Neuroprotective polyphenol analogs
A compound, optional technology, applied in the fields of preventing and alleviating neurodegenerative disorders, treating, preventing and alleviating diabetes and Huntington's disease, capable of solving problems of low lipophilicity, poor bioavailability, complexity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
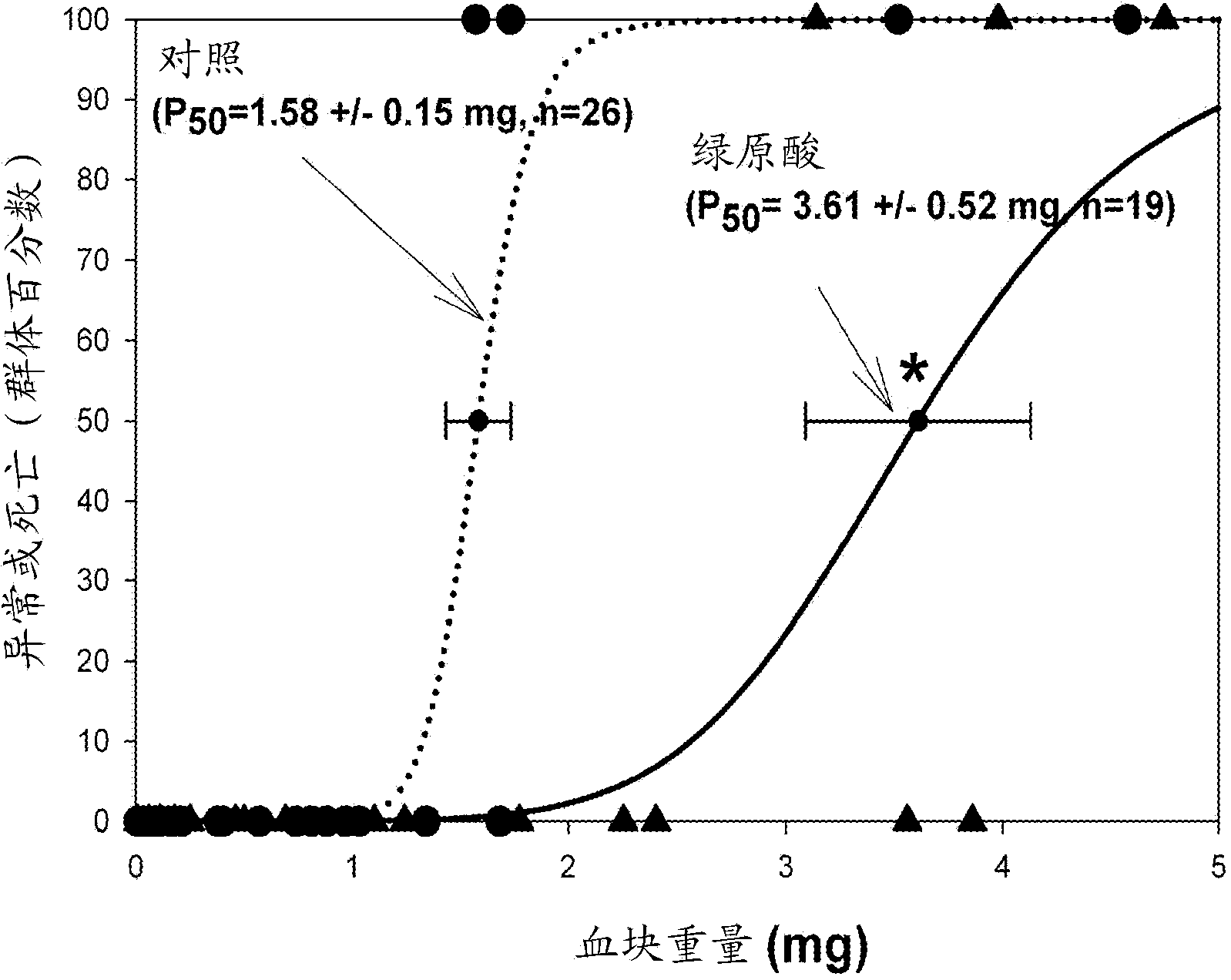
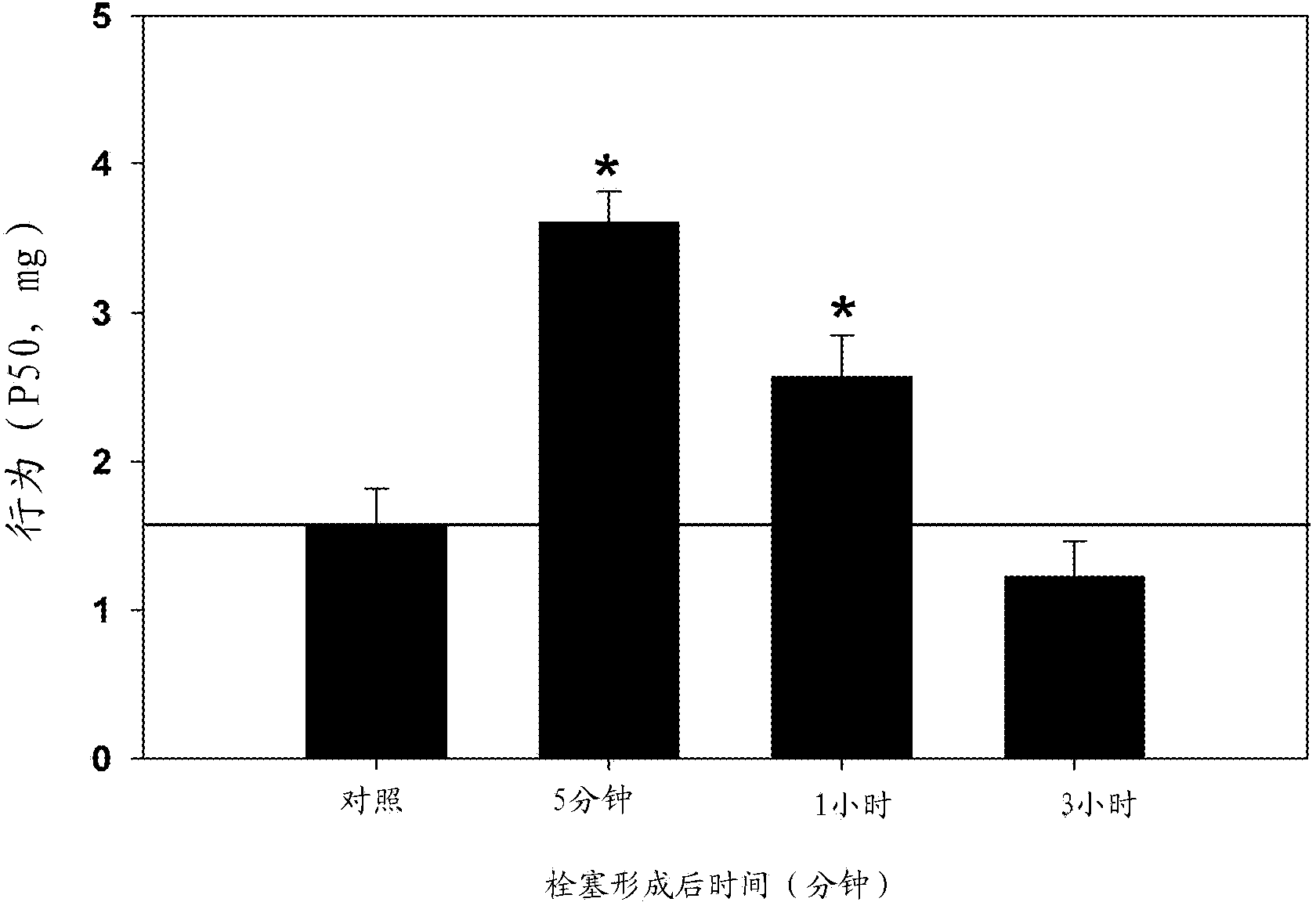
[0467] Example 1 - Chlorogenic Acid Improves Neurological Performance After Embolic Stroke in Rabbits
[0468] After a bolus of vehicle or chlorogenic acid was administered intravenously over 1 minute, embolization was initiated for 5 minutes using a suspension of small-sized clots. In this series of studies, CGA was given at 50 mg / kg [65]. Behavioral analyzes were performed 24 hours after treatment, which allowed the construction of quantitative dose-response curves. Figure 1 shows a graphical representation of the raw data superimposed with the theoretical quantitative analysis curves. For overlaid plots, normal animals are plotted at 0% of the y-axis and abnormal animals are plotted at 100%. The figure shows a positive correlation between the data (circles or triangles) and the statistically fitted quantity curve. In addition, compared with the vehicle control, CGA made P 50 Value increased (abnormal clot volume produced in 50% of treatment groups). For a detailed di...
Embodiment 2
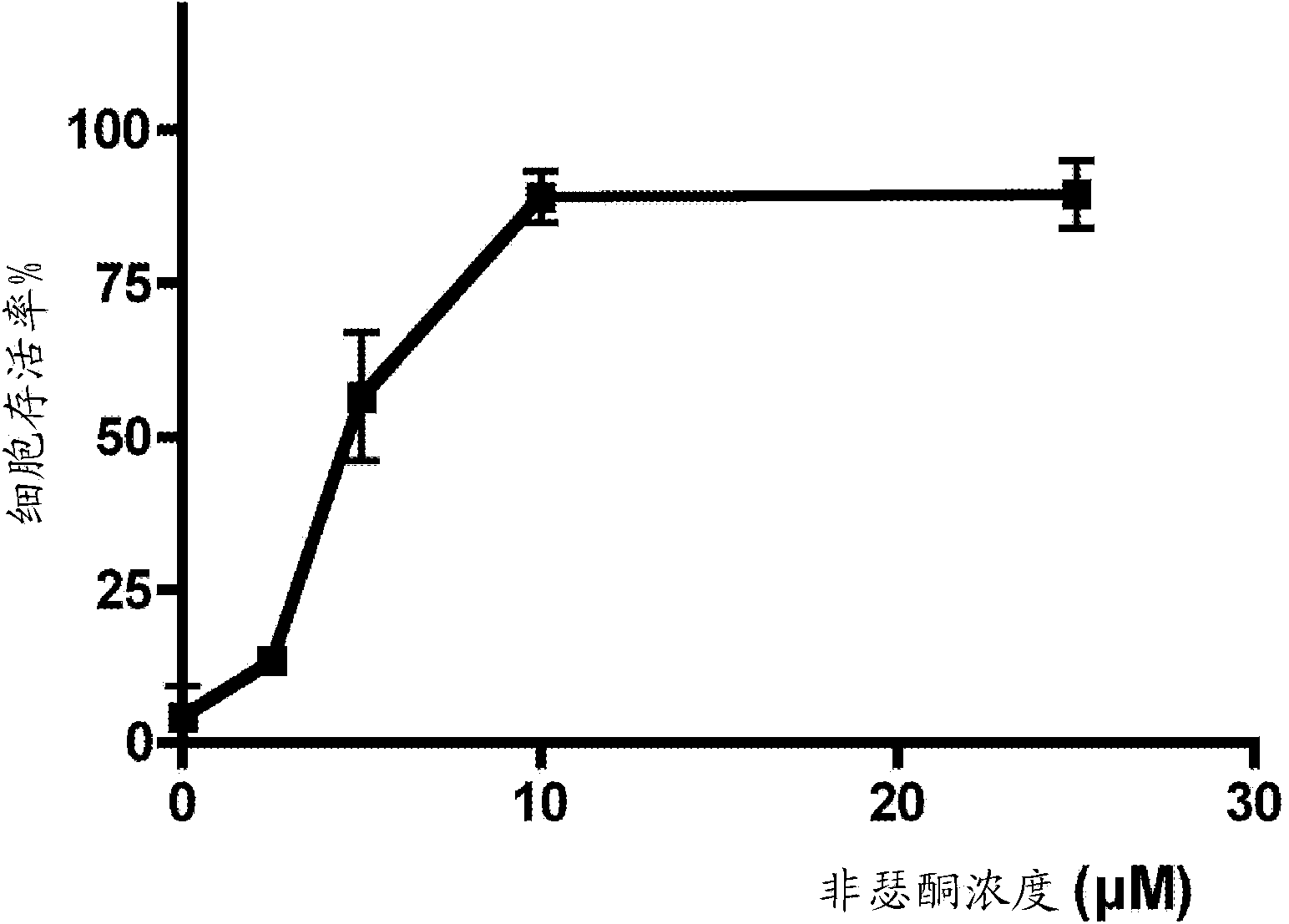
[0469] Example 2 - Fisetin is neuroprotective in vitro and in vivo
[0470]For these studies, cultured HT22 mouse hippocampal cells were used in an in vitro stroke assay [80]. HT22 cells were treated with iodoacetic acid (IAA, an irreversible inhibitor of glyceraldehyde 3-phosphate dehydrogenase (G3PDH)) alone or in the presence of different concentrations of fisetin for 2 h. G3PDH is an enzyme of the glycolytic pathway that catalyzes the synthesis of 1,3 diphosphoglycerate, a "high energy" intermediate for the synthesis of ATP. Cell viability was measured using the standard colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay for cell viability. MTT is a pale yellow substrate that is cleaved by living cells to yield a dark blue formazan product. Active mitochondria are required for this process, and even cells that have just died cannot cleave significant amounts of MTT. The graph (FIG. 3) shows that fisetin has a dose-dependent effect on...
Embodiment 3-12-L
[0474] The neuroprotective effect of embodiment 3-12-LOX inhibitor baicalein in vitro
[0475] In Figure 5, panels A-D show that baicalein was able to inhibit neuronal cell death in four additional neurotoxicity paradigms. These include trophic factor withdrawal (TFW), excitotoxicity, glucose starvation and most importantly in chemical ischemia models. Baicalein significantly enhanced the survival of freshly plated, low-density cultured rat cortical neurons in serum-containing media—a trophic factor withdrawal test [92] (Fig. 5A). Using a published excitotoxicity assay [53], baicalein rescued about 35% of the cells (Fig. 5B).
[0476] Baicalein was also neuroprotective in a glucose starvation assay [93], and these results were reproduced using PC12 cells. When these cells were starved of glucose, there was about 70% maximal survival in the presence of NGF. Baicalein increased the survival rate by more than 80% (Fig. 5C). Finally, baicalein prevented cell death in the che...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com