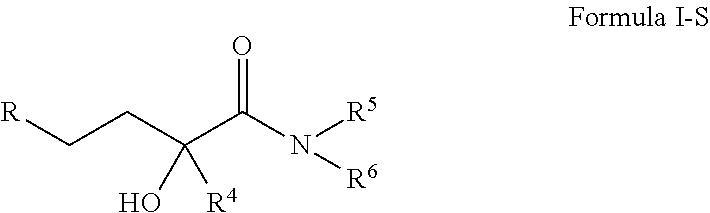
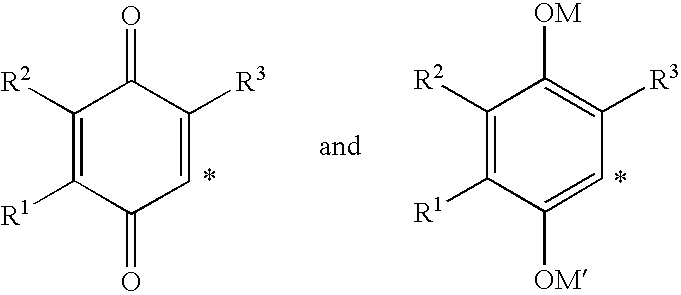
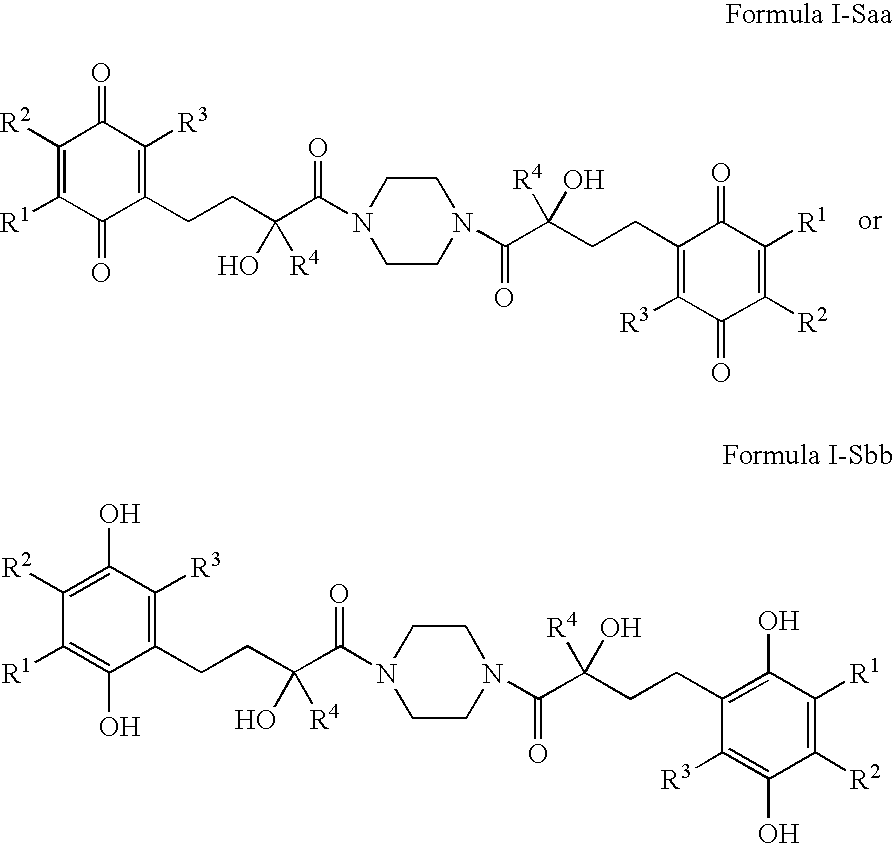
4-(p-quinonyl)-2-hydroxybutanamide derivatives for treatment of mitochondrial diseases
a technology of mitochondrial diseases and derivatives, which is applied in the direction of amide active ingredients, muscular disorders, drug compositions, etc., can solve the problems of affecting the health of patients, neurological symptoms, and often present symptoms of tissue or organ dysfunction, so as to improve the health of patients and raise the level of atp in individuals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-tert-Butyl-2-hydroxy-2-methyl-4-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienyl)butanamide
[0287]Following the amide coupling procedure described in protocol A, 500 mg 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (2.00 mmol), 355 mg CDI (2.20 mmol) and 160 mg t-butylamine (2.20 mmol) produced 125.1 mg of N-tert-butyl-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxamide as a white crystalline solid.
[0288]1H NMR (400 MHz, CDCl3) δ 6.40 (br s, 1H), 4.51 (s, 1H), 2.60 (m, 2H), 2.26 (m, 1H), 2.19 (s, 3H), 2.16 (s, 3H), 2.10 (s, 3H), 1.88 (m, 1H), 1.47 (s, 3H), 1.26 (m, 9H).
[0289]Oxidation as described in protocol B, using 95 mg (0.311 mmol) of N-tert-butyl-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxamide and 358 mg CAN (0.653 mmol) yielded 92.2 mg of N-tert-butyl-2-hydroxy-2-methyl-4-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienyl)butanamide as a yellow solid.
[0290]1H NMR (400 MHz, CDCl3) δ 6.61 (br s, 1H), 3.45 (s, 1H), 2.55 (m, 1H), 2.39 (m, 1H), 2.04-1.91 (m, 10H), 1.56 (m, 1...
example 2
2-Hydroxy-N,N,2-trimethyl-4-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienyl)butanamide
[0291]Following the amide coupling procedure described in protocol A, 504 mg 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (2.01 mmol), 361 mg CDI (2.23 mmol) and 1.1 mL of a 2.0 M solution of N,N-dimethylamine in THF (2.2 mmol) produced 412 mg of 6-hydroxy-N,N,2,5,7,8-hexamethylchroman-2-carboxamide as amorphous powder.
[0292]1H NMR (400 MHz, CDCl3) δ 4.31 (s, 1H), 3.26 (s, 3H), 2.85 (s, 3H), 2.80-2.41 (m, 3H), 2.16 (s, 6H), 2.08 (s, 3H), 1.70-1.60 (m, 4H).
[0293]Oxidation as described in protocol B, using 138.6 mg (0.50 mmol) of 6-hydroxy-N,N,2,5,7,8-hexamethylchroman-2-carboxamide and 560 mg CAN (1.02 mmol) yielded 139.9 mg of 2-hydroxy-N,N,2-trimethyl-4-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienyl)butanamide as a yellow oil.
[0294]1H NMR (400 MHz, CDCl3) δ 5.07 (s, 1H), 2.23 (br s, 3H), 3.07 (br s, 3H), 2.51 (m, 1H), 2.33 (m, 1H), 2.02 (m, 3H), 1.99-1.94 (m, 7H), 1.69 (m, 1H), 1.47 (s, 3...
example 3
N-Benzyl-2-hydroxy-2-methyl-4-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienyl)butanamide
[0295]Following the amide coupling procedure described in protocol A, 500 mg 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (2.0 mmol), 362 mg CDI (2.23 mmol) and 235 mg benzylamine (2.20 mmol) produced 507 mg of N-benzyl-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxamide as a brown oil.
[0296]1H NMR (400 MHz, CDCl3) δ 7.22 (m, 3H), 7.00 (m, 2H), 6.76 (br t, 1H), 4.81 (s, 1H), 4.50 (m, 1H), 4.35 (m, 1H), 2.62 (m, 2H), 2.45 (m, 1H), 2.16 (s, 3H), 2.11 (s, 6H), 1.92 (m, 1H), 1.58 (s, 3H).
[0297]Oxidation as described in protocol B, using 130 mg (0.383 mmol) of N-benzyl-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxamide and 441 mg CAN (0.805 mmol) yielded 119.7 mg of N-benzyl-2-hydroxy-2-methyl-4-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dienyl)butanamide as a yellow foam.
[0298]1H NMR (400 MHz, CDCl3) δ 7.26 (m, 6H), 4.42 (m, 2H), 3.57 (s, 1H), 2.56 (m, 1H), 2.36 (m, 1H), 2.04-1.93 (m, 10H), 1.59...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com