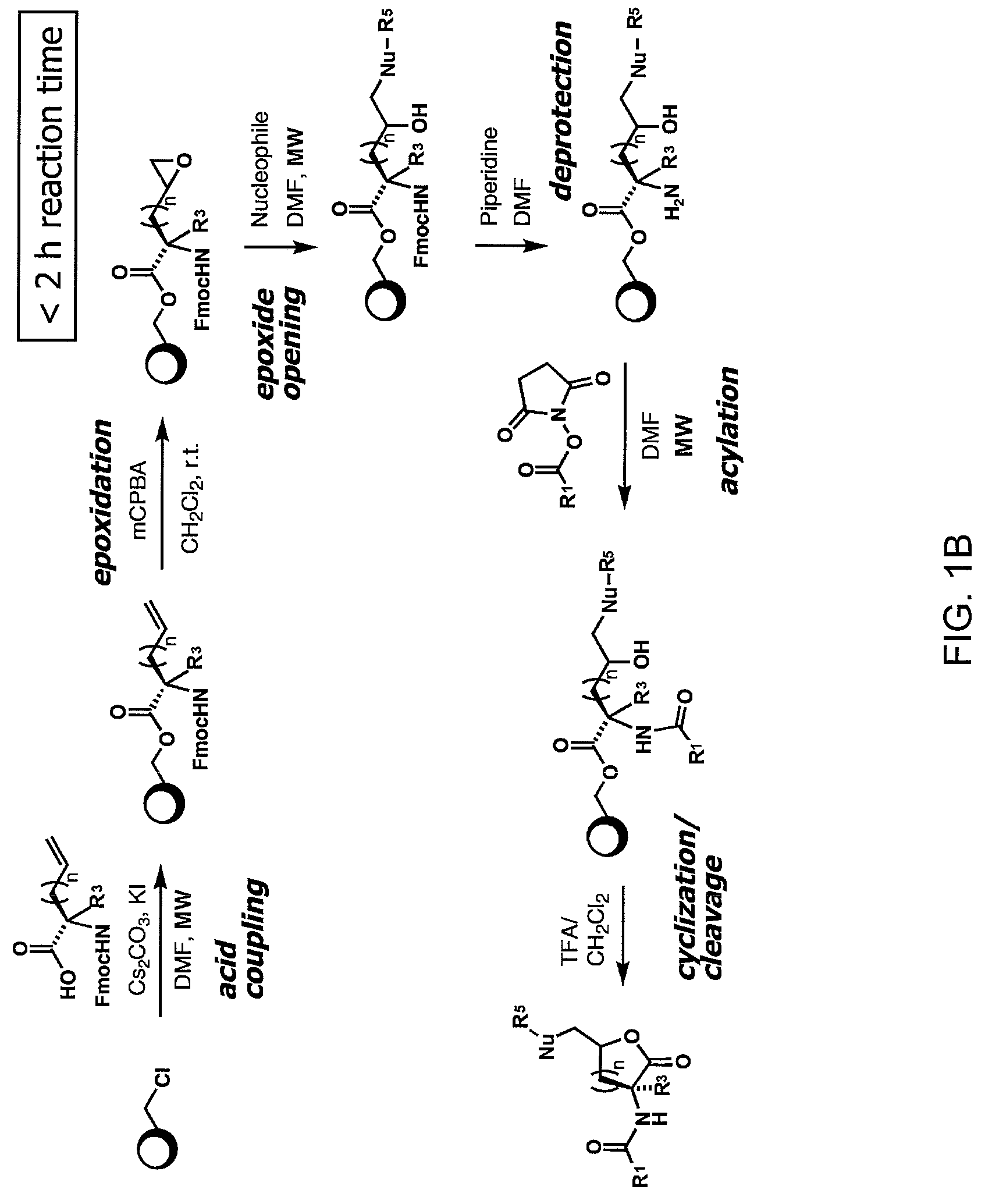
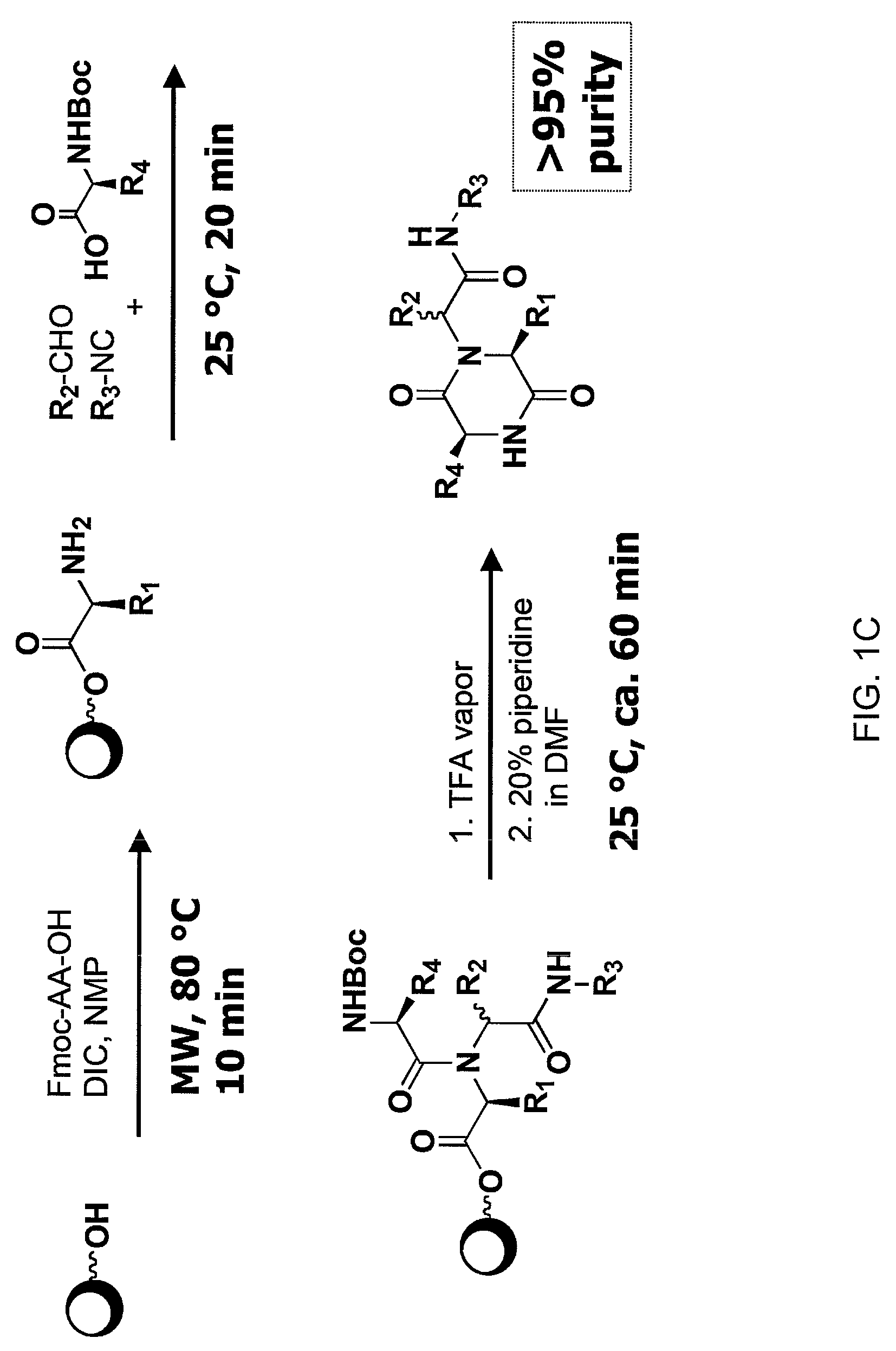
Patents
Literature
88 results about "Synthetic analogue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthetic Analogs of Naturally-Occurring Drugs Psychoactive drugs act by mimicking natural transmitters, or blocking them, or in some other way interfering with them or boosting their levels. Synthetic analogs of natural drugs are sometimes called designer drugs. Whatever they are called, the synthetic analogs pose a problem.
Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders
Disclosed herein are analogs of clozapine and pharmaceutically acceptable salts, esters, amides, or prodrugs thereof; methods of synthesizing the analogs; and methods of using the analogs for treating neuorpsychiatric disorders. In some embodiments, the analogs are amino substituted diaryl[a,d]cycloheptenes.
Owner:ACADIA PHARMA INC
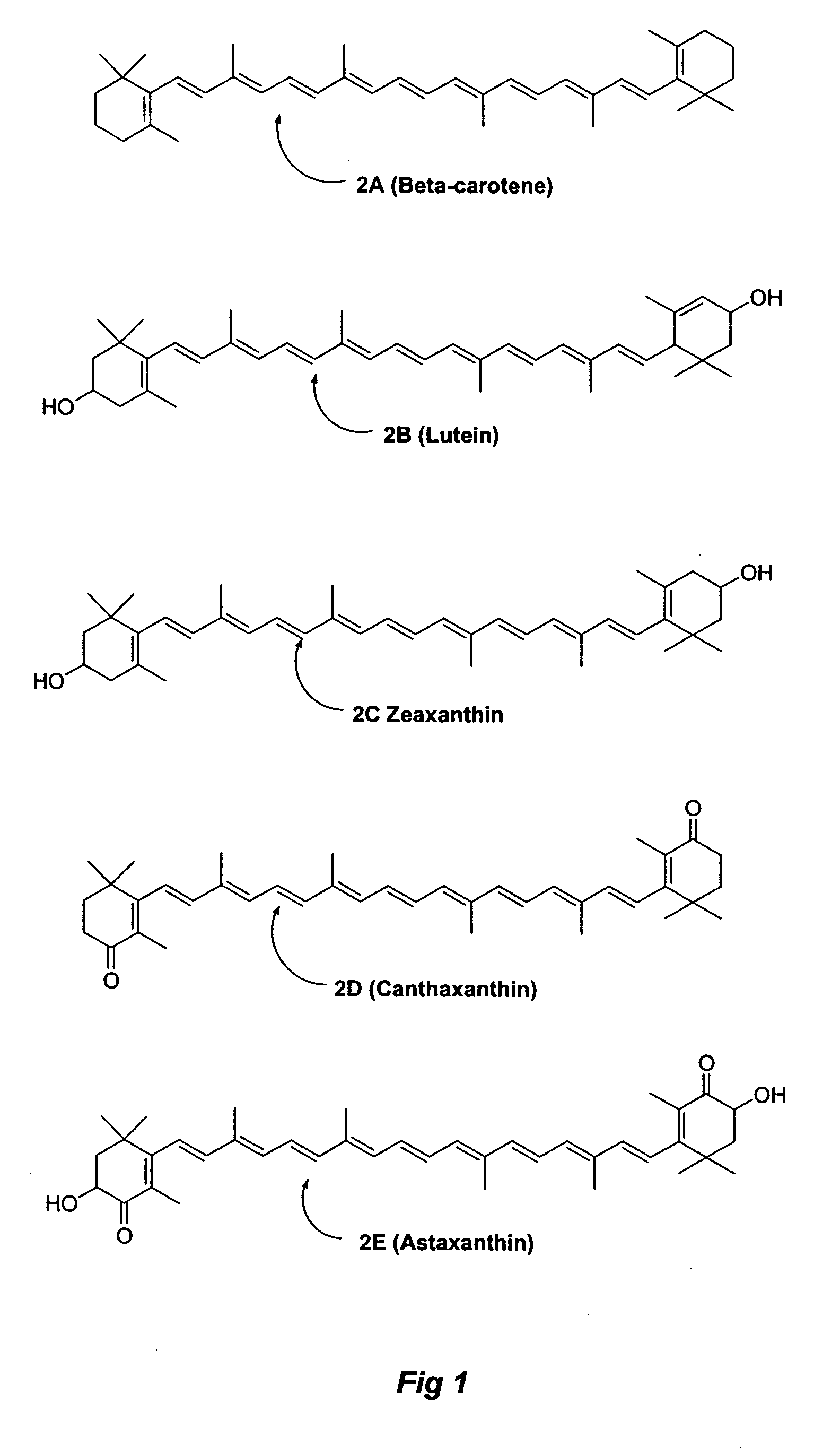
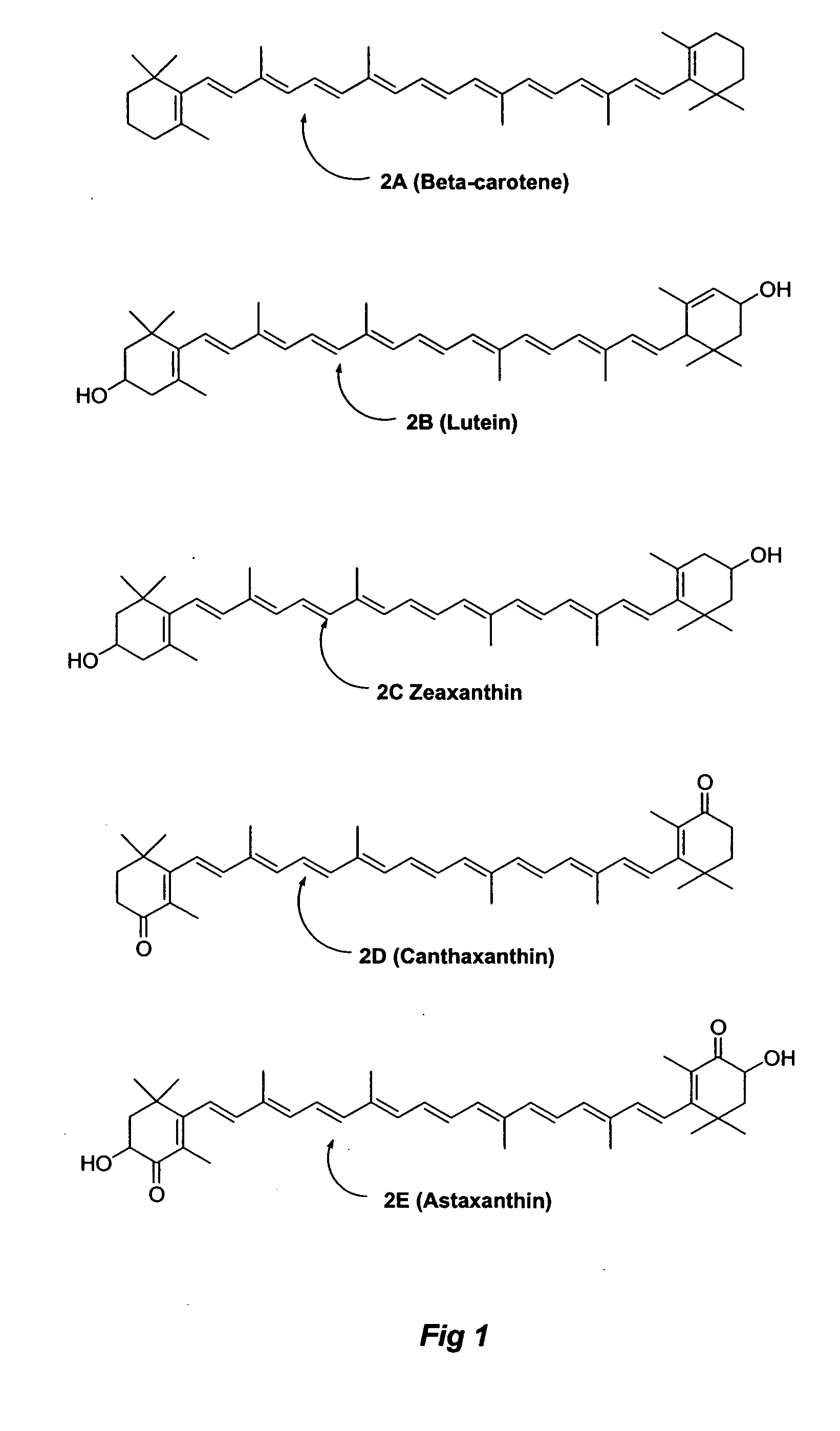
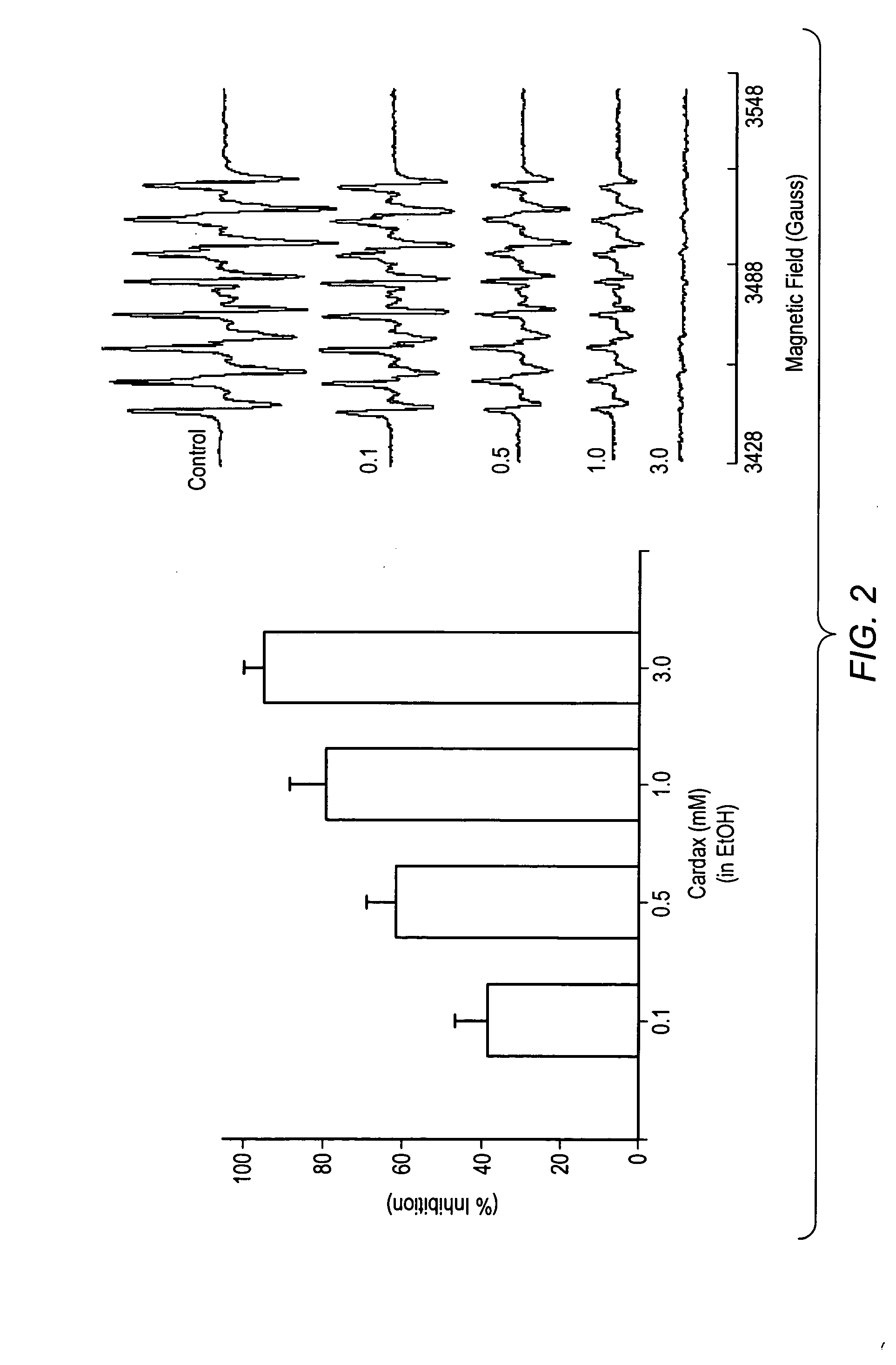
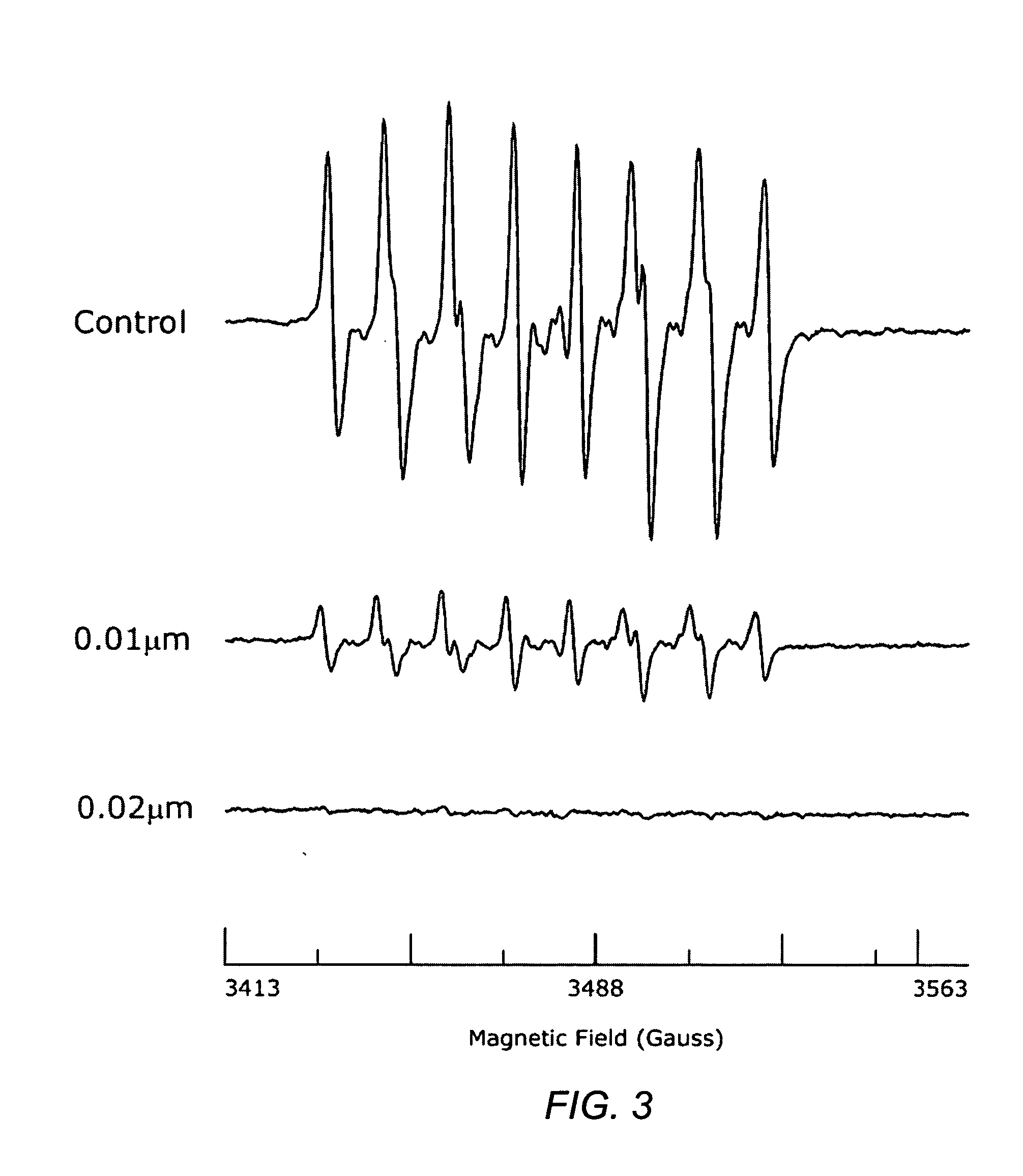
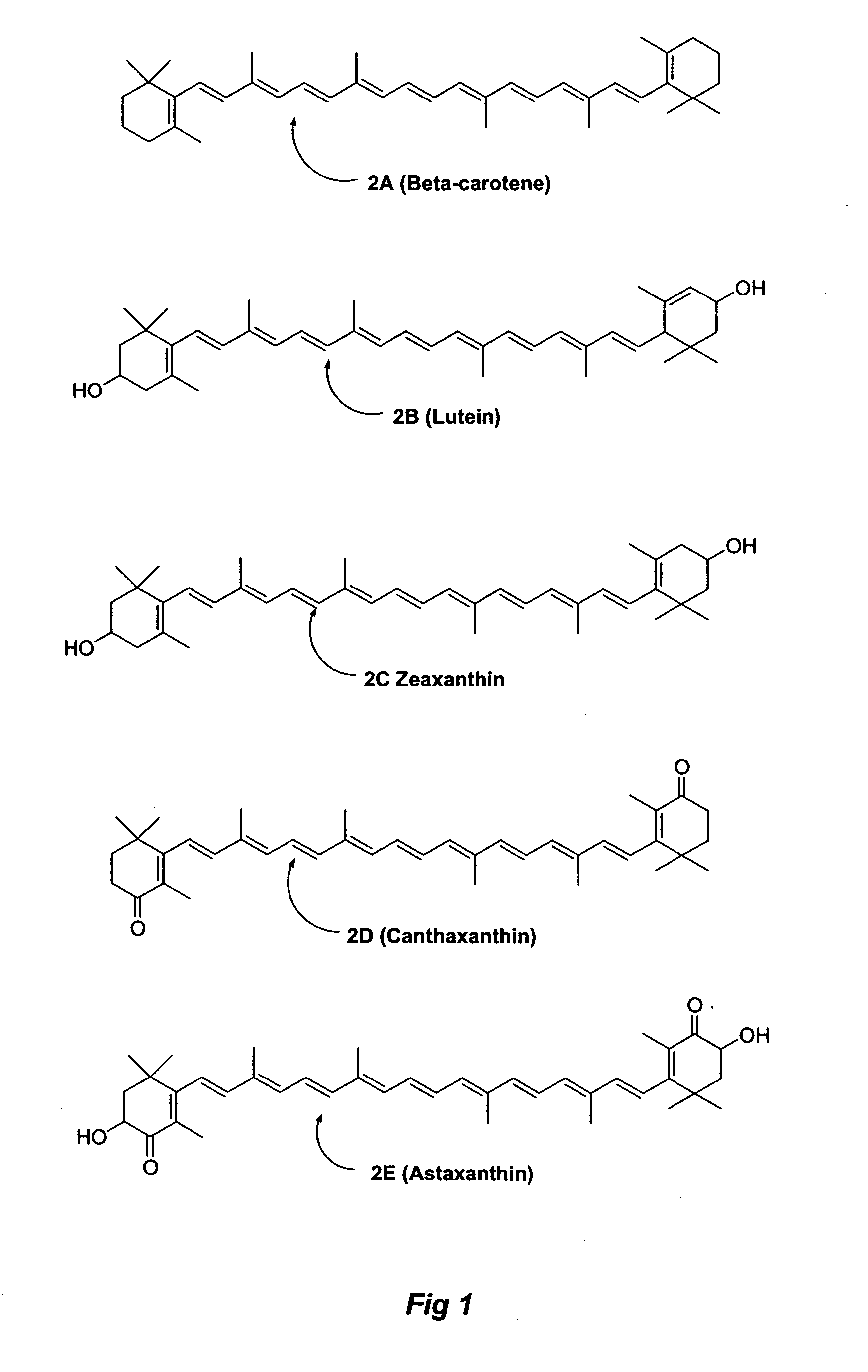
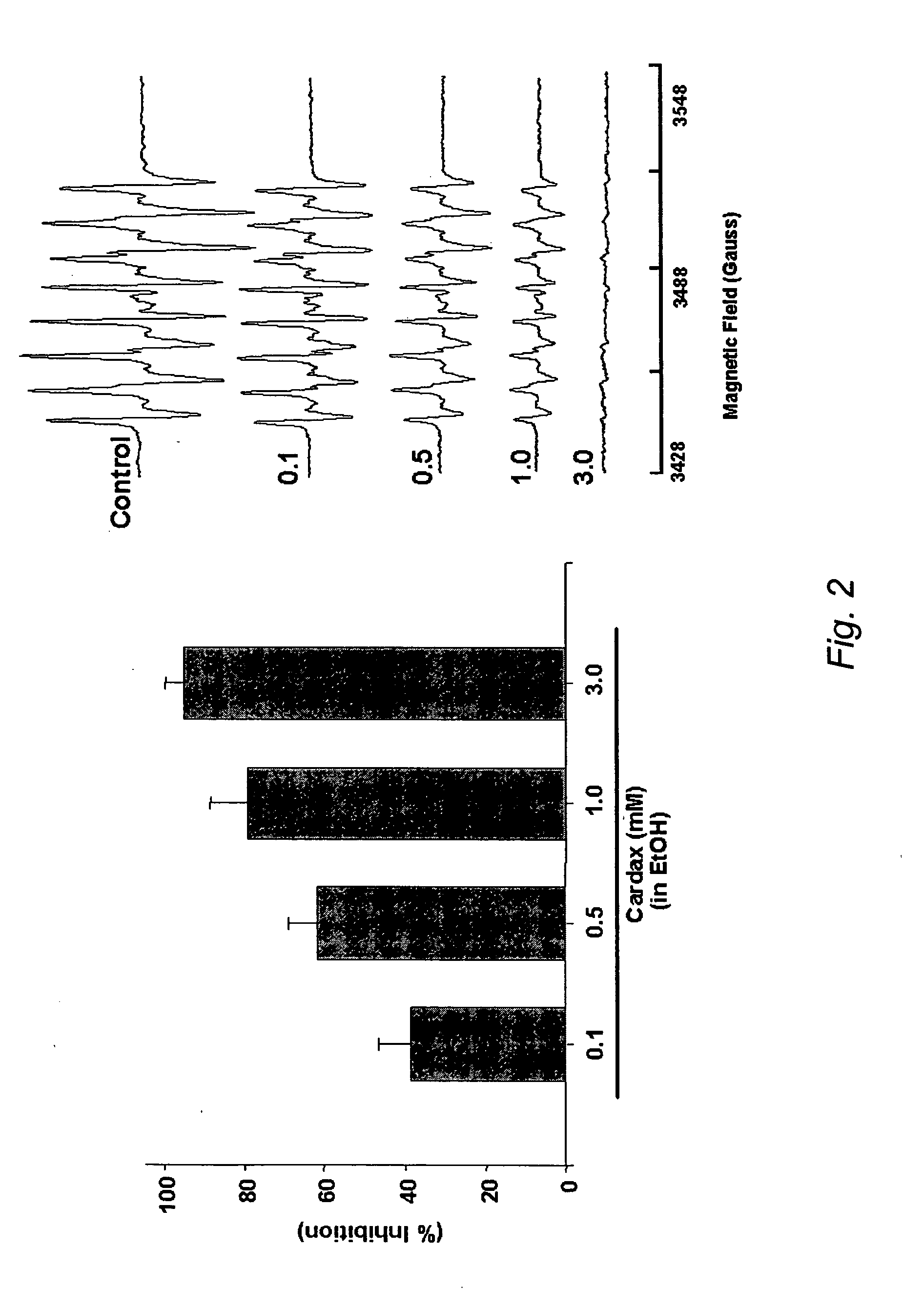
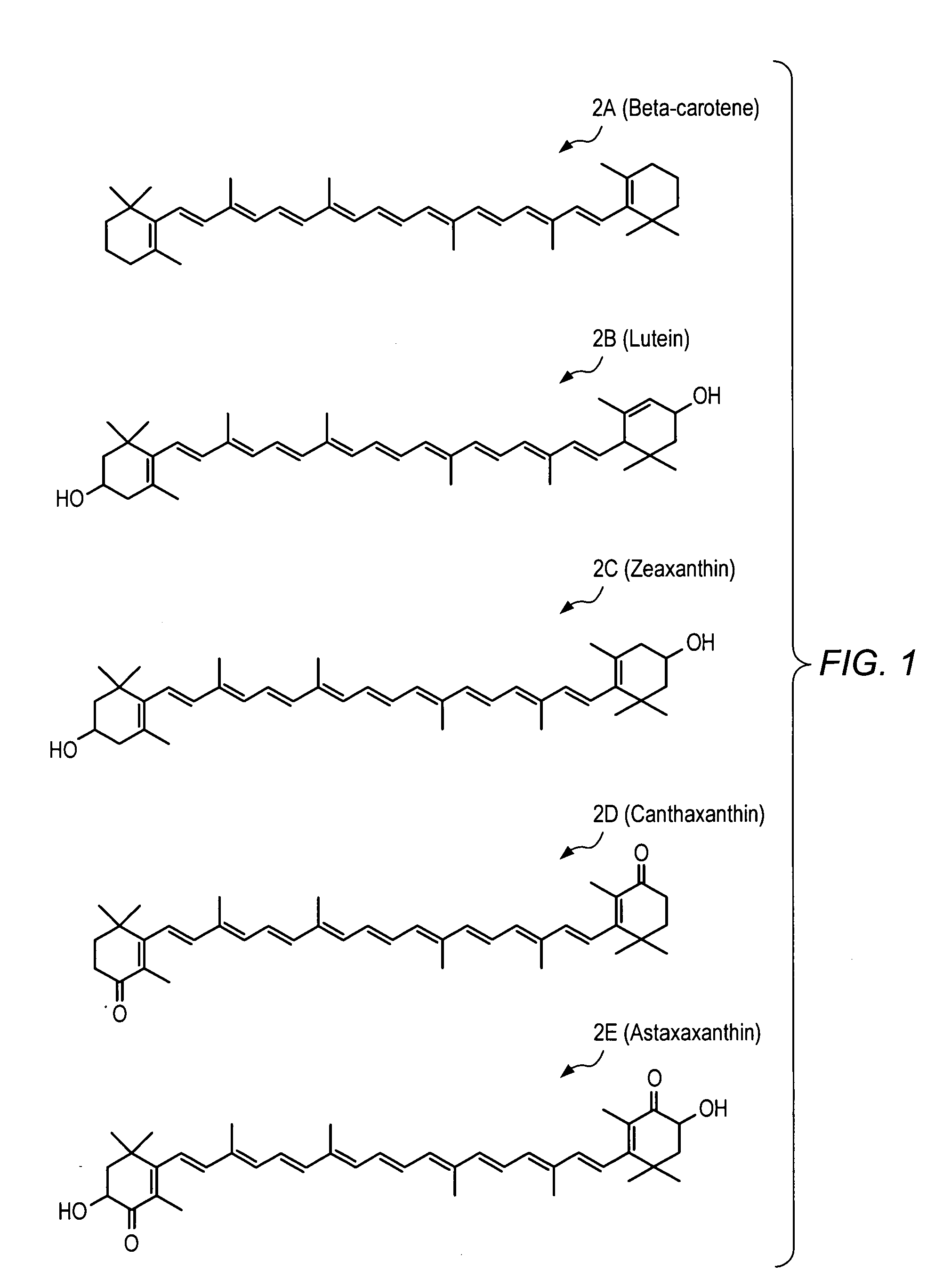
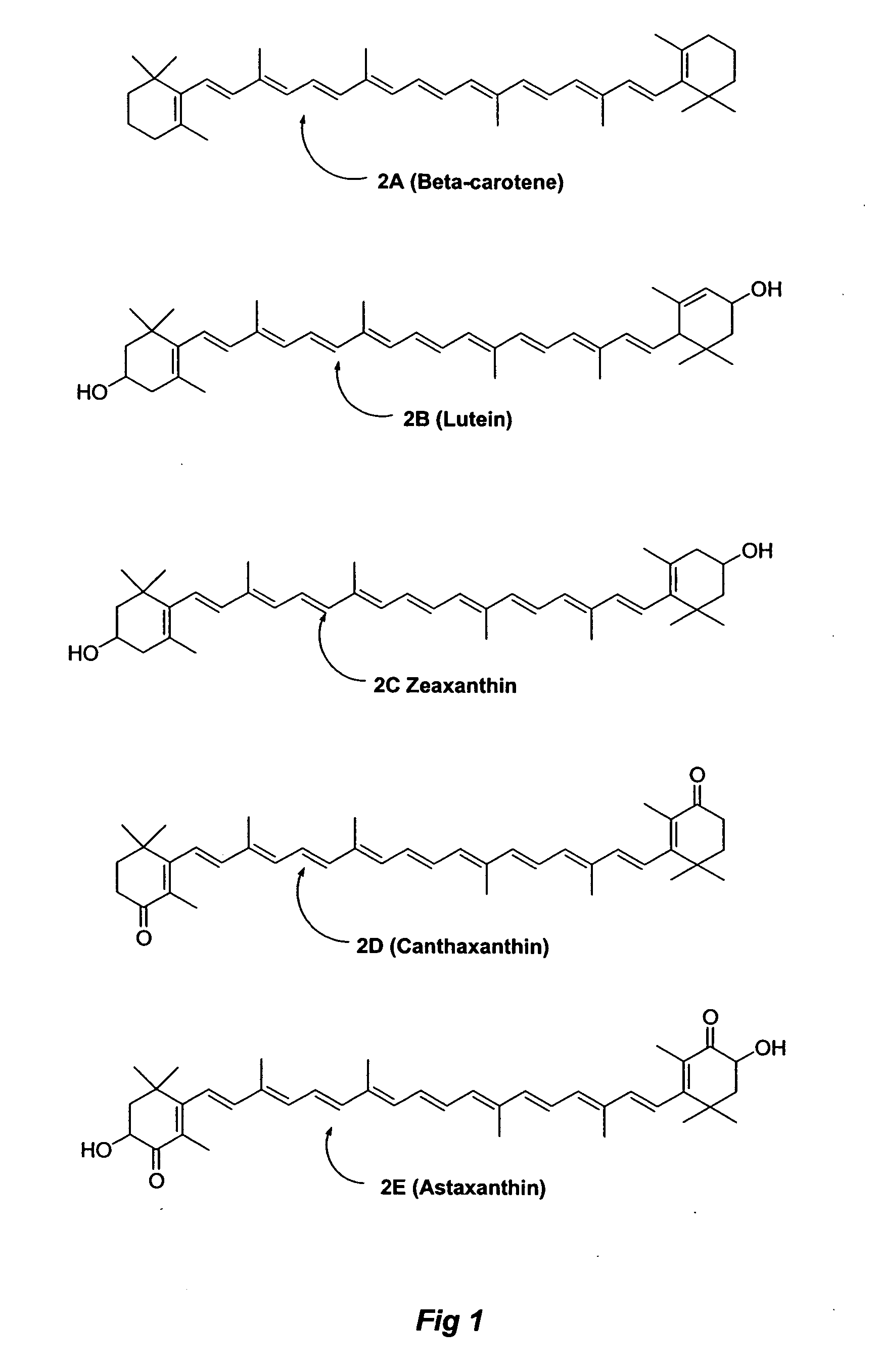
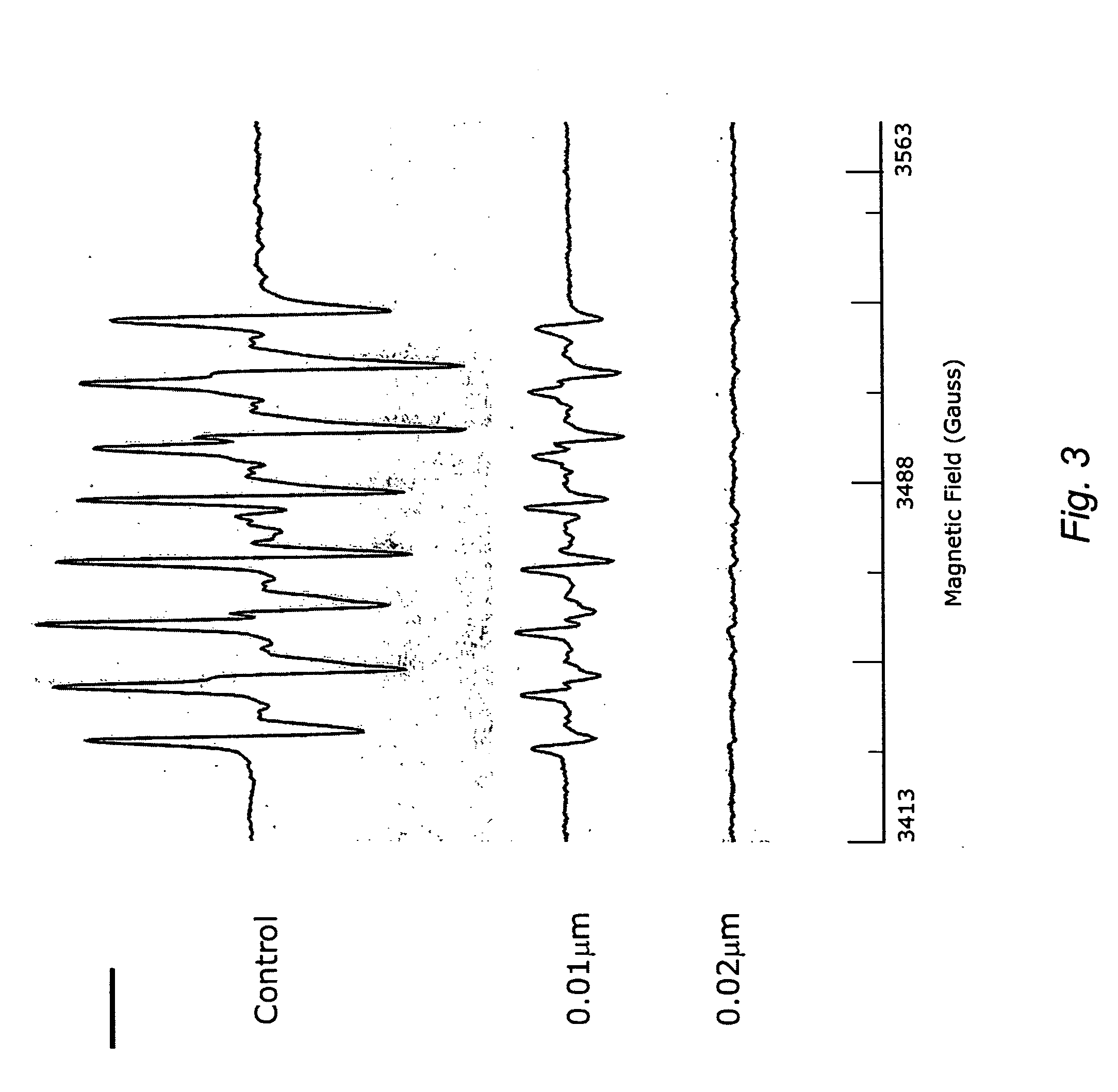
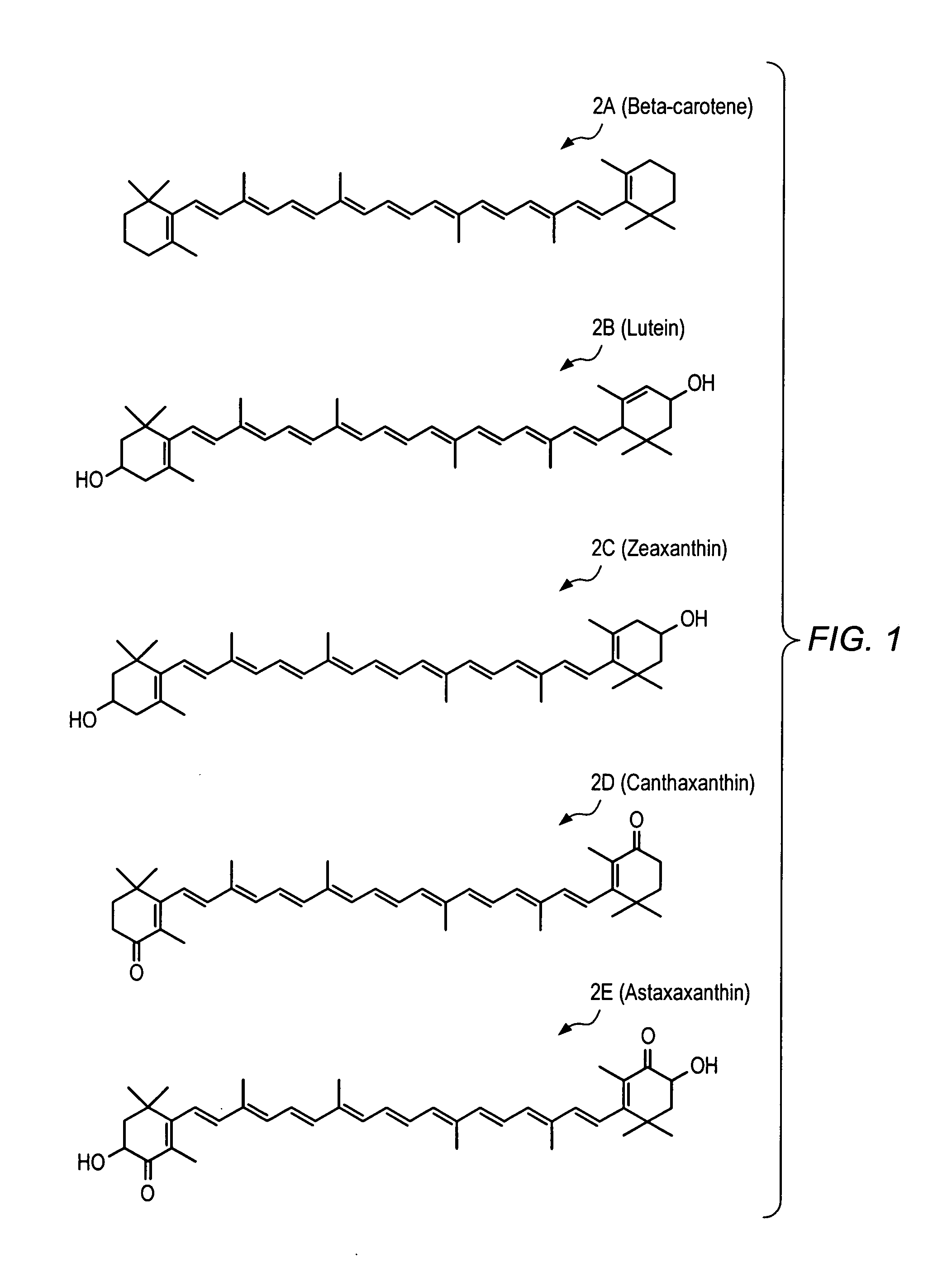
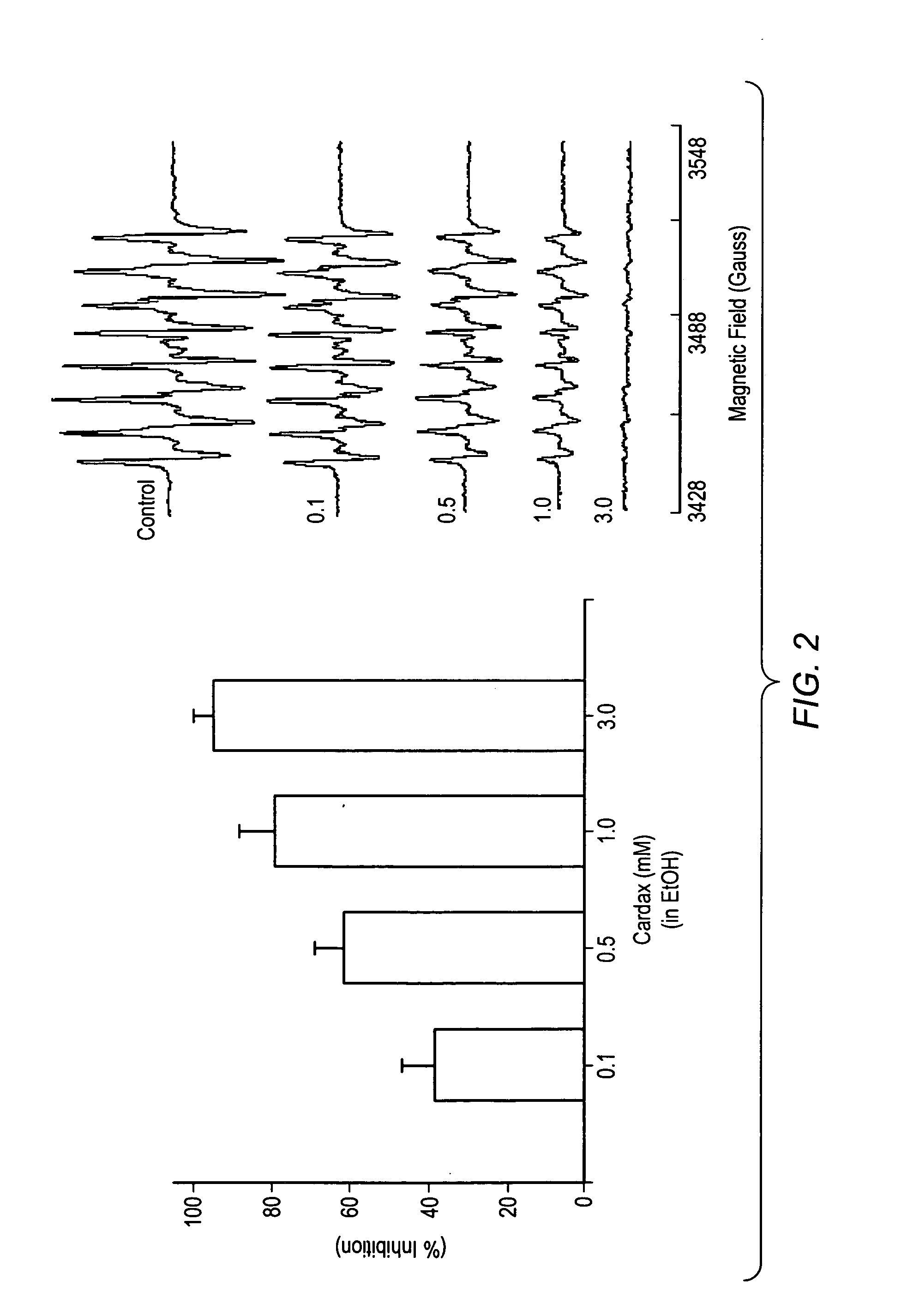
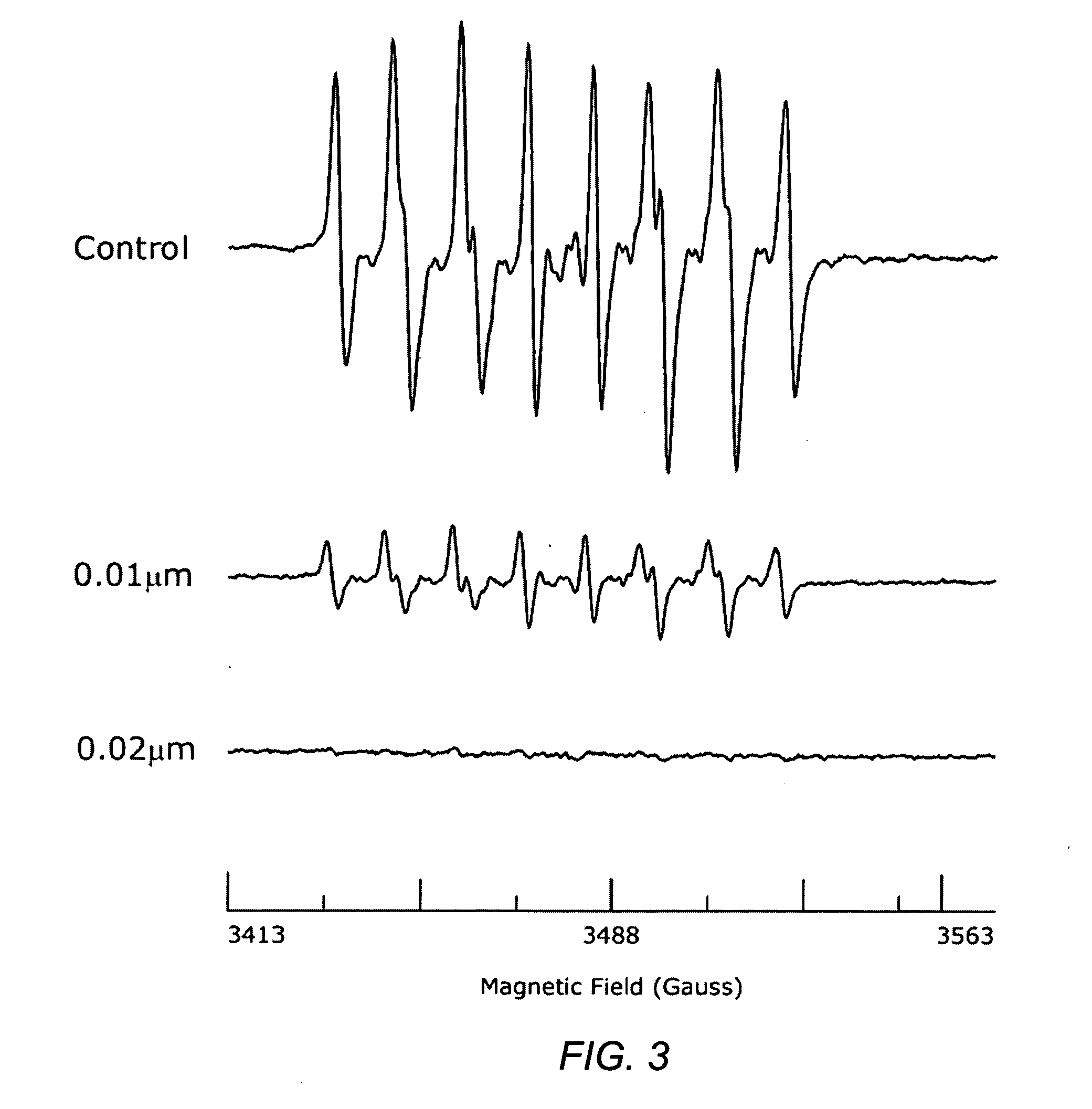
Carotenoid ester analogs or derivatives for controlling connexin 43 expression
InactiveUS20050009788A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsSynthetic analogueCancer cell
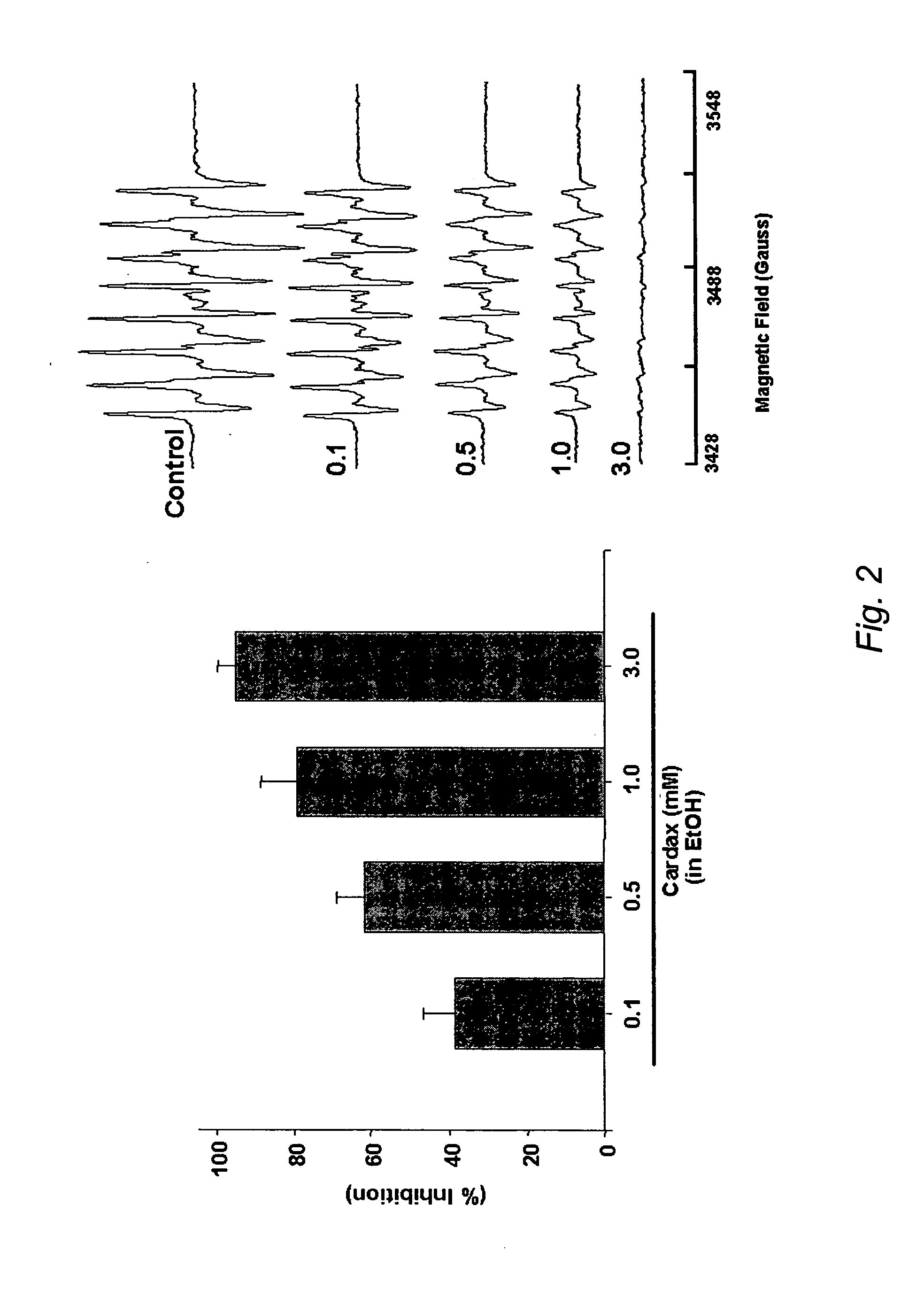
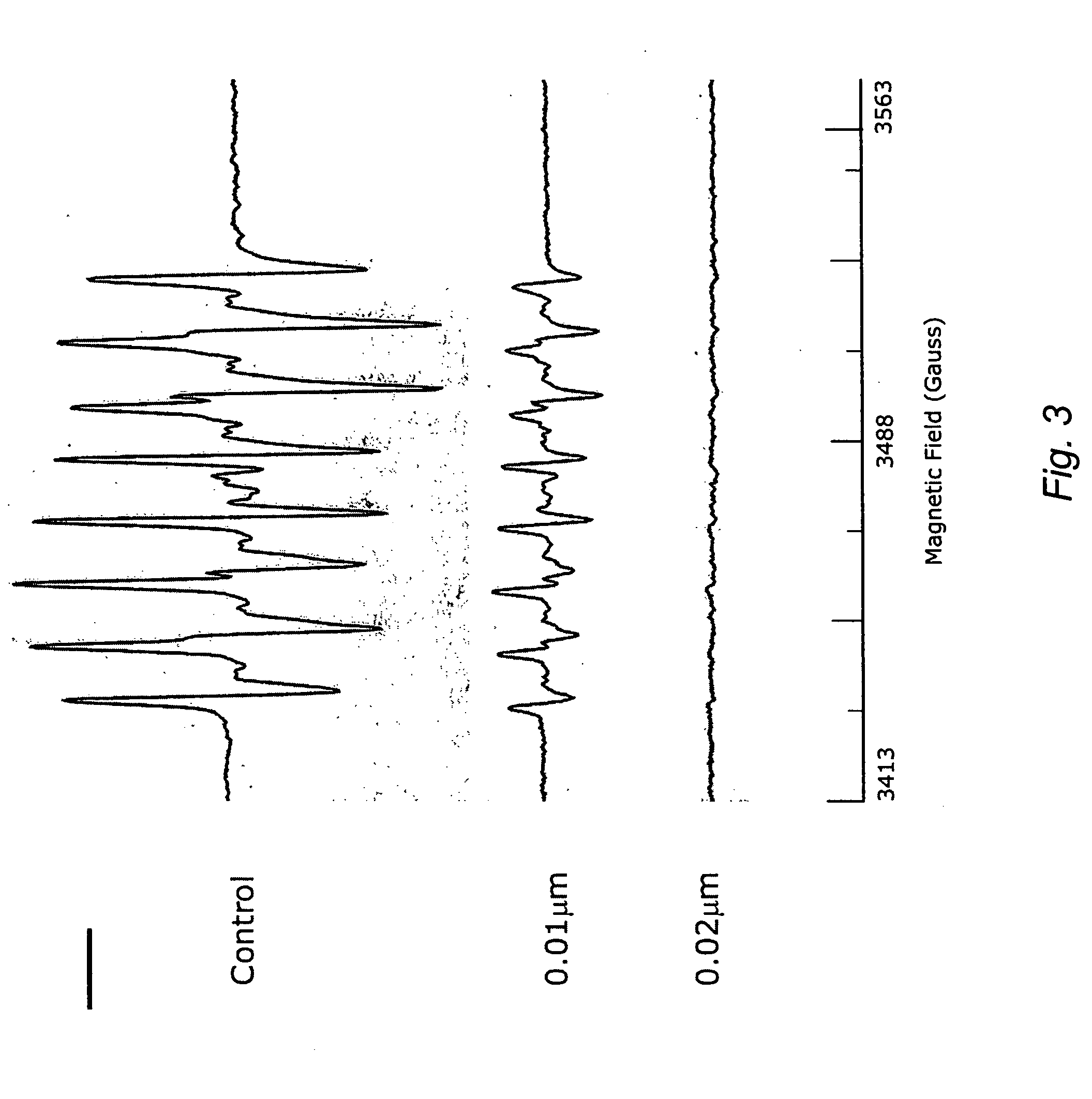
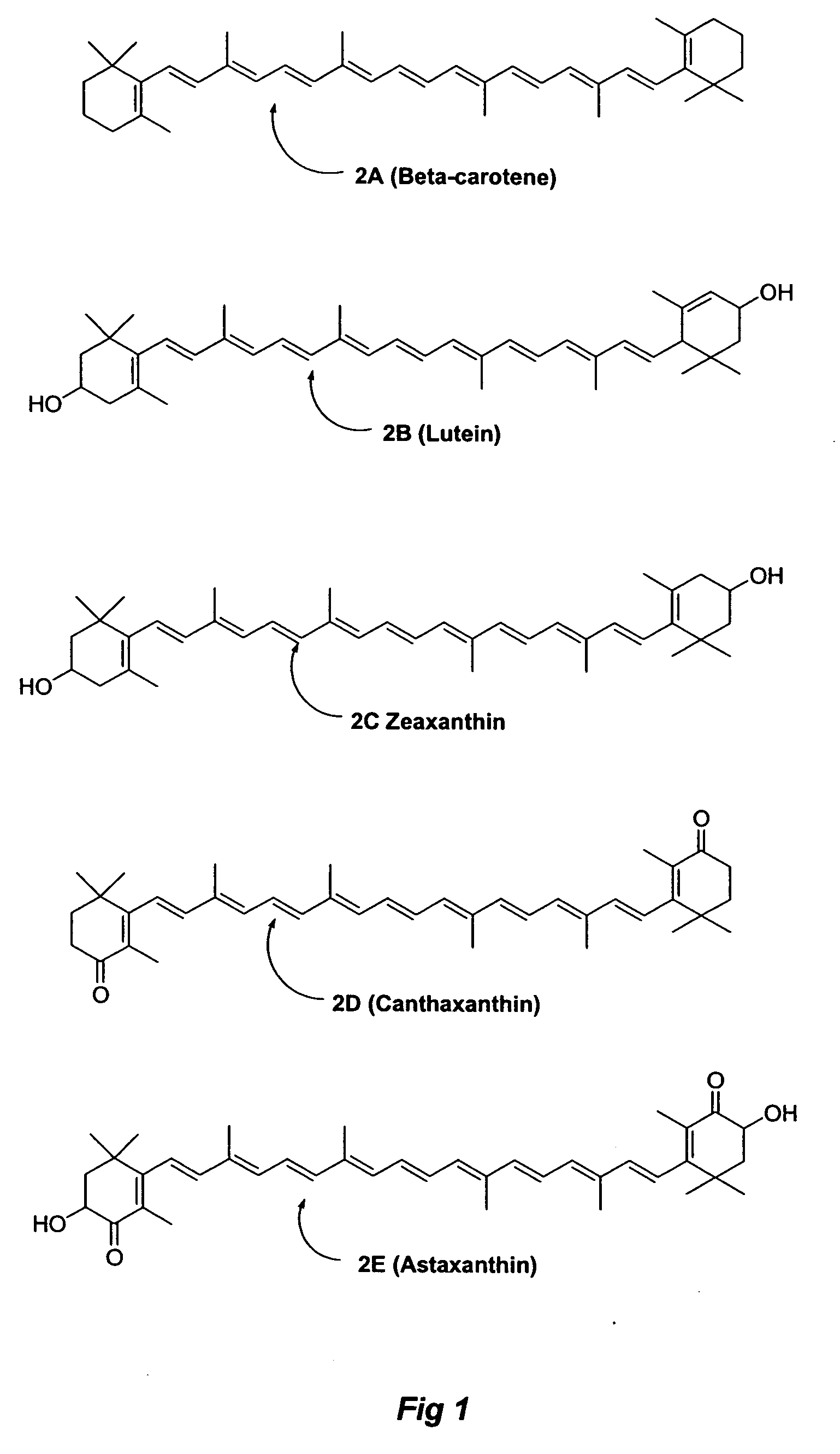
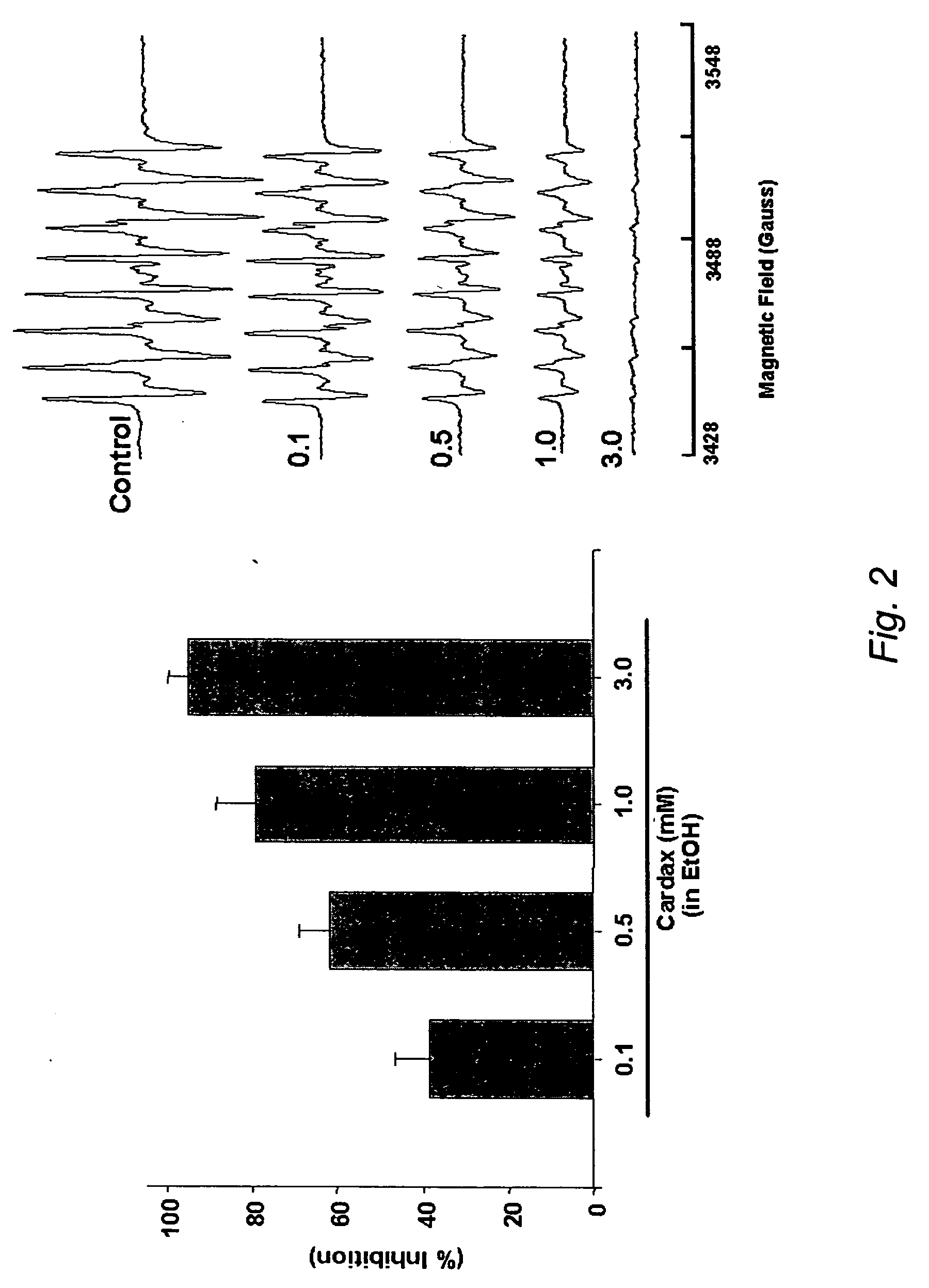
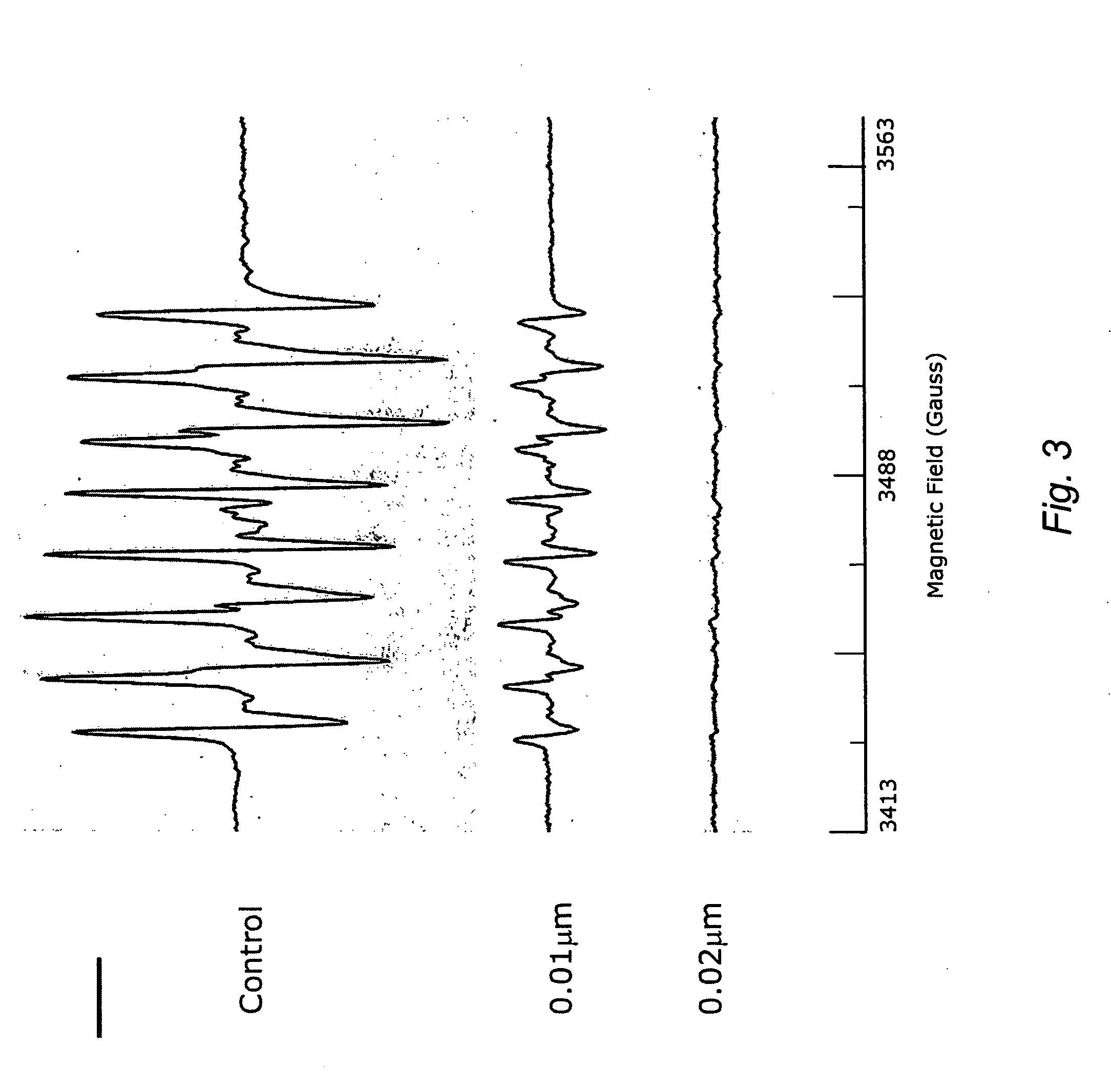
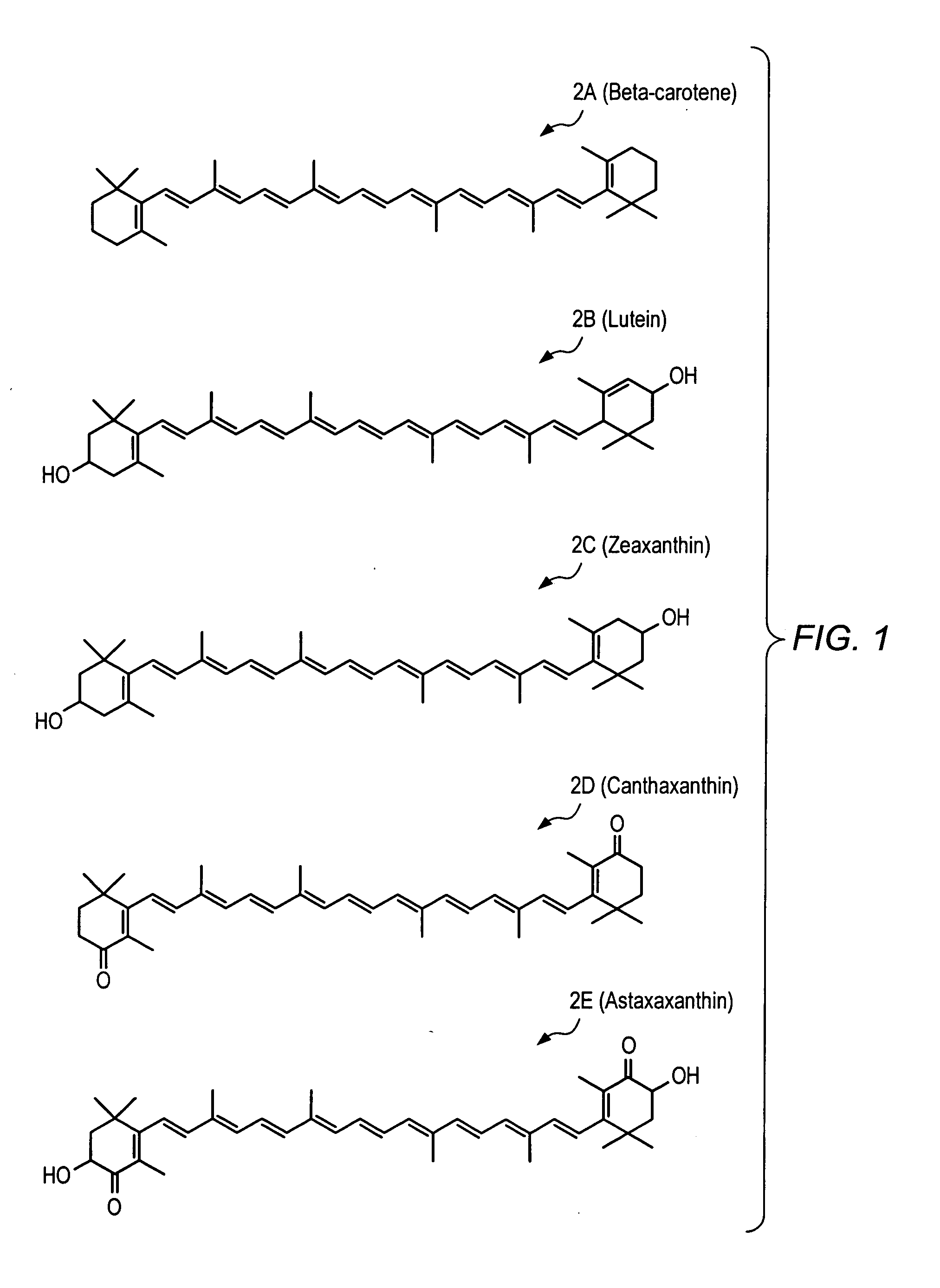
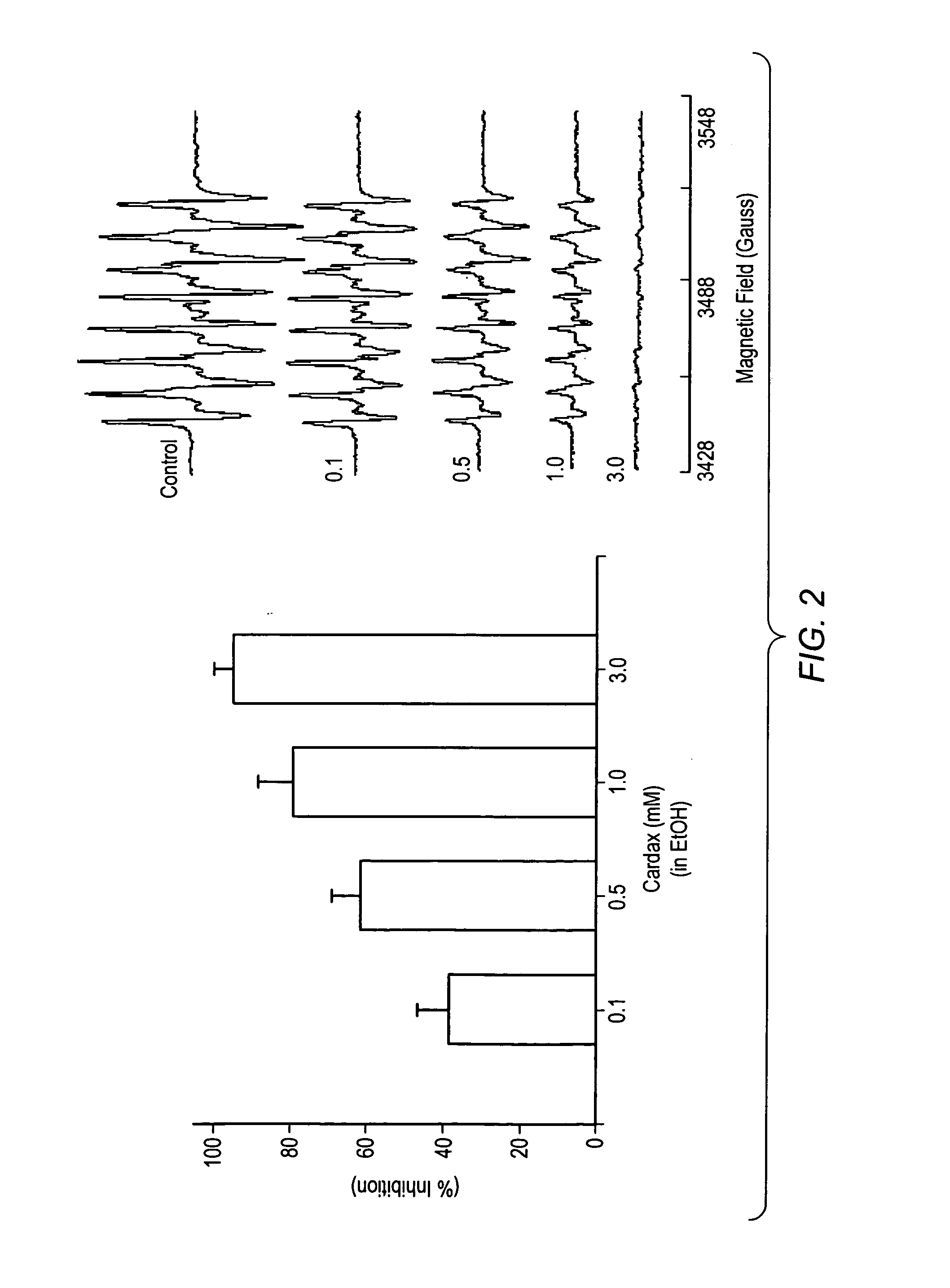
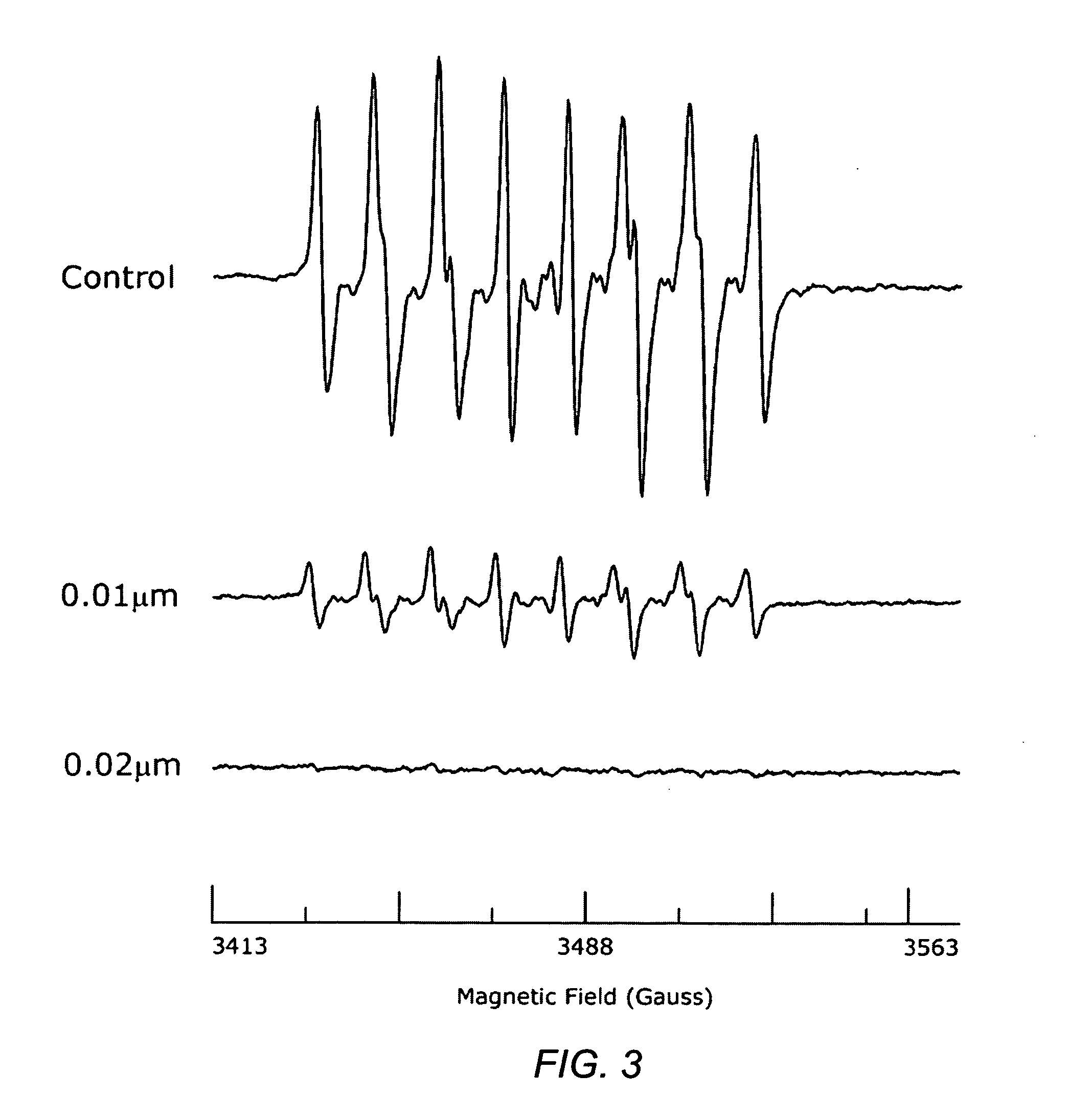
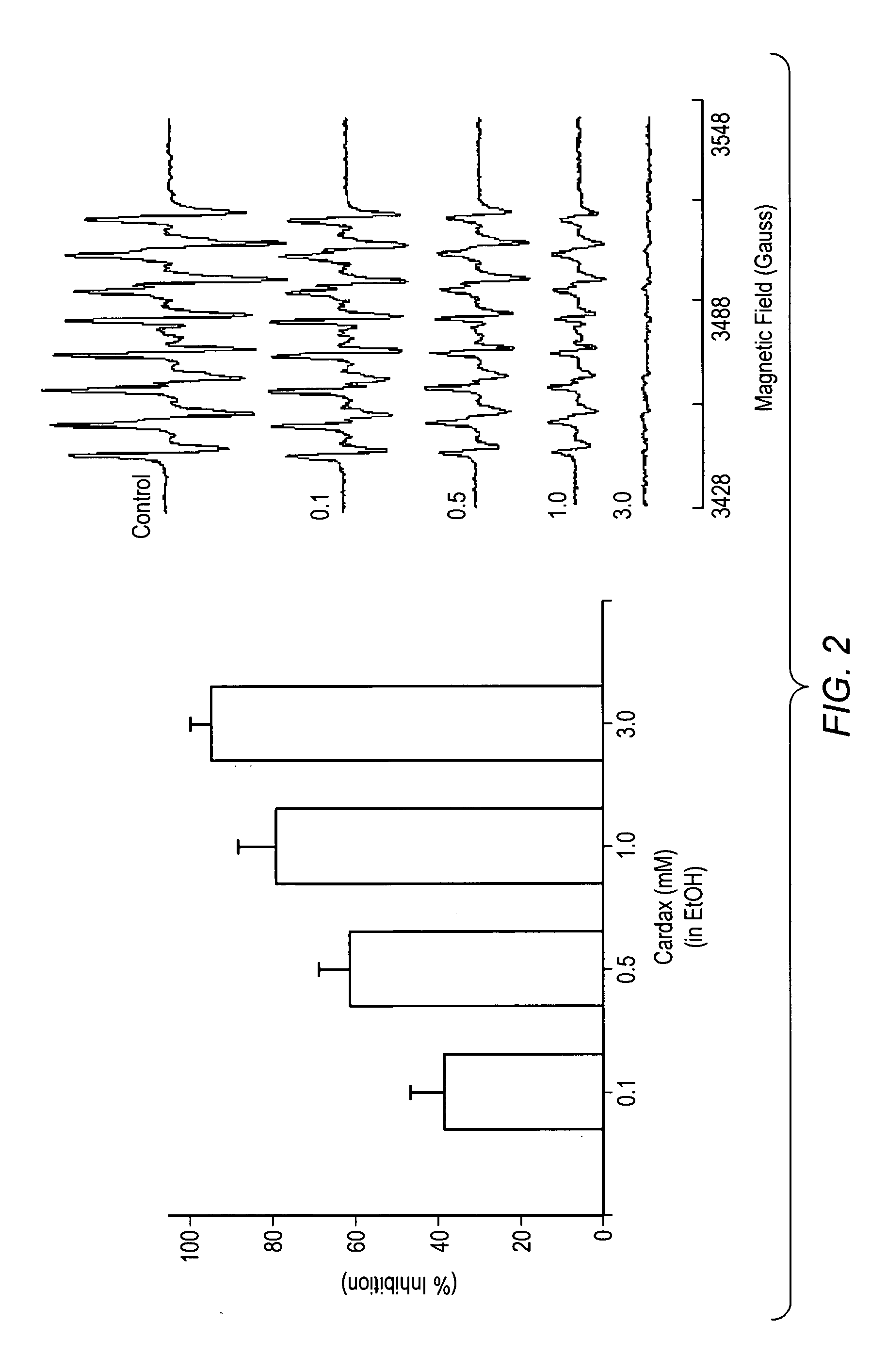
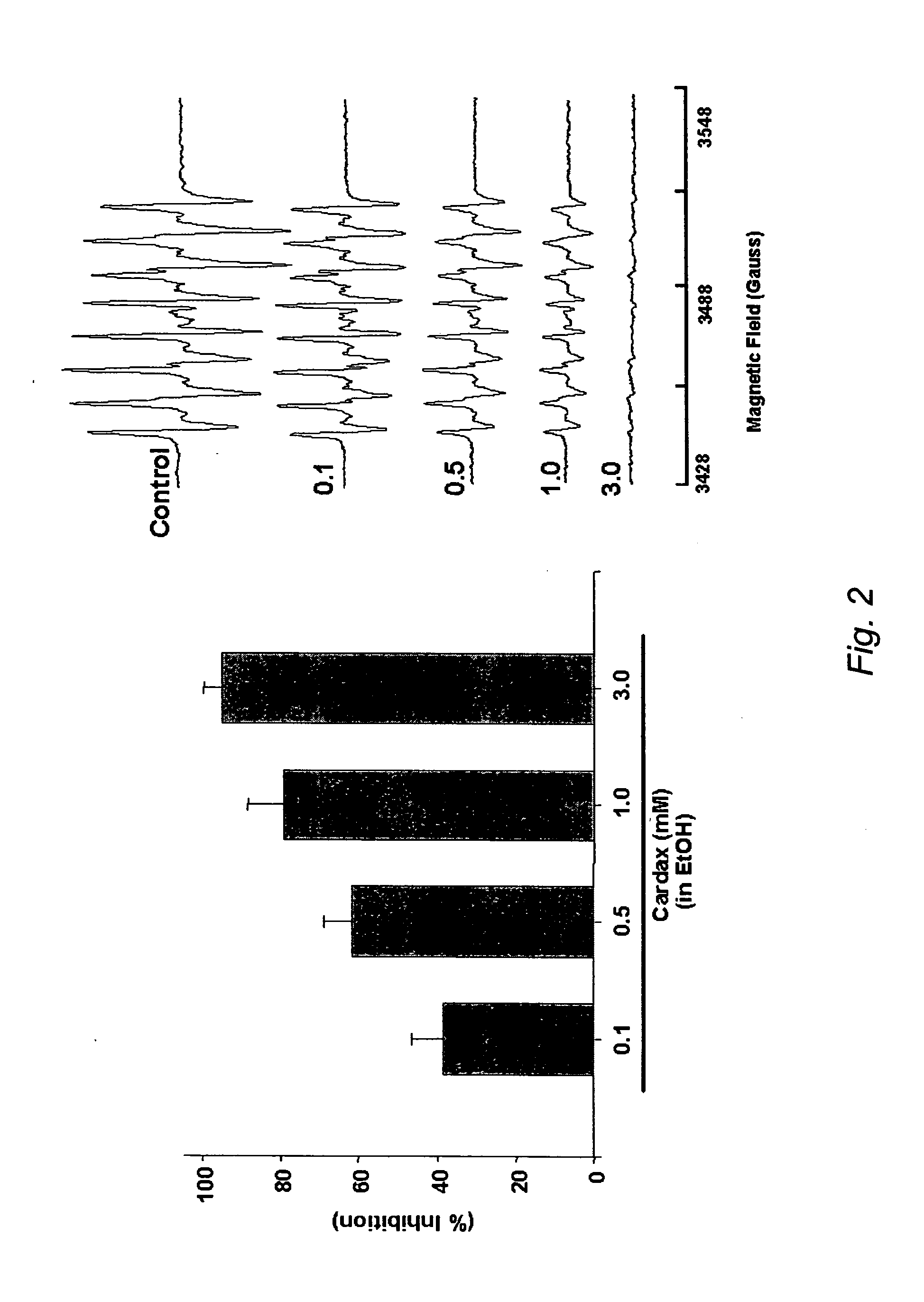
A method of controlling (e.g., influencing or affecting) connexin 43 expression in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. In some embodiments, controlling connexin 43 expression in a subject may effectively treat cardiac arrhythmia and / or cancerous and pre-cancerous cells in a subject. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ester functionality.
Owner:CARDAX PHARMA
Carotenoid ether analogs or derivatives for the inhibition and amelioration of liver disease
InactiveUS20050026874A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsDiseaseSynthetic analogue
A method of treating liver disease in a subject. The method may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ether functionality.
Owner:CARDAX PHARMA
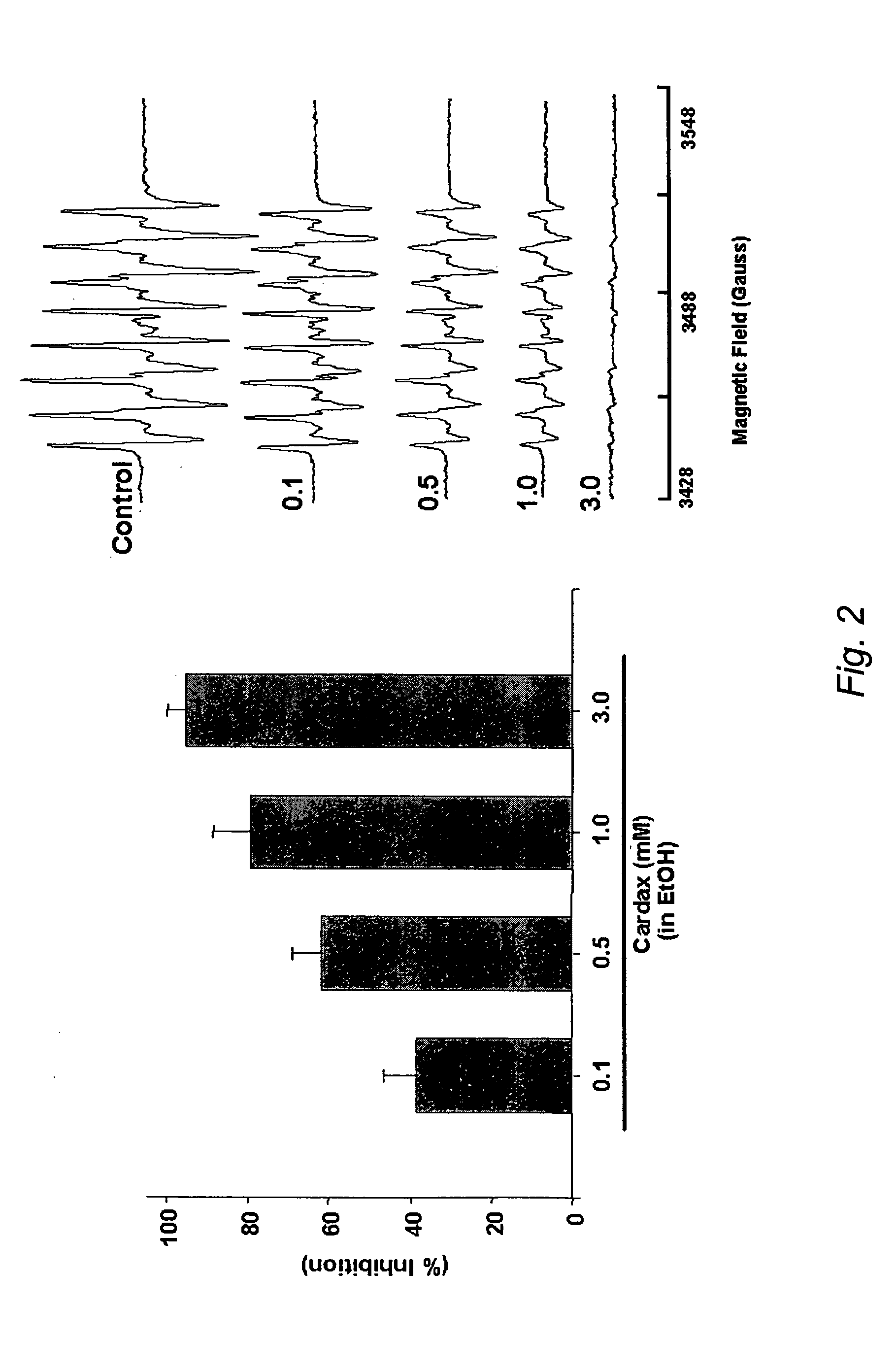
Carotenoid ester analogs or derivatives for the inhibition and amelioration of ischemic reperfusion injury
ActiveUS20050037995A1Increase connexin expressionAmeliorate proliferationAntibacterial agentsOrganic active ingredientsSynthetic analogueAntioxidant
A method of treating ischemic reperfusion injury in a subject. The method may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ester functionality.
Owner:CARDAX PHARMA
Methods and compounds for antimicrobial intervention
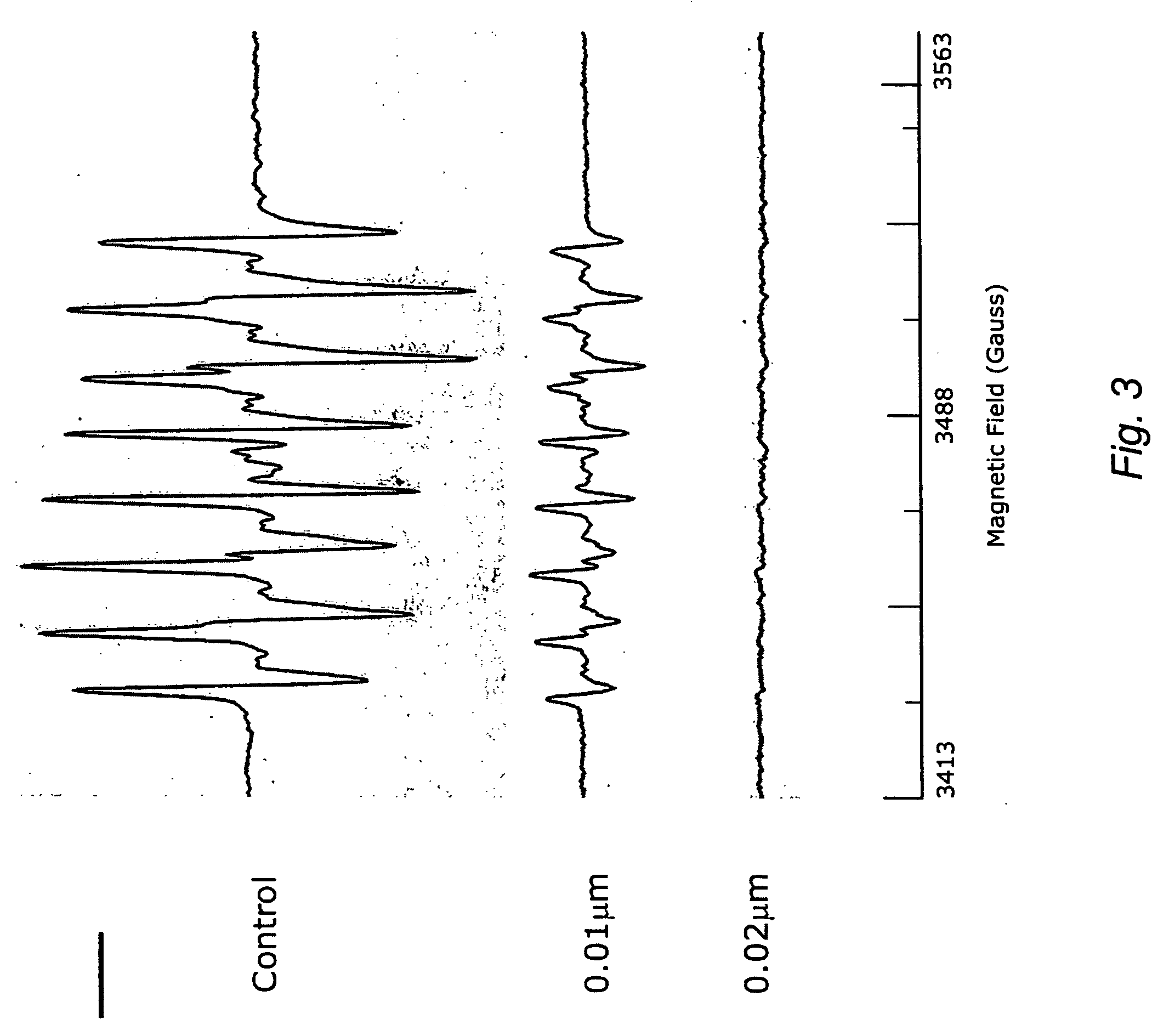
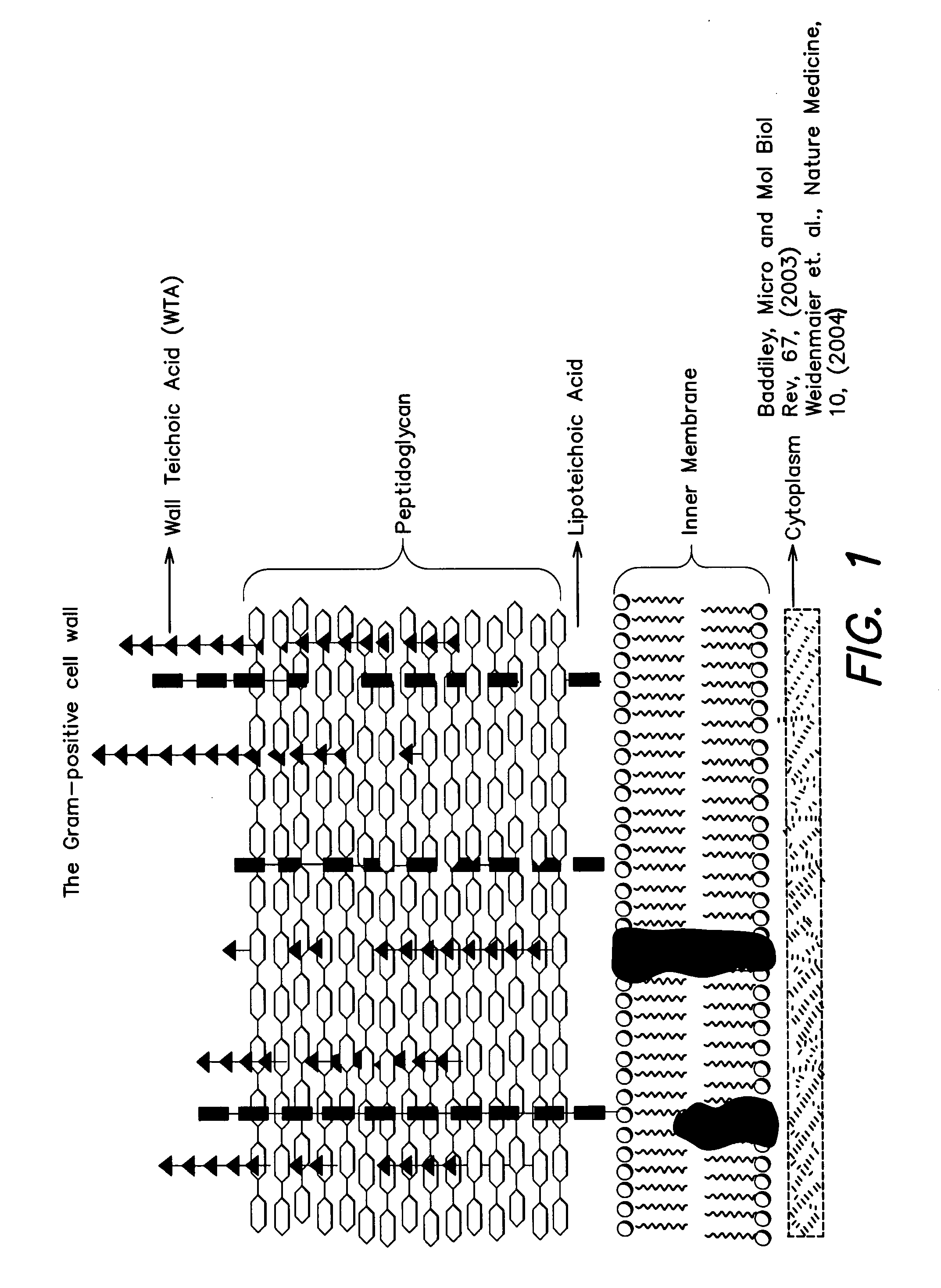
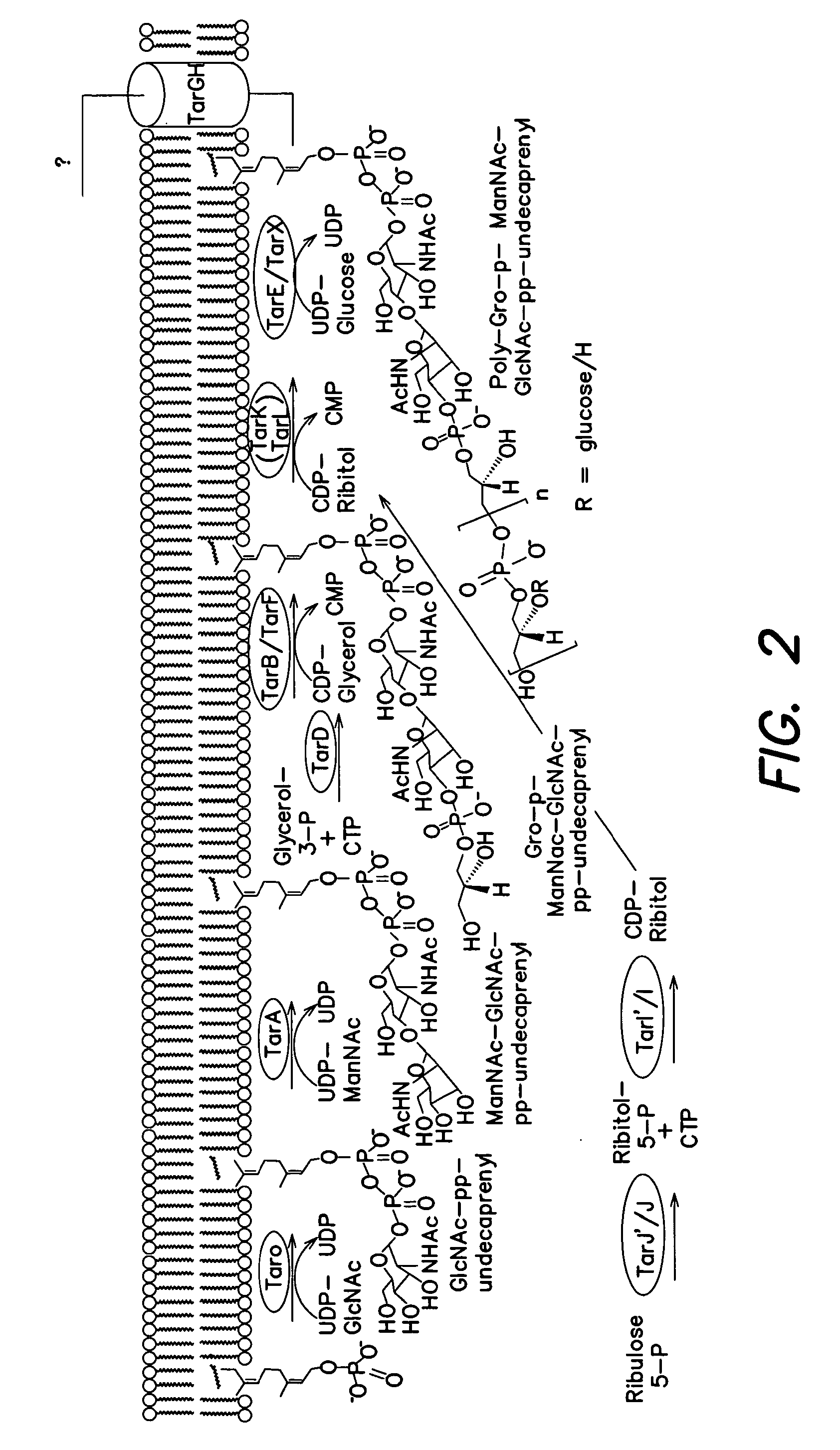
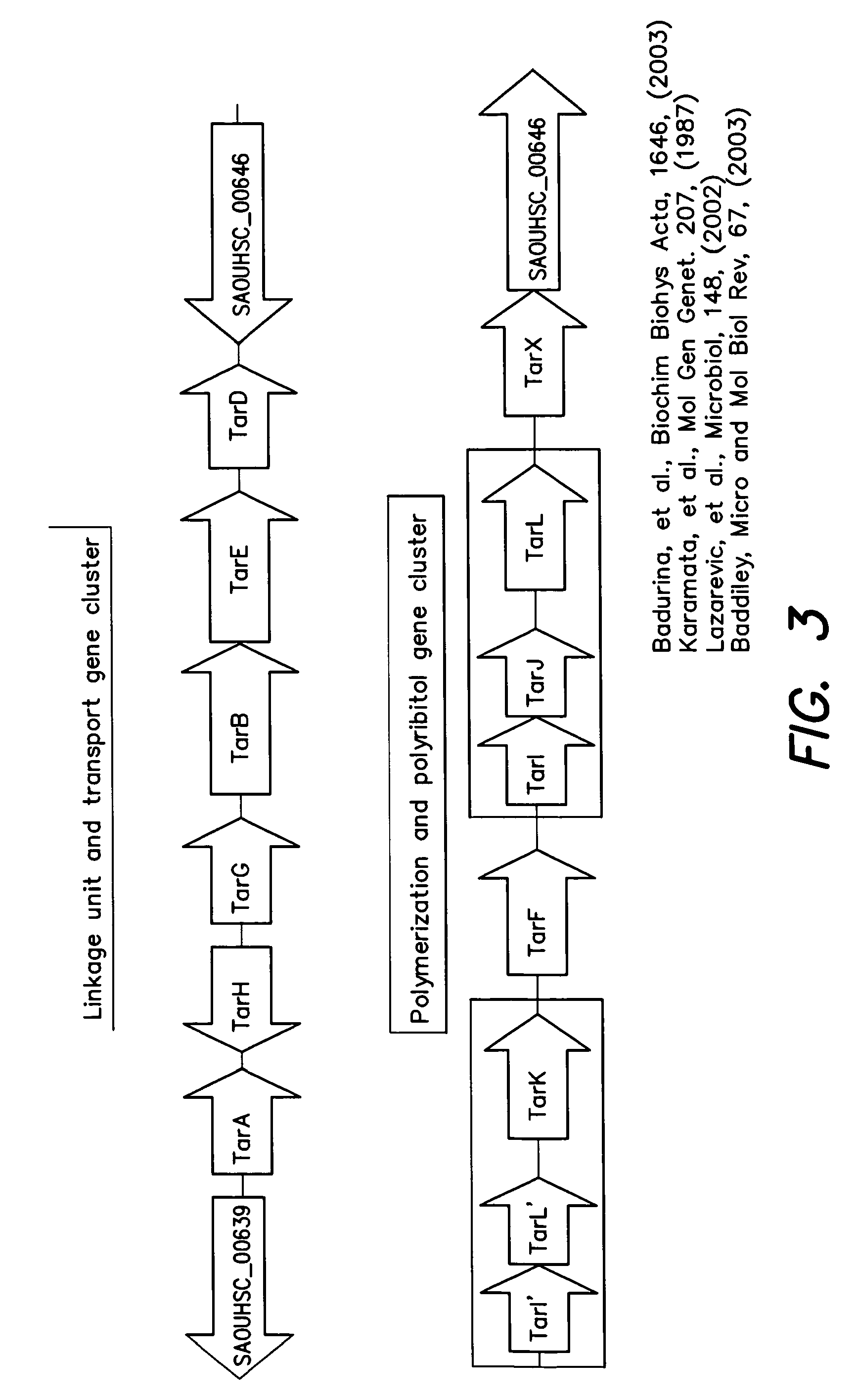
InactiveUS8598342B2Antibacterial agentsBiocideSynthetic analogueMethicillin-resistant Staphylococcus aureus
The present invention provides Wall Teichoic Acid biosynthesis inhibitors such as compound 1835F03 (targocil) and related synthetic analogs. The invention also provides pharmaceutical compositions thereof and methods for treating bacterial infection and the suppression of growth of bacterial cells by administering a Wall Teichoic Acid biosynthesis inhibitor. The invention is particularly useful for the treatment of Methicillin-resistant Staphylococcus aureus (MRSA). The invention further provides procedures for the syntheses of Wall Teichoic Acid biosynthesis inhibitors. The invention also provides methods for the identification of antibacterial therapeutic agents.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
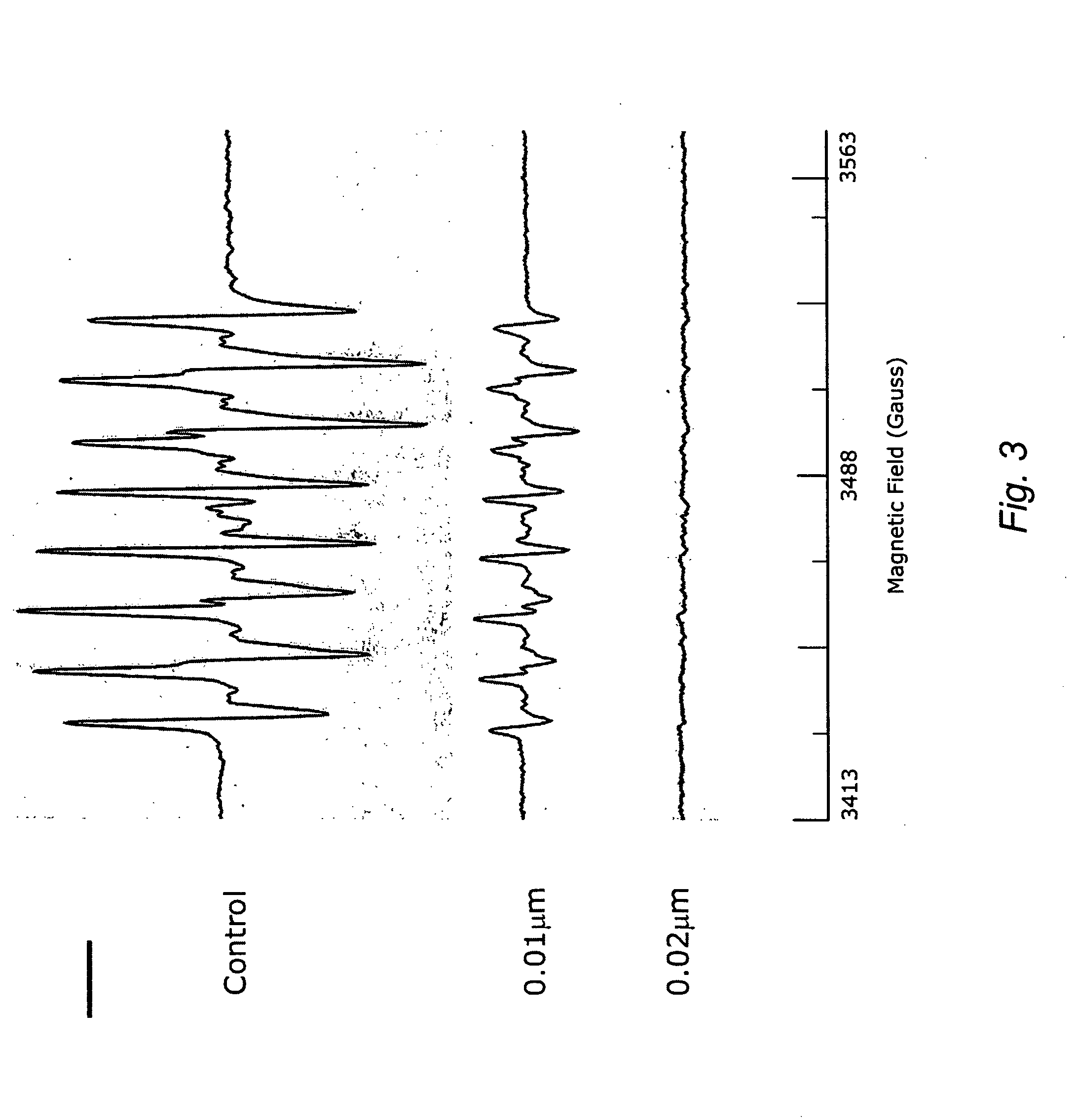
Carotenoid ether analogs or derivatives for the inhibition and amelioration of ischemic reperfusion injury
InactiveUS20050065097A1Inhibit and ameliorate some typeMaintain relatively stableAntibacterial agentsOrganic active ingredientsSynthetic analogueAntioxidant
A method of treating ischemic reperfusion injury in a subject. The method may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ether functionality.
Owner:CARDAX PHARMA
Carotenoid ester analogs or derivatives for the inhibition and amelioration of liver disease
InactiveUS20050090469A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsSynthetic analogueDisease
A method of treating liver disease in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ester functionality.
Owner:CARDAX PHARMA
Carotenoid analogs or derivatives for the inhibition and amelioration of liver disease
InactiveUS20050004235A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsDiseaseSynthetic analogue
A method of treating liver disease in a subject. The method may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include an acyclic alkene including at least one substituent and / or a cyclic ring including at least one substituent. In some embodiments, a carotenoid analog or derivative may include at least one substituent.
Owner:CARDAX PHARMA
Carotenoid analogs or derivatives for controlling C-reactive protein levels
InactiveUS20050059659A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsSynthetic analogueAntioxidant
A method of controlling (e.g., influencing or affecting) C-reactive protein levels in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include an acyclic alkene including at least one substituent and / or a cyclic ring including at least one substituent. In some embodiments, a carotenoid analog or derivative may include at least one substituent.
Owner:CARDAX PHARMA
Carotenoid ether analogs or derivatives for controlling C-reactive protein levels
InactiveUS20050049248A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsSynthetic analogueAntioxidant
A method of controlling (e.g., influencing or affecting) C-reactive protein levels in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one j e substituent. The substituent may be coupled to the cyclic ring with an ether functionality.
Owner:CARDAX PHARMA
Carotenoid analogs or derivatives for controlling connexin 43 expression
InactiveUS20050009930A1Inhibit and ameliorate some typeMaintain relatively stableBiocideHydrocarbon active ingredientsSynthetic analogueDouble bond
A method of controlling (e.g., influencing or affecting) connexin 43 expression in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. In some embodiments, controlling connexin 43 expression in a subject may effectively treat cardiac arrhythmia and / or cancerous and pre-cancerous cells in a subject. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include an acyclic alkene including at least one substituent and / or a cyclic ring including at least one substituent. In some embodiments, a carotenoid analog or derivative may include at least one substituent.
Owner:CARDAX PHARMA
Carotenoid ester analogs or derivatives for controlling C-reactive protein levels
InactiveUS20050059635A1Increase connexin expressionAmeliorate proliferationBiocideHydrocarbon active ingredientsSynthetic analogueAntioxidant
A method of controlling (e.g., influencing or affecting) C-reactive protein levels in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ester functionality.
Owner:CARDAX PHARMA
EXENDIN-4 analogue dimer and preparation method and application thereof
ActiveCN103833841AOvercoming the problem of short half-lifeReduce intakeHormone peptidesPeptide/protein ingredientsSolid phasesHalf-life
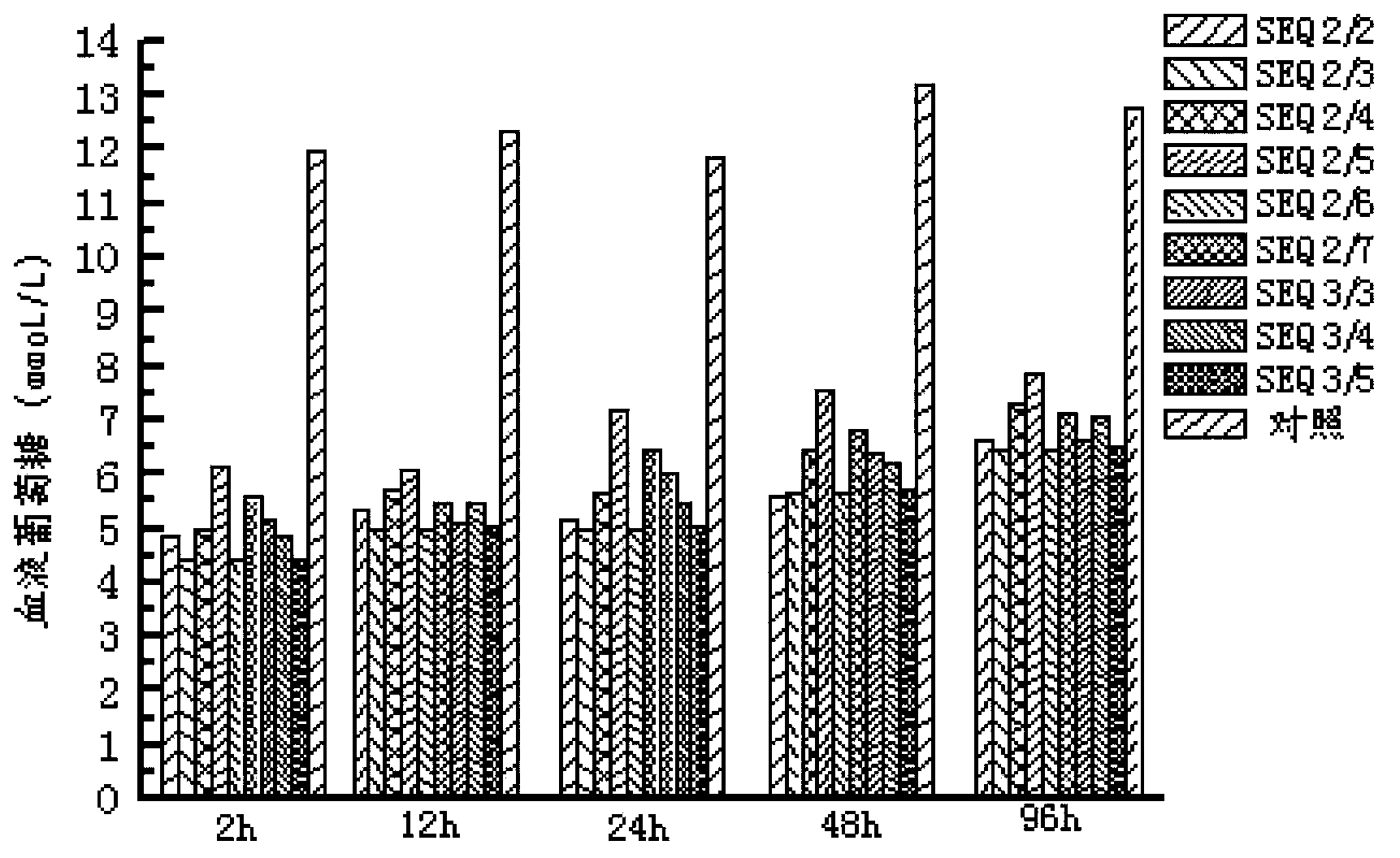
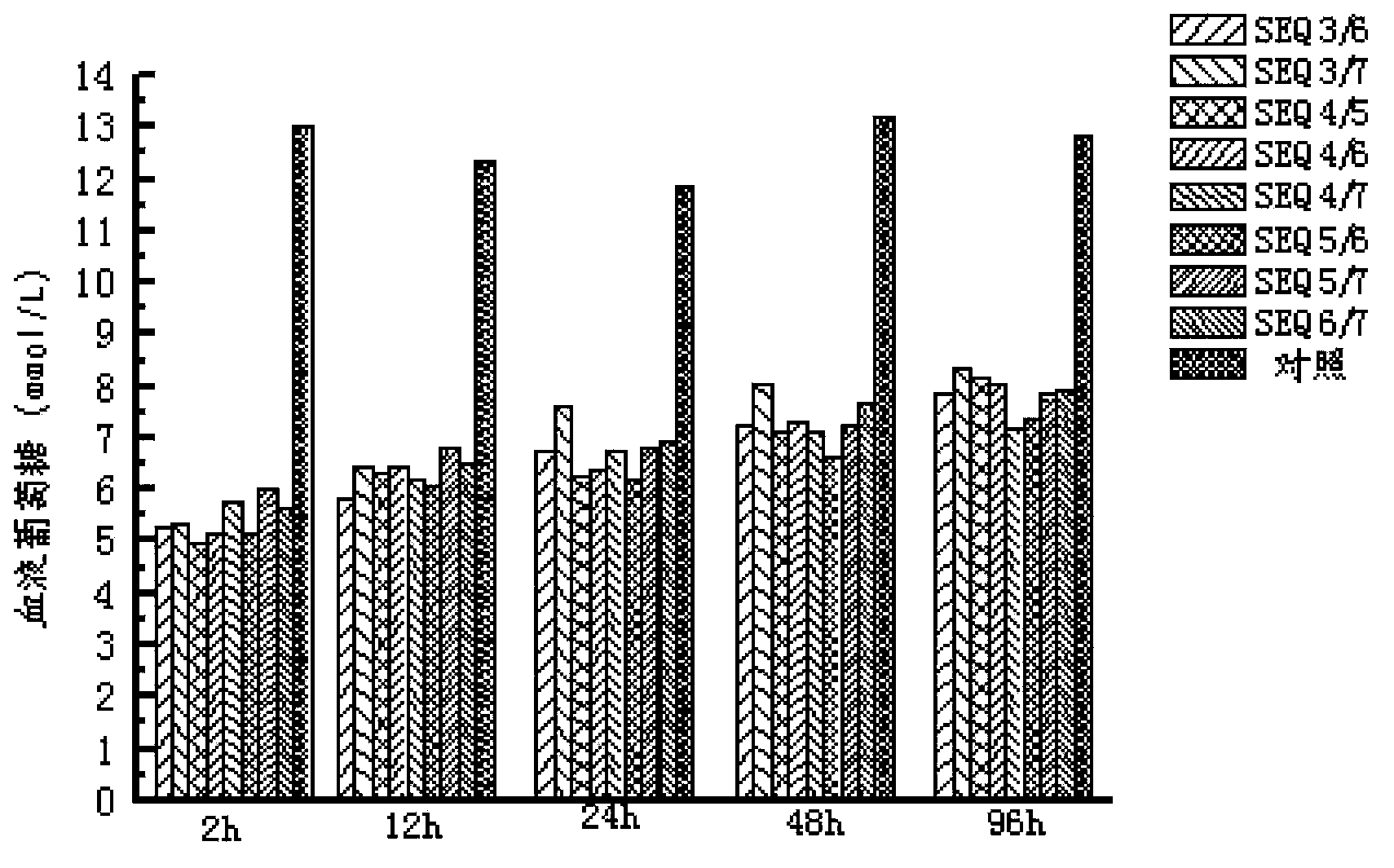
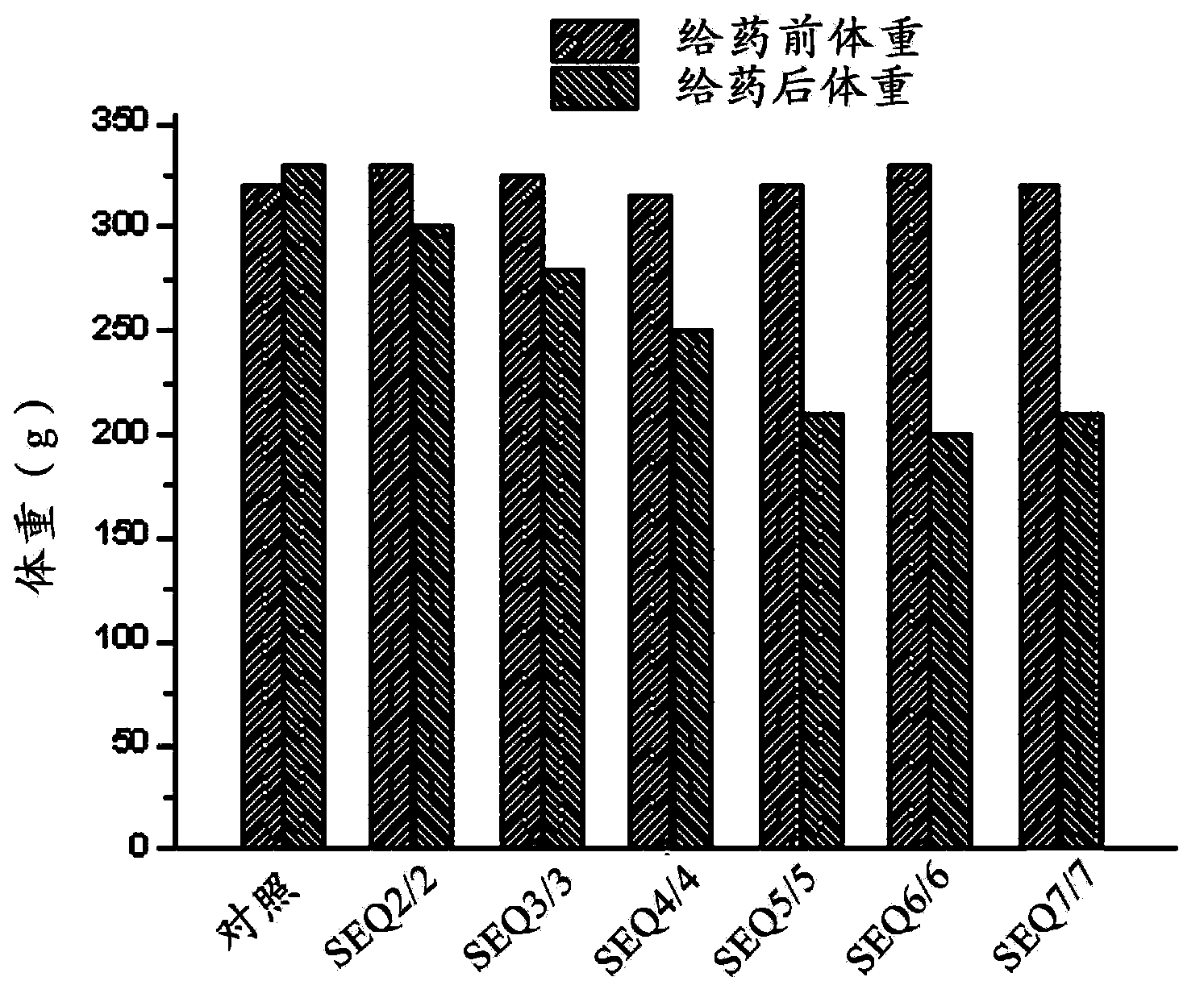
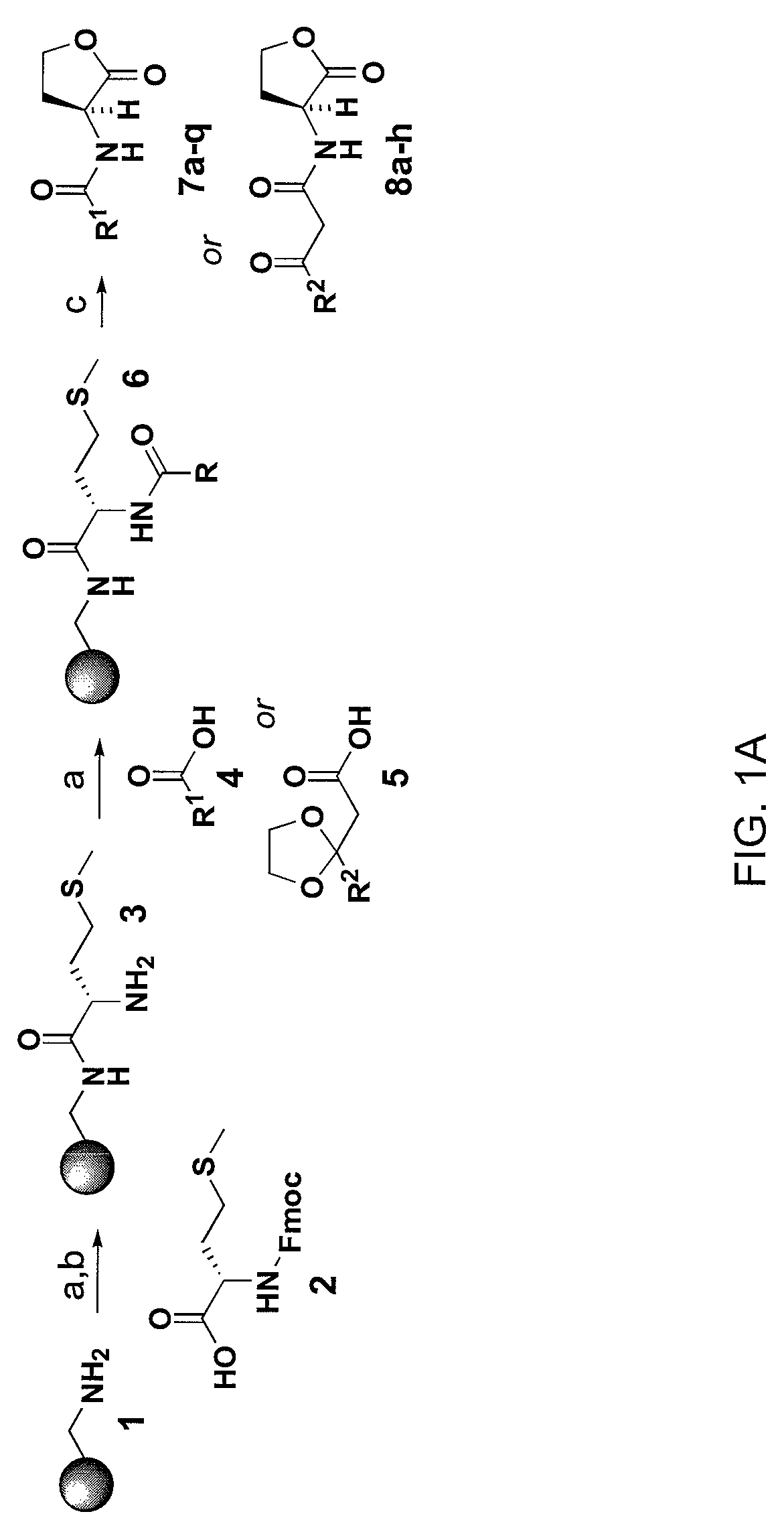
The invention provides EXENDIN-4 analogue dimer and a preparation method and application thereof. The dimer is prepared from two same or different EXENDIN-4 analogues by forming a disulfide bond between the monomers through cysteine. The preparation method of the EXENDIN-4 analogue dimer comprises the steps of synthesizing an EXENDIN-4 analogue monomer by an Fmoc solid-phase polypeptide synthesis method, and forming the dimer from the monomer. The invention also provides an application of the EXENDIN-4 analogue dimer in preparing a medicine for treating diabetes mellitus and treating and / or preventing obesity or obesity-related diseases. The in-vivo half-life period of the EXENDIN-4 analogue dimer formed from the monomer provided by the invention can exceed 14-96 hours, which is obviously prolonged as compared with the half-life period (only 2.4 hours) of EXENDIN-4 in separate administration, thereby greatly facilitating the clinical popularization and application.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Compounds and methods for modulating communication and virulence in quorum sensing bacteria
The present invention provides compositions and methods for modulating the communication and virulence of quorum sensing bacteria. In various exemplary embodiments, the invention provides a combinatorial library of quorum sensing compounds including synthetic analogs of naturally occurring and non-naturally occurring acyl-homoserine lactone (AHL) analogs, and methods of synthesizing and using these compounds.
Owner:WISCONSIN ALUMNI RES FOUND
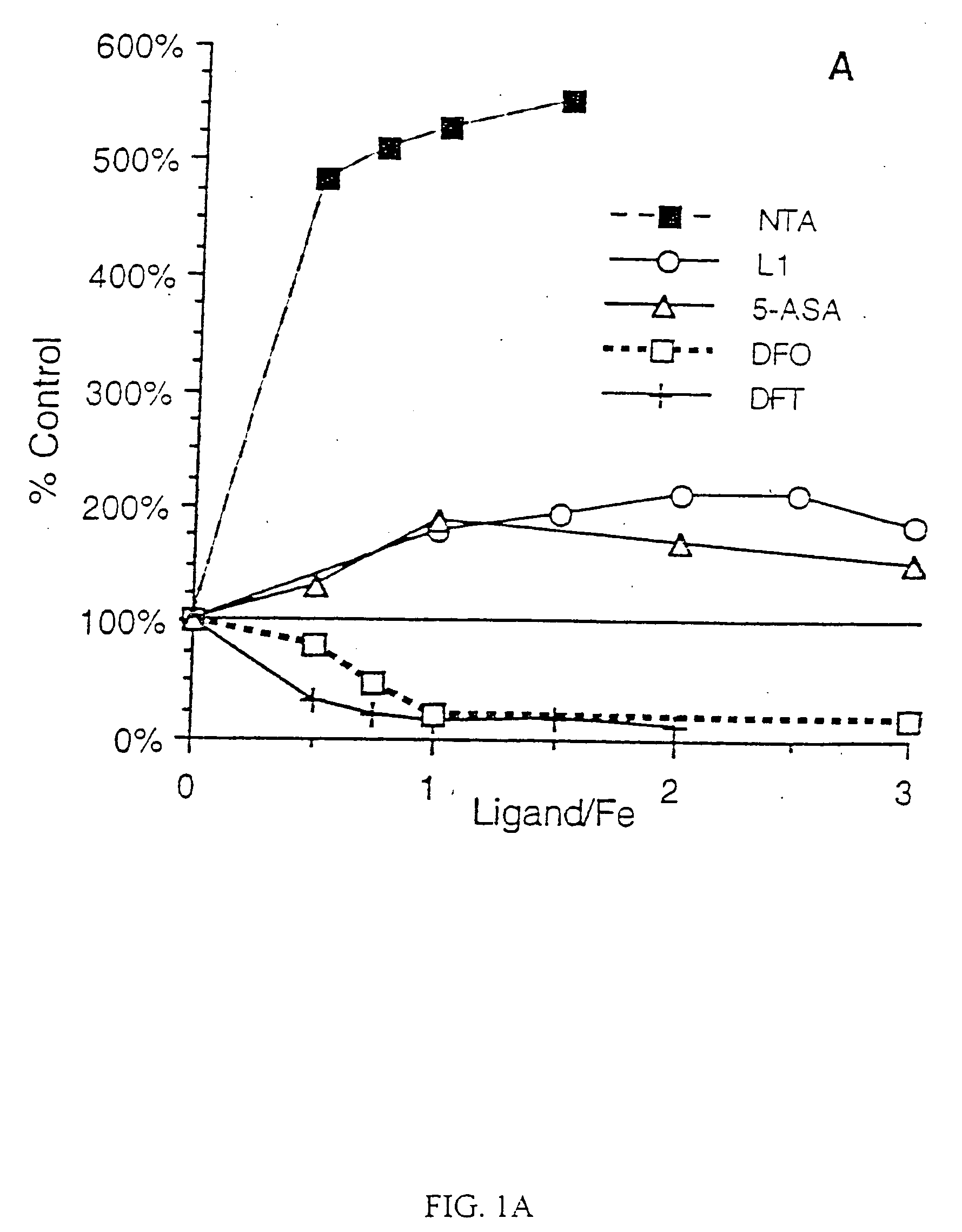
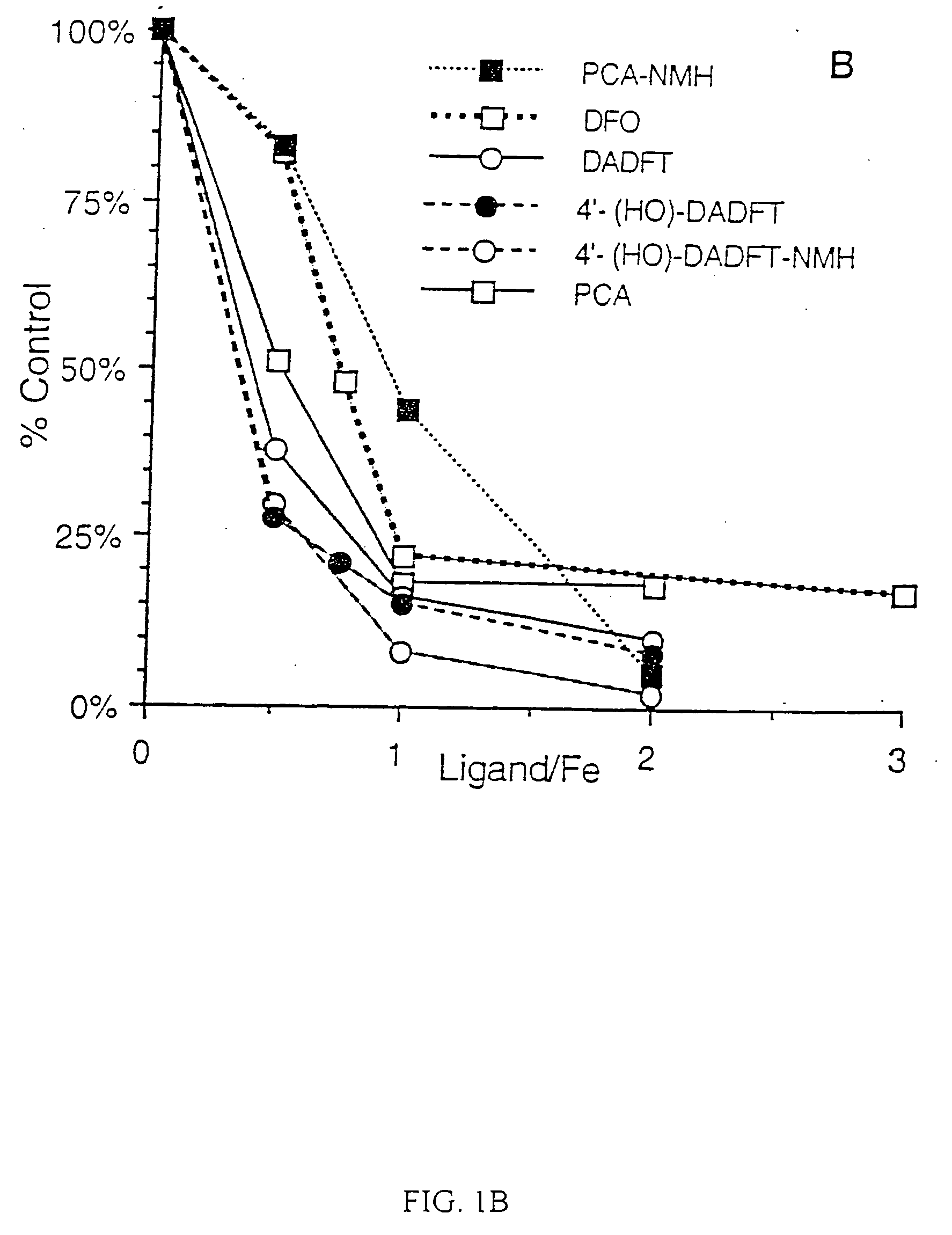
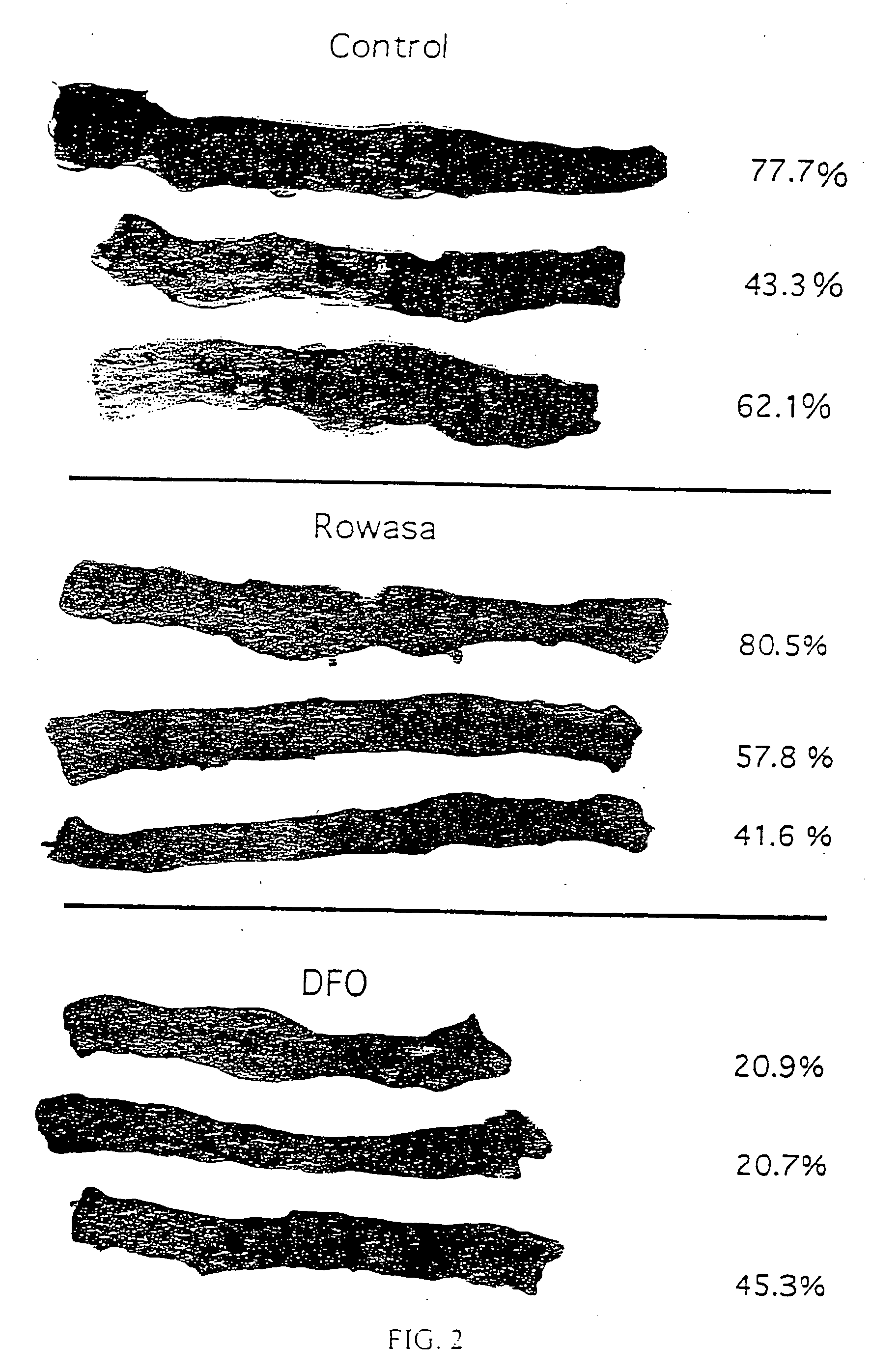
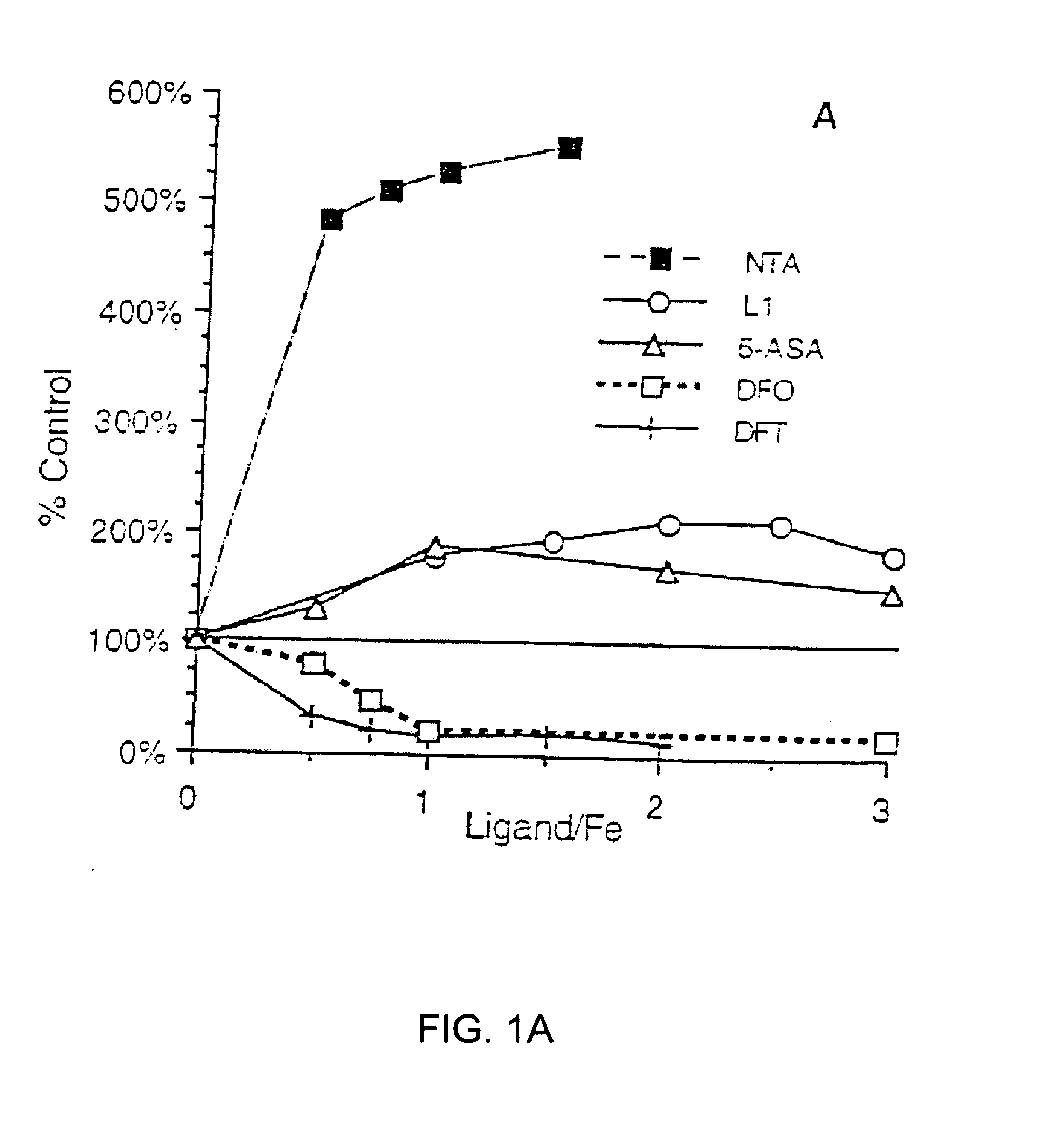
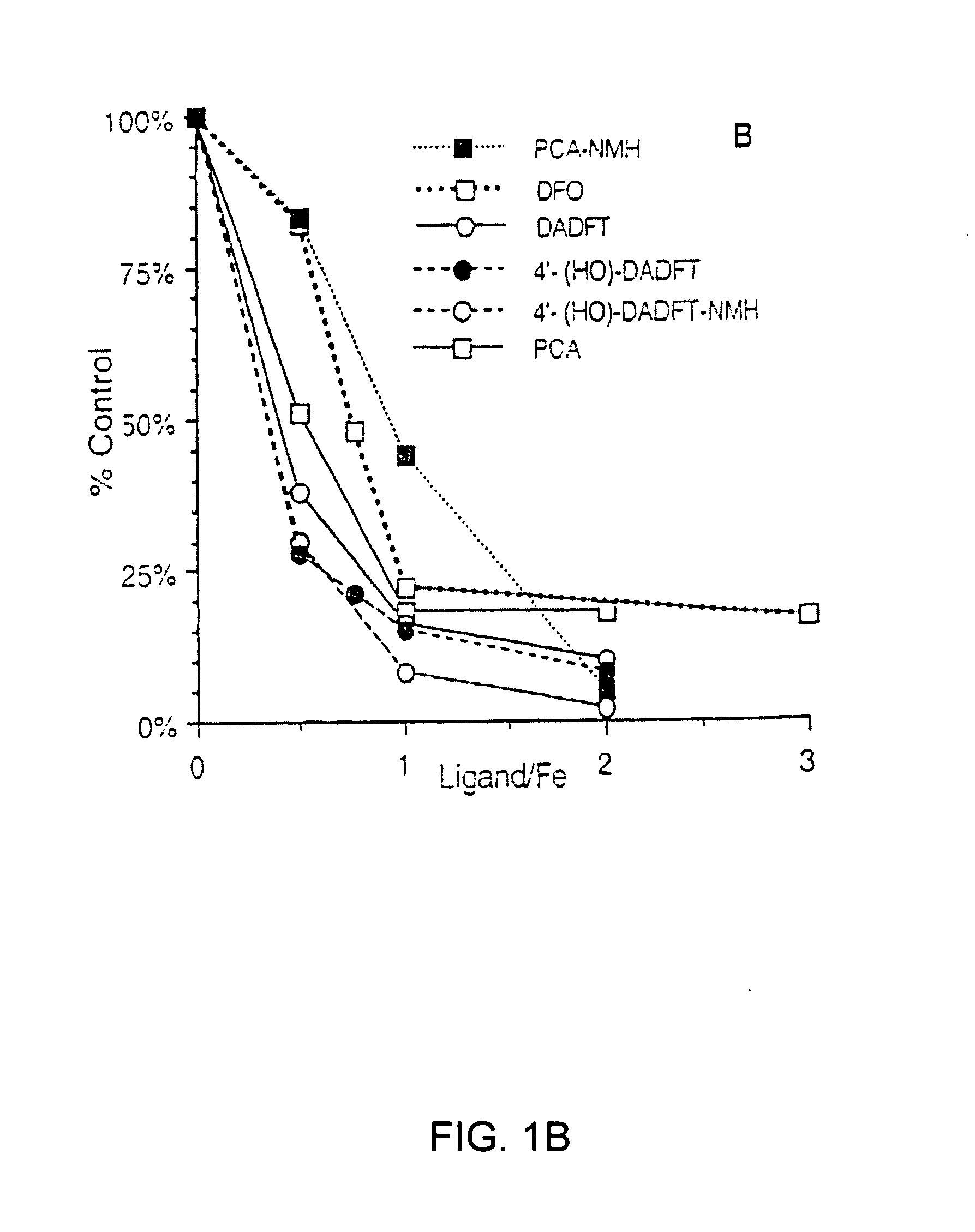
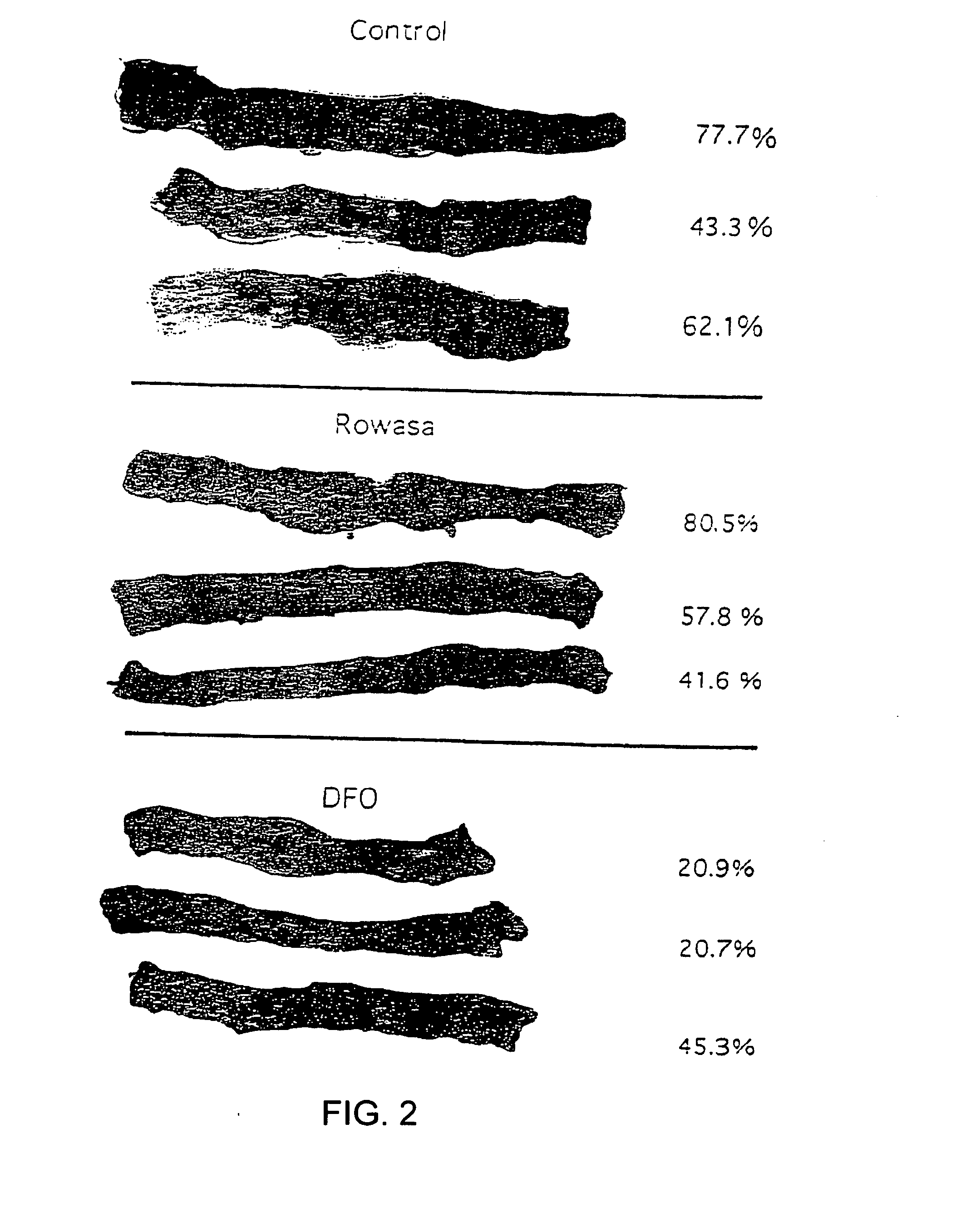
Antioxidant and radical scavenging activity of synthetic analogs of desferrithiocin
InactiveUS20050234113A1Limit formation of and damageAvoid attenuationBiocideDigestive systemCellular respirationAntioxidant
Free radicals and reactive oxygen species have the potential to damage a wide variety of organic molecules, typically by oxidizing certain moieties. These damaging species can, for example, be produced by an organism as a by-product of cellular respiration or by the reaction of iron(II) and peroxide. The present invention includes methods of using aryl-substituted heterocyclic iron chelating compounds as antioxidants, as well as preventing the reduction of iron(III) to iron(II). In addition, the present invention provides methods of treating conditions such as inflammatory disease, neoplastic disease, and ischemic episodes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Hybrid molecules having Factor VII/VIIa activity
InactiveUS7432352B2Same and increased activityIncreased serum half-lifePeptide/protein ingredientsMammal material medical ingredientsSynthetic analogueFactor ii
Owner:NOVO NORDISK HEALTH CARE AG
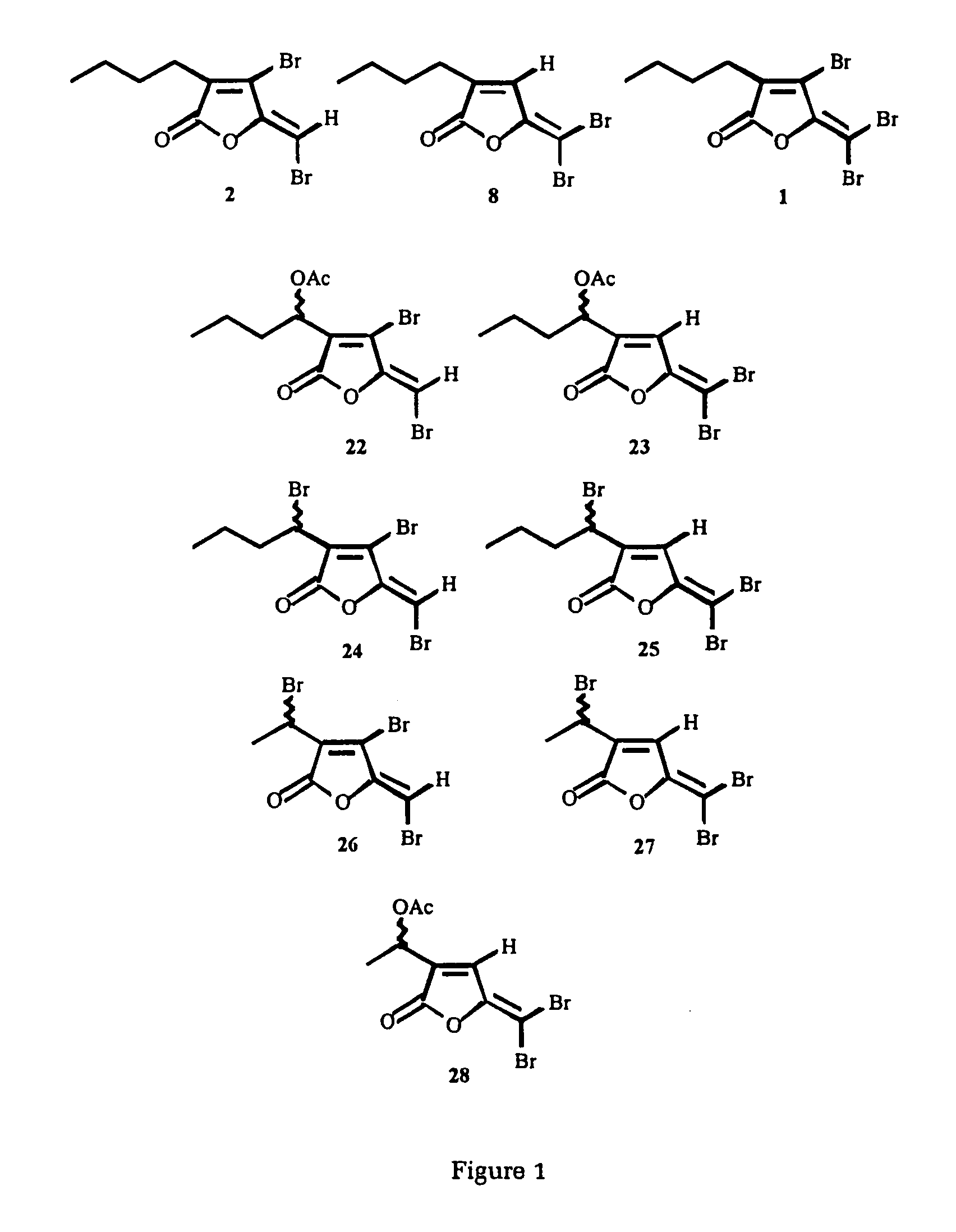
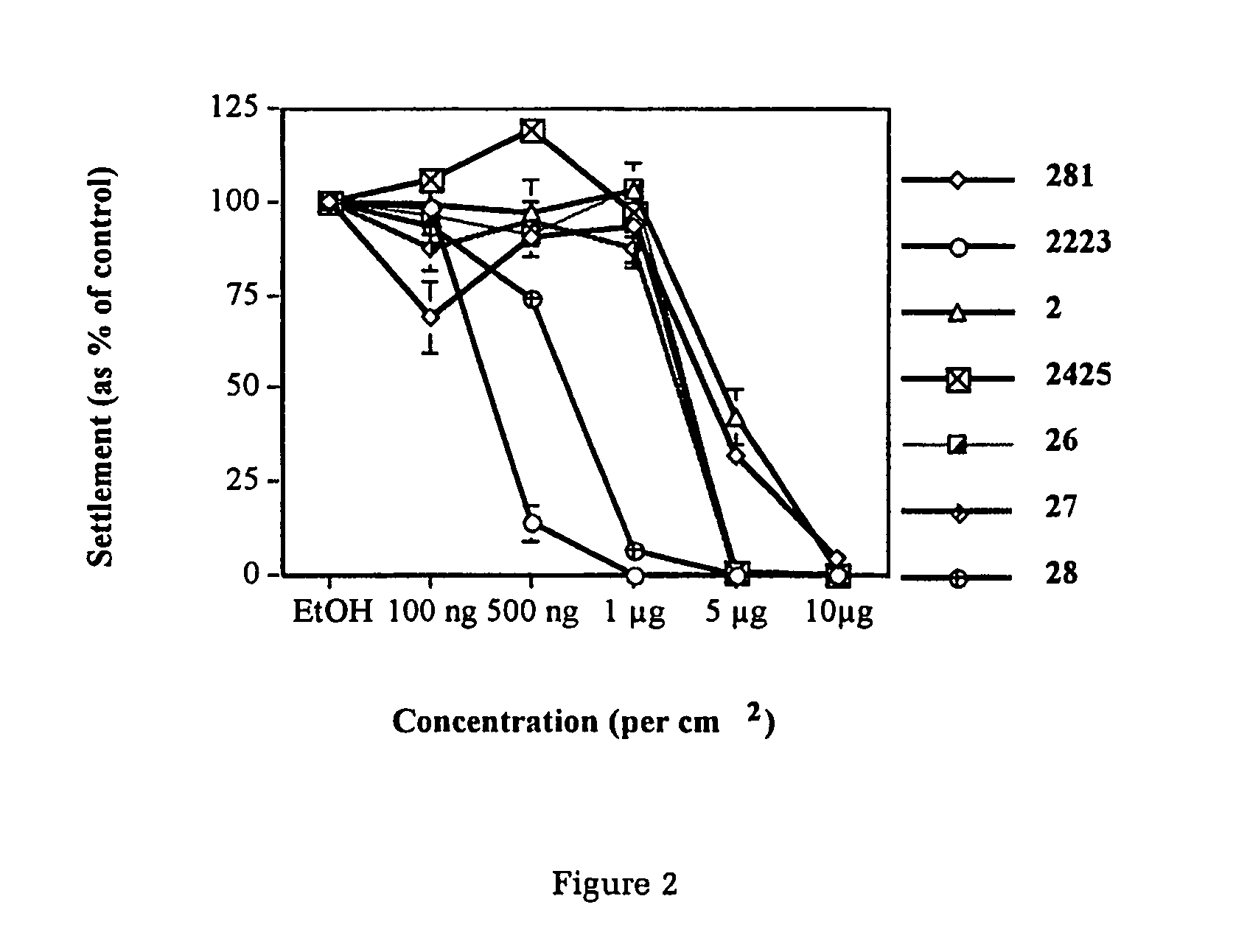
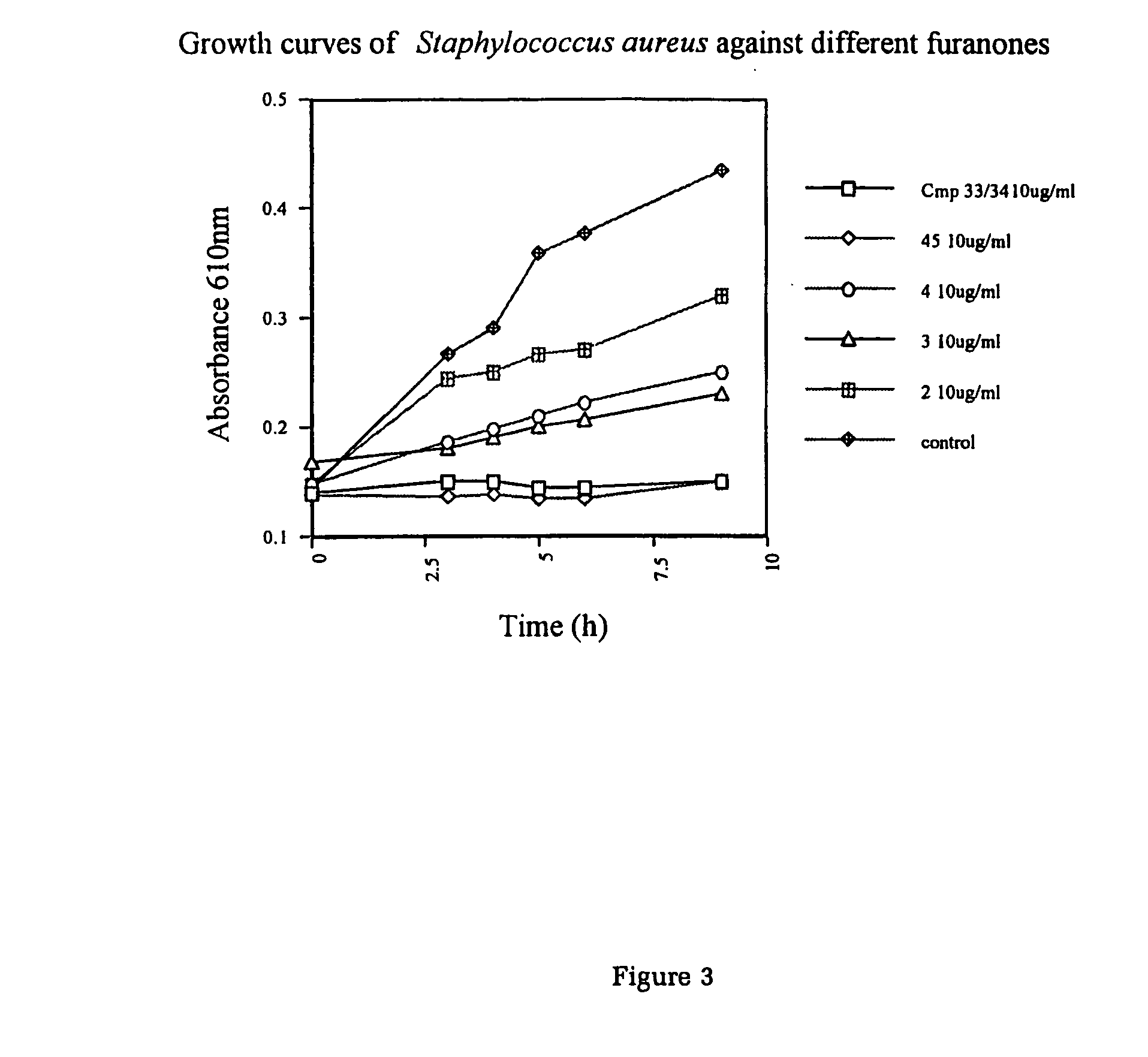
Production of furanones
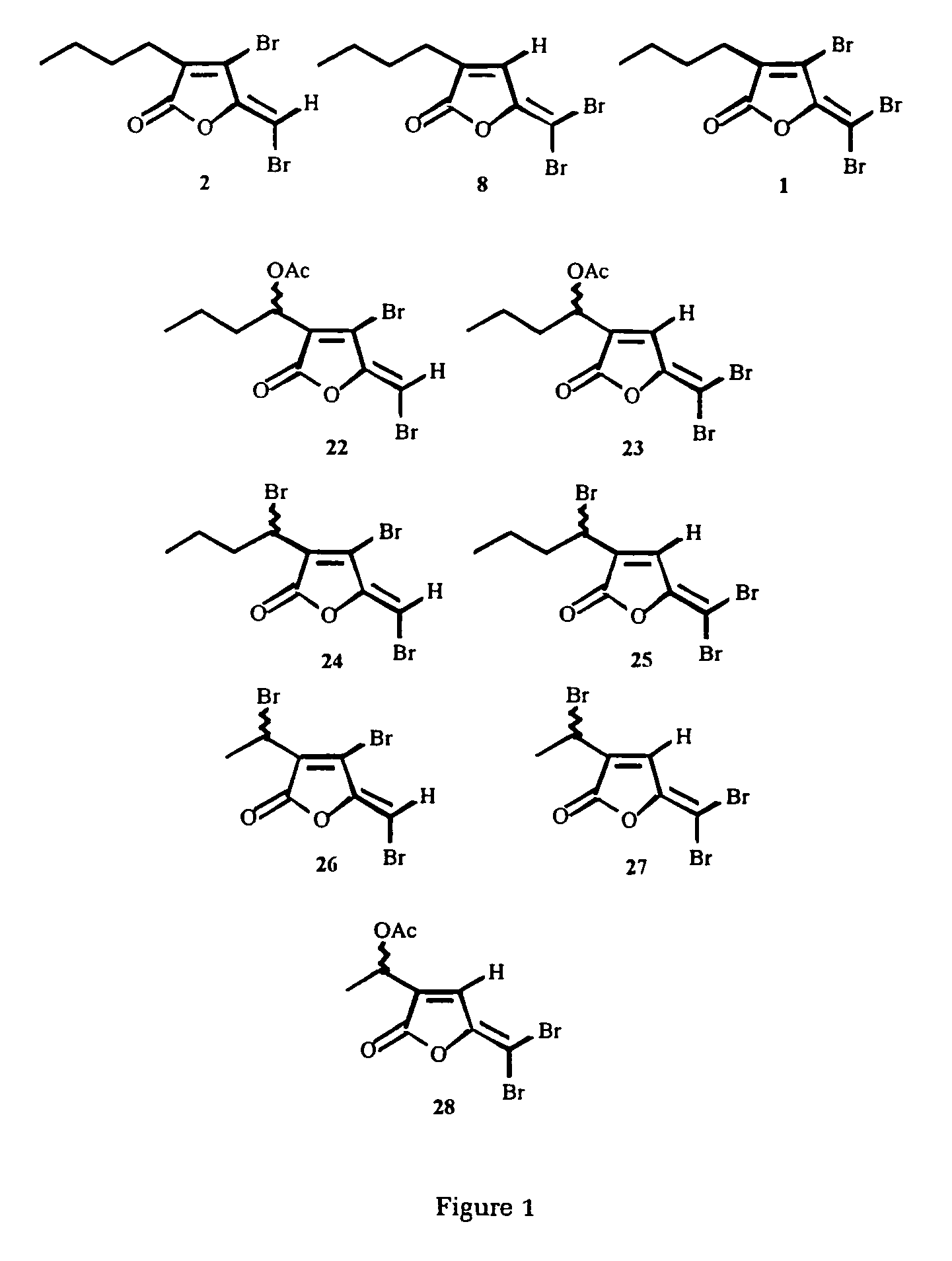
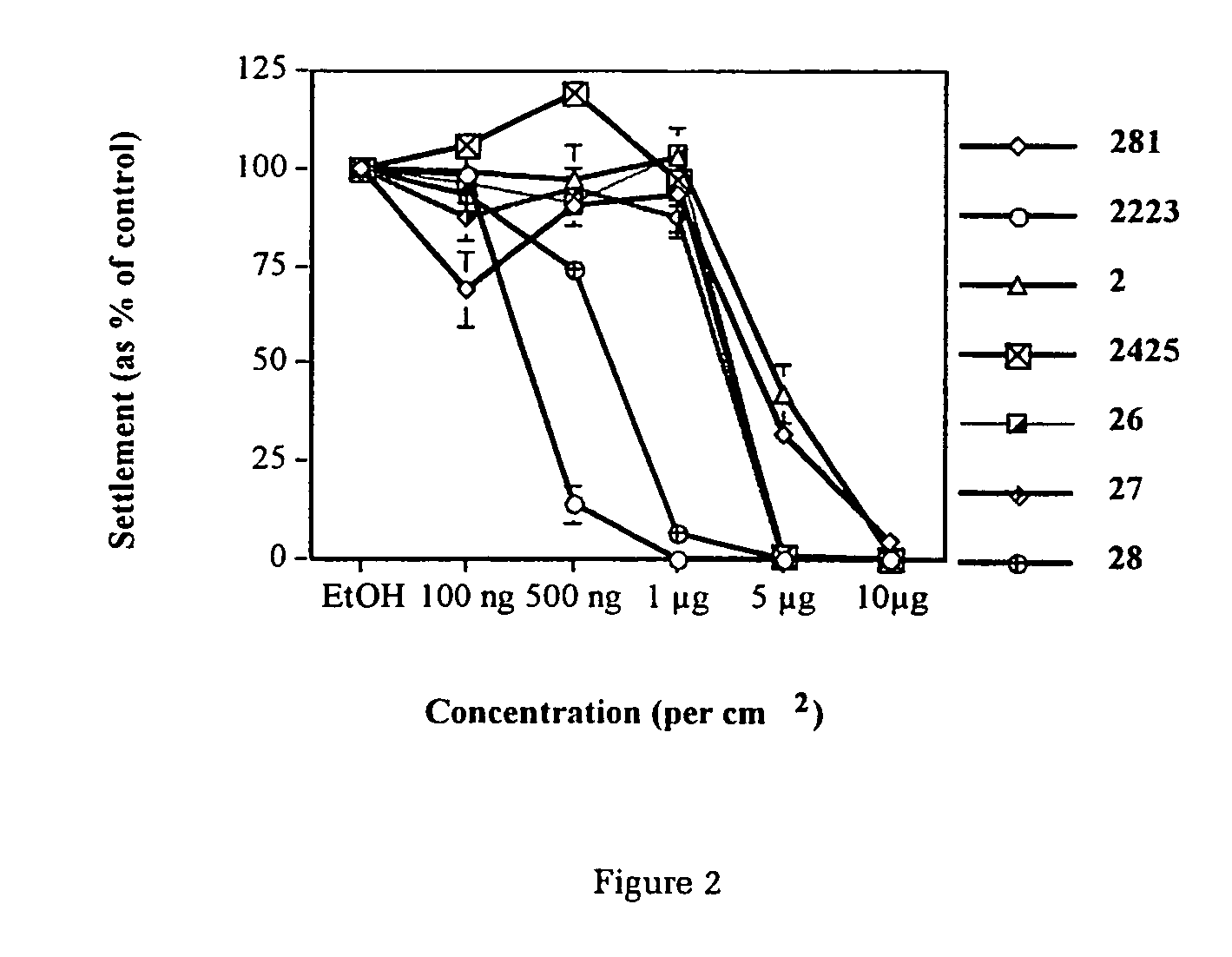
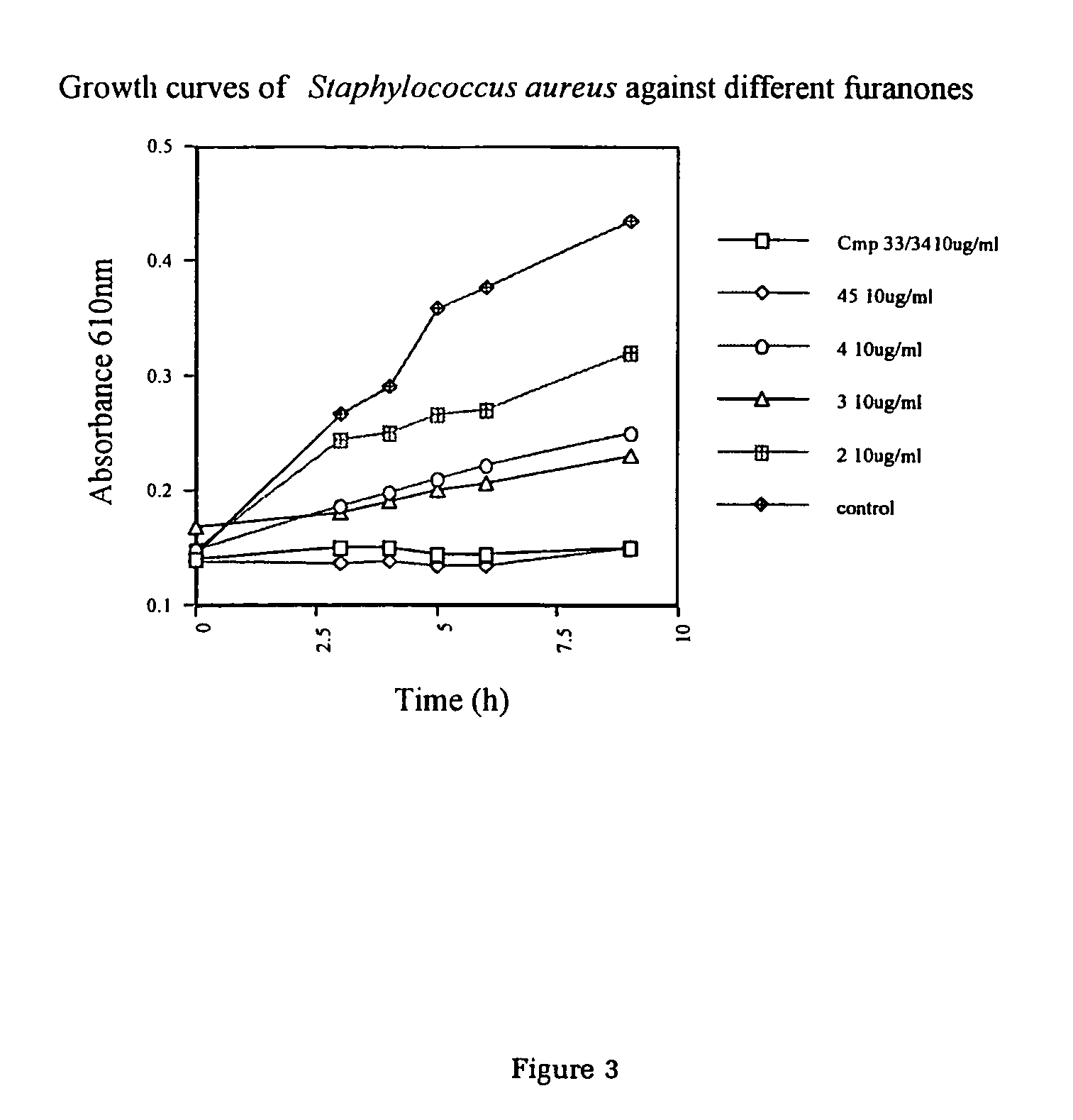
The present invention relates to the side chain functionalization of fimbrolides (halogenated 3-alkyl-5-methylene-2(5H)-furanones) and their synthetic analogues, that yields fimbrolides substituted with a halogen, an oxygen or a nitrogen functionality in the alkyl chain, especially fimbrolide alcohols, caboxylate and sulfinate and sulfonate esters, ethers, aldehydes, ketones, acids, amides, nitro derivatives, hydrophobic, hydrophilic and fluorophilic alkyl derivatives and polymers.
Owner:UNISEARCH LTD
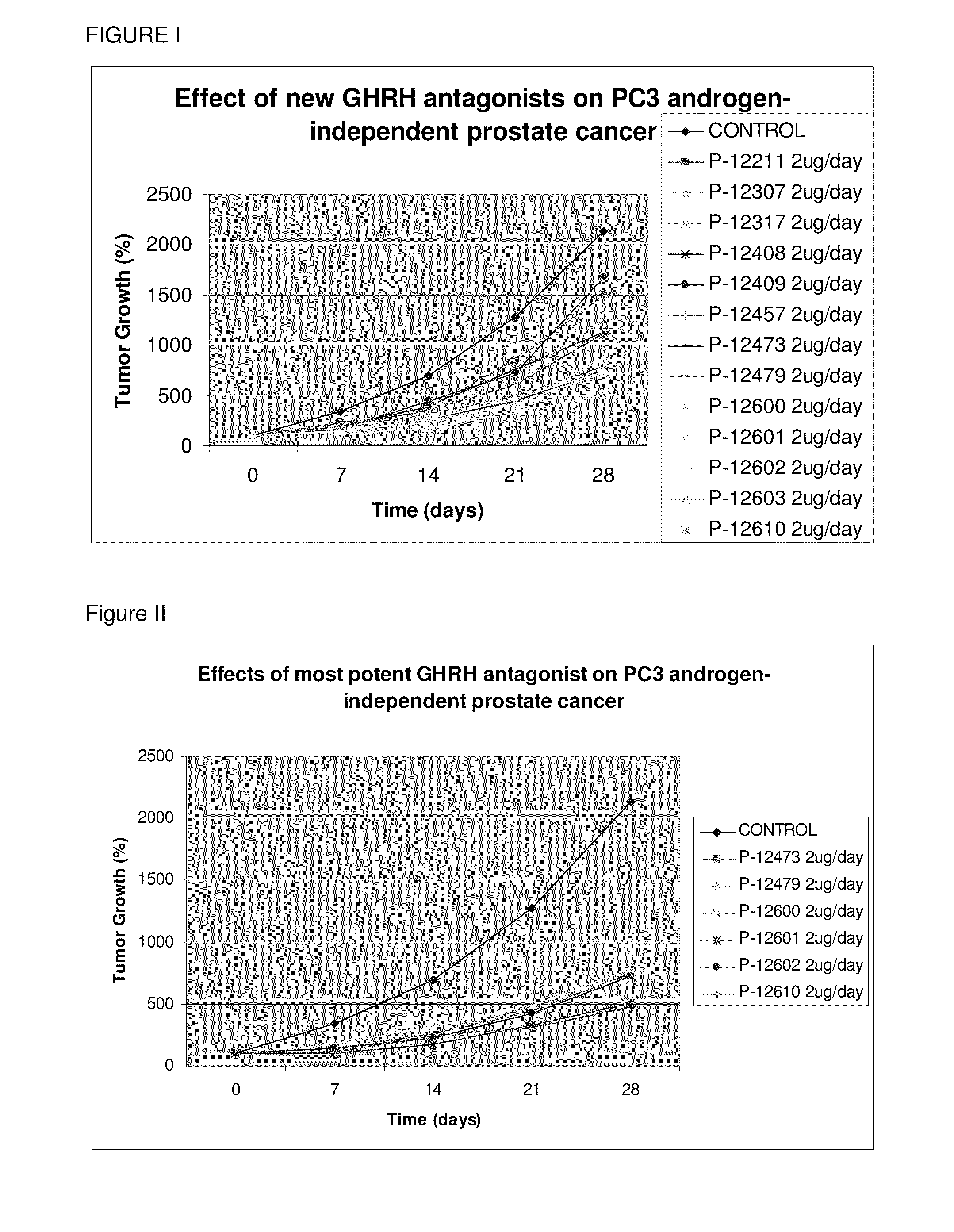
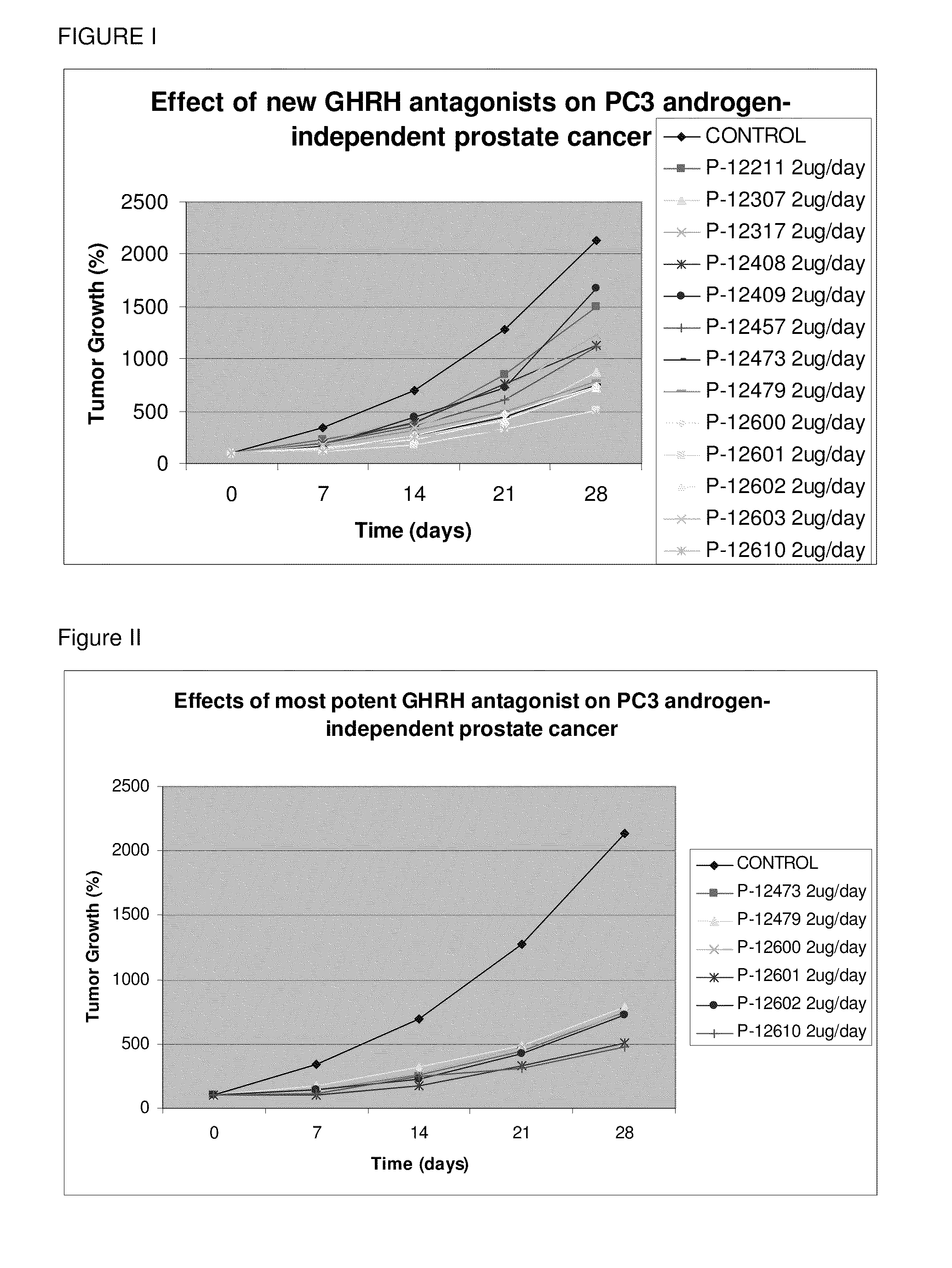
Fluorinated GHRH Antagonists
InactiveUS20110066230A1Inhibition releaseEnhanced inhibitory effectStentsPeptide/protein ingredientsSynthetic analogueHuman cancer
Novel fluorinated synthetic analogs of hGH-RH(1-30)NH2 that inhibit the release of growth hormone from the pituitary in mammals as well as inhibit the proliferation of human cancers through a direct effect on the cancer cells, and to therapeutic compositions containing these novel peptides and their use.
Owner:UNIV OF MIAMI +1
Method of detecting surrogate markers in a serum sample
ActiveUS20130137598A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCompetitive bindingCholesterol
The invention provides a method of detecting surrogate markers for active tuberculosis in a serum sample. The surrogate markers are selected from serum mycolic acid antigen, serum anti-mycolic acid antibodies or both. The method includes the steps of combining the serum sample with a labelled monoclonal immunoglobulin antibody or fragment thereof to mycolic acids to produce a combined serum sample, the antibody or fragment thereof not substantially cross-reacting with cholesterol and the label being selected so that binding of the labelled antibody to immobilized mycolic acid antigen of mycobacterial origin produces a detectable signal and combining a blank sample with the labelled monoclonal immunoglobulin antibody or fragment thereof to mycolic acid to produce a combined blank sample. The method includes exposing both samples to immobilised mycolic acid antigen of mycobacterial origin or a synthetic analogue or analogues thereof so that the labelled immunoglobulin antibodies or fragments thereof in each sample bind to the immobilised antigen to produce detectable signals. If the surrogate markers are present, the signal produced by the blank sample will be stronger than that produced by the serum sample because of inhibition of binding of the labelled antibody in the serum sample arising from prior binding of the labelled antibody with the mycolic acid antigen in the serum sample or by competitive binding of serum anti-mycolic acid antibodies in the serum sample to the immobilised mycolic acid antigen or both.
Owner:UNIVERSITY OF PRETORIA
Fluorinated GHRH Antagonists
ActiveUS20100092539A1Inhibition releaseEnhanced inhibitory effectSenses disorderPeptide/protein ingredientsHuman cancerSynthetic analogue
Novel fluorinated synthetic analogs of hGH-RH(1-30)NH2 that inhibit the release of growth hormone from the pituitary in mammals as well as inhibit the proliferation of human cancers through a direct effect on the cancer cells, and to therapeutic compositions containing these novel peptides and their use.
Owner:UNIV OF MIAMI +1
Neuroprotective polyphenol analogs
The present invention provides neuroprotective polyphenol compounds, which can be synthetic analogs of fisetin, baicalein or chlorogenic acid, that maintain neuroprotective, anti-inflammatory, glutathione promoting, and / or antioxidant properties. The neuroprotective polyphenol compounds are useful for promoting, enhancing and / or increasing neuron protection, growth and / or regeneration. The polyphenol compounds further find use for increasing and or maintaining intracellular glutathione (GSH) levels. The polyphenol compounds are also useful for treating, preventing, mitigating and / or delaying neurodegenerative conditions, including diabetes, Parkinson's disease, Huntington's disease, Alzheimer's disease, non-Alzherimer's dementias, multiple sclerosis, traumatic brain injury, spinal cord injury or ALS.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Immunomodulatory alkaloids
Immunotherapy comprises administration of an alkaloid at a dose sufficient to induce IL-2 production in dendritic cells in a patient. The alkaloid induces the production of IL-2 in dendritic cells. The alkaloids need not be naturally occurring, and may be synthetic analogues or derivatives of naturally occurring counterparts. Such analogues or derivatives are preferably pharmaceutically acceptable analogues, salts, isomers or derivatives as herein defined. However, preferred alkaloids are phytochemicals. Such phytochemicals may be isolated from natural sources or synthesised in vitro. Particularly preferred are alkaloids is selected from piperidine alkaloids; pyrrolin alkaloids; pyrrolidine alkaloids; pyrolizidine alkaloids: indolizidine alkaloids and nortropane alkaloids.
Owner:SUMMIT
Carotenoid ester analogs or derivatives for the inhibition and amelioration of liver disease
InactiveUS7723327B2Ameliorate some typeMaintain relatively stableBiocideHydrocarbon active ingredientsSynthetic analogueDisease
A method of treating liver disease in a subject may include administering to the subject an effective amount of a pharmaceutically acceptable formulation. The pharmaceutically acceptable formulation may include a synthetic analog or derivative of a carotenoid. The subject may be administered a carotenoid analog or derivative, either alone or in combination with another carotenoid analog or derivative, or co-antioxidant formulation. The carotenoid analog may include a conjugated polyene with between 7 to 14 double bonds. The conjugated polyene may include a cyclic ring including at least one substituent. In some embodiments, a cyclic ring of a carotenoid analog or derivative may include at least one substituent. The substituent may be coupled to the cyclic ring with an ester functionality.
Owner:CARDAX PHARMA
Antioxidant and radical scavenging activity of synthetic analogs of desferrithiocin
InactiveUS20080255081A1Limit formation of and damageAvoid attenuationBiocideDigestive systemCellular respirationDisease
Free radicals and reactive oxygen species have the potential to damage a wide variety of organic molecules, typically by oxidizing certain moieties. These damaging species can, for example, be produced by an organism as a by-product of cellular respiration or by the reaction of iron(II) and peroxide. The present invention includes methods of using aryl-substituted heterocyclic iron chelating compounds as antioxidants, as well as preventing the reduction of iron(III) to iron(II). In addition, the present invention provides methods of treating conditions such as inflammatory disease, neoplastic disease, and ischemic episodes.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
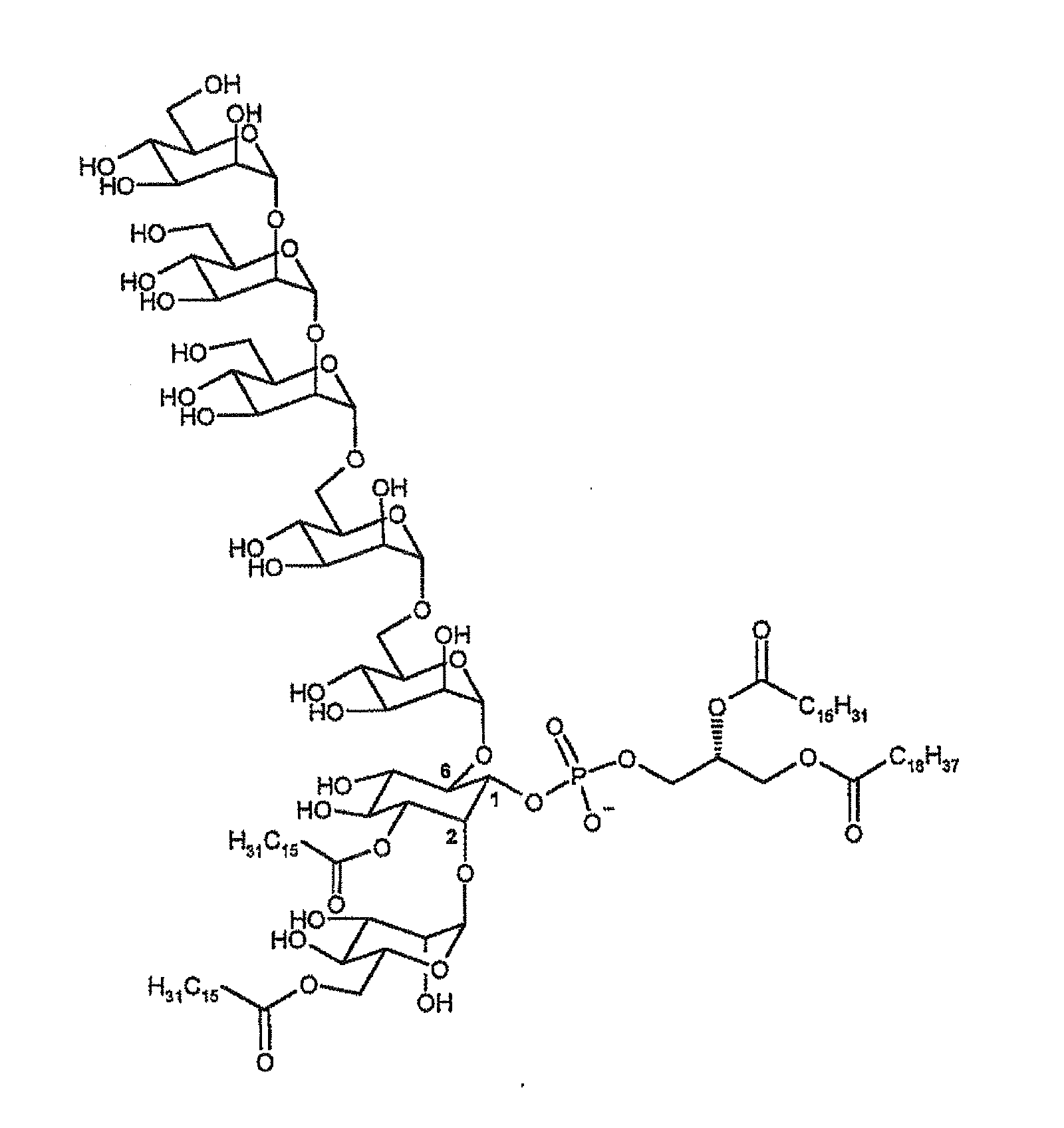
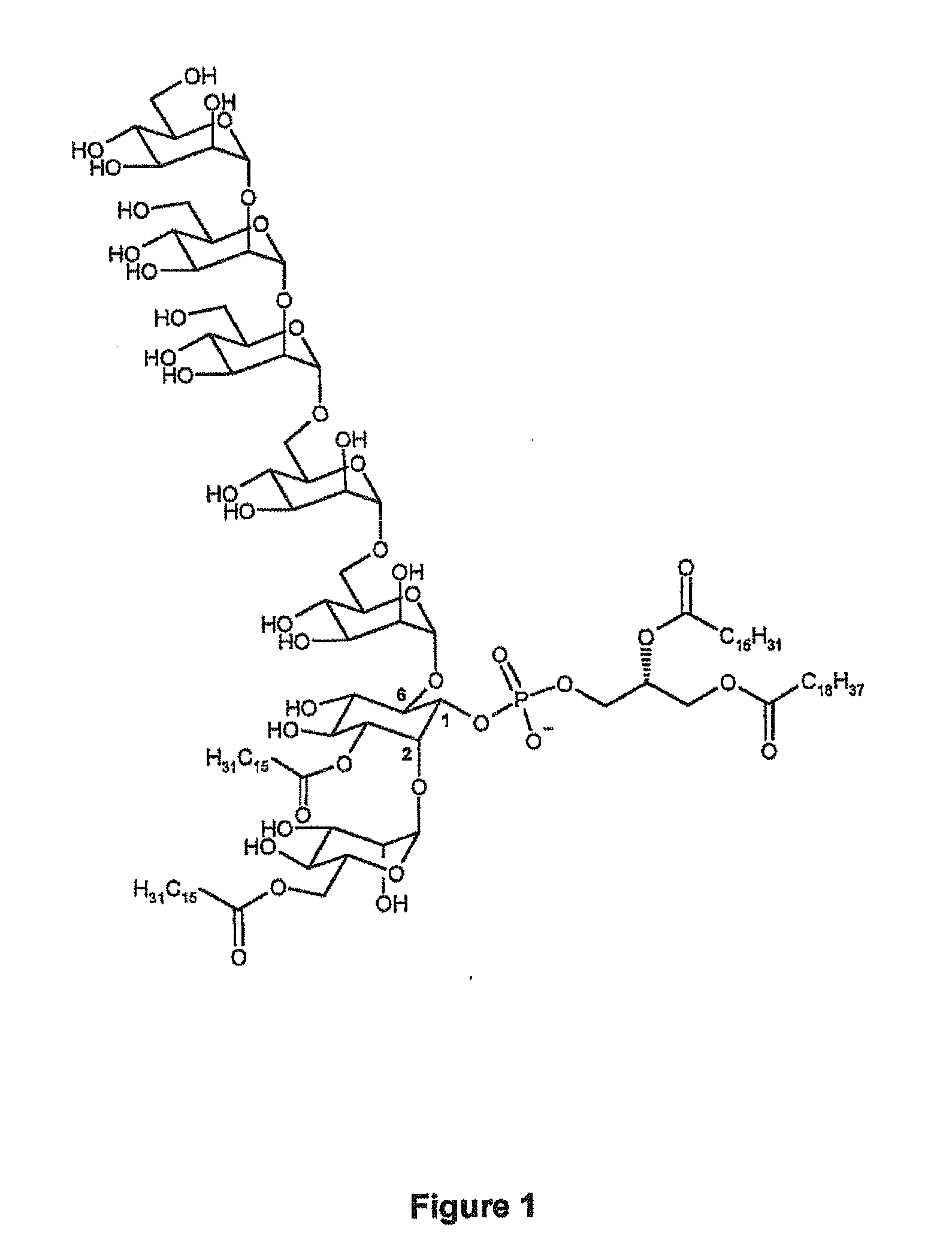
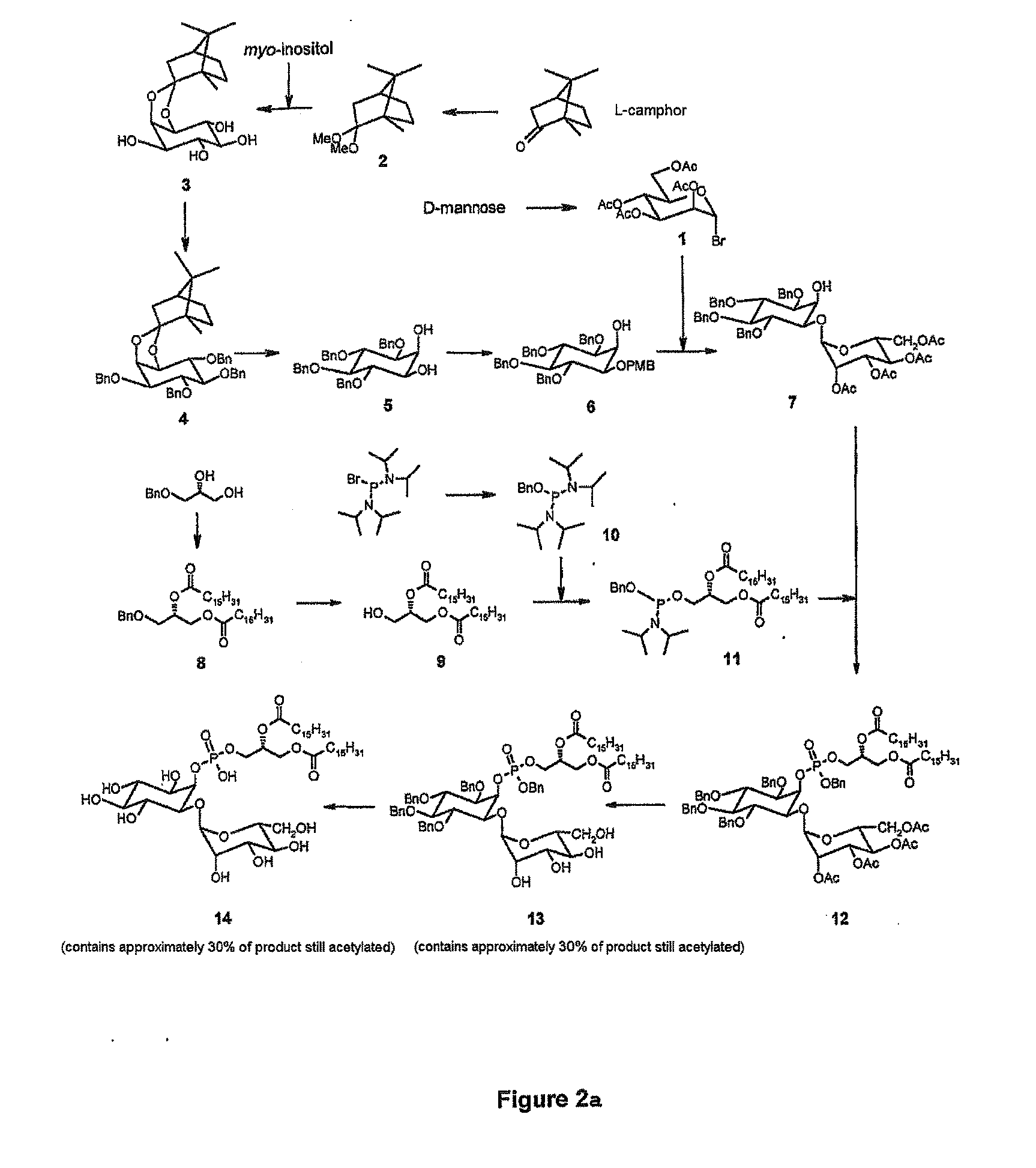
Synthetic analogues of phosphatidyl-myo-inositol mannosides with an inhibitory activity of the inflammatory response
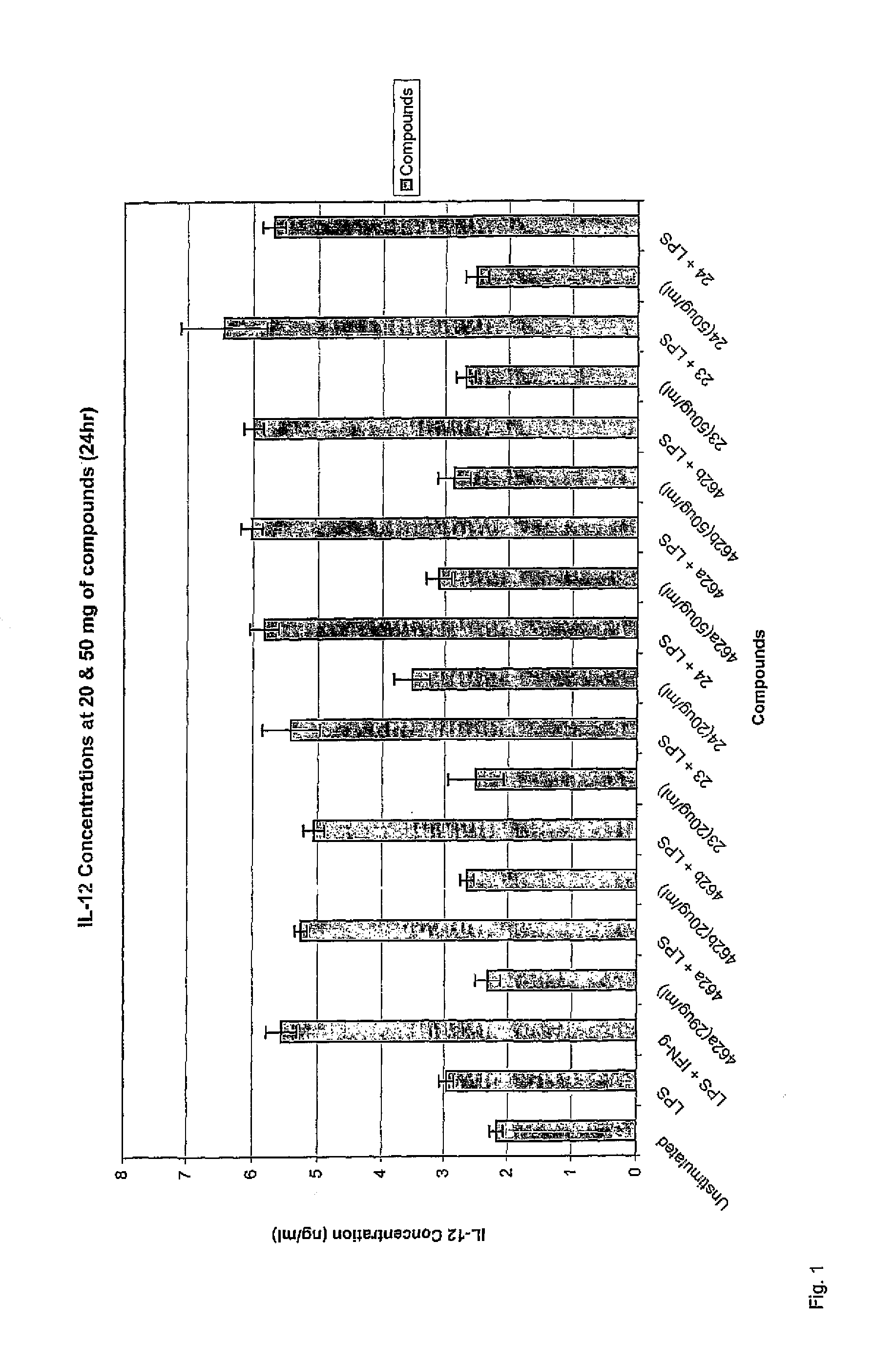
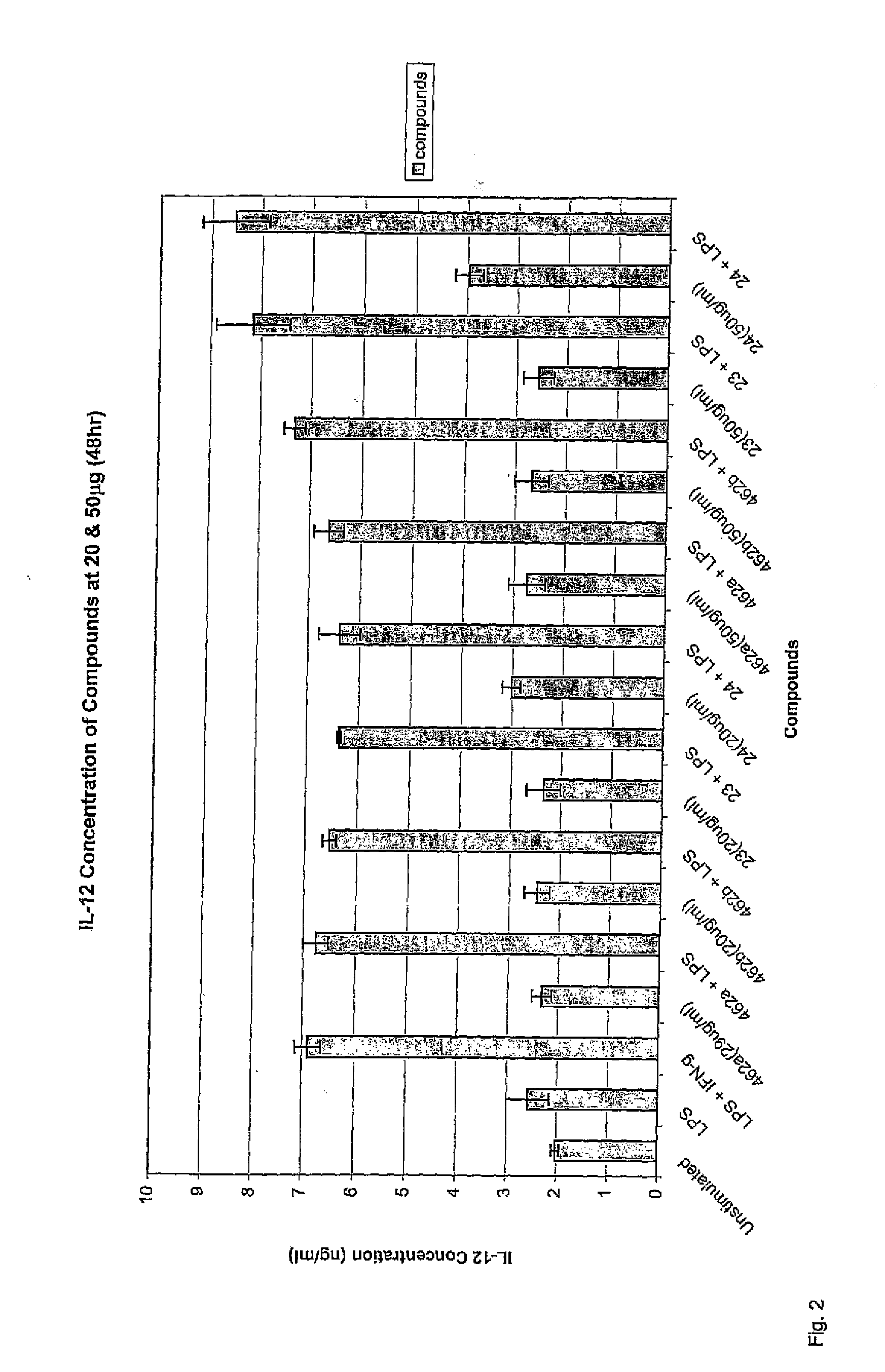
The present invention relates to novel synthetic analogues of phosphatidyl-myo-inositol mannosides (hereinafter referred to as PIMs) of general formula (I): or a pharmaceutically acceptable salt thereof, to the method for preparing same and to the use thereof in the prevention or treatment of a disease associated with the overexpression of cytokines or of chemokines, in particular of TNF and / or of IL-12. The invention also relates to a pharmaceutical composition comprising at least one synthetic derivative of PIM.
Owner:CENT NAT DE LA RECHERCHE SCI +1
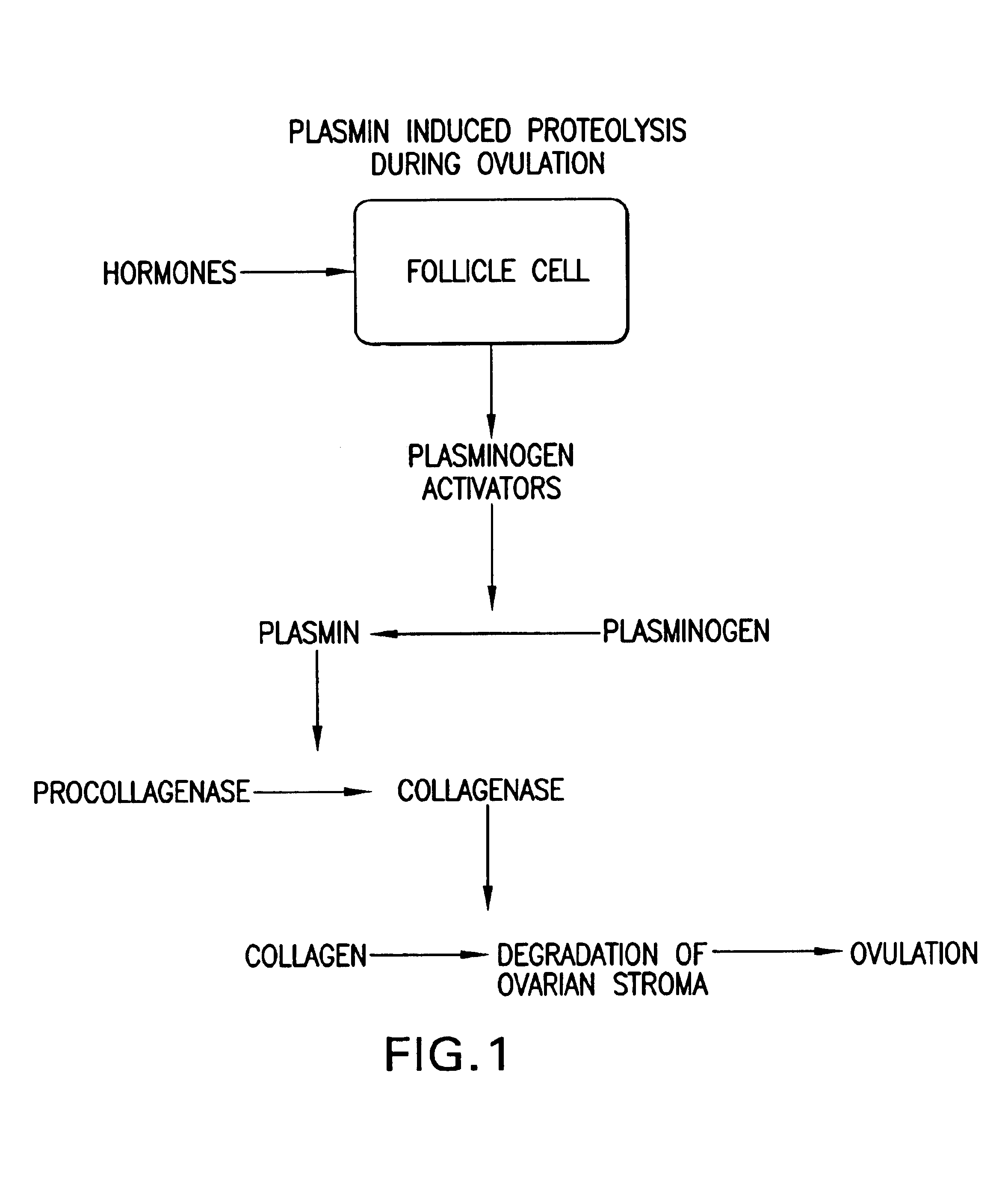
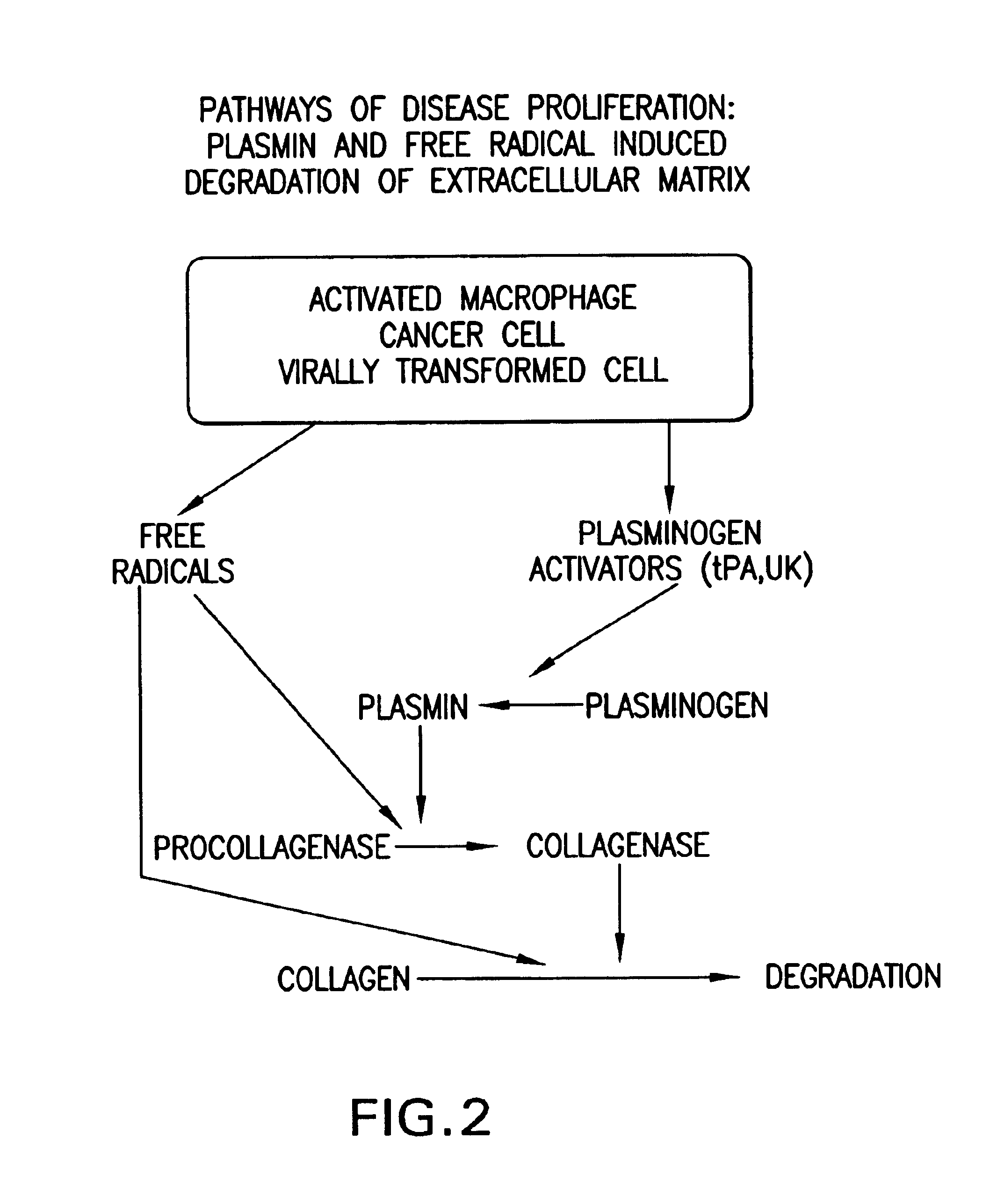
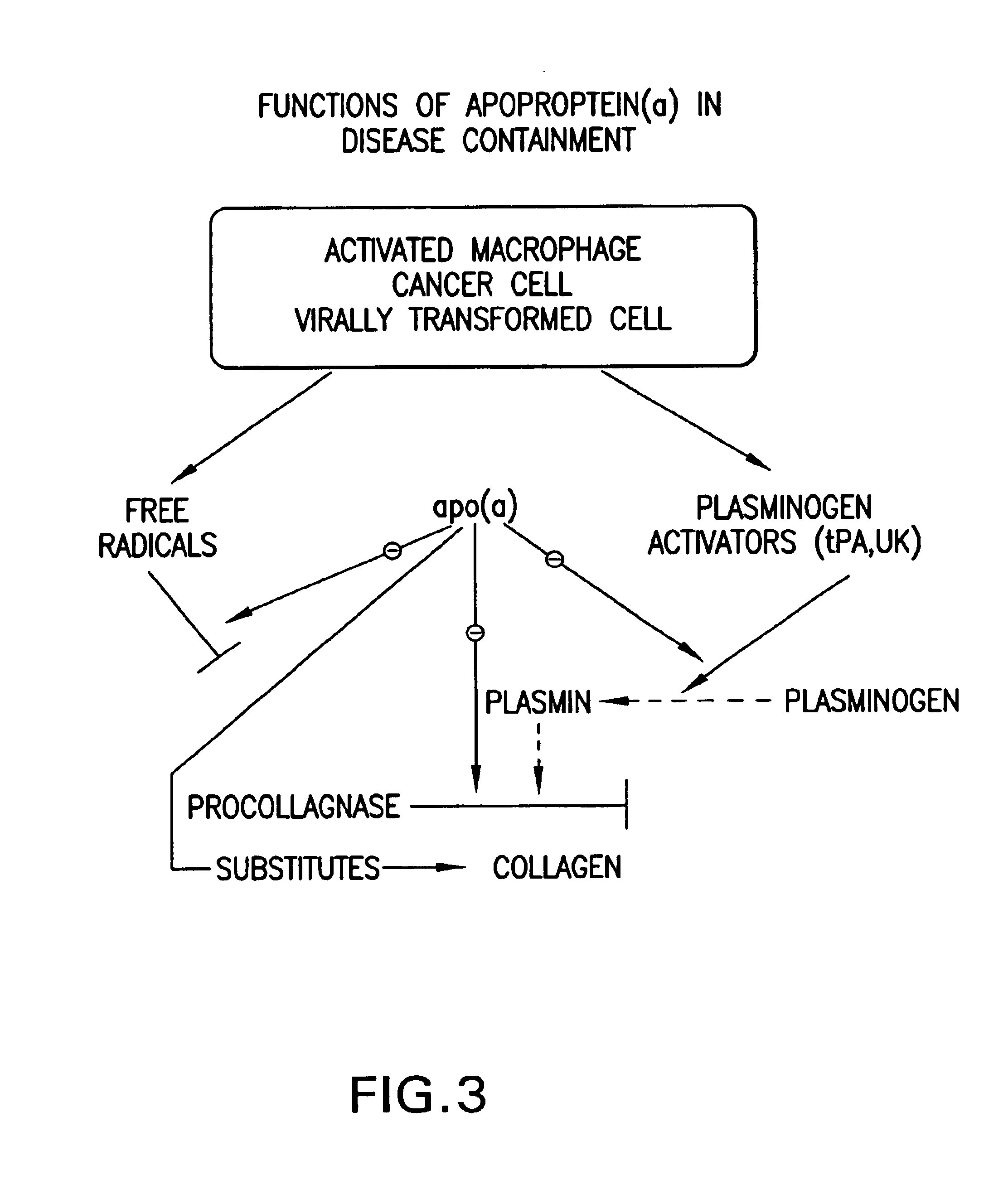
Synergistic compositions comprising ascobate and lysine for states related to extra cellular matrix degeneration
InactiveUS6974833B2Decrease plasmin-and free radical-mediated productionBiocidePeptide/protein ingredientsSynthetic analogueCell-Extracellular Matrix
Methods for the treatment of diseases or pathological states related to the degradation of the extracellular matrix, such as degenerative diseases atheriosclerosis, cancer, infection or other inflammatory diseases are disclosed, comprising administering compositions of lysine, proline, ascorbate, and their derivatives and synthetic analogues and vitamins, pro-vitamins and trace elements.
Owner:RATH MATTHIAS
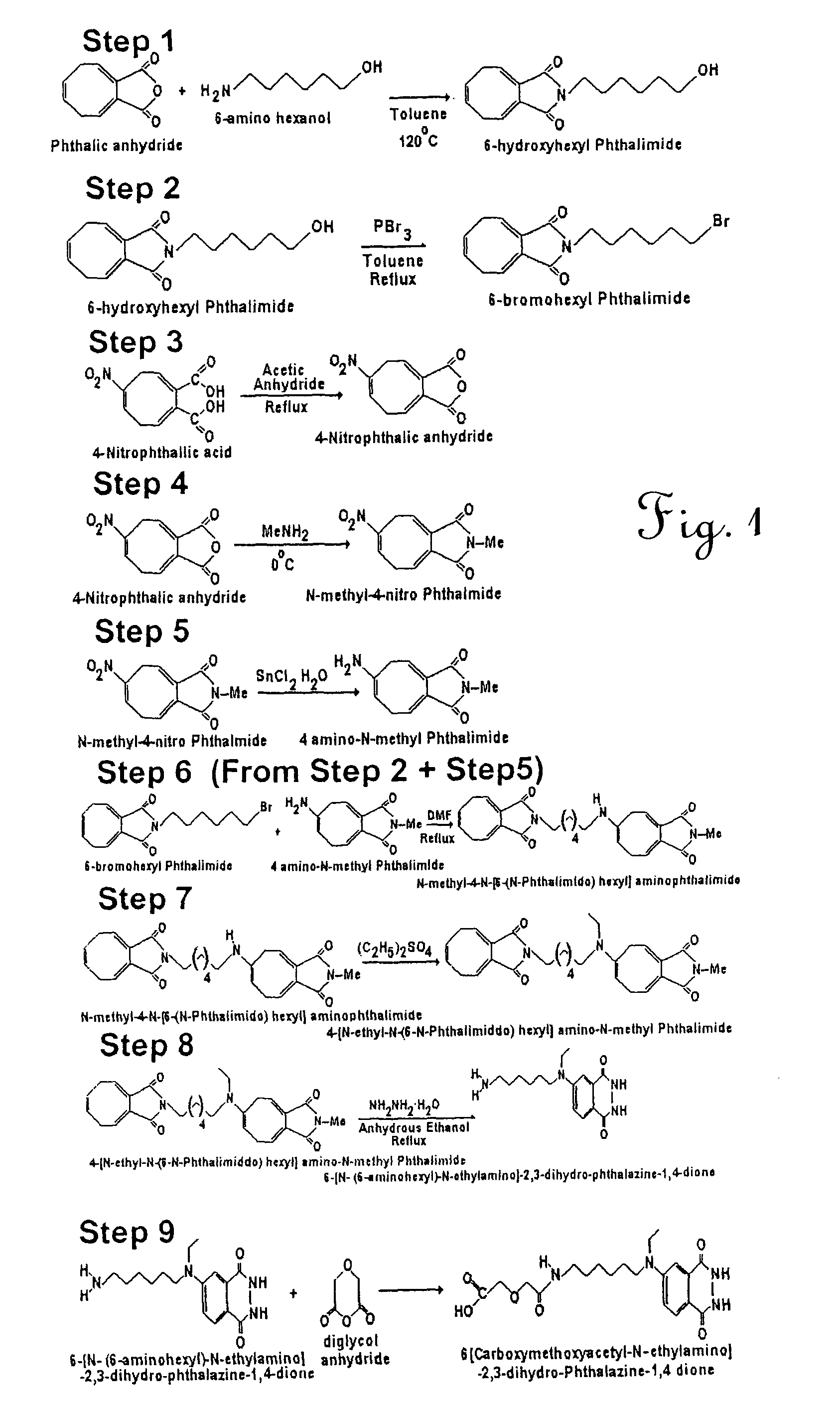
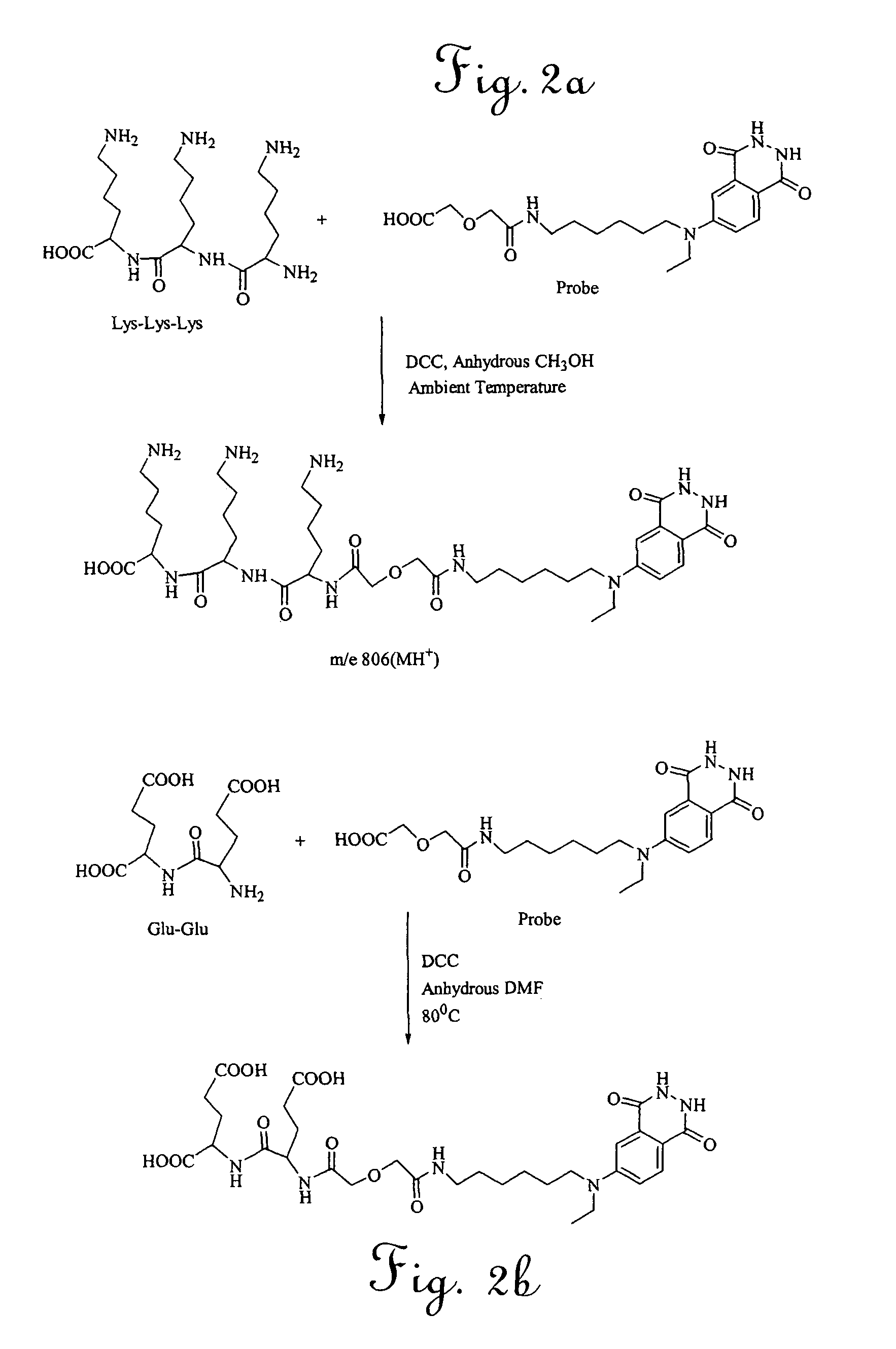
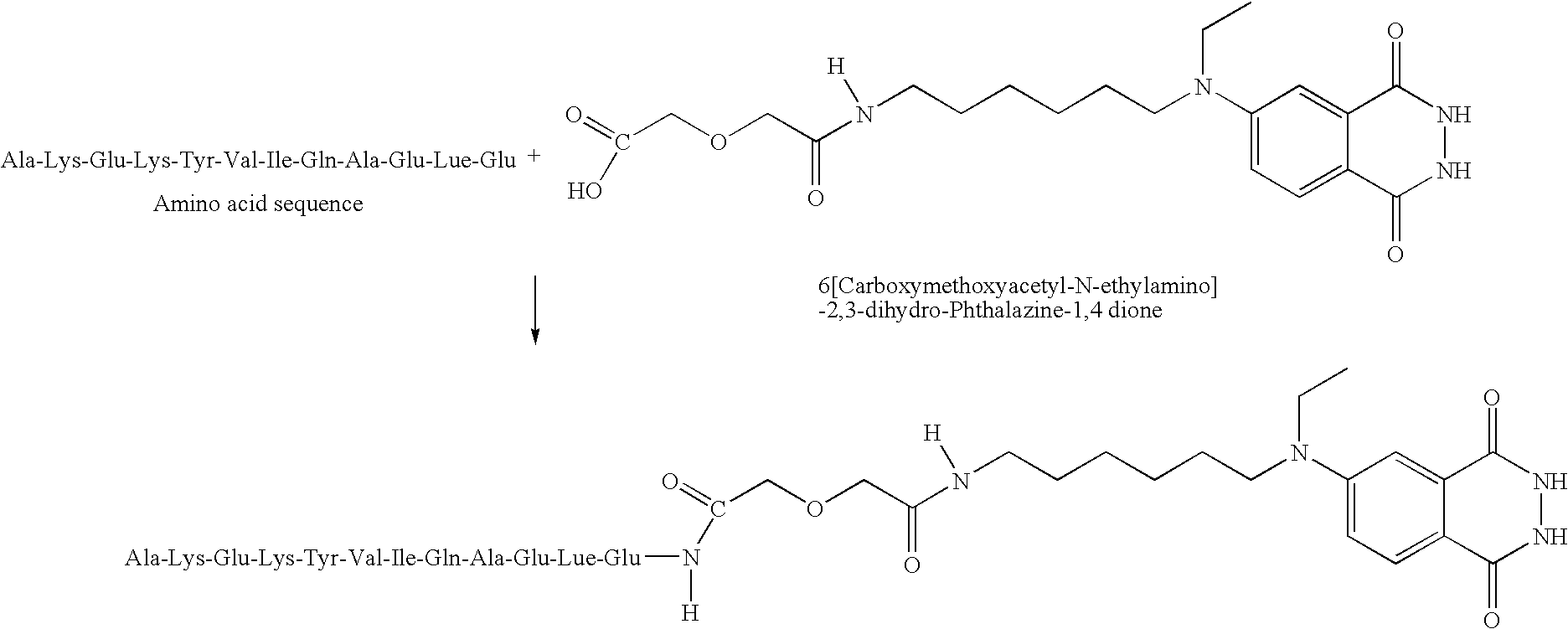
Bioactive peptide-based probes
A method for preparing a site-specific peptide probe, wherein the peptide is specific to a receptor, includes modifying a marker to include a tether molecule and covalently binding the tether molecule to the peptide. The present invention also provides a labeled probe, comprising a peptide specific for a receptor and a marker. The marker is modified to include a tether molecule capable of covalently binding to the peptide. The peptide is typically derived from a bacteriophage or is a synthetic analog or derivative of the peptide. The receptor will typically be found on a surface of a bacterial cell. The method and probe of the invention are suitable for a rapid assay for a bacteria in a complex mixture.
Owner:UNIV OF KENTUCKY RES FOUND
Pharmaceutical composition containing oleanolic acid acetate as an active ingredient for preventing or treating TLR- or IL-6-mediated diseases
ActiveCN103648502AInhibitory activityInhibit phosphorylationAntibacterial agentsSenses disorderDiseaseSide effect
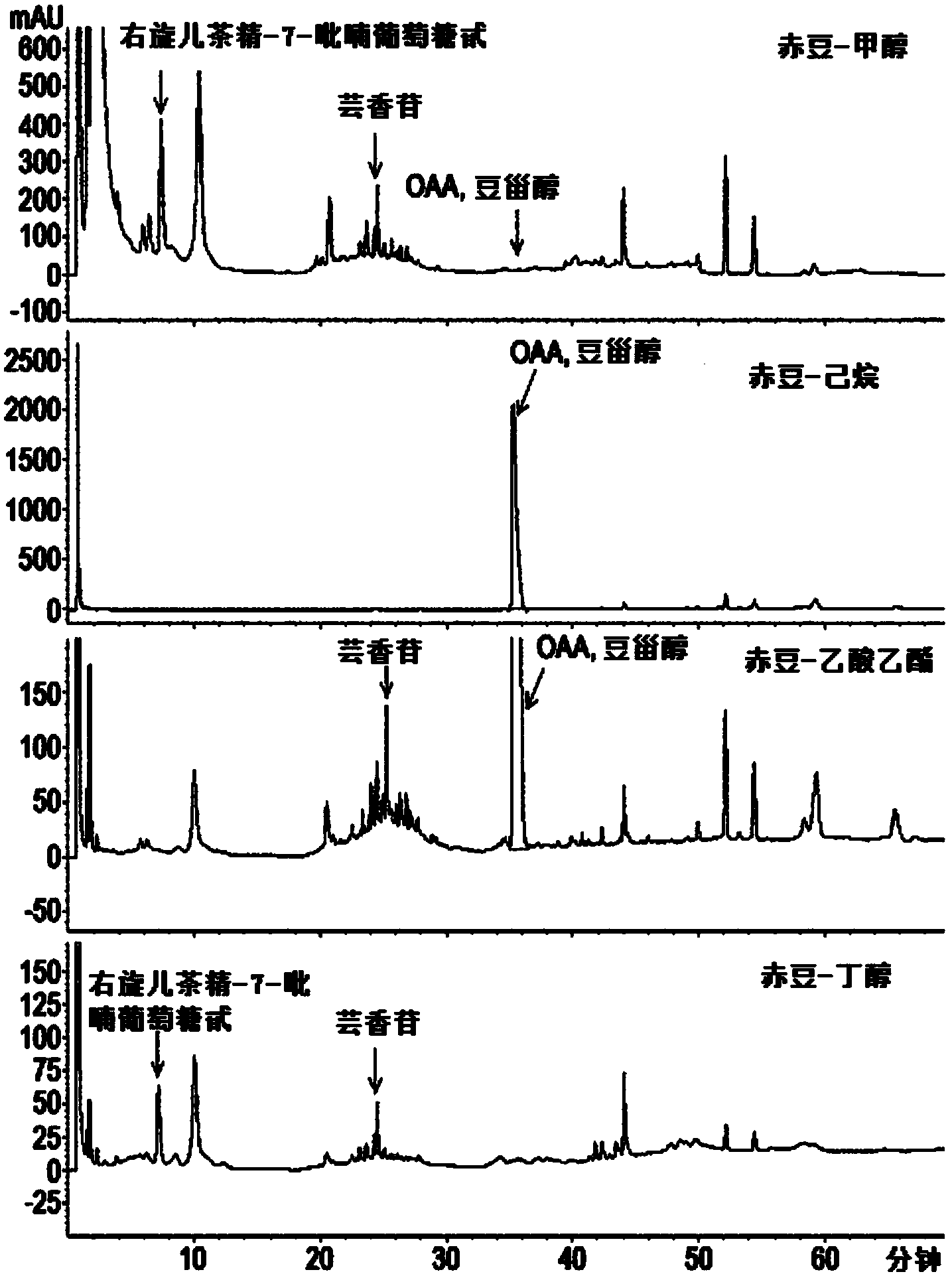
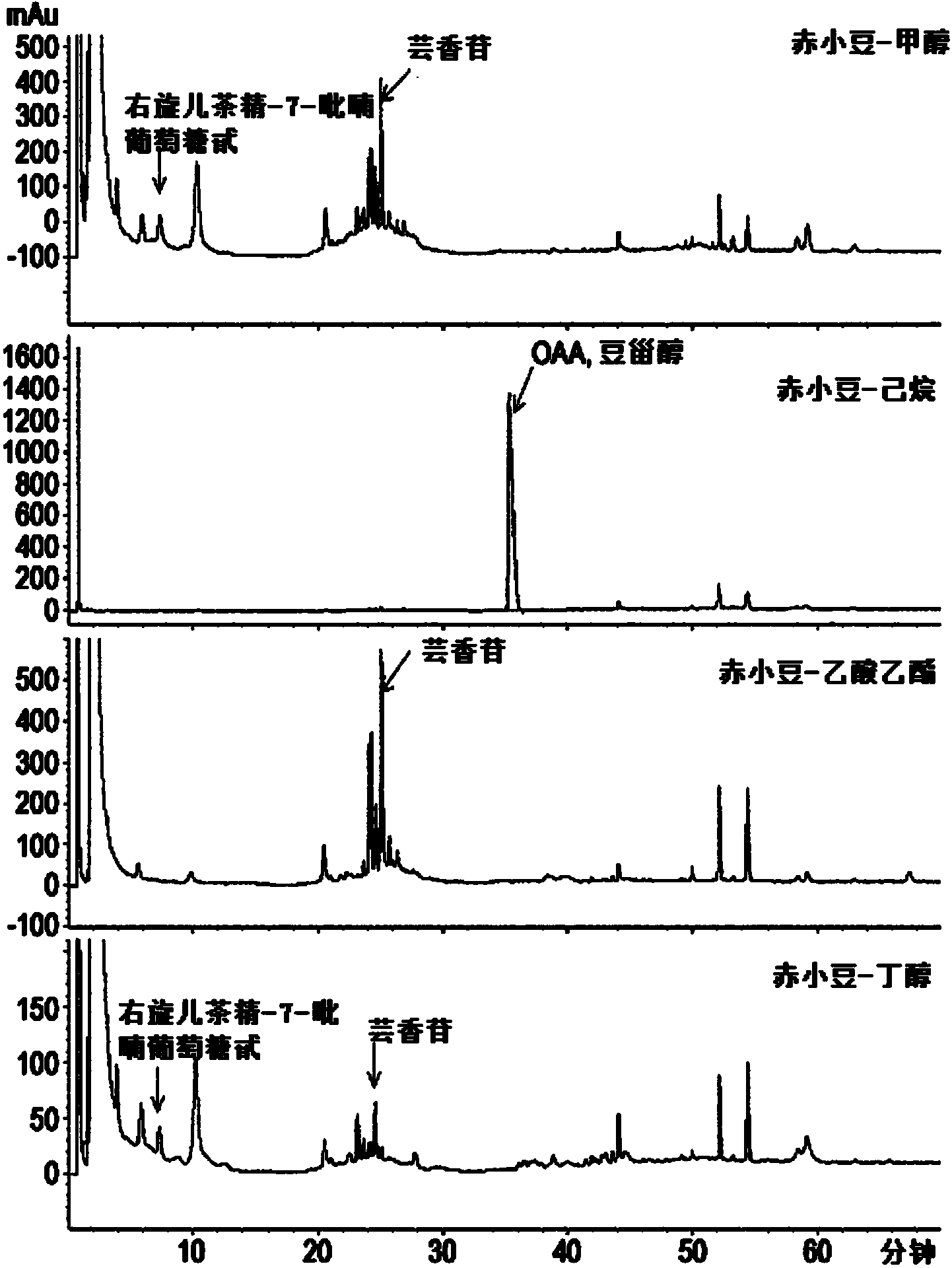
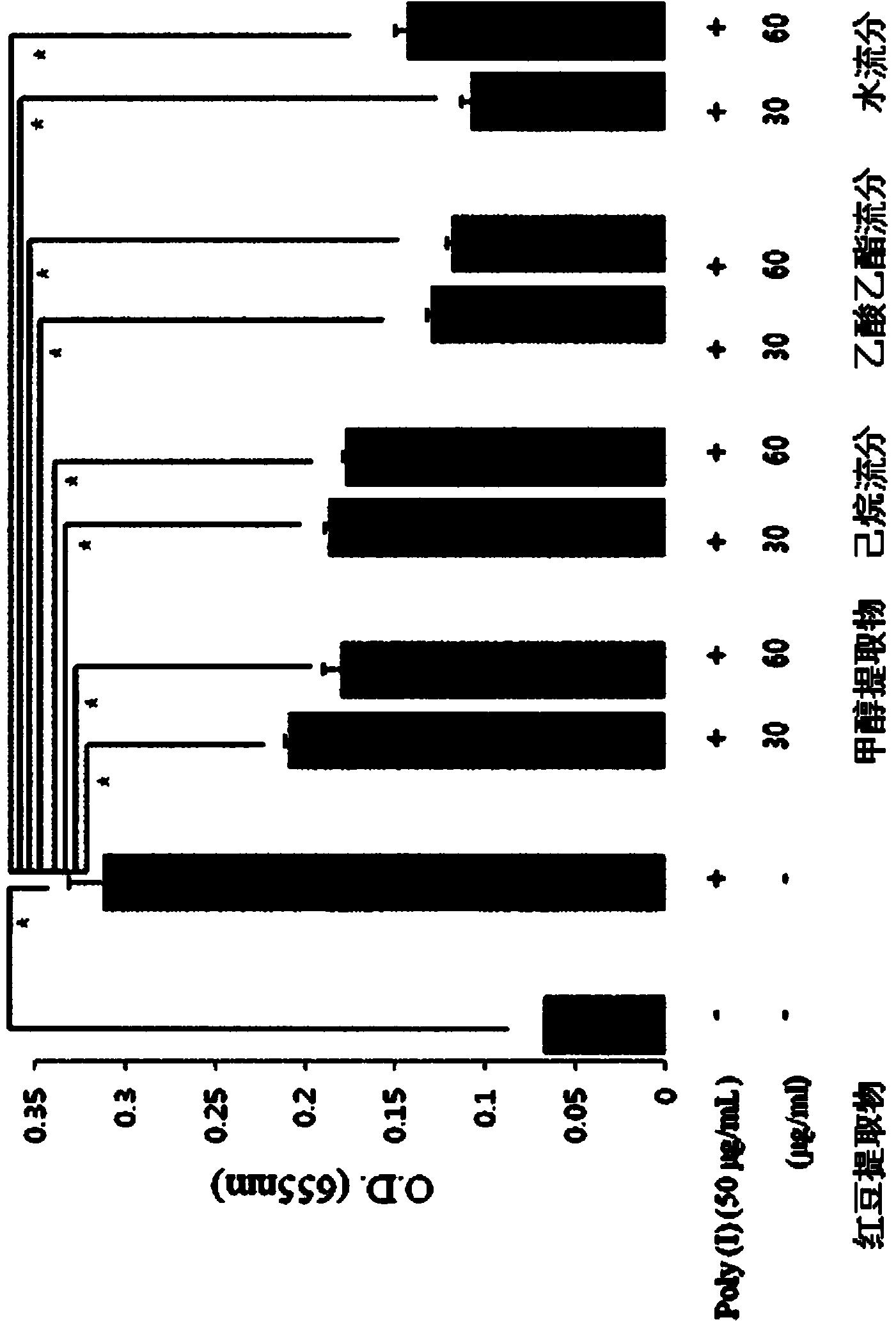
The present invention relates to a pharmaceutical composition for preventing or treating TLR- or IL-6-mediated diseases, containing oleanolic acid acetate or pharmaceutically acceptable salts thereof as an active ingredient, and to a pharmaceutical composition for preventing or treating TLR- or IL-6-mediated diseases and containing phaseoli semen extract, or a fraction thereof, which contains said compound or salts as an active ingredient. The phaseoli semen extract or compound according to the present invention is derived from a natural substance, which has long been used in natural medicine, and therefore causes no side effects. The phaseoli semen extract or compound inhibits the activity of TLR-3 and TLR-7 induced by Poly(I:C) and Poly(I), which are the synthetic analogues of dsRNA and ssRNA, blocks signaling pathways including NF-kappaB, and inhibits the transcriptional activity and phosphorylation of STAT3, which is an inflammation-related transcription factor activated by IL-6. Therefore, the phaseoli semen extract or compound of the present invention can be widely used in developing agents for preventing or treating TLR- and IL-6-mediated diseases, such as atopic dermatitis or arthritis.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Production of furanones
The present invention relates to the side chain functionalization of fimbrolides (halogenated 3-alkyl-5-methylene-2(5H)-furanones) and their synthetic analogues, that yields fimbrolides substituted with a halogen, an oxygen or a nitrogen functionality in the alkyl chain, especially fimbrolide alcohols, carboxylate and sulfinate and sulfonate esters, ethers, aldehydes, ketones, acids, amides, nitro derivatives, hydrophobic, hydrophilic and fluorophilic alkyl derivatives and a polymers.
Owner:UNISEARCH LTD
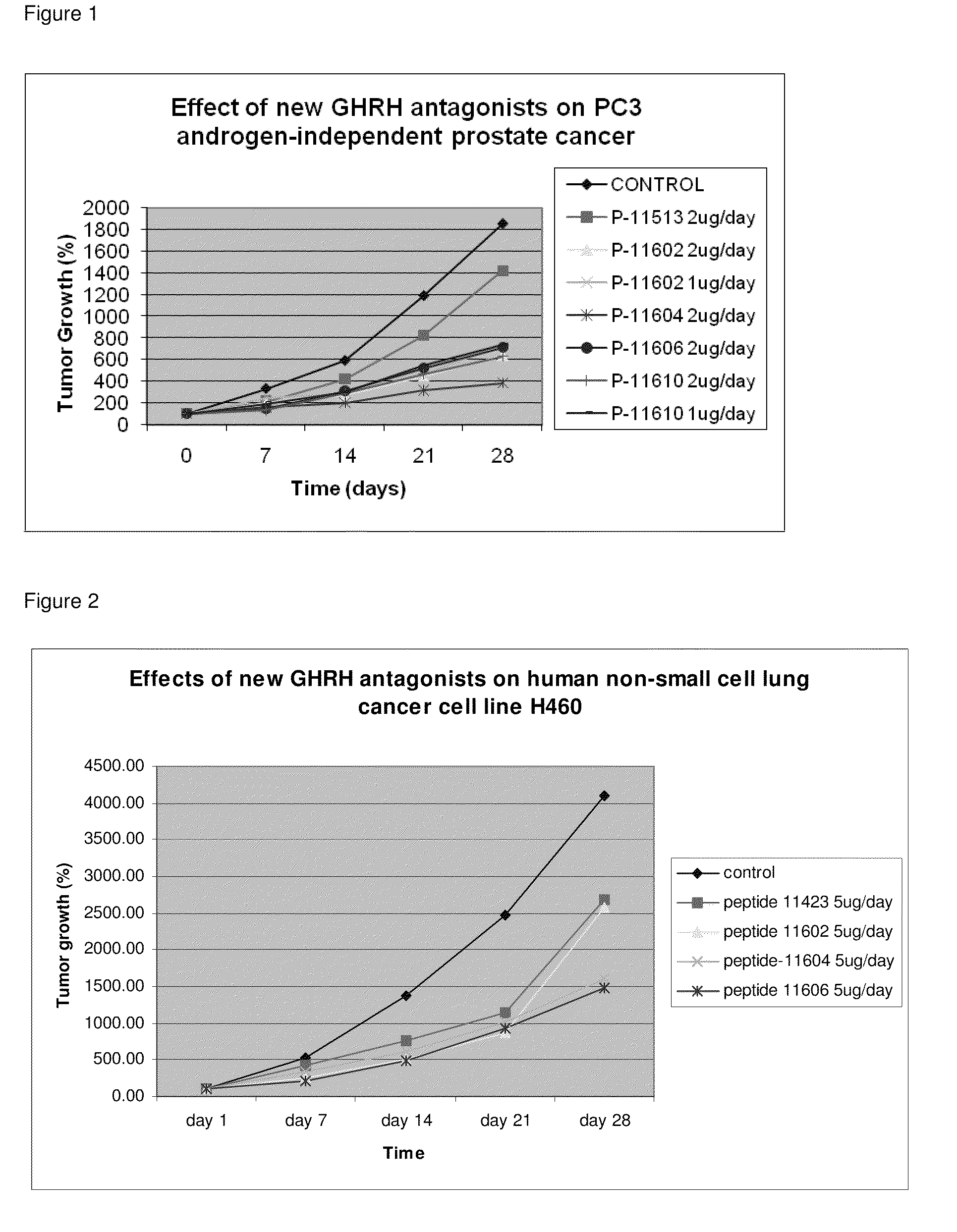
N- and C- terminal substituted antagonistic analogs of GH-RH
ActiveUS8691942B2Inhibition releaseEnhanced inhibitory effectPeptide/protein ingredientsSecretinsHuman cancerSynthetic analogue
There is provided a novel series of synthetic analogs of hGH-RH(1-29)NH2 (SEQ ID NO: 1) and hGH-RH(1-30)NH2. Of particular interest are those carrying PhAc, N-Me-Aib, Dca, Ac-Ada, Fer, Ac-Amc, Me-NH-Sub, PhAc-Ada, Ac-Ada-D-Phe, Ac-Ada-Phe, Dca-Ada, Dca-Amc, Nac-Ada, Ada-Ada, or CH3—(CH2)10—CO-Ada, at the N-Terminus and β-Ala, Amc, Apa, Ada, AE2A, AE4P, ε-Lys(α-NH2), Agm, Lys(Oct) or Ahx, at the C-terminus. These analogs inhibit the release of growth hormone from the pituitary in mammals as well as inhibit the proliferation of human cancers, and inhibit the hyperplastic and benign proliferative disorders of various organs, through a direct effect on the cancerous and non-malignant cells. The stronger inhibitory potencies of the new analogs, as compared to previously described ones, result from replacement of various amino acids.
Owner:MIAMI UNIVERISTY OF +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/c39d20f2-03bd-480c-bd6a-a01e1d9720ef/US20050192268A1-20050901-C00001.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/c39d20f2-03bd-480c-bd6a-a01e1d9720ef/US20050192268A1-20050901-C00002.png)
![Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders Amino substituted diaryl[a,d]cycloheptene analogs as muscarinic agonists and methods of treatment of neuropsychiatric disorders](https://images-eureka.patsnap.com/patent_img/c39d20f2-03bd-480c-bd6a-a01e1d9720ef/US20050192268A1-20050901-C00003.png)