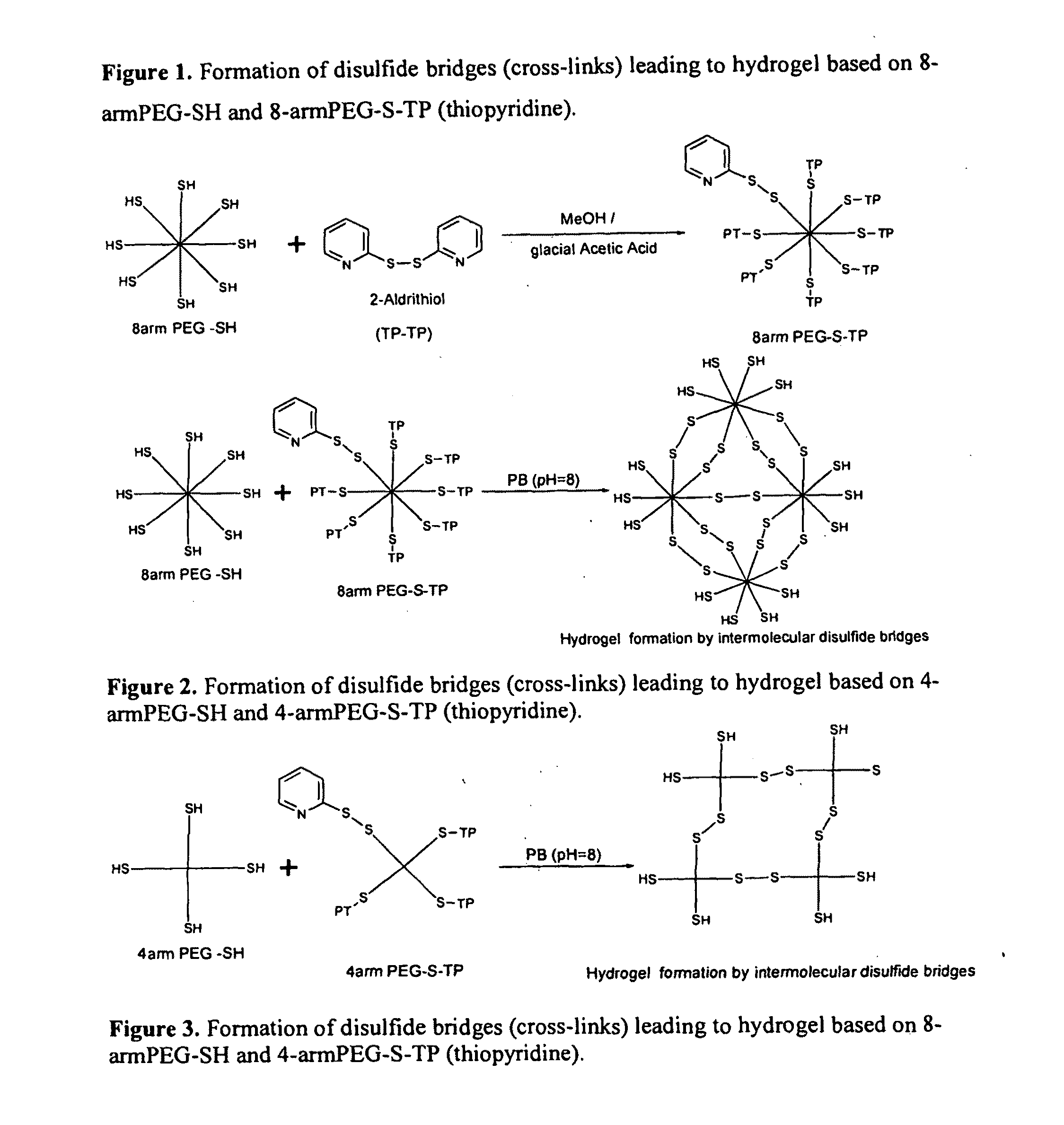
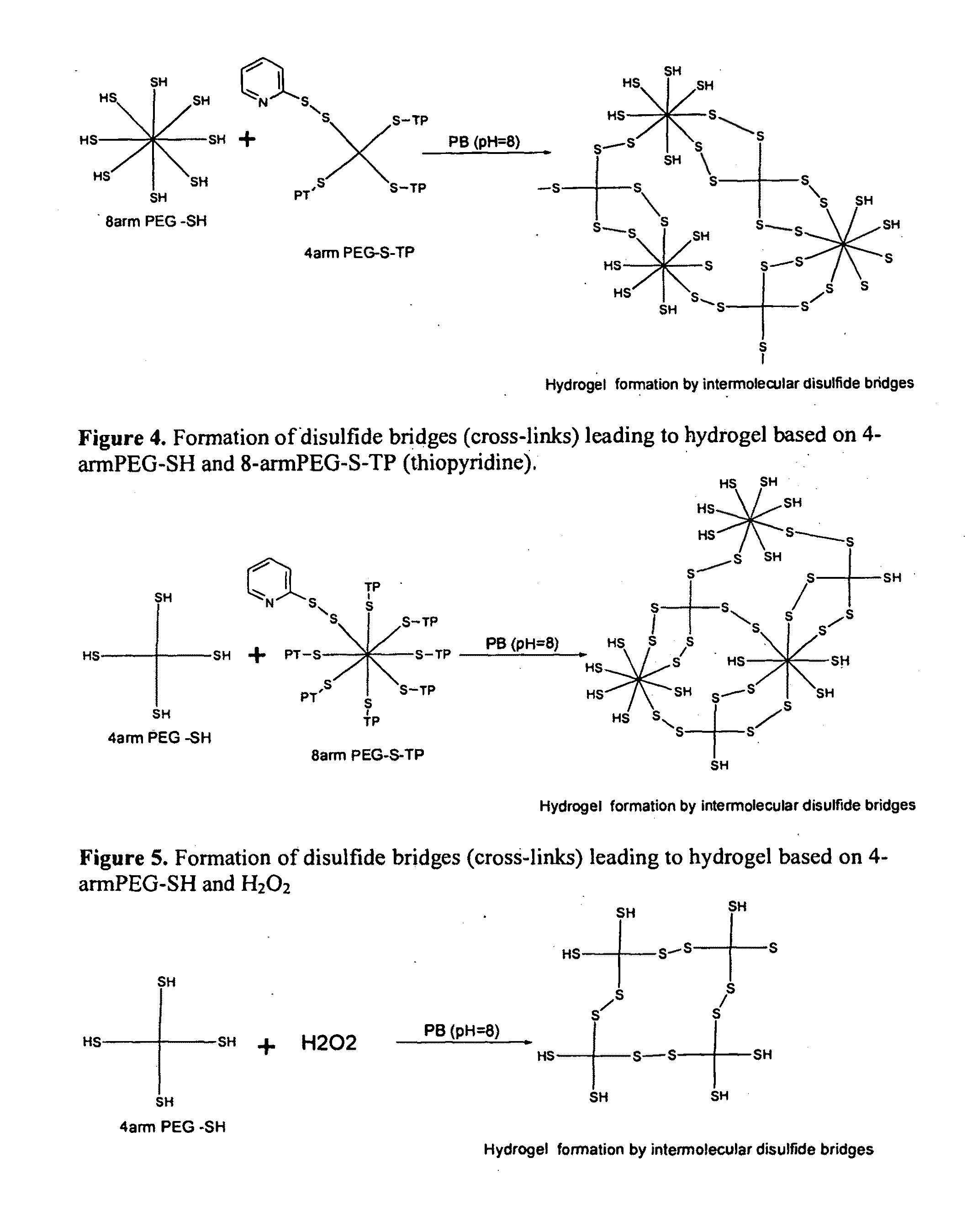
Patents
Literature
1352 results about "Body compartment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
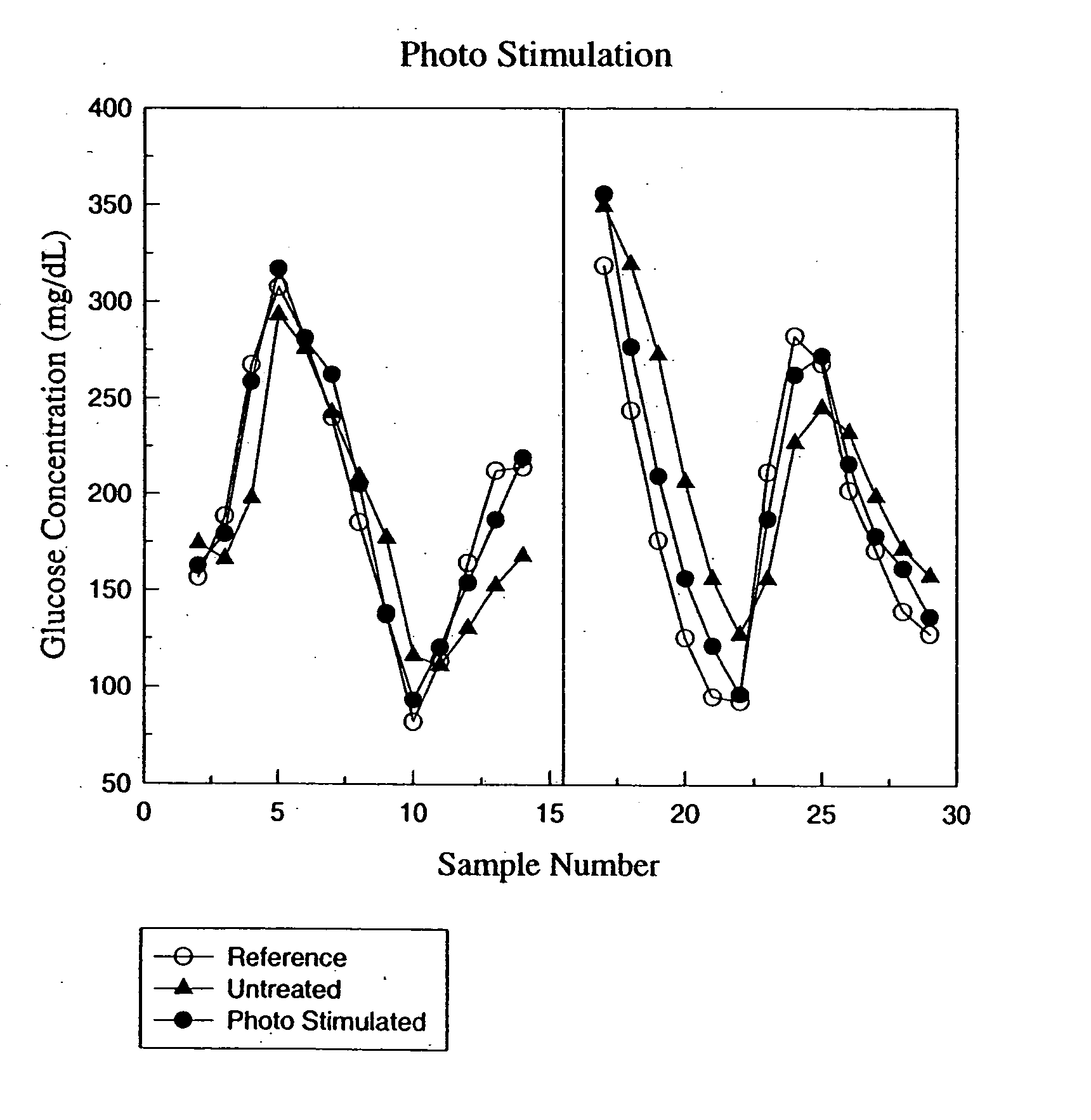
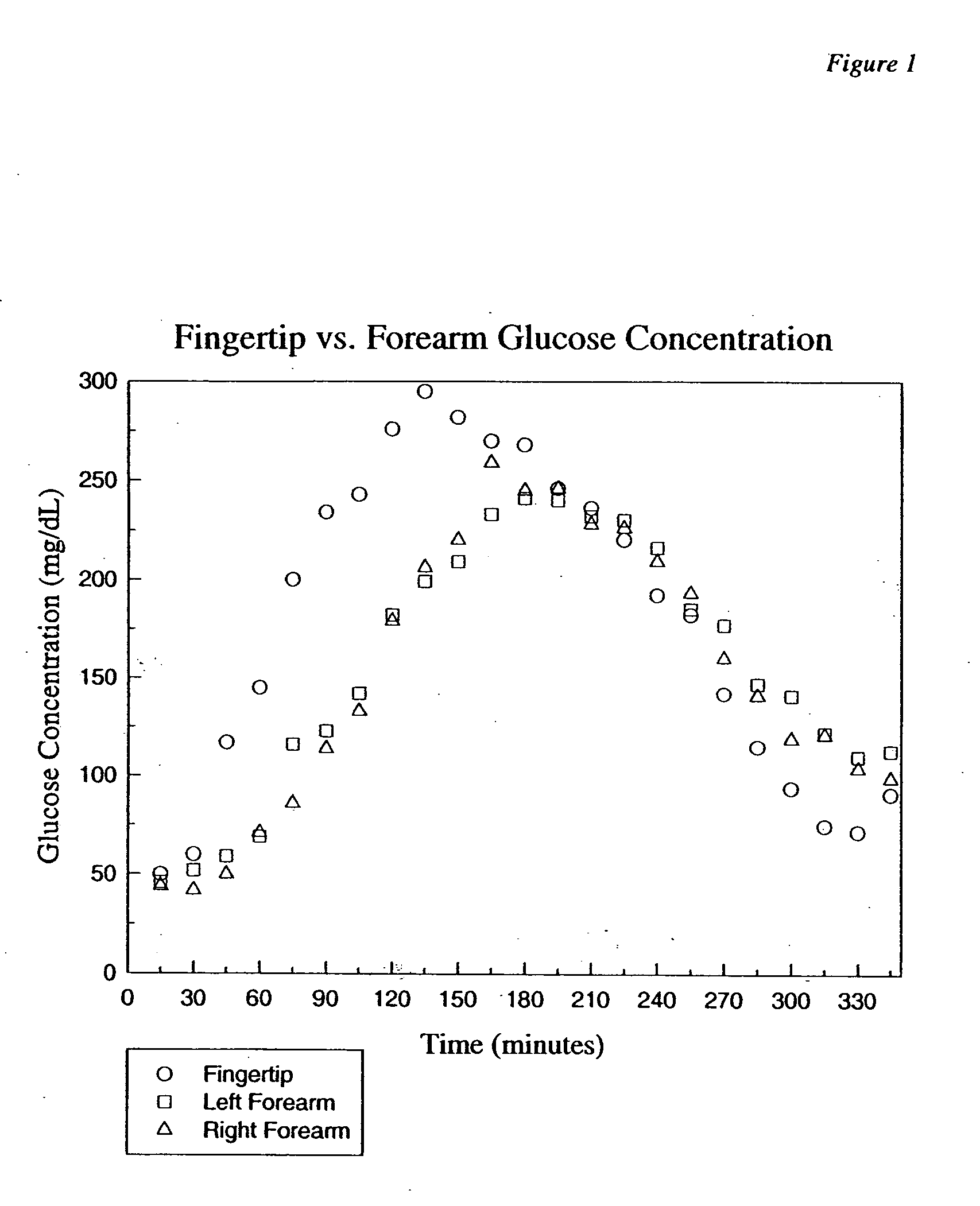
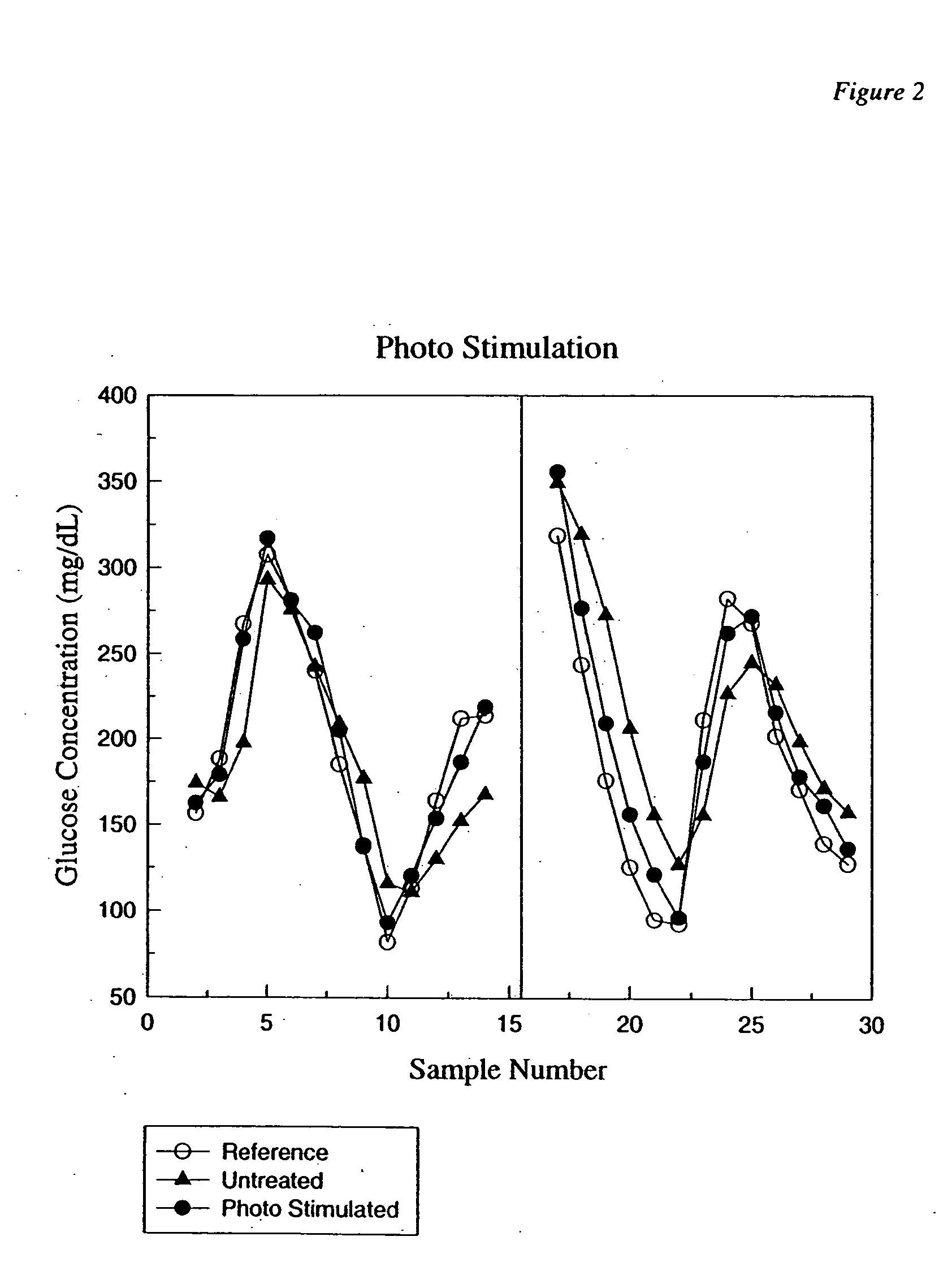
Photostimulation method and apparatus in combination with glucose determination
InactiveUS20050054908A1Increase perfusionReduce errorsDiagnostics using spectroscopyColor/spectral properties measurementsBody compartmentPerfusion
A method and apparatus using photo-stimulation to treat or pretreat a sample site prior to analyte concentration determination is presented. More particularly, photo-stimulation at or near at least one sample site is used to enhance perfusion of the sample site leading to reduced errors associated with sampling. Increased perfusion of the sample site leads to increased volume percentages of the target analyte and / or allows the blood or tissue constituent concentrations to more accurately and / or precisely track corresponding sample constituents in more well perfused body compartments or sites such as arteries, veins, or fingertips. In one embodiment, analysis of the photo-stimulated site is used in conjunction with glucose analyzers to determine the analyte concentration with greater ease, accuracy, or precision and may allow determination of the analyte concentration of another non-sampled body part or compartment.
Owner:GLENN PATENT GROUP
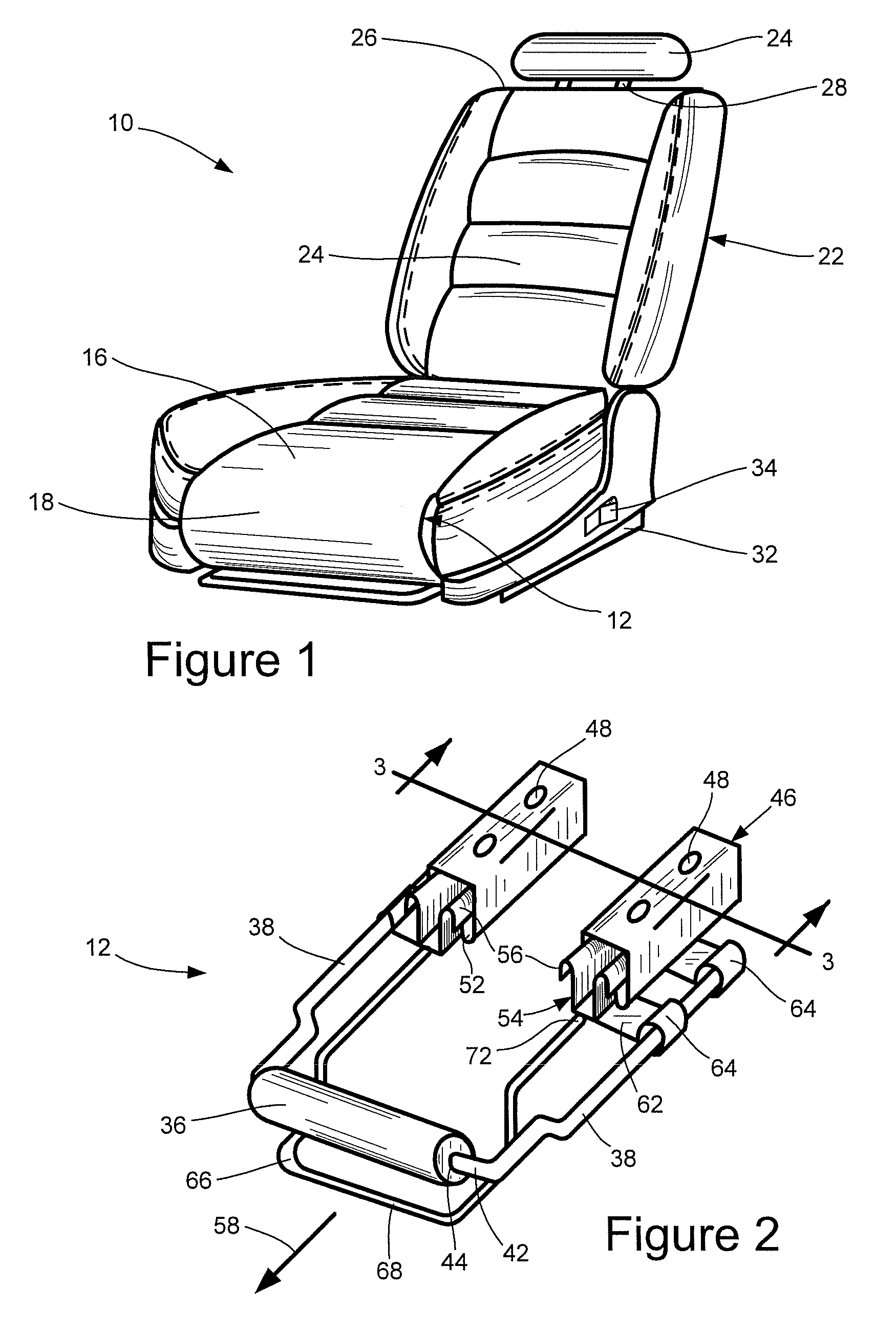
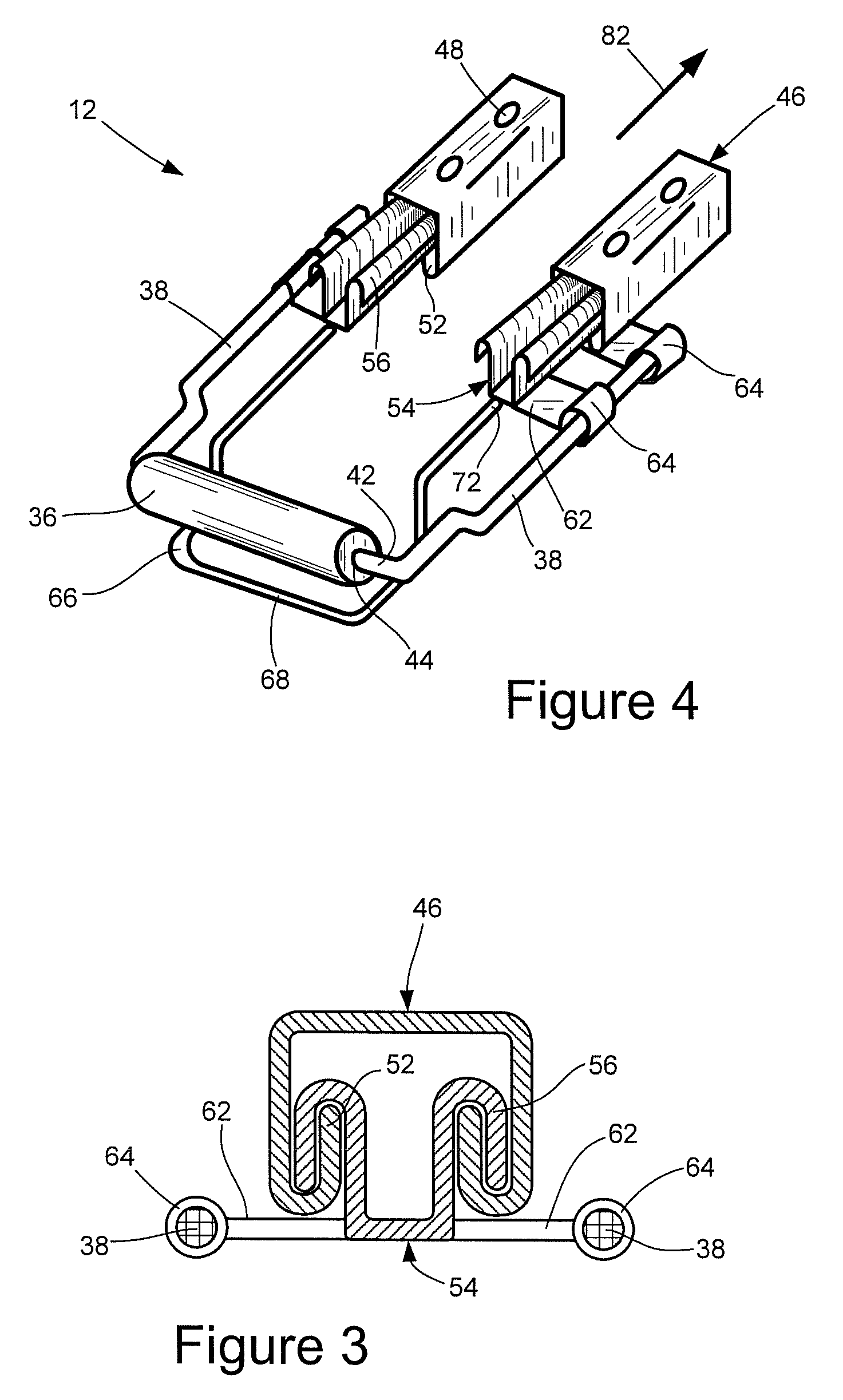
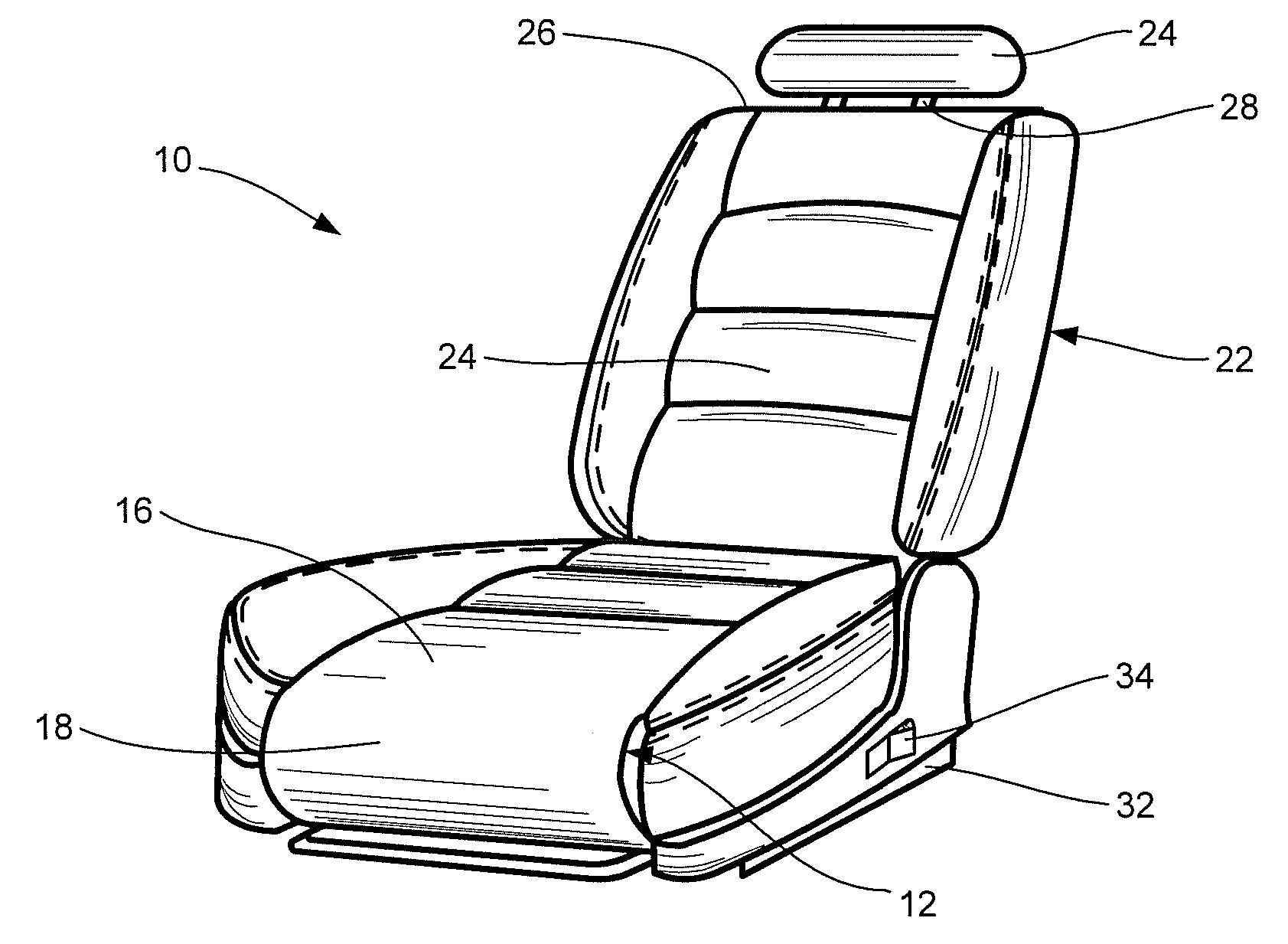
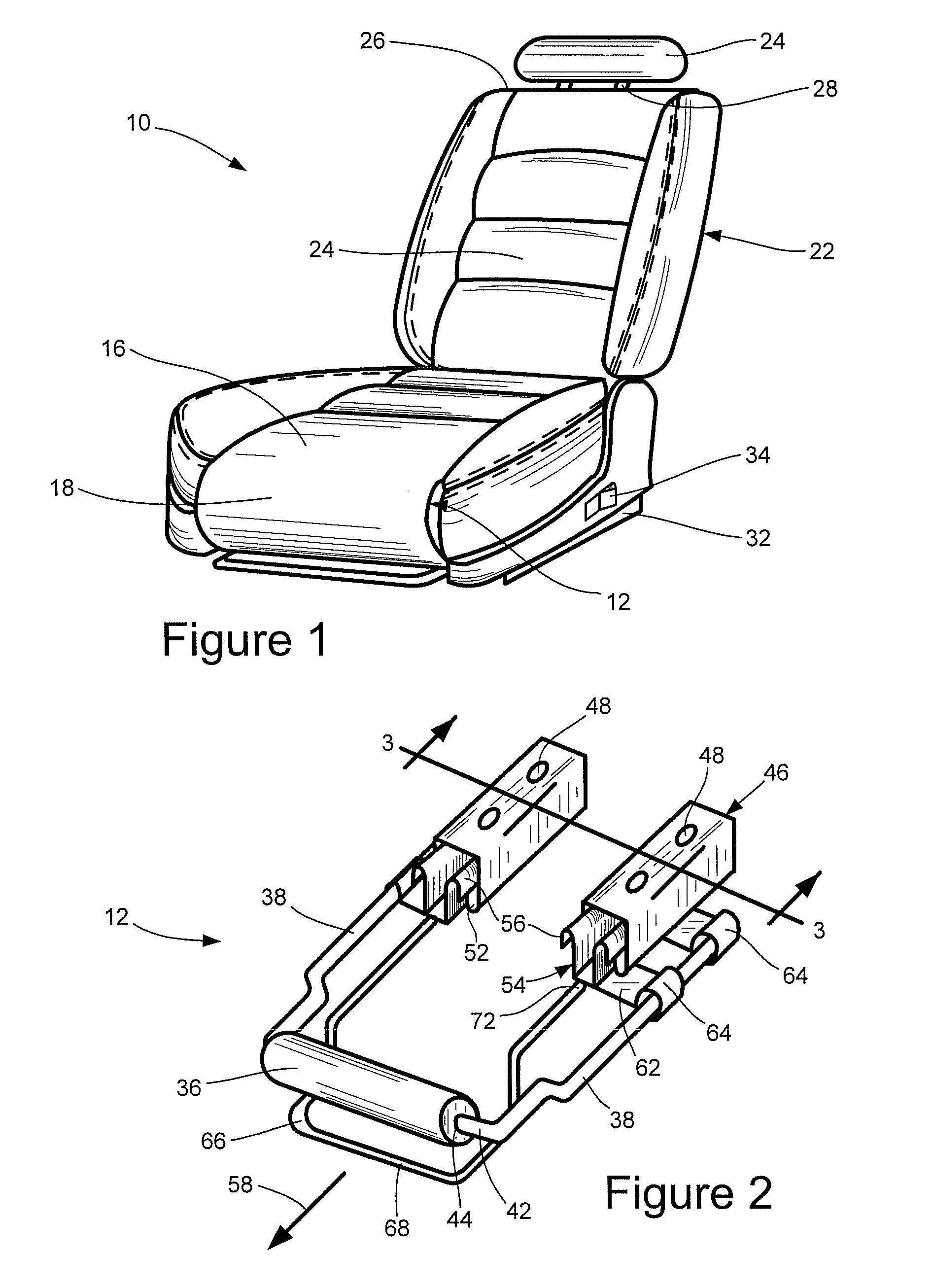
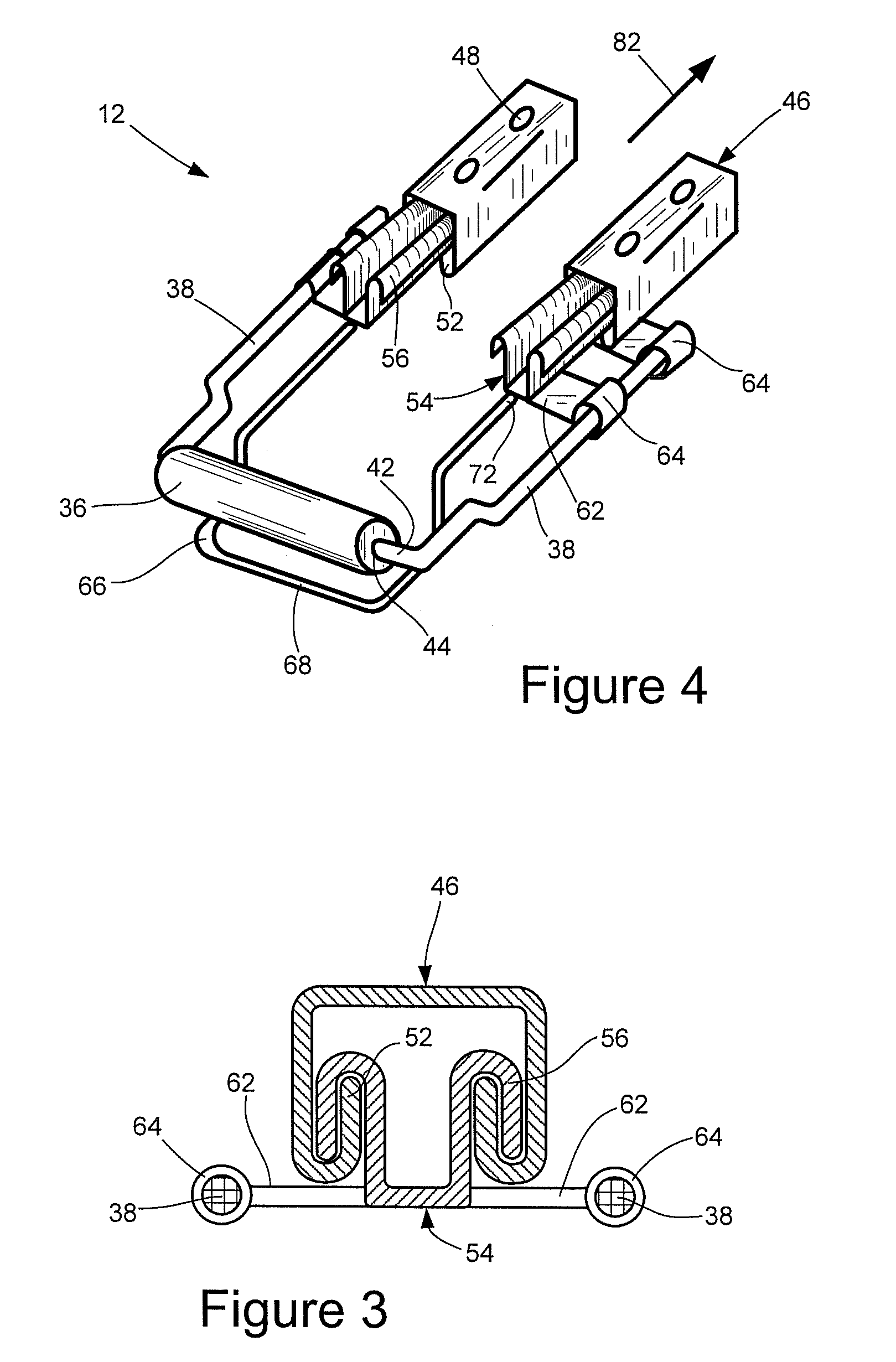
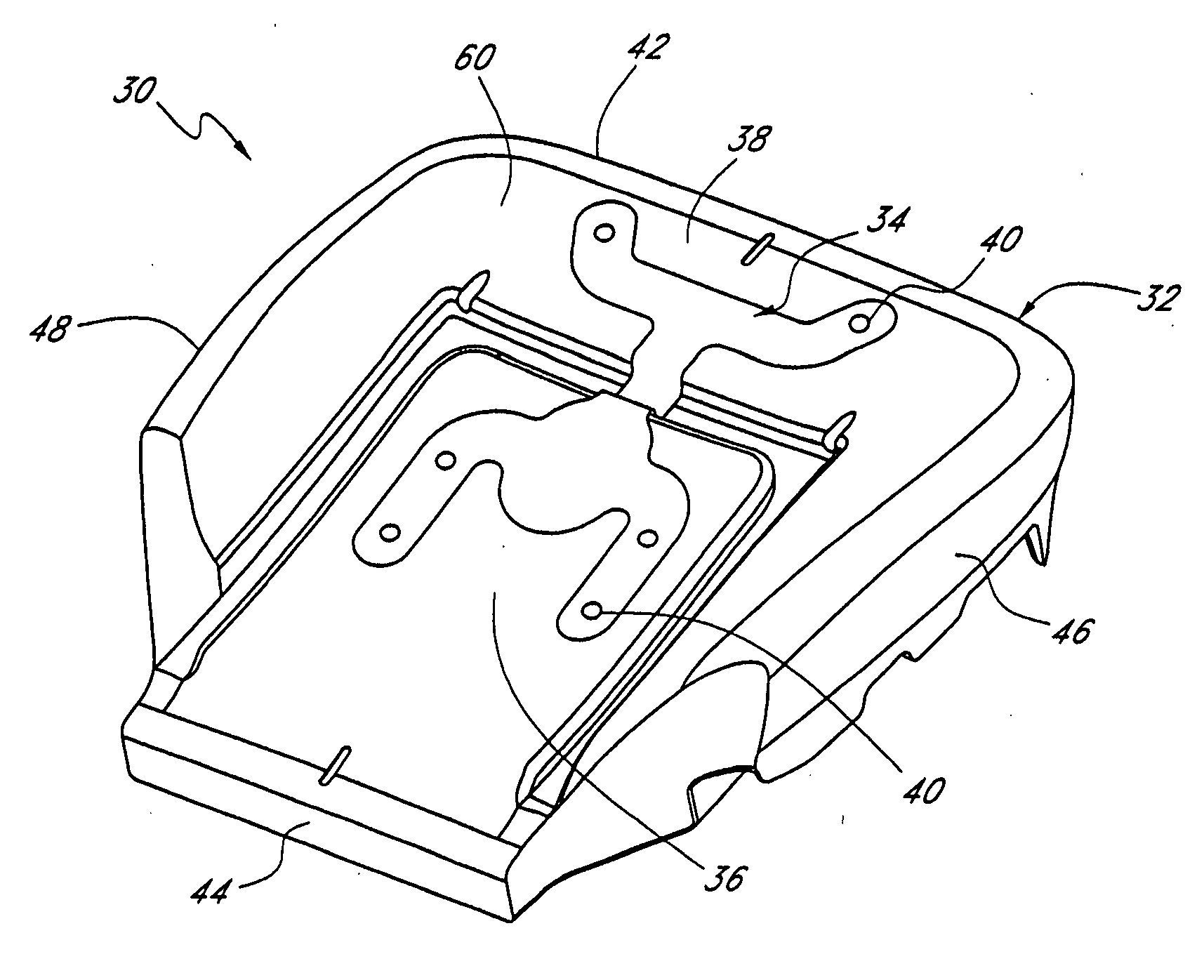
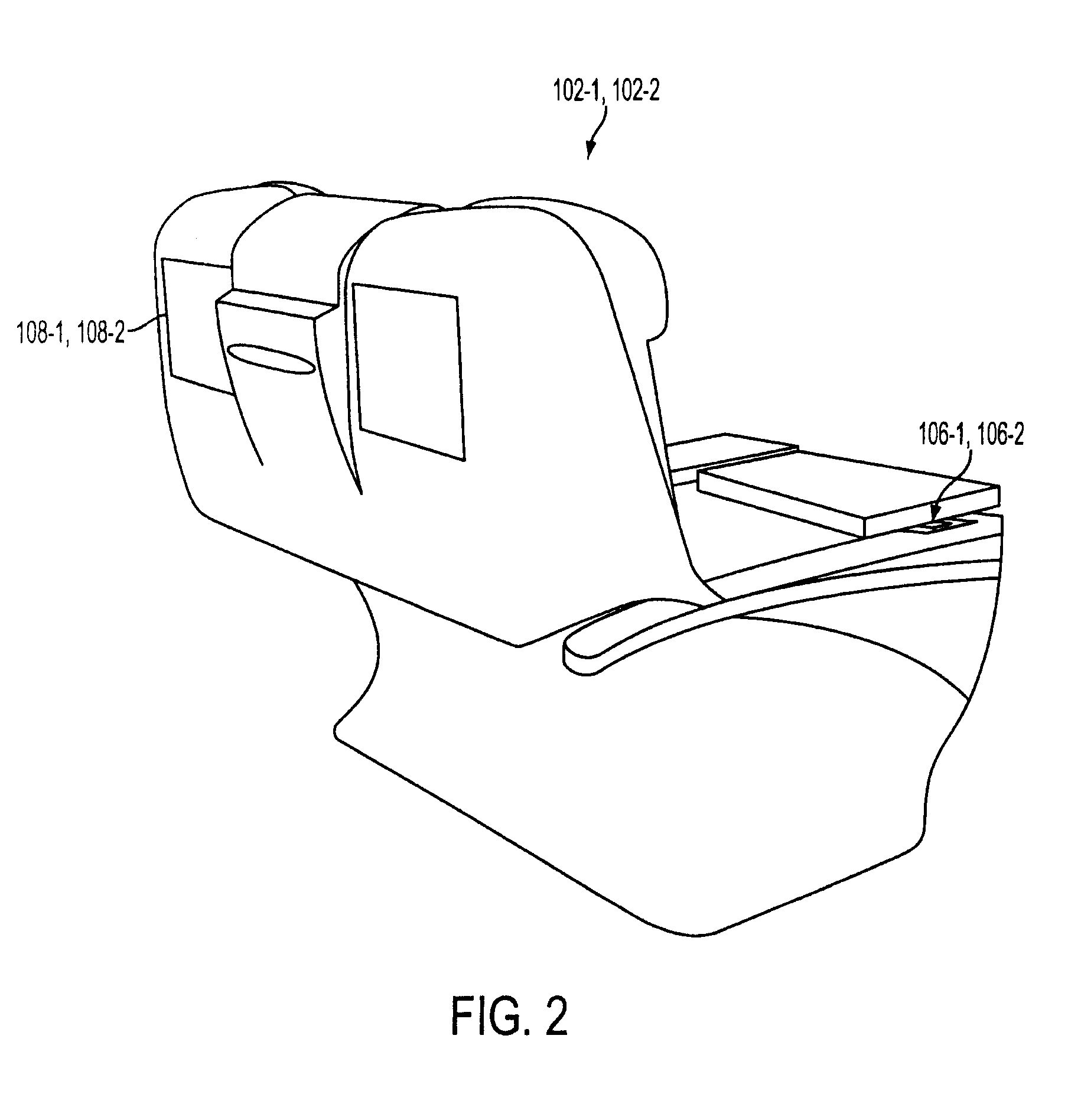
Vehicular seat with adjustable thigh support
A seat for installation in a vehicle that is adjustable to support an occupant's thighs. The seat includes a generally-horizontal seat frame supporting a seat cushion including a front edge that at least partially extends outwardly beyond the seat frame, a seatback coupled to the seat frame for providing back support to the occupant while seated, and a seat bracket coupled to the seat frame and compatible with a floor bracket supported above a floor of a vehicle cabin for coupling the seat to the vehicle. The seat further includes an adjustable thigh support adjacent to a front portion of the seat cushion. The thigh support includes a spool coupled to the seat frame that can be adjusted relative to the seat frame between a retracted position and an extended position, wherein the front edge of the seat cushion is at least partially wrapped around the spool while the spool is in the retracted position, and the front edge of the seat cushion is at least partially unwrapped from around the spool while the spool is in the extended position.
Owner:HONDA MOTOR CO LTD
Vehicle seat
InactiveUS20050285438A1Inhibit deteriorationAir-treating devicesSeat heating/ventillating devicesBody compartmentEngineering
A vehicle seat having: a seat cushion and a seat back formed by covering a surface of a seat pad with a top cover member; a plurality of air holes which penetrate the seat pad to reach a surface of the top cover member; a plurality of air outlets which project from a surface of a duct to be connected to the air holes; and an air blower to pressure and feed air inside a vehicle compartment to the air holes through the duct and the air outlets, wherein a member which has an elastic modulus larger than that of the seat pad forming the seat cushion is interposed between the seat cushion and the duct.
Owner:TS TECH CO LTD
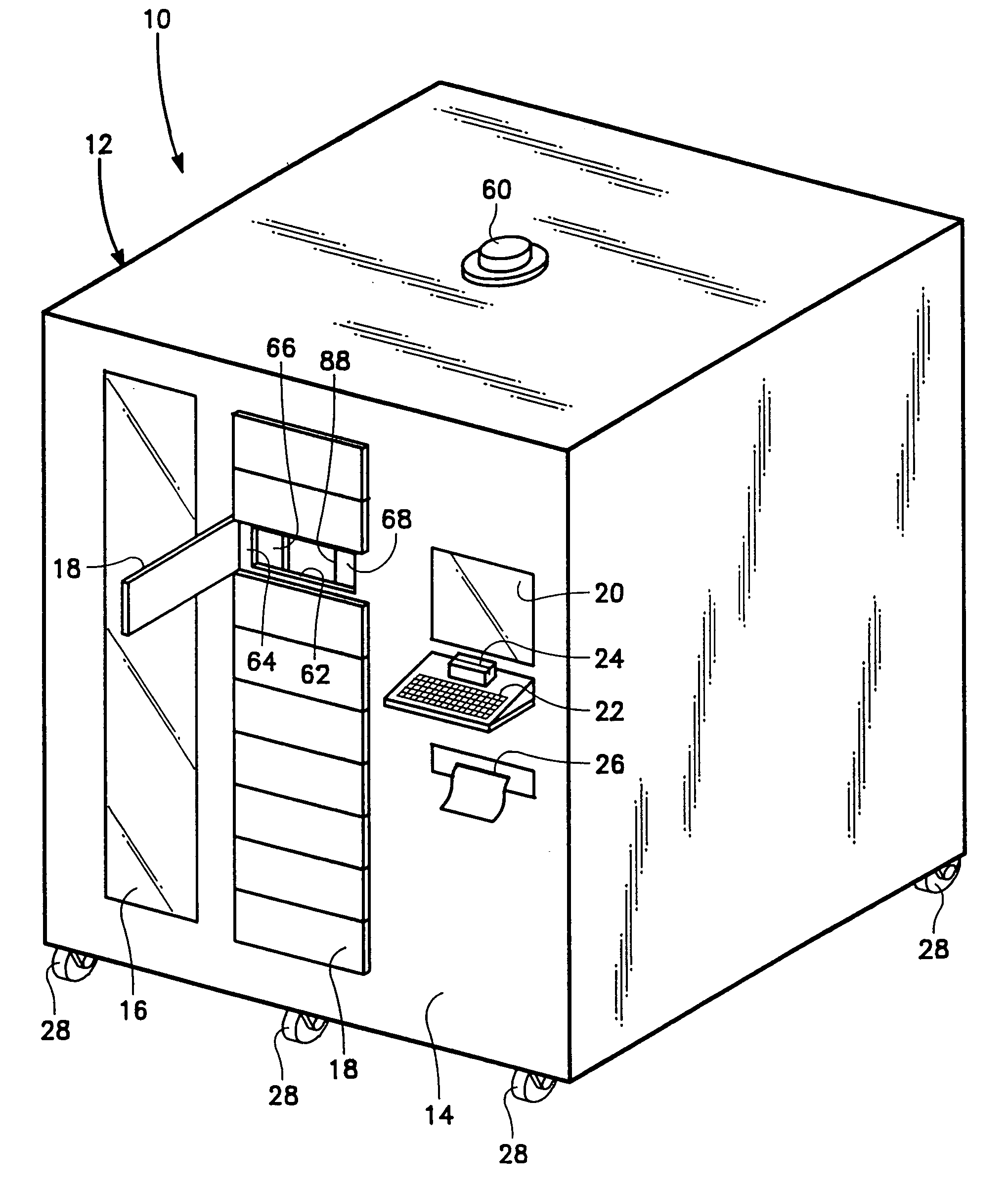
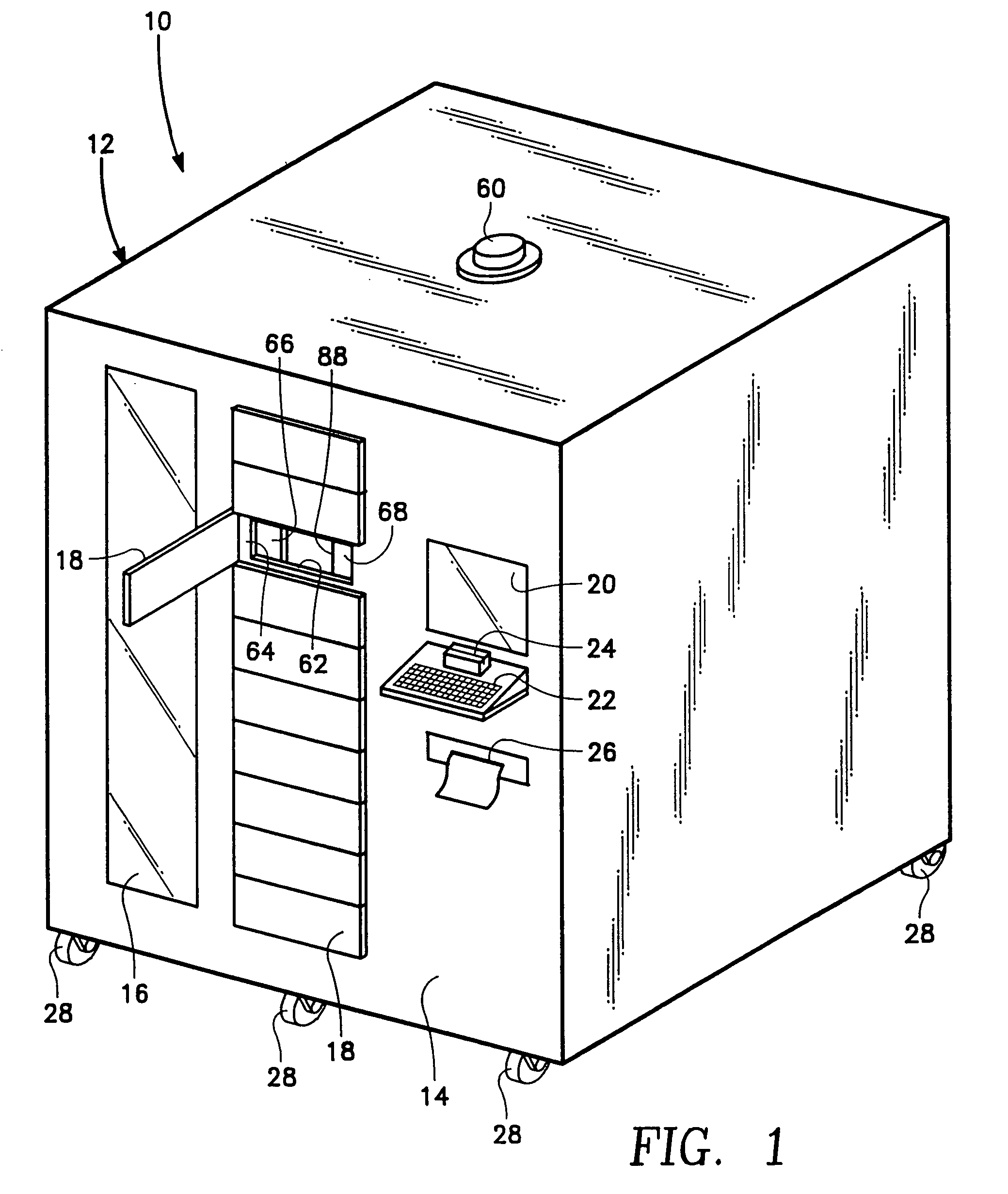
Controlled access supply cabinet and system
InactiveUS7809470B2Avoid disadvantagesDrug and medicationsDigital data processing detailsBody compartmentTransfer mechanism
Owner:S&S X RAY PRODS
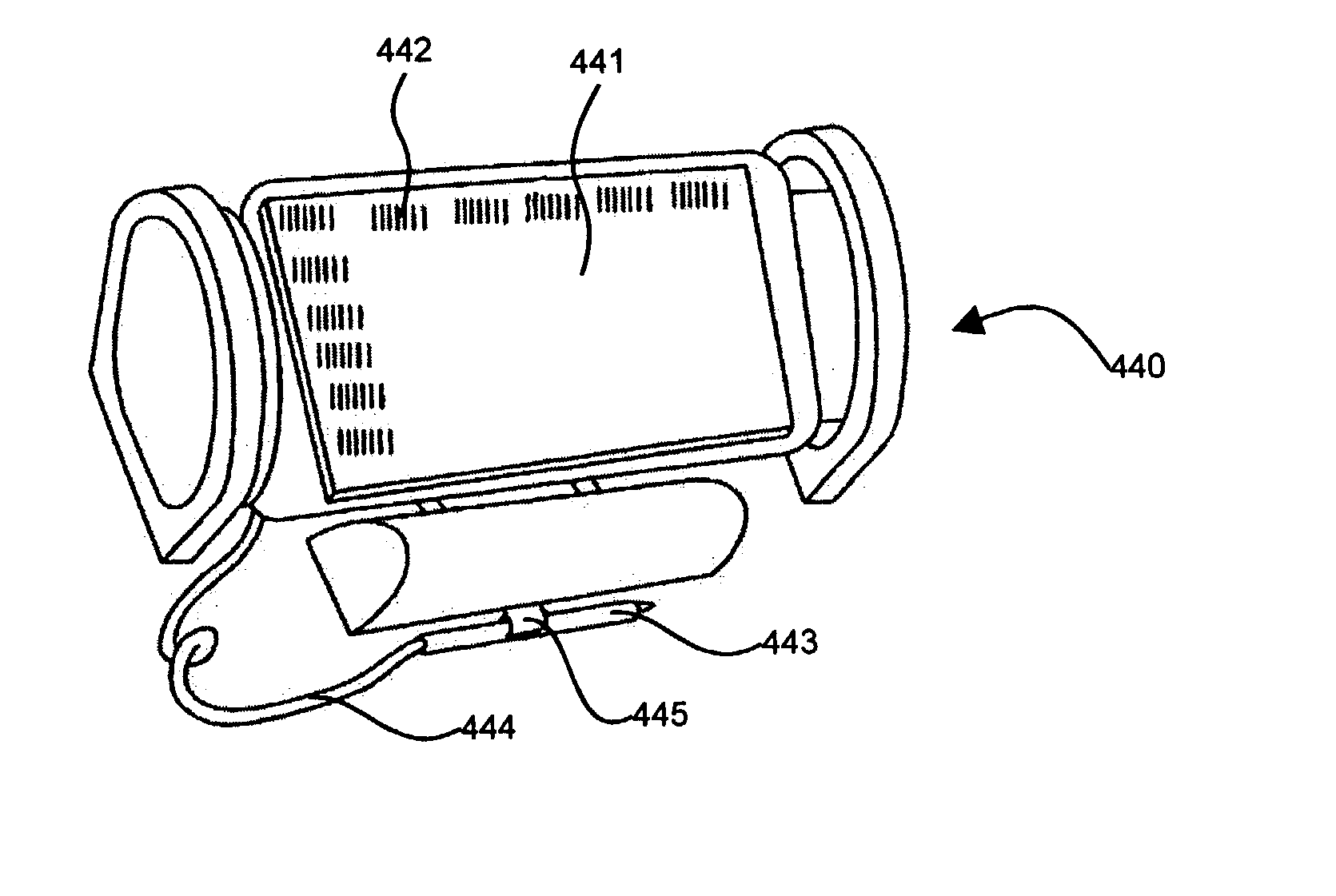
Wearable computing system, method and device
InactiveUS20030209604A1Input/output for user-computer interactionVisual indicationsAir filtrationDocking station
Disclosed is a wrist-wearable electronic interface movably mounted on an arm-attachment mechanism. Interface moves from under-sleeve wrist-adjacent position to palm-adjacent position where it can be manipulated by the hand of the arm wearing the device. Alternately adaptable to telephones, audio recorders, remote controls, auto ID equipment, telephone call-blocking, and more. Alternative embodiment provides a wrist-mounted docking station. Another alternative embodiment includes a superior carpal tunnel syndrome therapy device. The system also includes novel battery chargers: (1) window-mounted, solar-powered; (2) mounted in an automobile that also includes an improved odometer, license plate, and cabin air filtration system.
Owner:SEARCH & SOCIAL MEDIA PARTNERS
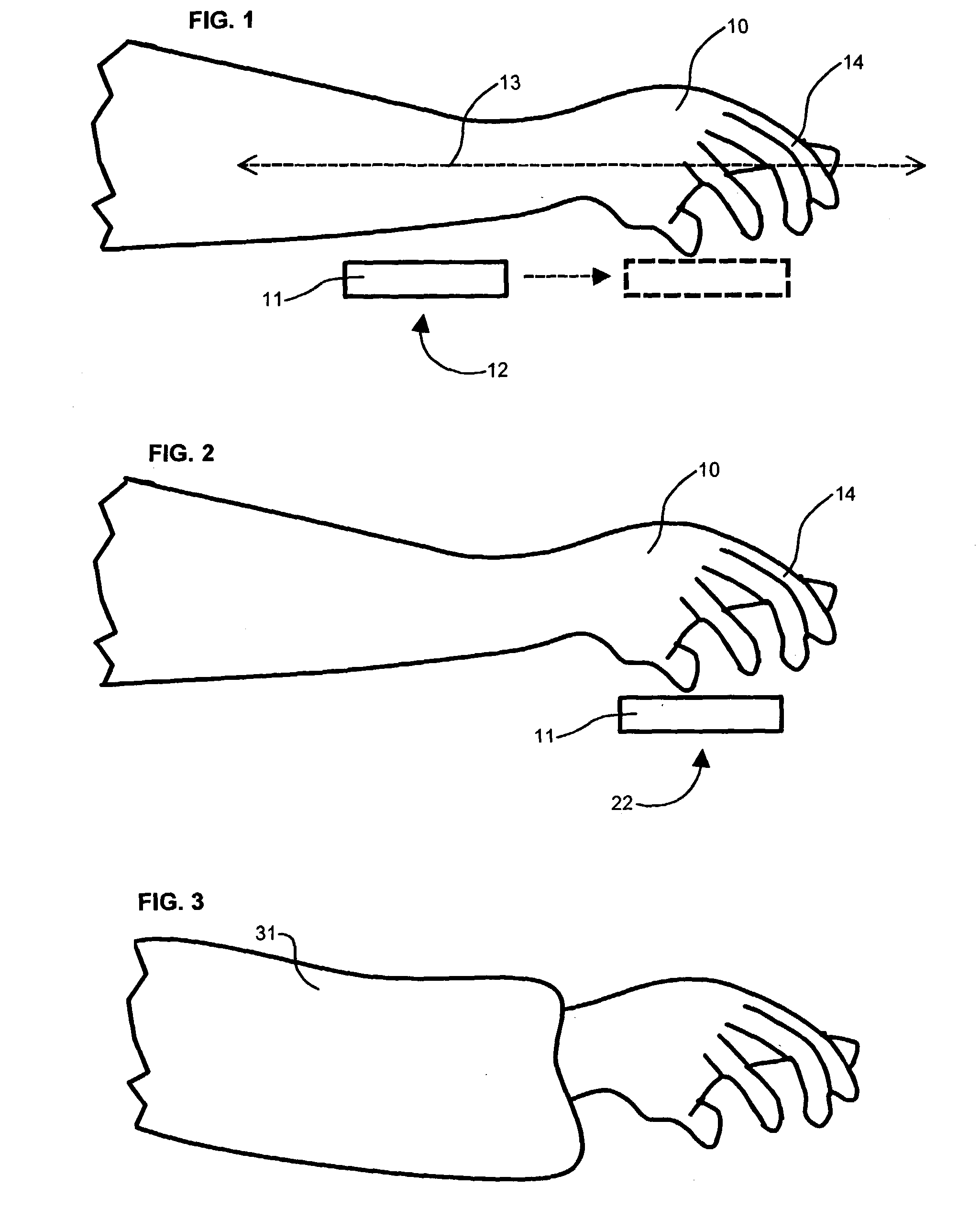
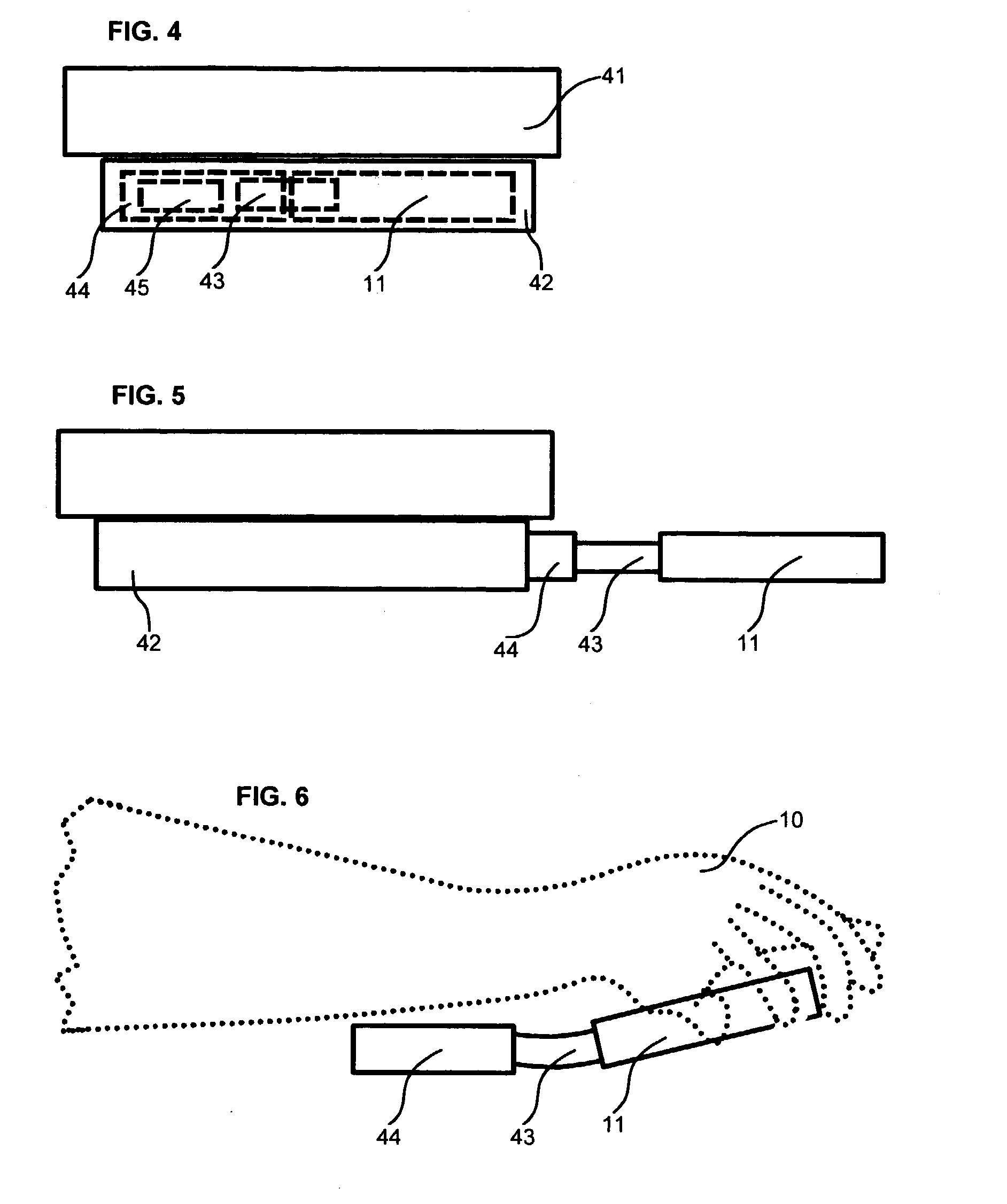
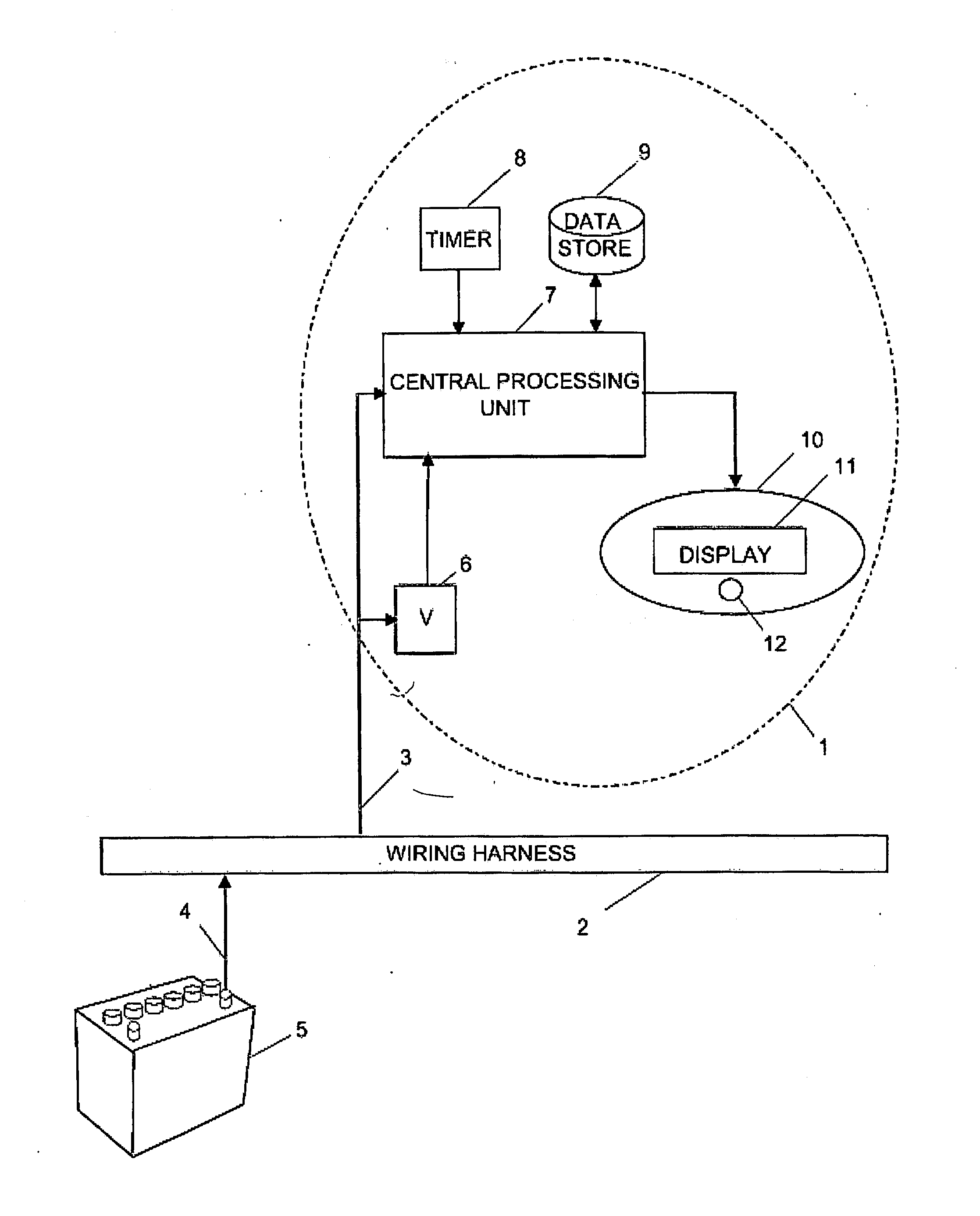
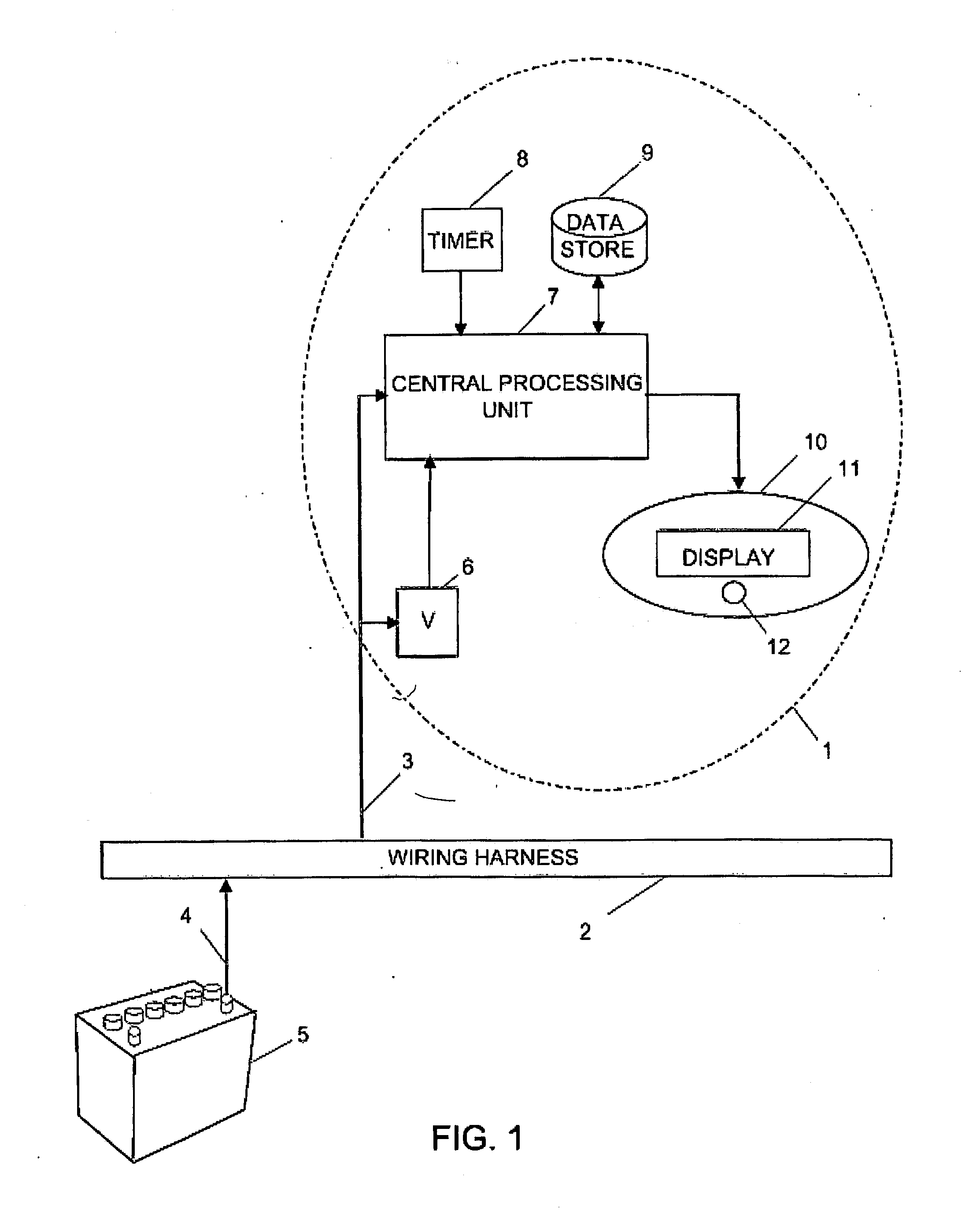
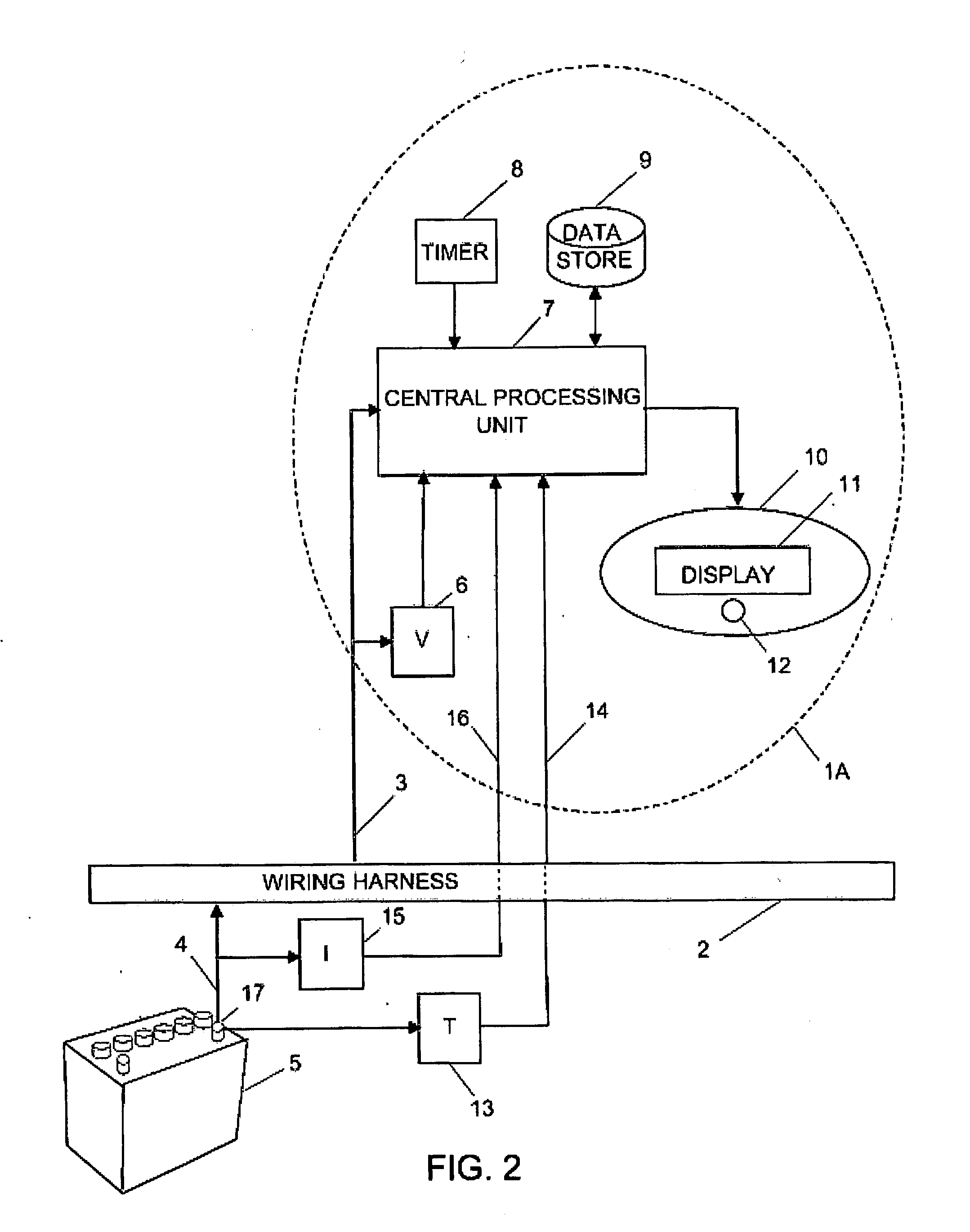
Battery monitor system attached to a vehicle wiring harness
InactiveUS20120029852A1Diagnose their healthEasy to viewTesting electric installations on transportSpecial data processing applicationsElectrical batteryBody compartment
A computer system that installs in the proximity of the vehicle's operator by attaching to the vehicle's wiring harness (e.g., via a power outlet in the vehicle cabin). The device, gathers data relating to the operational state of the vehicle's battery, calculates various health information of the battery from the gathered data, and provides the health and operational state of the battery to the vehicle's operator. To facilitate battery health calculations, the device receives input from a temperature sensor that is remote to the battery, such as a temperature sensor in the device's housing or in the vehicle cabin. The temperature reading can be used to approximate the temperature of the battery. The computer system can also support non-battery related functions, such as navigation, theft deterrence, etc. Algorithms utilizing battery health data over multiple load cycles to determine the health of a battery are also disclosed.
Owner:GOFF LONNIE C +2
Mobile computing device with modular expansion features
InactiveUS20070083298A1Improve acceleration performanceOptimize locationDigital data processing detailsVehicle componentsBody compartmentModularity
Owner:L 3 COMM CORP
Safety system for a compartment of a vehicle
InactiveUS7097226B2Guaranteed ease of operationEasily operableMechanical controlsElectrical locking circuitsControl signalBody compartment
A safety sensing and / or release system for a closed compartment of a vehicle is operable to detect an occupant within the vehicle compartment. The system may be operable to sense ambient conditions in the vehicle compartment, and may generate a control signal in response to the sensed conditions. The system may actuate indicators to notify operators of the vehicle that there is a person or animal detected in the compartment. Optionally, the system may open the vehicle compartment in response to a detection of an occupant and the sensed conditions. The safety system includes a false trigger protection means that limits or reduces false detections of a person or animal within the compartment.
Owner:DONNELLY CORP
Vehicular seat with adjustable thigh support
A seat for installation in a vehicle that is adjustable to support an occupant's thighs. The seat includes a generally-horizontal seat frame supporting a seat cushion including a front edge that at least partially extends outwardly beyond the seat frame, a seatback coupled to the seat frame for providing back support to the occupant while seated, and a seat bracket coupled to the seat frame and compatible with a floor bracket supported above a floor of a vehicle cabin for coupling the seat to the vehicle. The seat further includes an adjustable thigh support adjacent to a front portion of the seat cushion. The thigh support includes a spool coupled to the seat frame that can be adjusted relative to the seat frame between a retracted position and an extended position, wherein the front edge of the seat cushion is at least partially wrapped around the spool while the spool is in the retracted position, and the front edge of the seat cushion is at least partially unwrapped from around the spool while the spool is in the extended position.
Owner:HONDA MOTOR CO LTD
Climate controlled seat
InactiveUS20060197363A1Seat heating/ventillating devicesPedestrian/occupant safety arrangementBody compartmentEngineering
A vehicle seat has a seat bottom and seat back. The seat bottom can have a ventilated insert located between the seat upholstery and the seat cushion. The inset can be in fluid communication with the cabin space of the vehicle. In one arrangement, the insert has a cover with holes and a body. The cover and body cooperate to form a plurality of plenums for passing air through the insert. A fluid system can deliver air to the insert so that the air passes through the plenums and directed out of the holes in the cover. The air then flows out of the vehicle seat to control the climate around the person in the seat. The insert can also be used to remove air near the seat by drawing air proximate the seat into the insert. The air can then pass through the insert and is delivered to the fluid system.
Owner:LOFY JOHN +2
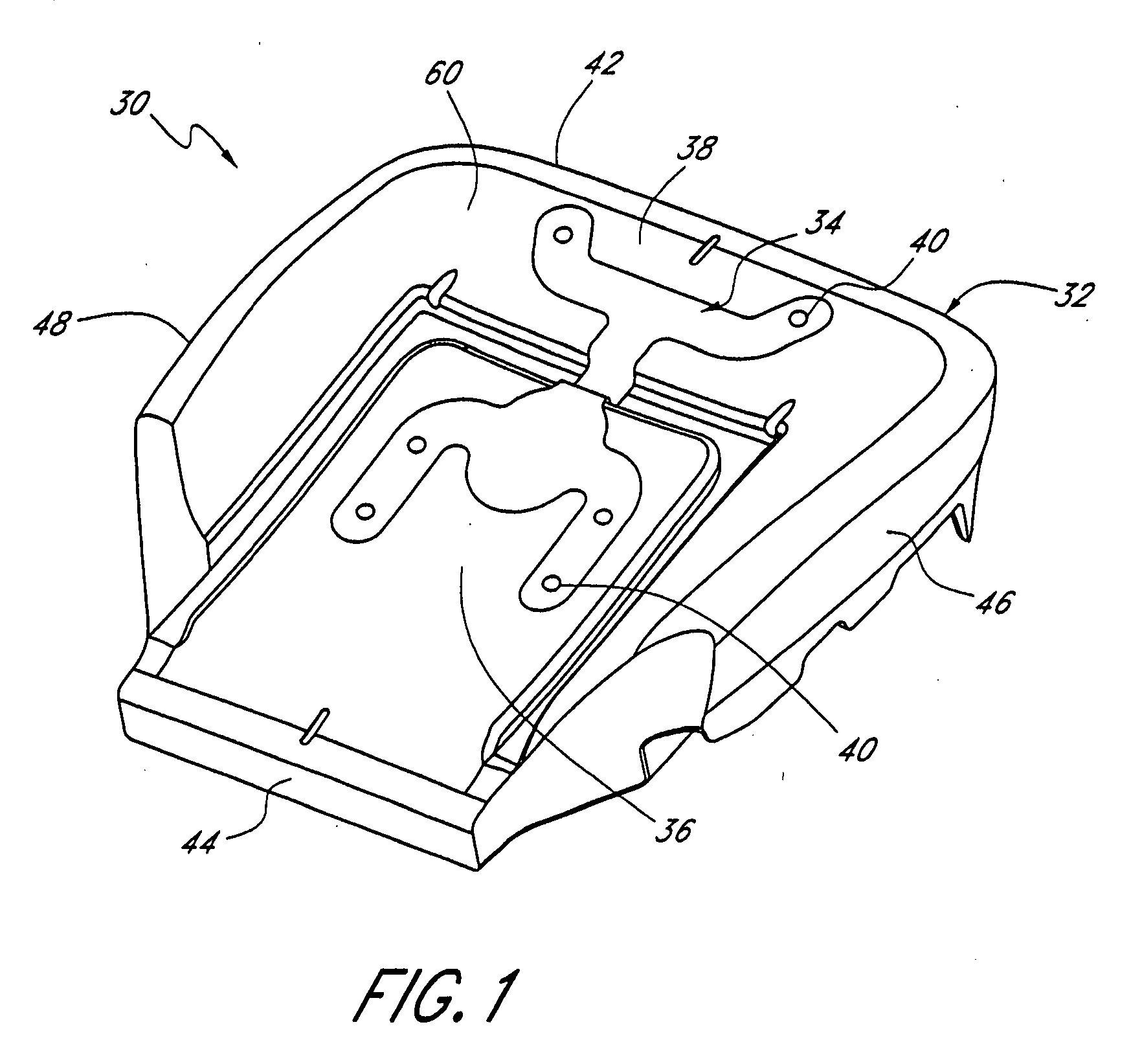
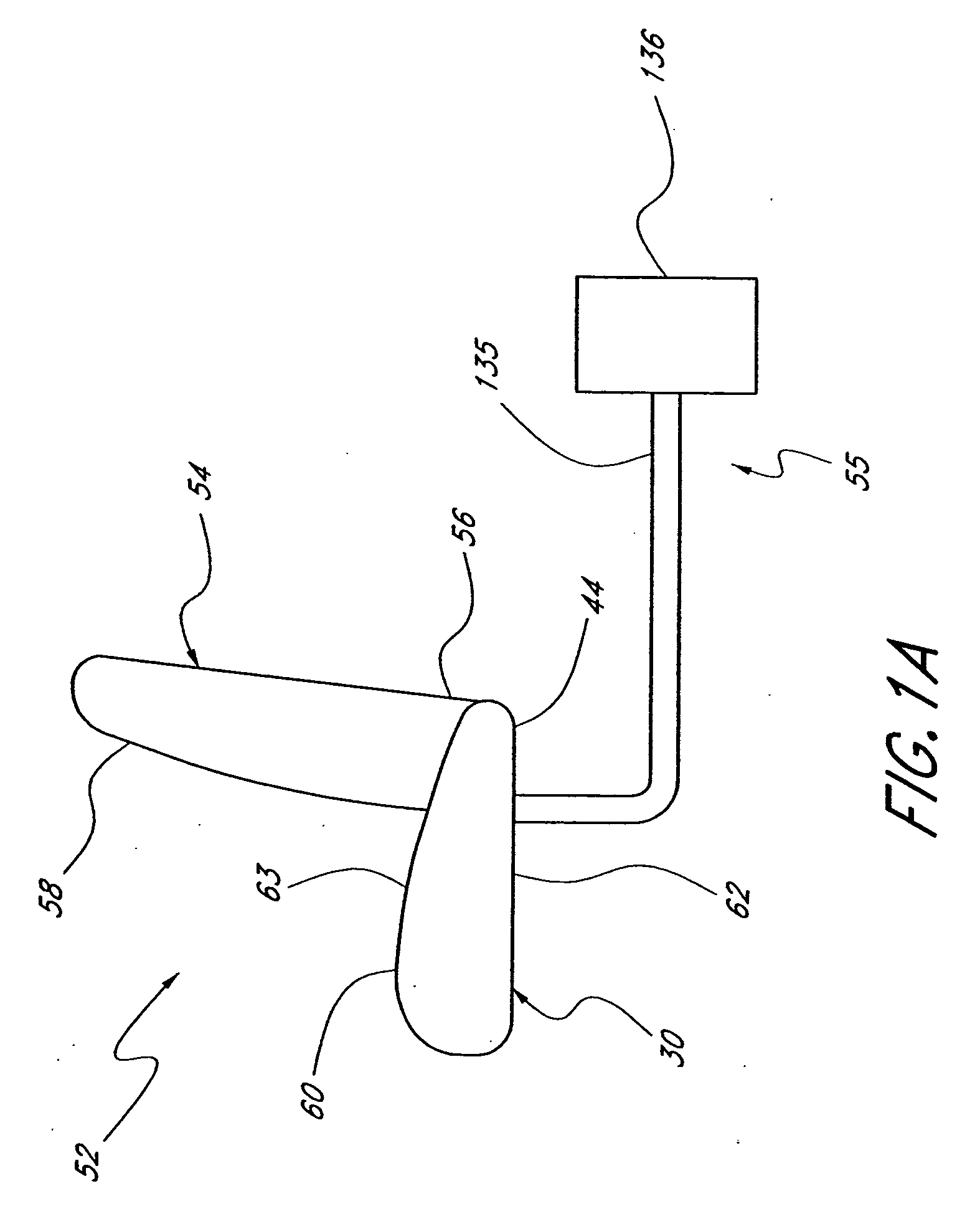
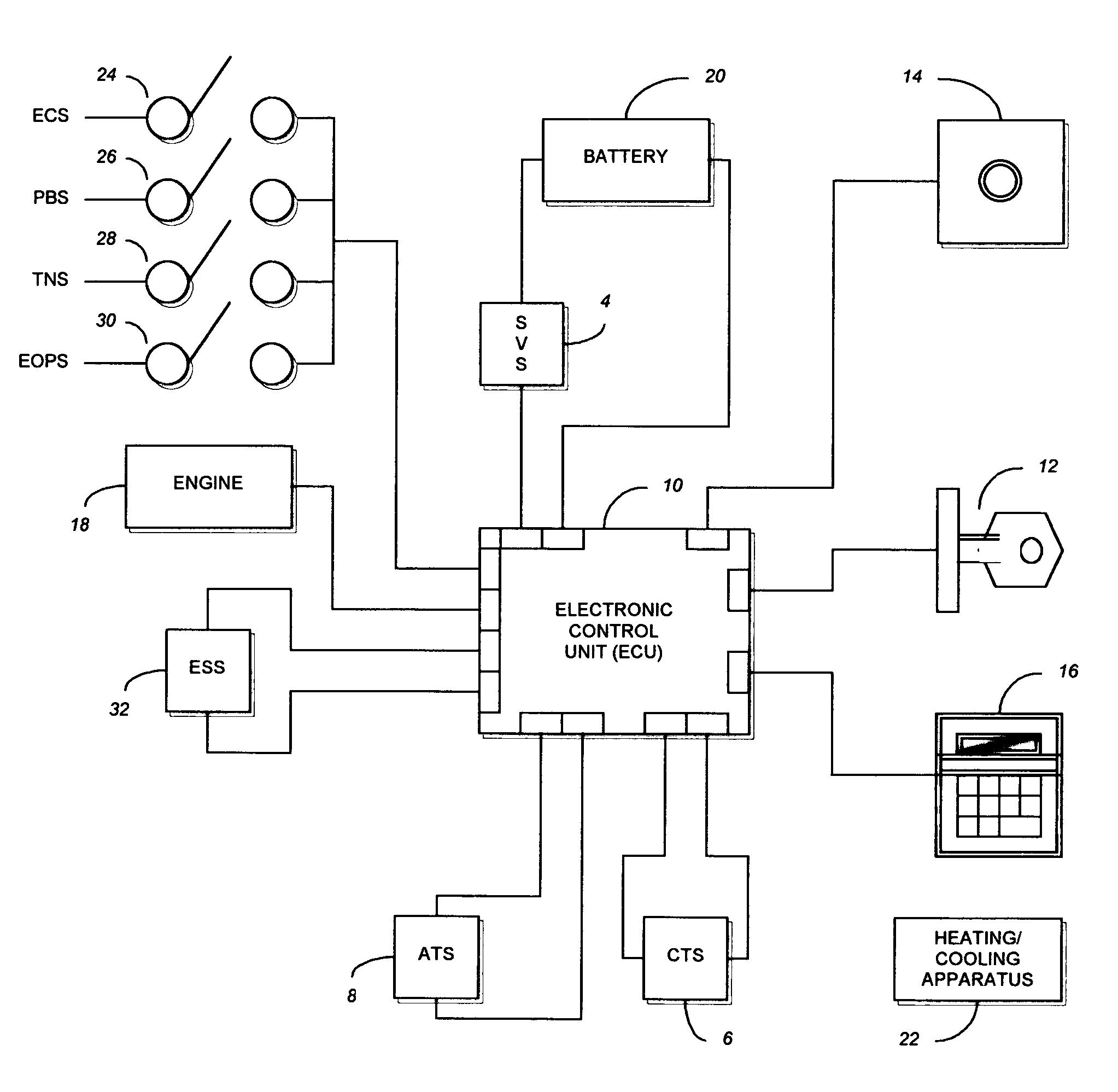
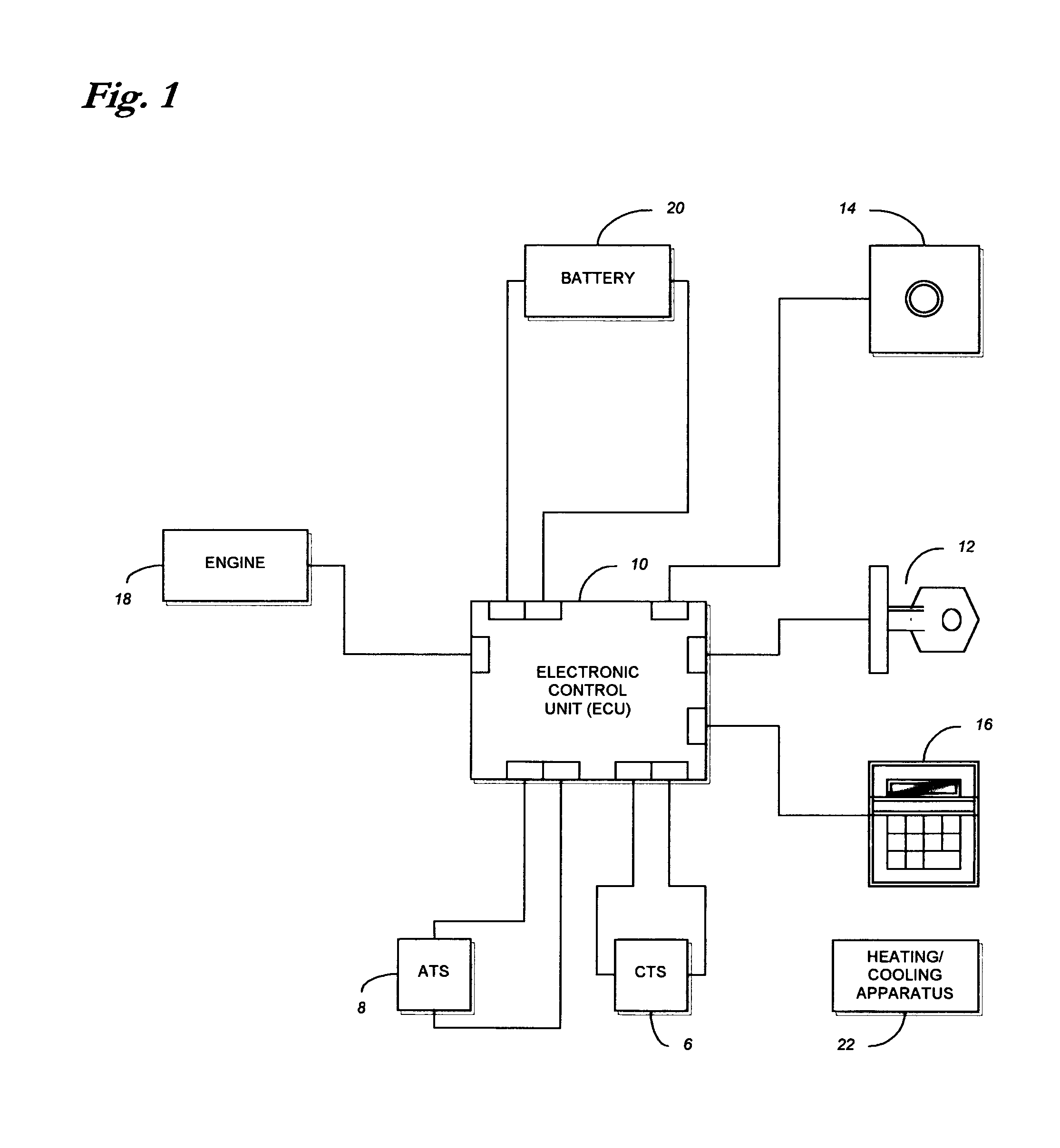
Method and system for controlling an engine to maintain a comfortable cabin temperature within a vehicle
ActiveUS7027912B1Minimizing engine idlingAnalogue computers for vehiclesMeasurement deviceBody compartmentTwo temperature
A method and system for controlling an engine in order to maintain a comfortable cabin temperature within a vehicle that is equipped with an engine, a battery and a heating / cooling apparatus including determining an acceptable range of cabin temperatures, monitoring cabin and outside air temperatures, controlling automatic starting of the vehicle engine when both temperatures are outside of the acceptable range and running the engine only as is minimally necessary to maintain the cabin temperature within the acceptable range.
Owner:METZGER WILLIAM R
Vehicle seat
ActiveUS7322643B2Easy to manufactureIncrease pressureAir-treating devicesSeat heating/ventillating devicesBody compartmentEngineering
A vehicle seat having: a seat cushion and a seat back formed by covering a surface of a seat pad with a top cover member; a plurality of air holes which penetrate the seat pad to reach a surface of the top cover member; a plurality of air outlets which project from a surface of a duct to be connected to the air holes; and an air blower to pressure and feed air inside a vehicle compartment to the air holes through the duct and the air outlets, wherein an engagement portion which projects in a radial direction of one of the air outlets is provided at a tip of the one of the air outlets.
Owner:TS TECH CO LTD
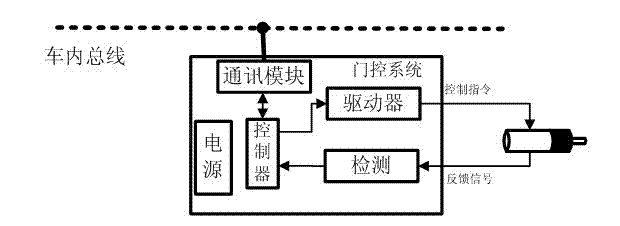
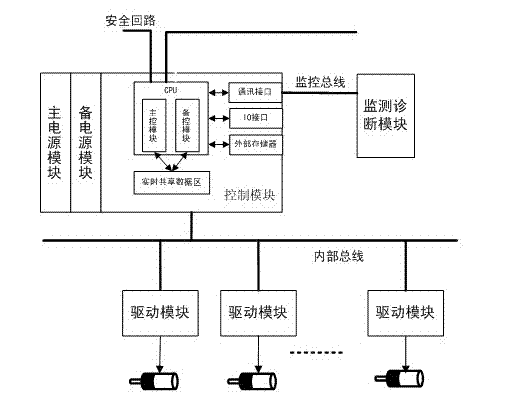
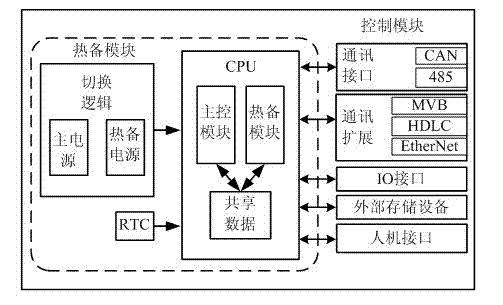
Intelligent door control system of rail train
InactiveCN102536033AHigh speedImprove reliabilityPower-operated mechanismArea networkBody compartment
The invention discloses an intelligent door control system of a rail train, which comprises control modules, driving modules and a monitoring and diagnosing module, wherein each carriage is controlled by a control module, a driving module is arranged on each door, and in each carriage, the control module and the driving modules are communicated by adopting a CAN (controller area network) bus so as to realize a control mode where one control module corresponds to multiple driving modules. The control modules are connected with a train bus. The operation processes are as follows: the control modules receive control signals such as door opening, door closing and the like transmitted on the train bus, send TCMS (train control and monitor system) communication commands to the driving module on each door by the CAN bus or in other communication manners according to the obtained control signals so as to control opening and closing actions of the doors by electric motors, and carry out collection and tentative diagnosis on real-time monitoring data of the driving modules on the electric motors. The intelligent door control system can carry out real-time monitoring and diagnosis on running states of the control modules, the driving modules and the electric motors, so as to estimate safety degree of a door system and improve running reliability of a train.
Owner:NANJING UNIV OF SCI & TECH
Tool vending machine and method therefore
ActiveUS7086558B1Coin-freed apparatus detailsApparatus for meter-controlled dispensingBody compartmentEngineering
A tool vending machine which permits human access to only a single size compartment of a plurality of different sized compartments at a time so that the user is only able to extract a tool that is contained within that single compartment and does not permit access to any other compartment. The particular compartment is to be manually selected by the human user.
Owner:AUTOCRIB
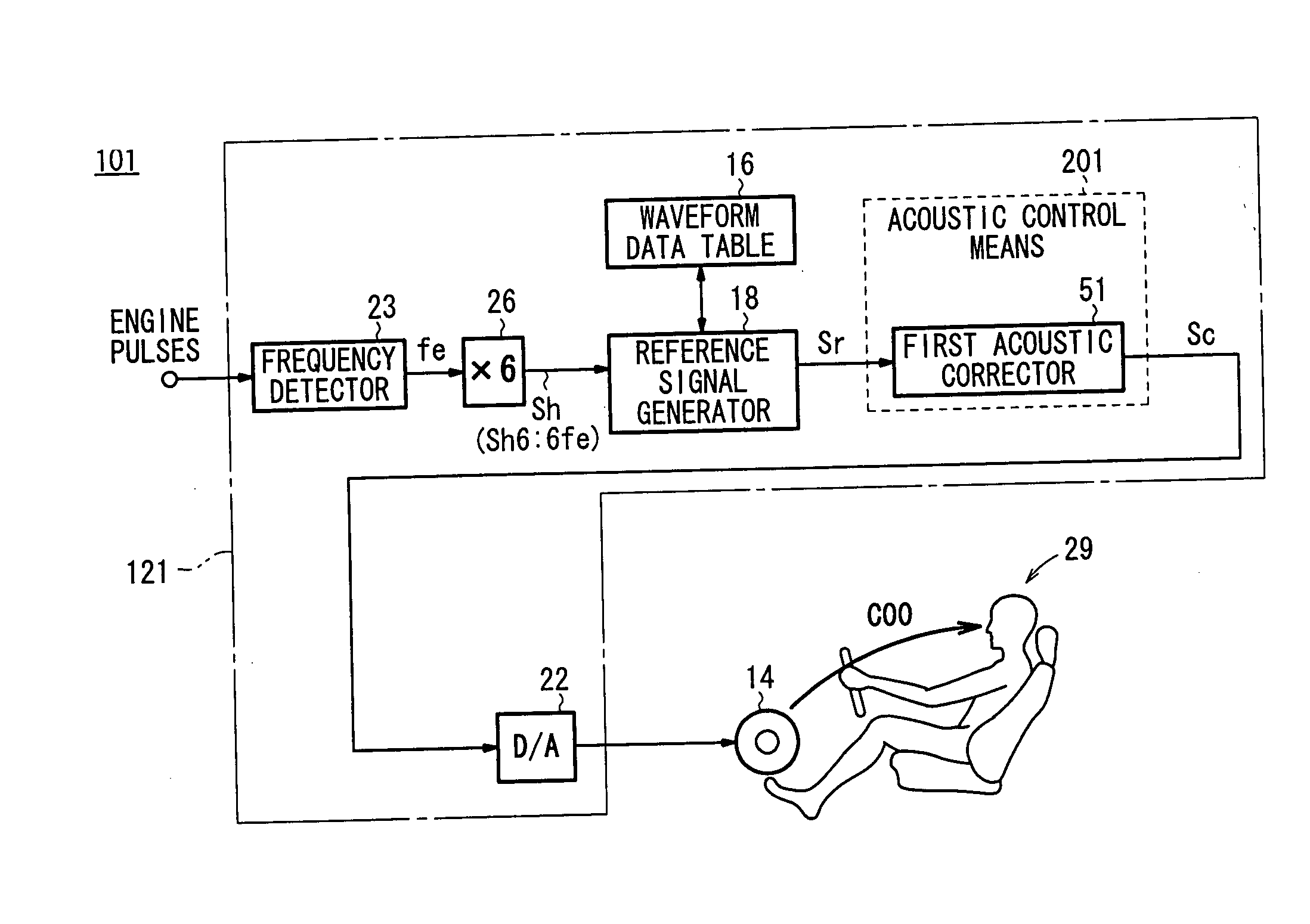
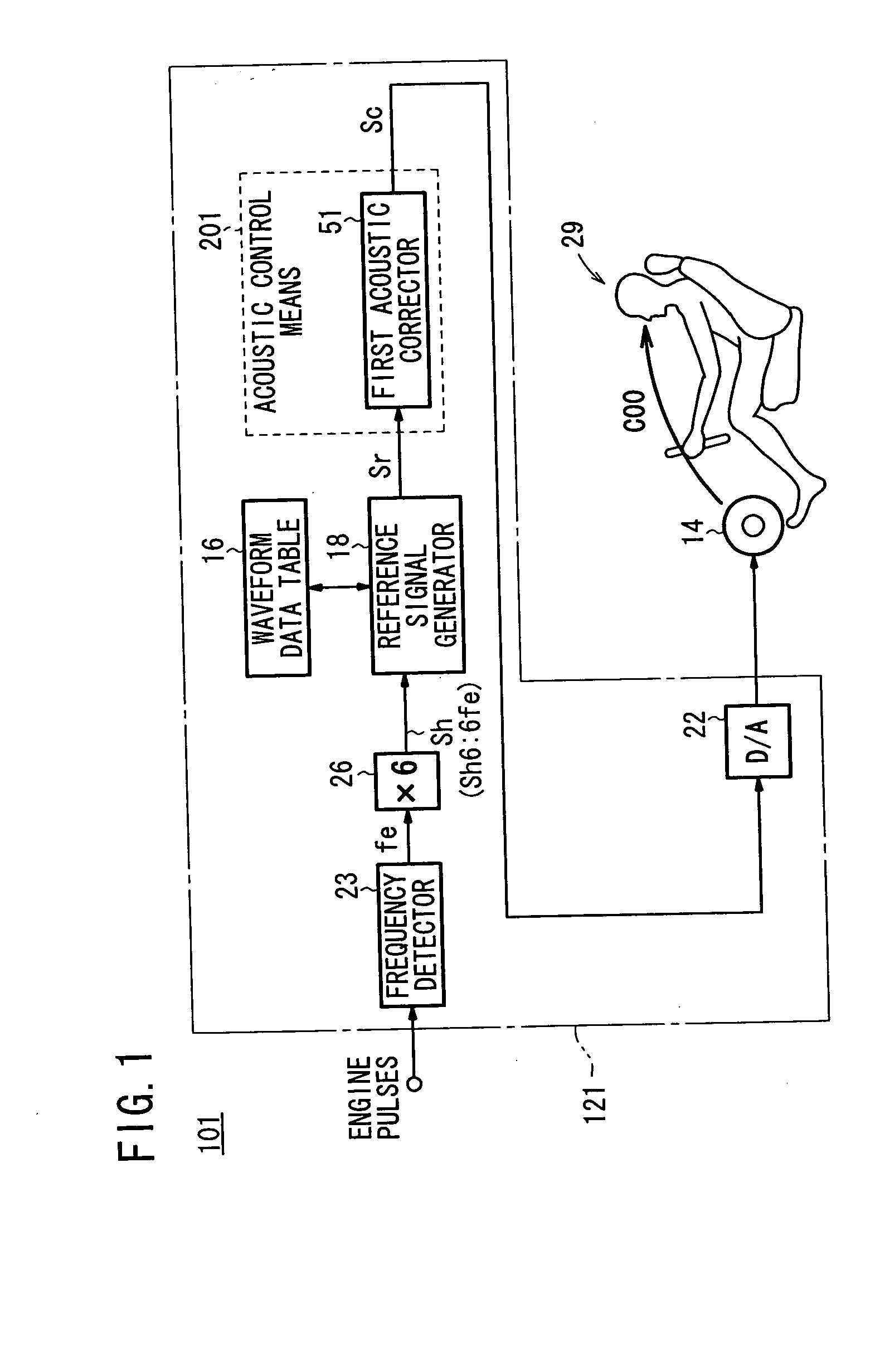
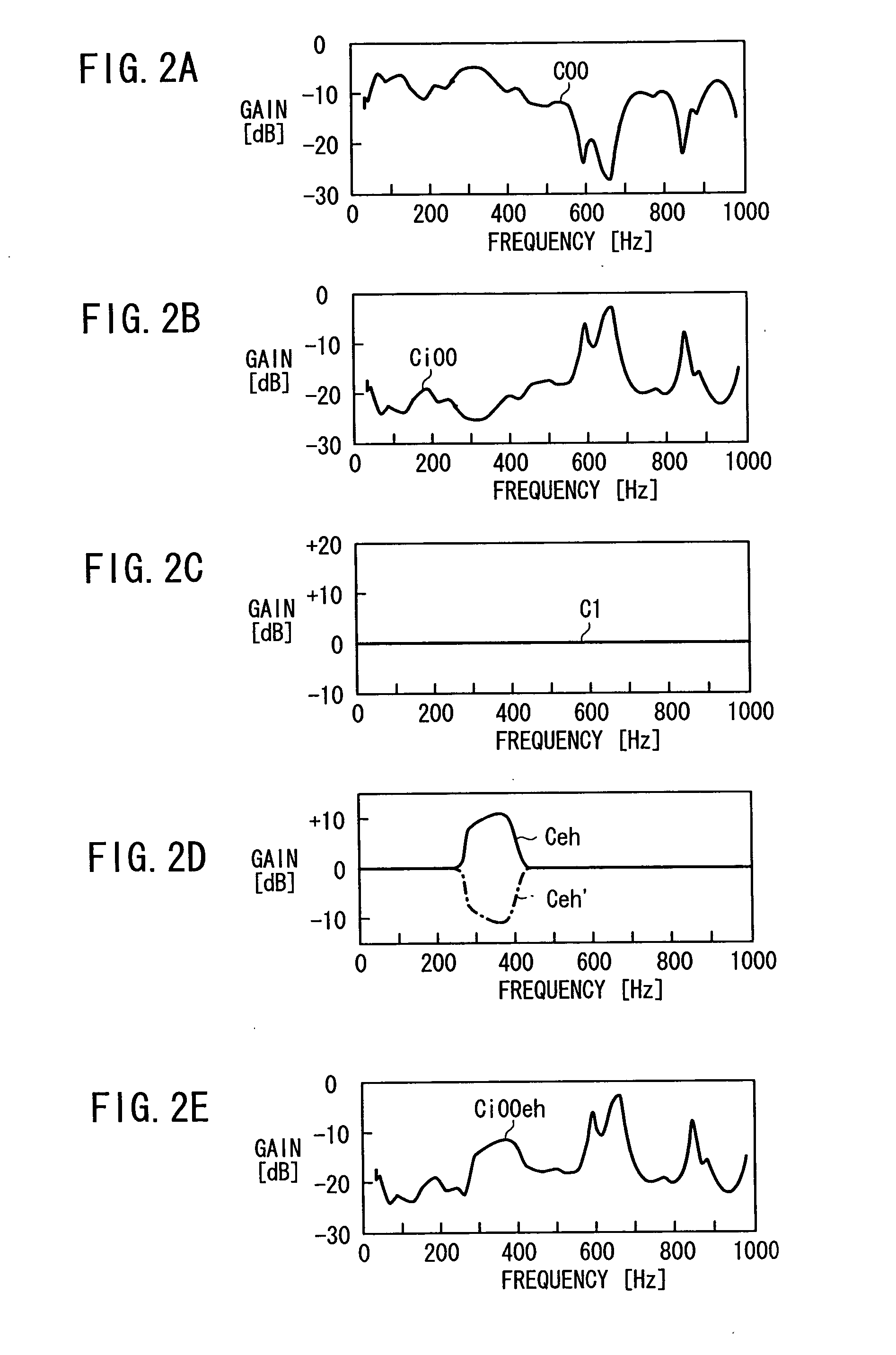
Apparatus for producing sound effect for mobile object
InactiveUS20060215846A1Improve acoustic performanceImprove linearityEar treatmentGain controlBody compartmentEngineering
Gain characteristics depending on the frequency of a reference signal from speakers to a passenger position in a motor vehicle, i.e., gain characteristics which are an inversion of vehicle cabin sound field characteristics, are set in a first acoustic corrector. At the passenger position, a gain characteristic curve that is flat at various frequencies is achieved to prevent gain peaks and dips from occurring at the passenger position. A sound effect generated at the passenger position is made linear depending on the state of a noise source, or more specifically, a noise source caused by an accelerating action on the motor vehicle.
Owner:HONDA MOTOR CO LTD
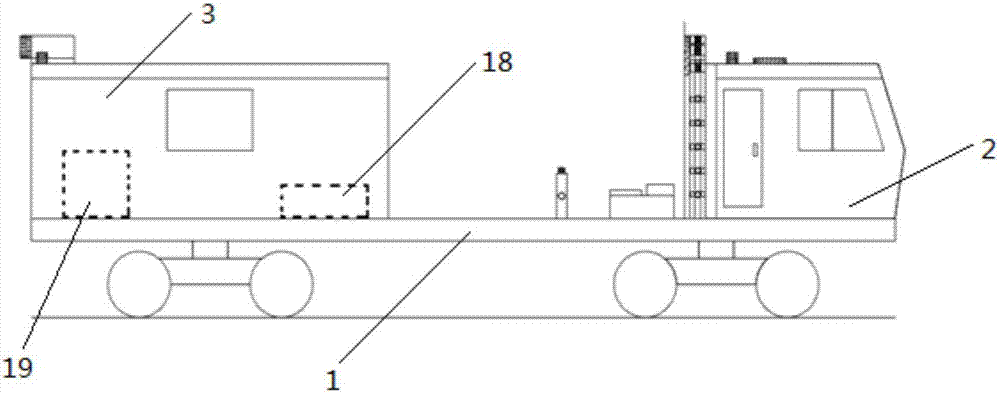
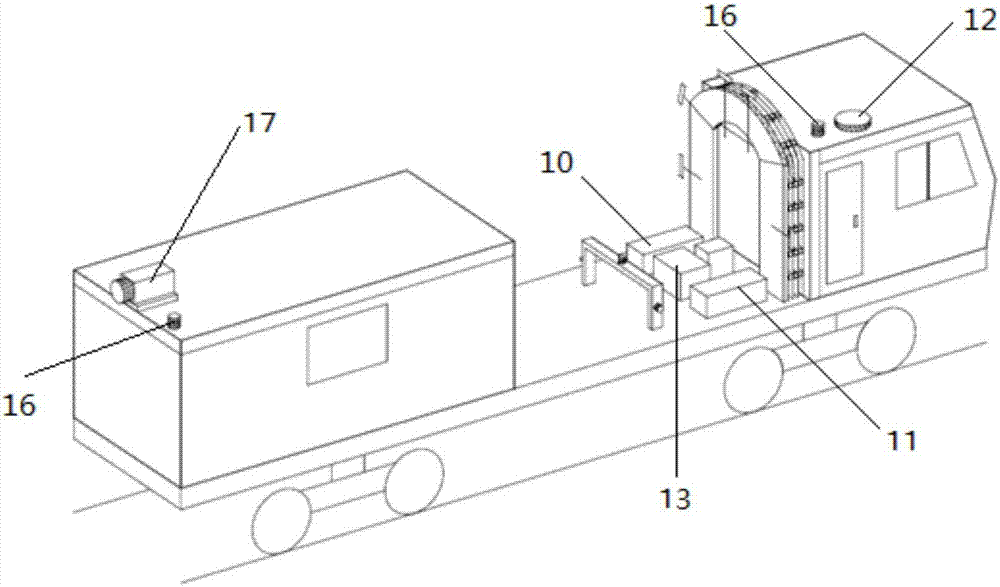
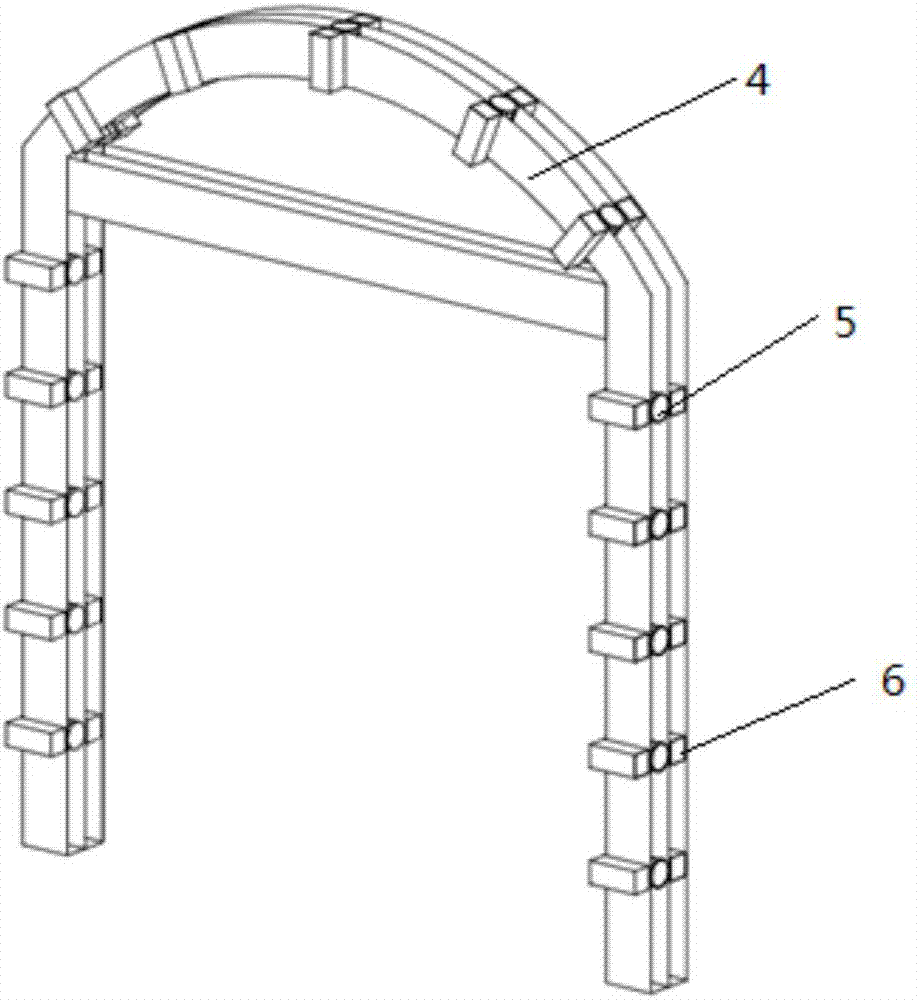
Method and rail vehicle for full-section comprehensive detection of railway tunnels
PendingCN107014352AAvoid time-consuming and inefficientDetection speedSatellite radio beaconingProfile tracingRailway tunnelBody compartment
The invention belongs to the technical field of tunnel detection of railway engineering systems and particularly relates to a method and a rail vehicle for full-section comprehensive detection of railway tunnels. The rail vehicle comprises a rail vehicle floor board, a cab and a carriage. Line-scan cameras are arranged on an arch frame which is arranged on the rail vehicle floor board, air-coupled shield antennas, and a GPS (global positioning system) mainframe, ground penetrating radar and infrared thermal imagers are arranged on the rail vehicle floor board. A GPS receiver is arranged at the top end of the cab, a laser scanner is arranged at the tail of the top plate of the carriage, and an industrial personal computer is arranged in the carriage. By multiple detection systems, one-time full-section detection of tunnel lining states and tunnel bottom damages can be realized, high detection accuracy, high efficiency, shortening of maintenance time and saving of maintenance cost are realized, influences on normal transportation of the railway tunnels are low, and loss caused by stopping of transportation is reduced.
Owner:CHINA RAILWAY SIYUAN SURVEY & DESIGN GRP
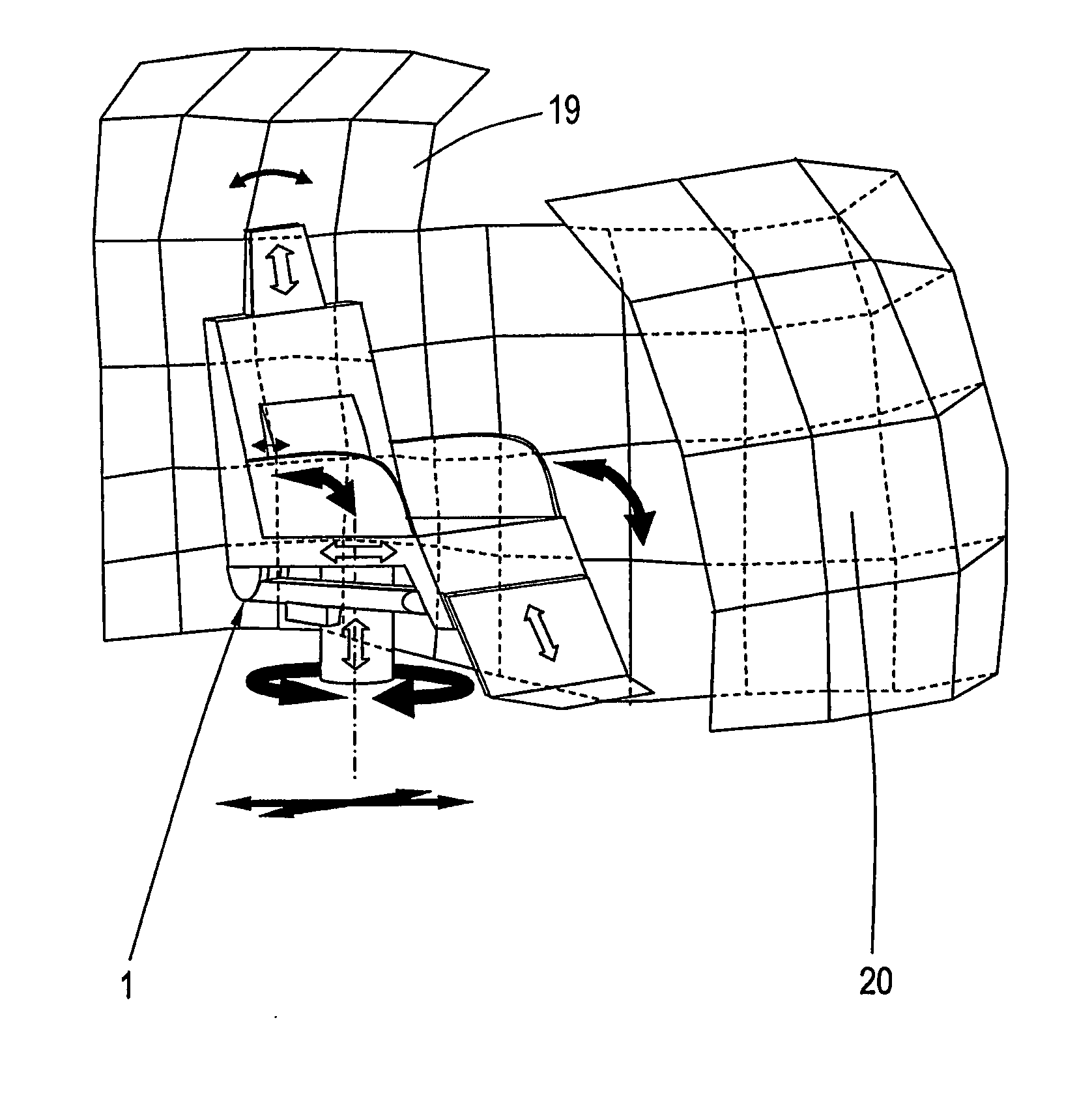
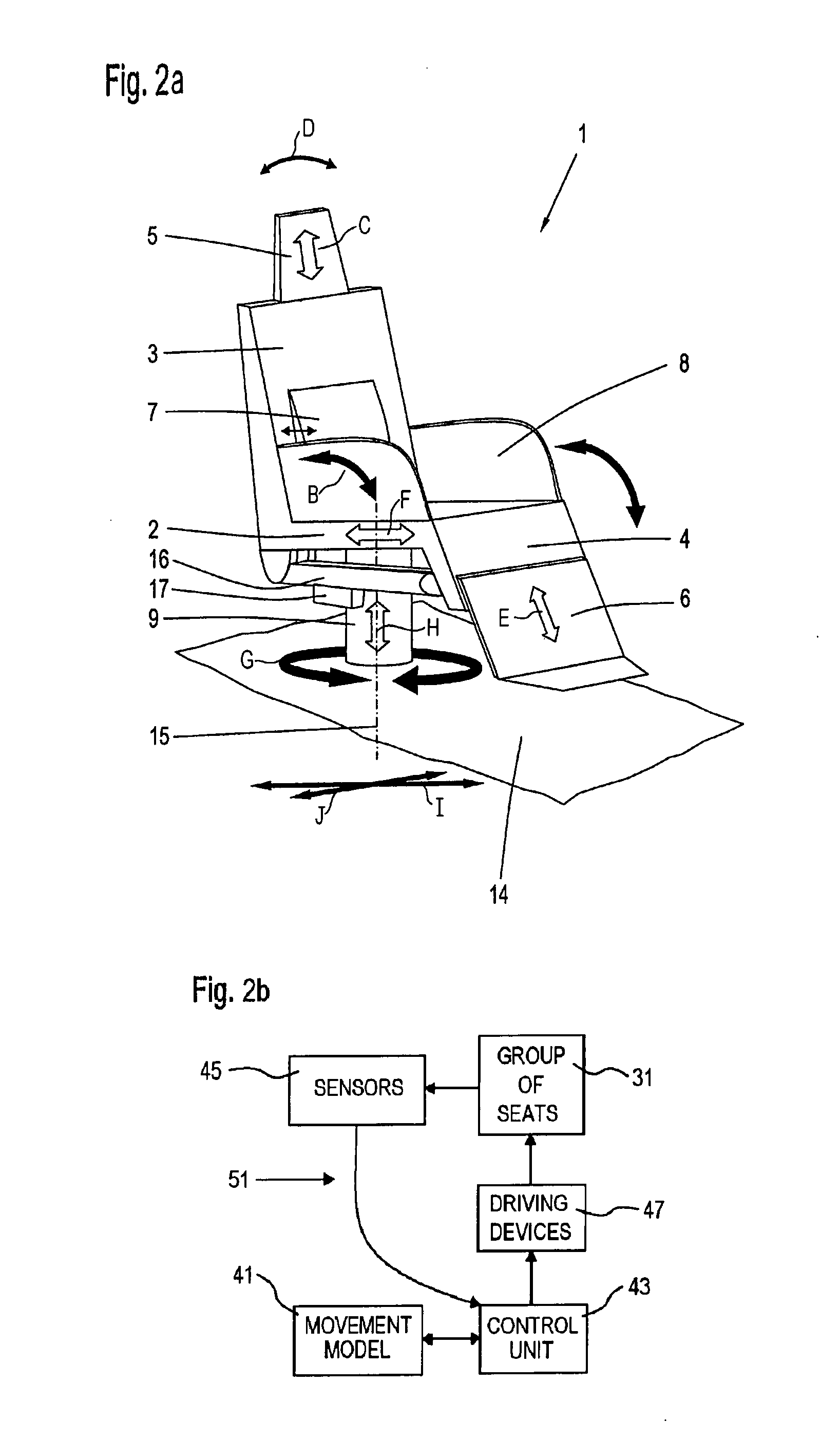
Adjusting system for moving a vehicle seat in a vehicle cabin
InactiveUS20080009958A1Easy to adaptAvoid disadvantagesVehicle seatsDigital data processing detailsBody compartmentEngineering
A vehicle seat, an adjusting system and a method for moving at least one vehicle seat arranged in a vehicle cabin, particularly an aircraft seat, comprising seat components that are coupled or guided together, such as a seat part, a backrest and a leg rest, the location and / or inclination of which can be adjusted by means of at least one or more adjusting units. The adjusting system allows a separation of the basic functions from driving devices, with an electronic device for collision-free movement and for adjusting custom-designed functions and facilitating the adaptation of the custom-designed functions. The movement sequences and movements of the respective individual seat components and the seat components in relation to one another and the movements of the components or seats within an environment or group of seats are accomplished by means of a movement model in a coordinate system.
Owner:BUHLER MOTOR GMBH
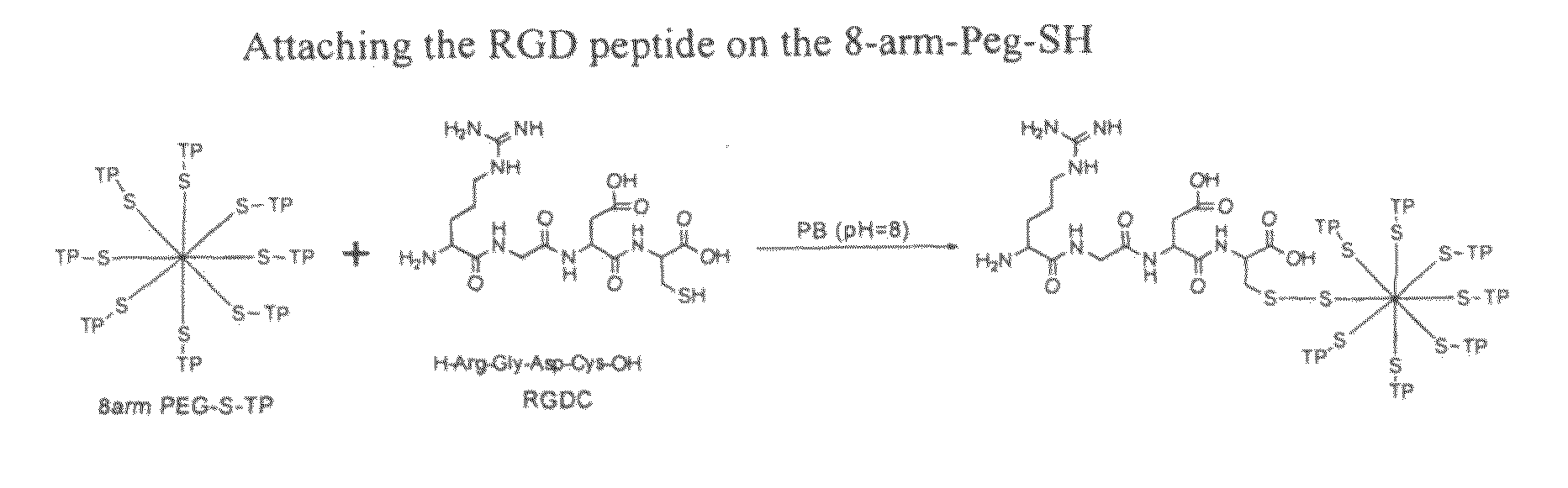
Dressing compositions and methods
ActiveUS20110033503A1Readily solubleEasy and painless to removeCosmetic preparationsBiocideDisulfide bondingWound dressing
Described is a spray-on hydrogel comprising water-soluble PEG polymers that cross-link in situ to form a hydrogel such that the cross-links are reversible. The hydrogel can be useful as a drug delivery composition, wound dressing or surgery adjuvant. Polyethylene glycol polymer and cross-linker solutions are sprayed simultaneously through a common orifice. Cross-linking via formation of thioether or disulfide bonds is initiated upon mixing, providing rapid gelation. The hydrogel components can be derivatized with RGD peptides or analogs thereof to promote retention in / on a body compartment such as the skin, surface of the eye, or a mucosa such as the vaginal mucosa. The cross-links are reversed using a reducing solution enabling easy removal of the hydrogel by dissolution. Processes for preparation of the cross-linker, RGD derivatized PEG and RGD-linked agents are also disclosed.
Owner:RUTGERS THE STATE UNIV
Fire simulation experiment system for railway tunnel rescue station
InactiveCN102306459ARealize measurementReal-time recording and objective reflection of heat insulation protection effectCosmonautic condition simulationsSimulatorsRailway tunnelBody compartment
The invention provides a fire simulation experiment system for a rail tunnel rescue station. The system comprises a simulation tunnel, a simulation rescue station platform, a simulation railway train, a smoke exhaust system, a fire monitoring system, a fire suppression system and a fire parameter measurement system. By the experiment system, the research of a series of basic theory problems and engineering practice problems of fire development, smoke flow, fire monitoring and suppression and the like generated when the fire of the tunnel rescue station is positioned on the top of a carriage, inside the carriage and at the bottom of the carriage can be comprehensively carried out. By the system, a whole set of solution is provided for the research on the effectiveness of a fire monitoring and suppression technology and a smoke control technology under the condition of different longitudinal wind speeds and the evolution law of characteristic parameters such as a temperature field, a poisonous and harmful gas concentration field, thermal radiation and the like in the scene that a train in an extra-long railway tunnel is stopped at the rescue station in the fire process.
Owner:UNIV OF SCI & TECH OF CHINA
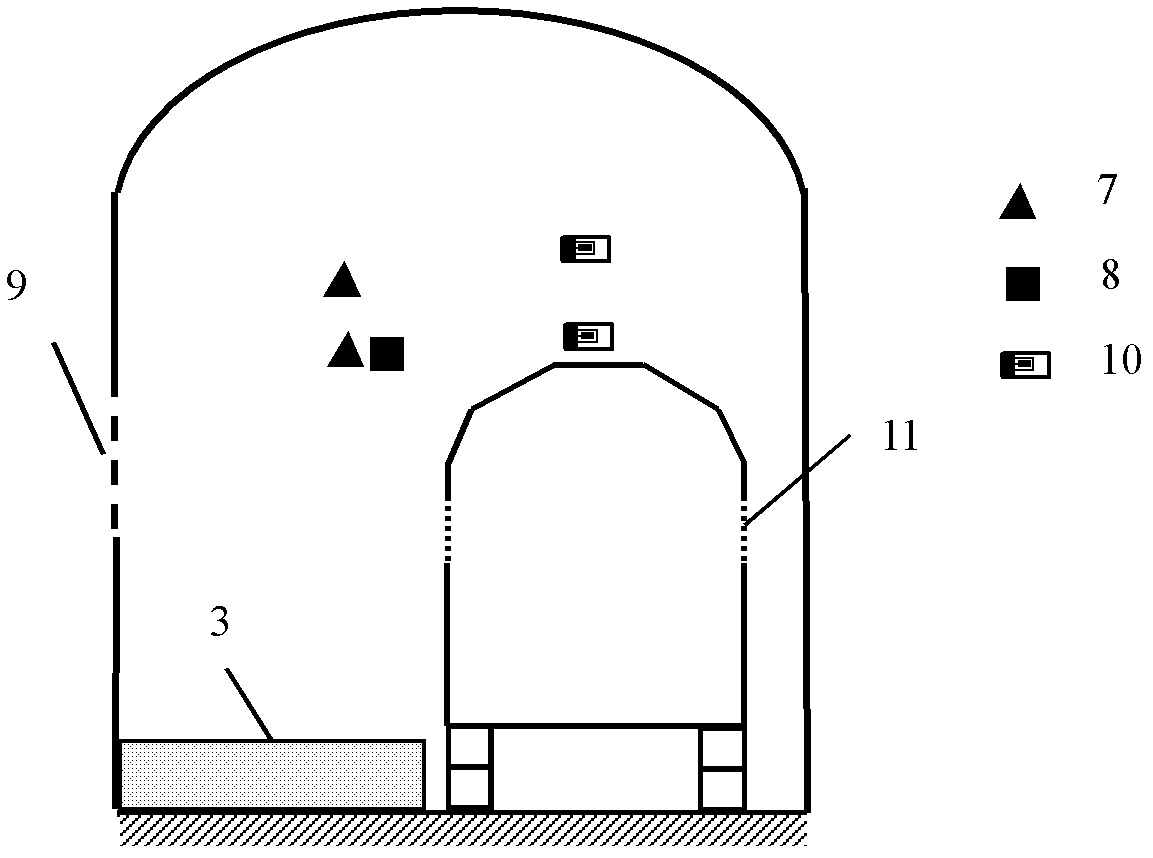
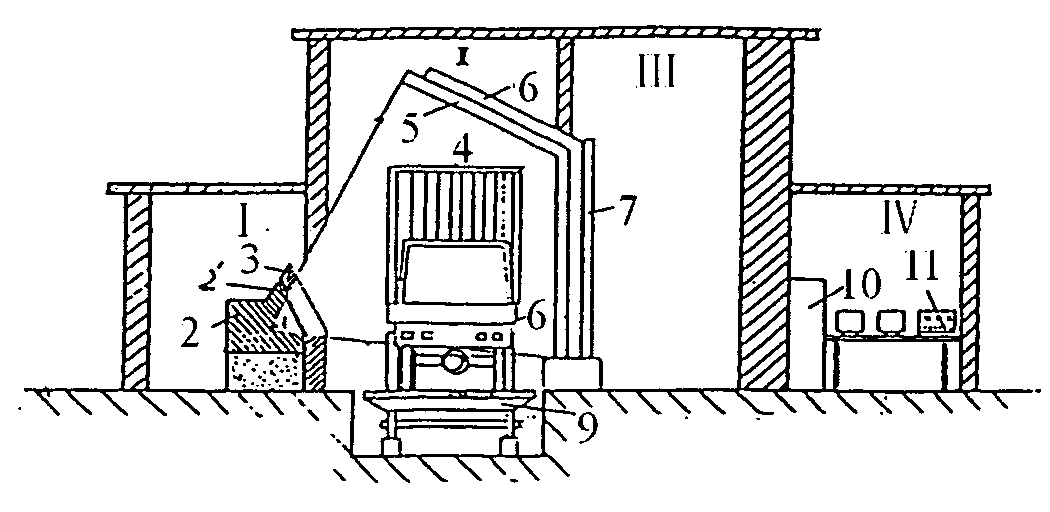
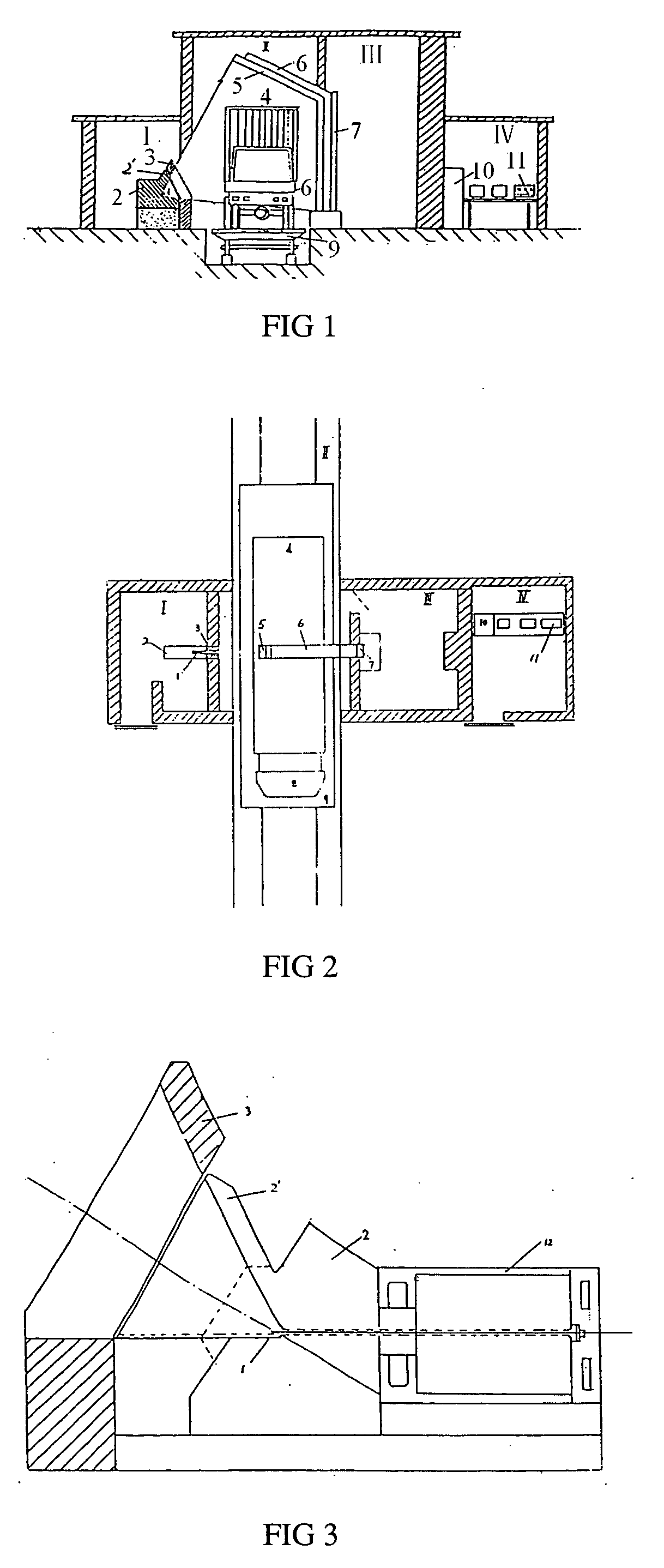
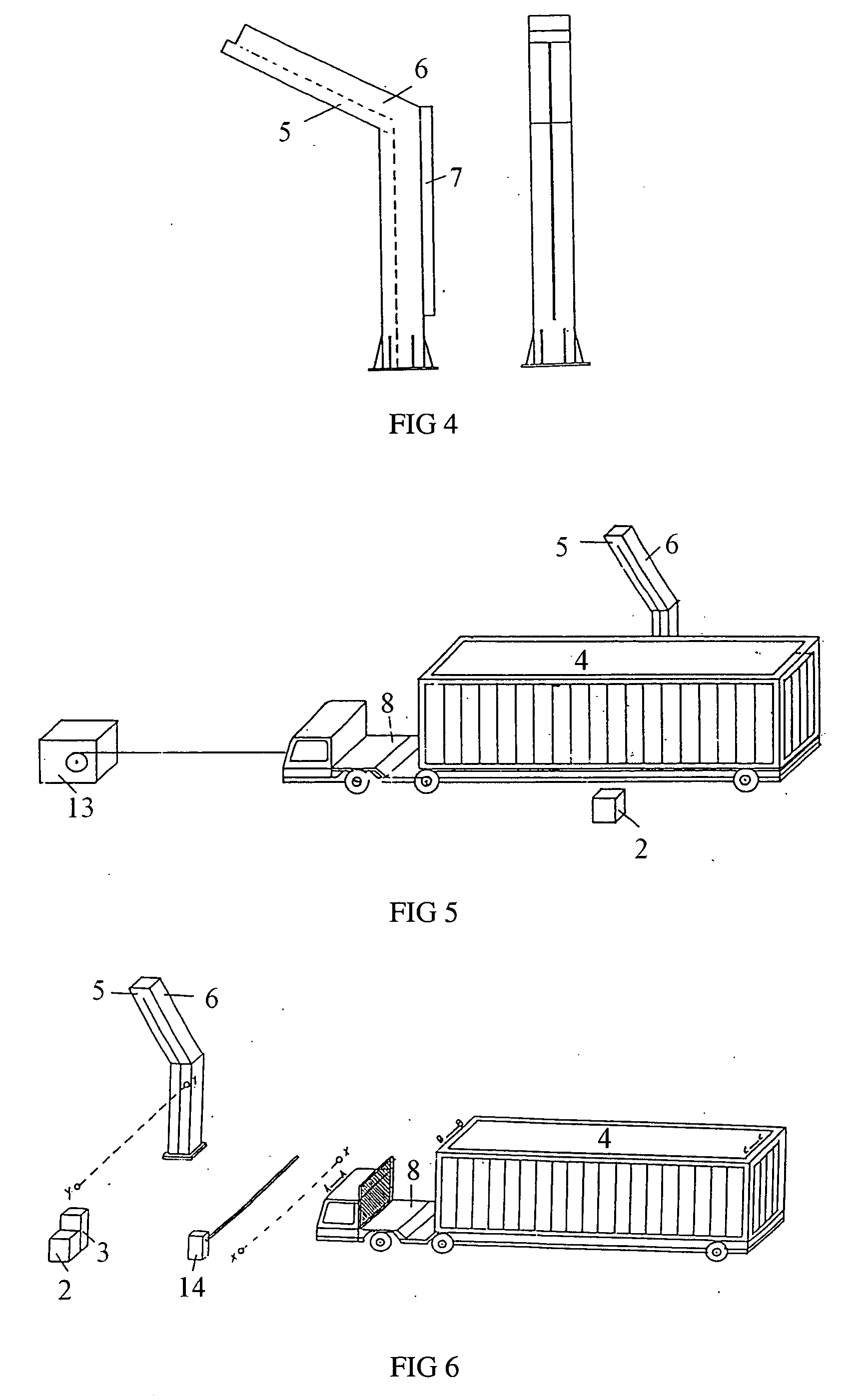
Contain inspection system using cobalt-60 gamma-ray source and cesium iodide or cadmium tungstate array detector
InactiveUS20040179647A1Easy to produceLow costHandling using diaphragms/collimetersX/gamma/cosmic radiation measurmentAutomatic controlBody compartment
A container inspection equipment with cobalt-60 gamma-ray source and cesium iodide or cadmium tungstate detector includes a cobalt-60 gamma-ray source, a cask, a front collimator, a rear collimator, a cesium iodide or cadmium tungstate detector, signal and image processing systems, container trailer system, and automatic control system. The cask of the cobalt-60 gamma-ray source, the beam shutter, and the front collimator are fixed on the same chassis to form an integration and placed in the source room. The rear collimator, cesium iodide or cadmium tungstate array detector and the radiation catcher are fixed on the same chassis to form an integration and placed in the detector room. A container inspection tunnel is formed between the source room and the detector room. The equipment is mainly used for inspecting smuggling goods and contrabands etc., in large containers, container trucks, train carriage and air containers. The equipment either can be installed in the sea port and land frontier customs or installed at the air port, vital communication line, and railway station.
Owner:BEIJING ISOTOPE NUCLEAR ELECTRONICS MACHINE
Extendable ramp for boats and vehicles
An extendable ramp apparatus is provided for automatically extending a ramp. The apparatus comprises a generally rectangular frame with a track for movement of a carriage pivotally connected to a ramp along the track. The ramp pivots downwardly as it is extends from the frame until it encounters a surface. A bidirectional motor is provided with a cabling arrangement to reciprocate the carriage and ramp along the track. Shock absorbers may slow the carriage movement as the ramp is extended so that hard contact of the ramp with a surface is minimized. An innovative capstan arrangement provides more rapid movement of the ramp as it retracts but provides more power during ramp extension, so that vertical movement of the ramp is slowed as the ramp is extended. The apparatus may find application in vehicles such as trucks, vans, and boats whenever it is desired to move goods or people between surfaces of different heights, such as a truck bed and the ground.
Owner:JONES BILLY GENE MR +1
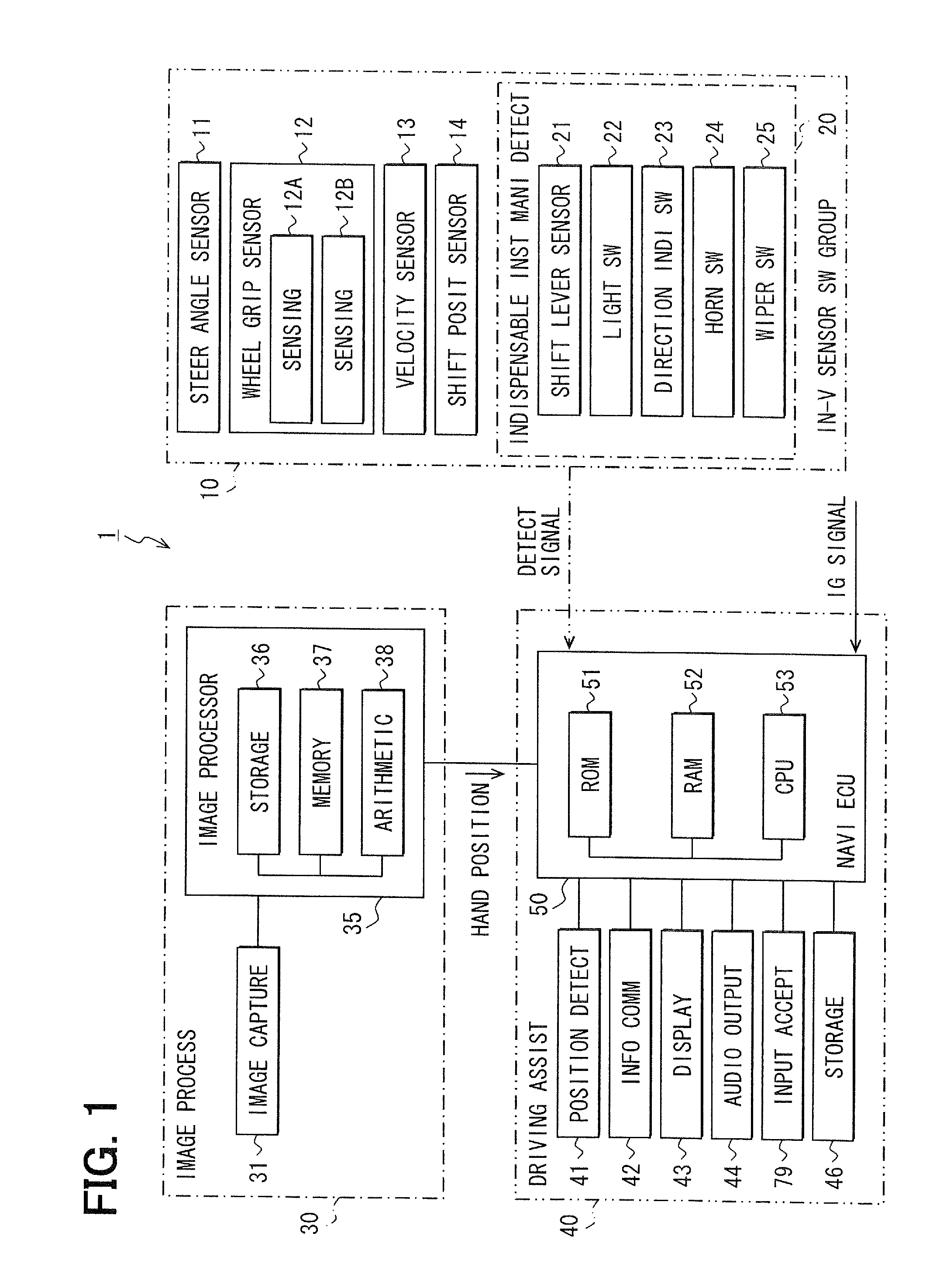
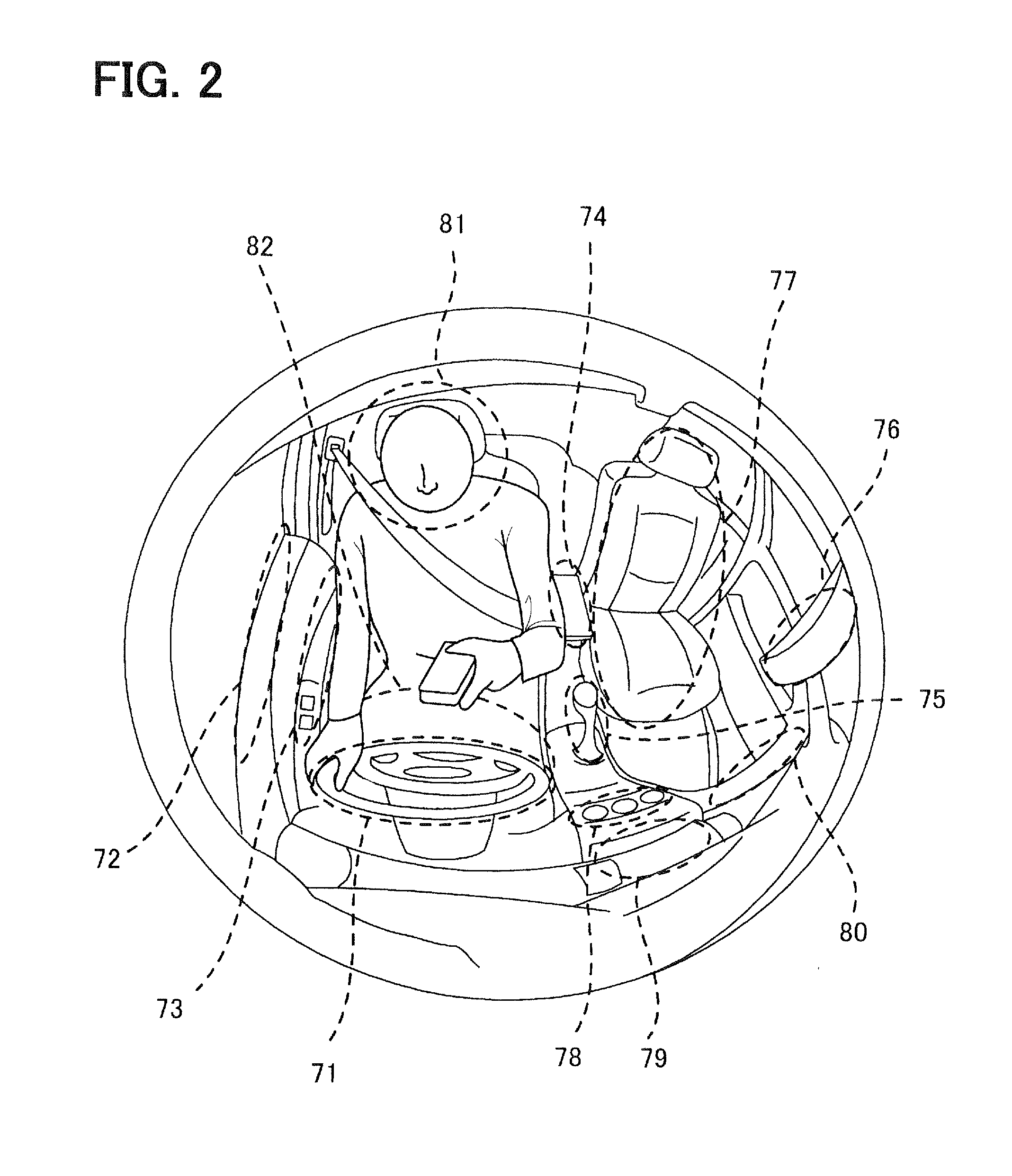
Driving assistance apparatus
InactiveUS20110254956A1Maintain concentrationStay focusedCharacter and pattern recognitionCathode-ray tube indicatorsHands freeEngineering
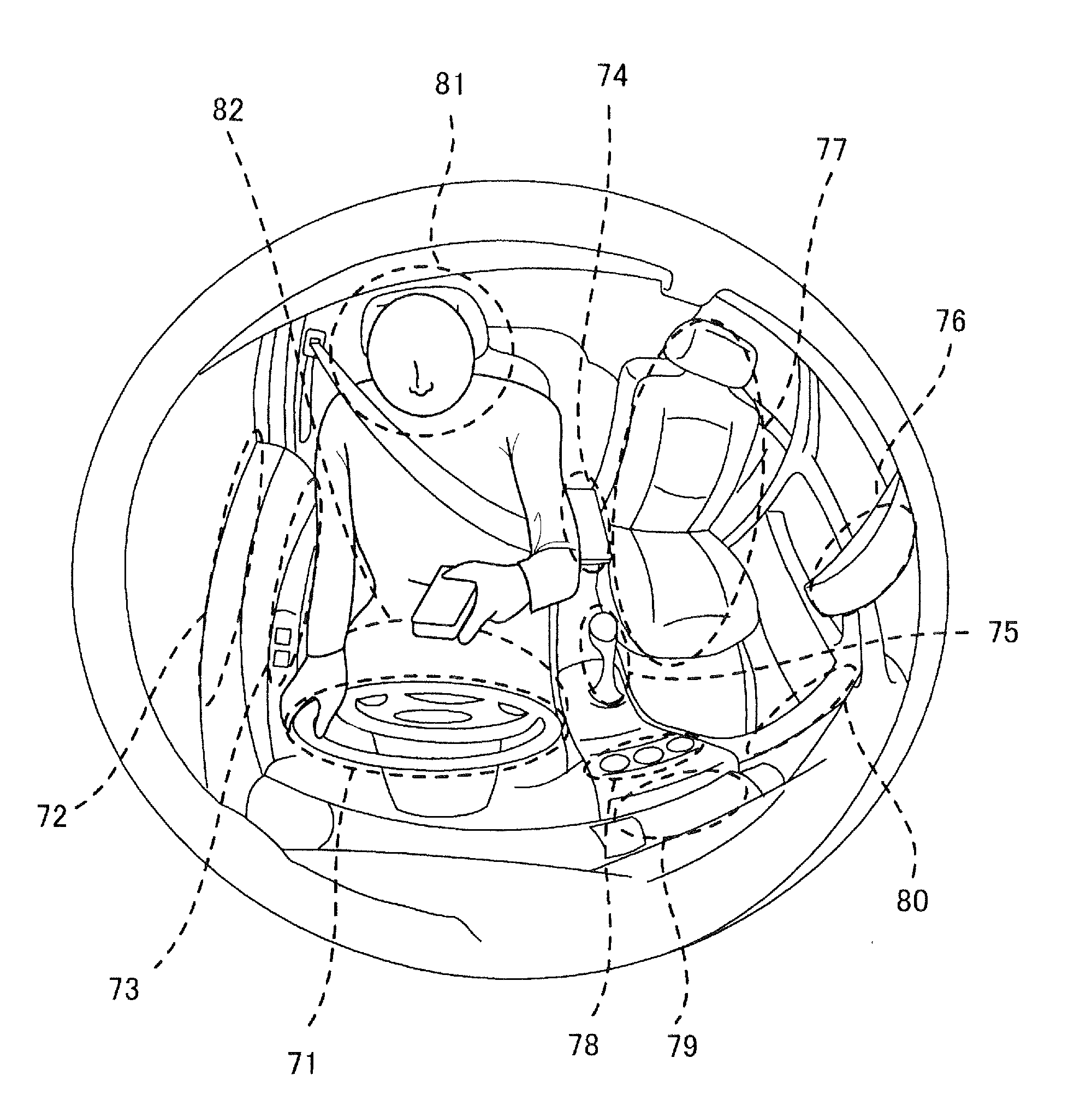
In a hand-free driving warning process for a vehicle, when a grip state of a steering wheel is in a hand-free driving state, and the vehicle is in a travel state stipulated previously, a danger degree is derived based on a position of a vehicle compartment where a non-grip hand exists. In deriving the danger degree, when the non-grip hand is in a high danger position, a high level is set; when the non-grip hand exists in a low danger position, a middle level or a low lever is set. In the low level, any warning is not outputted; in the middle level, a warning is outputted in a sound volume smaller than a usual sound volume; and in the high level, a warning is outputted in the usual sound volume or greater.
Owner:DENSO CORP
Server design and method
ActiveUS20080312778A1Low costImprove performanceDigital data processing detailsBroadcast specific applicationsCommunications systemOperational system
A communication system for a vehicle includes a server that includes a real time operating system, at least one cabin function application that runs on the real time operating system, and at least one in-flight entertainment application that runs on another operating system on top of the real time operating system. Thus, an in-operation entertainment system is capable of providing audio and / or visual content to a large number of locations in a cabin of the vehicle, and a cabin function system is capable of providing various cabin applications, e.g., lighting level control, attendant calling, air conditioning control, etc. at different locations in the vehicle cabin in a manner that is isolated and prioritized over the entertainment system.
Owner:THALES AVIONICS INC
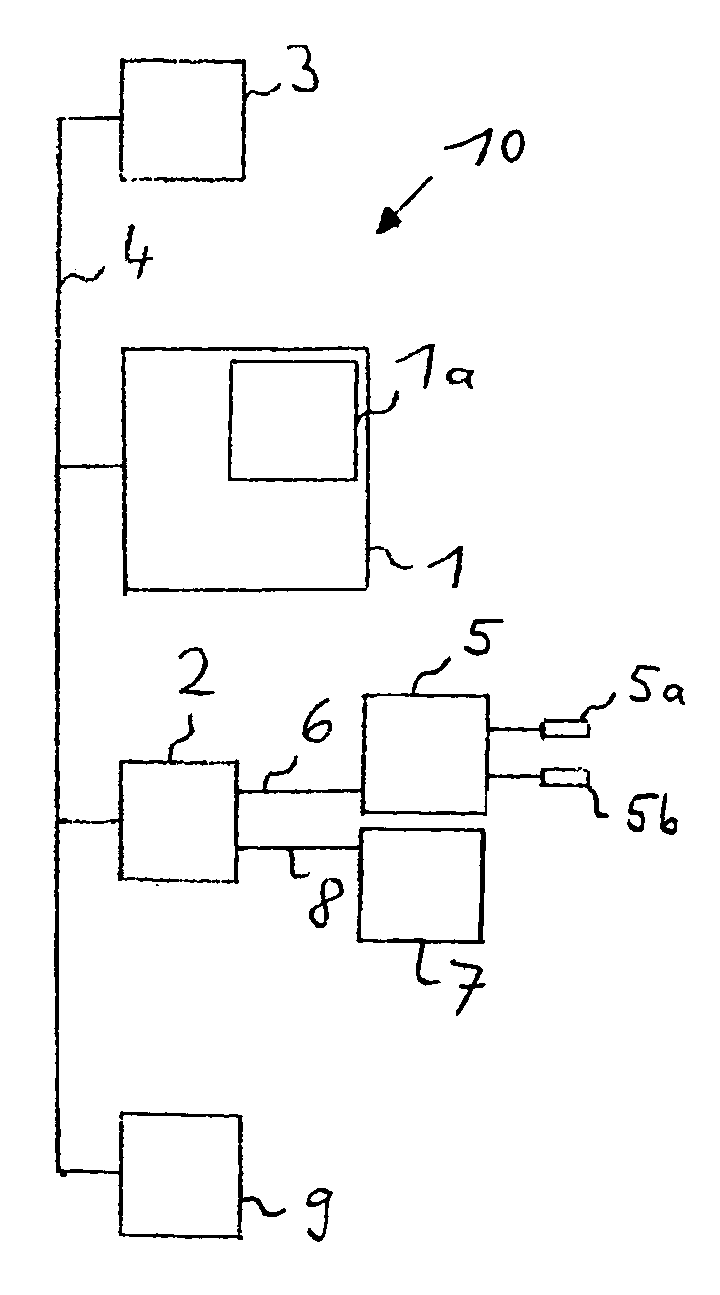
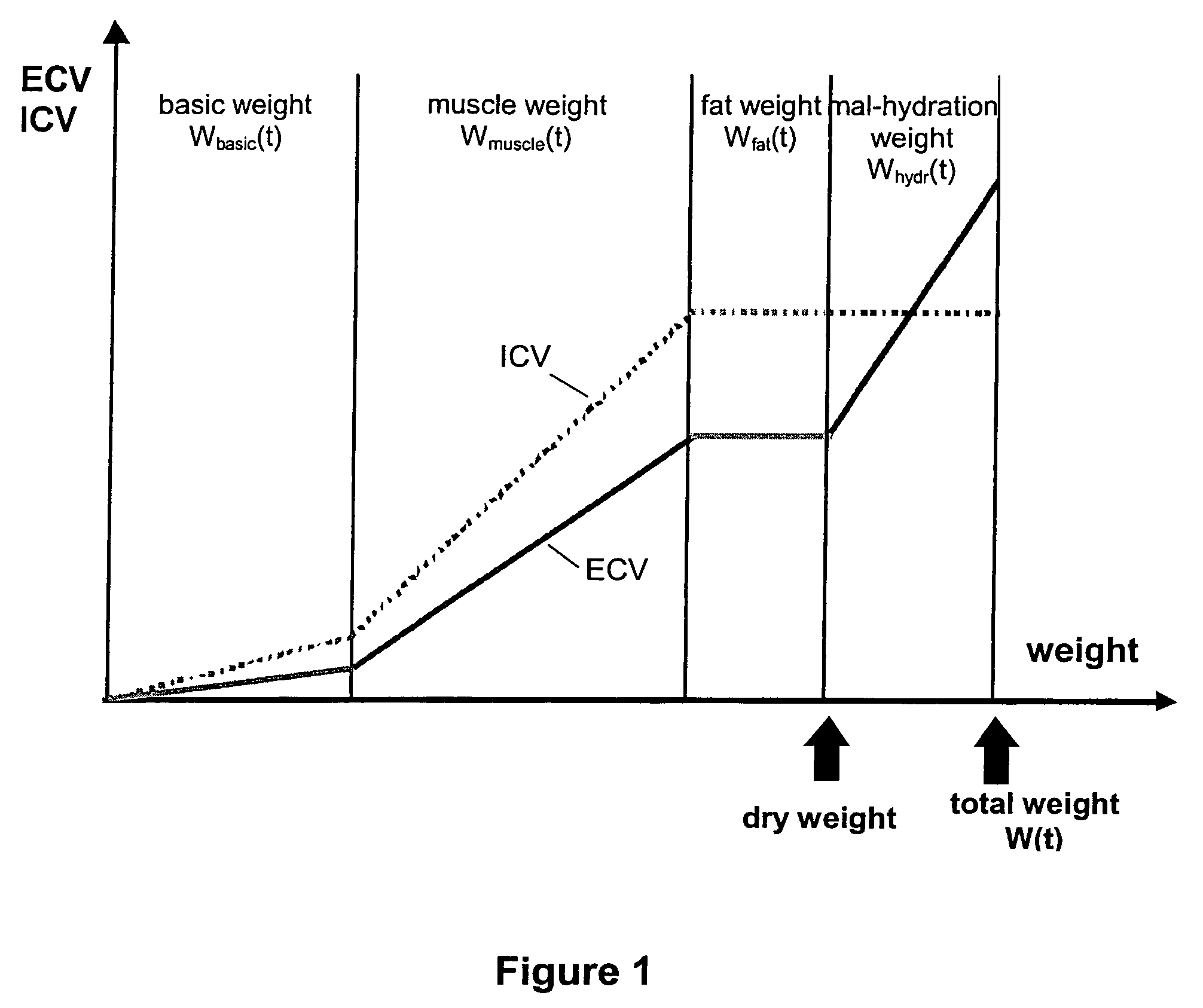
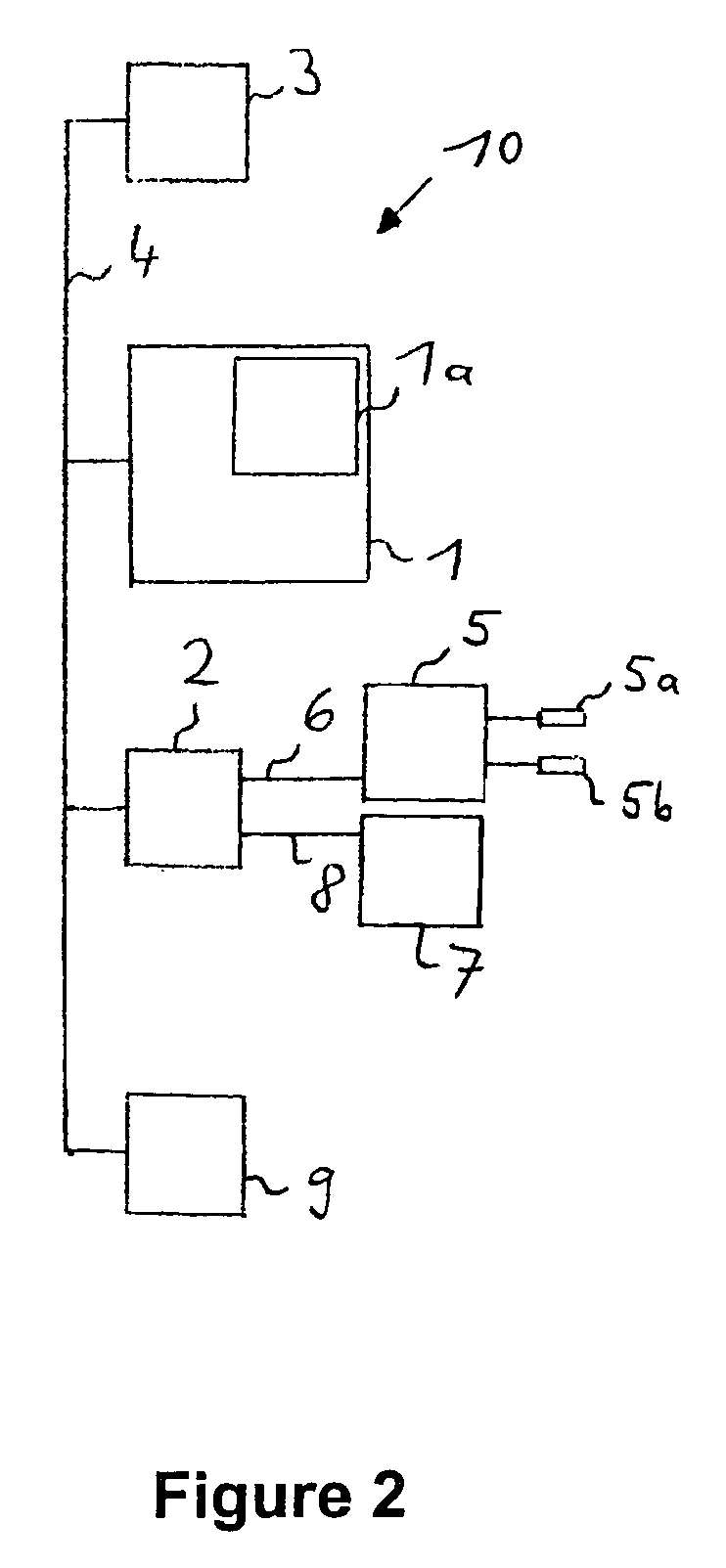
Determining the hydration status of a patient
InactiveUS7133716B2The process is simple and convenientSimple methodDiagnostic recording/measuringSensorsExtracellularWater volume
A device and method are provided for determining the volume ECVhydr(t) of a body compartment of a patient at a time t by conducting measurements at the time t of the patient to determine at least one anthropometric measure X(t), the extracellular water volume ECV(t), and the intracellular water volume ICV (t) of the patient. The extracellular water volume ECVbasic(t) of a first compartment with weight Wbasic(t) of the patient at the time t is derived by using X (t), the extracellular water volume ECVsec(t) of a second compartment of the patient at the time t is derived by using ICV (t), and ECVhydr(t) as the extracellular water volume of a third compartment of the patient is derived with weight Whydr(t). The extracellular volume ECVhydr(t) is a measure for the hydration status of the patient.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
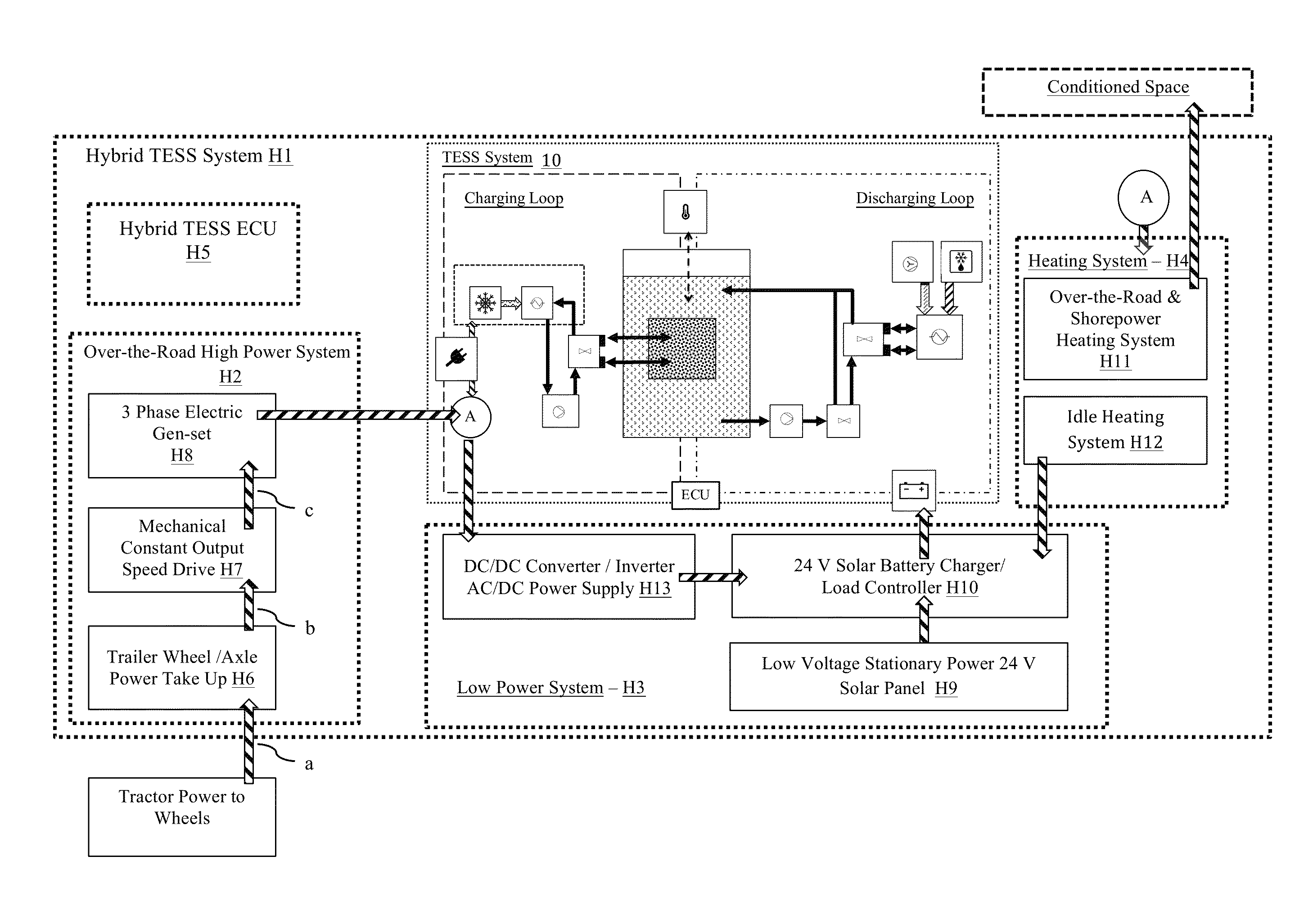
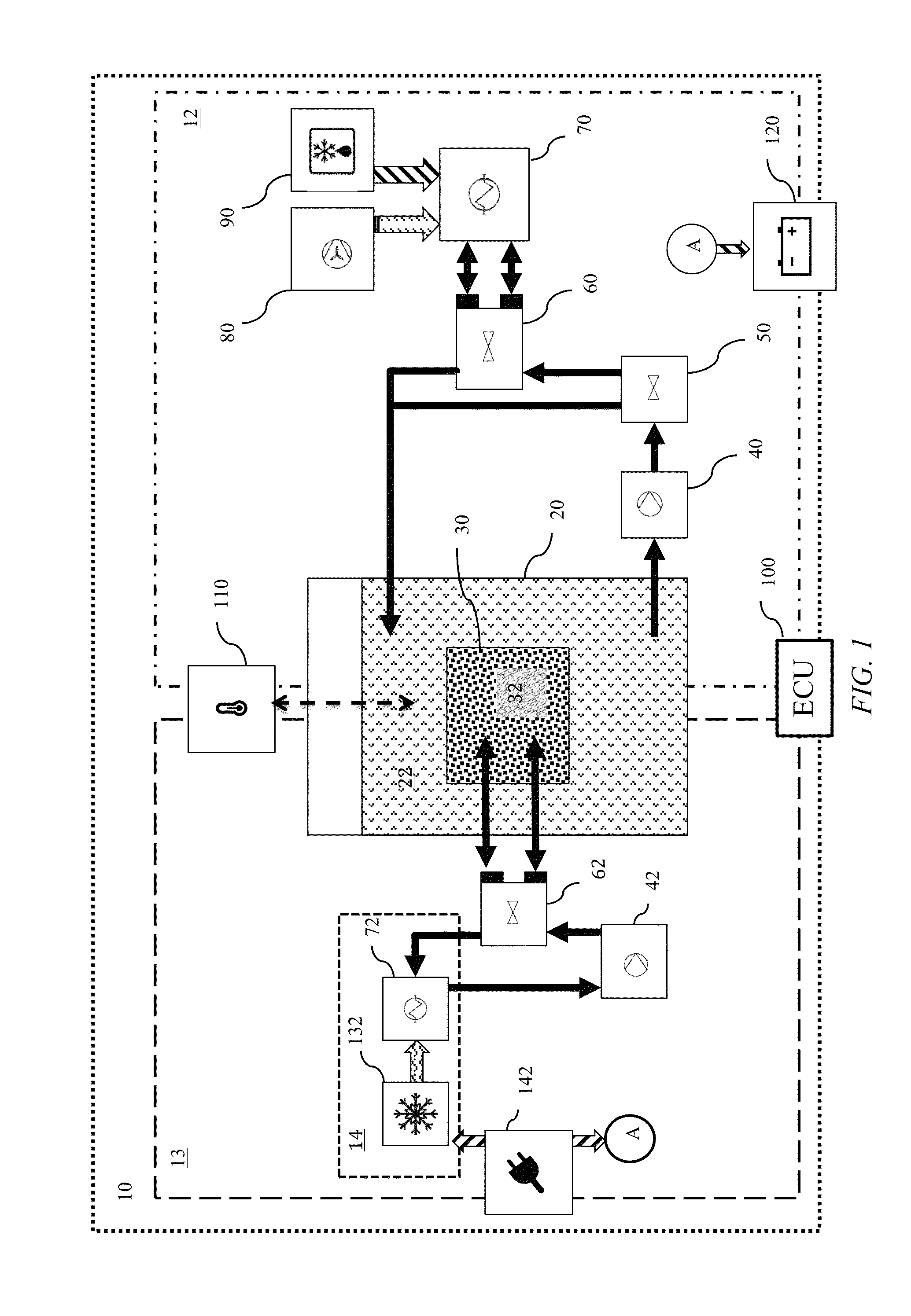
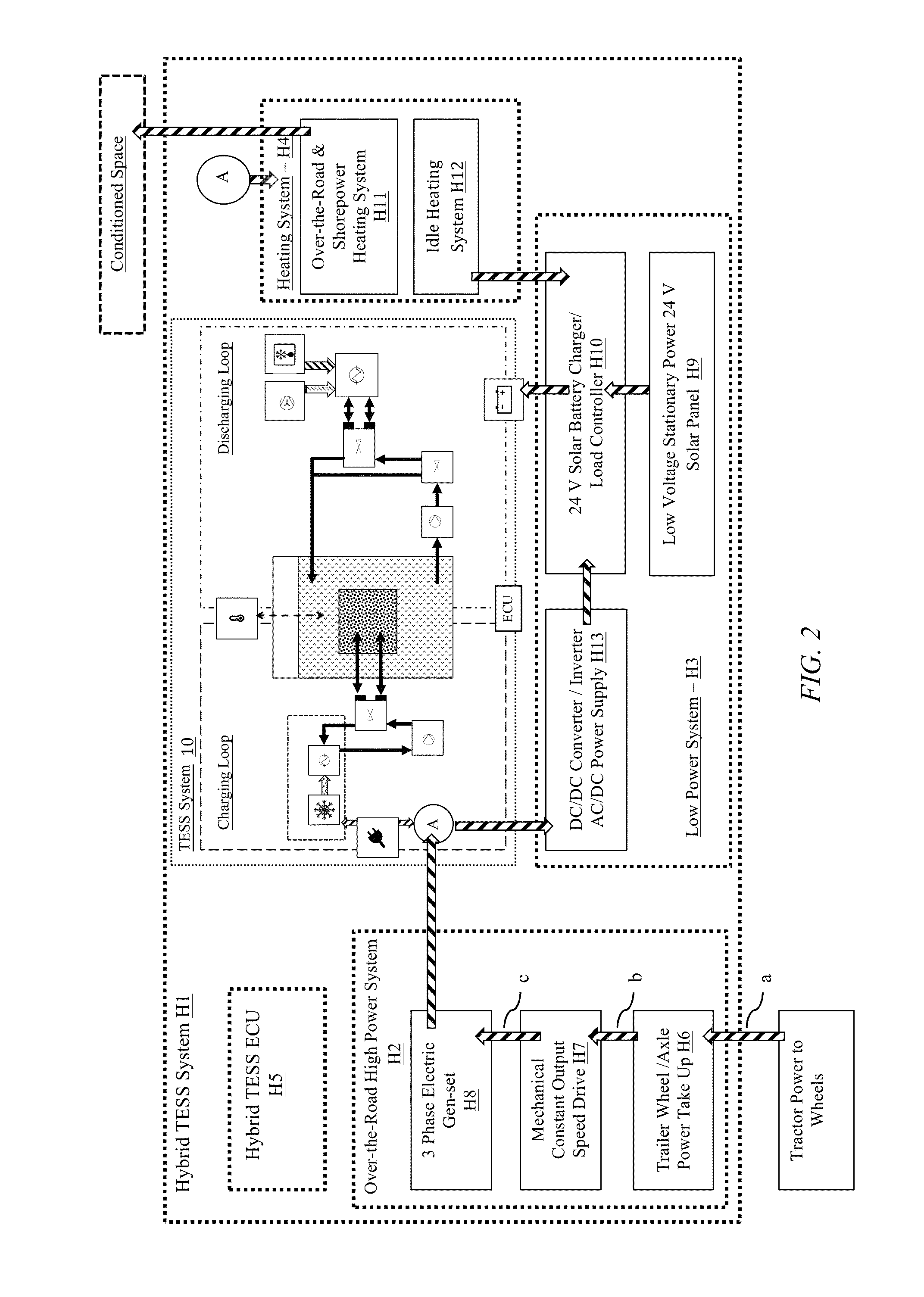
Transportation refrigeration system with integrated power generation and energy storage
InactiveUS9389007B1Avoids cost and weight and emission and regulatory impactMinimized in sizeAuxillary drivesRefrigerated goods vehicleThermal energyWorking fluid
A thermal energy storage system (TESS) that enables the discharge of refrigerated air for cooling cargo or passengers in large compartments, such as the trailer of a semi-truck, for a period of time well in excess of several hours. The TES system is able to provide refrigeration without operating a conventional VCC unit, the truck engine, or the TRU diesel APU engine during all or a significant portion of the period of the typical range of time that a 53 foot refrigerated the truck is traveling over the road. The TES system includes a phase change material (PCM) reservoir, a cooling system-to-WF heat exchanger in fluid communication with the PCM reservoir, and a PCM-to-target heat exchanger in fluid communication with the PCM reservoir. The PCM reservoir contains a phase change material, a working fluid (WF) and a working fluid-to-PCM heat exchanger.
Owner:NEW WEST TECH LLC
Stabilized reverse micelle compositions and uses thereof
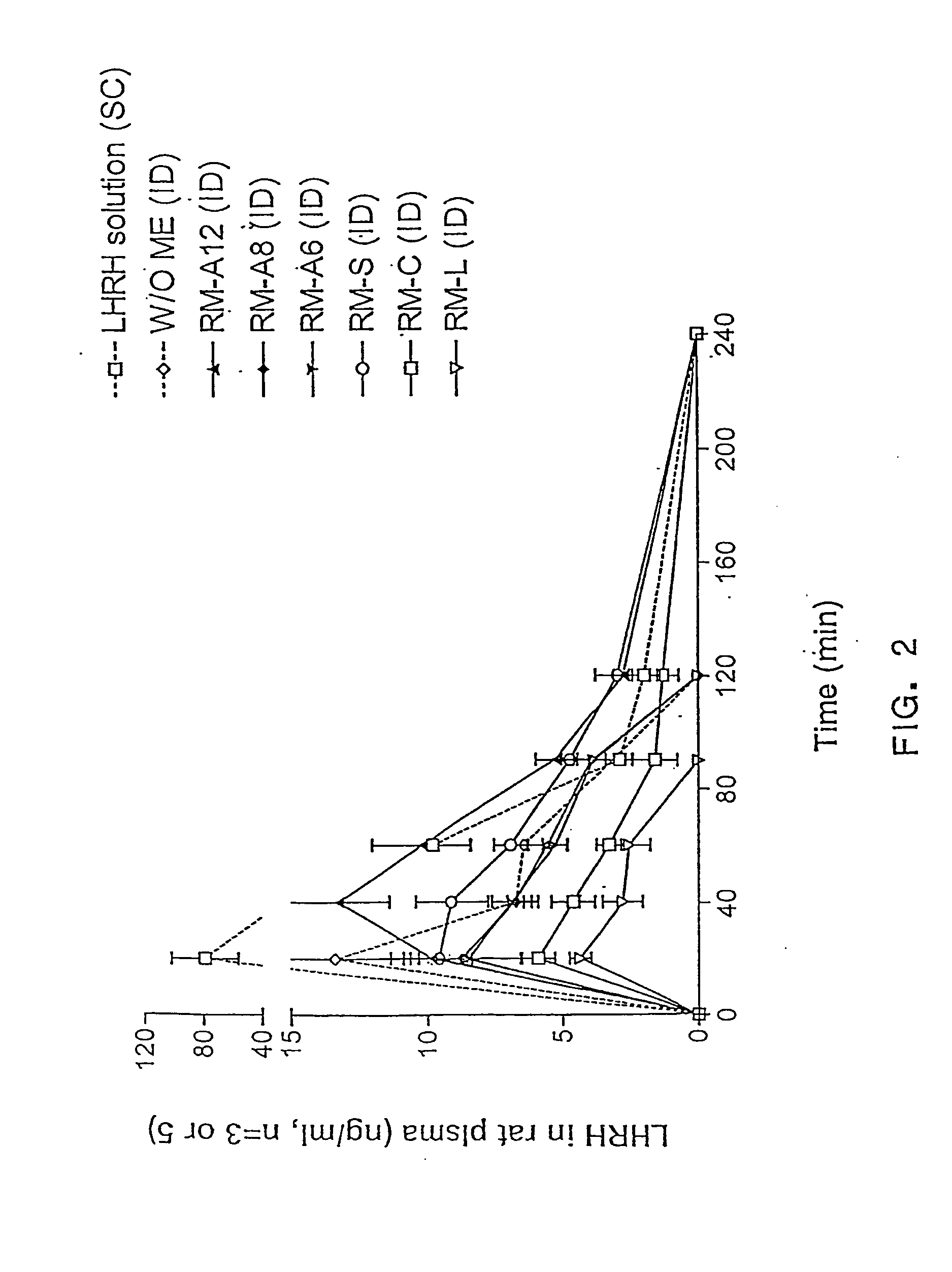
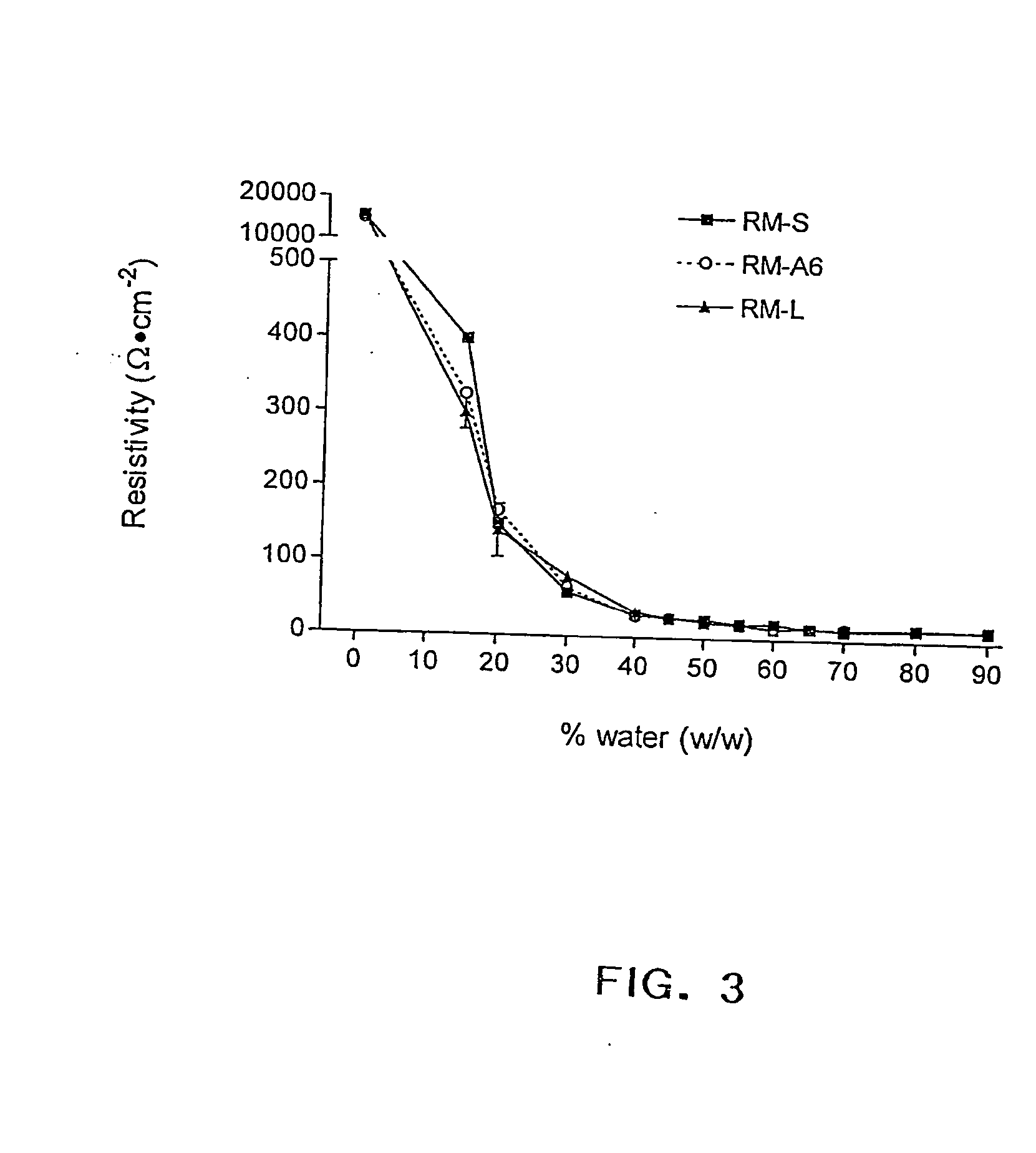
ActiveUS20050079145A1Promote absorptionReduce dosageCosmetic preparationsNervous disorderBody compartmentDrug release
The invention relates to compositions and methods for drug delivery suitable for promoting the transmucosal absorption of drugs, especially drugs with poor intrinsic bioavailability, such as peptides, proteins, vaccines, and nucleic acids. The delivery system of this invention preferably comprises fatty acid esters and their hydrophilic derivatives that associate with water and other polar solvents to form reverse micelles tha are physically stabilized in the presence of gastrointestinal fluid, water, and other hydrophilic solvents. Such stable reverse micelles are formed by suitable mixtures of polymeric or non-polymeric compounds with amphiphiles. Micelles made using these methods undergo phase transformation more slowly resulting in delayed drug release profiles and sustained absorption. When administered as a pharmaceutical to mucosal surfaces following oral ingestion or intranasal administration, therapeutic molecules principally solubilized in the aqueous phase are protected from digestion by mucosal enzymes and other mucosal degradative processes and are taken up by absorptive cell mechanisms and reach appropriate body compartments. The reverse micelle compositions may comprise mono-, di-glycerides and / or their transesterifed products containing C6-C12 fatty acids chains, wherein the transester groups consist of hydrohilic moieties.
Owner:SOLIGENIX INC
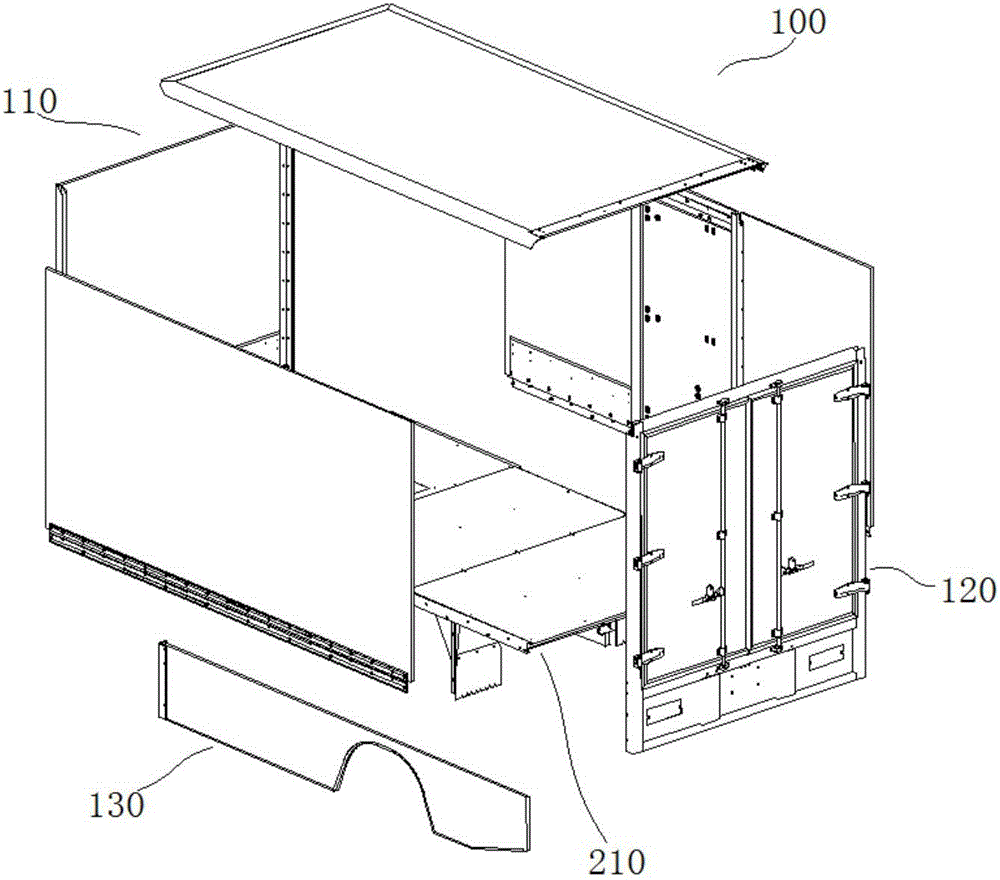
Logistics vehicle and container thereof
ActiveCN106394706AEasy to assembleLower skill requirementsDoorsLoading-carrying vehicle superstructuresEngineeringChassis
The invention relates to the technical field of van vehicles, in particular to a logistics vehicle and a container thereof. The container comprises a container body which comprises a container plate assembly, a chassis assembly, a skirt assembly and a back door plate assembly; and the container plate assembly is arranged on the chassis assembly, the skirt assembly is arranged under the chassis assembly, and the back door plate assembly is simultaneously connected with the container plate assembly, the chassis assembly and the skirt assembly. The logistics vehicle comprises the container. The container is simple in structure, small in dead load and convenient to assemble.
Owner:HANGZHOU HOLYCORE COMPOSITE MATERIAL
Sprinkler assembly
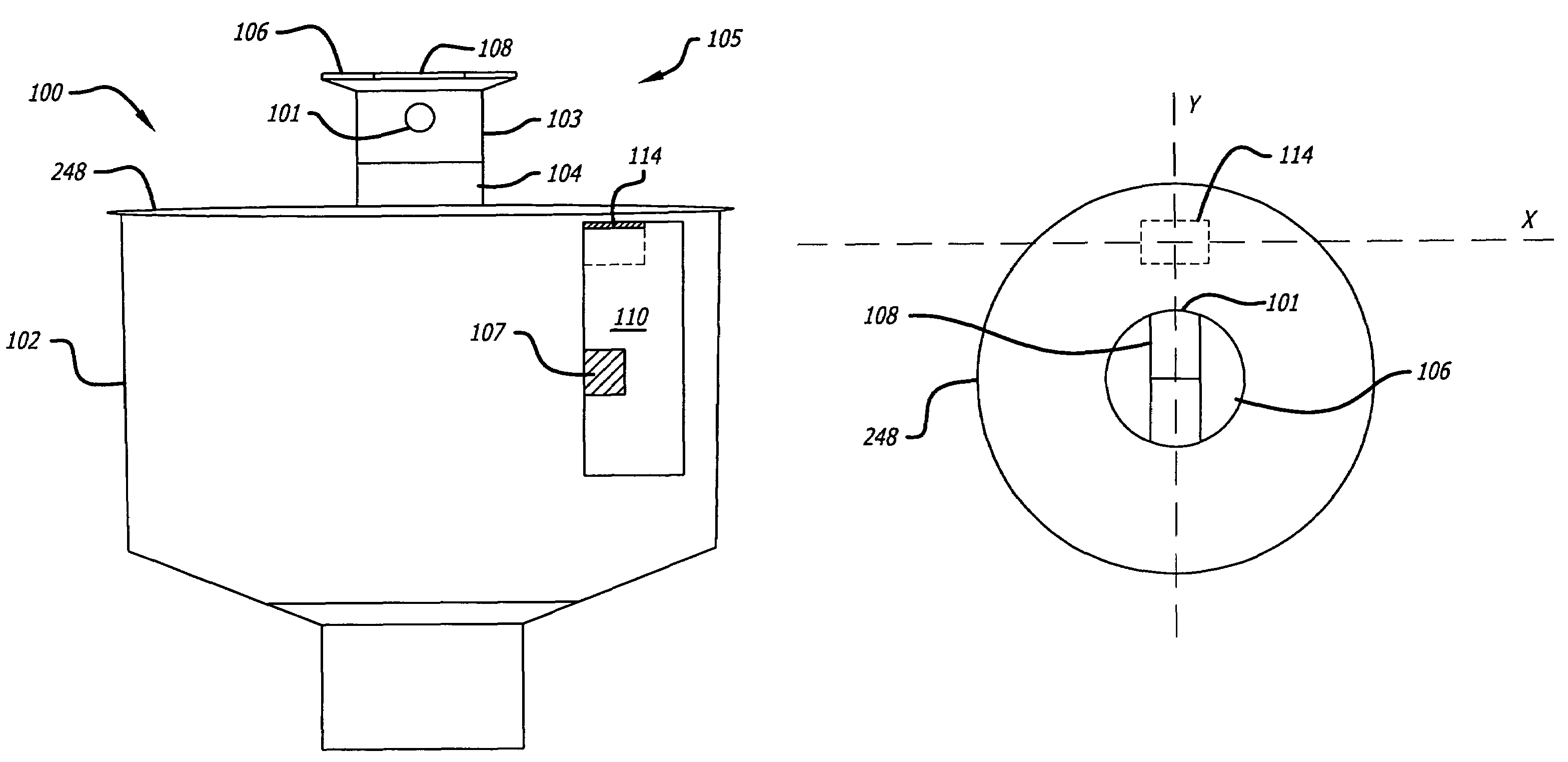
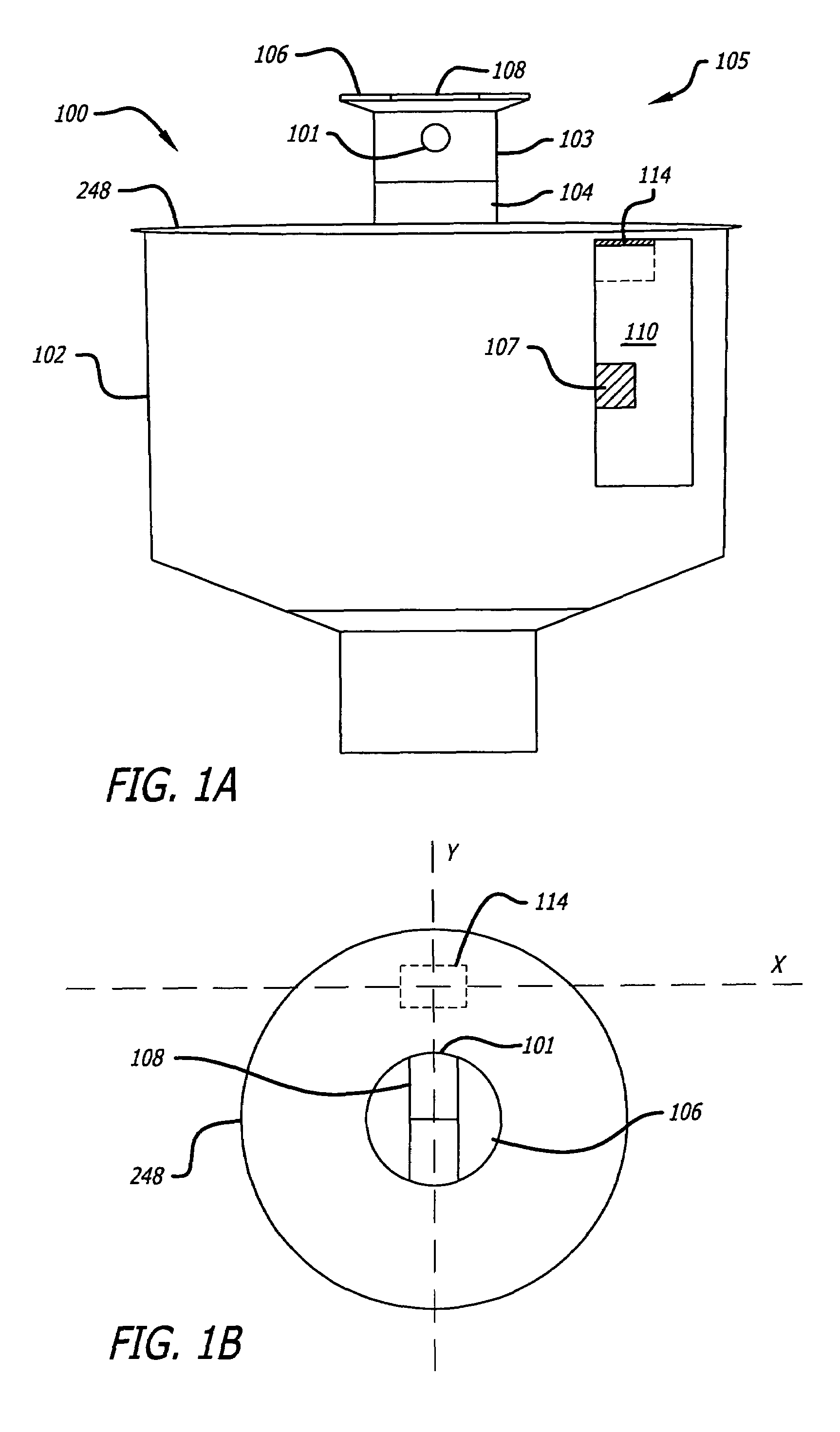
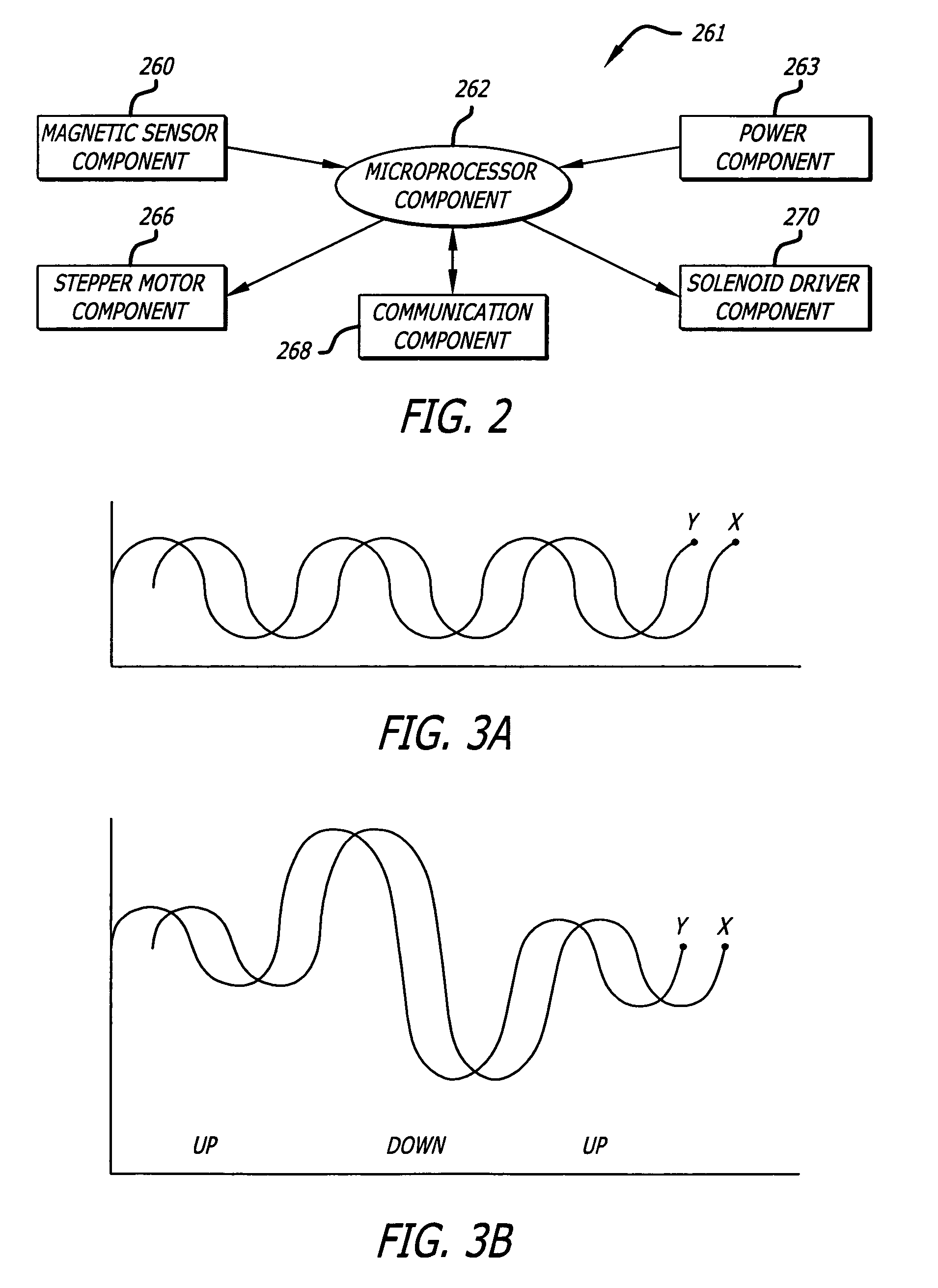
ActiveUS7631813B1Improved sprinkler body designEasy to disassembleValve arrangementsSelf-acting watering devicesSprinkler systemBody compartment
The present invention includes an improved sprinkler design having a magnetic sensing system for determining the position of the riser nozzle, a waterproofed motor housing and related cables, configurable sprinkler body compartments, and a pilot valve with a check valve assembly, both of which are located within the sprinkler body.
Owner:TORO CO THE
Endoscope transportation device
A device for in-hospital transportation of flexible medical endoscopes comprises: a re-usable tray having an inner compartment defined by a generally planar base, surrounding walls upstanding therefrom, and peripheral lip-portion(s) provided at least partially around said surrounding walls and extending outwardly therefrom; a single-use, disposable tray-liner having margins to embrace and to detachably-engage at least a major part of said peripheral lip-portion(s) thereof; an open-faced pouch provided centrally of said margins, such that in use said pouch is able to conform itself substantially to the inner compartment of the re-usable tray; and a pouch-closing protective cover which in use is capable of being extended from one edge across an open-face of the pouch supported in the inner compartment and detachably secured so as safely to enclose an endoscope when it is within the pouch within the inner compartment.
Owner:CANTEL UK LTD
Quinone derivatives for use in the modulation of redox status of individuals
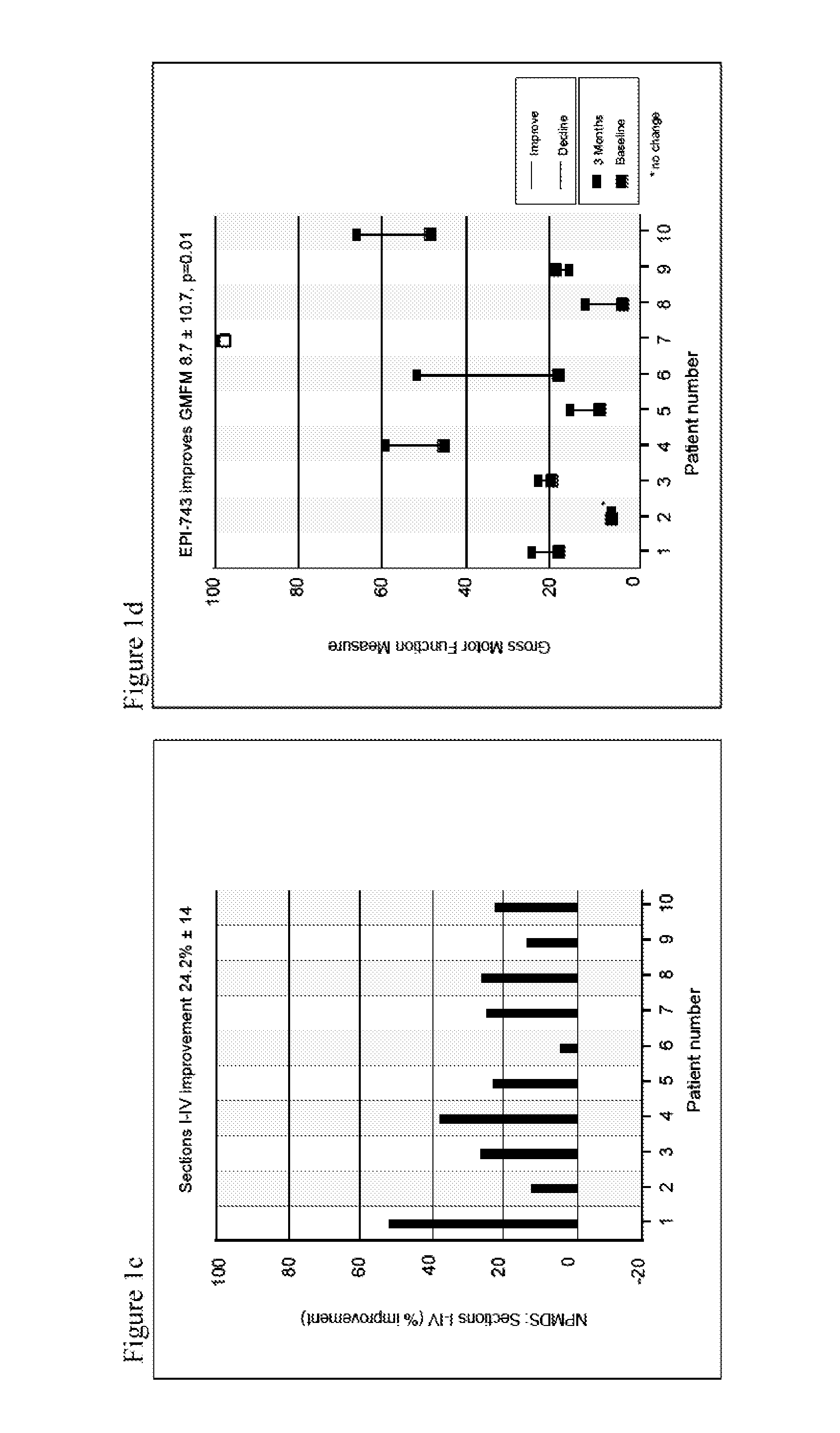
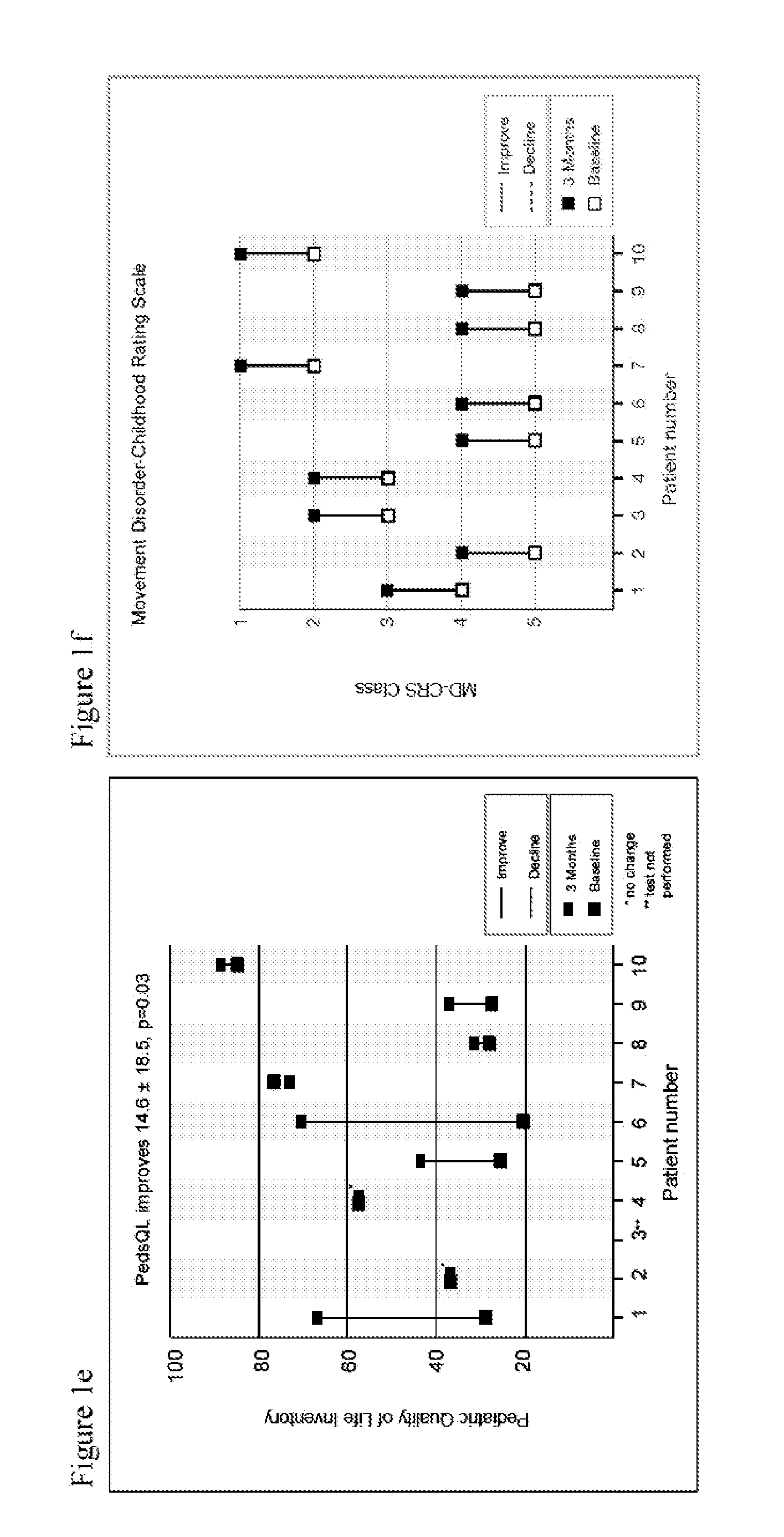
InactiveUS20150216820A1Restoration of balance controlRestoration of metabolic controlBiocideSenses disorderDiseaseQuinone
Methods of modulating, adjusting, and maintenance of the glutathione redox status of an individual, or cell, tissues, bodily fluids, or body compartments of the individual, are disclosed, as are compositions suitable for such modulating, adjusting, and maintenance. The modulation or adjustment is achieved by altering the amounts of reduced glutathione versus oxidized glutathione in the individual, or cells, tissues, bodily fluids, or body compartments of the individual. Modulation, adjustment, and maintenance of the redox status of an individual enables treatment, prevention or suppression of diseases or symptoms associated with diseases. These methods are achieved by using quinone derivatives.
Owner:BIOELECTRON TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com