Patents
Literature
1073 results about "Subcutaneous tissue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
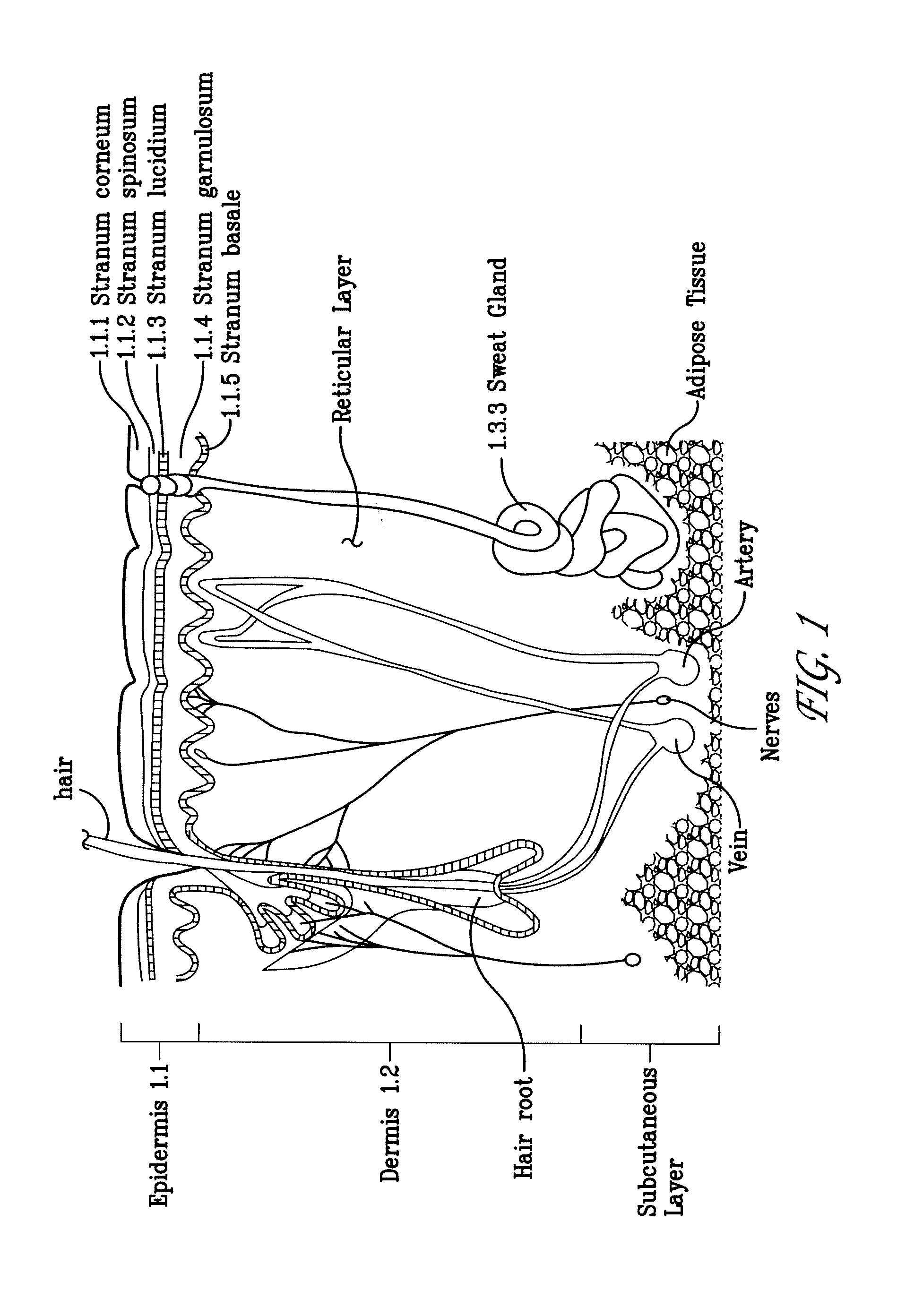
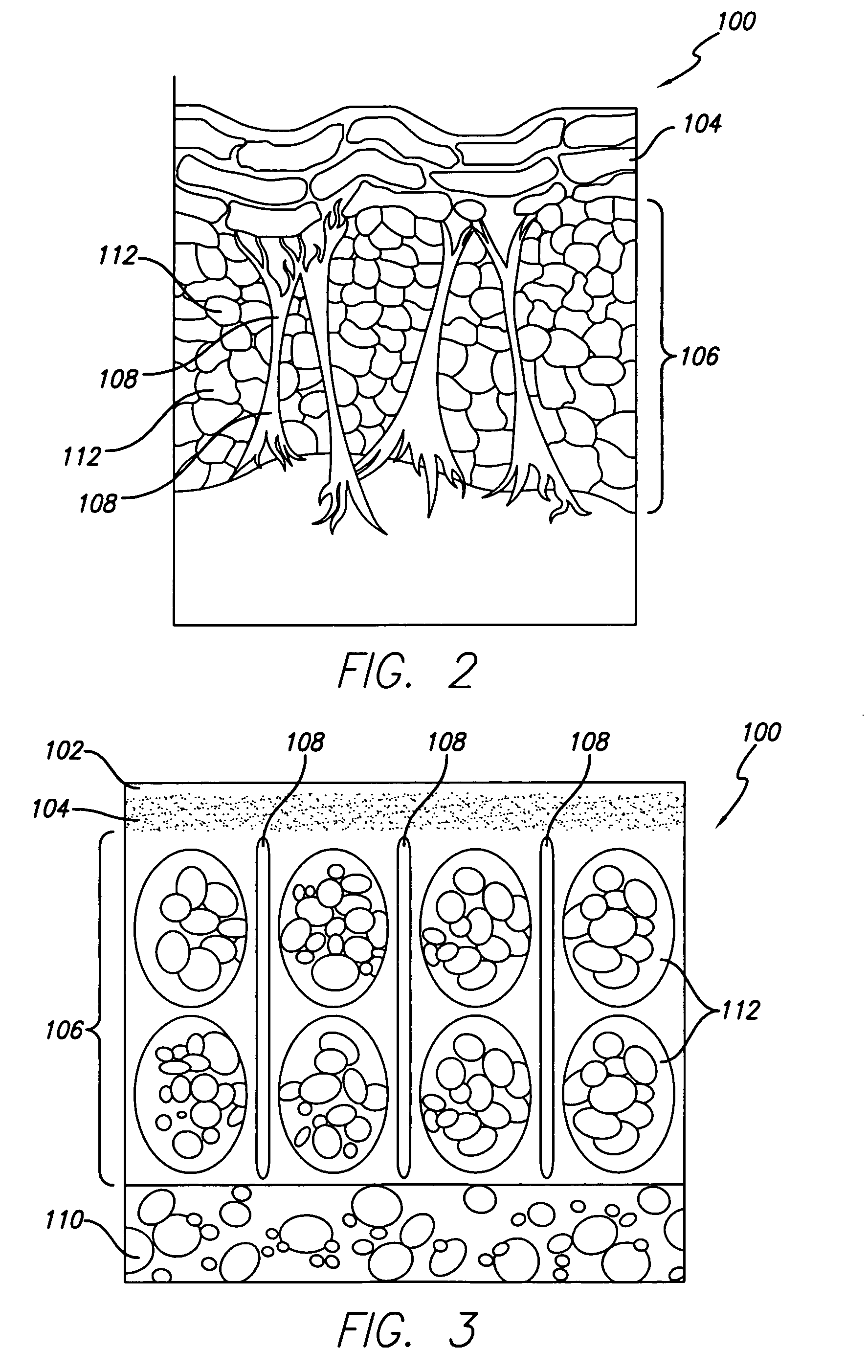
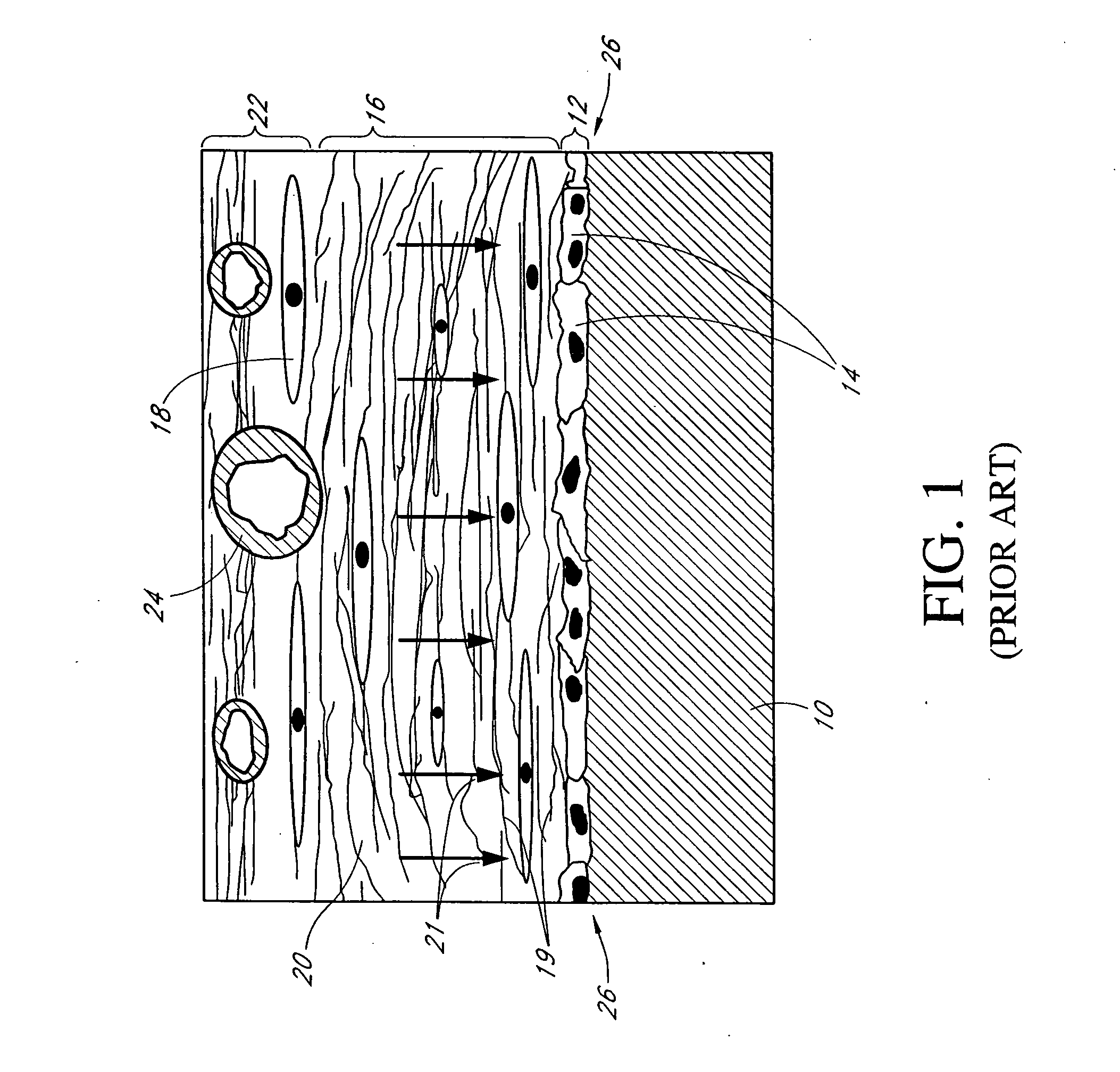
The subcutaneous tissue (from Latin subcutaneous, meaning 'beneath the skin'), also called the hypodermis, hypoderm (from Greek, meaning 'beneath the skin'), subcutis, or superficial fascia, is the lowermost layer of the integumentary system in vertebrates. The types of cells found in the hypodermis are fibroblasts, adipose cells, and macrophages. The hypodermis is derived from the mesoderm, but unlike the dermis, it is not derived from the dermatome region of the mesoderm. In arthropods, the hypodermis is an epidermal layer of cells that secretes the chitinous cuticle. The term also refers to a layer of cells lying immediately below the epidermis of plants.
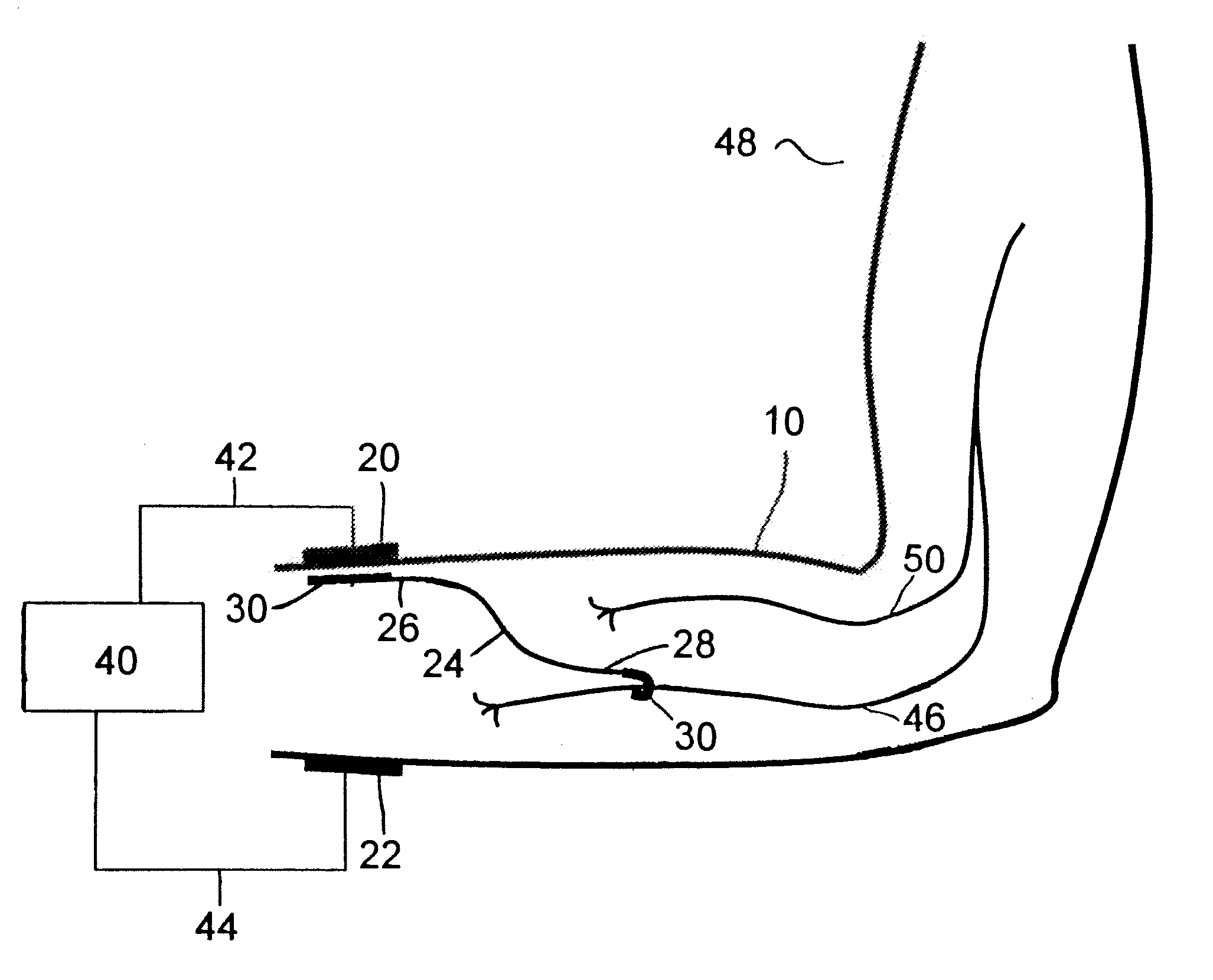
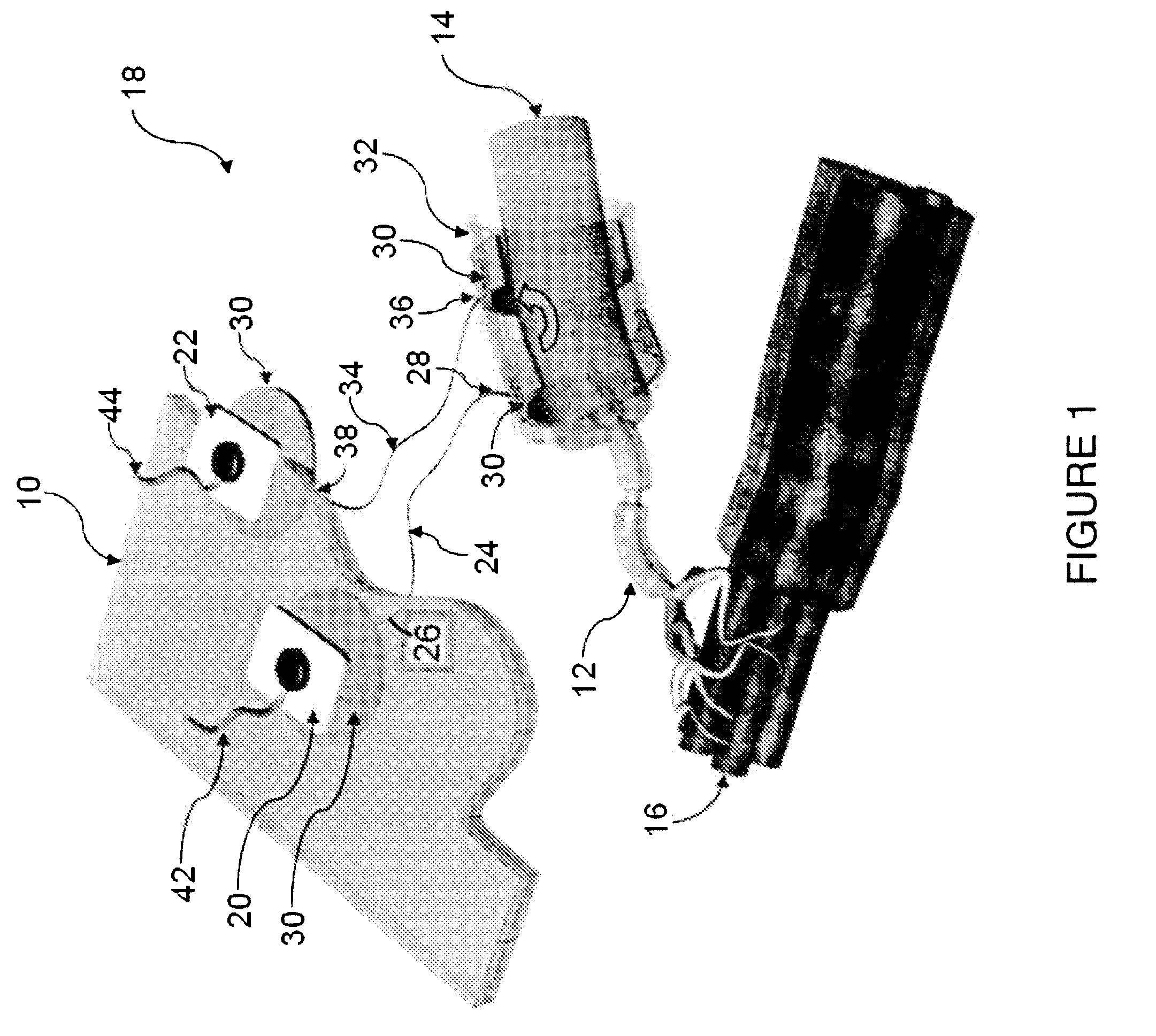
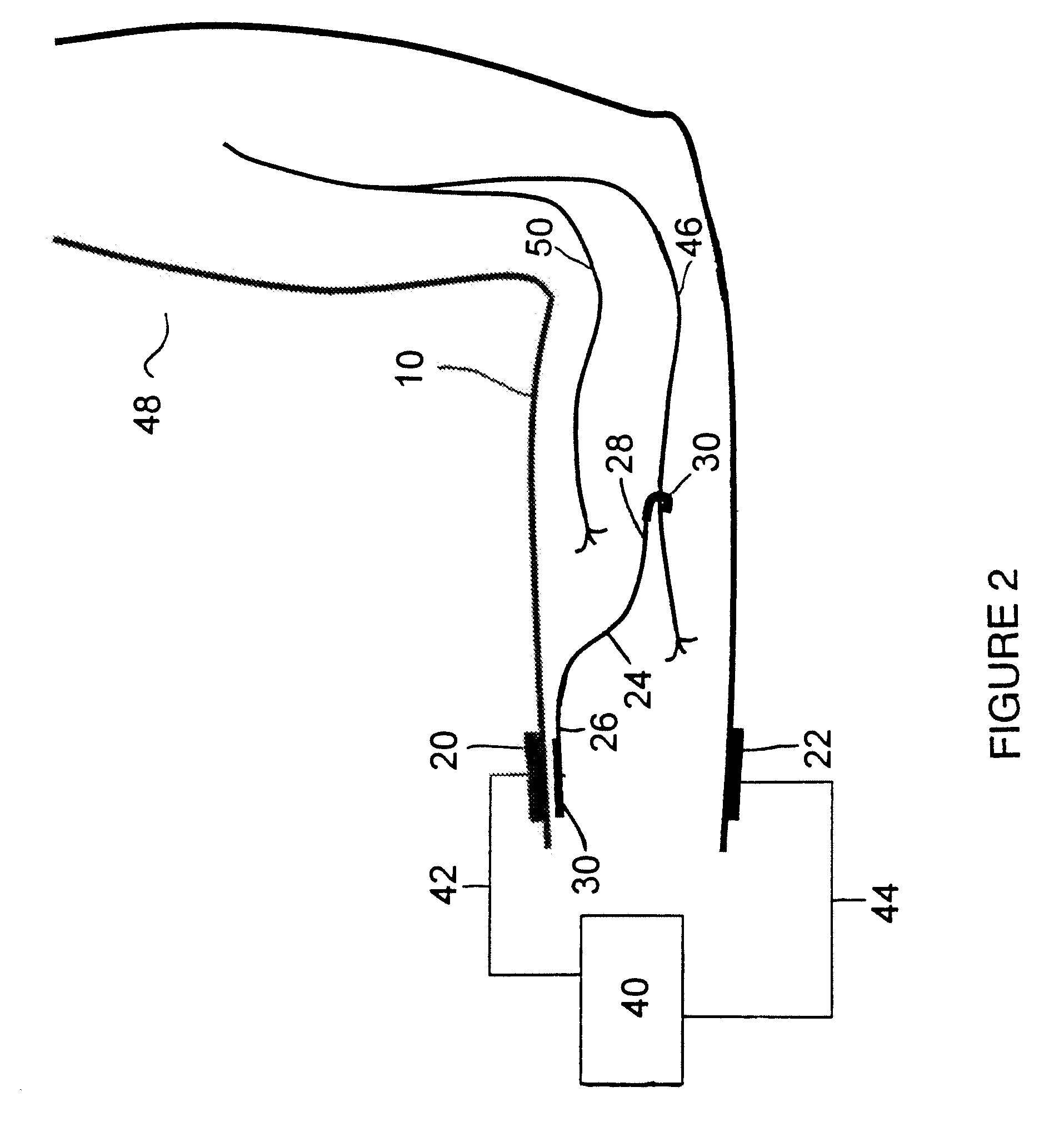
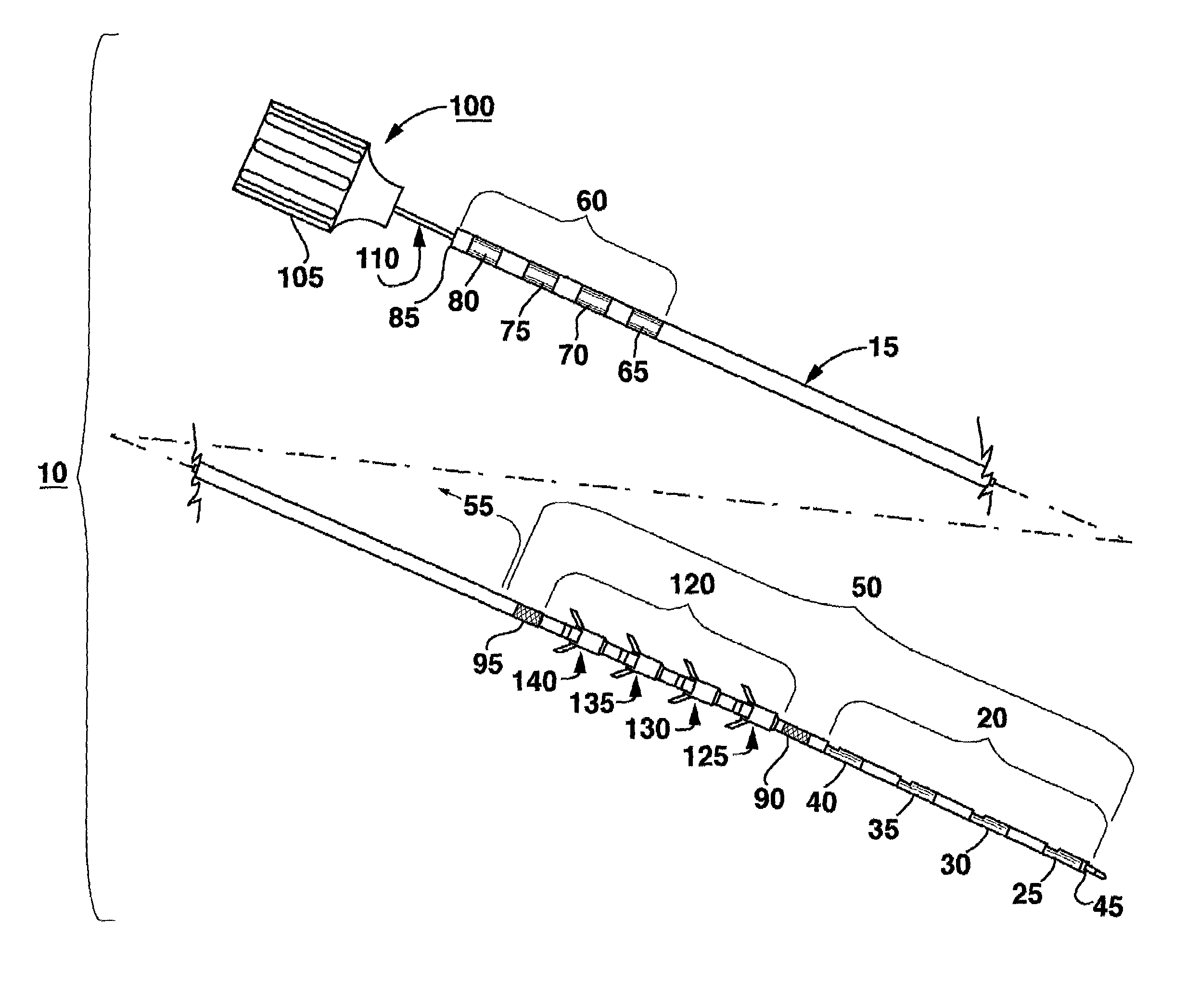
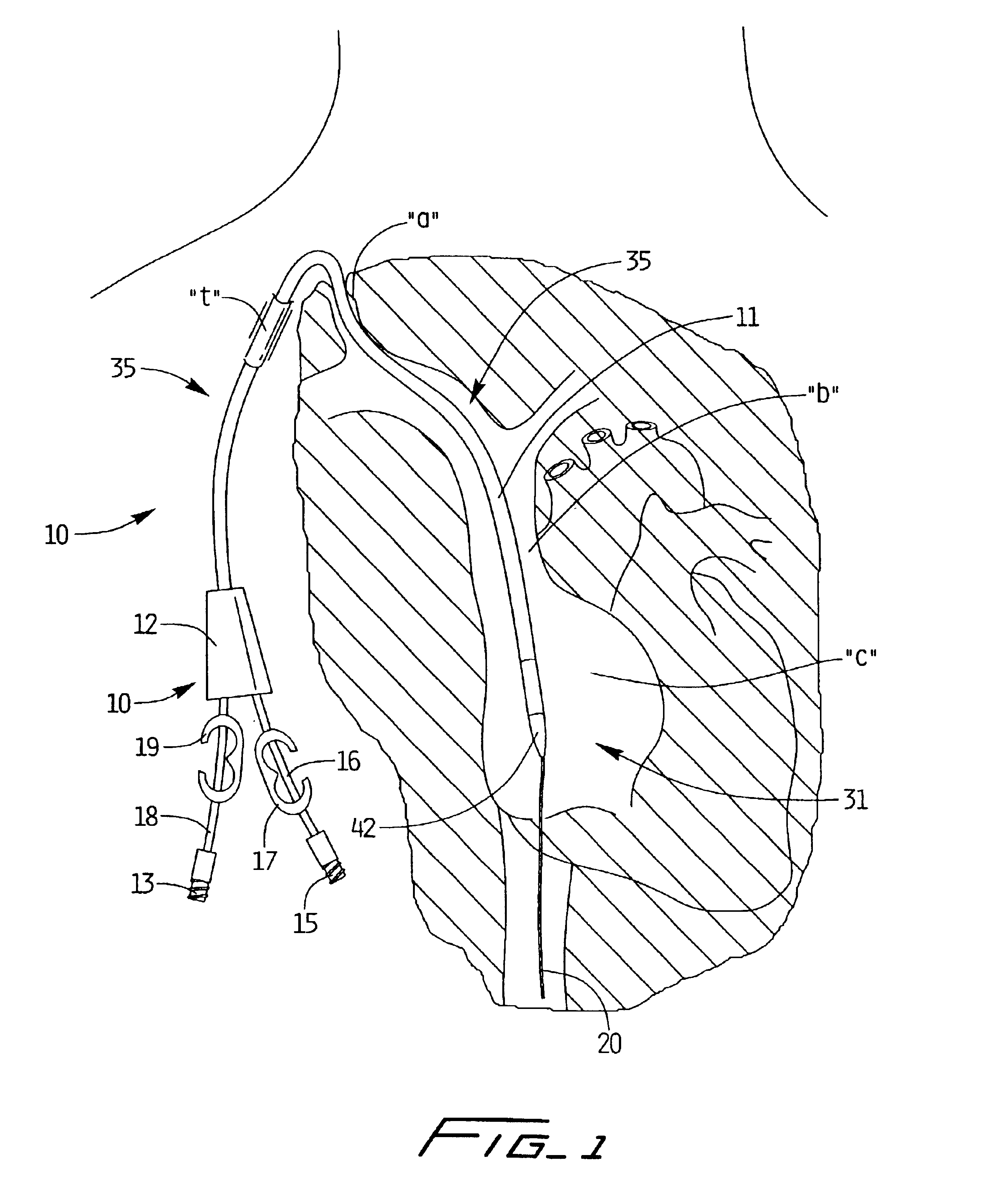
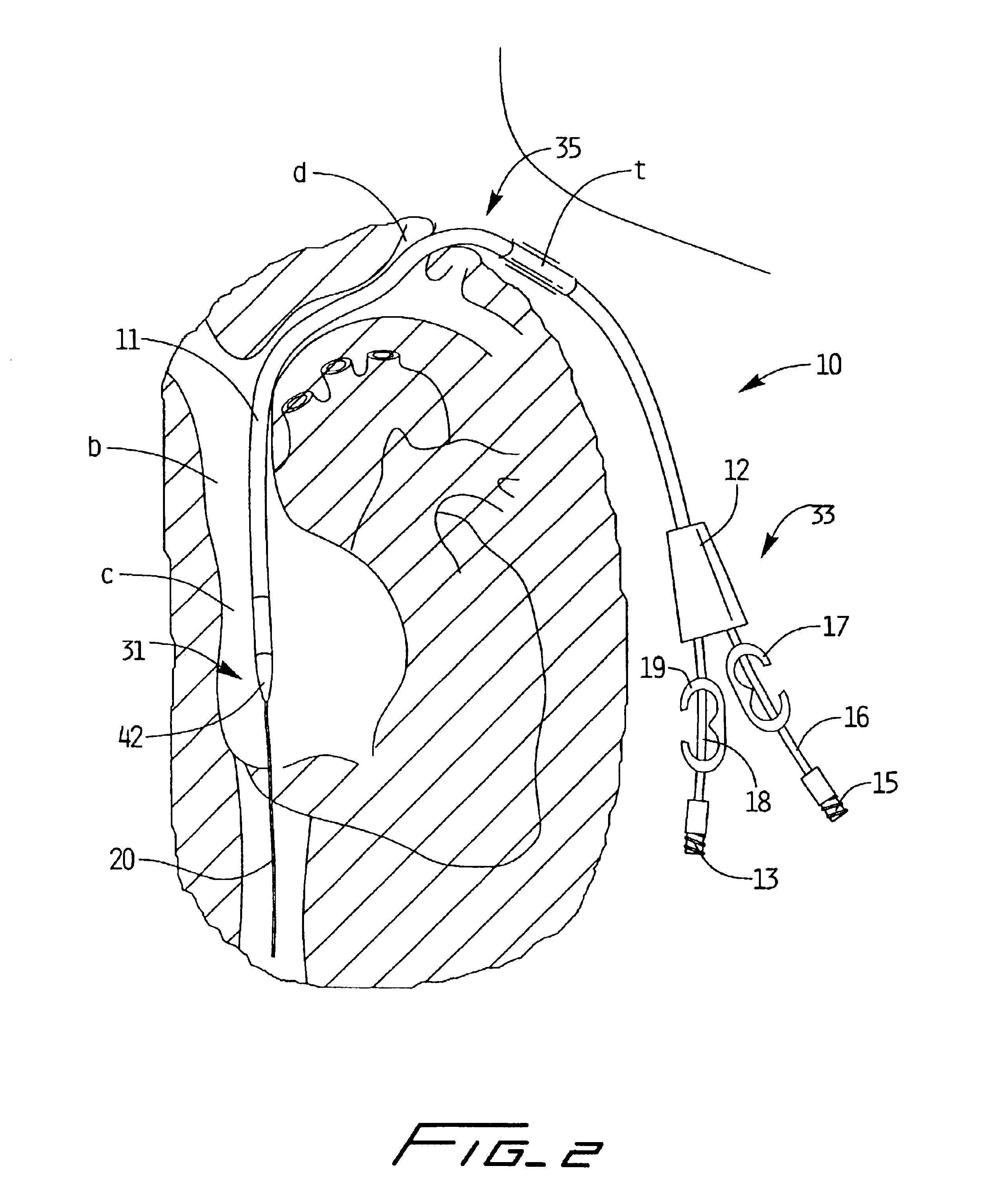
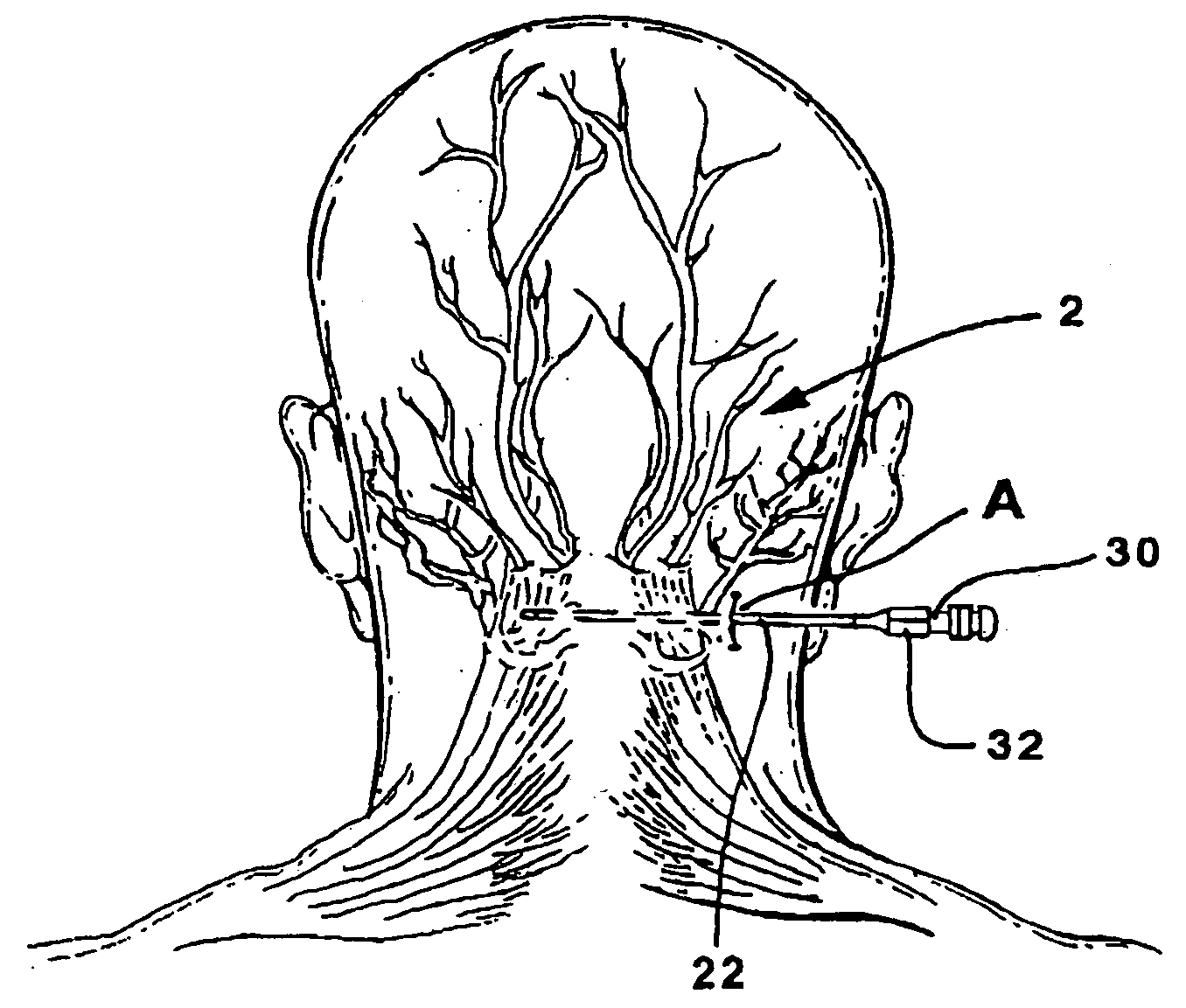
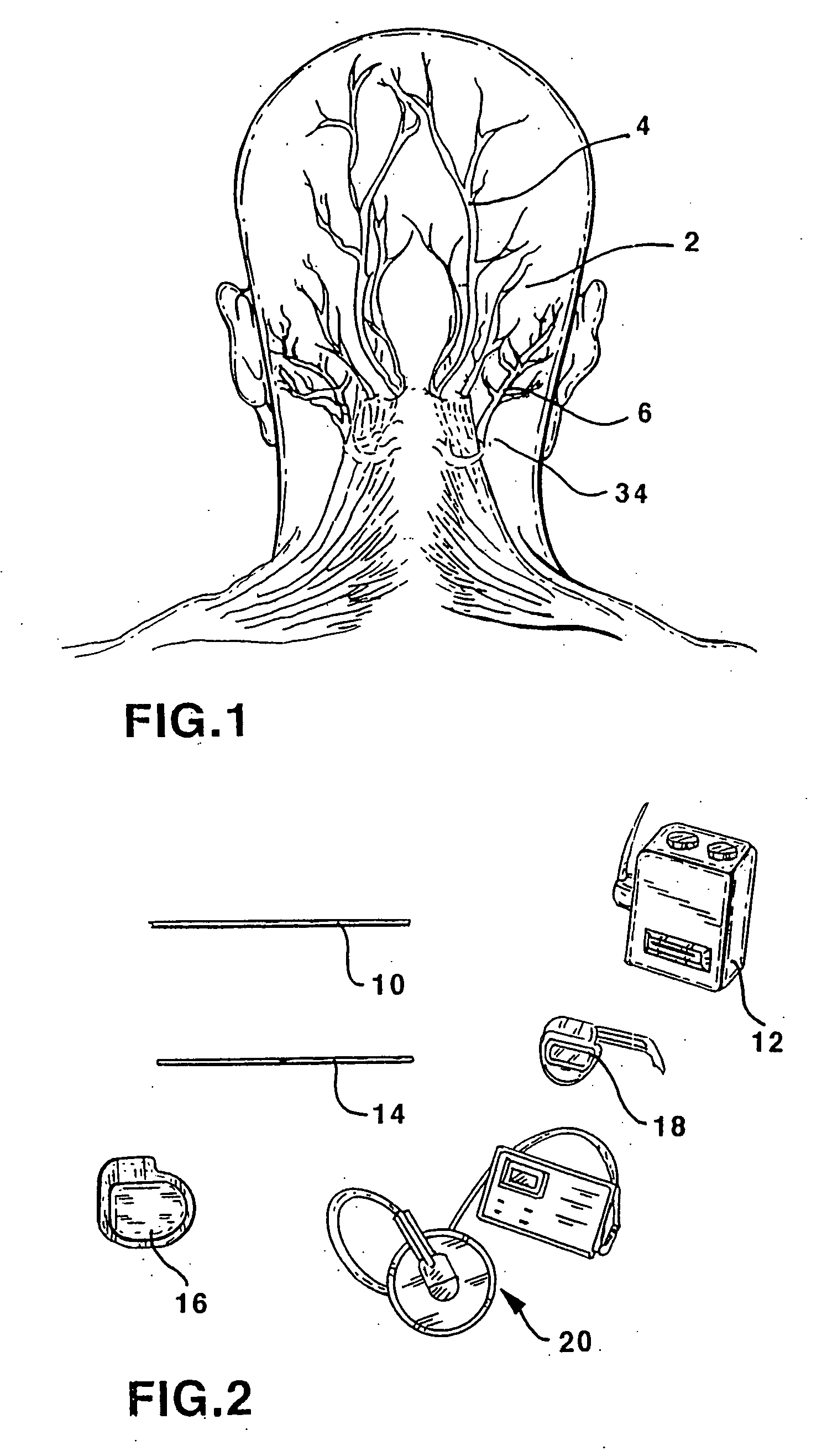
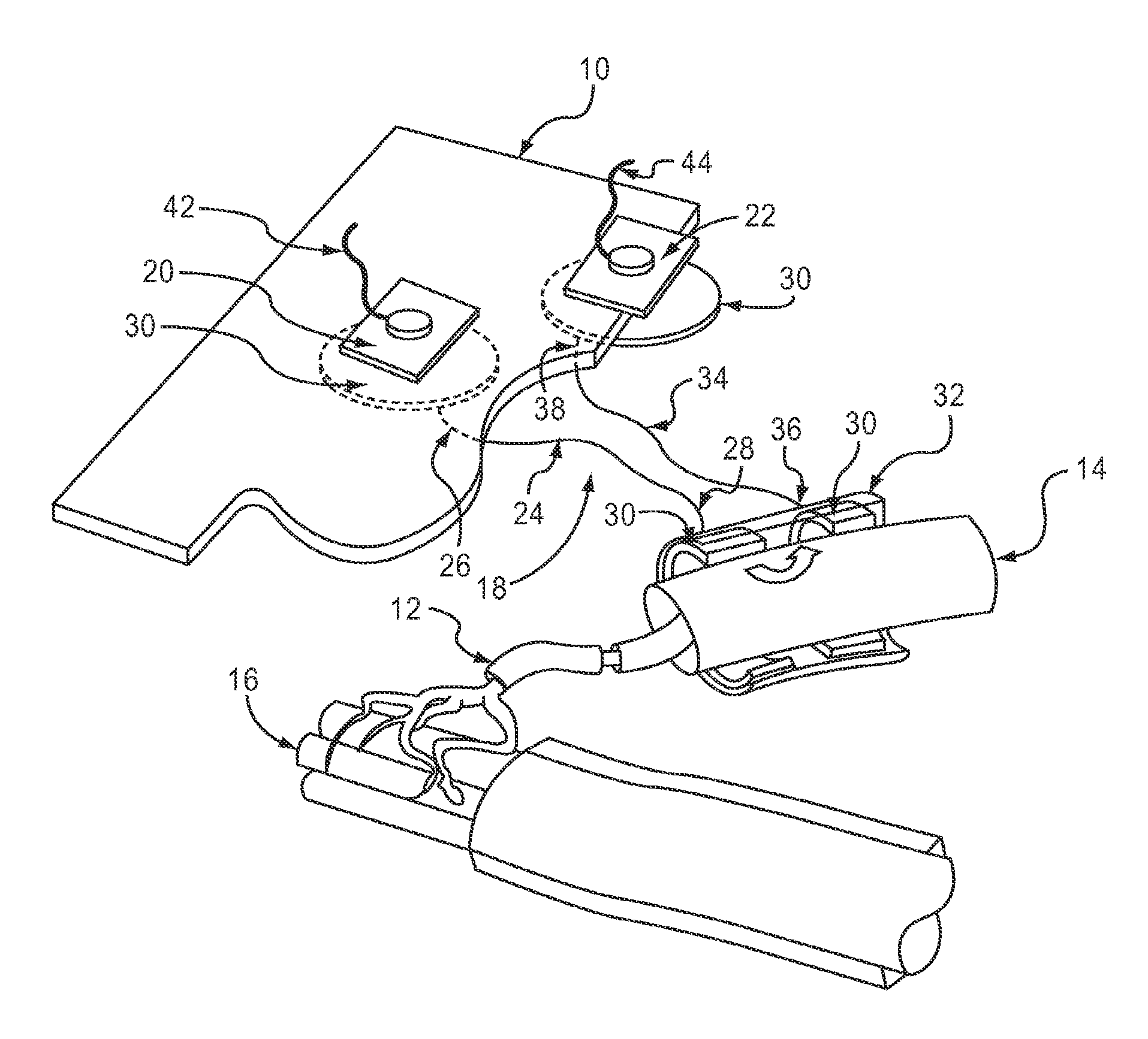
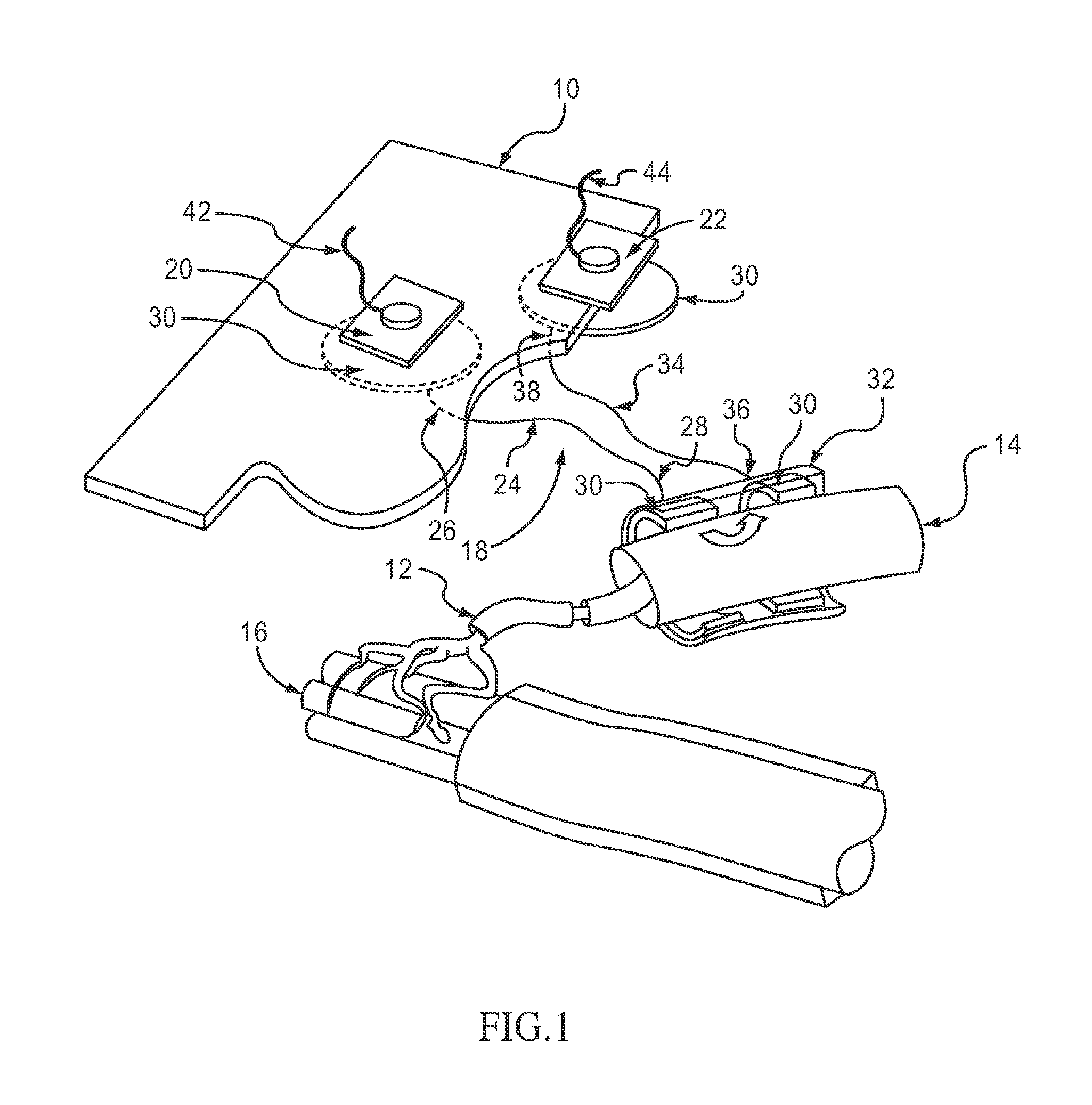
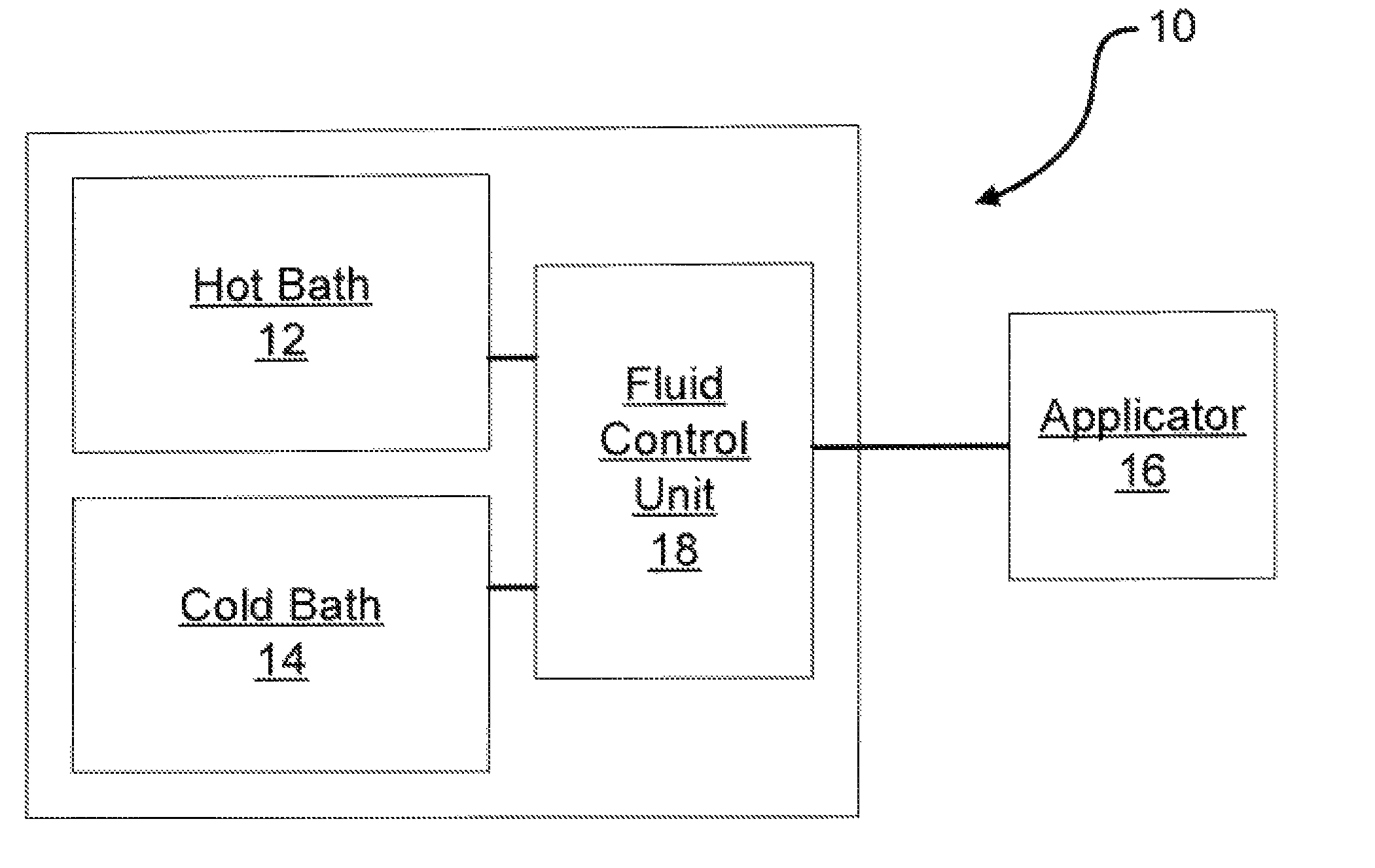
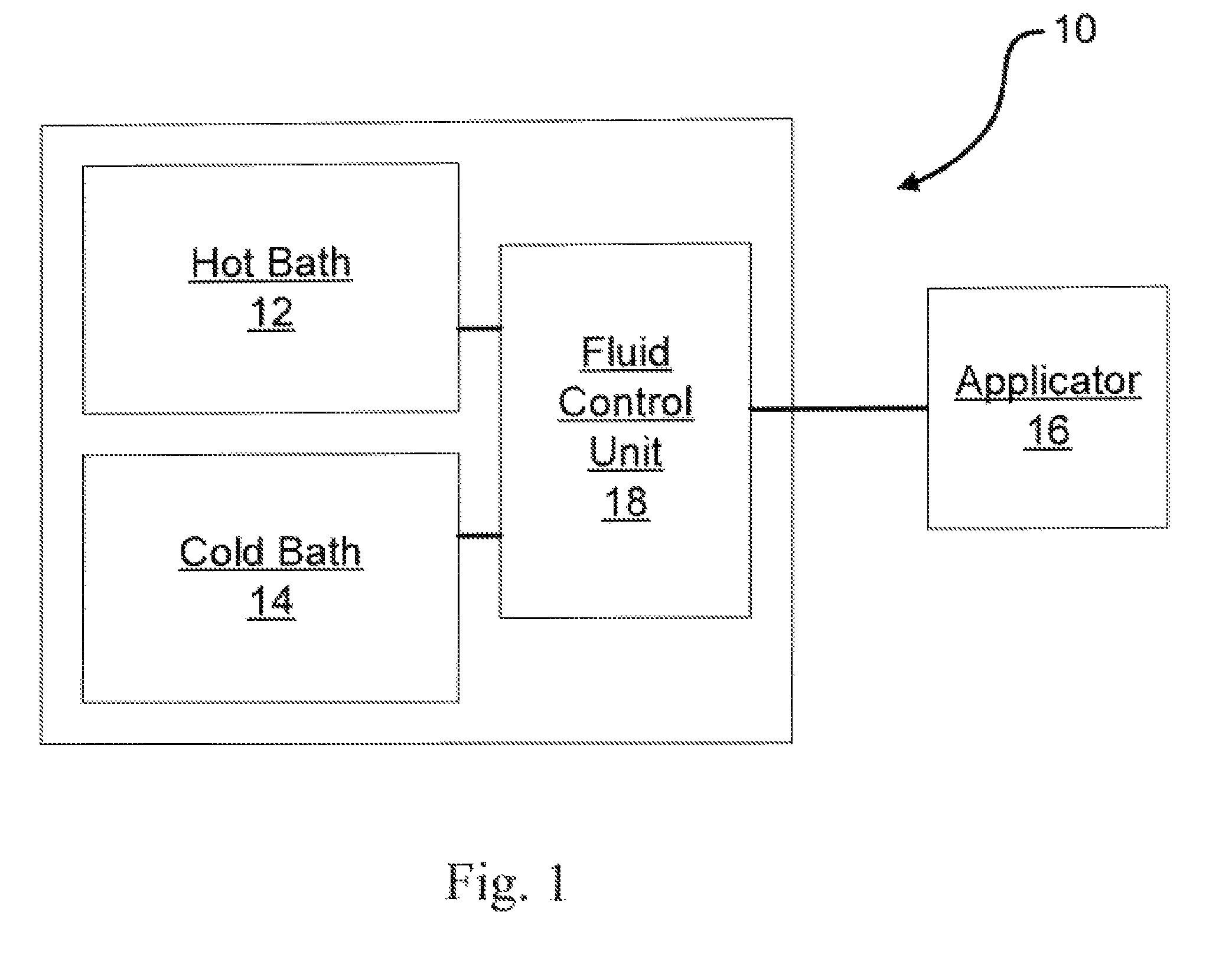
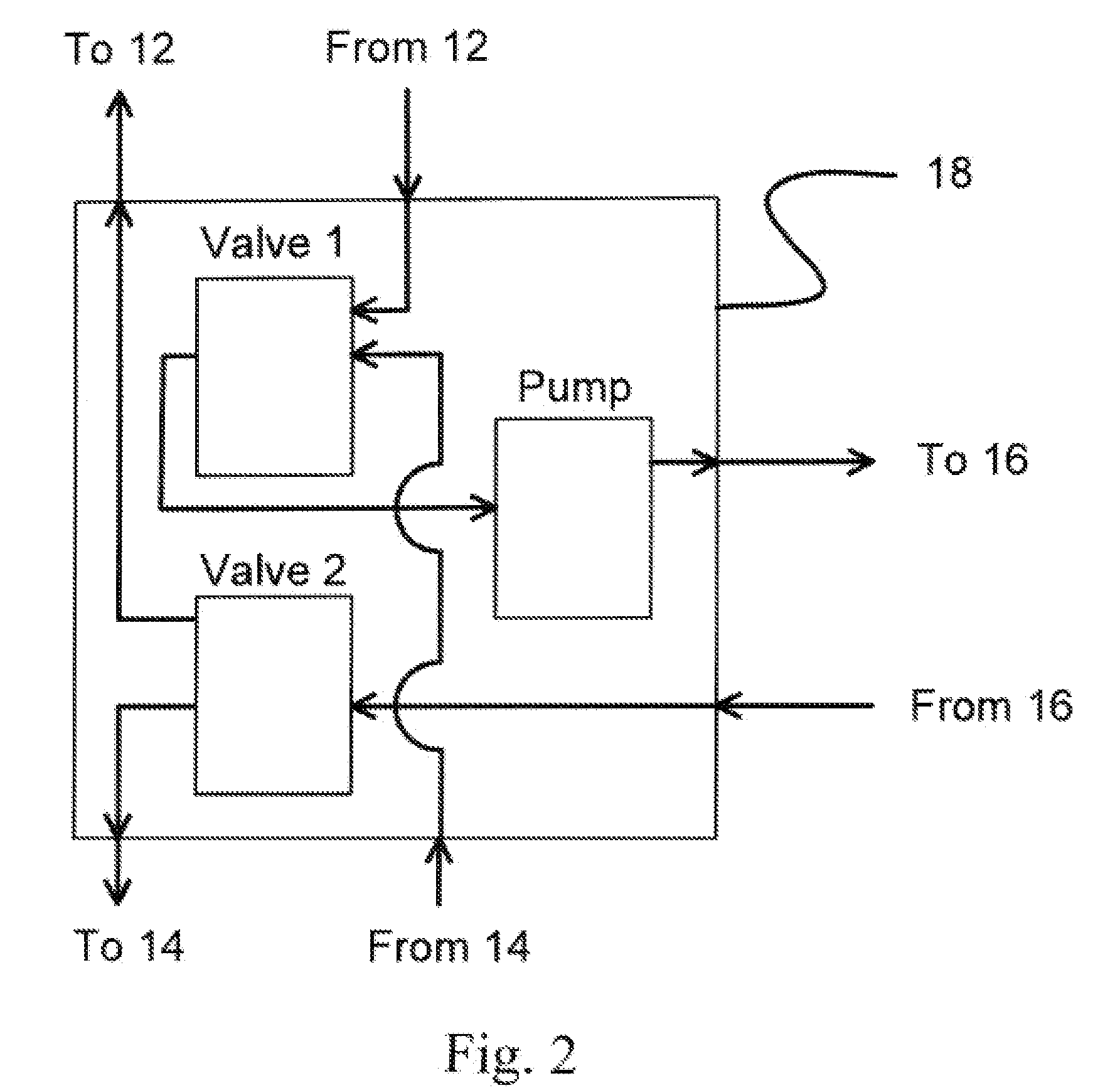
Method of routing electrical current to bodily tissues via implanted passive conductors
The invention provides an implant, system and method for electrically stimulating a target tissue to either activate or block neural impulses. The implant provides a conductive pathway for a portion of electrical current flowing between surface electrodes positioned on the skin and transmits that current to the target tissue. The implant has a passive electrical conductor of sufficient length to extend from subcutaneous tissue located below a surface cathodic electrode to the target tissue. The conductor has a pick-up end which forms an electrical termination having a sufficient surface area to allow a sufficient portion of the electrical current to flow through the conductor, in preference to flowing through body tissue between the surface electrodes, such that the target tissue is stimulated to either activate or block neural impulses. The conductor also has a stimulating end which forms an electrical termination for delivering the current to the target body tissue.
Owner:2249020 ALBERTA LTD
Implantable medical electrical stimulation lead fixation method and apparatus
InactiveUS6999819B2Optimize locationQuick placementSpinal electrodesExternal electrodesMuscle tissueMedicine
Owner:MEDTRONIC INC +1
Optimized sensor geometry for an implantable glucose sensor
InactiveUS20060200022A1Precise processAccurate measurementDiagnostic recording/measuringSensorsGlucose sensorsAnalyte
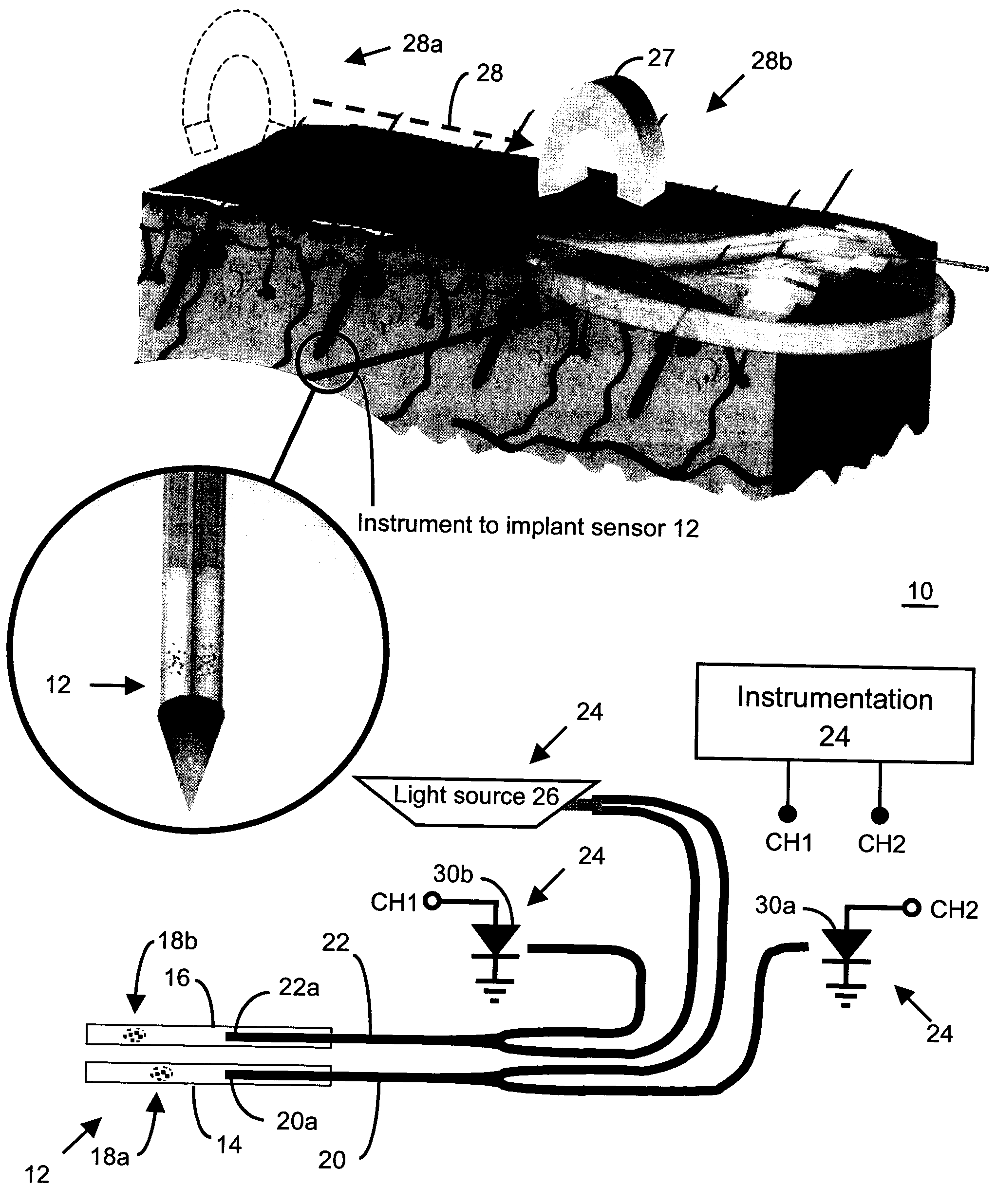
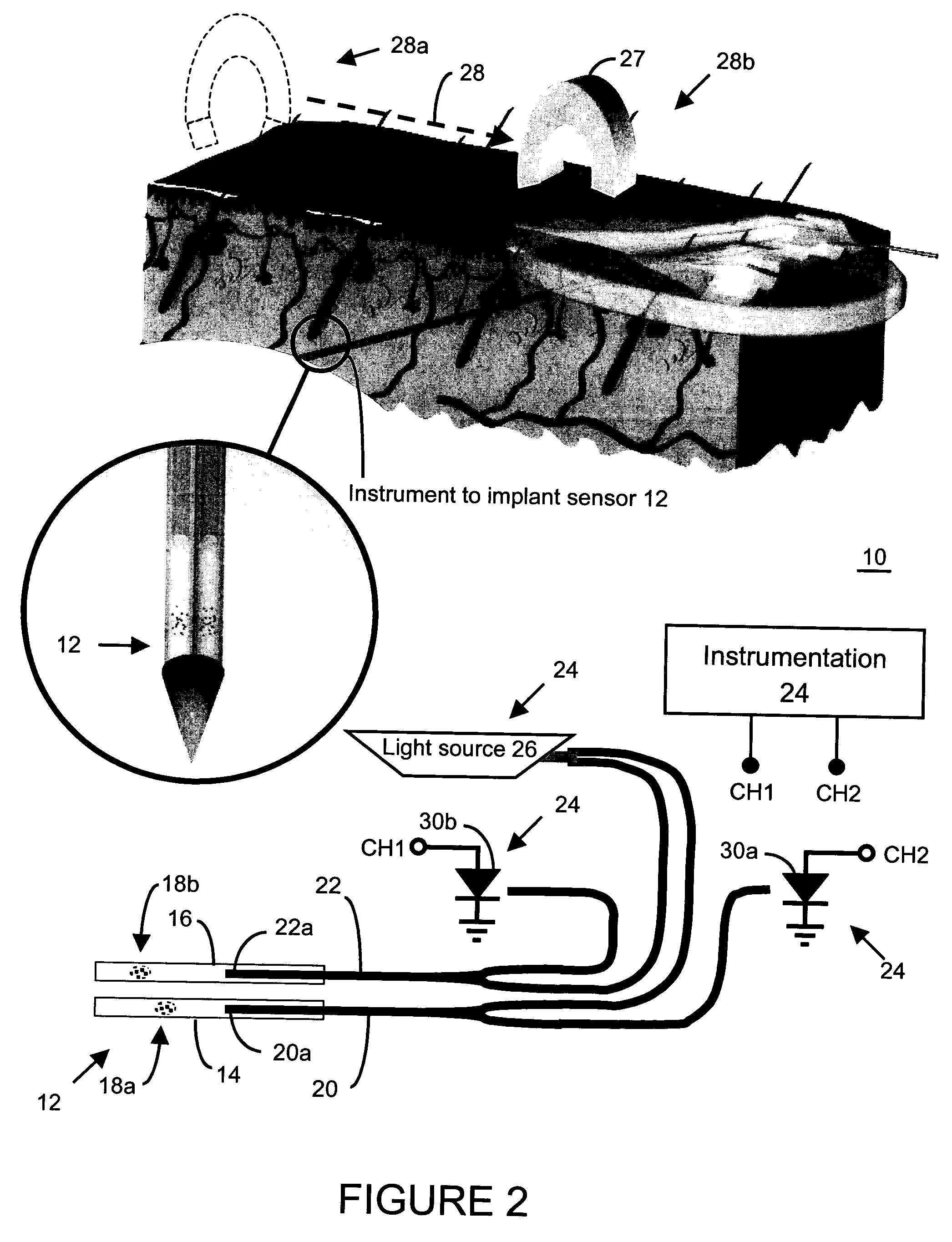
An implantable sensor for use in measuring a concentration of an analyte such as glucose in a bodily fluid, including a body with a sensing region adapted for transport of analytes between the sensor and the bodily fluid, wherein the sensing region is located on a curved portion of the body such that when a foreign body capsule forms around the sensor, a contractile force is exerted by the foreign body capsule toward the sensing region. The body is partially or entirely curved, partially or entirely covered with an anchoring material for supporting tissue ingrowth, and designed for subcutaneous tissue implantation. The geometric design, including curvature, shape, and other factors minimize chronic inflammatory response at the sensing region and contribute to improved performance of the sensor in vivo.
Owner:DEXCOM
Vascular insertion sheath with stiffened tip
InactiveUS20050096697A1High stiffness coefficientImprovement factorSurgical needlesSurgical veterinaryEngineeringSubcutaneous tissue
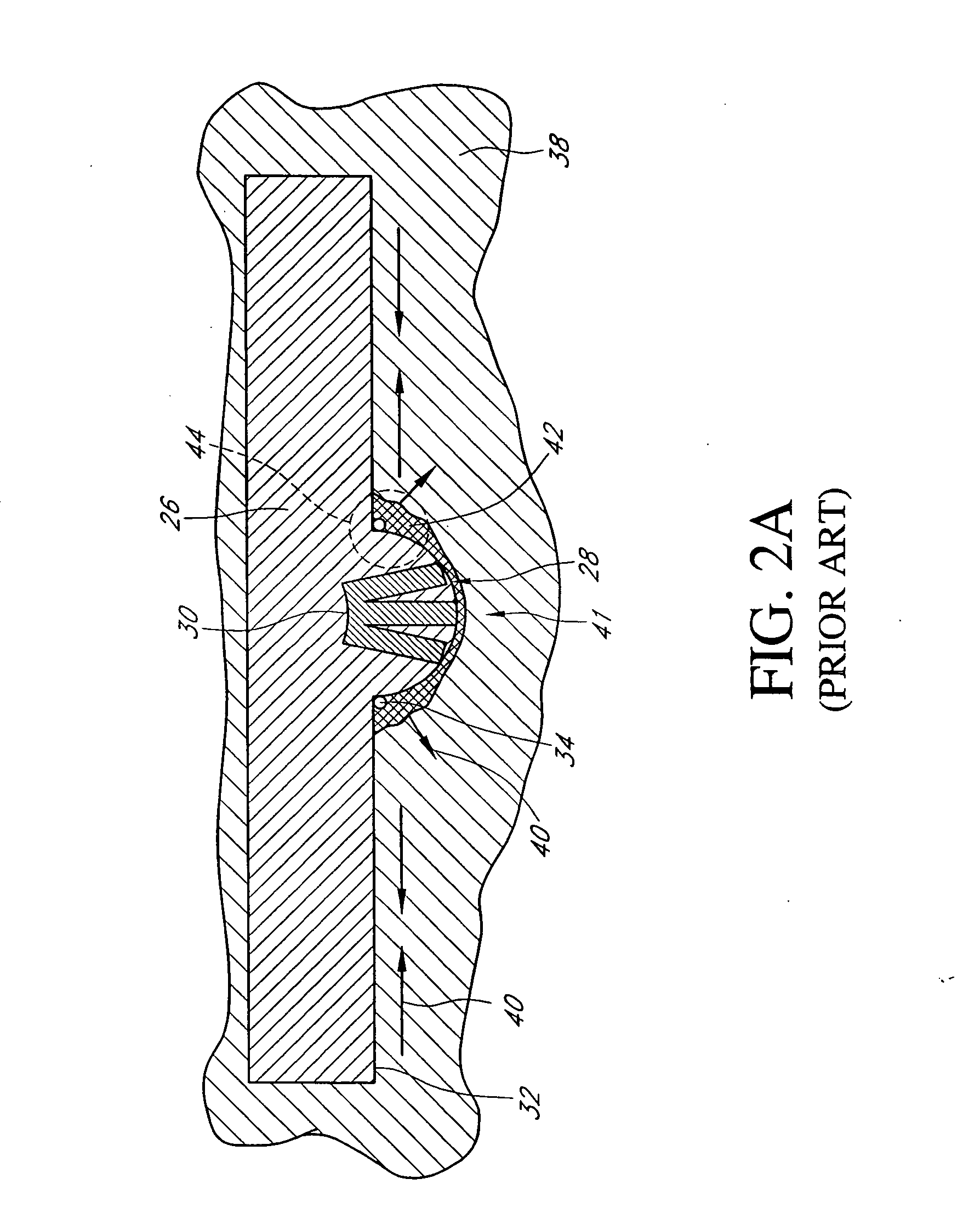
The present invention provides a method and apparatus for sealing a subcutaneous tissue puncture. The method and apparatus reduce the occurrence of anchor shuttling by stiffening a tip or end portion of an insertion sheath that acts as a one-way valve to a closure device anchor. The stiffening of the tip reduces shuttling by reducing or eliminating the tendency of prior insertion sheath tips from puckering and creating a gap into which the anchor may reenter. The method of stiffening may take on many different mechanisms, several of which are disclosed.
Owner:ST JUDE MEDICAL PUERTO RICO BV
Tissue Treatment Methods
ActiveUS20080077202A1Reduce and eliminate fat cellMinimize injuryChiropractic devicesSurgical instruments for heatingSubcutaneous adipose tissueReperfusion injury
Methods are provided herein for affecting a region of a subject's body, comprising exposing the region to a cooling element under conditions effective to cool subcutaneous adipose tissue in said region; and increasing the blood flow rate to the cooled tissue by exposing the tissue to an energy source. Methods are also provided for treating subcutaneous adipose tissue in a region of a subject's body, comprising exposing said region to a cooling element under conditions effective to cool said tissue; and exposing the tissue to an energy source to increase the blood flow rate to the cooled tissue, thereby stimulating reperfusion in, and / or causing an ischemia-reperfusion injury to, the cooled tissue.
Owner:ZELTIQ AESTHETICS INC
Vascular puncture seal anchor nest
The present invention provides a method and apparatus for sealing a subcutaneous tissue puncture. The method and apparatus increases the reliability of device function by creating a multi-level anchor nest in a carrier tube of a tissue puncture closure device. The multi-level nest allows an insertion sheath or other deployment member to slide between the carrier tube and the anchor to rotate and deploy the anchor within an artery or other lumen.
Owner:TERUMO PUERTO RICO L L C
Method for treating skin and underlying tissue
InactiveUS7189230B2Reduce the amount requiredSmall sizeElectrotherapyPneumatic massageSkin surfaceMedicine
A method for treating a tissue site couples an energy delivery surface of an electromagnetic energy delivery device with a skin surface. An underlying tissue area beneath the skin surface is created. The cooling creates a reverse thermal gradient, where a temperature of the skin surface is less than a temperature of the underlying tissue area. Energy is delivered from the energy delivery device to the underlying tissue area. The energy delivery modifies the underlying tissue area and decreases irregularities of the skin surface.
Owner:THERMAGE INC
Method and apparatus for determining local tissue impedance for positioning of a needle
InactiveUS20090036794A1Eliminate impedanceGood distinctionDiagnostic recording/measuringSensorsTissues typesSubcutaneous tissue
The invention relates to apparatus and methods for measuring local tissue impedance for subcutaneous tissue surrounding a needle tip inserted into a subject, impedance spectra and / or complex impedance values are determined. The invention applies a monopolar impedance measuring setup with a needle, a current-carrying electrode, an optional reference electrode. The setup is configured to eliminate contributions from the current-carrying electrode in order to measure local impedance of tissue in the close neighbourhood of the needle tip instead of an averaged value over the volume or current path between the needle and the electrode(s). The determined impedance can be correlated with either a tissue type or state, or with a position of the needle tip in the subject, and can thereby provide an insertion history to the operator in the form of impedance or corresponding tissue type as a function of insertion depth or time.
Owner:UNIV OSLO HF
Electroporation devices and methods of using same for electroporation of cells in mammals
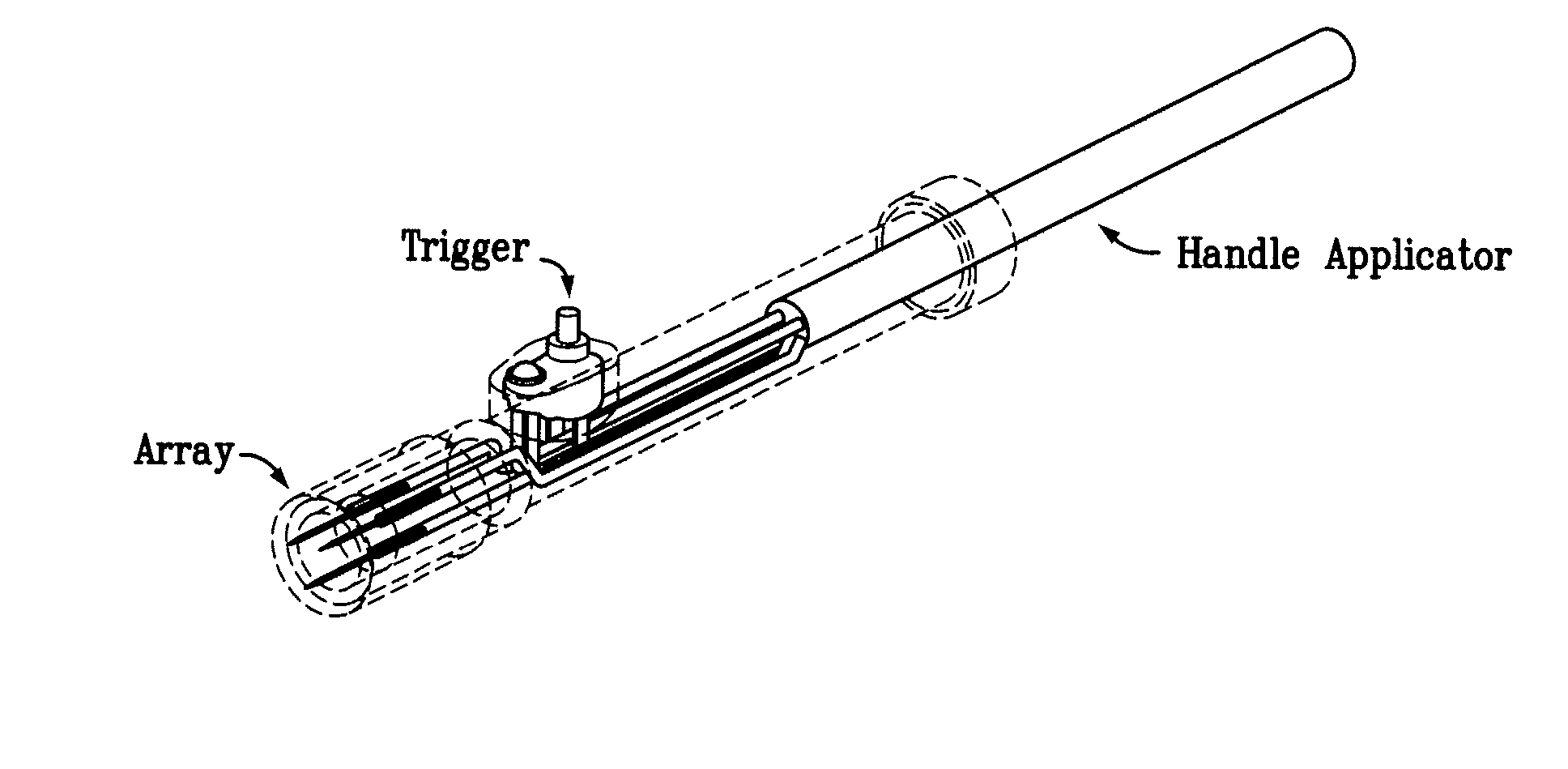
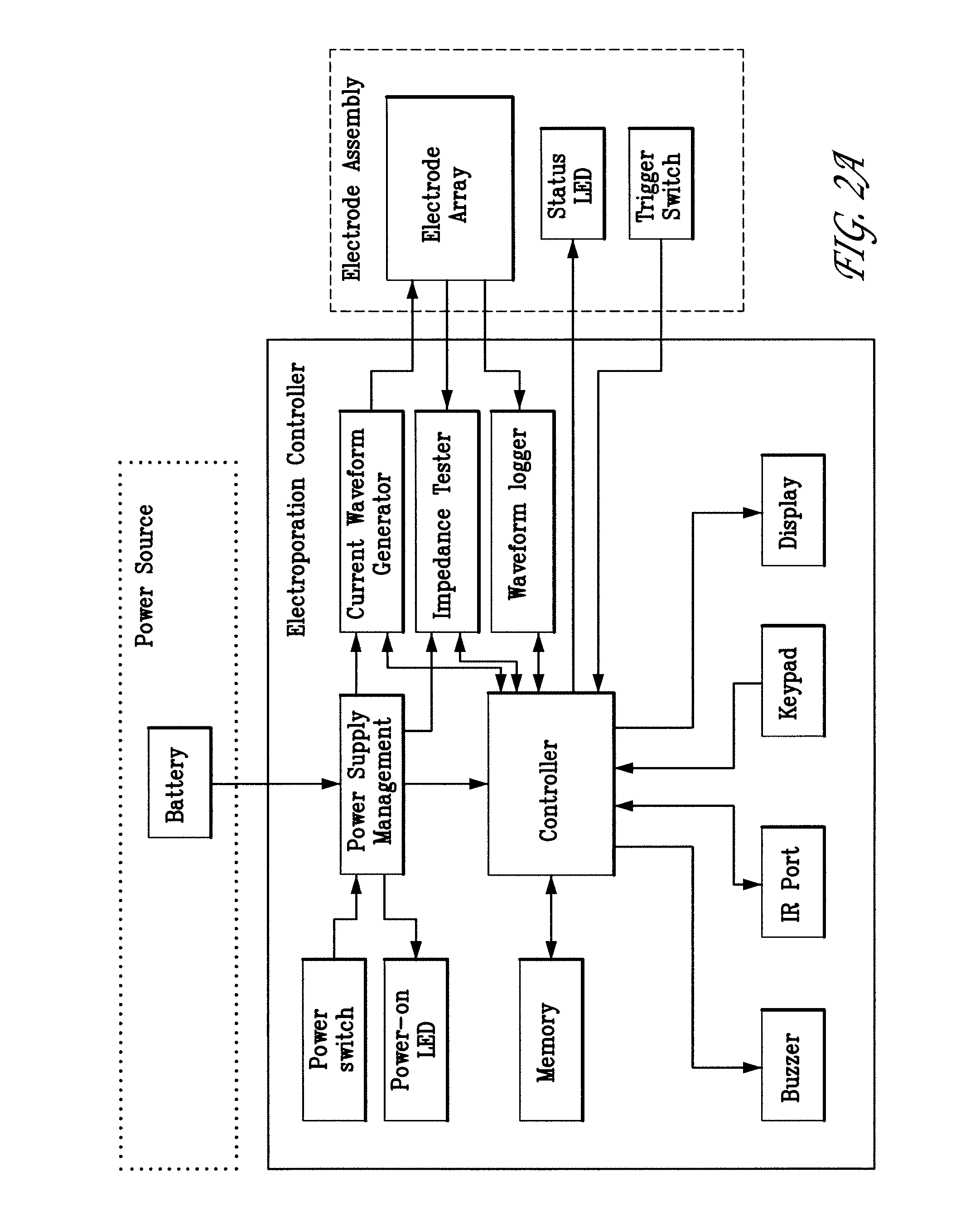
Aspects of the present invention relate to electroporation devices and methods of using same to effectively facilitate the introduction of a biomolecule into cells of a selected tissue in a body, in particular skin such as intradermic or subcutaneous tissue. In some aspects, the present invention is a skin EP device, which produces a pulse of energy and delivers same to the skin tissue using a skin electrode array and maintains a constant current in the same skin tissue based on user input, including a preset current, and allows the storage and acquisition of current waveform data.
Owner:INOVIO PHARMA
Dialysis catheter and methods of insertion
InactiveUS6858019B2Reduce overall outer diameterMulti-lumen catheterDiagnosticsVeinSuperior vena cava
A method of inserting a dialysis catheter into a patient comprising the steps of inserting a guidewire into the jugular vein of the patient through the superior vena cava and into the inferior vena cava, providing a trocar having a lumen and a dissecting tip, inserting the trocar to enter an incision in the patient and to create a subcutaneous tissue tunnel, threading the guidewire through the lumen of the trocar so the guidewire extends through the incision, providing a dialysis catheter having first and second lumens, removing the trocar, and inserting the dialysis catheter over the guidewire through the incision and through the jugular vein and superior vena cava into the right atrium.
Owner:ARGON MEDICAL DEVICES
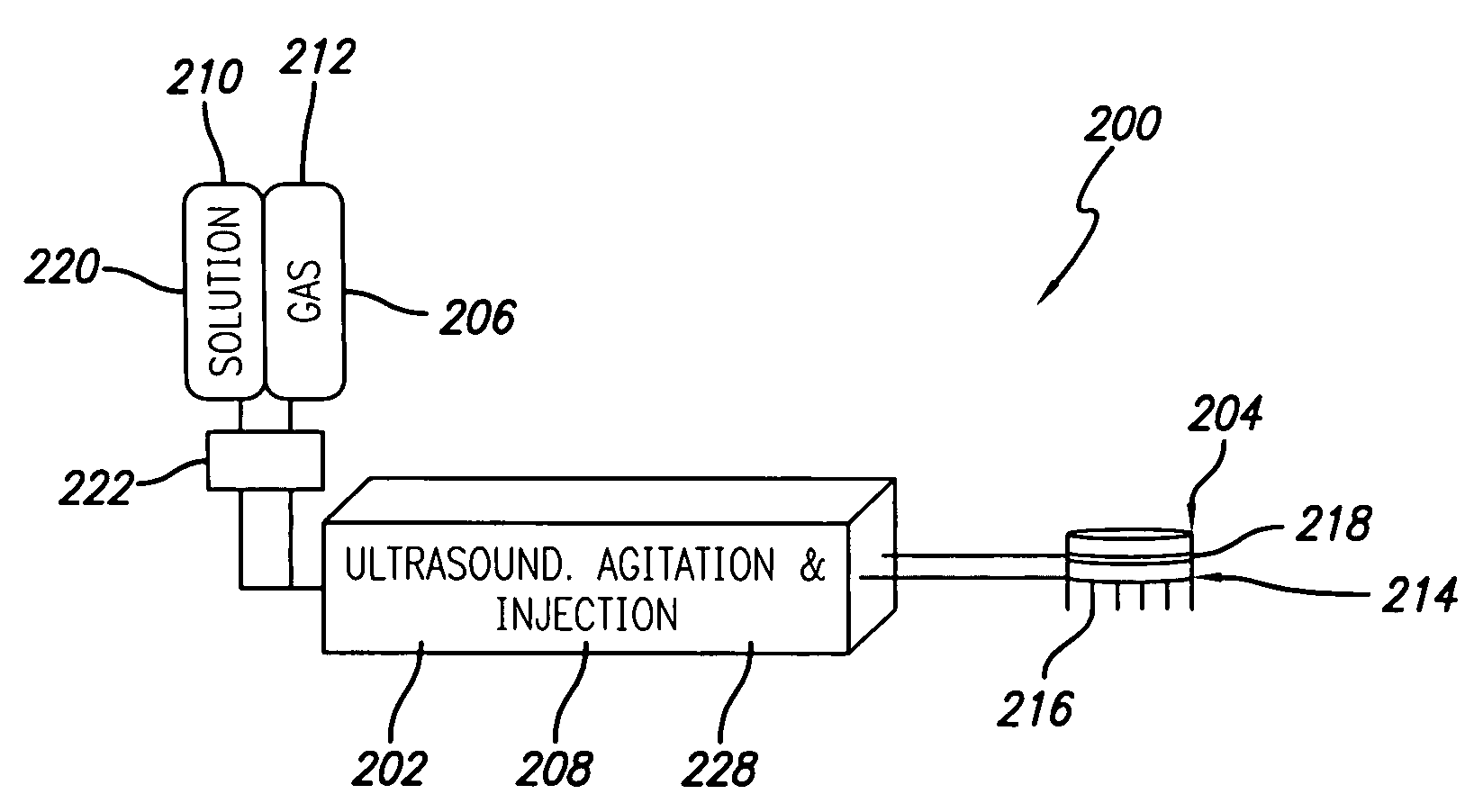
Method for treating subcutaneous tissues
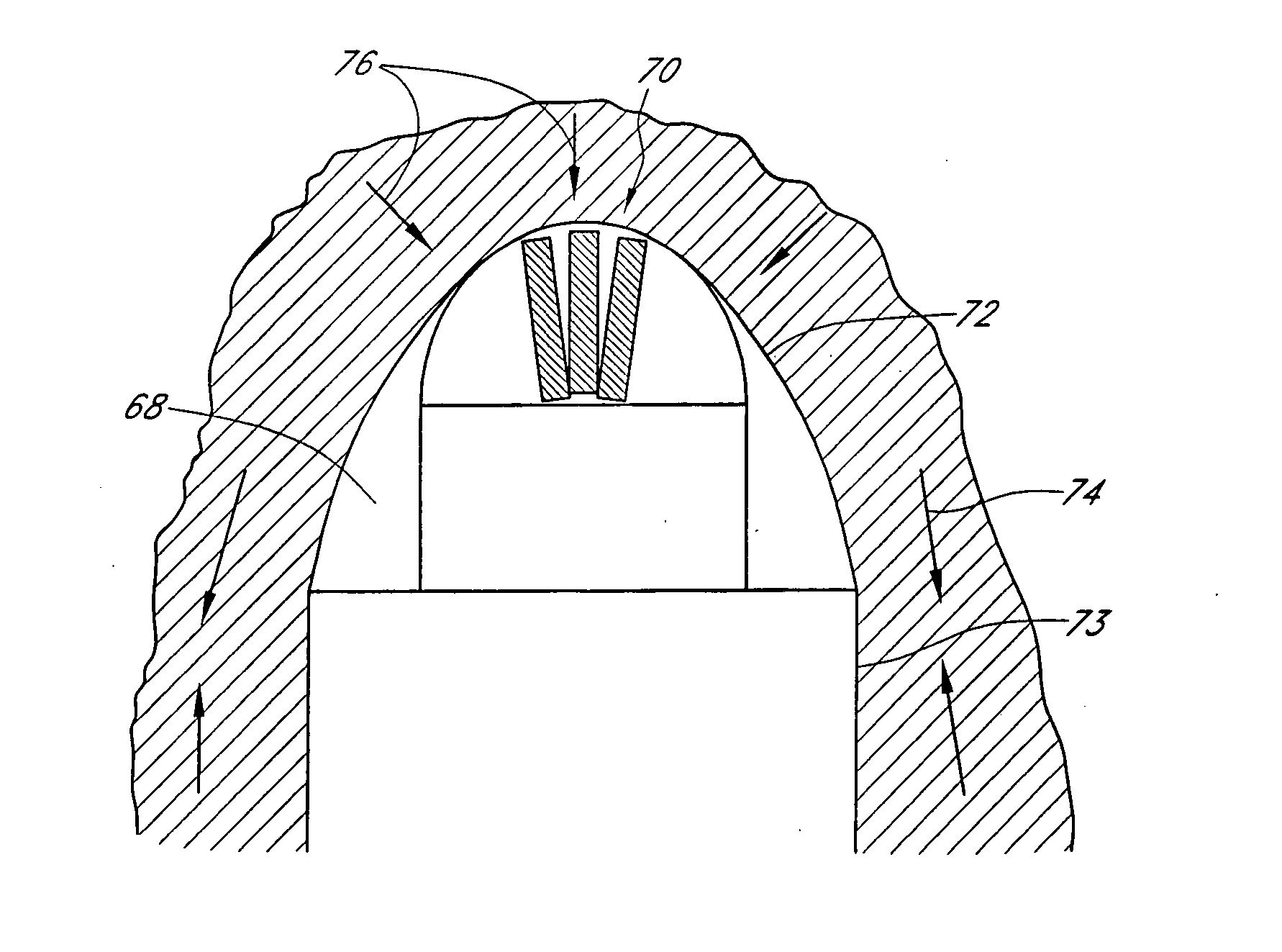
ActiveUS20070055179A1Sufficient powerEliminate riskUltrasonic/sonic/infrasonic diagnosticsElectrotherapyAbnormal tissue growthCavitation
An apparatus and method for treating subcutaneous tissues using acoustic waves in the range of low acoustic pressure ultrasound waves is disclosed. The method includes injections of enhancing agents, wherein disruption of subcutaneous tissues and subcutaneous cavitational bioeffects are produced by ultrasound waves having a power that will not produce tissue cavitation in the absence of the enhancing agents. The apparatus and method of use is useful for treatment of subcutaneous abnormalities including cellulite, lipomas, and tumors.
Owner:ULTHERA INC
Vascular puncture seal anchor nest
ActiveUS20050125030A1Reducing anchor shuttleSuture equipmentsSurgical veterinarySubcutaneous tissueBiomedical engineering
The present invention provides a method and apparatus for sealing a subcutaneous tissue puncture. The method and apparatus increases the reliability of device function by creating a multi-level anchor nest in a carrier tube of a tissue puncture closure device. The multi-level nest allows an insertion sheath or other deployment member to slide between the carrier tube and the anchor to rotate and deploy the anchor within an artery or other lumen.
Owner:TERUMO PUERTO RICO L L C
Peripheral nerve stimulation
An apparatus for treating pain by electrical stimulation is disclosed. A lead is placed subcutaneously in the region of pain. The subcutaneous tissue is electrically stimulated to cause paresthesia. The method encompasses subcutaneous placement of an electrical lead near the region of pain and subsequent electrical stimulation of the tissue to cause paresthesia. In particular, an apparatus for treating intractable occipital neuralgia using percutaneous electrostimulation techniques is disclosed.
Owner:WEINER RICHARD L
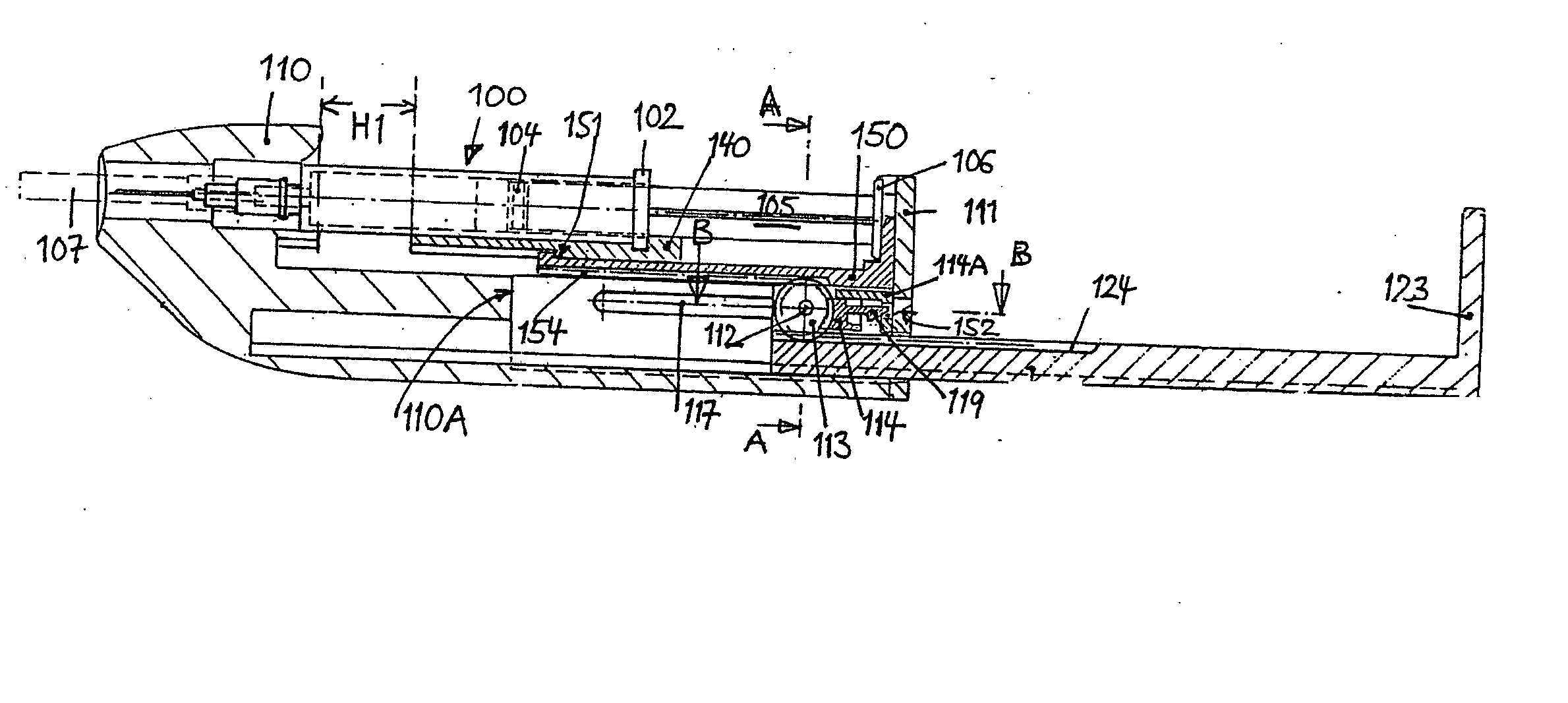
Injection device
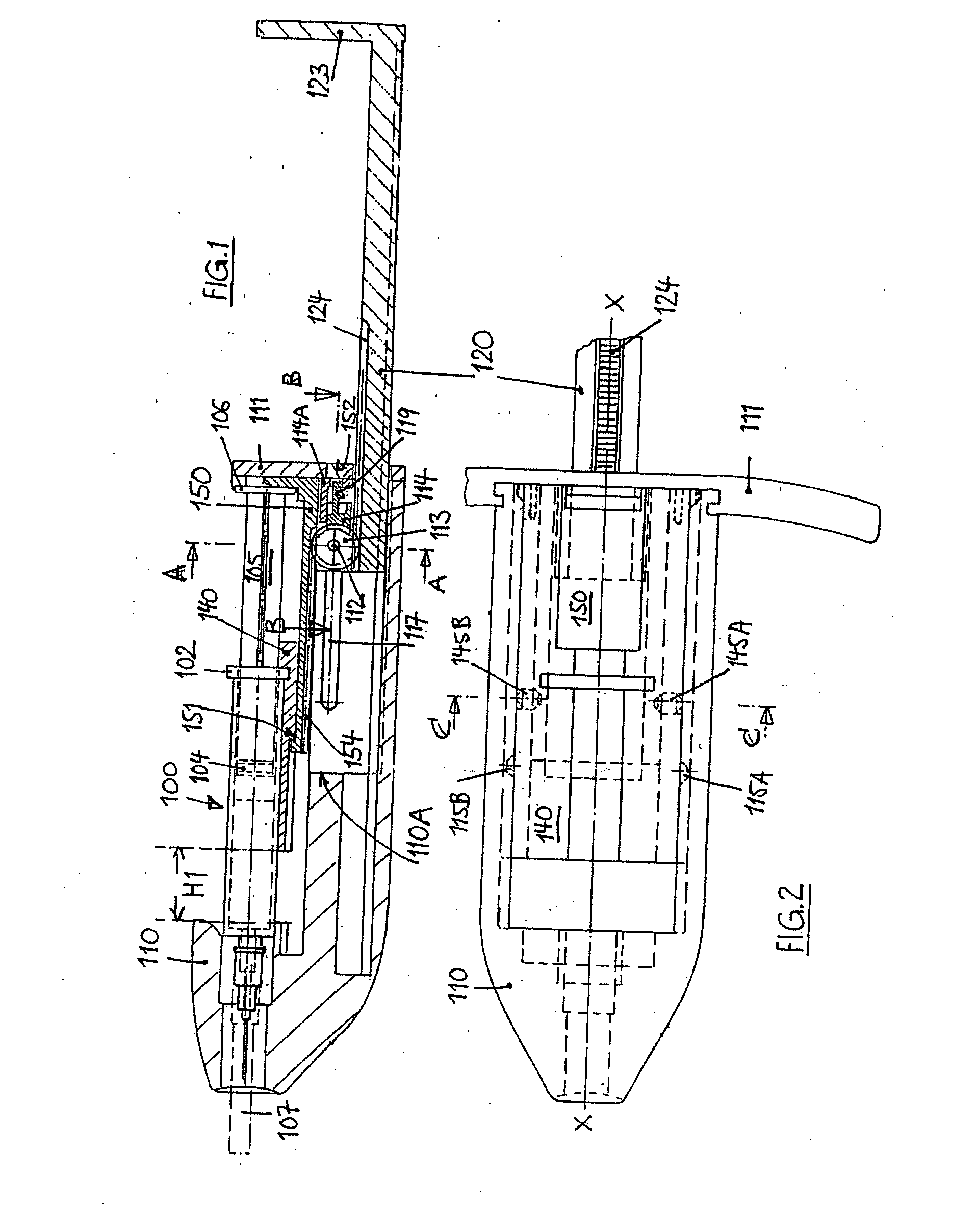
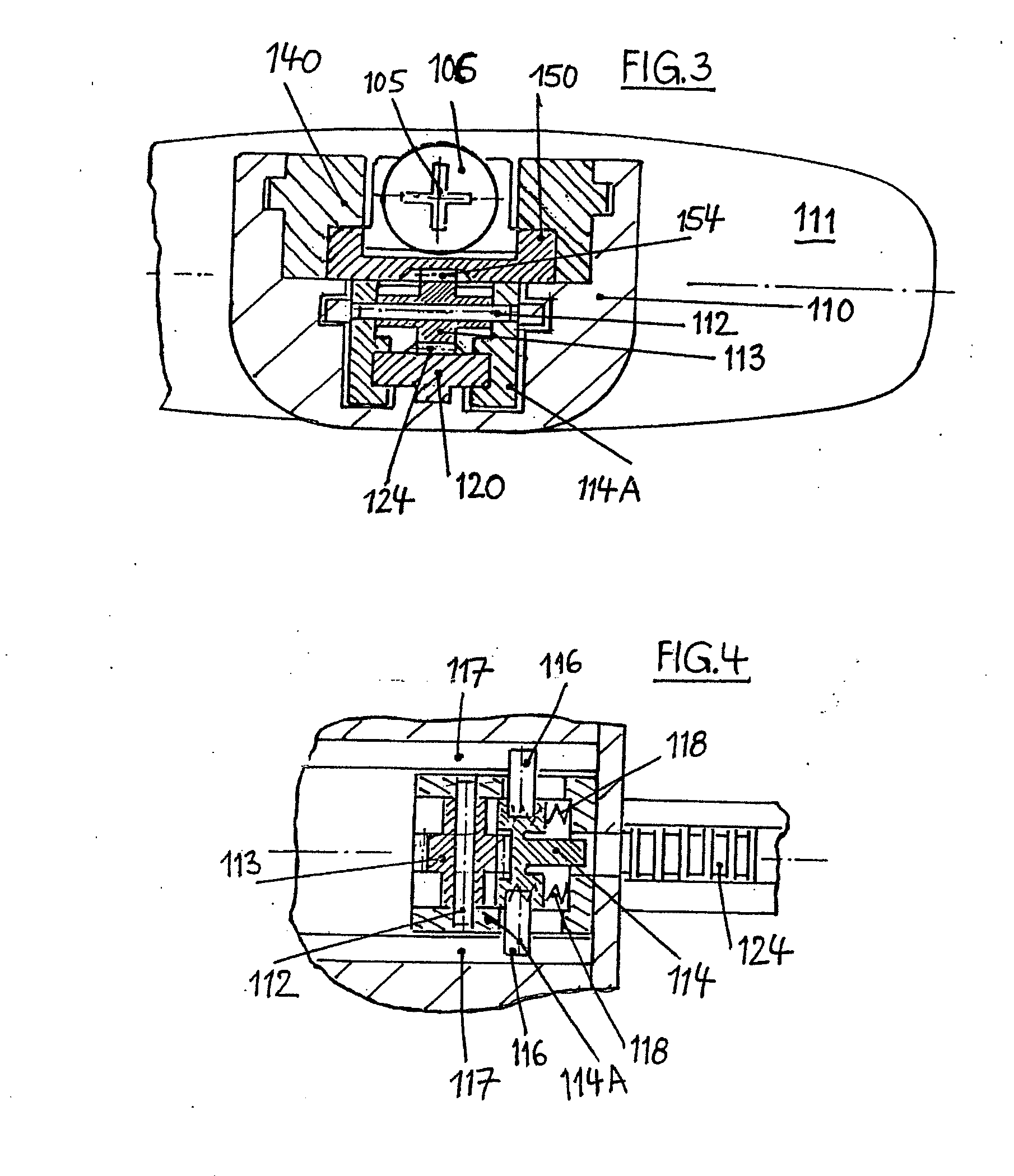
InactiveUS20060258990A1HandlingSimple and safe operationAutomatic syringesMedical devicesRisk strokeSubcutaneous tissue
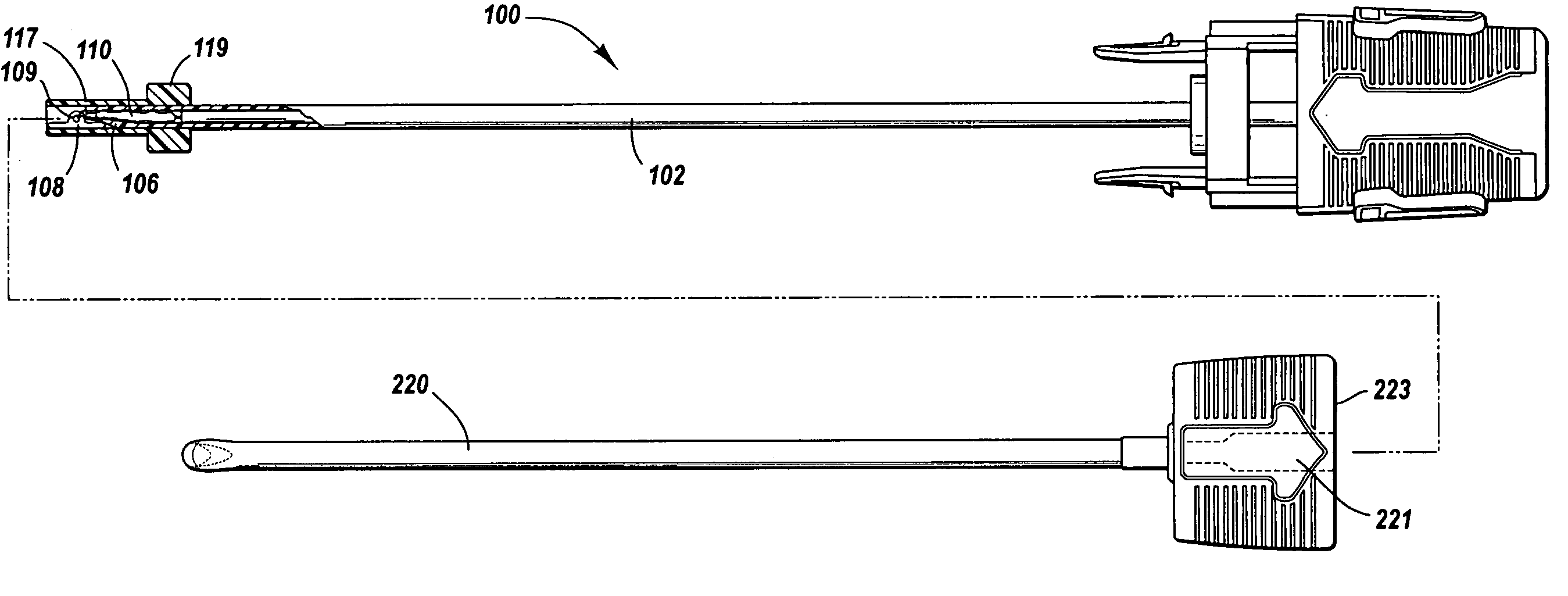
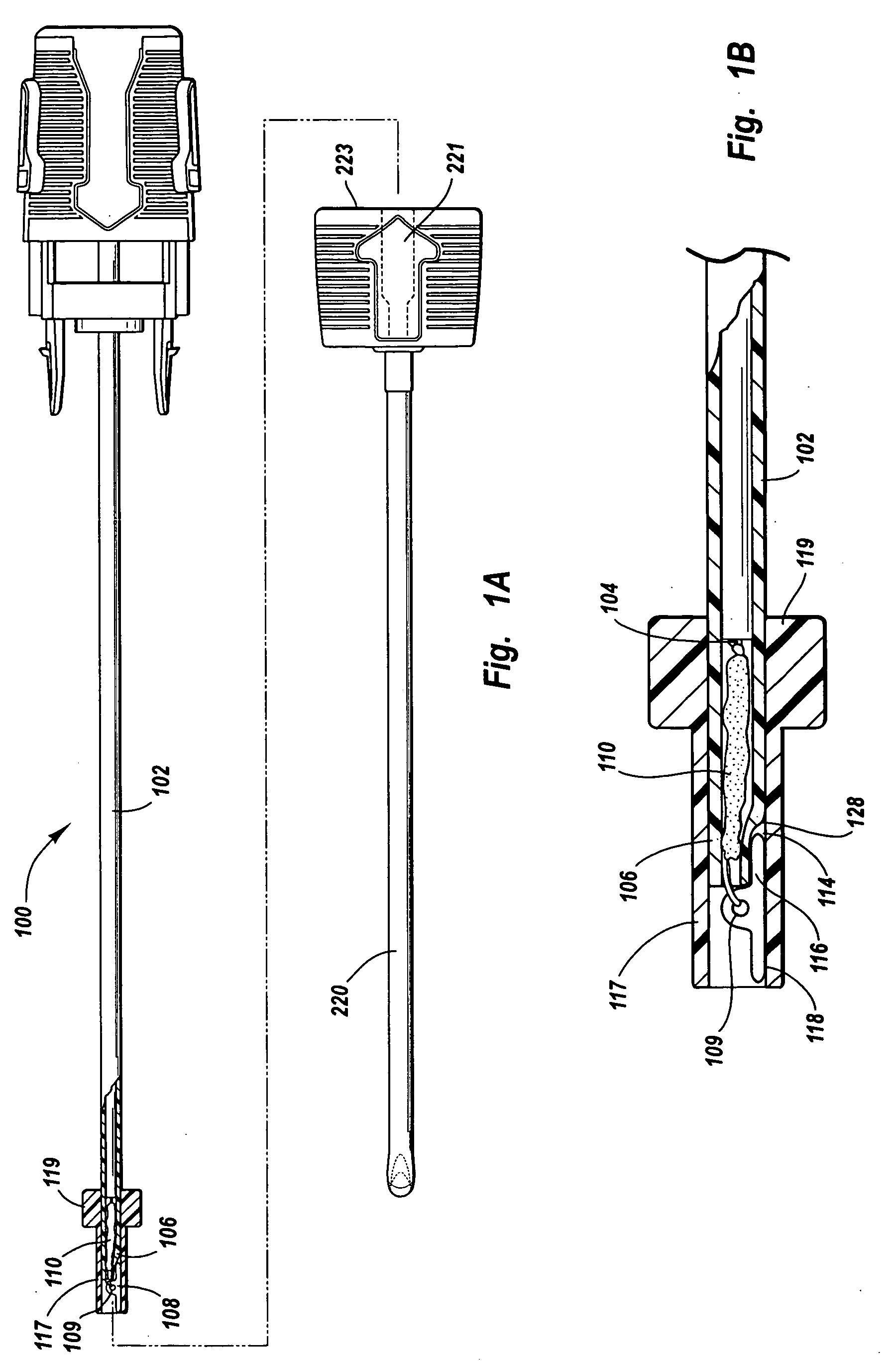
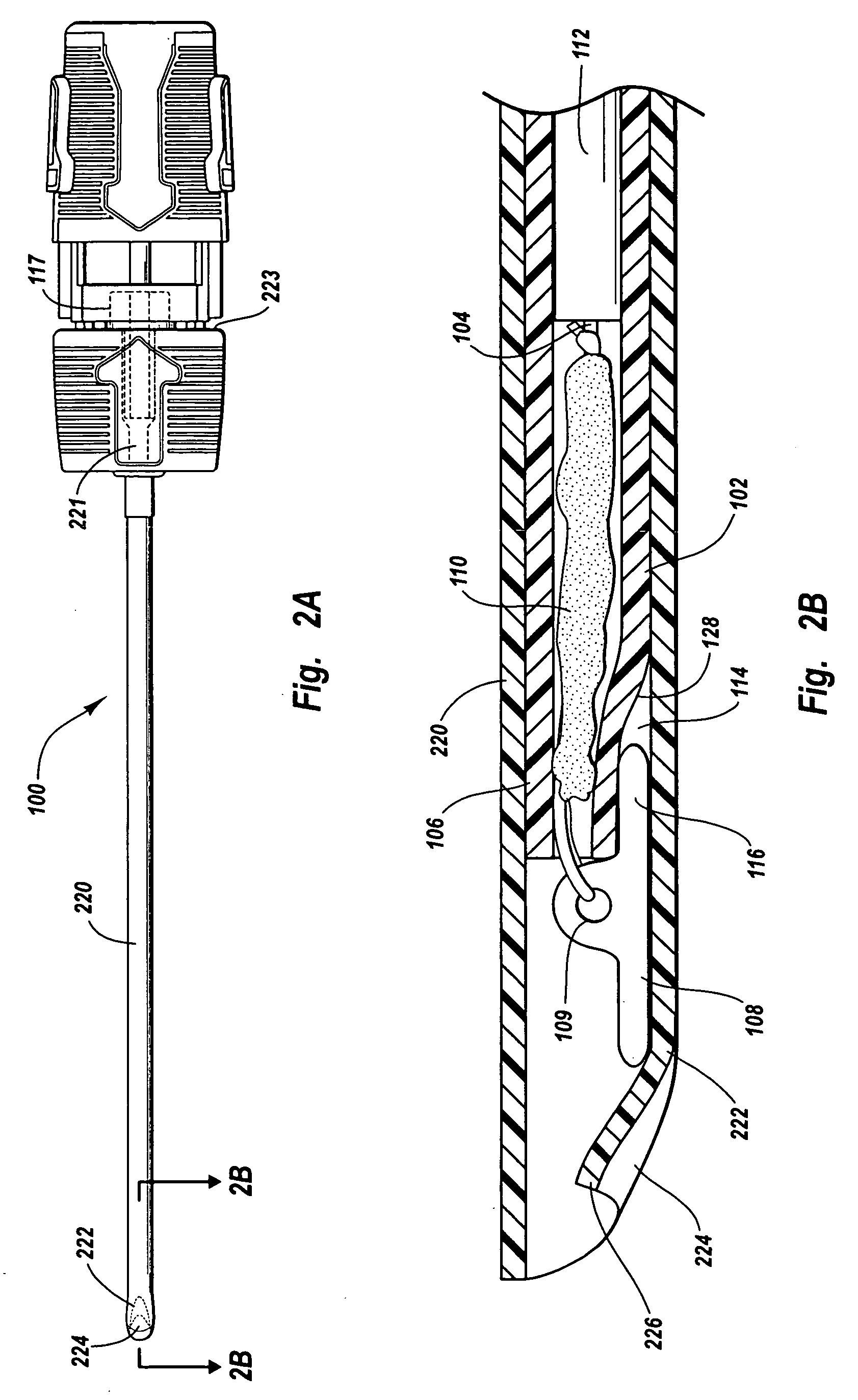
An injection device for a syringe, having a syringe body, a cannula with a needle, a plunger with a plunger rod, and an injection carriage for displacing the syringe body and the plunger, comprises at least one actuating element that acts on the injection carriage to carry out the injection procedure. The actuating element (120, 220, 320) cooperates with components which withdraw the needle (108, 208, 308) from the puncture site once the injection procedure has been completed, using a return stroke (H3) that is applied to the injection carriage. A single, targeted linear movement inserts the needle to a defined depth, injects the medicament and, once the injection has been completed, produces a return stroke which allows the needle to be withdrawn into the housing and thus out from the puncture site. The injection device is advantageously equipped with additional components which produce a delay (TV) between the completion of the injection stroke (H2) and the start of the return stroke (H3). The advantage of said delay is that the pressure that has been produced in the subcutaneous tissue by the injection of the medicament can subside before the needle is withdrawn, thus preventing to a great extent the penetration of the medicament into the insertion channel of the needle. A volume adapter (410) can advantageously be used to predetermine the injection stroke (H2) and thus the quantity of a medicament that is administered during the course of the injection stroke (H2).
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Method, kit and device for the treatment of cosmetic skin conditions
The present invention concerns a method and a kit, for the treatment of a selected area of the skin and / or subcutaneous tissue, and in particular for the cosmetic treatment of skin conditions such as regional fat deposits including cellulite. The method comprises heating the selected area to a sustained skin temperature of about 32 to about 50° C. for a desired period of time, using a device comprising either a heat source capable of conductively heating the selected area or, alternatively, an infra-red source for emitting infra-red with a wavelength from about 700 nm to about 15000 nm. The method also comprises administering, simultaneously or sequentially in either order, a composition containing an active agent selected from the group comprising a skin active, a nutrient absorption suppressant or a thermogenic agent, preferably a metabolic stimulating agent, more preferably a lipolytic agent, or a mixture thereof, the composition being either a topical composition for application to the selected area, or an area adjacent thereto, or an oral composition.
Owner:THE PROCTER & GAMBLE COMPANY
Method and apparatus for analyte sensing
InactiveUS7226414B2Reducing dispersion viscosityLow viscosityCatheterDiagnostic recording/measuringRare-earth elementEngineering
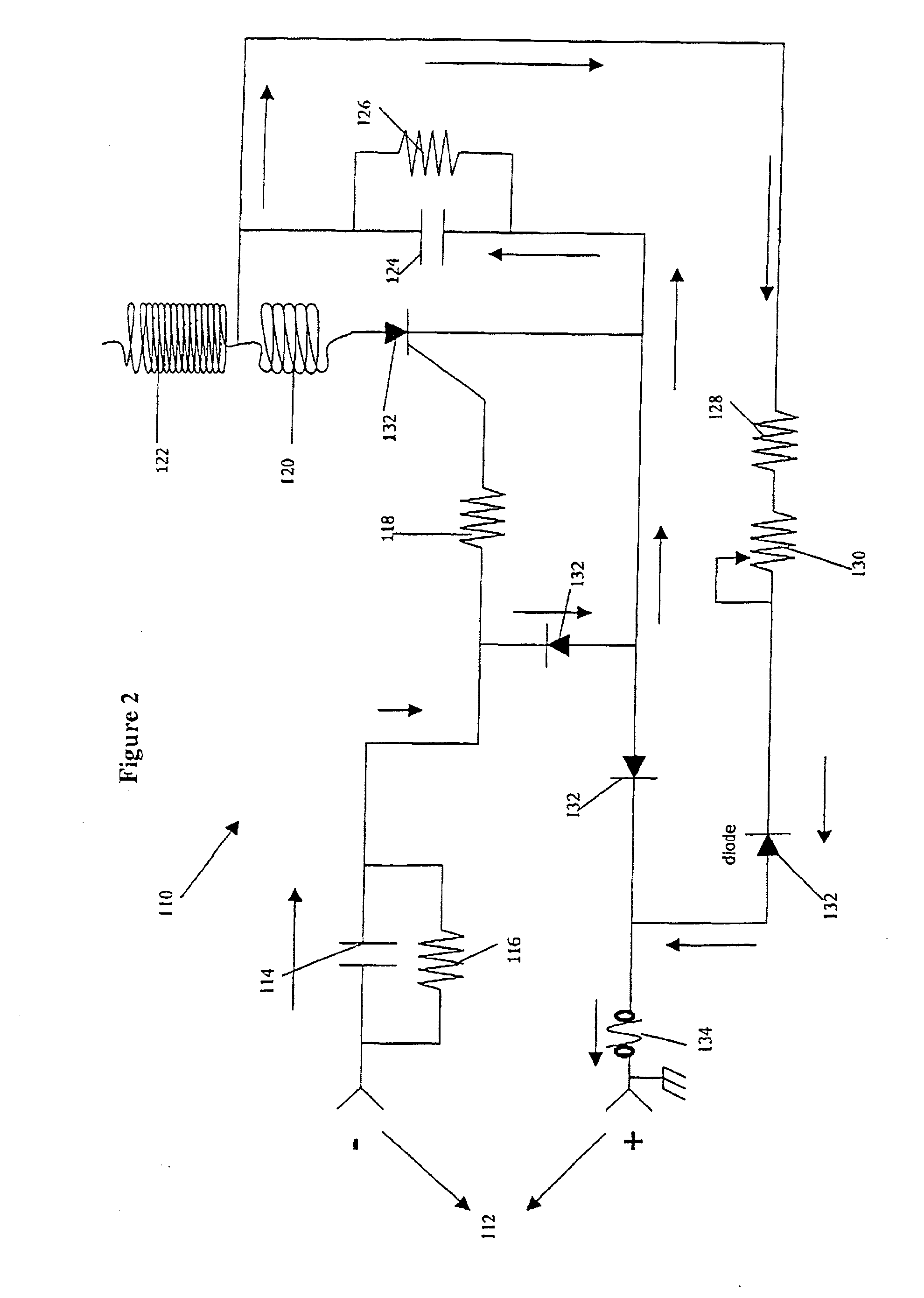
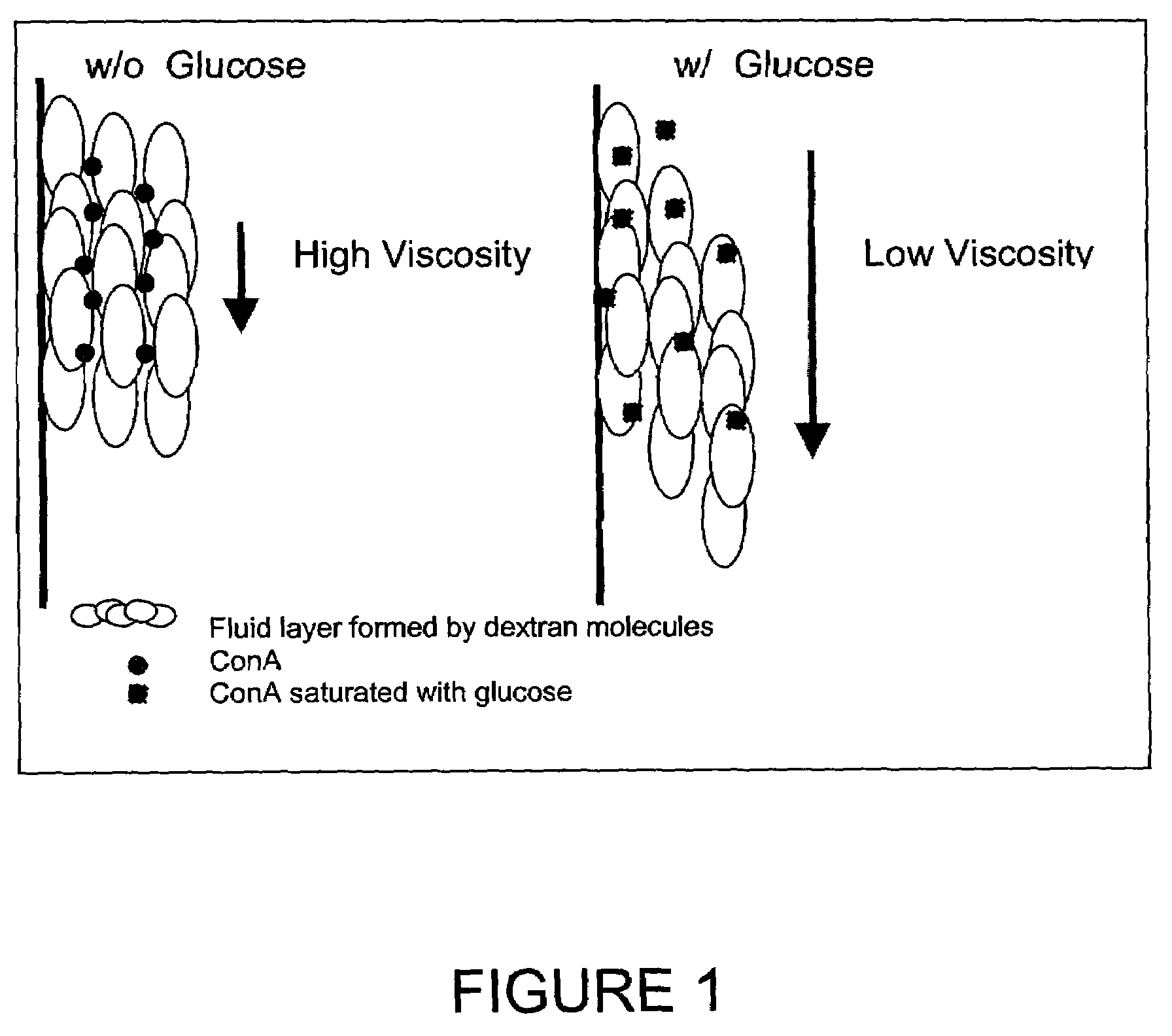
In one aspect, the present invention is directed to a glucose sensing device for implantation within subcutaneous tissue of an animal body. In one embodiment, the glucose sensing device includes a first chamber containing first magnetic particles and a hydrocolloid solution (for example, ConA-dextran hydrocolloid) wherein the first magnetic particles are dispersed in the hydrocolloid solution. In operation, glucose within the animal may enter and exit the first chamber and the hydrocolloid solution changes in response to the presence or concentration of glucose within the first chamber. The sensing device also includes a reference chamber containing second magnetic particles and a reference solution wherein the second magnetic particles are dispersed in the reference solution. The reference solution (for example, oil or alcohol compounds) includes a known or fixed viscosity. The reference solution may also be a hydrocolloid solution (for example, ConA-dextran hydrocolloid). The first and / or second magnetic particles may include amine-terminated particles, at least one rare earth element (for example, neodymium or samarium), and / or a ferromagnetic material.
Owner:BIOTEX
Method and system for treating muscle, tendon, ligament and cartilage tissue
InactiveUS20080071255A1Reduce the angleSmall sizeUltrasound therapyOrgan movement/changes detectionCuldoplastyLigament structure
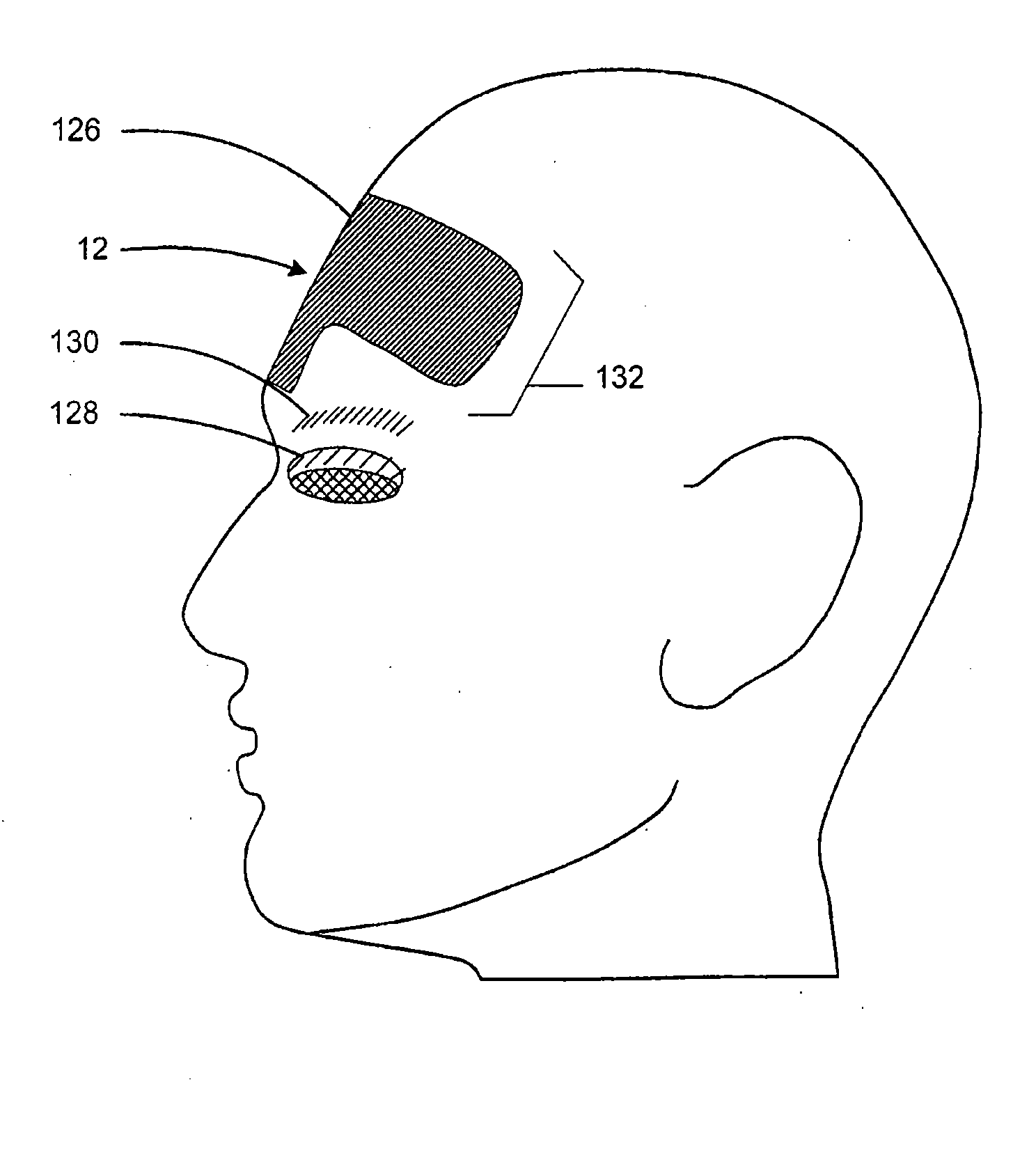
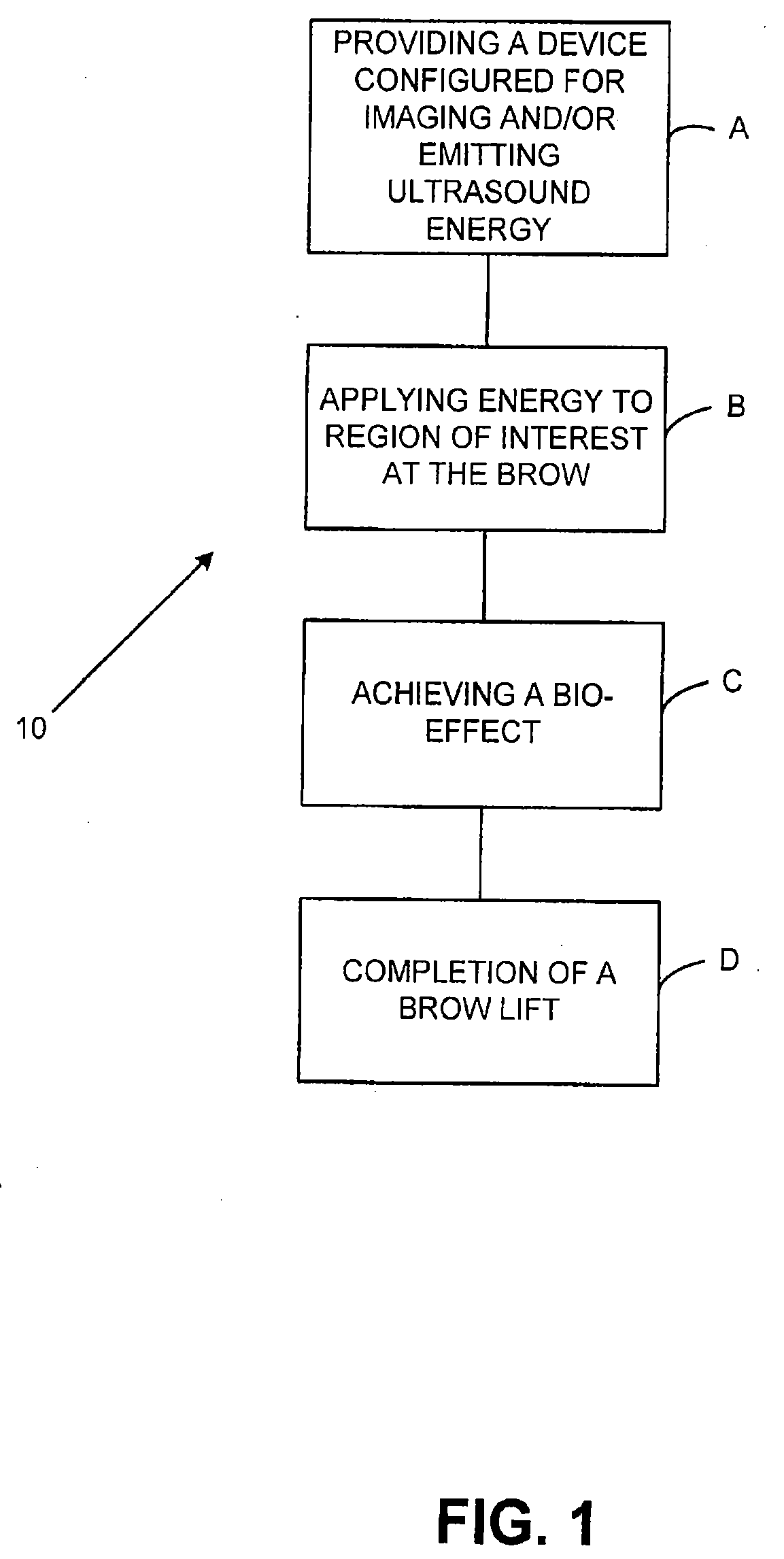
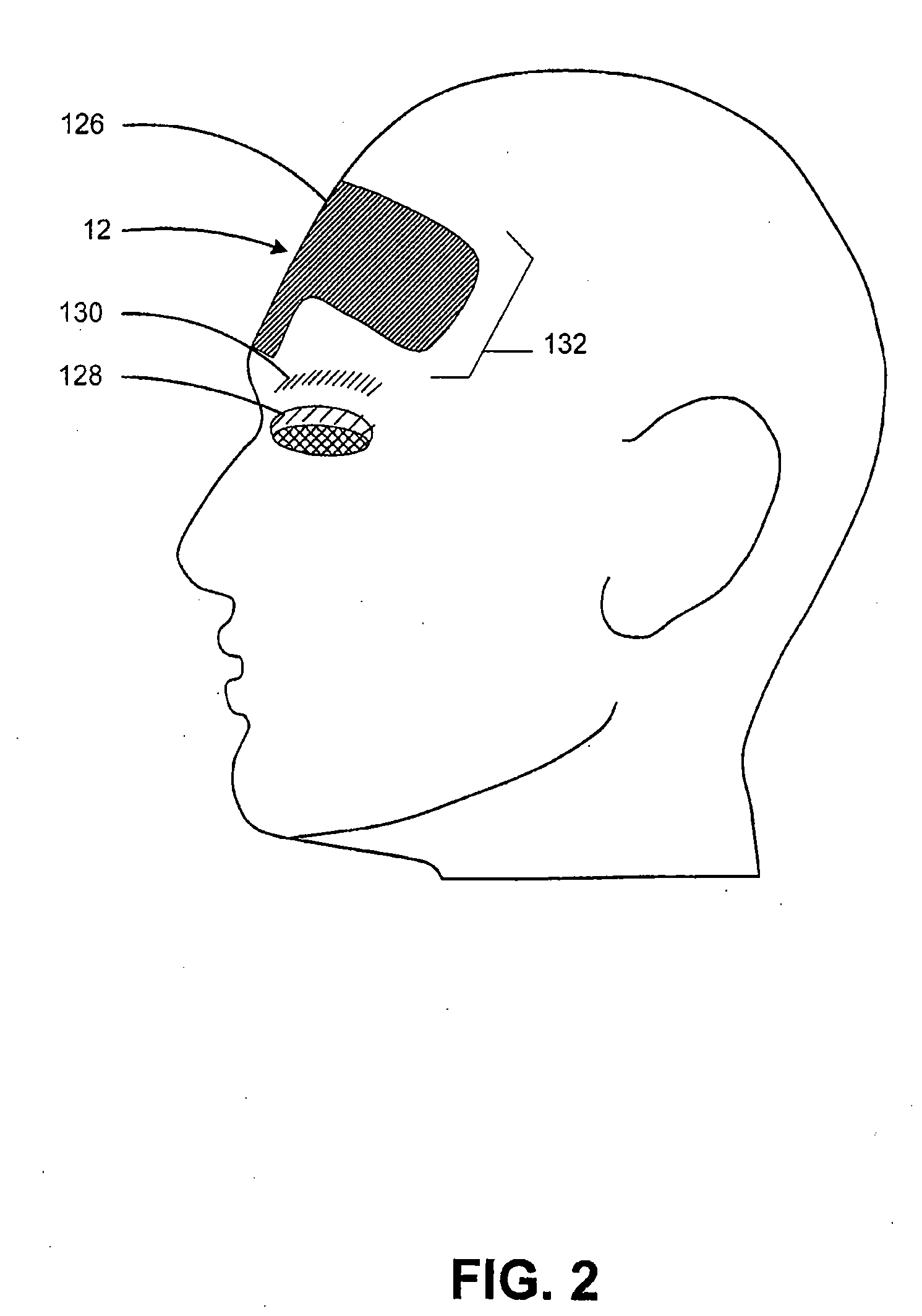
A method and system for treating subcutaneous tissue with energy such as ultrasound energy is disclosed. In various exemplary embodiments, ultrasound energy is applied at a region of interest to affect tissue by cutting, ablating, micro-ablating, coagulating, or otherwise affecting the subcutaneous tissue to conduct numerous procedures that are traditionally done invasively in a non-invasive manner. Certain exemplary procedures include a brow lift, a blepharoplasty, and treatment of cartilage tissue.
Owner:GUIDED THERAPY SYSTEMS LLC
Optimized sensor geometry for an implantable glucose sensor
InactiveUS20060211921A1Accurate measurementDiagnostic recording/measuringSensorsGlucose sensorsChronic inflammatory response
An implantable sensor for use in measuring a concentration of an analyte such as glucose in a bodily fluid, including a body with a sensing region adapted for transport of analytes between the sensor and the bodily fluid, wherein the sensing region is located on a curved portion of the body such that when a foreign body capsule forms around the sensor, a contractile force is exerted by the foreign body capsule toward the sensing region. The body is partially or entirely curved, partially or entirely covered with an anchoring material for supporting tissue ingrowth, and designed for subcutaneous tissue implantation. The geometric design, including curvature, shape, and other factors minimize chronic inflammatory response at the sensing region and contribute to improved performance of the sensor in vivo.
Owner:DEXCOM
Treatment of indications using electrical stimulation
ActiveUS20110282412A1Promote wound healingReduce joint painElectrotherapyArtificial respirationElectrical conductorElectrical stimulations
In one embodiment, a method includes implanting an implant entirely under the subject's skin. The implant includes a passive electrical conductor of sufficient length to extend from subcutaneous tissue located below one of a surface cathodic electrode and a surface anodic electrode to the tibial nerve. The surface electrodes are positioned in spaced relationship on the subject's skin, with one of the electrodes positioned over the pick-up end of the electrical conductor such that the portion of the current is transmitted through the conductor to the tibial nerve, and such that the current flows through the tibial nerve and returns to the other of the surface cathodic electrode and the surface anodic electrode. An electrical current is applied between the surface cathodic electrode and the surface anodic electrode to cause the portion of the electrical current to flow through the implant to stimulate the tibial nerve.
Owner:BIONESS
Apparatus and method for percutaneous sealing of blood vessel punctures
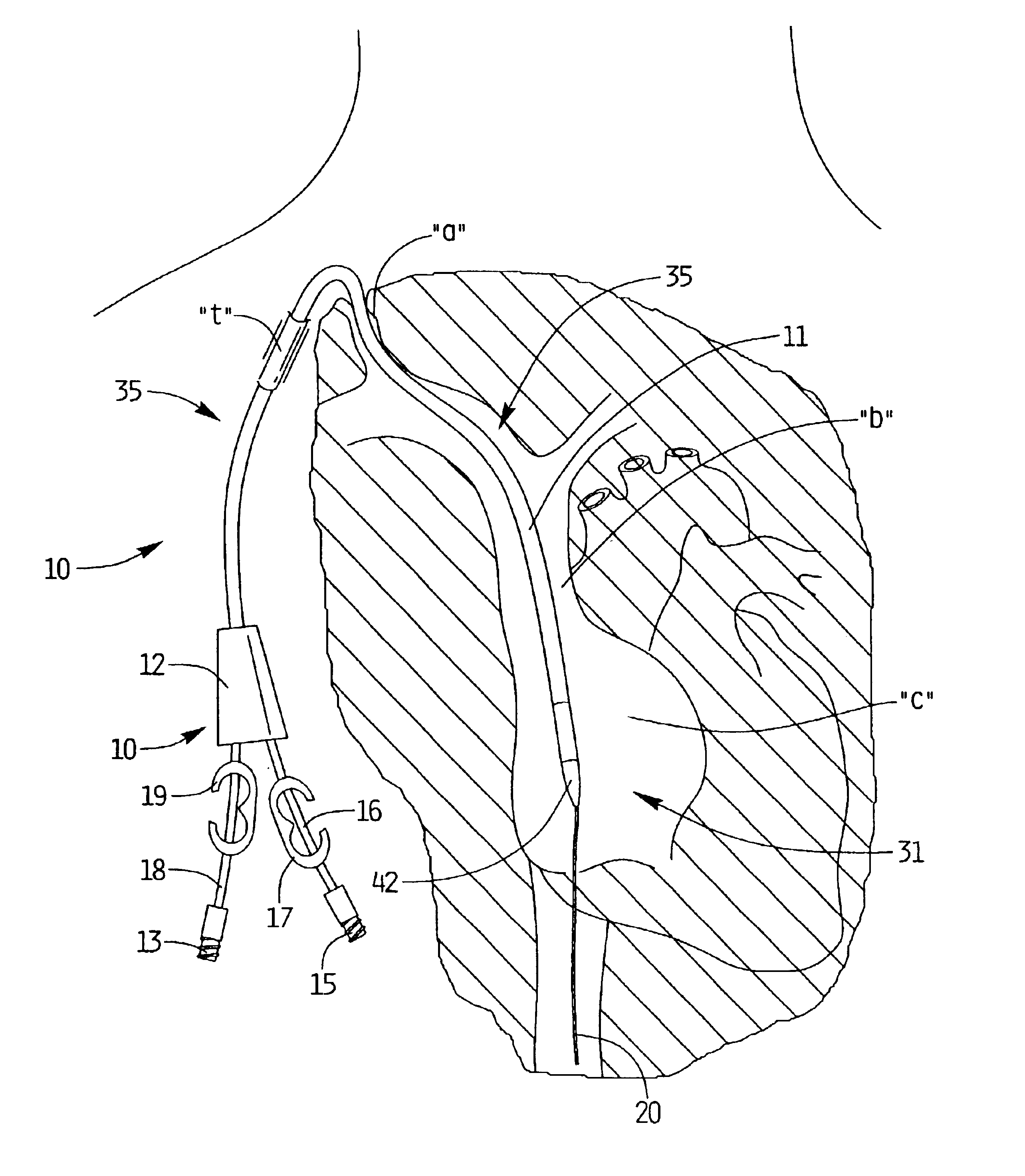
InactiveUS7175646B2Minimize timeFacilitate possible reentrySurgical needlesDilatorsVia incisionSubcutaneous tissue
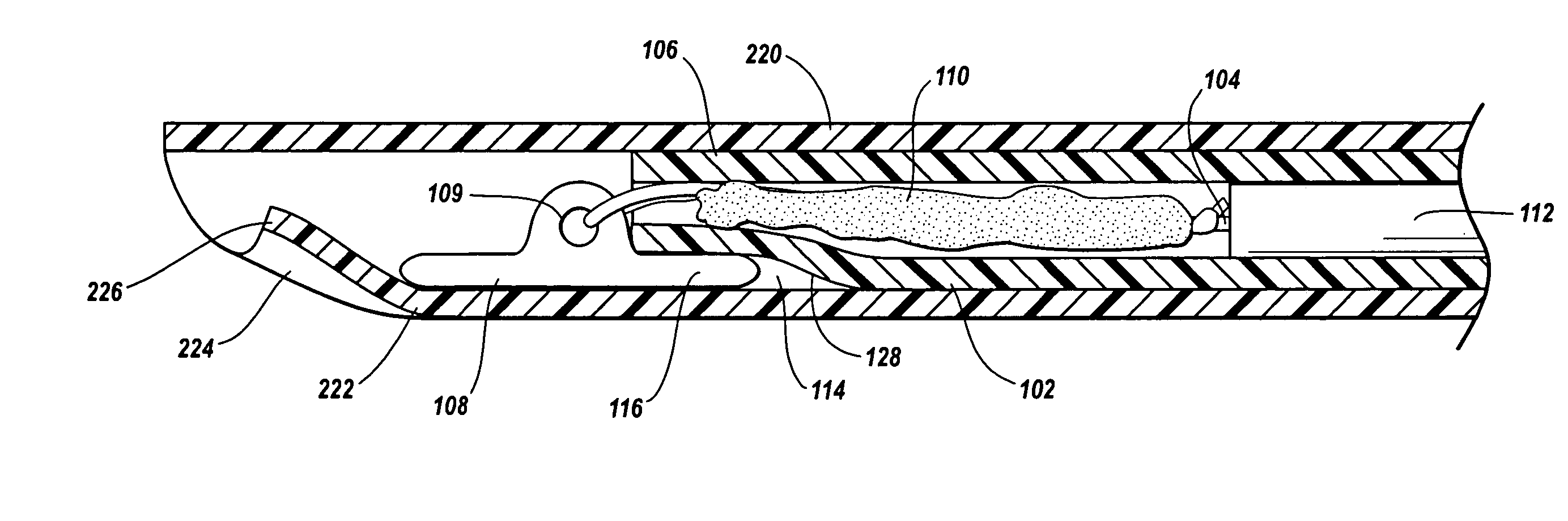
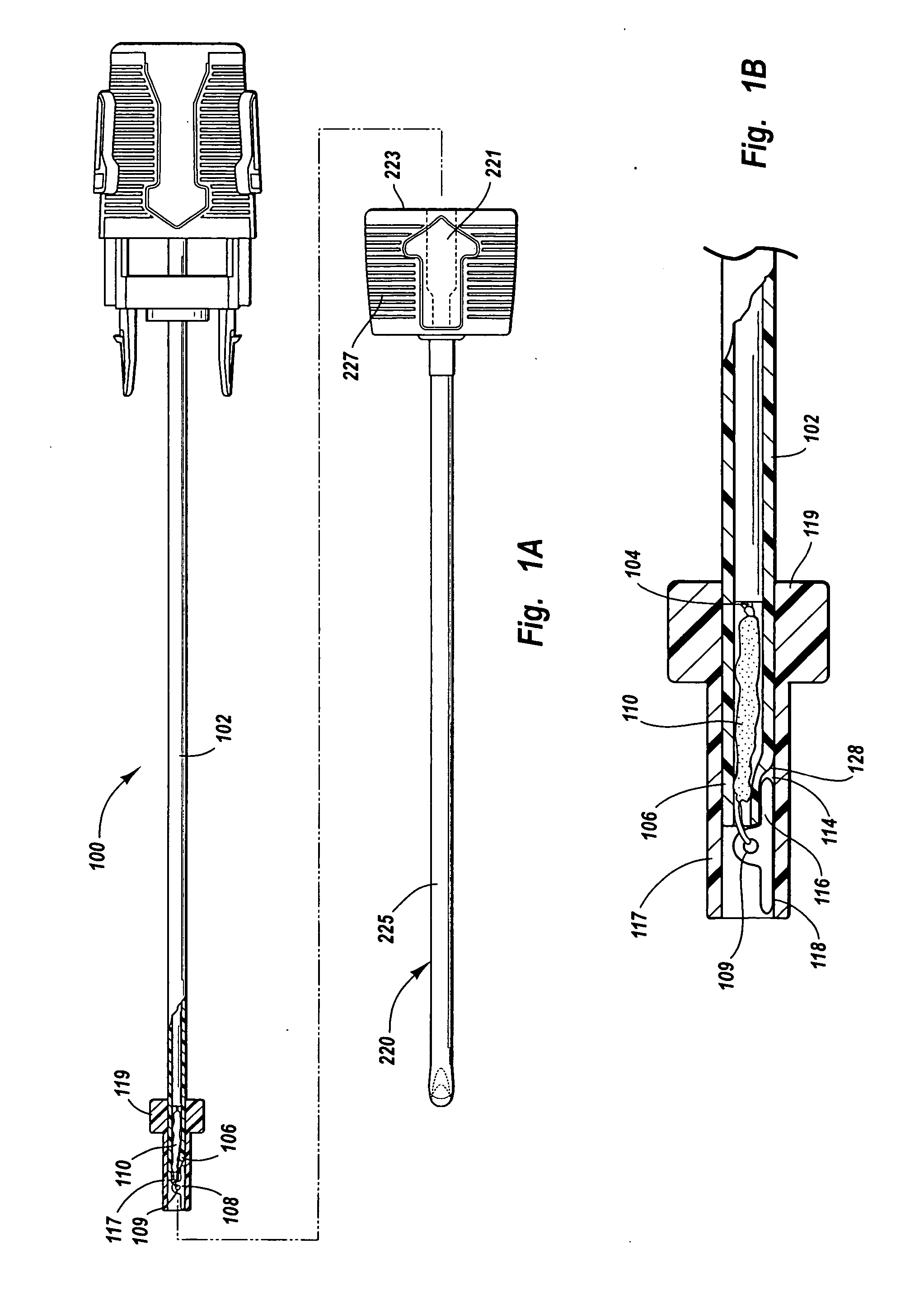
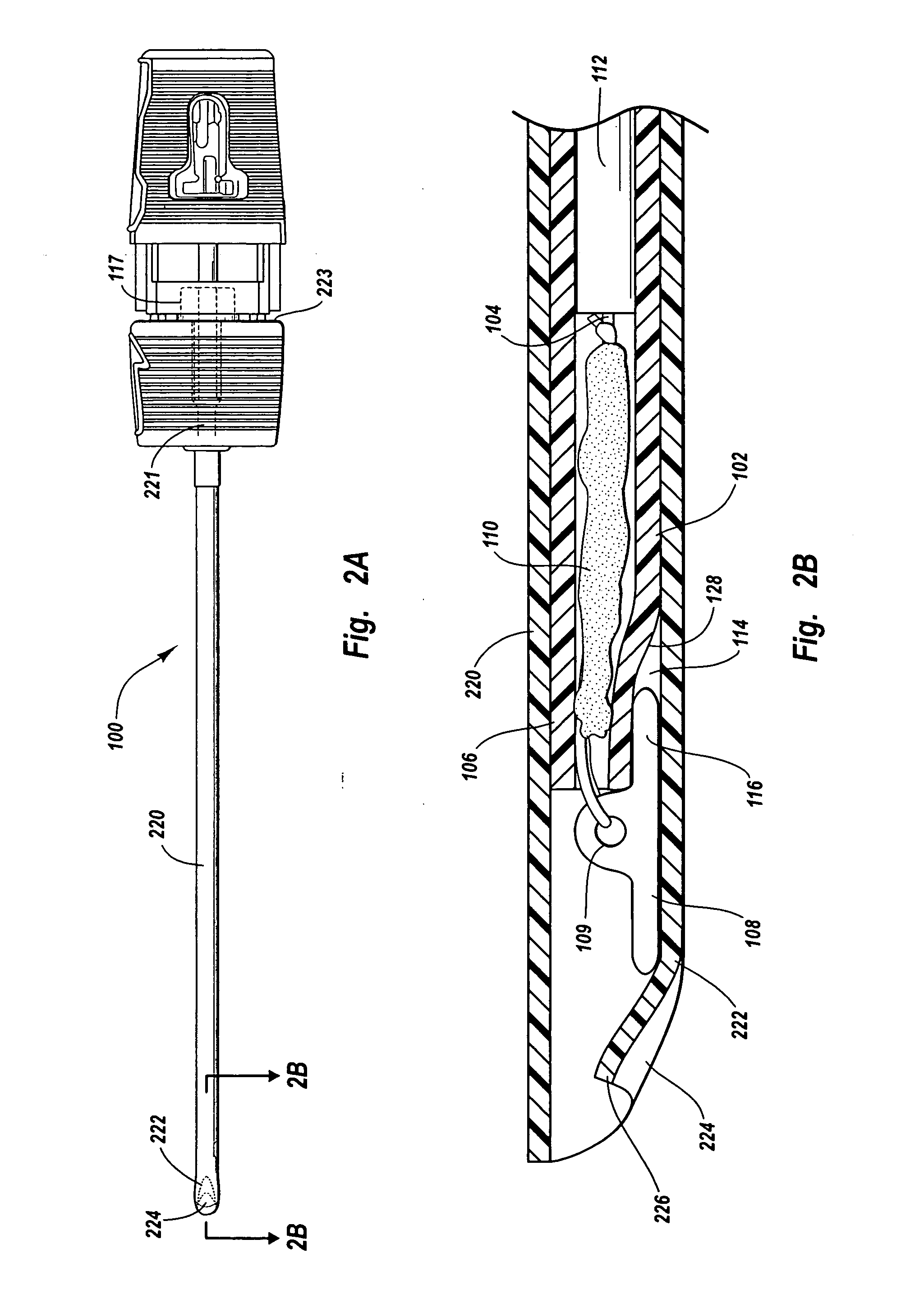
A device for promoting hemostasis in a blood vessel puncture is employed with an introducer that accesses the puncture through an incision. The introducer has an open distal end positionable at the puncture, an external portion with an open proximal end, and an axial channel therebetween. The device includes a hollow catheter, dimensioned to pass through the introducer channel, having a distal end to which is attached an expansible compression element, which may be an inflatable balloon, a collapsible prong assembly, or a resilient foam pad. The compression element is collapsed when the distal end of the catheter is enclosed within the introducer. When the catheter and the introducer are located at the desired distance from the puncture, the introducer is displaced axially relative to the catheter to expose the compression element to the subcutaneous tissue, whereupon the compression element is expanded.
Owner:BOSTON SCI CORP +11
Reduced-pressure surgical wound treatment systems and methods
ActiveUS20090299257A1Reduce pressureNon-adhesive dressingsChiropractic devicesPressure systemSubcutaneous tissue
A reduced-pressure system for treating tissue, such as damaged subcutaneous tissue, includes a shaped dressing bolster for placing on the patient's epidermis and substantially sized to overlay the damaged subcutaneous tissue. The system further includes a sealing subsystem for providing a fluid seal over the shaped dressing bolster and a portion of the patient's epidermis, and a reduced-pressure subsystem for delivering a reduced pressure to the sealing subsystem. The reduced-pressure system may develop a force, which may include a vertical force that is realized at tissue site deeper than the epidermis or a closing force directed towards the incision. The shaped dressing bolster is shaped to evenly distribute the force. Other methods and systems are included.
Owner:3M INNOVATIVE PROPERTIES CO
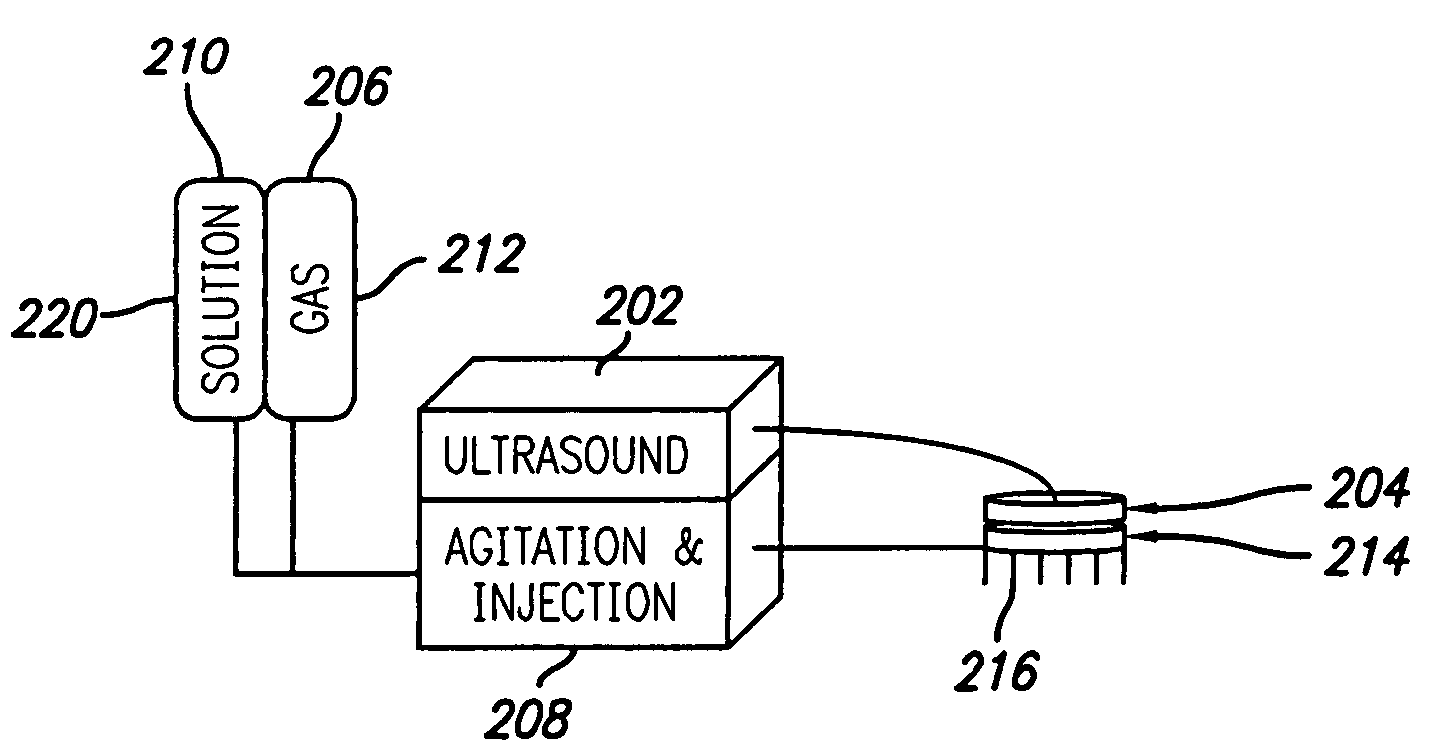
Interstitial microwave system and method for thermal treatment of diseases
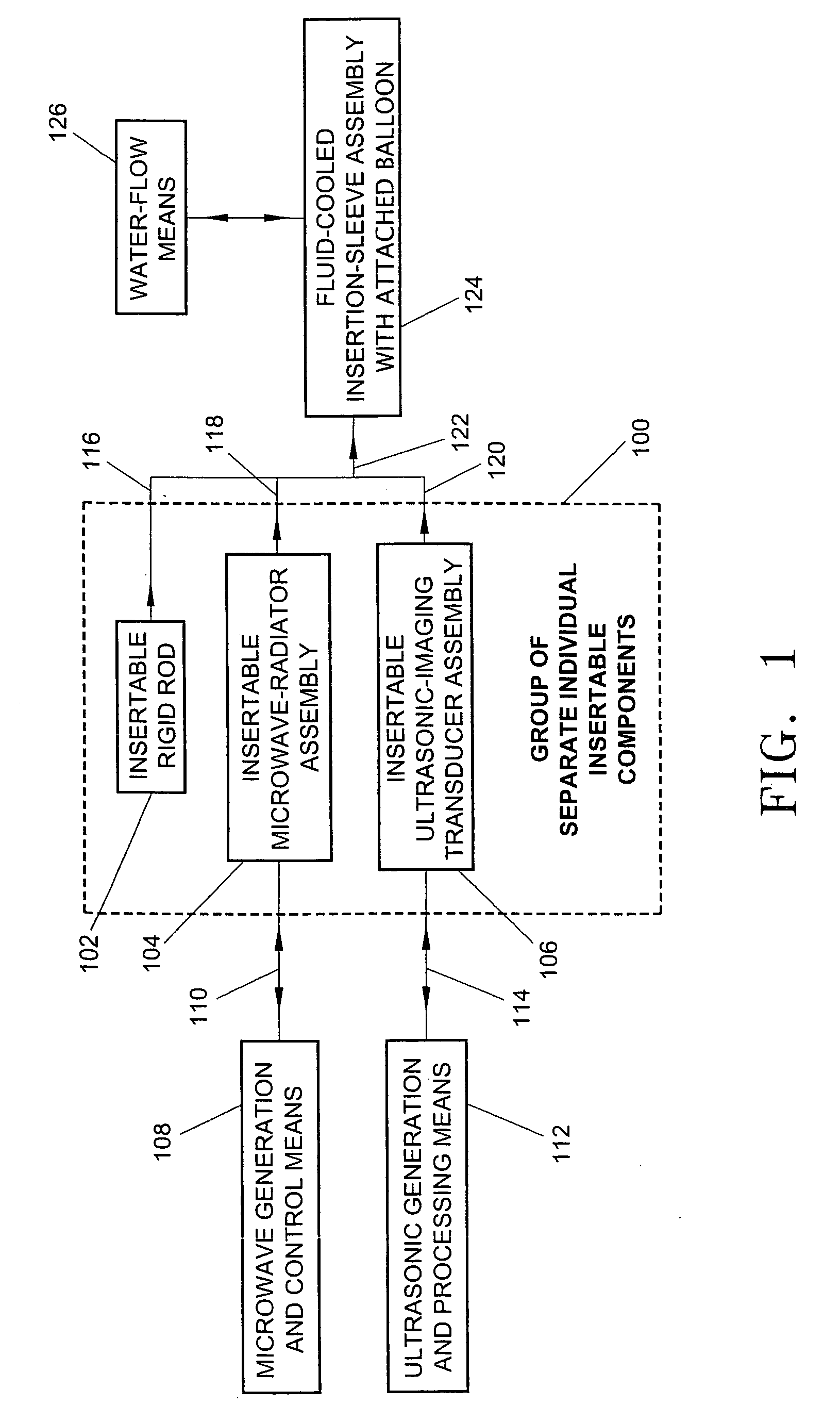
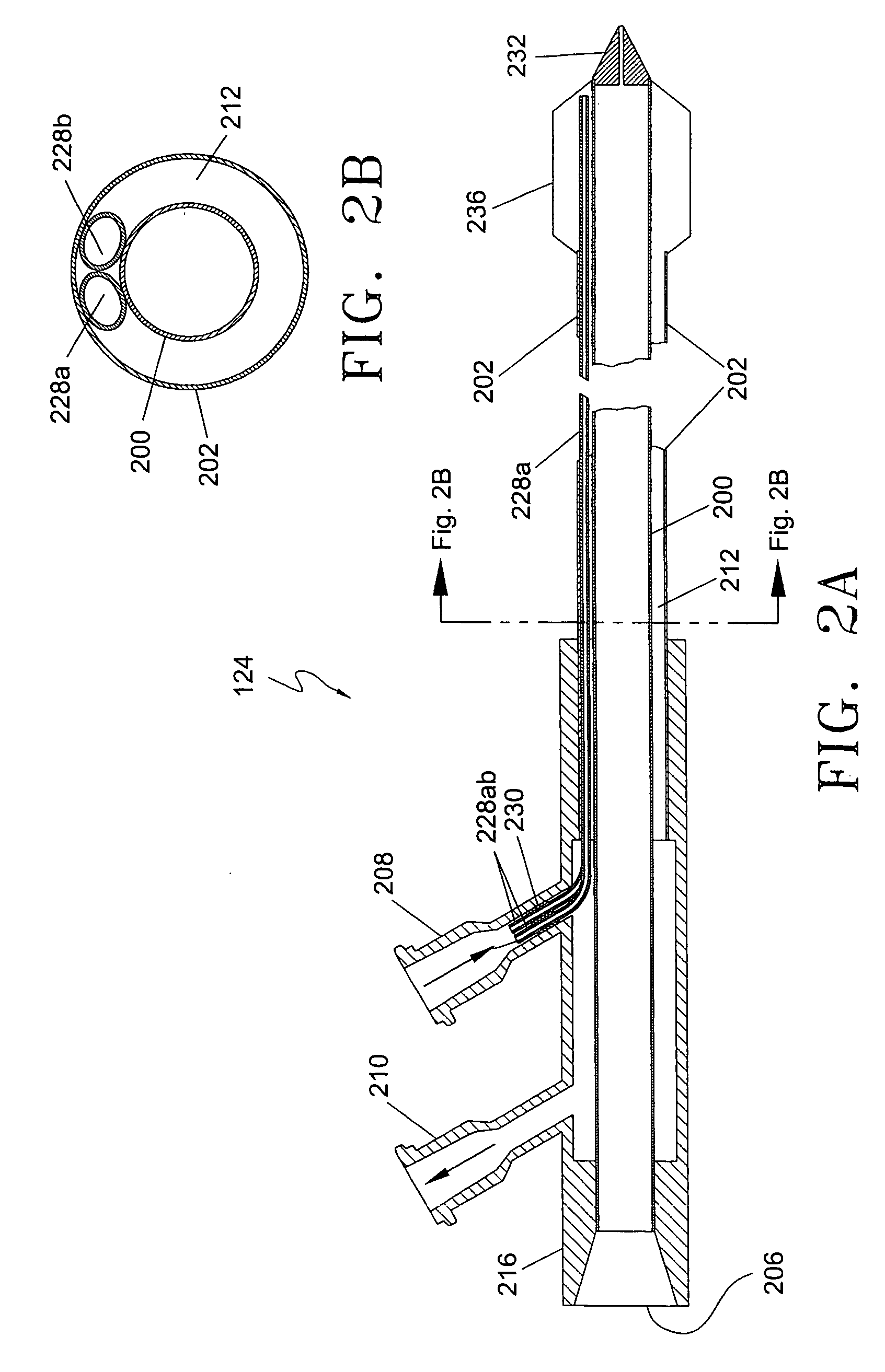
A minimally-invasive fluid-cooled insertion sleeve assembly, with an attached balloon and distally-located penetrating tip, into which sleeve any of a group comprising a rigid rod, a microwave-radiator assembly and an ultrasonic-imaging transducer assembly may be inserted, constitutes a probe of the system. The sleeve assembly comprises spaced inner and outer plastic tubes with two fluid channels situated within the coaxial lumen between the inner and outer tubes. The fluid coolant input flows through the fluid channels into the balloon, thereby inflating the balloon, and then exits through that coaxial lumen. An alternative embodiment has no balloon. The method employs the probe for piercing sub-cutaneous tissue and then ablating deep-seated tumor tissue with microwave-radiation generated heat.
Owner:MMTC LTD +1
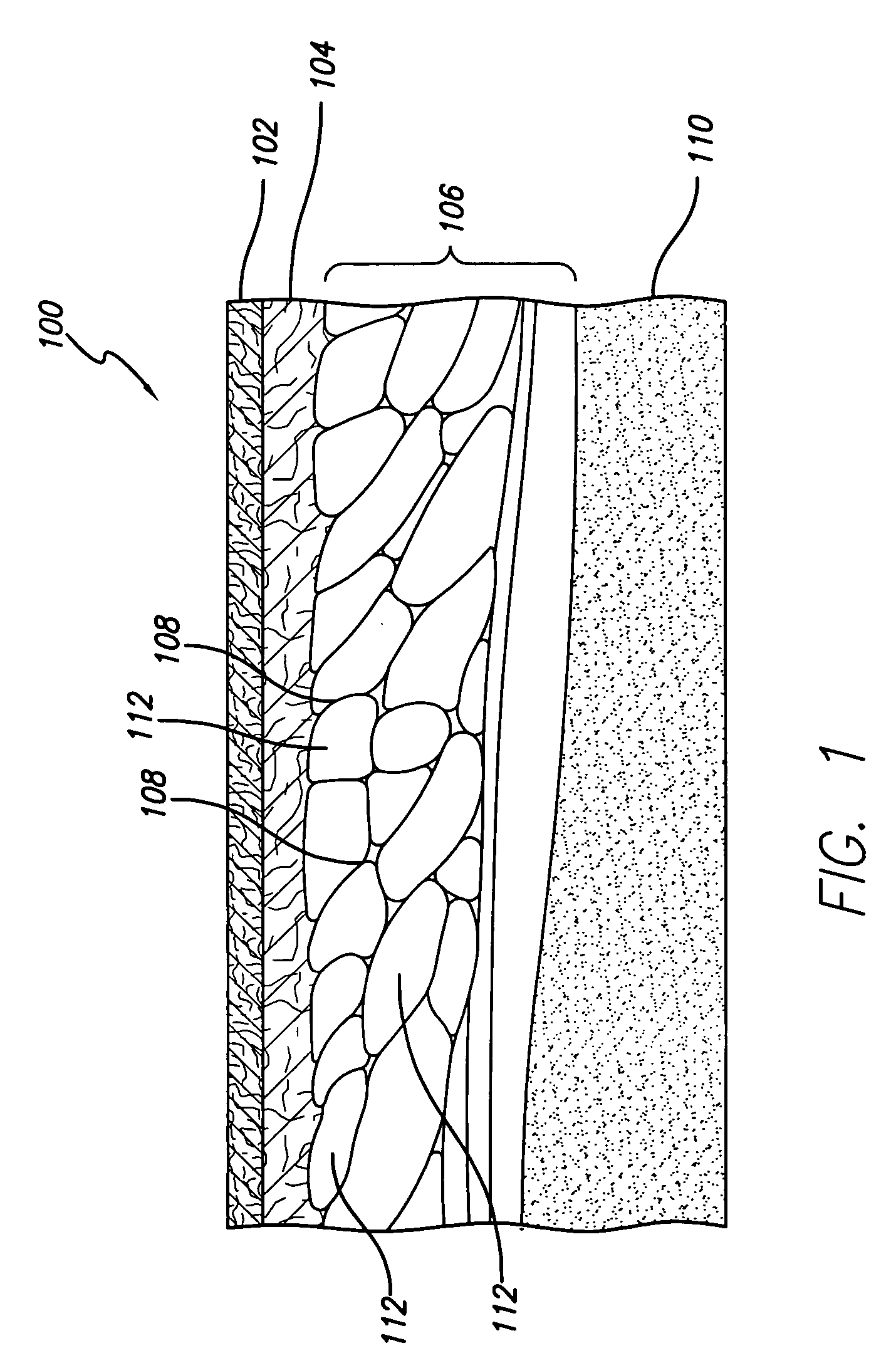
Tissue lockable connecting structures
InactiveUS7083648B2Strengthen the mechanical connectionInhibits tissueDental implantsSkin implantsSoft tissue infectionSubcutaneous tissue
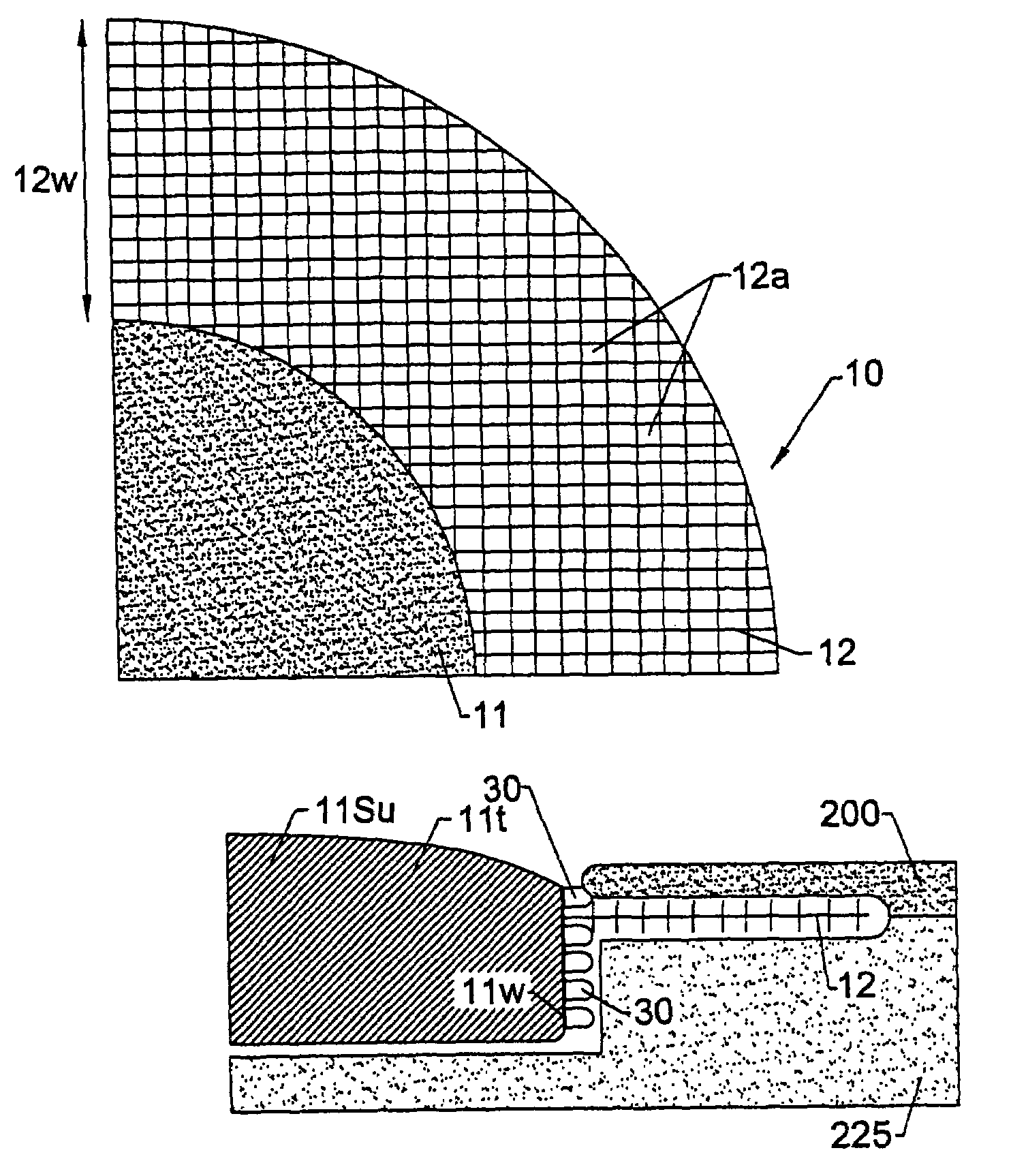
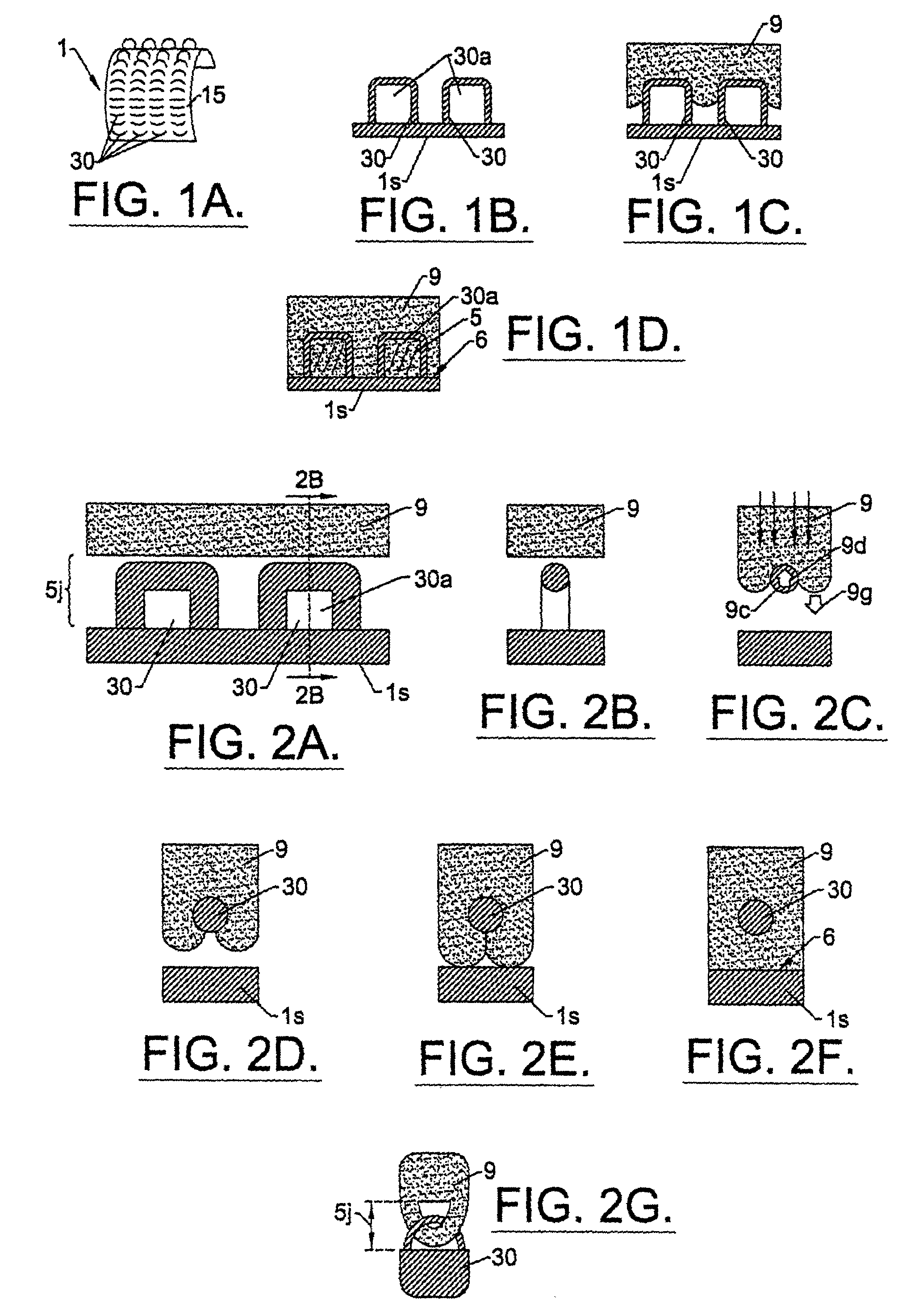
Percutaneous skin access devices include a plurality of locked connecting units mounted to the exterior surface of an implantable medical object which, in position, is configured to penetrate the skin of a subject. The locked connecting units may be mounted directly onto the desired surface of the exterior of the device or may be held on a substrate sheet, which is mounted to the exterior surface of the device. In position, the locked connecting units engage with soft tissue which can include the skin to form a bio-junction layer which includes mechanical and bio-sealing connection between the device body and the soft tissue. The configuration at the bio-junction layer secures the medical object in location in the subject even for long-term indwelling applications in a manner, which inhibits soft tissue infection.The locked connecting units may be rigid or semi-rigid for longer-term indwelling applications, and semi-rigid and / or resilient for shorter term indwelling applications. The locked connecting units may take on the form of rings, hooks, or loops having aperture or gap width / length sizes of from about 0.2–4 mm. The rings, loops, or hooks may connect with any soft tissue including skin as well subcutaneous tissue. The rings, hooks, or loops may be released from the skin / tissue without requiring surgical cutting procedures.The locked connecting units may be configured as a semi-rigid mesh collar arranged about the primary body providing access to the subject such that it resides in the subject and engages with the skin (epidermal / dermal layer). The mesh collar can be described as a particular type of ring or loop structure as the mesh defines the gap provided in individual loop configurations. The mesh collar may be used alone, or in combination with the loops, rings, or hooks. A skin stop collar having increased rigidity may be disposed under the mesh collar.
Owner:EAST CAROLINA UNIVERISTY
Method and apparatus for fractional deformation and treatment of cutaneous and subcutaneous tissue
InactiveUS20100036295A1Reduce decreaseTreating sebaceous glands can potentially improved and/or avoid acneChiropractic devicesEye exercisersSubcutaneous tissueThermal treatment
Devices and methods of treatment of tissue, such as skin tissue and subcutaneous tissue, with thermal control elements are disclosed. Some disclosed devices and methods employ local deformation of tissue in small areas. Devices and methods employing local deformation are used to produce fractional thermal treatments. Some devices and methods are external to a subject's skin others are beneath the subject's skin in the subcutaneous tissue.
Owner:PALOMAR MEDICAL TECH
Compositions and methods for the treatment of cancer
InactiveUS20020128228A1Reducing and avoiding adverse effectImprove toleranceBiocideAnimal repellantsIntestinal structureCancer prevention
This invention relates to compositions comprising temozolomide and thalidomide which can be used in the treatment or prevention of cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof. A particular composition comprises temozolomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof. The invention also relates to methods of treating or preventing cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof, which comprise the administration of temozolomide and thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of cancer chemotherapy or radiation therapy which comprise the administration of temozolomide and thalidomide to a patient in need of such reduction or avoidance.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
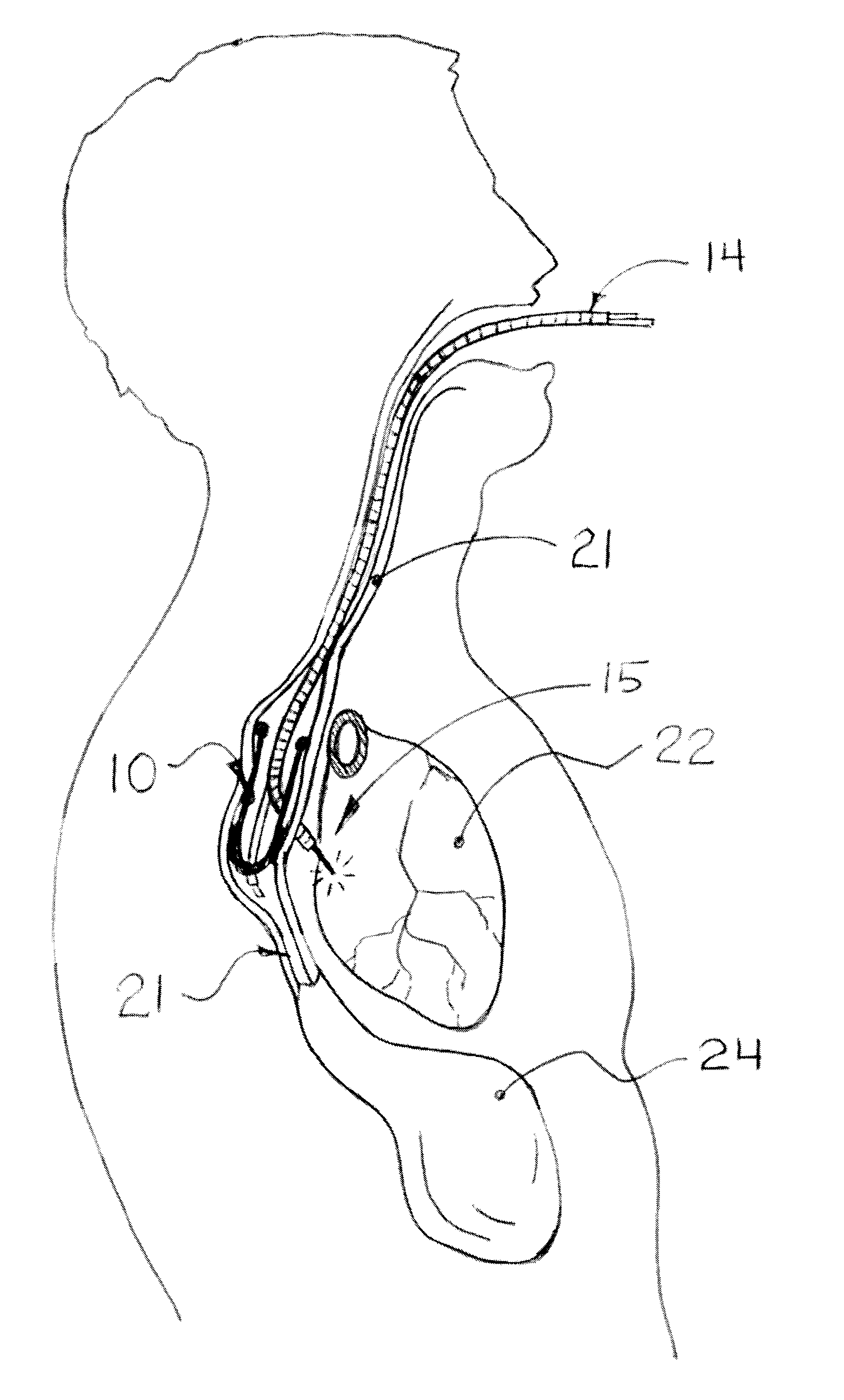
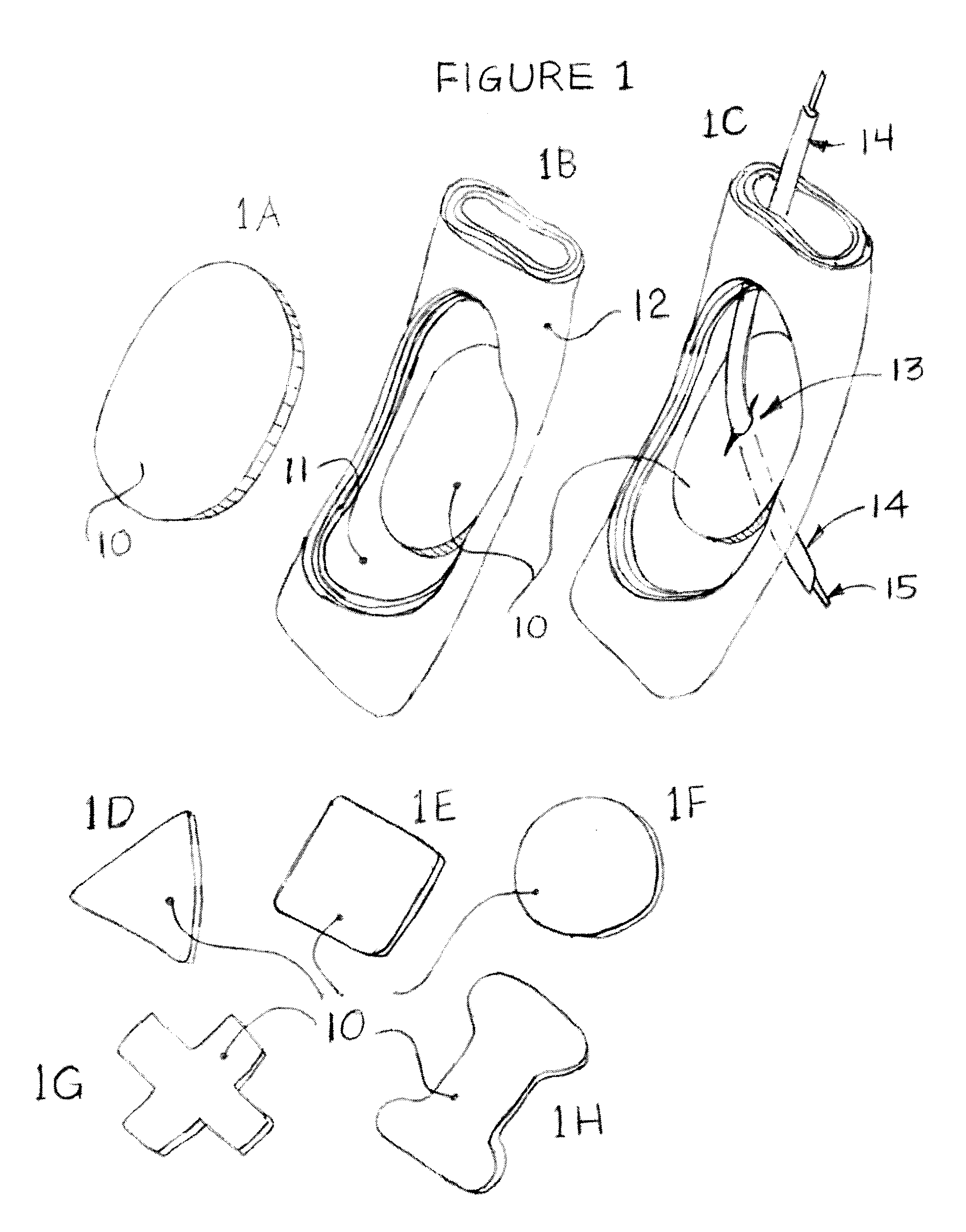
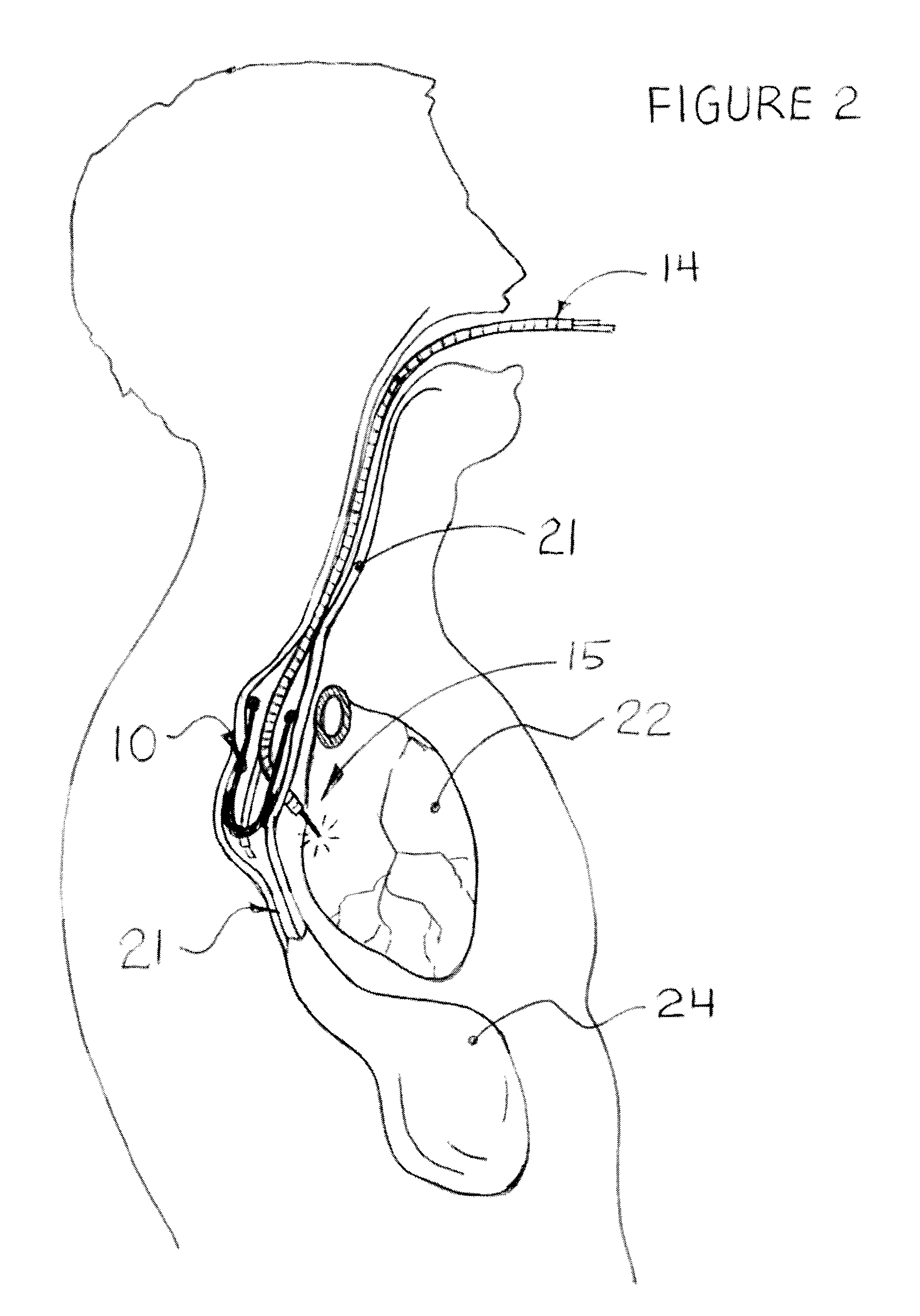
Methods and apparatus for transesophageal microaccess surgery
The current invention describes methods of transesophageal access to the neck and thorax to perform surgical interventions on structures outside the esophagus in both the cervical and the thoracic cavity. It describes a liner device made of a complete or partial tubular structure, or a flat plate, the liner having means to facilitate creation of a side opening, which may include a valve. The liner with its side opening form a port structure inside the esophageal lumen. The port structure allows elongated surgical devices to pass through a perforation across the full thickness of the esophageal wall to outside location, in a controlled way. The elongated surgical devices can be diagnostic scopes, therapeutic scopes, manual elongated surgical devices, robotic arms or the like. After being deployed outside the esophagus, the surgical devices can access structures outside the esophagus, in the neck and thorax in 360 degrees of freedom around the esophageal circumference. These structures can be bony, cartilaginous, spinal, vascular, soft tissue, deep tissues, lymph nodal, cardiac, pulmonary, tracheal, nervous, muscular or diaphragmatic, skin and subcutaneous tissues of the neck, skin and subcutaneous tissues of the anterior chest wall, skin and subcutaneous tissues of the skin of the back, and skin and layers of the breast.
Owner:MICROACCESS
Methods and system for treating subcutaneous tissues
ActiveUS7588547B2Eliminate riskFacilitate ablationUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyCavitationMedicine
An apparatus and method for treating subcutaneous tissues using acoustic waves in the range of low acoustic pressure ultrasound waves is disclosed. The method includes injections of enhancing agents, wherein disruption of subcutaneous tissues and subcutaneous cavitational bioeffects are produced by ultrasound waves having a power that will not produce tissue cavitation in the absence of the enhancing agents. The apparatus and method of use is useful for treatment of subcutaneous abnormalities including cellulite, lipomas, and tumors.
Owner:ULTHERA INC
Leadless Implantable Cardioverter Defibrillator
A leadless implantable cardioverter defibrillator (5) for treatment of sudden cardiac death includes a controller and at least one remote module. The defibrillator does not require transvenous / vascular access for intracardiac lead placement. The controller is leadless and uses subcutaneous tissue in proximity of the chest and abdomen for both sensing and defibrillation. The controller and one or more remote sensors sense a need for defibrillation and wireless communicate with the controller. The controller and one of the sensors discharge a synchronized defibrillation pulse to the surrounding subcutaneous tissue in proximity to the heart.
Owner:UNIVERSITY OF ROCHESTER
Near infrared risk assessment of diseases
The present invention provides an apparatus and a method for identifying the risk of a clinical condition in a human or animal by correlating Near Infrared (NIR) absorbance spectral data with one or several parameters including a concentration of one or more substances in the skin, a concentration of one or more substances in skin plus subdermal tissue, a score derived from one or more clinical tests like a stress test on a treadmill, coronary angiography, or intravascular coronary ultrasound. The method determines the concentration of a compound in the skin of a human or animal and comprises the steps of placing a part of the skin against a receptor, directing electromagnetic radiation (EMR) from the near-infrared spectrum onto the skin, measuring a quantity of EMR reflected by, or transmitted through, the skin with a detector; and performing a quantitative mathematical analysis of the quantity of EMR to determine the concentration of the compound, for example free and esterfied cholesterol. An example of a clinical condition is cardiovascular disease.
Owner:TYCO HEALTHCARE GRP LP
Tissue treatment methods
ActiveUS8192474B2Reduce and eliminate fat cellMinimize injuryChiropractic devicesSurgical instruments for heatingSubcutaneous adipose tissueReperfusion injury
Methods are provided herein for affecting a region of a subject's body, comprising exposing the region to a cooling element under conditions effective to cool subcutaneous adipose tissue in said region; and increasing the blood flow rate to the cooled tissue by exposing the tissue to an energy source. Methods are also provided for treating subcutaneous adipose tissue in a region of a subject's body, comprising exposing said region to a cooling element under conditions effective to cool said tissue; and exposing the tissue to an energy source to increase the blood flow rate to the cooled tissue, thereby stimulating reperfusion in, and / or causing an ischemia-reperfusion injury to, the cooled tissue.
Owner:ZELTIQ AESTHETICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com