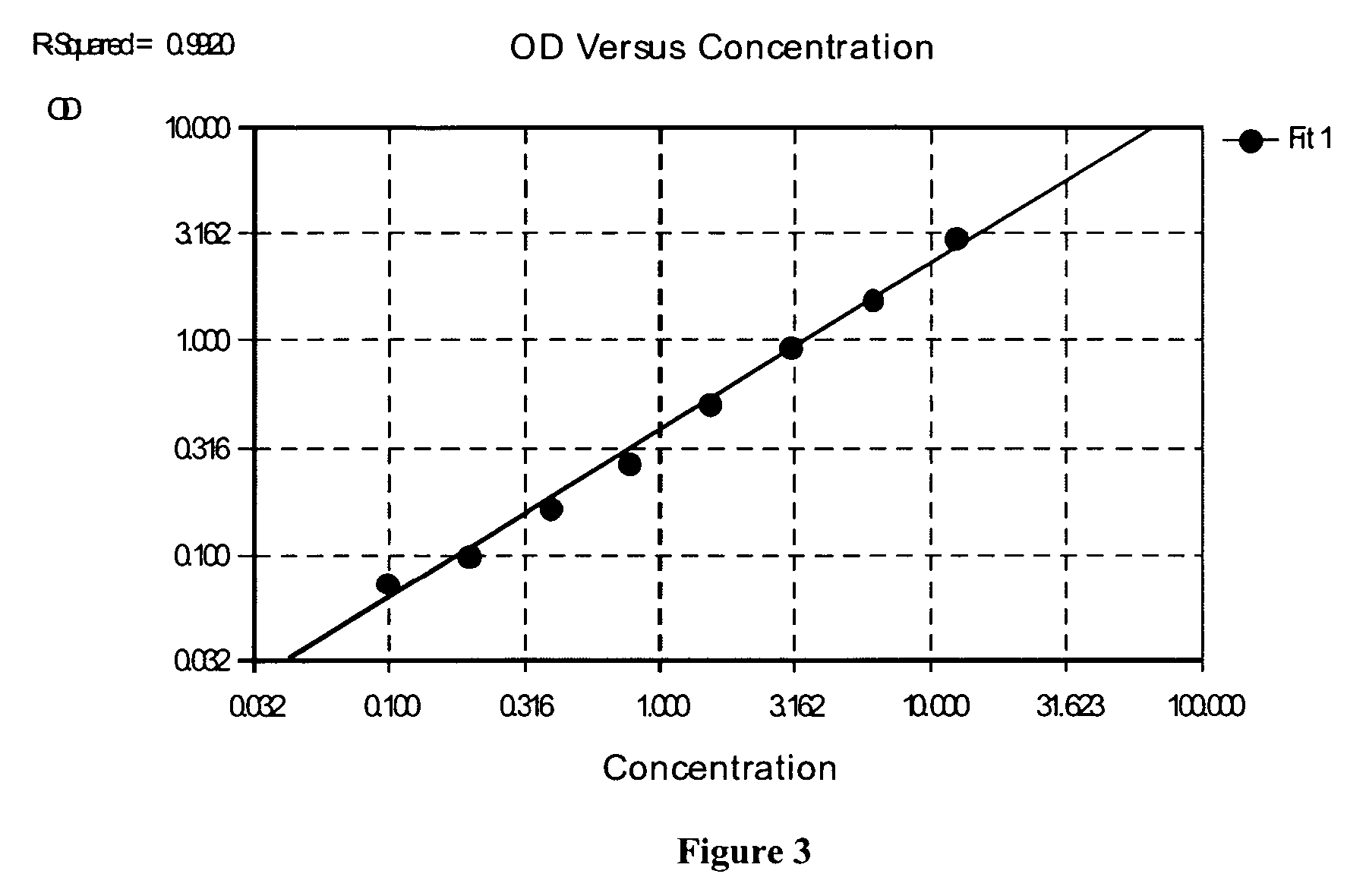
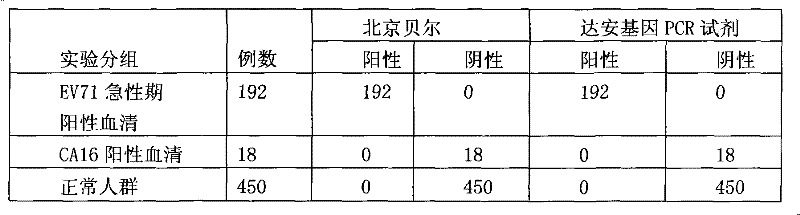
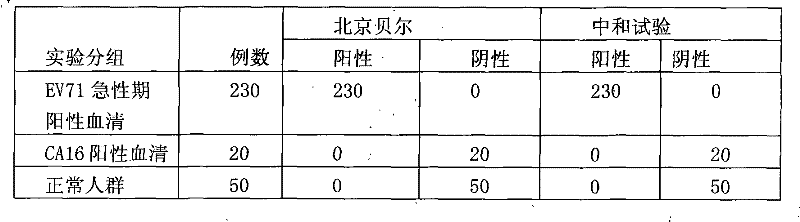
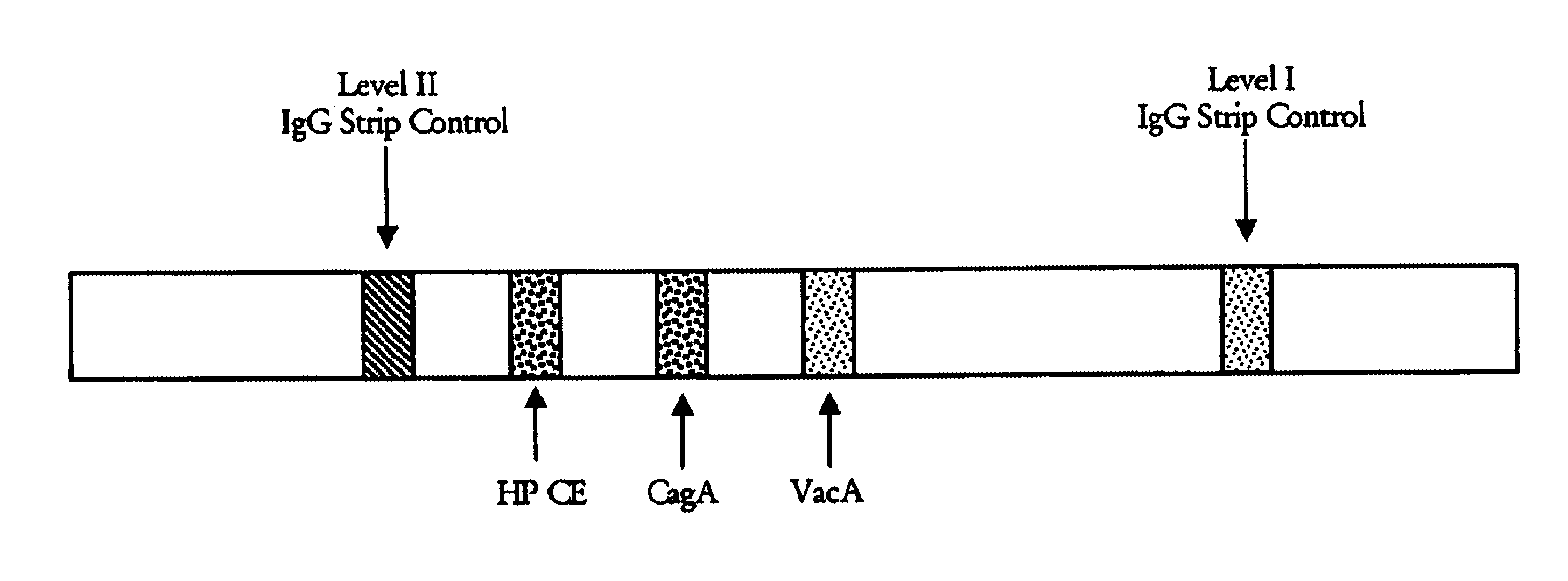
Patents
Literature
137 results about "Immunodiagnostics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
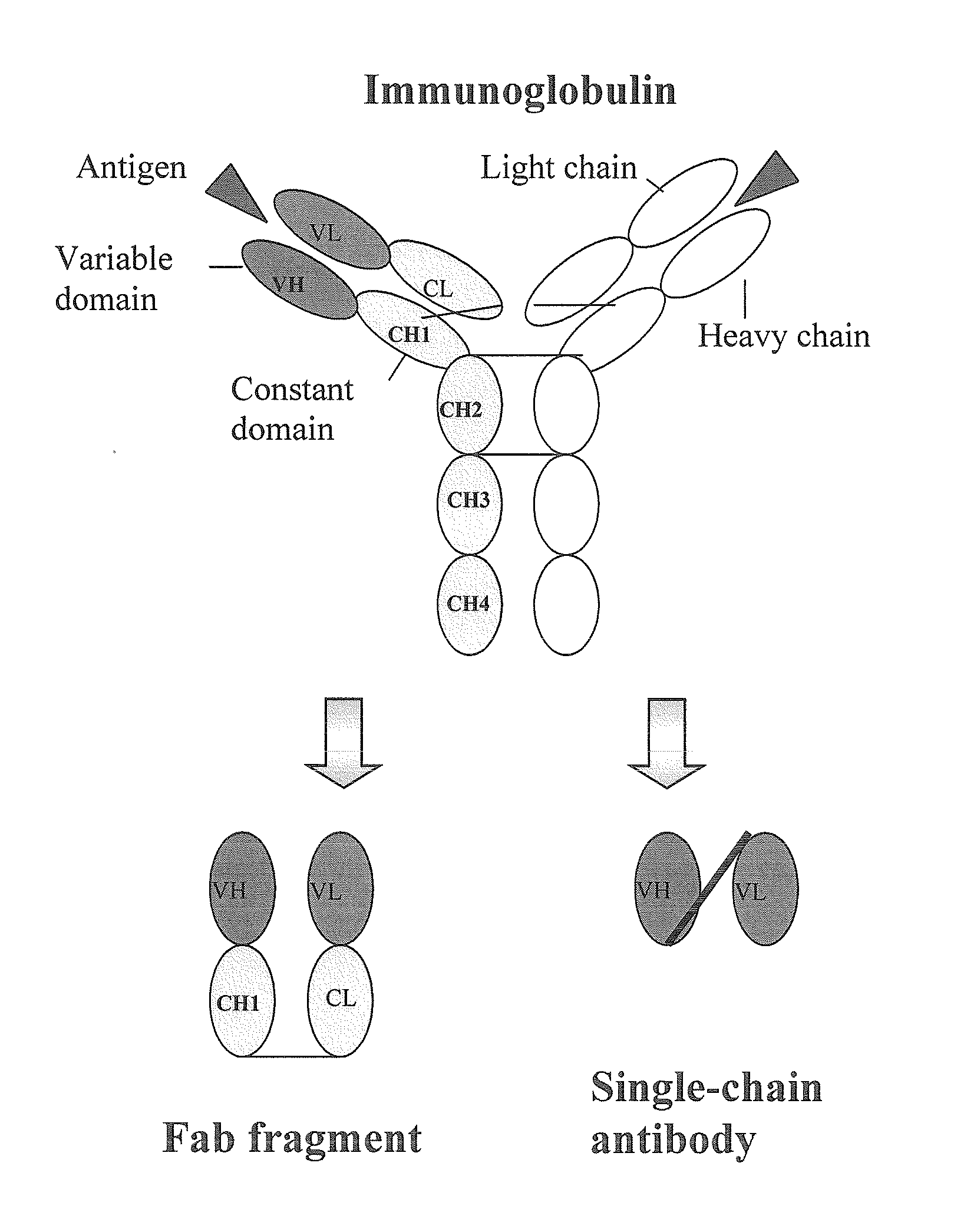
Immunodiagnostics is a diagnostic methodology that uses an antigen-antibody reaction as their primary means of detection. The concept of using immunology as a diagnostic tool was introduced in 1960 as a test for serum insulin. A second test was developed in 1970 as a test for thyroxine in the 1970s. It is well-suited for the detection of even the smallest of amounts of chemical substances. Antibodies specific for a desired antigen can be conjugated with a radiolabel, fluorescent label, or color-forming enzyme and are used as a "probe" to detect it. Well known applications include pregnancy tests, immunoblotting, ELISA and immunohistochemical staining of microscope slides. The speed, accuracy and simplicity of such tests has led to the development of rapid techniques for the diagnosis of disease, microbes and even illegal drugs in vivo. Such testing is also used to distinguish compatible blood types. The Enzyme-Linked ImmunoSorbent Assay or ELISA and the Lateral-Flow test, also known as the dipstick or rapid test, currently are the two predominant formats in immunodiagnostics.
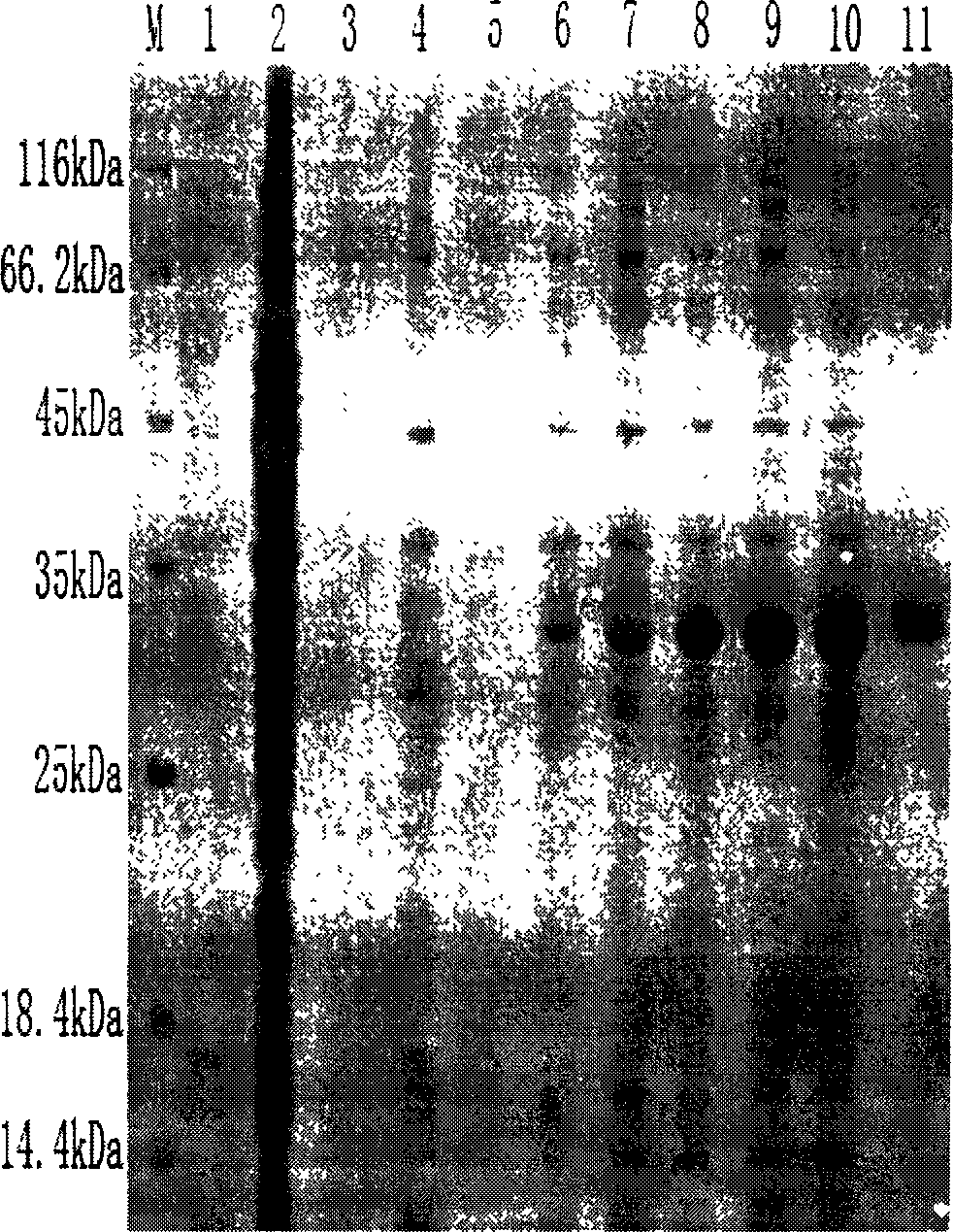
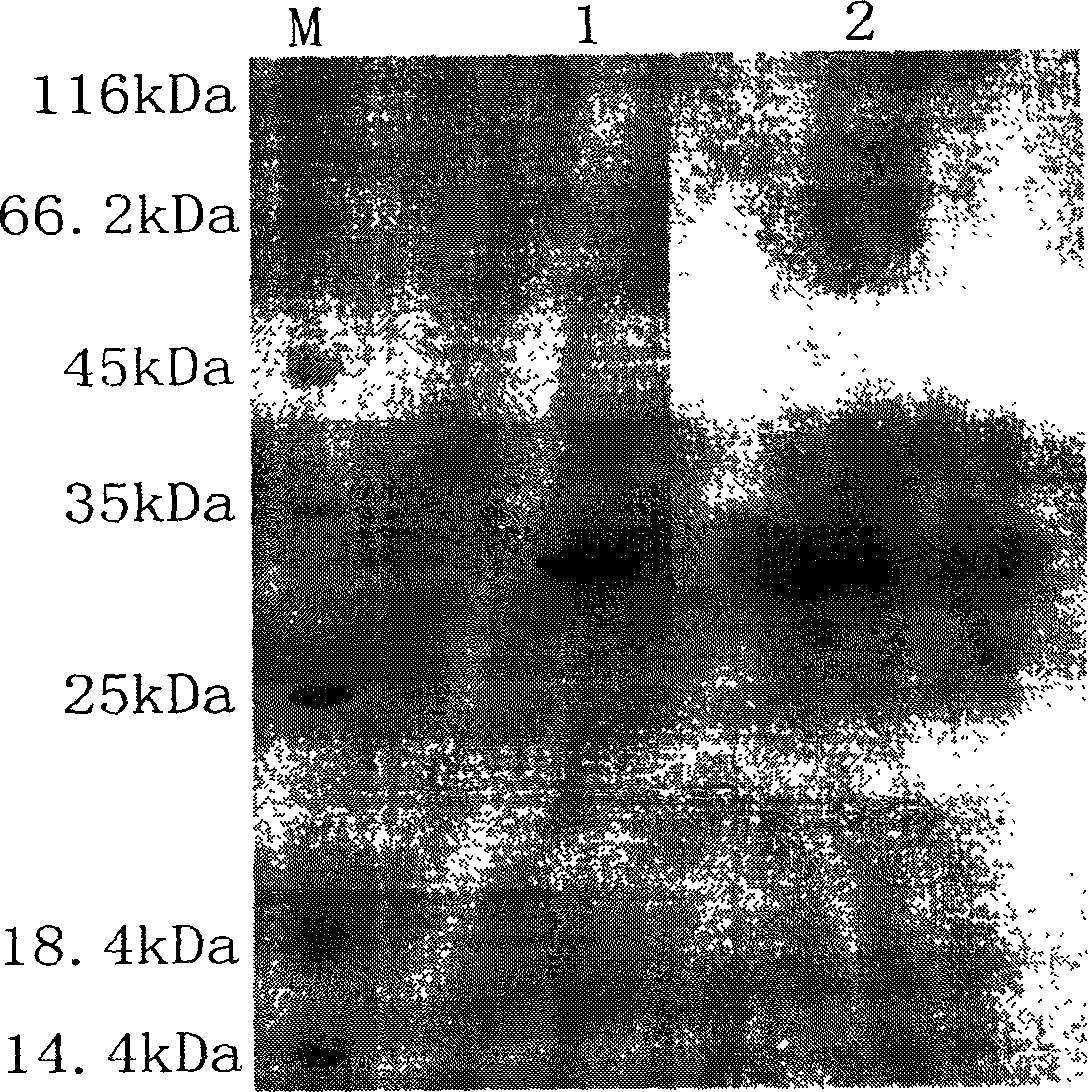
Microfluidic apparatus and methods for performing blood typing and crossmatching
ActiveUS20100112723A1Small dimensionIncrease ratingsBiocideBioreactor/fermenter combinationsAntigenGroup A - blood
Microfluidic cartridges for agglutination reactions are provided. The cartridges include a microfluidic reaction channel with at least two intake channels, one for an antigen-containing fluid and the other for an antibody-containing fluid, conjoined to a reaction channel modified by incorporation of a downstream flow control channel. At low Reynolds Number, the two input streams layer one on top of the other in the reaction channel and form a flowing, unmixed horizontally-stratified laminar fluid diffusion (HLFD) interface for an extended duration of reaction. Surprisingly, the design, surface properties, and flow regime of microfluidic circuits of the present invention potentiate detection of antibody mediated agglutination at the stratified interface. Antigen:antibody reactions involving agglutination potentiated by these devices are useful in blood typing, in crossmatching for blood transfusion, and in immunodiagnostic agglutination assays, for example.
Owner:PERKINELMER HEALTH SCIENCES INC
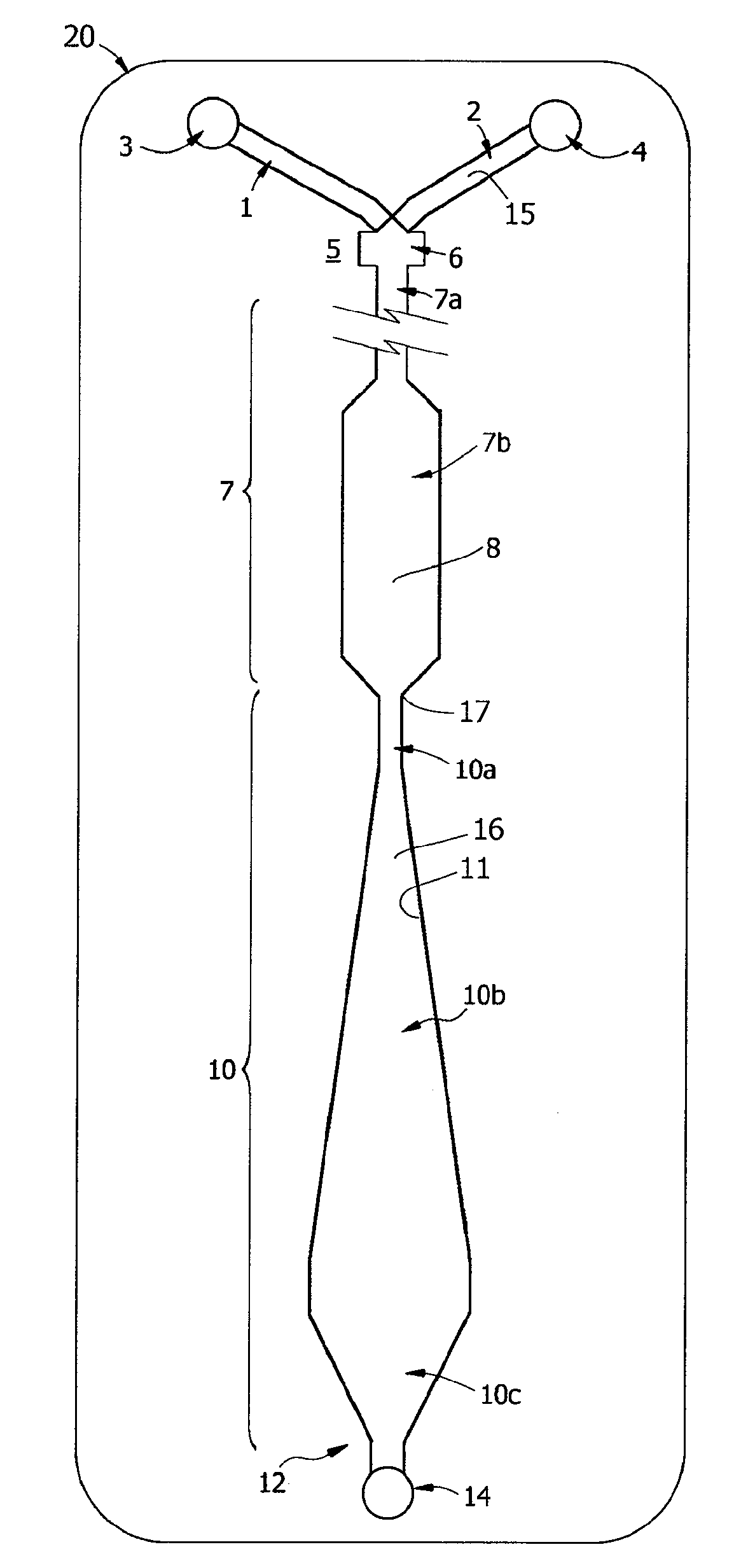
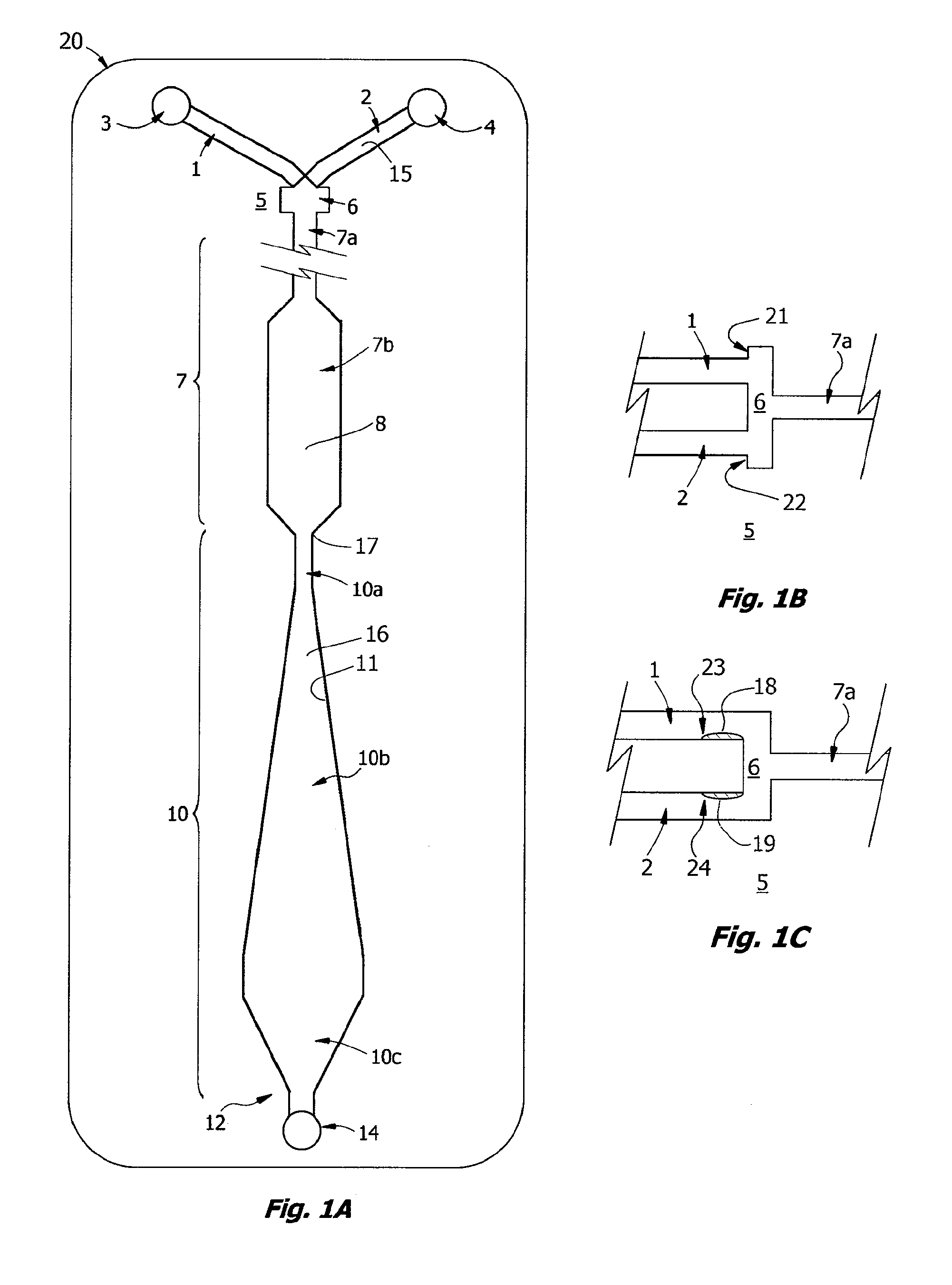
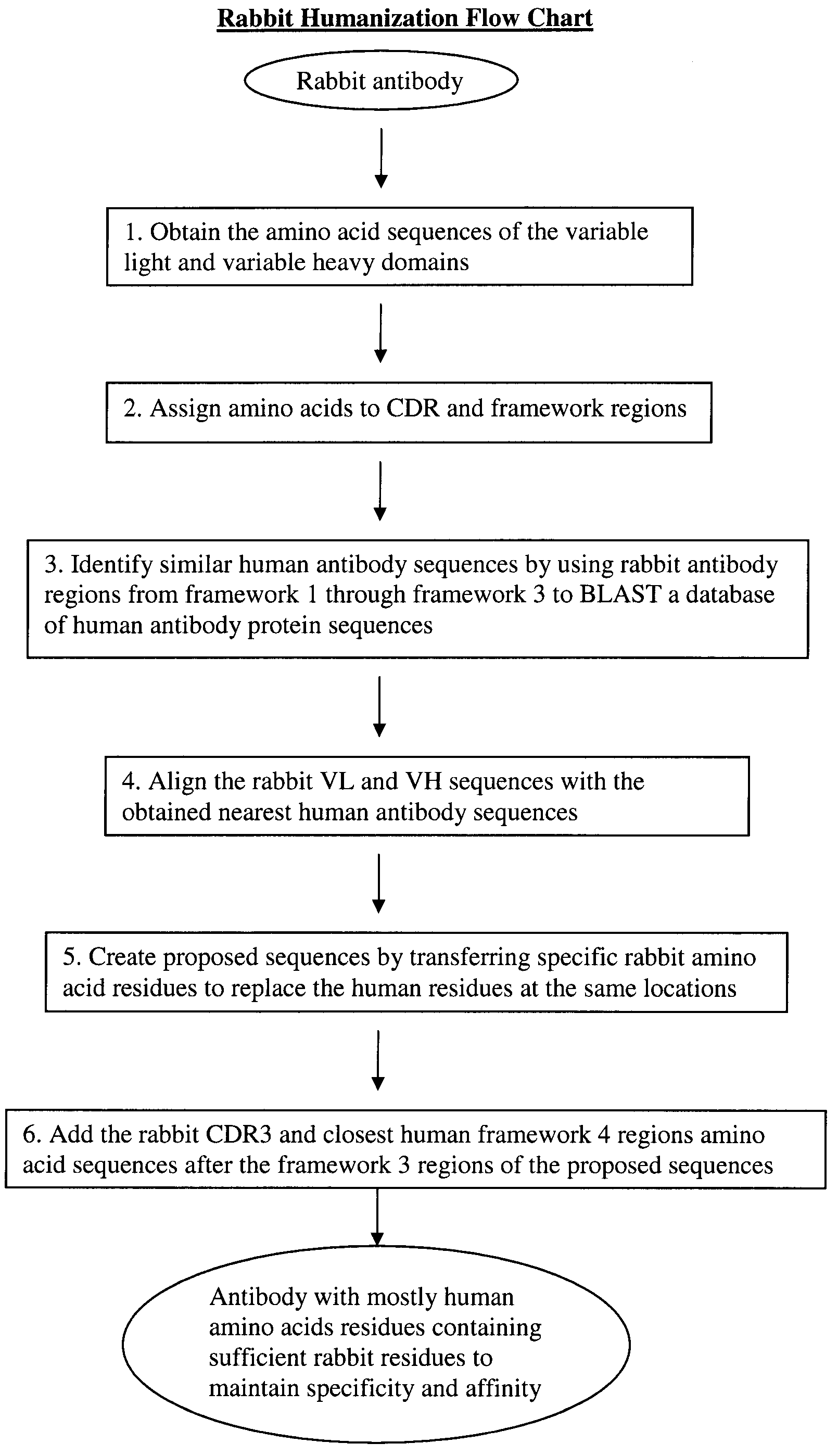
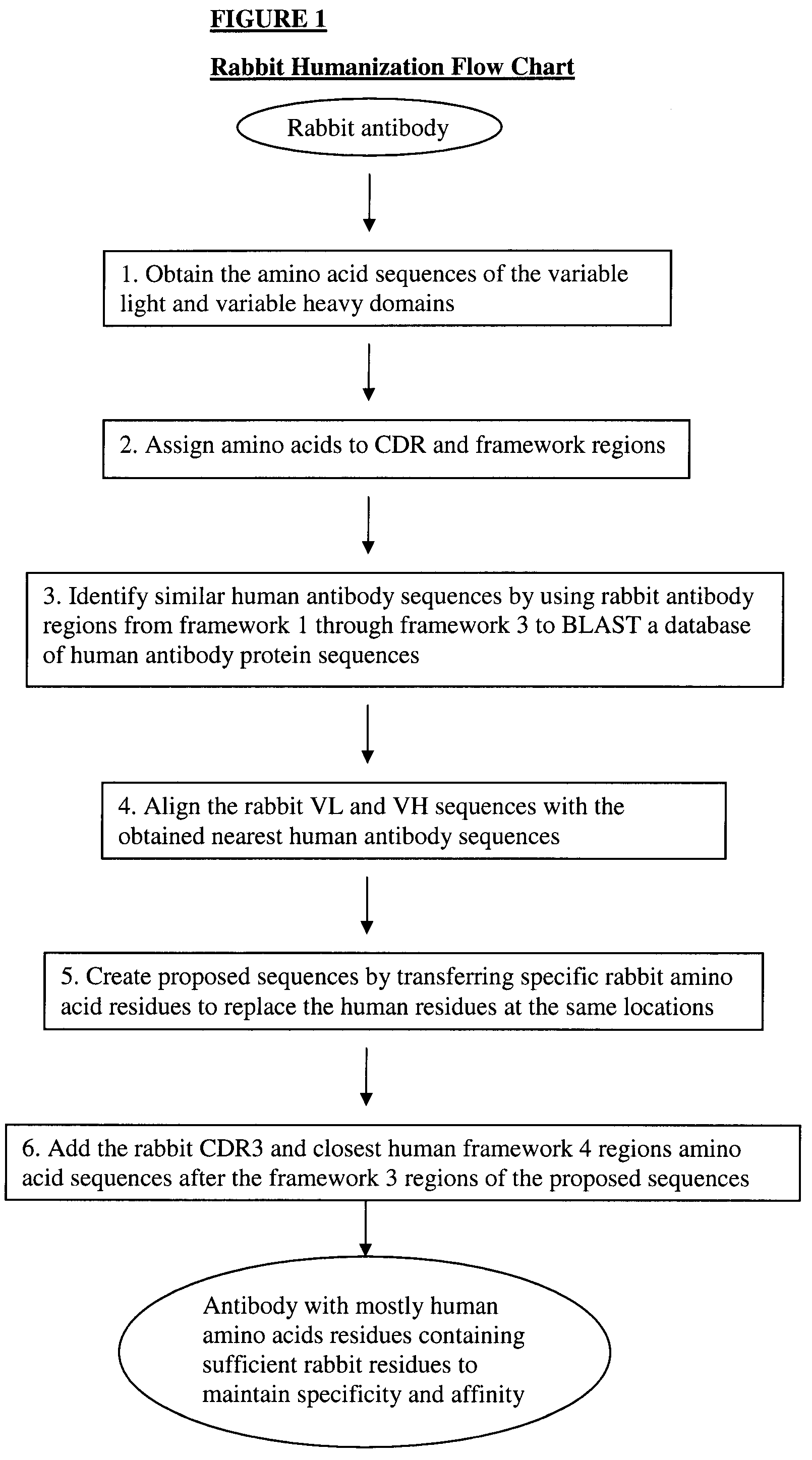
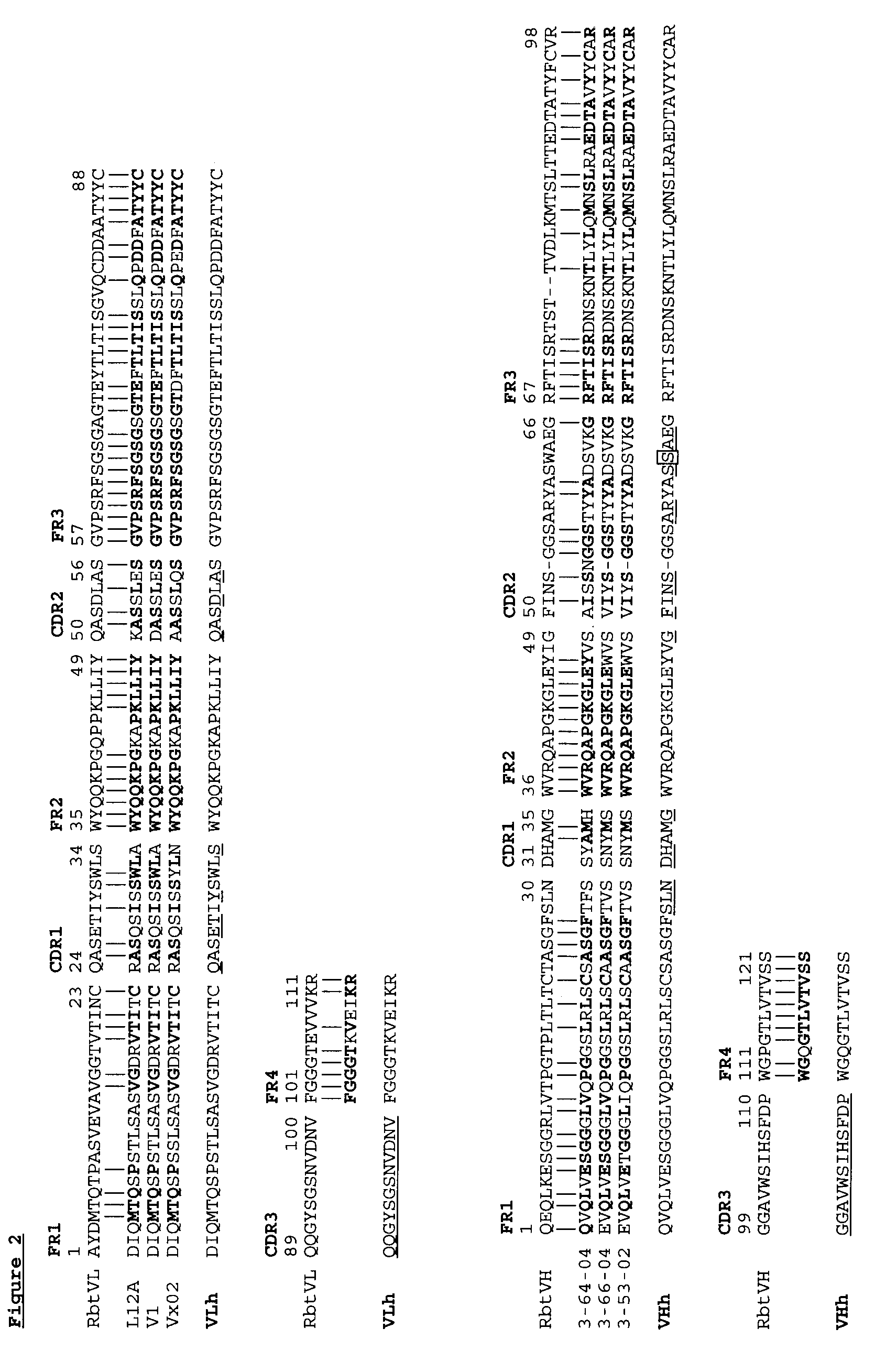
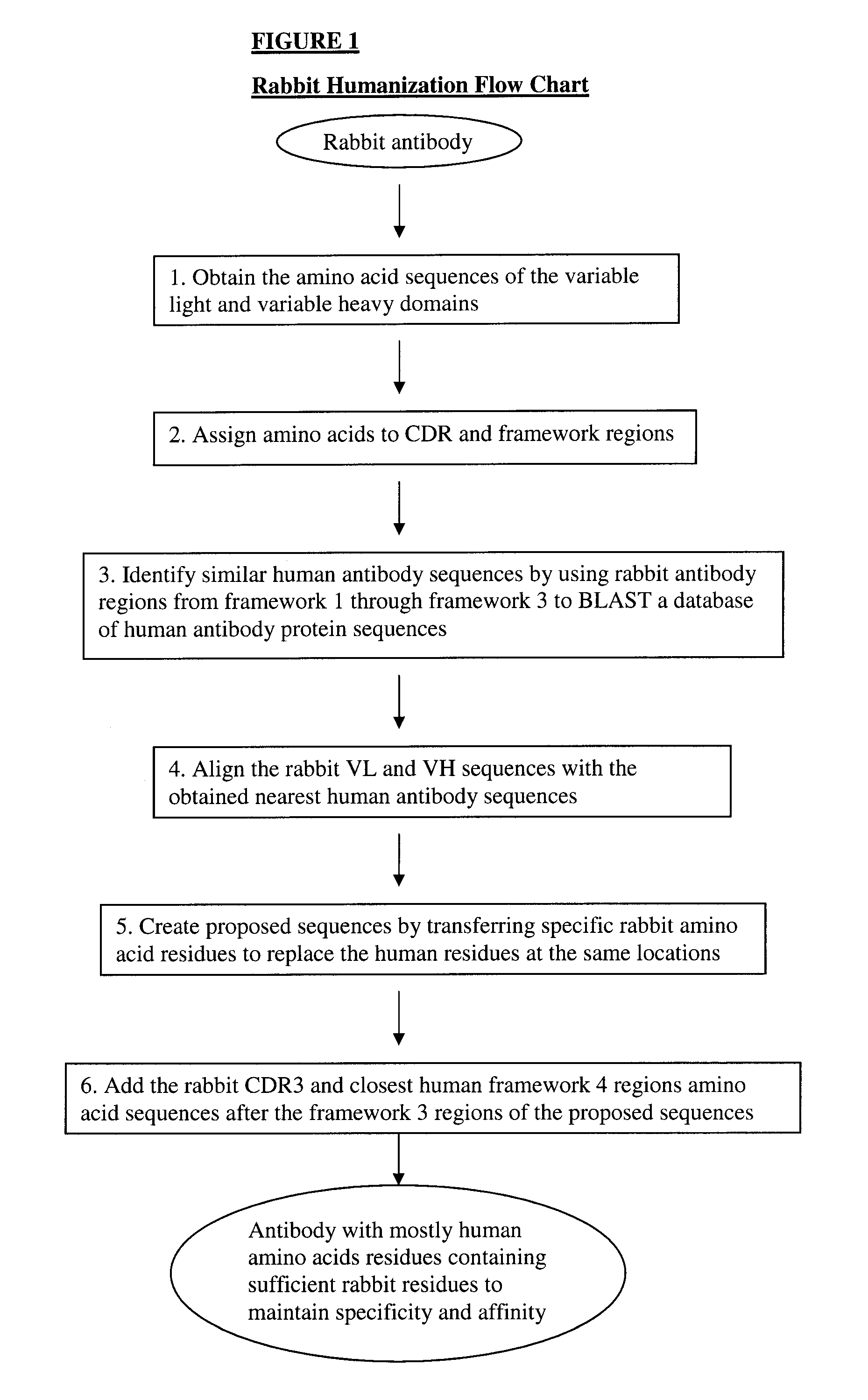
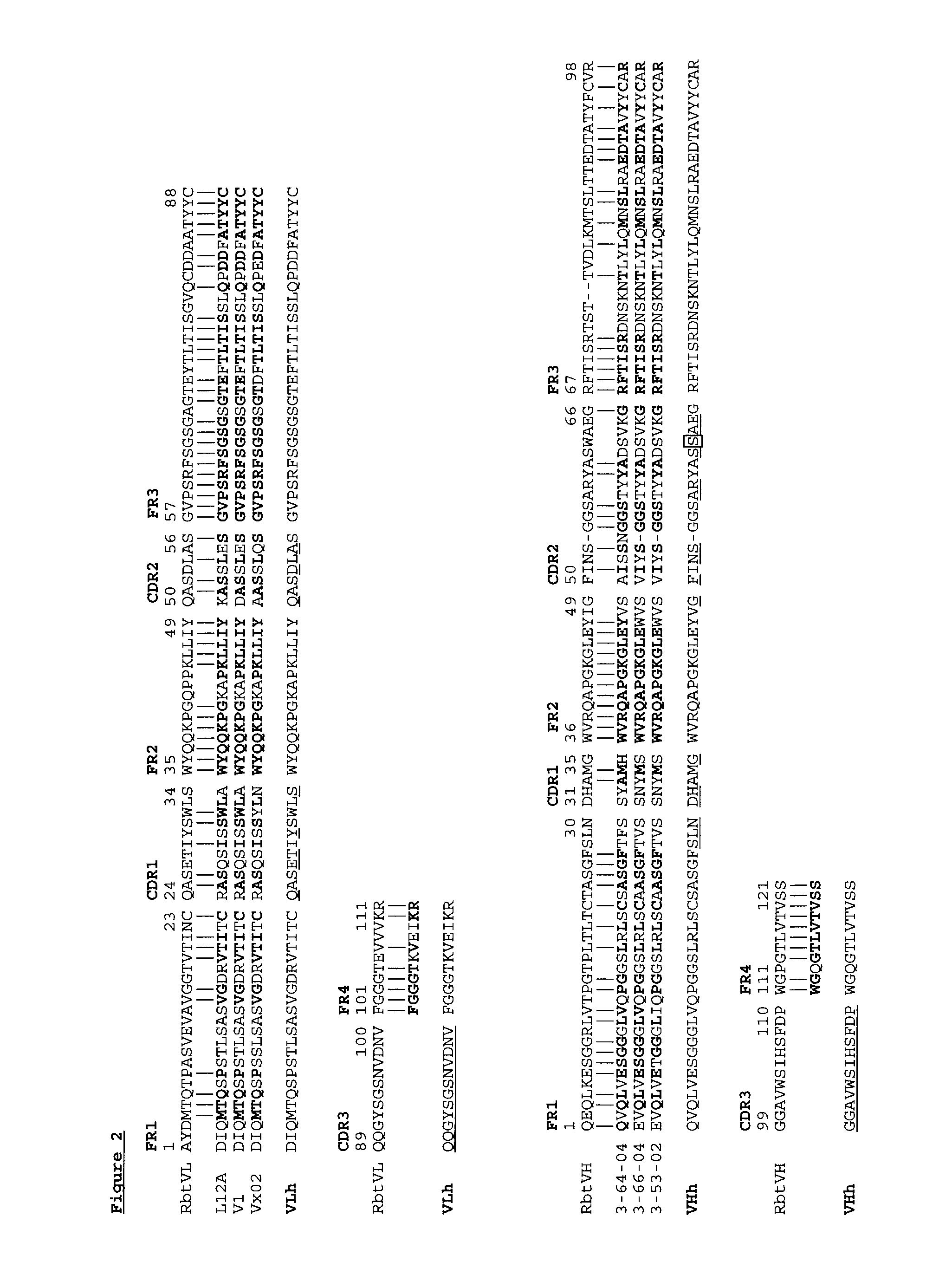
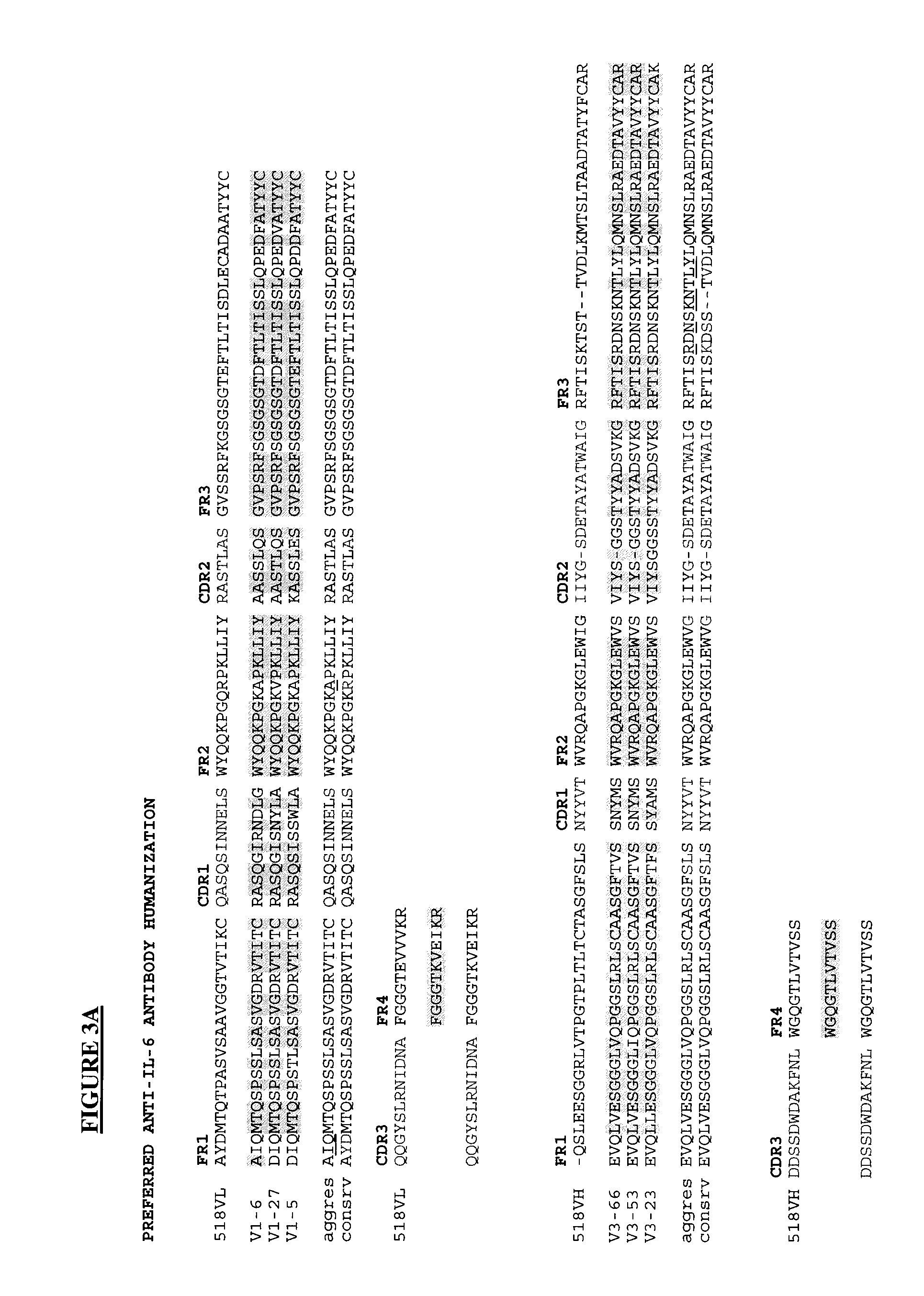
Novel Rabbit Antibody Humanization Methods and Humanized Rabbit Antibodies
The present invention is directed to novel and improved methods for humanizing rabbit heavy and light variable regions. The resulting humanized rabbit heavy and light chains and antibodies and antibody fragments containing are well suited for use in immunotherapy and immunodiagnosis as they retain the antigen binding affinity of the parent antibody and based on their very high level of sequence identity to human antibody sequences should be essentially non-immunogenic in humans. The invention exemplifies the protocol for the manufacture of therapeutic humanized anti-human TNF-alpha and anti-human IL-6 antibodies.
Owner:ALDERBIO HLDG LLC
Novel rabbit antibody humanization methods and humanized rabbit antibodies
The present invention is directed to novel and improved methods for humanizing rabbit heavy and light variable regions. The resulting humanized rabbit heavy and light chains and antibodies and antibody fragments containing are well suited for use in immunotherapy and immunodiagnosis as they retain the antigen binding affinity of the parent antibody and based on their very high level of sequence identity to human antibody sequences should be essentially non-immunogenic in humans. The invention exemplifies the protocol for the manufacture of therapeutic humanized anti-human TNF-alpha and anti-human IL-6 antibodies.
Owner:ALDERBIO HLDG LLC
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
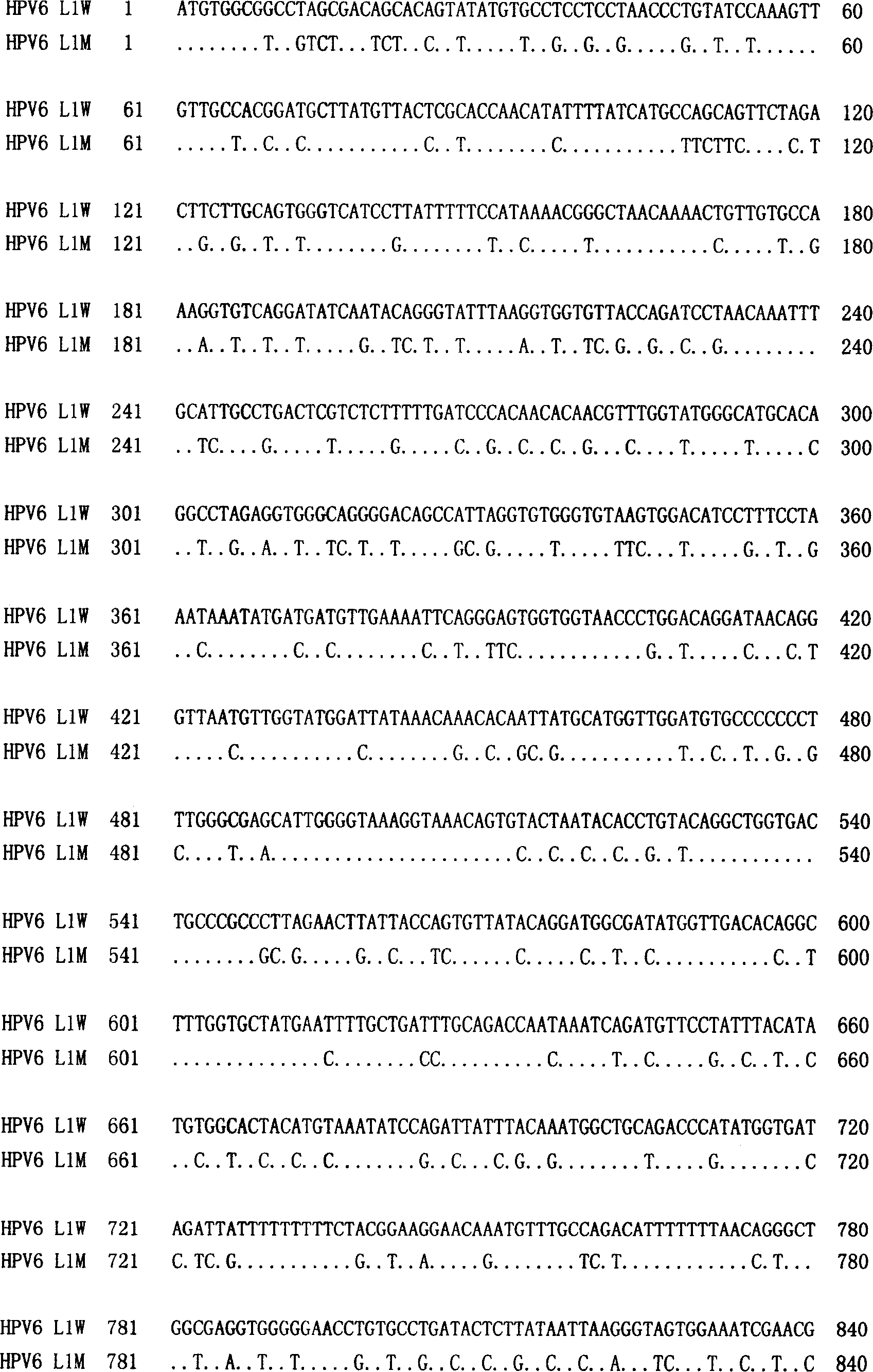
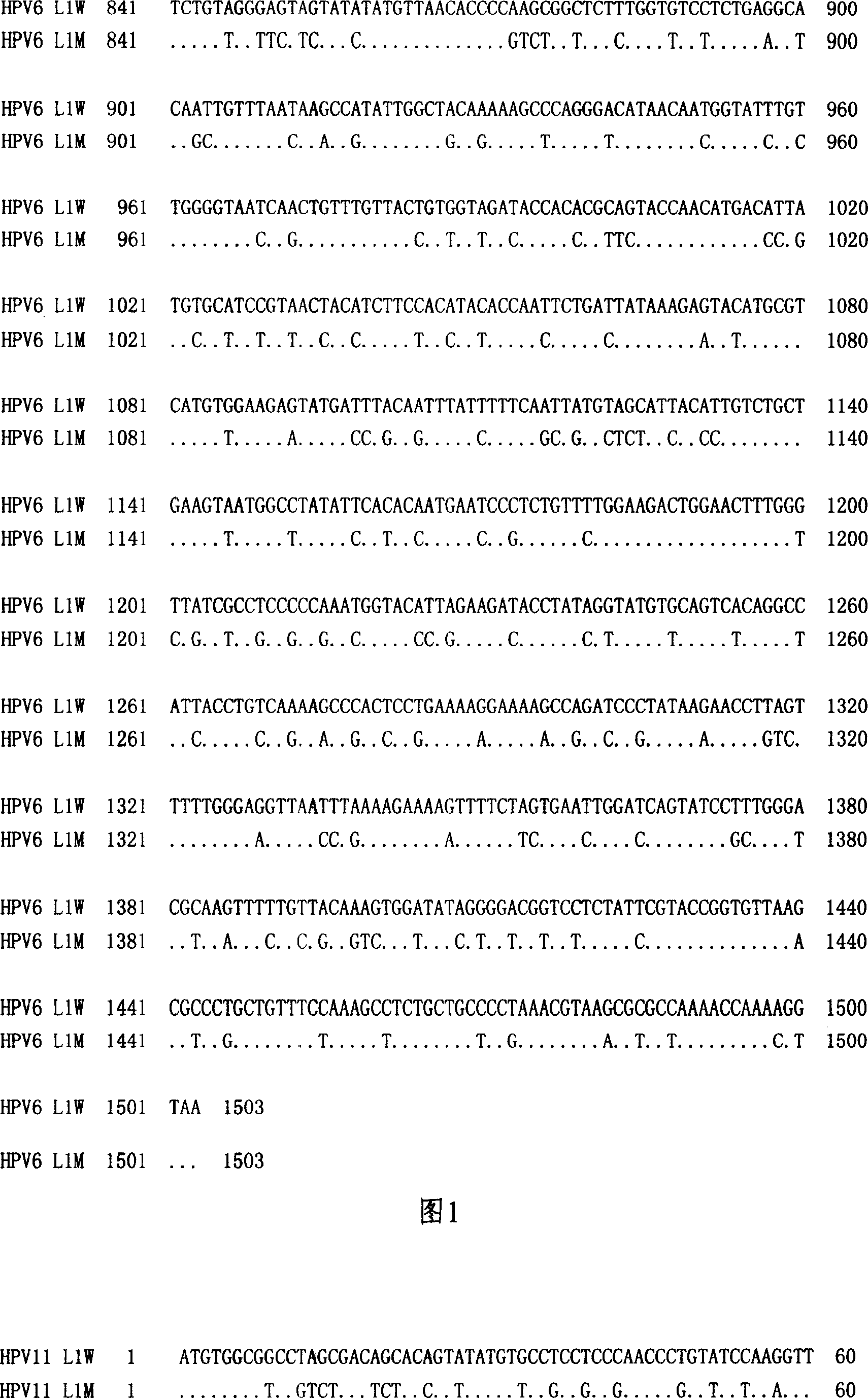
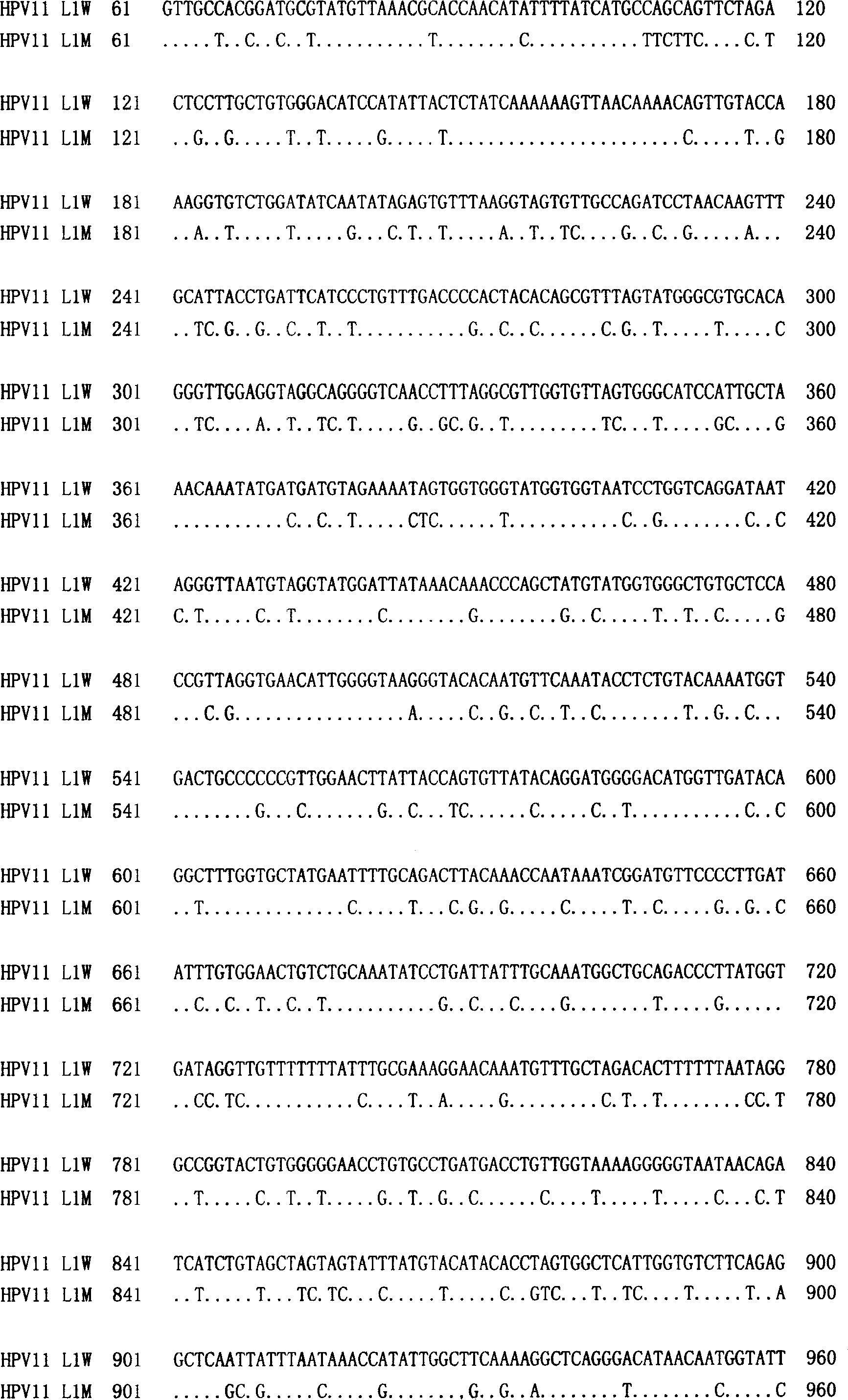
Method of increasing yield of human papilloma virus L1 albumen pronucleus expression
ActiveCN101016542ALow costHigh expressionViral antigen ingredientsAntiviralsTGE VACCINENucleic acid sequence
The invention discloses a method to increase human papilloma virus L1 protein pronucleus expression productivity and also discloses a encode HPV L1 protein codon majorizing nucleic acid sequence from this method, which is characterized by the following: supplying expression carrier and host cell and HPVL1 protein poly body of nucleic acid sequence; disclosing appliance in preparing vaccine, drug compound and immunodiagnosis or antibody. The nucleic acid sequence expressing quantity possesses distinctive improvement, which decreases the preparing cost effectively.
Owner:BEIJING HEALTH GUARD BIOTECH
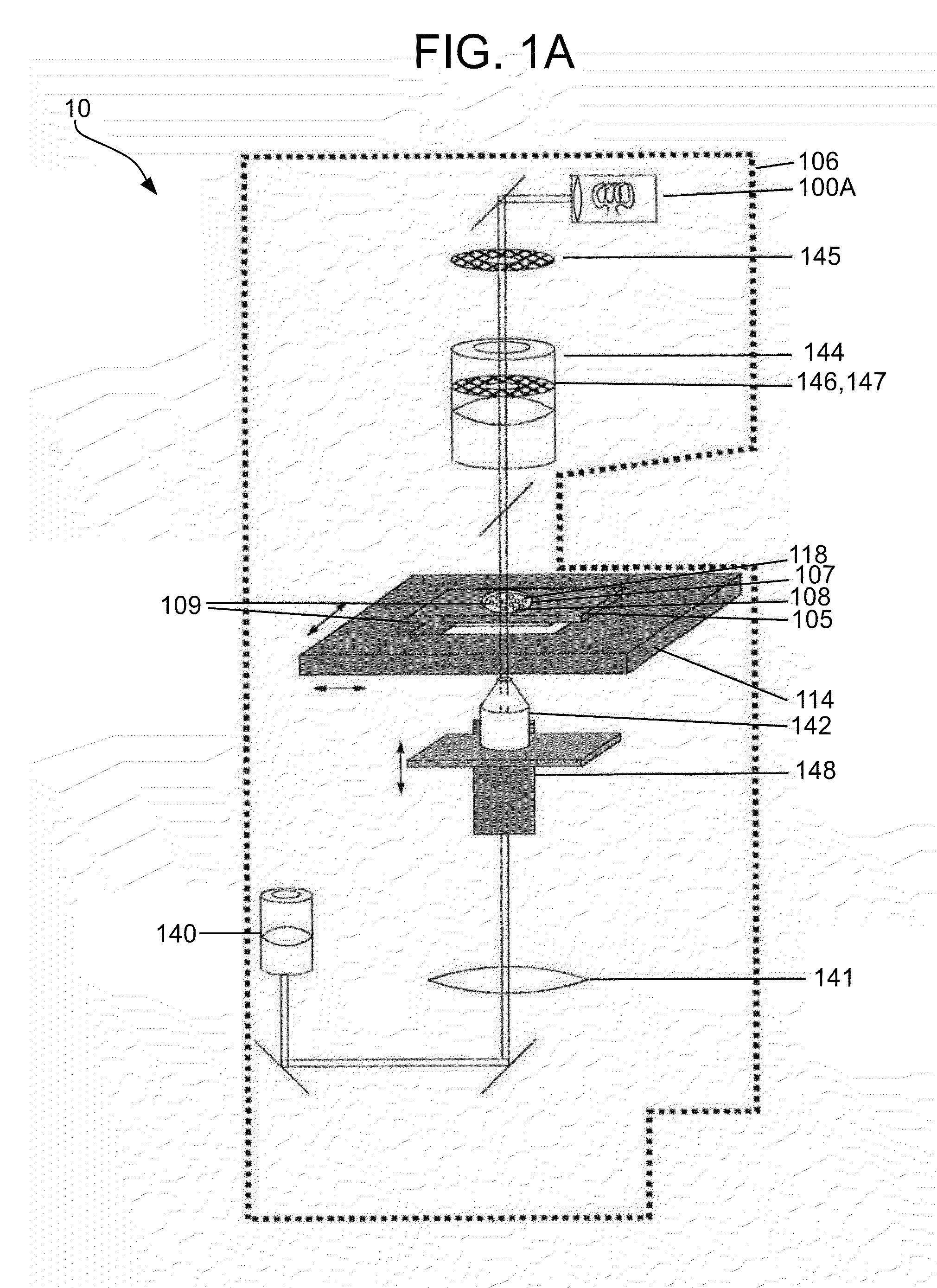
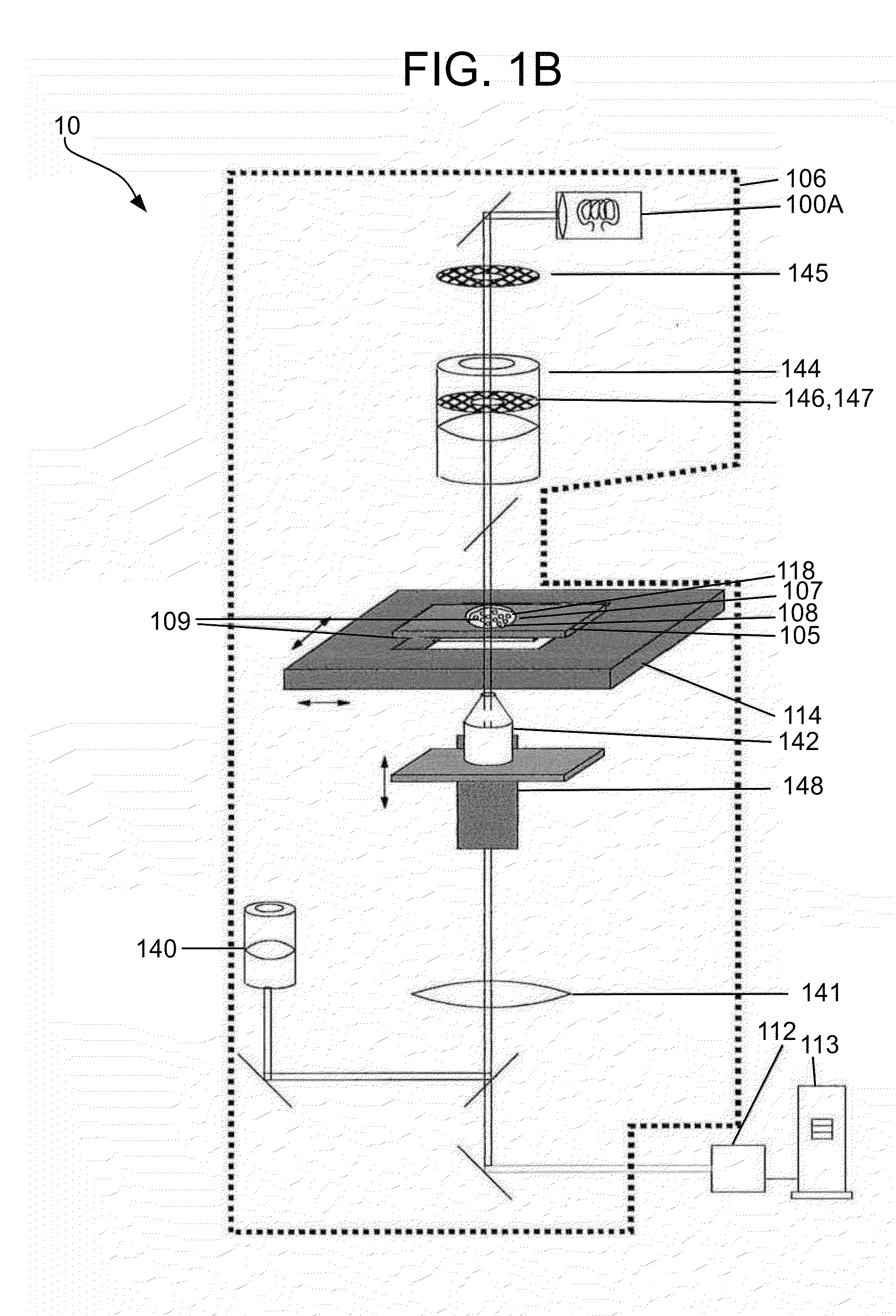
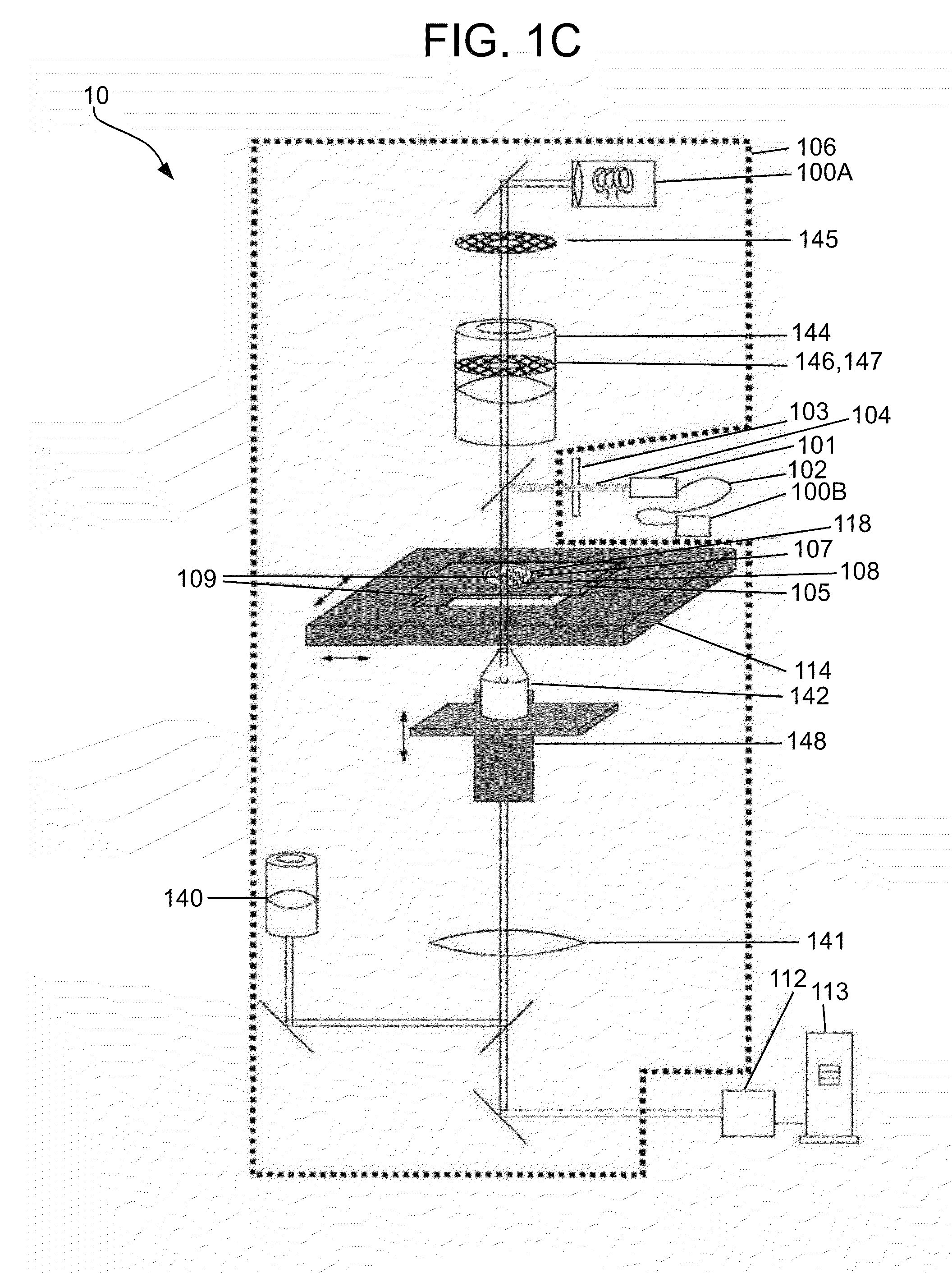
Methods and apparatuses for detection of positional freedom of particles in biological and chemical analyses and applications in immunodiagnostics
InactiveUS20130274119A1Improve discriminationLibrary screeningMaterial analysis by optical meansAnalyteImmunodiagnostics
The present invention relates to methods and apparatuses for the detection of positional freedom of particles used in biological, biochemical, physical, biophysical, and chemical analyses. In particular, the present invention relates to methods and apparatuses which can detect and characterize a population of particles / cells based upon their detected mobility. In one embodiment consistent with the invention, detection of certain cells is based on differences detected in populations of cells that bind to a substrate and those that exhibit weaker binding forces. Initially, cells are settled on the substrate, and in the presence of gravitational, natural thermodynamic pressure fluctuations, and other random or applied forces, some of the particles may exhibit translational movement. Particle movement is detected, and measurements are computed, according to the methods and apparatuses of the present invention, to determine the binding of specific analytes.
Owner:ARRYX INC
P153 and P156 antigens for the immunodiagnosis of canine and human ehrlichioses and uses thereof
Sequences encoding two immunoreactive glycoproteins were cloned from Ehrlichia canis (p153 gene) and Ehrlichia chaffeensis (p156 gene). These two glycoproteins are species-specific immunoreactive orthologs that are useful as subunit vaccines and for serologic and molecular diagnostics for E. canis and E. chaffeensis.
Owner:RES DEVMENT FOUND
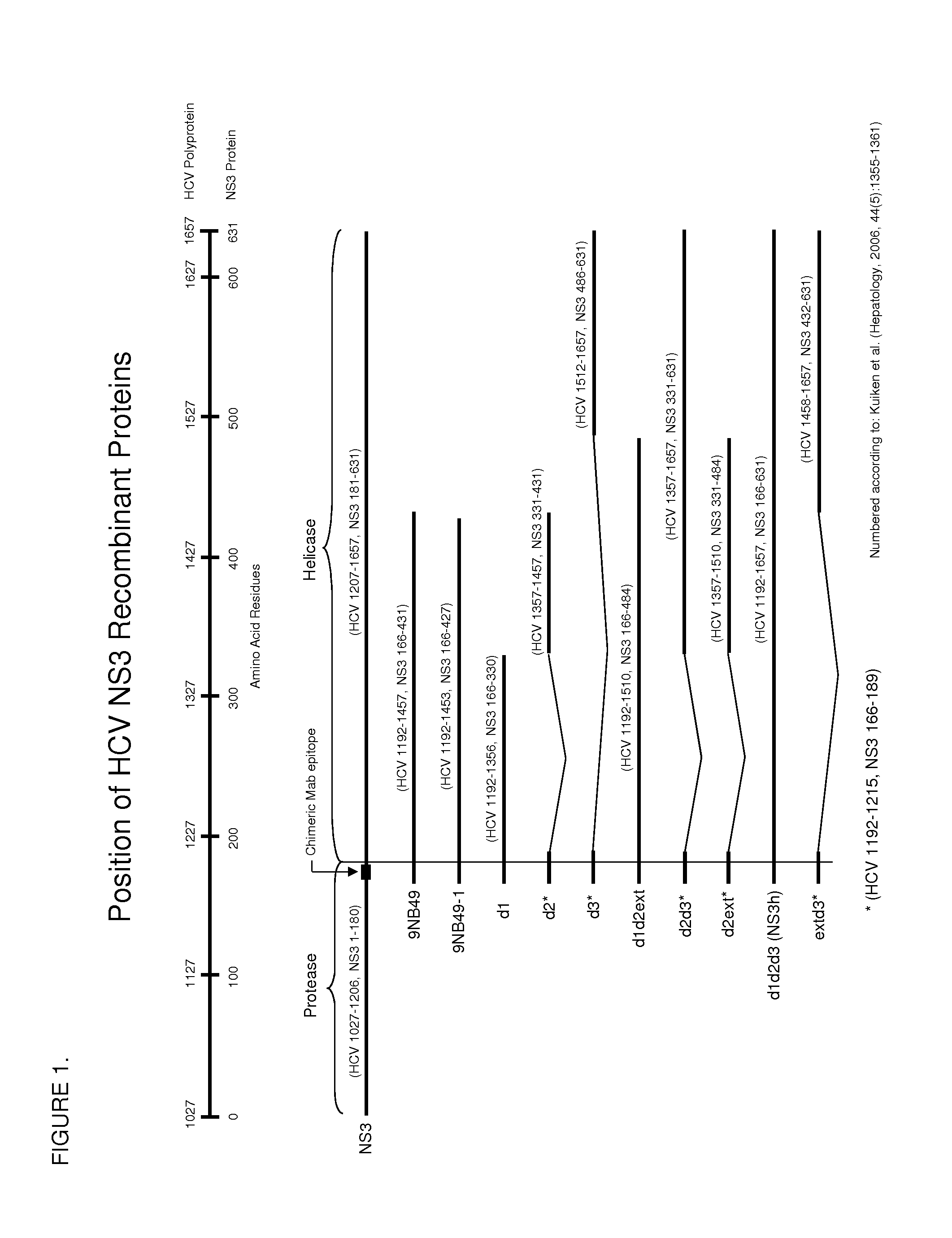
Hcv ns3 recombinant antigens and mutants thereof for improved antibody detection
ActiveUS20140272932A1Excellent redox stabilityImprove stabilitySsRNA viruses positive-senseBacteriaAnti hcv antibodyHCV Antibody
Owner:ABBOTT LAB INC
Stabilized two component system for chemiluminescent assay in immunodiagnostics
The present invention provides stabilized chemiluminescent formulations for use in in vitro diagnostics, including competitive as well as sandwich-type immunological assays. The stabilized assay system may be composed of two components, where the first component may contain a chemiluminescent organic compound, an enhancer, a homogenizing agent, and a suitable buffer with formulations having a pH range from about 7.2 to about 12, and optionally a solubilizing agent. The chemiluminescent system of the present invention is useful in immunoenzymatic analytical procedures, such as immunometric, competitive binding and sandwich type assays. In such immunoassays employing the chemiluminescent system of the present invention, the detectable light signal shows a proportional decay with time in the test samples and standards, so that the decay of the light emitted does not effect the concentration of the analyte measured over the entire analyte measurement range of the immunoassay. This allows accurate measurement of analyte concentrations in a test sample over extended periods of time.
Owner:KALRA BHANU +1
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
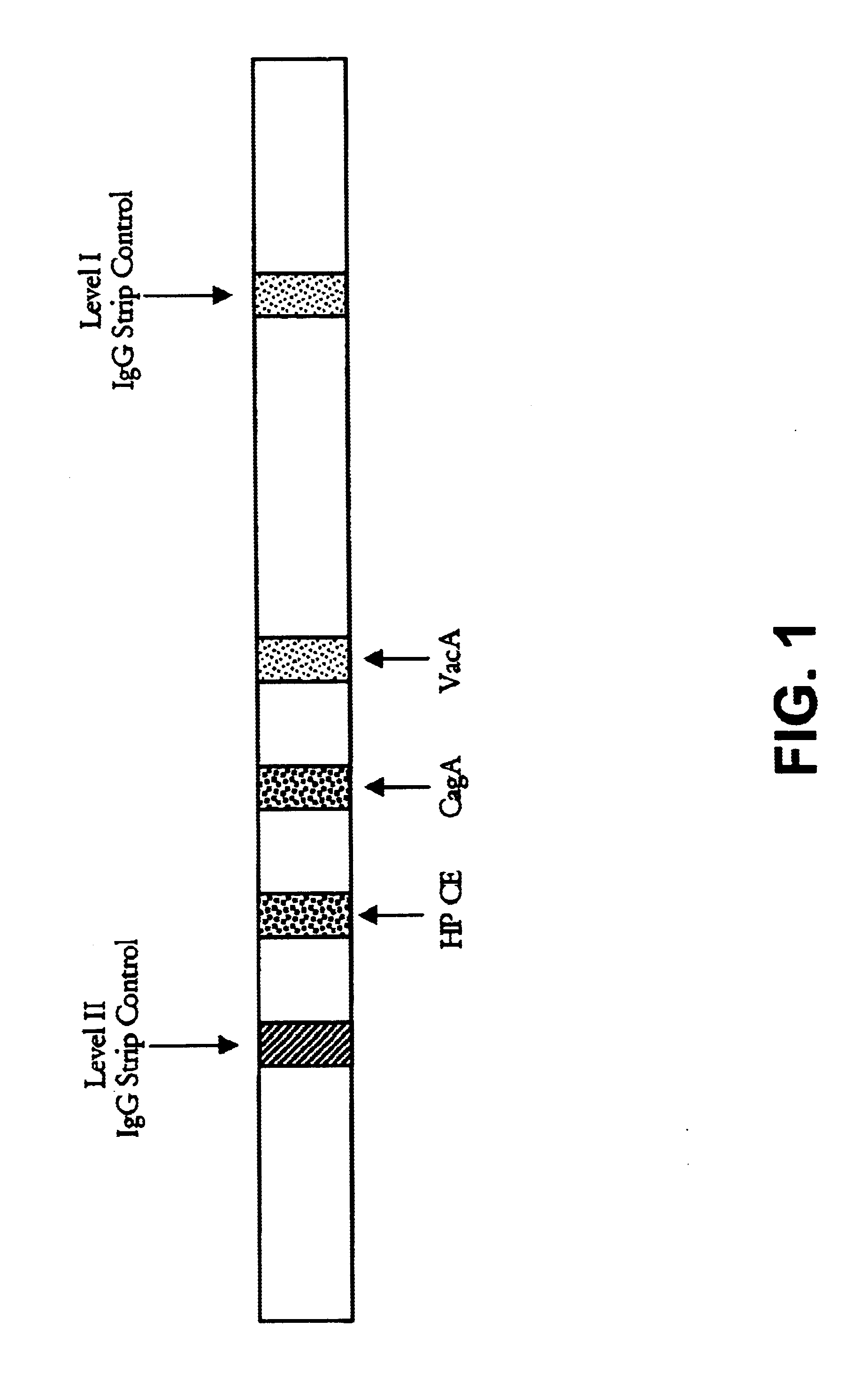
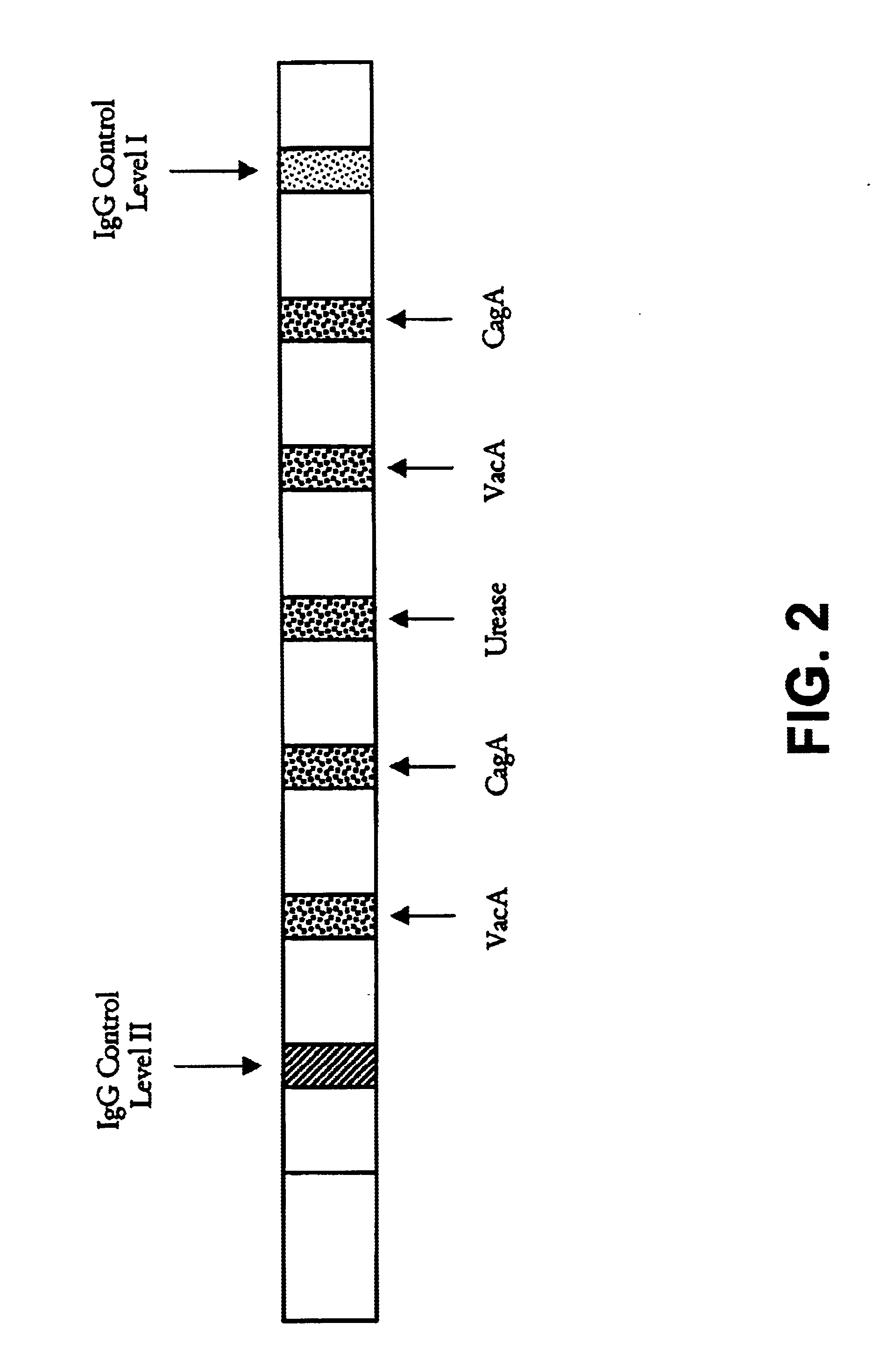
Helicobacter pylori diagnostics
InactiveUS6902903B1Simple and extremely accurate and efficient methodMonitoring usingSugar derivativesBacteriaImmunodiagnosticsAssay
Novel methods, membrane supports and immunodiagnostic test kits for diagnosing Helicobacter pylori infection, are disclosed. The methods can also be used to monitor the progress of treatment of an infection. The methods, supports and kits employ both type-common and type-specific H. pylori antigens and can conveniently be performed in a single-step assay format. The methods provide for highly accurate results and discriminate between H. pylori Type I and H. pylori Type II infection so that an accurate diagnosis can be accomplished.
Owner:CHIRON CORP
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562368AHigh sensitivityEasy to operateBiological material analysisImmunodiagnosticsNeutralising antibody
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, a first antigen and a second antigen ofS protein of SARS-CoV-2, and the second antigen is marked with a signal substance. Whether a tested person is infected by the new coronavirus or not and whether infection risks exist or not are judgedby detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
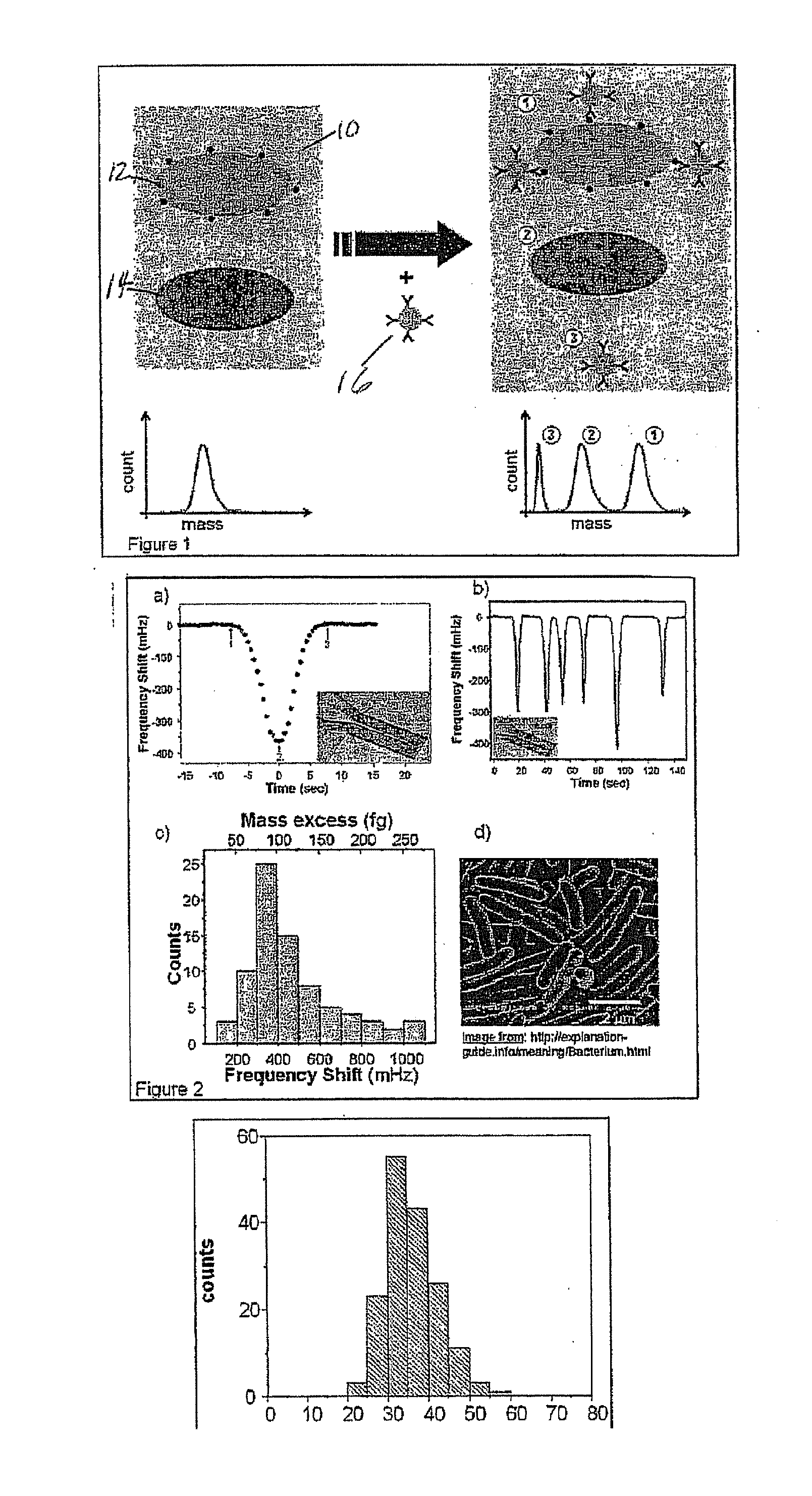
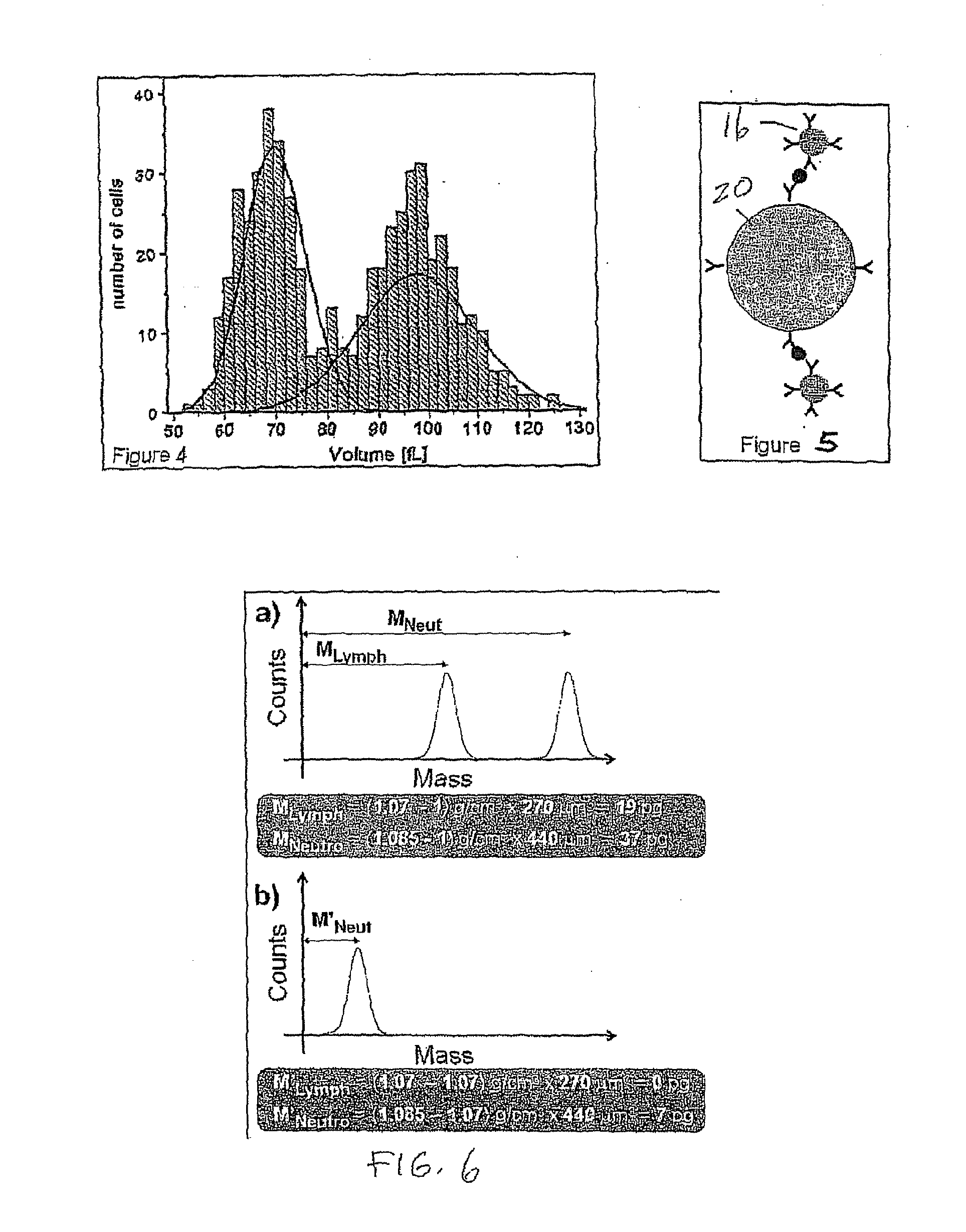
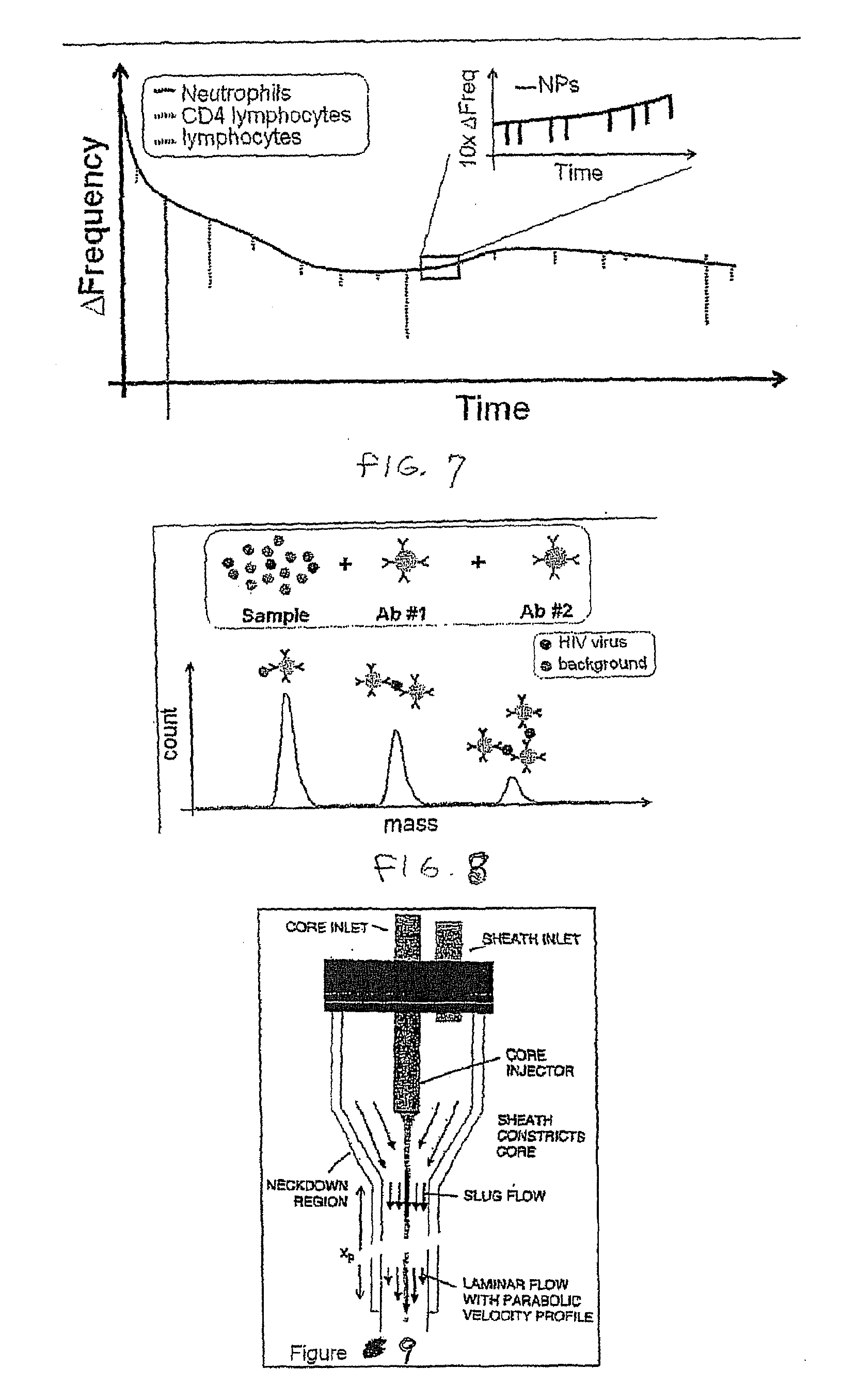
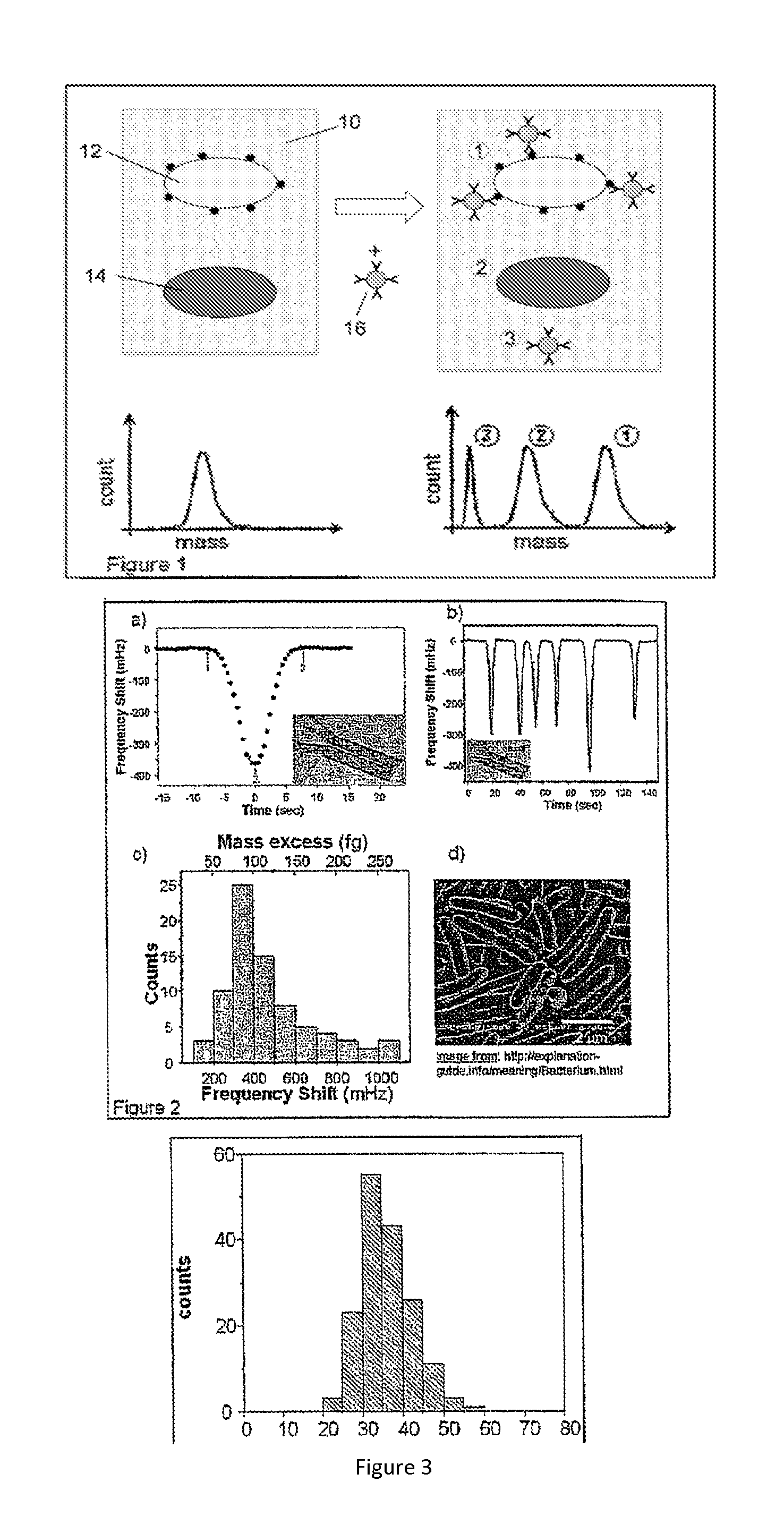
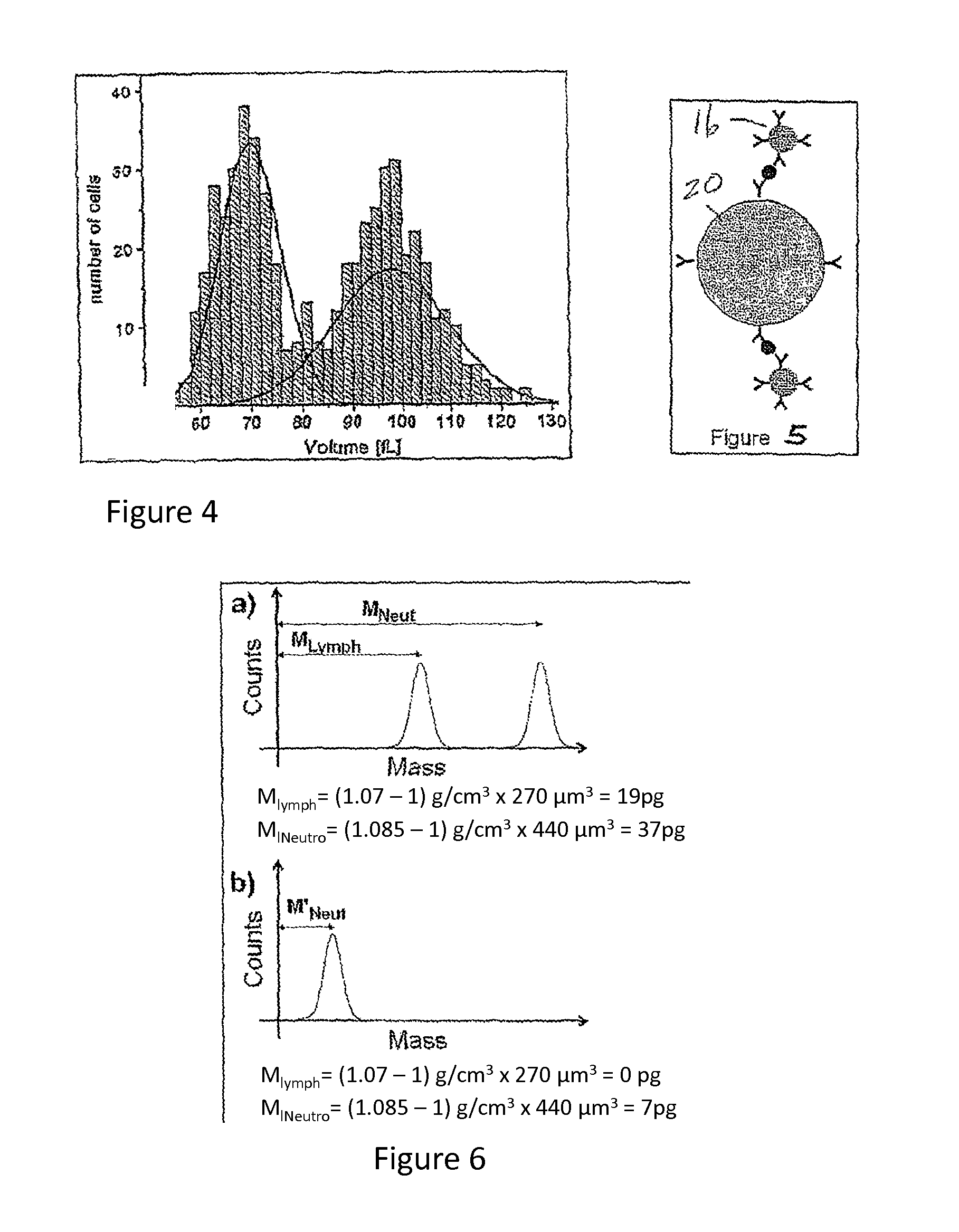
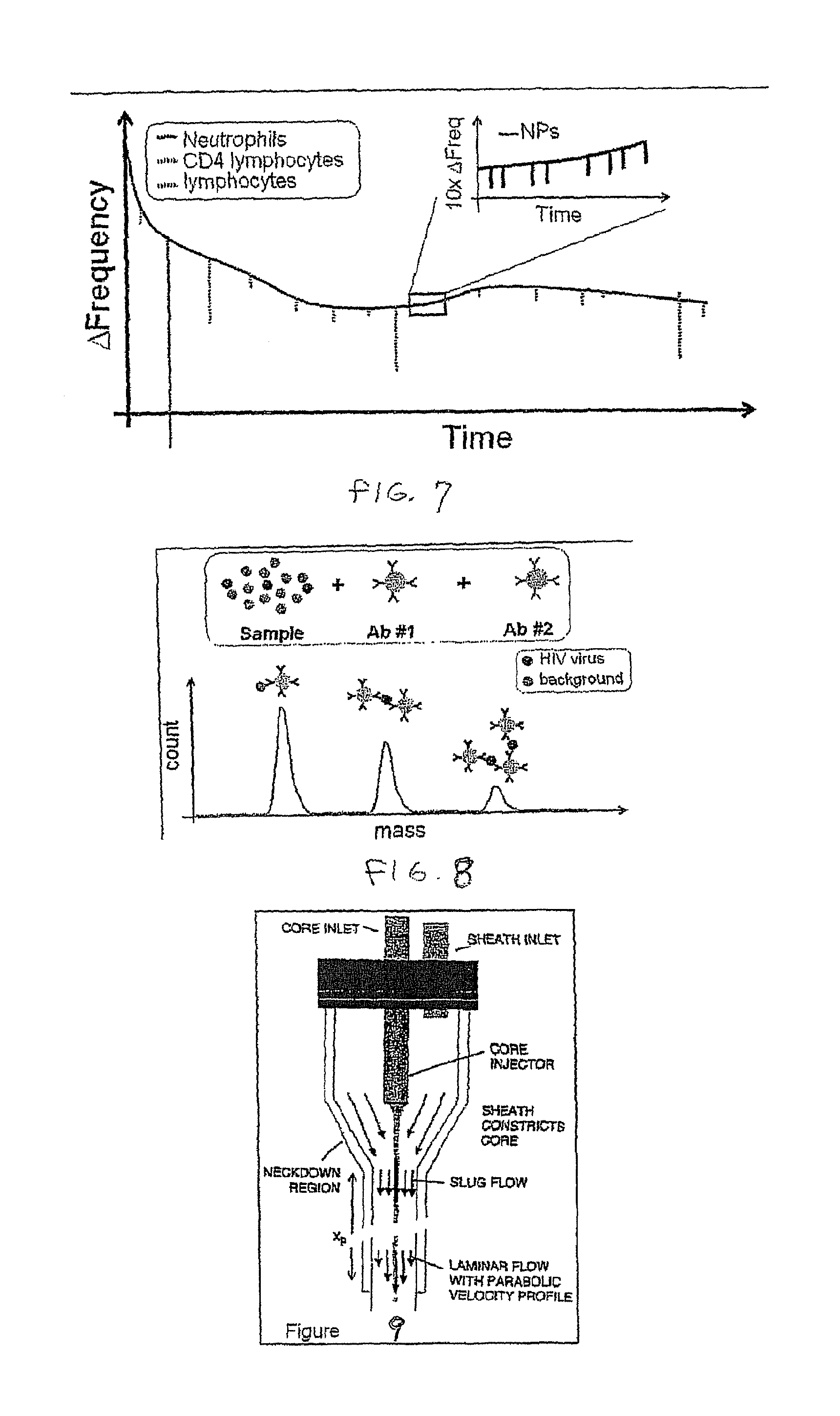
Flow cytometry methods and immunodiagnostics with mass sensitive readout
ActiveUS20100227310A1Packed tightlyImprove throughputMicrobiological testing/measurementBiological testingImmunodiagnosticsNanoparticle
Mass cytometry method. In one aspect, the method includes providing a sample having at least one cell type and mixing the sample with material such as nanoparticles functionalized with affinity molecules for the at least one cell type. The sample is transported through a suspended microchannel resonator to record a mass histogram and a cell count for the at least one cell type is determined from the histogram.
Owner:MASSACHUSETTS INST OF TECH
Anti-vibrio parahaemolyticus chicken yolk antibody, preparation method and application thereof
The invention discloses anti-vibrio parahaemolyticus chicken egg yolk antibody and the preparation method. The anti-vibrio parahaemolyticus chicken egg yolk antibody is prepared and obtained through the steps of preparation and inactivation of vibrio parahaemolyticus antigen, immunization of hens, collection of immunized eggs, coarse extraction of anti-vibrio parahaemolyticus chicken egg yolk antibody from the egg yolk liquid of the immunized eggs and purification. The invention also provides the application of the anti-vibrio parahaemolyticus chicken egg yolk antibody in food safety detection reagents and disease immune diagnostic reagents related with the preparation of vibrio parahaemolyticus and medicines and healthy products or feed additives for preventing and curing the related diseases of vibrio parahaemolyticus. The anti-vibrio parahaemolyticus chicken egg yolk antibody (IgY) prepared and provided by the invention has the advantages that the specificity and the purity are high, the anti-vibrio parahaemolyticus chicken egg yolk antibody has good effects when being used for preparing immunology testing reagents and medicines and feed additives for treating the related diseases of vibrio parahaemolyticus, the cost is low, the production volume is high, and the anti-vibrio parahaemolyticus chicken egg yolk antibody is easy to be industrialized.
Owner:SOUTH CHINA AGRI UNIV
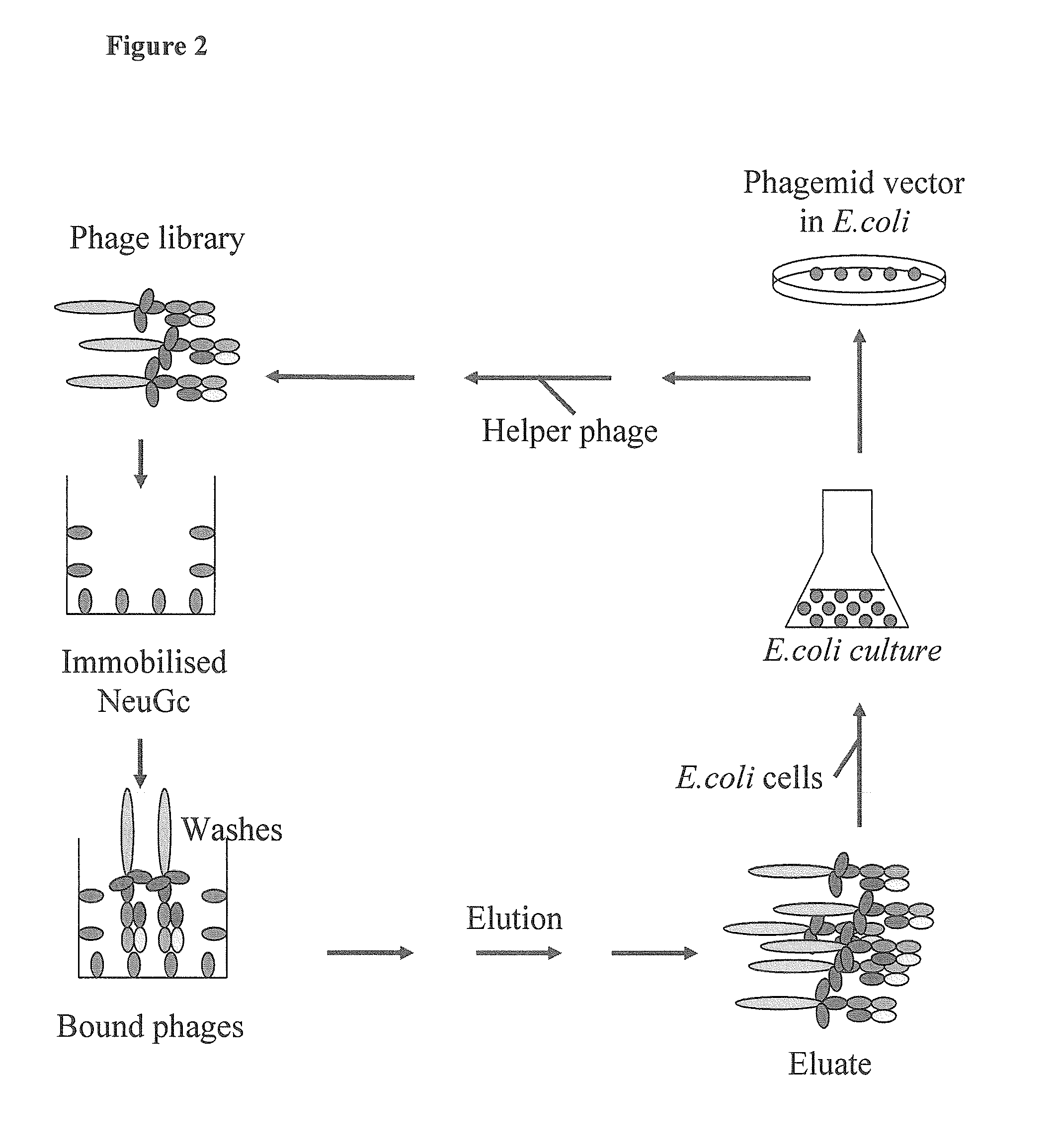
Human monoclonal antibodies directed to sialyl lewis c, sialyl tn and n glycolylneuraminic acid epitopes and a method of analysis of stem cells comprising said epitopes
InactiveUS20100292095A1Eliminate variationSugar derivativesLibrary screeningEpitopeImmunodiagnostics
This invention relates to antibody engineering technology. More particularly, the present invention relates to human IgM antibodies and derivatives thereof, which have novel binding specificity with regard to several oligosaccharide sequences and / or xenoantigenic sialic acid residue. The present invention also relates to processes for making and engineering such novel saccharide and / or NeuGc-binding monoclonal antibodies and to methods for using these antibodies and derivatives thereof in the field of immunodiagnostics, enabling qualitative and quantitative determination of xenoantigenic NeuGc in biological and raw material samples, as well as in immunotherapy, enabling blocking of xenoantigenic NeuGc in patients.
Owner:GLYKOS FINLAND
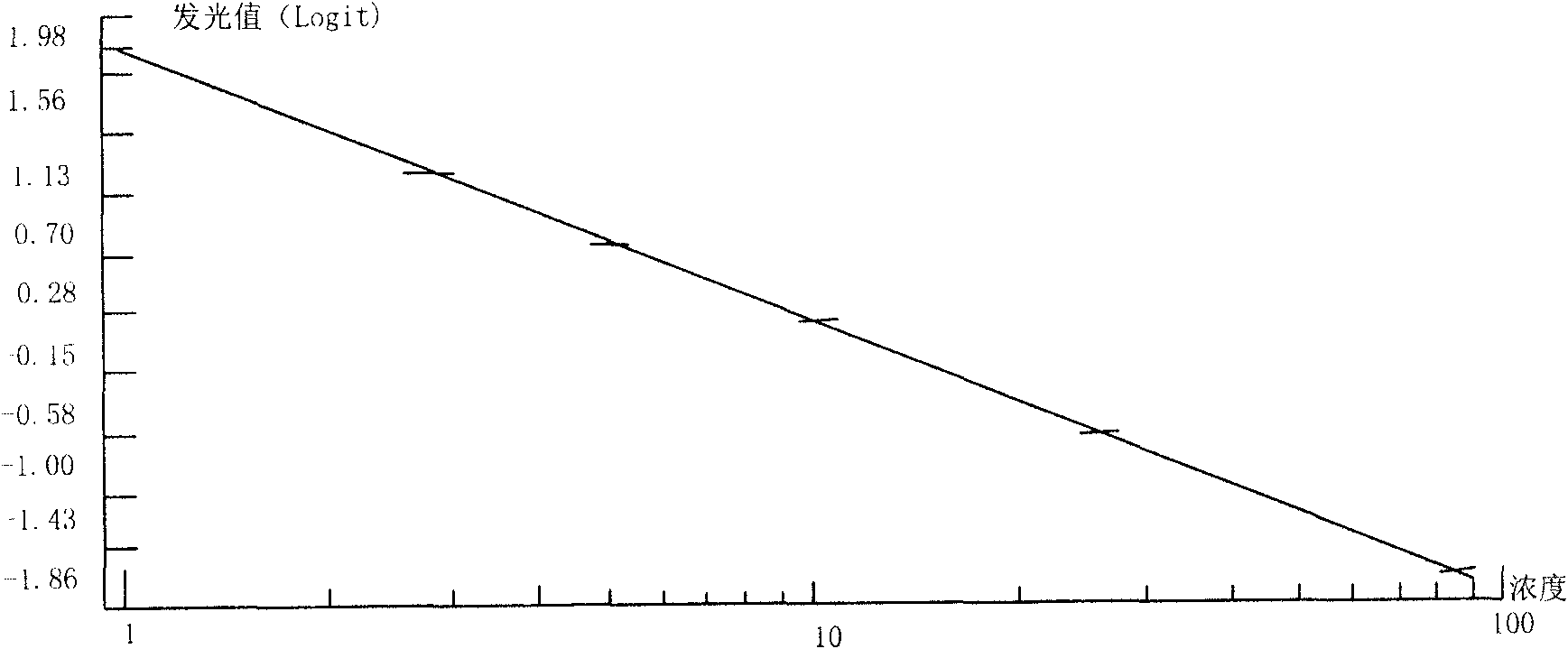
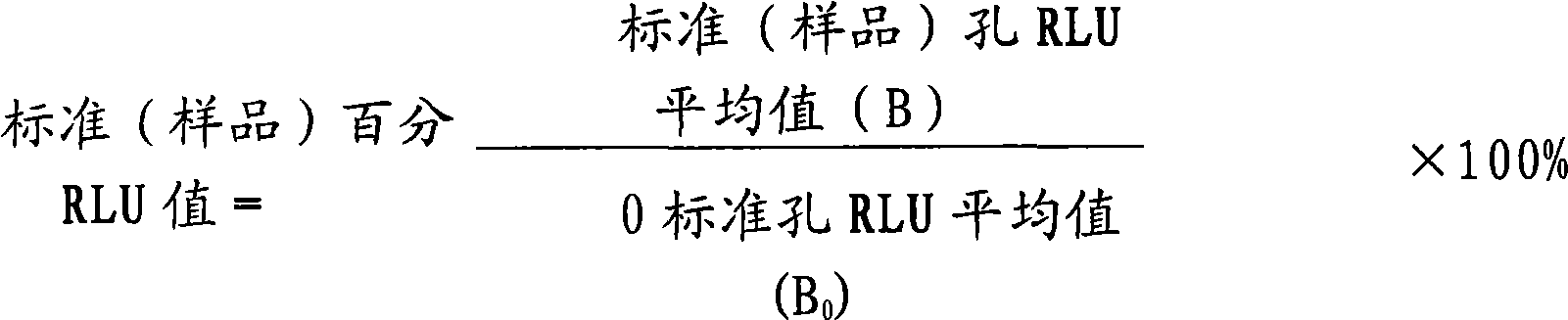
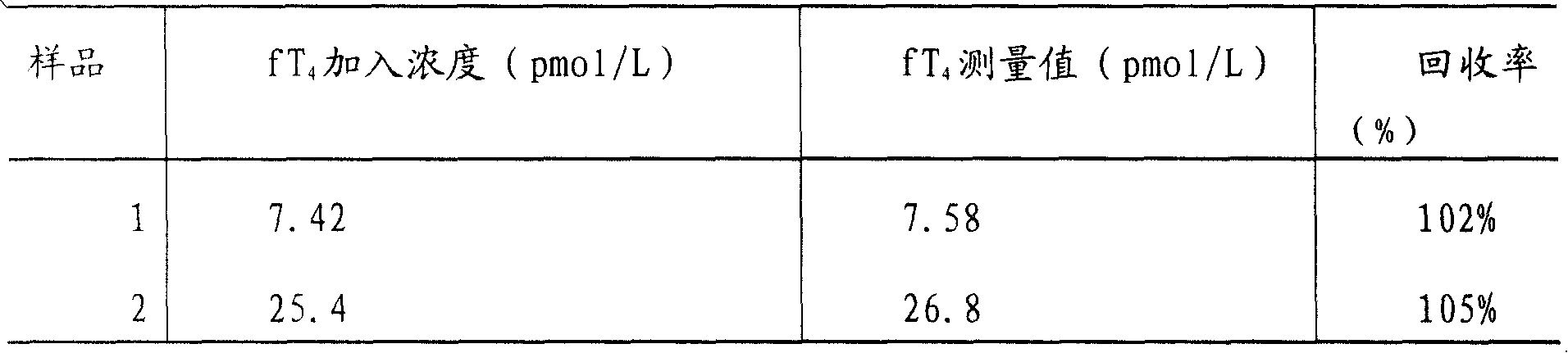
Magnetic particle chemiluminescence detection kit of free thyroxine and application thereof
InactiveCN101551389AReduce manufacturing costIncrease surface areaChemiluminescene/bioluminescenceAntigenMicrosphere
The present invention discloses a quantitative detection kit of free thyroxine (fT4) and a method for detecting the free thyroxine with the detection kit. The detection kit comprises a free thyroxine standard, a free thyroxine antigen marked by horseradish peroxidase, an antibody, a magnetic bead separating agent, luminescence substrate reagent and wash liquid. The method for detecting free thyroxine comprises the following steps: adding the free thyroxine standard and sample to be measured into the test tube, adding the free thyroxine antigen marked by horseradish peroxidase and antibody, after mixing to uniform, incubating for one hour in 37 DEG C, then adding magnetic bead separating agent for stewing for 5 minutes, separating for obtaining the immune magnetic microspheres, discarding the supernate, washing the deposit for two times; adding the luminescence substrate reagent, measuring and analyzing the concentration of free thyroxine in sample to be measured. The quantitative detection kit of free thyroxine and the detection method provided by the invention have the advantages of higher stability, higher sensitivity and higher accuracy, and belong to the technical field of immunodiagnosis.
Owner:天津九鼎医学生物工程有限公司 +1
Echinococcus granulosusglutathione transferase gene and application thereof
The invention belongs to the field of bioengineering and provides an Echinococcus granulosusglutathione transferase gene a base sequence of which is shown in SEQ ID NO: 1. The invention also provides a protein encoded by the Echinococcus granulosusglutathione transferase gene. The amino acid sequence of the protein encoded by the Echinococcus granulosusglutathione transferase gene is shown in SEQ ID NO: 2, or is the same as 1st-219th amino acid sequences as shown in SEQ ID NO: 3. The recombinant protein can be used for immunodiagnosis of cystic echinococcosis patients and can be used for coating a plate; and the serums of the patients having cystic echinococcosis, alveolitoid echinococcosis, cysticercosis and other parasite diseases and healthy people are detected by using an enzyme-linked immunosorbent assay (ELISA), and the obtained sensitivity and specificity are respectively 72.41% and 92.36%.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Recombinant constructs of Borrelia burgdorferi
InactiveUS20070020286A1Improve overall utilizationAvoid infectionBacteriaAntibody mimetics/scaffoldsImmunodiagnosticsBorreliella burgdorferi
Novel chimeric nucleic acids, encoding chimeric Borrelia proteins comprising OspC or an antigenic fragment thereof and OspA or an antigenic fragment thereof, are disclosed. Chimeric proteins encoded by the nucleic acid sequences are also disclosed. The chimeric proteins are useful as vaccine immunogens against Lyme borreliosis, as well as for immunodiagnostic reagents.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
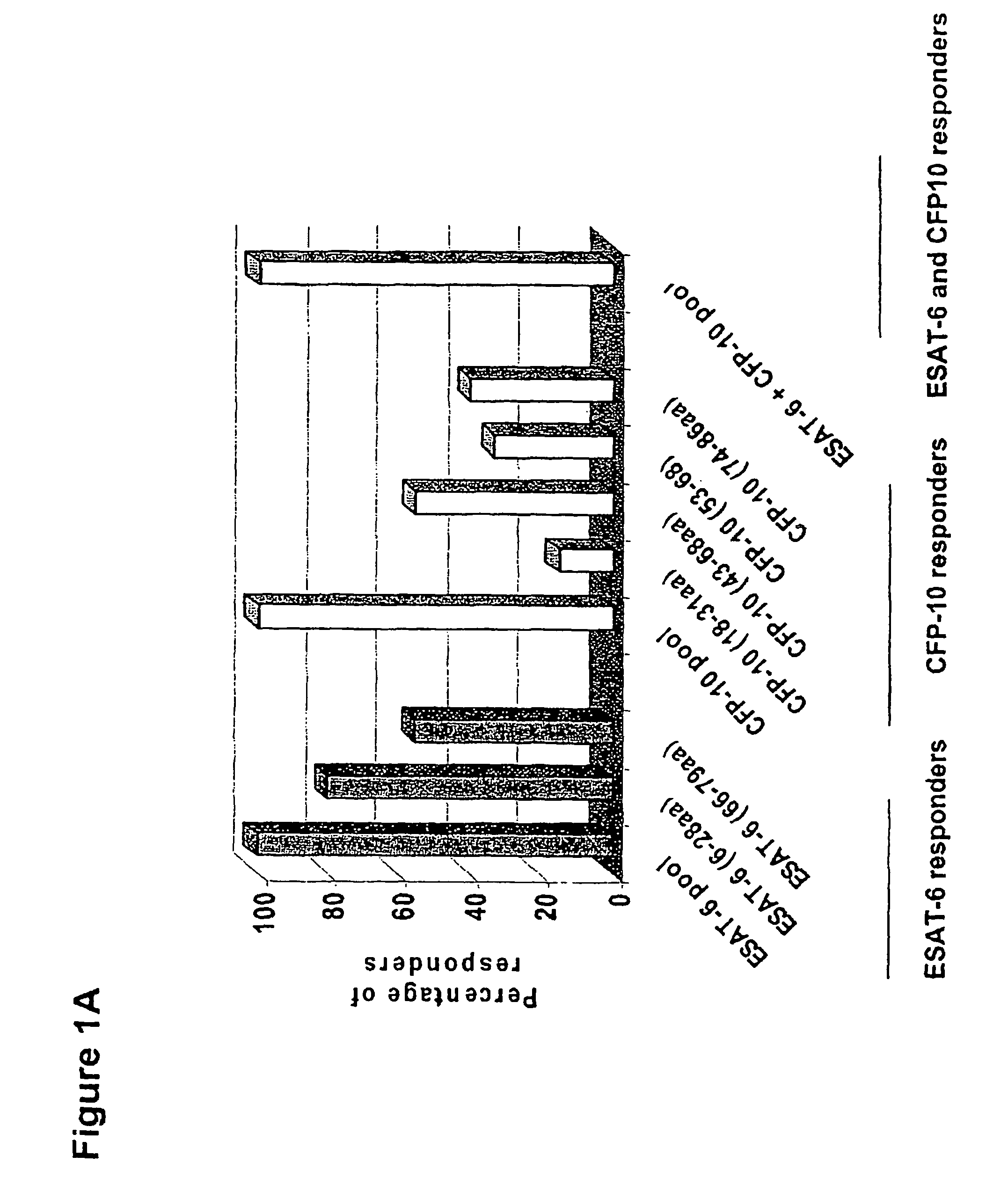
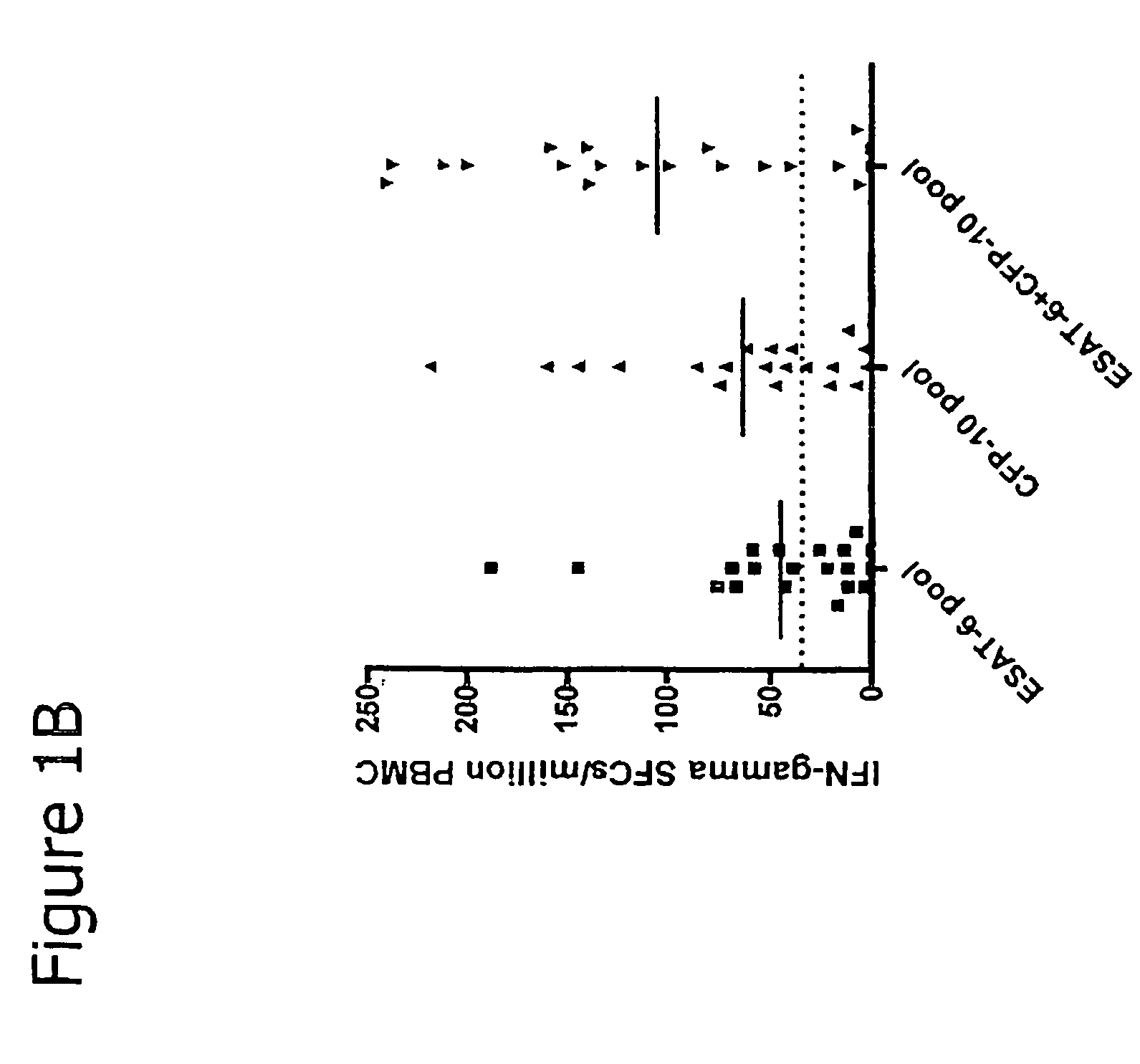
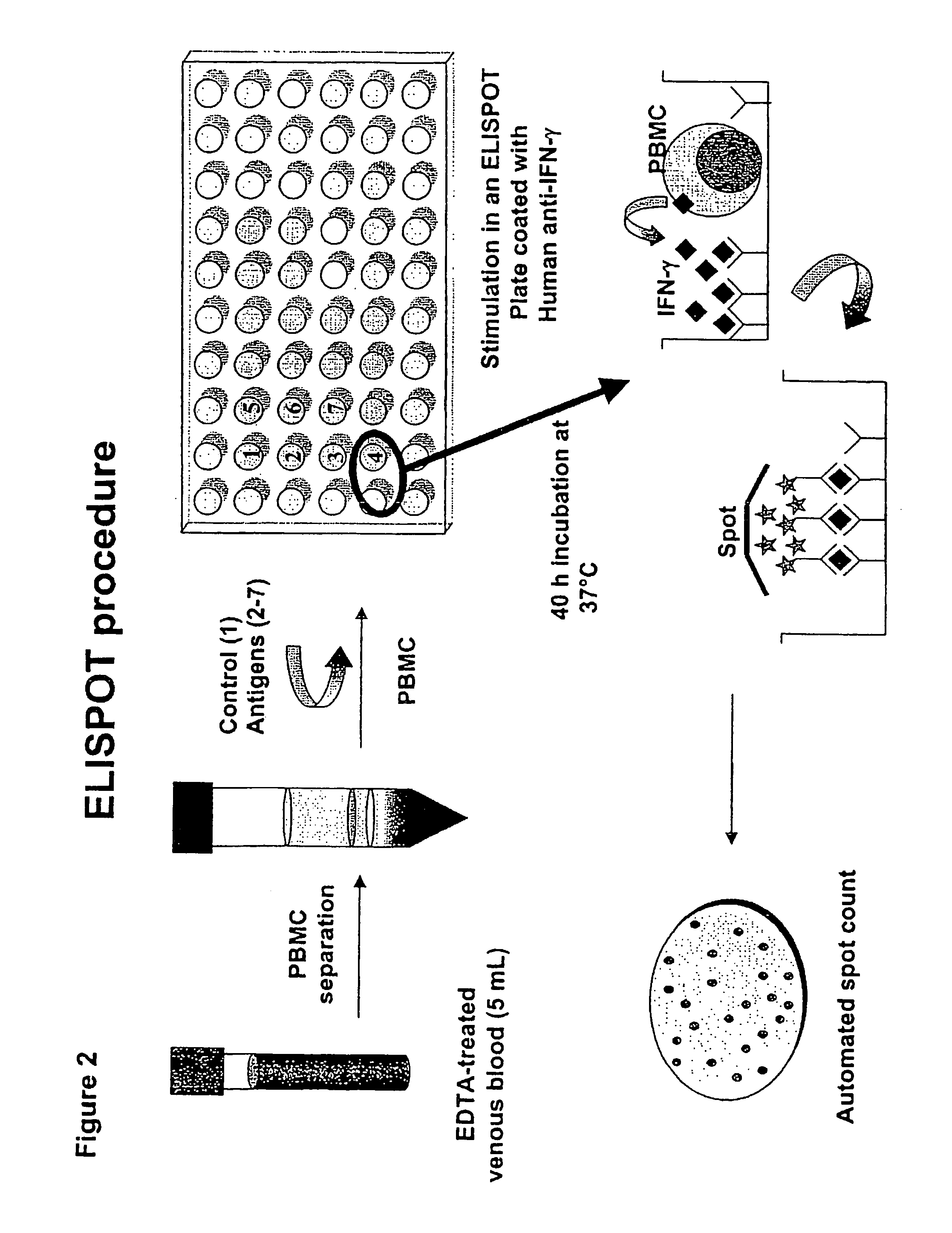
Immune diagnostic assay to diagnose and monitor tuberculosis infection
InactiveUS7785607B2High detection sensitivityHigh sensitivityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseT lymphocyte
The present invention relates to a method of diagnosing and monitoring various distinct presentations of tuberculosis: active tuberculosis disease, latent tuberculosis infection and recent tuberculosis infection. The rapid immune assay is based on the evaluation of the frequency of Interferon (IFN) gamma-producing antigen-specific T lymphocytes responding to selected peptide sequences from Mycobacterium tuberculosis, selected for their immunogenicity. The invention concerns also immunogenic and vaccine compositions based on these specific peptide sequences.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
Composite nano colloidal gold chitosan immune carrier and preparation method thereof
The invention provides a composite nano colloidal gold chitosan immune carrier, wherein, colloidal gold is uniformly dispersed in chitosan. The invention also provides a preparation method of the composite nano colloidal gold chitosan immune carrier and a labeled antigen-antibody. According to the invention, the characteristic that chitosan has good affinity for the antigen and antibody is applied in the colloidal gold, thus the adsorption capability of the colloidal gold for the antigen-antibody is increased, and the amount of the colloidal gold is reduced. The composite nano colloidal gold chitosan immune carrier can be widely used for immunodetection or immunodiagnosis in the fields of biomedicine or agriculture and the like.
Owner:JIANGSU YITONG BIOTECHNOLOGY CO LTD
Human and animal brucella antibody immunochromatography test paper and preparation method thereof
The invention relates to the field of zoonosis immune diagnosis and discloses a brucellosis antibody detection immunochromatography test strip based on colloidal gold as a marking material and a preparation method of the test strip. In a quick brucellosis antibody detection technology, the 40-nm colloidal gold labeled with staphylococcus aureus protein A (SPA) is sprayed on glass fibers to form a gold labeled pad. Genes OMP31 and BP26 are cloned from a brucella genome, form prokaryotic expression recombinant plasmids and are transformed in escherichia coli to express proteins omp31 and bp26, the two proteins as coating antigens are respectively coated on a nitrocellulose membrane to serve as detection lines, and the detection lines, the gold labeled pad, a specially treated sample loading pad and water absorption paper are assembled into an immunochromatography detection device. The test strip has the characteristics of strong specificity, high sensitivity, convenience, simplicity, economy and the like, can be applied to typing detection of brucellosis antibodies of sheep and cattle, and has very important meaning and practical application value for brucellosis monitoring and prevalence control.
Owner:SHIHEZI UNIVERSITY
Flow cytometry methods and immunodiagnostics with mass sensitive readout
ActiveUS8722419B2Packed tightlyImprove throughputBioreactor/fermenter combinationsBiological substance pretreatmentsImmunodiagnosticsNanoparticle
Mass cytometry method. In one aspect, the method includes providing a sample having at least one cell type and mixing the sample with material such as nanoparticles functionalized with affinity molecules for the at least one cell type. The sample is transported through a suspended microchannel resonator to record a mass histogram and a cell count for the at least one cell type is determined from the histogram.
Owner:MASSACHUSETTS INST OF TECH
Vmp-like sequences of pathogenic borrelia species and strains
The present invention relates to DNA sequences encoding Vmp-like polypeptides of pathogenic Borrelia, the use of the DNA sequences in recombinant vectors to express polypeptides, the encoded amino acid sequences, application of the DNA and amino acid sequences to the production of polypeptides as antigens for immunoprophylaxis, immunotherapy, and immunodiagnosis. Also disclosed are the use of the nucleic acid sequences as probes or primers for the detection of organisms causing Lyme disease, relapsing fever, or related disorders, and kits designed to facilitate methods of using the described polypeptides, DNA segments and antibodies.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
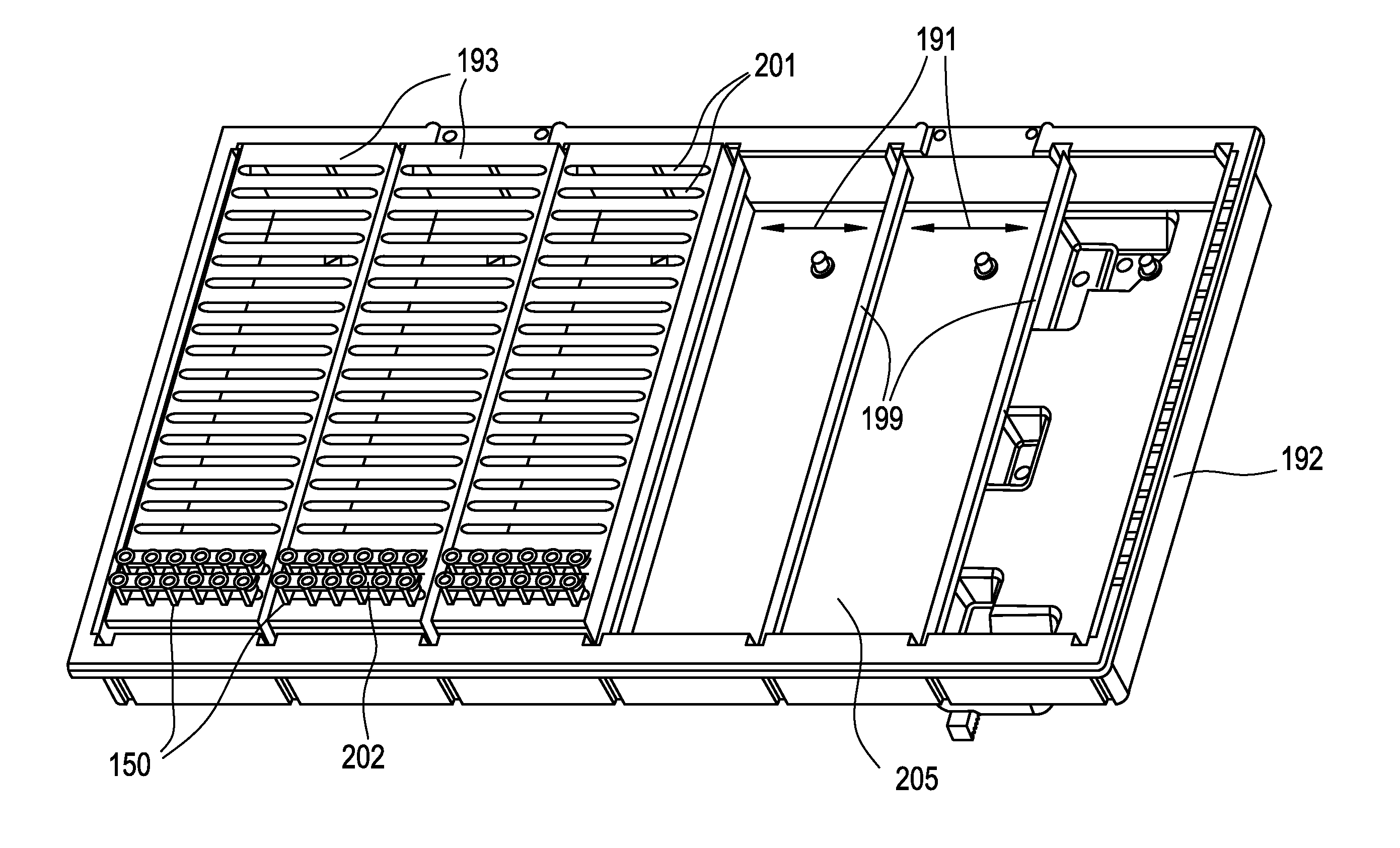
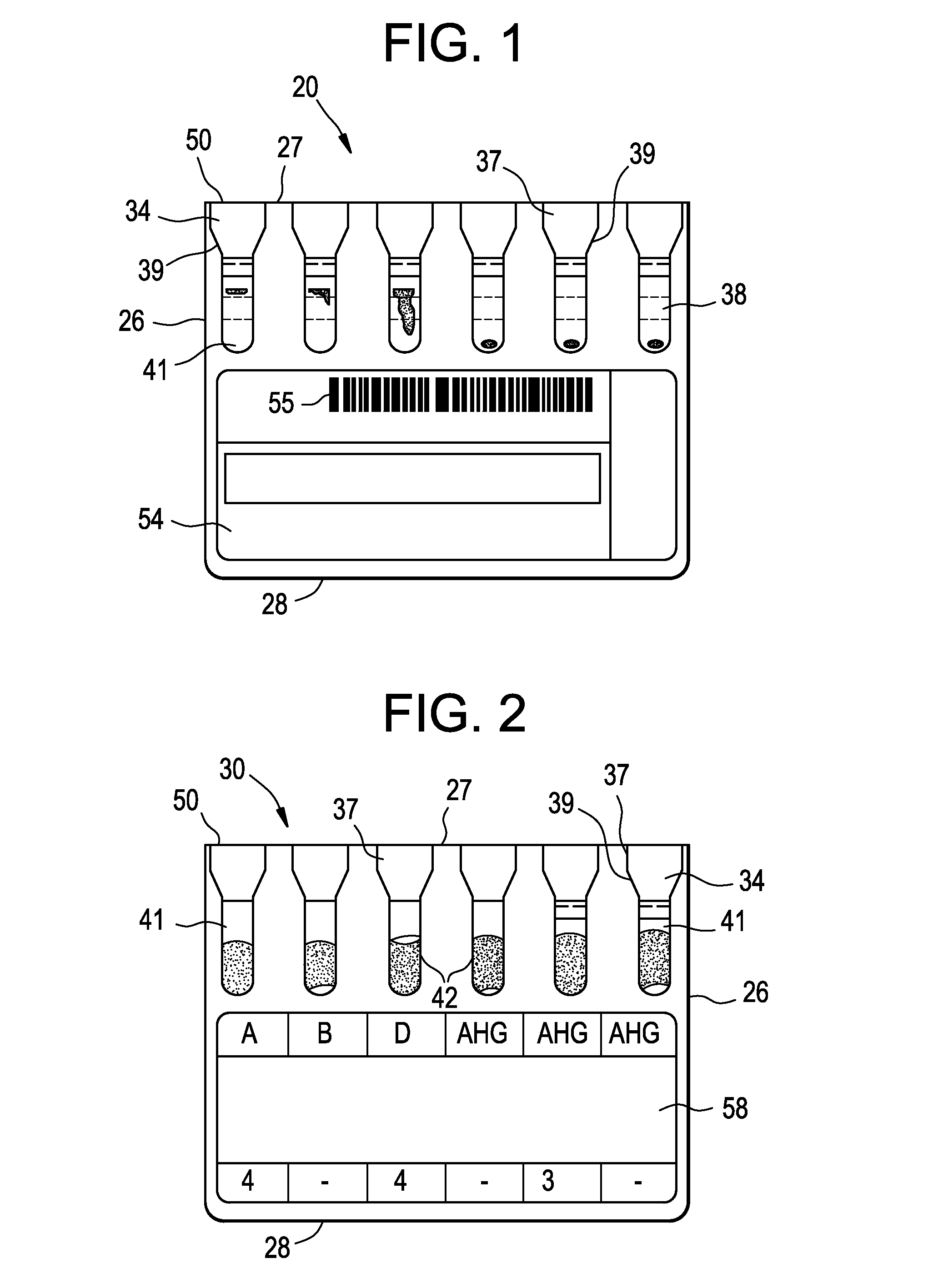
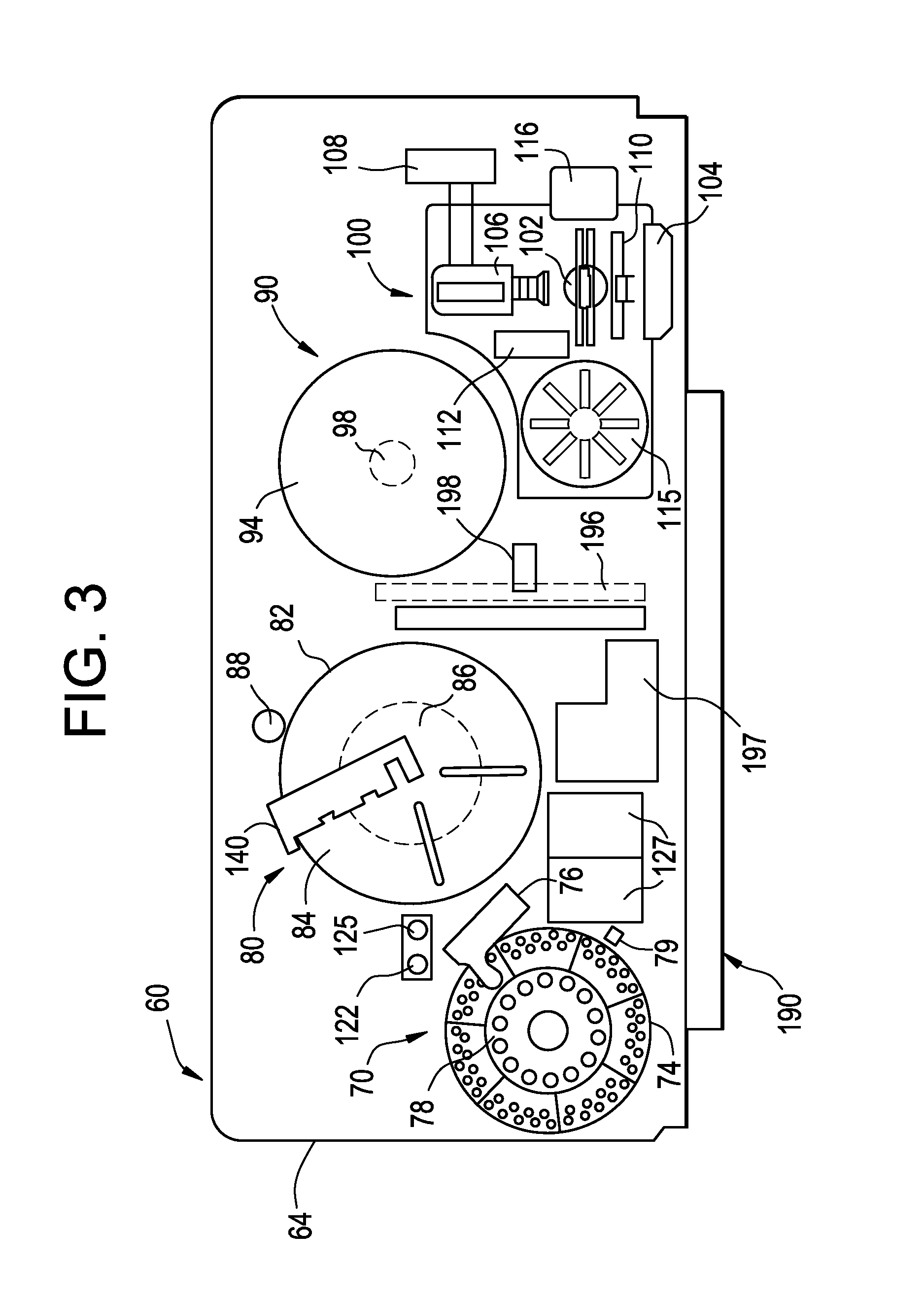
Method for holding multiple types of diagnostic test consumables in a random access single container
ActiveUS20140163920A1Test may changeConsiderable timeMeasurement arrangements for variableDigital computer detailsImmunodiagnosticsDiagnostic test
An immunodiagnostic test method includes holding a selection of immunological test elements or consumables in one or more containers attached to or positioned in the analyzer and providing random access to any test element therein. The container can hold multiple types of test elements in compartments or slots. Through sensing of a test element position in its slot, the detection mechanism of the invention provides for random access to multiple types of test elements in any sleeve and within a single sleeve, and provides efficient inventory control. The method increases the number of test element types that may be loaded onto an analyzer and maintains fast determination of inventory.
Owner:ORTHO-CLINICAL DIAGNOSTICS
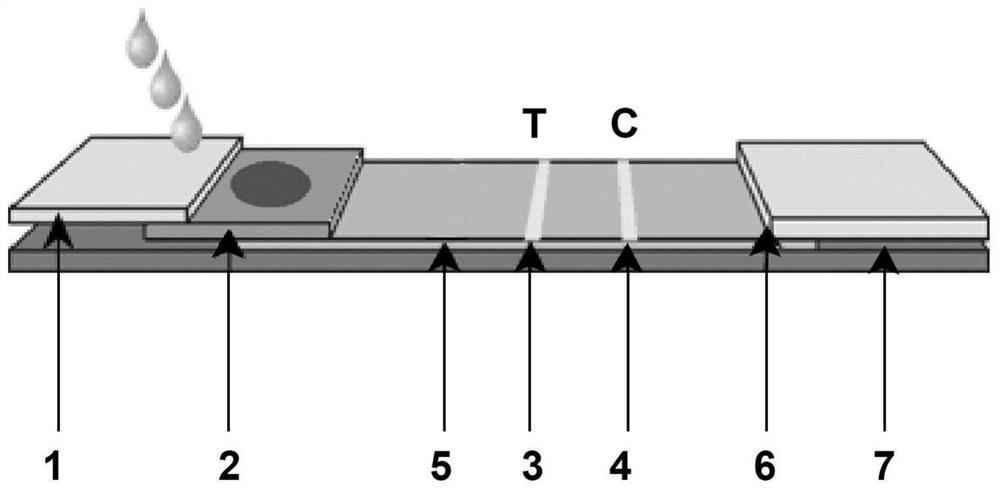
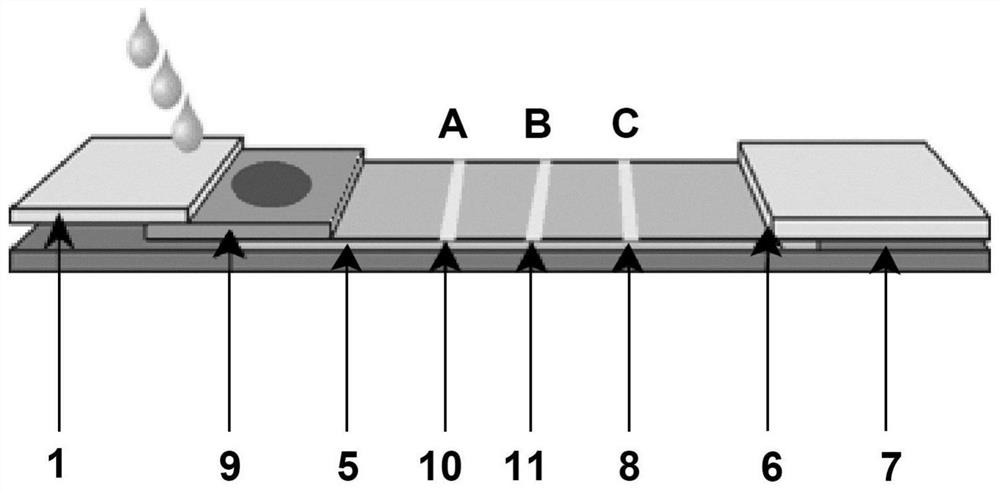
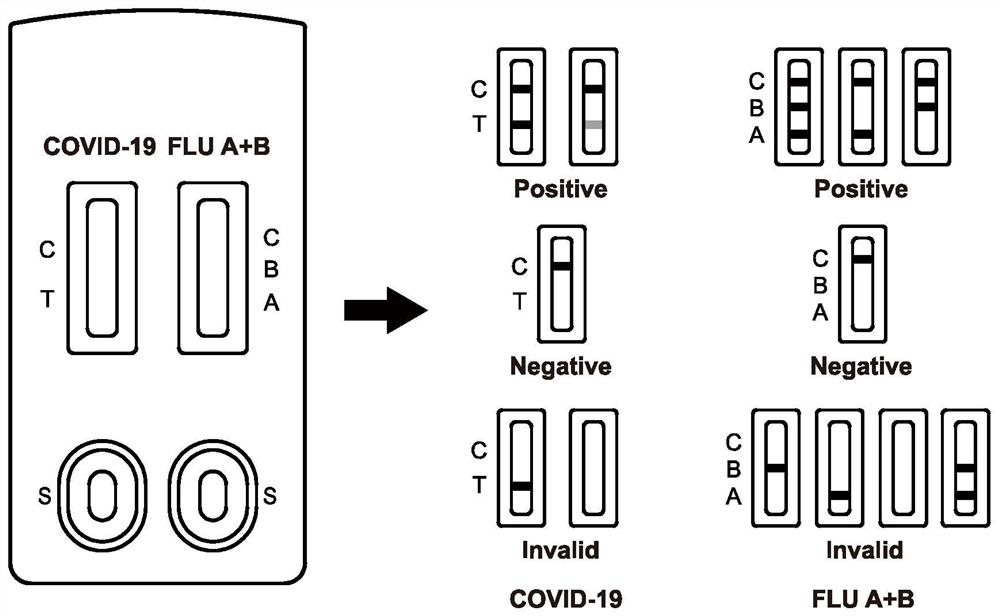
Immunochromatographic test paper for rapid combined diagnosis of neocoronavirus, influenza A and influenza B and preparation method thereof
PendingCN112129935AShort detection timeMeet the needs of on-site testingMaterial analysis by observing effect on chemical indicatorBiological testingNitrocelluloseMurine antibody
The invention relates to the field of immunodiagnosis, and provides immunochromatographic test paper for rapid combined diagnosis of neocoronavirus, influenza A and influenza B aiming at the problemsof long time consumption and single project of neocoronavirus detection. The immunochromatographic test paper comprises neocoronavirus test paper and influenza A / influenza B test paper, wherein each of the neocoronavirus test paper and the influenza A / influenza B test paper comprises a PVC bottom plate, a modified nitrocellulose membrane, a water absorption pad and a sample pad; the neocoronavirustest paper comprises a latex labeled treatment pad; a detection line T coated with an anti-neocoronavirus protein monoclonal antibody and a quality control line C coated with a streptavidin conjugateare arranged on the modified nitrocellulose membrane; a treatment pad of the influenza A / influenza B test paper is a gold-labeled probe treatment pad; and a detection line A coated with an anti-influenza A virus monoclonal antibody, a detection line B coated with an anti-influenza B virus monoclonal antibody and a quality control line C2 coated with a goat anti-mouse IgG antibody are arranged onthe modified nitrocellulose membrane. The detection efficiency of detecting antigens by using the antibodies is high. The invention also provides a preparation method of the test paper.
Owner:HANGZHOU ALLTEST BIOTECH
Anti-cryptococcal capsular polysaccharide monoclonal antibody and preparation and application of hybridoma cell strain thereof
ActiveCN109879961ANo cross reactionHigh affinityTissue cultureImmunoglobulins against fungi/algae/lichensColloidCell strain
The invention relates to an anti-cryptococcal capsular polysaccharide hybridoma cell strain and an anti-cryptococcal capsular polysaccharide monoclonal antibody secreted thereof. The antibody can be specifically bound to cryptococcal capsular polysaccharide and can be used for in vitro detection of cryptococcal infection. The antibody titer is up to more than one million, and the antibody has excellent affinity and good specific binding capability. The detection limit of a colloidal gold-labeled immunodiagnostic reagent developed with the antibody as the raw material is 0.5 ng / ml, and the antibody has excellent performance in sensitivity, specificity, stability and all other aspects.
Owner:GENOBIO PHARM CO LTD
Immunodiagnostic assays using reducing agents
InactiveUS20060263854A1Prevent and treat HCV infectionBioreactor/fermenter combinationsFungiCell lysatesCell
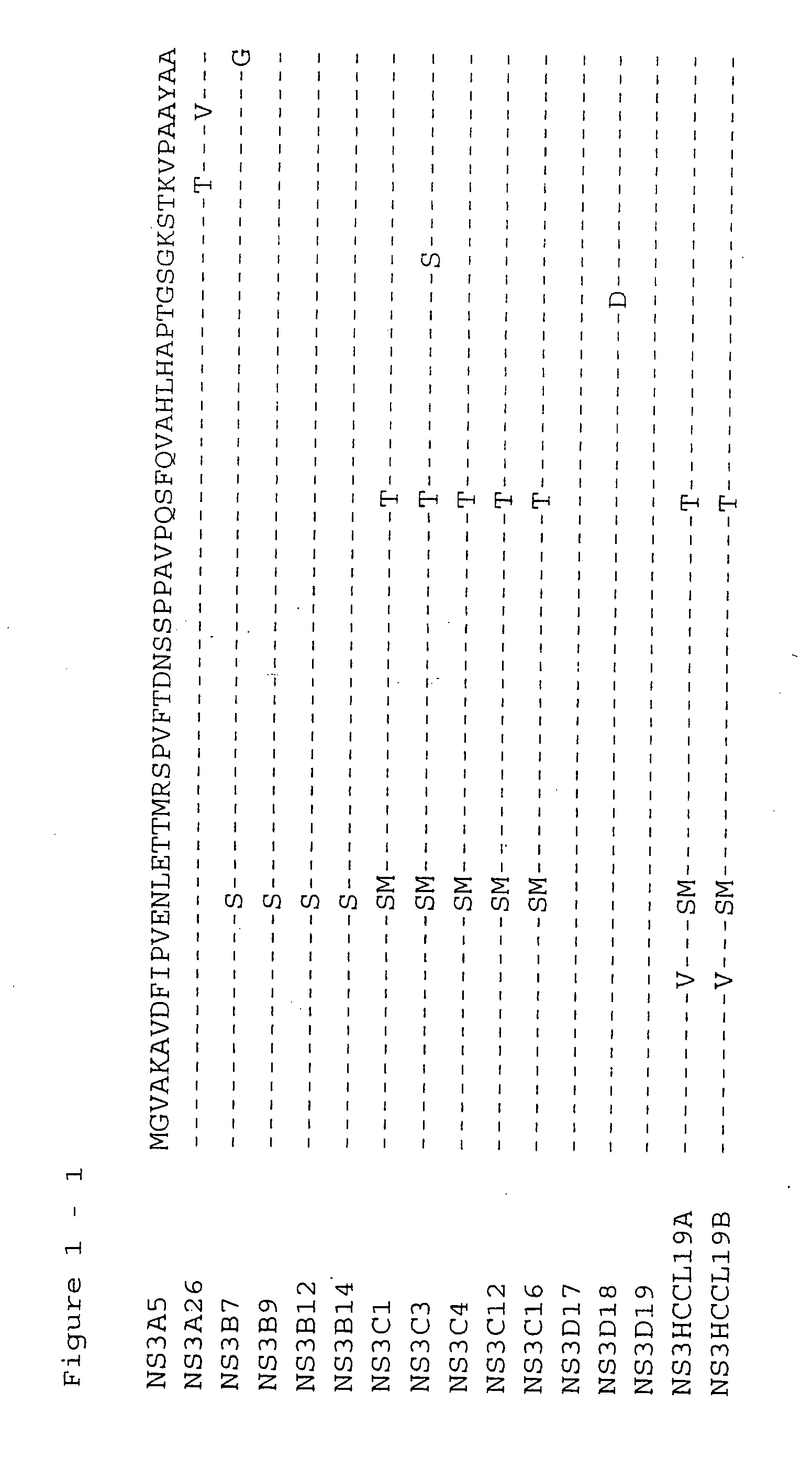
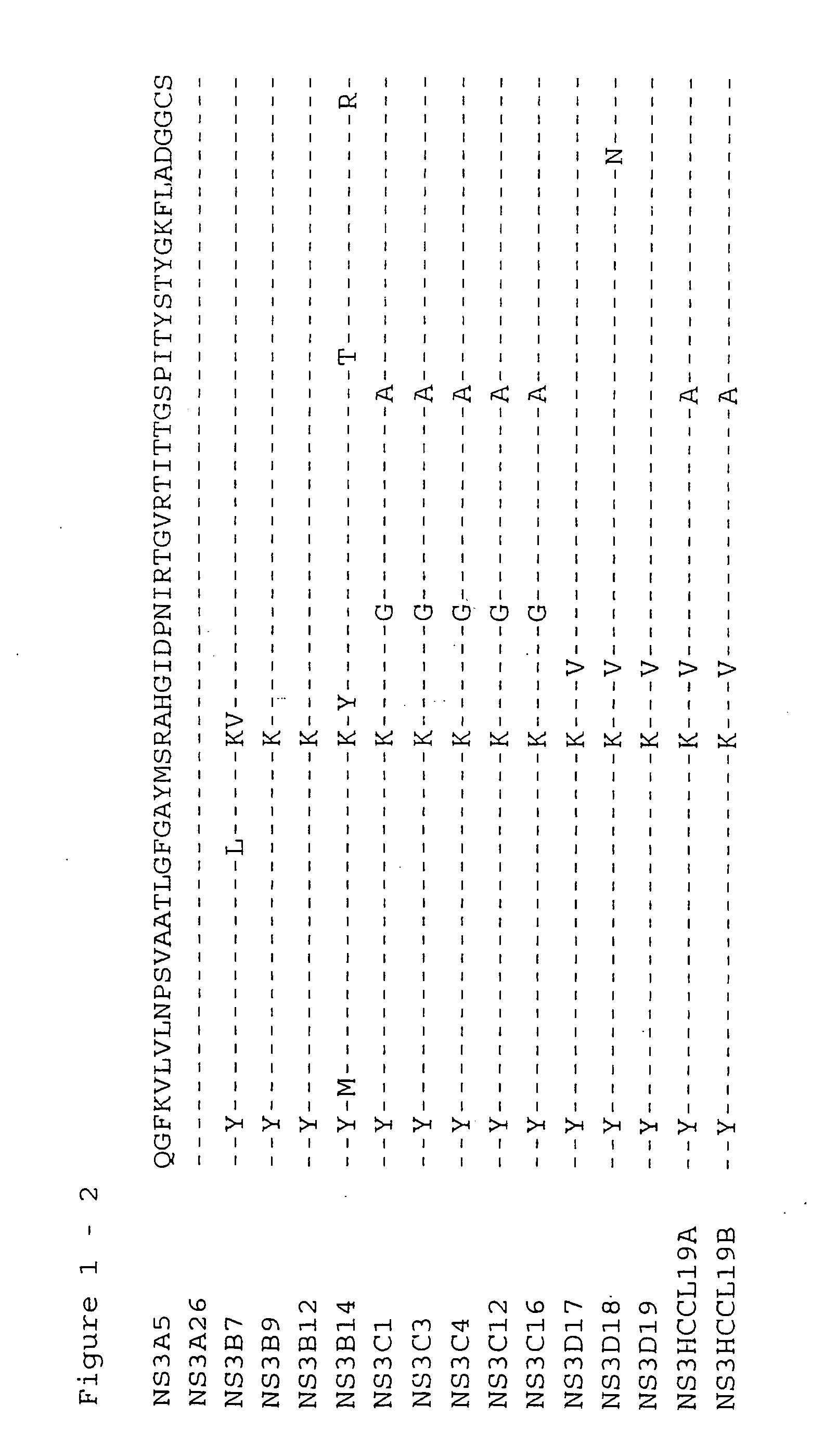
The present invention relates to a solid phase immunoassay comprising on said solid phase an antigen in the presence of a reducing agent. The present invention also relates to a method for purifying a cysteine containing recombinantly expressed protein comprising at least 2, preferably 3 or 4 and even more preferably all of the following steps: (a) sulphonation of a lysate from recombinant host cells or lysis of recombinant host cells in the presence of guanidinium chloride followed by a subsequent sulphonation of the cell lysate, (b) treatment with a zwitterionic detergent, preferably after removal of the cell debris, (c) purification of the sulphonated version of the recombinant protein or purification of the sulphonated version of the recombinant protein with subsequent removal of the zwitterionic detergent, with said purification being preferably chromatography, more preferably a Ni-IMAC chromatography with said recombinant protein being a His-tagged recombinant protein, (d) desulphonation of the sulphonated version of the recombinant protein, preferably with a molar excess of DTT, (e) storage in the presence of a molar excess of DTT. The present invention also relates to novel HCV NS3 sequences as depicted in FIGS. 1-8.
Owner:INNOGENETICS NV
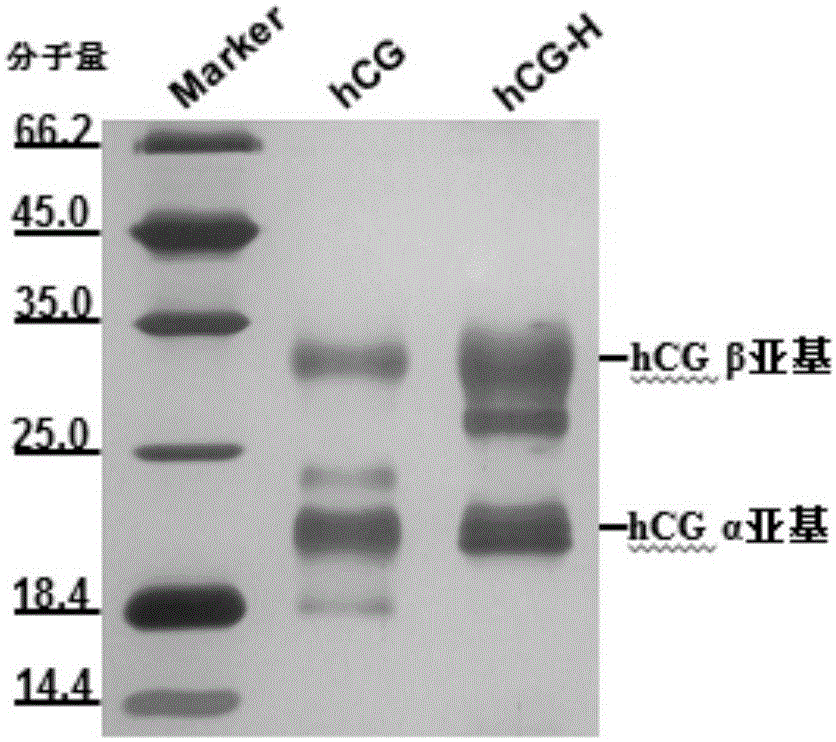
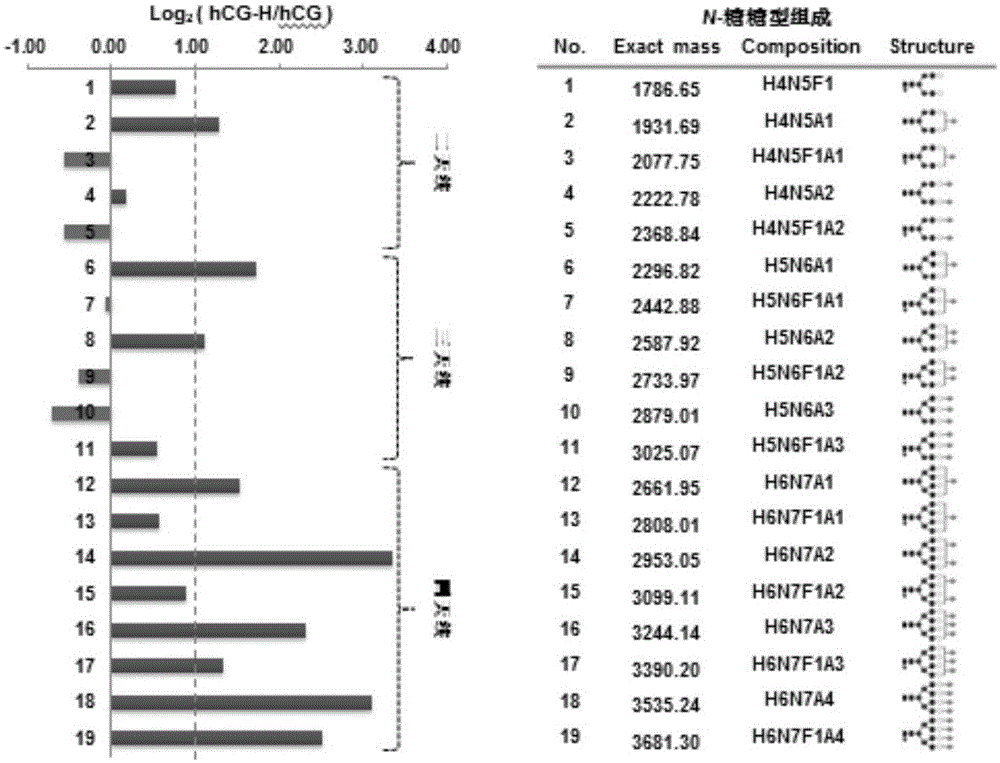
Detection kit for hyperglycosylated modification of hCG (human chorionic gonadotropin) tumor marker
InactiveCN105158485AHigh sensitivityImprove featuresDisease diagnosisBiological testingTumor BiomarkersImmunodiagnostics
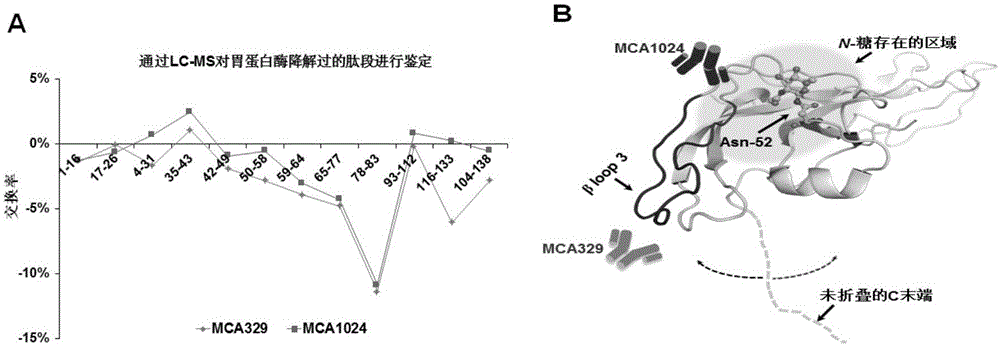
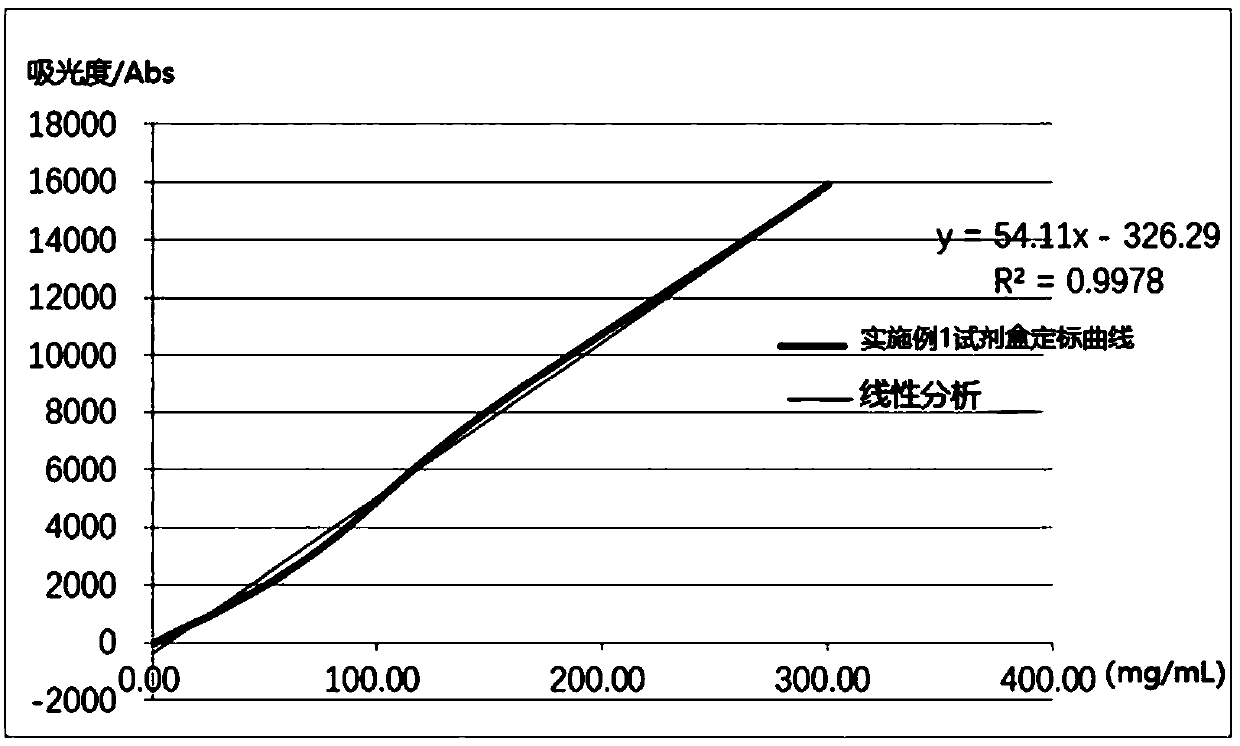
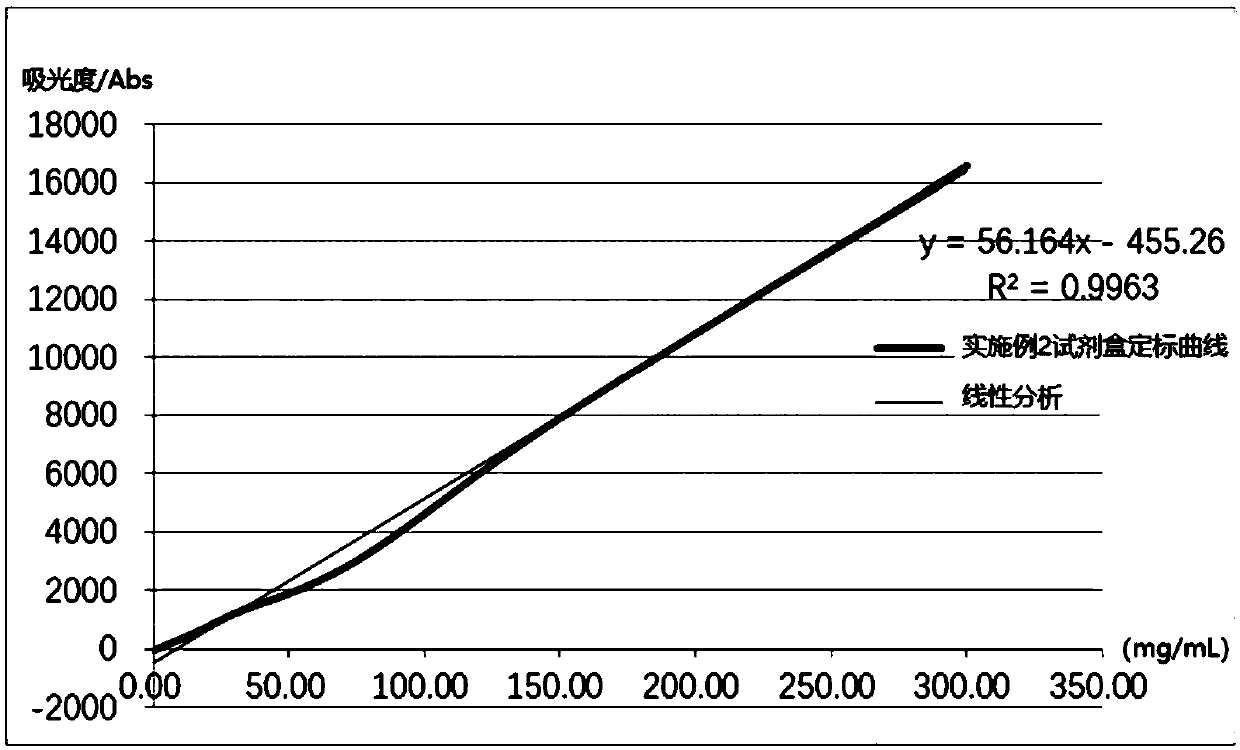
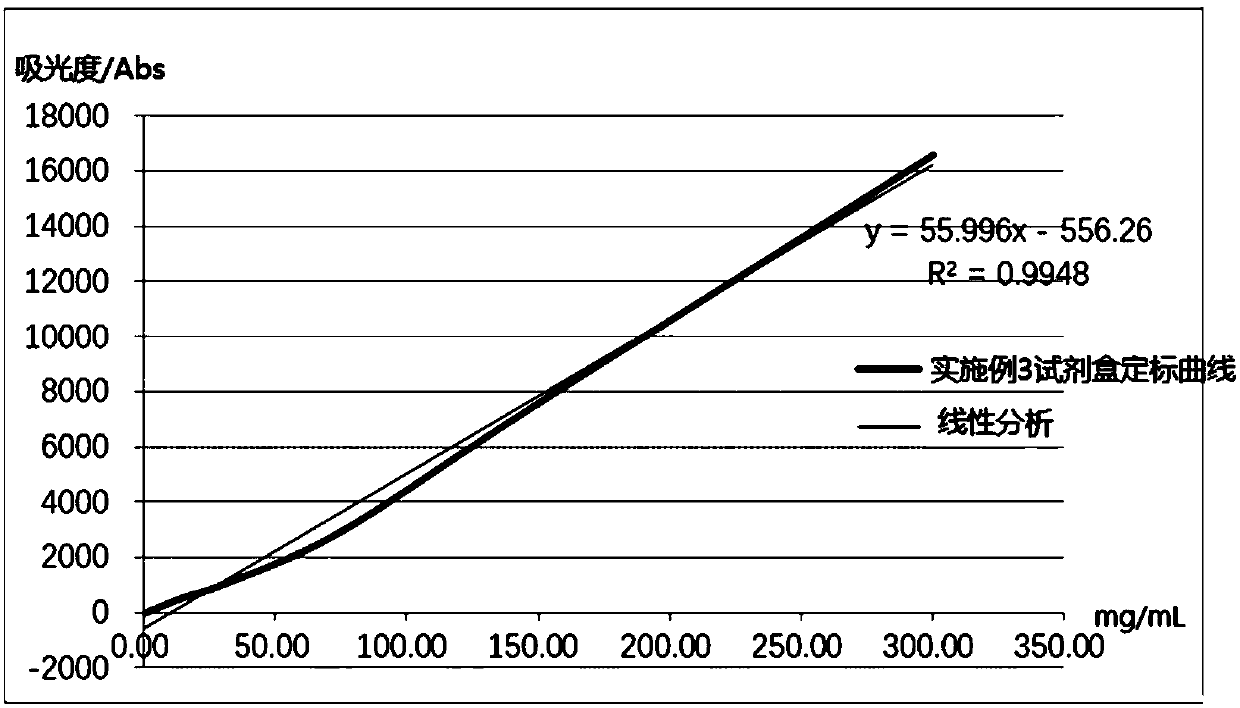
The invention relates to a detection kit for hyperglycosylated modification of an hCG (human chorionic gonadotropin) tumor marker. According to the detection kit, magnetic beads are combined with an antibody (MCA-A) to serve as a capture antibody to capture total hCG in a to-be-detected sample, wherein the affinity of the antibody (MCA-A) is least influenced by glycosylation change; an antibody (MCA-B) most influenced by glycosylation change and another antibody (MCA-C) less influenced by glycosylation are used for detecting the total protein content and the N-glycosylation modification degree of hCG in the sample simultaneously, so that a tumor biomarker is identified. The detection kit improves the accuracy, the sensitivity and the specificity of an immunodiagnosis method greatly and has great practical value for detection of hyperglycosylated modification of the tumor marker in the actual sample.
Owner:SHANDONG UNIV
Human alpha1-microglobulin (MG) determination kit with high sensitivity and wide detection range
InactiveCN109633166AWide detection rangeGuaranteed SensitivityBiological testingRenal tubular reabsorptionSingle sample
The embodiment of the invention relates to the field of immunodiagnosis, in particular to a human alpha1-microglobulin (MG) determination kit with high sensitivity and a wide detection range. The human alpha1-MG determination kit provided by the embodiment of the invention comprises a reagent 1, a reagent 2 and human alpha1-MG calibration products of different gradient concentrations, wherein thereagent 2 comprises carboxylated latex microspheres labeled with a human alpha1-MG monoclonal antibody only and carboxylated latex microspheres labeled with a human alpha1-MG polyclonal antibody only;and the average particle size of the carboxylated latex microspheres labeled with the human alpha 1-MG monoclonal antibody only is larger than that of the carboxylated latex microspheres labeled withthe human alpha 1-MG polyclonal antibody. The human alpha1-MG determination kit provided by the invention can complete the detection of a single sample within 10 minutes with excellent precision, accuracy and anti-interference performance, can well reflect the renal tubular reabsorption function and glomerular filtration function, is very suitable for clinical biochemical analyzers, and greatly facilitates clinical examination.
Owner:BEIJING BEIER BIOENG
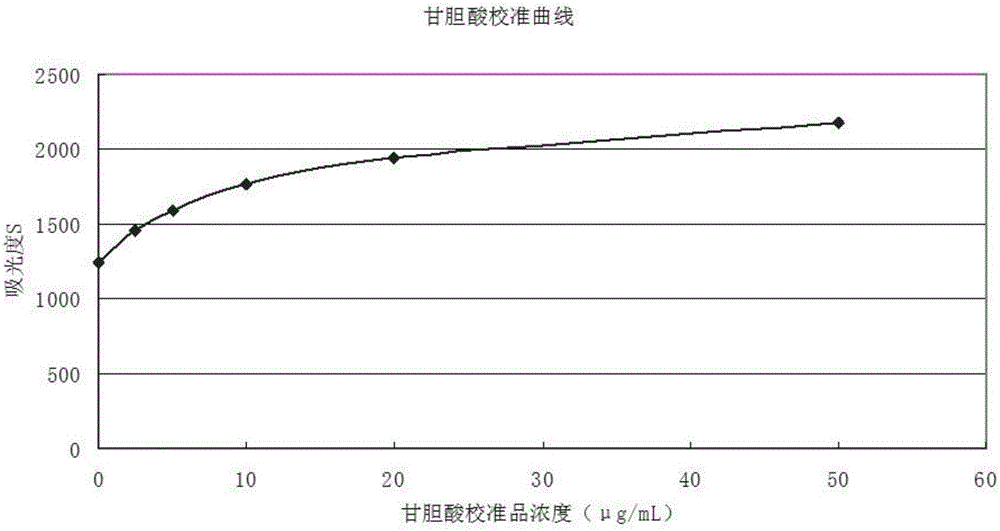
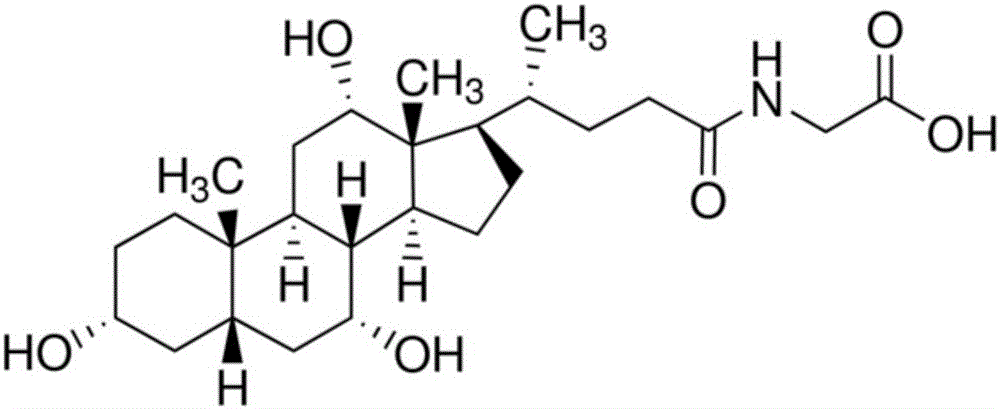
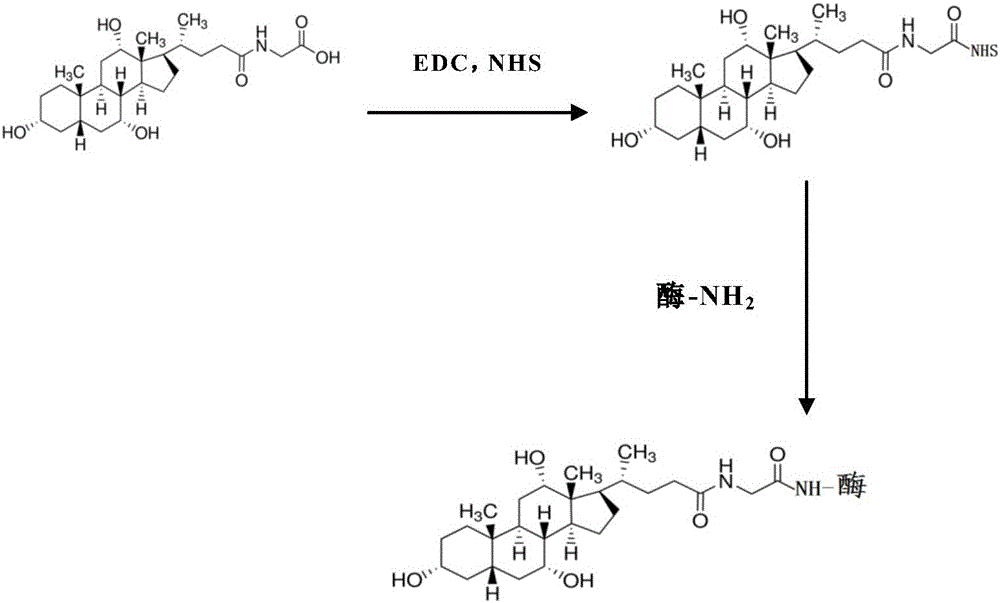
Preparation method for homogeneous enzyme immunodiagnosis reagent used for glycocholic acid
InactiveCN106405069AEasy to operateReliable responseColor/spectral properties measurementsBiological activationGlucose phosphate
The invention relates to a preparation method for a homogeneous enzyme immunodiagnosis reagent used for glycocholic acid, belonging to the technical field of biological medicine. The preparation method comprises the following steps: preparation of a glycocholic acid antibody solution; preparation of a glycocholic acid-enzyme conjugate solution; and preparation of a glycocholic acid calibrating substance. The glycocholic acid-enzyme conjugate solution is prepared by adding glycocholic acid into a MES buffer solution, adding a carboxyl activator for carboxyl activation, then adding 6-phosphogluconate dehydrogenase for a condensation reaction so as to obtain a crude glycocholic acid-enzyme conjugate product, carrying out dialysis and purification, adding the treated crude glycocholic acid-enzyme conjugate product into a Tris-HCl buffer solution, adding an auxiliary reagent and carrying out uniform mixing so as to obtain the glycocholic acid-enzyme conjugate solution. The homogeneous enzyme immunodiagnosis reagent for glycocholic acid prepared by using the method is safe, rapid, highly efficient and sensitive and can accurately detect the content of glycocholic acid in a to-be-detected sample.
Owner:李松羊
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com