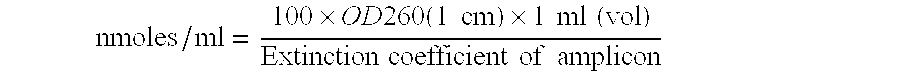Patents
Literature
39 results about "Plasmodium vivax" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly (a pathologically enlarged spleen). P. vivax is carried by the female Anopheles mosquito; the males do not bite.
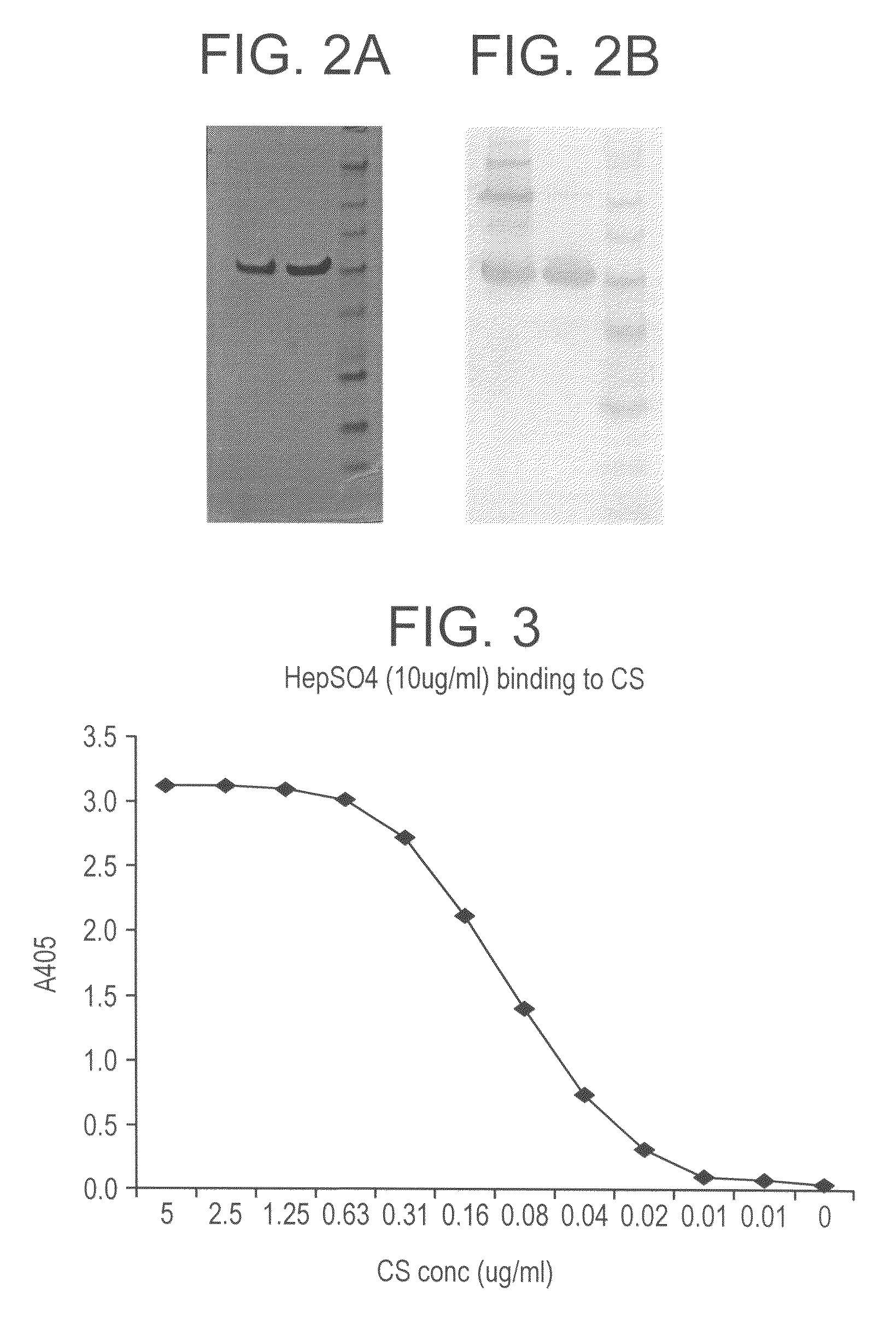
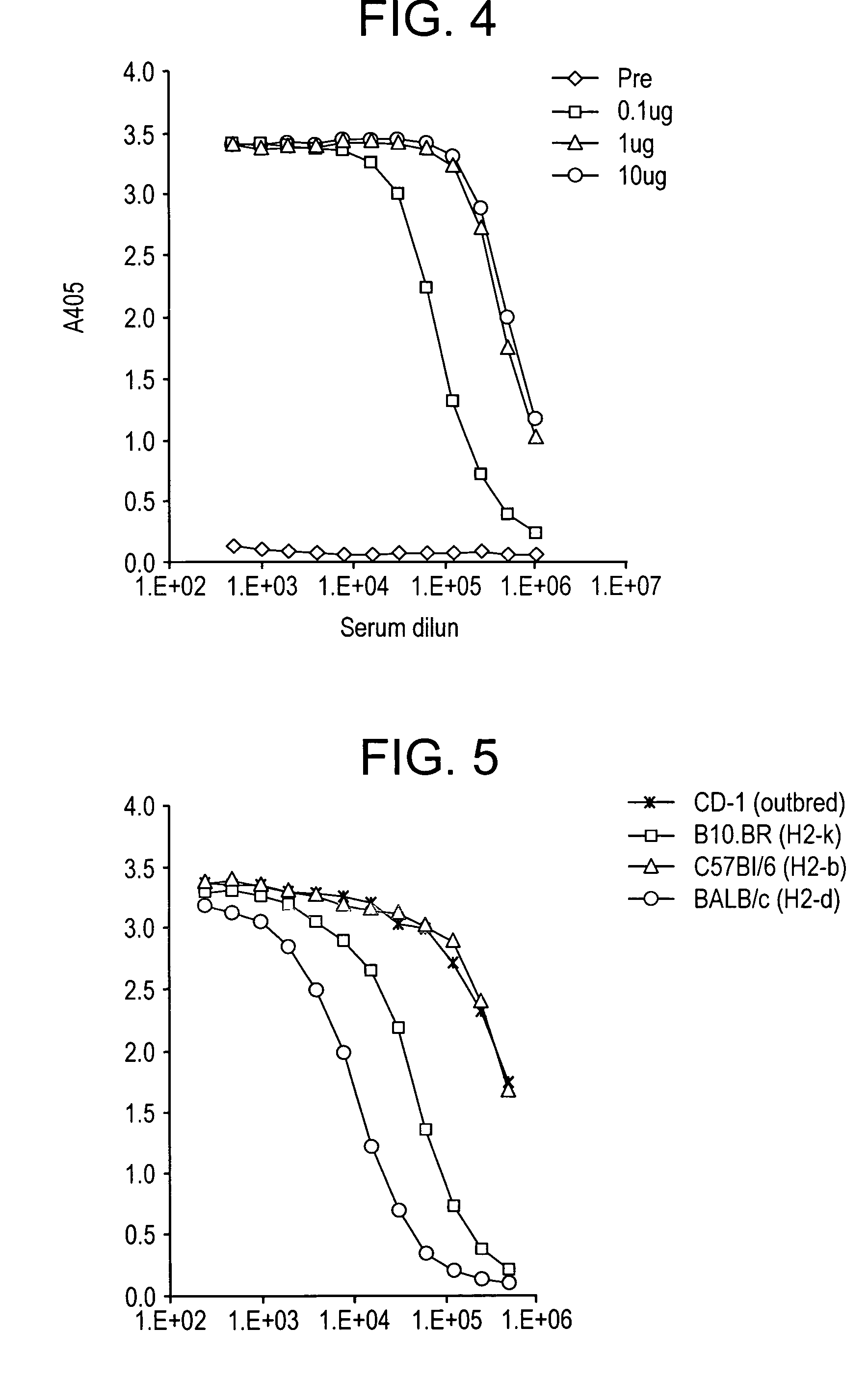
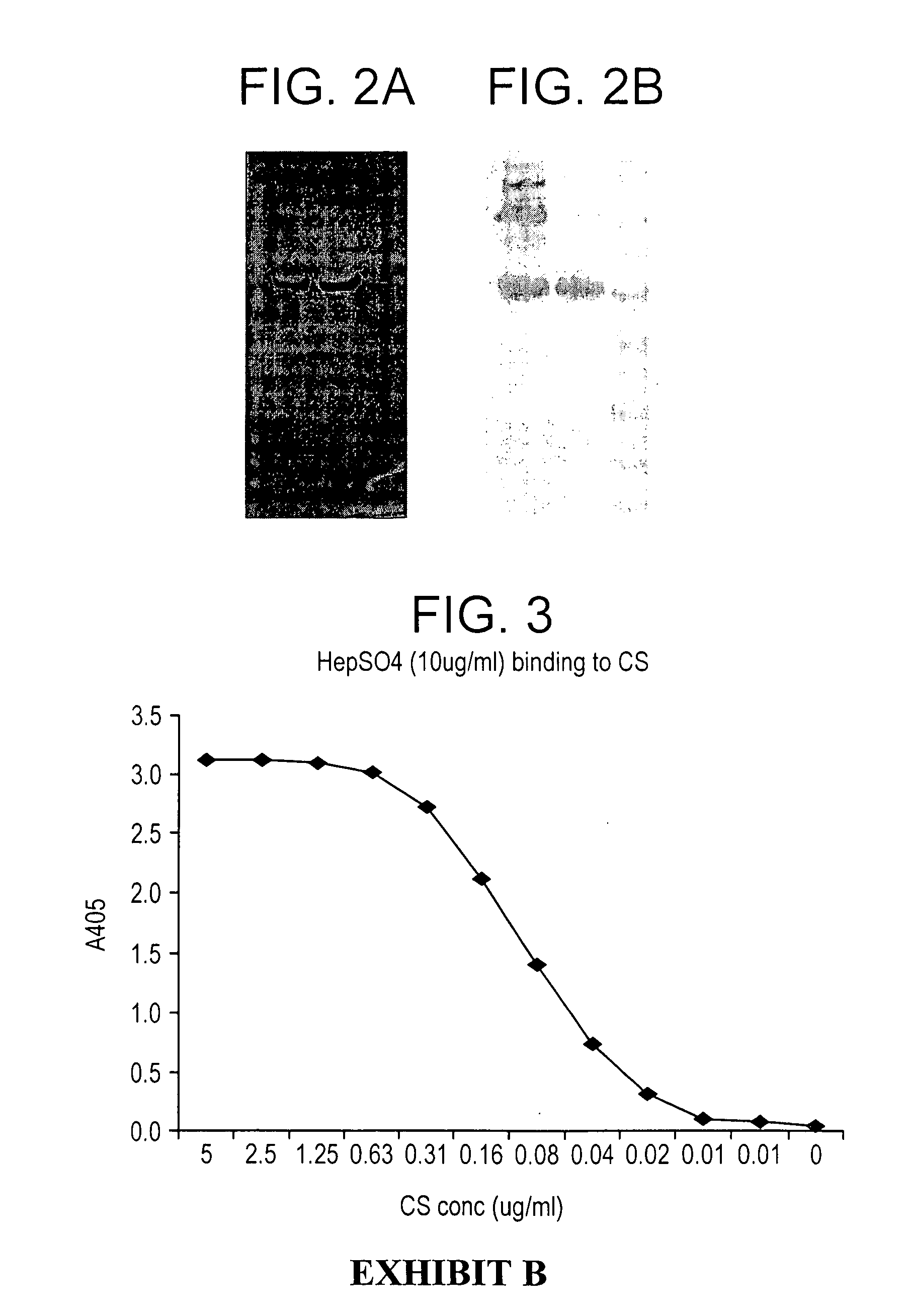
Fusion proteins comprising CD4 and the malaria parasite merozoite glycophorin binding protein 130 (GBP-130)
InactiveUS7585508B1Reduce infectivityRelieve symptomsFusions with soluble cell surface receptorAntiviralsGlycophorinBinding peptide
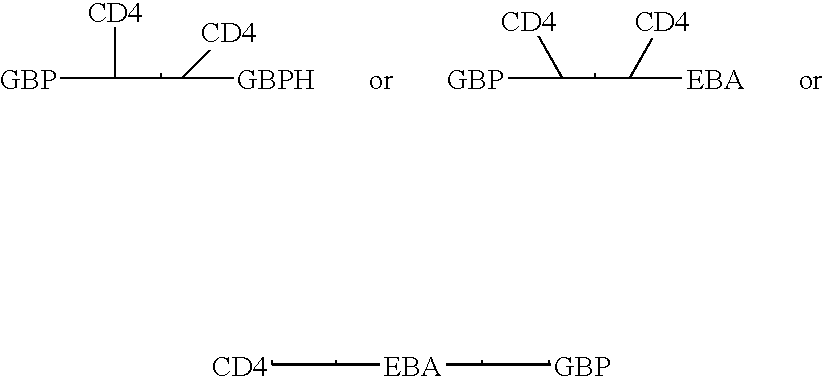
Novel hybrid fusion peptides are disclosed. The novel peptides are formed by the fusion of two or more components. One component is a peptide sequence or variant of a peptide sequence derived from a malaria parasite merozoite peptide which has affinity for and binding capability to red blood cells.In particular segments of the glycophorin binding peptide 130 (GBP130), are preferred for the first component. Also disclosed are alternative first components, the glycophorin binding peptide homologues (GBPH), or the erythrocyte binding antigen 175 (EBA175), or the plasmodium vivax Duffy receptor or the pre major merozoite surface antigen PMMSA or the (P200) peptide.The first component peptide is fused to all or part of a peptide segment derived from the CD4 molecule or part thereof or variant thereof which shows binding affinity for the HIV virus.The resulting fusion peptide being exemplified asNH2-CD4-GBP130-COOH1-371 201-774Also disclosed are the methods of manufacture and means to use the novel hybrid peptides as clinical agents to treat, prevent or test for HIV infection.
Owner:PRENDERGAST KENNETH F
Plasmodium vivax hybrid circumsporozoite protein and vaccine
InactiveUS7790186B2Decreased number of repeatMaximize possibility of generatingPeptide/protein ingredientsAntibody mimetics/scaffoldsCircumsporozoite proteinAntibody production
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Plasmodium vivax hybrid circumsporozoite protein and vaccine
InactiveUS20090196883A1Not painful to administerIntrinsically safePeptide/protein ingredientsAntibody mimetics/scaffoldsCircumsporozoite proteinAntibody production
Described in this application is a synthetic P. vivax circumsporozoite protein useful as a diagnostic reagent, for antibody production, and as a vaccine protective against infection with any strain of P. vivax.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Immunoassay and diagnostic reagent for malaria
InactiveCN1406284AHigh antigen sensitivityStrong specificityMicrobiological testing/measurementBiological material analysisMalarial parasiteProtozoa
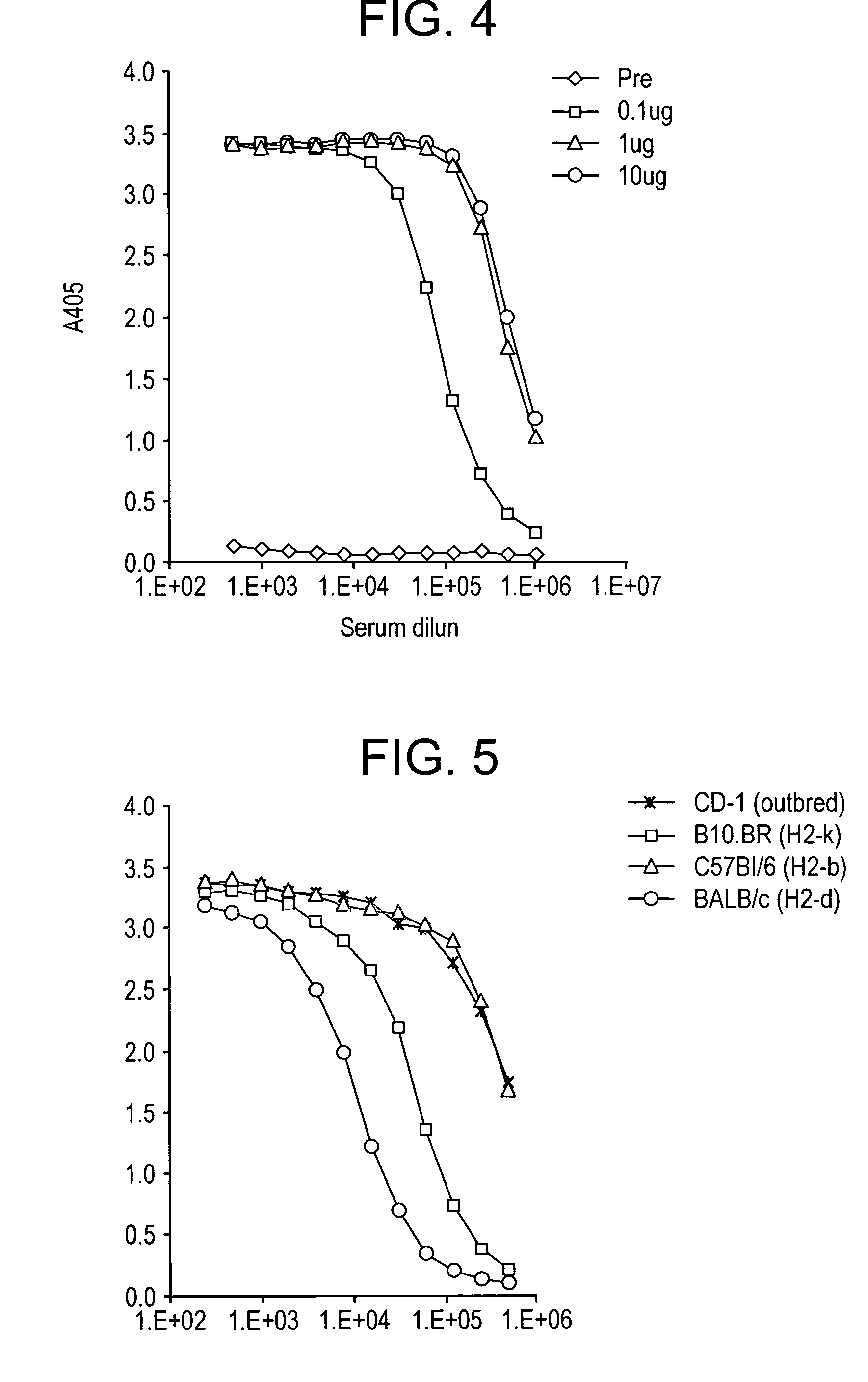
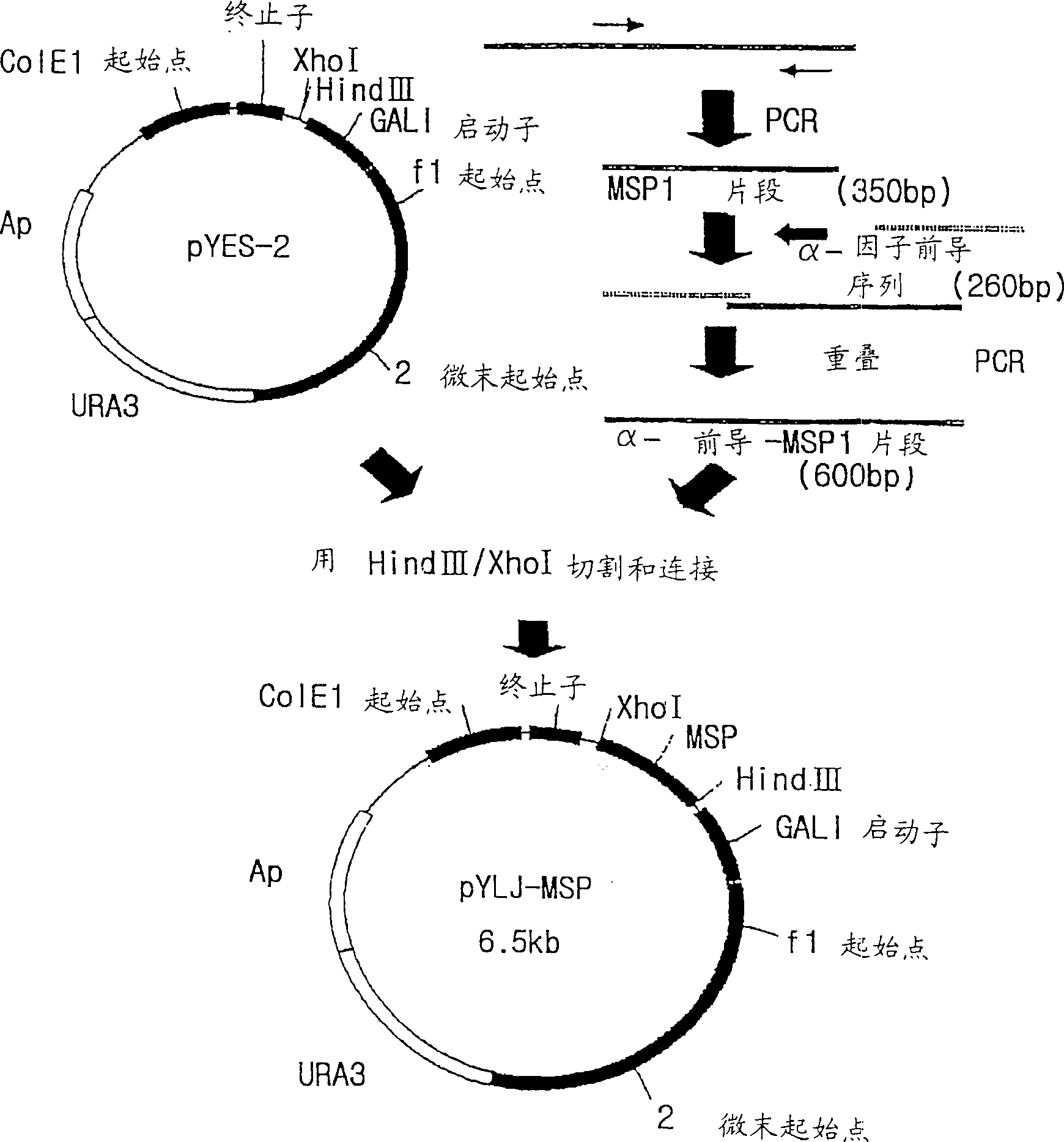
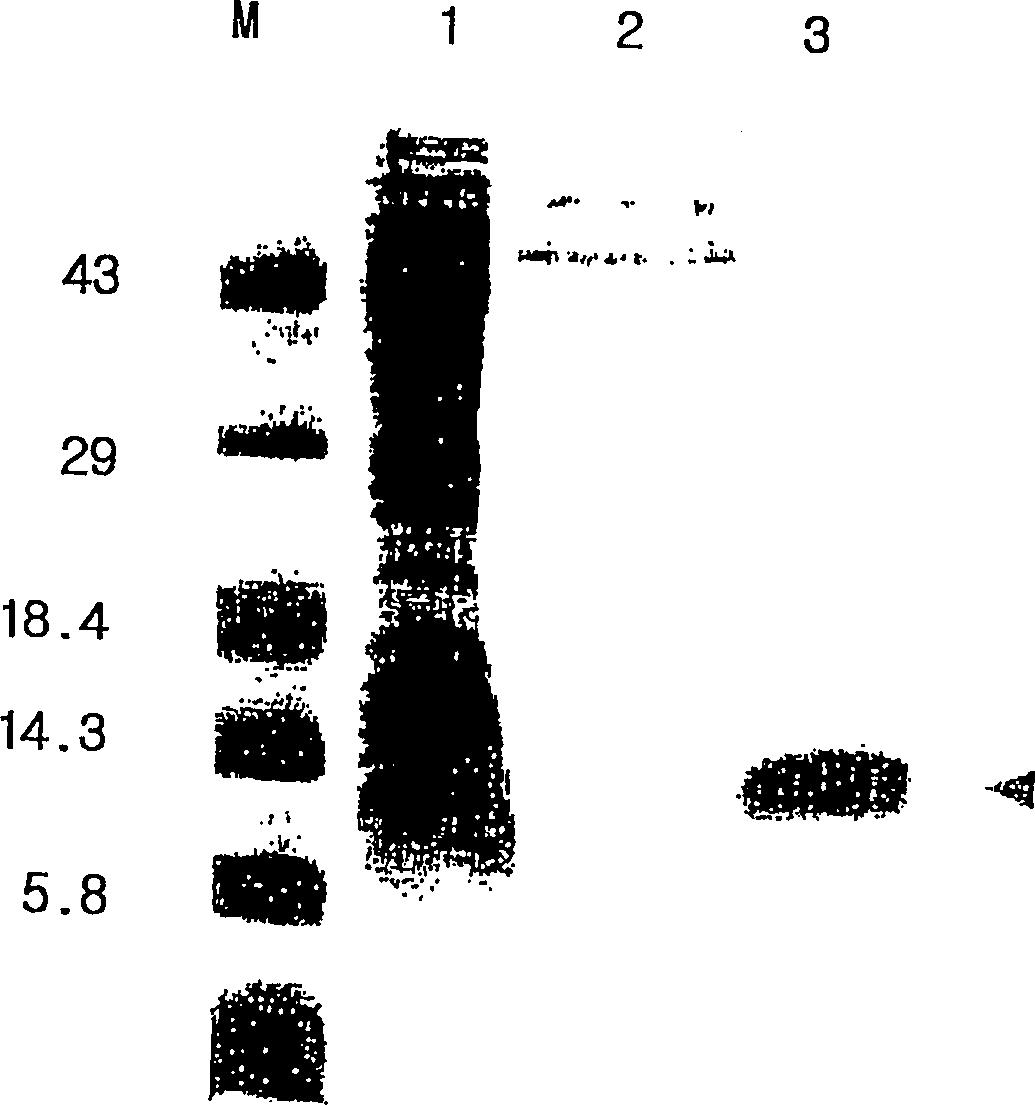
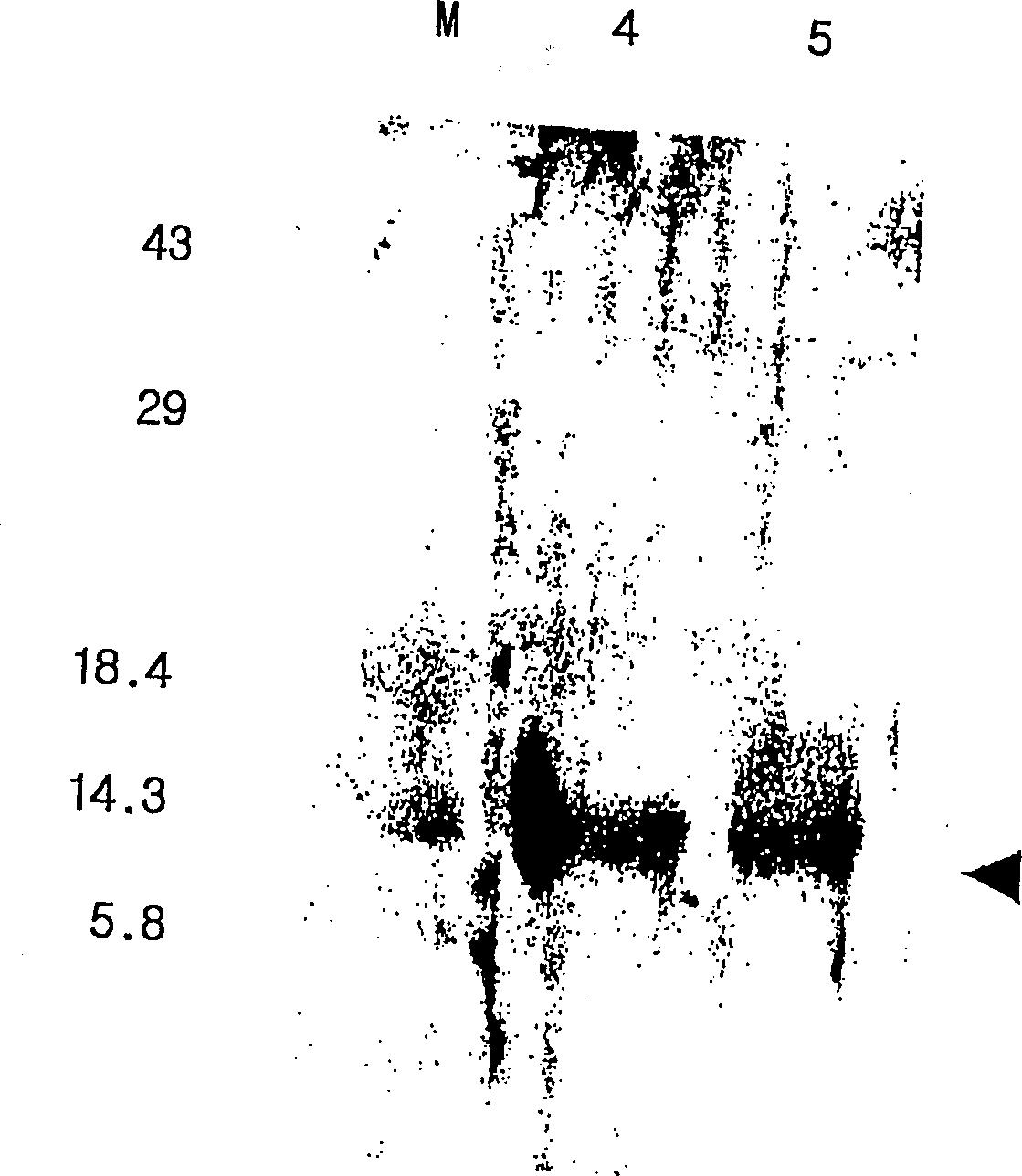
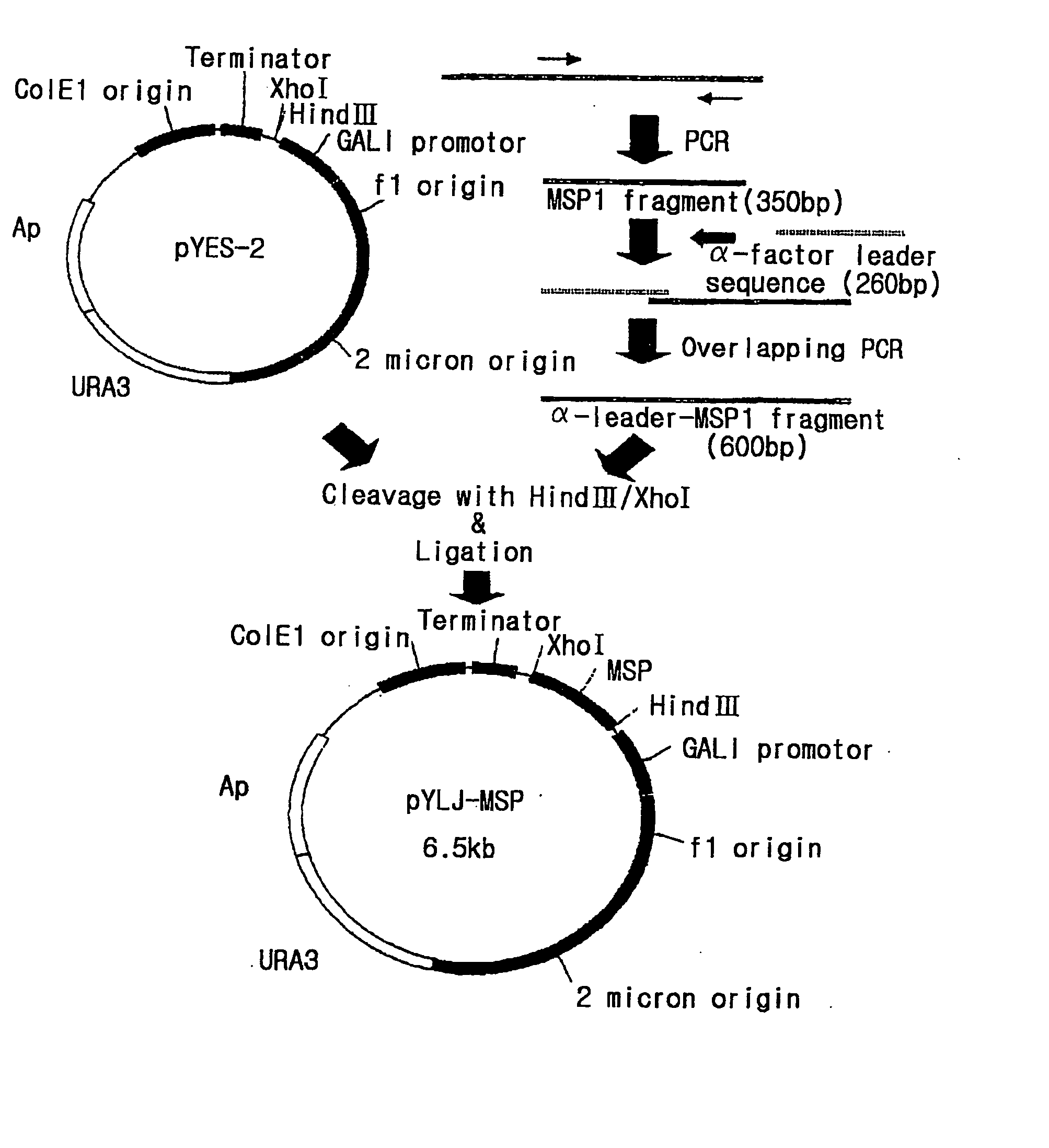
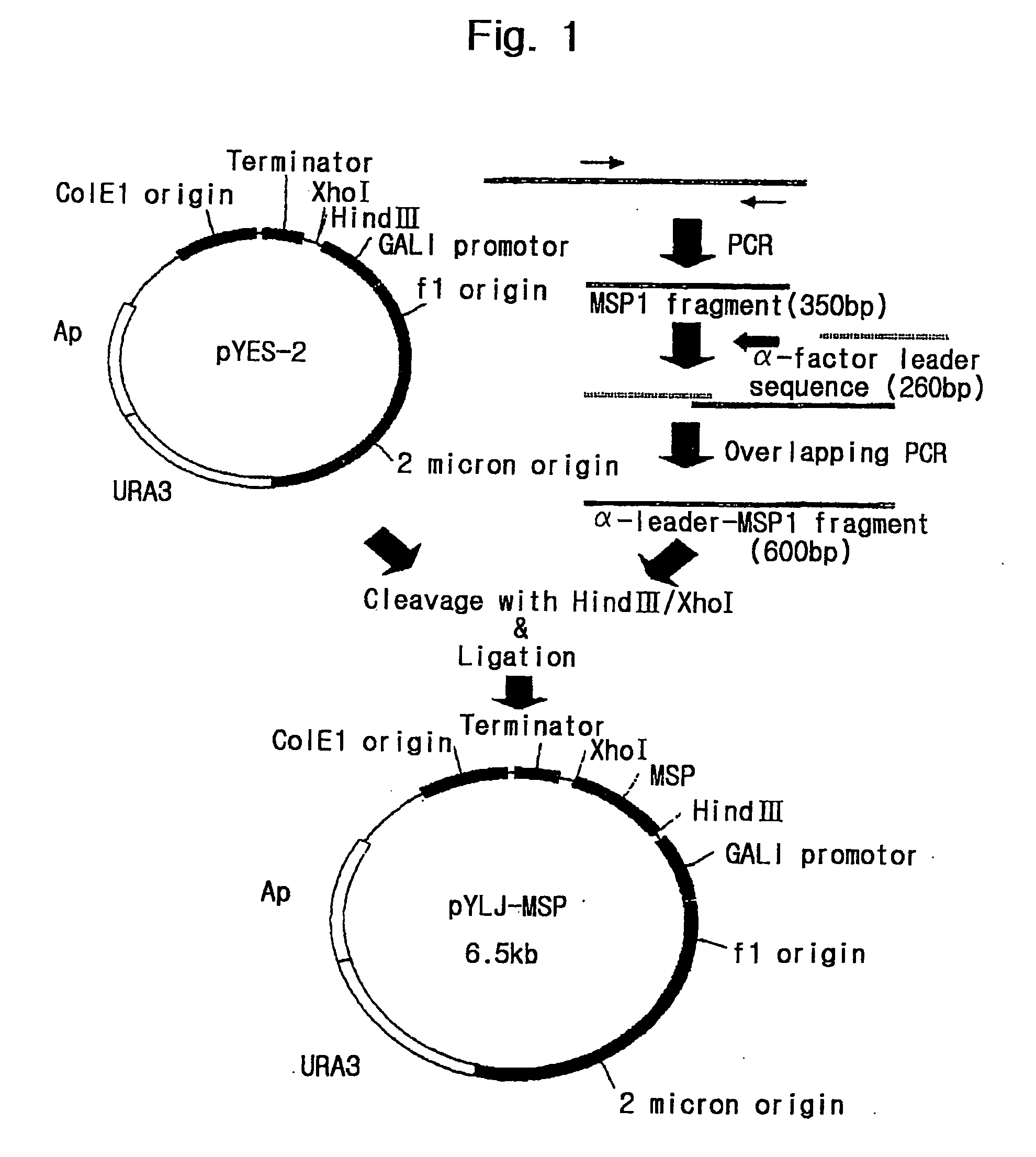
The present invention relates to an immunoassay and diagnostic reagent for malaria by using antigens of malarial Protozoa. More preferably, the present invention relates to an immunoassay and diagnostic reagent for malaria which detect malaria-specific antibodies in blood by using Merozoite Surface Protein of Plasmodium vivax. The immunoassay and diagnostic reagent detecting malaria-specific antibodies in blood according to the present invention have high specificity and sensitivity and are useful in diagnosing a type of malaria where latent period is long and number of Protozoa in blood if few. Also, the present invention relates to a preparation method of the surface protein of malarial Protozoa using yeast or E.Coli. Preferably, the present invention provides an expression vector comprising genes of Merozoite Surface Protein of Plasmodium Vivax and histidine residues, as well as transformants transformed with the expression vector. Also, the present invention provides a method for preparing Merozoite Surface Protein of malarial Protozoa by using the transformant. The surface protein Merozoite Surface Protein of malarial Protozoa prepared from yeast or E.Coli transformant according to the present invention has high sensitivity and specificity to antibody as well as high purity. Also, the surface protein prepared by the preparation method of the present invention has markedly low pseudo-positive signals, and is useful in diagnosing malaria.
Owner:LG CORP
Plasmodium vivax PvMSP1 recombinant antigenic protein as well as preparation method and application thereof
ActiveCN103570817AIncreased sensitivityImprove featuresBiological material analysisMicroorganism based processesEpidemiologic surveyGlycosylphosphatidylinositol
The invention discloses a plasmodium vivax PvMSP1 recombinant antigenic protein, which is protein of which the amino acid sequence is shown in SEQ ID NO:1 which has glycophosphatidylinositol (GPI) anchor and epidermal growth factor-like (EGF-like) structure domain. Furthermore, the invention also discloses a preparation method of the recombinant antigenic protein, which comprises the steps of amplifying a plasmodium vivax PvMSP1 gene sequence, constructing and identifying recombinant plasmid, inducibly expressing and purifying recombinant protein and the like. Experiments prove that the PvMSP1 recombinant antigenic protein has the advantages of high sensitivity, strong specificity and the like to assay of the serum antibody of a plasmodium vivax infected patient, and has a wide application prospect in the aspect of plasmodium vivax epidemiological investigation.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Geographically-specific Plasmodium vivax molecule marker, and its application in strain tracing
PendingCN103865979AGuaranteed to be scientificTo achieve the purpose of traceabilityMicrobiological testing/measurementBiological material analysisMicrosatelliteMosquito infection
The invention relates to a geographically-specific Plasmodium vivax molecule marker, and its application in strain tracing. The molecule marker includes a Plasmodium vivax circumsporozoite protein center replication region reflecting mosquito infection differences of different media, a height polymorphism microsatellite reflecting mutation, migration and genetic drift, and a drug resistance related gene mutation reflecting the population positivity selection of drug control. Results show that each of a Plasmodium vivax circumsporozoite protein center replication region sequence, dihydrofolate reductase SNP, dihydrobiopterin synthetase SNP, a neutral microsatellite and the like has a geographic specificity, and can be used as a classification index in tracing detection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
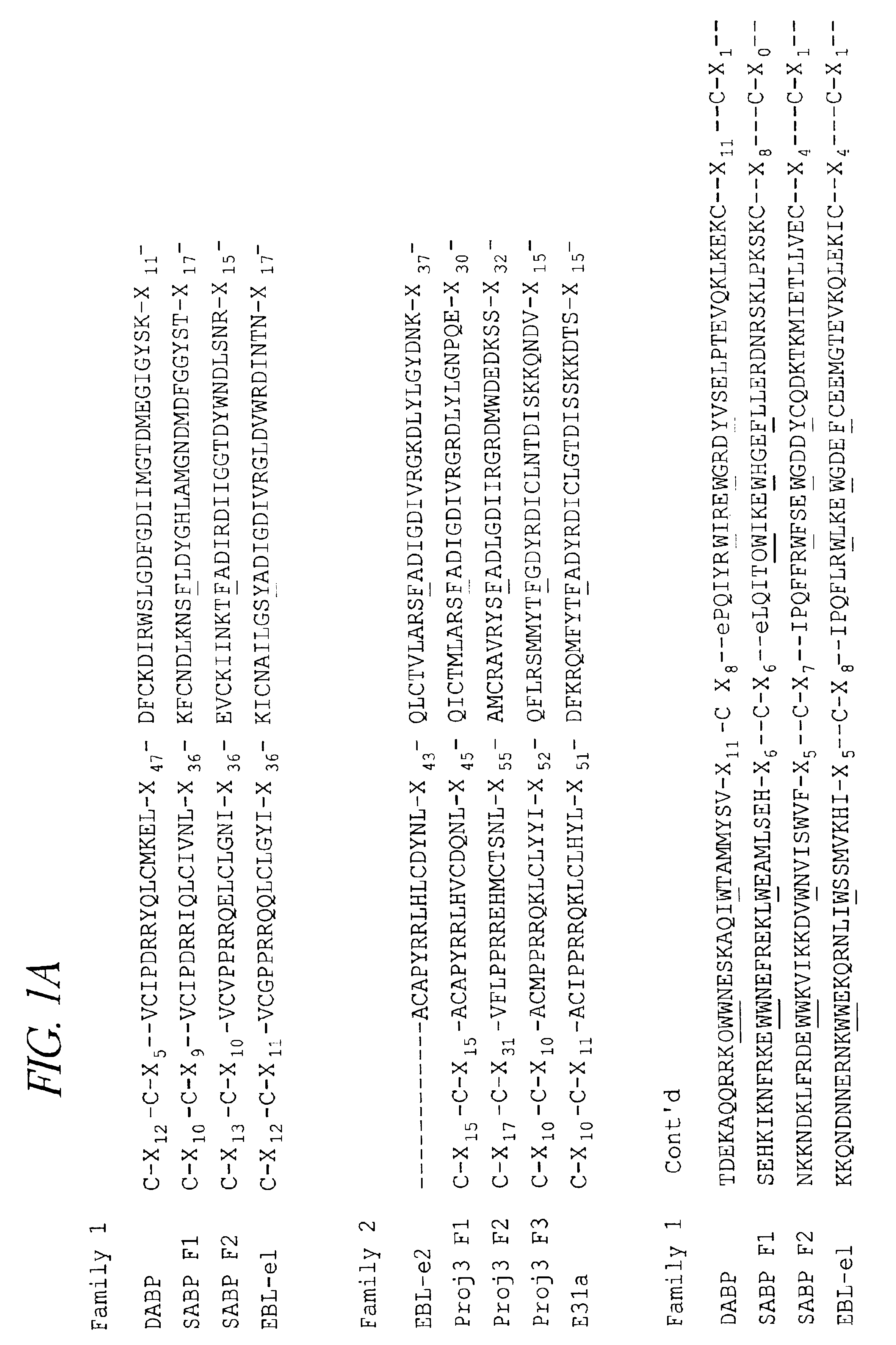
Binding domains from Plasmodium vivax and Plasmodium falciparum erythrocyte binding proteins
The present invention provides isolated polypeptides useful in the treatment and prevention of malaria caused by Plasmodium falciparum or P. vivax. In particular, the polypeptides are derived from the binding domains of the proteins in the EBL family as well as the sialic acid binding protein (SABP) on P. falciparum merozoites. The polypeptides may also be derived from the Duffy antigen binding protein (DABP) on P. vivax merozoites.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
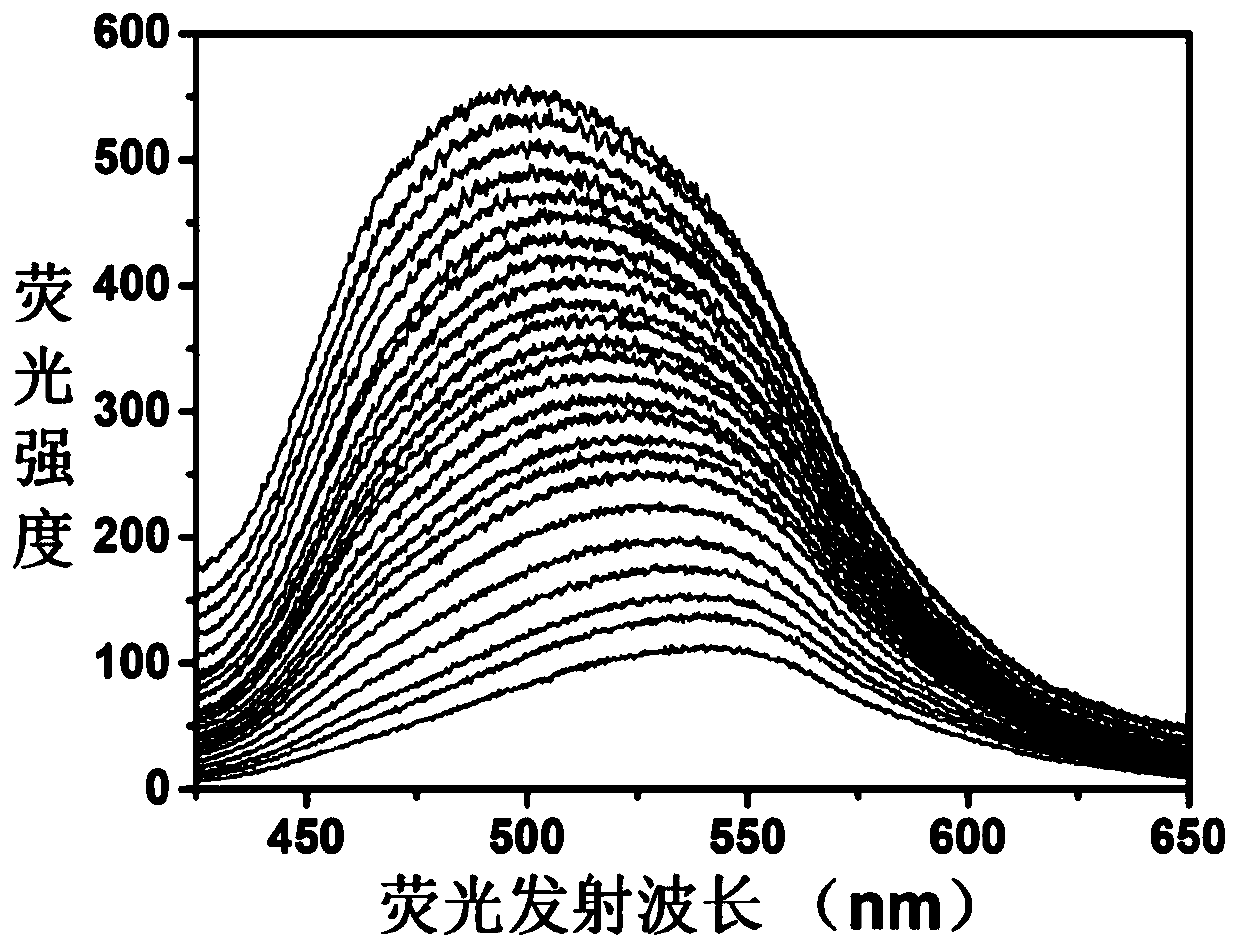
Adenosine monophosphate-protected gold-silver alloy nanocluster fluorescence probe, and application thereof in detection of Plasmodium vivax lactate dehydrogenase
ActiveCN110408380AImprove stabilityGood water solubilityMaterial nanotechnologyNanoopticsLactate dehydrogenaseAdjuvant
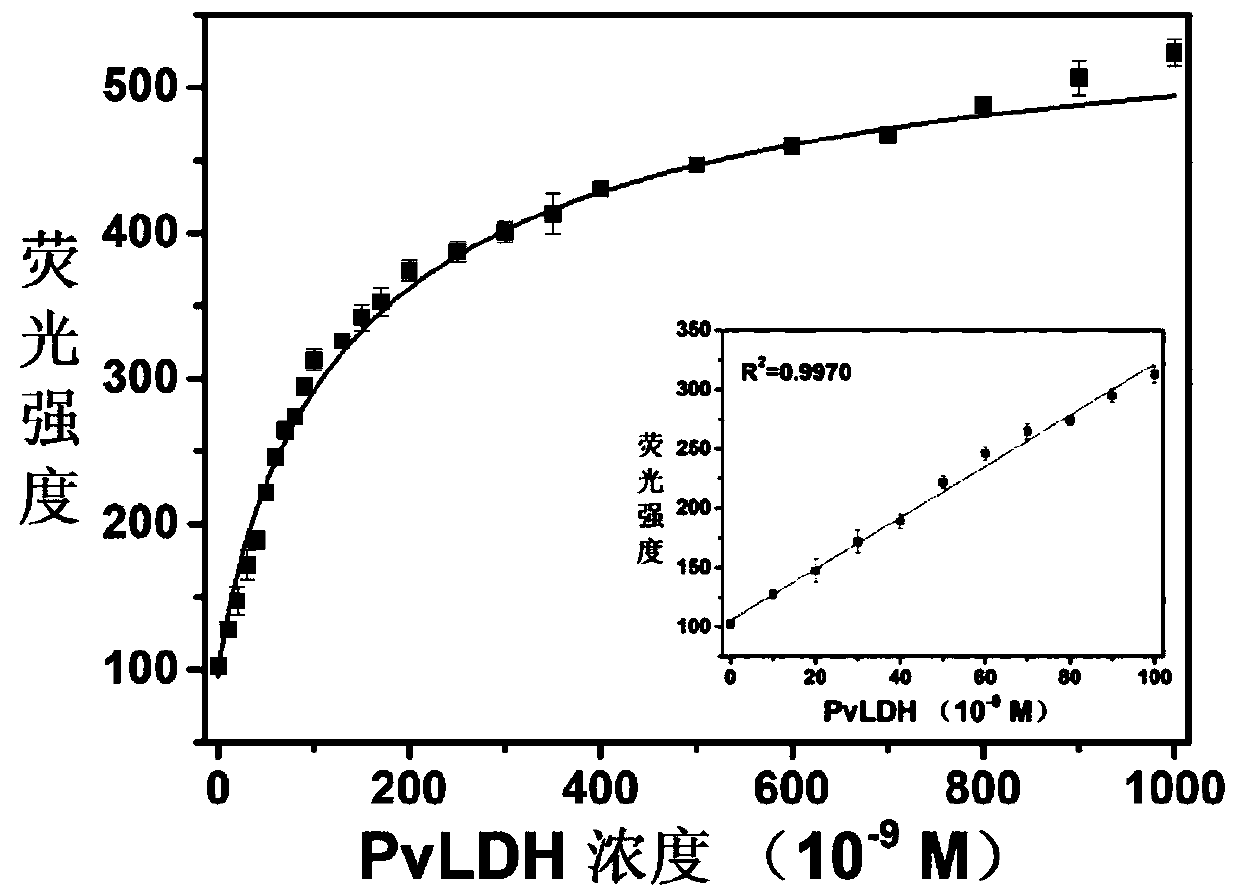
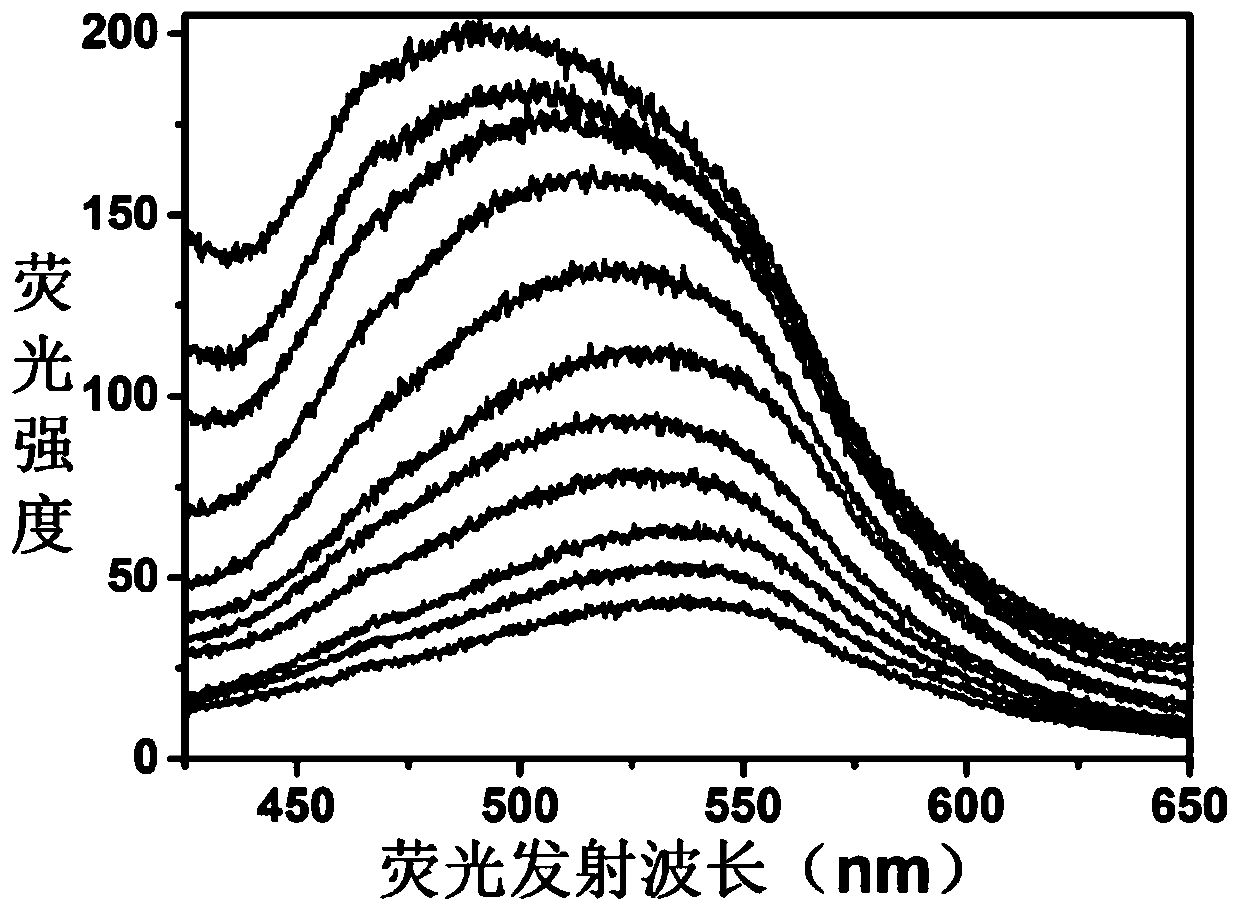
The invention discloses an adenosine monophosphate-protected gold-silver alloy nanocluster fluorescence probe, and an application thereof in the detection of Plasmodium vivax lactate dehydrogenase, and belongs to the technical field of fluorescence probes. The fluorescence probe has the advantages of simple structure, easiness in synthesis, strong stability and good biocompatibility, and can generate large linear fluorescence enhanced response to the Plasmodium vivax lactate dehydrogenase (PvLDH). The response has a wide detection range (0 to 1.0 *10<-6> mol / L) and a high sensitivity, and thedetection limit can reach 3.7 ng / mL. An adjuvant Al<3+> is introduced, so the probe can completely distinguish the Plasmodium vivax lactate dehydrogenase and other types of lactate dehydrogenases. Thedetection method of the invention has the advantages of high speed, simplicity in operation, stable signal, high sensitivity, no pretreatment, and no complicated detection instruments.
Owner:JILIN UNIV
Tet transactivator system
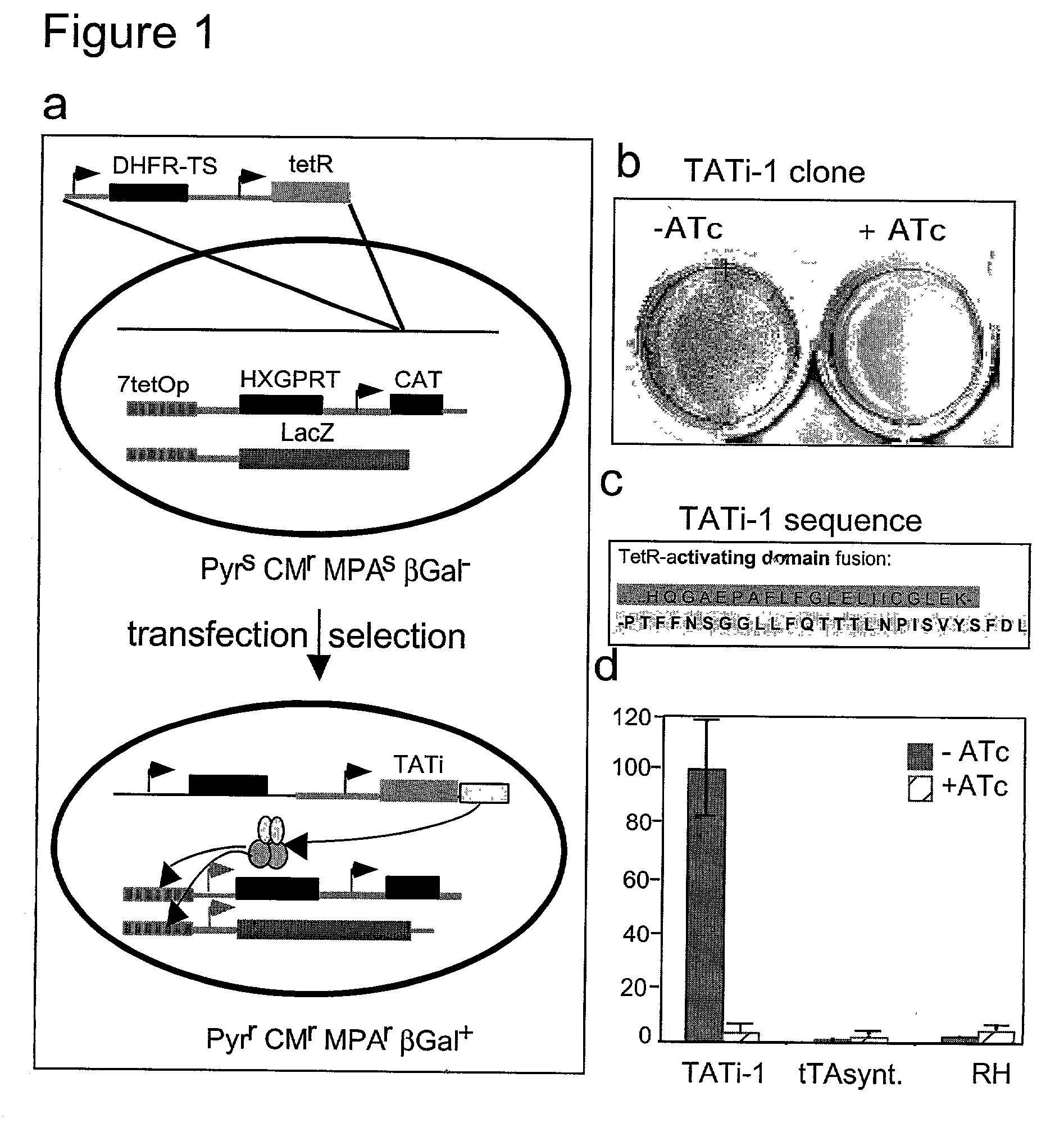
InactiveUS20030185851A1Prevent normal gene functionDisrupting functionOrganic active ingredientsProtozoa antigen ingredientsBiotechnologyTetR
A transcriptional activator of T. gondii is provided which comprises the tetracycline repressor (TetR) operatively linked to a transacting factor of T. gondii. Strains of T. gondii transformed with a vector containing such a transactivator may be used to prepare vaccine compositions or to identify essential genes in the parasite. The system provided may be useful in other Apicomplexan species such as Plasmodium falciparum, Plasmodium vivax, Plasmodium berghei, Plasmodium yoelii, Plasmodium knowlesi, Trypanosoma brucei, Entamoeba histolytica, and Giardia lambia.
Owner:IMPERIAL INNOVATIONS LTD
Immunoassay and diagnostic reagent for malaria
InactiveUS20040132117A1Effective diagnosisIncrease probabilityBiological material analysisDepsipeptidesEscherichia coliProtozoa
The present invention relates to an immunoassay and diagnostic reagent for malaria by using antigens of malarial Protozoa. More preferably, the present invention relates to an immunoassay and diagnostic reagent for malaria which detect malaria-specific antibodies in blood by using Merozoite Surface Protein of Plasmodium vivax. The immunoassay and diagnostic reagent detecting malaria-specific antibodies in blood according to the present invention have high specificity and sensitivity and are useful in diagnosing a type of malaria where latent period is long and number of Protozoa in blood if few. Also, the present invention relates to a preparation method of the surface protein of malarial Protozoa using yeast or E. Coli. Preferably, the present invention provides an expression vector comprising genes of Merozoite Surface Protein of Plasmodium Vivax and histidine residues, as well as transformants transformed with the expression vector. Also, the present invention provides a method for preparing Merozoite Surface Protein of malarial Protozoa by using the transformant. The surface protein Merozoite Surface Protein of malarial Protozoa prepared from yeast or E. Coli transformant according to the present invention has high sensitivity and specificity to antibody as well as high purity. Also, the surface protein prepared by the preparation method of the present invention has markedly low pseudo-positive signals, and is useful in diagnosing malaria.
Owner:LG CHEM INVESTMENT LTD
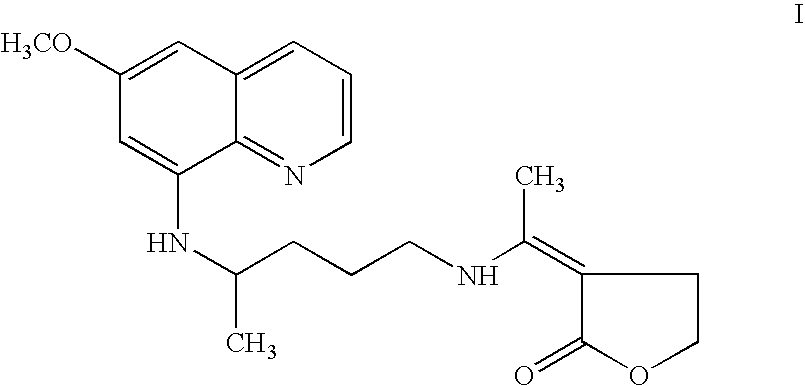
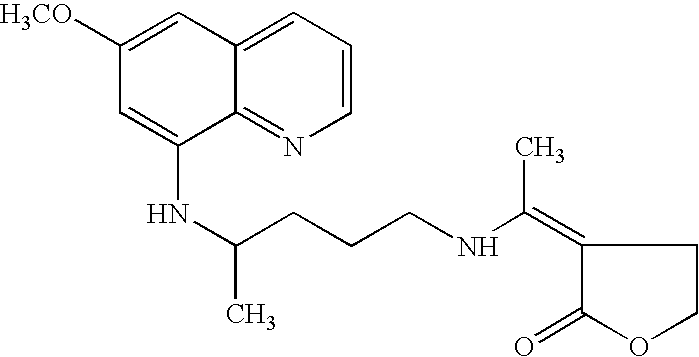
Combination kit used in the treatment of malaria
A combination kit for the treatment of malaria caused by Plasmodium vivax (P. vivax) having individual doses of an anti-malarial agent, 3-[1-[[4-[(6-methoxy-8-quinolinyl)amino]pentyl]amino]ethylidene]-dihydro-2(3H)-furanone (I) in the form of capsules; individual doses of the anti-malarial agent, chloroquine in the form of tablets; and instruction material for the administration of the two anti-malarial drugs. The combination kit is particularly suited for a 6 days treatment regimen where the treatment is rendered by five tablets containing 500 mg of chloroquine phosphate (equivalent to 300 mg base), three to be taken on day one and one each on days two and three; and five capsules containing 25 mg of 3-[1-[[4-[(6-methoxy-8-quinolinyl)amino]pentyl]amino]ethylidene]-dihydro-2(3H)-furanone (I), one each to be taken on days two to six.
Owner:NICHOLAS PIRAMAL INDIA LTD +1
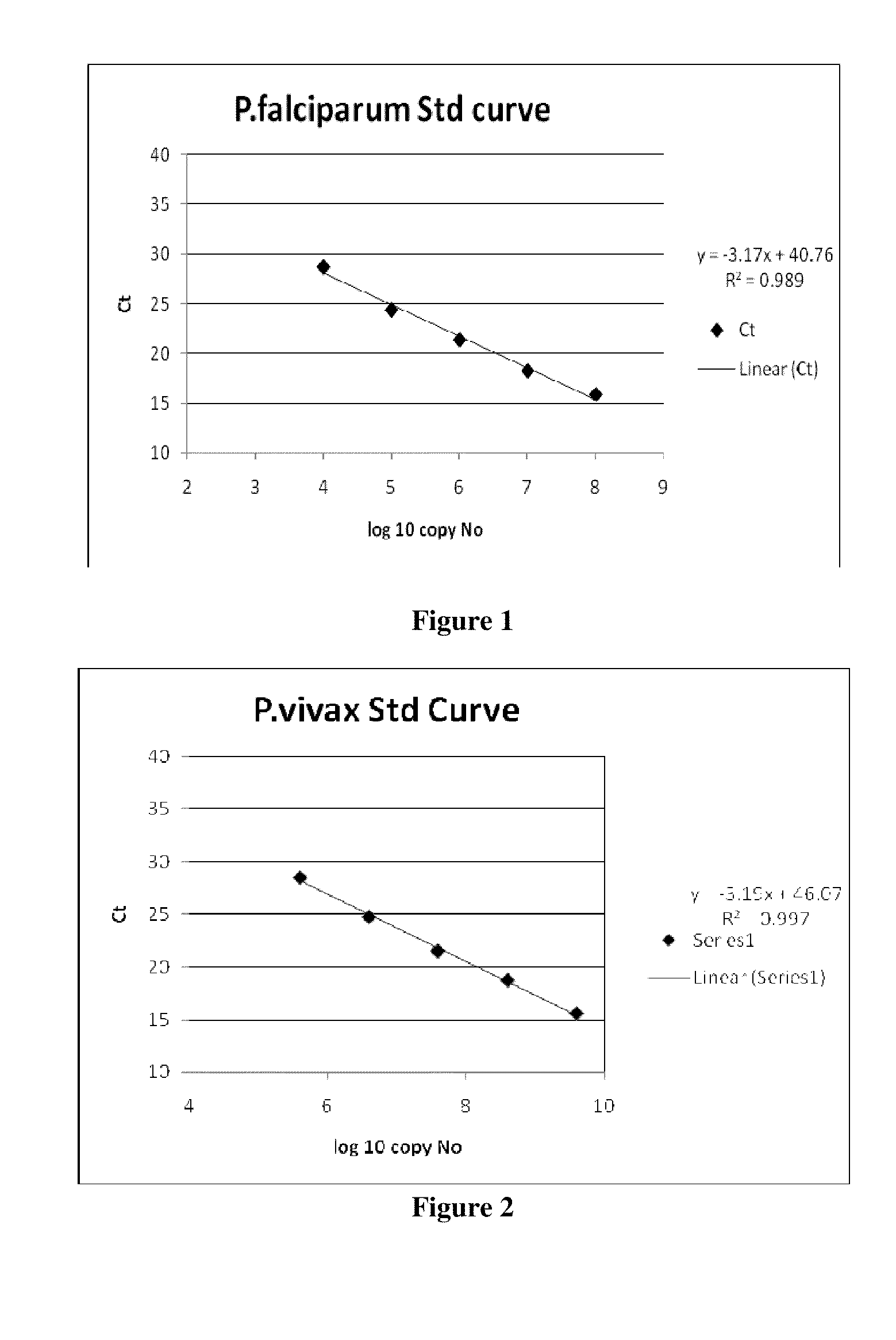
Probes and Primers for Detection of Malaria
InactiveUS20110306046A1Fluorescent signal enhancementSugar derivativesMicrobiological testing/measurementBiologyMalarial infection
The present disclosure gives description of a method used for the detection and quantification of malarial infection caused either by Plasmodium falciparum or Plasmodium vivax using nucleic acids isolated from blood samples by employing Oligonucleotide probes. The method employed here for detection is by Real time PCR.
Owner:BIGTEC PTE LTD
Vaccines for blocking transmission of plasmodium vivax
InactiveUS7407658B2Avoid spreadingEnhanced antigenic propertyImmunoglobulinsAntibody medical ingredientsMalariaMalarial parasites
Owner:UNITED STATES OF AMERICA
Conjugates of plasmodium falciparum surface proteins as malaria vaccines
InactiveUS20100183678A1Effective vaccineImprove the level ofCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsMedicineMalarial parasites
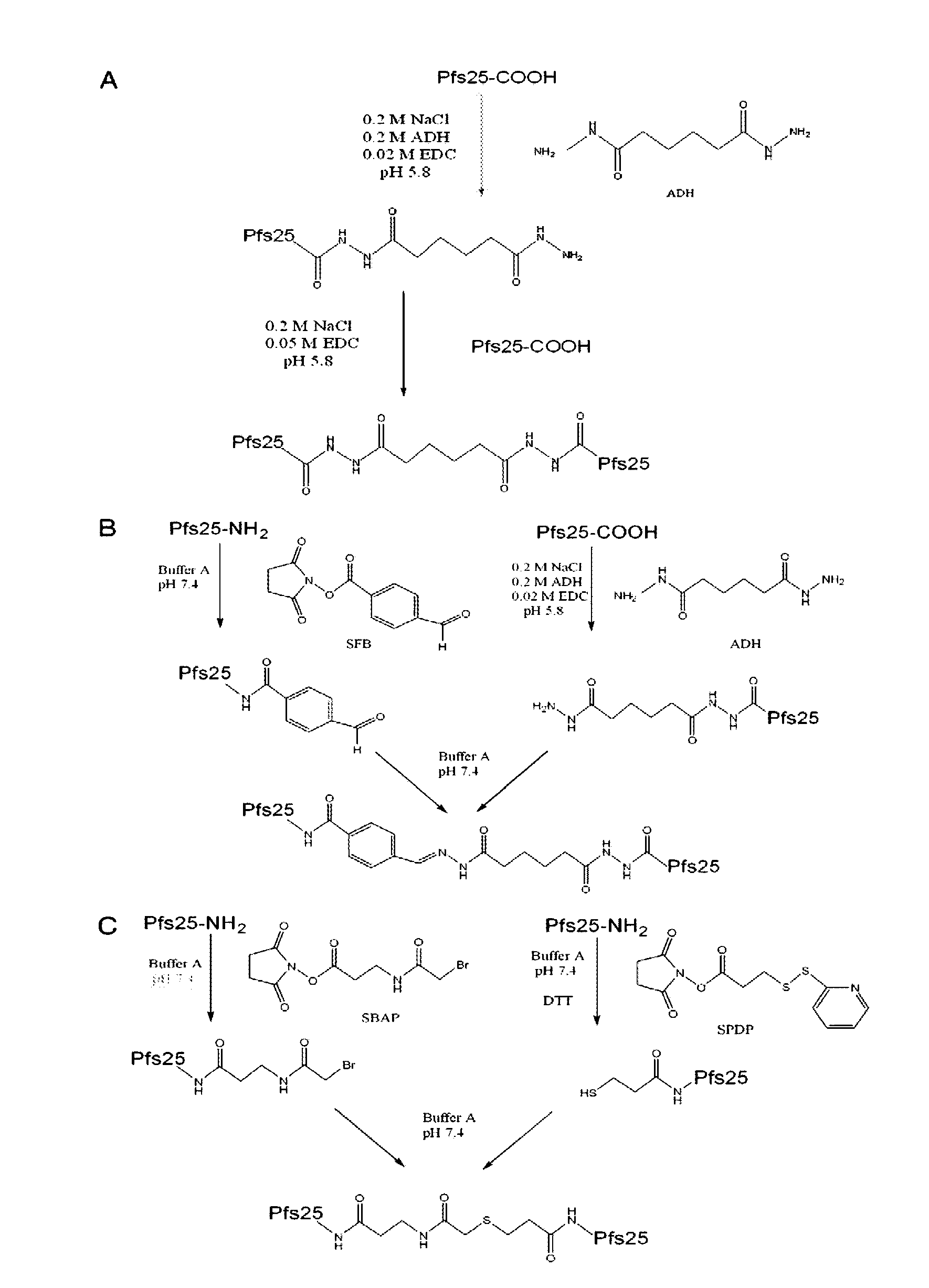
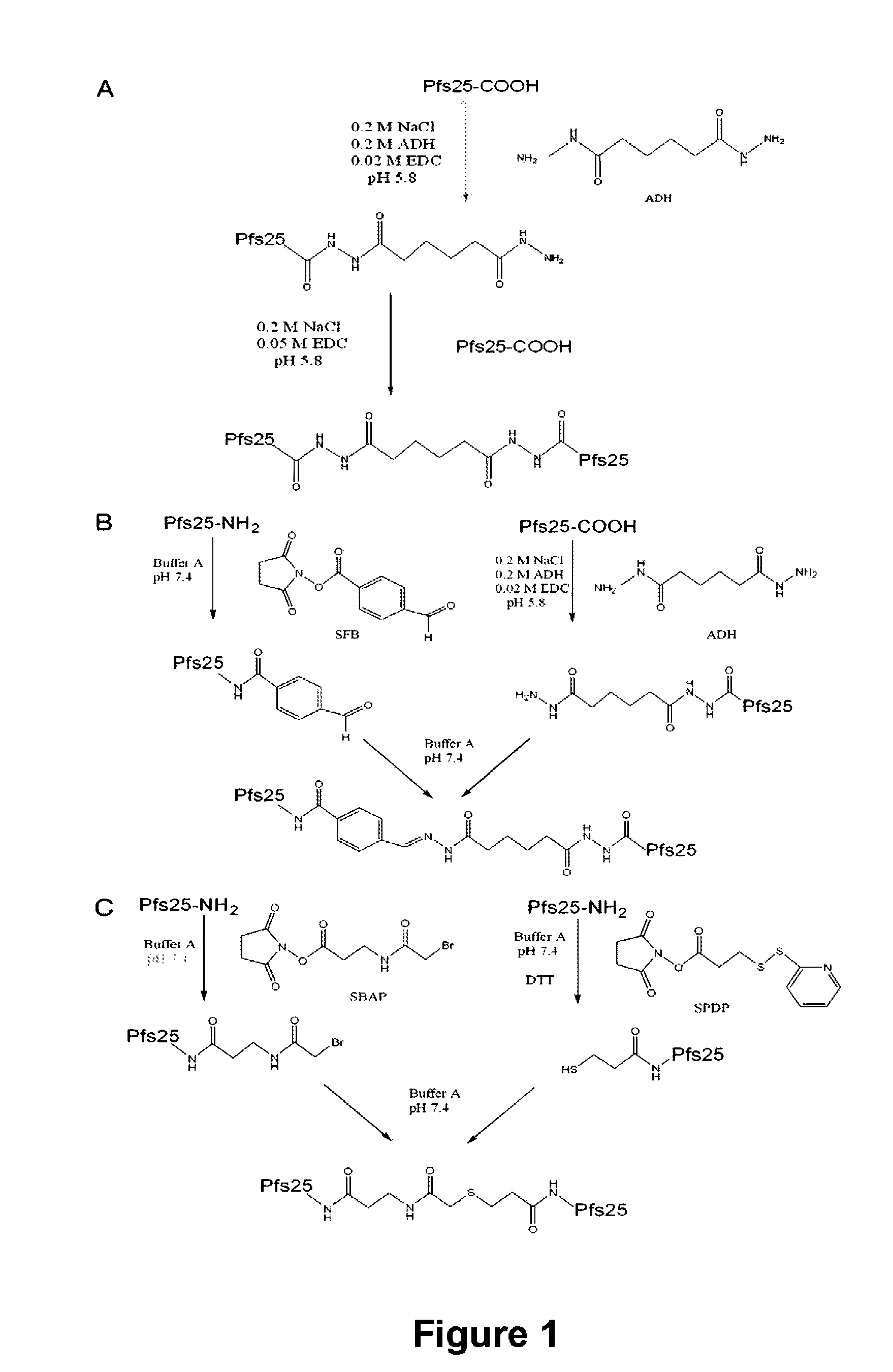
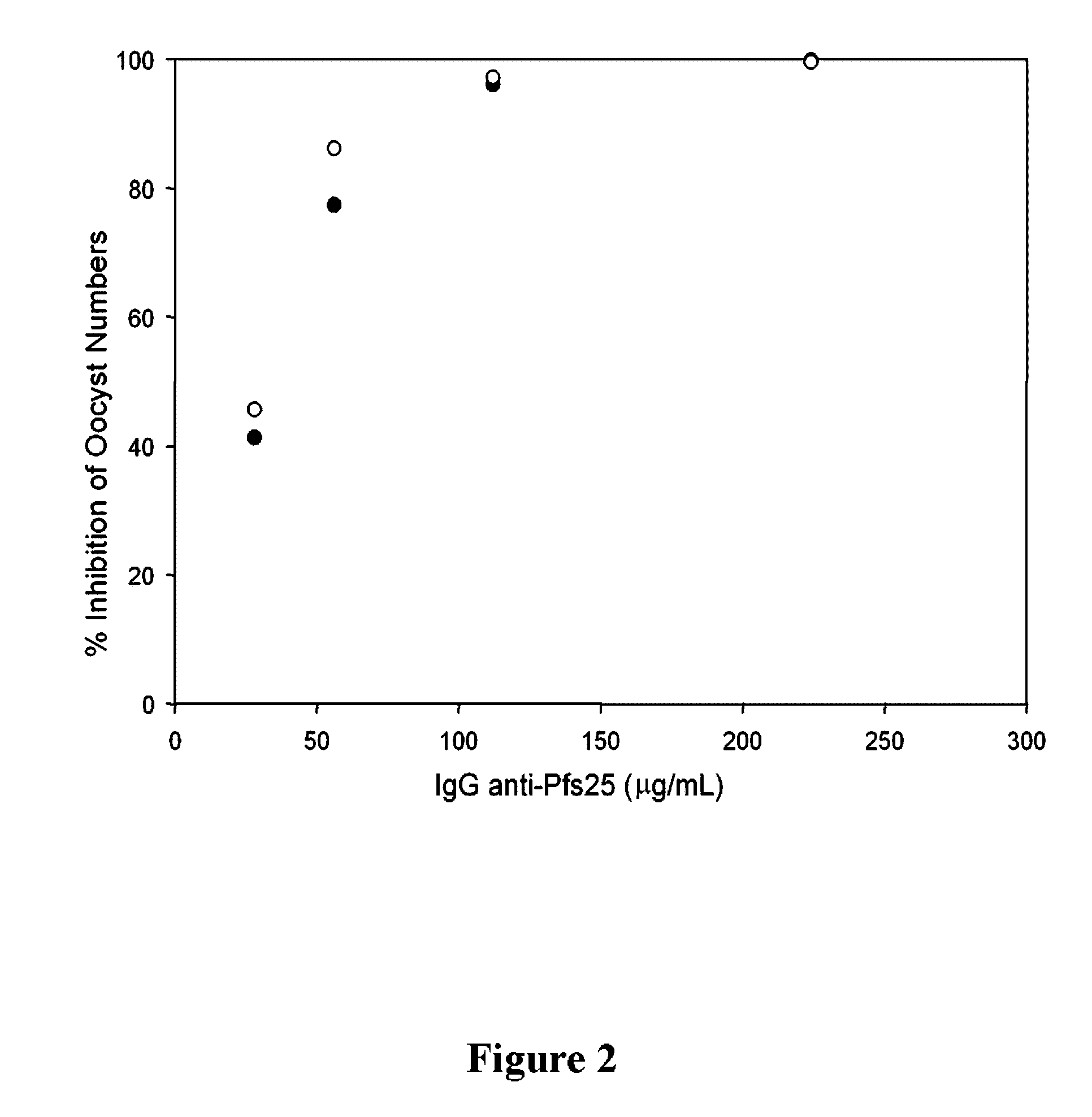
Conjugates of ookinete surface protein Pfs25 are provided that are efficacious as vaccines against Plasmodium falciparum, the most severe form of malaria. Conjugates of ookinete surface protein Pvs25 for use as a vaccine against Plasmodium vivax are also provided. Methods for preparing the conjugates, which comprise the ookinete surface protein bound onto itself or onto another protein by a linking group, are also provided.
Owner:UNITED STATES OF AMERICA
Probes and primers for detection of malaria
The present disclosure gives description of a method used for the detection and quantification of malarial infection caused either by Plasmodium falciparum or Plasmodium vivax using nucleic acids isolated from blood samples by employing oligonucleotide probes. The method employed here for detection is by real time PCR.
Owner:BIGTEC PTE LTD
Vaccines for blocking transmission of Plasmodium vivax
InactiveUS7192934B1Avoid spreadingEnhanced antigenic propertyBiocideProtozoa antigen ingredientsMalariaOrganism
The present invention relates to novel methods and compositions for blocking transmission of Plasmodium vivax which cause malaria. In particular, Pvs25 and Pvs28 polypeptides, variants, including deglycosylated forms, and fusion proteins thereof, are disclosed which, when administered to a susceptible organism, induce an immune response against a 25 kD and 28 kD protein, respectively, on the surface of Plasmodium vivax zygotes and ookinetes. This immune response in the susceptible organism can block transmission of malaria.
Owner:GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES THE
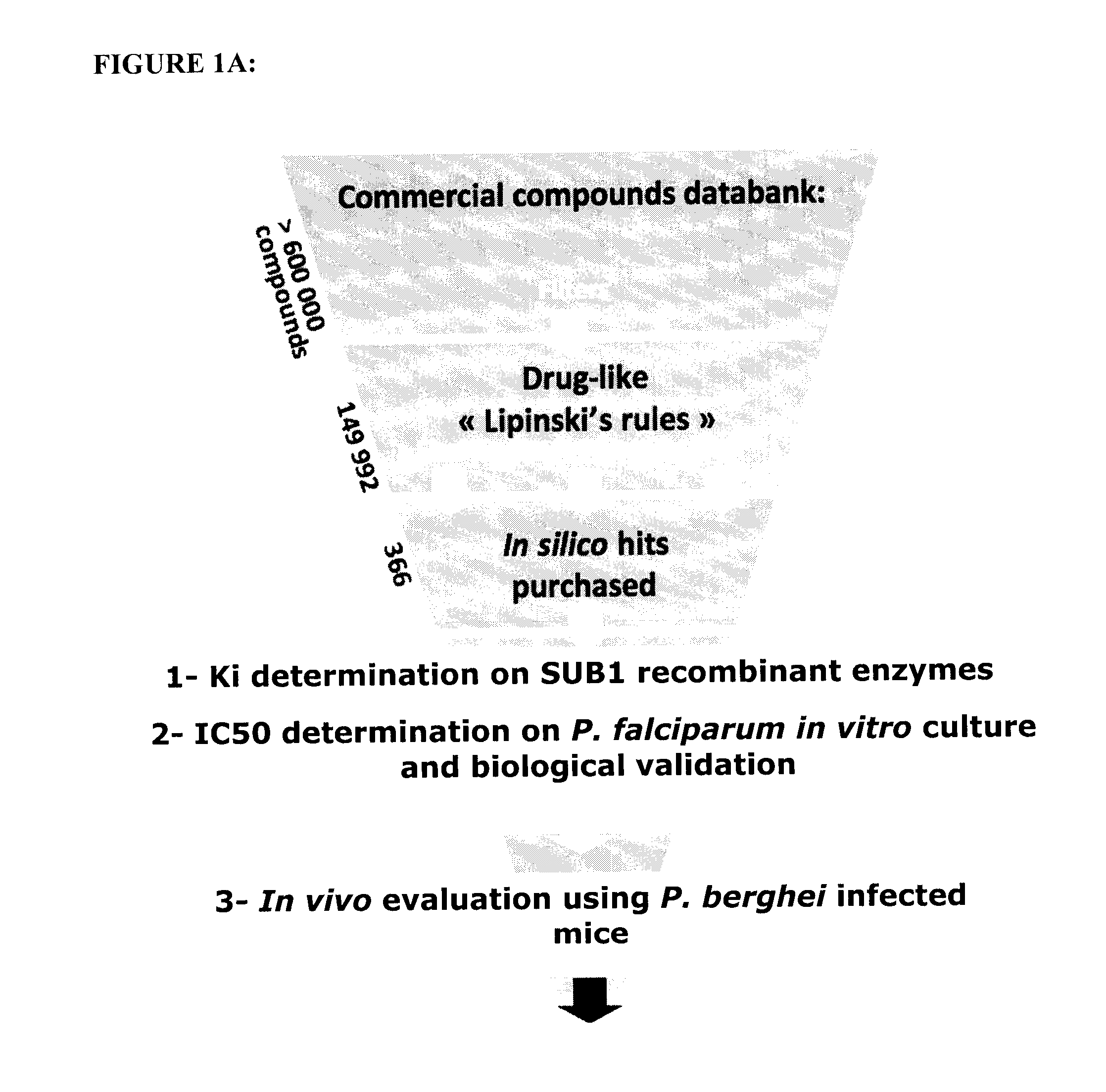
Screening methods for identifying plasmodium proteases inhibitors
ActiveUS20140134657A1Maximize efficacyRisk minimizationSugar derivativesPeptide/protein ingredientsPlasmodium wenyoniScreening method
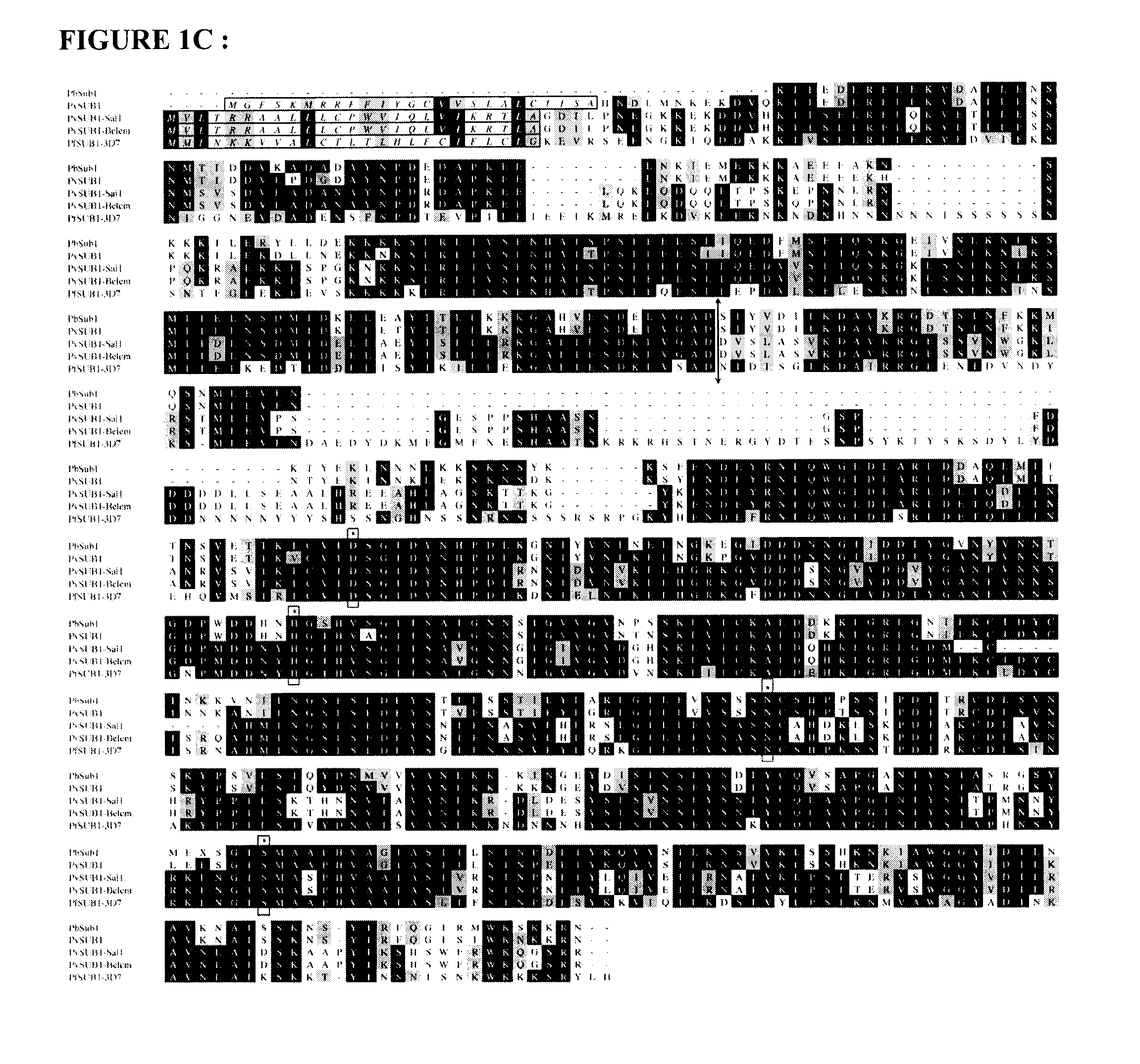
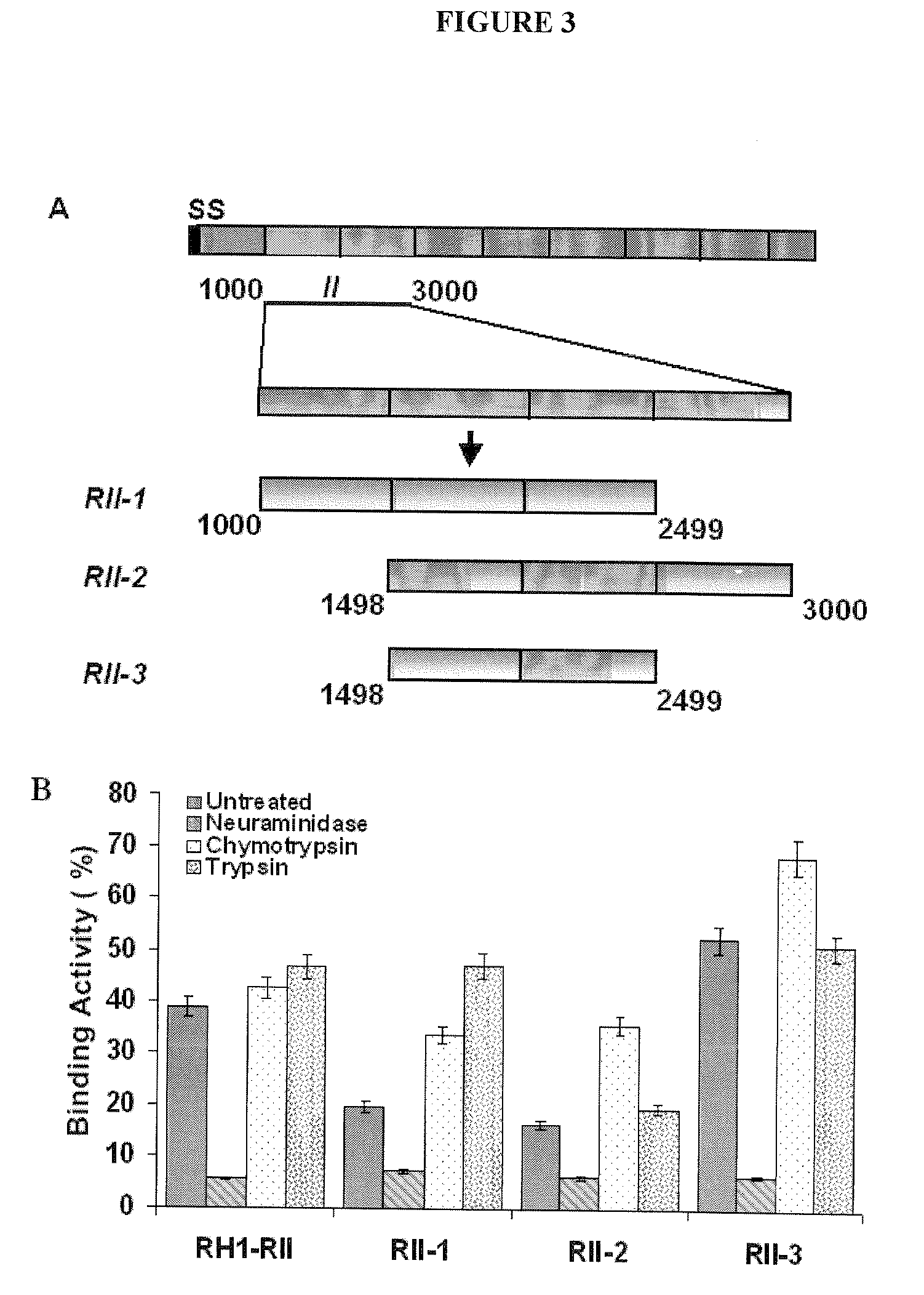
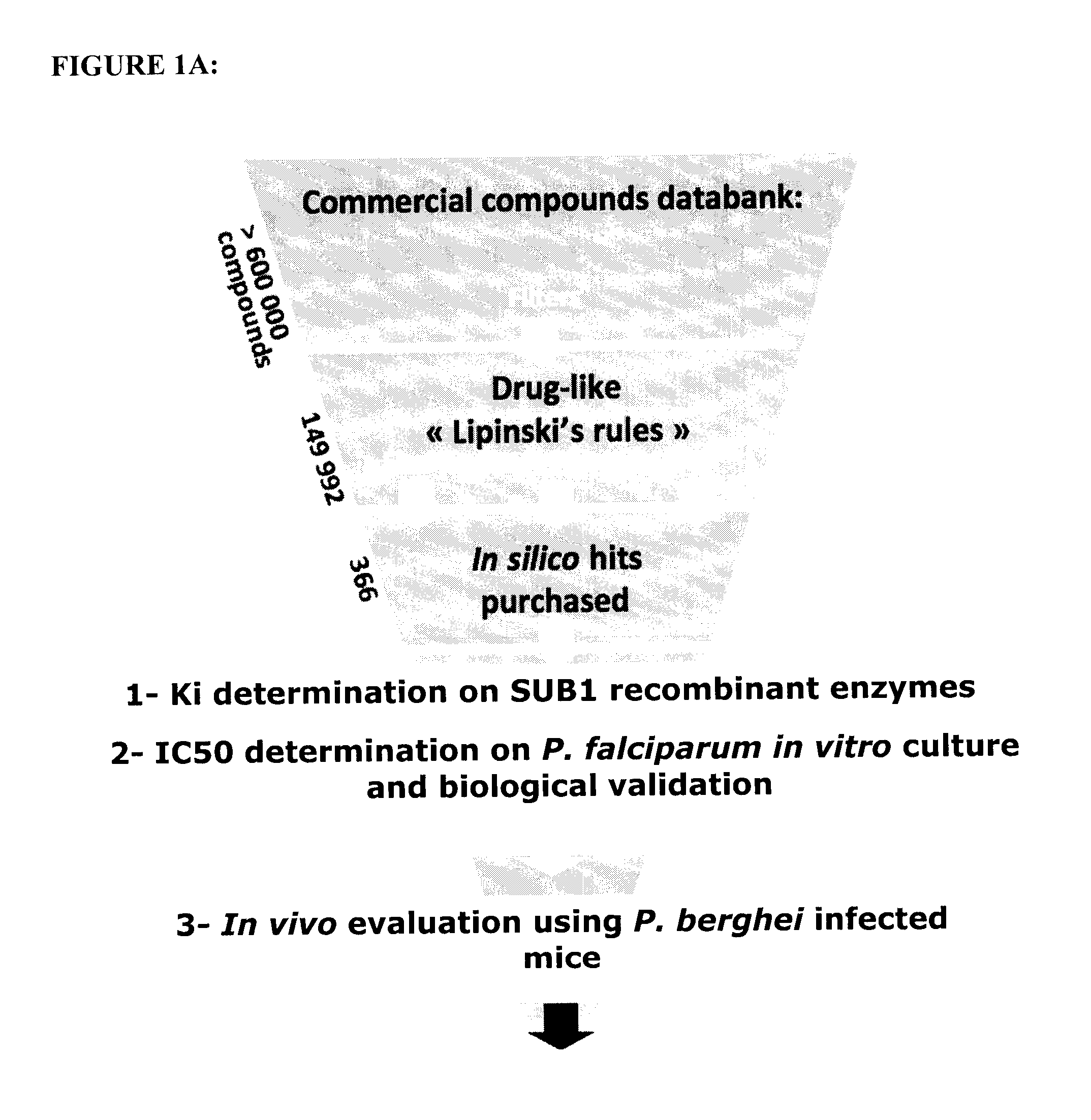
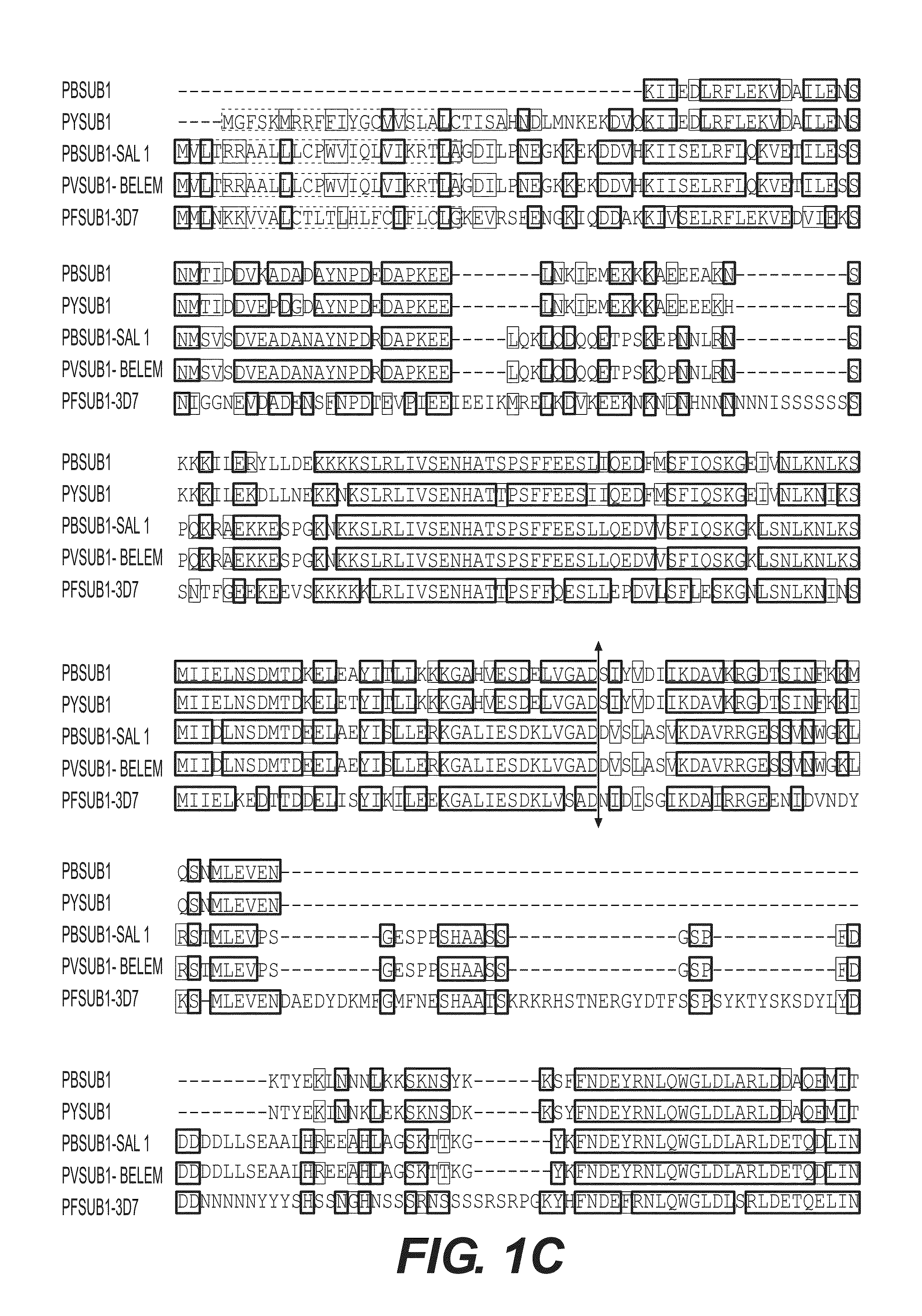
The invention relates to the field of parasitology. Methods and peptidic substrate for screening and identifying inhibitors of Plasmodium are described. Also described are compounds identified by these screening methods, including more particularly inhibitors of Plasmodium subtilisin-like proteases. The invention also concerns anti-malaria compounds, anti-malaria compositions, and uses thereof for preventing, treating, improving, and / or alleviating a Plasmodium infection in a subject, and more Plasmodium vivax and / or by Plasmodium falciparum human infections.
Owner:INST PASTEUR
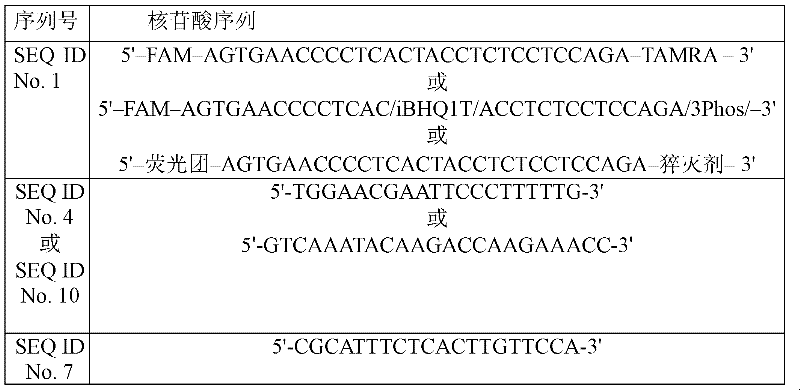
Binding protein combination for detecting plasmodium falciparum HRP2 and plasmodium vivax LDH and preparation method and application thereof
ActiveCN110343161AHigh affinityGood storage stabilityBiological material analysisDepsipeptidesFhit geneGenetic engineering
The invention discloses a binding protein combination for detecting plasmodium falciparum HRP2 and plasmodium vivax LDH and a preparation method and application thereof. The binding protein combination is composed of a binding protein 1 and a binding protein 2 which detect the plasmodium falciparum HRP2 and a binding protein 3 and a binding protein 4 which detect the plasmodium vivax LDH, and amino acid sequences of the binding protein 1, binding protein 2, binding protein 3 and binding protein 4 are shown in SEQ ID NO:5-8. According to the binding protein combination and the preparation method and application thereof, a phage display technology is adopted, the plasmodium falciparum HRP2 and the plasmodium vivax LDH are taken as target antigens, and the binding protein combination with high affinity and good specificity is selected from a phage display protein library. A genetic engineering technology is used for recombinant expression and purification, a binding protein combination reagent replaces an antibody reagent for malaria immunodetection, a colloidal gold chromatography rapid detection method is established, the storage stability of an obtained kit is higher than that of an existing clinical detection product, and the binding protein combination has the advantages of being reliable, accurate, safe, simple, convenient and stable.
Owner:JINAN UNIVERSITY +1
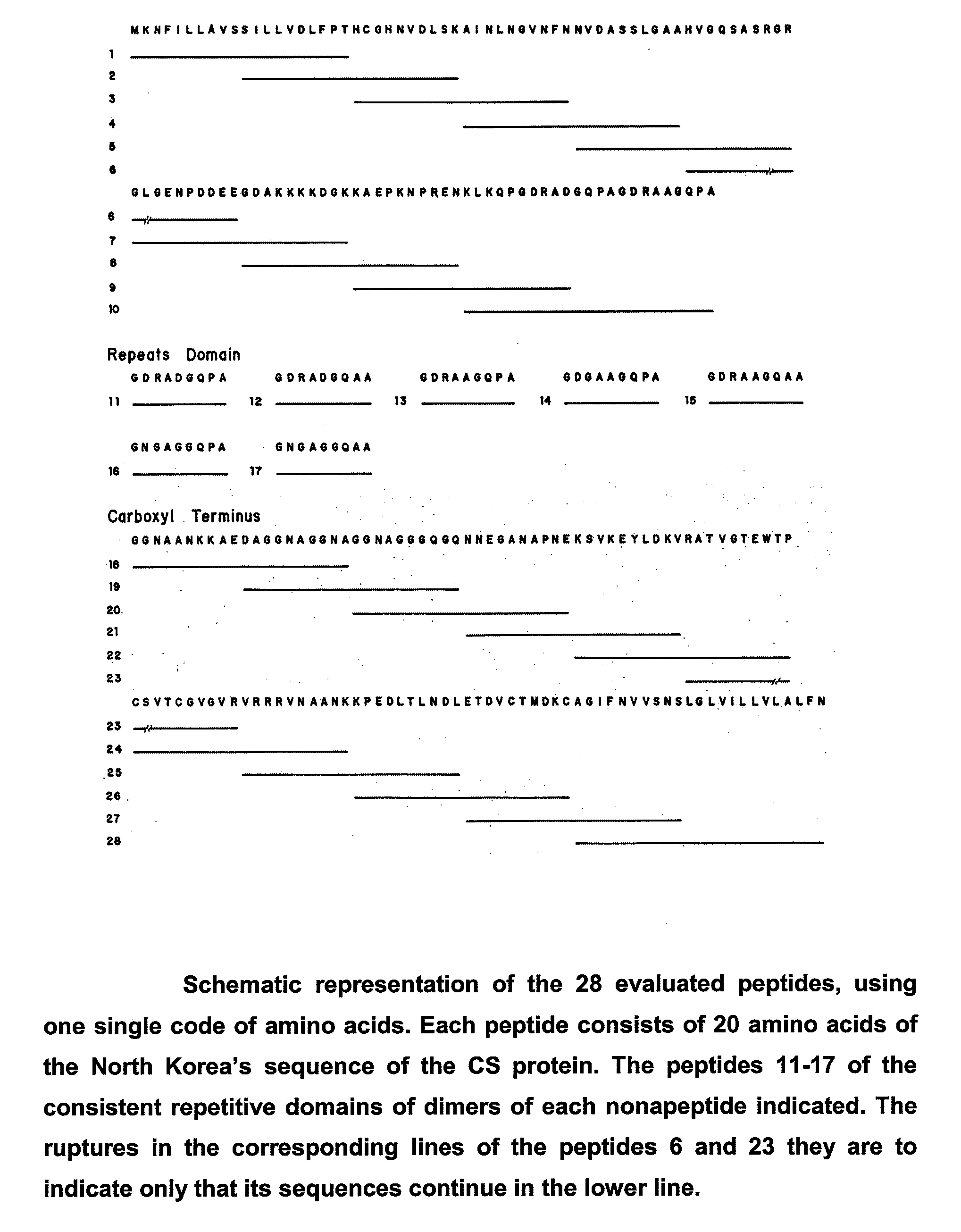
Malaria vaccine based on fragments and combination of fragments of the cs protein of plasmodium vivax
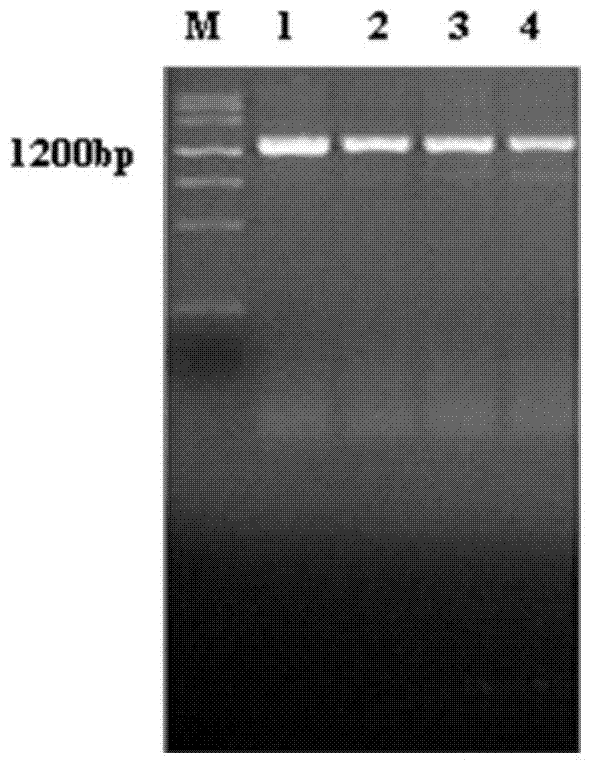
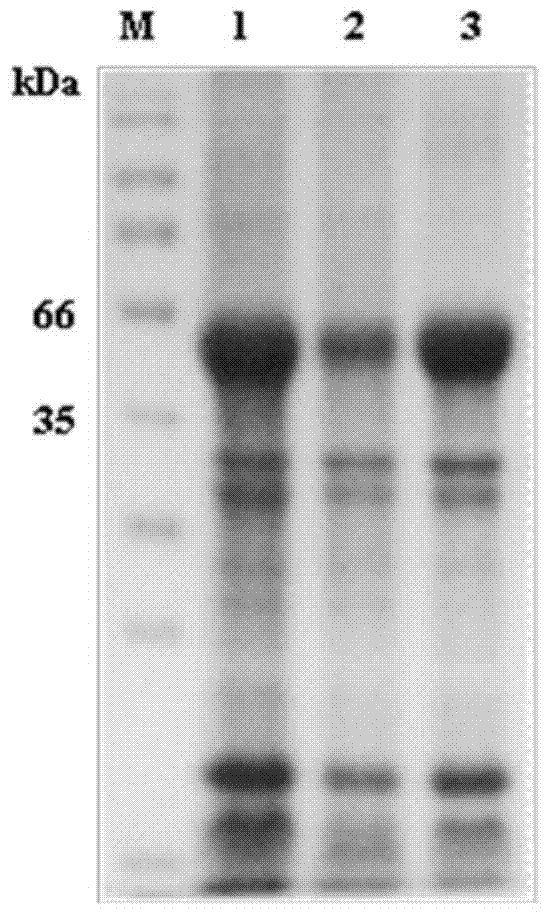
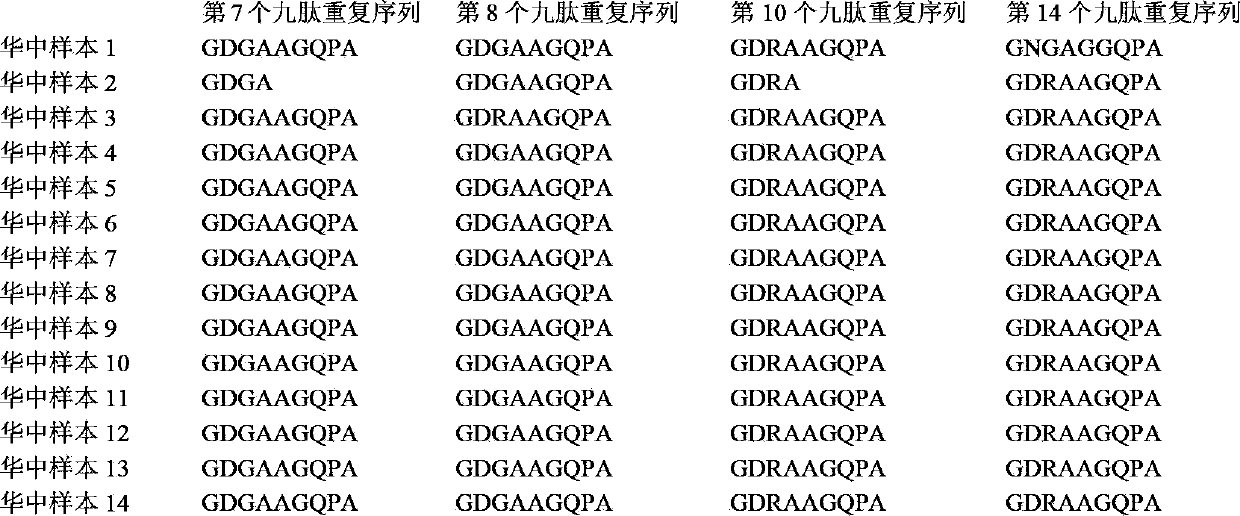
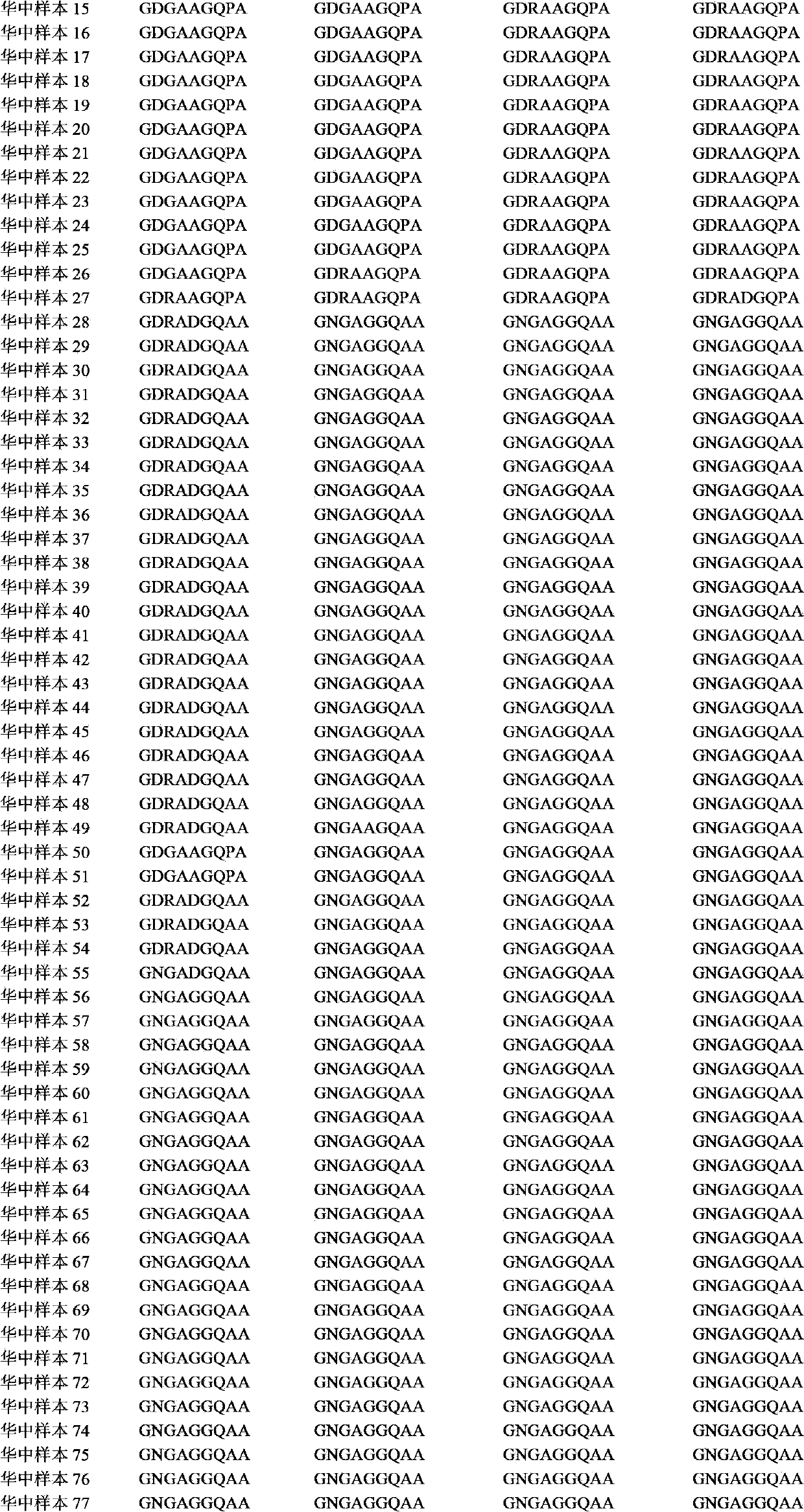
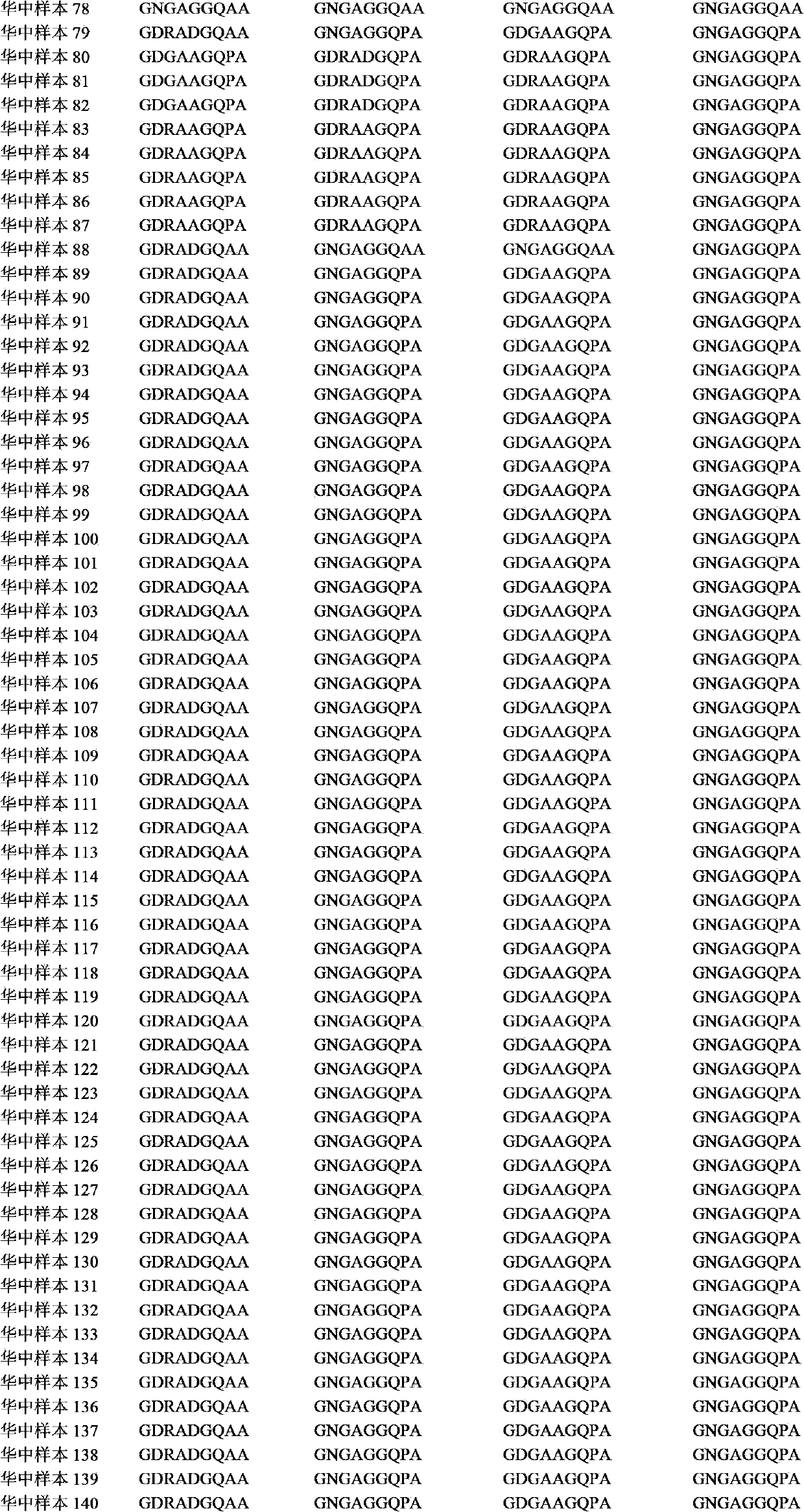
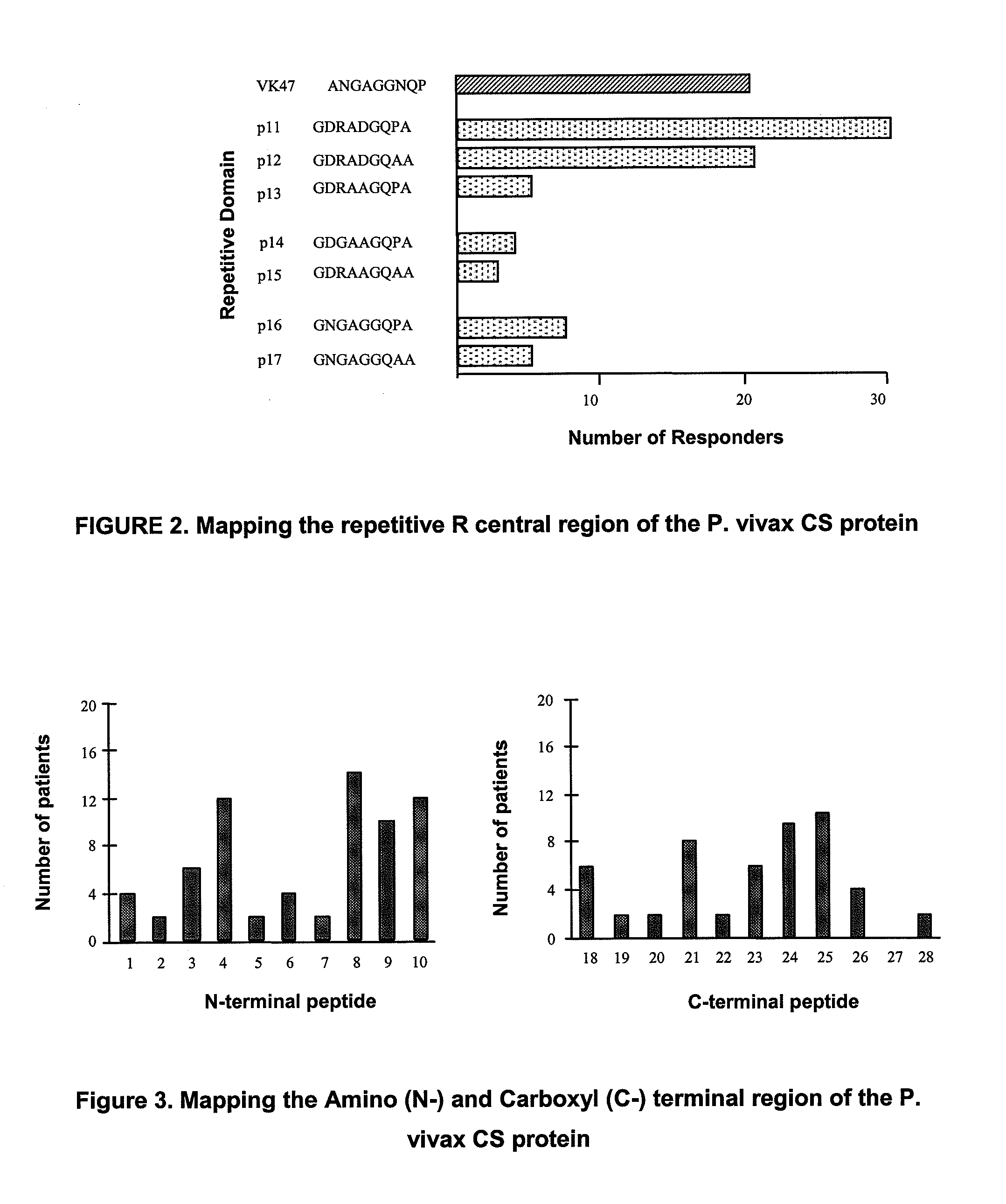
The invention relates to a recombinant or synthetic polypeptide characterised in that it includes at least three consecutive repetitions of nonapeptide A N G A G X1 Q X2 X3, in which X1 is selected from D and N, X2 is selected from P and A and X2 is selected from G and A. The inventive polypeptide also preferably includes at least two (2) consecutive repetitions of sequence GDRADGQPA and in an even more preferable embodiment the polypeptide includes an amino-terminal region, a C-terminal region and / or the ptt30 fragment. The invention also relates to malaria vaccines characterised in that they include said peptides.
Owner:CENT INT DE VACUNAS
Vaccine against malaria, based on the 200l subuniti of plasmodium vivax msp1 protein
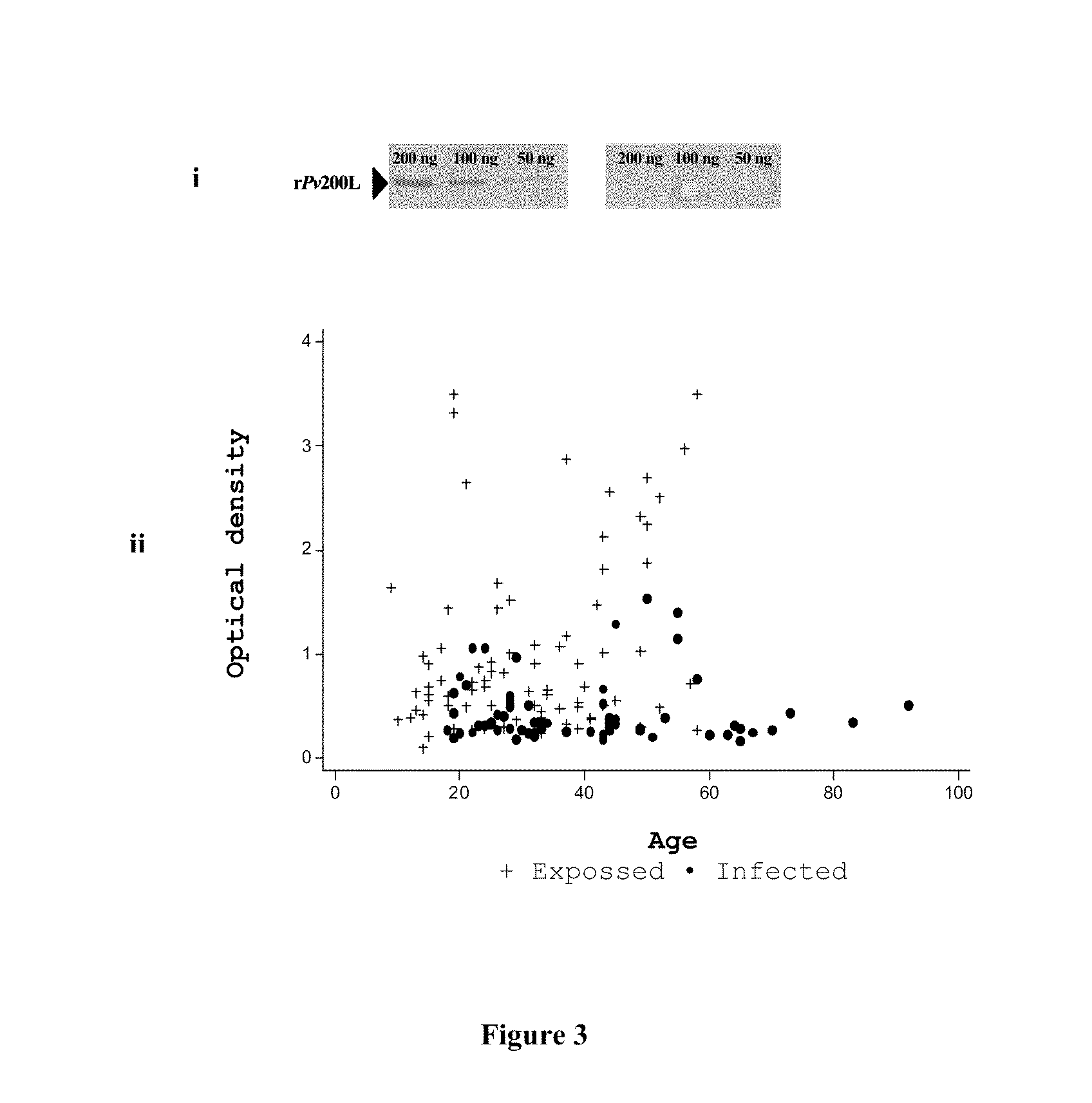
A candidate subunit for a vaccine against malaria caused by P. vivax, known as Pv200L, which is based on N-terminal end portions of the P. vivax MSP-1 protein is disclosed. The subunit is designed for use alone or in formulations, combined with other subunits. The production of recombinant prototypes of the subunit and the design of a production process that can be scaled up for mass production thereof is also disclosed.
Owner:CENT INT DE VACUNAS +1
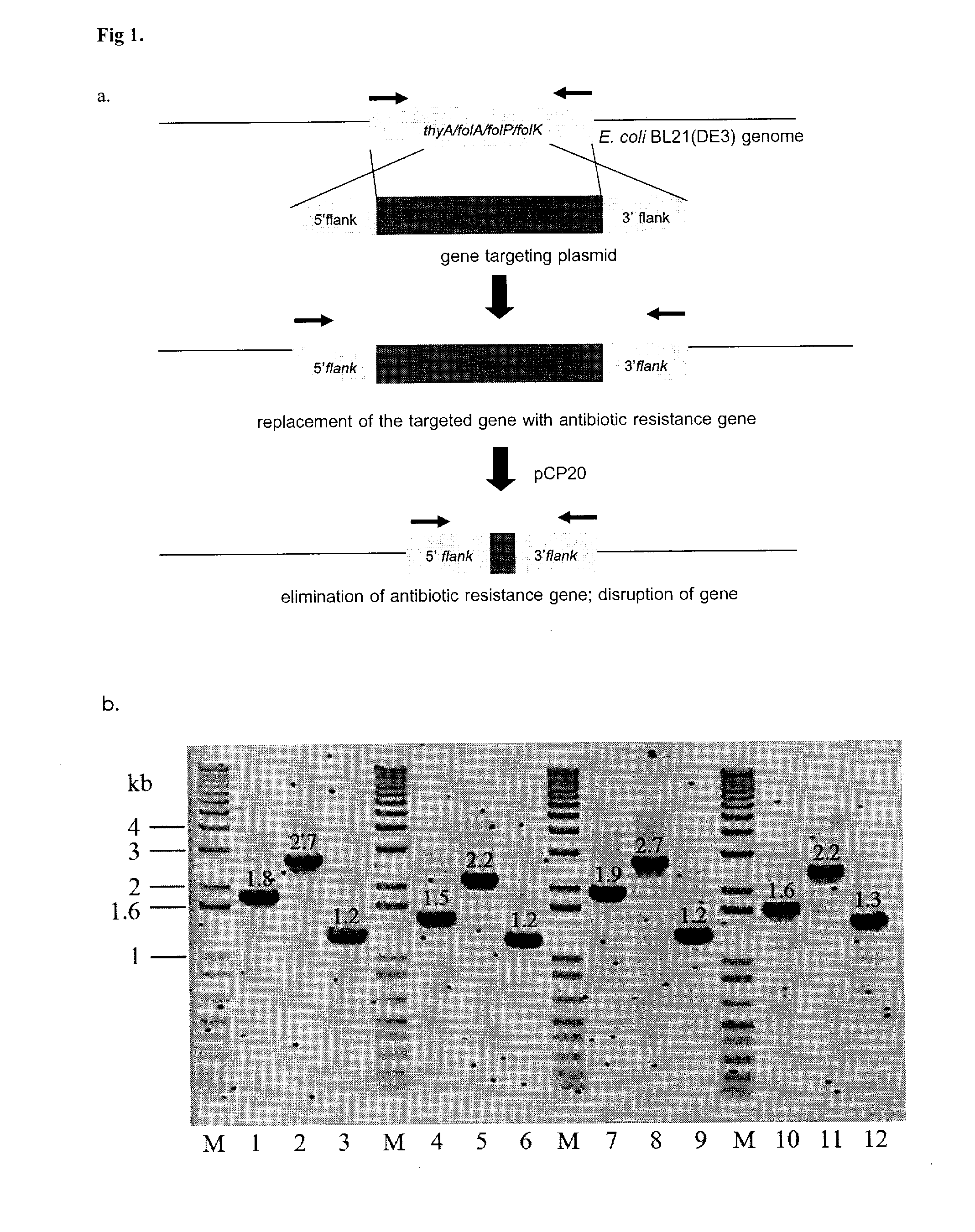
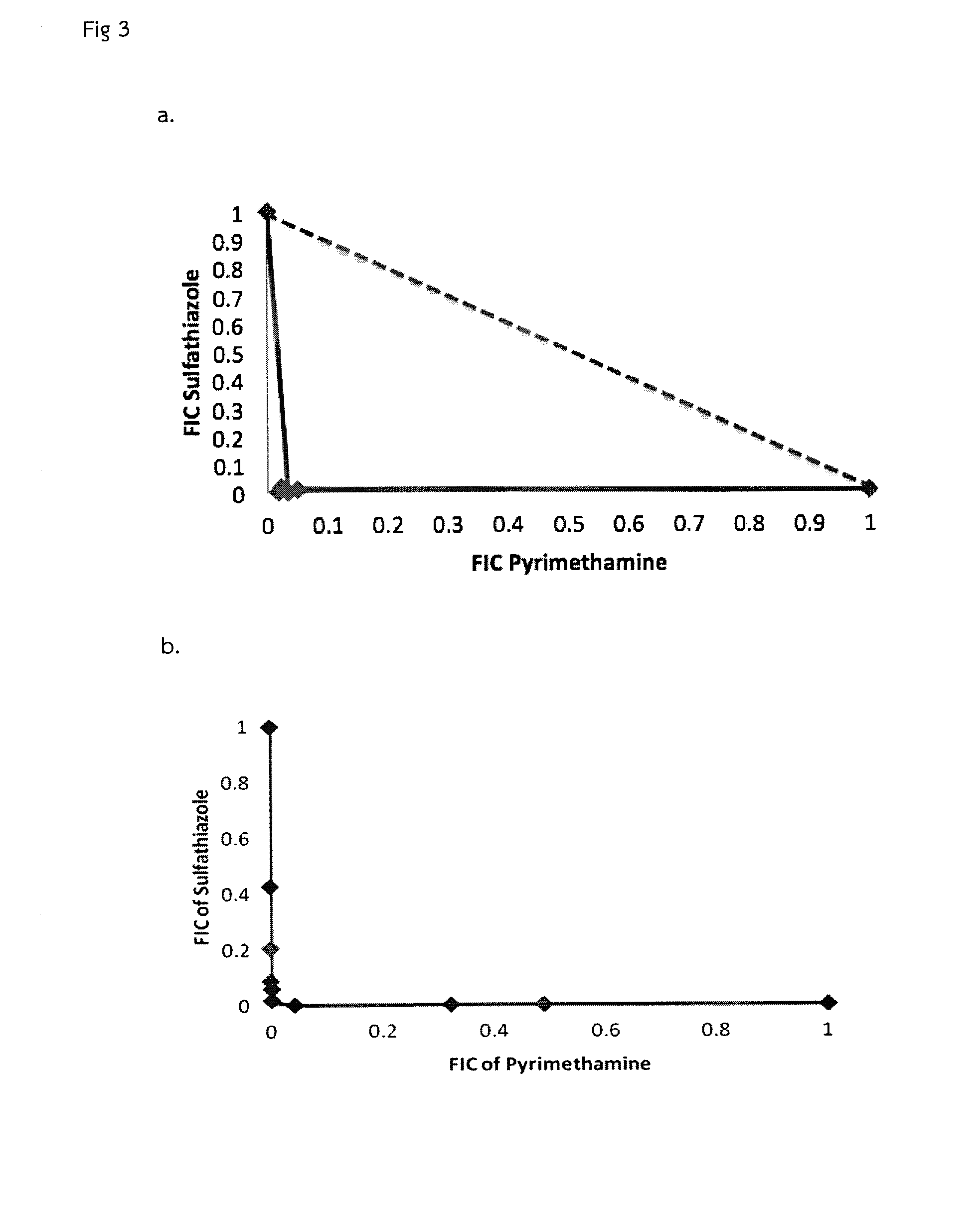
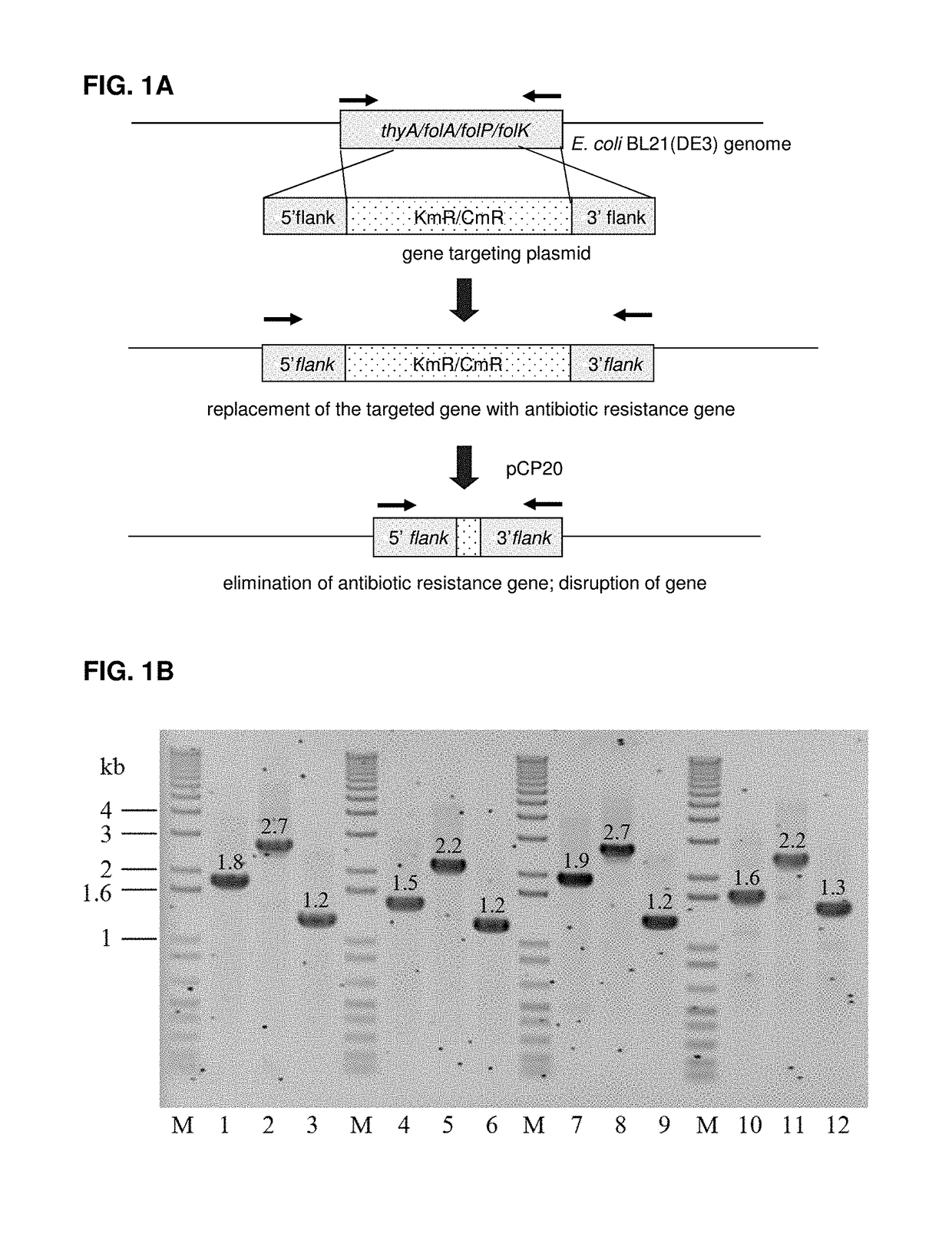
A bacterial surrogate for testing of antimalarials: thya knockout, fola knockout, folp knockout, and folk knockout bacteria for testing of inhibition of antifolate pathway
ActiveUS20160230178A1Microbiological testing/measurementBiological material analysisBacteroidesAntibiotic resistance
In this invention, cell lines are created for enzyme inhibitory testing of inhibitors against Plasmodium falciparum DHFR-TS and HPPK-DHPS. Provided the complementing DHFR-TS and HPPK-DHPS have sufficient activities to support growth of the surrogates in un-supplemented medium, the same surrogates could be used for screening inhibitors of targets against other parasite and pathogen species e.g. Plasmodium vivax, Trypanosoma brucei, Trypanosoma cruzi, Toxoplasma gondii or Mycobacterium tuberculosis. The cell lines in this invention are Escherichia coli strain whose thyA, folA, folK, and folP genes were disrupted using genetic knockout coupled with elimination of antibiotic resistance markers. The thyA KO, folP KO, folK KO, thyAfolA KO, folKfolP KO, thyAfolAfolP KO, thyAfolAfolK KO and thyAfolAfolKfolP KO E. coli cell lines are easy and convenient for testing single and combination drugs as plasmids bearing complementing parasite genes can be introduced simply by transformation using standard antibiotic selection.
Owner:NAT SCI & TECH DEV AGENCY
Escherichia coli cell line with thyA knockout, folA knockout, and one or both of folP knockout, and folK knockout
ActiveUS10011842B2Microbiological testing/measurementBiological material analysisAntibiotic resistanceDHPS
In this invention, cell lines are created for enzyme inhibitory testing of inhibitors against Plasmodium falciparum DHFR-TS and HPPK-DHPS. Provided the complementing DHFR-TS and HPPK-DHPS have sufficient activities to support growth of the surrogates in un-supplemented medium, the same surrogates could be used for screening inhibitors of targets against other parasite and pathogen species e.g. Plasmodium vivax, Trypanosoma brucei, Trypanosoma cruzi, Toxoplasma gondii or Mycobacterium tuberculosis. The cell lines in this invention are Escherichia coli strain whose thyA, folA, folK, and folP genes were disrupted using genetic knockout coupled with elimination of antibiotic resistance markers. The thyA KO, folP KO, folK KO, thyAfolA KO, folKfolP KO, thyAfolAfolP KO, thyAfolAfolK KO and thyAfolAfolKfolP KO E. coli cell lines are easy and convenient for testing single and combination drugs as plasmids bearing complementing parasite genes can be introduced simply by transformation using standard antibiotic selection.
Owner:NAT SCI & TECH DEV AGENCY
Vaccines for blocking transmission of plasmodium vivax
InactiveUS20080274132A1Enhanced antigenic propertyEasy to manufactureProtozoa antigen ingredientsPeptide/protein ingredientsMalariaOrganism
The present invention relates novel methods and compositions for blocking transmission of Plasmodium vivax which cause malaria. In particular, Pvs25 and Pvs28 polypeptides, variants, including deglycosylated forms, and fusion proteins thereof, are disclosed which, when administered to a susceptible organism, induce an immune response against a 25 kD and 28 kD protein, respectively, on the surface of Plasmodium vivax zygotes and ookinetes. This immune response in the susceptible organism can block transmission of malaria.
Owner:THE GOV OF THE USA REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Use of a cysteine protease of plasmodium vivax
ActiveUS9677063B2Unusual pH-dependent substrate specificityHighly unique substrate preferencePeptide/protein ingredientsMicrobiological testing/measurementParasitic diseaseBiology
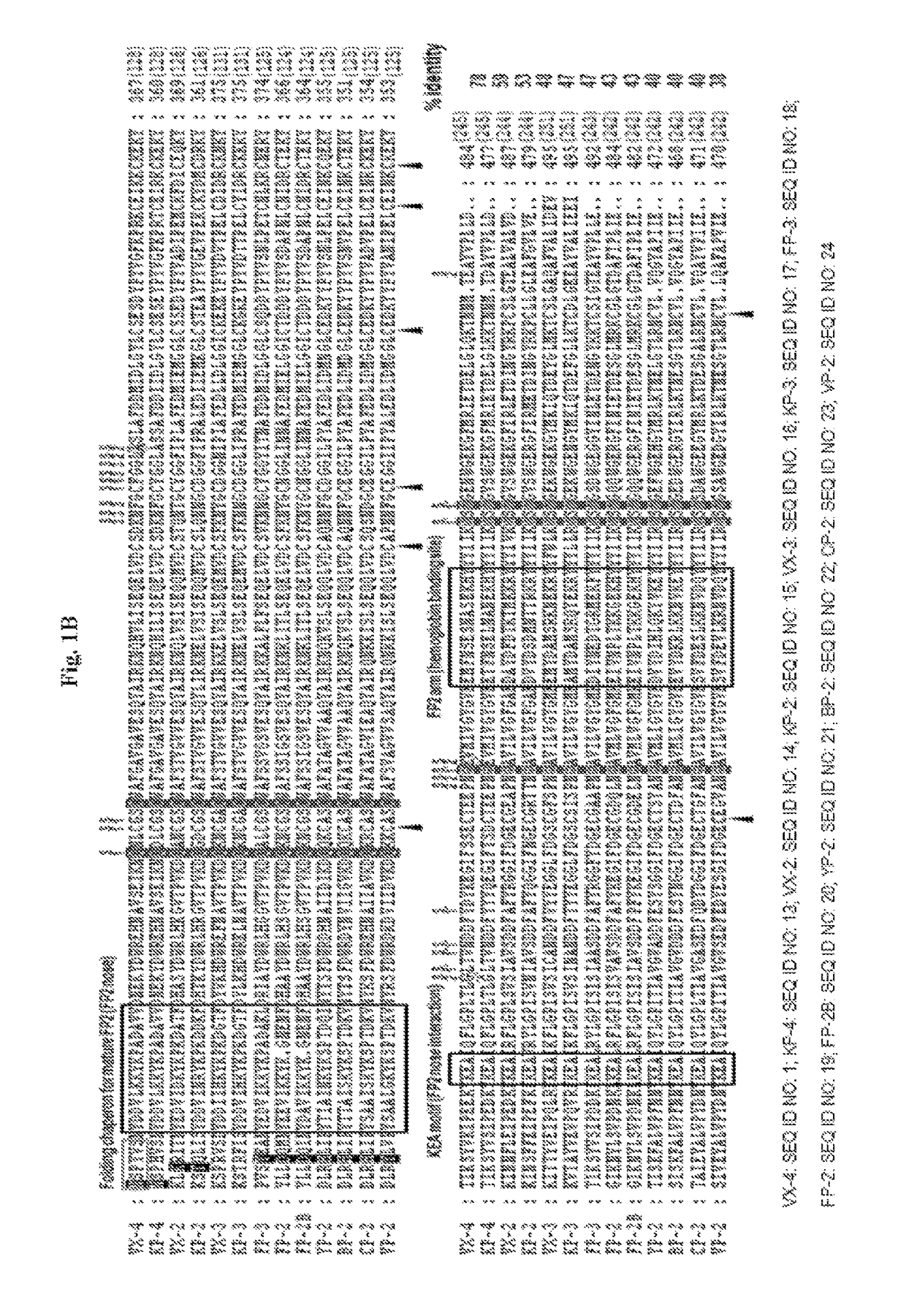
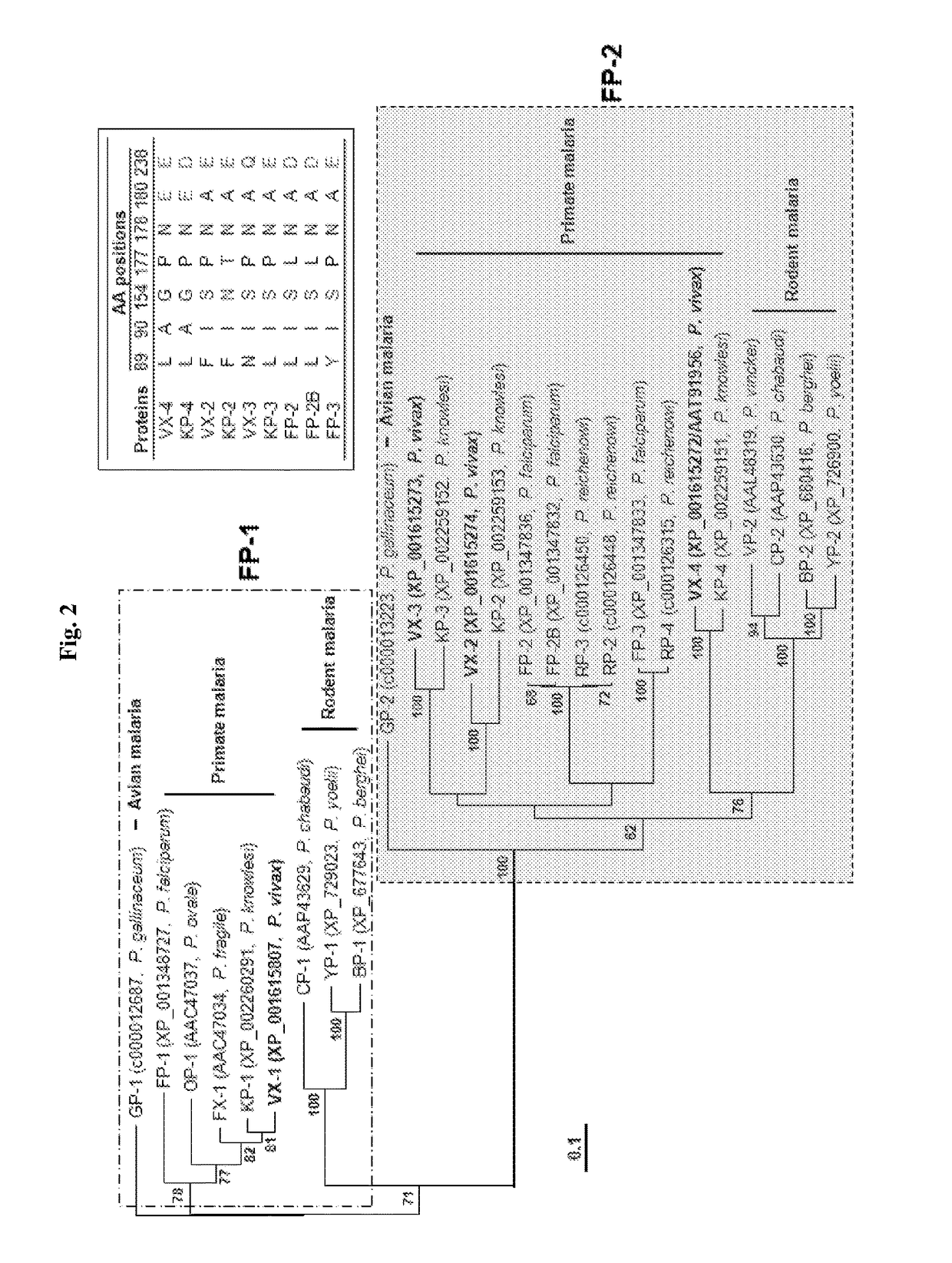
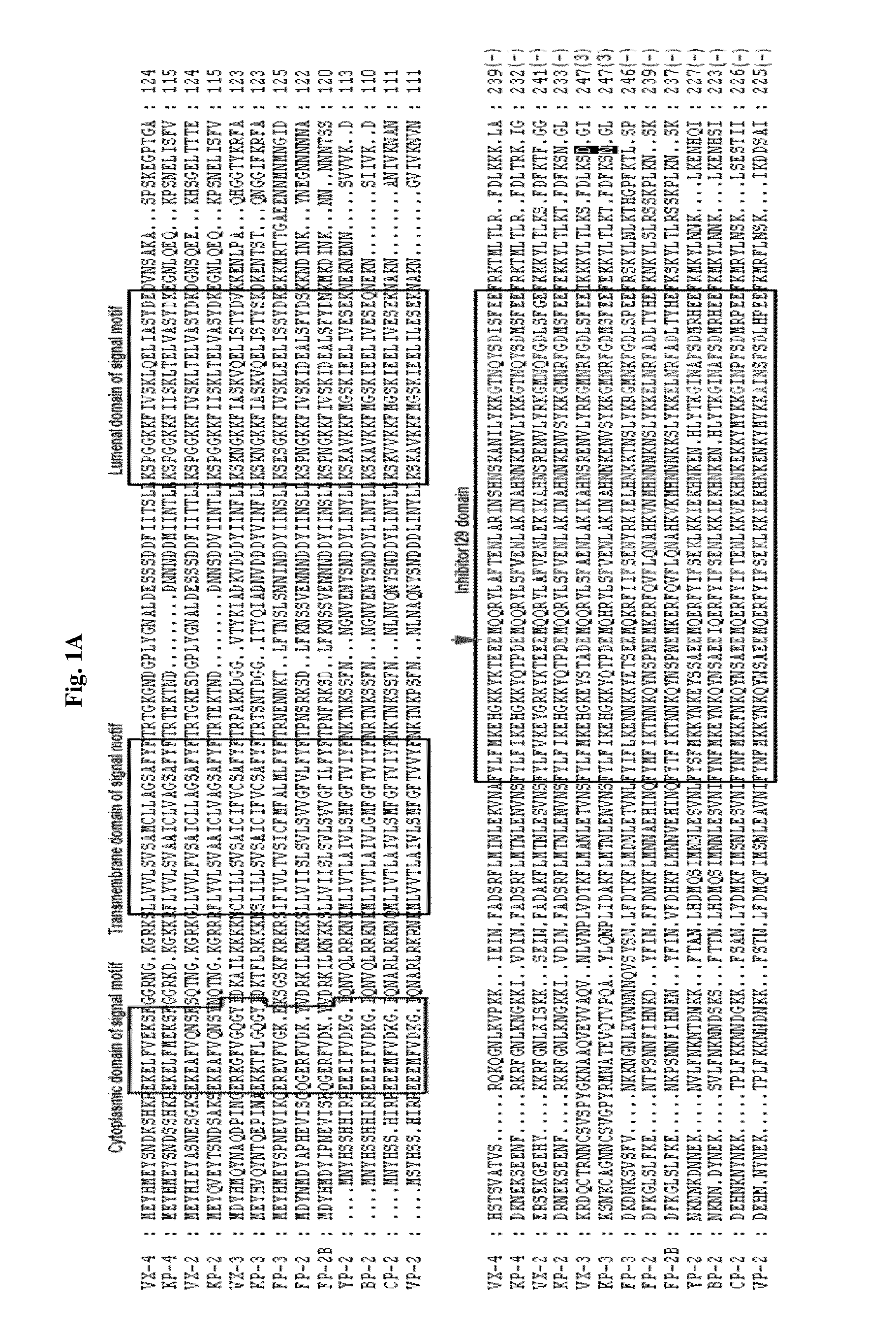
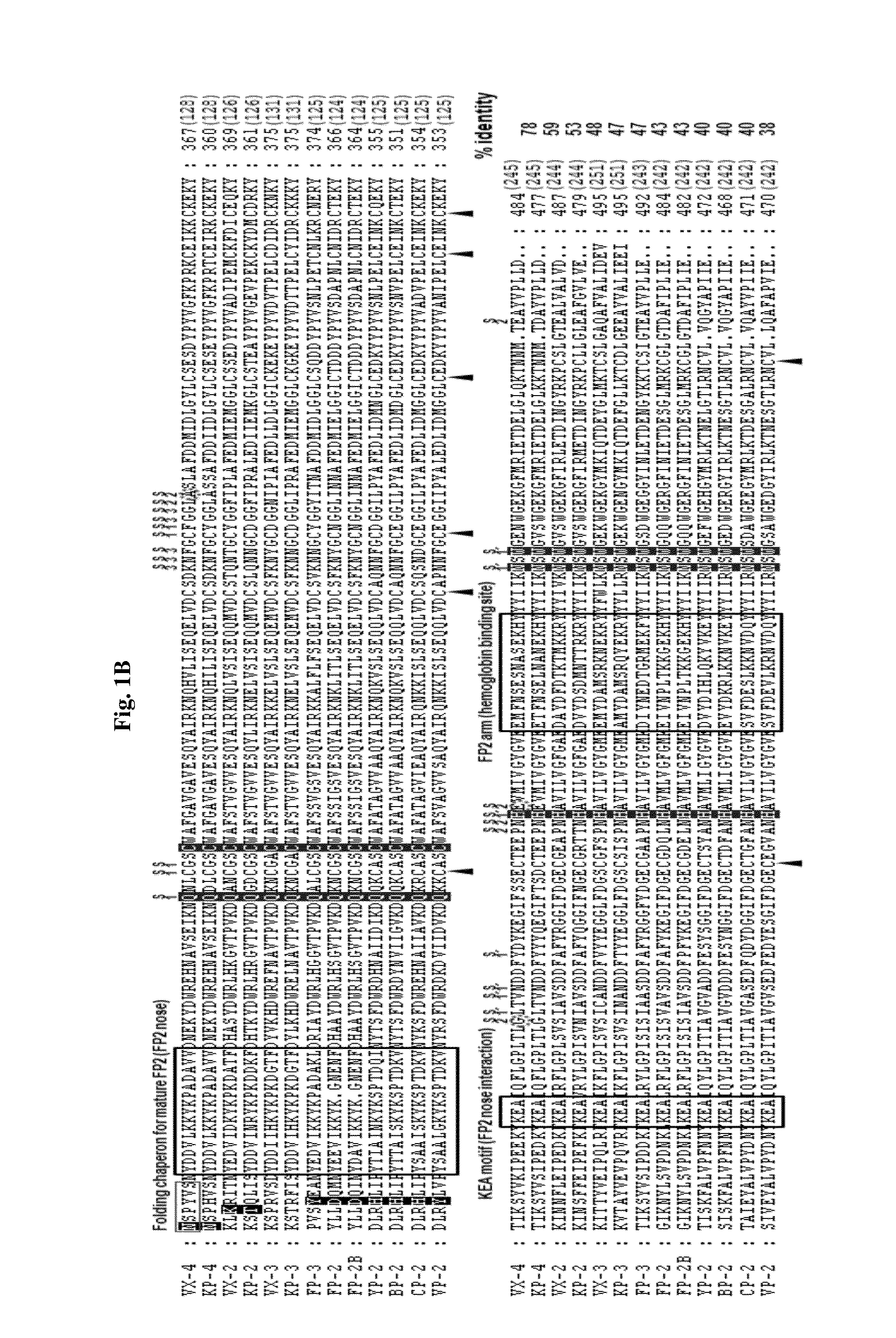
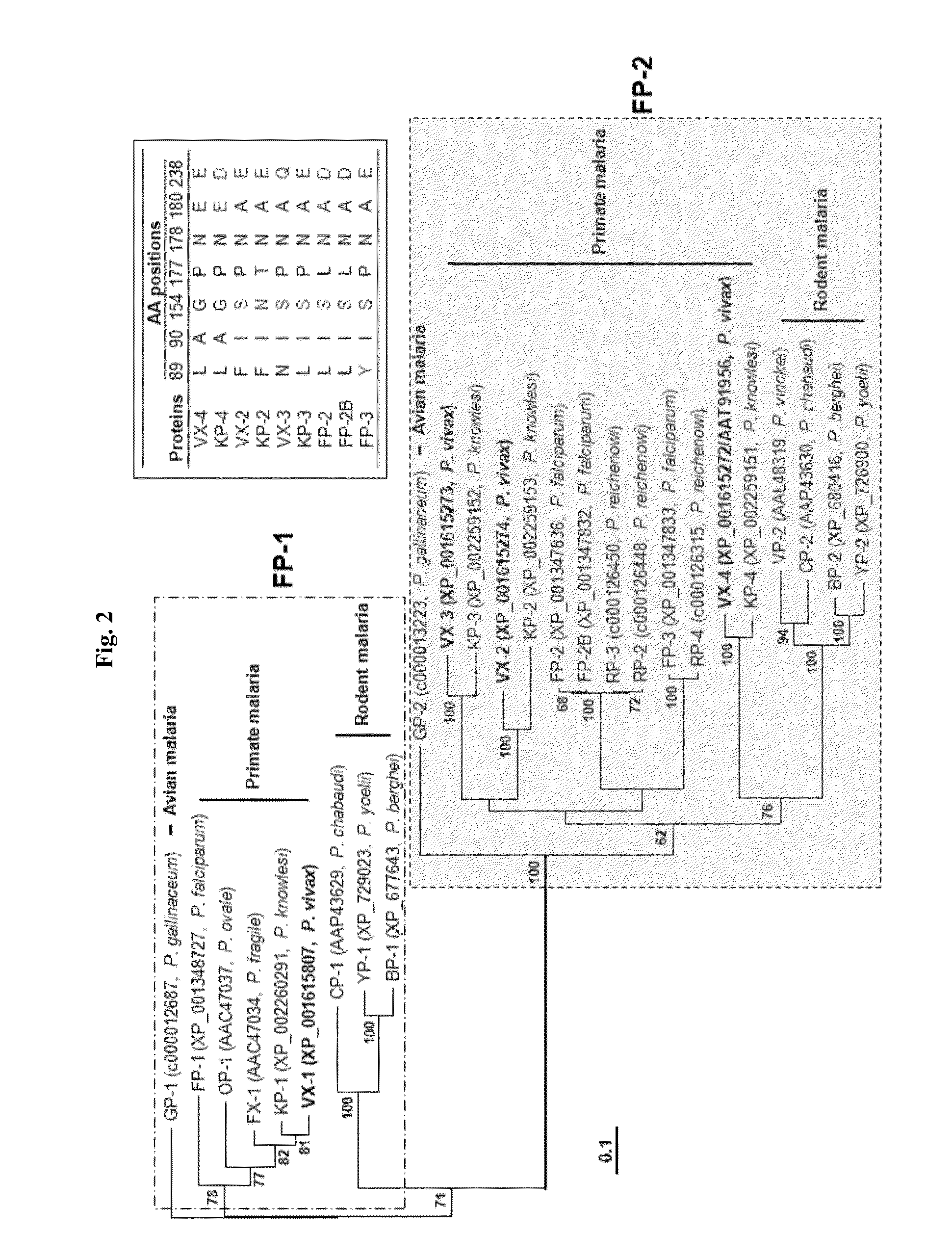
A use of vivapain-4 (VX-4), which is a cysteine protease of Plasmodium vivax, showing pH-dependent switching of substrate specificity, is provided. More specifically, a method of treating a parasitic disease caused by Plasmodium vivax by inhibiting VX-4; a method of screening a protease inhibitor acting on VX-4, wherein the protease inhibitor is useful as an anti-malarial agent acting on Plasmodium species, for example, Plasmodium vivax; and a method of identifying the activity of VX-4, are provided.
Owner:SAMSUNG ELECTRONICS CO LTD
Binding domain of Plasmodium reticulocyte binding proteins
ActiveUS8252293B2Efficient measurementBiocidePeptide/protein ingredientsErythrocyte bindingRecombinant vaccines
The present invention provides isolated polynucleotides, polypeptides, antibodies and / or vaccines for the prevention and / or treatment of malaria caused by Plasmodium falciparum and / or Plasmodium vivax. In particular, the polypeptide fragments are derived from the binding domain of the reticulocyte binding proteins of Plasmodium falciparum and / or Plasmodium vivax. The present invention also provides recombinant vaccines and their use in the prevention and / or treatment of malaria.
Owner:NANYANG TECH UNIV
Vaccines for blocking transmission of plasmodium vivax
InactiveUS20070110772A1Avoid spreadingEnhanced antigenic propertyGenetic material ingredientsImmunoglobulinsBiological bodyMalaria
The present invention relates novel methods and compositions for blocking transmission of Plasmodium vivax which cause malaria. In particular, Pvs25 and Pvs28 polypeptides, variants, including deglycosylated forms, and fusion proteins thereof, are disclosed which, when administered to a susceptible organism, induce an immune response against a 25 kD and 28 kD protein, respectively, on the surface of Plasmodium vivax zygotes and ookinetes. This immune response in the susceptible organism can block transmission of malaria.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Screening methods for identifying Plasmodium proteases inhibitors
ActiveUS9404142B2Risk minimizationMaximize efficacyMicrobiological testing/measurementDepsipeptidesPlasmodium wenyoniScreening method
The invention relates to the field of parasitology. Methods and peptidic substrate for screening and identifying inhibitors of Plasmodium are described. Also described are compounds identified by these screening methods, including more particularly inhibitors of Plasmodium subtilisin-like proteases. The invention also concerns anti-malaria compounds, anti-malaria compositions, and uses thereof for preventing, treating, improving, and / or alleviating a Plasmodium infection in a subject, and more Plasmodium vivax and / or by Plasmodium falciparum human infections.
Owner:INST PASTEUR
Use of a cysteine protease of Plasmodium vivax
ActiveUS20140370570A1Unusual pH-dependent substrate specificityHighly unique substrate preferencePeptide/protein ingredientsMicrobiological testing/measurementSubstrate SpecificitiesParasitic disease
Owner:SAMSUNG ELECTRONICS CO LTD
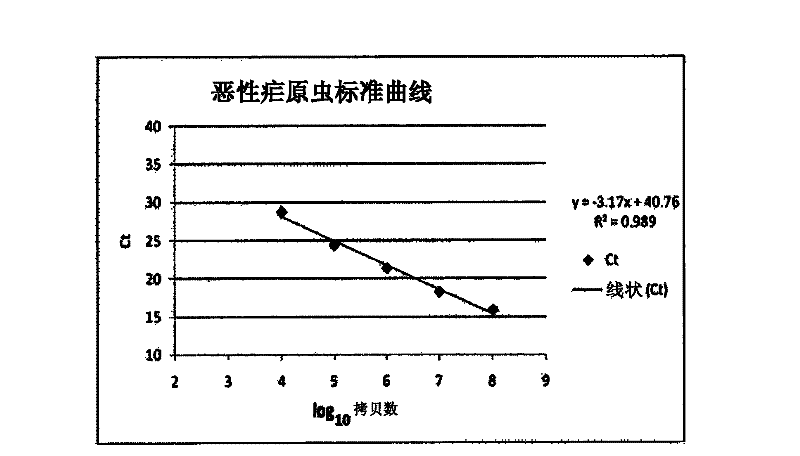
Rapid and sensitive detection method for plasmodium vivax
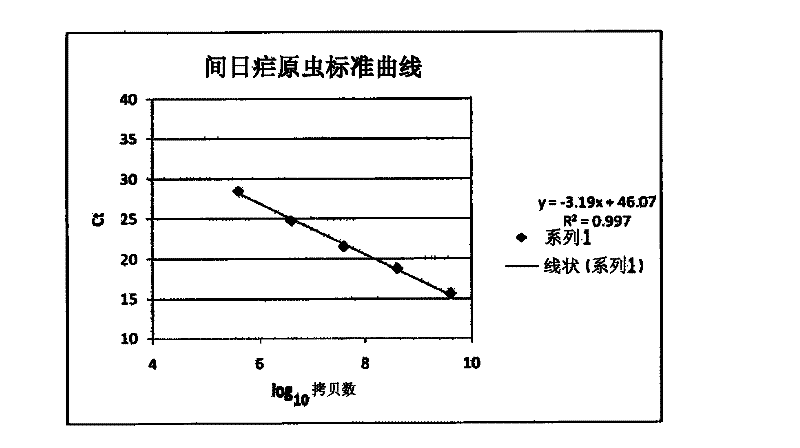
ActiveCN104561269AMeet the requirements of safety testingMeet the testing requirementsMicrobiological testing/measurementMicroorganism based processesA-DNAWorkload
The invention relates to a rapid and sensitive detection method for plasmodium vivax. The detection method integrates two powerful molecular biology techniques, namely, polymerase chain reaction (PCR) and microarray, a PCR-hybridized probe is directly fixed to hybridization cabins of the microarray and the probe and a PCR reaction chamber are located at the same chip. The detection method comprises the following steps of carrying out bacteria-proliferating treatment on a sample, extracting a DNA liquid, carrying out PCR amplification, hybridizing, cleaning and interpreting the results. By the detection method, plasmodium vivax can be rapidly and sensitively detected, the detection efficiency of frontline inspection and quarantine personnel of import and export ports can be greatly improved, the workload can be reduced, the problem of possibly missed positive inspections in the traditional detection methods can be furthest solved and thus the malaria parasite epidemic situation is furthest prevented.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Primers and method for identifying genetic polymorphism of Plasmodium vivax PvMSP-3alpha and application of primers
InactiveCN109295243AStrong specificityAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesGene typeWild type
The invention discloses primers and method for identifying genetic polymorphism of Plasmodium vivax PvMSP-3alpha. The primers include an upstream primer (shown as SEQ ID NO: 1) and a downward primer (shown as SEQ ID NO: 2). The specific primers are designed according to regional characteristics of genetic polymorphism; a DNA fragment containing a conserved region and a polymorphic region is acquired by PCR (polymerase chain reaction); whether PvMSP-3alpha gene mutates can be identified through sequencing and analysis; the gene type being wild or mutant can be distinguished then. In addition, the invention also discloses application of the specific primers for identifying genetic polymorphism of Plasmodium vivax PvMSP-3alpha in the preparation of detection tools for identifying genetic polymorphism of Plasmodium vivax PvMSP-3alpha.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com