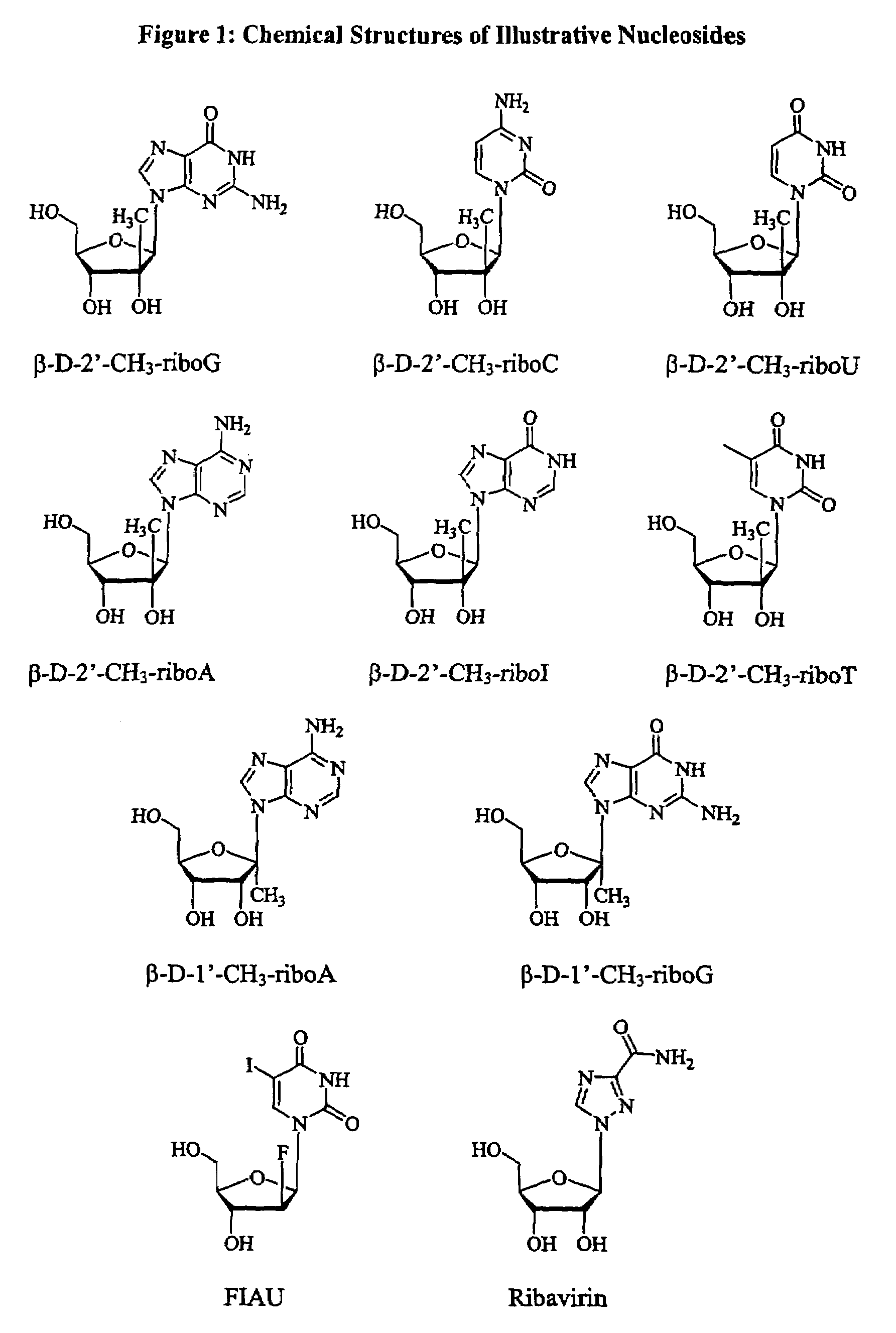
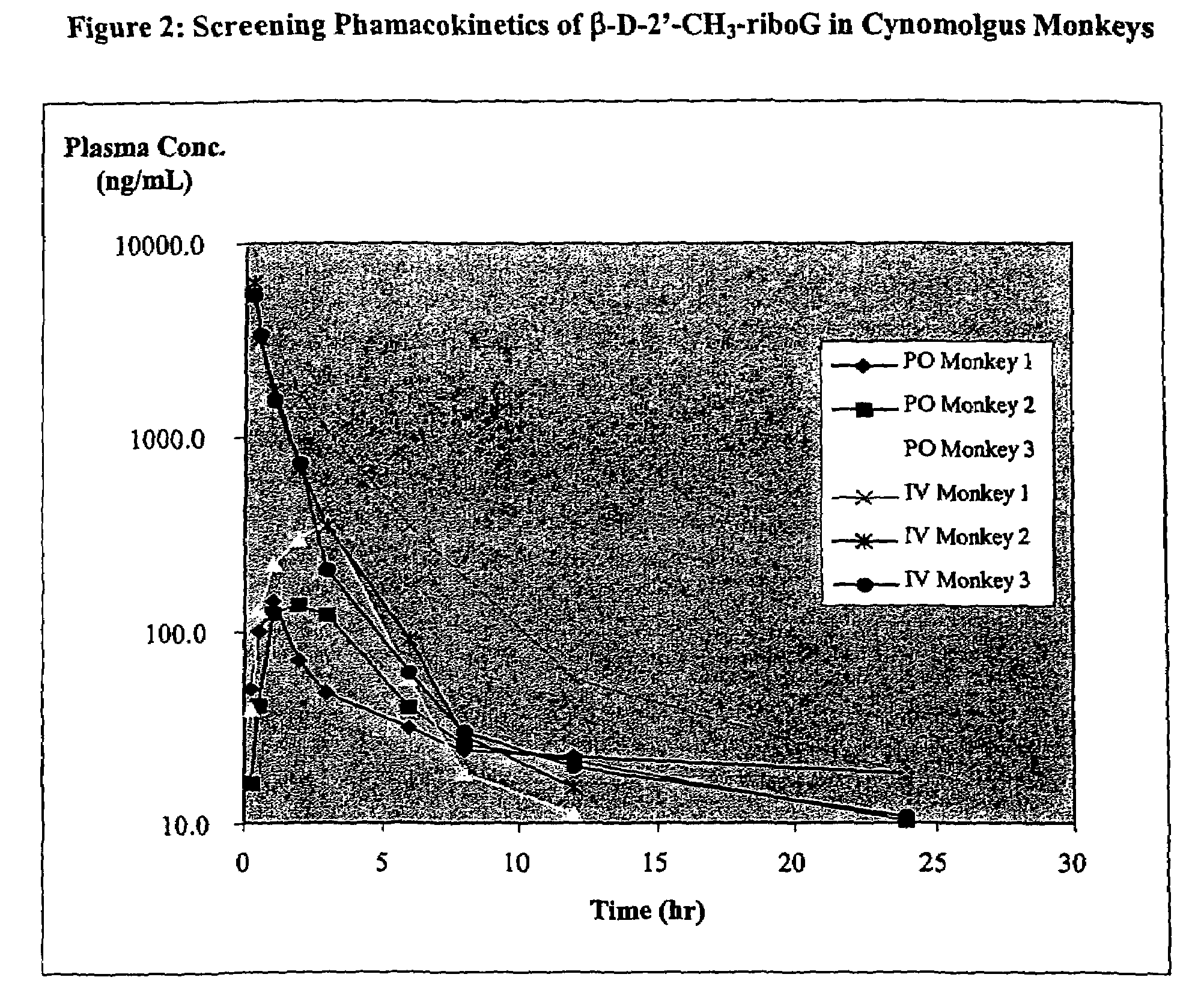
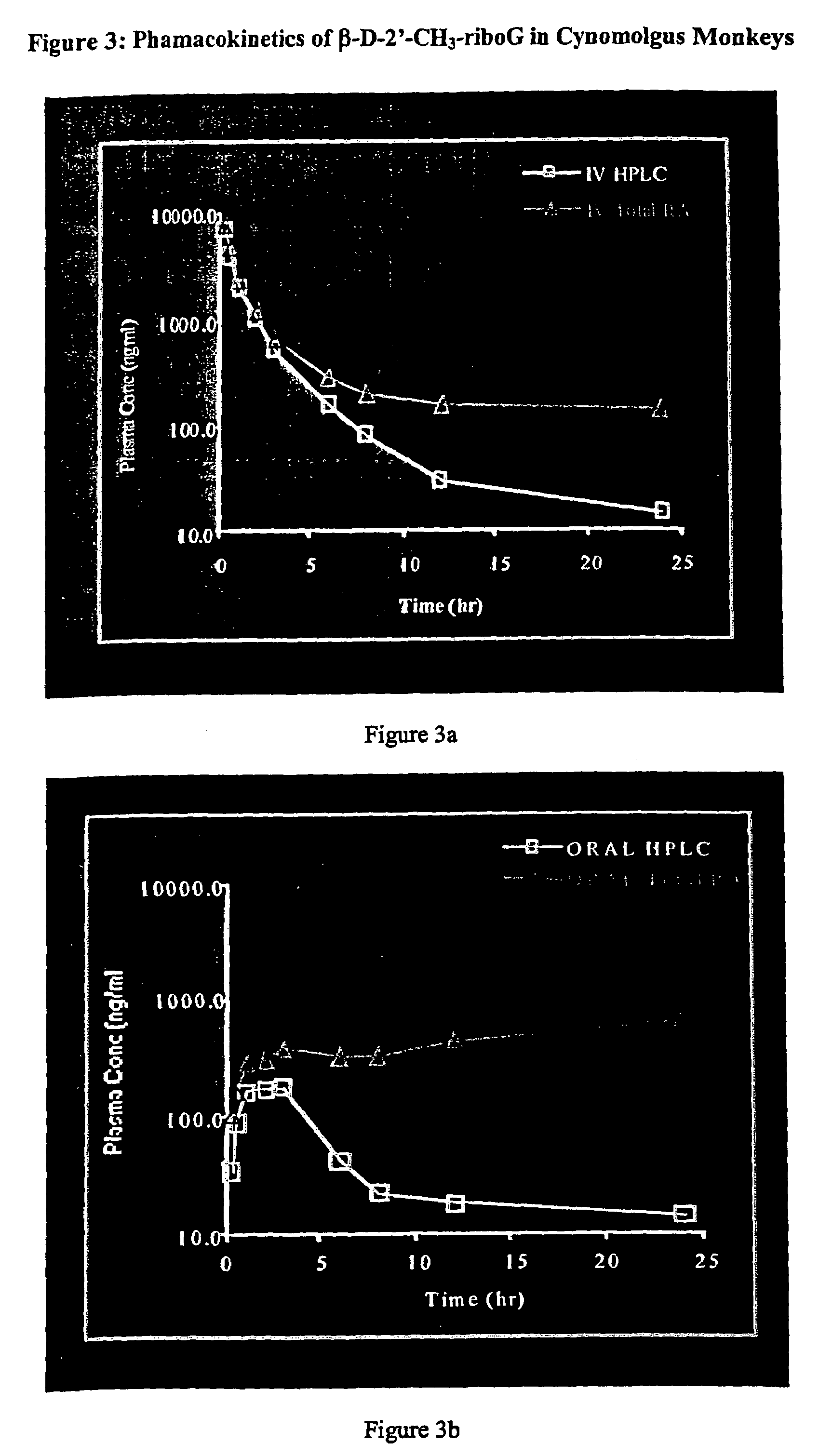
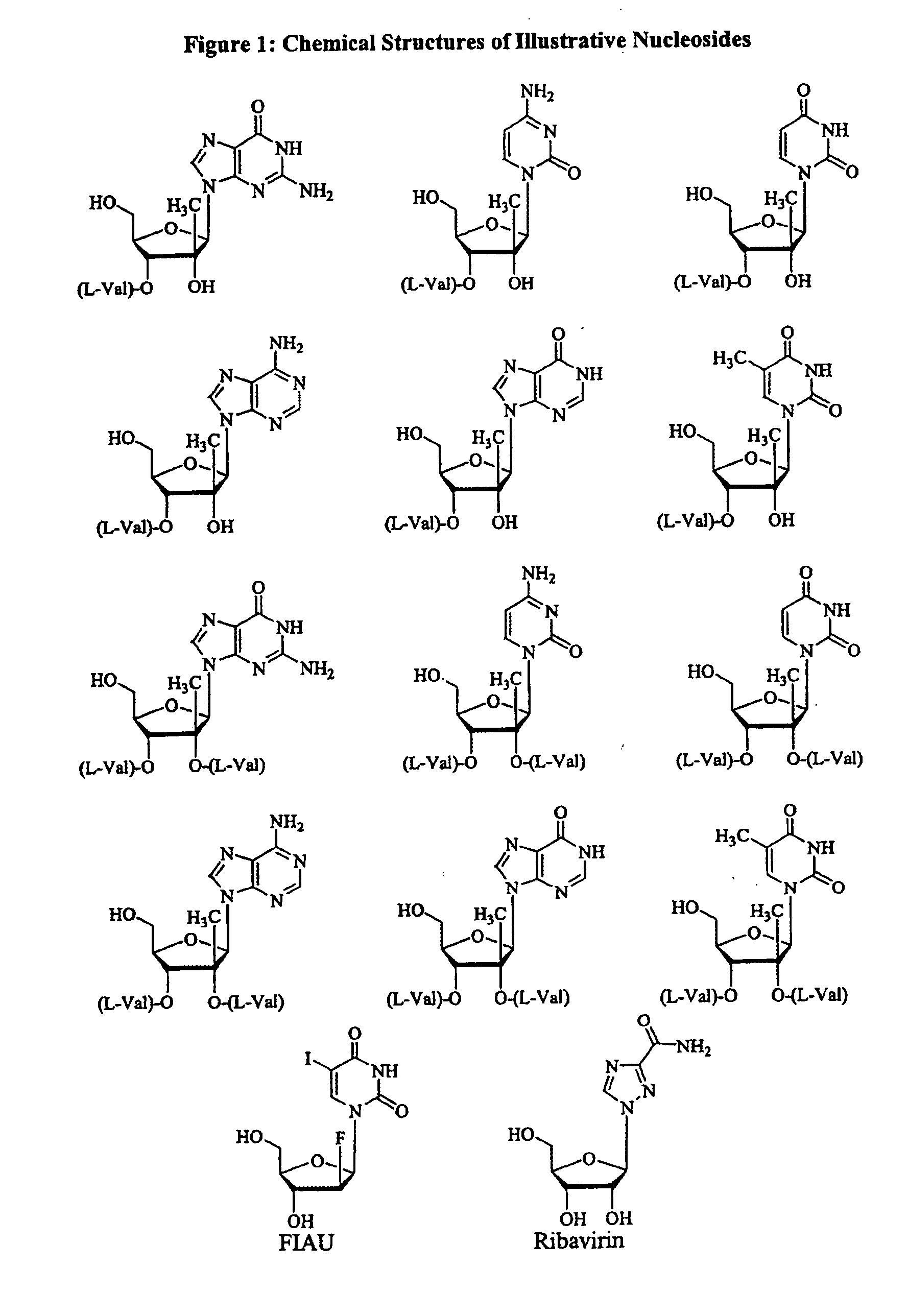
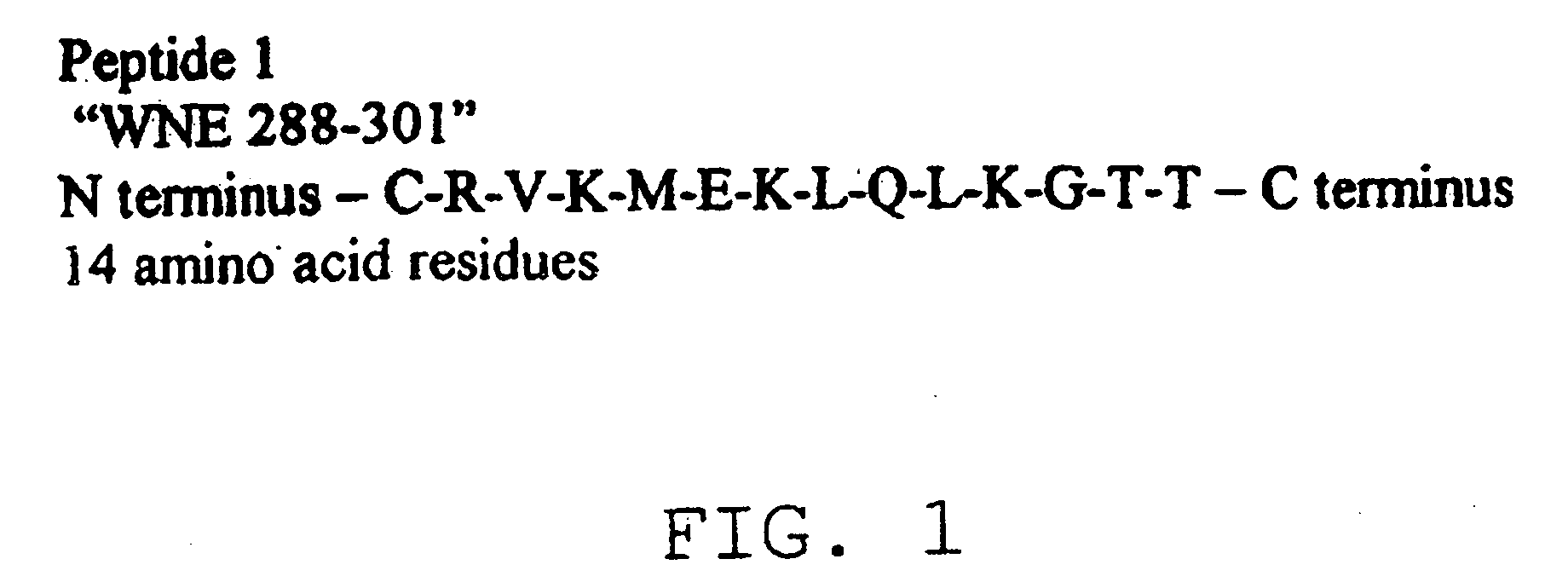
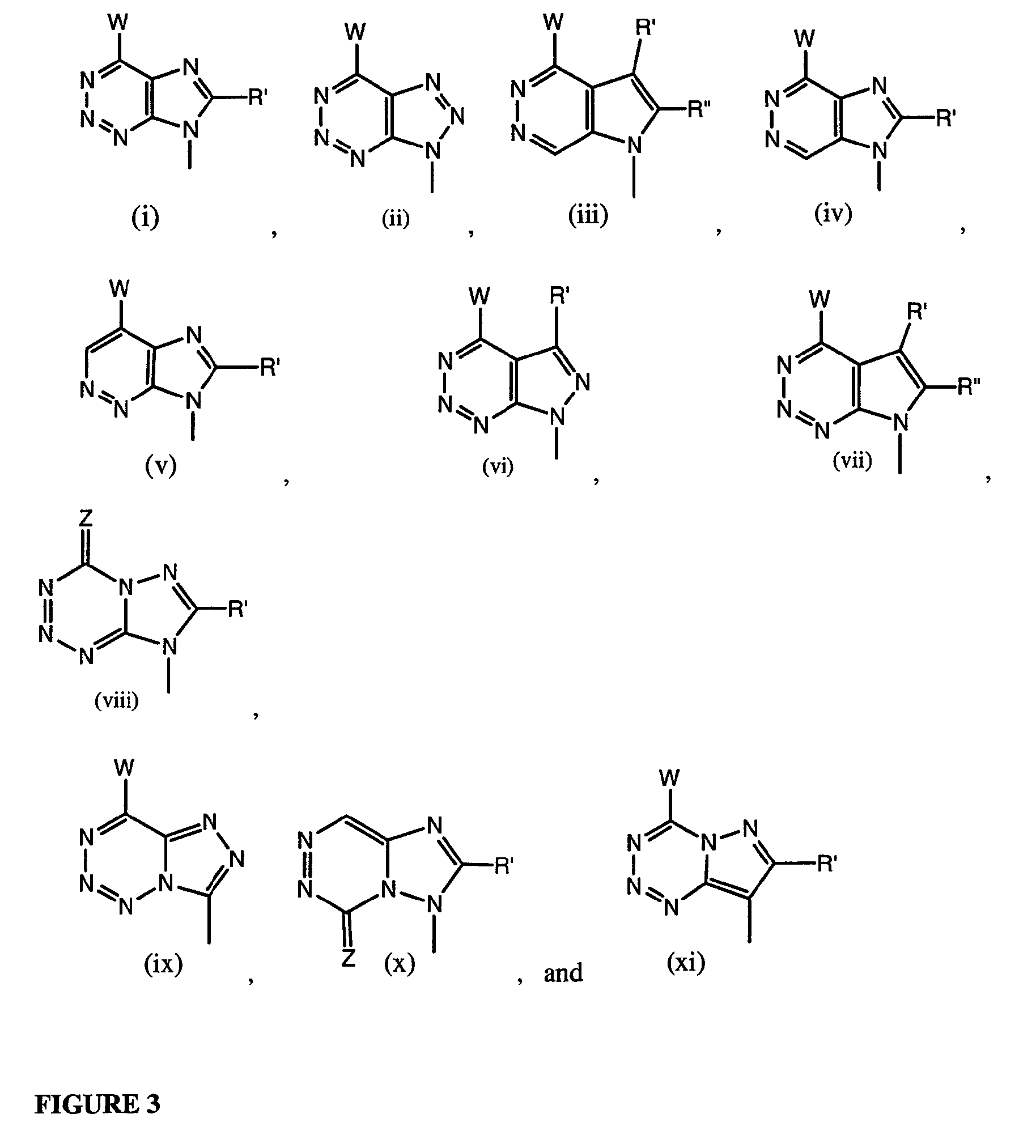
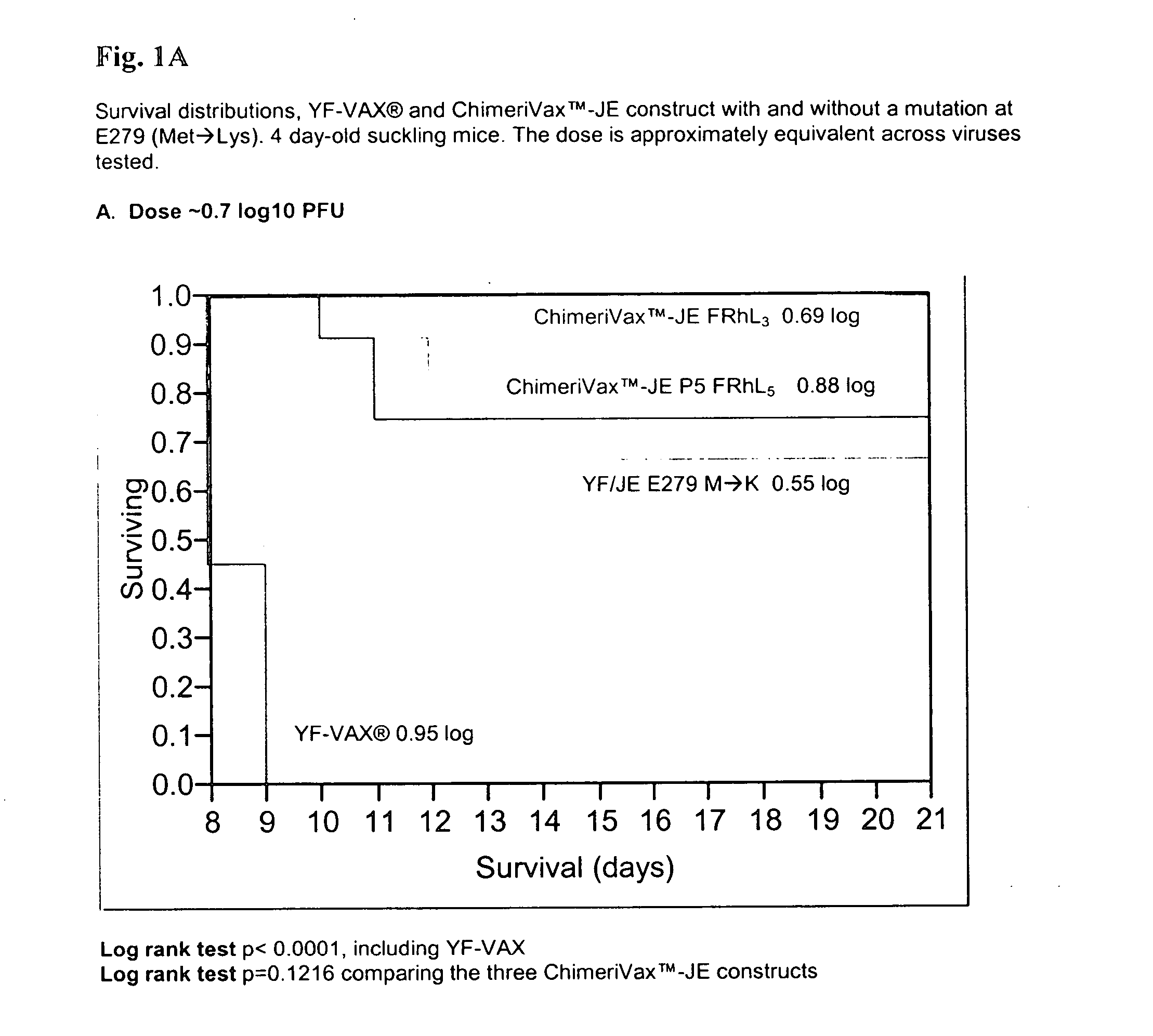
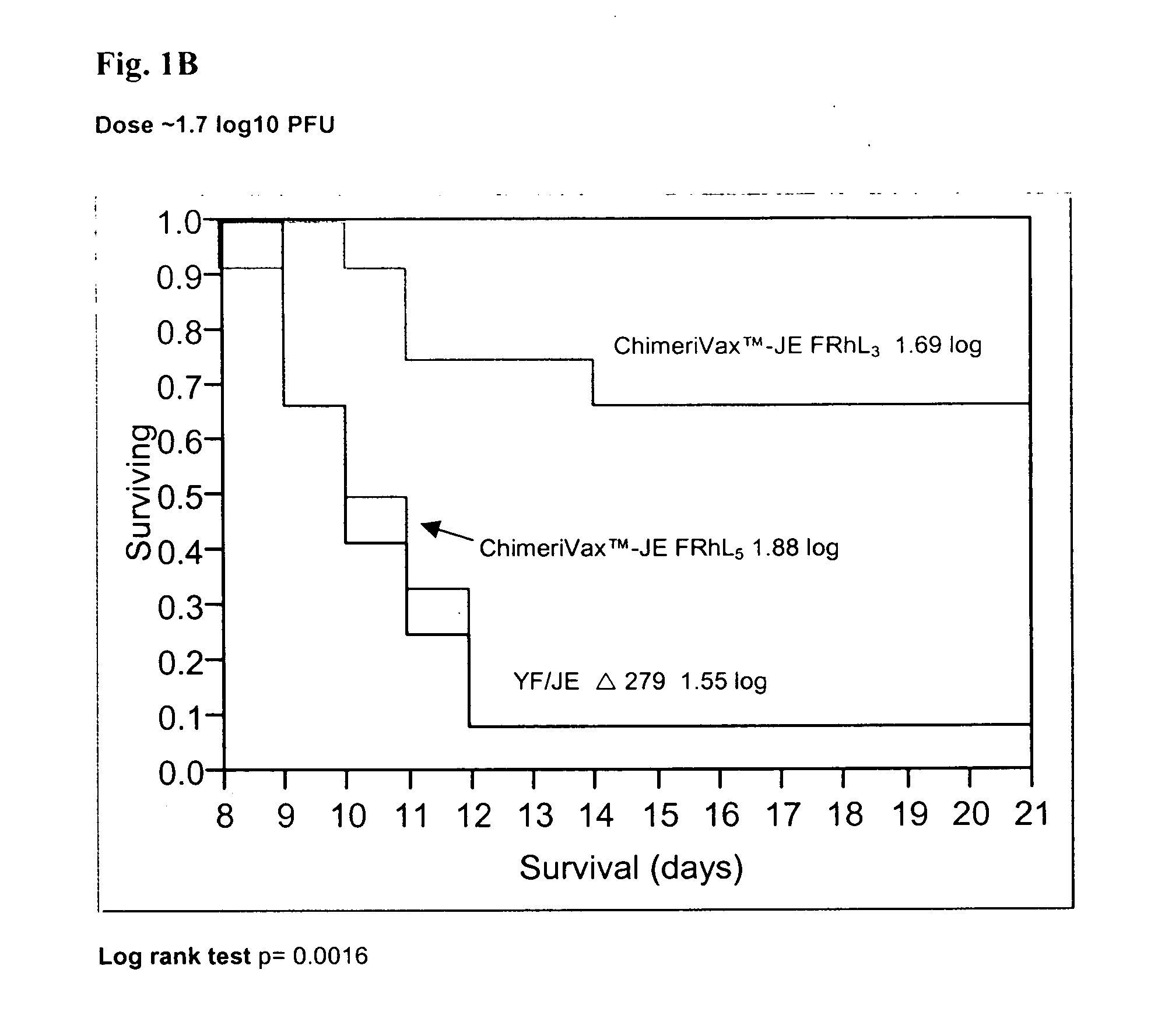
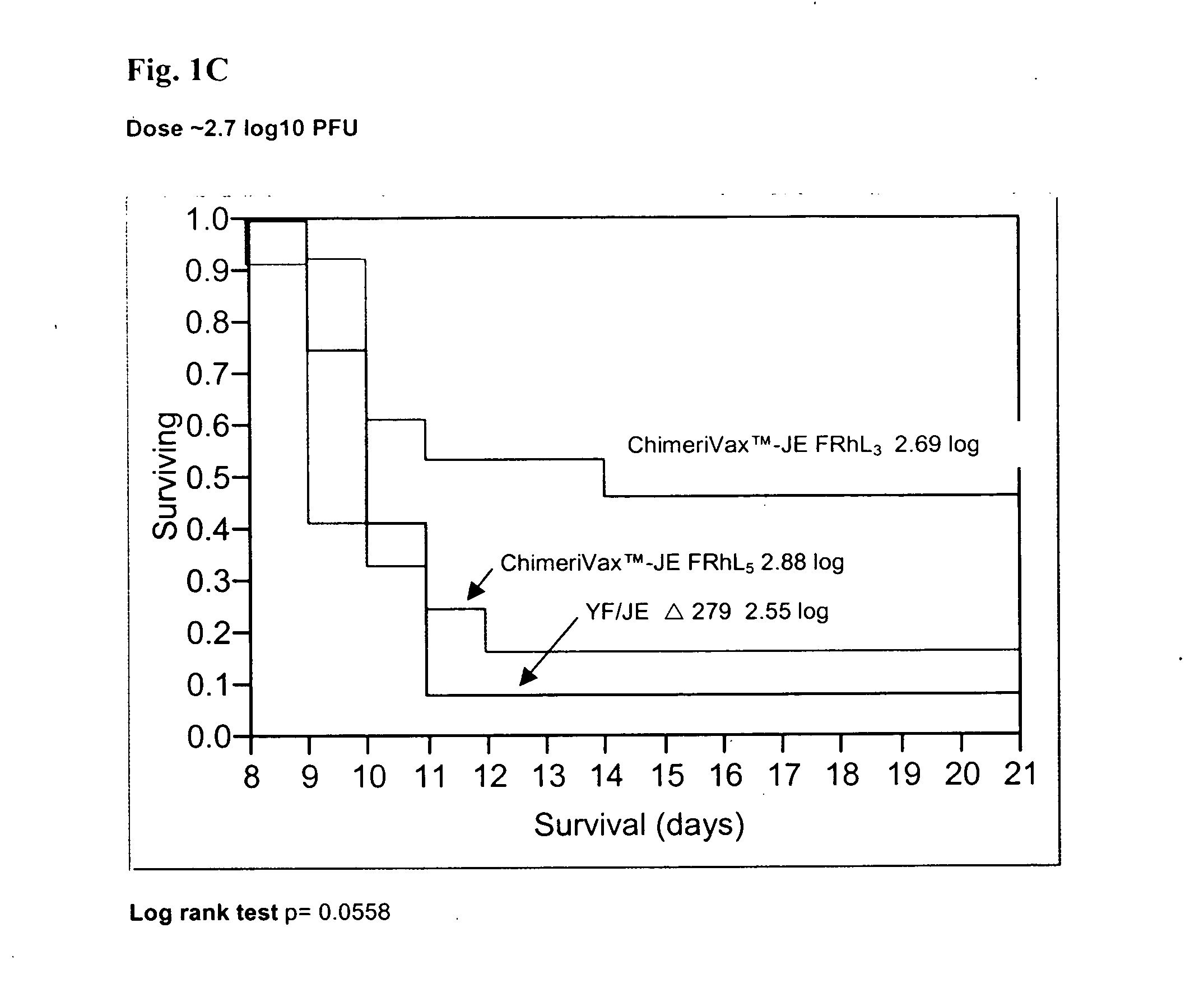
Patents
Literature
347 results about "Flavivirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
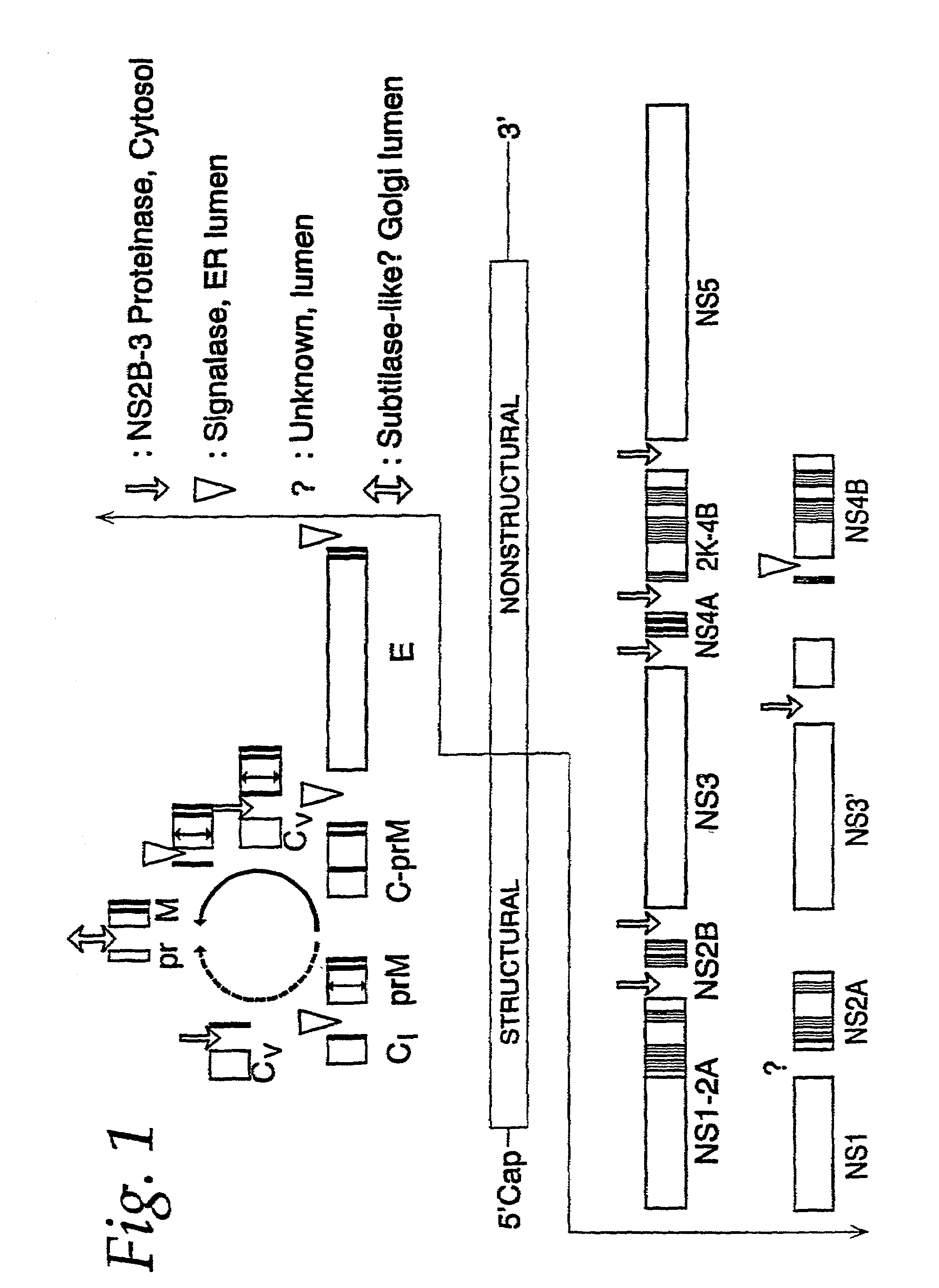
Flavivirus is a genus of viruses in the family Flaviviridae. This genus includes the West Nile virus, dengue virus, tick-borne encephalitis virus, yellow fever virus, Zika virus and several other viruses which may cause encephalitis, as well as insect-specific flaviviruses (ISFs) such as cell fusing agent virus (CFAV), Palm Creek virus (PCV), and Parramatta River virus (PaRV).
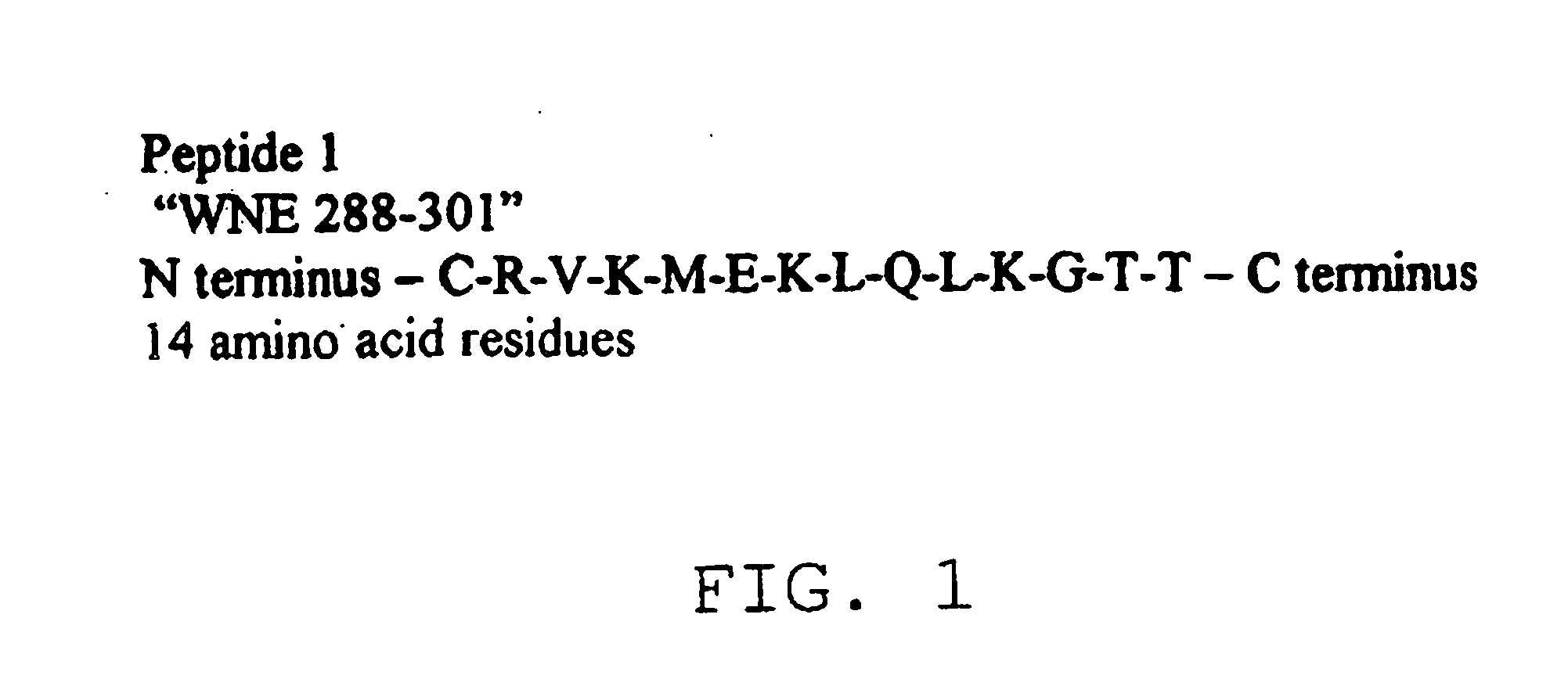
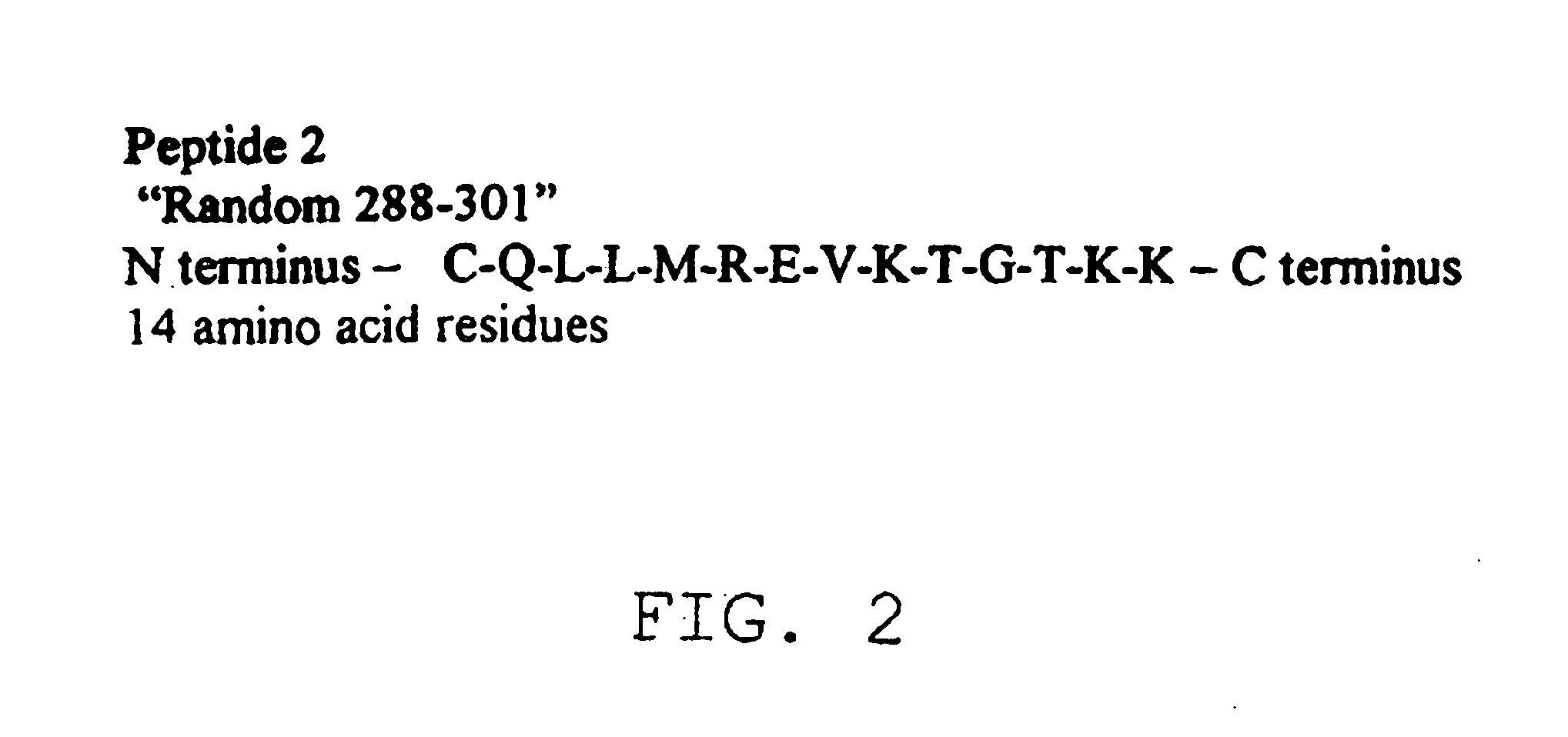
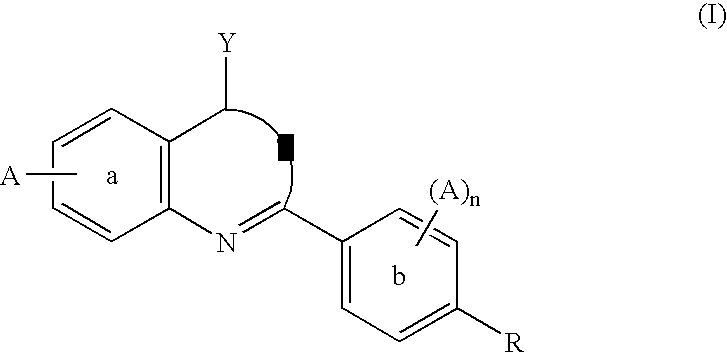
Compounds and methods for the treatment or prevention of Flavivirus infections
InactiveUS6881741B2Useful in therapyInhibit and reduce activityGroup 4/14 element organic compoundsBiocideMedicineViral infection
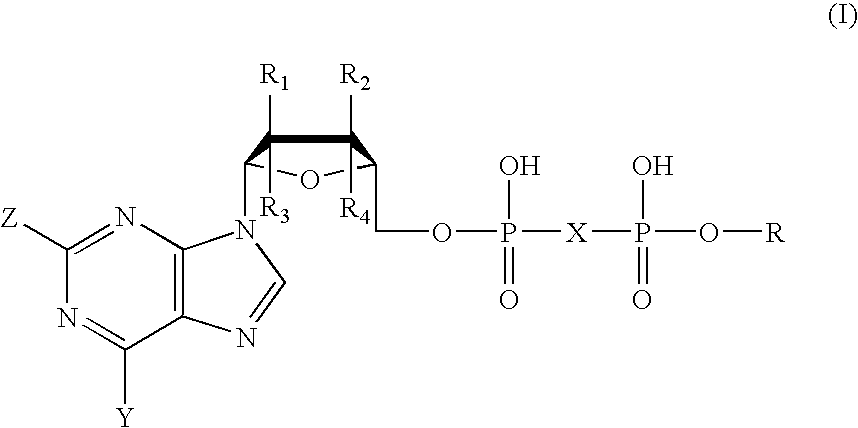
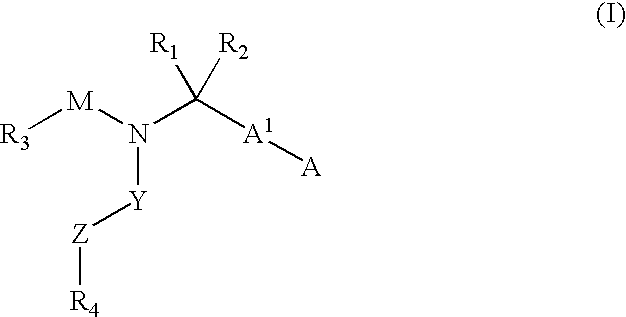
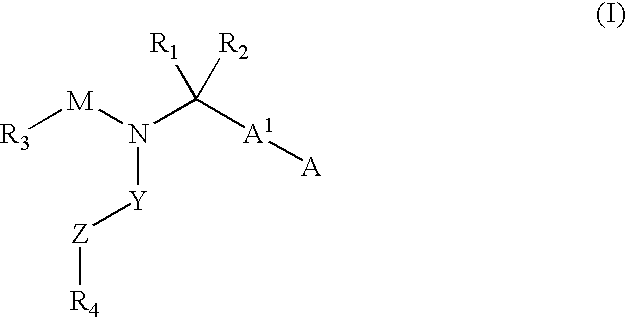
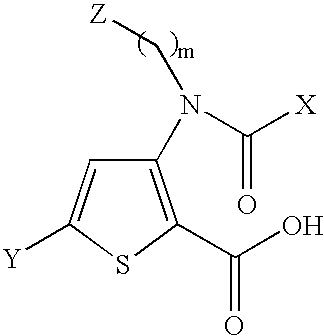
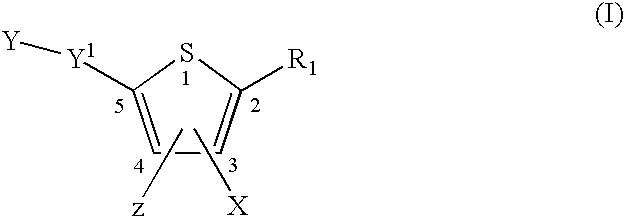
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating flaviviridae viral infection.
Owner:VERTEX PHARMA CANADA
Screening for west nile virus antiviral therapy
InactiveUS20050058987A1Improve efficiencySsRNA viruses positive-senseVectorsHigh-Throughput Screening MethodsImmunogenicity
The instant invention provides stable and novel lineage I WNV reverse genetics systems, and methods for making the reverse genetics systems, specifically, a fully-infectious lineage I WNV cDNA or replicon system engineered with one or more nucleotide sequences each encoding a reporter gene to be used in high throughput cell-based screening assays for the identification of novel antiflaviviral chemotherapeutics and / or vaccines effective to treat and / or immunize against infections by WNV and other emerging flaviviruses, such as, for example, JEV, SLEV, AV, KV, JV, CV, YV, TBEV, DENV-1, DENV-2, DENV-3, DENV-4, YFV and MVEV. The present invention further provides methods of high throughput screening of antiflaviviral compounds or improved derivatives thereof using novel lineage I WNV reverse genetics systems and / or cell lines stably containing the reverse genetics systems. Also, the invention provides novel pharmaceutical compositions comprising an attenuated lineage I WNV that is less virulent but similarly immunogenic as the parent WNV and is capable of providing a protective immune response in a host.
Owner:HEALTH RES INC
2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
InactiveUS20070015905A1Inhibit HCV polymerase activityInhibit polymerase activityBiocideSugar derivativesDiseaseReverse transcriptase
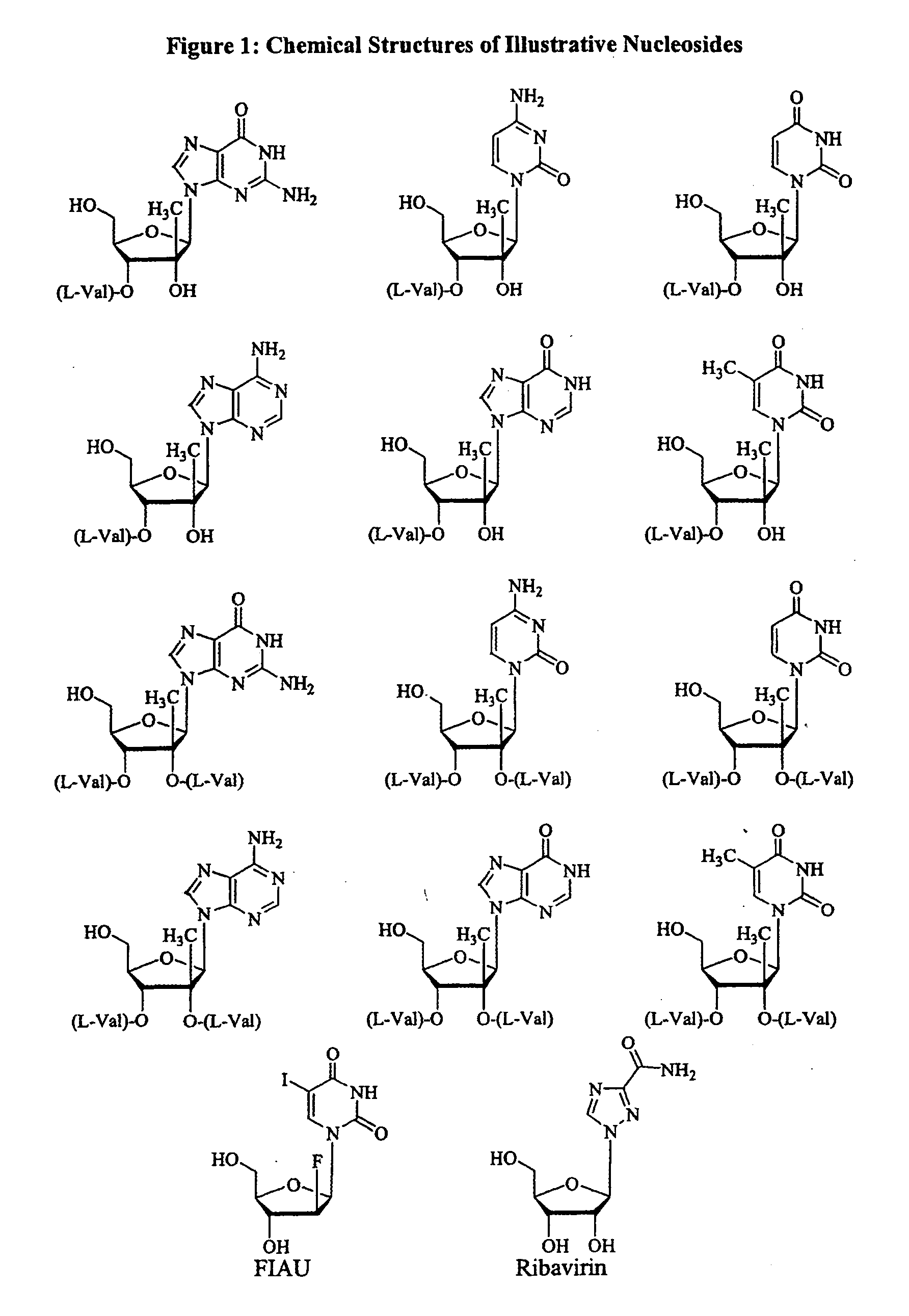
2′ and 3′-Prodrugs of 1′, 2′, 3′ or 4′-branched β-D or β-L nucleosides, or their pharmaceutically acceptable salts and derivatives are described, which are useful in the prevention and treatment of Flaviviridae infections and other related conditions. These modified nucleosides provide superior results against flaviviruses and pestiviruses, including hepatitis C virus and viruses generally that replicate through an RNA dependent RNA reverse transcriptase. Compounds, compositions, methods and uses are provided for the treatment of Flaviviridae infection, including HCV infection, that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:INDENIX PHARM LLC +3
Methods and compositions for treating flaviviruses and pestiviruses
A method and composition for treating a host infected with flavivirus or pestivirus comprising administering an effective flavivirus or pestivirus treatment amount of a described 1′, 2′ or 3′-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:INDENIX PHARM LLC
2' and 3'-nucleoside prodrugs for treating Flaviviridae infections
2′ and 3′-Prodrugs of 1′, 2′, 3′ or 4′-branched β-D or β-L nucleosides, or their pharmaceutically acceptable salts and derivatives are described, which are useful in the prevention and treatment of Flaviviridae infections and other related conditions. These modified nucleosides provide superior results against flaviviruses and pestiviruses, including hepatitis C virus and viruses generally that replicate through an RNA dependent RNA reverse transcriptase. Compounds, compositions, methods and uses are provided for the treatment of Flaviviridae infection, including HCV infection, that include the administration of an effective amount of the prodrugs of the present invention, or their pharmaceutically acceptable salts or derivatives. These drugs may optionally be administered in combination or alteration with further anti-viral agents to prevent or treat Flaviviridae infections and other related conditions.
Owner:THE CENT NAT DEL LA RECH SCIQUE +3
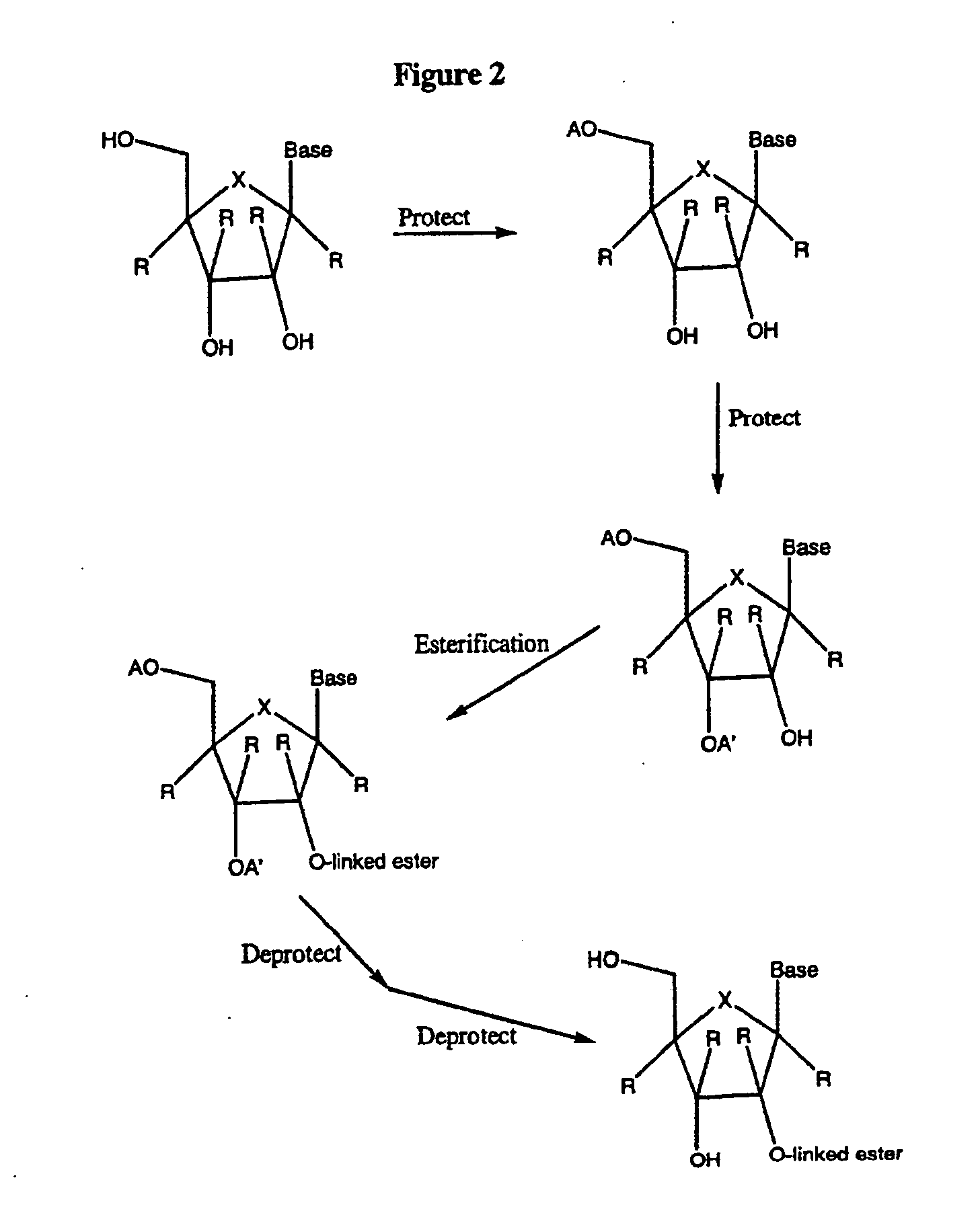
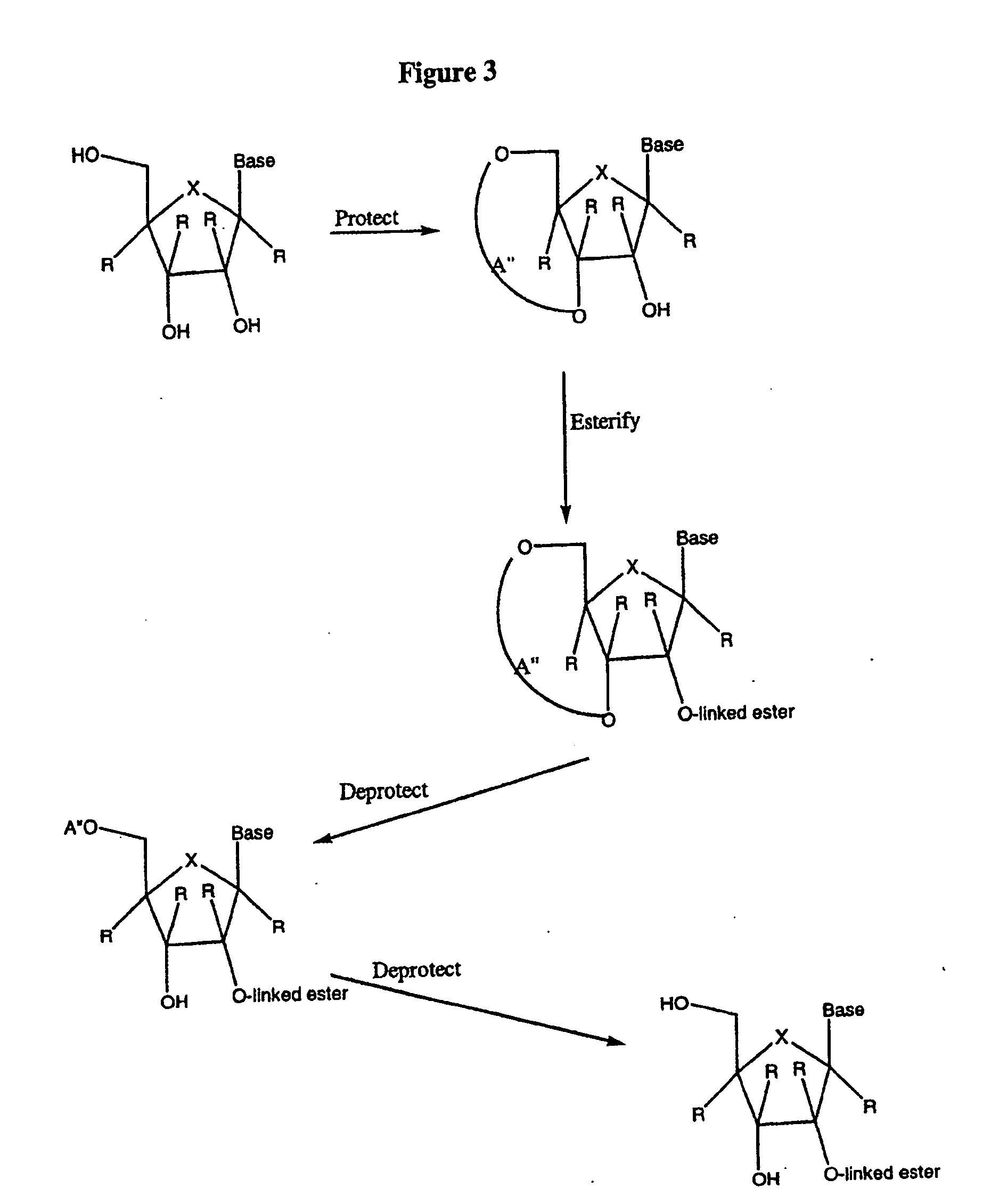
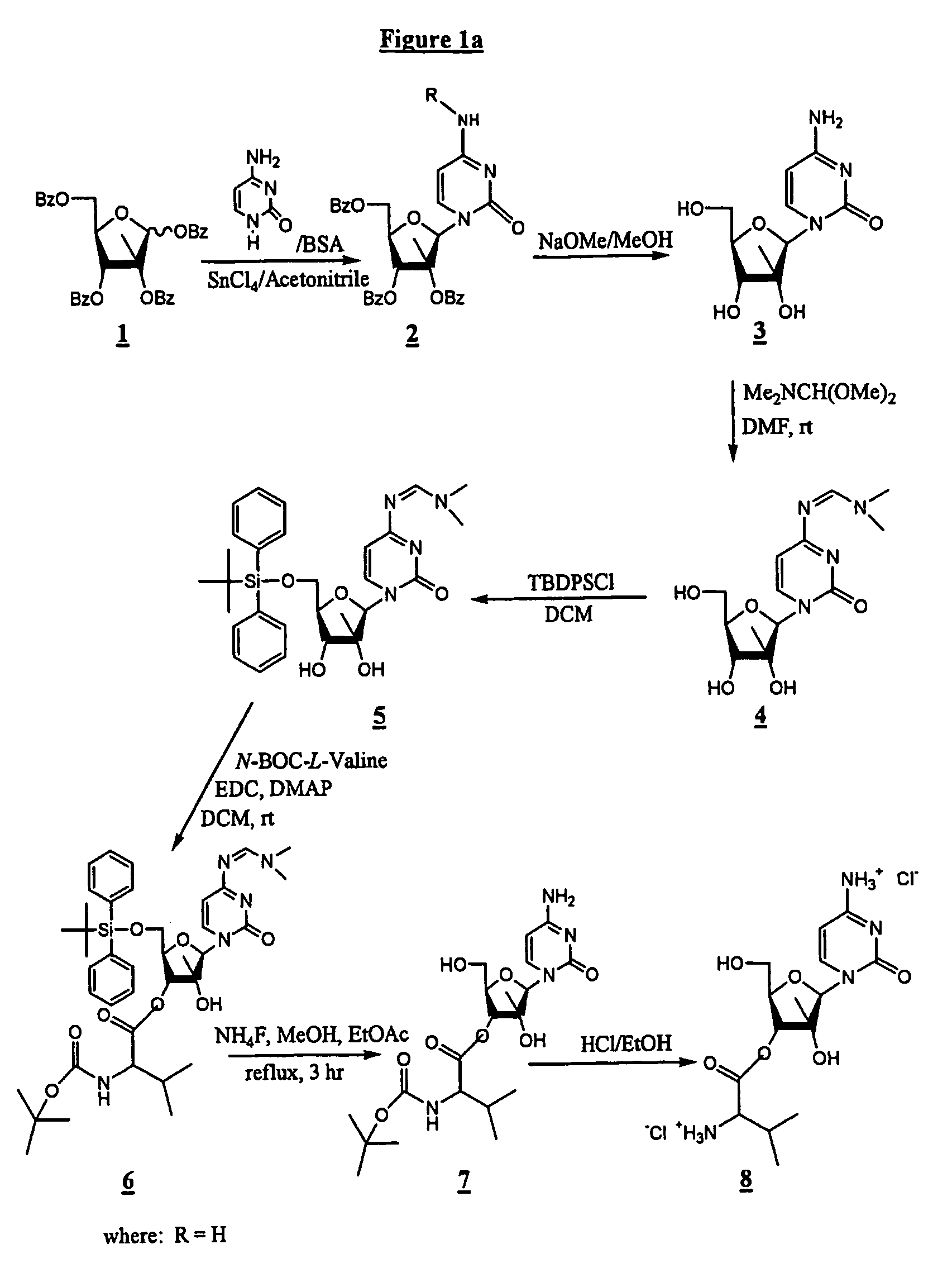
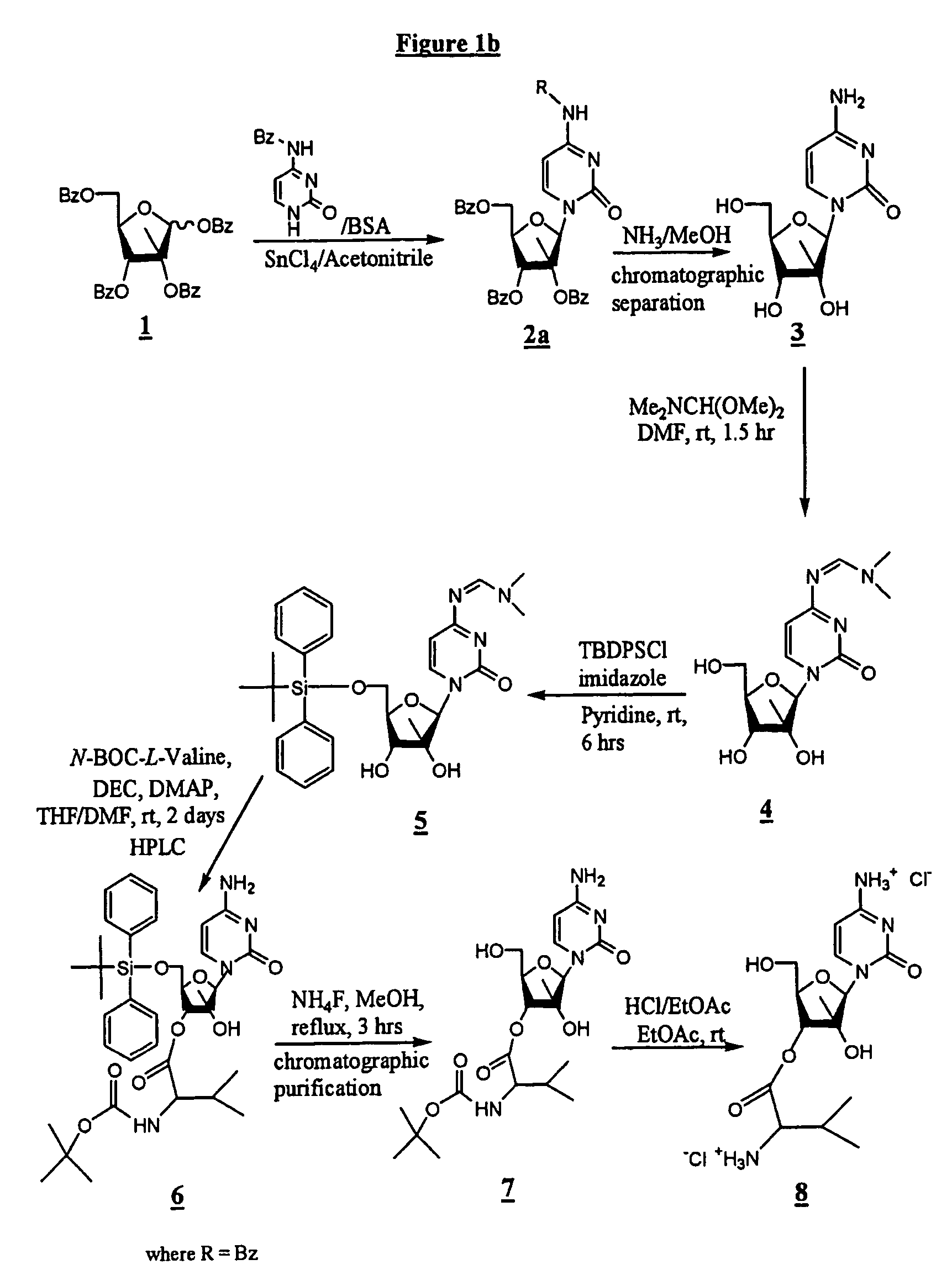
2'-C-methyl-3'-O-L-valine ester ribofuranosyl cytidine for treatment of flaviviridae infections
The 3′-L-valine ester of β-D-2′-C-methyl-ribofuranosyl cytidine provides superior results against flaviviruses and pestiviruses, including hepatitis C virus. Based on this discovery, compounds, compositions, methods and uses are provided for the treatment of flaviviridae, including HCV, that include the administration of an effective amount of val-mCyd or its salt, ester, prodrug or derivative, optionally in a pharmaceutically acceptable carrier. In an alternative embodiment, val-mCyd is used to treat any virus that replicates through an RNA-dependent RNA polymerase.
Owner:INDENIX PHARM LLC +3
Purine nucleoside analogues for treating Flaviviridae including hepatitis C
This invention is directed to a method for treating a host, especially a human, infected with hepatitis C, flavivirus and / or pestivirus, comprising administering to that host an effective amount of an anti-HCV biologically active pentofuranonucleoside where the pentofuranonucleoside base is an optionally substituted 2-azapurine. The optionally substituted pentofuranonucleoside, or a salt or prodrug thereof, may be administered alone or in combination with one or more optionally substituted pentofuranonucleosides or other anti-viral agents.
Owner:THE CENT NAT DEL LA RECH SCIQUE +2
Antiviral agents for treatment of Flaviviridae infections
InactiveUS20040266723A1Alleviating and preventing and delaying onsetEffective conditioningBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a composition for and a method of treating Flaviviridae (Hepacivirus, Flavivirus, Pestivirus) infections, including BVDV and HCV, in a host, including animals, and especially humans, using a small molecule or its pharmaceutically acceptable salt or prodrug.
Owner:PHARMASSET
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:INTERMUNE INC
Compounds and methods for the treatment or prevention of Flavivirus infections
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating Flaviviridae viral infection.
Owner:VIROCHEM PHARMA INC (CA)
Avirulent, immunogenic flavivirus chimeras
InactiveUS7094411B2Minimize and inhibit infectionStable maintenanceOrganic active ingredientsVirusesViral diseaseAmino acid mutation
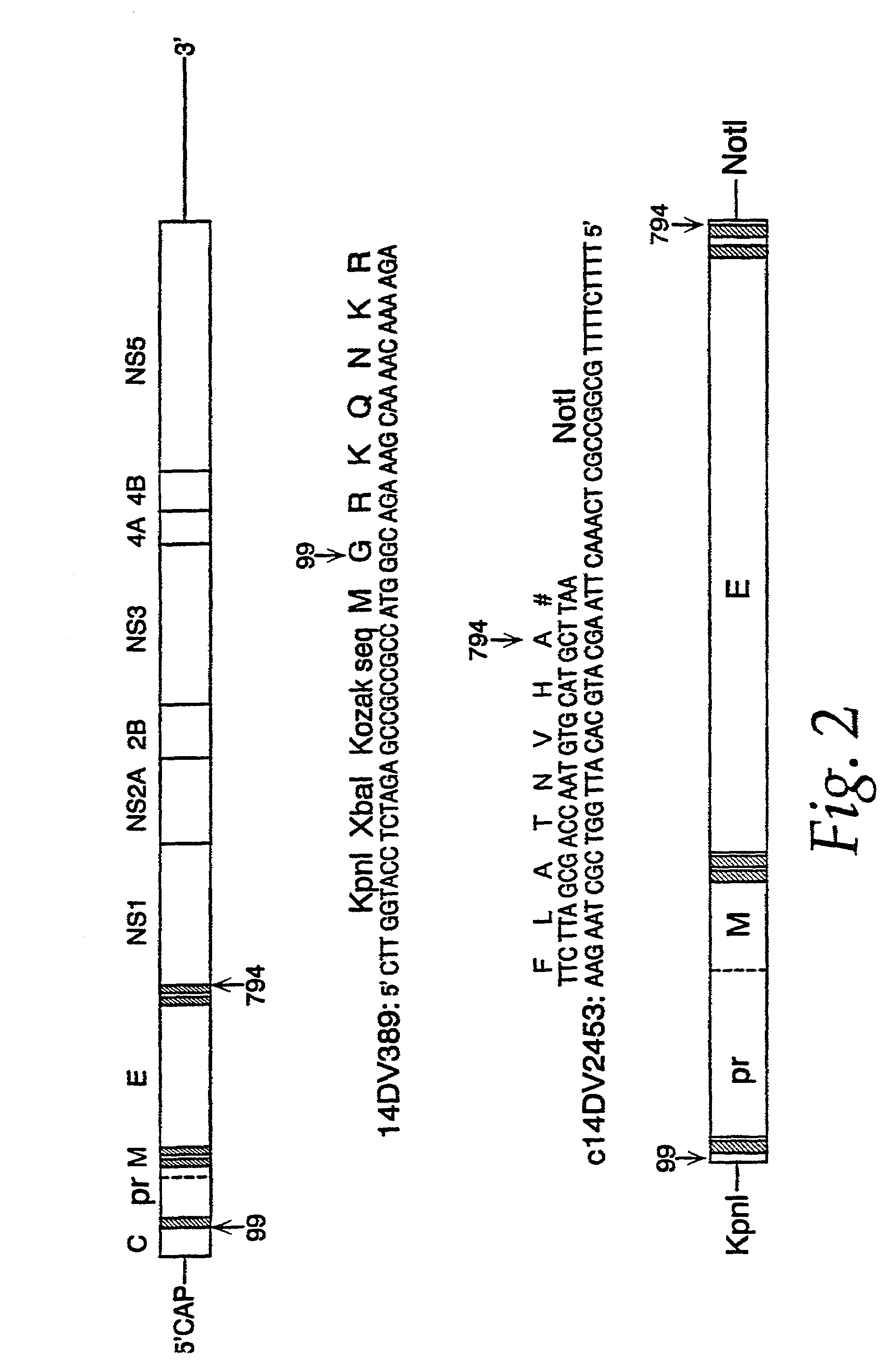
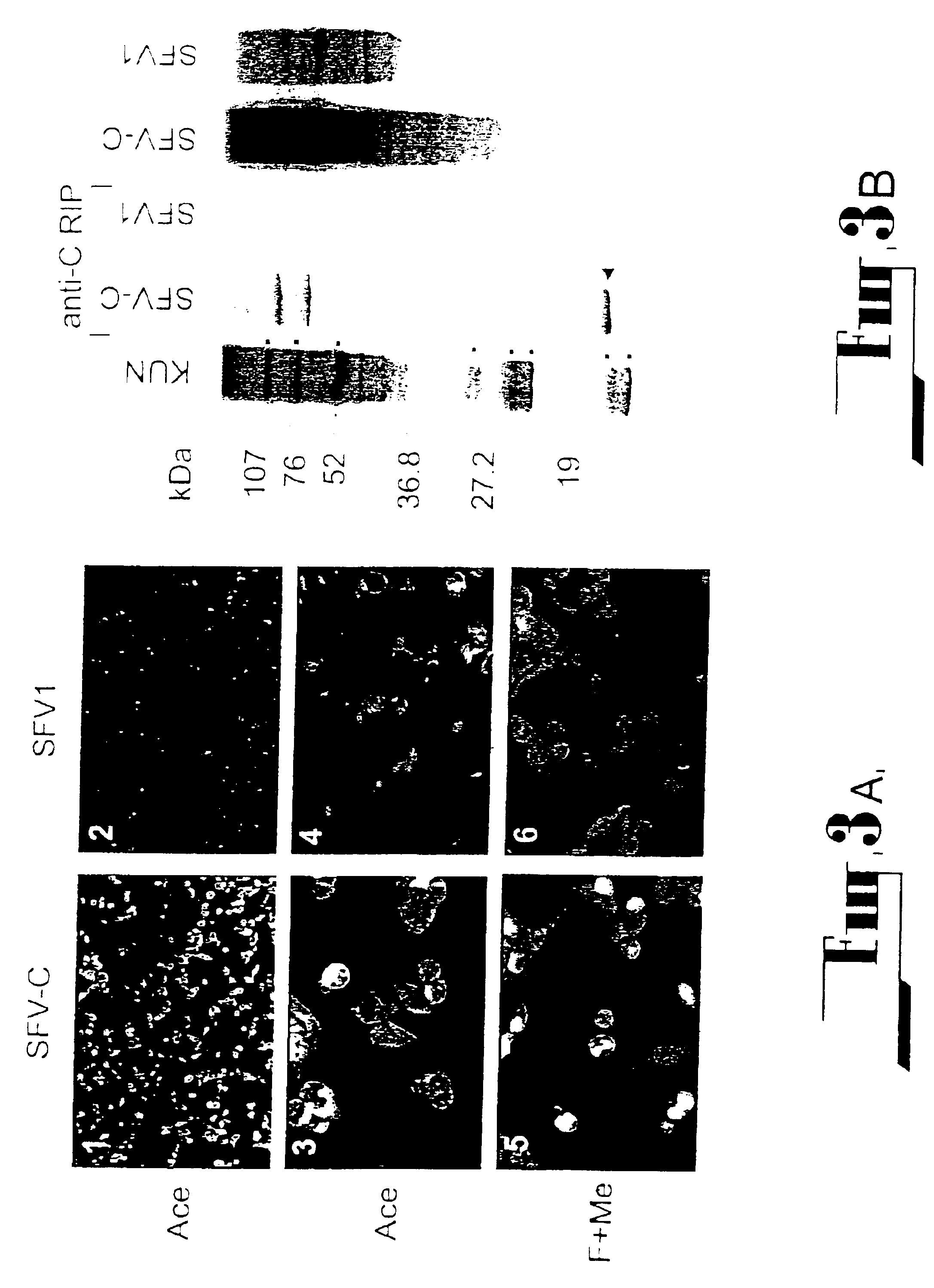
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
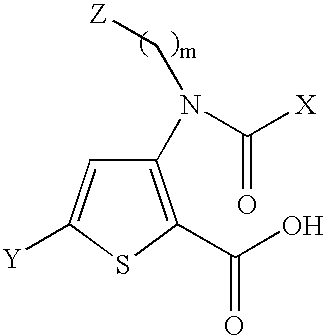
Compounds and methods for the treatment or prevention of Flavivirus infections
InactiveUS7402608B2Inhibiting and reducing activity of viralBiocideOrganic chemistryMedicineFlaviviridae
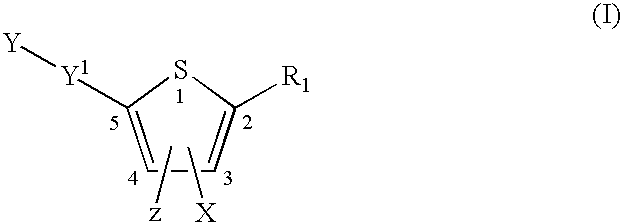
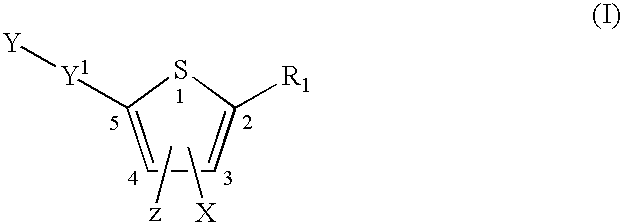
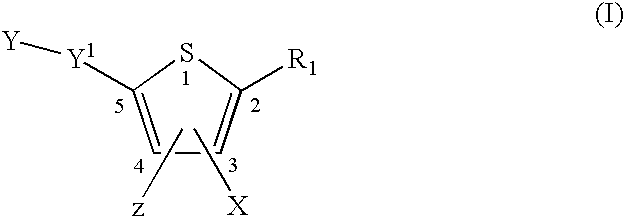
Compounds represented by formula:wherein X, Y and Z are as defined herein, pharmaceutically acceptable salts thereof, and related compounds, are suitable for use in treating or preventing a Flaviviridae viral infection in a host.
Owner:VERTEX PHARMA CANADA
Purine nucleoside analogues for treating flaviviridae including hepatitis C
This invention is directed to a method for treating a host, especially a human, infected with hepatitis C, flavivirus and / or pestivirus, comprising administering to that host an effective amount of an anti-HCV biologically active pentofuranonucleoside where the pentofuranonucleoside base is an optionally substituted 2-azapurine. The optionally substituted pentofuranonucleoside, or a salt or prodrug thereof, may be administered alone or in combination with one or more optionally substituted pentofuranonucleosides or other anti-viral agents.
Owner:INDENIX PHARM LLC +2
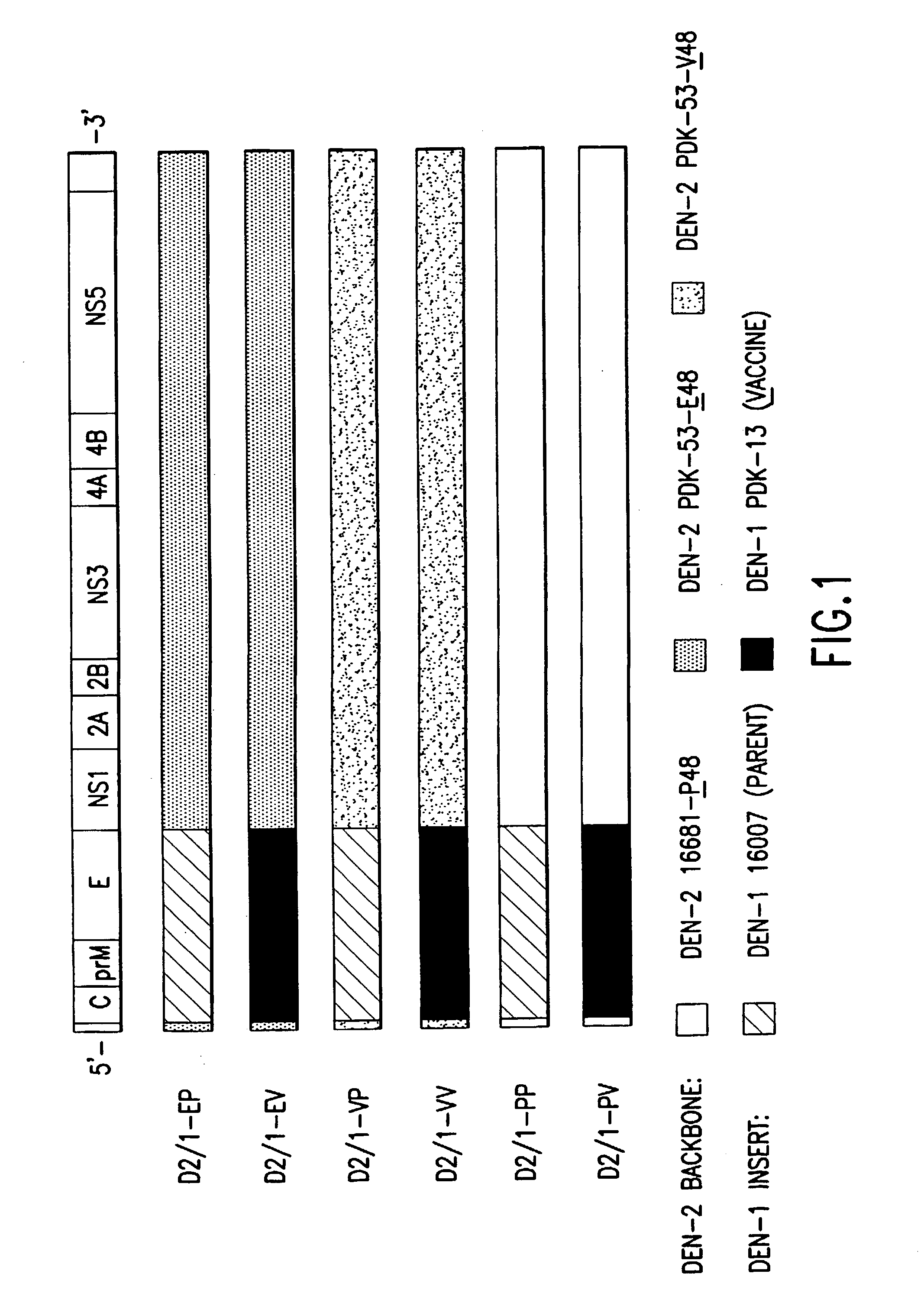
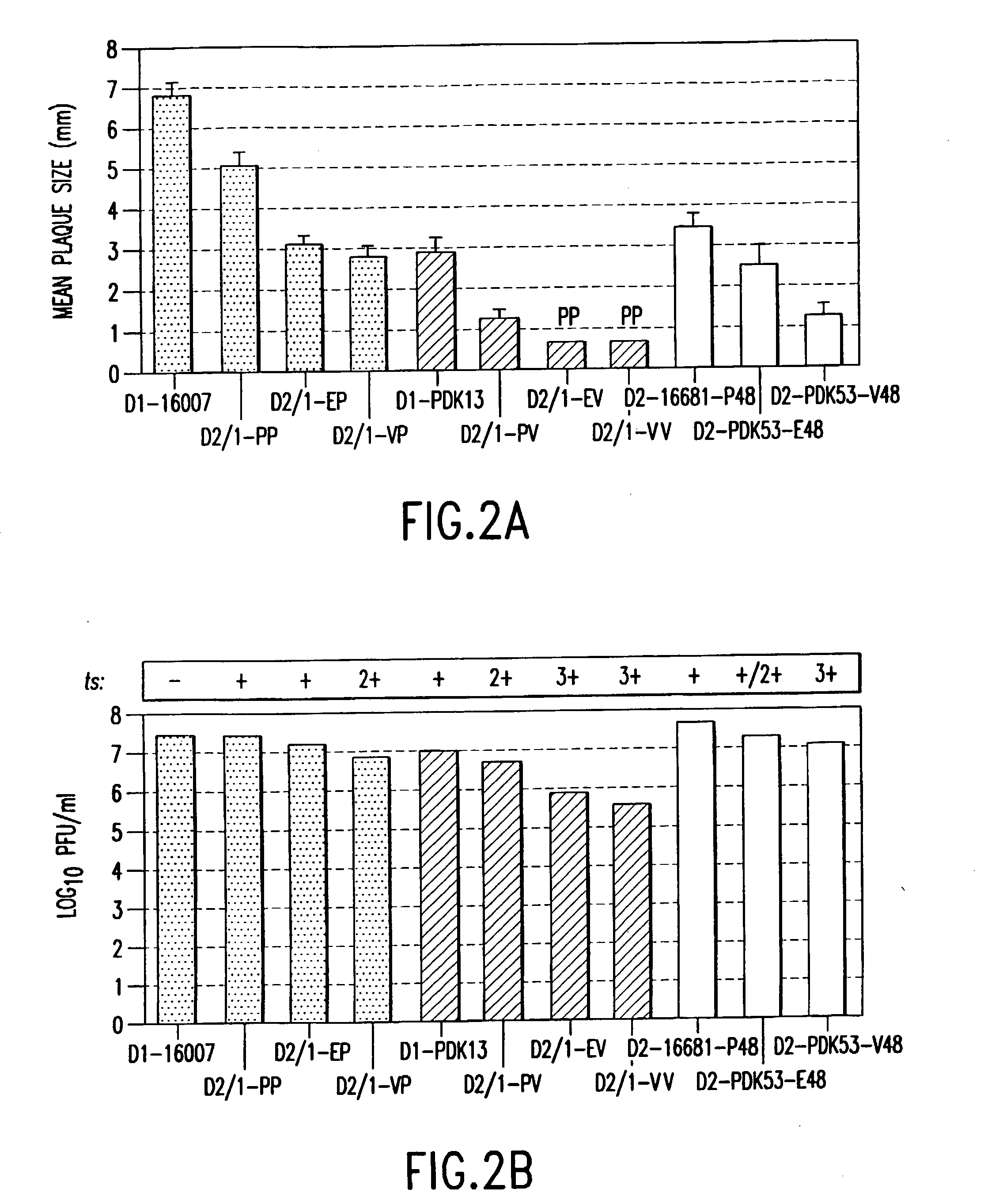
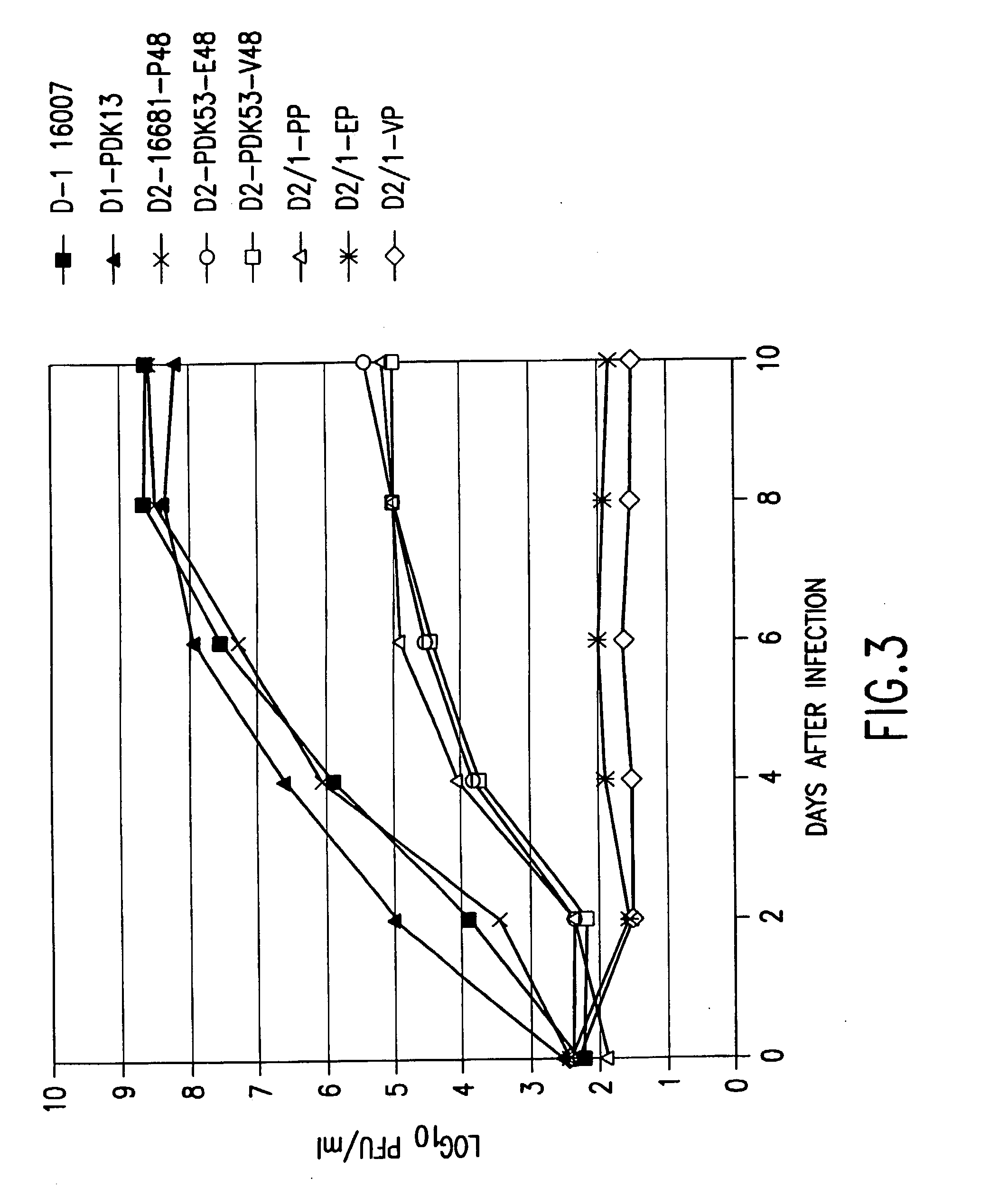
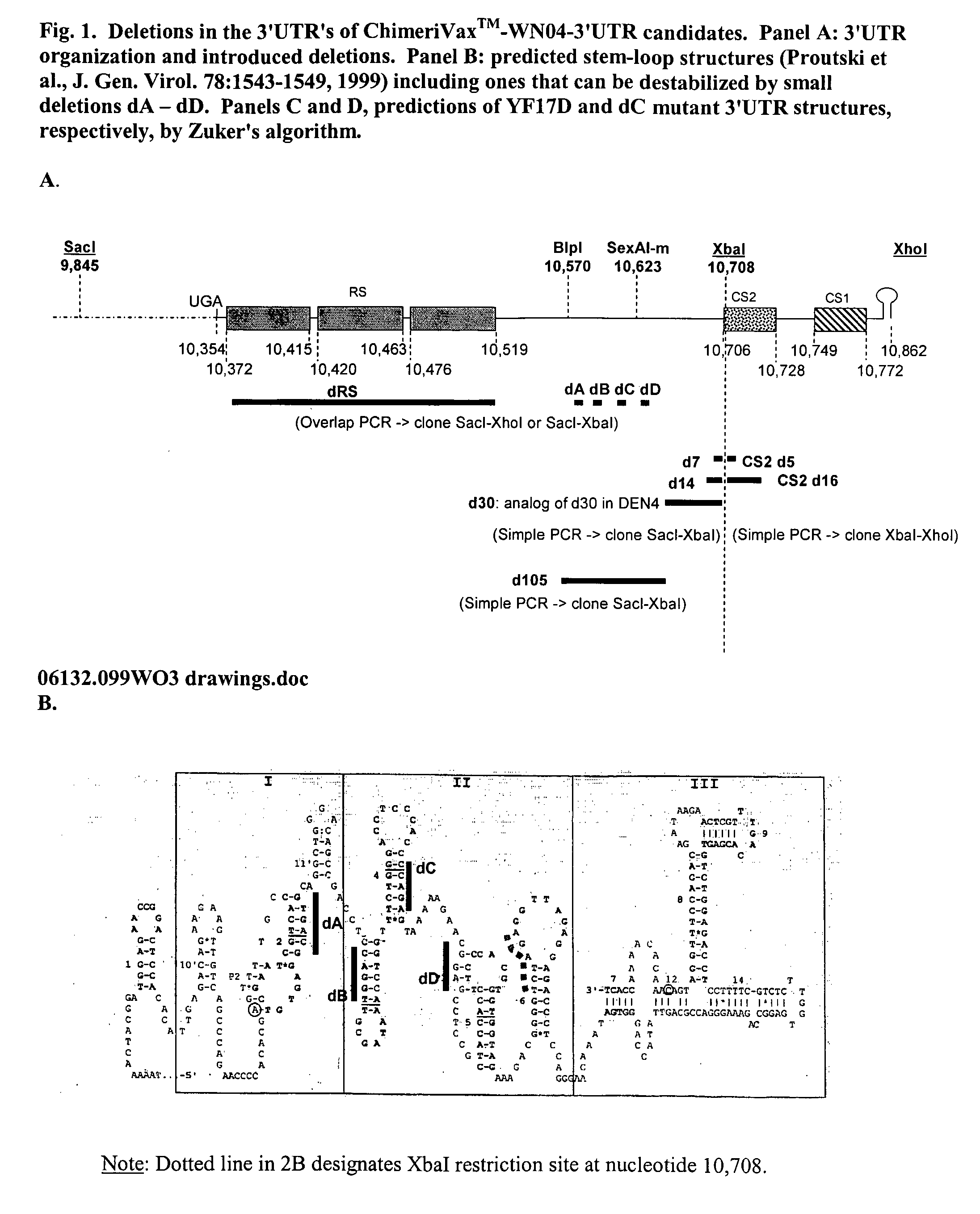
Flavivirus vaccines
InactiveUS20050002968A1Improve security levelDecreased viscerotropismSsRNA viruses positive-senseSugar derivativesVirologyFlavivirus
The invention provides attenuated flavivirus vaccines and methods of making and using these vaccines.
Owner:ACAMBIS INC
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
Oligonucleotide analog and method for treating flavivirus infections
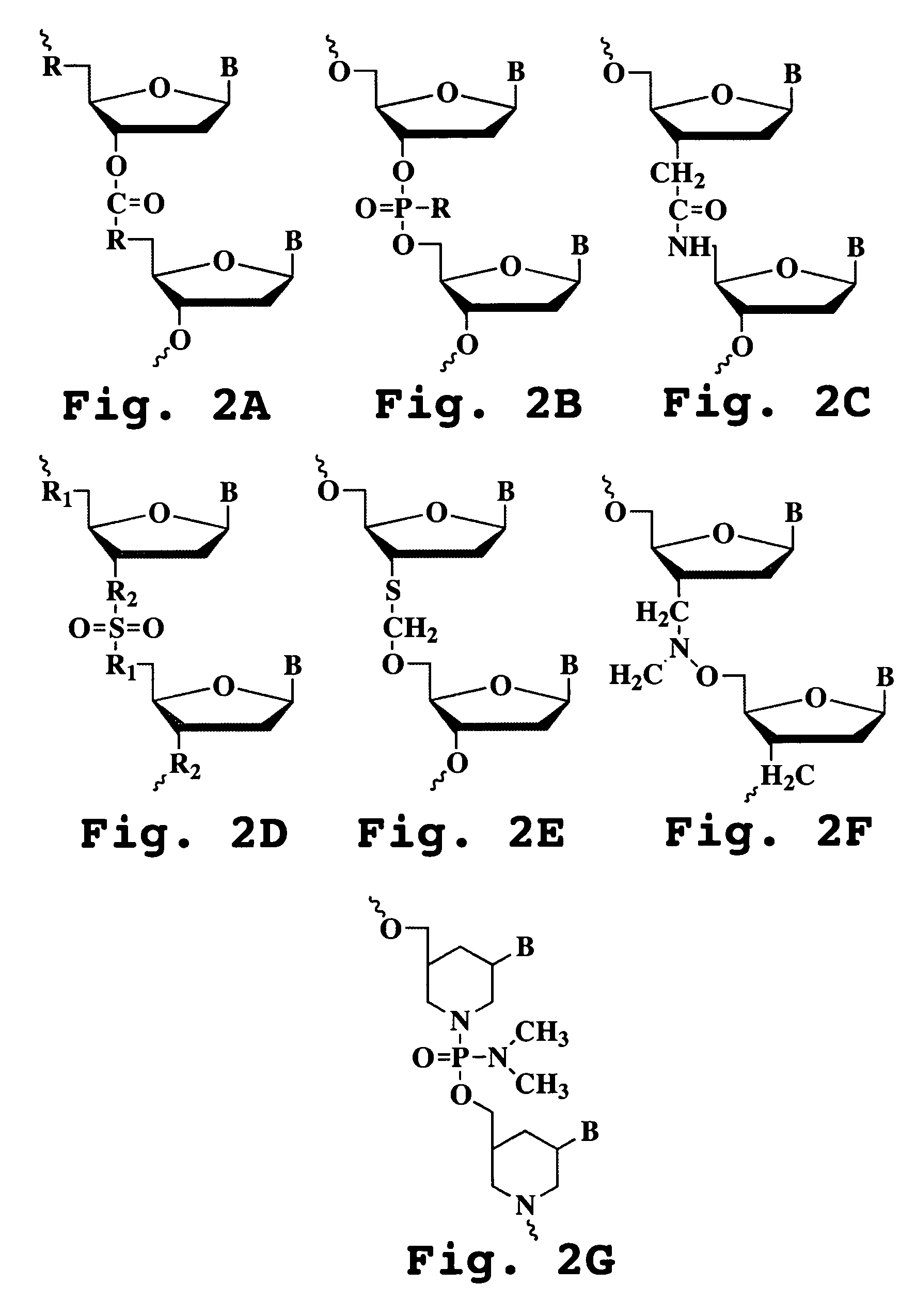
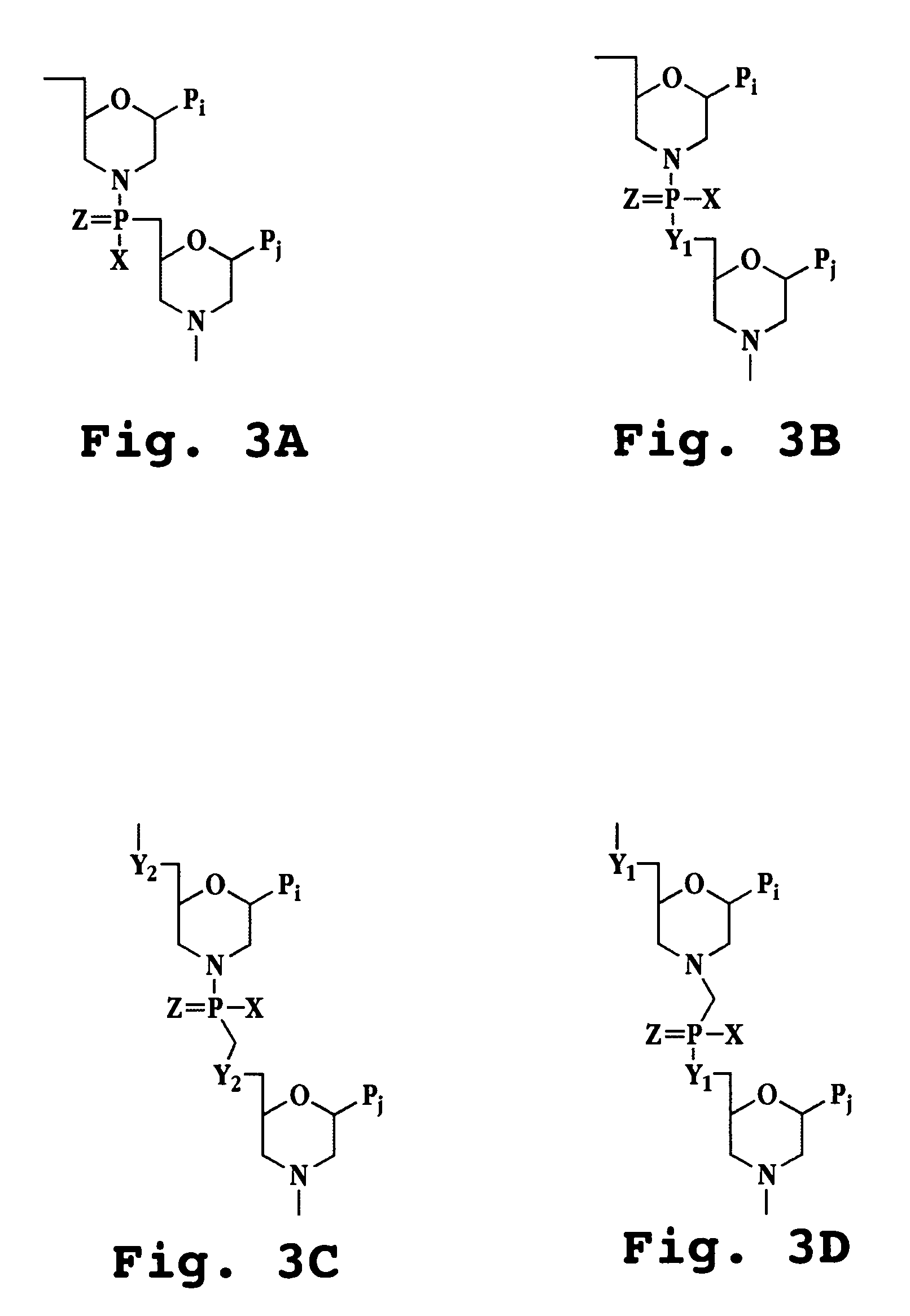
InactiveUS20050096291A1Inhibition of replicationSugar derivativesMicrobiological testing/measurementHeterologousHeteroduplex
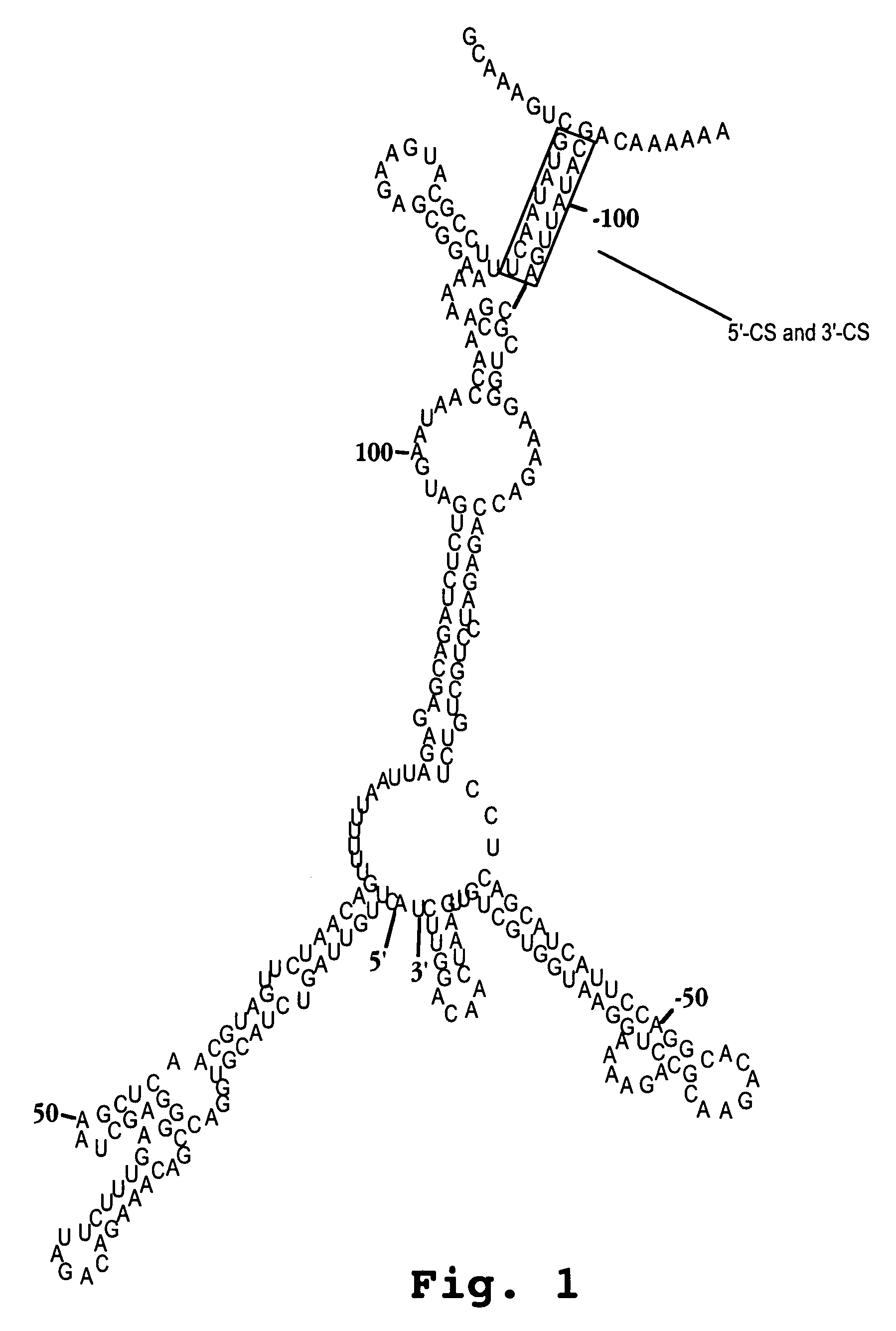
A method of inhibiting replication of a flavivirus in animal cells, and an oligonucleotide compound for use in the method are disclosed. The oligonucleotide analog (i) has a nuclease-resistant backbone, (ii) is capable of uptake by the cells, (iii) contains between 8-40 nucleotide bases, and (iv) has a sequence of at least 8 bases complementary to a region of the virus' positive strand RNA genome that includes at least a portion of SEQ ID NOS:1-4. Exposure of cells infected with a flavivirus to the analog is effective to form within the cells, a heteroduplex structure composed of the virus ssRNA and the oligonucleotide, characterized by a Tm of dissociation of at least 45 ° C., and having disrupted base pairing between the virus' 5′ and 3′ cyclization sequences.
Owner:SAREPTA THERAPEUTICS INC
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7227011B2Improve translationLarge structureOrganic active ingredientsFungiImmunogenicityVirology
The present invention encompasses isolated nucleic acids containing transcriptional units which encode a signal sequence of one flavivirus and an immunogenic flavivirus antigen of a second flavivirus. The invention further encompasses a nucleic acid and protein vaccine and the use of the vaccine to immunize a subject against flavivirus infection. The invention also provides antigens encoded by nucleic acids of the invention, antibodies elicited in response to the antigens and use of the antigens and / or antibodies in detecting flavivirus or diagnosing flavivirus infection.
Owner:HEALTH & HUMAN SERVICES THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC +1
Compounds and methods for the treatment or prevention of Flavivirus infections
InactiveUS20060142347A1Inhibit and reduce activityBiocideGroup 4/14 element organic compoundsMedicineViral infection
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating flaviviridae viral infection.
Owner:VERTEX PHARMA CANADA
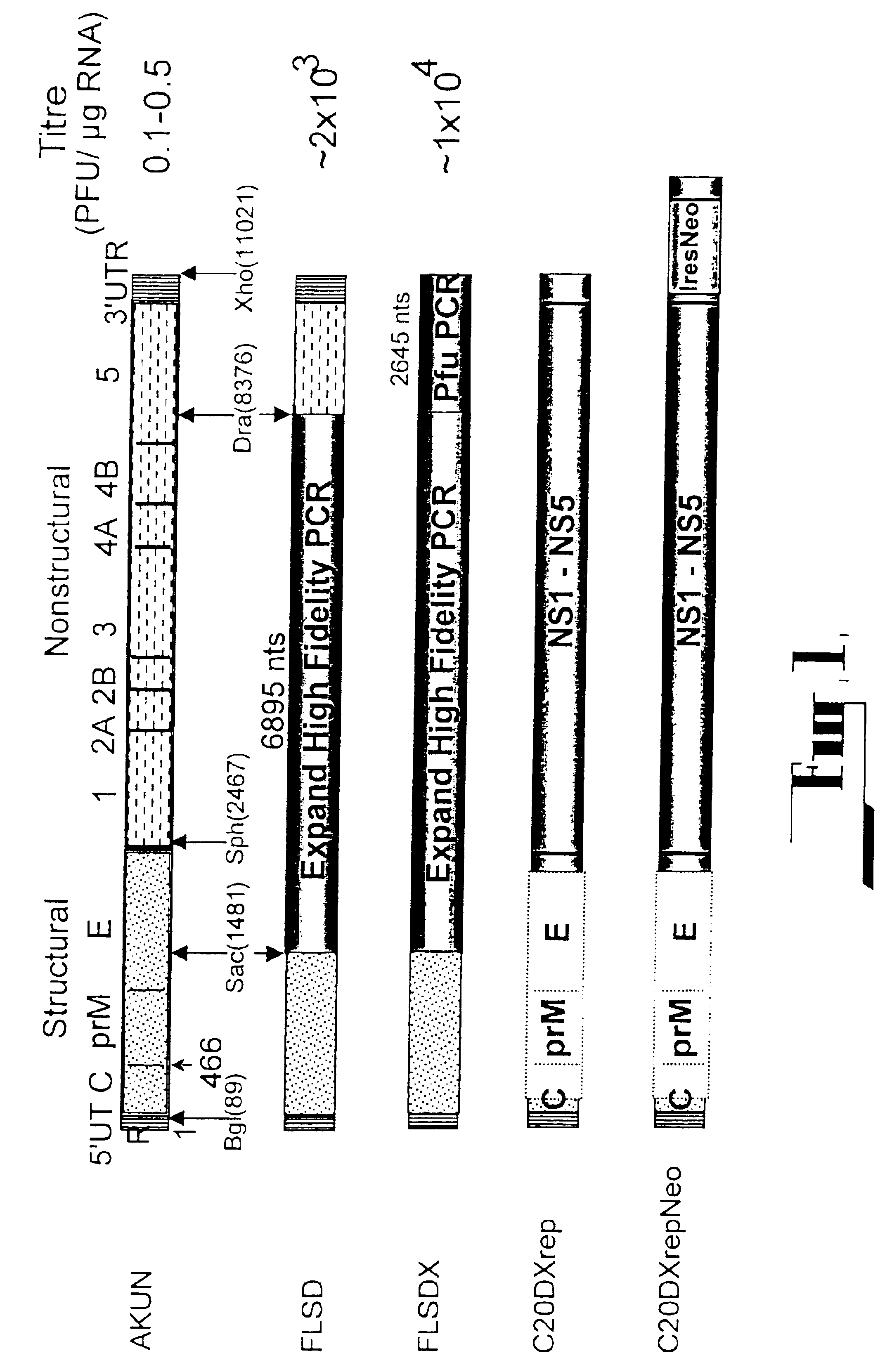
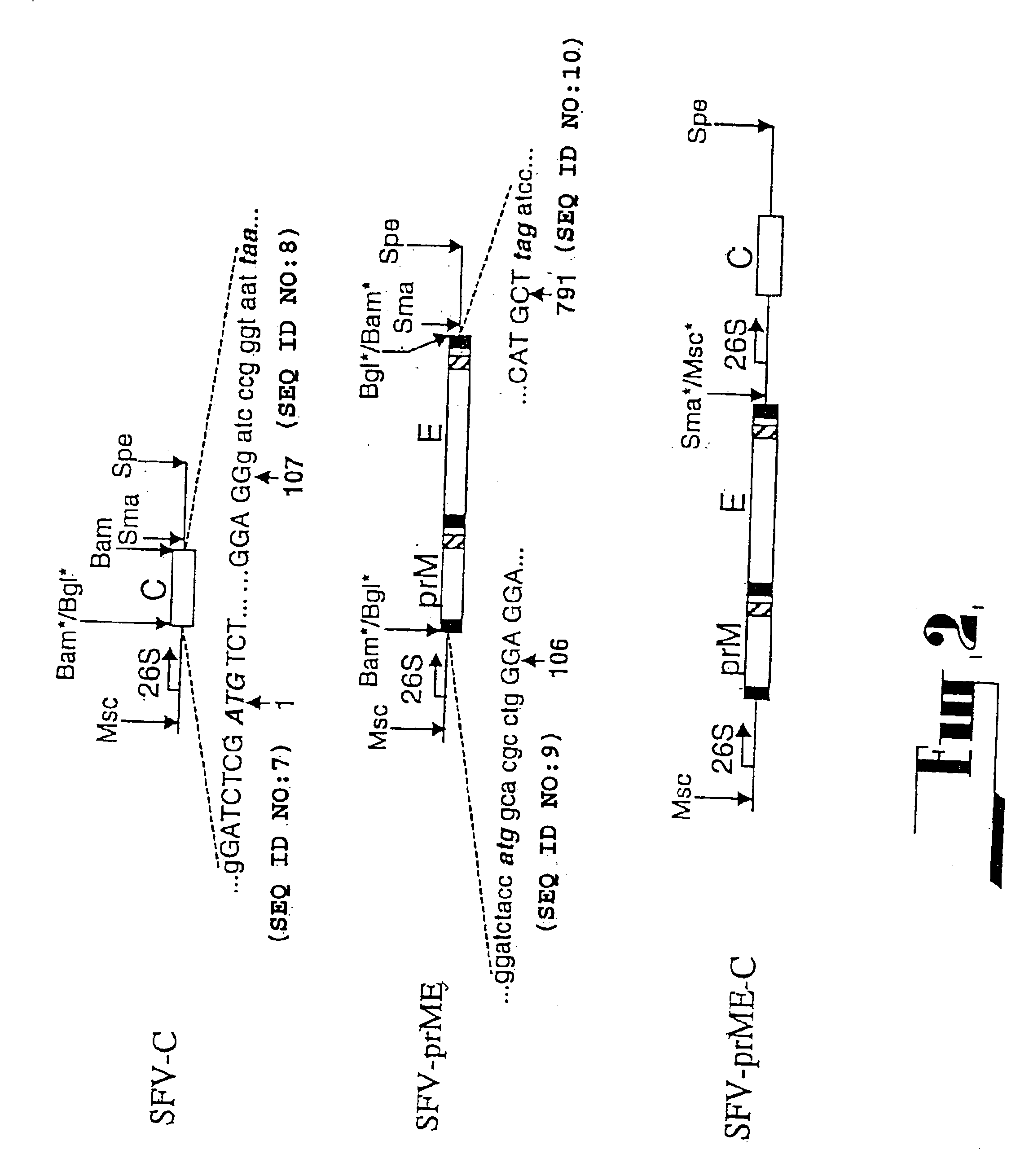
Flavivirus reporter virus and methods of making and using the same
The present invention relates to the production and uses of flavivirus replicons and flavivirus particles and reporter virus particles. The present invention relates to the production and uses of chimeric and codon-optimized flavivirus virus replicons and flavivirus virus particles and reporter virus particles.
Owner:INTEGRAL MOLECULAR
GeXP (Gene Expression Profiler) detection kit for differentiating 11 kinds of duck viral diseases
ActiveCN103773899AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationDiseaseDuck hepatitis A virus
The invention discloses a GeXP (Gene Expression Profiler) detection kit for differentiating 11 kinds of duck viral diseases. The invention provides a GeXP detection primer group for identifying or assisting to identify duck infectious disease pathogens, wherein the primer group consists of a primer pair A, a primer pair B, a primer pair C, a primer pair D, a primer pair E, a primer pair F, a primer pair G, a primer pair H, a primer pair I, a primer pair J, a primer pair K and a primer pair L. According to the GeXP detection kit, shown by experiments, the primer group, a PCR (Polymerase Chain Reaction) reagent and the primer pairs, provided by the invention, are used for simultaneously differentiating and detecting avian influenza viruses, H5, H7 and H9 subtype avian influenza viruses, duck hepatitis viruses, duck plague viruses, duck flaviviruses, newcastle disease viruses, egg drop syndrome viruses, muscovy duck reoviruses, muscovy duck parvoviruses and duck circoviruses and are good in specificity and high in sensitivity. The detection kit, which is simple and convenient and is high in flux, and a detection system are provided for the detection on common major duck infectious disease pathogens, so that the practical needs are better met, and application prospects are broad.
Owner:GUANGXI VETERINARY RES INST
Oligonucleotide analog and method for treating flavivirus infections
Owner:SAREPTA THERAPEUTICS INC
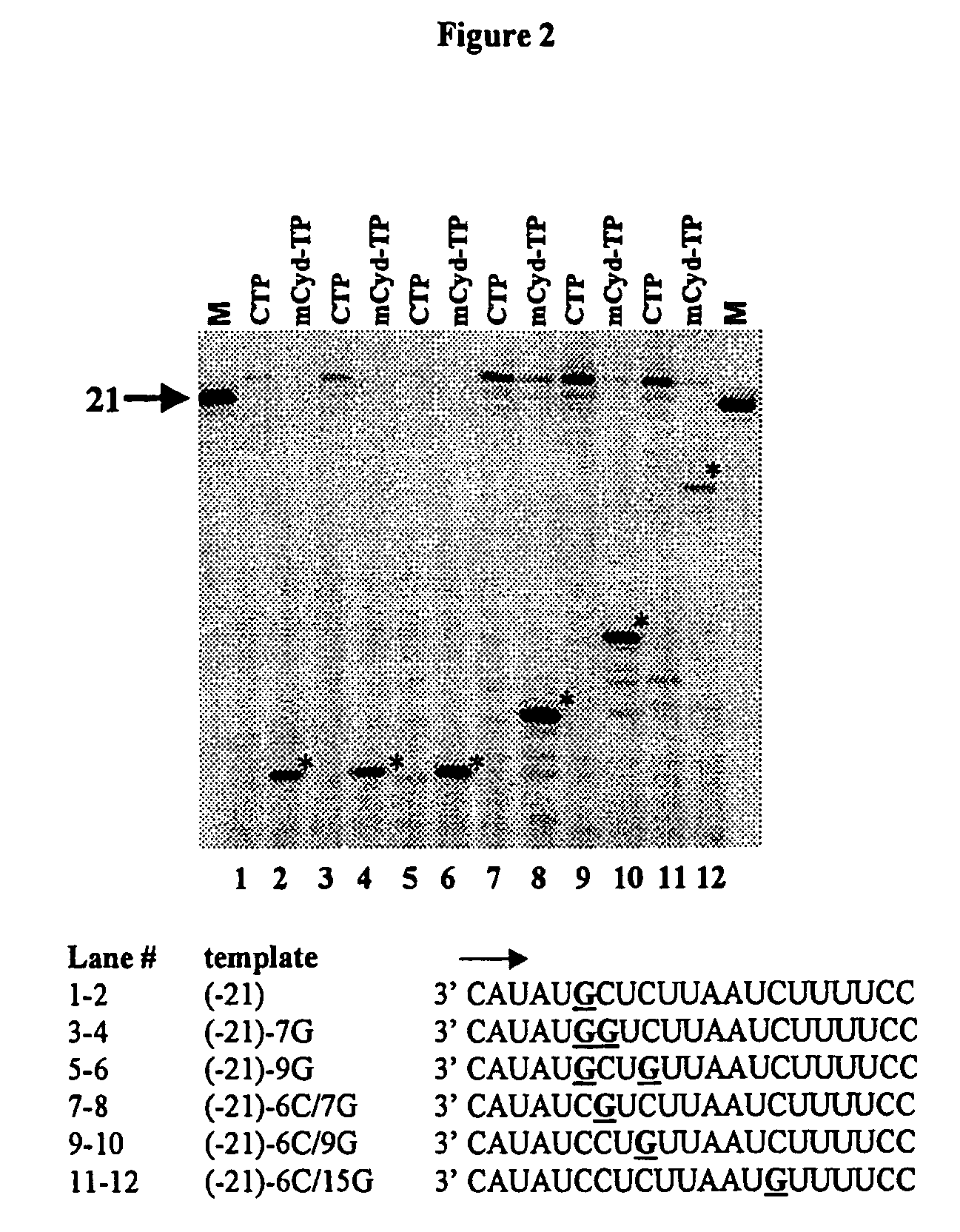
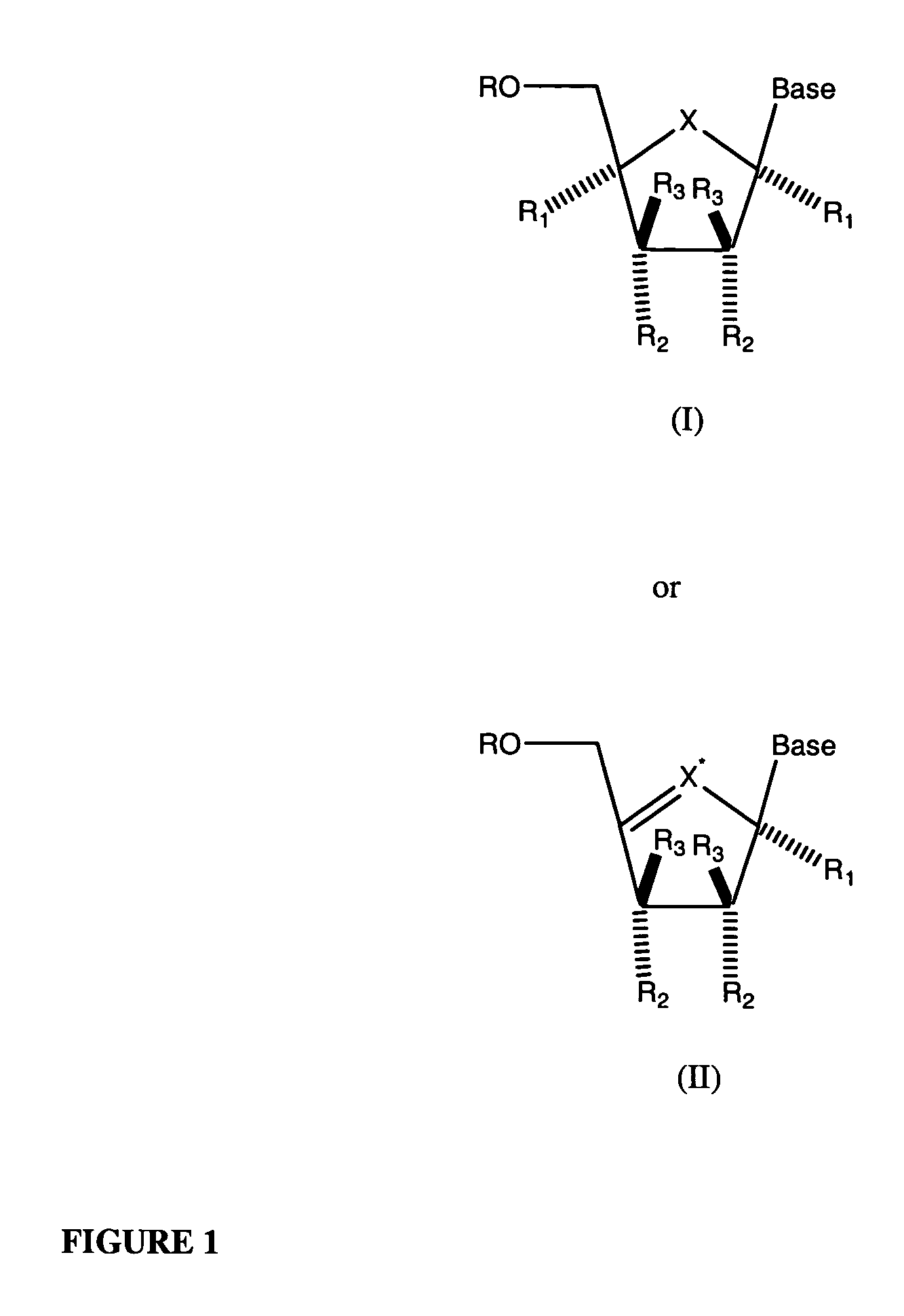
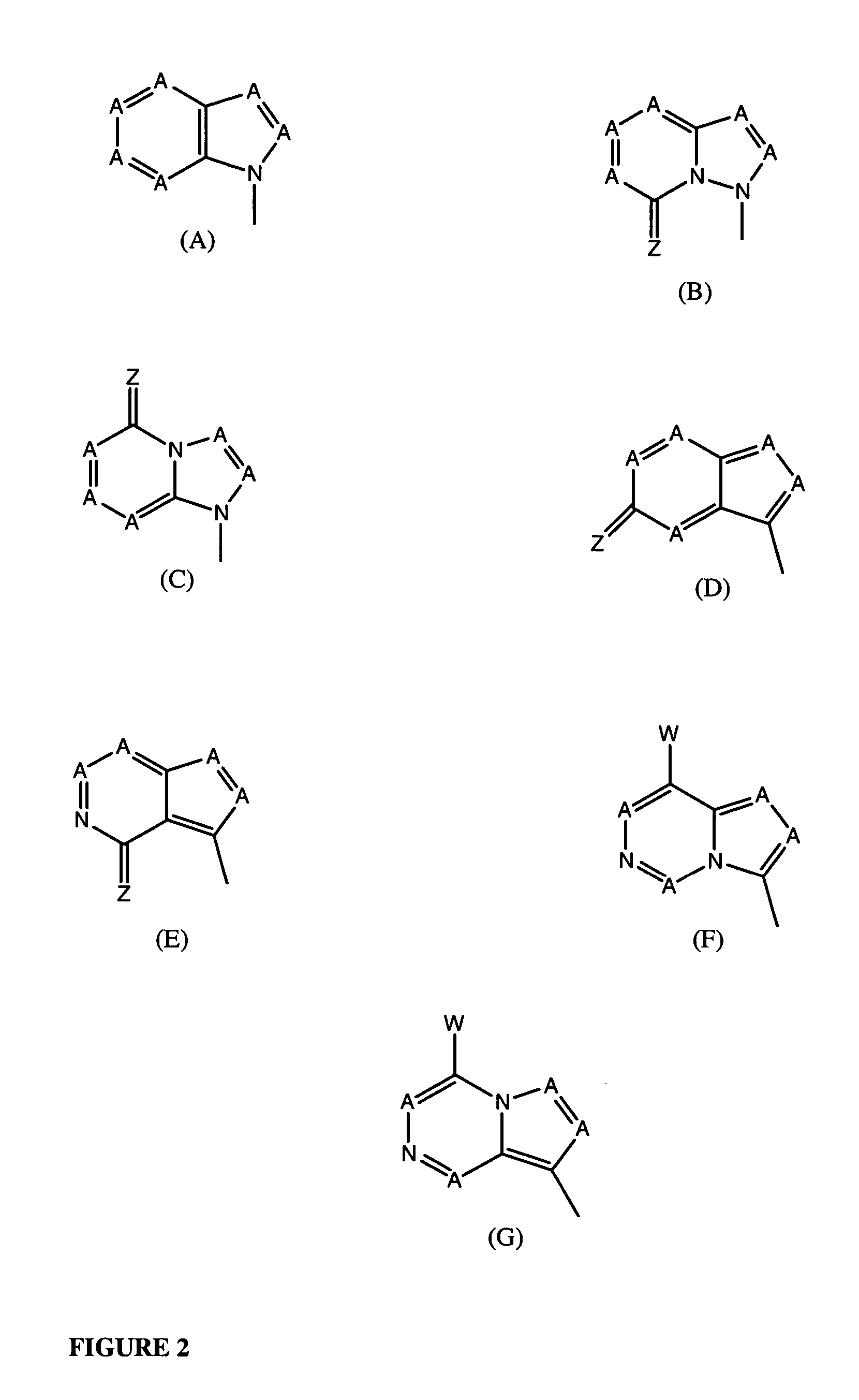
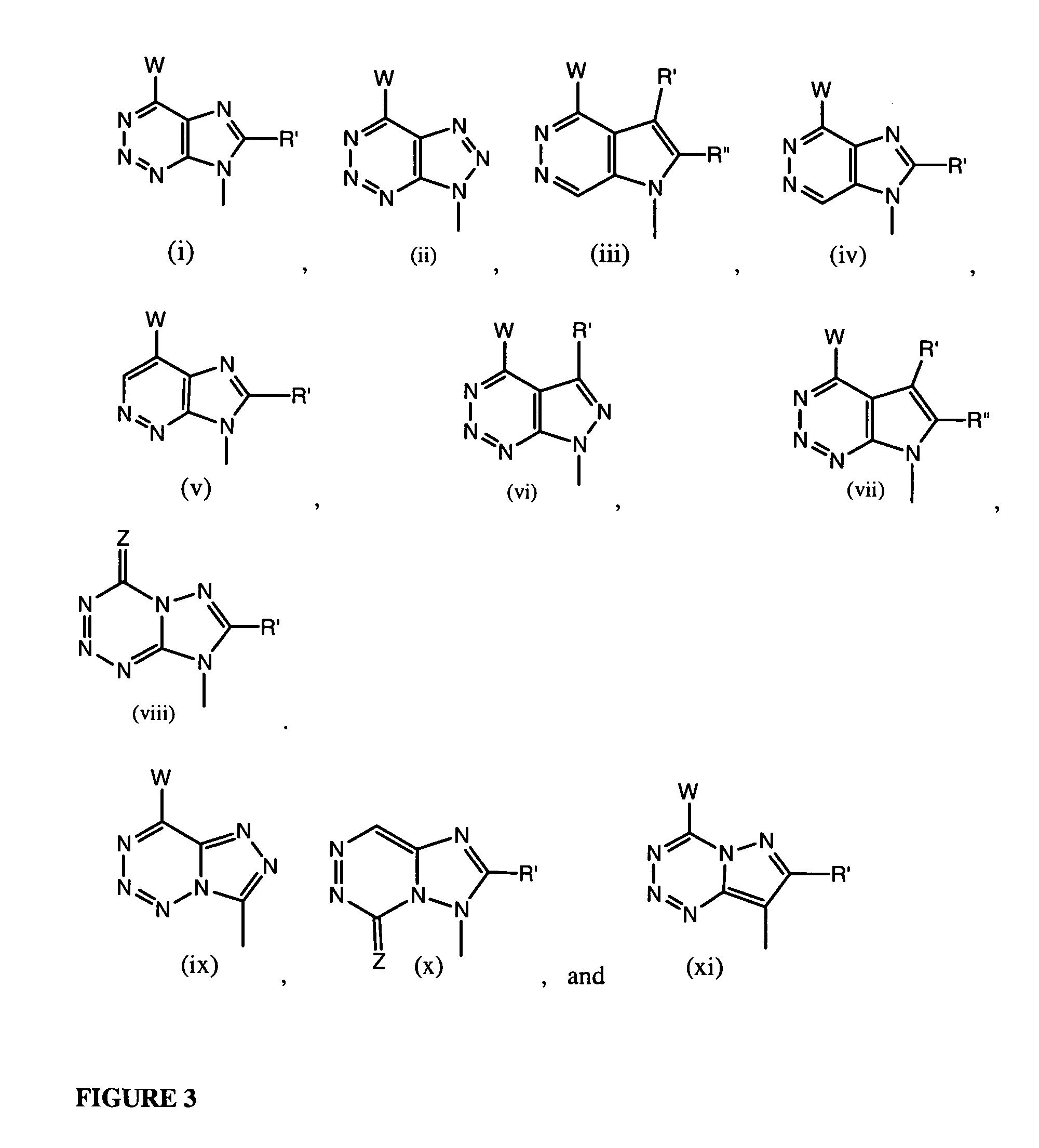
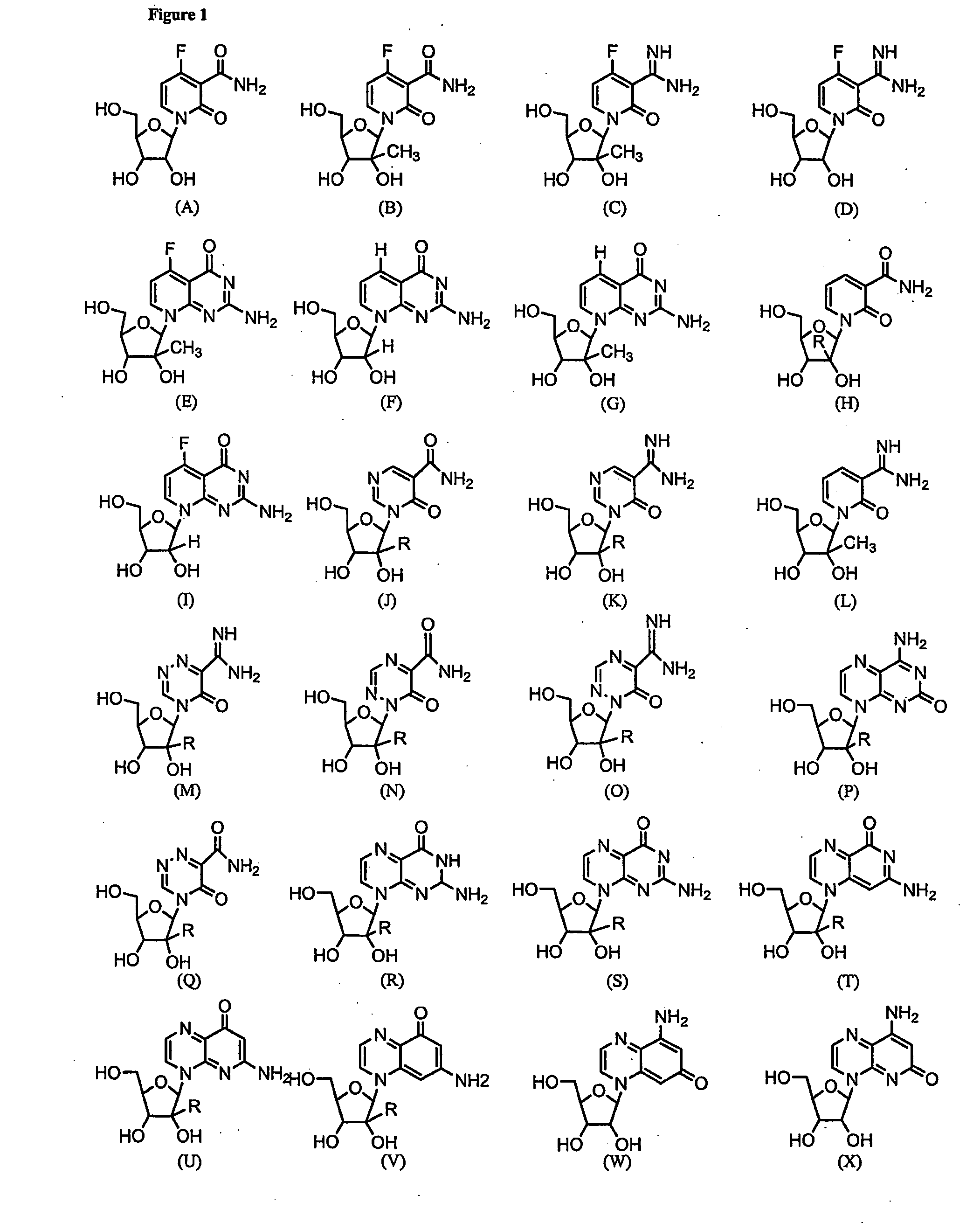
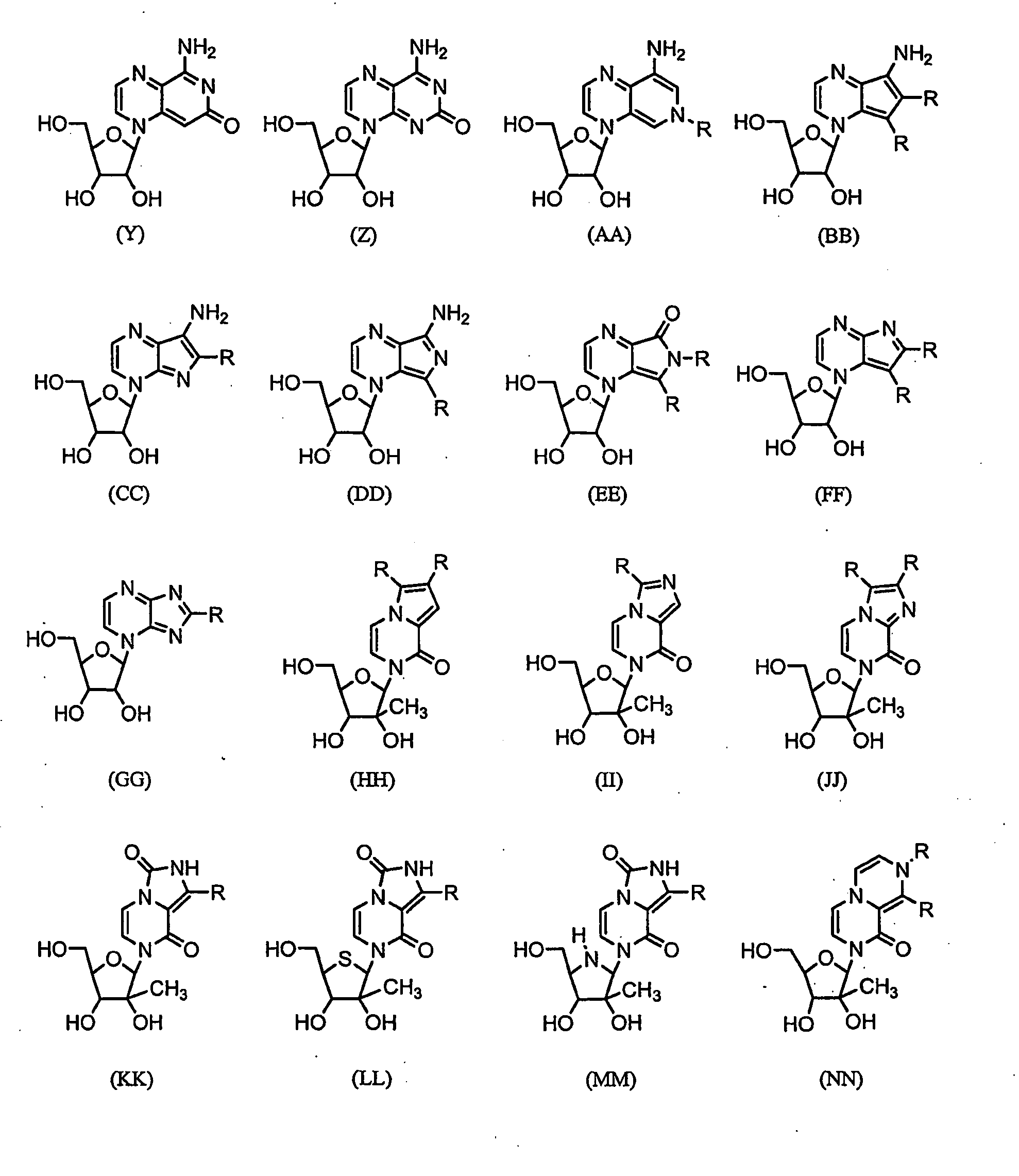
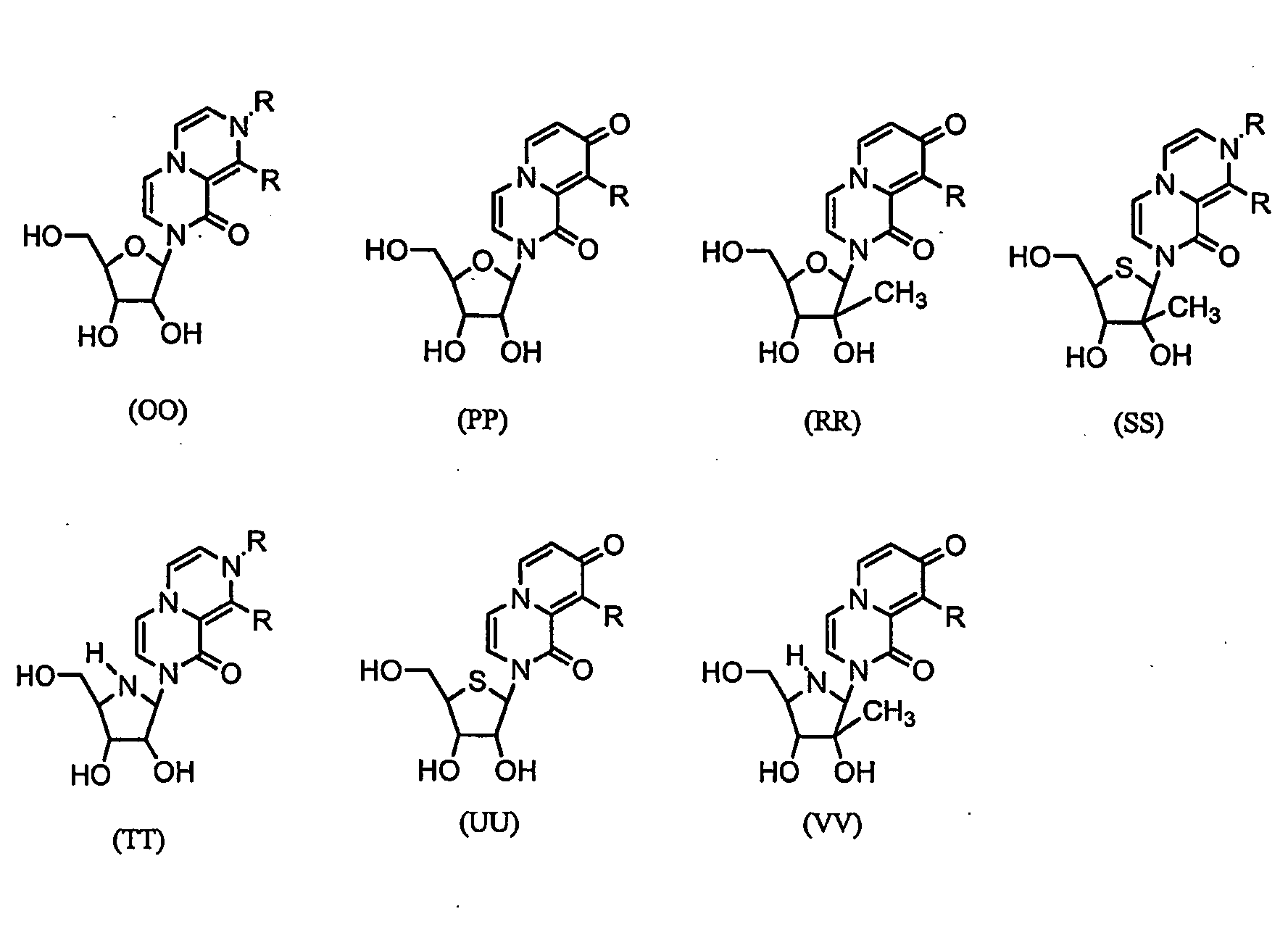
Nucleosides With Non-Natural Bases as Anti-Viral Agents
A method and composition for treating a host infected with flavivirus, pestivirus or hepacivirus comprising administering an effective flavivirus, pestivirus or hepacivirus treatment amount of a described base-modified nucleoside or a pharmaceutically acceptable salt or prodrug thereof, is provided.
Owner:INDENIX PHARM LLC +2
Flavivirus expression and delivery system
InactiveUS6893866B1High expressionImprove translation efficiencySsRNA viruses positive-senseVectorsNucleotideStructural protein
The present invention provides a gene expression system comprising: a) a self-replicating expression vector of flavivirus origin which includes the flavivirus 5′ untranslated region (UTR), at least a portion of the 5′ coding region for flavivirus core protein, the nucleotide sequence coding for the flavivirus non-structural proteins, and the complete or most of the 3′-terminal sequence of the flavivrus 3′UTR, required for self-replication of flavivirus genomic material, which vector is adapted to receive at least a nucleotide sequence without disrupting its replication capabilities; and b) at least a second vector that is capable of expressing flavivirus structural protein(s) and any other proteins required for packaging of the self-replicating expression vector into flavivirus viral particles which vector is engineered to prevent recombination with the self-replicating vector when in its presence.
Owner:REPLIKUN BIOTECH
Avirulent, immunogenic flavivirus chimeras
InactiveUS20060062803A1Inhibition effectPreserve immunogenicityBiocideOrganic active ingredientsViral diseaseFhit gene
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Method to Treat Flavivirus Infection with siRNA
InactiveUS20090047338A1Avoid infectionInhibit expressionOrganic active ingredientsSsRNA viruses positive-senseAntisense RNADisease
The present invention is directed to methods of treating flavivirus mediated diseases using siRNAs. The invention is based upon our findings in a mouse model that siRNAs directed against sequences conserved among multiple flaviviruses prevents and treats flavivirus infections. Accordingly, the present invention provides an isolated siRNA comprising a sense RNA and an antisense RNA strand or a single strand. The sense and the antisense RNA strands, or the single RNA strand, form an RNA duplex, and wherein the RNA strand comprises a nucleotide sequence identical to a target sequence of about 15 to about 30 contiguous nucleotides in flavivirus mRNA or mutant or variant thereof.
Owner:IMMUNE DISEASE INST INC
Vaccines Against Japanese Encephalitis Virus and West Nile Virus
ActiveUS20070269458A1Decrease viscerotropism/viremiaHigh genetic stabilitySsRNA viruses positive-senseSugar derivativesViral VaccineWest Nile virus RNA
The invention provides attenuated Flavivirus vaccines, such as vaccines against Japanese encephalitis virus and West Nile virus, as well as methods of making and using these vaccines.
Owner:SANOFI PASTEUR BIOLOGICS CO
Use of flavivirus for the expression of protein epitopes and development of new live attenuated vaccine virus to immunize against flavivirus and other infectious agents
InactiveUS20060159704A1Stable expressionSafe and effectiveSsRNA viruses positive-senseVirus peptidesEpitopeSpecific immunity
The present invention relates to a vaccine against infections caused by flavivirus. More particularly to the use of the YF vaccine virus (17D) to express at the level of its envelope, protein epitopes from other pathogens which will elicit a specific immune response to the parental pathogen.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com