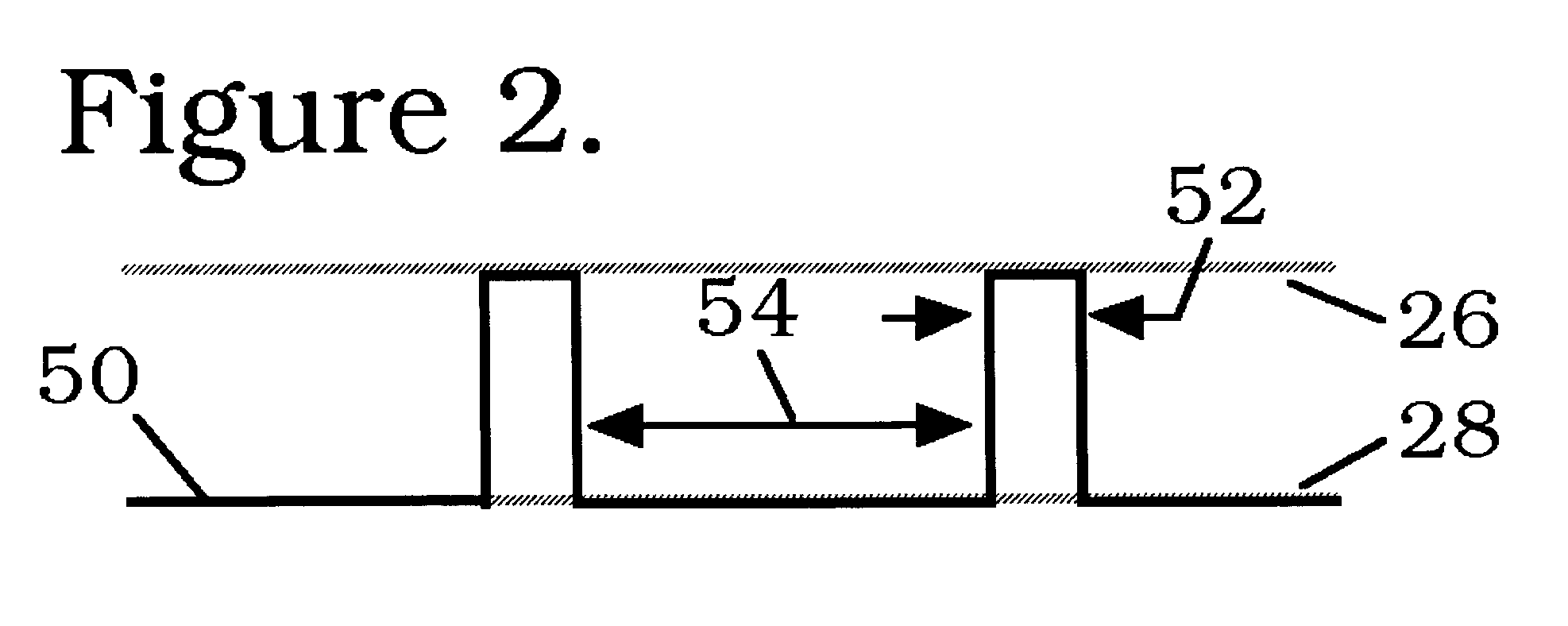
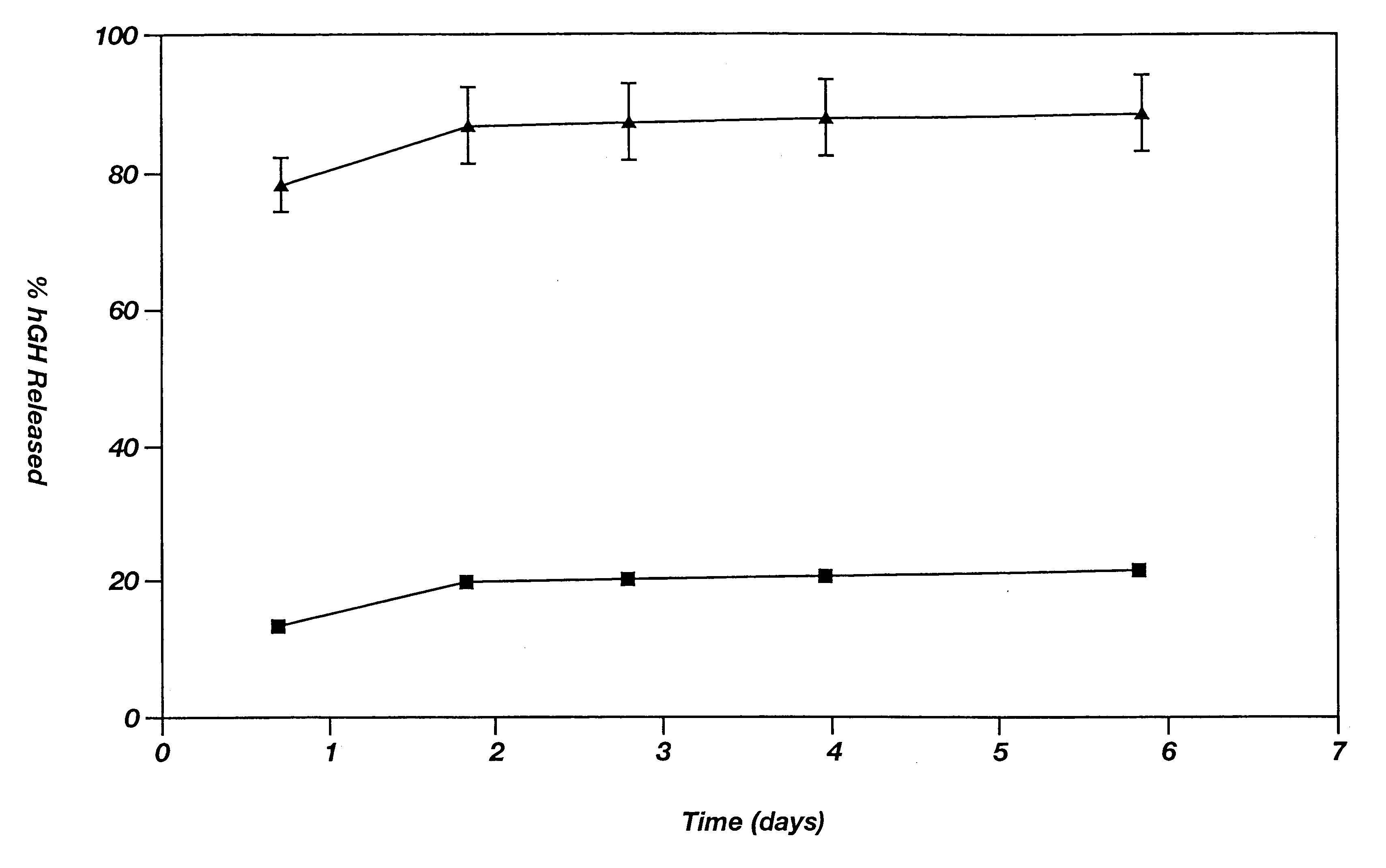
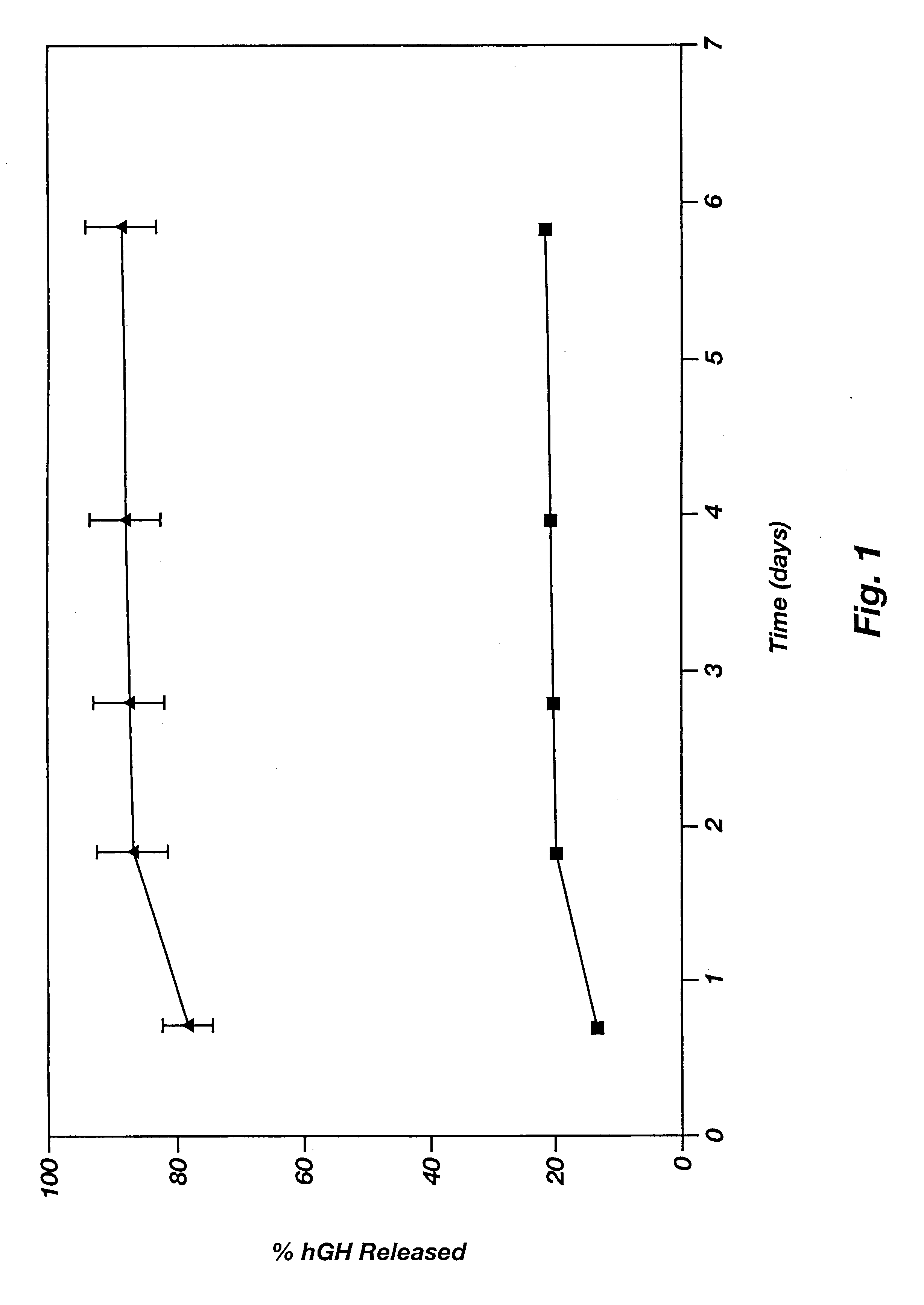
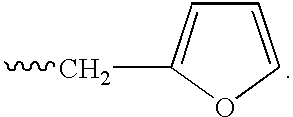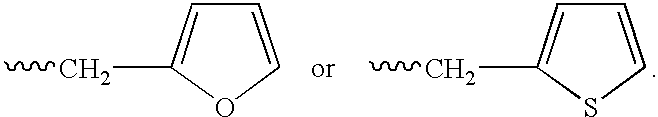Patents
Literature
88174 results about "Pharmaceutical drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A medication (also referred to as medicine, pharmaceutical drug, or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.
Simultaneous stimulation and concentration of cells
InactiveUS6867041B2Maximizes stimulationCulture processArtificial cell constructsDrug discoveryCells signal
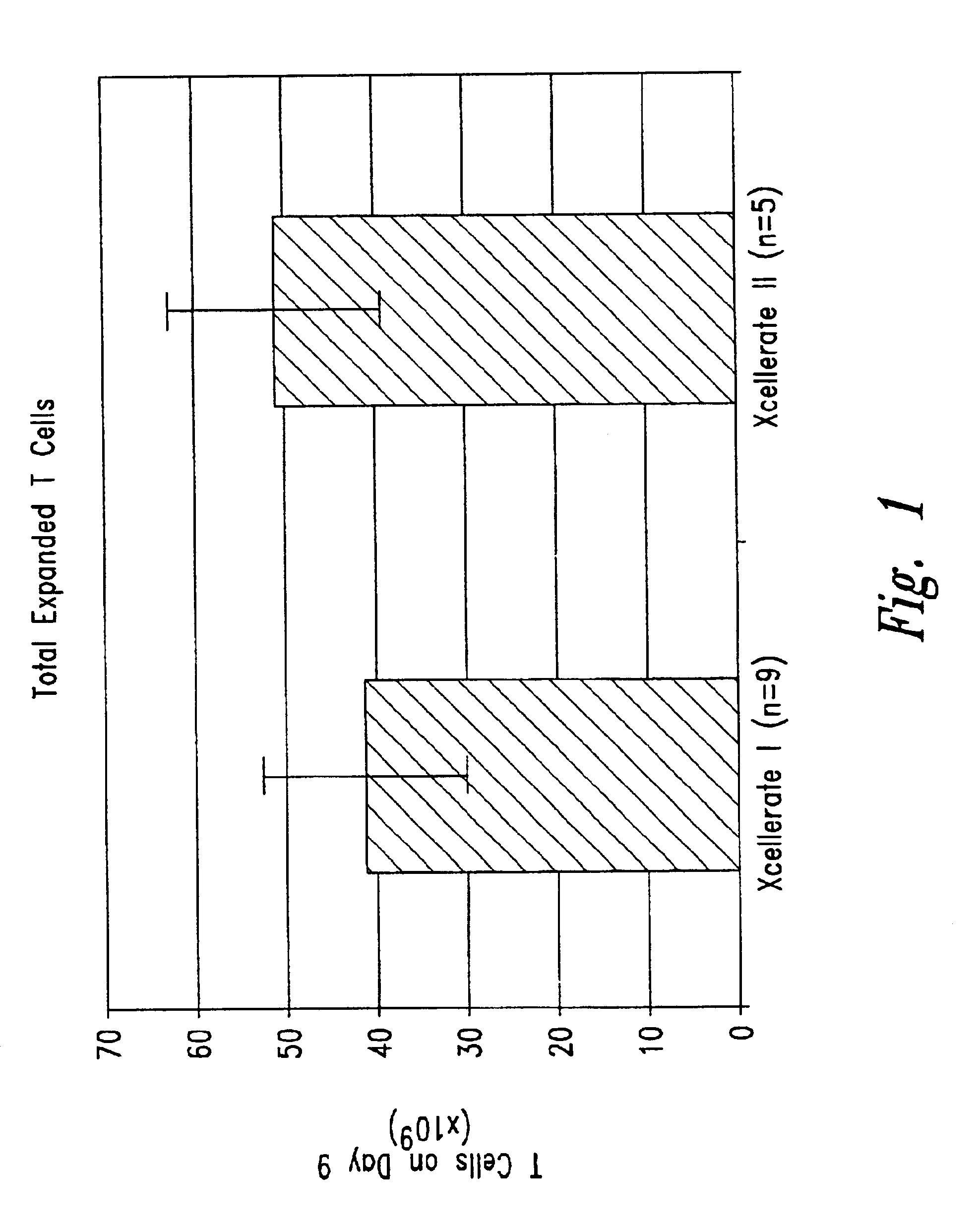
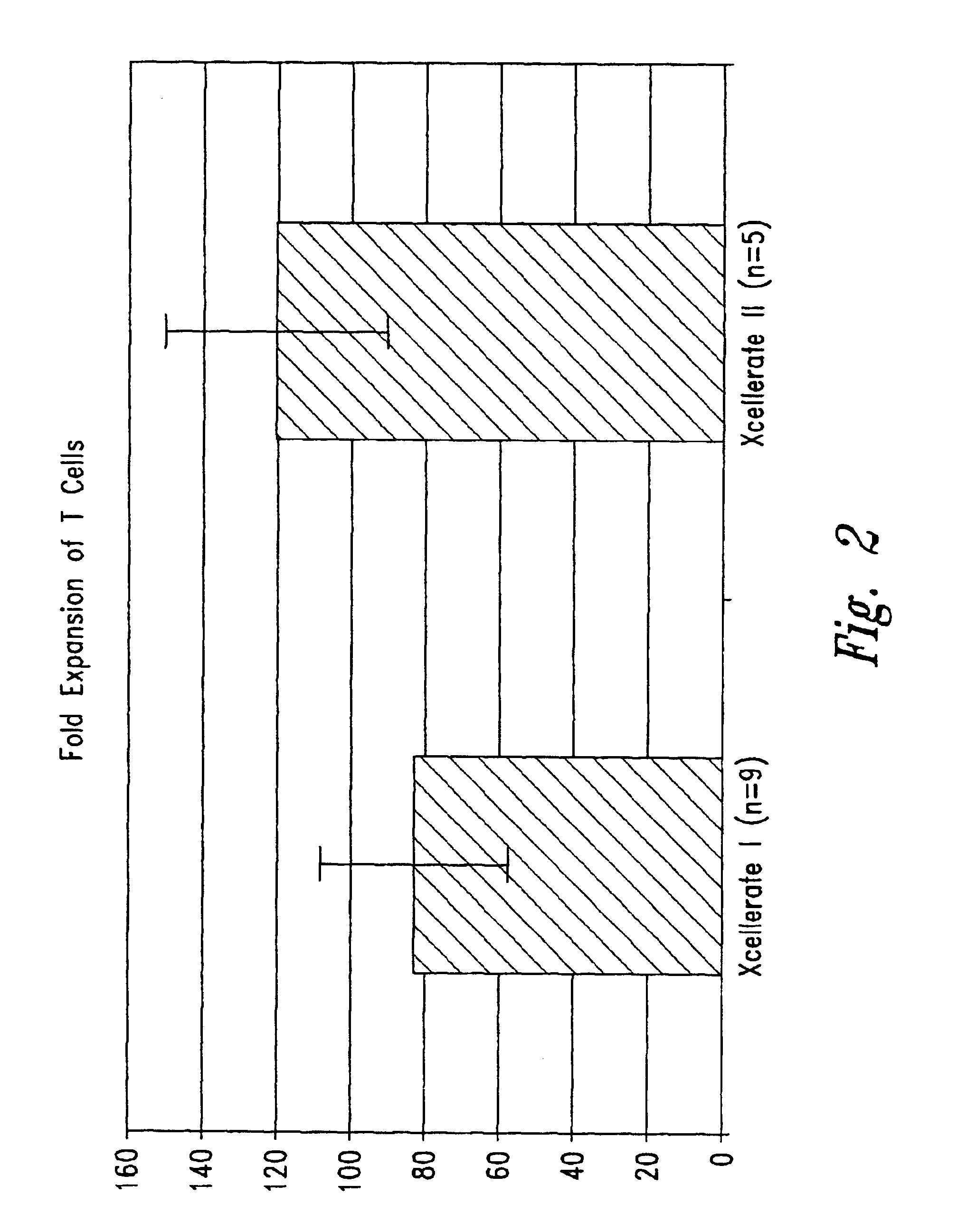
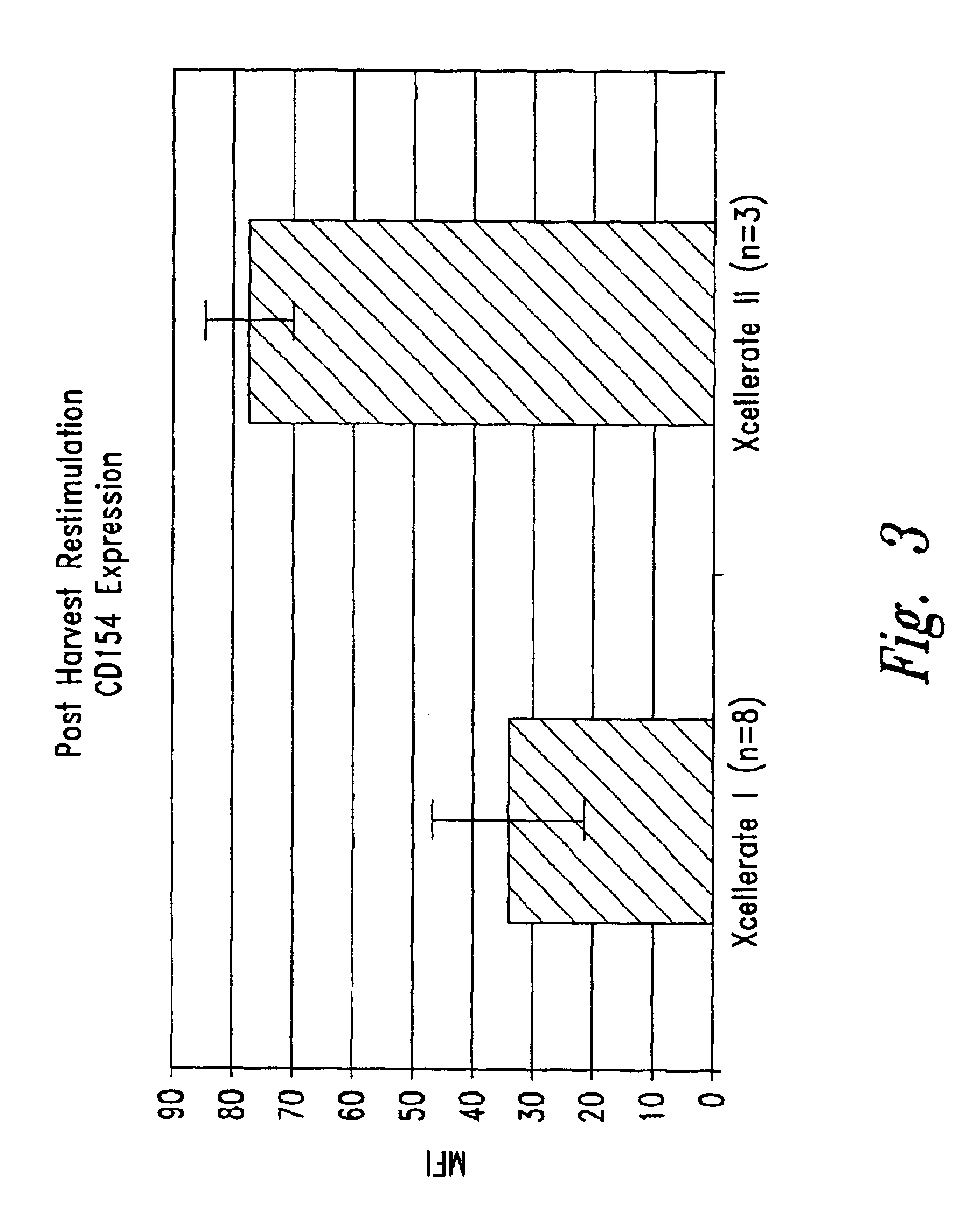
The present invention relates generally to methods for stimulating cells, and more particularly, to a novel method to concentrate and / or stimulate cells that maximizes stimulation and / or proliferation of such cells. In the various embodiments, cells are stimulated and concentrated with a surface yielding enhanced proliferation, cell signal transduction, and / or cell surface moiety aggregation. In certain aspects methods for stimulating a population of cells such as T-cells, by simultaneous concentration and cell surface moiety ligation are provided by contacting the population of cells with a surface, that has attached thereto one or more agents that ligate a cell surface moiety and applying a force that predominantly drives cell concentration and cell surface moiety ligation, thereby inducing cell stimulation, cell surface moiety aggregation, and / or receptor signaling enhancement. Also provided are methods for producing phenotypically tailored cells, including T-cells for the use in diagnostics, drug discovery, and the treatment of a variety of indications, including cancer, viral infection, and immune related disorders. Compositions of cells having specific phenotypic properties produced by these processes are further provided.
Owner:LIFE TECH CORP
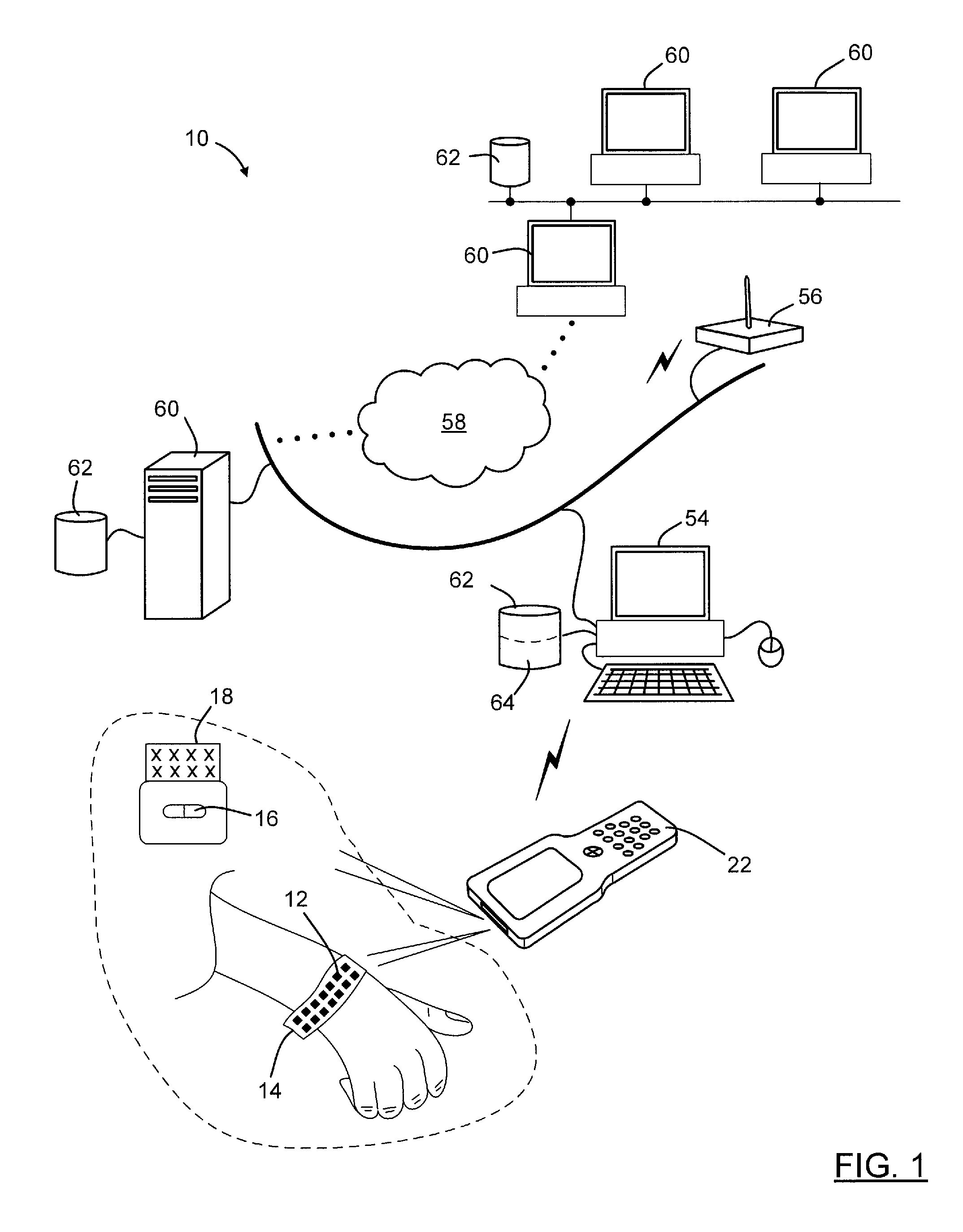
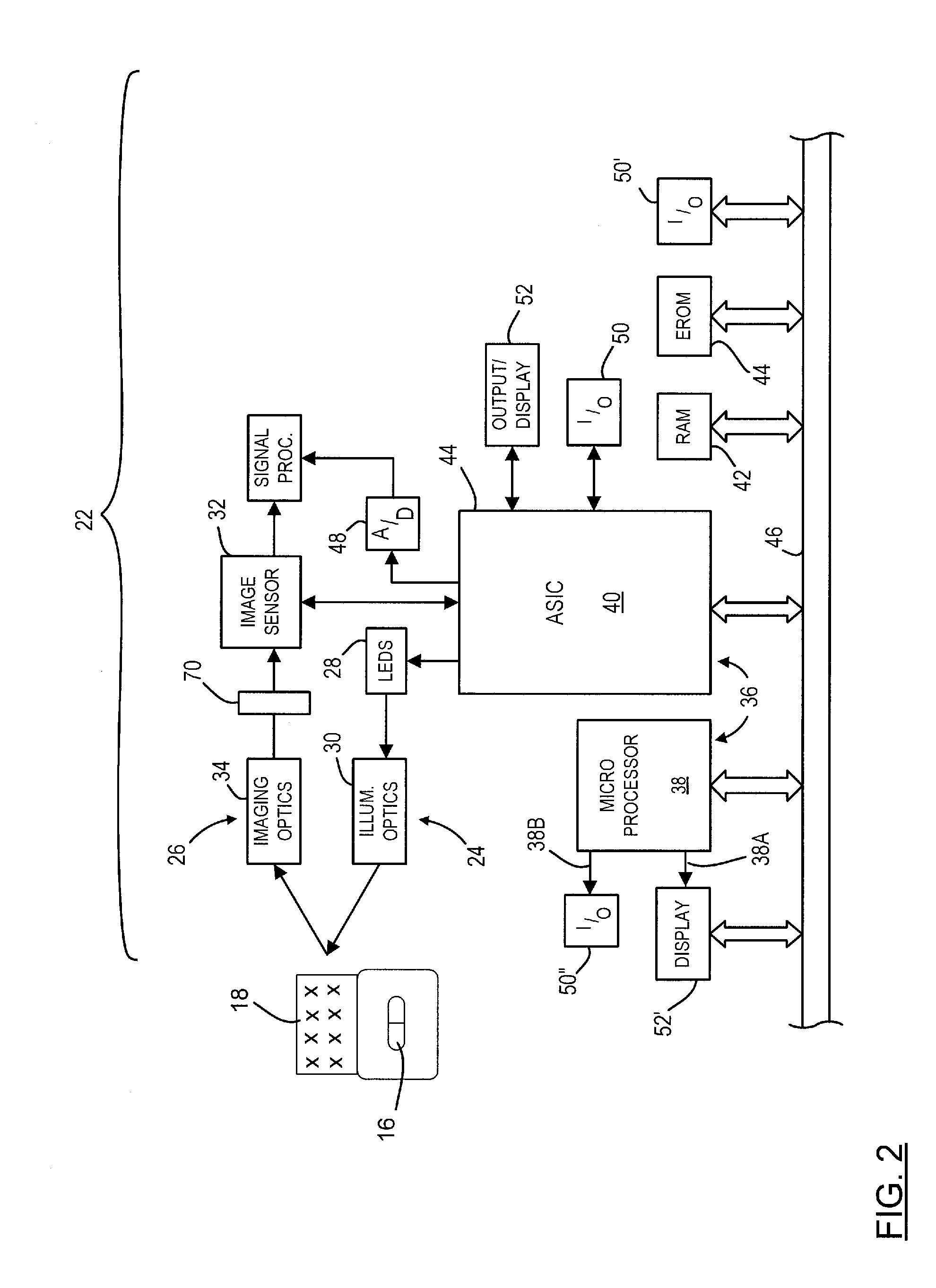
Optical imager and method for correlating a medication package with a patient
Owner:METROLOGIC INSTR
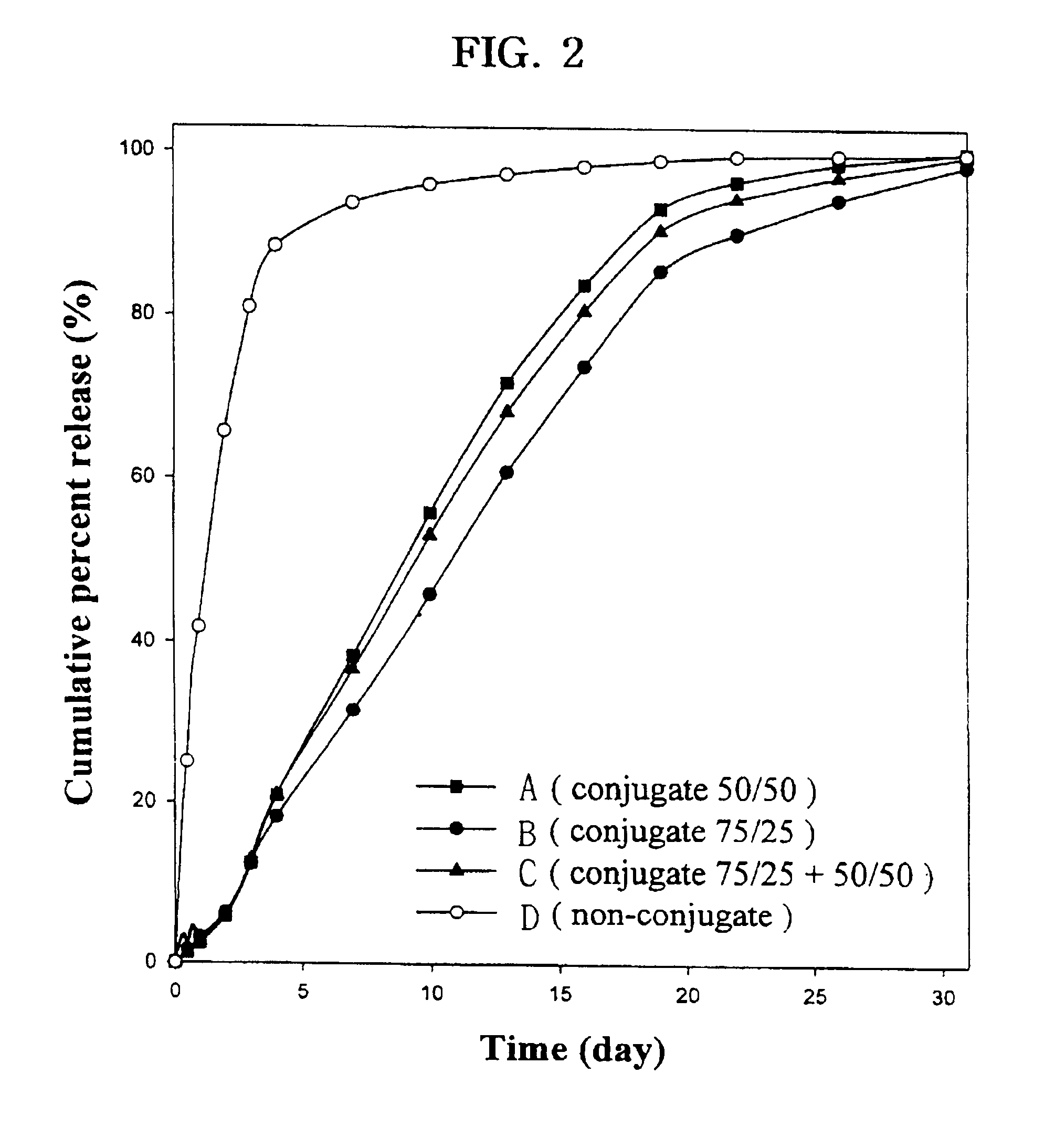
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
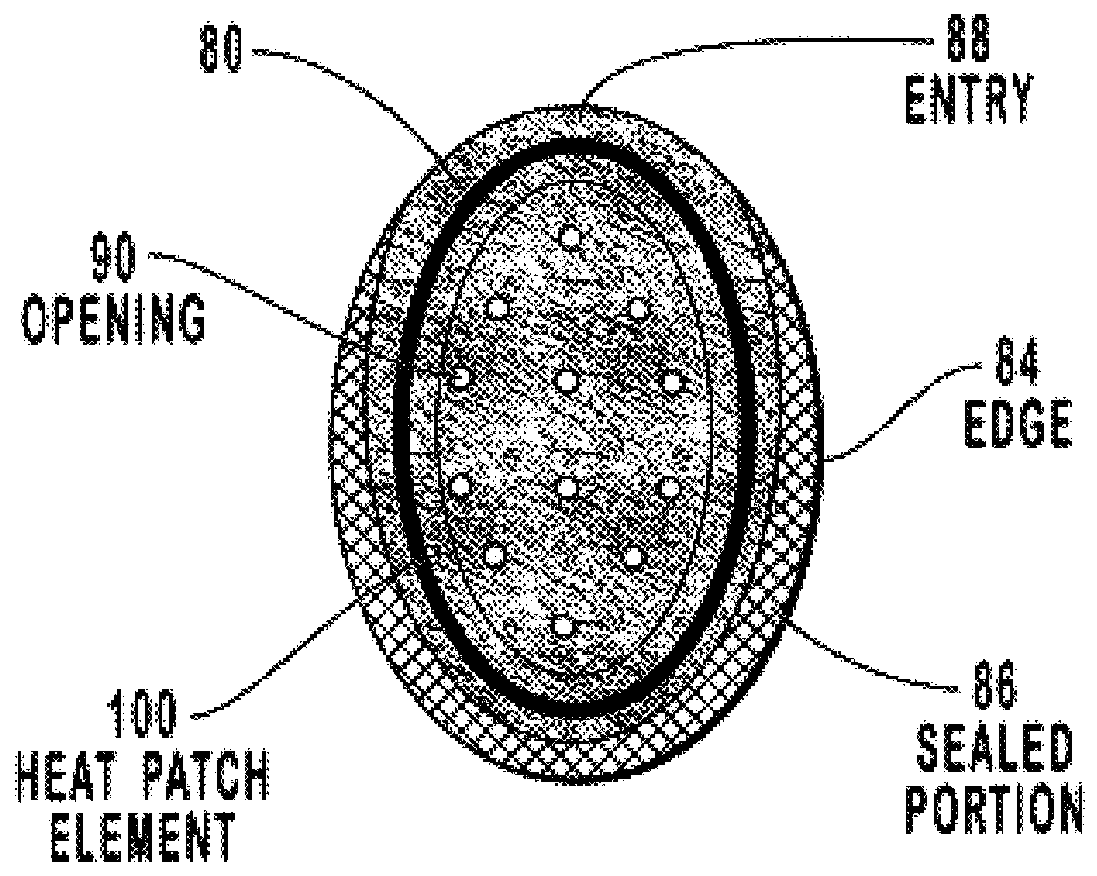
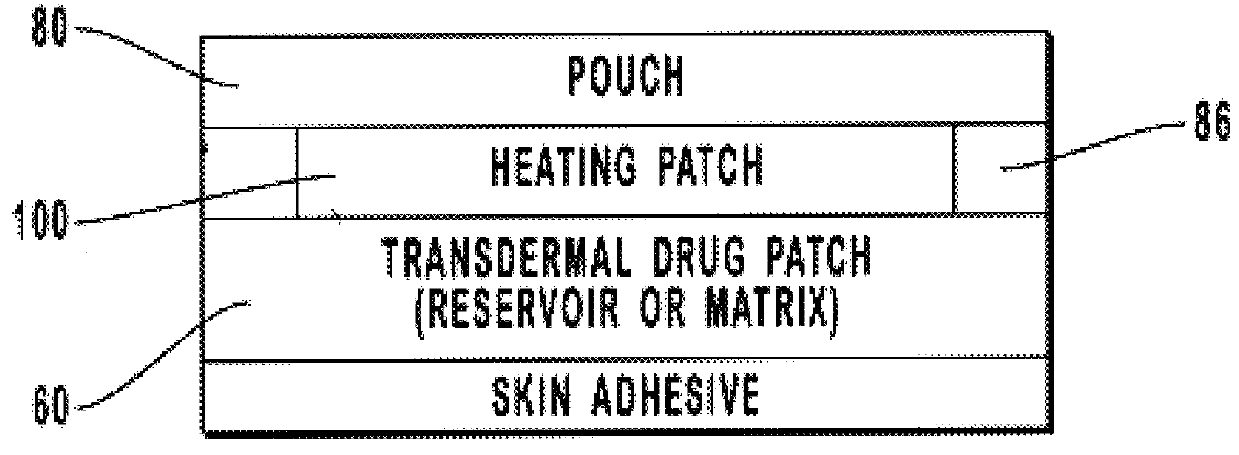
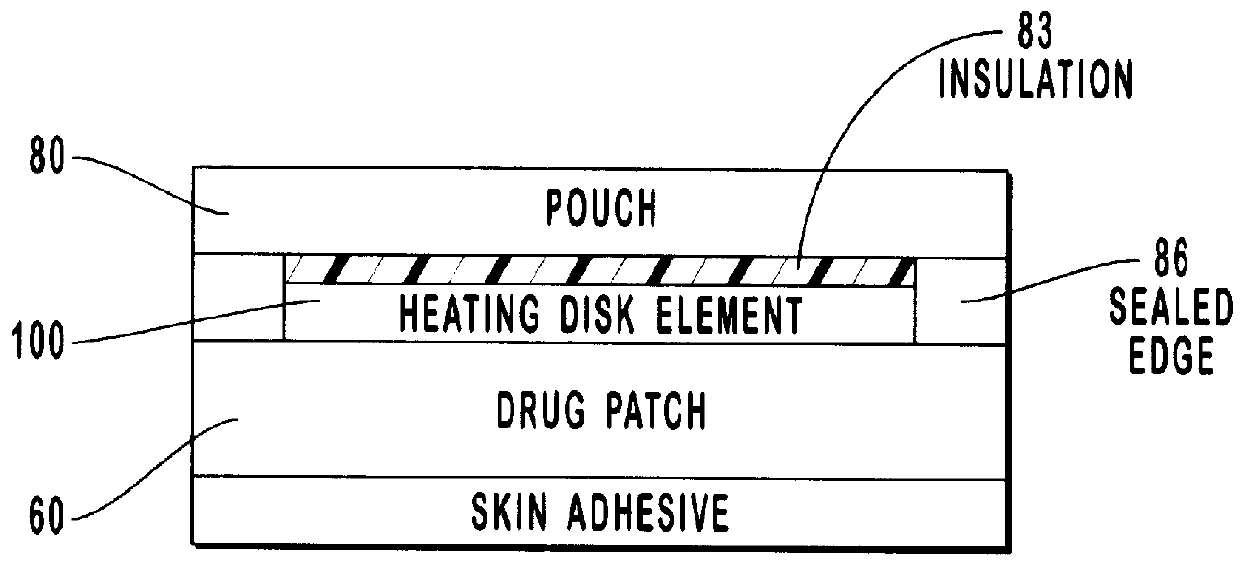
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease
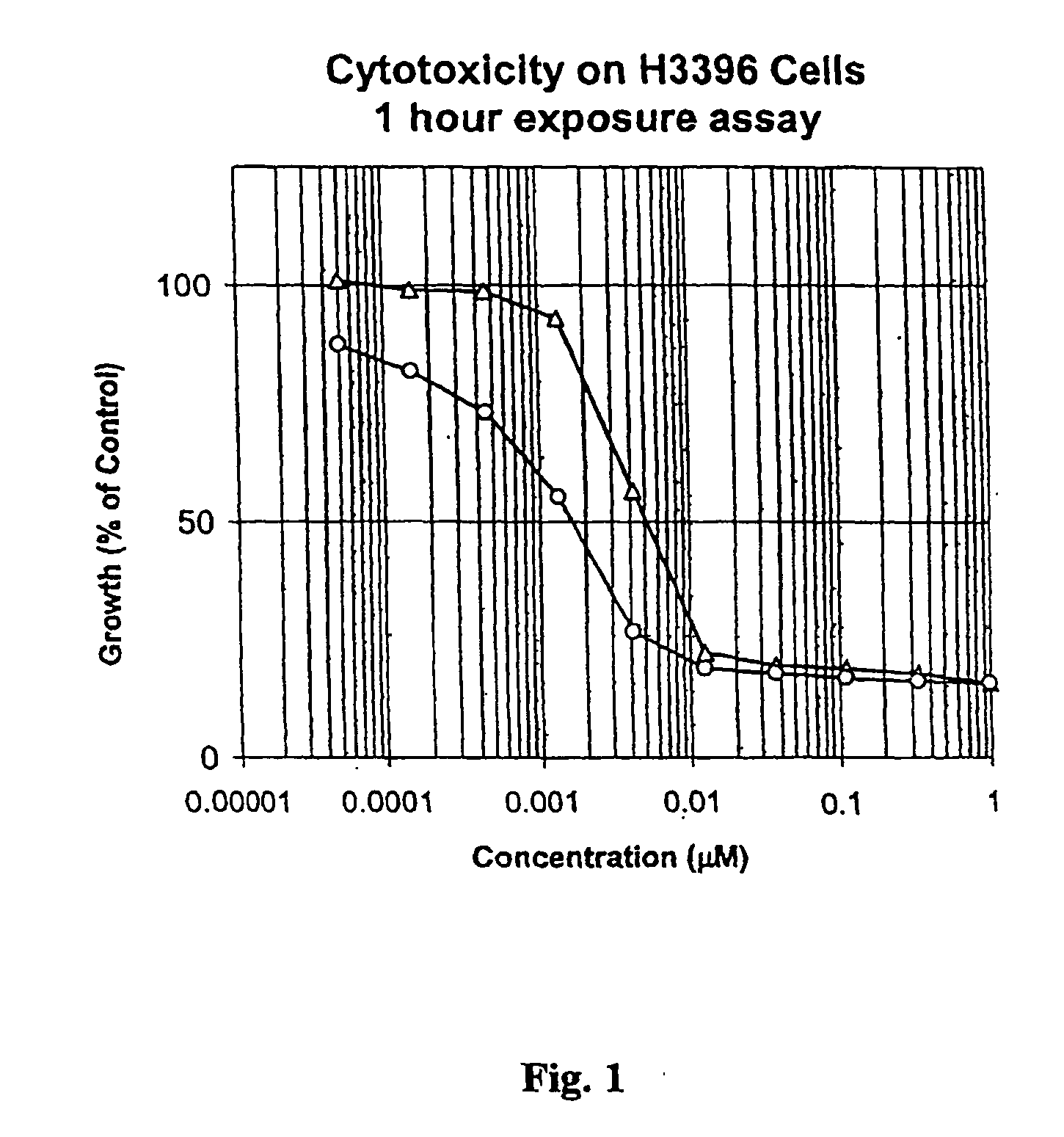
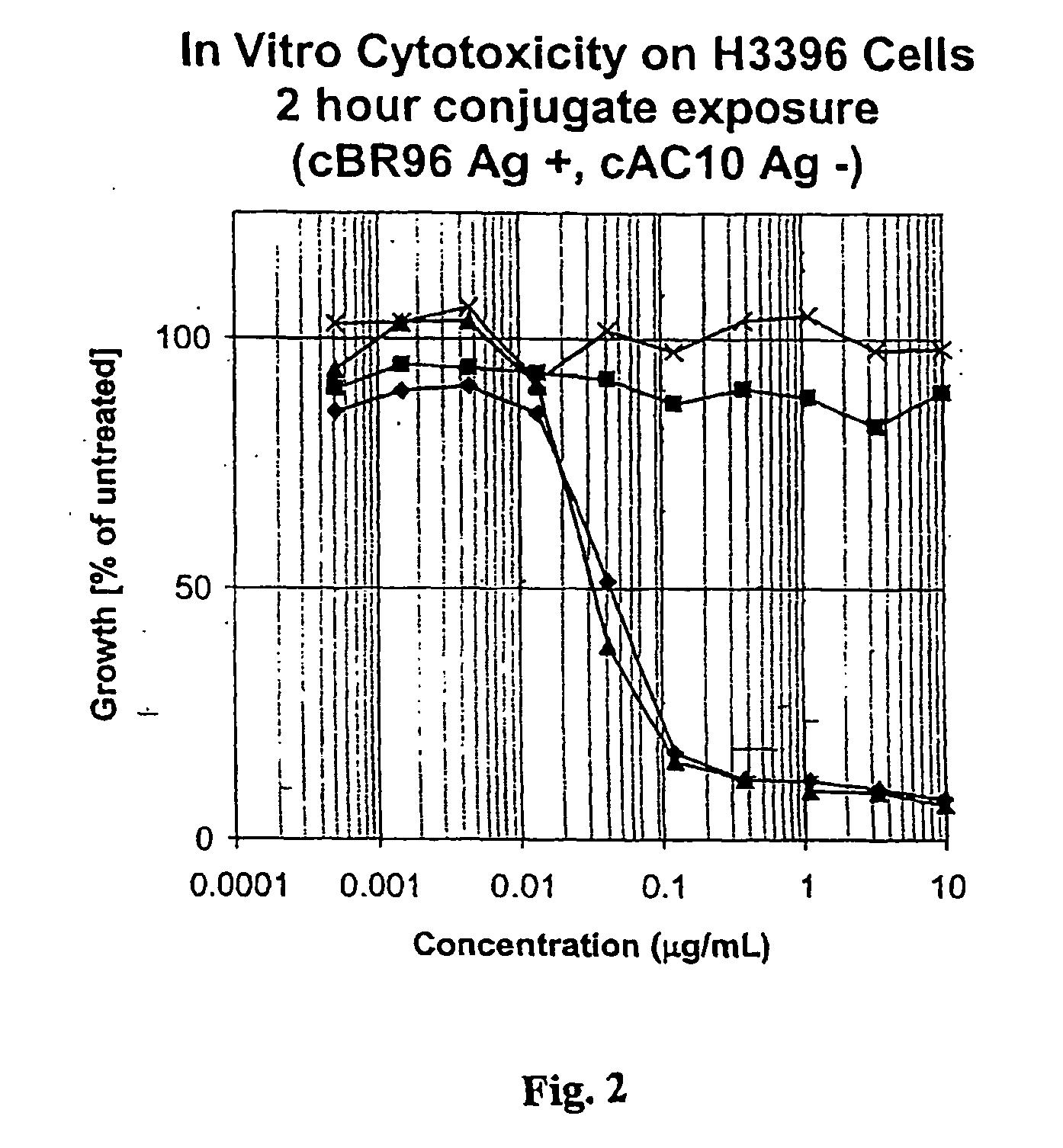
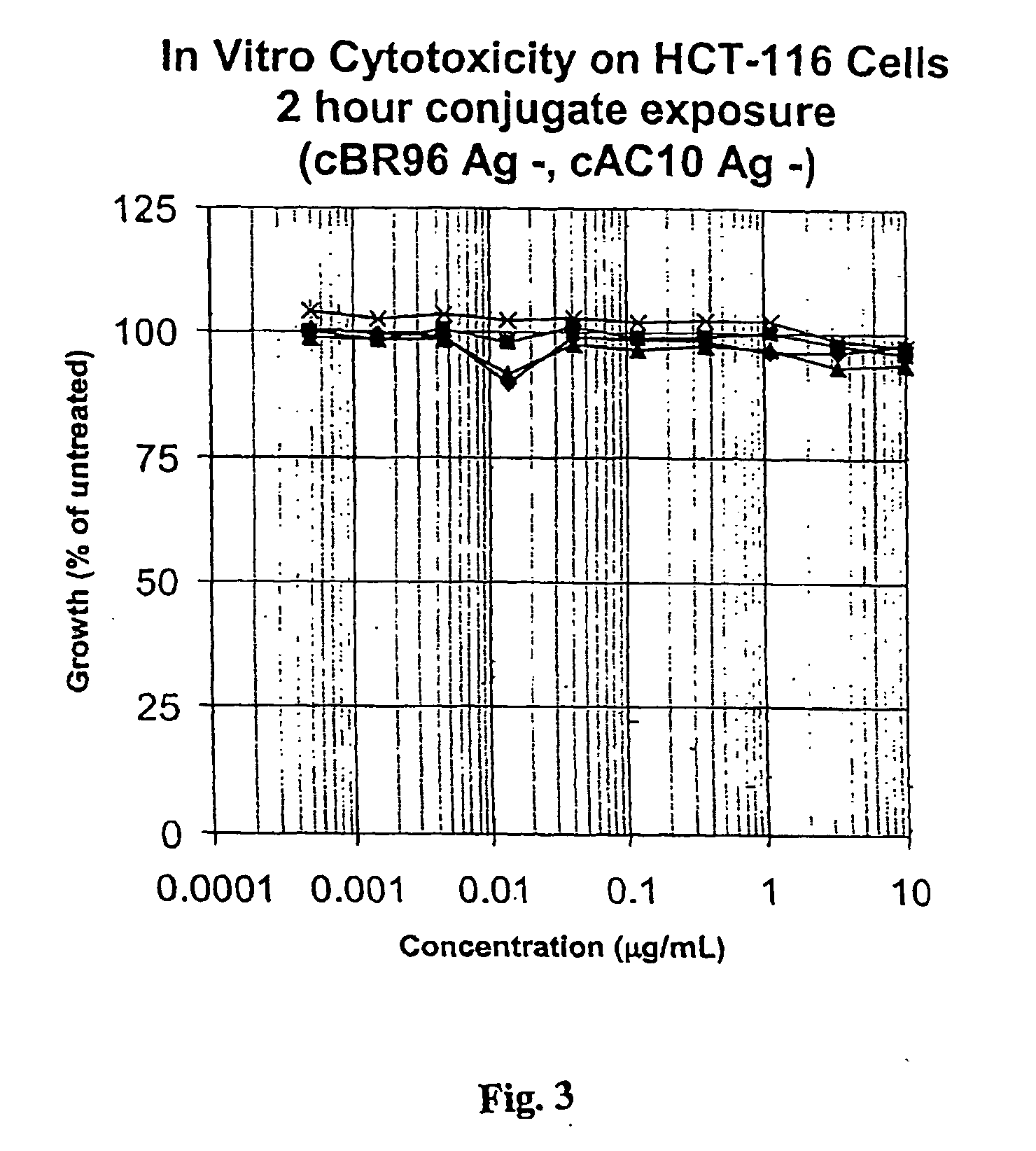
Drug-Linker-Ligand Conjugates are disclosed in which a Drug is linked to a Ligand via a peptide-based Linker unit. In one embodiment, the Ligand is an Antibody. Drug-Linker compounds and Drug compounds are also disclosed. Methods for treating cancer, an autoimmune disease or an infectious disease using the compounds and compositions of the invention are also disclosed.
Owner:SEAGEN INC
Methods for detecting and identifying single molecules
InactiveUS6287765B1Highly specific controlImprove securityNanotechSugar derivativesSynthetic nucleotideMolecular adsorption
Multimolecular devices and drug delivery systems prepared from synthetic heteropolymers, heteropolymeric discrete structures, multivalent heteropolymeric hybrid structures, aptameric multimolecular devices, multivalent imprints, tethered specific recognition devices, paired specific recognition devices, nonaptameric multimolecular devices and immobilized multimolecular structures are provided, including molecular adsorbents and multimolecular adherents, adhesives, transducers, switches, sensors and delivery systems. Methods for selecting single synthetic nucleotides, shape-specific probes and specifically attractive surfaces for use in these multimolecular devices are also provided. In addition, paired nucleotide-nonnucleotide mapping libraries for transposition of selected populations of selected nonoligonucleotide molecules into selected populations of replicatable nucleotide sequences are described.
Owner:MOLECULAR MACHINES
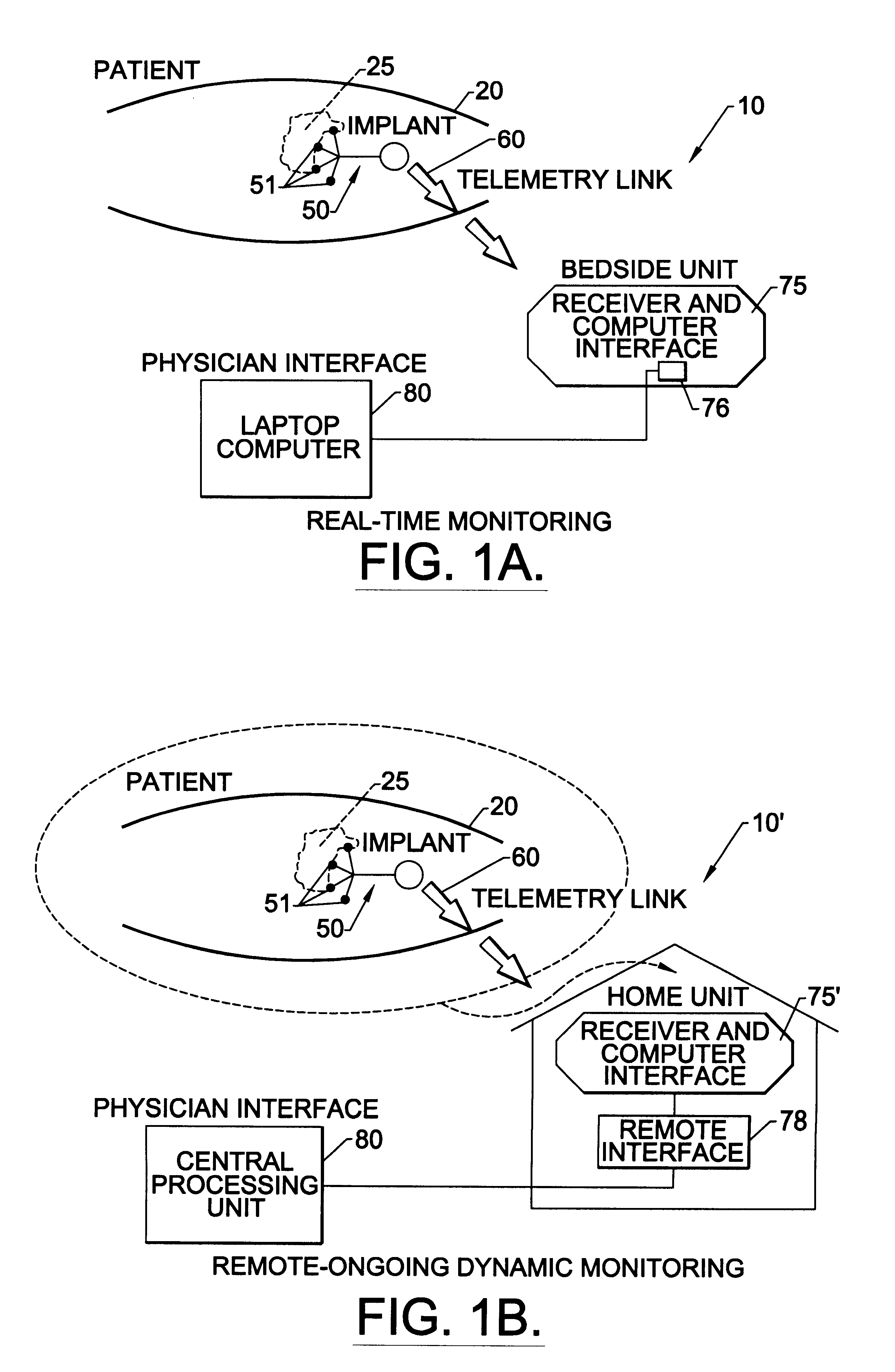
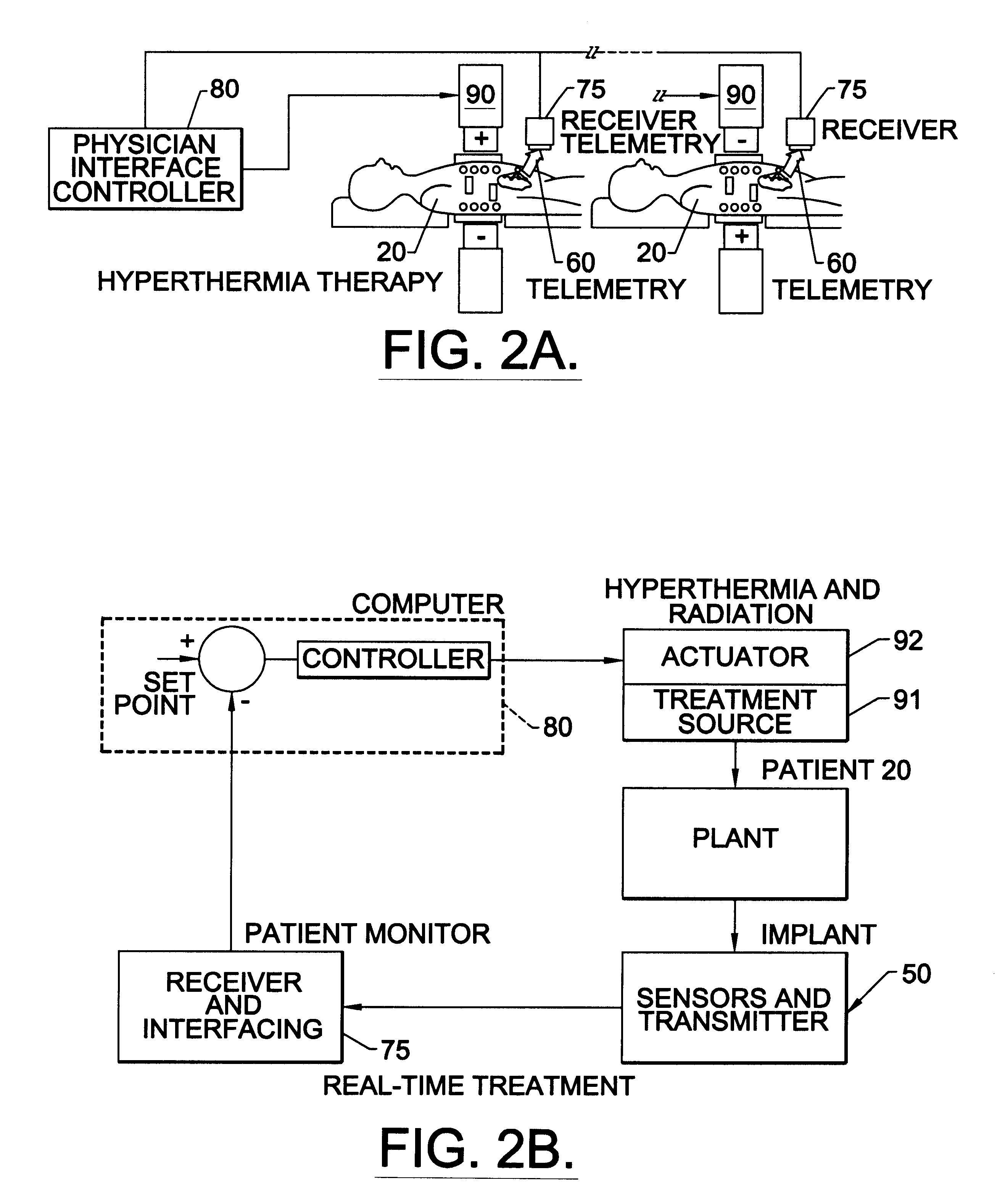
Methods, systems, and associated implantable devices for dynamic monitoring of physiological and biological properties of tumors
InactiveUS6402689B1Enhanced and favorable treatmentMinimize couplingMechanical/radiation/invasive therapiesSurgeryDynamic monitoringEngineering
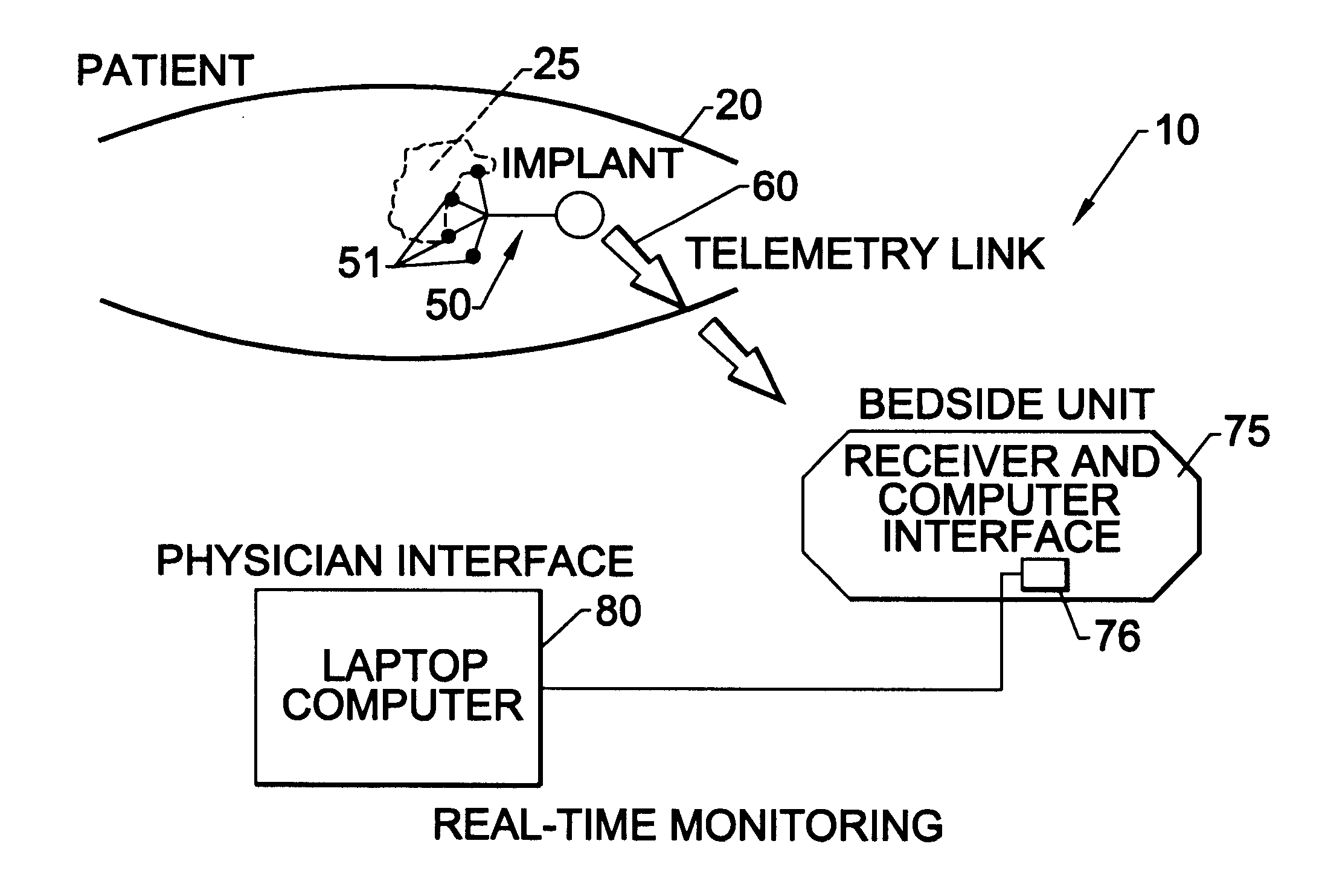
Methods of monitoring and evaluating the status of a tumor undergoing treatment includes monitoring in vivo at least one physiological parameter associated with a tumor in a subject undergoing treatment, transmitting data from an in situ located sensor to a receiver external of the subject, analyzing the transmitted data, repeating the monitoring and transmitting steps at sequential points in time and evaluating a treatment strategy. The method provides dynamic tracking of the monitored parameters over time. The method can also include identifying in a substantially real time manner when conditions are favorable for treatment and when conditions are unfavorable for treatment and can verify or quantify how much of a known drug dose or radiation dose was actually received at the tumor. The method can include remote transmission from a non-clinical site to allow oversight of the tumor's condition even during non-active treatment periods (in between active treatments). The disclosure also includes monitoring systems with in situ in vivo biocompatible sensors and telemetry based operations and related computer program products.
Owner:NORTH CAROLINA STATE UNIV +1
Apparatus and method for bioelectric stimulation, healing acceleration, pain relief, or pathogen devitalization
ActiveUS7117034B2Minimal stressPromote healingElectrotherapyArtificial respirationEngineeringAnimal body
An method and method for generating an electrical signal for use in biomedical applications, including two timing-interval generators, each optionally driving a multistep sequencer; analog, digital or hybrid means for combining the resulting timed signals into a complex electrical signal; optional filtering means for blocking direct current, removing selected frequency components from the resulting signal, and / or providing voltage step-up if needed; and conductive means for coupling the resulting signal to a human or animal body, food, beverage or other liquid, cell or tissue culture, or pharmaceutical material, in order to relieve pain, stimulate healing or growth, enhance the production of specific biochemicals, or devitalize selected types of organisms.
Owner:HEALTHONICS INC
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
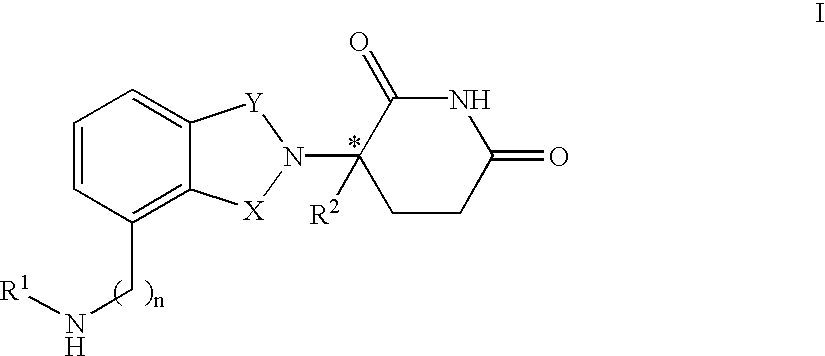
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
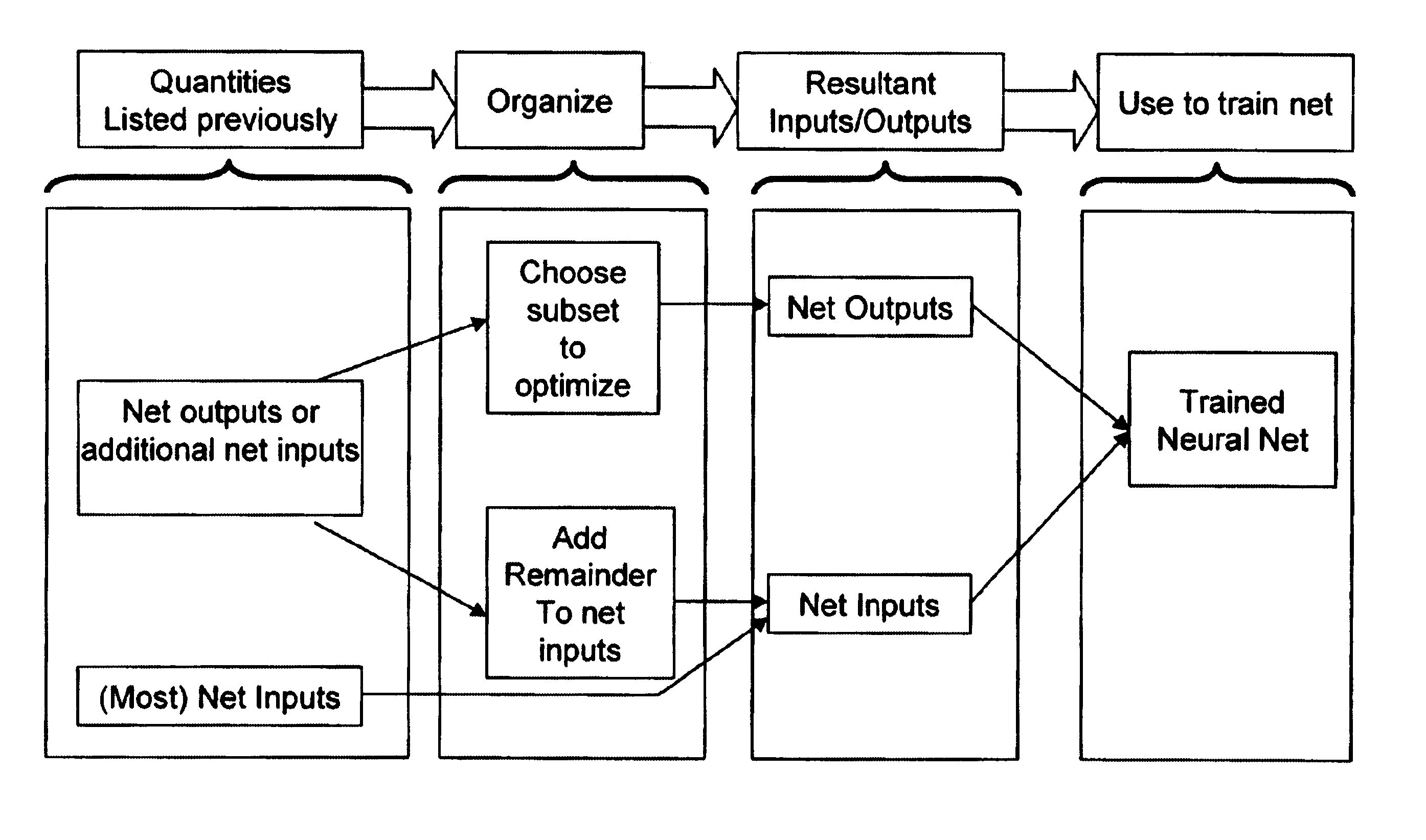
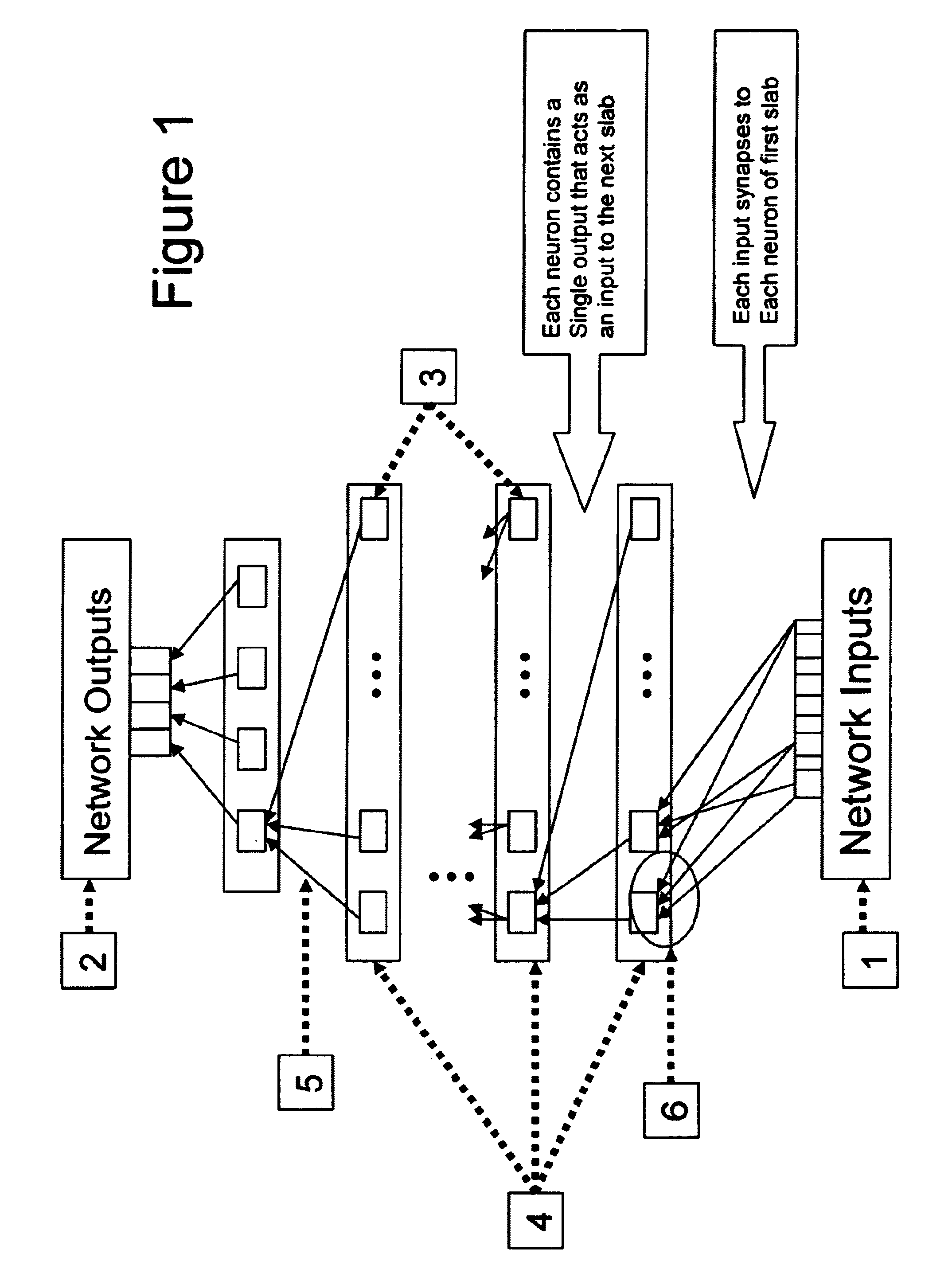
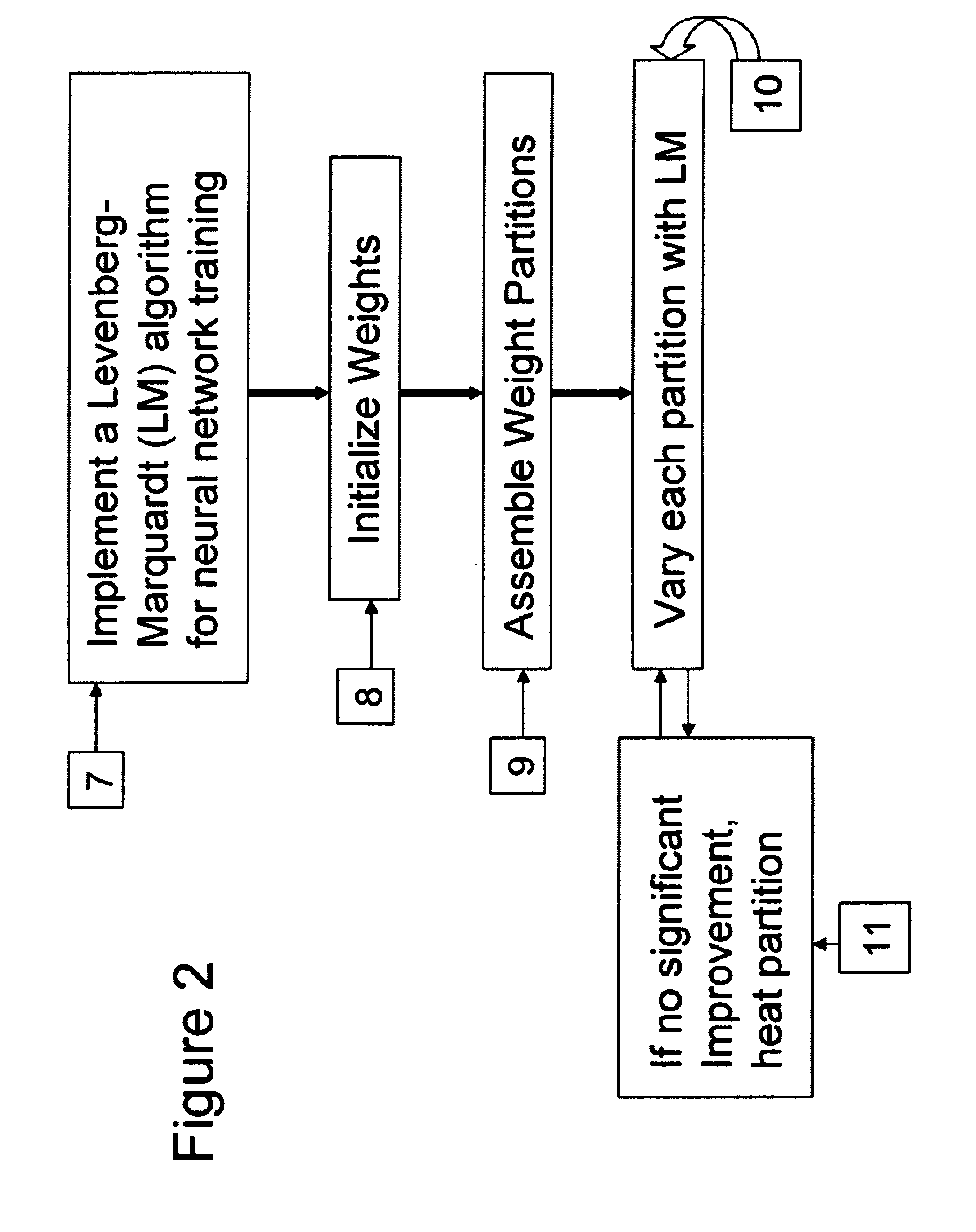
Neural network drug dosage estimation
InactiveUS6658396B1Improve accuracyGood precisionDrug and medicationsBiological neural network modelsNerve networkPatient characteristics
Neural networks are constructed (programmed), trained on historical data, and used to predict any of (1) optimal patient dosage of a single drug, (2) optimal patient dosage of one drug in respect of the patient's concurrent usage of another drug, (3a) optimal patient drug dosage in respect of diverse patient characteristics, (3b) sensitivity of recommended patient drug dosage to the patient characteristics, (4a) expected outcome versus patient drug dosage, (4b) sensitivity of the expected outcome to variant drug dosage(s), (5) expected outcome(s) from drug dosage(s) other than the projected optimal dosage. Both human and economic costs of both optimal and sub-optimal drug therapies may be extrapolated from the exercise of various optimized and trained neural networks. Heretofore little recognized sensitivities-such as, for example, patient race in the administration of psychotropic drugs-are made manifest. Individual prescribing physicians employing deviant patterns of drug therapy may be recognized. Although not intended to prescribe drugs, nor even to set prescription drug dosage, the neural networks are very sophisticated and authoritative "helps" to physicians, and to physician reviewers, in answering "what if" questions.
Owner:PREDICTION SCI
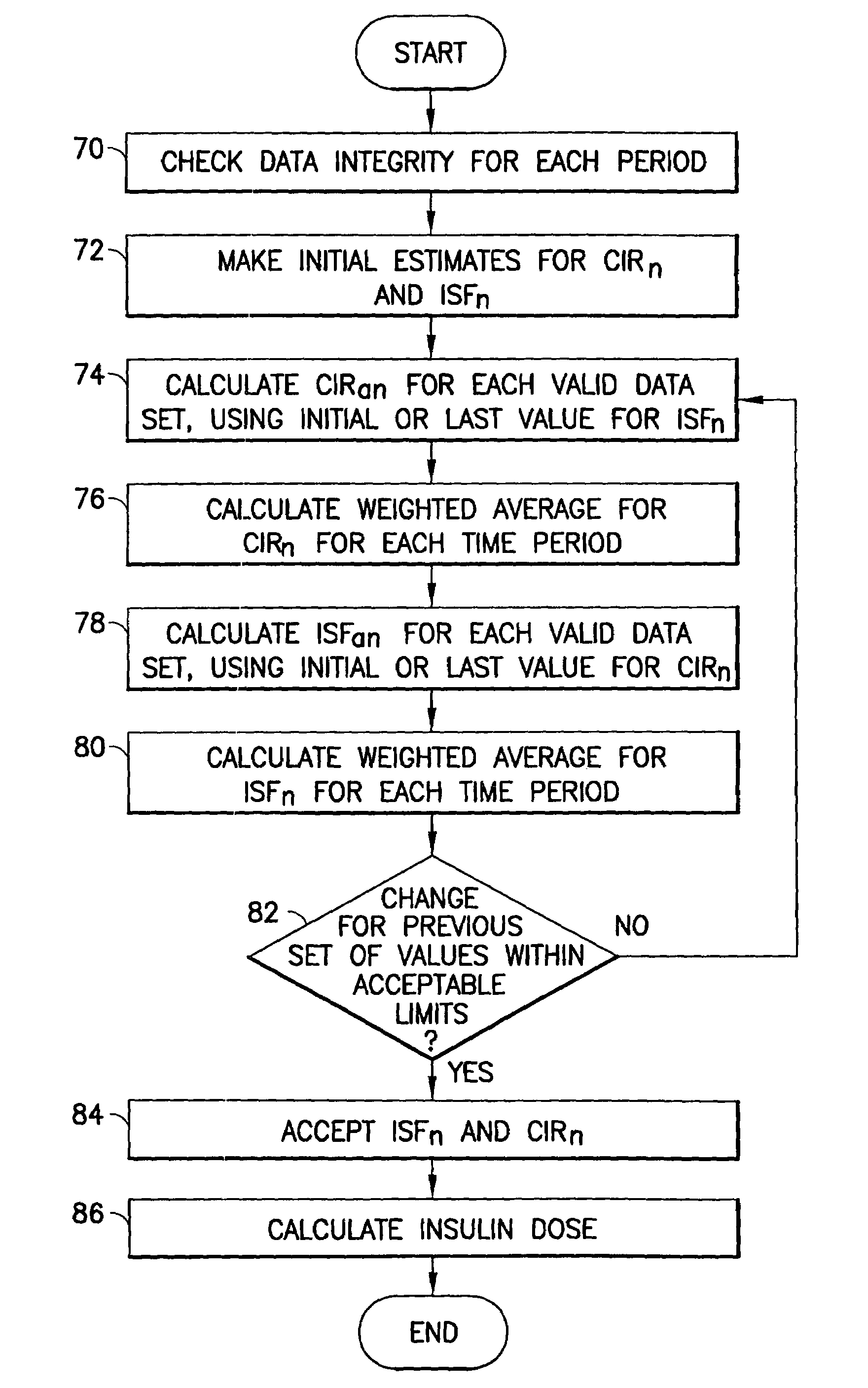
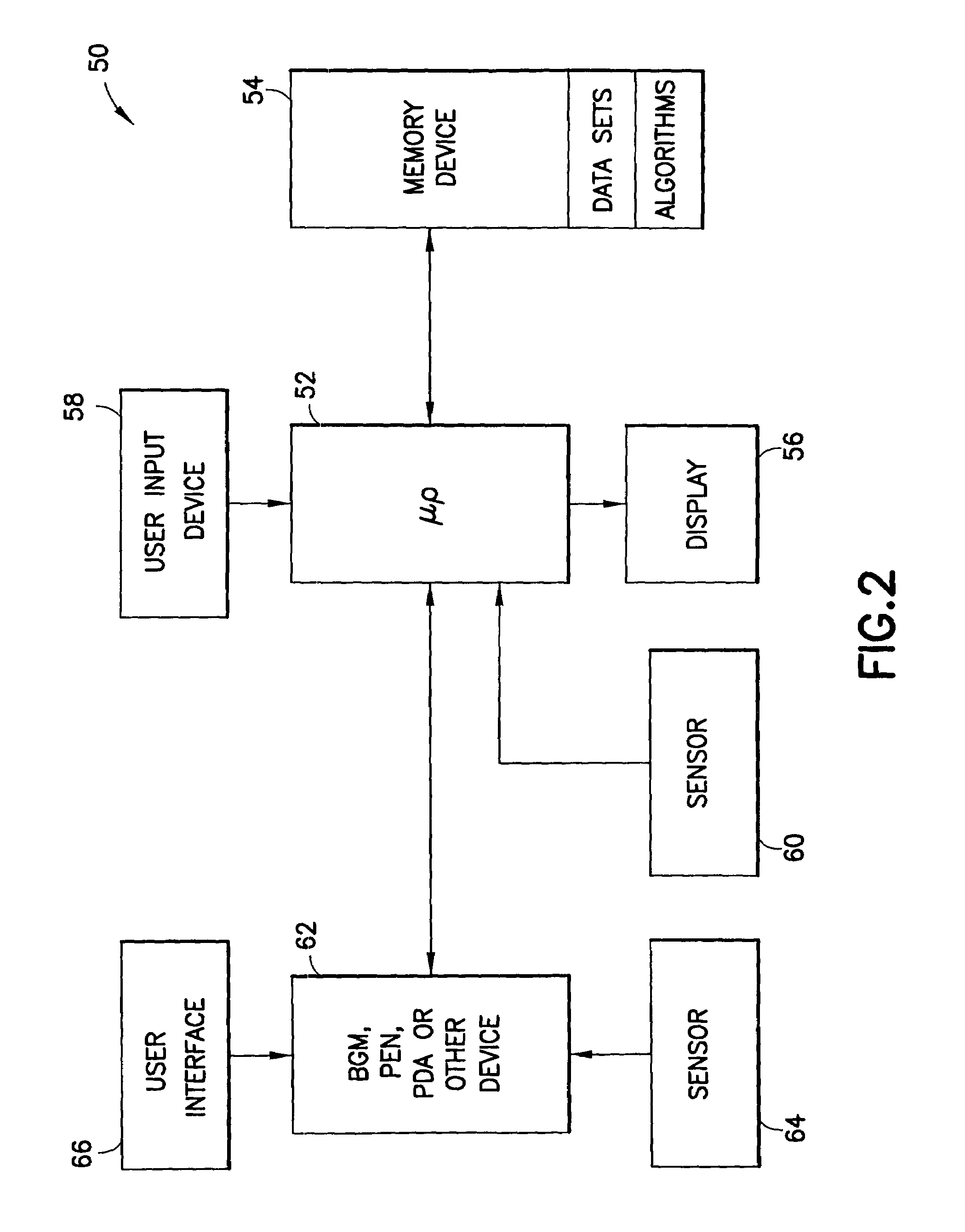
System for determining insulin dose using carbohydrate to insulin ratio and insulin sensitivity factor
Owner:EMBECTA CORP
System and method for building and manipulating a centralized measurement value database
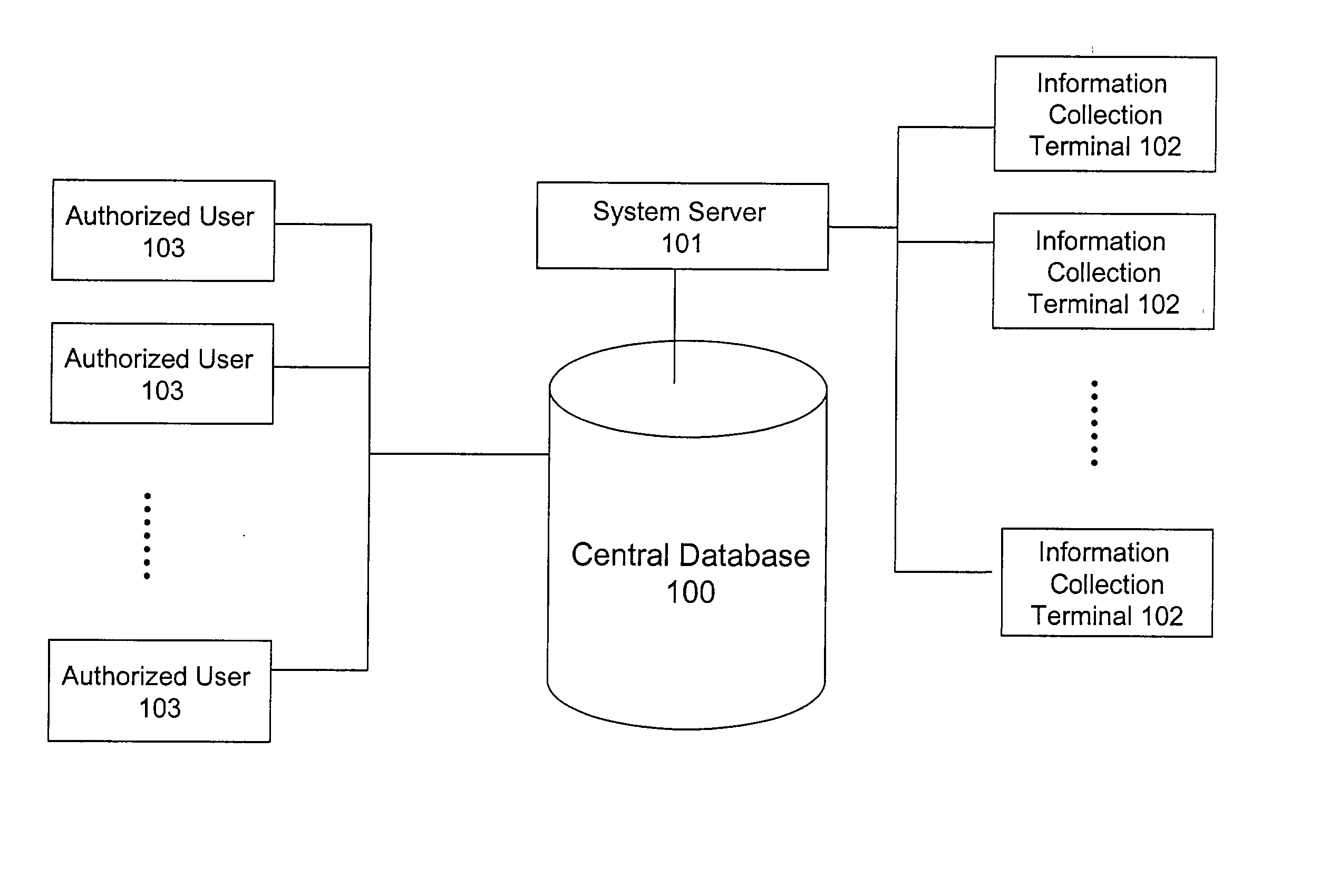
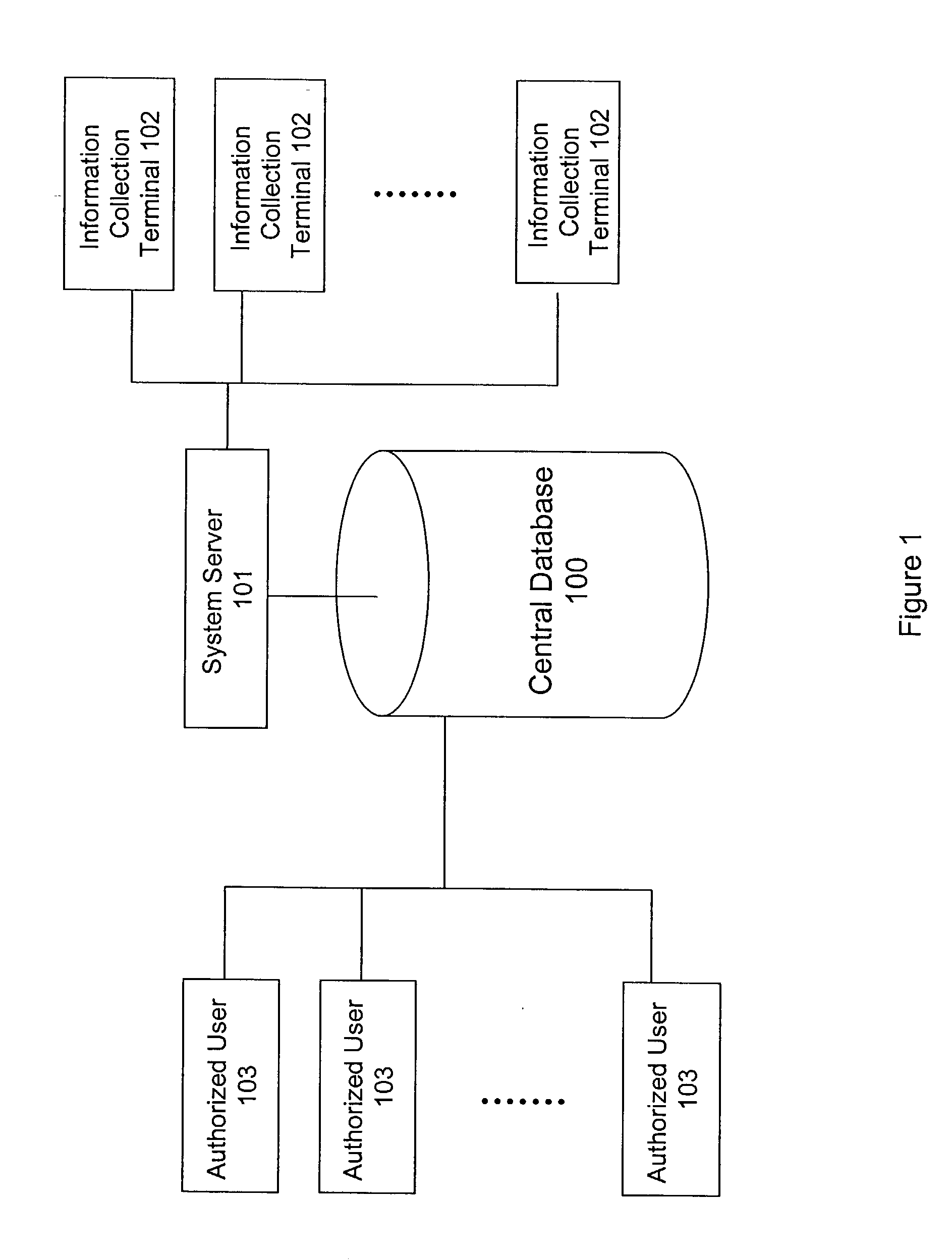
InactiveUS20020186818A1Low penetrationEasy to aimImage enhancementImage analysisMarket penetrationEfficacy
A system and method for building and / or manipulating a centralized medical image quantitative information database aid in diagnosing diseases, identifying prevalence of diseases, and analyzing market penetration data and efficacy of different drugs. In one embodiment, the diseases are bone-related, such as osteoporosis and osteoarthritis. Subjects' medical images, personal and treatment information are obtained at information collection terminals, for example, at medical and / or dental facilities, and are transferred to a central database, either directly or through a system server. Quantitative information is derived from the medical images, and stored in a central database, associated with subjects' personal and treatment information. Authorized users, such as medical officials and / or pharmaceutical companies, can access the database, either directly or through the central server, to diagnose diseases and perform statistical analysis on the stored data. Decisions can be made regarding marketing of drugs for treating the diseases in question, based on analysis of efficacy, market penetration, and performance of competitive drugs.
Owner:IMAGING THERAPEUTICS +1
Drug storage and dispensing devices and systems comprising the same
ActiveUS20070186923A1Minimizing saliva influxPowdered material dispensingDrug and medicationsDrug StorageBiomedical engineering
Drug storage and dispensing devices for dispensing a drug dosage form to a patient are disclosed. The dispensing device has a programmable lock-out feature for locking the dispensing device and is capable of detecting the identity of a user. The invention further provides a method for the treatment of subject, by administering to the subject a drug dosage form using a dispensing device of the invention.
Owner:ACEIRX PHARM INC
Disposable fluid injection module
An automated injection module is comprised of a housing, a piston drug capsule disposed within the housing, a piston core including a puncture seal membrane defining a reservoir for holding a drug between the puncture seal membrane and the piston drug capsule, an injection device having at least one sharp end for puncturing the puncture seal upon activation of the device, an end cap on a distal end of the housing, and a pressure source on a proximal end of the housing. The pressure source is preferably a propellant that ignites and forces the piston toward the distal end. Substantially simultaneously, the injection device pierces the puncture seal membrane, the piston core is forced into the piston drug capsule, and the drug is evacuated from the reservoir through the injection device and into a patient.
Owner:SARCOS LC
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
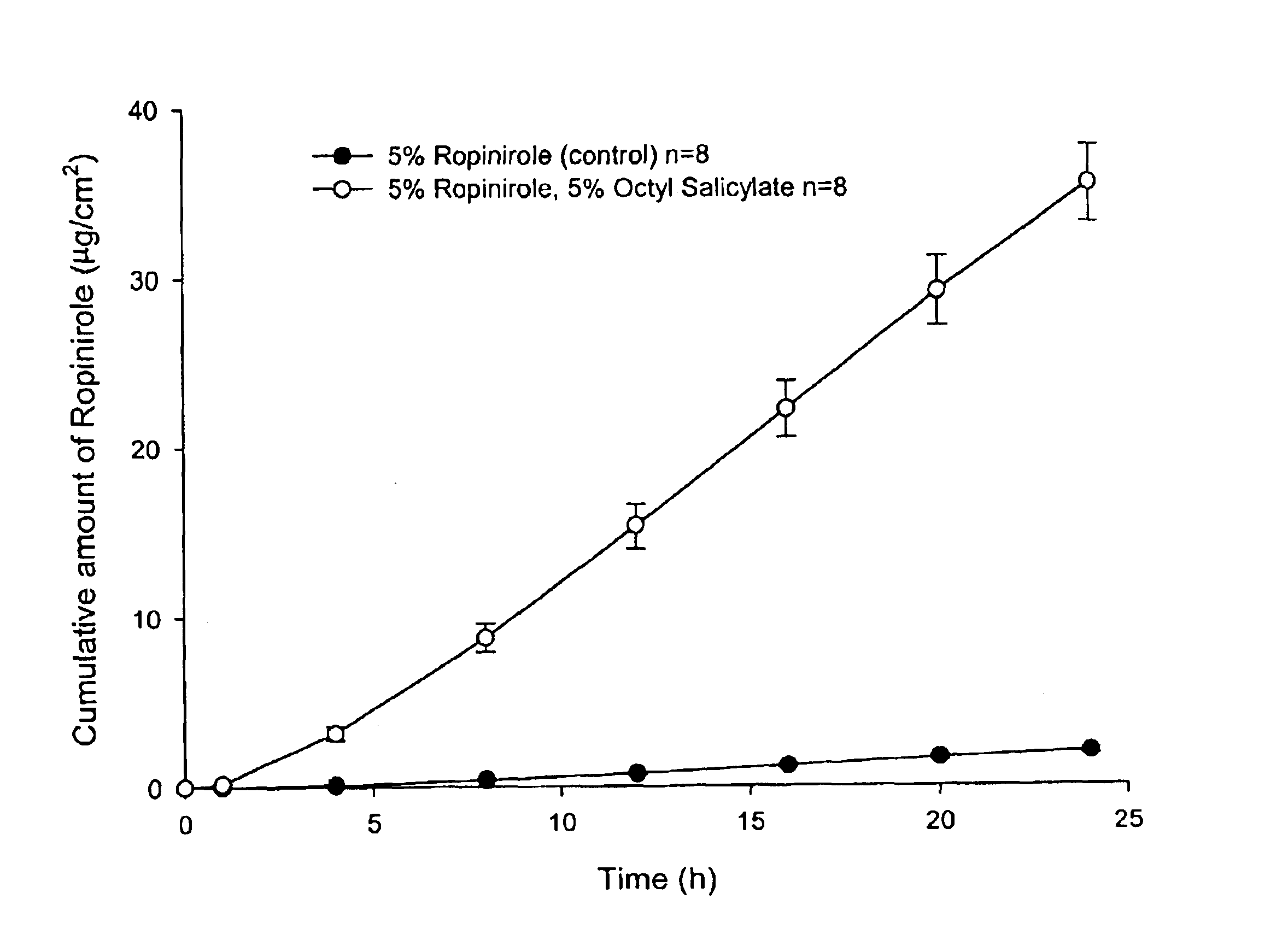
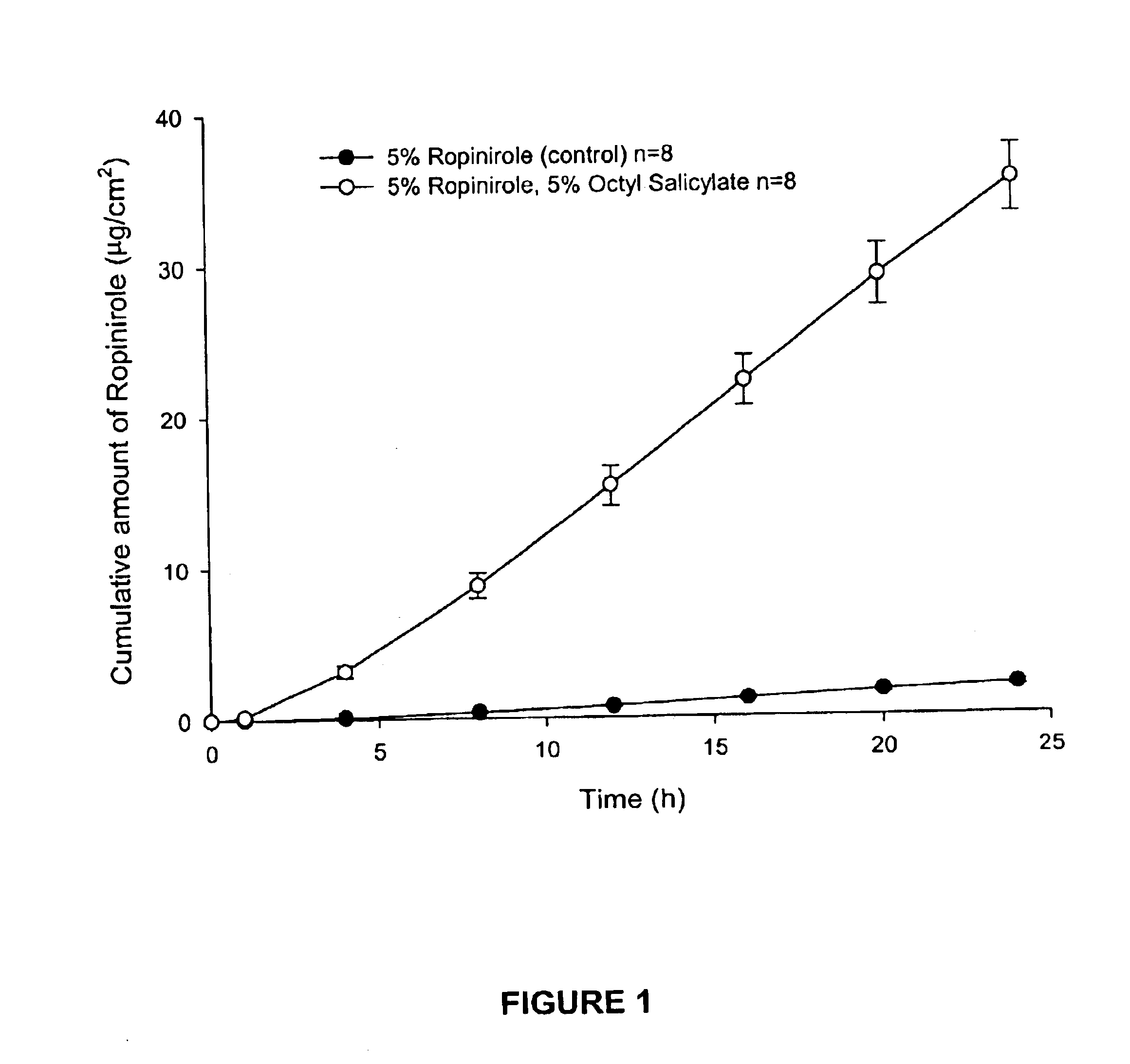
Transdermal delivery of antiparkinson agents
InactiveUS6929801B2Less complicated manufactureImprove dose uniformityCosmetic preparationsOrganic active ingredientsWhole bodyMedicine
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of an antiParkinson agent; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic acting antiParkinson agent to an animal which comprises applying an effective amount of the antiParkinson agent in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS
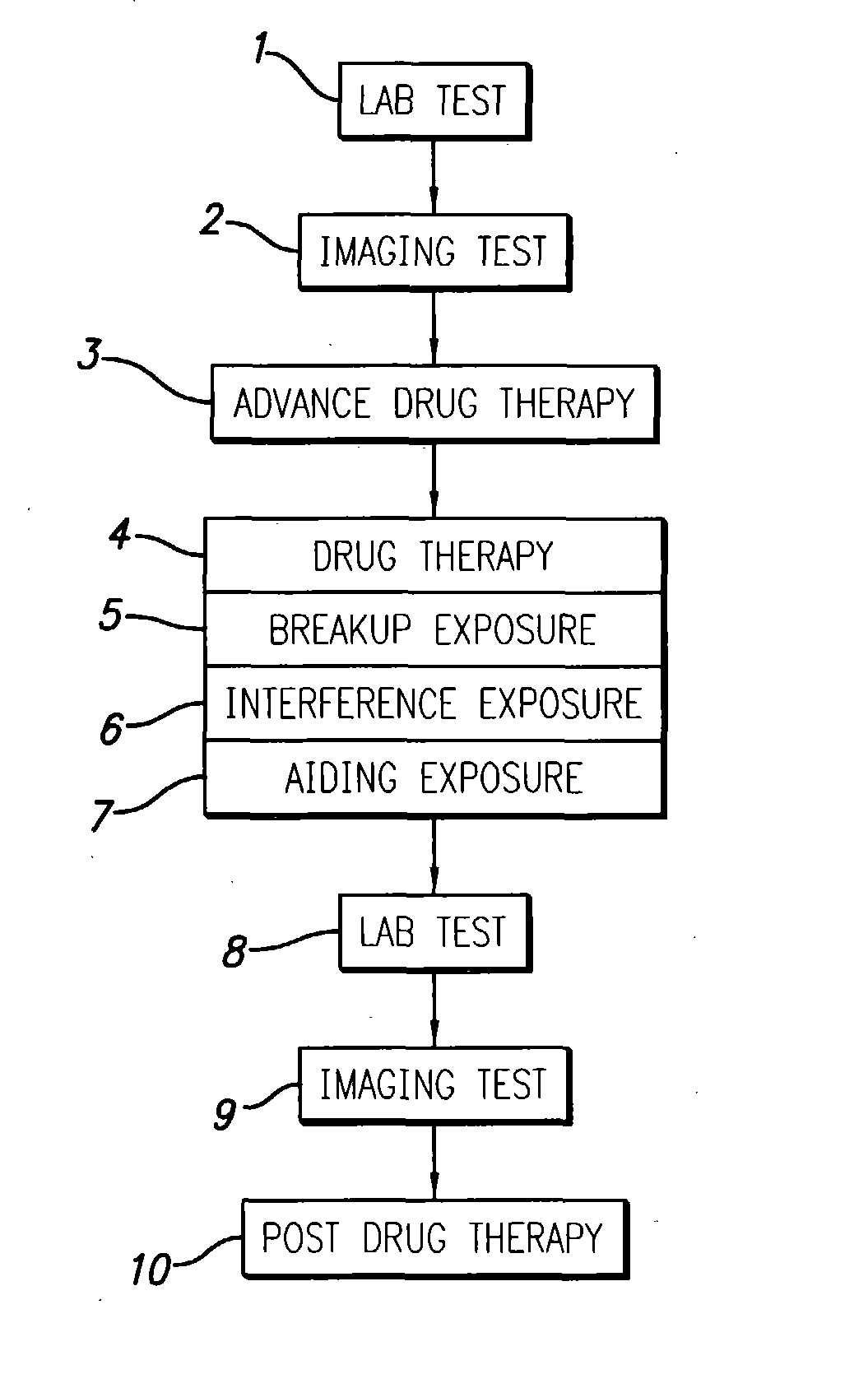
System and methods for treatment of alzheimer's and other deposition-related disorders of the brain
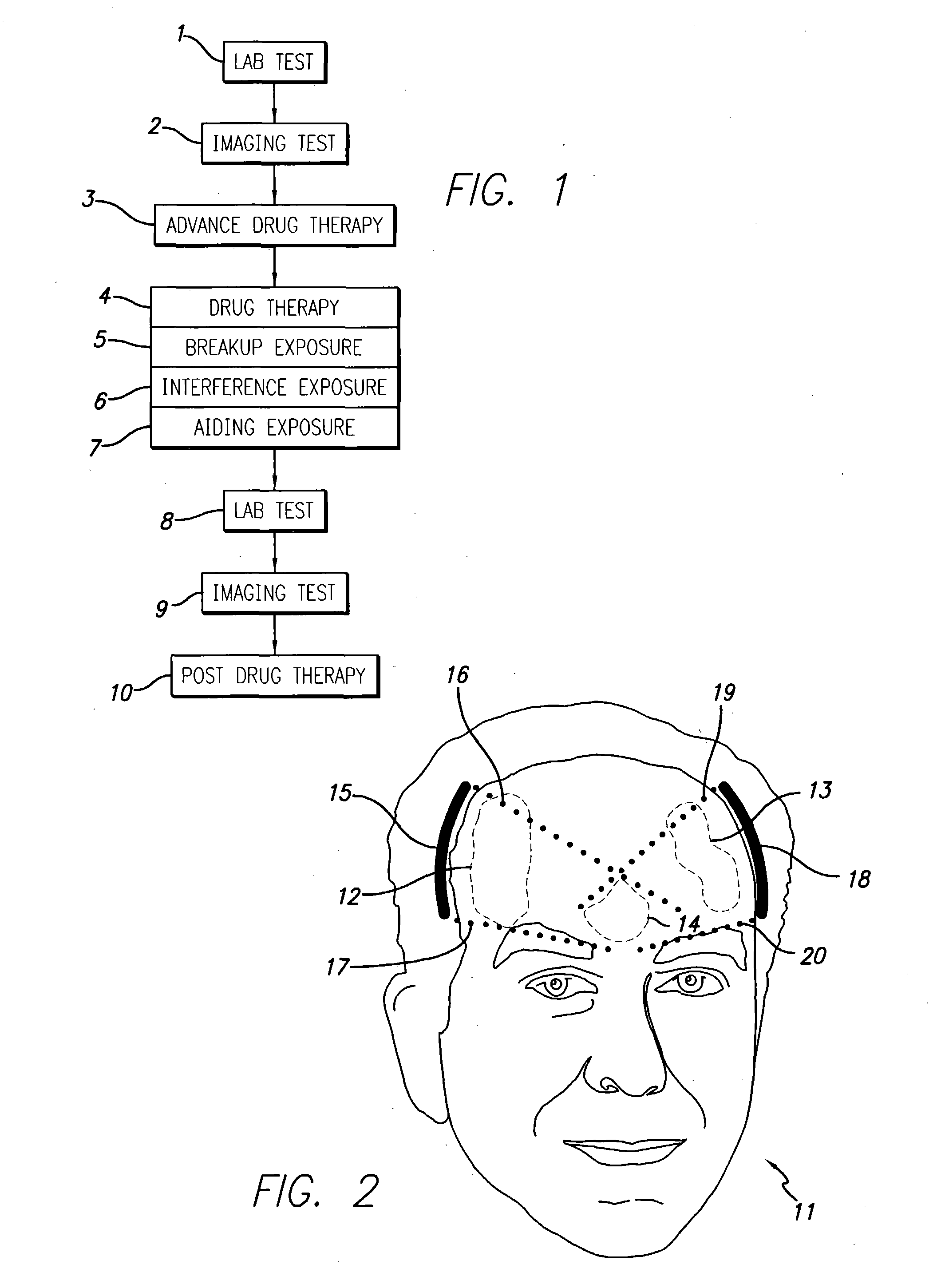
InactiveUS20040049134A1Slowing, stopping or avoiding a patient's cognitive lossesMinimal adverse side effectUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseSide effect
A system and methods are provided for the therapeutic treatment of brain-plaques, fibrils, abnormal-protein related or aggregation-prone protein related deposition-diseases. The system employs acoustic exposure therapy means for delivering therapeutic energy to at least one brain region. The therapy supports at least one of the following processes: (i) physical breakup, erosion, disentanglement, de-aggregation, dissolution, de-agglomeration, de-amalgamation or permeation of the deposits, (ii) interference in at least one deposit formation process, deposition related chemical reaction or biological or genetic pathway contributing to the deposits or deposition-related processes, and (iii) aiding the recovery, growth, regrowth or improved functionality of brain-related cells or functional pathways negatively impacted by, stressed by or disposed to the deposits, deposition-processes or deposition disease state, or supporting the growth of newly transplanted cells anywhere in the brain-related anatomy. The system and methods treat Alzheimer's and other deposition-related disorders of the brain, with minimal adverse side effects to the patient and may be used in cooperation with a drug.
Owner:TOSAYA CAROL A +1
Autonomous, ambulatory analyte monitor or drug delivery device
ActiveUS7004928B2Improve capillary forceMinimize coagulationAutomatic syringesMicroneedlesBiomedical engineeringElectronic equipment
The invention relates to analyte monitoring / drug (pharmaceutical agent) delivery device. The invention is suited for monitoring various blood constituents such as glucose. The device has a housing that at least partially encloses a plurality of microneedles disposed on a carrier and an electronics portion. Each microneedle is in fluid communication with a corresponding microchannel. Each microneedle is individually addressable. That is, each microneedle can be extended and retracted individually via an actuator. The electronics portion includes a processor and associated circuitry (e.g., memory, supporting electronics and the like), a motor or the like, a sensor, a power supply (e.g., battery) and optionally an interface. In general, the processor controls the operation of the device and is data communication with the actuator, motor, sensor and interface. The invention provides for autonomous operation, that is, without intervention of the user. The invention can optionally provide for calibration without intervention of the user. The invention can also provide for semi-continuous monitoring for day and night time. The invention can provide for up to four, or more, weeks of operation. The invention can provide for a device that is relative small in size, and therefore unobtrusive. The invention can also provide for device with remote control and interactive electronics. The invention may be also used for the delivery of various pharmaceutical agents including high potency drugs to minimize patient intervention and minimize discomfort.
Owner:INTUITY MEDICAL INC
Techniques for positioning therapy delivery elements within a spinal cord or brain
Apparatus and techniques to address the problems associated with lead migration, patient movement or position, histological changes, neural plasticity or disease progression. Disclosed are techniques for implanting a lead having therapy delivery elements, such as electrodes or drug delivery ports, within a vertebral or cranial bone so as to maintain these elements in a fixed position relative to a desired treatment site. The therapy delivery elements may thereafter be adjusted in situ with a position control mechanism and / or a position controller to improve the desired treatment, such as_electrical stimulation and / or drug infusion to a precise target. The therapy delivery elements may be positioned laterally in any direction relative to the targeted treatment site or toward or away from the targeted treatment site. A control system maybe provided for open- or closed-loop feedback control of the position of the therapy delivery elements as well as other aspects of the treatment therapy.
Owner:MEDTRONIC INC
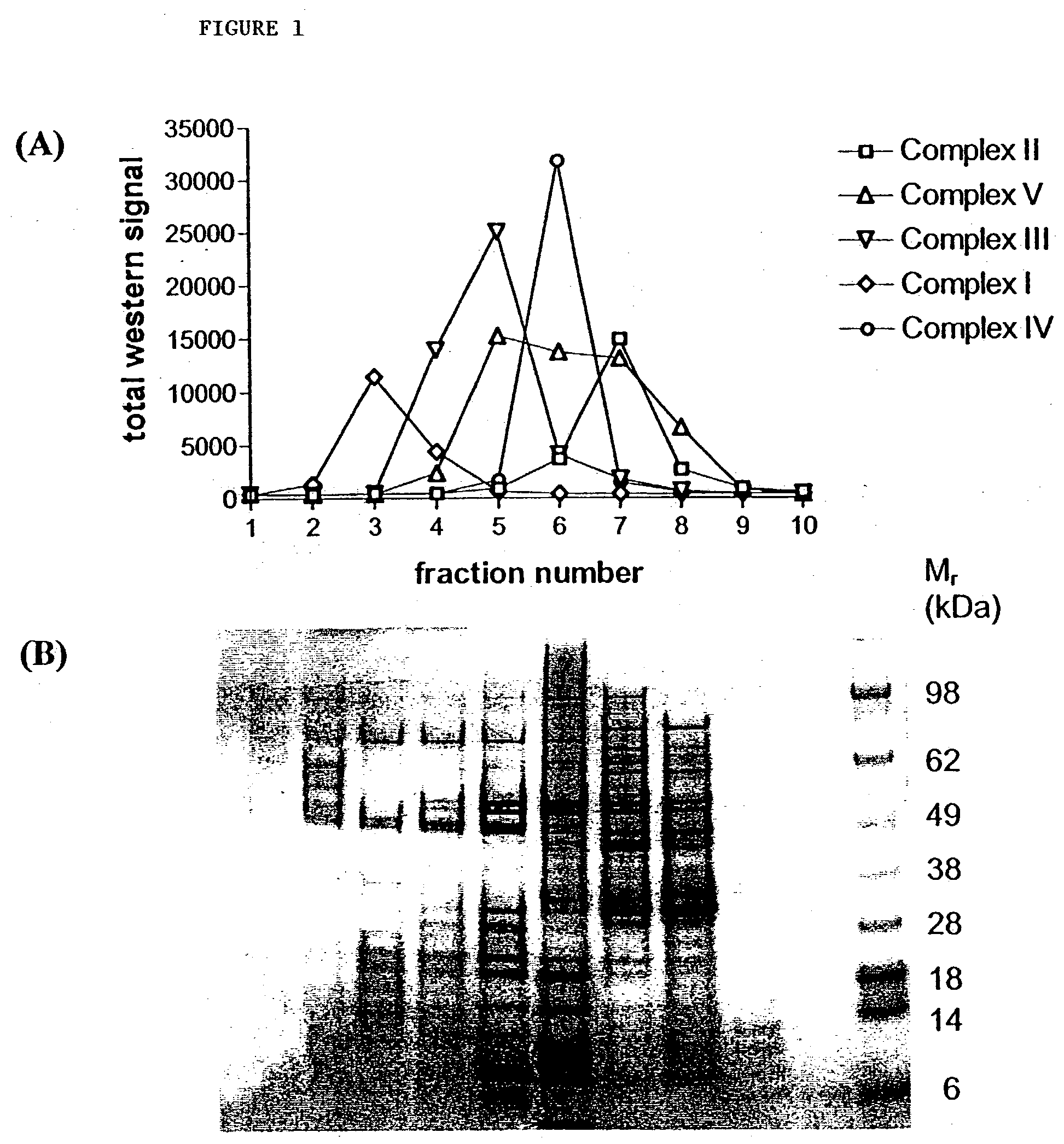
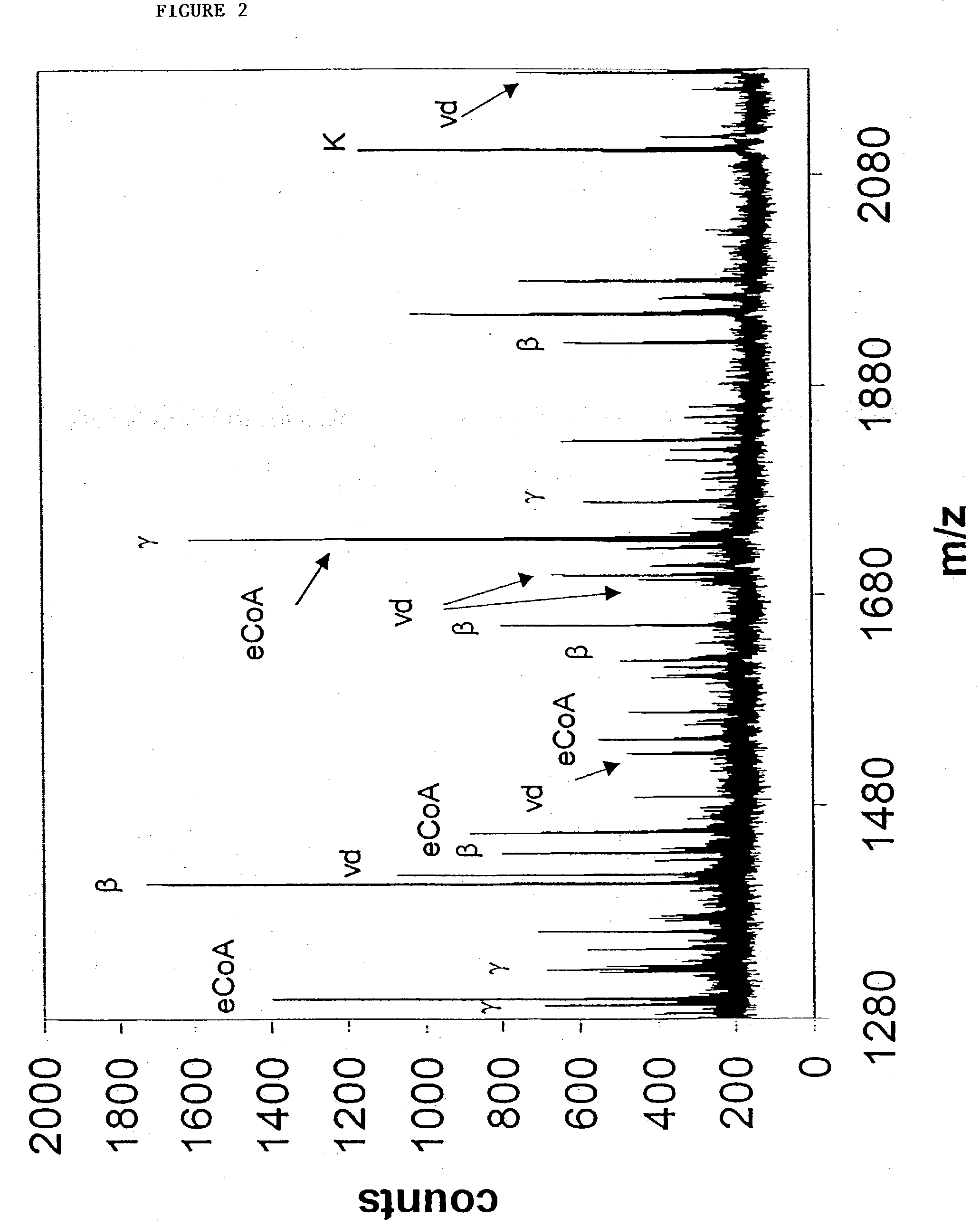
Targets for therapeutic intervention identified in the mitochondrial proteome
Mitochondrial targets for drug screening assays and for therapeutic intervention in the treatment of diseases associated with altered mitochondrial function are provided. Complete amino acid sequences [SEQ ID NOS:1-3025] of polypeptides that comprise the human heart mitochondrial proteome are provided, using fractionated proteins derived from highly purified mitochondrial preparations, to identify previously unrecognized mitochondrial molecular components.
Owner:THE BUCK INST FOR RES ON AGING +1
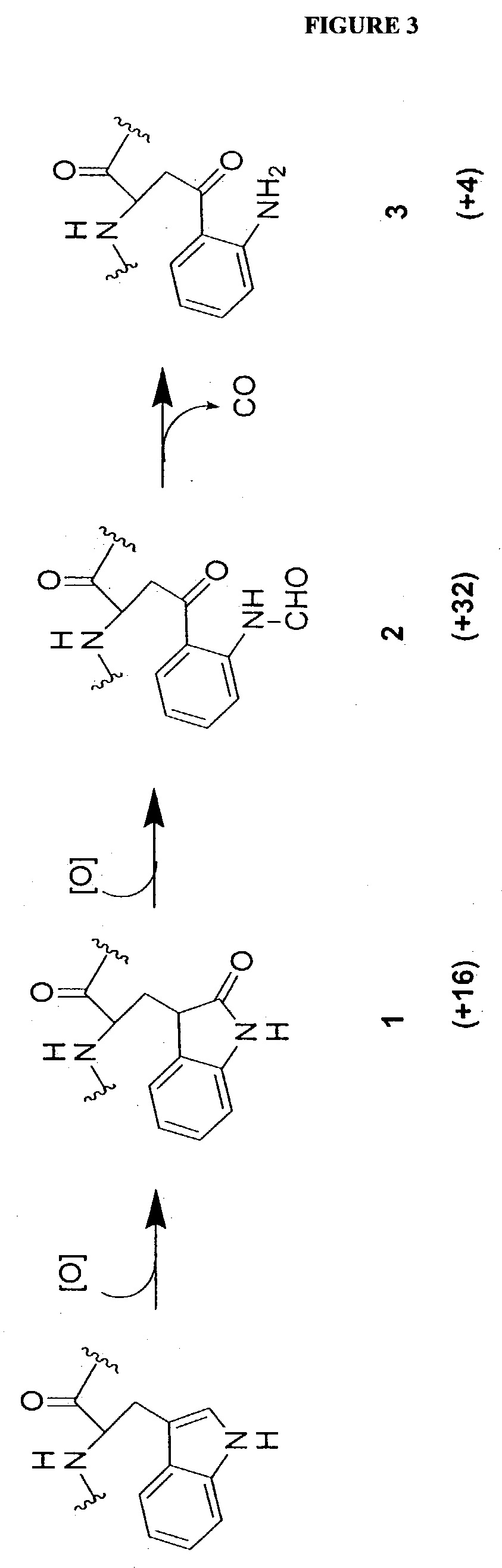
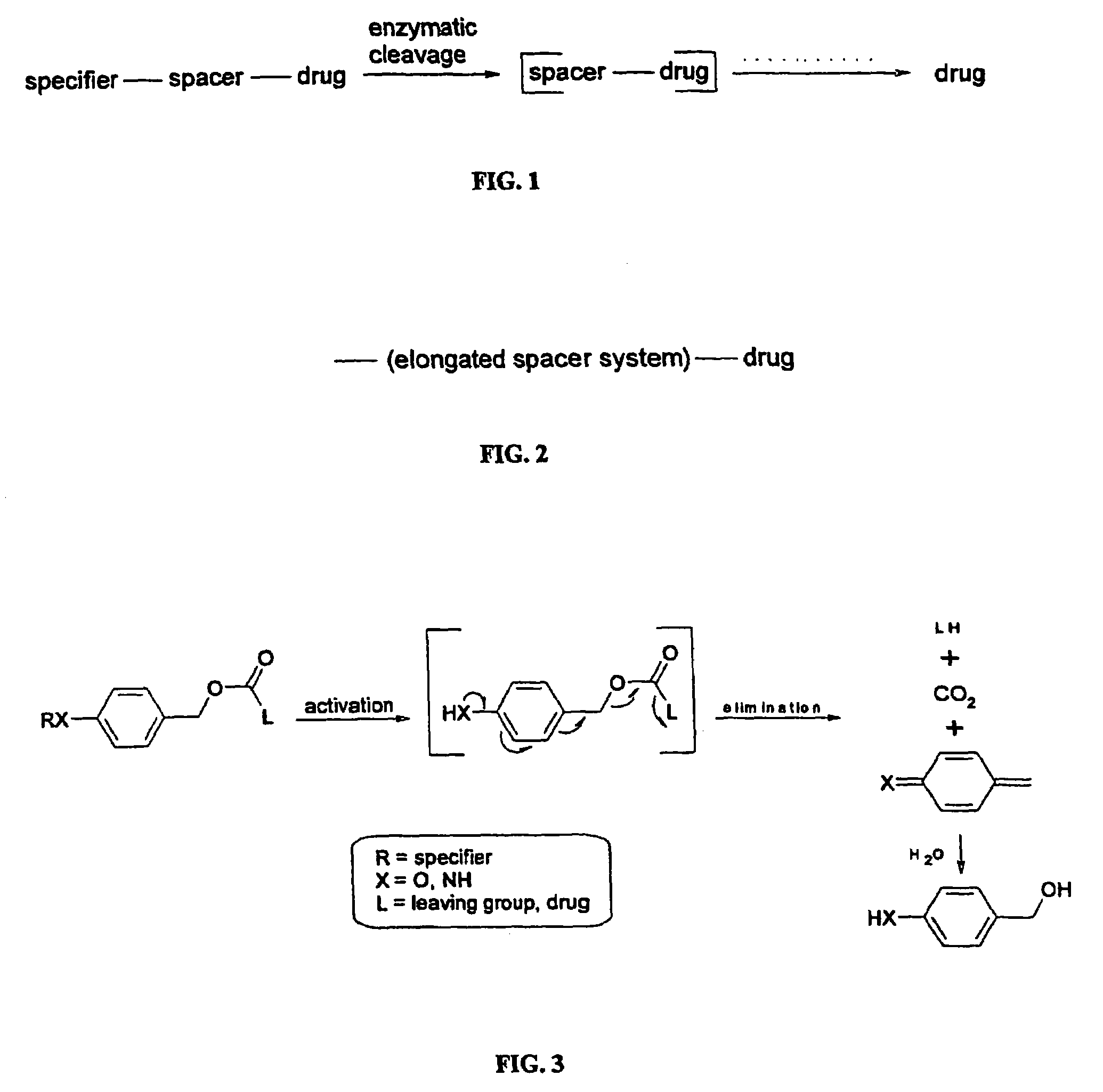
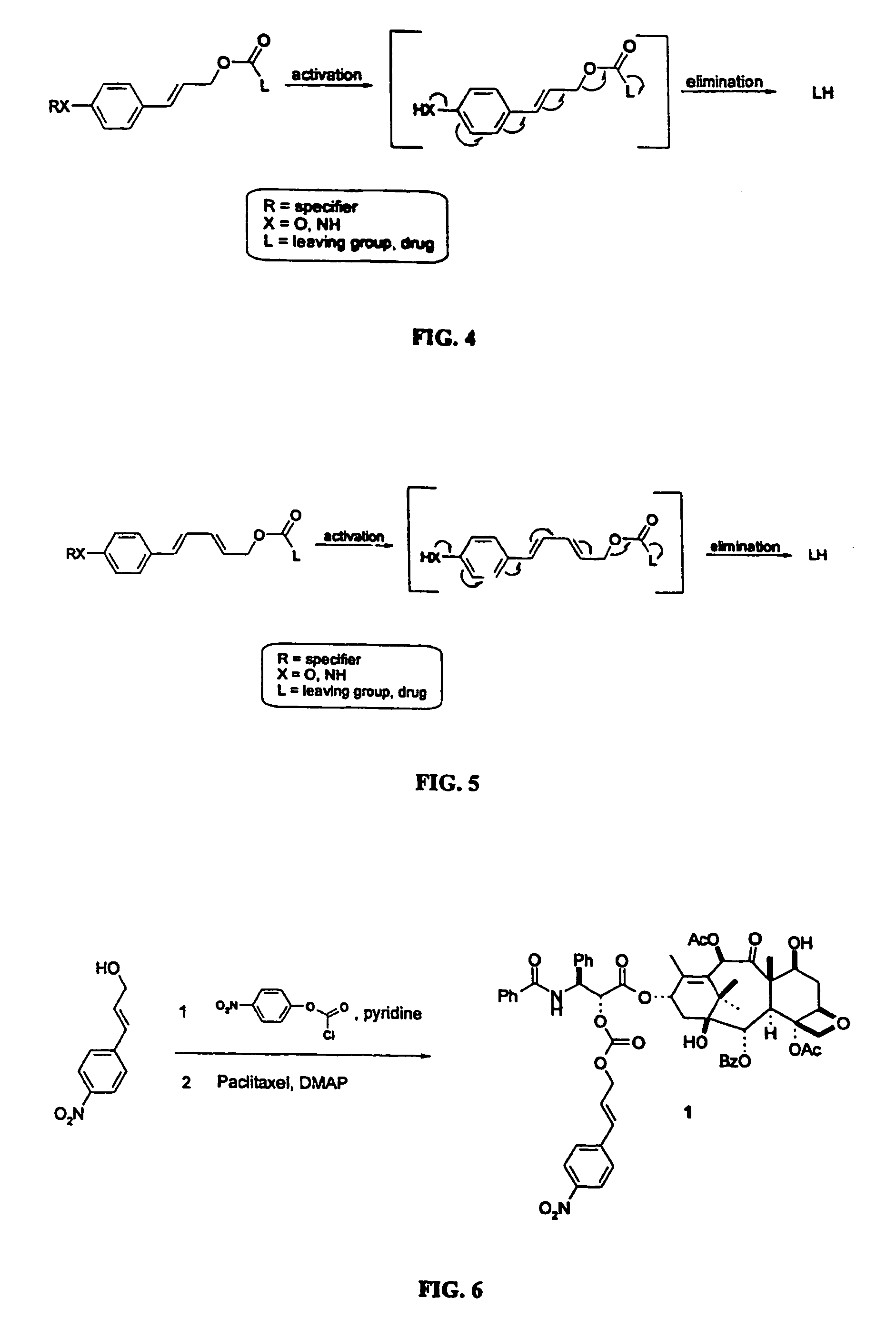
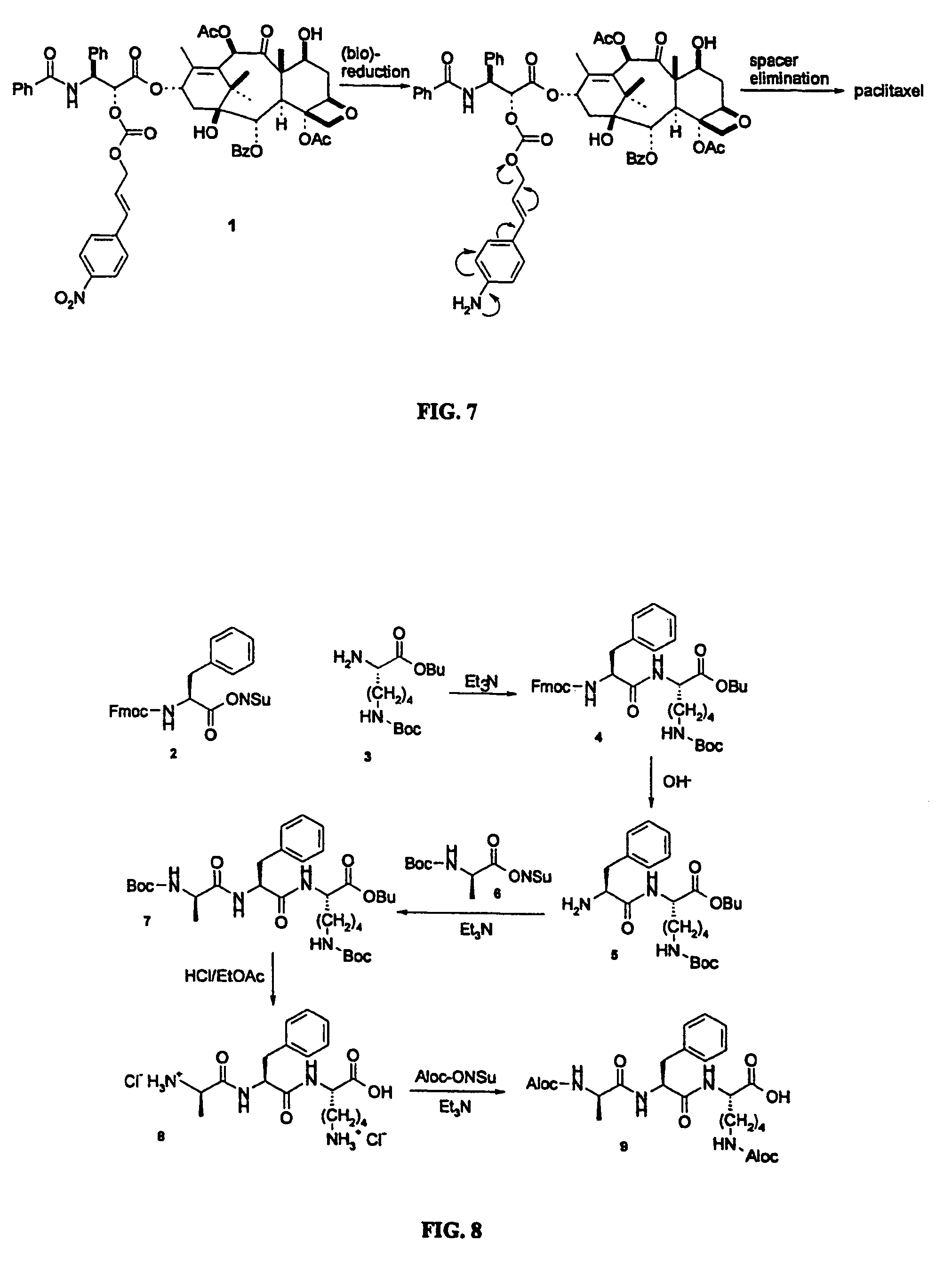
Elongated and multiple spacers in activatible prodrugs
InactiveUS7223837B2Improved kineticsFacilitate enzymatic cleavageAntibacterial agentsOrganic active ingredientsTumor cellsChemistry
This invention is directed to prodrugs that can be activated at the preferred site of action in order to selectively deliver the corresponding therapeutic parent drugs to target cells or to the target site. This invention will therefore primarily but not exclusively relate to tumor cells as target cells. More specifically the prodrugs are compounds of the formula V—(W)k—(X)l—A—Z, wherein: V is a specifier; (W)k—(X)l—A is an elongated self-elimination spacer system; W and X are each a 1,(4+2n) electronic cascade spacer, being the same or different; A is either a spacer group of formula (Y)m wherein: Y is a 1,(4+2n) electronic cascade spacer, or a group of formula U being a cyclization elimination spacer; Z is a therapeutic drug; k, l and m are integers from 0 (included) to 5 (included); n is an integer of 0 (included) to 10 (included), with the provisos that: —when A is (Y)m: k+l+m≧1, and if k+l+m=1; —when A is U: k+l≧1.
Owner:BYONDIS BV
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Immunoassay that provides for both collection of saliva and assay of saliva for one or more analytes with visual readout
InactiveUS6248598B1Eliminate riskBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteSaliva sample
A device that provides for both the collection of saliva and detection of at least one analyte therein, e.g., a drug, is provided. This device provides for rapid analysis of saliva samples, while also providing a convenient assay method that does not require the addition of extraneous reagents, or other materials. Thereby, this device can be used by non-laboratory personnel without risk of user introduced errors.
Owner:BOGEMA STUART C
4-amino substituted-2-substituted-1,2,3,4-tetrahydroquinolines
Cholesteryl ester transfer protein inhibitors, pharmaceutical compositions containing such inhibitors and the use of such inhibitors to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases in some mammals, including humans.
Owner:PFIZER INC
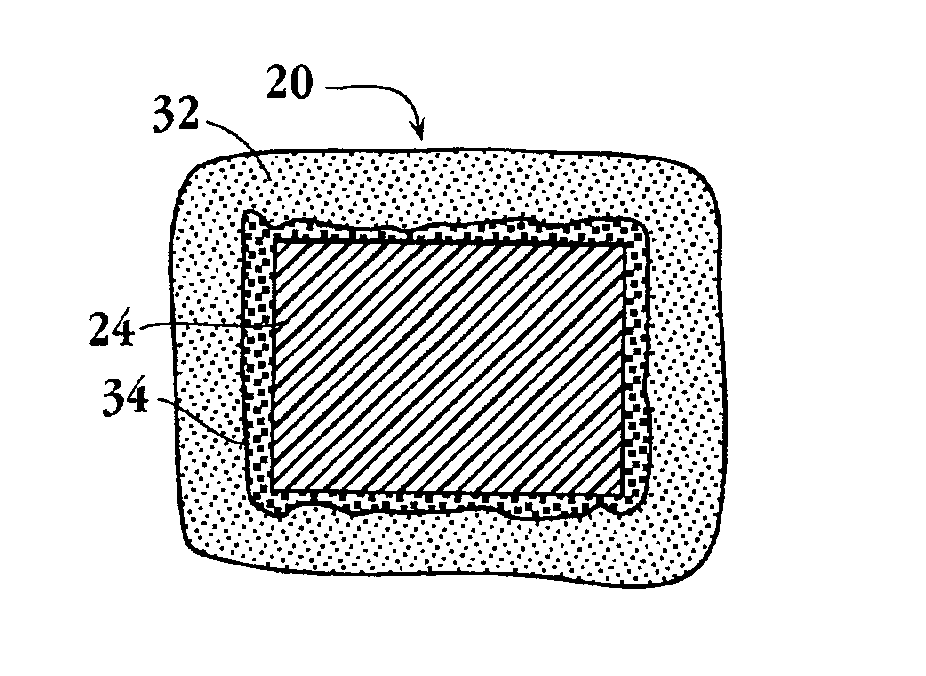
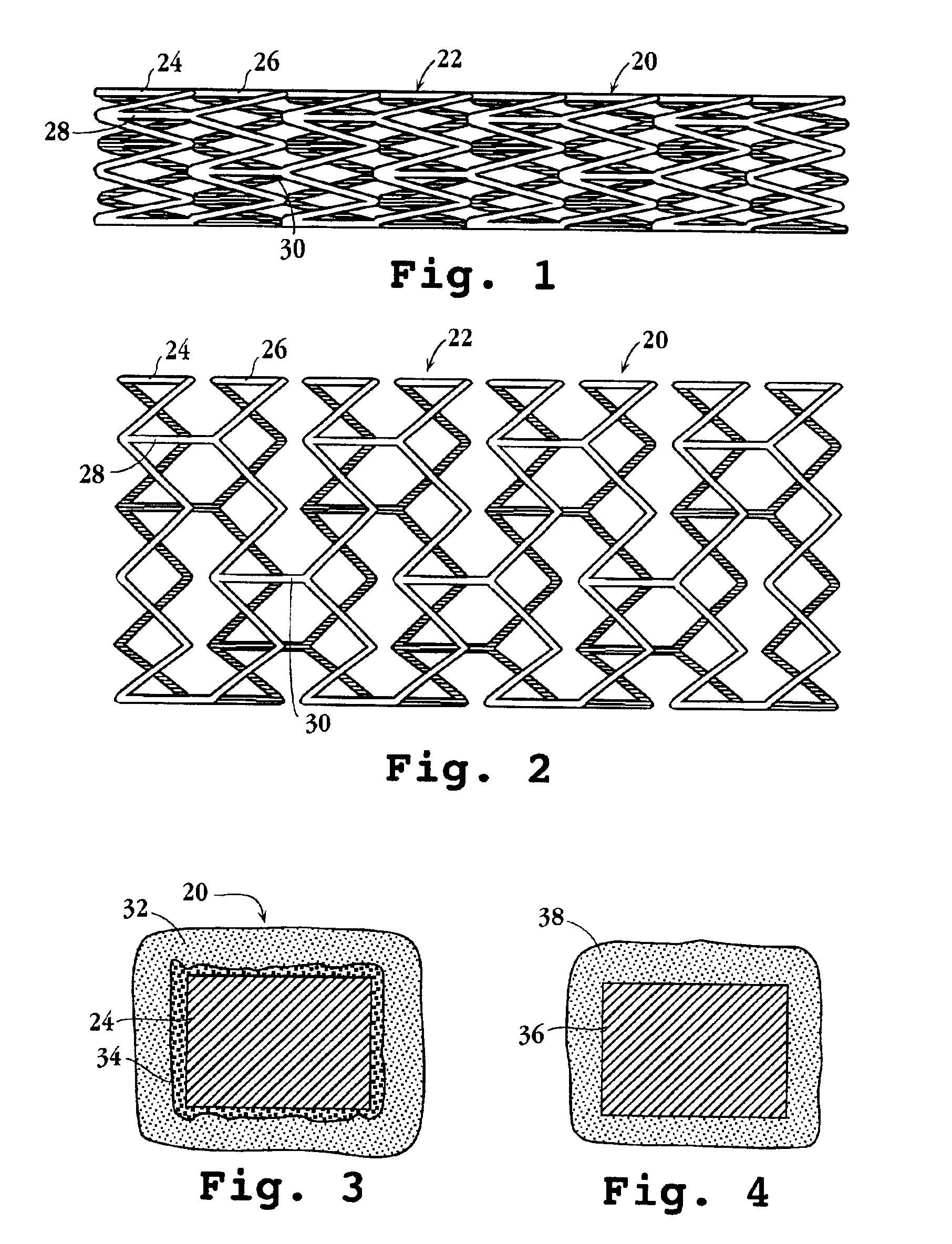
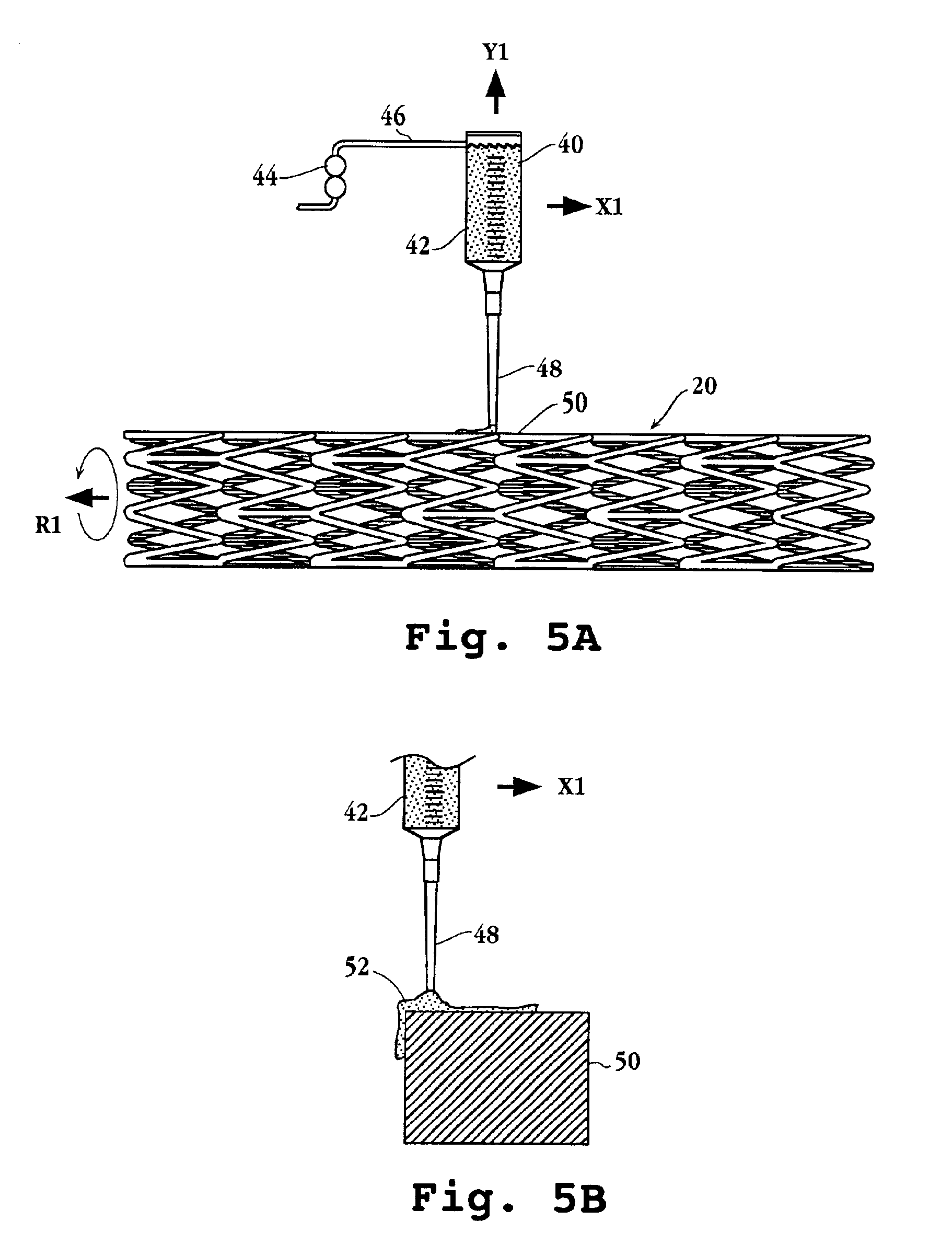
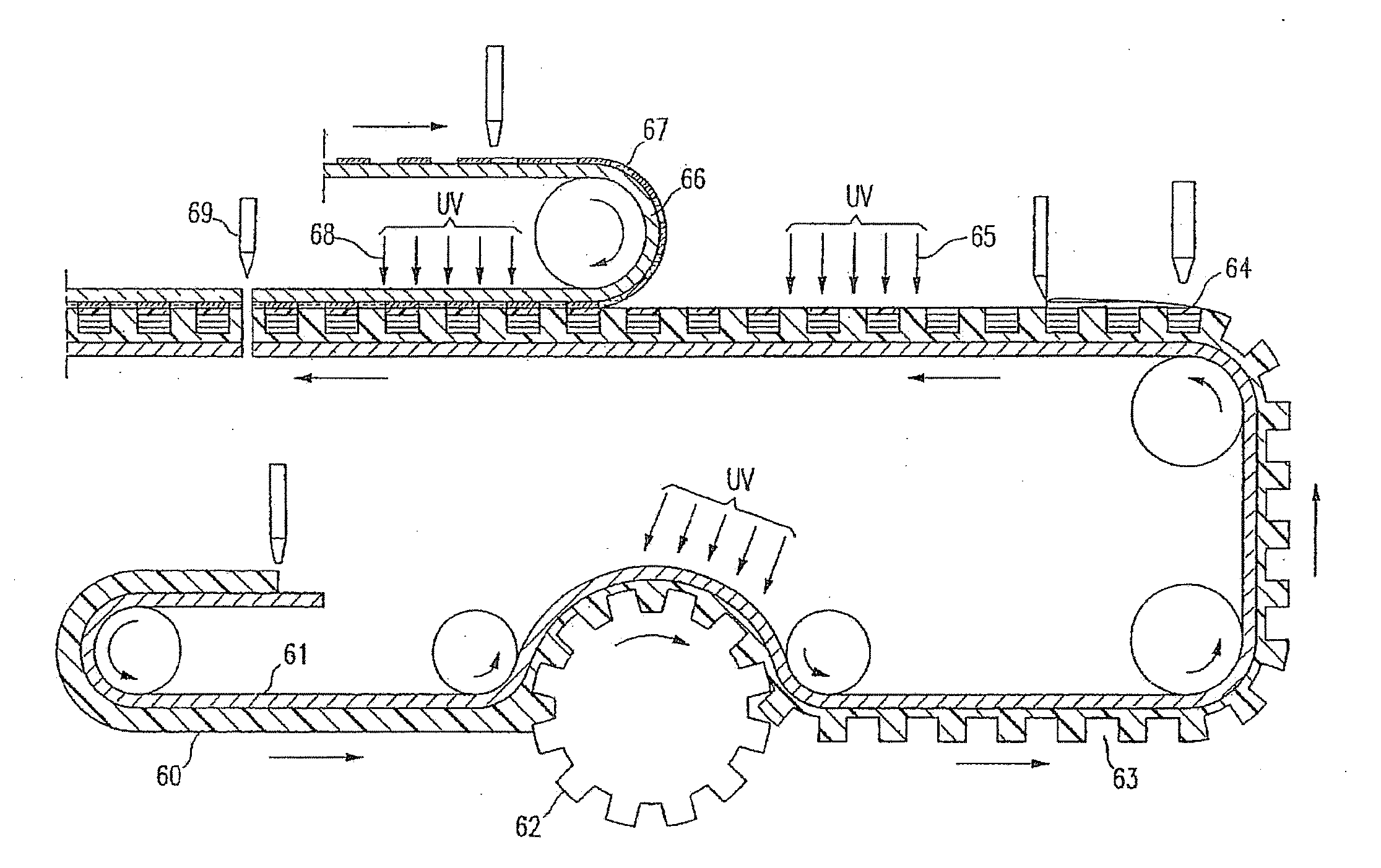
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
Electrophoretic display
InactiveUS20150005720A1Improve integrityImprove mechanical propertiesLiquid crystal compositionsCosmetic preparationsElectrophoresisDisplay device
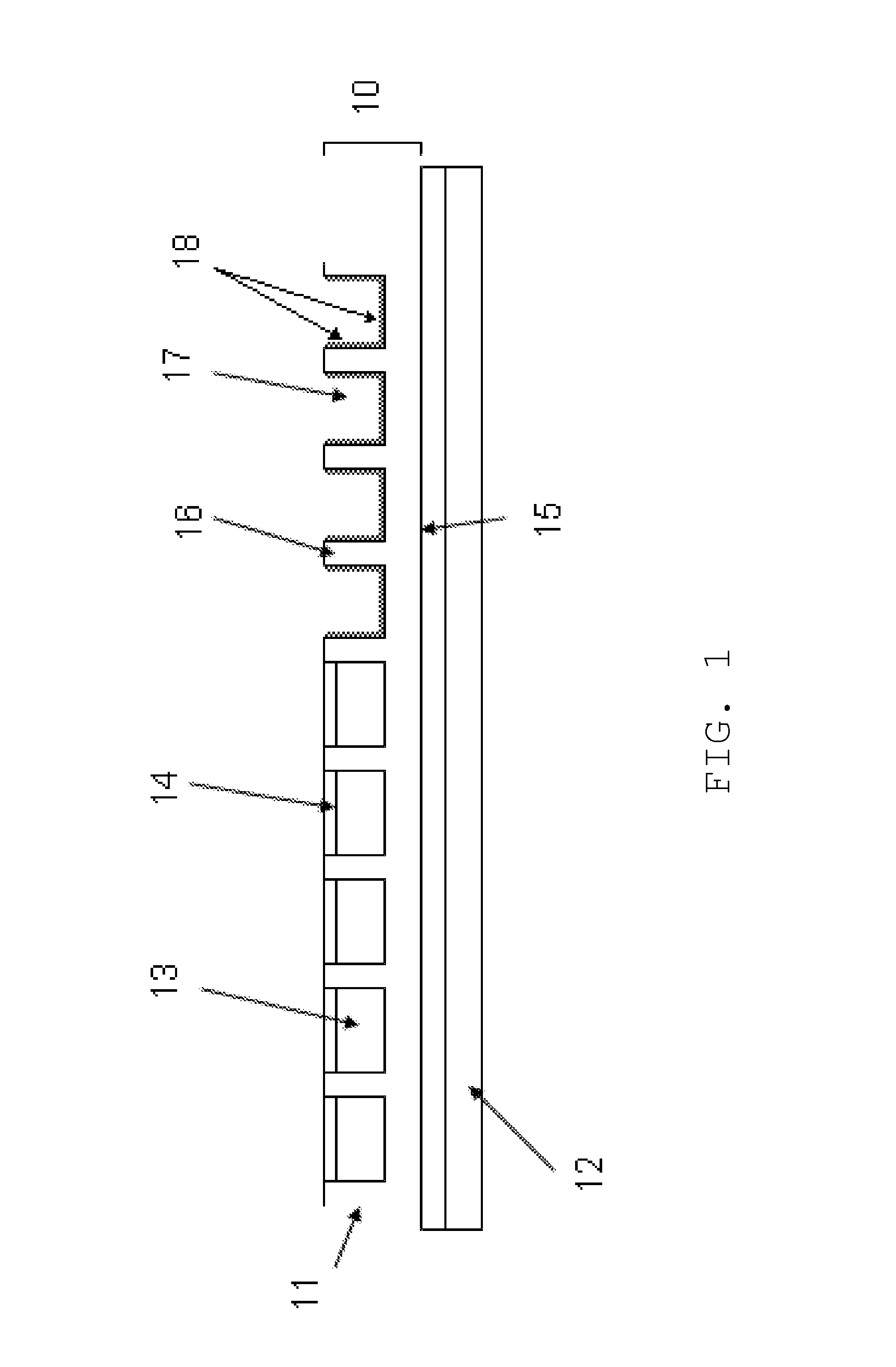
The present invention is directed to a transdermal delivery system for delivering a medicinal or cosmetic agent through the skin of a subject, which delivery system comprises (a) one or more display cells comprising partition walls and top-openings; (b) a liquid composition filled in the display cells wherein said liquid composition comprises the medicinal or cosmetic agent; and (c) a sealing layer to enclose the liquid composition within the microcups wherein said sealing layer is hardened in situ.
Owner:E INK CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com