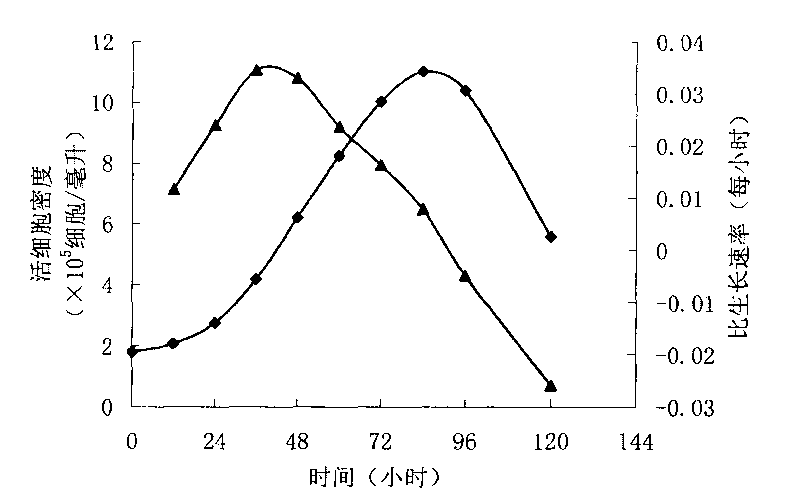
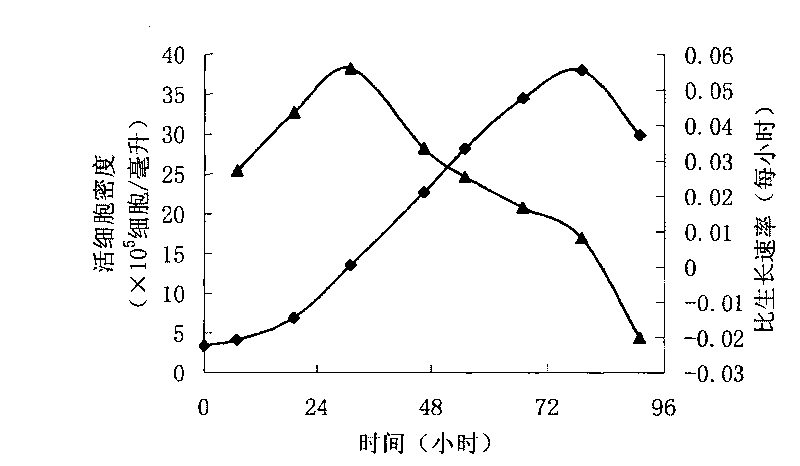
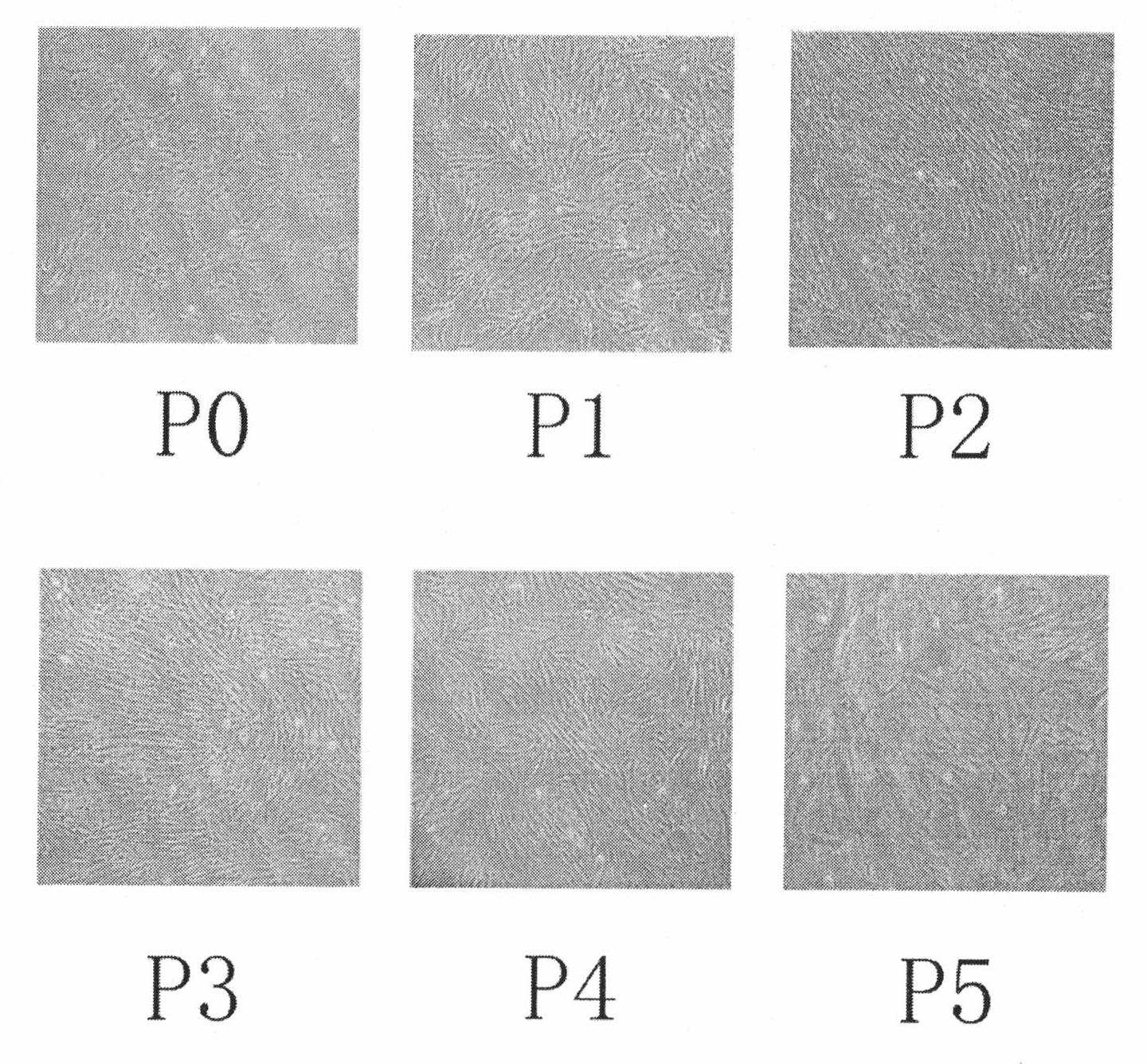
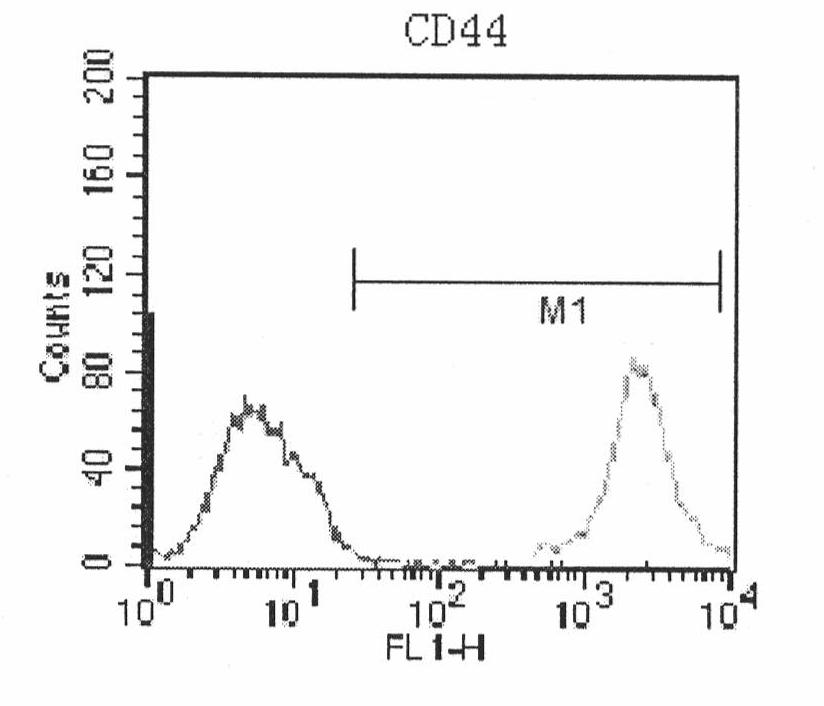
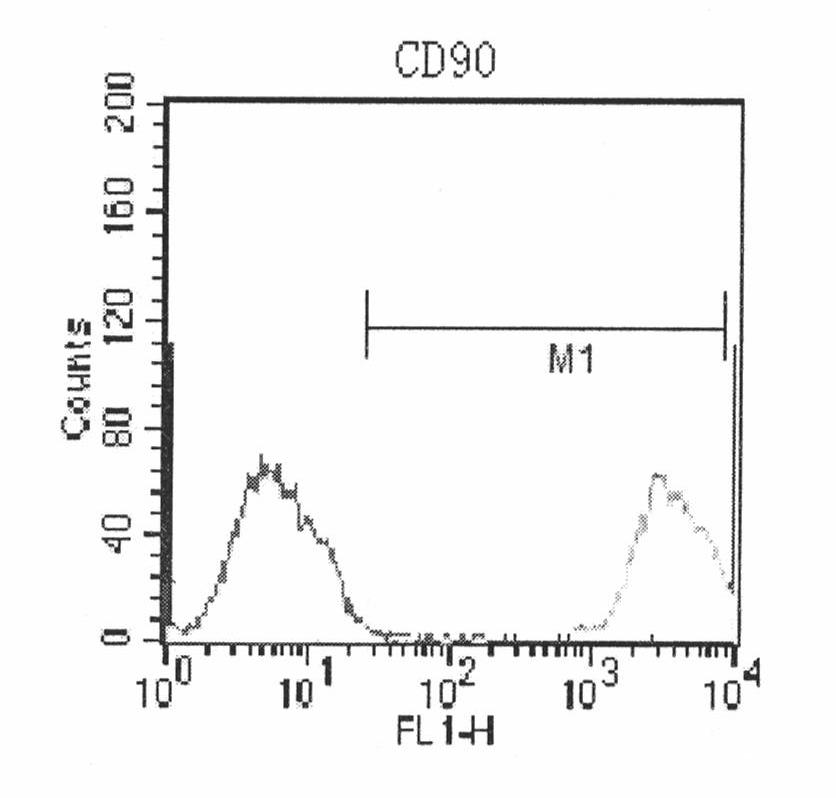
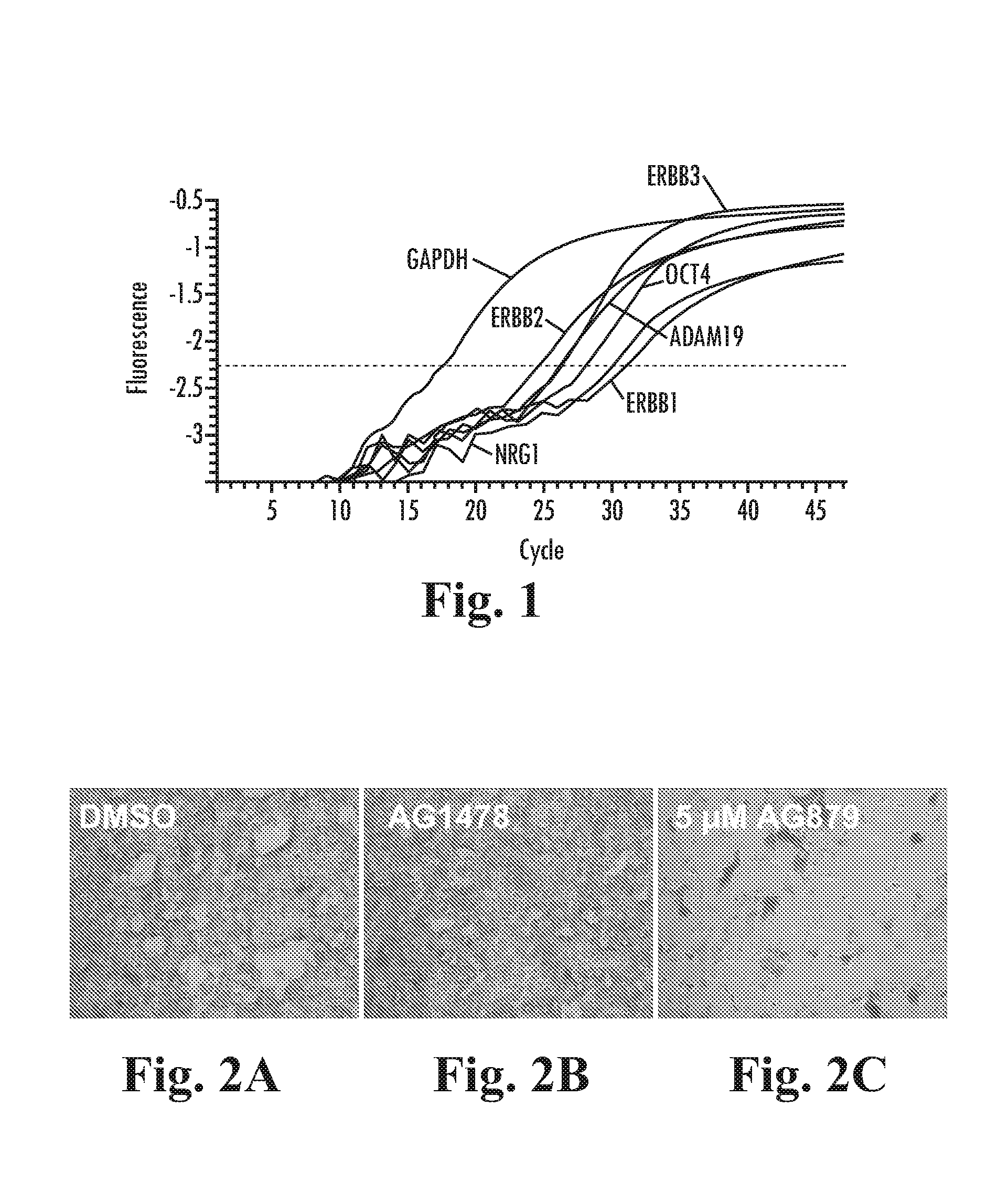
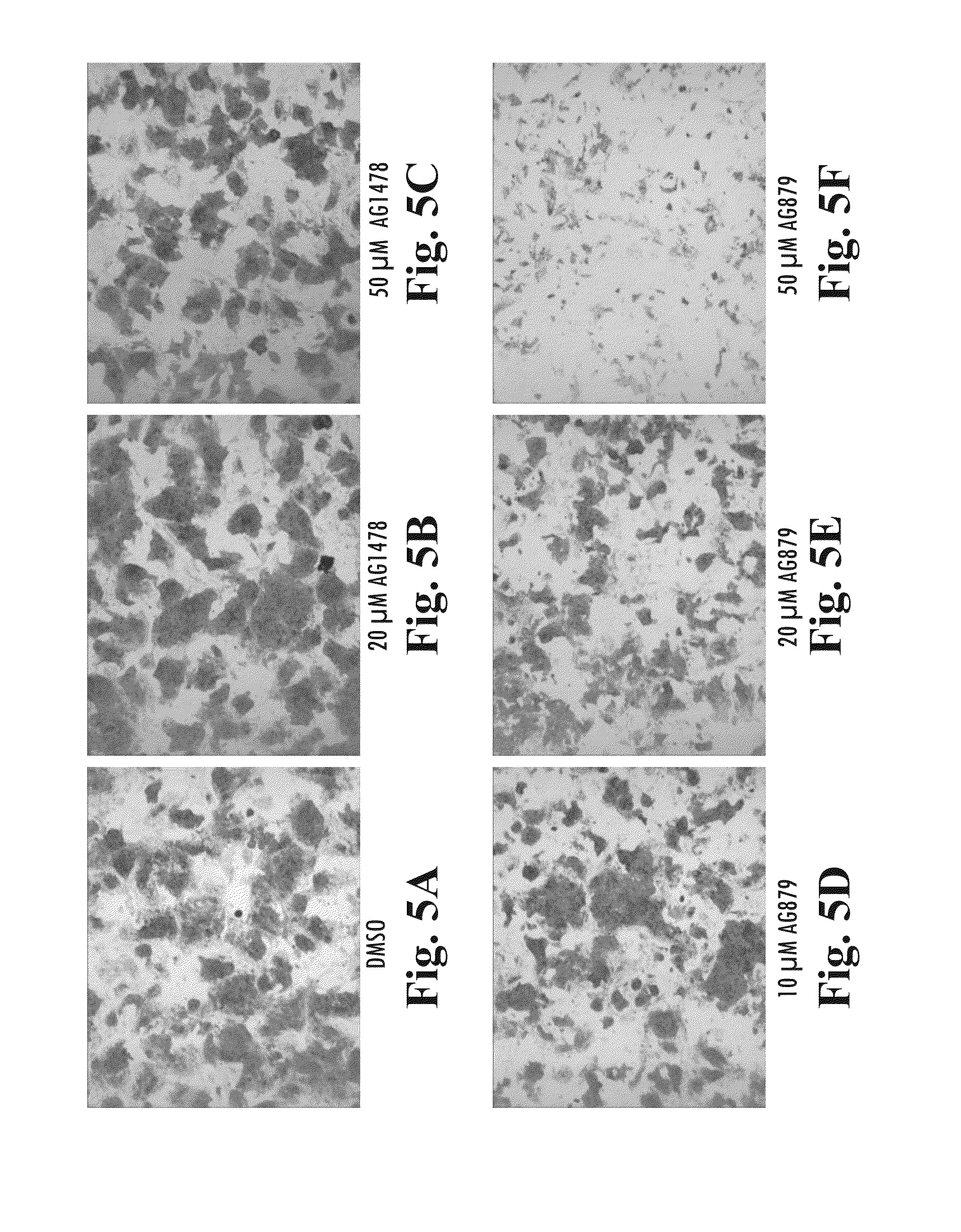
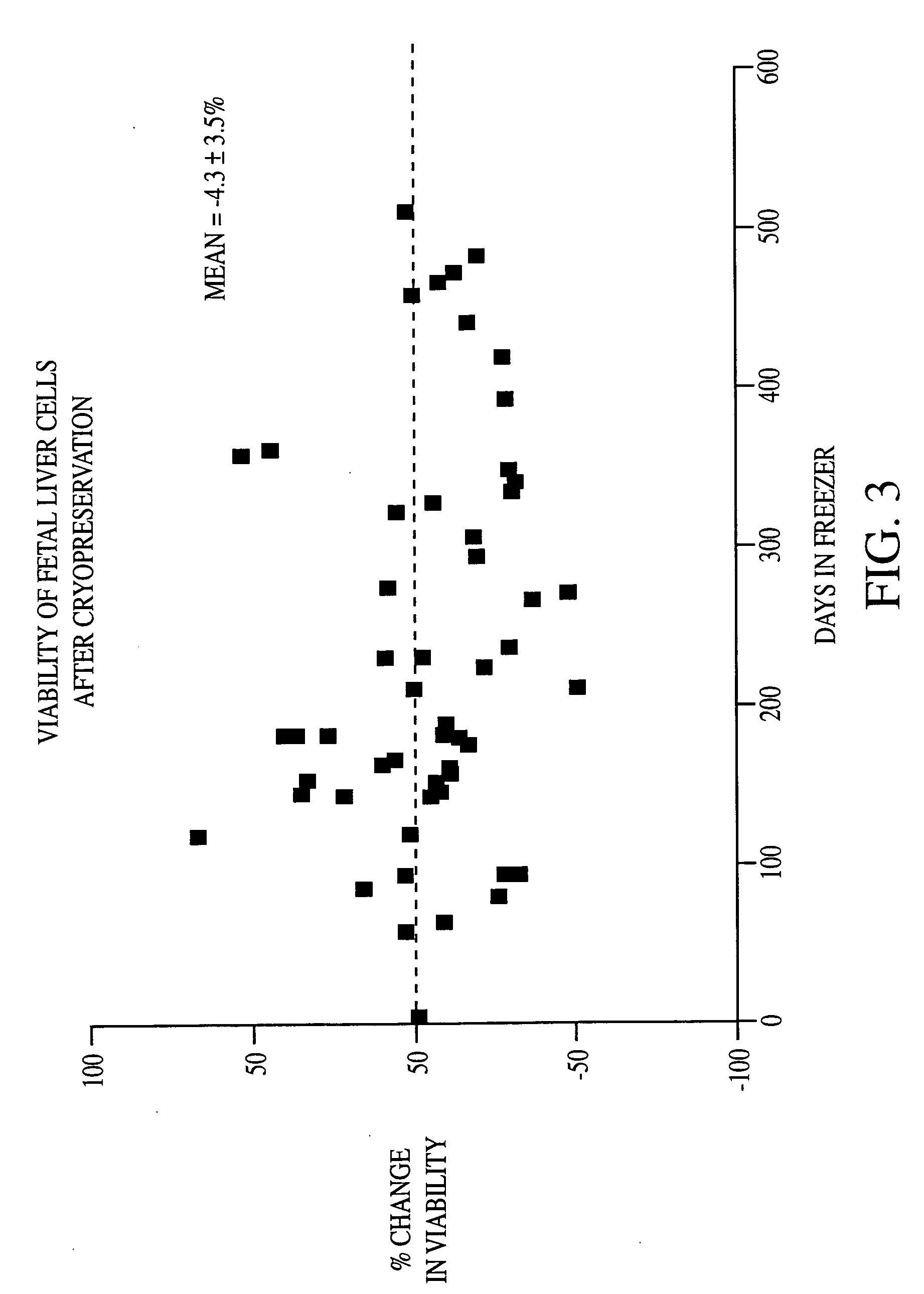
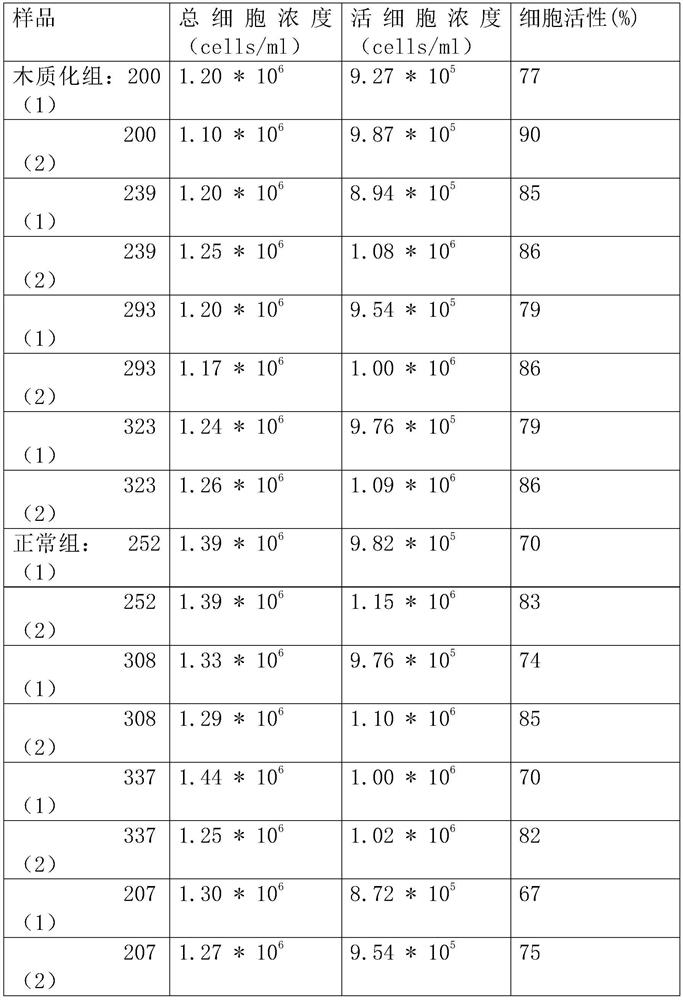
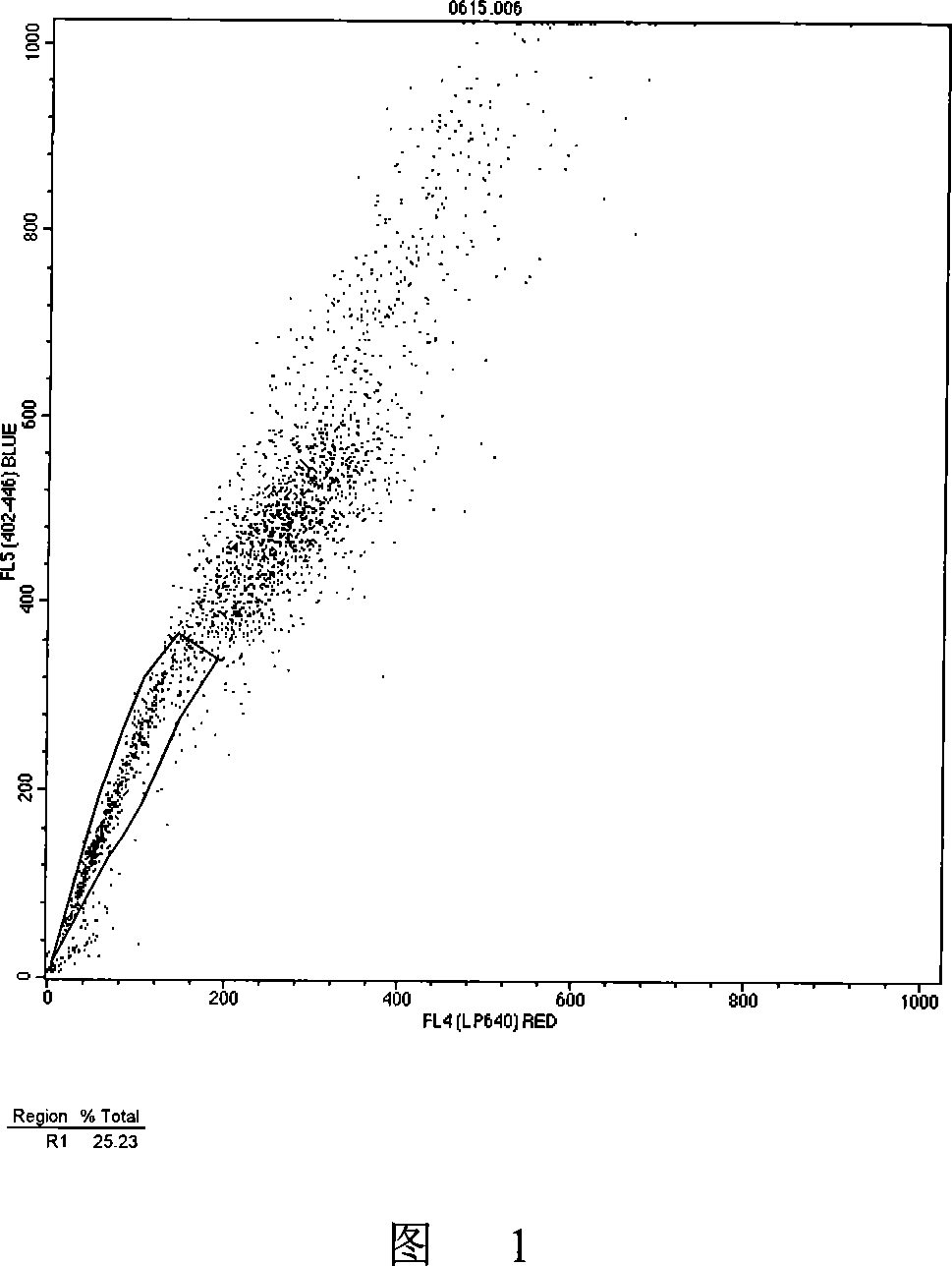
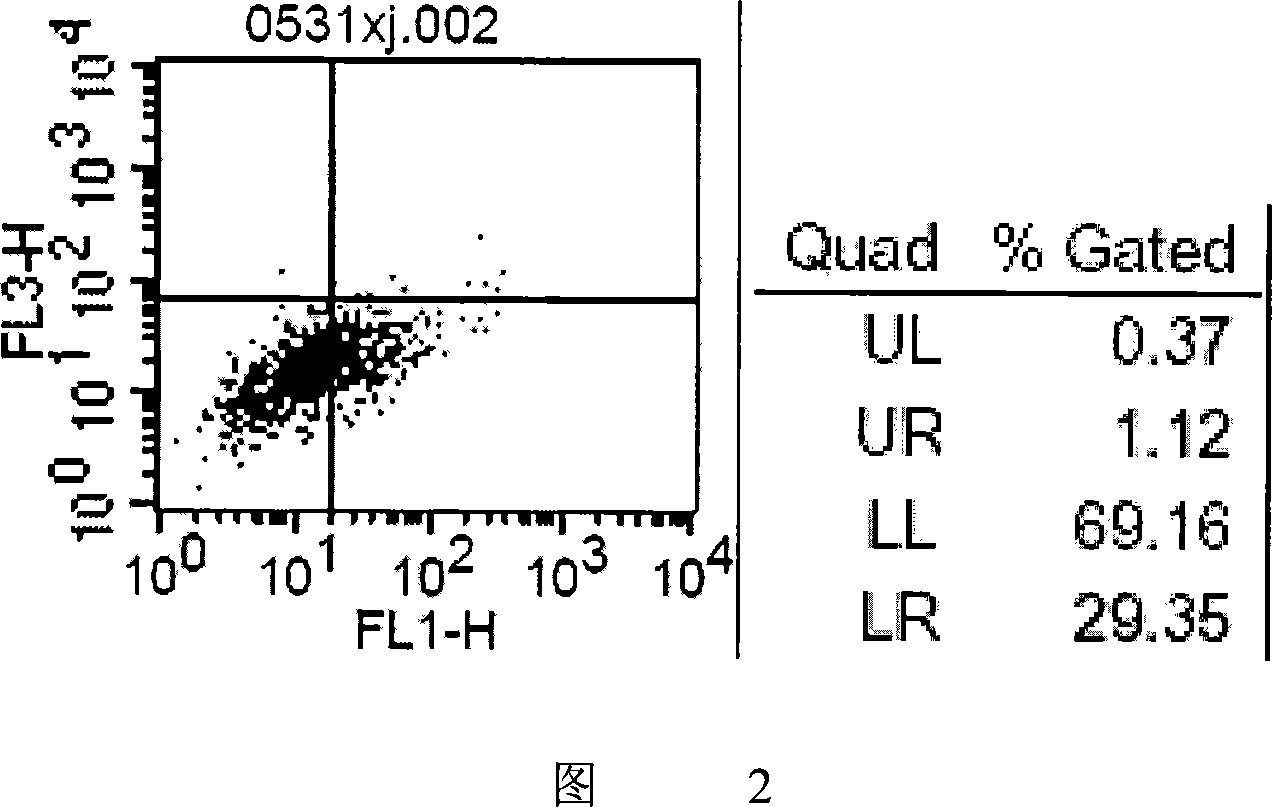
Patents
Literature
390 results about "Single cell suspension" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Single-cell suspensions are a prerequisite for experiments in cell separation, cell analysis and cell culture. To avoid tedious and often painful manual dissociations the gentleMACS Dissociator allows one to dissociate tissue very efficiently under controlled and reproducible conditions.
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
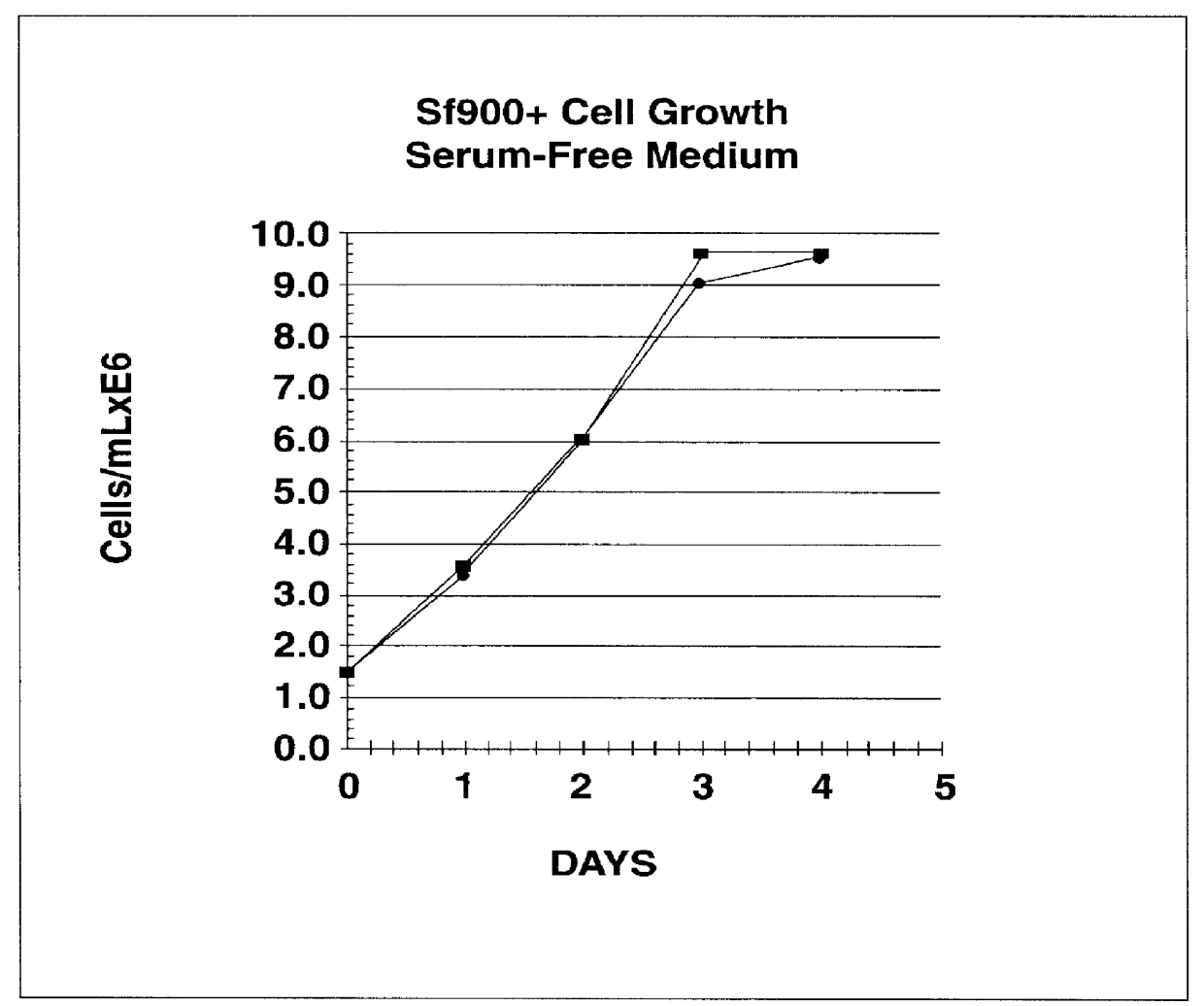
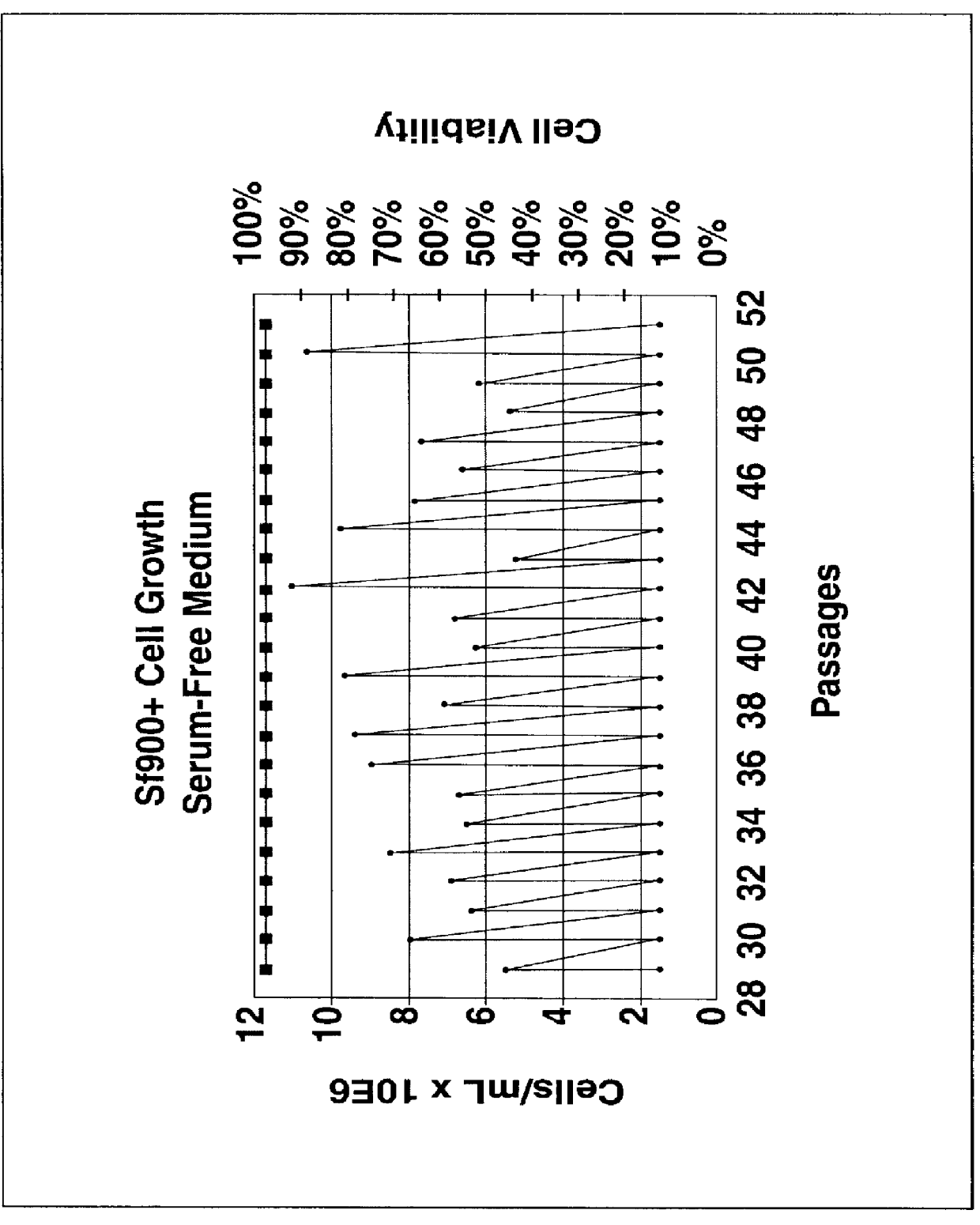
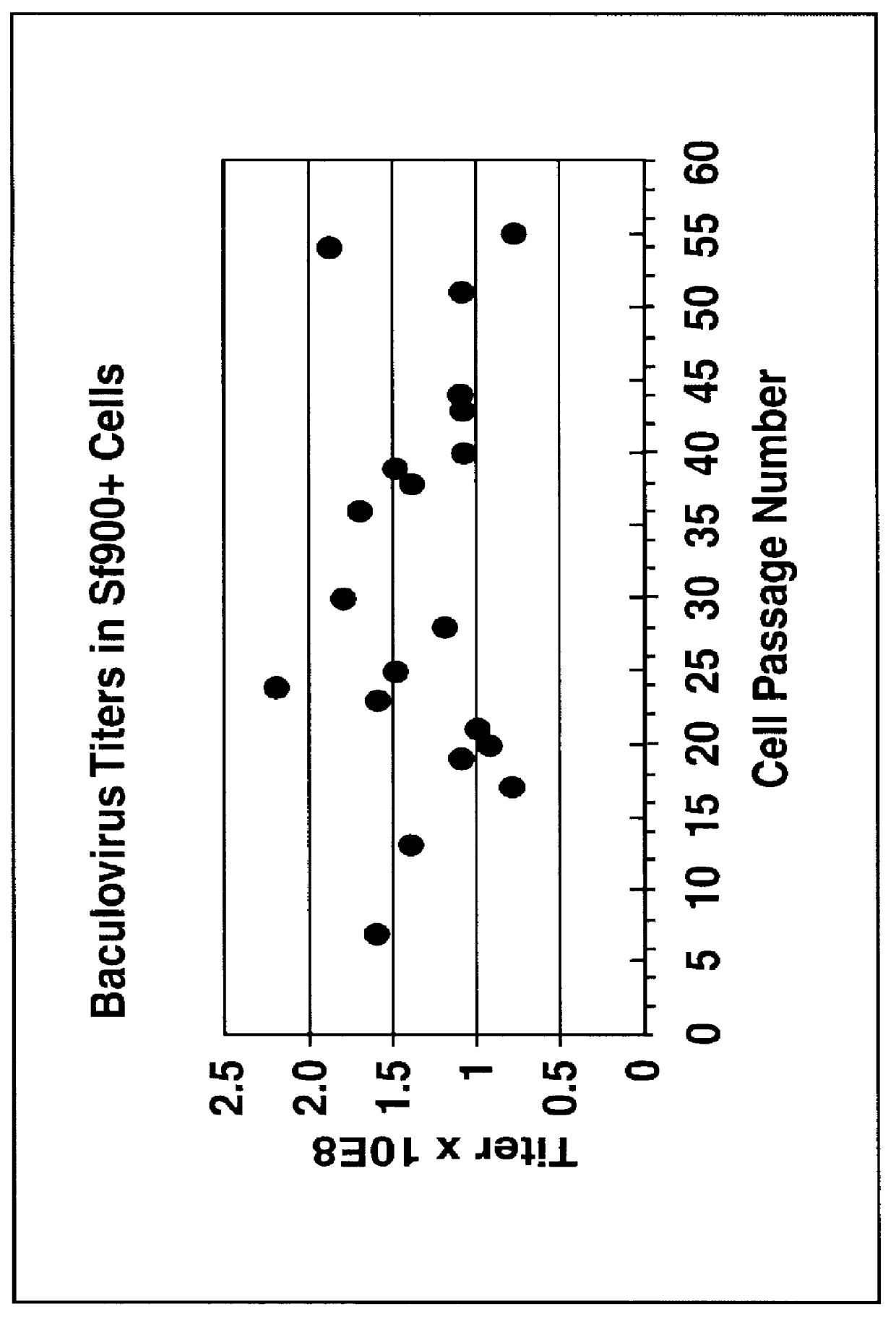
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Stem Cell Aggregate Suspension Compositions and Methods of Differentiation Thereof
The present invention relates to methods for production of undifferentiated or differentiated embryonic stem cell aggregate suspension cultures from undifferentiated or differentiated embryonic stem cell single cell suspensions and methods of differentiation thereof.
Owner:VIACYTE INC
Stem cell aggregate suspension compositions and methods of differentiation thereof
The present invention relates to methods for production of undifferentiated or differentiated embryonic stem cell aggregate suspension cultures from undifferentiated or differentiated embryonic stem cell single cell suspensions and methods of differentiation thereof.
Owner:VIACYTE INC
Mesenchyme stem cell preserving fluid and use thereof
ActiveCN101210232AImprove survival rateReduce adhesionDead animal preservationTissue culturePhosphate ionCell mass
The invention discloses a preservation solution for mesenchymal stem cells and application thereof. The cell preservation solution contains human albumin and heparin as the main components, and other auxiliary reagents such as human cytokine, phosphate ion, metal ions or monosaccharide are contained in a buffer solution for preserving human mesenchymal stem cells. The preservation solution can keep high survival rate of human mesenchymal stem cells in transportation process, reduce adhesion between cells and between the cell and the inner wall of a container, and reduce the possible occurrence of cell mass embolism in blood vessel while clinically infusing human mesenchymal stem cells. The mesenchymal stem cells can be maintained in a state of single-cell suspension at an environment temperature of 4 to 15 DEG C for 24 h, thus greatly enlarging the clinic application range of the human mesenchymal stem cells. The components used in the solution accord with the clinic application, and can meet the requirement for clinic use of the human mesenchymal stem cells.
Owner:TIANJIN AMCELLGENE ENG
Serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture
ActiveCN101760442ASupports adherent growthReduce the burden of separation and purification in the later stageVertebrate cellsArtificial cell constructsLipid formationSerum free media
The invention relates to the culture medium research and development technical field of modern biological technology and provides a serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture, which comprises 21 amino acids, 6 vitamins, 8 salts, 8 lipids, 4 trace elements, 2 buffers, 1 protein hydrolysate, 1 acid-base indicator and 6 other additives. The serum-free medium can be prepared by the conventional preparation method, and an application method thereof is the conventional method. The serum-free medium has the beneficial effects that: the serum-free medium does not contain serum, has clear components, is beneficial for separating and purifying the product and improves the product quality; the serum-free medium supports long-term subculture of MDCK cells and does not require long-term and complex adaptation process; and the serum-free medium can well support the adherent growth and single-cell suspension growth of the MDCK cells, has clear components and easy preparation and utilization, and is suitable for mass production of biological products.
Owner:EAST CHINA UNIV OF SCI & TECH
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Scalable primate pluripotent stem cell aggregate suspension culture and differentiation thereof
ActiveUS20130115695A1Little and no turbulenceGuarantee the environmentBioreactor/fermenter combinationsBiological substance pretreatmentsSingle cell suspensionSuspension culture
The present invention relates to methods for production of undifferentiated or differentiated embryonic stem cell aggregate suspension cultures from undifferentiated or differentiated embryonic stem cell single cell suspensions and methods of differentiation thereof.
Owner:VIACYTE INC
Stem cell regenerating surface cornea and its application in corneal transplantation
InactiveCN1398644AGuaranteed long-term effectivenessRich sourcesEye implantsArtificial cell constructsDiseaseCorneal disease
In the present invention an embryo or adult cornea epithelium is cut, digested with digesting liquid and centrifugated to prepare single cell suspension, and the cell suspension is cultured in culture dish or bottle with proper amount of culture medium and CO2 in 5% at 37 deg.c. The cultured cell is passed after cell converges to 80-90% and the second and sixth generation of cell is passed directly to amnion for culture for another 8-20 days to obtain the stem cell regenerating surface cornea of the present invention. The stem cell regenerating surface cornea may be used as material for treating corneal disease. The present invention provides a new material for treating corneal disease with rich material source, no danger of mouse-originated pollution, no or slight immunological rejection.
Owner:北京科宇联合干细胞生物技术有限公司
Scalable primate pluripotent stem cell aggregate suspension culture and differentiation thereof
ActiveUS8895300B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle cell suspensionSuspension culture
The present invention relates to methods for production of undifferentiated or differentiated embryonic stem cell aggregate suspension cultures from undifferentiated or differentiated embryonic stem cell single cell suspensions and methods of differentiation thereof.
Owner:VIACYTE INC
Separating and culturing process of human amnion mesenchyme stem cell and its medical composition
ActiveCN1810959AWide variety of sourcesUnrestricted by ethicsMammal material medical ingredientsSkeletal/connective tissue cellsSingle cell suspensionCulture mediums
The present invention is separating and culturing process of human amnion mesenchyme stem cell and its medical composition. The separating and culturing process includes digesting human amnion successively with trypsin, collagenase and deoxyribonuclease, and filtering to prepare single cell suspension; culturing in DMEM / F12 culture medium with VDMEM and VF12 in the equal ratio and containing ox embryo blood serum in 10-20 vol% and basic fibroblast growth factor of ultimate concentration 10-20 ng / ml inside a culture box at 37 deg.c, saturated humidity and CO2 in 5 vol%; and replacing liquid and culture passage to proliferate and purify human amnion mesenchyme stem cell. The process has wide material source no ethnic limitation and wide application foreground. The medical composition may be used in various kinds of treatment.
Owner:SHENZHEN BEIKE BIOTECH +4
Neural stem cell preparation, preparing method thereof and use of same
InactiveCN1435187AImprove proliferative abilityEffectively treats damageNervous disorderMuscular disorderSingle cell suspensionEmbryo
A neural stem cell preparation for treating human myelopathy, especially the motor neuron diseases, is prepared through digesting the brain tissue of human embryo, adding to culture medium, centrifugal processing, mechanical beating to obtain cell suspension, culturing in culture medium in CO2 atmosphere, screening neural stem cell balls, and passage by 3-6 generations.
Owner:北京科宇联合干细胞生物技术有限公司
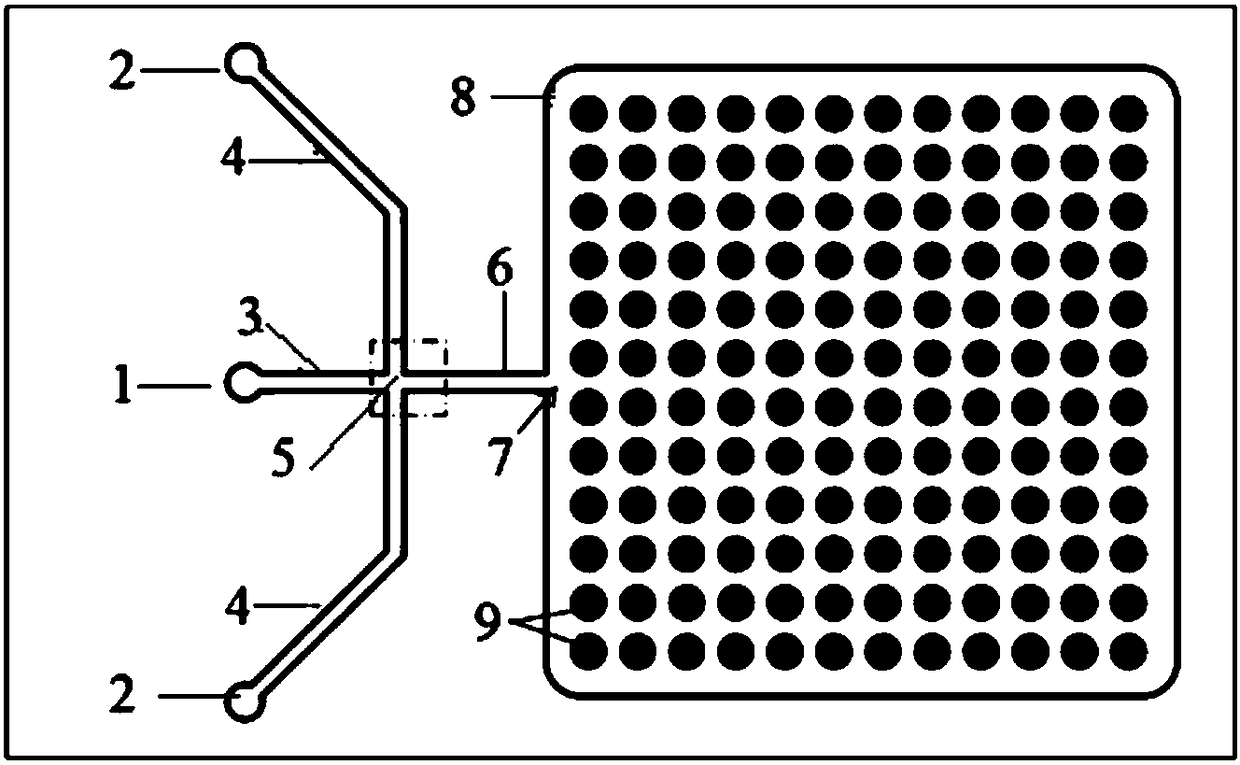
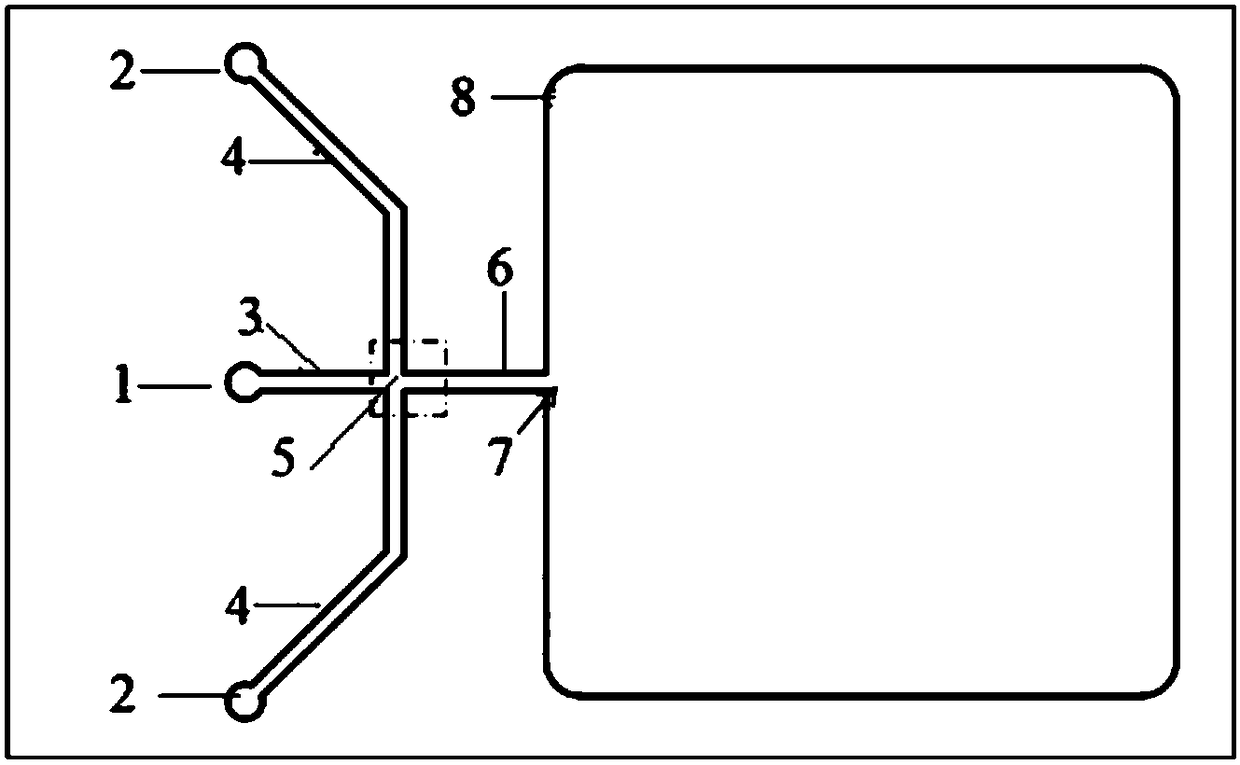
Single cell separation method based on droplet micro-fluidic chips
PendingCN108949496AReduce dosageReduce experiment costBioreactor/fermenter combinationsBiological substance pretreatmentsSingle cell suspensionOil phase
The invention provides a single cell separation method based on droplet micro-fluidic chips. The method concretely comprises the following steps: A, a single cell suspension flows into a dispersed phase inlet channel from a dispersed phase inlet; B, an oil phase liquid flows into a continuous phase inlet channel from a continuous phase inlet; C, the above two phases merge to form droplets enclosing single cells, the droplets flow over a droplet capturing unit with the generation of a large number of droplets in a liquid storage pool, and stand for 2-5 min, and the superfluous droplets are sucked out when the droplets slowly settle into the droplet capture unit; and D, the droplet chips which capture the single cells are cultured in a 37 DEG C incubator, then DAPI is added to carry out nuclear staining, and the single cell capture rate is detected. The method has the advantages of simplicity and rapidity in operation, small use amounts of the cells and reagents, low experiment cost, high integration and wide application range.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for primary culture of tumor cells
InactiveCN103865876AIncrease success rateGood removal effectTumor/cancer cellsSingle cell suspensionBovine serum albumin
The invention discloses a method for primary culture of tumor cells and relates to the field of cell biology. According to the method disclosed by the invention, a tumor sample is prepared into a single-cell suspension liquid by use of an enzyme method or a mechanical method, and then tumor cell bladders are prepared by use of a serum-free suspension culture method, wherein alkaline fibroblast growth factors, epidermal growth factors, insulin and bovine serum albumin need to be added during a culturing process, then the growth factors are removed, and the culture is implemented in a general culture medium to obtain adherent cells capable of realizing continuous passage. The method disclosed by the invention is short in culture period and has the effect of increasing the success rate of the primary culture of tumors.
Owner:NORTHWEST UNIVERSITY FOR NATIONALITIES
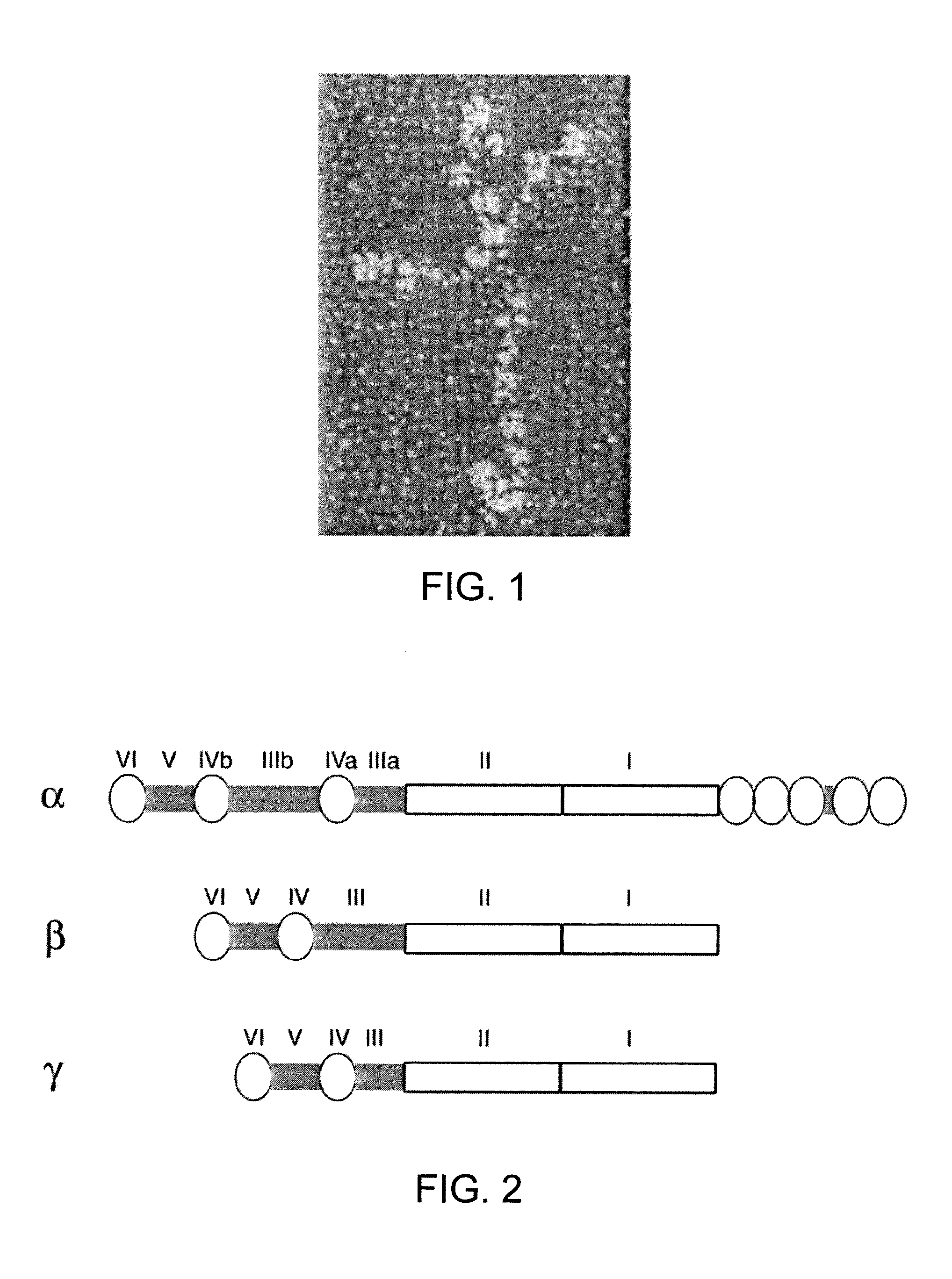
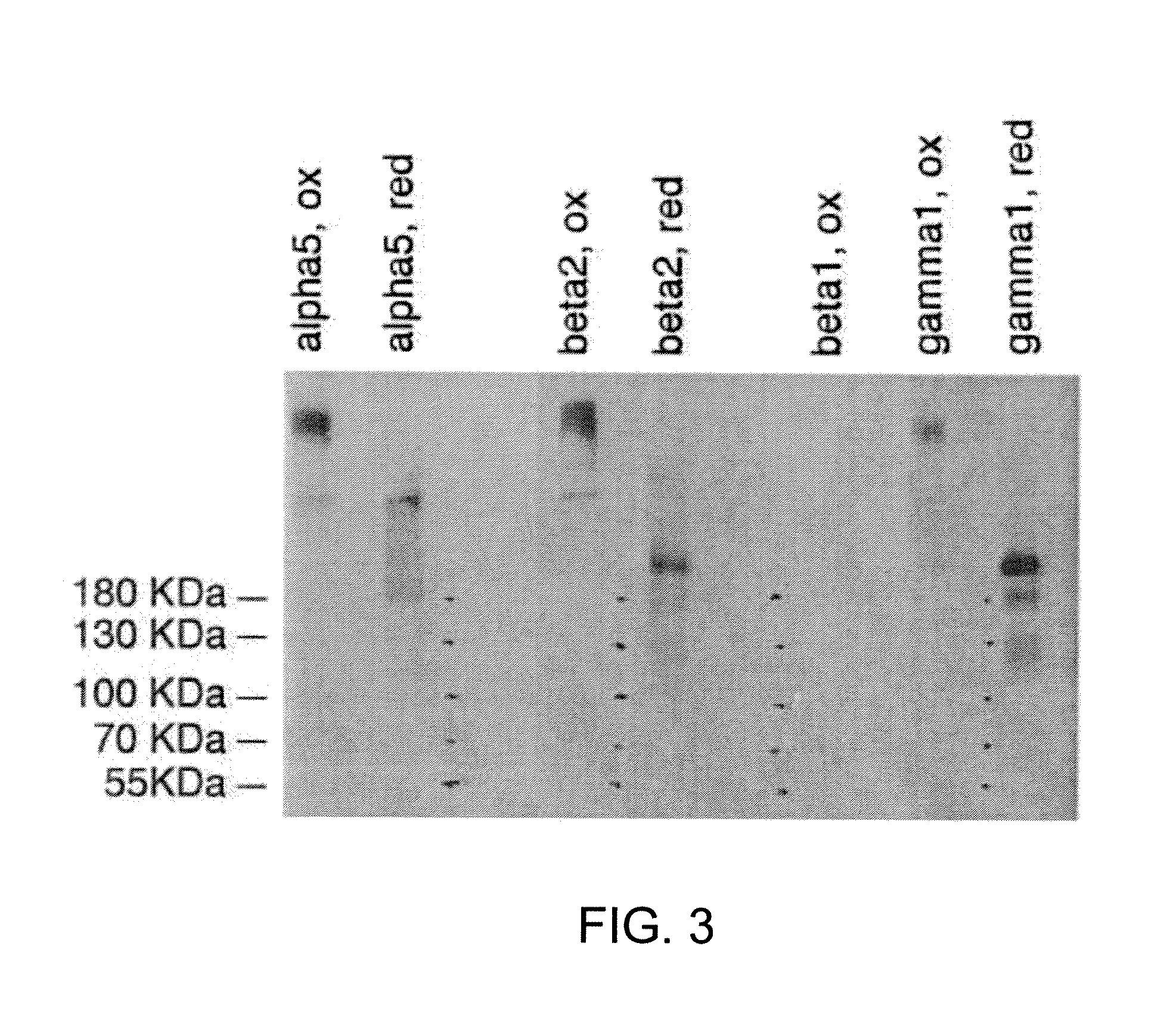
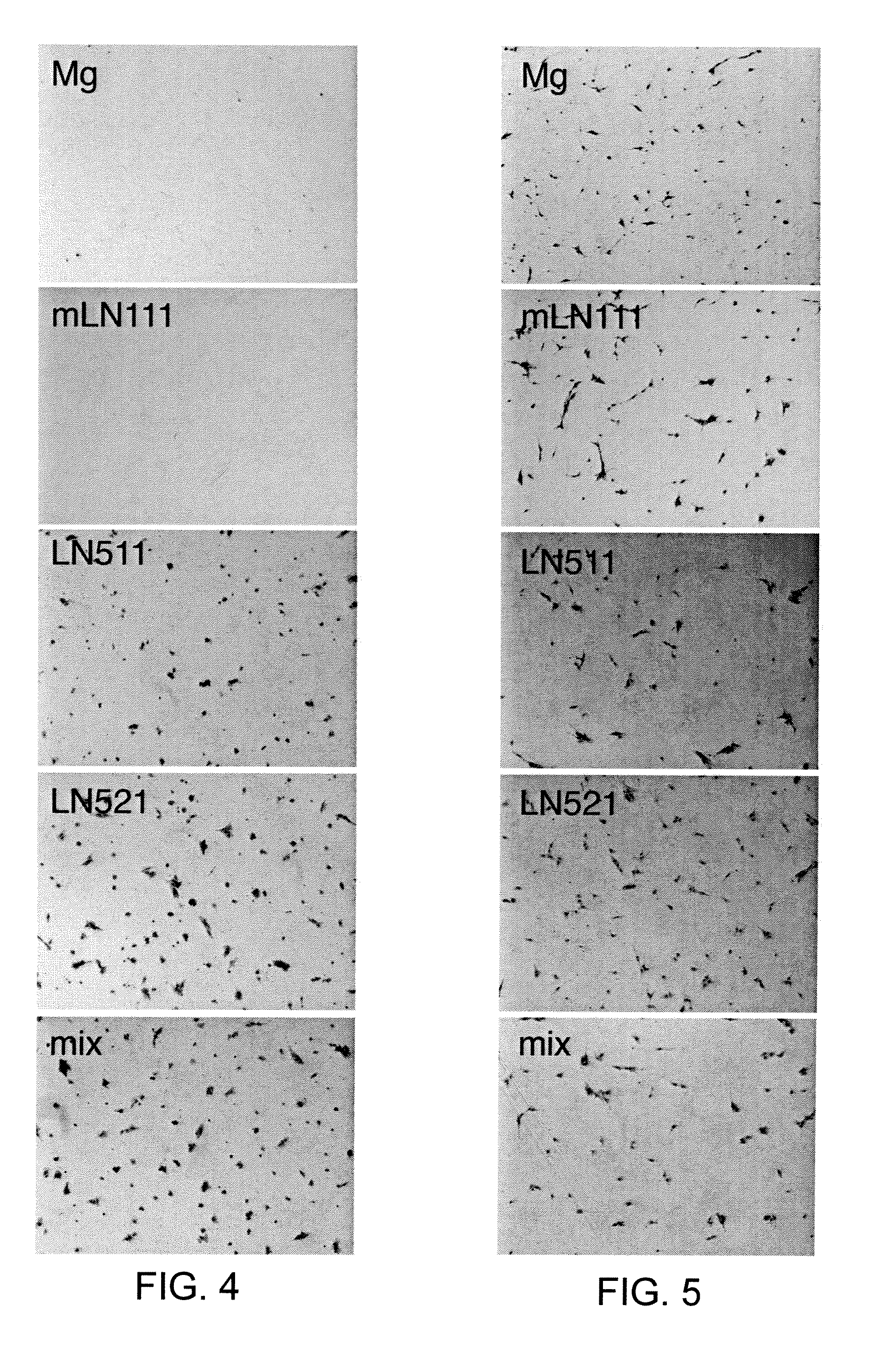
Recombinant laminin-521
ActiveUS20120156254A1Fast and economically efficient scale-upFacilitate scientificBioreactor/fermenter combinationsPeptide/protein ingredientsSingle cell suspensionCell culture media
The present disclosure related to isolated laminin-521, methods for making recombinant laminin-521, host cells that express recombinant laminin-521, and compositions containing laminin-521. Laminin-521 can maintain stem cells in vitro pluripotency, enable self-renewal, and enable single cell survival of human embryonic stem cells. When pluripotent human embryonic stem cells are cultured on plates coated with a matrix of recombinant laminin-521 (laminin 11), in the absence of differentiation inhibitors or feeder cells, the embryonic stem cells proliferate and maintain their pluripotency. It has also been discovered that human recombinant laminin-521 (laminin-11) provides single cell survival of stem cells after complete dissociation into a single cell suspension. Useful cell culture mediums containing at most 3.9 ng / ml of beta fibroblast growth factor (bFGF) are also described herein.
Owner:BIOLAMINA
Method for using umbilical stalk placenta to prepare mesenchyma stem cell
ActiveCN101492654AGood proliferative potentialConvenient sourceSkeletal/connective tissue cellsSingle cell suspensionMesenchymal stem cell
The invention discloses a method for preparing a mesenchymal stem cell by using umbilical cords and placenta, and mainly relates to a new source for the mesenchymal stem cell and a preparation method thereof. The umbilical cord or the placenta is employed by the stem cell as a cell source; tissues are assimilated at first; then a single cell suspension is prepared and inoculated into a culture bottle, a culture dish or a culture bag to amplify the mesenchymal stem cell, then amplified the mesenchymal stem cell is assimilated, collected and frozen. The mesenchymal stem cell prepared according to the technique of the invention contains a plurality of mesenchymal stem cells with excellent multiplication potentiality. The mesenchymal stem cell can be used in the experimental study, clinical treatment and tissue engineering, etc. The material source thereof is abundant; the separation successful rate and yield are high; the cell multiplication capacity is strong; and the contents of allergic and animal-originated substances are low.
Owner:UNION STEMCELL & GENE ENG
Preparation method and application of collagen scaffold composite bone marrow-derived mesenchymal stem cells (BMSCs)
ActiveCN103705984AGood biocompatibilityPromote degradationSurgeryIntrauterine deviceBiocompatibility Testing
The invention relates to a preparation method and application of collagen scaffold composite bone marrow-derived mesenchymal stem cells (BMSCs). The preparation method comprises the steps of preparing single cell suspension after trypsinizing the BMSCs, uniformly dropwise adding the single cell suspension to a collagen scaffold, putting the collagen scaffold into an incubator to be cultured, and adding an L-DMEM complete medium to continue culture, thus obtaining the collagen scaffold composite BMSCs. The preparation method has the advantages that the following defects are overcome: endometria are seriously injured as intrauterine adhesion is mechanically separated by adopting hysteroscopic surgery, intrauterine devices or anti-adhesion materials are put after the surgery and estrogens are given after the surgery to promote intima growth; the problem of intima scars can not be solved; functional intima repair can not be achieved; adhesion is very easy to happen again. As active ingredients for treating serious endometrium injury, the BMSCs are convenient to obtain, secrete growth factors to improve the local microenvironment and immunoregulation, have good biocompatibility, degradability and safety, promote scarred endometrium repair and increase the intima thickness and local blood vessel density.
Owner:YANTAI ZHENGHAI BIO TECH
Alginate poly-L-Lysine encapsulation as a technology for controlled differentiation of embryonic stem cells
ActiveUS20090311765A1Induce depolymerizationArtificial cell constructsMammal material medical ingredientsPolyelectrolyteSingle cell suspension
Alginate polyelectrolyte encapsulation is used for the controlled differentiation of embryonic stem cells. An isolated cell population is provided. The cell population includes a single cell suspension of ES cells encapsulated within an alginate polyelectrolyte microenvironment. The encapsulated ES cells are capable of differentiating within said microenvironment into hepatocyte lineage cells in the absence of embryoid body intermediates or growth factor supplementation.
Owner:RUTGERS THE STATE UNIV
Gastrointestinal stem cells and uses thereof
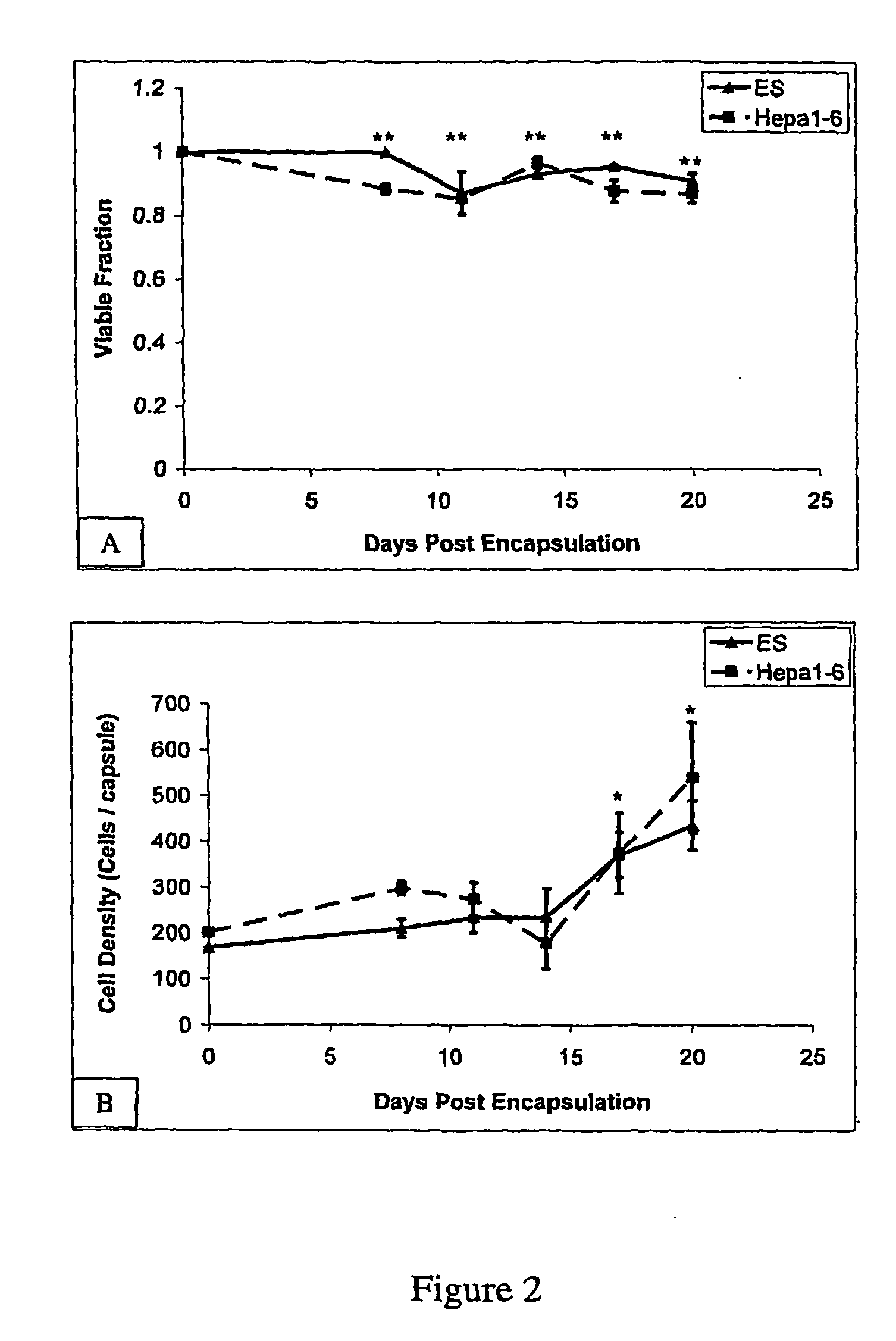
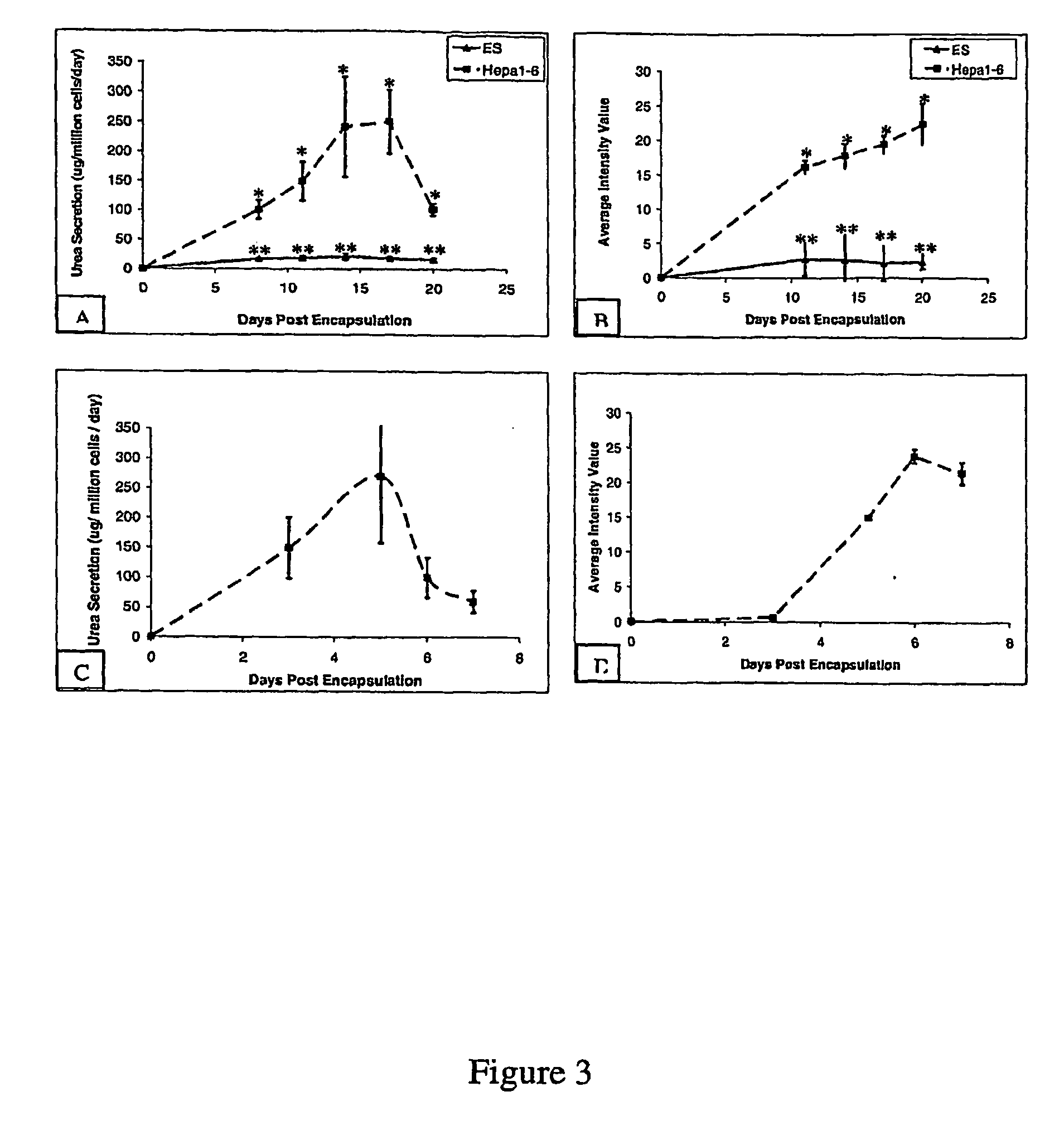
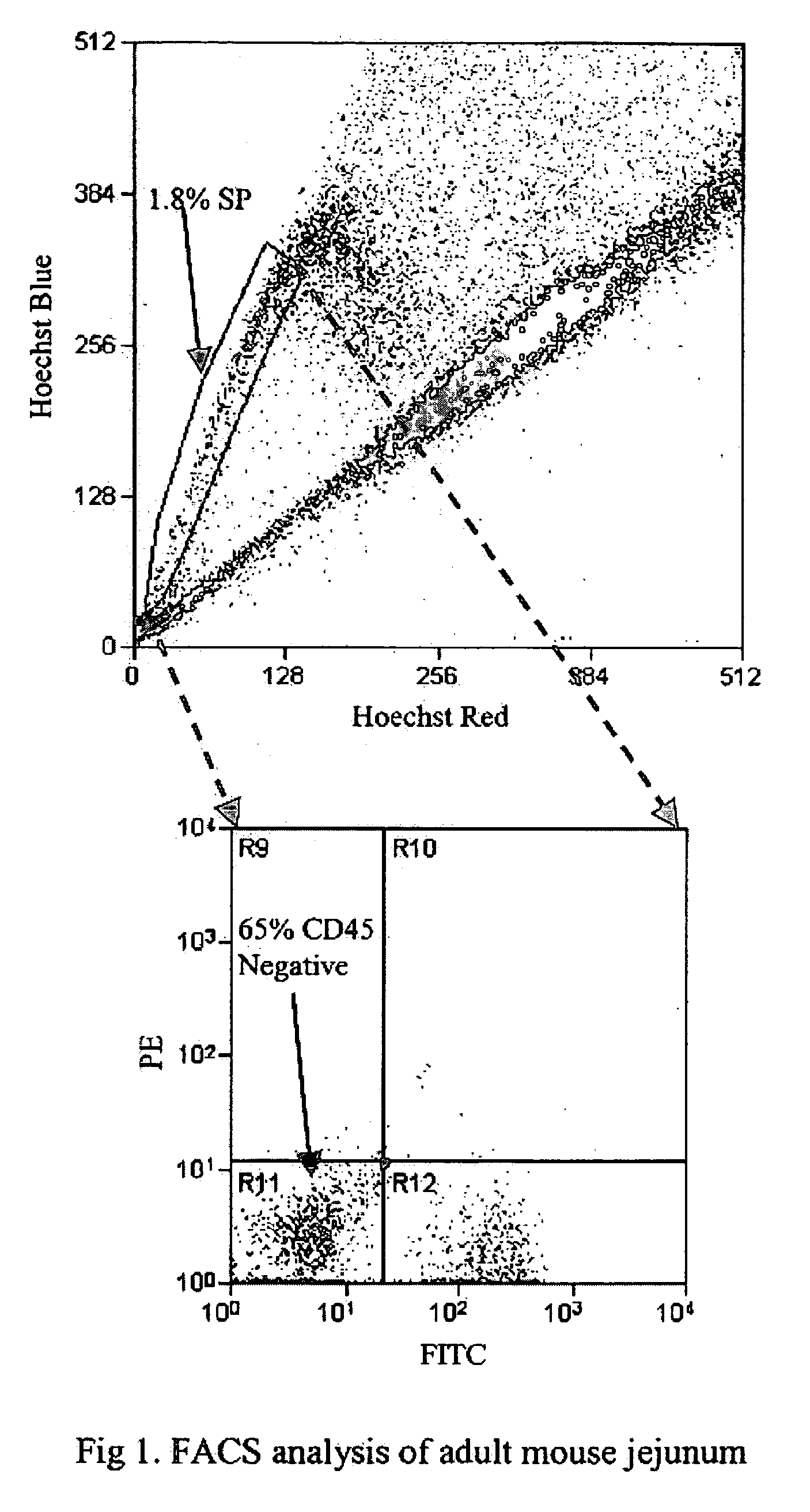
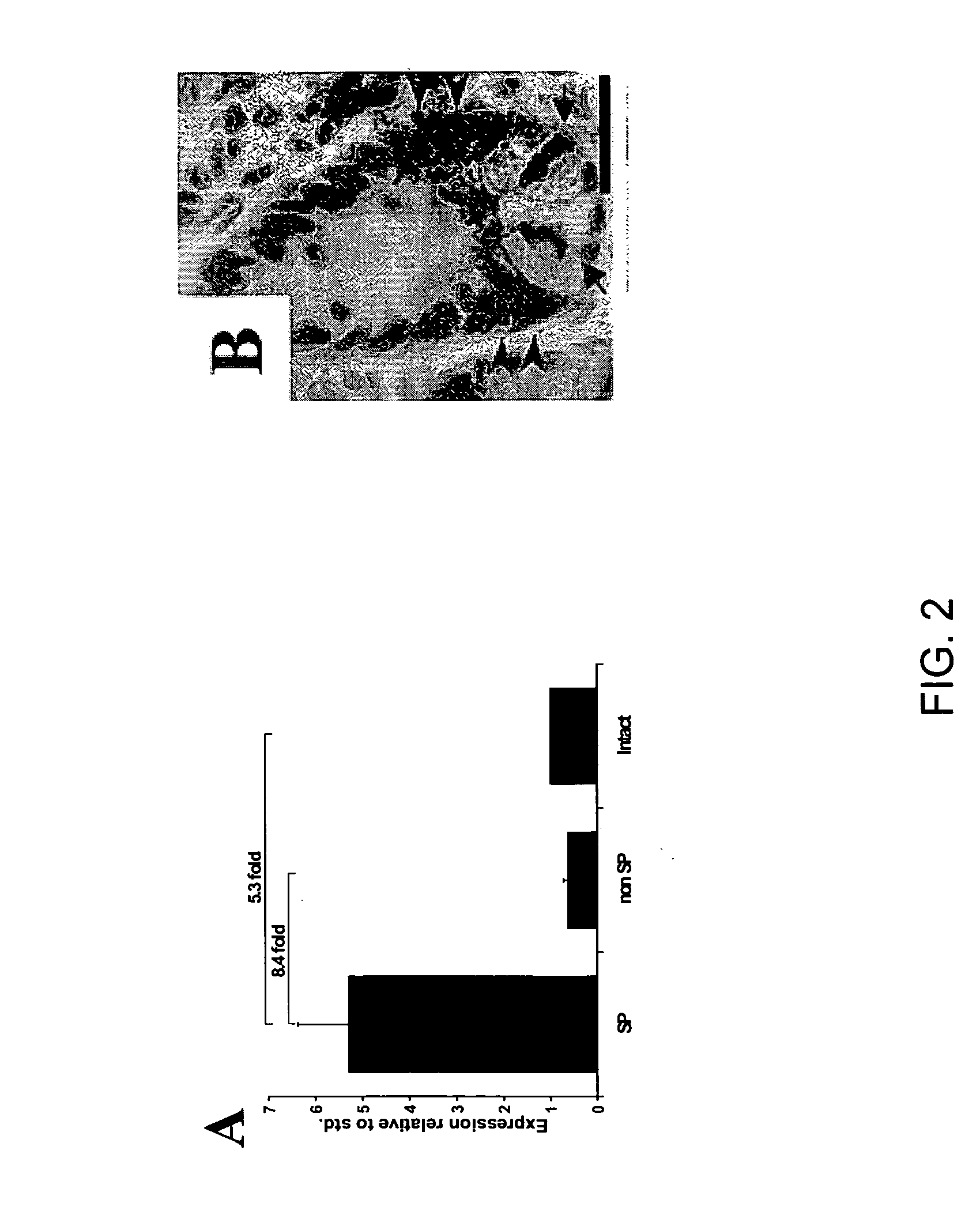
InactiveUS20050256077A1High turnover rateSlow turnoverCell dissociation methodsGastrointestinal cellsSingle cell suspensionTherapeutic intent
The present invention relates to compositions and methods concerning isolated gastrointestinal stem cells. Particularly, the invention provides isolated gastrointestinal stem cells comprising a CD45 negative marker, a collagen IV negative marker, and that is Msi-1 positive. In particular embodiments the isolated cells are comprised in a single cell suspension. In other particular embodiments, the isolated cells are utilized for therapeutic purposes, such as for a gene therapy vector and / or for replenishing stem cells in a gastrointestinal tract in need thereof.
Owner:BAYLOR COLLEGE OF MEDICINE
Placenta source filling dry cell and production thereof
InactiveCN1746297AEasy to obtainIncrease productionGenetic material ingredientsMammal material medical ingredientsSingle cell suspensionVolumetric Mass Density
A placenta original filled stem cell and its production are disclosed. The process is carried out by bleeding after delivering placenta from uterus, perfusing to obtain single-cell soliquoid from continuous perfusion method or collagenase digestion method and acquiring filled stem cell from gradient and density centrifugation or immune magnetic bead separation. It has better external enrichment potentiality and multidirectional differential potentiality and low immunogenicity.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Chemical dissociation of cell aggregates
InactiveUS20050221476A1Improve survivabilityReduce cell damageCell dissociation methodsNervous system cellsSingle cell suspensionCell biology
The invention provides a novel method of dissociating anchorage independent and dependent cell aggregates. The invention also includes the cells resulting from the methods of the invention and the use of the cells in various applications requiring the generation of a single cell suspension.
Owner:SEN ARINDOM +2
Culture solution for primary culture of newly born rat hippocampal neuron and preparation method and application thereof
InactiveCN102533654AMetabolic recovery and improvementAvoid damageCulture processNervous system cellsNutritionSingle cell suspension
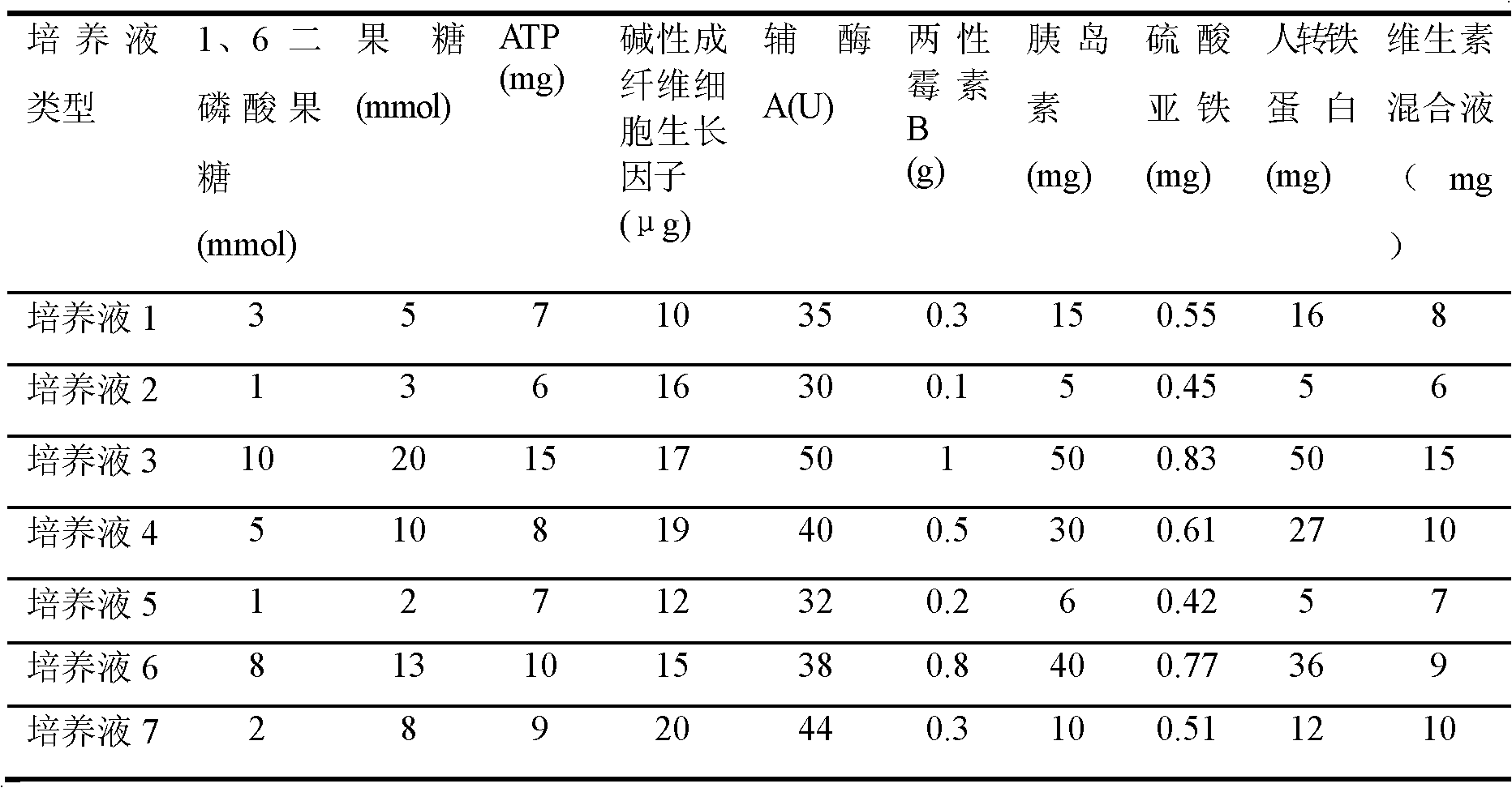
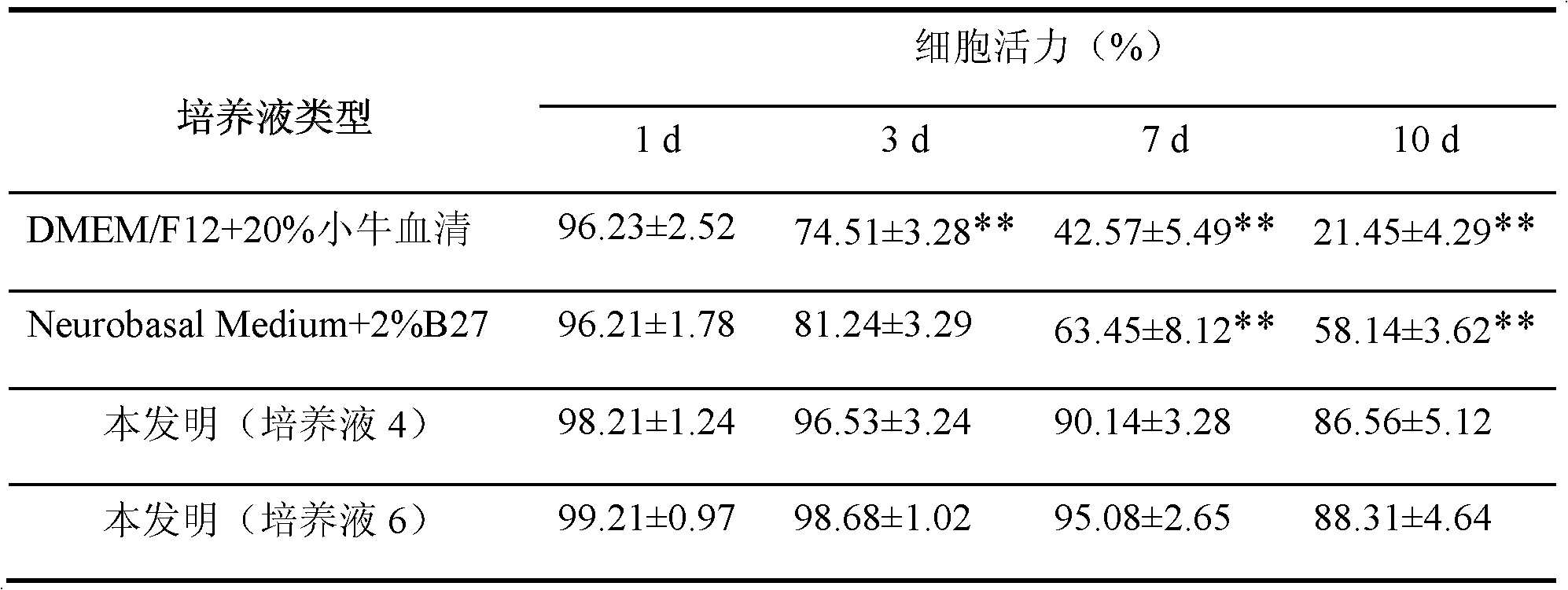
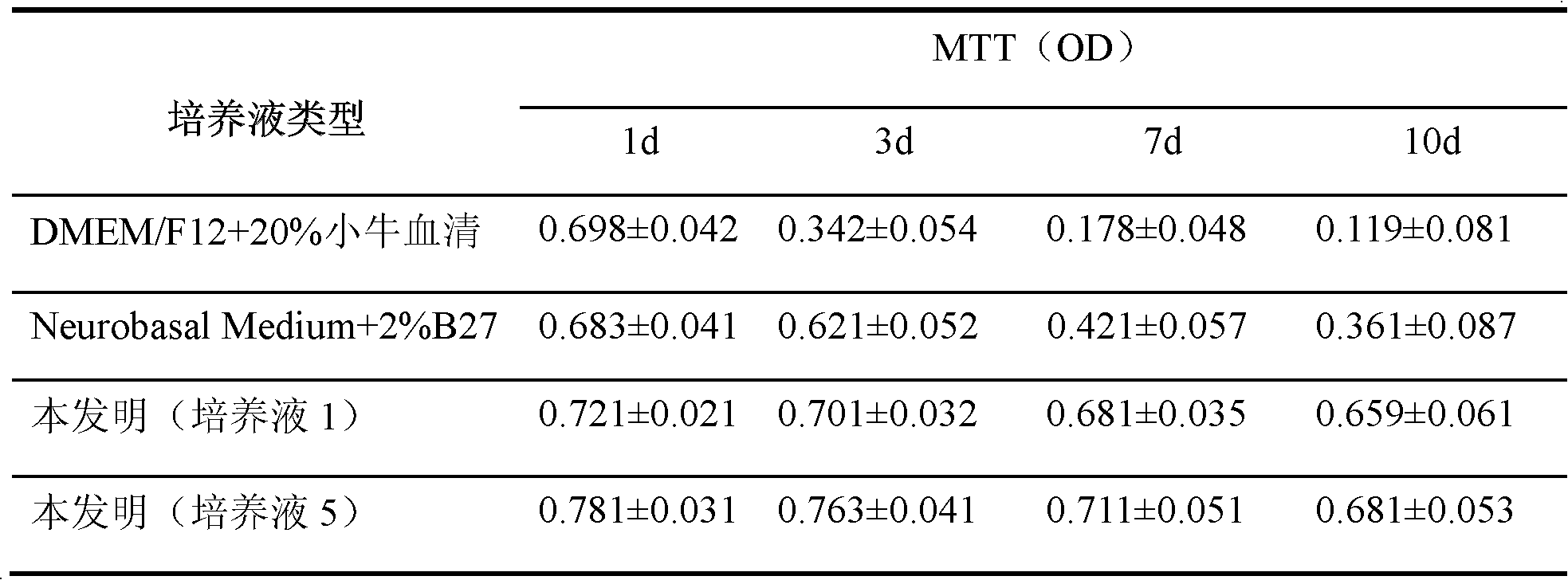
The invention discloses culture solution for primary culture of newly born rat hippocampal neuron and a method for primarily culturing the rat hippocampal neuron. The culture solution is formed by adding 1,6 diphosphonic acid fructose, fructose and nutrition additive into base culture solution Neurobasalmedium. The method for primarily culturing the rat hippocampal neuron comprises the following steps: 1 preparing hippocampal neuron culture solution; 2 drawing materials and digesting the materials; 3 preparing single cell suspension; and 4 conducting inoculating and cultivating. The method for primarily culturing the rat hippocampal neuron is simple, convenient and capable of obtaining hippocampal neuron with good growth state and high purity by adopting the culture solution and has important application value and economical value.
Owner:温州医学院附属第二医院
Method of inducing and differentiating human umbilical cord derived mesenchymal stem cells into cartilage cells
The invention relates to a method of inducing and differentiating human umbilical cord derived mesenchymal stem cells into cartilage cells. The method includes: in order to solve the problem that the prior art is low in differentiation efficiency, cleaning umbilical cord tissue to remove blood stain, cutting the same into small sections, dissecting the small sections to remove vascular tissue, cutting the small sections into pieces, and digesting to obtain a single mesenchymal stem cell; culturing the obtained mesenchymal stem cell in an amplification manner to the seventh generation to obtain a unicellular suspension, inoculating the unicellular suspension into a serum-free culture medium containing a cartilage differentiation inducer to continue culturing, replacing a fresh culture medium every other 3.5 days, and inducing for 14 days to obtain the cartilage cells. The method can efficiently differentiate the umbilical cord mesenchymal stem cells into the cartilage cells and is of important significance in the aspects of restoring cartilage tissue in human bodies and treating bone injury.
Owner:中卫华医(北京)生物科技有限公司 +1
Test method for detecting cytotoxicity of buccal tobacco products
ActiveCN104789633AEasy to handleAccurate and effective detectionMicrobiological testing/measurementFiberPretreatment method
The invention provides a test method for detecting cytotoxicity of buccal tobacco products. The test method comprises the following steps: sample pretreatment, single-cell suspension preparation, cell concentration calculation, cell inoculation, test substance addition, test substance incubation, dye incubation, light absorption value measurement, cell inhibition rate calculation and cell half lethal dose calculation. According to the test method provided by the invention, the exposure means and action manners of the buccal tobacco products are comprehensively surveyed, a sample pretreatment method of the buccal tobacco products is established, human oral mucosa fibroblasts hoMF are selected as the cells for cytotoxicity test of the buccal tobacco products according to the acting target cells, the sample detection dosage is determined and a cytotoxicity test method suitable for the buccal tobacco products is formed.
Owner:CHINA TOBACCO YUNNAN IND
Construction method of human amniotic mesenchymal stem cell bank
ActiveCN103422176AWide variety of sourcesUnrestricted by ethicsSkeletal/connective tissue cellsLibrary creationSingle cell suspensionPancreatic hormone
The invention relates to a construction method of a human amniotic mesenchymal stem cell bank. The construction method comprises the following steps: taking a human amnion for detection, flushing and washing the human amnion with a phosphate buffered solution, then smashing the human amnion, diluting with the phosphate buffered solution, digesting with trypsin, digesting with collagenase IV and deoxyribonuclease I, and filtering to obtain a single-cell suspension; by adding human serum albumin, transferring, insulin and sodium selenite into a DMEM / F12 basal culture medium with the ratio of VDMEM to VF12 being 1 to 1, placing human amnion mesenchymal stem cells in an incubator under the serum-free condition, and then performing liquid exchange and culture transfer; subjecting the mesenchymal stem cells obtained through in vitro culture and proliferation to liquid nitrogen refrigeration, and preserving the cells according to the gender of newborn infants, the ABO / Rh type and the HLA type; establishing cell information files, so that the human amniotic mesenchymal stem cell bank is constructed. The method has the characteristics of no other animal derivation, wide source range and no ethic limitation. With adoption of the method, the human amniotic mesenchymal stem cells can be provided for cell therapy and other application.
Owner:沈阳艾米奥生物工程技术研发中心有限公司
Tumor stem cell suspension culture method
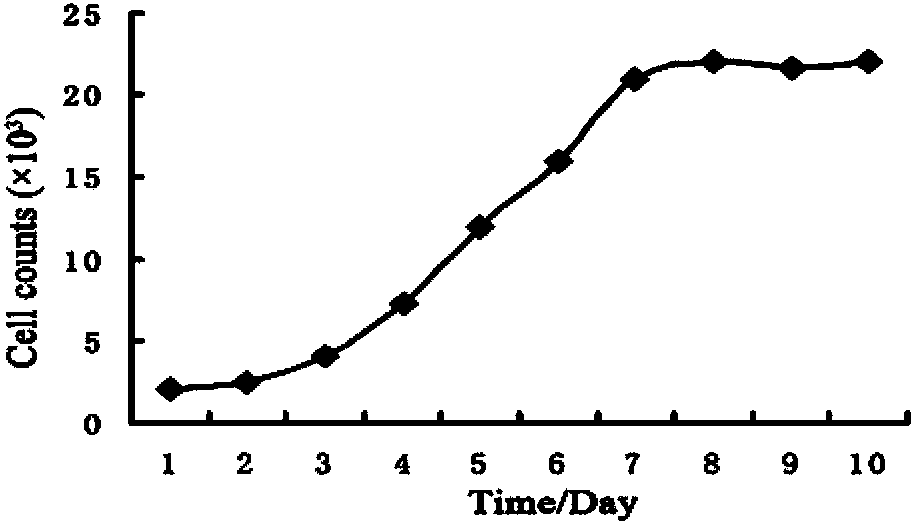
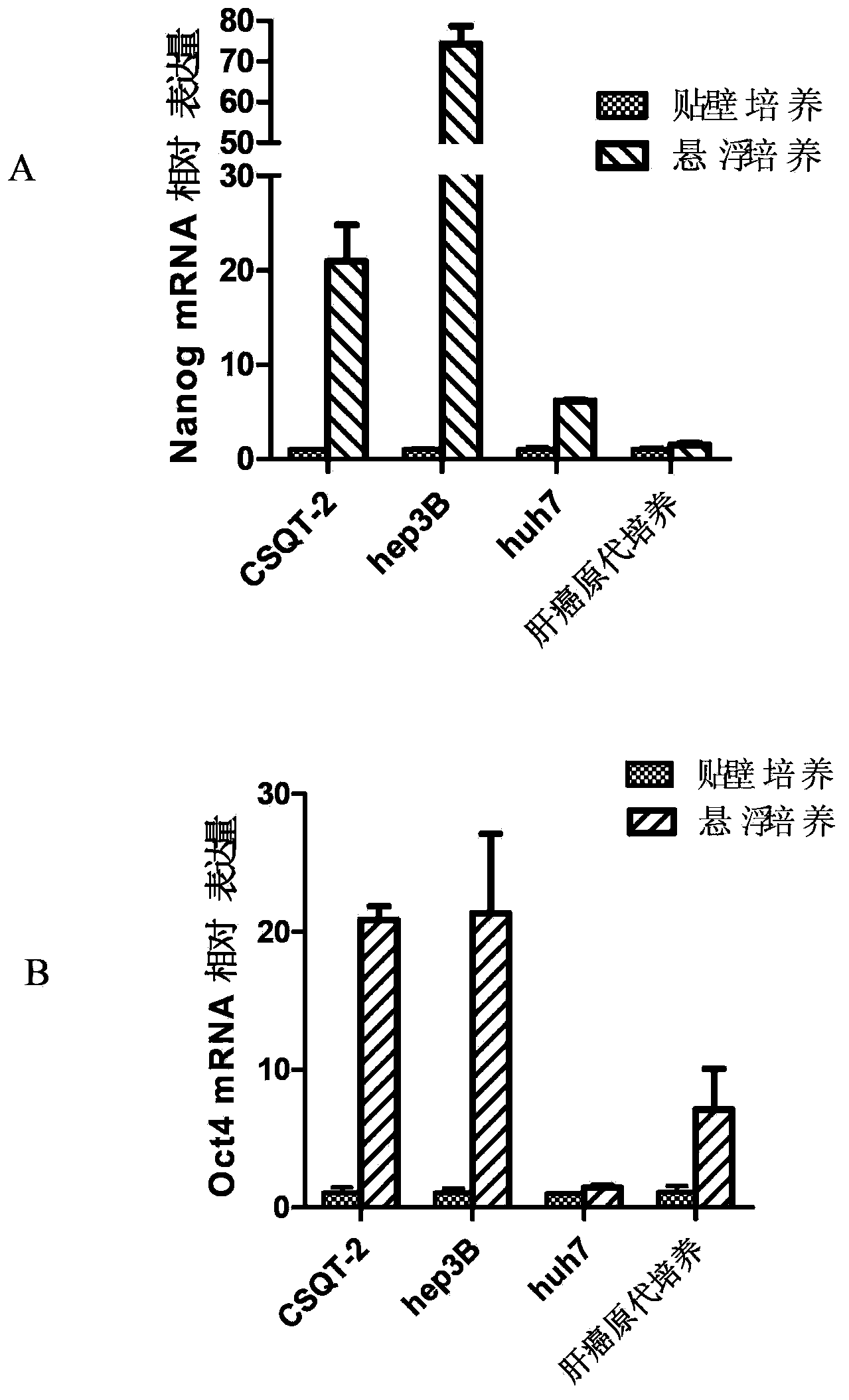
The invention belongs to the technical field of modern biotechnology cell culture. A purpose of the invention is to provide a tumor stem cell suspension culture method, which can be used for culture of tumor cells with different tissue sources, and tumor stem cells obtained through the culture method have characteristics of self-renewal capacity and enrichment. The tumor stem cell suspension culture method comprises: carrying out digestion centrifugation collection on tumor cells requiring culture, adopting PBS to carry out resuspending centrifugation, adding to a prepared suspension culture medium of the invention, carrying out beating-up into a single cell suspension, inoculating into a paved ultra-low absorption culture dish, and carrying out cell culture. The tumor stem cell suspension culture method has the following characteristics that: ball forming rates of various in vitro cultured tumor cells are high, multi-function factors are expressed, the OCT4 proportion and the NANOG proportion are increased, rich and comprehensive experimental materials are provided for tumor stem cell researches, and broad application prospects are provided in basic researches and clinical applications.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Human liver progenitors
InactiveUS20050148072A1Low densityReduce in quantityHepatocytesGenetic material ingredientsProgenitorGlycophorin
Methods of isolating and cryopreserving progenitors from human liver are disclosed which include processing human liver tissue to provide a substantially single cell suspension comprising progenitors and non-progenitors of one or more cell lineages found in human liver; subjecting the suspension to a debulking step, which reduces substantially the number of non-progenitors in the suspension, and which provides a debulked suspension enriched in progenitors exhibiting one or more markers associated with at least one of the one or more cell lineages; and selecting from said debulked suspension those cells, which themselves, their progeny, or more mature forms thereof express one or more markers associated with at least one of the one or more cell lineages. Among these markers are CD14, CD34, CD38, CD45, and ICAM. Hepatic progenitors are characterized as being 6-15μ in diameter, diploid, glycophorin A−, CD45−, AFP+++, ALB+, ICAM+, and with subpopulations varying in expression of CD14+. CD34++, CD38++, CD117+. These progenitor subpopulations have characteristics expected for cells that are particularly useful in liver cell and gene therapies and for establishing bioartificial organs.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Preparation method of tissue single-cell suspension
PendingCN112574941AEasy accessEfficiently obtainedAnimal cellsCell dissociation methodsSingle cell suspensionLiving cell
The invention discloses a preparation method of tissue single-cell suspension. The preparation method comprises the following steps: (1) preparing a reagent and a culture medium; (2) pretreating tissues; (3) primarily digesting and dissociating the tissues; (4) secondarily digesting and thoroughly dissociating the tissues; (5) filtering the liquid thorugh a micron-scale cell filter; (6) producingthe target tissue single-cell suspension; and (7) detecting living cell yield. According to the method for preparing the tissue single-cell suspension, the tissue single-cell suspension can be rapidlyand efficiently obtained with low cost, the dissociated cells can keep good vitality, so that the tissue single-cell suspension can be applied to dissociation, separation and purification of large-scale animal cells and establishment of a cell line, thus providing important reference materials for single cell, gene function and genetic breeding research of animals.
Owner:SHANGHAI PASSION BIOTECHNOLOGY CO LTD
Method for separating stem cell of breast cancer
This invention relates to a method for separating breast cancer stem cells. The method comprises: digesting breast cancer cells into single cell suspension, preparing Percoll discontinuous density gradient separating medium, mixing with single cell suspension, centrifuging to separate cell subsets, detecting by comparing the experimental tube and the control tube, adjusting the density of Percoll discontinuous density gradient separating medium until the content of the cell subsets related to the breast cancer stem cells reaches predetermined enrichment degree, and screening with a flow cytometry. The method utilizes Percoll screening or serum-free culture to enrich breast cancer stem cells, and then screens with a flow cytometry to ensure the screening efficiency. The ratio of the breast cancer stem cells after primary enrichment is higher than 25%, while that after low cytometry screening is higher than 99%.
Owner:JIANGSU PROVINCE HOSPITAL
Preparation method of deciduous tooth mesenchymal stem cells and used kit
InactiveCN105907711AReduce the risk of infectionEasy to useCulture processDead animal preservationSingle cell suspensionMesenchymal stem cell
The invention discloses a preparation method of deciduous tooth mesenchymal stem cells, comprising the steps of: cleaning and sterilizing the tissue surfaces of deciduous teeth with normal saline and 75% alcohol, preserving the deciduous teeth in deciduous teeth preserving fluid; acquiring dental pulp from the deciduous teeth, adding dental pulp digestive fluid to the dental pulp for digestion; obtaining a unicell suspension; adding cell culture fluid, carrying out centrifuging and cleaning, adding precipitated cells into cell culture fluid, putting the cell culture fluid in a carbon dioxide incubator for culturing; when the primary cell fusion reaches 70%, adding cell washing fluid, shaking the culture bottle for washing cells, sucking and abandoning the cell washing fluid, adding cell digestive fluid for digestion, adding cell culture fluid to terminate digestion, repeatedly beating upon the bottle bottom until the cells completely fall off, adding cell culture fluid, inoculating into a culture bottle, putting the culture bottle in the carbon dioxide incubator for culturing, regarding the cells as P1 generation mesenchymal stem cells; when the P1 generation cell fusion reaches 70-80%, carrying out trypsin digestion, collecting digestive cells, carrying out centrifuging and inoculation; after 3d, carrying out trypsin digestion again, collecting digestive cells, carrying out centrifuging, counting and inoculation, and when the P3 generation cell growth reaches 80%, gathering and cryopreserving the cells.
Owner:ANHUI NEW LIFE STEM CELL TECH CO LTD
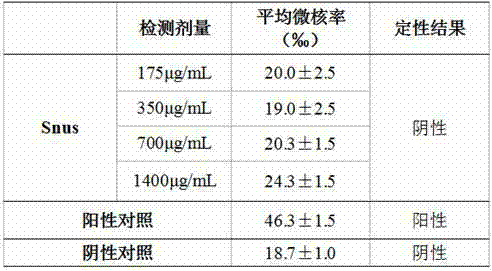
Method for detecting influence of buccal tobacco product on micronucleus rates of cells
ActiveCN104749353AAccurate and effective detectionCells are used for detection, and the sample detection is confirmed to be accurate and effectiveBiological testingPretreatment methodSingle cell suspension
The invention provides a method for detecting influence of a buccal tobacco product on micronucleus rates of cells. The method comprises the following steps of performing a sample pretreatment method, performing preparation of single-cell suspension, calculating cell concentration, performing cell inoculation, adding a test object, incubating the test object, harvesting the cells, performing hypotension, dropping to a slider, dyeing, performing micronucleus calculation, performing result determination and the like. According to the method provided by the invention, by comprehensively surveying the exposure way and the action mode of the buccal tobacco product, the sample pretreatment method for the buccal tobacco product is established; moreover, human oral mucosal fibroblasts hOMF are selected according to action target cells of the buccal tobacco product to serve as cells for in vitro micronucleus test detection on the buccal tobacco product, and the sample detection dosage is determined, and therefore an in vitro micronucleus test detection method suitable for the buccal tobacco product is formed.
Owner:CHINA TOBACCO YUNNAN IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com