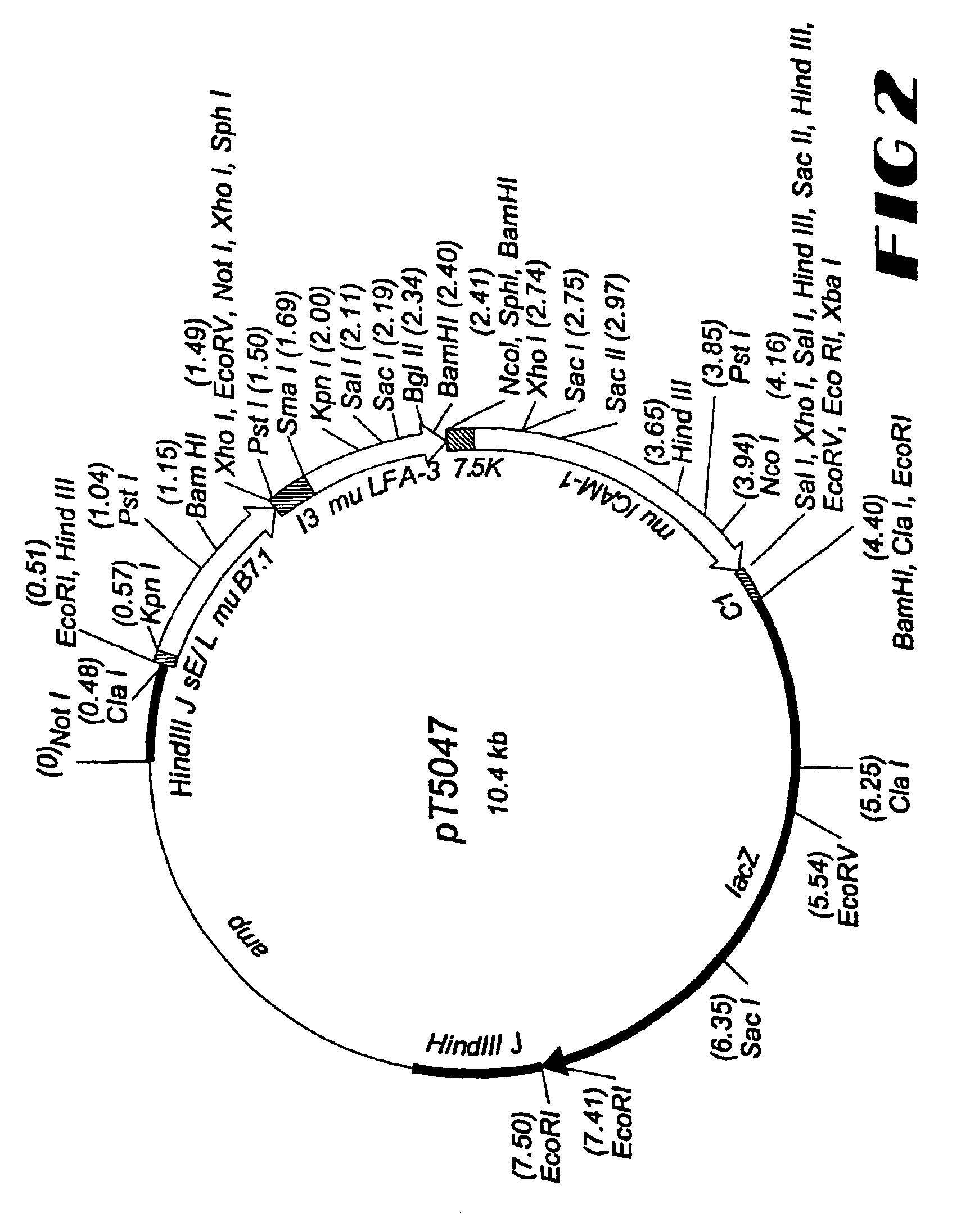
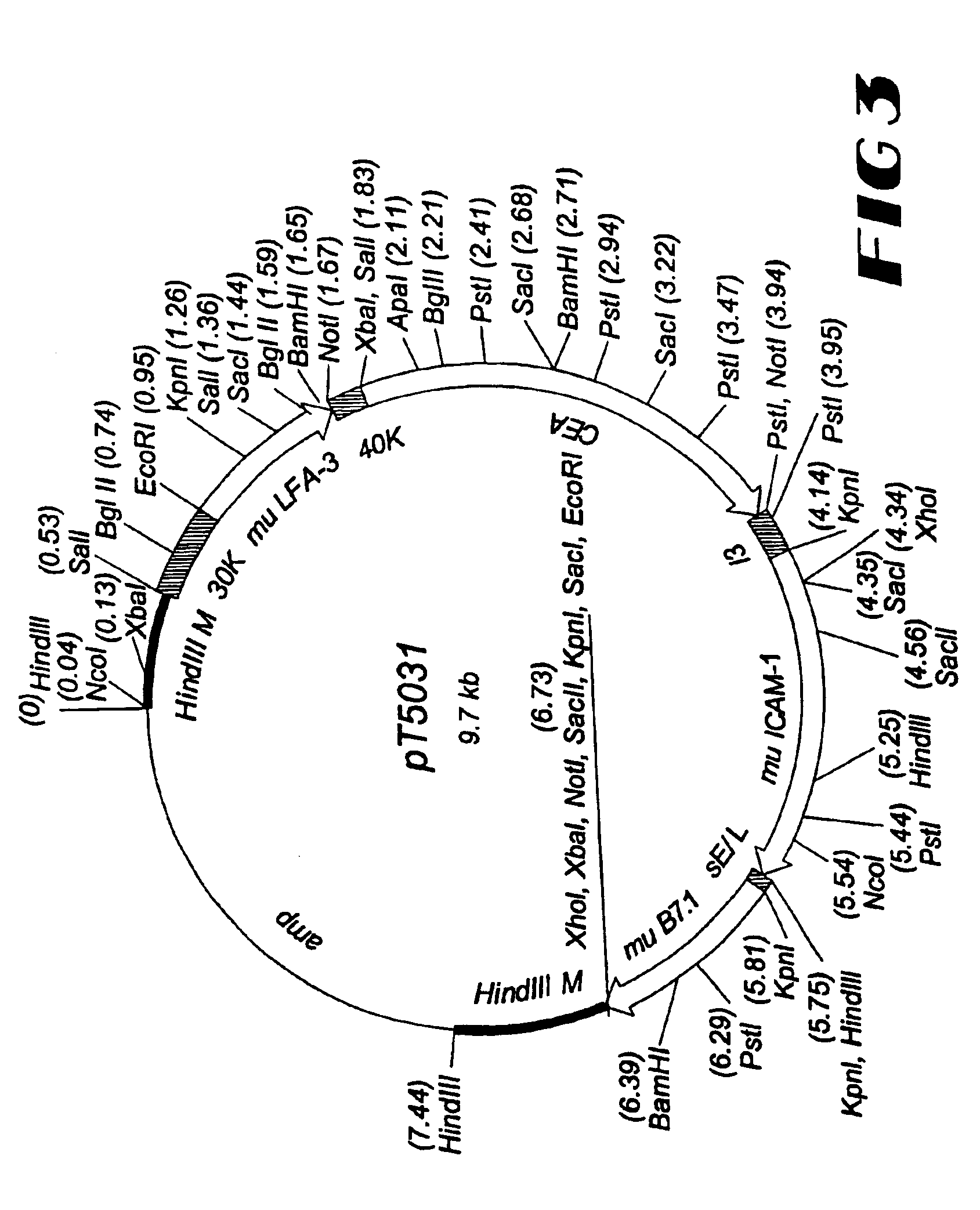
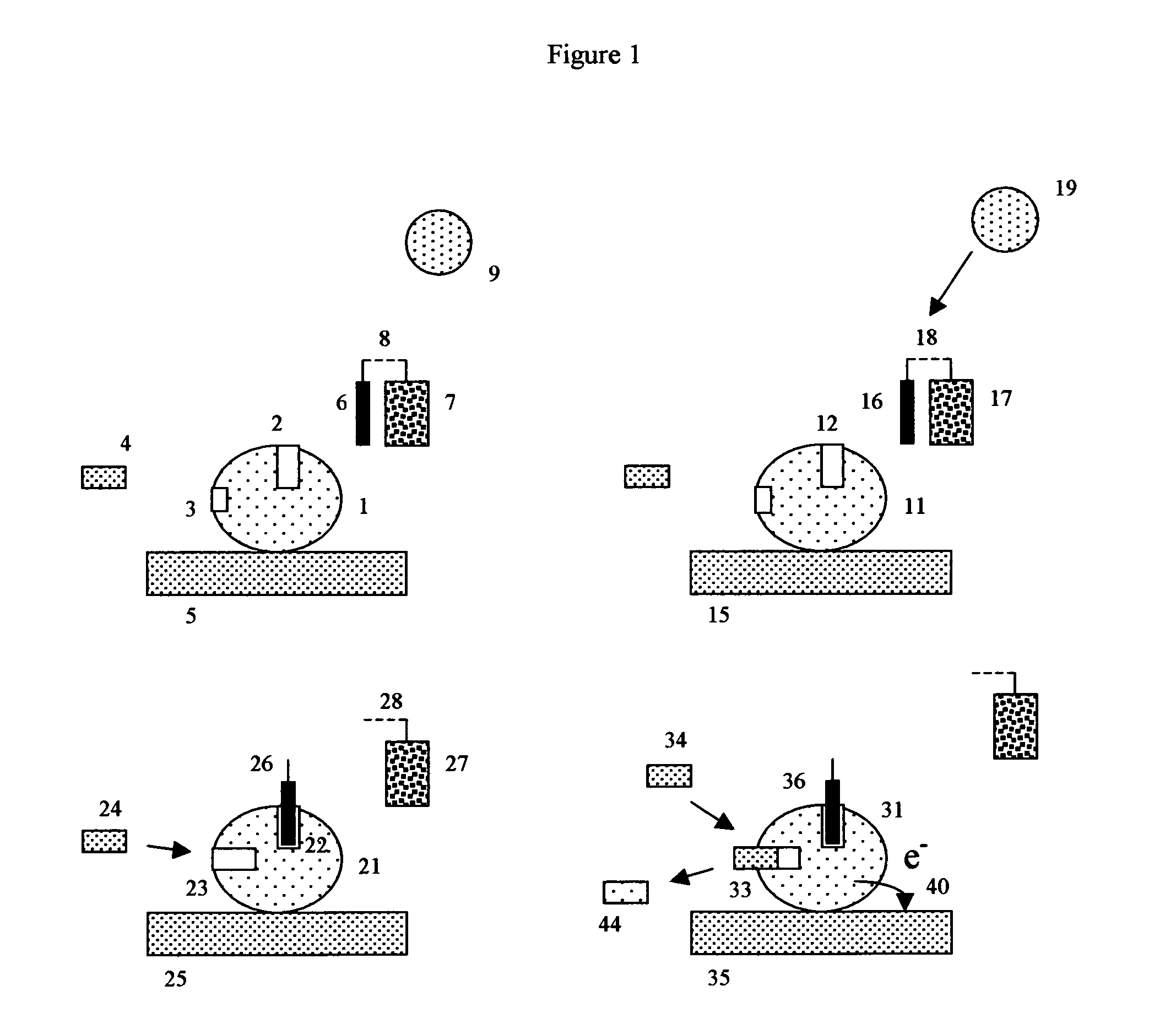
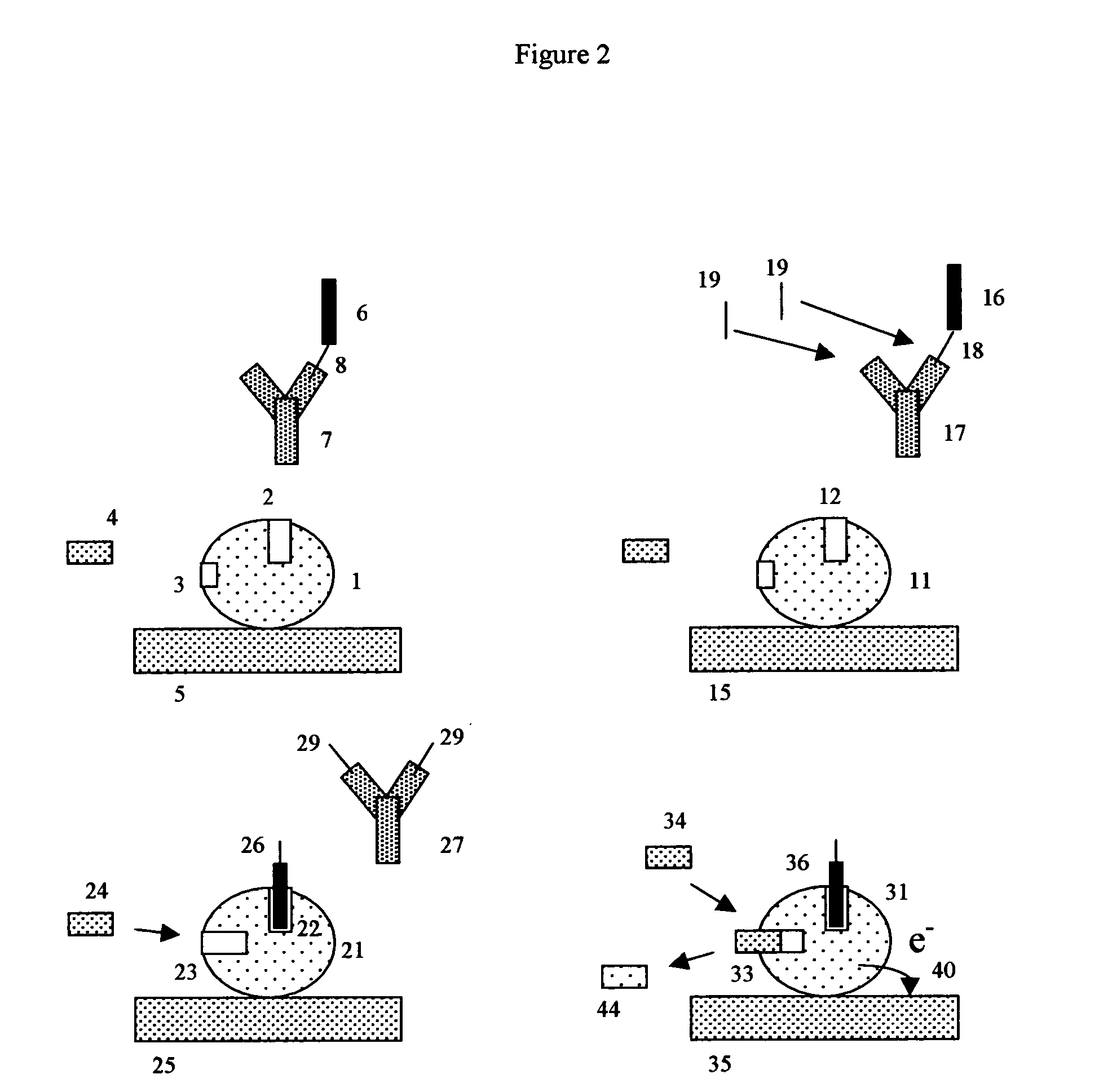
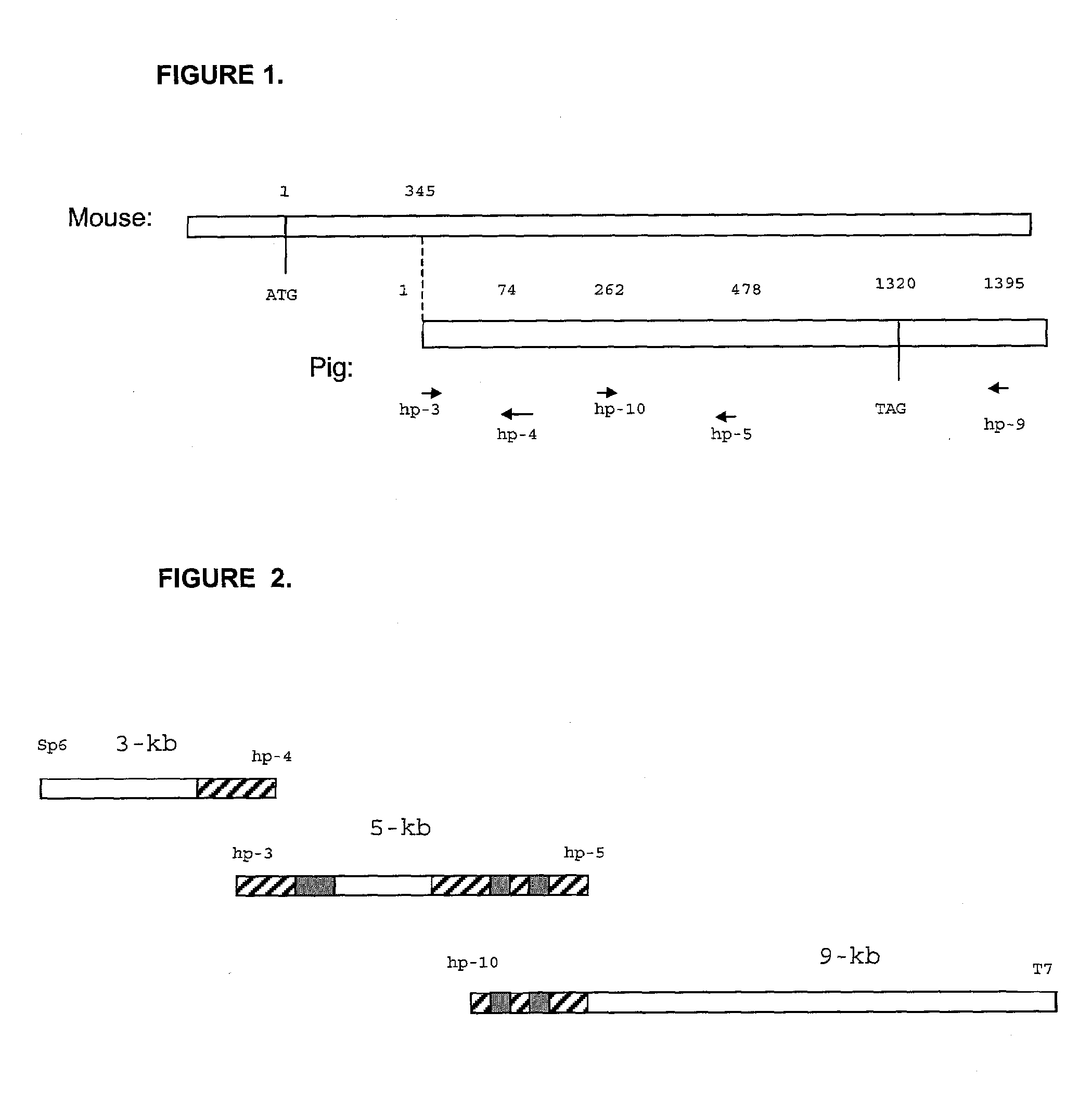
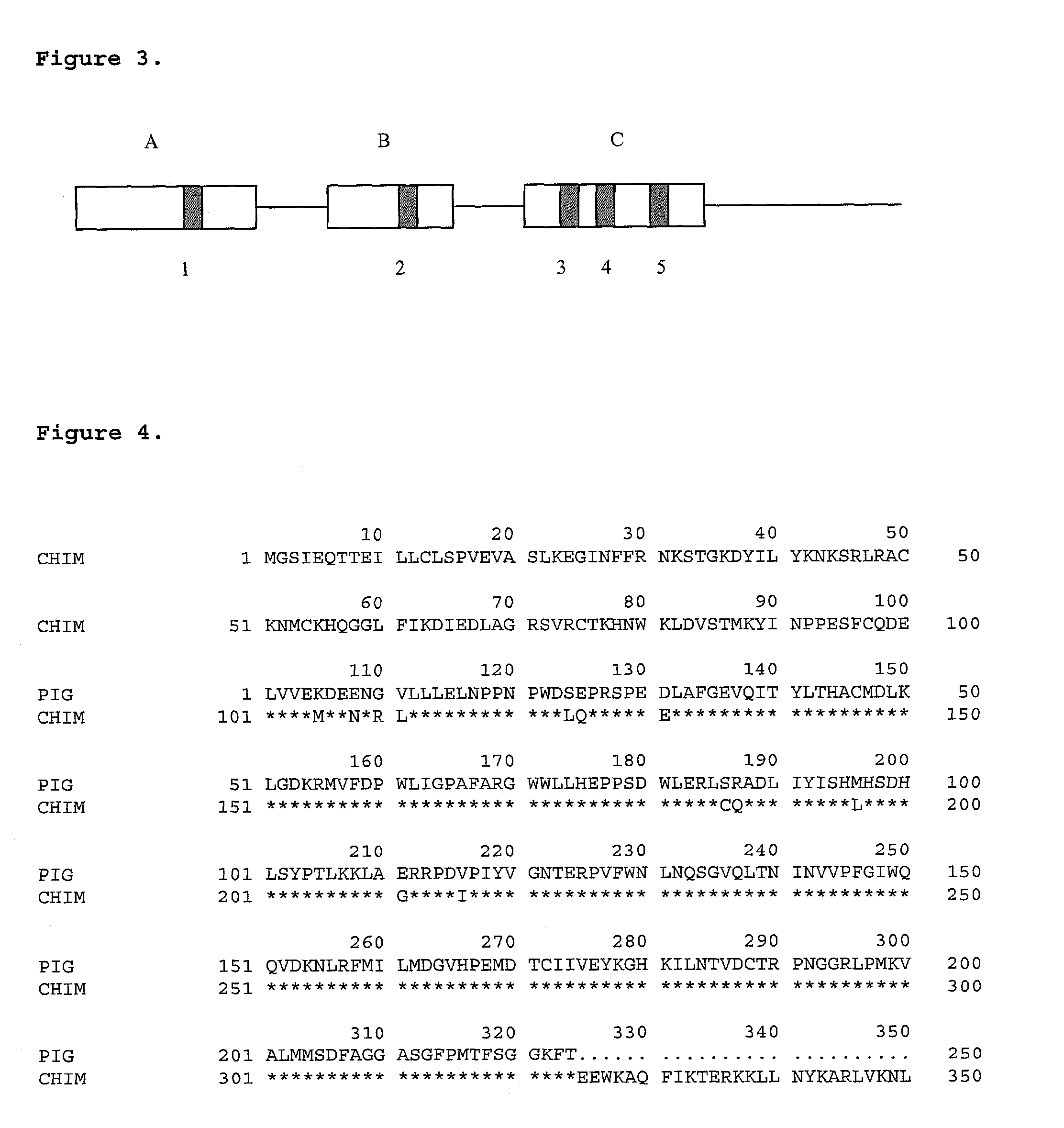
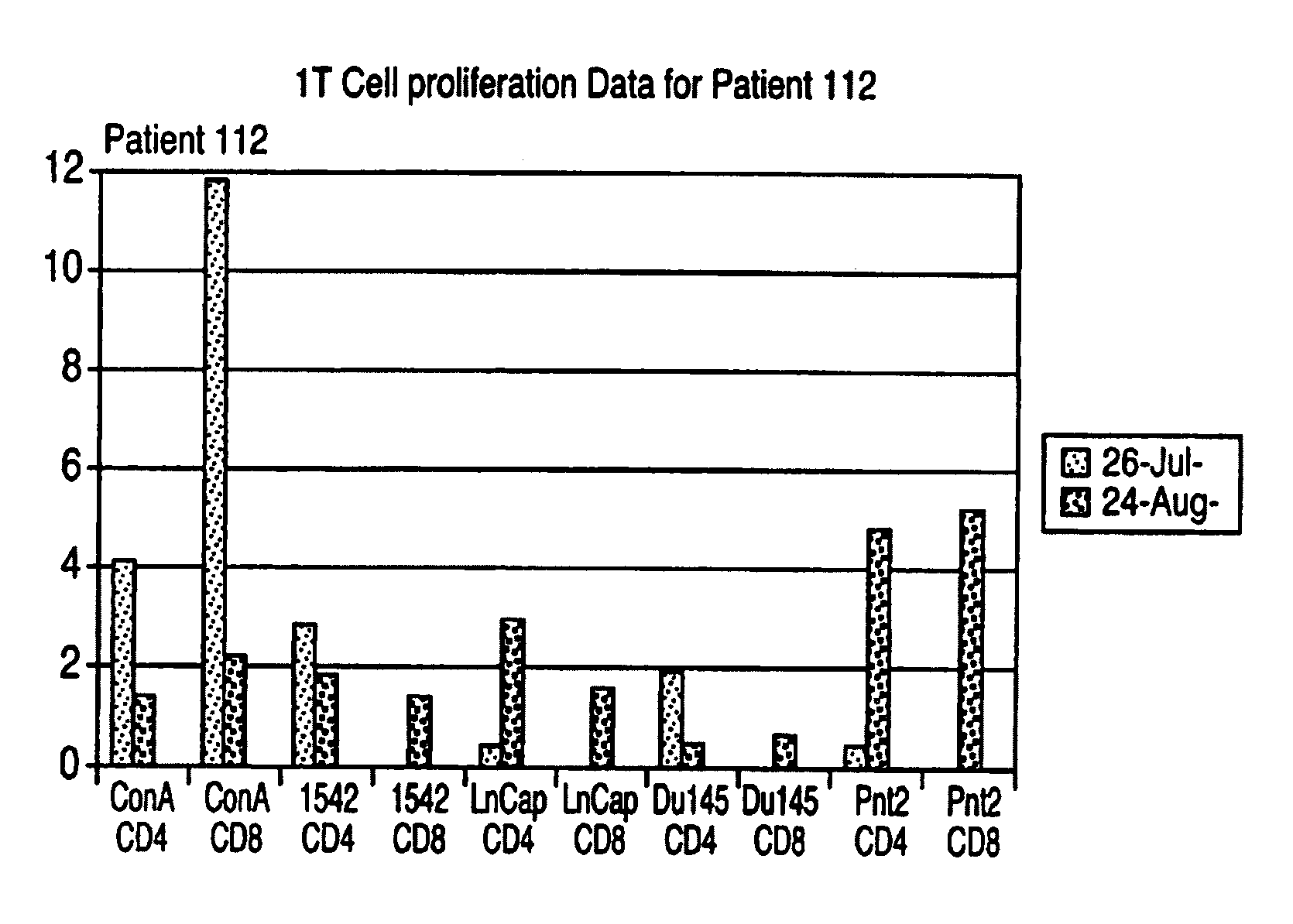
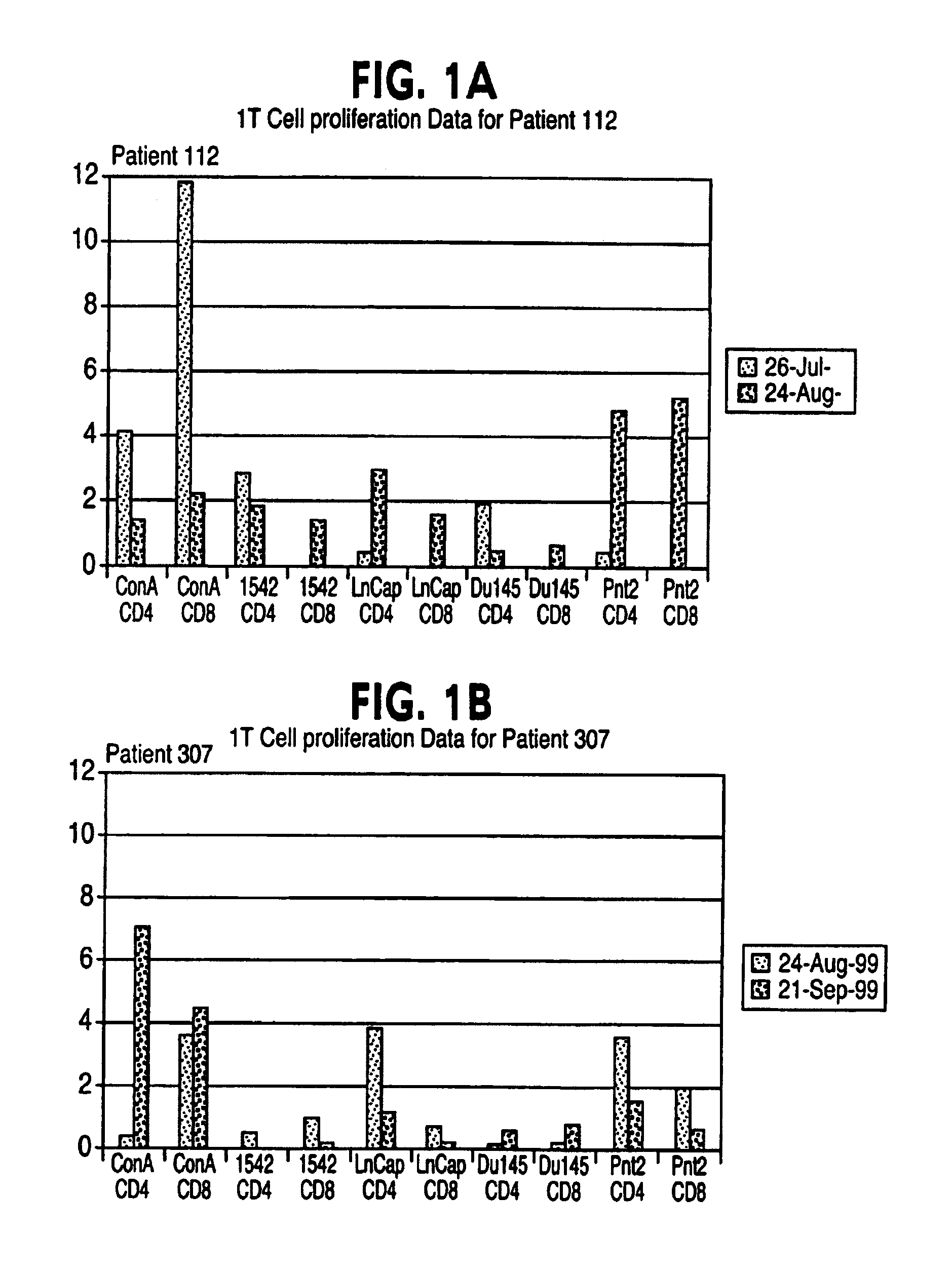
Patents
Literature
211 results about "Antigens present" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The ABO blood group system involves two antigens and two antibodies found in human blood. The two antigens are antigen A and antigen B. The two antibodies are antibody A and antibody B. The antigens are present on the red blood cells and the antibodies in the serum.
Artificial antigen presenting cells and methods of use thereof
InactiveUS20020131960A1Palliating their conditionReduce riskBiocideCompound screeningEpitopeAccessory molecule
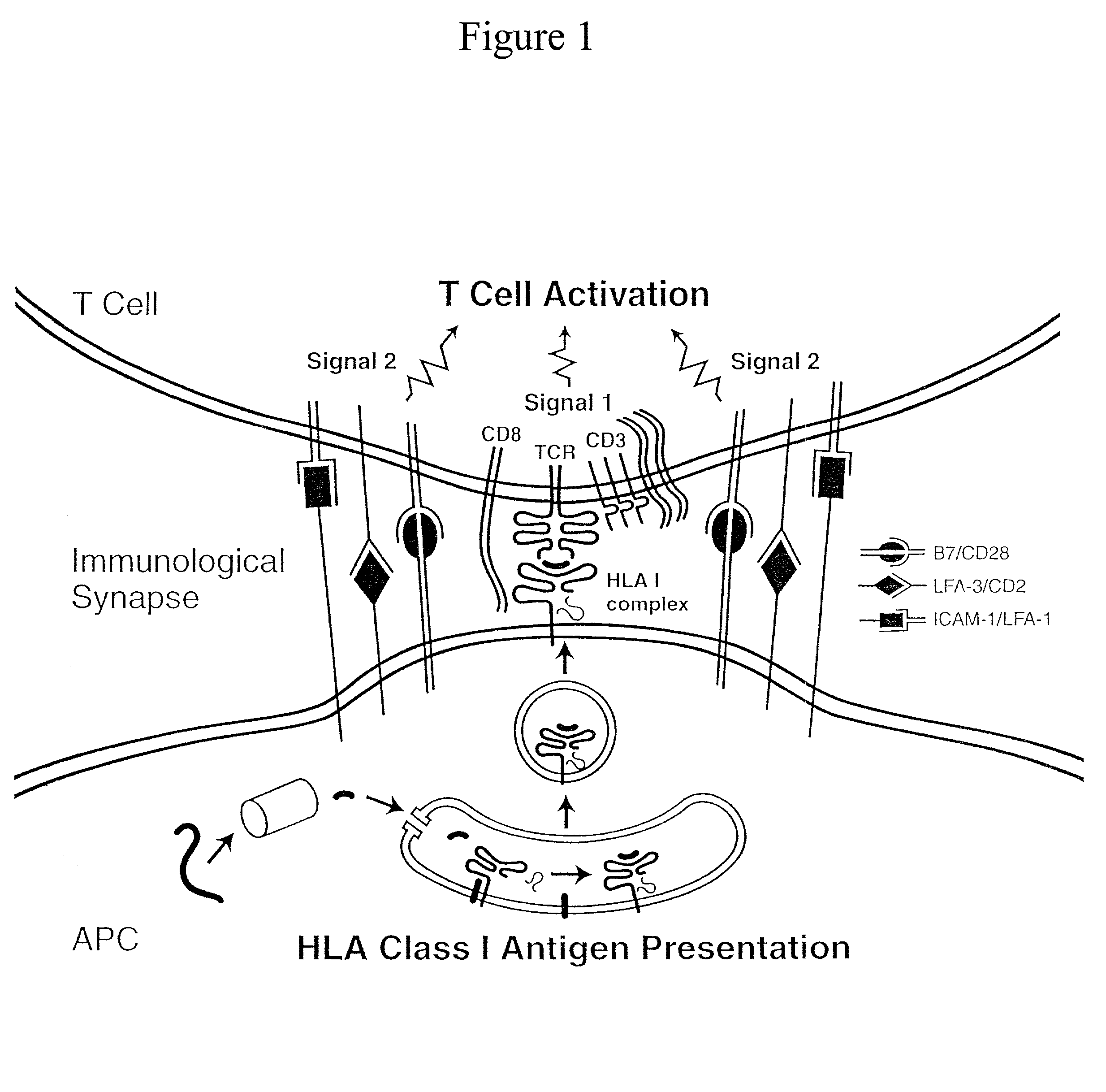
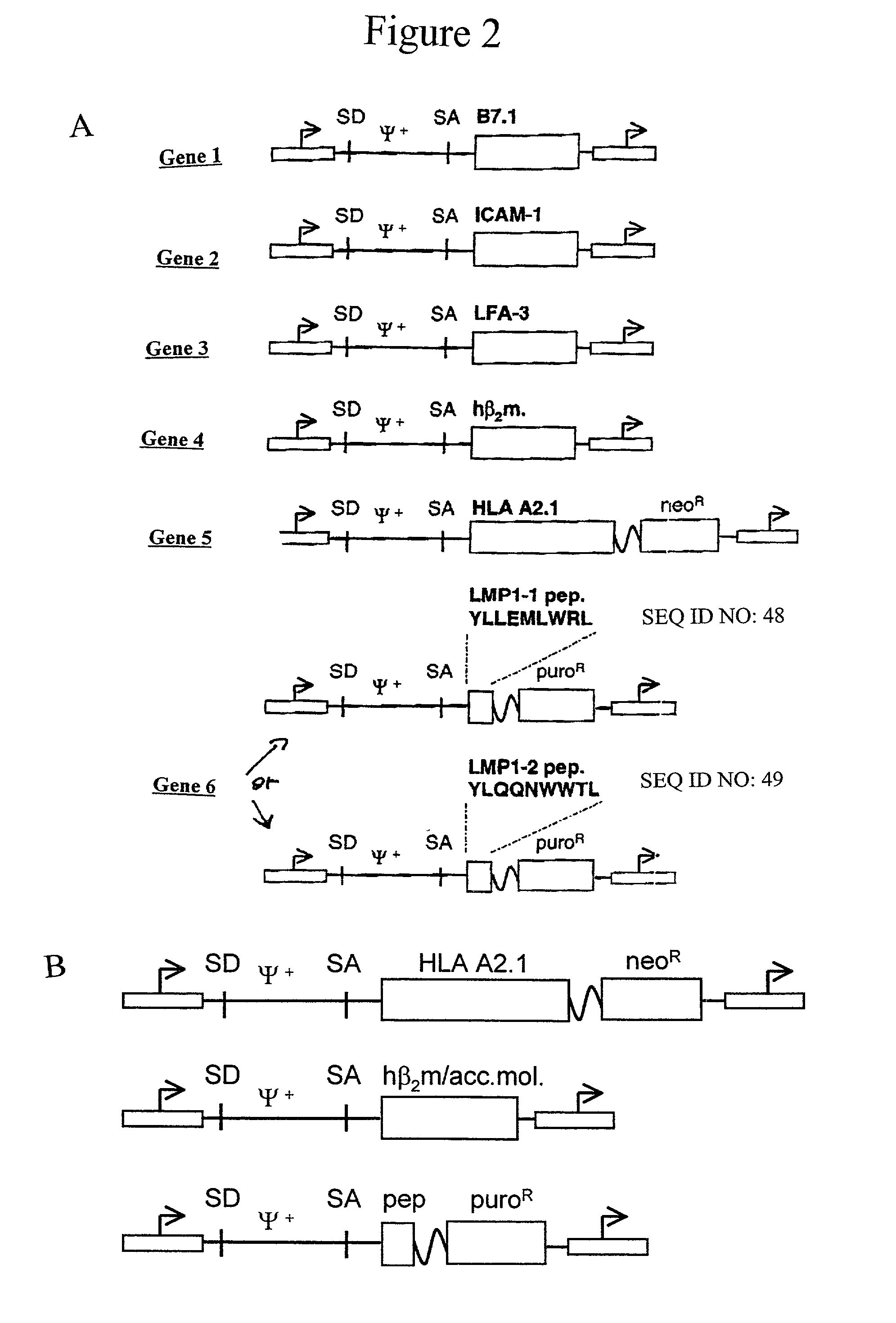
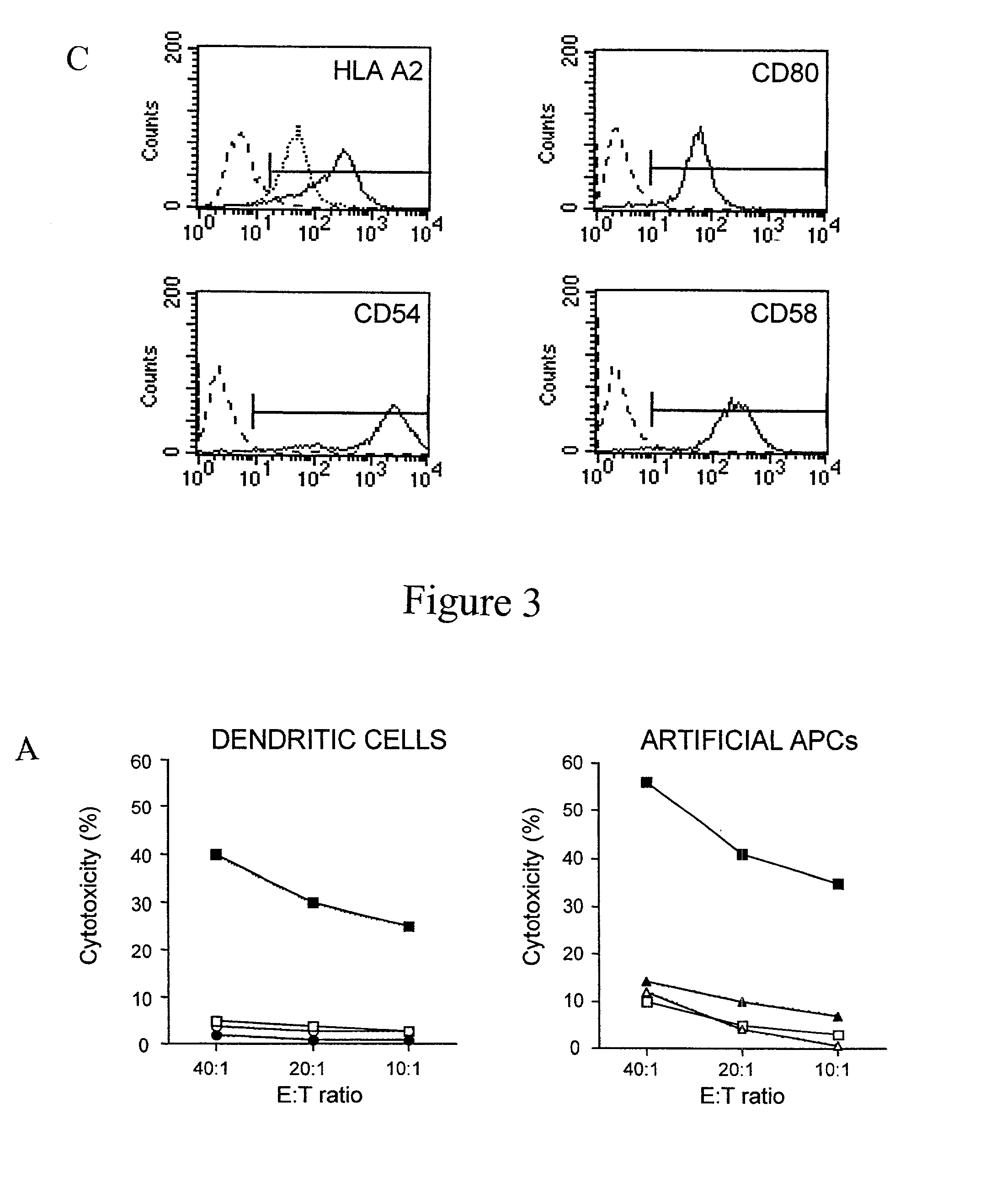
The invention provides an artificial antigen presenting cell (AAPC) comprising a eukaryotic cell expressing an antigen presenting complex comprising a human leukocyte antigen (HLA) molecule of a single type, at least one exogenous accessory molecule and at least one exogenous T cell-specific epitope. Methods of use for activation of T lymphocytes are also provided.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Apoenzyme reactivation electrochemical detection method and assay
InactiveUS7166208B2Immobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorAssay
Owner:ZWEIG STEPHEN ELIOT
Compositions capable of specifically binding particular human antigen presenting molecule/pathogen-derived antigen complexes and uses thereof
Owner:TECHNION RES & DEV FOUND LTD
Mammalian antigen-presenting T cells and bi-specific T cells
InactiveUS20050129671A1Guaranteed functionImproving in vivo survivalBiocideGenetic material ingredientsAbnormal tissue growthAutoimmune condition
The present invention is directed to mammalian bi-specific T cells and methods for using these bi-specific T cells. More specifically, the invention relates to viral specific T cells that express chimeric anti-tumor receptors. These bi-specific T cell clones are a source of effector cells that persist in vivo in response to stimulation with viral antigen, leading to long-term function after their transfer to patients with cancer and autoimmune diseases.
Owner:CITY OF HOPE
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS7211432B2Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of these costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater than the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+ and CD8+ T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced antigen-presenting functions.
Owner:UNITED STATES OF AMERICA
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS6969609B1Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of them costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater that the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+and CD8+T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced and antigen-presenting functions.
Owner:THE GOVERMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES (SEE PF37)
Apoenzyme reactivation electrochemical detection method and assay
InactiveUS20050196820A1Immobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorAnalyte
The invention discloses a methods in which dry reagent enzyme based electrochemical biosensors, which are in a relatively mature form due to the extensive amount of development pioneered by the blood glucose monitoring industry, may be simply adapted to perform tests for blood coagulation, enzymatic activity, or immunochemical assays for antigens present in a fluid sample. In particular, the utility of combining apoenzyme based dry reagent electrochemical biosensors with apoenzyme reactivation technology is taught. This combination creates a novel combination dry reagent test technology capable of detecting a wide range of different analytes.
Owner:ZWEIG STEPHEN ELIOT
Antibodies against cancer
InactiveUS7318924B2Improve permeabilityPeptide/protein ingredientsGenetic material ingredientsAnticarcinogenCripto
An isolated binding partner of a Cripto-1 protein, Pim-1 protein or an antigen present in a colon cancer cell lysate is described. The binding partner inhibits growth of one or more cancer cell types and may be used in an anti-cancer agent for treating cancer in a subject. The binding partner may also be used in a method of inducing apoptosis in a cancer cell, as well as in a method of sensitizing a cancer cell to a cytotoxic compound. In addition, a cancer vaccine is described wherein the vaccine comprises a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate or, alternatively, comprises an expressible DNA molecule encoding a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate.
Owner:THE MACFARLANE BURNET INST FOR MEDICAL RES & PUBLIC HEALTH LTD
Labeled cell sets for use as functional controls in rare cell detection assays
InactiveUS7282350B2Great severity of diseaseImprove severityNanomagnetismPreparing sample for investigationRare cellFluorescence
Methods and kits for used as an internal or external control in rare cell analysis are disclosed. A plurality of fluorescently distinct sets of cells is used to define a range to assess the isolation of rare target cells from a sample. Thus, a known number of cells, expressing the surface and intracellular antigens present in the targeted rare cells, are stabilized and modified in such a way that they can be discriminated from the targeted rare cells. In addition, these cells are separated into at least two sets based upon the number. These sets are detectably distinct from each other and provide an upper and lower indication of the detection ability of the rare cell assay.
Owner:MENARINI SILICON BIOSYSTEMS SPA
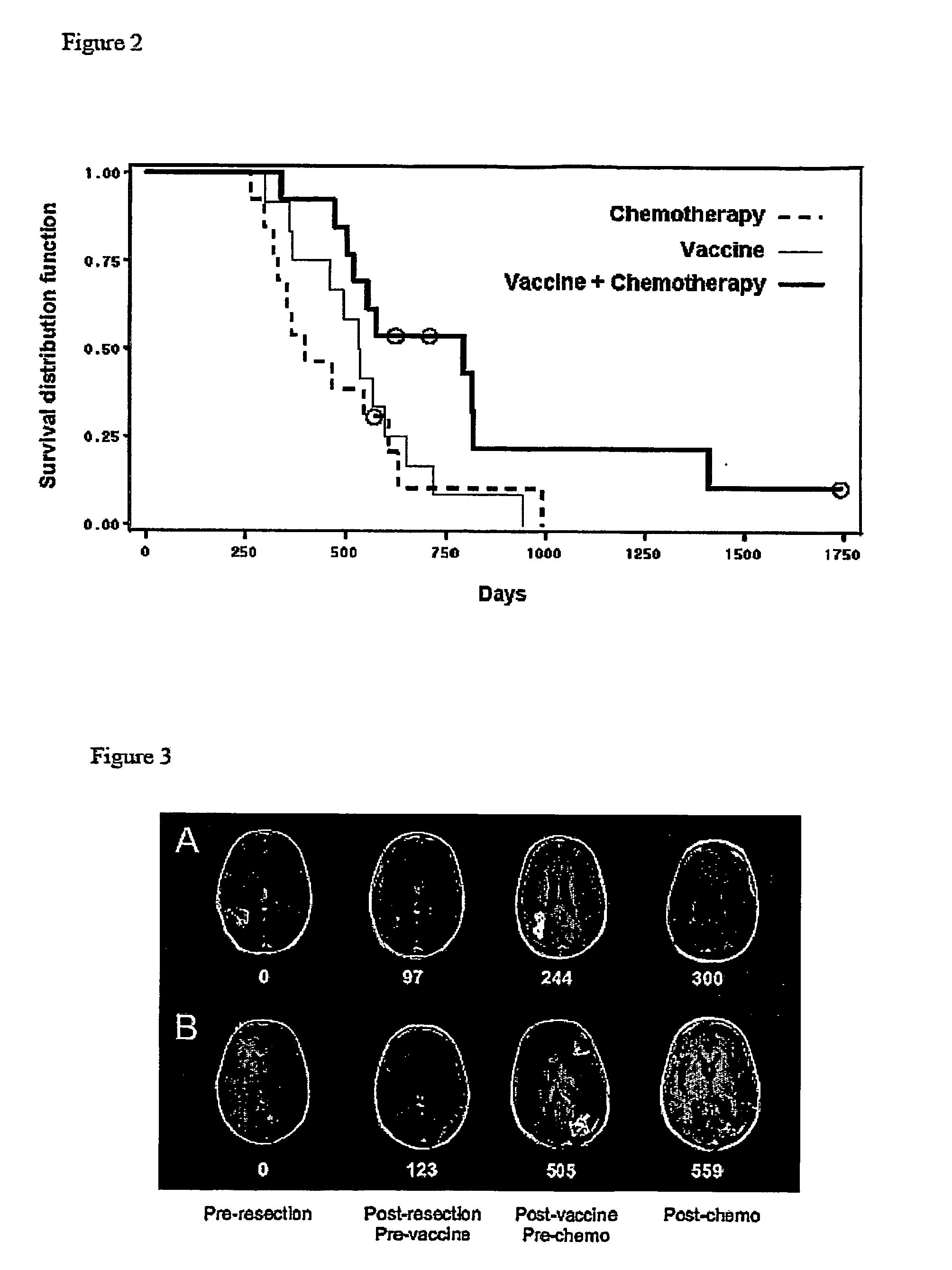
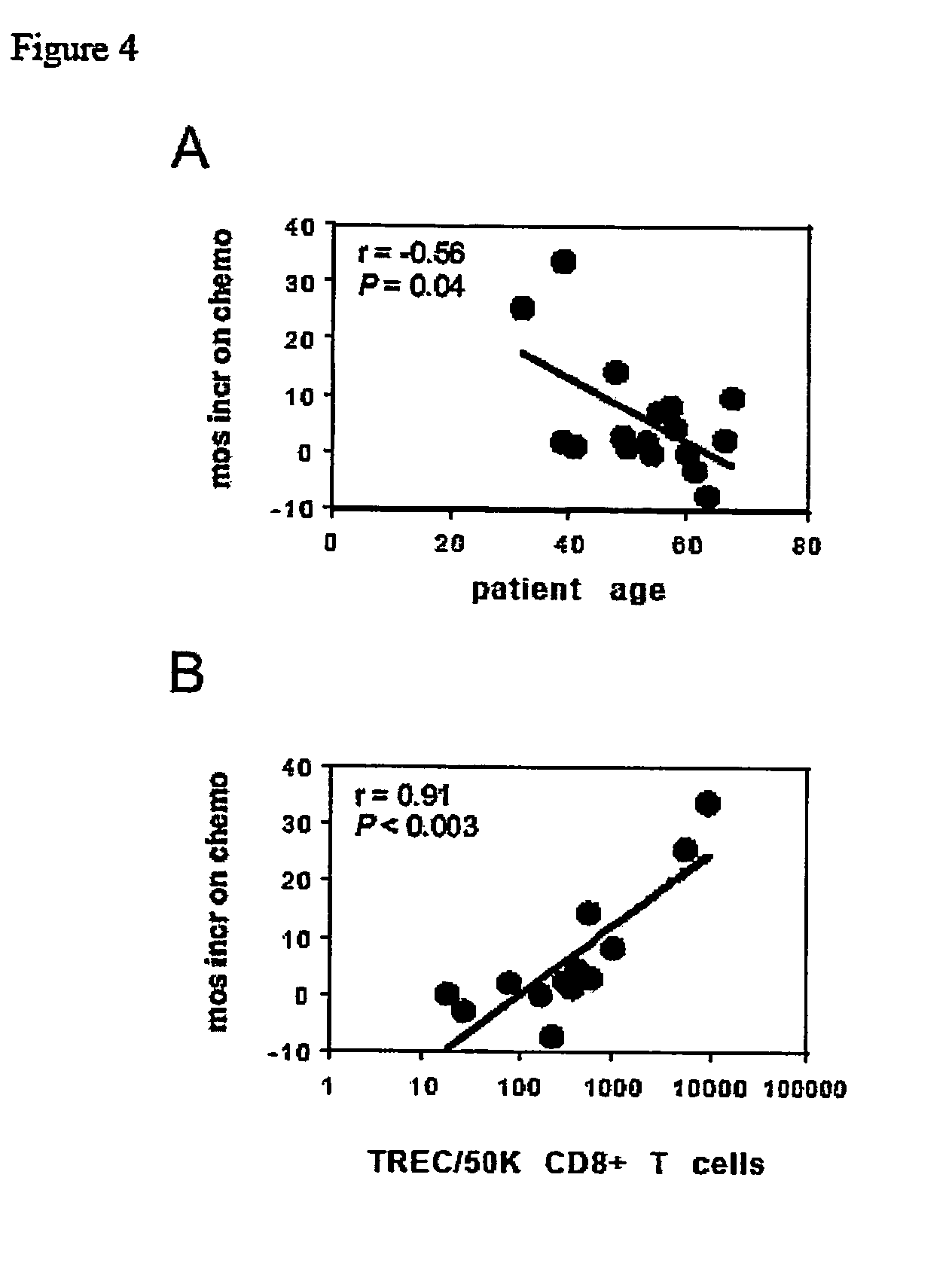
System and method for the treatment of cancer, including cancers of the central nervous system
The invention relates to the treatment of cancer, and particularly to the treatment of cancers of the central nervous system, such as glioblastoma multiforme. A dual therapeutic approach is provided, including the administration of a dendritic cell-based cancer vaccine and a regimen of chemotherapy. The two therapies may be administered concurrently with one another and / or with an initial vaccination preceding chemotherapy. In various embodiments, the dendritic cell-based cancer vaccine includes either primed or unprimed dendritic cells; for instance, the dendritic cells may be autologous tumor antigen-presented dendritic cells. The dual therapeutic approach of the instant invention beneficially influences the chemosensitivity of a mammal with cancer.
Owner:CEDARS SINAI MEDICAL CENT
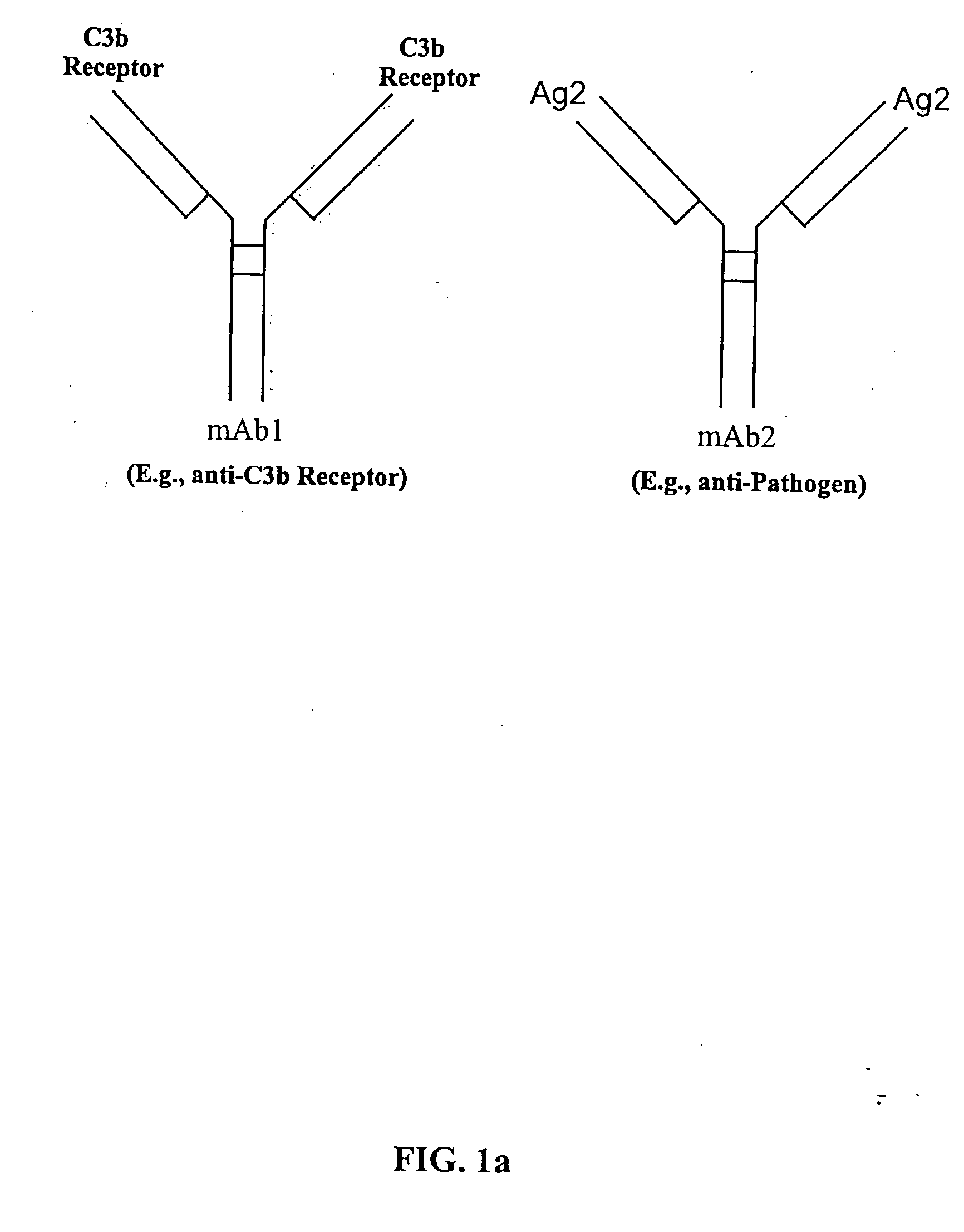
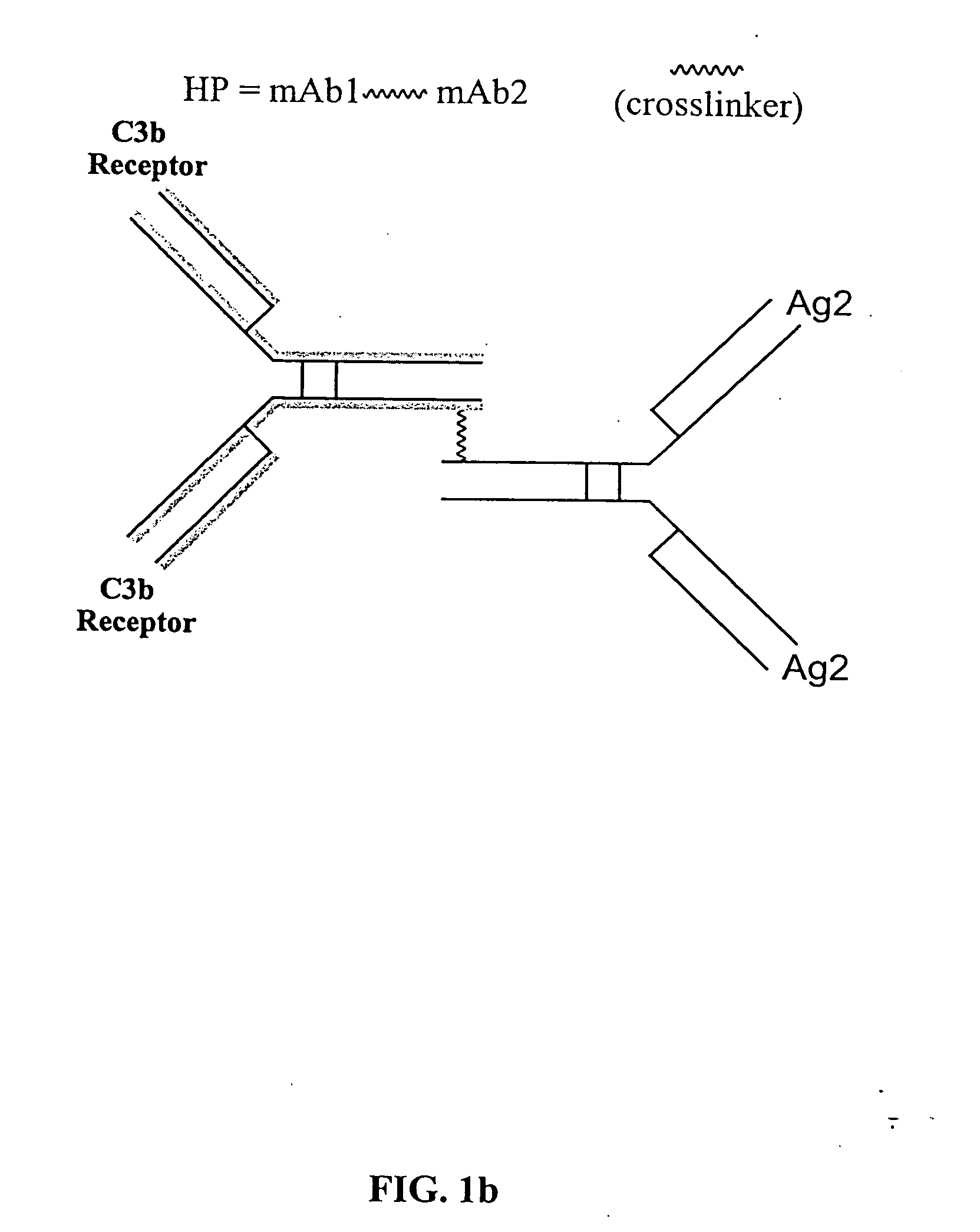
Bispecific molecules and uses thereof
InactiveUS20040180046A1Reduce clearanceRapidly and efficiently clearedFungiBacteriaCross-linkPrimate
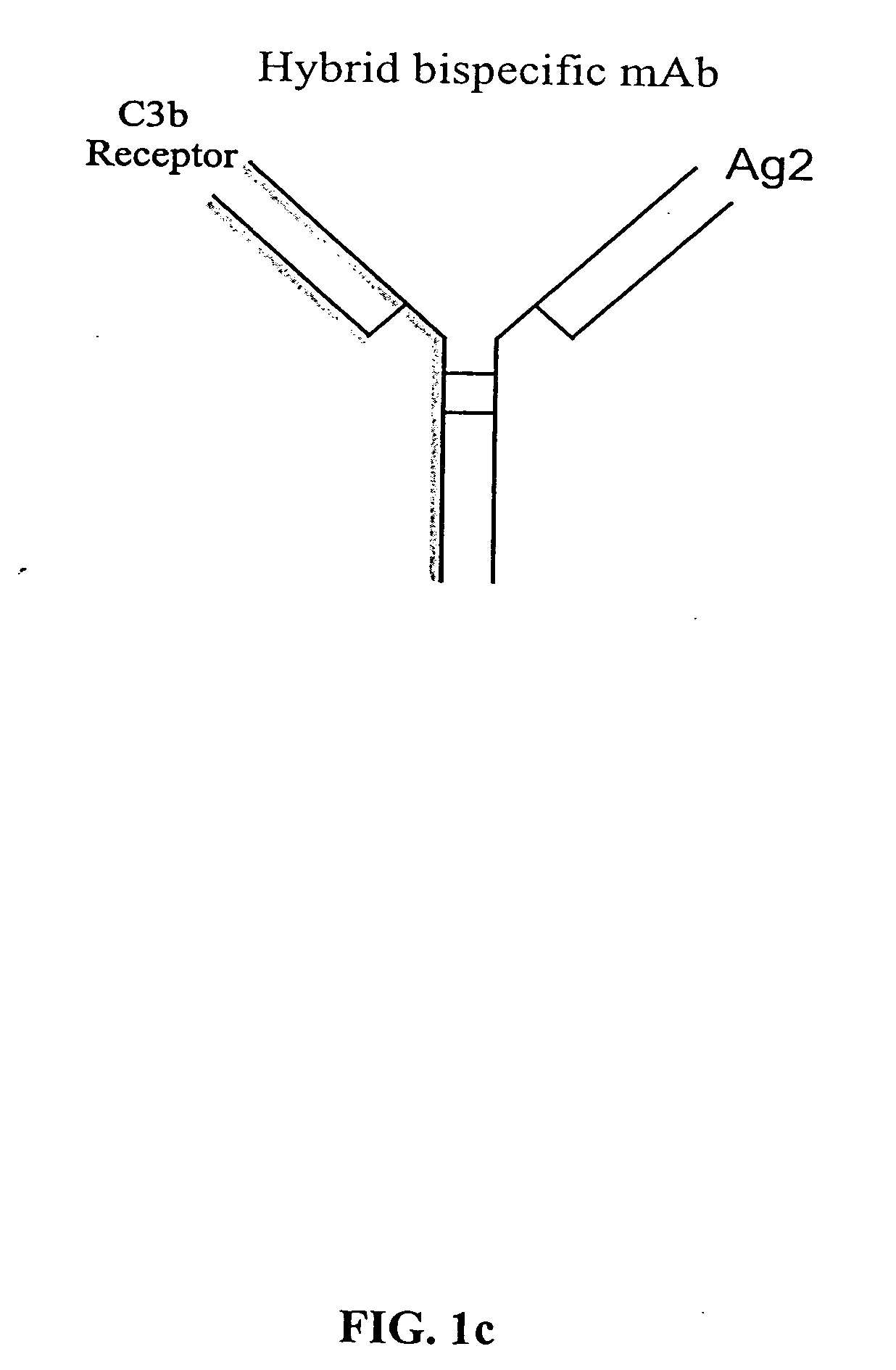
The present invention relates to bispecific molecules that are characterized by having a first binding domain which binds an antigen present in the circulation of a mammal and a second binding domain which binds the C3b-like receptor (known as complement receptor 1 (CR1) or CD35 in primates). The bispecific molecules do not consist of a first monoclonal antibody to CR1 that has been chemically cross-linked to a second monoclonal antibody. The invention also relates to methods of making the bispecific molecules and therapeutic uses thereof, as well as to kits containing the bispecific molecules. The invention further provides polyclonal populations of bispecific molecules, which comprise populations of bispecific molecules with different antigen recognition specificities. Such polyclonal populations of bispecific molecules can be used for targeting multiple epitopes of a pathogenic antigenic molecule and / or multiple variants of a pathogenic antigenic molecule.
Owner:ELUSYS THERAPEUTICS
Apoenzyme reactivation electrochemical detection method and assay
InactiveUS20070289880A1Immobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorIn vivo
The invention discloses a device and method by which dry reagent enzyme based electrochemical biosensors, which are in a relatively mature form due to the extensive amount of development pioneered by the blood glucose monitoring industry, may be simply adapted to perform tests for blood coagulation, enzymatic activity, or immunochemical assays for antigens present in a fluid sample. In particular, the utility of combining apoenzyme based dry reagent electrochemical biosensors with apoenzyme reactivation technology is taught. This combination creates a novel combination test technology capable of detecting a wide range of different analytes, and operating in a wide variety of wet or dry, in vivo or in vitro environments.
Owner:ZWEIG STEPHEN ELIOT
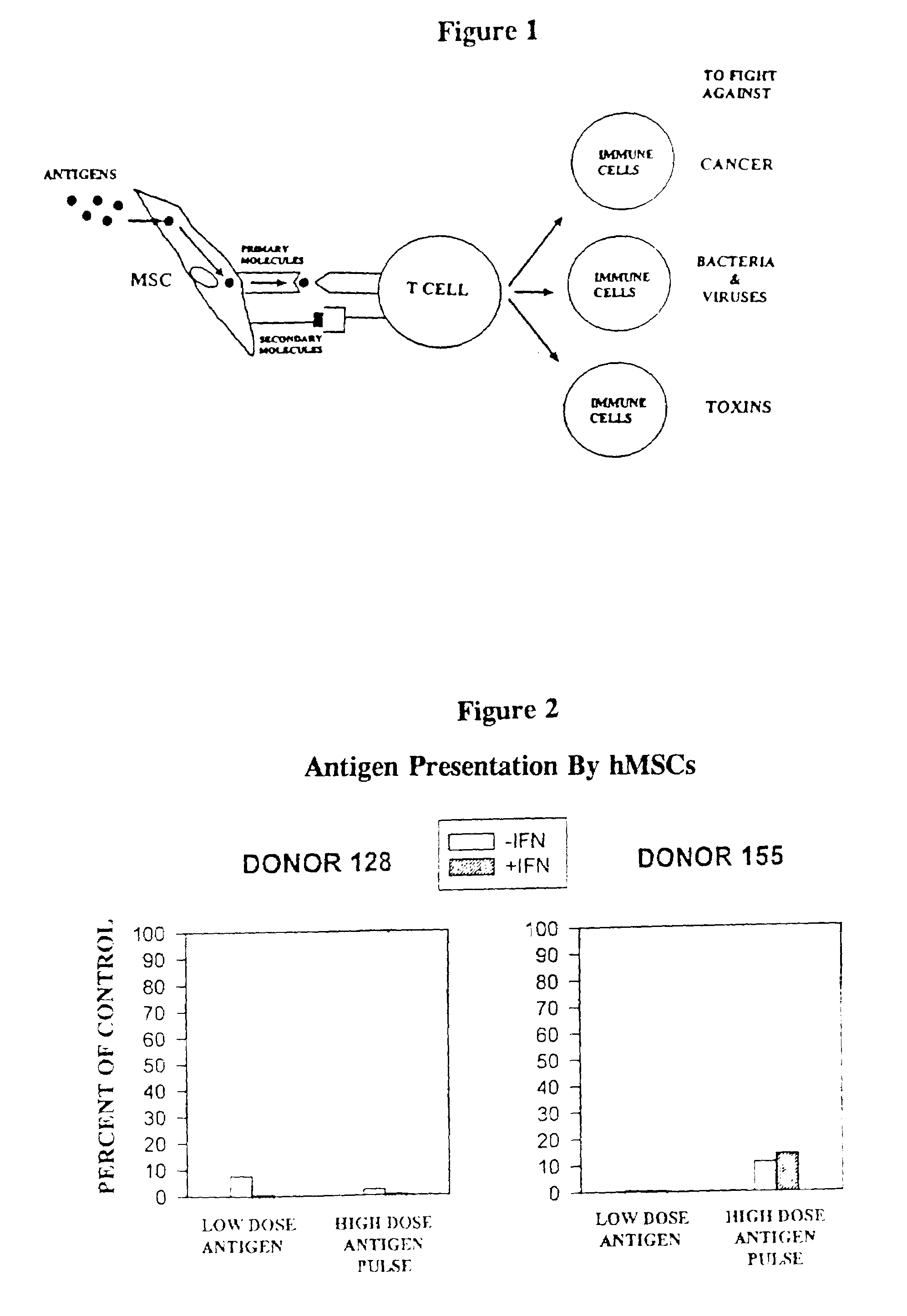
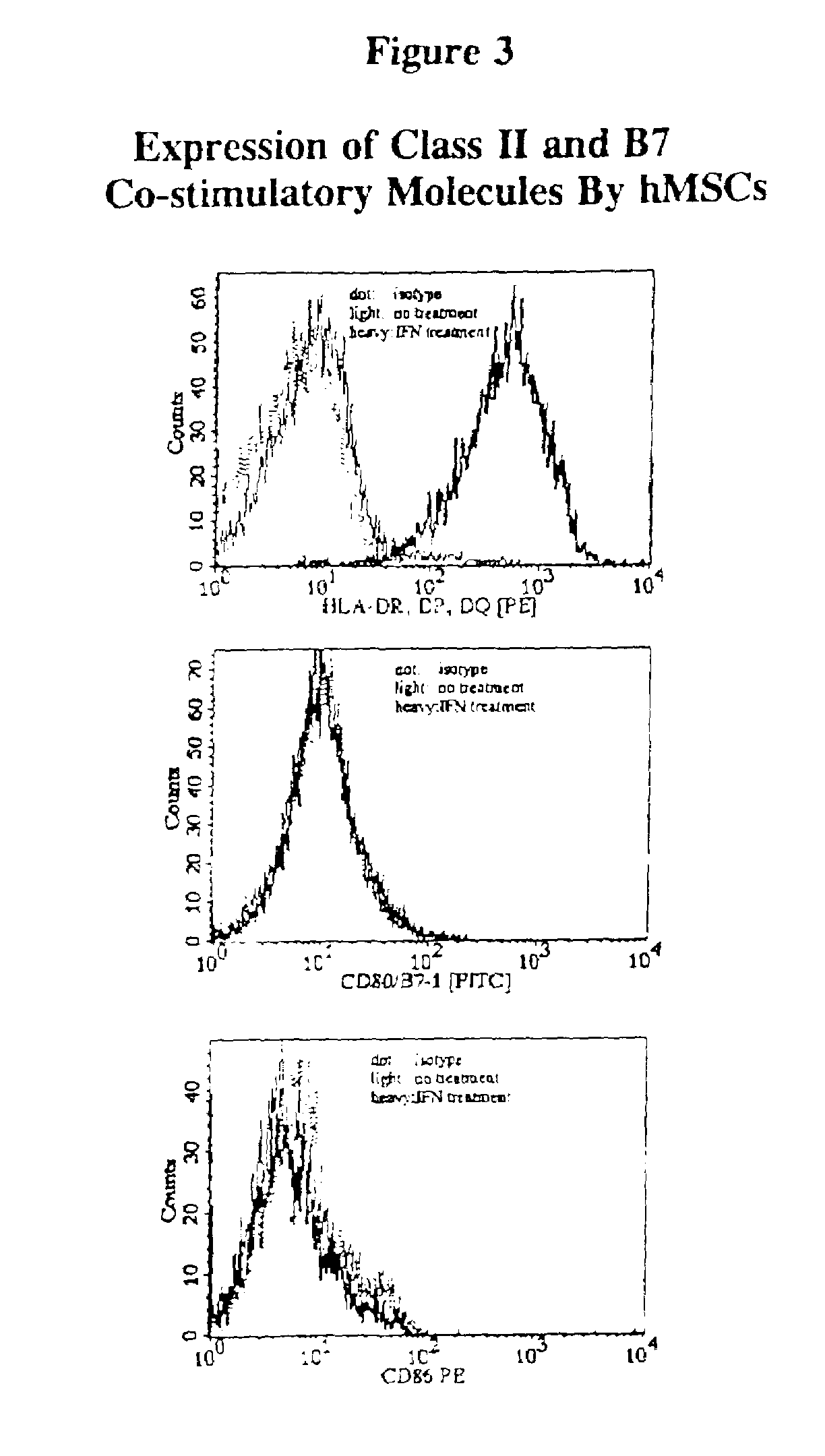
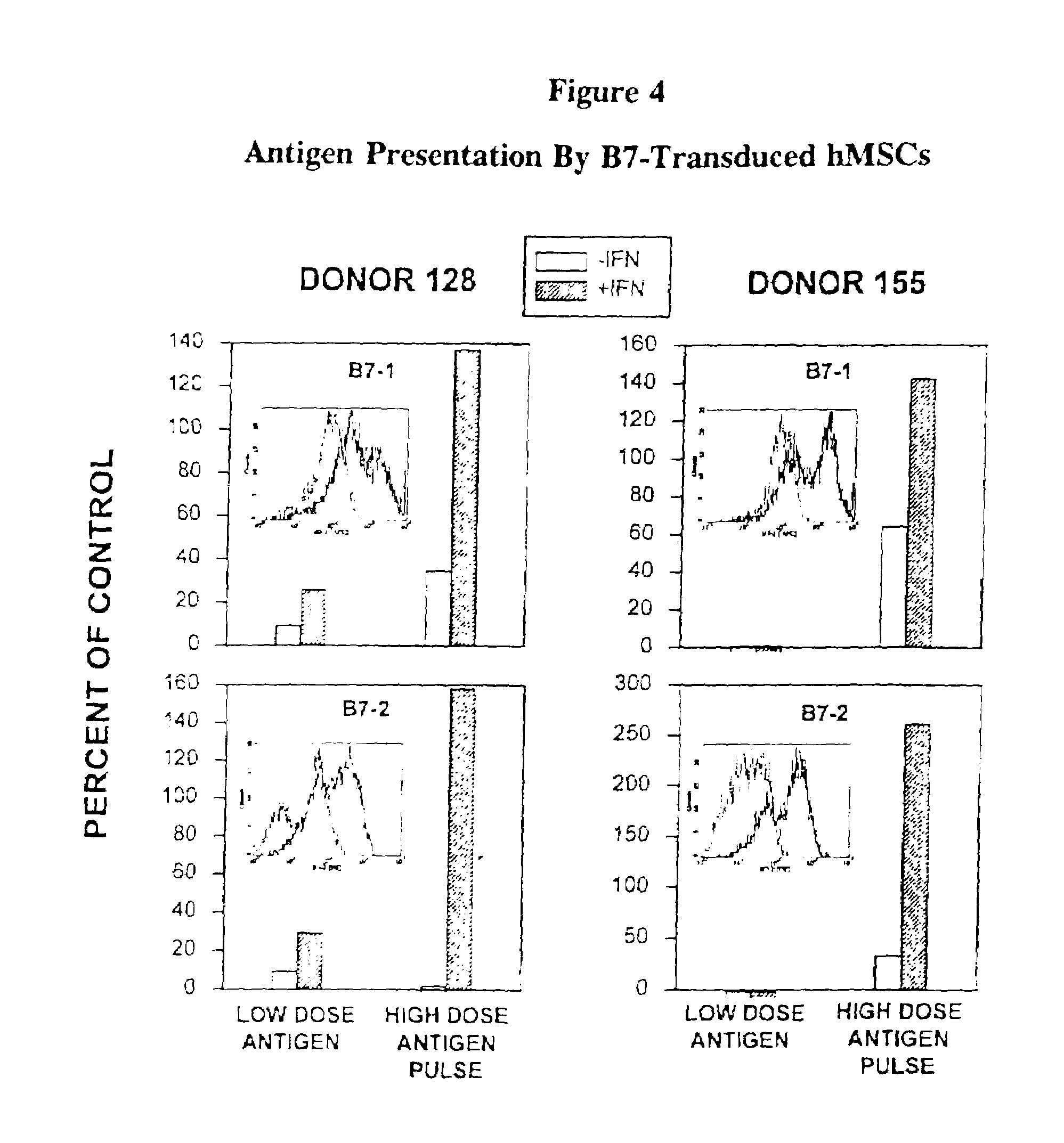
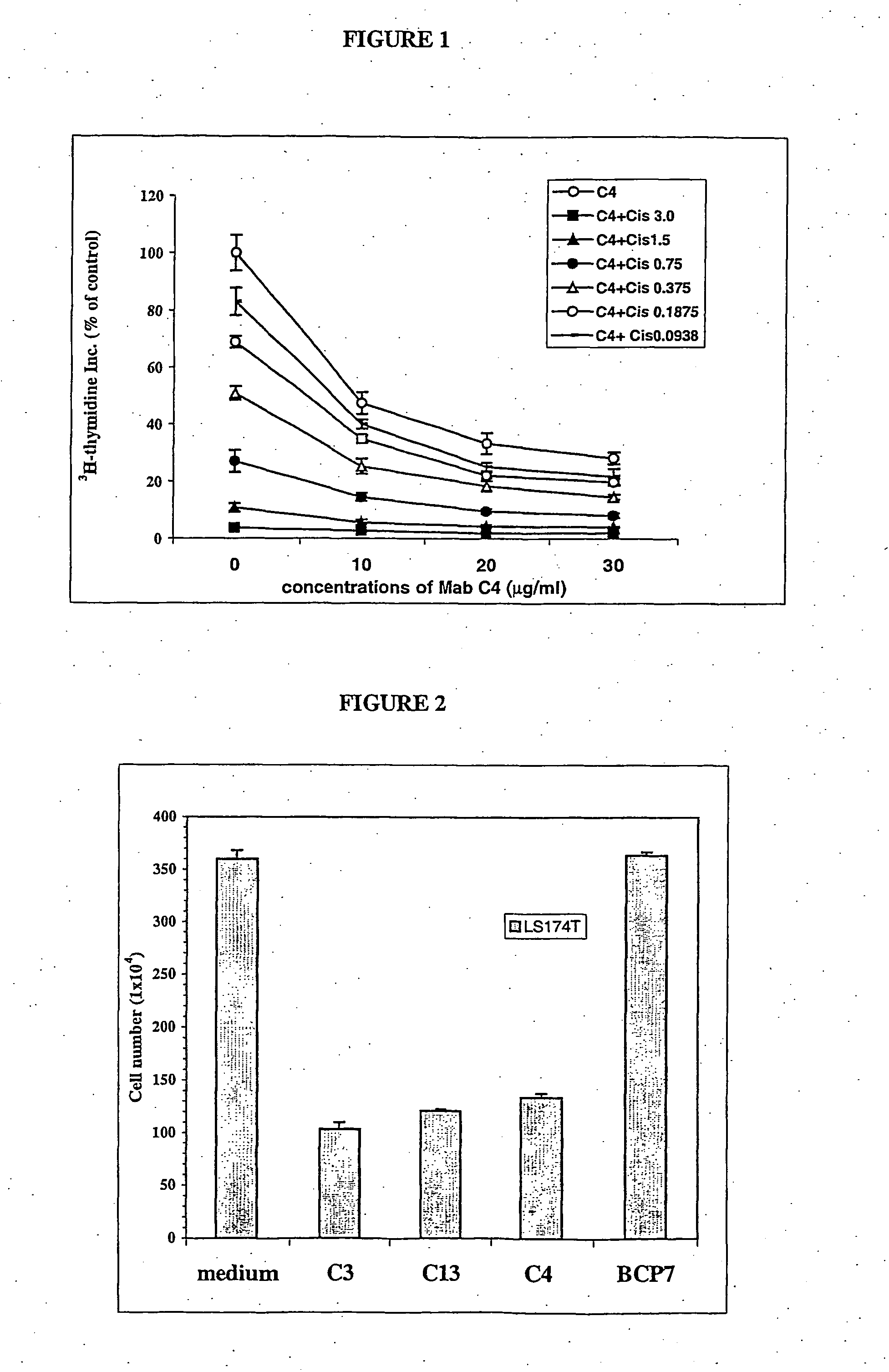
Antigen presenting mesenchymal stem cells
Disclosed is a mesenchymal stem cell and / or cell of the adipocyte lineage that (i) has been modified to have at least one exogenous antigen bound to at least one primary surface molecule of said cell such that said at least one antigen can initiate an immune response and (ii) also expresses at least one co-stimulatory molecule. The antigen is preferably a protein, polypeptide, lipid or glycolipid. The primary surface molecule is MHC I, MHC II or CD1. Also disclosed is a method for stimulating presentation of at least one exogenous antigen fragment on a mesenchymal stem cell primary surface molecule by contacting a mesenchymal stem cell that is capable of expressing at least one co-stimulatory molecule with (i) an exogenous antigen or (ii) genetic material that codes for the exogenous antigen which the mesenchymal stem cell processes into at least one antigen fragment. The method can further include contacting the mesenchymal stem cell with interferon-γ. Also disclosed are a method for determining the state of activation of a T lymphocyte population and a method for the treatment or prevention of a disease in an animal.
Owner:MESOBLAST INT
Antibodies against cancer
InactiveUS20040176576A1Reduce the number of cellsEnhanced inhibitory effectPeptide/protein ingredientsGenetic material ingredientsAnticarcinogenCripto
An isolated binding partner of a Cripto-1 protein, Pim-1 protein or an antigen present in a colon cancer cell lysate is described. The binding partner inhibits growth of one or more cancer cell types and may be used in an anti-cancer agent for treating cancer in a subject. The binding partner may also be used in a method of inducing apoptosis in a cancer cell, as well as in a method of sensitising a cancer cell to a cytotoxic compound. In addition, a cancer Vaccine is described wherein the vaccine comprises a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate or, alternatively, comprises an expressible DNA molecule encoding a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate.
Owner:THE MACFARLANE BURNET INSTITUTE FOR MEDICAL RESEARCH AND PUBLIC HEALTH LTD
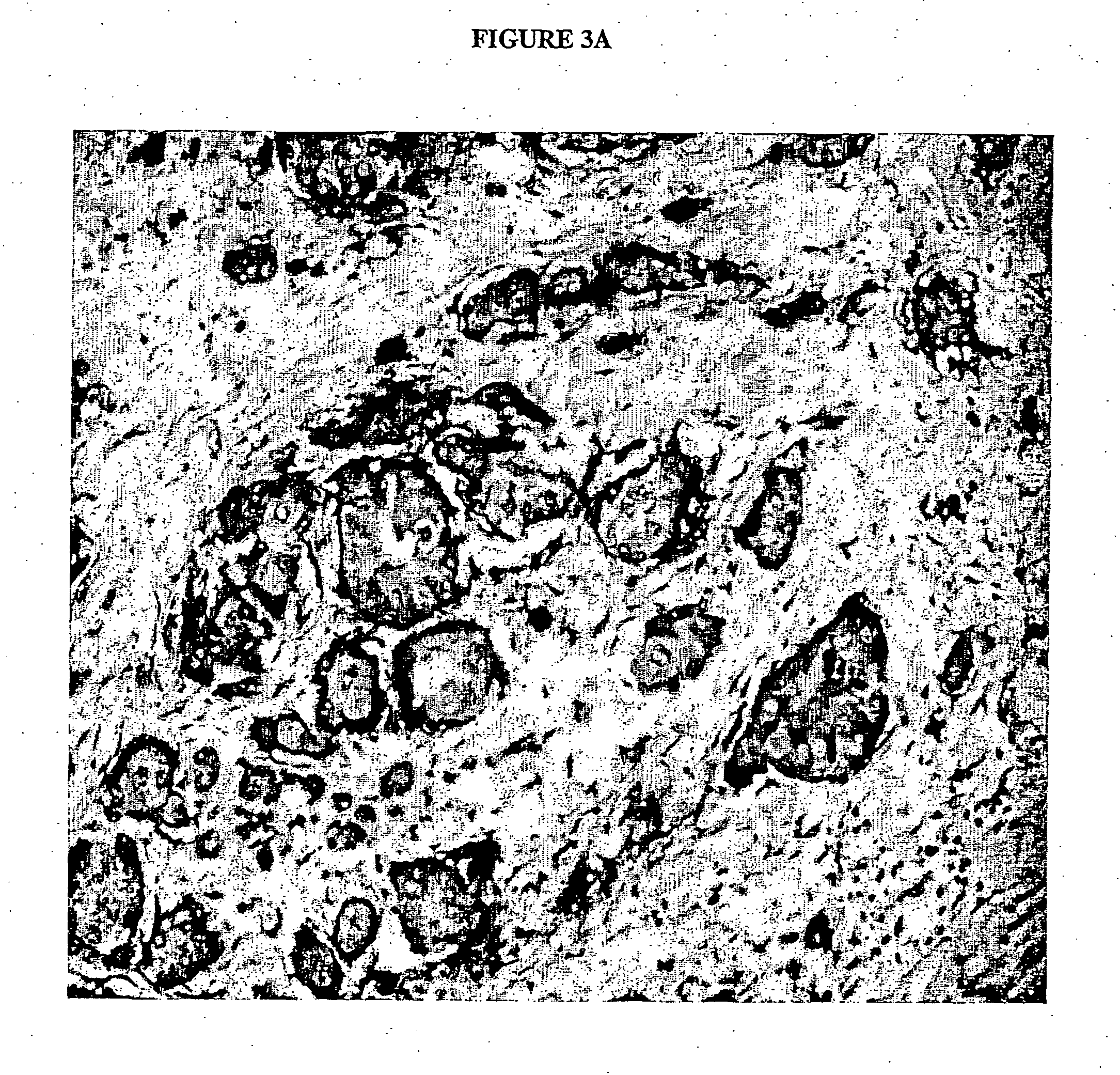
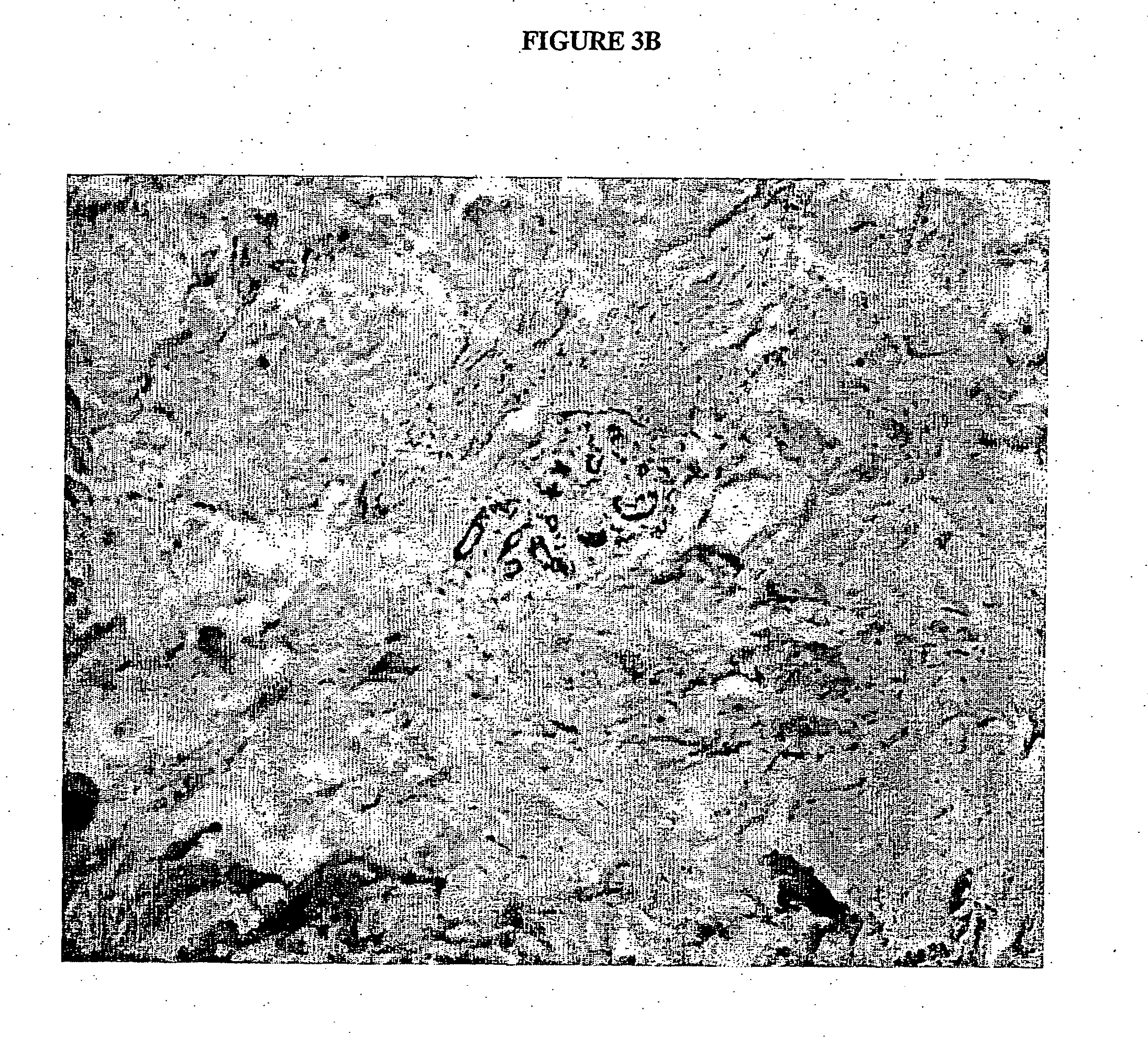
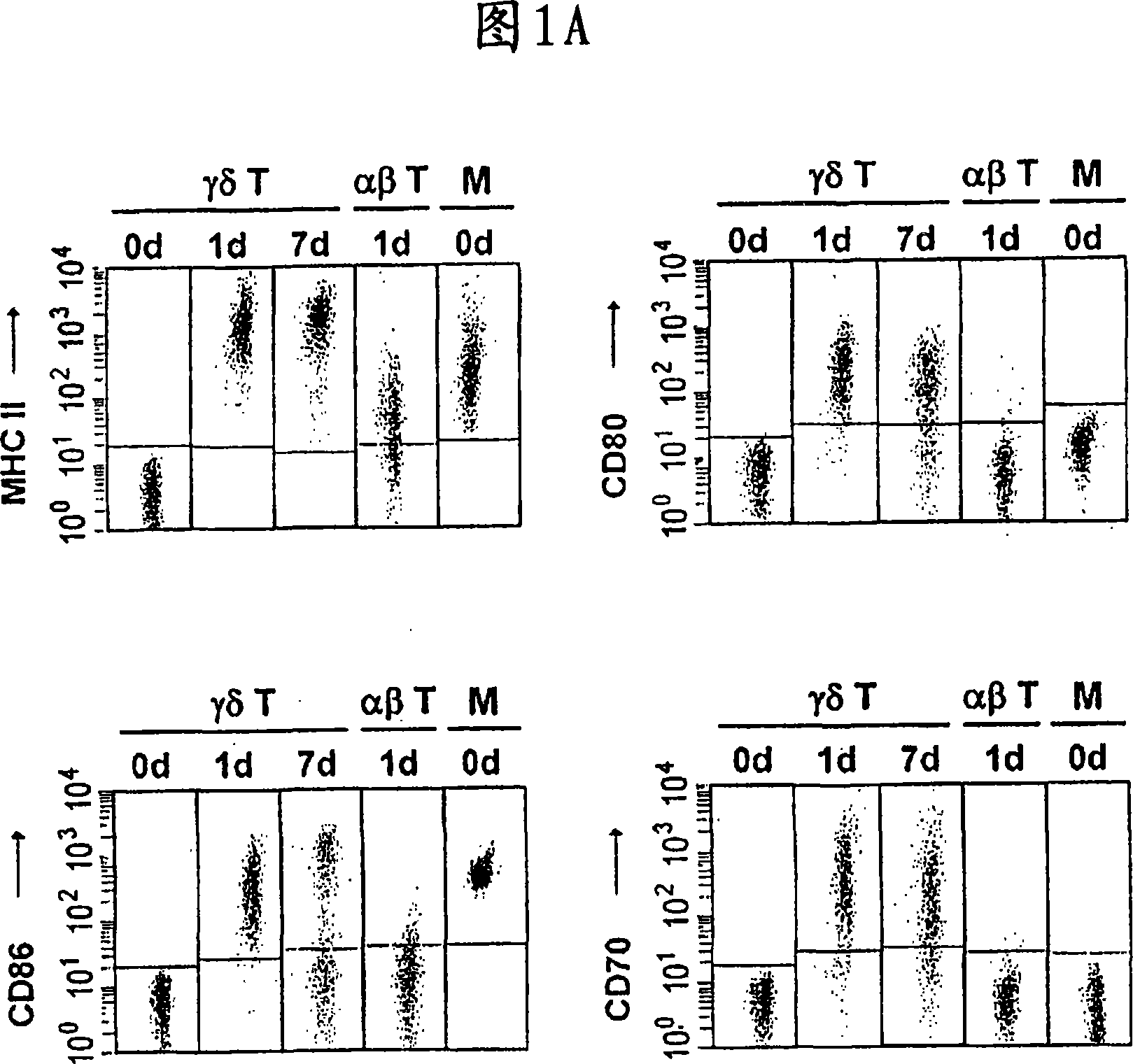
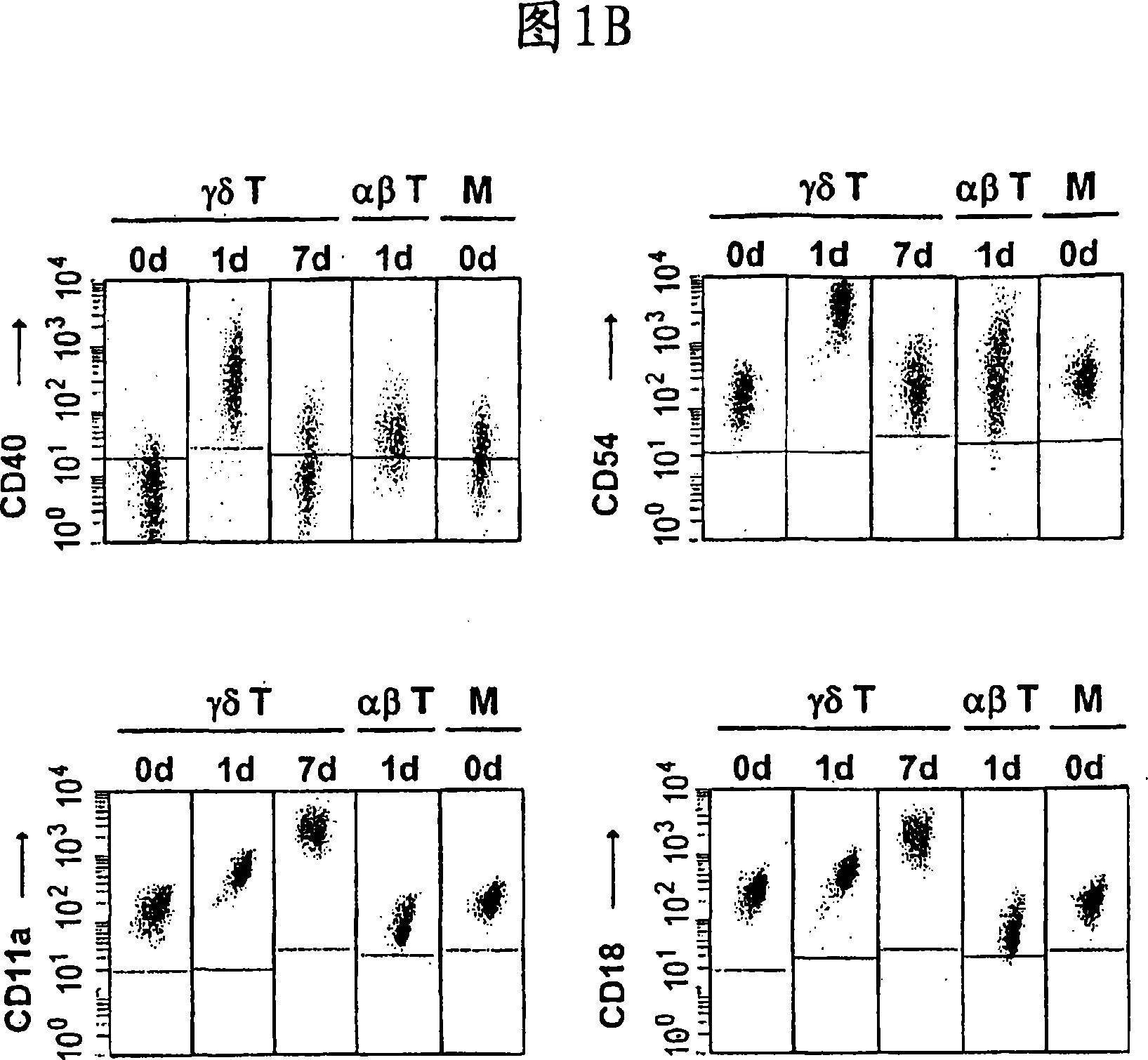
Preparation of antigen-presenting human gamma delta t cells and use in immunotherapy
InactiveCN101031641AGood effectEffective antigen uptakeTissue cultureVaccinationEpidermal Dendritic Cells
The invention relates to a method for the preparation of efficient antigen-presenting human Gamma Delta T cells, to the Gamma Delta T cells prepared by such a method, and to their use in immunotherapy, vaccination, vaccine development and diagnostics. Similar to dendritic cells (DCs) in potency and efficacy, these human Gamma Delta T cells process antigens and present antigenic peptides to Alpha Beta T cells and induce antigen-specific responses (proliferation and differentiation) in nave Alpha Beta T cells. Gamma Delta T cells are easily purified from peripheral blood, acquire''maturation'' status (expression of essential adhesion, co-stimulatory and major histocompatibility complex molecules) within 1 day of in vitro culture under stimulation and induce strong primary and secondary T helper cell and cytotoxic T cell responses. The Gamma Delta T cells may be used in a method of treatment of tumors or chronic or recurrent infectious diseases, in identification of novel tumor or pathogen-derived antigens, and in the diagnosis of the immune competence of a patient.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
System and methods for detection of Bacillus anthracis related analytes in biological fluids
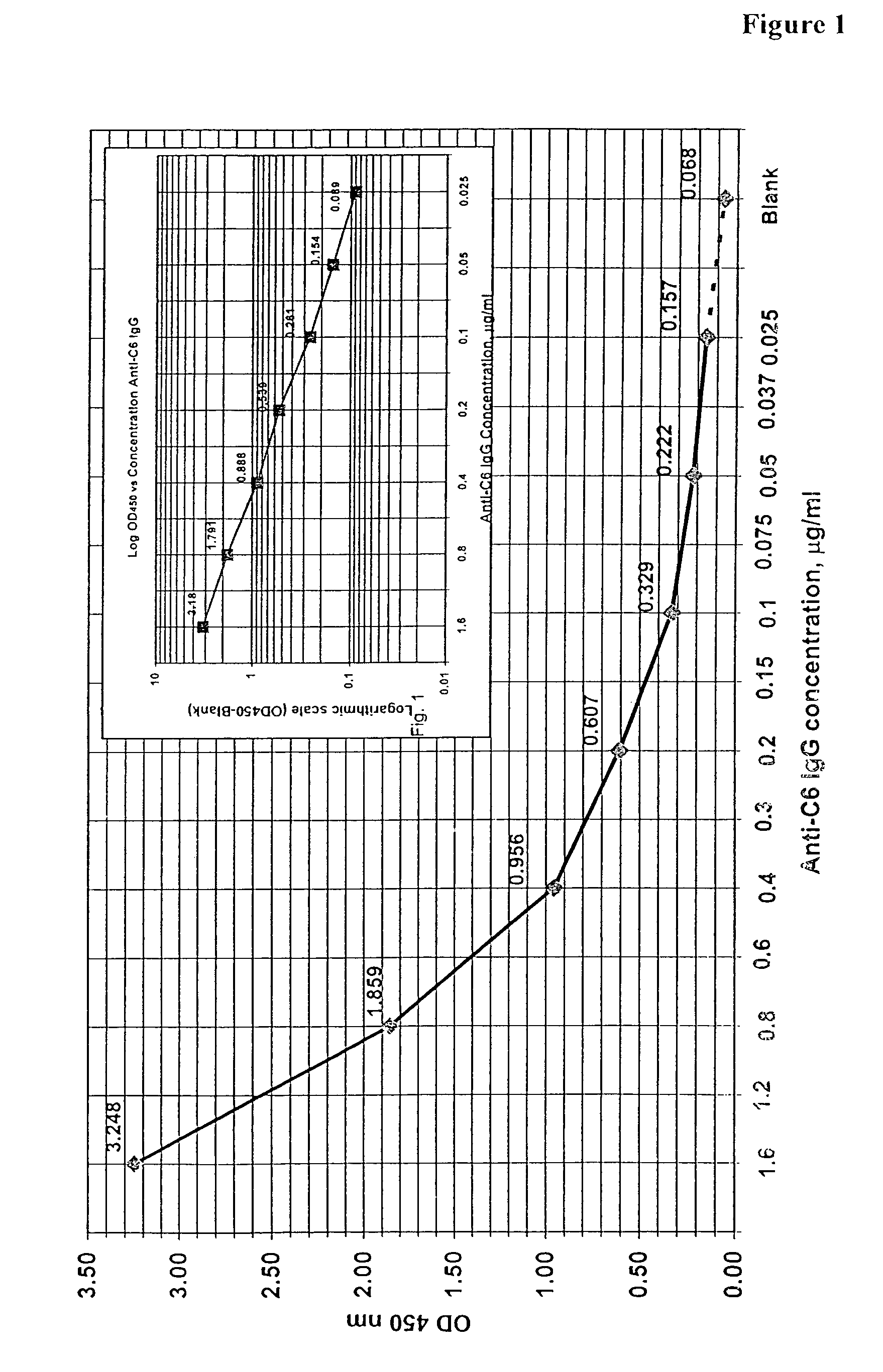
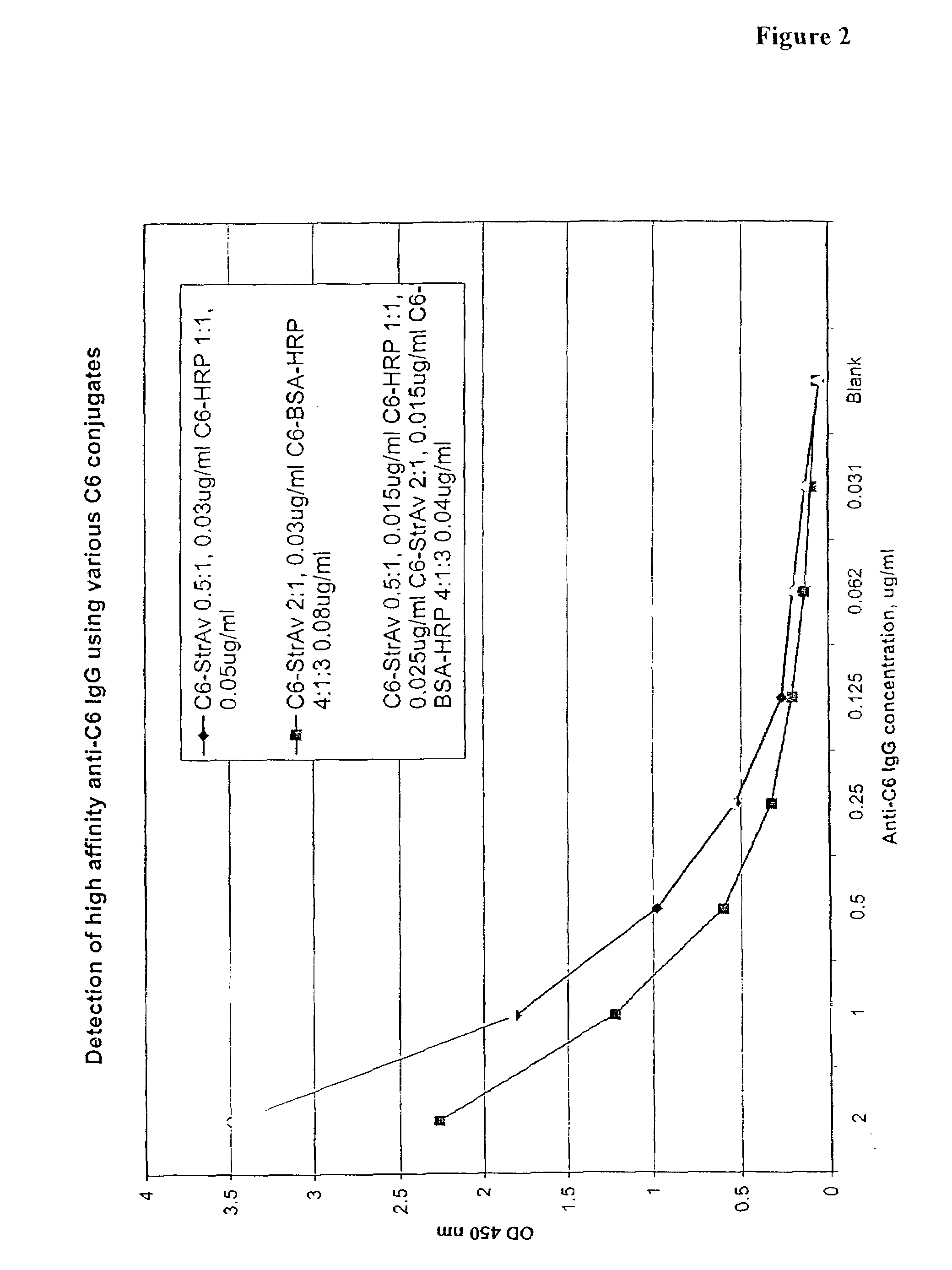
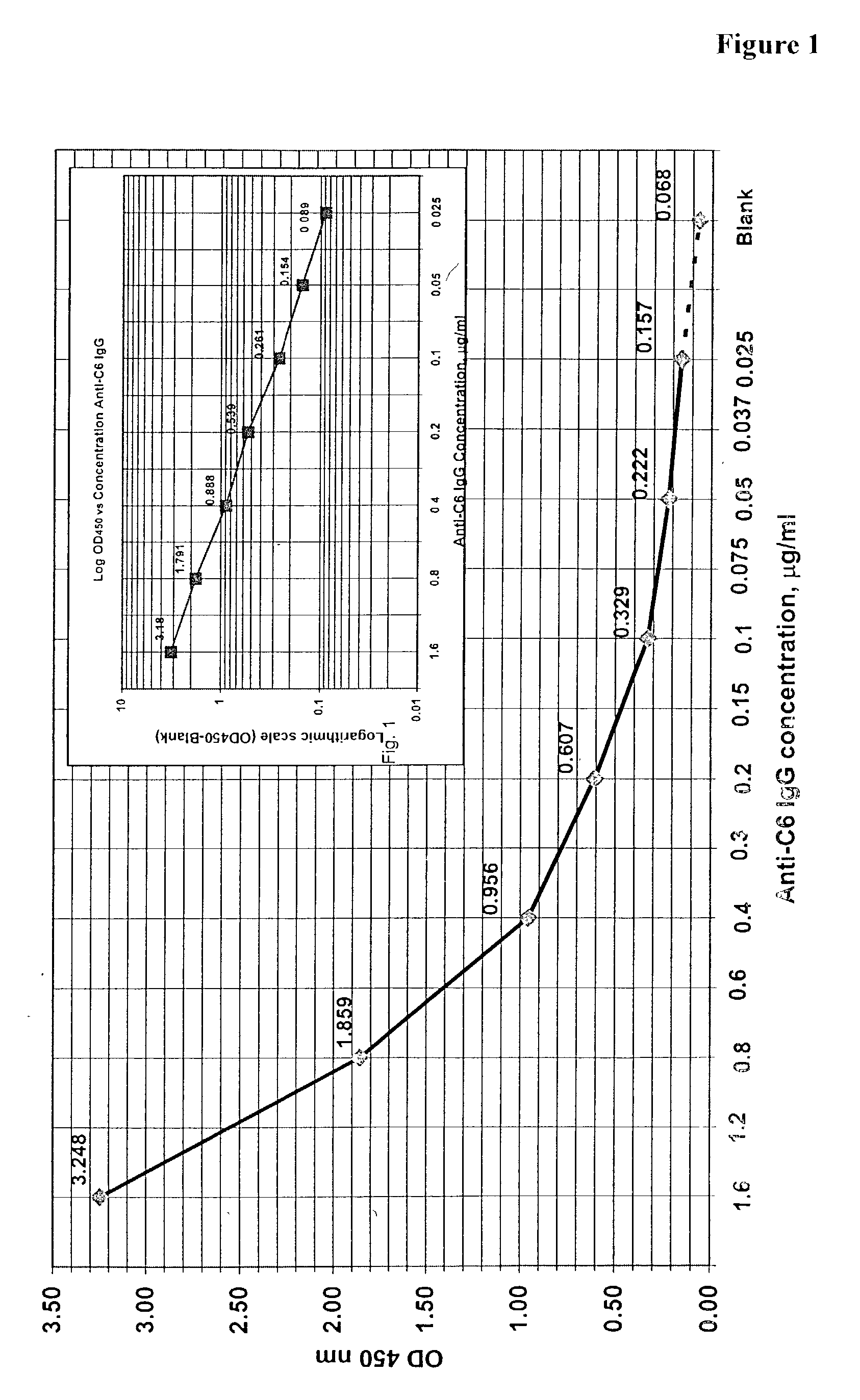
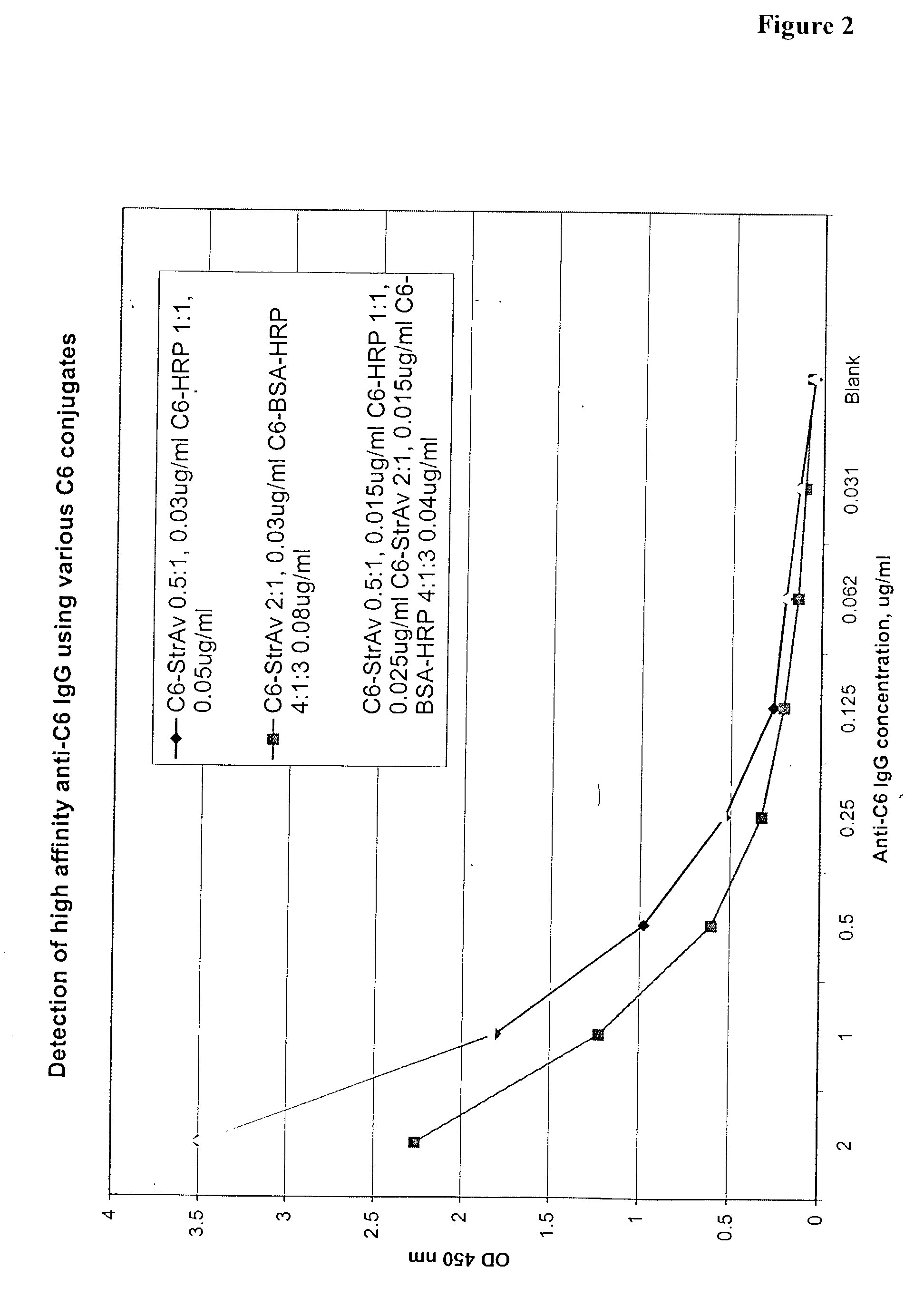
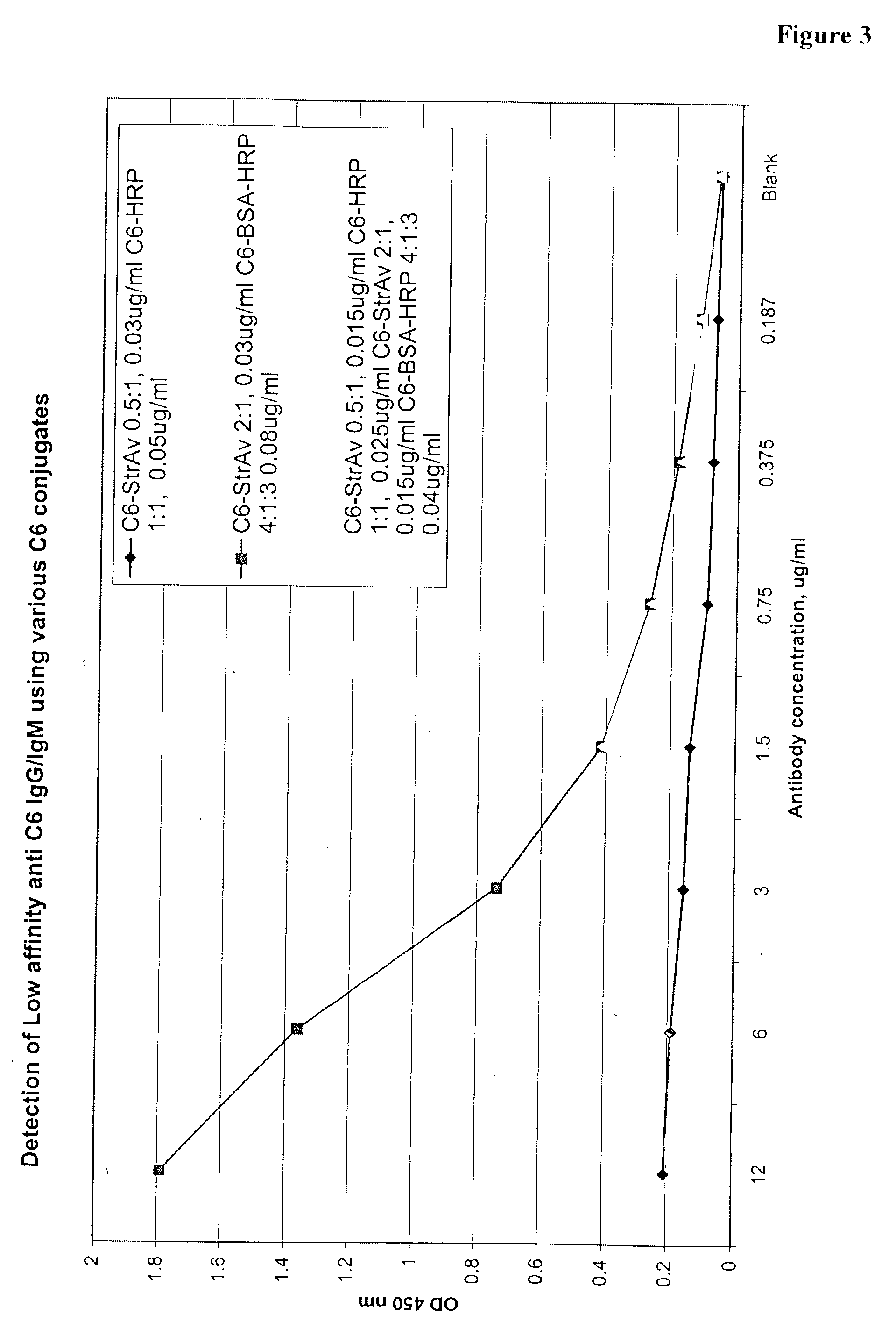
The invention provides a heterogeneous immunoassay for detection of antibodies and antigens based on specific antigen-antibody immune complex formation with multiple antigen-bearing conjugate components. The invention further provides means for optimizing the assay format for the detection of both low and high-affinity antibodies, and provides means for quantitative detection of both antibody and the corresponding antigen present in a sample. In preferred embodiments, the invention can be practiced to detect presence in biological fluid samples of antibodies to Bacillus anthracis related antigens.
Owner:IMMUNETICS
Methods for producing functional antigen presenting dendritic cells using biodegradable microparticles for delivery of antigenic materials
InactiveUS20050158856A1Efficient targetingIncrease probabilityArtificial cell constructsCell culture supports/coatingDendritic cellMicroparticle
Methods are provided for producing functional antigen presenting dendritic cells. The dendritic cells are produced by treating an extracorporeal quantity of a subject's blood to induce differentiation of blood monocytes into dendritic cells. The dendritic cells may be exposed to cellular material encapsulated within a biodegradable polymer material to produce the antigen presenting dendritic cells.
Owner:EDELSON RICHARD L +3
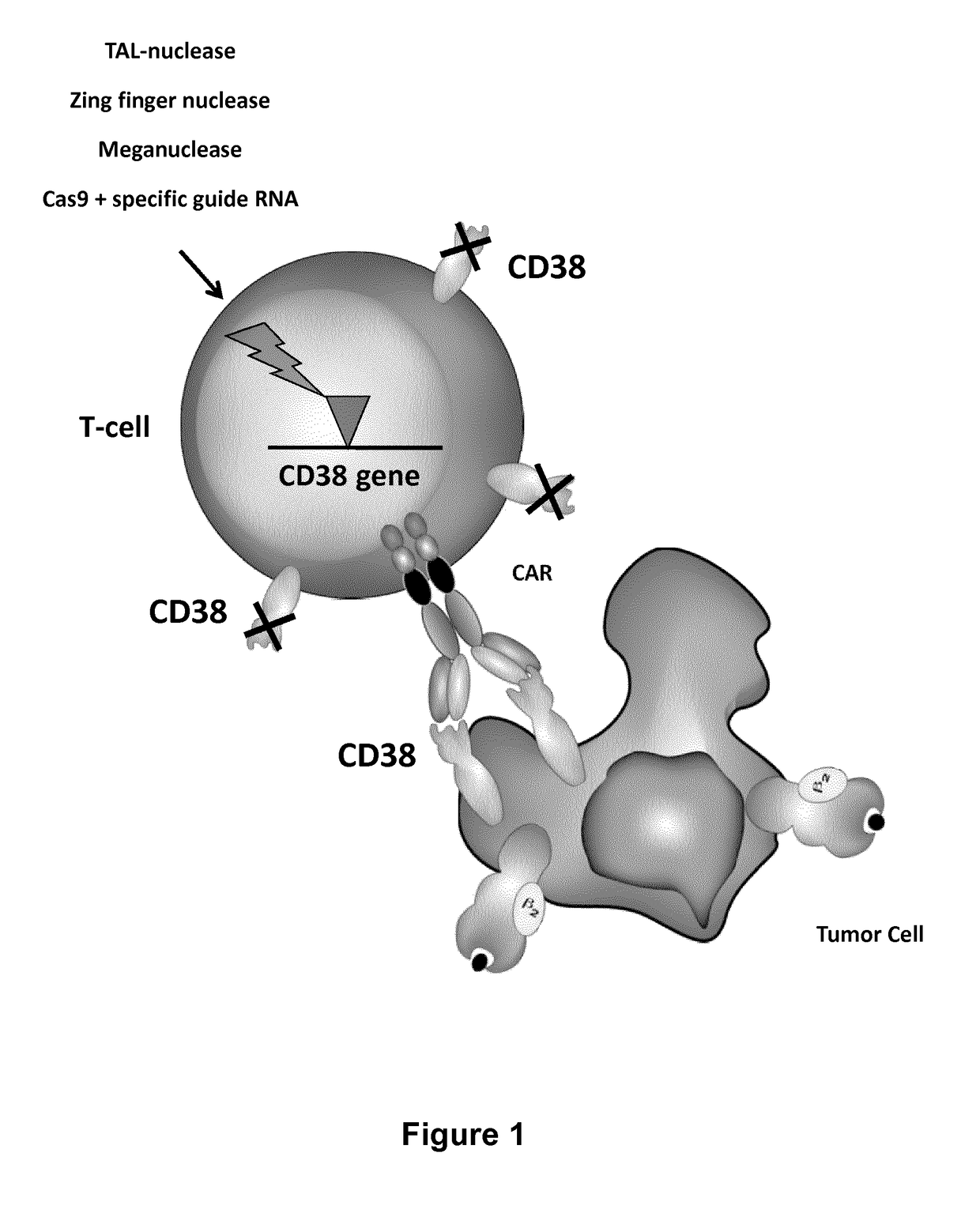
Cells for immunotherapy engineered for targeting antigen present both on immune cells and pathological cells
ActiveUS20180201901A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigens present
Methods of developing genetically engineered immune cells for immunotherapy, which can be endowed with Chimeric Antigen Receptors targeting an antigen marker that is common to both the pathological cells and said immune cells (ex: CD38, CS1 or CD70) by the fact that the genes encoding said markers are inactivated in said immune cells by a rare cutting endonuclease such as TALEN, Cas9 or argonaute.
Owner:CELLECTIS SA
Compositions capable of specifically binding particular human antigen presenting molecule/pathogen-derived antigen complexes and uses thereof
Owner:TECHNION RES & DEV FOUND LTD
Administration of dendritic cells partially matured in vitro for the treatment of tumors
InactiveUS20060057120A1Efficient up takeEasy to handleBiocideCulture processAbnormal tissue growthDendritic cell
The present invention provides populations of cells comprising partially matured dendritic cells that can be used for administration individuals having a tumor. Partially matured dendritic cells, those contacted with a dendritic cell maturation agent for about 1 to about 10 hours, or more, efficiently take up and process tumor antigens in the area of the tumor site, complete maturation, and can subsequently migrate to the lymph nodes of a treated individual. Once in the lymph node the now fully mature antigen presenting dendritic cells secrete the appropriate cytokines (e.g., TNFα and IL-12) and contact T cells inducing a substantial anti-tumor immune response.
Owner:NORTHWEST BIOTHERAPEUTICS INC
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS20090041804A1Enhance immune responseFacilitated DiffusionImmunoglobulin superfamilyGenetic material ingredientsDendritic cellOrganism
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of these costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of Tell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater than the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+ and CD8+ T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced antigen-presenting functions.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY DEPARTMENT OF HEALTH AND HUMAN SERVICES
Systems and methods for detection of analytes in biological fluids
The invention provides a heterogeneous immunoassay for detection of antibodies and antigens based on specific antigen-antibody immune complex formation with multiple antigen-bearing conjugate components. The invention further provides means for optimizing the assay format for the detection of both low and high-affinity antibodies, and provides means for quantitative detection of both antibody and the corresponding antigen present in a sample.
Owner:IMMUNETICS
Antigen presenting vesicles
The present invention relates to an antigen presenting membrane vesicle comprising on its surface a composition of either relevant molecules for antigen-specific activation or deactivation of T lymphocytes and which is present in the form of an artificially induced lipid vesicle budded from a plasma membrane of a eukaryotic, preferably human, cell, and wherein the composition of said relevant molecules for activation or deactivation present on the vesicle surface is adjustable to a recipient's needs or requirements independently of any blood or tissue cells of said recipient. The invention further relates to a method of manufacture of the vesicles and to compositions containing the vesicles as well as to the use of the vesicles for various purposes including medical and diagnostic applications.
Owner:WINFRIED PICKL +1
Modified Organs and Cells for Xenotransplantation
InactiveUS20070089178A1Maintain integrityMaintaining viabilityBiocideGenetic material ingredientsHuman bodyGenetically engineered
Owner:RBC BIOTECH
Detection of analytes in fecal samples
A method for detecting a parasitic worm infection of the digestive tract of a mammal. The method includes detecting the binding of a worm antibody to a worm antigen present in the soluble portion of a fecal sample of infected mammals.
Owner:IDEXX LABORATORIES
Modified organs and cells for xenotransplantation
InactiveUS7166278B2Maintain integrityMaintaining viabilityBiocidePeptide/protein ingredientsHeterograftsEngineered genetic
It has been discovered that there are at least two significant antigens present on the cells of animal species such as pigs that elicit an immune or inflammatory response immediately upon implantation into humans or contact with human serum. The first is an α-galactosyl (Gal) epitope, for example, Galα(1->3)Galβ(1->4)GlcNac (linear B type 2) or Galα(1->3) Galβ(1->4)Glc (linear B type 6). The second is an N-glycolylneuraminic acid (NeuGc) structure. By eliminating these epitopes, preferably by genetically engineering the animal so that the epitope is either not produced or is greatly reduced, or by chemical or enzymatic treatment of the animal's cells to remove the epitopes, it is possible to produce organs, tissues and cells suitable for xenotransplantation into humans. Cells can be rendered even more compatible by genetically engineering the animal to express a human complement regulatory protein (inhibitor), such as CD59, on its cells, or to express an excess of a pig complement regulatory protein.
Owner:RBC BIOTECH
Use of human prostrate cell lines in prostate cancer treatment
InactiveUS6972128B1Promote growthSufficient characteristicBiocidePeptide/protein ingredientsGamma irradiationProstate cancer
The invention here relates to a product comprised of a cell line or lines intended for use as an allogeneic immunotherapy agent for the treatment of cancer in mammals and humans. All of the studies of cell-based cancer vaccines to date have one feature in common, namely the intention to use cells that contain at least some TSAs and / or TAAs that are shared with the antigens present in patients' tumour. In each case, tumour cells are utilised as the starting point on the premise that only tumour cells will contain TSAs or TAAs of relevance, and the tissue origins of the cells are matched to the tumour site in patients. A primary aspect of the invention is the use of immortalised normal, non-malignant cells as the basis of an allogeneic cell cancer vaccine. Normal cells do not possess TSAs or relevant concentrations of TAAs and hence it is surprising that normal cells are effective as anti-cancer vaccines. For prostate cancer, for example, a vaccine may be based on one or a combination of different immortalised normal cell lines derived from the prostate. The cell lines are lethally irradiated utilising gamma irradiation at 50–300 Gy to ensure that they are replication incompetent prior to use in the mammal or human.
Owner:ONYVAX
Assays for autoantibodies
A method and kit for screening a sample of body fluid for at least one autoantibody to at least one antigen. A source of at least one antigen to the autoantibody is provided. A substrate having immobilized thereto at least one antibody to the antigen is also provided. The antigen source is contacted with the sample of body fluid, so as to obtain a mixture wherein the antigen is allowed to substantially bind with the autoantibody, when the latter is present in the sample of body fluid. The mixture is allowed to flow relative to the substrate so as to allow the mixture to contact the antibody immobilized to the substrate. Labeling means are provided to permit monitoring of binding of the autoanitbody and the antigen present in the mixture, so as to provide an indication of the presence of the autoanitbody in the sample of body fluid.
Owner:R S R
Multiple antigen presenting immunogenic composition, and methods and uses thereof
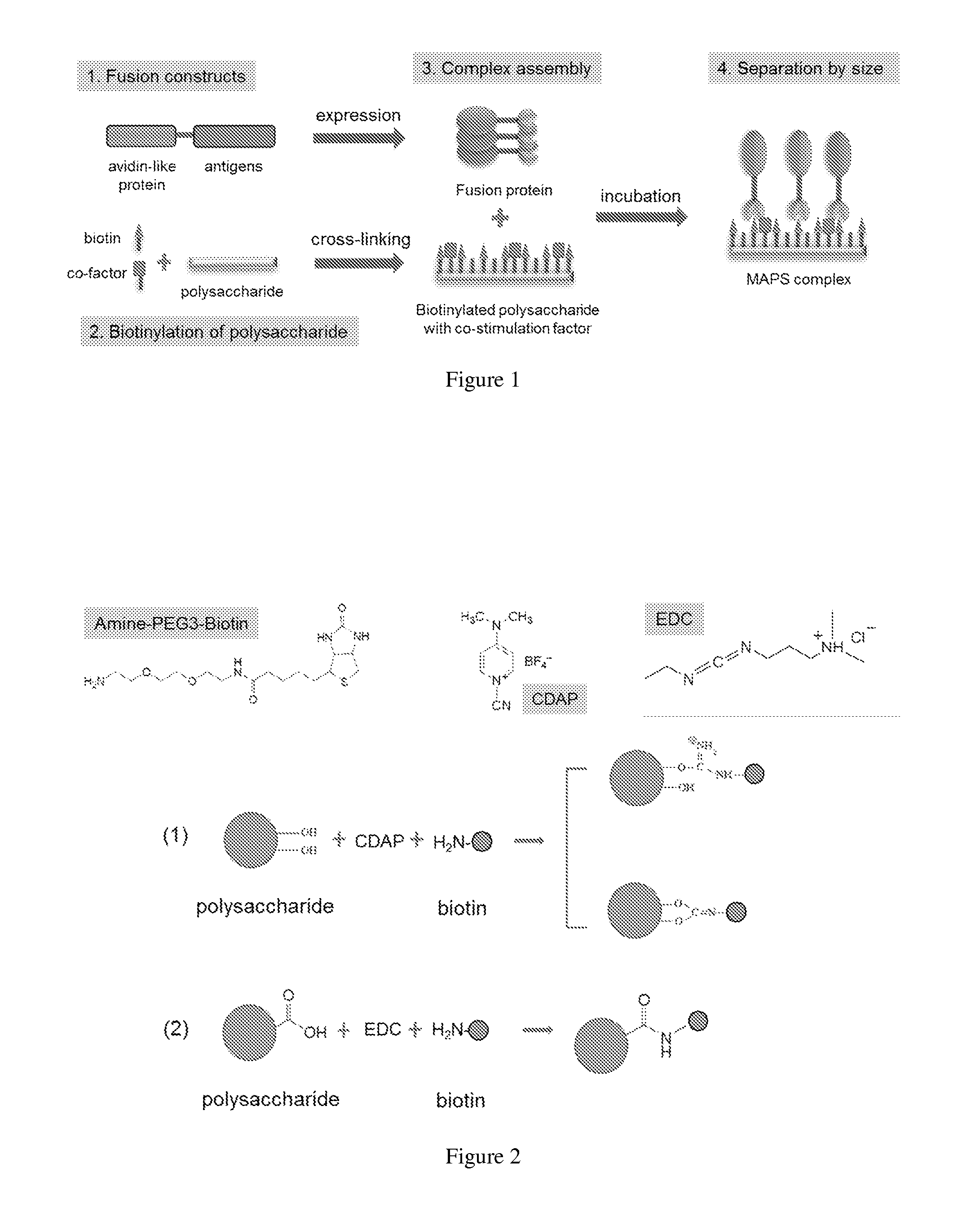
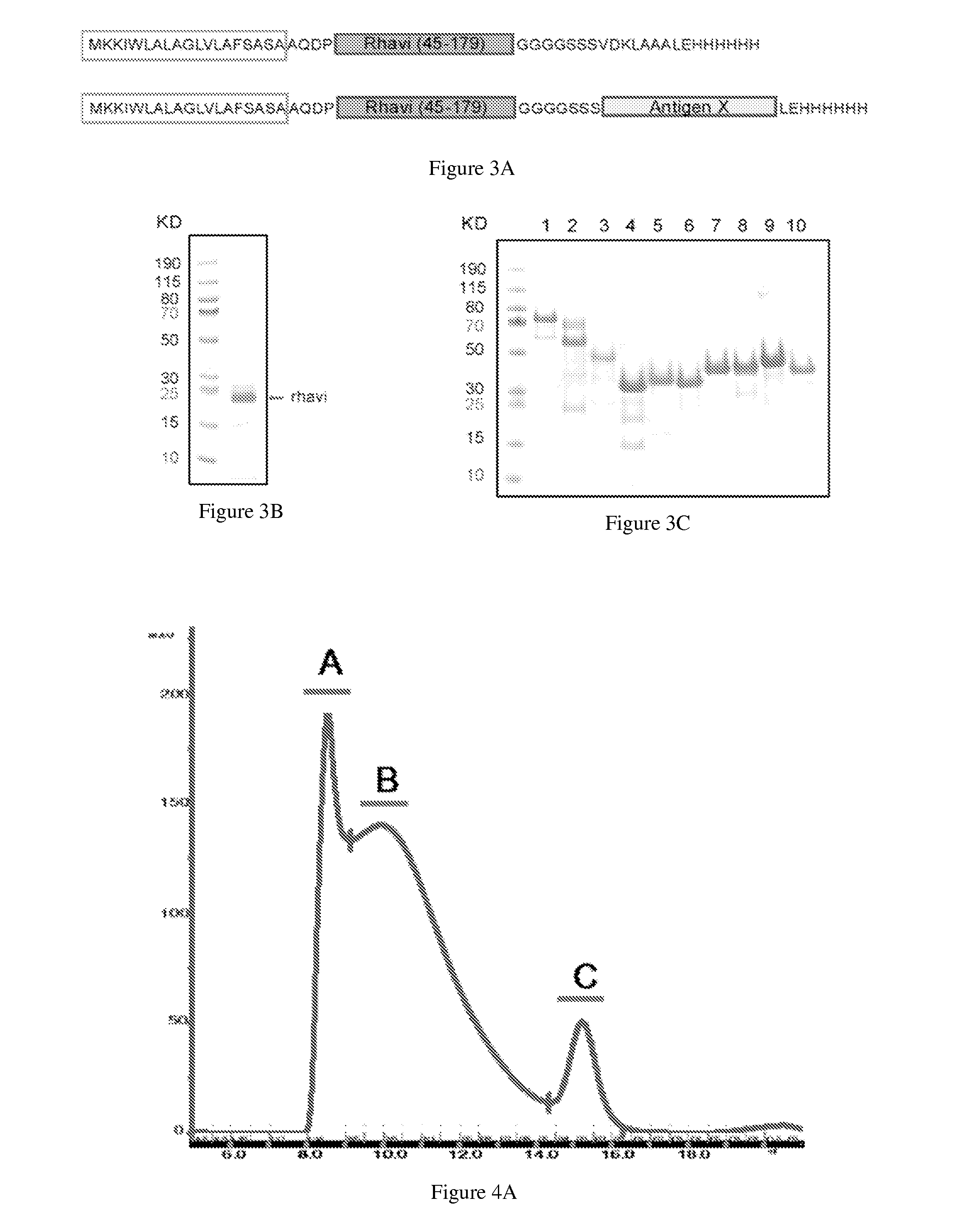
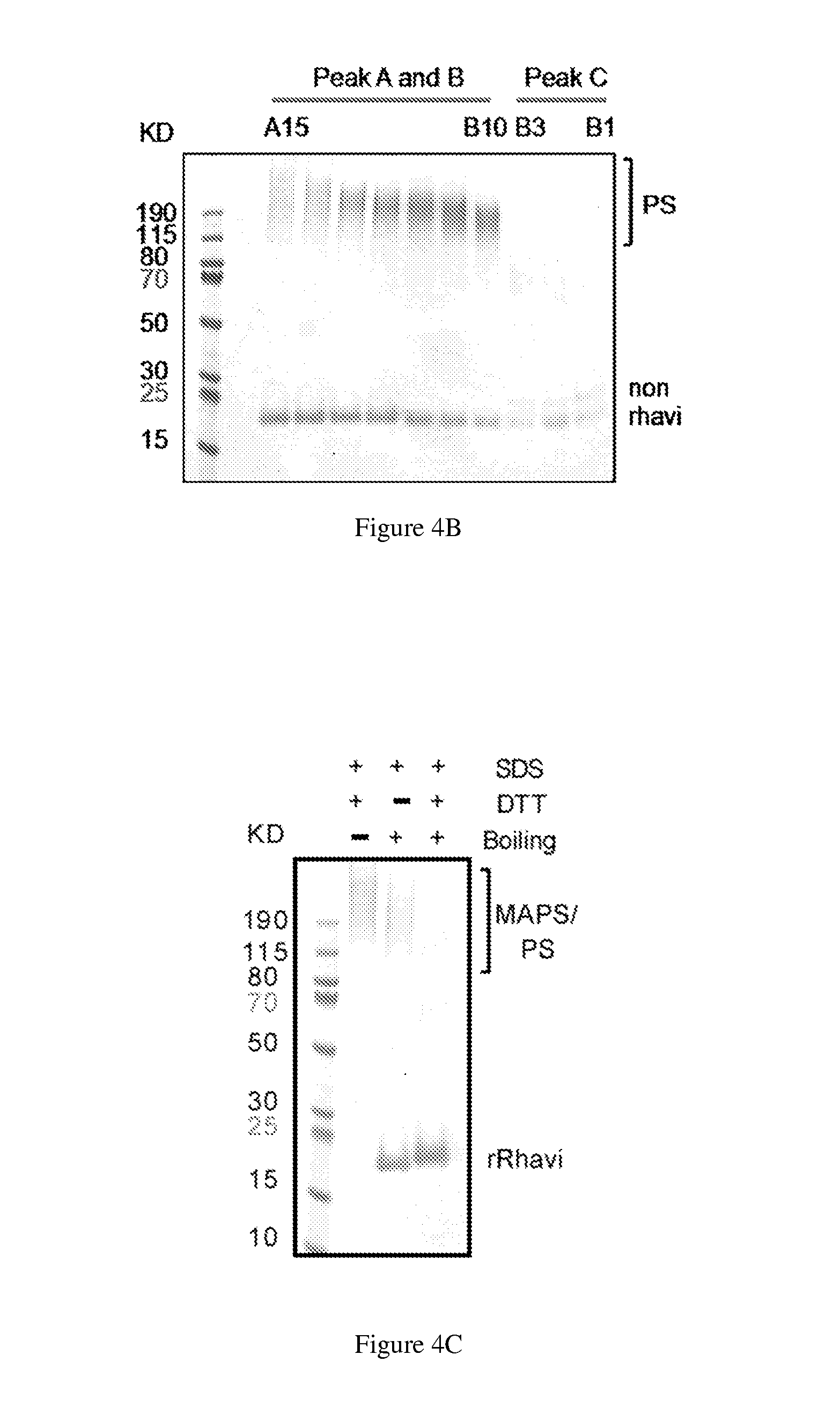
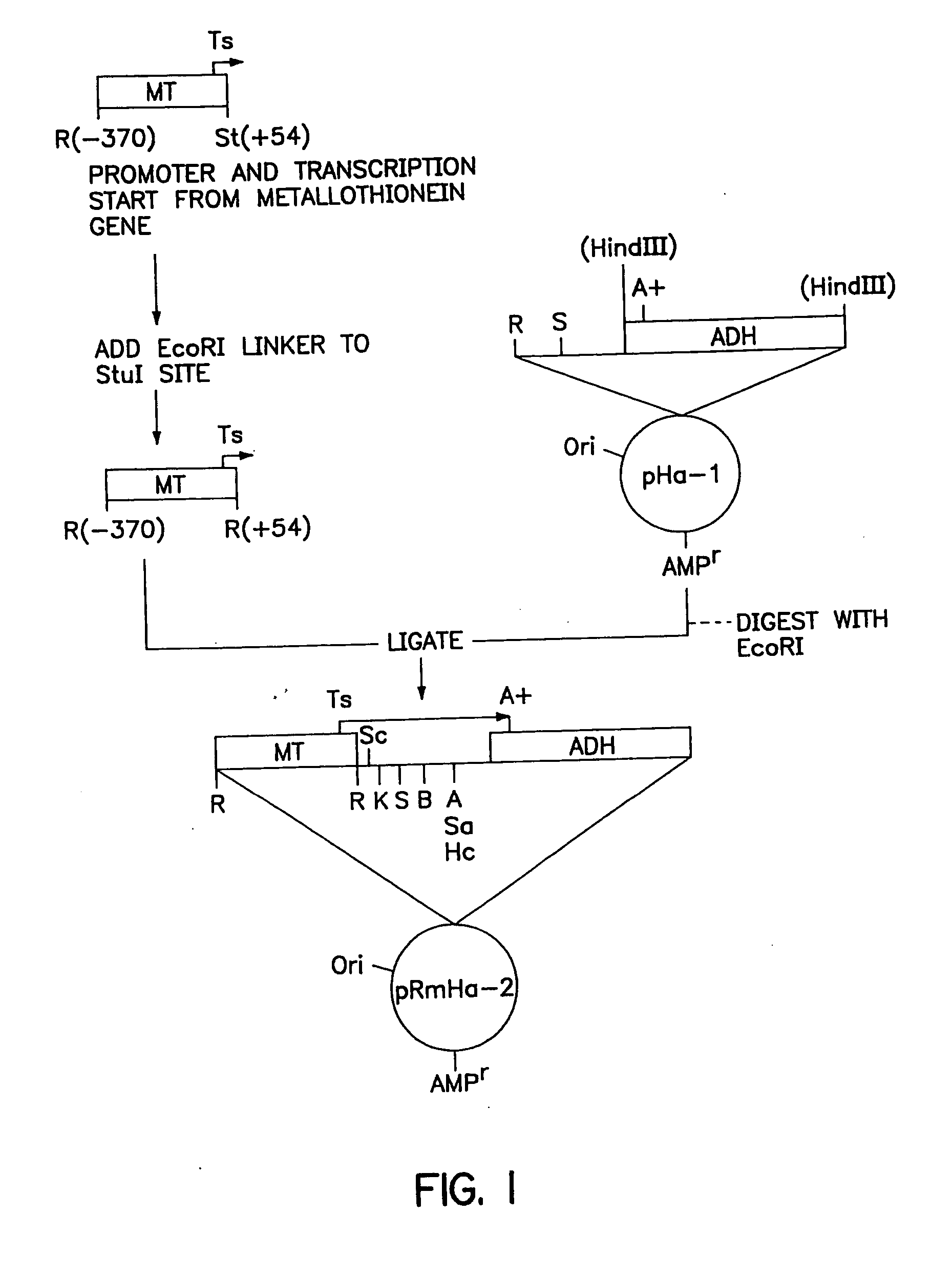
ActiveUS20140154287A1High potencyRobust productionAntibacterial agentsAntibody mimetics/scaffoldsPeptide antigenAntigens present
Owner:CHILDRENS MEDICAL CENT CORP
Antigen presenting system and methods for activation of T-cells
InactiveUS20050152916A1Great T-cell activationPromote activationVirusesPeptide/protein ingredientsCytotoxicityCD8
The present invention relates to synthetic antigen-presenting matrices, their methods of making and their methods of use. One such matrix is cells that have been transfected to produce MHC antigen-presenting molecules and assisting molecules such as co-stimulatory molecules. The matrices can be used to activate CD8+ T-cells to produce cytokines and become cytotoxic.
Owner:THE SCRIPPS RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com