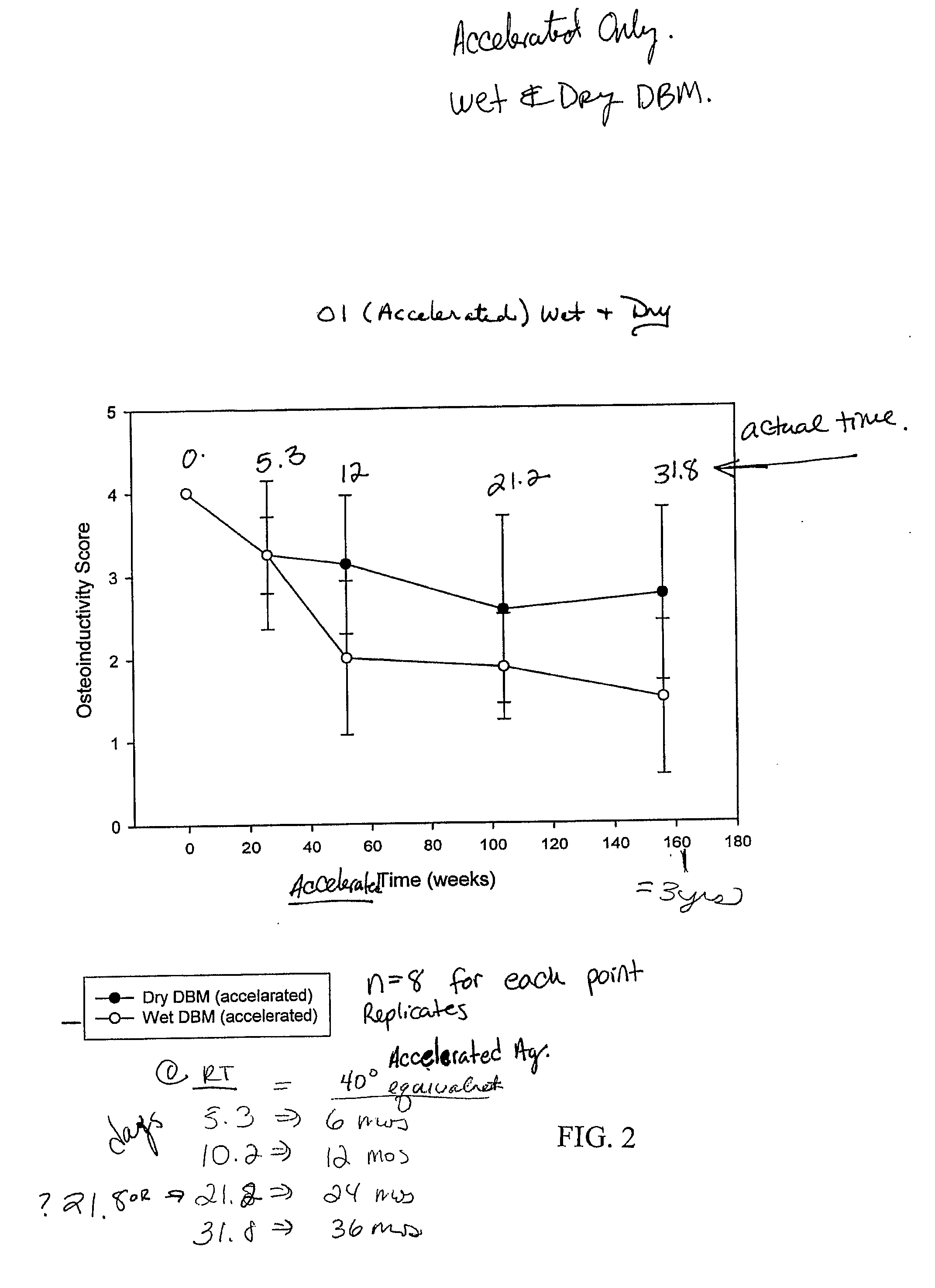
ActiveUS20070178158A1Good osteoinductivityImprove stabilityOrganic active ingredientsBiocideProteinase activityGlycerol
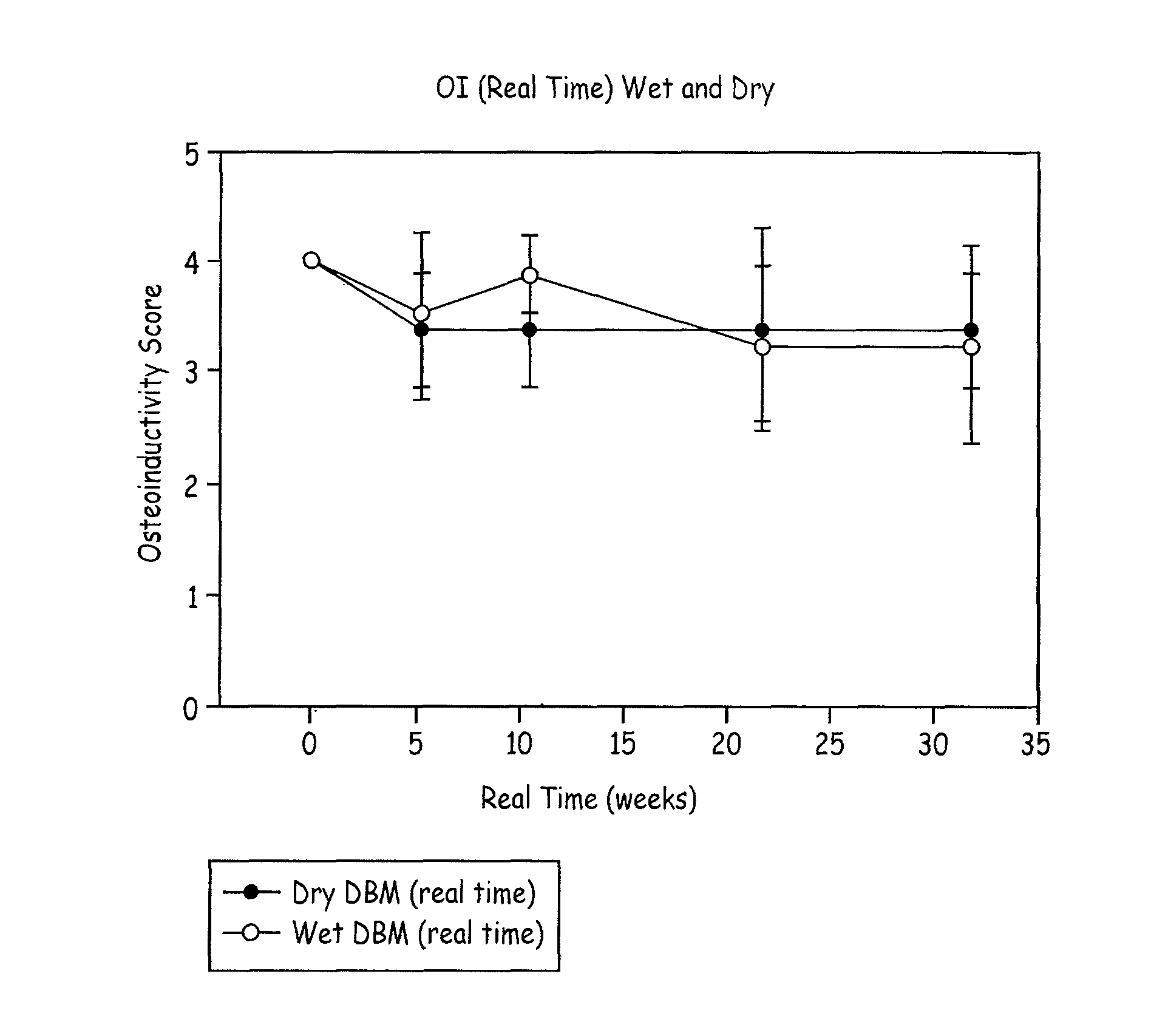
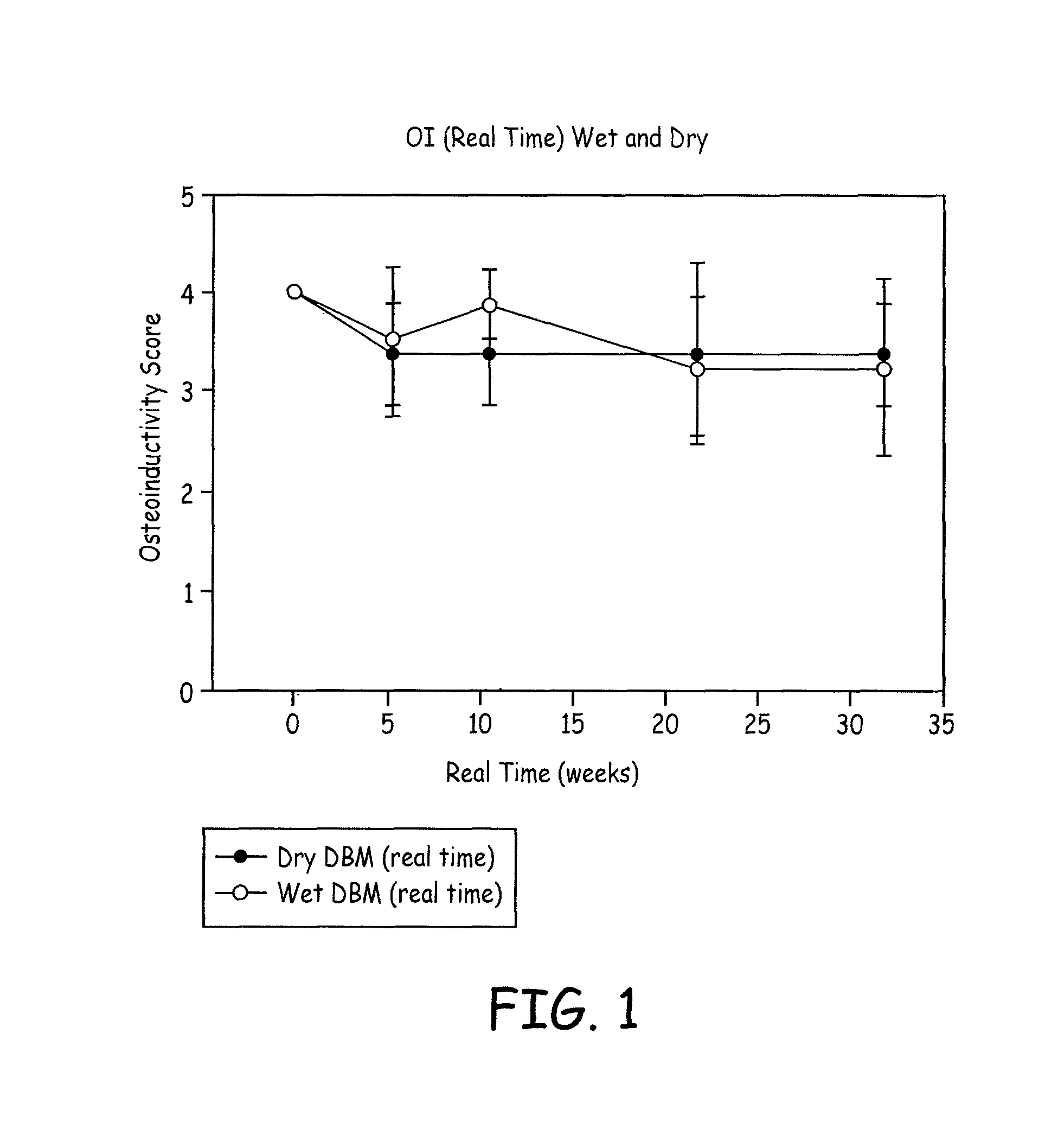
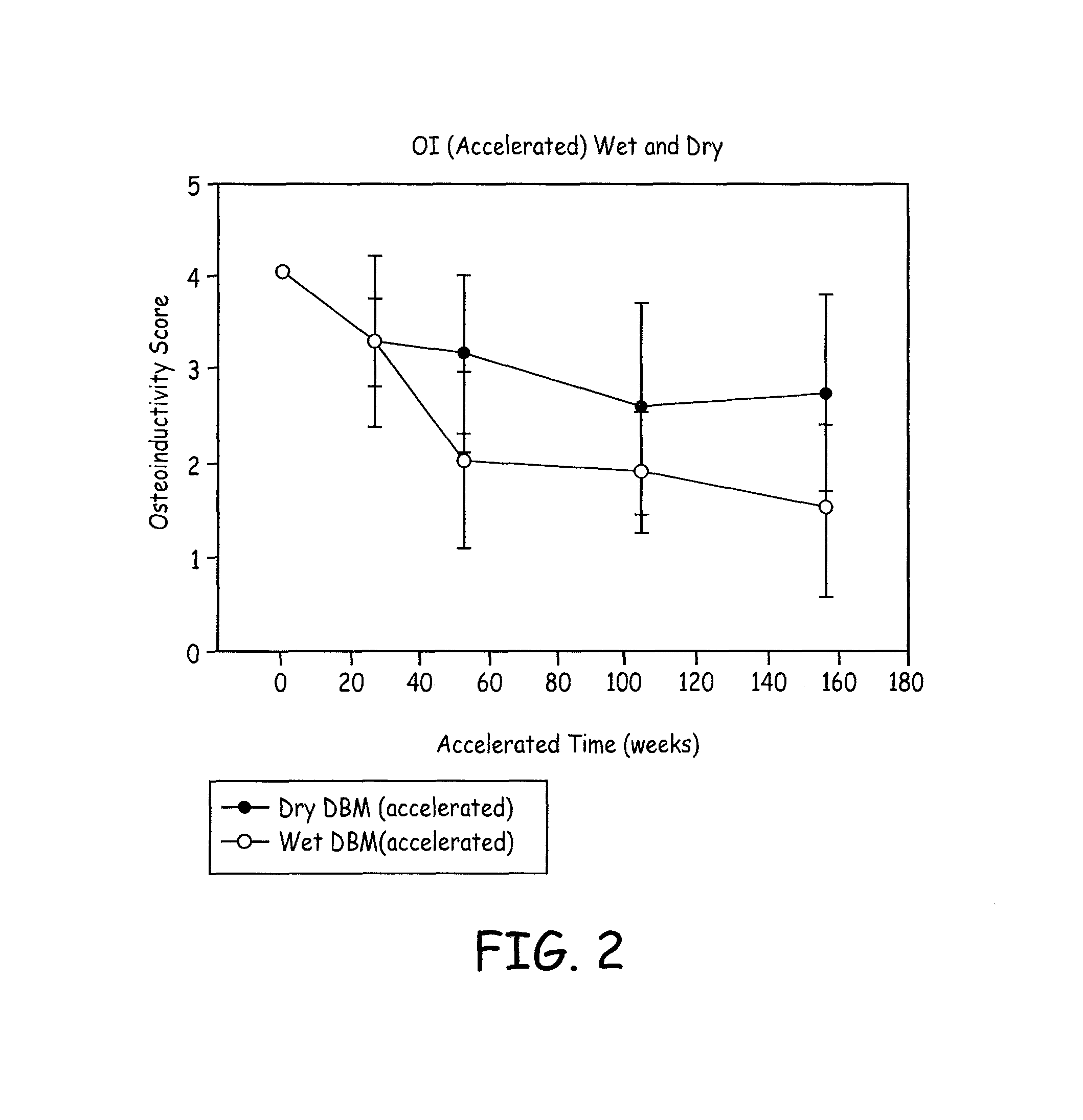
A demineralized bone matrix (DBM) or other matrix composition is provided that has been stabilized by lowering the pH of the composition, reducing the water content, adding water substitutes, and / or increasing the amount of deuterated water present in the composition in order to reduce the activity of endogenous degrading enzymes such as proteases. A hydrated form of a stabilized DBM composition may be stable up to a year at room temperature at acidic pH. The acidified DBM compositions may be further stabilized by the addition of a stabilizing agent such as deuterated water, water substitutes, polymers, protease inhibitors, glycerol, hydrogels, etc. The invention also provides methods of preparing, testing, and using the inventive stabilized osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
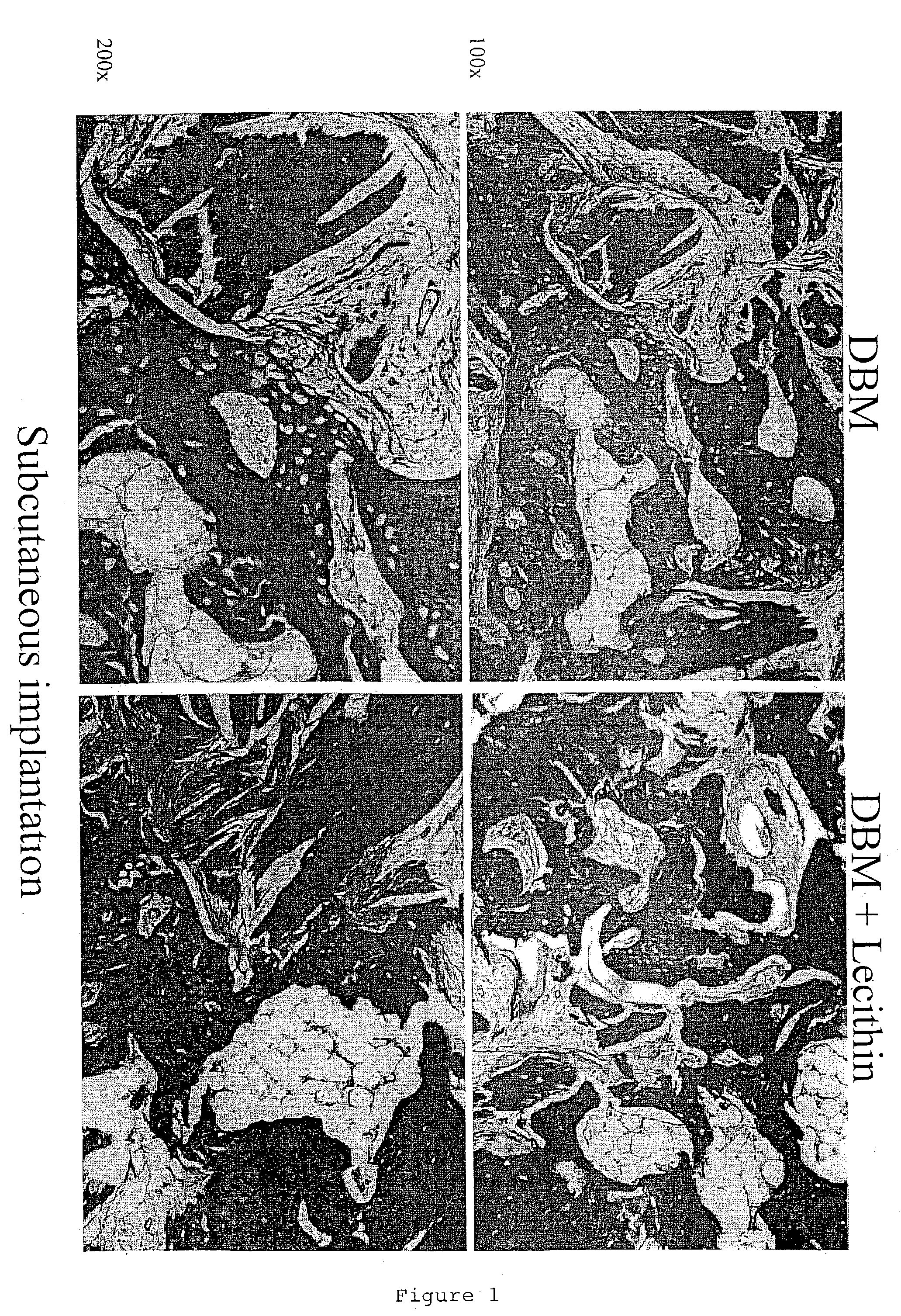
Bone graft material incorporating demineralized bone matrix and lipids
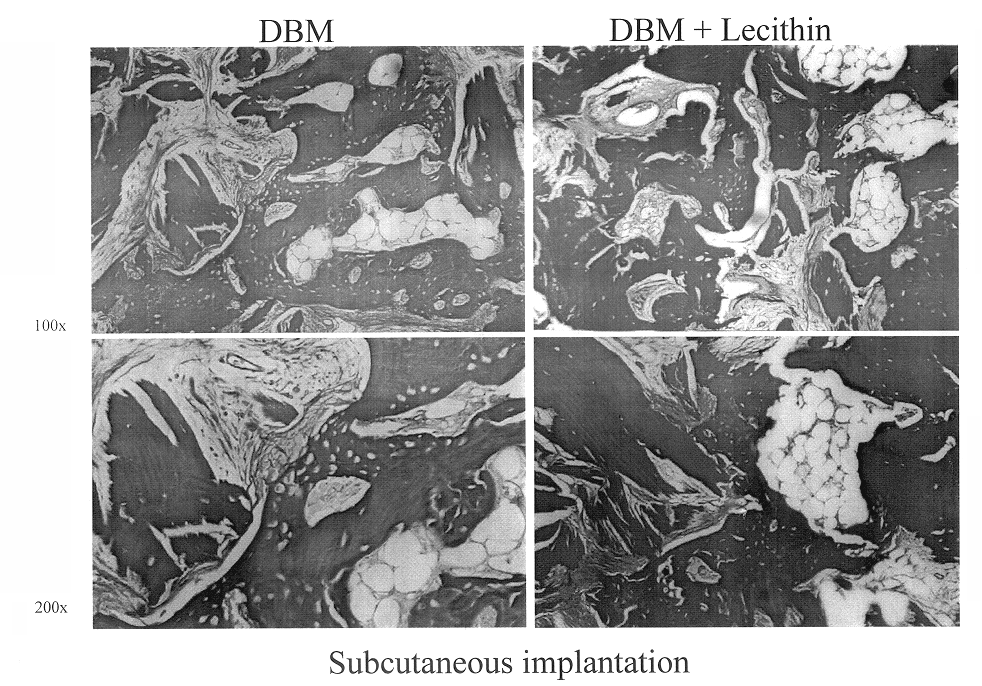
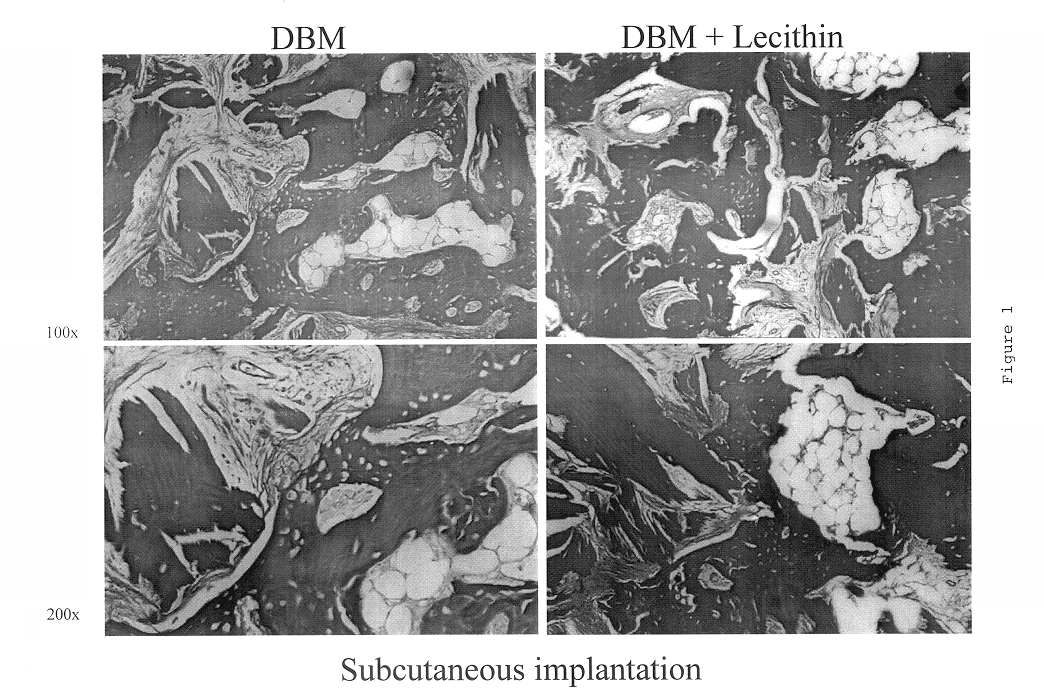
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
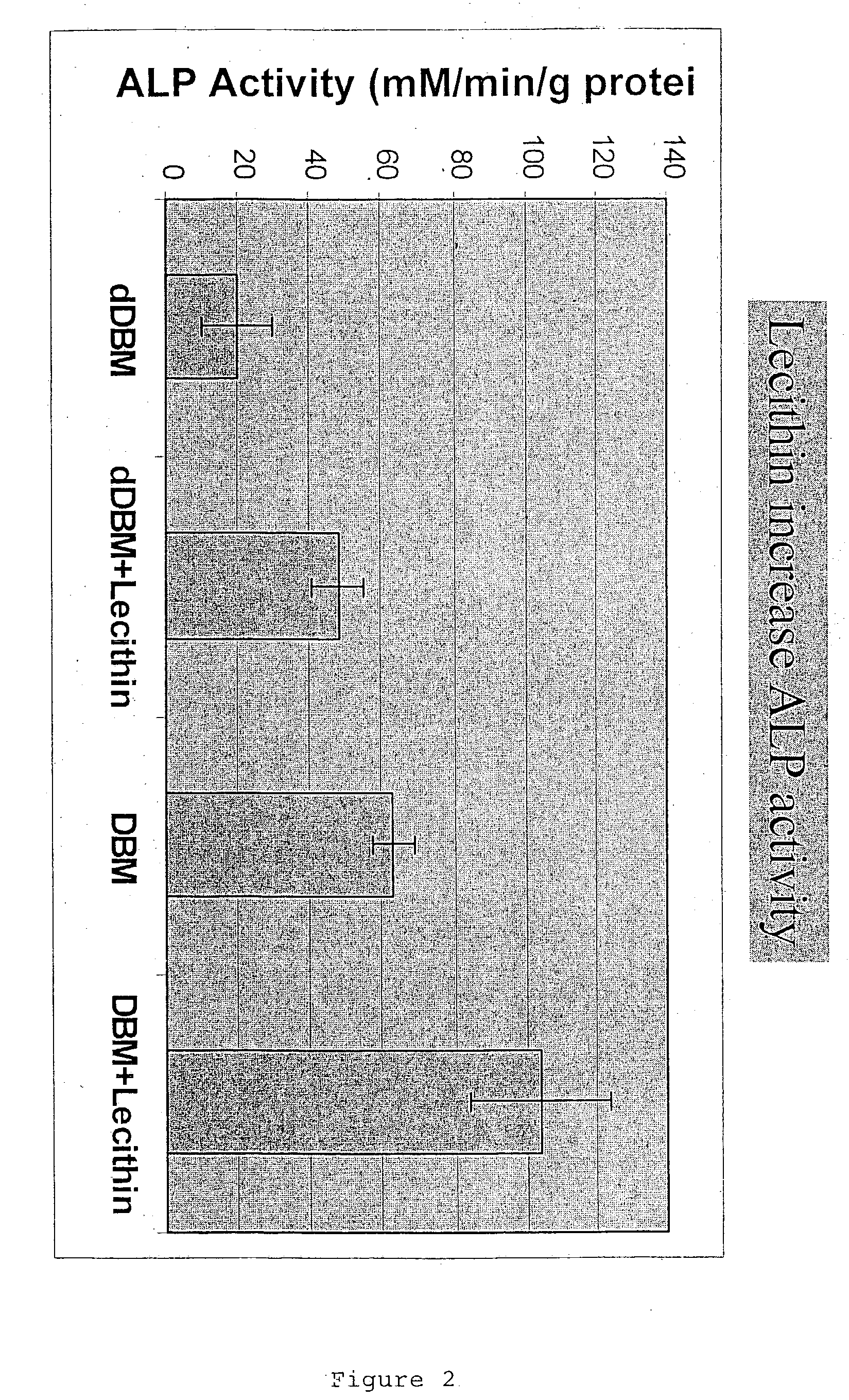
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS20030049326A1Augment its volume and biocompatibilityEfficient interactionBiocidePowder deliveryHydroxypropylmethyl celluloseBone graft materials
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Bracket material for bone tissue engineer and preparation method thereof
InactiveCN101417145AImprove mechanical propertiesGood biocompatibilityProsthesisDrug biological activityProtein C
The invention relates to a bracket material used for an osseous tissue project, and a preparation method thereof. Firstly, the bracket of a type I collagen is extracted from a small fresh pigskin by a mature extracting technique. Then, the bracket is further expanded in a Tris cushion liquid, the pH of which is equal to 8.8 to obtain a natural porous collagen bracket by the treatments of freezing and drying. The bracket is respectively and repeatedly mineralized in a CaCl2 liquid and in (NH4)2HPO4 liquid or mineralized in simulated body fluid for a long period to lead the weakly crystallized HA to be uniformly settled into the collagen bracket; and then a pigskin collagen-hydroxyapatite ossein is obtained by the treatments of freezing and drying to replace the natural bracket material. The invention not only maintains the natural bracket structure of the collagen in an organism, but also has the advantages of low material cost, simple devices, short period and easy operation. The obtained compound bracket material used for the pigskin collagen-hydroxyapatite osseous tissue project has the characteristics of high intensity, large toughness, non-antigenicity, higher biological activity as well as degradation and releasing control.
Owner:SHANDONG UNIV
Bone restoration body with composite porous structure and preparation method thereof
InactiveCN102512267AGood mechanical compatibilityGood bone conductionBone implantFreeze-dryingReticular formation
A bone restoration body with a composite porous structure and a preparation method of the bone restoration body. The bone restoration body comprises a porous metal bracket and an infill body with a porous structure, wherein the porous metal bracket is of a three-dimensional net structure, a plurality of pores are arranged in the inner part of the porous metal bracket, and the infill body with the porous structure is fully filled in all the pores. The preparation method combines the direct metal rapid prototyping technology and the freeze drying technology and comprises the steps of preparing the porous metal bracket by a structural design and the direct metal rapid prototyping technology, pouring uniformly-mixed polymer solution or polymer / biological ceramics mixing solution into the porous metal bracket, carrying out freezing treatment, and then forming the infill body with the porous structure through freeze drying so as to obtain the bone restoration body with the composite porous structure, wherein the infill body with the porous structure has micropore characteristics. The bone restoration body has good mechanics compatibility, can obtain good bone conduction performance and bone induction performance, improves bone integration efficiency and can be used for clinical treatment of segmental bone defect of a bearing part.
Owner:SHANGHAI JIAO TONG UNIV
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS20040091459A1Good osteoinductivityEasy to operateBiocidePowder deliveryAntioxidantMaterials science
One embodiment of the invention is demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, insoluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose, a methylcellulose derivative, carboxymethyl cellulose, or hydroxypropyl methylcellulose. A second embodiment of the invention is a demineralized bone paste composition comprising: (1) about 15% to about 75% of an emulsion carrier, such as an aqueous phase; and (2) a bone-material-containing phase comprising: (a) demineralized bone matrix (DBM); and (b) an emulsifier component that is compatible with lipids. This bone paste composition is moldable, biocompatible, slowly resorbable, miscible with bone graft materials, soluble or partially soluble in tissue fluids, and extrudable.
Owner:BIOMET MFG CORP
Non-glycerol stabilized bone graft
ActiveUS8333985B2Slows proteolysisExtended shelf lifeBiocideOrganic active ingredientsProteinase activityGlycerol
A demineralized bone matrix (DBM) or other matrix composition is provided that has been stabilized by lowering the pH of the composition, reducing the water content, adding water substitutes, and / or increasing the amount of deuterated water present in the composition in order to reduce the activity of endogenous degrading enzymes such as proteases. A hydrated form of a stabilized DBM composition may be stable up to a year at room temperature at acidic pH. The acidified DBM compositions may be further stabilized by the addition of a stabilizing agent such as deuterated water, water substitutes, polymers, protease inhibitors, glycerol or hydrogels.
Owner:WARSAW ORTHOPEDIC INC
Method for polylactic acid high polymer material surface modified hydroxyapatite coating
InactiveCN102504311AAchieve chemical bondingGood osteoinductivityCoatingsProsthesisPolymer scienceApatite
The invention discloses a method for a polylactic acid high polymer material surface modified hydroxyapatite coating, and aims to design a method for the polylactic acid high polymer material surface modified hydroxyapatite coating, which has the advantages of simple and feasible preparation method, low cost and suitability for surface modification of various degradable polylactic acid high polymer material scaffolds. The method for the polylactic acid high polymer material surface modified hydroxyapatite coating comprises the following steps of: soaking a polylactic acid high polymer material with the molecular weight of 10,000-200,000 in an activating solution for activating, covalently introducing -OH, -COOH or -NH2 active groups onto the surface, soaking the activated polylactic acid high polymer material in a Ca<2+> ionic solution, soaking in a PO4<3-> alkaline solution, and repeatedly circulating the steps for 3-50 times to grow a layer of uniform and compact hydroxyapatite coating on the surface of the material finally. The method is environment-friendly, has the advantages that the coating preparation period is short and the cost is low, and is suitable for preparing degradable biological material hydroxyapatite coatings.
Owner:HENAN NORMAL UNIV
Bone tissue regeneration guiding membrane and preparation method thereof
A bone tissue regeneration guiding membrane. The invention employs an antigen-extracted mammal tissue membrane, which has a double layer structure of a compact layer and a loosening layer. The compact layer is formed by tightly arranged collagen fibers, and an external side of the compact layer is composited with a collagen coating containing active polypeptides; the loosening layer is formed by interlaced collagen fibers and is composited with ossification active factors. The prepared guiding membrane can prevent soft tissue from evolving and promote new bone formation, has good biological compatibility, hydrophilism and bone induction capability, and can be completely degraded in body without generation of harmful substances. Besides, the guiding membrane has an effect of isolating cell evolving, a characteristic of slow degradation and a longer retaining time in body than a current guiding membrane prepared from natural high-molecular polymer, and can provide enough time and space for new bone growth. The method of the invention is simple, and raw materials have wide sources and low prices, so that the method is suitable for large-scale industrial production and beneficial to lowering medical costs and mitigating patient burdens.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
Method for preparing biological composite coating on surface of magnesium-based material
ActiveCN102268711AGood biocompatibilityImprove biological activityAnodisationProsthesisMicro arc oxidationPlasma electrolytic oxidation
The invention discloses a method for preparing a biological composite coating on the surface of a magnesium-based material, and the method comprises the following steps of: after the magnesium-based material is pre-treated, placing the magnesium-based material in an electrolyte containing Na3PO4 and Ca(NO3)2 for micro-arc oxidation treatment to obtain a ceramic film layer; then placing the material with the micro-arc oxidation film layer in an electrodeposition solution to carry out electrodeposition reaction at a constant voltage for 0.1-2 hours, wherein the electrodeposition solution comprises 0.1 mol / L Ca(NO3)2, 0.06 mol / L NH4H2PO4, 0.04 mol / L NaNO3 and 3-5% chitosan solution, and the pH value is adjusted to be 3.8-4.9; and after electrodeposition, taking out the material, rinsing and drying. In the method disclosed by the invention, chitosan is added to calcium phosphate ceramic coated on the micro-arc oxidation film layer, thereby the structure of the calcium phosphate deposited layer can be significantly changed, and ultimately a calcium phosphate / chitosan composite and hybrid is formed. The obtained biological composite coating has excellent corrosion resistance, biological activity and compatibility as well as good adhesion with the magnesium metal substrate, and can be used as a new bone substitute material.
Owner:BEIJING JINGCI PERMANENT MAGNETIC TECH DEV CO LTD
Polydopamine-modified halloysite nanotube / polylactic acid composite material and preparation and application thereof
ActiveCN105566872ASolving Dispersion ProblemsSolve interface compatibility issuesMonocomponent polyesters artificial filamentArtifical filament manufactureHydroxyapatite crystalNanotube
The present invention discloses a polydopamine-modified halloysite nanotube / polylactic acid composite material and preparation and application thereof. The polydopamine-modified halloysite nanotube / polylactic acid composite material comprises 0.05 to 60% by mass of polydopamine-modified halloysite nanotubes and 40 to 99.95% by mass of polylactic acid. By surface modification of the halloysite nanotubes, dispersion of the halloysite nanotubes in a polylactic acid matrix and interface compatibility of the halloysite nanotubes and the polylactic acid matrix can be solved, and the polylactic acid matrix can be effectively enhanced by the halloysite nanotubes; excellent cell affinity and osteogenic activity can be given to the polydopamine-modified halloysite nanotube / polylactic acid composite material, and more significantly, by the use of biological mineralization of a polydopamine layer on the surface of the halloysite nanotubes for formation of hydroxyapatite crystals, eventually good osteo inductivity can be given to the polydopamine-modified halloysite nanotube / polylactic acid composite material. The polydopamine-modified halloysite nanotube / polylactic acid composite material is simple in preparation method, mild in reaction conditions, low in price, and suitable for industrial production.
Owner:JINAN UNIVERSITY
Porous, controllable and low-modulus bone defect repair bracket and preparation method thereof
ActiveCN103751840APromote ingrowthSolve the interface stress problemElectrolytic inorganic material coatingProsthesisApatiteRapid prototyping
The invention discloses a porous, controllable and low-modulus bone defect repair bracket which consists of a porous, controllable and low-modulus titanium alloy bracket and a biological activity coating on the surface of the titanium alloy bracket. The invention further discloses a preparation method of the bone defect bracket. The method comprises the steps that the low-modulus and porous titanium alloy bracket in a controllable internal microstructure is prepared by a quick forming technique; a uniform hydroxyapatite coating and a strontium doped hydroxyapatite coating are prepared on the surface of the bracket in the controllable microstructure; an electrolyte with certain concentration is prepared; a potential is controlled in an electrolytic bath of a three-electrode system; and pulse electrochemical deposition is performed. The elastic modulus of the prepared titanium alloy bracket is equivalent to that of a bone; the prepared hydroxyapatite coating or strontium doped hydroxyapatite coating is combined with a titanium alloy well; the concentration of strontium is controllable; and an implant provided with the biological activity coating can be used for a bone defect of a massive bearing part clinically.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Silk fibroin base integrated osteochondral two-layer bracket, preparation and application thereof
ActiveCN103028145AImprove adhesionNutrient penetrationSurgeryCoatingsDipotassium phosphatePhosphoric acid
The invention discloses a silk fibroin base integrated osteochondral two-layer bracket, preparation and application thereof. The preparation of the silk fibroin base integrated osteochondral two-layer bracket is carried out by the following steps of: respectively soaking parts of 1 / 2-3 / 4 height of a three-dimensional silk fibroin bracket in a calcium chloride ethanol solution, absolute ethyl alcohol, a dipotassium phosphate aqueous solution and de-ionized water; repeating the above operation for 1-15 times, taking the silk fibroin bracket finally soaked by the de-ionized water to soak in the calcium chloride ethanol solution for 5-30 min and subsequently using the de-ionized water to soak the silk fibroin bracket for 3-10 min and pre-calcify the three-dimensional silk fibroin bracket; then soaking the pre-calcified part of the three-dimensional silk fibroin bracket in a bionic calcium ion buffer solution and culturing for 4-8 days at a constant temperature of 37 DEG C to obtain the silk fibroin base integrated osteochondral two-layer bracket. In the pre-calcification process of the invention, the silk fibroin bracket can obtain a nucleating locus of hydroxyapatite crystals; and evenly dispersed weak crystallization nano hydroxyapatites are formed on the surfaces of the porous channels of the bracket by culturing the bracket in the bionic calcium ion buffer solution.
Owner:ZHEJIANG UNIV
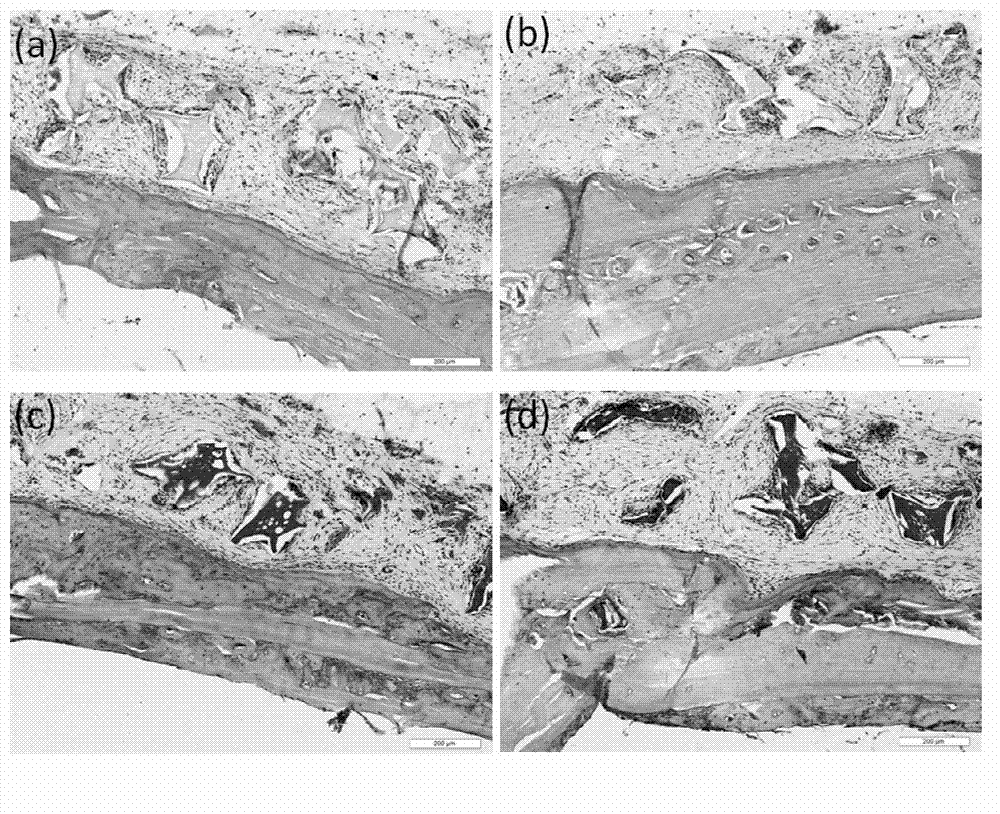
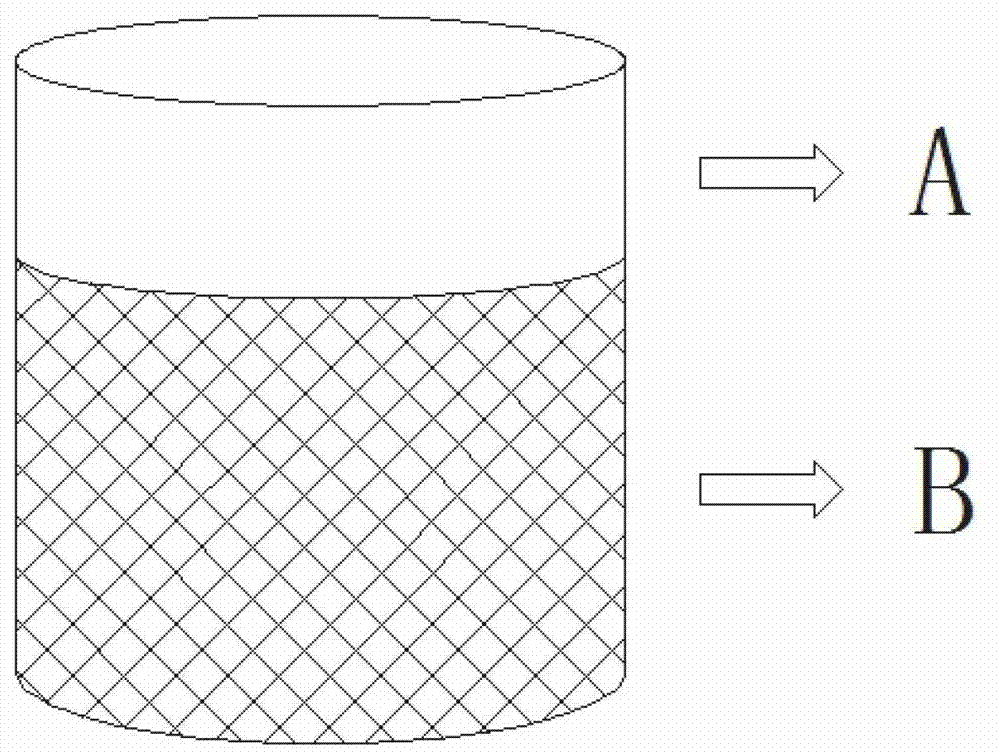
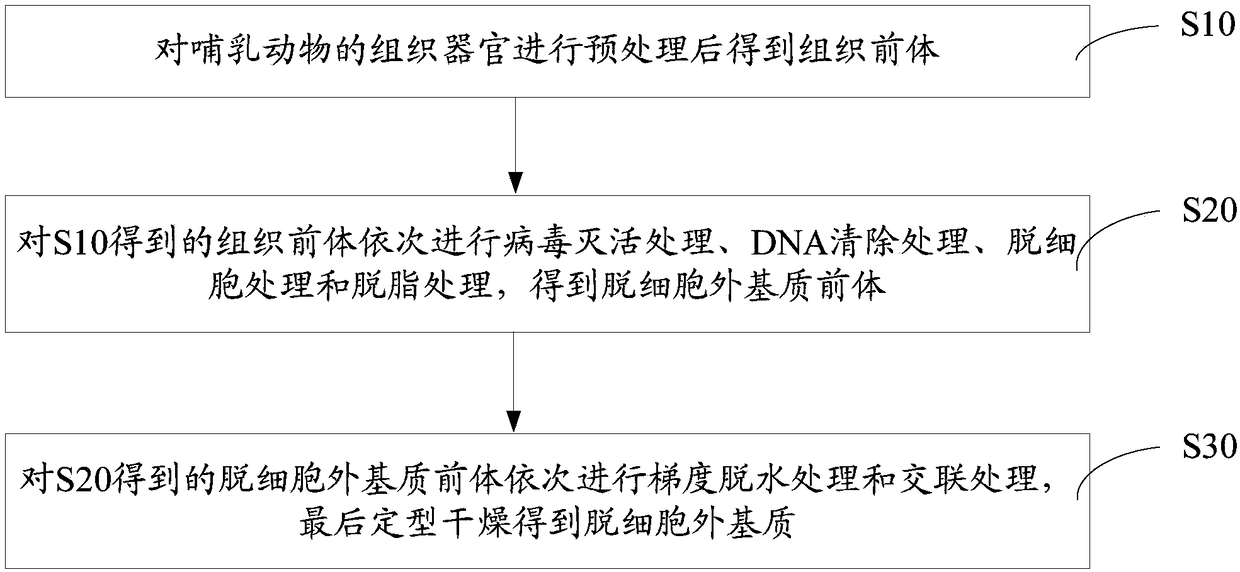
Accellular epimatrix and preparation method and application thereof
InactiveCN108261564AGood flexibilityGood biocompatibilityTissue regenerationProsthesisVirus inactivationCollagen fiber
The invention discloses a preparation method of an accellular epimatrix, the accellular epimatrix prepared by the preparation method and application of the accellular epimatrix in the field of preparation of medical instruments. The preparation method of the accellular epimatrix comprises the following steps: carrying out pretreatment on tissues and organs of mammals to obtain an analogy tissue precursor; separately carrying out virus inactivation treatment, DNA eliminating treatment, accellular treatment and defatting treatment on the analogy tissue precursor to obtain an accellular epimatrixprecursor; and successively carrying out gradient dewatering treatment and crosslinking treatment on the accellular epimatrix precursor, and finally shaping and drying to obtain the accellular epimatrix. By the gradient dewatering treatment, the accellular epimatrix precursor is soaked in organic matter solutions of which the concentrations are successively increased successively. According to the preparation method of the accellular epimatrix, the tissue precursor is treated through gradient dewatering, and thus, the orderly structure of collagenous fibers of the finally prepared accellularepimatrix is not damaged.
Owner:SHENZHEN LANDO BIOMATERIALS
Bone regeneration materials based on combinations of monetite and other bioactive calcium and silicon compounds
InactiveUS20120058152A1Promote bone regenerationImprove osteoconductivityBiocideInorganic phosphorous active ingredientsDental surgeryCalcium silicate
The present invention incorporates new materials for bone regeneration, methods for their manufacture, and application in traumatology surgery, maxillo facial surgery, dental surgery, orthognatic surgery, endodontics, ophthalmology, neurosurgery and / or osteoporotic processes, and other indications where bone regeneration is required. In particular, the present invention incorporates synthetic materials with a 20% to a 95%, preferably between 40% and 90% in mass of monetite [Ca1-XMXHPO4, where 0≦x≦0.05, and where M can be a divalent metallic ion], and which in their final composition incorporate between 5% and 80%, preferably between 0% and 60%, in mass of bioactive calcium compounds chosen from calcium phosphates and between 5% and 80% in total mass of bioactive silicon compounds chosen from calcium silicates and / or bioactive silica glasses and gels.
Owner:HELICON MEDICAL SL
Bone regeneration materials based on combinations of monetite and other bioactive calcium and silicon compounds
InactiveUS8506985B2Promote bone regenerationImprove osteoconductivityTissue regenerationProsthesisCalcium silicateDental surgery
The present invention incorporates new materials for bone regeneration, methods for their manufacture, and application in traumatology surgery, maxillofacial surgery, dental surgery, orthognatic surgery, endodontics, ophthalmology, neurosurgery and / or osteoporotic processes, and other indications where bone regeneration is required. In particular, the present invention incorporates synthetic materials with a 20% to a 95%, preferably between 40% and 90% in mass of monetite [Ca1-XMXHPO4, where 0≦x≦0.05, and where M can be a divalent metallic ion], and which in their final composition incorporate between 5% and 80%, preferably between 0% and 60%, in mass of bioactive calcium compounds chosen from calcium phosphates and between 5% and 80% in total mass of bioactive silicon compounds chosen from calcium silicates and / or bioactive silica glasses and gels.
Owner:HELICON MEDICAL SL
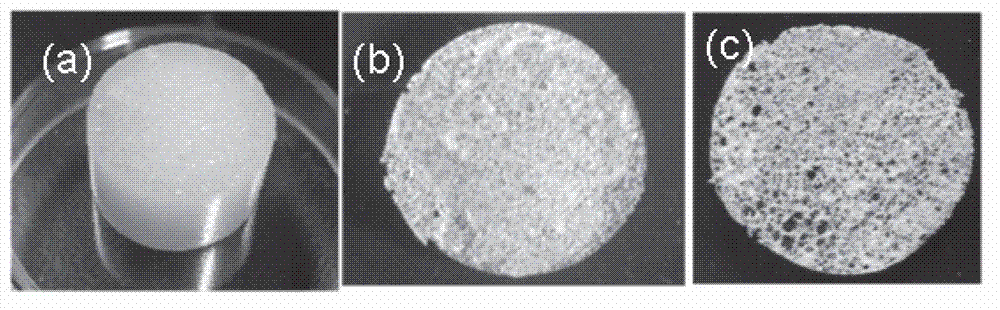
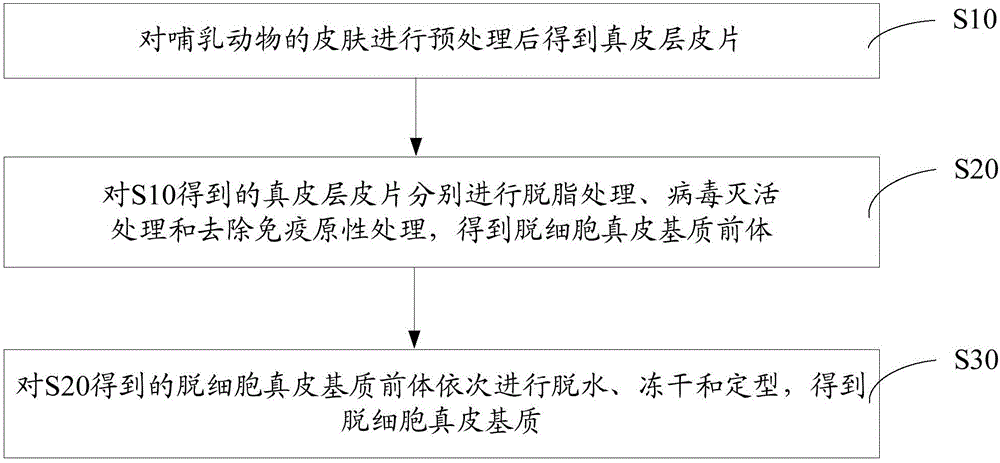
Acellular dermal matrix and preparing method of acellular dermal matrix
The invention discloses an acellular dermal matrix and a preparing method of the acellular dermal matrix. The preparing method of the acellular dermal matrix comprises the following steps of: performing pre-processing on the skin of a mammal to obtain a skin corium flap; respectively performing degreasing processing, virus inactivation processing and immunogenicity removal processing on the skin corium flap to obtain an acellular dermal matrix precursor (the immunogenicity removal processing operation comprises the step of alternately soaking the skin corium flap into a prolease solution and a surfactant solution); and sequentially performing dewatering, freeze-drying and shaping on the acellular dermal matrix precursor to obtain the acellular dermal matrix. The preparing method of the acellular dermal matrix has the advantages that the skin corium flap is alternately soaked in the prolease solution and the surfactant solution, so that the immunogenicity of the acellular dermal matrix can be reduced to the maximum degree; the normal collagen structure of the acellular dermal matrix is also remained; the prepared acellular dermal matrix has the proper degradation speed, good biocompatibility and good bone induction capability.
Owner:SHENZHEN LANDO BIOMATERIALS
Mixed porous structure interbody fusion cage and preparation method thereof
InactiveCN102440852AGood mechanical compatibilityGood bone conductionSpinal implantsFreeze-dryingReticular formation
Disclosed are a mixed porous structure interbody fusion cage and a preparation method thereof. The interbody fusion cage comprises a porous metal support and porous structure filling bodies, the porous metal support is a three-dimensional net-shaped structure, a plurality of holes are arranged in the porous metal support, and the porous structure filling bodies are fully filled in the holes. The preparation method includes steps that the metal rapid forming technology is directly combined with the freeze drying technology, the porous metal support is manufactured via a structural design and the direct metal rapid forming technology, then uniformly mixed polymer gel or polymer / biological ceramic compound gel is poured in the porous metal support to realize freeze treatment, so that the porous structure filling bodies with the micropore feature are formed after freeze drying, and the mixed porous structure interbody fusion cage is obtained. Mechanical compatibility is good, contact area between the mixed porous structure interbody fusion cage and natural centrum is further increased, instant stability is good, fusion rate is improved, and the mixed porous structure interbody fusion cage and the preparation method thereof can be used for treating clinical degenerative disc diseases.
Owner:SHANGHAI JIAO TONG UNIV
Periodontal tissue regeneration using composite materials comprising phosphophoryn
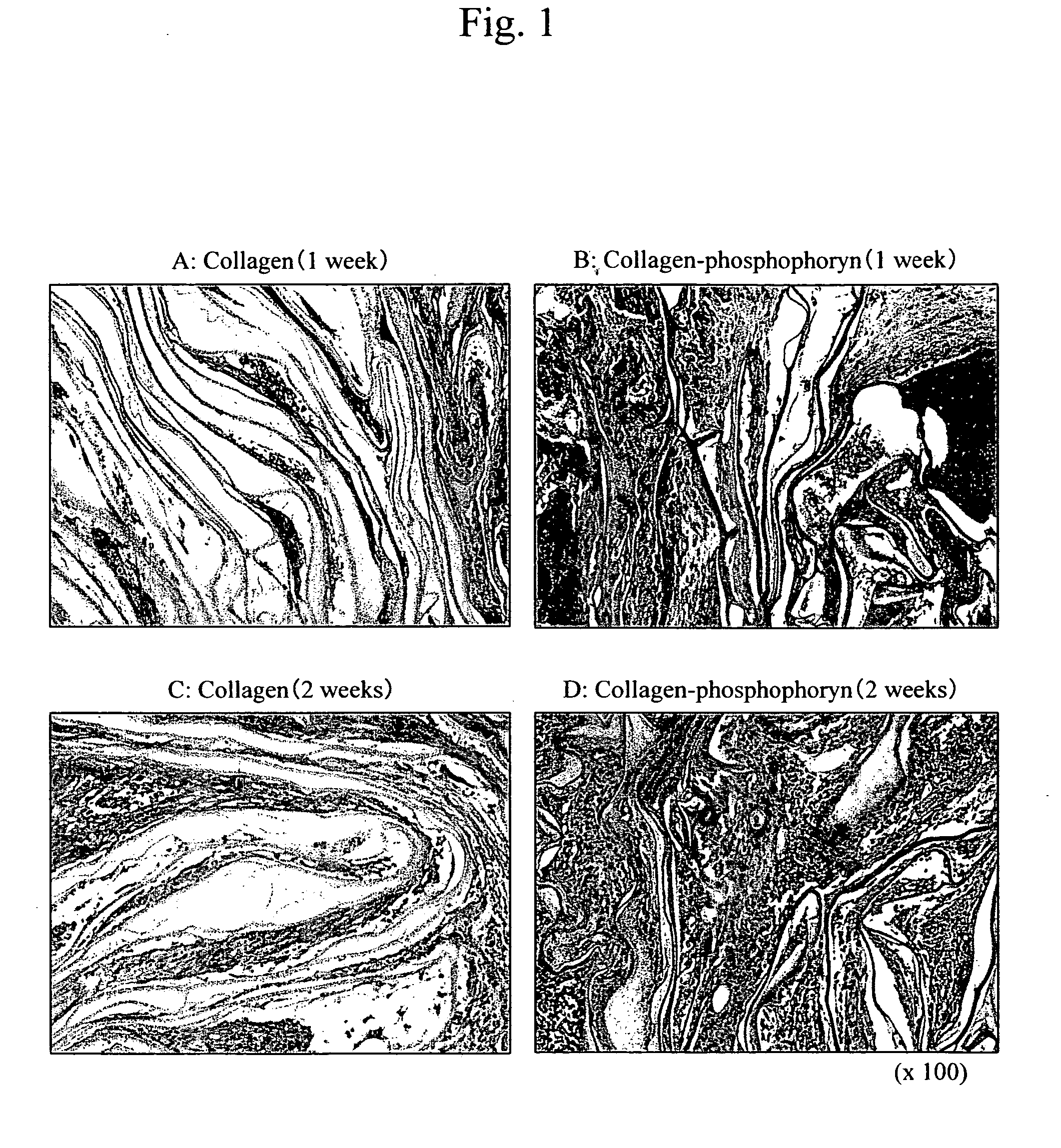
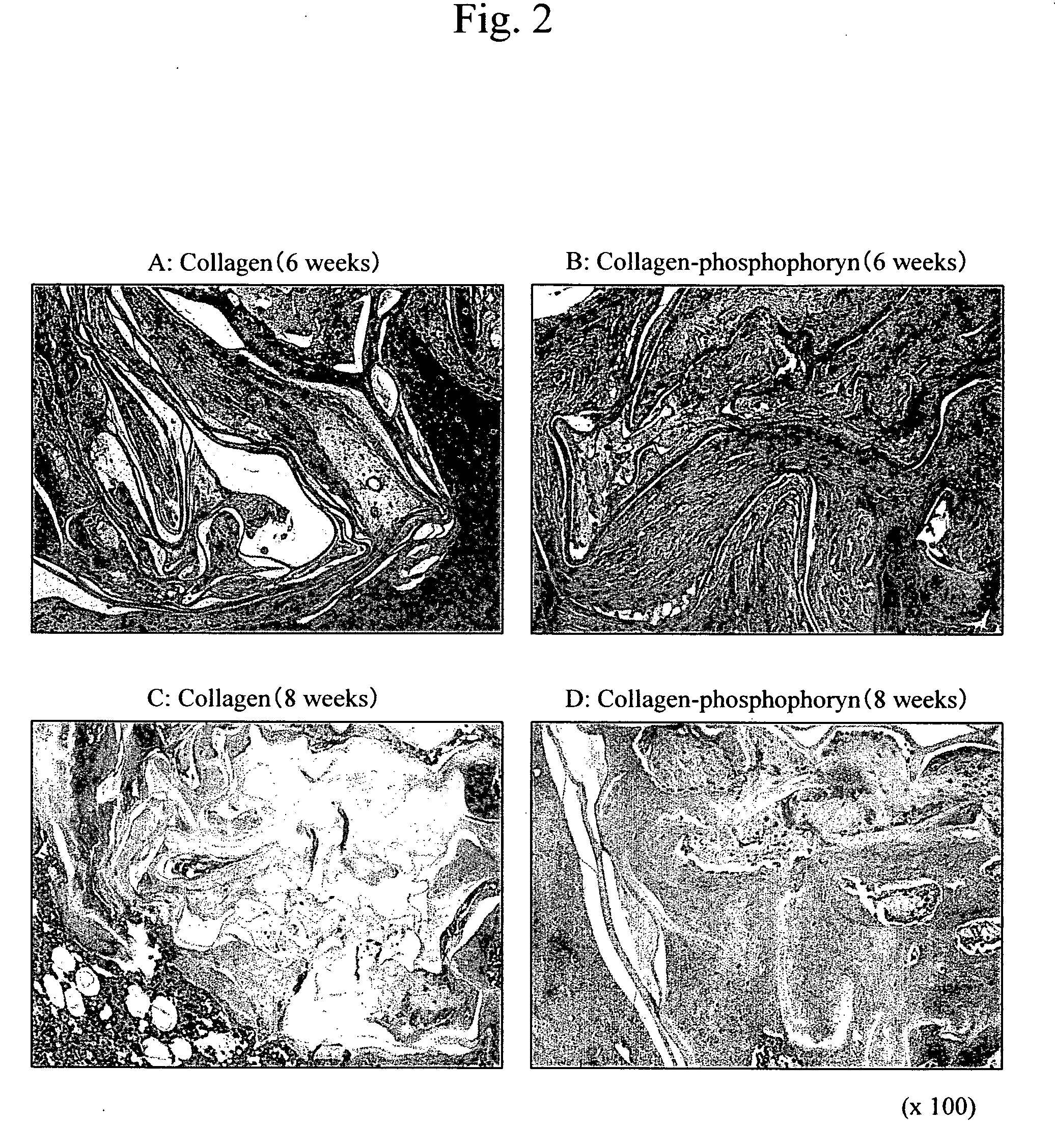
InactiveUS20060188544A1Easy to shapeCost-effectiveVertebrate cellsArtificial cell constructsPeriodontal tissueSponge
This invention relates to composite biomaterials having a sponge-like structure and comprising phosphophoryn and collagen, and to periodontal tissue regeneration.
Owner:SAITO TAKASHI
Composite bone cement and preparation method thereof
ActiveCN105536070AEasy injectionImprove mechanical propertiesTissue regenerationProsthesisMass ratioHigh pressure
The invention relates to composite bone cement for repairing bone tissues and a preparation method thereof. The bone cement is prepared from the following components in percentage by mass: 90-99.9 percent of calcium sulfate / calcium phosphate composite and 0.1-10 percent of additives. The method comprises the following steps: uniformly mixing raw materials, drying in an oven at 70 DEG C, grinding, and performing a crystal transformation reaction in a high-pressure kettle at a temperature of 120-200 DEG C and a pressure of 0.1-1MPa for 3-8 hours; after reaction, drying in an oven at 80-100 DEG C for 12 hours, and uniformly grinding to obtain bone cement powder; and weighing the bone cement powder, adding the powder into a liquid in a powder-liquid mass ratio of (1-3):1, uniformly stirring, and curing to obtain the bone cement. The prepared bone cement has the advantages of high tensile strength, good injection performance, good water scattering resistance, controllable degradation and the like, has excellent biological activity, compatibility and safety, and is used for fixing multiple bone fractures and filling bone defects.
Owner:山东明德生物医学工程有限公司
Medical porous polyether-ether-ketone with drug loading function and preparing method and application thereof
ActiveCN106178104AGood mechanical propertiesExcellent bone transportTissue regenerationProsthesisPorosityPressure casting
The invention relates to medical porous polyether-ether-ketone with a drug loading function and a preparing method and application thereof. The medical porous polyether-ether-ketone has a uniform porous structure or gradient change type porous structure, pore size is 0.05-2 mm, and porosity is 10-90%. During preparation, molten polyether-ether-ketone is extruded into preform pores through pressure casting, and then preform is etched off with the chemical method to form the porous polyether-ether-ketone. The material has excellent mechanical property, and pore size and porosity are controllable. The porous structure of the material can be loaded with drugs to prevent infection and promote bone growth and healing. Compared with the prior art, the medical porous polyether-ether-ketone with the drug loading function and the preparing method and application thereof have the advantages that operation is easy, structure is controllable, drug loading is convenient, drug binding is firm, and cost is low.
Owner:SHANGHAI JIAO TONG UNIV
Hydrophobic composite biological activity coating on surface of pure-magnesium or magnesium alloy and preparation method of hydrophobic composite biological activity coating
InactiveCN105420789AImprove compactnessGood biocompatibilityAnodisationProsthesisMicro arc oxidationMagnesium phosphate
The invention relates to the technical field of surface treatment of bio-medical metal materials, in particular to a hydrophobic composite biological activity coating on the surface of pure-magnesium or magnesium alloy and a preparation method of the hydrophobic composite biological activity coating. The method includes the technical processes of preparing a micro-arc oxidation coating on the surface of a magnesium matrix material firstly, preparing a hydroxyapatite coating on the basis of the micro-arc oxidation coating to form a composite active coating, and carrying out hydrophobic treatment on the composite active coating finally to form the hydrophobic composite biological activity coating. The hydrophobic composite biological activity coating on the surface of the magnesium alloy is composed of magnesium oxide, magnesium phosphate and hydroxyapatite and has a compact layer ranging from 5 micrometers to 10 micrometers and a band-shaped hydroxyapatite array, the contact angle of the coating and simulated body fluid is larger than 90 degrees, and the coating shows hydrophobicity. The hydrophobic composite biological activity coating has the beneficial effects of high corrosion resistance, good biocompatibility, good bone induction capacity and the like, and has the wide application prospect.
Owner:FUZHOU UNIV
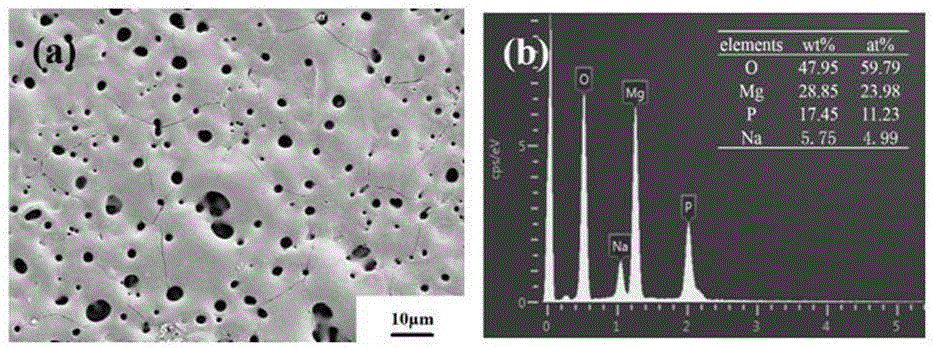
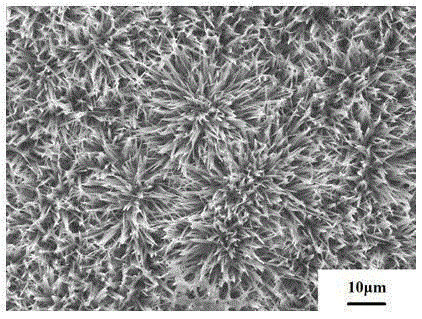
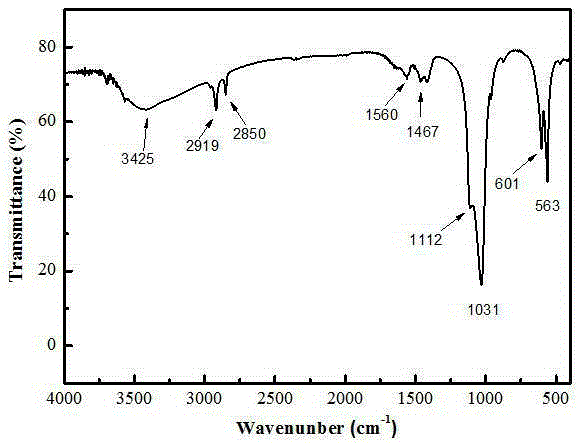
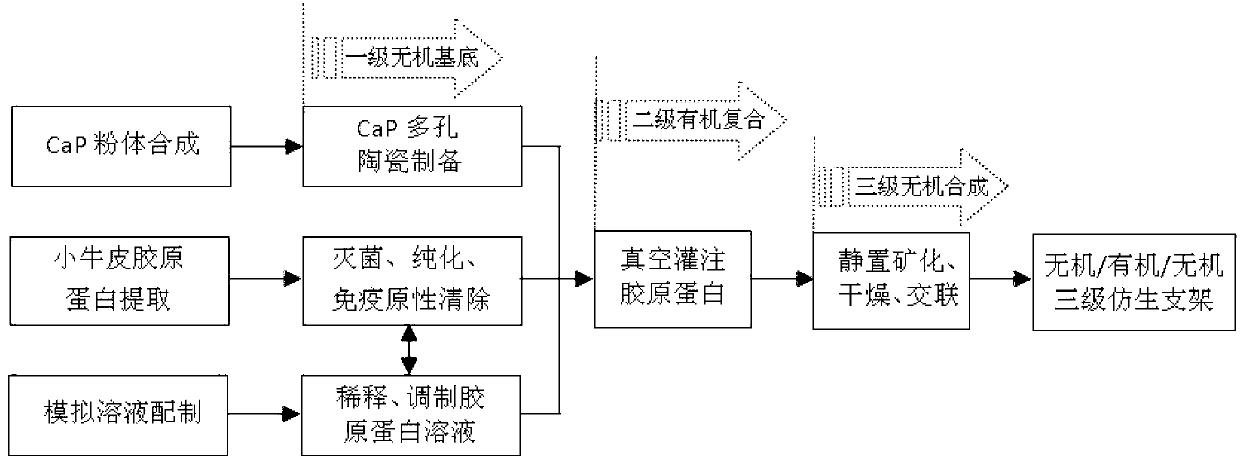
Calcium phosphate/collagen/bone-like apatite three-level bionic bone tissue engineering scaffold and preparation method thereof
InactiveCN103341206APromote repair and regenerationGood biocompatibilityProsthesisThree levelApatite
The invention provides a calcium phosphate / collagen / bone-like apatite three-level bionic bone tissue engineering scaffold and a preparation method thereof. The material of the scaffold possesses components similar to natural bone tissues, and an inorganic / organic / inorganic three-level bionic bone-like netted three-dimensional structure. The preparation method comprises: customizing a calcium phosphate ceramic which possesses a bionic porous structure similar to the natural bone micropore structure, filling collagen prepared by simulated body fluid (SBF) into the porous calcium phosphate ceramic base, low-temperature aging to simulate a biomineralization process, in-situ nucleating on the collagen organic macro-molecule matrix, and self-assembly crystallizing to form a third level bone-like apatite layer structure. The composite scaffold material has bionic material components and bionic microstructure both similar to the natural bone tissues, the mechanical properties and the biological activity of the material are substantially improved, and the composite scaffold material has a wide application prospect.
Owner:SICHUAN UNIV
Bone matrix compositions and methods
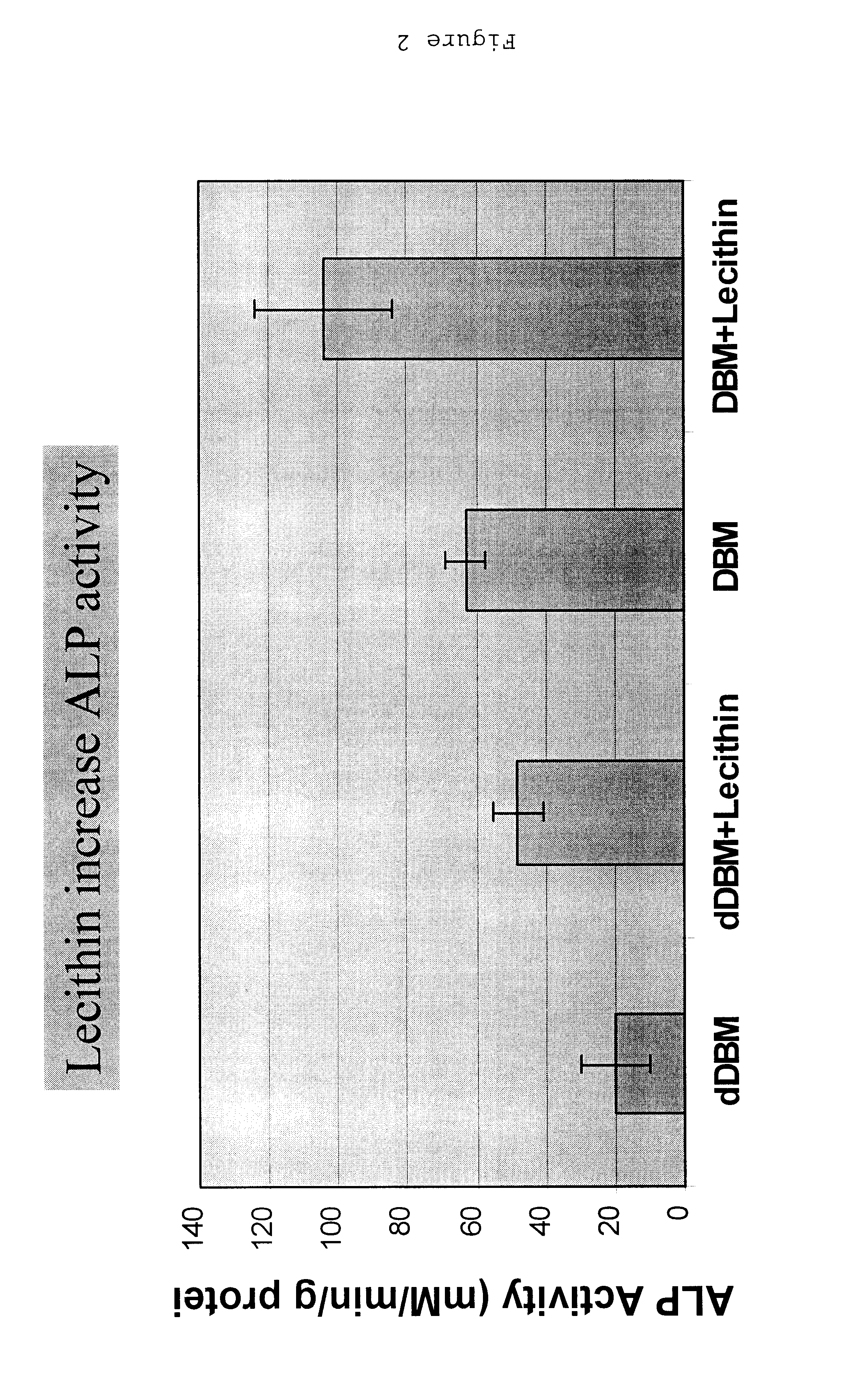
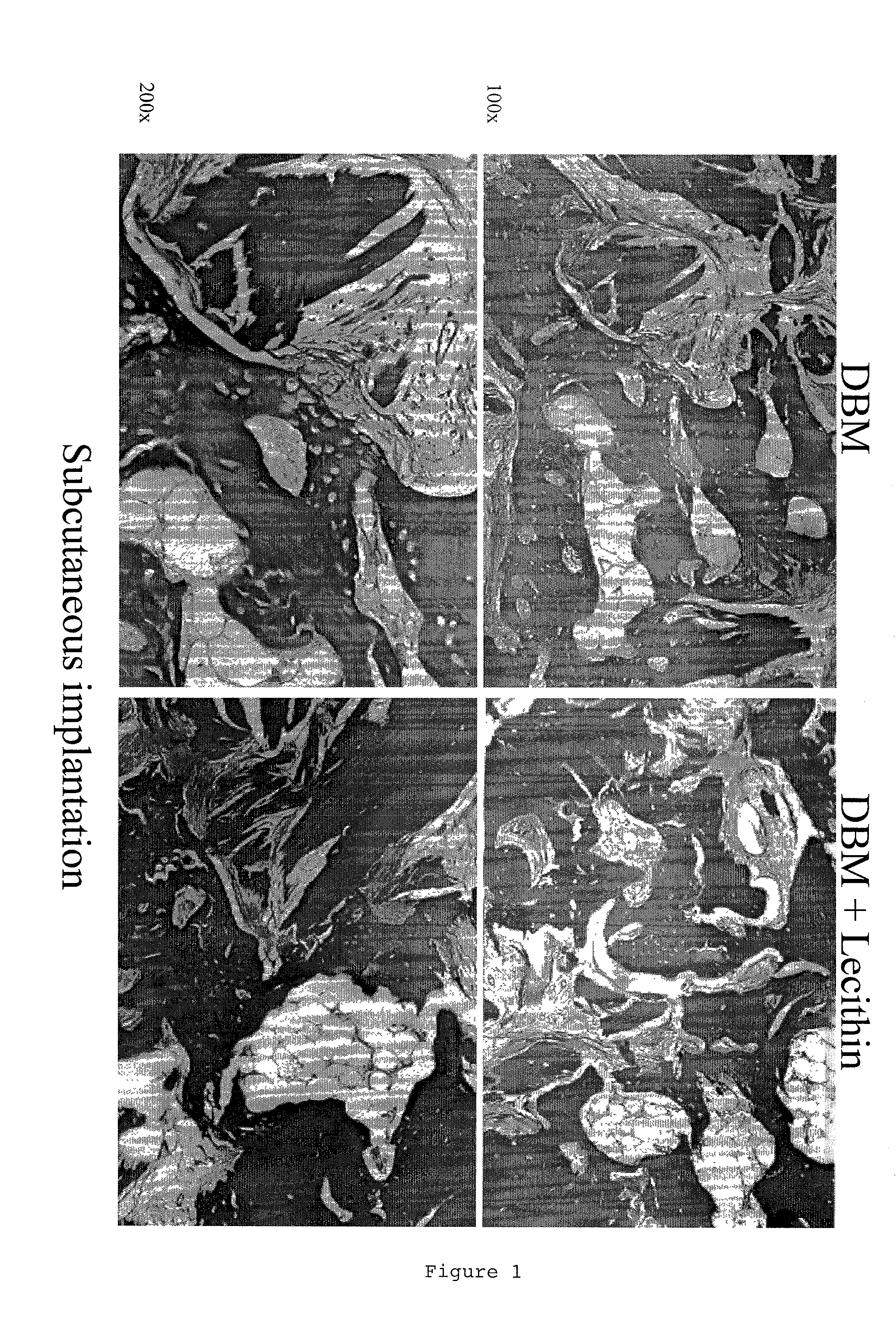
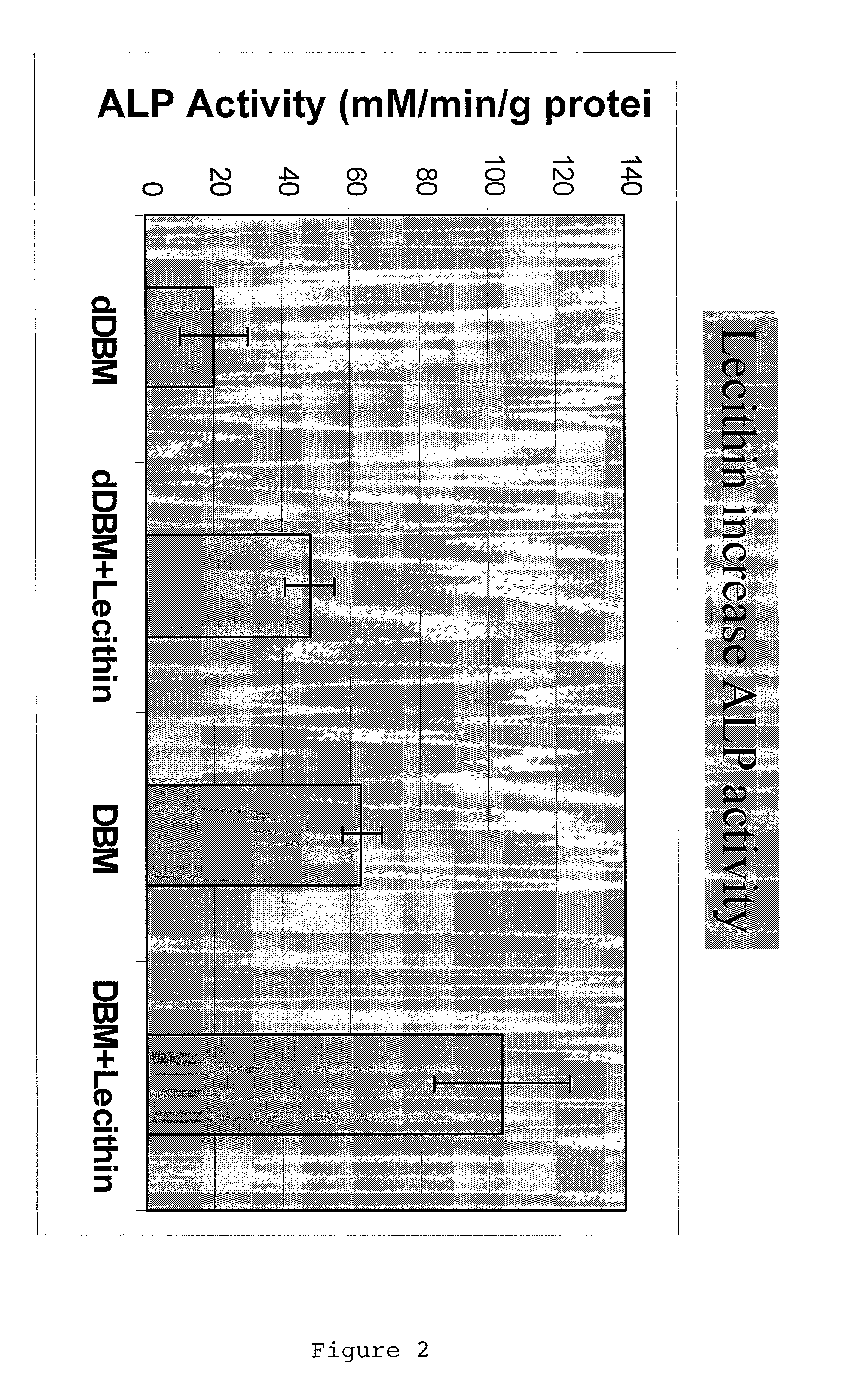
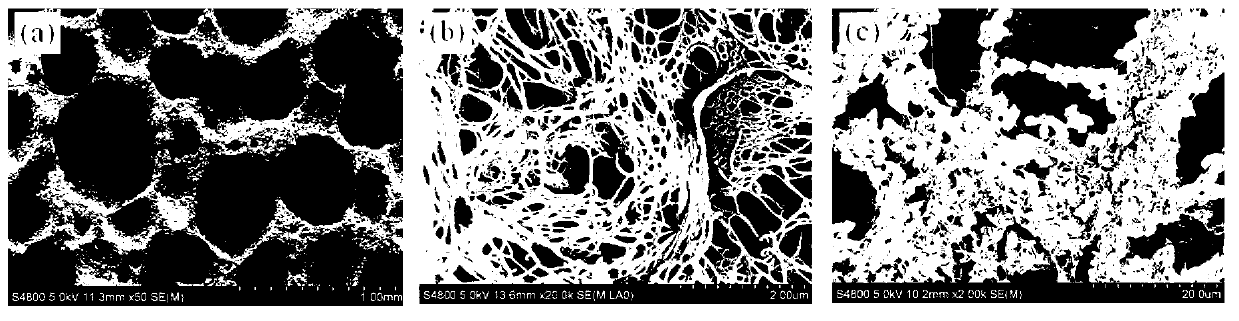
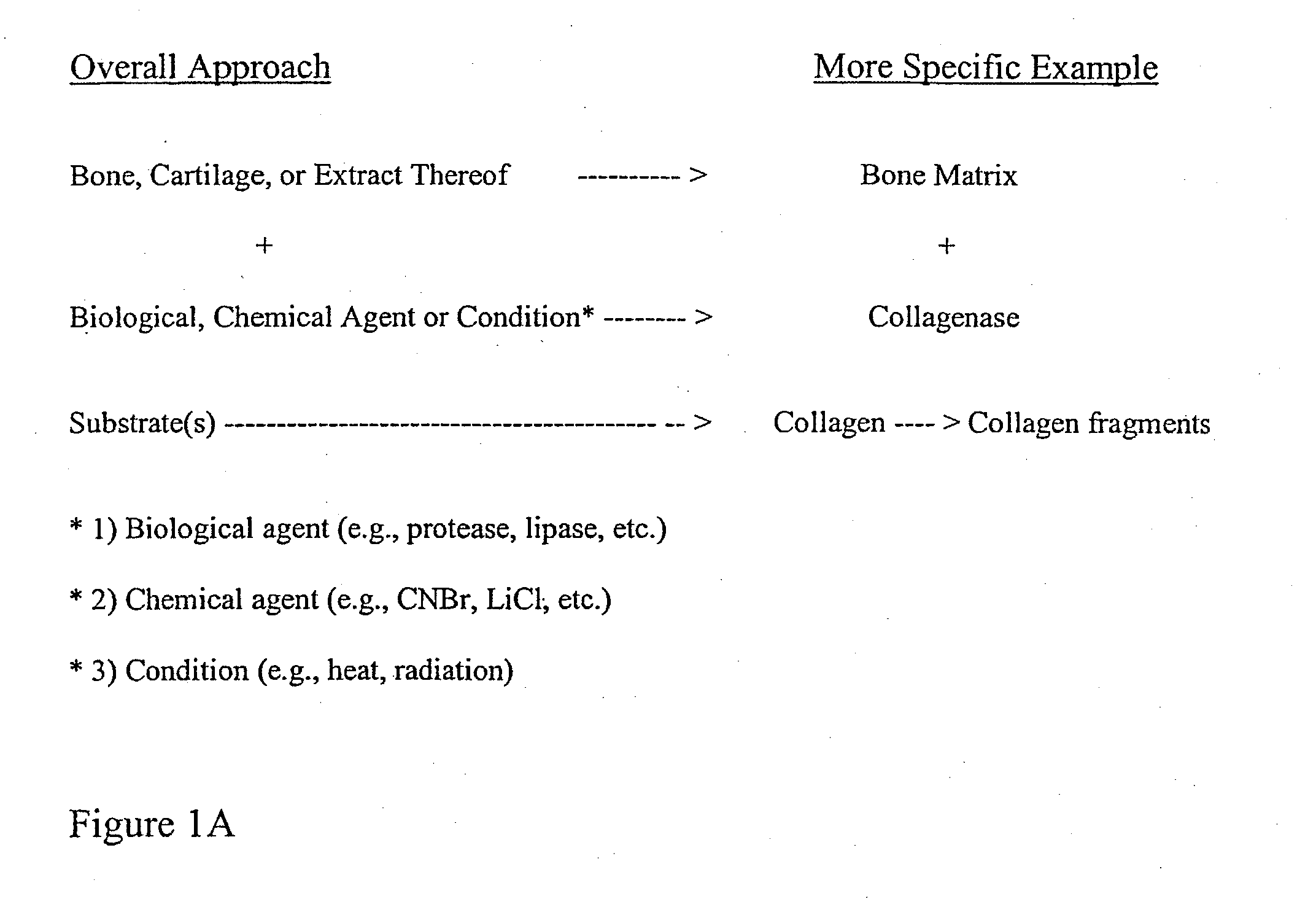
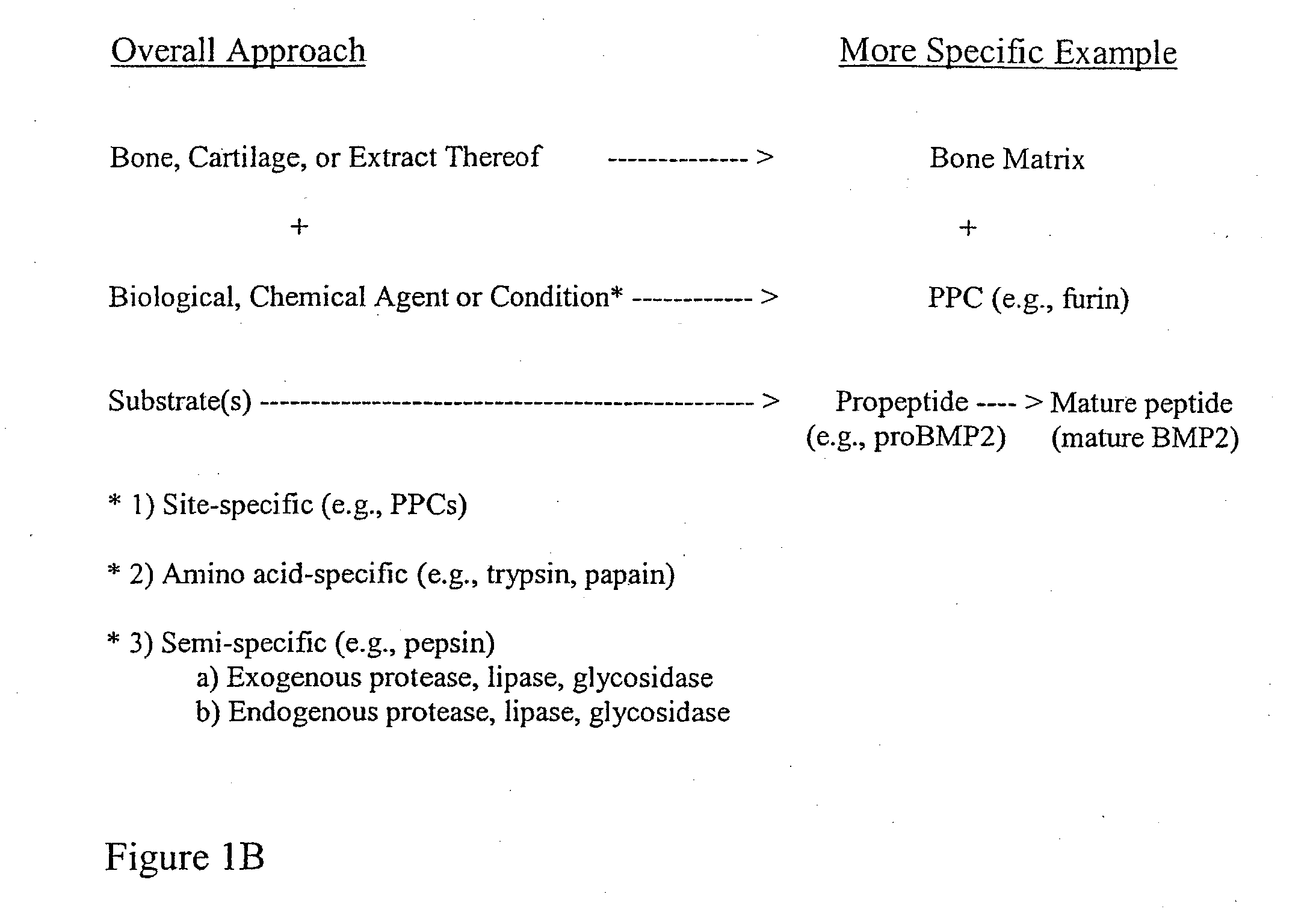
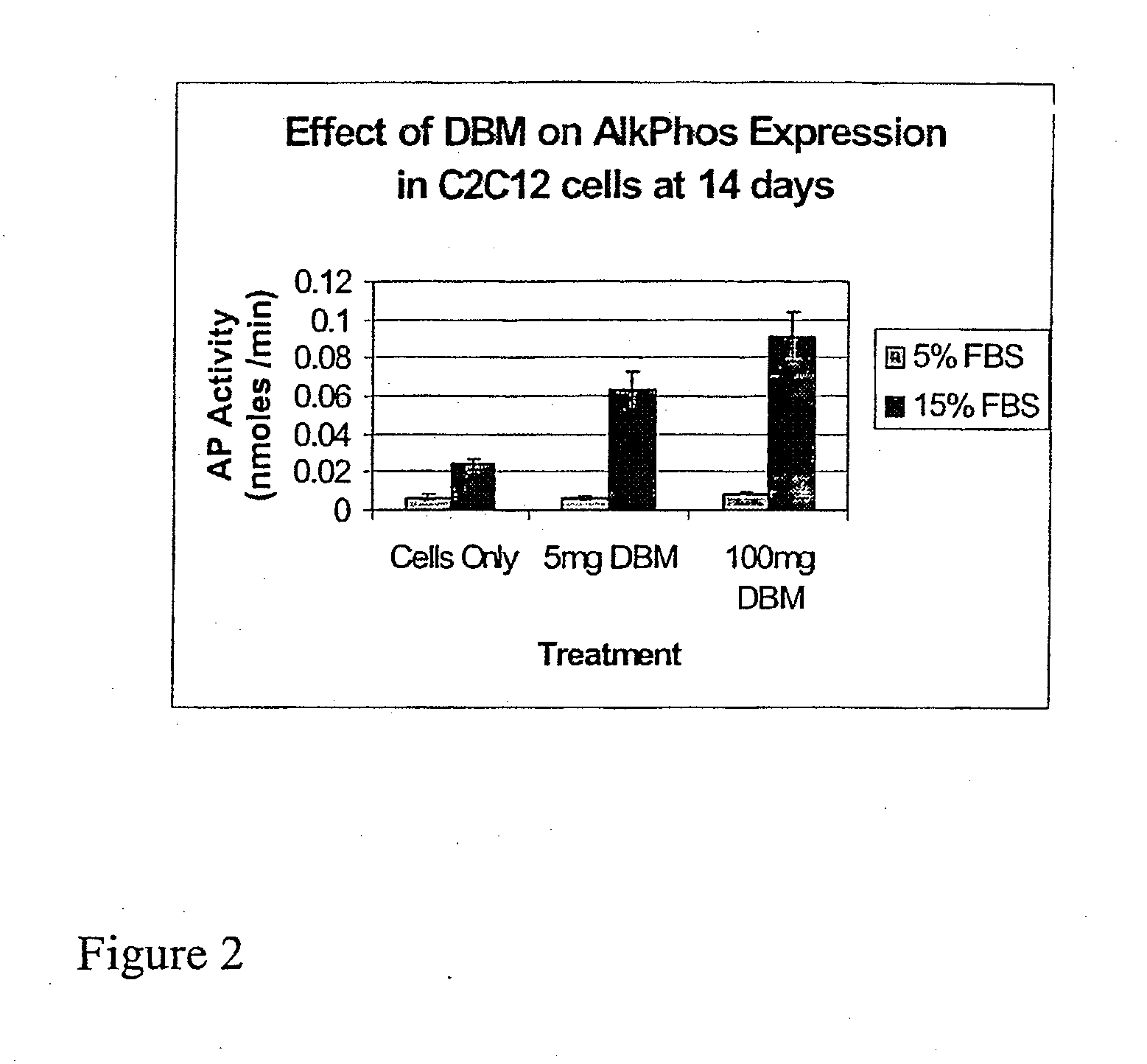
ActiveUS20110195052A1Improve biological activityFacilitated releaseBiocideHydrolysed protein ingredientsOsteoblastSpecific protein
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. On a assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Nanometer bionic scaffold material and preparation method thereof
InactiveCN101628130AGood biocompatibilityGood bone conductionProsthesisChemistryBiocompatibility Testing
The invention specifically relates to a nanometer bionic scaffold material and a preparation method thereof. The nanometer bionic scaffold material is assembled in synergy with a dimolecular template, wherein the dimolecular template comprises a protein and protein template, a protein and polyoses template, a polyoses and polyoses molecule template, a synthesis molecule and synthesis molecule template, a synthesis molecule template and a natural molecule template. The preparation method comprises the following steps: mixing one template with solution containing calcium ion; mixing the other template with solution containing phosphate radical ion; adding the solution containing phosphate radical ion into the solution containing calcium ion after evenly mixing the two plates; regulating thepH value of the mixed solution to be 7.4 and then transferring to the water at a constant temperature of 37 DEG C to obtain sediment, i.e, the nanometer bionic scaffold material. The bionic scaffold material not only has favorable biocompatibility and degradability, but also has bionic multi-layer assembling structure of highly-approximate natural scaffold, thereby being a bionic scaffold material in true sense and having wide application prospect in clinical scaffold repair.
Owner:HUAZHONG UNIV OF SCI & TECH
Bioactive glass-modified gelatin composite hydrogel as well as preparation method thereof
ActiveCN106730021AGood biocompatibilityGood osteoinductivityPharmaceutical delivery mechanismTissue regenerationBone tissue engineeringBioactive glass
The invention discloses bioactive glass-modified gelatin composite hydrogel as well as a preparation method thereof, and belongs to the field of biomedical materials. The bioactive glass-modified gelatin composite hydrogel is prepared by the following steps: the bioactive glass powder is added into a solution of modified gelatin containing a photoinitiator, ultrasonic treatment and stirring are carried out, uniformly dispersed materials are poured into a die, and after UV-irradiation is carried out for a period of time, the bioactive glass-modified gelatin composite hydrogel is obtained. The bioactive glass-modified gelatin composite hydrogel has good biological activity, osteoinductivity and high water content (which is 80% or above), and the product is used for the fields of non-bearing bone defect repair, slow release of medicament, bone tissue engineering, and the like.
Owner:SOUTH CHINA UNIV OF TECH
Bone repair composite material based on acellular biological tissue matrix material and preparation method of bone repair composite material
ActiveCN105412989AImprove adsorption capacityVarious ingredientsPharmaceutical delivery mechanismSkeletal disorderAcellular matrixBiocompatibility Testing
The invention relates to a bone repair composite material based on an acellular biological tissue matrix material and a preparation method of the bone repair composite material. According to the bone repair composite material disclosed by the invention, a micro-fibrotic animal tissue acellular matrix material is taken as an organic component, and a calcium salt biological ceramic material or other inorganic bioglass is taken as an inorganic component. According to the bone repair composite material prepared by the method disclosed by the invention, additional physical crosslinking or chemical crosslinking is not needed, and the bone repair composite material has a complete three-dimensional porous net structure; the protein component in the biological tissue matrix material has the natural triplex structure; the bone repair composite material has the excellent biocompatibility, the complete biodegradability, the excellent osteoconduction, as well as the excellent osteoinductivity and the excellent osteogenesis, also has certain mechanical strength and shape memory function, and can be used as a bone filling material with bioactivity or a repair material for large-area bone defect.
Owner:HANGZHOU HUAMAI MEDICAL DEVICES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com