Patents
Literature
83 results about "Endoderm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
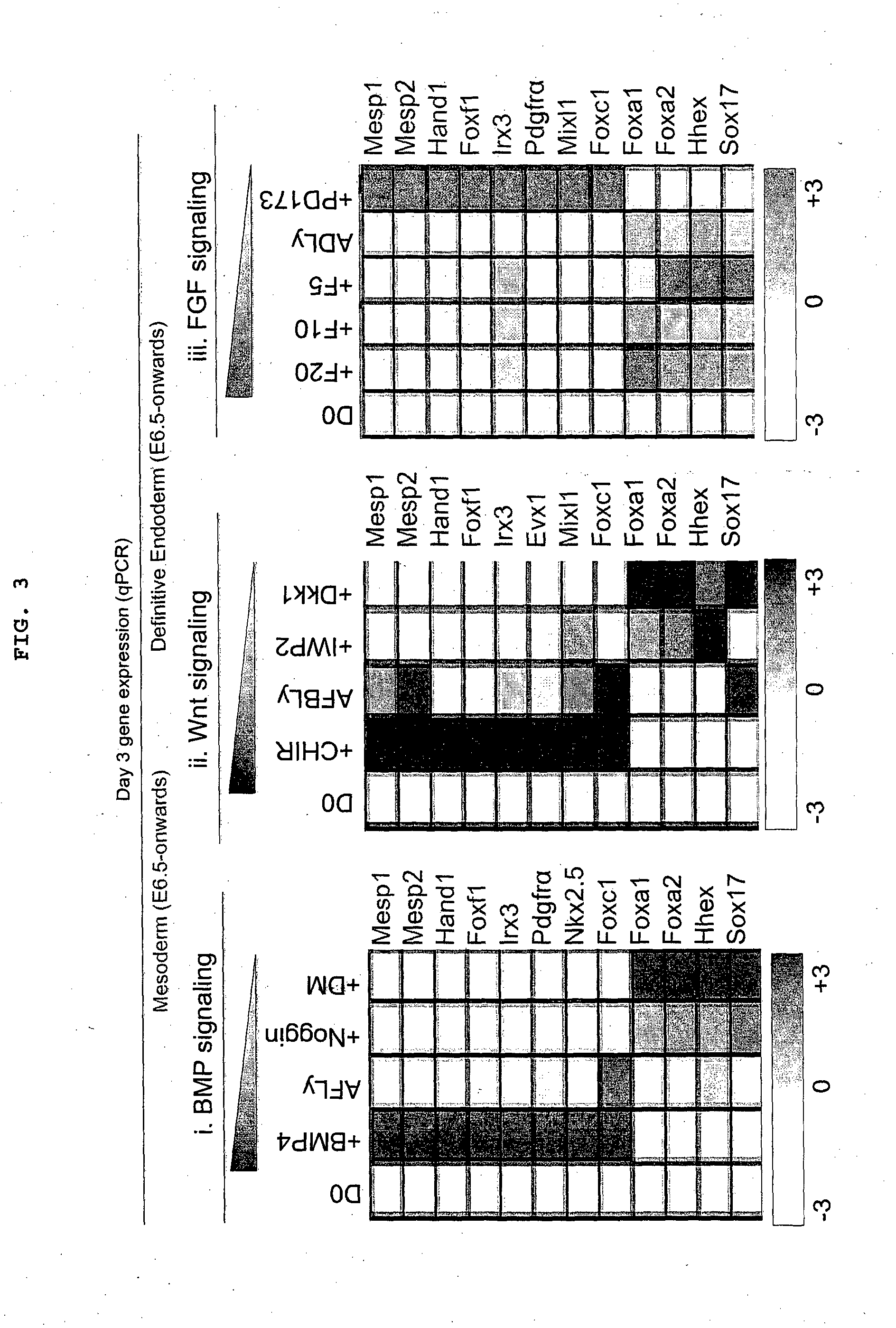
Endoderm is one of the three primary germ layers in the very early embryo. The other two layers are the ectoderm (outside layer) and mesoderm (middle layer), with the endoderm being the innermost layer. Cells migrating inward along the archenteron form the inner layer of the gastrula, which develops into the endoderm.
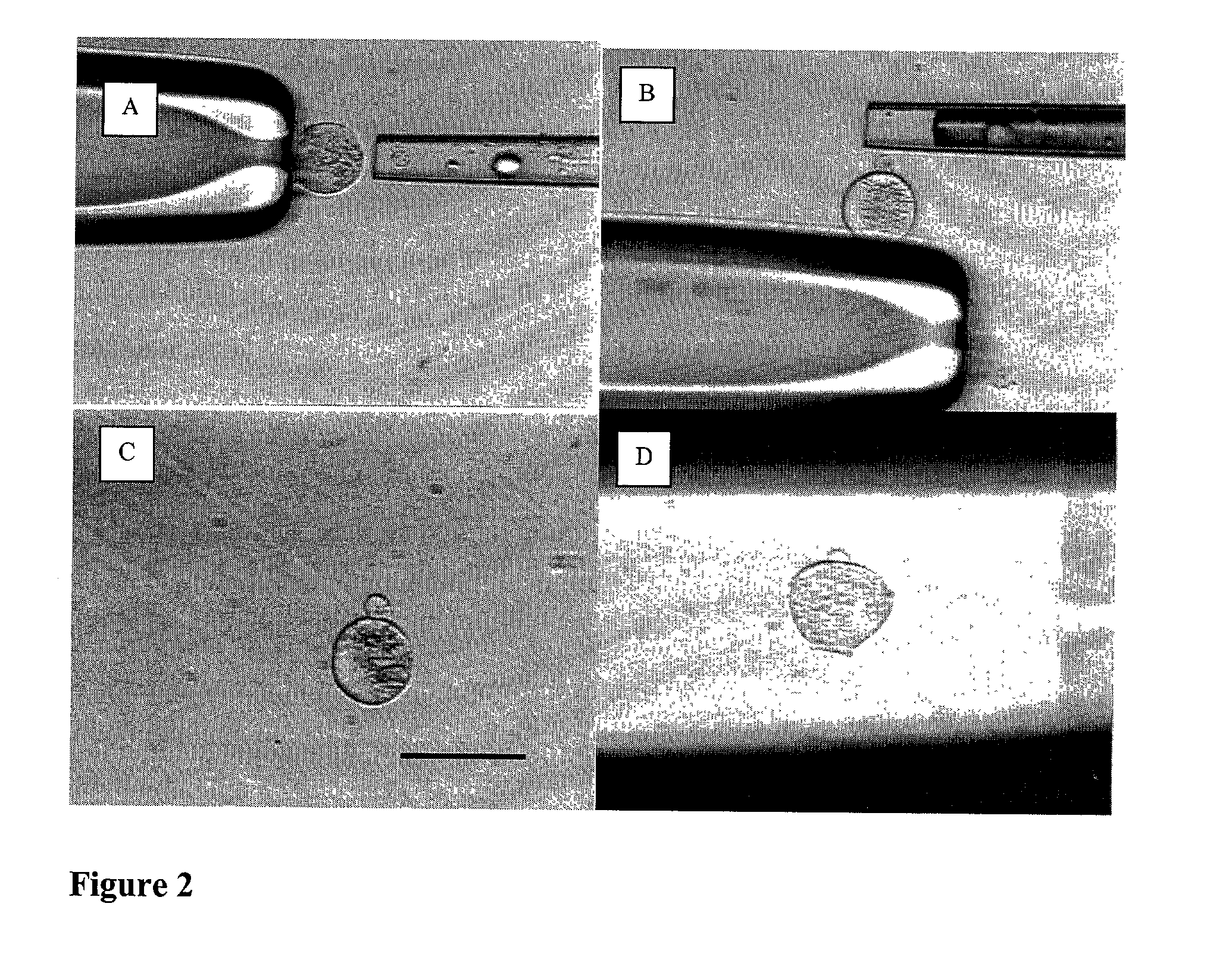
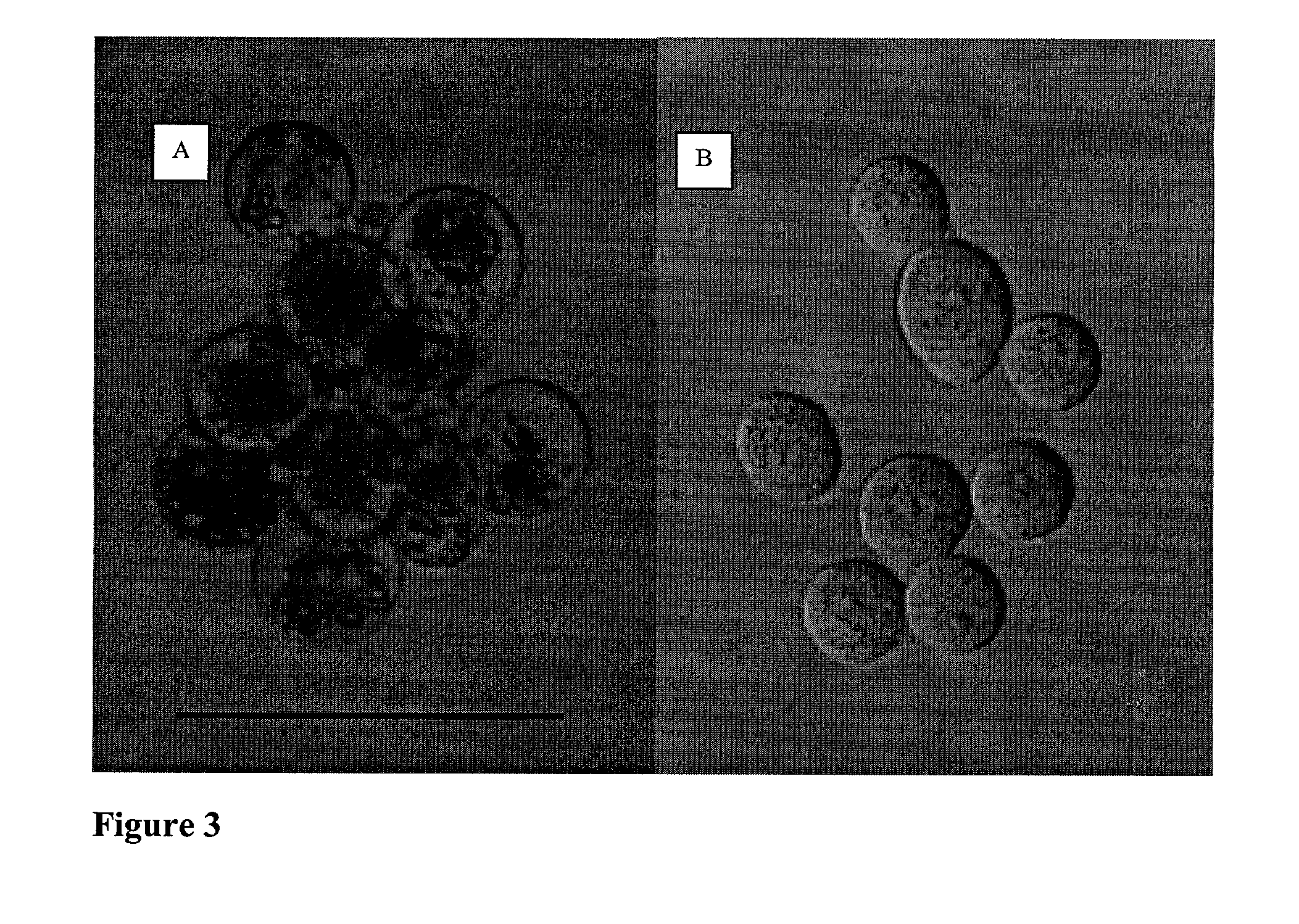
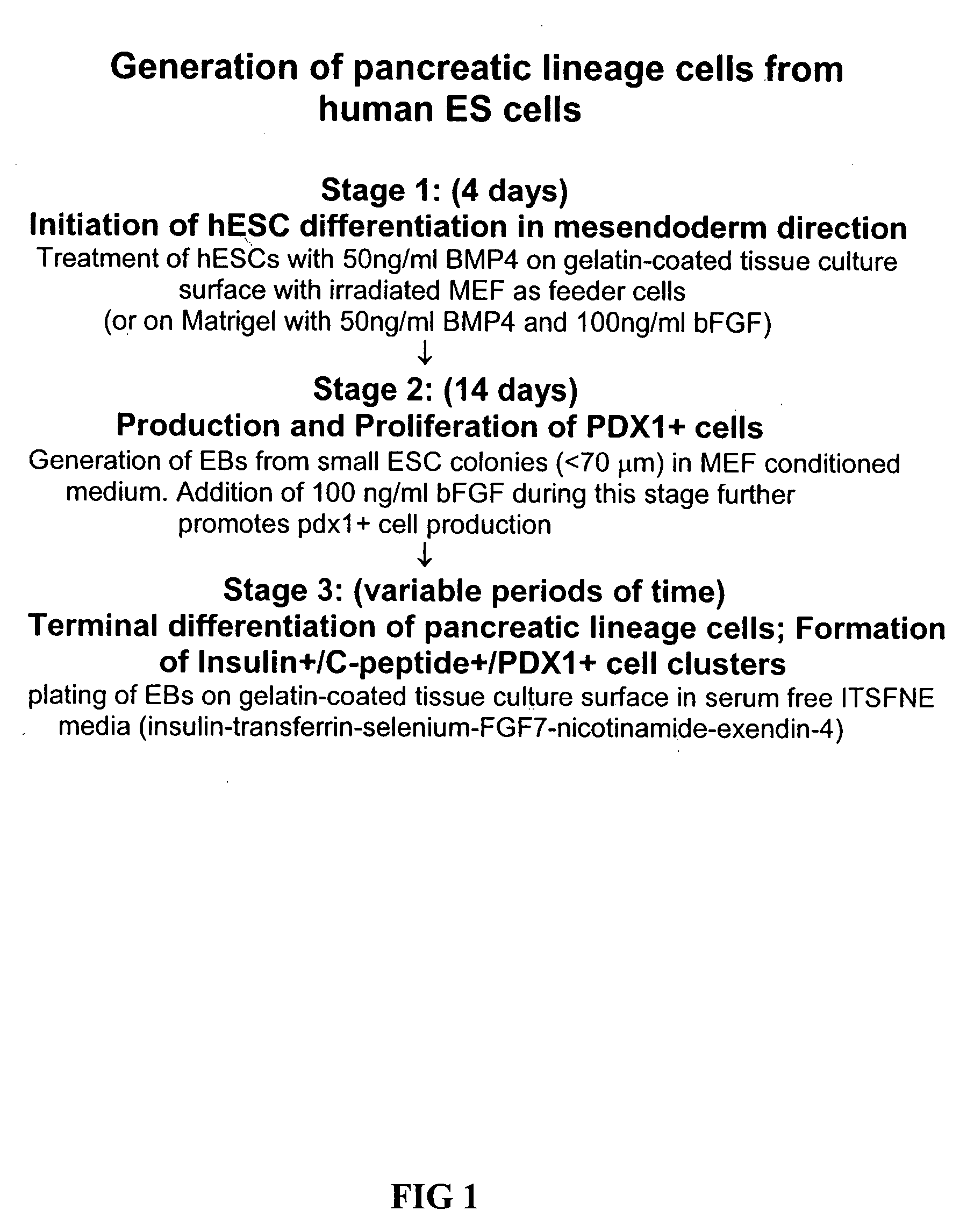
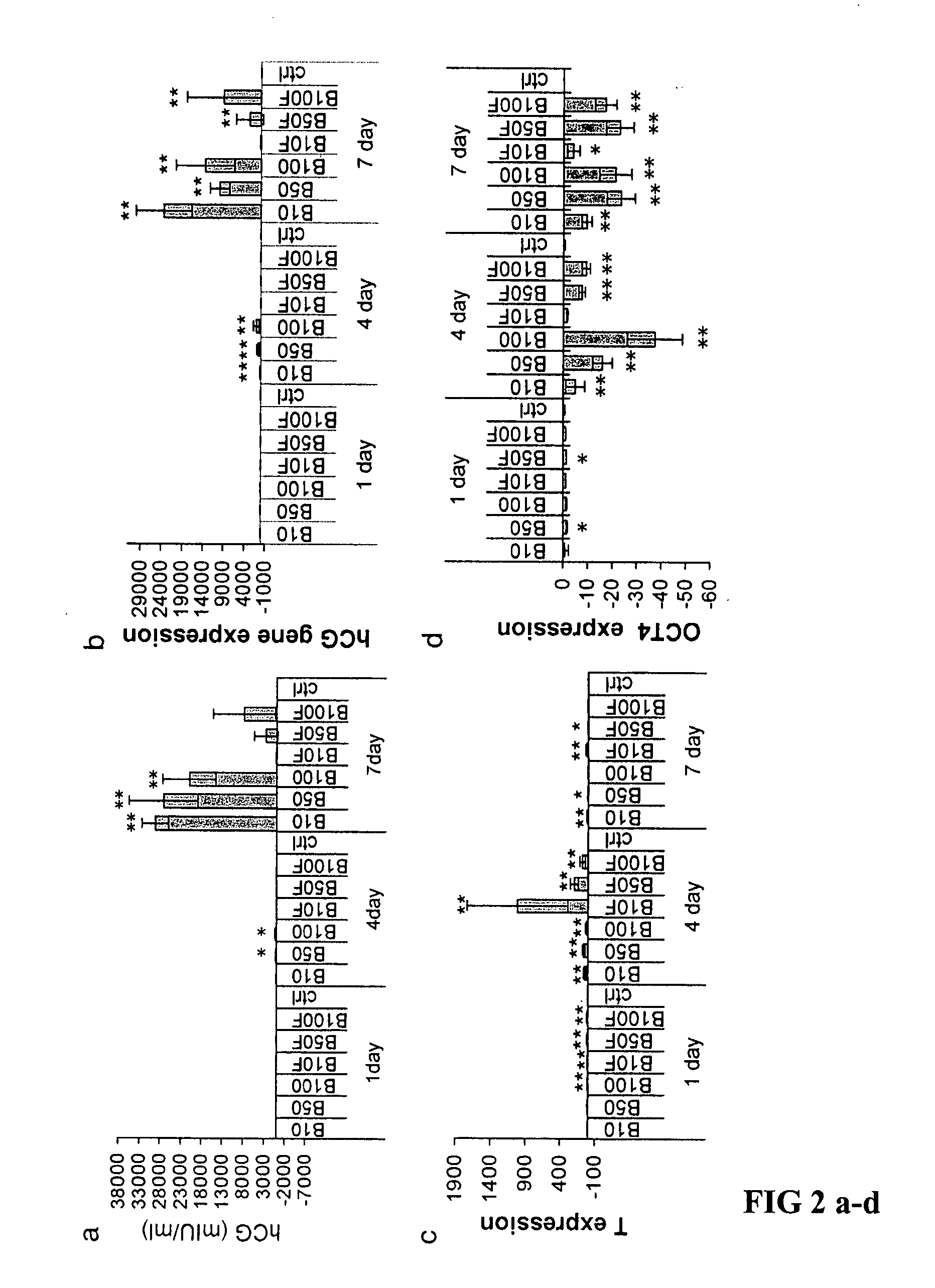
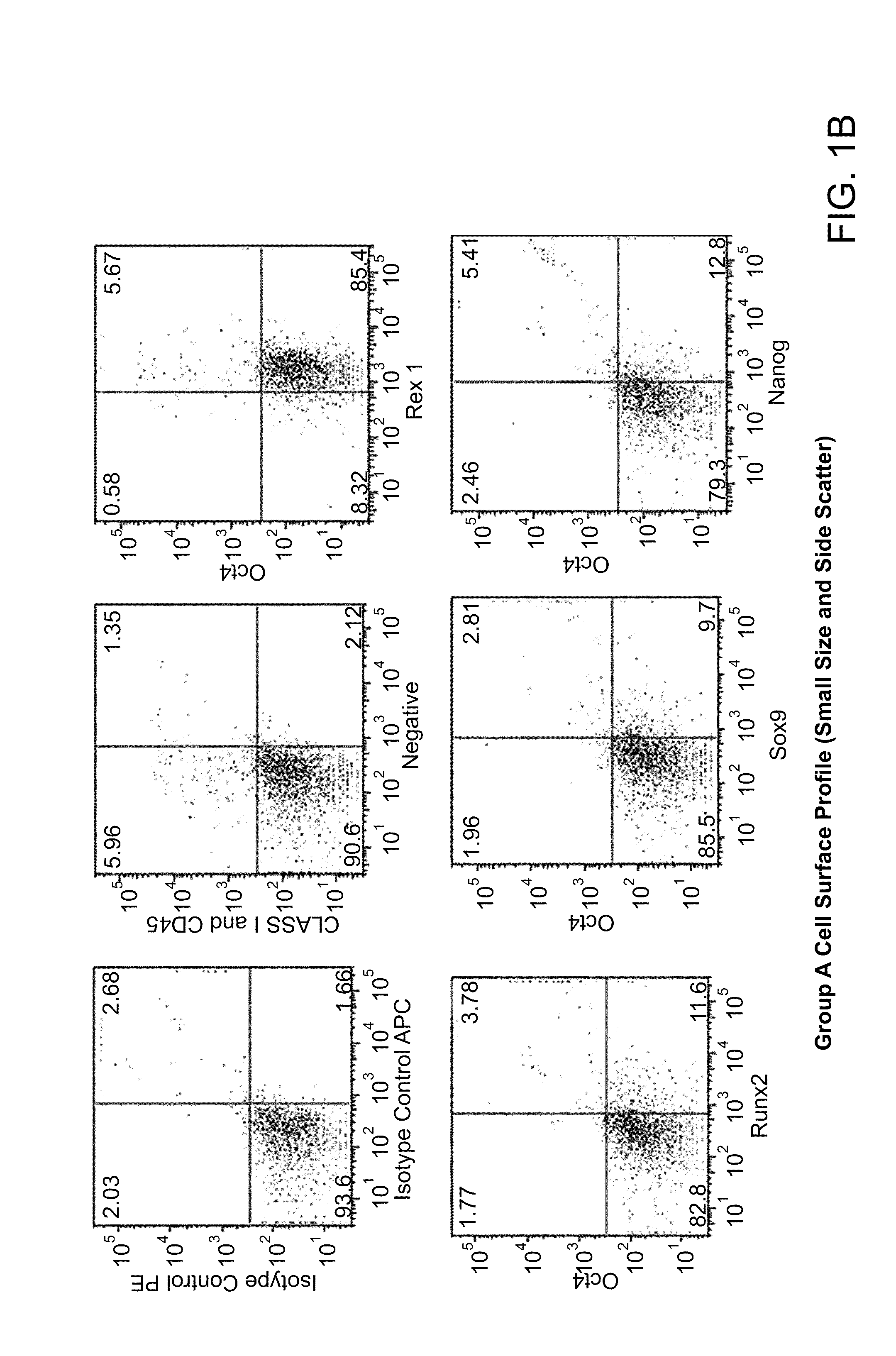
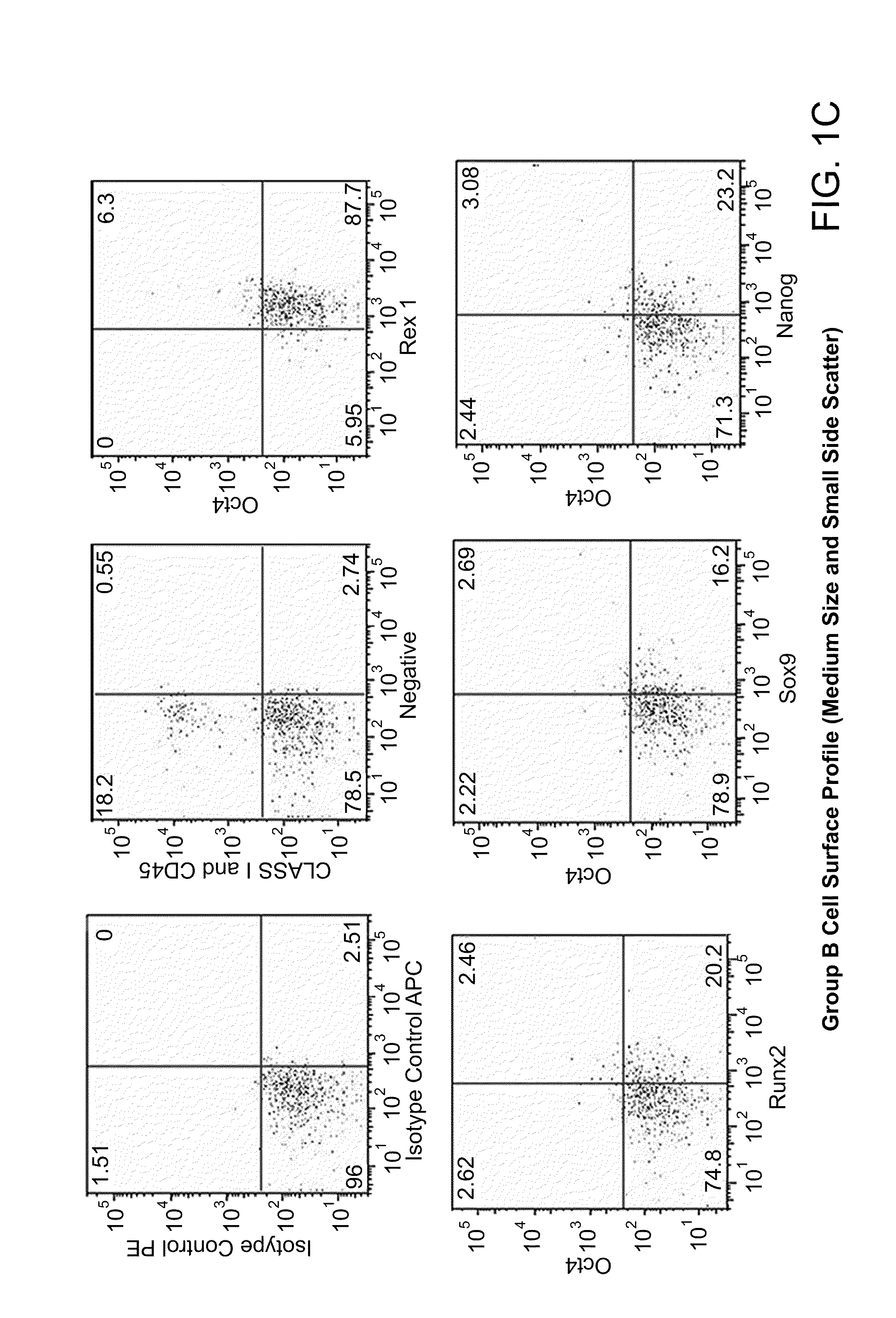
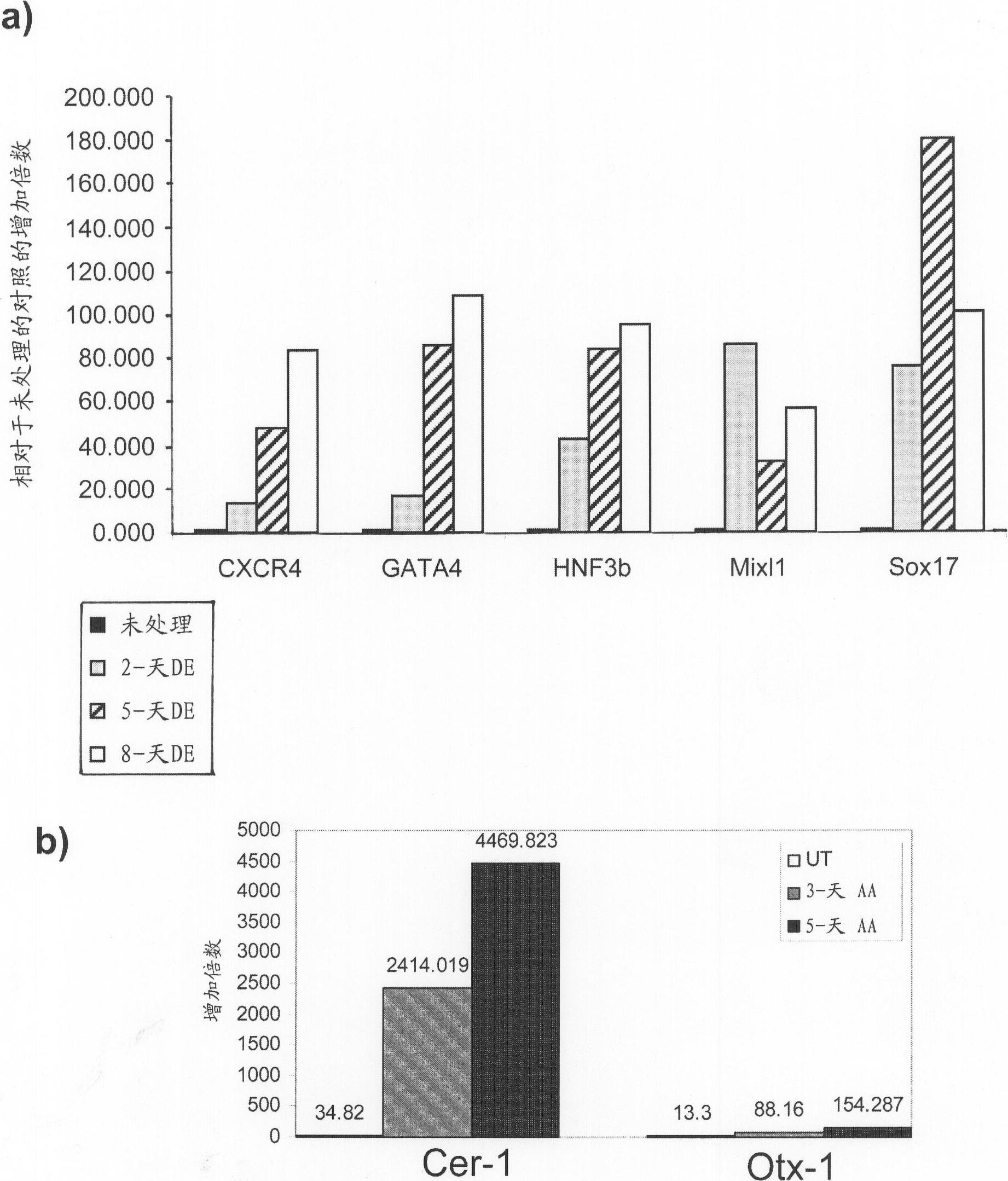
Differentiation of human embryonic stem cells
ActiveUS20070254359A1Inhibits Notch signalingPancreatic cellsArtificial cell constructsGerm layerPluripotential stem cell
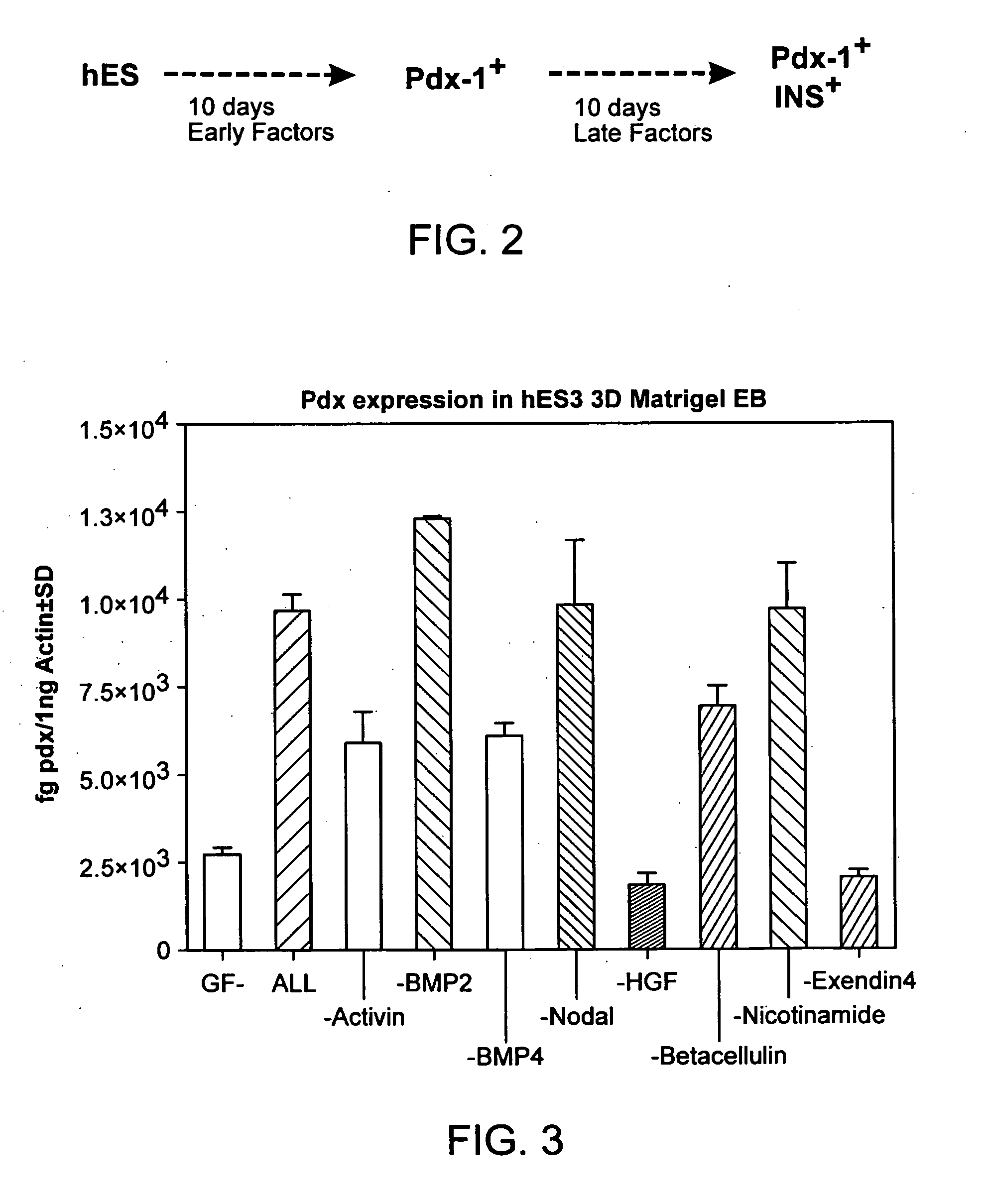
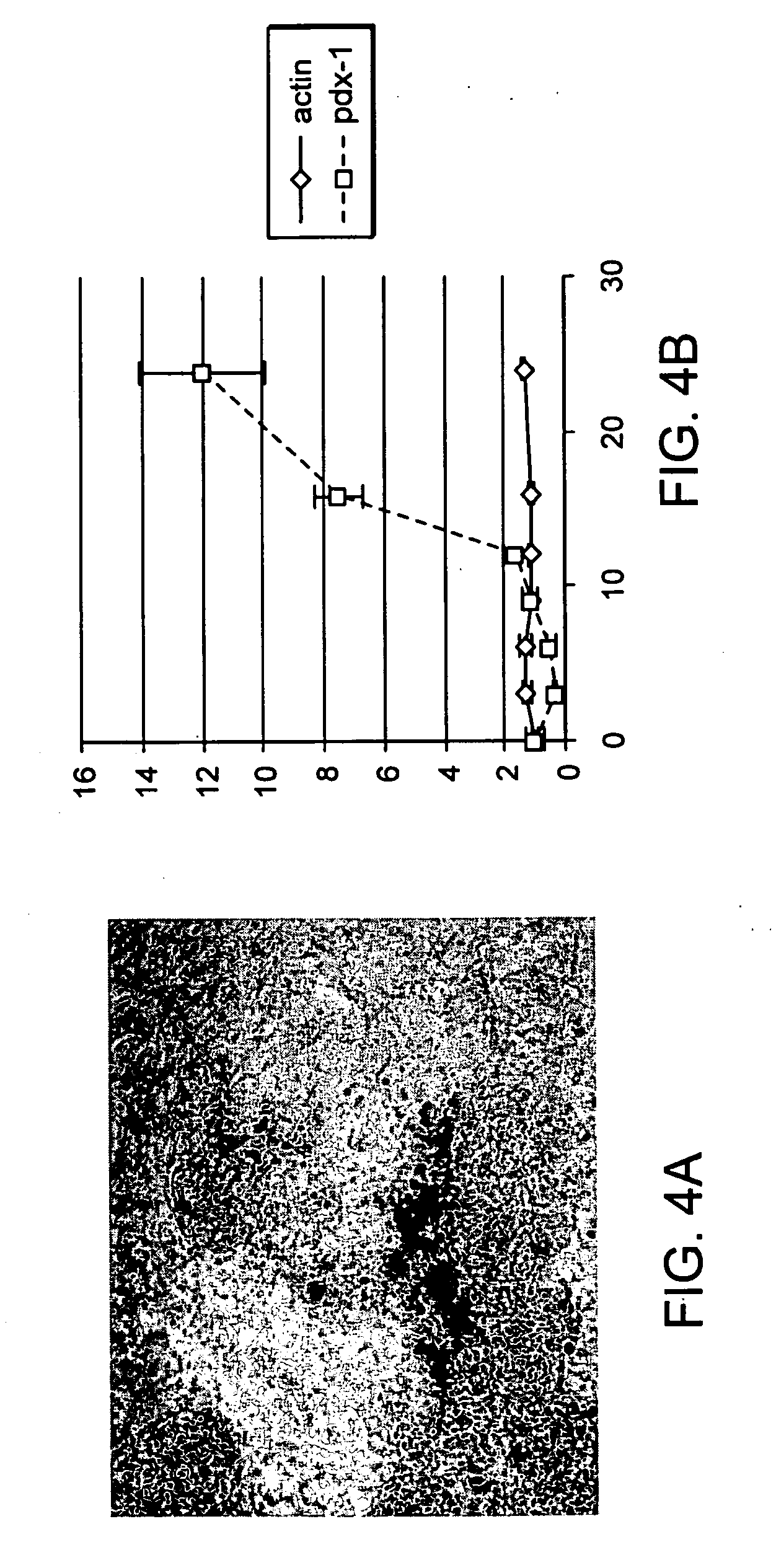
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
Pluripotent stem cells derived without the use of embryos or fetal tissue
InactiveUS20030113910A1New breed animal cellsArtificial cell constructsPluripotential stem cellGerm layer
Owner:STEMA
Method of differentiating stem cells into cells of the endoderm and pancreatic lineage
InactiveUS20070259423A1Promote differentiationMass productionPancreatic cellsCulture processPluripotential stem cellGerm layer
Methods are described to more efficiently produce cells of the endoderm and pancreatic lineage from mammalian pluripotent stem cells. These methods provide a simple, reproducible culture protocol using defined media components to enable consistent, large-scale production of pancreatic cell types for research or therapeutic uses.
Owner:WISCONSIN ALUMNI RES FOUND
Directed differentiation of embryonic stem cells and uses thereof
The present invention provides methods for the directed differentiation of embryonic stem cells along the endodermal lineage, especially the pancreatic lineage.
Owner:ES CELL INT
Differentiation of Human Embryonic Stem Cells
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
Ex vivo progenitor and stem cell expansion for use in the treatment of disease of endodermally-derived organs
Methods of ex-vivo expansion of endodermally-derived and non-endodermally-derived progenitor and stem cells, expanded populations of renewable progenitor and stem cells and to their uses in therapeutic applications such as the production of endocrine hormones and the prevention and treatment of liver and pancreatic disease.
Owner:GAMIDA CELL
Pancreatic and liver endoderm cells and tissue by differentiation of definitive endoderm cells obtained from human embryonic stems
The invention relates to methods that allow for the efficient differentiation to form pancreatic endoderm cells from pluripotent stem cells such as human embryonic stem cells and definitive endoderm cells. The invention is directly applicable to the ultimate generation of pancreatic beta cells that could be used as part of a therapy to treat or even cure diabetes. Additionally, the present invention may be used to generate liver endoderm cells from human embryonic stem cells and definite endoderm cells as well. This invention relates to a method for generating definitive endoderm and pancreatic endoderm cells from stem cells, preferably human embryonic stem cells using defined media in the absence of feeder cells. A simply two step procedure to provide pancreatic endoderm cells from embryonic stem cells represents further embodiments of the present invention.
Owner:UNIV OF GEORGIA RES FOUND INC
Multipotent stem cells derived from placenta tissue and cellular therapeutic agents comprising the same
InactiveUS20070243172A1Negative immunological responseBiocideArtificial cell constructsGerm layerDisease
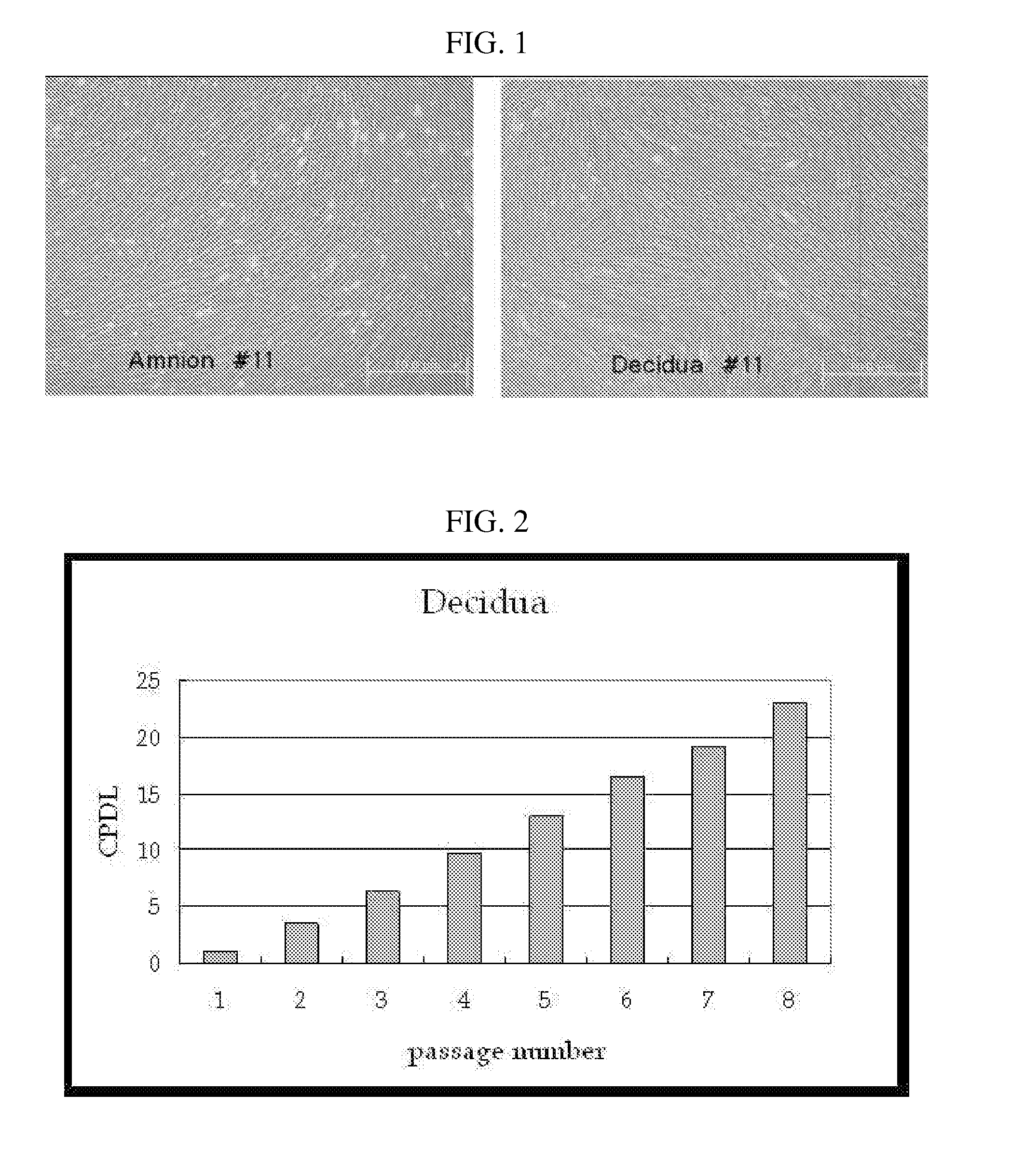
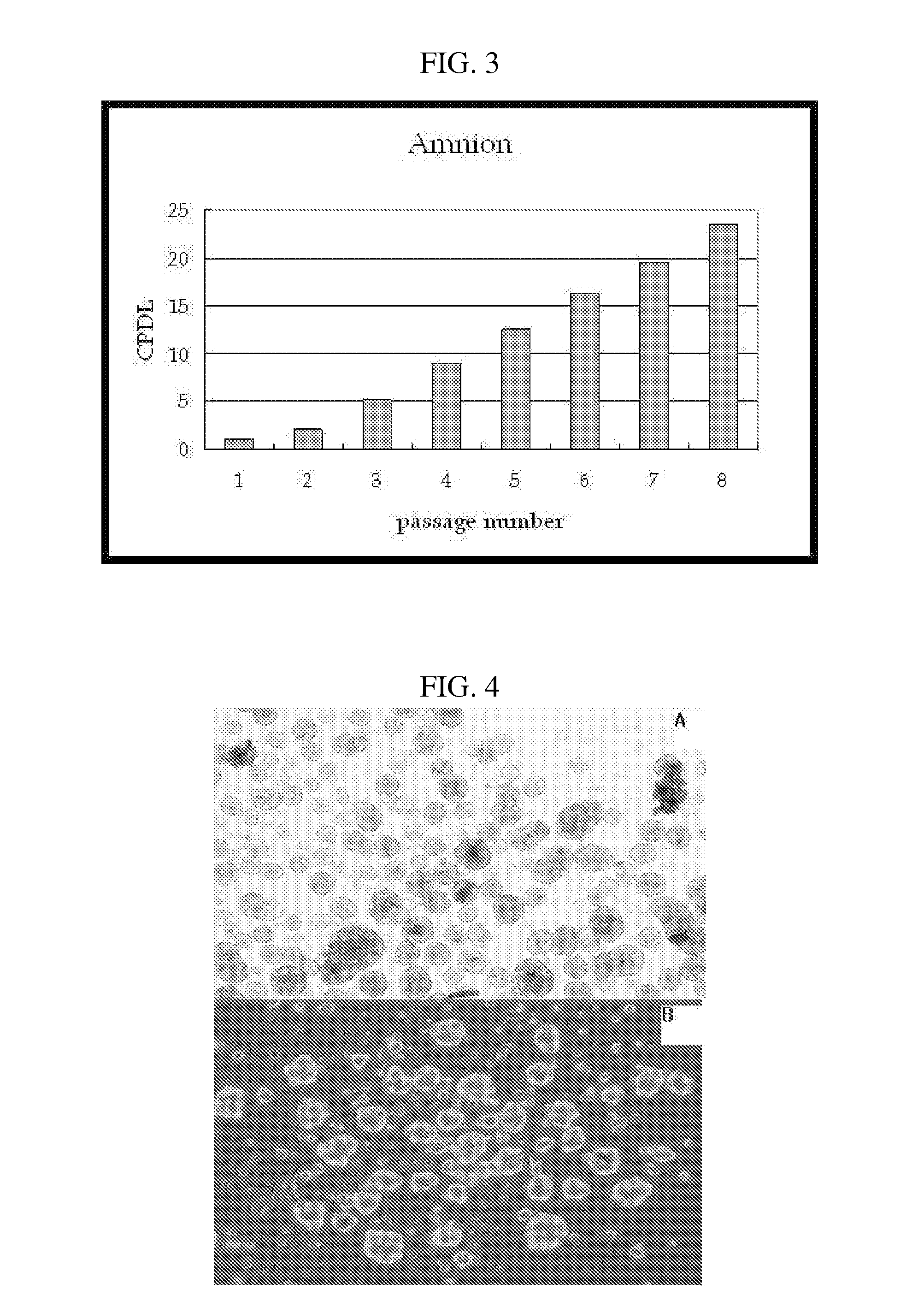
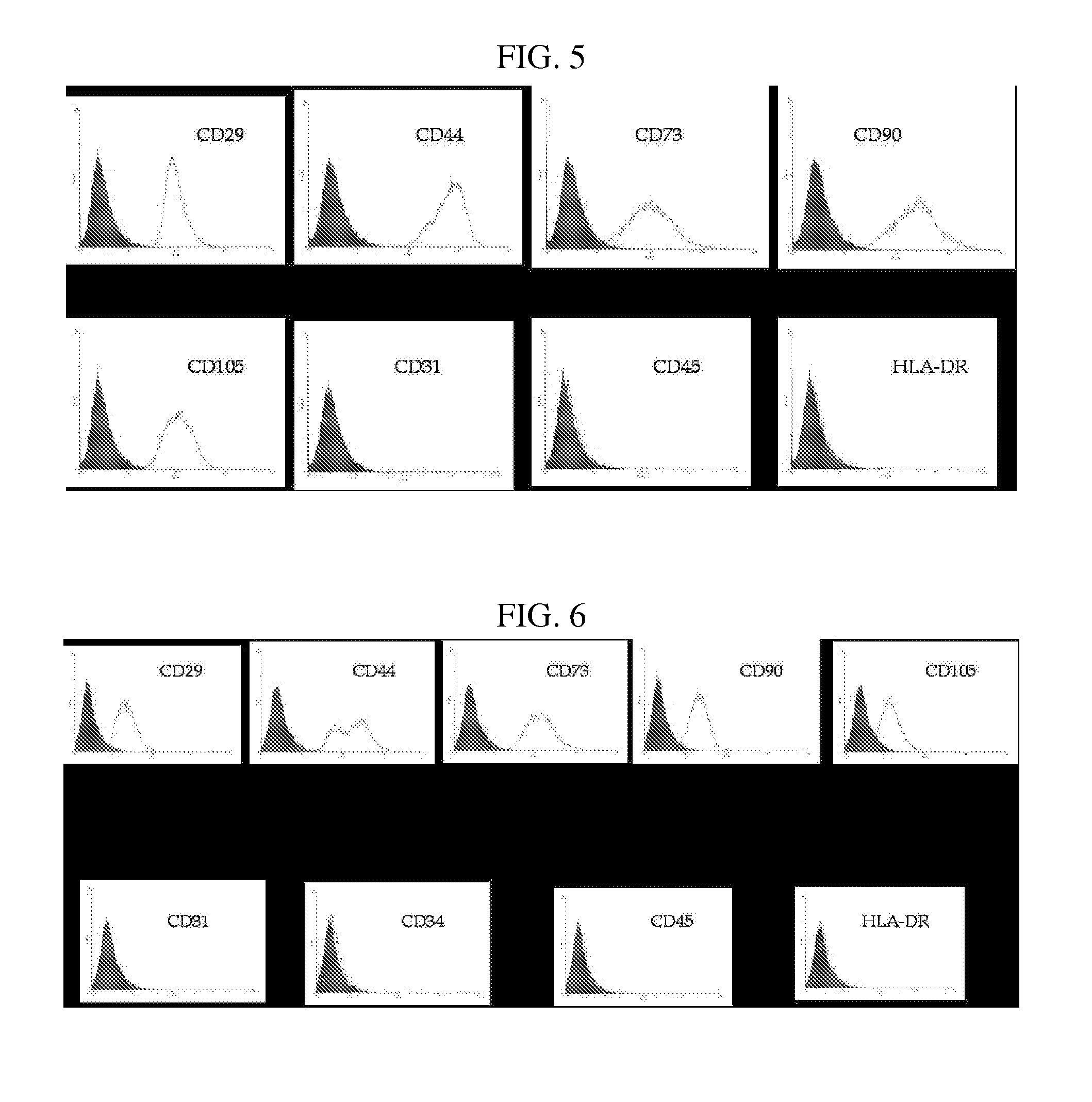
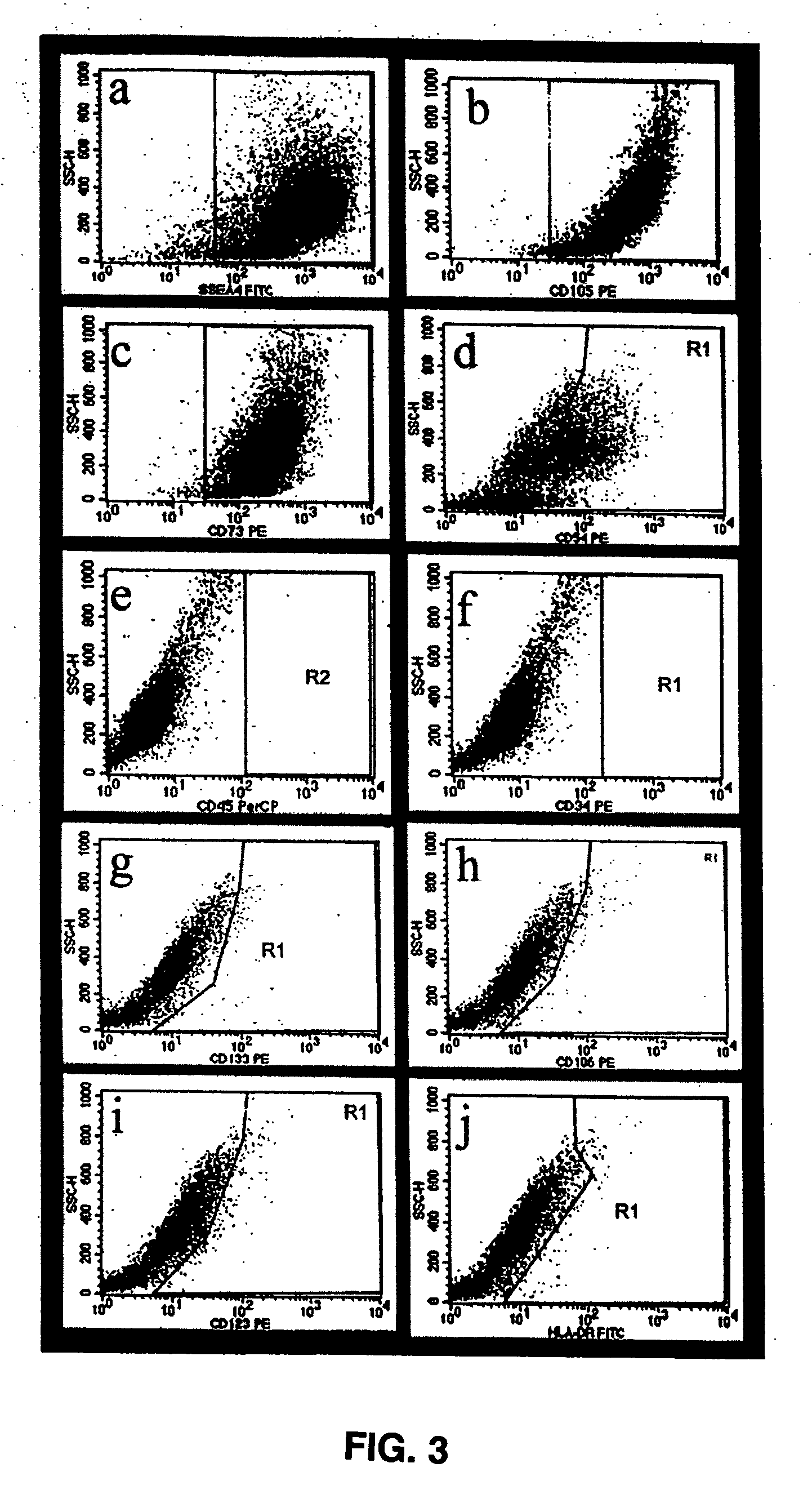
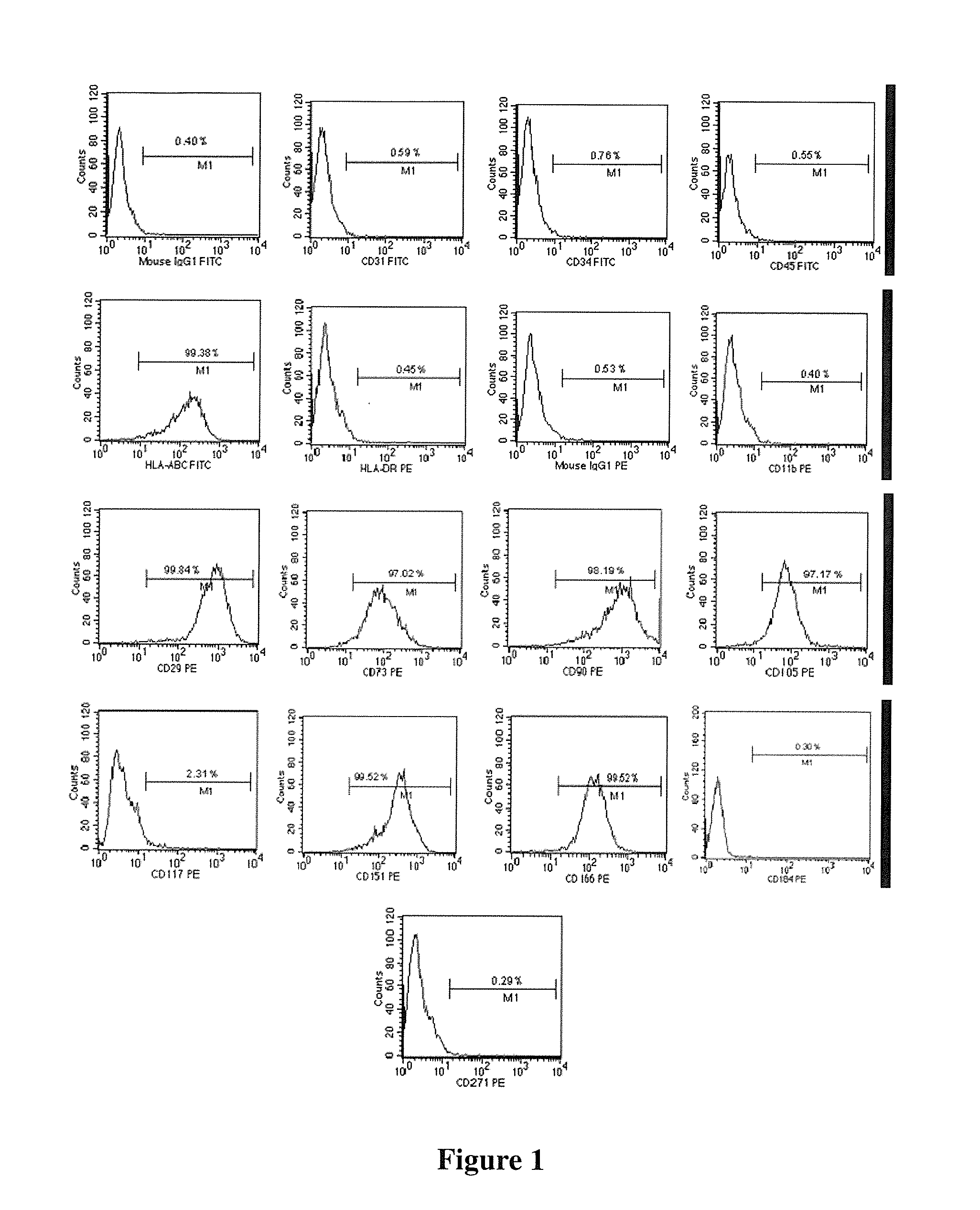
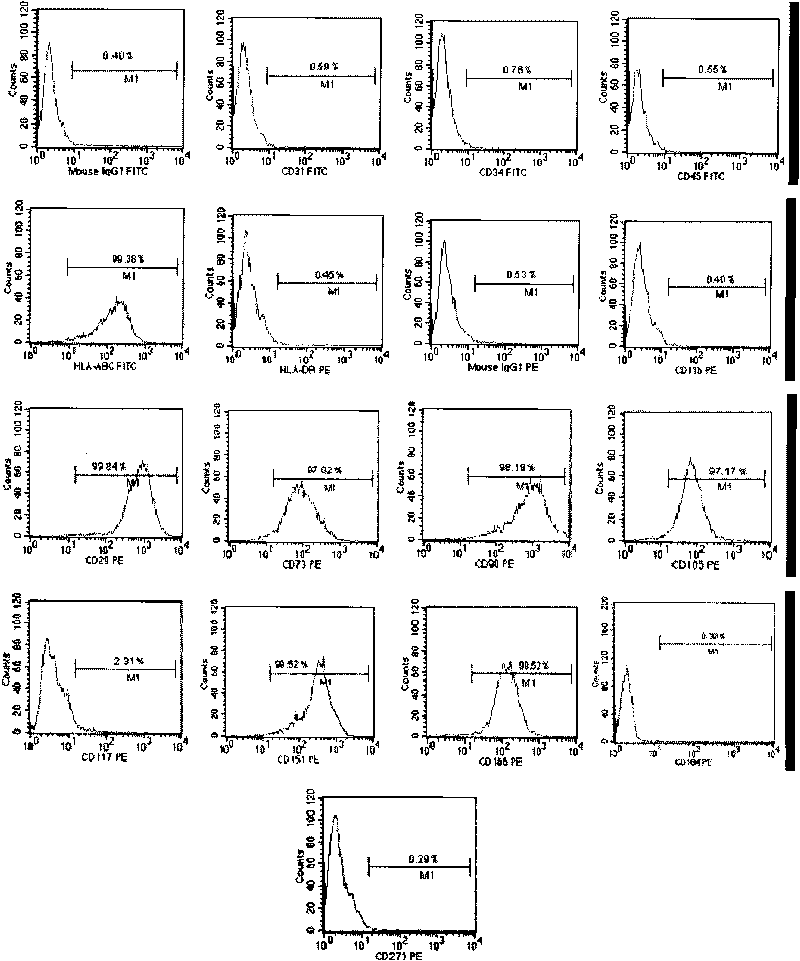
The present invention relates to placenta tissue-derived multipotent stem cells and cell therapeutic agents containing the same. More specifically, to a method for producing placenta stem cells having the following characteristics, the method comprising culturing amnion, chorion, decidua or placenta tissue in a medium containing collagenase and bFGF and collecting the cultured cells: (a) showing a positive immunological response to CD29, CD44, CD73, CD90 and CD105, and showing a negative immunological response to CD31, CD34, CD45 and HLA-DR; (b) showing a positive immunological response to Oct4 and SSEA4; (c) growing attached to plastic, showing a round-shaped or spindle-shaped morphology, and forming spheres in an SFM medium so as to be able to be maintained in an undifferentiated state for a long period of time; and (d) having the ability to differentiate into mesoderm-, endoderm- and ectoderm-derived cells. Also the present invention relates to placenta stem cells obtained using the production method. The inventive multipotent stem cells have the ability to differentiate into muscle cells, vascular endothelial cells, osteogenic cells, nerve cells, satellite cells, fat cells, cartilage-forming cells, osteogenic cells, or insuline-secreting pancreatic β-cells, and thus are effective for the treatment of muscular diseases, osteoporosis, osteoarthritis, nervous diseases, diabetes and the like, and are useful for the formation of breast tissue.
Owner:RNL BIO
Induction of pluripotent stem cells into mesodermal lineages
InactiveUS20080038820A1Promotes commitment and survivalGenetic material ingredientsSkeletal/connective tissue cellsProgenitorGerm layer
The present invention provides a method of inducing mesoderm derived cells from pluripotent stem cells. In contrast to methods known in the art that are often designed to replicate in vivo events of mesoderm induction, the present invention provides a unique, yet simple, method whereby pluripotent stem cells are mesodermally primed in the presence of factors that concomitantly inhibit the spontaneous differentiation of endoderm and ectoderm during expansion and suspension steps. Exposure and / or adherence of primed aggregates to a extracellular matrix that promotes the commitment and survival of induced mesoderm progenitors, followed by exposure to various mesoderm associated factors, allows for the subsequent induction of such cells into terminally differentiated lineages, such as cardiomyocytes. End products of this induction system will ultimately provide an unlimited source of mesoderm-derived cell types for therapeutic and pharmacological purposes.
Owner:RUDY REIL DIANE ELIZABETH DR +1
Pluripotent embryonic-like stem cells derived from corneal limbus, methods of isolation and uses thereof
InactiveUS20060216821A1Increase telomerase activityCulture processNervous system cellsGerm layerDisease
The present disclosure describes mammalian pluripotent embryonic-like stem cells (ELSCs) isolated from corneal limbal tissue, a non-embryonic tissue. The ELSCs of the present disclosure are capable of proliferating in an in vitro culture, maintain the potential to differentiate into cells of endoderm, mesoderm, and ectoderm lineage in culture, and are capable of forming embryoid-like bodies when placed in suspension culture. Thus, these cells possess multi-lineage differentiation potential and self-renewing capability. ELSCs may be a promising therapeutic tool, and may provide new therapeutic alternatives for various diseases, conditions, and injuries.
Owner:RELIANCE LIFE SCI PVT
Pluripotent stem cells, method for preparation thereof and uses thereof
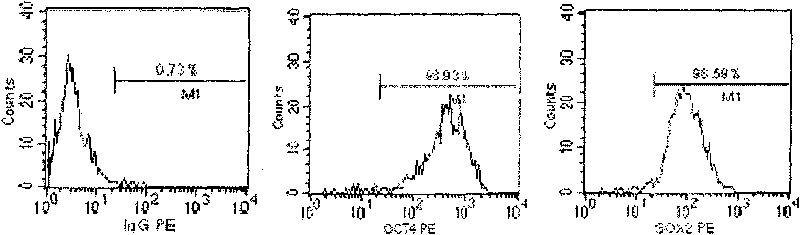
Human pluripotent stem cells which are isolated from cut human umbilical cord or placenta and characteristic of cell surface marker CD151+, OCT4+ and CD184−, can adhere to tissue culture plastic and have the potential to differentiate into three germ layers: endoderm, mesoderm and ectoderm. Methods of isolating, purifying and culturally expanding of a population of human pluripotent stem cells and uses for treating diseases caused by cell damage or cell aging, and as a kind of carrier cells in gene therapy are provided.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Non-Embryonic Totipotent Blastomere-Like Stem Cells And Methods Therefor
Non-embryonic blastomere-like totipotent stem cells are disclosed. Most preferably, such cells are obtained from various tissues of postnatal mammals (e.g., using tissue biopsied from the mammal), are smaller than 1 μm, have normal karyotype, and do not spontaneously differentiate in serum-free medium without differentiation inhibitors. These non-embryonic blastomere-like totipotent stem cells typically express CD66e, CEA-CAM-1 and telomerase, but do not typically express CD10, SSEA-1, SSEA-3, and SSEA-4. Such blastomere-like totipotent cells can be differentiated into ectodermal, mesodermal, or endodermal tissues, including placental tissues and germ cells. Moreover, when implanted into a mammal, such cells will not be teratogenic.
Owner:MORAGA BIOTECH CORP
Homogeneous differentiation of hepatocyte-like cells from embryonic stem cells
InactiveUS20100143313A1Rapid and direct differentiationImprove survivalBiocideHepatocytesLiver morphologyBioartificial liver device
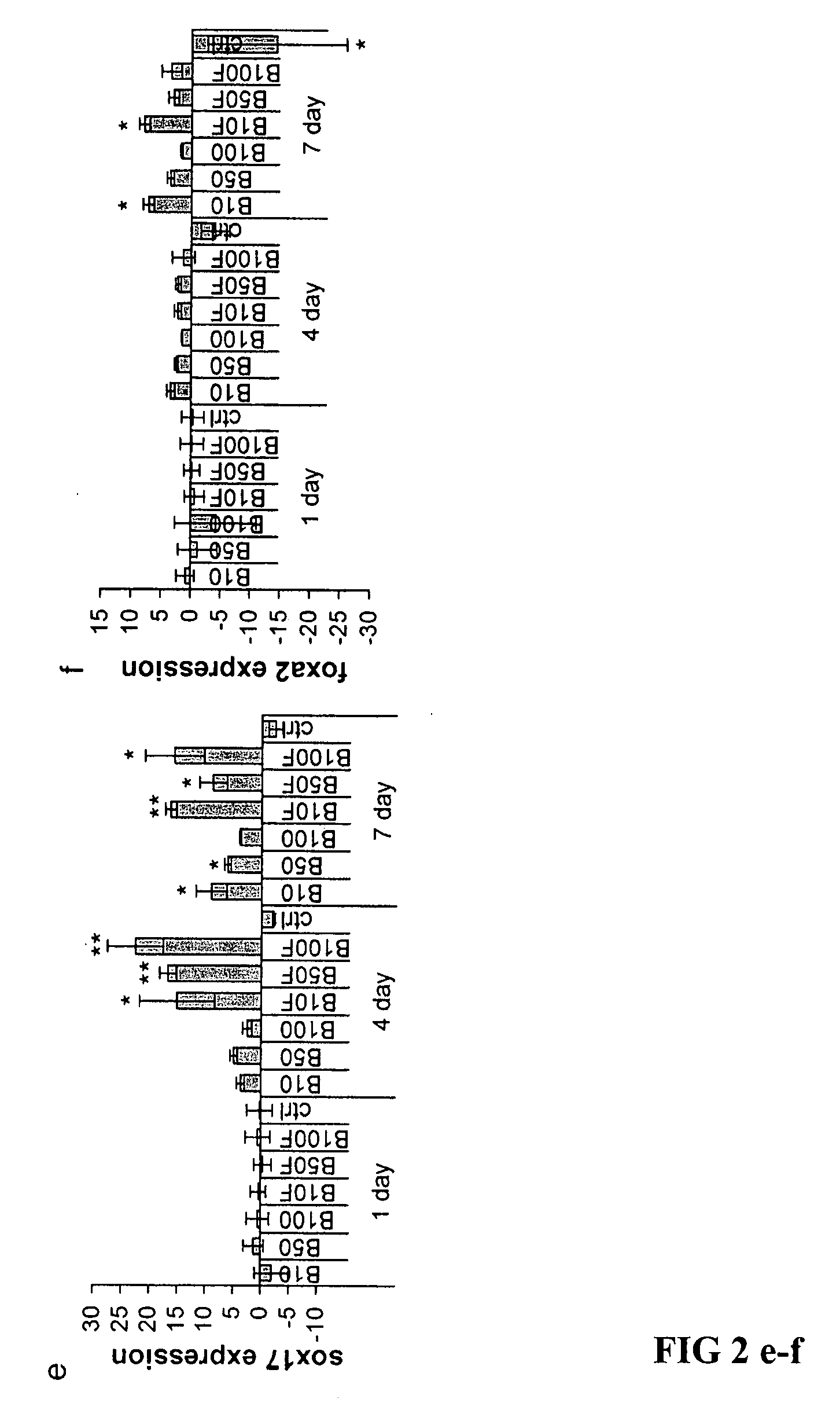
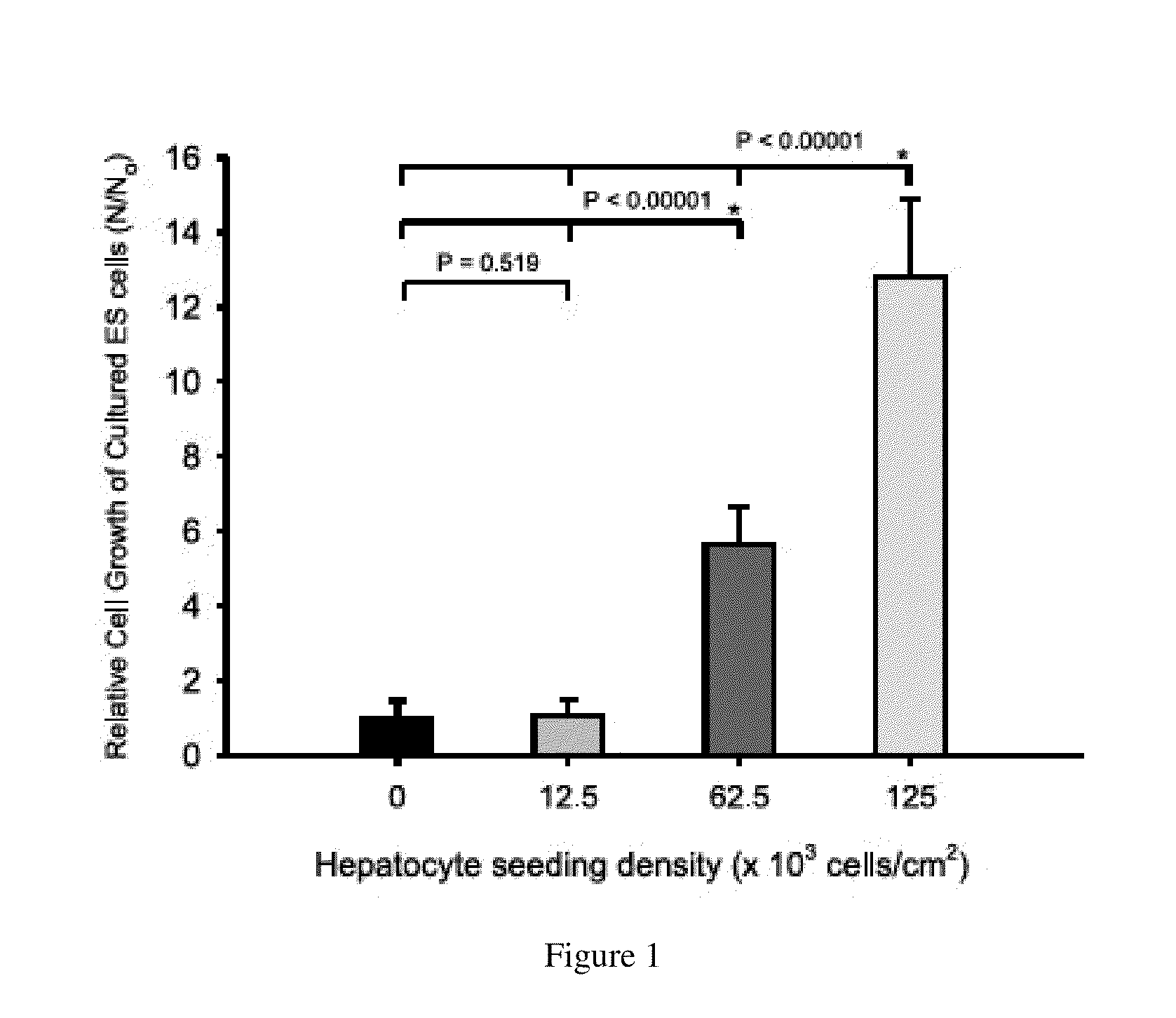
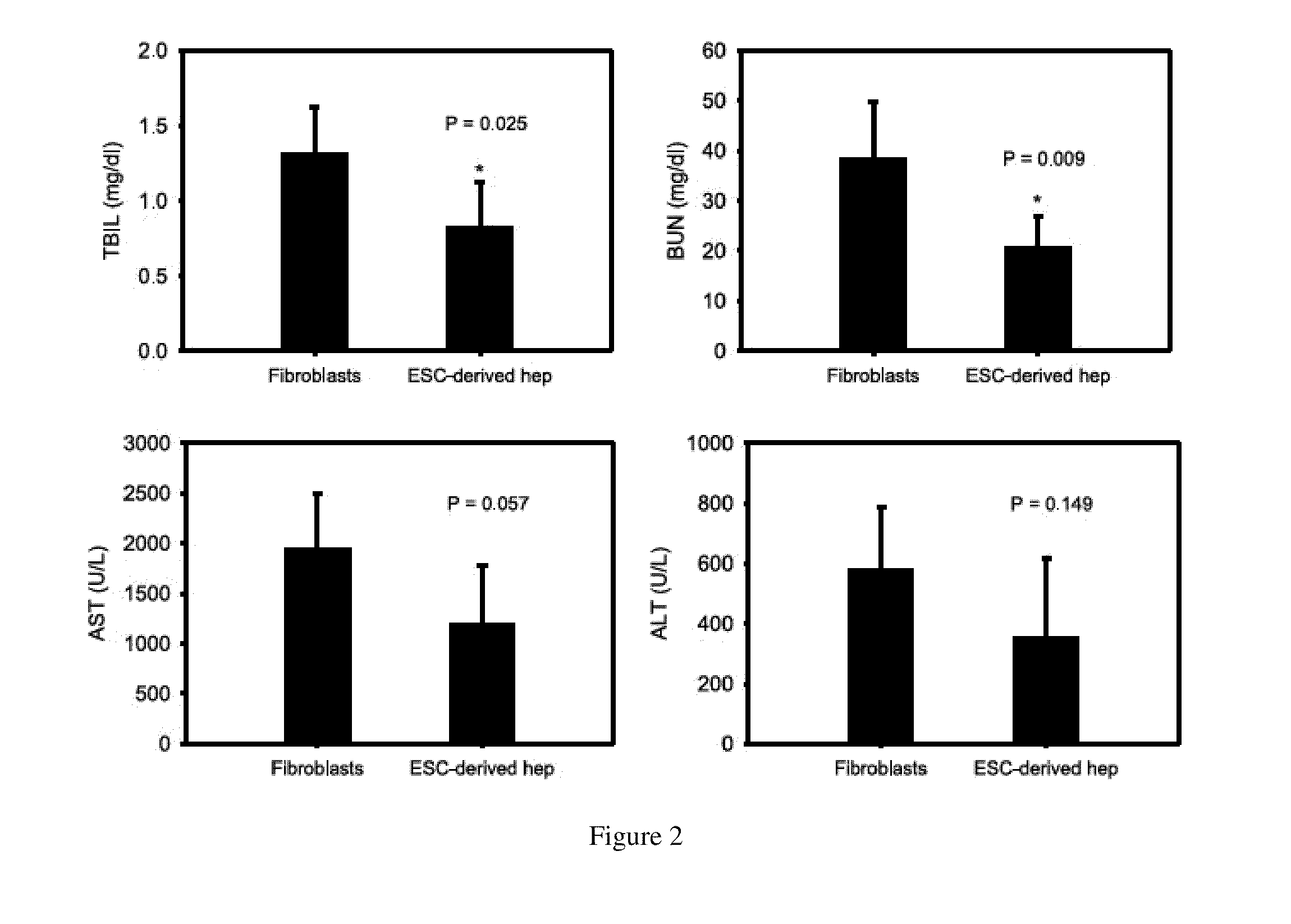
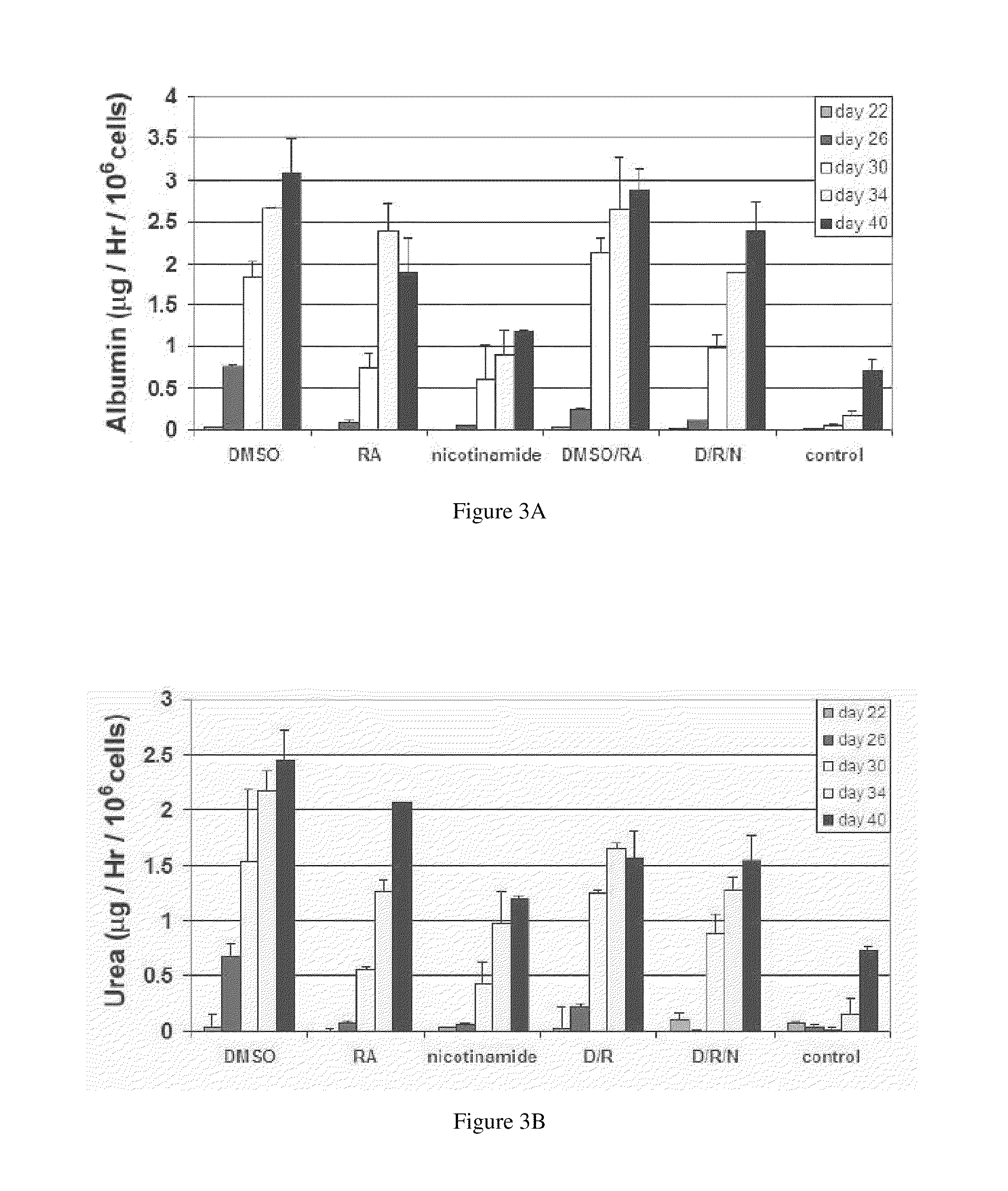
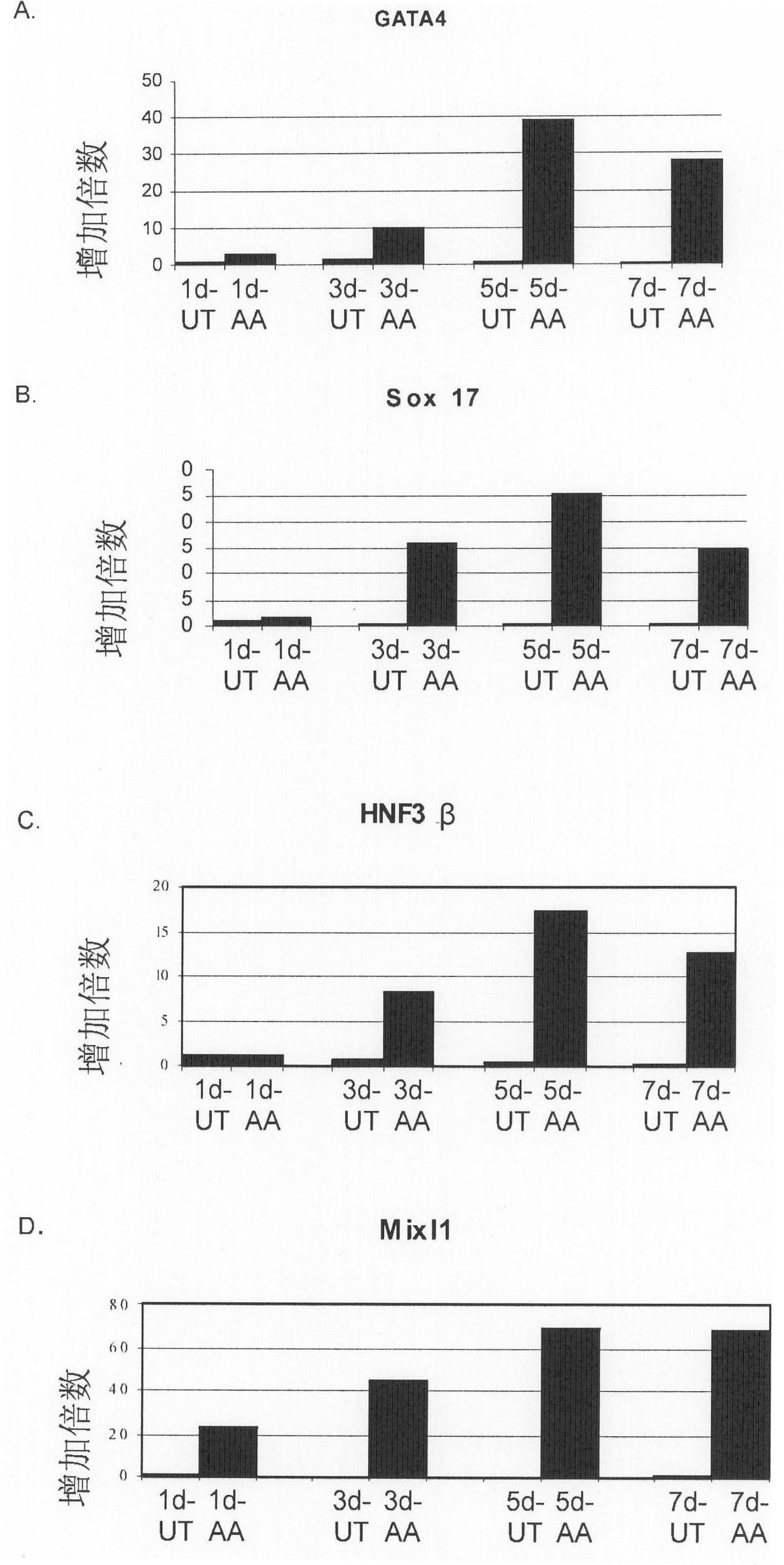
One of the major hurdles of cellular therapies for the treatment of liver failure is the low availability of functional human hepatocytes. Although embryonic stem (ES) cells represent a potential cell source for therapy, current methods for differentiation result in mixed cell populations or low yields of the cells of interest. The present invention provides for a rapid, direct differentiation method that yields a homogeneous population of endoderm-like cells with 95% purity. In one embodiment, mouse ES cells cultured on top of collagen-sandwiched hepatocytes differentiate and proliferate into a uniform and homogeneous cell population of endoderm-like cells. The endoderm-like cell population was positive for Foxa2, Sox17 and AFP, and could further differentiate into hepatocyte-like cells that demonstrate hepatic morphology, functionality, and gene and protein expression. Incorporating the hepatocyte-like cells into a bioartificial liver device to treat fulminant hepatic failure improved animal survival, thereby underscoring the therapeutic potential of these cells.
Owner:THE GENERAL HOSPITAL CORP
Methods of differentiating stem cells into one or more cell lineages
The present disclosure provides an understanding of the regulation of the developmental phases of stem cells and their induction into relevant cell lineages, such as primitive streak, endoderm, mesoderm, or subterrotories of endoderm's. In particular, the present disclosure provides methods, culture medium and kits for the maintenance and differentiation of stem cells into relevant cell lineages.
Owner:AGENCY FOR SCI TECH & RES
Sub totipotential stem cell and preparation method and application thereof
The invention discloses a method for preparing a population of?human pluripotent stem cells and the application thereof. The preparation of stem cells is characterized by comprising the following steps: CD151<+>, CD31<->, Sox<2+> pluripotent stem cells are separated and collected from human umbilical cord and or placenta tissues; the cells adhere to grow in a culture vessel under a predetermined condition and expand through passage 20 or above to be still stable in gene expression. The population of cells of this invention do not form teratoma after injection into animals. The human pluripotent stem cells highly express CD151, OCT4 and Sox-2 as specific markers of embryonic stem cells, as well as specific markers of epidermic cells, endothelial cells, thrombocytes, dendritic cells, while lack expression of CD31, CD34, CD45 and HLA-II. The pluripotent stem cells are also characterized as being able to adhere to tissue culture plastic and having the potential to differentiate into three germ layers: endoderm, mesoderm and ectoderm. These pluripotent stem cells are able to be used as carrier cells of gene therapy and for the treatment of diseases caused by cell damage or cell aging. The present invention provides a method of isolating, purifying and culturally expanding of a population of human pluripotent stem cell for preparing the high purity injection preparation. The preparation of stem cells has a good therapeutic effect on the treatment of diseases caused by cell damage or cell aging in animal and human clinical trials. The preparation also has no toxic side effect and no immune rejection.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Multipotent stem cells and uses thereof
ActiveUS20110070205A1Easily attainable embryonic-likePromote wound healingBiocideNervous disorderGerm layerMHC class I
The invention provides a quiescent stem cell having the capacity to differentiate into ectoderm, mesoderm and endoderm, and which does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 CD90, CD66A, CD66E, CXCR4, CD133 or an SSEA. The invention further provides a proliferative stem cell, which expresses genes including Oct-4, Nanog, Sox2, GDF3, P16INK4, BMI, Notch, HDAC4, TERT, Rex-1, TWIST, KLF-4 and Stella but does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105, CD90, CD66A, CD66E, CXCR4, CD133 or an SSEA. The cells of the invention can be isolated from adult mammals, have embryonic cell characteristics, and can form embryoid bodies. Methods for obtaining the stem cells, as well as methods of treating diseases and differentiated the stem cells, are also provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Multipotent stem cells and uses thereof
InactiveUS20100291042A1Easily attainable embryonic-likePromote wound healingBiocidePancreatic cellsDiseaseMHC class I
The invention provides a quiescent stem cell having the capacity to differentiate into ectoderm, mesoderm and endoderm, and which does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 and CD90. The invention further provides a proliferative stem cell, which expresses genes including Oct-4, Nanog, Sox2, GDF3, P16INK4, BMI, Notch, HDAC4, TERT, Rex-1 and TWIST but does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 and CD90. The cells of the invention can be isolated from adult mammals, have embryonic cell characteristics, and can form embryoid bodies. Methods for obtaining the stem cells, as well as methods of treating diseases and differentiated the stem cells, are also provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
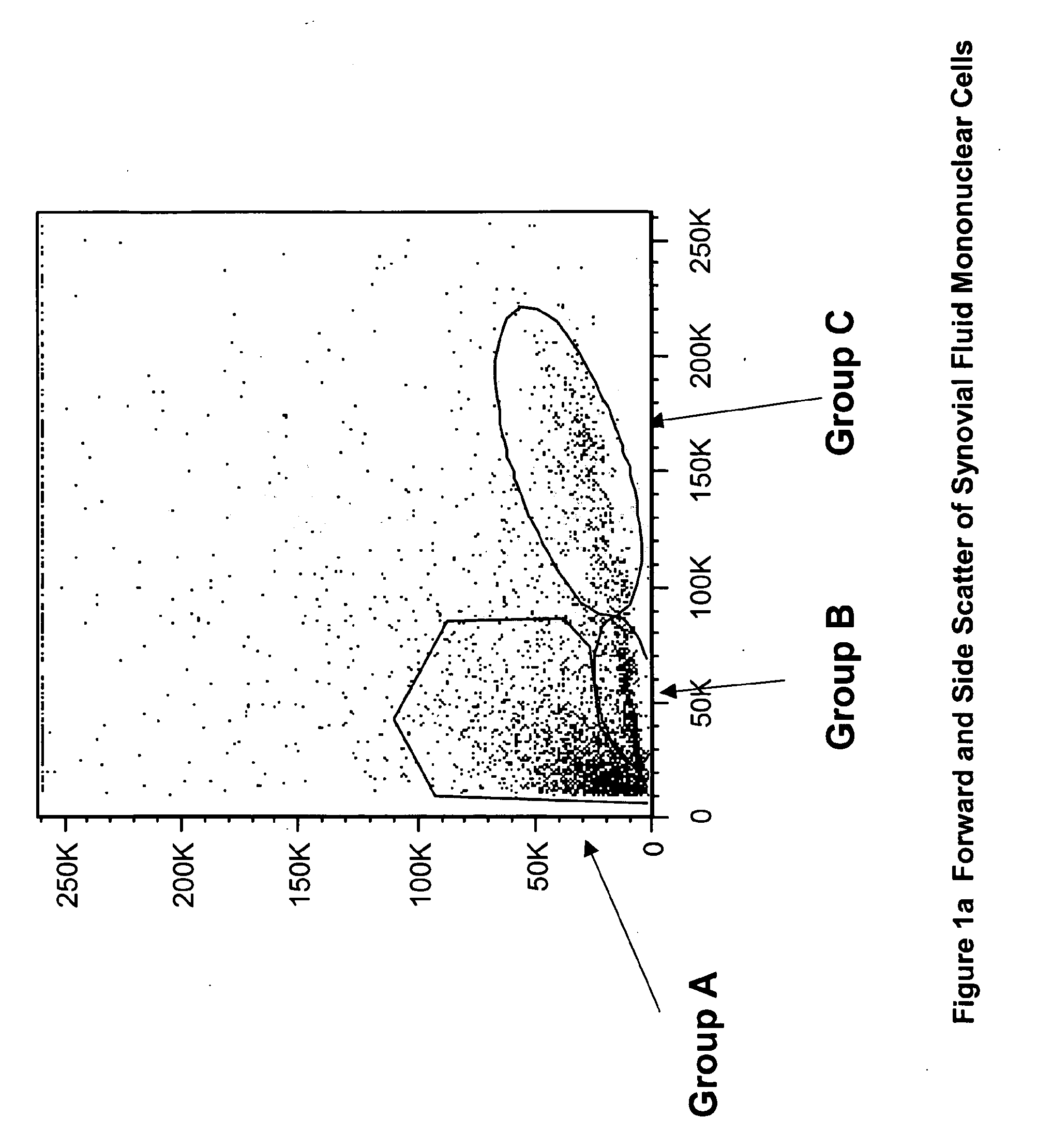
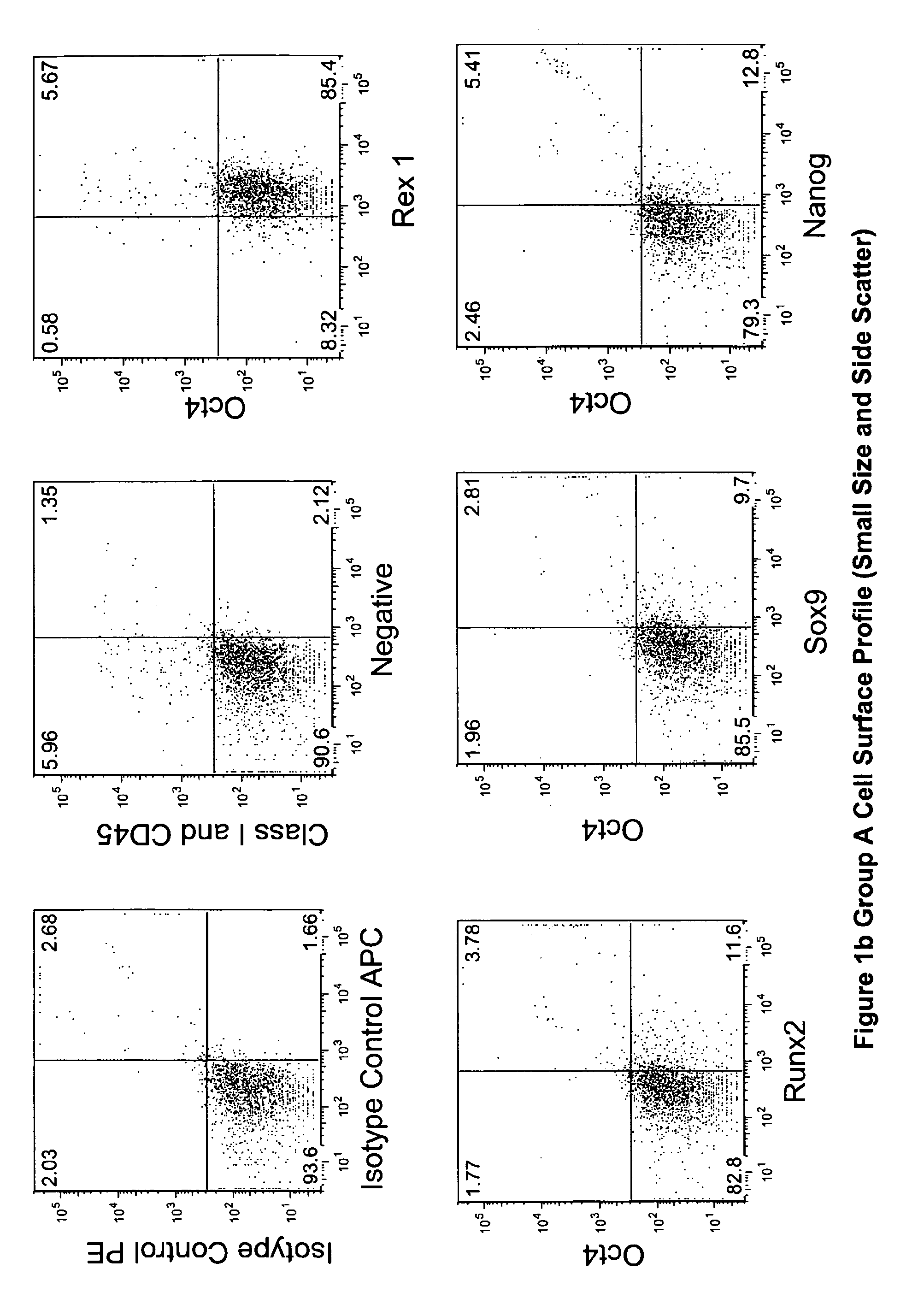
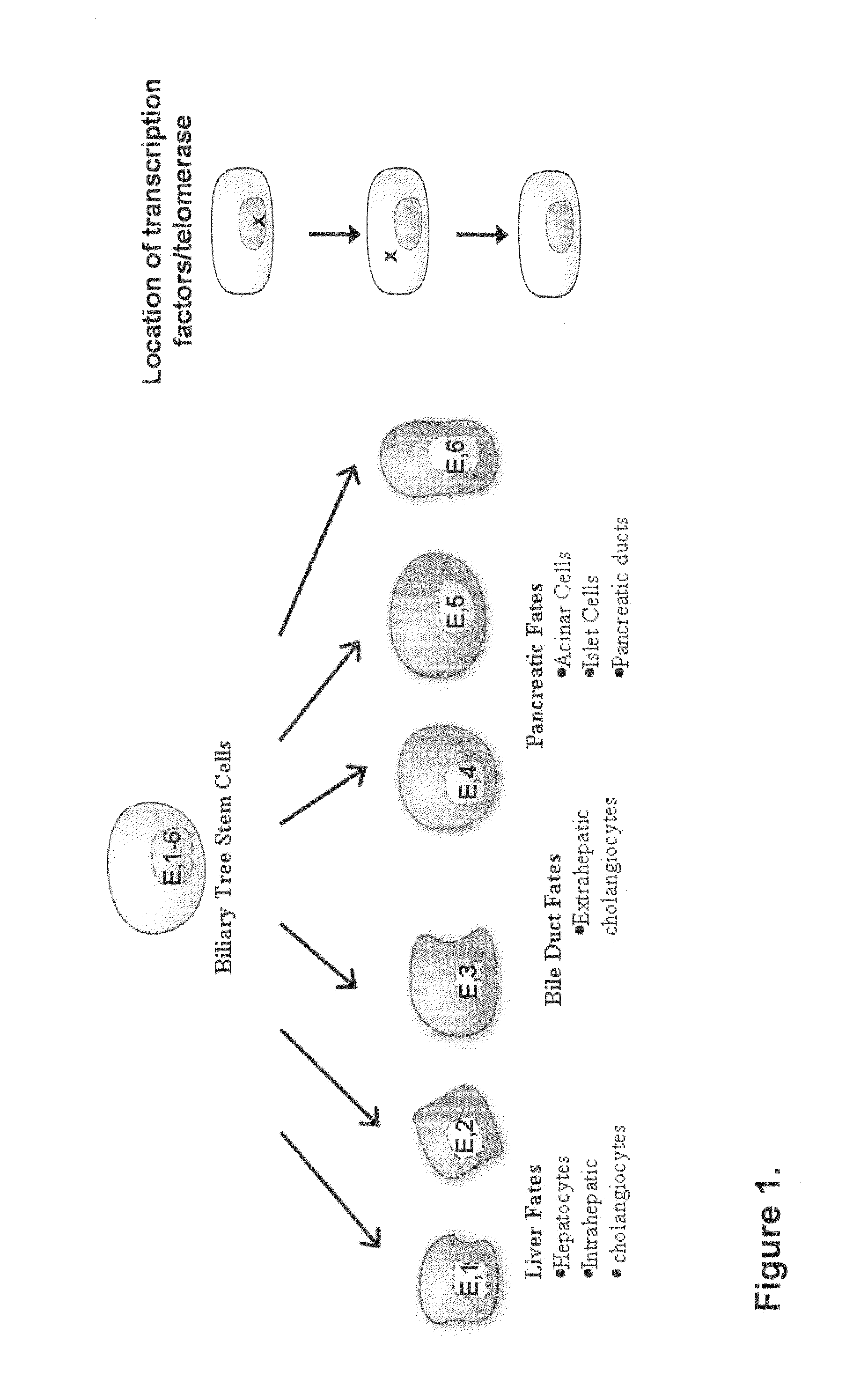
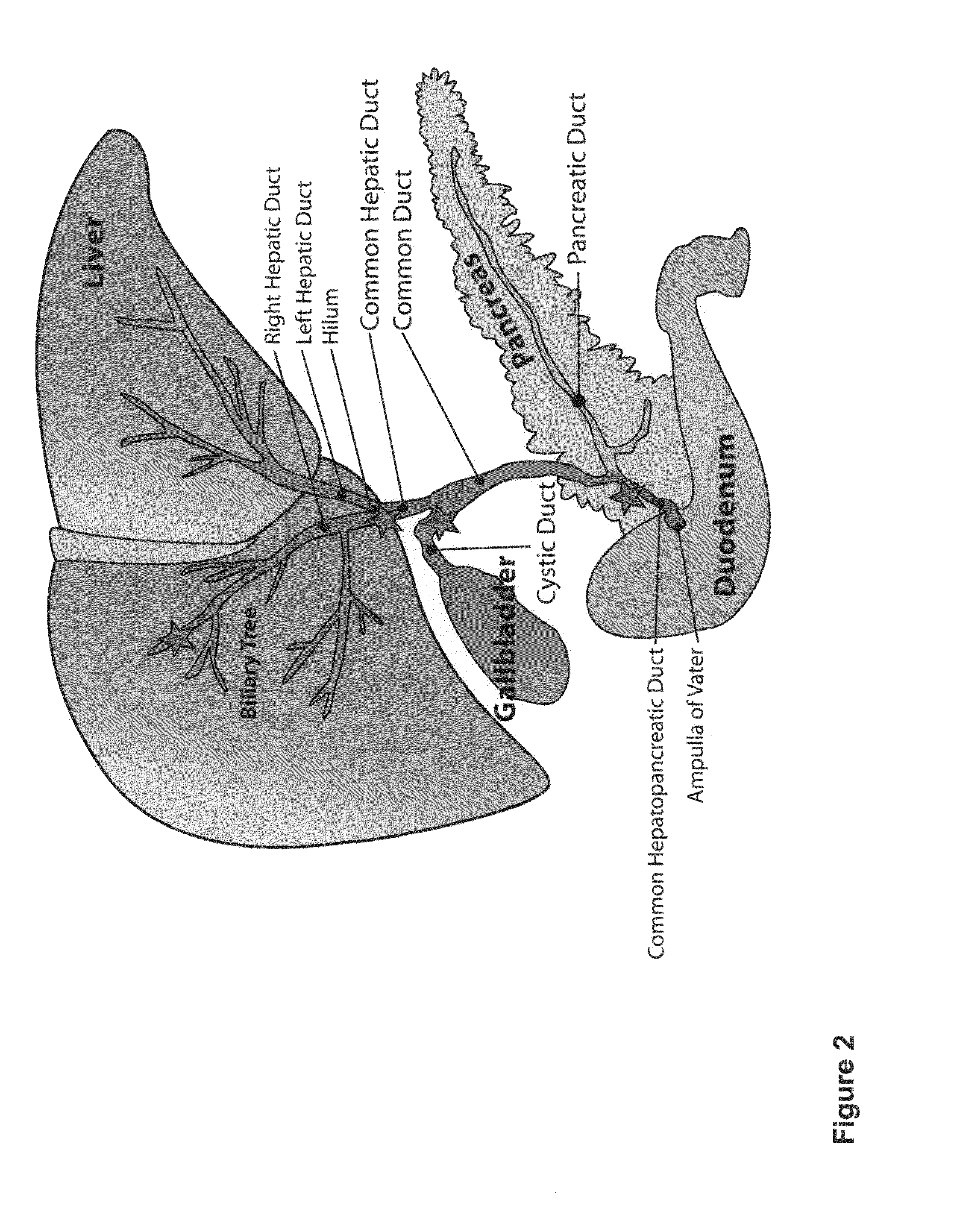
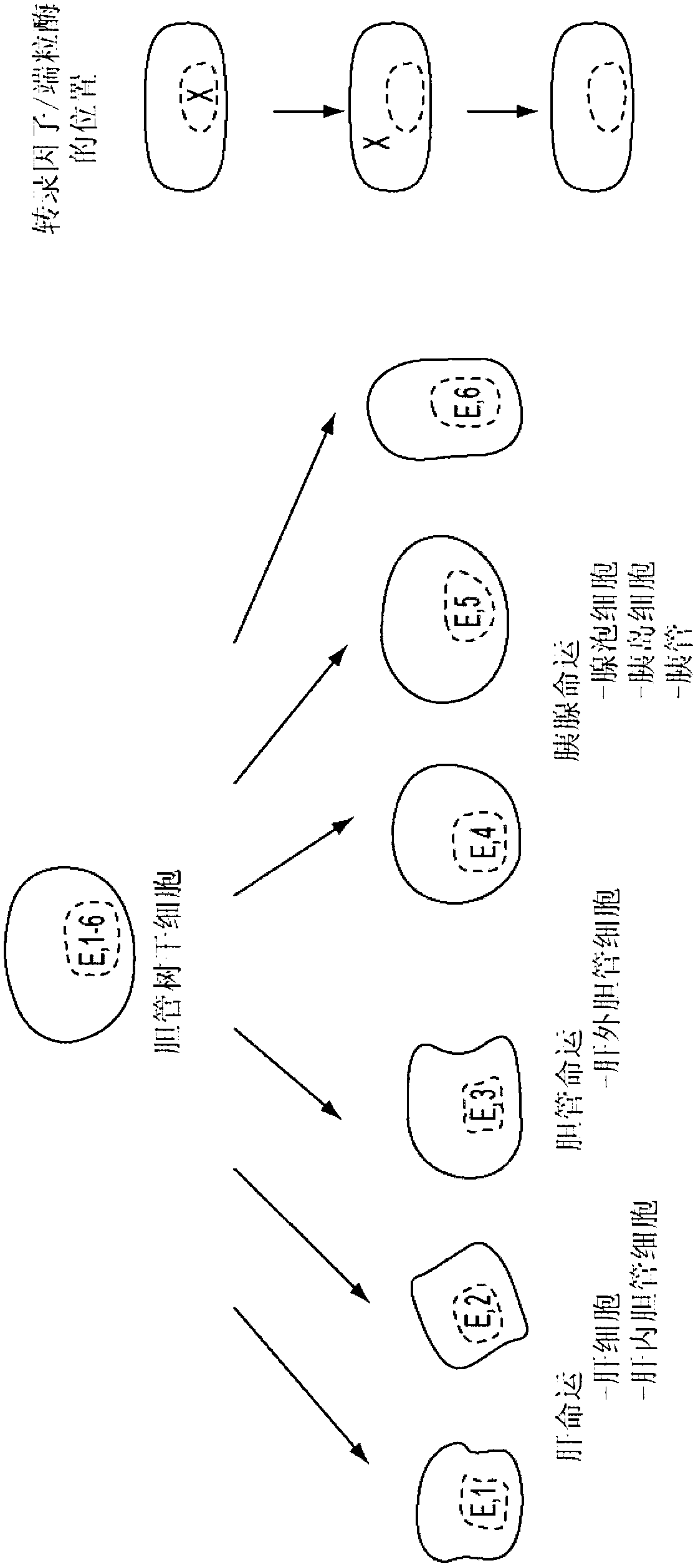
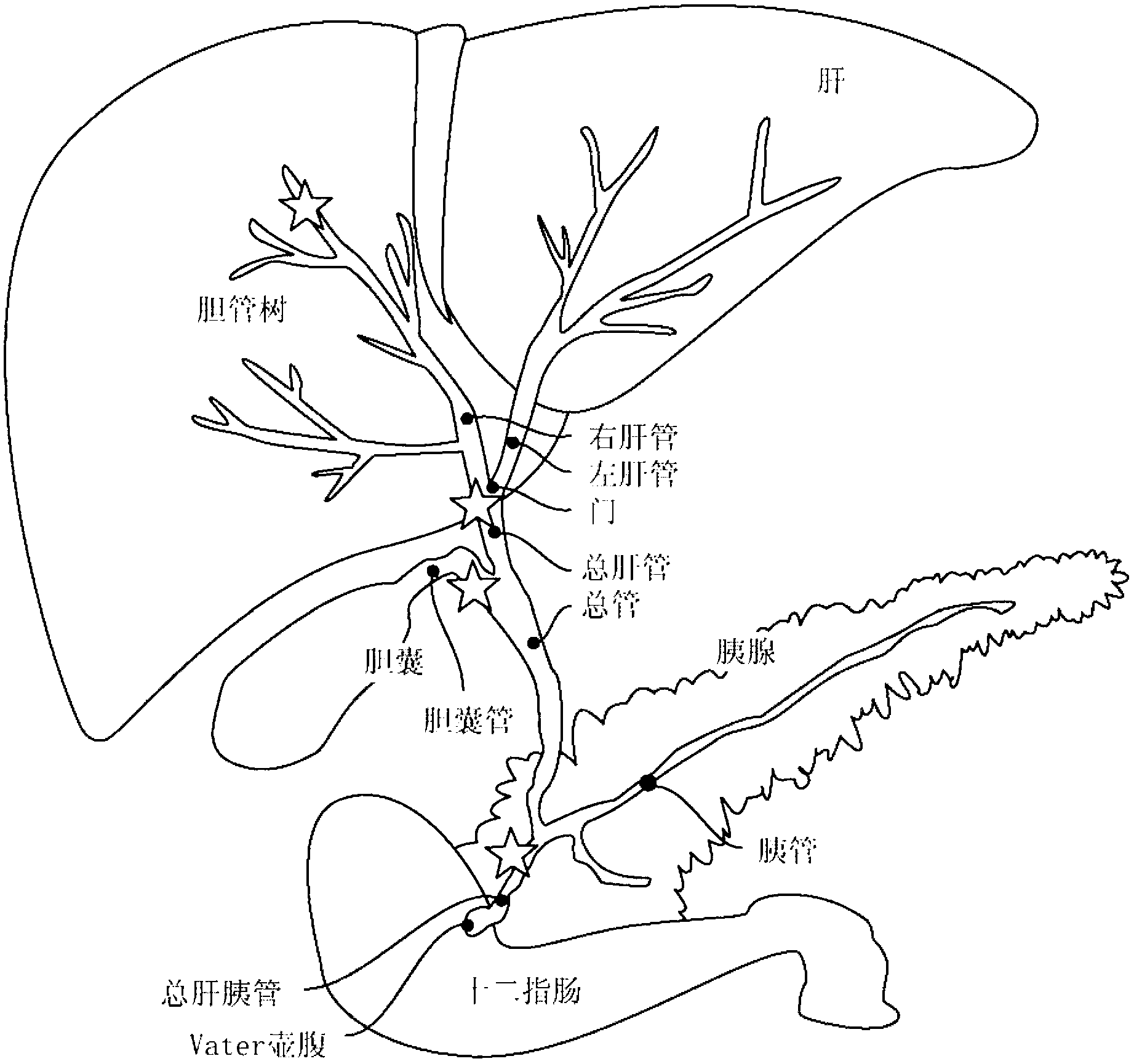
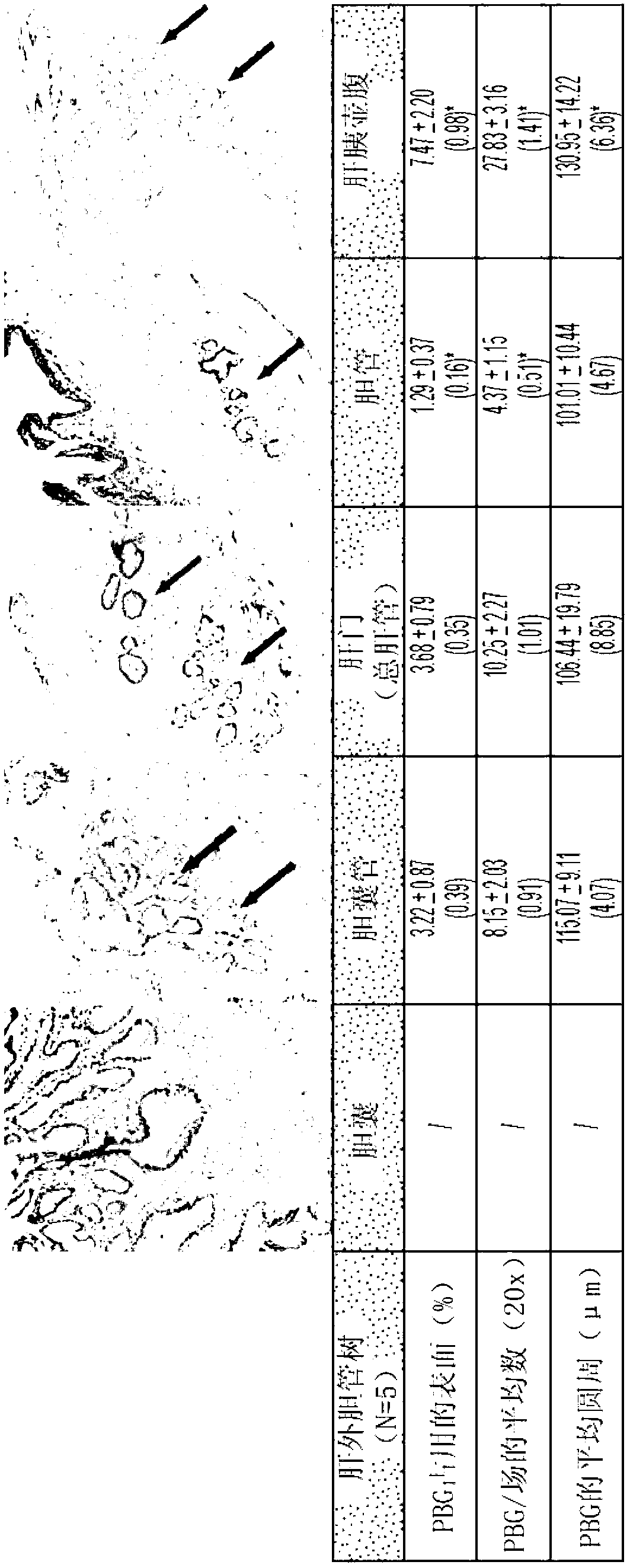
Multipotent stem cells from the extrahepatic biliary tree and methods of isolating same
PendingUS20110135610A1Differentiate faster and moreBiocideHepatocytesPluripotential stem cellGerm layer
The present invention relates to a multipotent stem cell, multipotent cell populations, and an enriched multipotent cell population, each found in fetal, neonatal, pediatric, and adult biliary tree tissue and up to 72 hours post mortem (although preferentially, within 10 hours post mortem) and capable of maturing into multiple endodermal tissues that include liver, biliary and pancreatic tissues. The multipotent stem / progenitor cell and cell populations are found in peribiliary glands, and progenitors descending from them are present throughout the biliary tree including in the gallbladder. High numbers of the peribiliary glands are found in the branching locations of the biliary tree such as hilum, common hepatic duct, cystic duct, common duct, common hepato-pancreatic duct and gallbladder. Related multipotent cells, multipotent cell populations and their descendent progenitors are found throughout the biliary tree including in the gall bladder, which does not have peribiliary glands. Compositions comprising same, methods of identifying and isolating same, maintaining same in culture, expanding same in culture and differentiating or lineage restricting the same in vitro or in vivo to hepatic, biliary or pancreatic fates (e.g., as hepatocytes, cholangiocytes, and / or pancreatic islet cells) are also provided. Methods of using the multipotent cells and / or multipotent cell populations are also provided.
Owner:SAPIENZA UNIV DE ROMA +1
Transgenic cell overlapped vascular inner rack and manufacture method thereof
InactiveCN101269242AAccelerated surface endothelializationPrevent restenosisStentsSurgeryHigh level expressionVascular endothelium
The invention provides an intravascular stent covered by transgene cells, which is characterized in that: a low-temperature plasma treatment layer, an adhesive interstitial substance coat and a cell coat are orderly arranged on the surface of the naked metal stent. The adhesive interstitial substance coat is a protein coat or a peptide sequence coat; the cell coat is obtained by stabilizing transfected cells by target gene. Preparing the intravascular stent includes the following steps: obtaining the target gene; constructing and amplifying the eukaryotic expression carrier of the target gene; obtaining cells; modifying the surface of the metal stent; preparing the adhesive interstitial substance coat; rotating, cultivating, and preparing the intravascular stent covered by transgene cells. On the one hand, by expressing the target gene at high-level, the rehabilitating of the injured endovascular endoderm is enhanced and the multiplication of smooth muscle cells is inhibited; one the other hand, by the multiplication of external-source cells, the surface endothelialization of the intravascular stent is accelerated; the coating is close applied on the surface of the stent, the biocompatibility is fine, and the intravascular stent can be inhibited from turning narrower ultimately.
Owner:CHONGQING UNIV
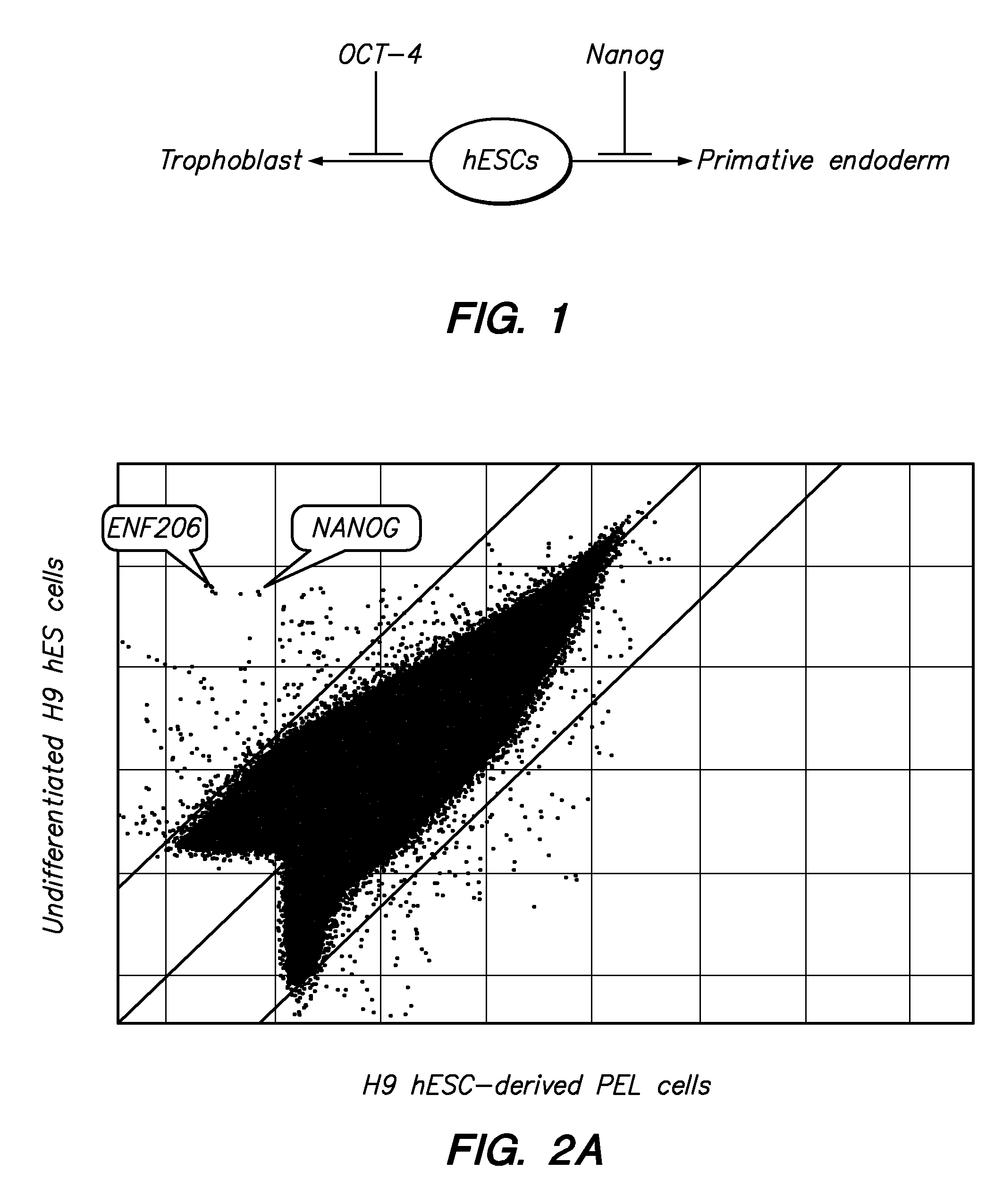
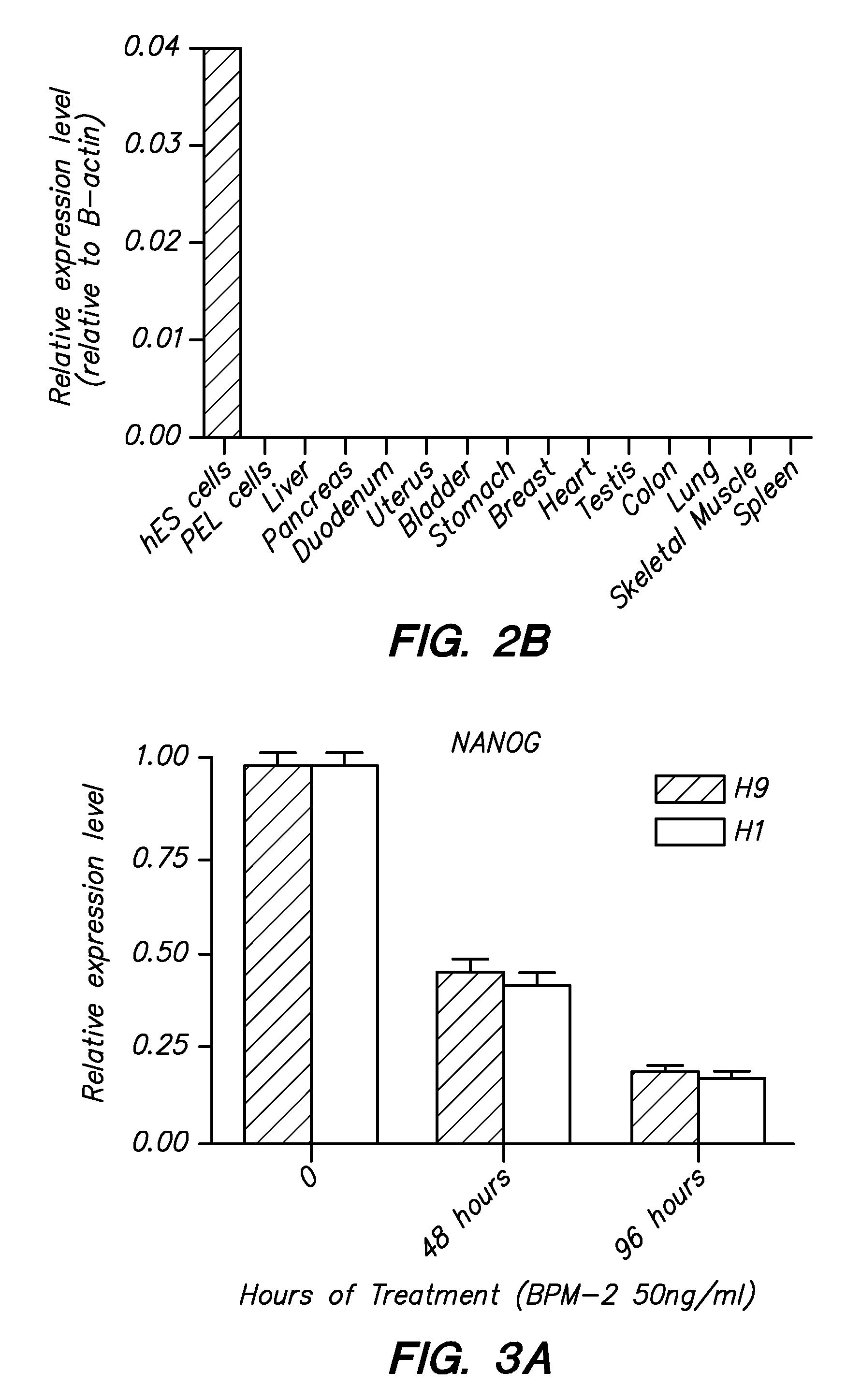
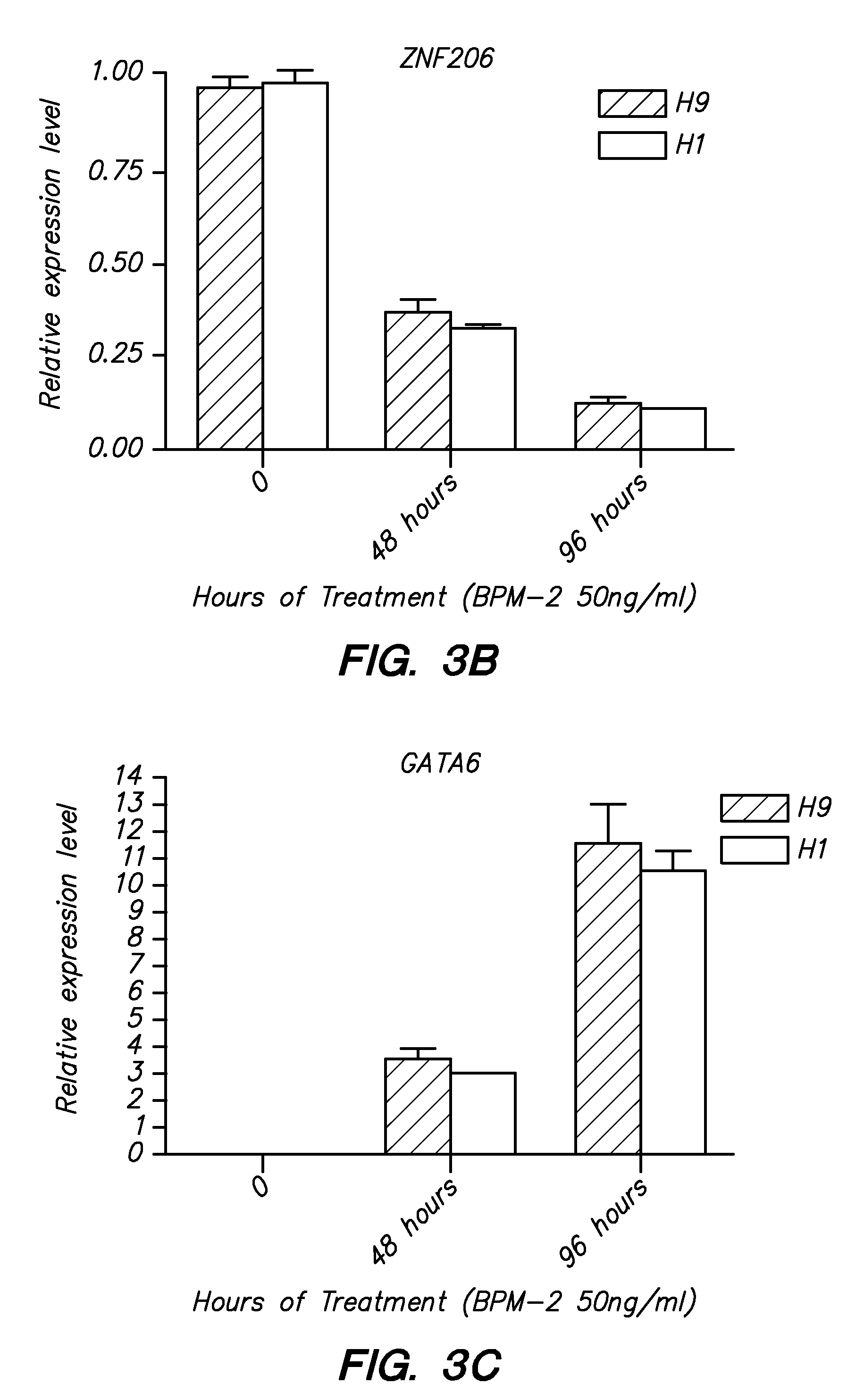
ZNF206: A Novel Regulator of Embryonic Stem Cell Self-renewal and Pluripotency
Owner:BURNHAM INST FOR MEDICAL RES
Establishment of a human embryonic stem cell line using mammalian cells
InactiveUS7811817B2Improve matchIncrease valueBiocideMicrobiological testing/measurementGerm layerFGF4
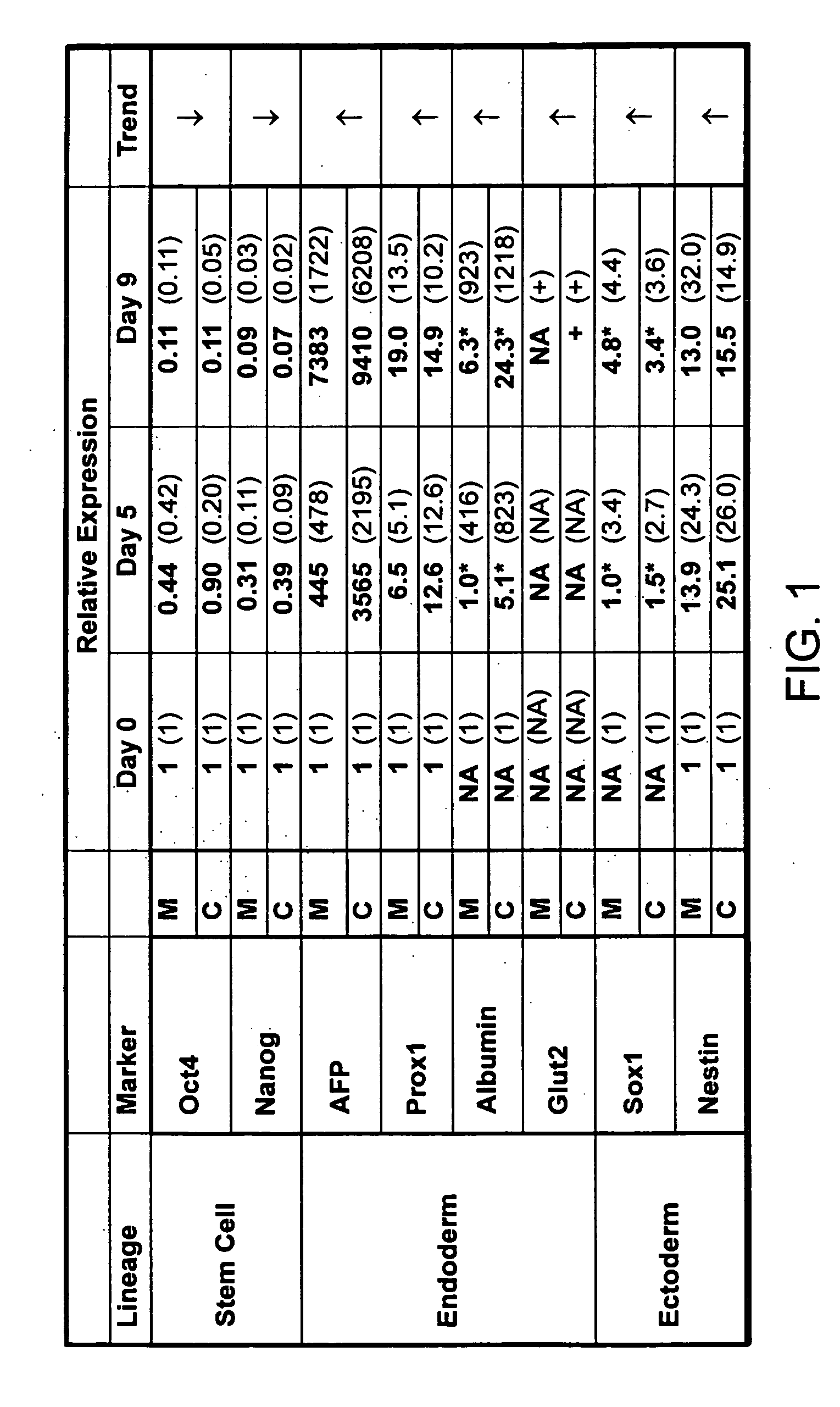
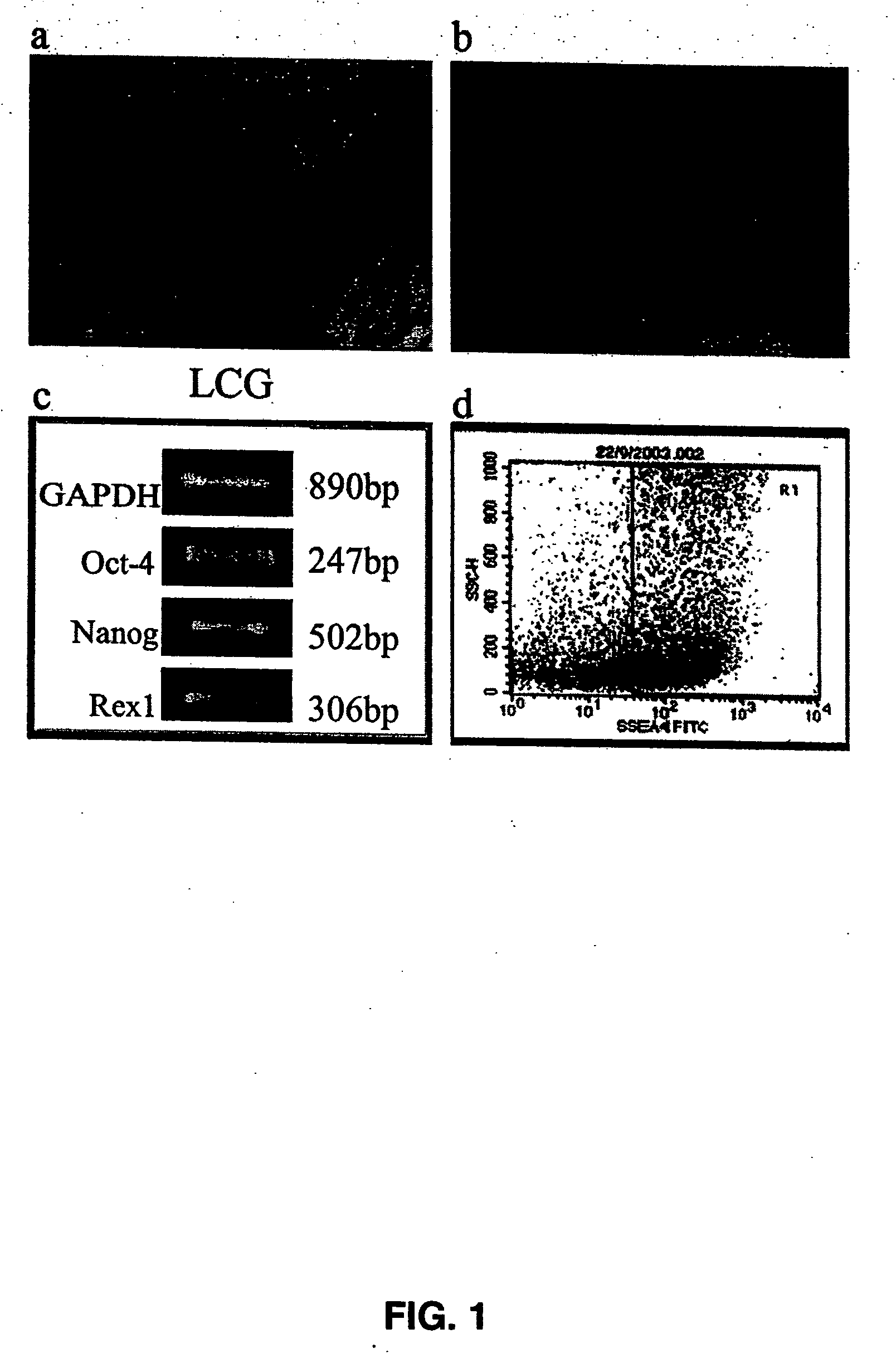
Purified preparations of human embryonic stem cells with certain population-specific characteristics are disclosed. This preparation is characterized by the positive expression of the following pluripotent cell surface markers: SSEA-1 (−); SSEA-4 (+); TRA-1-60 (+); TRA-1-81 (+); alkaline phosphatase (+), as well as a set of ES cell markers including Oct-4, Nanog, Rex1, Sox2, Thy1, FGF4, ABCG2, Dppa5, UTF1, Cripto1, hTERT, Connexin-43 and Connexin-45. The cells of the preparation are negative for lineage specific markers like Keratin 8, Sox-1, NFH (ectoderm), MyoD, brachyury, cardiac-actin (mesoderm), HNF-3 beta, albumin, and PDX1 (endoderm). The cells of the preparation are human embryonic stem cells, have normal karyotypes, exhibit high telomerase activity and continue to proliferate in an undifferentiated state after continuous culture for over 40 passages. The embryonic stem cell line Relicell™ hES1 also retains the ability, throughout the culture, to differentiate into cell and tissue types derived from all three embryonic germ layers (endoderm, mesoderm and ectoderm). Methods for isolating a human embryonic stem cell line are also disclosed.
Owner:RELIANCE LIFE SCI PVT
Differentiation of human embryonic stem cells
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
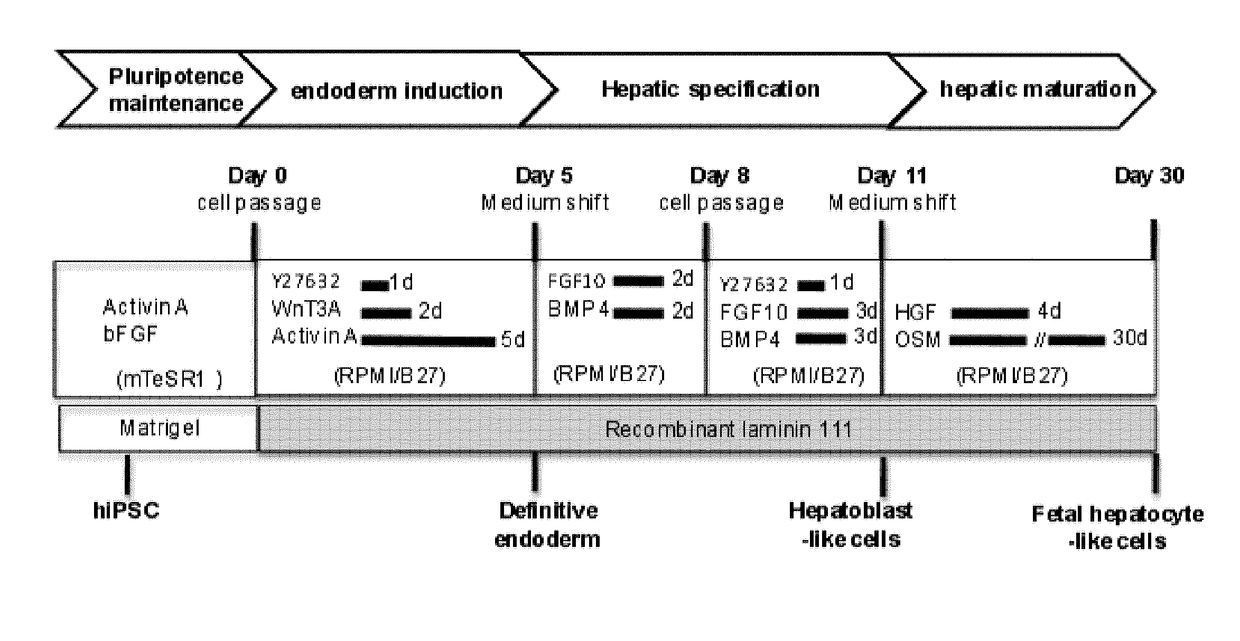
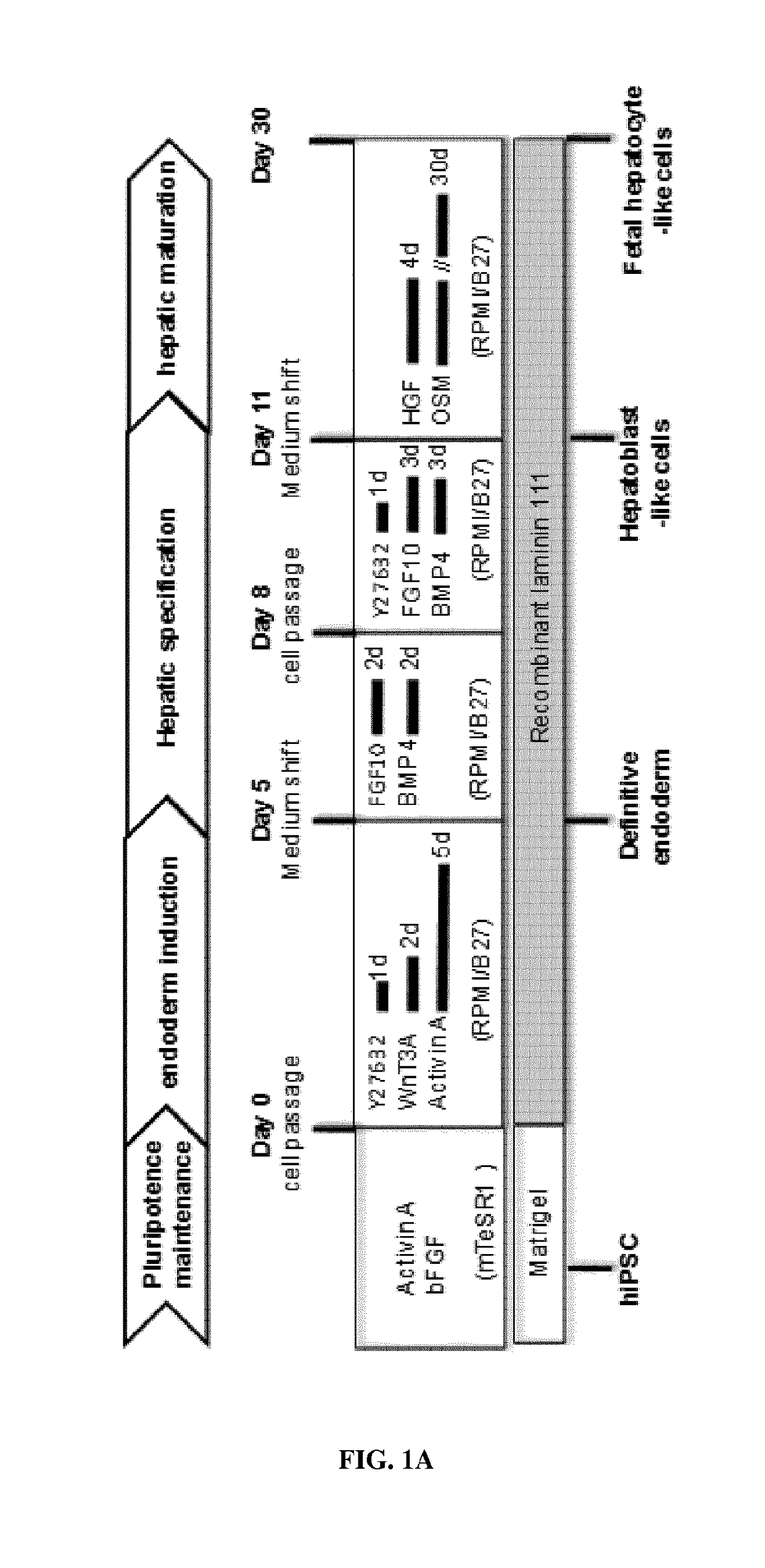
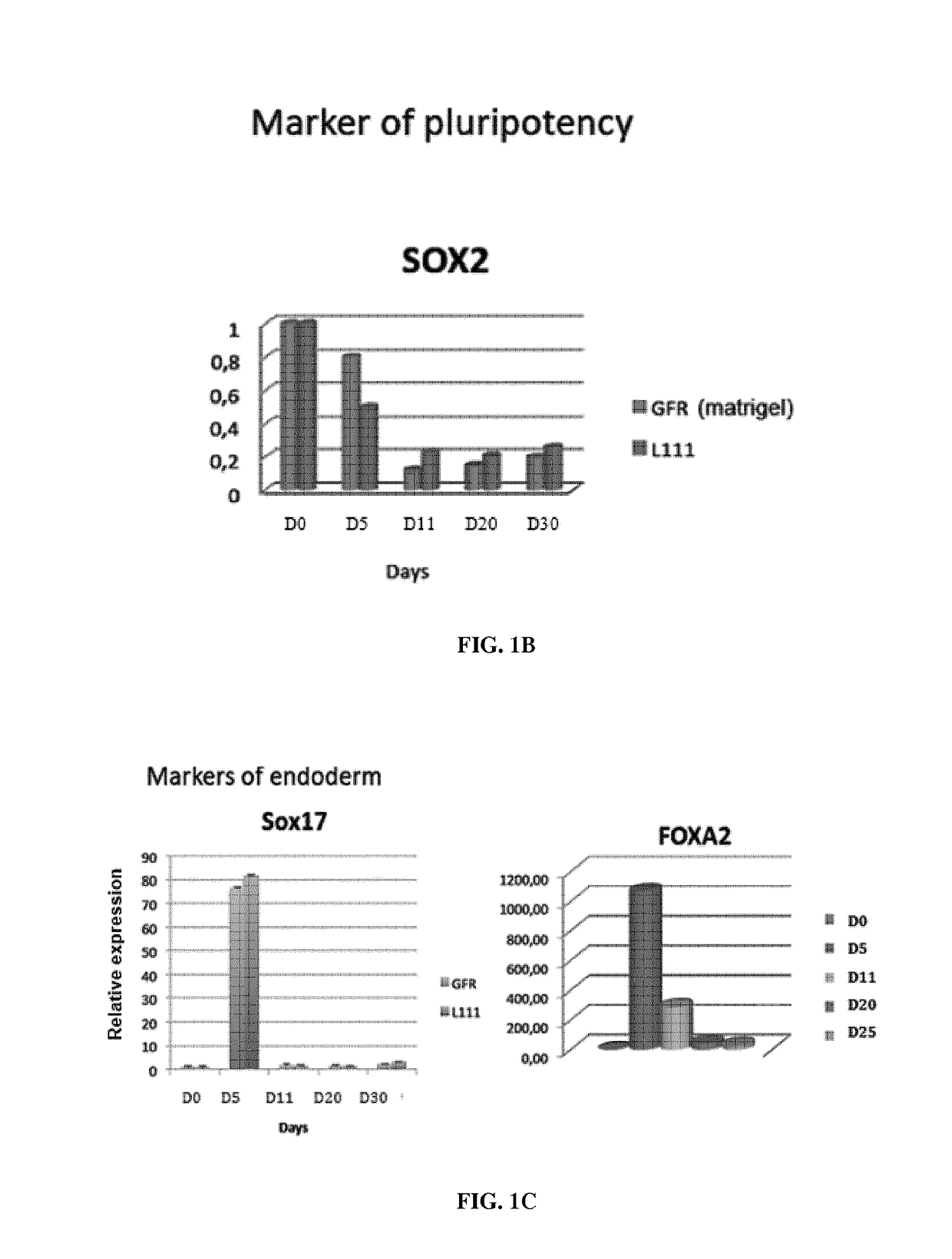
Use of a laminin for differentiating pluripotent cells into hepatocyte lineage cells
The invention relates to the use of a laminin (LN) as a matrix for hepatic differentiation. The invention also relates to a method for inducing hepatic differentiation comprising the steps of: (i) providing a population of human pluripotent cells, (ii) culturing the population on a support coated with a laminin in a endoderm induction medium to produce a population of human DE cells, (iii) culturing said population of human DE cells on a support coated with a laminin in a hepatic induction medium to produce a population of human hepatoblasts-like cells, and (iv) optionally culturing said population of human hepatoblasts-like cells on a support coated with a laminin in a hepatic maturation medium to produce a population of human hepatocyte-like cells. The invention further relates to a population of human hepatoblasts-like cells or human fetal hepatocyte-like cells obtained by the method of the invention. The invention further relates to a population of human hepatoblasts-like cells expressing HNF4α and expressing substantially AFP for use in a method of treatment of the human body.
Owner:UNIV DE NANTES +1
Multipotent stem cells and uses thereof
ActiveUS8574567B2Easily attainable embryonic-likePromote wound healingBiocideNervous disorderMHC class ISurface marker
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Differentiation of human embryonic stem cells
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
Primate embryonic stem cells
InactiveUS20050158854A1High nucleus/cytoplasm ratioNervous disorderMetabolism disorderGerm layerSurface marker
A purified preparation of primate embryonic stem cells is disclosed. This preparation is characterized by the following cell surface markers: SSEA-1 (−); SSEA-4 (+); TRA-1-60 (+); TRA-1-81 (+); and alkaline phosphatase (+). In a particularly advantageous embodiment, the cells of the preparation are human embryonic stem cells, have normal karyotypes, and continue to proliferate in an undifferentiated state after continuous culture for eleven months. The embryonic stem cell lines also retain the ability, throughout the culture, to form trophoblast and to differentiate into all tissues derived from all three embryonic germ layers (endoderm, mesoderm and ectoderm). A method for isolating a primate embryonic stem cell line is also disclosed.
Owner:THOMSON
Multipotent stem cells from the extrahepatic billary tree and methods of isolating same
The present invention relates to a multipotent stem cell, multipotent cell populations, and an enriched multipotent cell population, each found in fetal, neonatal, pediatric, and adult biliary tree tissue and up to 72 hours post mortem (although preferentially, within 10 hours post mortem) and capable of maturing into multiple endodermal tissues that include liver, biliary and pancreatic tissues. The multipotent stem / progenitor cell and cell populations are found in peribiliary glands, and progenitors descending from them are present throughout the biliary tree including in the gallbladder. High numbers of the peribiliary glands are found in the branching locations of the biliary tree such as hilum, common hepatic duct, cystic duct, common duct, common hepato-pancreatic duct and gallbladder. Related multipotent cells, multipotent cell populations and their descendent progenitors are found throughout the biliary tree including in the gall bladder, which does not have peribiliary glands. Compositions comprising same, methods of identifying and isolating same, maintaining same in culture, expanding same in culture and differentiating or lineage restricting the same in vitro or in vivo to hepatic, biliary or pancreatic fates (e.g., as hepatocytes, cholangiocytes, and / or pancreatic islet cells) are also provided. Methods of using the multipotent cells and / or multipotent cell populations are also provided.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Primate embryonic stem cells
A purified preparation of primate embryonic stem cells is disclosed. This preparation is characterized by the following cell surface markers: SSEA-1 (−); SSEA-4 (+); TRA-1-60 (+); TRA-1-81 (+); and alkaline phosphatase (+). In a particularly advantageous embodiment, the cells of the preparation are human embryonic stem cells, have normal karyotypes, and continue to proliferate in an undifferentiated state after continuous culture for eleven months. The embryonic stem cell lines also retain the ability, throughout the culture, to form trophoblast and to differentiate into all tissues derived from all three embryonic germ layers (endoderm, mesoderm and ectoderm). A method for isolating a primate embryonic stem cell line is also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Multi-lineage directed induction of bone marrow stromal cell differentiation
Methods of inducing differentiation of mammalian bone marrow stromal cells into cells of multiple embryonic lineages by contacting marrow stromal cells with precursor differentiation-inducing compounds followed by contacting the partially differentiated precursor cells with specific cell type differentiation-inducing compounds. In one embodiment, the MSC derived precursor cell cultures comprise cells, at least some of which simultaneously express markers that are characteristic of endodermal and ectodermal cell types. In another embodiment, the differentiated cells are insulin-secreting pancreatic islet cells. Precursor differentiation-inducing compounds of the invention include anti-oxidants such as, but not limited to, beta-mercaptoethanol, dimethylsulfoxide, butylated hydroxyanisole, butylated hydroxytoluene, ascorbic acid, dimethylfumarate, and n-acetylcysteine. Endodermal cell differentiation-inducing compounds of the invention include but are not limited to anti-oxidants and growth factors including basic fibroblast growth factor. Once induced to differentiate into a particular cell type, the cells can be used for cell therapy, gene therapy, or both, for treatment of diseases, disorders, or conditions associated with tissues of multiple embryonic origins.
Owner:RUTGERS THE STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com