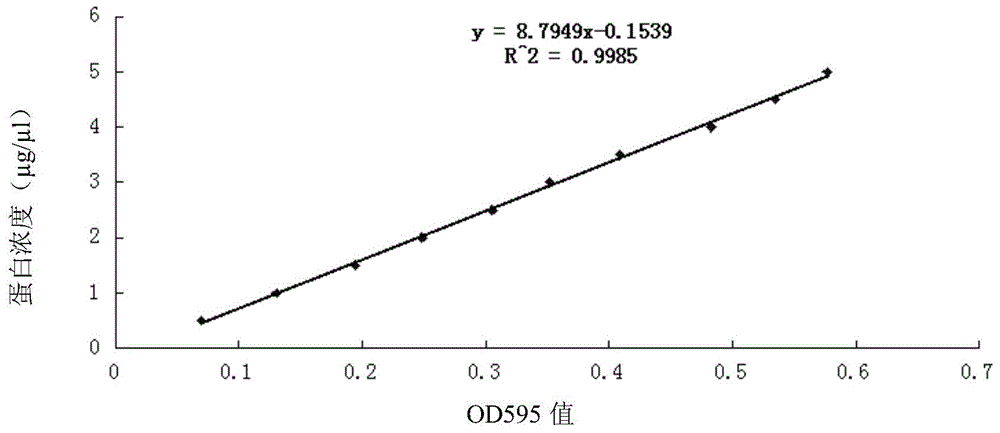
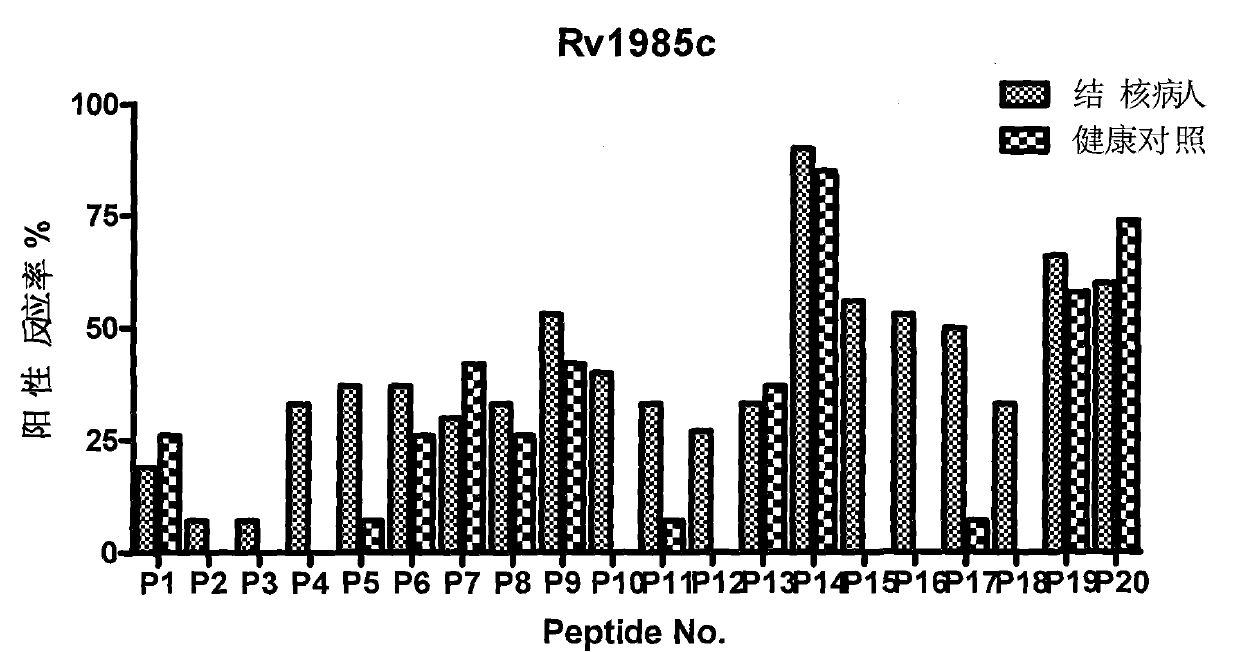
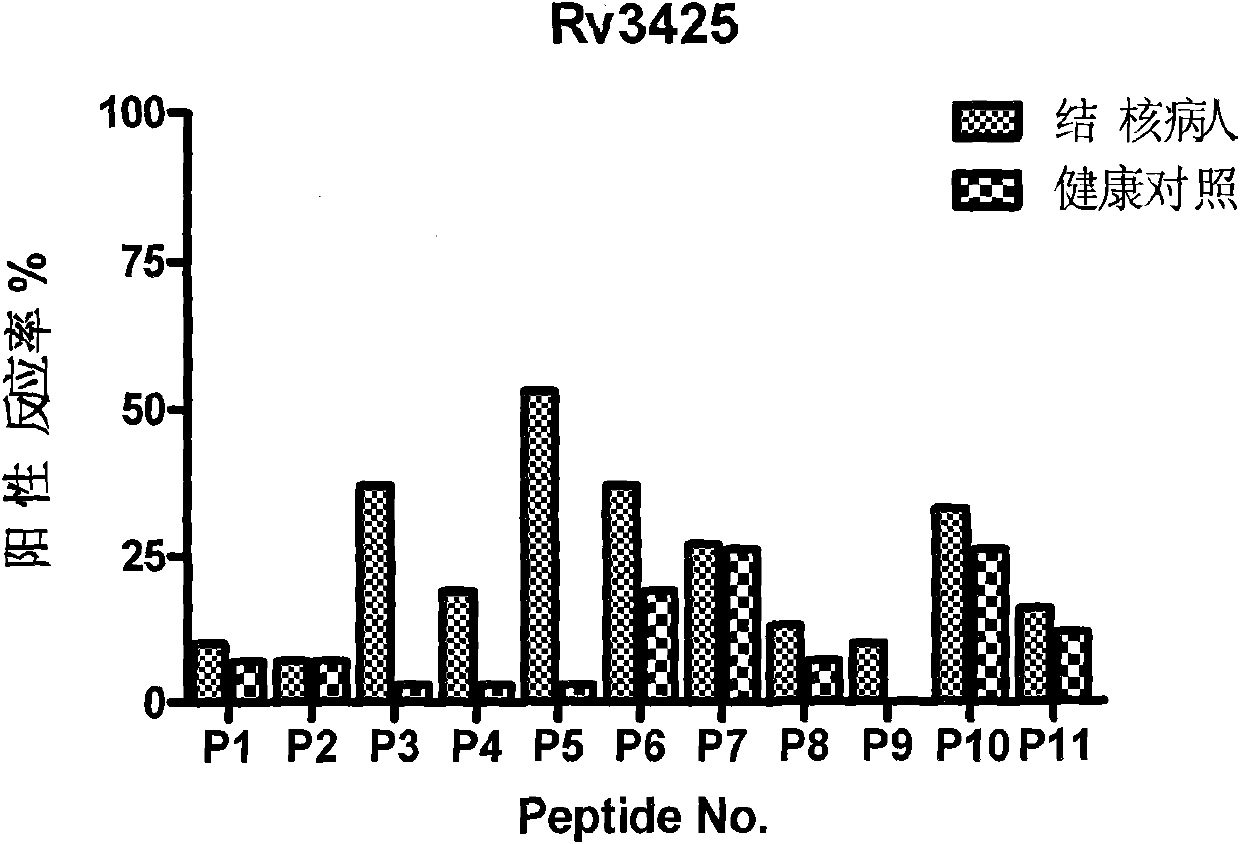
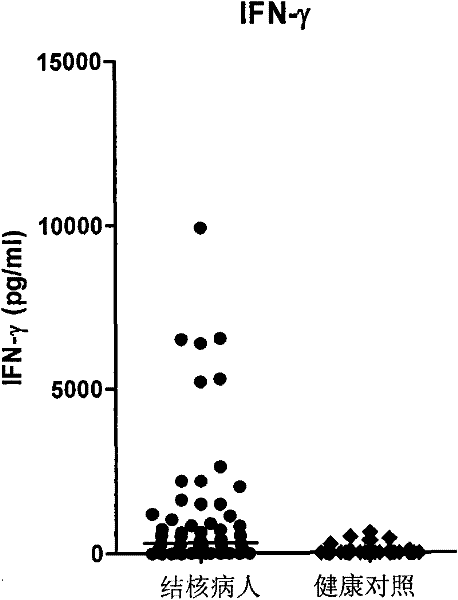
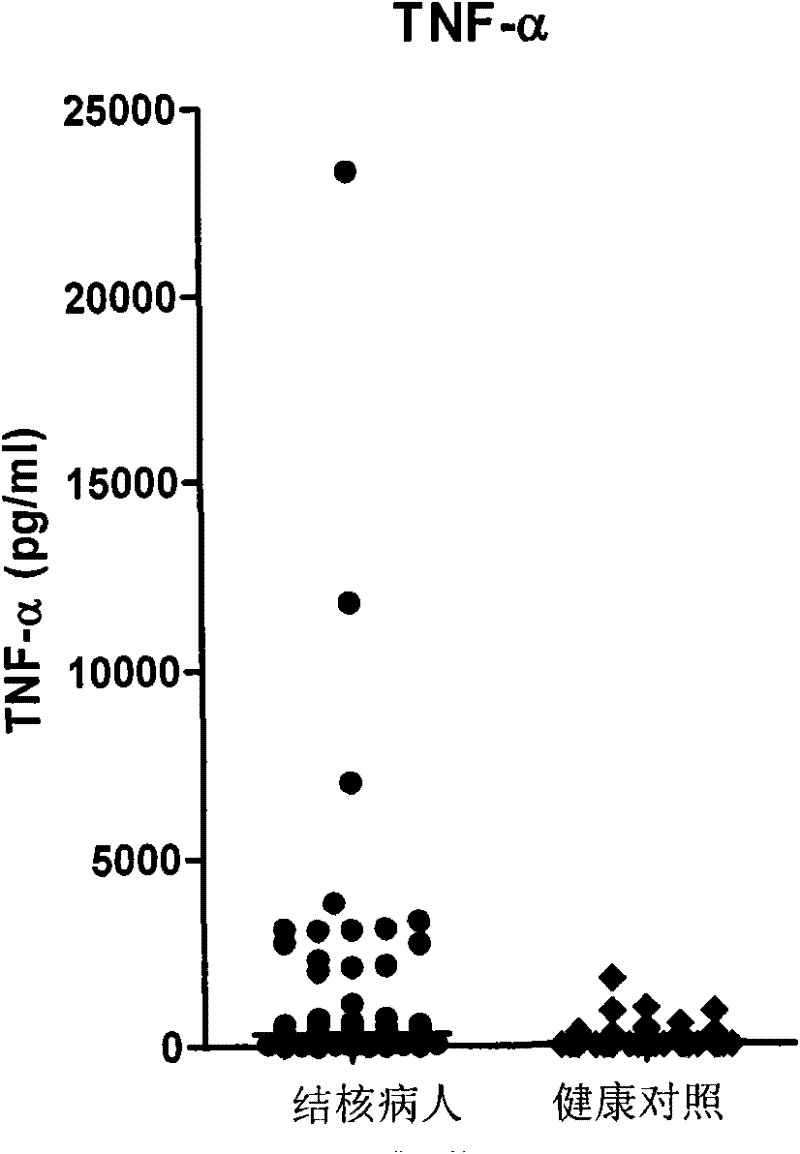
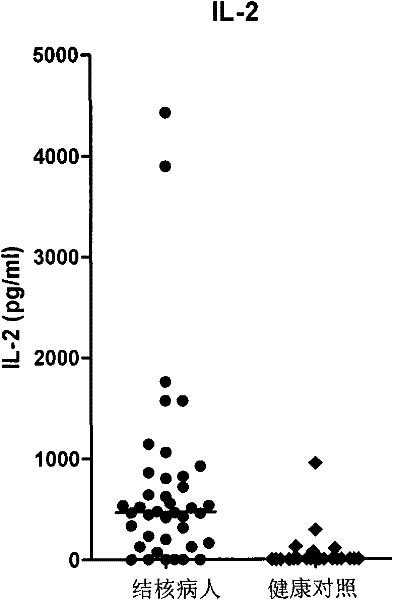
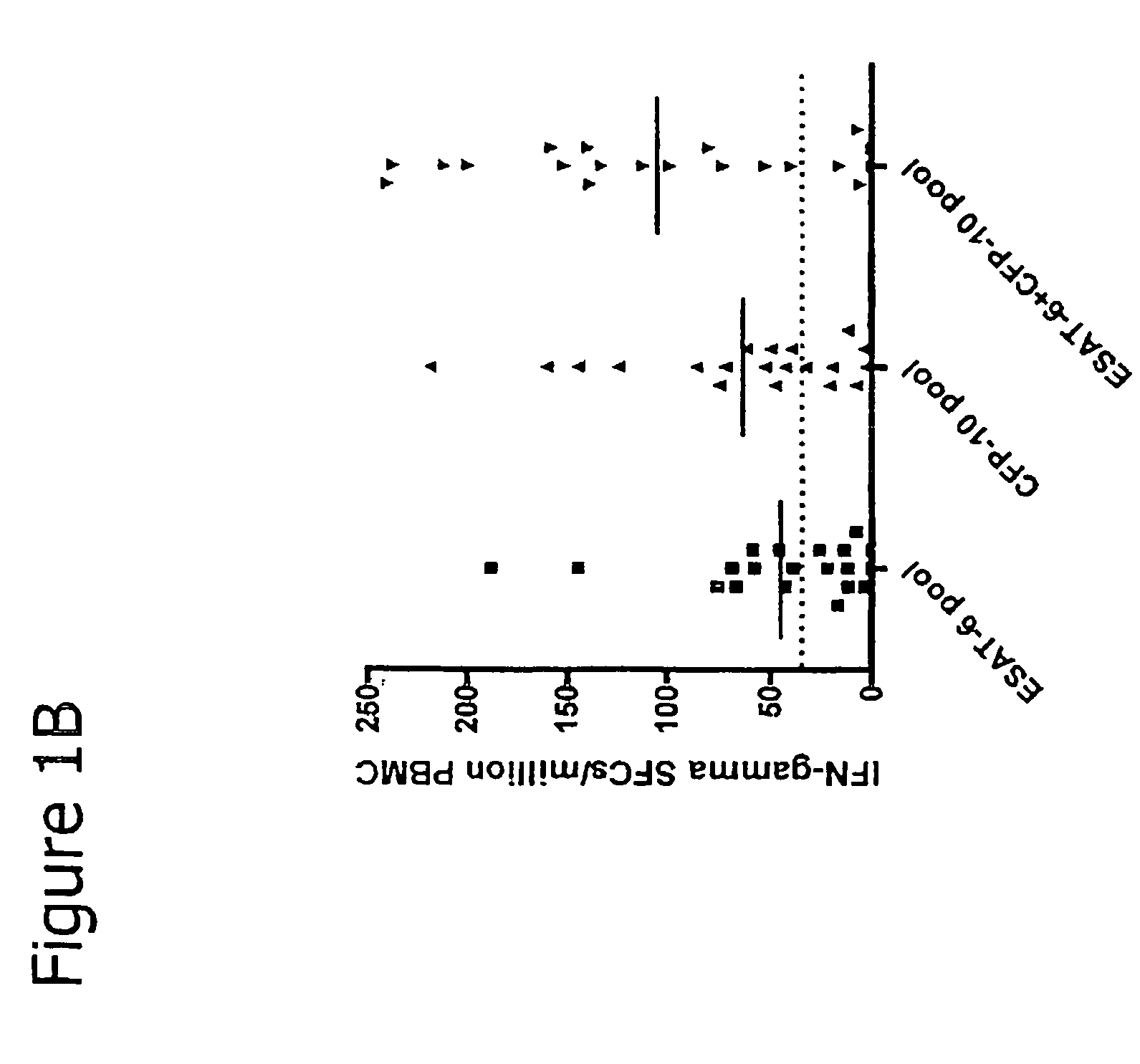
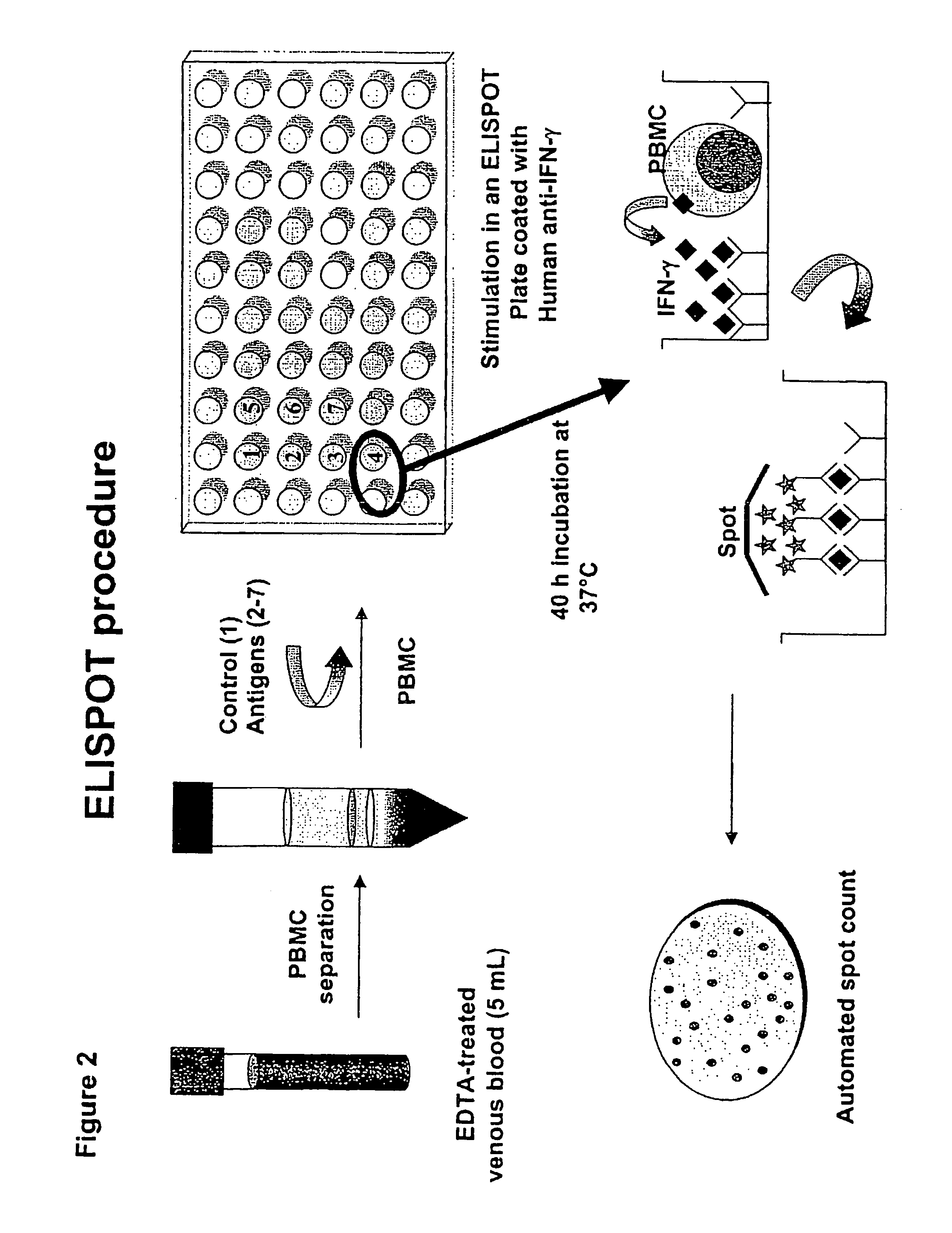
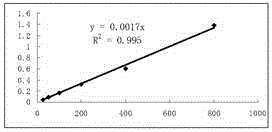
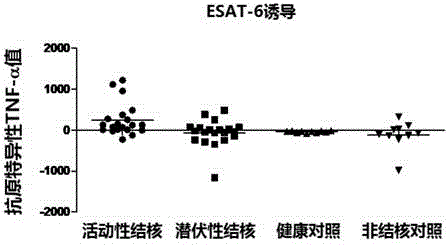
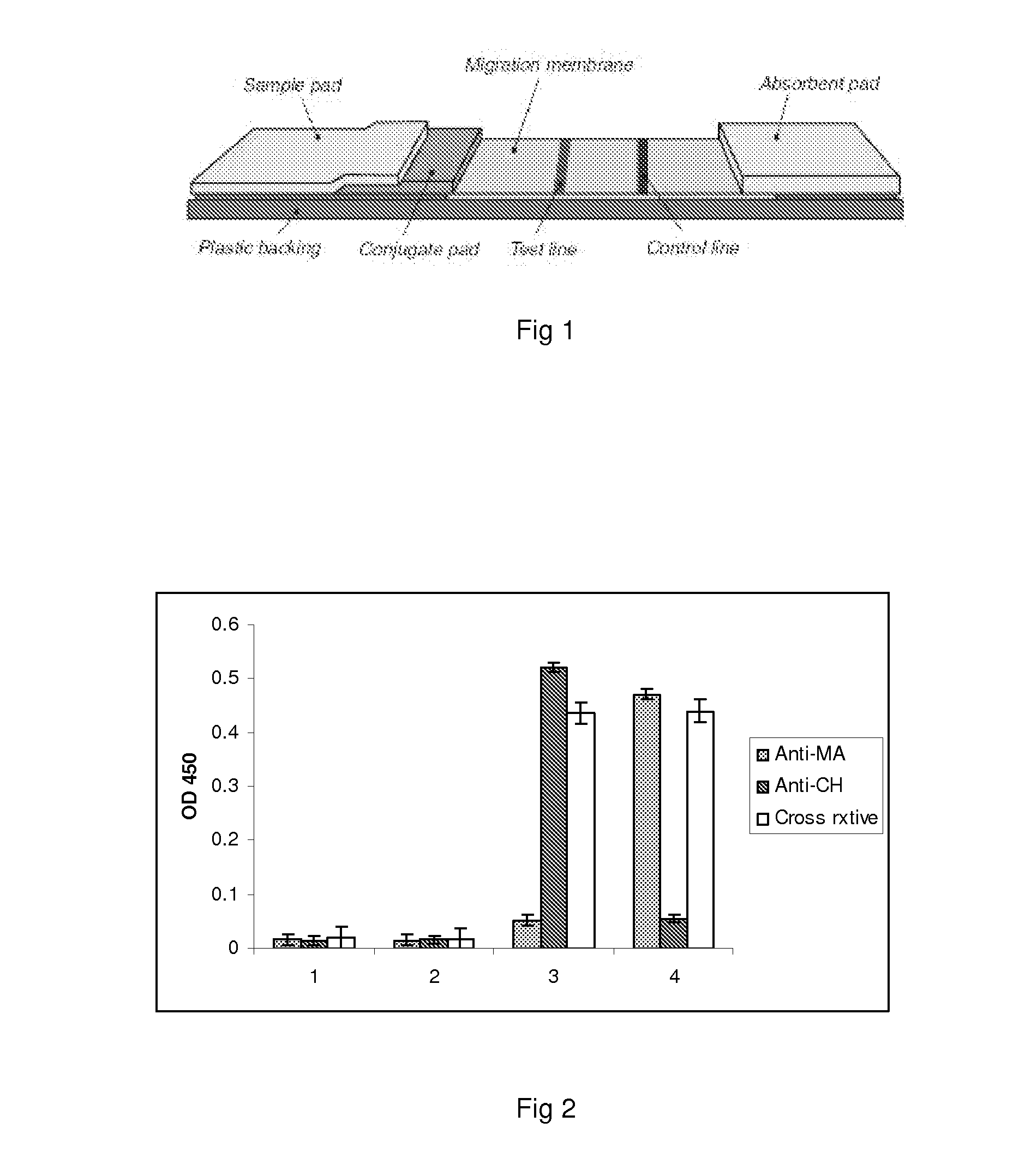
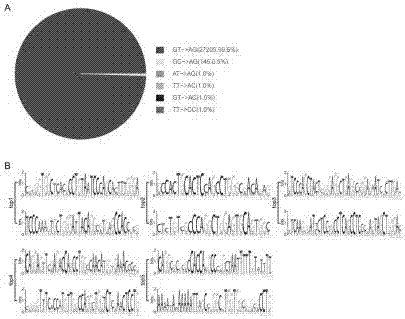
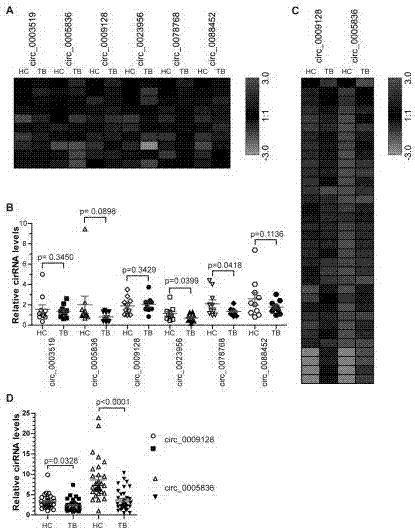
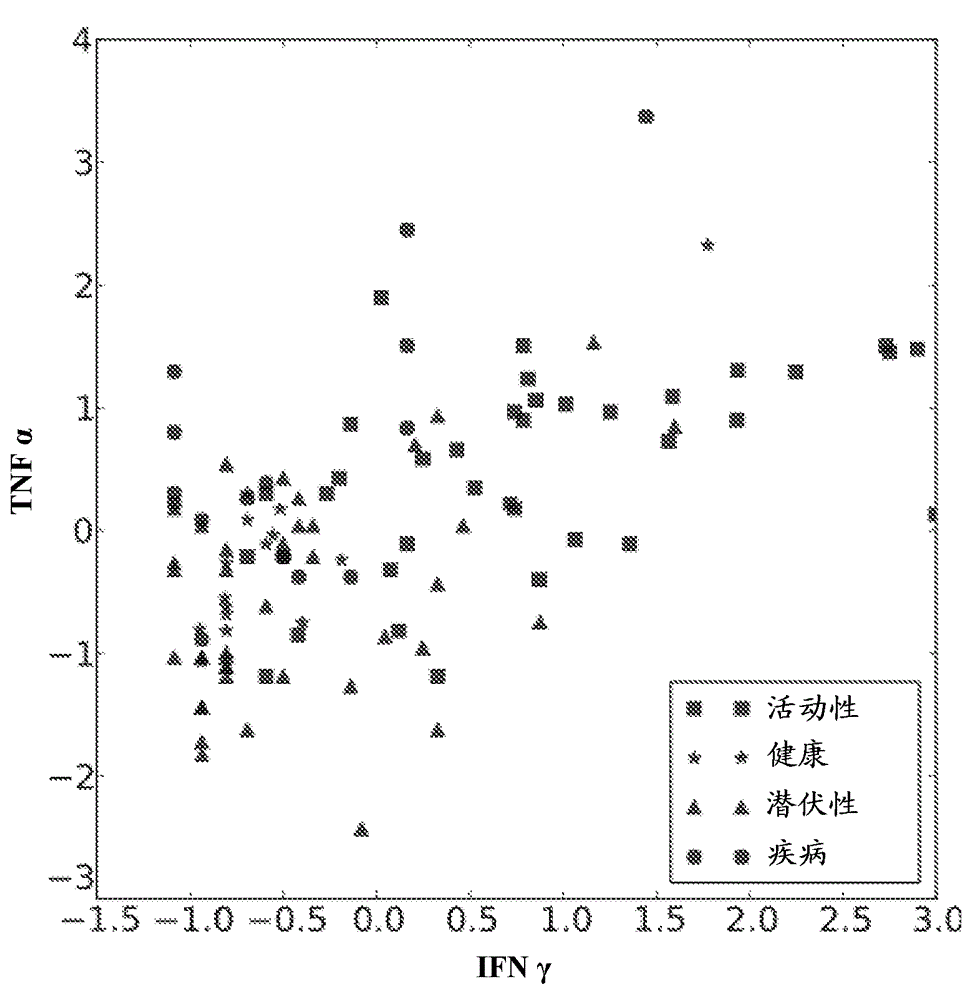
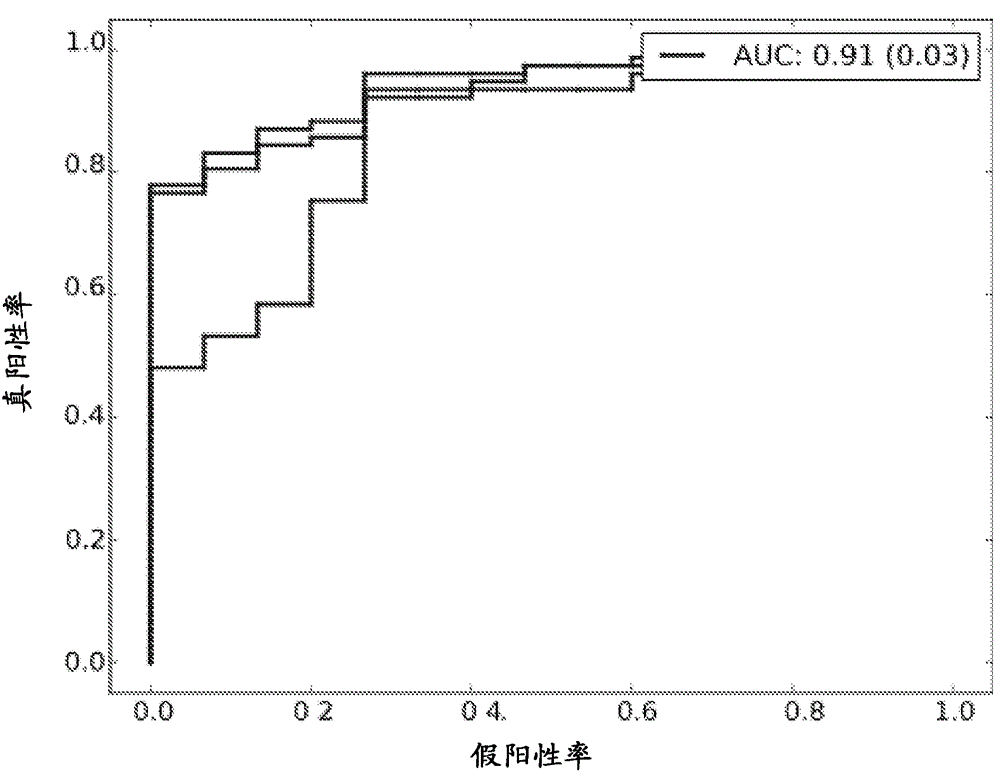
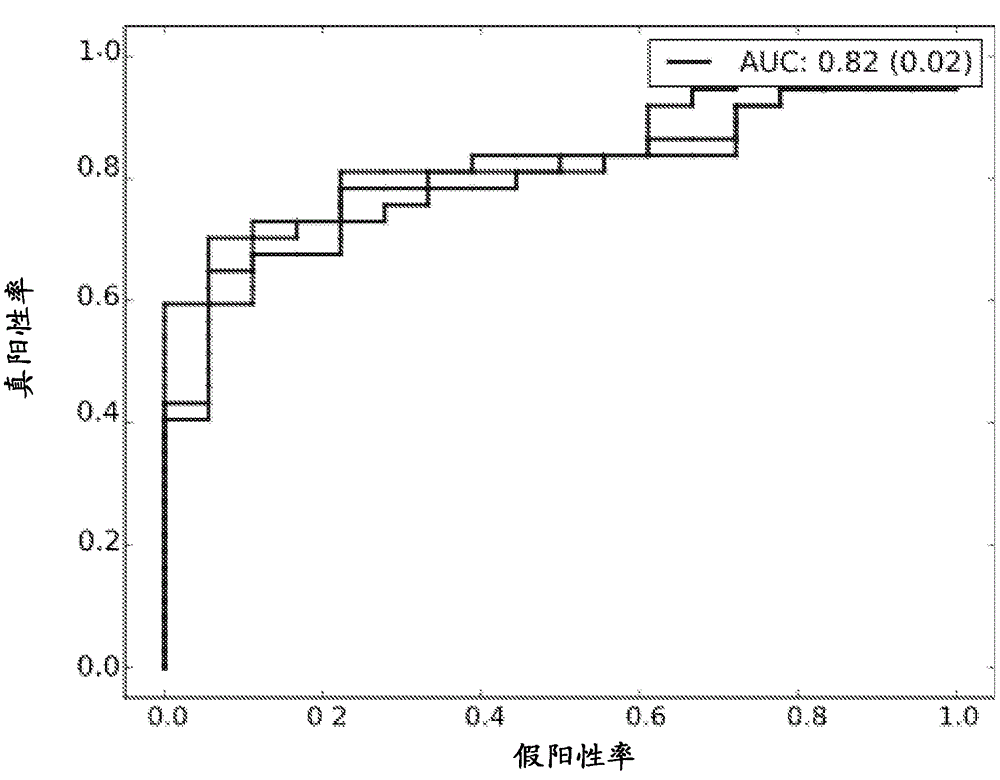
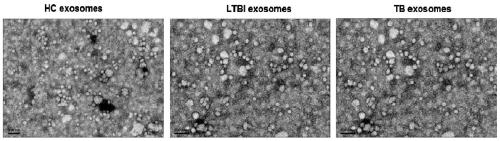
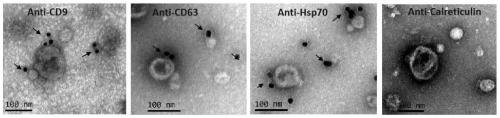
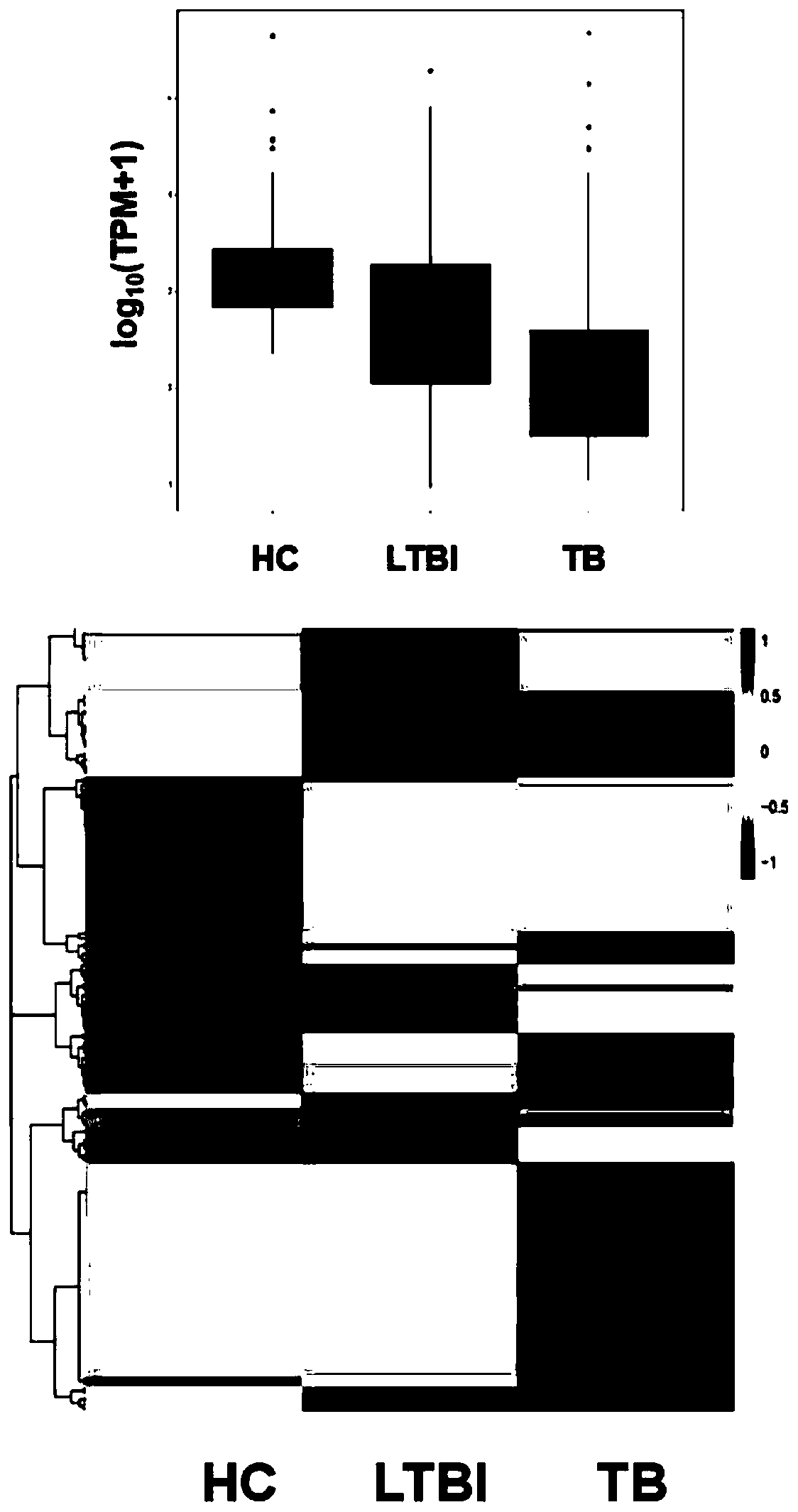
Patents
Literature
190 results about "Active tuberculosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein characteristic spectrum of active tuberculosis in children and method for creating protein characteristic spectrum
The invention discloses a protein characteristic spectrum of active tuberculosis in children and a method for creating the protein characteristic spectrum. The protein characteristic spectrum of the active tuberculosis in the children is obtained by comparing proteomics difference of an active tuberculosis group and a healthy control group. In addition, the invention further relates to the method for creating the protein characteristic spectrum of the active tuberculosis in the children, protein in blood plasma can be effectively identified and relatively quantified by utilizing a non-marked tandem mass spectrum technology of relative and absolute quantification, and a protein expression difference mass spectrum in the blood plasma of a patient suffering from the active tuberculosis in the children can be obtained by adopting the technology. A series of discovered proteins provide a foundation and resources for searching new more ideal markers; compared with a conventional blood plasma detection method, the method has relatively high sensitivity and specificity, and can be used for screening drugs for the active tuberculosis in the children; at the same time, a new way is provided for a mechanism for exploring occurrence and development of diseases.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Kit and method for detecting mycobacterium tuberculosis infection and application
ActiveCN102004155ALow costQuality assuranceBiological testingMycobacterium InfectionsLatent tuberculosis
The invention belongs to the field of biomedicine examination, and particularly relates to a kit and a method for detecting mycobacterium tuberculosis infection and application. The invention discloses a novel mycobacterium tuberculosis detection reagent by screening specific T cell epitope of mycobacterium tuberculosis, wherein the reagent contains polypeptide or analog thereof represented by SEQ ID No.1-10. The method detects cell factors released from T cells by using single or more SEQ ID No.1-10 polypeptides to contact the T cells of mycobacterium tuberculosis infected individuals. The method can effectively detect active tuberculosis or latent tuberculosis infection, and is free from disturbance of Bacilli Calmette Guerin (BCG) inoculation vaccines. The invention also discloses a diagnostic kit and other application based on the polypeptide and the method. Compared with the gamma interferon release experiments in the prior art, the method can obviously improve the detection rate without reducing the specificity and has high clinical application value.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Kits for Auxiliary Diagnosis of Tuberculosis
InactiveCN102297968AIncreased sensitivityReduce positive rateImmunoglobulins against cytokines/lymphokines/interferonsDepsipeptidesAIDS diagnosisIfn gamma
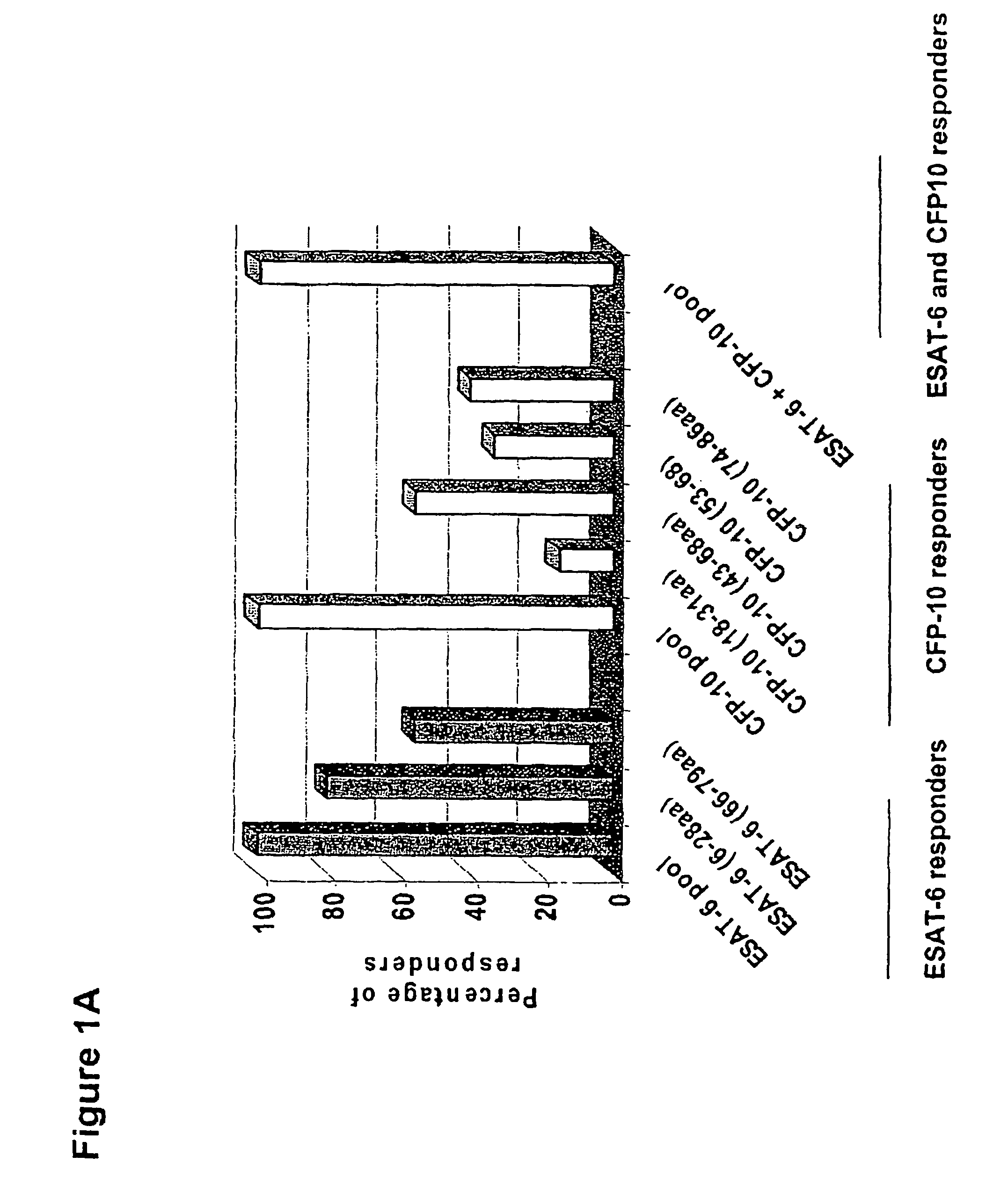
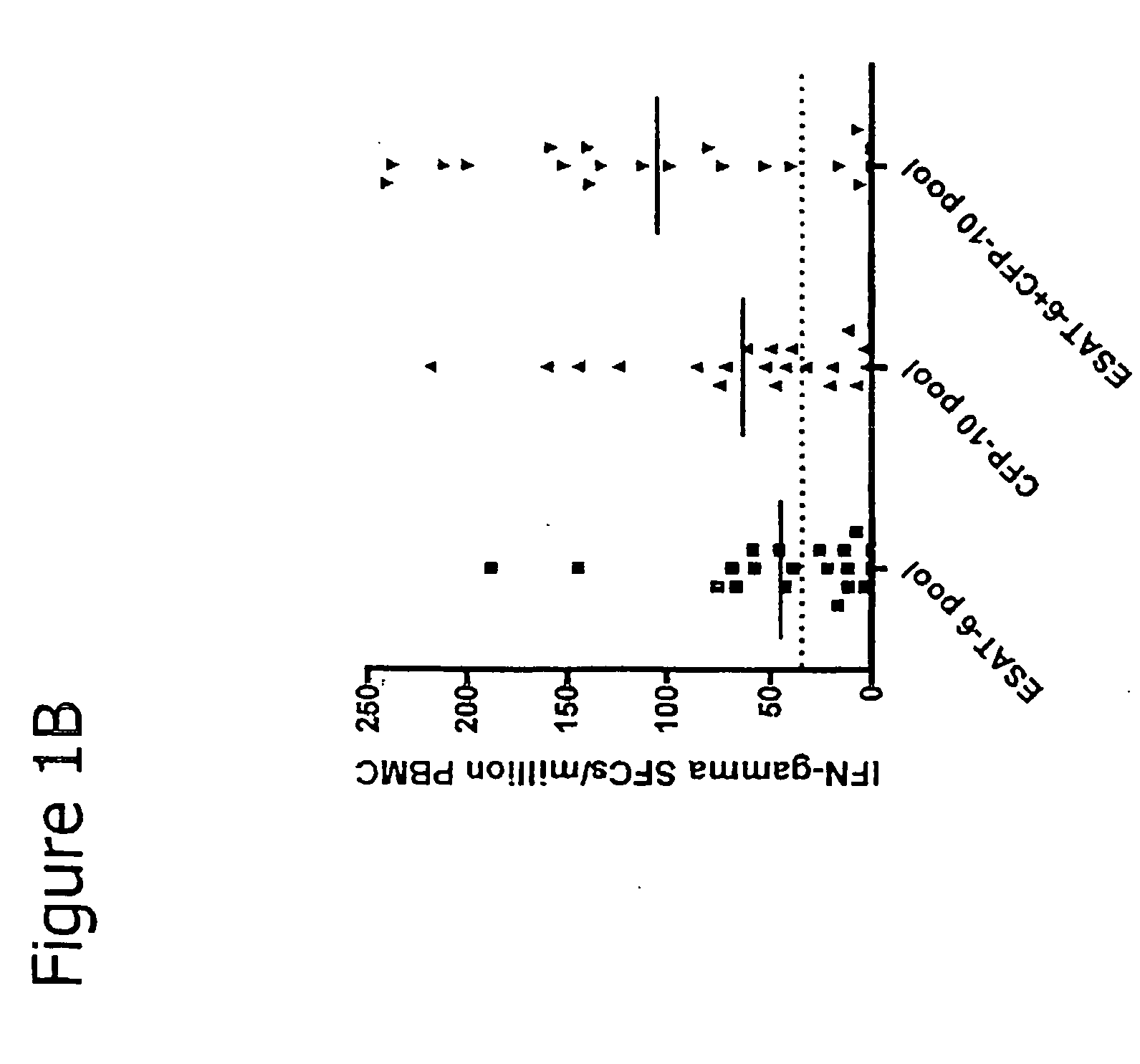
The invention discloses a kit for assisted diagnosis of tuberculosis, which comprises a specific antibody composition, wherein the specific antibody composition comprises the following five antibodies: (1) IFN-gamma antibody, (2) TNF-alpha antibody, (3) IL-2 antibody, (4) MIG antibody and (5) IP-10 antibody. The kit disclosed by the invention can distinguish a Mycobacterium tuberculosis infected person from a BCG vaccinee. Compared with the ELISPOT tuberculosis diagnosis method, the tuberculosis diagnosis kit based on multi-molecular marker detection has obviously enhanced sensitivity to active tuberculosis (from 72% to 89%), and the positive rate for normal healthy persons is obviously reduced (from 27% to 16%).
Owner:程小星 +3
Reagent and method for detecting active tuberculosis and tuberculosis dormant infection
The invention belongs to the biomedical detection field, in particular relates to a reagent and method used for detecting activity tuberculosis and latent tuberculosis infection; based on the genomic principle, the invention discloses a novel detection reagent for mycobacterium tuberculosis, containing protein or polypeptide which is represented by SEQ ID1-2, 4-5, 8-28; the method uses one or a plurality of SEQ ID 1-28 protein or polypeptide to contact T cells of a mycobacterium tuberculosis host, and detects cytokine released from the T cells; the method can detect the tuberculosis and latent infection effectively and is not interfered by BCG vaccine at the same time; the invention also discloses a diagnostic reagent kit and other application based on the protein or polypeptide and the method; compared with the T-SPOT of the prior art, the invention can improve detectable rate obviously under the condition that the specificity is not reduced; the reagent kit has cheap price, and the cost is 1 / 5 to 1 / 10 of that of the T-SPOT reagent, thus being beneficial to being popularized in the developing countries and poor areas.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
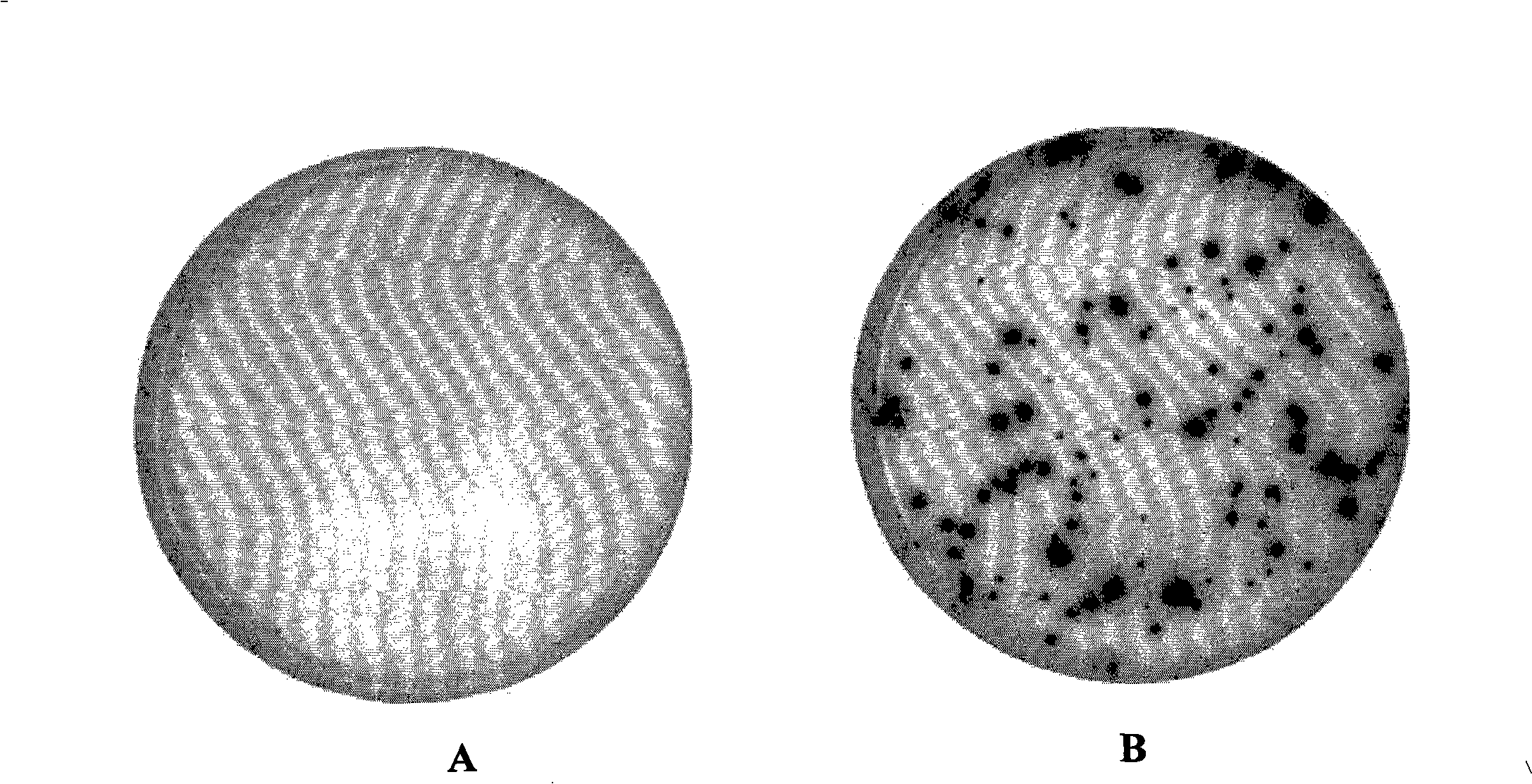
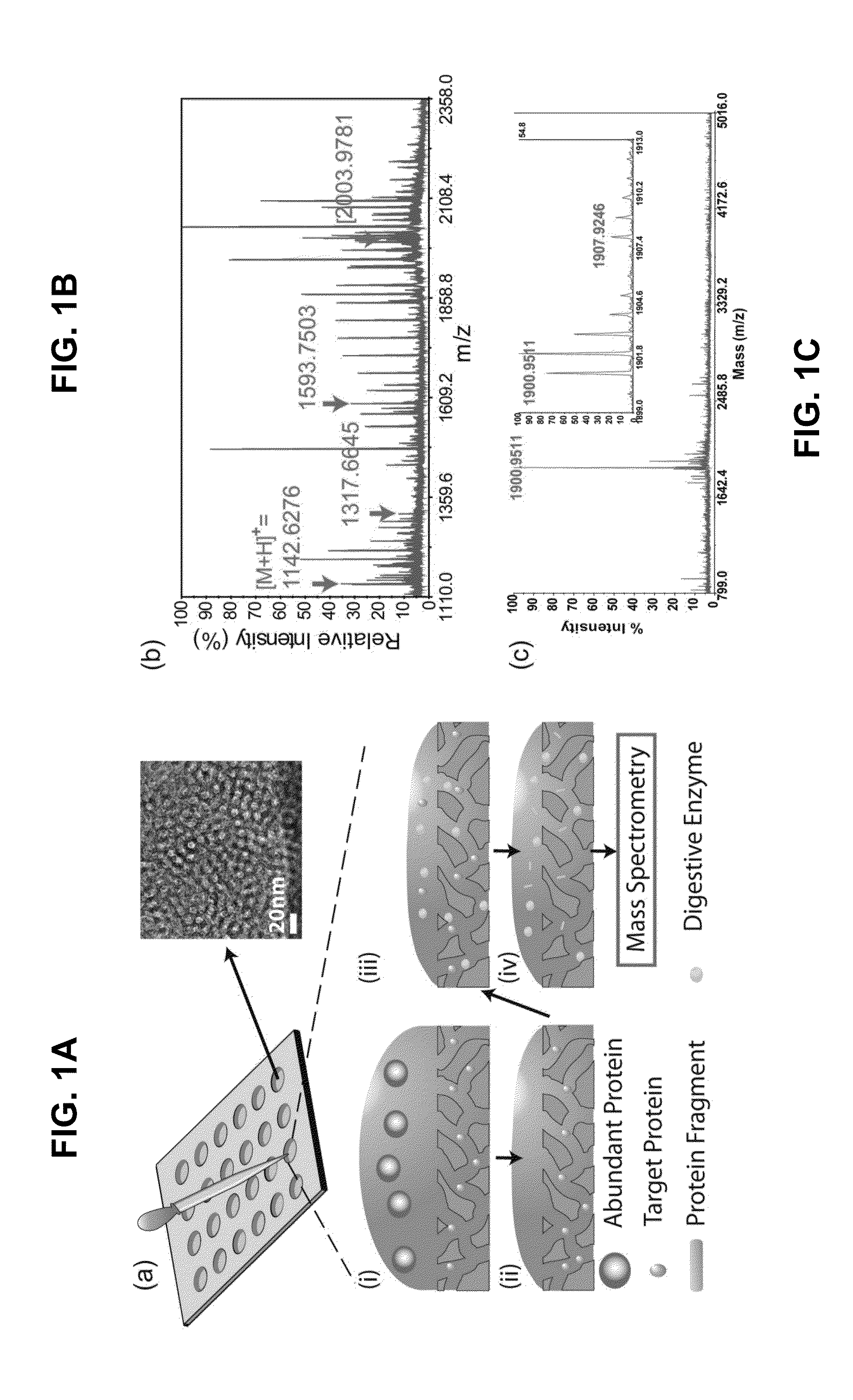
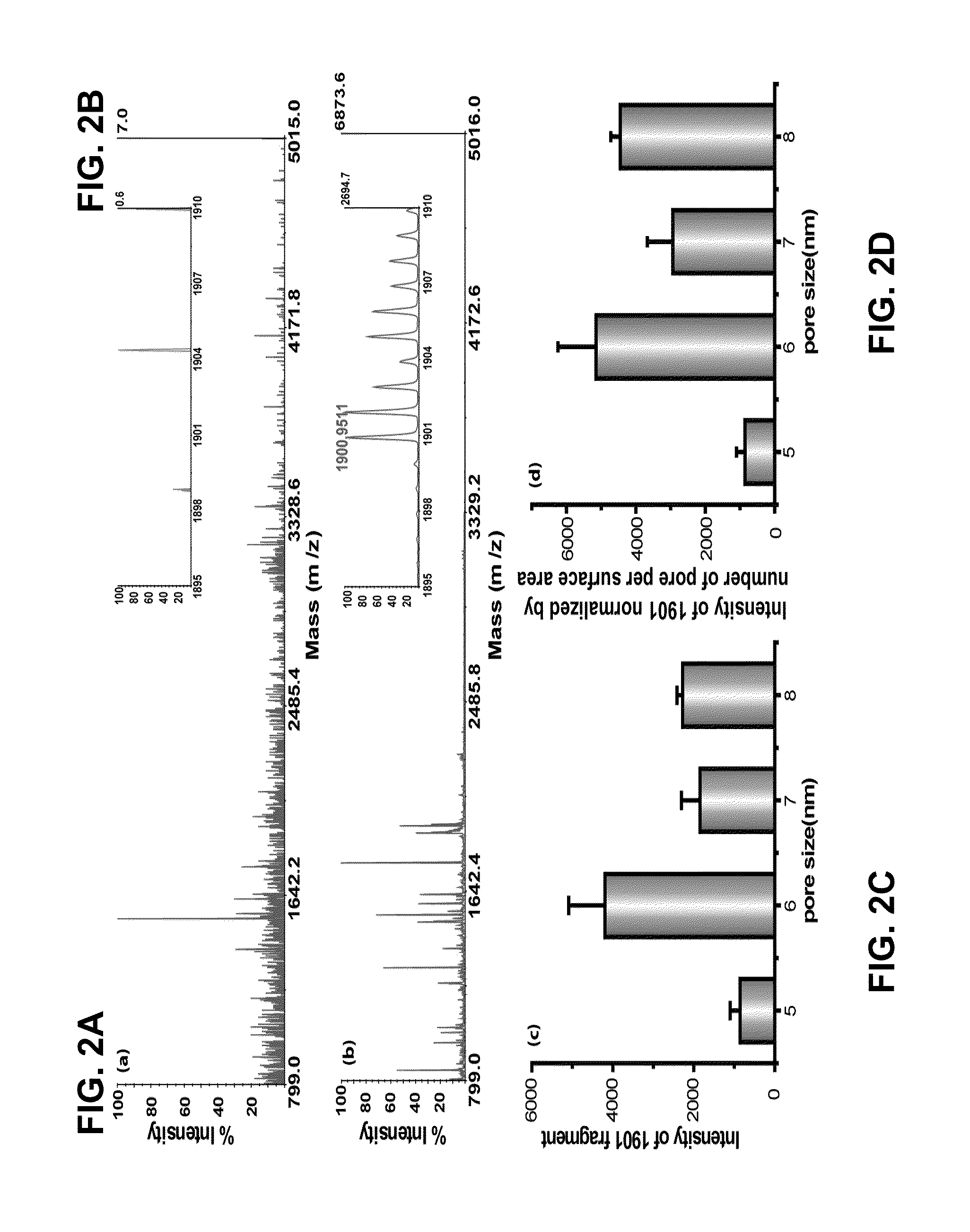
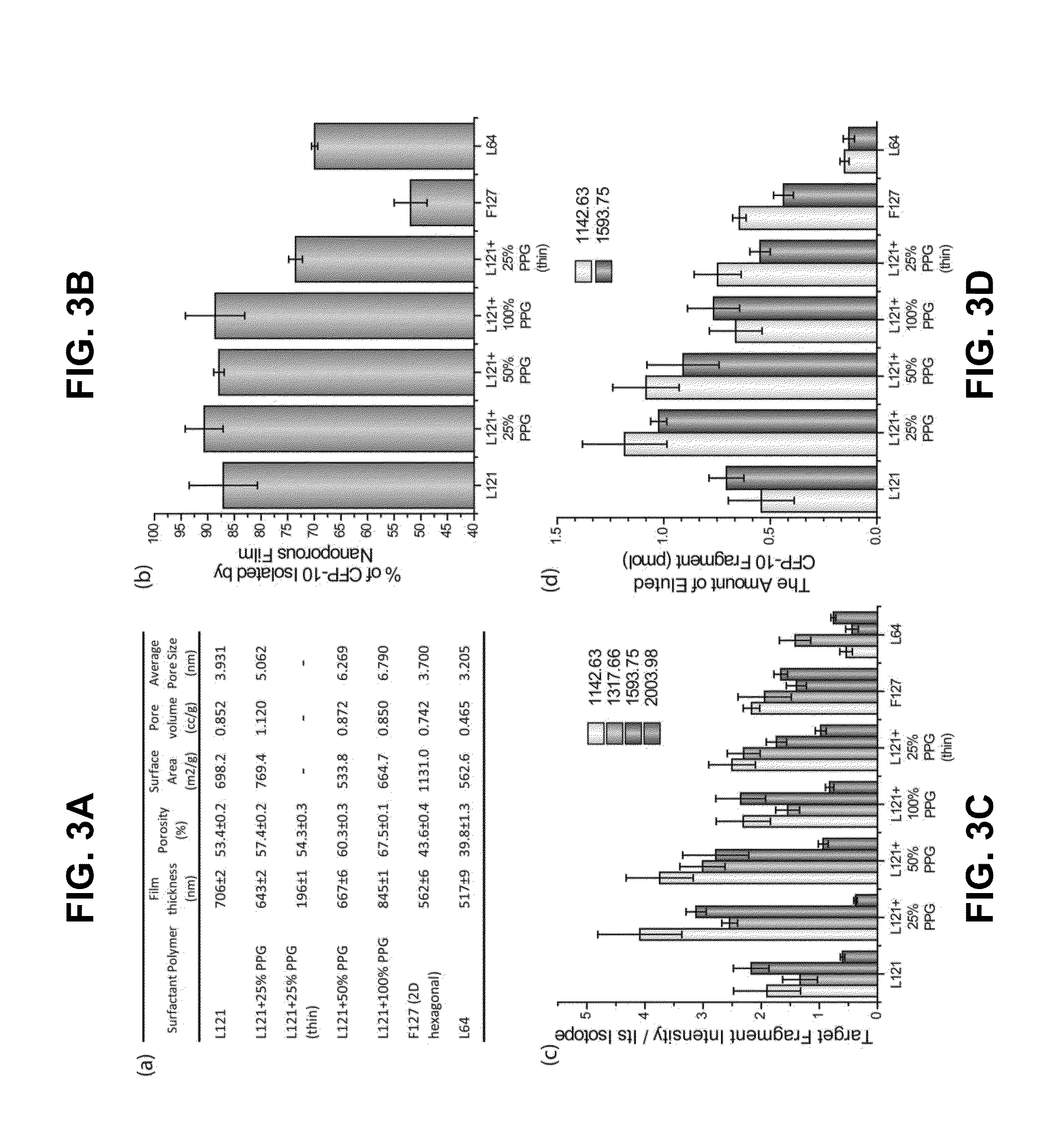
Rapid detection and quantitation of pathogen-specific biomarkers using nanoporous dual- or multi-layer silica films
InactiveUS20150260715A1Cost-effectiveRapid diagnosisMicrobiological testing/measurementLibrary screeningNanoporous membraneDigestion
Improved methods for detecting active tuberculosis are disclosed. A method comprises enriching at least one M. tuberculosis-specific biomolecule from a sample by contacting the sample with a nanoporous film; and detecting the presence of the M. tuberculosis-specific biomolecule or fragment(s) thereof. The method may further comprise digesting the enriched M. tuberculosis-specific biomolecule with an enzyme to produce a digestion product comprising at least one fragment of the M. tuberculosis-specific biomolecule. Improved sensitivity and speed achieved.
Owner:THE METHODIST HOSPITAL
Kit for detecting mycobacterium tuberculosis infection and monitoring clinical treatment effect and application of kit
ActiveCN104020297AIncreased sensitivityImprove featuresDisease diagnosisBiological testingTherapeutic effectSpecific antibody
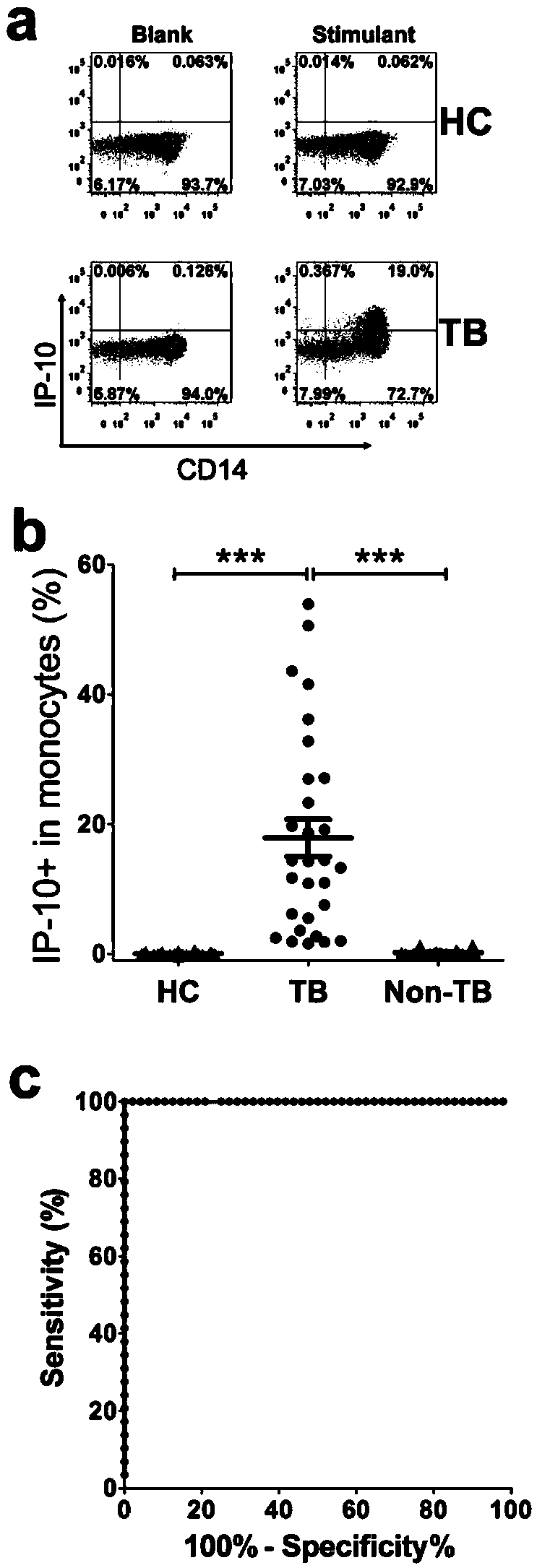
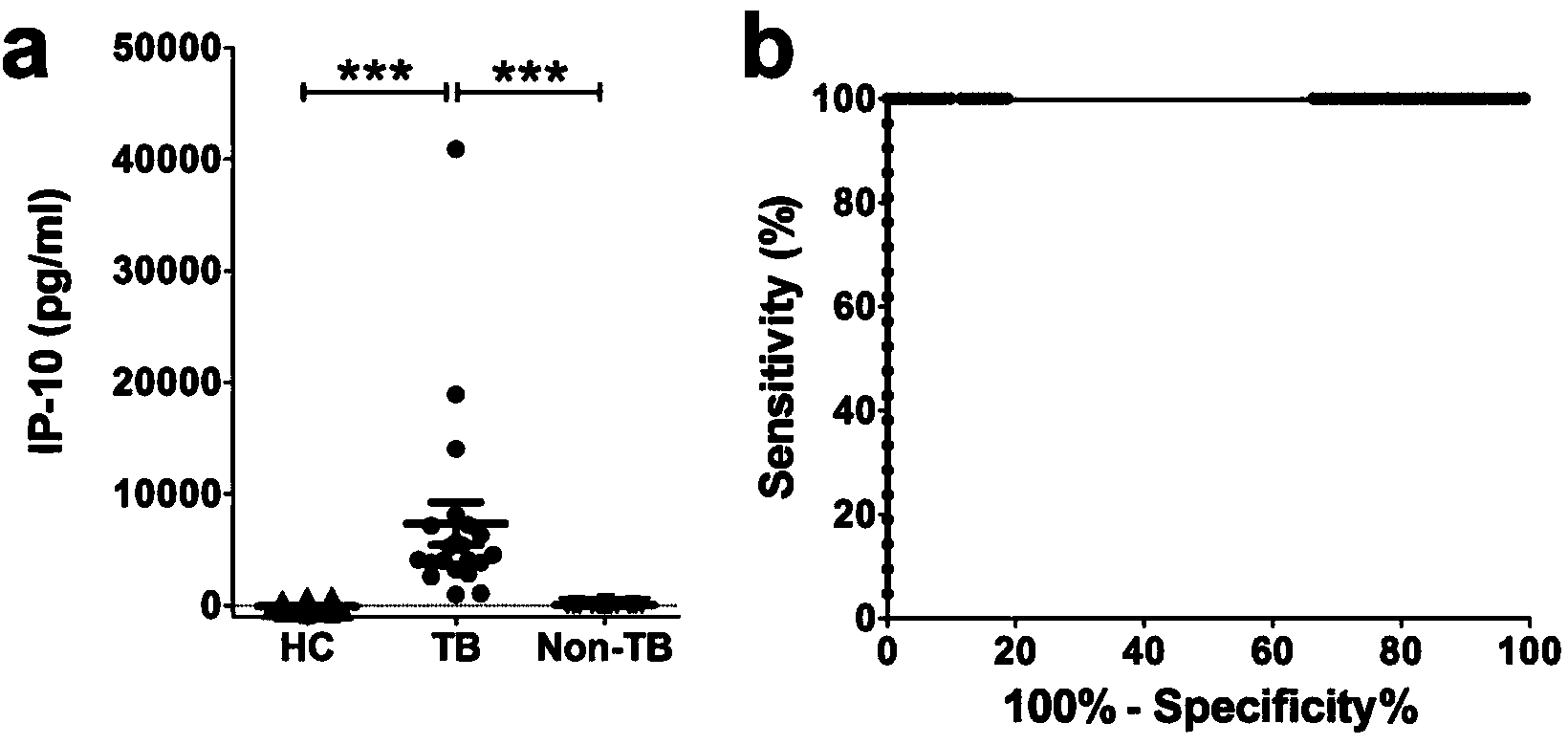
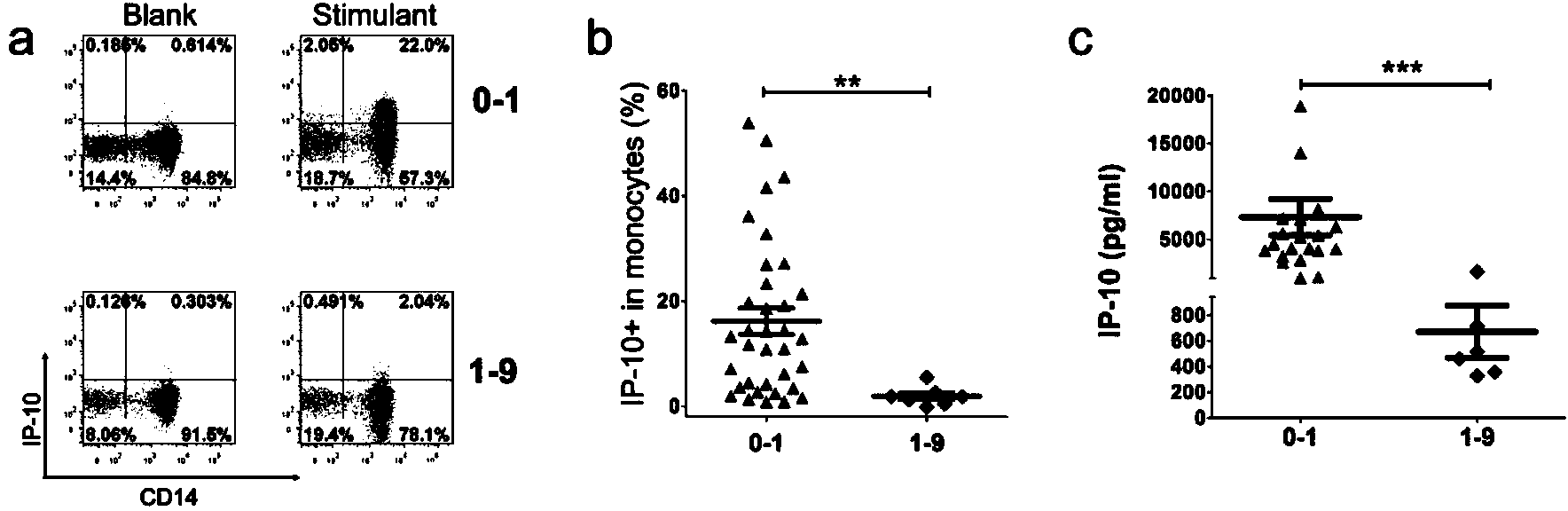
The invention discloses a kit for detecting the mycobacterium tuberculosis infection and monitoring a clinical treatment effect. The kit provided by the invention comprises a specific antibody, namely an IP-10 antibody and / or CD14 antibody, an antigen irritant and a positive contrast irritant. The kit disclosed by the invention can be used for diagnosing an active tuberculosis patient or a tuberculosis latent infection patient and is not affected by BCG (bacillus calmette-guerin) inoculation. The sensitivity and the specificity of the kit for diagnosing the active tuberculosis patient are higher than those of a commercial T-SPOT.TB kit and can be up to 100 percent. After the antituberculosis therapy is performed for 1 month, the detection rates of over 80 percent of clinical tuberculosis patients are converted to be negative, so that the kit can be used for detecting the clinical antituberculosis treatment effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Immune diagnostic assay to diagnose and monitor tuberculosis infection
InactiveUS7785607B2High detection sensitivityHigh sensitivityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseT lymphocyte
The present invention relates to a method of diagnosing and monitoring various distinct presentations of tuberculosis: active tuberculosis disease, latent tuberculosis infection and recent tuberculosis infection. The rapid immune assay is based on the evaluation of the frequency of Interferon (IFN) gamma-producing antigen-specific T lymphocytes responding to selected peptide sequences from Mycobacterium tuberculosis, selected for their immunogenicity. The invention concerns also immunogenic and vaccine compositions based on these specific peptide sequences.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
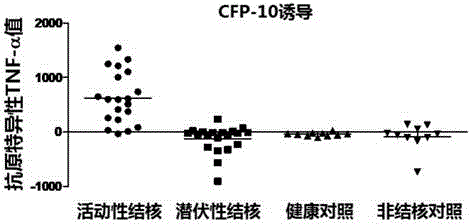
Kit for detecting active tuberculosis based on antigen-specific TNF-alpha-ELISA (enzyme linked immunosorbent assay) and application thereof
InactiveCN103604933AGood performance parametersStrong specificityDisease diagnosisBiological testingPeripheral blood mononuclear cellHorseradish peroxidase
The invention discloses a kit for detecting active tuberculosis based on antigen-specific TNF-alpha-ELISA (enzyme linked immunosorbent assay) and an application thereof. The kit comprises an ELISA plate coating a TNF-alpha antibody, a TNF-alpha protein standard, protein liquid of ESAT-6 and CFP-10, a biotin-marked TNF-alpha antibody and avidin-marked horseradish peroxidase. By adopting the kit for detecting active tuberculosis based on TNF-alpha-ELISA, a method for distinguishing active tuberculosis infection from latent tuberculosis infection is established; the TNF-alpha released by the peripheral blood mononuclear cell under the stimulus of tuberculosis-specific antigens ESAT-6 and CFP-10 and the background TNF-alpha released by the peripheral blood mononuclear cell without stimulus are quantitatively detected through the ELISA technology, and the value of the antigen-specific TNF-alpha is obtained by subtracting the two so as to perform direct diagnosis on the active tuberculosis.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
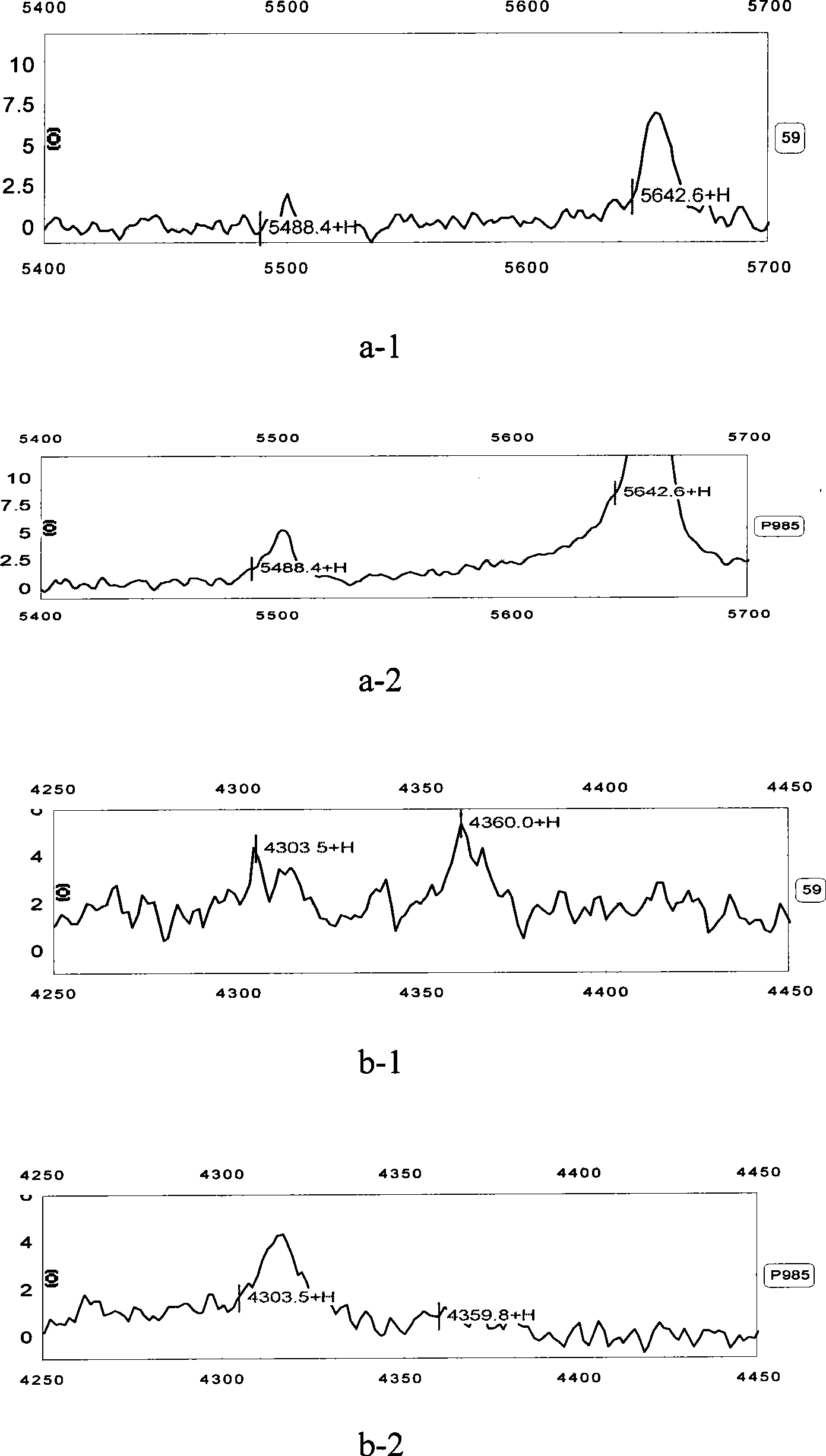
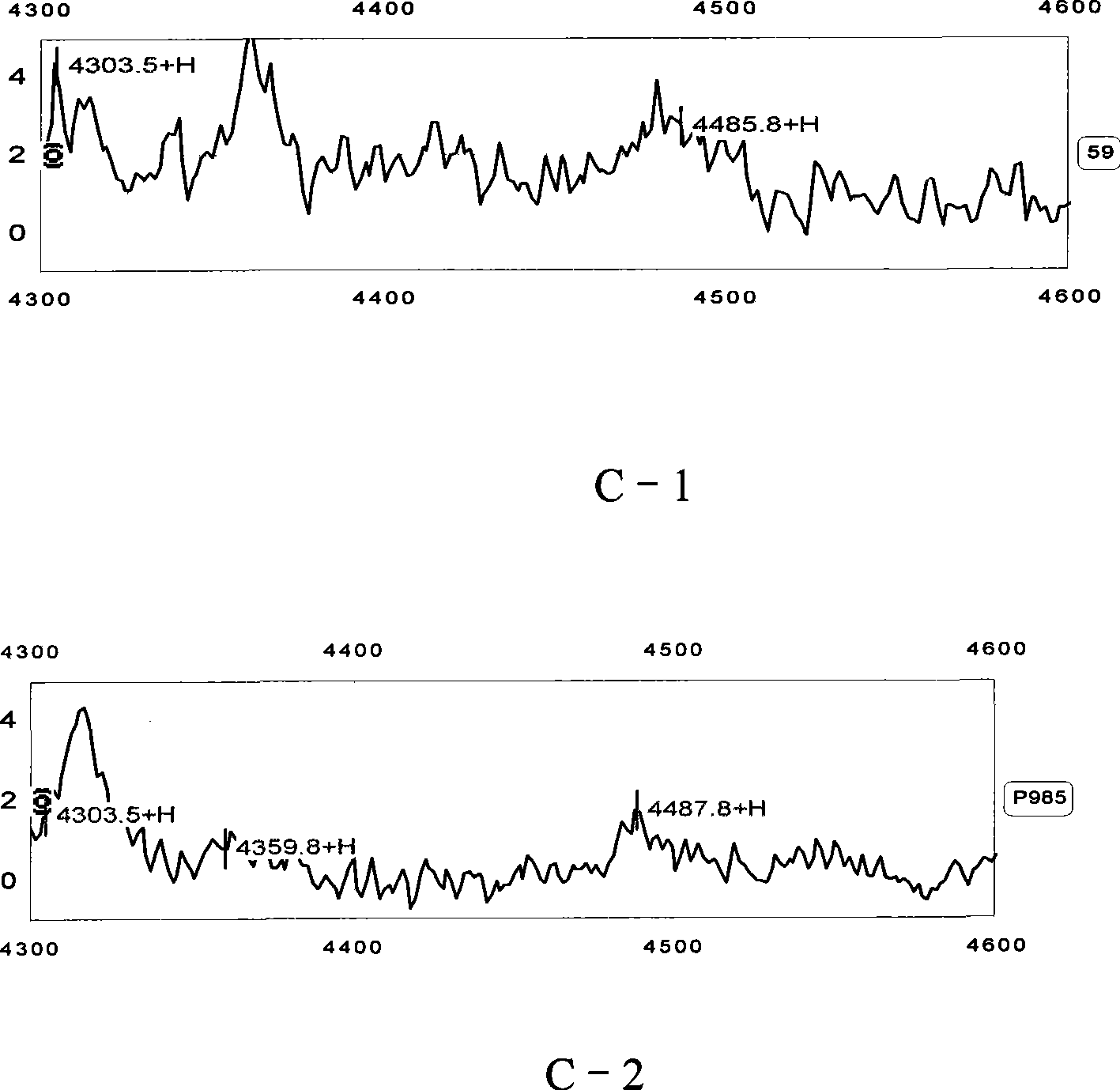
Serodiagnosis model establishing method for active tuberculosis disease
InactiveCN101424661AEasy to set upCreate method quicklyMaterial analysis by electric/magnetic meansVenous bloodData acquisition
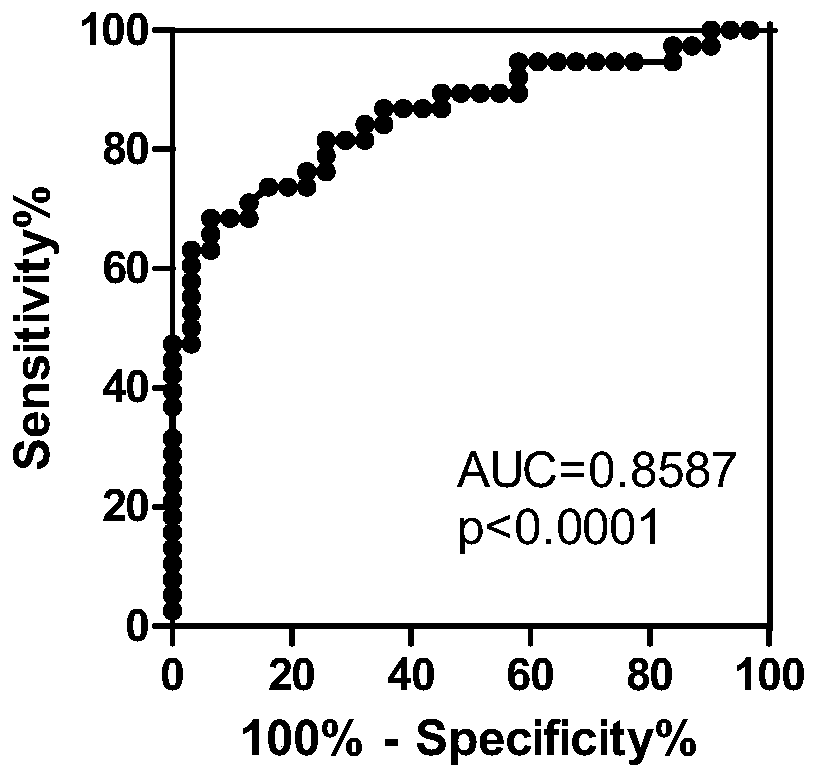
The invention relates to an establishing method of a serum diagnosing model of active tuberculosis, which has the following steps: step 1 is a serum source taking and preparing step which comprises an experiment group and a control group, the venous blood of a checked person is collected, serum and the rest are separately stored after serum separation at the temperature of -80 DEG C in a refrigerator for spare use; step 2 is the step of the mass spectrometric analysis of the serum and comprises sample treatment, protein plate check, spectrometric analysis and data acquisition; step 3 is the step of statistic analysis and the difference protein screening; step 4 is the step of establishing the diagnosing model, and Biomarker Pattern 5.0 software is used for establishing the model of diagnosing active tuberculosis. When the model is used for identifying the active tuberculosis and the control group, the sensitivity is 96.9 percent, the specificity is 97.8 percent, the positive predicating value and the negative predicating value are 97.7 percent and 97.1 percent, and the total accurate rate is 97.3 percent.
Owner:中国人民解放军总医院第二附属医院 +1
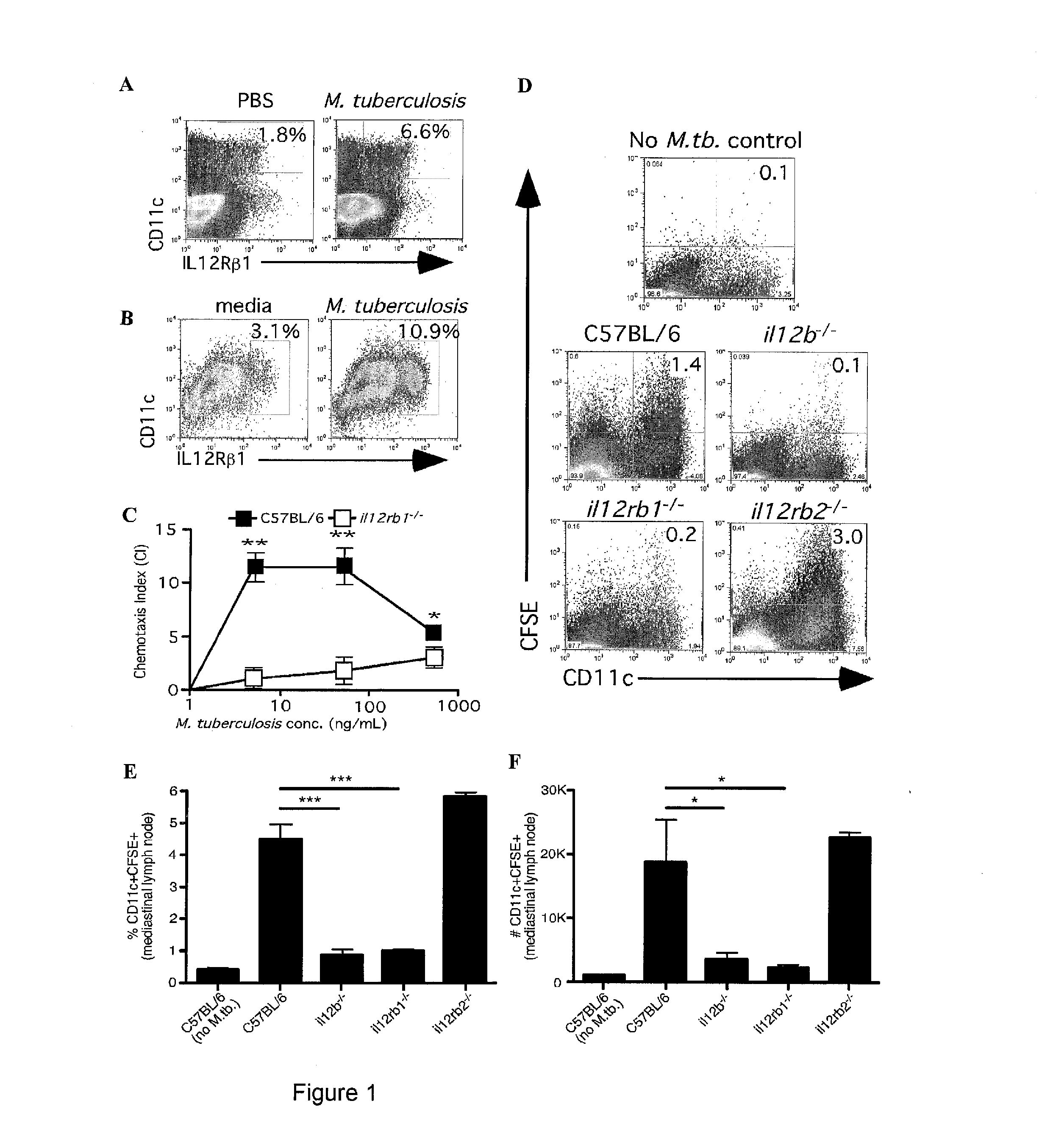
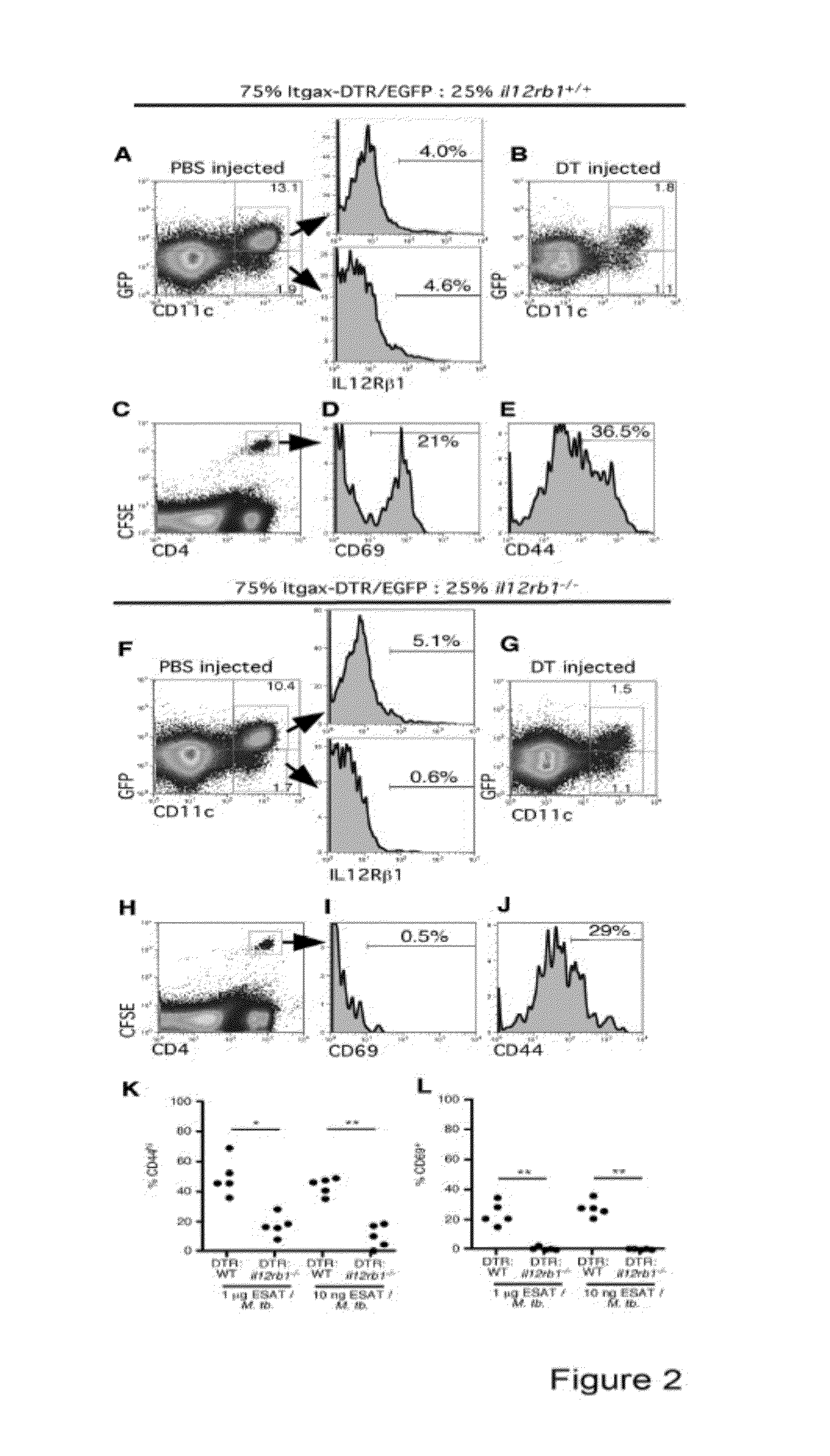
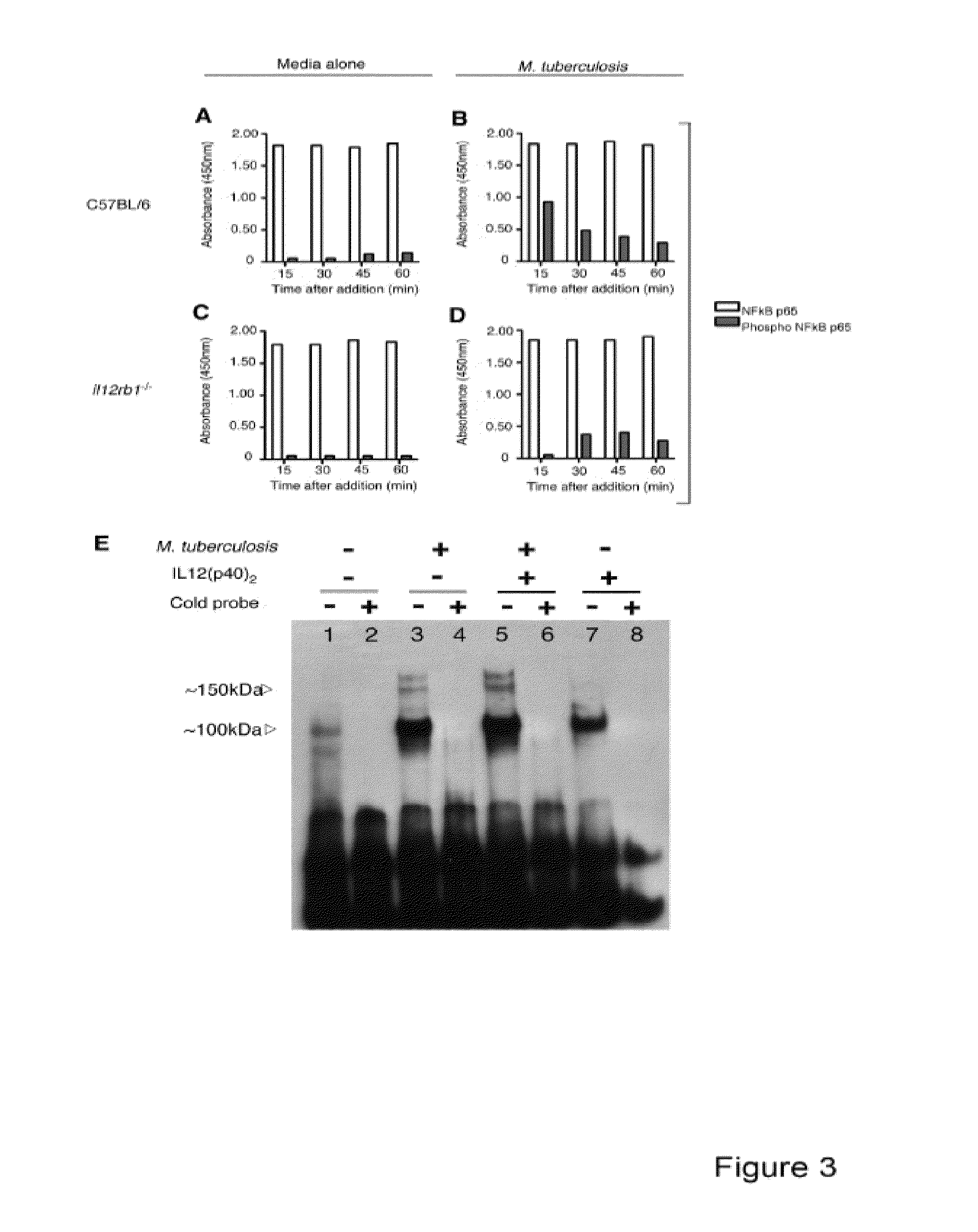
Use of an il12 receptor-beta 1 splice variant to diagnose active tuberculosis
InactiveUS20140227324A1Improve responseAltered functionBacterial antigen ingredientsMicrobiological testing/measurementMycobacterium InfectionsLatent tuberculosis
The present invention describes compositions for both diagnostic and therapeutic applications. In one embodiment, the present invention contemplates a method of identifying an active M. tuberculosis infection. In another embodiment, the present invention contemplates a method of monitoring a M. tuberculosis infection. In yet another embodiment, the present invention contemplates a method of monitoring a patient's response to treatment for an active M. tuberculosis infection. In a further embodiment, the present invention contemplates a method of monitoring a patient's response to treatment for an active M. tuberculosis infection.
Owner:TRUDEAU INST INC
Immune diagnostic assay to diagnose and monitor tuberculosis infection
InactiveUS20070196878A1High detection sensitivityHigh sensitivityBacterial antigen ingredientsSugar derivativesT lymphocyteLatent tuberculosis
The present invention relates to a method of diagnosing and monitoring various distinct presentations of tuberculosis: active tuberculosis disease, latent tuberculosis infection and recent tuberculosis infection. The rapid immune assay is based on the evaluation of the frequency of Interferon (IFN) gamma-producing antigen-specific T lymphocytes responding to selected peptide sequences from Mycobacterium tuberculosis, selected for their immunogenicity. The invention concerns also immunogenic and vaccine compositions based on these specific peptide sequences.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
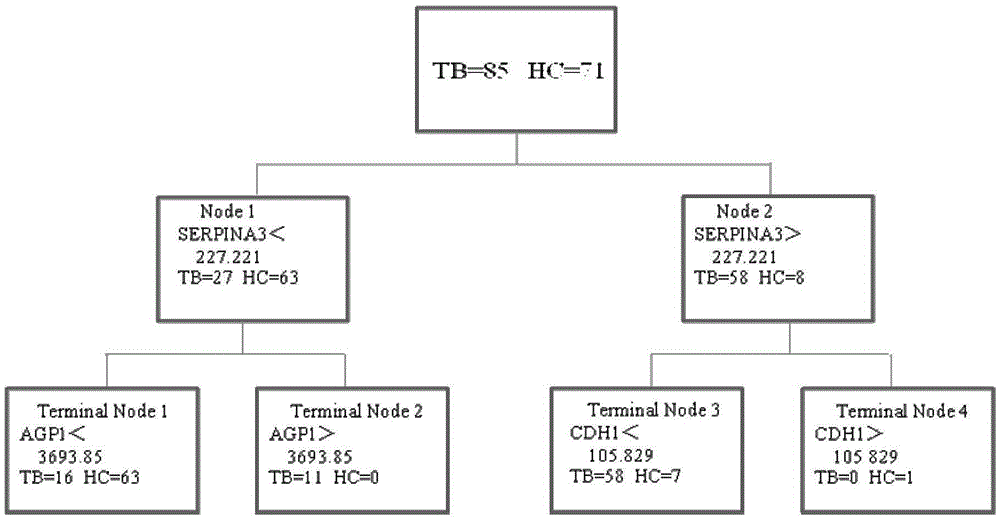
Application of AGP1, SERPINA3 and CDH1 content detection system in screening active tuberculosis patients
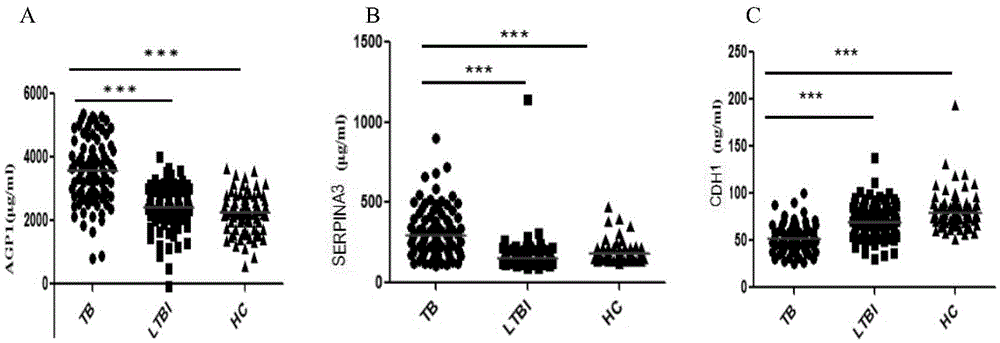
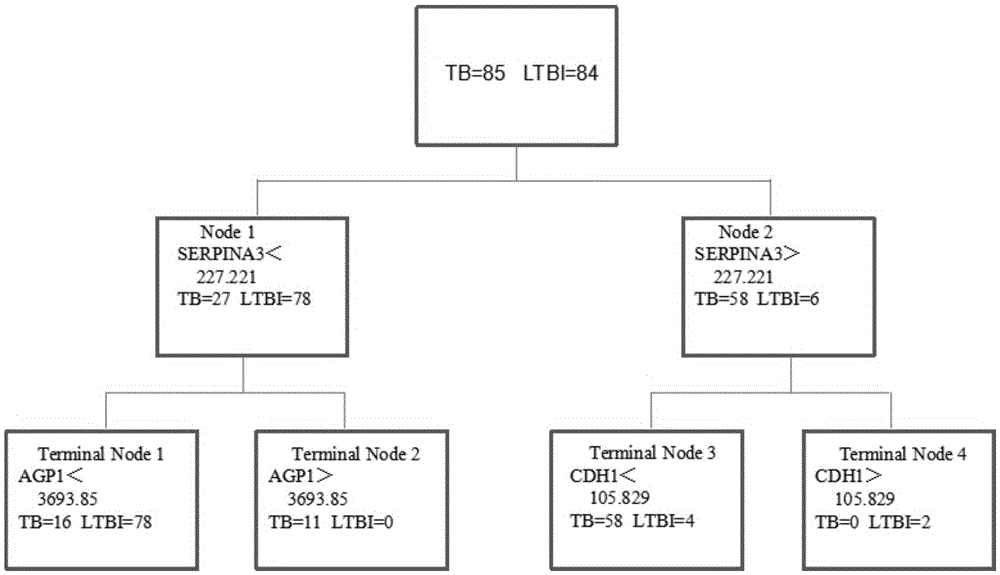
The invention discloses application of an AGP1, SERPINA3 and CDH1 content detection system in screening active tuberculosis patients. The inventor of the application discovers that three proteins with specific expression, namely AGP1, SERPINA3 and CDH1, in the active tuberculosis patients and establishes an active tuberculosis patient screening model with sensitivity of 81.18%, specificity of 96.15% and accuracy of 88.17% by use of content of three proteins in plasma. By use of the content of three proteins in plasma and the active tuberculosis patient screening model, the active tuberculosis patients can be screened, and active tuberculosis patient screening products can be prepared by use of substances for detecting the content of three proteins in plasma.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV +1
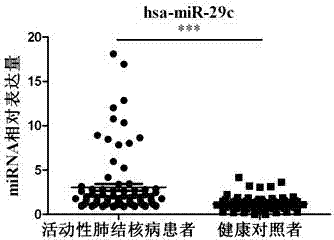
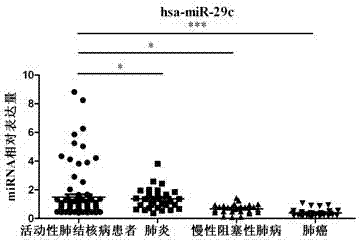
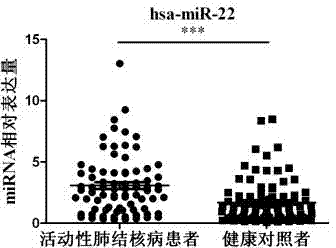
Kit for detecting active pulmonary tuberculosis
ActiveCN103045723AEarly Detection ImprovesEarly detection to improve the early diagnosis of active tuberculosisMicrobiological testing/measurementMicroorganism based processesSerum mirnaPulmonary tb
The invention provides a diagnostic kit for detecting active pulmonary tuberculosis. The kit consists of an RNA extract buffer solution, a specific serum miRNA composition for the active pulmonary tuberculosis, internal reference reverse transcription primers, polymerase chain reaction (PCR) primers and a fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) reaction solution, wherein the specific serum miRNA composition for the active pulmonary tuberculosis consists of four differential expression serum miRNAs, namely hsa-miR-29c, hsa-miR-22, hsa-miR-320b and hsa-miR-101. By using the specific serum miRNAs composition for the active pulmonary tuberculosis to detect the active pulmonary tuberculosis on the serum miRNAs level, the sensitivity is 90.2 percent, and the specificity is 75.0 percent; and the kit has early-stage diagnostic value for the active pulmonary tuberculosis, and can realize early warning and early diagnosis of the active pulmonary tuberculosis.
Owner:ZHEJIANG UNIV
Method of detecting surrogate markers in a serum sample
ActiveUS20130137598A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCompetitive bindingCholesterol
The invention provides a method of detecting surrogate markers for active tuberculosis in a serum sample. The surrogate markers are selected from serum mycolic acid antigen, serum anti-mycolic acid antibodies or both. The method includes the steps of combining the serum sample with a labelled monoclonal immunoglobulin antibody or fragment thereof to mycolic acids to produce a combined serum sample, the antibody or fragment thereof not substantially cross-reacting with cholesterol and the label being selected so that binding of the labelled antibody to immobilized mycolic acid antigen of mycobacterial origin produces a detectable signal and combining a blank sample with the labelled monoclonal immunoglobulin antibody or fragment thereof to mycolic acid to produce a combined blank sample. The method includes exposing both samples to immobilised mycolic acid antigen of mycobacterial origin or a synthetic analogue or analogues thereof so that the labelled immunoglobulin antibodies or fragments thereof in each sample bind to the immobilised antigen to produce detectable signals. If the surrogate markers are present, the signal produced by the blank sample will be stronger than that produced by the serum sample because of inhibition of binding of the labelled antibody in the serum sample arising from prior binding of the labelled antibody with the mycolic acid antigen in the serum sample or by competitive binding of serum anti-mycolic acid antibodies in the serum sample to the immobilised mycolic acid antigen or both.
Owner:UNIVERSITY OF PRETORIA
Biomarker of active pulmonary tuberculosis
ActiveCN107022605AImprove developmentAntibacterial agentsMicrobiological testing/measurementPulmonary tuberculosisBiomarker (petroleum)
The invention relates to the technical field of biomarkers, in particular to a biomarker of active pulmonary tuberculosis. The biomarker of active pulmonary tuberculosis is selected from at least one of the following six circRNAs: hsa_circ_0005836, hsa_circ_0009128, hsa_circ_0003519, hsa_circ_0023956, hsa_circ_0078768, hsa_circ_0088452. Specifically, hsa_circ_0005836 has the most obvious down-regulated expression. The biomarker of active pulmonary tuberculosis can be used as a therapeutic target, and is beneficial to development of new drugs.
Owner:GUANGDONG MEDICAL UNIV
Biomarkers for diagnosing and/or monitoring tuberculosis
InactiveCN104823052APromote or inhibit the production ofDisease diagnosisAntimycobacterialCausative organism
The invention relates to biomarkers for diagnosing and / or monitoring tuberculosisin both immunocompetent and immunocompromised individuals, monitoring the responses of individuals to anti-mycobacterial chemotherapy, monitoring the progression of latent tuberculosis to active tuberculosis, differentiating active tuberculosis from latent tuberculosis, and from other clinical conditions that mimic tuberculosis (TB). The invention also relates to methods for diagnosing, treating and monitoring tuberculosis using said biomarkers. The above pertain in all aspects both to pulmonary and extrapulmonary Mycobacterium.tuberculosis infections, with Mycobacterium.tuberculosis being the causative organism in tuberculosis.
Owner:PROTEINLOGIC
miRNA marker for assistant diagnosis of tuberculosis and application thereof
The invention discloses a miRNA marker for assistant diagnosis of tuberculosis and application thereof. The invention requires the protection of the application of a substance for detecting miRNAs inserum exosomes in the preparation of products used for diagnosing or assisting the diagnosis of tuberculosis patients or for diagnosing or assisting the diagnosis of active tuberculosis patients, wherein each miRNA refers to one or a combination of the following five kinds of miRNAs: miR-28-3p, miR-193b-5p, miR-370-3p, miR-1246 and miR-2110. The invention also requires the application of the substance for detecting miRNAs in serum exosomes in the preparation of products used for diagnosing or assisting the diagnosis of patients with latent tuberculosis infections, wherein each miRNA refers toone or a combination of the following three kinds of miRNAs: let-7e-5p, let-7d-5p and miR-140-5p. A foundation is laid for further development of the early rapid diagnosis technology and method for tuberculosis.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV
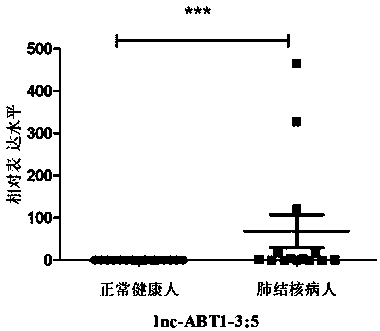
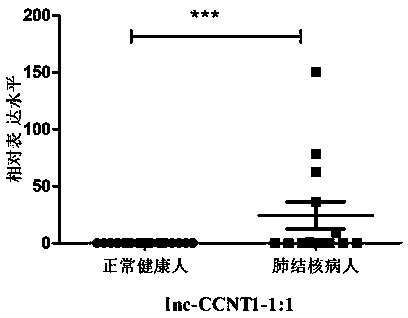
Application of lncRNAs as active tuberculosis specific markers
ActiveCN107653315AMicrobiological testing/measurementDNA/RNA fragmentationPhases of clinical researchWhole blood sample
The invention discloses application of lncRNAs as active tuberculosis specific markers. Due to tissue specificity and spatial-temporal specificity of lncRNAs, different tissues are different in lncRNAs expression quantity, and the same tissue or organ varies in lncRNAs expression quantity in different growth stages. Two lncRNAs serving as the active tuberculosis specific markers are disclosed forthe first time, a whole blood sample which can be acquired easily, directly and clinically is combined with designed primer pairs to verify lncRNAs differential expression through RT-qPCR, and quickness, simplicity, time saving, high detection sensitivity and low sample consumption are realized. The method is quite suitable for active tuberculosis diagnosis by lncRNAs in differential expression, and important significances to researching of tuberculosis propagation control and reduction of the tuberculosis occurrence rate are achieved.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
Biomarker for diagnosing mycobacterium tuberculosis infection and related kit
InactiveCN106501530AImprove diagnostic efficiencyEasy to operateBiological material analysisBiological testingAntigenInfection diagnosis
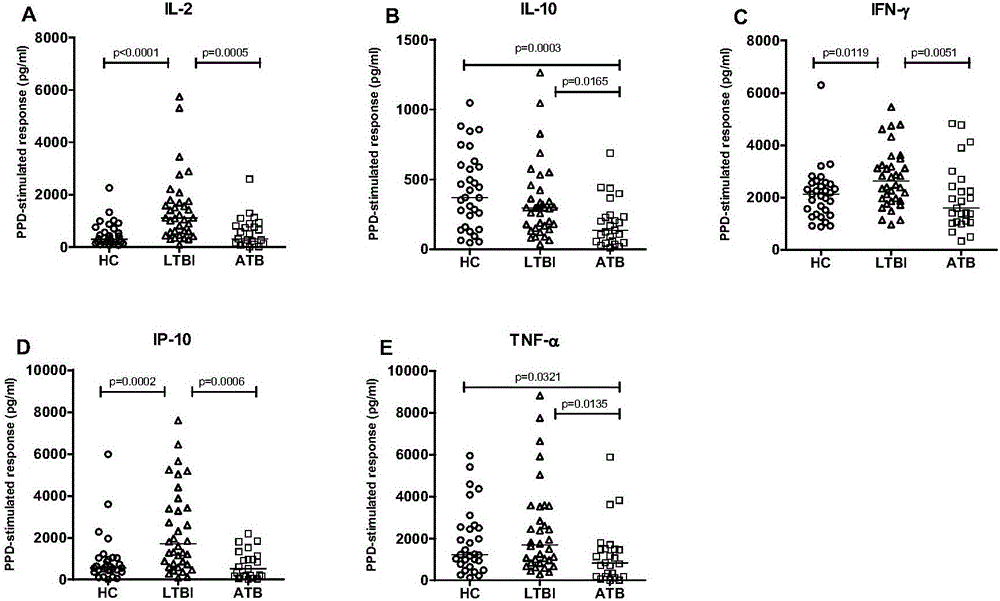
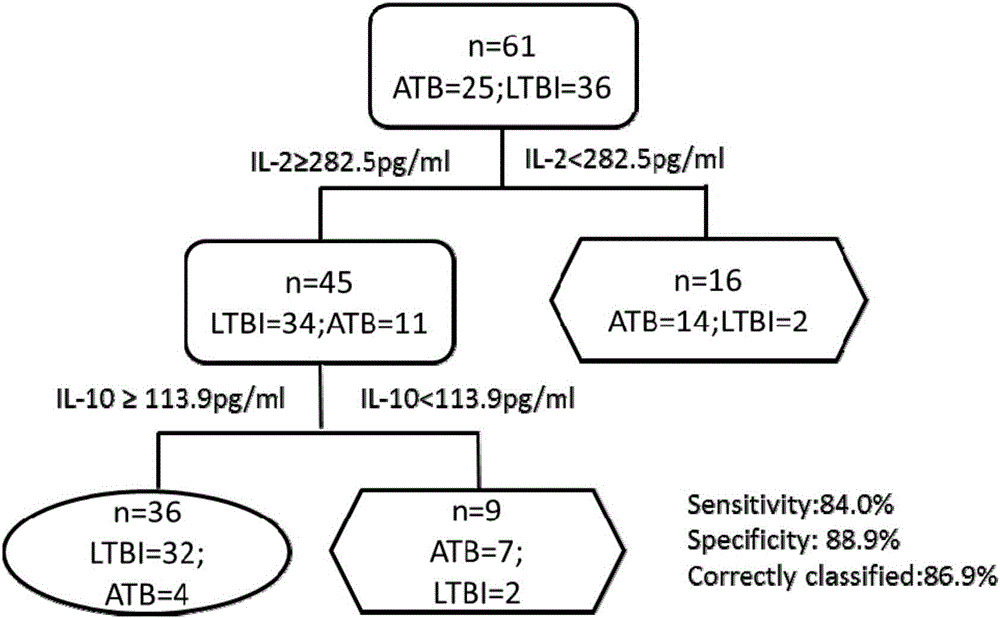
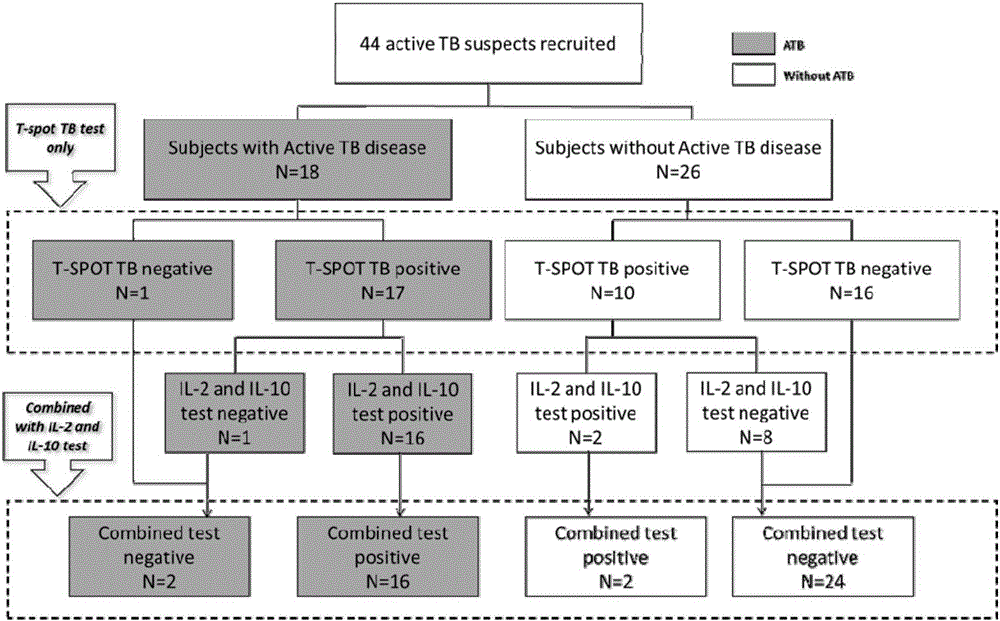
The invention discloses a biomarker for diagnosing mycobacterium tuberculosis infection. The biomarker comprises IL-2 and IL-10. The invention proves that cell factors IL-2 and IL-10 can serve as markers for tuberculosis infection diagnosis for the first time, the tuberculosis infection can be diagnosed by measuring the concentration of the IL-2 and the IL-10 after peripheral blood monouclear cells are stimulated by tuberculosis antigen, and active tuberculosis and latent tuberculosis infection are further distinguished. A tuberculosis infection blood detection reagent and a kit which are prepared on the basis of the two cell factors provide new technological basis for quick diagnosis of the active tuberculosis, have the advantages of high sensitivity, high specificity and the like, are quick in operation, reasonable and practical, and can obviously improve the diagnosis efficiency of the active tuberculosis.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
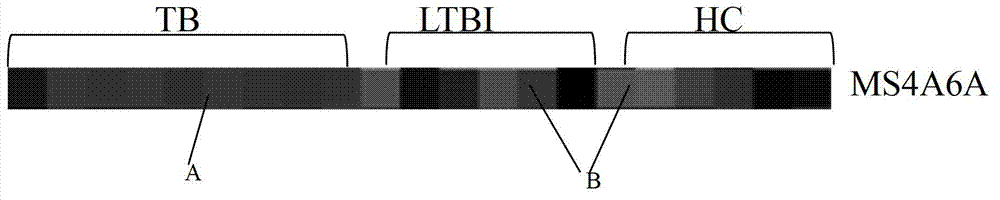
MS4A6A gene application
InactiveCN103074422AMicrobiological testing/measurementDNA/RNA fragmentationCrowdsLatent tuberculosis
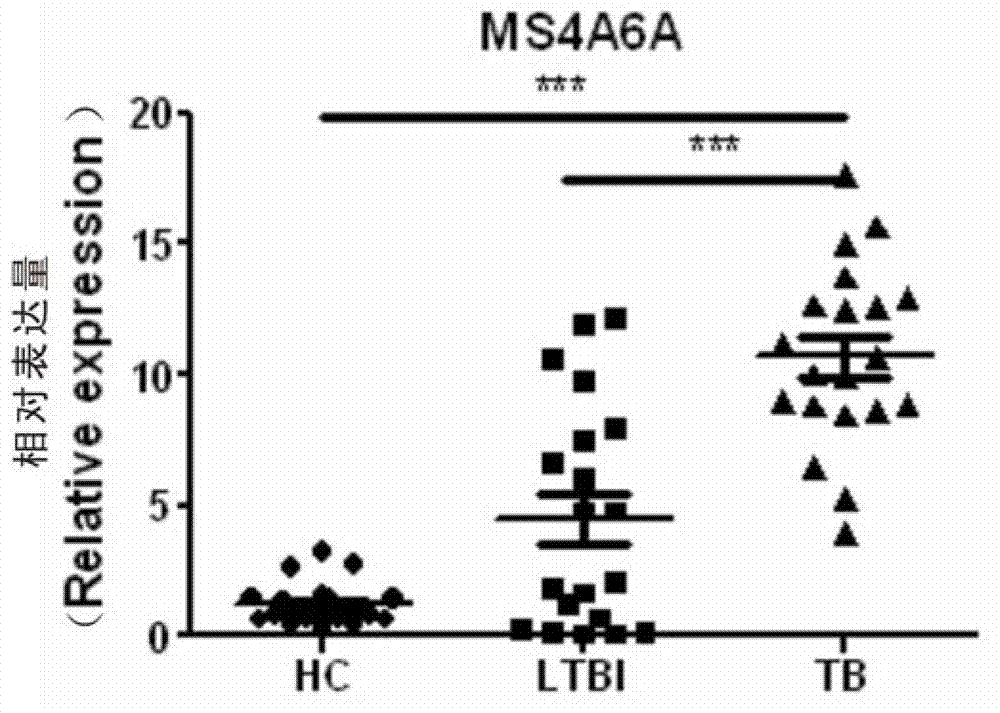
The invention provides an MS4A6A gene application, and relates to the preparation of products to distinguish latent tuberculosis infection and active tuberculosis. The preferred products include the products utilizing real-time quantitative PCR or gene chip detection to distinguish the latent tuberculosis infection and the active tuberculosis. According to the experimental results, the expression of the MS4A6A gene is obviously higher in the blood of tuberculosis patients than in healthy people or latent infection crowds, and therefore, the MS4A6A gene can serve as a special marking gene for the diagnosis of tuberculosis, so as to allow the tuberculosis diagnosis to be more accurate and quicker.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
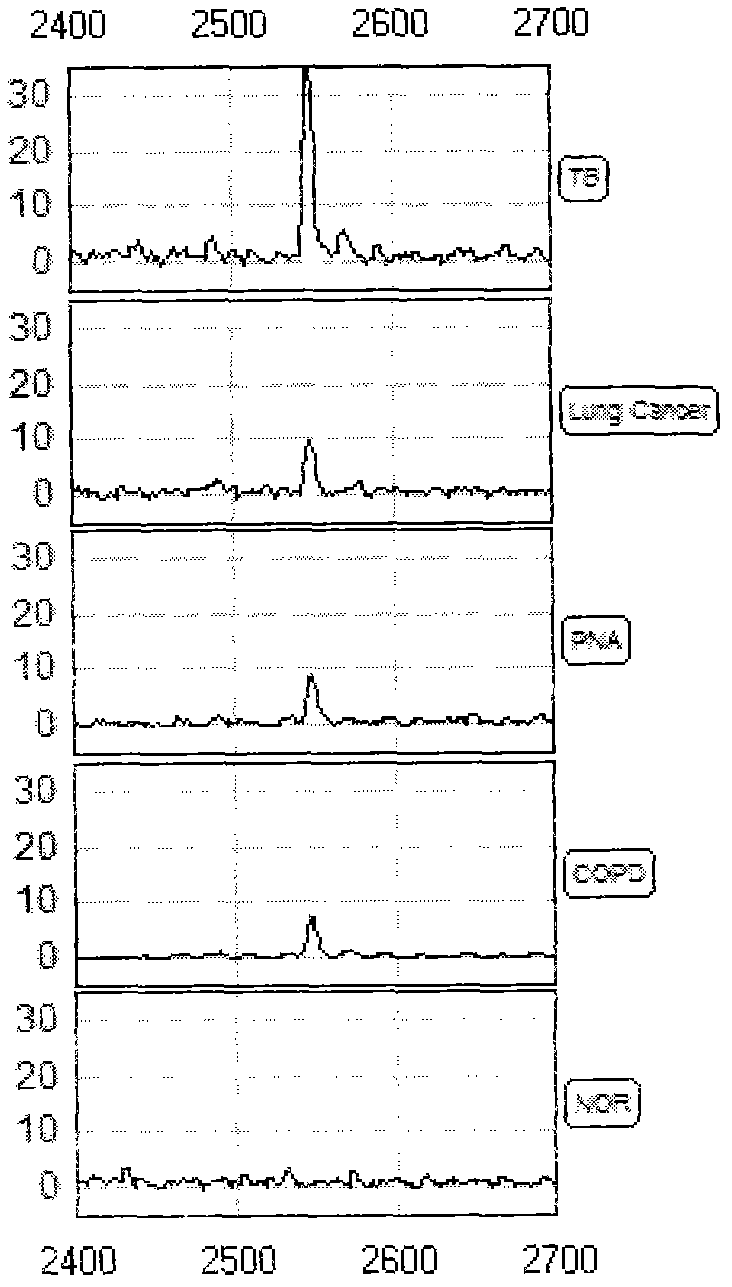
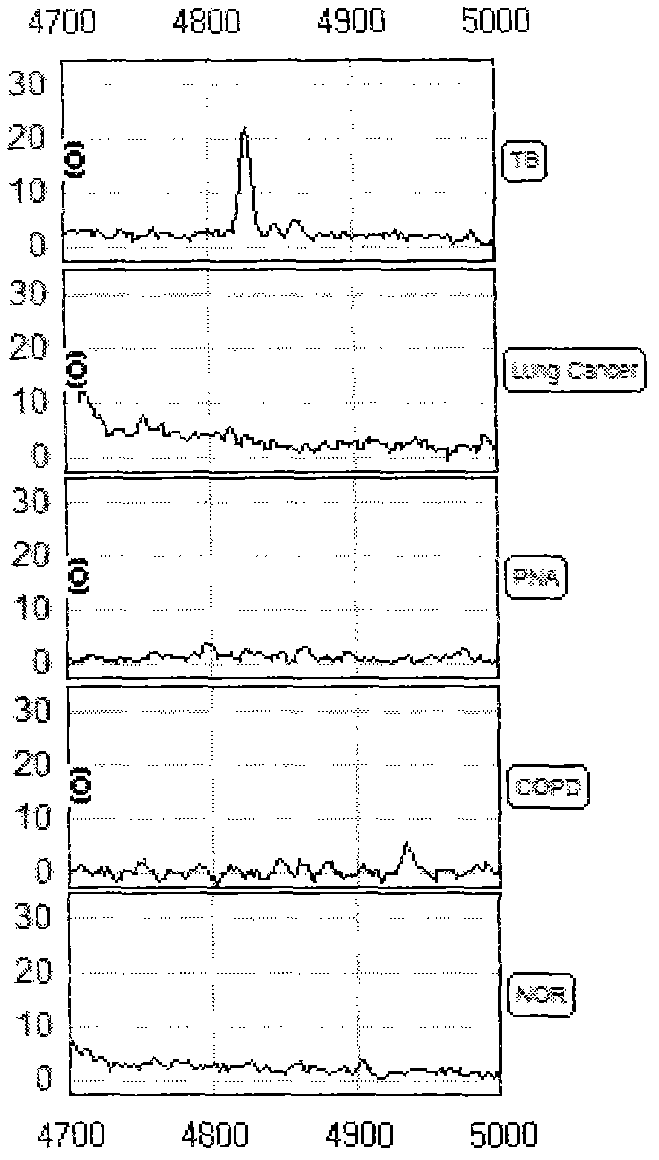
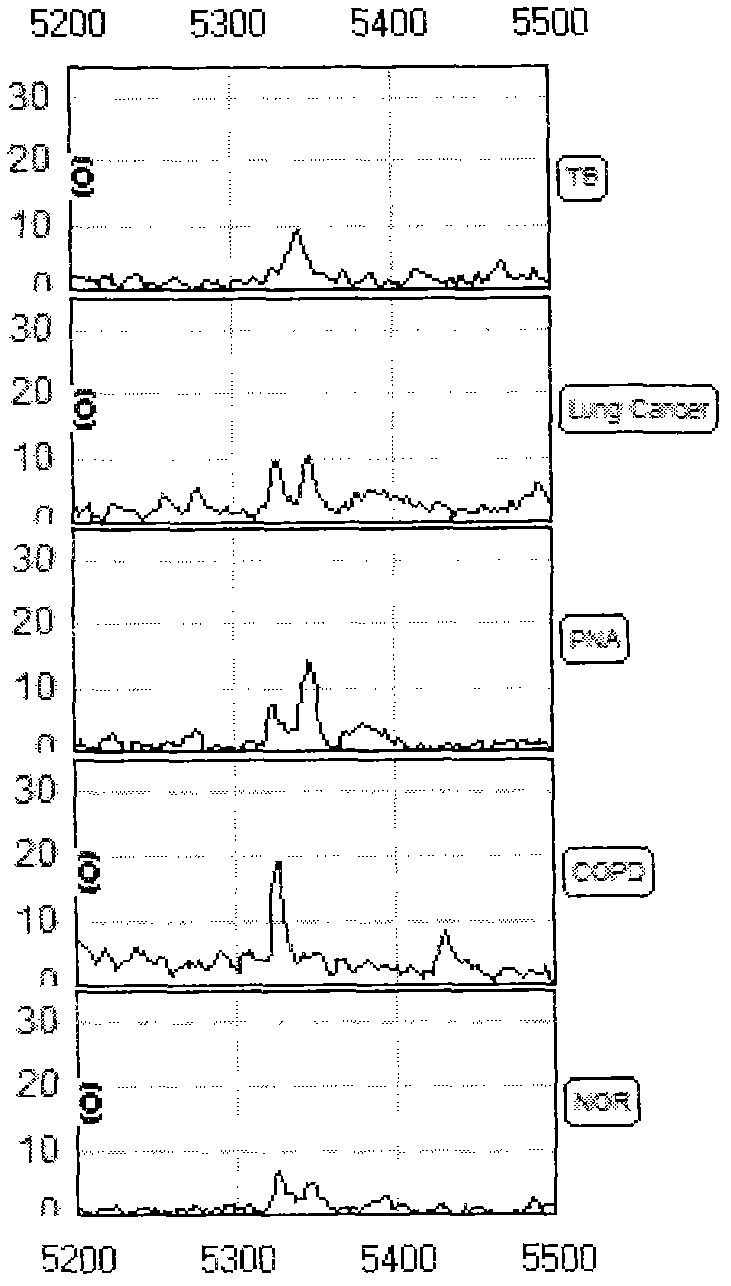
Sample containing tuberculosis serum characterized protein and preparation method thereof
ActiveCN102661884APreparing sample for investigationMaterial analysis by electric/magnetic meansObstructive Pulmonary DiseasesNon invasive
The invention relates to a sample containing a tuberculosis serum characterized protein and a preparation method thereof. The sample comprises the serum of an untreated patient suffering from active tuberculosis, and the serum protein of a patient suffering from lung cancer, a patient suffering from pneumonia, a patient suffering from chronic obstructive pulmonary disease or a healthy person who is ensured to be normal by physical examination, wherein the serum protein comprises three up-regulated proteins and one down-regulated protein; the proteins exit independently or any or all of the proteins are mixed together; and the sample is suitable to be detected by using mass spectrum. The sample provides a basis for further discovering new active tuberculosis biomarkers. By using the sample, the detection of the active tuberculosis is superior to any single detection method adopted currently; a non-invasive technology for early discovery and early treatment of the active tuberculosis is provided; and therefore, a new method for reducing the infectious rate of the active tuberculosis and improving the recovery rate of the tuberculosis is provided.
Owner:ZHEJIANG UNIV
Method for Screening of Active Tuberculosis
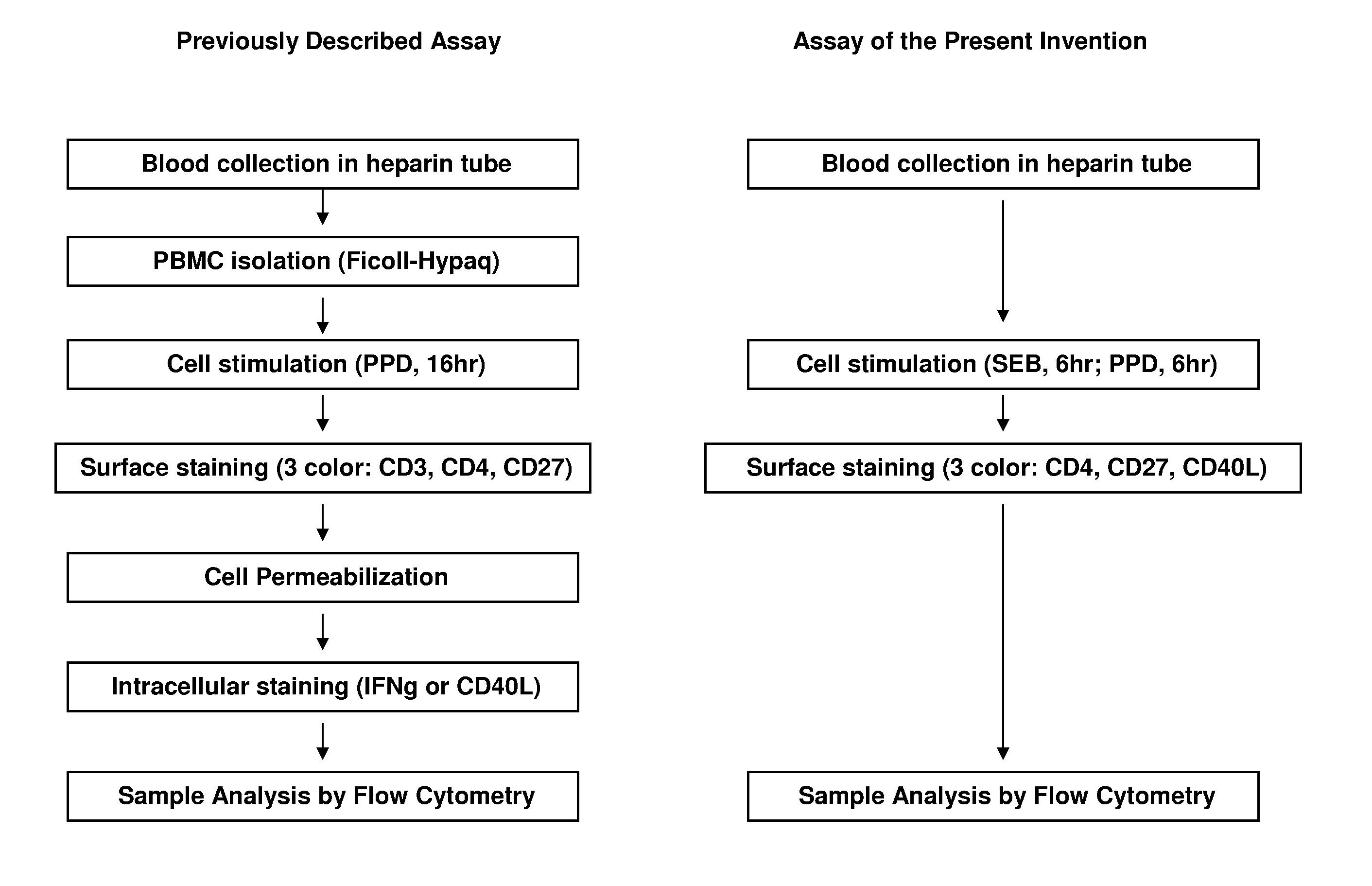
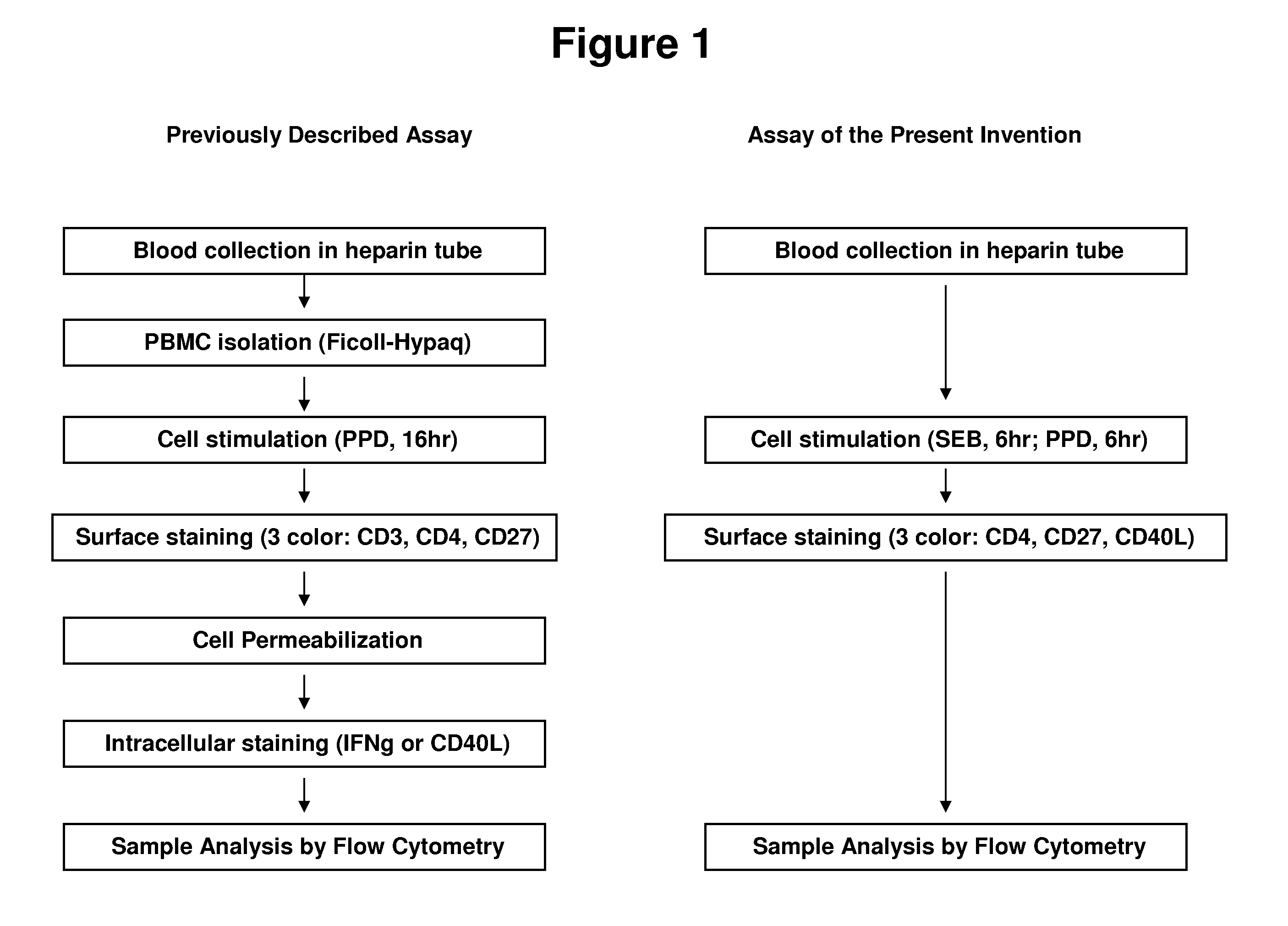
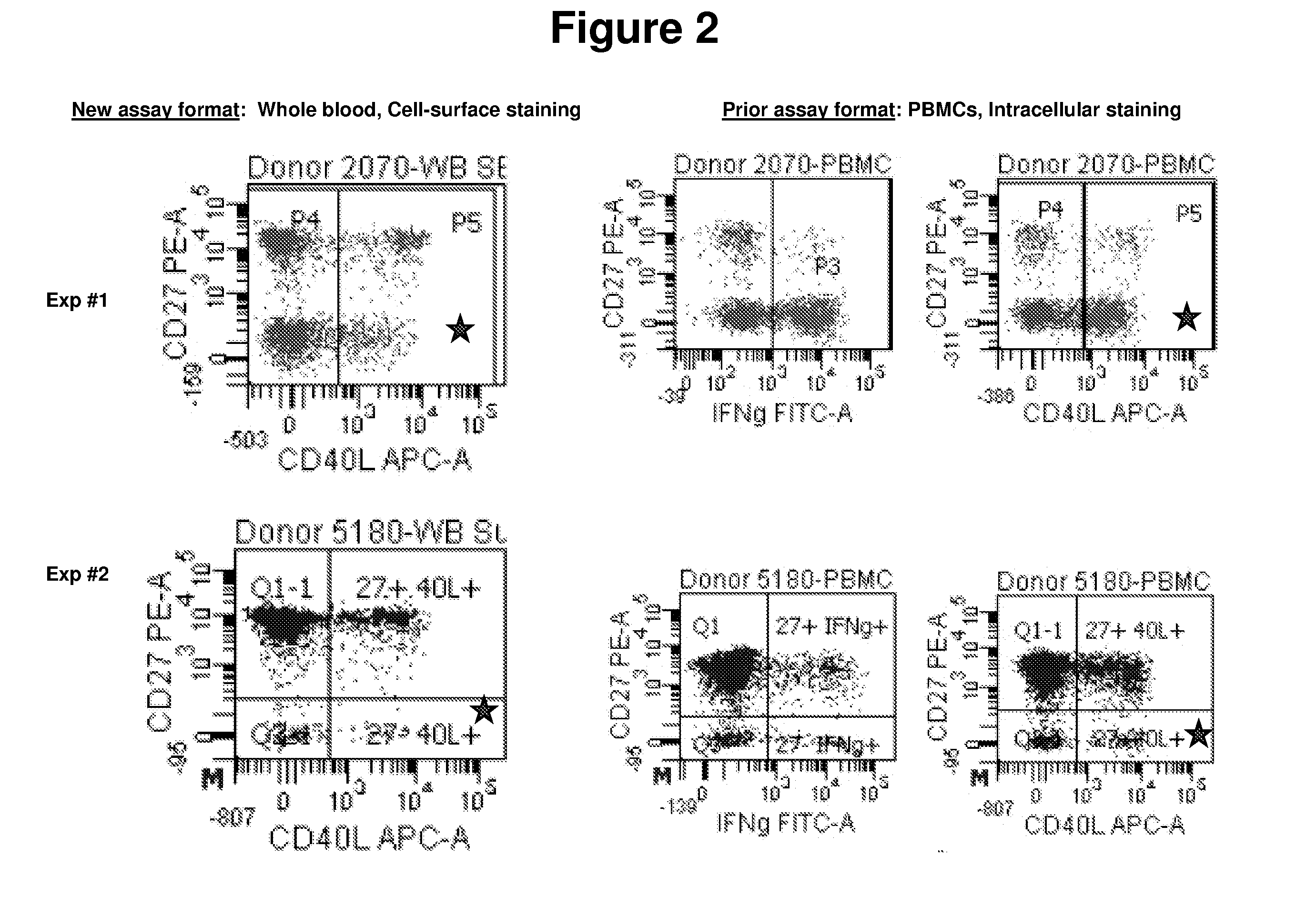
InactiveUS20110070599A1Improved and simplifiedSimplifying assayBiological testingImmunoassaysCell surface stainingT cell
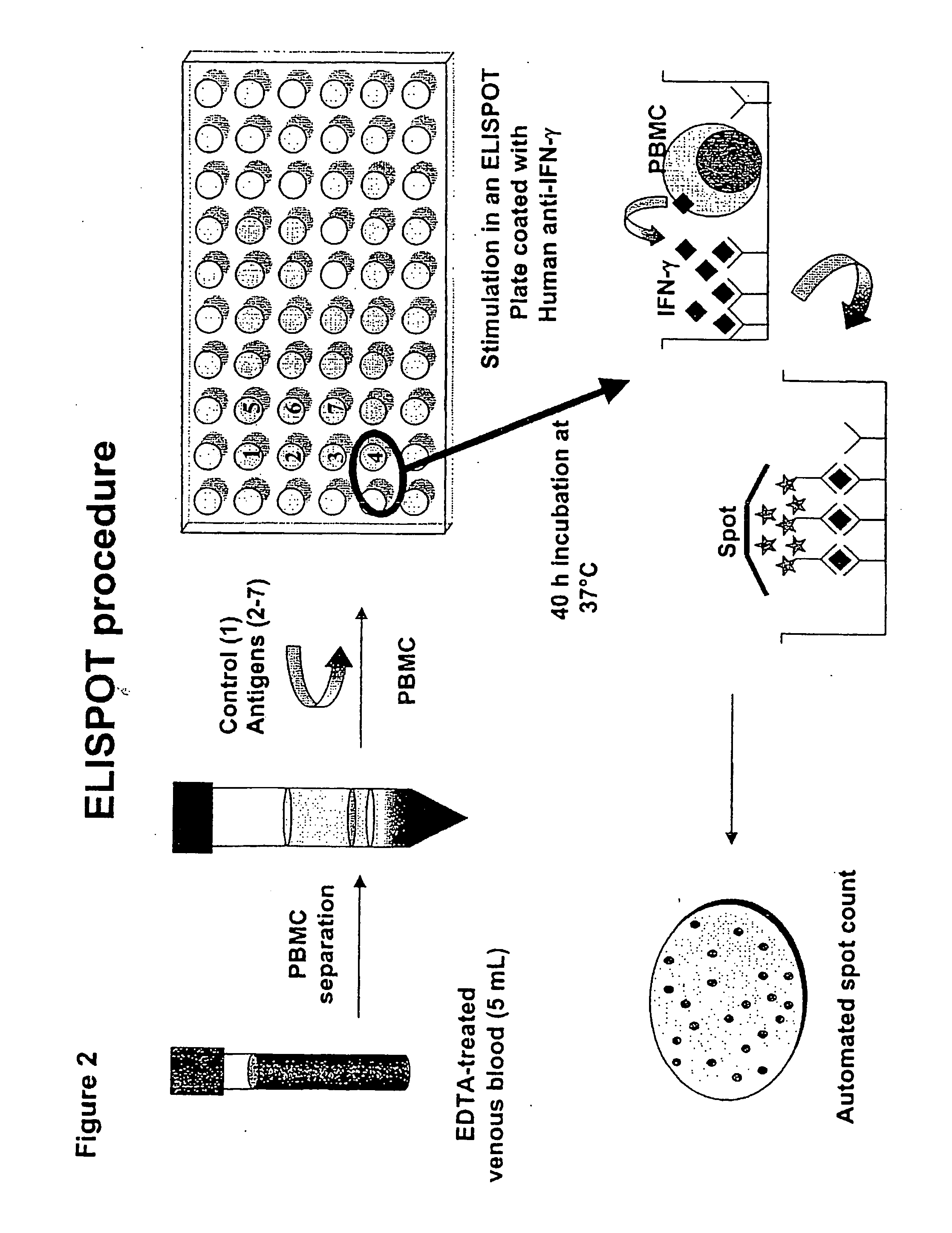
The present invention provides an improved and simplified assay for use in diagnosing active Tuberculosis infections. The present assay is carried out using a sample of whole blood, does not require separating the blood into components, such as the isolation of peripheral blood mononucleocytes (PBMC), and is carried out using only cell-surface staining of T-cells.
Owner:BECTON DICKINSON & CO
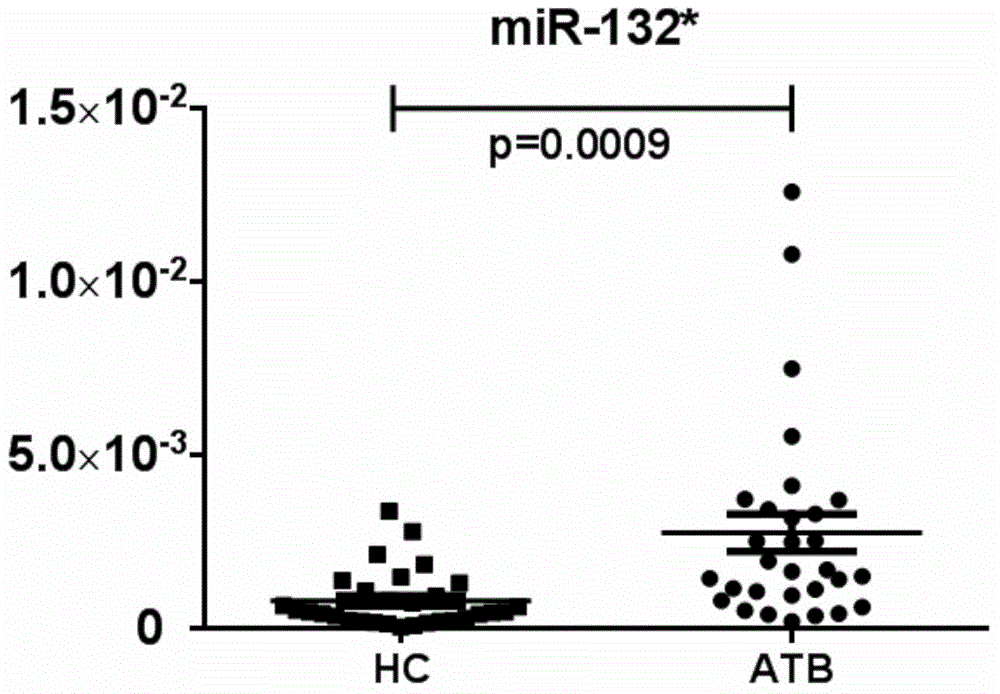
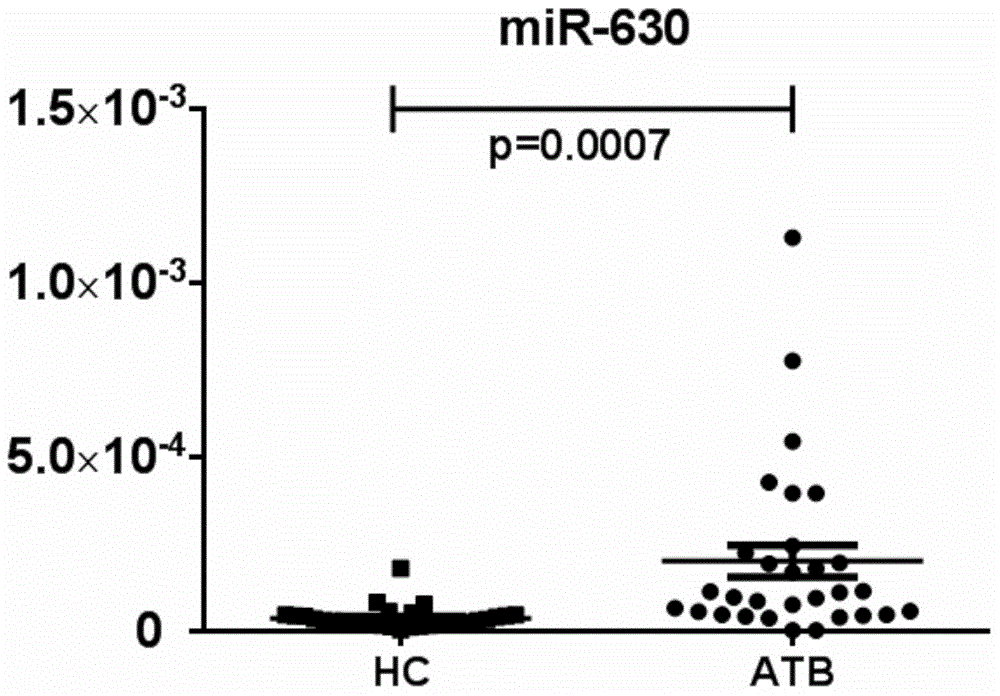
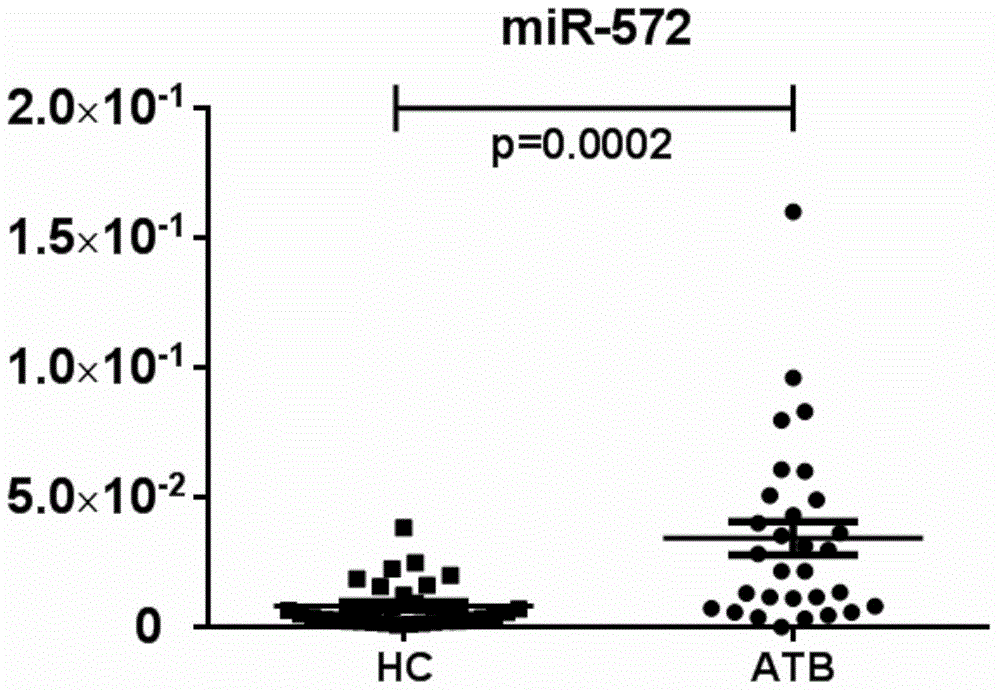
MicroRNA (Ribose Nucleic Acid) and application thereof in preparation of active tuberculosis detection reagent
ActiveCN104017806ARapid diagnosisHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMicroRNABuffer solution
The invention further discloses a microRNA (Ribose Nucleic Acid) sequenced as SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4 and an application of the microRNA in preparation of an active tuberculosis detection reagent. The active tuberculosis detection kit comprises an amplification specificity primer, a real-time PCR (Polymerase Chain Reaction) kit and an internal reference primer, and can further comprise a microRNA reverse transcription system and a buffer solution. The content of the microRNA is specially detected via a real-time PCR relative quantitative method; and the microRNA is compared with the internal reference microRNA so as to detect the tuberculosis. The microRNA disclosed by the invention has the high sensitivity and the high accuracy for detecting the active tuberculosis and is rapid in detection speed, so that the diagnostic efficiency of the active tuberculosis can be greatly improved.
Owner:FUDAN UNIV +1
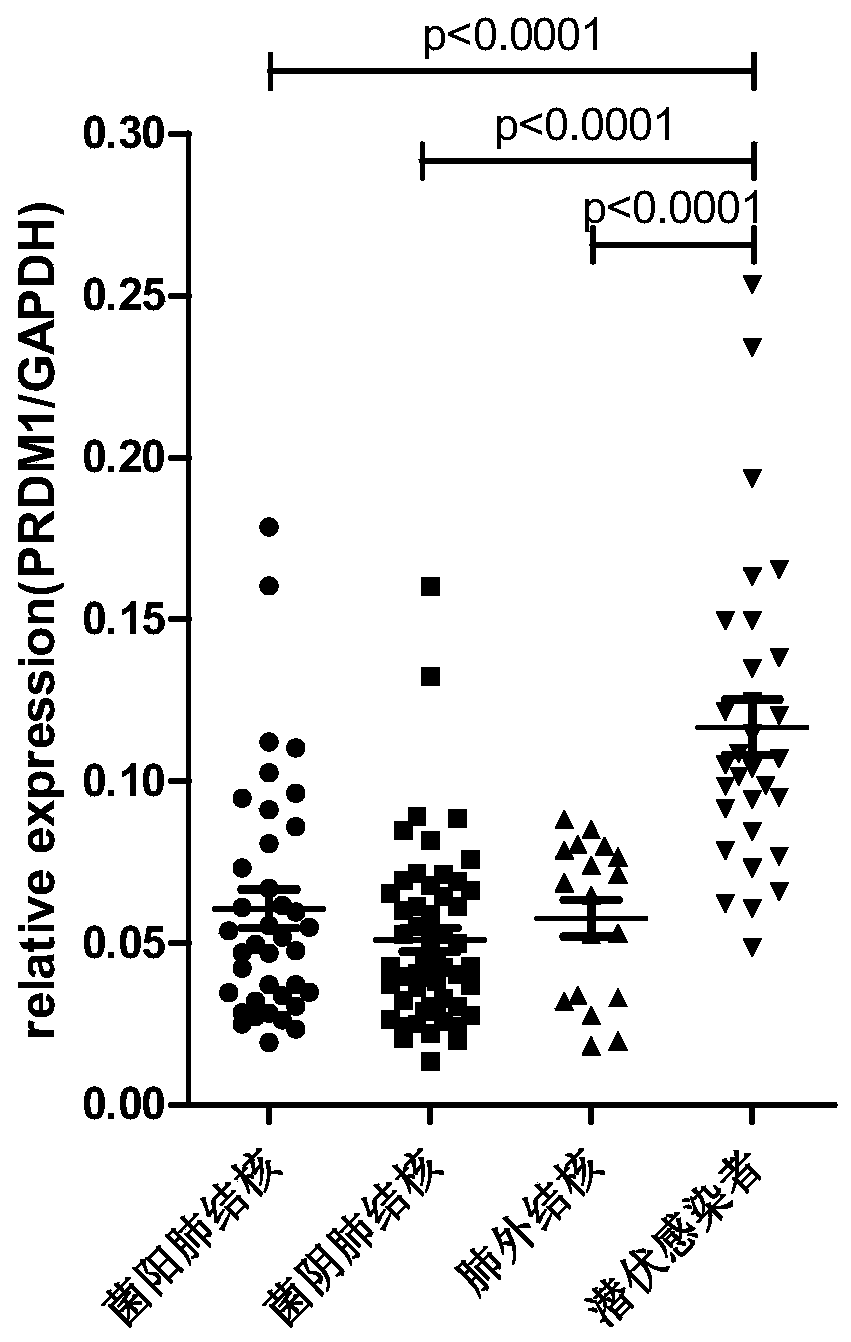
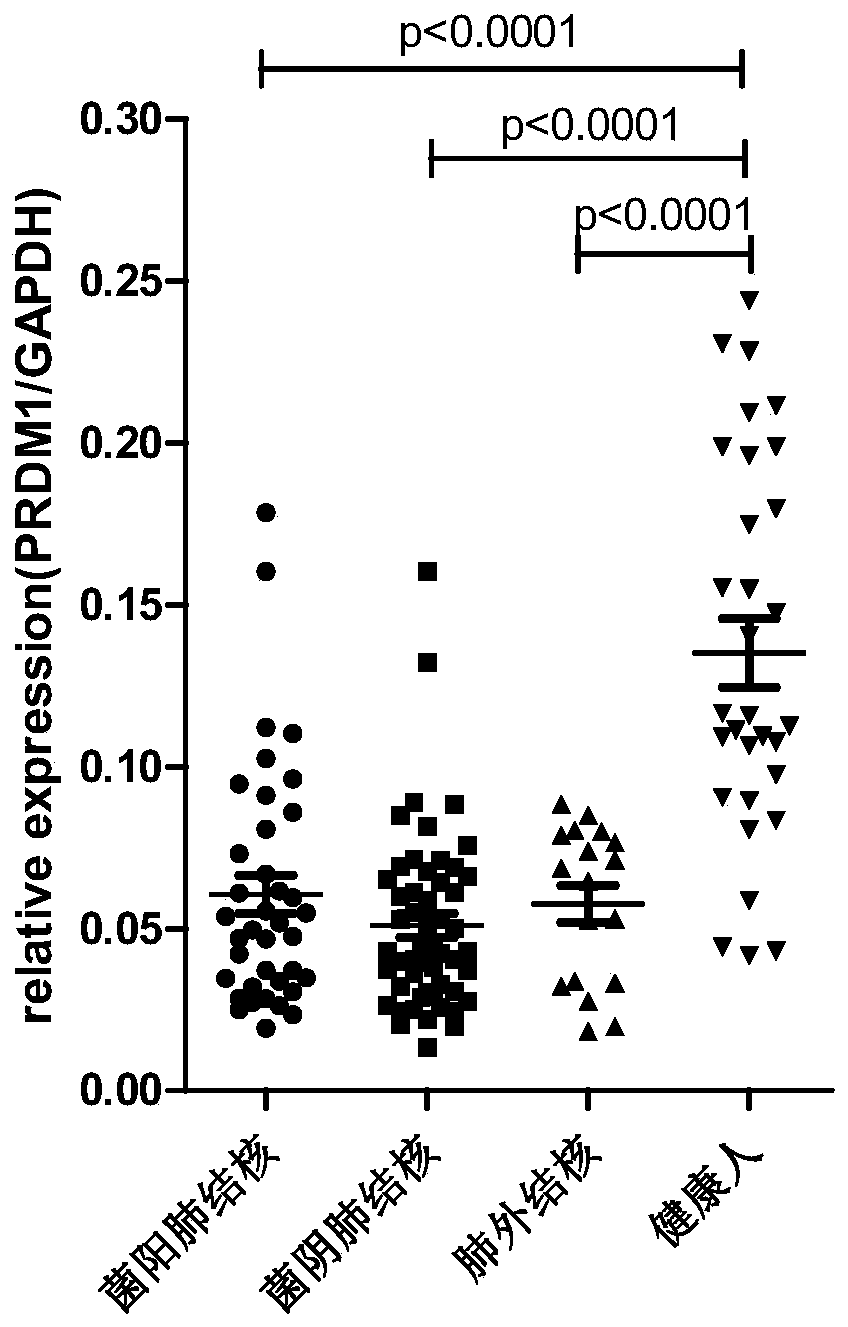
Application of PRDM1 as marker for preparing product for diagnosing active tuberculosis
InactiveCN110295229AMicrobiological testing/measurementDisease diagnosisFluorescenceLatent tuberculosis
The invention discloses application of PRDM1 as a marker for preparing a product for diagnosing active tuberculosis. Fluorescence quantitative PCR is adopted for detecting expression quantity of a PRDM1 gene in a tuberculosis patient PBMCs. A result shows that the relative expression quantity of PRDM1 in smear positive tuberculosis patients, smear negative tuberculosis patients and extrapulmonarytubereulosis patients PBMCs is remarkably lowered that that of latent tuberculosis infected persons and healthy control. A subject working characteristic curve analysis result shows that PRDM1 can distinguish active tuberculosis and latent tuberculosis infection, and becomes the diagnosis target for diagnosing the active tuberculosis.
Owner:中国人民解放军总医院第八医学中心
Co-expression system and construction method of polyvalent bacteriophage lyase genes, live vaccine of carrying system and preparation and application of live vaccine
InactiveCN104673821AEnables cheap scalingNo autolysisAntibacterial agentsBacterial antigen ingredientsMycobacterium smegmatisLatent tuberculosis
The invention discloses a co-expression system and construction method of polyvalent bacteriophage lyase genes, a live vaccine of a carrying system and preparation and application of the live vaccine. Eukaryotic expression plasmids are used as an expression vector, and LysinA, LysinB and Holin gene segments are directionally inserted into the plasmids to simultaneously express the co-expression system of the bacteriophage lyase genes of three kinds of targeted mycobacterium tuberculosis. Mycobacterium smegmatis with good targeting property of macrophages or genetically-modified recombinant BCG is used as a live vector, the co-expression system simultaneously carrying three kinds of genes is electrically transformed into the mycobacterium smegmatis or the genetically-modified recombinant BCG, and then the recombinant therapeutic tuberculosis live vaccine is obtained through expansion in vitro. The live vaccine has a good effect on the field of curing active tuberculosis or latent tuberculosis infection caused by proliferative mycobacterium tuberculosis, dormant mycobacterium tuberculosis and drug-resistant mycobacterium tuberculosis.
Owner:伊正君
Pulmonary tuberculosis variation activity marker, kit, method and model construction method
PendingCN113178263AGood clinical valueOvercoming low diagnostic detection ratesMedical simulationProteomicsLung tuberculosisBlood plasma
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Active tuberculosis marker, kit, detection method and model construction method
PendingCN113192552AOvercoming low diagnostic detection ratesOvercome time-consuming and other shortcomingsMedical simulationBiostatisticsAPOA4Blood plasma
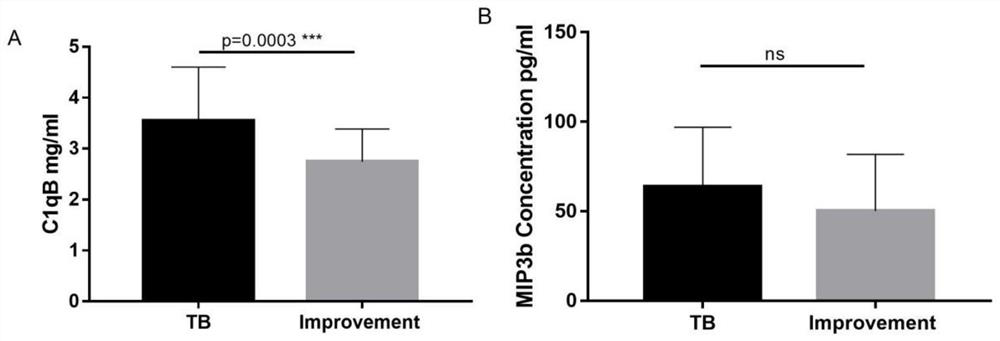
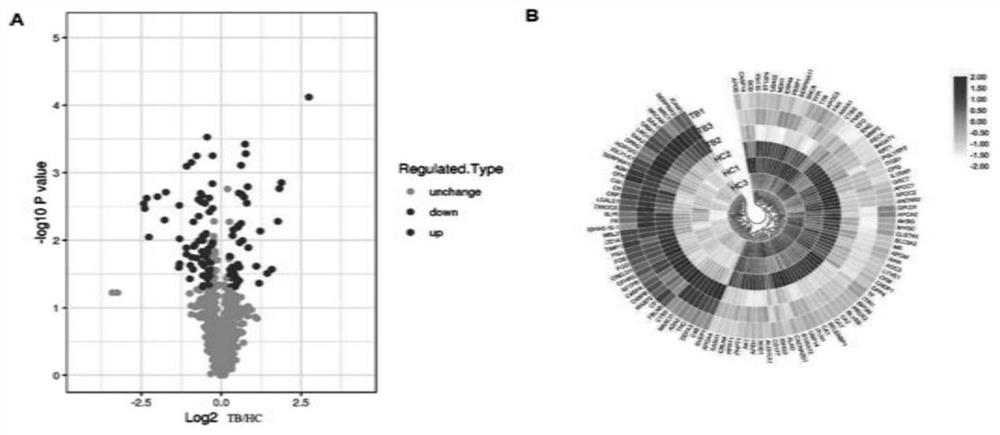
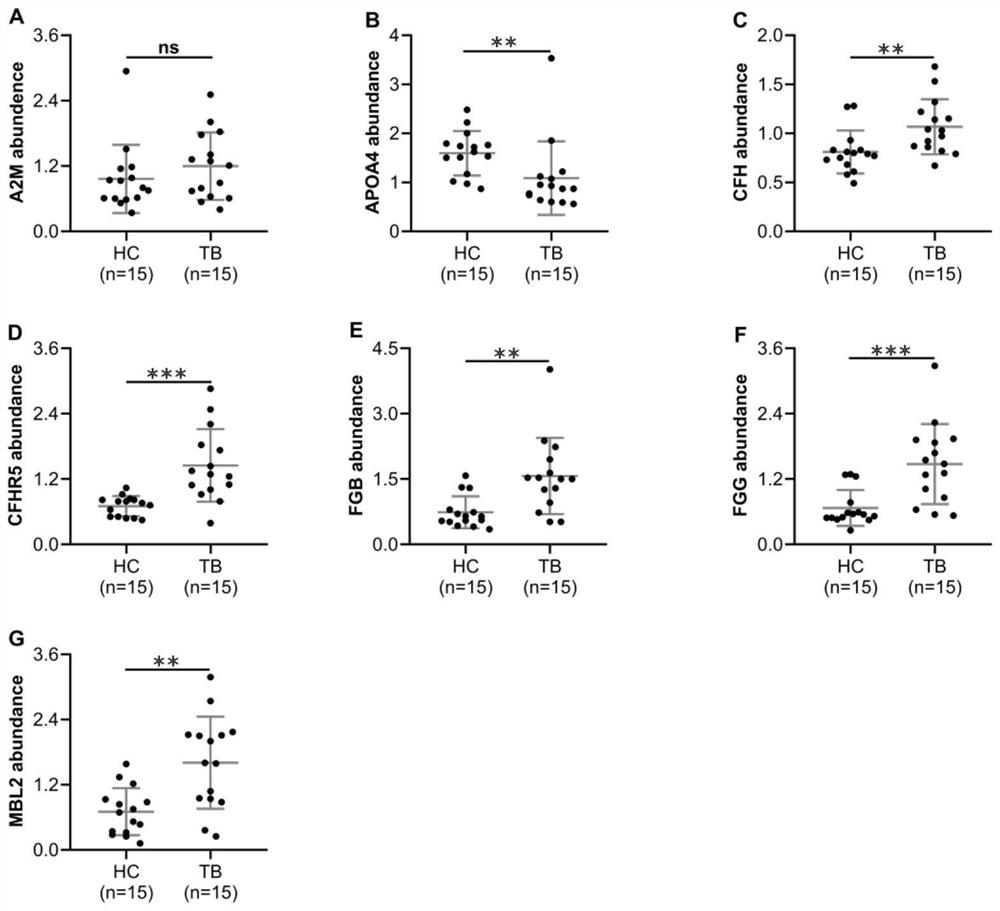
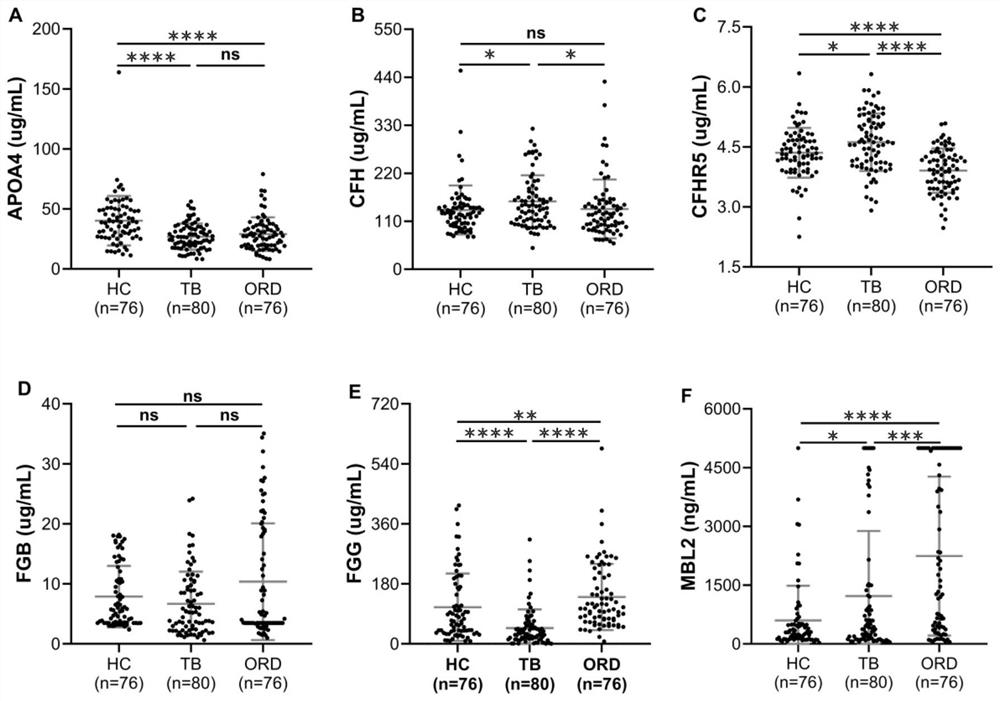
The invention provides an active tuberculosis marker, a kit, a detection method and a model construction method. An active tuberculosis diagnostic marker is a plasma protein biomarker in a blood sample, wherein the plasma protein biomarker is a combined marker comprising one or more of APOA4 protein, CFH protein, CFHR5 protein, FGG protein and MBL2 protein. According to the invention, the combined marker of APOA4, CFH, CFHR5 and FGG derived from plasma protein is used as the biomarker for active tuberculosis detection for the first time, a rapid diagnosis model for active tuberculosis detection is constructed, and a new direction is provided for clinical diagnosis of tuberculosis. Therefore, the invention overcomes the defects of low detection rate, long time consumption and the like of the existing active tuberculosis diagnosis, has the characteristics of good specificity and high sensitivity, and has good clinical application value for the auxiliary diagnosis of the active tuberculosis.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Screening and application of active tuberculosis diagnosis molecules
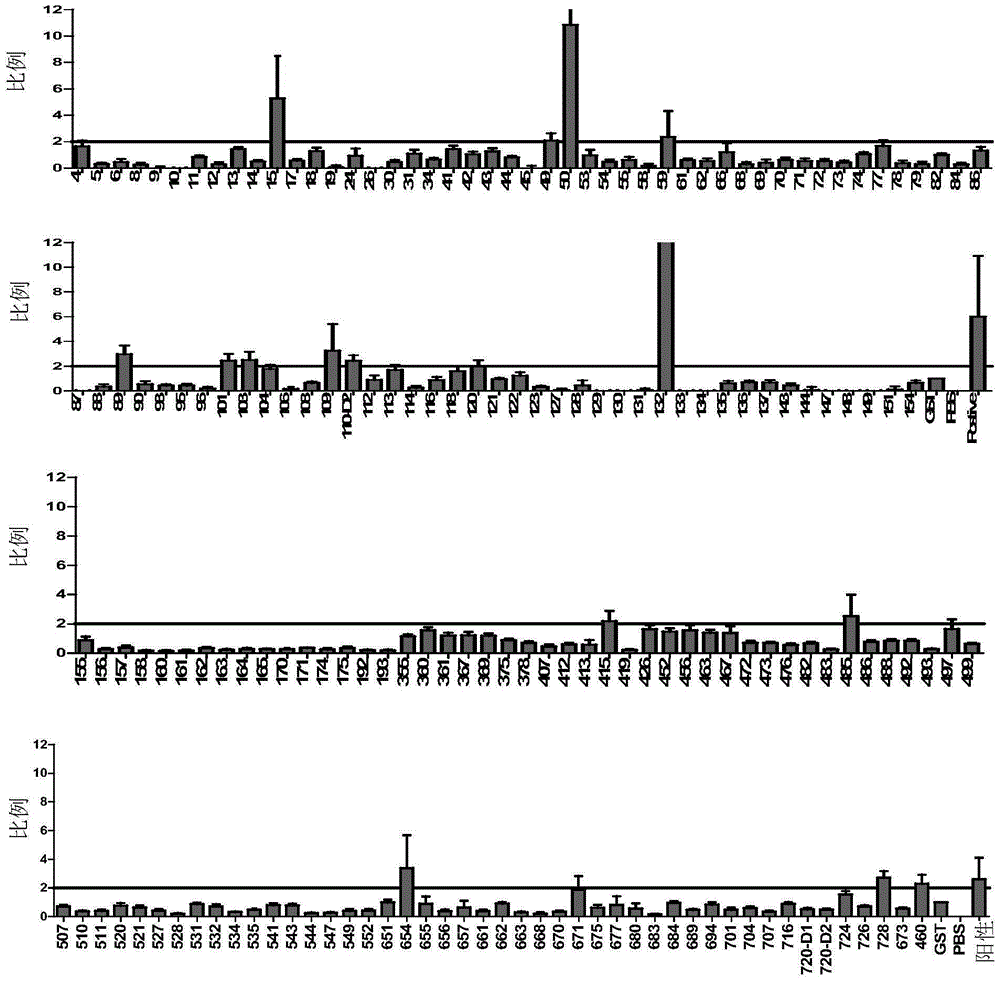
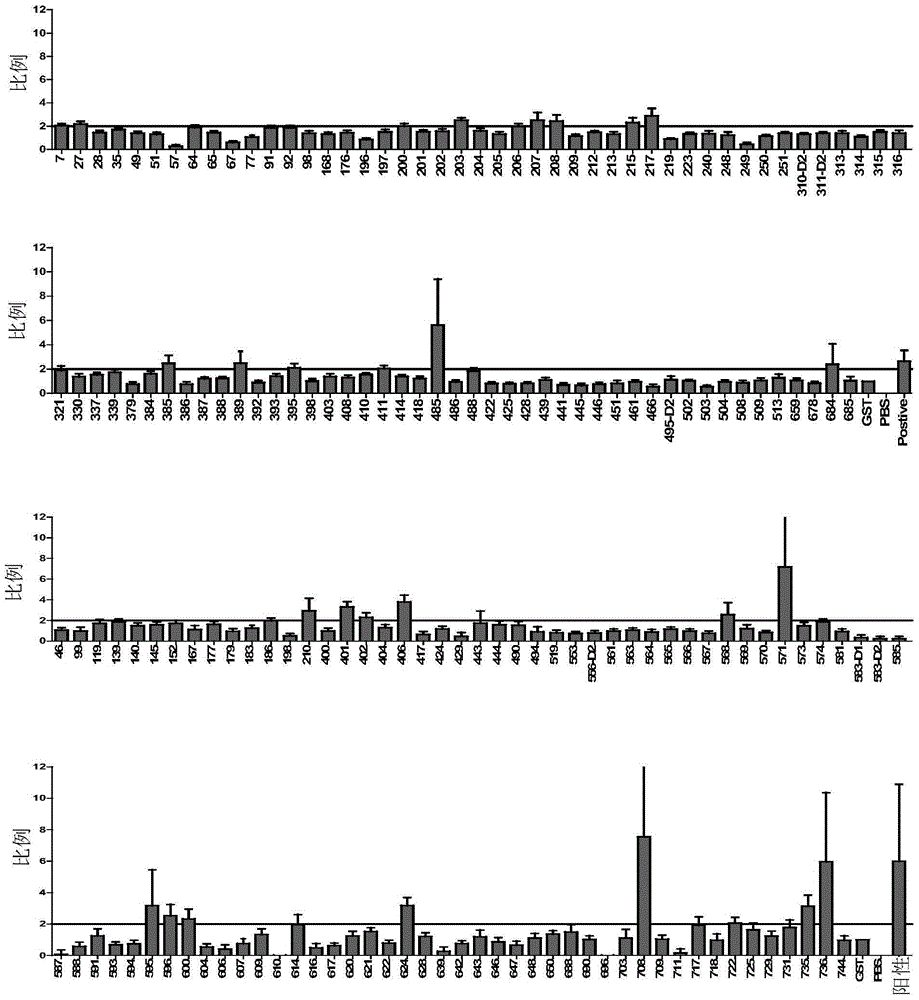
ActiveCN106589082AHigh sensitivityStrong specificityAntibody mimetics/scaffoldsImmunoglobulins against bacteriaHigh-Throughput Screening MethodsSerological assay
The invention provides screening and application of active tuberculosis diagnosis molecules. In particular, the invention discloses high throughput screening of mycobacterium tuberculosis's important antigens and application thereof in active tuberculosis diagnosis. Based on a high throughput functional protein screening technology of GST fusion expression, 92 positive antigens recognizable by tuberculosis patients' serum are screened out, wherein 14 antigens present strongly positive reaction. Active tuberculosis serologic detection shows that the positive antigens have high sensitivity and specificity in detection of active tuberculosis. Specifically, TBGP1, TBGP2, TBGP3, TBGP4, TBGP5 and TBGP6 6 proteins form an antigen combination for tuberculosis serologic detection, and laboratory verification shows that the combination has high sensitivity and specificity in detection of mycobacterium tuberculosis, and has application value in tuberculosis diagnosis and monitoring.
Owner:TONGJI UNIV
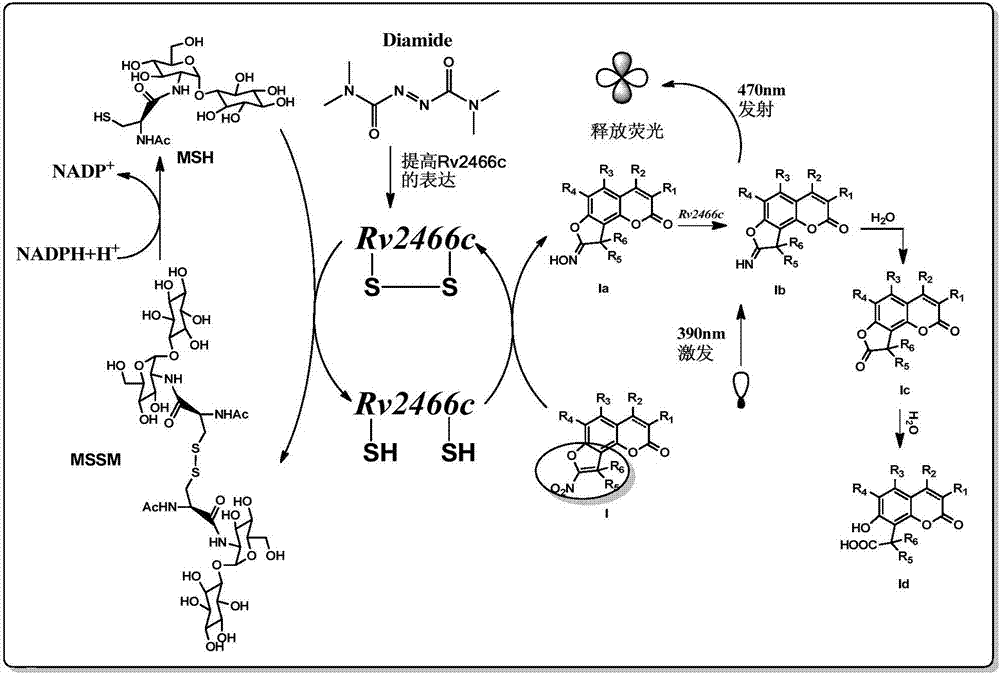
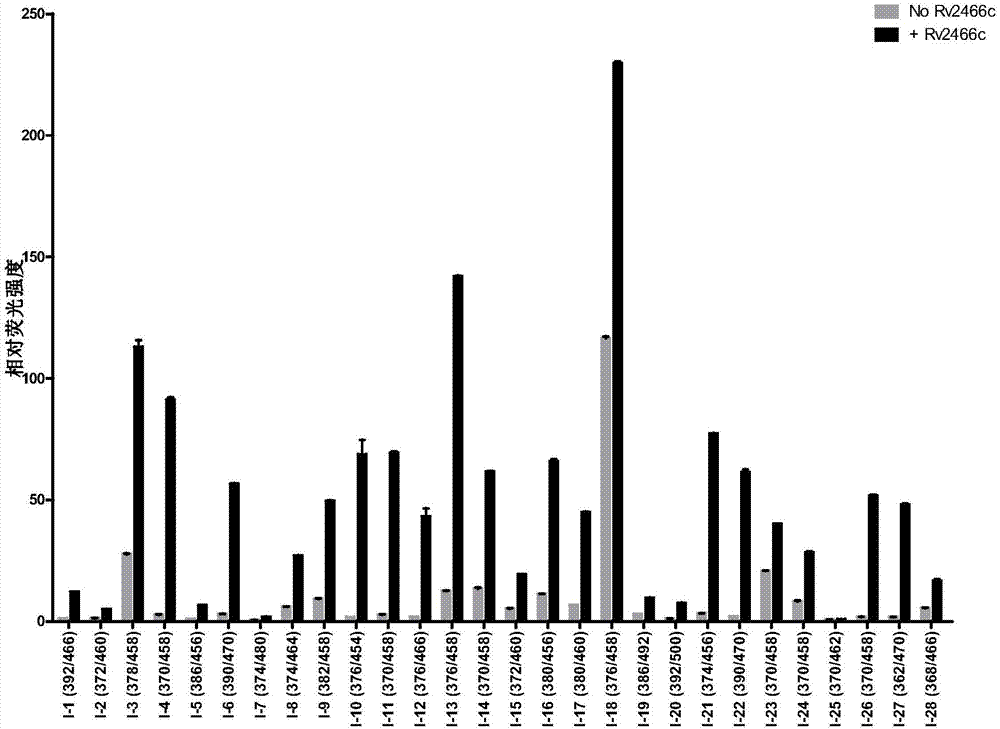
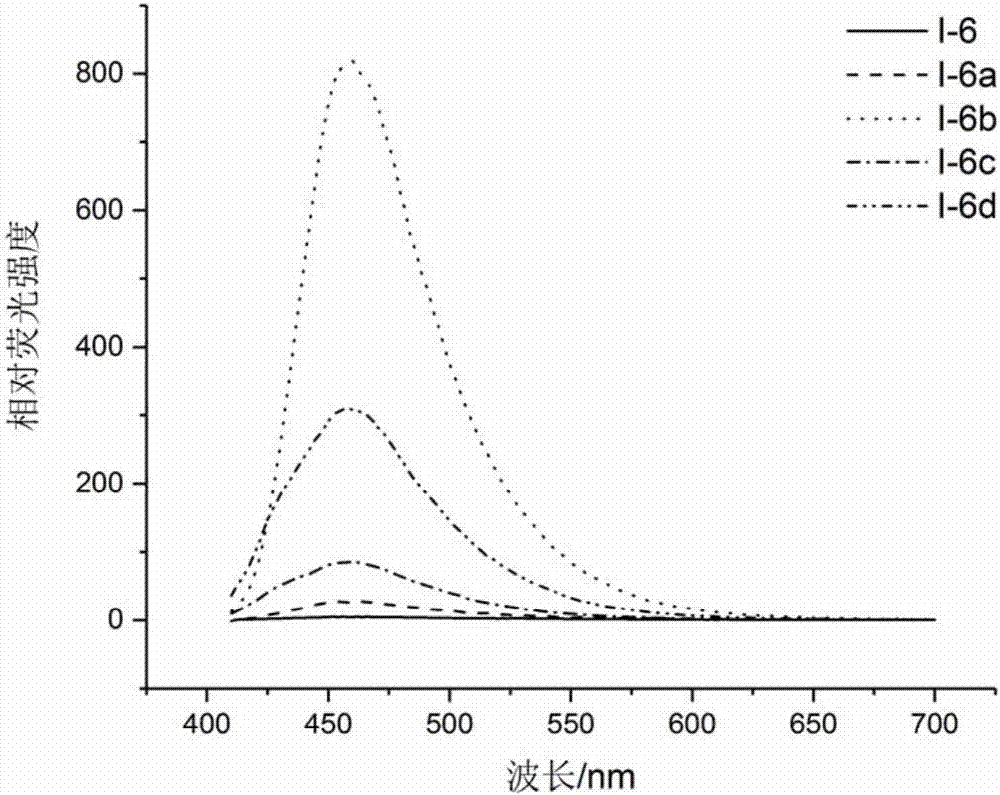
Compound and application thereof to detection of mycobacterium tuberculosis
The invention provides a compound and an application thereof to detection of mycobacterium tuberculosis. The compound is a compound or derivative thereof shown in the formula I as in the description. The compound or derivative thereof shown in the formula I is taken as a detection reagent and contacts a to-be-detected sample. Based on fluorescence change of the to-be-detected sample before or after the contact, whether mycobacterium tuberculosis exists in the to-be-detected sample can be effectively determined, and the result is accurate and reliable. Further, the detection information can be effectively used for diagnosing whether a suspected patient, where the to-be-detected sample comes, suffers from diseases related to mycobacterium tuberculosis. Therefore, tuberculosis positive patients (active tuberculosis patients) and mycobacterium tuberculosis carriers can be detected.
Owner:TSINGHUA UNIV
Mycobacterium tuberculosis combined antigen for diagnosing pulmonary tuberculosis
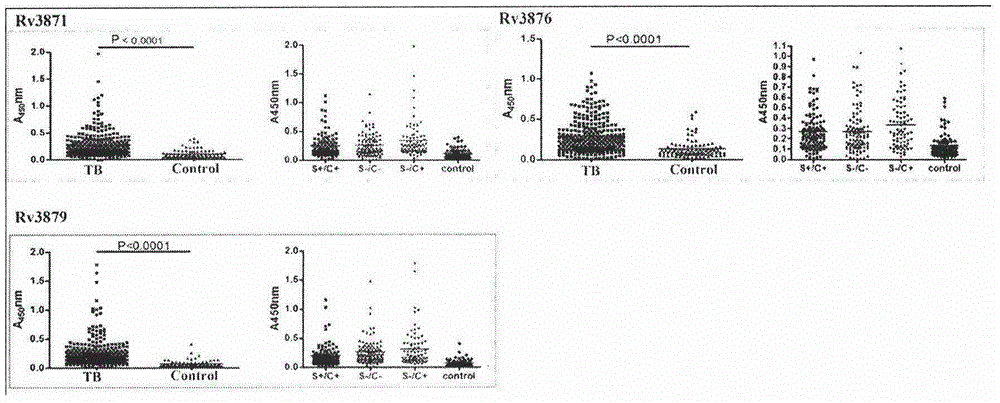
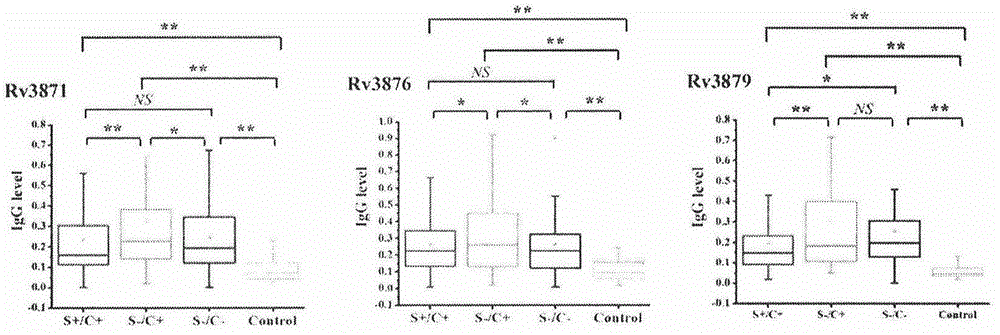
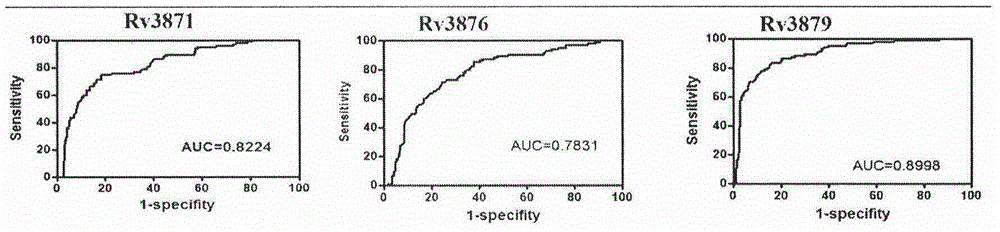
ActiveCN104678097AHigh diagnostic efficiencyHigh sensitivityDepsipeptidesDisease diagnosisOrganismAntibody level
The invention provides a combined antigen comprising three mycobacterium tuberculosis antigens and an application of the combined antigen to preparation of a reagent for diagnosing active tuberculosis. The combined antigen comprises Rv3871, Rv3876 and Rv3879. Corresponding antibody detection methods are established by the aid of the three antigens and used for detecting corresponding antibody levels in biological samples, and the three detected antibody levels are particularly higher in sputum smear negative / sputum culture positive tuberculosis patients. A pulmonary tuberculosis diagnosing method is established by the aid of the combined antigen, and a positive sample is defined in such a manner that antibody levels corresponding to two or more antigens reach a positive judgment value. Verification of a lot of clinical samples indicates that the diagnosing method has the high sensitivity and the high specificity for sputum smear positive / sputum culture positive, sputum smear negative / sputum culture positive and sputum smear negative / sputum culture negative tuberculosis patients.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com