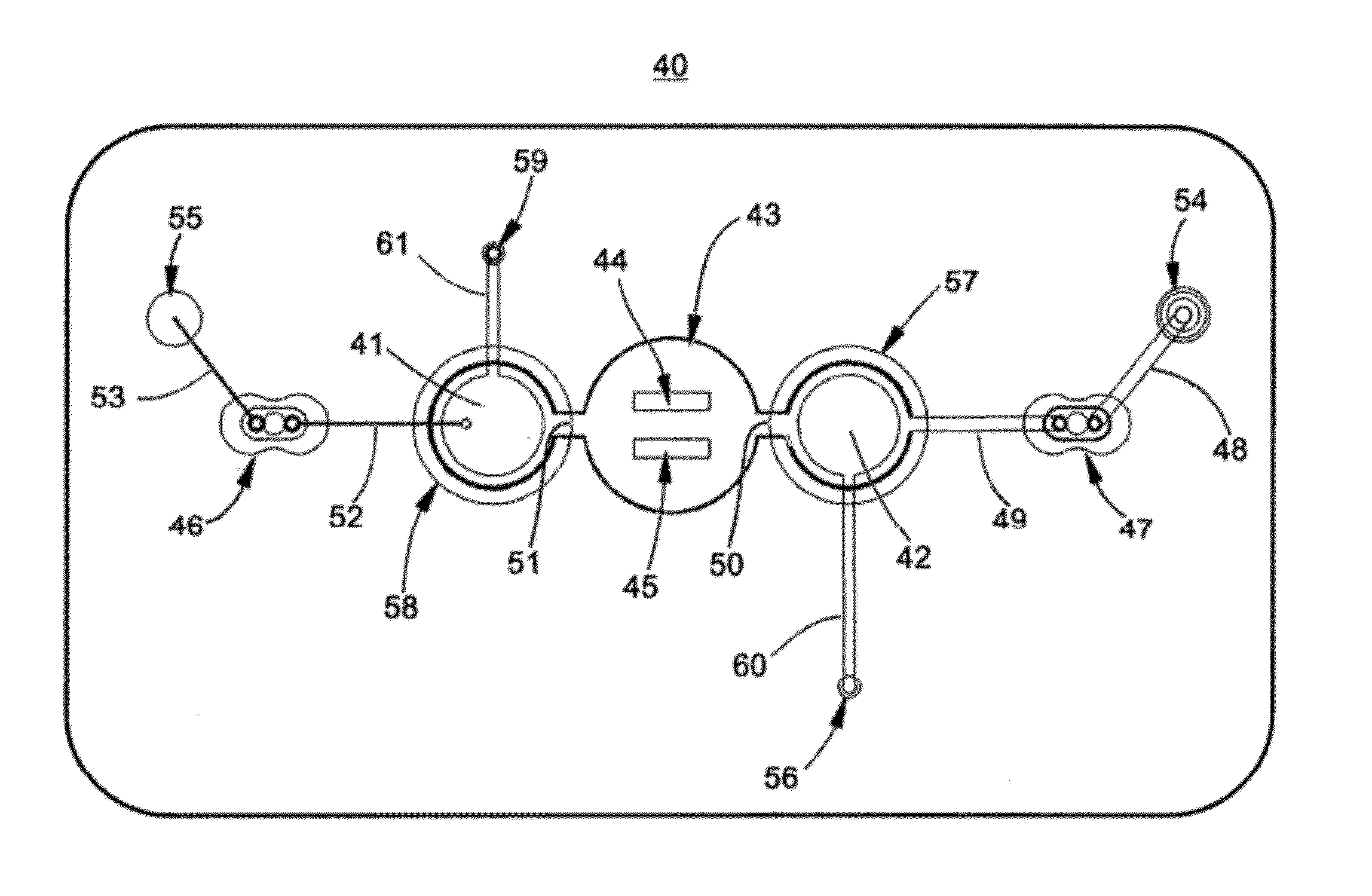
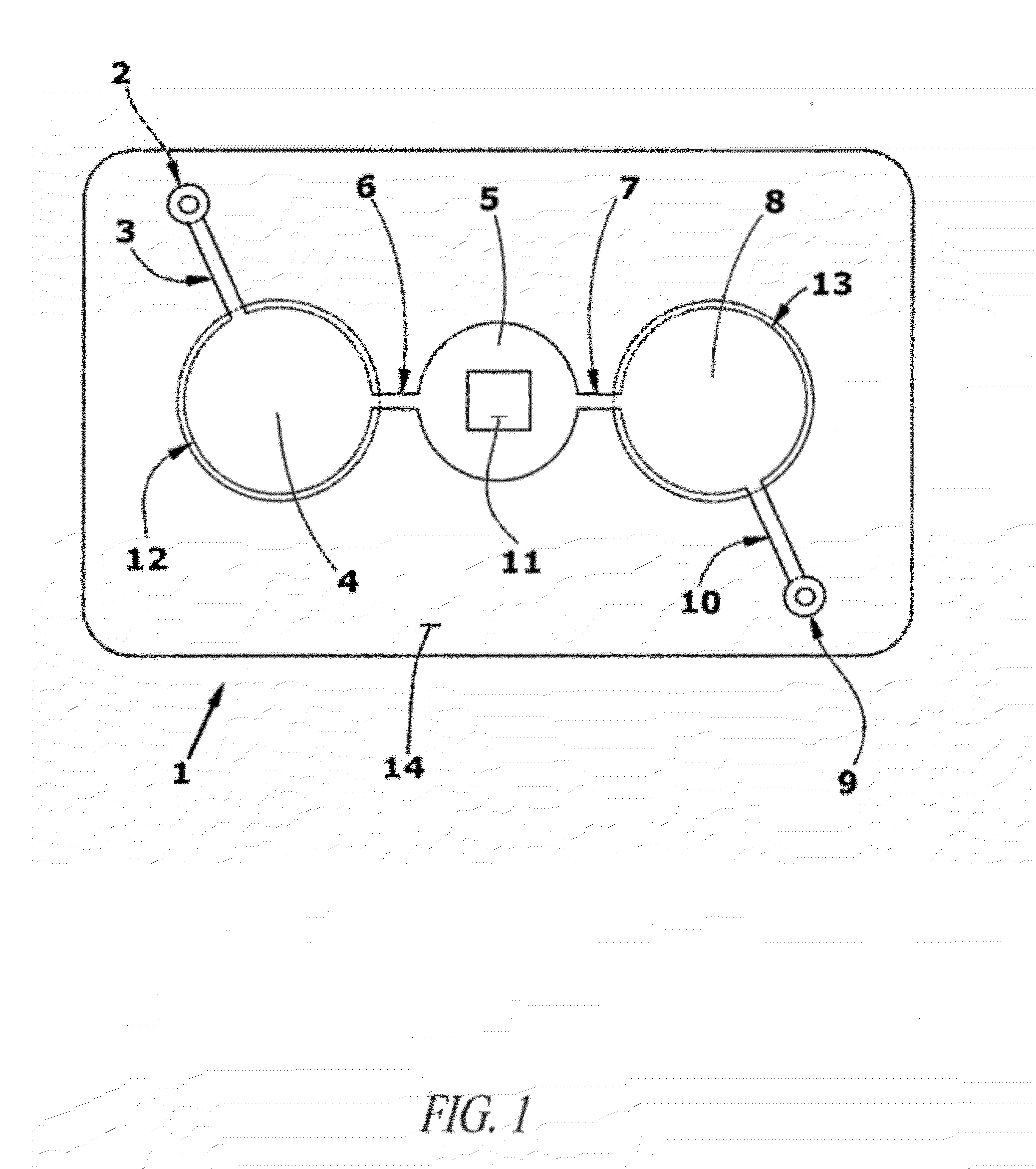
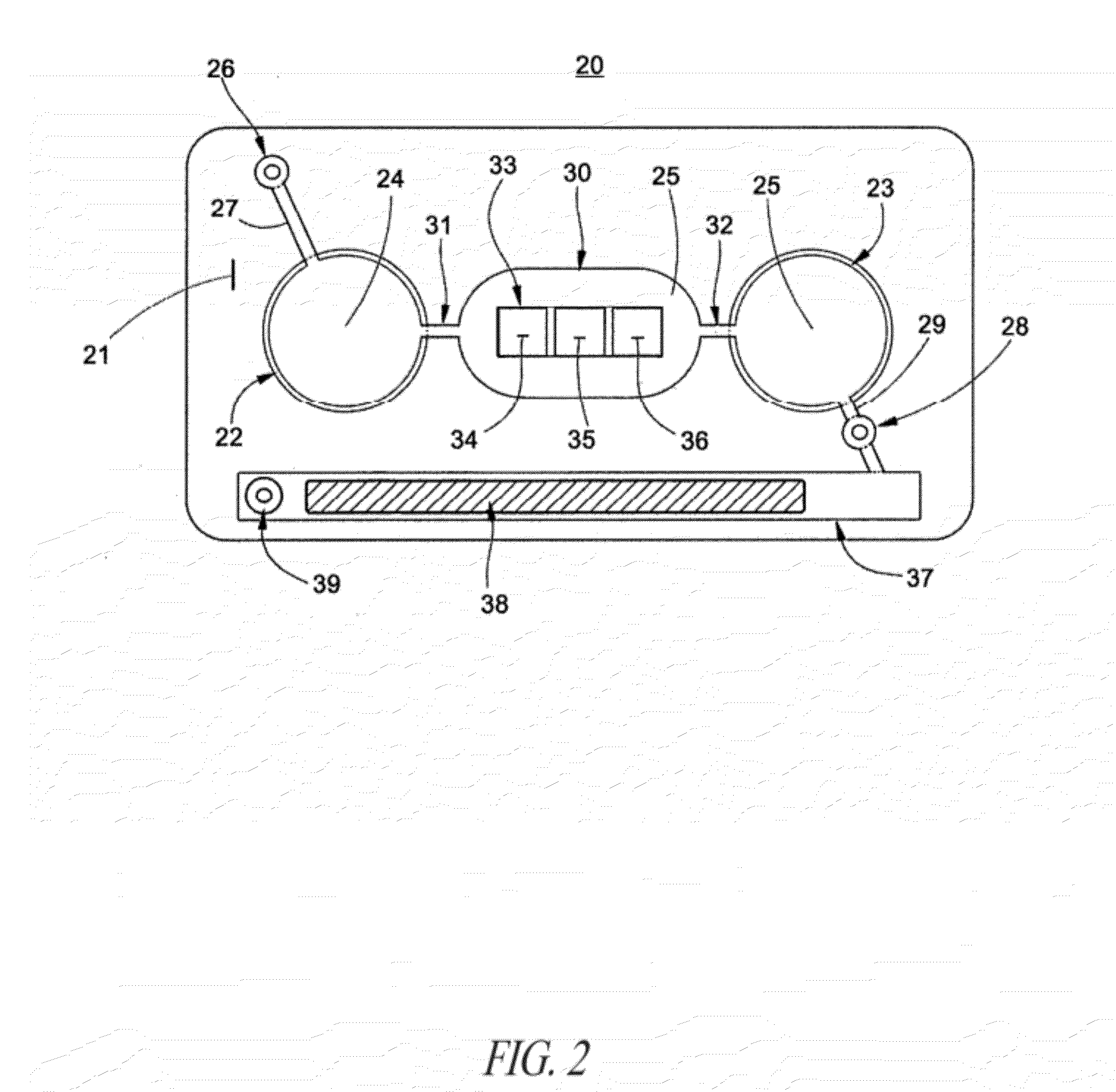
Methods and devices for microfluidic point-of-care immunoassays
a technology of immunoassays and microfluidic devices, applied in the field of clinical assays performed in microfluidic devices, can solve the problems of large hybridization chambers, inconvenient or complicated equipment not readily adaptable, and the mixing of small volumes is not without unique problems, so as to reduce the incubation time and the mean flow velocity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Assembly of a Microfluidic Assay Device
[0141]A microfluidic device for ELISA immunoassay was prepared as follows:[0142]1. Human IgG (0.5 ug in bicarbonate binding buffer pH 9) was deposited onto a plasma-treated test field of a PET sheet. Human IgM was applied as a negative control to a second test field. The proteins were then fixed to the plastic by drying for 5 minutes at 60° C.[0143]2. After fixation, the test fields were blocked for 30 minutes with casein buffer (biotin free) and washed twice with PBST (phosphate buffered saline with 0.1% TWEEN 20).[0144]3. After drying, the treated PET sheets were then assembled between adhesive layers in the microfluidic device of FIG. 3 with integrated pneumatic circuitry for pressurizing the bellows pumps and opening and closing sanitary valves.
example 2
Performance of an ELISA Immunoassay in a Microfluidic Device
[0145]A standard assay for ELISA, useful as a benchmark in method development, involves detection of immobilized human IgG on a solid substrate, followed by blocking and detection of the IgG with biotin-labelled anti-human antibody. The biotin in turn is detected with enzyme-labelled streptavidin.
[0146]Procedure:[0147]1. Referring to the microfluidic device prepared as described in Example 1, anti-human IgG biotin (Pierce, 180 uL, 1:10,000 in casein buffer) was added through the sample port, and the device was incubated for 1 min with slow mixing. The test fields were then rinsed with PBST, with slow mixing for 1 min before removing the rinse. A Microflow microfluidics assay instrument (Micronics, Redmond Wash. USA) was used to perform mixing and washing.[0148]2. Detection reagent (Poly SA-HRP) was added with incubation for 1 min with slow mixing. Poly SA-HRP is streptavidin labeled with horseradish peroxidase. The test fie...
example 3
Assembly of a Microfluidic Device for Immunoassay of Antibodies to Capsular Polysaccharides of Streptococcus pyogenes
[0152]Assembly of test card:[0153]1. Capsular mucosaccharide antigen purified from Group A Streptococcus pyogenes is immobilized on a N2 plasma activated polyester sheet by co-polymerization with acrylamide monomer. The sheet is previously masked to define the test field area.[0154]2. The plasma treated test field and surrounding areas are then blocked for 30 minutes with casein buffer and washed twice with PBST (phosphate buffered saline with 0.1% TWEEN 20).[0155]3. After drying, a stack of laminate layers including the antigen-treated polyester sheet are assembled in a microfluidic device generally dimensioned as described in FIG. 9 and assembled with fluidic circuitry per the pattern of FIG. 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com