Antigen presenting system and methods for activation of T-cells
a technology of t-cells and presenting systems, applied in the field of t-cell activation systems and presenting systems, can solve the problems of inability to specifically activate cytotoxic t-cells, few successful attempts to use cytotoxic t-cells, and the cost of eliminating a viral infection is the accompanying loss of infected cells, so as to achieve greater t-cell activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of Human Class I MHC Molecules
A. Preparation of pRmHa-3 Expression Vector
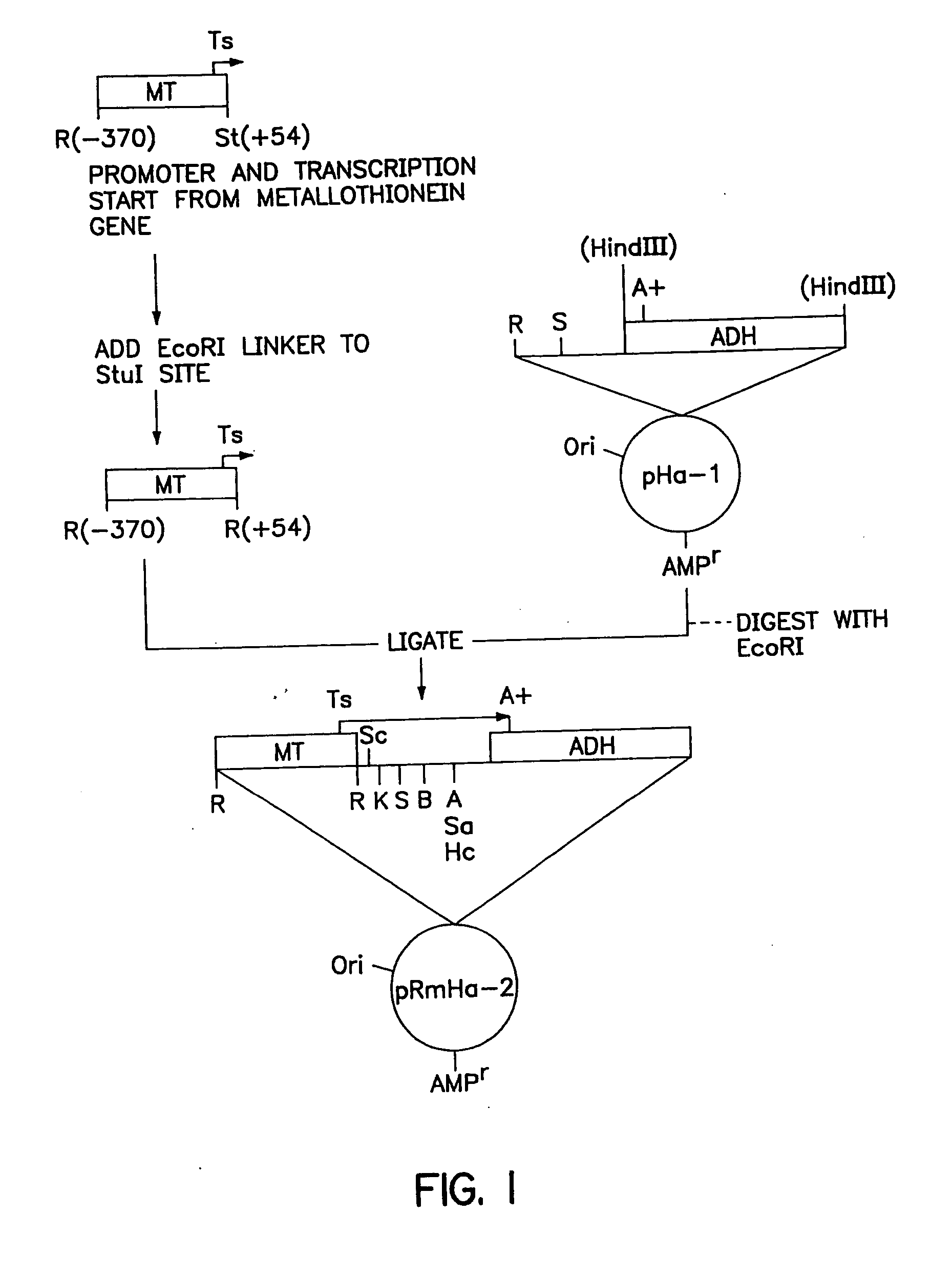
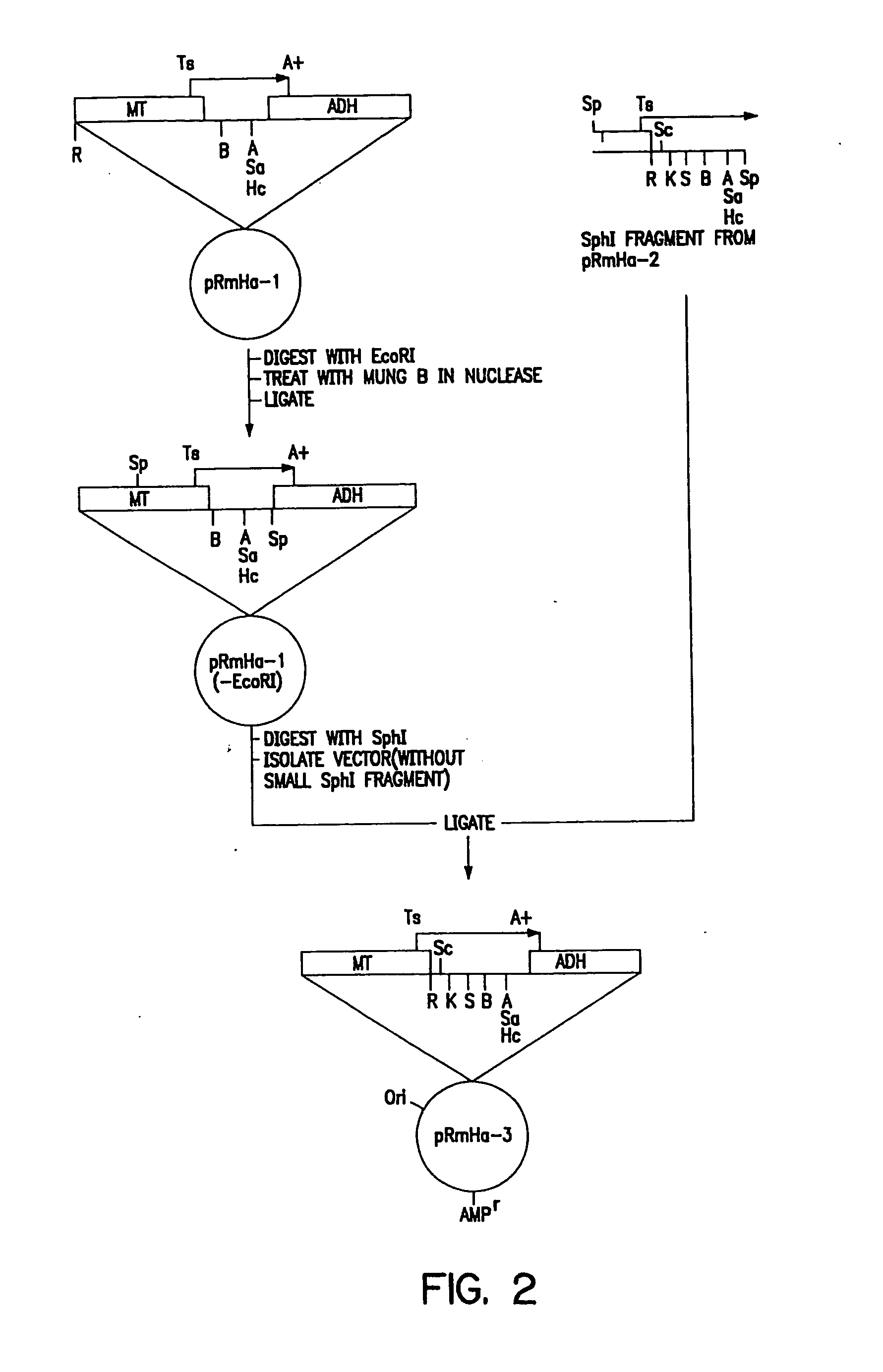
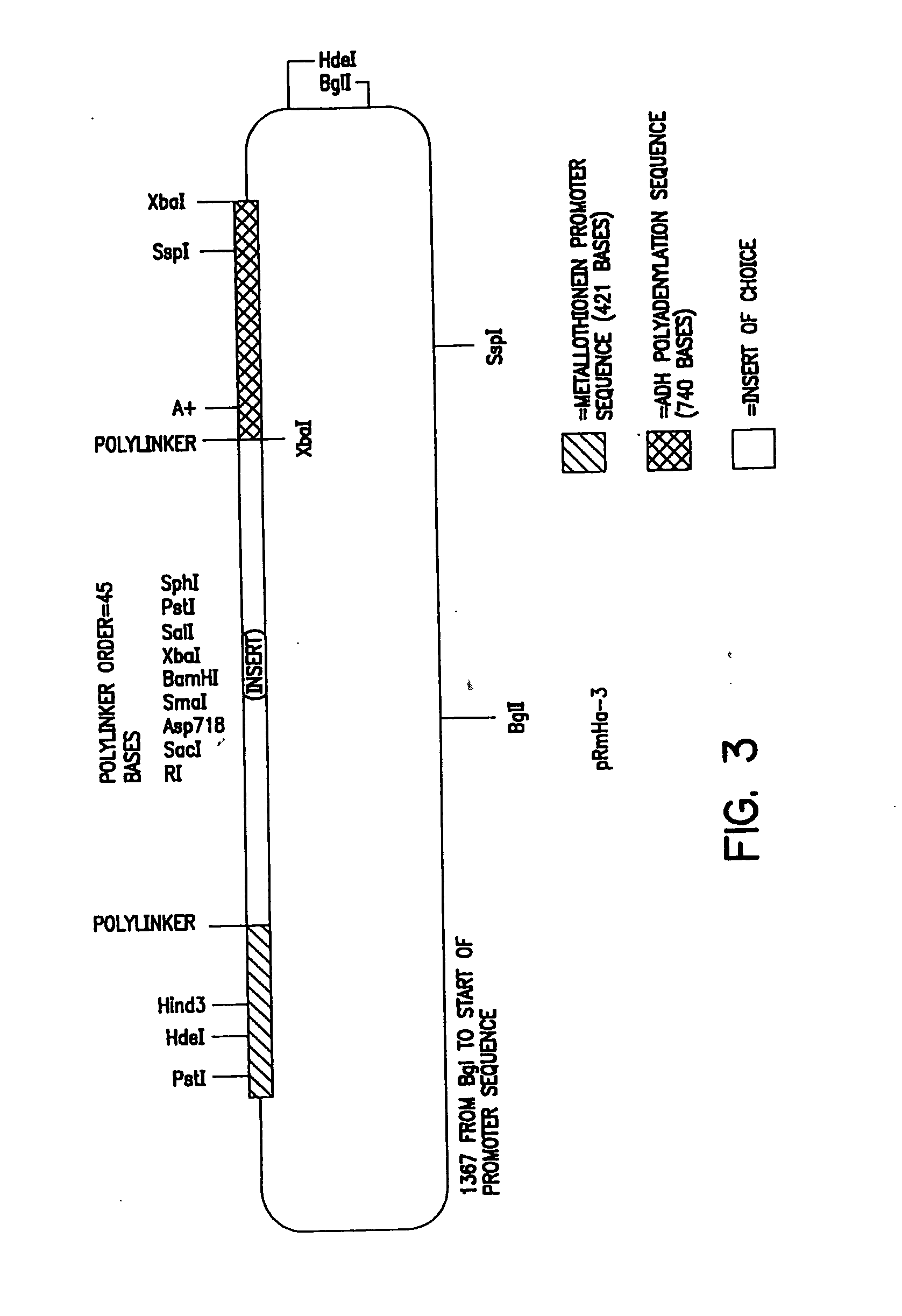
[0150] The pRmHa-3 expression vector for use in expressing MHC proteins in Drosophila Schneider 2 (S2) cells as described in this invention was constructed by ligating a Sph I linearized pRmHa-1 DNA expression vector with a DNA fragment resulting from a Sph I restriction digest of a pRmHa-2 expression vector as described below. The ligating of pRmHa-1 with the pRmHa-2 fragment in this manner was performed to remove one of two Eco RI restriction endonuclease cloning sites present in pRmHa-1. Thus, the resultant pRmHHa-3 expression vector contained only one Eco RI restriction site in the multiple cloning site (polylinker) into which various MHC-encoding DNA fragments were inserted as described in the Examples.
1. Preparation of pRmHa-1 Expression Vector
[0151] The pRmHa-1 expression vector, containing a metallothionein promoter, metal response consensus sequences (designated MT) and an alcohol dehy...
example 2
Preparation of Synthetic Antigen-Presenting Cells
A. Osmotic Loading
[0202] Osmotic loading of SC2 and 3T3 cells with ovalbumin protein was carried out as described by Moore, et al., Cell 54: 777-785 (1988). The assay procedure is as follows. In a 96-well dish, 1×105 Drosophila cells (with or without peptide / protein loaded) or 3T3 cells were cocultured with 1×105 B3 / CD8 T-cell hybridoma cells in 200 μl of RPMI media supplemented with 10% fetal bovine serum. After 24 hours of incubation, 100 μl of the supernatant from these cultures was added to 100 μl of RPMI containing 5,000 CTLL cells. The cells were cocultured for 24 hours at 37° C. when 1 μCi of 3H thymidine (Amersham) was added. After a further incubation of 15 hours at 37° C., the incorporation of radiolabel into the CTLL cells was determined by scintillation counting.
[0203] Assays conducted with murine MHC also verified that the insect cells are capable of loading peptide onto the Class I molecules. Cells expressing as few ...
example 3
Stimulation of Proliferation and Differentiation of Armed Effector T-Cells
[0208] We have found that Drosophila S2 cells transfected with MHC class I molecules and specific assisting molecules are able to stimulate primary responses from T-cells in vitro. We present data below in this example from a mouse model system. In this example, constructs coding for mouse MHC class I (Ld), β2 microglobulin, specific assisting molecules were used and tested with CD8+ cells from lymph nodes of T-cell receptor transgenic mice.
[0209] The data in FIG. 7 provides evidence that the transfected Drosophila S2 cells express the protein products of the transfected murine genes. Flow cytometry using a fluorescence-activated cell sorter (FACS) and fluorescently labeled antibodies were used to demonstrate the expression of Ld (MHC molecule which includes heavy chain and β2) and the specific assisting molecules B7.1 (CD80) and ICAM-1 (CD54) molecules by transfected Drosophila S2 cells. Transfected cells w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| Eco RI | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com