Sub totipotential stem cell and preparation method and application thereof
A technology of sub-totipotent stem cells and uses, applied in the field of sub-totipotent stem cells, the preparation of sub-totipotent stem cells, can solve the problems of poor understanding of stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
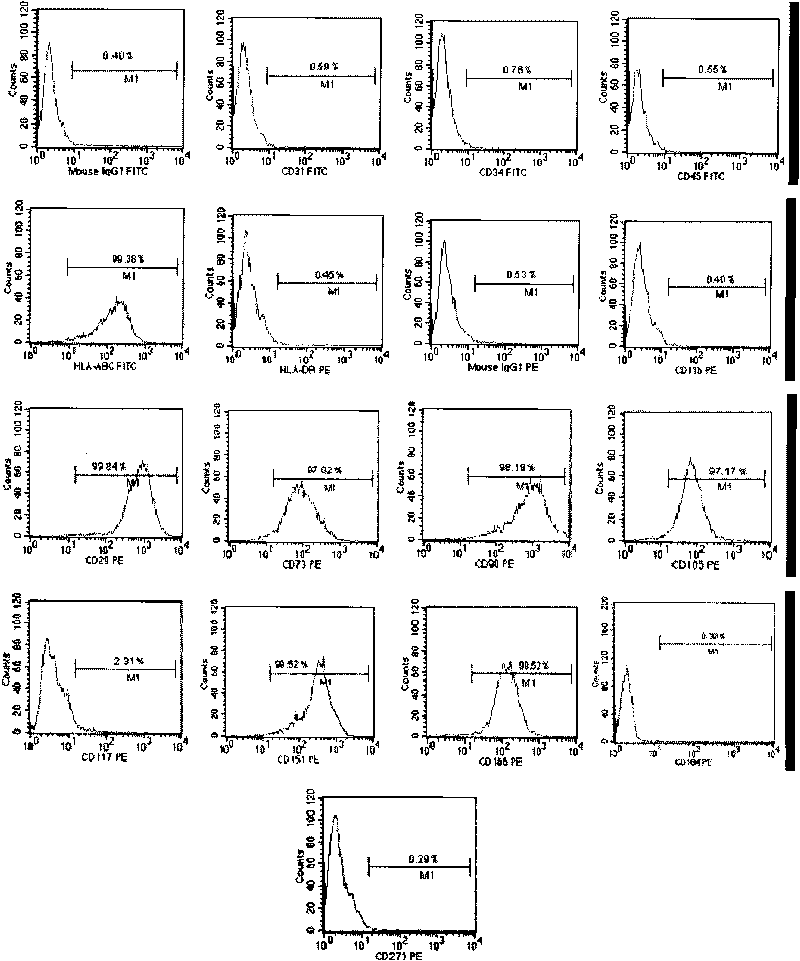
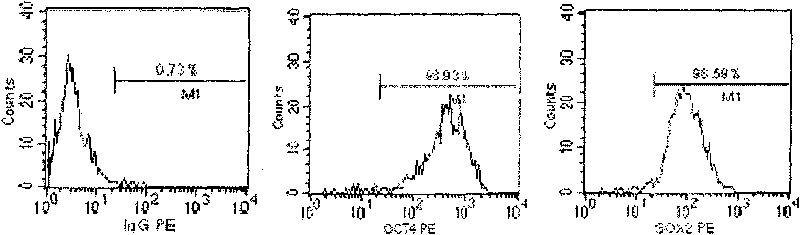
[0072] Isolation of CD151 by adherent subculture + CD184 - Oct4 + subtotipotent stem cells
[0073] Take cut human placenta, carry out CD151 + CD184 - Oct4 + Preparation of stem cell products. The specific steps are as follows: 1) Aseptically collect the cut human placenta; 2) Cut to small fragments of 3 Small tissue pieces were digested with 0.5mg / mL collagenase I at 37°C for 1 hour, and 1mg / mL trypsin at 37°C for 30 minutes, with gentle shaking 5 times during the period, sieved, centrifuged to collect the cell suspension, and inoculated in T75cm 2 In the culture flask, cultured in cell culture solution A for 5 to 7 days, the spindle-like cells adhered to the wall; 3) The adherent cells were digested with trypsin and further cultured, passaged, purified, expanded and passaged for 6 generations, and the cells were harvested, After concentrating and preparing a cell suspension with cell preservation solution, some cells are sent for quality inspection, and the rest are st...
Embodiment 2
[0077] Isolation of CD151 by adherent subculture + CD184 - Oct4 + subtotipotent stem cells
[0078] Same as Example 1, except that the cell culture medium used is cell culture medium B.
[0079] The results obtained were substantially the same as in Example 1.
Embodiment 3
[0081] Isolation of CD151 by adherent subculture + CD184 - Oct4 + subtotipotent stem cells
[0082] Same as Example 1, except that the cell culture medium used is cell culture medium C.
[0083] The results obtained were substantially the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com