Application of Remdesivir in preparation of anti-bovine parainfluenza virus 3 medicine
A technology of remdesivir and drugs, which is applied in the field of application of remdesivir in the preparation of anti-bovine parainfluenza virus type 3 drugs, and can solve problems such as prevention or treatment of bovine parainfluenza virus type 3.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] virus-TCID 50 Determination of
[0055] MDBK cells (preserved by Dairy Cow Research Center, Shandong Academy of Agricultural Sciences) were digested and mixed with 1 × 10 per well. 5 Cells / mL were seeded into 96-well cell culture plates, placed in 37°C, 5% CO 2 After being cultured into a single layer of cells in a cell culture box, the cell growth solution in the well was discarded, and the virus dilution solution of BPIV3 serial 10-fold dilution (dilutions were 10 -1 ~10 -10 ) inoculated in a 96-well plate full of monolayer cells, 100 μL per well, placed in 37°C, 5% CO 2 Continue to culture in the incubator, observe the CPE of the cells day by day, and record the number of cells with pathological changes in detail. At the same time, a normal cell control group and a blank control group were set up, with 8 replicates in each group, and the results were judged when no further cell lesions occurred. The cell pathological well is the cell hole corresponding to the ab...
Embodiment 2
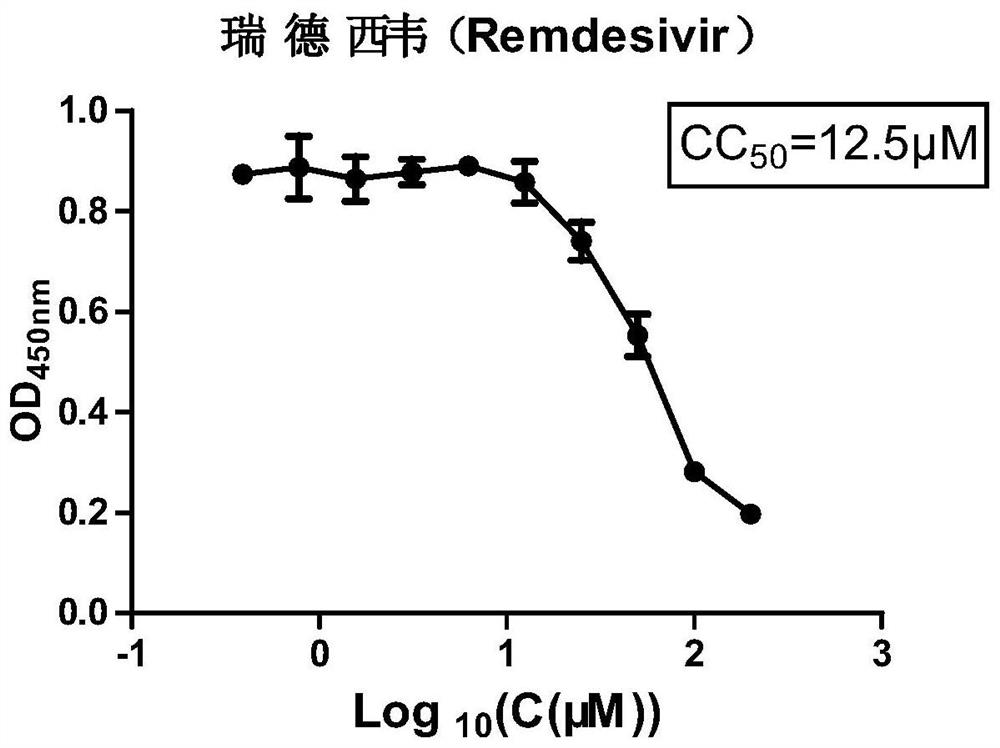
[0066] Toxicity test of Remdesivir on MDBK cells:
[0067] MDBK cells are susceptible to BPIV3. Therefore, the cytotoxicity of Remdesivir to MDBK cells was first detected, and the specific experimental steps were as follows:
[0068] (1) Inoculate 100 μL cells (MDBK 1×10 4 pieces / hole).
[0069] (2) After cultured to the MDBK monolayer, the next step of drug addition analysis was performed. Discard the medium, add 100 μL of 2% FBS DMEM containing different drug concentrations to each well, and make 3 parallels for each concentration. At the same time as the control well: add 100 μL of 2% FBS DMEM medium. Zero well: no cells are plated.
[0070] (3) At 37°C, 5% CO 2 After culturing under the conditions for 48 hours, operate according to the instructions of the CCK-8 kit, and measure the OD value at 450nm with a microplate reader.
[0071] (4) 37°C, 5% CO 2 After culturing for 2 h under the same conditions, the absorbance was measured at 450 nm. A450 nm of normal growin...
Embodiment 3
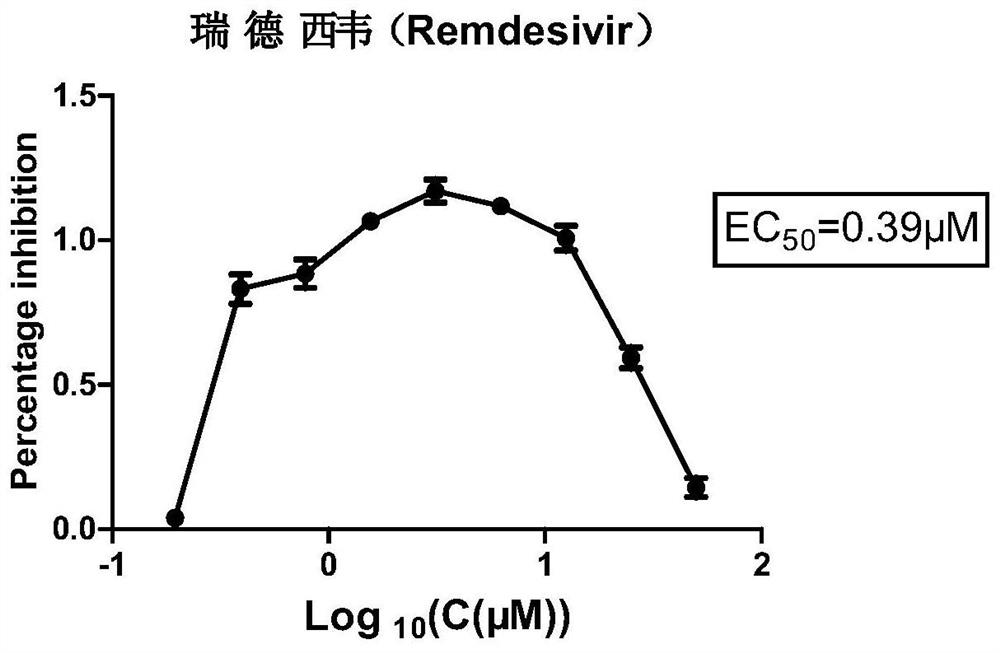
[0075] Inhibition experiment of Remdesivir on BPIV3:
[0076] (1) Inoculate 1×10 in each well of a 96-well plate 4 MDBK cells, 37°C, 5% CO 2 Cultivate overnight in an incubator;
[0077] (2) Discard the medium and add 100 μL 1000TCID to each well 50 The BPIV3 dilution solution (use 2% FBS DMEM to add the virus dilution solution after the cells are overgrown, according to the initial concentration of 25μM, double the concentration gradient dilution and dosing, 5% CO 2 Cultivated in an incubator;
[0078] (3) After 48 hours, operate according to the instructions of the CCK-8 kit, and measure the OD value at 450 nm with a microplate reader.
[0079] (4) Analyze data, virus inhibition rate (%)=(drug treatment group D450nm value-virus control group D450 nm value) / (normal cell control group D450 nm value-virus control group D450nm value) * 100%, use GraphPad Prism5 The half effective concentration (EC) of the compound obtained by the software 50 )value. The result is as ima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com