Patents
Literature
3037 results about "Clinical tests" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
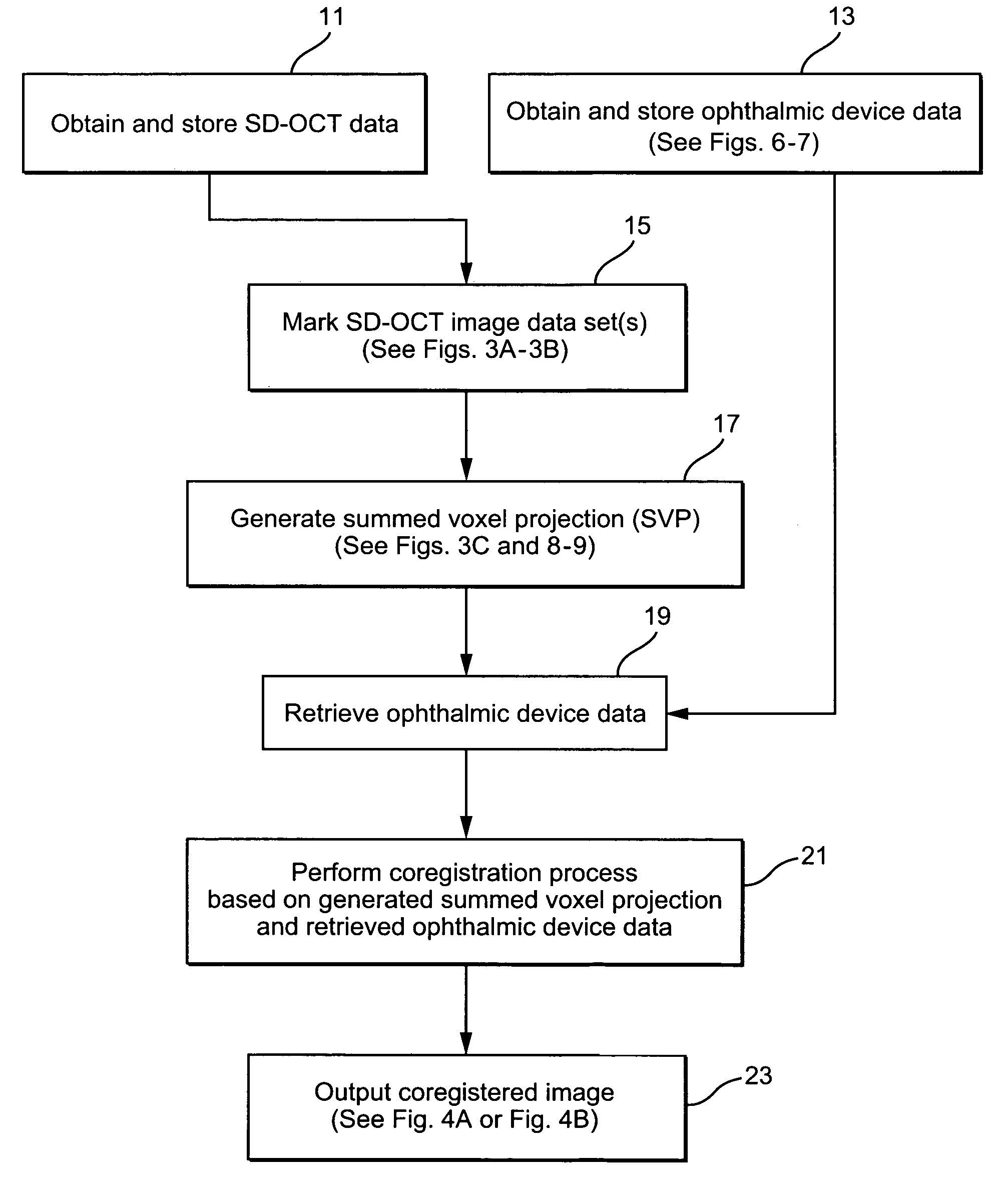
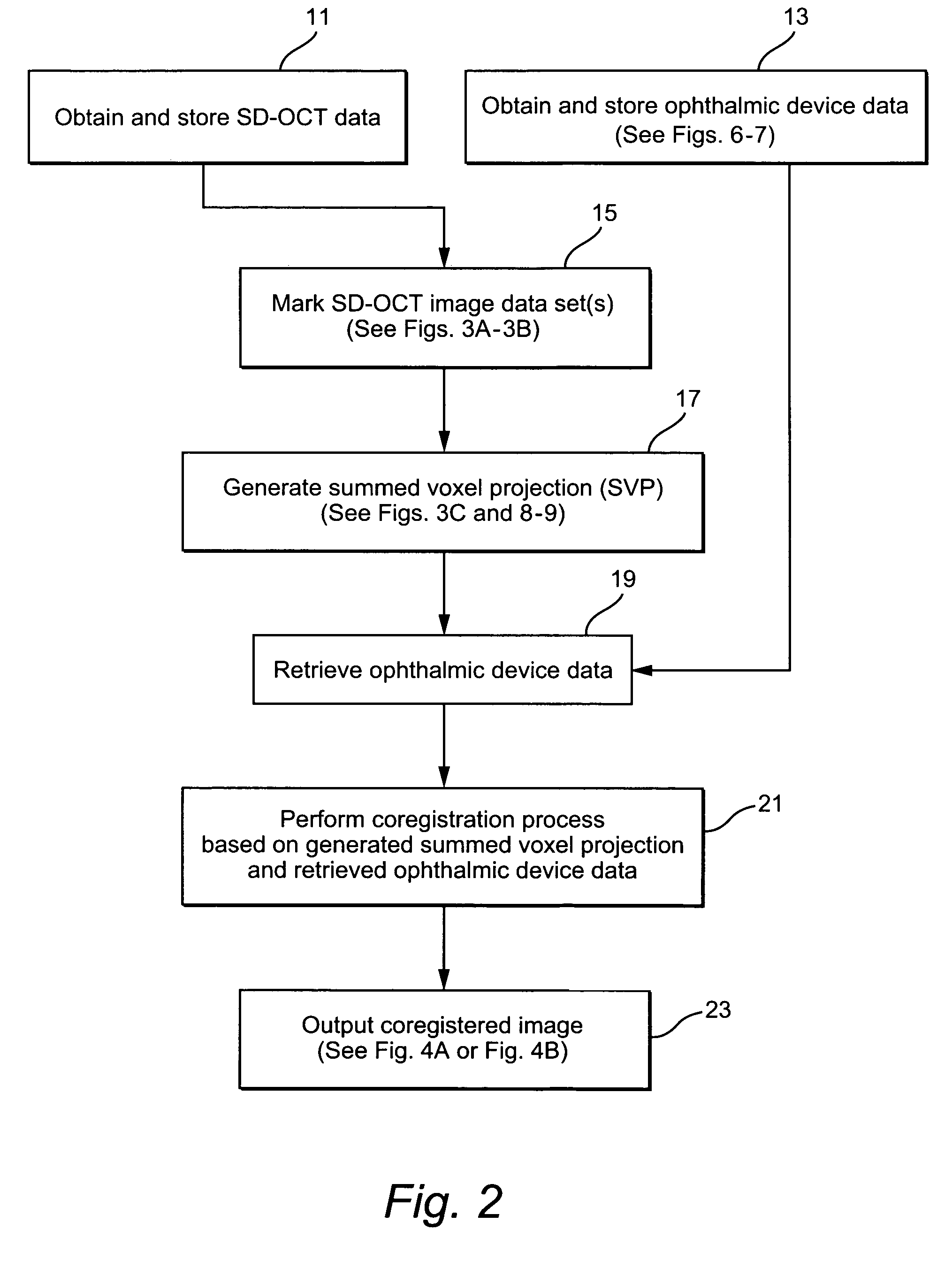
Patent Type
Patent Status
Application Year
Inventor
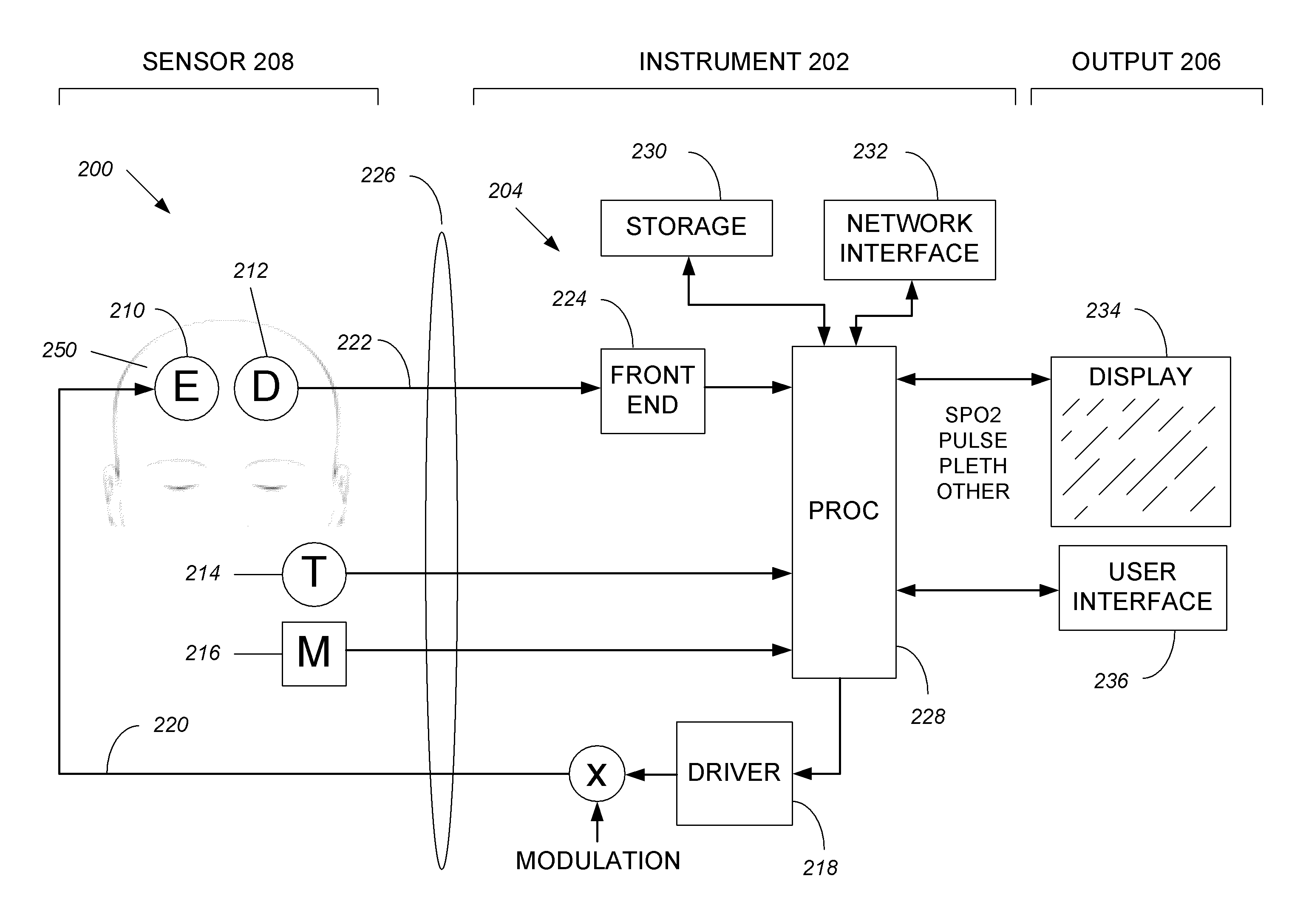
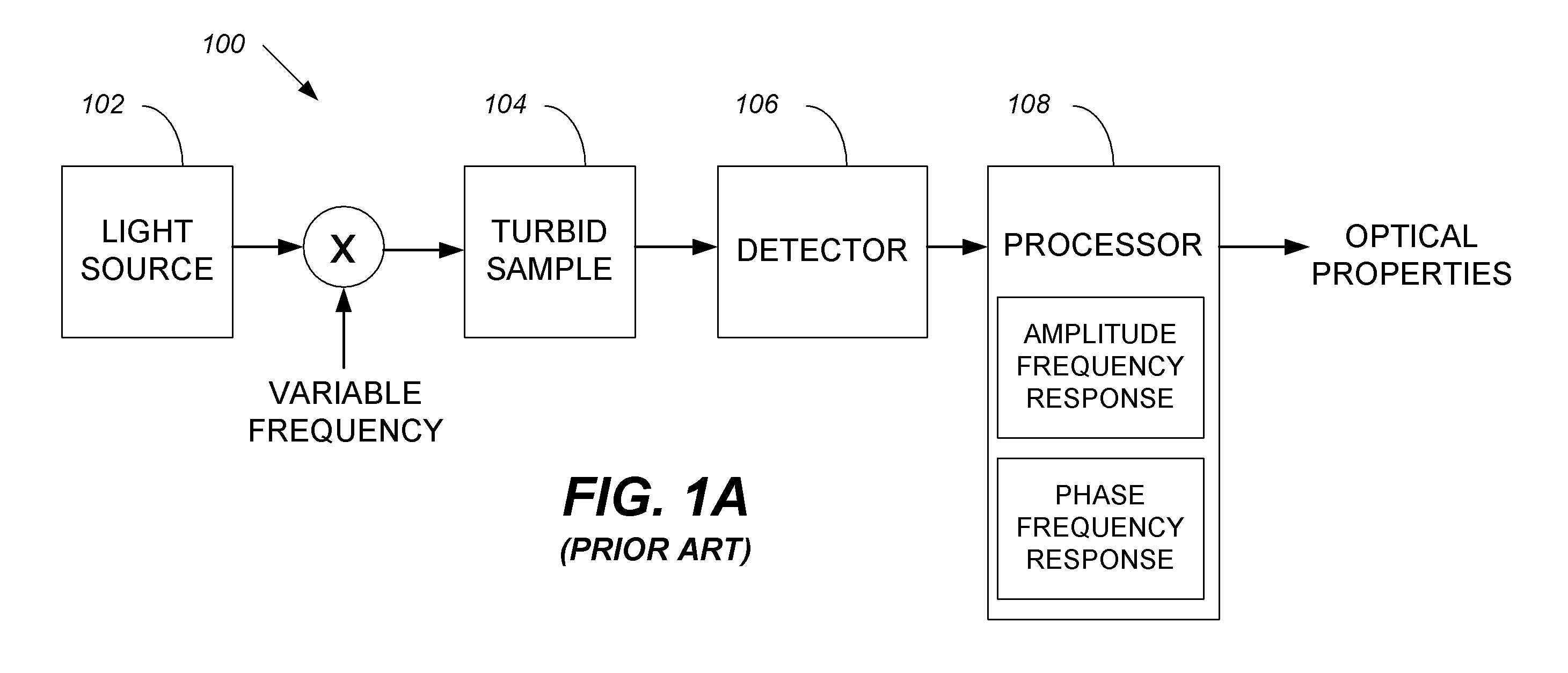
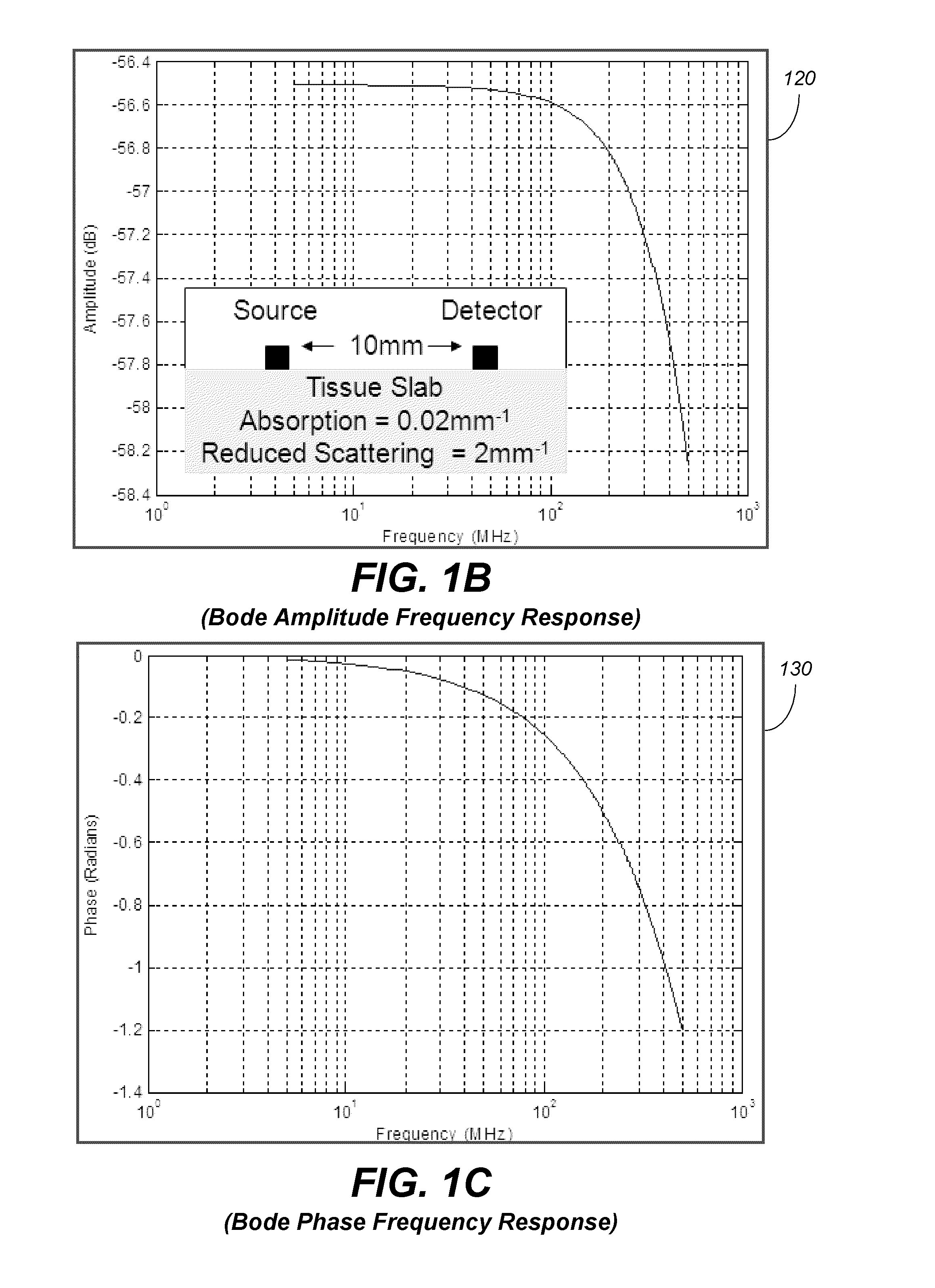
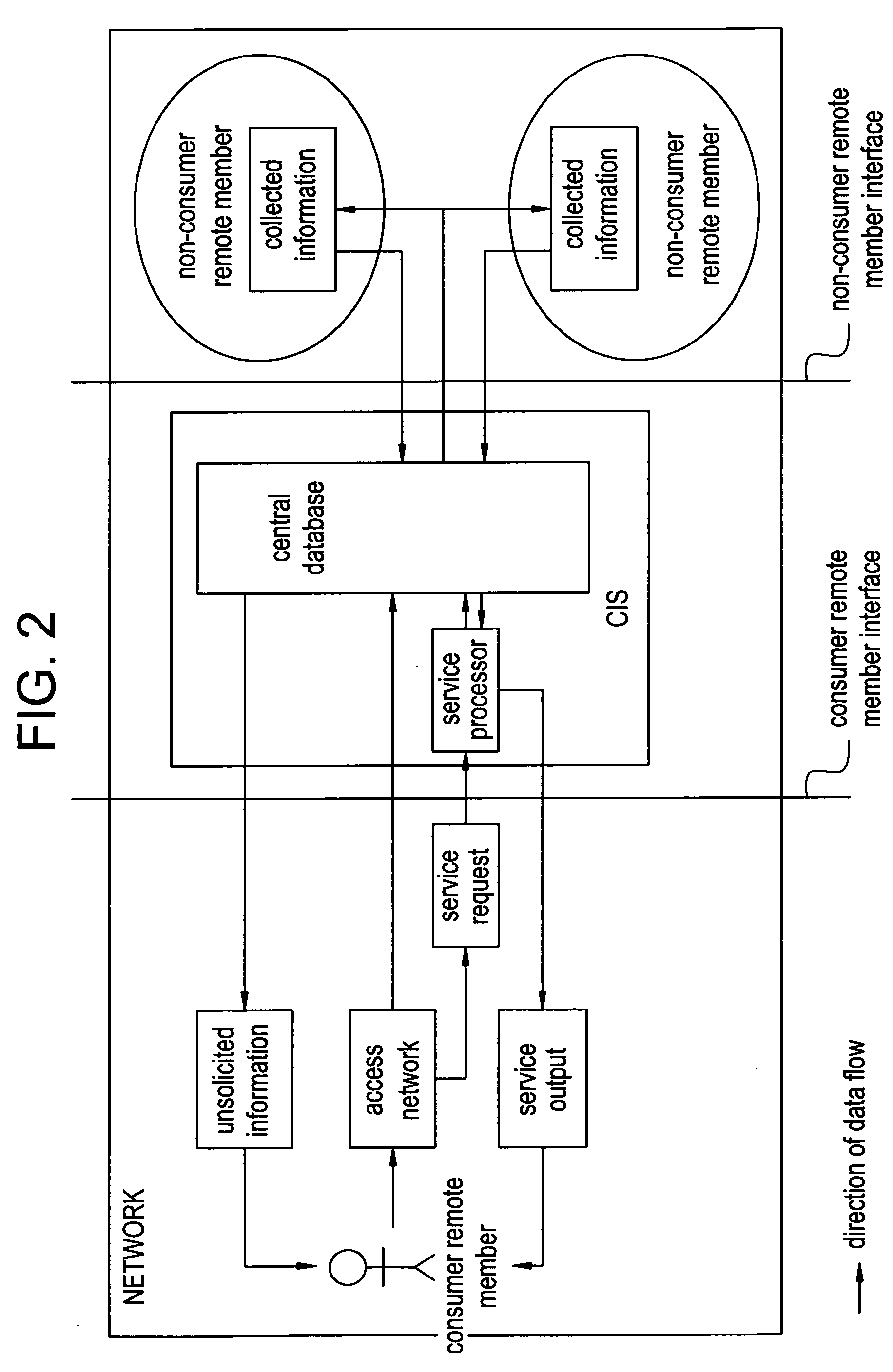
Systems and methods of monitoring a patient through frequency-domain photo migration spectroscopy
InactiveUS20120165629A1Reduce signalingReduce dependenceCatheterDiagnostic recording/measuringSpectroscopyClinical tests
FDPM processing provides an amplitude signal and a phase signal at a modulation frequency to improve measurement fidelity during measurement of one or more blood parameters. In an embodiment, a light source modulates light at a modulation frequency around 200 MHz to produce an amplitude and phase plethysmograph, usable to access clinical test data.
Owner:MASIMO CORP
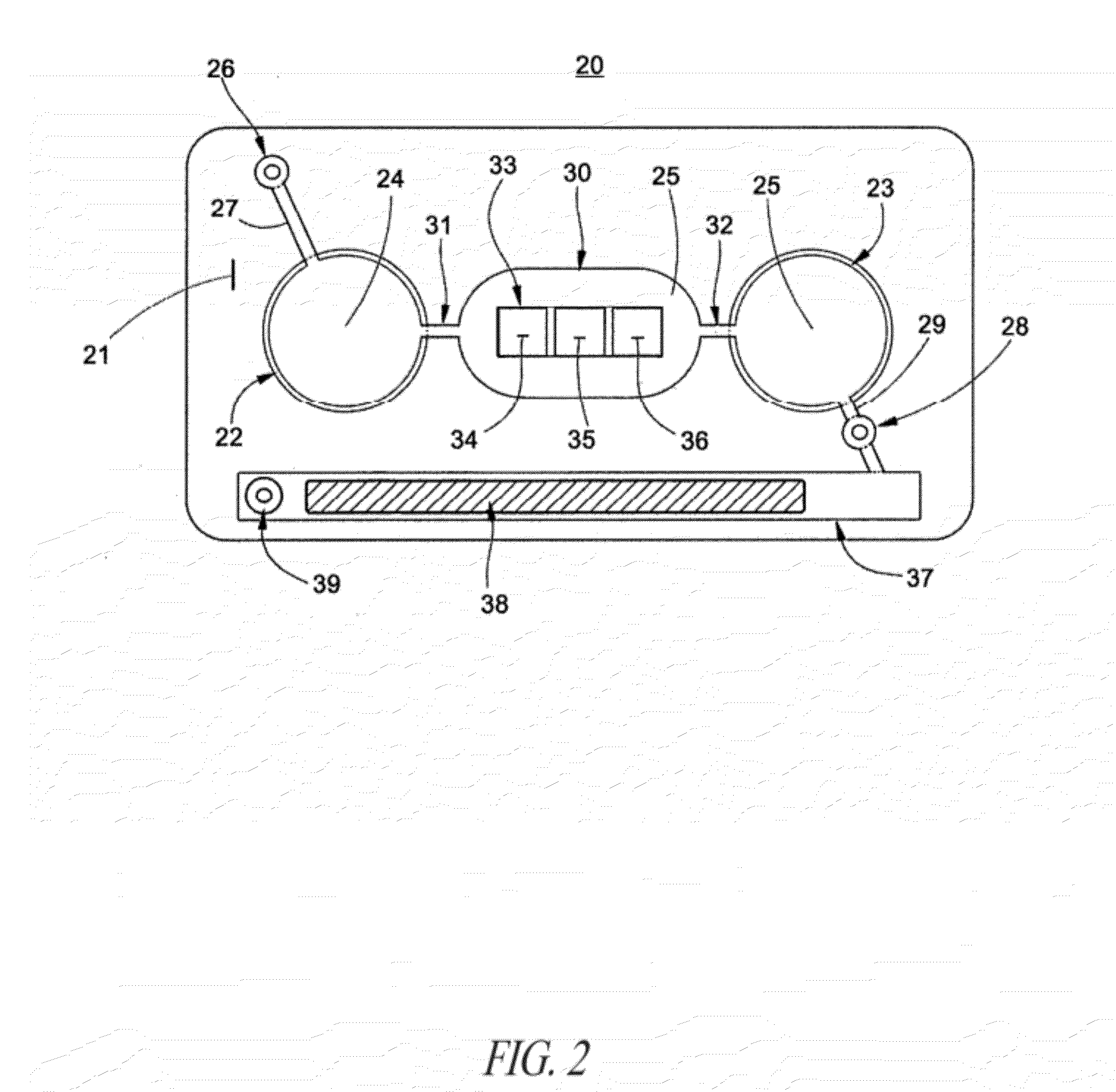
Methods and devices for microfluidic point-of-care immunoassays
ActiveUS20090181411A1Endpoint detectionReduce incubation timeBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
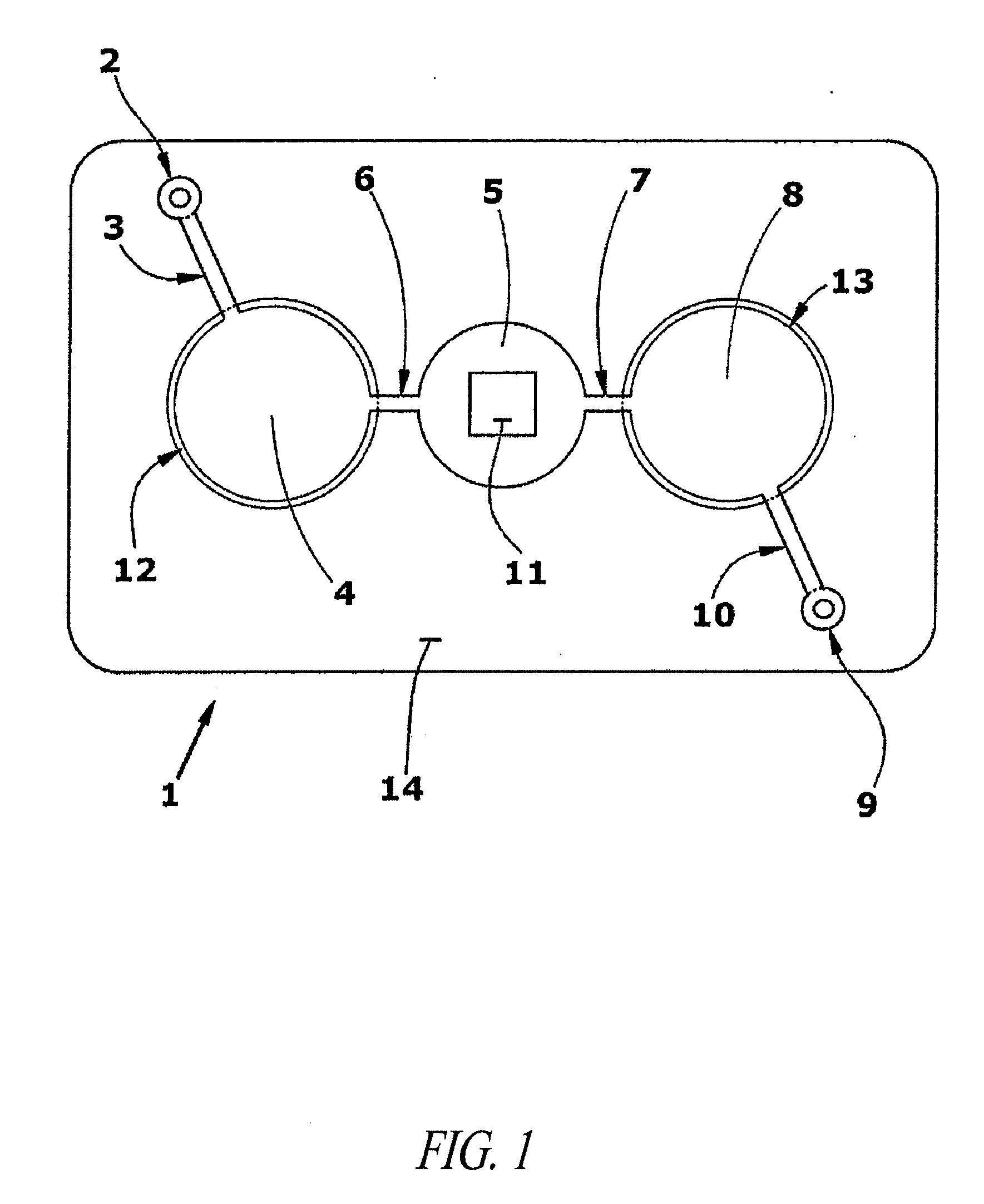
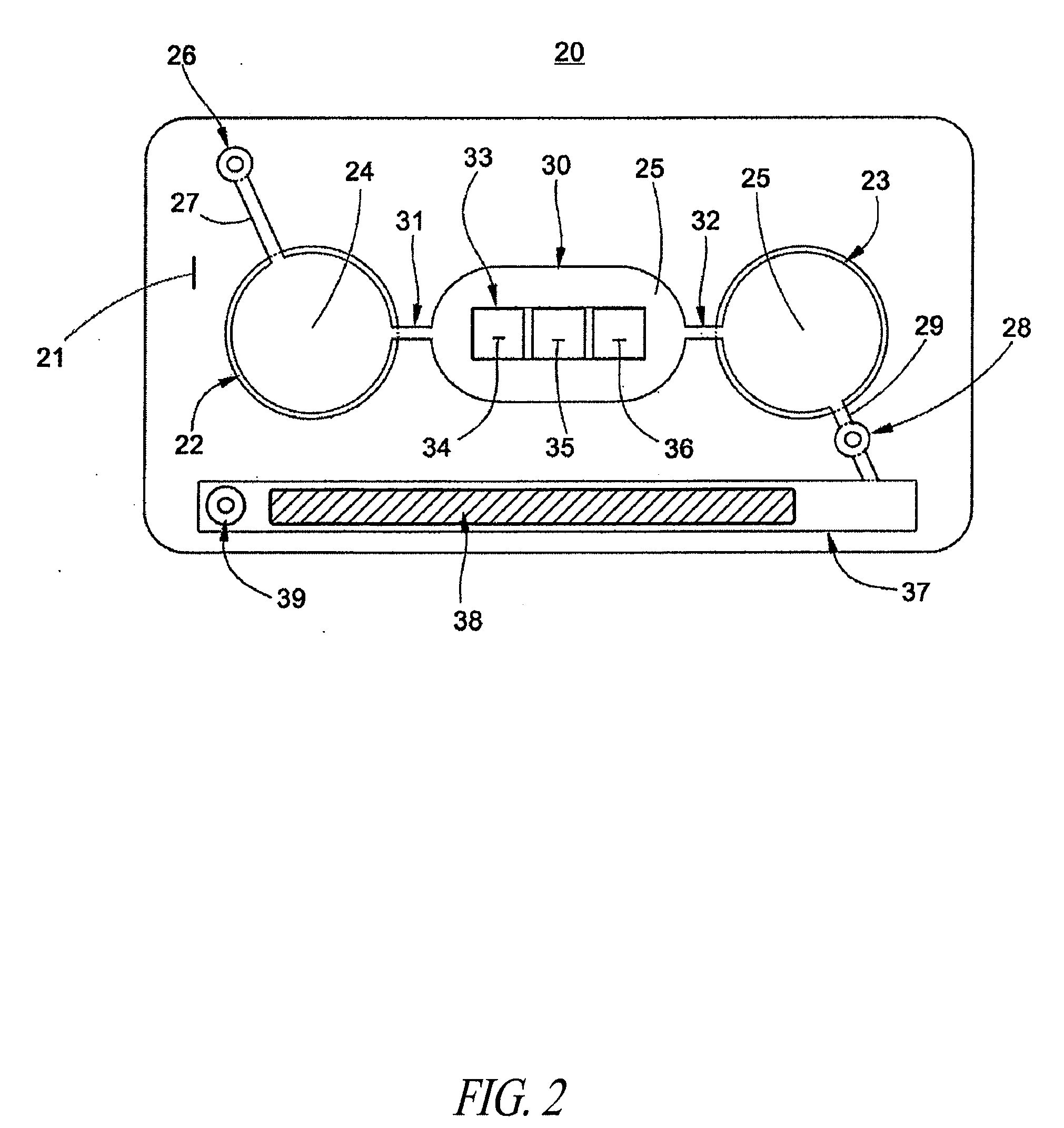
Network and methods for integrating individualized clinical test results and nutritional treatment
Owner:BODYBIO INC
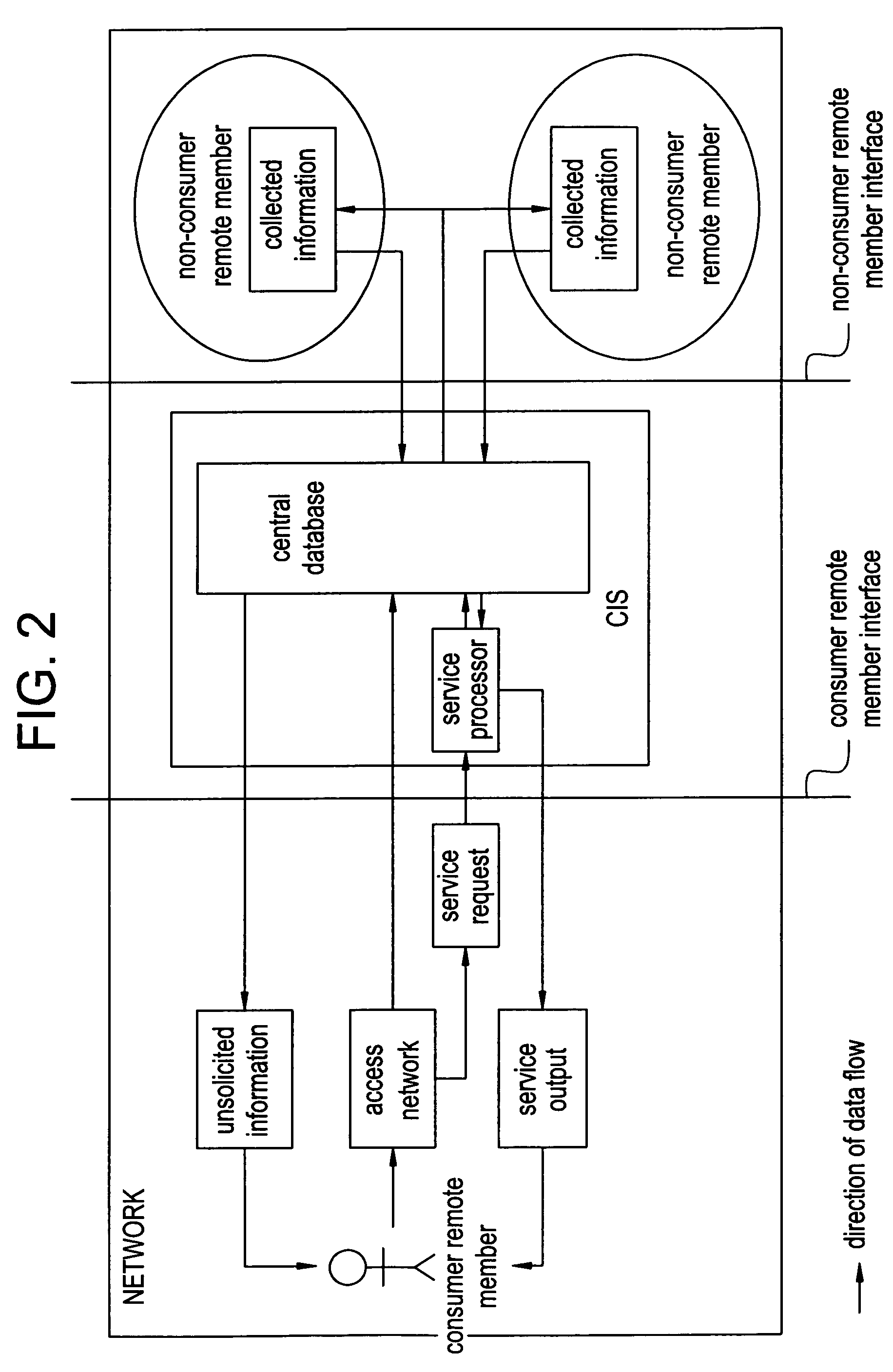
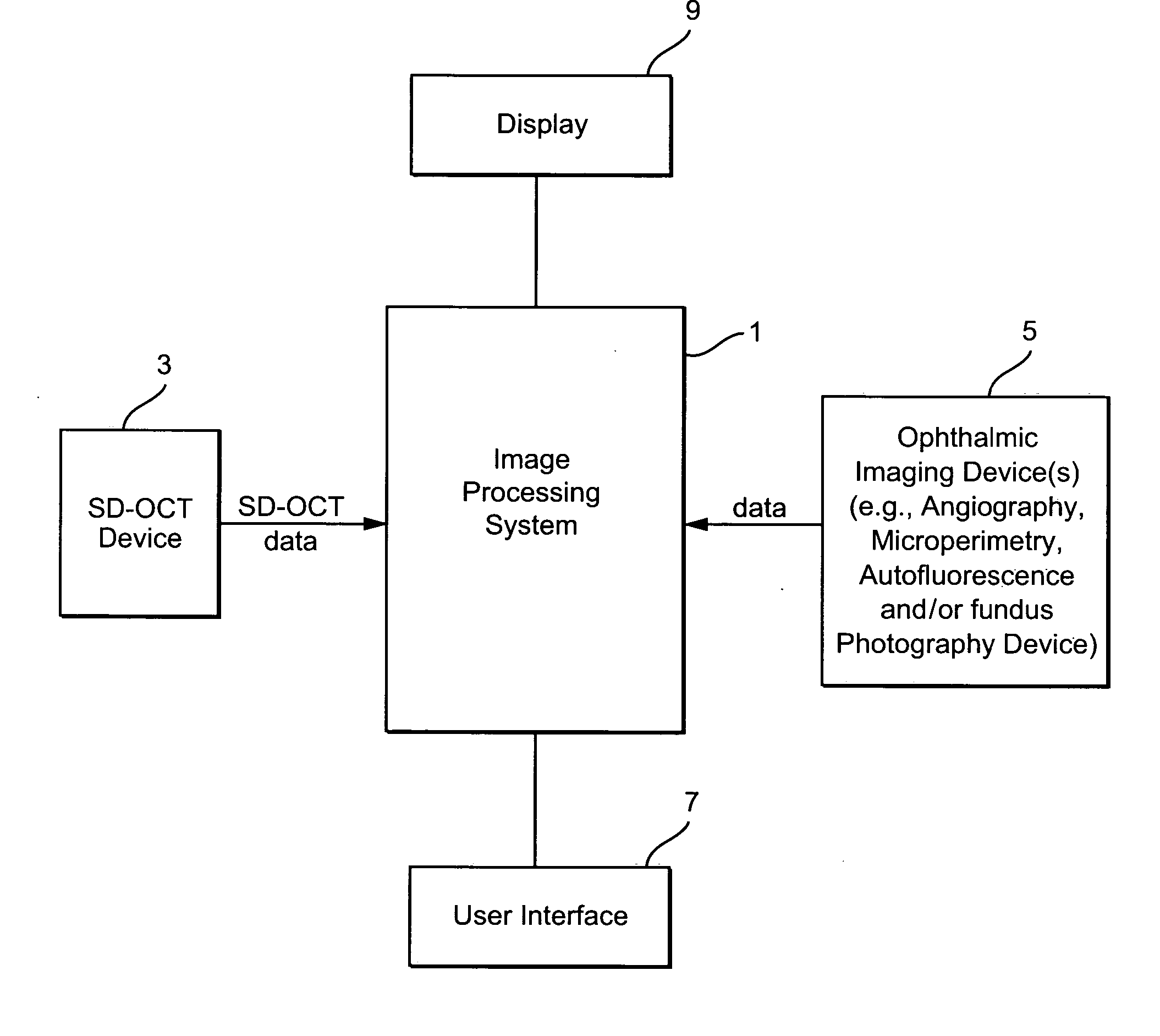
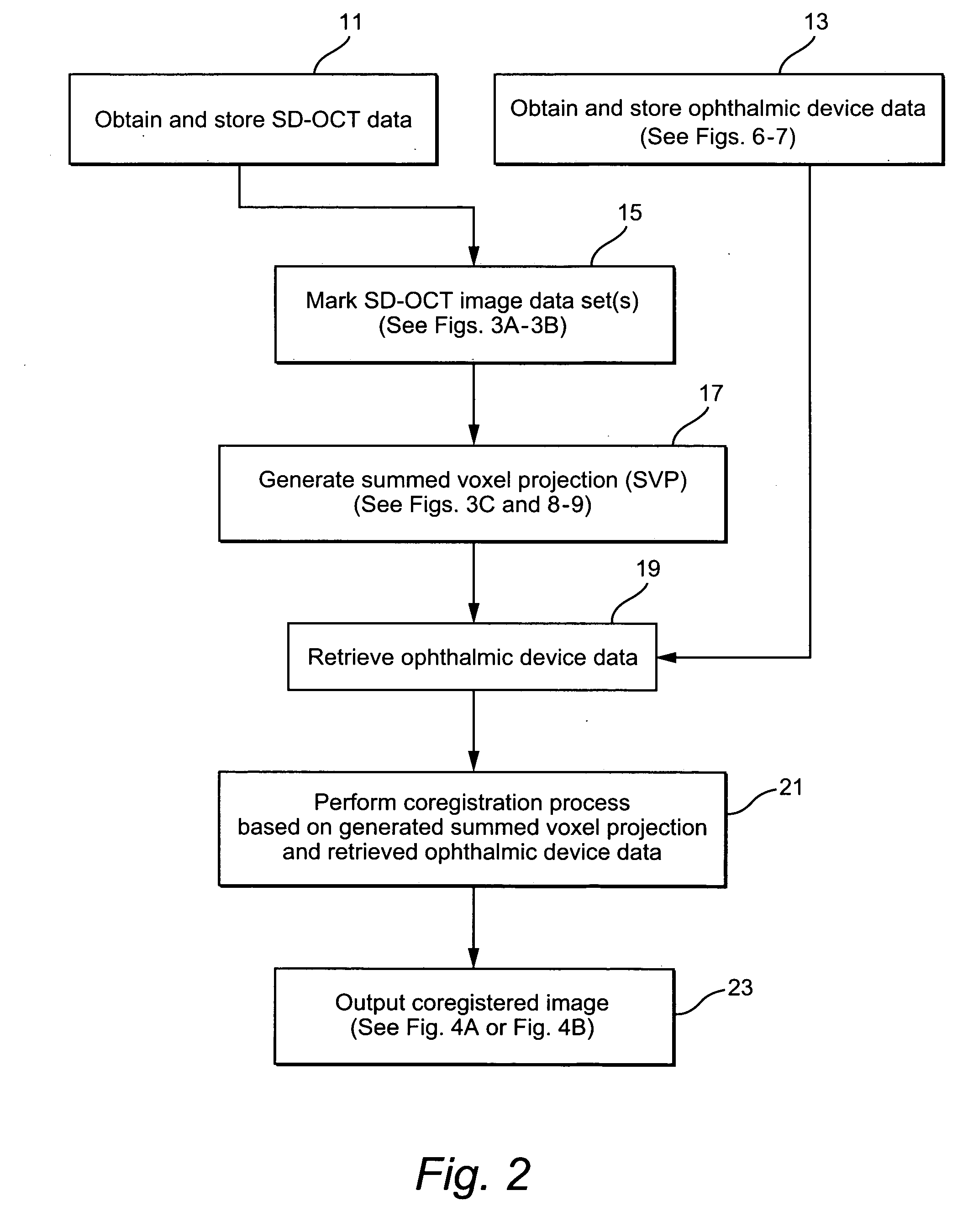
Method and system of coregistrating optical coherence tomography (OCT) with other clinical tests
ActiveUS20070115481A1Precise maintenanceImprove signal-to-noise ratioMaterial analysis using wave/particle radiationRadiation/particle handlingClinical testsTomography
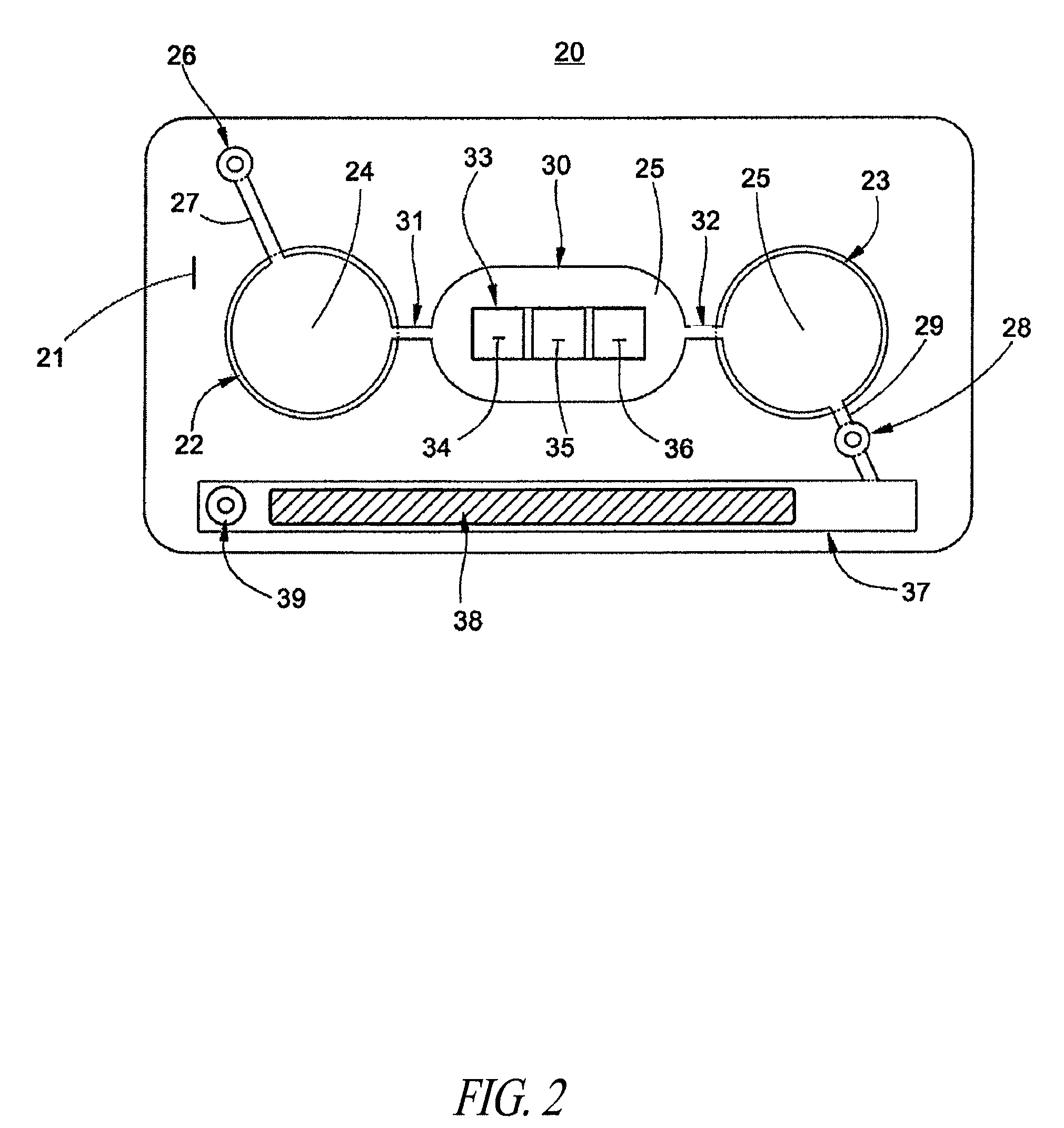
A method / system preserves annotations of different pathological conditions or changes that are recognized on cross-sections within a three dimensional volume of a patient's eye so that the annotations are maintained in a visible state in an en face projection produced with a SVP technique. It is thus possible to coregister the annotated conditions or changes with other types of two dimensional en face images such as images from other ophthalmic devices (e.g., angiography device, microperimetry device, autofluorescence device, fundal photography device.). The annotations are also maintained in a visible state in the coregistered image.
Owner:DUKE UNIV
Near infrared risk assessment of diseases
The present invention provides an apparatus and a method for identifying the risk of a clinical condition in a human or animal by correlating Near Infrared (NIR) absorbance spectral data with one or several parameters including a concentration of one or more substances in the skin, a concentration of one or more substances in skin plus subdermal tissue, a score derived from one or more clinical tests like a stress test on a treadmill, coronary angiography, or intravascular coronary ultrasound. The method determines the concentration of a compound in the skin of a human or animal and comprises the steps of placing a part of the skin against a receptor, directing electromagnetic radiation (EMR) from the near-infrared spectrum onto the skin, measuring a quantity of EMR reflected by, or transmitted through, the skin with a detector; and performing a quantitative mathematical analysis of the quantity of EMR to determine the concentration of the compound, for example free and esterfied cholesterol. An example of a clinical condition is cardiovascular disease.
Owner:TYCO HEALTHCARE GRP LP
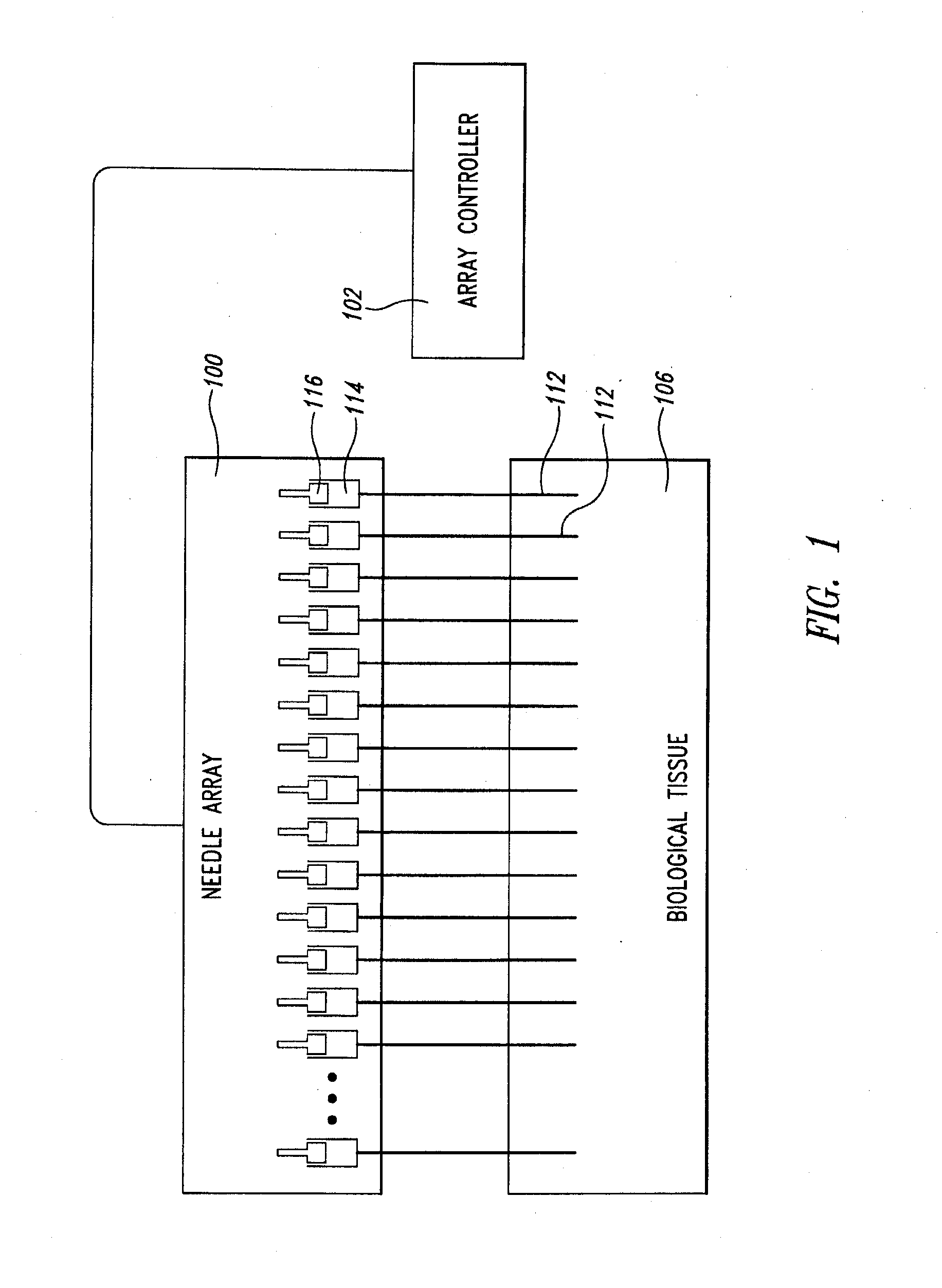
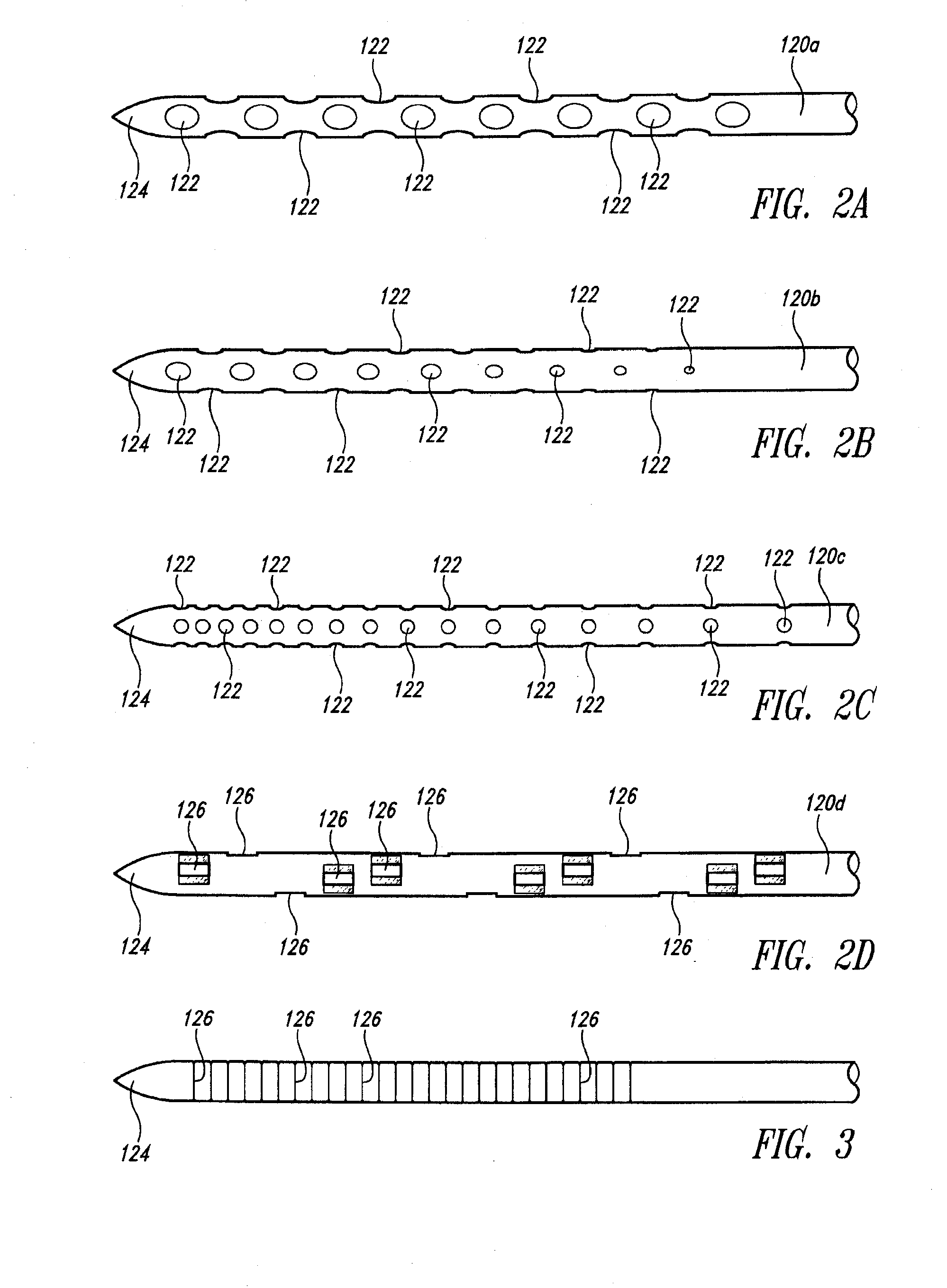
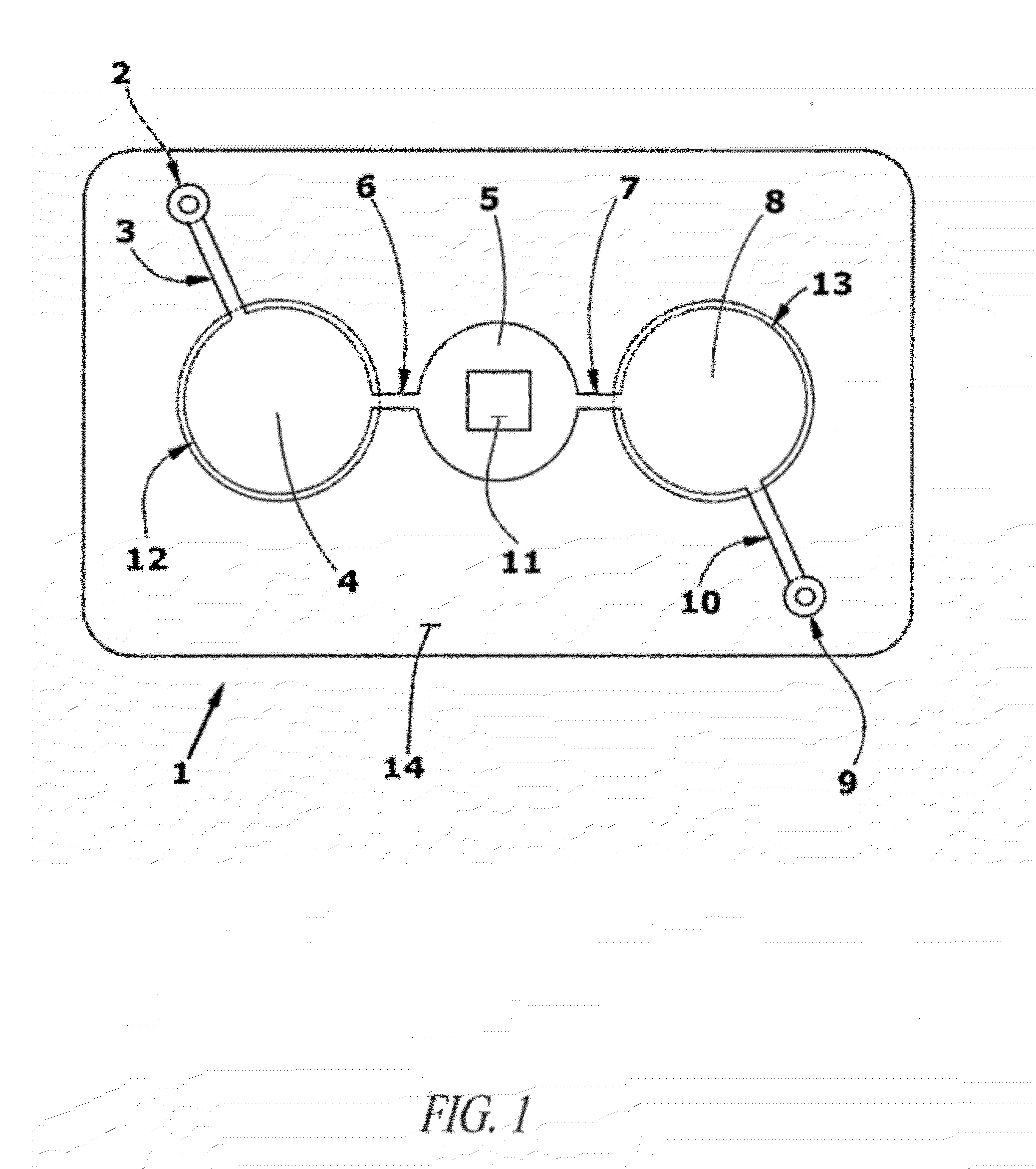
Needle Array Assembly and Method for Delivering Therapeutic Agents
A fluid delivery device includes an array of needles, each in fluid communication with a respective reservoir. Respective actuators are coupled so as to be operable to drive fluid from the reservoirs via needle ports. Each needle can have a plurality of ports, and the ports can be arranged to deliver a substantially equal amount of fluid at any given location along its length. A driver is coupled to the actuators to selectively control the rate, volume, and direction of flow of fluid through the needles. The device can simultaneously deliver a plurality of fluid agents along respective axes in solid tissue in vivo. If thereafter resected, the tissue can be sectioned for evaluation of an effect of each agent on the tissue, and based on the evaluation, candidate agents selected or deselected for clinical trials or therapy, and subjects selected or deselected for clinical trials or therapeutic treatment.
Owner:FRED HUTCHINSON CANCER RES CENT
Methods and devices for microfluidic point-of-care immunoassays
ActiveUS8110392B2Reduce incubation timeMean flow velocityBioreactor/fermenter combinationsShaking/oscillating/vibrating mixersPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
Method and system of coregistrating optical coherence tomography (OCT) with other clinical tests
ActiveUS7593559B2Improve signal-to-noise ratioReduce artifactsMaterial analysis using wave/particle radiationRadiation/particle handlingClinical testsClinical trial
Owner:DUKE UNIV
Near infrared risk assessment of diseases
The present invention provides an apparatus and a method for identifying the risk of a clinical condition in a human or animal by correlating Near Infrared (NIR) absorbance spectral data with one or several parameters including a concentration of one or more substances in the skin, a concentration of one or more substances in skin plus subdermal tissue, a score derived from one or more clinical tests like a stress test on a treadmill, coronary angiography, or intravascular coronary ultrasound. The method determines the concentration of a compound in the skin of a human or animal and comprises the steps of placing a part of the skin against a receptor, directing electromagnetic radiation (EMR) from the near-infrared spectrum onto the skin, measuring a quantity of EMR reflected by, or transmitted through, the skin with a detector; and performing a quantitative mathematical analysis of the quantity of EMR to determine the concentration of the compound, for example free and esterfied cholesterol. An example of a clinical condition is cardiovascular disease.
Owner:TYCO HEALTHCARE GRP LP
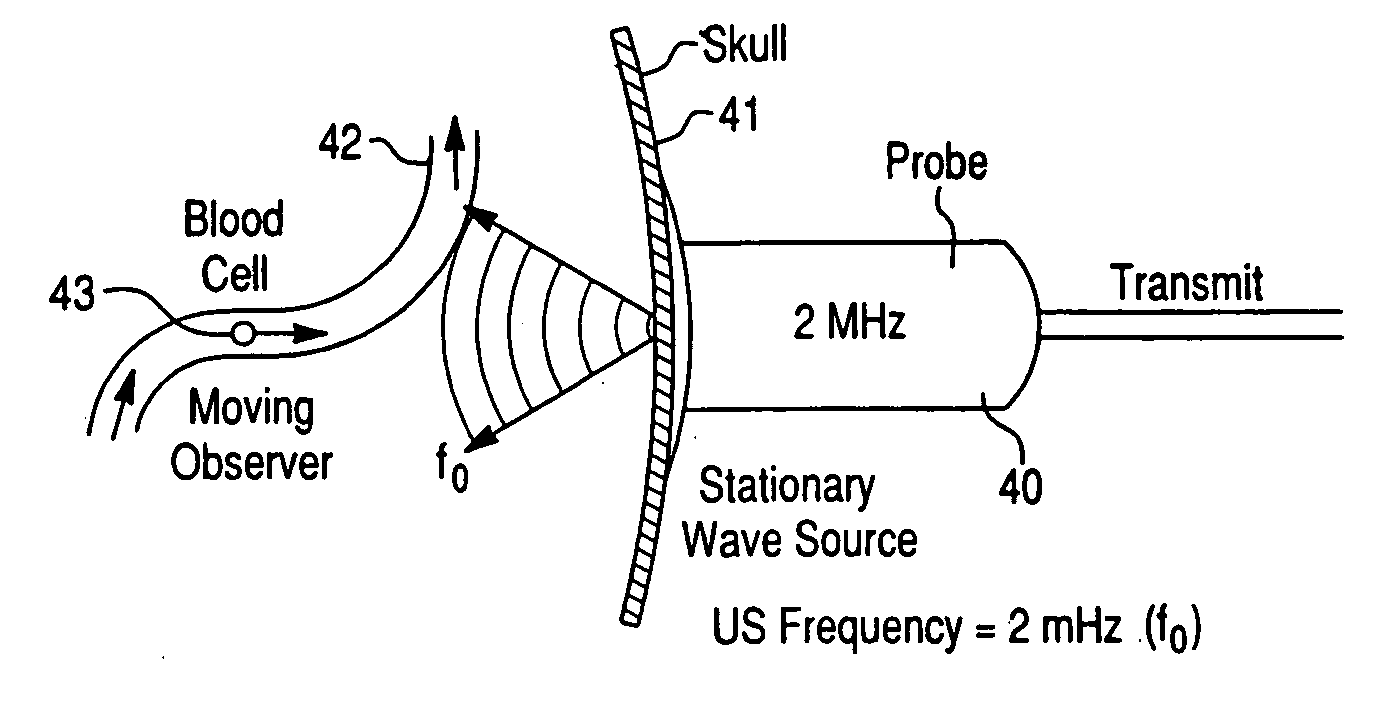
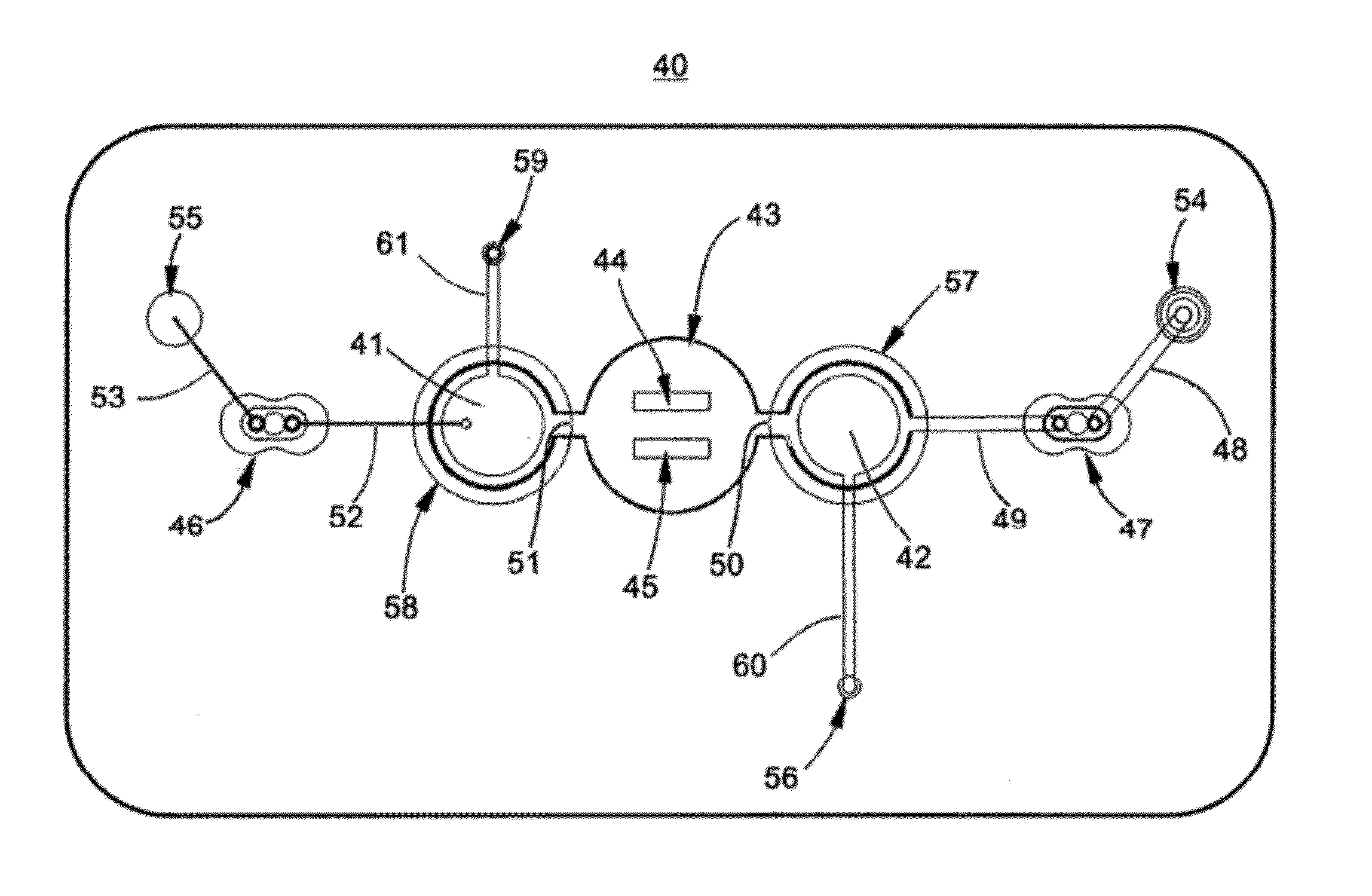
Systems and methods for using dynamic vascular assessment to improve vascular stent placement, application, design and marketing
InactiveUS20050038342A1Rapid and inexpensive to performEfficient deliveryBlood flow measurement devicesMedical automated diagnosisClinical testsPre operative
The invention relates to systems and methods for assessing blood flow in single or multiple vessels and segments, for assessing vascular health, for conducting clinical trials, for screening therapeutic interventions for effect, for assessing risk factors, for evaluating intracranial pressure and for analyzing the results in a defined manner. The invention enables direct monitoring of therapies, substances and devices on blood vessels, especially those of the cerebral vasculature. Relevant blood flow parameters include mean flow velocity, systolic acceleration, and pulsatility index. Measurement and analysis of these parameters, and others, provides details regarding the vascular health of individual and multiple vessels and a global analysis of an individual's overall vascular health. The invention is applicable as both a system and method for monitoring and improving the design, performance, marketing and use of vascular stents. In particular, the invention enables DVA-assisted stenting procedures, including both pre-operative and post-operative management.
Owner:NEW HEALTH SCI
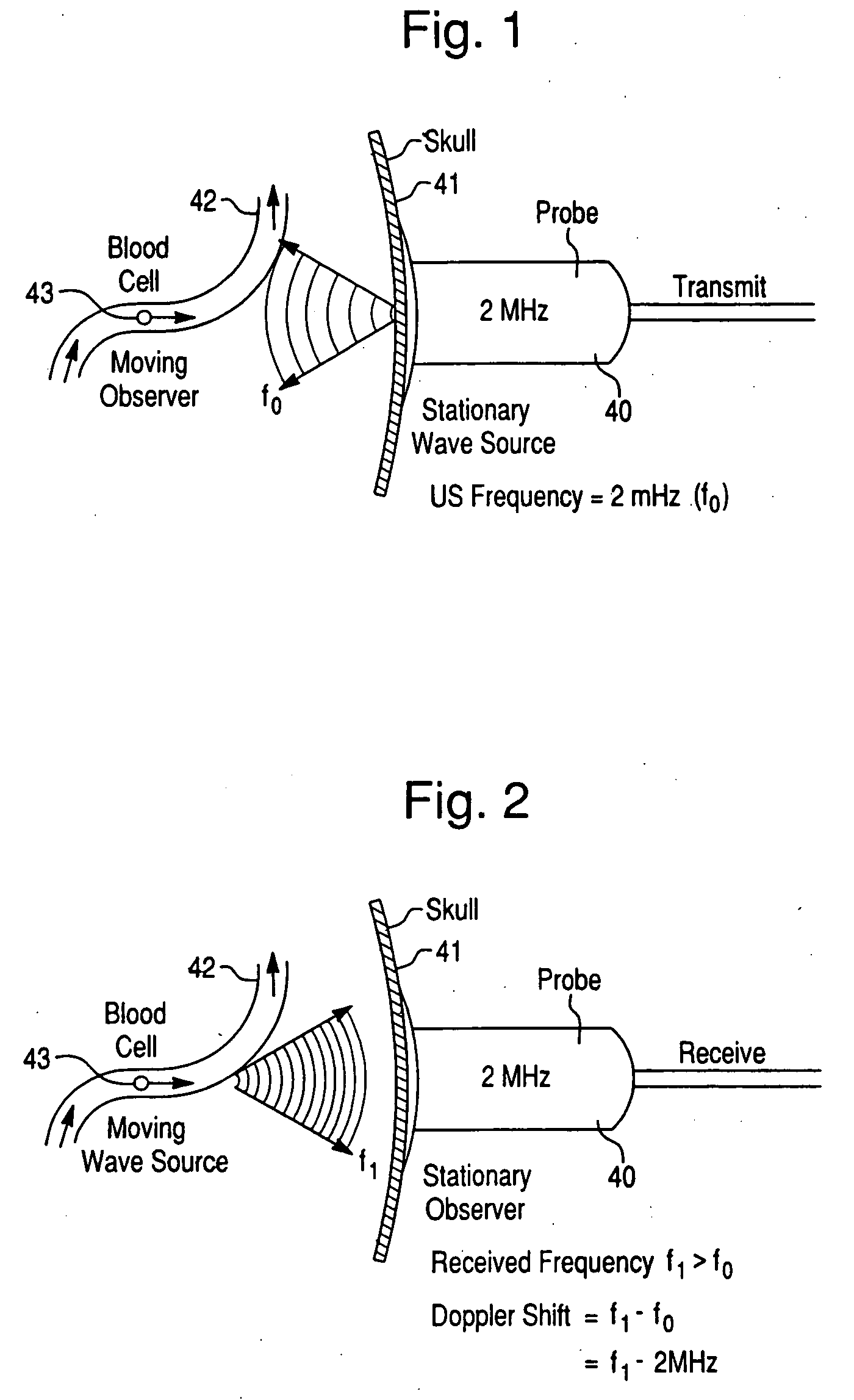
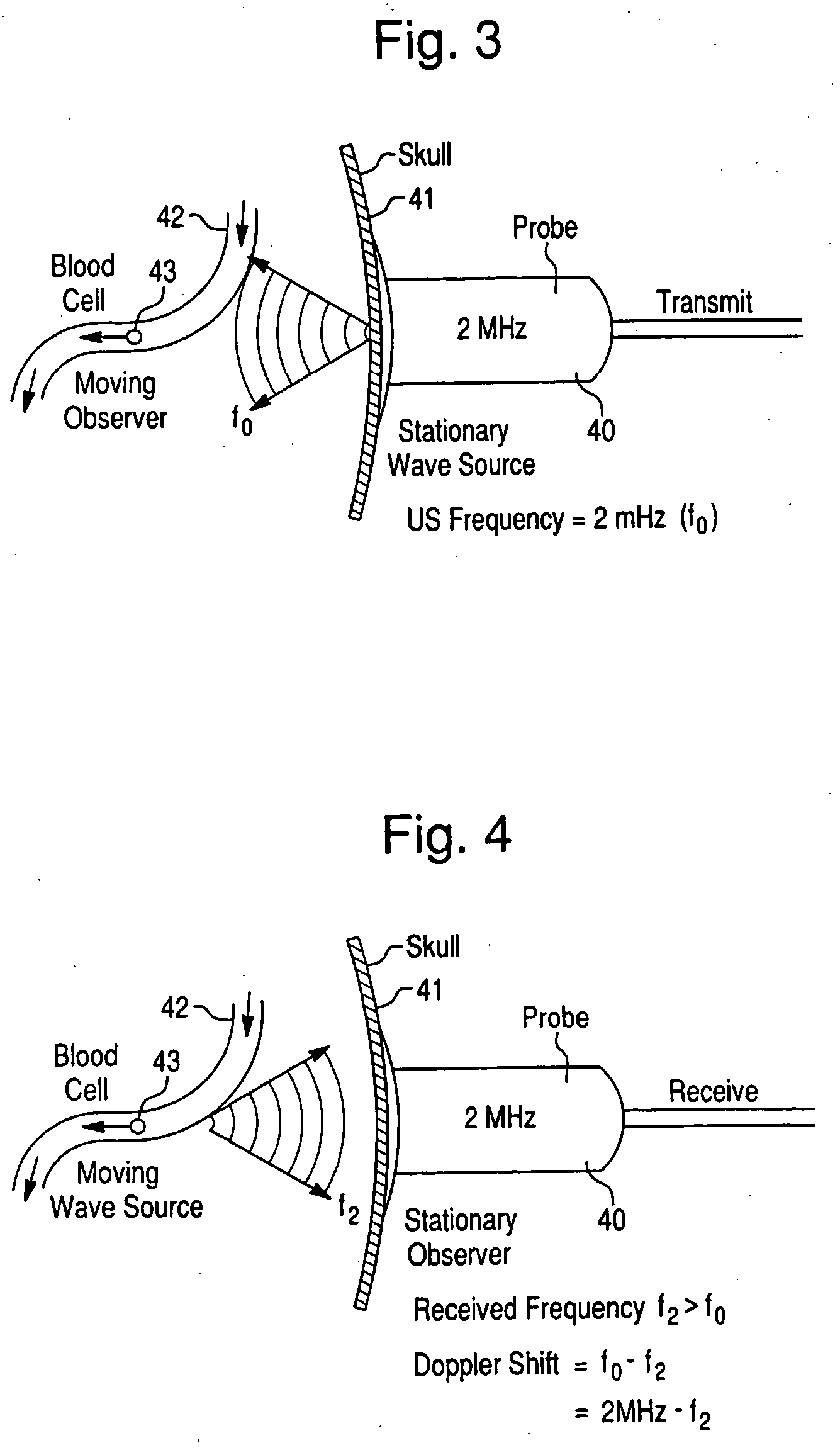
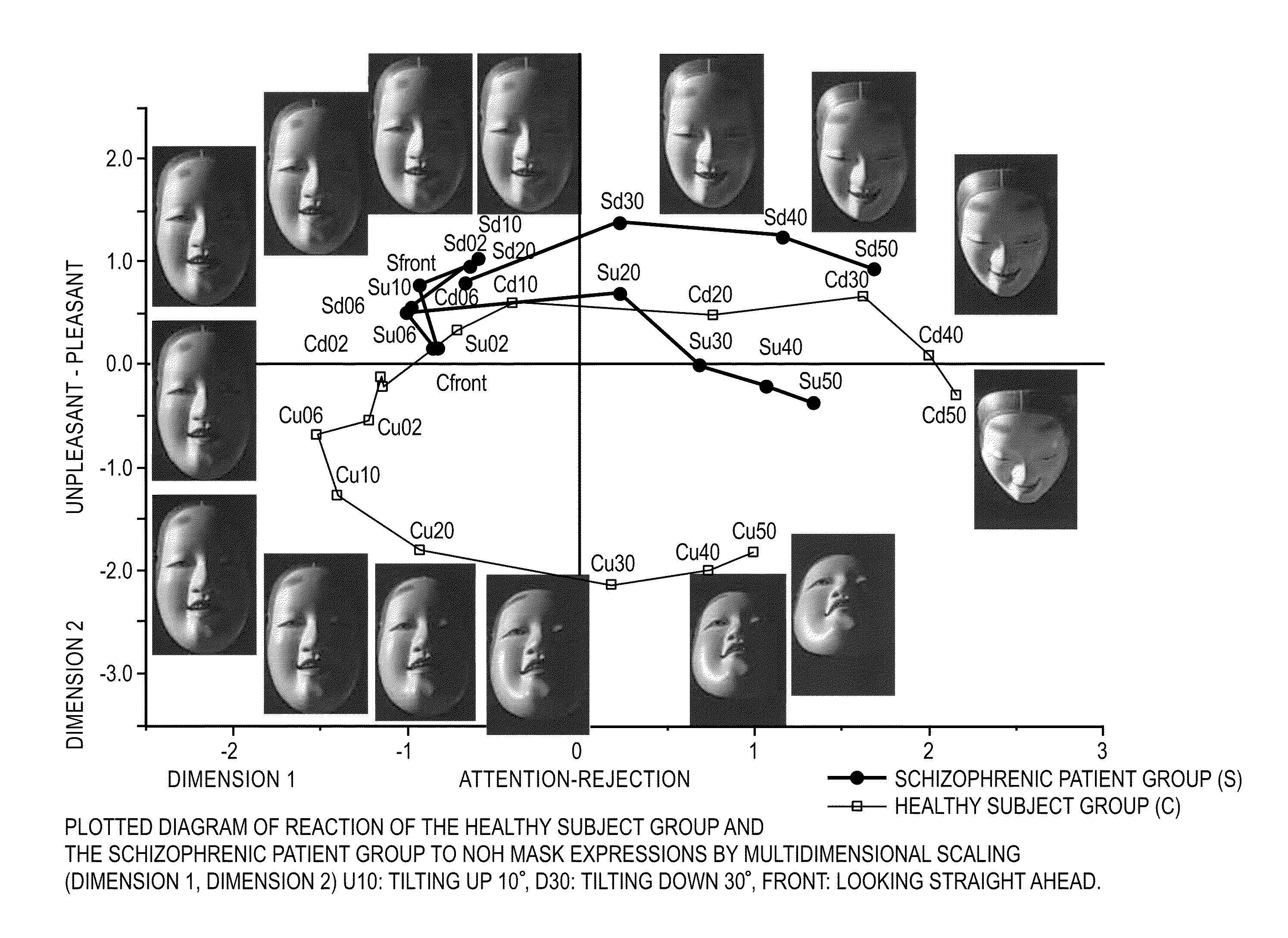
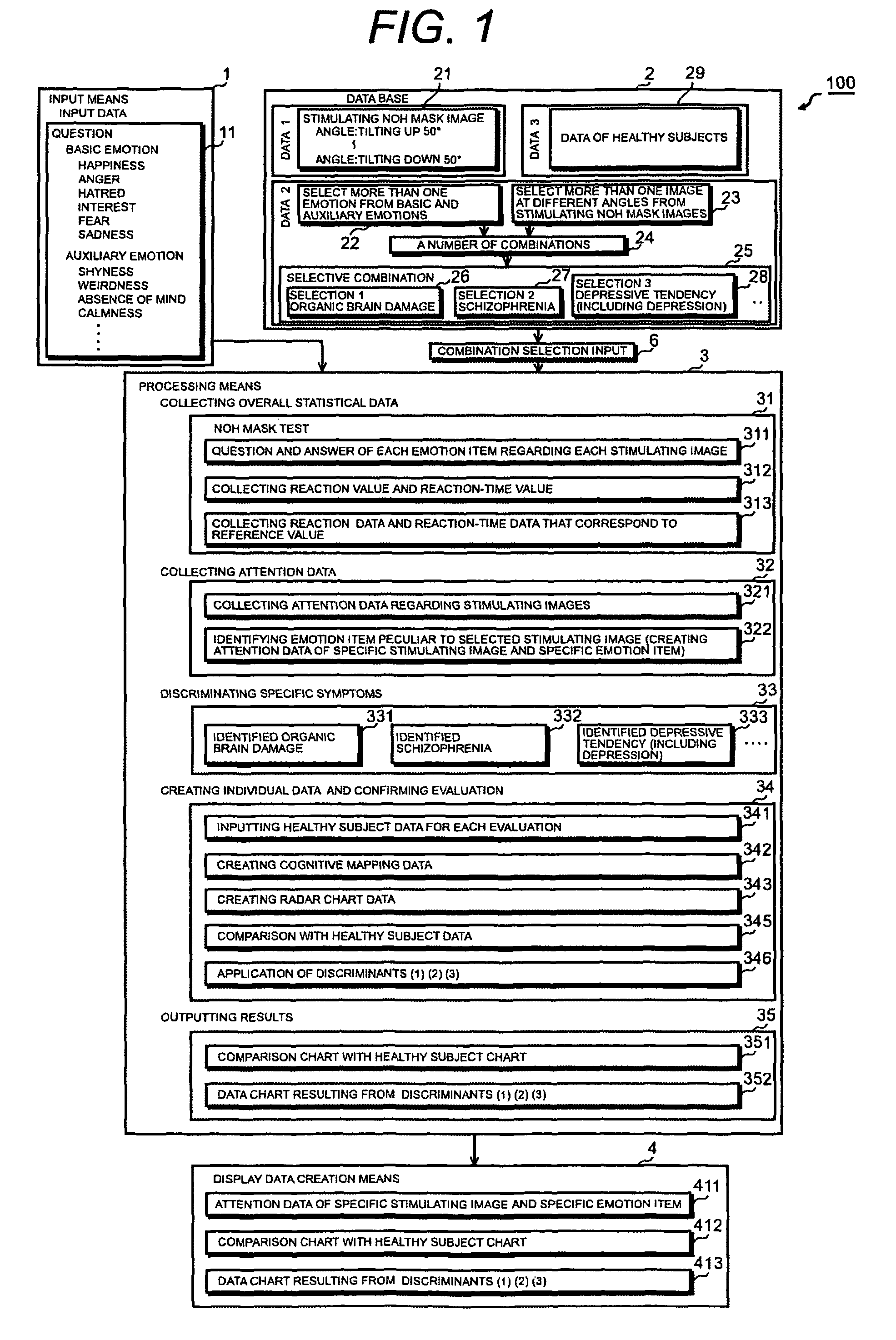
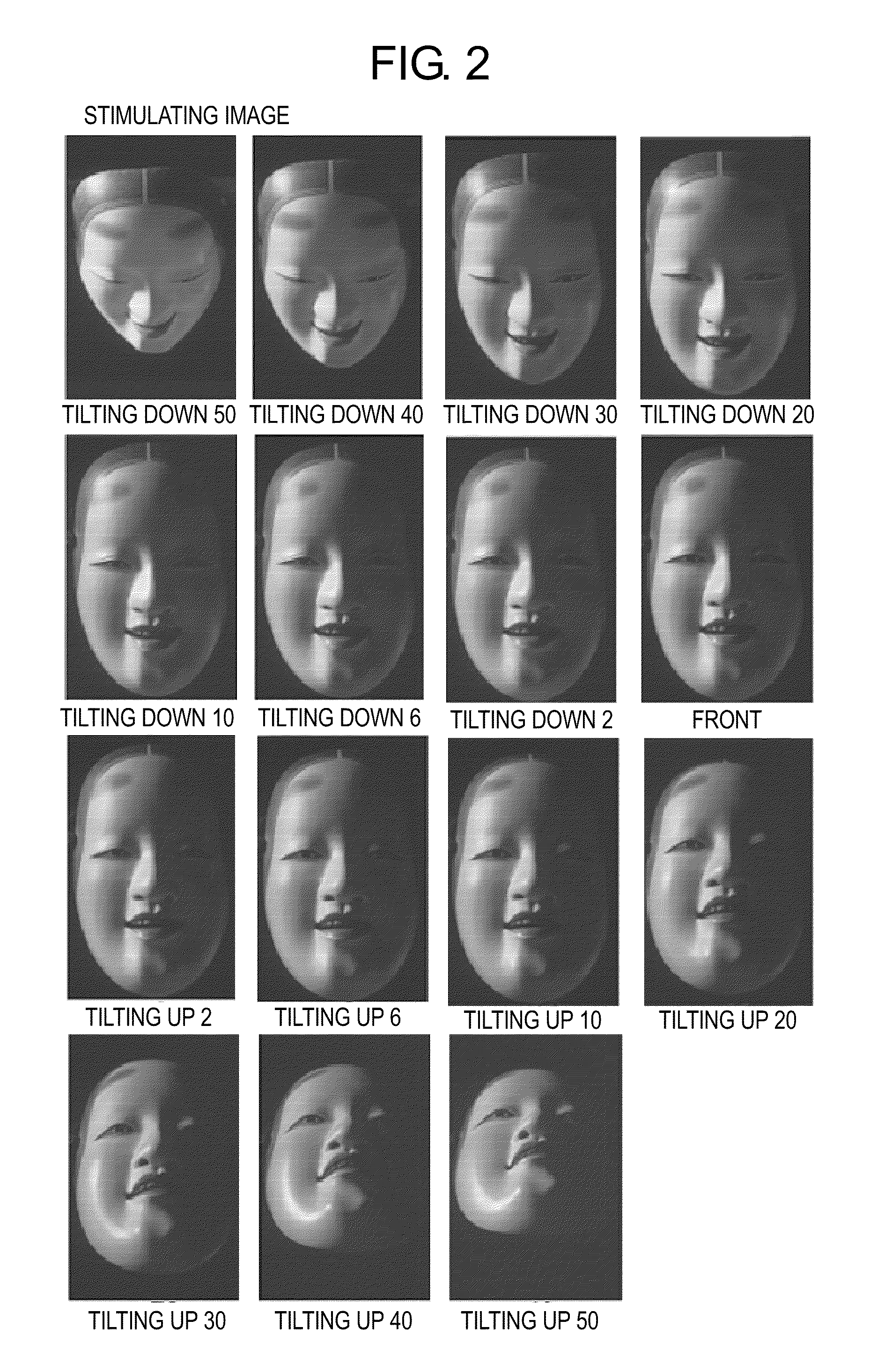
Psychotic manifestation and mental state evaluation apparatus and evaluation method
ActiveUS7942816B2Increase probabilityMedical data miningMental therapiesMental stateClinical psychology
A psychotic manifestation and mental state evaluation apparatus and method which can individually discriminate among symptoms by taking advantage of stimulating Noh mask images and also evaluate with a high probability whether a person is suffering from a specific symptom at the time of diagnosing, clinical examination, assessment, or counseling.
Owner:SATOH SHINJI +1
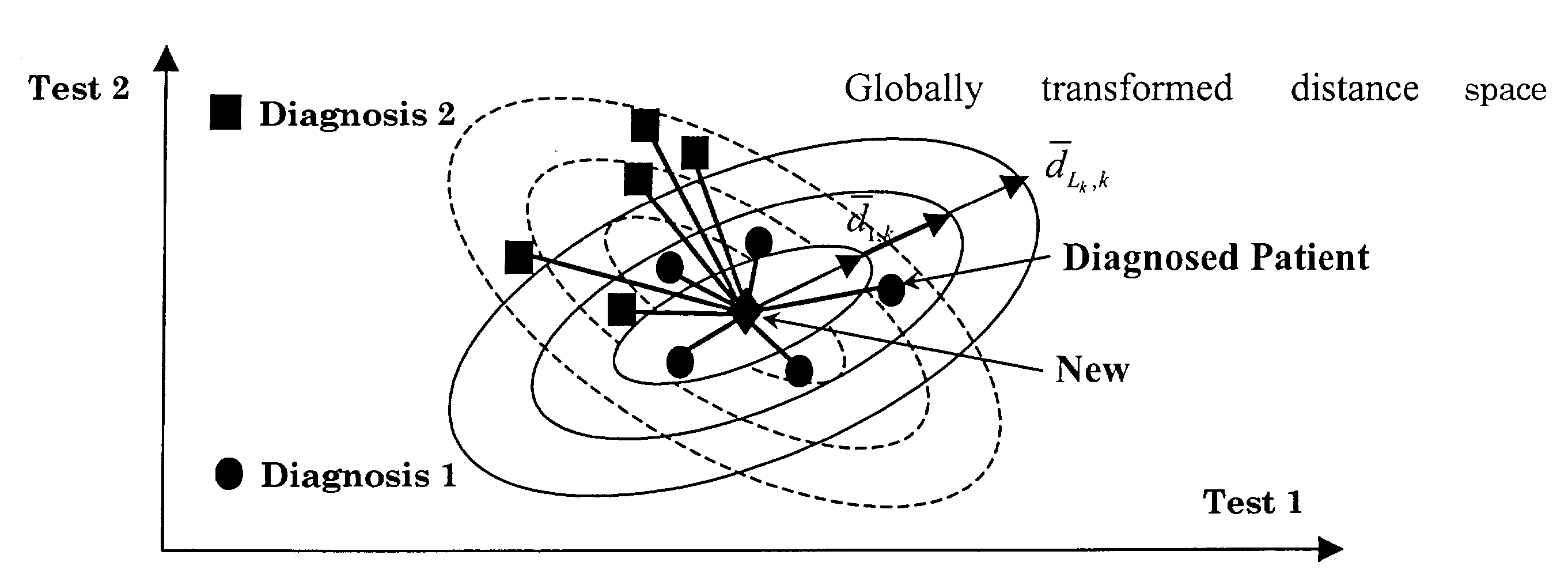
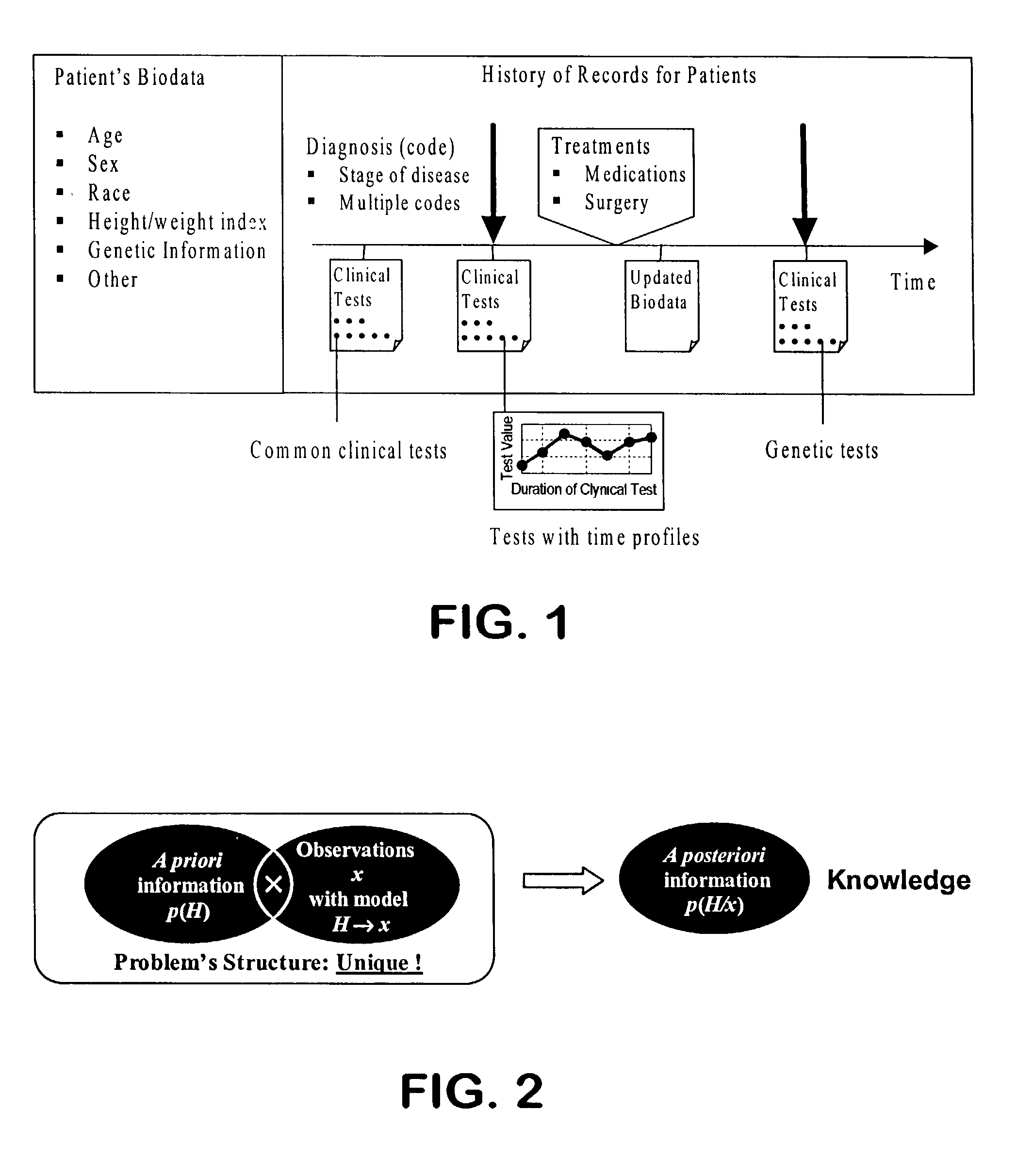
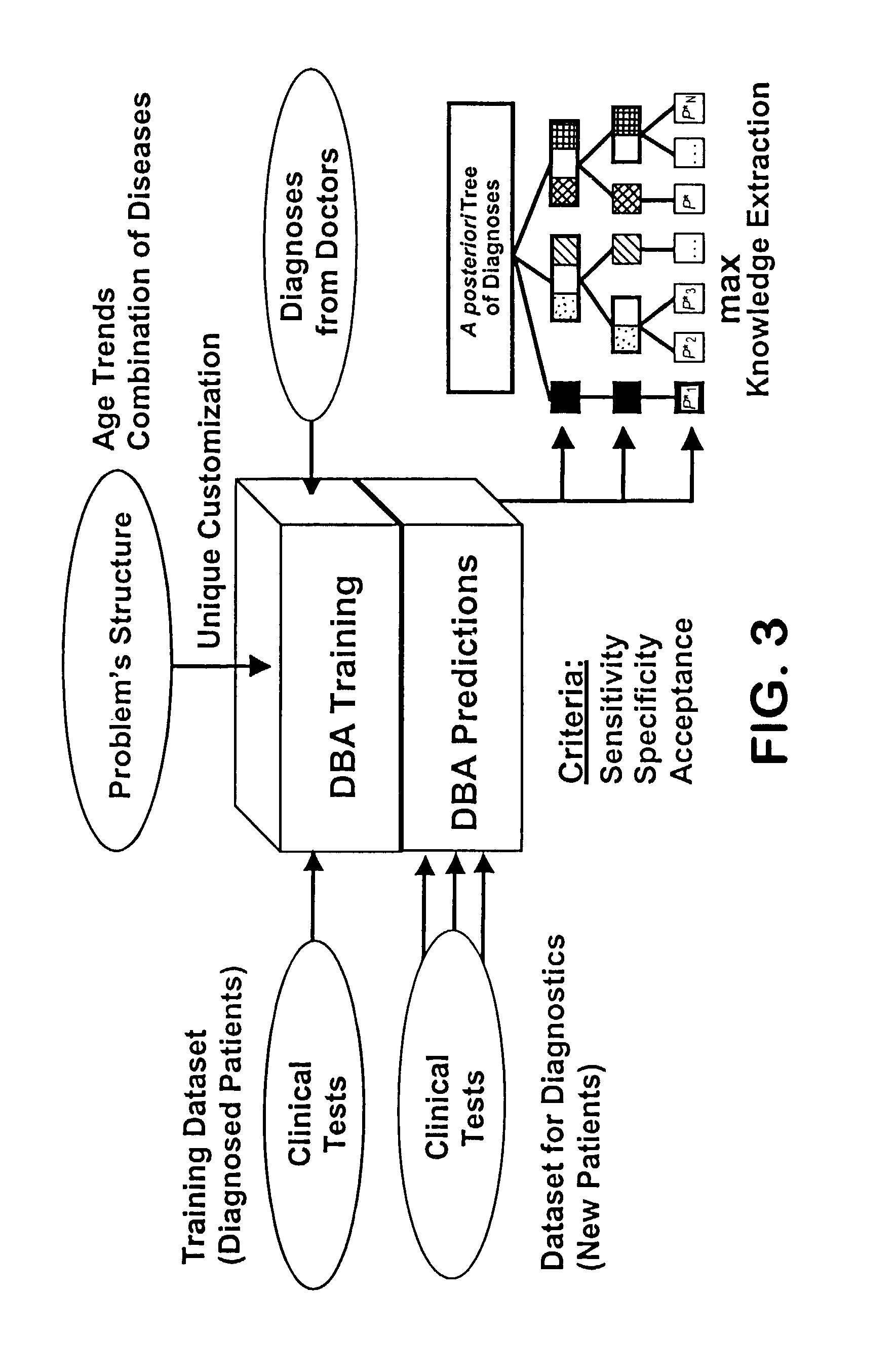
Diagnosing inapparent diseases from common clinical tests using Bayesian analysis
InactiveUS7392199B2Strong specificityHigh sensitivityComputer-assisted medical data acquisitionDiagnostic recording/measuringMedicineClinical tests
A system and method of diagnosing diseases from biological data is disclosed. A system for automated disease diagnostics prediction can be generated using a database of clinical test data. The diagnostics prediction can also be used to develop screening tests to screen for one or more inapparent diseases. The prediction method can be implemented with Bayesian probability estimation techniques. The system and method permit clinical test data to be analyzed and mined for improved disease diagnosis.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Methods and devices for microfluidic point-of-care immunoassays
InactiveUS20120164627A1Reduce incubation timeMean flow velocityBioreactor/fermenter combinationsShaking/oscillating/vibrating mixersPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
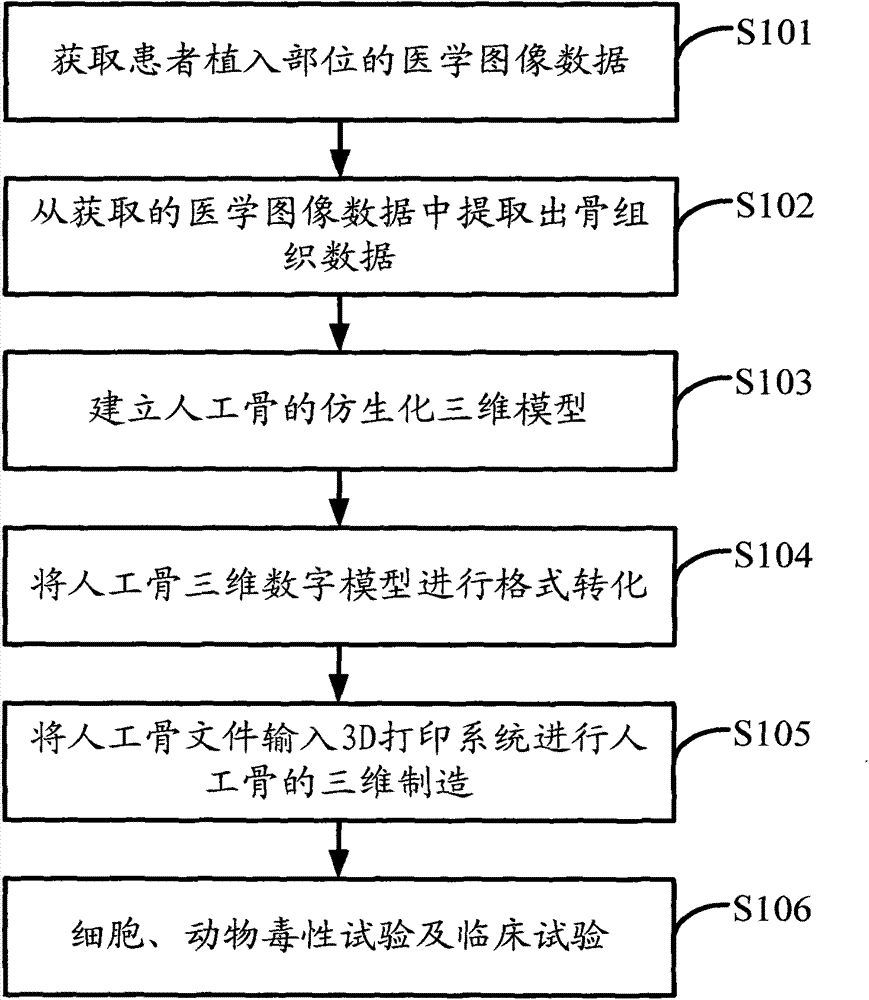
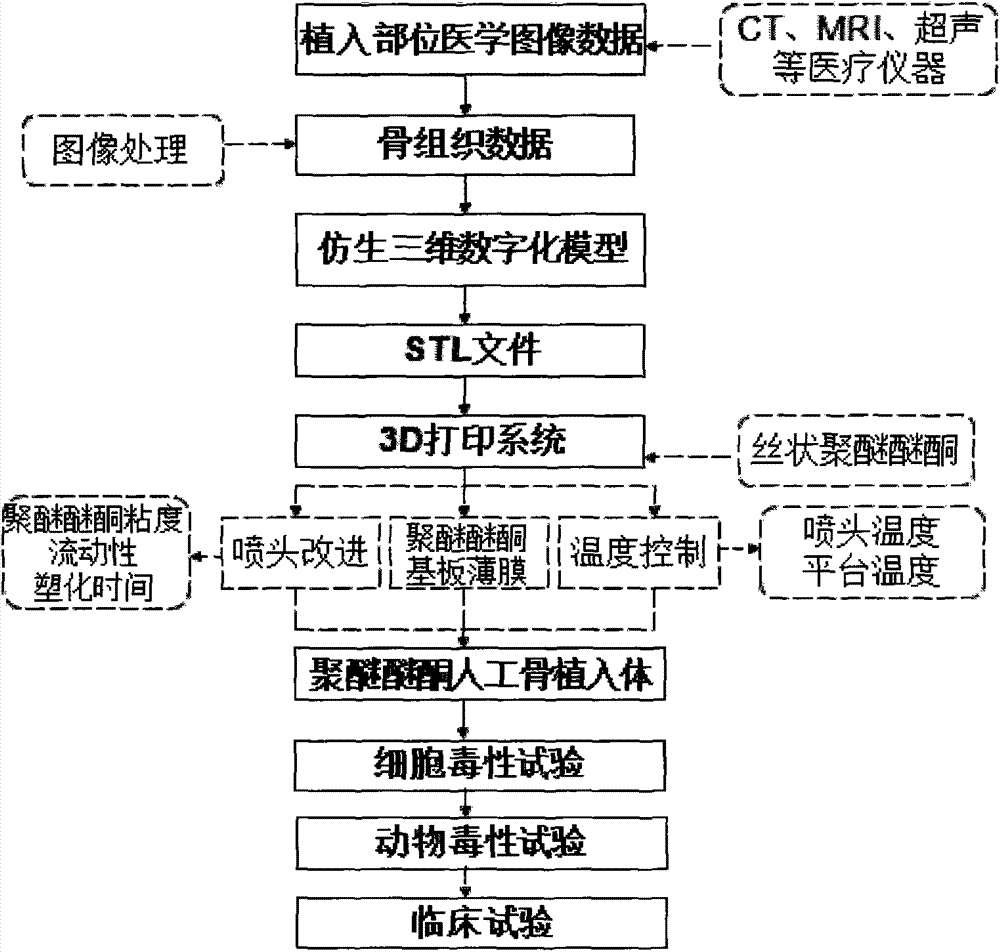
Polyether-ether-ketone biomimetic artificial bone 3D printing manufacturing method
ActiveCN103707507ASave timeSave costSpecial data processing applications3D modellingBiocompatibility TestingArtificial bone
The invention discloses a polyether-ether-ketone biomimetic artificial bone 3D printing manufacturing method, wherein the artificial bone can replace metal and has an excellent biocompatibility. The method comprises the following steps: first, collecting the bone tissue image data of the part, which is about to be implanted with an artificial bone, of a patient by using a medical instrument; secondly, establishing a three-dimensional digital model of the artificial bone on the basis of the collected data; thirdly, carrying out a format conversion on the three-dimensional digital model of artificial bone, inputting the converted file into a 3D printing system to manufacture the artificial bone; and finally carrying out cell toxicity tests, animal tests, and clinical tests. The invention utilizes a self-made polyether-ether-ketone 3D printing system to manufacture artificial bones, thus the time and cost for manufacturing moulds are saved, the manufacture period is shortened; at the same time, the shape of parts can be adjusted at any time according to the setting of the forming software; so that an crystalline polymer polyether-ether-ketone artificial bone, which has excellent biocompatibility, can be implanted into the human body, and has the advantages of high melting point, large viscosity, and bad fluidity, can be manufactured through the 3D printing method.
Owner:JILIN UNIV
Diagnosing inapparent diseases from common clinical tests using bayesian analysis
InactiveUS20090024332A1High sensitivityStrong specificityNoise figure or signal-to-noise ratio measurementComputer-assisted medical data acquisitionClinical testsDiagnoses diseases
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
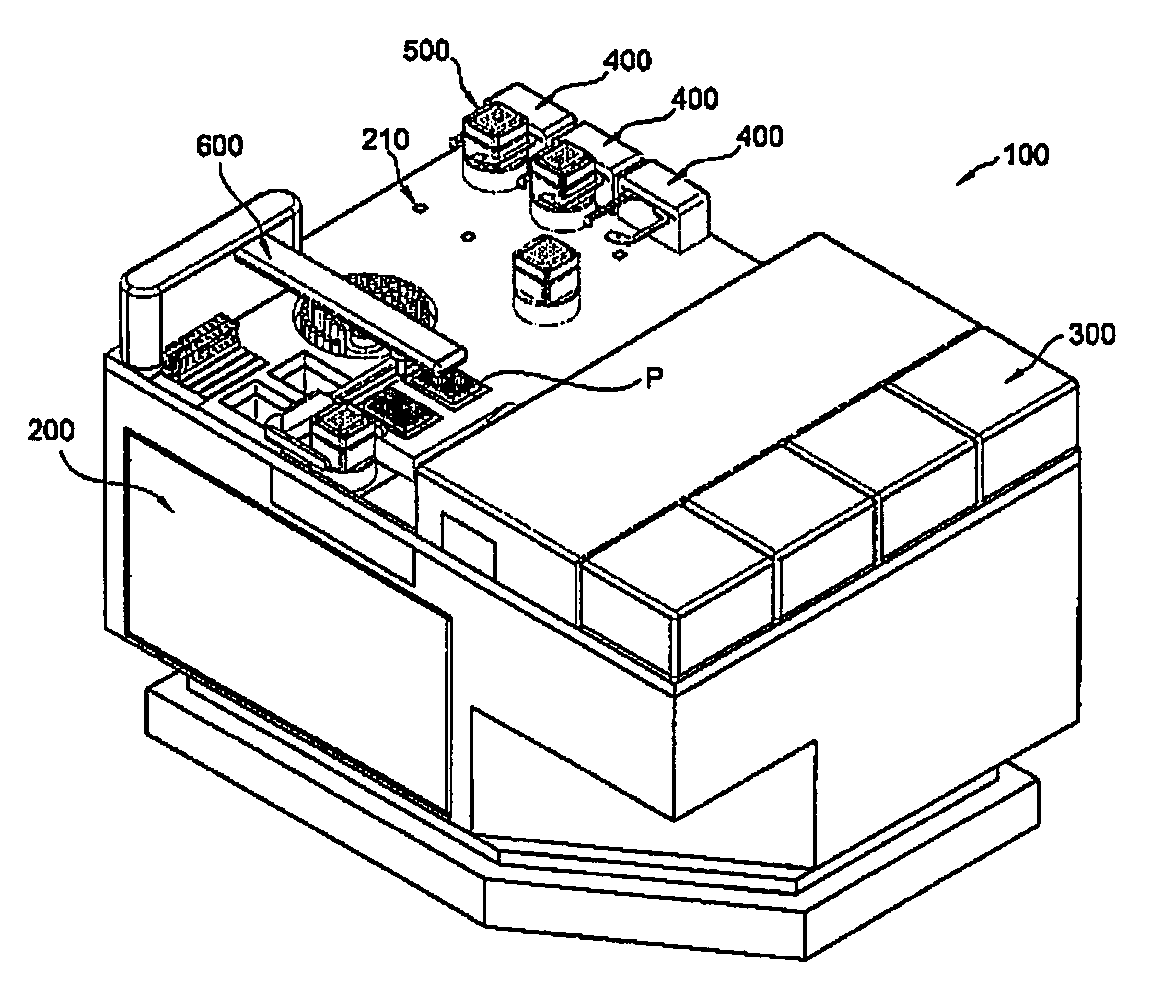
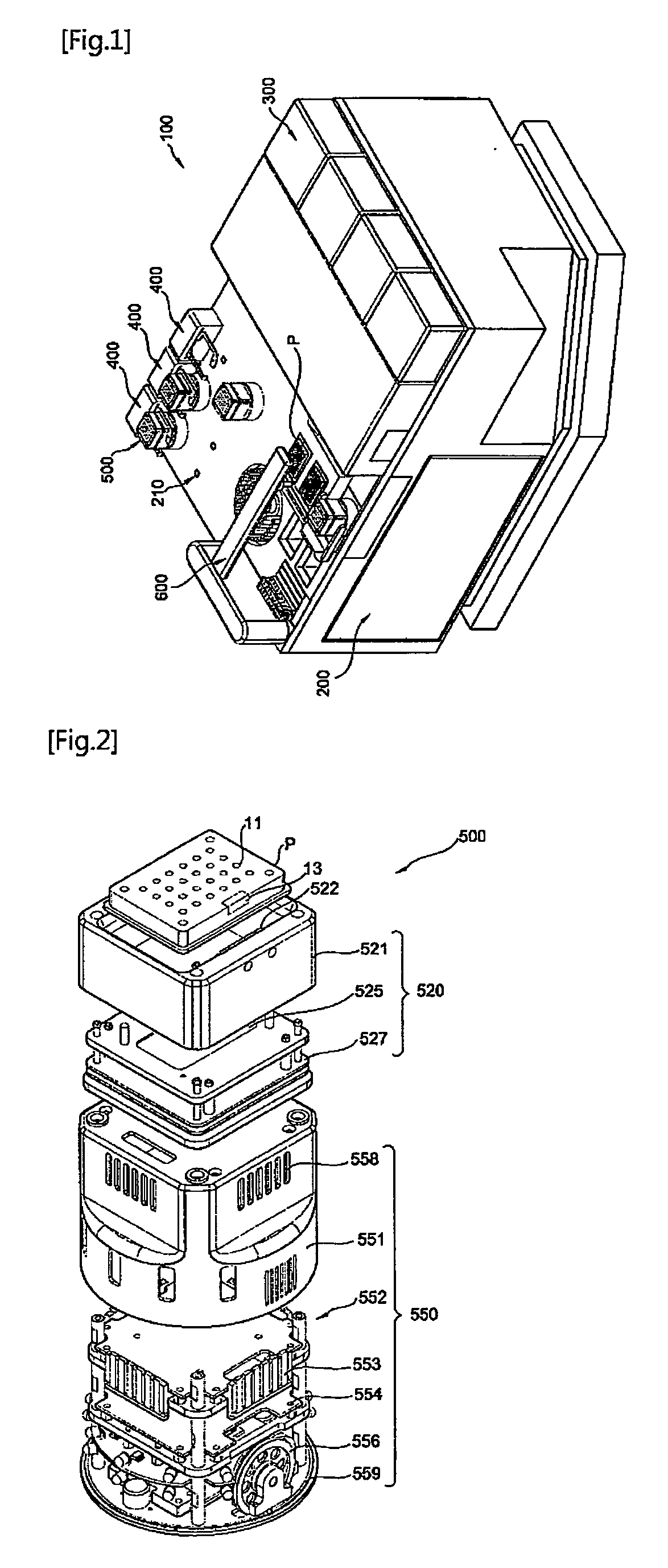
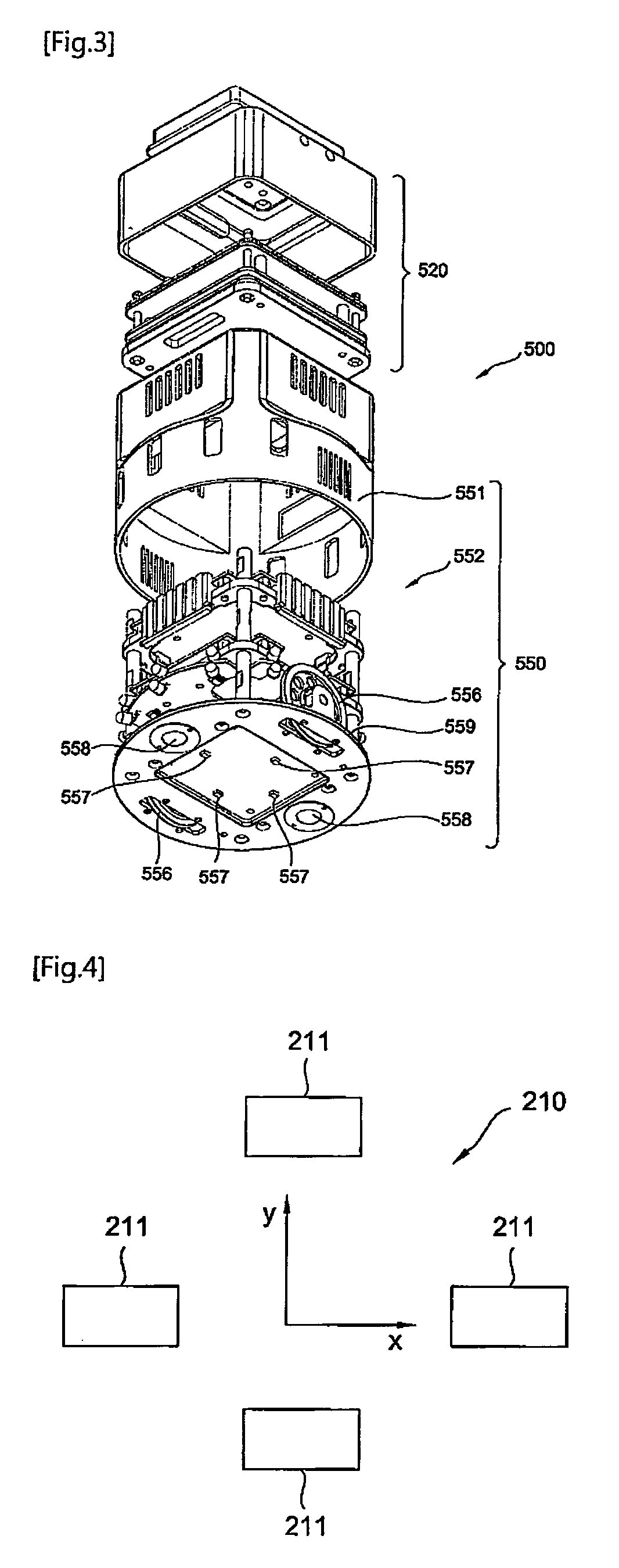
Mobile robot and clinical test apparatus using the same
InactiveUS20090035181A1Shorten the timeLower the volumeDiagnostic recording/measuringSensorsClinical testsClinical trial
A clinical test apparatus employing a mobile robot is provided. The clinical test apparatus includes a stage unit, a test station provided in the stage unit and configured to perform a clinical test, a mobile robot configured to move on a top surface of the stage unit and to transfer a plate on which samples and reagents are loaded to the test station, and a docking unit disposed in the stage unit and configured to reset a position of the mobile robot. A variety of test stations for a clinical test can be integrated, and multiple tests can be performed at the same time through a plurality of mobile robots.
Owner:POSTECH ACAD IND FOUND
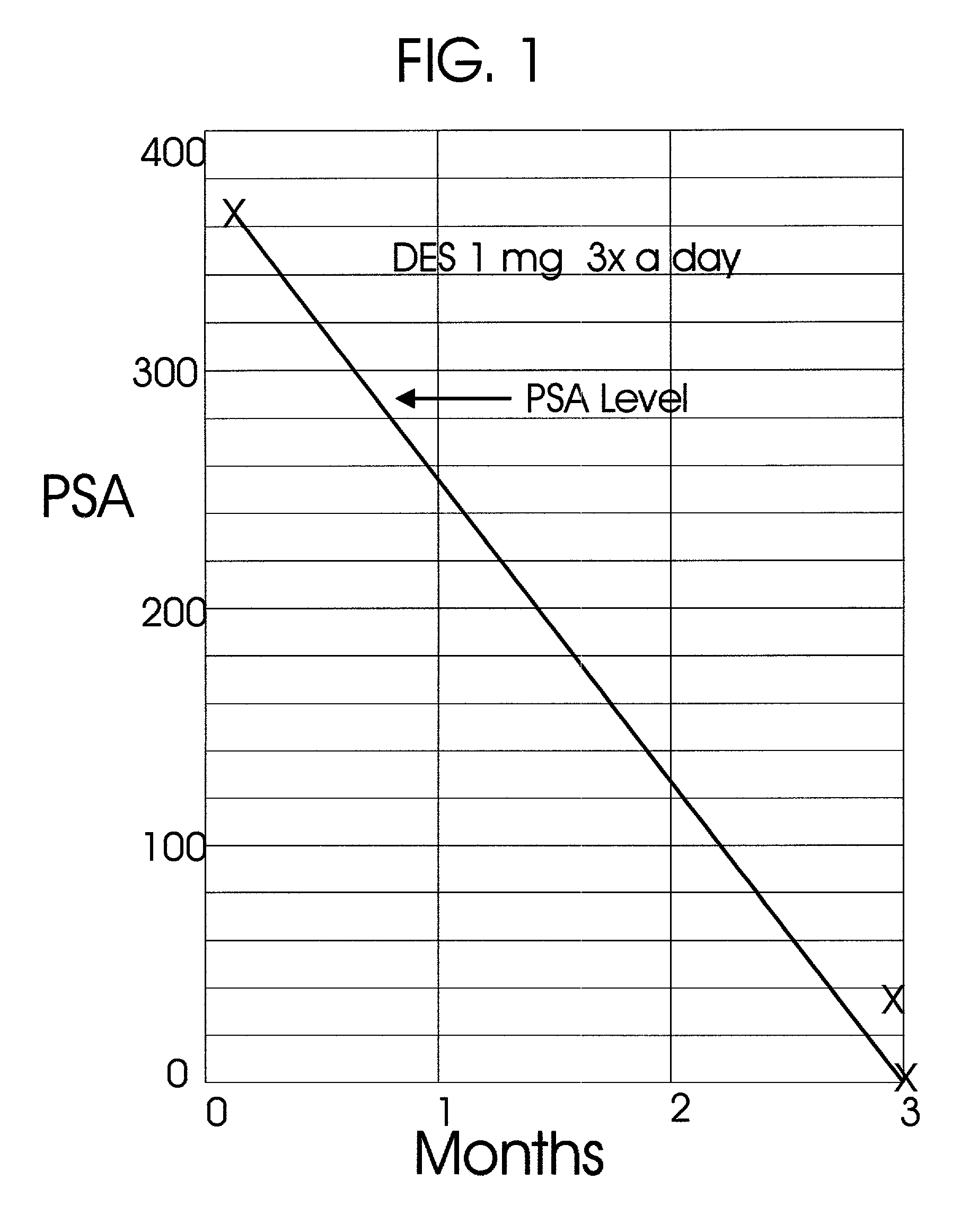
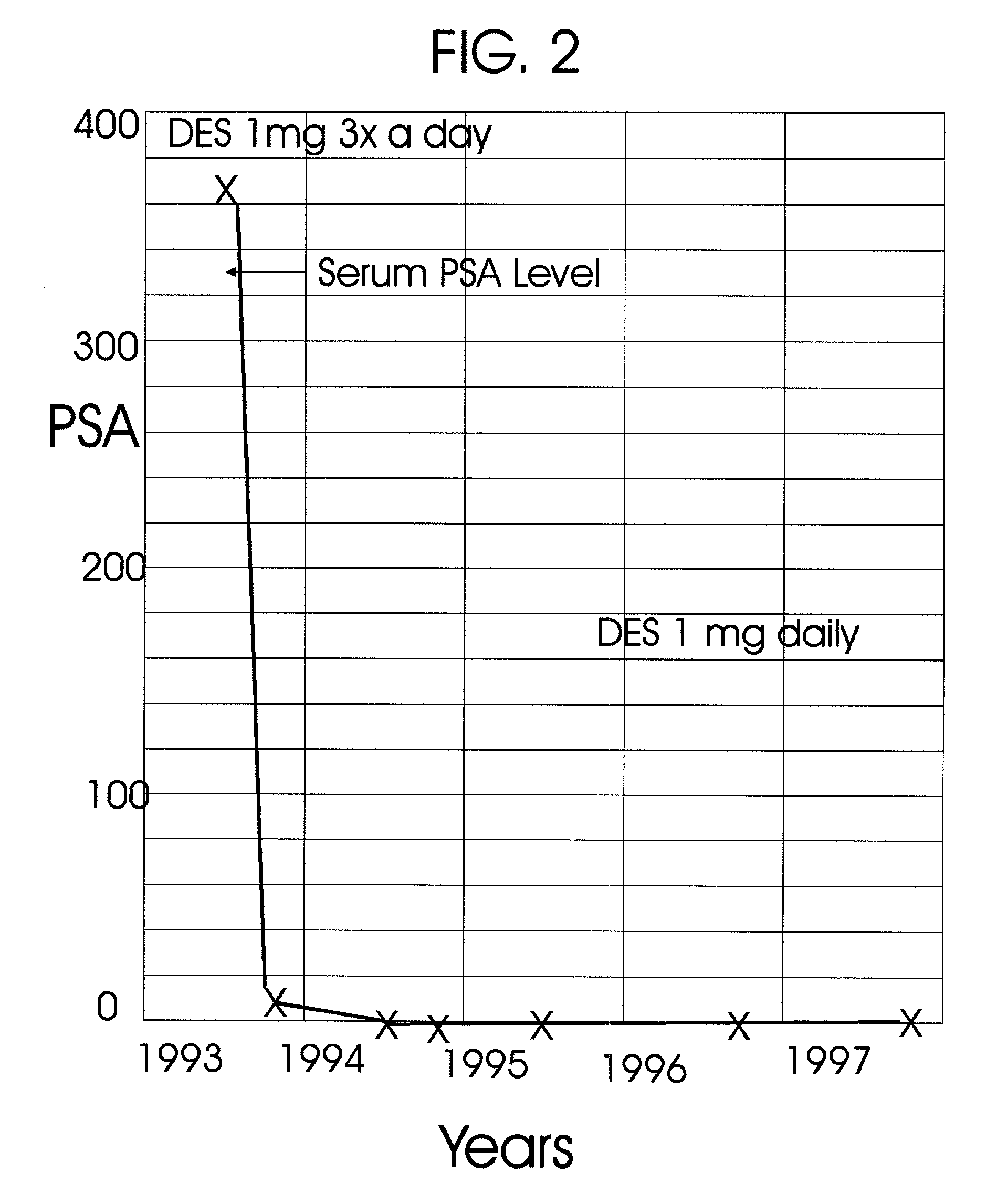
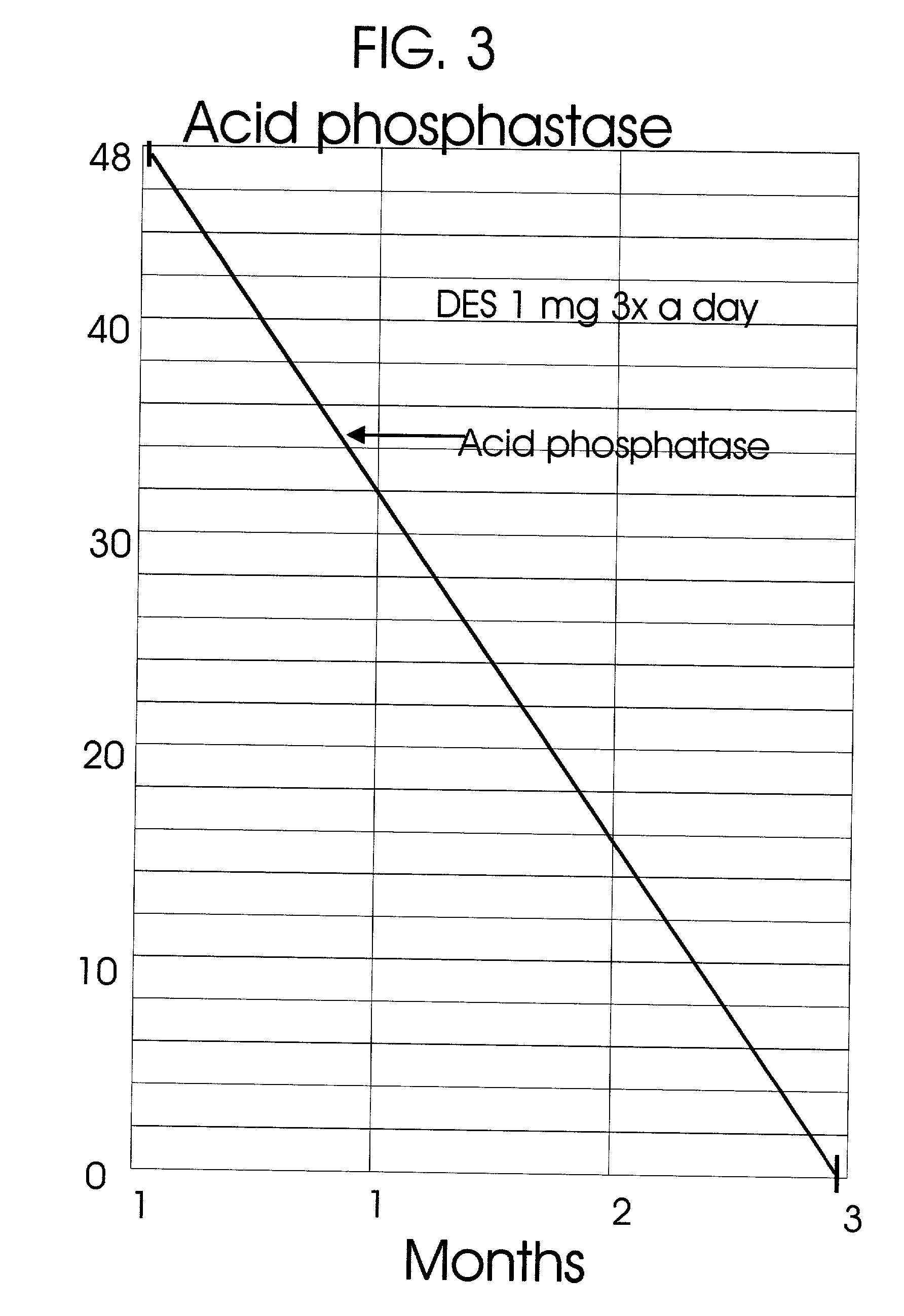
Prostatic hormonal implants treatment of prostate cancer
An improved method and products for the primary hormonal treatment of early stage, low and intermediate risk prostate cancers by prostatic implants of androgen suppressive drugs formulated as fused with a lipoid carrier or encapsulated in microcapsules or in Silastic capsules is provided. Such prostatic implants renders a constant slow-release of their contents to the prostate for extended periods by biodegradation and diffusion. It facilitates higher prostatic and lower systemic concentrations of androgen suppressive hormones. Because of their high prostatic and lower systemic concentrations, tumor control is much improved and the their systemic toxicity is minimized. Tumor control after such primary hormonal implant treatment is followed by clinical examinations and the biochemical tumor control is followed by periodic estimations of serum levels of PSA and acid phosphatase. More complex and expensive surgery or radiation therapy for this group of good prognostic early stage prostate cancer is reserved for those patients failing to this primary hormonal treatment. It will preserve potency more than by surgery or radiation therapy. Furthermore, it would reduce the cost of treatment for early stage prostate cancer significantly. Androgen suppressive hormonal implants to the prostate before, during or after lower dose conventional radiation therapy would also facilitate equal or better cure rates of localized prostate cancer as compared to the more complex and toxic higher dose radiation therapy.
Owner:SAHADEVAN VELAYUDHAN
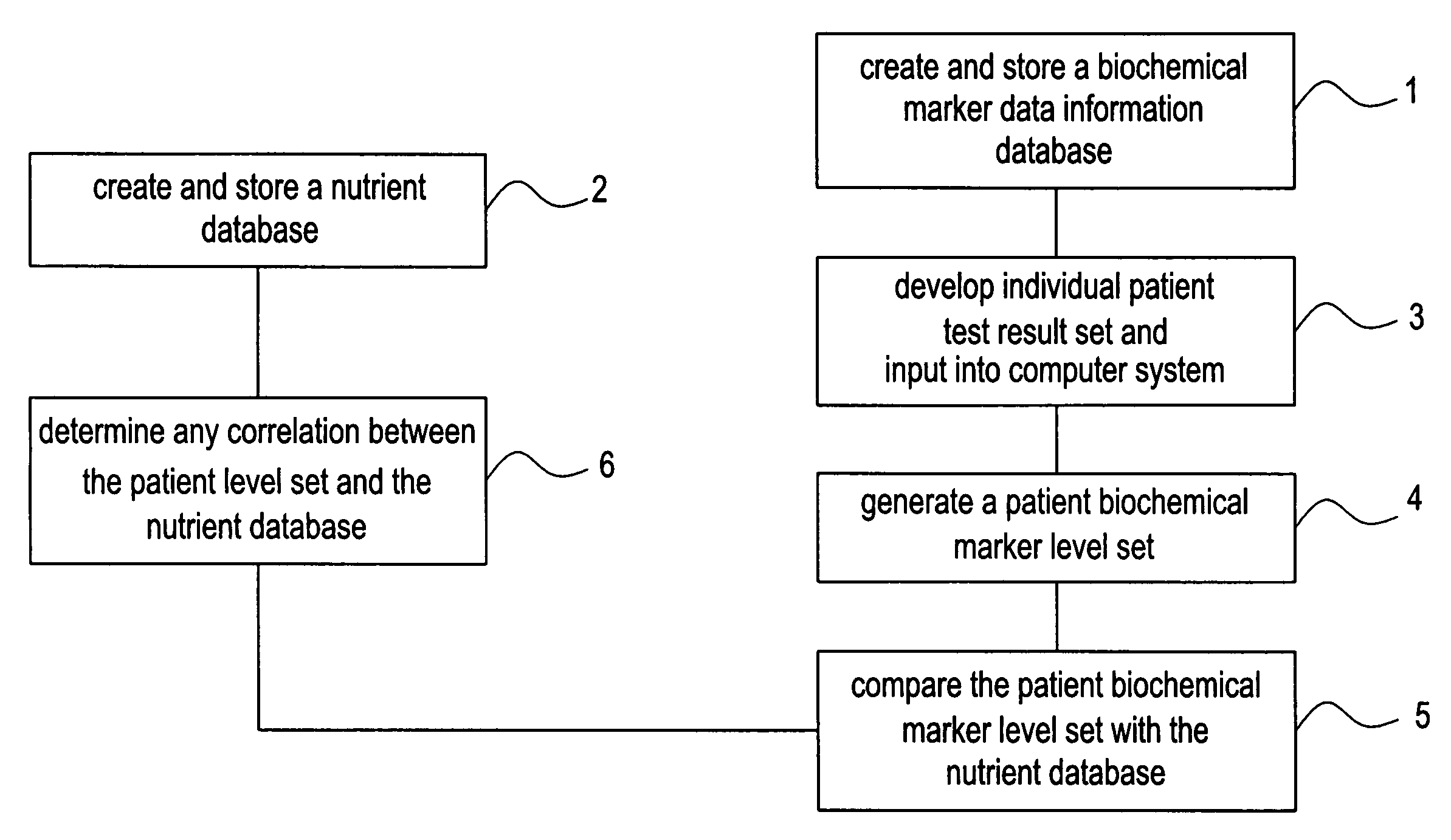
Network and methods for integrating individualized clinical test results and nutritional treatment
The present invention provides networks and method for linking consumers and nutritional pharmacologists offering personalized nutritional information through a central network site. The network includes a central integration site through which network members communicate with each other. The central integration site stores two or more databases in the storage medium. The databases store biochemical marker data information, nutritional and / or drug data information including a record for association and effect of nutrients with a particular biochemical marker, and / or drug. The network of the invention provides individualized nutritional diagnostic and treatment to consumers on the basis of their clinical test results.
Owner:BODYBIO INC
Formula for Chinese medicine for preventing and treating chemotherapeutic toxic and side reaction and preparation method for Chinese medicinal pills
ActiveCN102327390AEasy to prepareThe preparation method is simple and environmentally friendlyHydroxy compound active ingredientsAntinoxious agentsMedicinal herbsMonkshoods
The invention relates to a formula of a Chinese medicine for preventing and treating chemotherapeutic toxic and side reaction and a preparation method for Chinese medicinal pills. The Chinese medicine consists of donkey-hide gelatin, ginseng, cassia bark, Szechuan lovage rhizome, prepared rehmannia root, tuckahoe, largehead atractylodes rhizome, astragalus, Chinese angelica, white paeony root, south dodder seed, medlar, medicinal cyathula root, tangerine peel, glossy ganoderma, desertliving cistanche, hairy antler, musk, stalactite, medicinal indianmulberry root, prepared common monkshood daughter root, sea cucumber, liquoric root, deerhorn glue and yellow wine in a certain weight ratio. The preparation method comprises the following steps of: weighing the deerhorn glue in the weight ratio, adding into the yellow wine in the weight ratio, decocting, and cooling to room temperature to obtain glue solution; weighing the rest medicinal materials in the weight ratio, mixing uniformly, and crushing to form medicinal powder; and adding the medicinal powder into the glue solution, mixing uniformly, and pelletizing to form pills, wherein each pill is 5 grams. Clinical tests prove that the Chinese medicine can prevent and treat chemotherapeutic toxic and side reaction safely and effectively; and the preparation method for the pills is simple, convenient, easy and environment-friendly, and is suitable for production and clinical application.
Owner:LAVIANA TAIZHOU PHARMACHEM
Formula milk powder for promoting absorption of fatty acid and calcium and preparation method thereof
The invention discloses a formula milk powder for promoting the absorption of fatty acid and calcium and a preparation method thereof. Raw cow milk, lactose, 1,3-Dioleoyl 2-palmitoyl triglyceride and demineralized whey powder as main materials are added with concentrated whey albumen powder, Alpha-lactalbumin powder, oligosaccharide, walnut oil, casein phosphopeptide, docosahexaenoic acid, arachidonic acid, nucleotide, lutein, inositol, carnitine and the like as well as vitamins, mineral substances and other nutrients needed for strengthening infants, and fat humanization, protein humanization and carbohydrate humanization are realized. The powdery product is produced by the processes of blending, homogenization, concentration, spray-drying, packaging and the like. According to the physiological characteristics and nutritional demand of the infants, the invention reinforces the calcium, the 1,3-Dioleoyl 2-palmitoyl triglyceride, other nutrient ingredients and the like, and aiming at the oversea clinical test conclusion of the 1,3-Dioleoyl 2-palmitoyl triglyceride, the final test conclusion of comparison with breast milk and infant formula milk powder sold on the market in the process of a clinical feeding test is that the feeding result of the designed formula approximates the feeding result of the breast milk and is better than the feeding result of an infant formula milk powder group sold on the market.
Owner:HEILONGJIANG FEIHE DAIRY
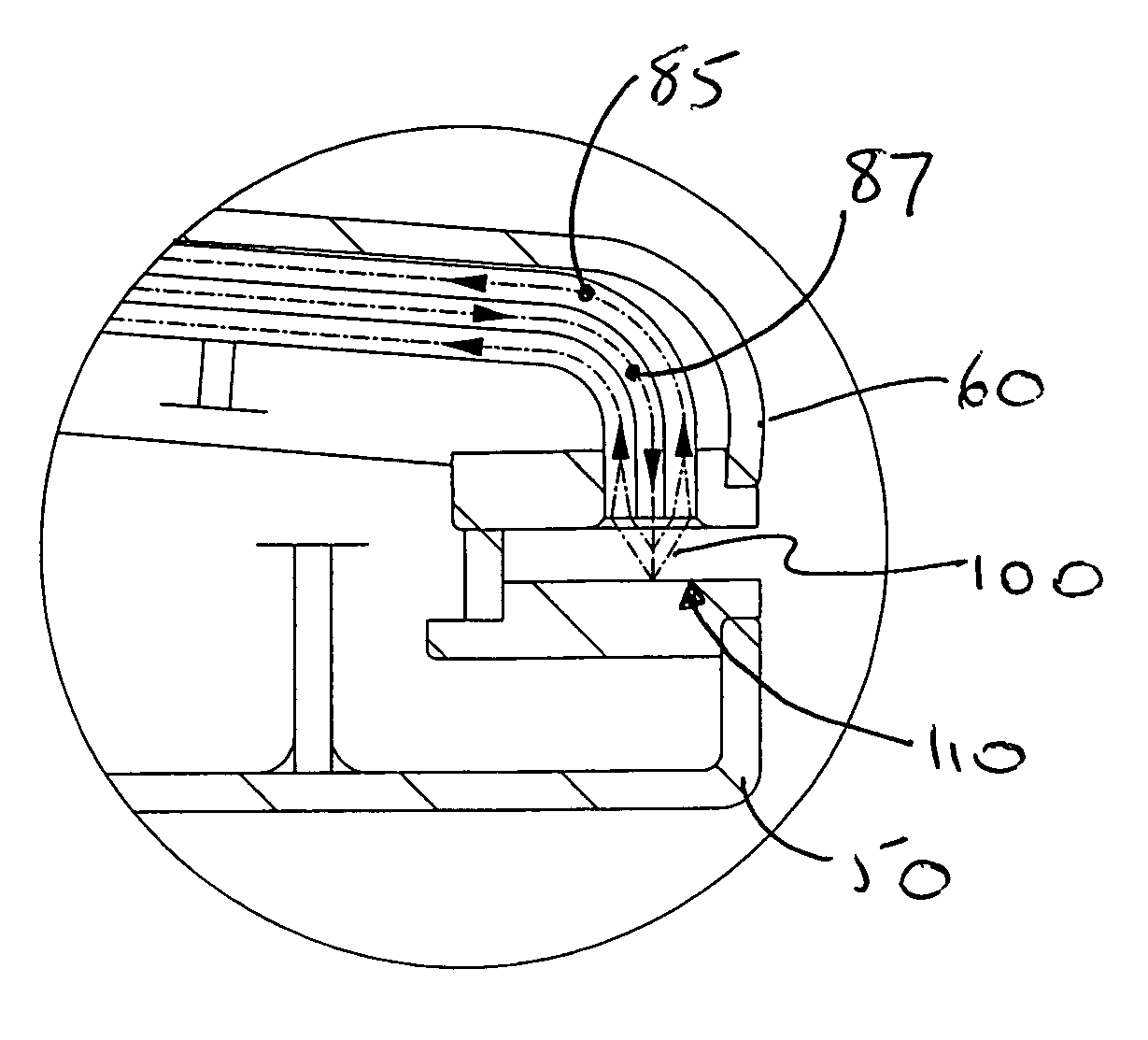
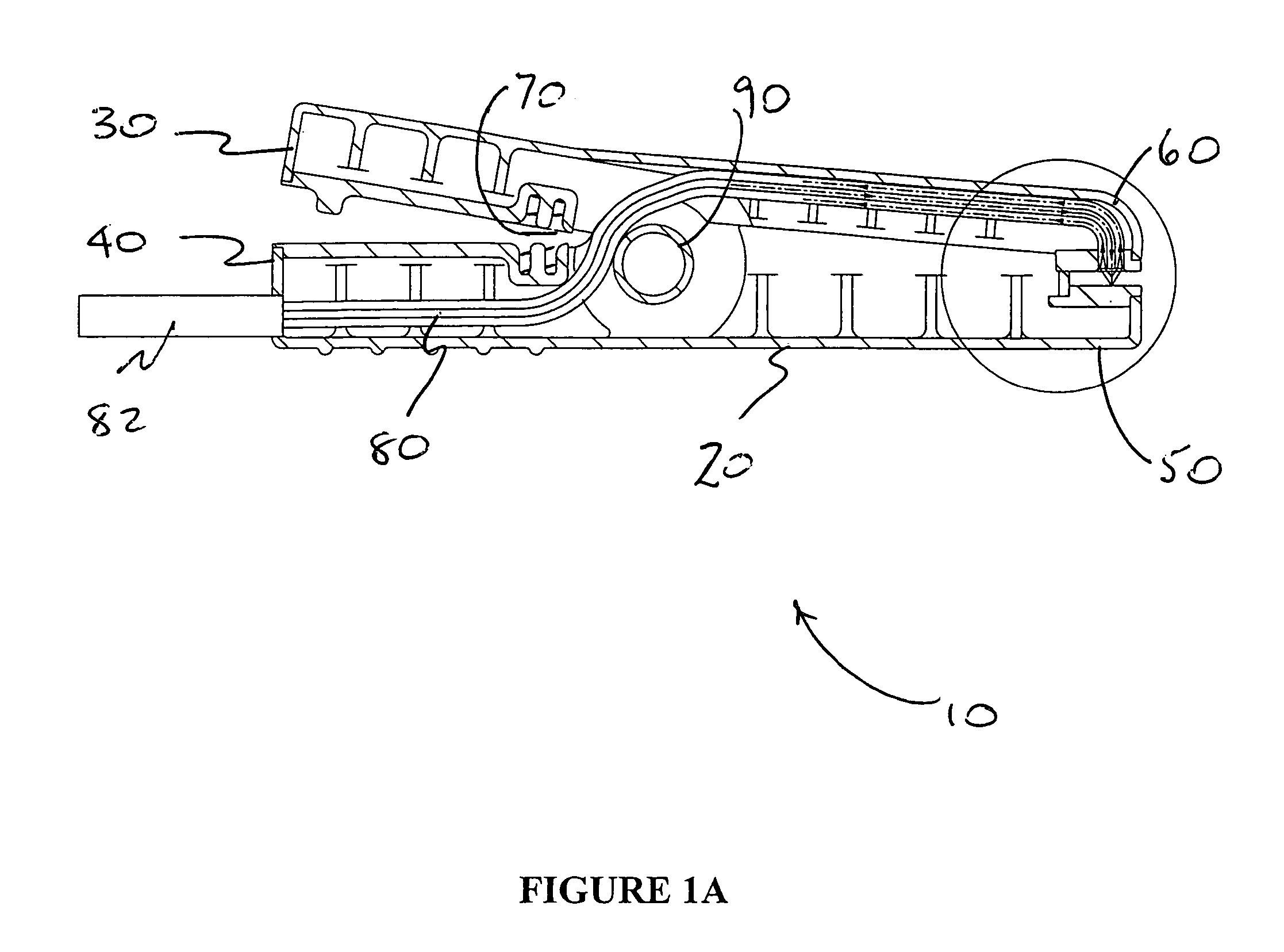
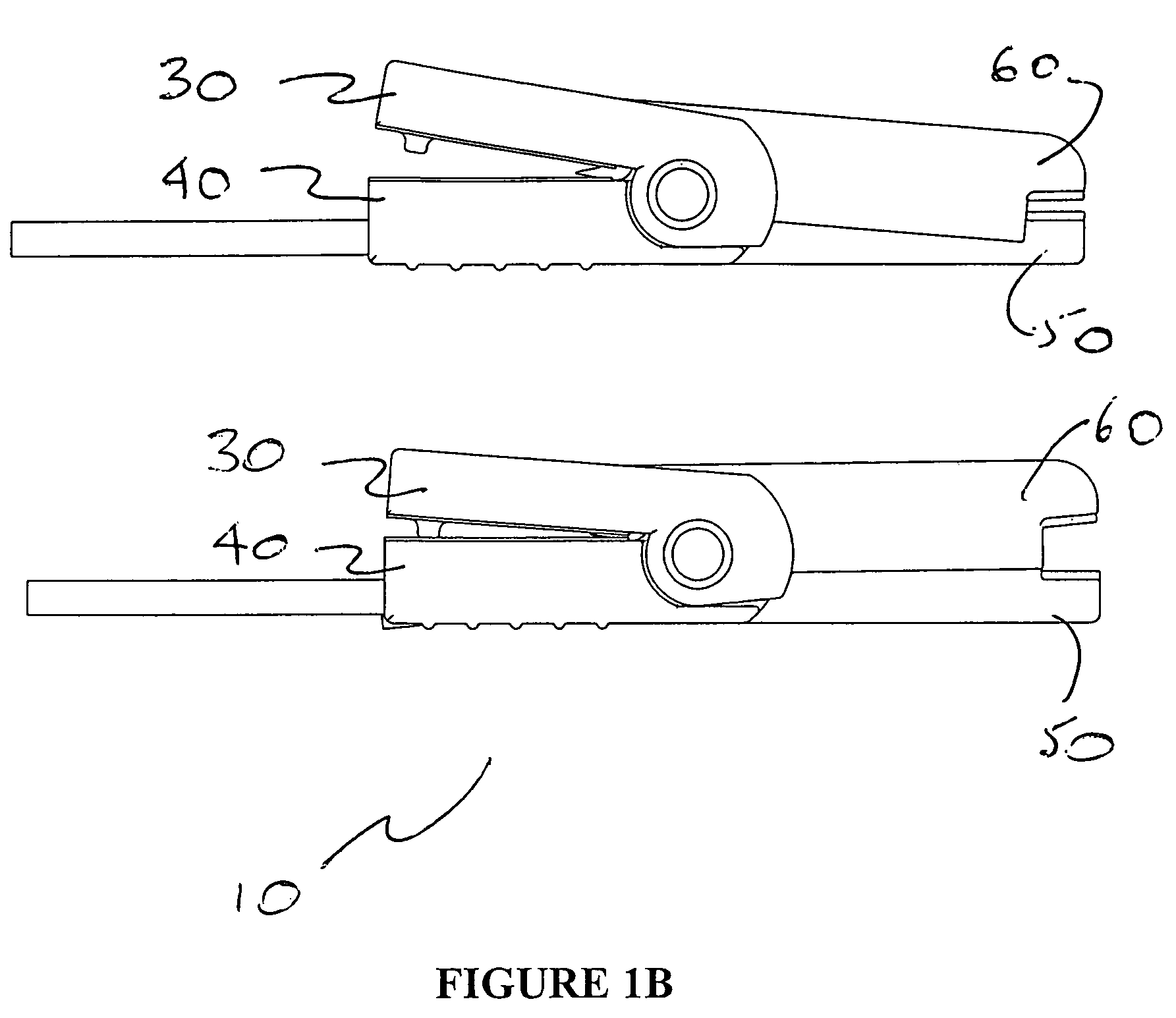
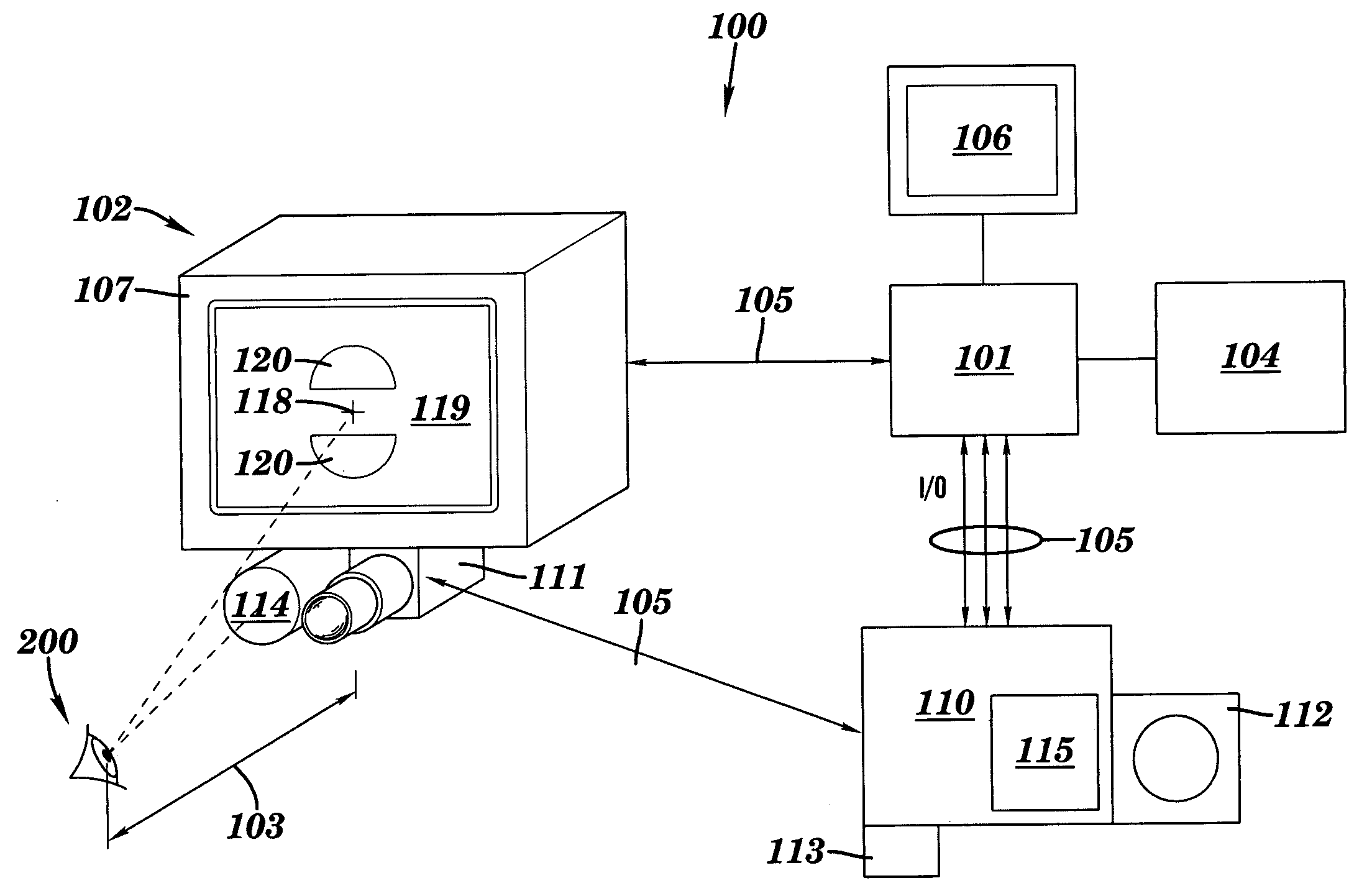
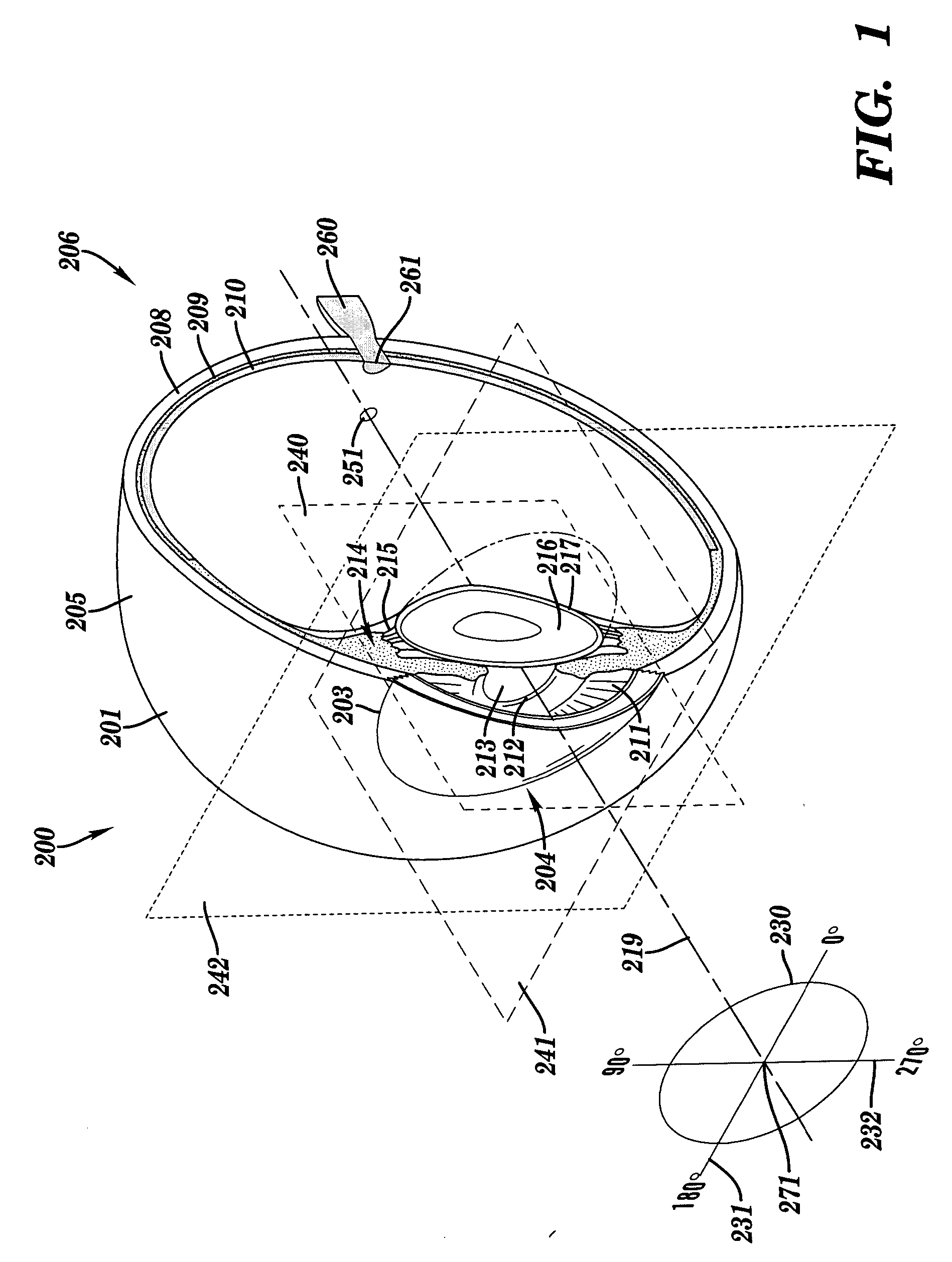
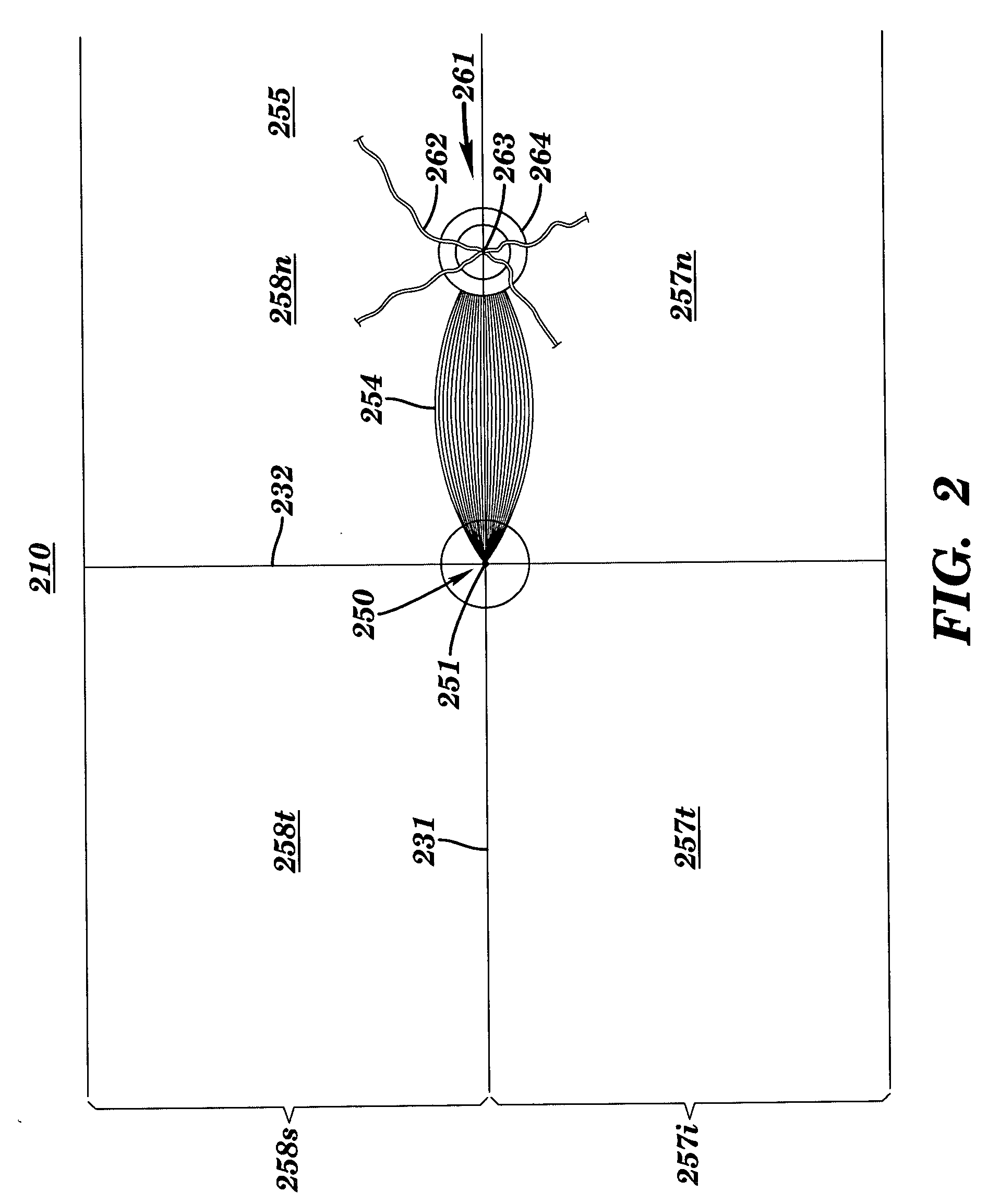
Apparatus and method for assessing retinal damage
InactiveUS20050200808A1Excessive duration of testShorten the construction periodEye diagnosticsVisual field lossSigmoid function
The invention administers an objective clinical test to an eye that measures the visual sensitivity of the superior retina and the inferior retina, by alternately presenting a stimulus pair comprising a shaped superior light stimulus and a shaped inferior light stimulus that are horizontal mirror images of one another and have shapes encompassing visual field defects. The shaped superior and inferior light stimuli stimulate pupillary responses whose amplitudes are measured. A cycle-averaged pupillary response balance and a luminance ratio are computed for each presentation of a stimulus pair. A stimulus pair response curve is computed by fitting cycle-averaged pupillary response balances to a sigmoid function of the luminance ratios. A balanced luminance ratio at which the cycle-averaged pupillary response balance is equal to about zero is computed from the sigmoid function. The balanced luminance ratio is indicative of the presence and location of retinal nerve damage.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
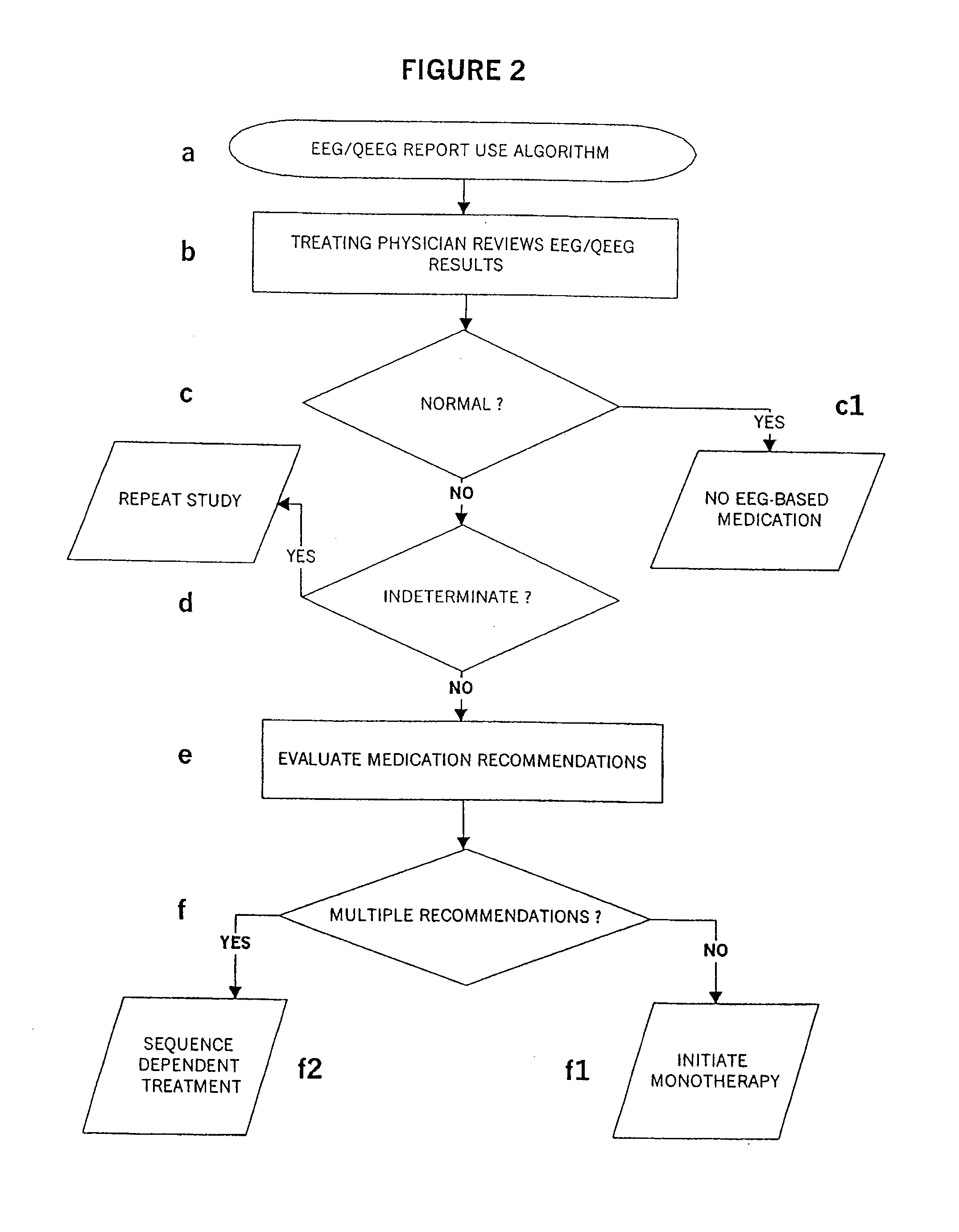
Methods For Classifying And Treating Physiologic Brain Imbalances Using Quantitative EEG
Owner:CNS RESPONSE
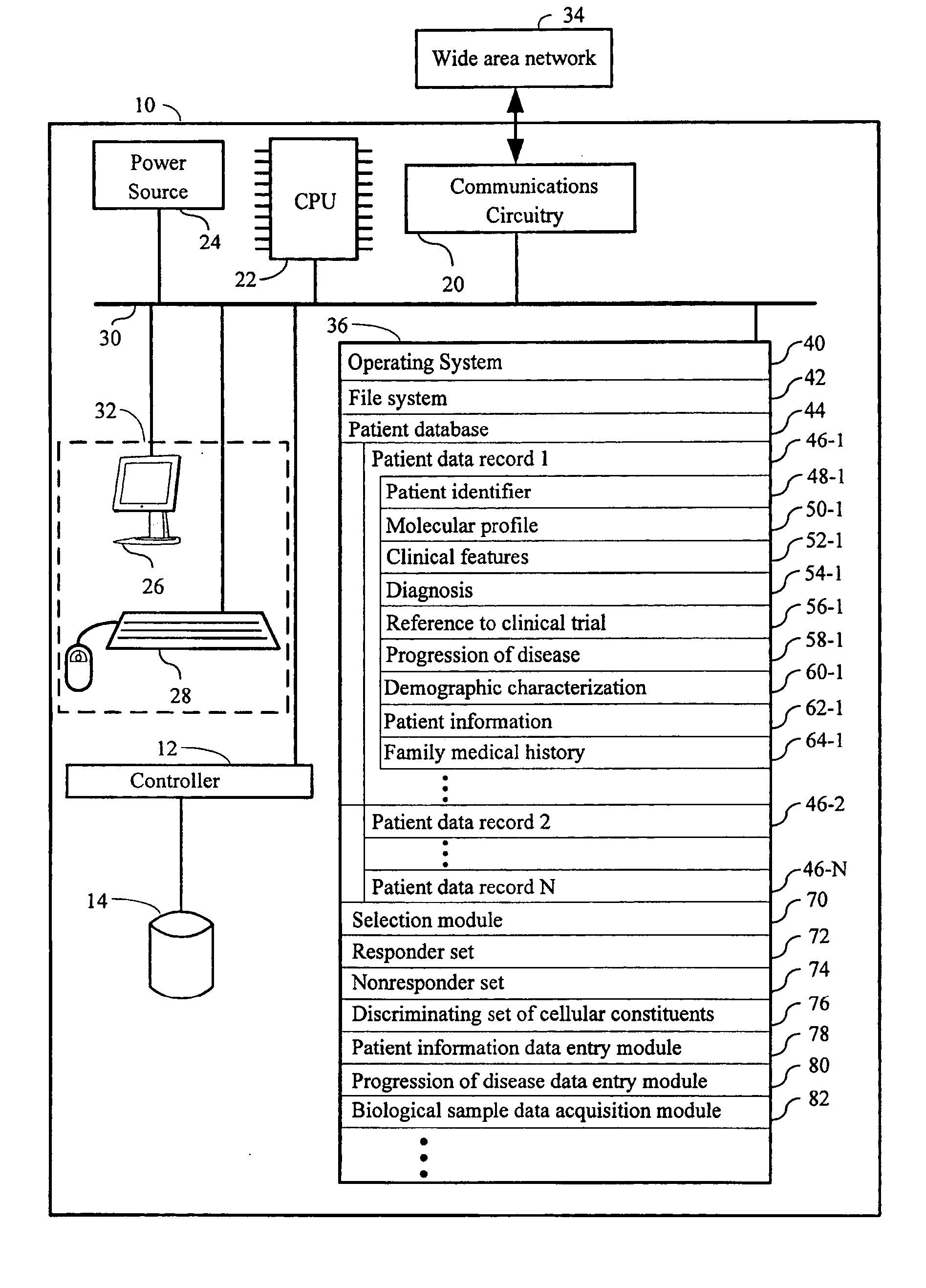
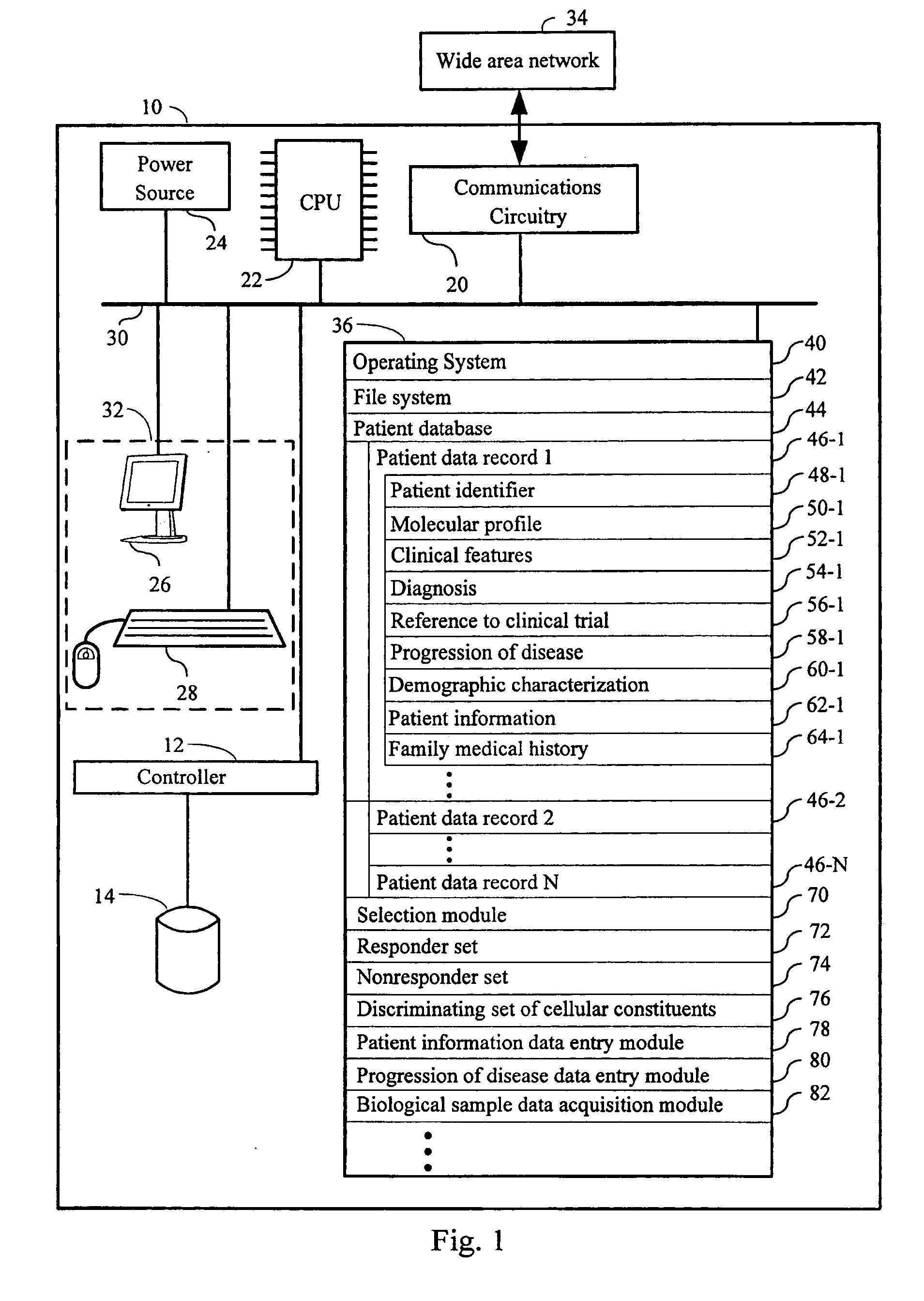
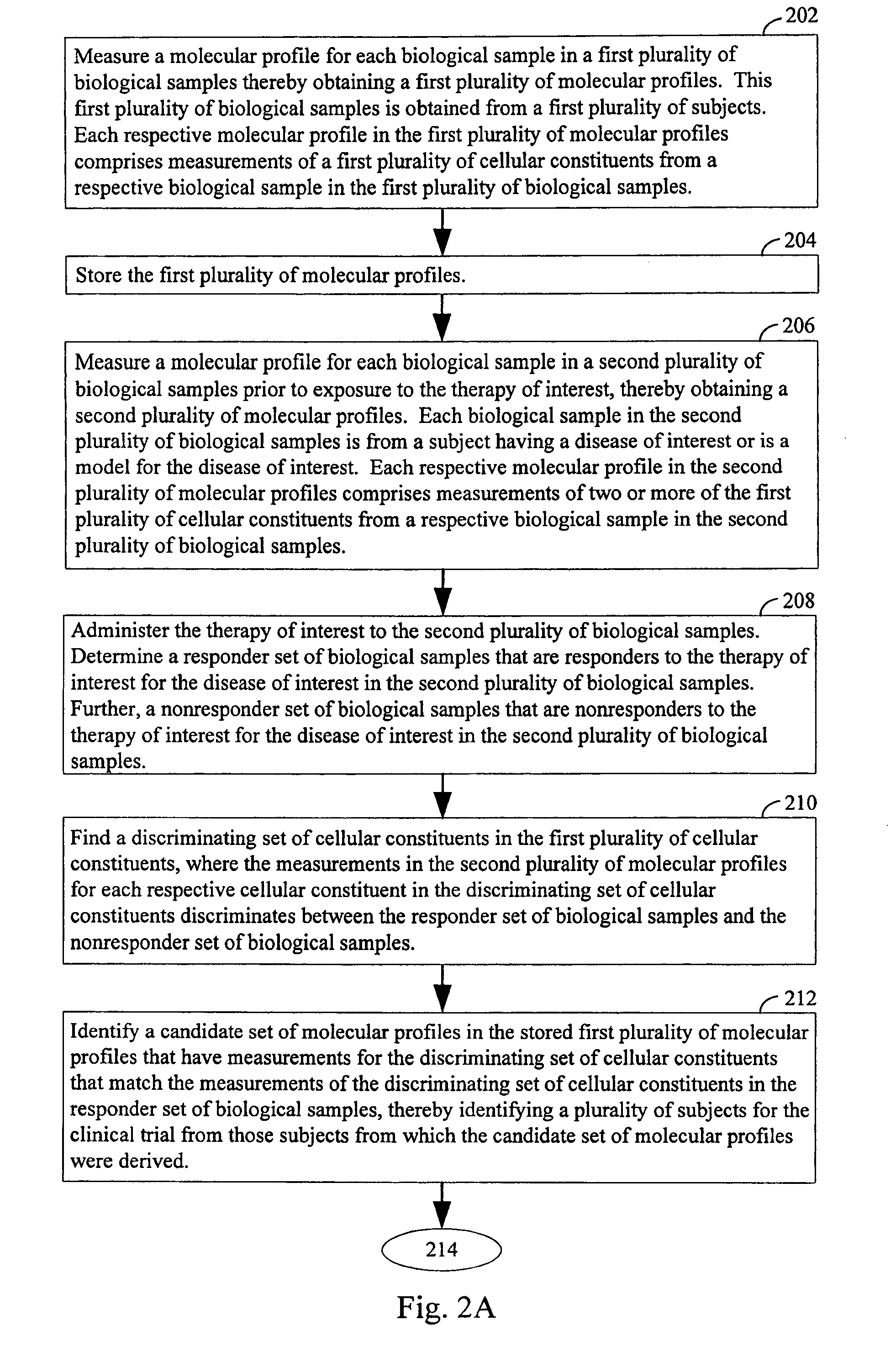
Computer systems and methods for selecting subjects for clinical trials
ActiveUS20080033658A1Data processing applicationsMechanical/radiation/invasive therapiesMolecular ProfileCellular component
Computers, computer program products, and methods for identifying a plurality of subjects for a clinical trial are provided. A candidate set of molecular profiles in a stored plurality of molecular profiles are identified. Each such profile has measurements for a discriminating set of cellular constituents that match the measurements of corresponding cellular constituents in a responder set of biological samples, thereby identifying the plurality of subjects for the trial from those subjects from which the candidate set of molecular profiles were derived. Each respective molecular profile in the stored plurality of profiles has measurements of a plurality of cellular constituents from a respective biological sample in a plurality of samples obtained from a first plurality of subjects. The discriminating set of cellular constituents is identified from those cellular constituents in the plurality of cellular constituents whose measurement values discriminates between the responder and nonresponder sets of biological samples.
Owner:UNIV OF SOUTH FLORIDA +1
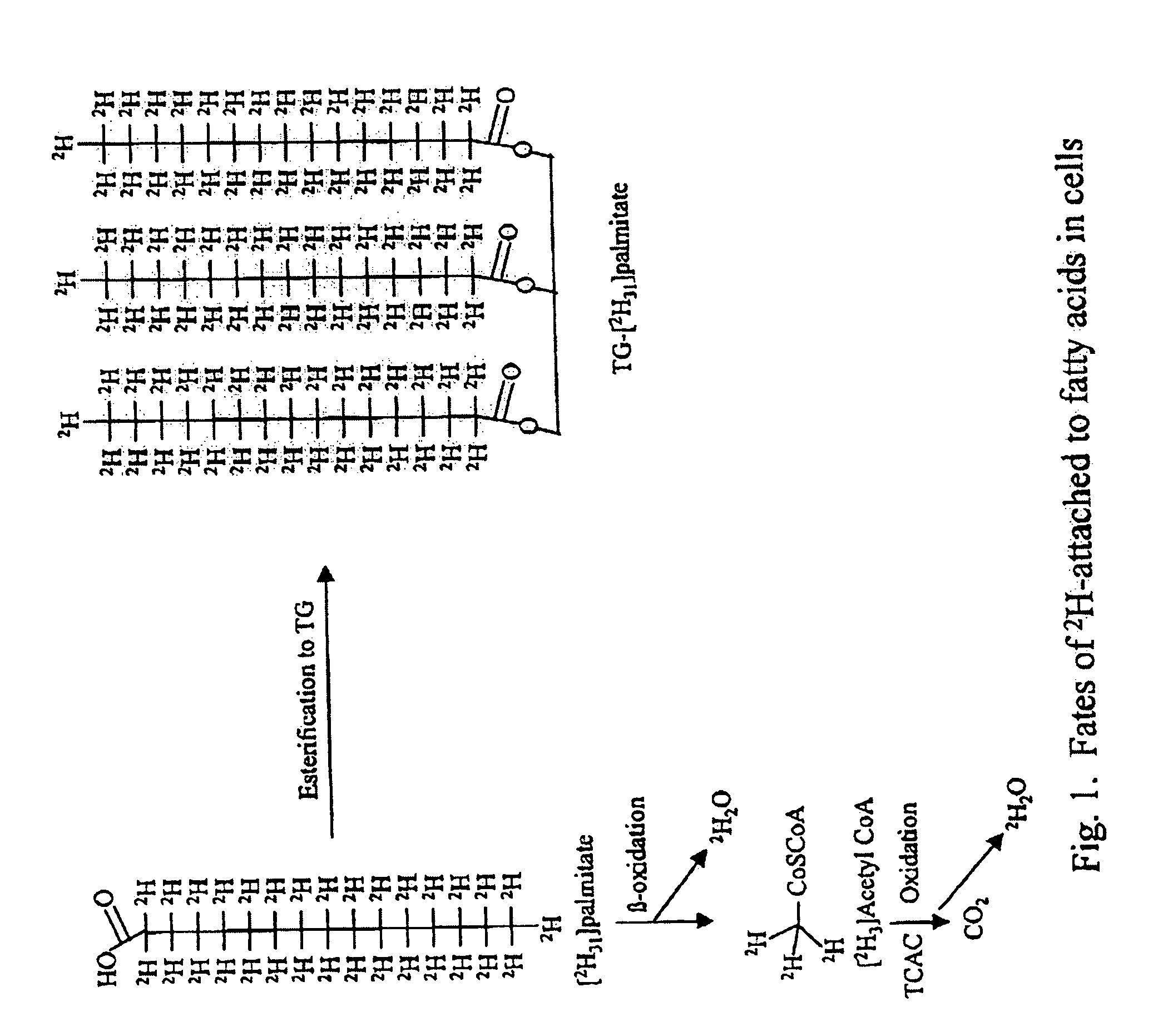
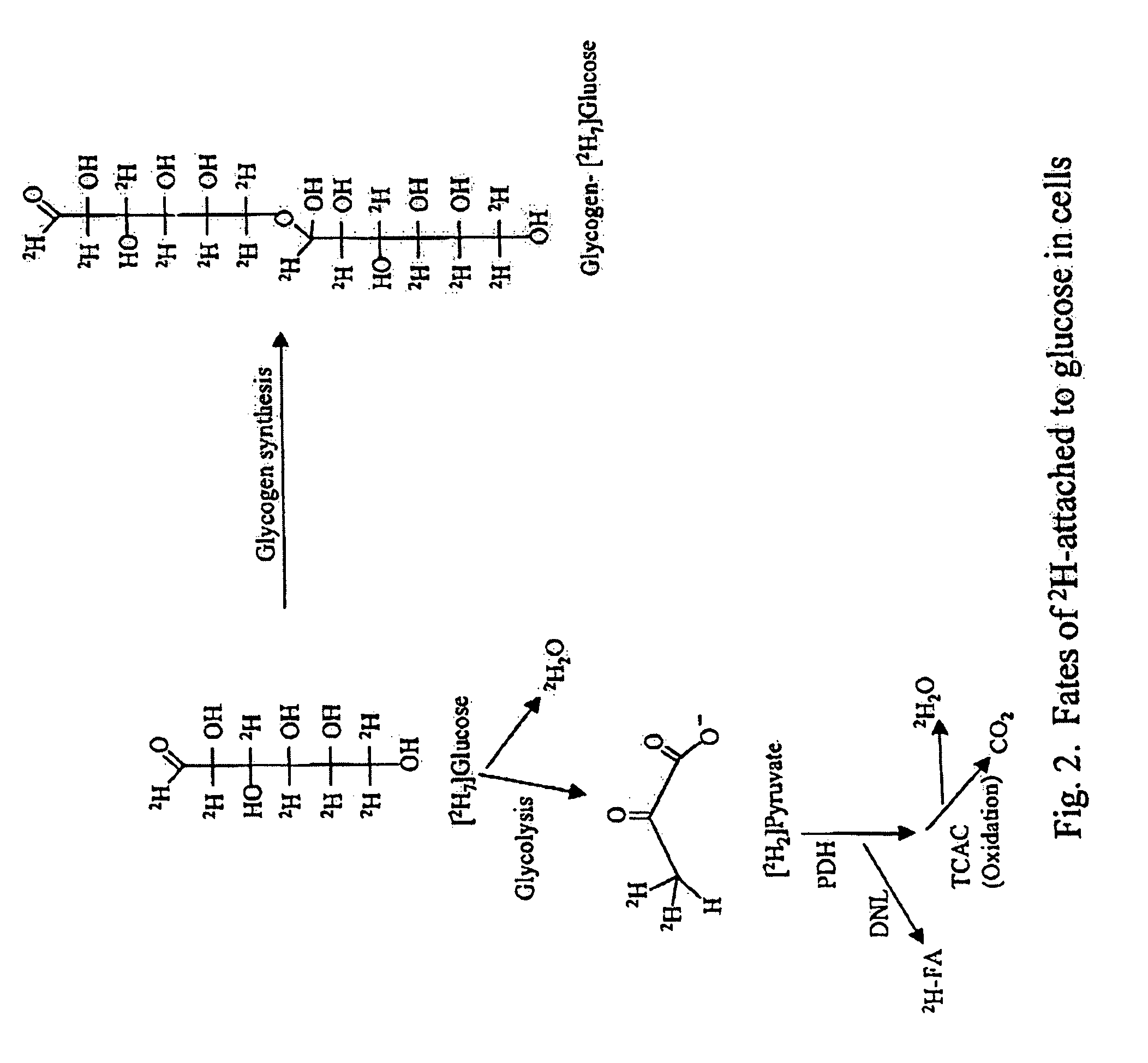
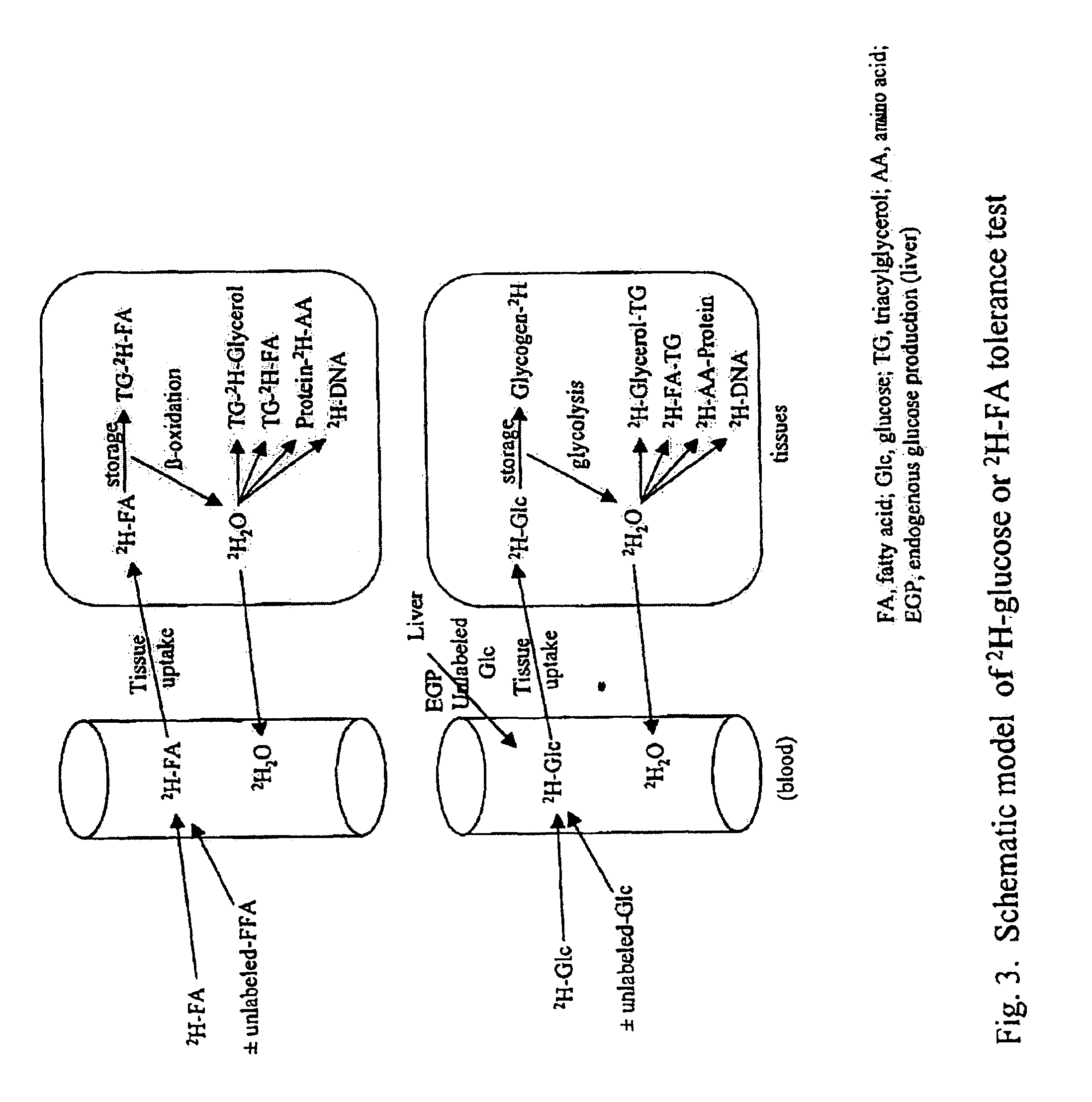
Methods for determining the metabolism of sugars and fats in an individual
ActiveUS7504233B2Compounds screening/testingIn-vivo radioactive preparationsTrial drugPreclinical testing
Provided herein are methods for determining the metabolism of one or more sugars and / or fatty acids, and applications thereof. Such applications include determining the rate of glycogen synthesis and glycolysis, which are believed to be early markers for predicting elevated risk of diabetes and cardiovascular disease. Other applications include methods for screening drugs that effect sugar and / or fatty acid metabolism. The methods are useful for at least partially characterizing drugs for desirable or undesirable (toxic) characteristics. Drugs that are at least partially characterized using the methods of the invention can then be further developed in pre-clinical testing and clinical trials. Such drugs may be found to be useful in treating obesity, diabetes, cardiovascular disease, and other disorders of metabolism.
Owner:RGT UNIV OF CALIFORNIA
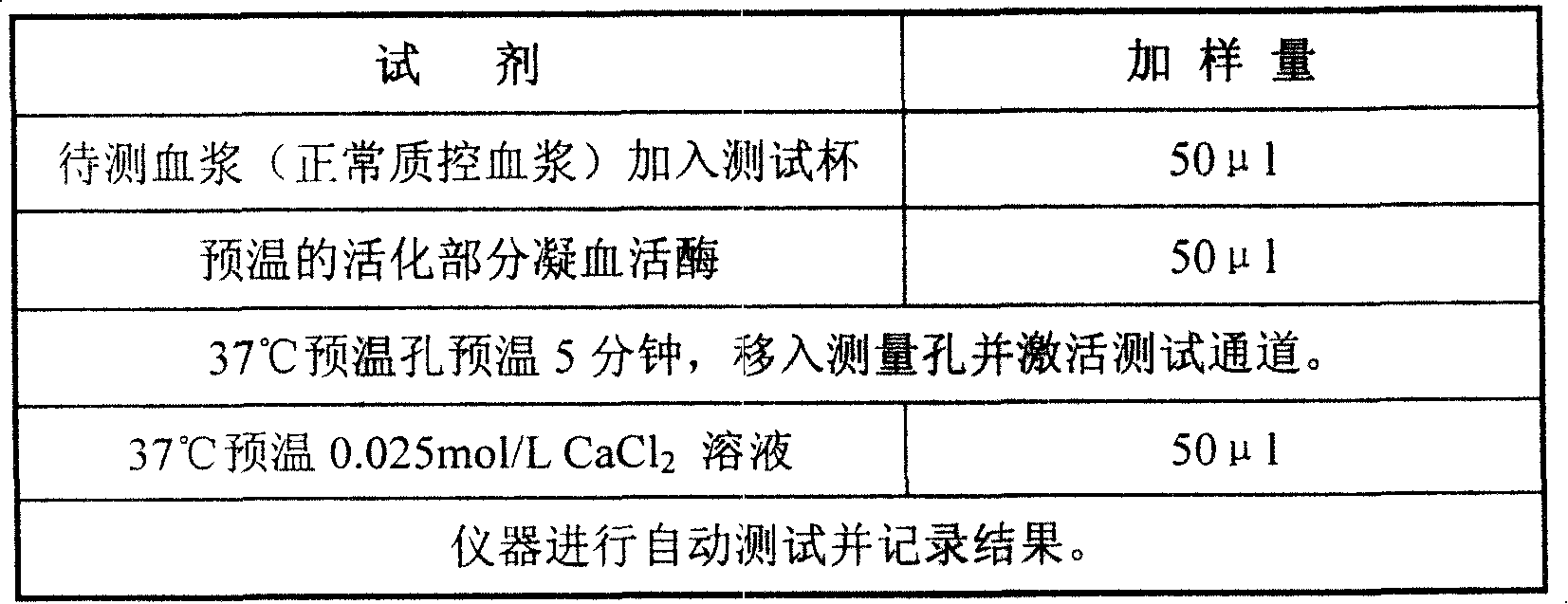
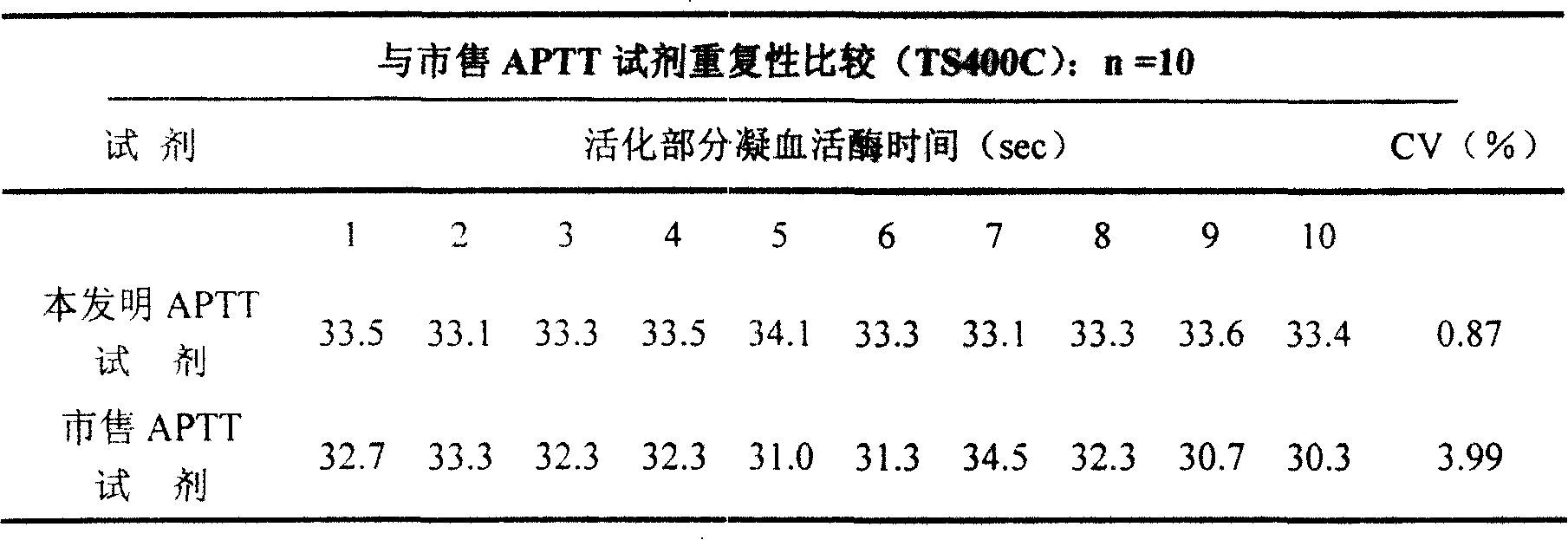
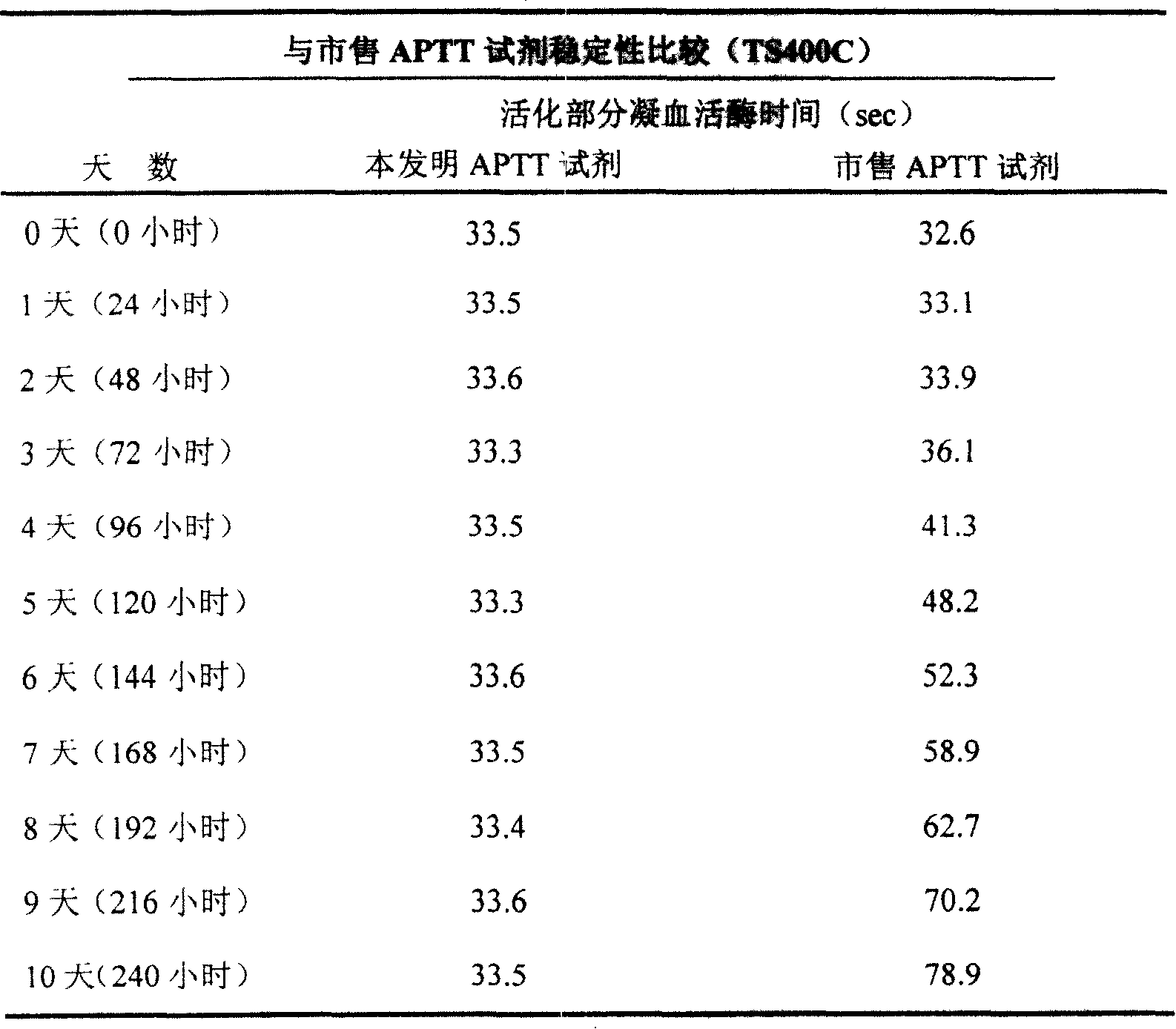
External diagnostic reagent kit used for measuring activated partial thromboplastin time
ActiveCN101221189AImprove stabilityGood repeatabilityMicrobiological testing/measurementBiological testingDisease causeAPTT - reference
The invention relates to an in vitro diagnostic kit for the determination of activated partial thromboplatin time (APTT) in clinical test. The invention consists of a partial thromboplatin reagent and a calcium chloride solution, which is used for the detection of the defects of the intrinsic coagulation pathway factors and the screening test of the related inhibitors, and the invention is also a primary means for the current coagulation factor and heparin anticoagulant treatment and the detection of lupus anticoagulant. The invention has the advantages of long stability time and good repeatability of the partial thromboplatin reagent after a re-dissolution, at the same time, the invention has better consistency of the measurement results of a blood coagulation analyzer by using an optical method and a magnetic bead method, therefore, the invention is applicable to large, medium and small hospitals, and the test results of different hospitals have comparability, therefore the invention has important meaning for implementing the one general report of test reports, provides the reliable experimental data for the clinical diagnosis and the treatment of diseases and improves the efficiency and value of the basic studies of thrombosis and hemostasis.
Owner:SHANGHAI SUNBIO TECH
Visualization interactive clinical test and clinical follow-up visit system and method
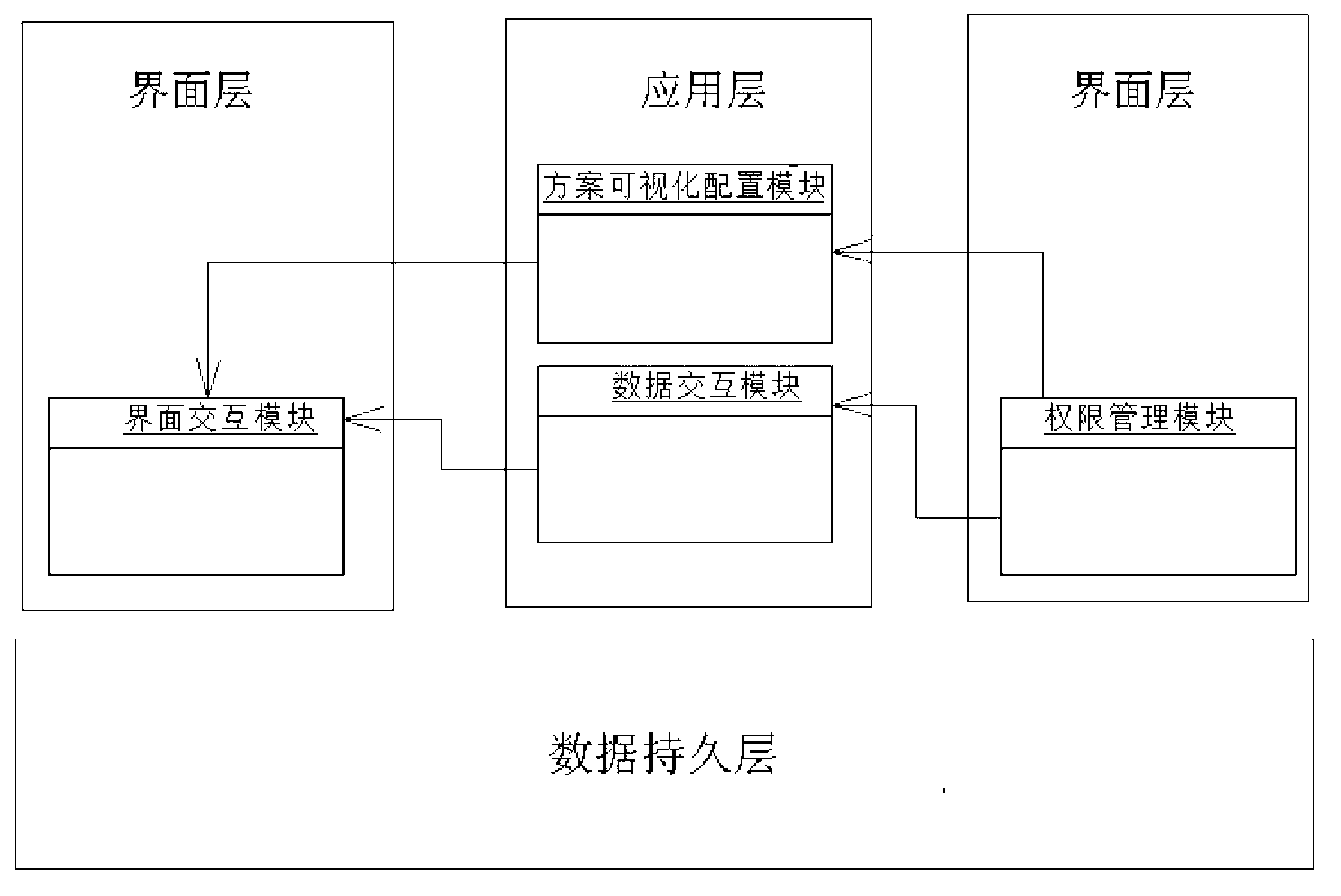
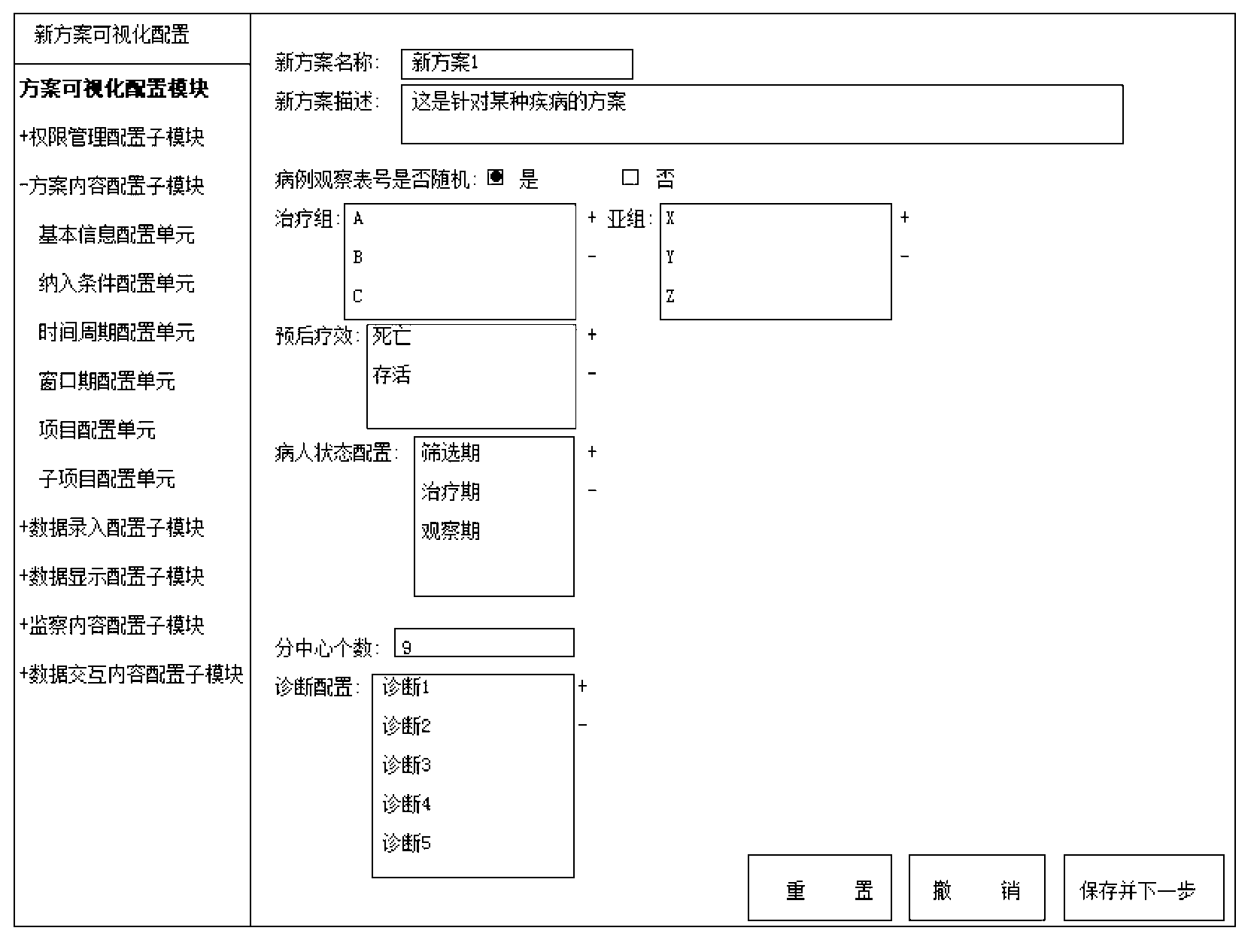
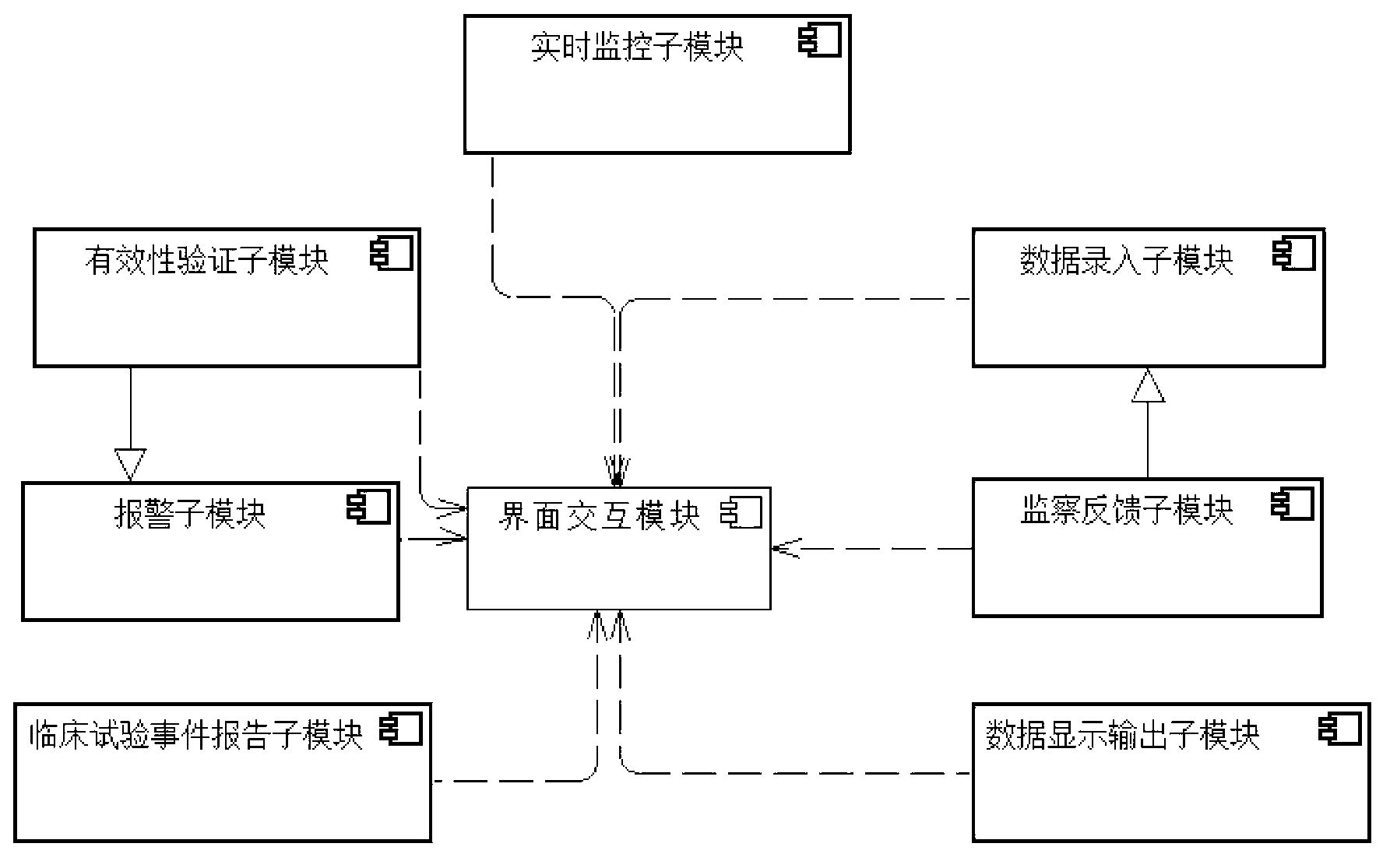
InactiveCN103218540AMeet the needs of self-adaptive transformation of the systemEasy to applySpecial data processing applicationsClinical testsClinical trial
The invention discloses a visualization interactive clinical test and clinical follow-up visit system. The system comprises a right management module, a scheme visualization configuration module, an interface interaction module and a data interaction module. On the basis of the system, the invention also provides a clinical test and clinical follow-up visit method. The method comprises the following steps of: defining the right of a user in a clinical test and clinical follow-up visit treatment process by utilizing the right management module; completing the configuration of contents of the interface interaction module and the data interaction module in the scheme visualization configuration module; completing the collection, supervision, display and output of clinical test and clinical follow-up visit data in the interface interaction module according to a self-defined scheme; and completing the data interaction among the modules inside the system or between the internal module and other external systems in the data interaction module. Due to the adoption of the system, the visualized self-defined configuration for a clinical test and clinical follow-up visit research scheme can be realized, a complete interface interaction and data interaction method can be provided, and the increasingly urgent clinical test and clinical follow-up visit data management demand can be satisfied.
Owner:夏琦 +2
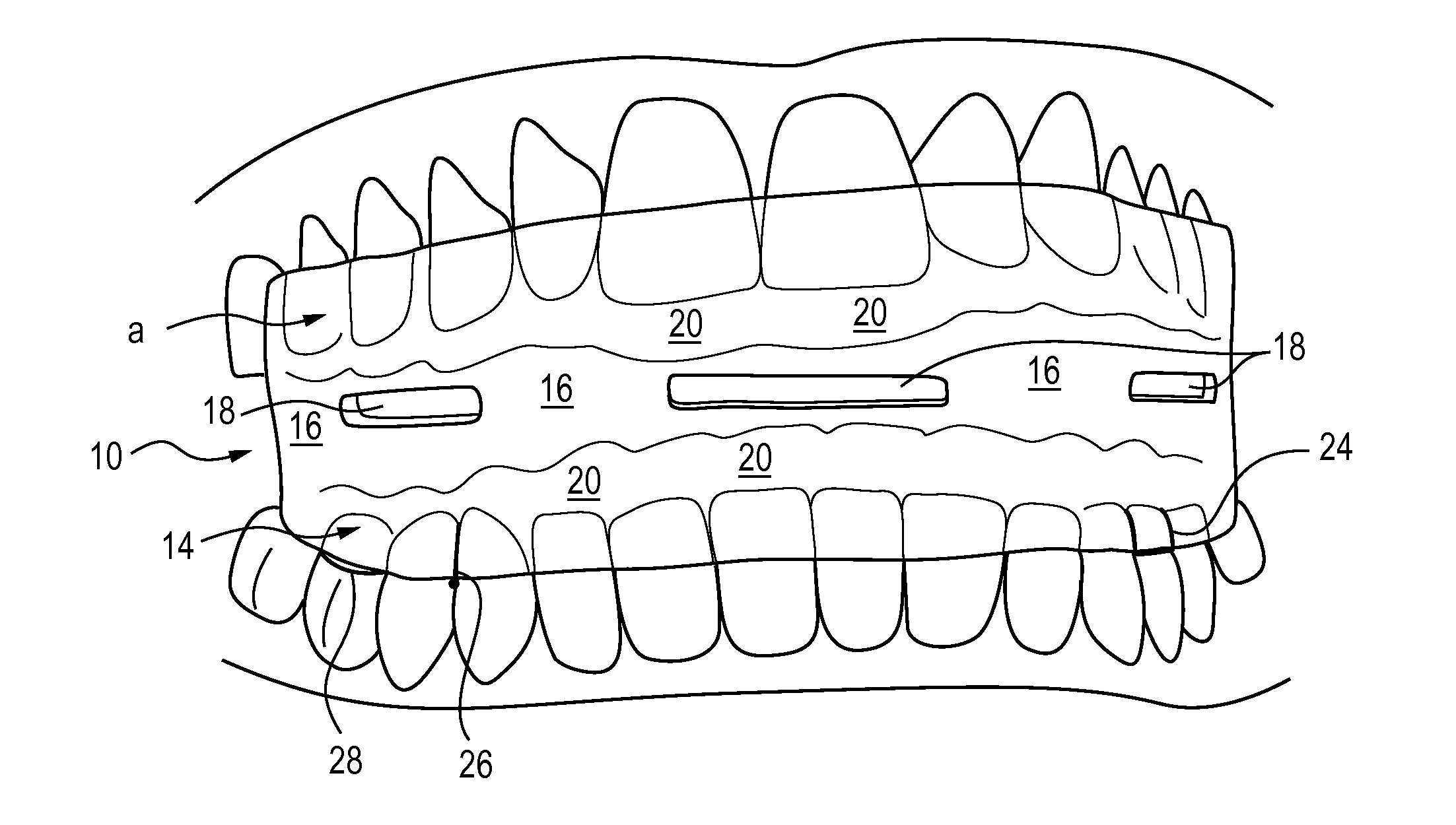
Apparatus and method for treatment of sleep apnea and snoring
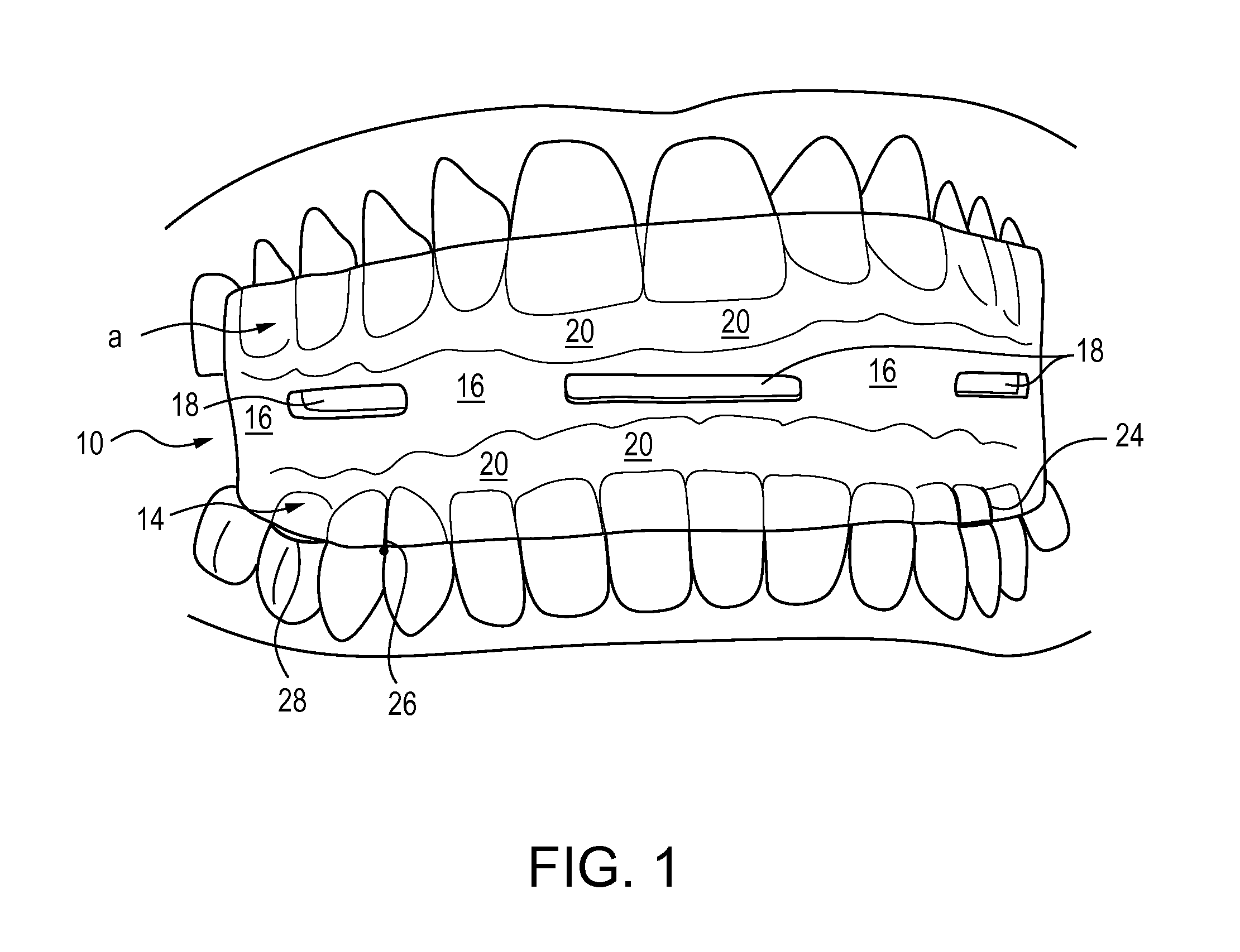
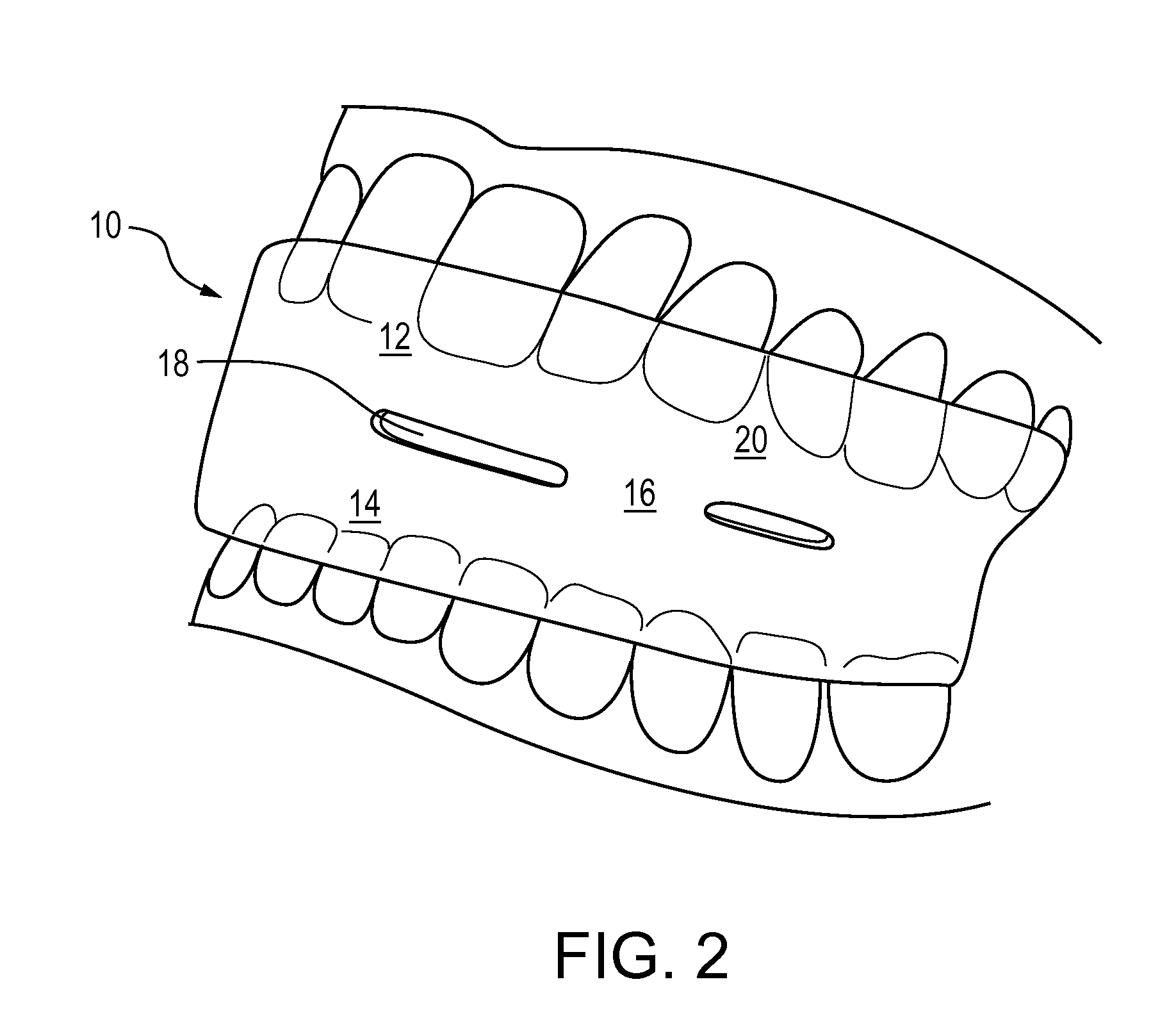
Methods and apparatus for treating snoring and sleep apnea may include an oral appliance based on an extensive, three-dimensional radiological and clinical examination. The oral appliance may position the patient's lower jaw to a position that may prevent or eliminate sleep apnea. Several parameters, often over 40 parameters, may be examined to determine the proper placement and orientation of the jaw, via the oral appliance. The oral appliance may have a soft insert on the inside for cushioning the patient's teeth while the outside of the oral appliance may be hard, so as to maintain airflow through the oral appliance. The oral appliance may be a “full coverage” piece, fitting over 70 percent, typically over 80 percent, of the patient's teeth.
Owner:SINGH PANKAJ P
Traditional Chinese medicine preparation for treating fractures and bone injuries
InactiveCN101623415AReactivity NoneNo side effectsPowder deliveryHeavy metal active ingredientsMyrrhSide effect
The invention relates to a traditional Chinese medicine preparation for treating fractures and bone injuries. The traditional Chinese medicine preparation is characterized by being prepared from the following raw material medicines: radix notoginseng, draconis sanguis, woodlouse, safflower, peach kernel, eucommia bark, pyrite, teasel root, rhizoma cyperi, mastic, myrrh, white paeony root, rhizoma ligustici wallichii, angelica, earthworm, twotooth achyranthes root, prepared rehmannia root, astragalus root and rhizoma drynariae. The preparation method comprises the following steps: mixing the raw material medicines together, grinding the raw material medicines into a powder shape, sieving the powder into the granularity of 180 meshes, and preparing traditional Chinese medicine medicinal powder. By the clinical test of many years, the invention forms a treating prescription for treating the fractures and the bone injuries by researching the function and indication of each kind of medicine according to modern traditional Chinese medicine pharmacodynamics, carries out the clinical tests on more than one thousand cases of patients with the fractures and the bone injuries and has the effects of promoting blood circulation, relieving the pain, decreasing swelling, eliminating blood stasis, recovering wounds, promoting tissue regeneration, setting bone and treating injuries. The invention can dissipate blood stasis, promote blood circulation, diminish inflammation and alleviate pain, also has the function of tranquilizing the mind and has short treatment course, high curative effect, low treating expense and no adverse reaction and side effect, the cure rate reaches more than 90%, and the curative effect is very satisfactory to people.
Owner:葛超顺
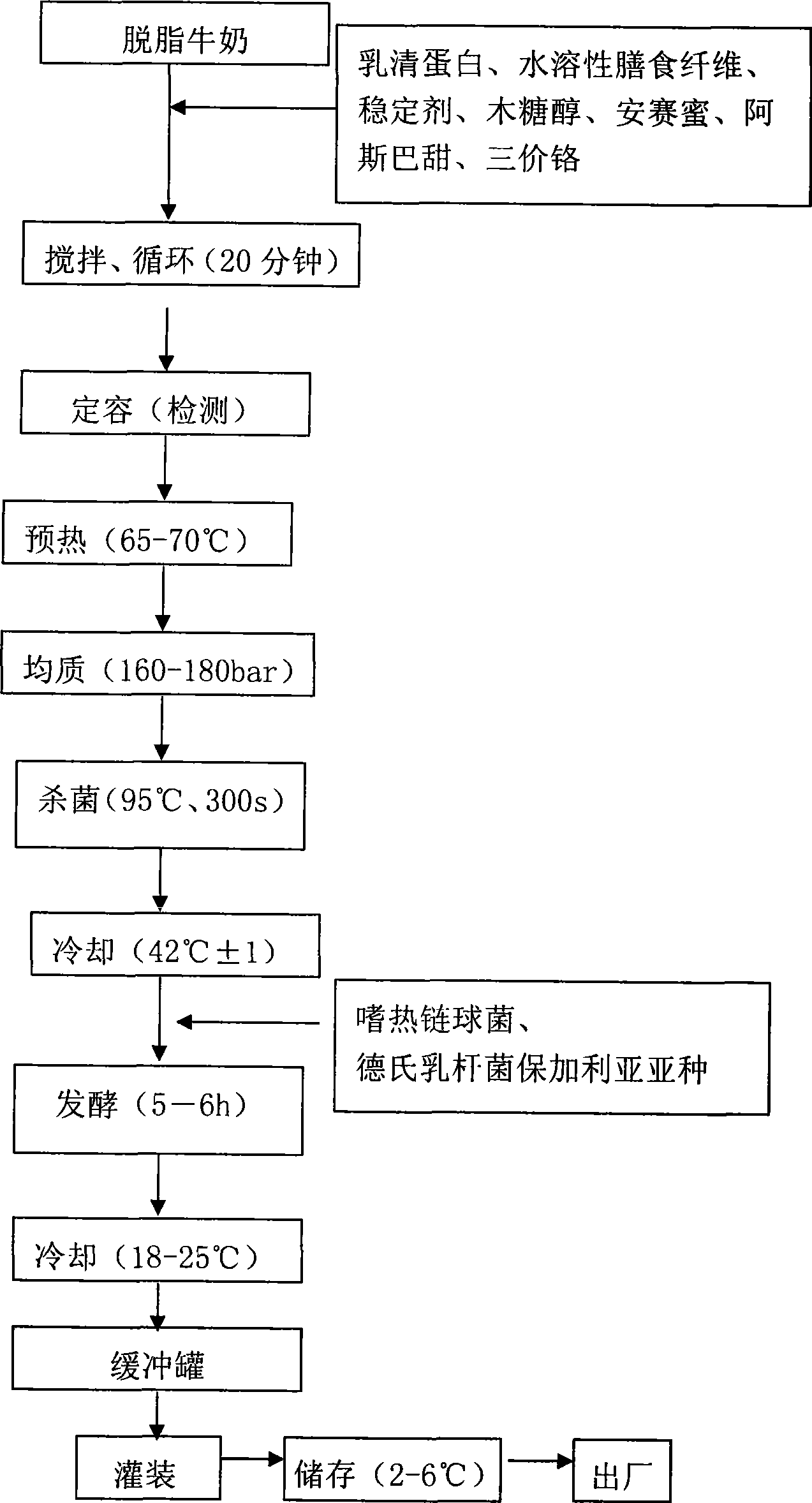
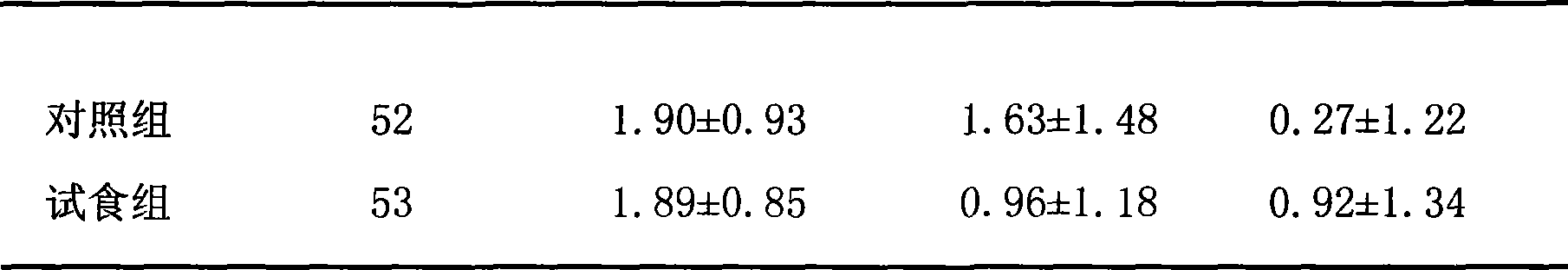
Yoghourt for reducing blood sugar and method for producing the same
The invention relates to a food, in particular to a yogurt manufacturing method, more particularly to a yogurt for assisting to reduce the blood sugar, and a production method thereof. The product comprises skimmed fresh milk, water-soluble dietary fiber, xylitol, lactoalbumin, stabilizer, aspartame, acesulfame potassium, trivalent chromium, streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus. The trivalent chromium for reducing the blood sugar is added in the yogurt, thereby the product plays the role of reducing the blood sugar, and not only has the abundant nutrition, but also plays the role of health care for assisting to reduce the blood sugar when the consumers with diabetes drink the yogurt. The product is effective after clinical test and has higher security.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Beta-carboline ruthenium compound as well as preparation method and application thereof
InactiveCN101845060AGood effectOrganic active ingredientsGroup 8/9/10/18 element organic compoundsCisplatinRuthenium Compounds
The invention discloses a beta-carboline ruthenium compound. The molecular formula of the beta-carboline ruthenium compound is [Ru (N^N) 2 (Nh) XClY] (A^A)z, wherein N^N is selected from bpy or phen; A^A is selected from PF6 or SO3CF3; X is equal to 1 or 2, Y is equal to 2-X, and Z is equal to 1 or 2; or the molecular formula of the beta-carboline ruthenium compound is [Ru (N^N) 2 (1-Py-betaC)] (PF^)2, wherein N^N is selected from bpy, phen or DIP. On a molecular mechanism, the invention proves that the beta-carboline ruthenium compound can be used for treating cancers and has a better effectthan the effects of that of the traditional metal compound cisplatin with wide application and ruthenium compounds NAMI-A in clinical tests; . Thethe beta-carboline ruthenium compound for treating the cancers as an autophagic cell inducer fills the blank of the prior art and develops a new line for developing a new generation of high-efficiency medicaments for treating malignant tumors.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com