Patents
Literature
42682results about "Unknown materials" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
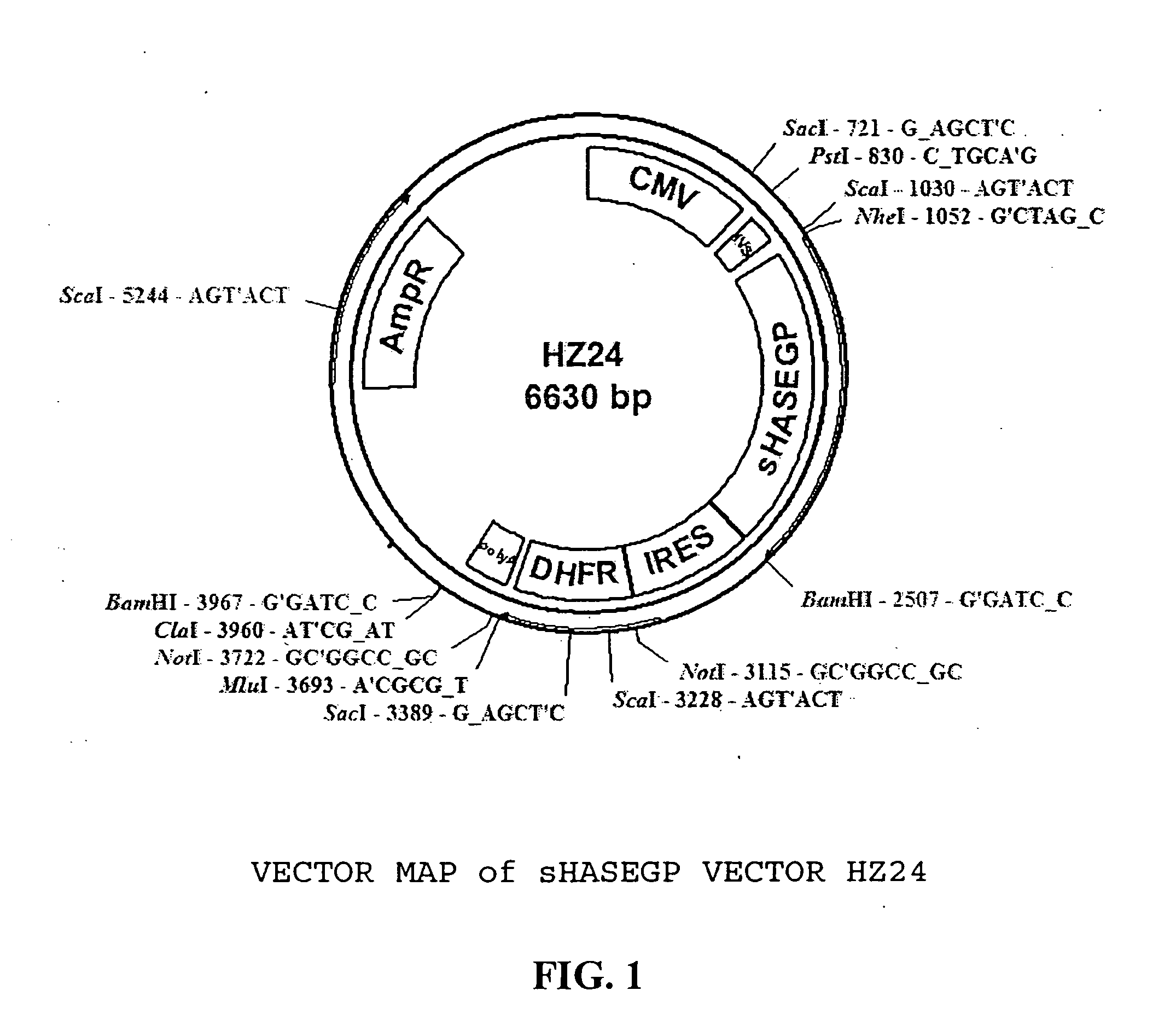
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
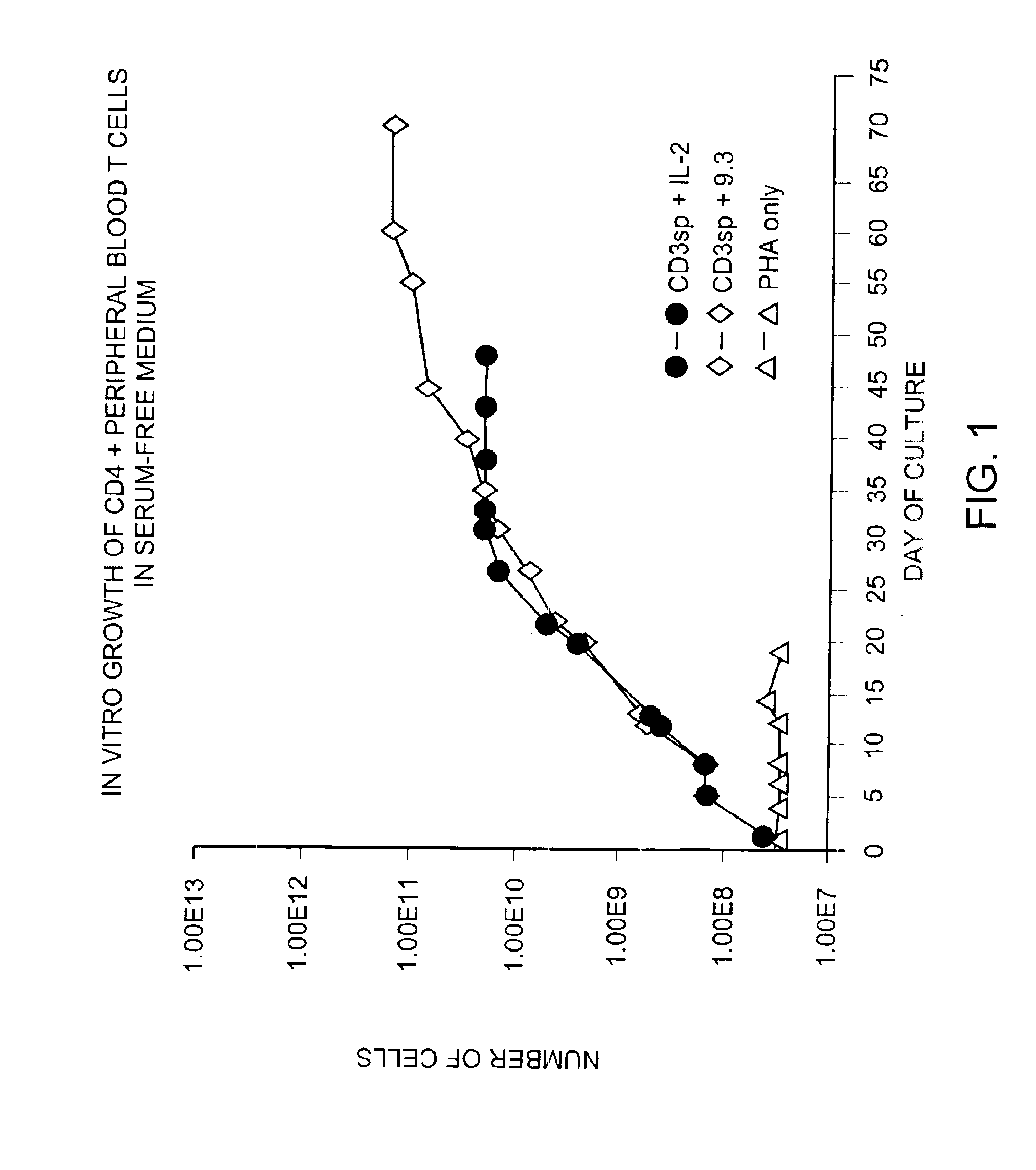
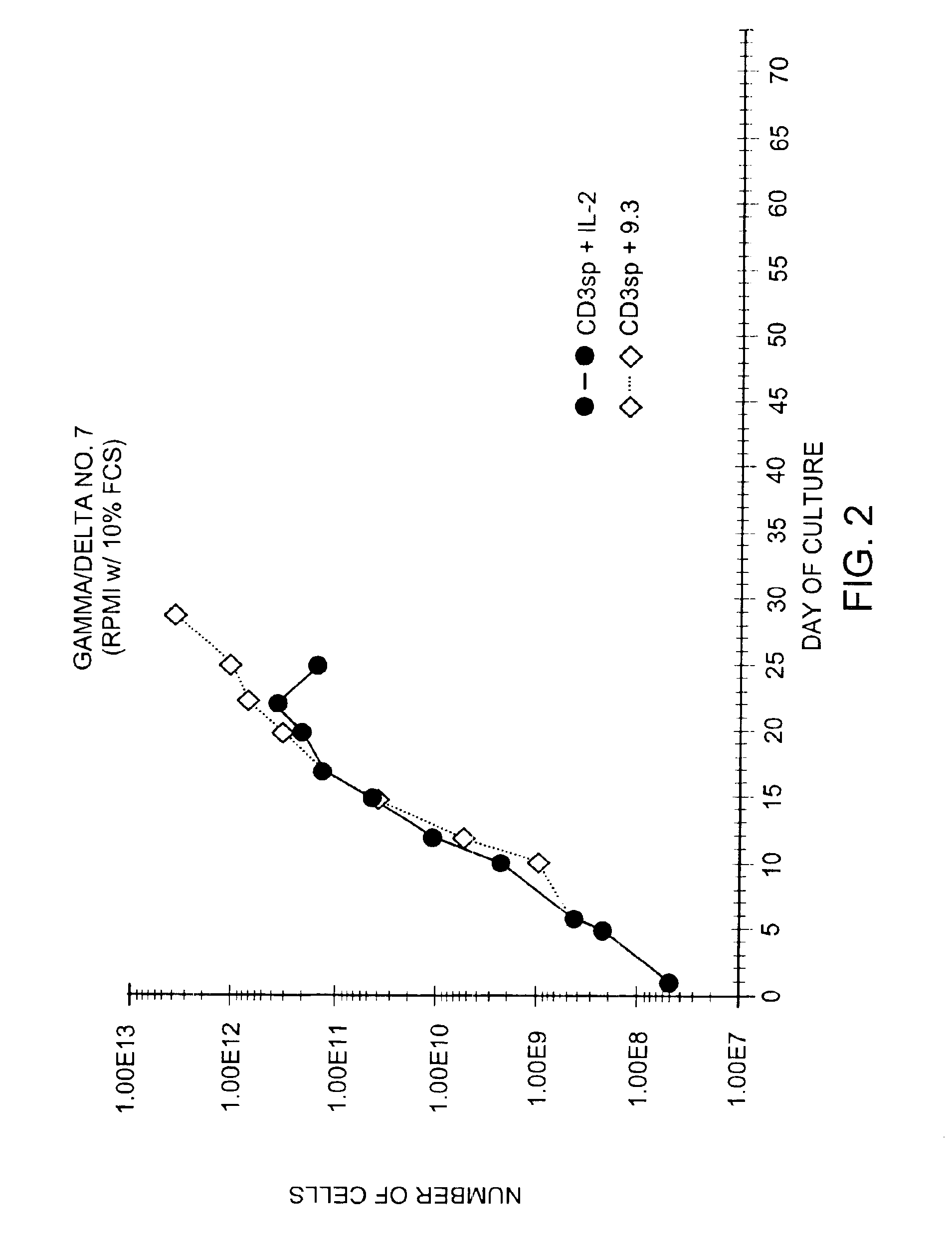
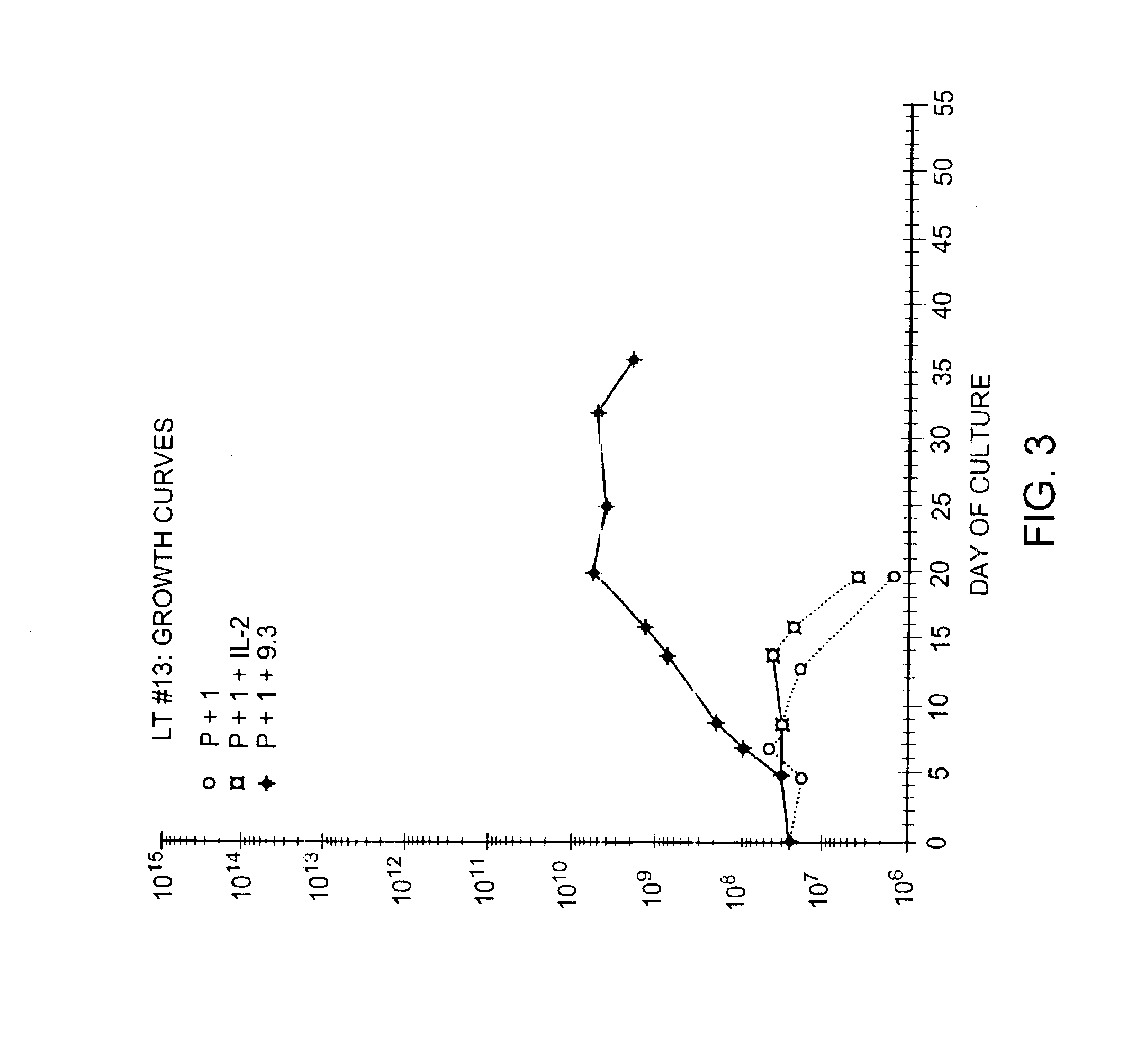
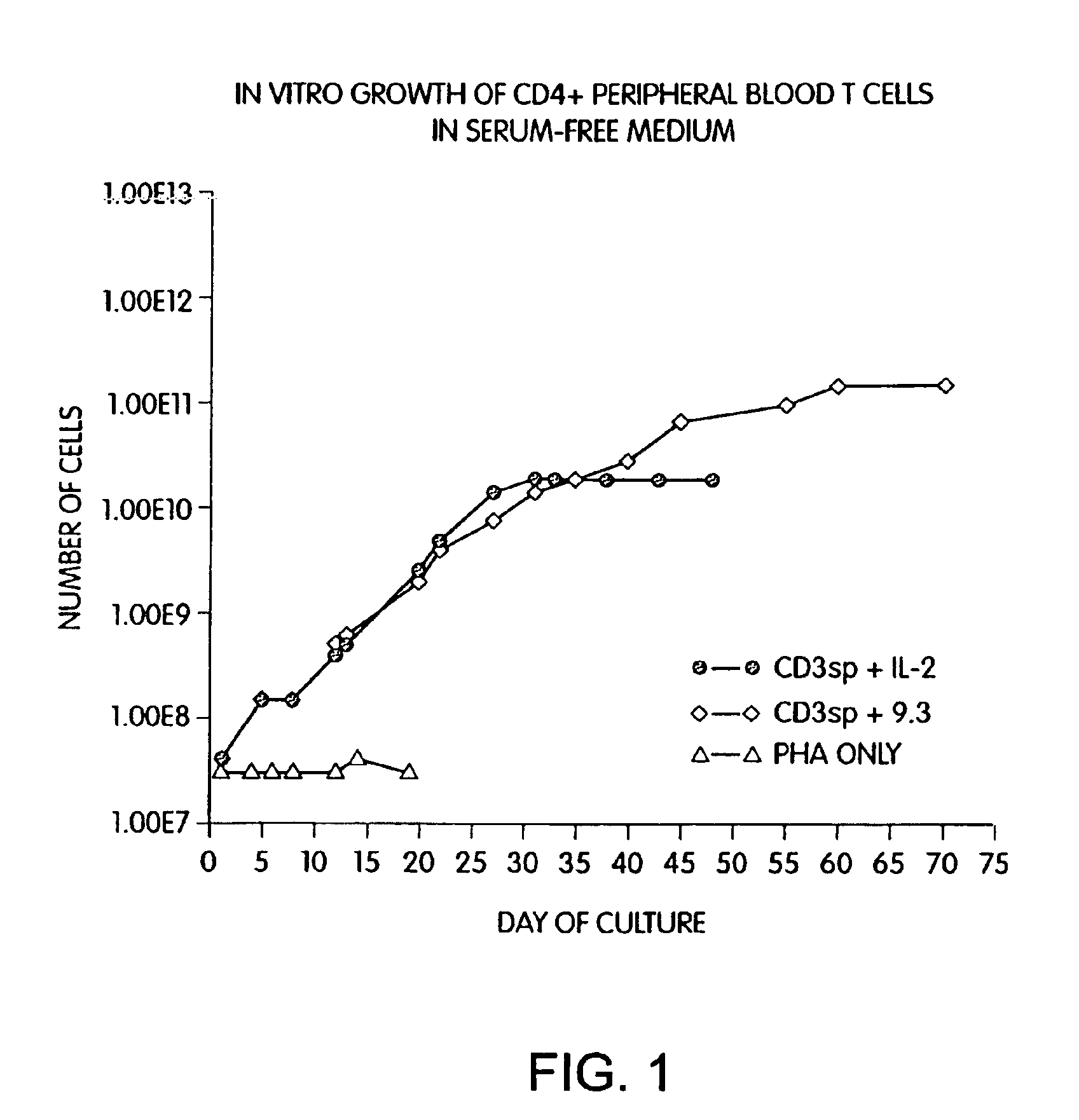
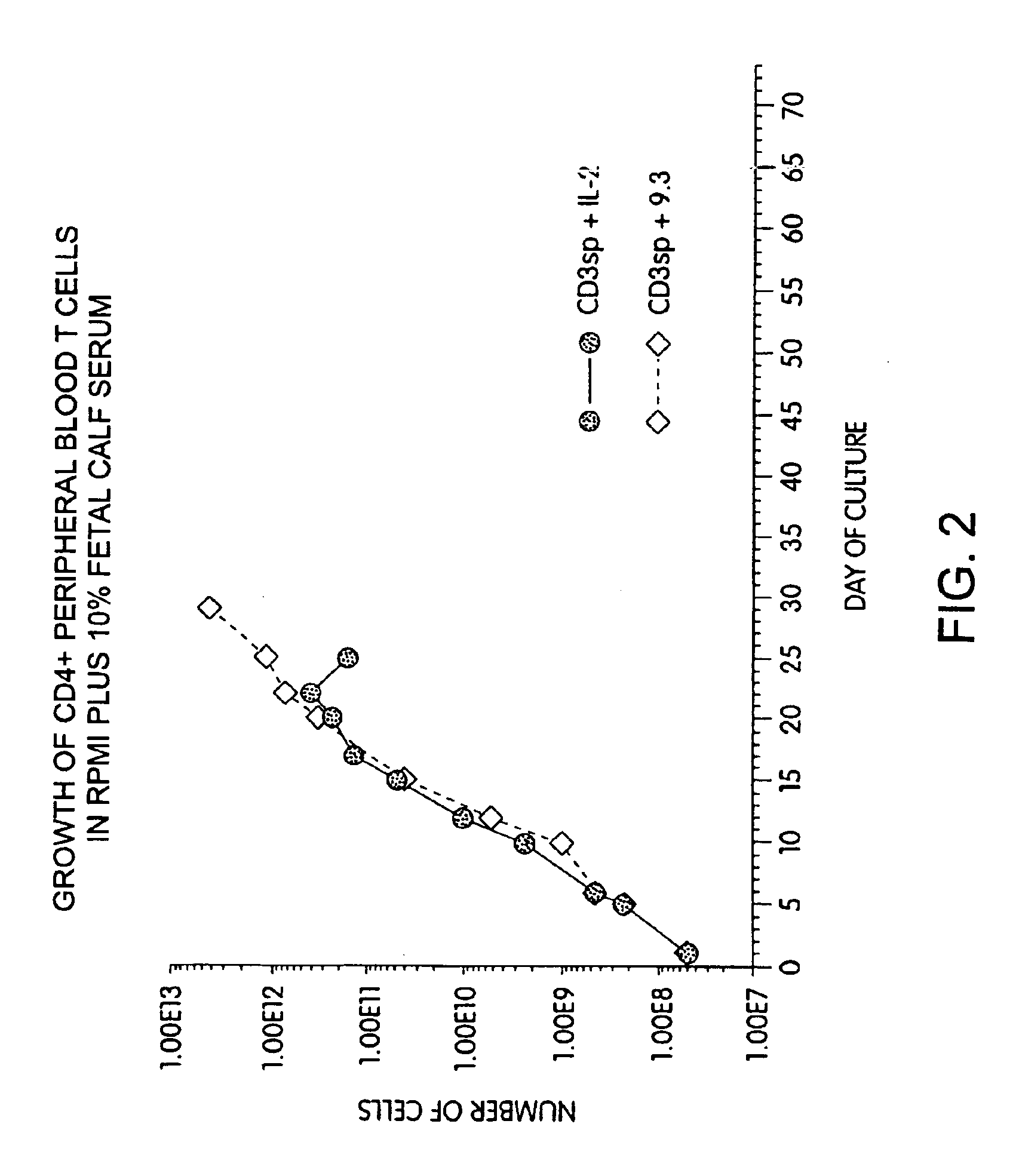
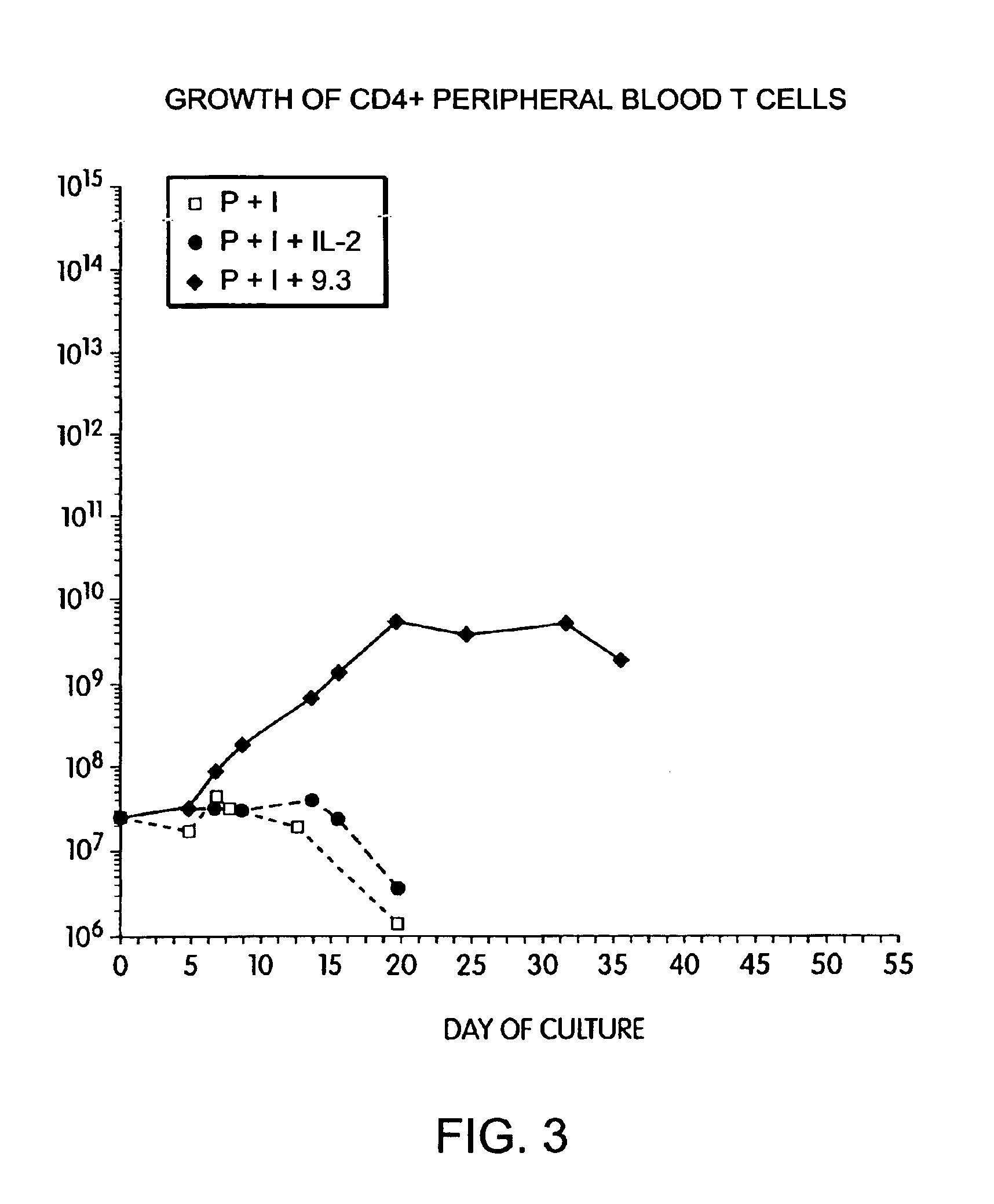
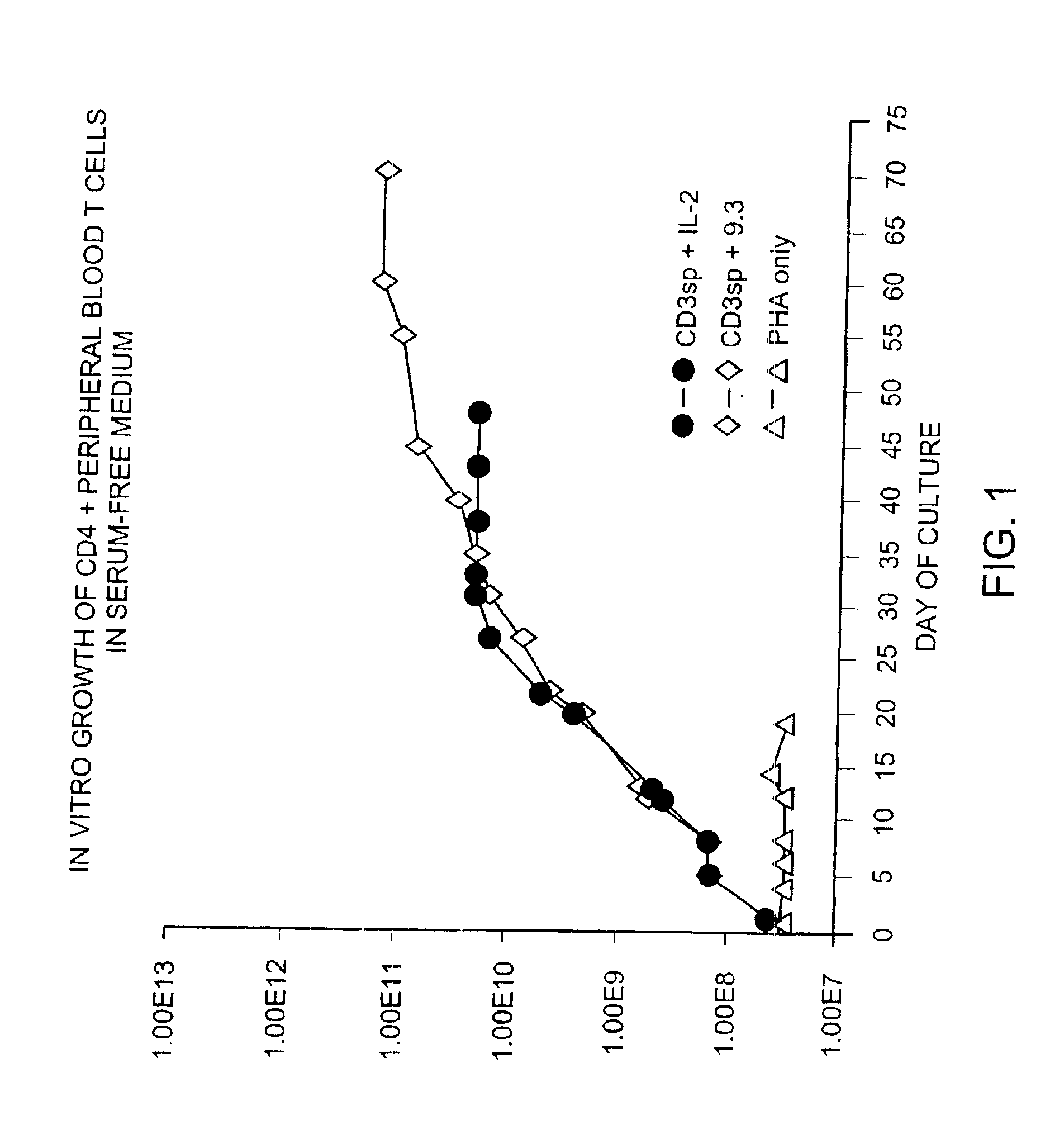
Methods for selectively stimulating proliferation of T cells
InactiveUS6905681B1Increase the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS6887466B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
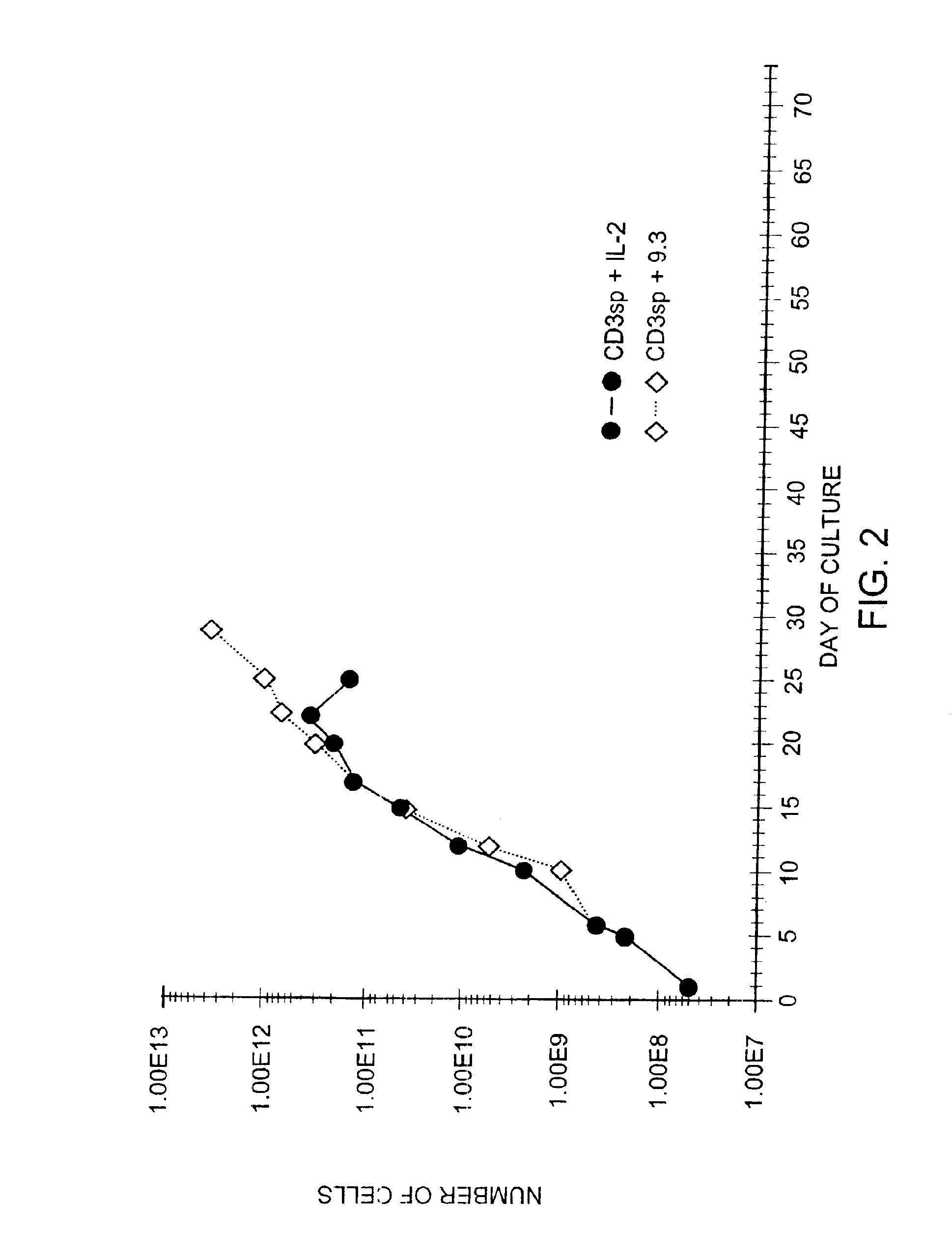
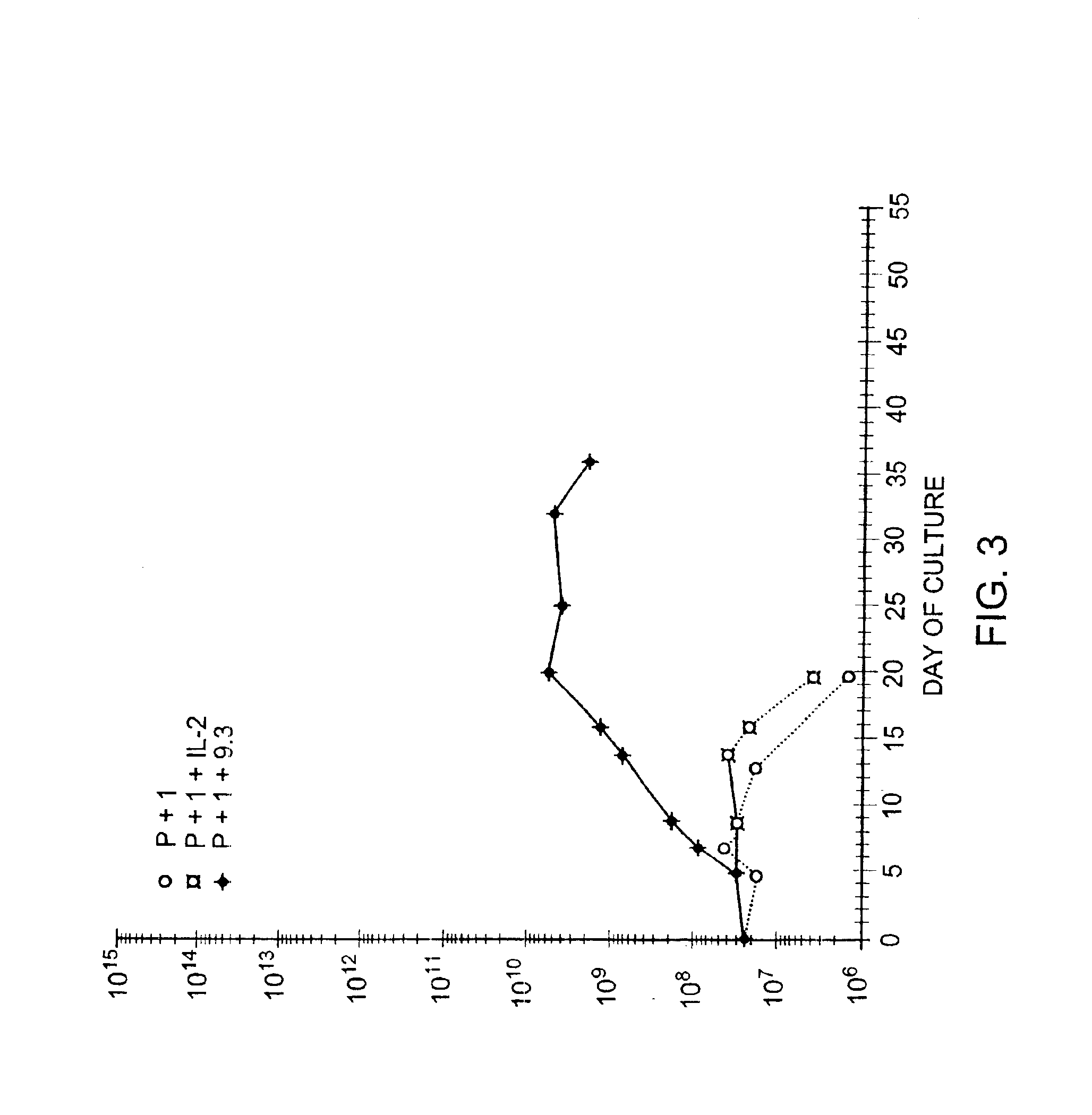
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods of treating HIV infected subjects
InactiveUS6905680B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
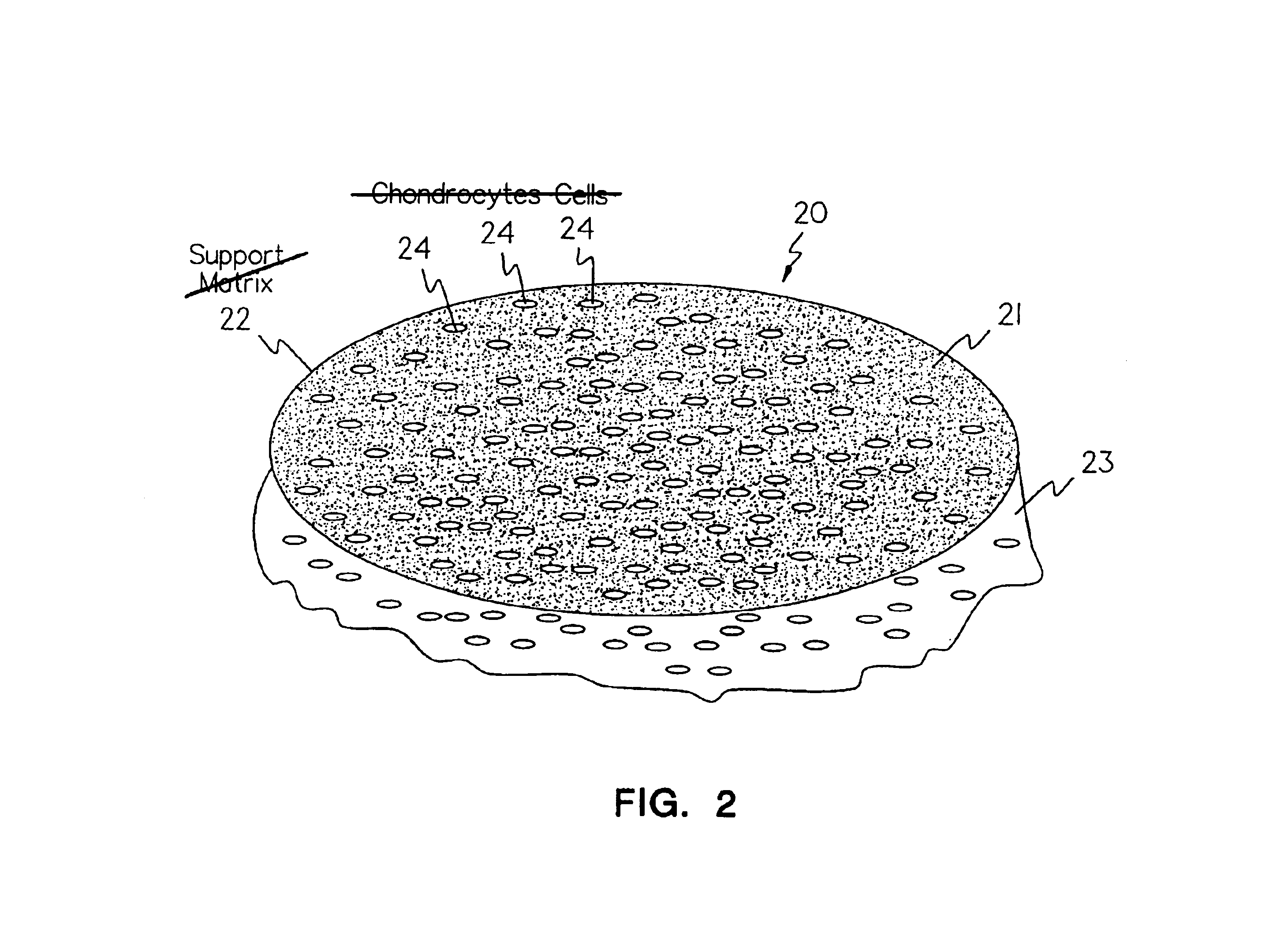
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
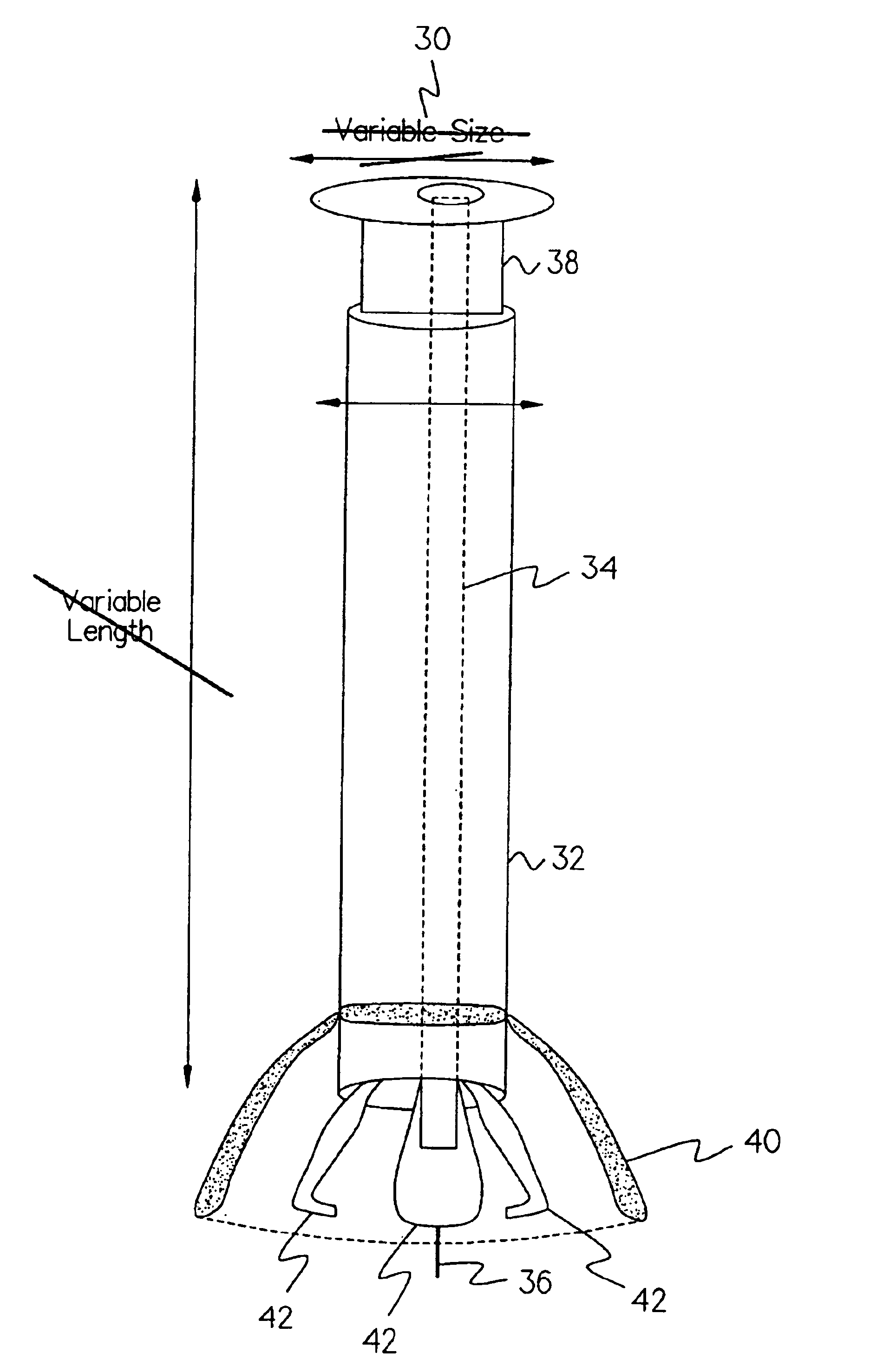
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
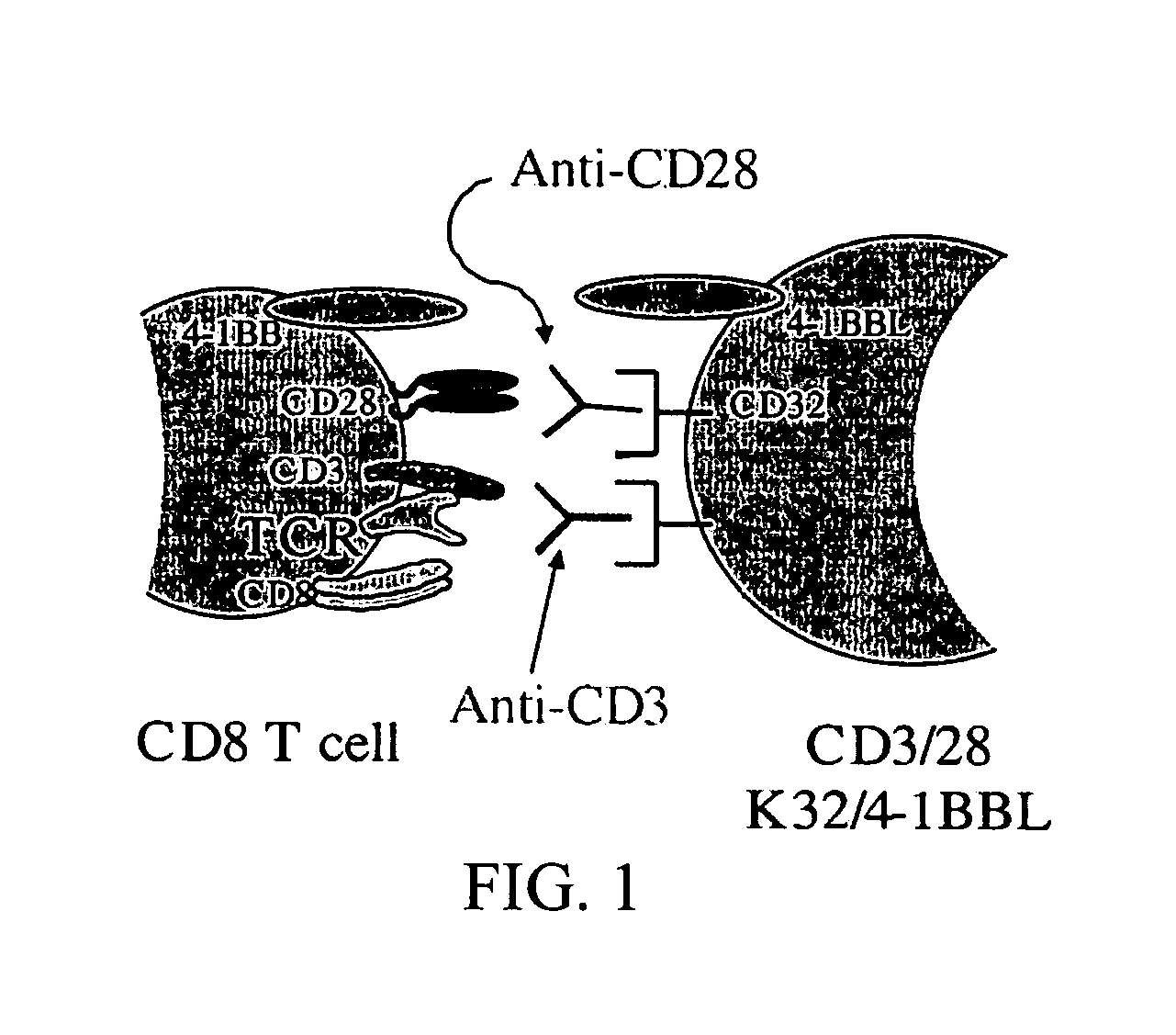
Novel artificial antigen presenting cells and uses therefor
The invention relates to novel artificial antigen presenting cells (aAPCs). The aAPC comprises at least one stimulatory ligand and at least one co-stimulatory ligand where the ligands each specifically bind with a cognate molecule on a T cell of interest, thereby mediating expansion of the T cell. The aAPC of the invention can further comprise additional molecules useful for expanding a T cell of interest. The aAPC of the invention can be used as an “off the shelf” APC that can be readily designed to expand a T cell of interest. Also, the aAPC of the invention can be used identify the stimulatory, co-stimulatory, and any other factors that mediate growth and expansion of a T cell of interest. Thus, the present invention provides powerful tools for development of novel therapeutics where activation and expansion of a T cell can provide a benefit.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
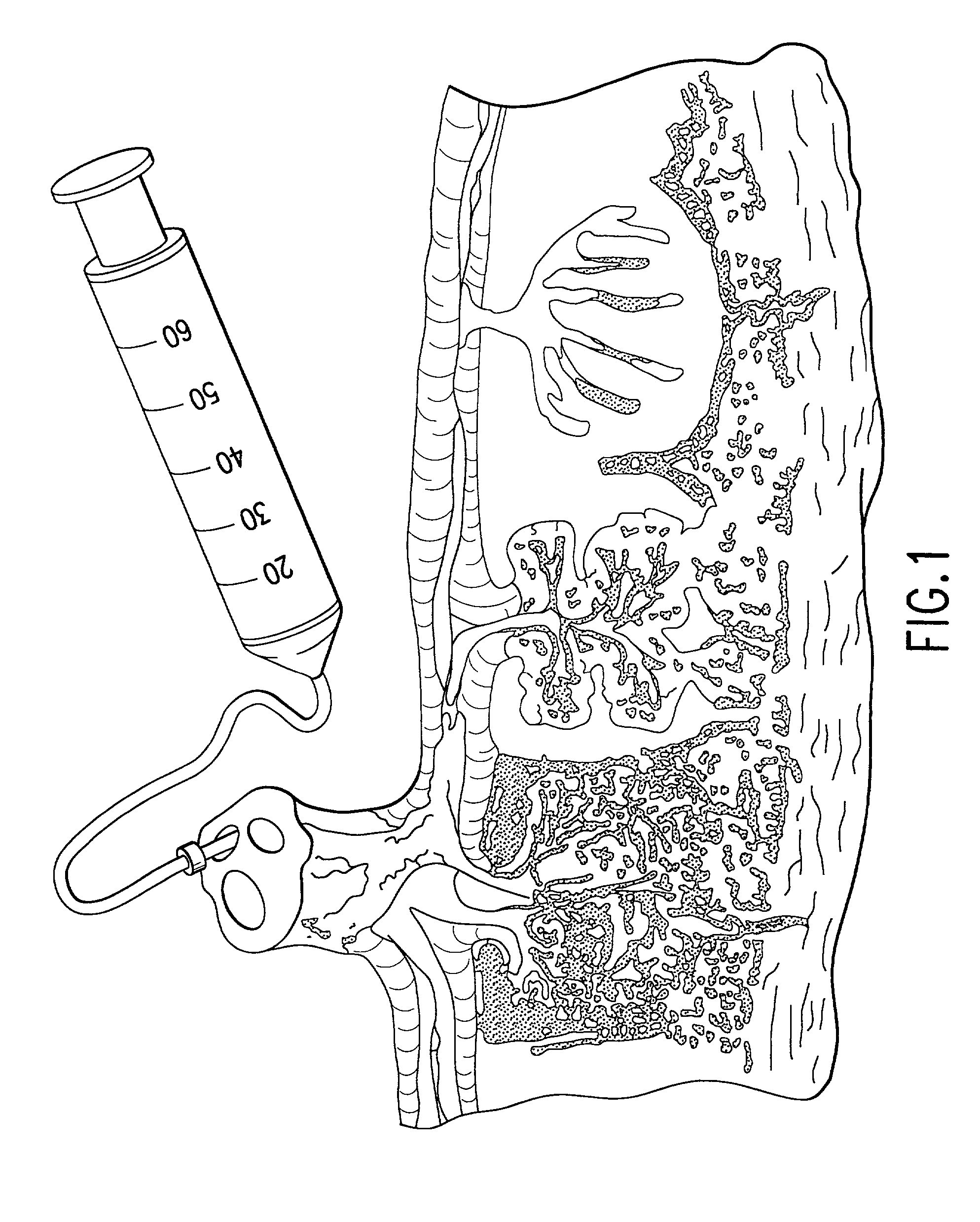
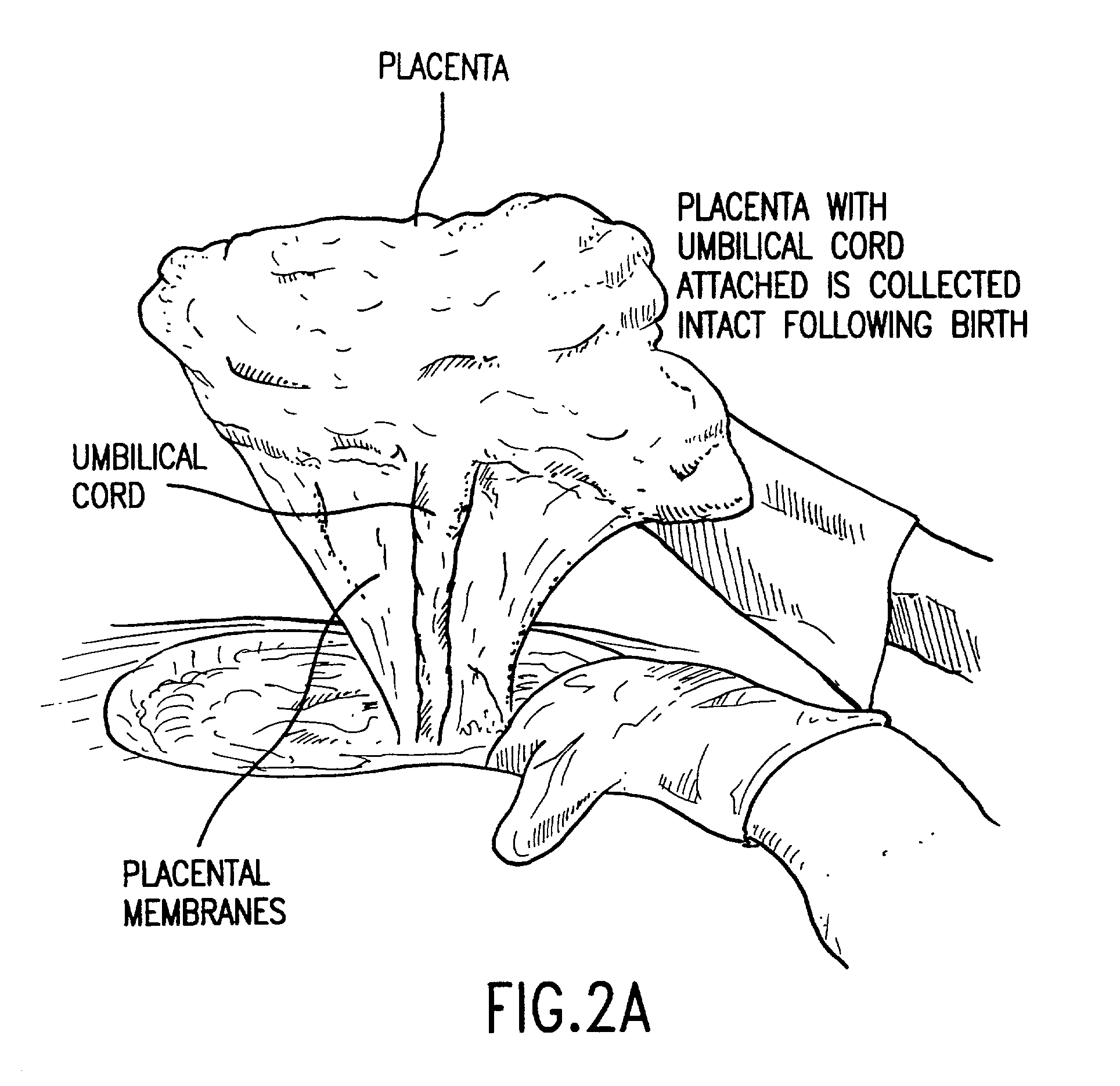
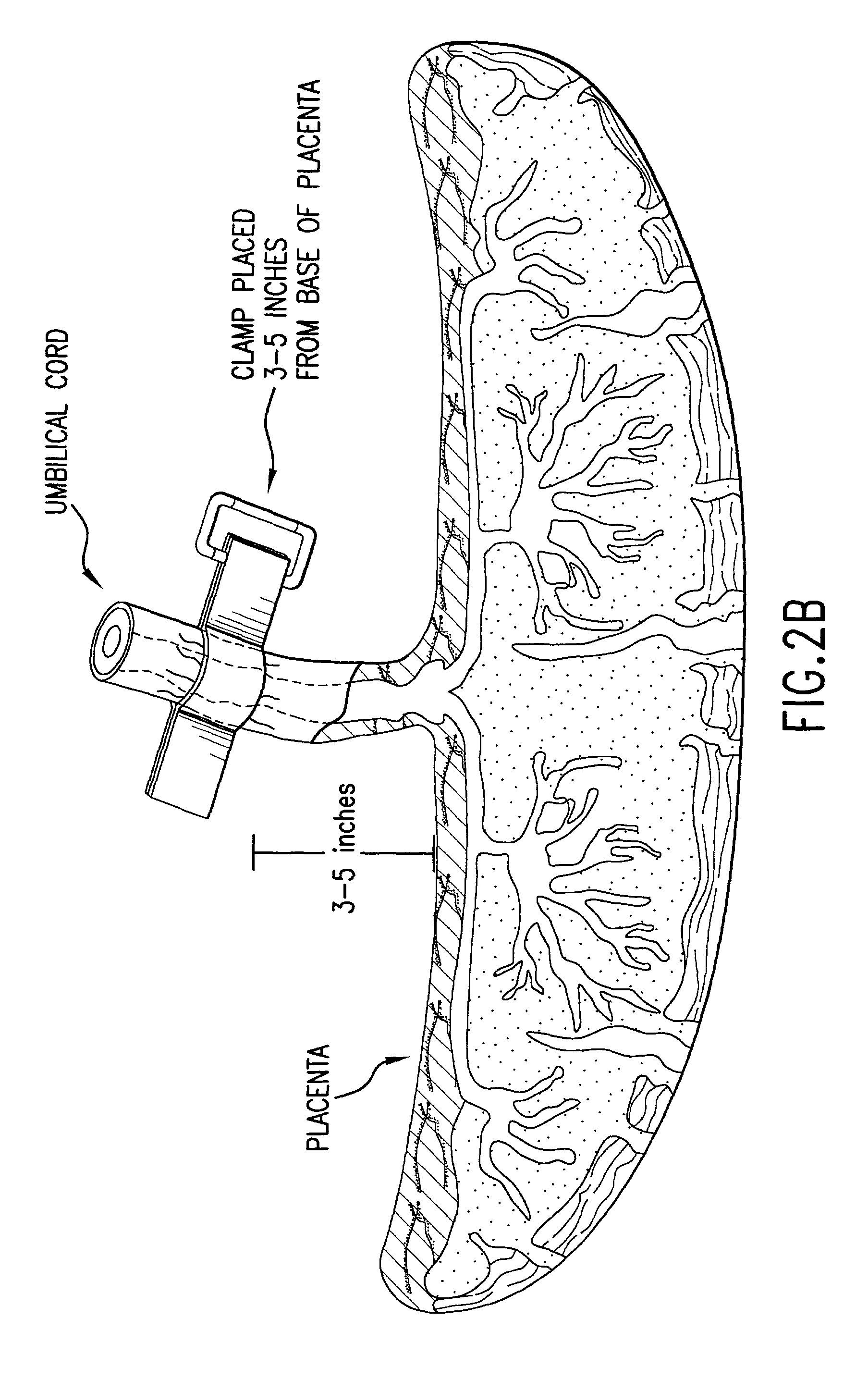
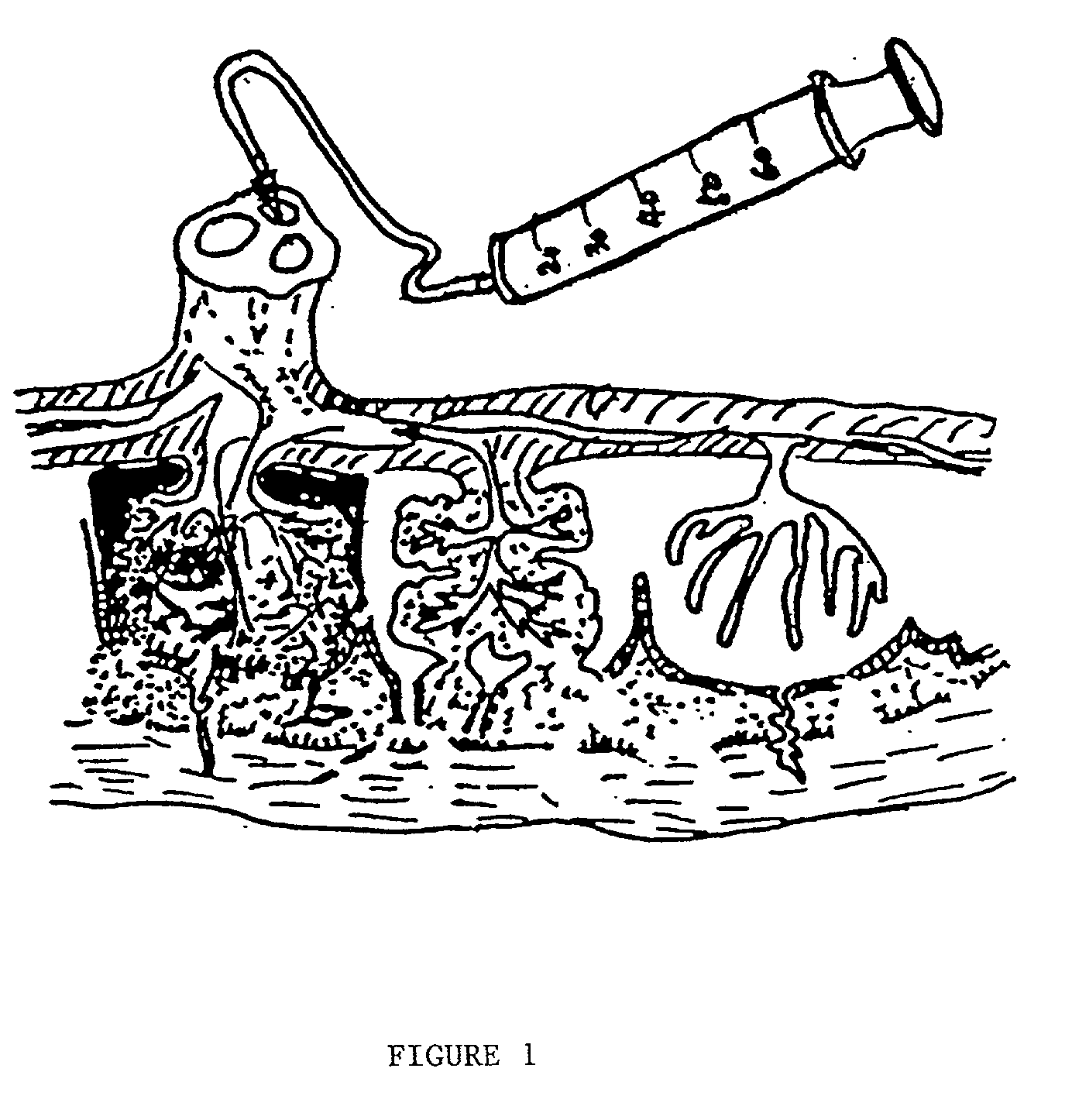
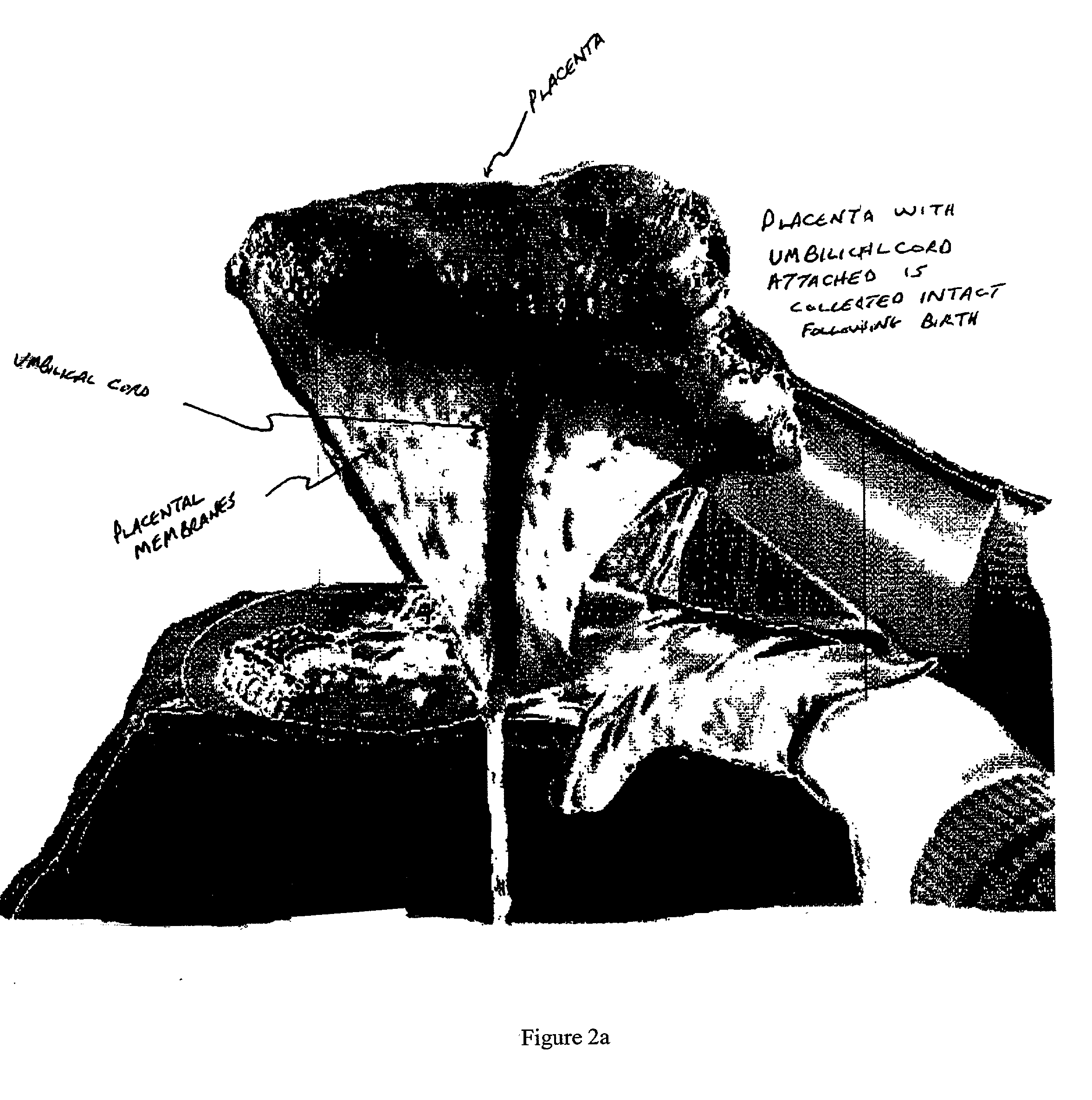
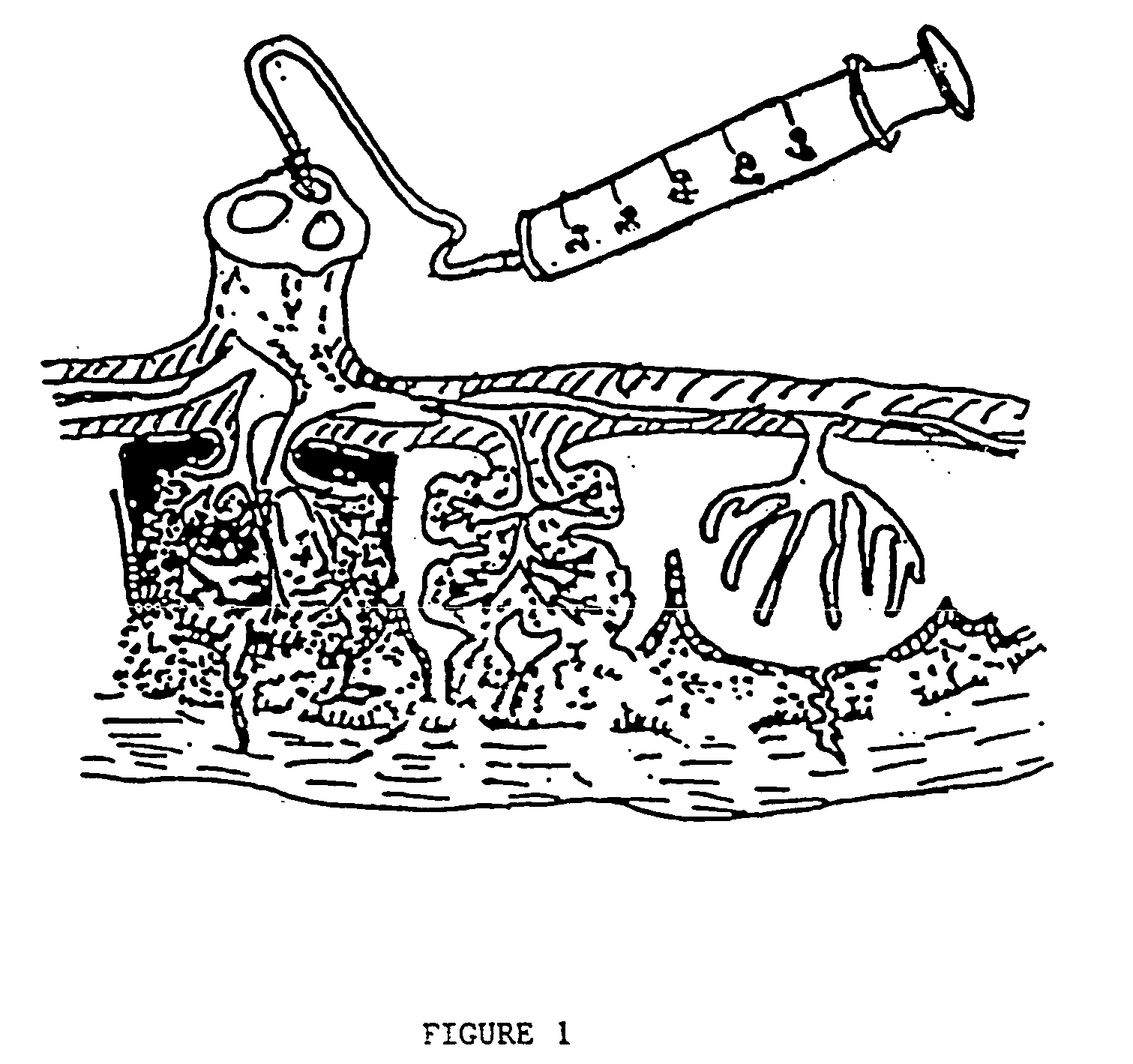
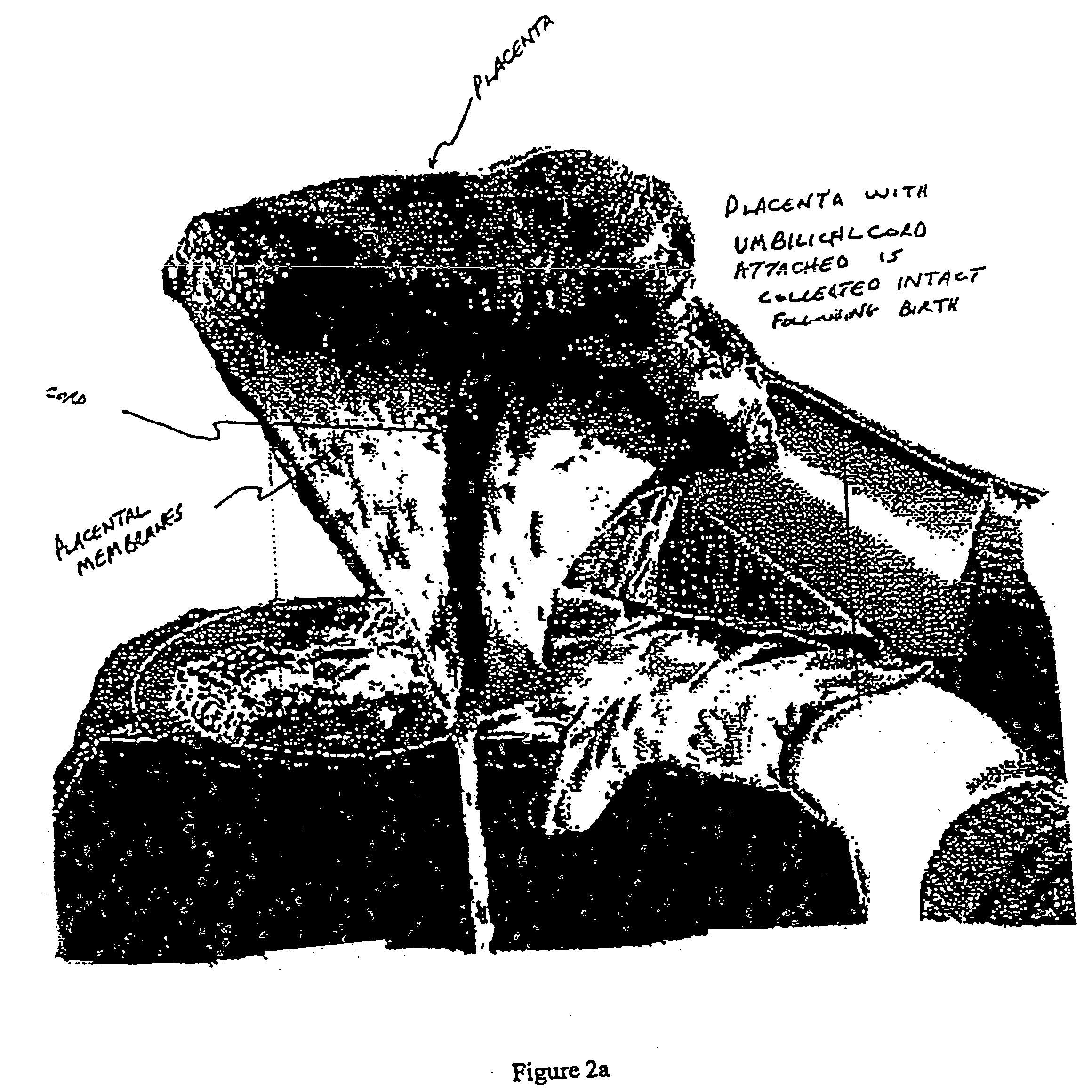
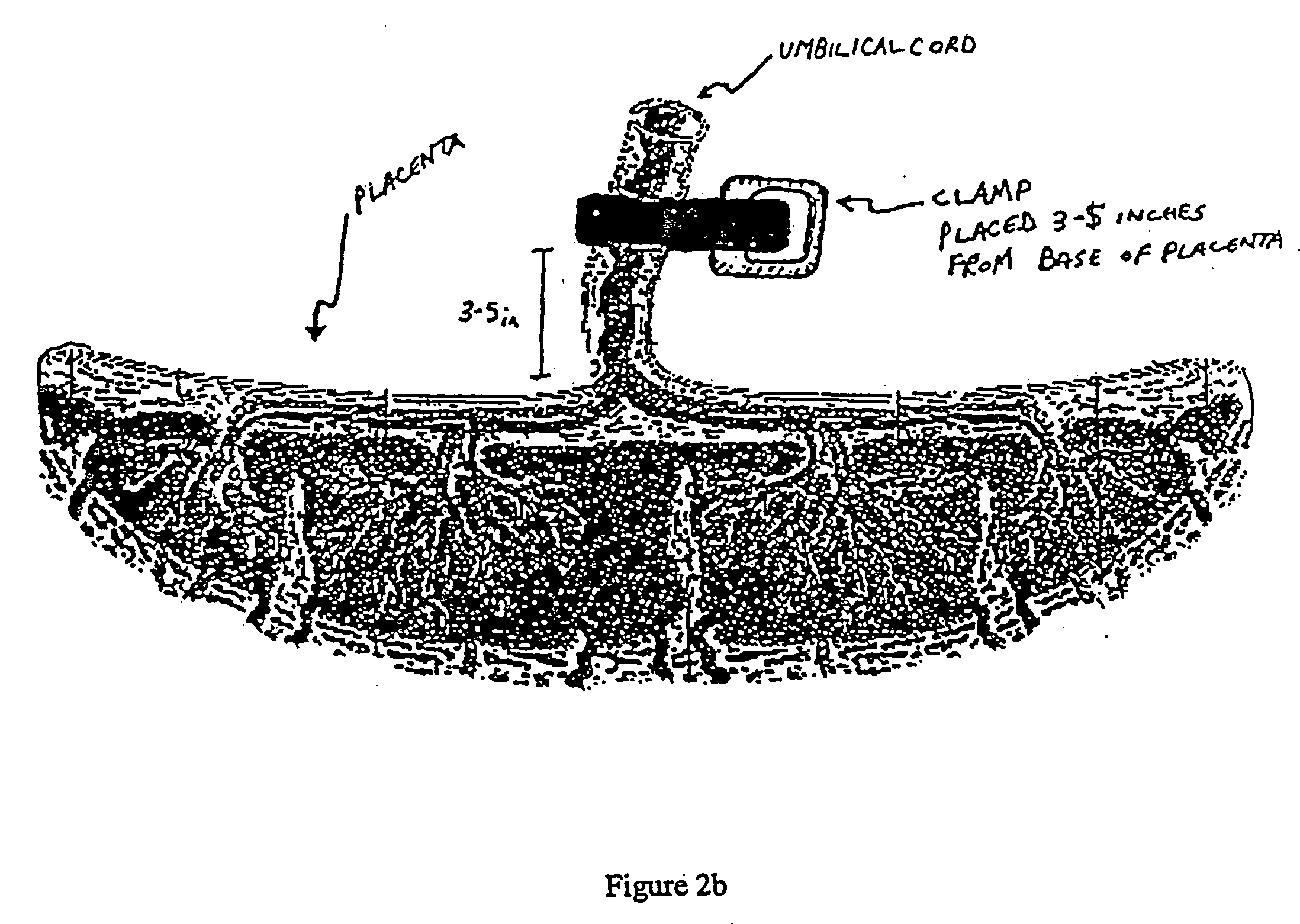
Post-partum mammalian placenta, its use and placental stem cells therefrom
InactiveUS20030032179A1Enhance exsanguinationEnhance sterile conditionSenses disorderAntipyreticAnticoagulant AgentEmbryo
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Three-dimensional culture of pancreatic parenchymal cells cultured living stromal tissue prepared in vitro
InactiveUS6022743AIncrease surface areaIncreased proliferationImmobilised enzymesSurgical needlesLigament structureIn vivo
A stromal cell-based three-dimensional cell culture system is prepared which can be used to culture a variety of different cells and tissues in vitro for prolonged periods of time. The stromal cells and connective tissue proteins naturally secreted by the stromal cells attach to and substantially envelope a framework composed of a biocompatible non-living material formed into a three-dimensional structure having interstitial spaces bridged by the stromal cells. The living stromal tissue so formed provides the support, growth factors, and regulatory factors necessary to sustain long-term active proliferation of cells in culture and / or cultures implanted in vivo. When grown in this three-dimensional system, the proliferating cells mature and segregate properly to form components of adult tissues analogous to counterparts in vivo, which can be utilized in the body as a corrective tissue. For example, and not by way of limitation, the three-dimensional cultures can be used to form tubular tissue structures, like those of the gastrointestinal and genitourinary tracts, as well as blood vessels; tissues for hernia repair and / or tendons and ligaments; etc.
Owner:REGENEMED
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Modulation of stem and progenitor cell differentiation, assays, and uses thereof
InactiveUS20030235909A1Modulate their differentiationIncrease speedOrganic active ingredientsSenses disorderAssayPlacenta
The present invention relates to methods of modulating mammalian stem cell and progenitor cell differentiation. The methods of the invention can be employed to regulate and control the differentiation and maturation of mammalian, particularly human stem cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem or progenitor cell populations along specific cell and tissue lineages, and in particular, to the differentiation of embryonic-like stem cells originating from a postpartum placenta or for the differentiation of early progenitor cells to a granulocytic lineage. Finally, the invention relates to the use of such differentiated stem or progenitor cells in transplantation and other medical treatments.
Owner:SIGNAL PHARMA LLC +2
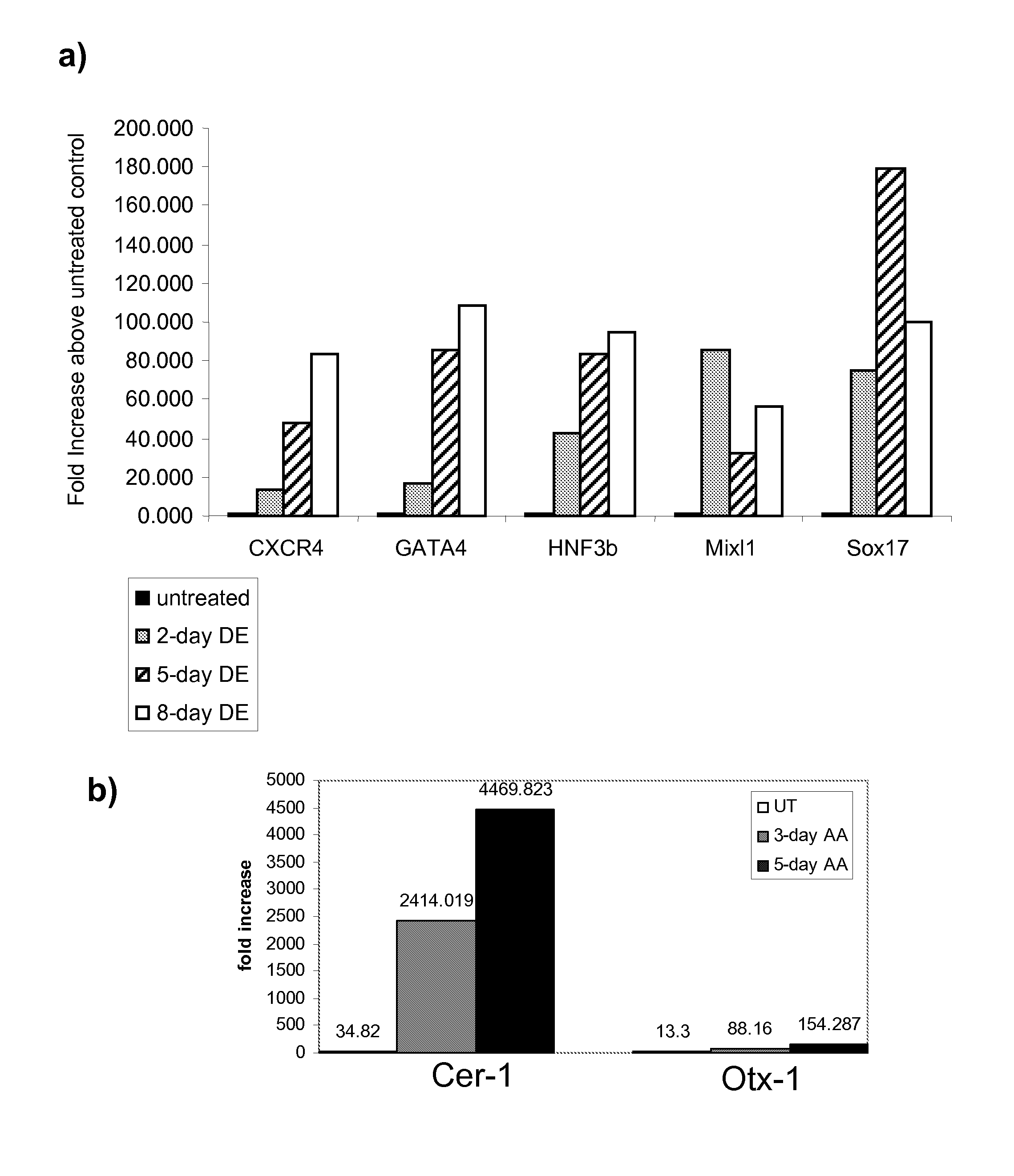
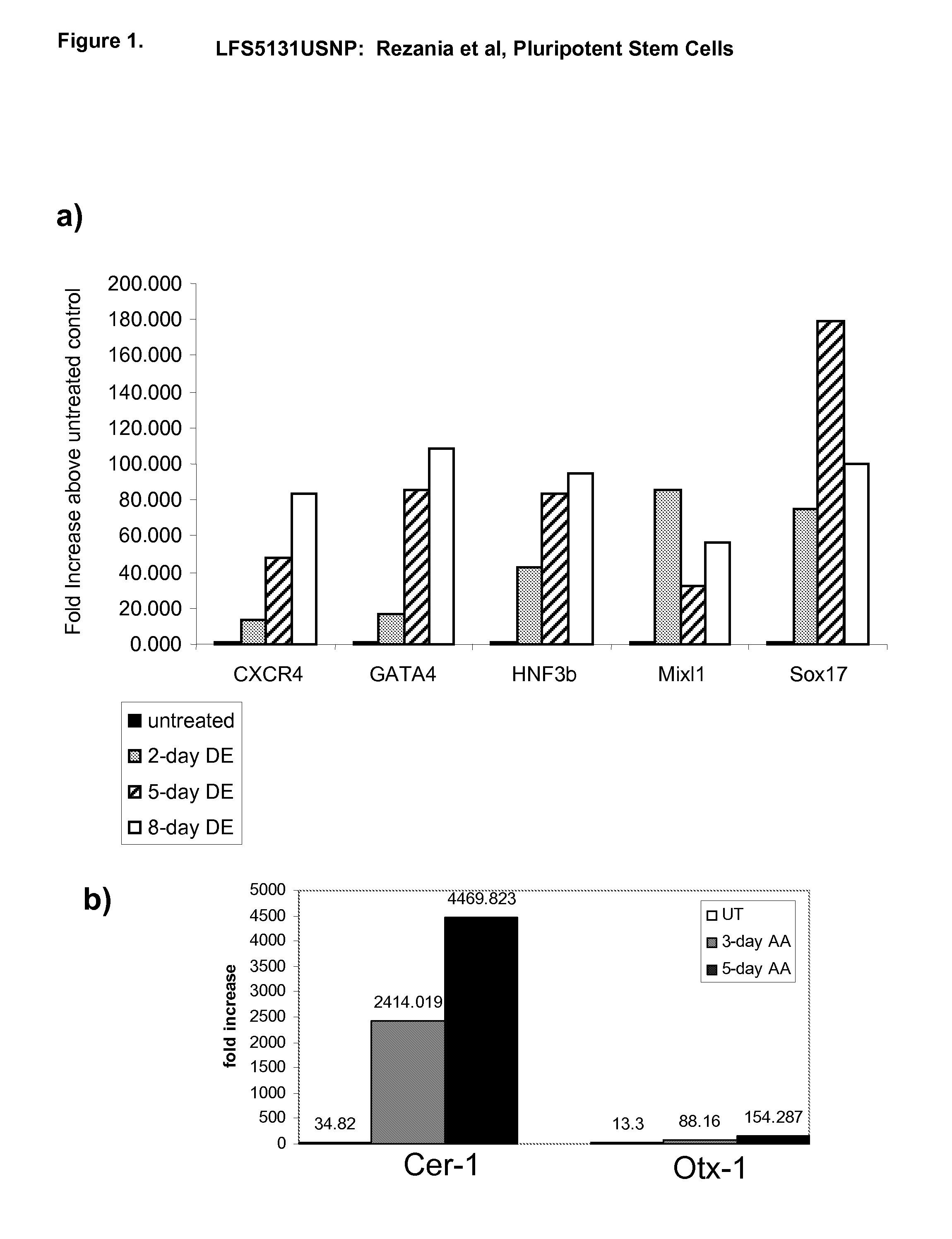
Differentiation of human embryonic stem cells
ActiveUS20070254359A1Inhibits Notch signalingPancreatic cellsArtificial cell constructsGerm layerPluripotential stem cell
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
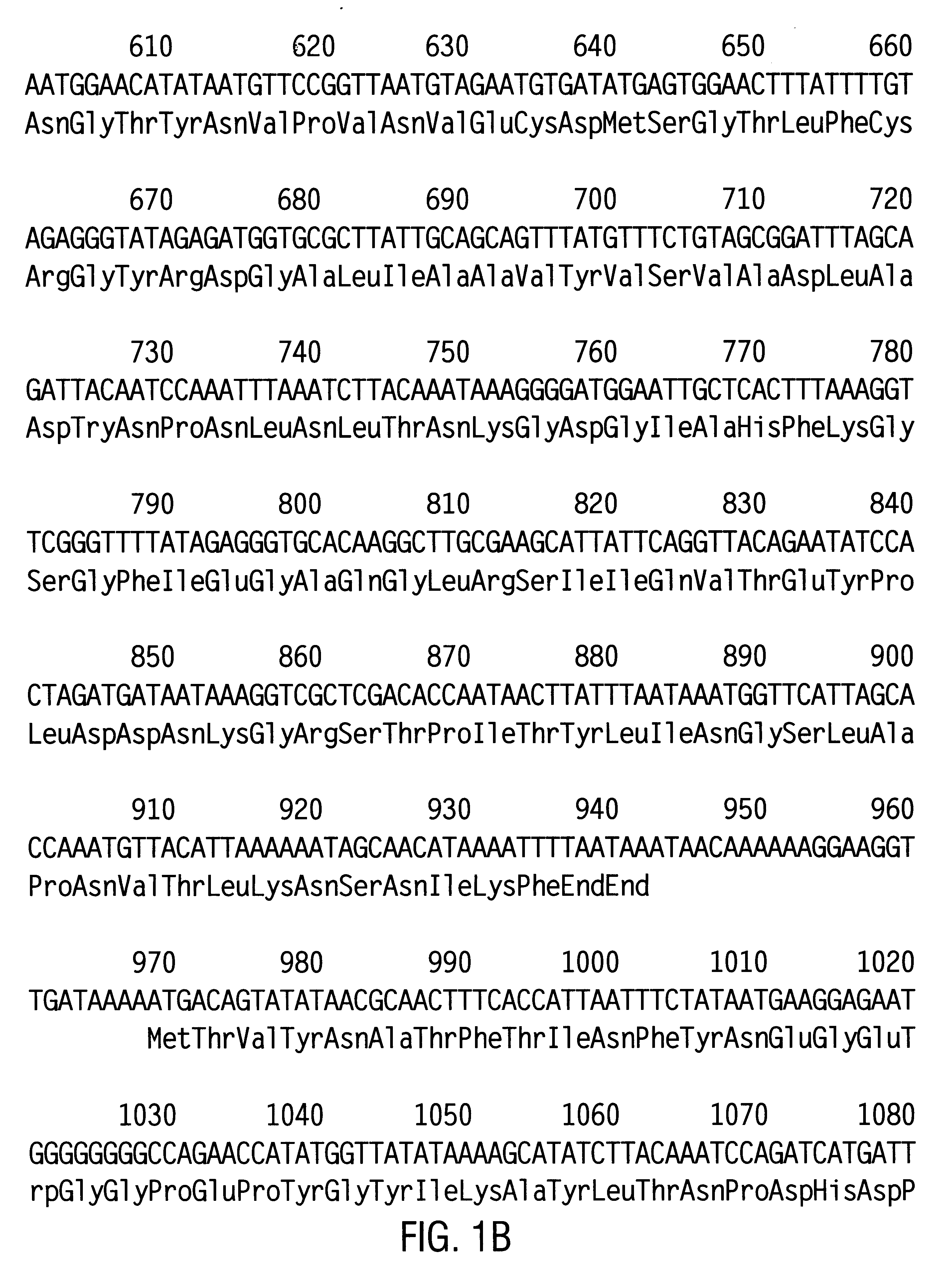
Axmi-031, axmi-039, axmi-040 and axmi-049, a family of novel delta-endotoxin genes and methods for their use
Compositions and methods for conferring pesticidal activity to bacteria, plants, plant cells, tissues and seeds are provided. Compositions comprising a coding sequence for a delta-endotoxin polypeptide are provided. The coding sequences can be used in DNA constructs or expression cassettes for transformation and expression in plants and bacteria. Compositions also comprise transformed bacteria, plants, plant cells, tissues, and seeds. In particular, isolated delta-endotoxin nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the polynucleotides are encompassed, and antibodies specifically binding to those amino acid sequences. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequence shown in SEQ ID NO:2, 4, 6, 8, 10, 12, 15, 17, 19, 21, 23, 25, 27, 29, 31, 33, 35, or 38, or the nucleotide sequence set forth in SEQ ID NO:1, 3, 5, 7, 9, 11, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, or 37, as well as variants and fragments thereof.
Owner:BASF AGRICULTURAL SOLUTIONS SEED LLC
Skin Firming Anti-Aging Cosmetic Mask Compositions
InactiveUS20040161435A1Promote excess fat reductionPromote cellulite controlBiocideCosmetic preparationsAdditive ingredientPhase mask
I have discovered cosmetic mask compositions suitable for face, neck, chin or body applications. These compositions synergistically combine at least one skin beneficial cosmetic or drug composition with at least one composition to promote excess fat reduction, cellulite control, or muscle toning benefits. The mask composition also contains at least one binder composition that binds with other beneficial ingredients by electrostatic, atomic, or ionic charges to synergistically enhance their topical site-specific benefits. These mask compositions are suitable for a variety of delivery system methods that include peel-off mask, leave-in mask, moisturizing mask, exfoliating mask, prosthetic mask, soaking mask, depilatory mask, foaming mask, rinse-off mask, sloughing mask, rub-off mask, two-phase mask, dual-chamber mask, and self-heating (heat releasing) mask.
Owner:GUPTA SHYAM K
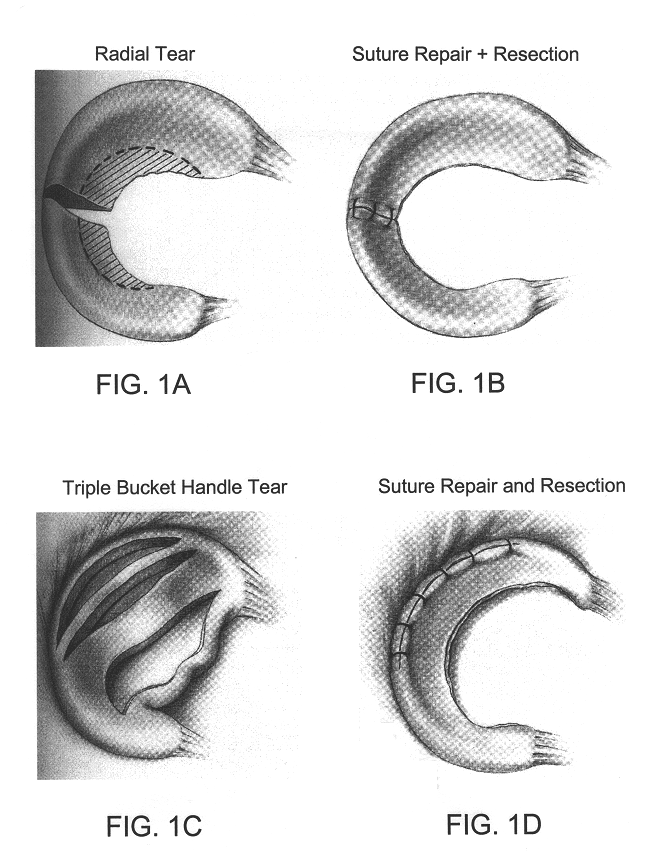
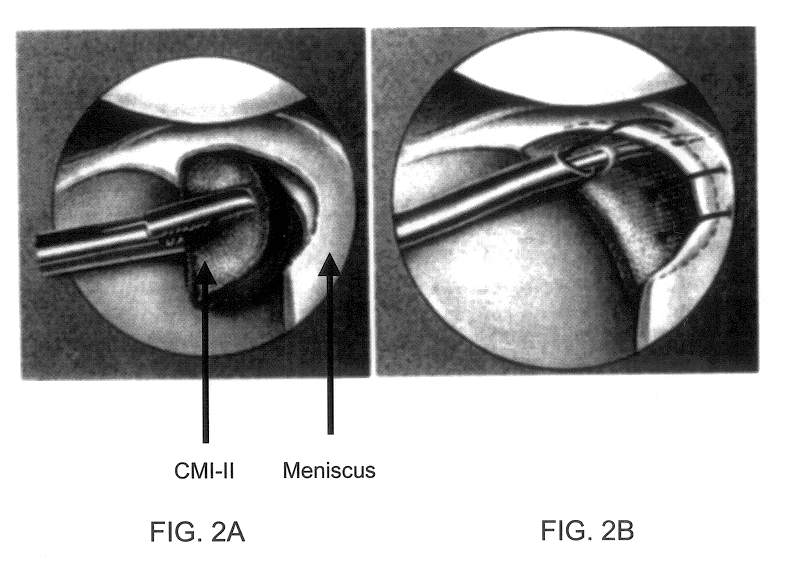
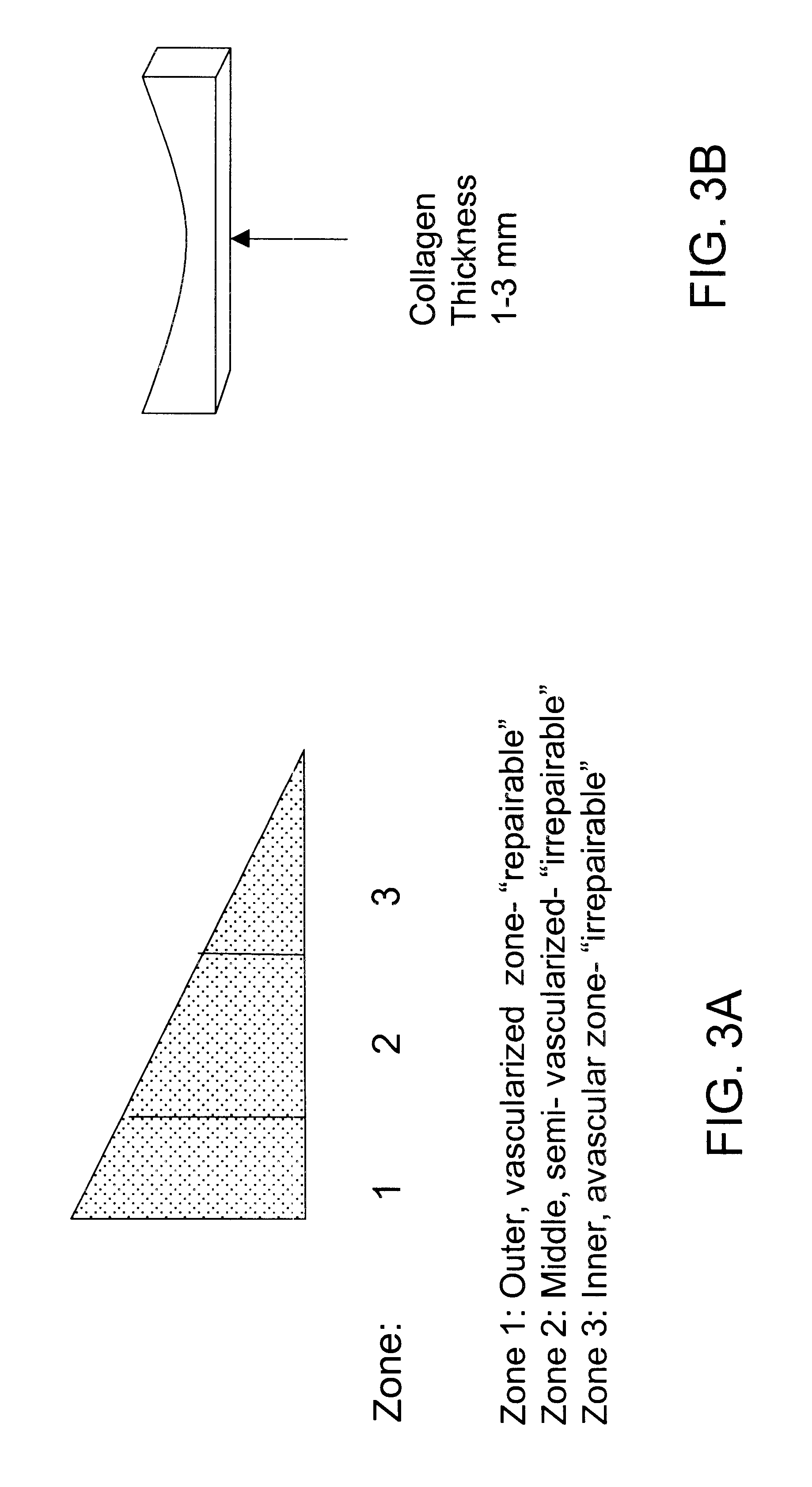
Compositions for regeneration and repair of cartilage lesions
InactiveUS6511958B1Increase ratingsImprove repair qualitySuture equipmentsPowder deliveryMedicineCartilage lesion
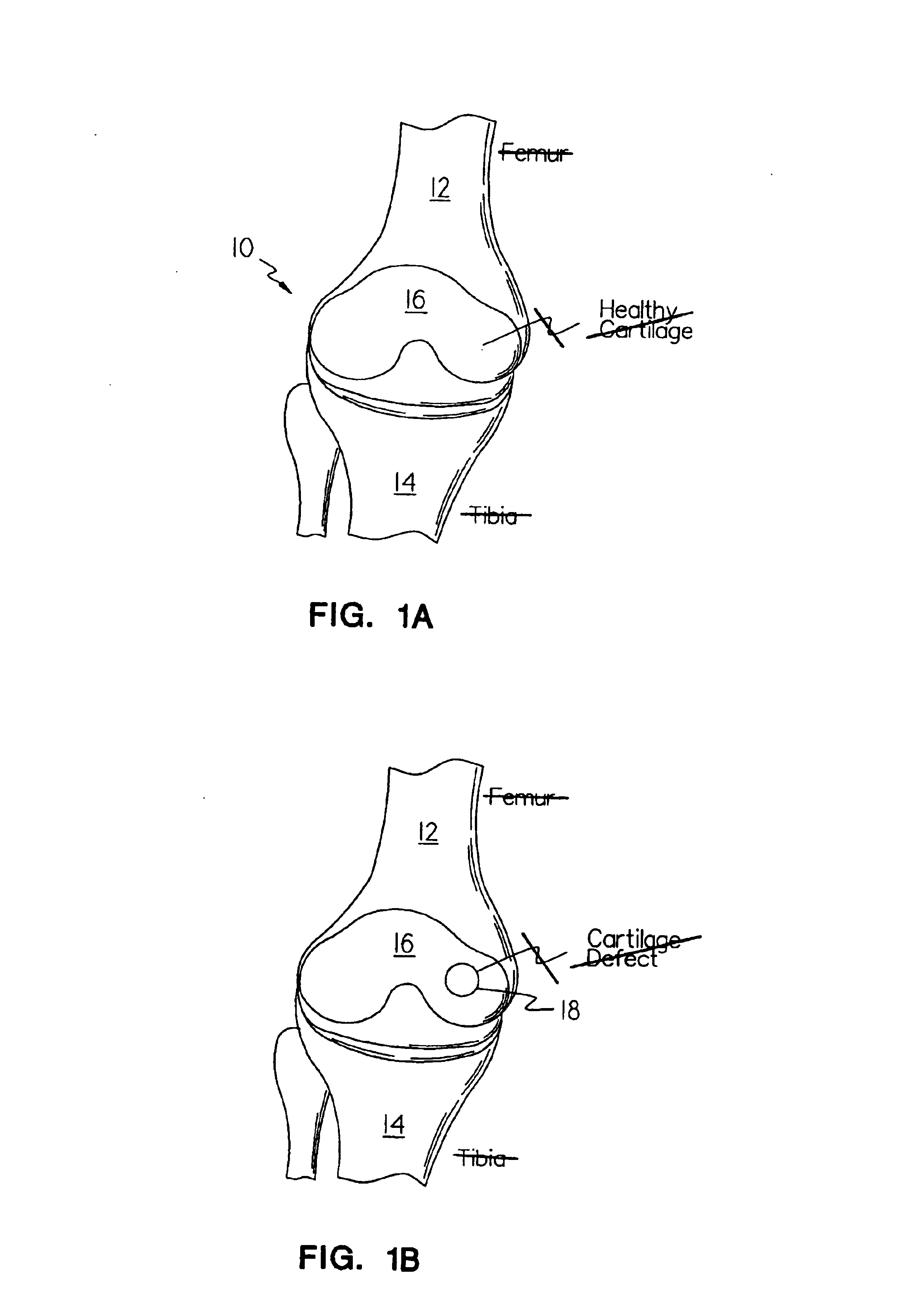
Disclosed is a cartilage repair product that induces both cell ingrowth into a bioresorbable material and cell differentiation into cartilage tissue. Such a product is useful for regenerating and / or repairing both vascular and avascular cartilage lesions, particularly articular cartilage lesions, and even more particularly mensical tissue lesions, including tears as well as segmental defects. Also disclosed is a method of regenerating and repairing cartilage lesions using such a product.
Owner:ZIMMER ORTHOBIOLOGICS
Bacillus thuringiensis CryET33 and CryET34 compositions and uses therefor
Disclosed are Bacillus thuringiensis strains comprising novel crystal proteins which exhibit insecticidal activity against coleopteran insects including red flour beetle larvae (Tribolium castaneum) and Japanese beetle larvae (Popillia japonica). Also disclosed are novel B. thuringiensis crystal toxin genes, designated cryET33 and cryET34, which encode respectively the coleopteran-toxic proteins, CryET33 (29-kDa) crystal protein, and CryET34 (14-kDa) crystal protein. Also disclosed are methods of making and using transgenic cells comprising the novel nucleic acid sequences of the invention.
Owner:MONSANTO TECH LLC
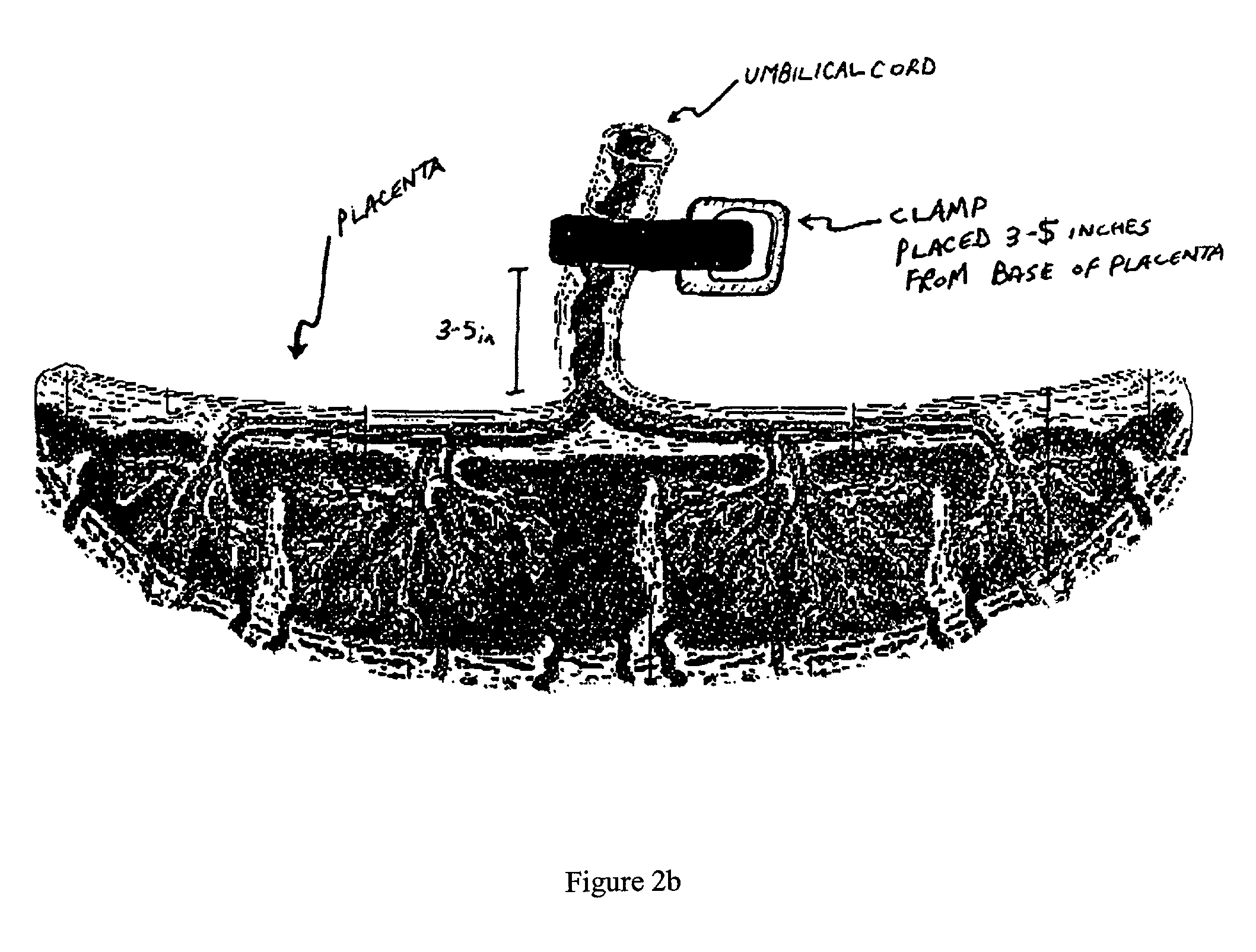
Method of collecting placental stem cells
InactiveUS20020123141A1Increase concentrationImprove the environmentSenses disorderAntipyreticCord blood stem cellEmbryo
A method of collecting embryonic-like stem cells from a placenta which has been treated to remove residual cord blood by perfusing the drained placenta with an anticoagulant solution to flush out residual cells, collecting the residual cells and perfusion liquid from the drained placenta, and separating the embryonic-like cells from the residual cells and perfusion liquid. Exogenous cells can be propagated in the placental bioreactor and bioactive molecules collected therefrom.
Owner:CELULARITY INC
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
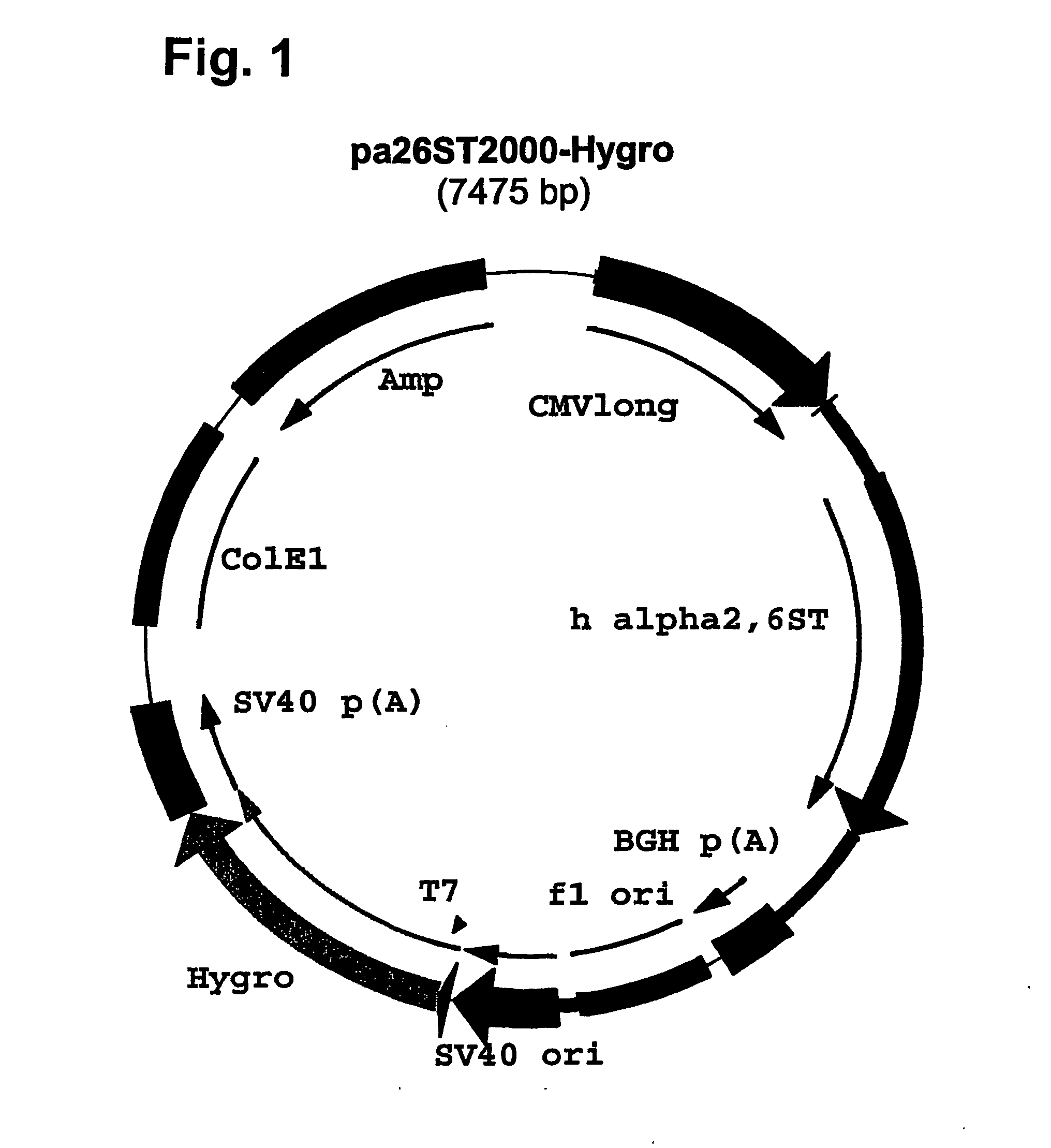
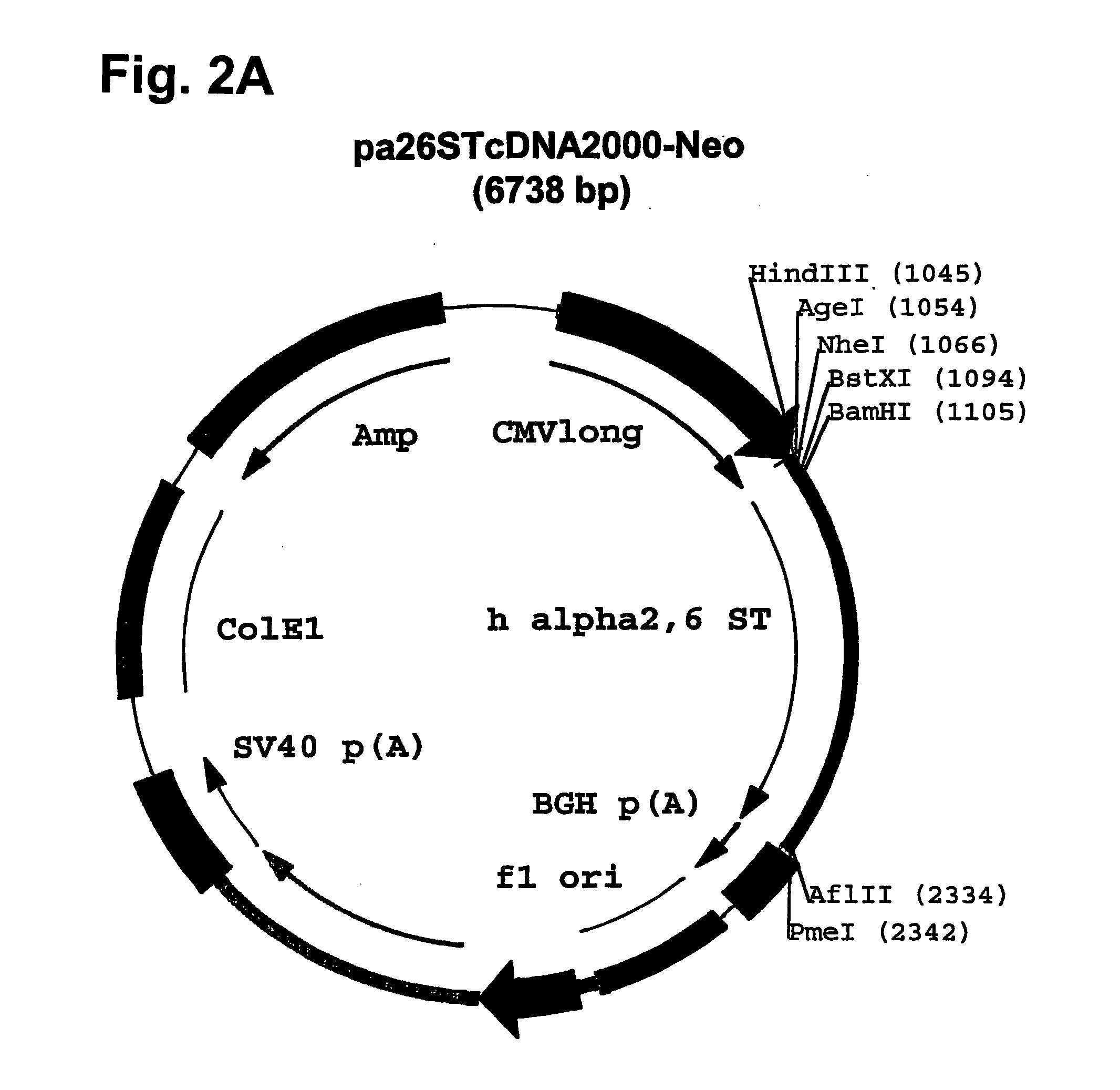
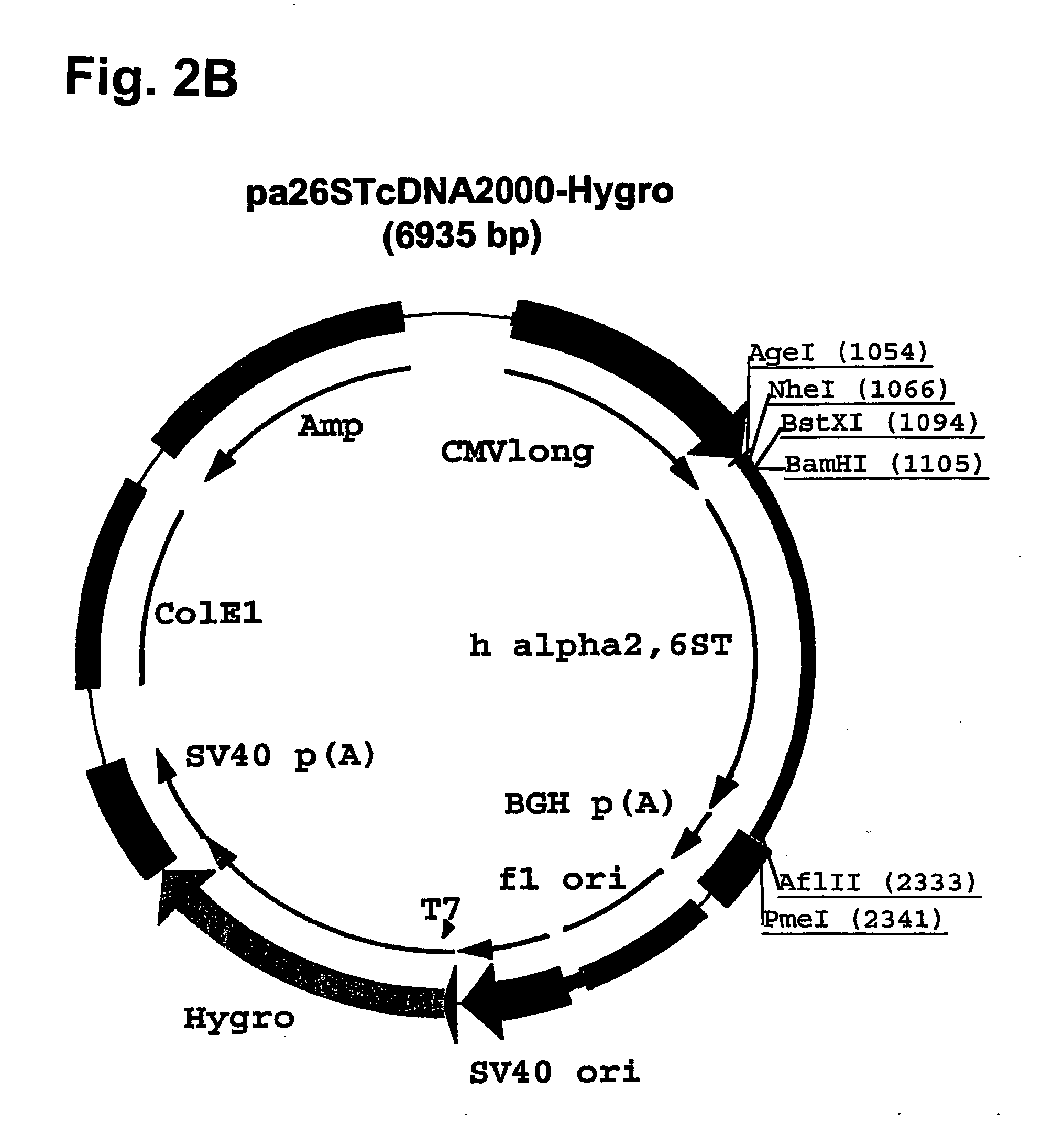
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
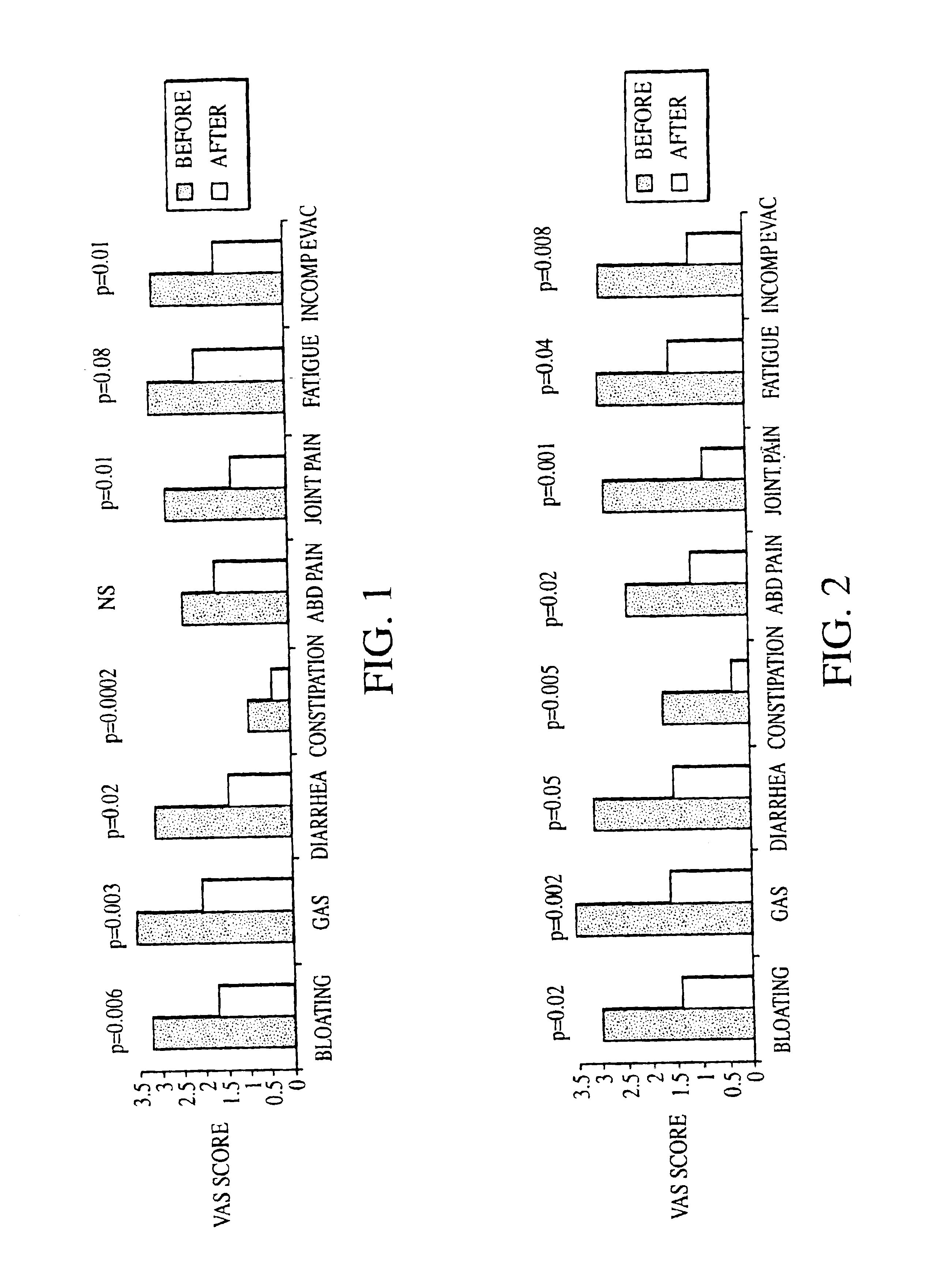
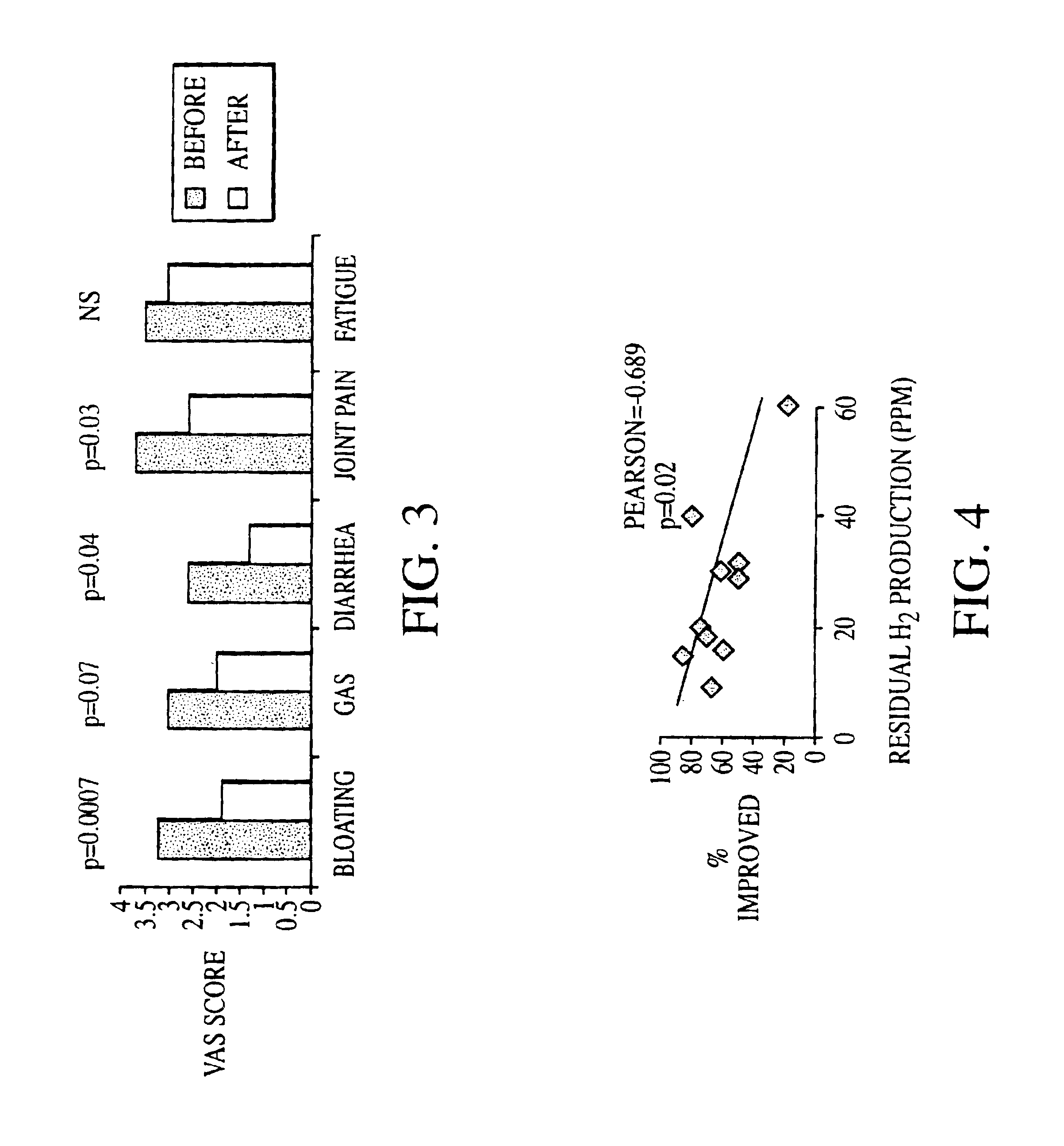
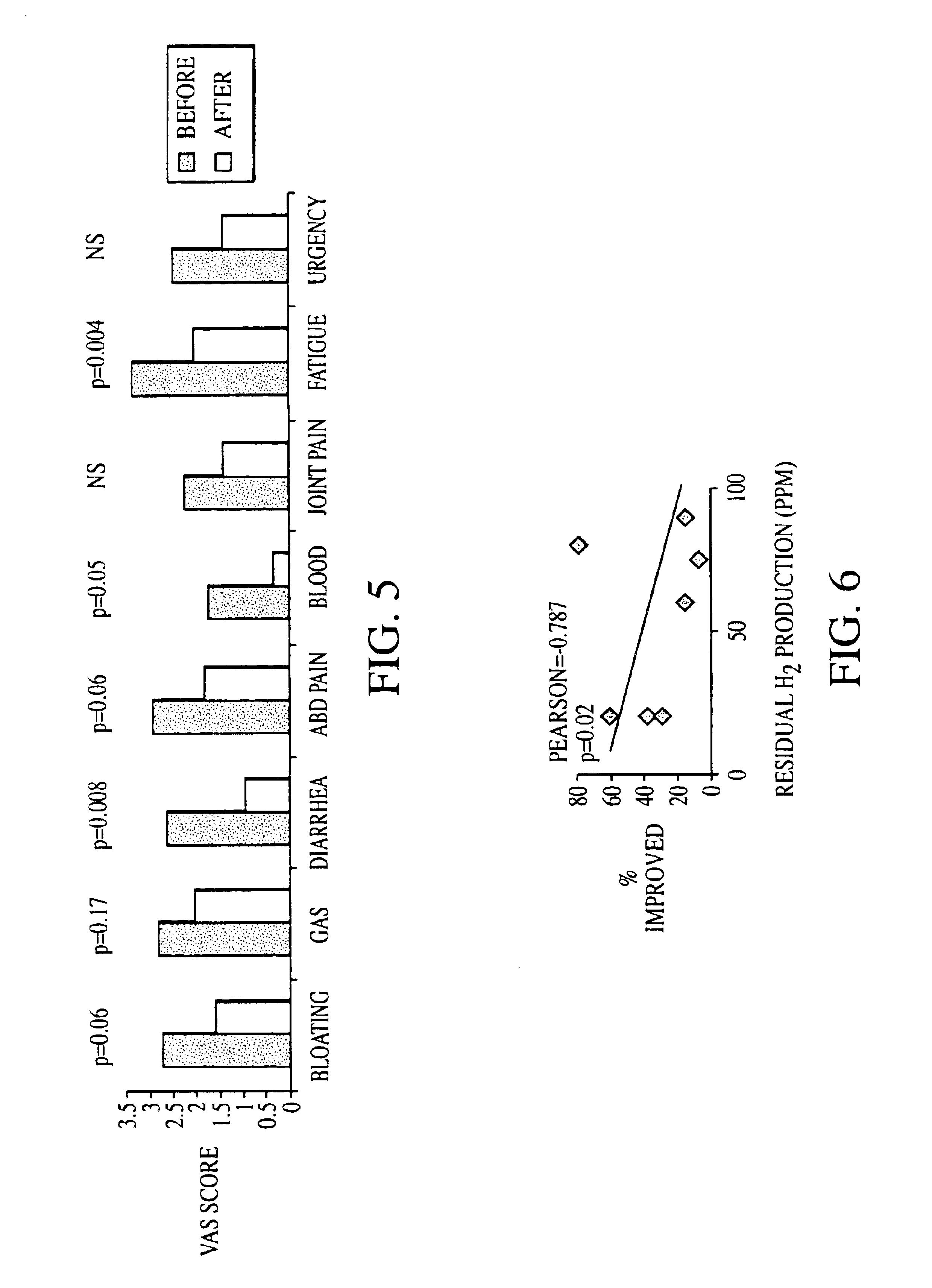
Methods of diagnosing or treating irritable bowel syndrome and other disorders caused by small intestinal bacterial overgrowth
InactiveUS6861053B1Eradicate small intestinal bacterial overgrowthSymptoms improvedAntibacterial agentsOrganic active ingredientsBacteroidesAutoimmune responses
Disclosed is a method of diagnosing irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, such as multiple sclerosis and systemic lupus erythematosus, or Crohn's disease, which involves detecting the presence of small intestinal bacterial overgrowth (SIBO) in a human subject having at least one symptom associated with a suspected diagnosis of any of those diagnostic categories. Also disclosed is a method of treating these disorders, and other disorders caused by SIBO, that involves at least partially eradicating a SIBO condition in the human subject. The method includes administration of anti-microbial or probiotic agents, or normalizing intestinal motility by employing a prokinetic agent. The method improves symptoms, including hyperalgesia related to SIBO and disorders caused by SIBO. Also disclosed is a kit for the diagnosis or treatment of irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, or Crohn's disease.
Owner:CEDARS SINAI MEDICAL CENT
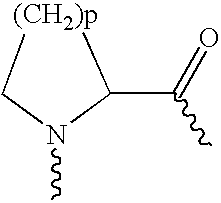
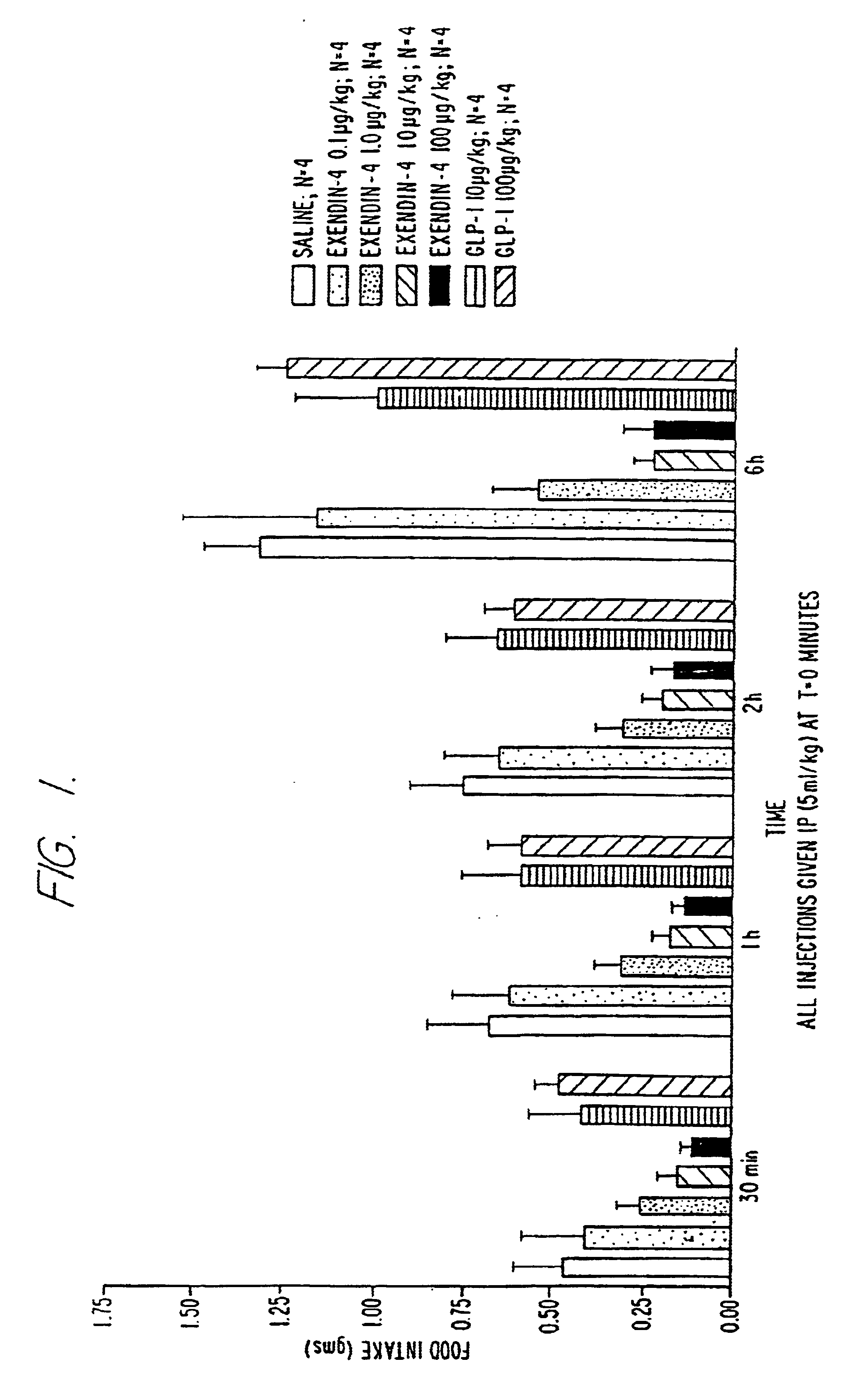
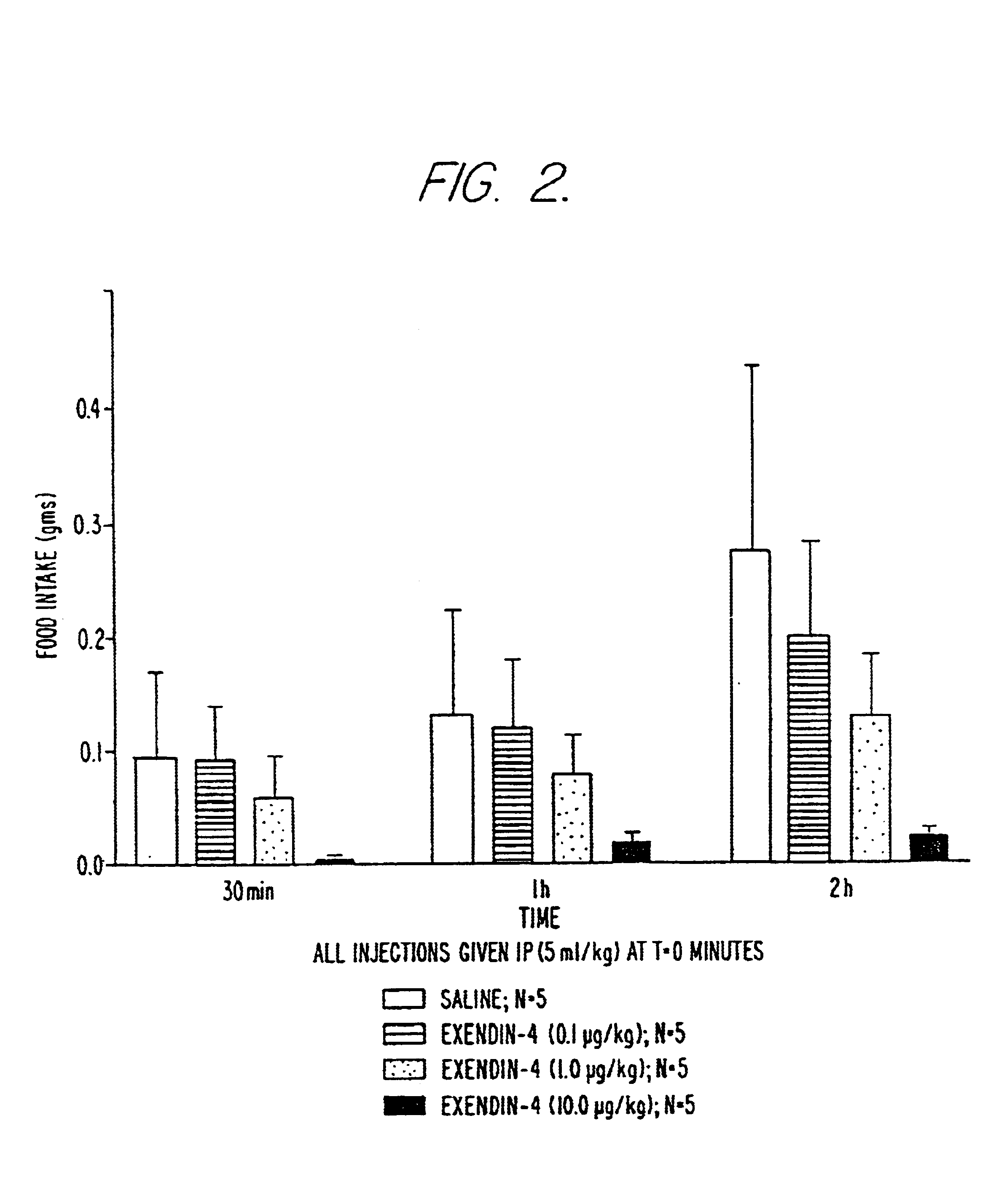
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
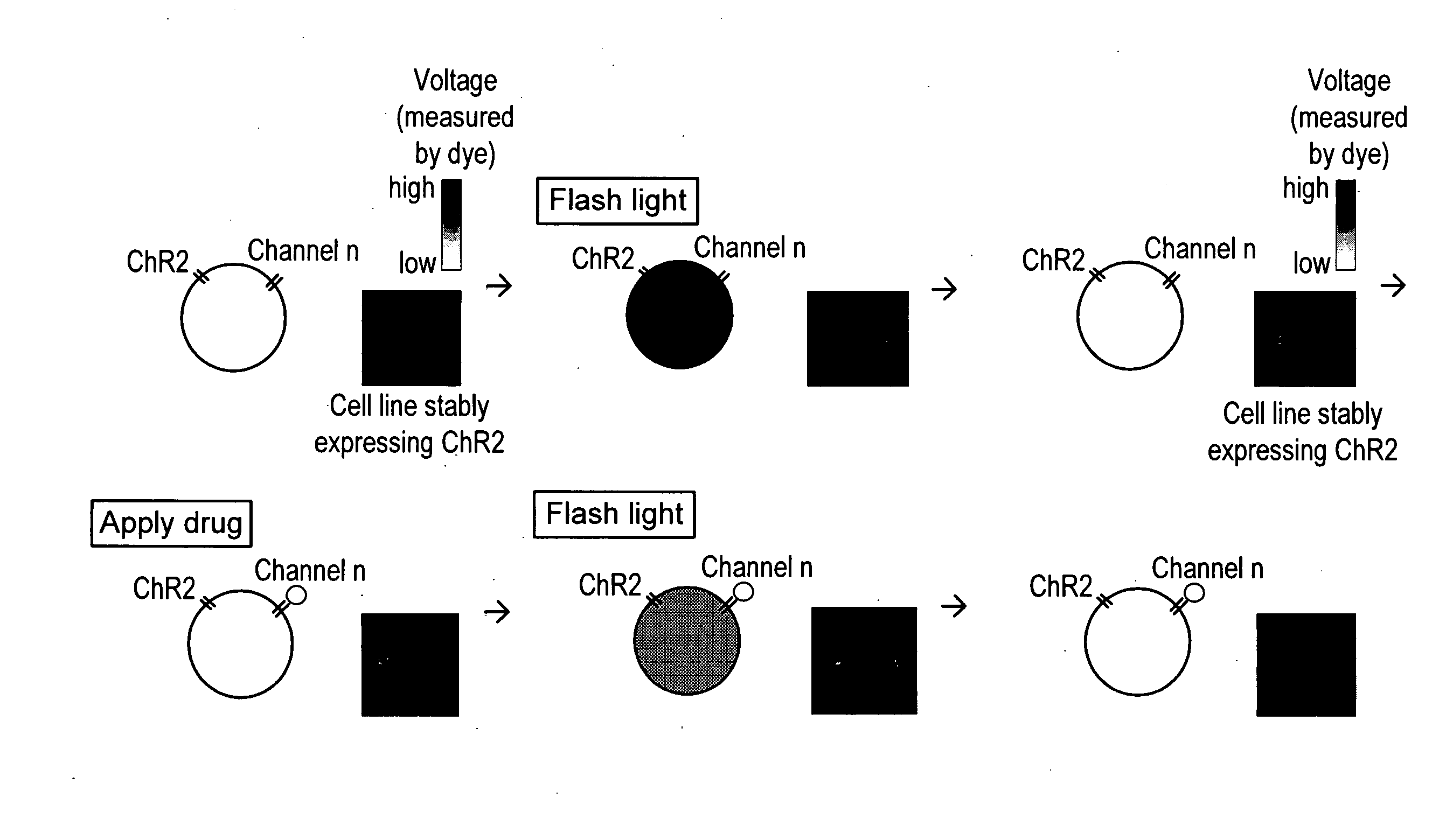
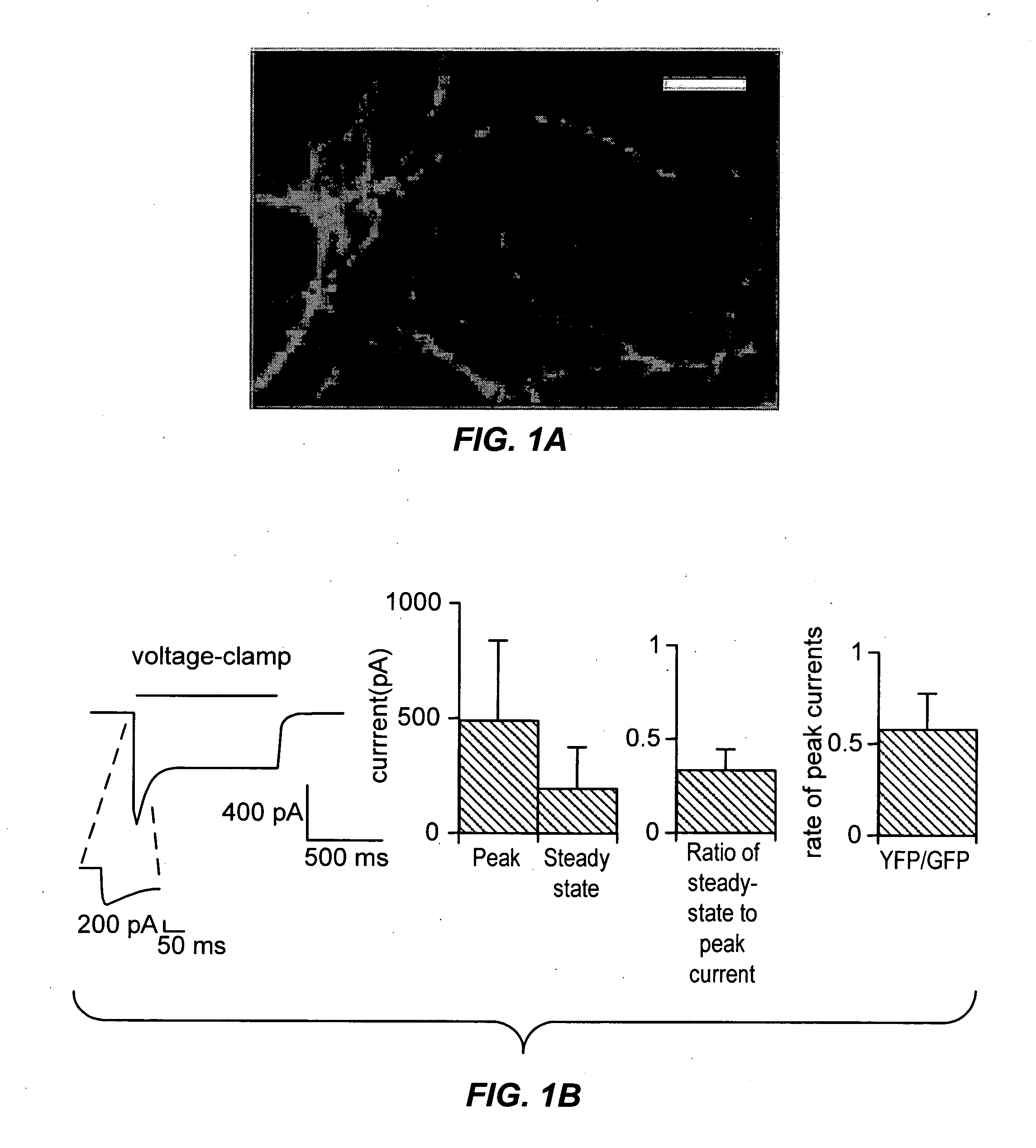
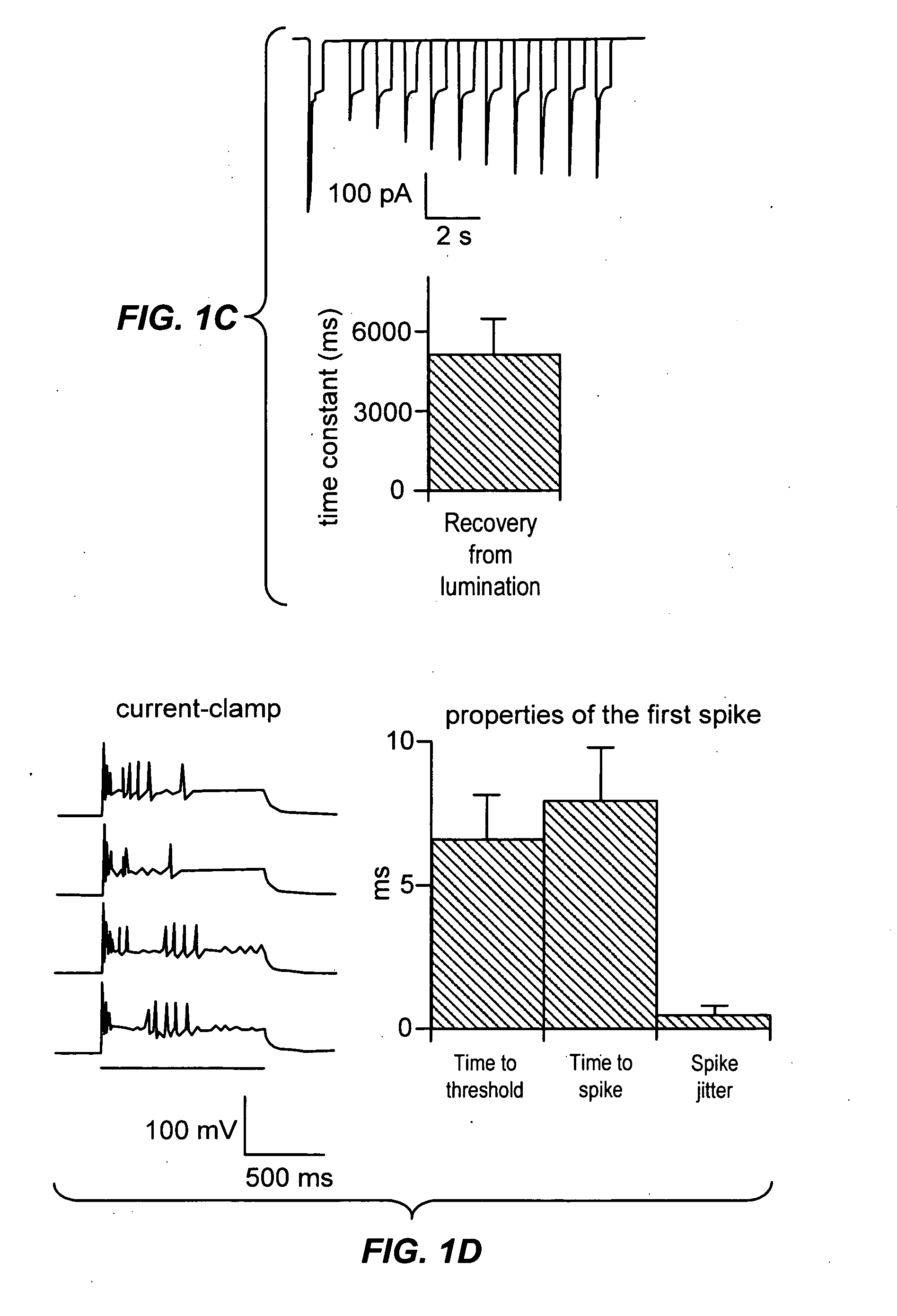
Light-activated cation channel and uses thereof
ActiveUS20070261127A1Improve abilitiesOrganic active ingredientsSenses disorderCell membraneExcitable cell
The present invention provides compositions and methods for light-activated cation channel proteins and their uses within cell membranes and subcellular regions. The invention provides for proteins, nucleic acids, vectors and methods for genetically targeted expression of light-activated cation channels to specific cells or defined cell populations. In particular the invention provides millisecond-timescale temporal control of cation channels using moderate light intensities in cells, cell lines, transgenic animals, and humans. The invention provides for optically generating electrical spikes in nerve cells and other excitable cells useful for driving neuronal networks, drug screening, and therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method of preparing kakadu plum powder
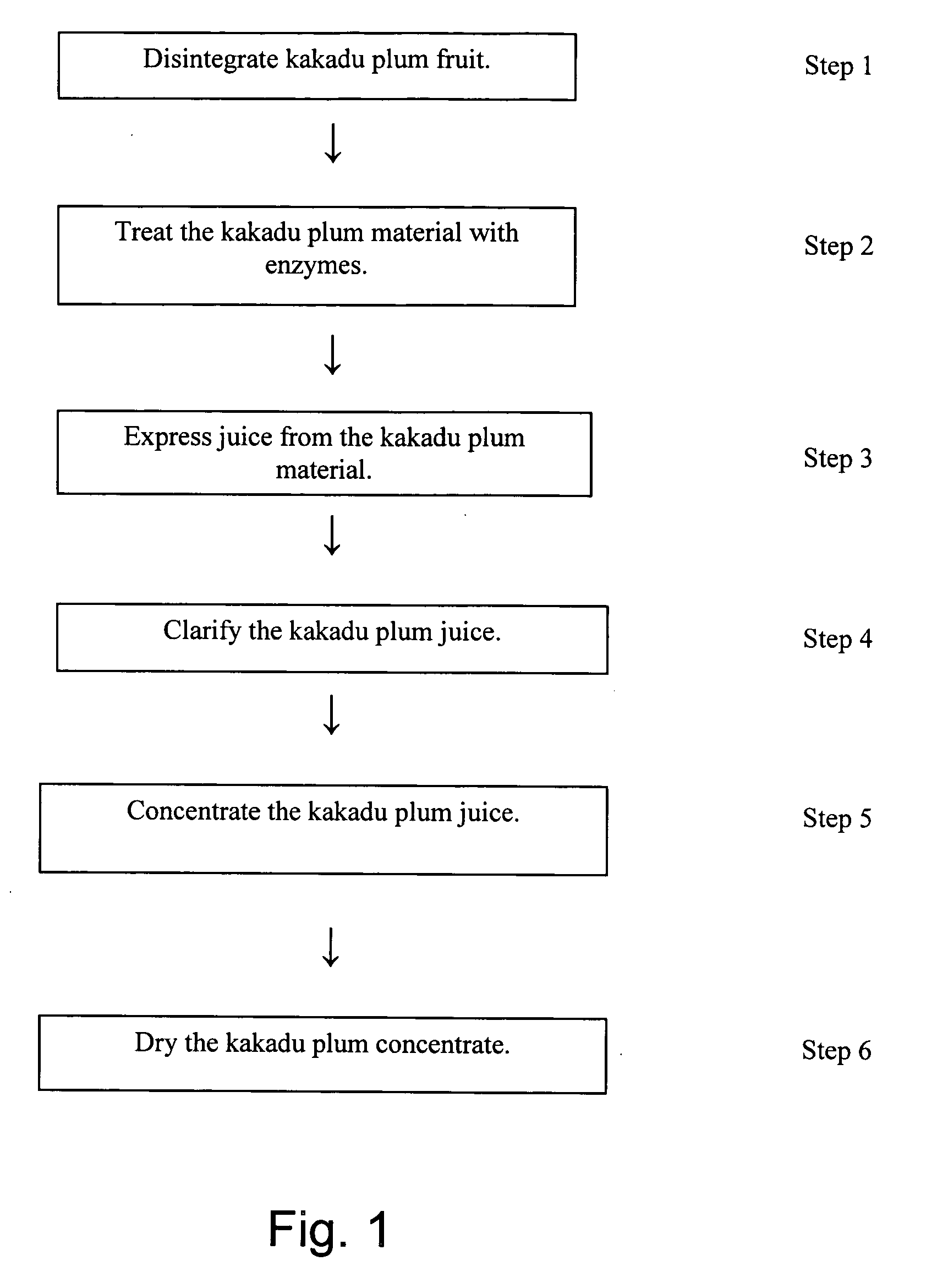
ActiveUS20050163880A1High viscosityImpede efficient processingBiocideUnknown materialsAbsorption capacityUltrafiltration
A process for producing a kakadu plum powder having an increased amount of naturally occurring ascorbic acid and high ORAC value. The process of preparing the extract includes the following: disintegrating kakadu plum fruit; treating the disintegrated kakadu plum material with enzymes to at least partially digest the material; juicing the kakadu plum material and drying the juice to produce a powder. In a preferred embodiment, the kakadu plum juice is further clarified with ultrafiltration and concentrated by performing reverse osmosis on the kakadu plum juice. The resultant kakadu plum powder has a natural ascorbic acid content of at least about 15% and a naturally occurring Oxygen Reduction Absorption Capacity value of at least 1500.
Owner:ACCESS BUSINESS GRP INT LLC +1
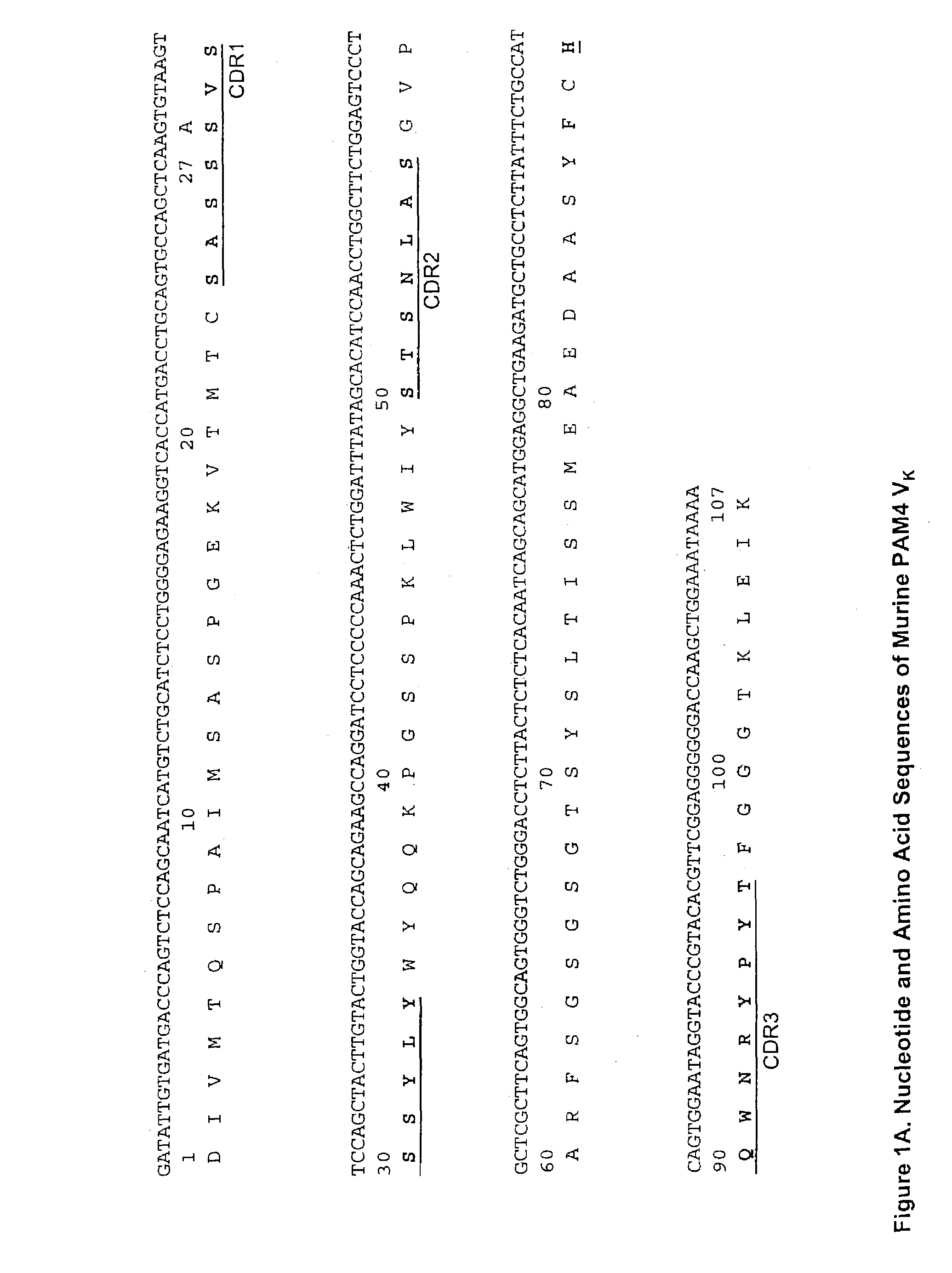
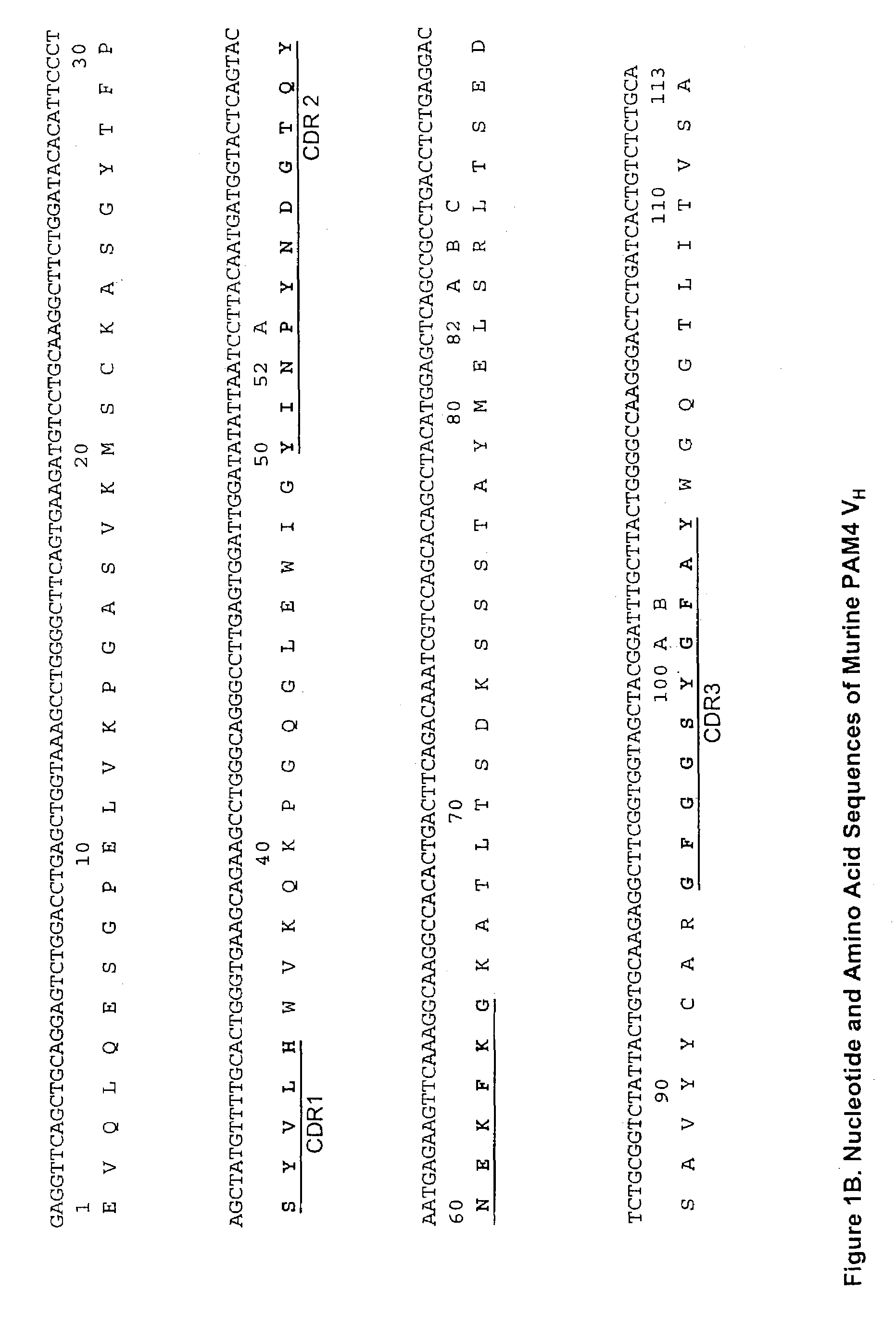
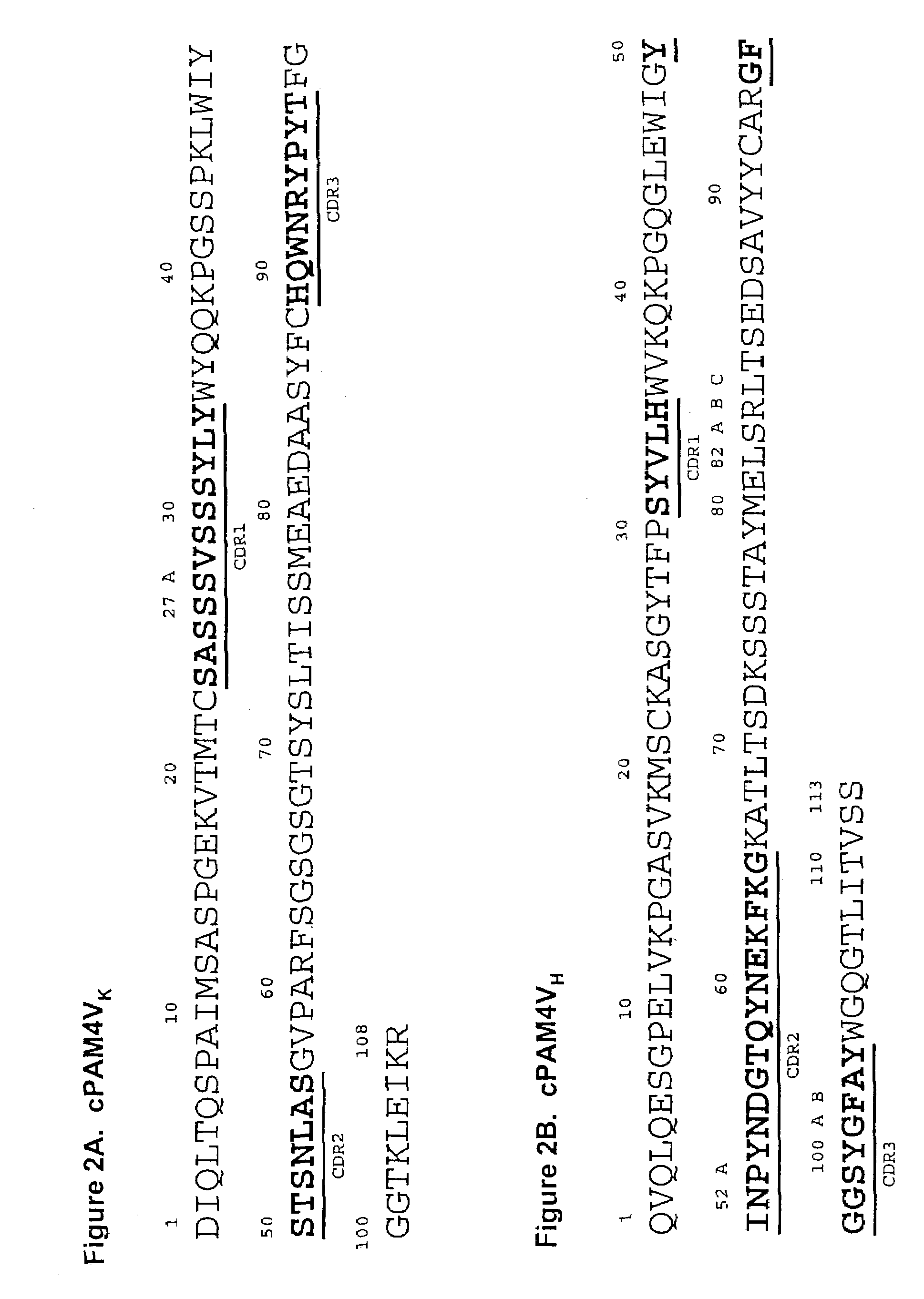
Monoclonal antibody hPAM4
This invention relates to monovalent and multivalent, monospecific antibodies and to multivalent, multispecific antibodies. One embodiment of these antibodies has one or more identical binding sites where each binding site binds with a target antigen or an epitope on a target antigen. Another embodiment of these antibodies has two or more binding sites where these binding sites have affinity towards different epitopes on a target antigen or different target antigens, or have affinity towards a target antigen and a hapten. The present invention further relates to recombinant vectors useful for the expression of these functional antibodies in a host. More specifically, the present invention relates to the tumor-associated antibody designated PAM4. The invention further relates to humanized and human PAM4 antibodies, and the use of such antibodies in diagnosis and therapy.
Owner:IMMUNOMEDICS INC
Post-partum mammalian placenta, its use and placental stem cells therefrom
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
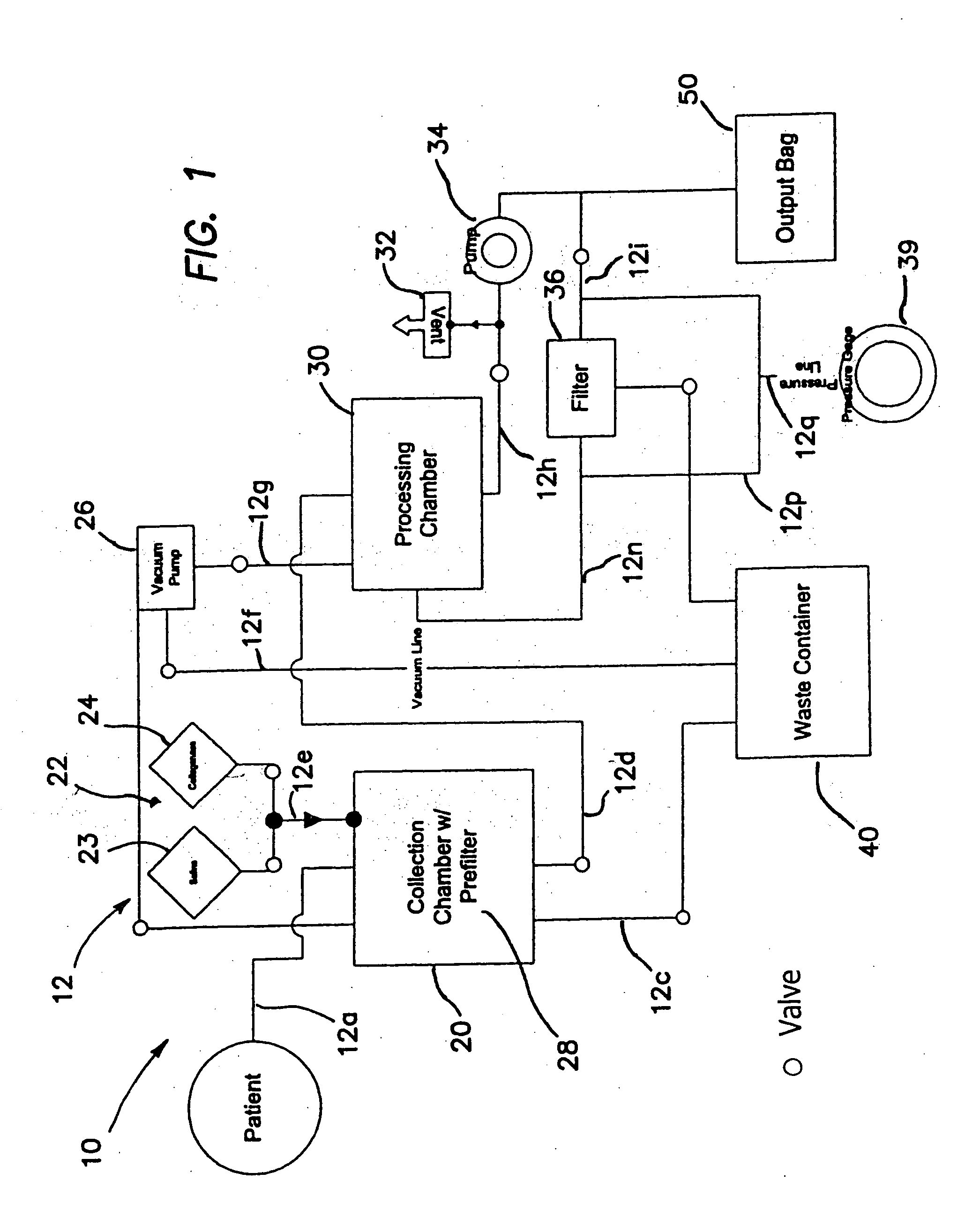
Systems and methods for separating and concentrating regenerative cells from tissue
ActiveUS20050084961A1High yieldImprove consistencyBioreactor/fermenter combinationsBiocideBiomedical engineeringAdipose tissue
Systems and methods are described that are used to separate cells from a wide variety of tissues. In particular, automated systems and methods are described that separate regenerative cells, e.g., stem and / or progenitor cells, from adipose tissue. The systems and methods described herein provide rapid and reliable methods of separating and concentrating regenerative cells suitable for re-infusion into a subject.
Owner:LOREM VASCULAR PTE LTD
Bacillus thuringiensis CryET29 compositions toxic to coleopteran insects and ctenocephalides SPP
InactiveUS6093695ARemarkable insecticidal activityGood reproducibilityBiocideBacteriaBacillus thuringiensisCtenocephalides felis felis
Disclosed is a novel delta -endotoxin, designated CryET29, that exhibits insecticidal activity against siphonapteran insects, including larvae of the cat flea (Ctenocephalides felis), as well as against colcopteran insects, including the southern corn rootworm (Diabrotica undecimpunctata), western corn rootworm (D. virgifera), Colorado potato beetle (Leptinotarsa decemlineata), Japanese beetle (Popillia japonica), and red flour beetle (Tribolium castaneur). Also disclosed are nucleic acid segments encoding CryET29, recombinant vectors, host cells, and transgenic plants comprising a cryET29 DNA segment. Methods for making and using the disclosed protein and nucleic acid segments are disclosed as well as assays and diagnostic kits for detecting cryET29 and CryET29 sequences in vivo and in vitro.
Owner:MONSANTO TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com