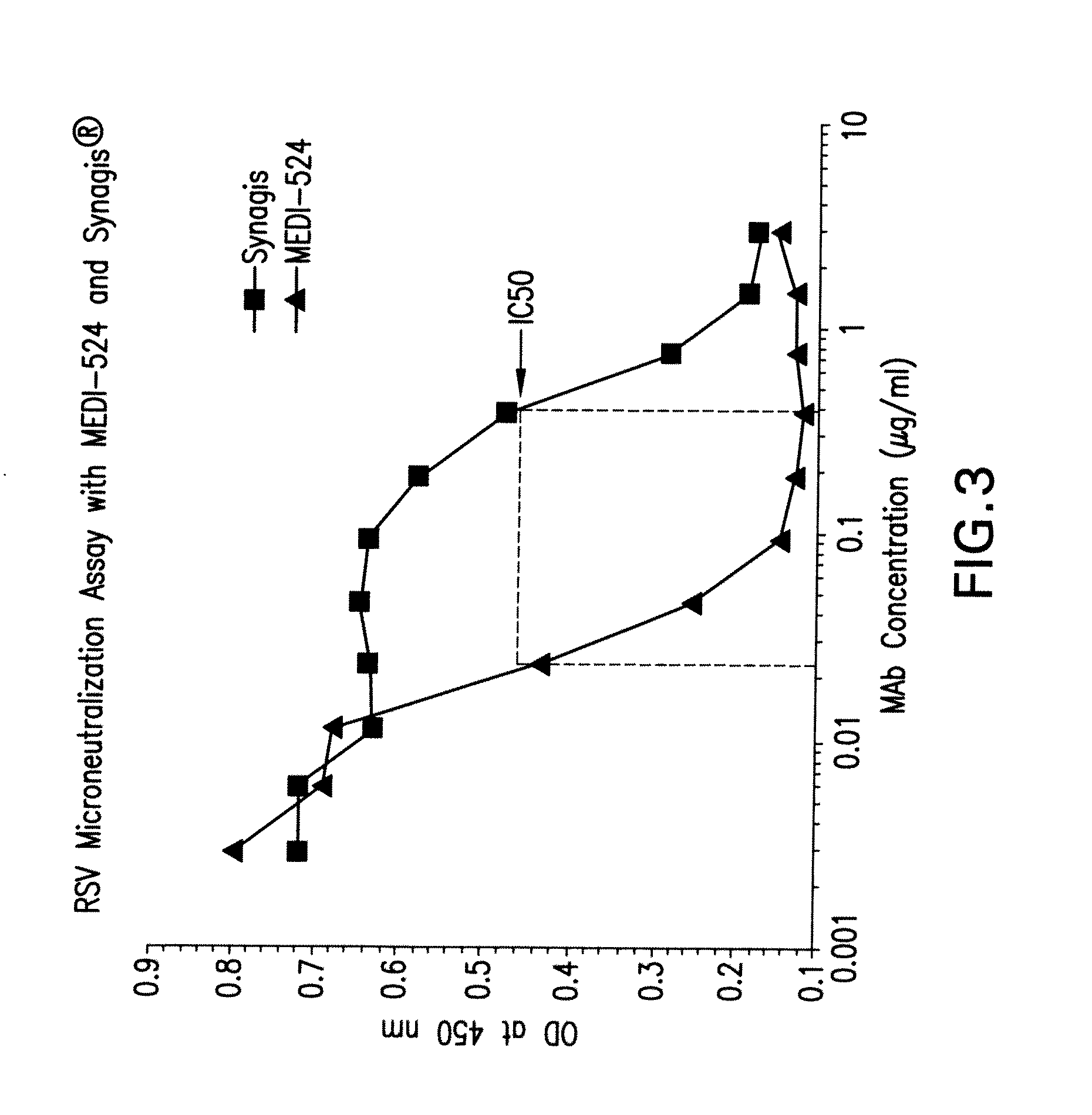
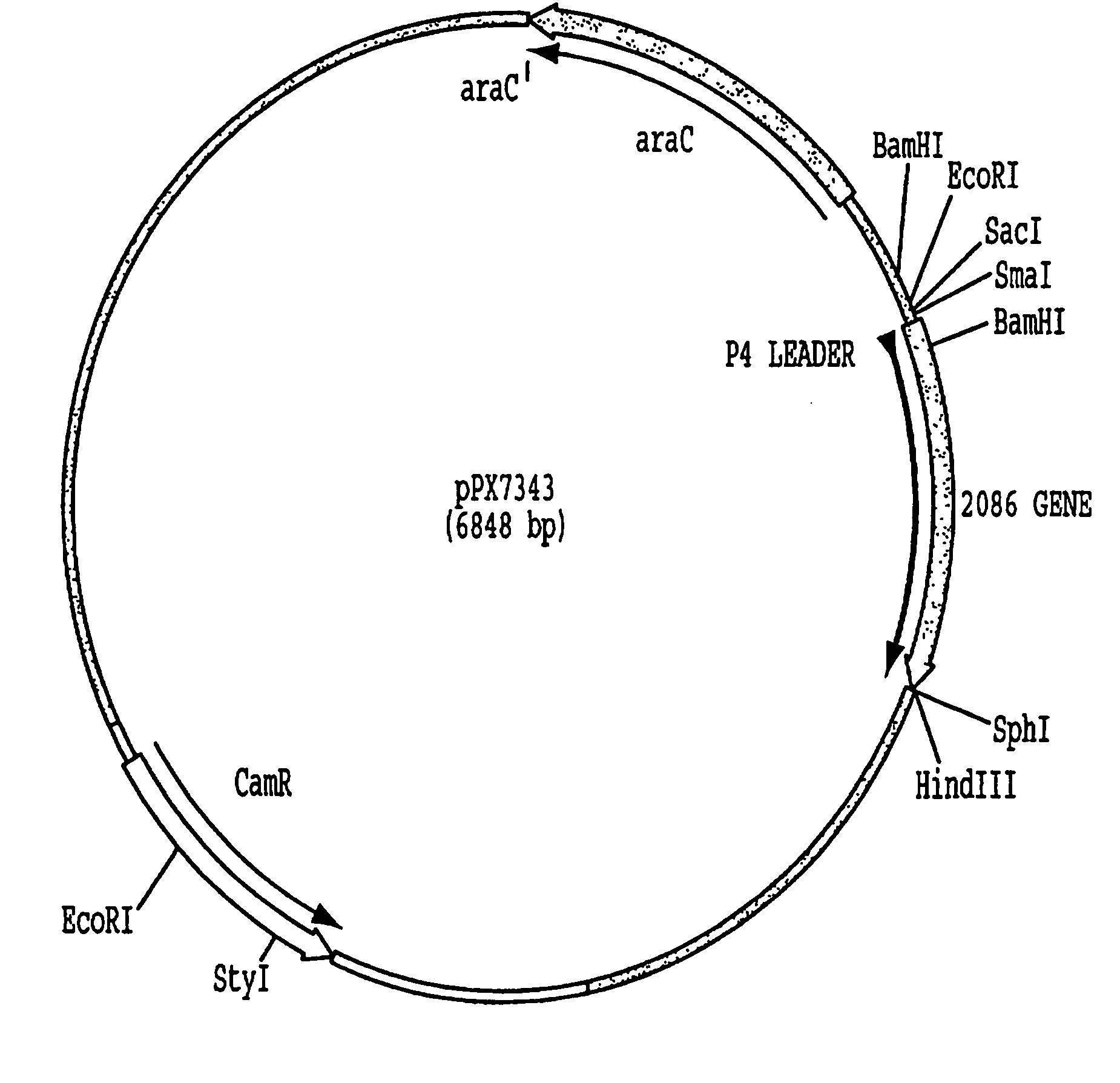
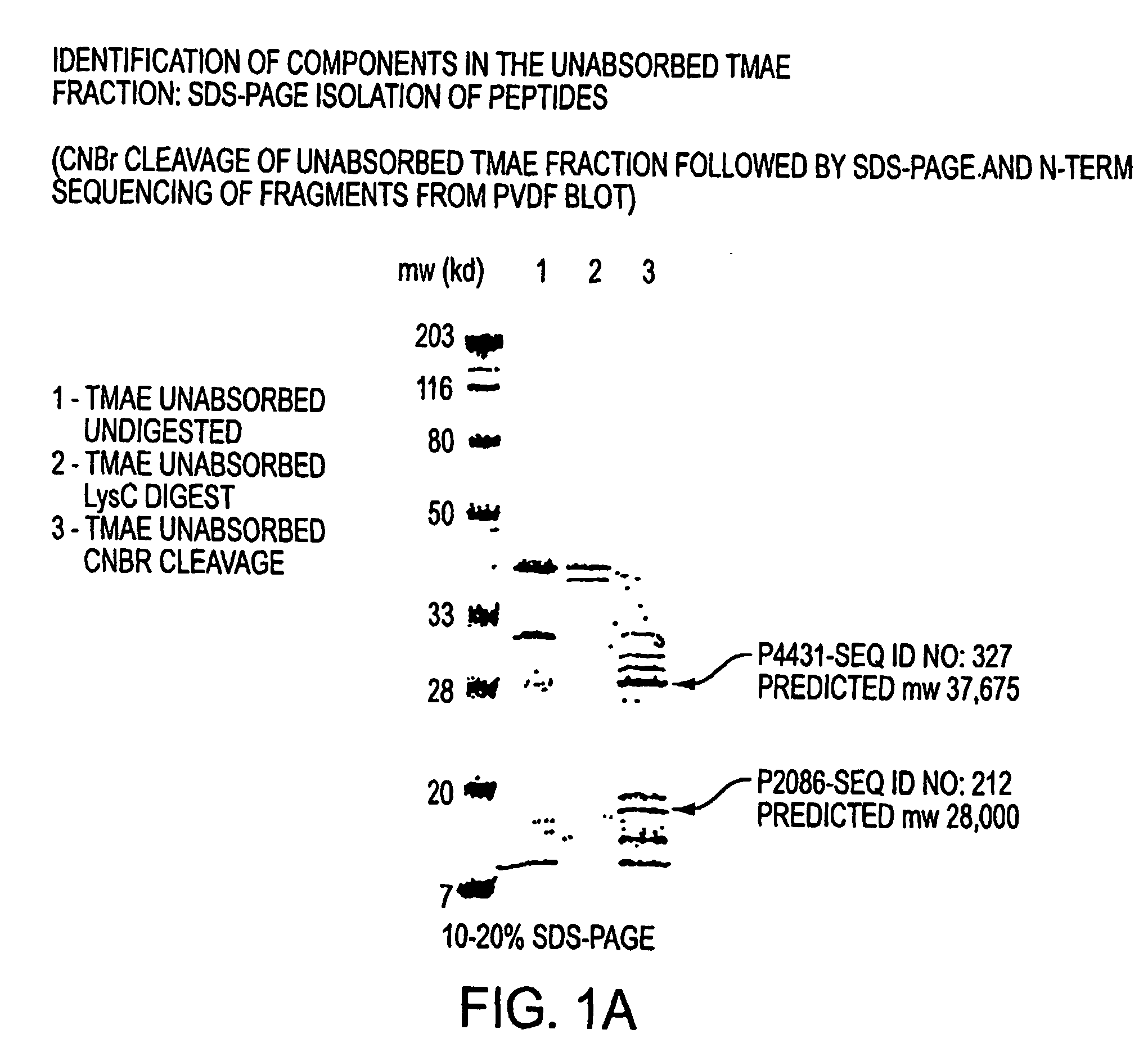
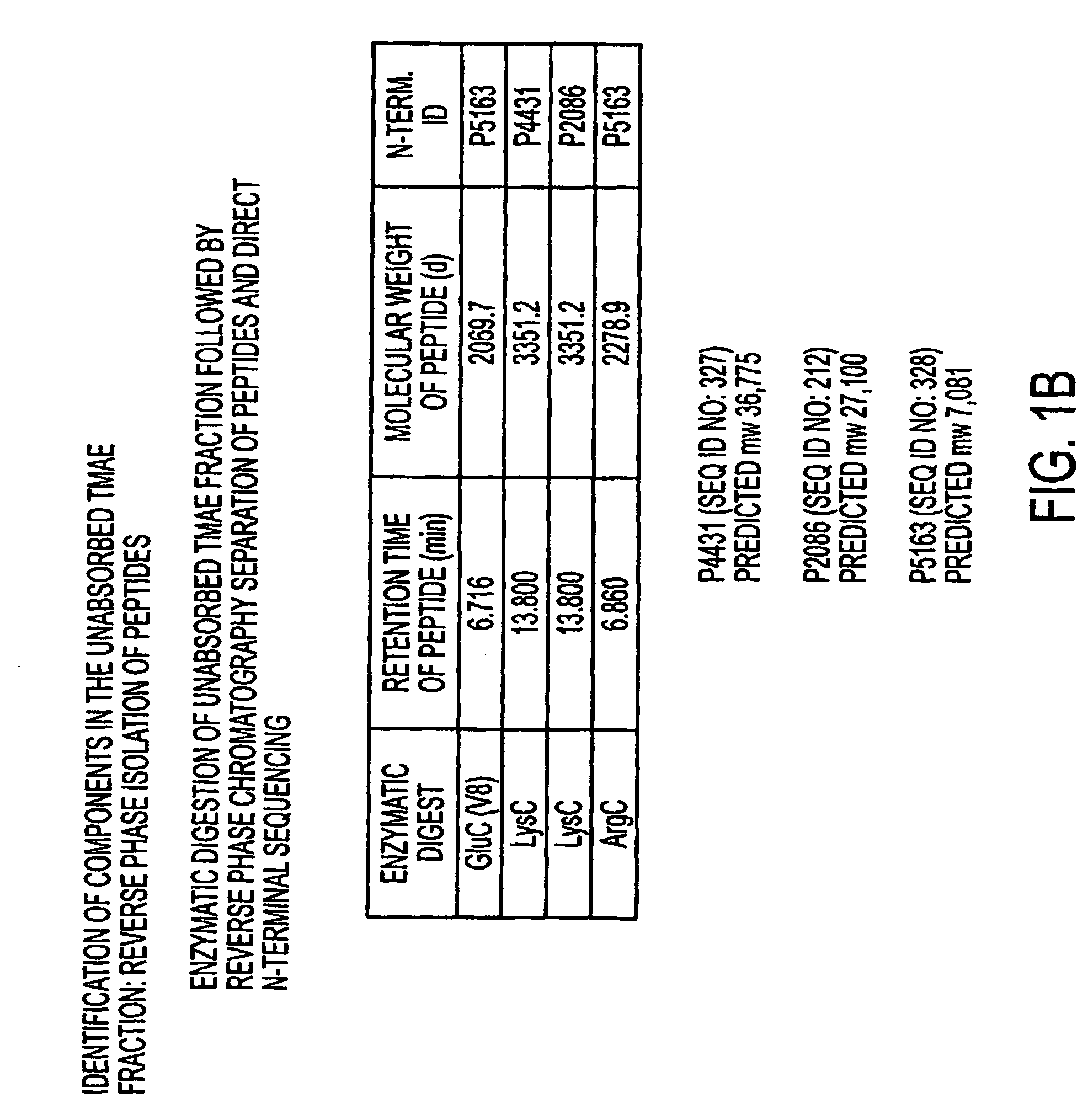
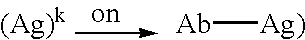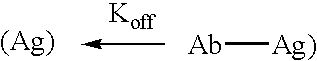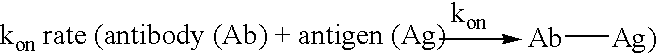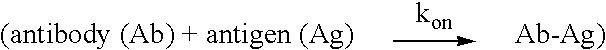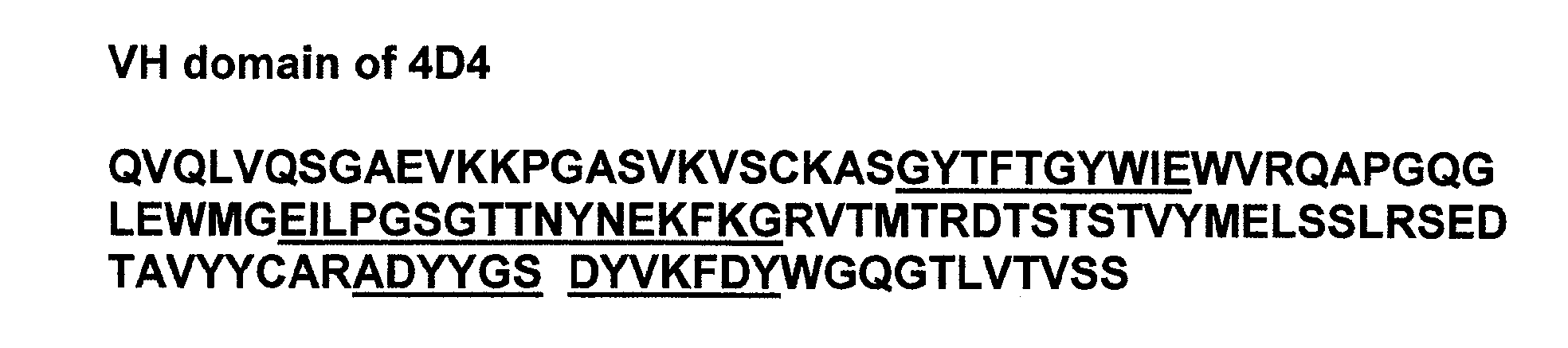Patents
Literature
174 results about "Immunologic specificity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
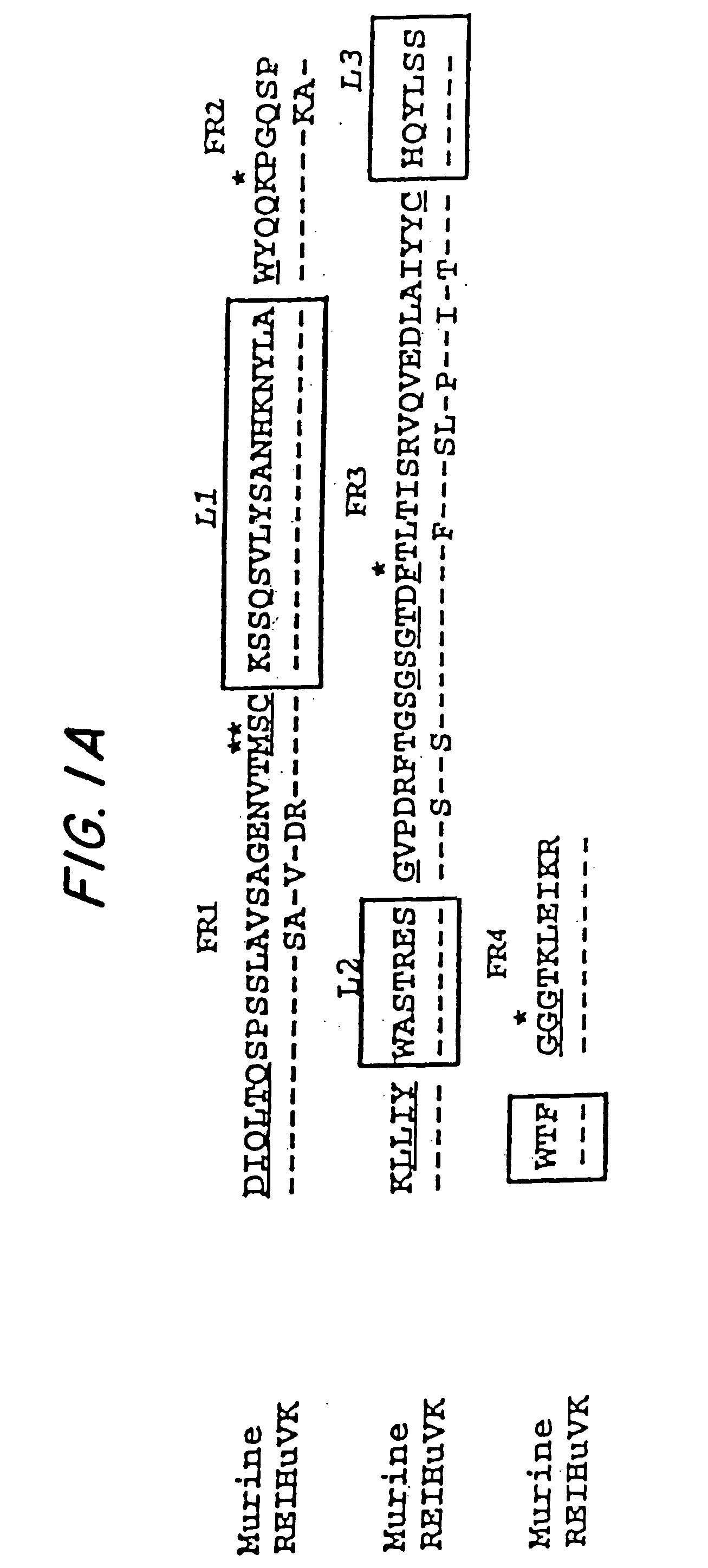
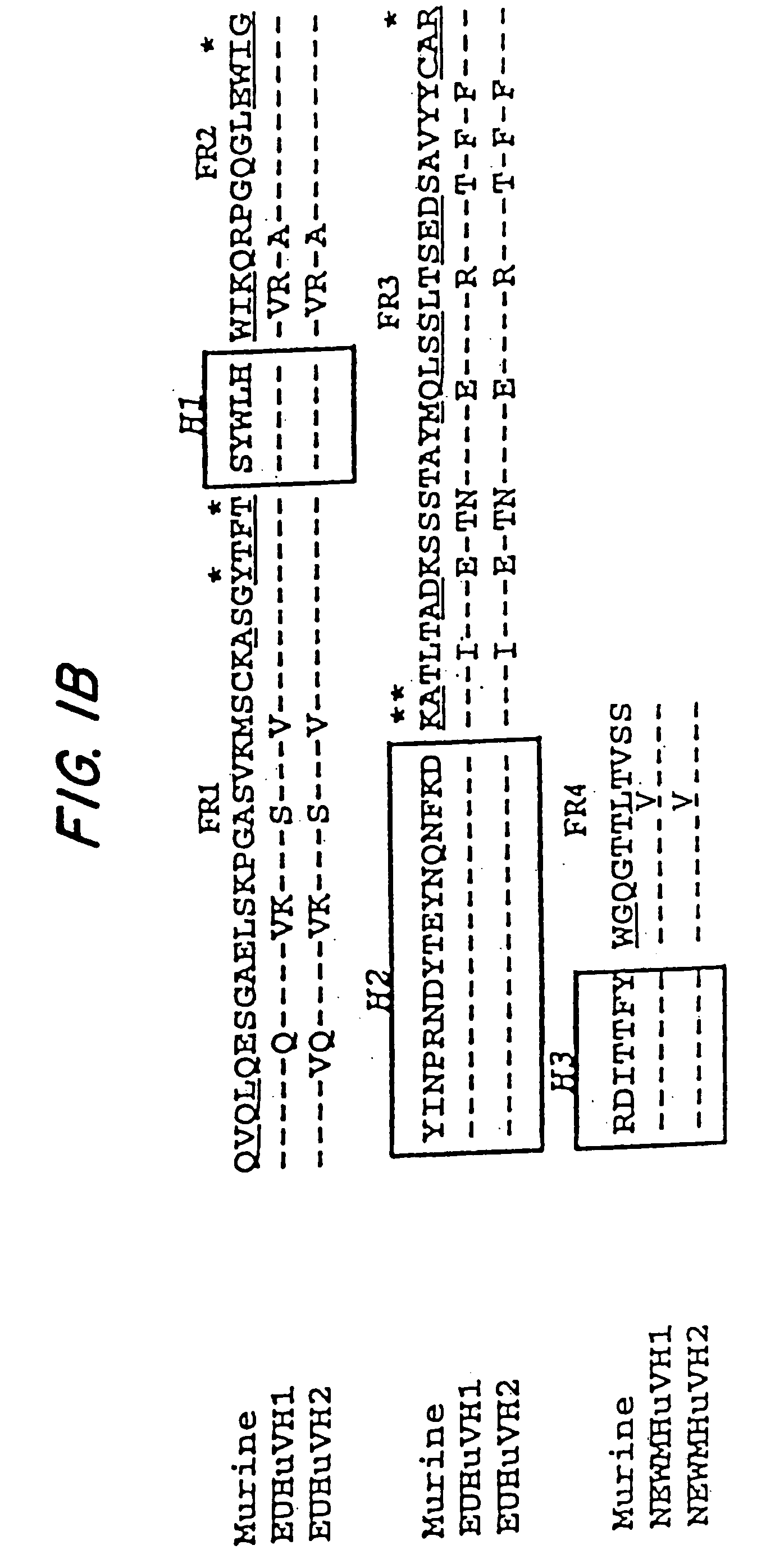
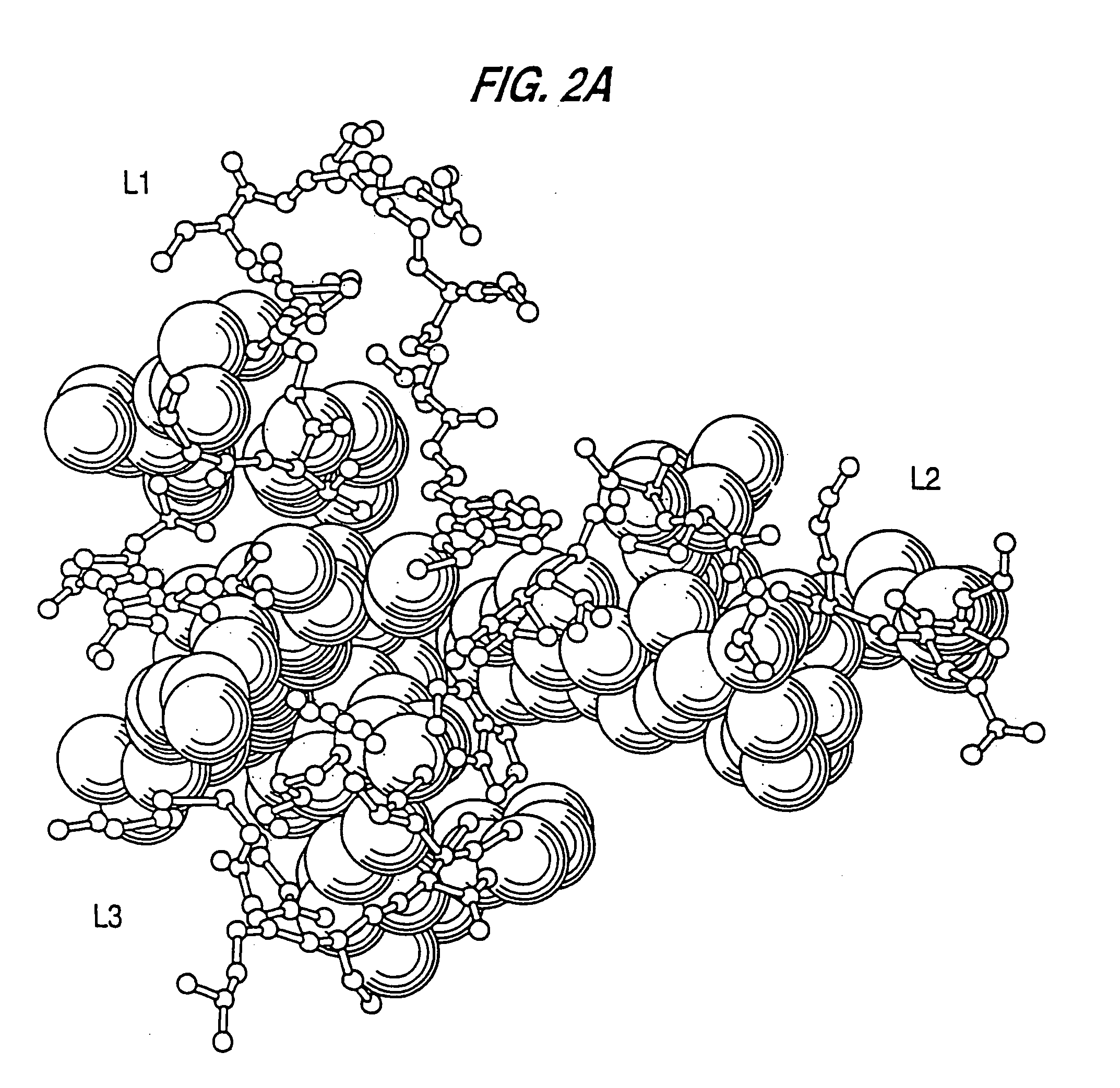
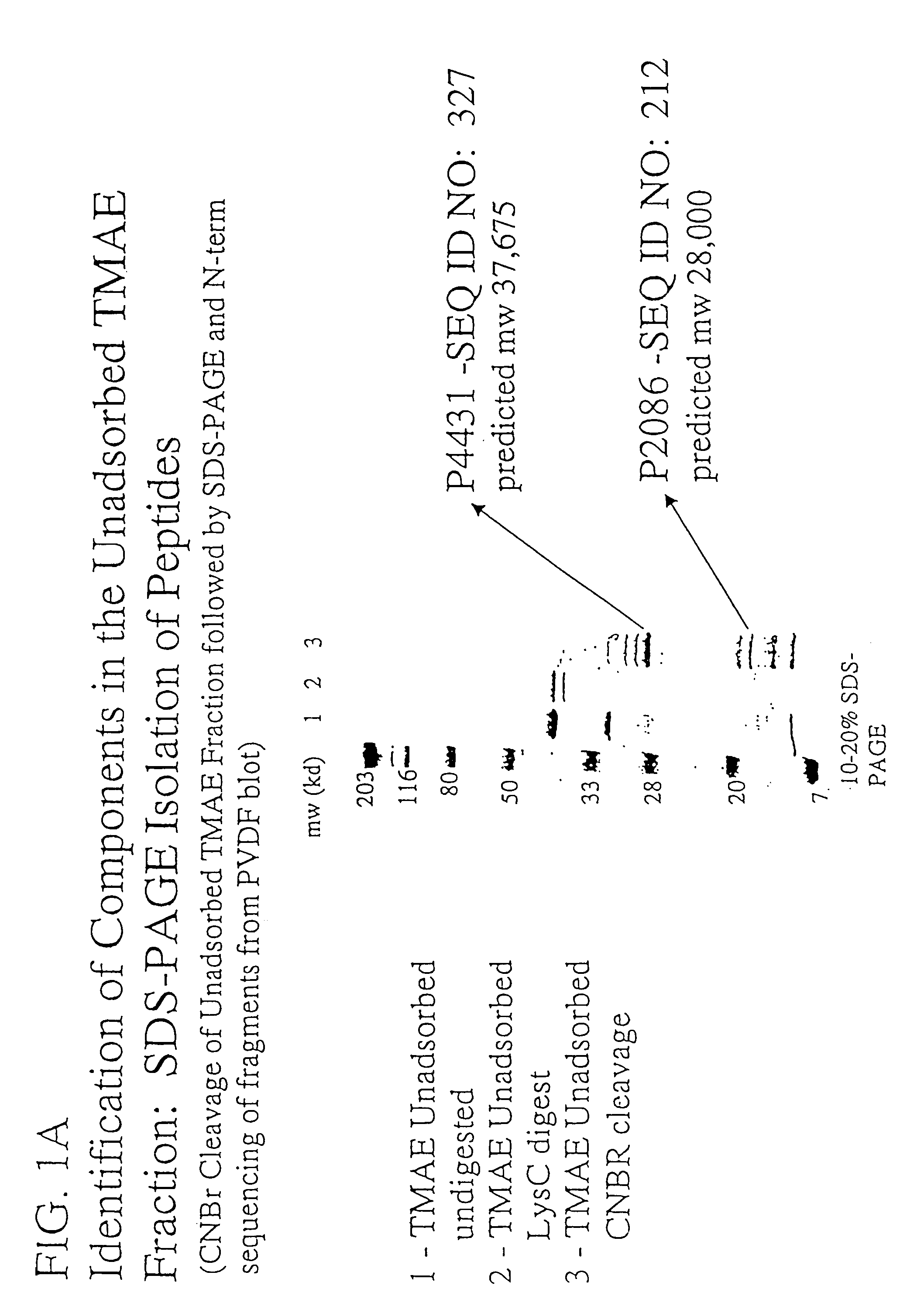
The concept of immunological specificity based on a unique combination of natural globulins is an attractive alternative to the classical concept of unique globulin molecules for each possible antigen.
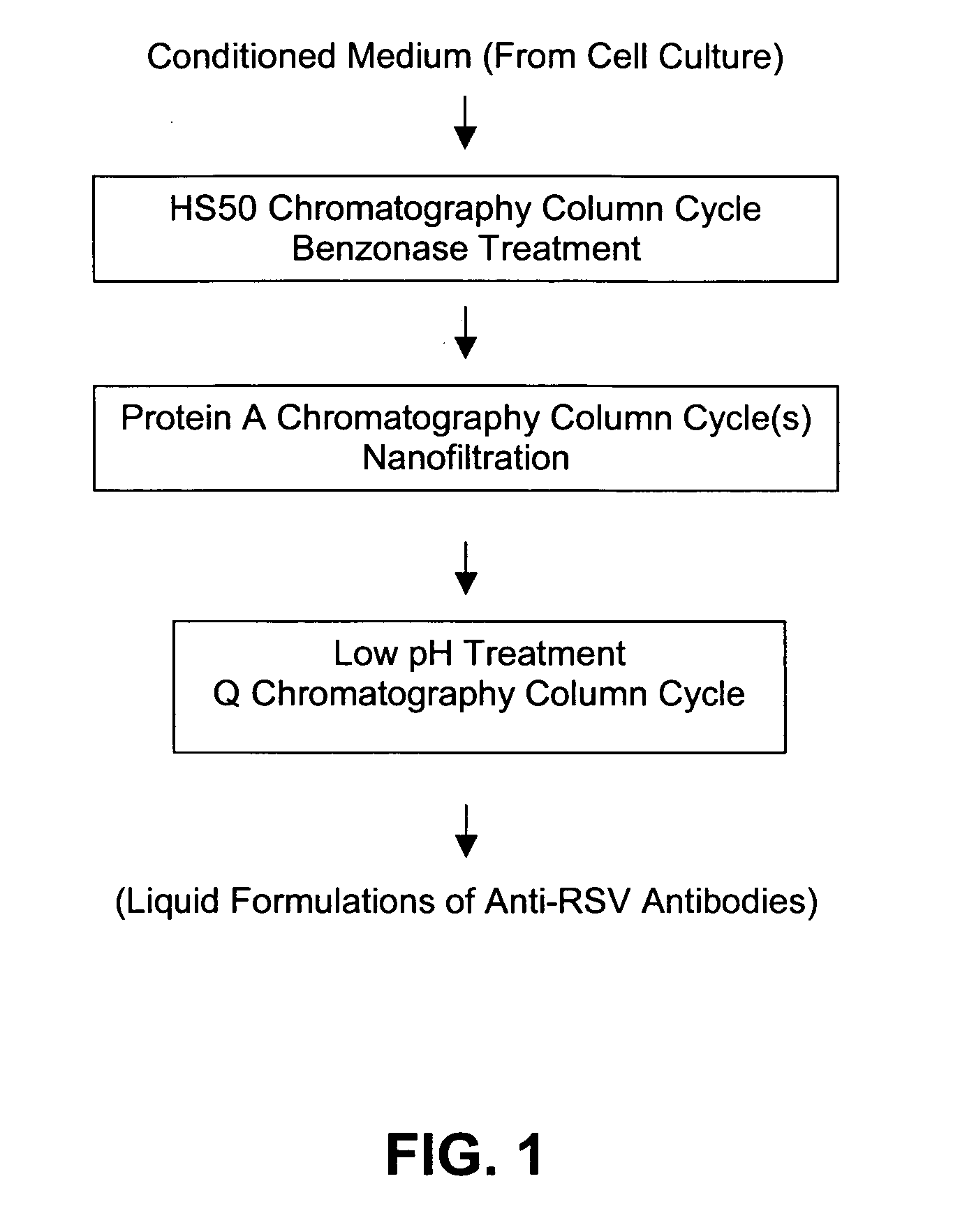
Anti-RSV antibodies
InactiveUS6818216B2Reduce dosing frequencyReduce dosageAnimal cellsSugar derivativesSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS7229619B1Reduce dosing frequencyReduce dosageImmunoglobulins against virusesAntibody ingredientsSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Recombinant IL-9 antibodies and uses thereof
InactiveUS20050002934A1Reduces function and activity and expressionReduced activityAntibacterial agentsNervous disorderAutoimmune diseaseImmunologic specificity
The present invention provides novel antibodies that immunospecifically bind to an IL-9 polypeptide and compositions comprising said antibodies. The present invention also provides methods and compositions preventing, treating, managing, and / or ameliorating diseases and disorders associated with aberrant expression and / or activity of IL 9 or IL-9 receptor or subunits thereof, autoimmune diseases, inflammatory diseases, proliferative diseases, and infections comprising administration of one or more antibodies thereof that immunospecifically bind to an IL-9 polypeptide. The invention also encompasses methods and compositions for diagnosing, monitoring, and prognosing these disorders. The present invention further relates to articles of manufacture and kits comprising antibodies that immunospecifically bind to an IL-9 polypeptide.
Owner:MEDIMMUNE LLC
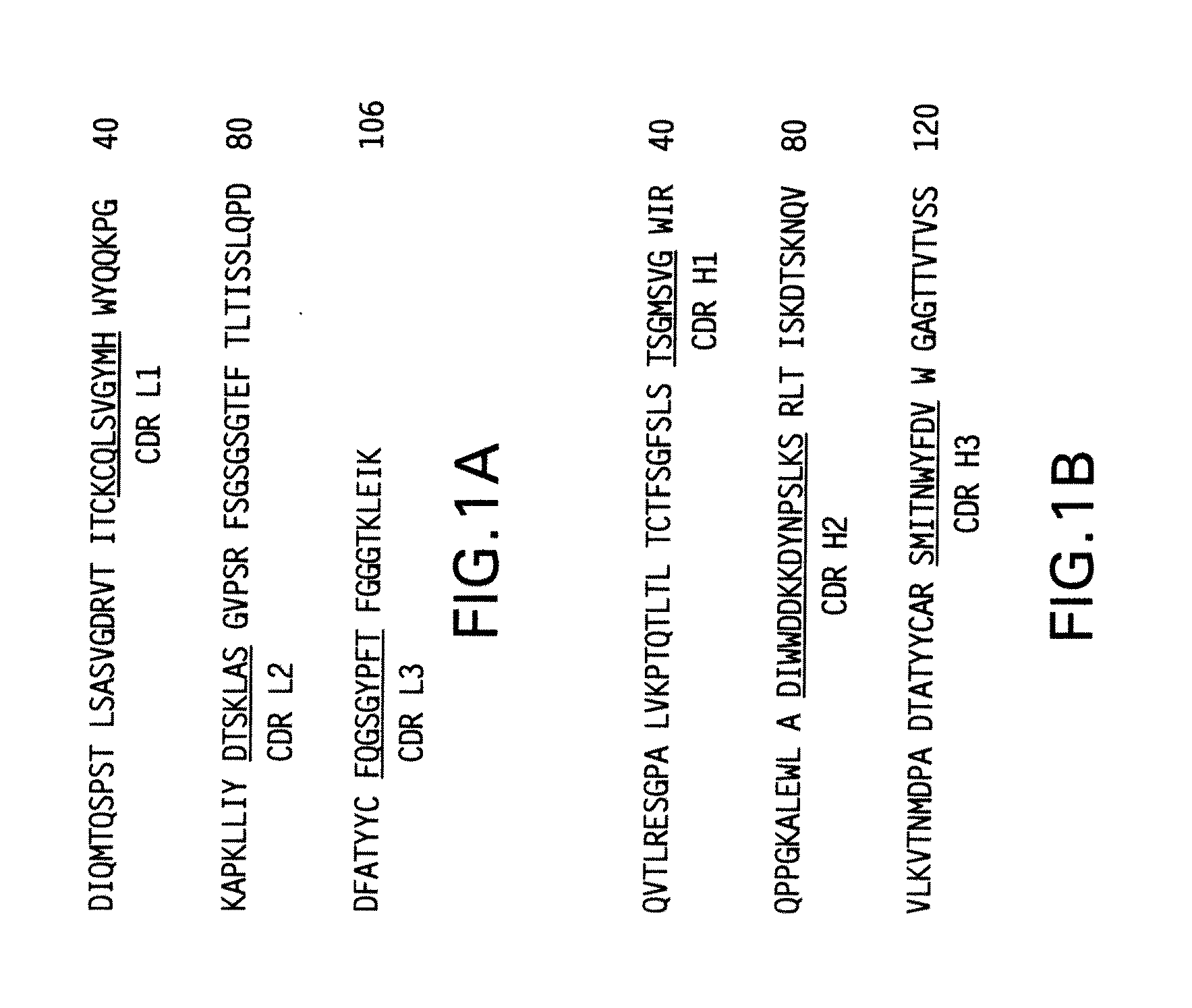
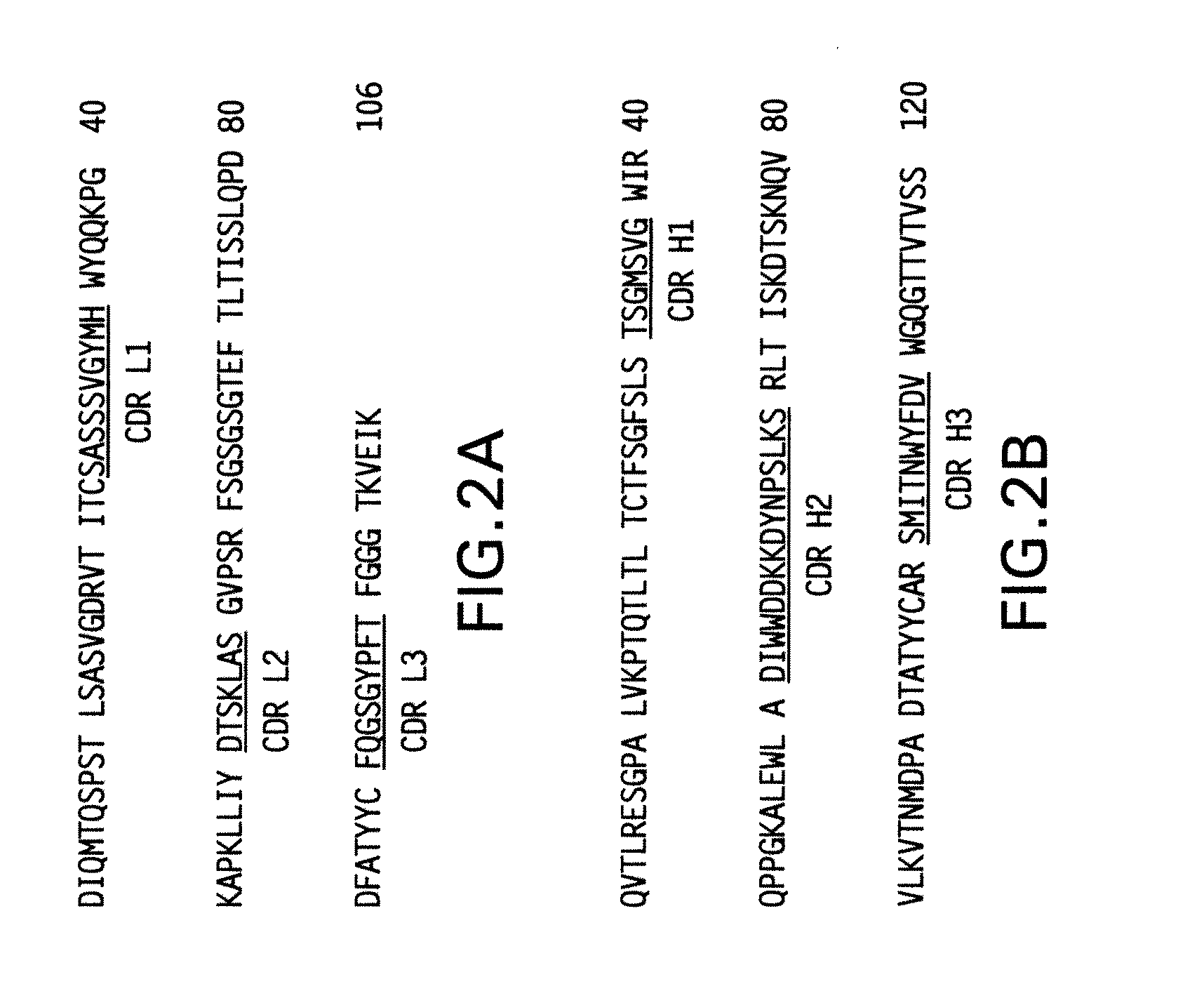
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
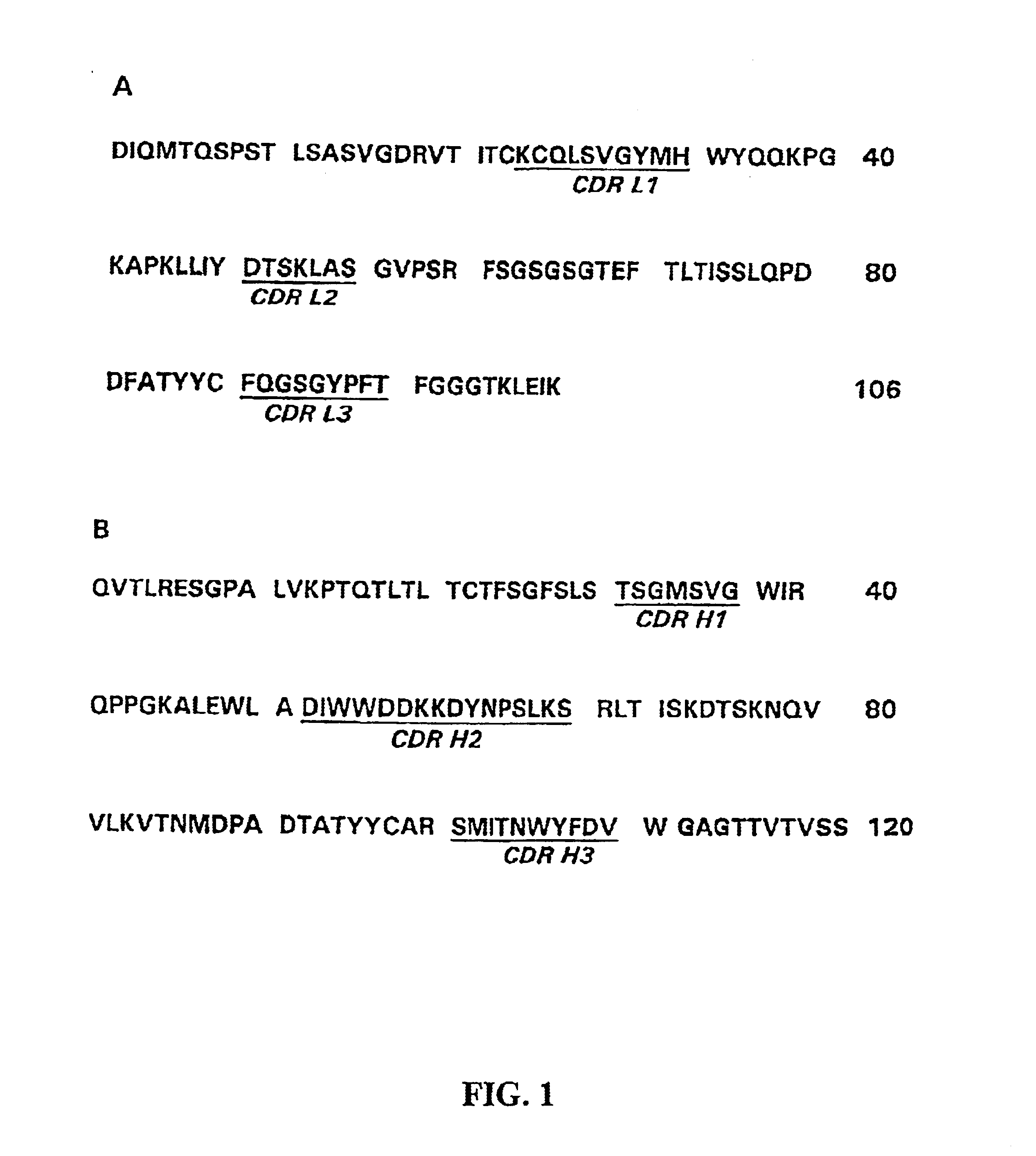
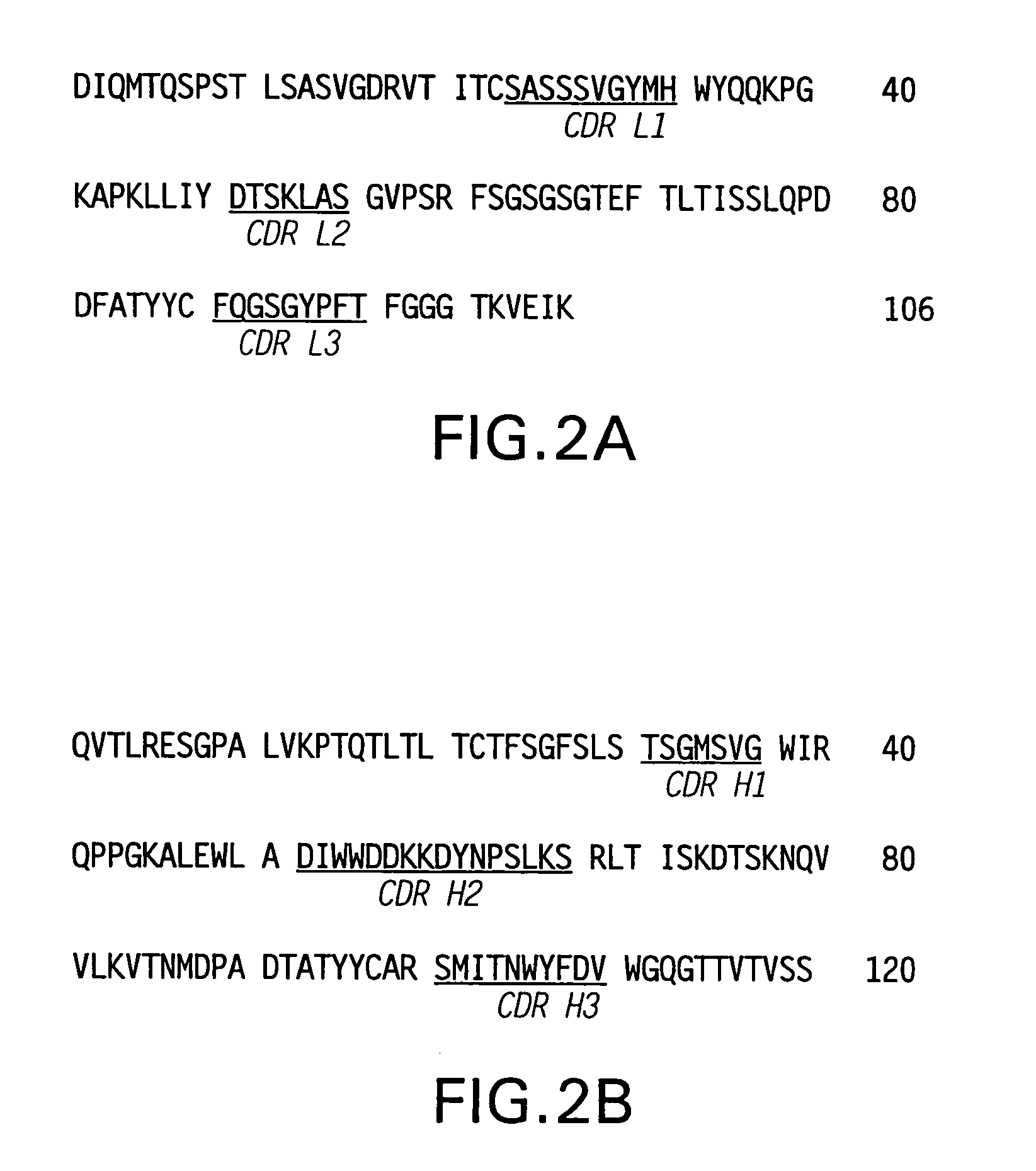
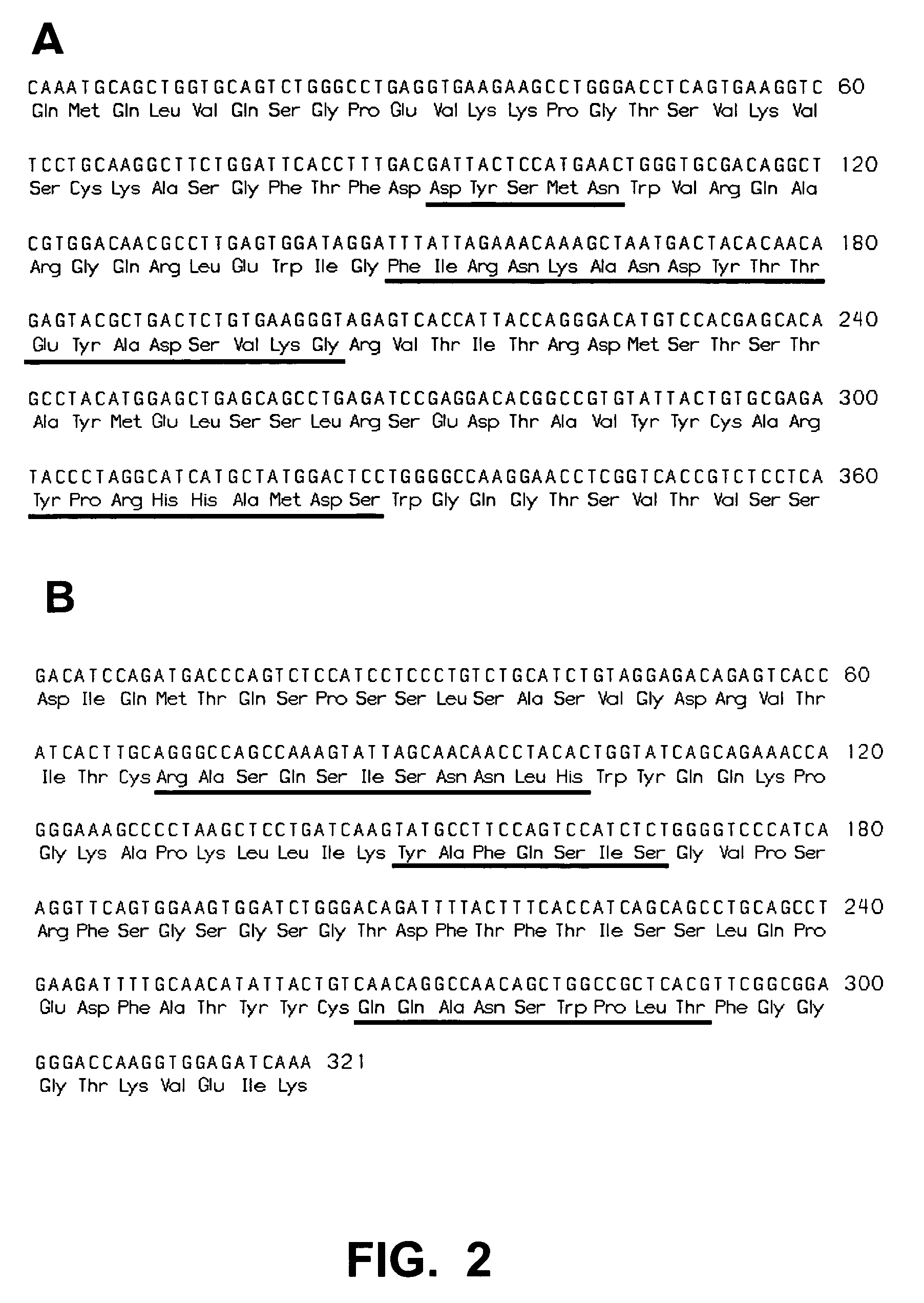
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Immunogenic compositions for the prevention and treatment of meningococcal disease
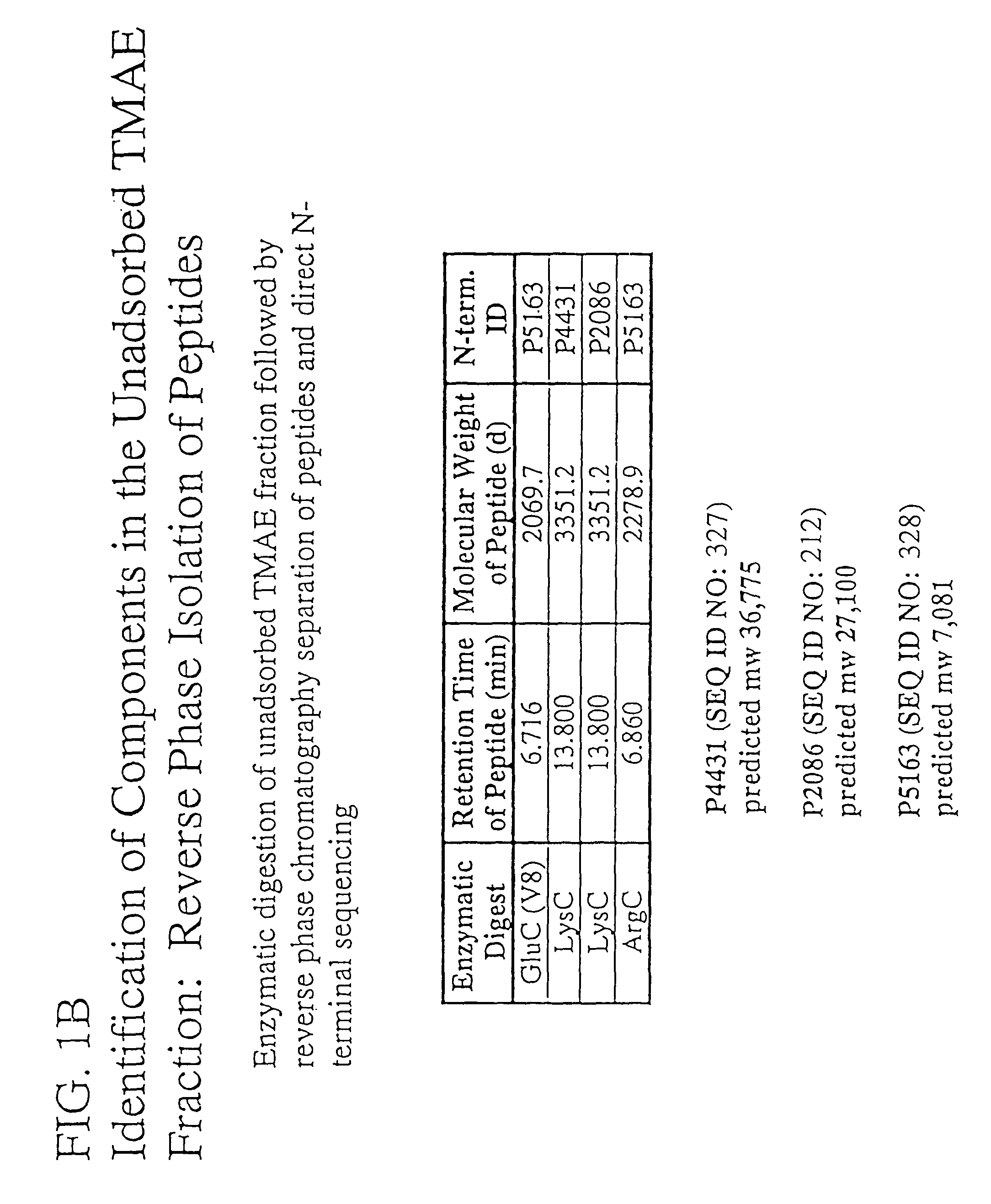
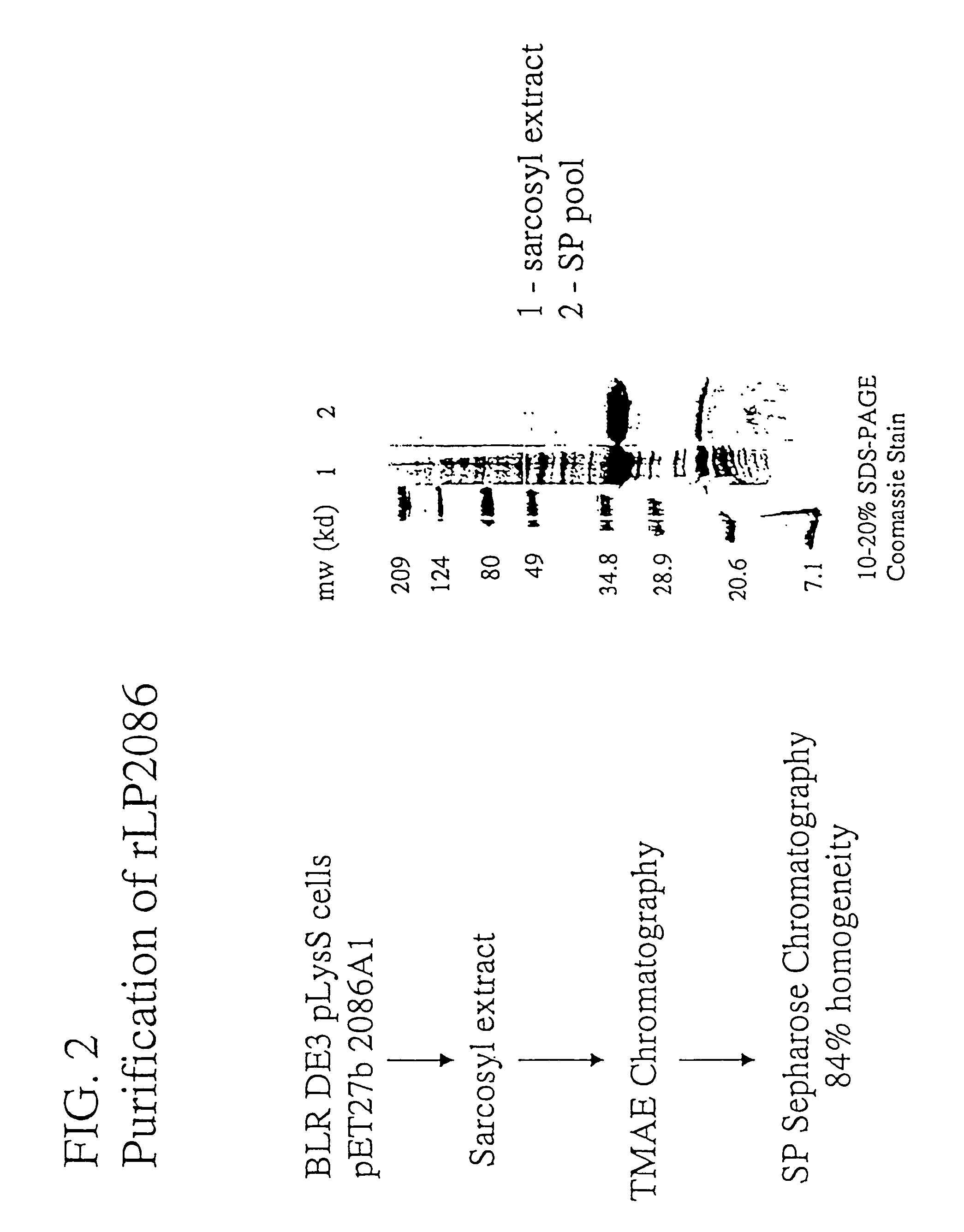
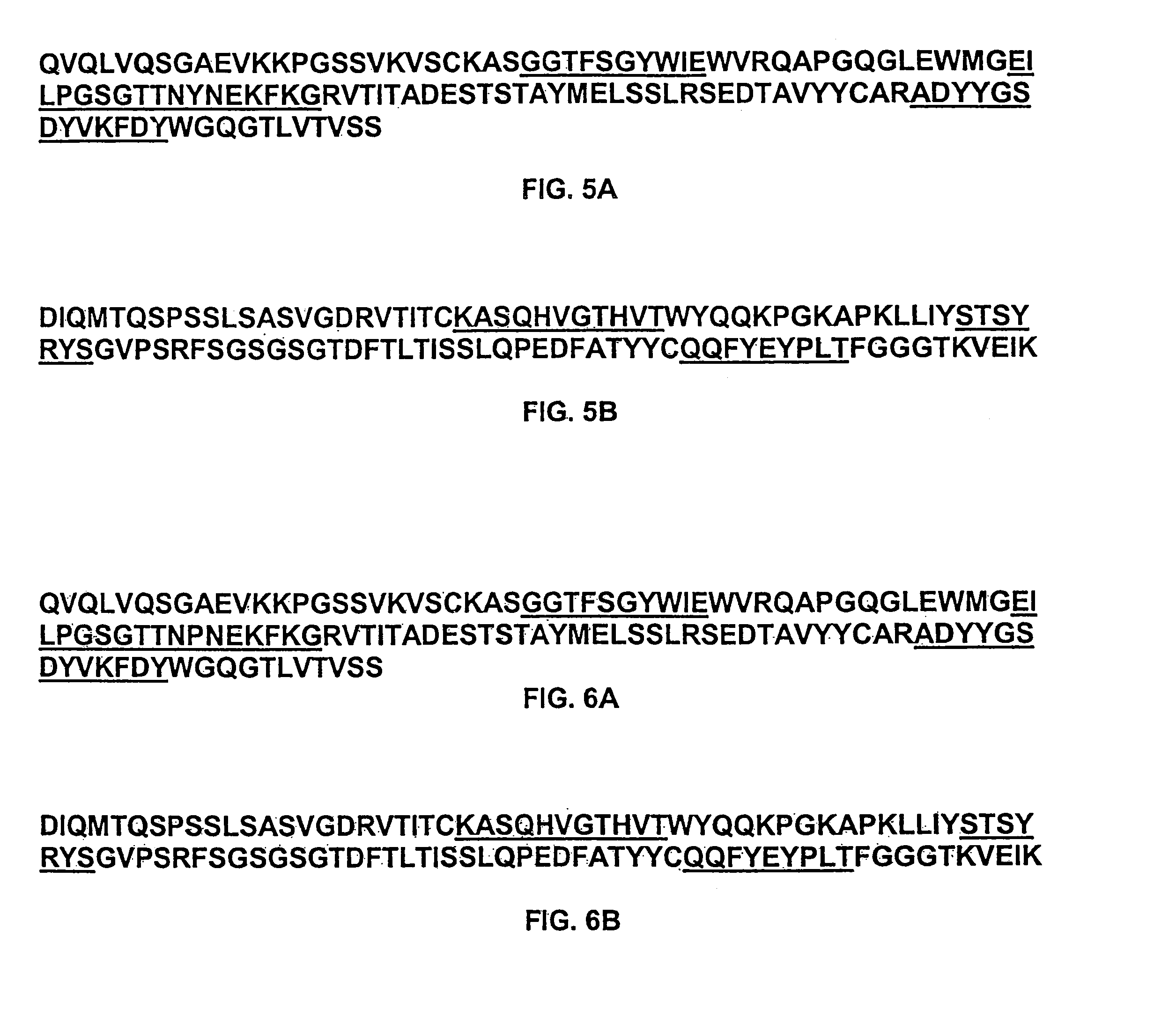
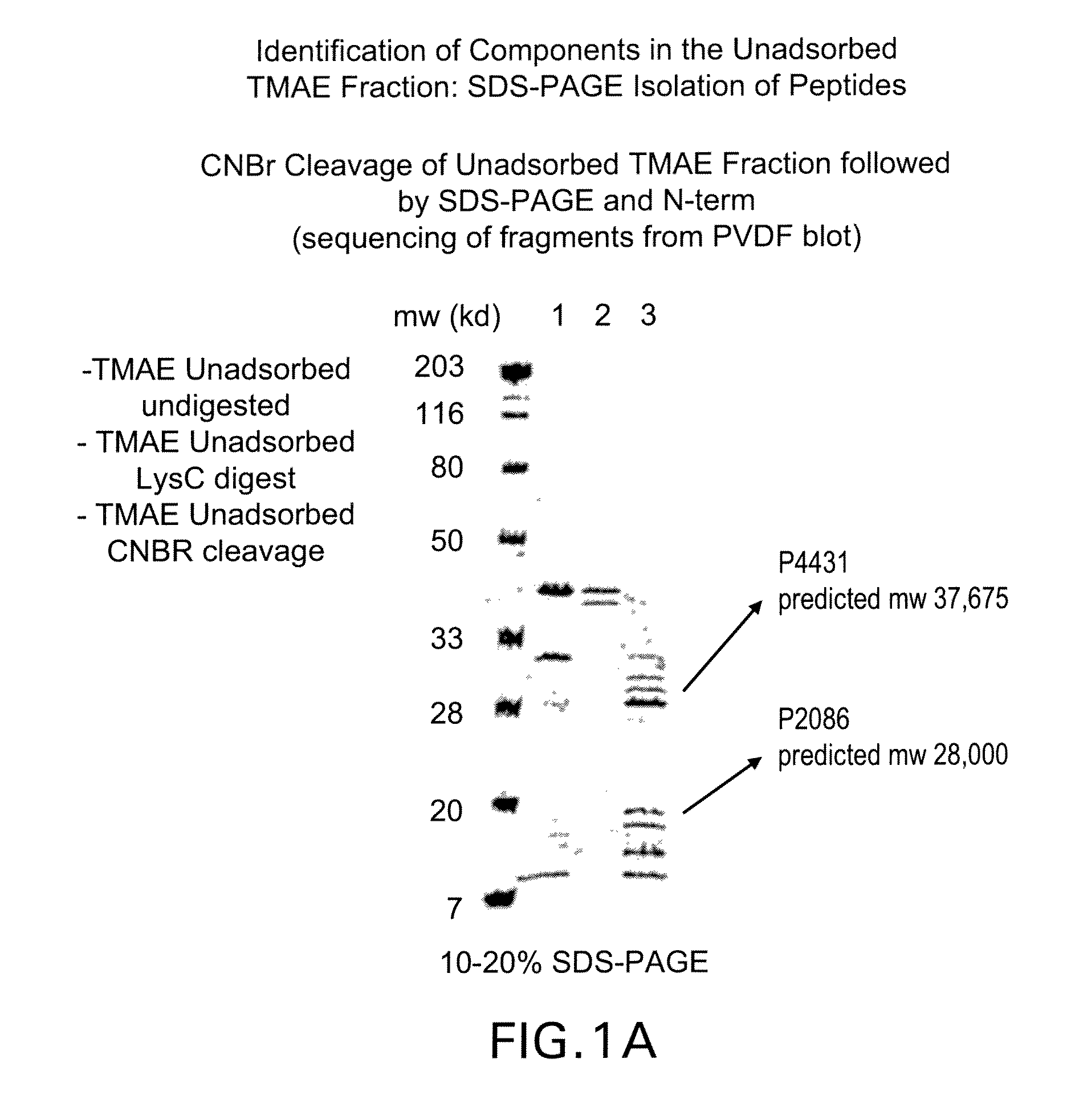
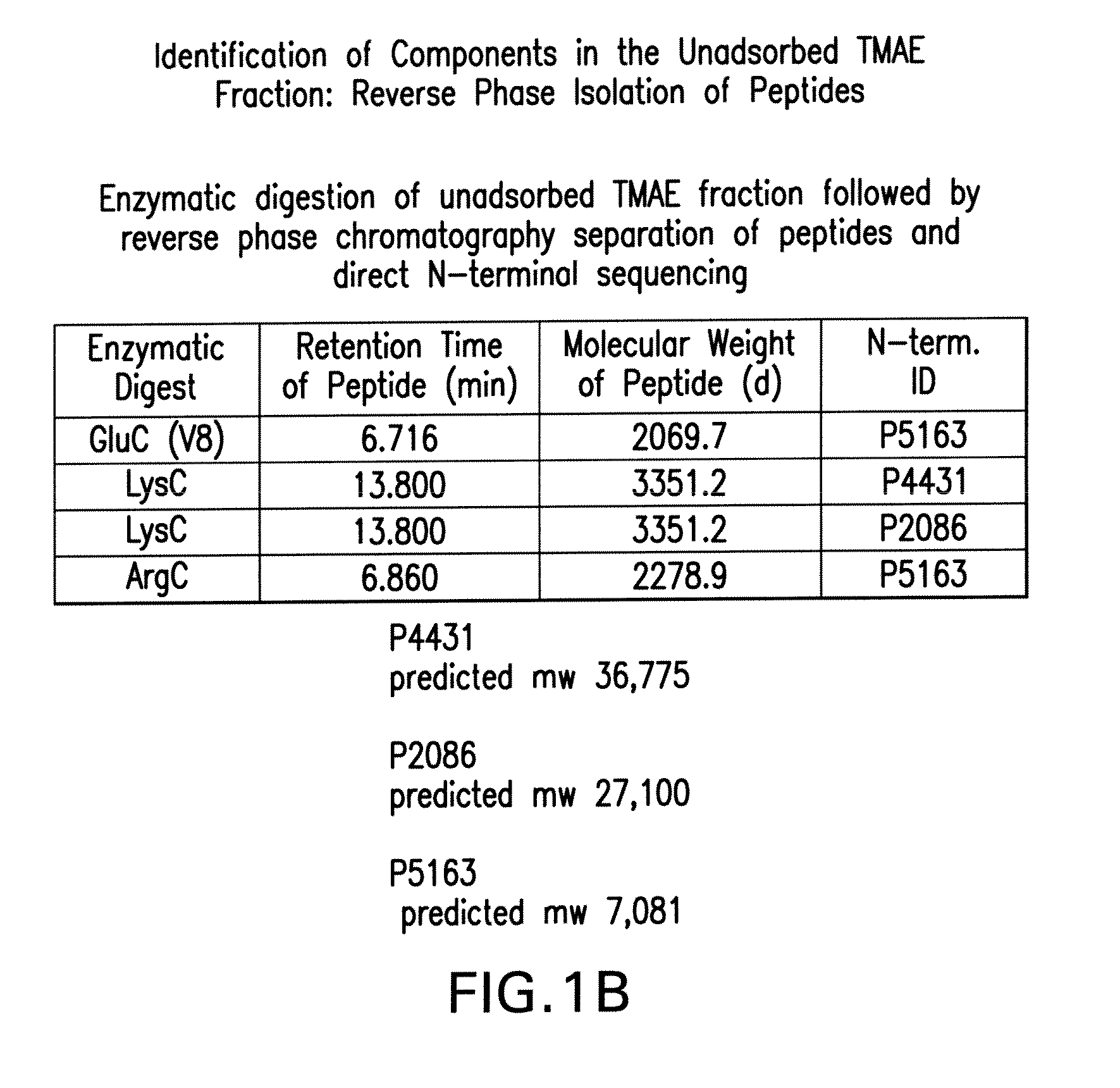
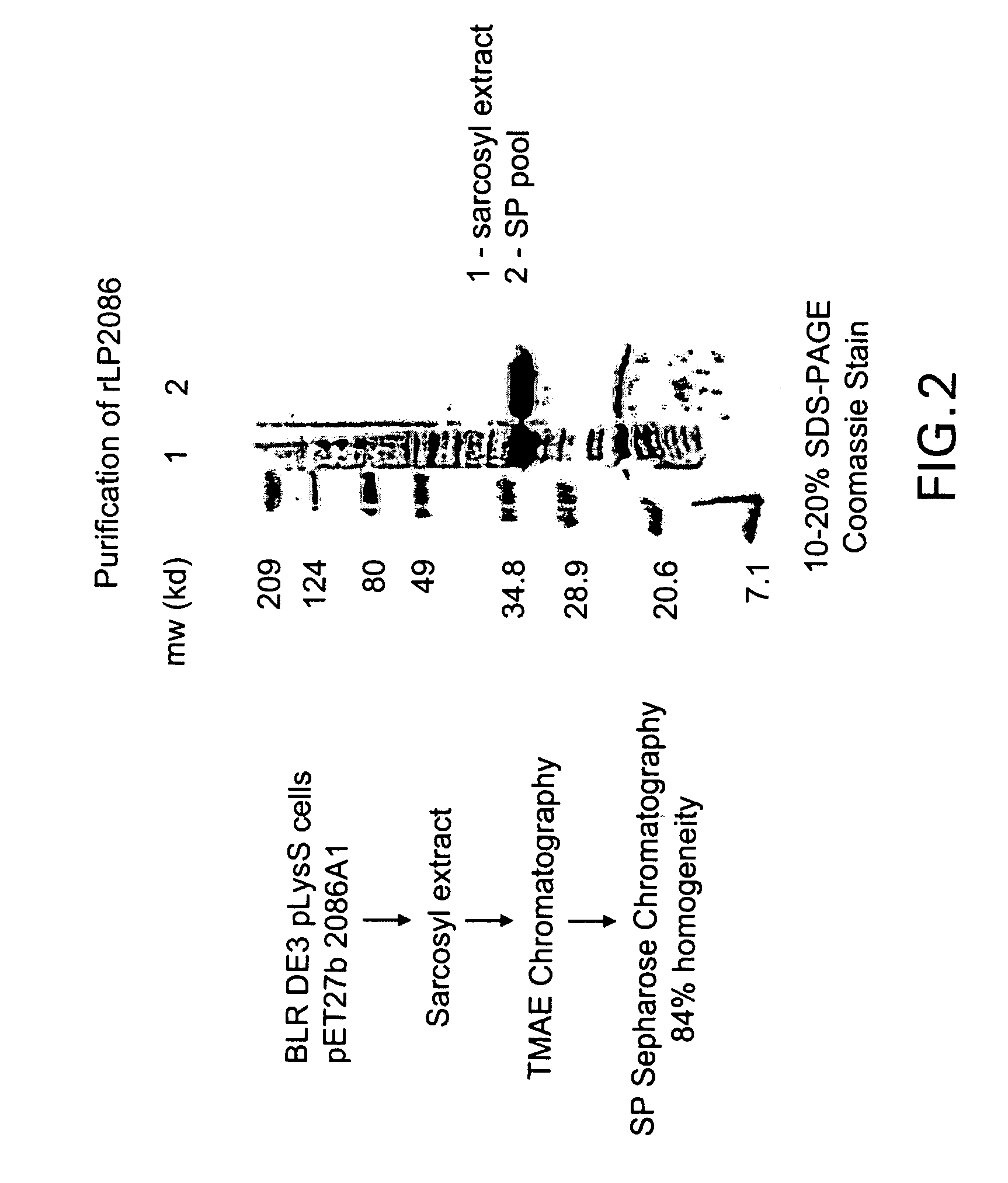
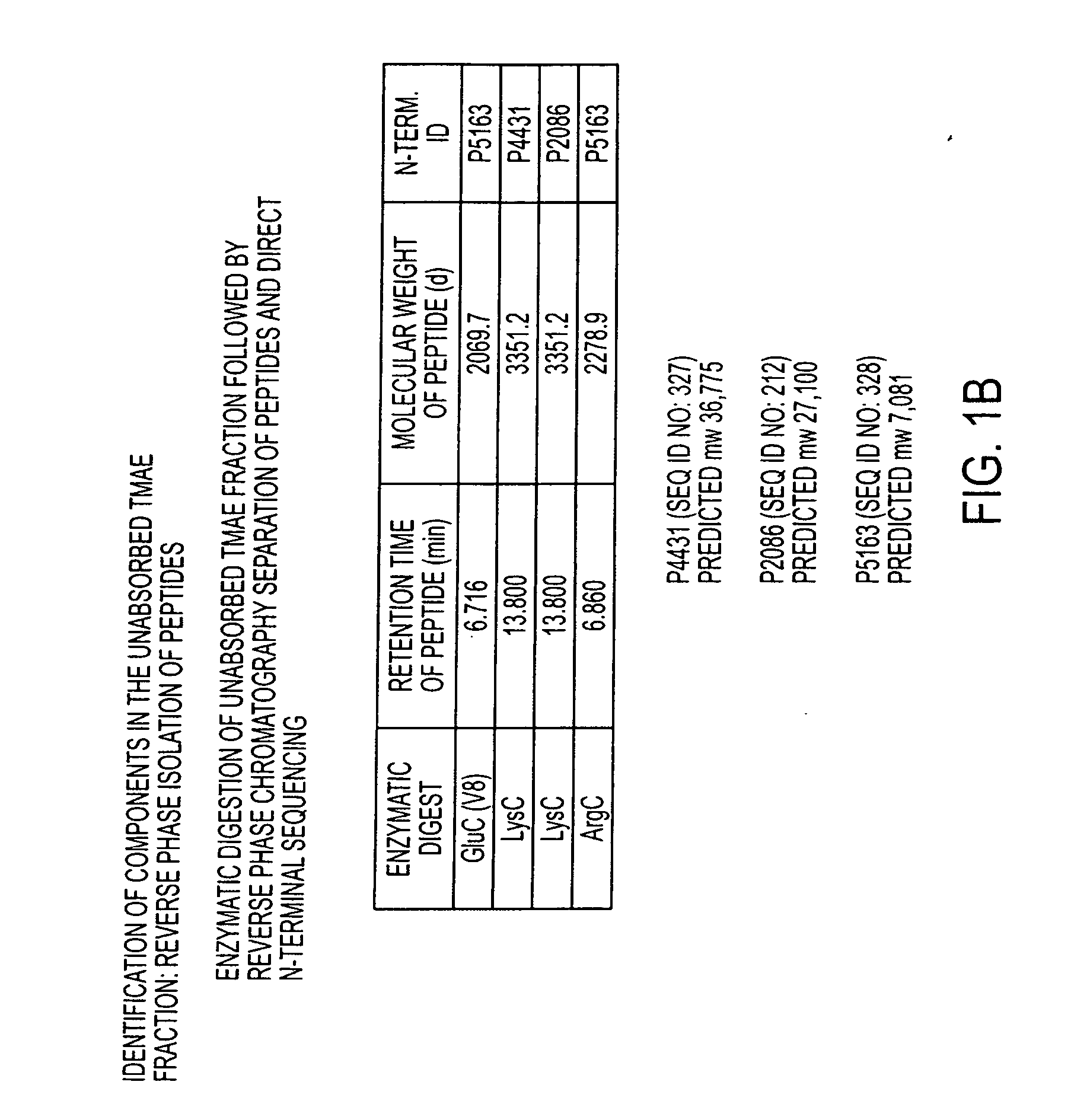
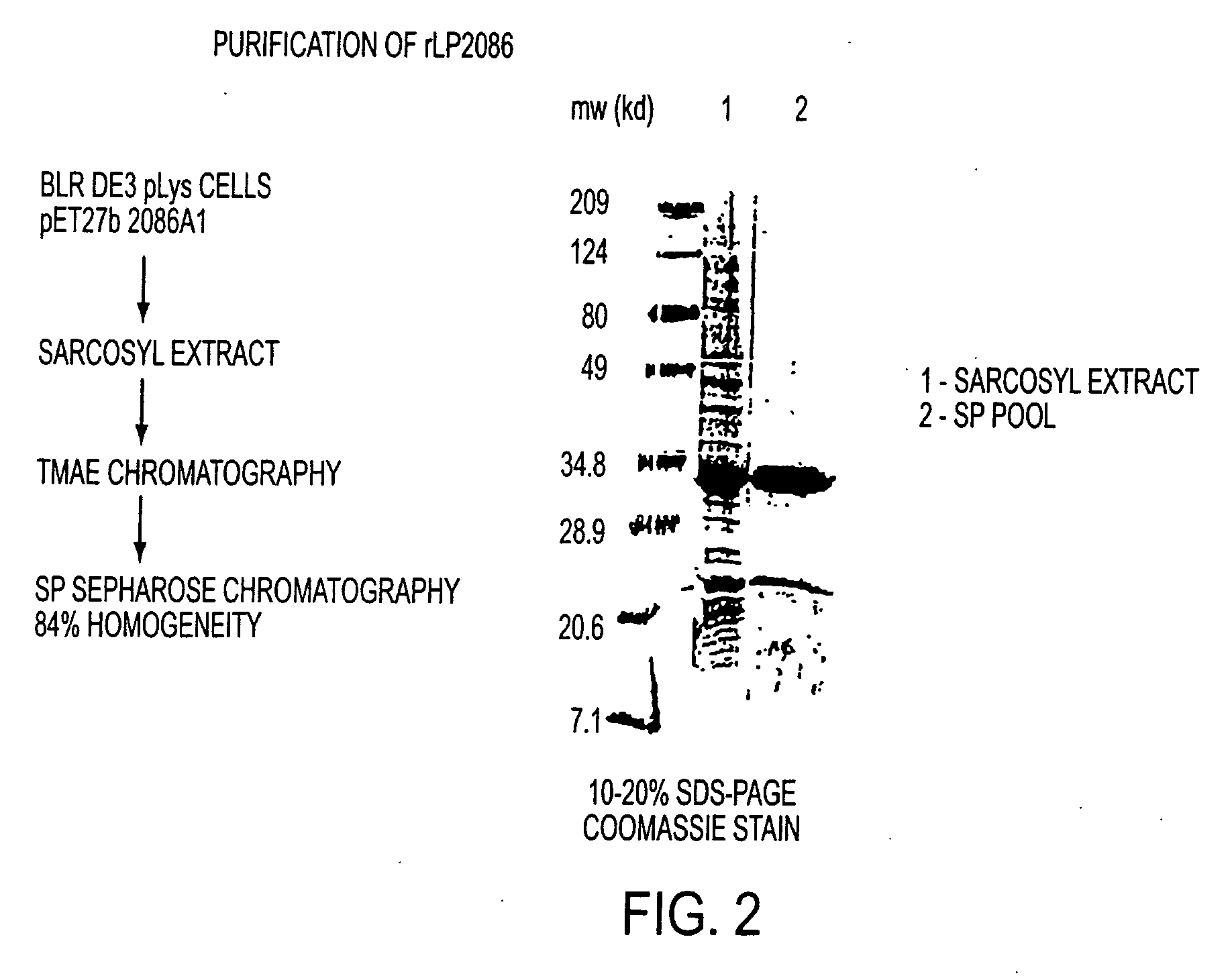
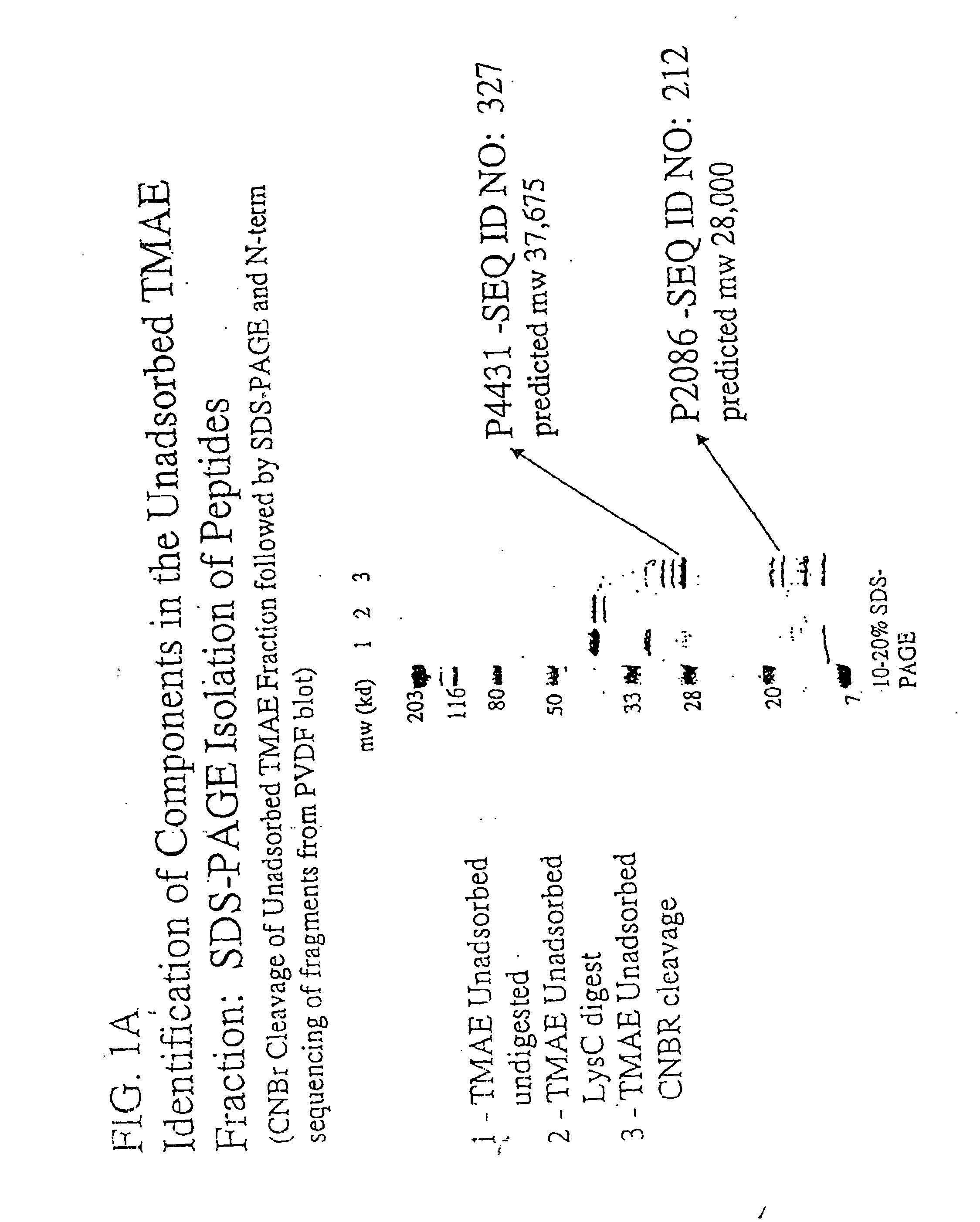
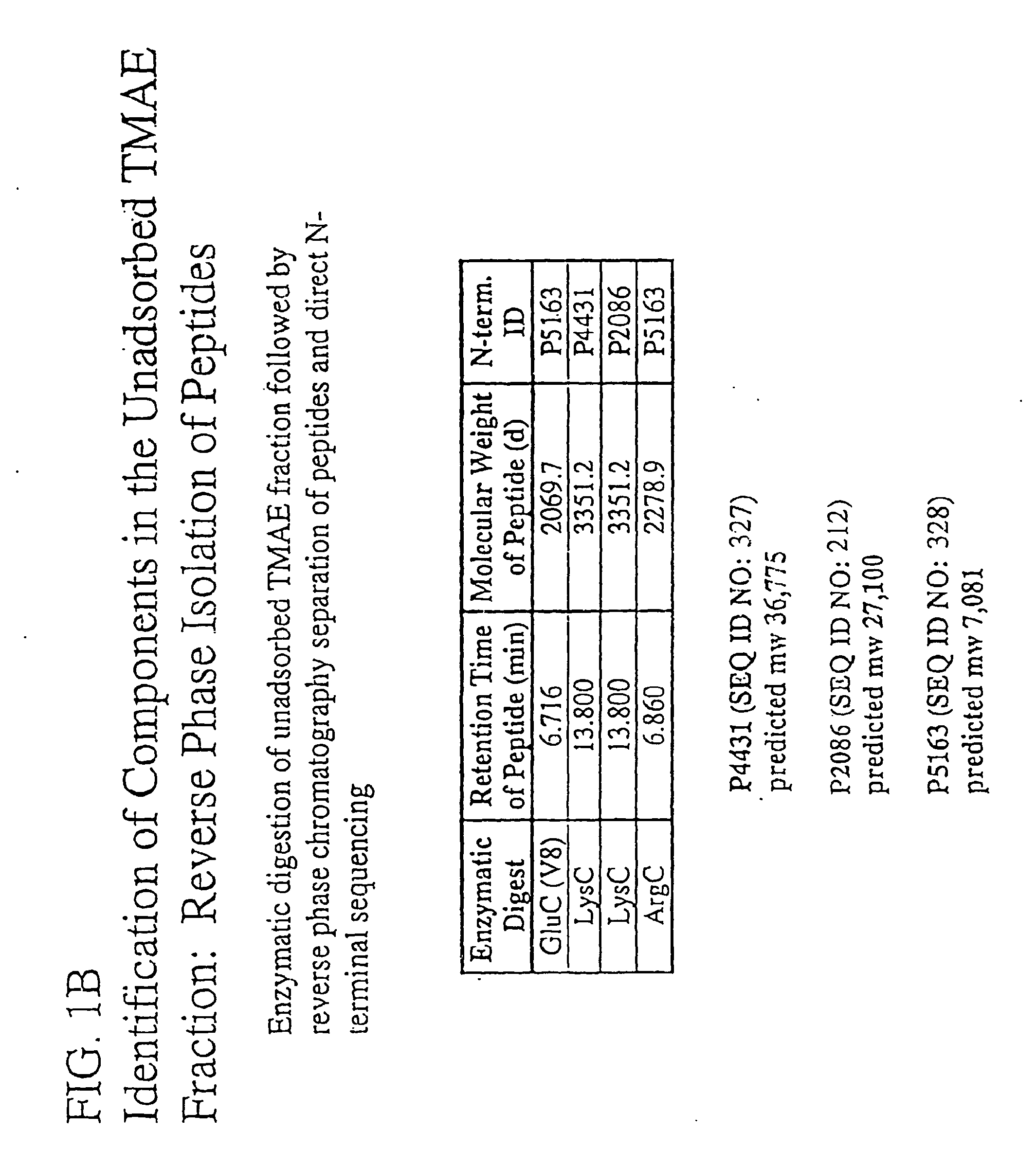
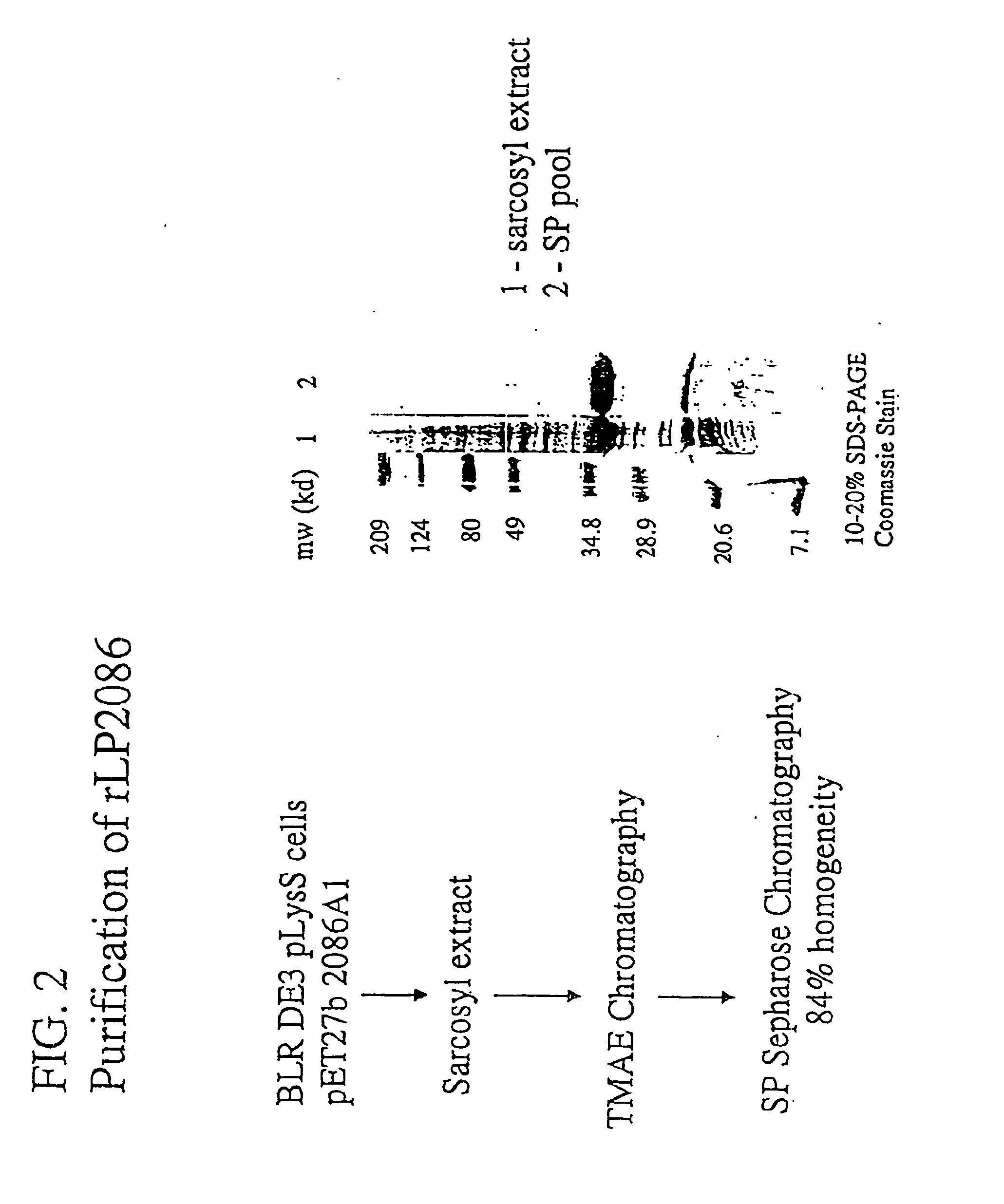
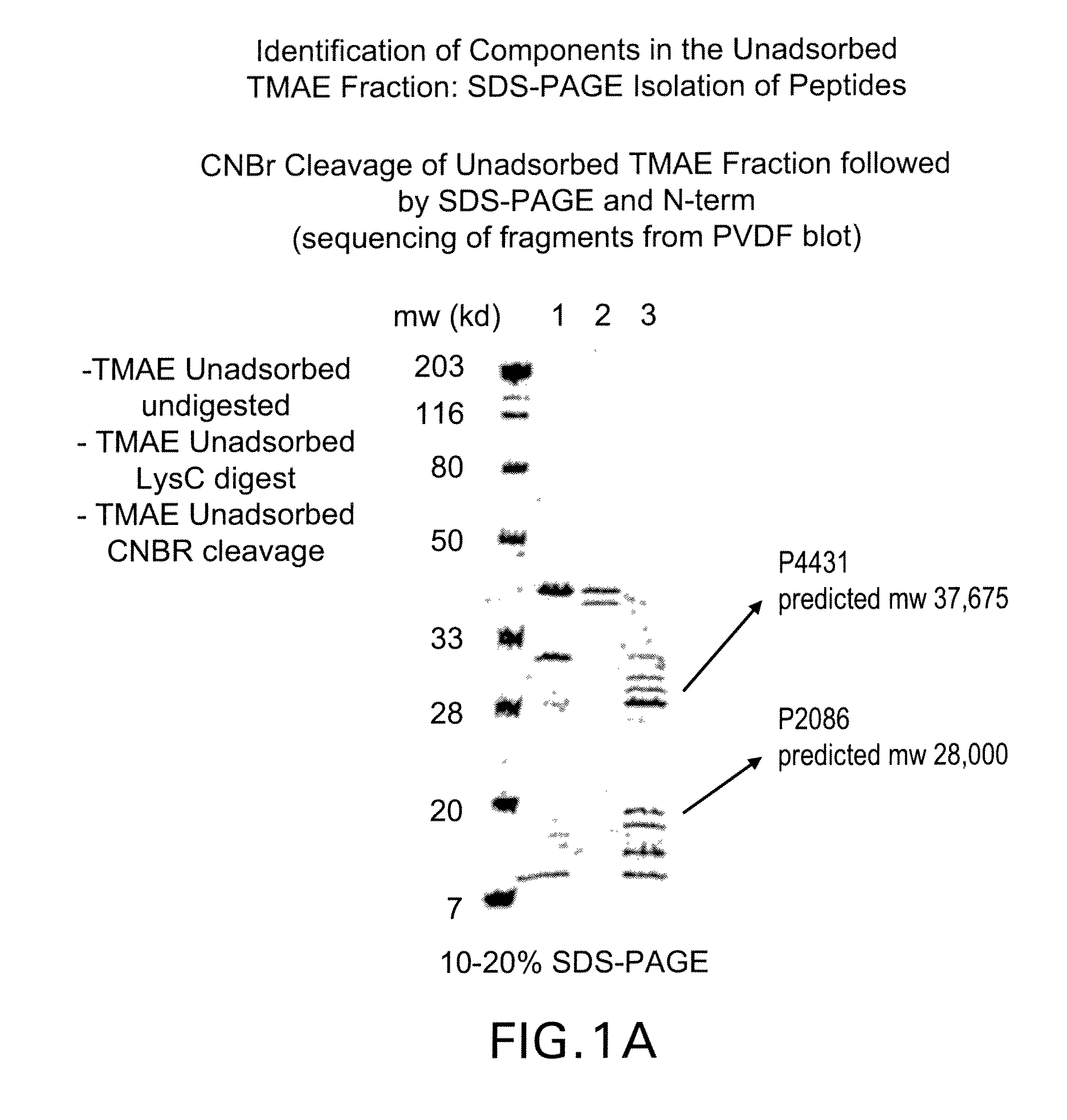
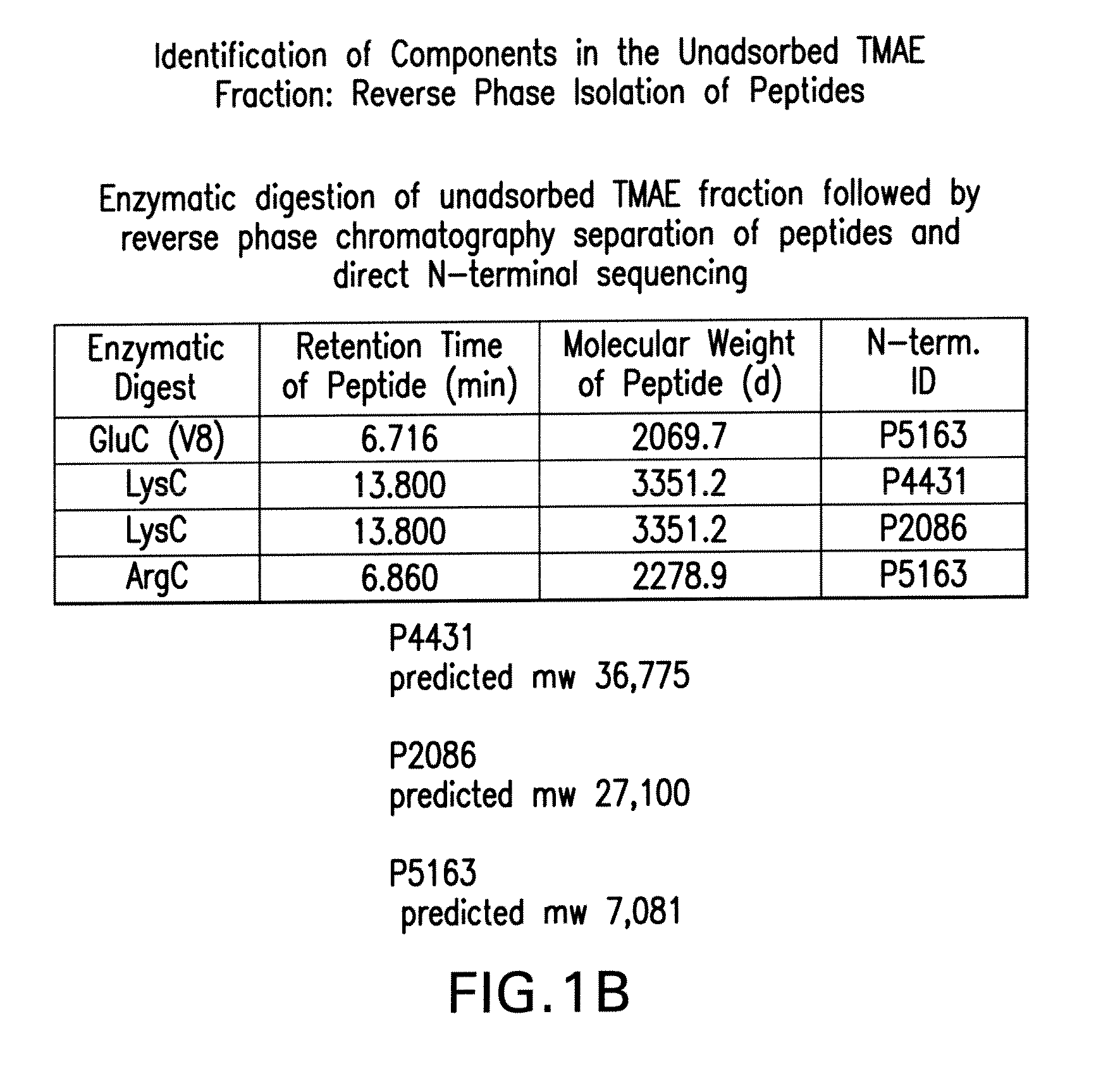
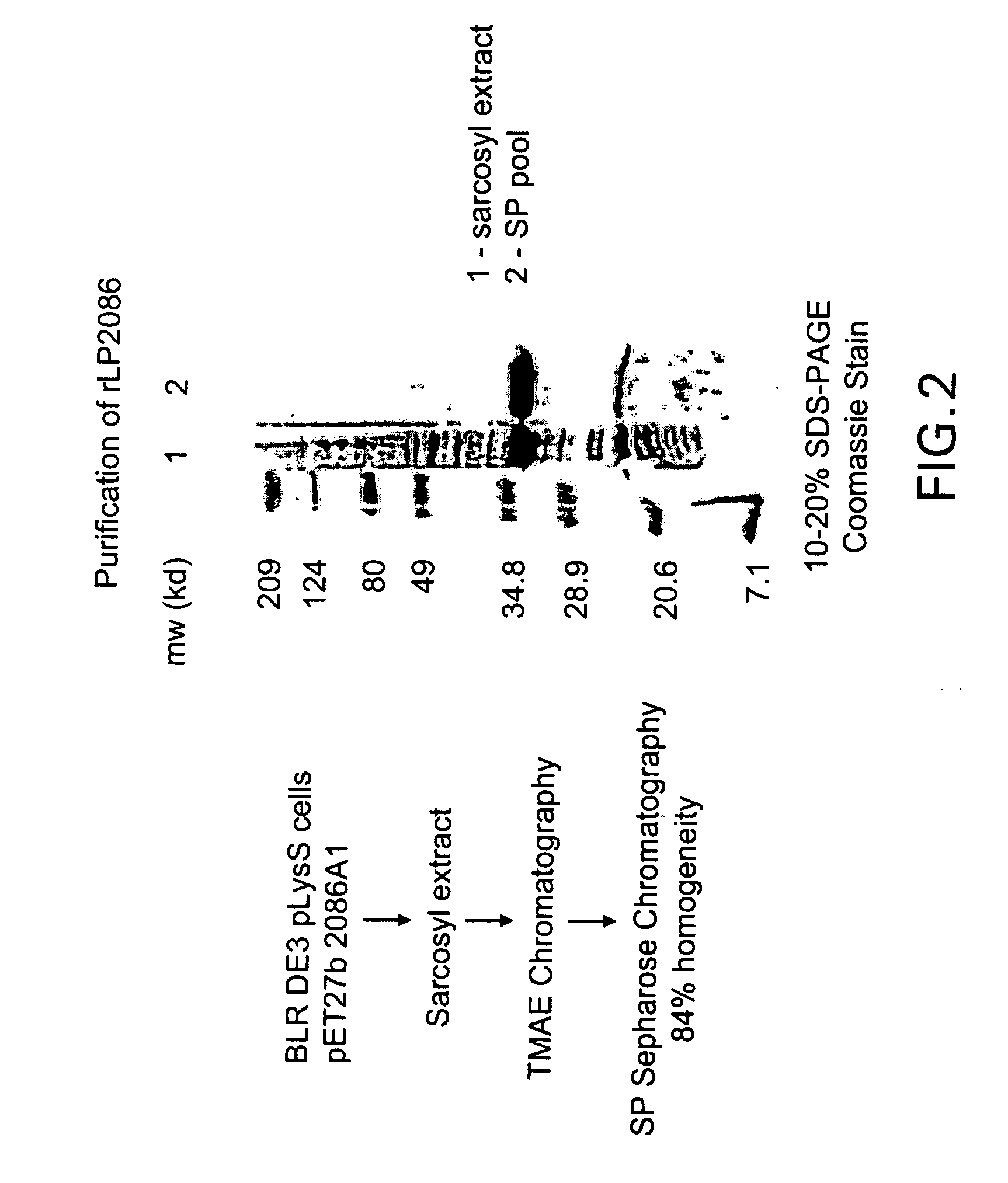
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
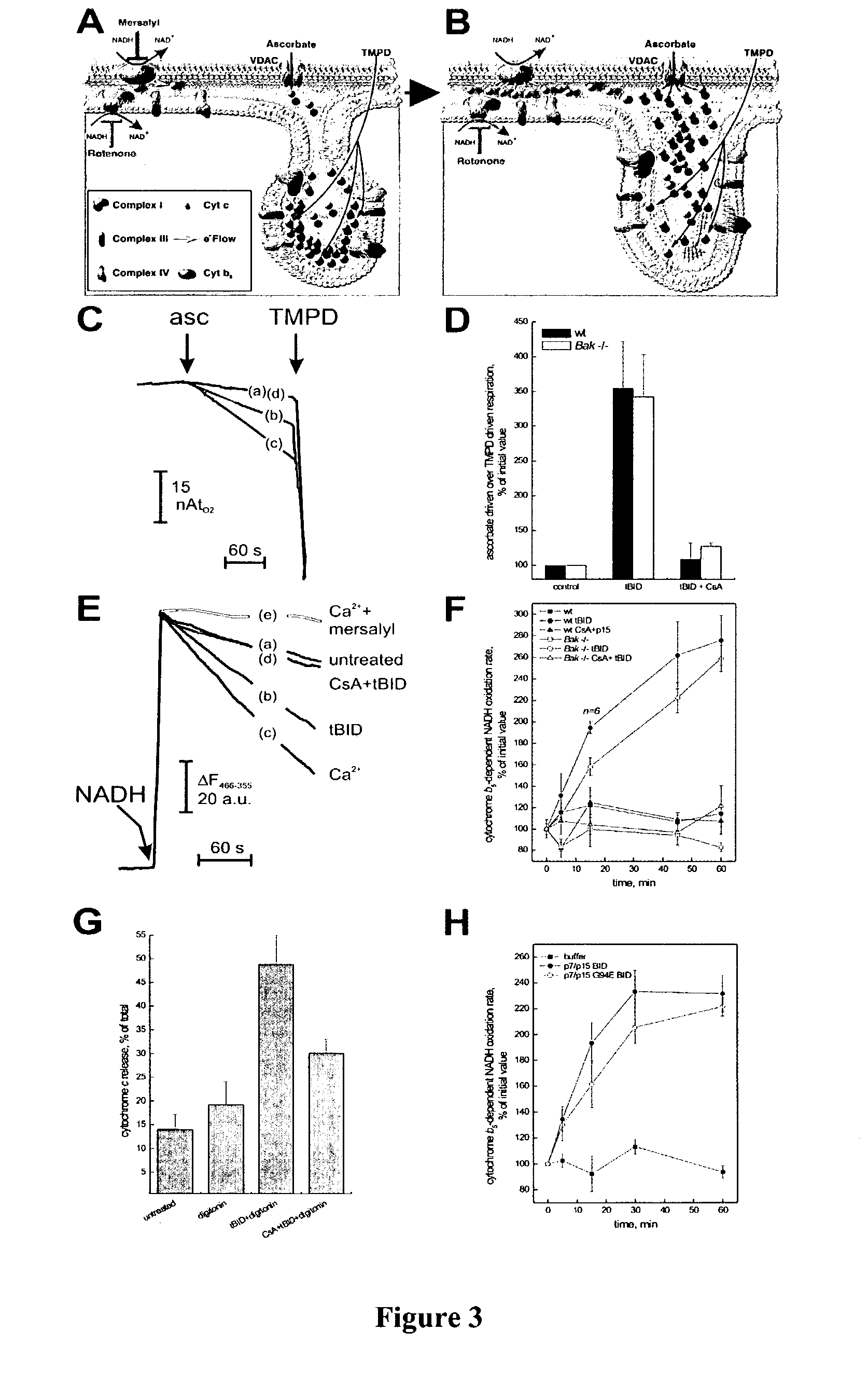
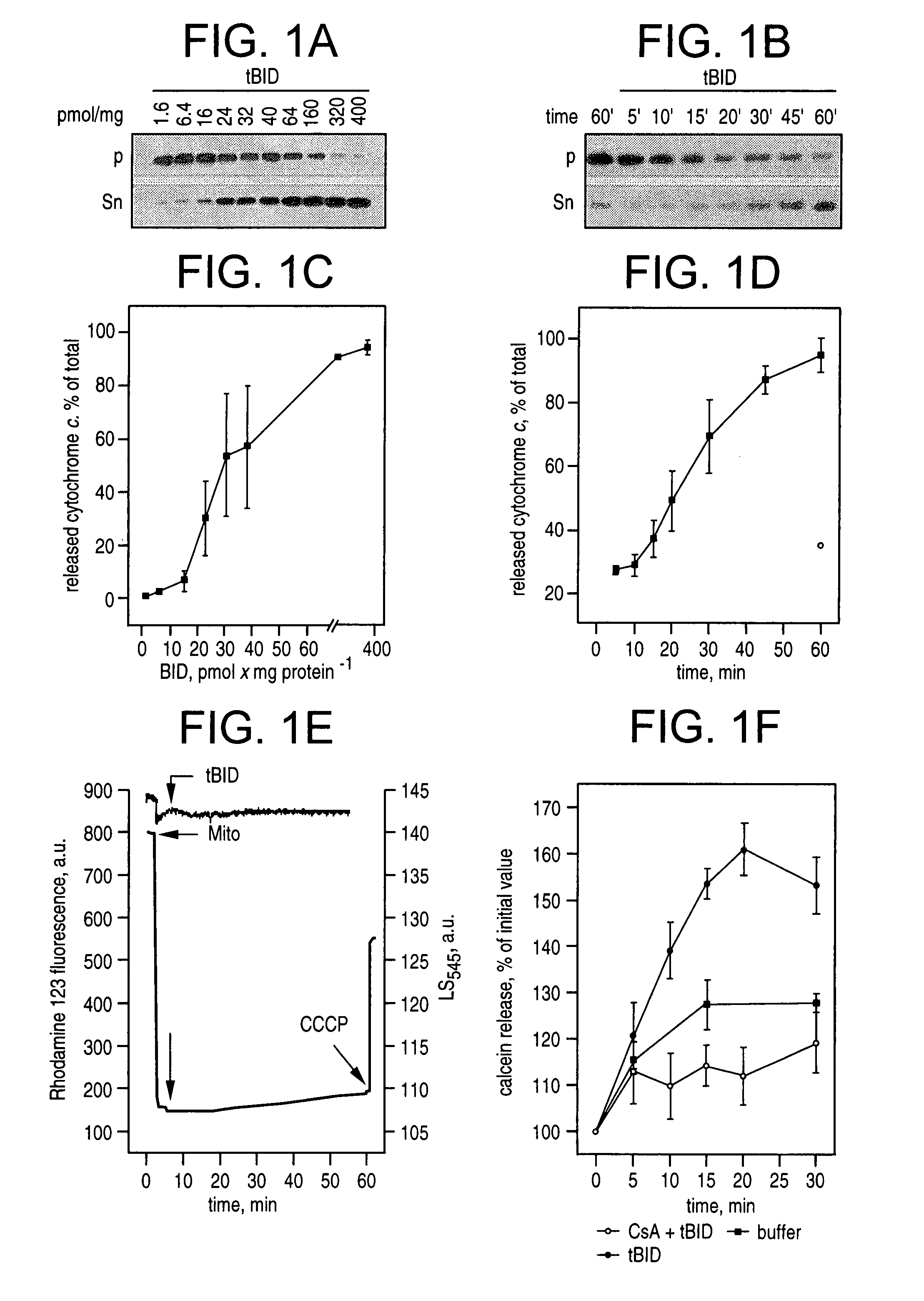
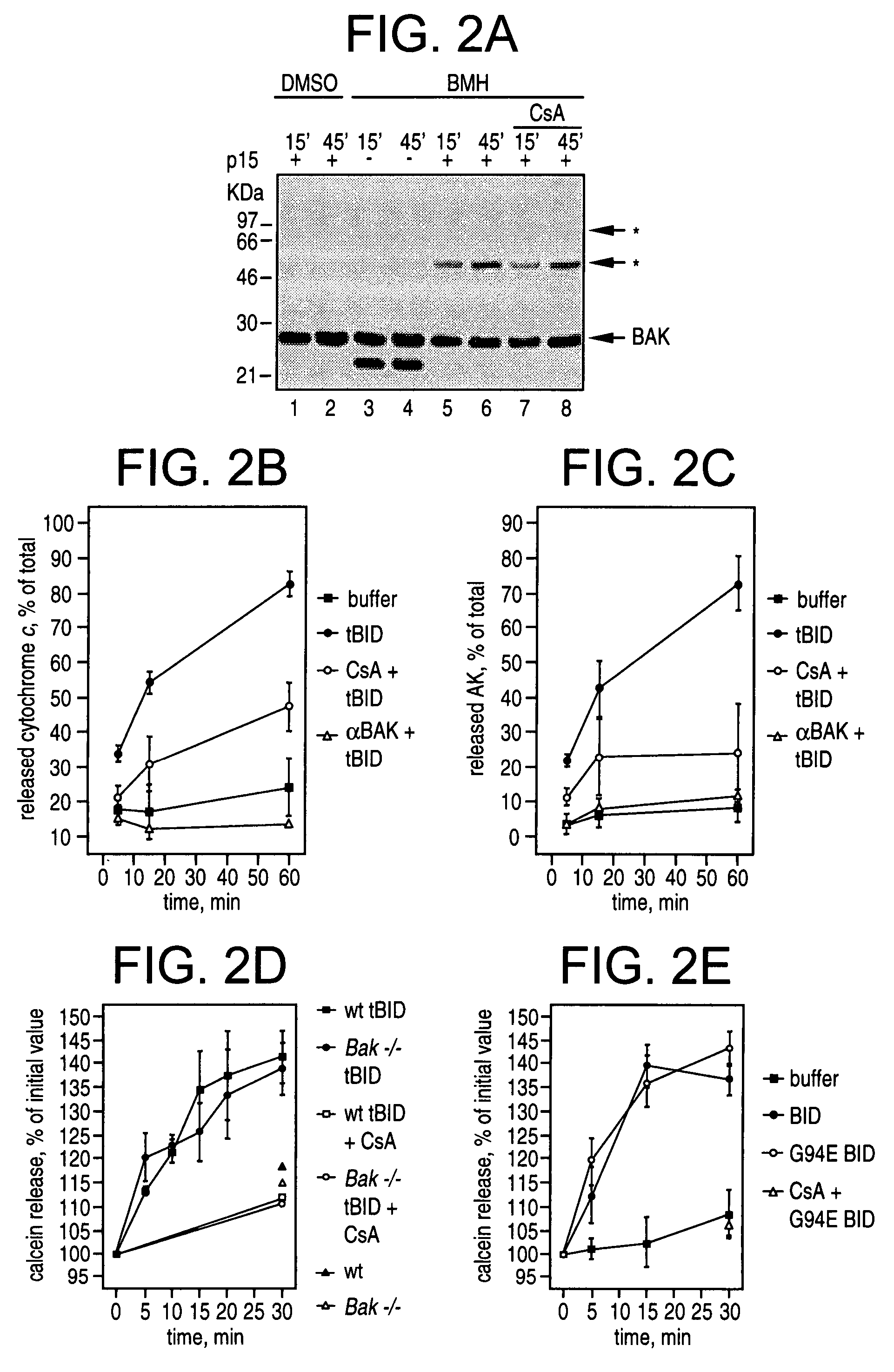
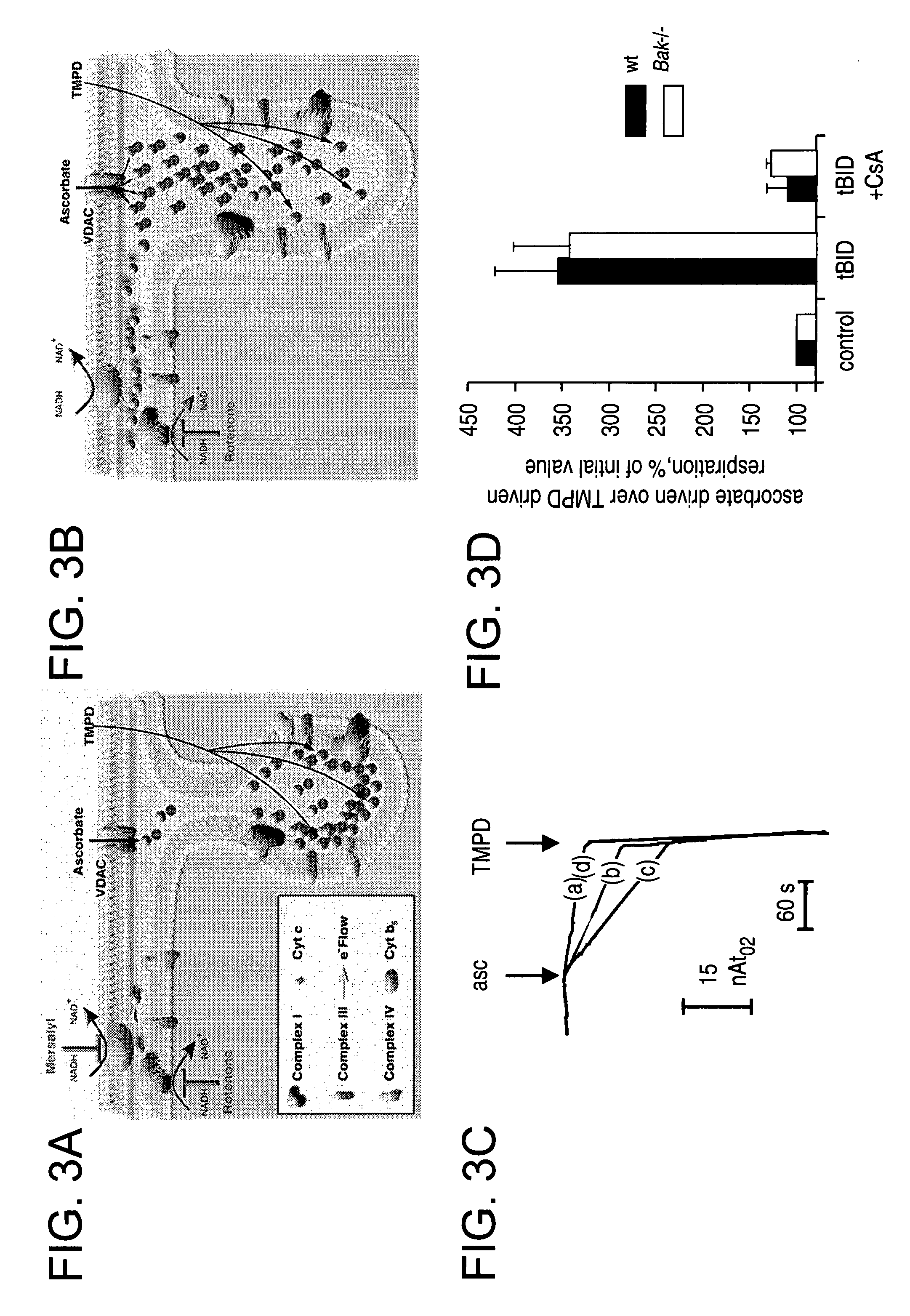
BID polypeptides and methods of inducing apoptosis
ActiveUS7247700B2Reduce triggersPeptide/protein ingredientsHydrolasesApoptosisNucleic acid sequencing
Disclosed herein are novel polypeptides and the nucleic acid sequences that encode them. Also disclosed are antibodies that immunospecifically bind to the polypeptide, as well as derivatives, variants, mutants, or fragments of the novel polypeptide, polynucleotide, or antibody specific to the polypeptide. Vectors, host cells, antibodies and recombinant methods for producing the polypeptides and polynucleotides, as well as methods for using same are also included. The invention further discloses therapeutic, diagnostic and research methods for diagnosis, treatment, and prevention of apoptosis associated disorders involving these novel human nucleic acids and proteins.
Owner:DANA FARBER CANCER INST INC
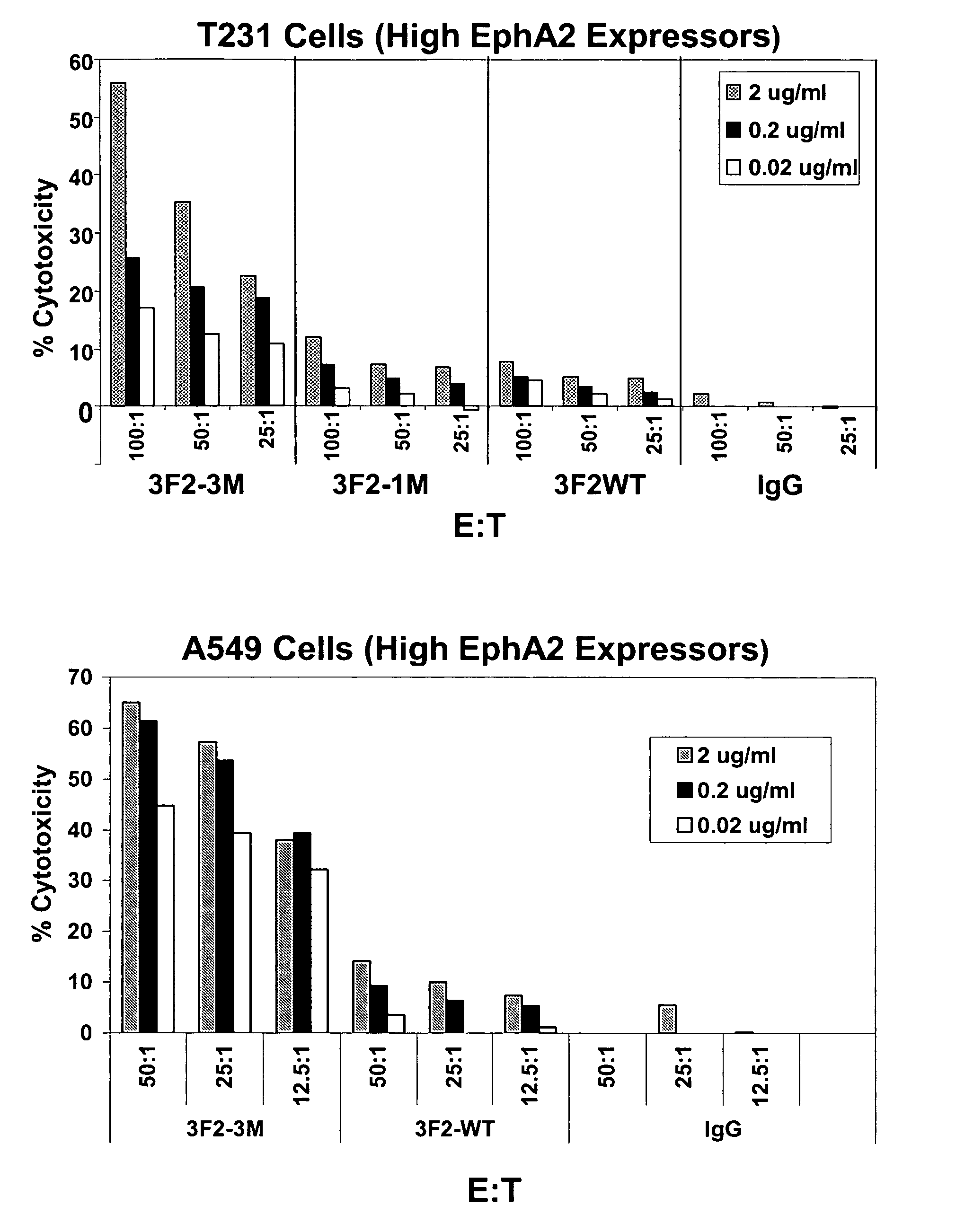
EPH receptor Fc variants with enhanced antibody dependent cell-mediated cytotoxicity activity
InactiveUS20060039904A1Altered binding affinityHigh binding affinityCompound screeningApoptosis detectionComplement-dependent cytotoxicityReceptor
The present invention relates to novel Fc variants that immuno-specifically bind to an Eph receptor. The Fc variants comprise a binding region that immunospecifically binds to an Eph receptor and an Fc region that further comprises at least one novel amino acid residue which may provide for enhanced effector function. More specifically, this invention provides Fc variants that have modified binding affinity to one or more Fc ligand (e.g., FcγR, C1q). Additionally, the Fc variants have altered antibody-dependent cell-mediated cytotoxicity (ADCC) and / or complement dependent cytotoxicity (CDC) activity. The invention further provides methods and protocols for the application of said Fc variants that immunospecifically bind to an Eph receptor, particularly for therapeutic purposes.
Owner:MEDIMMUNE LLC
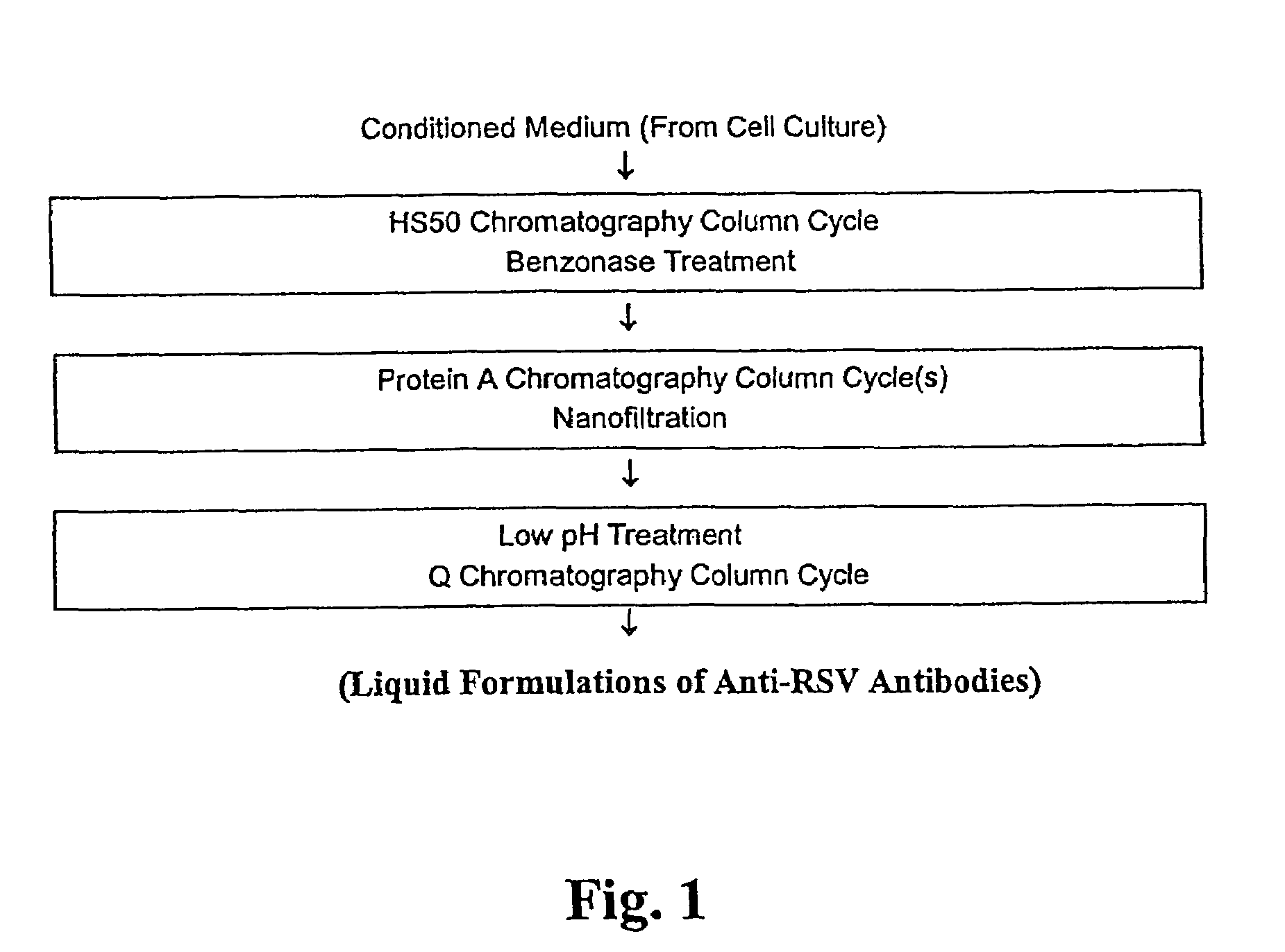
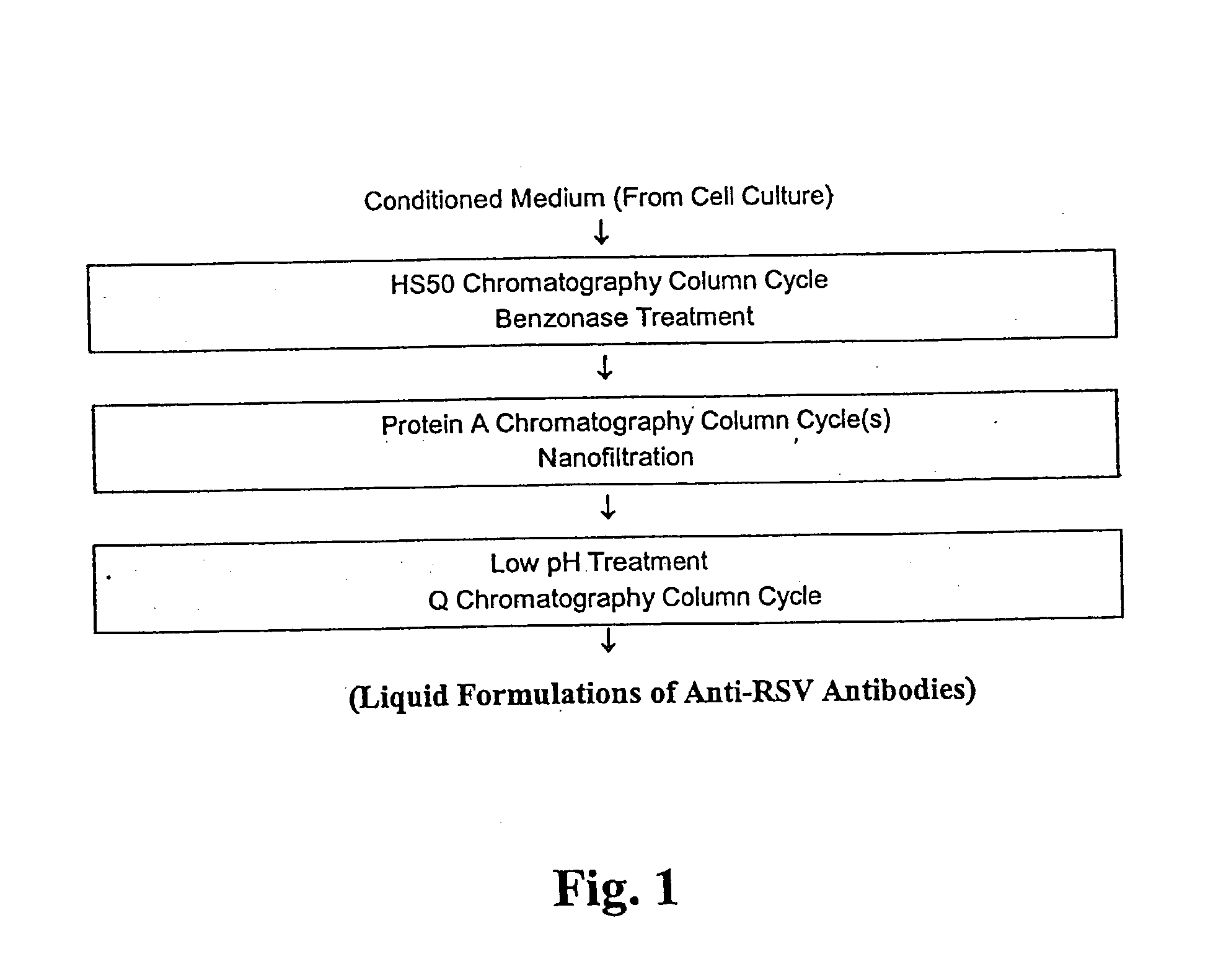
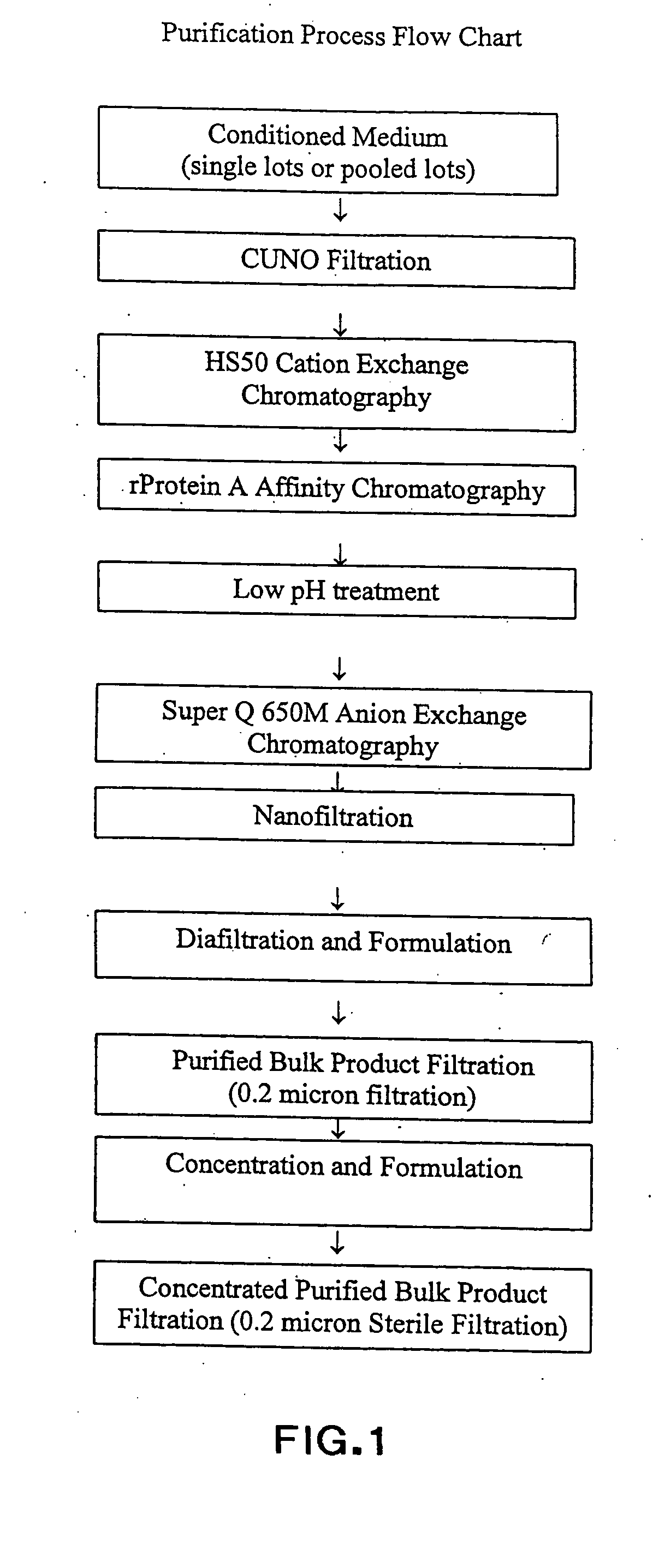
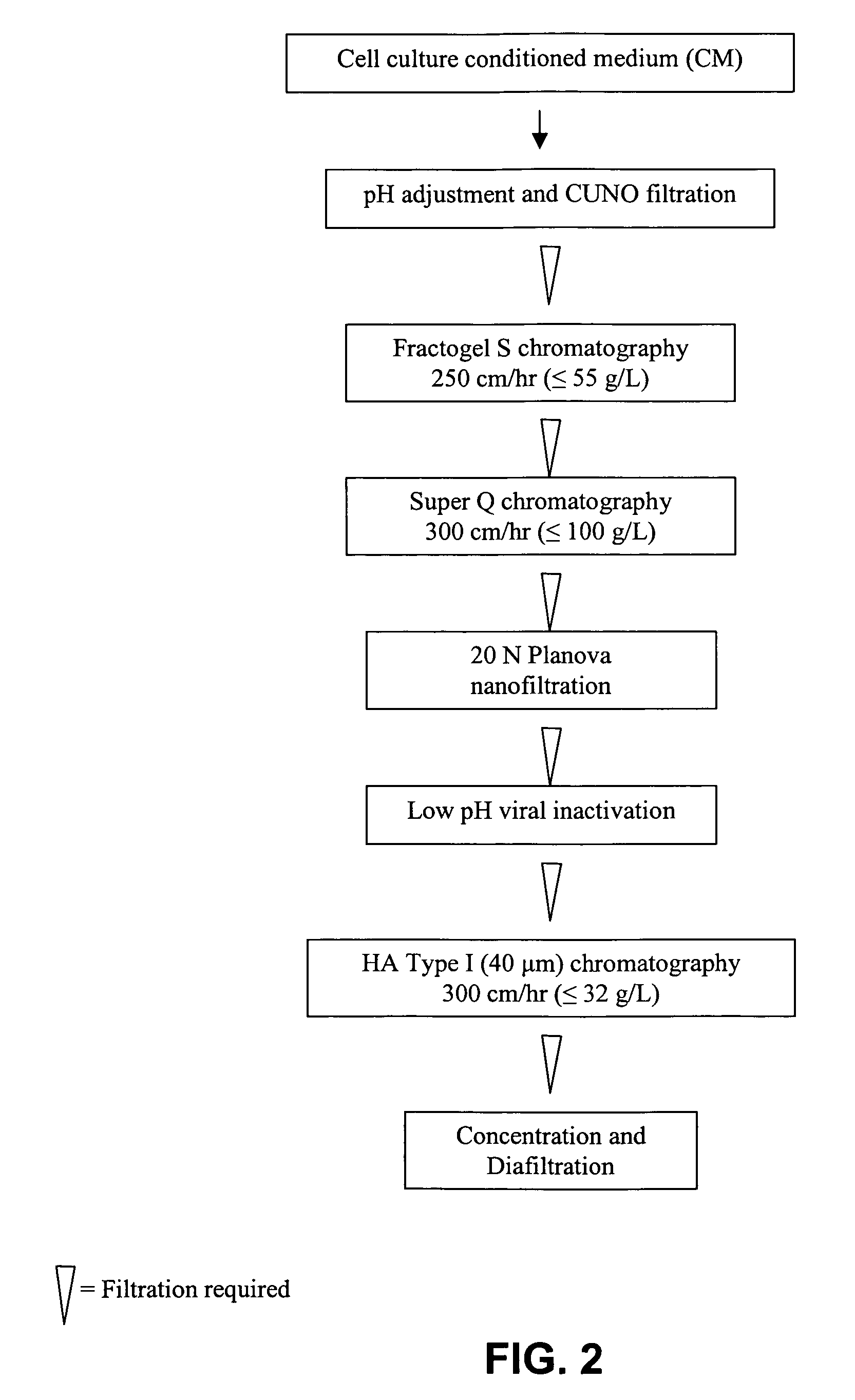
Stabilized anti-respiratory syncytial virus (RSV) antibody formulations
InactiveUS7425618B2Low to undetectable levelEasy to manageImmunoglobulins against virusesPharmaceutical delivery mechanismInorganic saltsAntibody fragments
The present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to a respiratory syncytial virus (RSV) antigen, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to a RSV antigen, which formulations are substantially free of surfactant, inorganic salts, and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating one or more symptoms associated with RSV infection utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
Stabilized anti-respiratory syncytial virus (RSV) antibody formulations
InactiveUS20040018200A1Low to undetectable levelImproved pharmacokinetic profileImmunoglobulins against virusesPharmaceutical delivery mechanismInorganic saltsAntibody fragments
The present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to a respiratory syncytial virus (RSV) antigen, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to a RSV antigen, which formulations are substantially free of surfactant, inorganic salts, and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating one or more symptoms associated with RSV infection utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
Integrin antagonists with enhanced antibody dependent cell-mediated cytoxicity activity
InactiveUS20060040325A1Enhance ADCCAltered binding affinityCompound screeningApoptosis detectionIntegrin antagonistComplement-dependent cytotoxicity
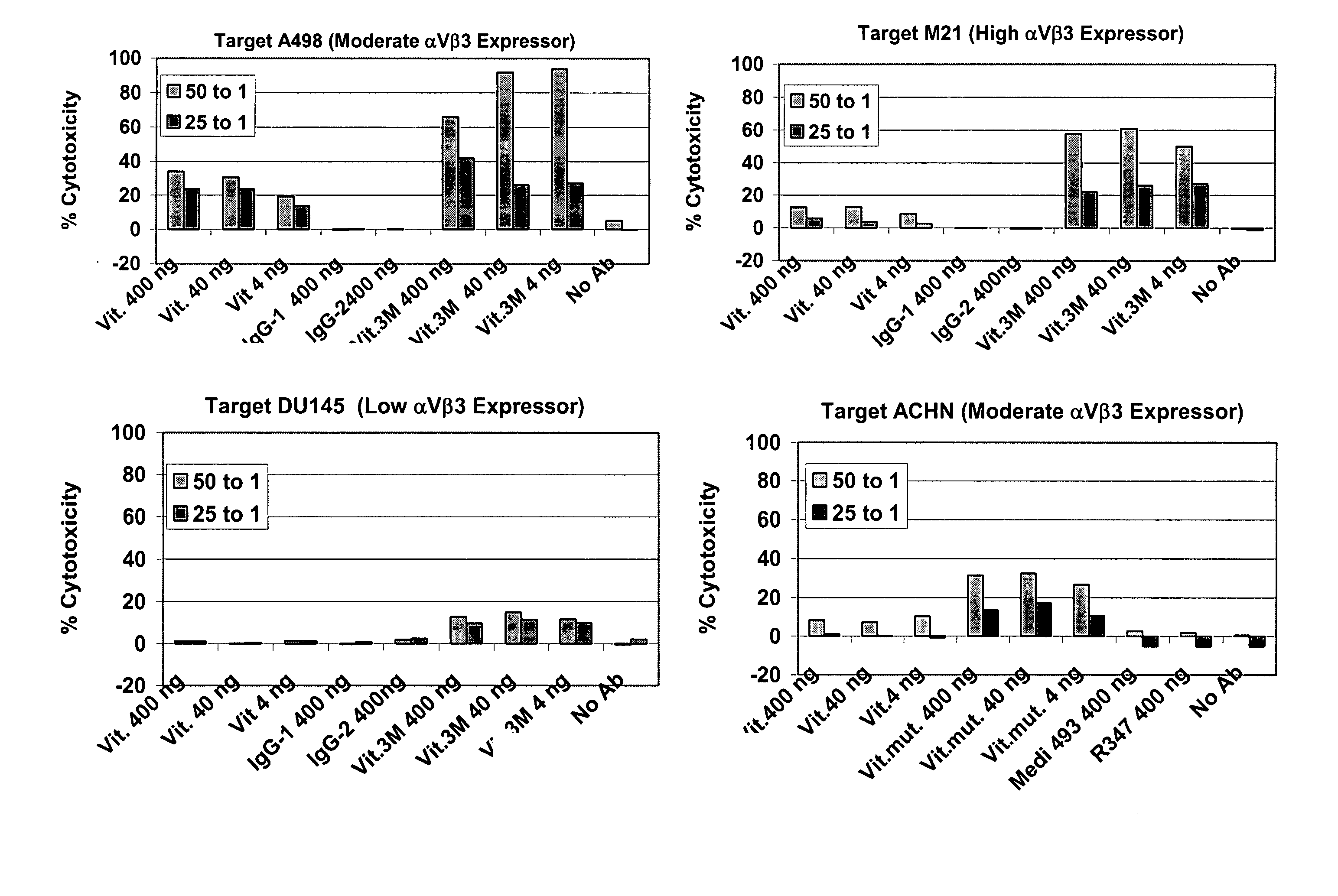
The present invention relates to novel Fc variants of antibodies that immunospecifically binds to Integrin αvβ3. The Fc variants comprise a variable region that immunospecifically binds to Integrin αvβ3 and a Fc region that further comprises at least one novel amino acid residue which may provide for enhanced effector function. More specifically, this invention provides Fc variants that have modified binding affinity to one or more FcγR and / or C1q. Additionally, the Fc variants have altered antibody dependent cell-mediated cytotoxicity (ADCC) and / or complement dependent cytotoxicity (CDC) activity. The invention further provides methods and protocols for the application of said Fc variants of an antibody that immunospecifically binds to Integrin αvβ3, particularly for therapeutic purposes.
Owner:MEDIMMUNE LLC
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS20030091584A1Reduce dosageLess frequent administrationViral antigen ingredientsMicrobiological testing/measurementSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection
Owner:MEDIMMUNE LLC
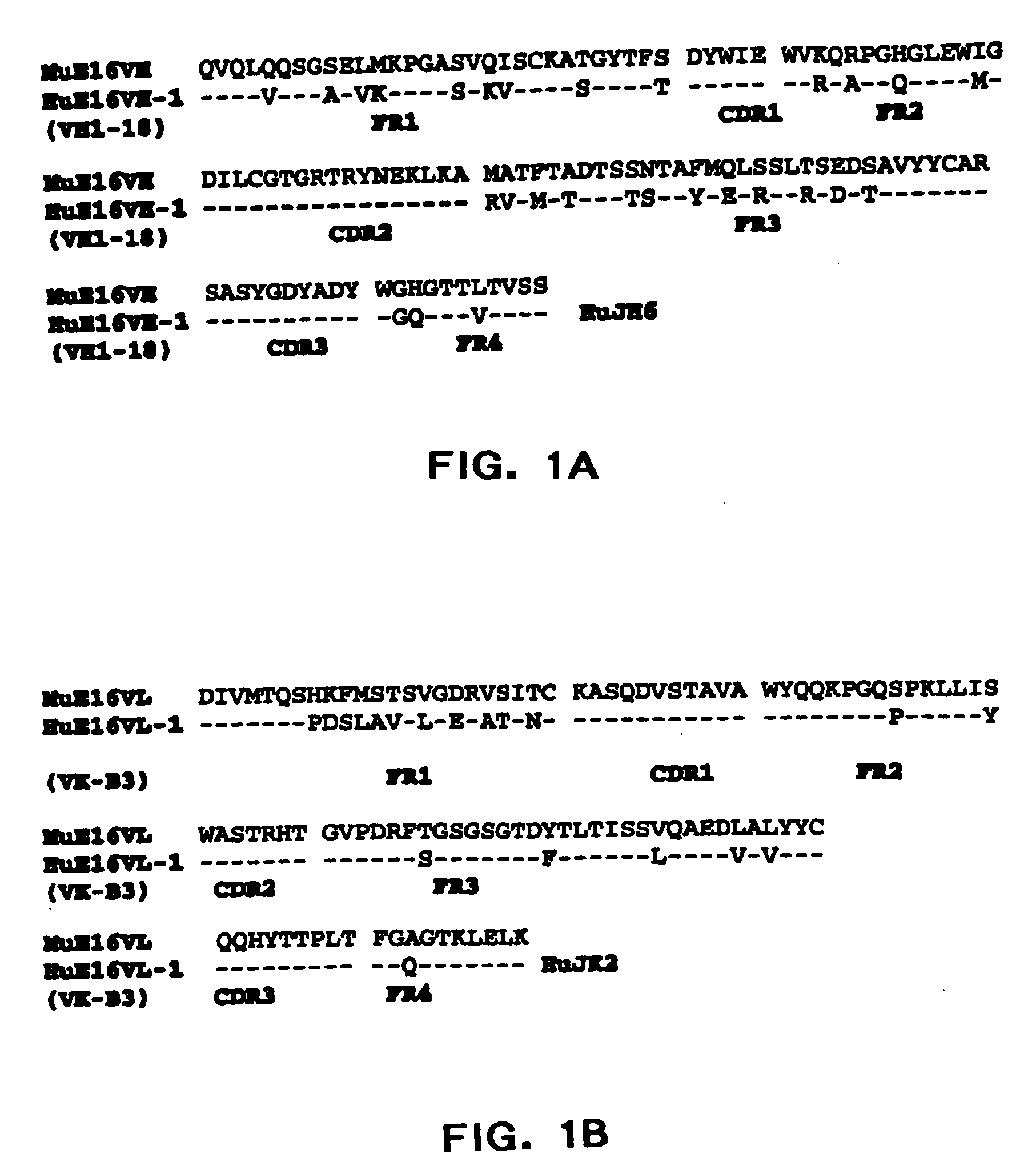
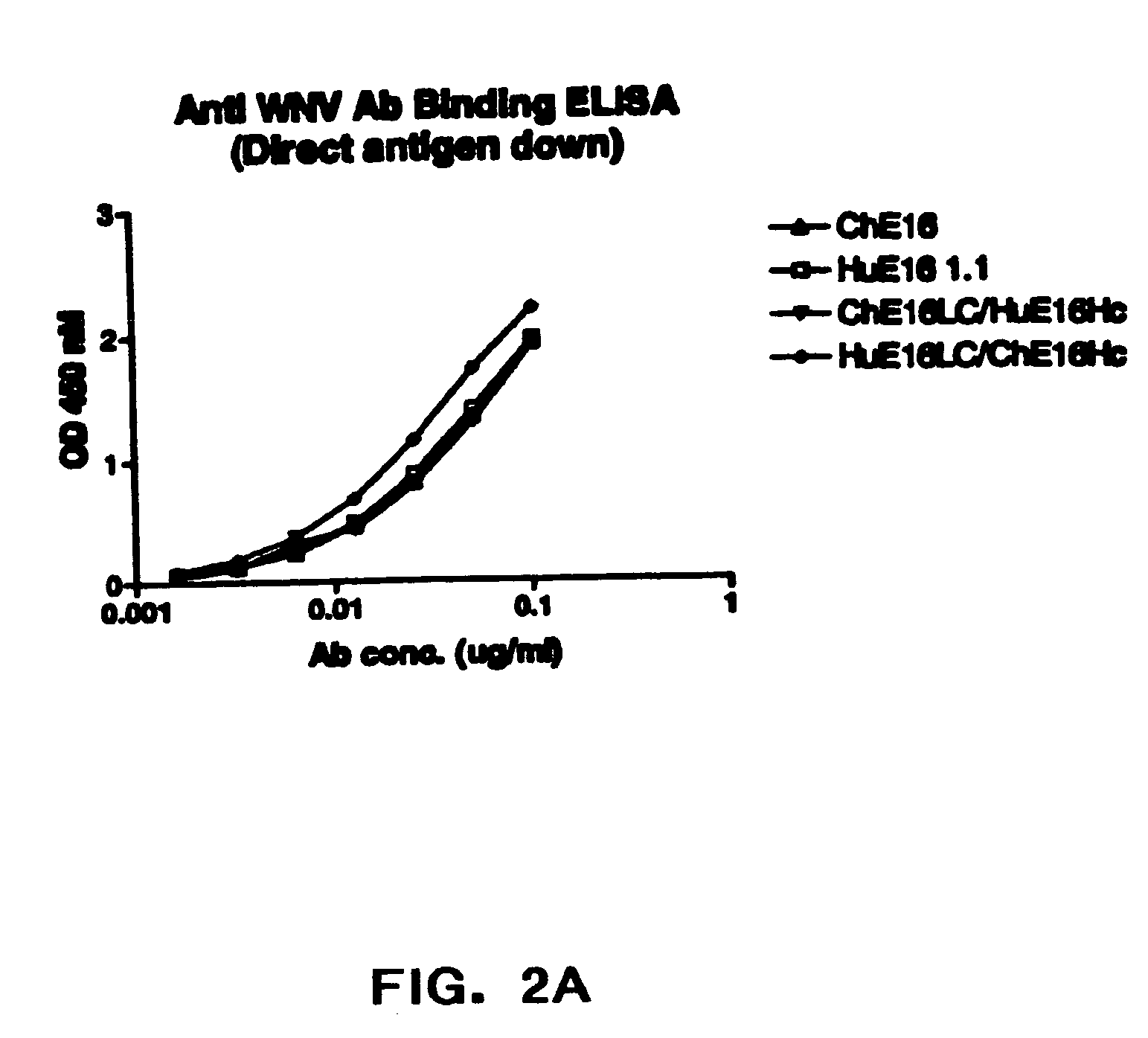
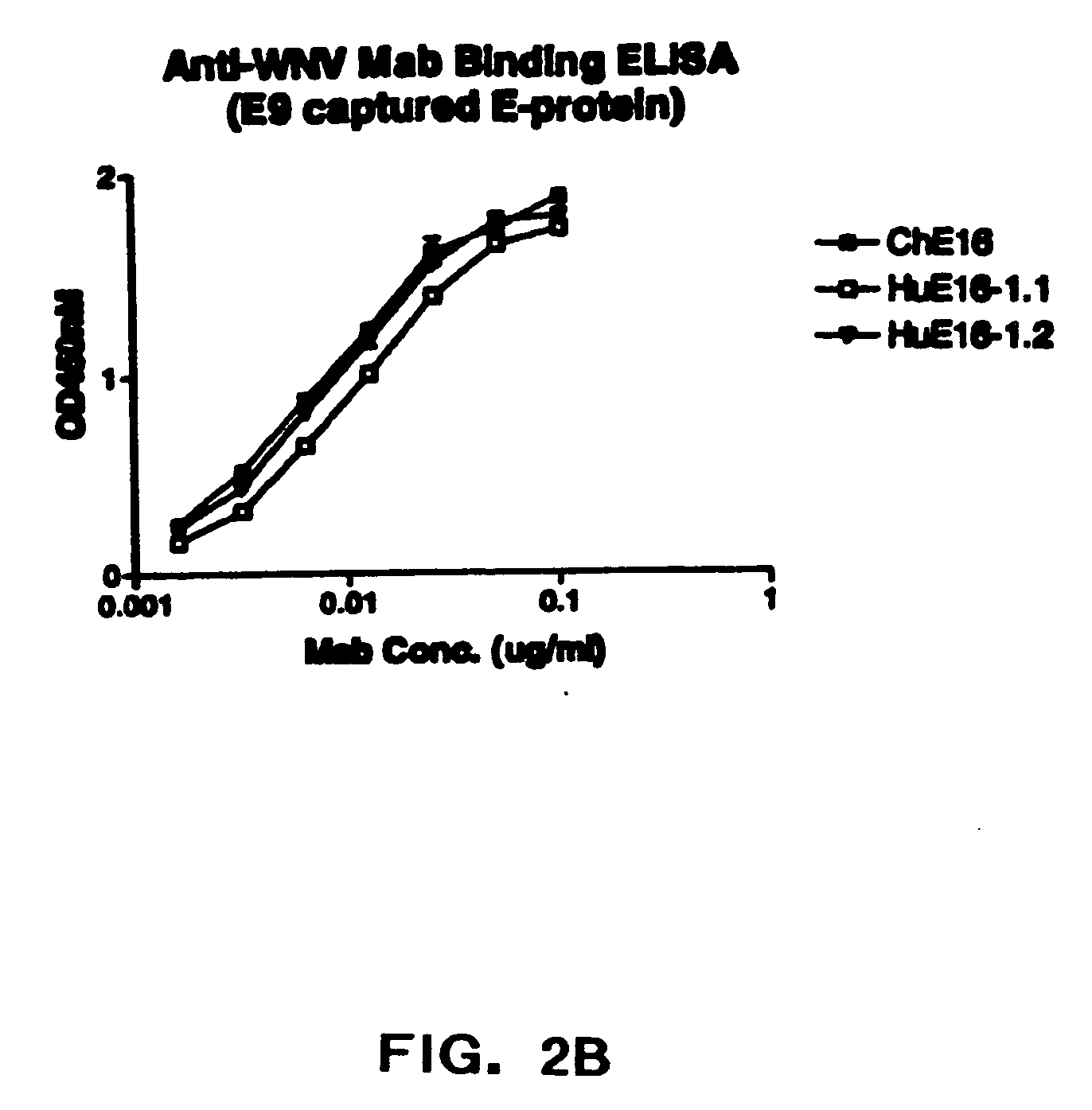
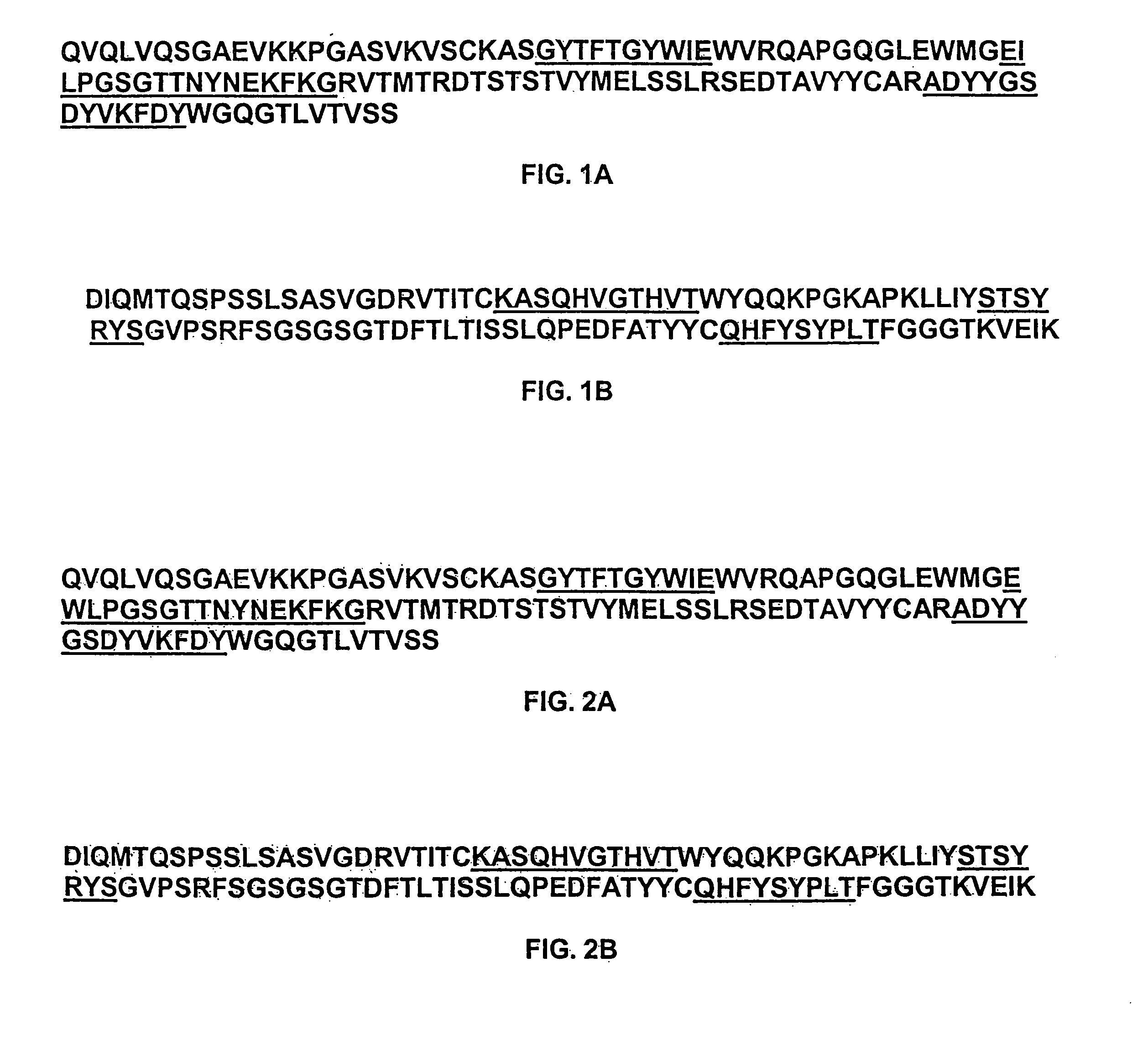
Humanized antibodies against West Nile Virus and therapeutic and prophylactic uses thereof
InactiveUS20060057149A1Easy to neutralizeEasy to removeCosmetic preparationsToilet preparationsHumanized antibodyViral infection
The present invention relates to compositions comprising humanized antibodies or fragments thereof that immunospecifically bind to one or more antigens of a flavivirus, particularly of West Nile Virus (WNV) and methods for preventing, treating or ameliorating symptoms associated with a flavivirus, particularly of West Nile Virus (WNV) infection utilizing said compositions. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with WNV infection, said methods comprising administering to a human subject an effective amount of one or more humanized antibodies or fragments thereof that immunospecifically bind to a WNV antigen. The present invention also relates to detectable or diagnostic compositions comprising humanized antibodies or fragments thereof that immunospecifically bind to a WNV antigen and methods for detecting or diagnosing WNV infection utilizing said compositions.
Owner:MACROGENICS INC
Recombinant IL-9 antibodies
InactiveUS7354584B2Reduces function and activity and expressionReduced activityAntibacterial agentsAntimycoticsAutoimmune diseaseImmunologic specificity
The present invention provides novel antibodies that immunospecifically bind to an IL-9 polypeptide and compositions comprising said antibodies. The present invention also provides methods and compositions preventing, treating, managing, and / or ameliorating diseases and disorders associated with aberrant expression and / or activity of IL 9 or IL-9 receptor or subunits thereof, autoimmune diseases, inflammatory diseases, proliferative diseases, and infections comprising administration of one or more antibodies thereof that immunospecifically bind to an IL-9 polypeptide. The invention also encompasses methods and compositions for diagnosing, monitoring, and prognosing these disorders. The present invention further relates to articles of manufacture and kits comprising antibodies that immunospecifically bind to an IL-9 polypeptide.
Owner:MEDIMMUNE LLC
Immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS7179900B2Reduce dosing frequencyReduce dosageSugar derivativesMicrobiological testing/measurementSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis of a RSV infection.
Owner:MEDIMMUNE LLC
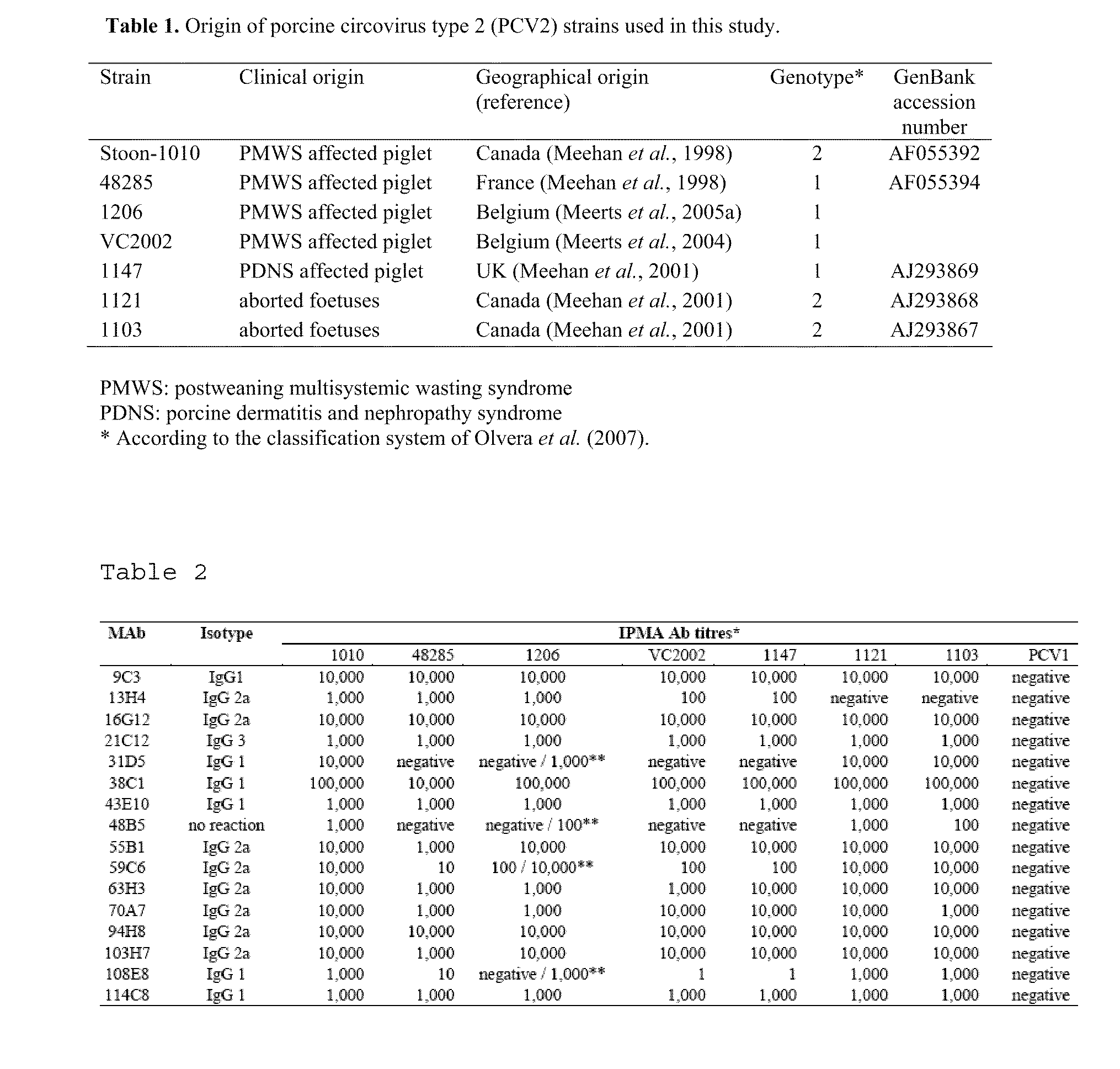
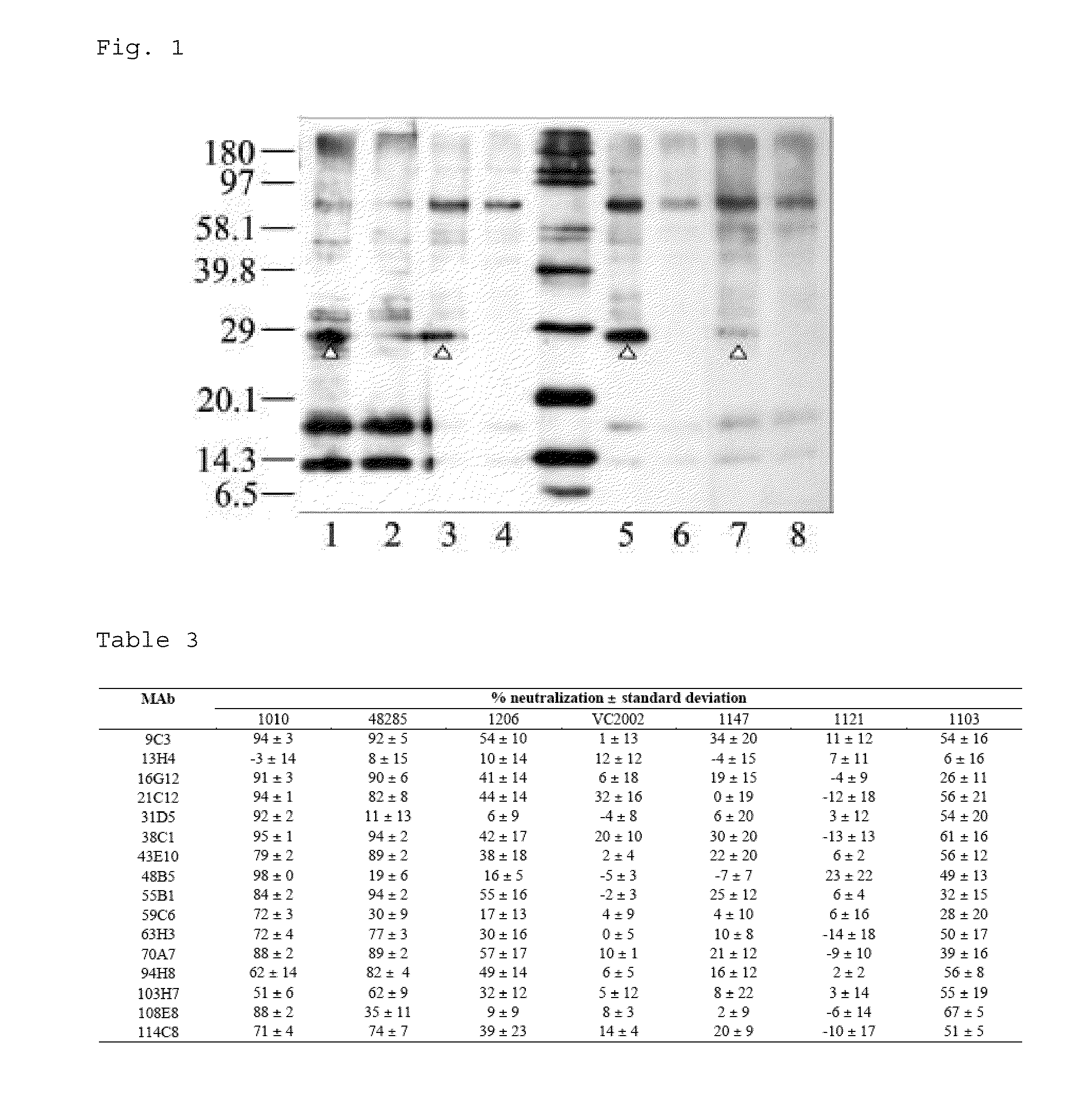
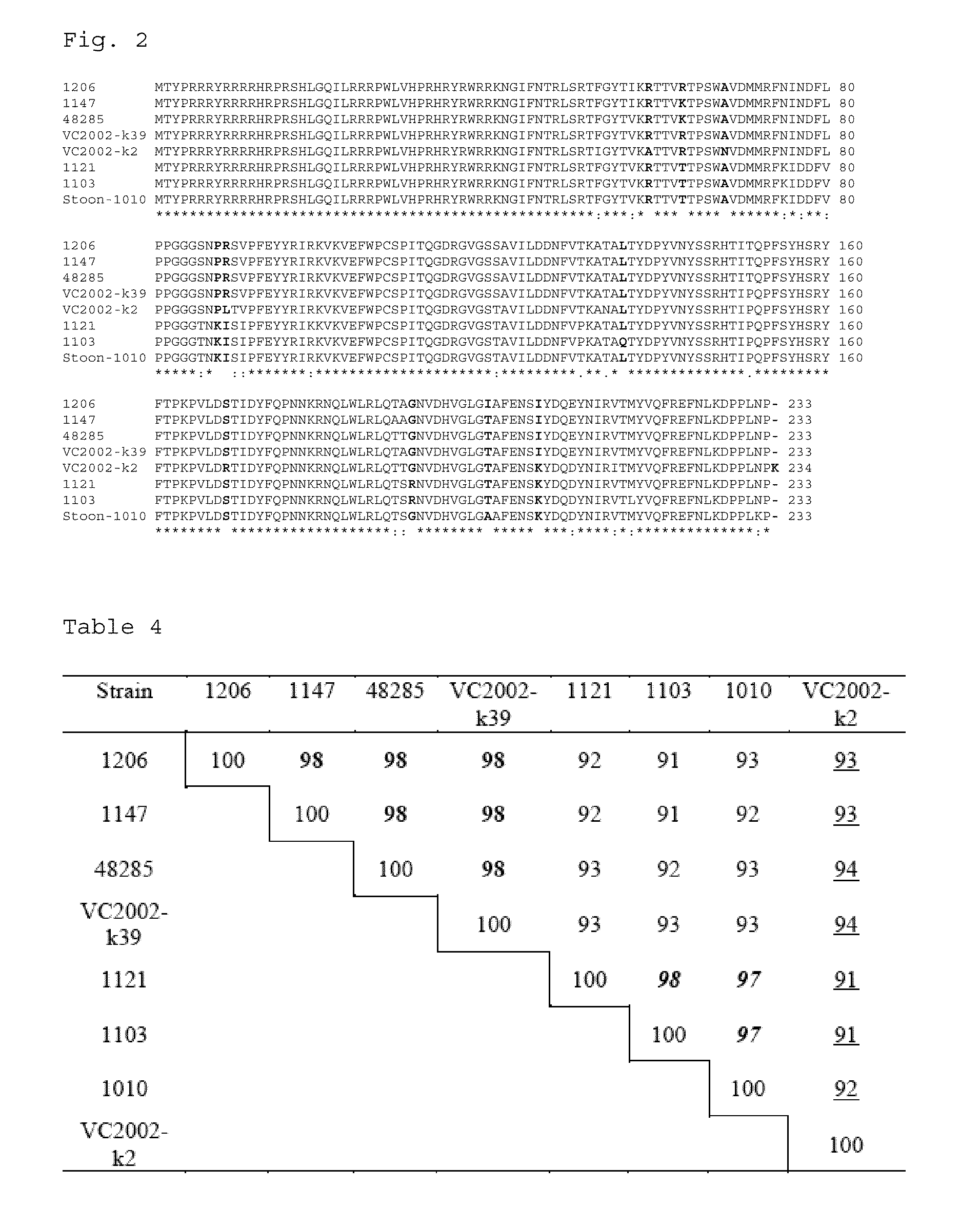
Methods and compositions in the treatment of porcine circoviral infection
InactiveUS20100184016A1Viral antigen ingredientsMicrobiological testing/measurementMammalPorcine circovirus
The invention relates generally to the field of virology. More particularly, the present invention relates to methods of diagnosing, prognosis, treatment and prevention of porcine circoviral infection in mammals, in particular of porcine circovirus type 2 (PCV2).Methods of using a nucleic acid(s) and / or a protein(s), which are immunogenic in said mammal, and antibodies immunospecific for said protein(s), to treat, diagnose and / or prevent said porcine circoviral infection, are provided for by the present invention.
Owner:UNIV GENT
Stabilized antibody formulations and uses thereof
InactiveUS20100129379A1Reduce decreaseEasy to manageAntipyreticAnalgesicsImmunologic specificityDrug biological activity
The present invention provides methods of optimizing certain stable liquid formulations of antibodies that immunospecifically bind to antigens of interest. Such formulations are suitable for parenteral administration to a subject, and exhibit increased stability, low to undetectable levels of aggregation, low to undetectable levels of antibody fragmentation / degradation, and very little to no loss of the biological activities of the antibodies, even during long periods of storage. The methods of the invention provide formulations that offer multiple advantages over formulations produced by non-optimized methods, including less stringent or more readily available transportation and storage conditions, less frequent dosing, and / or smaller dosage amounts in the therapeutic, prophylactic and diagnostic uses of such formulations. The invention further provides methods of identifying antibodies exhibiting certain phase behaviors such that the antibodies can be formulated by the methods of the invention. Also provided are prophylactic, therapeutic, and diagnostic uses of such antibody formulations.
Owner:MEDIMMUNE LLC +1
Stabilized high concentration anti-integrin alphanubeta3 antibody formulations
InactiveUS20040208870A1Low to undetectable levelEasy to manageBiocideOrganic active ingredientsHigh concentrationAntibody fragments
The present invention provides liquid formulations of antibodies or antibody fragments that immunospecifically bind to integrin alphaVbeta3, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to integrin alphaVbeta3, which formulations are substantially free of surfactant, inorganic salts, and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating an inflammatory disorder, an autoimmune disorder, a disorder associated with aberrant expression and / or activity of integrin alphaVbeta3, a disorder associated with abnormal bone metabolism, a disorder associated with aberrant angiogenesis or cancer utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
Antibody formulations having optimized aggregation and fragmentation profiles
InactiveUS20070110757A1Reduce aggregationReduce fragmentationAntibacterial agentsSenses disorderBiochemistryImmunologic specificity
The present invention provides methods of optimizing the production and purification of antibody formulations that immunospecifically bind to antigens of interest and are suitable for parenteral administration to a subject, which formulations exhibit increased stability due to reduced degradation and aggregation of the antibody component on long term storage. Such methods provide formulations that offer multiple advantages over formulations produced by non-optimized methods including less stringent or more readily available transportation / storage conditions, and less frequent dosing or smaller dosage amounts in the therapeutic, prophylactic and diagnostic use of such formulations. The invention further provides methods of utilizing the formulations of the present invention.
Owner:MEDIMMUNE LLC
Recombinant il-9 antibodies and uses thereof
InactiveUS20090047277A1Reduces function and activity and expressionReduced activityAntibacterial agentsNervous disorderAutoimmune conditionAutoimmune disease
The present invention provides novel antibodies that immunospecifically bind to an IL-9 polypeptide and compositions comprising said antibodies. The present invention also provides methods and compositions preventing, treating, managing, and / or ameliorating diseases and disorders associated with aberrant expression and / or activity of IL 9 or IL-9 receptor or subunits thereof, autoimmune diseases, inflammatory diseases, proliferative diseases, and infections comprising administration of one or more antibodies thereof that immunospecifically bind to an IL-9 polypeptide. The invention also encompasses methods and compositions for diagnosing, monitoring, and prognosing these disorders. The present invention further relates to articles of manufacture and kits comprising antibodies that immunospecifically bind to an IL-9 polypeptide.
Owner:MEDIMMUNE LLC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20070082007A1Avoid infectionInhibition of colonizationAntibacterial agentsNervous disorderDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:ZLOTNICK GARY W +5
Composition and method for treating lupus nephritis
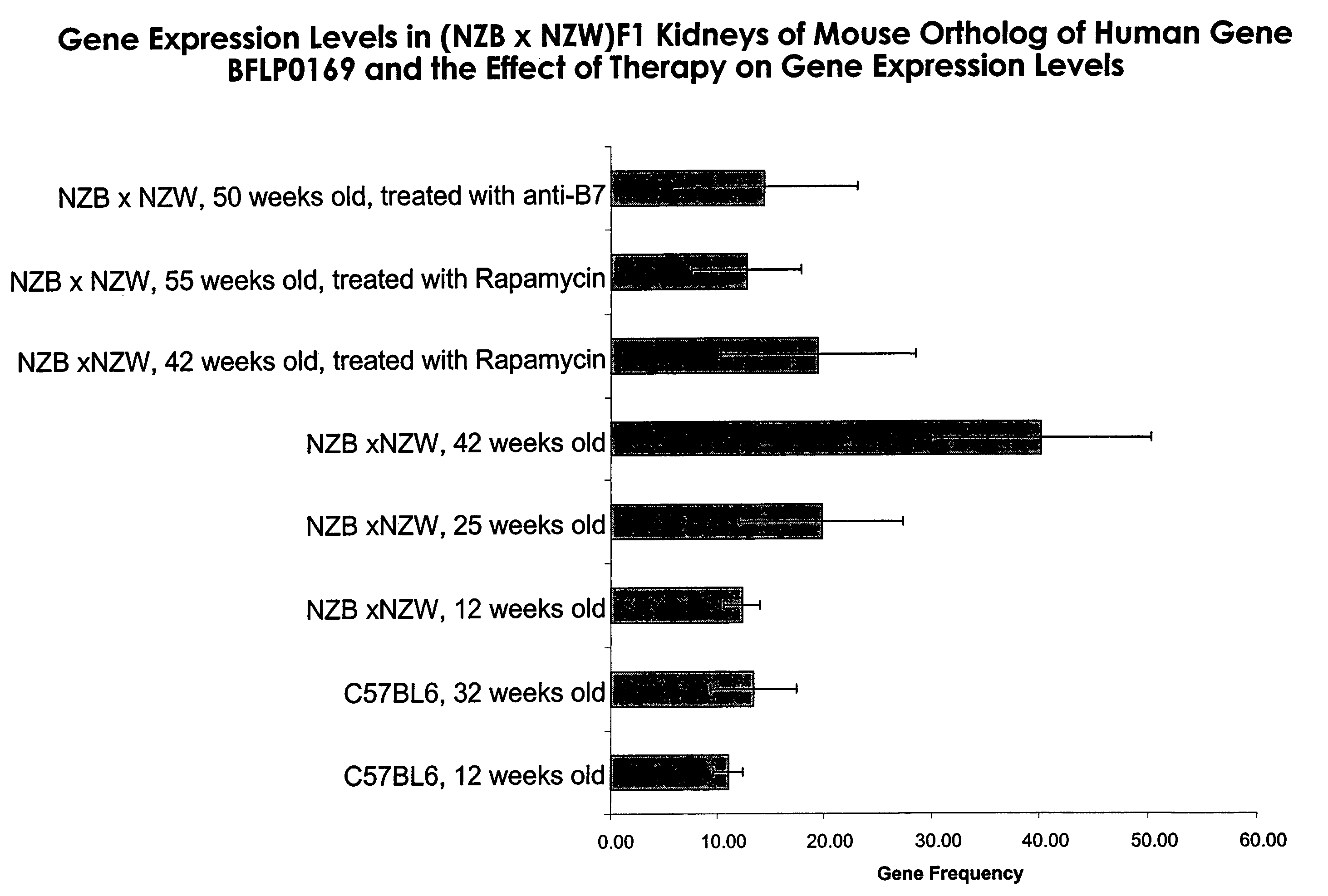
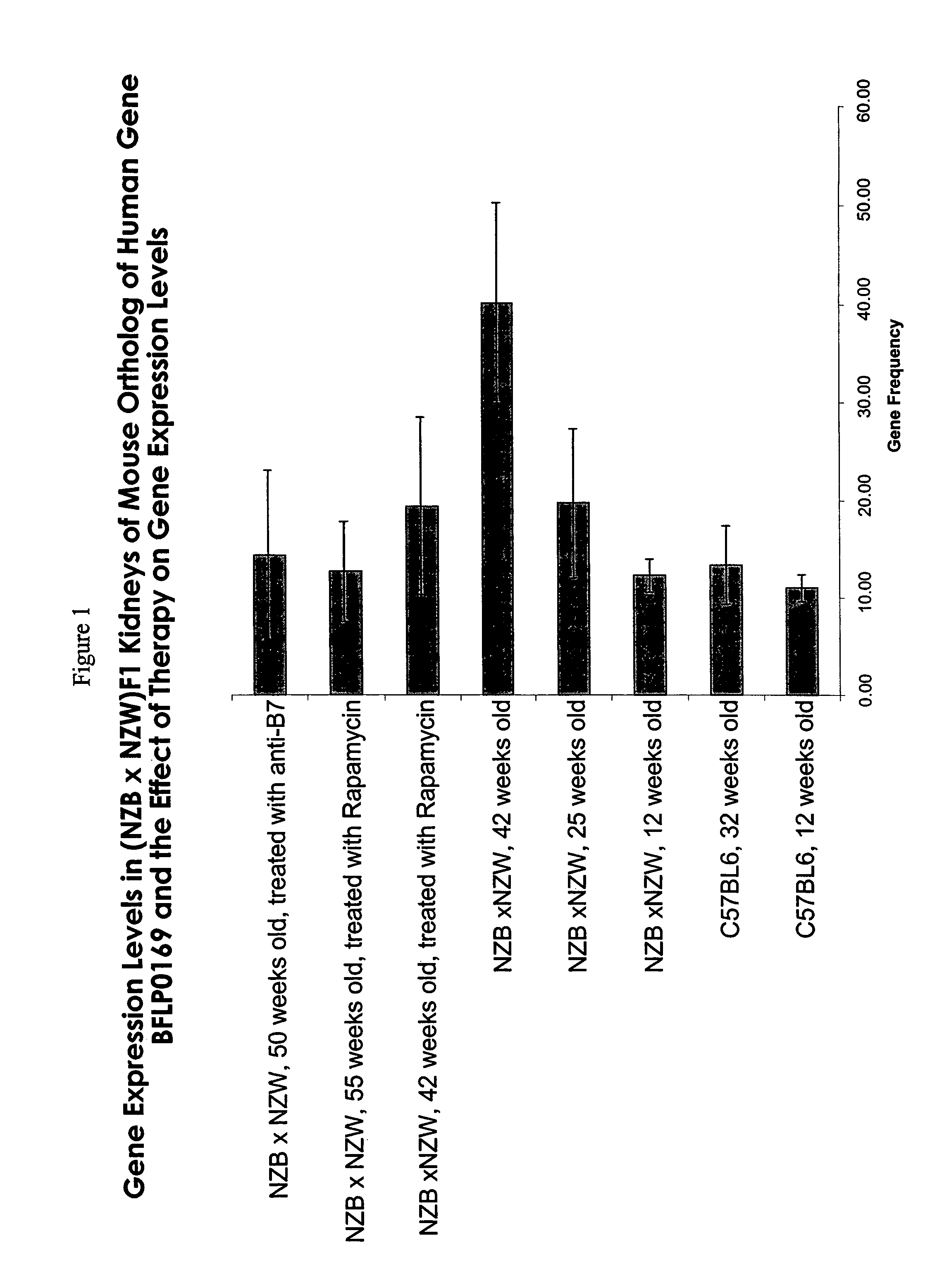
InactiveUS7060797B2Symptoms improvedImprove expression levelPeptide/protein ingredientsTissue culturePolynucleotideImmunologic specificity
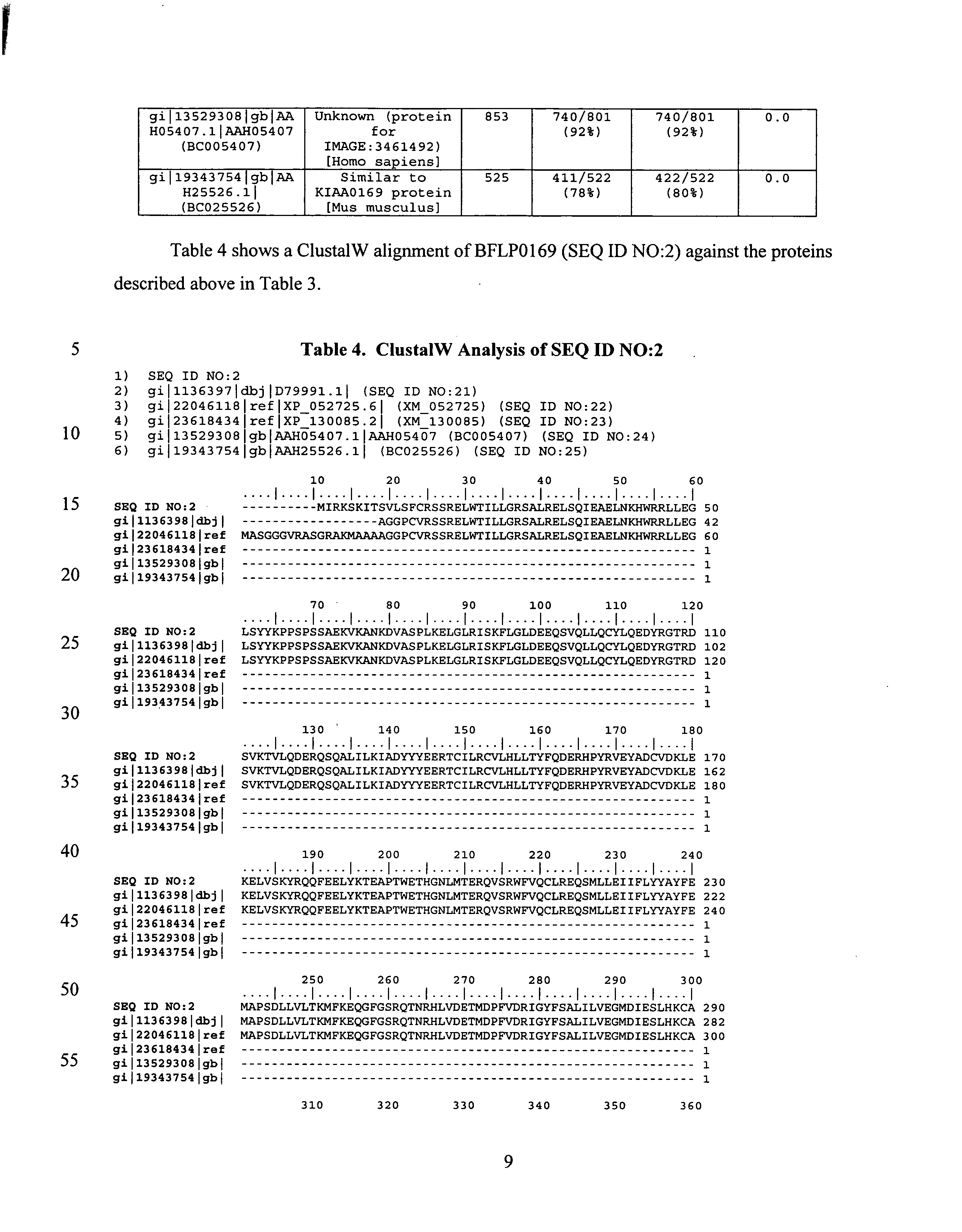
The present invention provides novel isolated BFLP0169 polynucleotides and polypeptides encoded by the BFLP0169 polynucleotides. Also provided are the antibodies that immunospecifically bind to a BFLP0169 polypeptide or any derivative (including fusion derivative), variant, mutant or fragment of the BFLP0169 polypeptide, polynucleotide or antibody. The invention additionally provides methods in which the BFLP0169 polypeptide, polynucleotide and antibody are utilized in the detection and treatment of a broad range of pathological states, as well as to other uses.
Owner:WYETH
Antibodies against and methods for producing vaccines for respiratory syncytial virus
ActiveUS20060099220A1Reduce fusionPrevents RSV infectionSsRNA viruses negative-sensePeptide/protein ingredientsRSV InfectionsRespiratory syncytial virus (RSV)
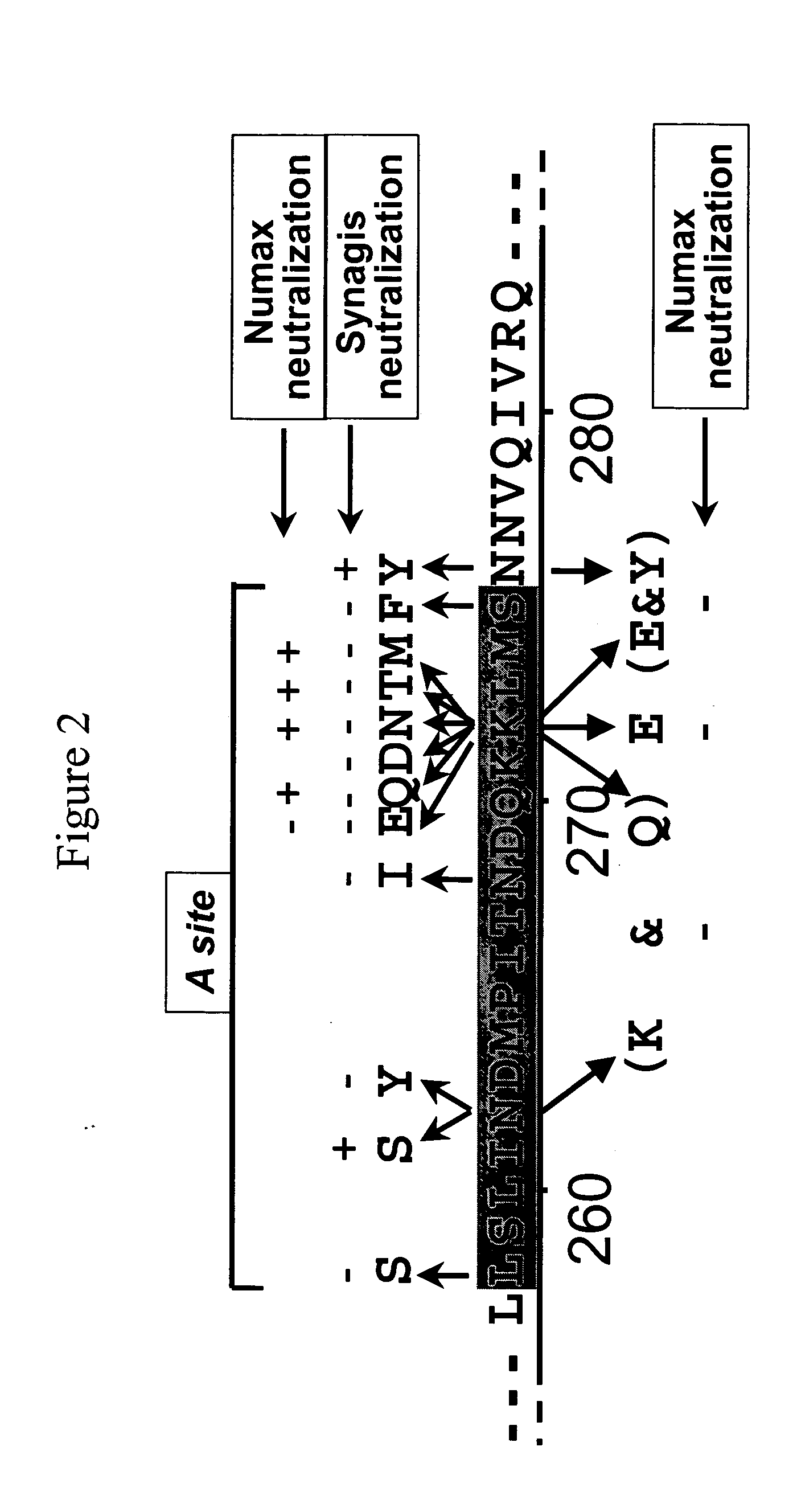
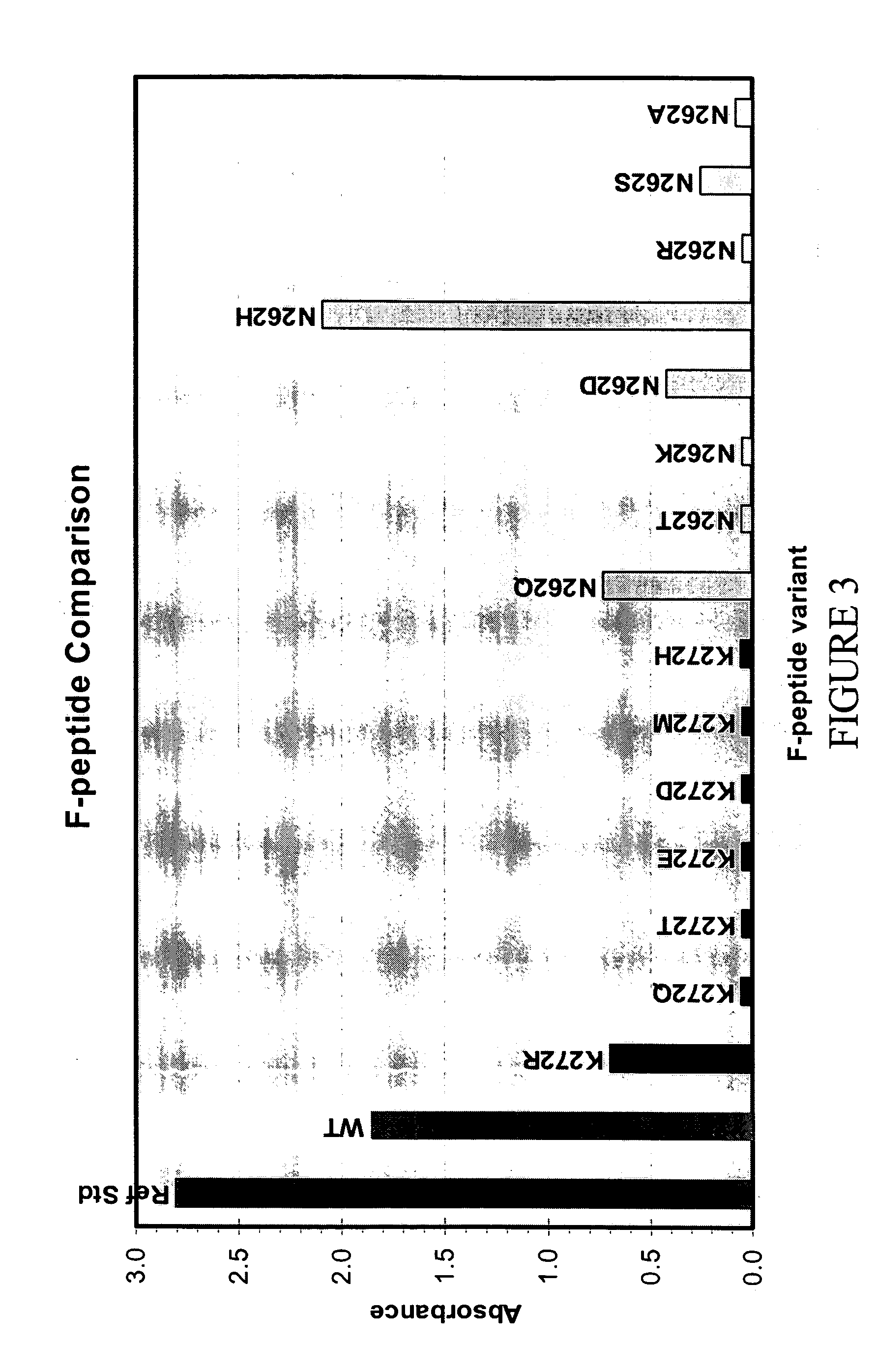
The present invention relates to novel respiratory syncytial virus (RSV) F peptides and compositions comprising them. The present invention also relates to methods of evaluating anti-RSV antibody binding to F peptides. The present invention also relates to antibodies that immunospecifically bind to an F peptide of the present invention. The invention further provides methods and protocols for the administration of F peptides and / or antibodies that immunospecifically bind to F peptides for the prevention, neutralization, treatment of RSV infection. Additionally, the methods of the invention may be useful for the treatment, prevention and the amelioration of symptoms associated with RSV infection.
Owner:AREXIS
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20110076299A1Antibacterial agentsOrganic active ingredientsDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Eph receptor Fc variants with enhanced antibody dependent cell-mediated cytotoxicity activity
InactiveUS7659374B2Altered binding affinityHigh binding affinityCompound screeningApoptosis detectionComplement-dependent cytotoxicityImmunologic specificity
The present invention relates to novel Fc variants that immuno-specifically bind to an Eph receptor. The Fc variants comprise a binding region that immunospecifically binds to an Eph receptor and an Fc region that further comprises at least one novel amino acid residue which may provide for enhanced effector function. More specifically, this invention provides Fc variants that have modified binding affinity to one or more Fc ligand (e.g., FcγR, C1q). Additionally, the Fc variants have altered antibody-dependent cell-mediated cytotoxicity (ADCC) and / or complement dependent cytotoxicity (CDC) activity. The invention further provides methods and protocols for the application of said Fc variants that immunospecifically bind to an Eph receptor, particularly for therapeutic purposes.
Owner:MEDIMMUNE LLC
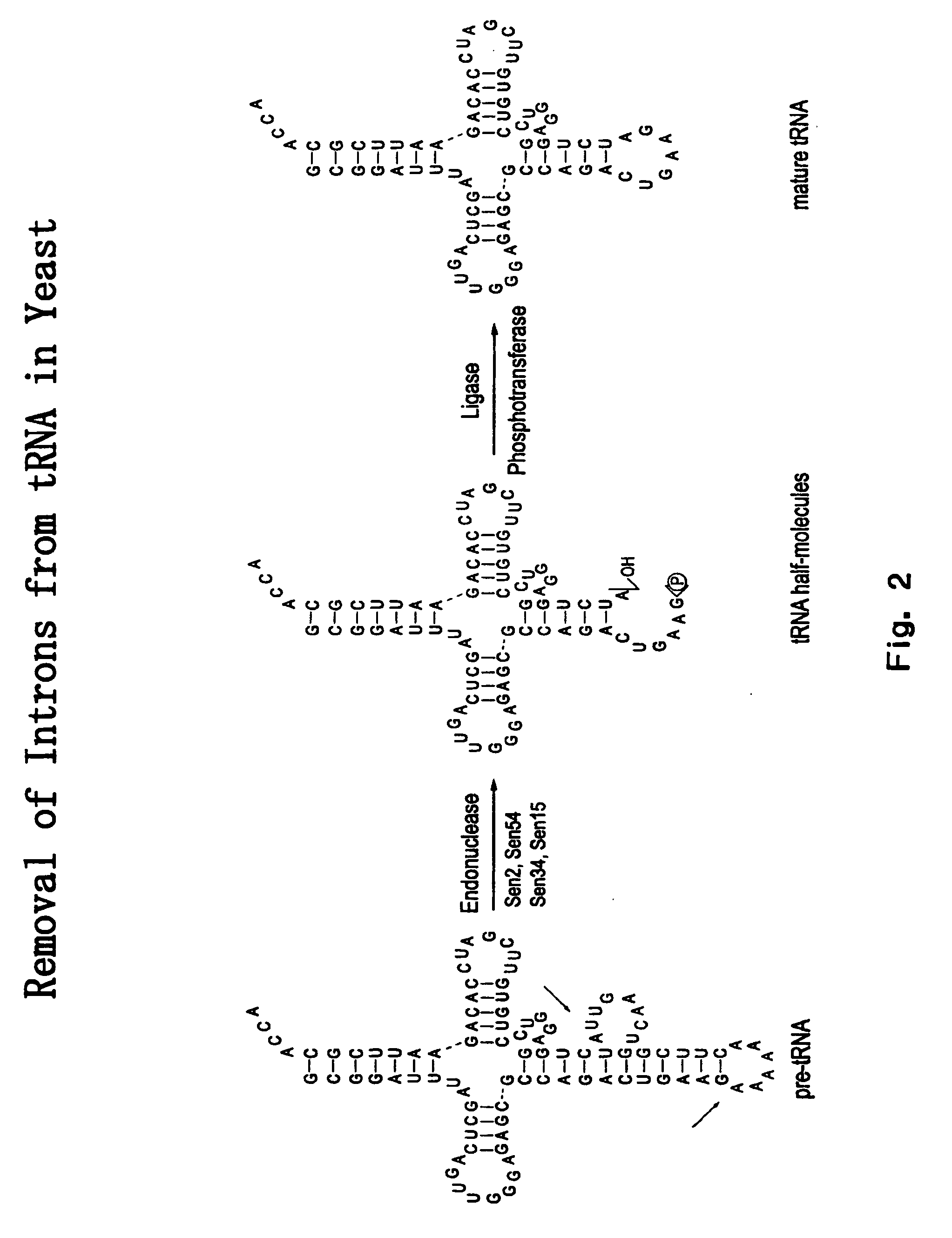
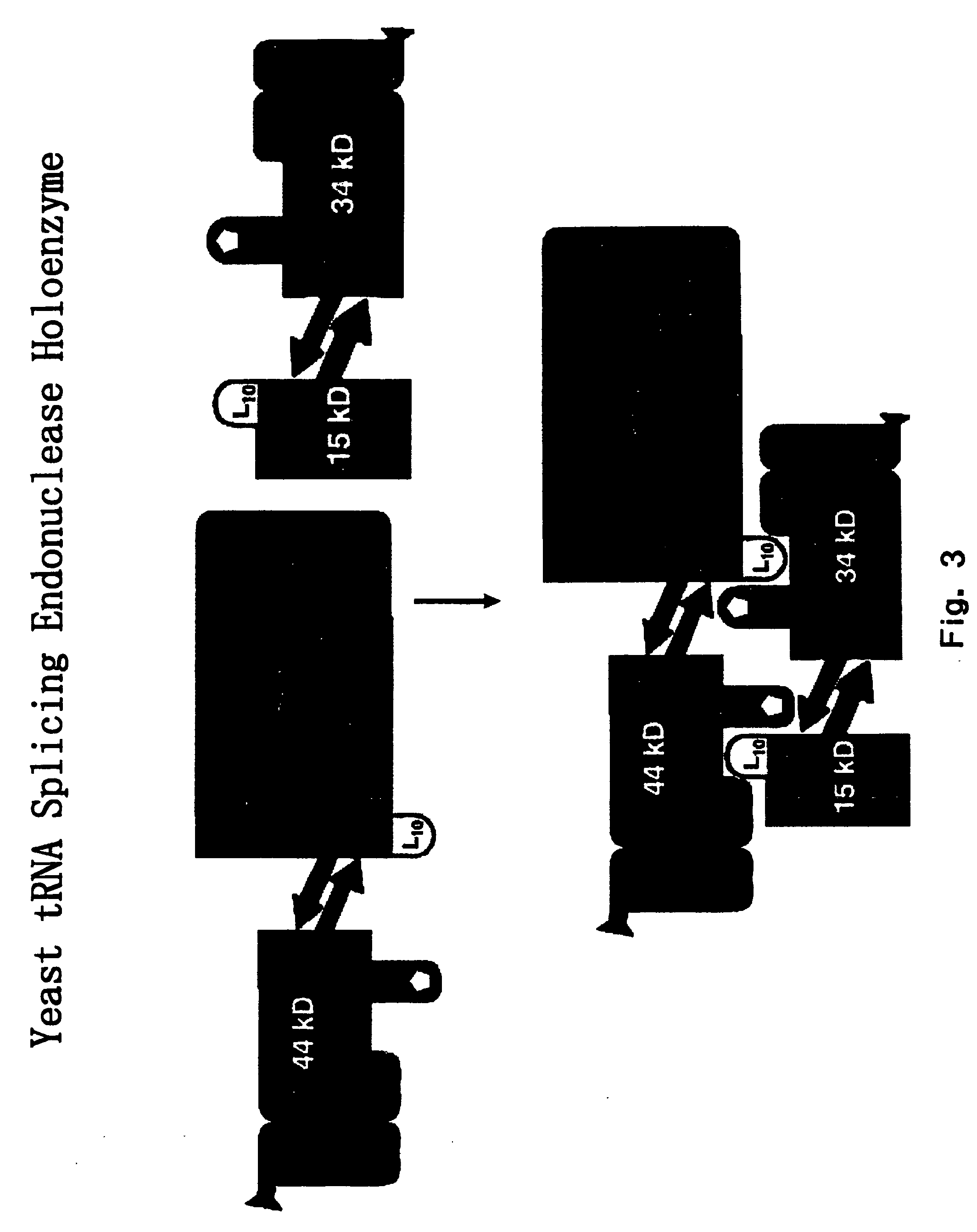
RNA processing protein complexes and uses thereof
InactiveUS20100136710A1Improve accuracyFidelity of of reducedSugar derivativesHydrolasesDiseaseProtein-protein complex
The invention provides human protein complexes with endonuclease activity. In particular, the invention provides human protein complexes with tRNA splicing endonuclease activity and / or 3′ end pre-mRNA endonuclease activity. The invention also provides a splice variant of human Sen2, namely human Sen2deltaEx8, and human protein complexes comprising human Sen2deltaEx8. The human Sen2deltaEx8 complexes have pre-tRNA cleavage activity and / or 3′ end pre-mRNA endonuclease activity. The invention also provides human protein complexes with pre-ribosomal RNA cleavage activity. The invention also provides antibodies that immunospecifically bind to a complex described herein or a component thereof, and methods of diagnosing, preventing, treating, managing or ameliorating a disorder utilizing such antibodies. The present invention also provides methods utilizing the complexes described herein, inter alia, in screening, diagnosis, and therapy. The invention further provides methods of preparing and purifying the complexes. The present invention further provides methods of identifying a compound that modulates the expression of a component of a complex described herein, the formation of a complex described herein or the activity of a complex described herein, and methods of preventing, treating, managing or ameliorating a disorder, such as a proliferative disorder, or a symptom thereof utilizing a compound identified in accordance with the methods.
Owner:PTC THERAPEUTICS INC
Methods of preventing and treating rsv infections and related conditions
Owner:MEDIMMUNE LLC
Novel Immunogenic Compositions for the Prevention and Treatment of Meningococcal Disease
InactiveUS20070253964A1Antibacterial agentsPeptide/protein ingredientsNucleic acid sequencingImmunogenicity
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from neisserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
Method of treating apoptosis and compositions thereof
InactiveUS7829662B2Peptide/protein ingredientsPeptide preparation methodsApoptosisNucleic acid sequencing
Disclosed herein are novel polypeptides and the nucleic acid sequences that encode them. Also disclosed are antibodies that immunospecifically bind to the polypeptide, as well as derivatives, variants, mutants, or fragments of the novel polypeptide, polynucleotide, or antibody specific to the polypeptide. Vectors, host cells, antibodies and recombinant methods for producing the polypeptides and polynucleotides, as well as methods for using same are also included. The invention further discloses therapeutic, diagnostic and research methods for diagnosis, treatment, and prevention of apoptosis associated disorders involving these novel human nucleic acids and proteins.
Owner:DANA FARBER CANCER INST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com