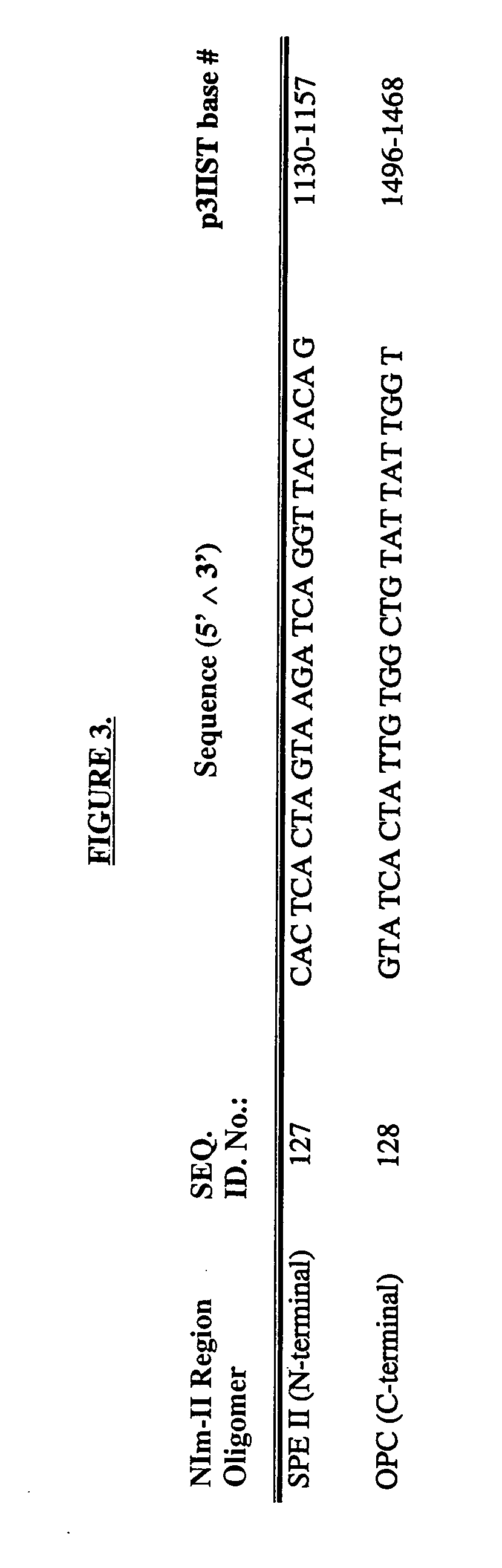
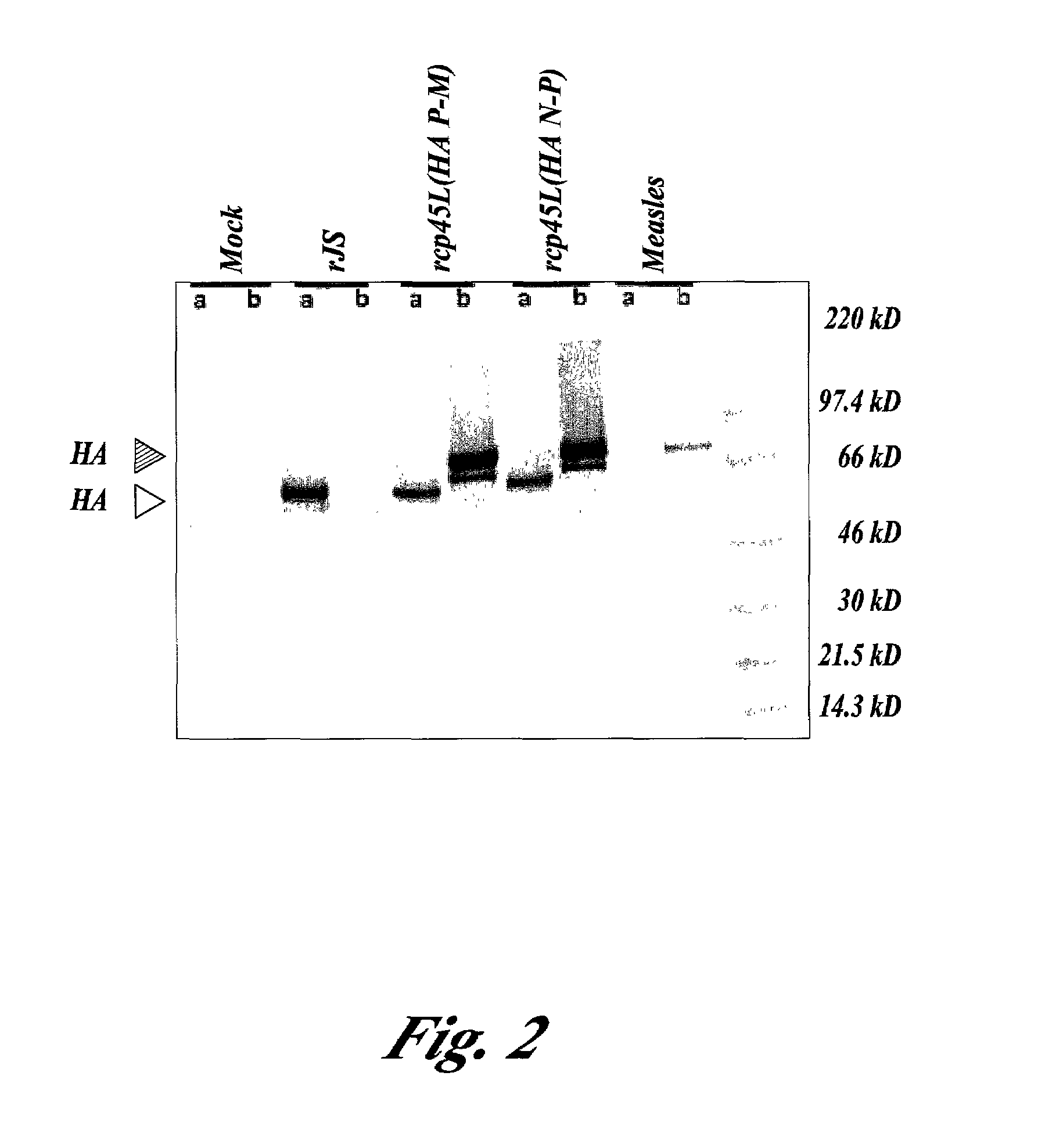
Patents
Literature
162 results about "Chimeric virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
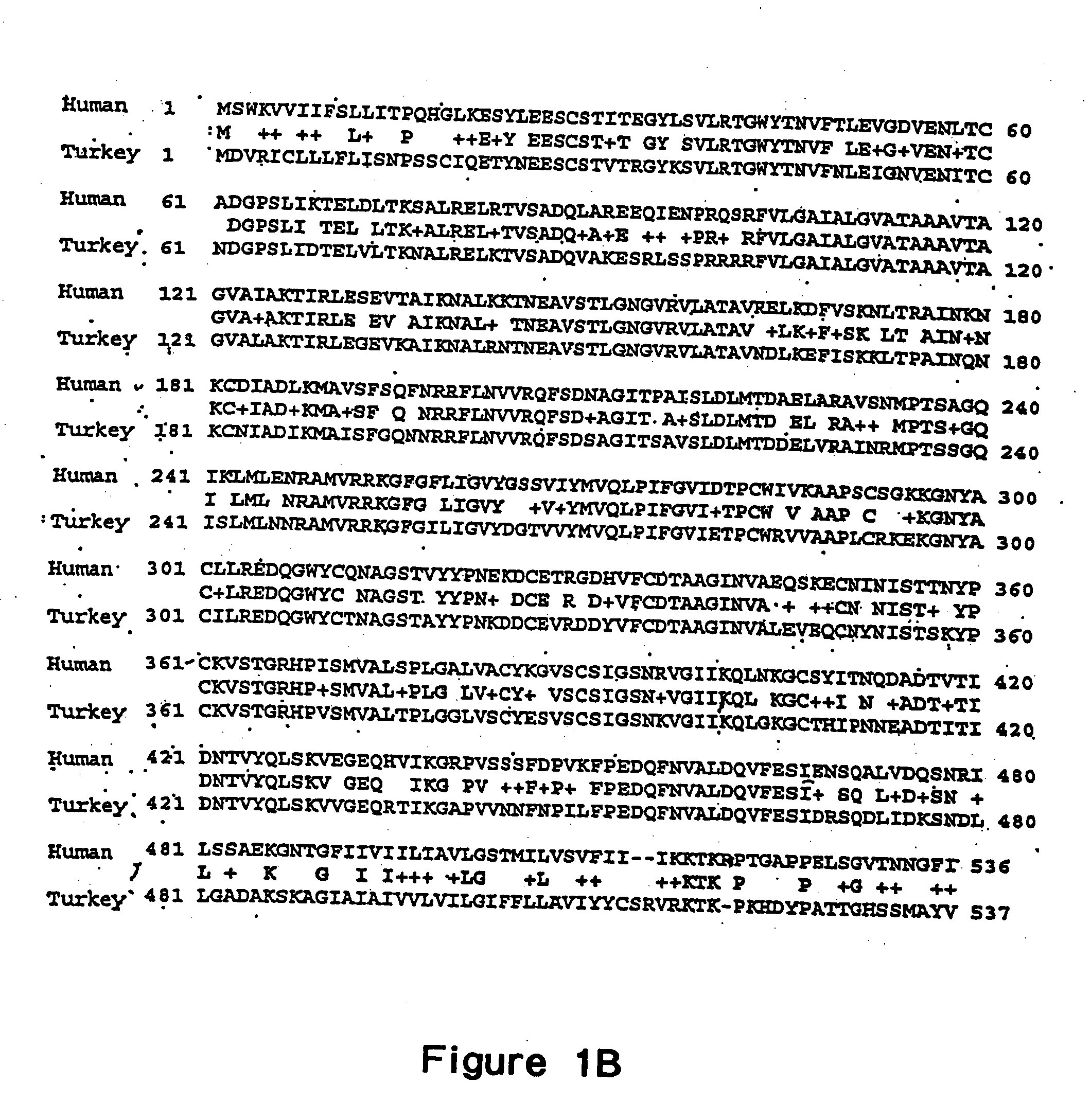
In mythology, a chimera is a creature such as a hippogriff or a gryphon formed from parts of different animals, thus the name for these viruses. Chimeric flaviviruses have been created in an attempt to make novel live attenuated vaccines.
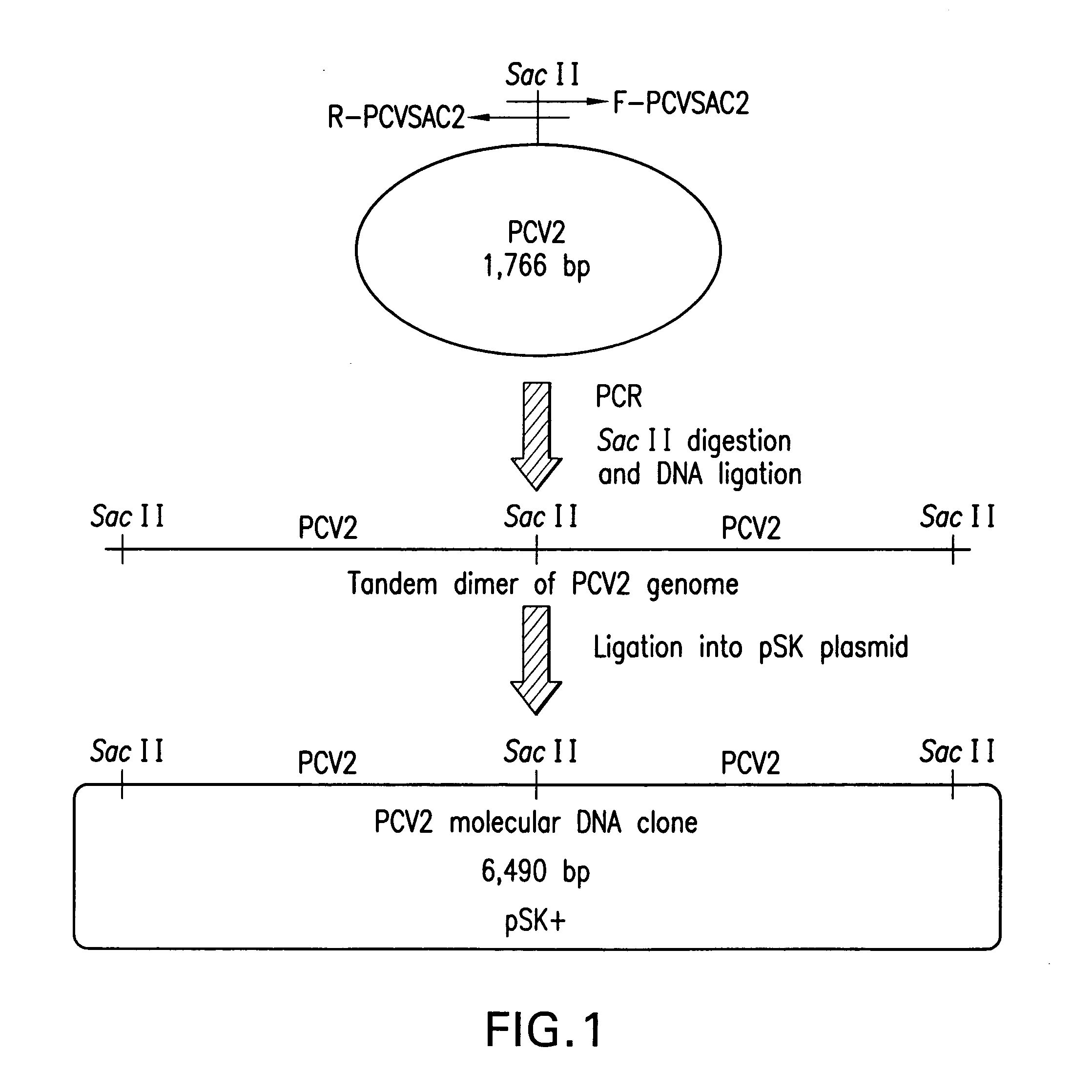
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products.
Owner:IOWA STATE UNIV RES FOUND +1
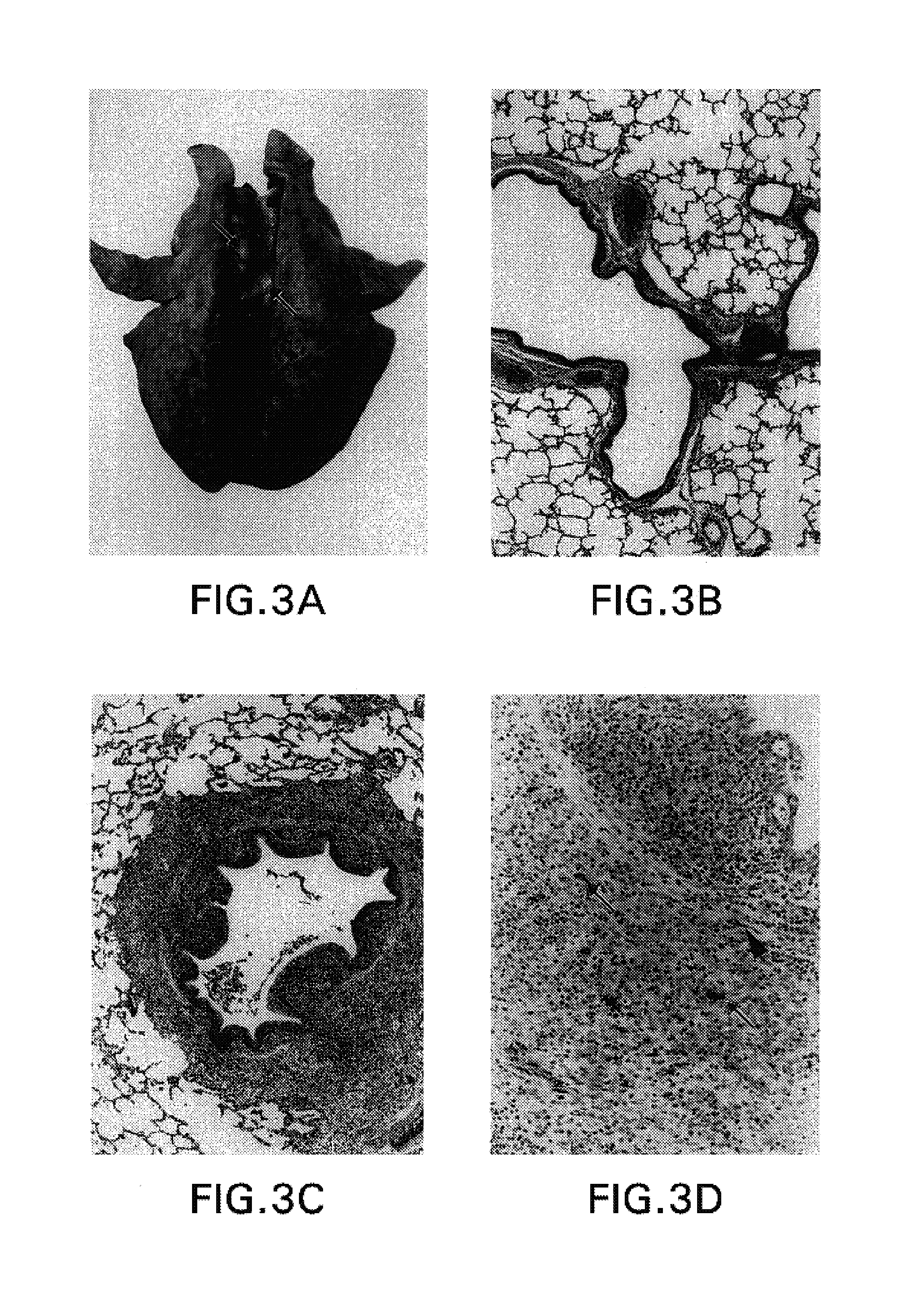
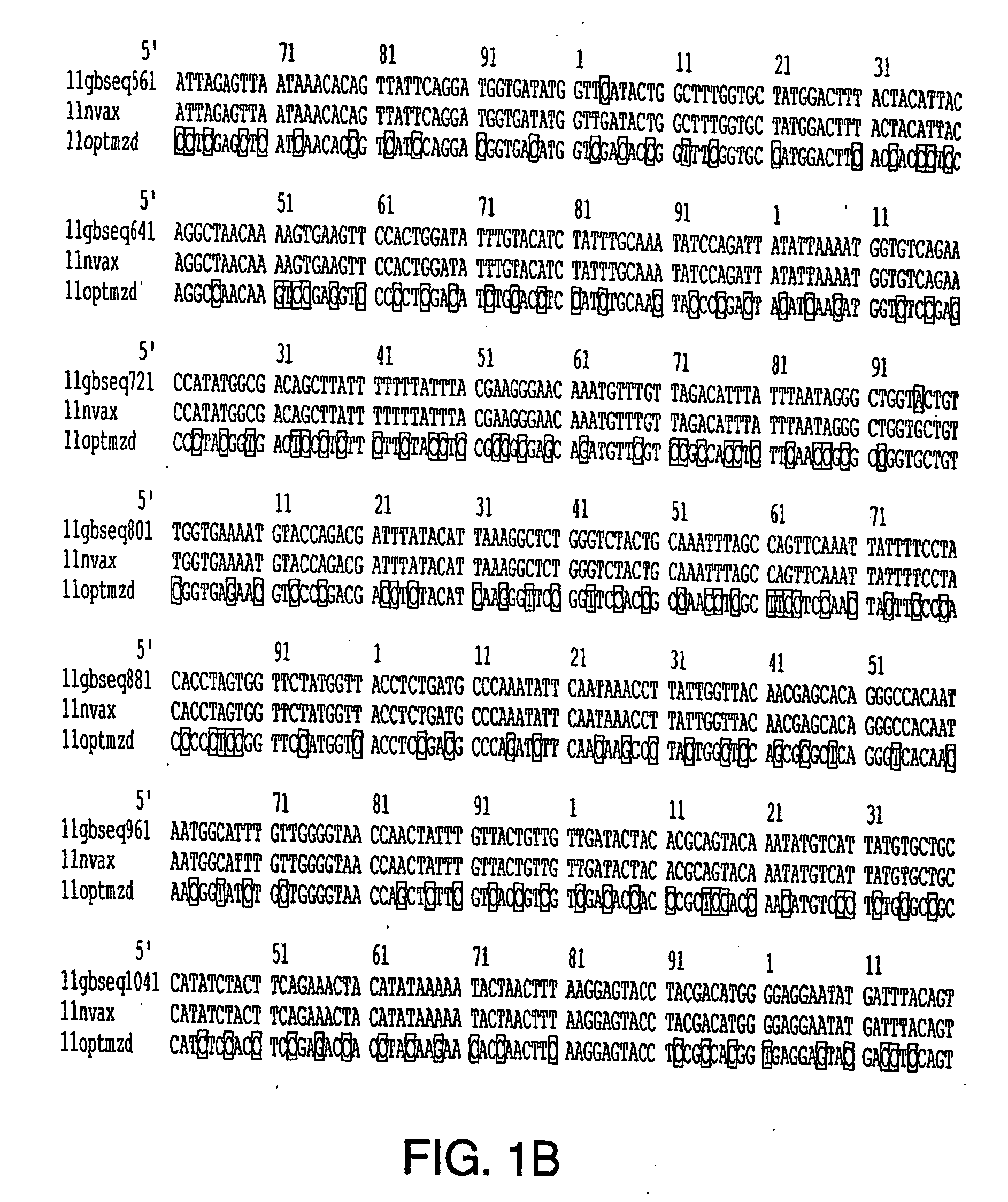
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
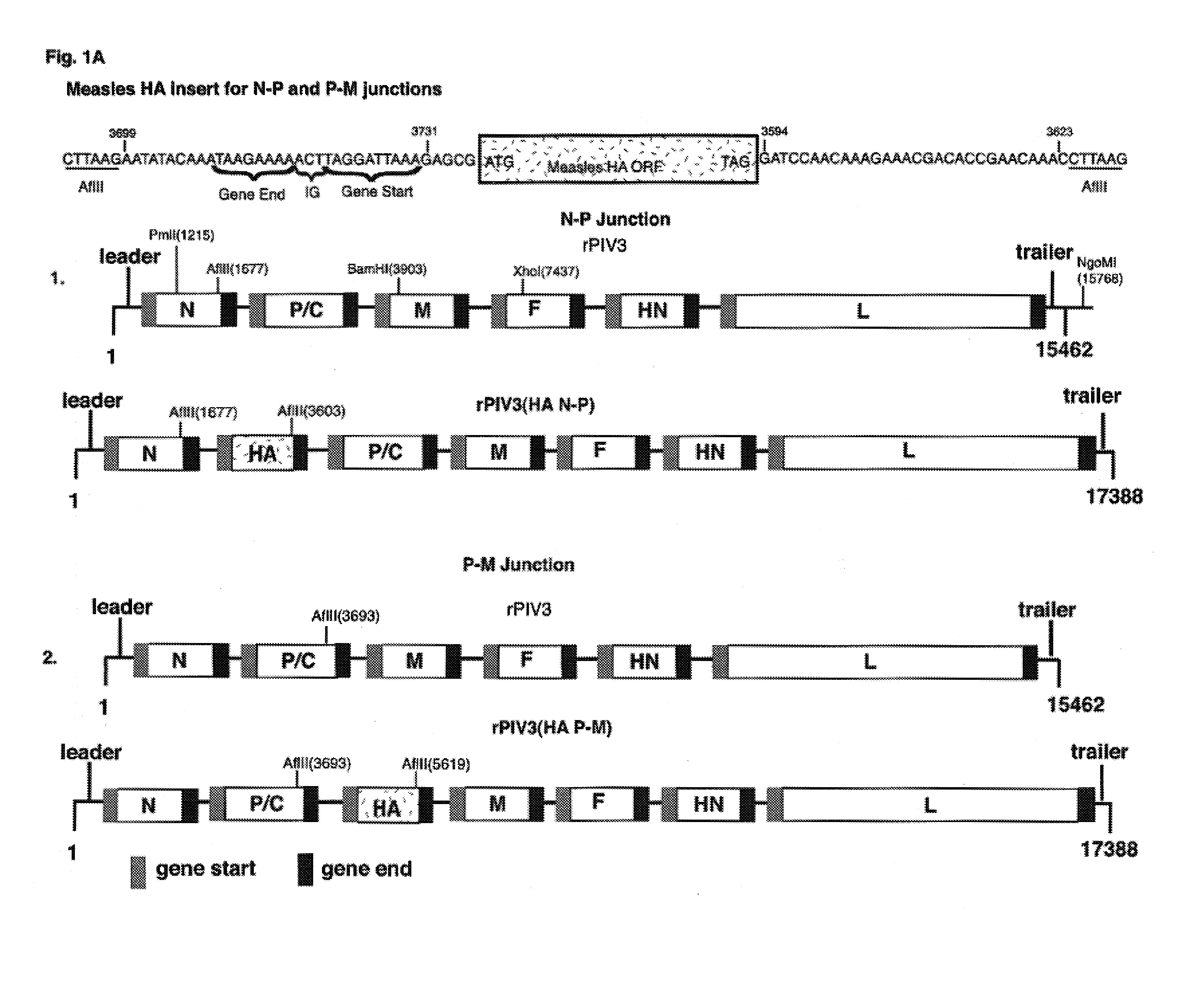
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
InactiveUS20030232061A1Optimization orderImprove scalabilitySsRNA viruses negative-senseVectorsNegative strandAntigen
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
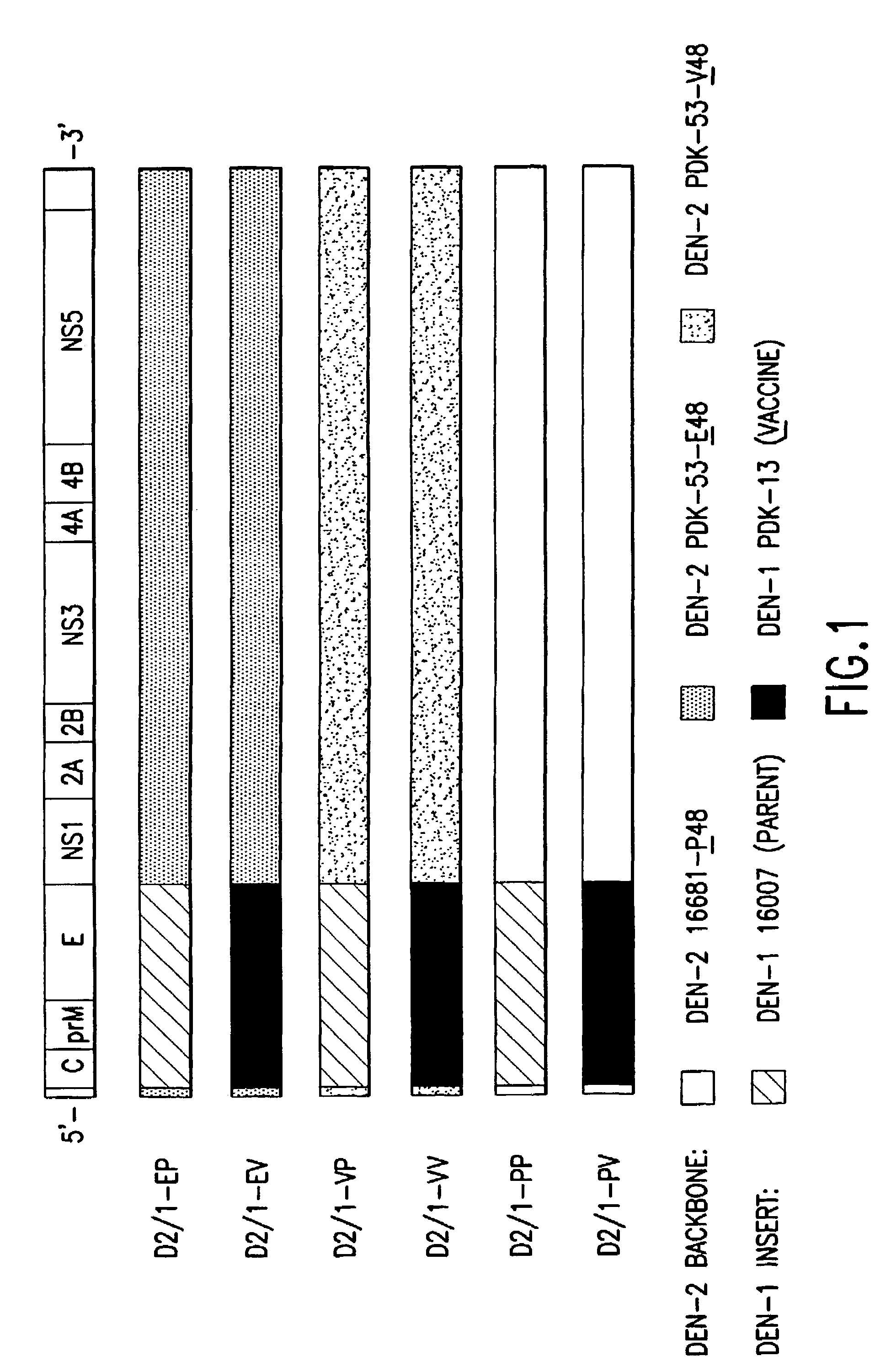
Avirulent, immunogenic flavivirus chimeras
InactiveUS7094411B2Minimize and inhibit infectionStable maintenanceOrganic active ingredientsVirusesViral diseaseAmino acid mutation
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Construction and use of recombinant parainfluenza viruses expressing a chimeric glycoprotein
InactiveUS7250171B1Prone to infectionHigh titerSsRNA viruses negative-senseOrganic active ingredientsHeterologousEpitope
Chimeric parainfluenza viruses (PIVs) are provided that incorporate a PIV vector genome or antigenome modified to encode a chimeric glycoprotein incorporating one or more heterologous antigenic domains, fragments, or epitopes of a second, antigenically distinct HPIV. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting an immune responses against one or more PIVs, and, optionally against respiratory syncytial virus (RSV). Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a HPIV vector genome or antigenome combined or integrated with one or more heterologous genome segment(s) encoding one or more antigenic determinant(s) of a heterologous PIV to encode a chimeric glycoprotein. In preferred aspects of the invention, the chimeric virus is attenuated for use as a vaccine agent by additional mutations or nucleotide modifications introduced into the chimeric genome or antigenome.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE
Recombinant negative strand virus rna expression systems and vaccines
ActiveUS20050221489A1Improving immunogenicityAttenuated phenotypeSsRNA viruses negative-senseAntibacterial agentsHeterologousNegative strand
The present invention relates to recombinant RNA virus templates derived from and applicable to negative strand naturally non-segmented viruses, including the families Bornaviridae, Filoviridae, and Paramyxoviridae, and methods for generating such recombinant RNA virus templates, wherein the templates are RNA generated from two or more recombinant RNA molecules. The invention relates to the use of segmented recombinant RNA virus templates for naturally non-segmented RNA viruses to express heterologous gene products in appropriate host cell systems and / or to construct recombinant viruses taken from that family and that express, package, and / or present the heterologous gene product. The invention includes the expression products and recombinant and chimeric viruses thus prepared and vaccine and therapeutic formulations comprising the recombinant RNA viruses.
Owner:MT SINAI SCHOOL OF MEDICINE
Modified factor viii and factor ix genes and vectors for gene therapy
The present invention relates to a modified and optimized Factor VIII or Factor IX nucleic acid for inclusion in a chimeric virus vector. Use of such vector can be used for treatment of hemophilia.
Owner:ASKLEPIOS BIOPHARMACEUTICAL INC
Avirulent, immunogenic flavivirus chimeras
InactiveUS20060062803A1Inhibition effectPreserve immunogenicityBiocideOrganic active ingredientsViral diseaseFhit gene
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Chimeric virus vaccine
InactiveUS20060088549A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsHeterologousNucleotide
The present invention provides an immunogenic composition comprising: (a) an isolated recombinant chimeric human rhinovirus, wherein the recombinant chimeric human rhinovirus comprises (i) a nucleic acid having a nucleotide sequence of a human rhinovirus encoding at least a portion of a human rhinovirus capsid; (ii) a heterologous nucleic acid having a nucleotide sequence encoding a chimeric region, wherein the chimeric region is expressed on the surface of the chimeric rhinovirus and is capable of participating in an immune reaction; and (iii)a pharmaceutically acceptable carrier.
Owner:ARNOLD GAIL +1
Use of recombinant parainfluenza viruses (PIVs) as vectors to protect against infection and disease caused by PIV and other human pathogens
InactiveUS7192593B2Prone to infectionHigh titerSsRNA viruses negative-senseSugar derivativesAntigenDisease
Chimeric parainfluenza viruses (PIVs) incorporate a PIV vector genome or antigenome and one or more antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting an immune responses against one or more PIVs, or against a PIV and non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric Ply genome or antigenome which includes a partial or complete PIV vector genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) encoding antigenic determinant(s) of a heterologous PIV or non-PIV pathogen.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE DEPT OF
Modified factor VIII and factor IX genes and vectors for gene therapy
The present invention relates to a modified and optimized Factor VIII or Factor IX nucleic acid for inclusion in a chimeric virus vector. Use of such vector can be used for treatment of hemophilia.
Owner:ASKLEPIOS BIOPHARMACEUTICAL INC
Chimeric virus-like particles
InactiveUS20100047266A1SsRNA viruses negative-senseAntibacterial agentsVirus-like particleChimeric virus
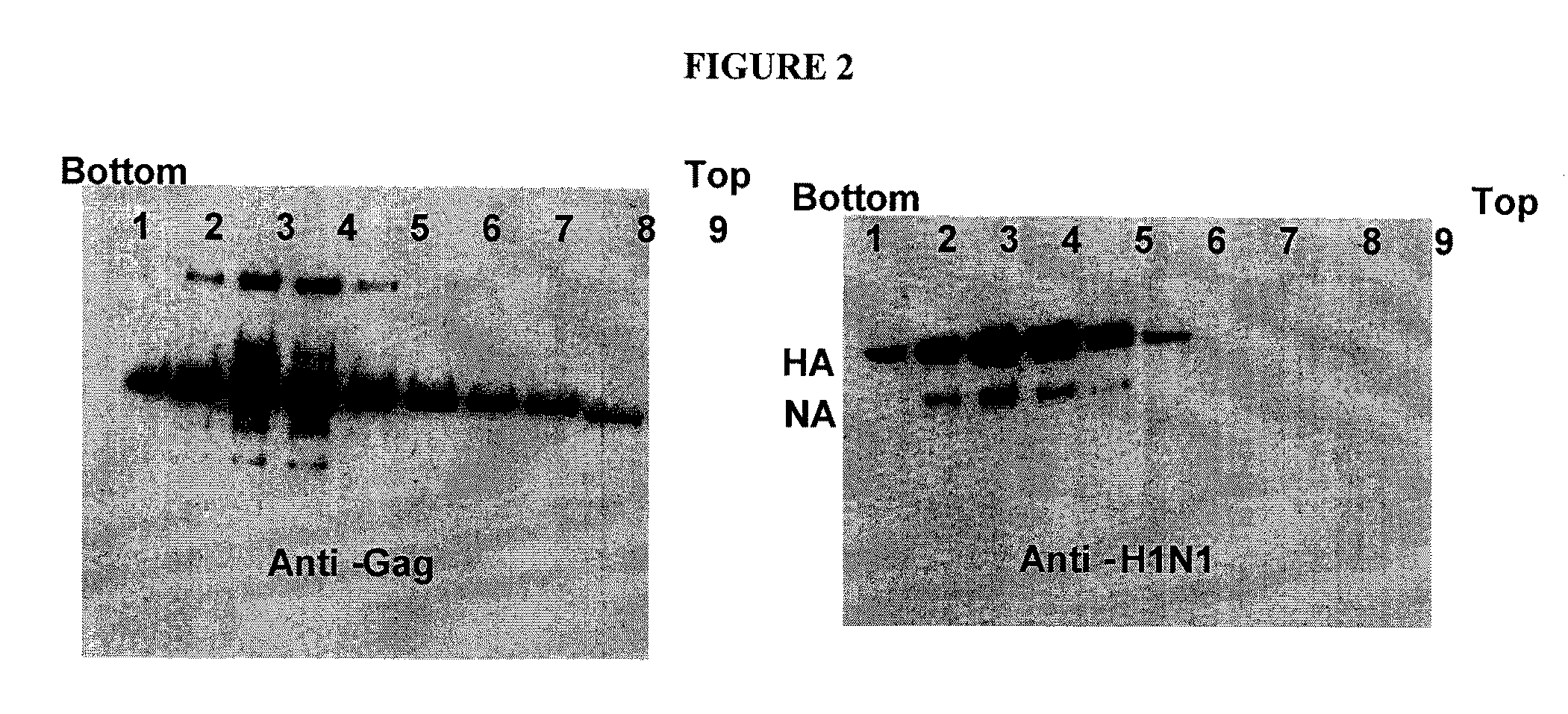
Chimeric virus-like particles including gag polypeptides are described. Virus-like particles are generated with a gag polypeptide and lipid raft-associated polypeptide linked to an antigen that is not naturally associated with a lipid raft. Preferred methods of generation include expression in insect cells.
Owner:TAKEDA VACCINES INC
Use of recombinant live-attenuated parainfluenza virus (PIV) as a vector to protect against disease caused by PIV and respiratory syncytial virus (RSV)
InactiveUS7314631B1Prone to infectionHigh titerSsRNA viruses negative-senseSugar derivativesAntigenDisease
Chimeric parainfluenza viruses (PIVs) are provided that incorporate a PIV vector genome or antigenome and one or more antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting and immune responses against one or more PIVs, or against a PIV and non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a partial or complete PIV vector genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) encoding antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. In preferred aspects of the invention, chimeric PIV incorporate a partial or complete human PIV vector genome or antigenome combined with one or more heterologous gene(s) or genome segment(s) from a heterologous PIV or non-PIV pathogen, wherein the chimeric virus is attenuated for use as a vaccine agent by any of a variety of mutations and nucleotide modifications introduced into the chimeric genome or antigenome.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE
Mosaic type virus-like particle DNA vaccine
InactiveCN1903363AIncreased ability to elicit a complete immune responseResist infectionGenetic material ingredientsAntiviralsHepatitis B virus core AntigenT cell
A chimeric virus-like granular DNA vaccine able to generate stronger B cell activity, exciting high antibody level, improving cell's immunizing level by cross presentation, and activating T cell, toxic T cell and complete immunoreaction is a recombinant eucaryotic expression carrier of coding gene with chimeric protein. Its preparing process is also disclosed.
Owner:CHINA AGRI UNIV
Recombinant parainfluenza virus expression systems and vaccines
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Chimeric protein, virus-like particle and application thereof
ActiveCN104693310AImproving immunogenicityFast and Efficient CarryBacteriaViral antigen ingredientsCircovirusVirus-like particle
The invention provides a chimeric protein, a virus-like particle and application thereof and belongs to the field of molecular biology. The chimeric protein is prepared by the following steps: replacing one, two or three of four sites in a porcine circovirus 2 type Cap protein by a main B cell epitope of a porcine O-shaped foot-and-mouth disease virus VP1 protein to obtain 58th-66th amino acid residues, 72nd-94th amino acid residues, 122nd-147th amino acid residues and 162nd-197th amino acid residues. The chimeric protein can form the chimeric virus-like particle after soluble expression, shows the main B cell epitope of the porcine O-shaped foot-and-mouth disease virus VP1 protein on the surface of the chimeric virus-like particle, has very good immunogenicity to both PCV2 and FMDV and can generate a high antibody to the PCV2 and the FMDV, by once immune.
Owner:江苏省苏农科技术转移中心有限公司
Classical swine fever virus virulence determinant and a novel classical swine fever vaccine
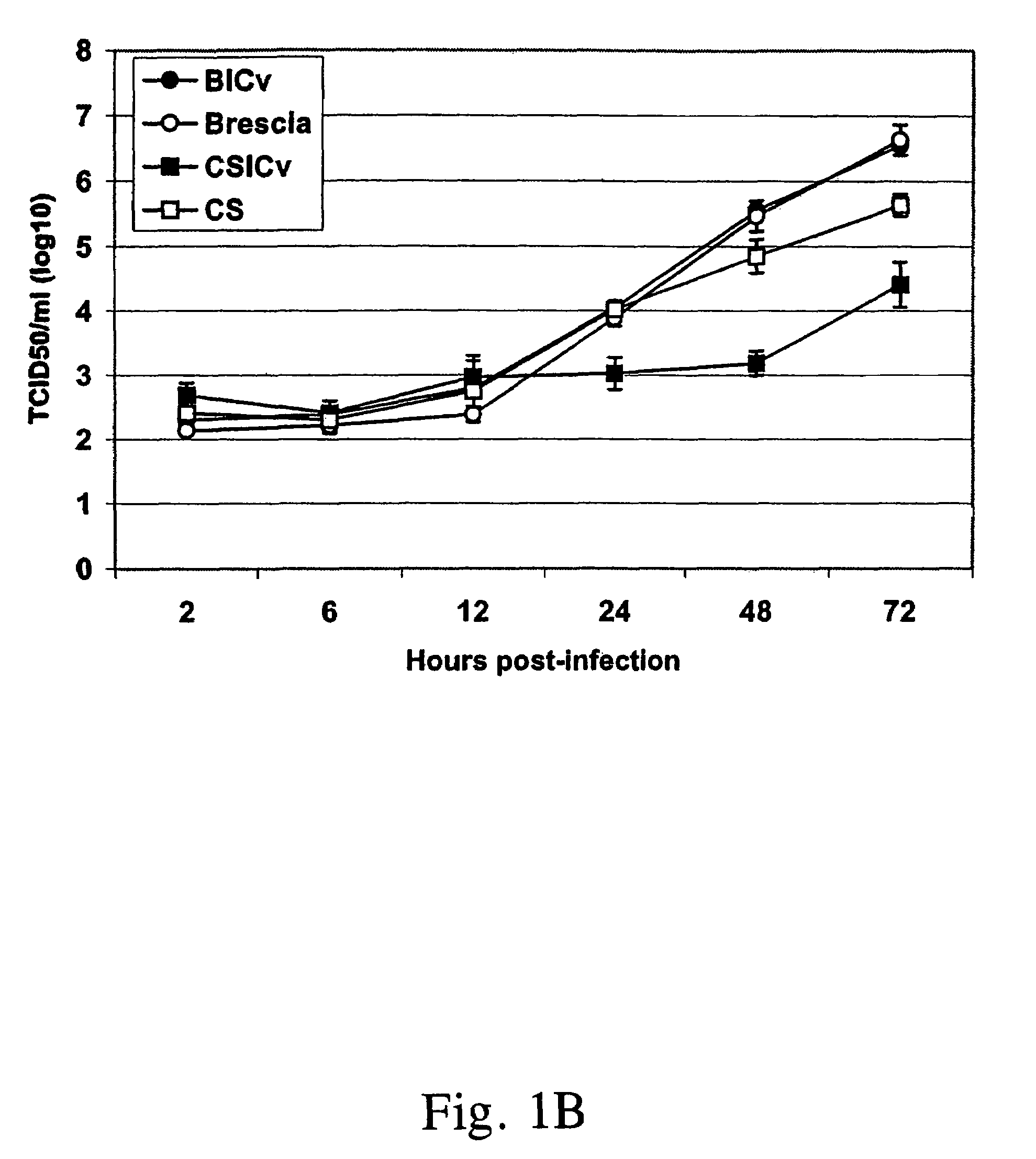
InactiveUS7332170B1Readily apparentSsRNA viruses positive-senseViral antigen ingredientsHighly pathogenicVirulent characteristics
Transposon linker insertion mutagenesis of a full-length infectious clone of the highly pathogenic classical swine fever virus (CSFV) isolate Brescia (pBIC) was used to identify genetic determinants of CSFV virulence and host range. A virus mutant, RB-C22 (RB-C22v), possessing a 19-residue tag insertion at the carboxyl end of E1 was constructed. RB-C22v and the parental virus pBIC (pBICv) exhibited similar growth characteristics on primary porcine macrophage cell cultures although RB-C22v produced significantly smaller plaques on SK6 cell cultures. In vivo, RB-C22v was markedly attenuated in swine. In contrast with pBIC infection, where mortality was 100%, all RB-C22v-infected pigs survived infection remaining clinically normal. Additionally, chimeras of the Brescia strain and the attenuated vaccine strain CS were constructed and evaluated for viral virulence in swine. Chimeras 138.8v and 337.14v, chimeras containing the E2 glycoprotein of CS and chimeric virus 319.1v, which contained only the CS E2 glycoprotein in the Brescia background, were attenuated in swine. Chimeras encoding all Brescia structural proteins in a CS genetic background remained attenuated, indicating that additional mutations outside the structural region are important for CS vaccine virus attenuation. The combined results indicate a significant role for E1 glycoprotein and E2 glycoprotein in swine virulence.
Owner:UNITED STATES OF AMERICA AS RESPRESENTED BY THE SEC OF AGRI THE
Ordinary/chimeric virus-like particle of H7 subtype influenza virus H7N9, preparation method and application of ordinary/chimeric virus-like particle, and vaccine
ActiveCN108329379AGood effectFix security issuesSsRNA viruses negative-senseViral antigen ingredientsVirus-like particleViral Vaccine
The invention provides an ordinary / chimeric virus-like particle of an H7 subtype influenza virus H7N9, a preparation method and application of the ordinary / chimeric virus-like particle, and a vaccine,and belongs to the technical field of bioengineering and viral vaccines. The ordinary / chimeric virus-like particle of the H7 subtype influenza virus is used for preparing the vaccine, and accordingly, the safety problems of existing vaccines are well solved; the obtained vaccine has high antibody valence and obvious effect; the ordinary / chimeric virus-like particle has wide application range andhigh actual application value.
Owner:NOVARTIS BIOTECH WUHAN +1
Novel coronavirus vaccine based on chimeric virus-like particles
PendingCN111620952AEnhance immune responseGood immune protectionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationReceptor
Owner:SUZHOU MIDI BIOTECH CO LTD
Newcastle disease chimeric virus marker vaccine strain as well as construction method and application thereof
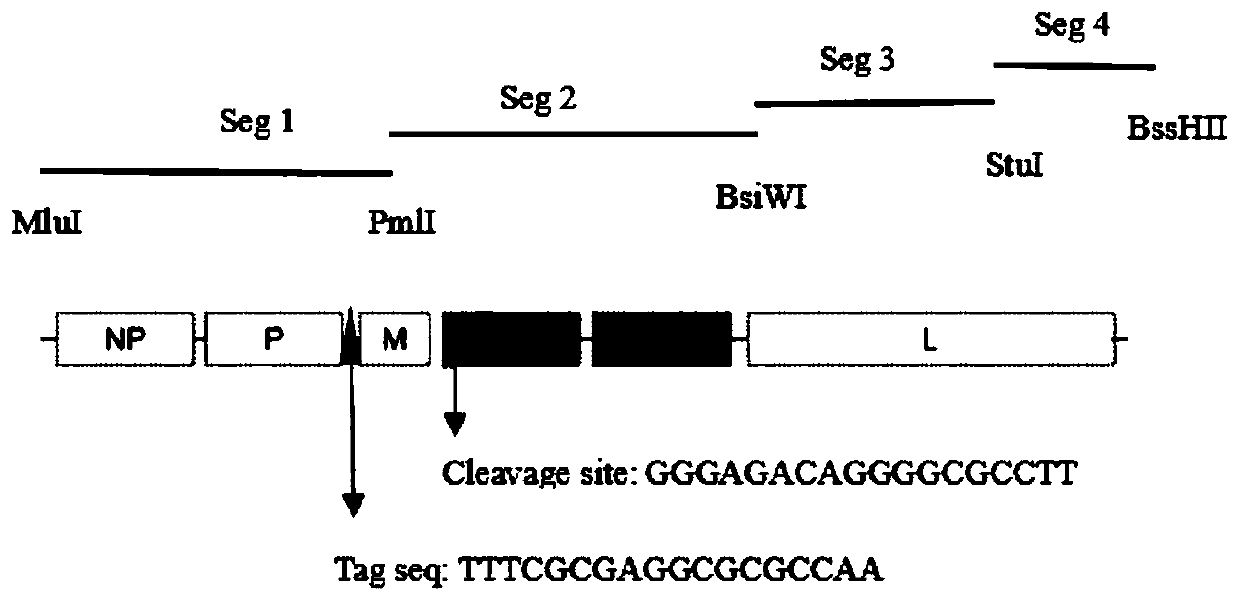
ActiveCN111575247AImprove growth characteristicsImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinChick embryos
The invention relates to a Newcastle disease chimeric virus marker vaccine strain as well as a construction method and application thereof, and belongs to the field of rescue and application of Newcastle disease chimeric vaccine strains. A Newcastle disease virus reverse genetic operation platform is utilized to mutate an F protein cleavage site of a Newcastle disease gene GVII type strain into acleavage site of an attenuated strain, F and HN genes of a mutated gene VII type Newcastle disease strain and NP, P, M and L of a gene II type NDV La Sota strain construct a full-length chimeric cDNAsequence, and a 18bp nucleotide marker sequence is inserted into a non-coding region between P and M. A Newcastle disease chimeric virus NDV DC strain is obtained through transfection cell rescue. Theconstructed chimeric virus can reach relatively high culture titer in chick embryos and cells. The chimeric strain contains envelope surface glycoprotein of the gene VII type Newcastle disease strain, contains a skeleton of the gene II type strain, and has immunogenicity of the Newcastle disease gene VII type strain and high reproduction and high safety characteristics of the gene II type La Sotastrain.
Owner:ZHEJIANG VBIOSCI INC +1
Recombinant parainfluenza virus expression system and vaccines
InactiveUS20030095987A1Suitable for useSsRNA viruses negative-sensePharmaceutical delivery mechanismAntigenNegative strand
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Vaccine for chimeric virus-like particles and preparation method thereof
ActiveCN102153656AStrong immune-inducing activityInactivation/attenuationAntiviralsEpitopeChimerin Proteins
The invention discloses chimeric protein. The chimeric protein is obtained by inserting an epitope E7 of a human papillomavirus type 16 at a ring position of human papillomavirus type 16 (HPV 16) protein L1, namely between the 140th amino acid and the 141st amino acid, wherein the epitope E7 of the human papillomavirus type 16 has genes of protein (a) with the following codes: the protein is formed by an amino acid sequence shown by an MLDLQPETT epitope (12-20E7 for short), a RAHYNIVTF epitope(49-57E7 for short), an LLMGTLGIV epitope (82-90E7 for short) or a TLGIVCPI epitope (86-93E7 for short). The invention also discloses virus-like particles formed by aggregating the chimeric protein, a mixture formed by the virus-like particles and application of the virus-like particles and the mixture of the virus-like particles to the preparation of the vaccine for HPV 16 L1-virus-like particles.
Owner:SOUTH CHINA UNITED VACCINE INST
Japanese encephalitis/dengue chimeric virus and application thereof
InactiveCN101560520AStable passageViral antigen ingredientsAntiviralsJapanese encephalitisWestern blot
The invention provides a Japanese encephalitis / dengue chimeric virus, which is obtained by replacing a prM / E gene in Japanese encephalitis cDNA clone with the prM / E gene of dengue virus type II. IFA and Western blot confirm that the chimeric virus can express E protein of a dengue virus and can stabilize passage in C6 / 36 cells. The invention lays a foundation for developing a dengue virus vaccine. The chimeric virus can be taken as a vaccine to immunize animals so as to enable the animals to obtain immune protection against the dengue virus.
Owner:中国疾病预防控制中心病毒病预防控制所
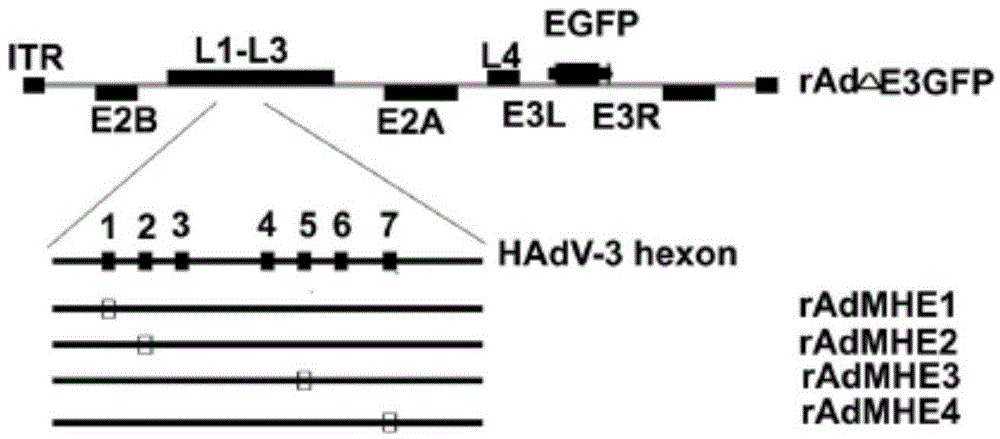
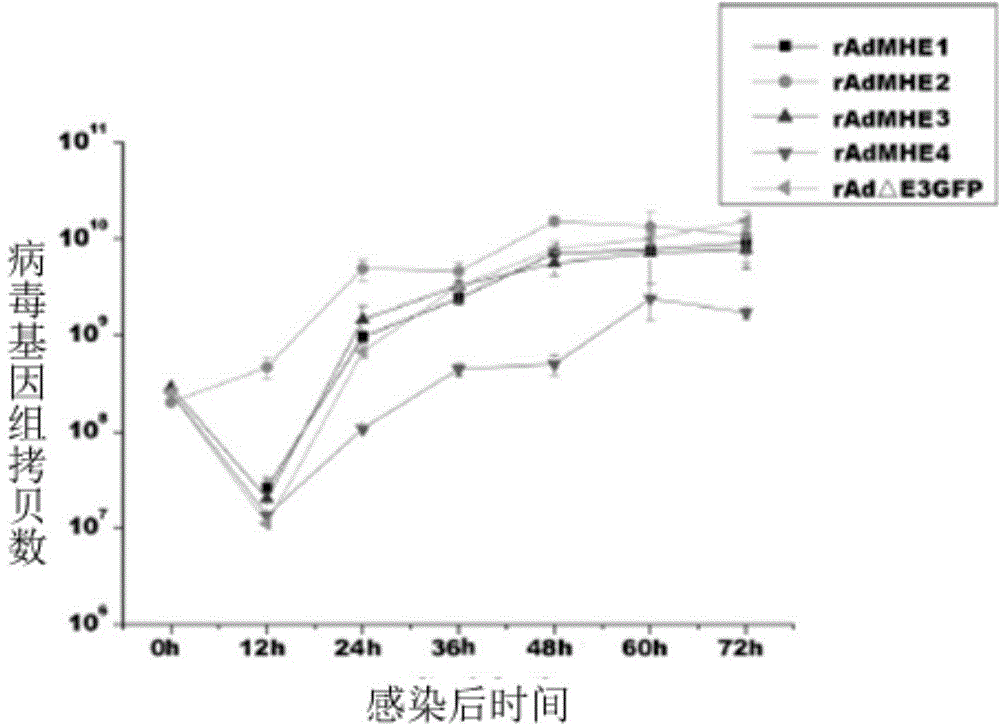
Neutralizing monoclonal antibody in human adenovirus 7 and preparation method and application thereof
ActiveCN104086650AEffective displayEnhance immune responseMicroorganism based processesImmunoglobulins against virusesResponse effectInfective disorder
The invention discloses a method for preparing a neutralizing monoclonal antibody in a human adenovirus 7. The hexon fifth hypervariable region of a human adenovirus 3 vector is substituted by a hexon fifth hypervariable region gene of the human adenovirus 7 to construct a chimeric virus which is taken as an immunogen. A murine human adenovirus 7 neutralizing monoclonal antibody and a hybridoma cell strain 5MH5 / 3G5 prepared by using the method are further provided. A humanized and chimeric monoclonal antibody prepared by using the method and a medicinal composition comprising the humanized or human-mouse chimeric monoclonal antibody serving as an effective component are further provided. An exogenous neutralizing epitope is embedded into the hypervariable region of the human adenovirus 3 vector to construct a recombinant adenovirus showing the exogenous neutralizing epitope. By using the recombinant adenovirus as an immunogen, the exogenous neutralizing epitope can be shown effectively, the immune response effect is enhanced, and the research of a monoclonal antibody for treating infectious diseases is facilitated.
Owner:广州瑞发一号健康投资中心(有限合伙)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com