Patents
Literature
51 results about "Glycophorin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A glycophorin is a sialoglycoprotein of the membrane of a red blood cell. It is a membrane-spanning protein and carries sugar molecules. It is heavily glycosylated (60%). Glycophorins are rich in sialic acid, which gives the red blood cells a very hydrophilic-charged coat. This enables them to circulate without adhering to other cells or vessel walls.
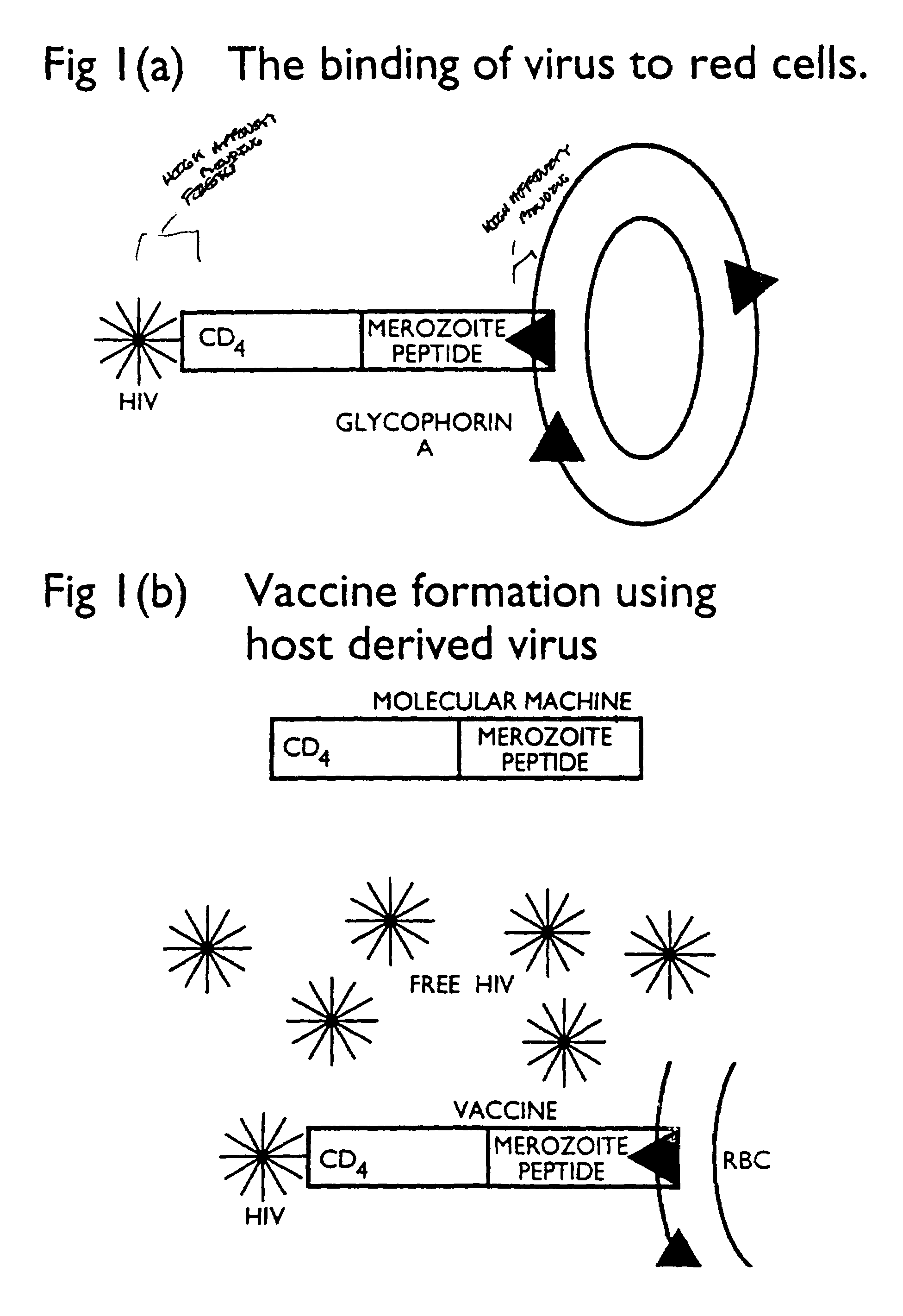
Fusion proteins comprising CD4 and the malaria parasite merozoite glycophorin binding protein 130 (GBP-130)
InactiveUS7585508B1Reduce infectivityRelieve symptomsFusions with soluble cell surface receptorAntiviralsGlycophorinBinding peptide
Novel hybrid fusion peptides are disclosed. The novel peptides are formed by the fusion of two or more components. One component is a peptide sequence or variant of a peptide sequence derived from a malaria parasite merozoite peptide which has affinity for and binding capability to red blood cells.In particular segments of the glycophorin binding peptide 130 (GBP130), are preferred for the first component. Also disclosed are alternative first components, the glycophorin binding peptide homologues (GBPH), or the erythrocyte binding antigen 175 (EBA175), or the plasmodium vivax Duffy receptor or the pre major merozoite surface antigen PMMSA or the (P200) peptide.The first component peptide is fused to all or part of a peptide segment derived from the CD4 molecule or part thereof or variant thereof which shows binding affinity for the HIV virus.The resulting fusion peptide being exemplified asNH2-CD4-GBP130-COOH1-371 201-774Also disclosed are the methods of manufacture and means to use the novel hybrid peptides as clinical agents to treat, prevent or test for HIV infection.
Owner:PRENDERGAST KENNETH F
Stem Cell Populations and Methods of Use
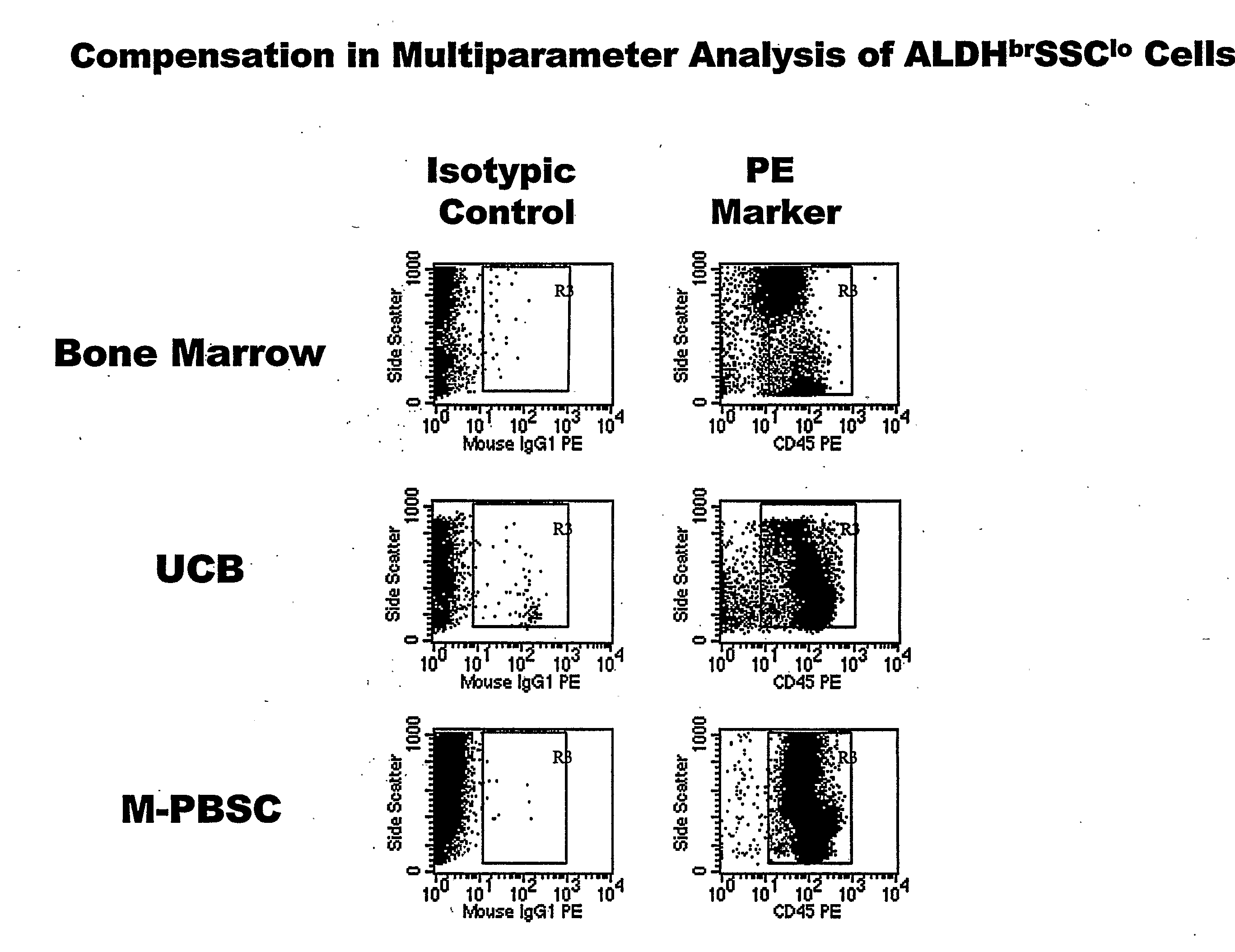
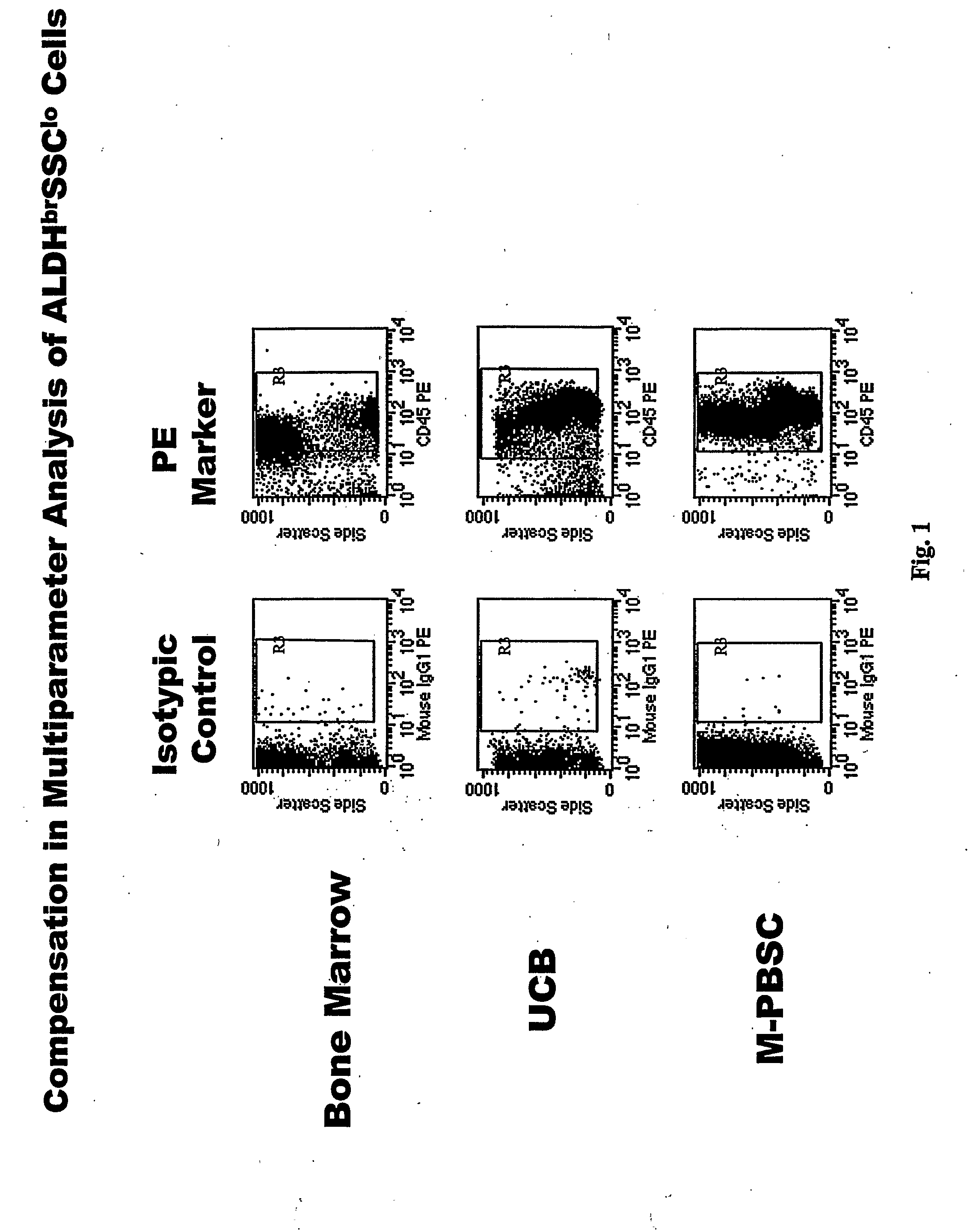
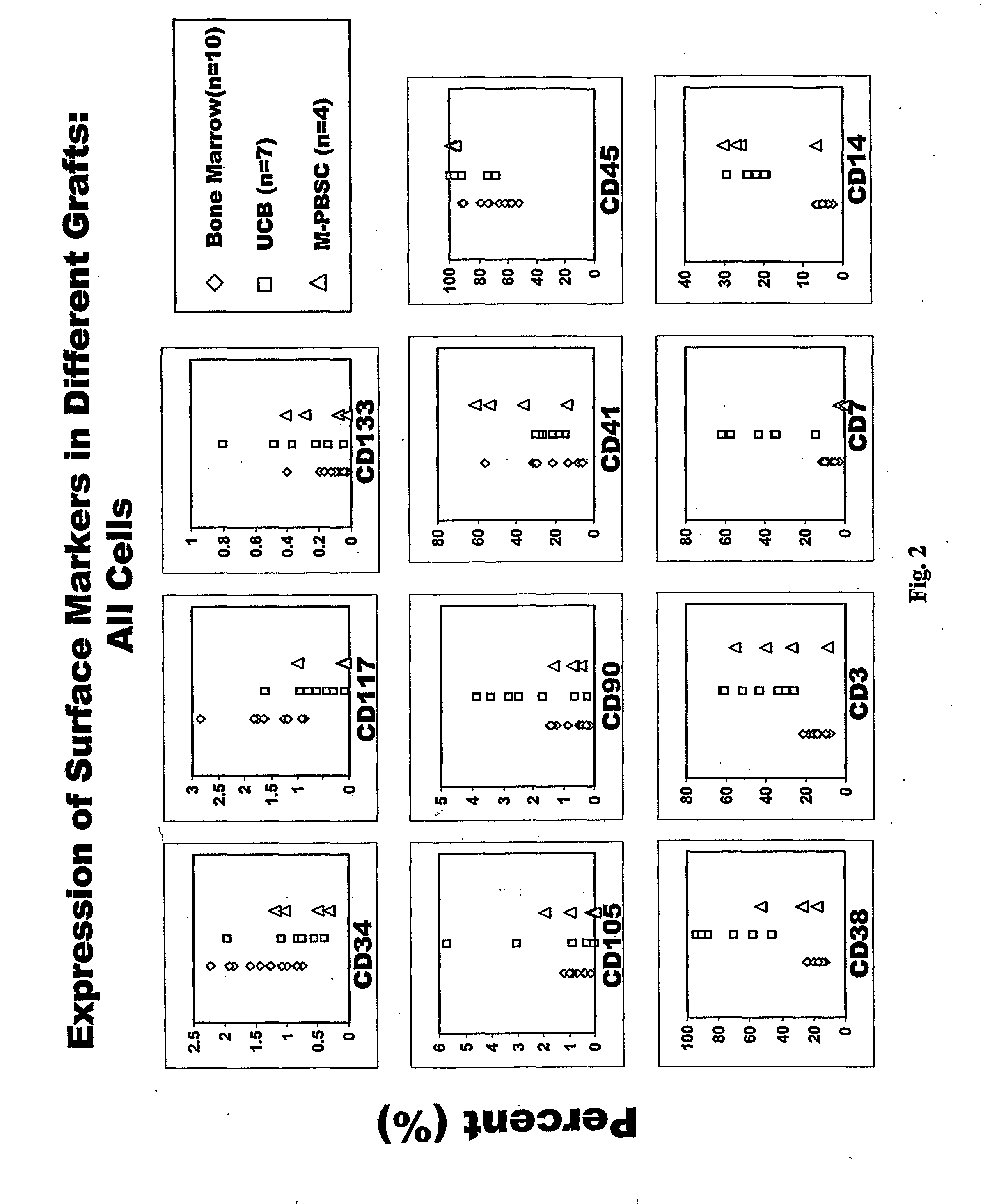
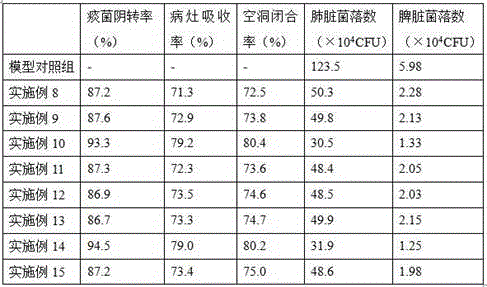
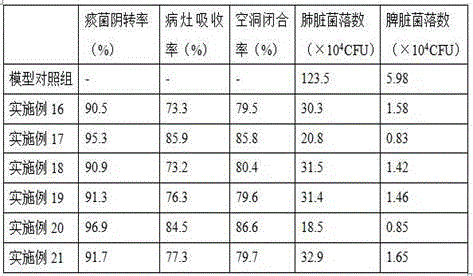
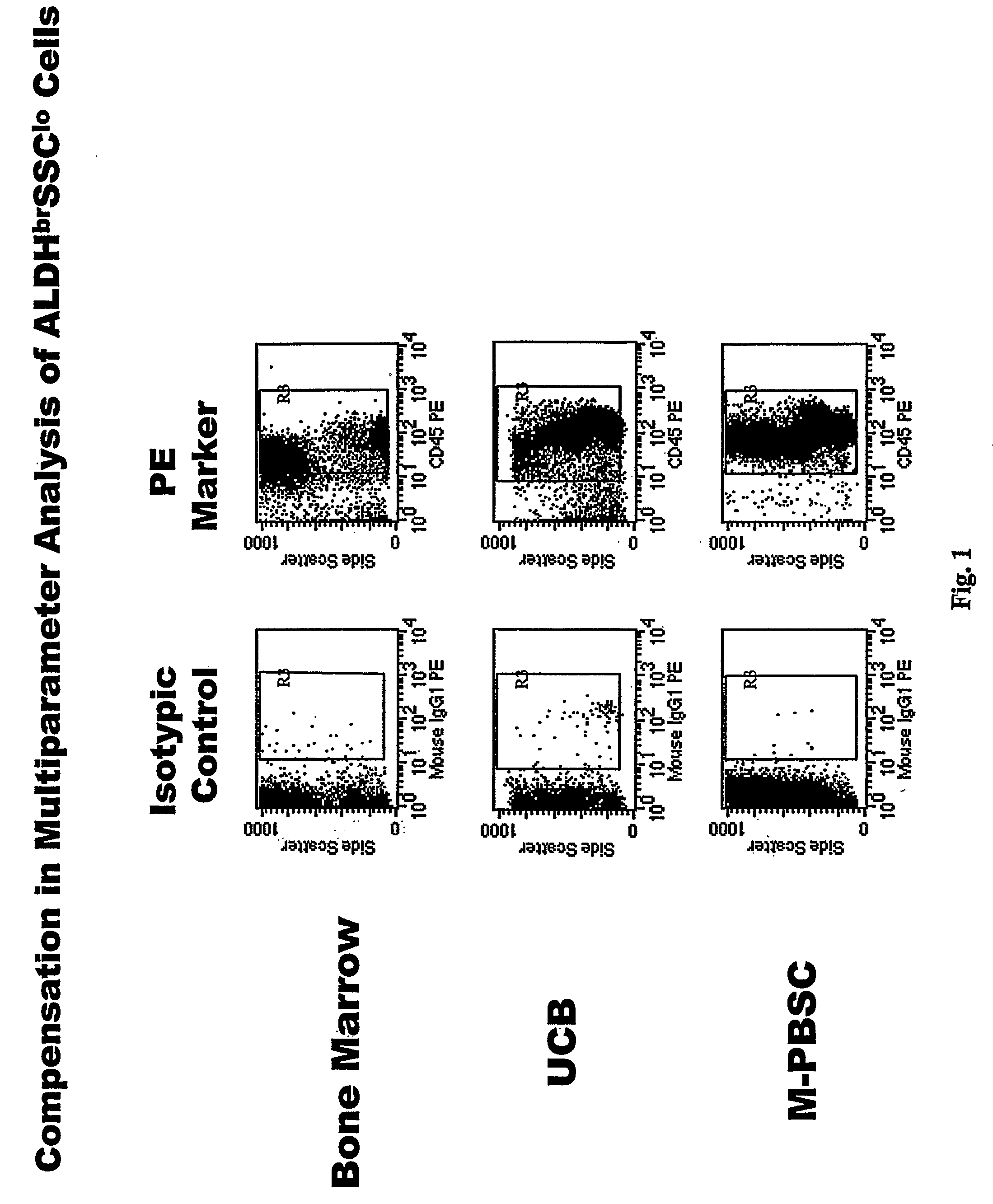
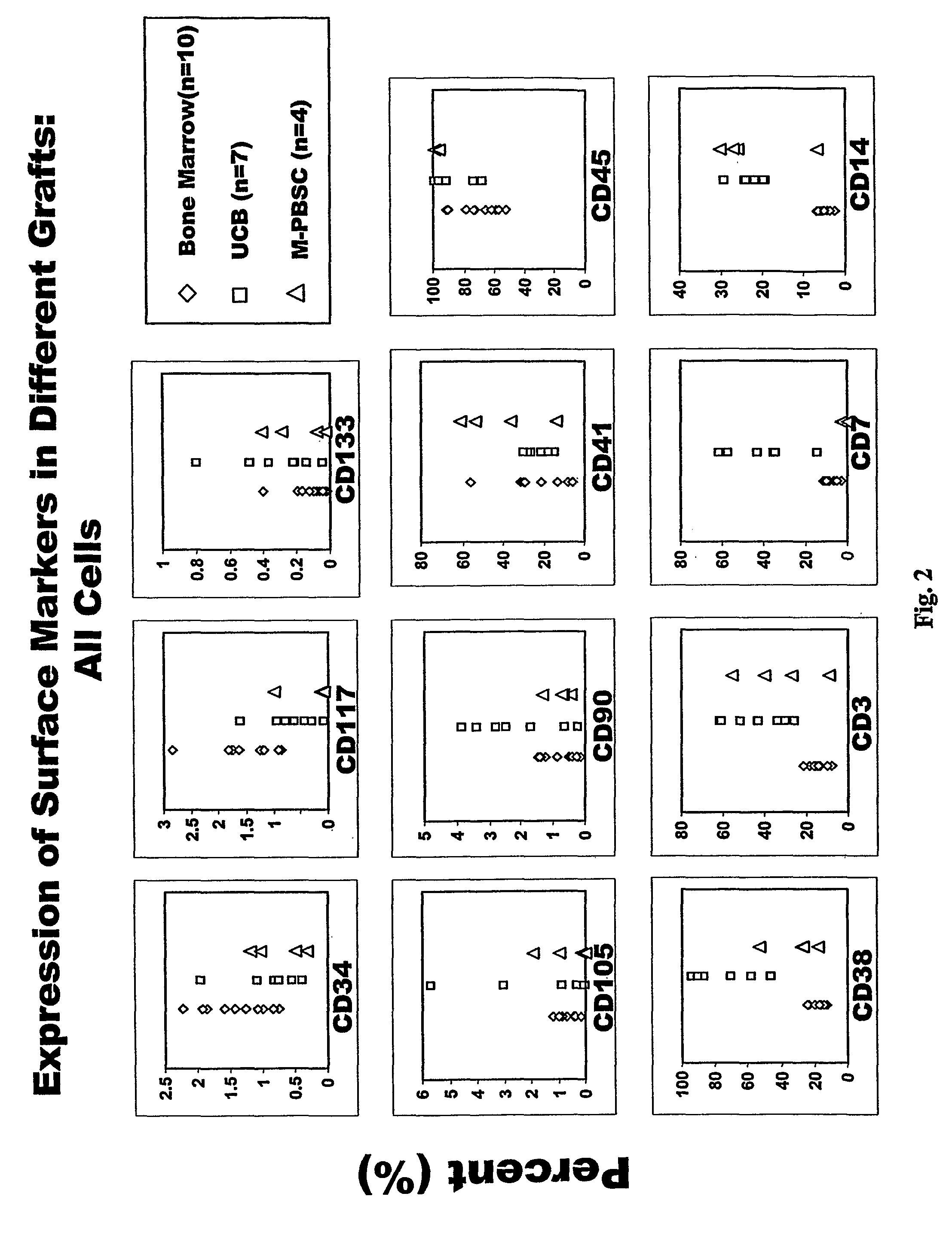
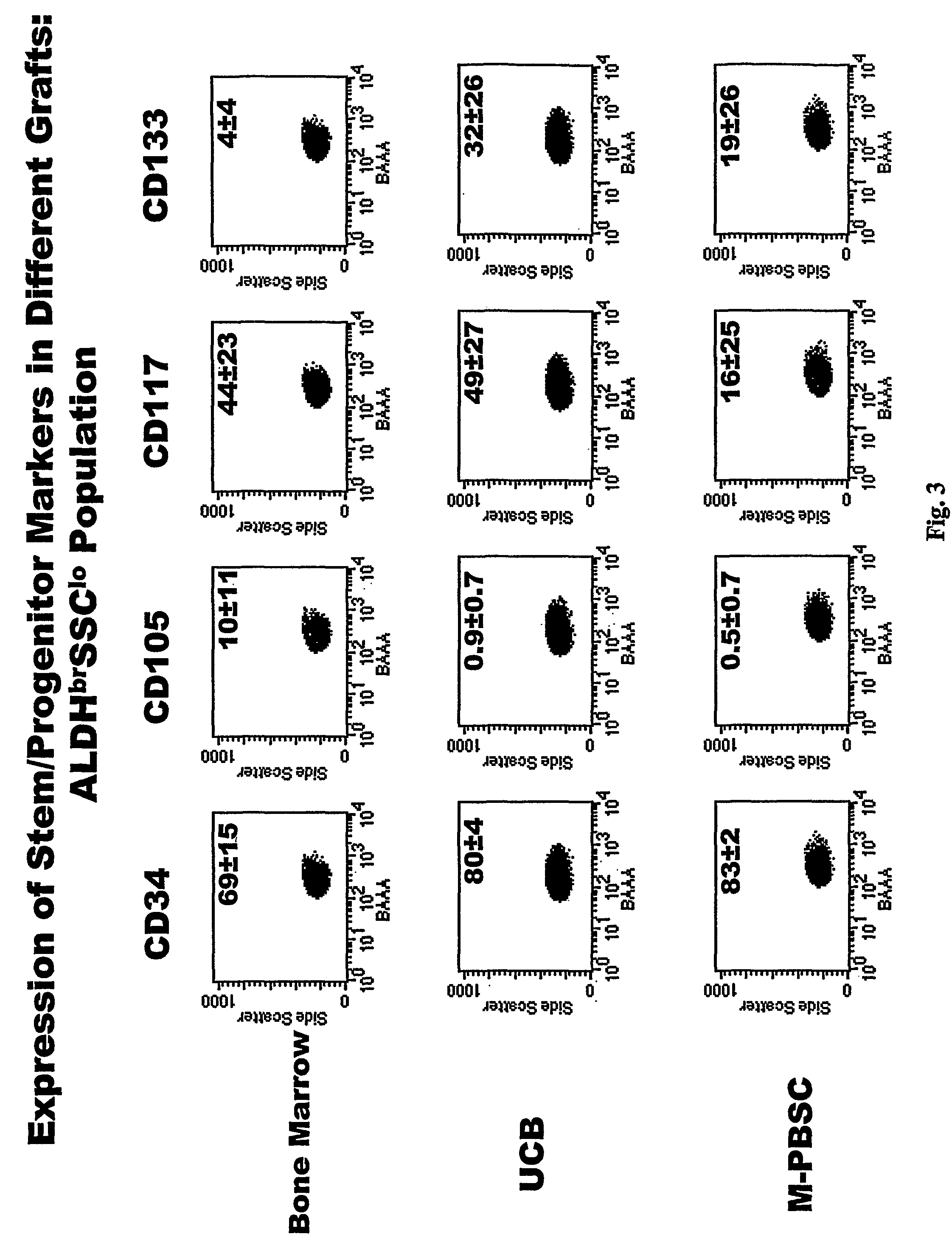
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
1-phenylalcoxy-2-beta-phenylethyl derivatives as p-glycoprotein (p-gp) inhibitors useful in drug resistance events
The invention relates to a new class of compounds, which are 1-phenylalcoxy-2-β-phenylethyl derivatives, as P-glycoprotein (P-GP) inhibitors. These compounds are useful in drug resistance events. They have been shown able to inhibit in a dose-dependent manner Glycoprotein-P (P-gp) activity in cell lines in which the expression of said glycoprotein is very high, like Caco-2 (human colon cancer) cells and MCF7 / Adr (adriamycin-resistant human breast carcinoma) cells. The invention also relates to methods of production and the utilization of such compounds as medicaments useful in the treatment of states linked to the difficulty for some drugs to cross the blood-brain barrier (BBB) and generally within the context of the problems of drug resistance induced by chemotherapy agents.
Owner:UNIV DEGLI STUDI DI BARI 60 +1
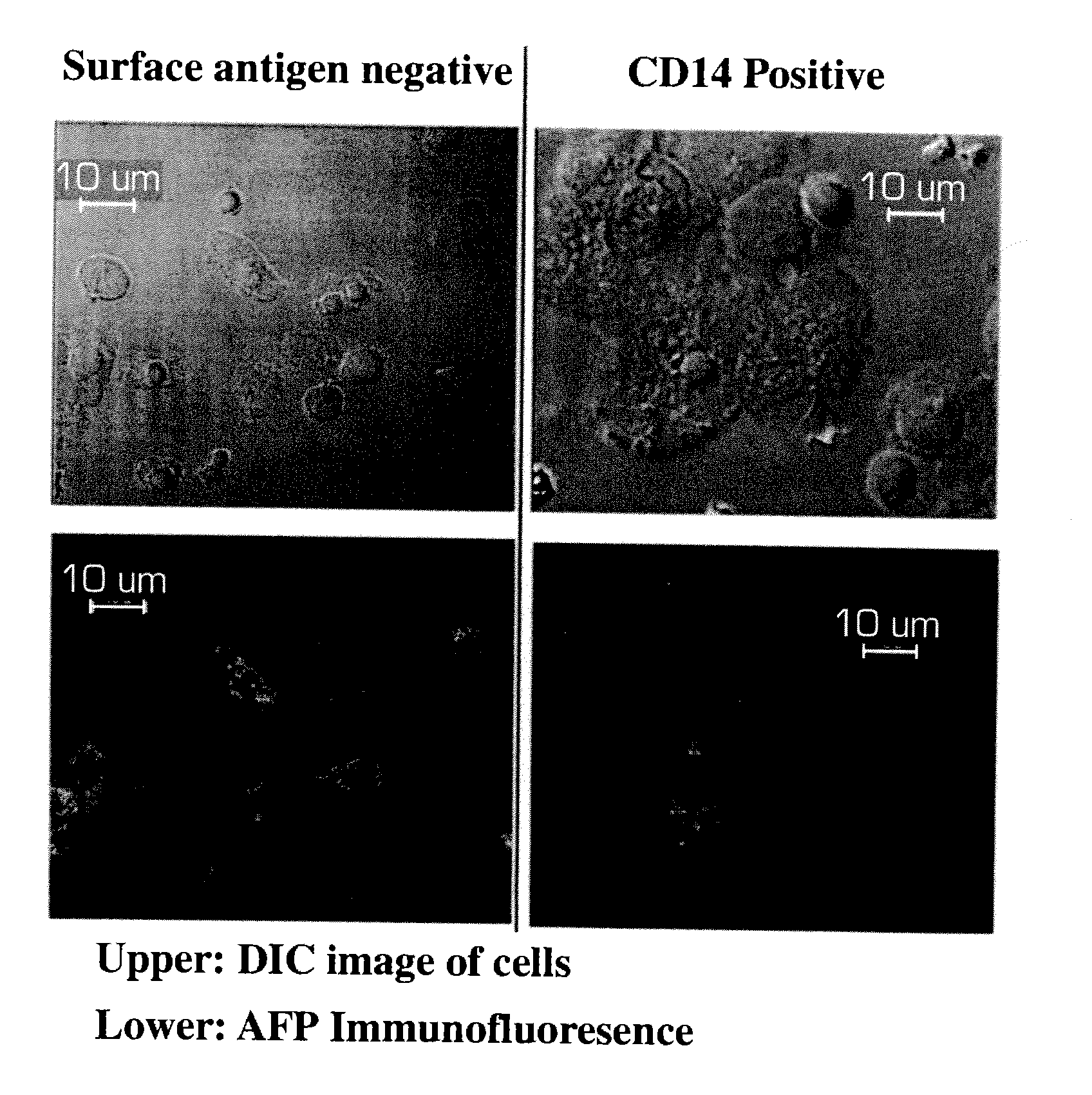
Human liver progenitors
InactiveUS20050148072A1Low densityReduce in quantityHepatocytesGenetic material ingredientsProgenitorGlycophorin
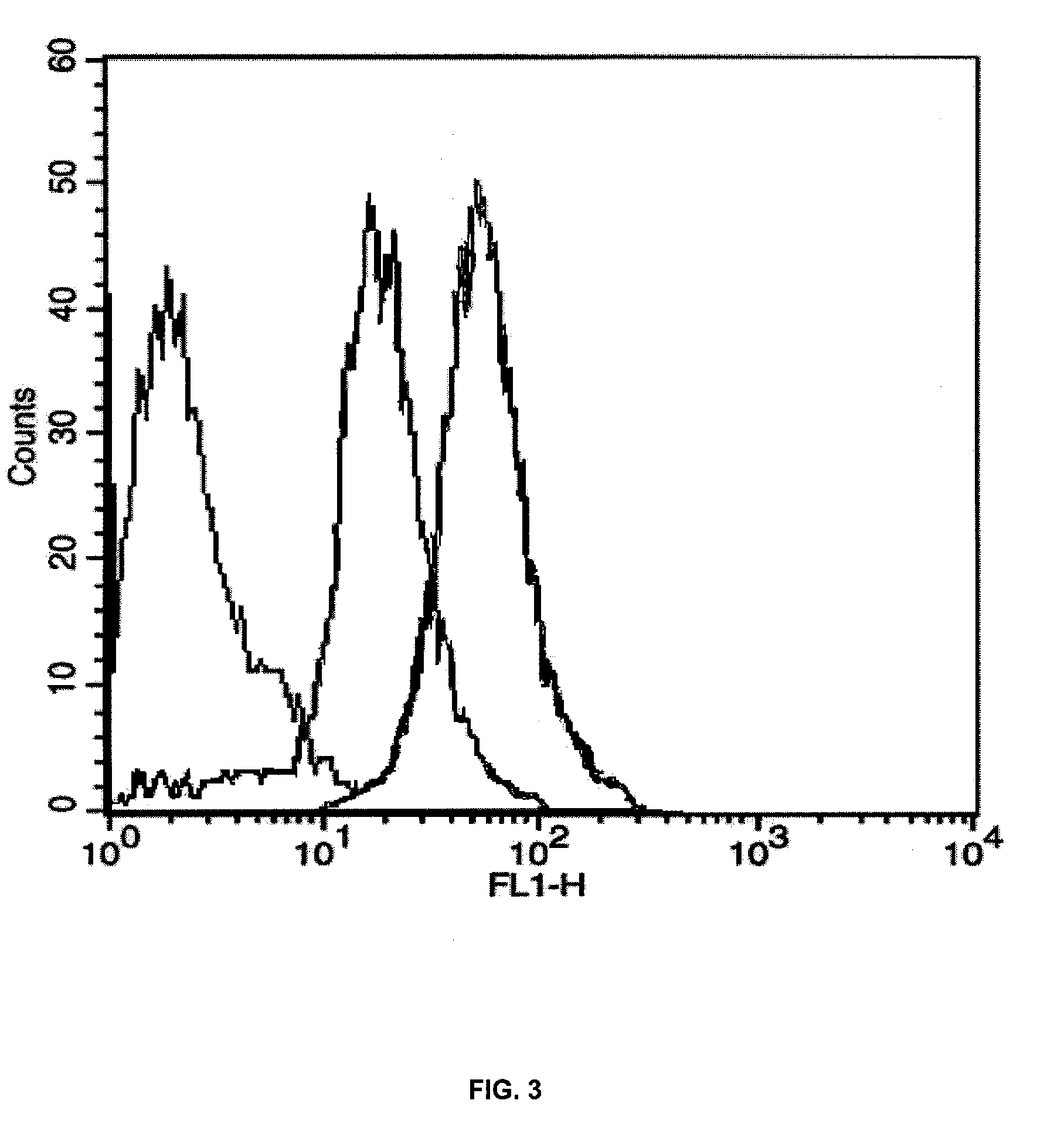
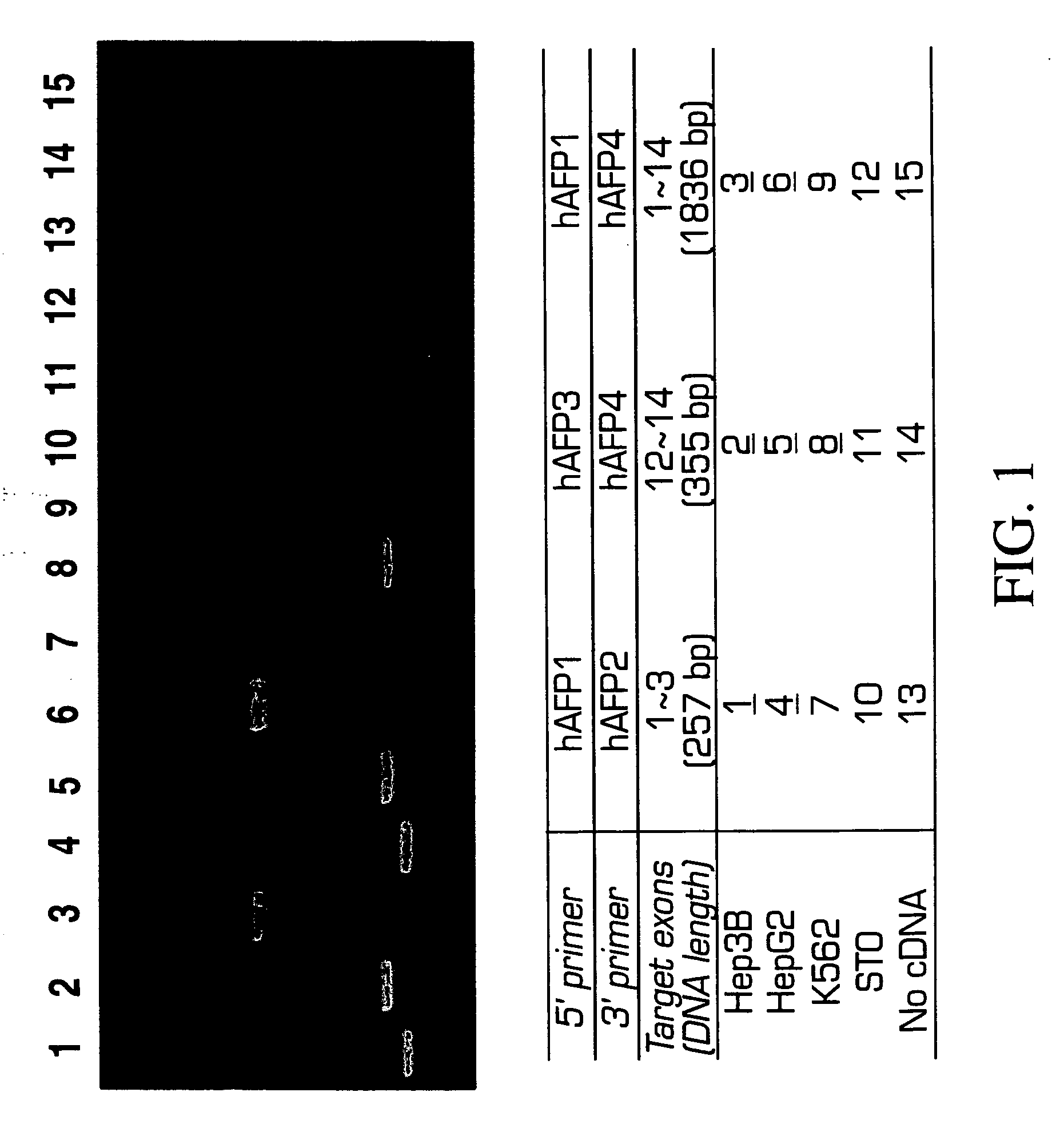
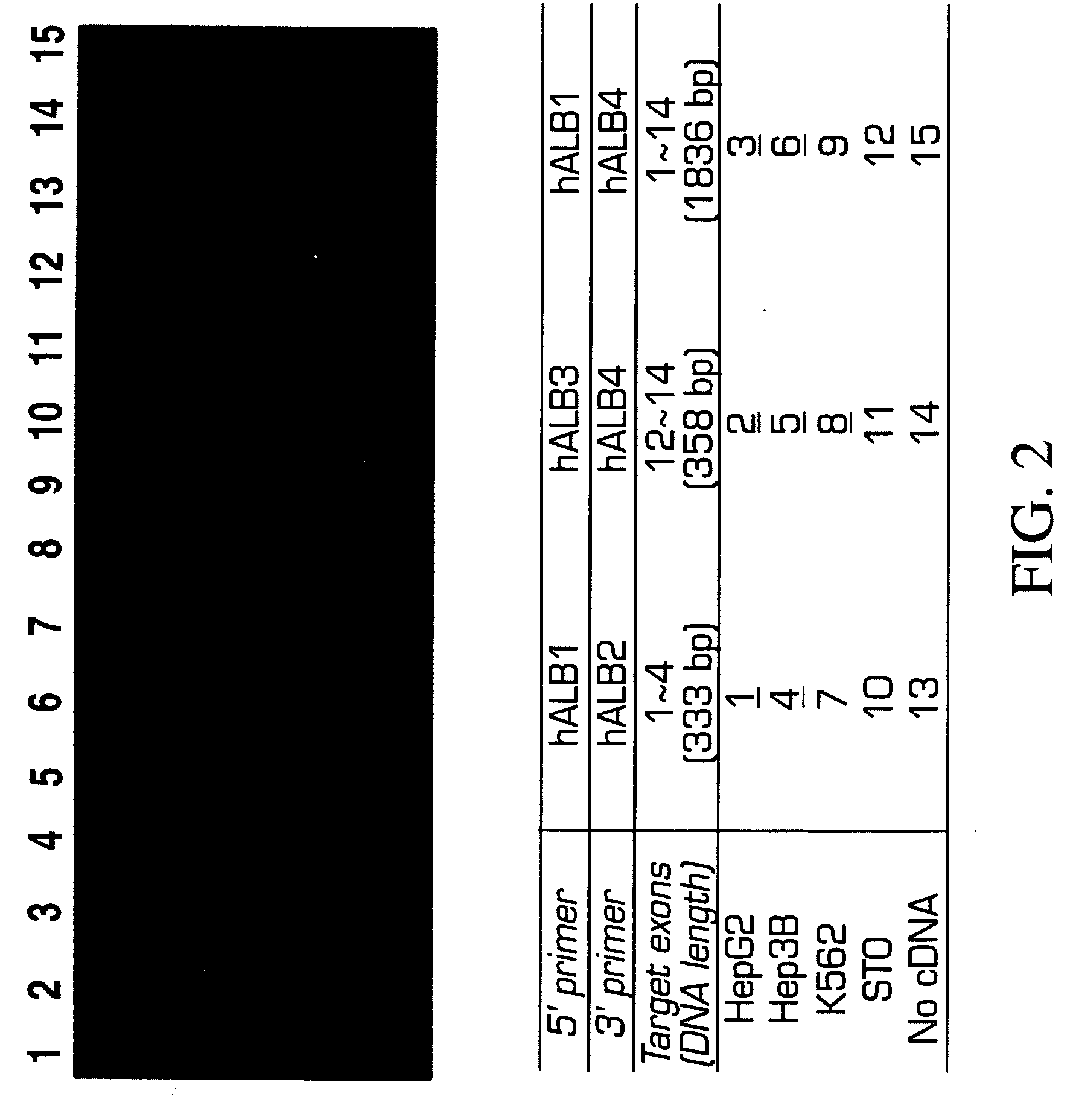
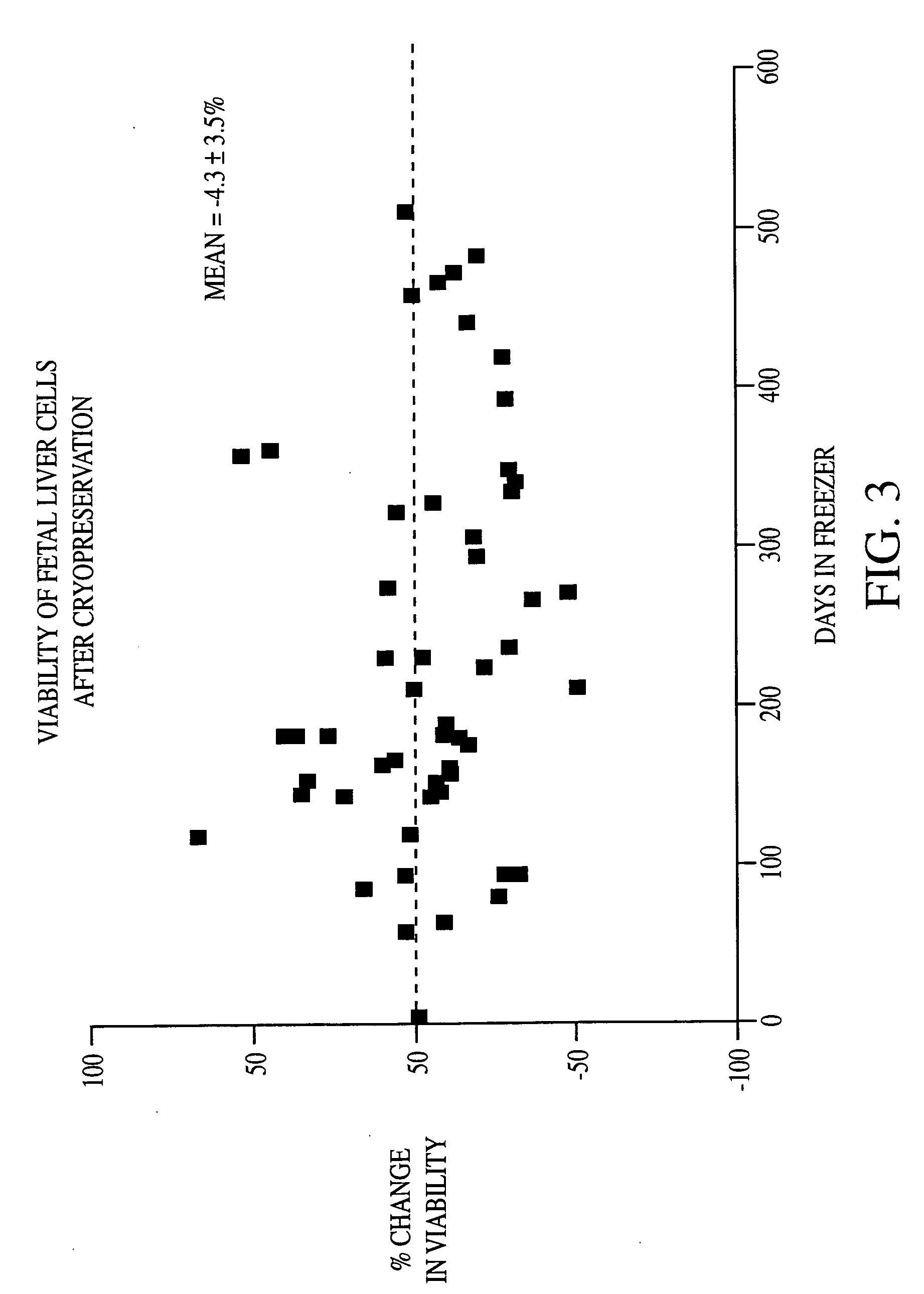
Methods of isolating and cryopreserving progenitors from human liver are disclosed which include processing human liver tissue to provide a substantially single cell suspension comprising progenitors and non-progenitors of one or more cell lineages found in human liver; subjecting the suspension to a debulking step, which reduces substantially the number of non-progenitors in the suspension, and which provides a debulked suspension enriched in progenitors exhibiting one or more markers associated with at least one of the one or more cell lineages; and selecting from said debulked suspension those cells, which themselves, their progeny, or more mature forms thereof express one or more markers associated with at least one of the one or more cell lineages. Among these markers are CD14, CD34, CD38, CD45, and ICAM. Hepatic progenitors are characterized as being 6-15μ in diameter, diploid, glycophorin A−, CD45−, AFP+++, ALB+, ICAM+, and with subpopulations varying in expression of CD14+. CD34++, CD38++, CD117+. These progenitor subpopulations have characteristics expected for cells that are particularly useful in liver cell and gene therapies and for establishing bioartificial organs.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
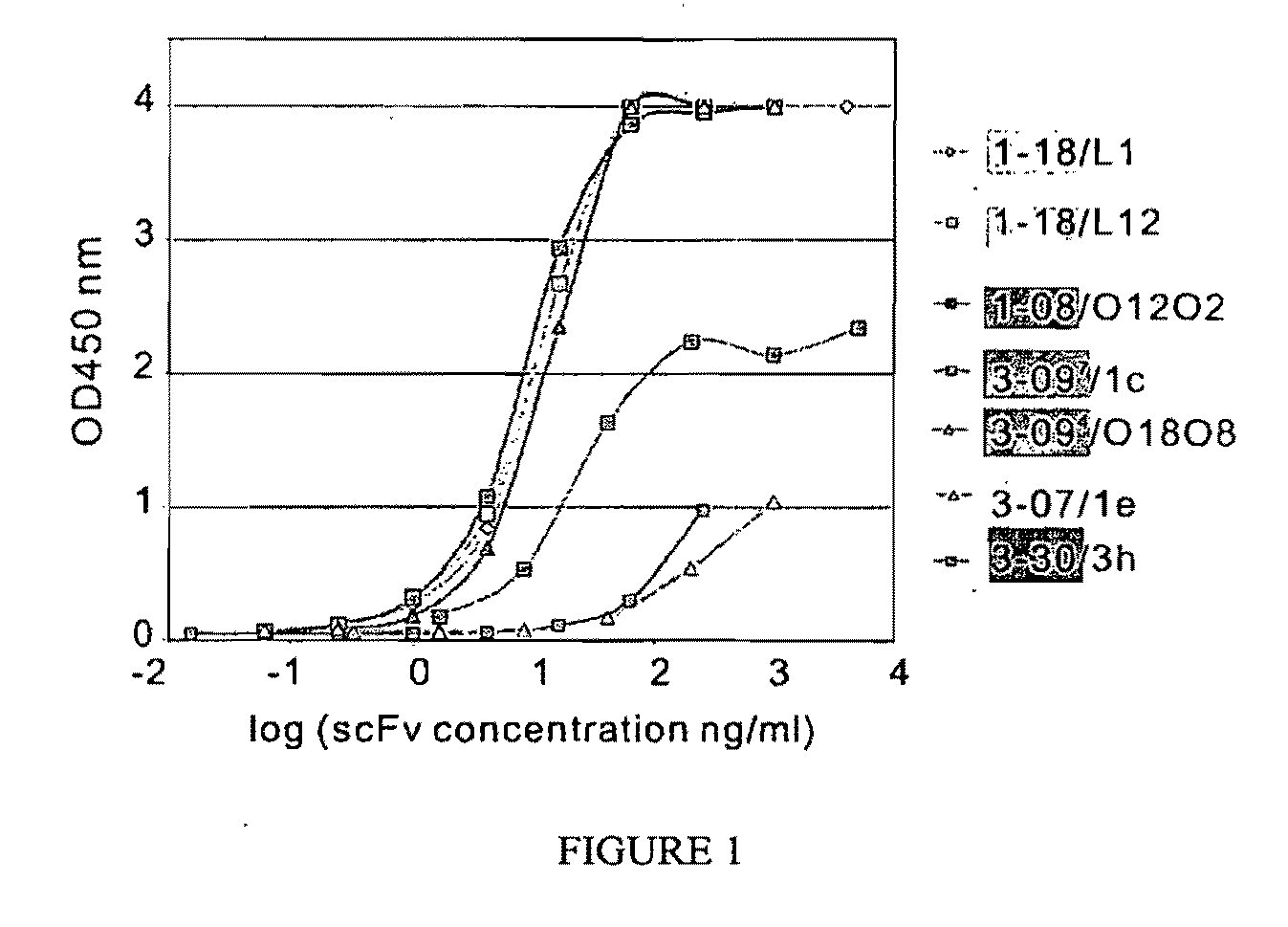
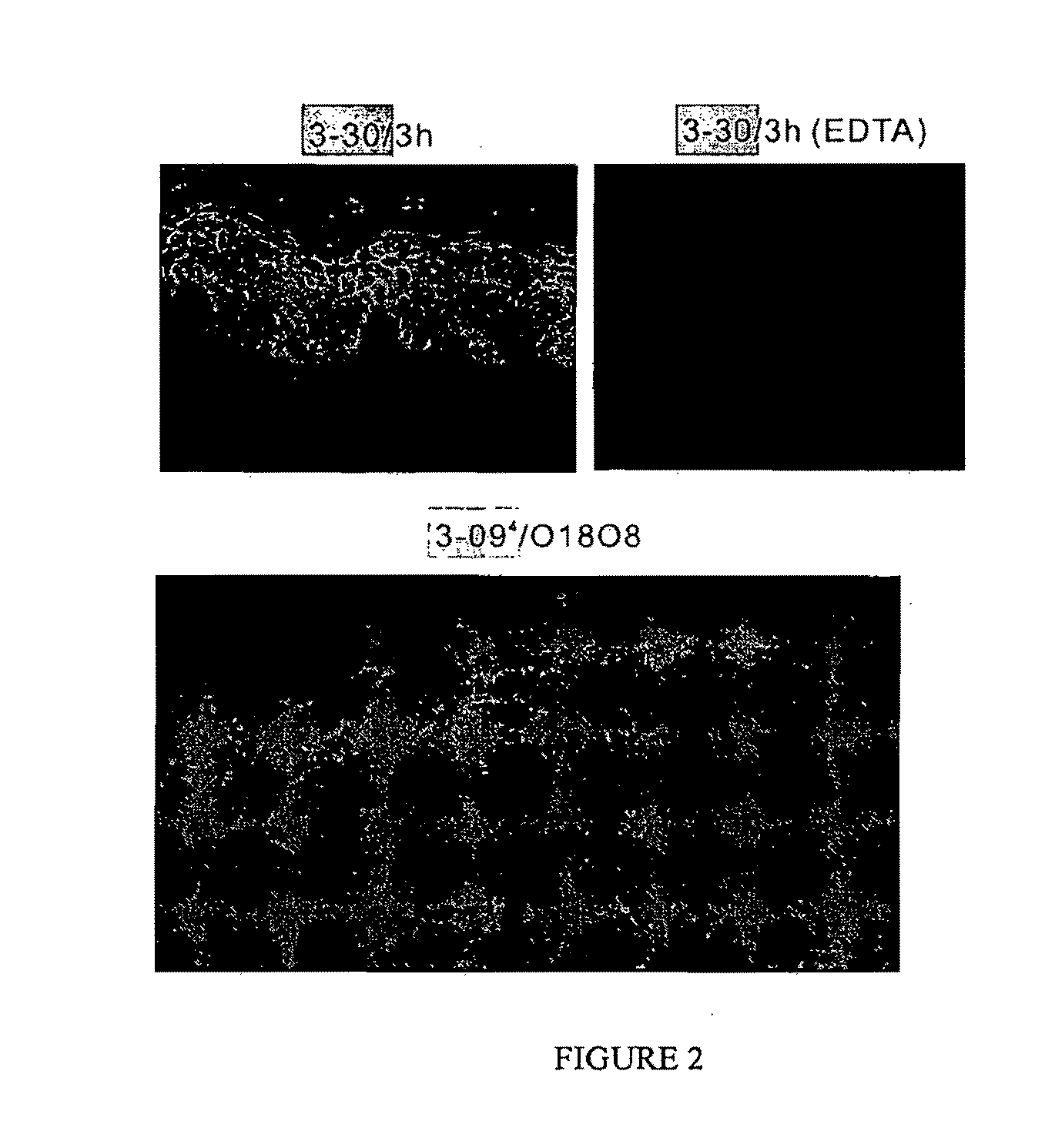
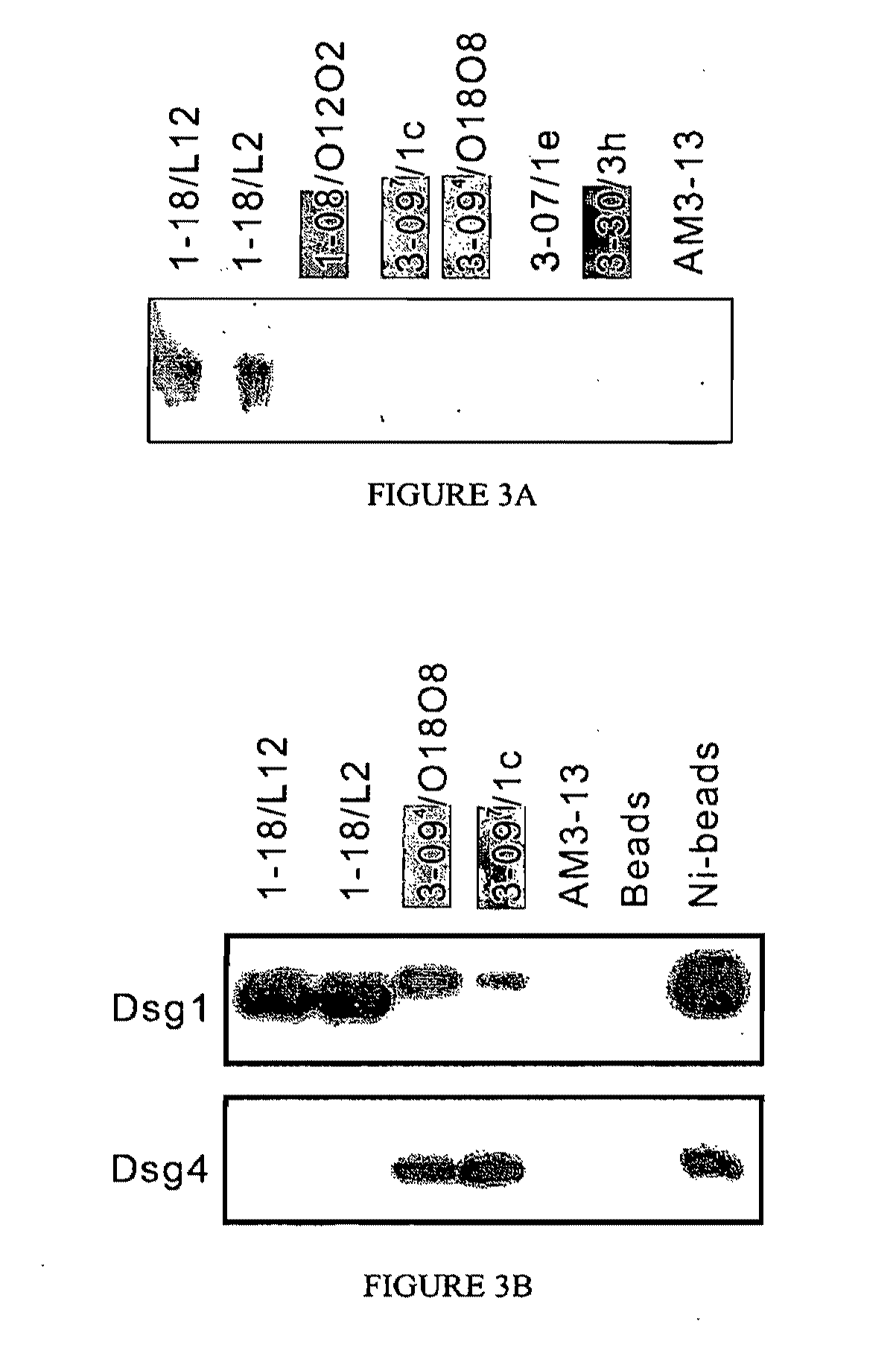
Isolation of Anti-Desmoglein 1 Antibodies by Phage Display of Pemphigus Foliaceus Autoantibodies
ActiveUS20110091449A1Inhibit expressionInhibit bindingOrganic active ingredientsPeptide/protein ingredientsGlycophorinAnaplasma phagocytophilum
This invention relates to compositions and methods for the use of anti-autoimmune reagents that specifically bind to anti-desmoglein antibodies, which are responsible for pemphigus foliaceus. In addition, the invention relates to methods and compositions for inhibiting the expression or function of a variable region of an anti-desmoglein (anti-Dsg) pathogenic autoantibody.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
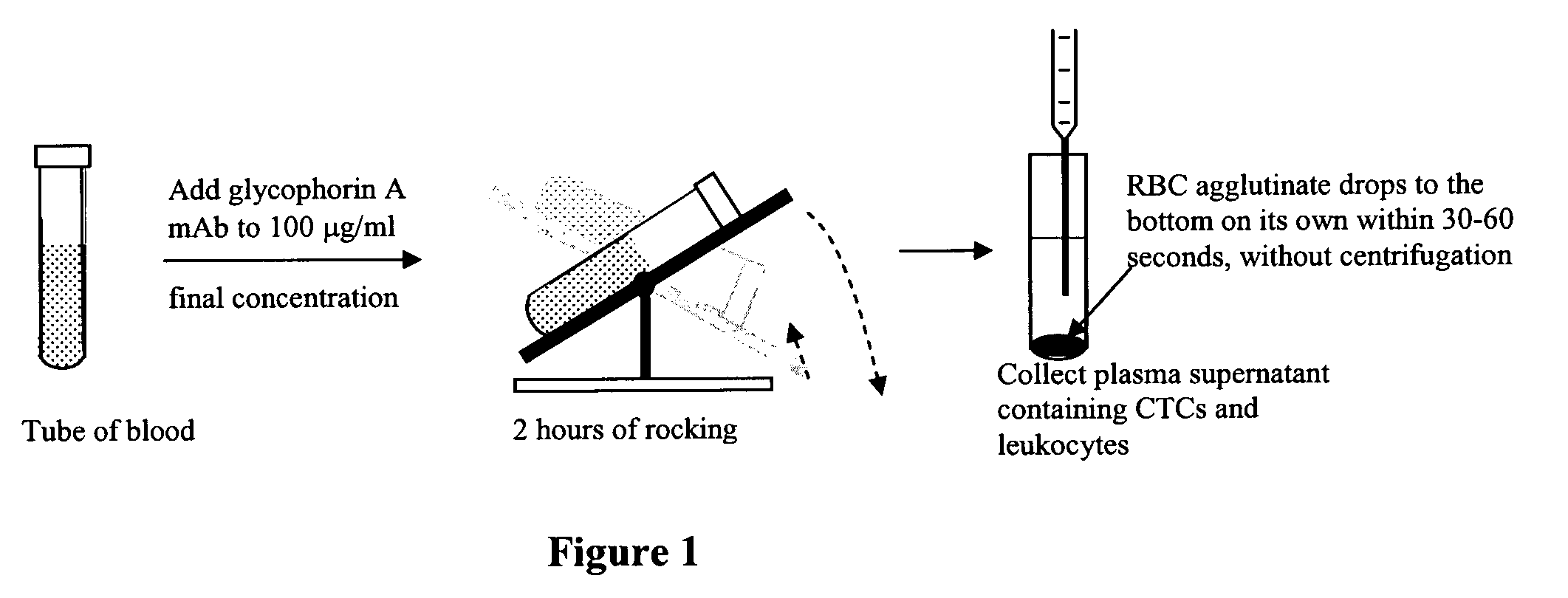
Method for enriching rare cell subpopulations from blood
ActiveUS8263404B2Increase cellular recoveryHighly reproducibleDead animal preservationBiological testingAntigenGlycophorin
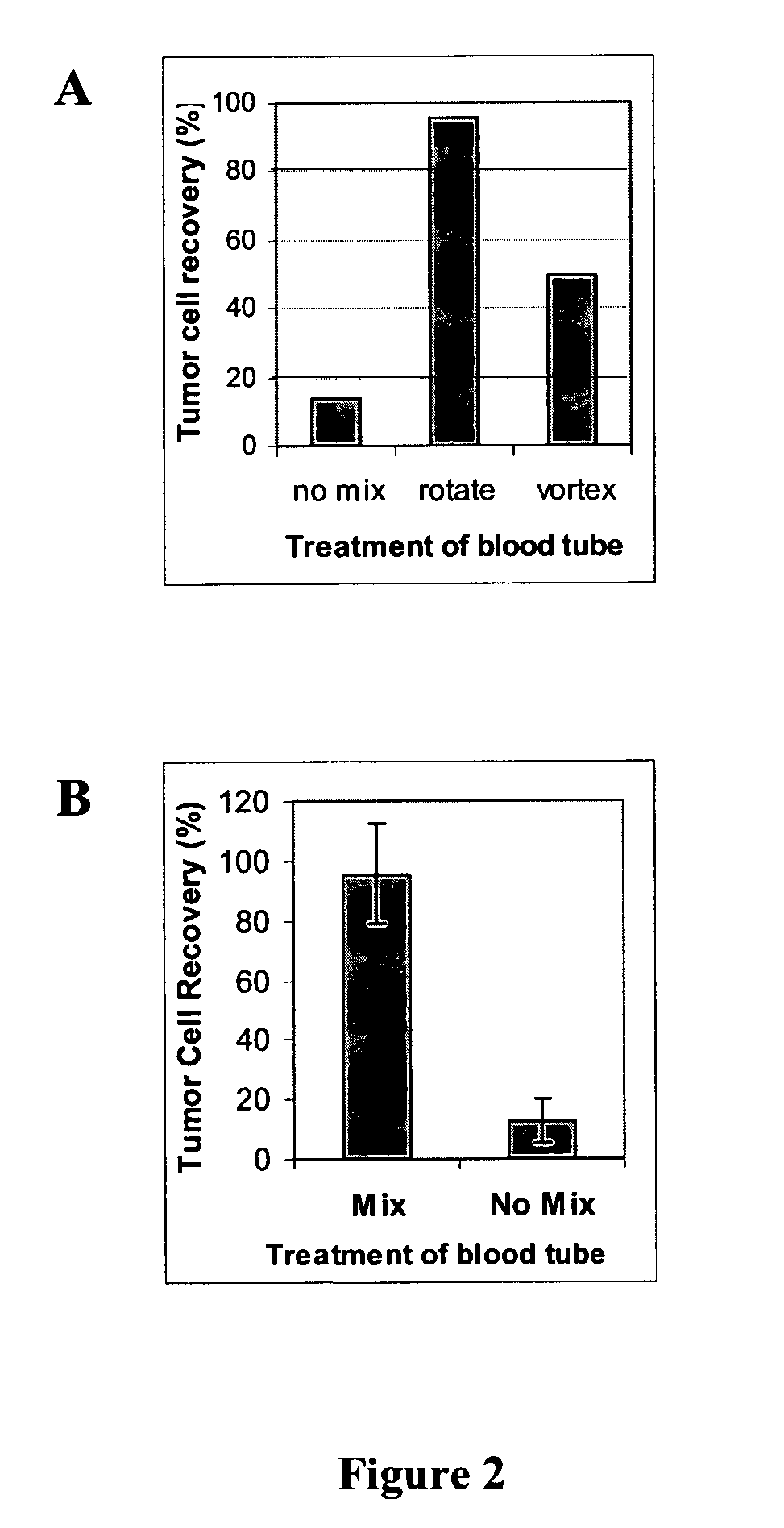
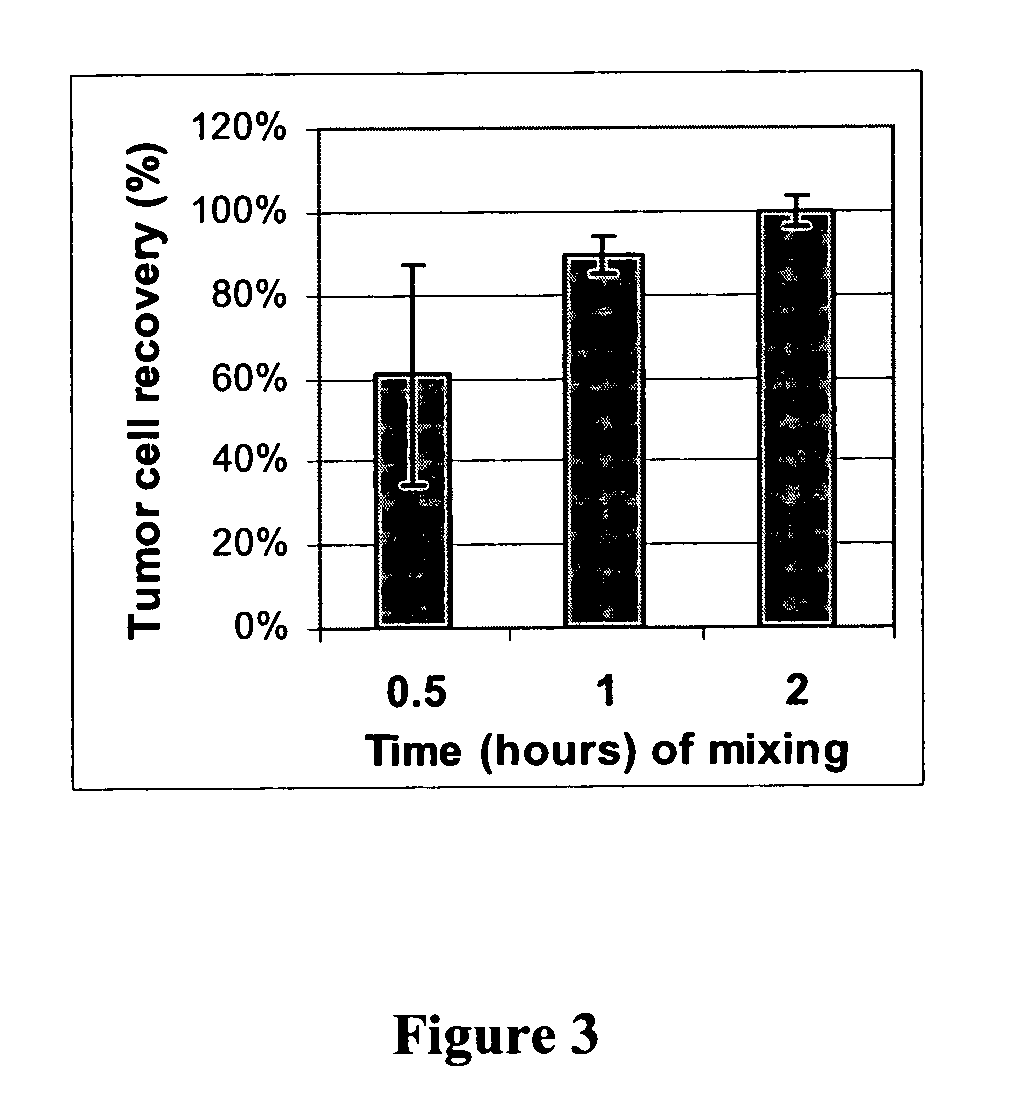
An antigen-dependent negative selection blood cell separation method is described. Rare circulating epithelial cells can be separated from blood by depleting erythrocytes from a blood sample. Erythrocytes are depleted by agglutination. The new method comprises the use of an agglutinating agent, such as an anti-glycophorin A or glycophorin B antibody, as glycophorin A or B are present on erythrocytes and not on the desired epithelial cells. With regular mixing, desired rare circulating epithelial cells do not become entrapped in the red cell agglutinate.
Owner:MEDICAL DISCOVERY PARTNERS
Insect cell line for production of recombinant glycoproteins with sulfated complex n-glycans
Owner:UNIVERSITY OF WYOMING
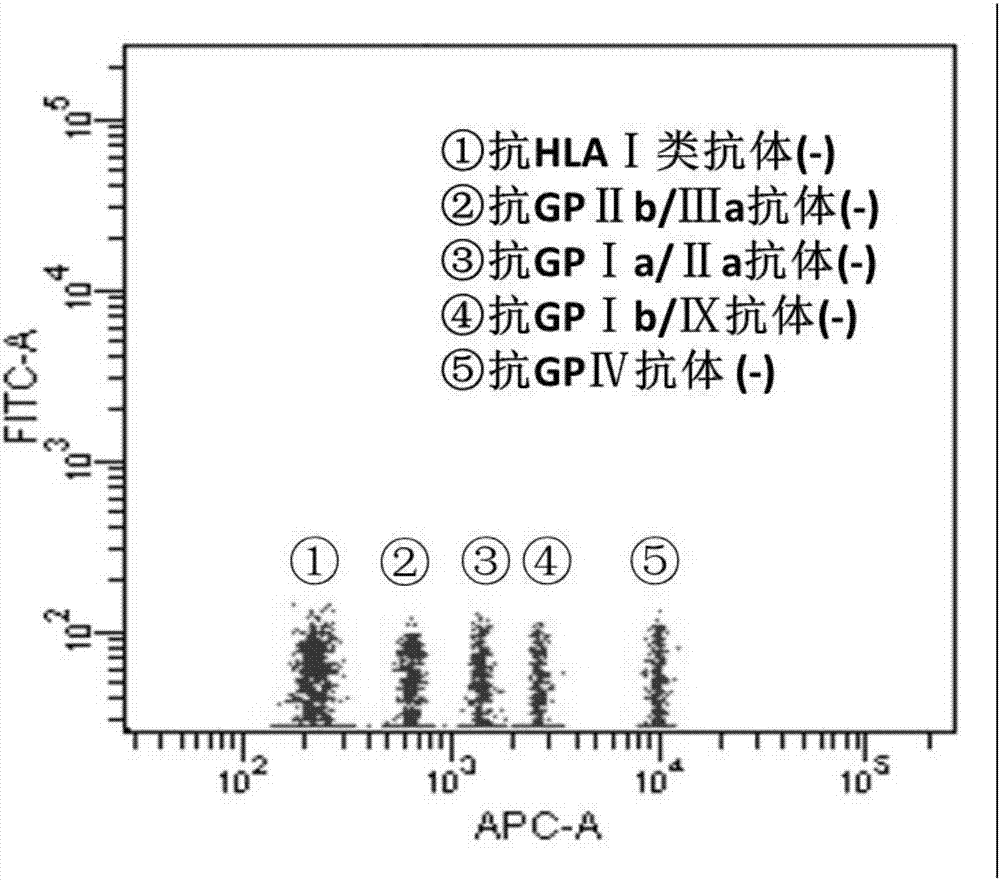
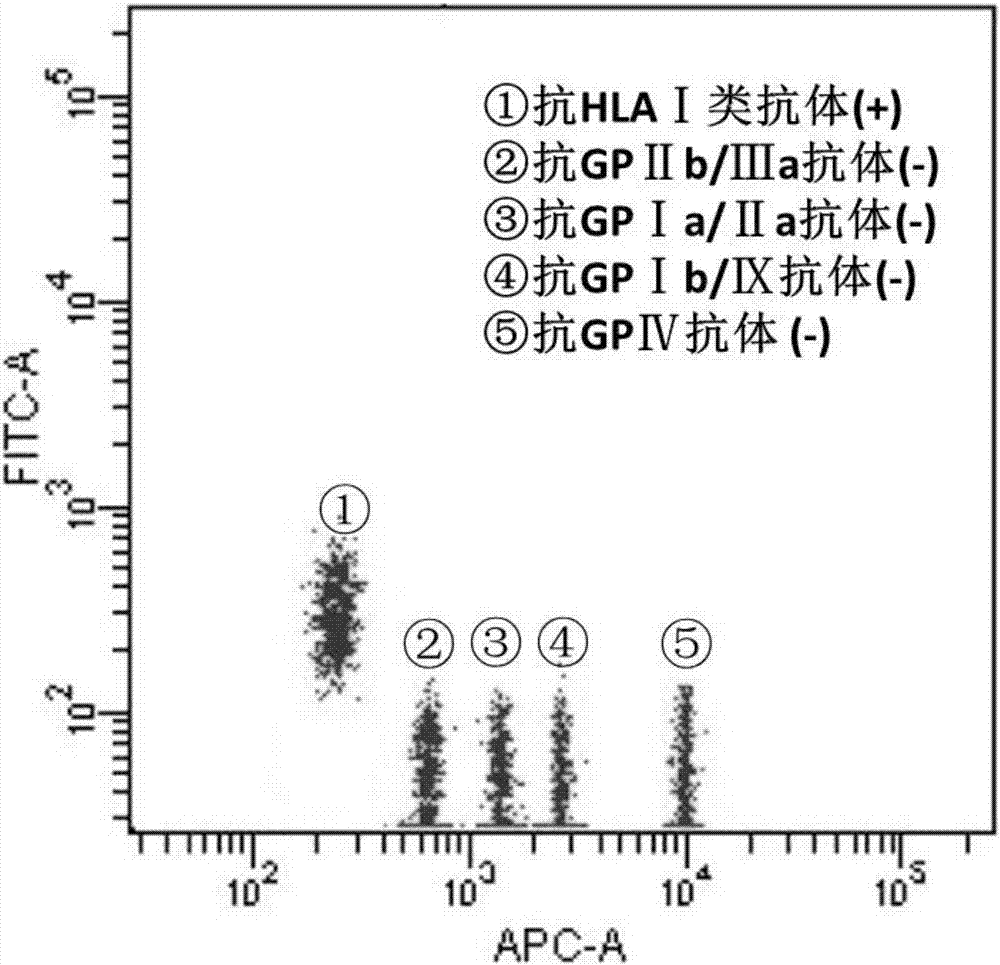
Blood platelet antibody specificity identification method and kit thereof
InactiveCN106872705AThe result is accurateTroubleshoot imprecise resultsIndividual particle analysisSerum igeGlycophorin
The invention provides a blood platelet antibody specificity identification method and a kit thereof. The method comprises the following steps: (1) connecting different blood platelet specificity glycoprotein to different fluorescent microsphere surfaces to obtain antigen specificity fluorescent microspheres for standby use; (2) adding the antigen specificity fluorescent microspheres into a sample to be detected to perform an incubation reaction, and washing for standby use; and (3) adding a fluorescent marked anti-human IgG into step (2) to perform the incubation reaction, washing, and performing flow cytometry detection. The blood platelet antibody specificity identification method solves the problems that the blood platelet antibody detection in the prior art mainly targets at an antigen-self blood platelet antibody complex on the surface of the blood platelet and does not detect free blood platelet antibodies in plasma or serum, and the detection result of the blood platelet antibody is imprecise; and moreover, the method is simple in steps, easy in operation, short in time comsuming and higher in efficiency.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
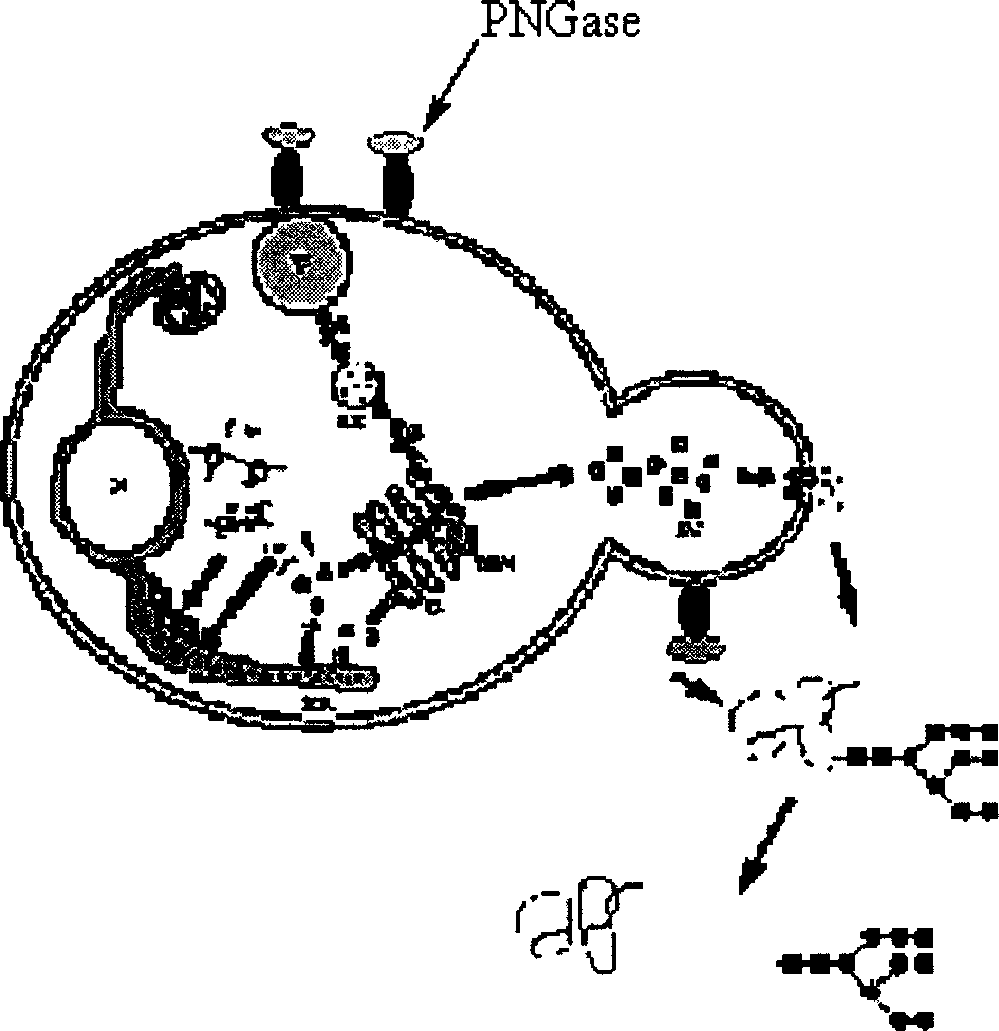
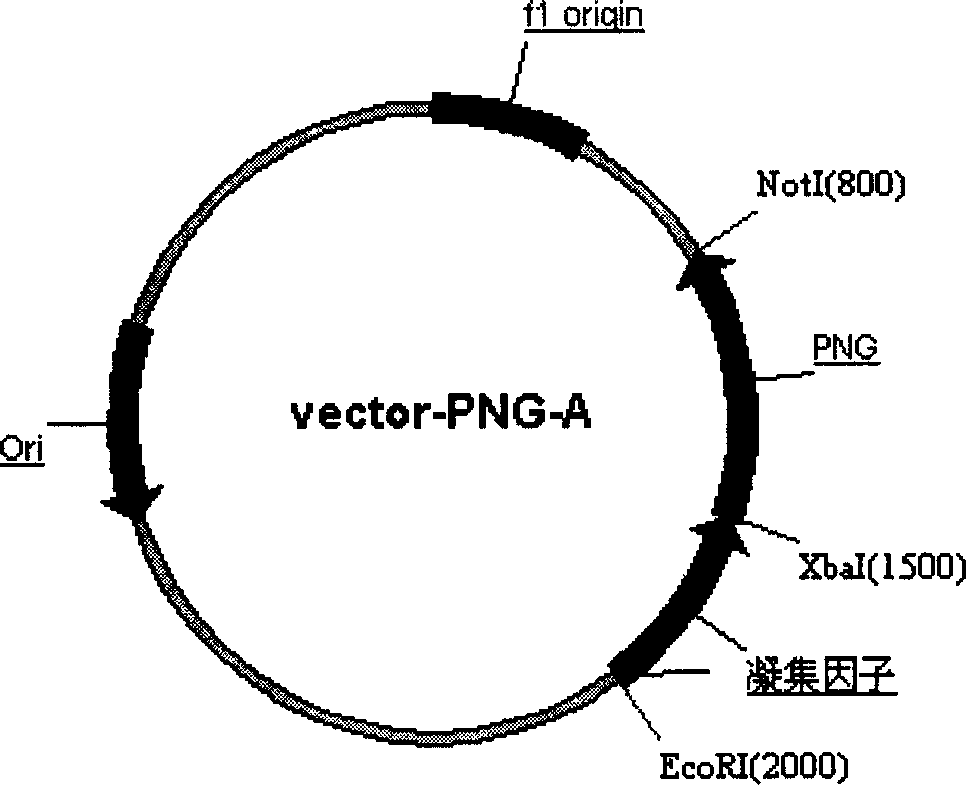
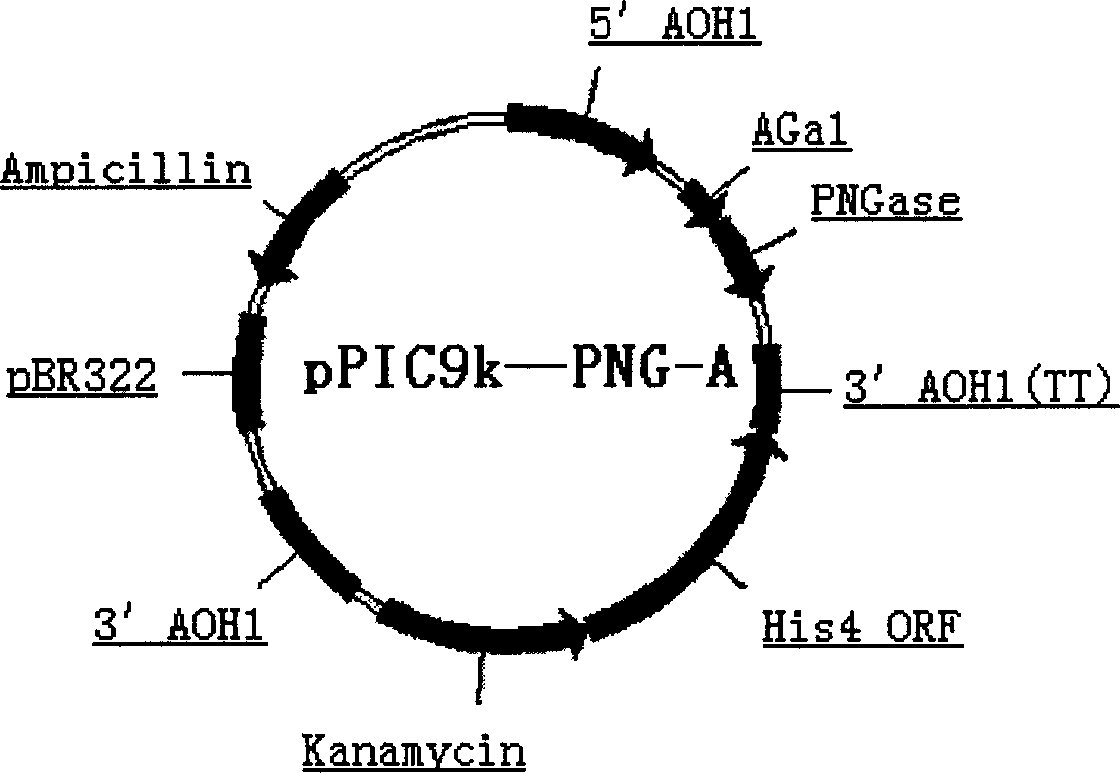
Production of Non-N glycosylated protein from yeast
InactiveCN1746302AEasy constructionSimple methodFermentationVector-based foreign material introductionHuman bodyGlycophorin
Production of non-glycosylated protein from yeast is carried out by constructing expression carrier containing N-glycoamidase and purposive protein gene from molecular biological technology, transferred into yeasts from electric transformation, expressing N- glycoamidase on yeast cell surface, excretory expressing purposive protein into culture medium, removing chain of glucoprotein excretory expressed from N- glycoamidase surface expressed and obtaining non-glycosylated protein. It has better expression protein activity and correct tertiary structure and no immunogenicity to human body.
Owner:SHANDONG UNIV
In vitro mass production of human erythroid cells from the blood of normal donors and thalassemic patients
InactiveUS20040229356A1FavorInhibit productionArtificial cell constructsBlood/immune system cellsGlycophorinThalassemia
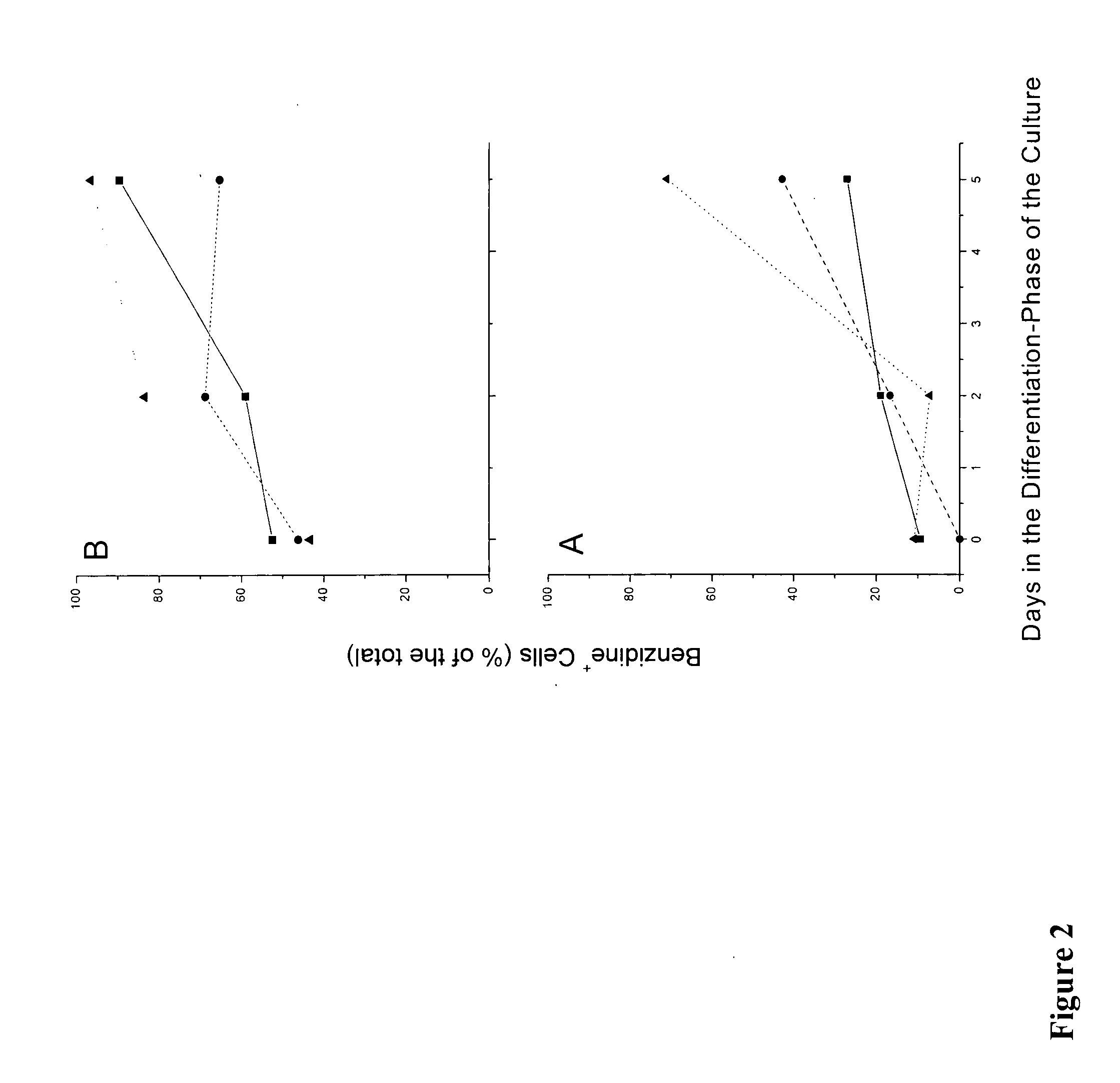
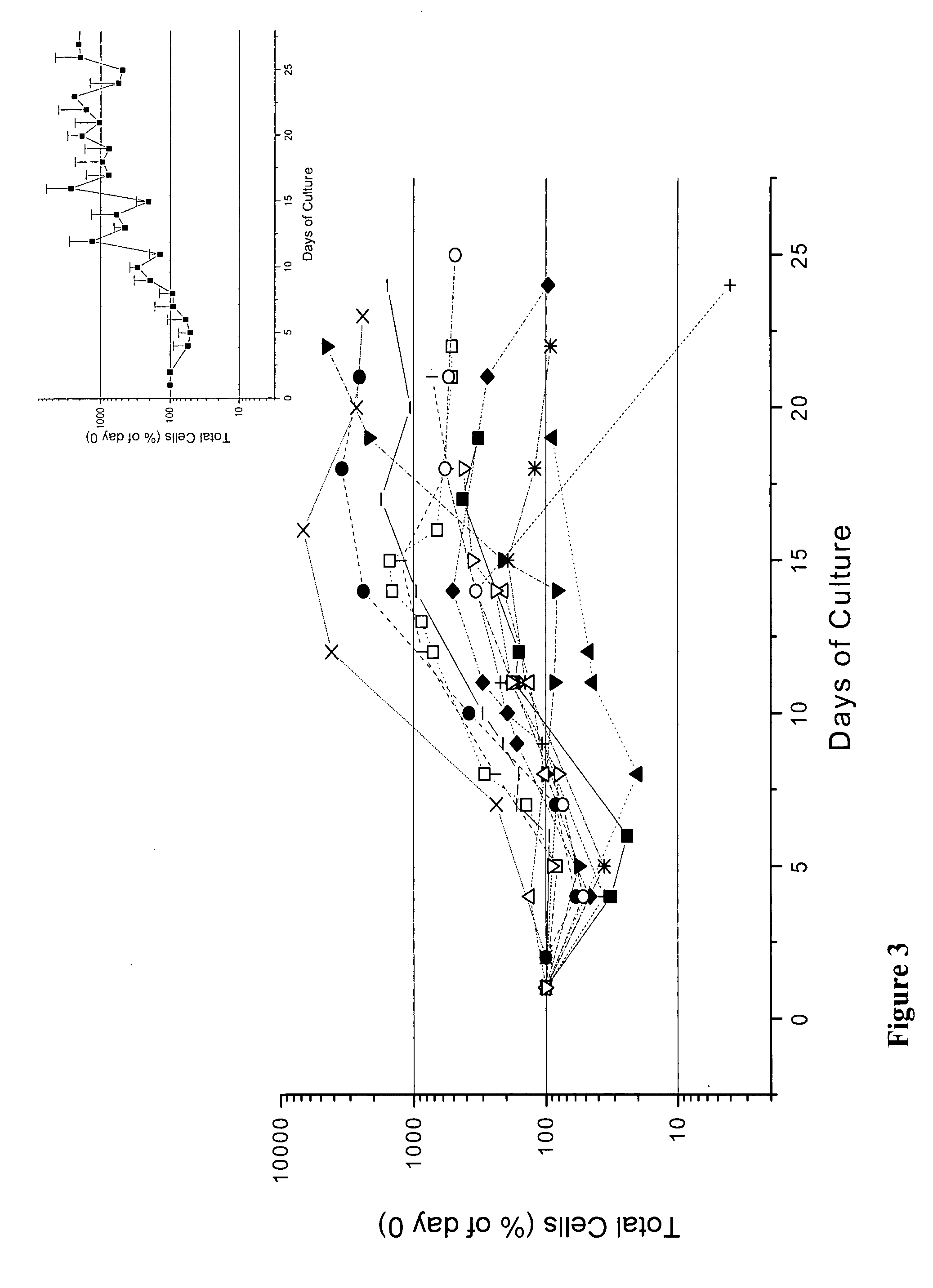
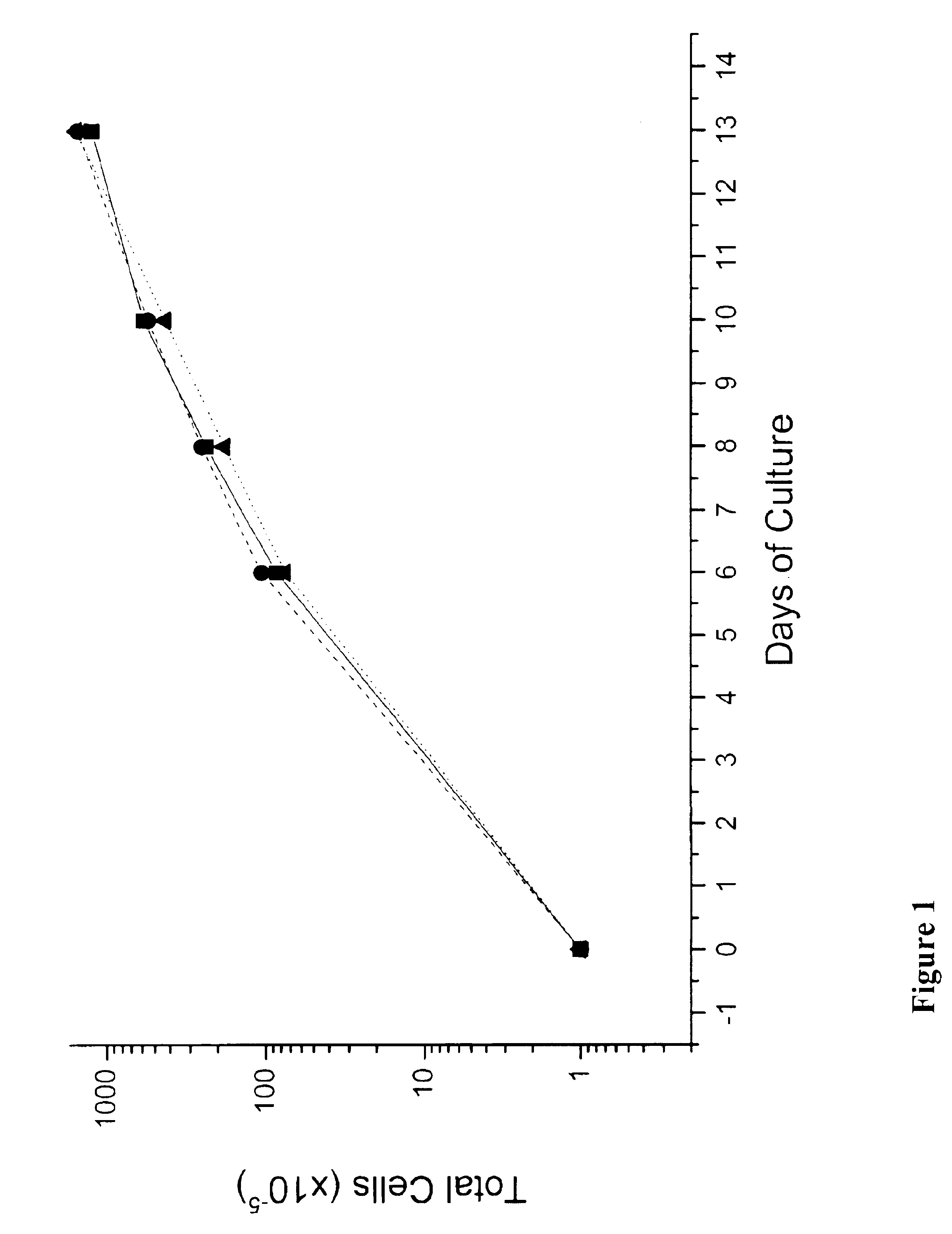
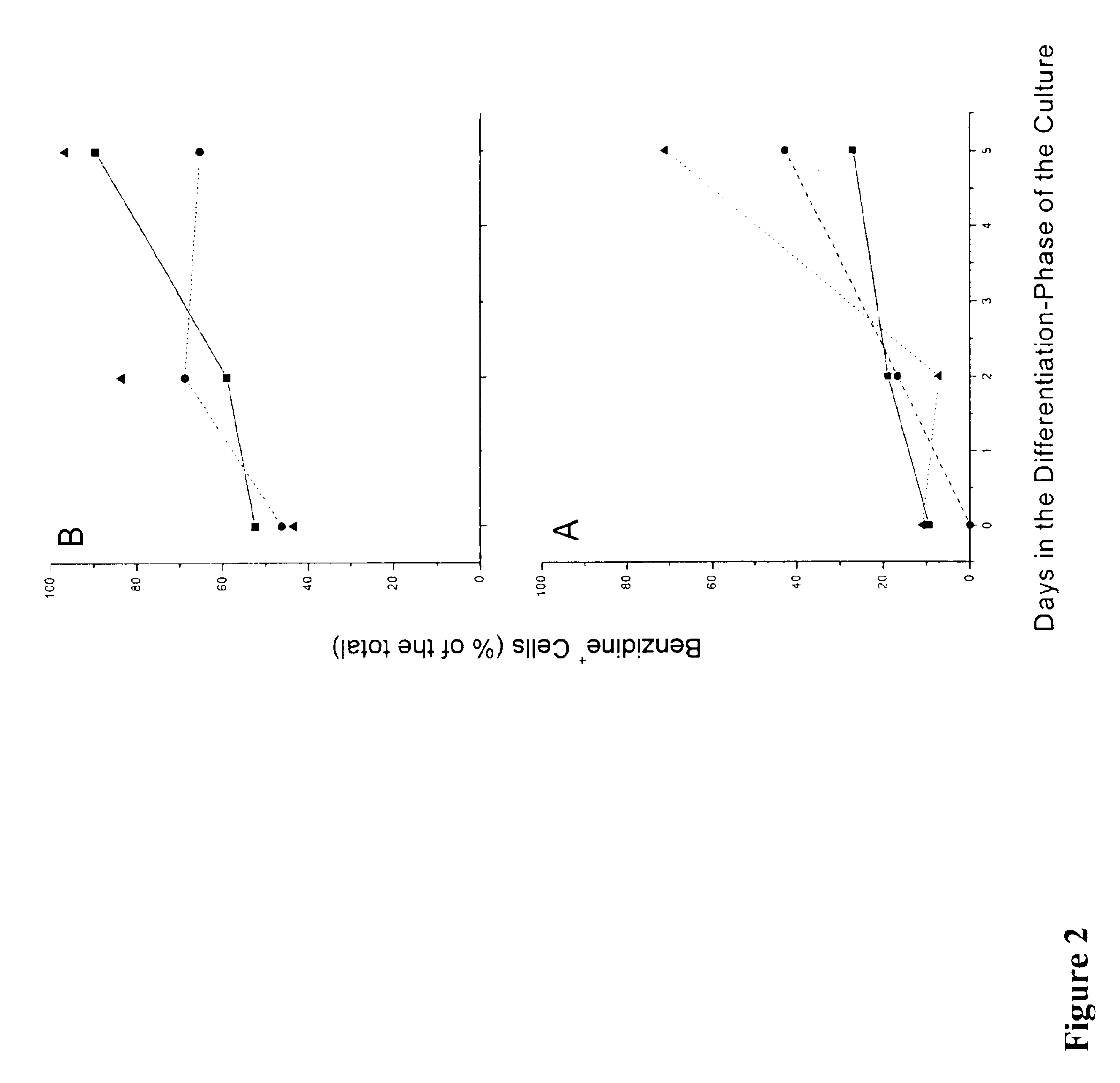
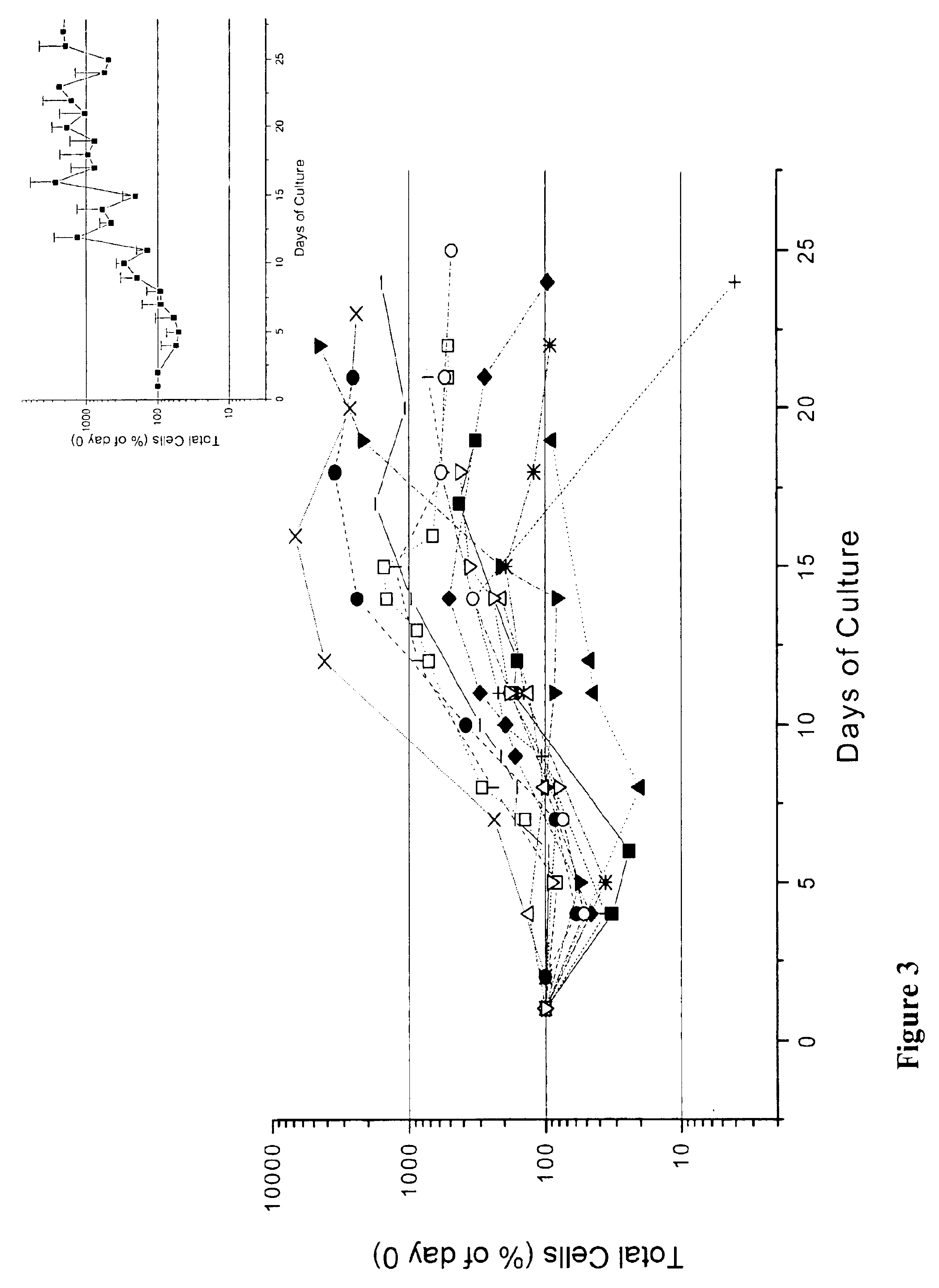
We describe a new two-step culture method for mass production in vitro of erythroid cells from either CD34<+> (10<5 >cells / mL) or light-density (10<6 >cells / mL) cells purified from the blood of normal donors and thalassemic patients. The method includes (i) culture of the cells in the presence of dexamethasone and estradiol (10<-6 >M each) and (ii) the growth factors SCF (50 ng / mL), IL-3 (1 ng / mL), and EPO (1 U / mL). In their proliferative phase, these cultures generated about 1-2x10<7 >erythroblasts for each milliliter of blood collected from normal donors or thalassemic patients. They were composed mostly (90%) of CD45<low> / glycophorin (GPA)<neg> / CD71<low >cells at day 7, 50-60% of which became CD45<neg> / GPA+ / CD71<high >by days 15-20. However, when cells from days 7 to 12 of the proliferative phase were transferred in differentiation medium containing EPO and insulin, they progressed to mature erythroblasts (>90% benzidine<pos >and CD45<neg> / GPA<+> / CD71<medium>) in 4 days. Because of the high number of erythroid cells that are generated from modest volumes of blood, this method will prove useful in donor-specific studies of erythroid differentiation.
Owner:INST SUPERIORE DI SANITA
In vitro mass production of human erythroid cells from the blood of normal donors and thalassemic patients
We describe a new two-step culture method for mass production in vitro of erythroid cells from either CD34+ (105 cells / mL) or light-density (106 cells / mL) cells purified from the blood of normal donors and thalassemic patients. The method includes (i) culture of the cells in the presence of dexamethasone and estradiol (10−6 M each) and (ii) the growth factors SCF (50 ng / mL), IL-3 (1 ng / mL), and EPO (1 U / mL). In their proliferative phase, these cultures generated about 1-2×107 erythroblasts for each milliliter of blood collected from normal donors or thalassemic patients. They were composed mostly (90%) of CD45low / glycophorin (GPA)neg / CD71low cells at day 7, 50-60% of which became CD45neg / GPA+ / CD71high by days 15-20. However, when cells from days 7 to 12 of the proliferative phase were transferred in differentiation medium containing EPO and insulin, they progressed to mature erythroblasts (>90% benzidinepos and CD45neg / GPA+ / CD71medium) in 4 days. Because of the high number of erythroid cells that are generated from modest volumes of blood, this method will prove useful in donor-specific studies of erythroid differentiation.
Owner:INST SUPERIORE DI SANITA
Method for diagnosing liver diseases
ActiveUS7575938B2Easy to detectEasily and rapidlyMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseGlycophorin
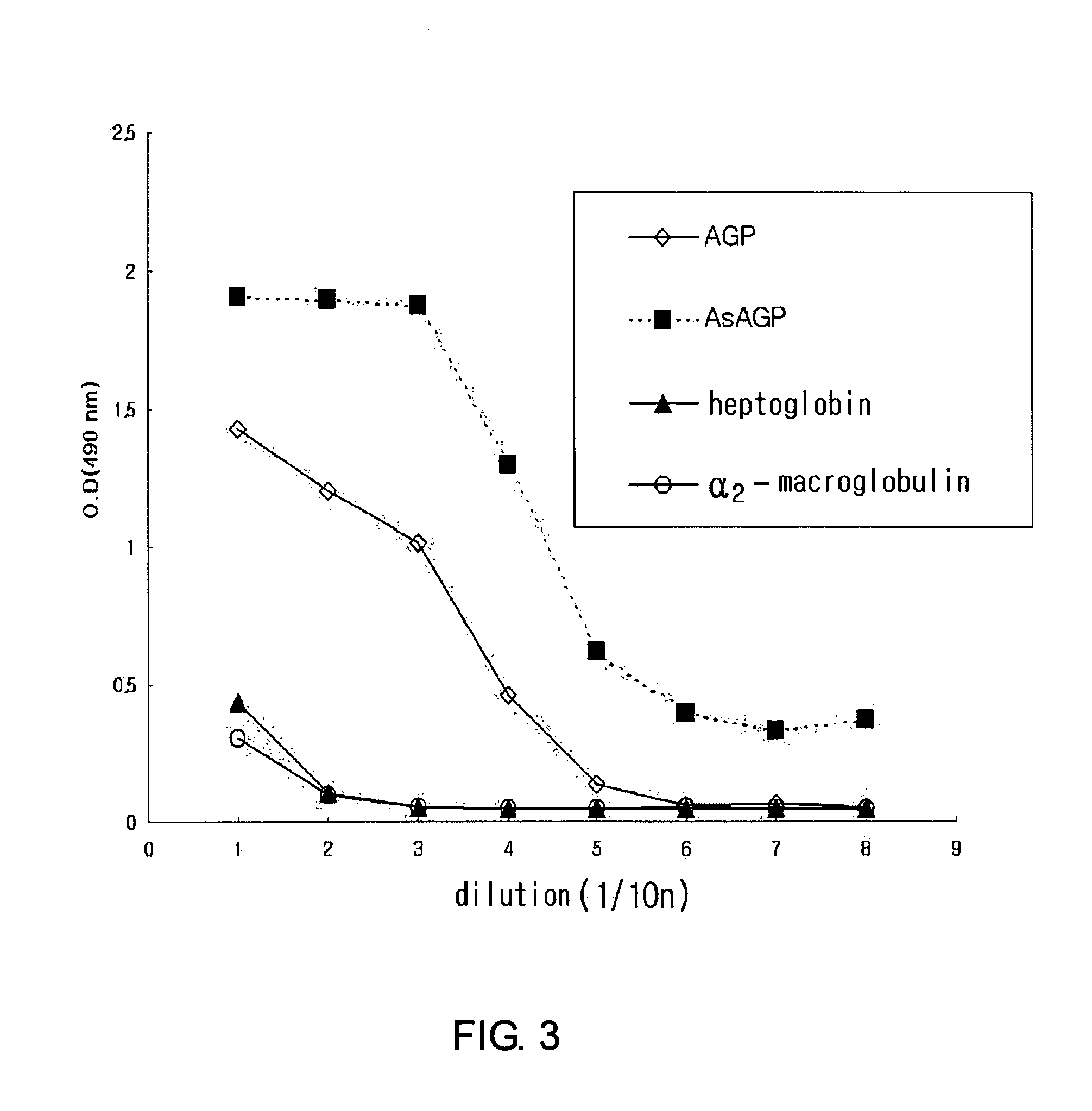
The present invention relates to a method for diagnosing a liver disease rapidly in an early stage. More particularly, the present invention relates to a monoclonal antibody against asialo α1-acid glycoprotein; a method for diagnosing a liver disease which evaluates asialo α1-acid glycoprotein in a test sample by using said monoclonal antibody; and an diagnostic strip for immunochromatography composed of said monoclonal antibody against asialo α1-acid glycoprotein and Ricinus communis agglutinin (RCA). The diagnostic device of the present invention is convenient to measure the concentration of asialo α1-acid glycoprotein and to diagnose a liver disease rapidly.
Owner:NEOBIODIGM +1
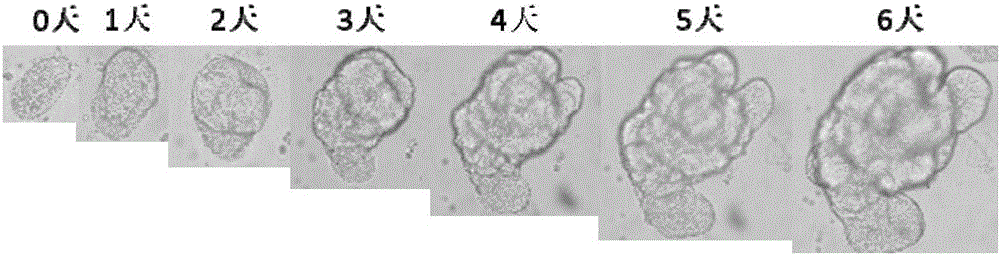
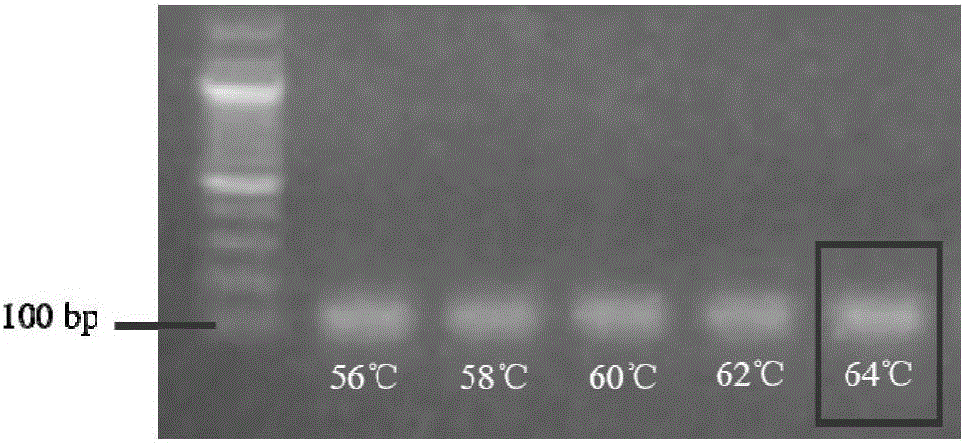
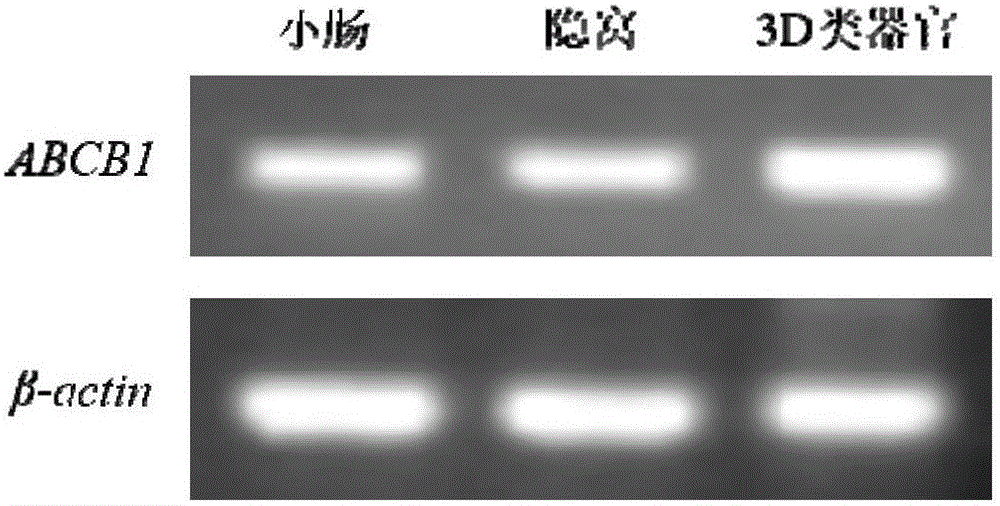
Method for building P-glycoprotein research models based on human small intestine 3D (three-dimensional) organoid and application of P-glycoprotein research models based on human small intestine 3D organoid
ActiveCN105950539AEfficient methodFast wayGastrointestinal cellsMicrobiological testing/measurementHigh-Throughput Screening MethodsMatrigel
The invention discloses a method for building P-glycoprotein (P-gp) research models based on human small intestine 3D (three-dimensional) organoid. The method includes inoculating human small intestine crypts in matrigel at first, adding ADMEM / F12 culture media with specific growth factors into the matrigel and cultivating the human small intestine crypts to form 3D organoids; carrying out morphological observation and detecting expression of P-gp from mRNA [messenger RNA (ribonucleic acid)] and protein level; researching influence of Verapamil and Mitotane on Rh123 transportation by the aid of a co-incubation process by Rhodamine 123 (Rh123) which is used as a substrate. The method has the advantages that the P-glycoprotein research models based on human small intestine 3D organoid can be applied to P-gp-mediated medicine transport research and also can be widely applied to in-vitro high-throughput screening on P-gp inhibitors, and the method is high in efficiency and speed.
Owner:EAST CHINA NORMAL UNIVERSITY
Taccalonolide microtubule stabilizing agents
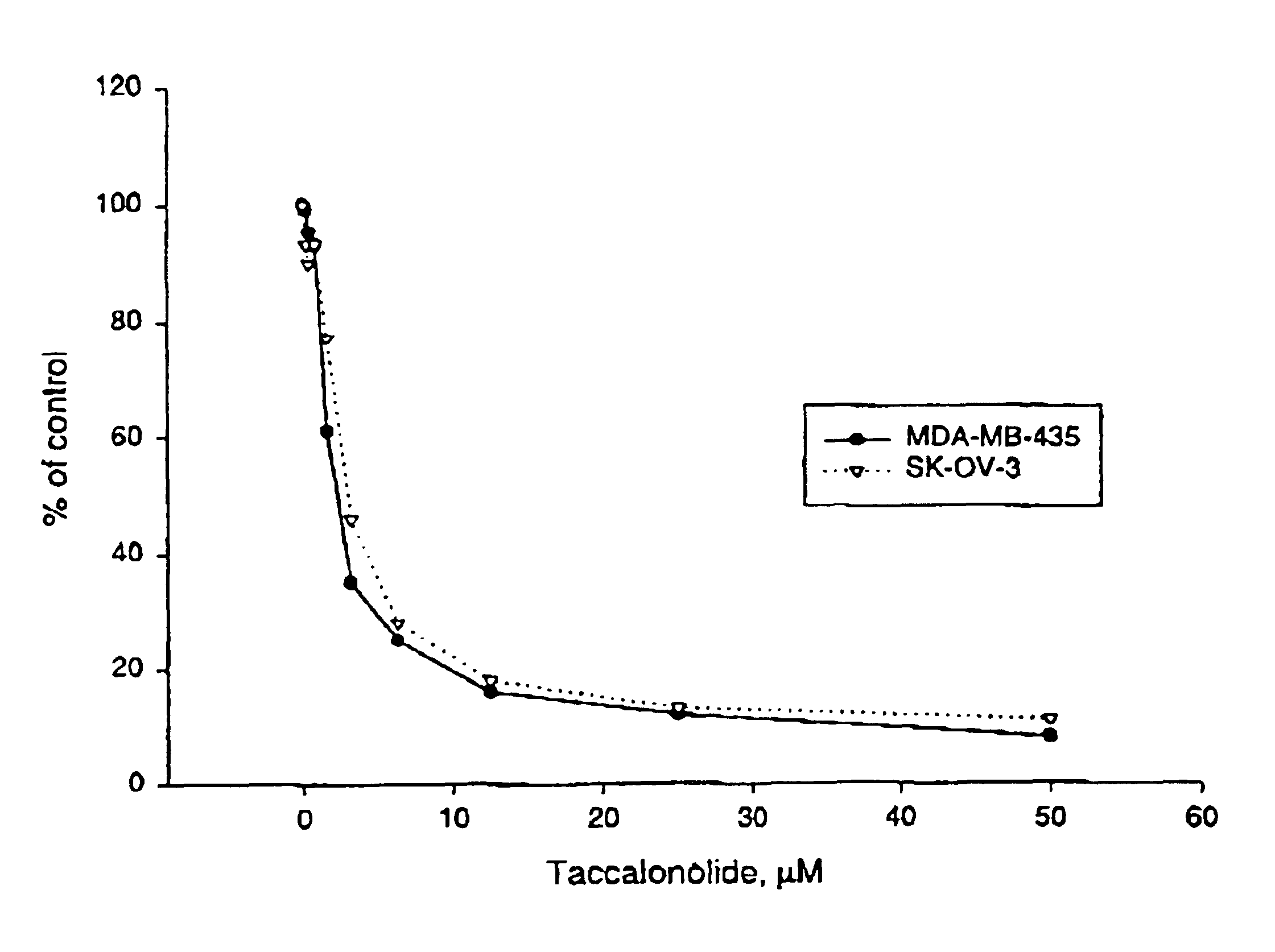
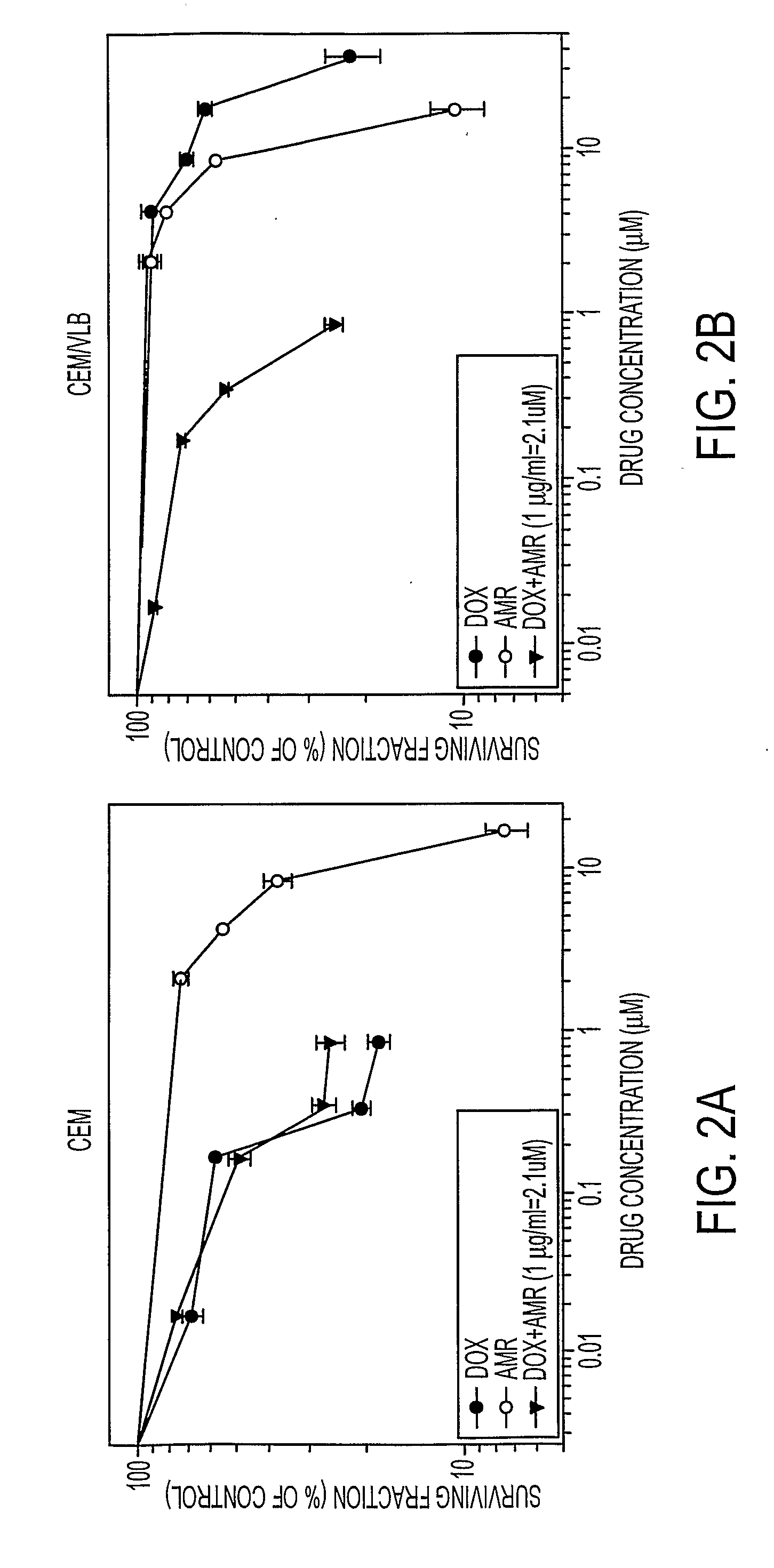
InactiveUS6878699B1Promote efficient proliferationTransport becomes poorOrganic active ingredientsSteroidsGlycophorinMDA-MB-435
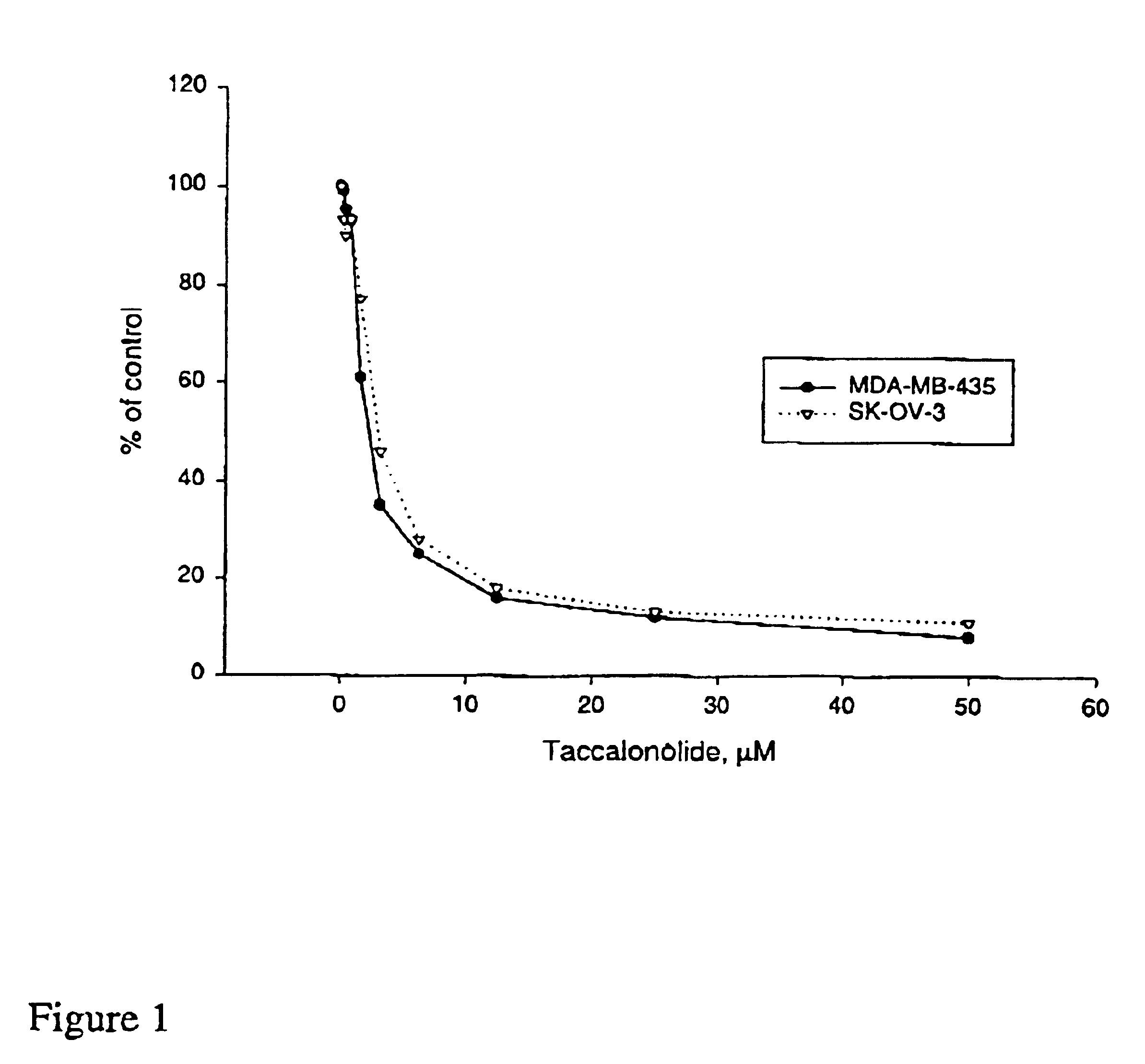
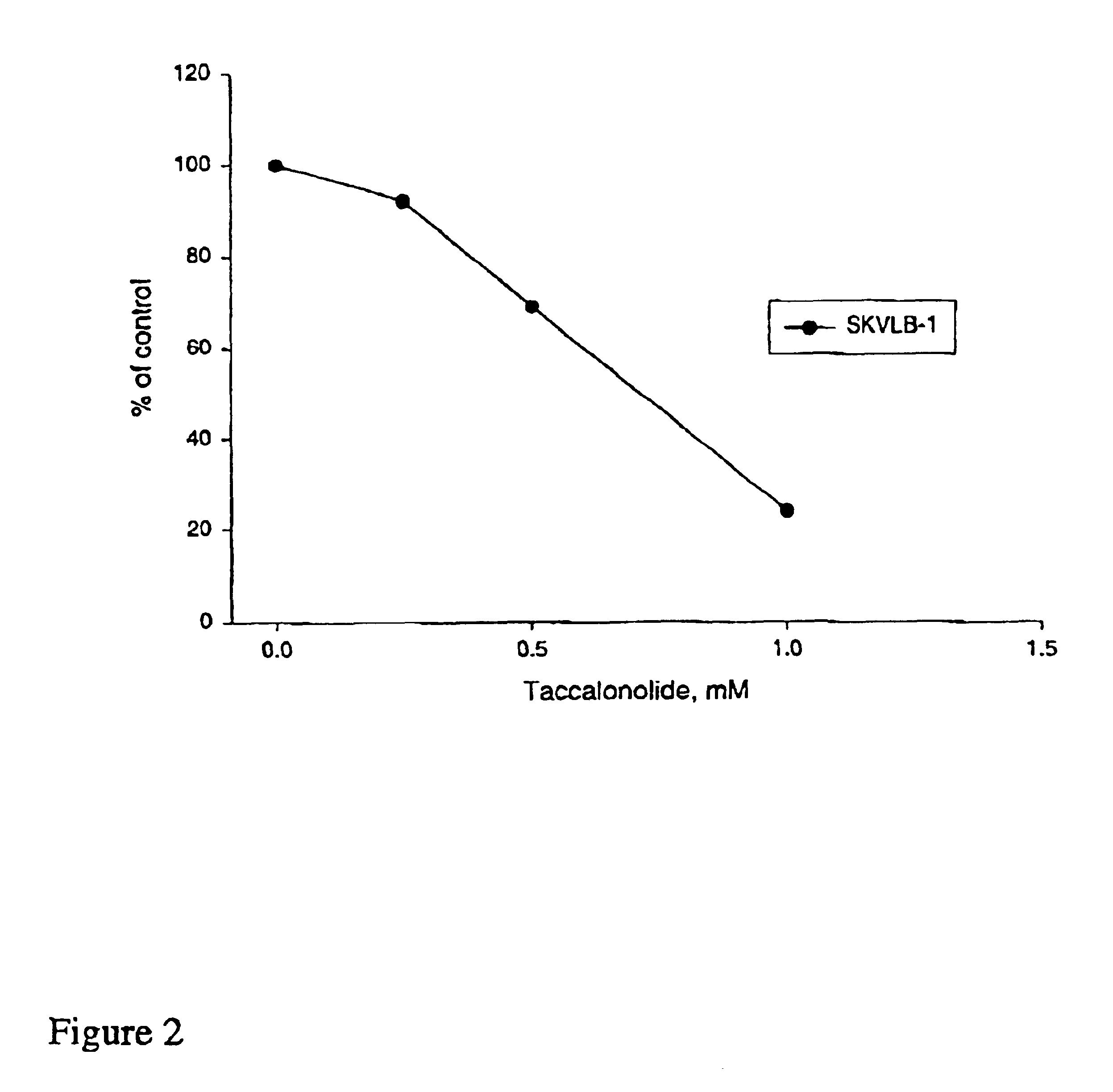
In accordance with the present invention, there have been identified extracts from a tropical plant that initiate paclitaxel-like microtubule bundling. Bioassay-directed purification yields the steroid taccalonolide A. Taccalonolide, like paclitaxel, initiates the formation of abnormal interphaes and mitotic microtubules. Cells treated with taccalonolide exhibit thick bundles of microtubules that appear to nucleate independent of the microtubule organizing center. Abnormal mitotic spindles consisting of multi-polar spindles are initiated by taccalonolide and resemble abnormal mitotic spindles found in the presence of paclitaxel. Like paclitaxel, taccalonolide causes the breakdown of the nucleus into micronuclei. Taccalonolide causes G2 / M arrest, Bc1-2 phosphorylation and initiates an apoptotic cascade that includes the activation of caspase 3. Taccalonolide is an effective inhibitor of proliferation against both SK-OV-3 and MDA-MB-435 cell with IC50 values of 2.3 μM and 2.1 μM respectively. In contrast to paclitaxel, taccalonolide is effective against the multidrug resistant SKVLB-1 cellline and thus appears to be a poor substrate for P-glycoprotein-mediated transport. Although taccalonolide is almost equipotent with paclitaxel in its effects on cellular microtubules, it is much less potent than paclitaxel in its ability to initiate the polymerization of purified tubulin and microtubule protein. Taccalonolide A is the first microtubule stabilizing agent to be discovered from a plant since identification of the mechanism of action of paclitaxel and it is the first natural product steroid identified to have these cellular effects.
Owner:UNIV OF HAWAII
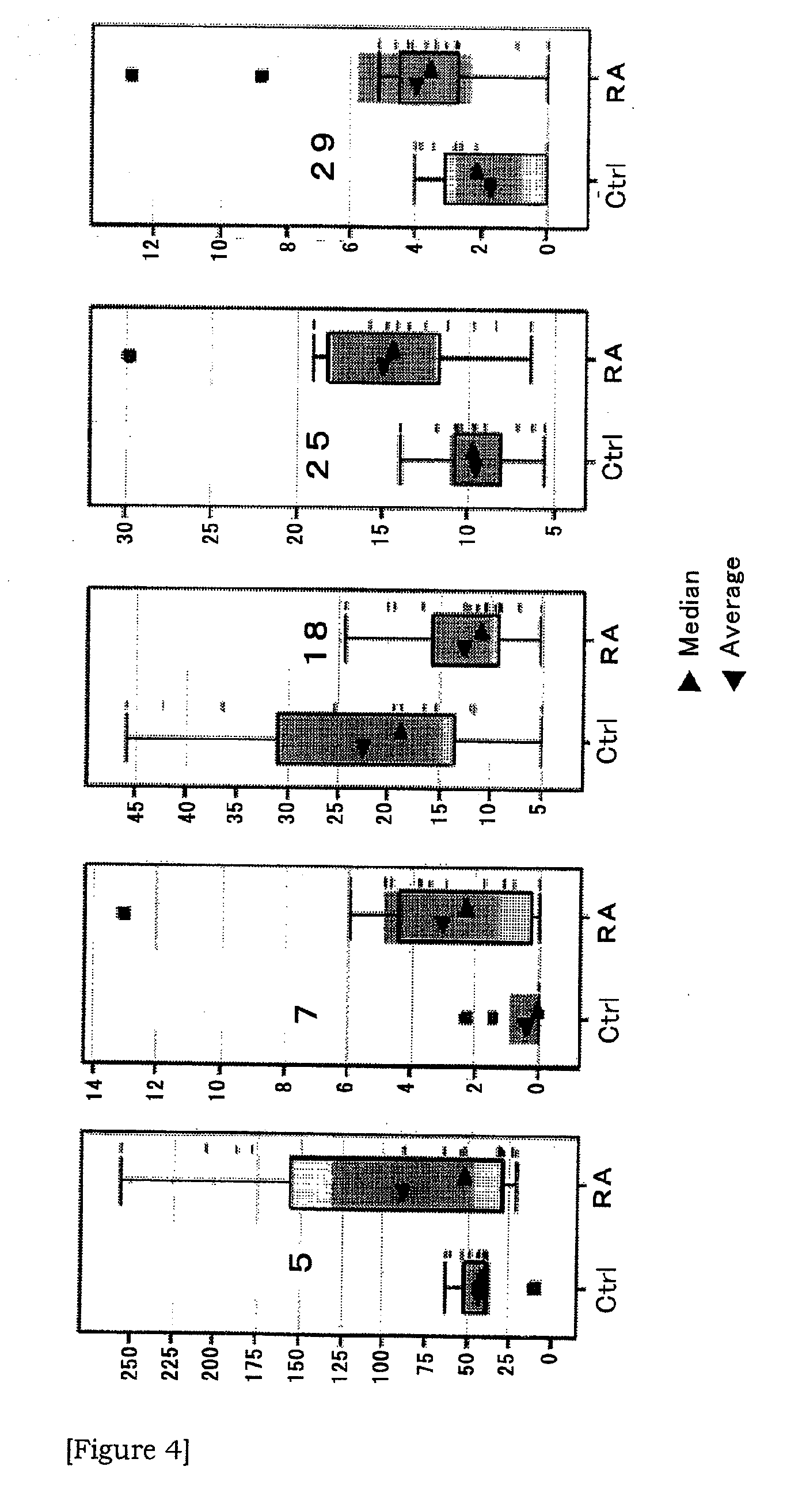
Method of diagnostic rheumatoid arthritis by sugar chain analysis
InactiveUS20100297682A1High sensitivityStrong specificitySugar derivativesMicrobiological testing/measurementGlycophorinQuantitative expression
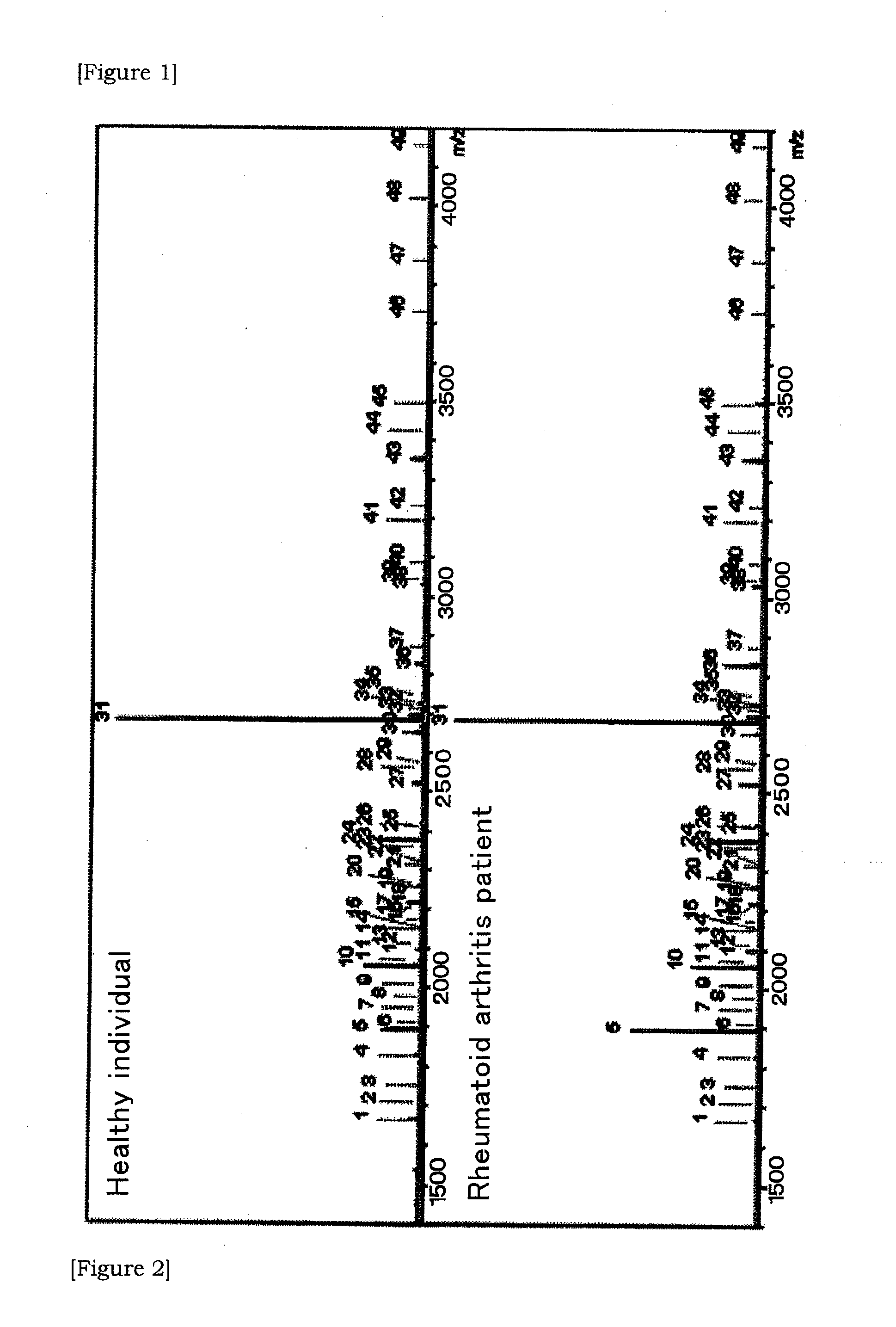
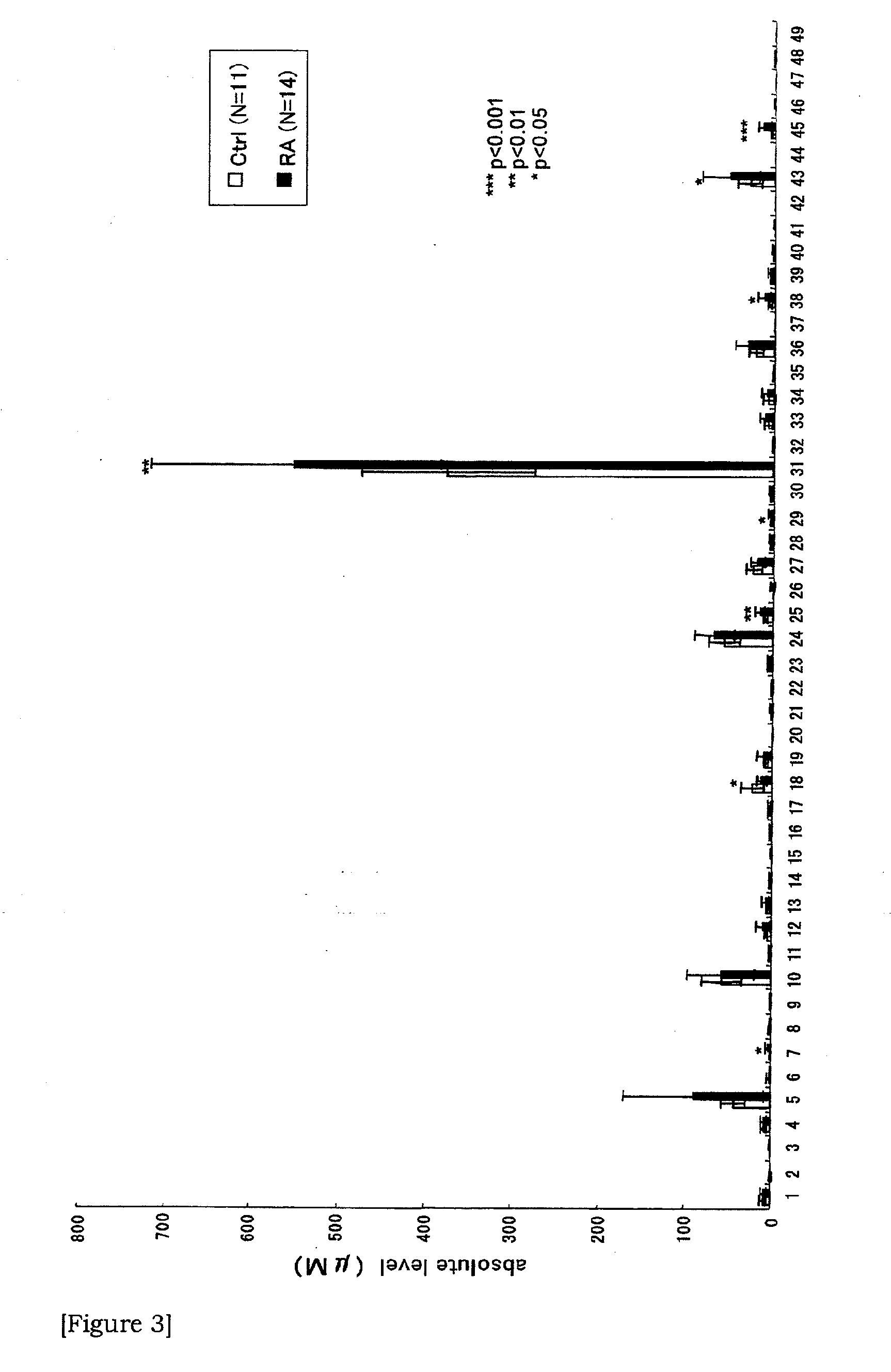
Provided is a novel method of diagnosing rheumatoid arthritis. Based on the quantitative expression profile of sugar chain expression amount in the serum (whole serum, HAP or LAP), the relevancy thereof to rheumatoid arthritis is analyzed. As a result, a sugar chain and a glycoprotein showing a change depending on the onset of rheumatoid arthritis are found out and thus a serum sugar chain and a glycoprotein usable as a novel biomarker are provided.
Owner:HOKKAIDO UNIVERSITY +1
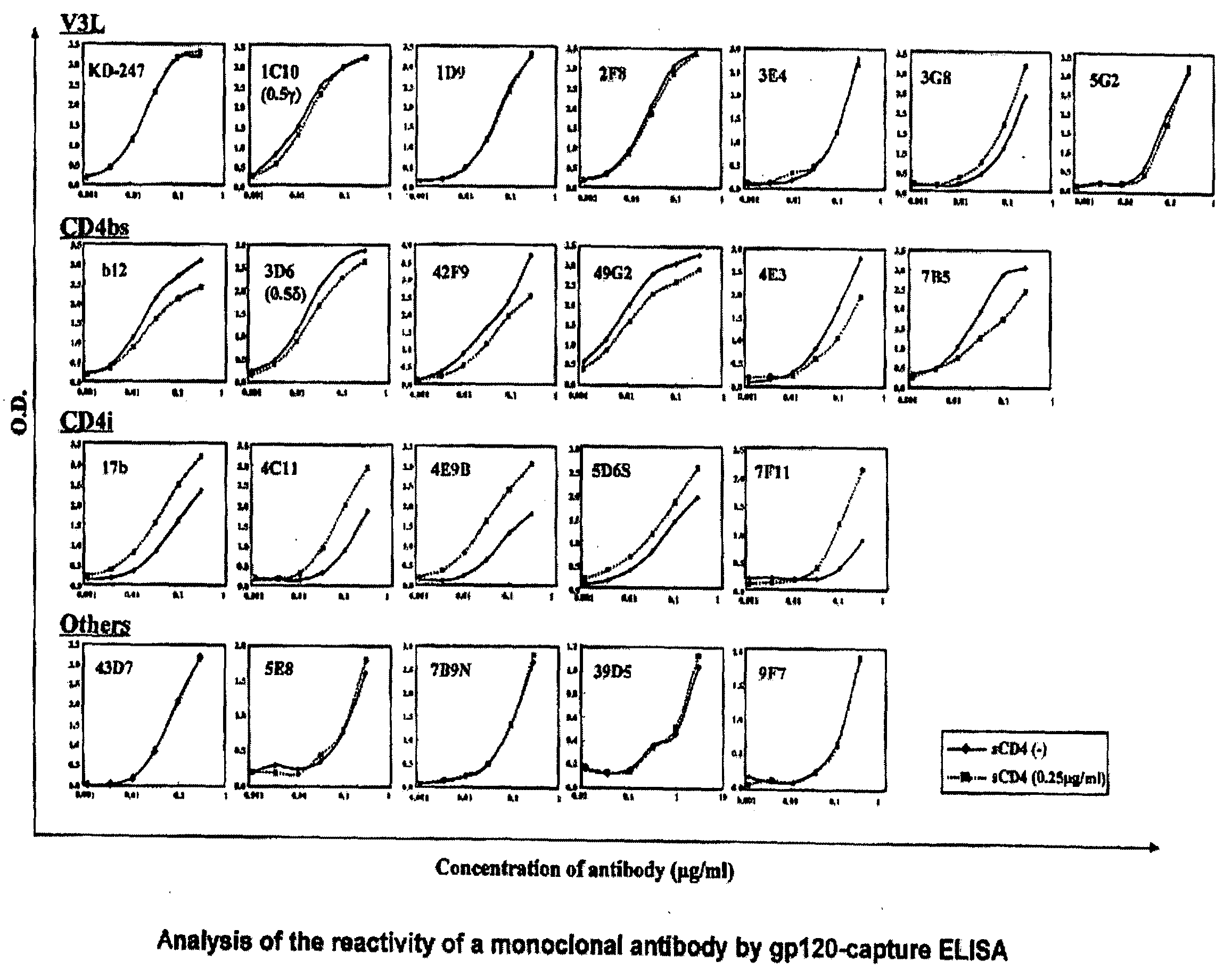
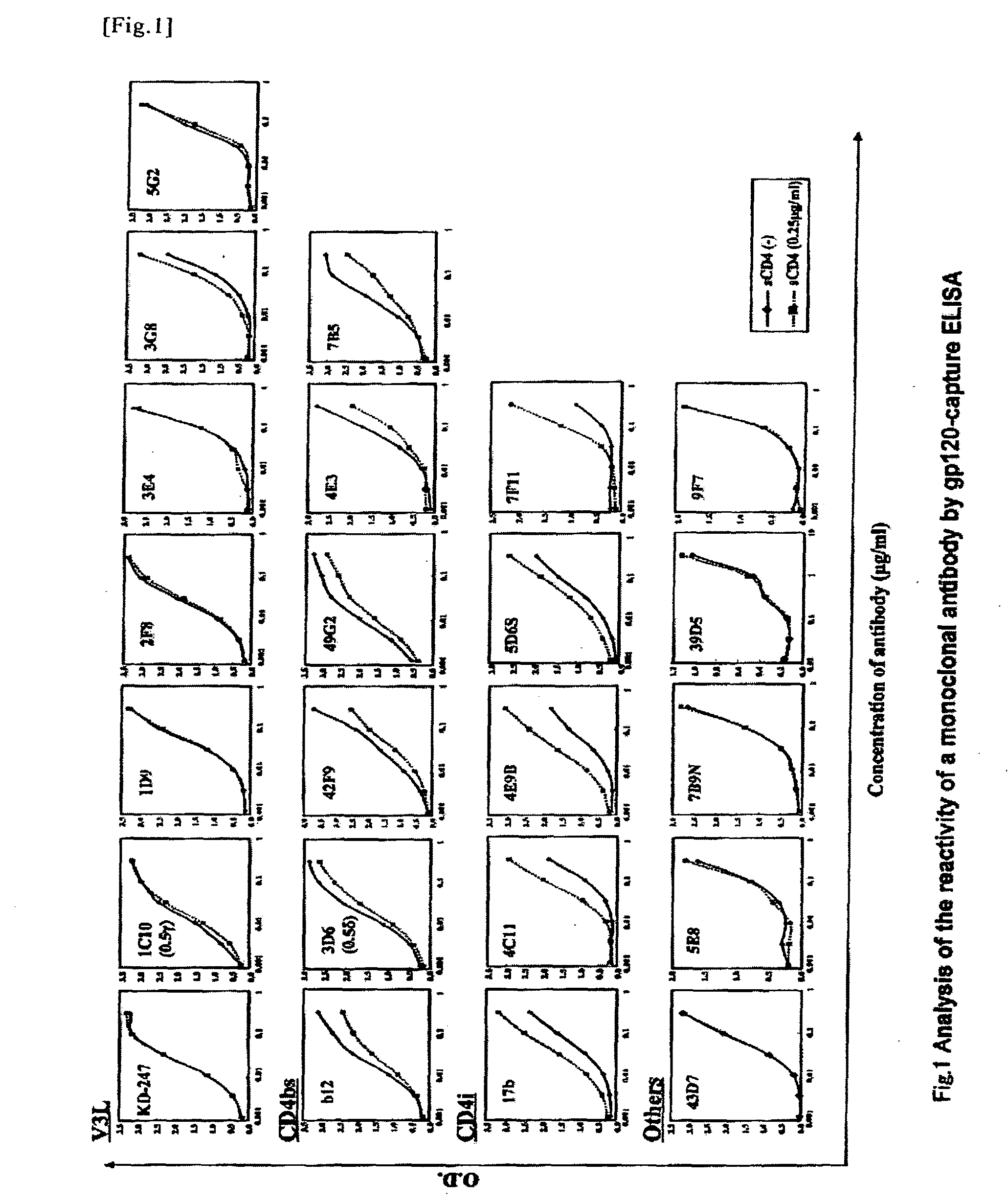
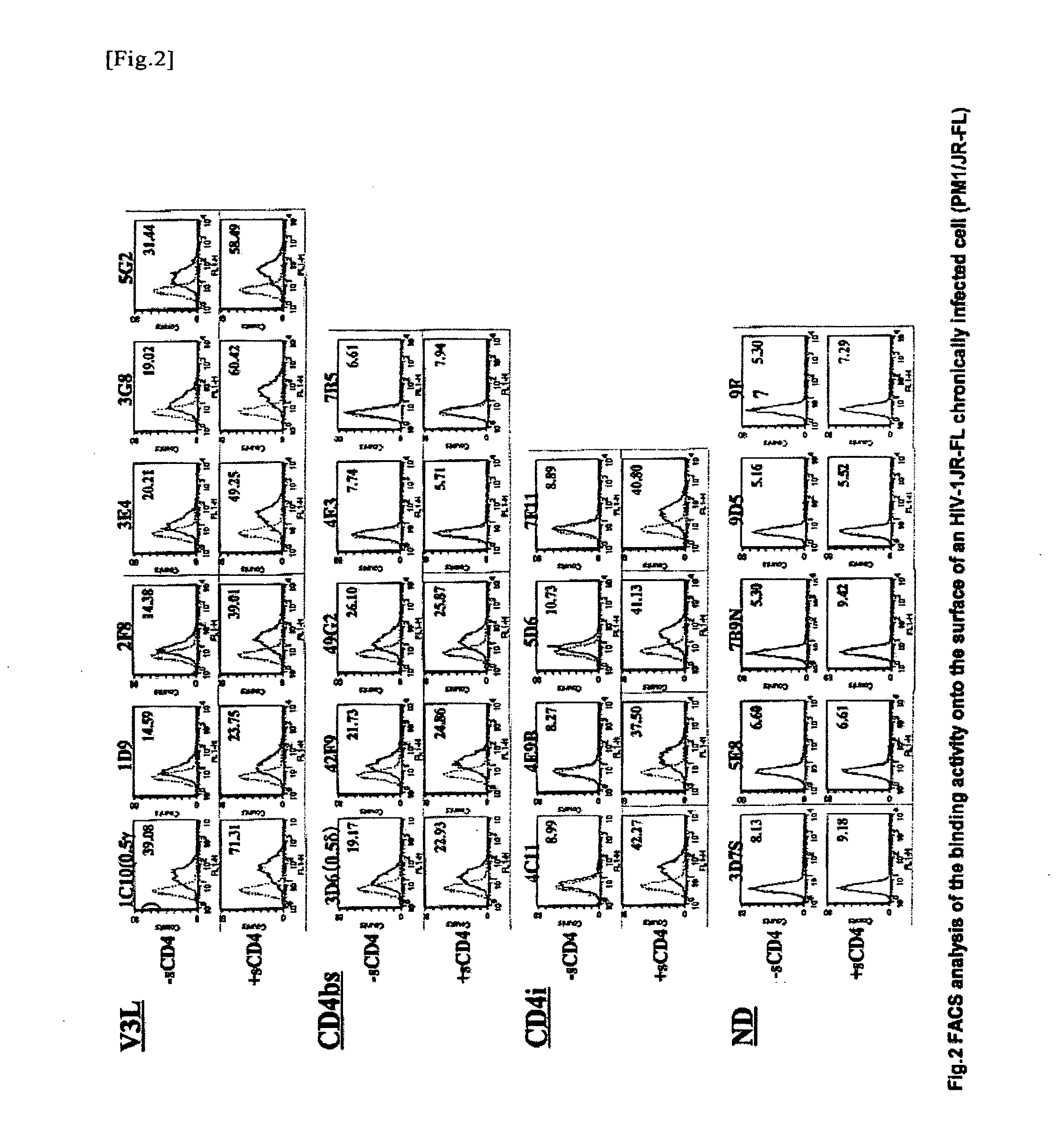
Anti-hiv monoclonal antibody
ActiveUS20110044995A1Microbiological testing/measurementImmunoglobulins against virusesGlycophorinAgricultural science
The present invention provides a monoclonal antibody that recognizes the V3 loop of the envelope glycoprotein gp120 of AIDS virus, which is any one selected from the following antibodies:(a) an antibody having the amino acid sequence shown in SEQ ID NO: 1 as the amino acid sequence of a H chain variable region (VH), and having the amino acid sequence shown in SEQ ID NO: 2 as the amino acid sequence of a L chain variable region (VL); and(b) an antibody having the amino acid sequence shown in SEQ ID NO: 3 as the amino acid sequence of a H chain variable region (VH), and having the amino acid sequence shown in SEQ ID NO: 4 as the amino acid sequence of a L chain variable region (VL).
Owner:NAT UNIV CORP KUMAMOTO UNIV
HUMAN iNKT CELL ACTIVATION USING GLYCOLIPIDS WITH ALTERED GLYCOSYL GROUPS
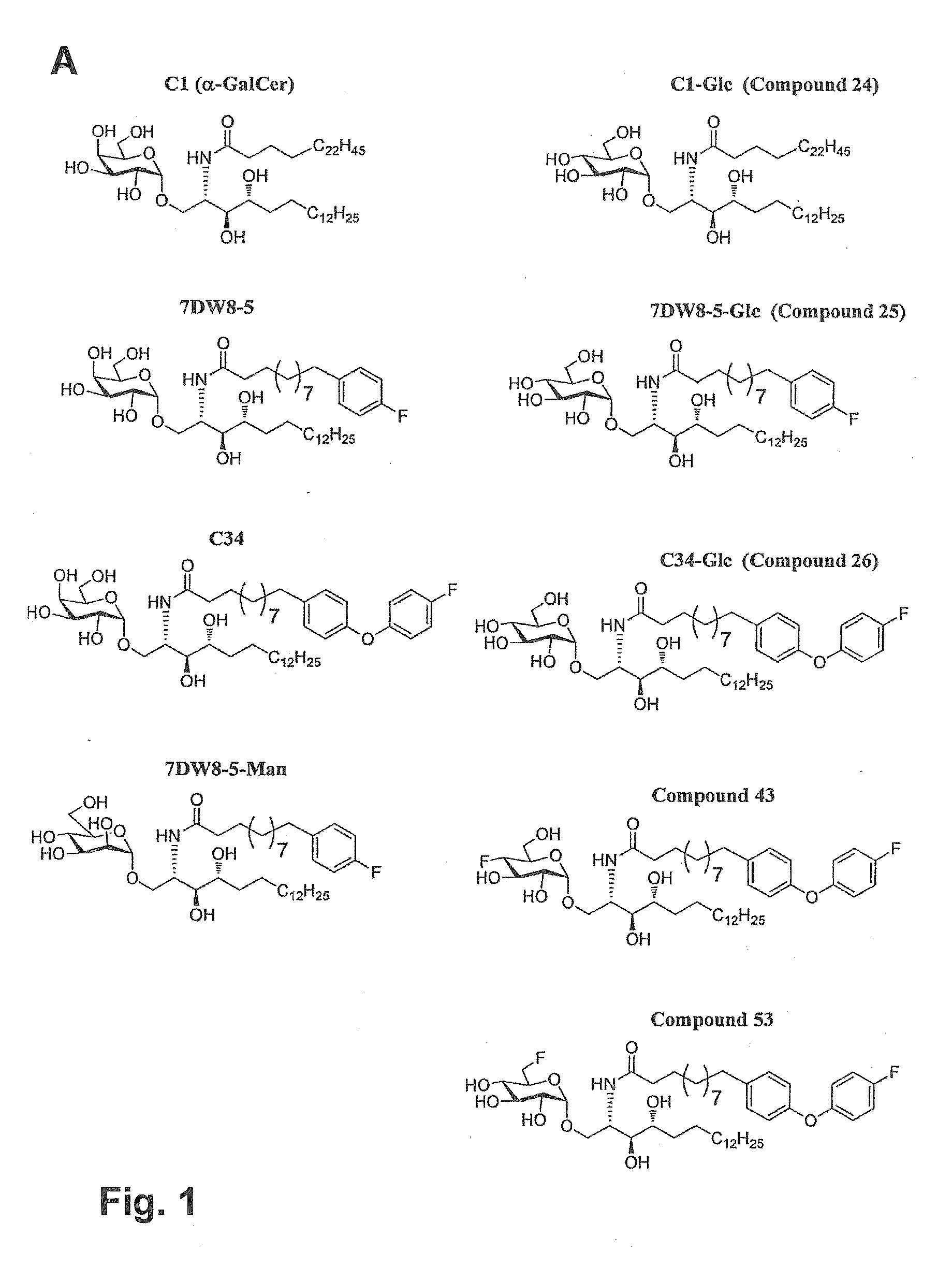
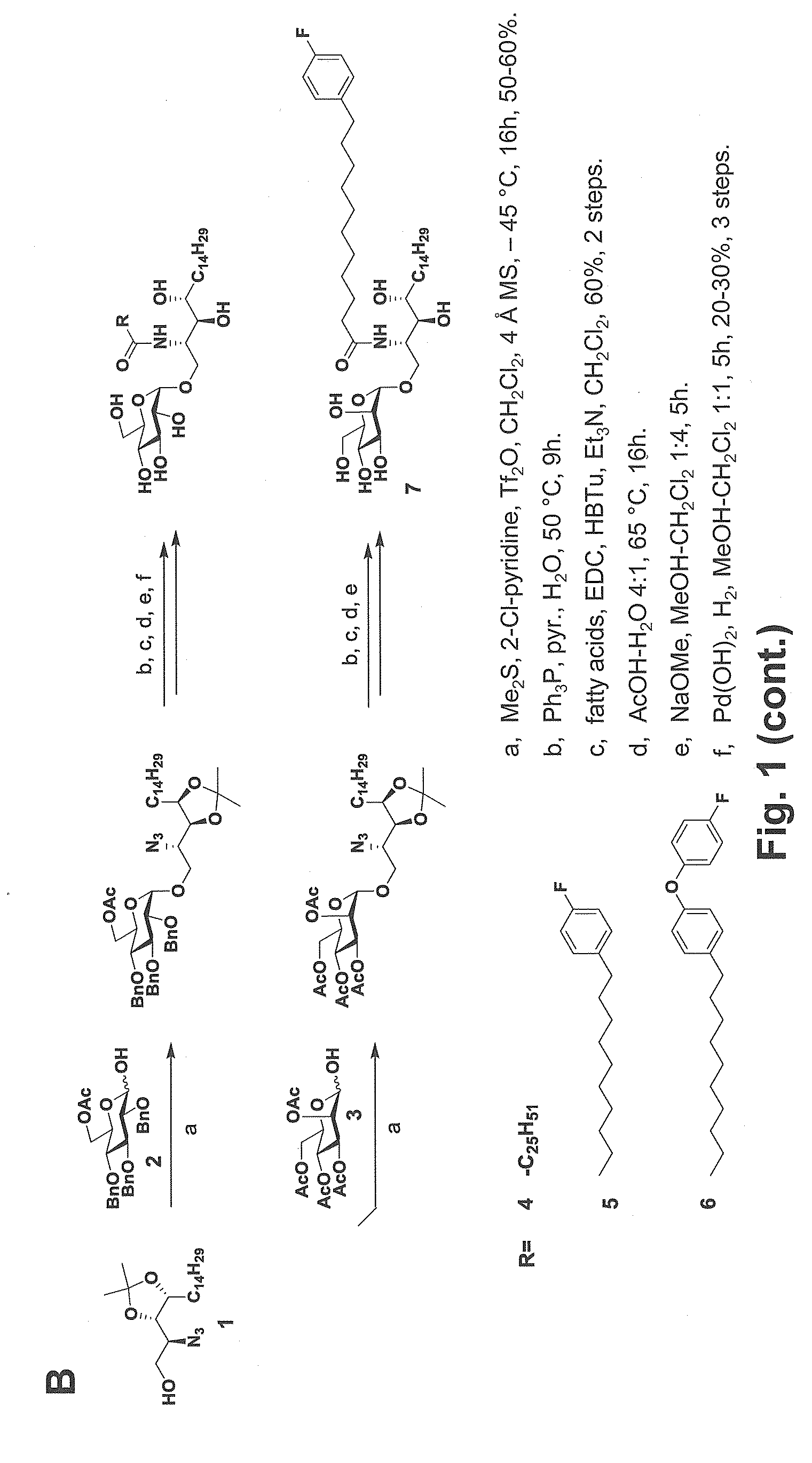
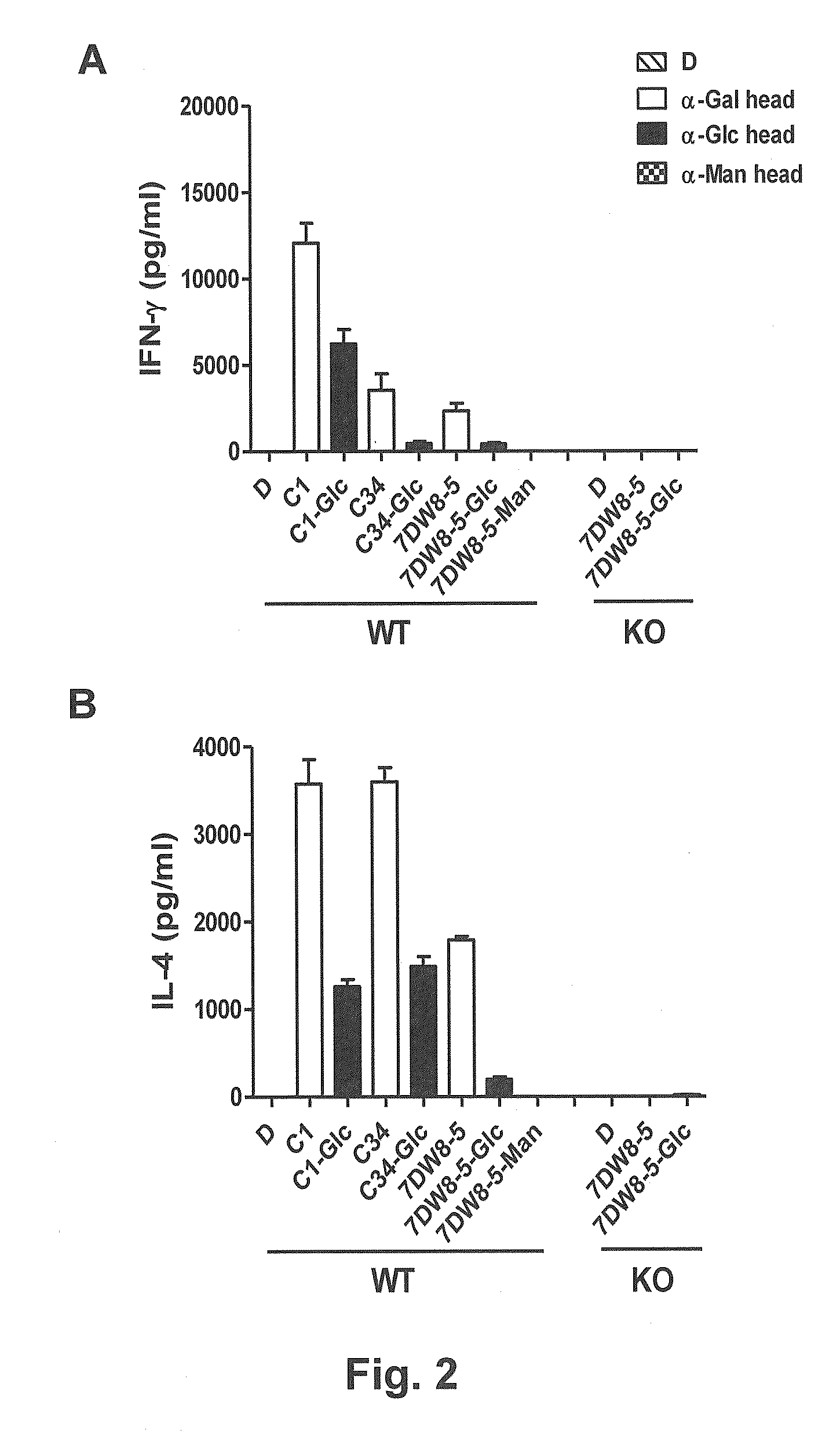
ActiveUS20150071960A1Superior tumour protectionGood choiceAntibacterial agentsBiocideHuman useGlycophorin
Glycosphingolipids (GSLs) bearing α-glucose (α-Glc) that preferentially stimulate human invariant NKT (iNKT) cells are provided. GSLs with α-glucose (α-Glc) that exhibit stronger induction in humans (but weaker in mice) of cytokines and chemokines and expansion and / or activation of immune cells than those with α-galactose (α-Gal) are disclosed. GSLs bearing α-glucose (α-Glc) and derivatives of α-Glc with F at the 4 and / or 6 positions are provided. Methods for iNKT-independent induction of chemokines by the GSL with α-Glc and derivatives thereof are disclosed. Methods for immune stimulation in humans using GSLs with α-Glc and derivatives thereof are provided.
Owner:ACAD SINIC
Medicine for treating pulmonary tuberculosis
PendingCN105582522AGood treatment effectIncrease the number ofAntibacterial agentsOrganic active ingredientsGlycophorinSide effect
The invention provides a medicine for treating pulmonary tuberculosis. The medicine is mixture of glucoprotein or polysaccharide and protein or is polypeptide or protein. The medicine provided by the invention has a favorable curative effect on pulmonary tuberculosis, is capable of obviously improving the sputum negative conversion rate, the focal absorption rate and the cavity closing rate, reducing the number of colonies of the lung and spleen, increasing the quantity of T lymphocytes CD4<+>T, increasing the thymus index and increasing the IFN-gamma content after being used for treating pulmonary tuberculosis for three months, is safe, efficient and free of side effect, and has obvious effects on preventing and treating pulmonary tuberculosis.
Owner:SHANDONG ZHONGHAI PHARMA CO LTD
Stem cell populations and methods of use
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
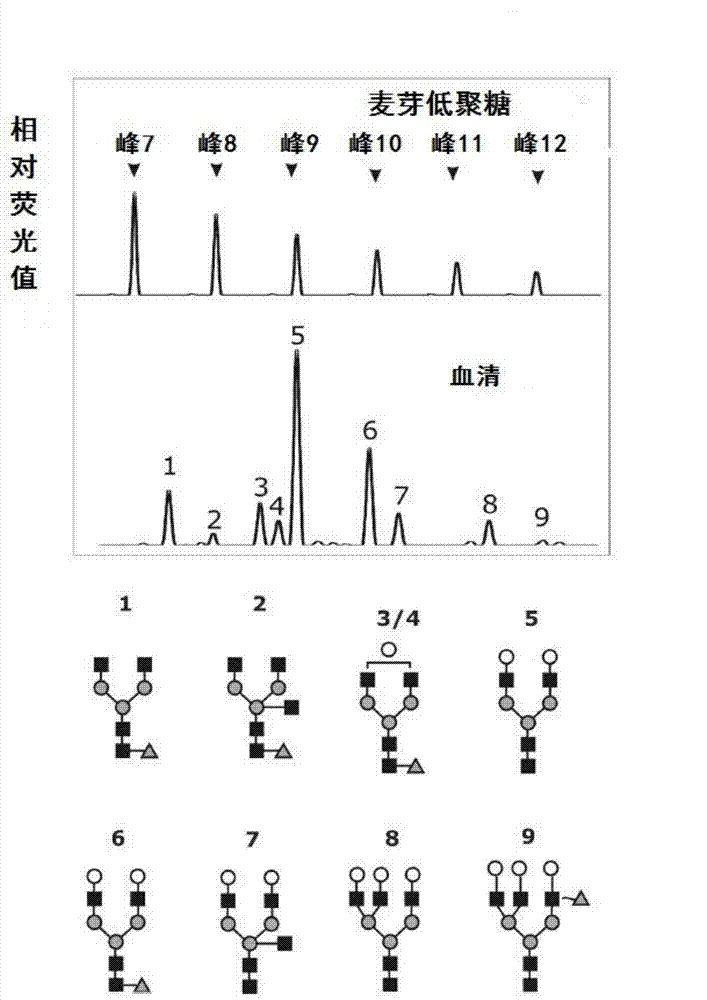
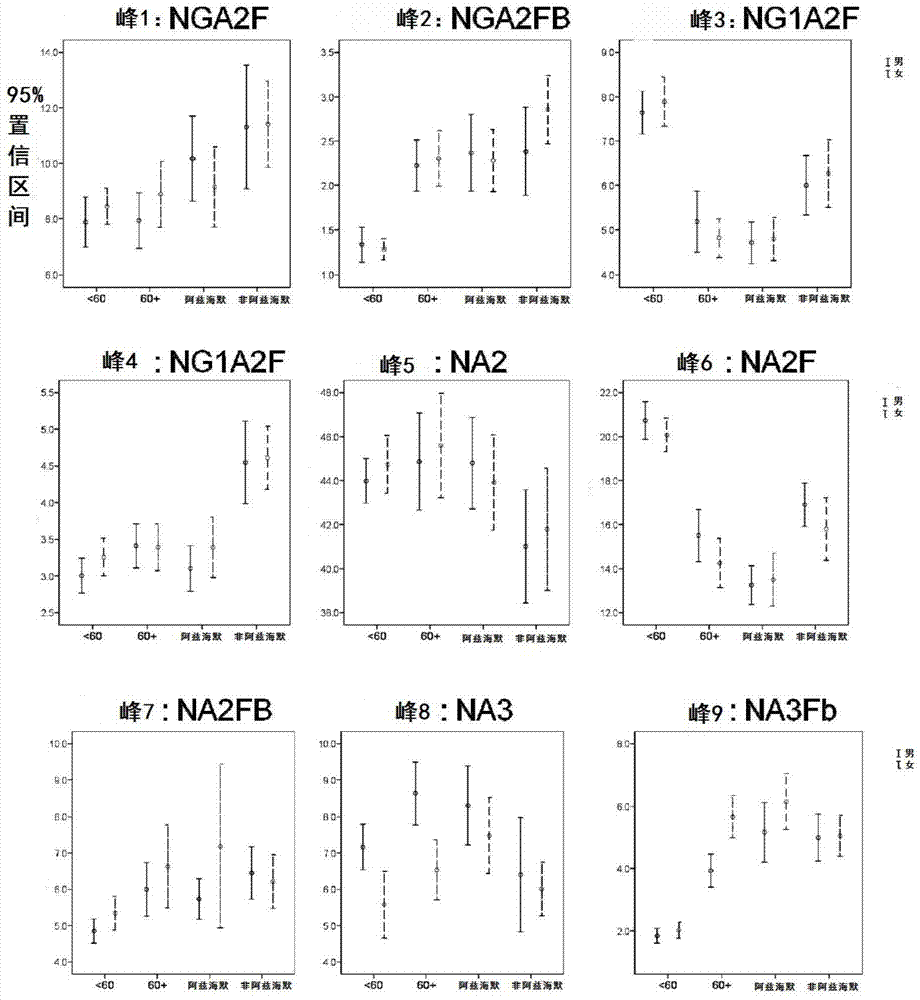
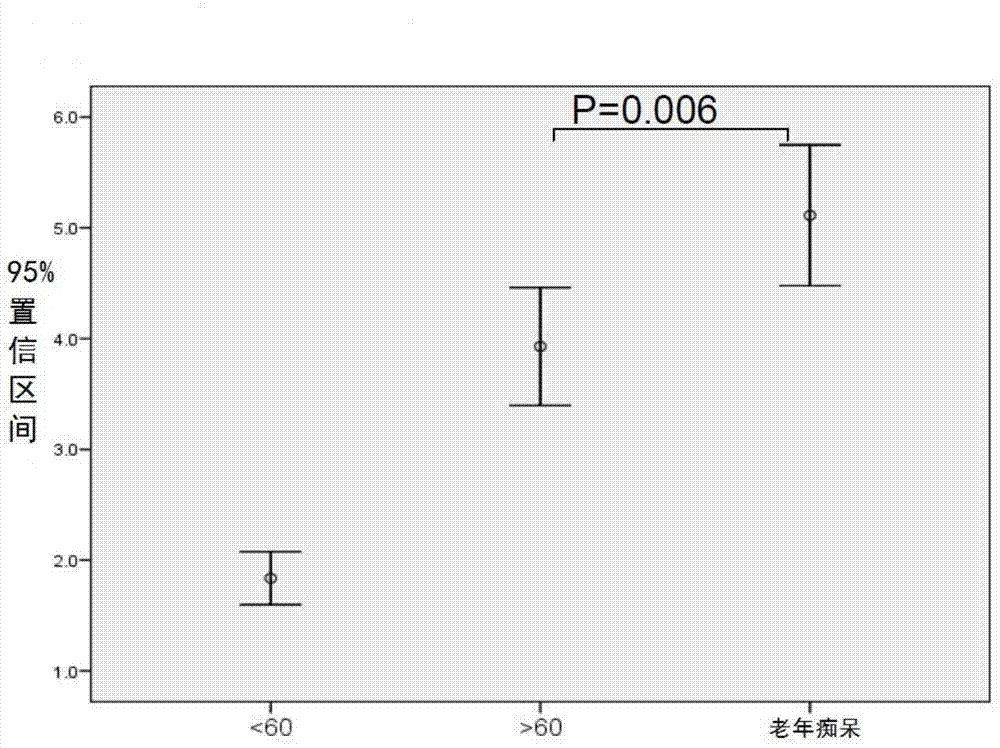
Biomark detection method of Alzheimer's disease
The invention relates to a biomark detection method of Alzheimer's disease, which comprises the following steps: (1) collecting and processing a serum sample; (2) performing fluorescence marking on an oligosaccharide in the serum sample with a fluorophore-assisted carbohydrate electrophoresis (DSA-FACE) technology through a DNA-based sequencer; (3) measuring content of the oligosaccharide subjected to fluorescence marking in the sample by ionophortic separation and obtaining an oligosaccharide fingerprint spectrum; (4) analyzing the oligosaccharide fingerprint spectrum. The difficult detection of the Alzheimer's disease is solved, the Alzheimer's disease can be quantitatively detected and a pathogenetic degree can be quantitatively evaluated.
Owner:XIANSIDA NANJING BIOTECH CO LTD
Peptides for enhancing protein expression
InactiveUS20150353959A1Increase production rateHigh yieldPeptide/protein ingredientsMammal material medical ingredientsFusion Protein ExpressionGlycophorin
The present invention pertains to the field of recombinant protein production. Novel peptides derived from the extracellular region of a glycophorin protein are provided which enhance the expression rate of proteins or peptides of interest when expressed as fusion protein together with said novel peptides.
Owner:GLYCOTOPE GMBH
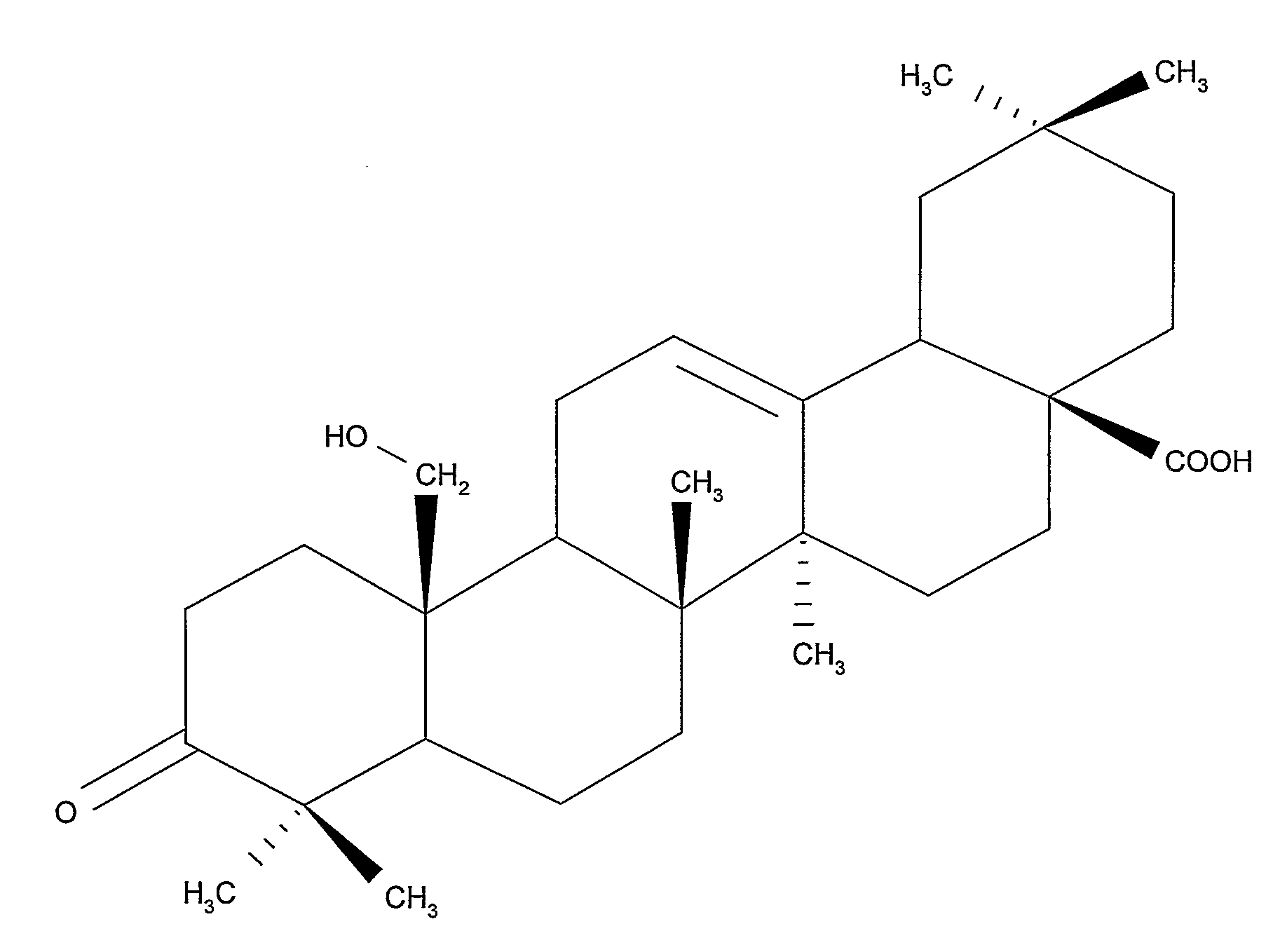
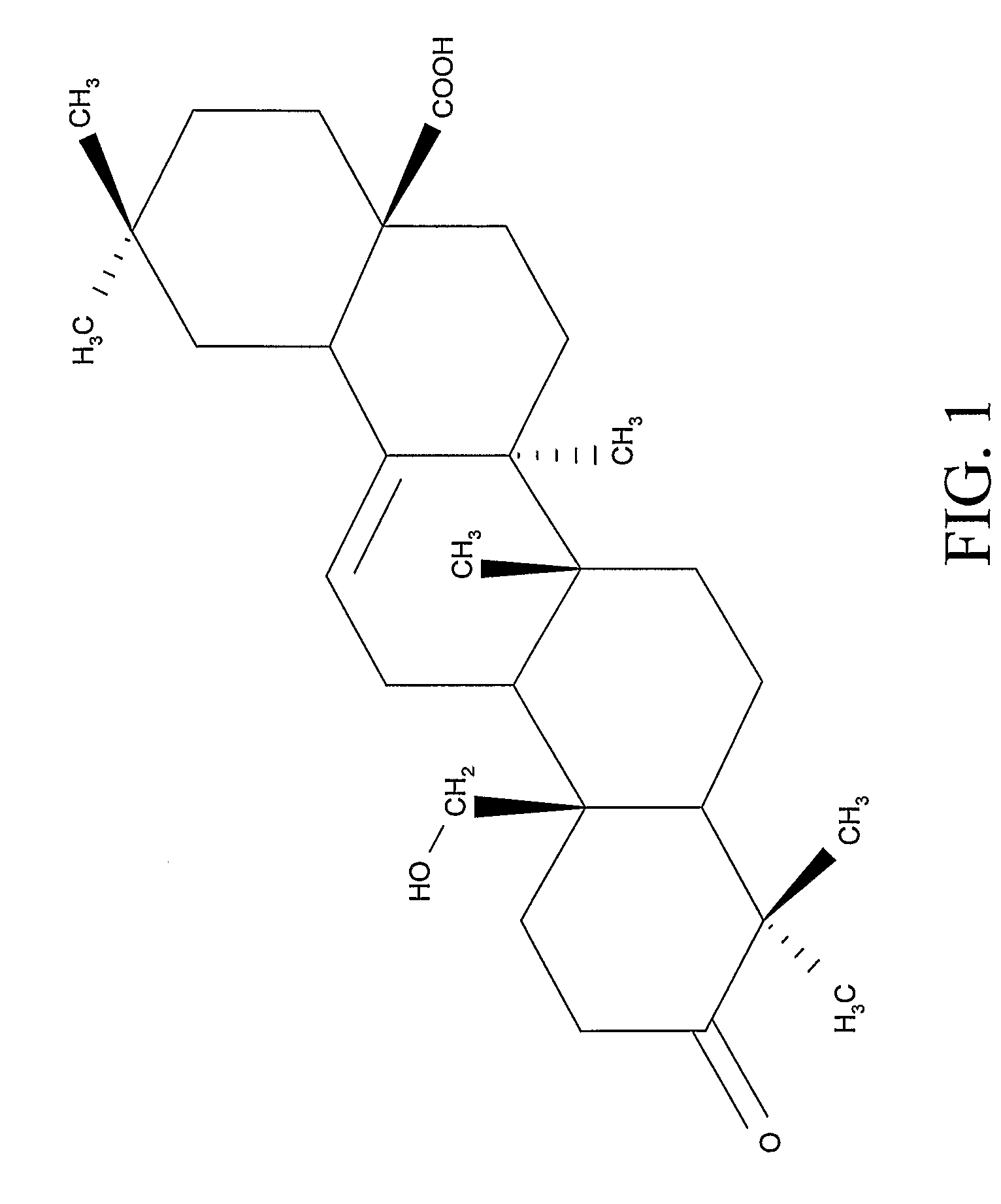
Compositions and Uses of Amooranin Compounds
InactiveUS20090203634A1Good anticancer effectReduce multidrug resistanceBiocidePeptide/protein ingredientsDiseaseAbnormal tissue growth
Amooranin (AMR) has been found to cause tumor cell death through G2 / M cell cycle arrest, caspase activation, and apoptosis. Furthermore, it has been demonstrated that AMR is a substrate for P-glycoprotein. Based on these activities, AMR compounds, including AMR analogs, can be used in the treatment of a number of diseases in which aberrant cellular proliferation occurs such as drug-sensitive and drug-resistant cancers, autoimmune disorders, and inflammatory diseases.
Owner:VARIETY CHILDRENS HOSPITAL MIAMI CHILDRENS HOSPITAL
Human liver progenitors
InactiveUS20100197015A1Low densityReduce in quantityHepatocytesGenetic material ingredientsProgenitorSingle cell suspension
Methods of isolating and cryopreserving progenitors from human liver are disclosed which include processing human liver tissue to provide a substantially single cell suspension comprising progenitors and non-progenitors of one or more cell lineages found in human liver; subjecting the suspension to a debulking step, which reduces substantially the number of non-progenitors in the suspension, and which provides a debulked suspension enriched in progenitors exhibiting one or more markers associated with at least one of the one or more cell lineages; and selecting from said debulked suspension those cells, which themselves, their progeny, or more mature forms thereof express one or more markers associated with at least one of the one or more cell lineages. Among these markers are CD14, CD34, CD38, CD45, and ICAM. Hepatic progenitors are characterized as being 6-15μ in diameter, diploid, glycophorin A−, CD45−, AFP+++, ALB+, ICAM+, and with subpopulations varying in expression of CD14+. CD34++, CD38++, CD117+. These progenitor subpopulations have characteristics expected for cells that are particularly useful in liver cell and gene therapies and for establishing bioartificial organs.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
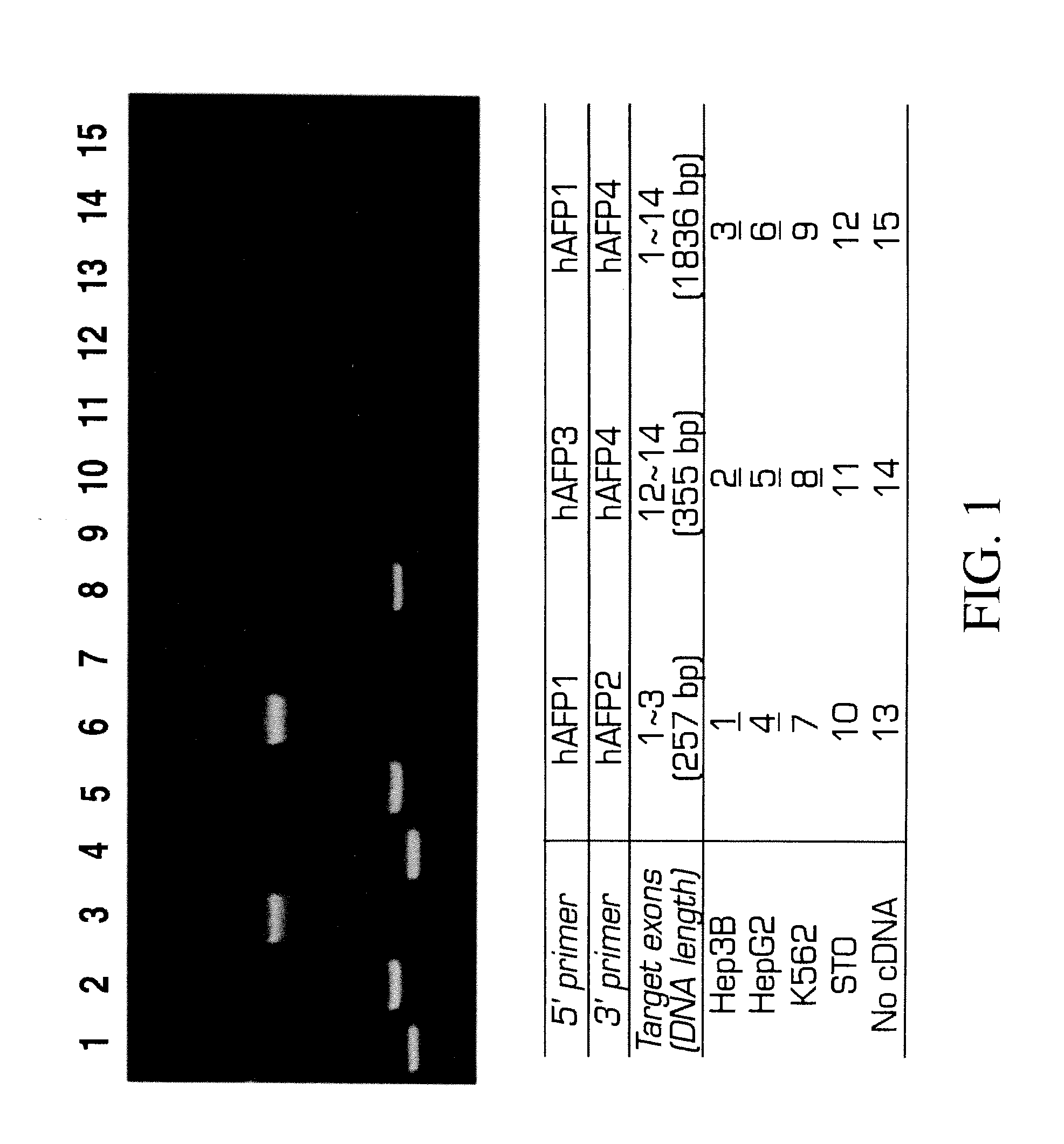
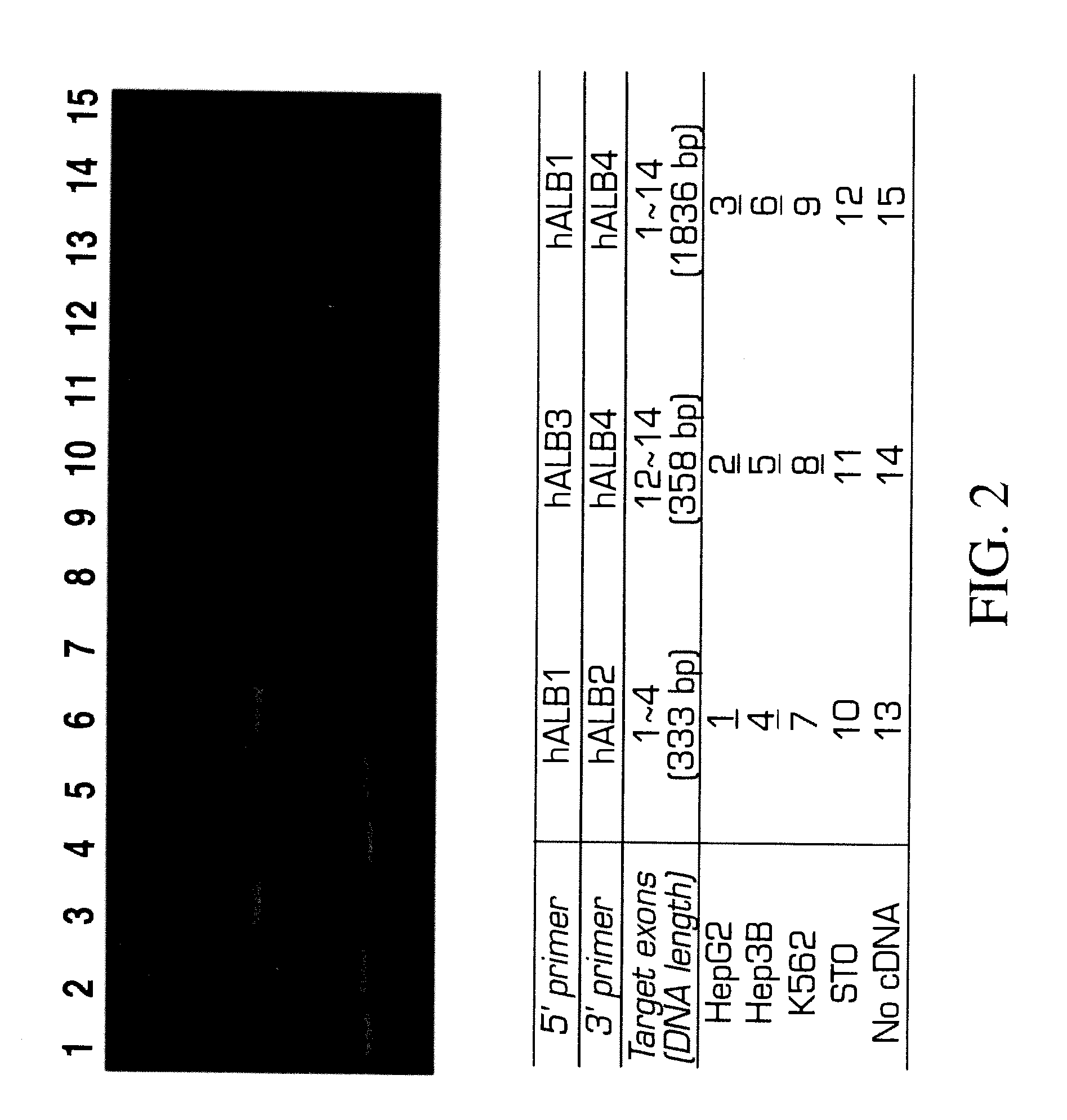
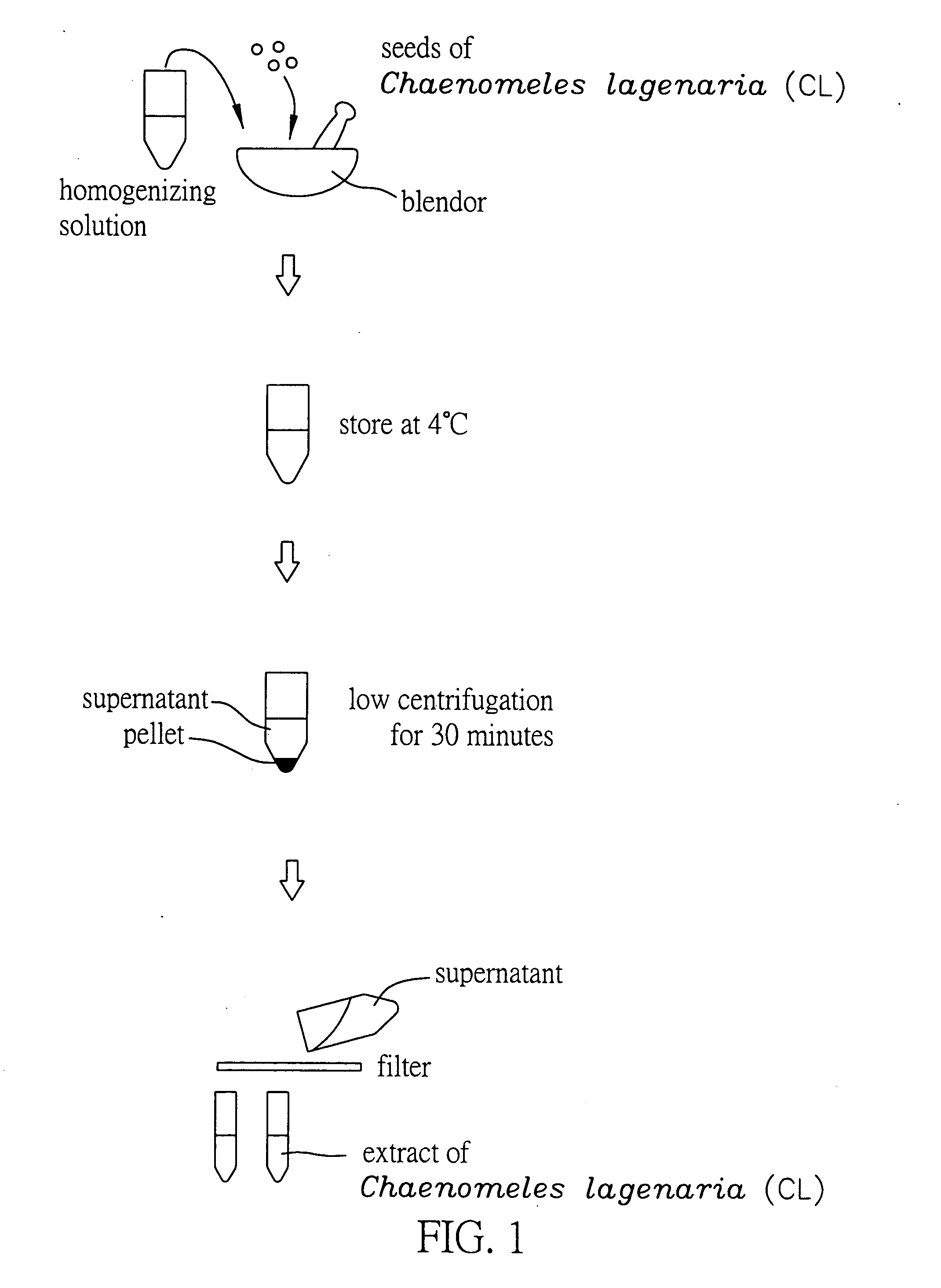
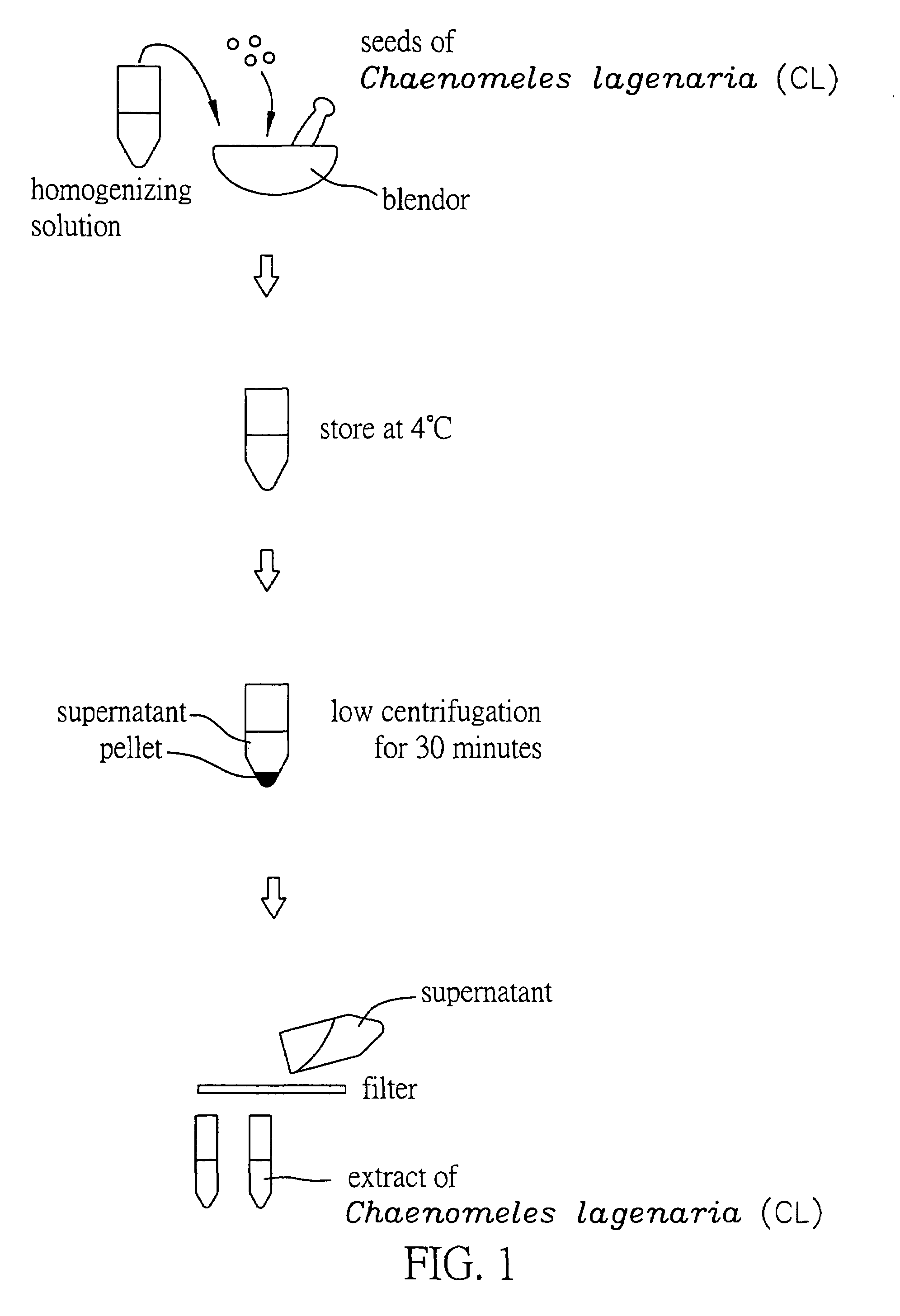
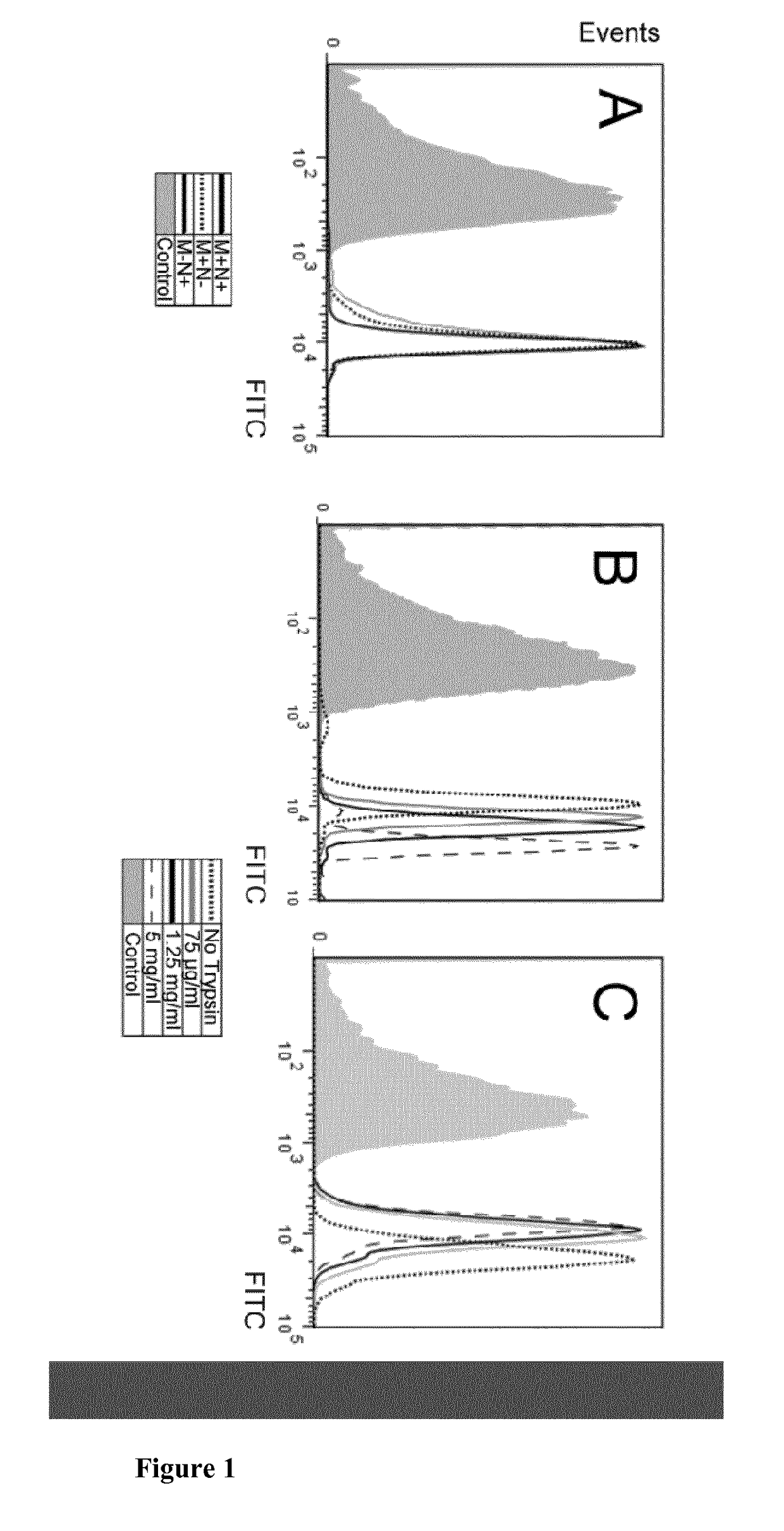
Method for extracting extract containing lectin from Chaenomeles lagenaria, Chaenomeles lagenaria extract, medical composition for inhibiting tumor growth, biological reagent for detecting glycophorin A on cell surface, and blood typing kit
InactiveUS20050260292A1Inhibition is effectiveGrowth inhibitionBiocideDepsipeptidesGlycophorinLagenaria
A Chaenomeles lagenaria extract having a lectin specific for glycophorin A and method for extracting the same are proposed. The extract is prepared by homogenizing seeds of Chaenomeles lagenaria with a homogenizing agent into a mixture, and the mixture is further processed to produce the extract both effective in inhibiting tumor growth and applicable to a blood typing test.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
Kit-of-parts to identify mother's milk missing fucosyltransferase-2 dependent glycans and feeding doses with said glycans
Described is a matrix to identify mother's milk missing fucosyltransferase-2 (FUT2) dependent glycans which comprise a line of a glycan binding protein such as a lectin or antibody suitable to bind 2'fucosyl-glycans and a line of a positive control to bind a mother's milk protein. For instance, this matrix is porous and allows for transport by capillary force of compounds of mother's milk such asglycoproteins. Especially, the glycan binding protein is the plant lectin from Ulex europaeus. A diagnostic kit comprising one or more of such matrices and a device suitable to receive such a matrix provided with a milk application window and a read-out window has been described as well. Finally a kit-of-parts comprising such a diagnostic kit and one or more feeding doses of 2'fucosyl-glycans hasbeen disclosed.
Owner:SOC DES PROD NESTLE SA
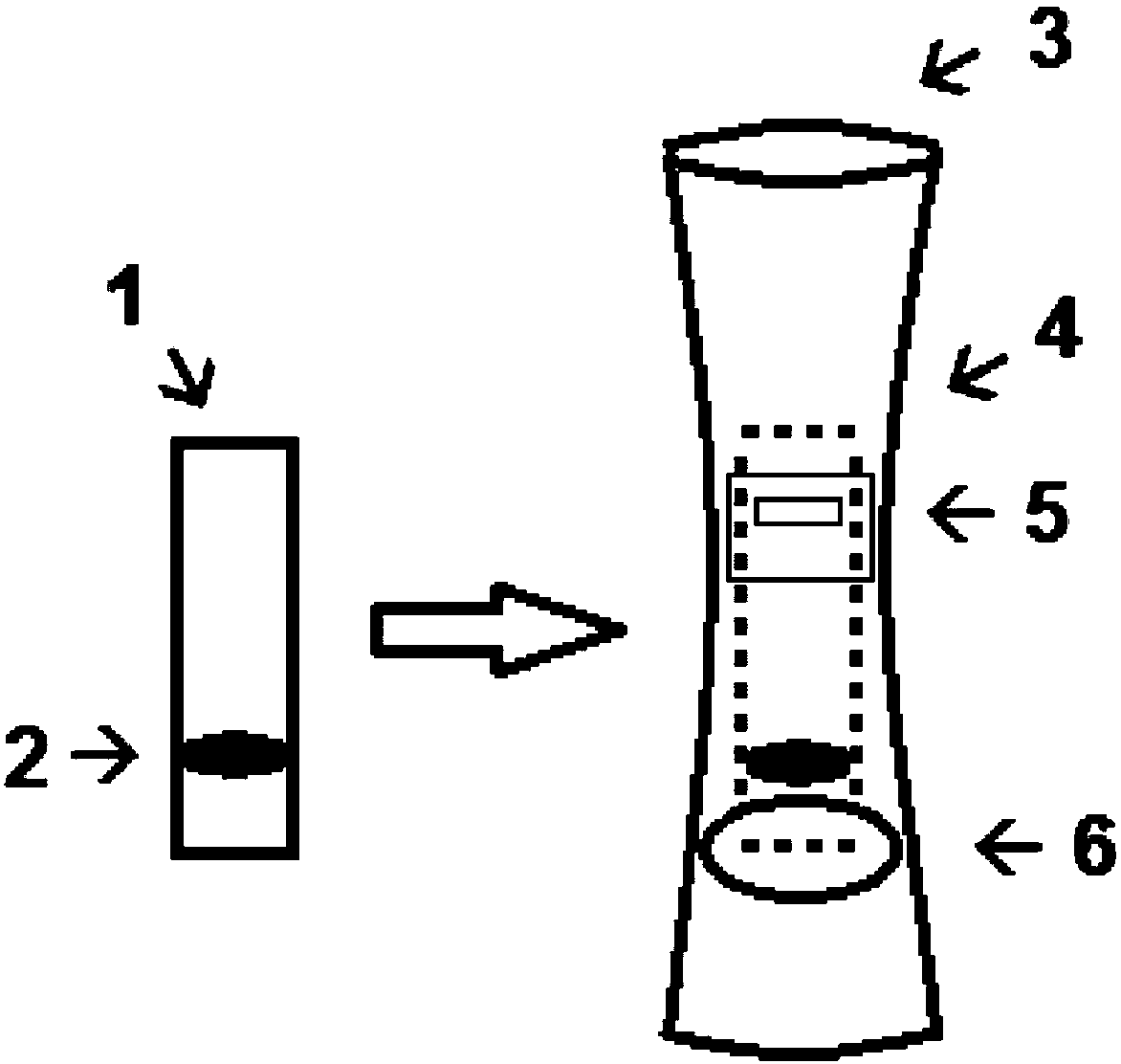
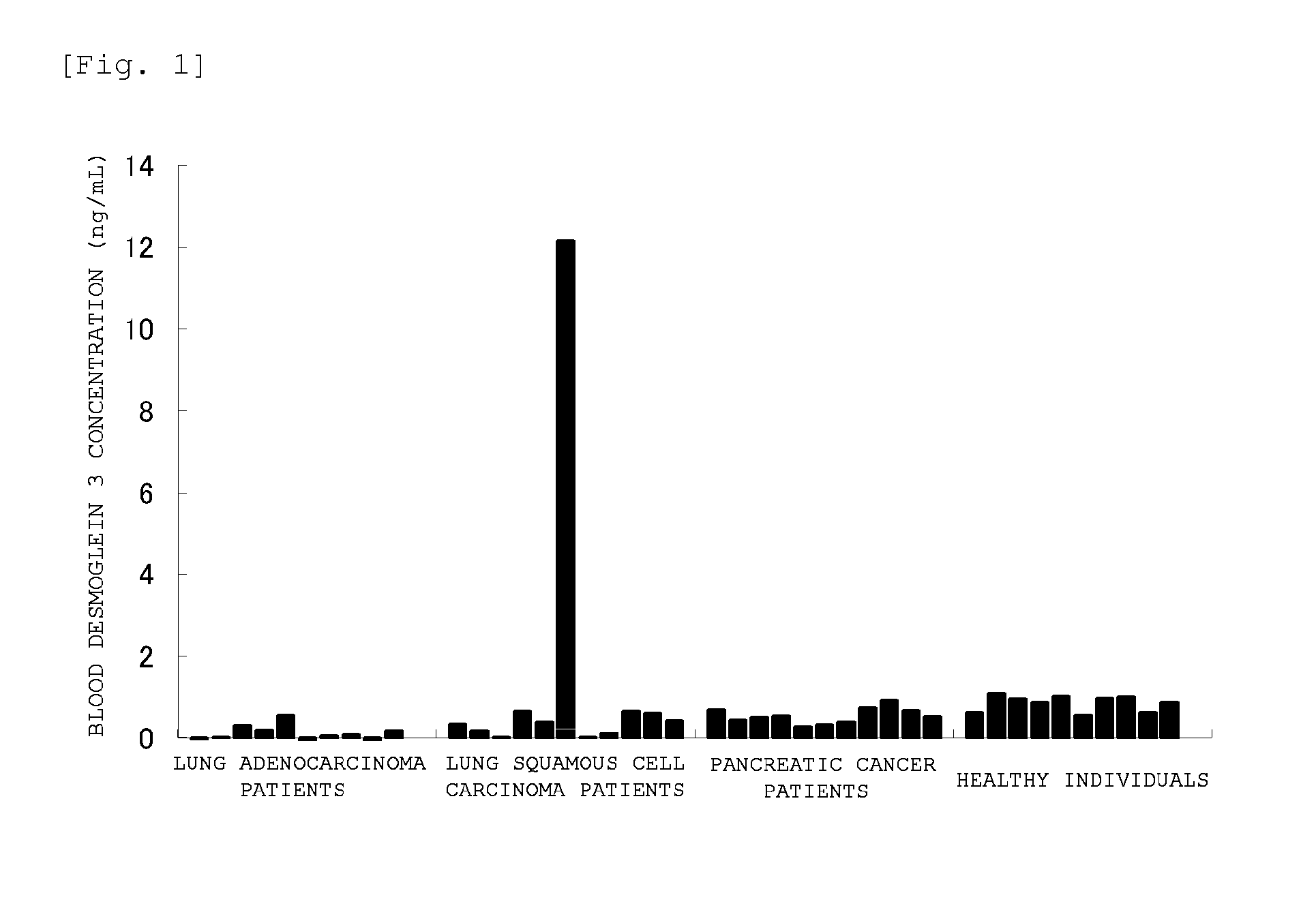
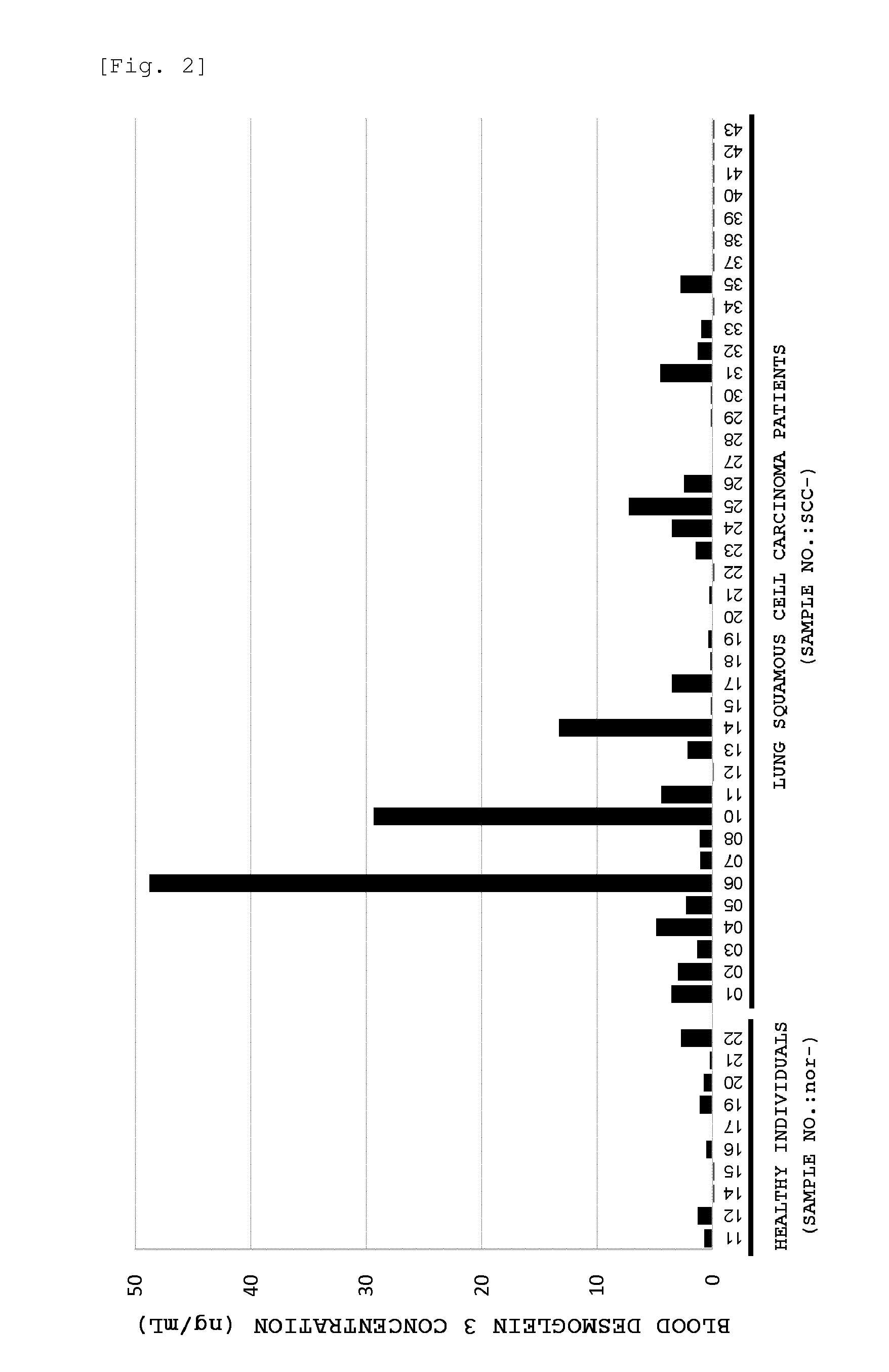
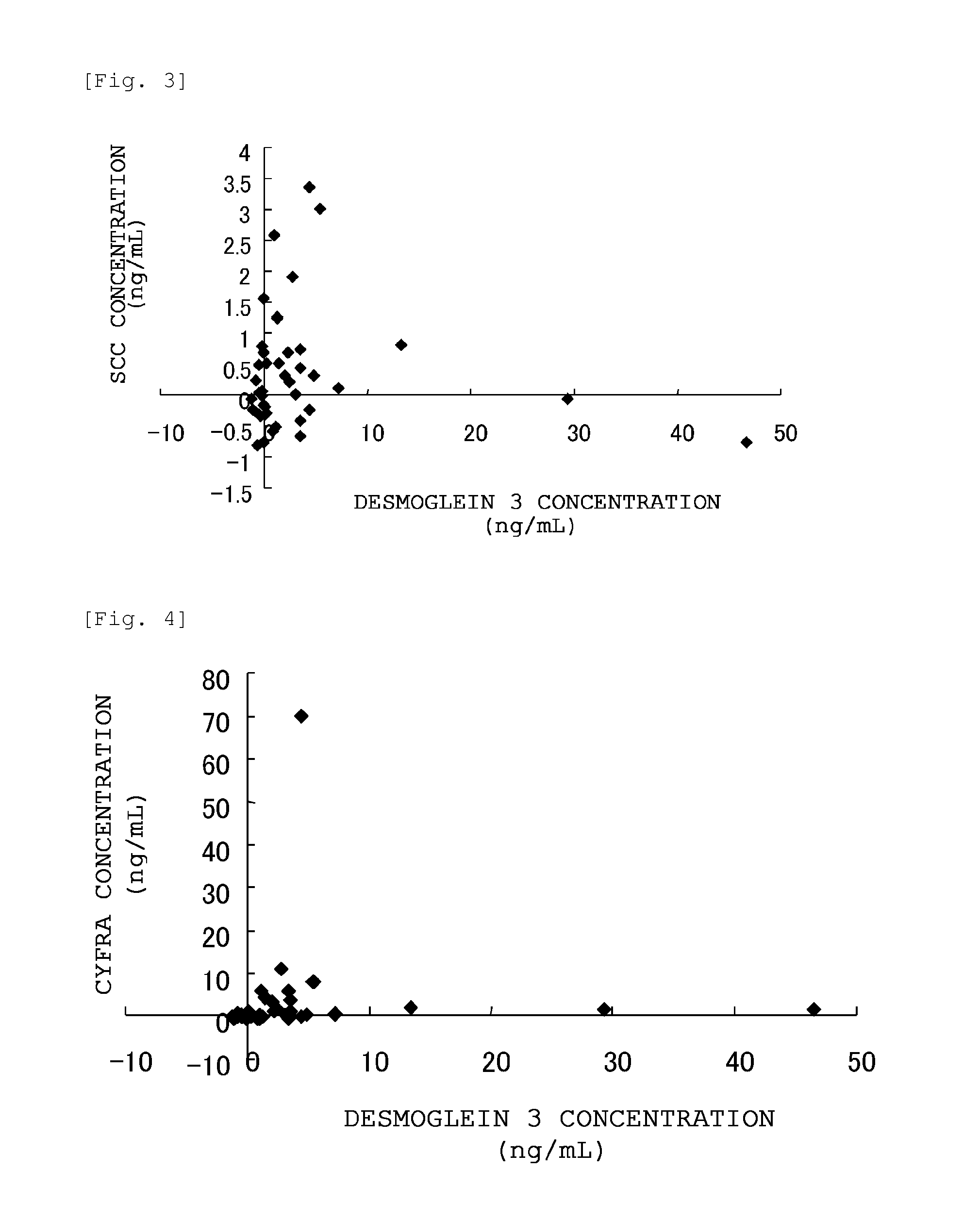
Method for detecting lung squamous cell carcinoma
ActiveUS20150276749A1The method is simple and fastHigh sensitivityCell receptors/surface-antigens/surface-determinantsBiological material analysisLung squamous cell carcinomaGlycophorin
The present invention provides a method by which lung squamous cell carcinoma can be detected in a simple and prompt manner with high detection performance; and the like. The method according to the present invention detects lung squamous cell carcinoma by an assessment including the steps of: (1) performing measurement of the desmoglein 3 content in a blood sample collected from a subject; and (2) comparing the desmoglein 3 content determined by the measurement with the desmoglein 3 content in a blood sample collected from a healthy individual so as to estimate the presence of lung squamous cell carcinoma in the subject when the desmoglein 3 content is higher in the blood sample collected from the subject.
Owner:CHUGAI PHARMA CO LTD +1
SPG-(Streptococcal Protein G) antibody polymer and preparation method as well as application thereof
InactiveCN102167746AHigh purityIncrease the number of cellsSkeletal/connective tissue cellsBlood/immune system cellsHigh cellProgenitor
The invention discloses SPG-(Streptococcal Protein G) antibody polymer and a preparation method as well as application thereof. The SPG-antibody polymer is formed by coupling anti-glycophorin A monoclonal antibody, an SPG antibody and a target antigen antibody. One end of the polymer is coupled with red blood cells through the anti-glycophorin A monoclonal antibody, and the other end of the polymer is coupled with a target antigen through antibodies of a surface antigen of a target cell and other antigens; then standing is performed to detect the target antigen through red blood cell precipitation, or mature nucleated cells and red cells in blood are removed through density gradient centrifugation; and all stem cells / progenitor cells are reserved. In the invention, a combined method of negative selection and density gradient centrifugation is adopted for separating and extracting the stem cells and the progenitor cells, and markers are not available on the surfaces of the collected progenitor / stem cells, so that the antibody polymer has the advantages of high cell activity, simple and practical method, industrial production and easiness in popularization, can be widely applied to research and treatment of laboratory and clinical cell damage diseases and has broad prospect.
Owner:姜东成
Synthetic peptides and kits for diagnosis of anti-phospholipid syndrome
InactiveUS7053178B2Rapid diagnosisSpecific treatmentApolipeptidesPeptide/protein ingredientsGlycophorinMonoclonal antibody
Synthetic peptides and derivatives thereof capable of inhibiting the biological activity of anti-beta-2-glycoprotein 1 (β2GPI) monoclonal antibodies (mAbs) in vitro, and of inhibiting induction of experimental anti-phospholipid syndrome (APS) in mice by anti-β2GPI mAbs, are provided for the diagnosis and treatment of anti-phospholipid syndrome in humans.
Owner:YEDA RES & DEV CO LTD
Method for extraction lectin from Chaenomeles lagenaria, medical composition for inhibiting tumor growth, biological reagent for detecting glycophorin A on blood and blood typing kit
A Chaenomeles lagenaria extract having a lectin specific for glycophorin A and method for extracting the same are proposed. The extract is prepared by homogenizing seeds of Chaenomeles lagenaria with a homogenizing agent into a mixture, and the mixture is further processed to produce the extract both effective in inhibiting tumor growth and applicable to a blood typing test.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
Fusion proteins and immunoconjugates and uses thereof which are specific for glycophorin A
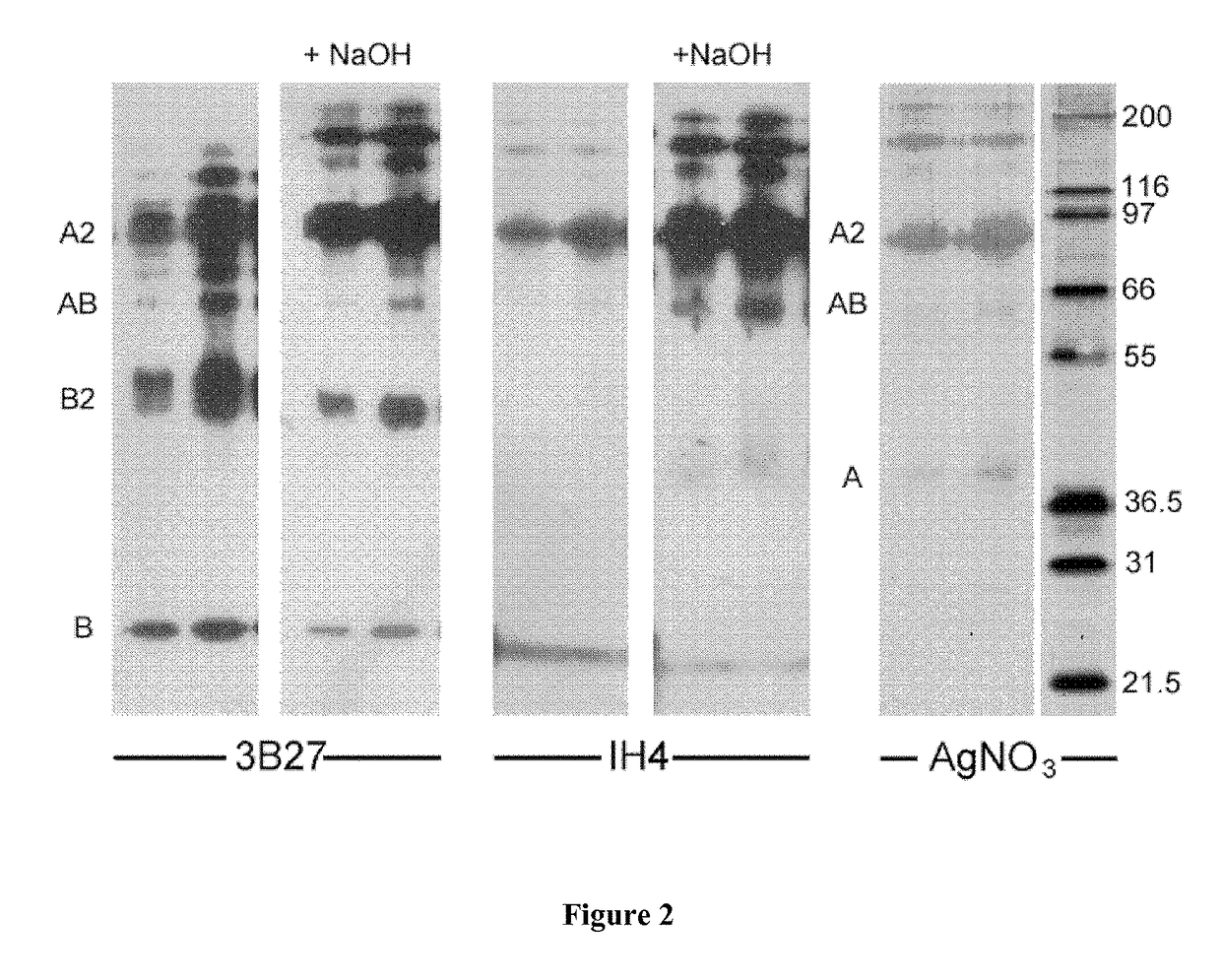
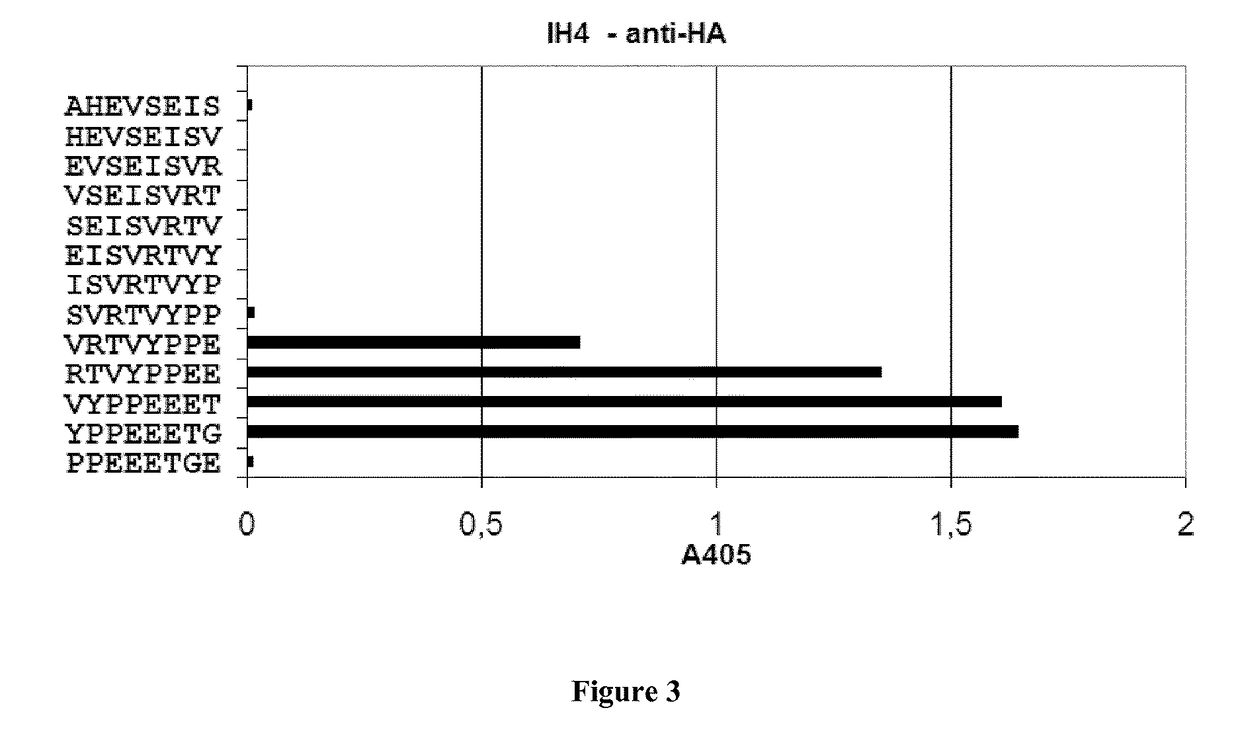
ActiveUS9879090B2Prevent steric hindrancesEnhanced couplingImmunoglobulins against blood group antigensPeptide/protein ingredientsHeterologousGlycophorin
Owner:UNIVERSITÉ PARIS CITÉ +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com