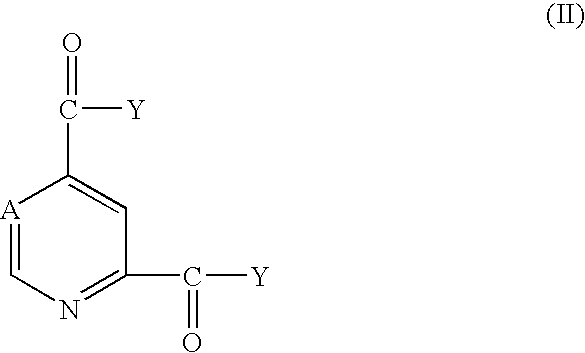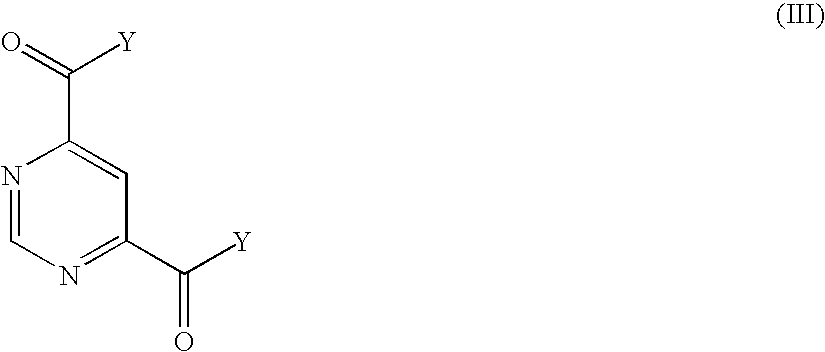Patents
Literature
874 results about "Collagenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This product is used to help the healing of burns and skin ulcers.
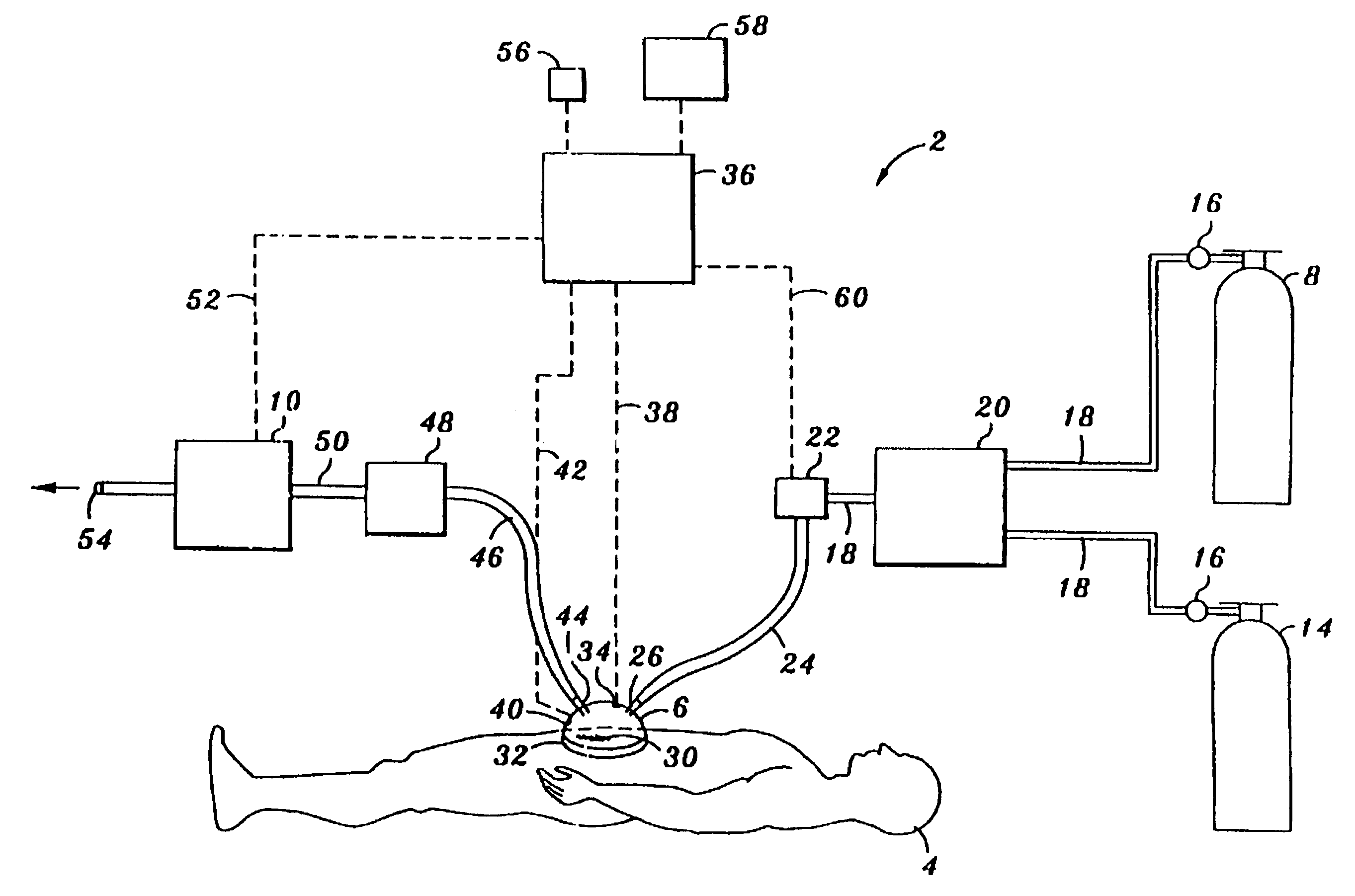
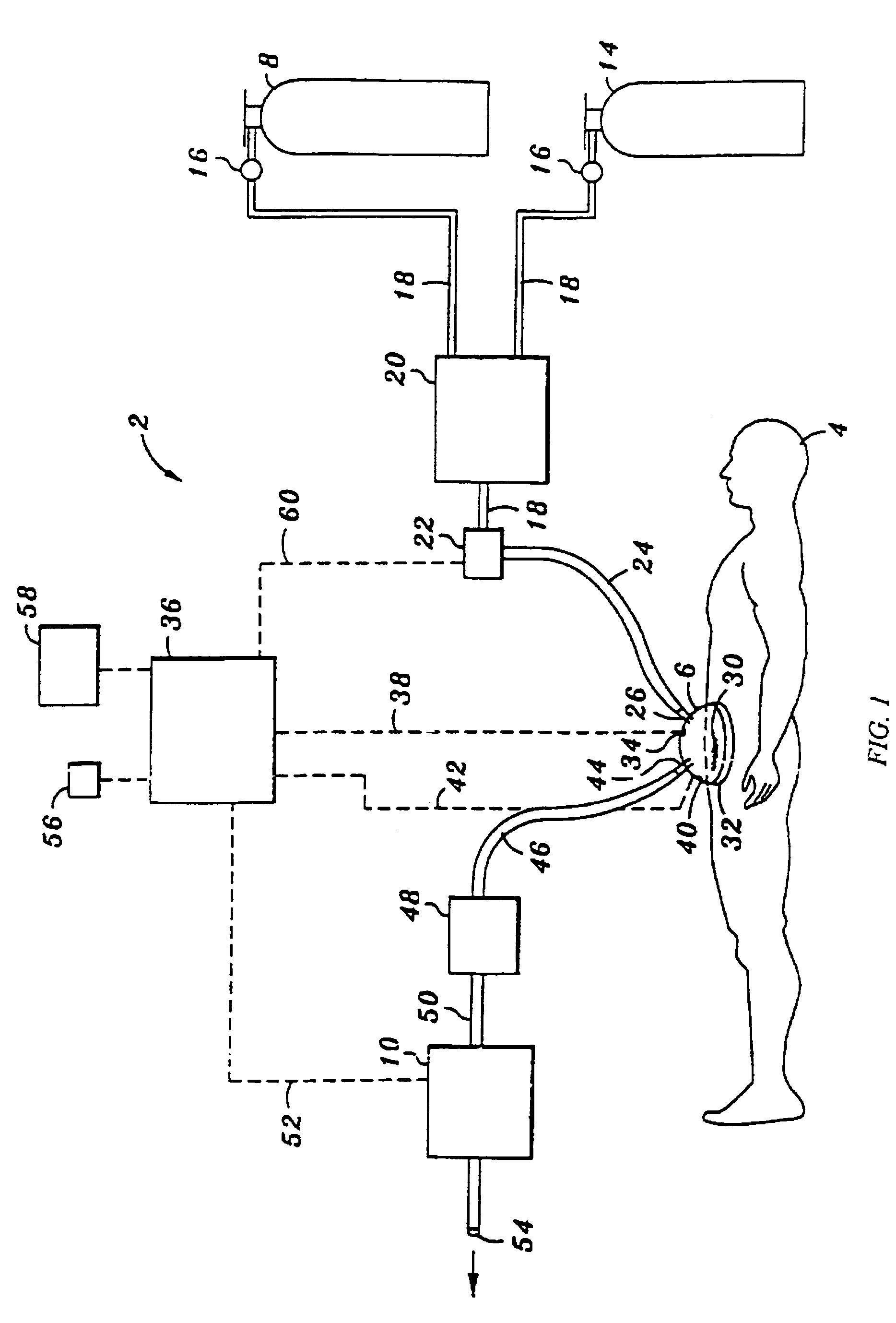
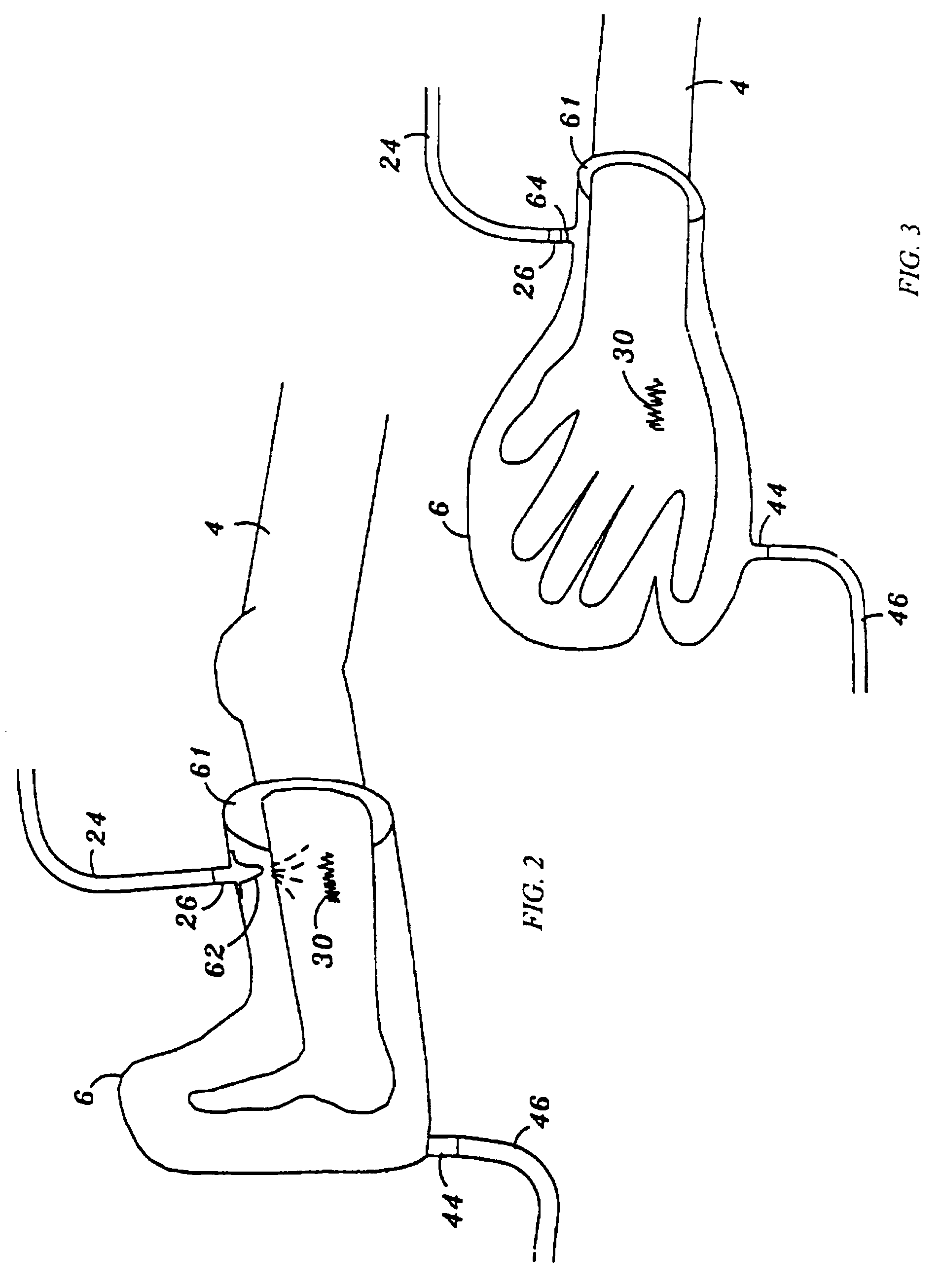
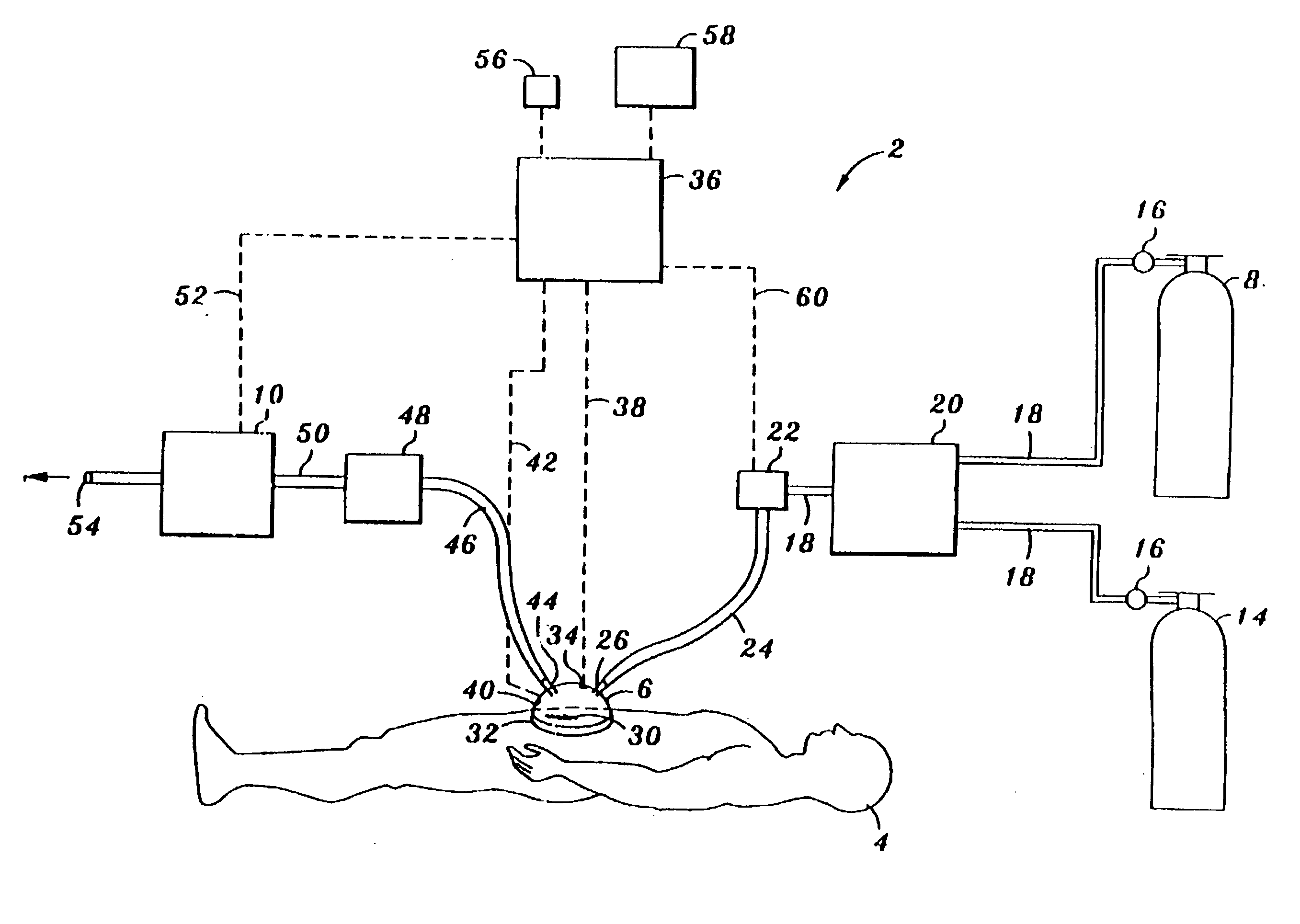
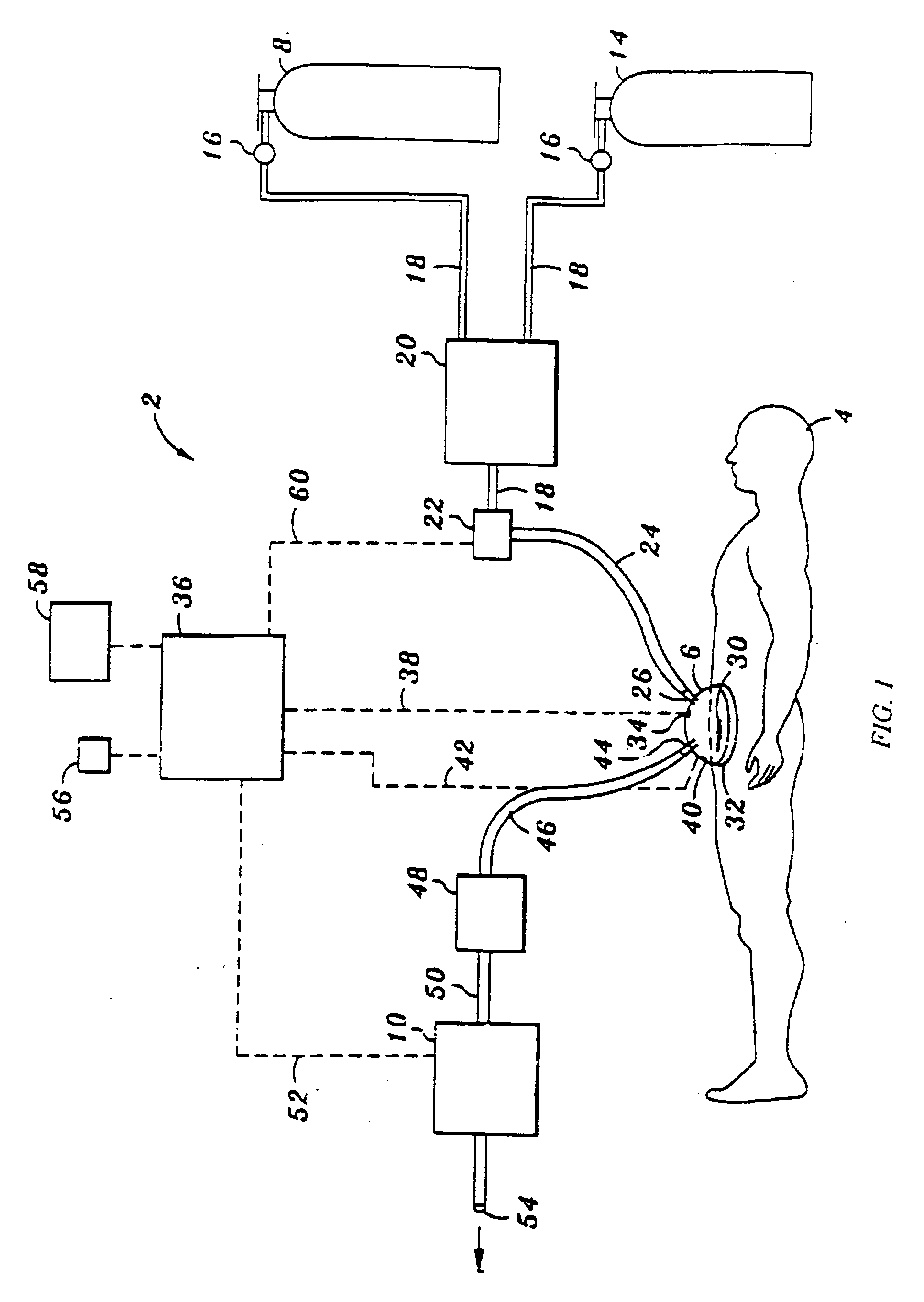
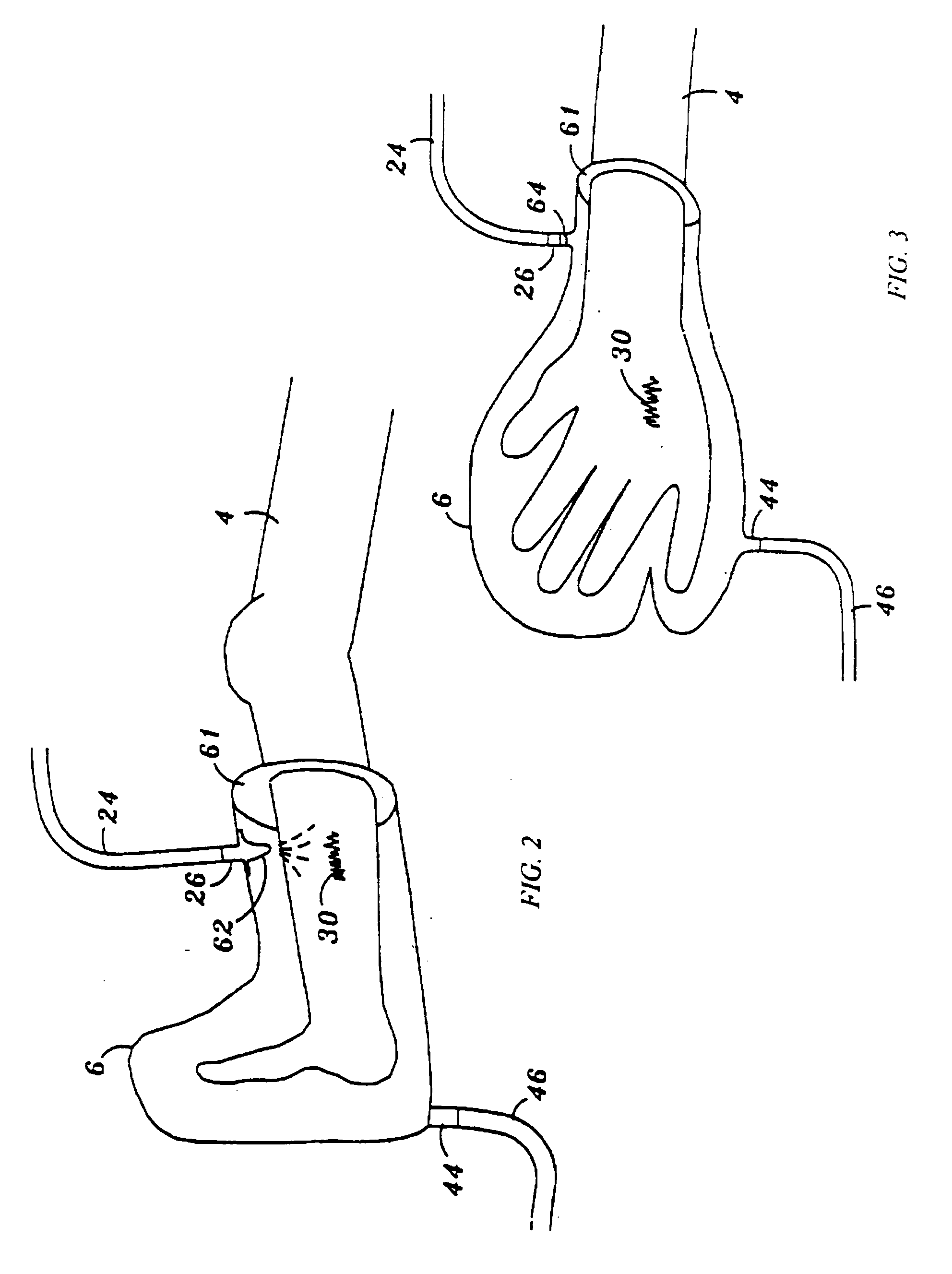
Device and method for treatment of wounds with nitric oxide
InactiveUS7122018B2Promote wound healingReduce the burden onBiocideOther blood circulation devicesHigh concentrationNitric oxide gas
Topical exposure of nitric oxide gas to wounds such as chronic non-healing wounds may be beneficial in promoting healing and preparing the wound bed for further treatment and recovery. Nitric oxide gas may be used to reduce the microbial infection, manage exudates secretion by reducing inflammation, upregulate expression of endogenous collagenase to locally debride the wound, and regulate the formation of collagen. High concentration of nitric oxide ranging from 160–400 ppm may be used without inducing toxicity in the healthy cells around a wound site. Exposure to the high concentration for a first treatment period reduces the microbial burden and inflammation, and increases collagenase expression to debride necrotic tissue at the wound site. After a first treatment period, a second treatment period at a lower concentration of nitric oxide, preferably ranging from 5–20 ppm may be used to restore the balance of nitric oxide and induce collagen expression aiding in the wound closure.
Owner:SENSORMEDICS +1
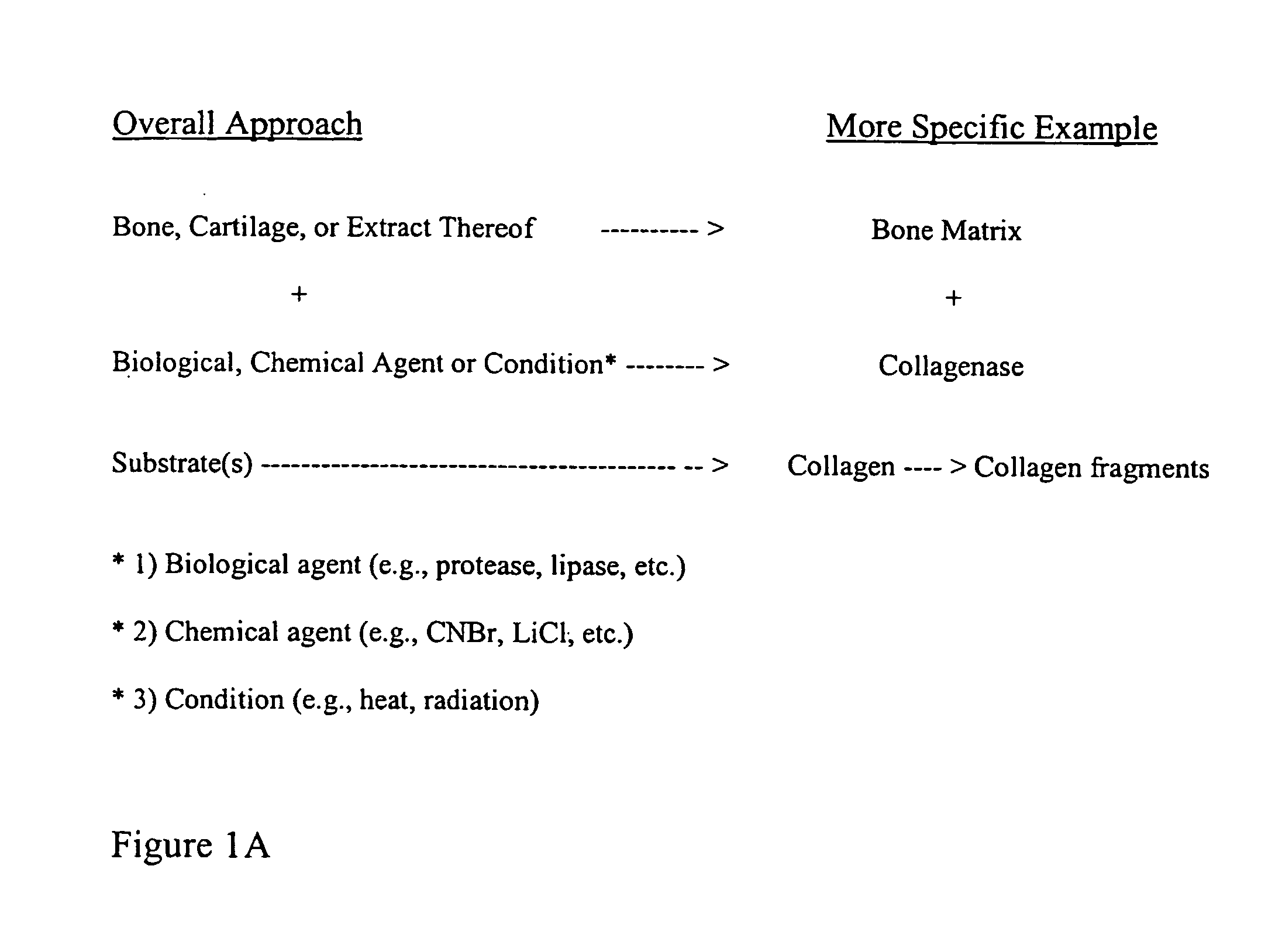
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
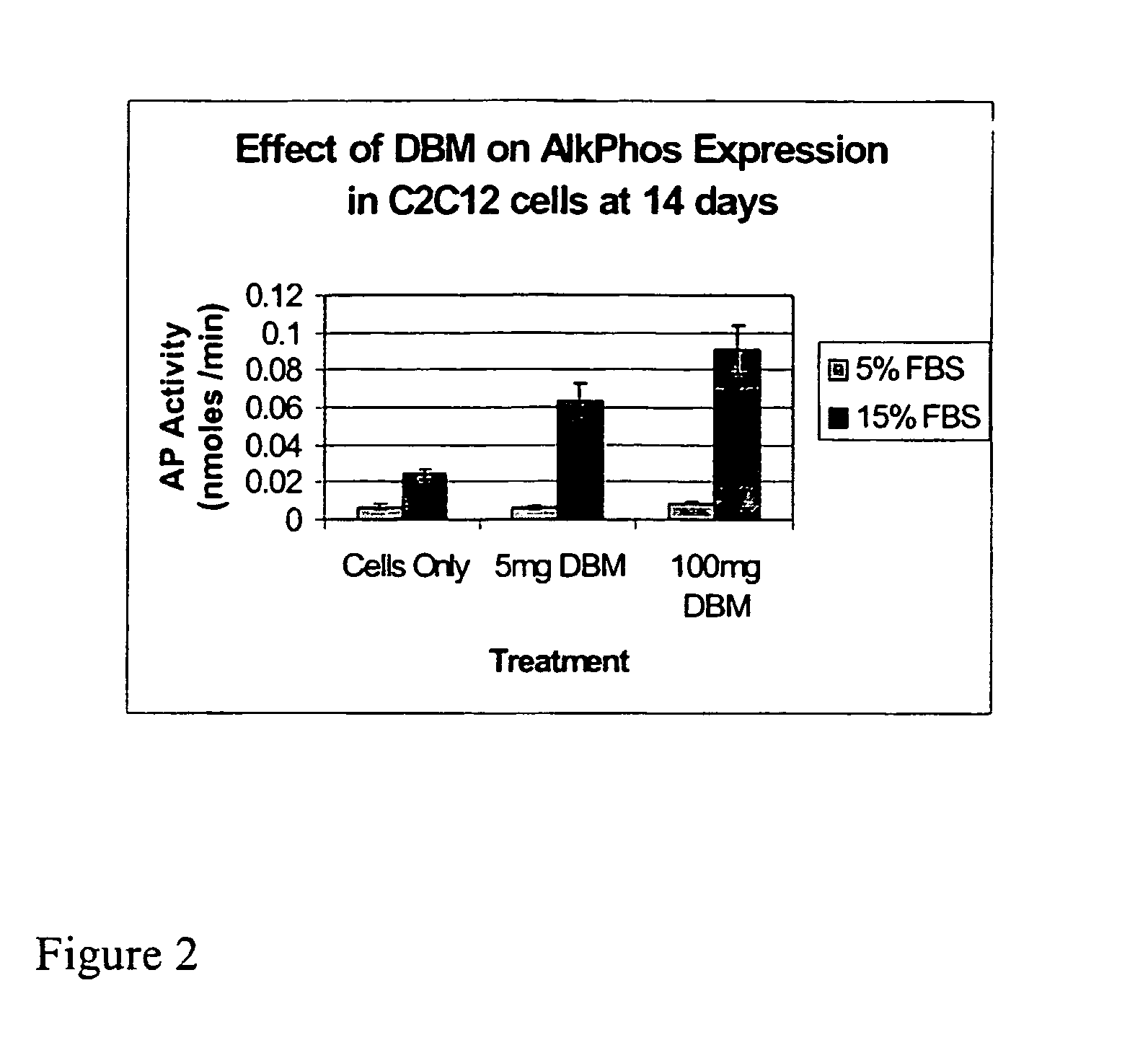
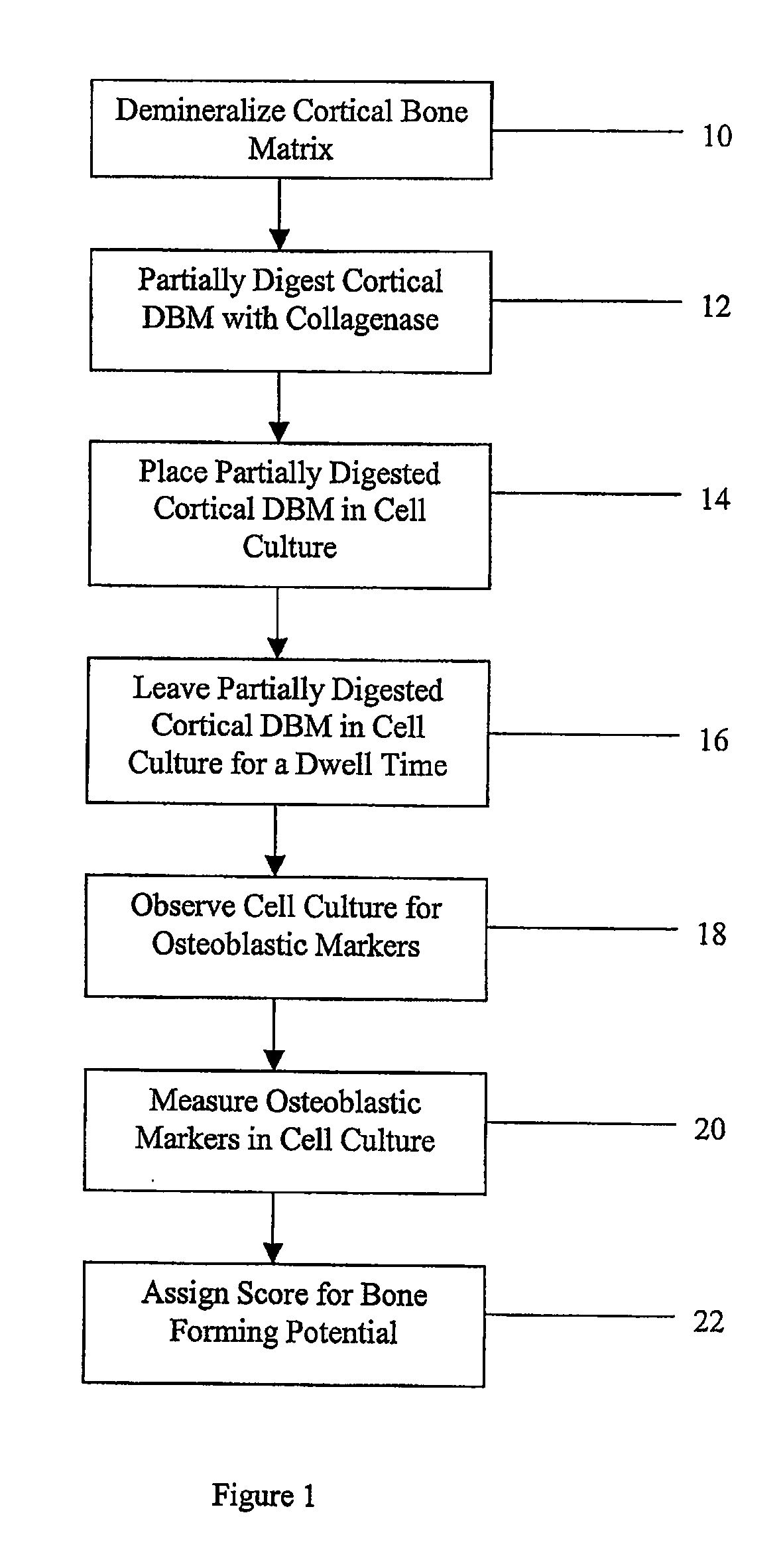
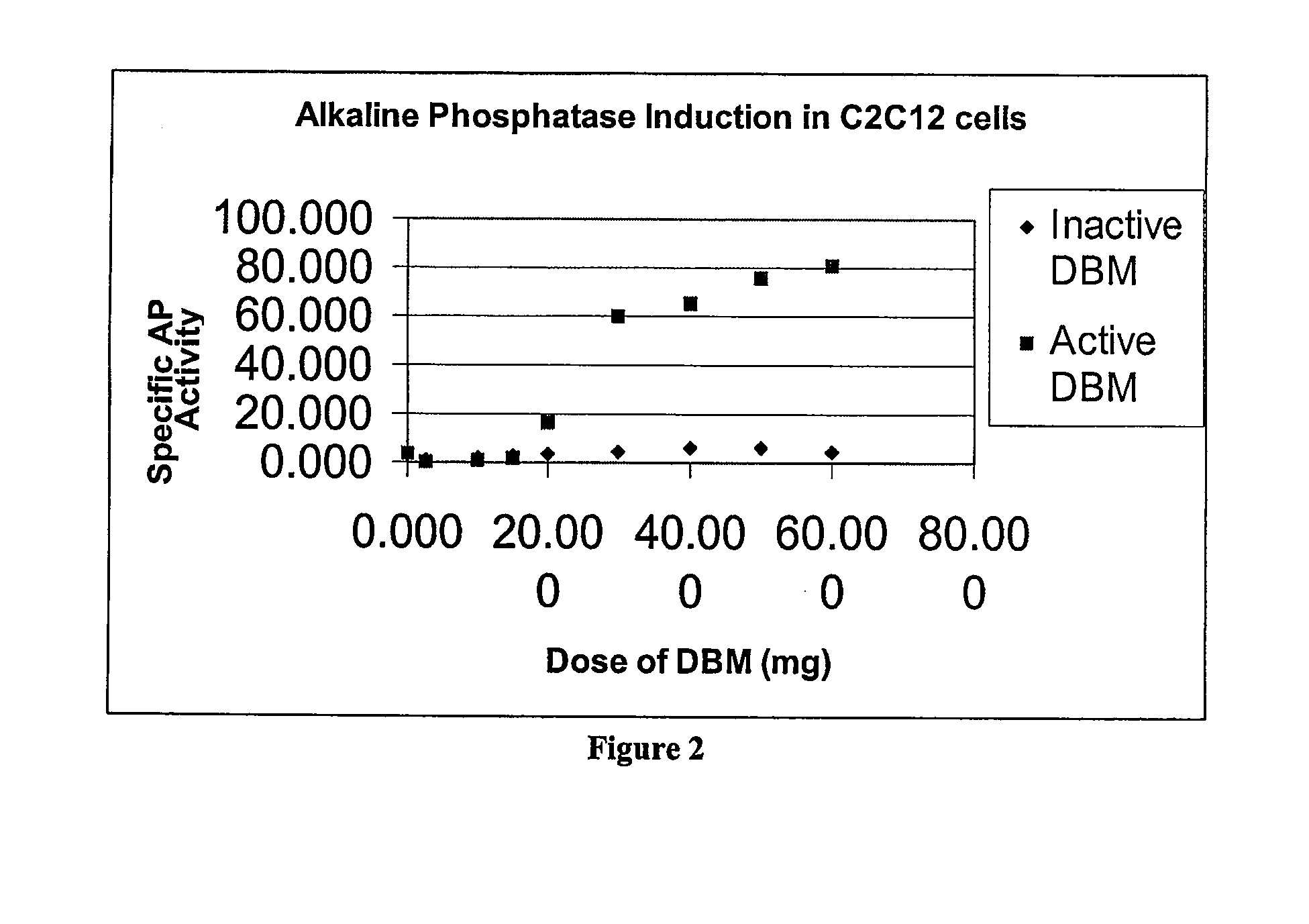
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Enzyme-activated anti-tumor prodrug compounds
Disclosed are enzyme-activated anti-tumor and anti-metastatic prodrug compounds. The specific enzymes are collagenase(IV) and elastase. Also disclosed are methods of making and using such compounds.
Owner:BOEHRINGER INGELHEIM PHARMA INC
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
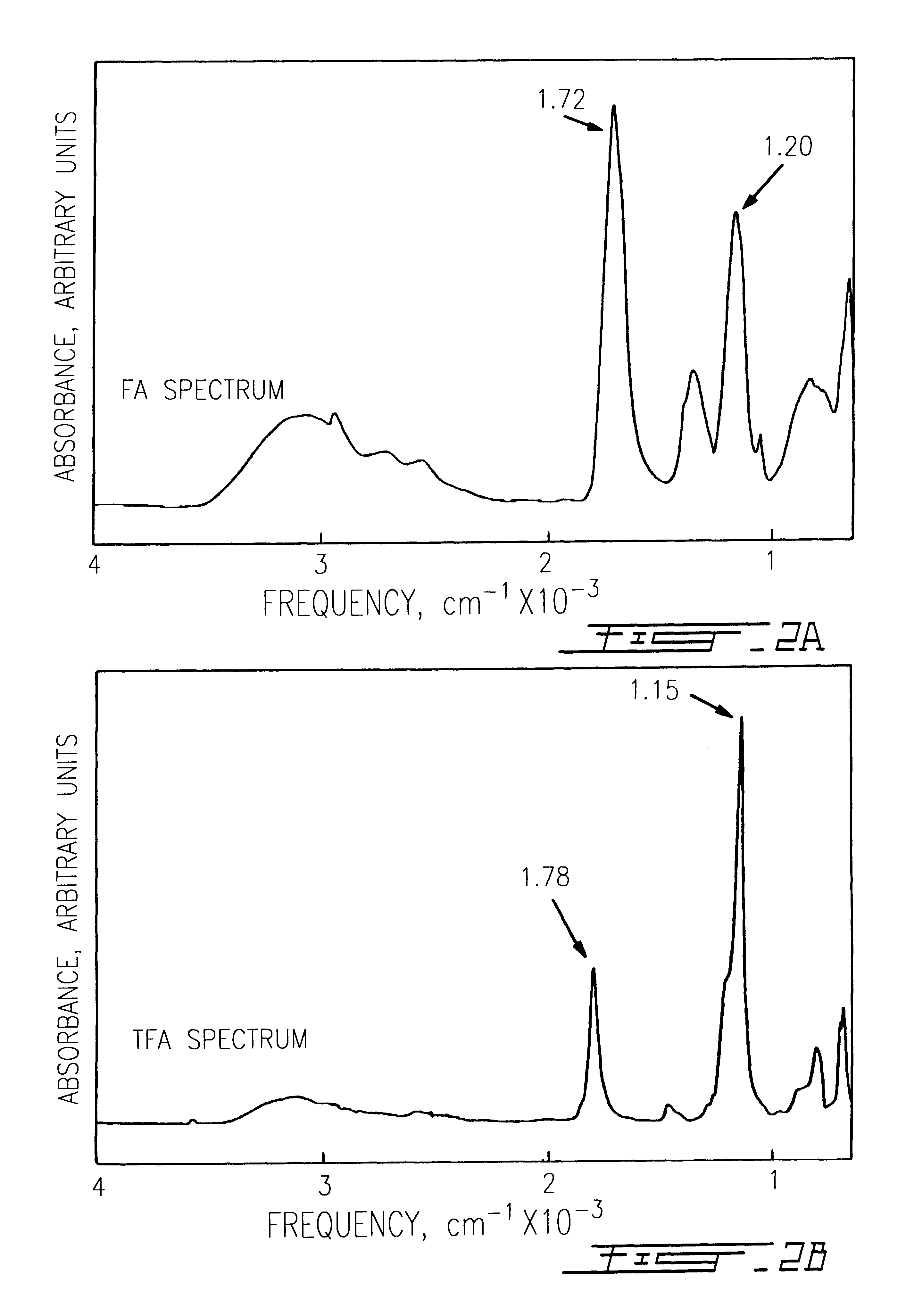
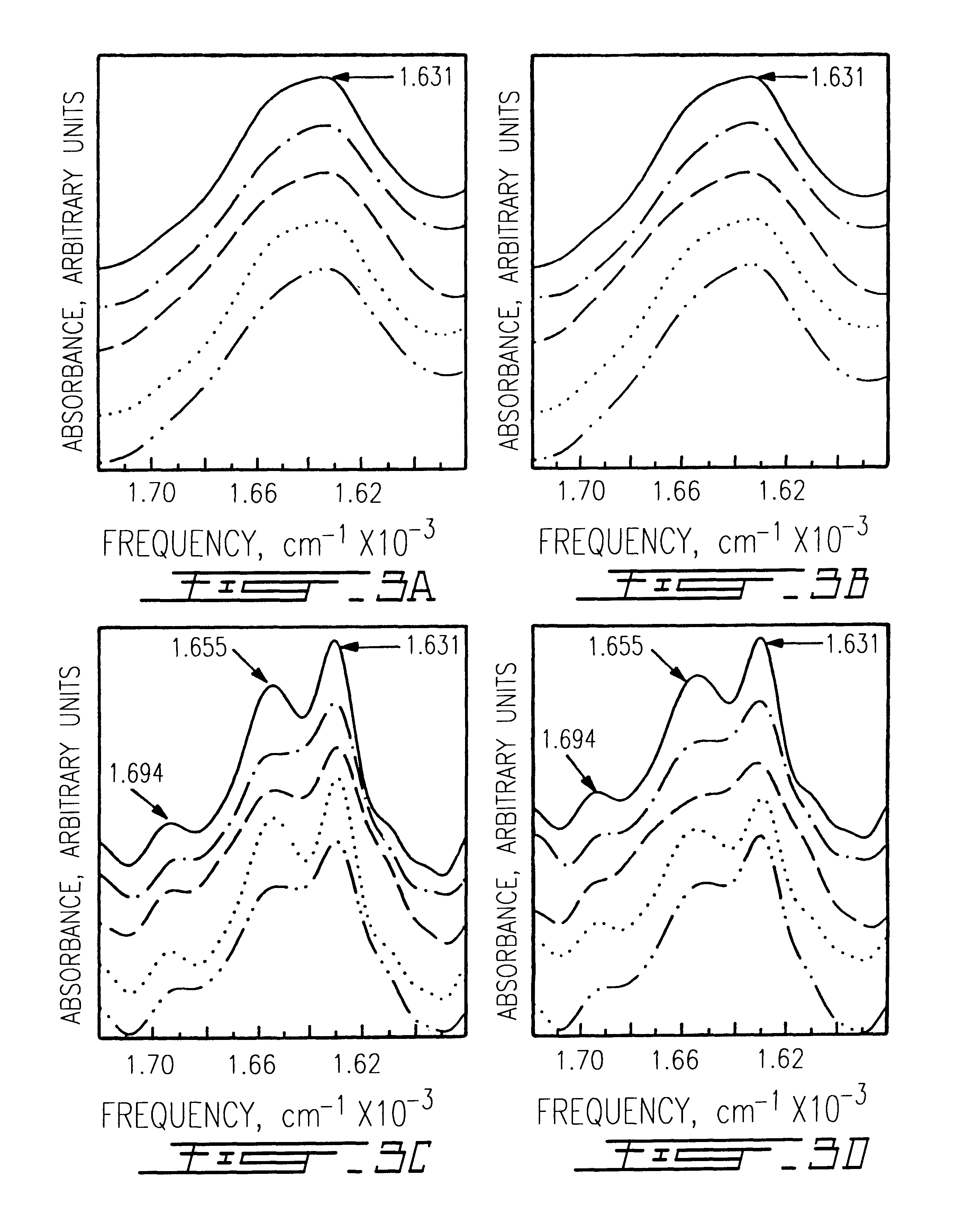
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Methods for designing agents that interact with MMP-13
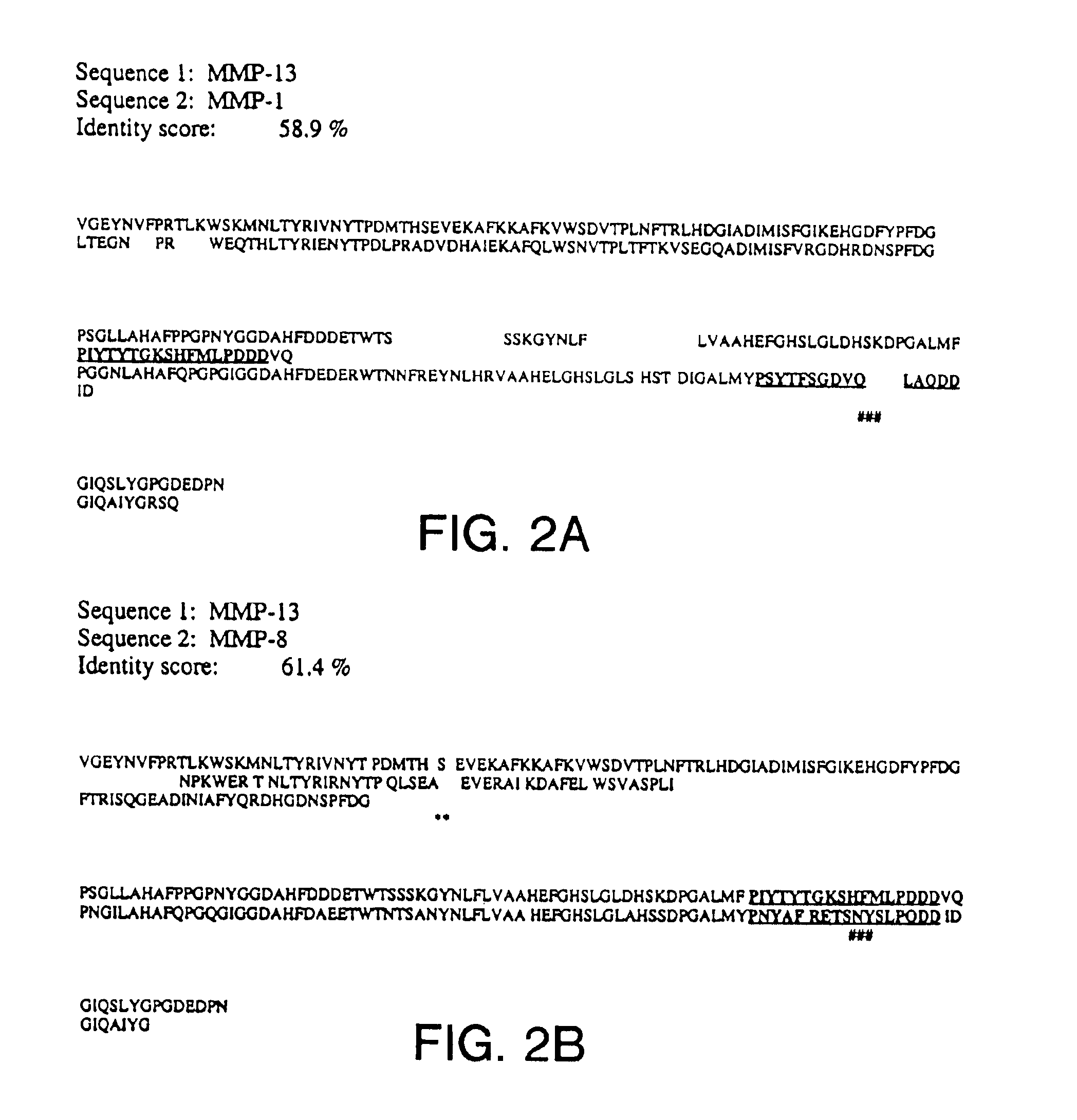
InactiveUS6934639B1High potencyStrong specificityOrganic active ingredientsHydrolasesCrystallographyCollagenase
The present invention relates to the three dimensional structure of human collagenase 3 (MMP-13), as well as to (i) methods of using the MMP-13 structure to rationally design or identify compounds or molecules that inhibit or activate MMP-13 activity, and (ii) compounds identified using said methods.
Owner:WYETH
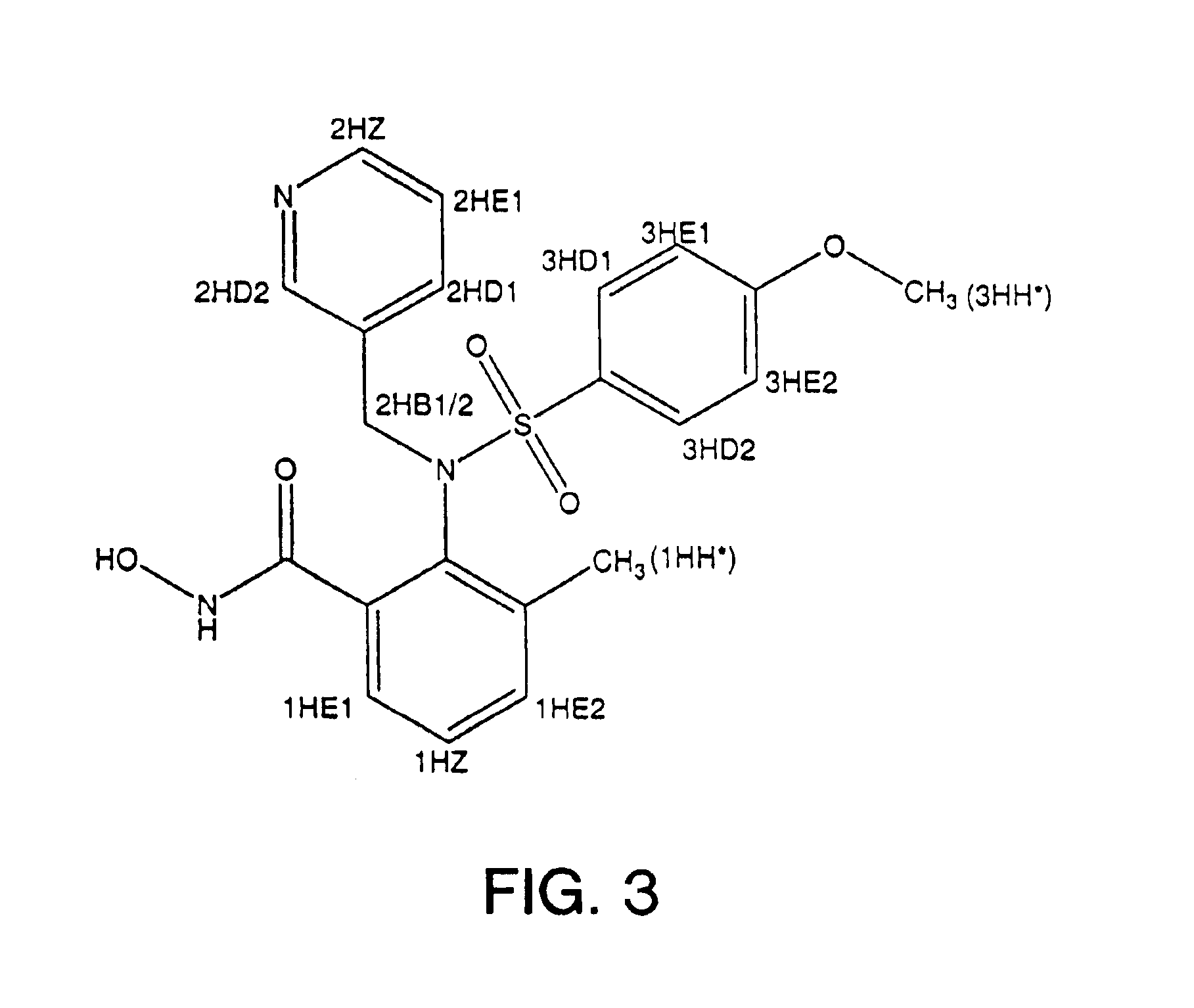
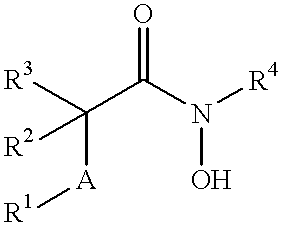
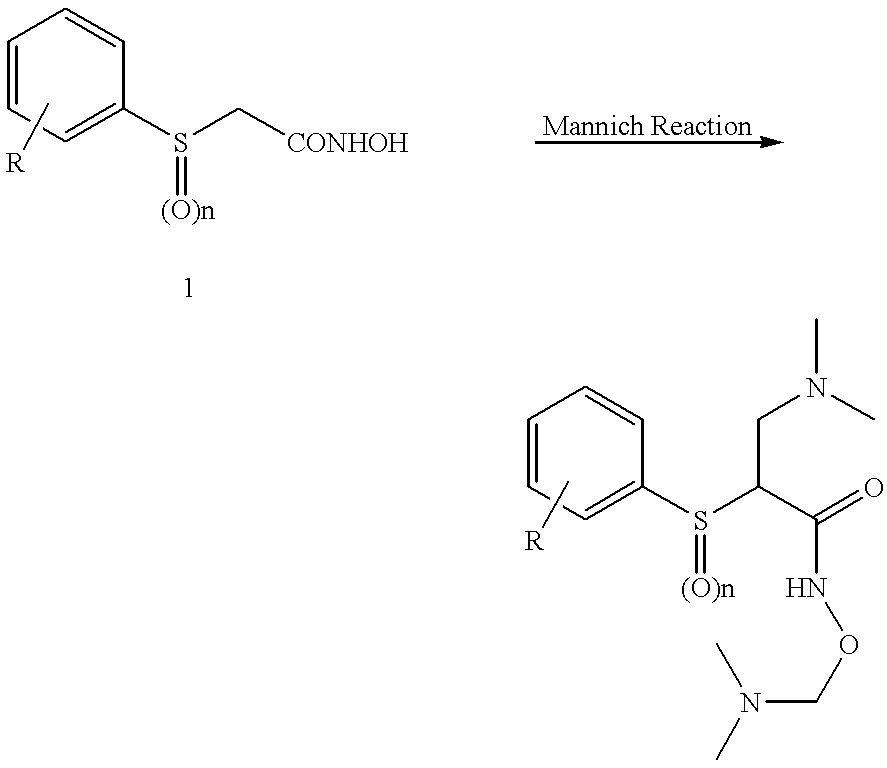
N-hdroxy-2-(alkyl, aryl, or heteroaryl, sulfanyl, sulfinyl or sulfonyl)-3-substituted alkyl, aryl or heteroarylamides as matrix metalloproteinase inhibitors
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formulawherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
Human placenta, umbilical cord mesenchyma stemcell stock and its construction method
ActiveCN1786154AActivity without lossEasy to operateSkeletal/connective tissue cellsDiseaseMesenchyme
The invention discloses a human placenta, umbilical cord mesenchyme stem cell bank and its making method. The method includes the following steps: the first is taking human placenta, umbilical cord to test; smashing after cleaning by phosphate buffer; adding phosphate buffer to dilute; the second is adding collagenase to digest; the third is adding phosphate buffer; the fourth is adding trypsinization; the fifth is mixing the cell from the second and fourth, centrifuging, removing supernatant, cleaning by the phosphate buffer, centrifuging, and removing supernatant; the mesenchyme stem cell are gained; the sixth is freezing them in liquid nitrogen; saving by ABO / Rh subtype and HLA subtype; setting recallable cell information file. The invention is newborn storage placenta and umbilical cord mesenchyme stem cell. It can offer mesenchyme stem cell to treat the disease for personal, family and others.
Owner:TIANJIN AMCELLGENE ENG
Method for separating and extracting stem cells from placenta, umbilical cord or adipose tissue
ActiveCN101693884AEasy accessSpecification acquisitionSkeletal/connective tissue cellsArtificially induced pluripotent cellsFicollDisease
The invention relates to a method for separating and extracting stem cells from a placenta, umbilical cord or adipose tissue, which comprises the following process steps: firstly mixing the placenta, umbilical cord or adipose tissue and a cell maintenance fluid according to the proportion by weight of 2.5-4:1, putting the mixture into a tissue crushing barrel, adding collagenase after crushing, uniformly mixing, hatching at the temperature of 37 DEG C, filtering, adding a precipitator, sucking a supernatant fluid after settling, centrifuging, removing the supernatant fluid, adding concentrated cells into a liquid of diatrizoate sodium-ficoll 400#, then centrifuging, collecting 10-15 ml of intermediate cell layer, washing with the cell maintenance fluid, counting the collected cells, and providing the cells for clinical use when the cell survival ratio is more than or equal to 95 percent. The invention not only realizes the separation and the extraction of all stem cells from the placenta, umbilical cord or adipose tissue, but also realizes industrialized production, and enables doctors to conveniently, safely and canonically obtain the adult stem cells in clinic and use the adult stem cells for treating the diseases of patients as using medicaments, thereby solving the bottleneck problem of difficult obtainment of adult stem cells in clinic and popularizing a cell treatment technology.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
Device and method for treatment of wounds with nitric oxide
InactiveUS20050191372A1Promote healingPrevent leakageBiocideOther blood circulation devicesHigh concentrationNitric oxide gas
Topical exposure of nitric oxide gas to wounds such as chronic non-healing wounds may be beneficial in promoting healing of the wound and in preparing the wound bed for further treatment and recovery. Nitric oxide gas may be used, for example, to reduce the microbial infection and burden on these wounds, manage exudate secretion by reducing inflammation, upregulate expression of endogenous collagenase to locally debride the wound, and regulate the formation of collagen. High concentration of nitric oxide ranging from about 160 to 400 ppm may be used without inducing toxicity in the healthy cells around a wound site. Additionally, exposure to the high concentration for a first treatment period reduces the microbial burden and inflammation at the wound site and increase collagenase expression to debride necrotic tissue at the wound site. After a first treatment period with high concentration of nitric oxide, a second treatment period at a lower concentration of nitric oxide preferably ranging from about 5-20 ppm may to provided to restore the balance of nitric oxide and induce collagen expression to aid in the closure of the wound.
Owner:SENSORMEDICS +1
Multipotent stem cells derived from placenta tissue and cellular therapeutic agents comprising the same
InactiveUS20070243172A1Negative immunological responseBiocideArtificial cell constructsGerm layerDisease
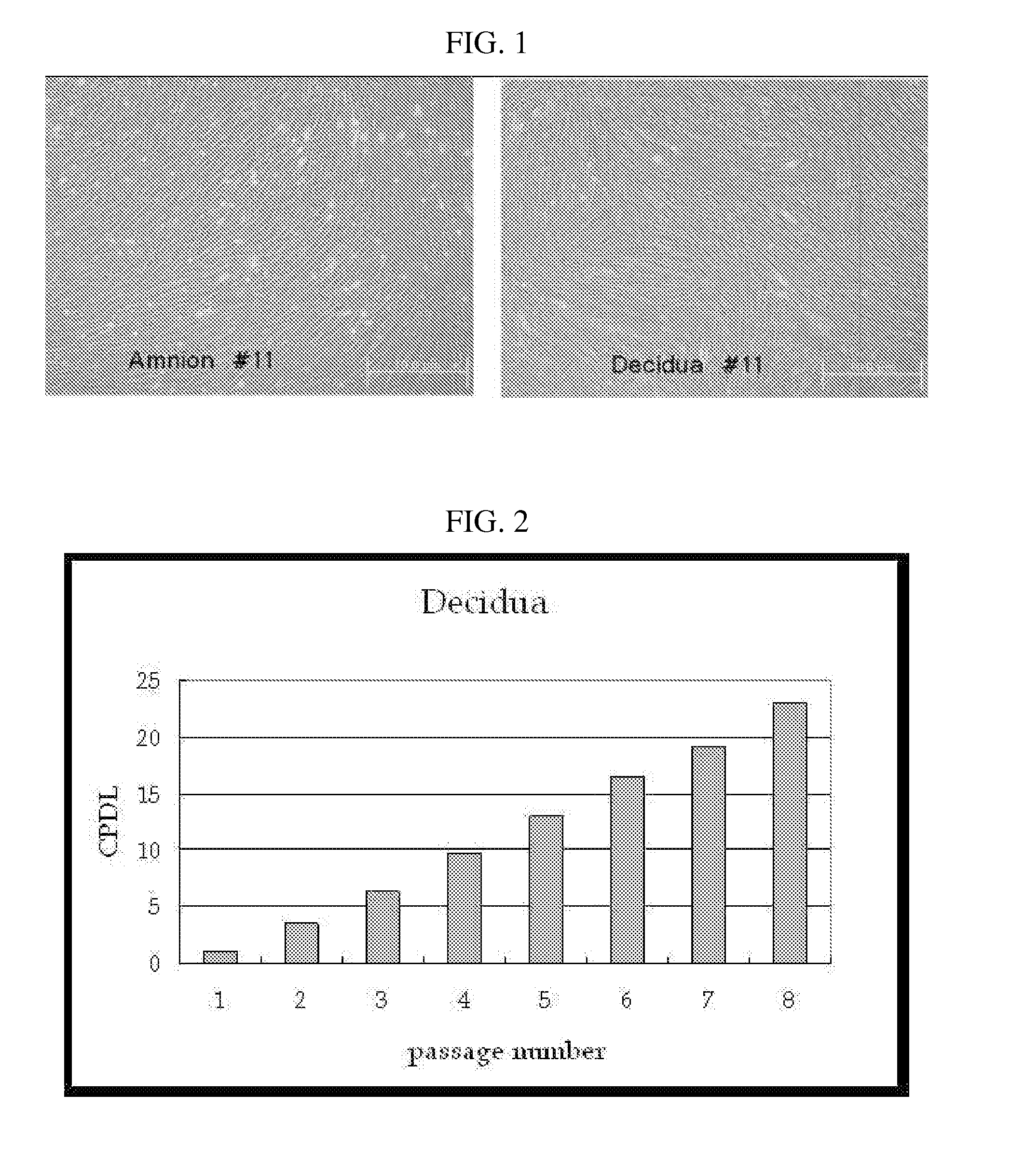
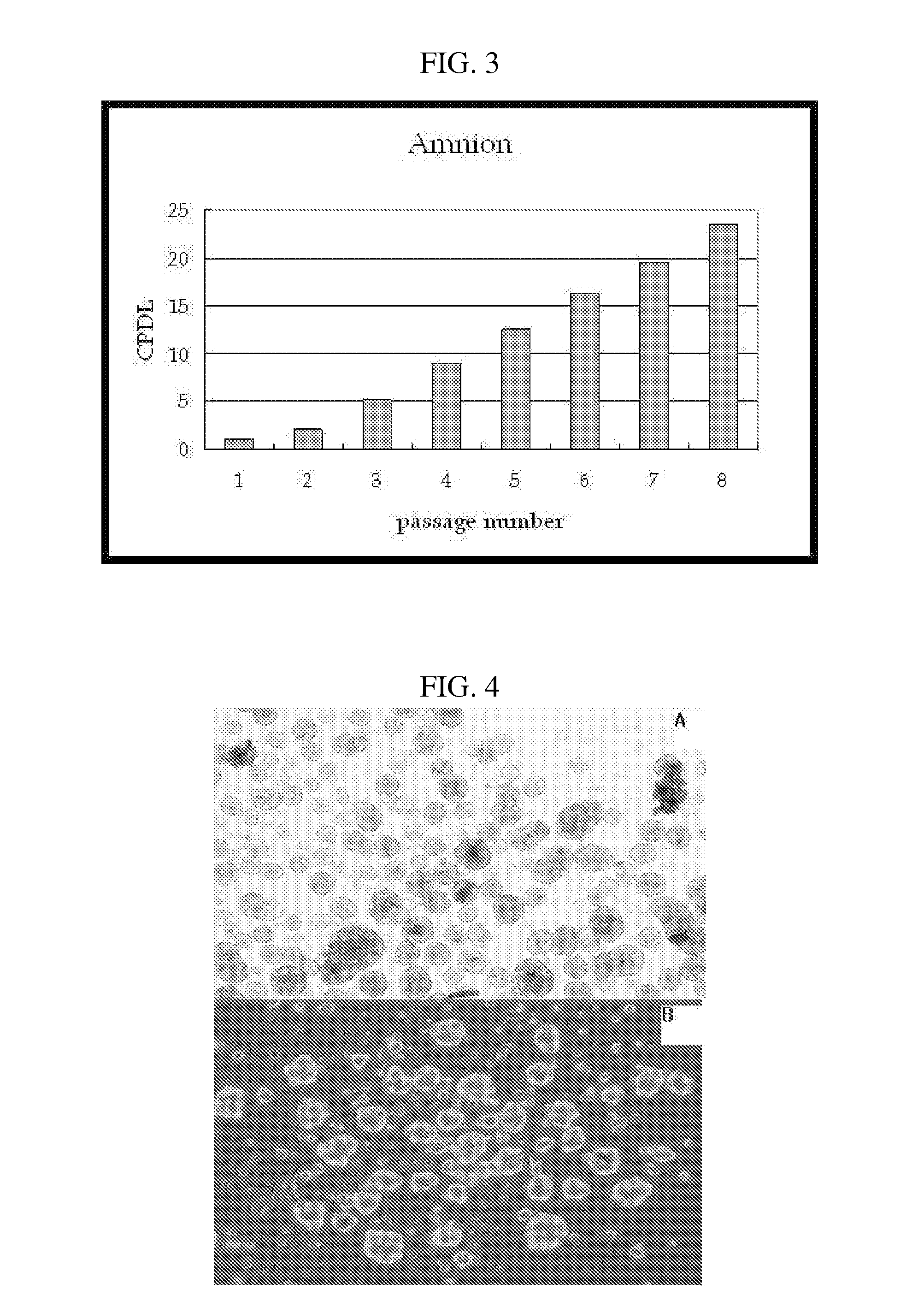
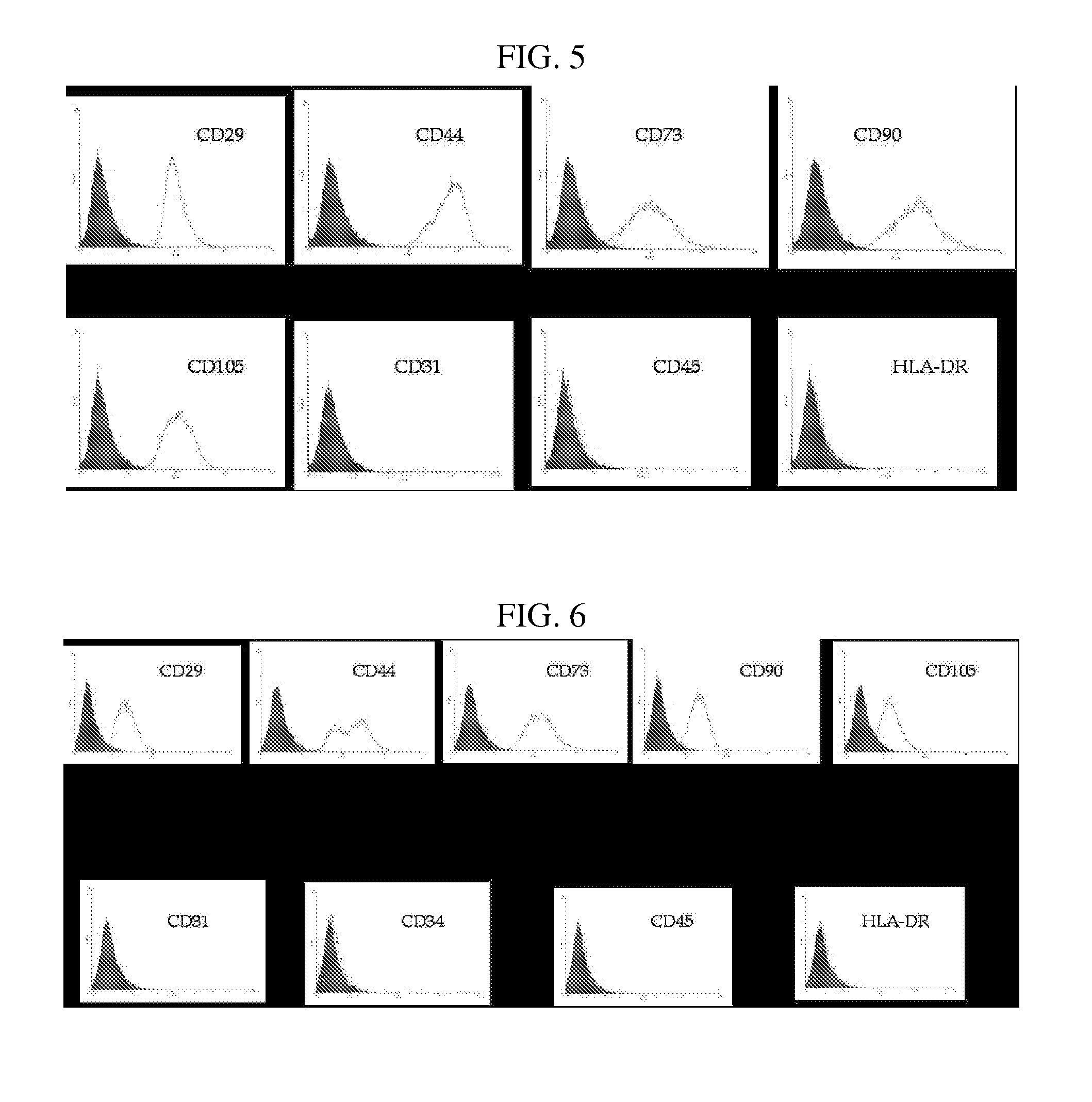
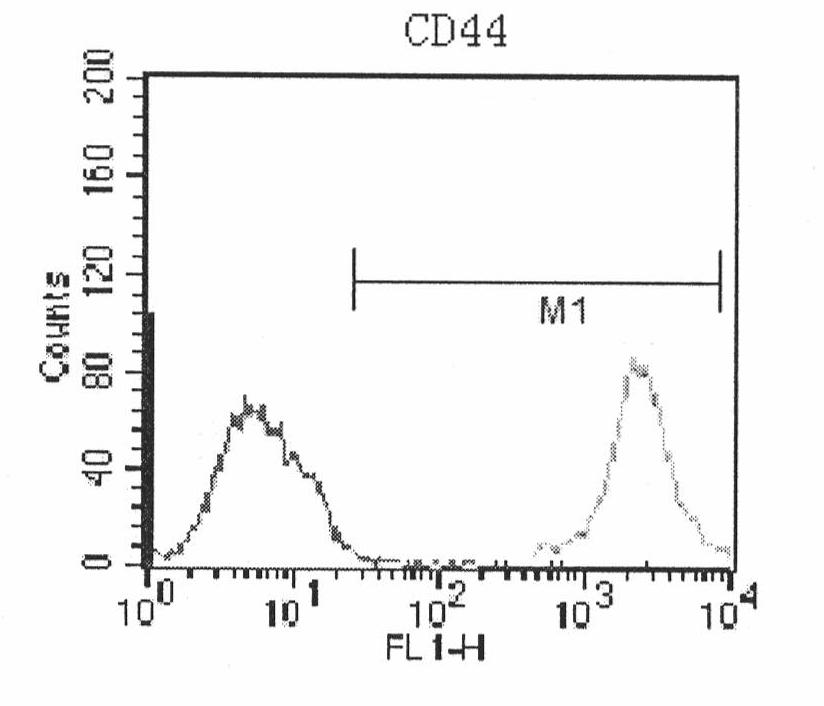
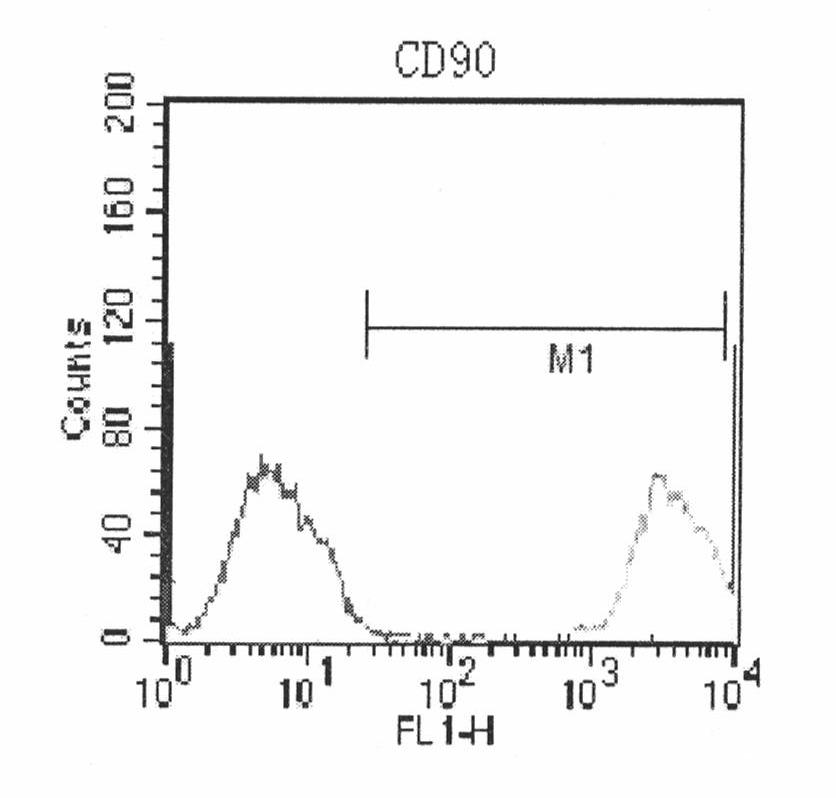
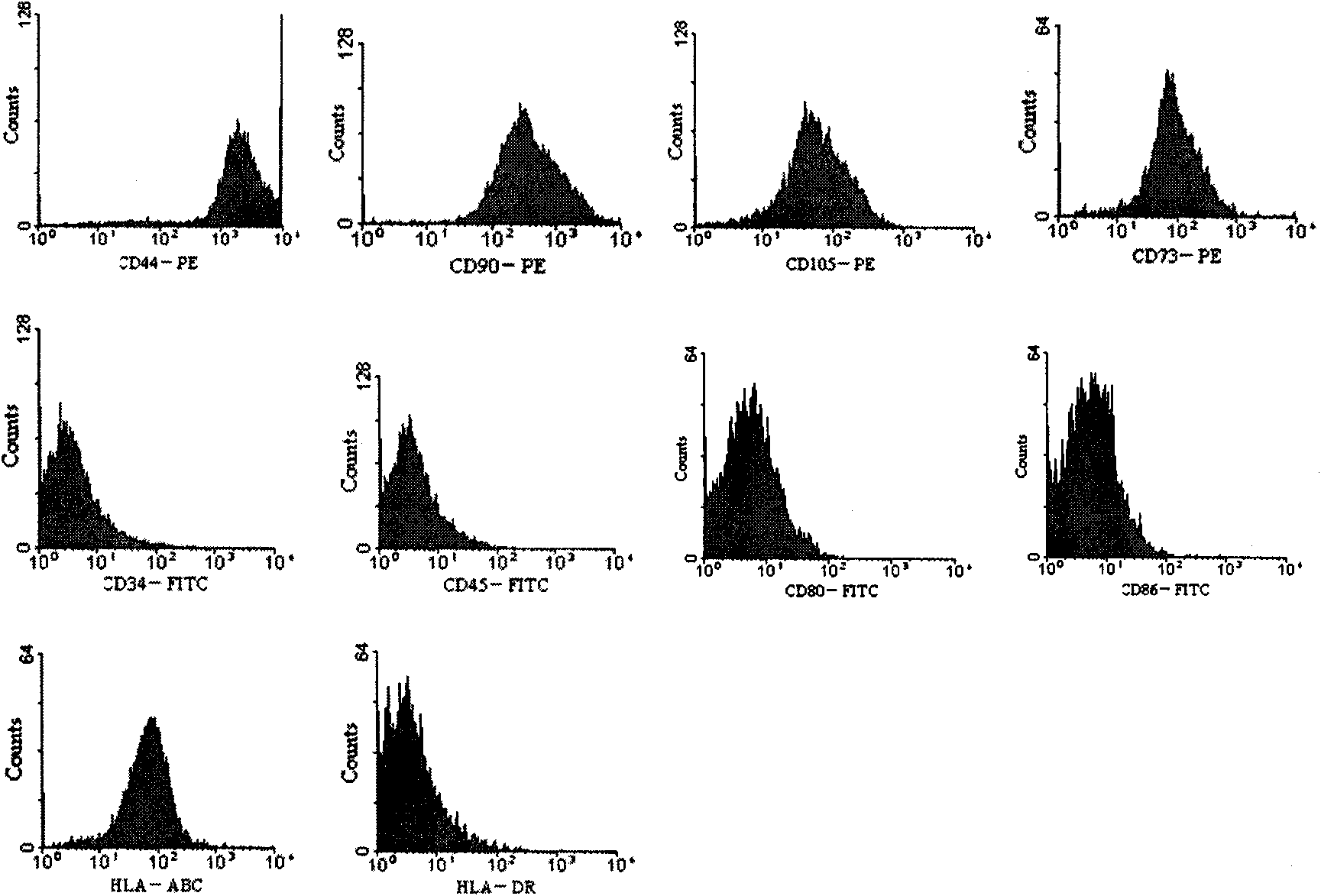
The present invention relates to placenta tissue-derived multipotent stem cells and cell therapeutic agents containing the same. More specifically, to a method for producing placenta stem cells having the following characteristics, the method comprising culturing amnion, chorion, decidua or placenta tissue in a medium containing collagenase and bFGF and collecting the cultured cells: (a) showing a positive immunological response to CD29, CD44, CD73, CD90 and CD105, and showing a negative immunological response to CD31, CD34, CD45 and HLA-DR; (b) showing a positive immunological response to Oct4 and SSEA4; (c) growing attached to plastic, showing a round-shaped or spindle-shaped morphology, and forming spheres in an SFM medium so as to be able to be maintained in an undifferentiated state for a long period of time; and (d) having the ability to differentiate into mesoderm-, endoderm- and ectoderm-derived cells. Also the present invention relates to placenta stem cells obtained using the production method. The inventive multipotent stem cells have the ability to differentiate into muscle cells, vascular endothelial cells, osteogenic cells, nerve cells, satellite cells, fat cells, cartilage-forming cells, osteogenic cells, or insuline-secreting pancreatic β-cells, and thus are effective for the treatment of muscular diseases, osteoporosis, osteoarthritis, nervous diseases, diabetes and the like, and are useful for the formation of breast tissue.
Owner:RNL BIO
Device and method for treatment of wounds with nitric oxide
InactiveUS20070088316A1Promote wound healingReduce the burden onBiocideOther blood circulation devicesHigh concentrationNitric oxide gas
Topical exposure of nitric oxide gas to wounds such as chronic non-healing wounds may be beneficial in promoting healing of the wound and in preparing the wound bed for further treatment and recovery. Nitric oxide gas may be used, for example, to reduce the microbial infection and burden on these wounds, manage exudate secretion by reducing inflammation, upregulate expression of endogenous collagenase to locally debride the wound, and regulate the formation of collagen. High concentration of nitric oxide ranging from about 160 to 400 ppm may be used without inducing toxicity in the healthy cells around a wound site. Additionally, exposure to the high concentration for a first treatment period reduces the microbial burden and inflammation at the wound site and increase collagenase expression to debride necrotic tissue at the wound site. After a first treatment period with high concentration of nitric oxide, a second treatment period at a lower concentration of nitric oxide preferably ranging from about 5-20 ppm may to provided to restore the balance of nitric oxide and induce collagen expression to aid in the closure of the wound.
Owner:ADVANCED INHILATION THERAPIES AIT LTD +1
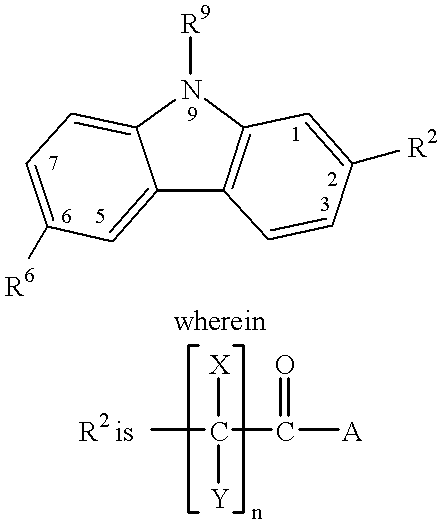
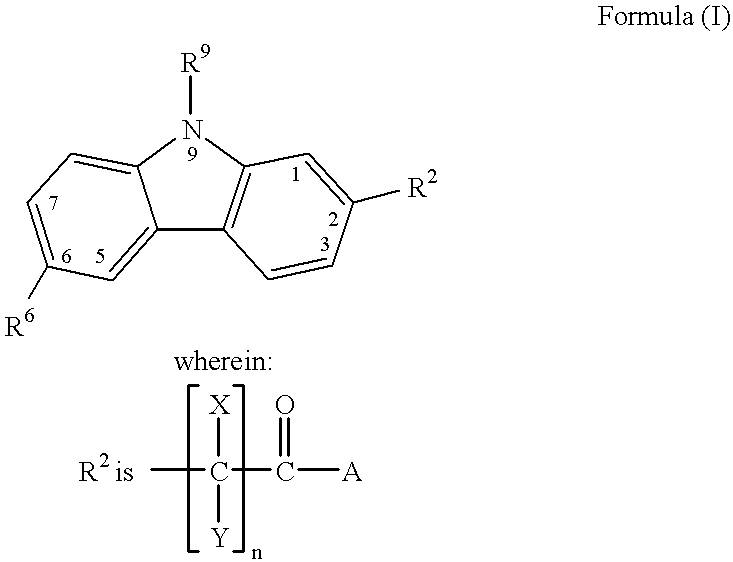
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
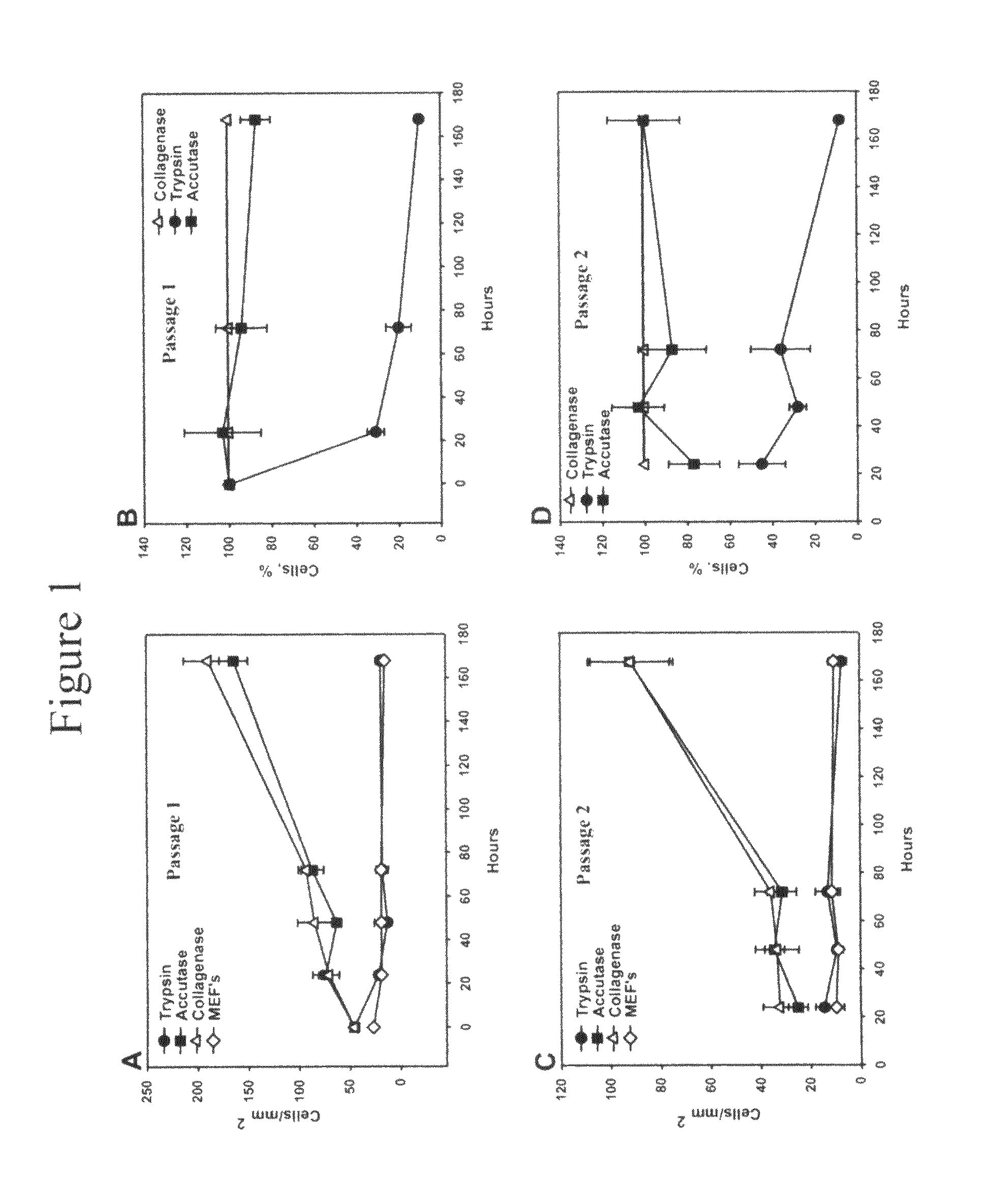
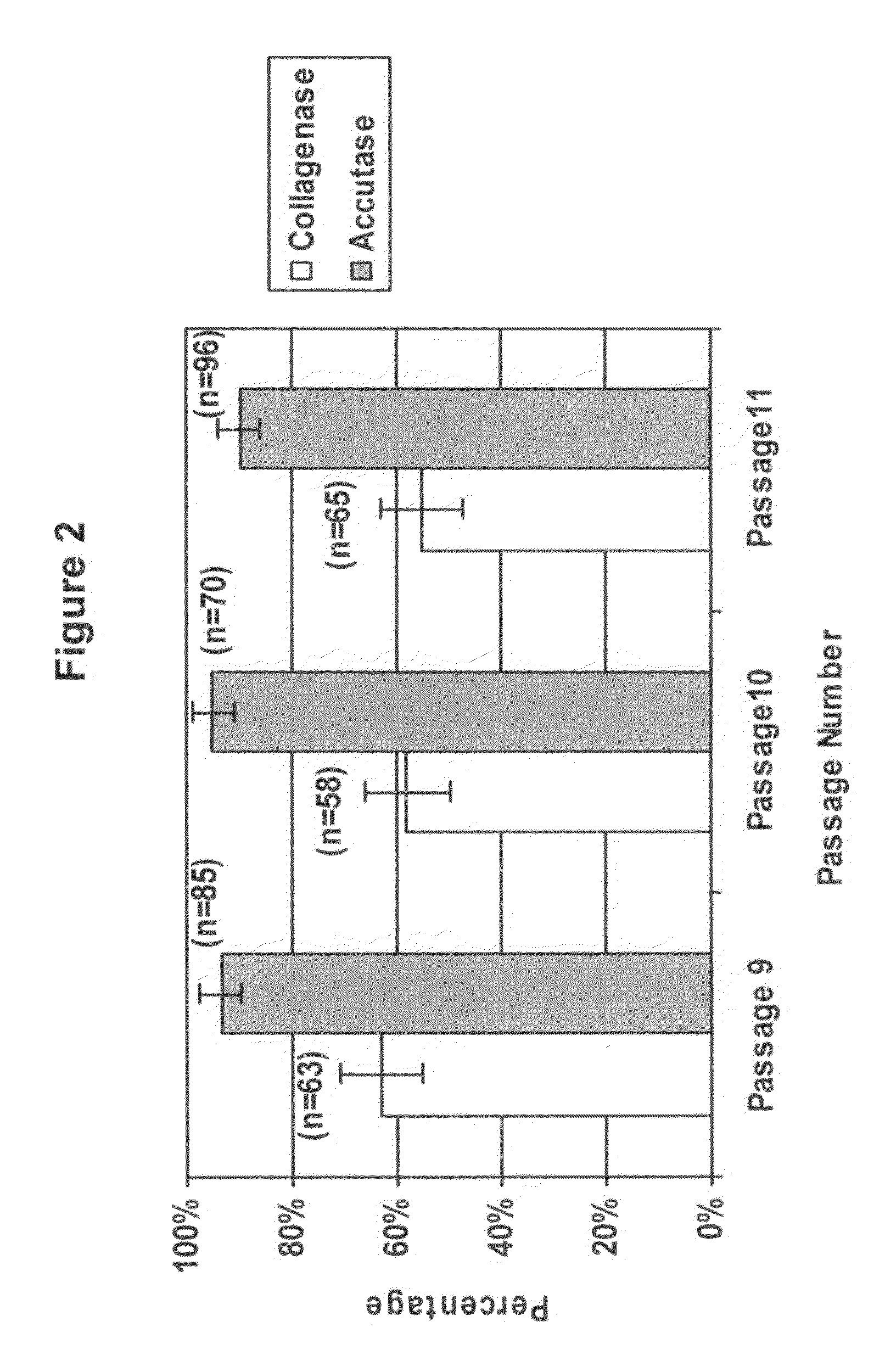
Methods for culture and production of single cell populations of human embryonic stem cells
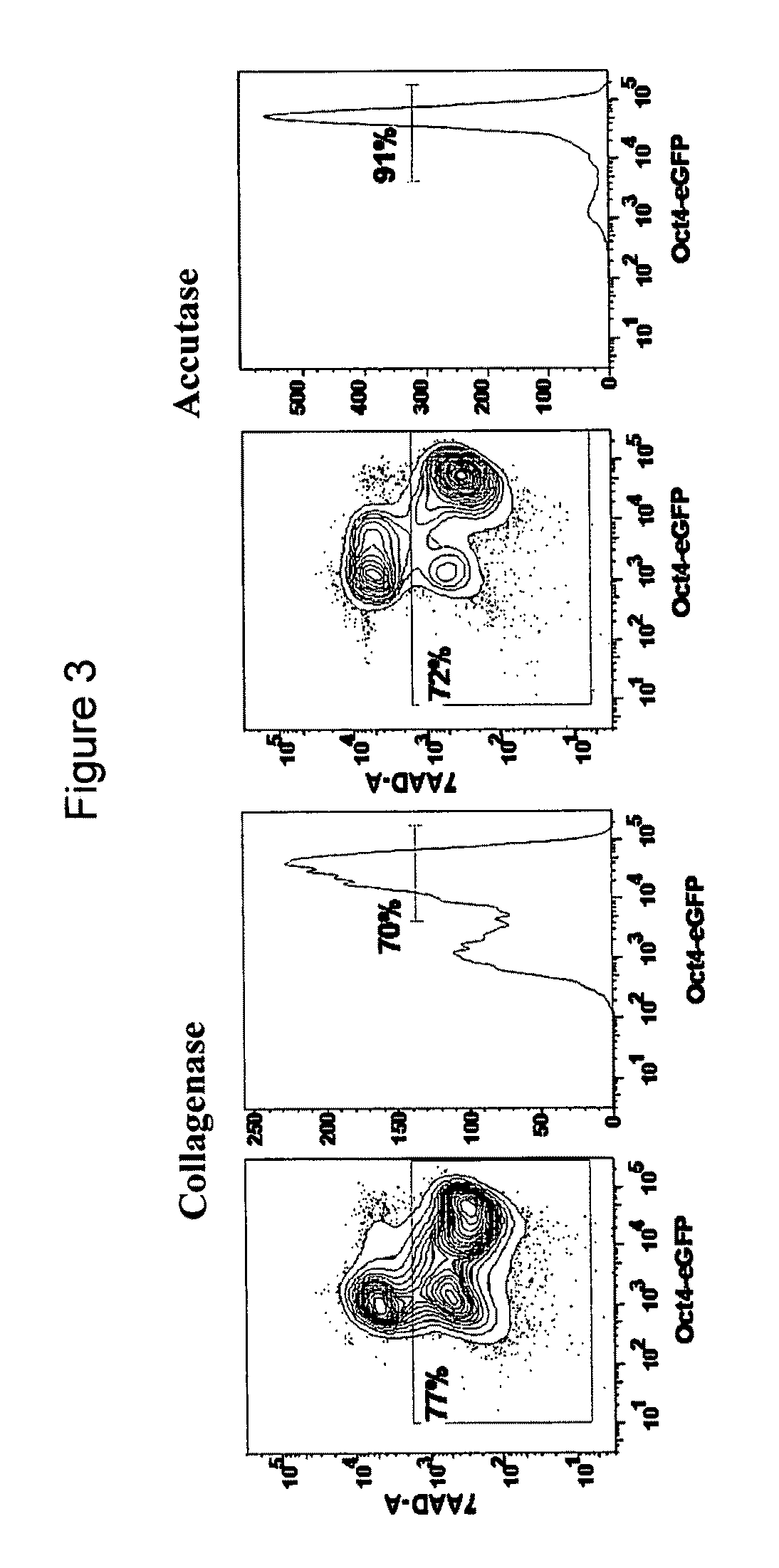
We used Accutase™, a commercially available cell detachment solution, for single cell propagation of pluripotent hESCs. Unlike trypsin dissociation, Accutase treatment does not significantly affect the plating efficiency of hESC dissociation into single cells. Cultures dissociated with Accutase to single cells at each passage maintain a higher proportion of pluripotent cells as compared to collagenase-passaged hESCs. Accutase-treated hESCs can be grown to a high density as monolayers, and yet retain their pluripotency.
Owner:BURNHAM INST FOR MEDICAL RES
Synthetic polypeptide-containing bioapplicable material and film-forming material
InactiveUS20070207180A1Improve securityHigh bioaffinityTripeptide ingredientsImmunoglobulinsCotton effectLength wave
The present invention provides a bioapplicable material (or composition) or film-forming material (or composition), which is free from a risk of an infection by a pathogenic organism or a transmission of a causative factor, has a high safety. The material (or composition) comprises a collagen-like synthetic polypeptide having at least an amino acid sequence represented by the formula -Pro-Y-Gly- (wherein Y represents Pro or Hyp). The polypeptide may show positive Cotton effect at a wavelength in range of 220 to 230 nm and negative Cotton effect at a wavelength in range of 195 to 205 nm in a circular dichroism spectrum. At least part of the polypeptide may be capable of forming a triple helical structure. The polypeptide may be degradable with a collagenase.
Owner:PHG
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Topical Skin Compositions, Their Preparation, and Their Use
InactiveUS20080081082A1Inhibit the causative factorsCosmetic preparationsBiocideBarrier functionDermatology
Owner:ACCESS BUSINESS GRP INT LLC
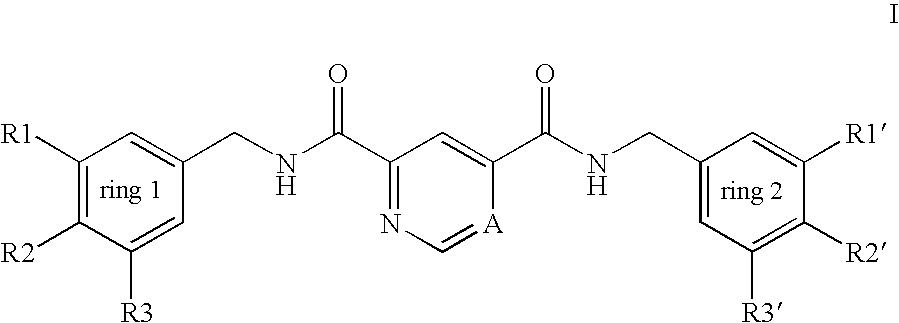
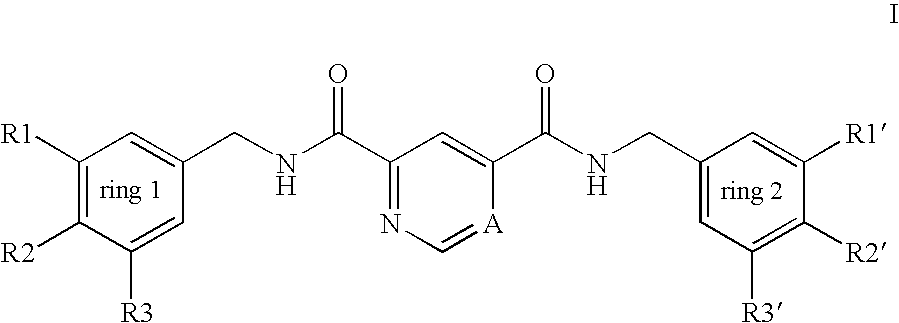
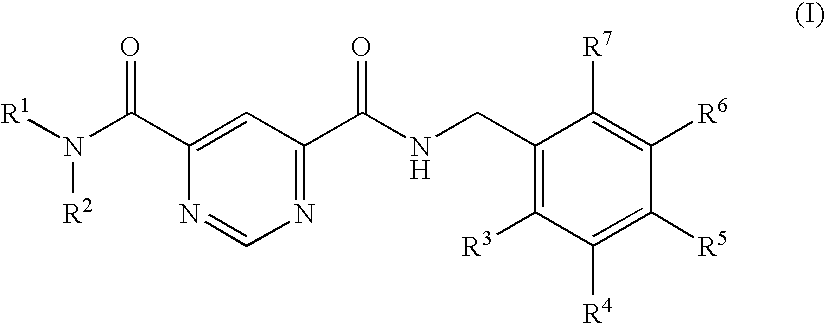
Pyridine-2,4-dicarboxylic acid diamides and pyrimidine-4,6-dicarboxylic acid diamides and the use thereof for selectively inhibiting collagenases
Abstract of Disclosure Pyridine-2,4-dicarboxylic acid diamides and pyrimidine-4,6-dicarboxylic acid diamides of formula I. These compounds are found to possess the property of selectively inhibiting collagenase (MMP). They may therefore be useful in prophylaxis and therapy of diseases whose course involves an increased activity of matrix metalloproteinase 13.
Owner:SANOFI AVENTIS DEUT GMBH
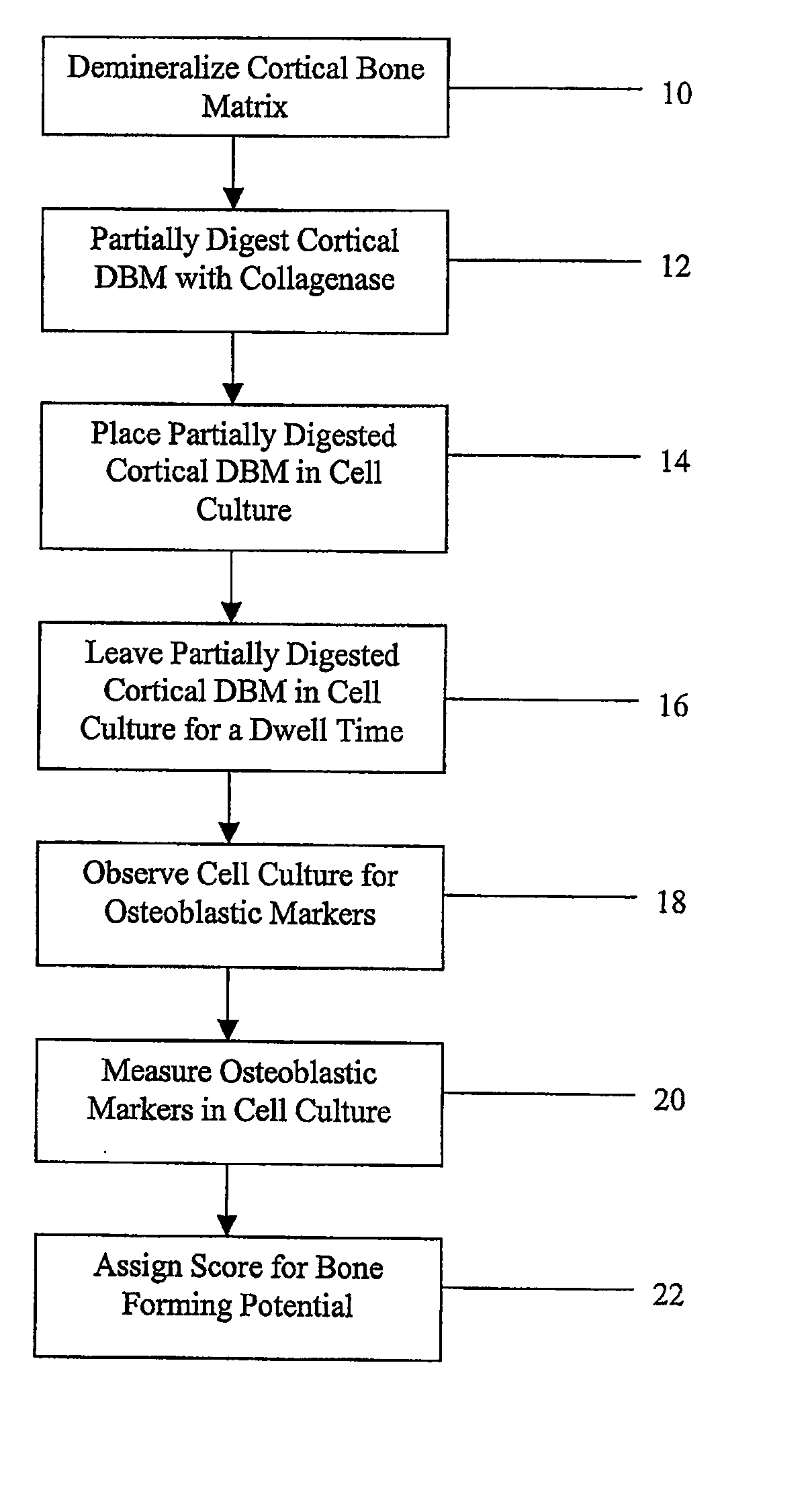
Method for In Vitro Assay of Demineralized Bone Matrix
A sensitive, quantitative method to assess bone forming potential of demineralized bone matrix (DBM) is provided. Cortical DBM is treated with collagenase. The treated cortical DBM is placed in a cell culture for a dwell time. After the dwell time, the cell culture is observed for osteoblastic markers. The cortical bone so treated exhibits the same bone forming potential in vivo as untreated cortical bone.
Owner:WARSAW ORTHOPEDIC INC
Construction method of human umbilical cord mesenchyma stem cell
ActiveCN101608174AAvoid damageActivity without lossDead animal preservationTissue cultureUmbilical cord tissueCell damage
The invention discloses a construction method of an umbilical cord mesenchyma stem cell bank utilizing newborn umbilical cords as resources, aiming at causing the steps to be less and the operation to be simpler and easier when constructing the umbilical cord mesenchyma stem cell bank. The invention comprises the following steps: (1), taking a human umbilical cord for detection, using a buffer solution to wash and remove residual blood, shearing to pieces, thus obtaining human umbilical cord tissue; (2), adding collagenase for digestion; (3), adding the buffer solution for diluting the digested tissue followed by organizing, mixing evenly and centrifuging; (4), abandoning the supernatant, adding the buffer solution for heavy suspension precipitation, screening, collecting filtrate and centrifuging, and repeating twice to obtain the human umbilical cord mesenchyma stem cell; (5), saving the obtained human umbilical cord mesenchyma stem cell in liquid nitrogen according to ABO / Rh parting and HLA parting thereof, establishing a cell information archive for retrieval, thus constructing the human umbilical cord mesenchyma stem cell bank. Compared with the existing method, the invention is more fast and convenient, and has less damage to the cell.
Owner:章毅 +7
Plant based formulations for improving skin moisture, texture, and appearance
ActiveUS20060198810A1Increase moistureImprove textureCosmetic preparationsBiocideLipid formationAdditive ingredient
The present invention relates to formulations of ingredients that are useful for improving the appearance, texture and / or moisture of skin. In particular, the formulations of the present invention stimulate collagen, elastin, and lipid synthesis and / or inhibit or minimize the loss of collagen, elastin, and lipids in the skin. Additionally, the formulations of the present invention inhibit matrix metalloproteases, such as MMP-1, MMP-9, collagenase, or elastase.
Owner:ACCESS BUSINESS GRP INT LLC
Methods for introducing genes into mammalian subjects
InactiveUS7067496B2Increase successAntibacterial agentsOrganic active ingredientsDelivery vehicleMammal
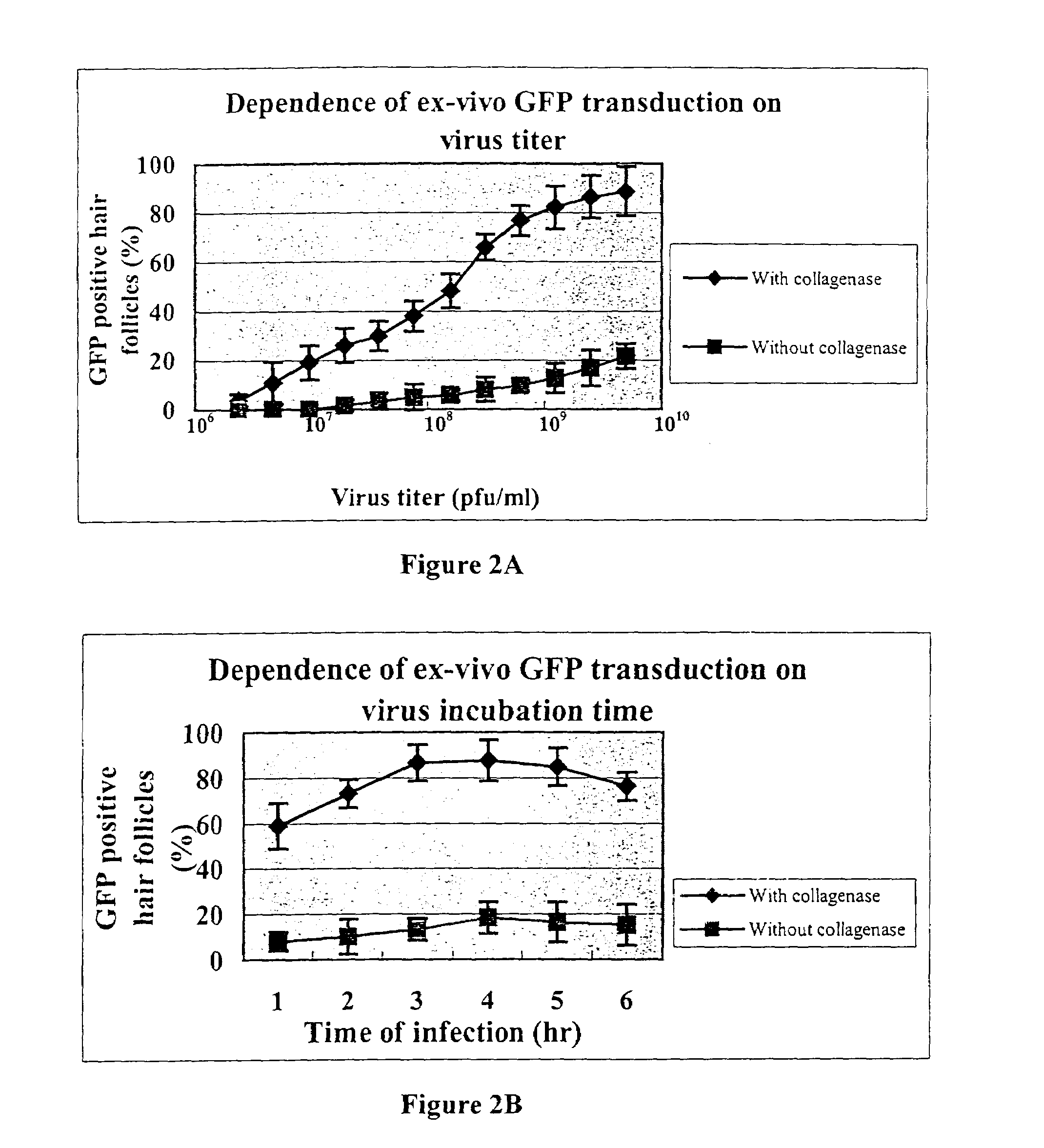
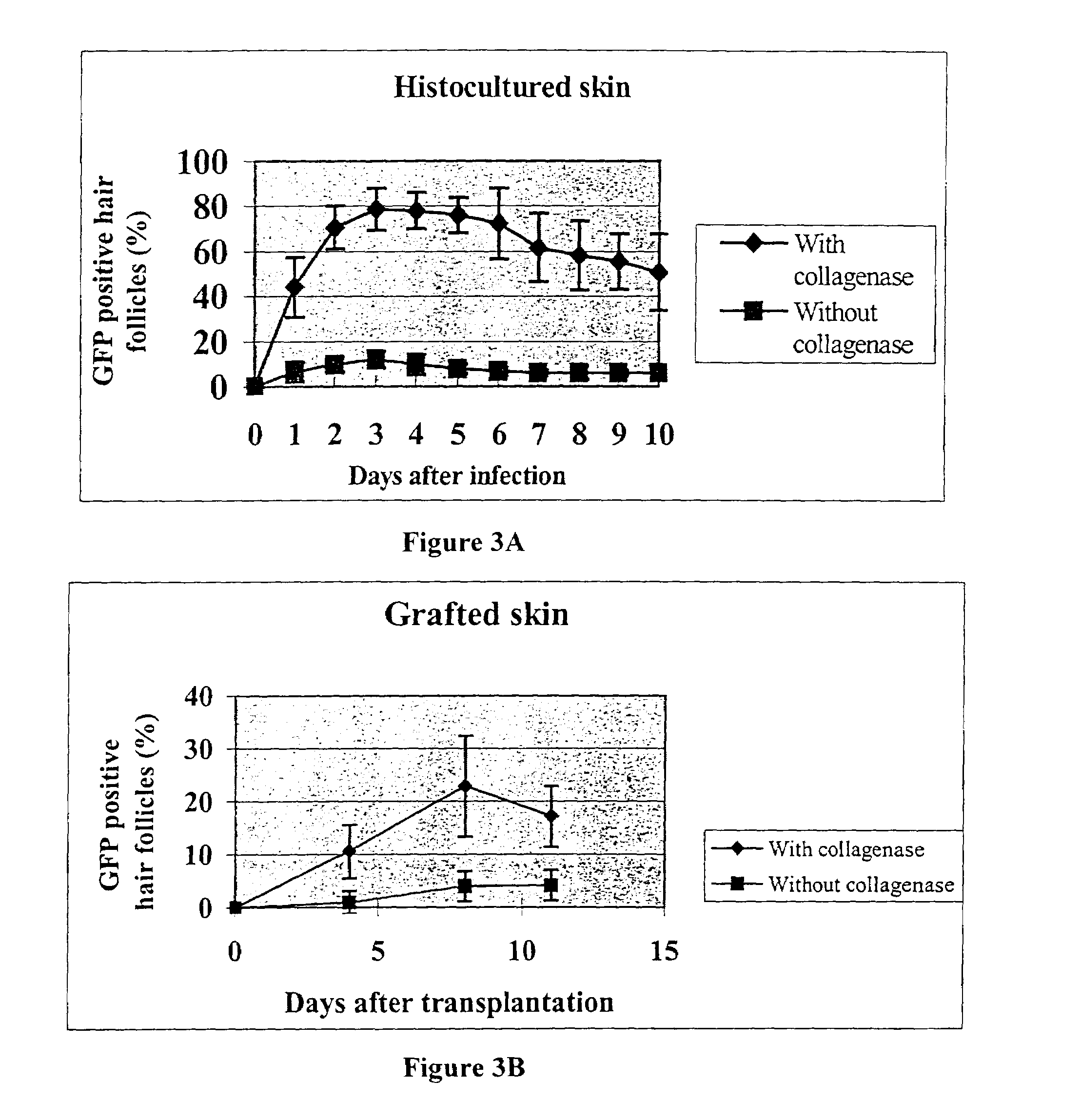
Methods to obtain genetic modifications of cells in histoculture are described. Modification is assisted by treating the histoculture with collagenase prior to contacting the histoculture with the delivery vehicle for the desired gene. Hair follicles and other organized tissues can be modified in this was and then transplanted into intact recipients.
Owner:ANTICANCER
Preparations Derived From Placental Materials and Methods of Making and Using Same
Preparations derived from placental materials and methods of making and using same, the preparations including a first preparation composed of placental membranes digested in collagenase, a second preparation composed of multipotent cells derived from the collagenase digested placental membranes and that are grown in adherent culture beyond confluence and a third preparation composed of ground placental membranes re-suspended in a fluid containing hyaluronic acid. The preparations can be used for regenerating damaged or defective tissue including connective tissue, nerve tissue, muscle tissue, skin tissue, cartilage tissue and bone tissue. The preparations can also be used as dermal fillers in cosmetic and plastic surgery applications.
Owner:NUTECH MEDICAL
Preparation method of umbilical cord Huatong glue mesenchymal stem cells and product thereof
The invention discloses a preparation method of original mesenchymal stem cells. In the invention, the mesenchymal stem cells are prepared by adopting fresh human umbilical cord, extracting Huatong glue, digesting by collagenase, and separating and cultivating, and the mesenchymal stem cells are suitable for cryopreservation. The invention also relates to the original mesenchymal stem cells prepared by the method, and the original mesenchymal stem cells are verified as mesenchymal stem cells by the identification of biological phenotype according to standard formulated by the International Society of Cytotherapy. The multiplication capability and the purity of the mesenchymal stem cell are both higher than those of bone mesenchymal stem cells; and the amount of the released growth factorsand cell factors is higher than that of the bone mesenchymal stem cells.
Owner:PLA NAVY GENERAL HOSIPTAL
Small molecule bovine bone collagen peptide and preparation method thereof
ActiveCN105018554AHigh yieldPracticalPeptide preparation methodsFermentationCrystallographyProteinase activity
The invention relates to a small molecule bovine bone collagen peptide and a preparation method thereof. The preparation method comprises the following steps in sequence: grinding, extraction separation, enzymolysis, composite filtration, concentration and drying, wherein in the enzymolysis process, bone collagenase is added to a bovine bone collagen extracting solution to carry out primary enzymolysis; after primary enzymolysis, the pH value of a primary enzymolysis solution is adjusted to be 6-7 with a mixed acid and then proline protease is added to carry out secondary enzymolysis for 2-4 hours; the addition of proline protease is 0.1-0.3% of the mass of bovine bone collagen. The small molecule bovine bone collagen peptide and the preparation method have the beneficial effects that according to the specificity of bovine bone collagen, proline protease and bone collagenase are adopted for the first time to carry out composite enzymolysis, so that the obtained product has strong practicability and the yield of the small molecule active peptide is as high as 90%.
Owner:北京善肽堂生物科技有限公司
Topical Skin Compositions, Their Preparation, and Their Use
InactiveUS20080124409A1Inhibit causative factorInhibit the causative factorsCosmetic preparationsBiocideBarrier functionDermatology
Topical skin compositions include a complex containing components to provide a defense against the various pathway mechanisms of free radicals, reactive oxygen species, reactive nitrogen species, and other oxidizing species on the human body including the skin. The compositions may be administered by topically applying them in an amount to inhibit those mechanisms. The compositions and methods are directed to the prevention of the adverse or detrimental effects of free radicals, reactive oxygen species, reactive nitrogen species, and other oxidizing species on the human body including the skin. Thus, the compositions according to the invention improve barrier function, inhibit elastase and collagenase, and / or promote synthesis of collagen and elastin.
Owner:ACCESS BUSINESS GRP INT LLC
Plant based formulations for improving skin moisture, texture, and appearance
ActiveUS7348034B2Increase moistureImprove textureCosmetic preparationsBiocideLipid formationAdditive ingredient
The present invention relates to formulations of ingredients that are useful for improving the appearance, texture and / or moisture of skin. In particular, the formulations of the present invention stimulate collagen, elastin, and lipid synthesis and / or inhibit or minimize the loss of collagen, elastin, and lipids in the skin. Additionally, the formulations of the present invention inhibit matrix metalloproteases, such as MMP-1, MMP-9, collagenase, or elastase.
Owner:ACCESS BUSINESS GRP INT LLC
Composition for preventing or treating periodontal diseases comprising extract from Achyranthis radix or Ulmus cortex
InactiveUS6045800AThorough understandingGood treatment effectCosmetic preparationsBiocideMedicineSuperoxide
The present invention provides a composition for preventing or treating periodontal diseases comprising an extract of Achyranthis radix, Ulmus cortex or a mixture thereof which inhibits the productions of superoxide, prostaglandin, and interleukin (IL-1.beta.) which are inducers for periodontal diseases and inhibits the enzyme activity of collagenase which decomposes collagen protein which is a substrate for the periodontal tissues, and at the same time promotes collagen protein synthesis, thereby treating periodontal diseases efficiently.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
Topical Skin Compositions, Their Preparation, and Their Use
InactiveUS20080081034A1Inhibit the causative factorsOrganic active ingredientsCosmetic preparationsBarrier functionDermatology
Topical skin compositions include a complex containing components to provide a defense against the various pathway mechanisms of free radicals, reactive oxygen species, reactive nitrogen species, and other oxidizing species on the human body including the skin. The compositions may be administered by topically applying them in an amount to inhibit those mechanisms. The compositions and methods are directed to the prevention of the adverse or detrimental effects of free radicals, reactive oxygen species, reactive nitrogen species, and other oxidizing species on the human body including the skin. Thus, the compositions according to the invention improve barrier function, inhibit elastase and collagenase, and / or promote synthesis of collagen and elastin.
Owner:ACCESS BUSINESS GRP INT LLC
Method for protecting and restoring skin using selective MMP inhibitors
InactiveUS20020119107A1Type of reductionEliminate the effects ofCosmetic preparationsToilet preparationsUltravioletSolar ultraviolet radiation
The invention is based on selective inhibition of the enzyme (MMP-1), which causes the dermal matrix damage in humans, while sparing the enzyme(s) (MMP-9 and perhaps MMP-2) which not only do not cause the damage (based on extrapolation from our in vitro collagen gel system to real skin) but actually "clear away" the damage produced by MMP-1 to restore normal function to the skin. Matrix metalloproteinase-1 (MMP-1; fibroblast collagenase) is induced by UV radiation from the sun and is naturally elevated in old age. Human fibroblasts exposed to the degradation products of MMP-1 contract collagen, but when this debris is removed from their environment, the fibroblasts behave normally. Inhibiting MMP-1 but sparing enzymes that remove the debris improves human skin after onslaught from solar UV radiation, old age, and acne.
Owner:RGT UNIV OF MICHIGAN
Selective MMP-13 inhibitors
The invention relates to a pyrimidine-4,6-dicarboxylic acid diamide compound, pharmaceutical preparation comprising it, process for preparing it and method for its pharmaceutical use. Particularly, the pyrimidine-4,6-dicarboxylic acid diamide compound is useful for selectively inhibiting collagenase matrix metalloproteinase (MMP) 13, or for treating a degenerative joint disease.
Owner:SANOFI AVENTIS DEUT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com