Immunoassay and diagnostic reagent for malaria
A technology for diagnostic reagents and immunodetection methods, which is applied in the field of preparation of Plasmodium surface proteins, can solve the problems of increased false positive signals, ineffective false positive signals, and increased preparation costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0128] The invention will be further illustrated by the following examples. It is clear to those skilled in the art that these embodiments are only a more detailed description of the present invention, but the present invention is not limited to these embodiments. Example 1-1 prepares cDNA with malaria positive blood
[0129] RNA from malaria-positive blood was purified using TRI Reagent™ (Sigma Co.). The following purification methods were provided by Sigma Co.
[0130] At room temperature, let 0.1ml of TRI reagent TM and 0.1ml of malaria-positive blood stand for 5 minutes, add 20 microliters of BCP to the mixture, let the final mixture stand for 5 minutes at room temperature, and centrifuge at 12,000 rpm and 4°C for 15 minutes , the centrifuged product was divided into 3 layers. Transfer the top layer containing RNAs to a new test tube, then add 50 μl of isopropanol to the test tube, let it stand at room temperature for 5 minutes, then centrifuge the resulting mixture at ...
Embodiment 1-2
[0132] 4 microliters of RT buffer, 2 microliters of 0.1M DTT (DL-dithiothreitol) (Sigma, Cal No. D5545), 1 microliter of 10mM dNTP, 1 microliter of reverse transcriptase (Gibco Co), 1 Microliter RNase inhibitor (Promega) and microliter deionized distilled water were added to the above mixture, reacted at 42° C. for 1 h, and then reacted at 70° C. for 10 minutes to prepare cDNA. Embodiment 1-2 prepares the recombinant plasmid containing gene encoding PV200C
[0133] Using the cDNAs prepared in Example 1-1 as a template and a pair of the following primers SEQ.ID.NO: 2 and SEQ.ID.NO: 4, polymerase chain reaction was carried out as follows.
[0134] The reaction mixture contains 5 μl of PCR buffer, 5 μl of 2.5mM dNTP, 1 μl of sensitive primer, 1 μl of anti-sensitive primer, 2 μl of cDNA template and 35 μl of deionized distilled water, and react at 94°C for 30 seconds . Then 1 microliter of Vent polymerase (BioLab) was added to the mixture, and the reaction was repeated 36 times ...
Embodiment 1-3
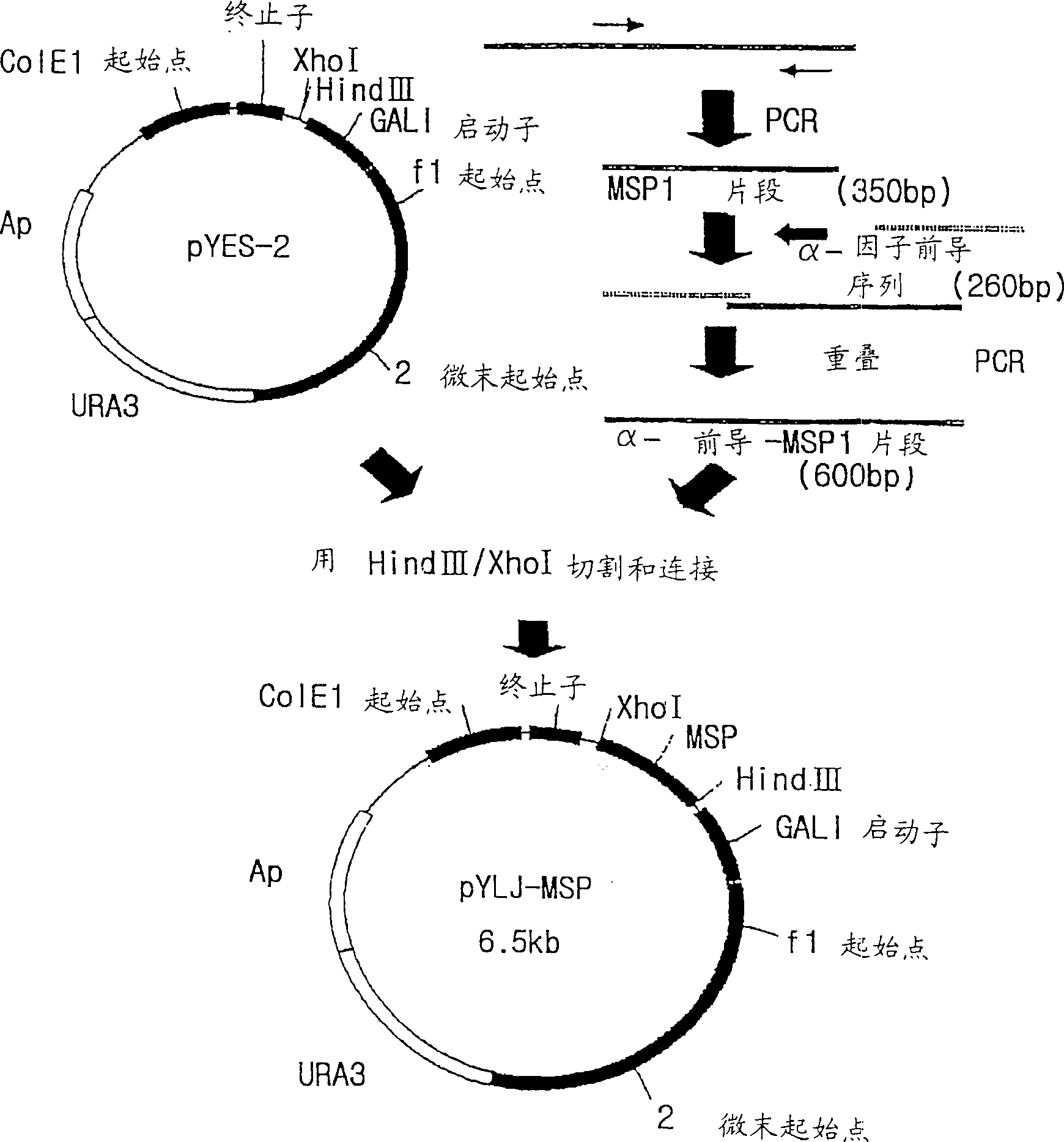
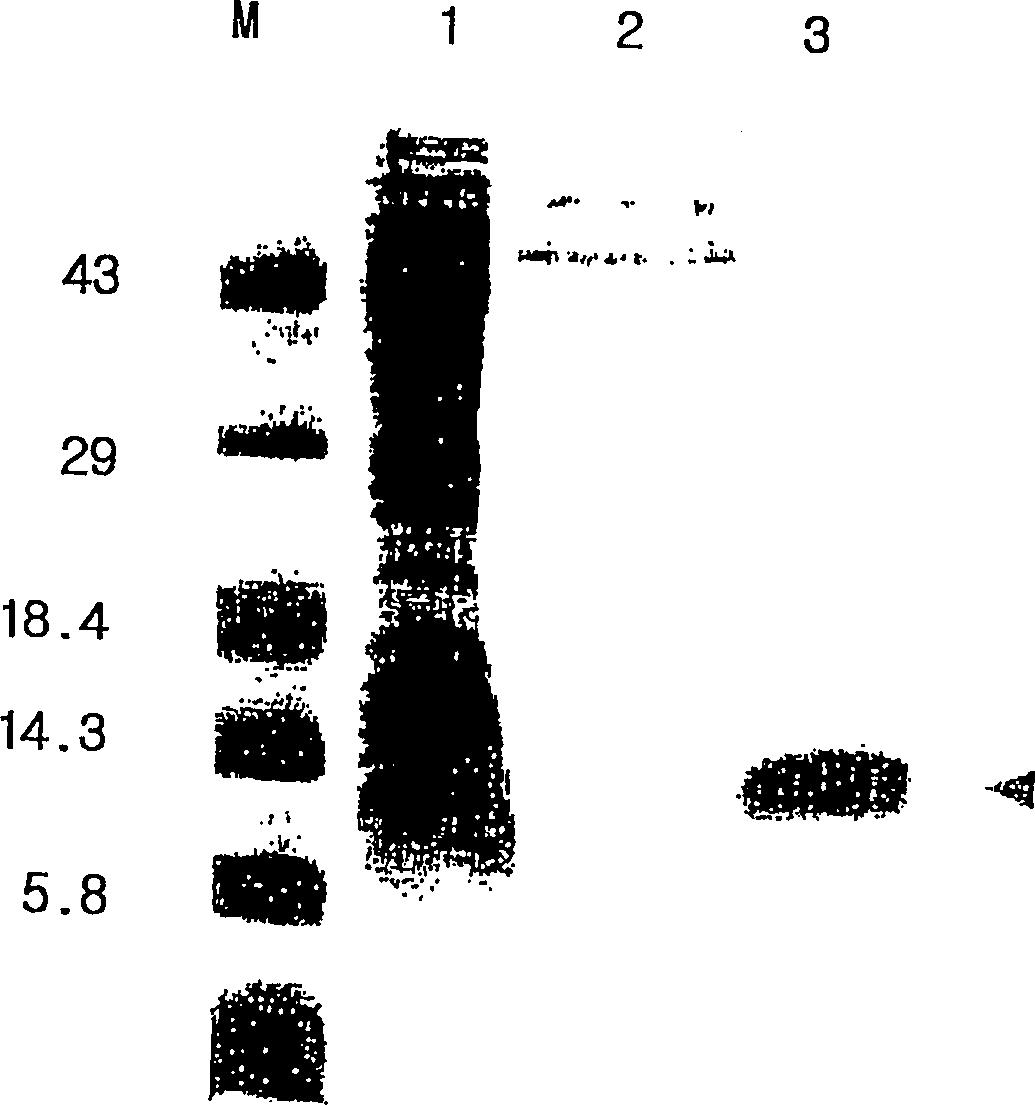
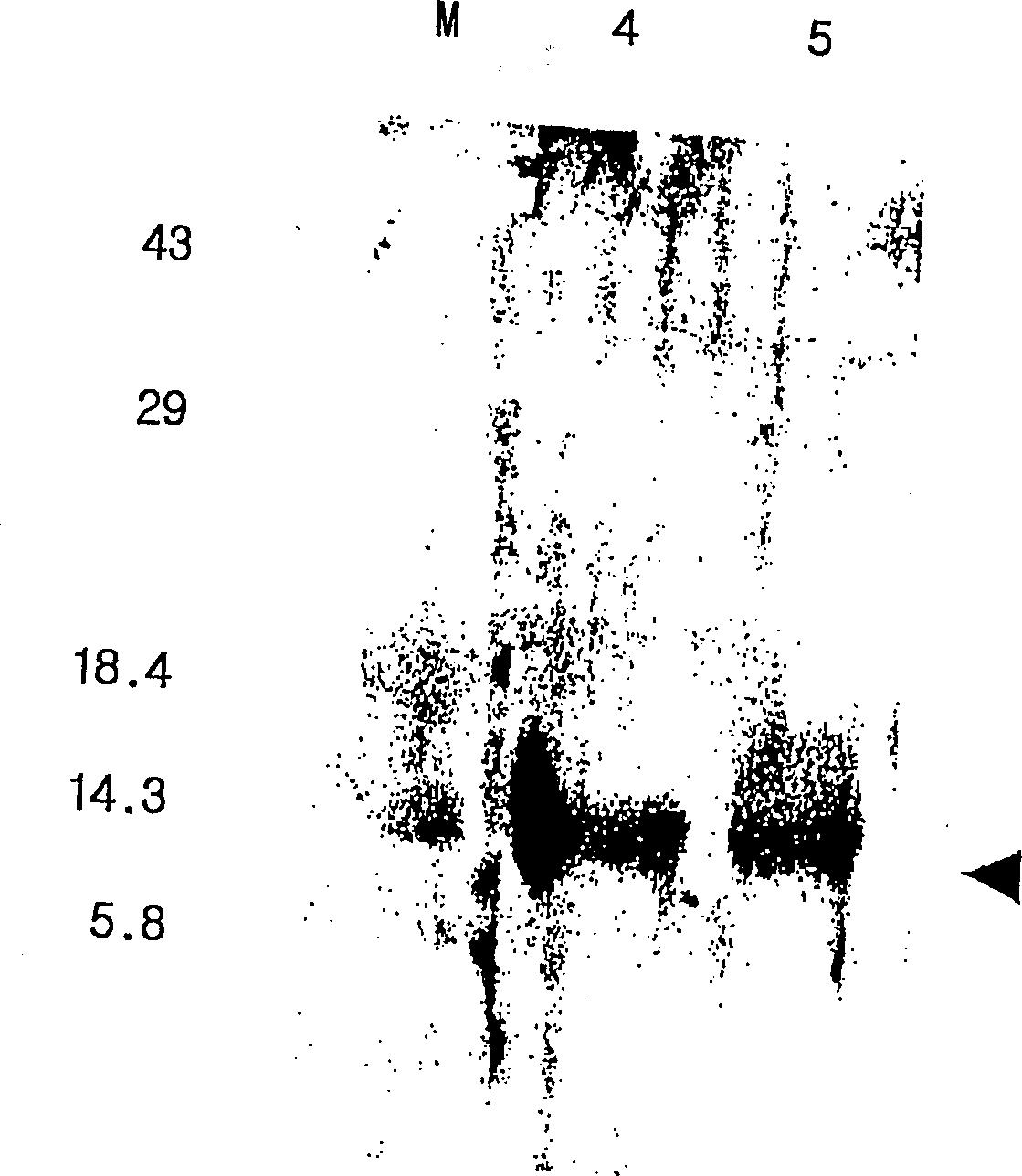
[0135] Vent polymerase (BioLab) PCR was performed under the same conditions as above. The enhanced DNAs can be confirmed by electrophoresis in 1% agarose gel, and the enhanced DNAs (referring to Pv200-ct657) and pBluscript KS(+) (Stratagene) vector are cut with restriction enzymes, using T4 DNA ligase (Promega ). EcoR V and Pv200-ct657 were embedded in pBluscript KS(+) vector. As a result, a recombinant plasmid (ie, pBC-Pv200ct657) was obtained. E. coli strain JM109 was then transformed with pBC-Pv200ct657. The preparation of embodiment 1-3 expression vector pYLJ-MSP
[0136] Using pBC-Pv200-ct657 prepared in Example 1-2 as a template, and a pair of primers SEQ.ID.NO: 5 and SEQ.ID NO: 6 for polymerase chain reaction. PCR was carried out under the same conditions as above. The enhanced DNAs (ie PV200-19) were confirmed by electrophoresis in a 1% agarose gel.
[0137] In order to obtain the leader peptide sequence of yeast α factor linked to the N-terminus of PV200C polype...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com