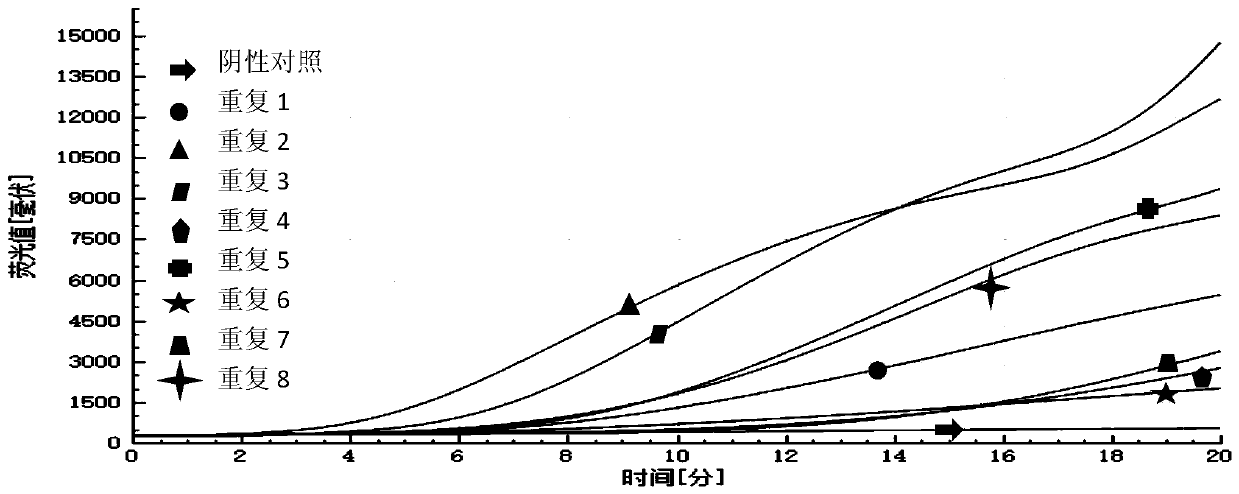
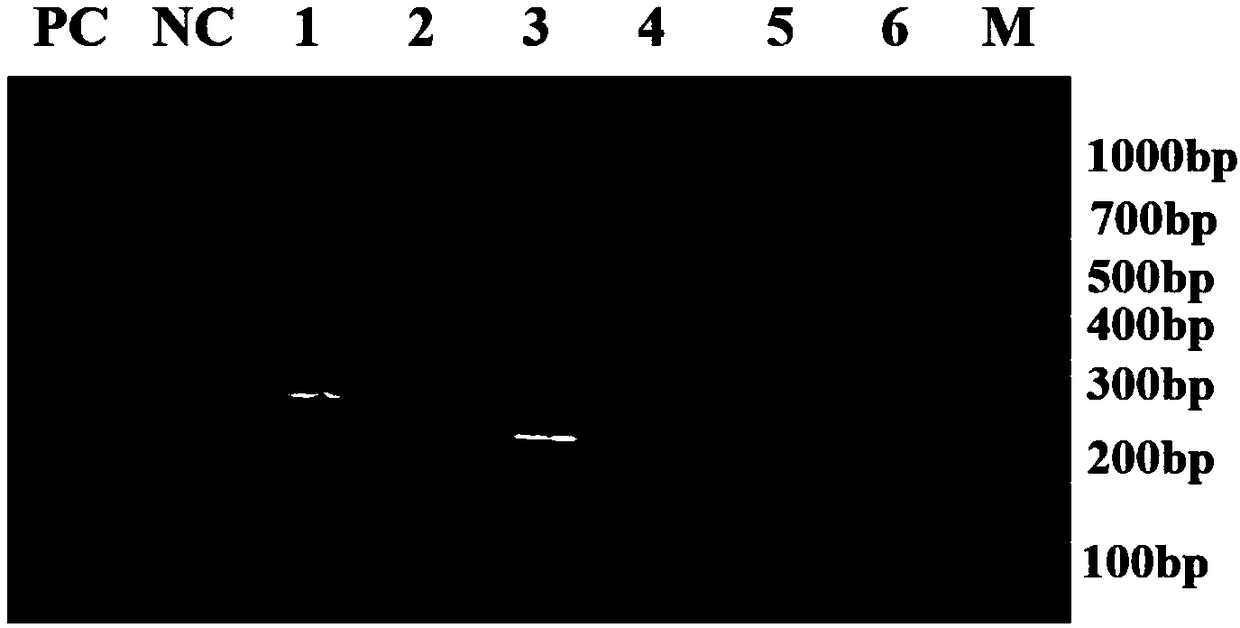
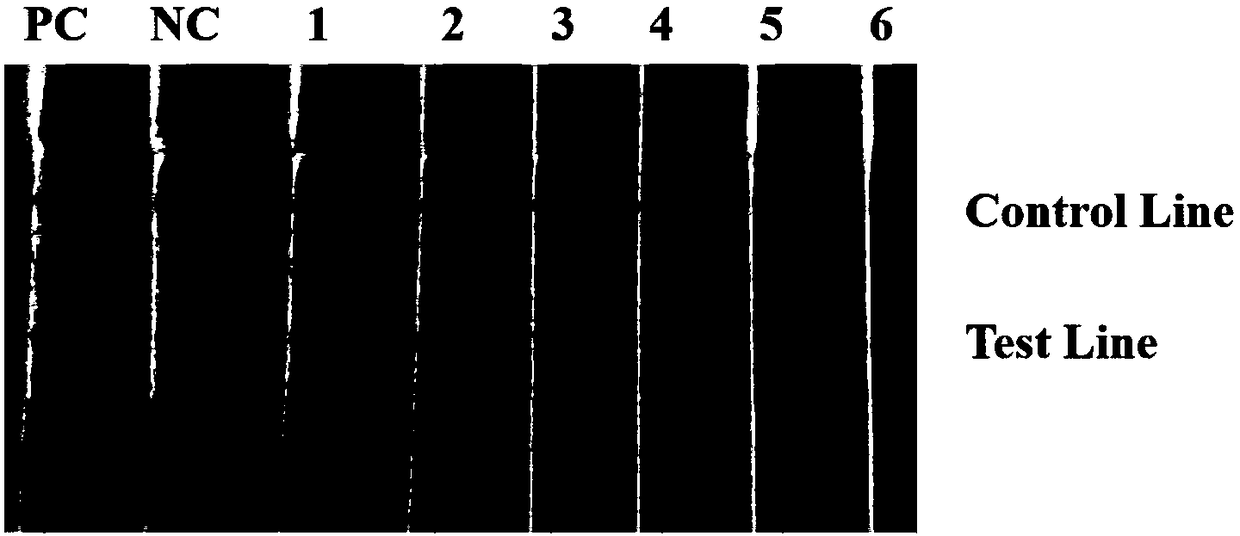
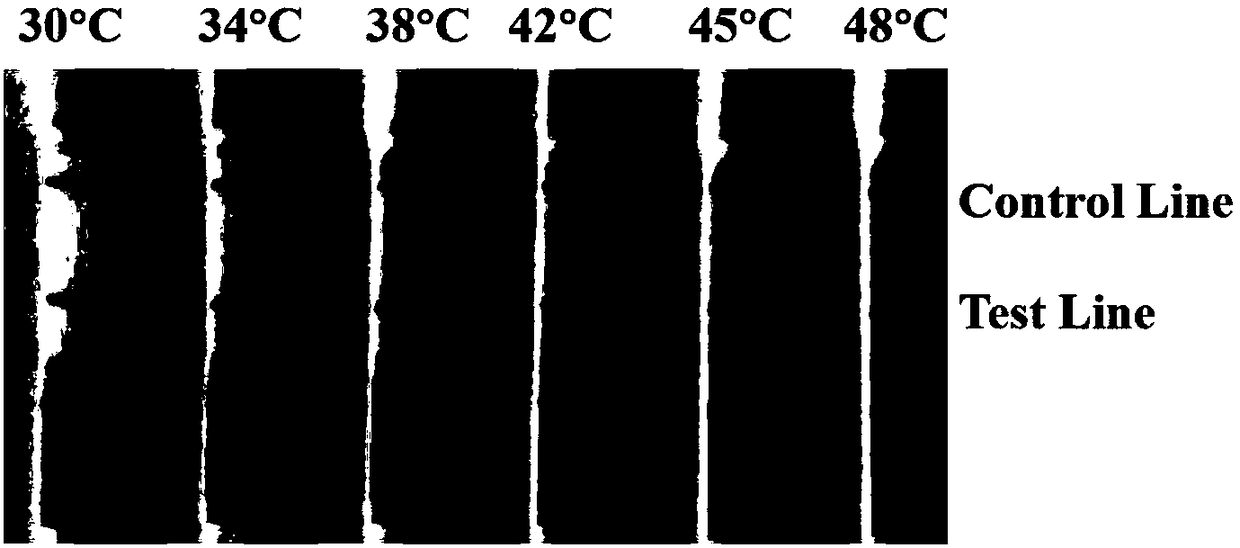
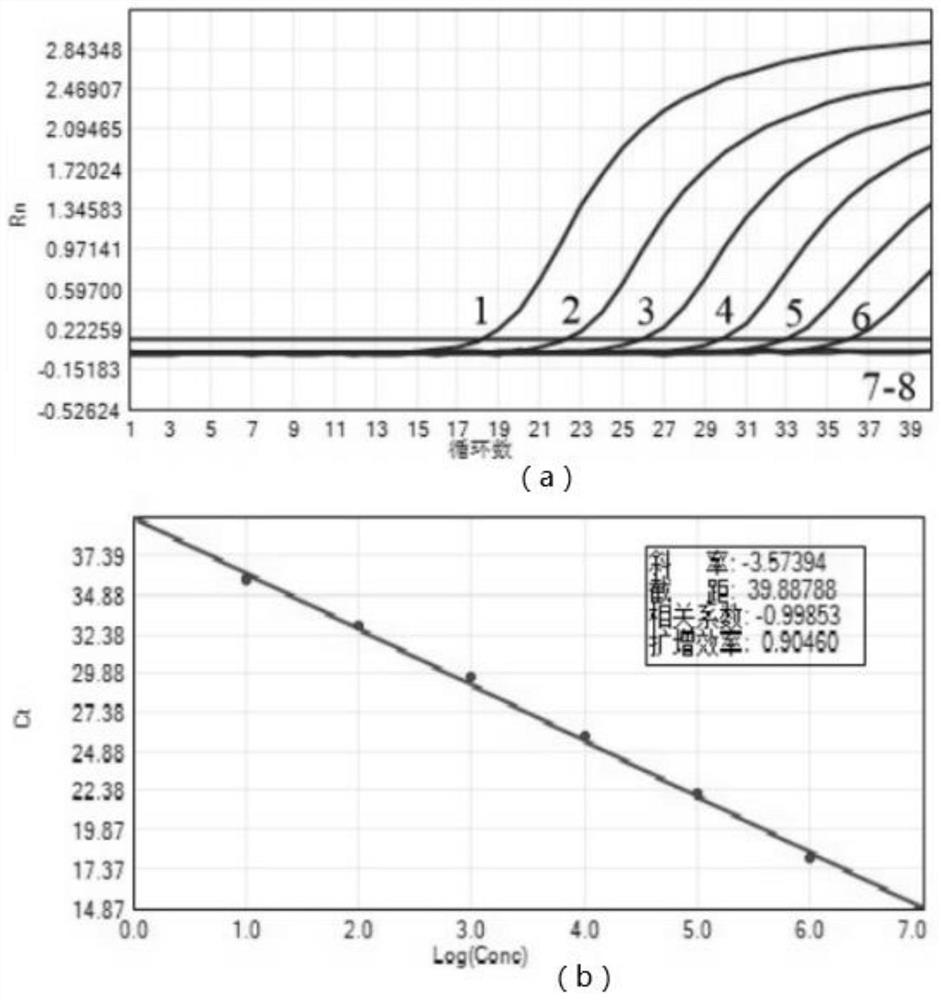
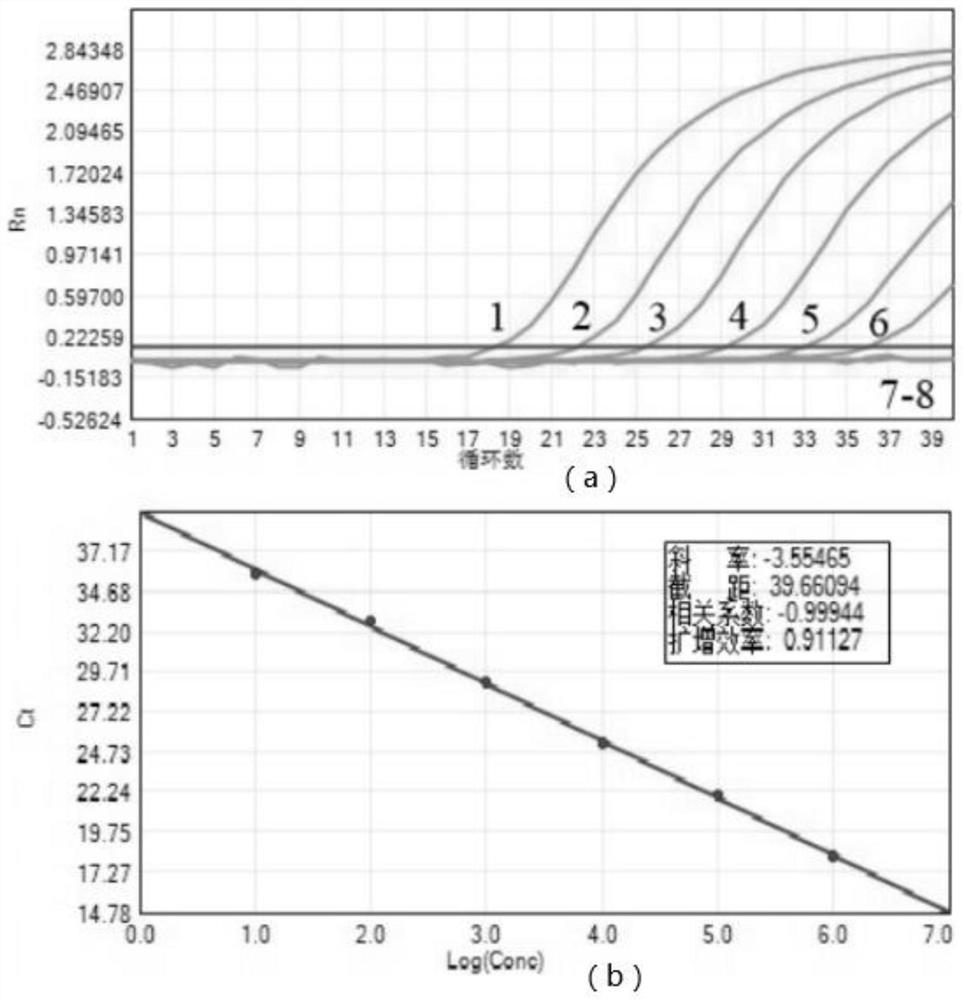
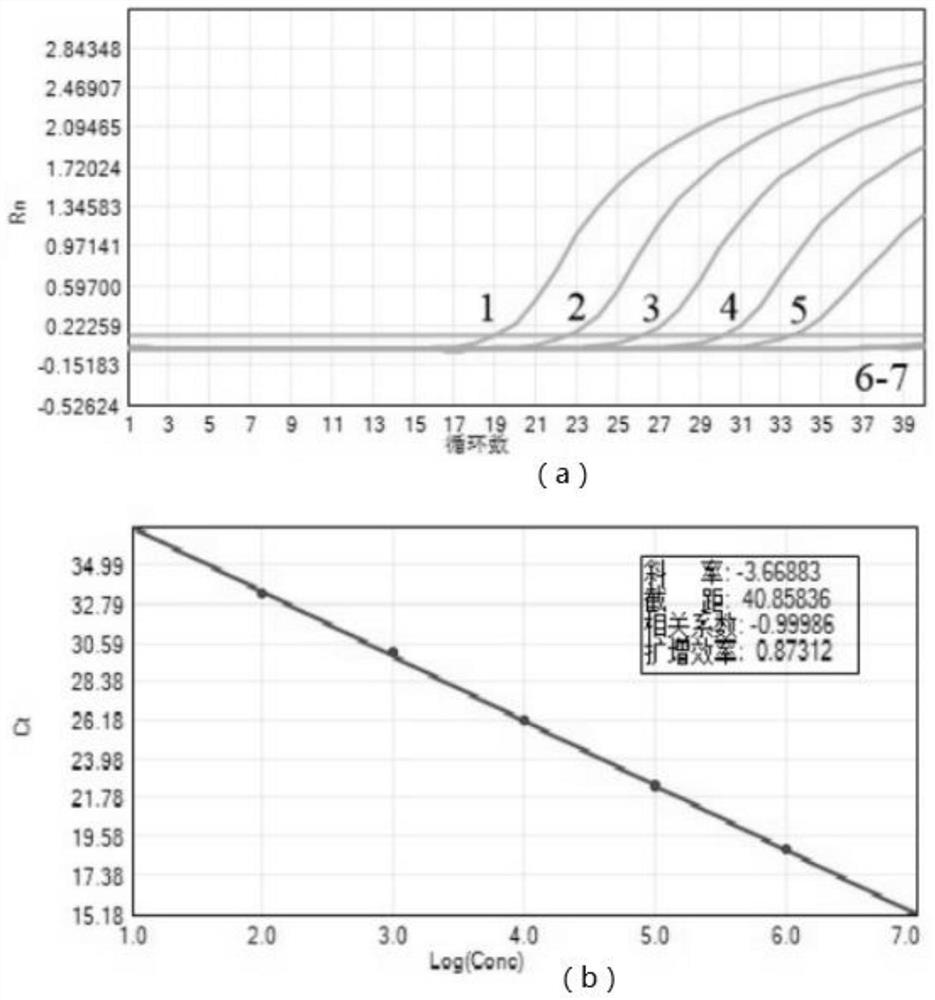
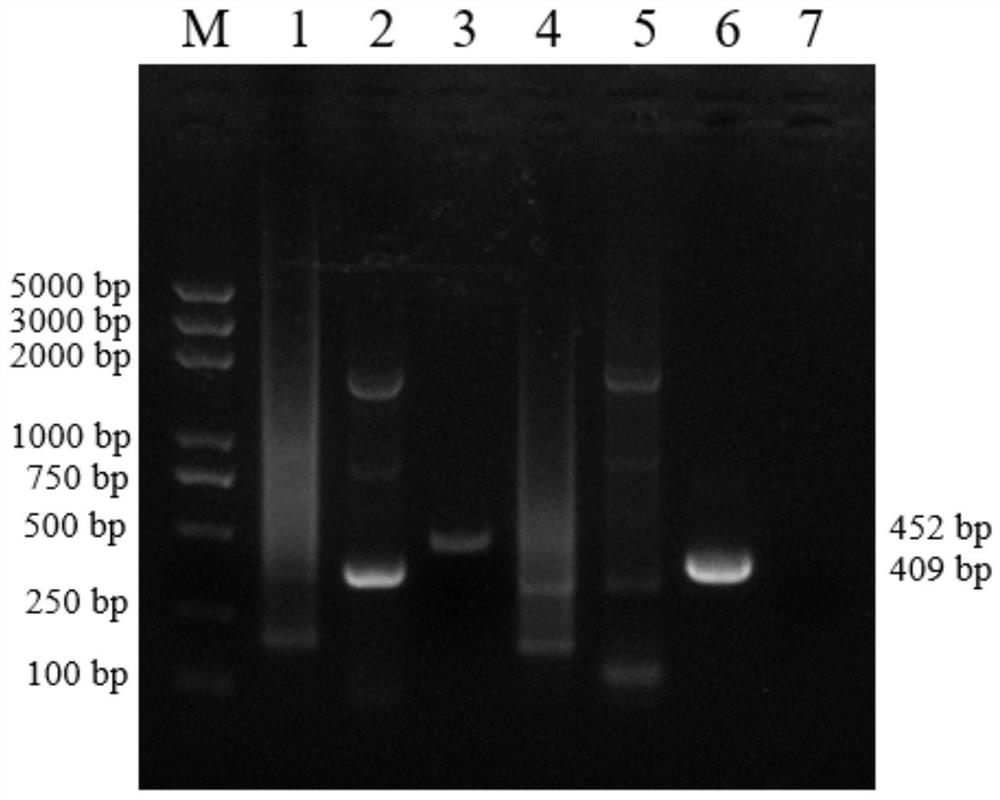
Patents
Literature
41 results about "Bovine parainfluenza virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bovine Respiratory Myxovirus Parainfluenza-3 This disease is caused by a virus from the paramyxovirus family and is known as the bovine parainfluenza virus type 3 (PI3). The disease is observed mostly in cattle. In the majority of cases, calves are said to be more susceptible to contracting the disease.
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
InactiveUS20030232061A1Optimization orderImprove scalabilitySsRNA viruses negative-senseVectorsNegative strandAntigen
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression system and vaccines
InactiveUS20030095987A1Suitable for useSsRNA viruses negative-sensePharmaceutical delivery mechanismAntigenNegative strand
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
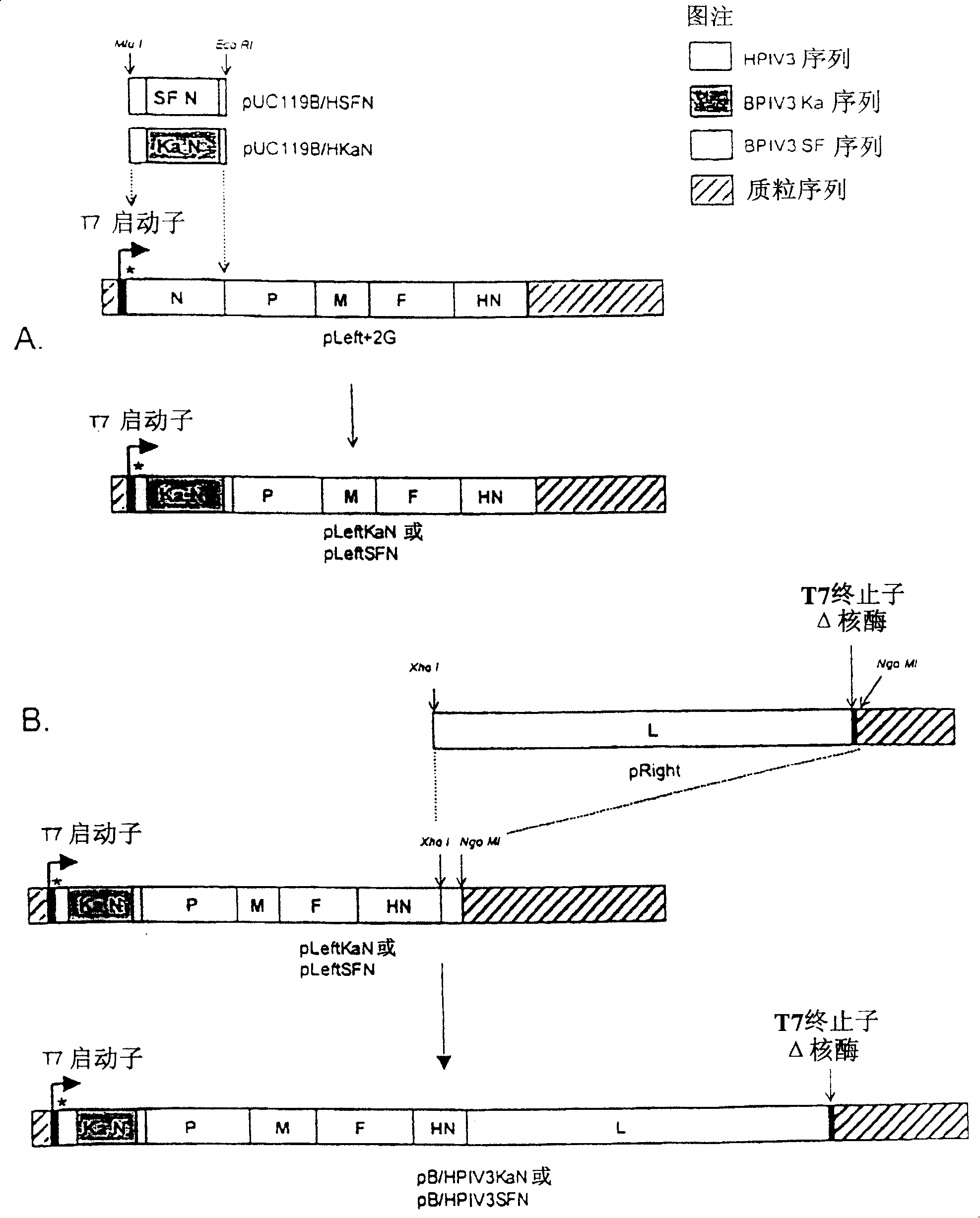
Attenuated human-bovine chimeric parainfluenza virus (PIV) vaccines
InactiveUS7622123B2High levelDesired characteristicSsRNA viruses negative-senseViral antigen ingredientsHeterologousBovine parainfluenza virus
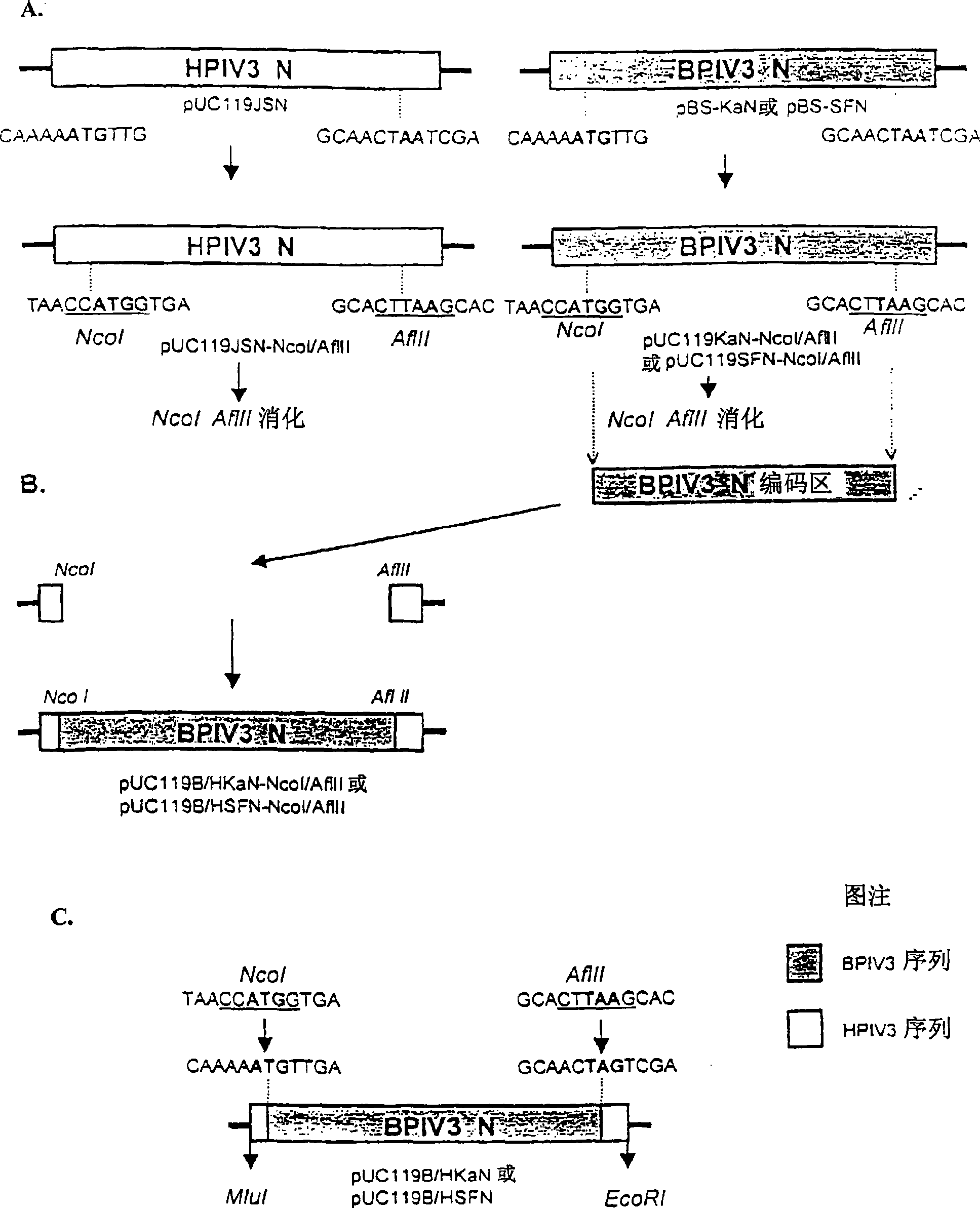
Chimeric human-bovine parainfluenza viruses (PIVs) are infectious and attenuated in humans and other mammals and useful individually or in combination in vaccine formulations for eliciting an anti-PIV immune response. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a partial or complete human or bovine PIV “background” genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) of a different PIV. Chimeric human-bovine PIV of the invention include a partial or complete “background” PIV genome or antigenome derived from or patterned after a human or bovine PIV virus combined with one or more heterologous gene(s) or genome segment(s) of a different PIV virus to form the human-bovine chimeric PIV genome or antigenome.
Owner:UNITED STATES OF AMERICA
Recombined cattle parainfluenza carrier for expressing protein VP1 of porcine O type foot-and-mouth disease virus
InactiveCN103773803ACapable of copyingVector-based foreign material introductionAntigen epitopeHeterologous
The invention relates to a recombined cattle parainfluenza carrier pcDNA-NM09-VP1 for expressing the protein VP1 of a porcine O type foot-and-mouth disease virus. The recombined cattle parainfluenza carrier is characterized in that an RNA (ribonucleic acid) extracted from a strain BPIV3NM09 is used as a template; a virus total-length gene group is segmentally amplified through RT-PCR (reverse transcription-polymerase chain reaction); the total-length cDNA of cattle parainfluenza is subjected to primary modification, namely AgeI is introduced between P and M, so that insertion and replacement of exogenous antigen gene fragments are facilitated; due to secondary modification, antigen epitope which is inserted into a heterologous virus in a modified manner is the protein VP1 of the O type foot-and-mouth disease virus; the amino acid sequence is SEQIDNO1, and the nucleotide sequence is SEQIDNO2. The foundation is laid for further development of a gene engineering recombined vaccine for preventing and controlling the porcine foot-and-mouth disease and research on the III-type toxicity factor and the molecular pathogenesis of the cattle parainfluenza; the recombined cattle parainfluenza carrier has the replication capacity.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
Attenuated human-bovine chimeric parainfluenza virus (PIV) vaccines
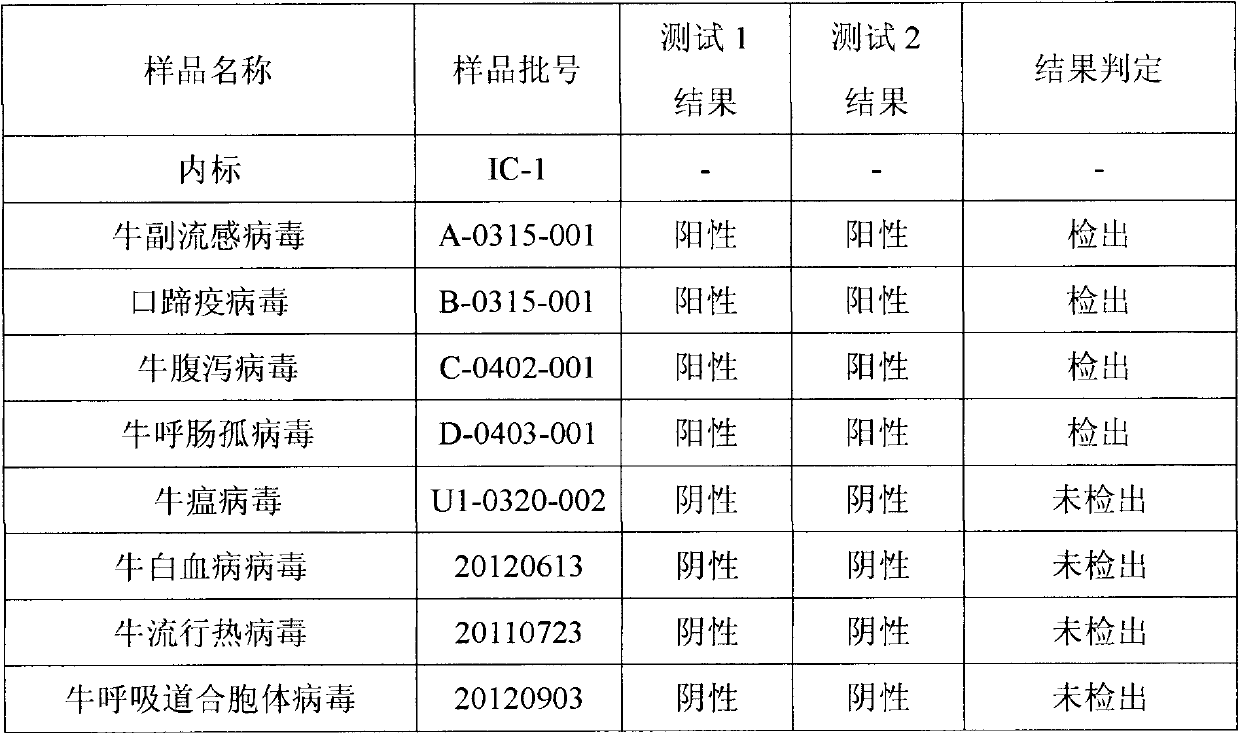
Reverse transcription-polymerase chain reaction (RT-PCR) detection method and kit for quickly diagnosing bovine parainfluenza virus 3 (BPIV-3)
InactiveCN102212622AShorten the timeReduce testing costsMicrobiological testing/measurementMicroorganism based processesBovine parainfluenza virusMultiplex polymerase chain reaction
The invention discloses a reverse transcription-polymerase chain reaction (RT-PCR) detection method and a kit for quickly diagnosing bovine parainfluenza virus 3 (BPIV-3), and belongs to the field of biomedical detection. The invention adopts the technical scheme that the method comprises acquisition of a sample, extraction of virus RNA, design optimization of a specific primer, RT-PCR amplification, agarose gel electrophoresis and result judgment; the specific primer is designed for a specific fragment of BPIV-3 nucleoprotein (NP) genes, and the upstream and downstream sequences of the specific primer are respectively P1: 5'-GGATGTTTGGGAGTGATCTTGAGTA-3' and P2: 5'-TGTGTTGAAAAATGAAGCAAGACCT-3'; the quick RT-PCR diagnosing kit for detecting the BPIV-3 comprises a sample virus RNA extracting reagent, a reverse transcription reagent, a PCR reagent, a positive reference substance and a negative reference substance; and by verifying, the method is sensitive and specific and has applicationprospect.
Owner:HUAZHONG AGRI UNIV
Attenuated human-bovine chimeric parainfluenza virus (PIV) vaccines
InactiveUS20060099226A1Easy to operateIncrease capacitySsRNA viruses negative-senseViral antigen ingredientsHeterologousGenomic Segment
Chimeric human-bovine parainfluenza viruses (PIVs) are infectious and attenuated in humans and other mammals and useful individually or in combination in vaccine formulations for eliciting an immune response to PIV or other pathogens. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a partial or complete human or bovine PIV “background” genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) of a different PIV. Chimeric human-bovine PIV of the invention include a partial or complete “background” PIV genome or antigenome derived from or patterned after a human or bovine PIV virus combined with one or more heterologous gene(s) or genome segment(s) of a different pathogen, including different PIV virus to form the human-bovine chimeric PIV genome or antigenome.
RAA primer, probe and method for detecting knopvelsiekte virus
ActiveCN110592286AAccurate detectionNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationBovine parainfluenza virusBovine Viral Diarrhea Viruses
The invention discloses a primer, a probe and a method for detecting knopvelsiekte virus by a RAA fluorescence method. The primer and the probe are suitable for the detection by the RAA fluorescence method, can accurately detect knopvelsiekte virus plasmids without cross reaction with mycoplasma, bovine infectious rhinotracheitis virus, bovine viral diarrhea virus, bovine parainfluenza virus, bovine respiratory syncytial virus, goat pox virus and sheep pox virus, and have a specificity of 100%. The method is fast and easy to achieve high throughput, and can reduce time and cost for detection.The method for rapid detection of DNA of the knopvelsiekte virus based on the RAA fluorescence method has high sensitivity reaching 10 copies / reaction.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT +1
RPA (Recombinase Ploymerase Amplification)-lateral flow assay detection primer of BPIV3 (Bovine parainfluenza virus type 3), probe and kit
InactiveCN108315491AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationForward primerBovine parainfluenza virus
The invention discloses a primer and a probe for detecting BPIV3 (Bovine parainfluenza virus type 3) aerosol through a RPA (Recombinase Ploymerase Amplification)-lateral flow assay, a kit and a detection method. The kit contains primer probe liquid formed by a RPA primer and a probe for detecting the BPIV3, wherein the forward primer sequence is shown as SEQ ID No.1; the reverse primer sequence isshown as SEQ ID No.2; the probe sequence is shown as SEQ ID No.3. The used primer probe group has a good RPA effect; the strip specificity is high; no cross reaction with other viruses exists; strongpositive reaction exists in a detection region; the detection sensitivity is enhanced. The RPA-lateral flow assay technology is used for building a BPIV3 fast detection method for the first time; through specificity and sensitivity evaluation, the method can be used for clinical field detection; a flexible and reliable novel method is provided for BPIV3 field detection.
Owner:SHANDONG NORMAL UNIV
Recombinant parainfluenza virus expression systems and vaccines
InactiveUS20040208895A1SsRNA viruses negative-senseMicroorganism based processesHeterologousNegative strand
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE VACCINES
Inactivation inspection method suitable for BVDV, IBRV, PIV3 and BRSV inactivated vaccines
InactiveCN108152498AGuaranteed infection timeImprove the detection rateMaterial analysisFreeze thawingAntigen
The invention discloses an inactivation inspection method suitable for BVDV, IBRV, PIV3 and BRSV inactivated vaccines, and relates to the field of biological medicines, in particular to an inactivation inspection method for BVDV, IBRV, PIV3 and BRSV inactivated vaccines. The inactivation inspection method comprises the following steps: (1) adsorbing an inactivated virus liquor on an appropriate cell for 40-60 min, and shaking for 2-3 times in the period; (2) after adsorption, adding a certain volume of a cell maintenance fluid in a cell spinner bottle, continuously performing culture, observing cytopathy, as for the virus fluid free from cytopathy, harvesting a cell culture fluid, and performing freeze thawing for 2-3 times; and (3) taking 10-15 ml of the frozen and thawed cell culture fluid, inoculating the taken cell culture fluid on the appropriate cell for adsorption, and continuously performing culture. Observation and fluorescence detection are performed, and if no specific fluorescence appears, complete inactivation is manifested. According to the technical scheme disclosed by the prevention, the anti-gen concentration and the virus infection time are guaranteed. The virus detection rate of the inactivation inspection method is high, and the bio-safety of the inactivated vaccines can be effectively ensured.
Owner:华威特(江苏)生物制药有限公司
Indirect competitive enzyme linked immunosorbent assay aptamer method used for detecting bovine parainfluenza virus 3 antibodies
InactiveCN106771184AOptimizing Coating ConcentrationImprove positive detection rateColor/spectral properties measurementsImmunoassaysBovine parainfluenza virusDisease
The invention relates to an indirect competitive enzyme linked immunosorbent assay aptamer method used for detecting bovine parainfluenza virus 3 antibodies, and belongs to the field of bioengineering. The indirect competitive enzyme linked immunosorbent assay aptamer method comprises following steps: coating; plate washing; blocking; adding of a nucleic acid aptamer and bovine serum; plate washing; adding of second antibodies; color developing; reaction killing; reading; determining of a negative and positive critical value; Ic-ELAA method specific detection; and clinical bovine serum detection. The indirect competitive enzyme linked immunosorbent assay aptamer method is high in specificity; compared with commercially available ELISA kits, the positive detection rate of Ic-ELAA is higher, and is more suitable for clinical serological investigation of bovine parainfluenza virus.
Owner:BEIJING UNIV OF AGRI
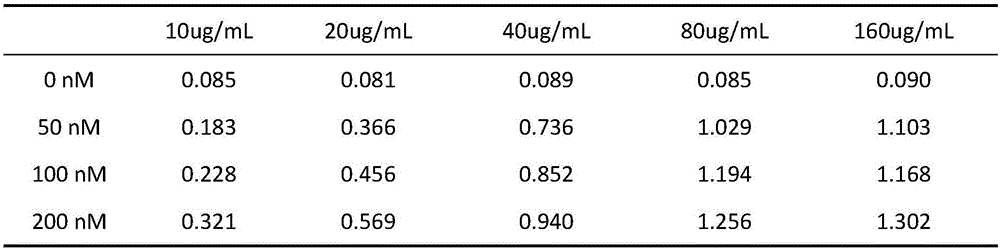
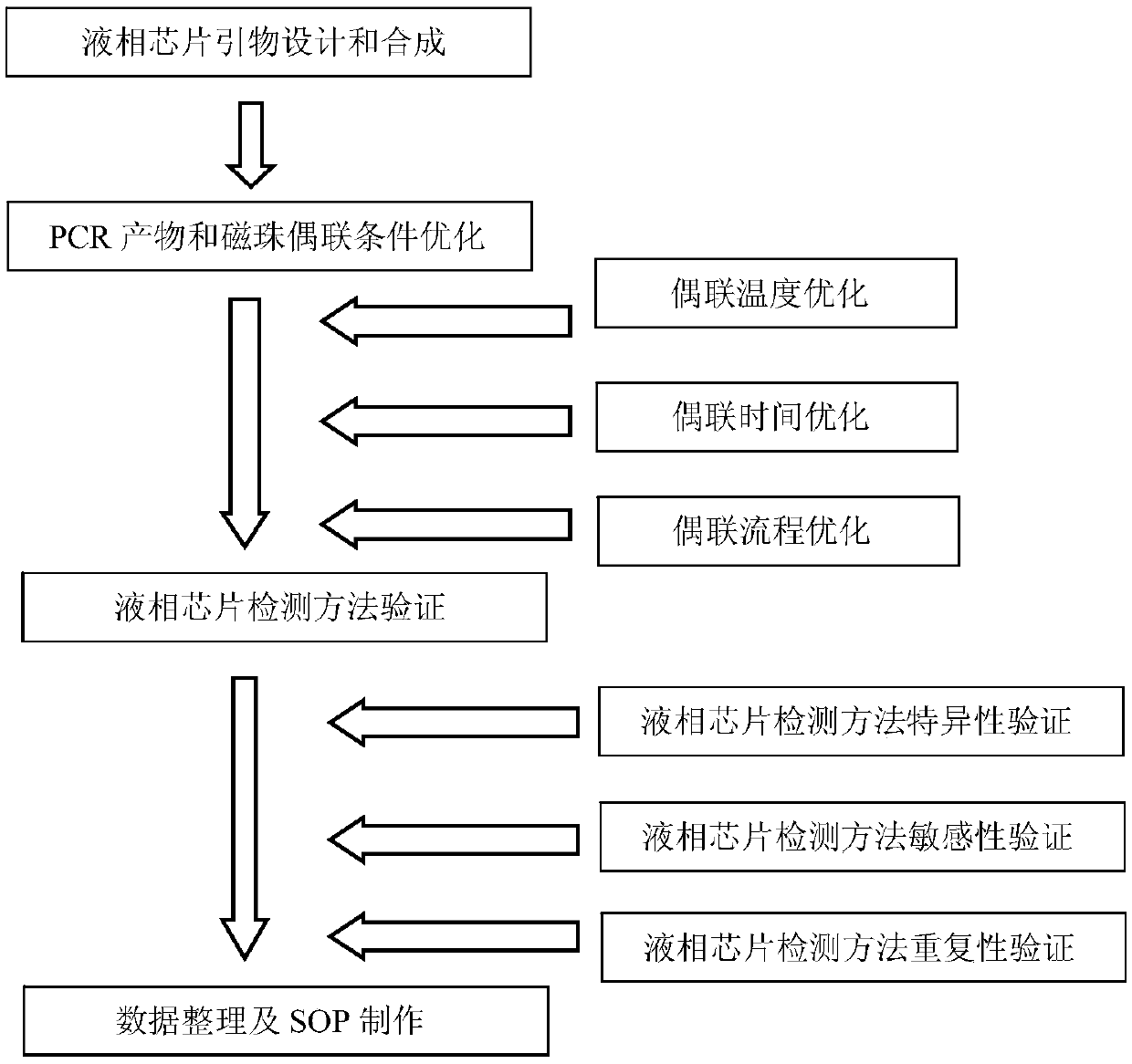
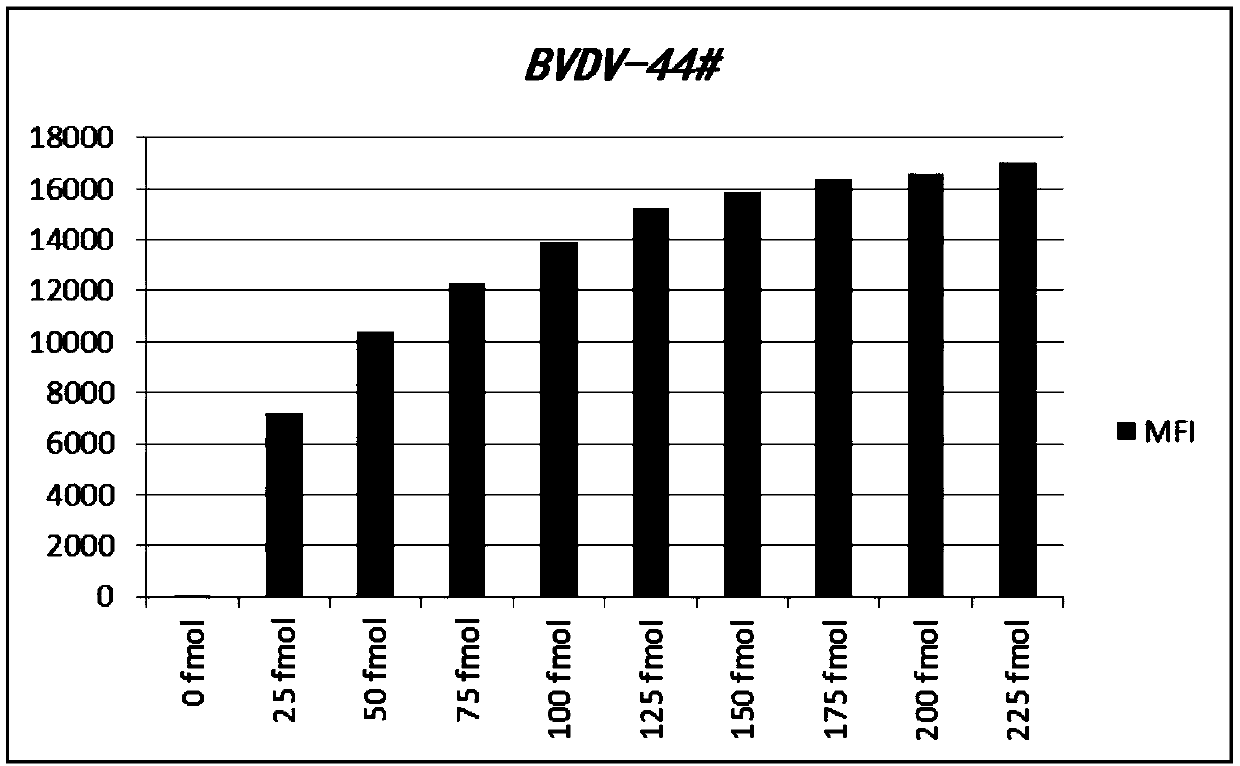
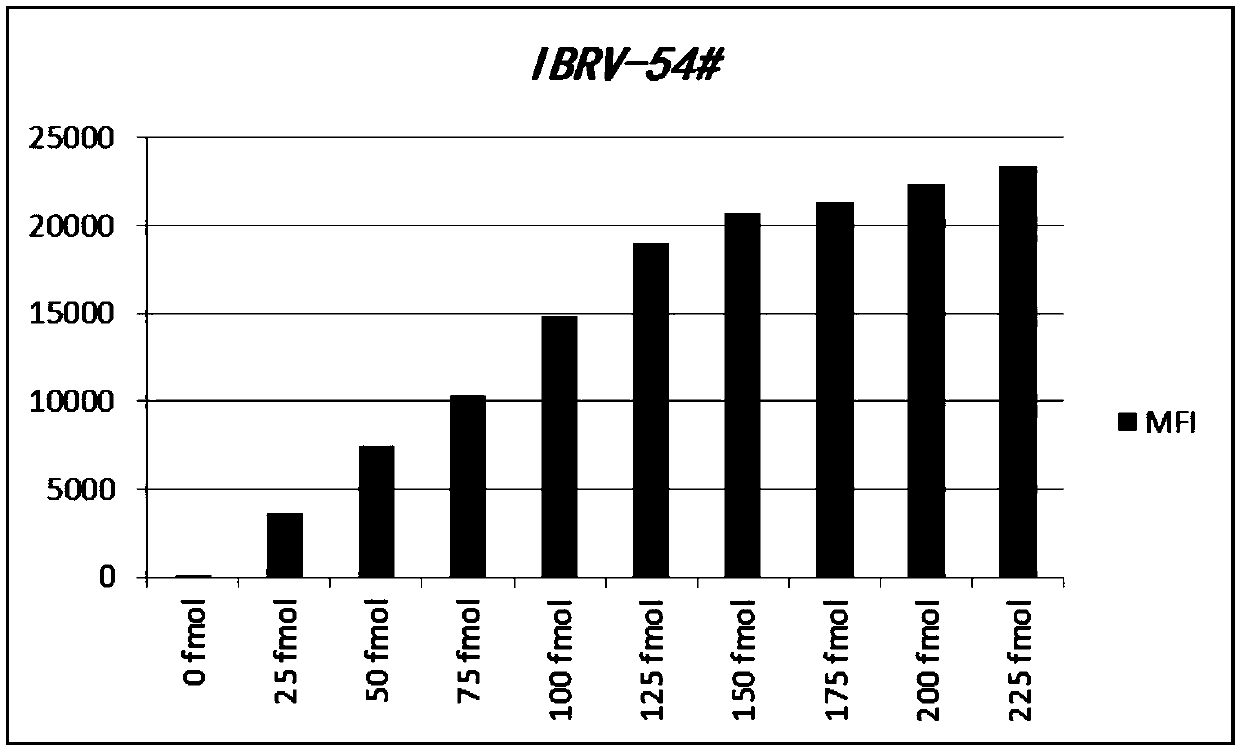
Liquid-phase chip detection kit for simultaneously detecting BVDV, IBRV and BPIV, and special primer and coupling probe thereof
ActiveCN110607393AImprove throughputHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesBovine parainfluenza virusCoupling
The invention provides a liquid-phase chip detection kit for simultaneously detecting bovine viral diarrhea-mucosal disease virus, infectious bovine rhinotracheitis virus and bovine parainfluenza virus as well as a special primer and a coupling probe thereof. The kit disclosed by the invention is simple and convenient to detect BVDV, IBRV and BPIV, strong in specificity, high in sensitivity and good in repeatability, and can be applied to the fields of virus detection, vaccine production, calf product quality inspection and the like.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Attenuated human-bovine chimeric parainfluenza virus (piv) vaccines
InactiveCN101392239AMaintain immunogenic activityReduce duplicationSsRNA viruses negative-senseAntibody mimetics/scaffoldsHeterologousBovine parainfluenza virus
The invention provides attenuated human-bovine parainfluenza viruses (PIVs), mainly comprising a nucleoprotein (N), phosphoprotein (P), and a large polymerase protein (L) and one or more heterogeny genome or antigenome section(s) combining different PIVs, to form partial or integrated PIV background genome or antigenome of human PIV or bovine PIV of human-bovine PIV genome or antigenome, and at least one heterogeny genome or gene section code PIV P protein. The invention also provides separated polynucleotide for coding the virus genome or antigenome, a method of preparing the virus through expressing polynucleotide, and PIV immunogenic compositions including plenty immunity.
Owner:UNITED STATES OF AMERICA
Attenuated human-bovine chimeric parainfluenza virus (PIV) vaccines
InactiveUS20070134271A1Desired characteristicHigh levelSsRNA viruses negative-senseSugar derivativesGenomic SegmentHeterologous
Chimeric human-bovine parainfluenza viruses (PIVs) are infectious and attenuated in humans and other mammals and useful individually or in combination in vaccine formulations for eliciting an anti-PIV immune response. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a partial or complete human or bovine PIV “background” genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) of a different PIV. Chimeric human-bovine PIV of the invention include a partial or complete “background” PIV genome or antigenome derived from or patterned after a human or bovine PIV virus combined with one or more heterologous gene(s) or genome segment(s) of a different PIV virus to form the human-bovine chimeric PIV genome or antigenome. In certain aspects of the invention, chimeric PIV incorporate a partial or complete human PIV background genome or antigenome combined with one or more heterologous gene(s) or genome segment(s) from a bovine PIV, whereby the resultant chimeric virus is attenuated by virtue of host-range restriction. In alternate embodiments, human-bovine chimeric PIV incorporate a partial or complete bovine PIV background genome or antigenome combined with one or more heterologous gene(s) or genome segment(s) from a human PIV gene that encode a human PIV immunogenic protein, protein domain or epitope, for example encoded by PIV HN and / or F glycoprotein gene(s) or genome segment(s). Human-bovine chimeric PIV of the invention are also useful as vectors for developing vaccines against other pathogens. A variety of additional mutations and nucleotide modifications are provided within the human-bovine chimeric PIV of the invention to yield desired phenotypic and structural effects.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
PCR (Polymerase Chain Reaction) method for simultaneously detecting four bovine infectious disease RNA (Ribose Nucleic Acid) viruses by single tube
InactiveCN103468828AGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceBovine parainfluenza virusPcr method
The invention discloses a PCR (Polymerase Chain Reaction) method for simultaneously detecting four bovine infectious disease RNA (Ribose Nucleic Acid) viruses by a single tube. The PCR method is used for detecting a bovine parainfluenza virus, a foot and mouth disease virus, a bovine diarrhea virus and a bovine reovirus. The PCR method is rapid in operation without one step. In addition, the invention also relates to a reagent related in the PCR method, which is used in a detection kit of the method and preparation and application of the detection kit.
Owner:SHANGHAI ZHUORUN BIOTECH
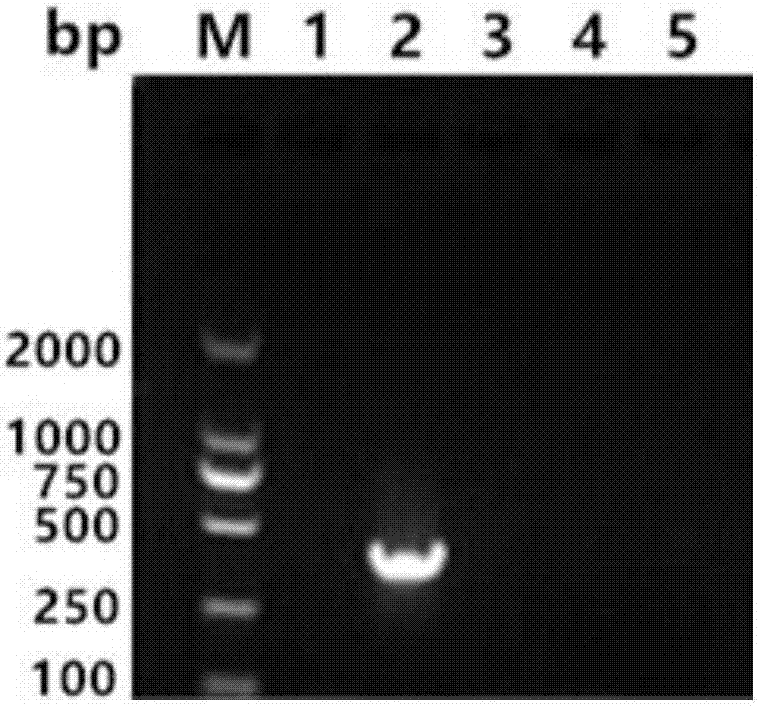
Bovine-para-influenza virus 3 nano-PCR (polymerase chain reaction) detection kit and preparation method thereof
InactiveCN106906307ASpecific and accurateAccurate specificityMicrobiological testing/measurementMicroorganism based processesBovine parainfluenza virusPositive control
The invention discloses a bovine-para-influenza virus 3 nano-PCR (polymerase chain reaction) detection kit and a preparation method thereof, relates to the field of bovine para-influenza virus detection, and solves the problems that an existing bovine-para-influenza virus 3 detection method is low in sensitivity, serological detection is high in false positive and foreign importing reagents are expensive. The kit comprises a MightyAmp enzyme, 2xBuffer Mix solution, gold nanoparticle sol, aseptic double-distilled water, a pair of specific primers P1 (5'-GCTCTTCTCTTTTTGTCCCATTCTT-3') and P2 (5'-AACCCCTTCCTCAATCCTGATATAC-3') and a bovine-para-influenza virus 3 positive control plasmid. The sequence of the bovine-para-influenza virus 3 positive control plasmid is as shown in SEQ ID NO: 2. The kit is high in sensitivity, simple, convenient, rapid, efficient and low in cost.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Primers, probes and kit for real-time fluorescent quantitative PCR detection of bovine epidemic diseases
InactiveCN113249517AQuick checkMicrobiological testing/measurementMicroorganism based processesBovine rotavirusDisease
The invention discloses primers, probes and a kit for real-time fluorescent quantitative PCR (polymerase chain reaction) detection of bovine epidemic diseases. The kit at least comprises primers and probes for detecting BHV (Bovine herpesvirus), BPIV (bovine parainfluenza virus), SC type MmmSC (Mycoplasma mycoides subsp mycoides SC), BRSV (Bovine respiratory syncytial virus), BTV (bluetongue virus), BVDV (bovine viral diarrhea virus), BRV (bovine rotavirus), PPRV (peste des petits ruminants virus), RPV (Rinderpestvirus), VSV (vesicular stomatitis virus), FMDV (foot-and-mouth disease virus) and IC. Compared with the prior art, the four TaqMan multiple real-time fluorescent quantitative PCR systems of BHV, BPIV, MmmSC and IC, BRSV, BVDV, BTV and IC, BRV, PPRV, RPV and IC, FMDV, VSV and IC are constructed, and the specificity, repeatability and sensitivity of the systems all reach expected effects, so that the 11 pathogens can be rapidly detected at the same time.
Owner:ACADEMY OF MILITARY MEDICAL SCI
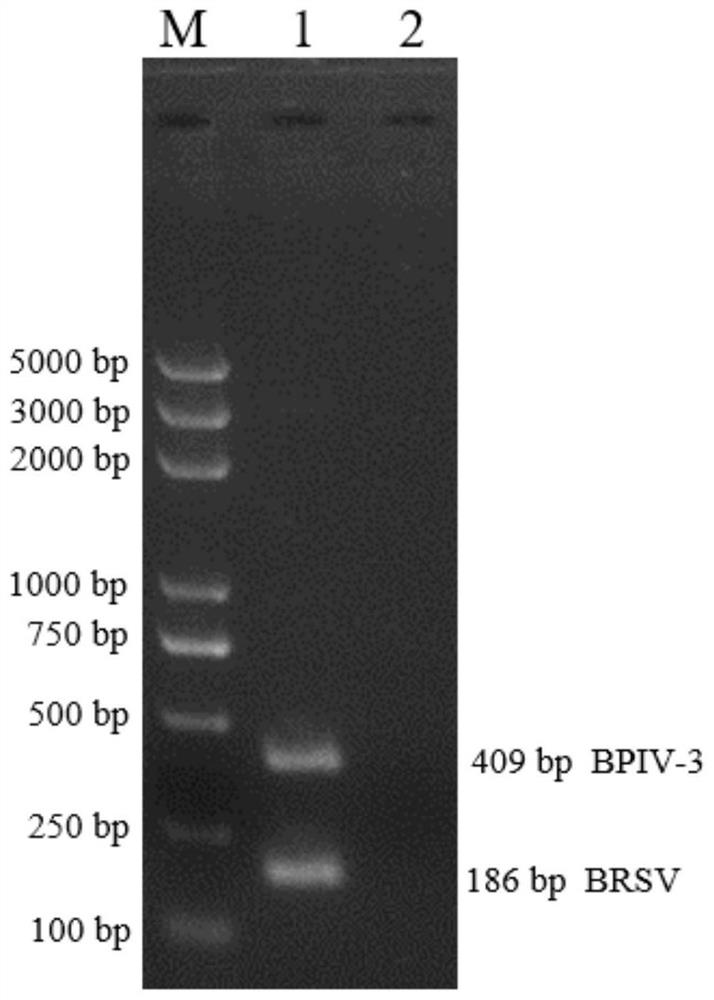
Dual RPA detection kit for bovine parainfluenza virus (BPIV) and bovine respiratory syncytial virus (BRSV)
ActiveCN112593013ARealize double RPA detectionThe detection process is fastMicrobiological testing/measurementAgainst vector-borne diseasesBovine parainfluenza virusNucleotide
The invention discloses a dual RPA detection kit for bovine parainfluenza virus (BPIV) and bovine respiratory syncytial virus (BRSV). The dual RPA detection kit comprises aBPIV RPA detection primer group consisting of nucleotide sequences shown in SEQ. ID. NO.2 and SEQ. ID. NO.5, and a BRSV RPA detection primer group consisting of nucleotide sequences shown in SEQ. ID. NO.8 and SEQ. ID. NO.10. Thekit is based on a recombinase polymerase amplification method, cDNA formed by RNA reverse transcription is used as a template, an amplification reaction is carried out at an optimized reaction temperature and time, and a detection result is obtained by nucleic acid gel electrophoresis. The kit can be used for simultaneously detecting the bBPIV and the BRSV, is simple to operate, has higher sensitivity and specificity, and provides a rapid detection reagent for on-site screening of bovine pathogens.
Owner:NORTHWEST A & F UNIV
Blocking ELISA kit for detecting bovine parainfluenza virus type 3 antibody and application thereof
PendingCN111650375AStrong responsivenessStrong specificityImmunoassaysBovine parainfluenza virusElisa kit
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
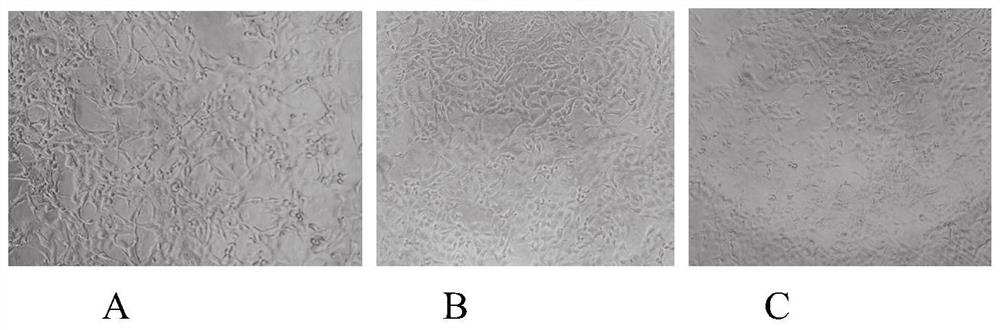
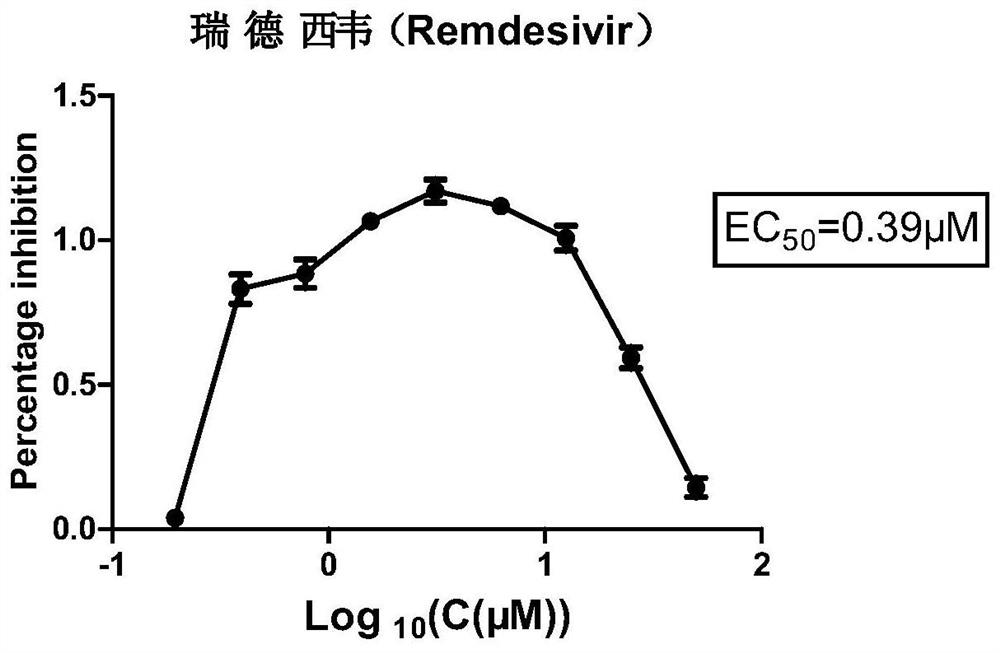
Application of Remdesivir in preparation of anti-bovine parainfluenza virus 3 medicine
PendingCN112843073APrevent proliferationLow toxicityOrganic active ingredientsAntiviralsBovine parainfluenza virusIntracellular
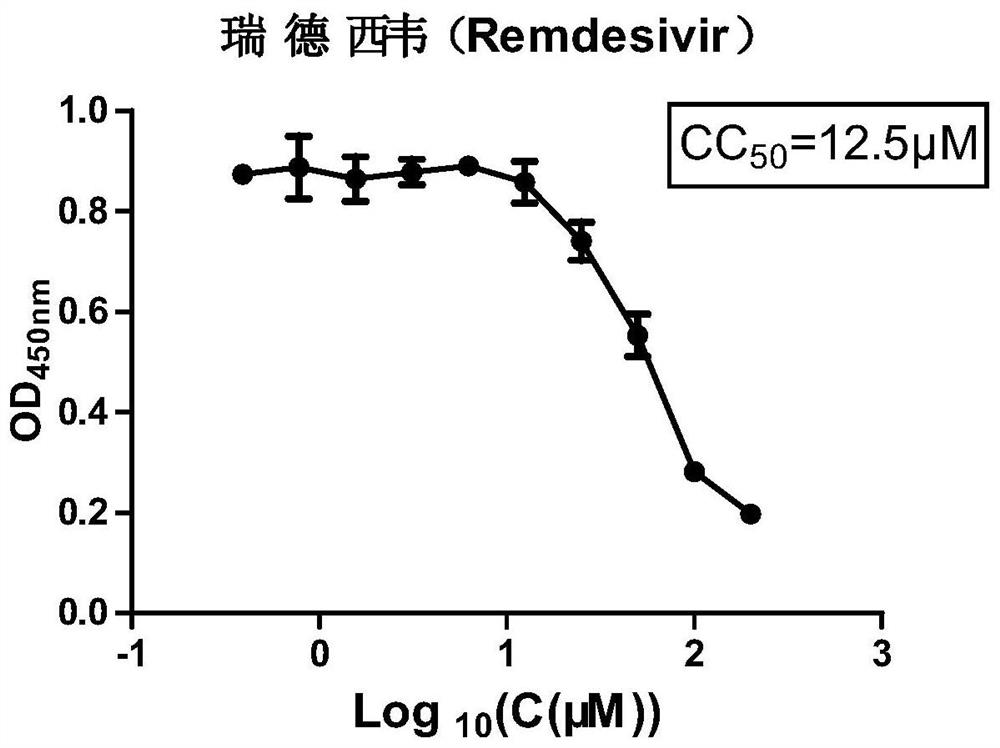
The invention relates to the technical field of medicines, and particularly relates to application of Remdesivir in preparation of an anti-bovine parainfluenza virus 3 medicine. The anti-BPIV3 comprises one or more of the following effects: proliferation of the BPIV3 virus is inhibited; the BPIV3 virus is inactivated; the adsorption effect of BPIV3 on cells is prevented; and the replication of the BPIV3 in cells is blocked. According to the application, it is found for the first time that the compound, namely, the Remdesivir, can effectively inhibit proliferation of the BPIV3 and is small in toxicity to cells, experiments prove that the Remdesivir can effectively inhibit and kill the BPIV3 on an in-vitro experiment MDBK cell model, invasion and replication of the BPIV3 can be effectively inhibited, the Remdesivir can be used as a novel anti-BPIV3 medicine, a novel medicine application is developed for the Remdesivir, an experimental foundation is laid for developing efficient and specific anti-BPIV3 medicines, and a new visual field is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI +1
Immunofluorescence reagent for detecting bovine parainfluenza virus type 3 and detection kit thereof
PendingCN110763838AIncreased sensitivityHigh potencyBiological material analysisBiological testingBovine parainfluenza virusAntigen
The invention belongs to the technical field of biology and particularly relates to an immunofluorescence reagent for detecting bovine parainfluenza virus type 3 and a detection kit of the immunofluorescence reagent. The immunofluorescence reagent is an anti-bovine parainfluenza virus type 3 monoclonal antibody marked by fluorescent dye, and the monoclonal antibody is prepared by taking virus particles obtained by concentrating and purifying the bovine parainfluenza virus type 3 as an antigen and adopting a hybridoma cell monoclonal antibody technology. The fluorescent dye is fluorescein isothiocyanate (FITC). The subtype of the monoclonal antibody is IgG2. An immune reagent in an immunofluorescence kit for detecting the bovine parainfluenza virus type 3 is an immunofluorescence reagent for detecting the bovine parainfluenza virus type 3. The invention has the advantages of high sensitivity, strong specificity, good stability, high diagnosis speed and the like.
Owner:华威特(江苏)生物制药有限公司
Attenuated human-bovine chimeric parainfluenza virus (PIV) vaccines
InactiveCN100402651CMaintain immunogenic activityReduce duplicationSsRNA viruses negative-senseAntibody mimetics/scaffoldsBovine parainfluenza virusHeterologous
Chimeric human-bovine parainfluenza viruses (PIVs) are infectious and attenuated in humans and other mammals and useful individually or in combination in vaccine formulations for eliciting an anti-PIV immune response. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a partial or complete human or bovine PIV "background" genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) of a different PIV.
Owner:UNITED STATES OF AMERICA
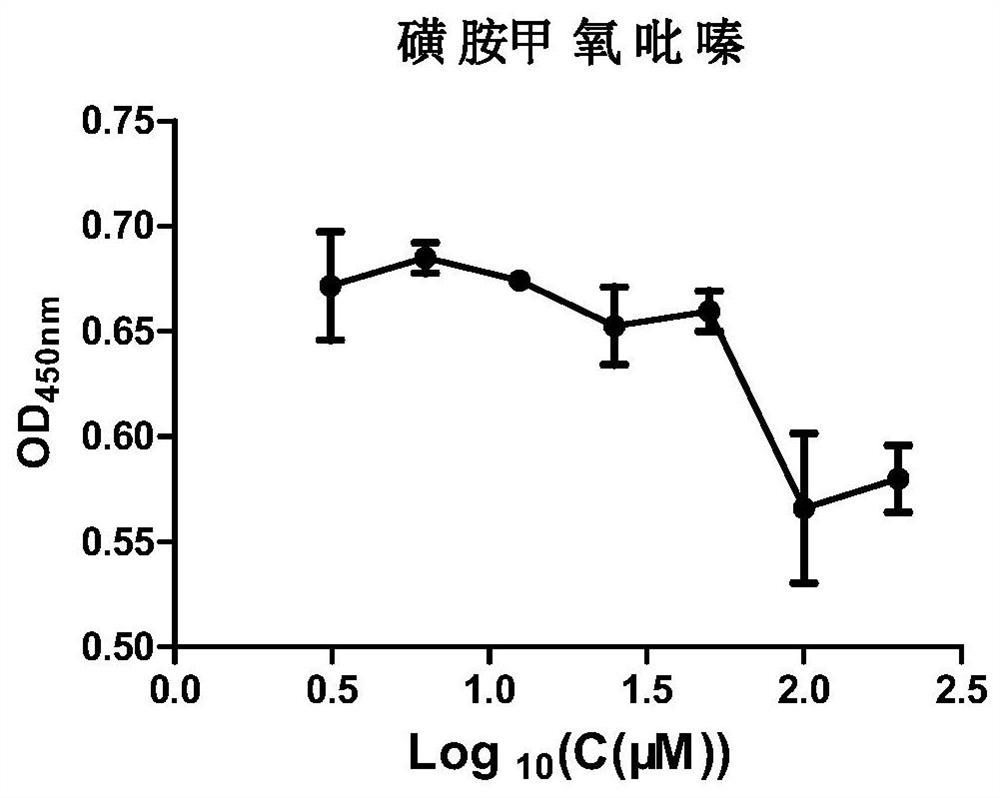
Application of sulfamethoxypyrazine in preparation of product for preventing and/or treating bovine parainfluenza virus
ActiveCN113304158APrevent proliferationLow toxicityOrganic active ingredientsAccessory food factorsBovine parainfluenza virusSulfanilamide
The invention particularly relates to application of sulfamethoxypyrazine in preparation of a product for preventing and / or treating bovine parainfluenza virus. Bovine parainfluenza virus type 3 is an important pathogen causing cattle respiratory syndrome in a livestock farm, and the transmission route comprises respiratory tract and reproductive organ transmission which may cause serious pneumonia and even infertility. The research result provides the application of sulfamethoxypyrazine in development of products for preventing and / or treating bovine parainfluenza virus, and verification shows that in a safe dosage range of normal cells, sulfamethoxypyrazine shows an inhibition effect on bovine parainfluenza virus type 3, blocks an adsorption effect of the virus on the normal cells, and can be used for preventing and / or treating the bovine parainfluenza virus type 3. Sulfamethoxypyrazine is expected to be applied to development of veterinary drugs or feed additives.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI +1
Reverse transcription-polymerase chain reaction (RT-PCR) detection method and kit for quickly diagnosing bovine parainfluenza virus 3 (BPIV-3)
InactiveCN102212622BEasy to copyHas nuclear transport functionMicrobiological testing/measurementMicroorganism based processesBovine parainfluenza virusMultiplex polymerase chain reaction
The invention discloses a reverse transcription-polymerase chain reaction (RT-PCR) detection method and a kit for quickly diagnosing bovine parainfluenza virus 3 (BPIV-3), and belongs to the field of biomedical detection. The invention adopts the technical scheme that the method comprises acquisition of a sample, extraction of virus RNA, design optimization of a specific primer, RT-PCR amplification, agarose gel electrophoresis and result judgment; the specific primer is designed for a specific fragment of BPIV-3 nucleoprotein (NP) genes, and the upstream and downstream sequences of the specific primer are respectively P1: 5'-GGATGTTTGGGAGTGATCTTGAGTA-3' and P2: 5'-TGTGTTGAAAAATGAAGCAAGACCT-3'; the quick RT-PCR diagnosing kit for detecting the BPIV-3 comprises a sample virus RNA extracting reagent, a reverse transcription reagent, a PCR reagent, a positive reference substance and a negative reference substance; and by verifying, the method is sensitive and specific and has applicationprospect.
Owner:HUAZHONG AGRI UNIV
RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification primer for quickly detecting bovine parainfluenza 3 virus, and application thereof
InactiveCN107988431AHigh sensitivityShort timeMicrobiological testing/measurementMicroorganism based processesBovine parainfluenza virusAgricultural science
The invention discloses a RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification primer for quickly detecting a bovine parainfluenza 3 virus, and application thereof, which belong to the field of animal bacteriology and molecular biology. The RT-PCR amplification primer is formed by an upstream primer with a nucleotide sequence as shown in an SEQ ID NO:1 and a downstream primer with a nucleotide sequence as shown in an SEQ ID NO:2; the primer is used for preparing a RT-PCR amplification kit for detecting the bovine parainfluenza 3 virus. An RT-PCR detection method provided by the kit has high specificity and sensibility, good repeatability, and high reliability, can specifically detect the bovine parainfluenza 3 virus and quickly and accurately obtain a detection result, islow in price, simple and convenient to operate, and suitable for a basic level at the same time, and can be used as a quick, accurate, simple and convenient detection tool for quick identification ina bovine parainfluenza 3 virus laboratory and large-scale epidemiological investigation.
Owner:GUANGXI VETERINARY RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com