Acellular dermal matrix and preparing method of acellular dermal matrix
An acellular dermis and matrix technology, applied in the field of medical materials, can solve the problems of limited scope of application and application, the preparation method is not described in detail, and the components of cell debris cannot be effectively removed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
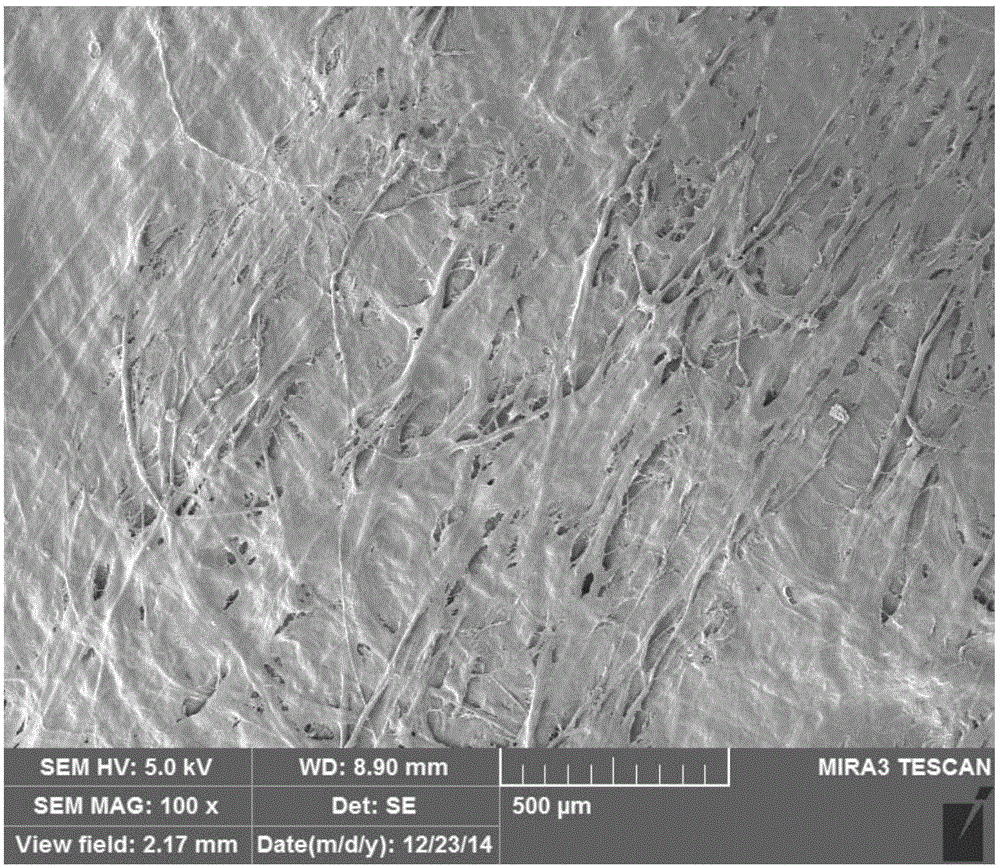
Image
Examples
preparation example Construction
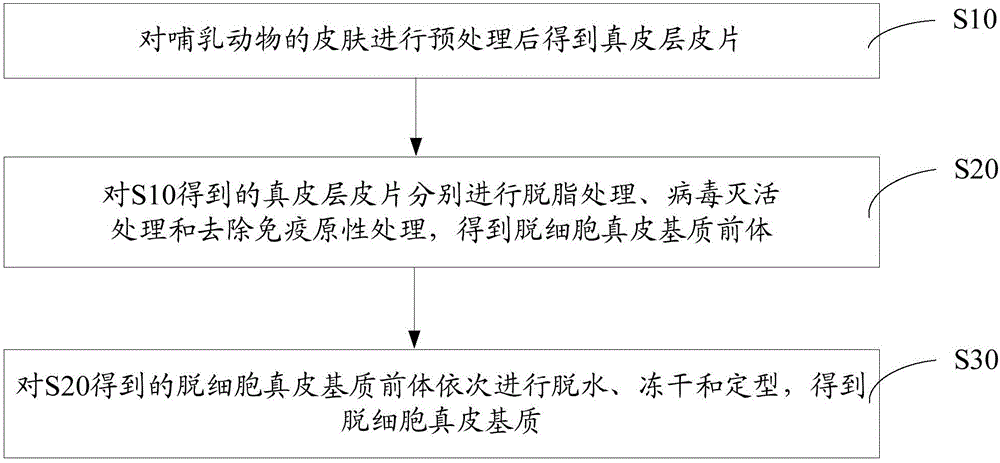
[0027] Such as figure 1 The method for preparing acellular dermal matrix includes the following steps:
[0028] S10, pretreating the skin of the mammal to obtain a dermal layer skin sheet.
[0029] The operation of pretreating the mammalian skin to obtain a dermal layer skin sheet is: mechanically degreasing and depilating the mammalian skin, and then using a skin machine to remove the 0.15mm~0.4mm epidermis from the mammalian skin After being combined with the subcutaneous tissue, a dermal skin sheet with a thickness of 0.3mm to 1.0mm is formed.
[0030] The skin of a mammal includes fur, epidermal layer, dermis layer and subcutaneous tissue layered in sequence.
[0031] Specifically, the skin of a mammal is the skin of a pig, cow, sheep, or horse.
[0032] The obtained dermal layer skin sheet can be stored at low temperature for later use. Specifically, the obtained dermal layer skin sheet can be stored at -20°C for later use.
[0033] S20: Perform degreasing treatment, virus inactiv...
Embodiment 1
[0057] (1) Preparation of dermal layer skin: the whole abdomen and back skin of healthy pigs are preliminarily degreased by mechanical method, the hair is removed with tweezers, and the subcutaneous tissue of the pig skin is removed with an electric peeling machine, and then the epidermis is removed 0.4mm, made into a dermal skin sheet with a thickness of 0.3mm, stored at -20℃ for later use;
[0058] (2) Degreasing treatment: primary degreasing is to use sodium hydroxide with a mass concentration of 1.0% for degreasing for 24 hours, and the volume ratio of the skin sheet to the degreasing agent is 1:3; the second degreasing is to place the skin sheet in the organic solvent n-ethane, Perform secondary degreasing in ethanol, with normal ethane treatment time of 60min and ethanol treatment time of 30min; the decellularized dermal matrix after degreasing is washed with phosphate buffered saline solution for 2h, and the phosphate buffered saline solution is changed every 20min;
[0059]...
Embodiment 2
[0068] (1) Preparation of dermal layer skin: the whole abdomen and back skin of healthy pigs are preliminarily degreased by mechanical method, the hair is removed with tweezers, and the subcutaneous tissue of the pig skin is removed with an electric peeler, and then the epidermis is removed 0.16mm, made into a dermis layer with a thickness of 0.7mm, stored at -20℃ for later use;
[0069] (2) Decellularization treatment: The dermis prepared in step (1) is treated with pepsin for 12 hours, and then treated with sodium dodecyl sulfate (SDS) for 24 hours, and this step is repeated 3 times;
[0070] (3) Degreasing treatment: primary degreasing is to use sodium bicarbonate with a mass concentration of 3.0% for degreasing for 12 hours, and the volume ratio of the skin piece to the degreasing agent is 1:4; the second degreasing is to put the skin piece in the organic solvent isopropanol, Perform secondary degreasing in ethanol, isopropanol treatment time is 60min, ethanol treatment time is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com