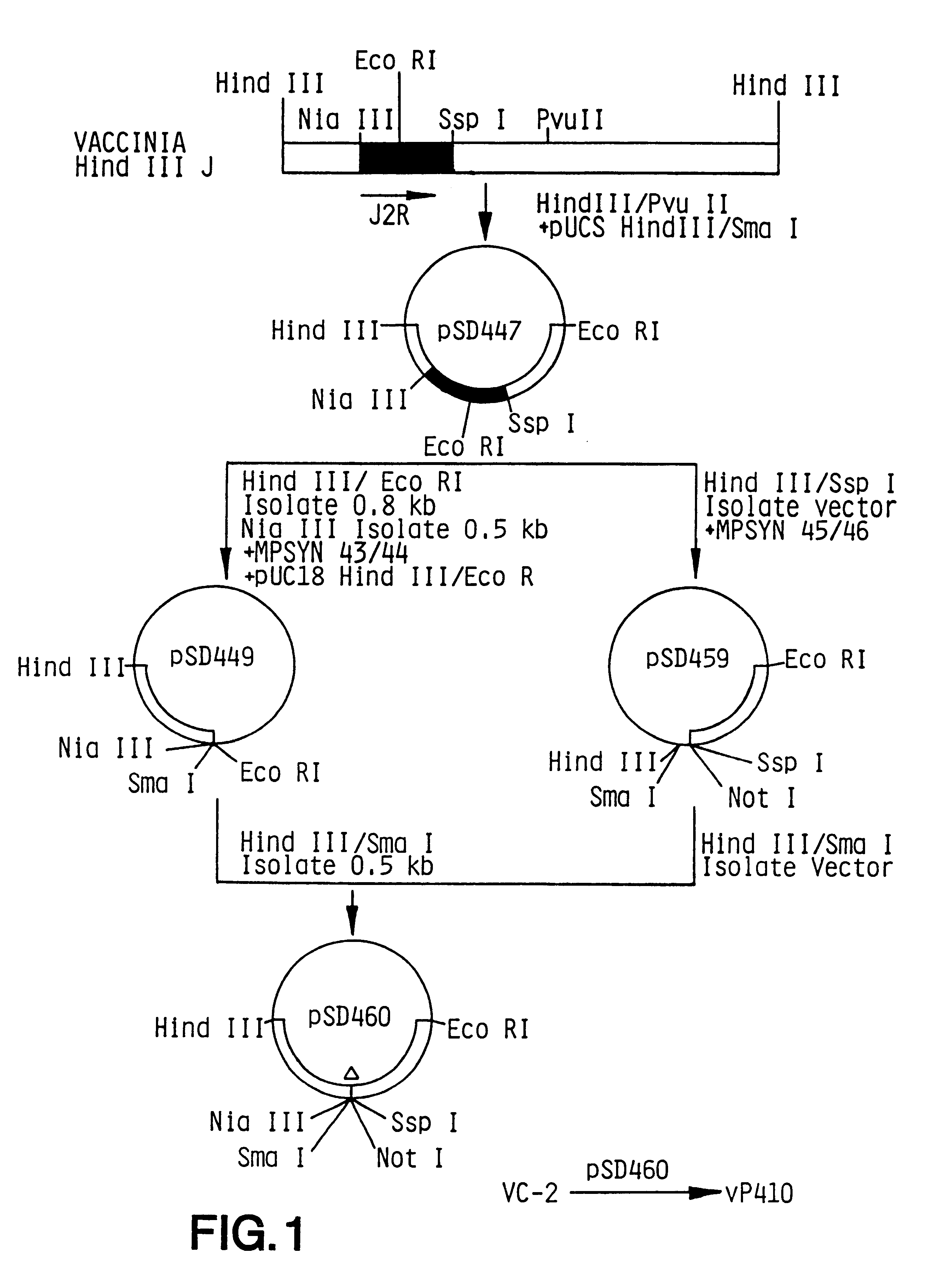
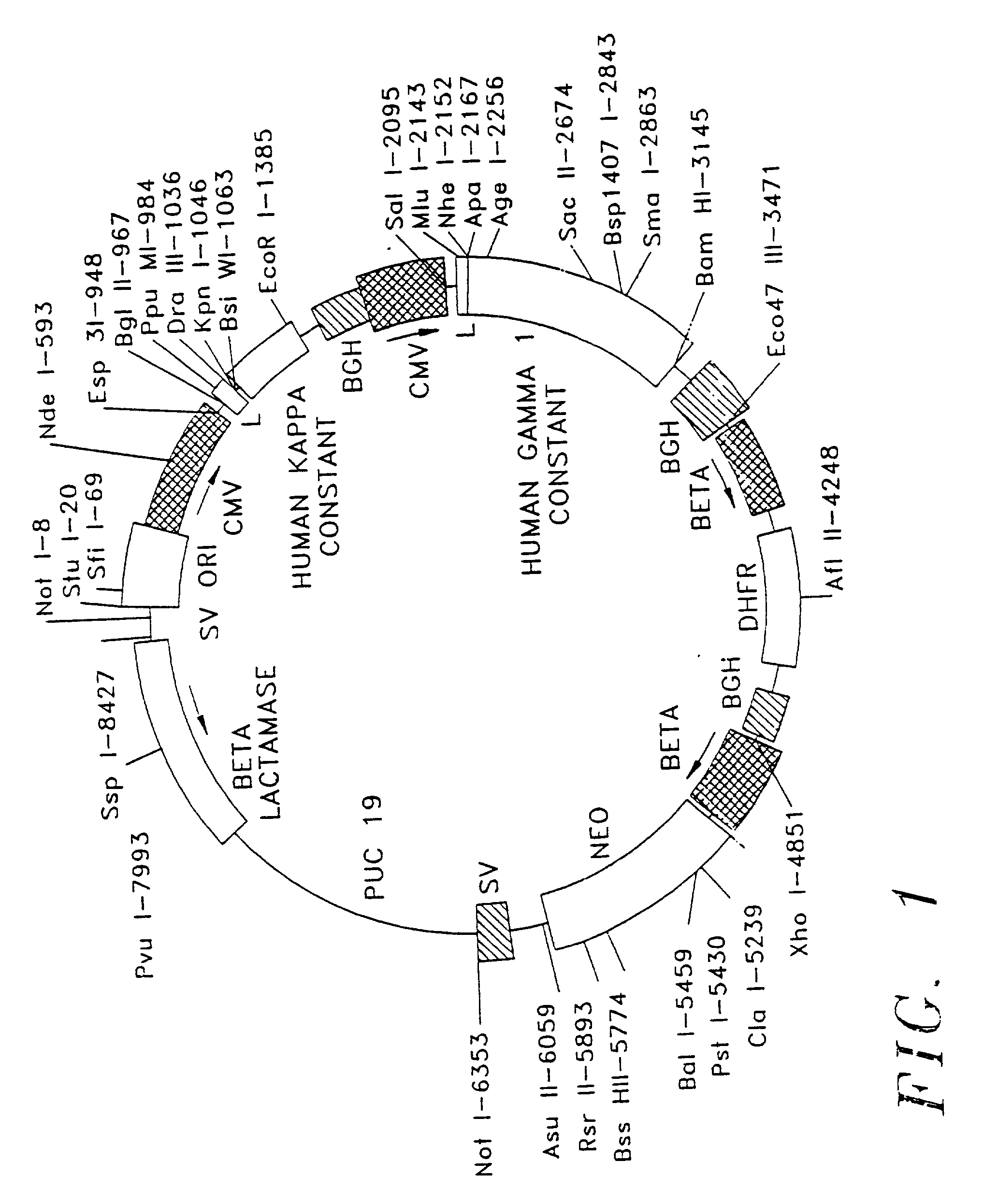
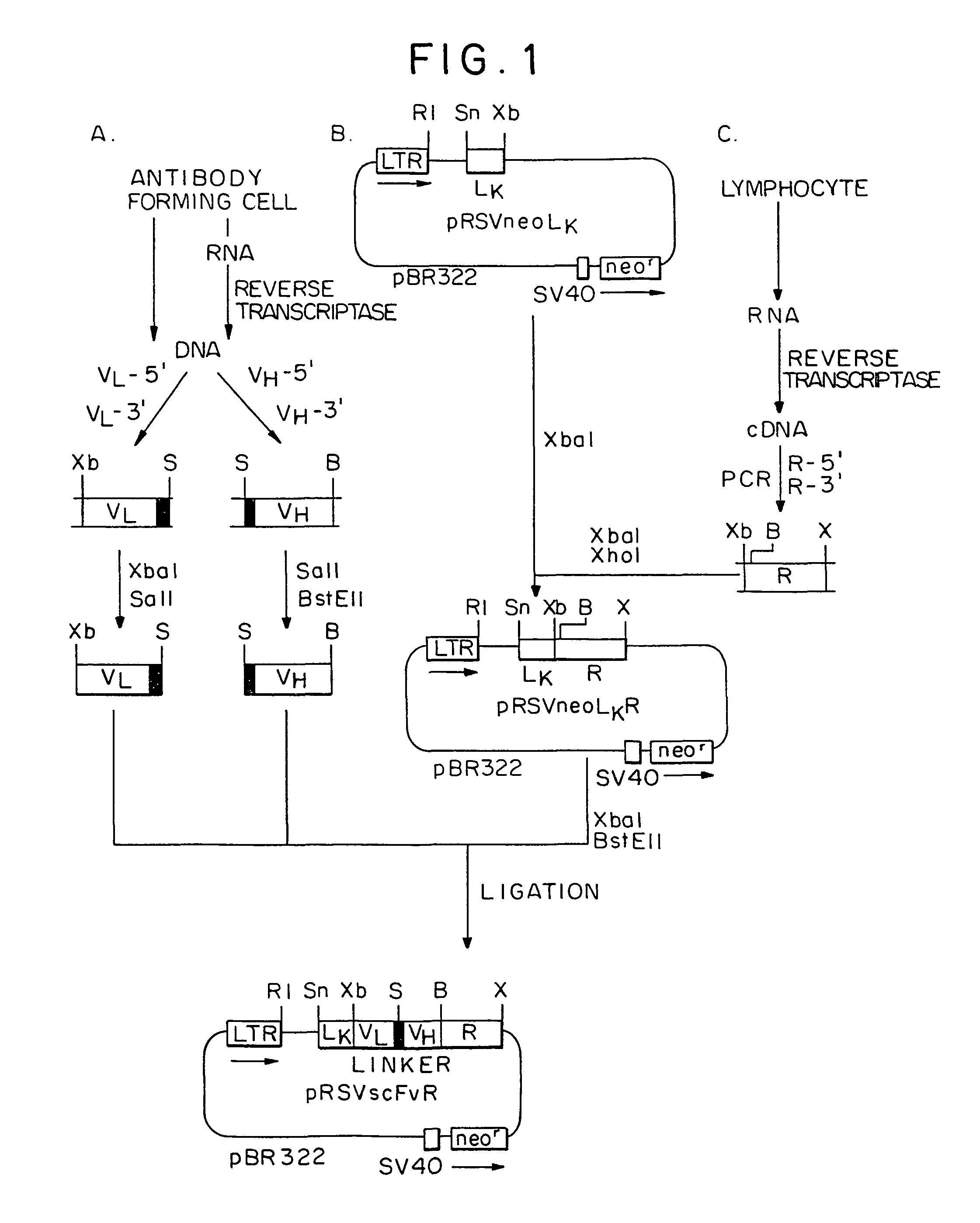
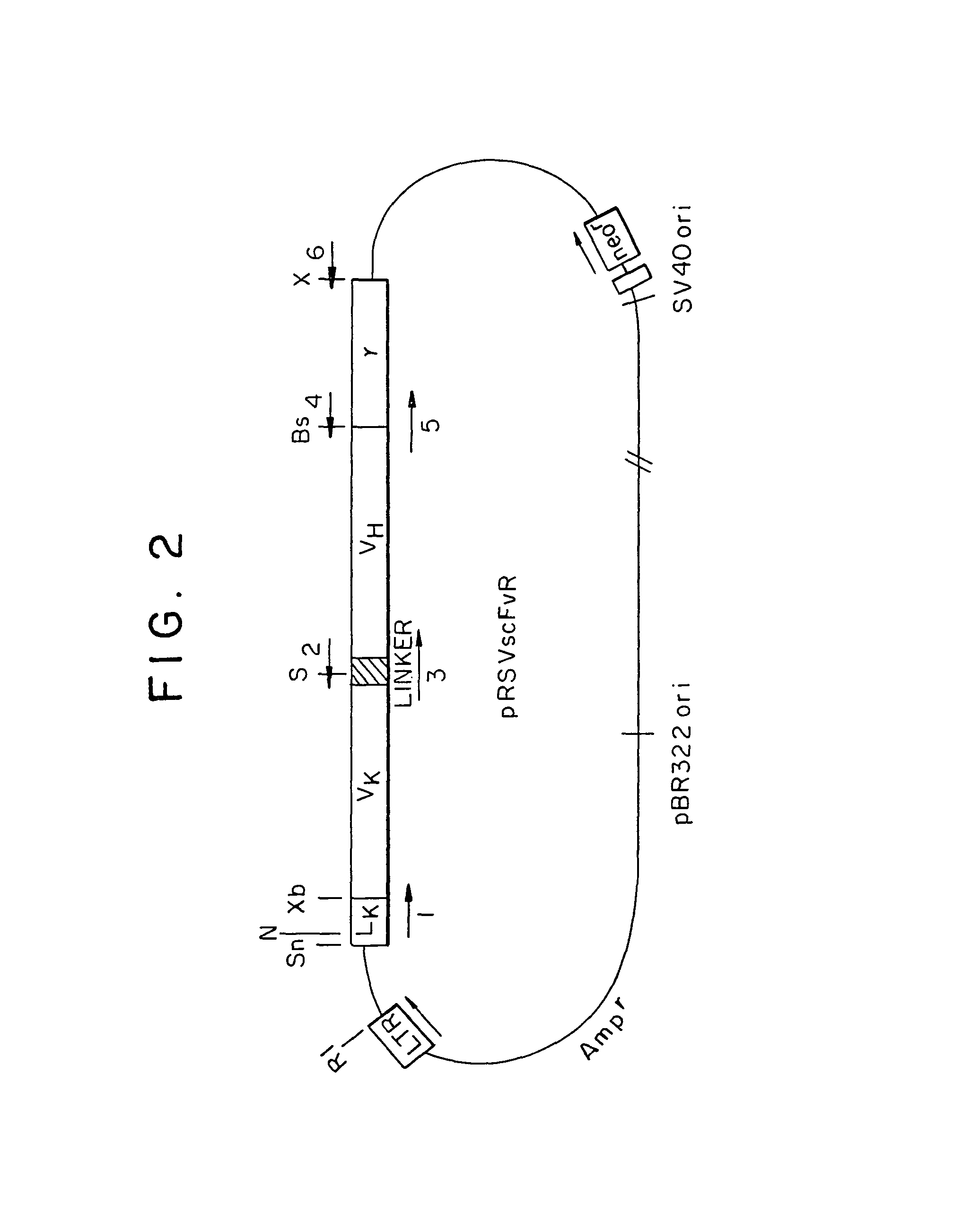
Patents
Literature
2253results about "Viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
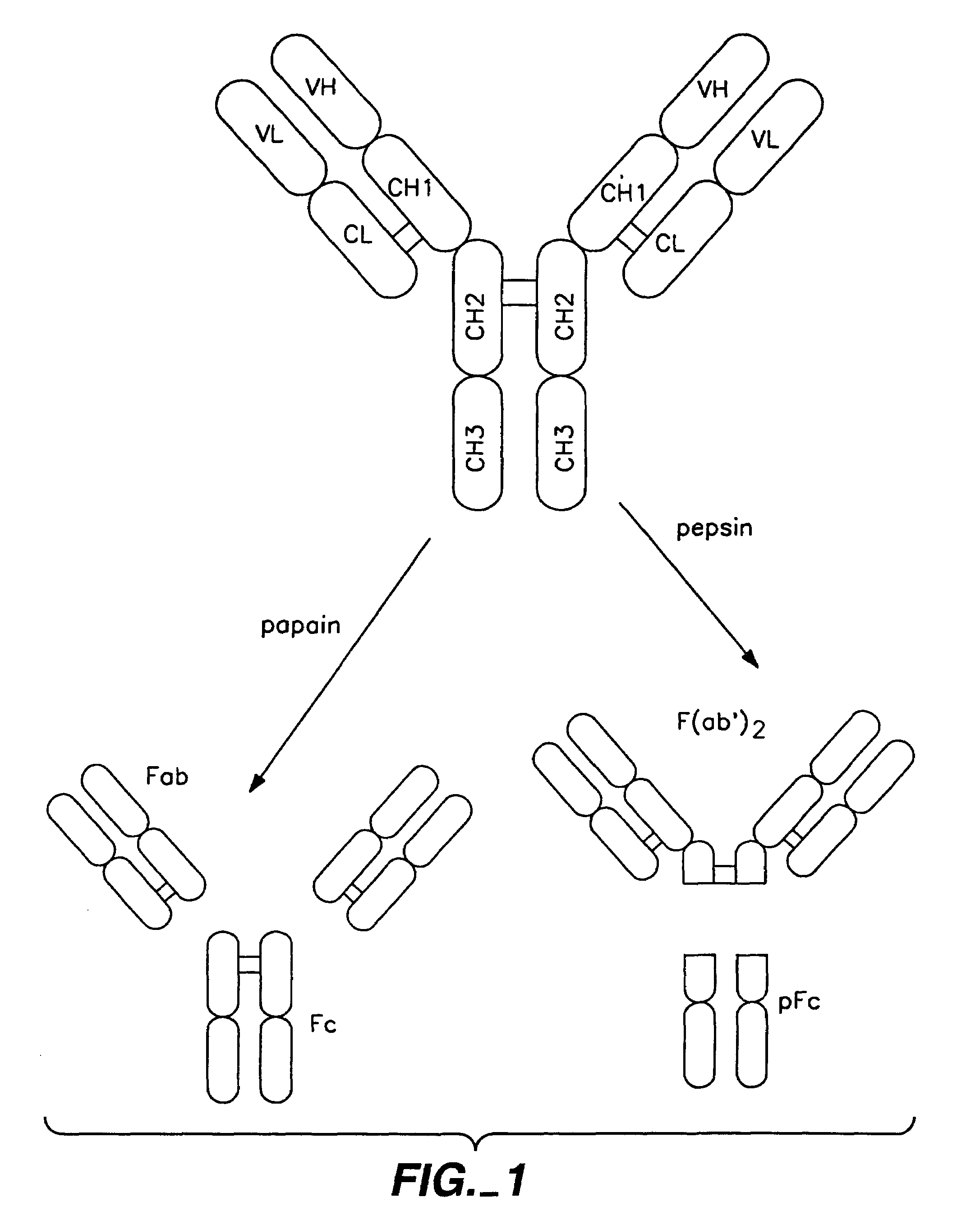
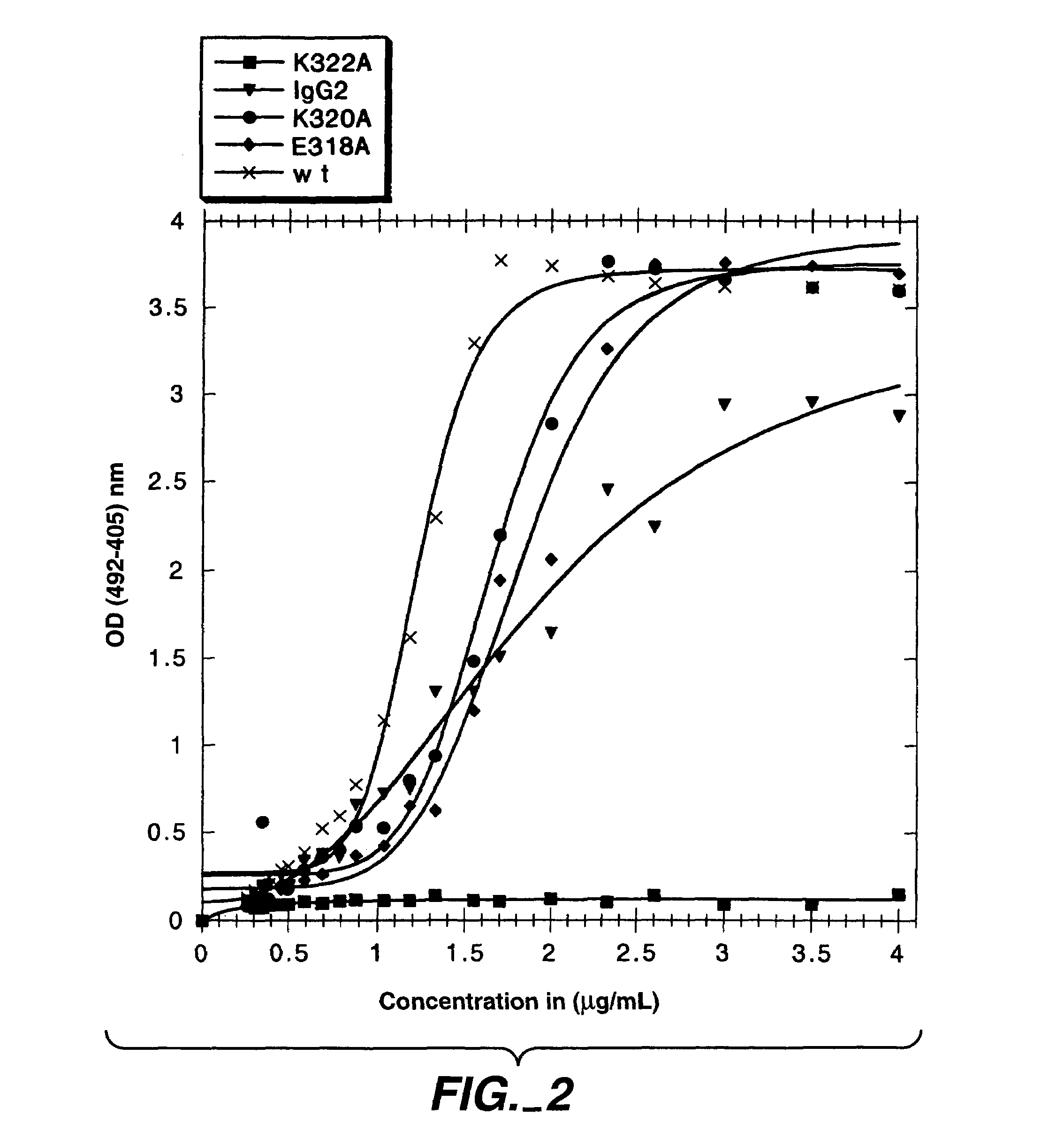
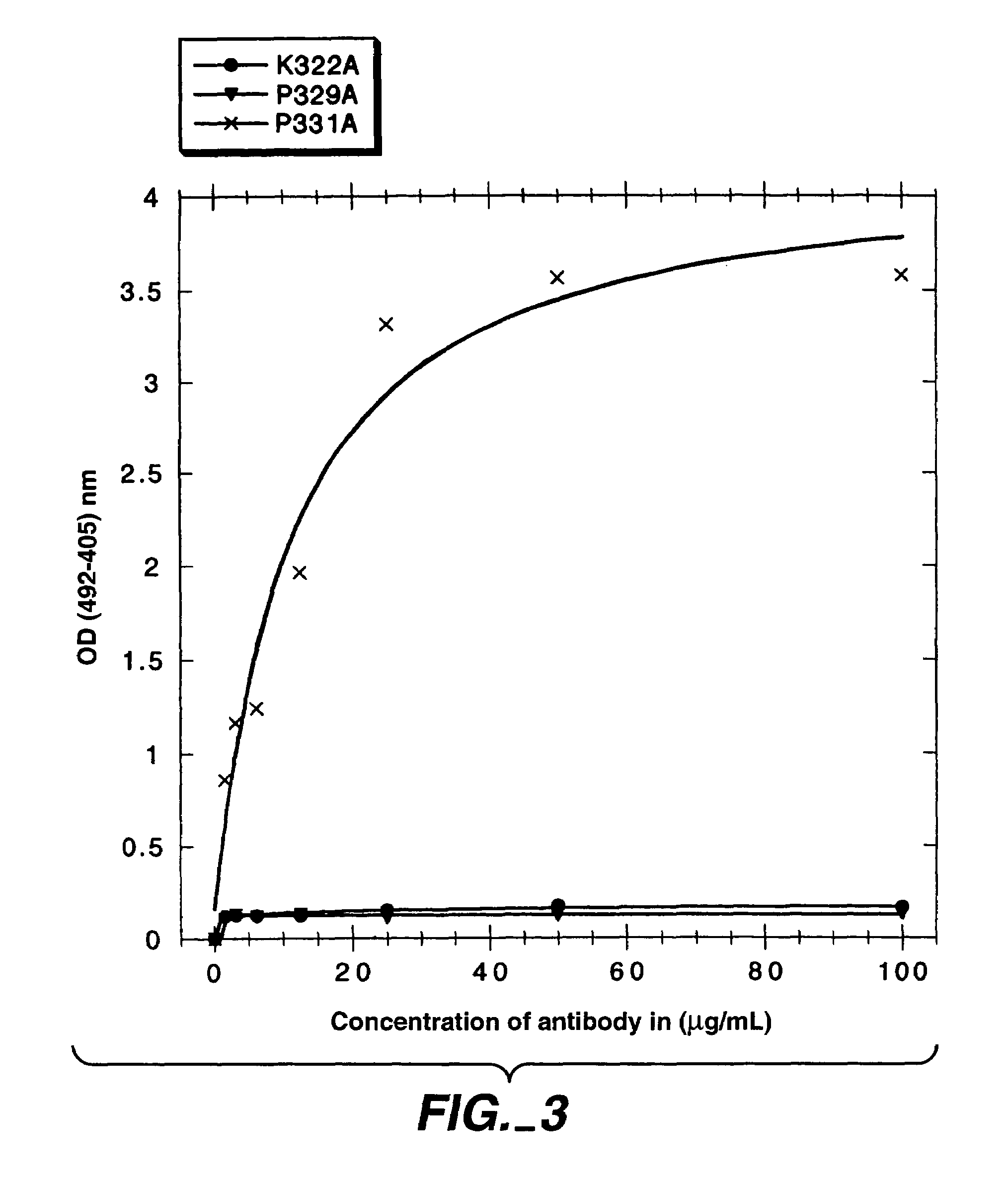
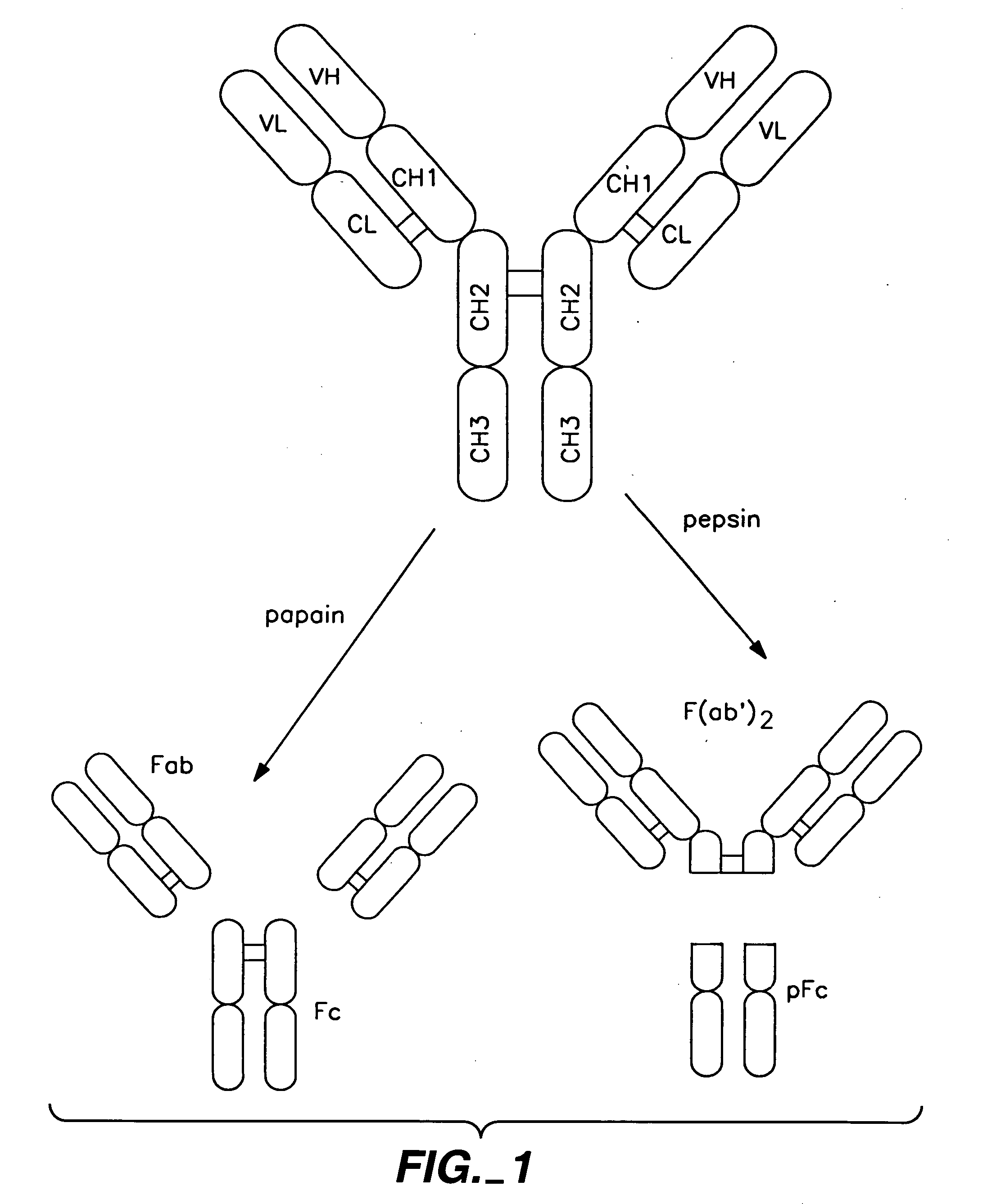
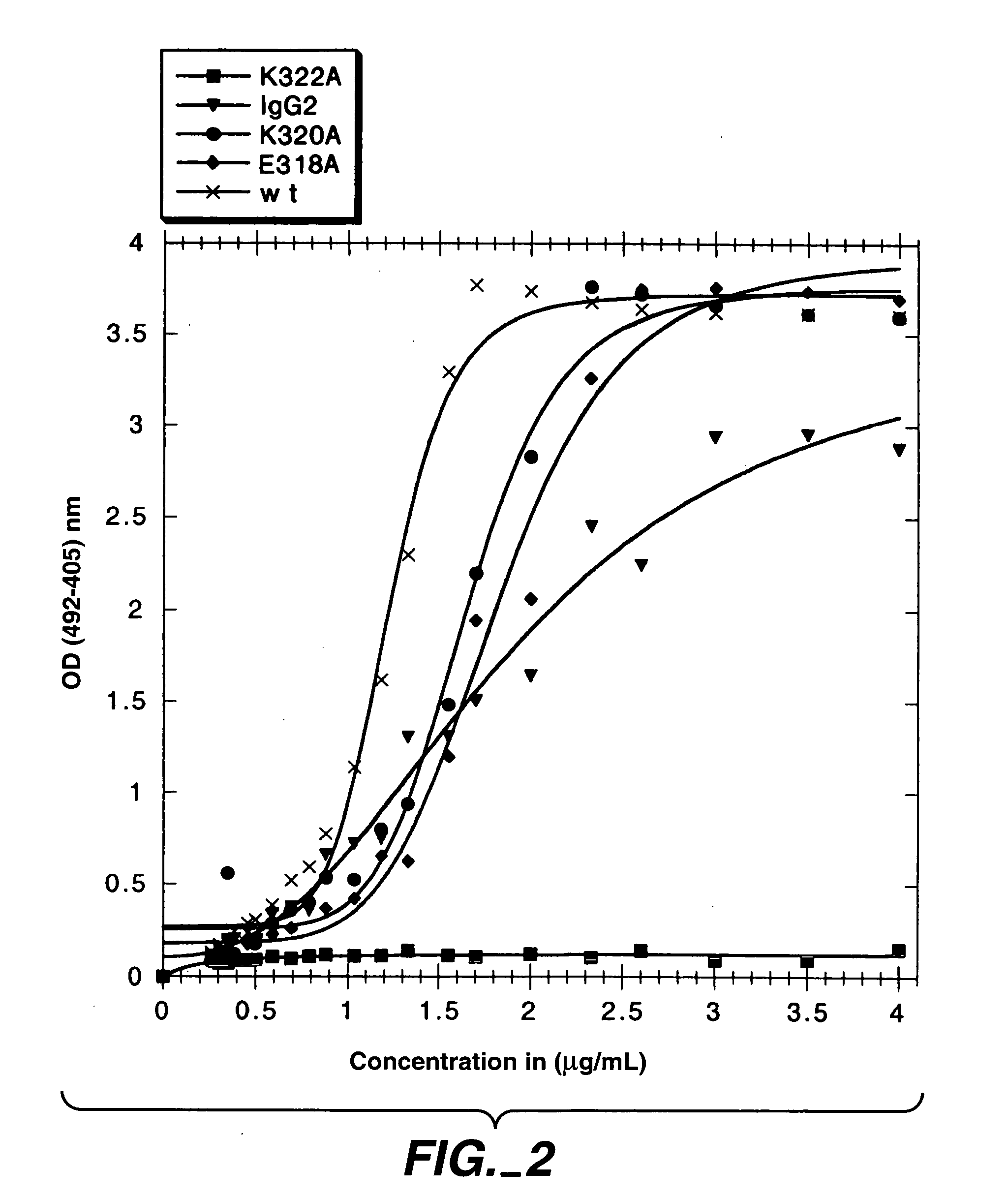
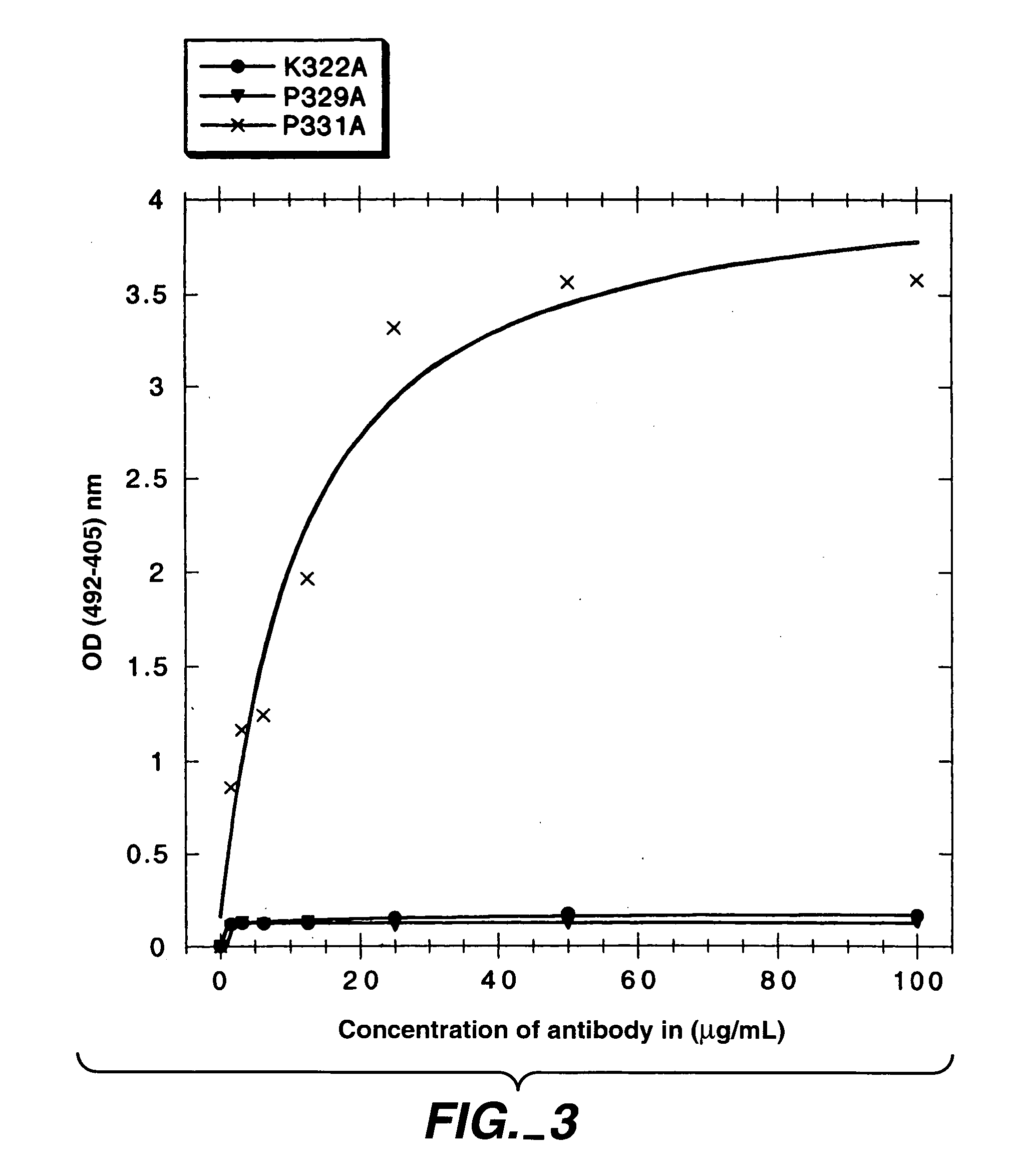
Polypeptide variants with altered effector function
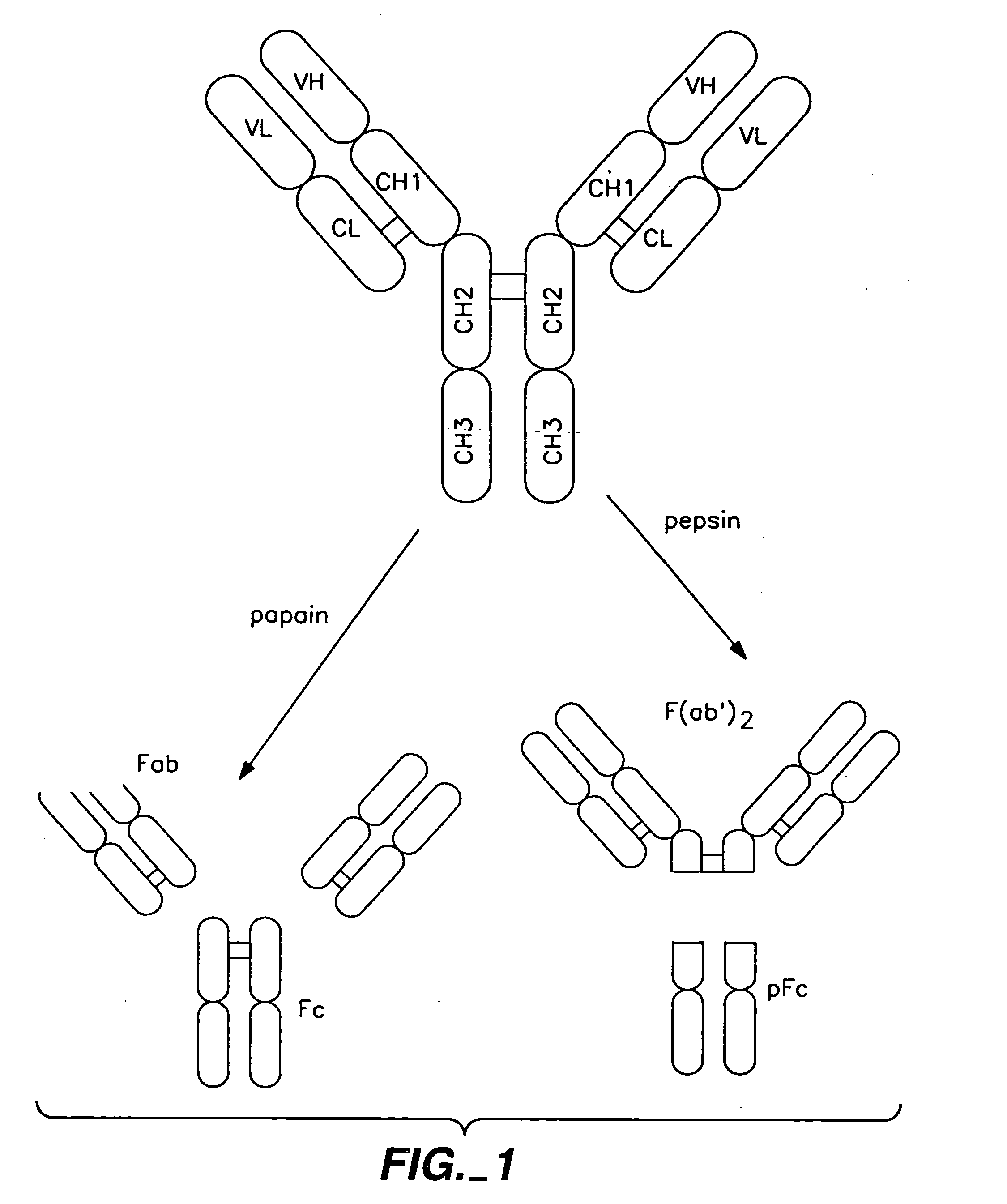
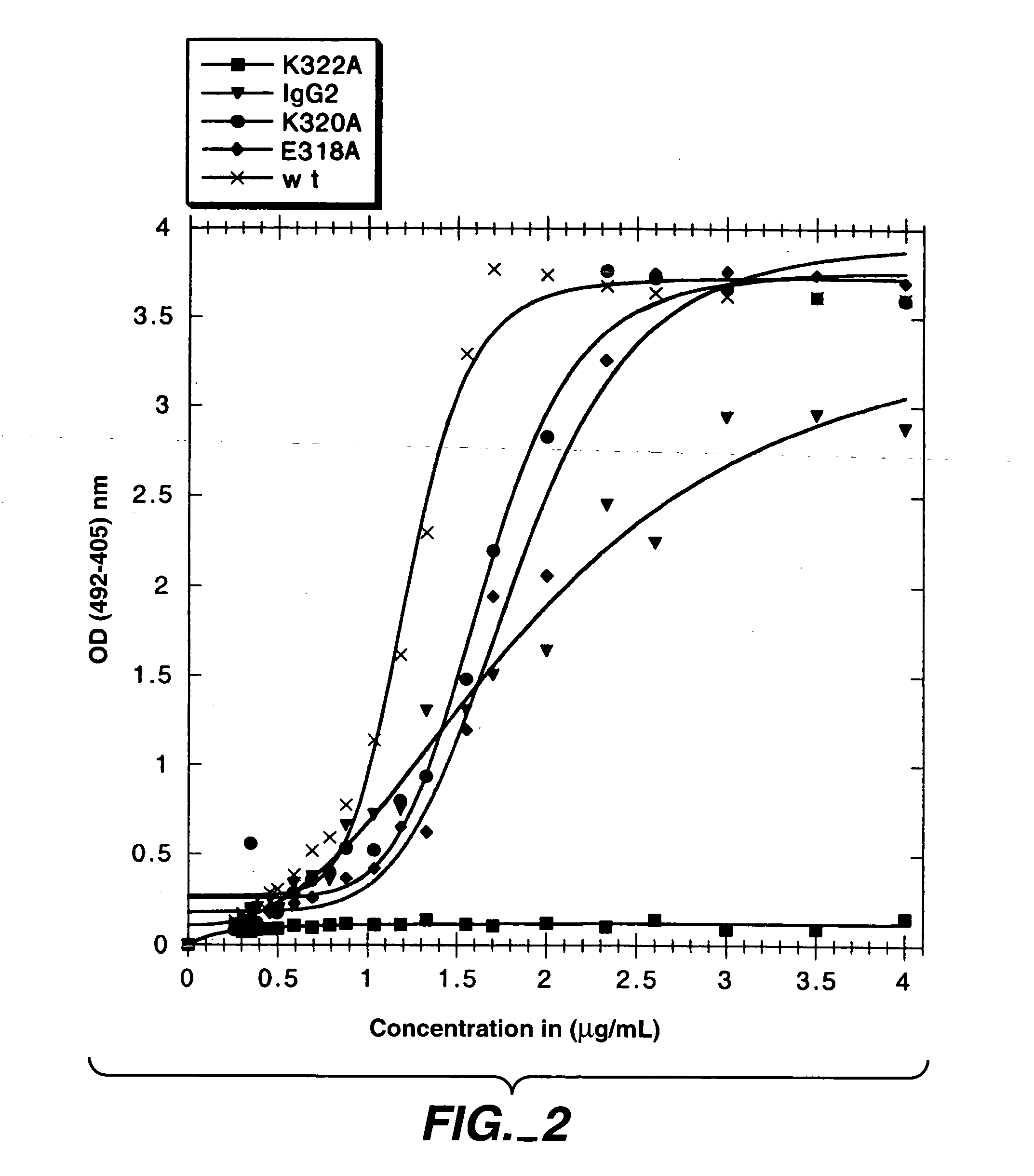
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
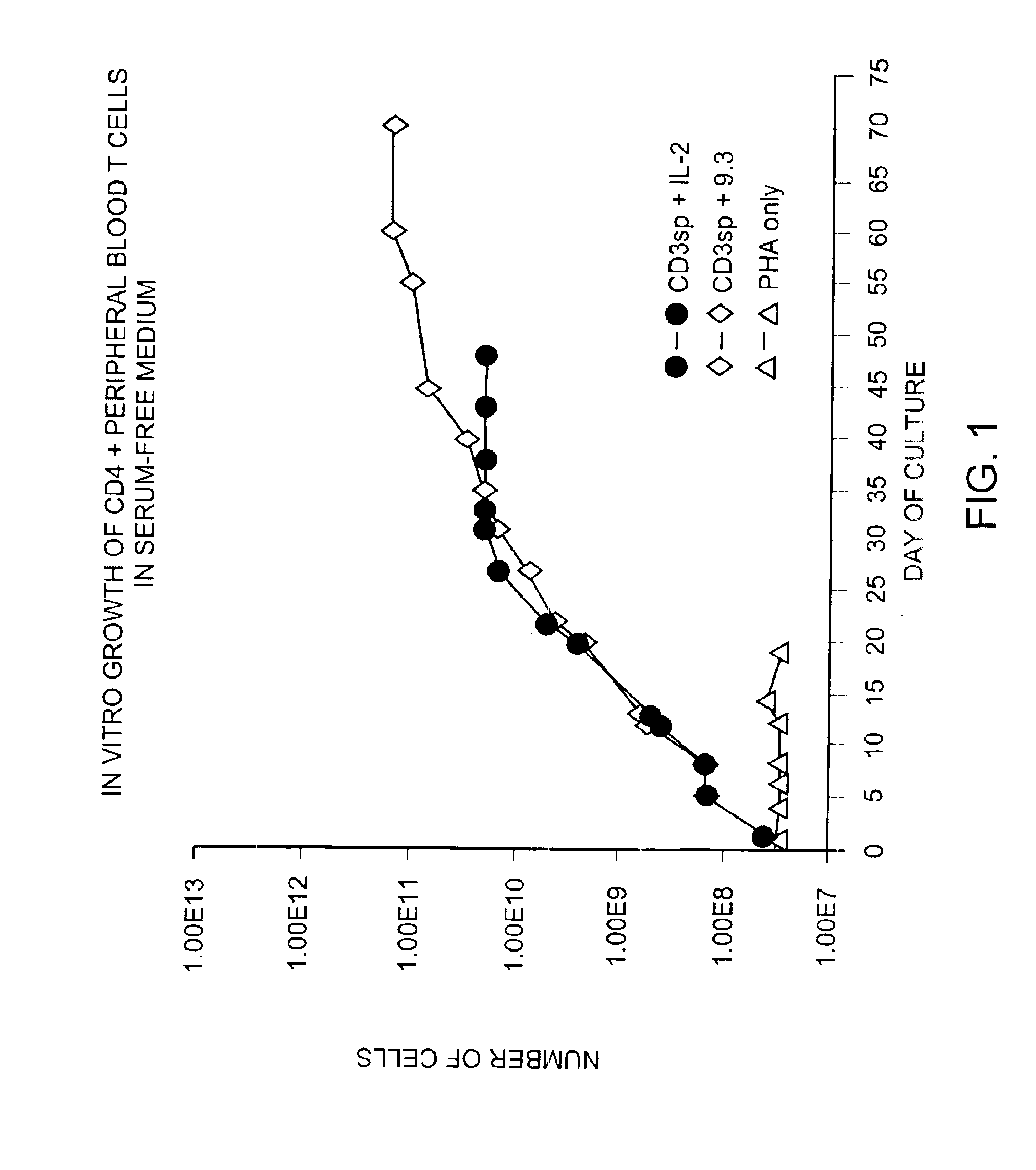
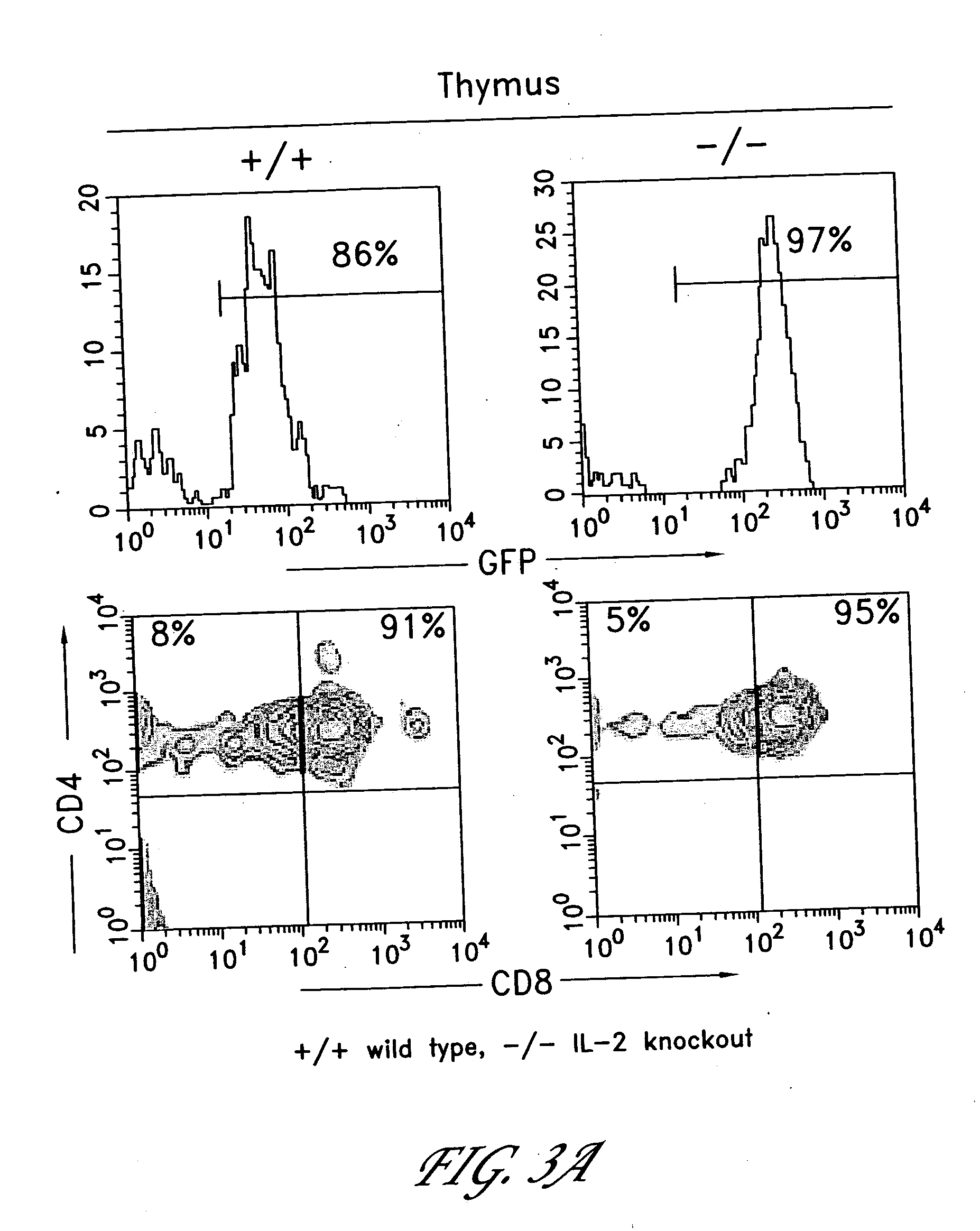
Methods for selectively stimulating proliferation of T cells
InactiveUS6905681B1Increase the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS6887466B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods of treating HIV infected subjects
InactiveUS6905680B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
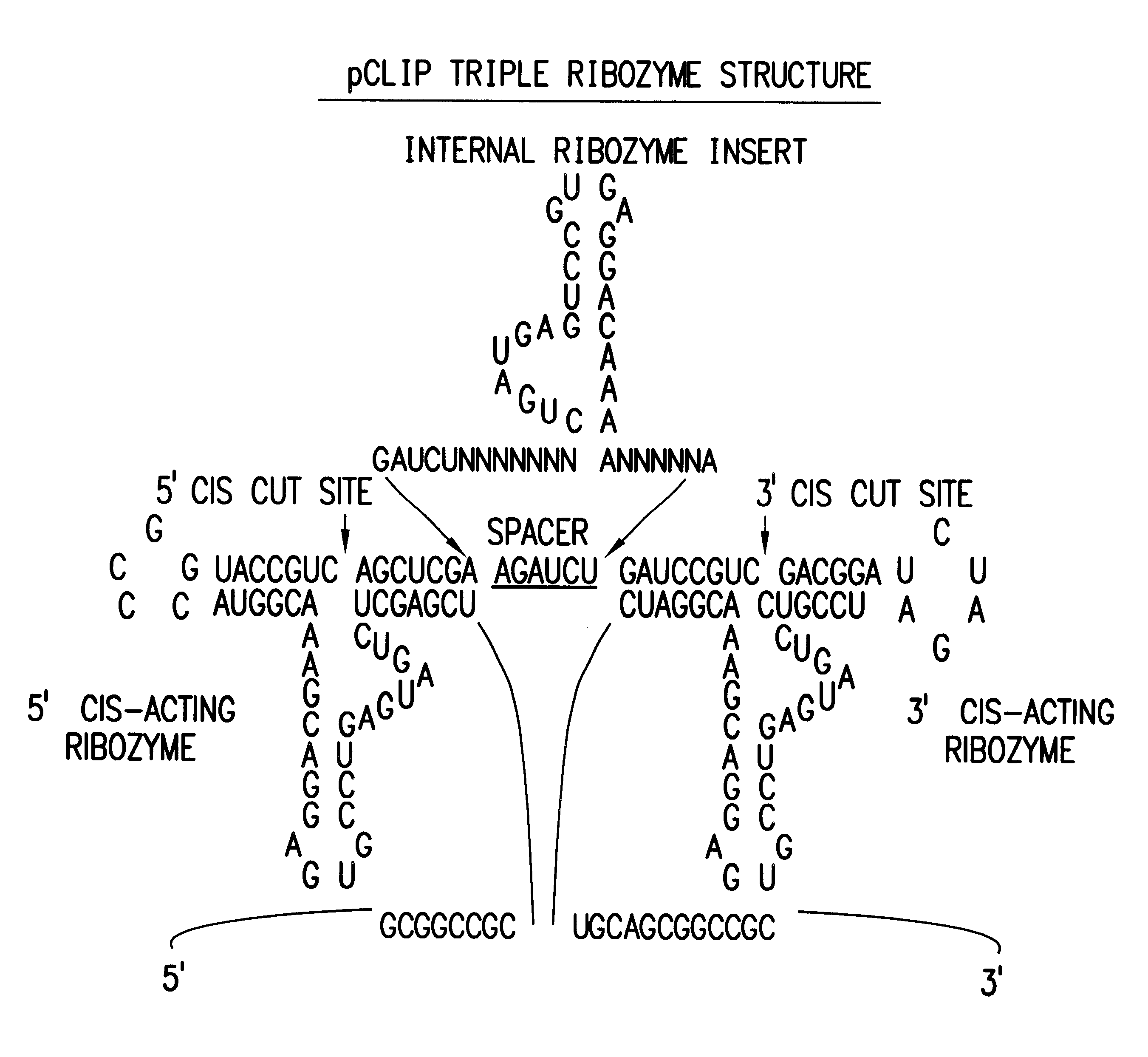
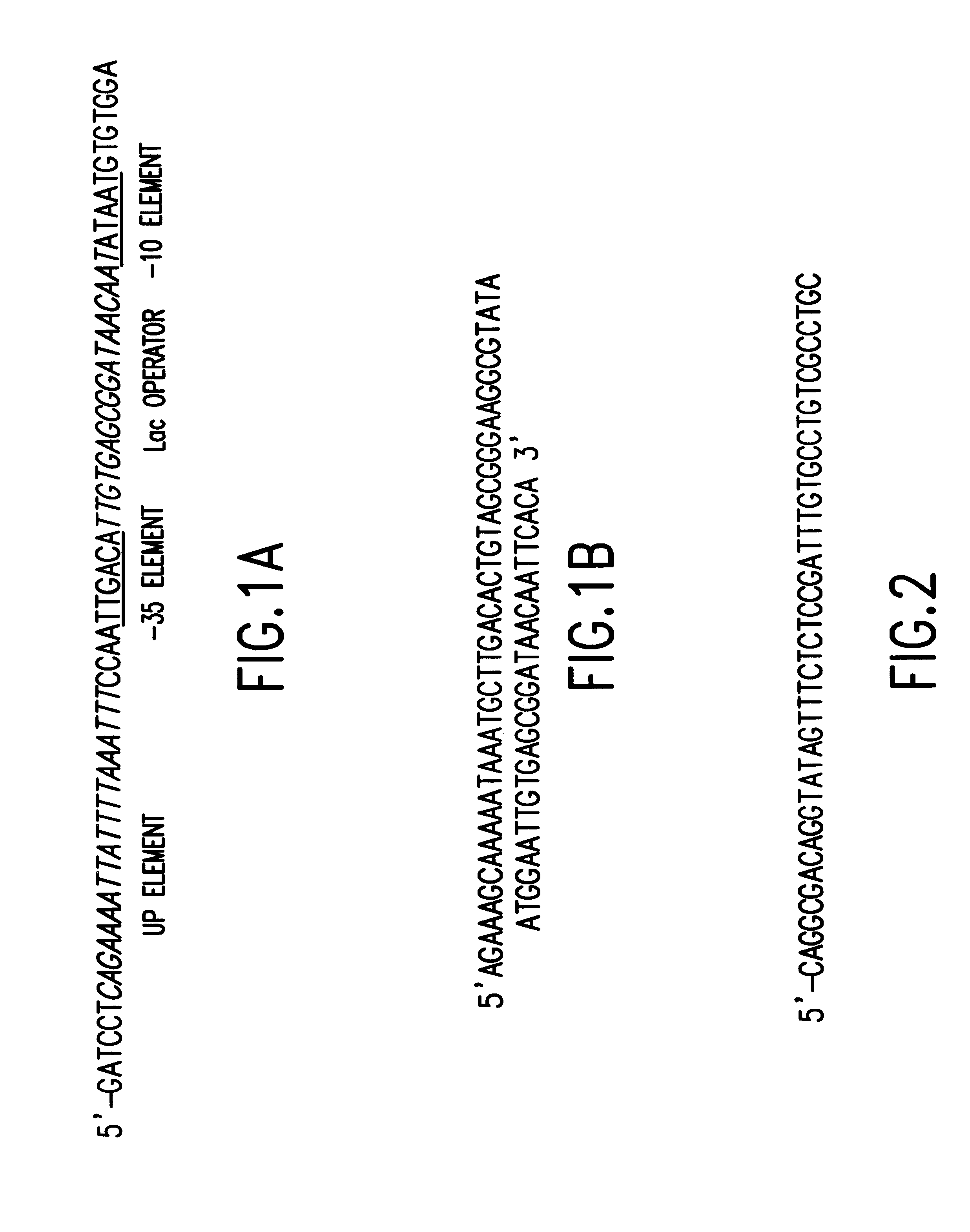
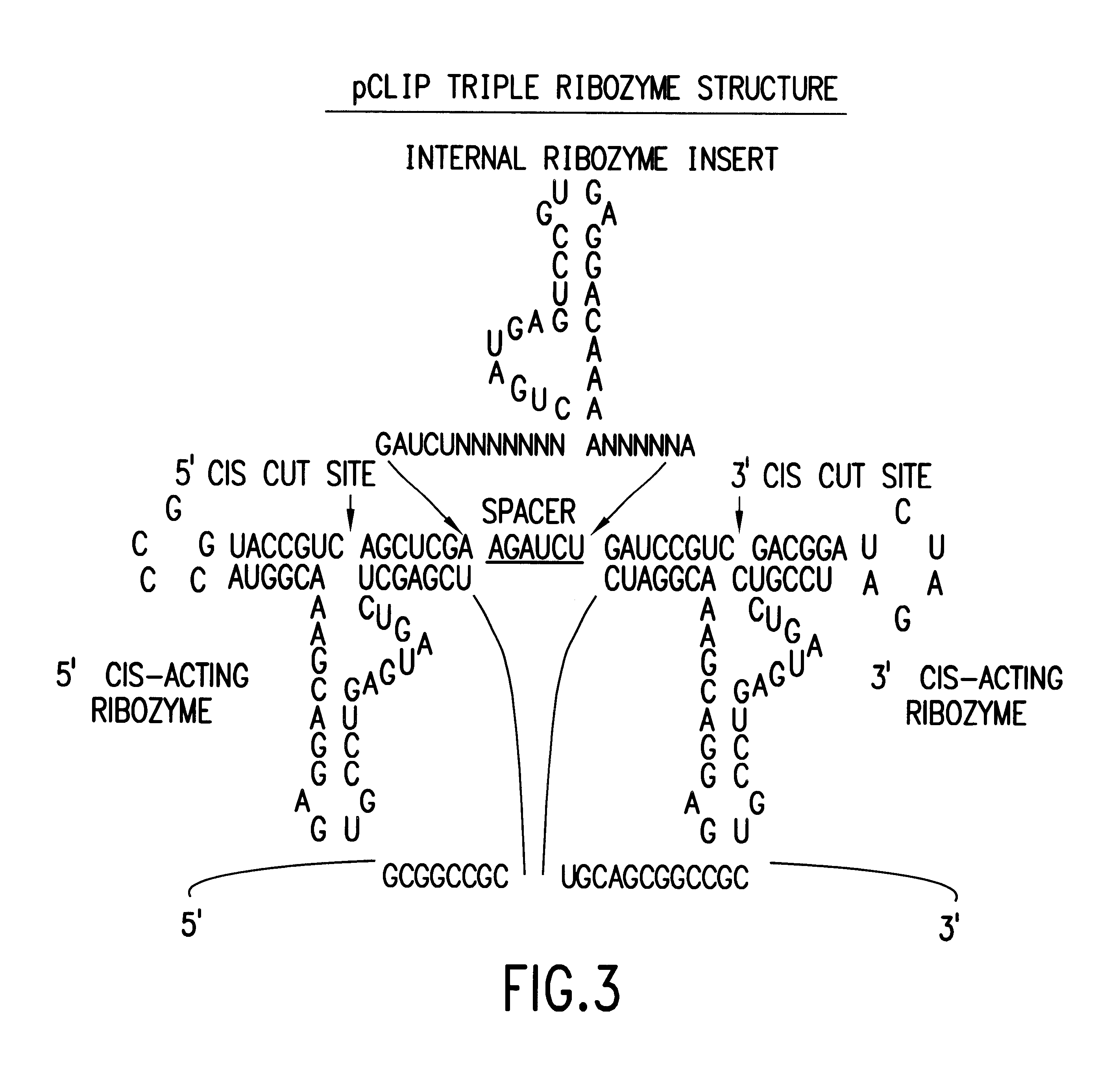
Tissue-specific and pathogen-specific toxic agents and ribozymes
InactiveUS6271359B1Rapidly and effectively expressedImprove stabilityVirusesSugar derivativesCancer cellBiology
The present invention relates to the discovery, identification and characterization of toxic agents which are lethal to pathogens and methods for targeting such toxic agents to a pathogen or pathogen infected cells in order to treat and / or eradicate the infection. In particular, the present invention relates to toxic agents which target bacteria at different stages of the bacterial life cycle, which are delivered alone or in combination to bacteria or bacteria-infected cells. The invention relates to toxic agents which are lethal to diseased cells and methods for targeting such toxic agents to a diseased cell in order to treat and / or eradicate the disease. The present invention relates to promoter elements which are pathogen-specific or tissue-specific and the use of such promoter elements to achieve pathogen-specific or tissue-specific expression of the toxic agent(s) and / or ribozyme(s) of the present invention. Specifically, the invention relates to the delivery of one or more toxic gene products, antisense RNAs, or ribozymes, or combination thereof. The invention provides a novel system by which multiple pathogenic targets may be simultaneously targeted to cause the death of a pathogen, or cell infected with a pathogen. Further, the invention has important implications in the eradication of drug-resistant bacterium and bacterial pathogens. The invention provides a novel system by which multiple targets may be simultaneously targeted to cause the death of a diseased cell. The invention also has important implications in the eradication of drug-resistant pathogens and drug-resistant diseased cells (such as cancer cells).
Owner:MUSC FOUND FOR RES DEV +1
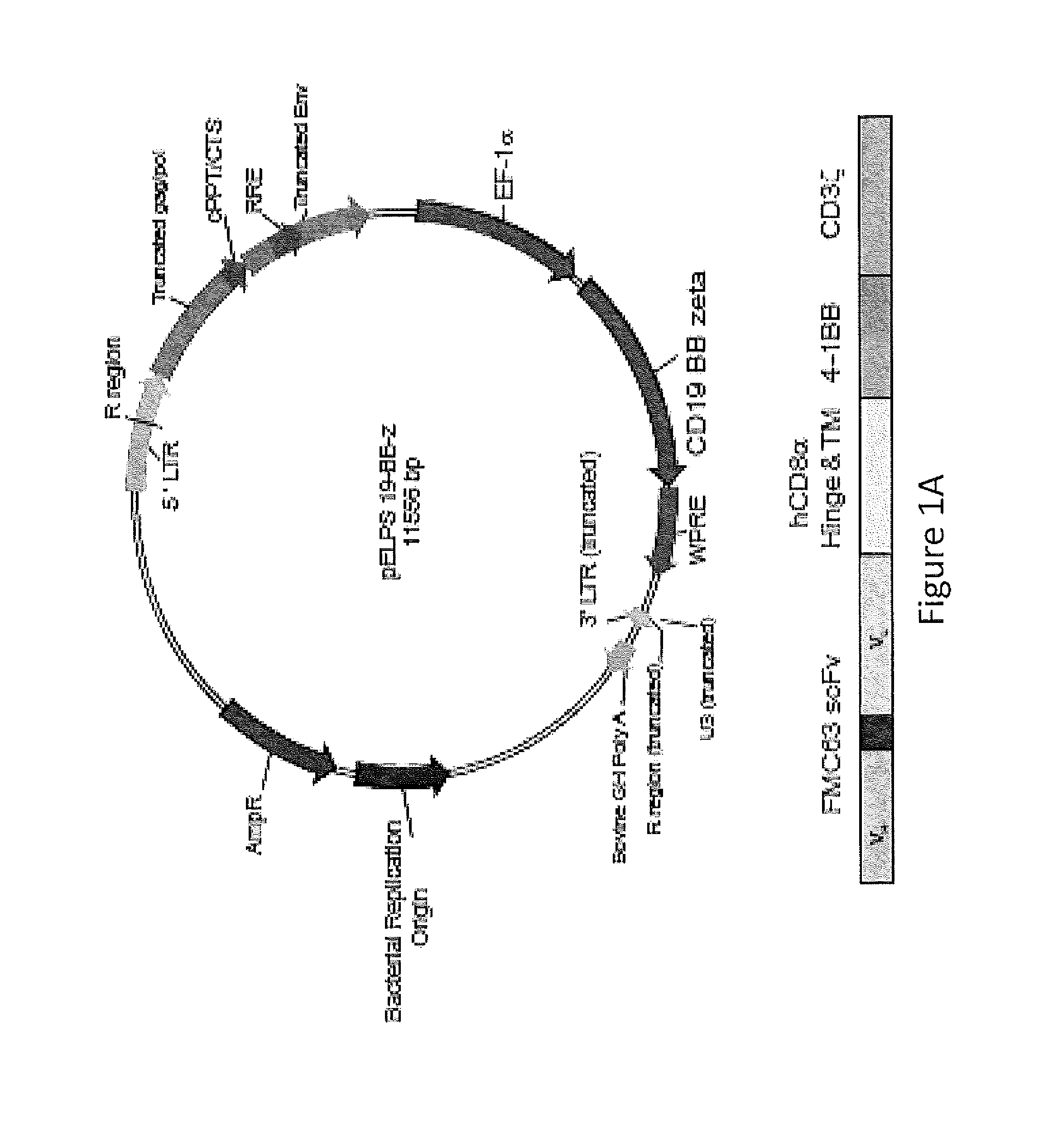
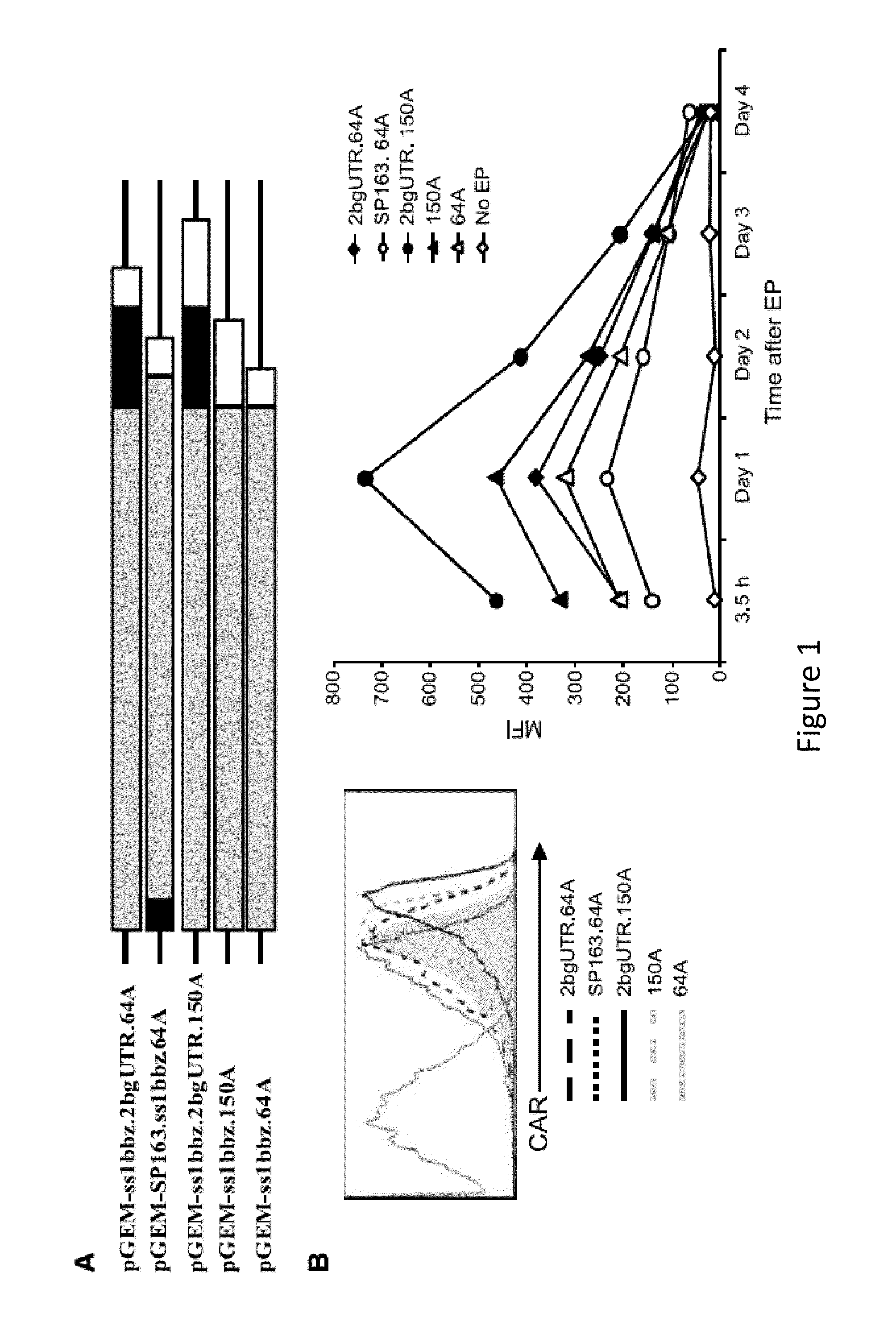
Use of Chimeric Antigen Receptor-Modified T-Cells to Treat Cancer
ActiveUS20130287748A1Stimulate immune responseVirusesPeptide/protein ingredientsBinding domainAntigen binding
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell to express a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Novel artificial antigen presenting cells and uses therefor
The invention relates to novel artificial antigen presenting cells (aAPCs). The aAPC comprises at least one stimulatory ligand and at least one co-stimulatory ligand where the ligands each specifically bind with a cognate molecule on a T cell of interest, thereby mediating expansion of the T cell. The aAPC of the invention can further comprise additional molecules useful for expanding a T cell of interest. The aAPC of the invention can be used as an “off the shelf” APC that can be readily designed to expand a T cell of interest. Also, the aAPC of the invention can be used identify the stimulatory, co-stimulatory, and any other factors that mediate growth and expansion of a T cell of interest. Thus, the present invention provides powerful tools for development of novel therapeutics where activation and expansion of a T cell can provide a benefit.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Pharmaceutical composition containing a stabilised mRNA optimised for translation in its coding regions
ActiveUS20050032730A1Overcome disadvantagesImprove efficiencyAntibacterial agentsVirusesTranslational efficiencyCoding region
The present invention relates to a pharmaceutical composition comprising a modified mRNA that is stabilised by sequence modifications and optimised for translation. The pharmaceutical composition according to the invention is particularly well suited for use as an inoculating agent, as well as a therapeutic agent for tissue regeneration. In addition, a process is described for determining sequence modifications that promote stabilisation and translational efficiency of modified mRNA of the invention.
Owner:CUREVAC SE
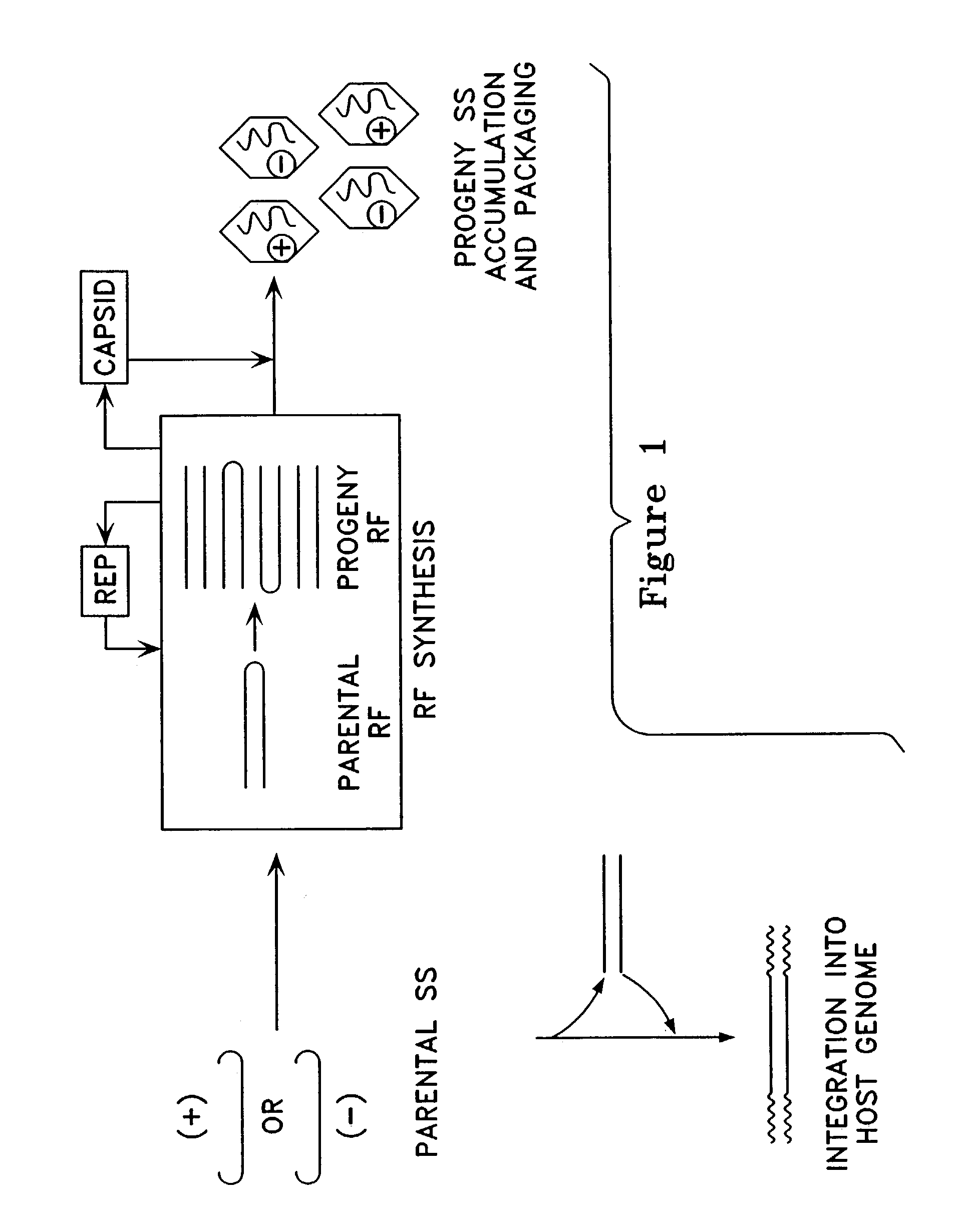
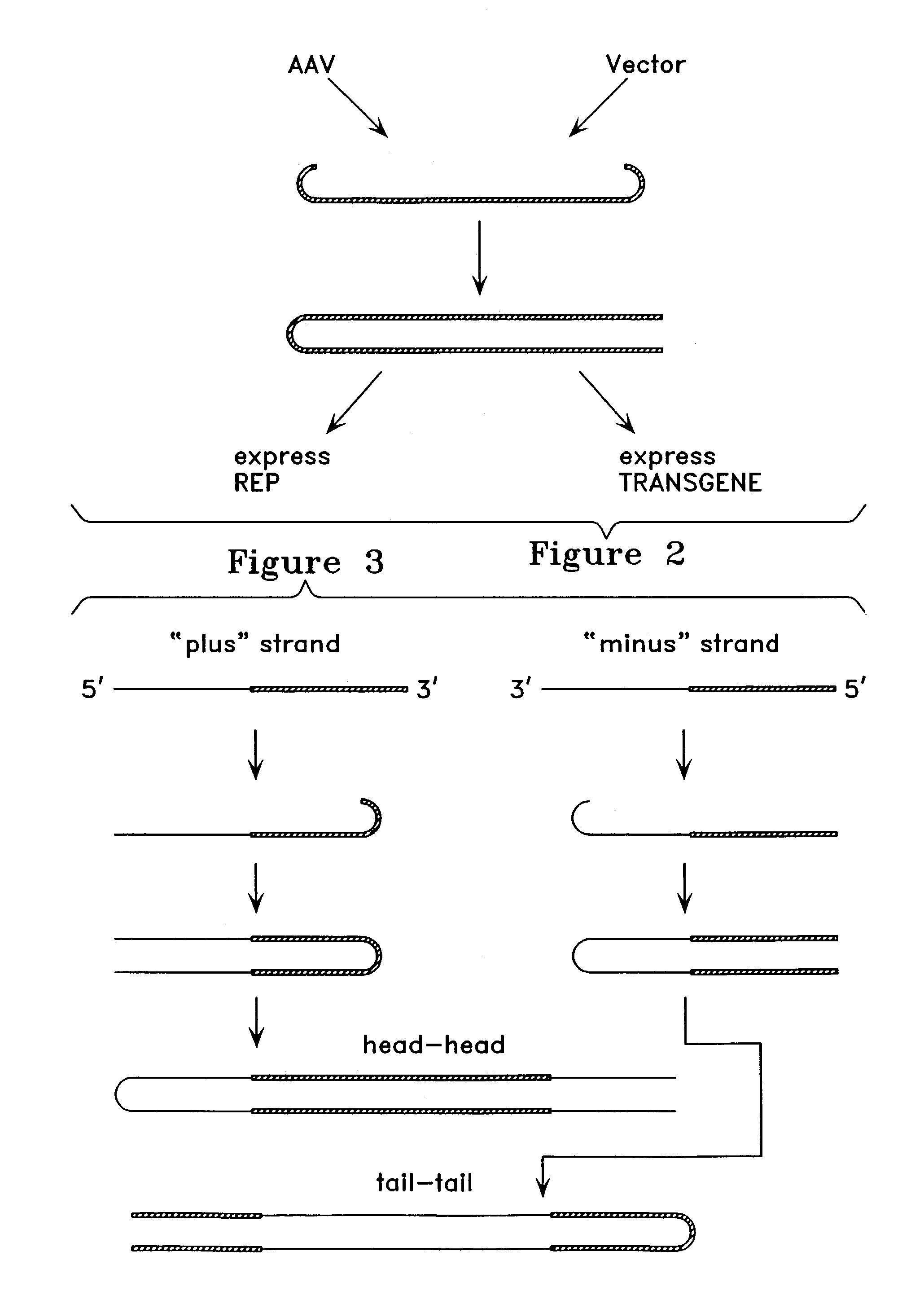
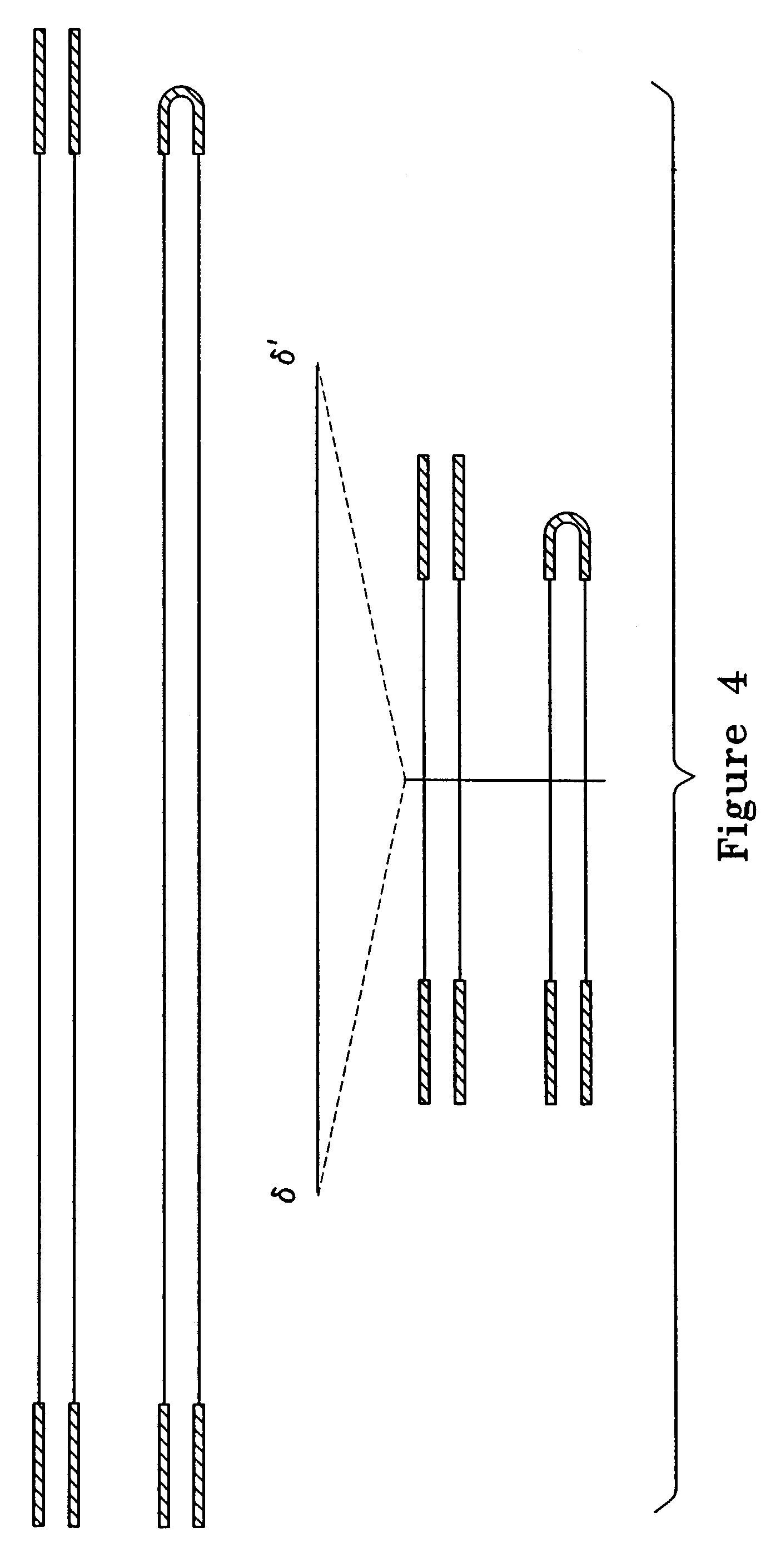
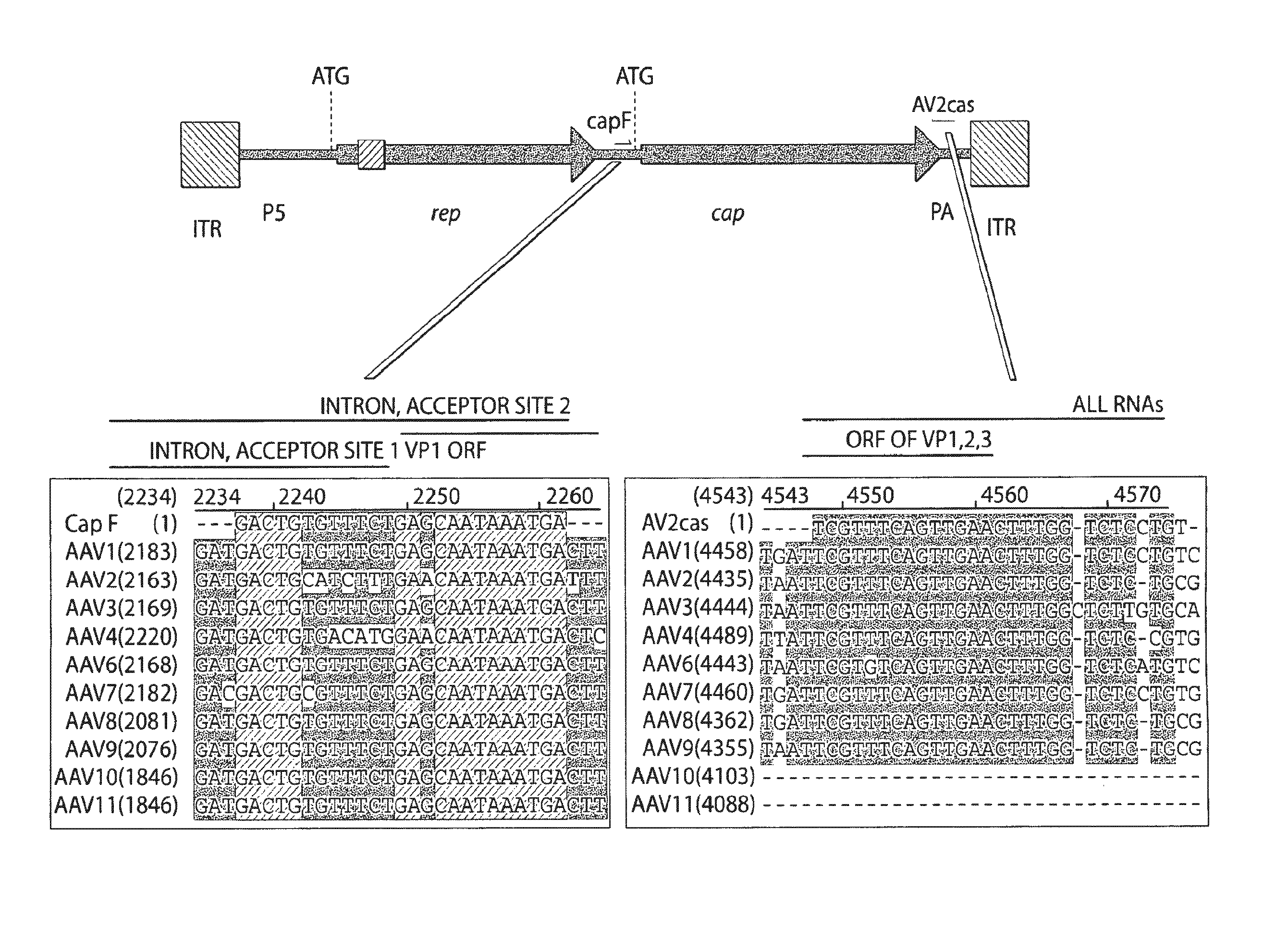
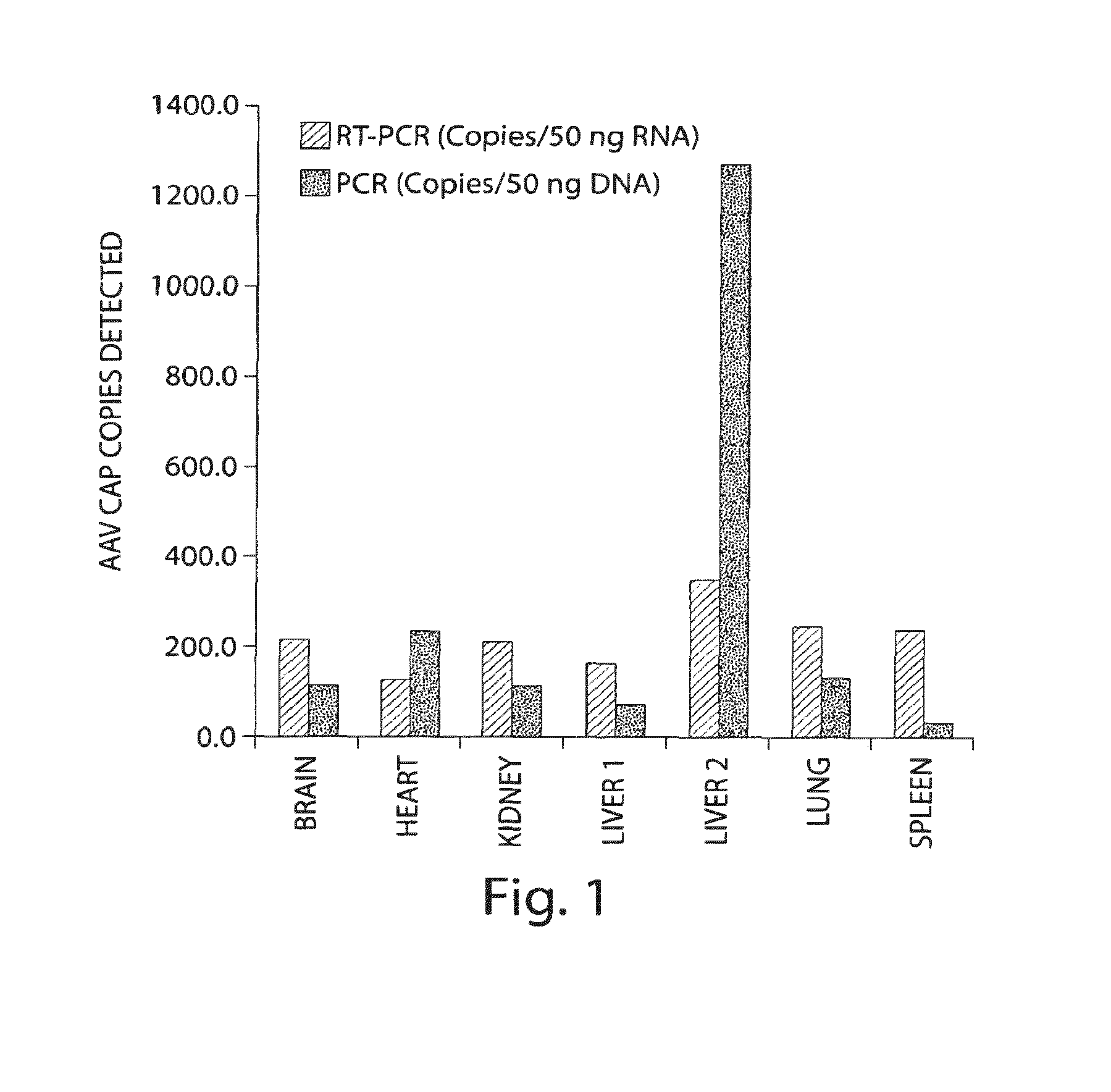
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Metabolically activated recombinant viral vectors and methods for their preparation and use
Recombinant viral vectors, especially parvovirus vectors such as adeno-associated virus (AAV) vectors, capable of enhanced expression of heterologous sequences, and methods for their construction and use, are provided. The vectors have a structure, or are capable of rapidly adopting a structure, which involves intrastrand base pairing of at least one region in a heterologous sequence.
Owner:GENZYME CORP
RNA engineered t cells for the treatment of cancer
ActiveUS20140227237A1Reduce riskLimited half-lifeBiocideVirusesAntigen receptorChimeric antigen receptor
The present invention relates to compositions and methods for generating RNA Chimeric Antigen Receptor (CAR) transfected T cells. The RNA-engineered T cells can be used in adoptive therapy to treat cancer.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Megakaryocytic protein tyrosine kinases
InactiveUS6326469B1Reduced megakaryocyte growthReduce differentiationVirusesPeptide/protein ingredientsDiseaseProtein-Tyrosine Kinases
The present invention relates to novel cytoplasmic tyrosine kinases isolated from megakaryocytes (megakaryocyte kinases or MKKs) which are involved in cellular signal transduction pathways and to the use of these novel proteins in the diagnosis and treatment of disease. The present invention further relates to specific megakaryocyte kinases, designated MKK1, MKK2 and MKK3, and their use as diagnostic and therapeutic agents.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV +1
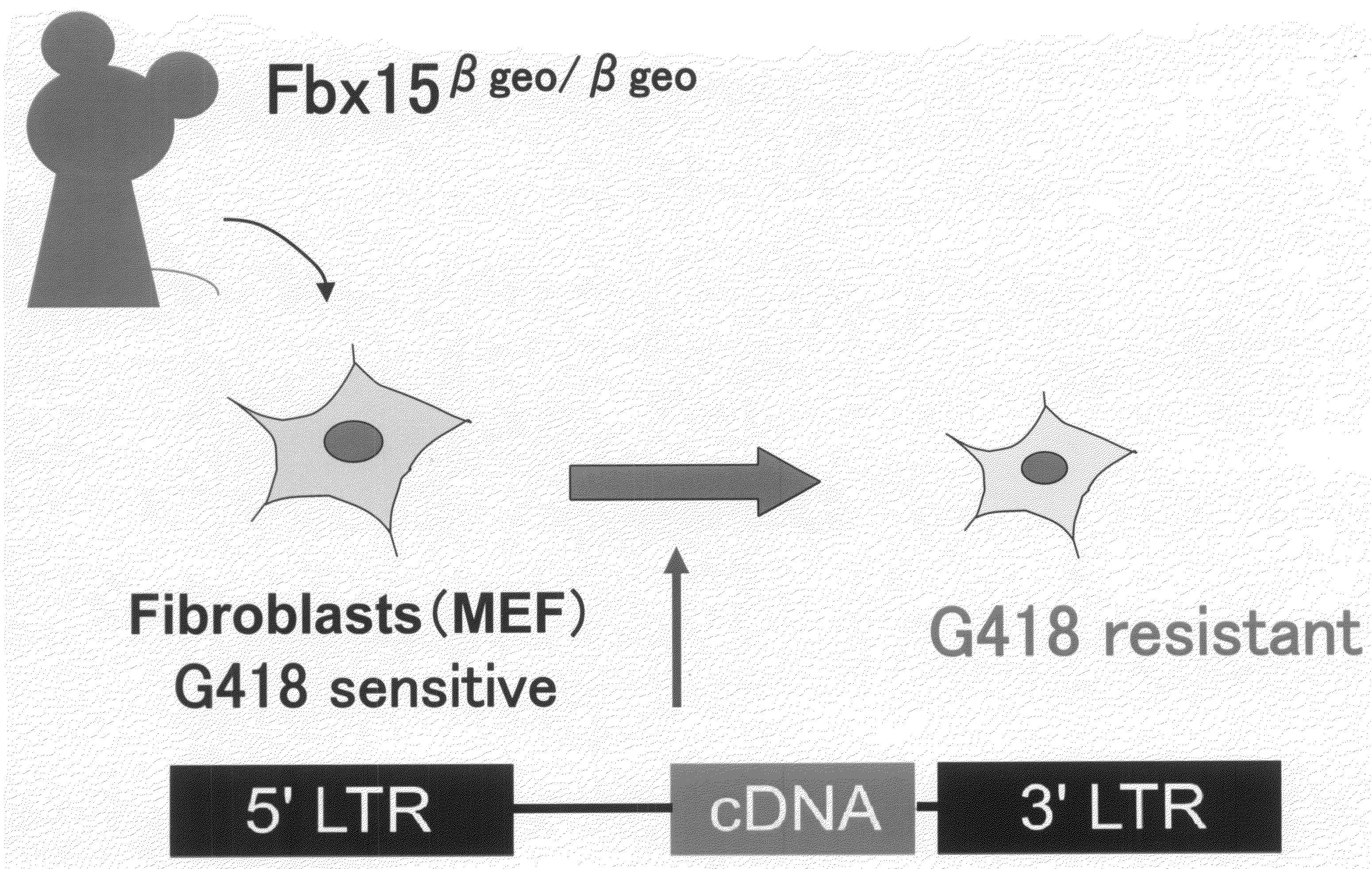
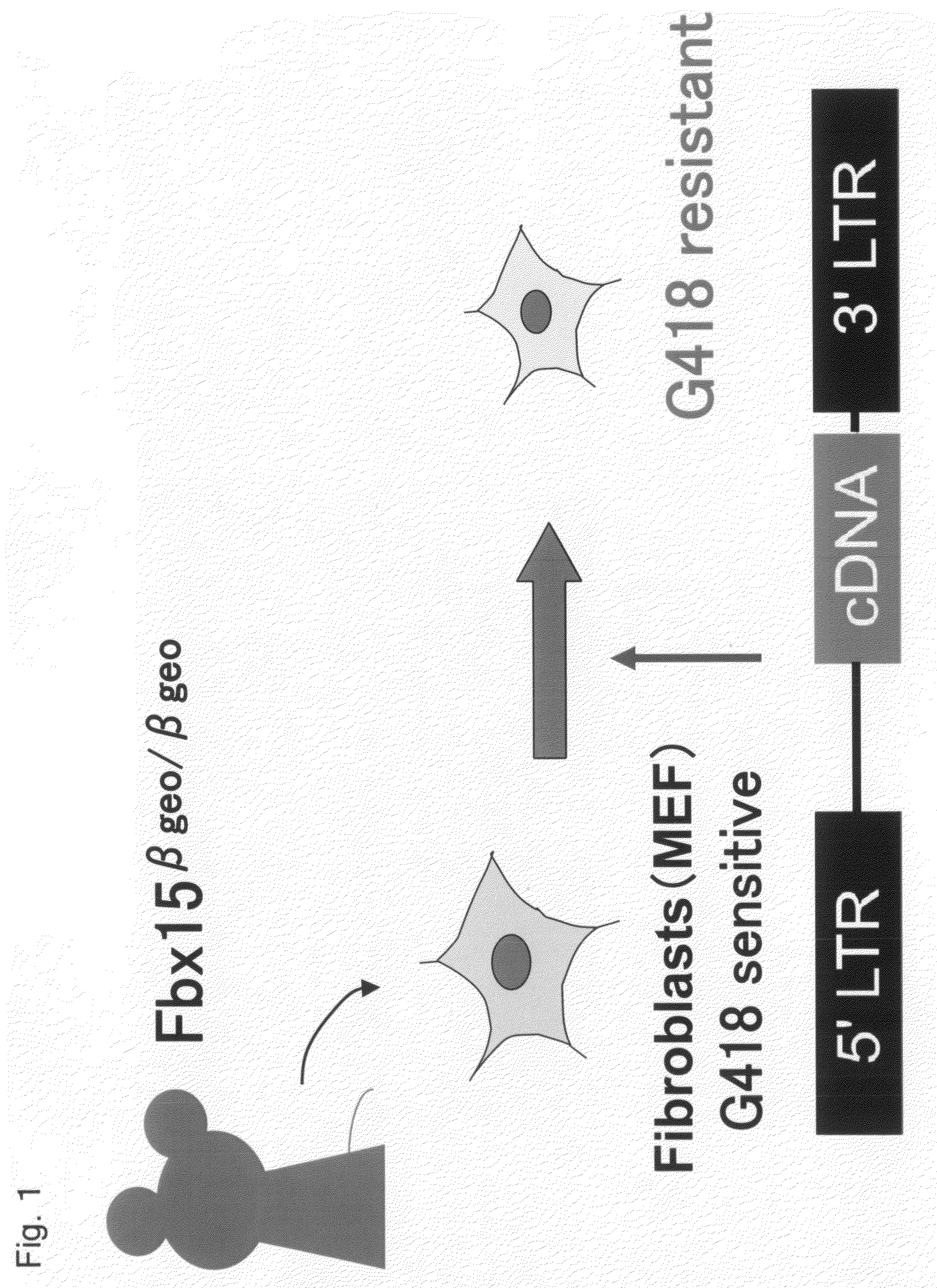
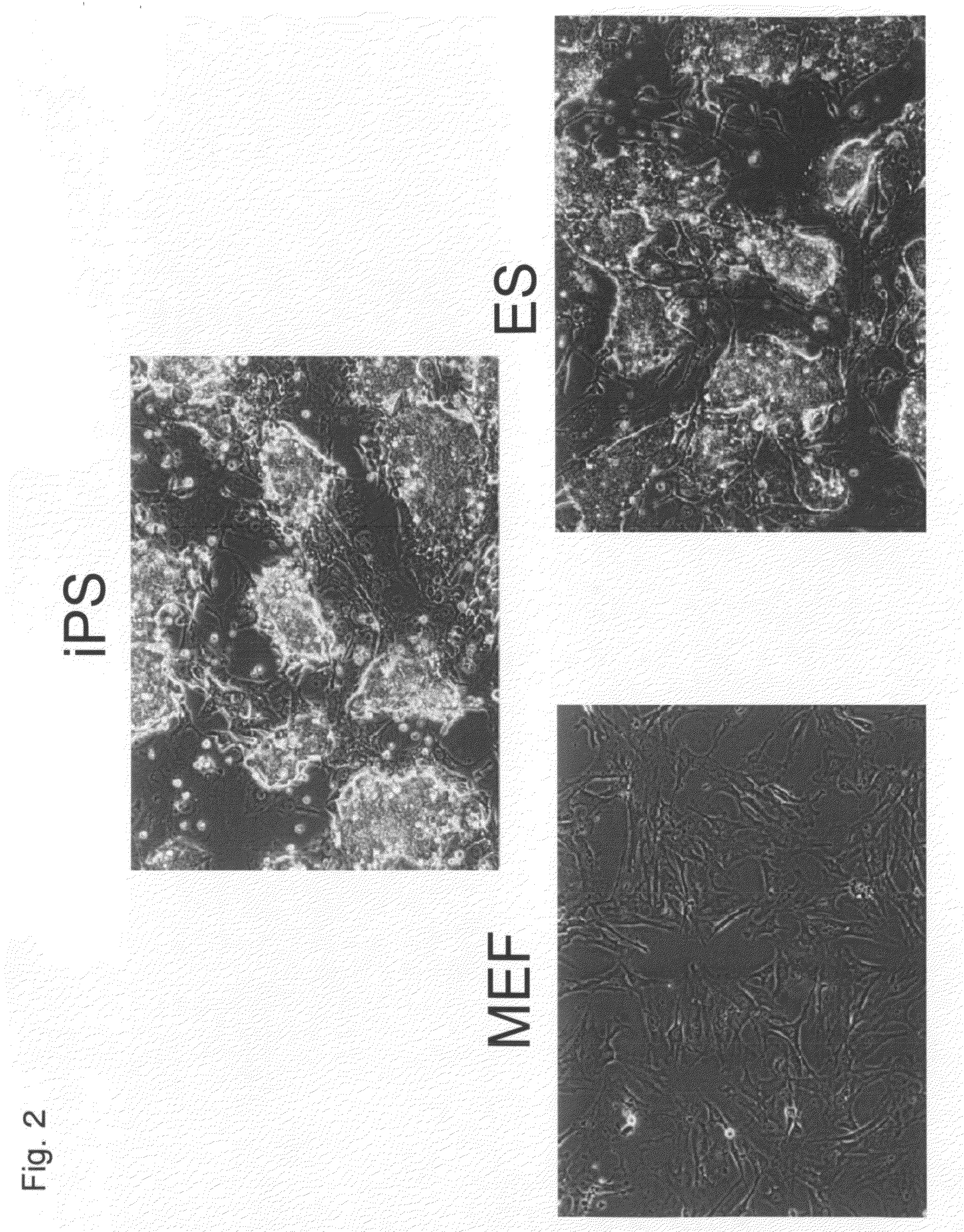
Nuclear Reprogramming Factor
There is provided a nuclear reprogramming factor for a somatic cell, which comprises a gene product of each of the following three kinds of genes: an Oct family gene, a Klf family gene, and a Myc family gene, as a means for inducing reprogramming of a differentiated cell to conveniently and highly reproducibly establish an induced pluripotent stem cell having pluripotency and growth ability similar to those of ES cells without using embryo or ES cell.
Owner:KYOTO UNIV
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Methods and compounds for controlled release of recombinant parvovirus vectors
InactiveUS7201898B2Prevent relapseImproved pulmonary mechanicsSuture equipmentsAntibacterial agentsControlled releaseSupport matrix
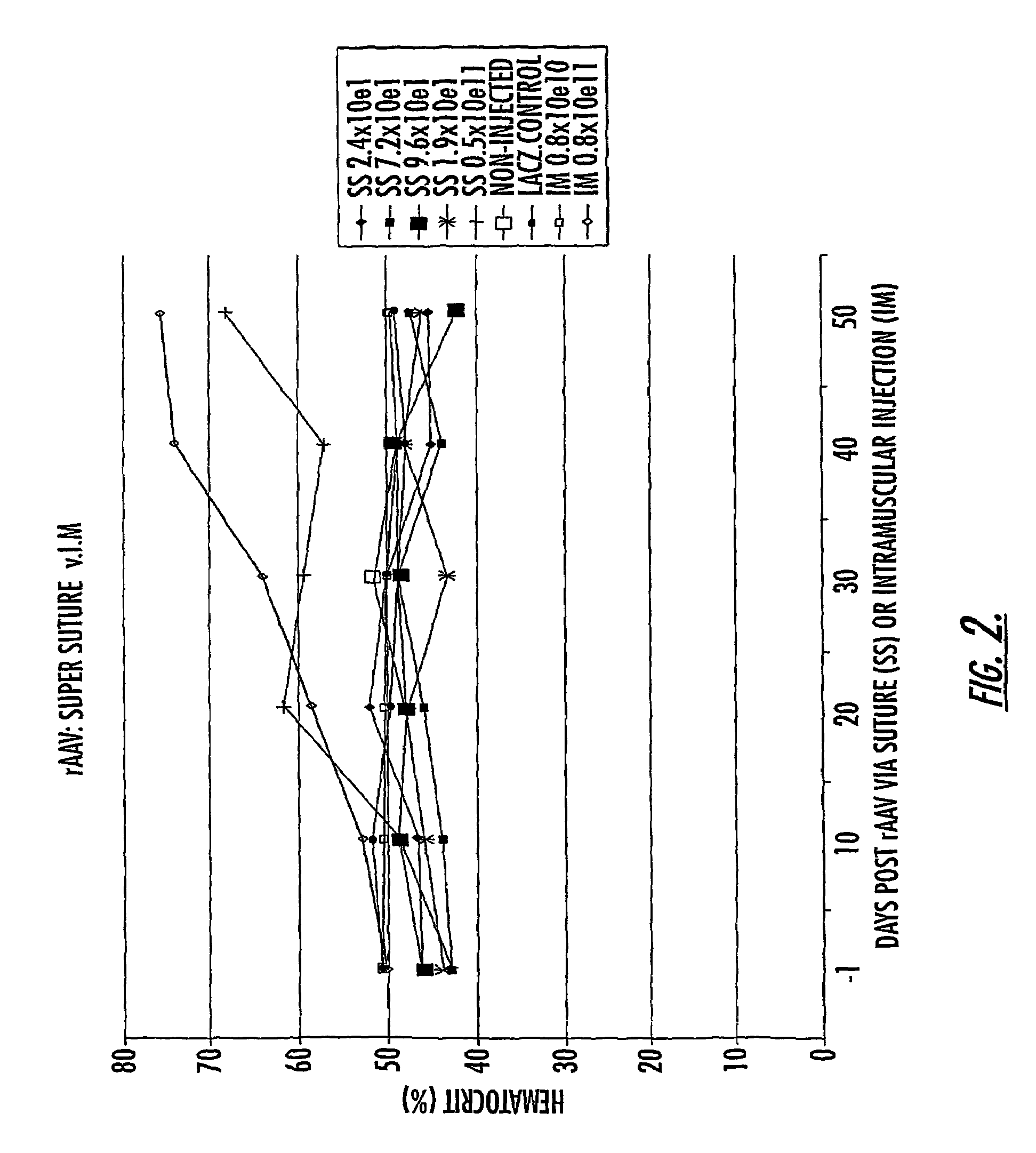
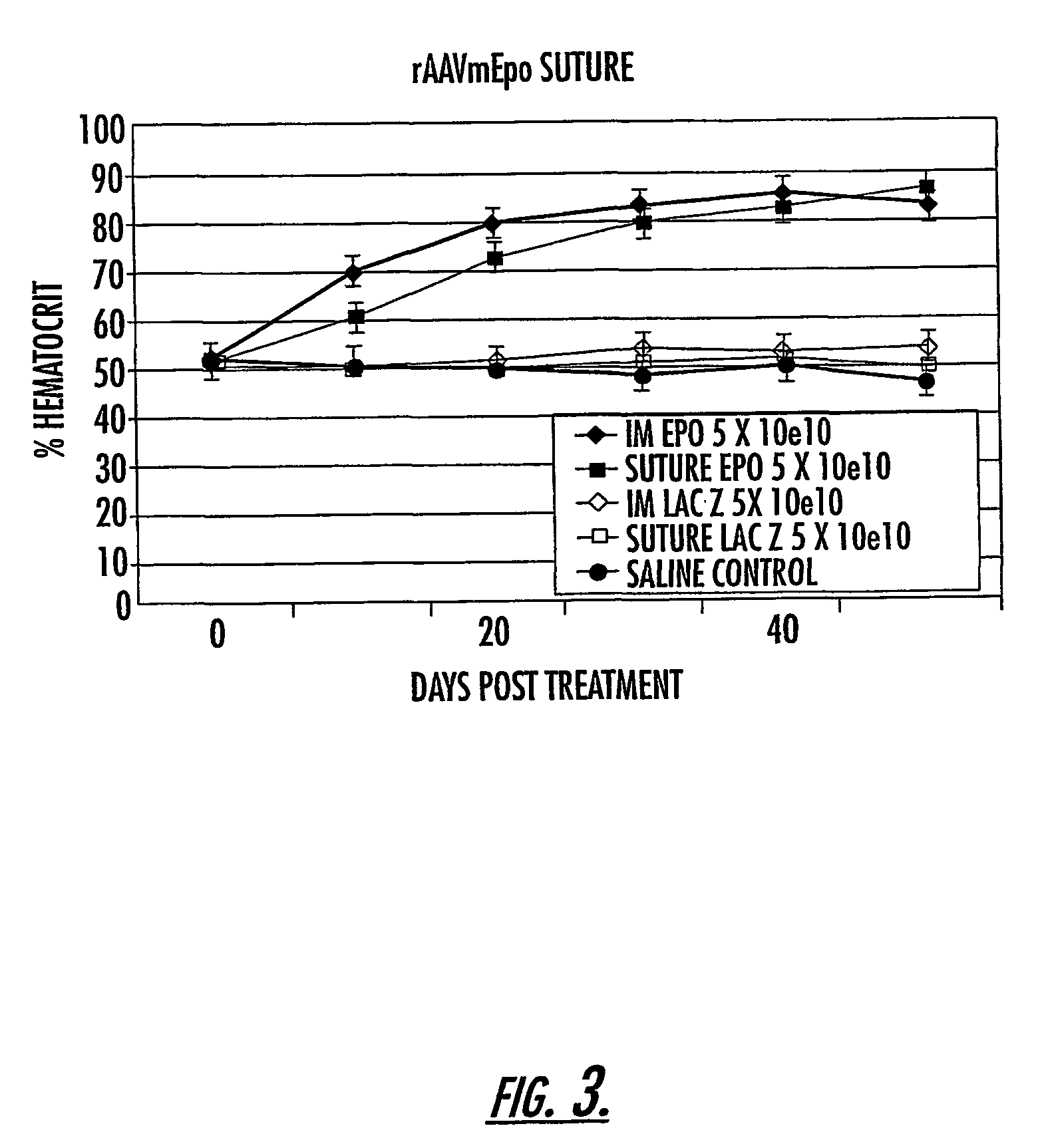
The invention uses recombinant parvoviruses, and particularly recombinant adeno-associated virus (rAAV) to deliver genes and DNA sequences for gene therapy following manipulation of the therapeutic virus for packaging and transport. The invention delivers therapeutic viral vectors via rAAV affixed to support matrixes (i.e., sutures, surgically implantable materials, grafts, and the like).
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
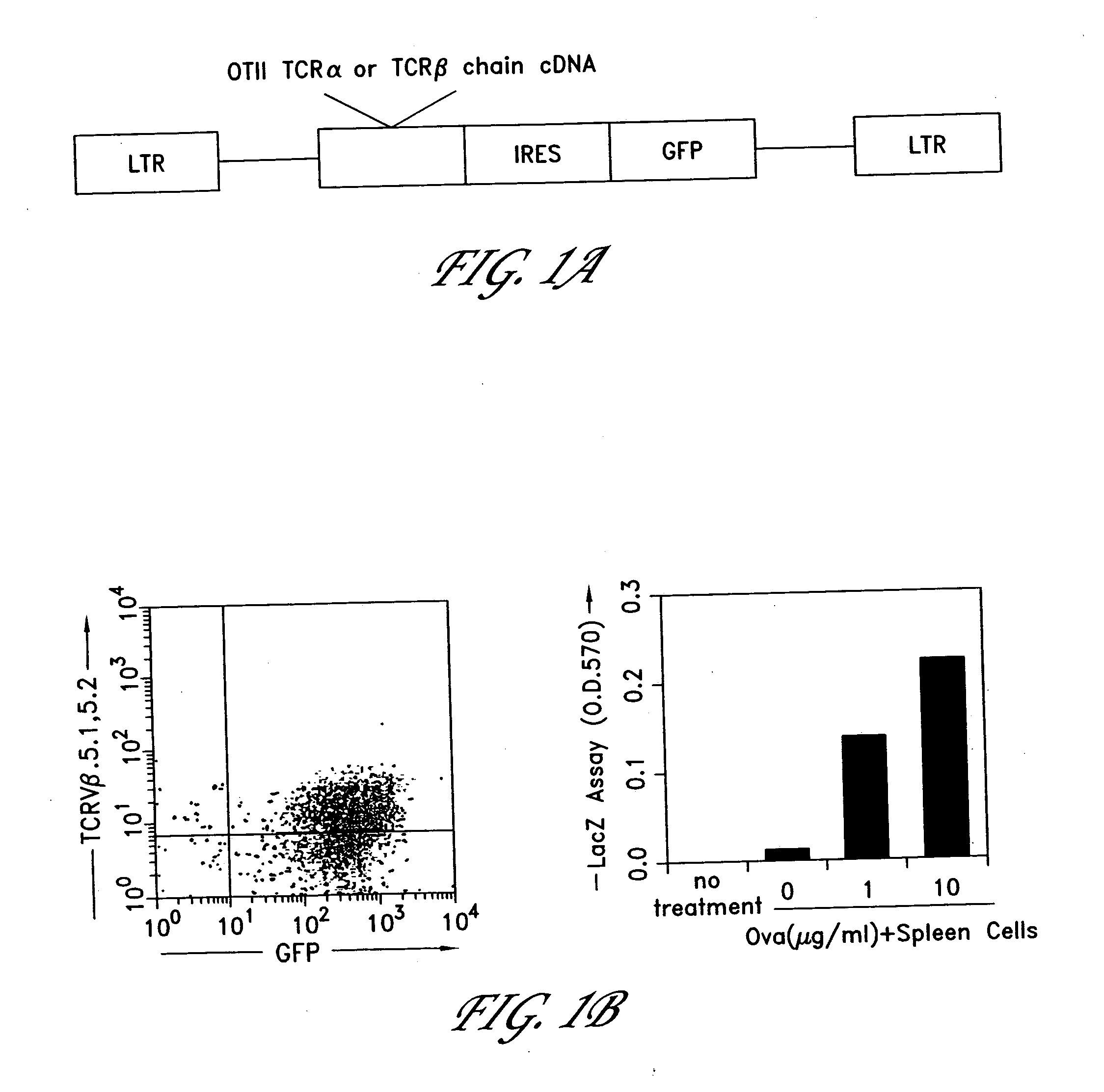
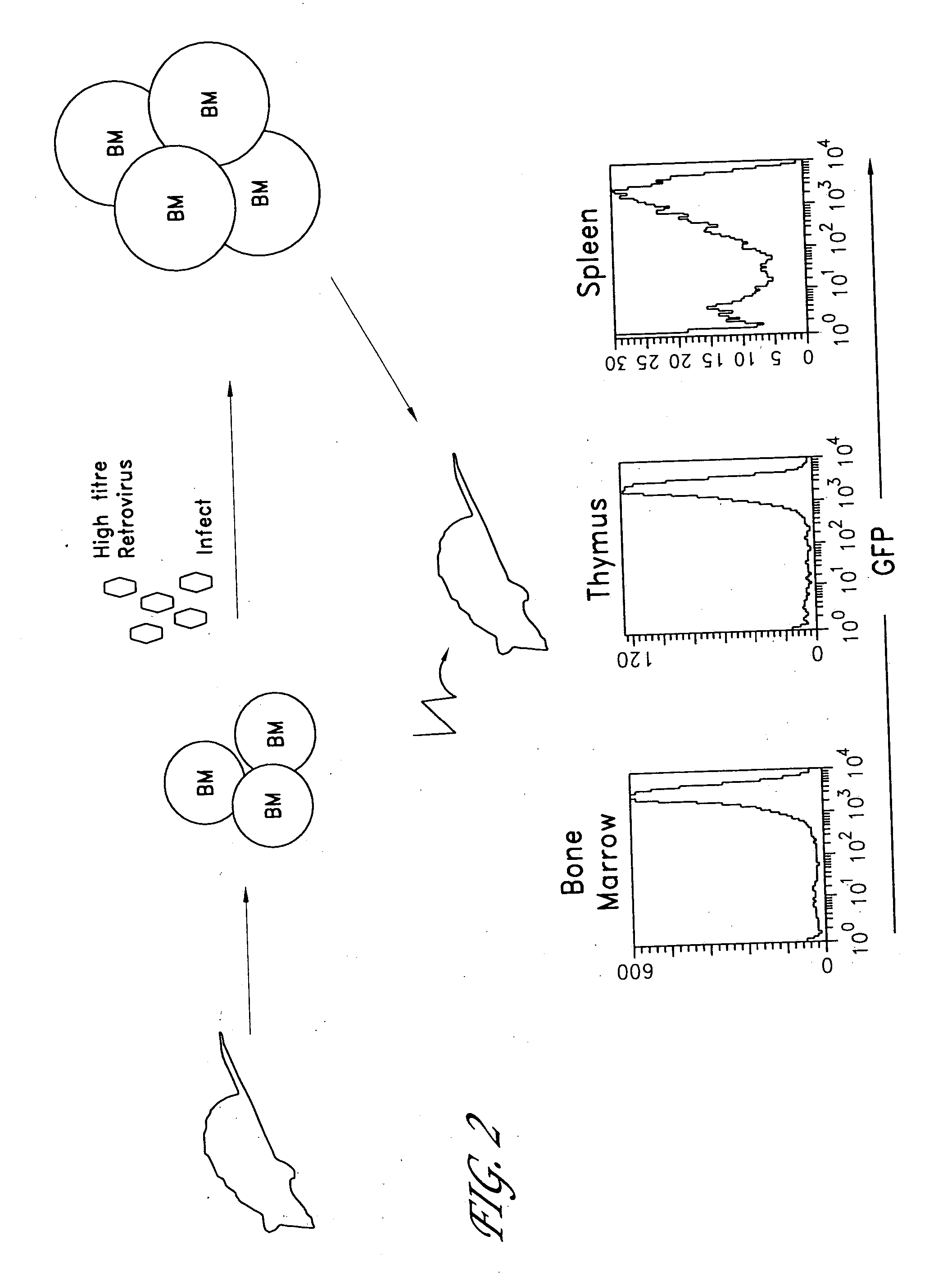
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
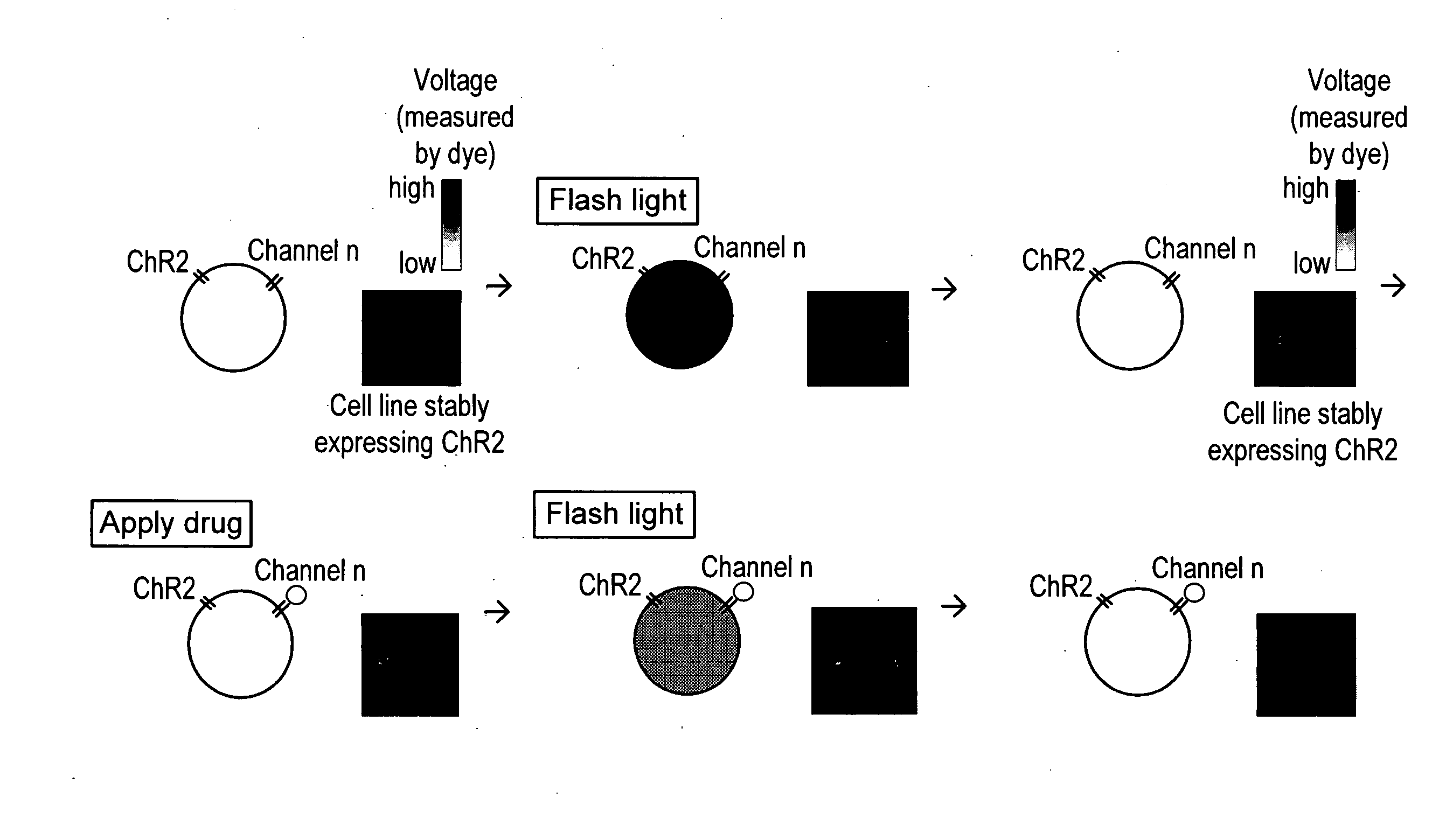
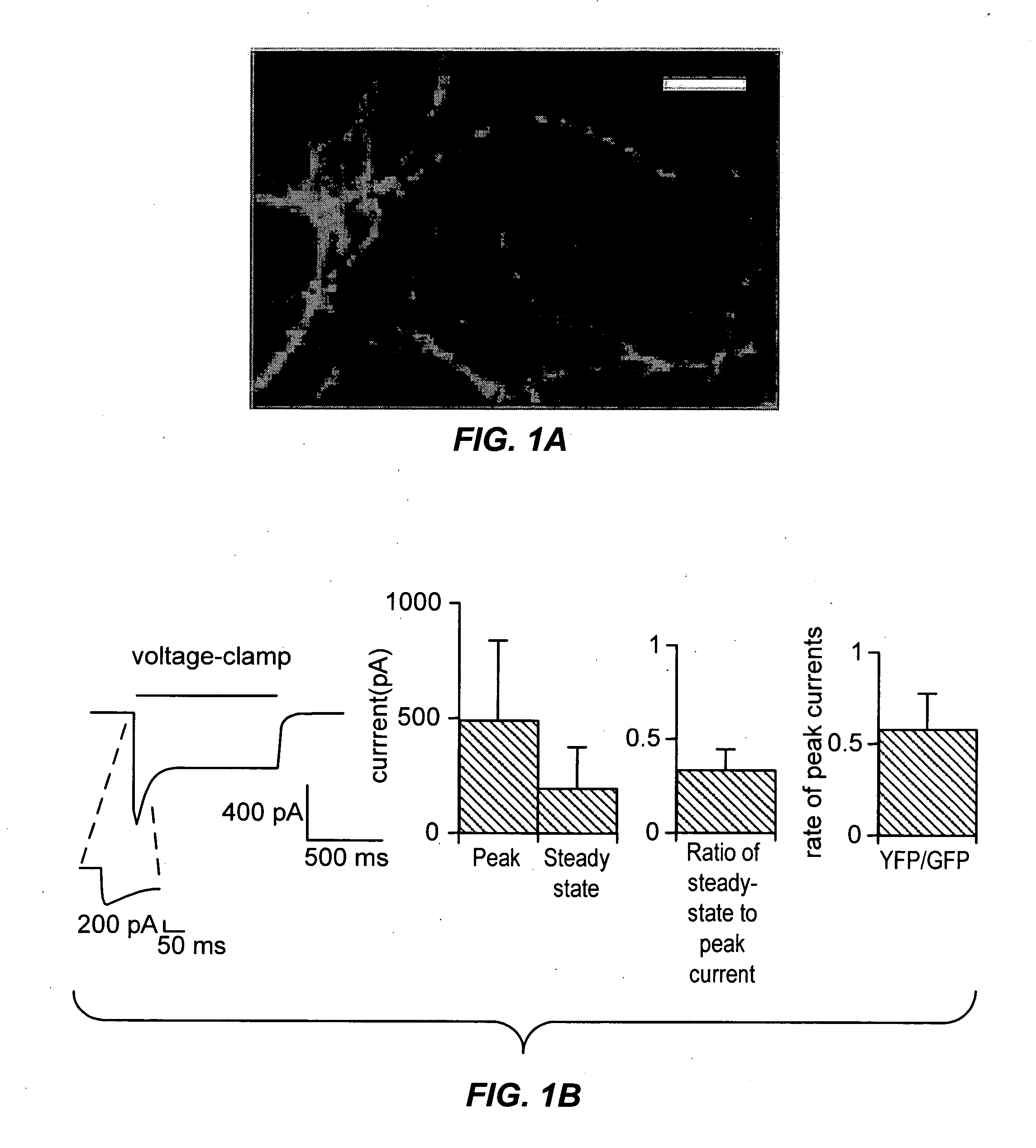
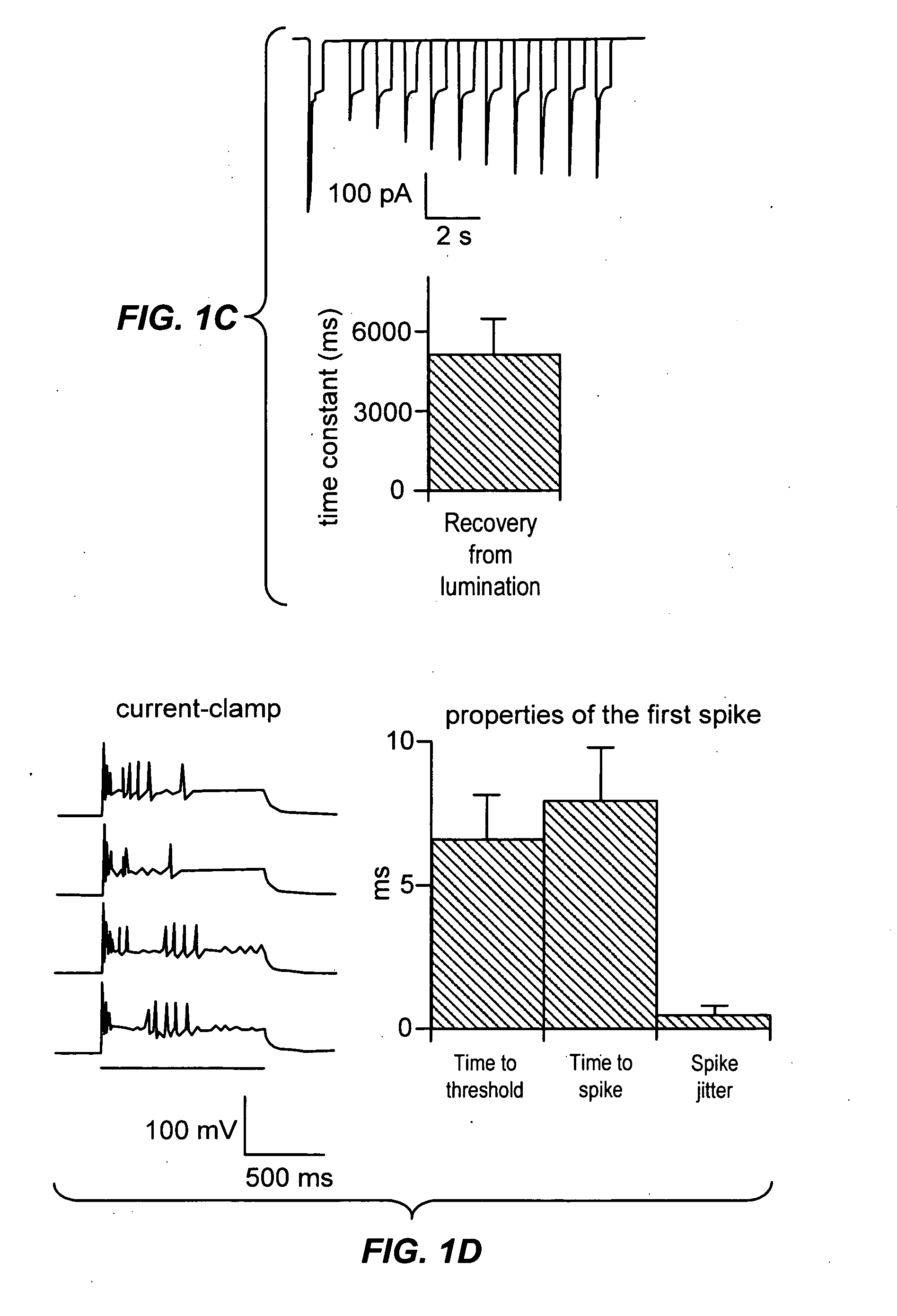
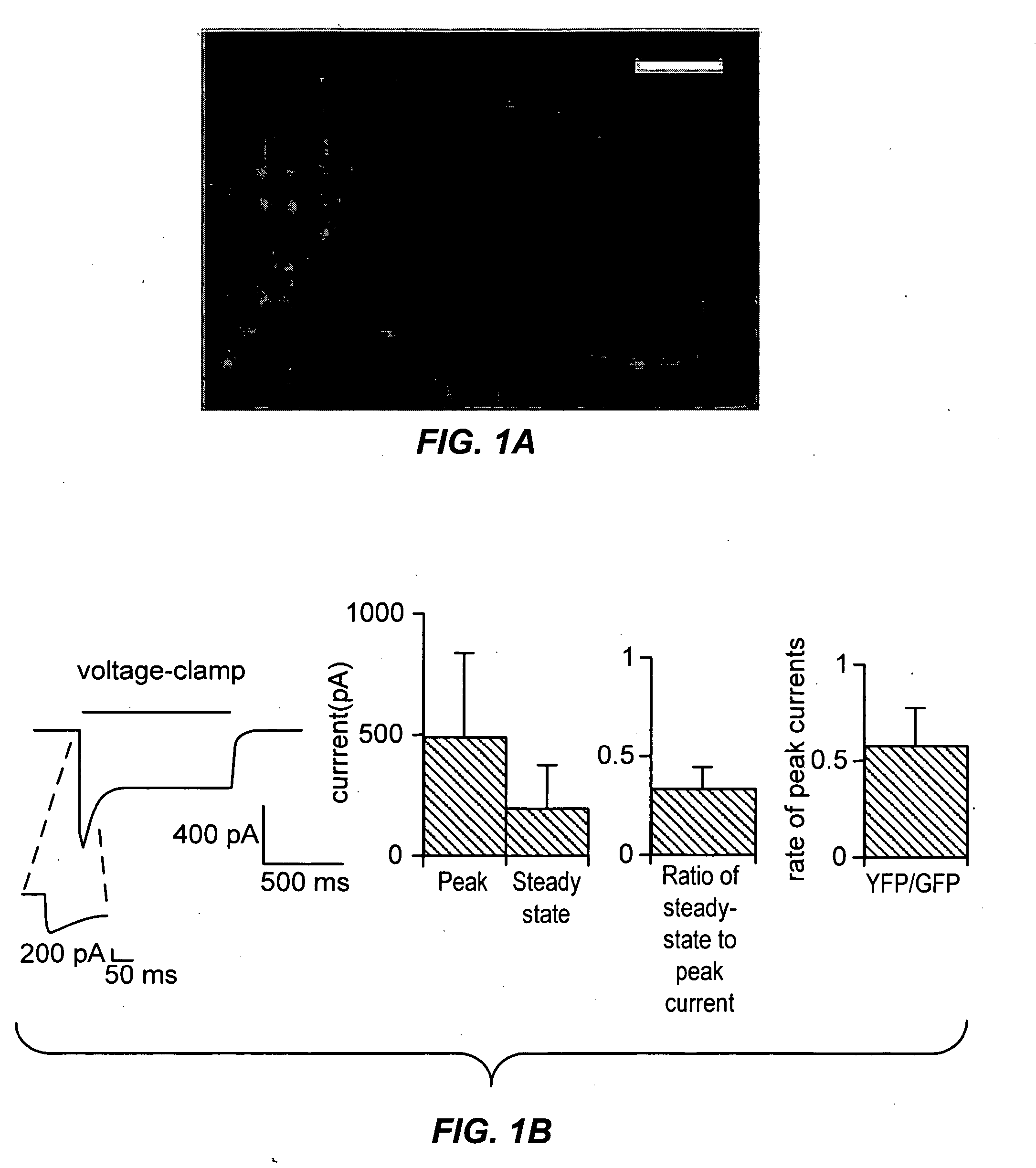
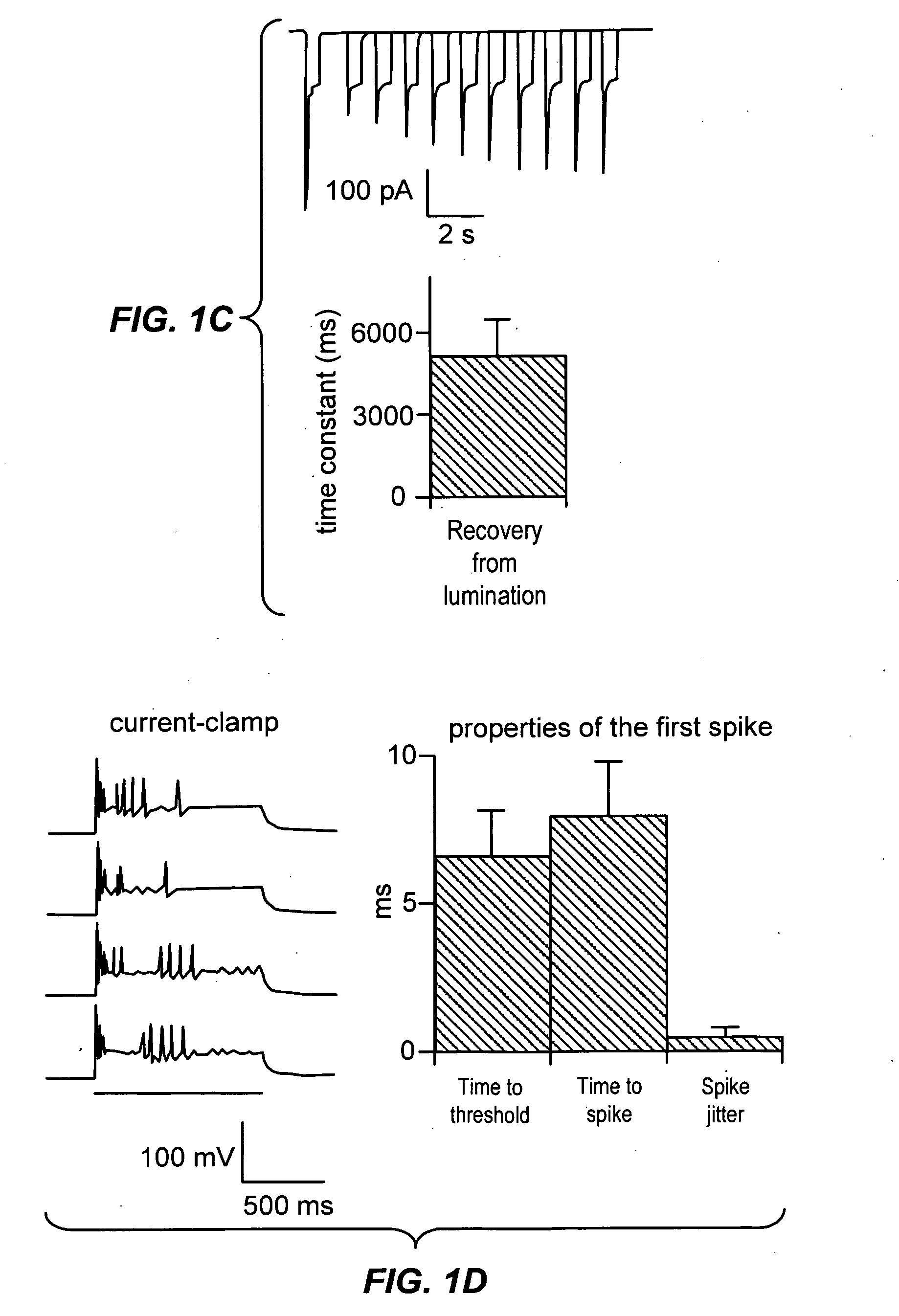
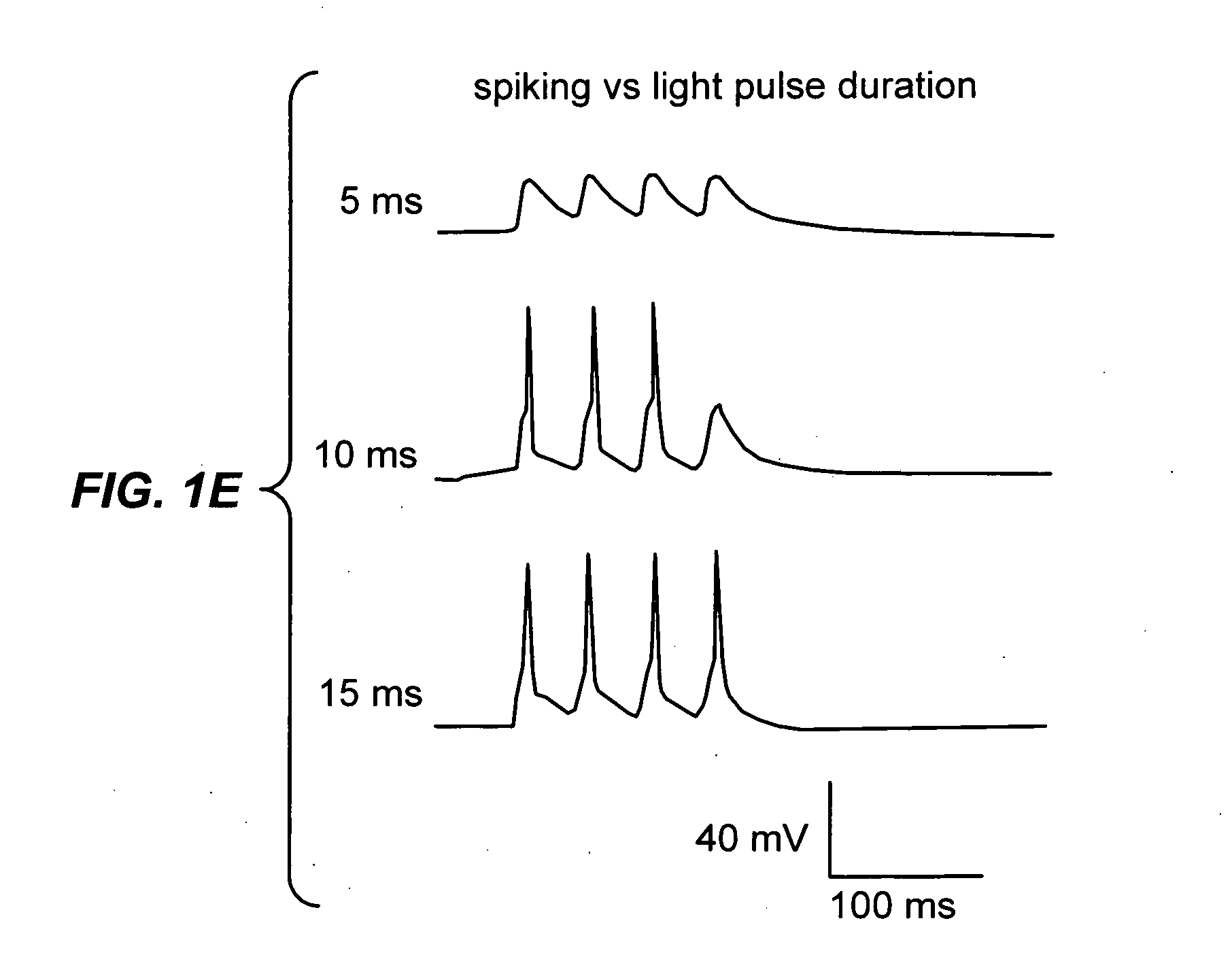
Light-activated cation channel and uses thereof
ActiveUS20070261127A1Improve abilitiesOrganic active ingredientsSenses disorderCell membraneExcitable cell
The present invention provides compositions and methods for light-activated cation channel proteins and their uses within cell membranes and subcellular regions. The invention provides for proteins, nucleic acids, vectors and methods for genetically targeted expression of light-activated cation channels to specific cells or defined cell populations. In particular the invention provides millisecond-timescale temporal control of cation channels using moderate light intensities in cells, cell lines, transgenic animals, and humans. The invention provides for optically generating electrical spikes in nerve cells and other excitable cells useful for driving neuronal networks, drug screening, and therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
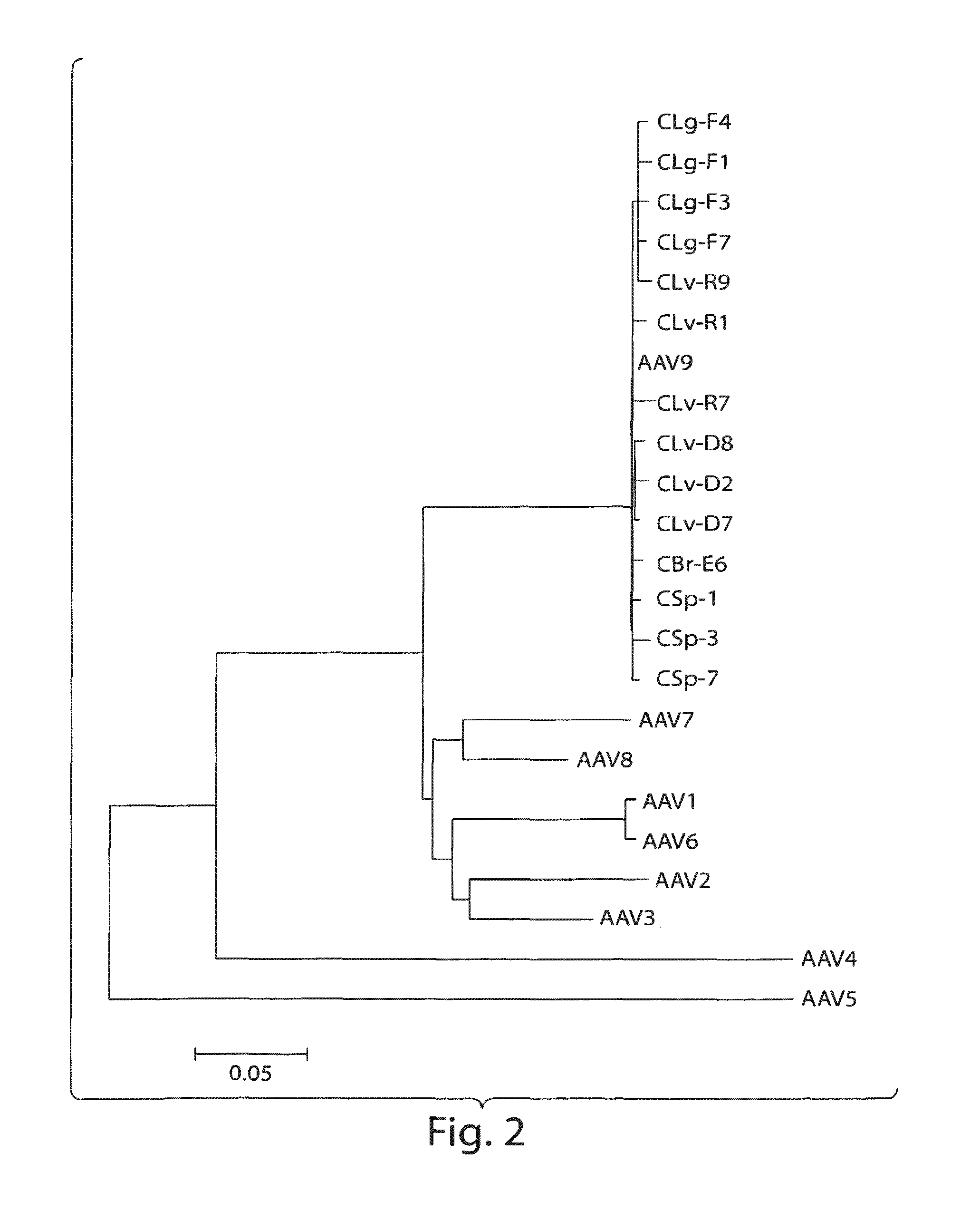
AAV's and uses thereof
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
Methods of spray-drying a drug and a hydrophobic amino acid
InactiveUS6372258B1High level of stabilityEasy to manufacturePowder deliveryOrganic active ingredientsMoistureParticle-size distribution
According to the subject invention, dispersible dry powder pharmaceutical-based compositions are provided, including methods for their manufacture and dry powder dispersion devices. A dispersible dry powder pharmaceutical-based composition is one having a moisture content of less than about 10% by weight (% w) water, usually below about 5% w and preferably less than about 3% w; a particle size of about 1.0-5.0 mum mass median diameter (MMD), usually 1.0-4.0 mum MMD, and preferably 1.0-3.0 mum MMD; a delivered dose of about >30%, usually >40%, preferably >50%, and most preferred >60%; and an aerosol particle size distribution of about 1.0-5.0 mum mass median aerodynamic diameter (MMAD), usually 1.5-4.5 mum MMAD, and preferably 1.5-4.0 MMAD. Such composition are of pharmaceutical grade purity.
Owner:NOVARTIS FARMA
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM') of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM' allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
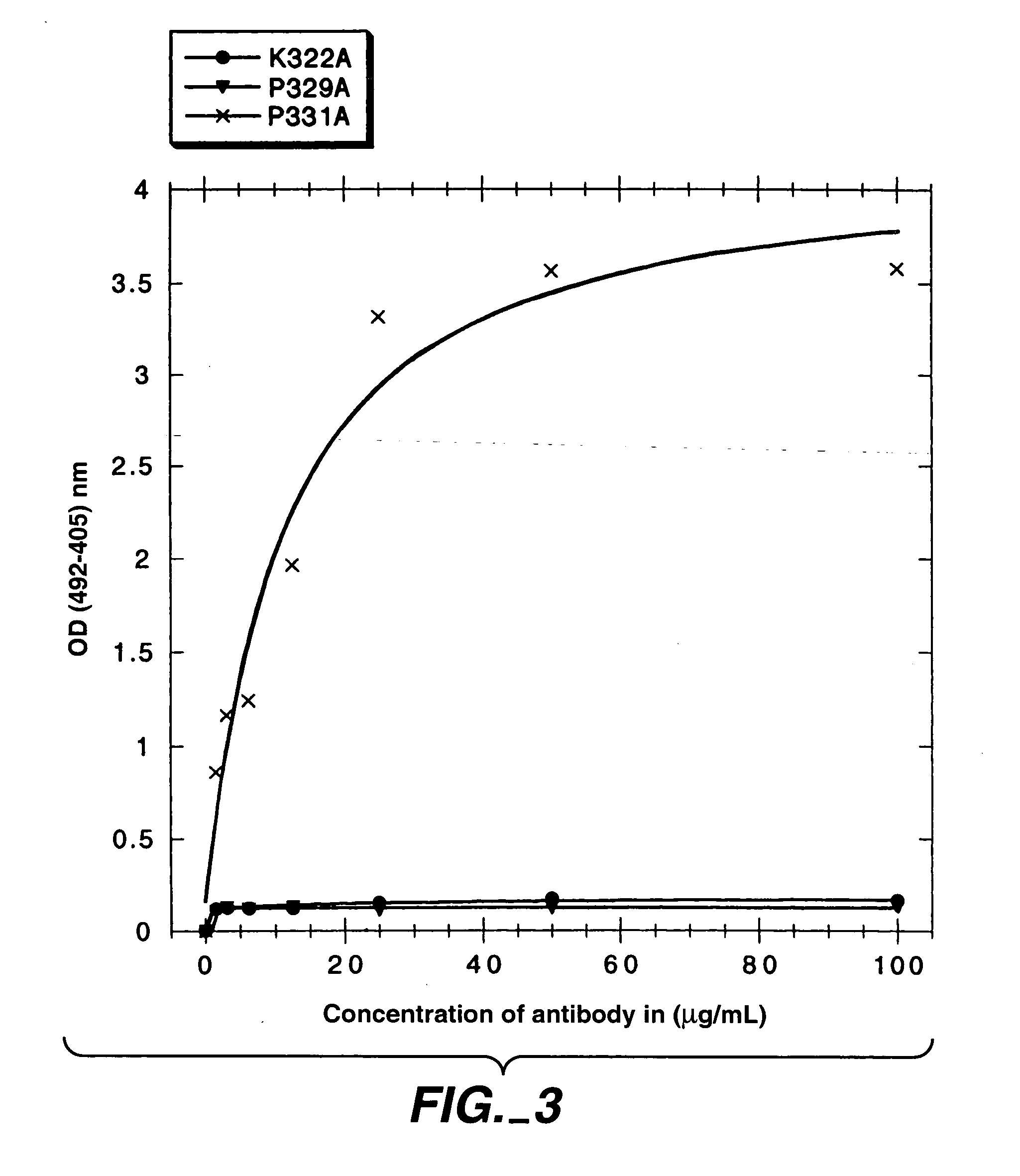
Polypeptide variants with altered effector function
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
Light-activated cation channel and uses thereof
ActiveUS20070054319A1Improve abilitiesOrganic active ingredientsSenses disorderCell membraneExcitable cell
The present invention provides compositions and methods for light-activated cation channel proteins and their uses within cell membranes and subcellular regions. The invention provides for proteins, nucleic acids, vectors and methods for genetically targeted expression of light-activated cation channels to specific cells or defined cell populations. In particular the invention provides millisecond-timescale temporal control of cation channels using moderate light intensities in cells, cell lines, transgenic animals, and humans. The invention provides for optically generating electrical spikes in nerve cells and other excitable cells useful for driving neuronal networks, drug screening, and therapy.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Biologic replacement for fibrin clot
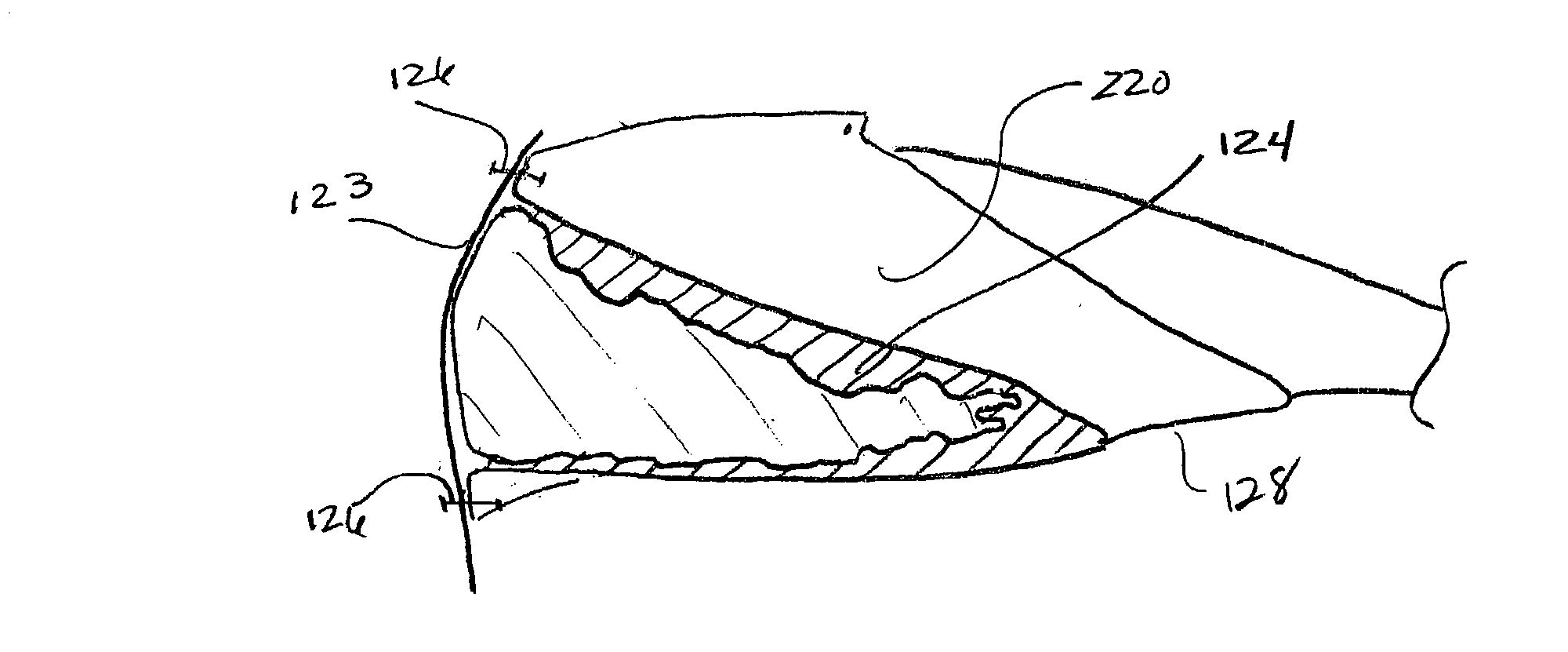
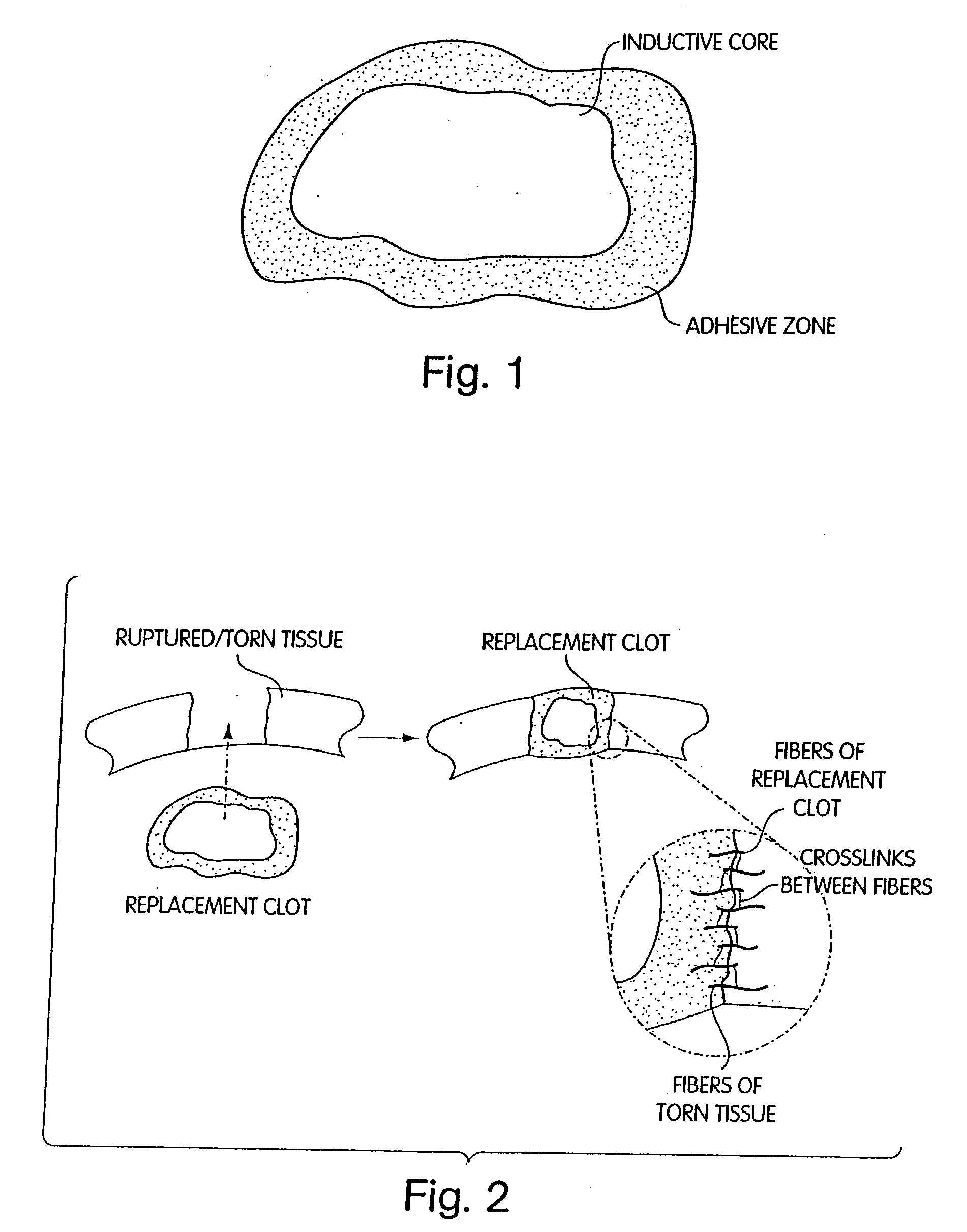
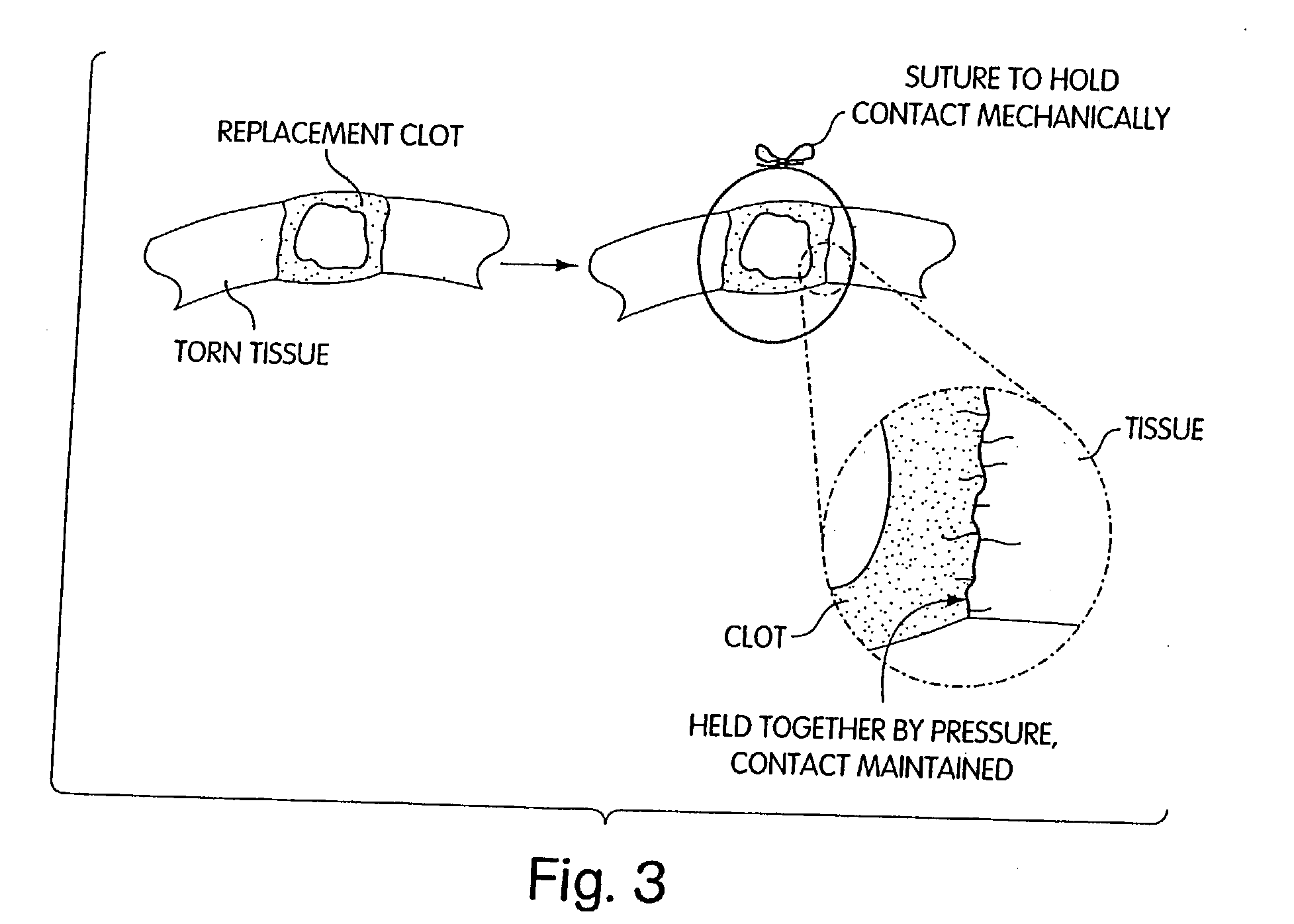
InactiveUS20040059416A1Promote regenerationWound closure is subsequentlySuture equipmentsVirusesLigament structureBiomedical engineering
Owner:CHILDRENS MEDICAL CENT CORP +1
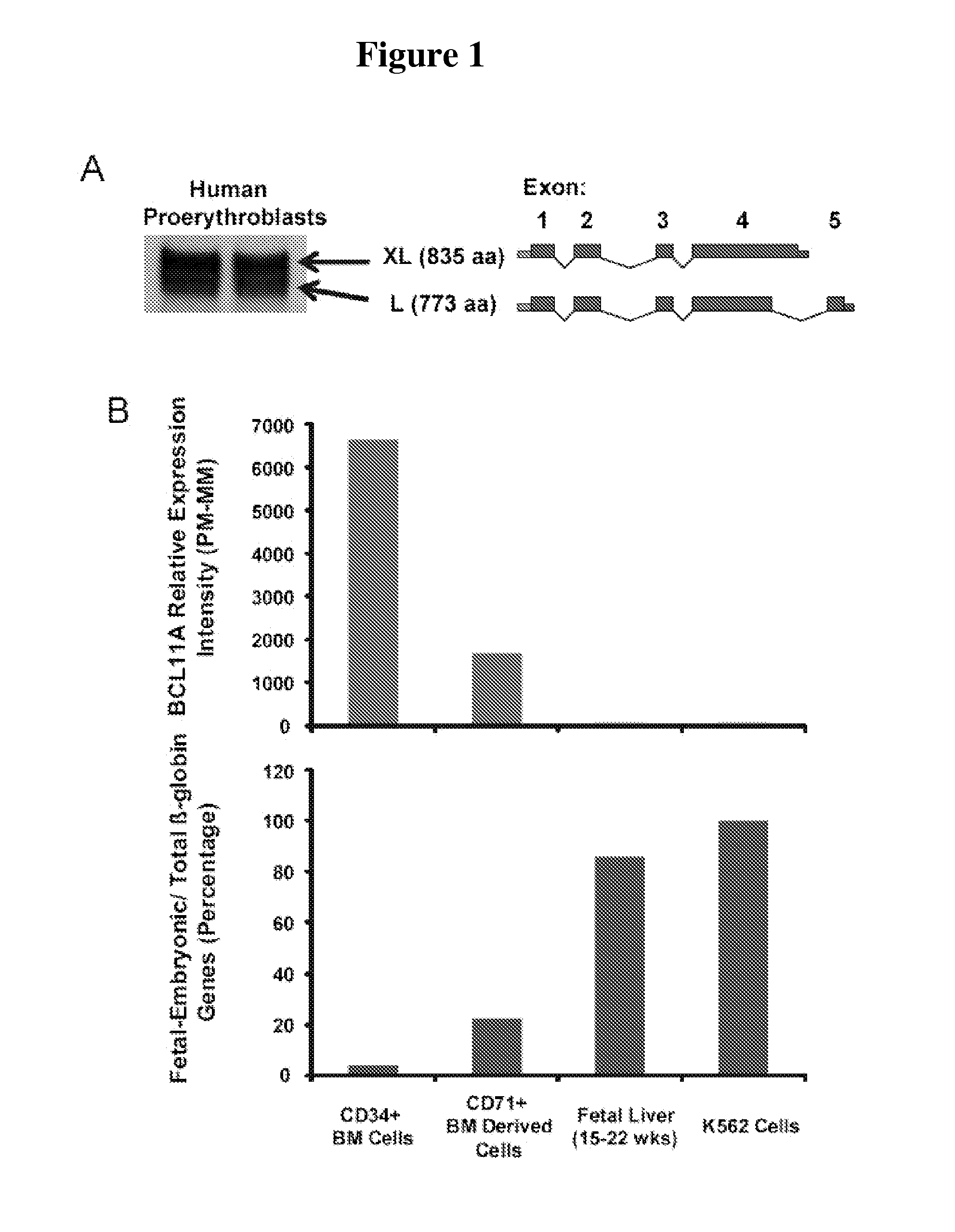
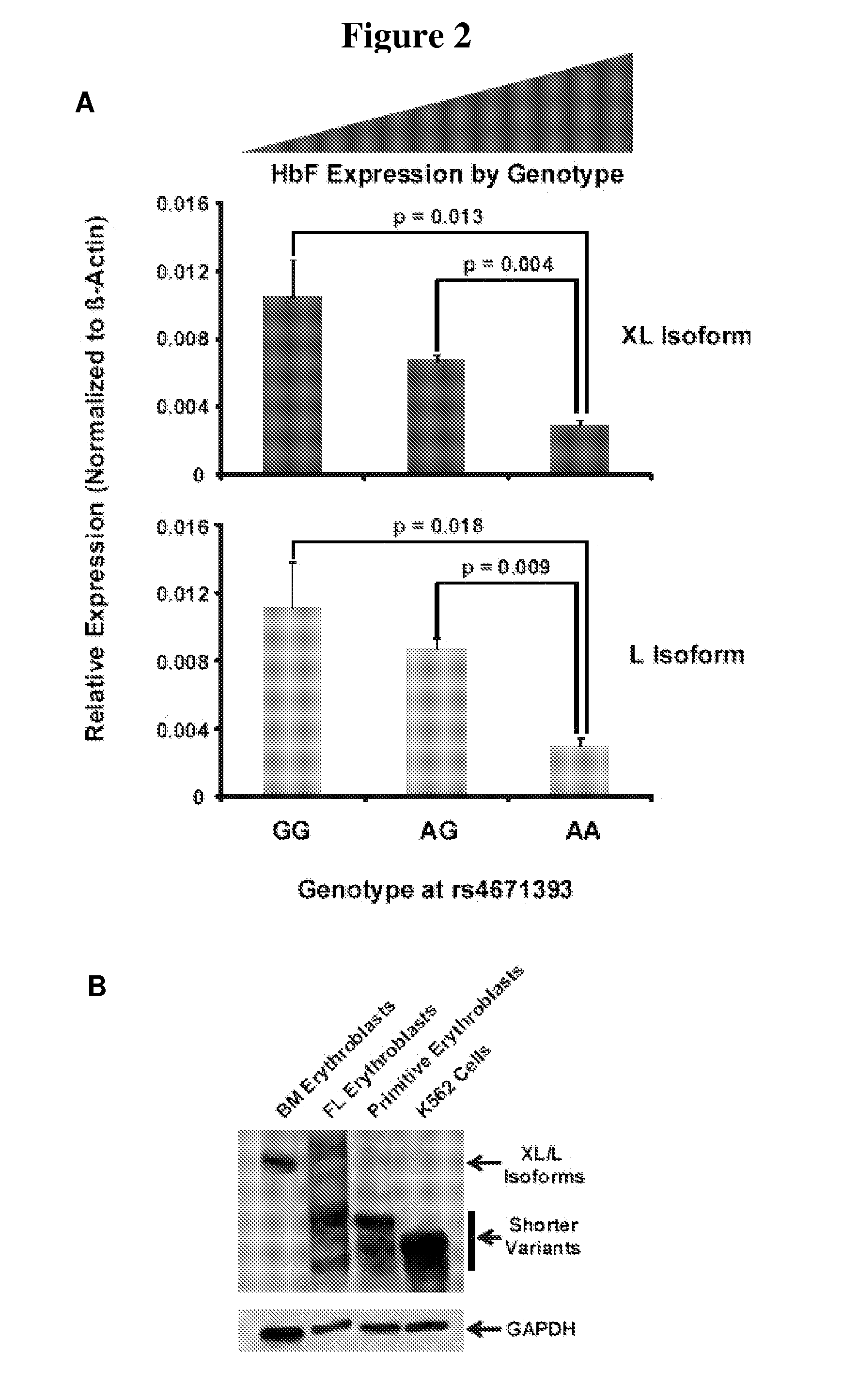
Modulation of bcl11a for treatment of hemoglobinopathies
ActiveUS20110182867A1Increasing fetal hemoglobin levelHigh expressionOrganic active ingredientsVirusesHemoglobinopathyFetal hemoglobin
The invention relates to methods and uses of modulating fetal hemoglobin expression (HbF) in a hematopoietic progenitor cells via inhibitors of BCL11A expression or activity, such as RNAi and antibodies.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
Chimeric receptor genes and cells transformed therewith
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:UNITED STATES OF AMERICA +1
Somatic cell reprogramming
ActiveUS20080233610A1High nucleus to cytoplasm ratioImprove effectivenessNervous disorderVirusesSomatic cellCell biology
The present invention relates to methods for reprogramming a somatic cell to pluripotency by administering into the somatic cell at least one or a plurality of potency-determining factors. The invention also relates to pluripotent cell populations obtained using a reprogramming method.
Owner:WISCONSIN ALUMNI RES FOUND
Polypeptide variants with altered effector function
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com