Quinone derivatives for use in the modulation of redox status of individuals
a technology of redox status and derivatives, which is applied in the direction of peptide/protein ingredients, extracellular fluid disorder, metabolic disorder, etc., can solve the problem of not clear treatment of diseases involving oxidative stress, and achieve the effects of restoring redox balance and metabolic control, reducing glutathione, and increasing the glutathione charge coupl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HPLC Determination of Free GSH, Protein Bound GSH (GS-Pro) and GSSG in Lymphocytes
[0152]The determination of the different forms of GSH obtained from lymphocytes can be performed in a manner similar to the method previously reported by Pastore et al. (Clin. Chem. (Washington, D.C.) 44, 825-832 (1998)). Briefly, 30 μl of 4 M NaBH4, 20 μl of 2 mM EDTA / DTT, 10 μl of 1-octanol and 20 μl of 1.8 M HCl are placed in the derivatization vial containing 30 μl of sample. After the mixture is incubated for three min, 100 μl of 1.5 M N-ethylmorpholine buffer, pH 8.0, 400 μl of distilled water, and 20 μl of 25 mM bromobimane are added. After an additional three-min incubation, 40 μl of acetic acid are added and 20 μl (for free GSH) or 80 μl (for GSSG and GS-Pro) of this mixture are injected into the column. The thiol derivatives are quantified by HPLC (Agilent Technologies 1100 HPLC with a fluorescence detector-excitation at 390 nm and an emission at 478 nm). The analytes are separated for detect...
example 2
Modulation of Glutathione Levels in Treatment of Leigh Disease
[0153]Study Overview:
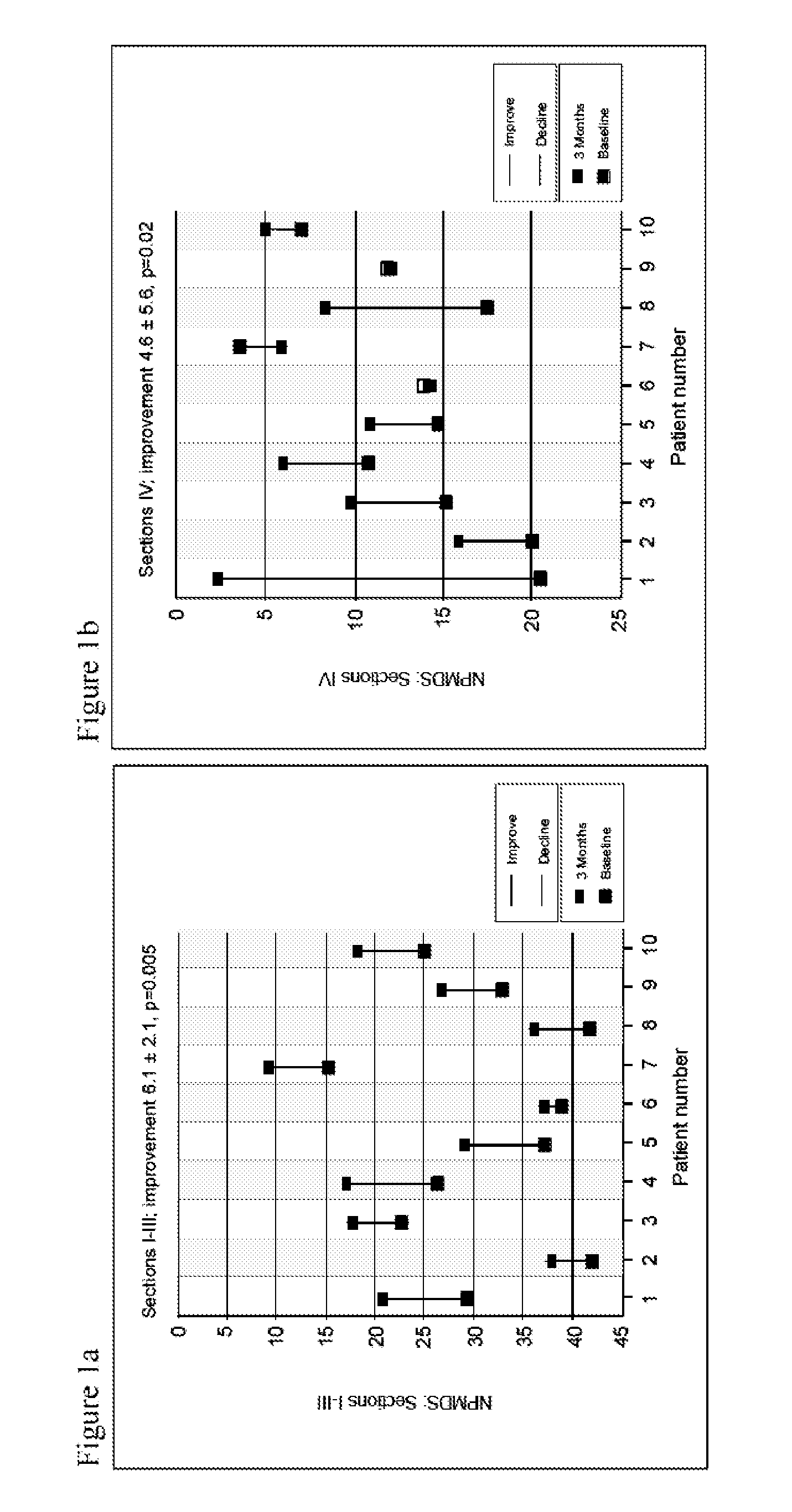
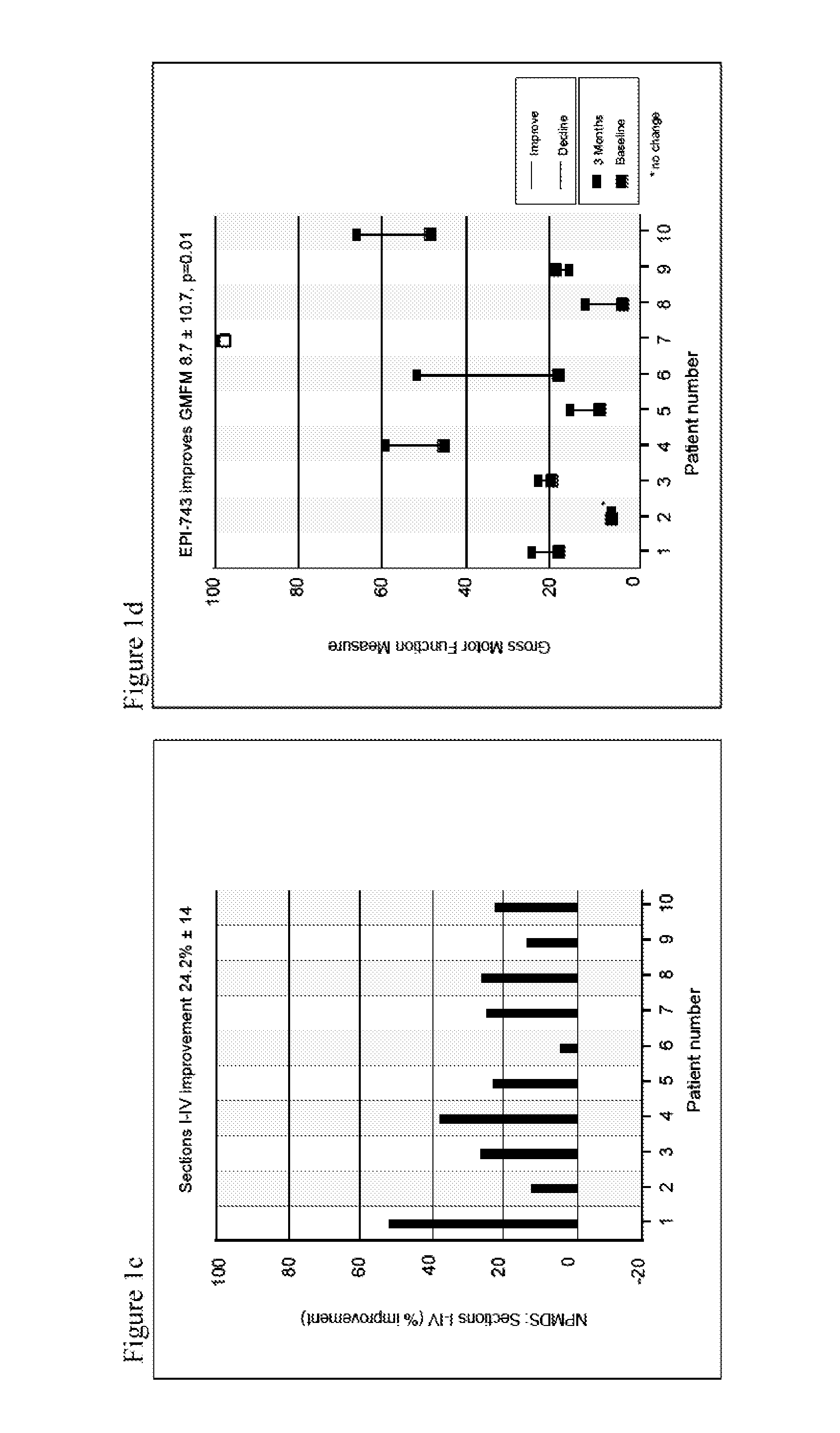
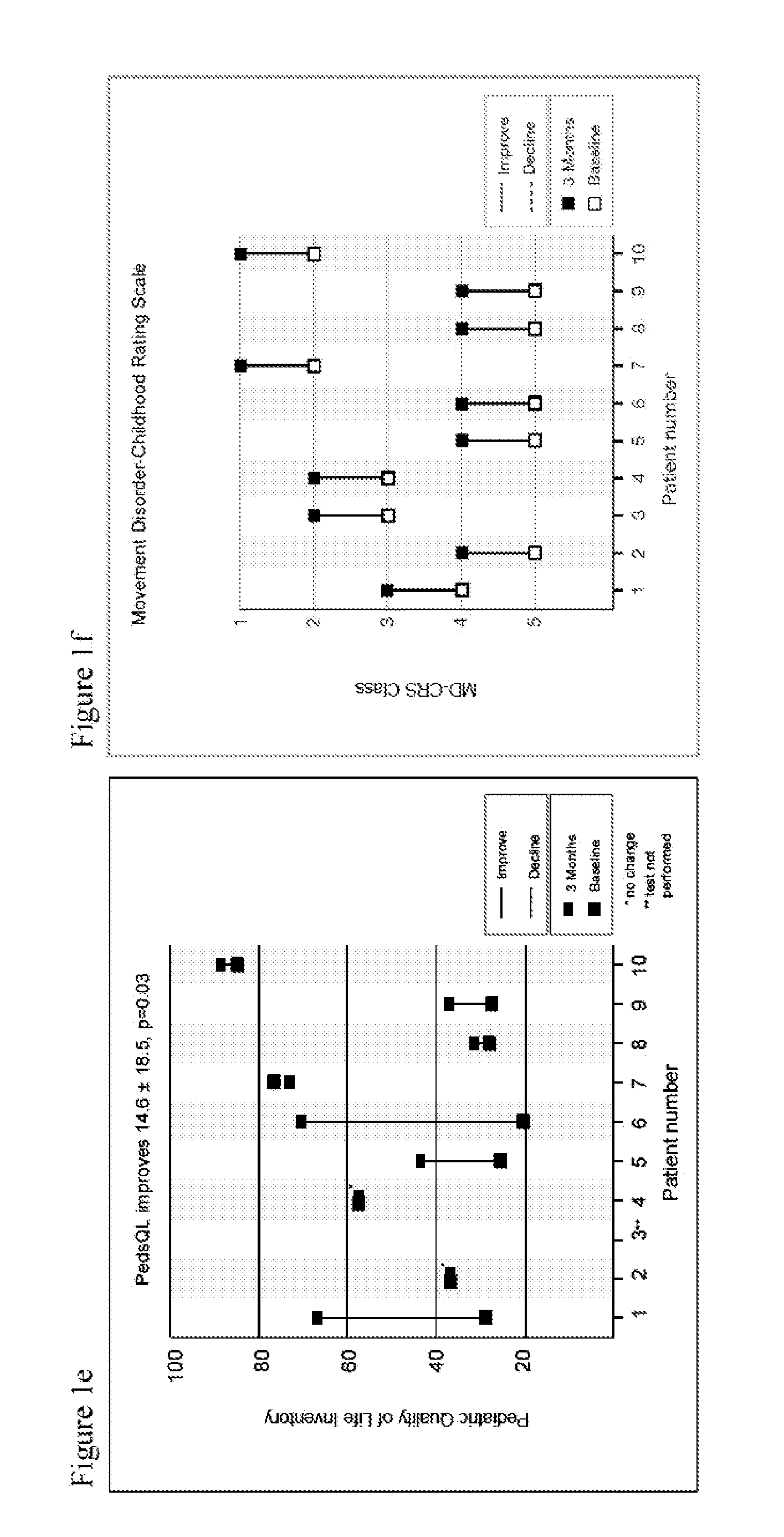
[0154]A prospective single arm subject-controlled trial of alpha-tocotrienol quinone was performed in children with genetically-confirmed Leigh syndrome (Table 1, FIG. 5). All subjects were treated for three months and evaluated using a battery of disease-relevant functional, neurologic, physiologic and biomarker assessments. This study was conducted at the Ospedale Pediatrico Bambino Gesù in Rome, Italy. Institutional Review Board approval was obtained prior to study initiation.
TABLE 1Baseline patient characteristicsBaselineAge atDefect and % ofNPMDSWeightSubjectenrollmentMutationmutated mtDNAScoreSex(kg)19ND1-G3697ABlood: homoplasmic49.4F2126SUCLA2c.850C > T / c.850C > T62M9.331ETHE1Del Exo4; c.375 + 5G > A38F7.748ND5-G13513AMuscle: 65%36.8F2156EARS2c.502 > G;51.6M16.2c.1279_1280insTCC;c.322C > T613SURF1c.784 delACCC / not found53.1F28.578ND5-G13513ABlood: 61%, Fibroblast: 75%18.7M21.383ND1-G3697AMuscle...
embodiment 1
[0173]A method of treating a subject having a glutathione redox potential disorder, comprising the steps of: a) altering the redox potential of glutathione in the subject by administering a compound to the subject at an initial dosage level that alters the concentration of reduced glutathione in the subject, the concentration of oxidized glutathione in the subject, or both the concentration of reduced glutathione and the concentration of oxidized glutathione in the subject; b) subsequent to administering the compound to the subject, measuring the concentration of reduced glutathione and oxidized glutathione in the subject; c) calculating the redox potential of glutathione in the subject; d) adjusting the dosage of the compound administered to the subject; and e) repeating steps b), c), and d) until a ratio of oxidized glutathione concentration to reduced glutathione concentration between about 0.01 and about 0.5 is attained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| reduction potential | aaaaa | aaaaa |
| reduction potential | aaaaa | aaaaa |
| reduction potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com