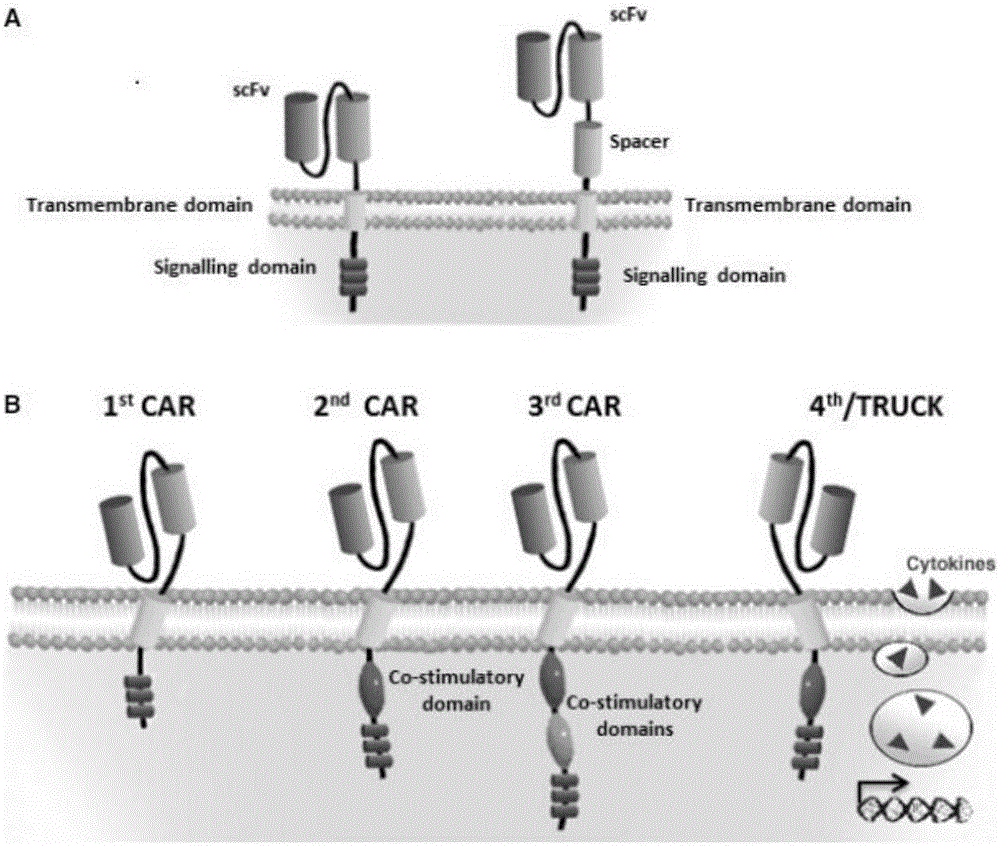
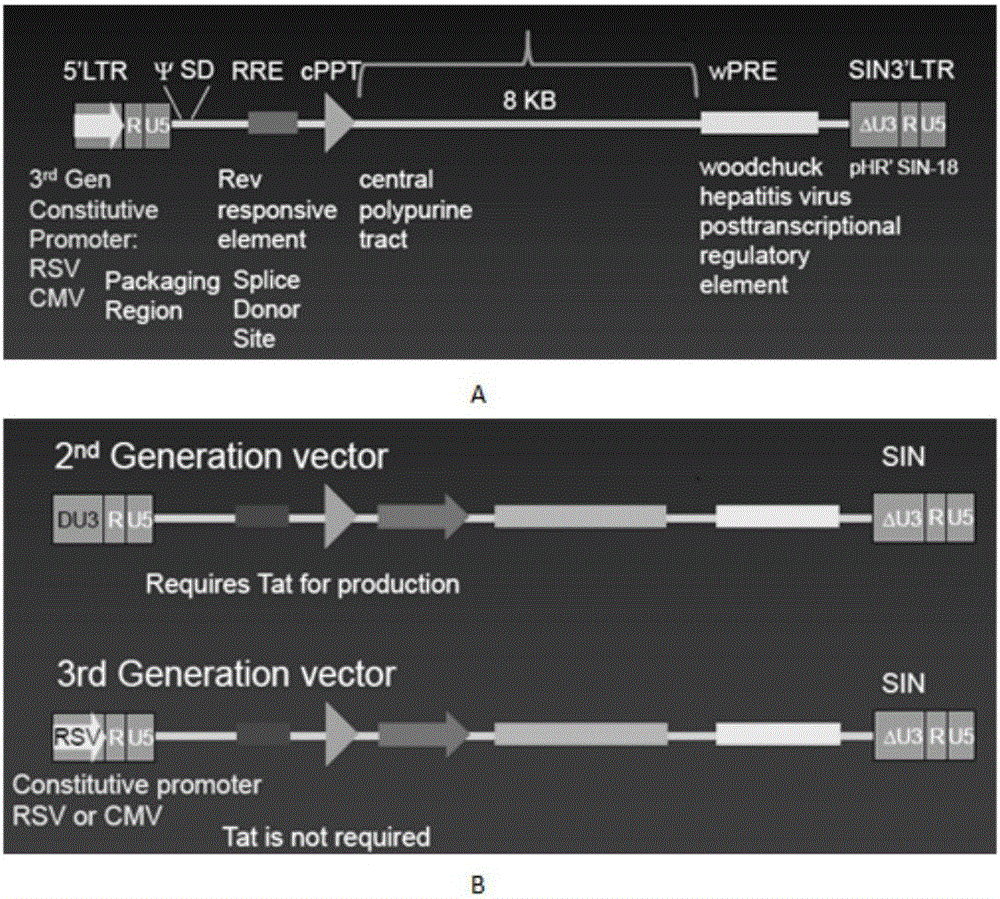
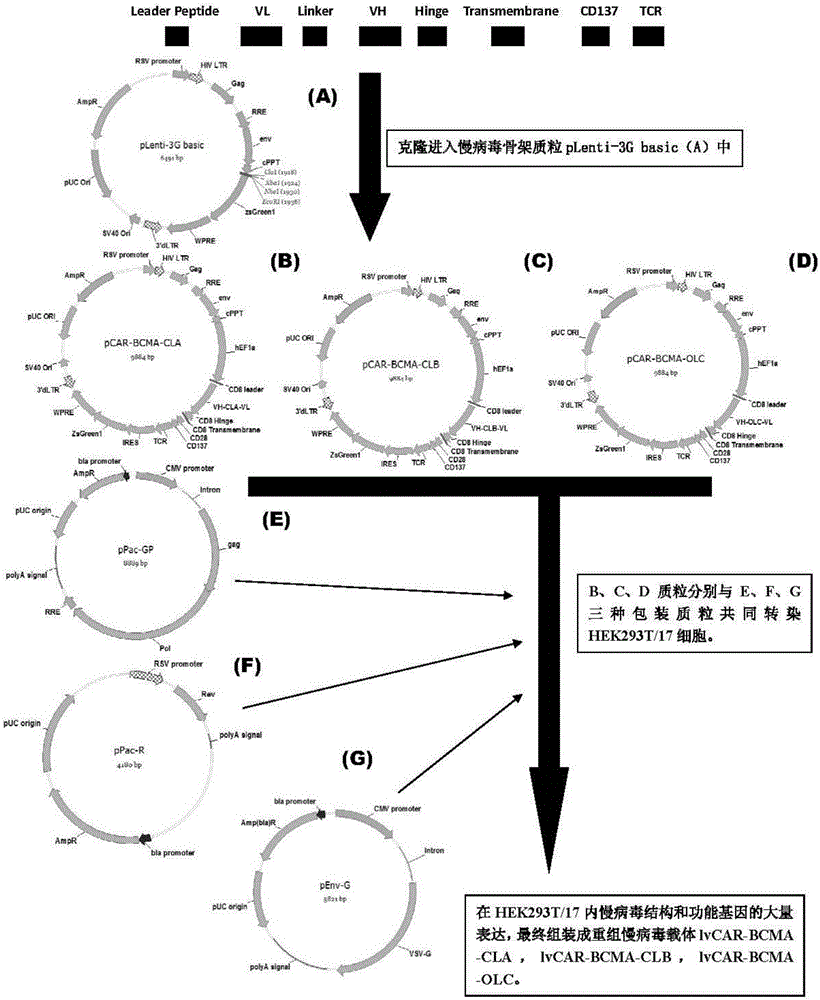
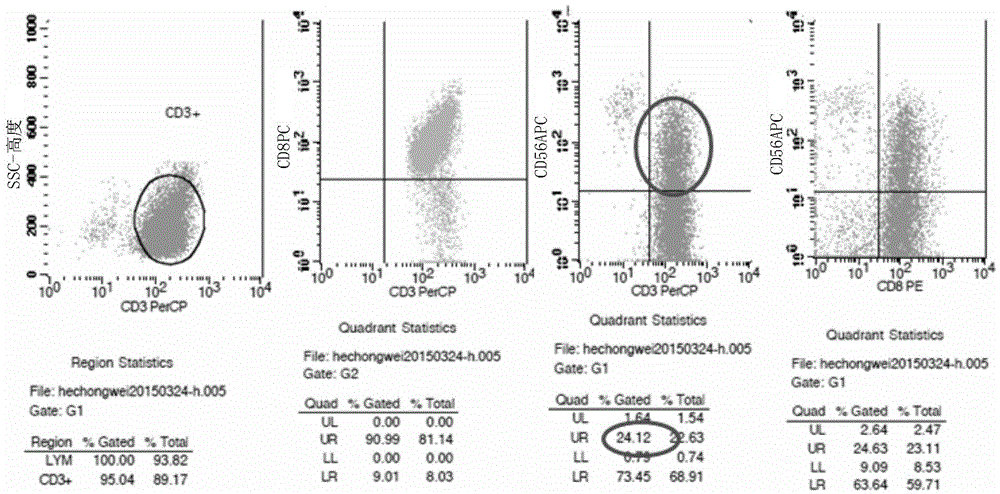
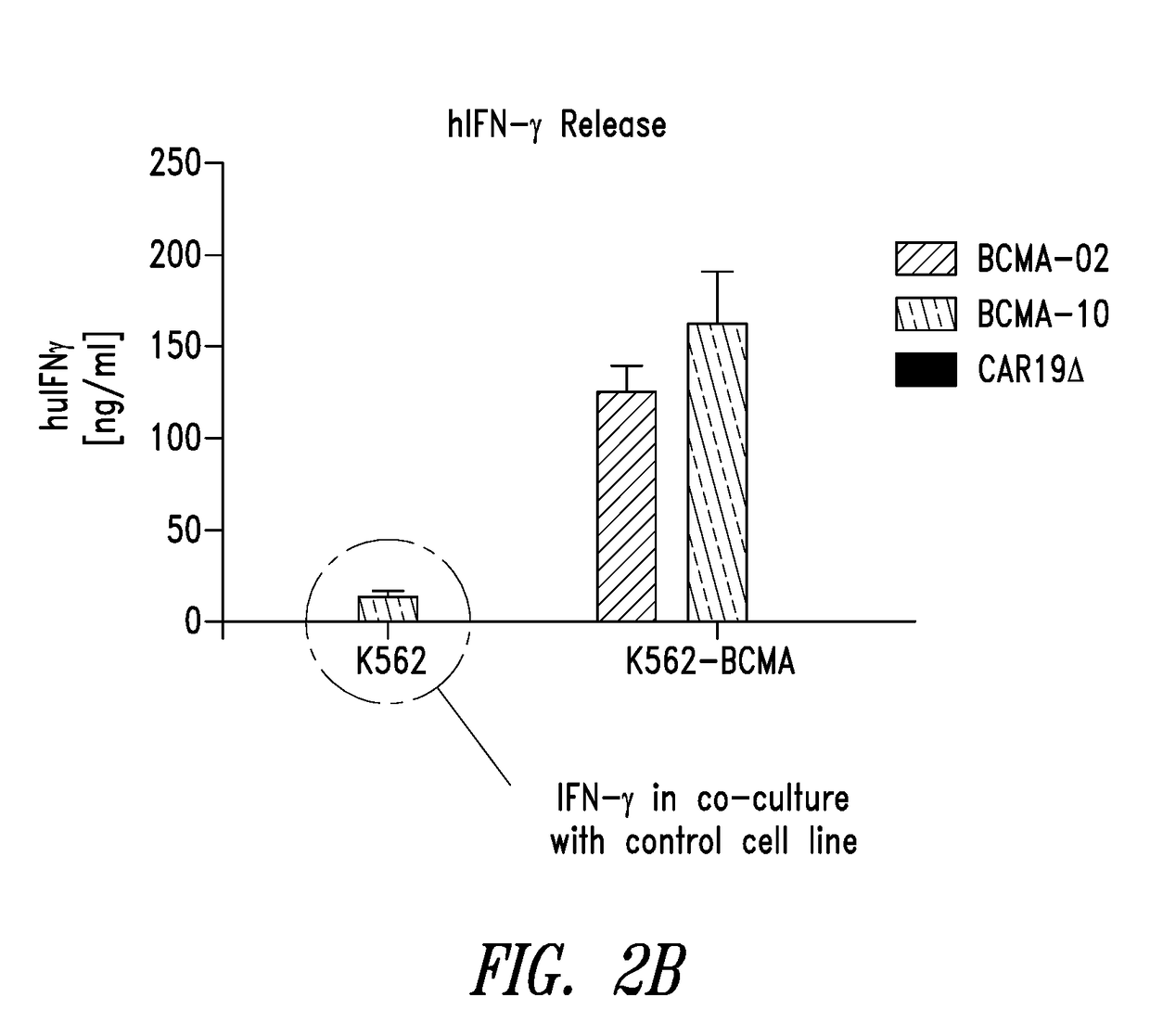
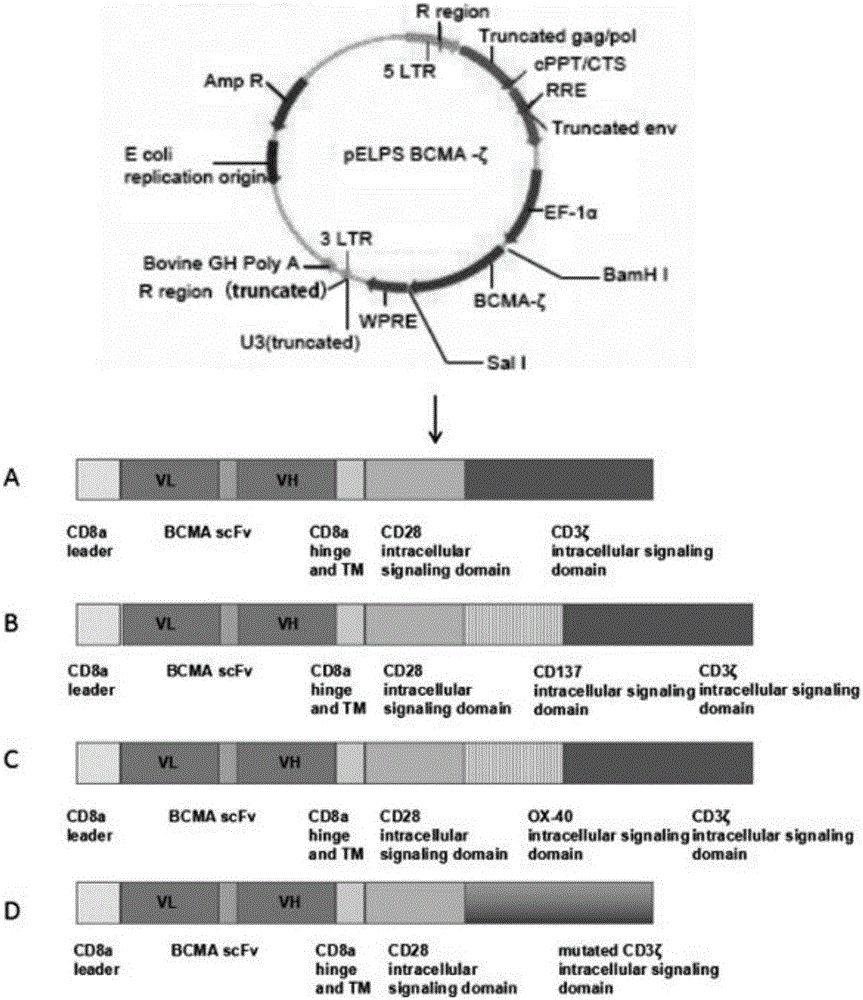
Patents
Literature
1169 results about "Antigen receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
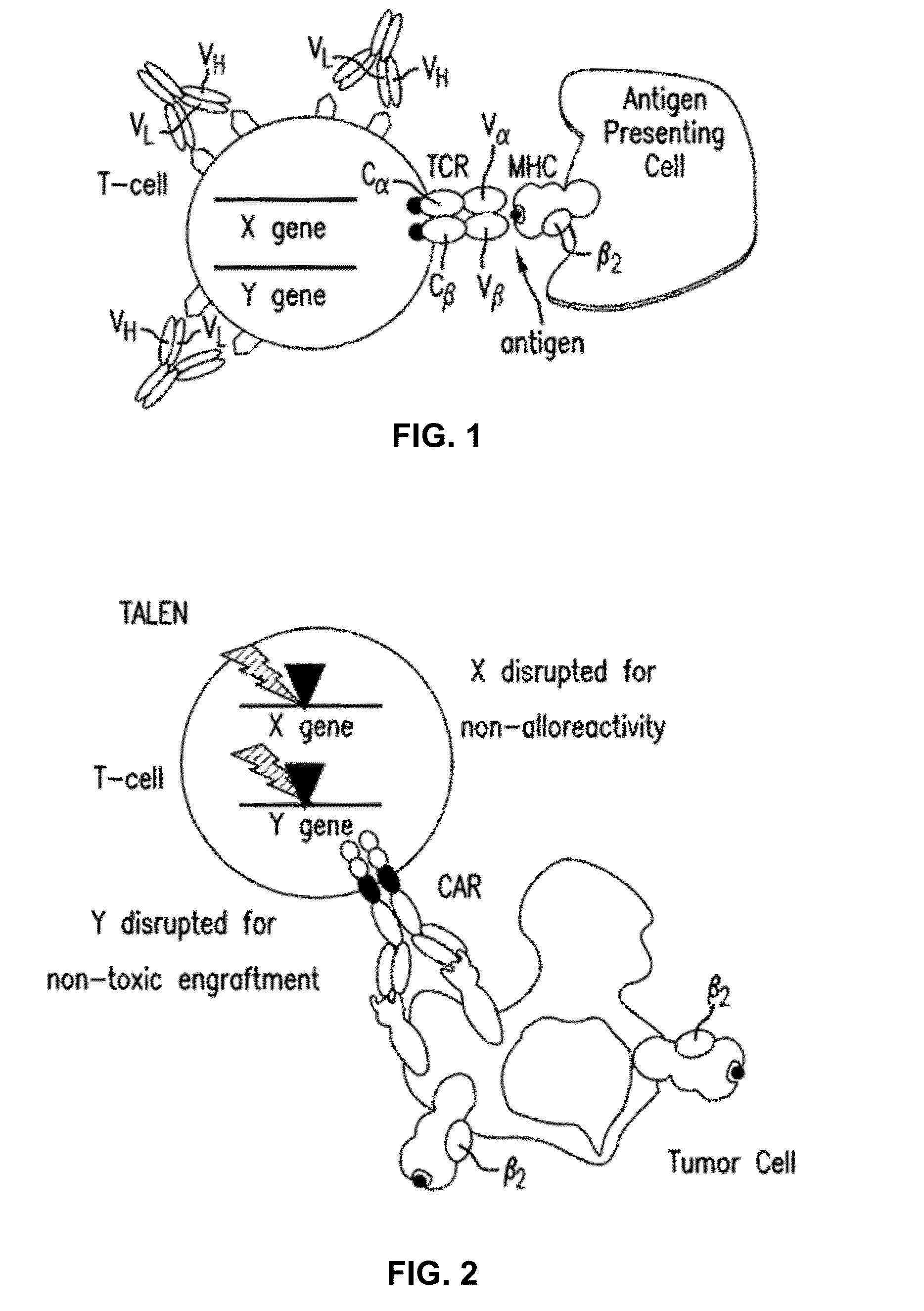
An antigen receptor is basically an antibody protein that is not secreted but is anchored to the B-cell membrane. All antigen receptors found on a particular B cell are identical, but receptors located on other B cells differ.
RNA engineered t cells for the treatment of cancer
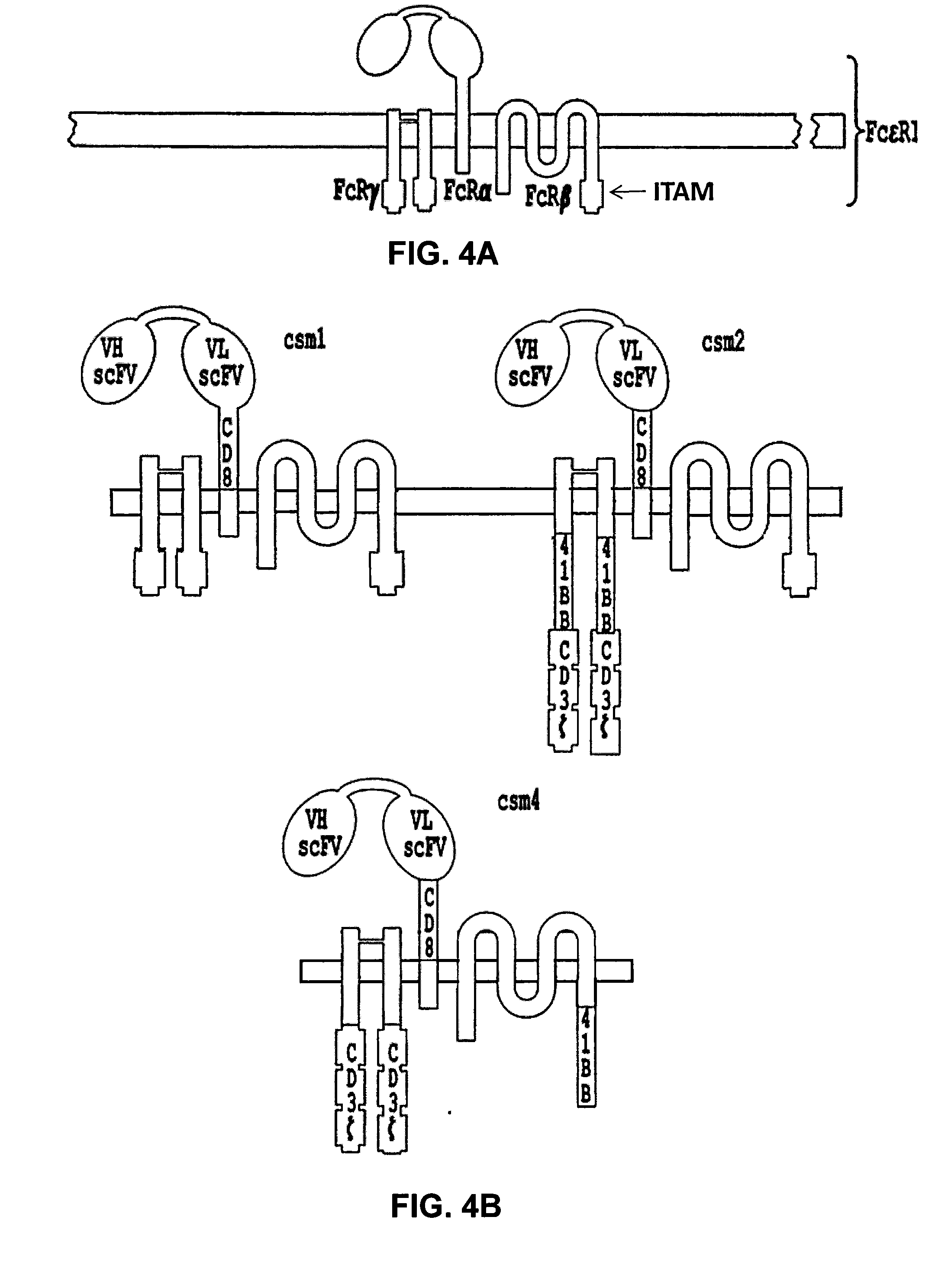
ActiveUS20140227237A1Reduce riskLimited half-lifeBiocideVirusesAntigen receptorChimeric antigen receptor
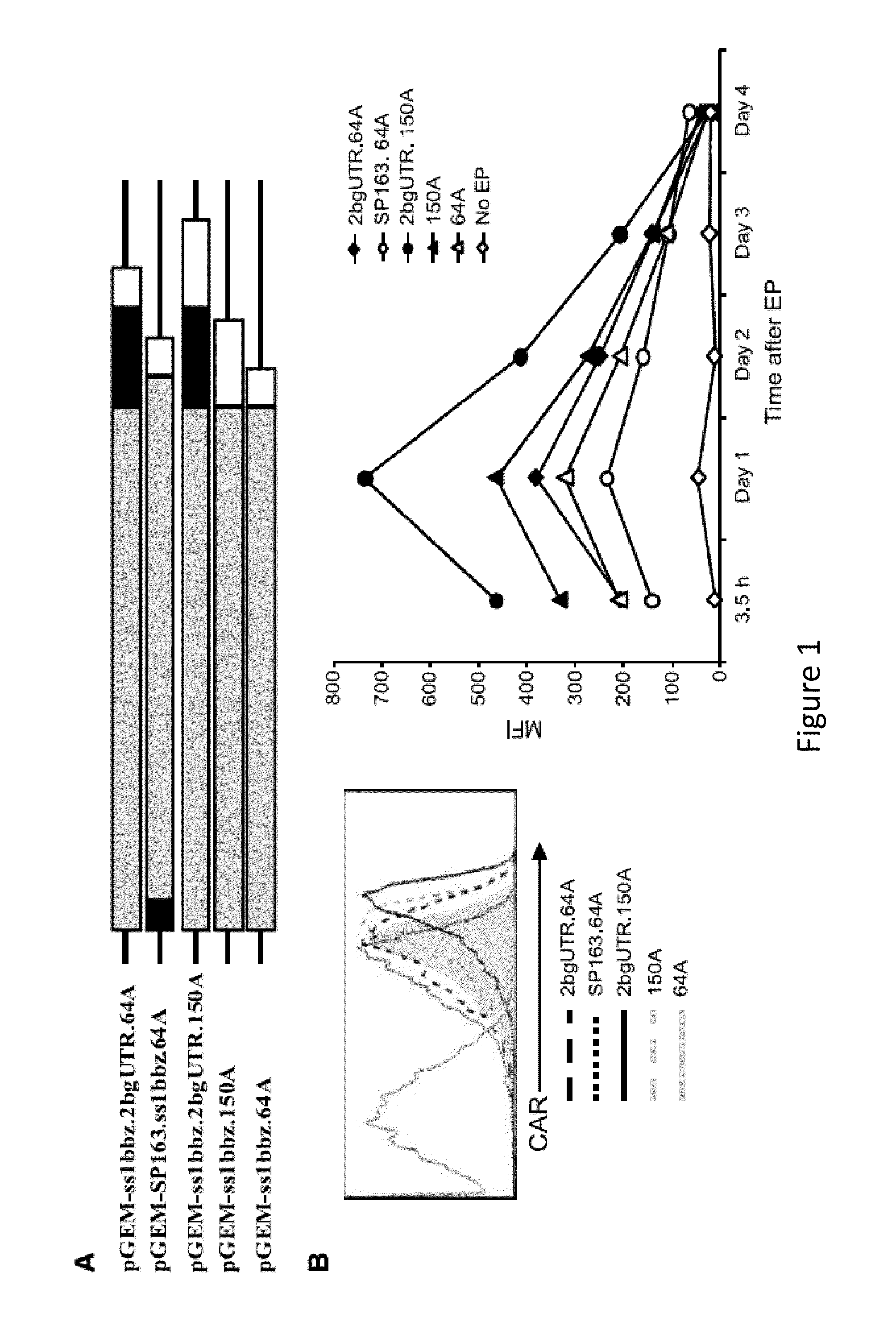
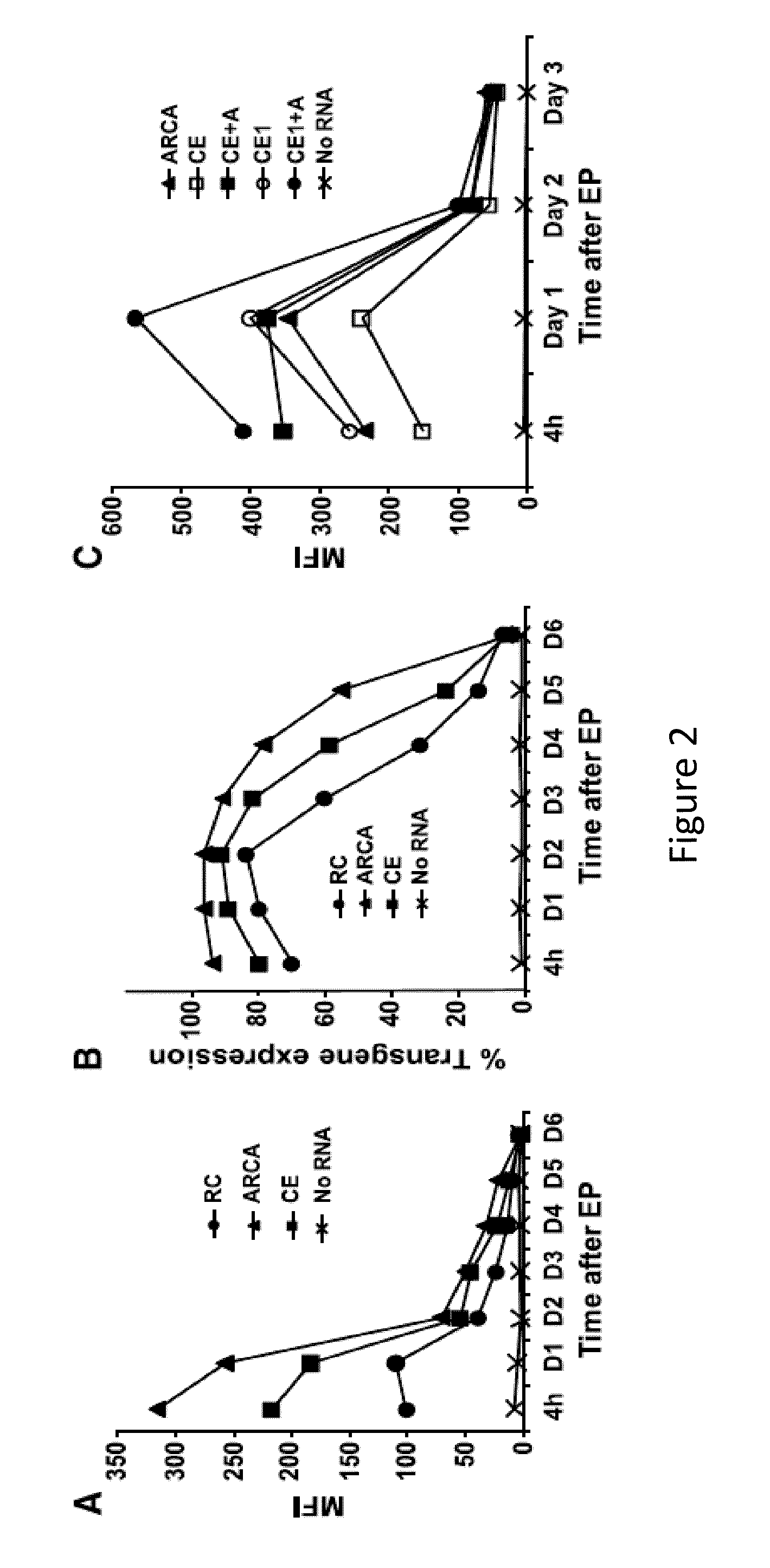
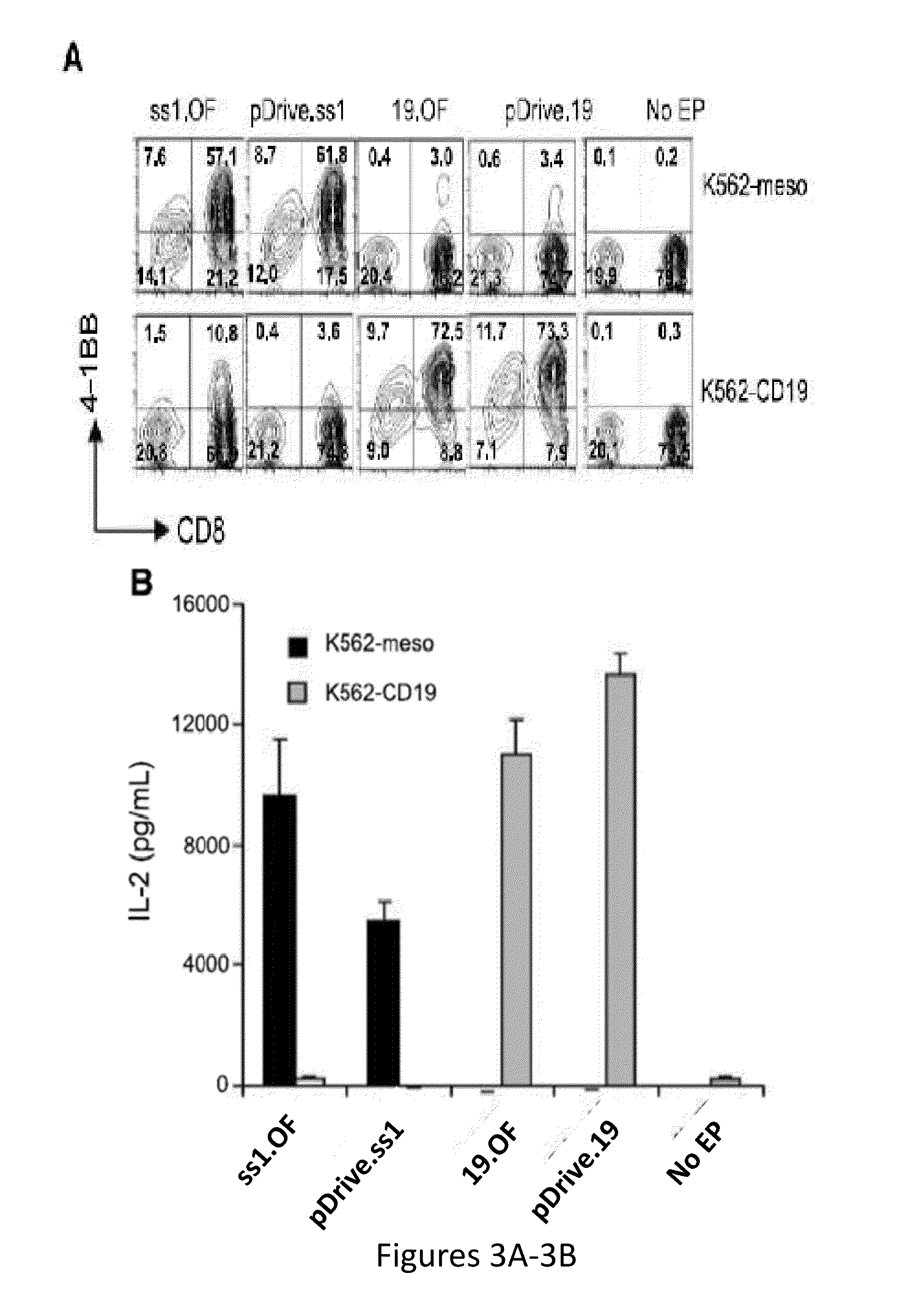
The present invention relates to compositions and methods for generating RNA Chimeric Antigen Receptor (CAR) transfected T cells. The RNA-engineered T cells can be used in adoptive therapy to treat cancer.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Chimeric antigen receptor
ActiveUS20140242701A1High expressionHigh cytotoxic activityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntigen receptor
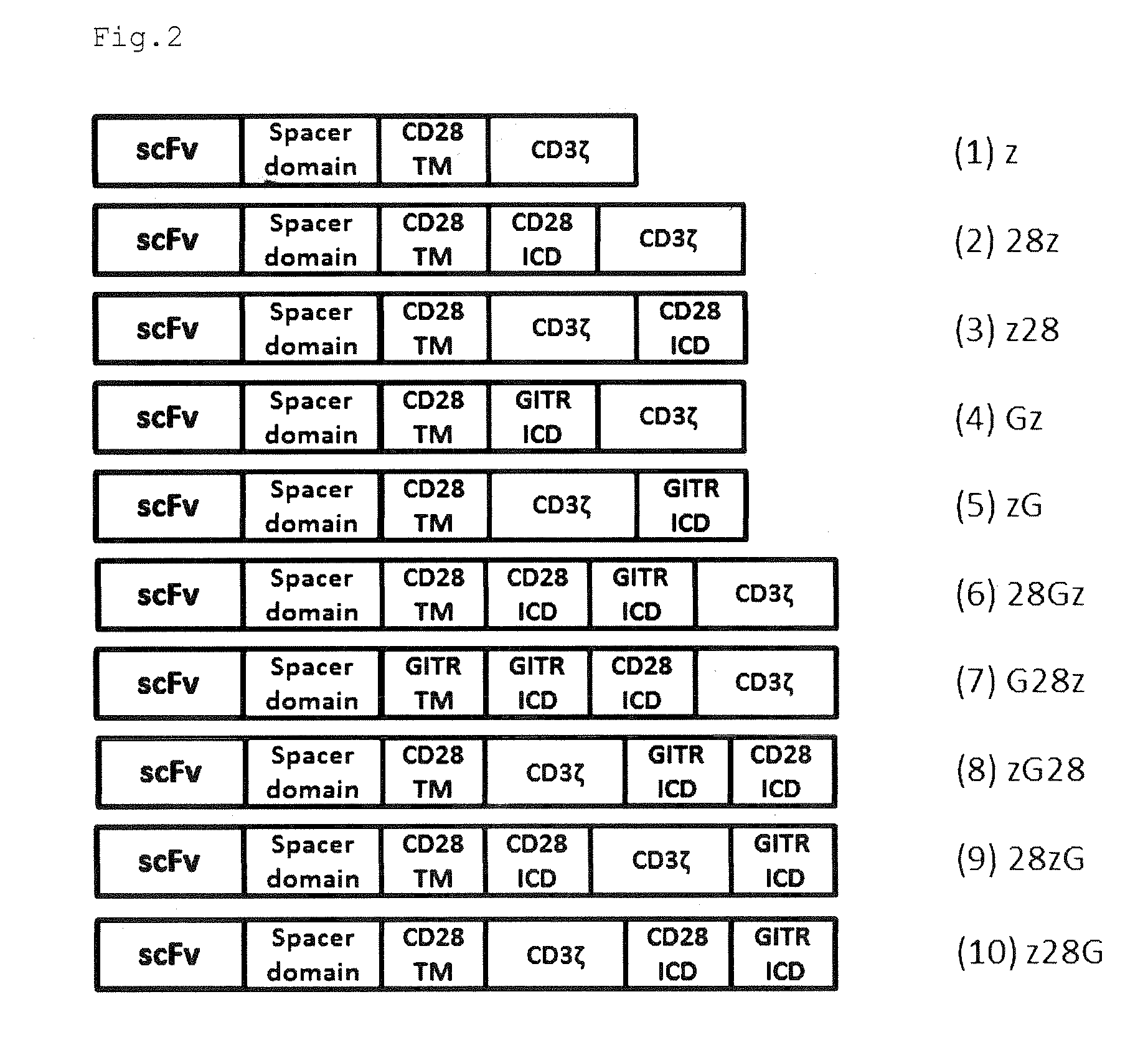
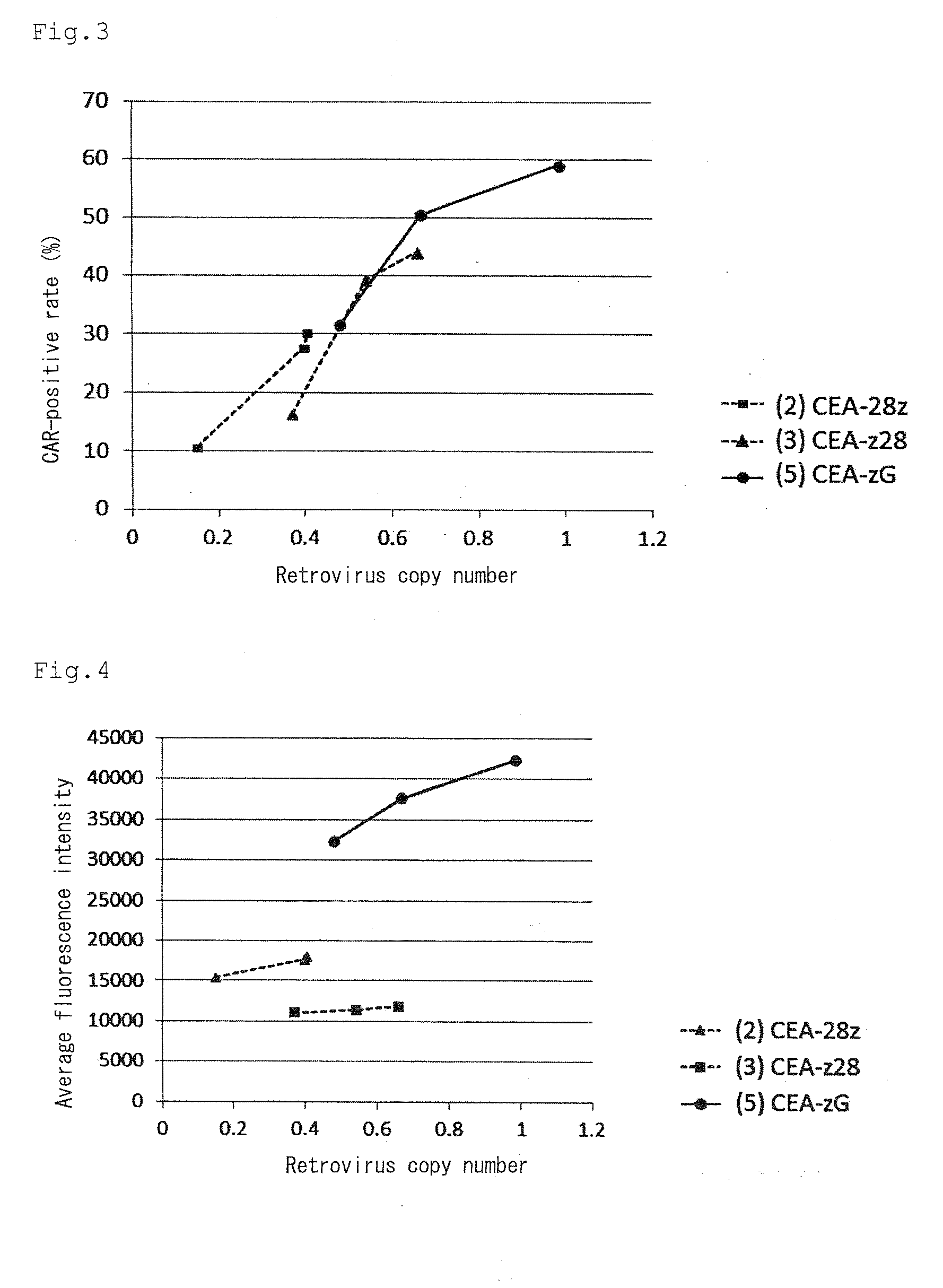
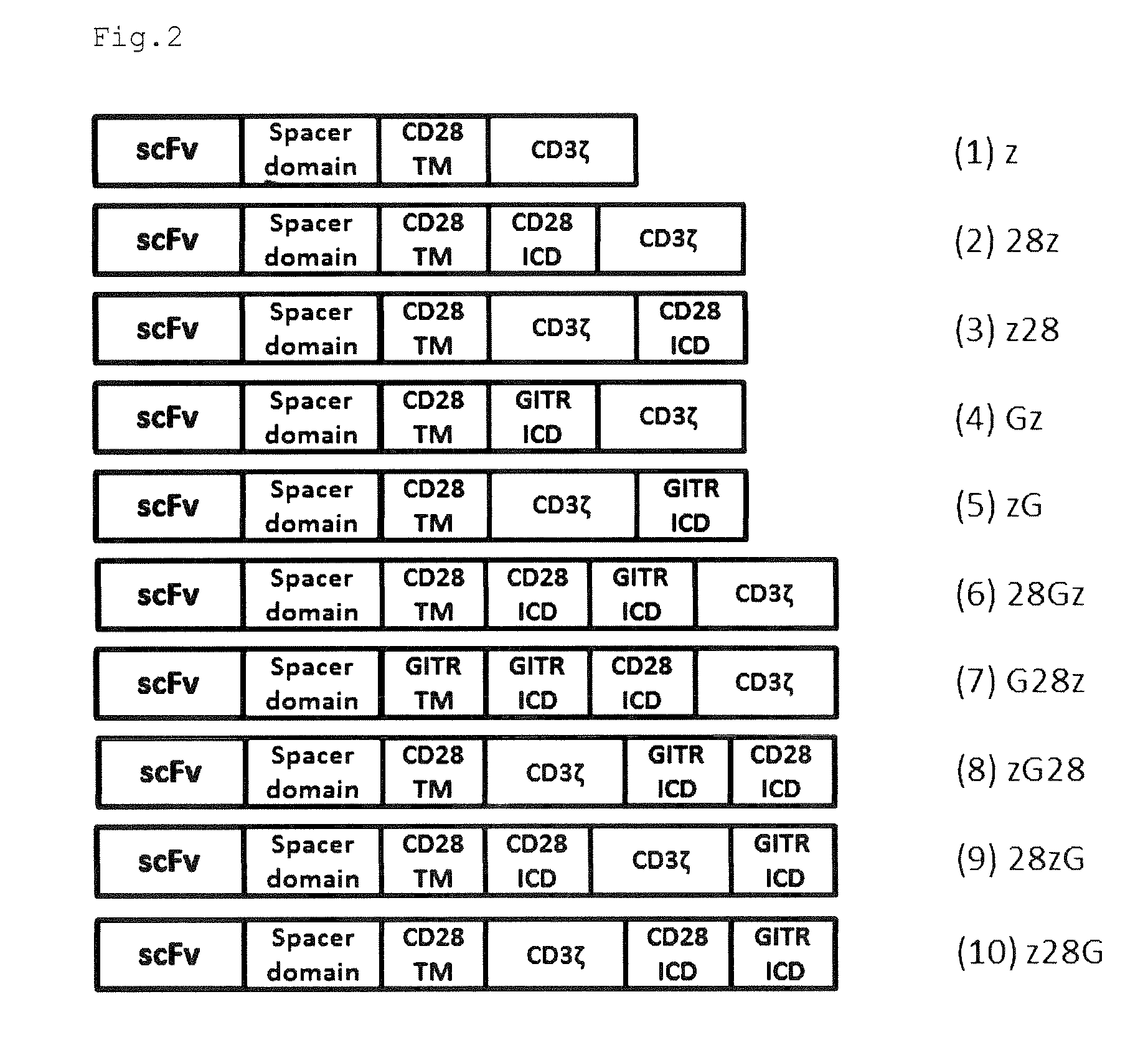
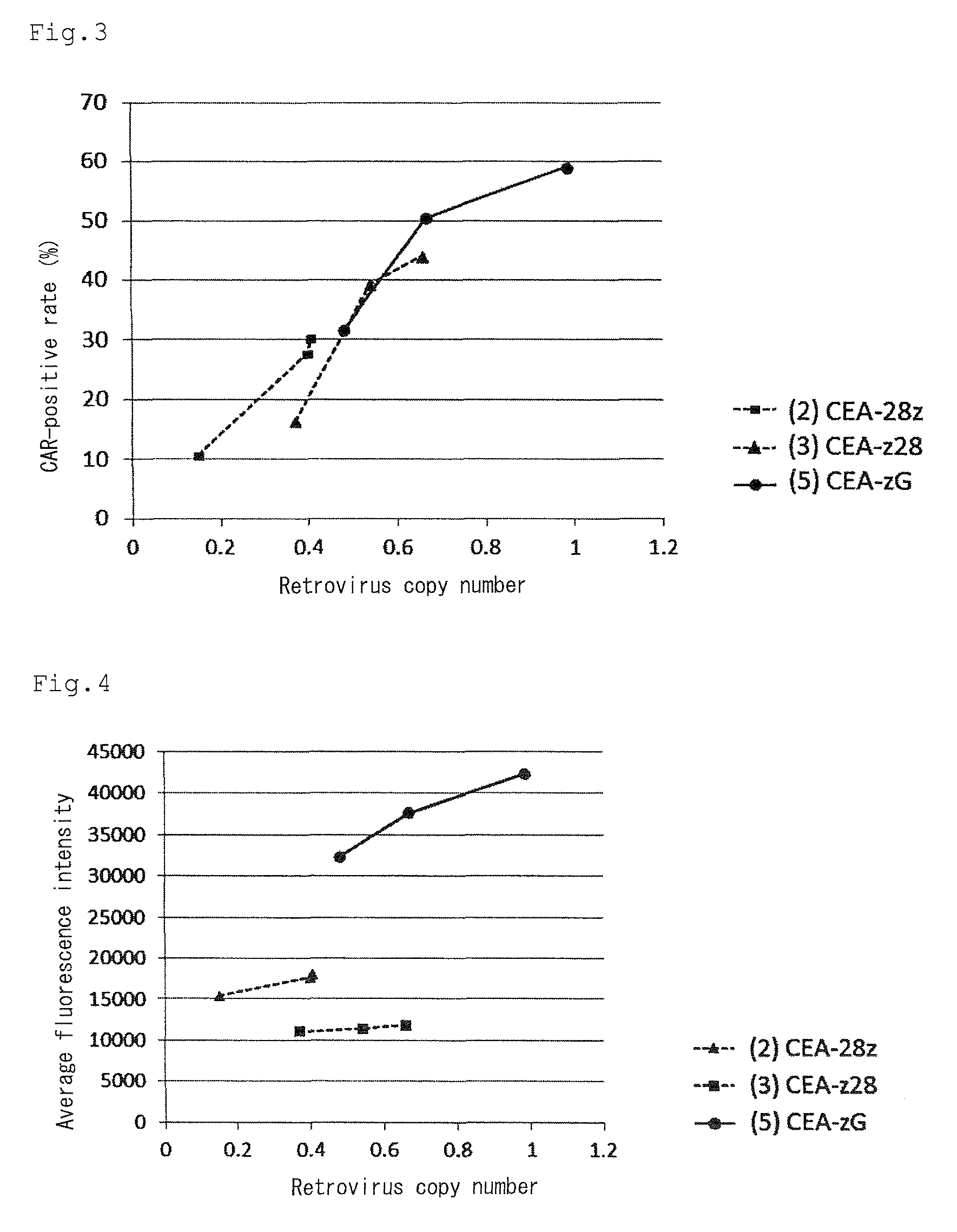
Provided are a chimeric antigen receptor comprising an extracellular domain capable of binding to an antigen, a transmembrane domain and at least one intracellular domain, the chimeric antigen receptor being characterized in that an intracellular domain of a glucocorticoid-induced tumor necrosis factor receptor (GITR) is contained as the intracellular domain; a nucleic acid encoding the chimeric antigen receptor; a cell expressing the chimeric antigen receptor; and a method for producing the cell.
Owner:MIE UNIVERSITY
Multi-Chain Chimeric Antigen Receptor and Uses Thereof
ActiveUS20140134142A1Modulate activityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorReceptor
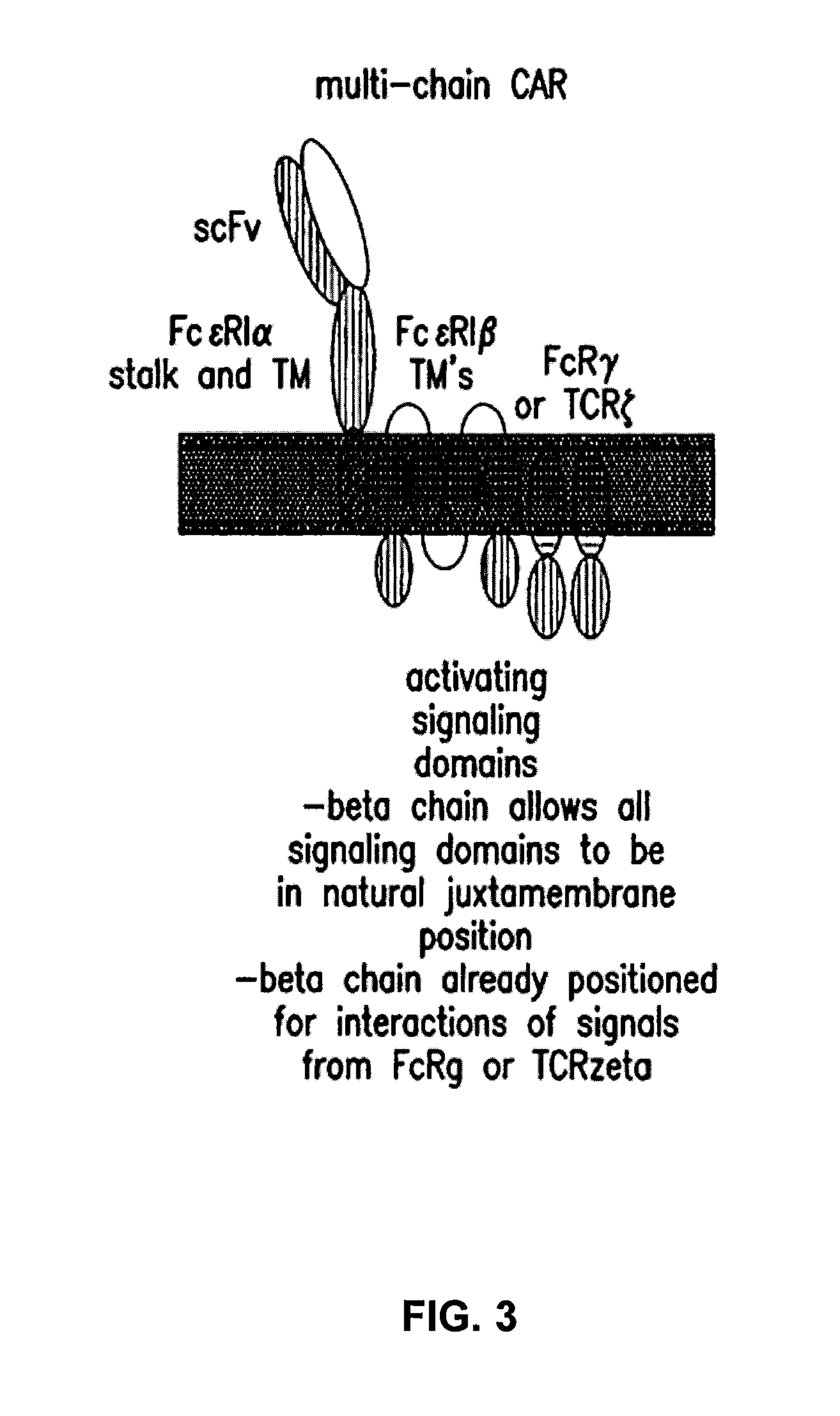
The present invention relates to a new generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi-chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
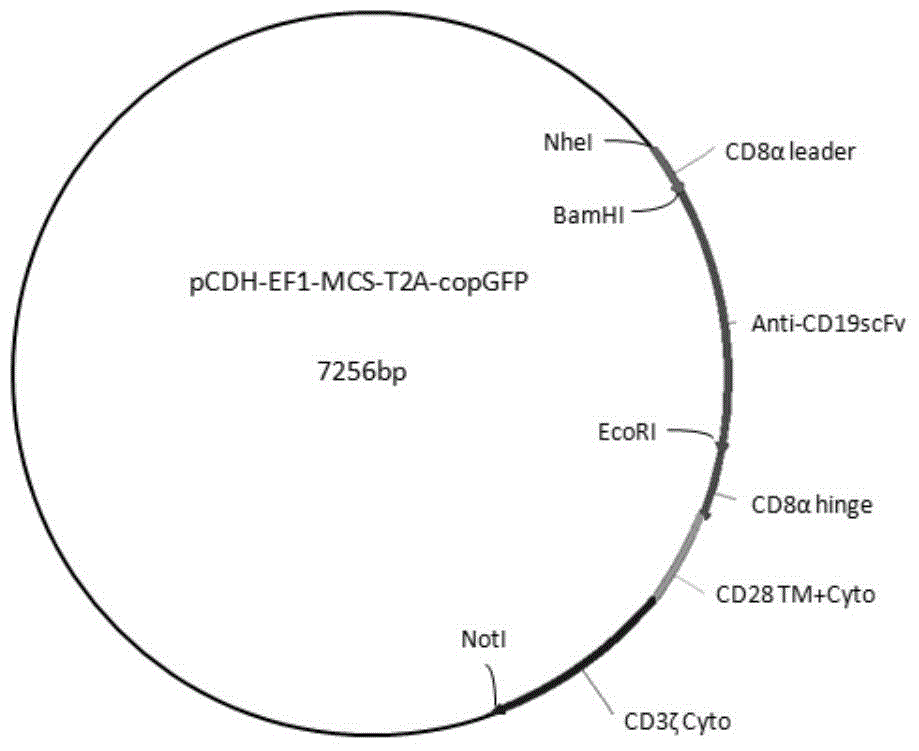
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
Chimeric antigen receptor and methods of use thereof
The present disclosure provides a heterodimeric, conditionally active chimeric antigen receptor (CAR), and a nucleic acid comprising a nucleotide sequence encoding the CAR. The present disclosure provides cells genetically modified to produce the CAR. A CAR of the present disclosure can be used in various methods, which are also provided.
Owner:RGT UNIV OF CALIFORNIA
Fully human, Anti-mesothelin specific chimeric immune receptor for redirected mesothelin-expressing cell targeting
The present invention relates to compositions and methods for treating diseases, disorders or conditions associated with dysregulated expression of mesothelin. In one embodiment, the invention relates to a fully human chimeric antigen receptor (CAR) wherein the CAR is able to target mesothelin.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
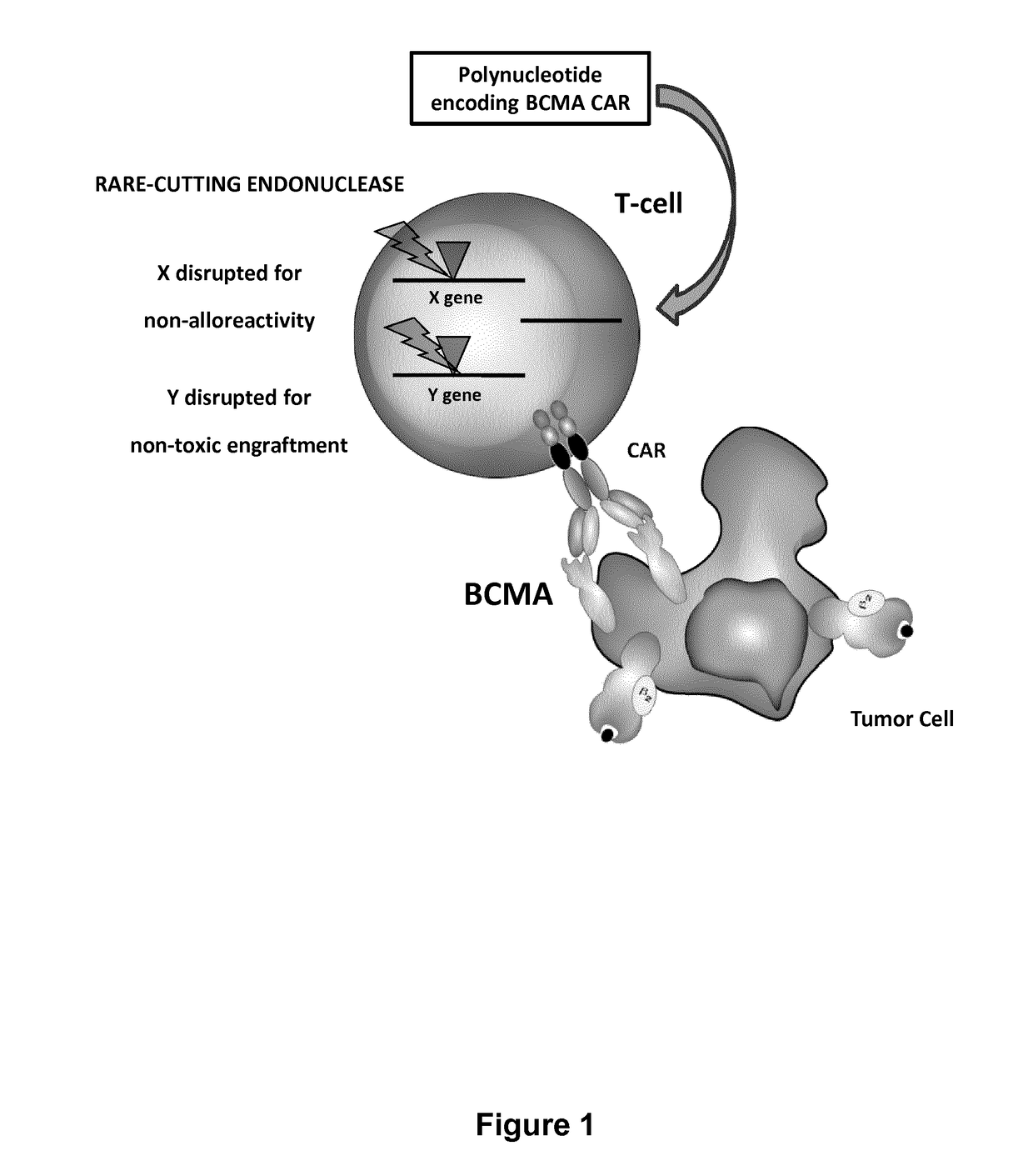
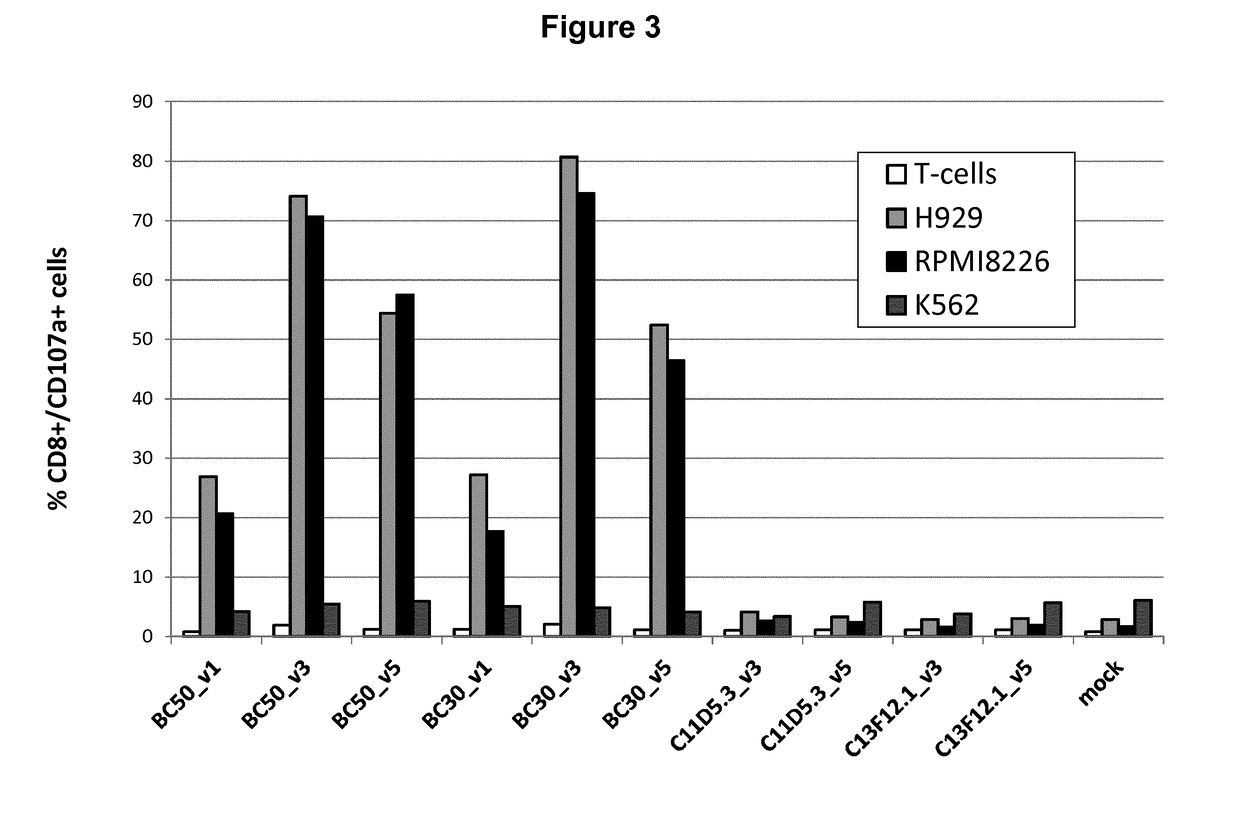
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
Chimeric antigen receptor with stable antigen binding units, method for preparing chimeric antigen receptor and application thereof
ActiveCN104829733APeptide/protein ingredientsPharmaceutical non-active ingredientsTumor targetAntigen receptors
The invention relates to a chimeric antigen receptor with stable antigen binding units. The chimeric antigen receptor comprises the extracellular antigen binding units, trans-membrane domains and intracellular co-stimulation signal domains. The antigen binding units can be stably expressed as compared with specific tumor targets and comprise heavy chains and light chains which are connected with one another by hinges of SEQ ID NO.2 (sequence identity number.2) amino acid sequence codes. The invention further relates to cells for expressing the receptor and application of the receptor.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Compositions and methods for immunotherapy
ActiveUS20160045551A1High activityAntibacterial agentsImmunological disordersAntigen receptorAntigen
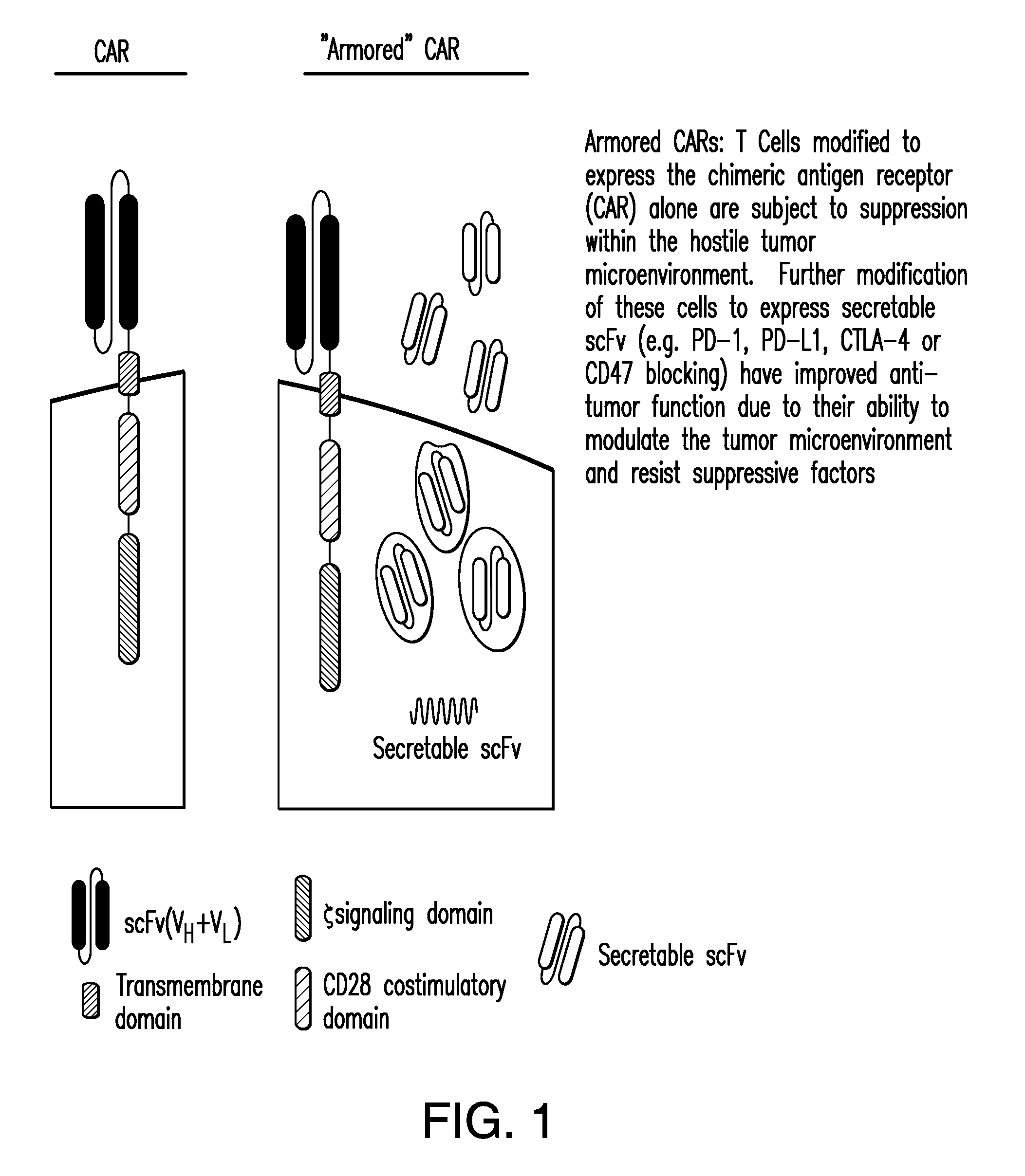
The present invention provides for methods and compositions for enhancing the immune response toward cancers and pathogens. It relates to immunoresponsive cells bearing antigen receptors, which can be chimeric antigen receptors (CARS), which express introduced ligands for immunomodulatory molecules. In particular embodiments, engineered immunoresponsive cells are antigen-directed and resist immunosuppression and / or have enhances immune-activating properties.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
M971 chimeric antigen receptors
ActiveUS20150299317A1Increase of tumor burdenBacteriaAntibody mimetics/scaffoldsPopulationAntigen binding
The invention provides a chimeric antigen receptor (CAR) comprising an antigen binding domain comprising SEQ ID NOs: 1-6, a transmembrane domain, and an intracellular T cell signaling domain. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Engineered CD20 targeting NKT cell and its preparation method and application
ActiveCN103820393AHigh transfection rateProlong survival timePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Tumor antigen
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Chimeric antigen receptor-targeting monoclonal antibodies
ActiveUS20160096902A1Easy to understandBacteriaImmunoglobulinsMonoclonal antibodyChimeric antigen receptor
Provided are monoclonal antibodies that detect CD 19 CAR-modified immune cells and CAR-modified immune cells irrespective of the tumor associated antigen they target. Methods of using these functional monoclonal antibodies include, but are not limited to, detection, quantification, activation, and selective propagation of CAR-modified immune cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
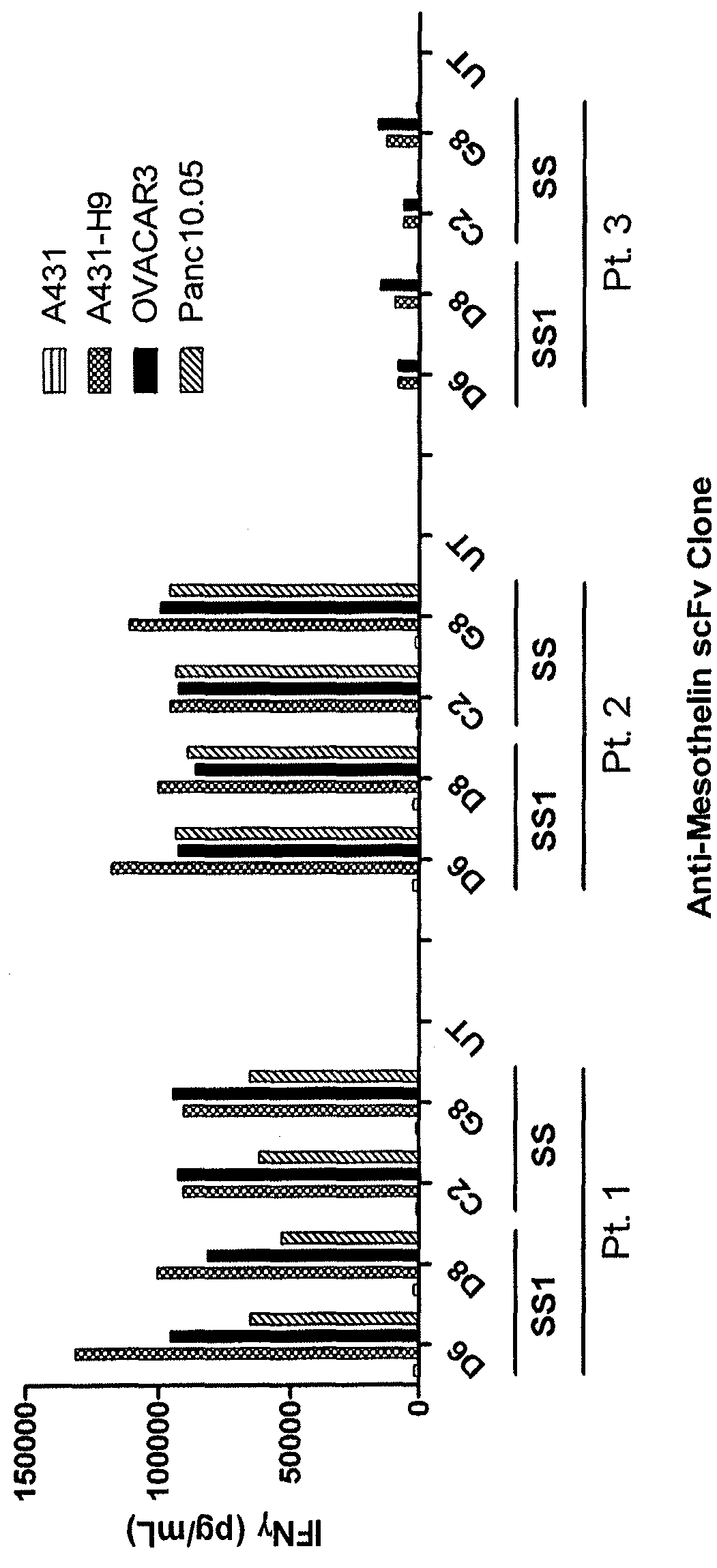
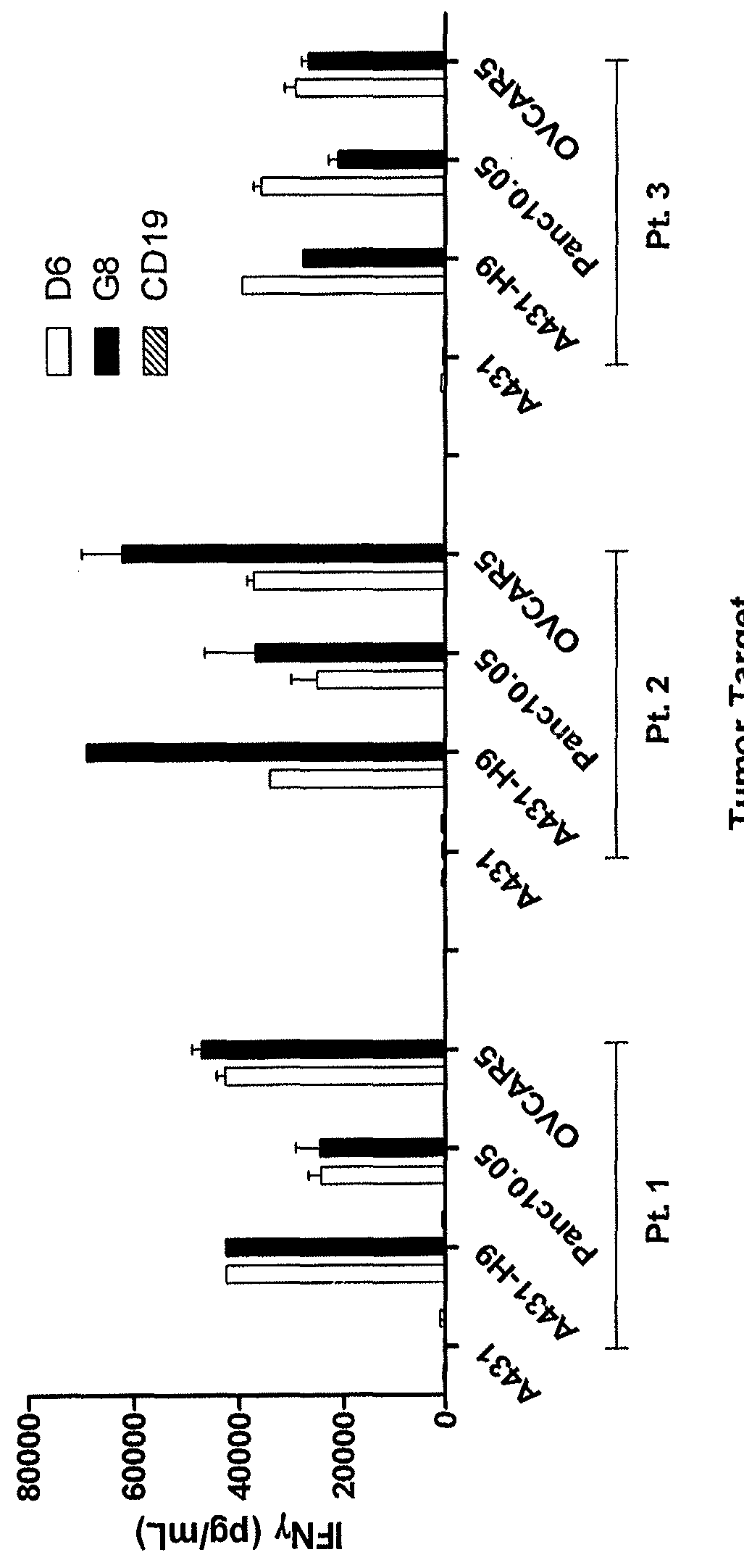
Anti-mesothelin chimeric antigen receptors
The invention provides a chimeric antigen receptor (CAR) (a) an antigen binding domain of HN1 or SS, a transmembrane domain, and an intracellular T cell signaling domain, or (b) an antigen binding domain of SS1, a transmembrane domain, an intracellular T cell signaling domain, and a granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor 2 leader. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Methods of preparation and composition of peptide constructs useful for treatment of autoimmune and transplant related host versus graft conditions
InactiveUS20060257420A1Effectively eliminate set and subsetTreating or preventing inappropriate autoimmune responsePeptide/protein ingredientsImmunoglobulinsAutoimmune diseaseApoptosis
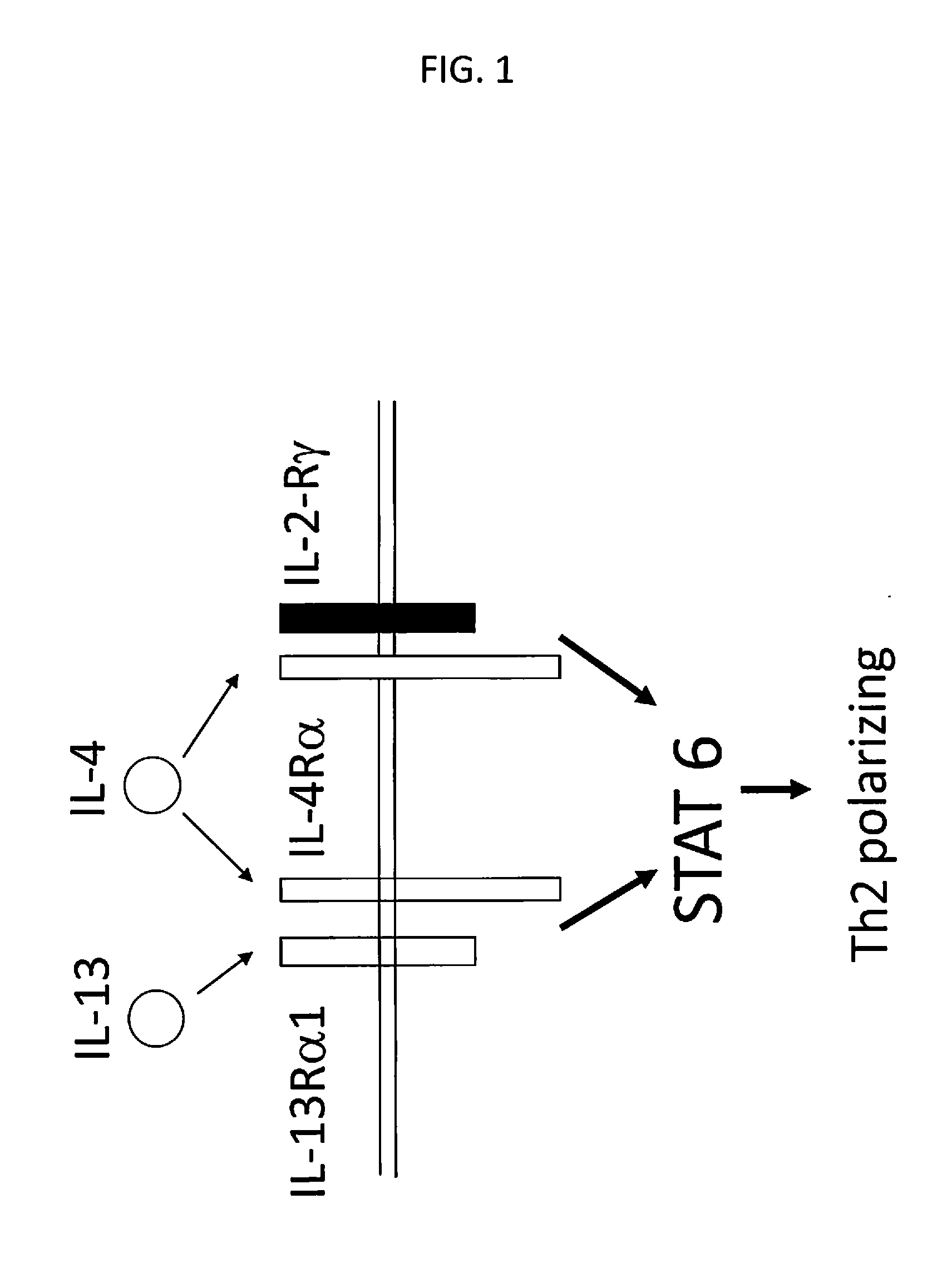
The invention is related to peptide constructs, i.e., polypeptides obtained by linking together two or more peptides based on or derived from different molecules, which are useful in the treatment or prevention of autoimmune diseases, asthma, allergies, and host versus graft (or graft versus host) rejection, as well as to compositions containing same, methods for producing same and methods for using same; wherein the peptide constructs have the formula P1-x-P2 where P1 is a peptide associated with autoimmune disease, allergy, asthma, host-versus-graft rejection, myocarditis, diabetes, and immune-mediated disease, which binds to an antigen receptor on a set or subset of T cells; P2 is a peptide which will cause a Th2 directed immune response by the set or subset of T cells to which the peptide P1 is attached or which will bind to a T cell receptor which will cause the set or subset of T cells to which the peptide P1 is attached to initiate, but not complete, an immune response causing the set or subset of T cells to undergo anergy and apoptosis; and x is a direct bond or linker for covalently bonding P1 and P2.
Owner:CEL SCI CORP
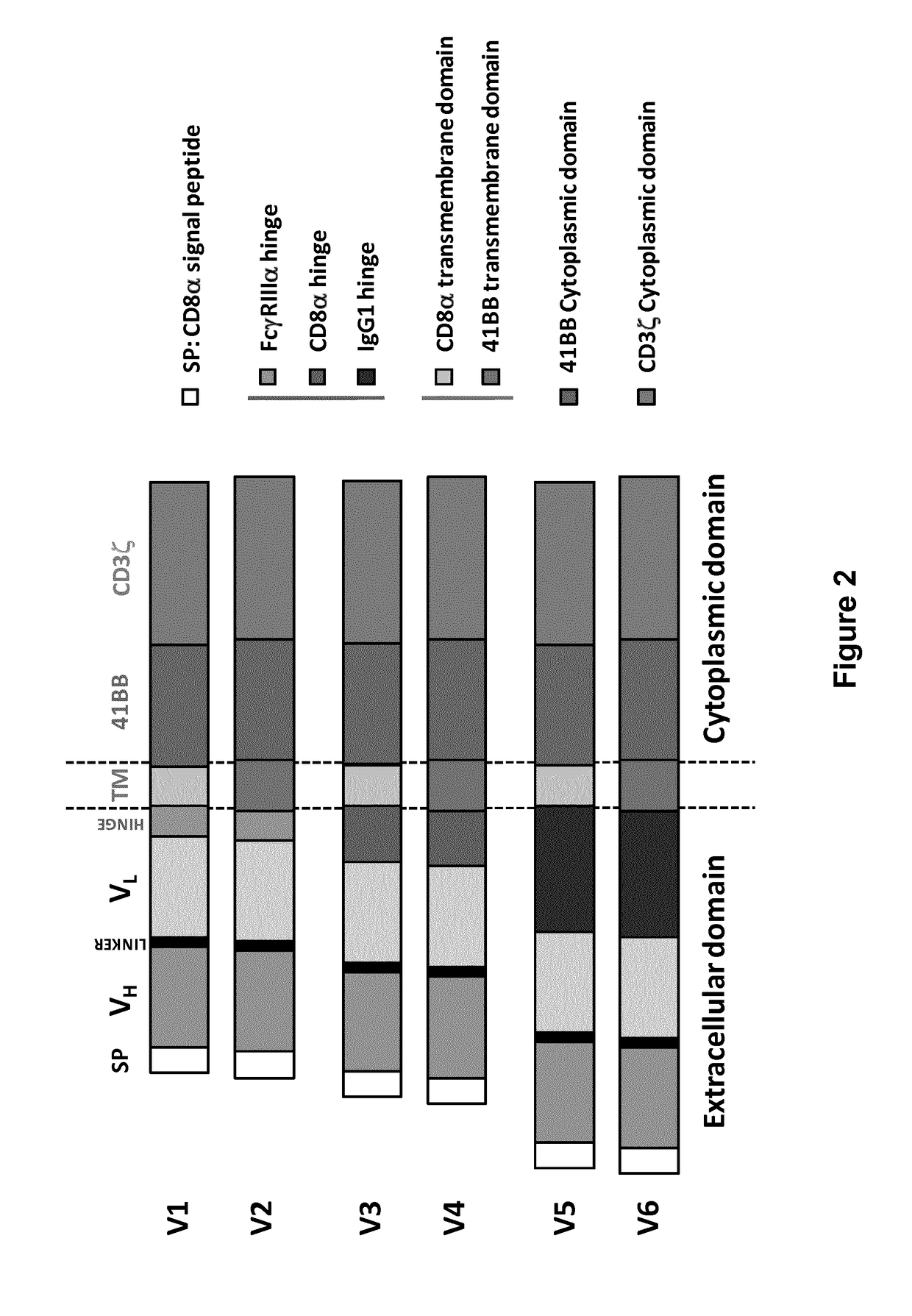
Anti-BCMA chimeric antigen receptor, encoding gene, recombinant expression vector and establishing method and application of anti-BCMA chimeric antigen receptor, encoding gene and recombinant expression vector
ActiveCN105777911AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalSingle-Chain Antibodies
The invention discloses an anti-BCMA chimeric antigen receptor, an encoding gene, a recombinant expression vector and an establishing method and application of the anti-BCMA chimeric antigen receptor, the encoding gene and the recombinant expression vector. The receptor comprises a CD8 leader chimeric receptor signal peptide, a BCMA single-chain antibody heavy chain VH, an Optimal Linker C, a BCMA single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulatory factor and a TCR chimeric receptor T cell activating domain which are sequentially connected in series. In addition, the invention further discloses the encoding gene and the recombinant expression vector of the anti-BCMA chimeric antigen receptor and the establishing method and application of the encoding gene and the recombinant expression vector. The secretion of cell factors and the cytotoxicity in vitro of CAR-T cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Chimeric antigen receptor dendritic cell (car-dc) for treatment of cancer
InactiveUS20170151281A1High proliferation rateIncrease productionPolypeptide with localisation/targeting motifPeptide/protein ingredientsIntracellular signallingDendritic cell
The current invention provides monocytic cells transfected with chimeric antigen receptor (CAR) to selectively home to tumors and upon homing differentiate into dendritic cells capable of activating immunity which is inhibitory to said tumor. In one embodiment of the invention, monocytic cells are transfected with a construct encoding an antigen binding domain, a transcellular or structural domain, and an intracellular signaling domain. In one specific aspect of the invention, the antigen binding domain interacts with sufficient affinity to a tumor antigen, capable of triggering said intracellular domain to induce an activation signal to induce monocyte differentiation into DC.
Owner:MYELOID THERAPEUTICS INC
CD19 targeting chimeric antigen receptor and NKT cell, and preparation method thereof and applications thereof
ActiveCN105418765AEnhance specific killing activityProlong survival timePeptide/protein ingredientsGenetic material ingredientsAntigenHinge region
The present invention discloses a chimeric antigen receptor, a gene and a recombinant expression vector thereof, an engineered CD19 targeting NKT cell and applications thereof. The chimeric antigen receptor is CD19ScFv-CD8-CD137-CD3 zeta, and consists of a hinge region and a transmembrane region of the CD19ScFv and CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta, and the the hinge region, the transmembrane region, the intracellular signal structural domain of CD137 and the intracellular signal structural domain of CD3 zeta are connected in series. When used to treat advanced stage CD19-positive B-cell acute lymphocytic leukemia, the NKT cell modified by the chimeric antigen receptor CD19ScFv-CD8-CD137-CD3 zeta provided by the present invention has good specific killing activity on leukemic cells, and has certain therapeutic effect on advanced stage CD19-positive B-cell acute lymphocytic leukemia patients who are repeatedly subjected to therapy such as radiotherapy, chemotherapy and symptomatic therapy by other drugs but cannot recover obviously.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
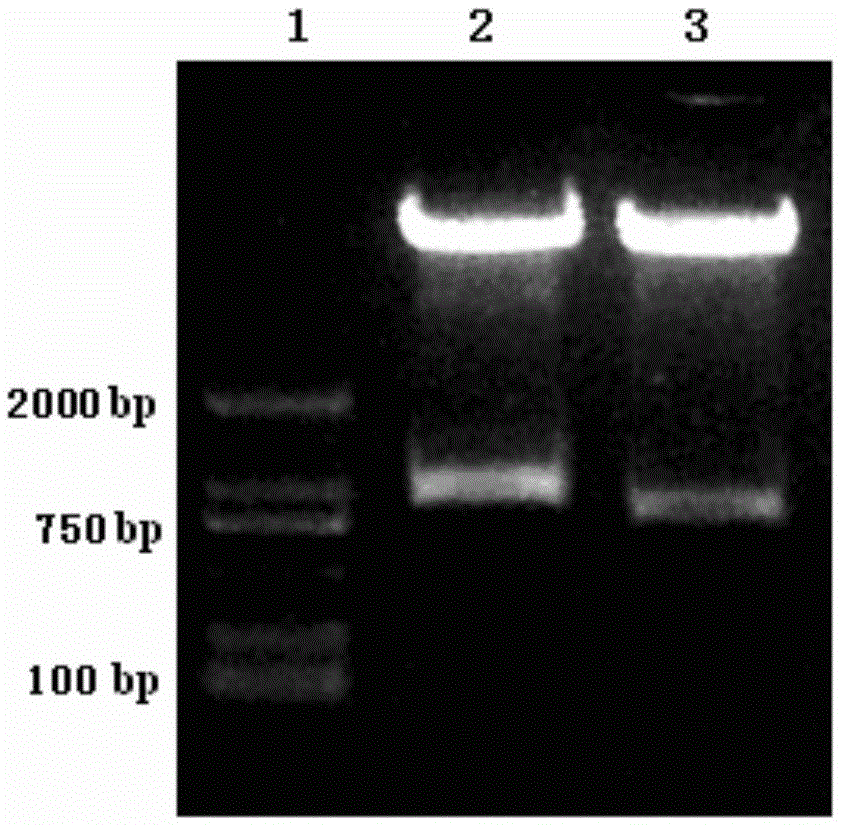
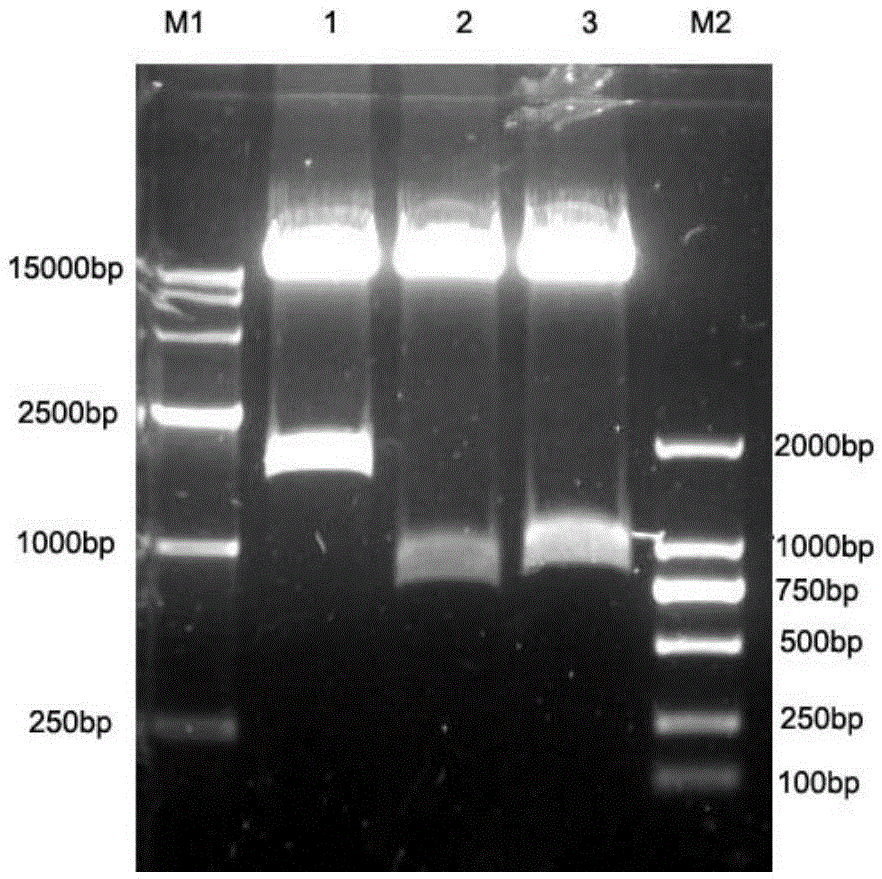
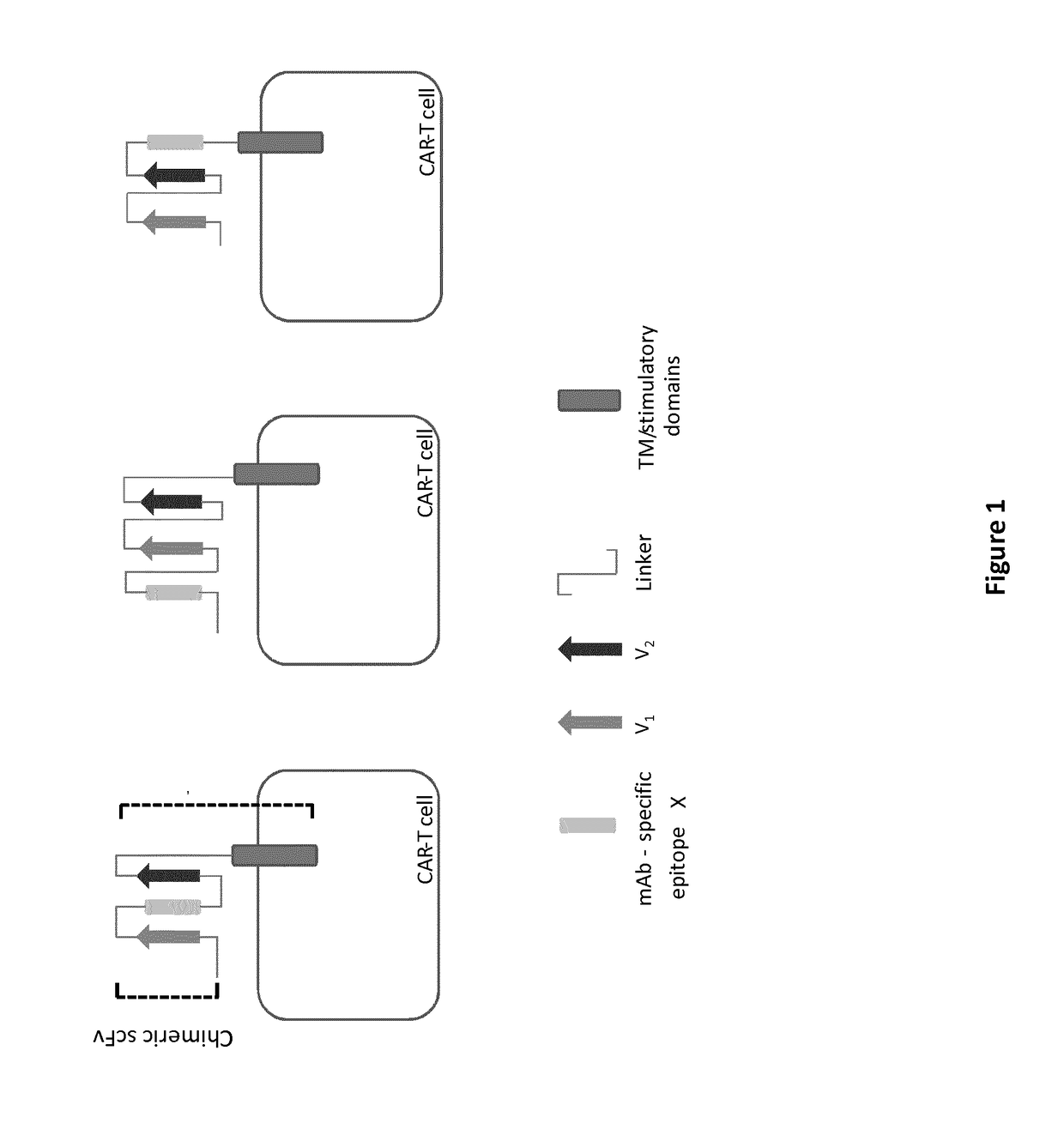
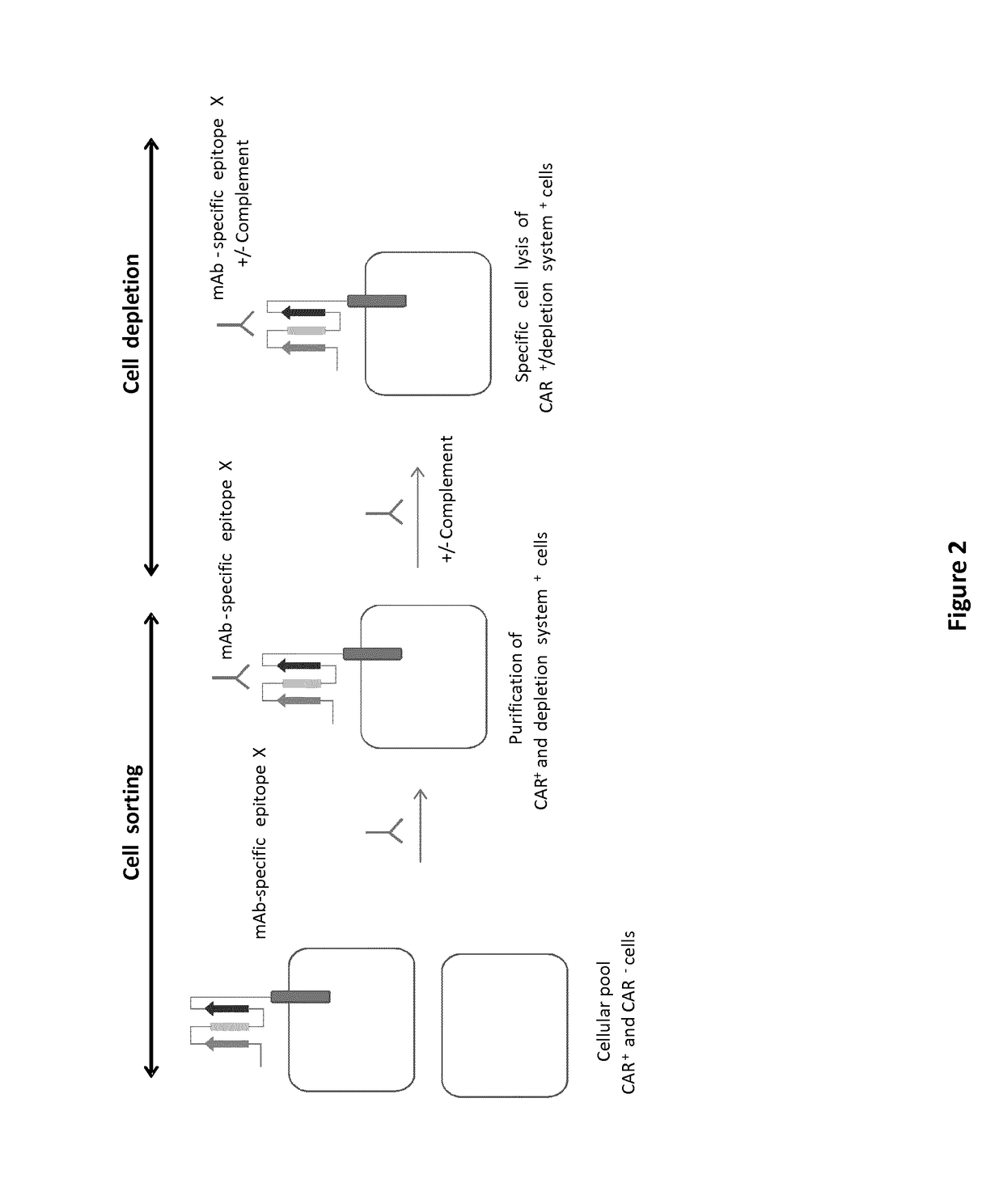
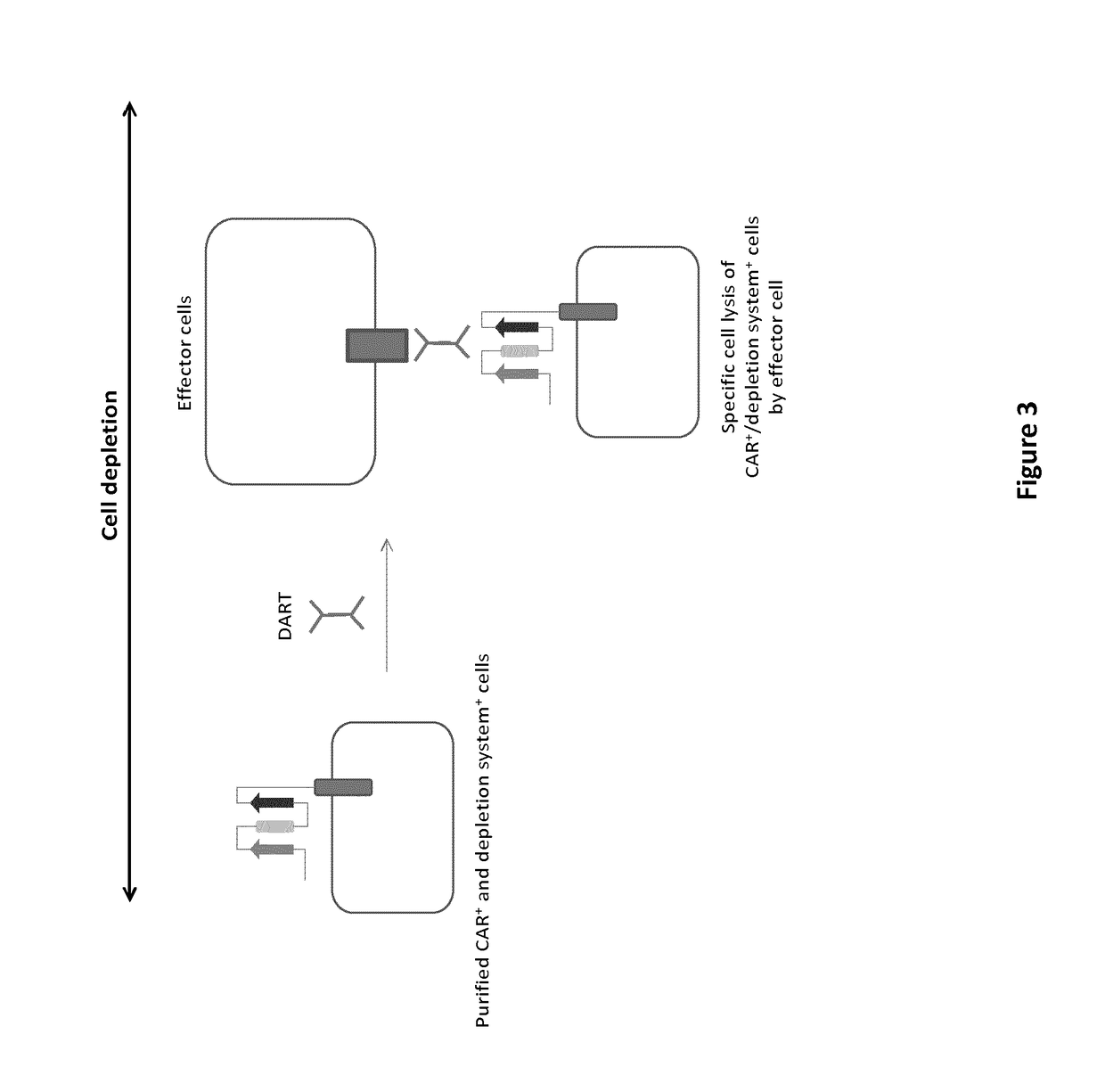
mAb-DRIVEN CHIMERIC ANTIGEN RECEPTOR SYSTEMS FOR SORTING/DEPLETING ENGINEERED IMMUNE CELLS
PendingUS20180002435A1Strong cytotoxicityPromote CDC cytotoxicityAnimal cellsAntibody mimetics/scaffoldsEpitopeBinding domain
A polypeptide encoding a chimeric antigen receptor (CAR) comprising at least one extracellular binding domain that comprises a scFv formed by at least a VH chain and a VL chain specific to an antigen, wherein said extracellular binding domain comprises at least one mAb-specific epitope.
Owner:CELLECTIS SA +1
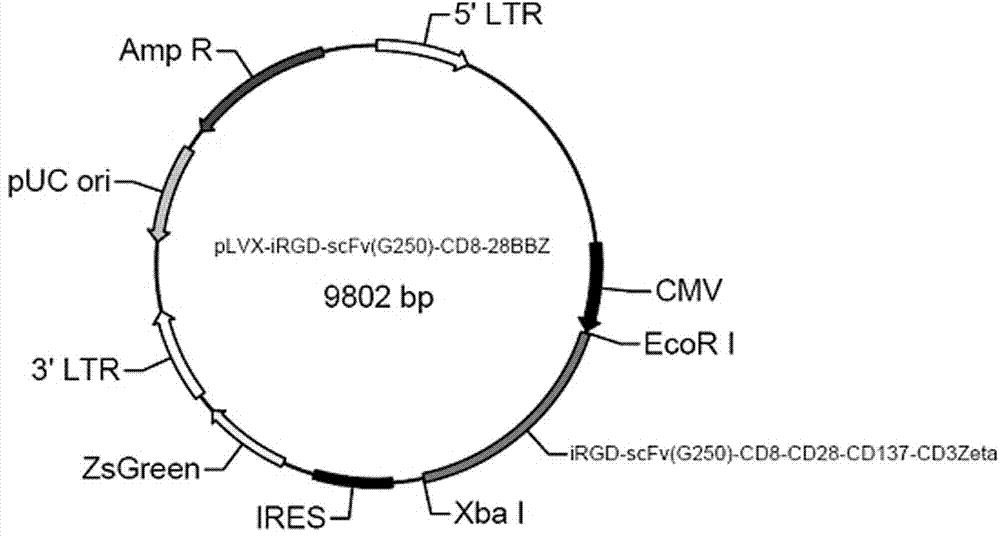
Chimeric antigen receptor iRGD-scFv (G250)-CD8-CD28-CD137-CD3zeta and application thereof
InactiveCN102775500APeptide/protein ingredientsPharmaceutical non-active ingredientsAntigen receptorSingle-Chain Antibodies
The invention belongs to the technical field of biology and new medicines and discloses a chimeric antigen receptor (CAR) iRGD-scFv (G250)-CD8-CD28-CD137-CD3zeta and application thereof. The chimeric antigen receptor is formed by series connection of tumor penetrating peptide iRGD, a single-chain antibody of a humanized anti-human renal carcinoma antigen G250, a hinge region and a transmembrane region of CD8, and intracellular signal structural domains of CD28, CD137 and CD3zeta. The chimeric antigen receptor can be used for modifying T lymphocytes, and the modified T lymphocytes can be used for renal carcinoma treatment.
Owner:郑骏年
Anti-mesothelin chimeric antigen receptors
ActiveUS9359447B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer preventionAntiendomysial antibodies
Owner:UNITED STATES OF AMERICA
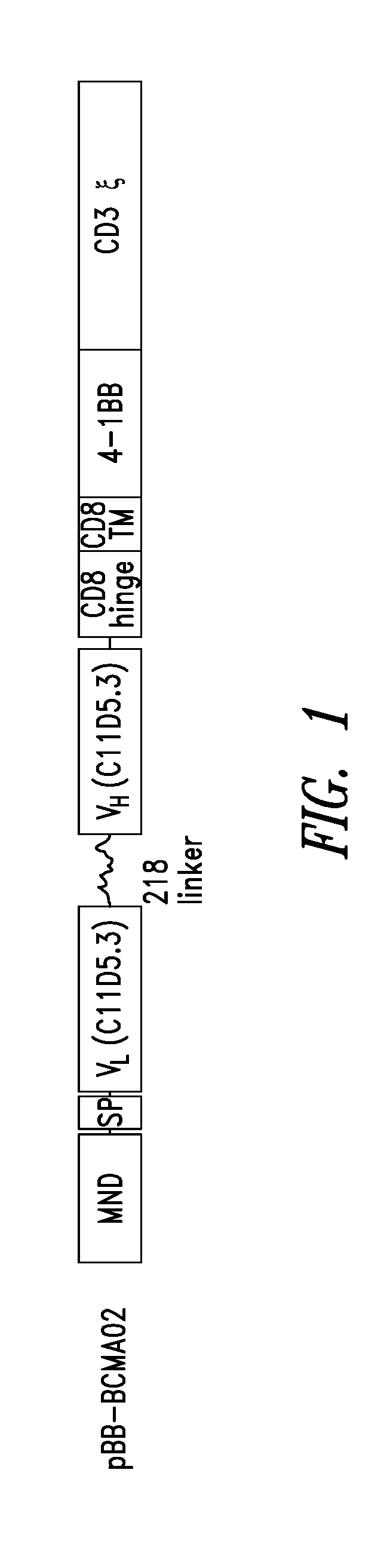
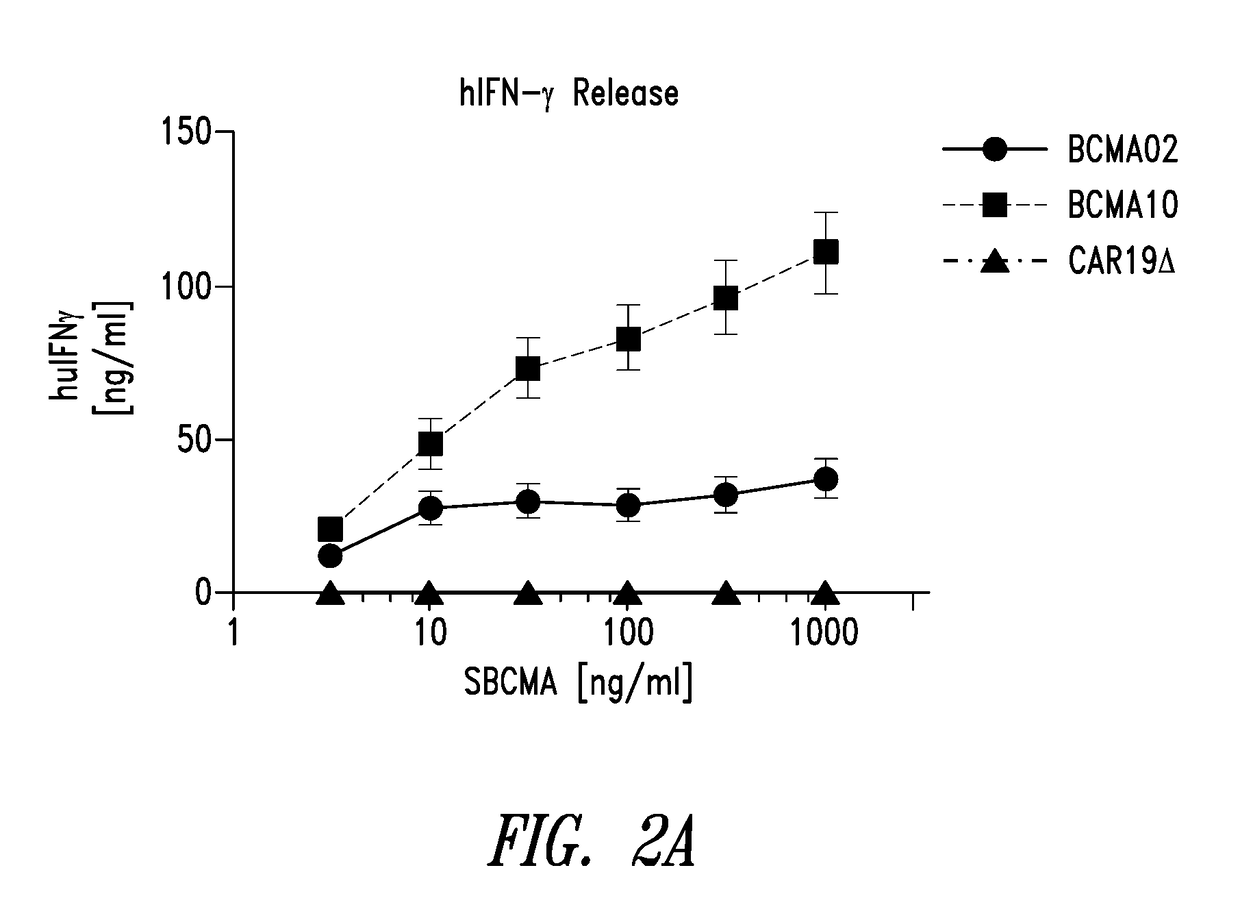
Bcma chimeric antigen receptors
ActiveUS20180085444A1Maintaining proliferationSenses disorderVirusesAntigen receptorChimeric antigen receptor
Owner:2SEVENTY BIO INC
BCMA-based (B cell maturation antigen-based) chimeric antigen receptor and preparation method and application thereof
InactiveCN105837693AImprove anti-apoptotic abilityImprove bindingAntibody mimetics/scaffoldsMammal material medical ingredientsTumor targetAntigen
The invention provides a BCMA-based (B cell maturation antigen-based) chimeric antigen receptor, comprising following cis-form cascade domains: CD8a leader region, scFv fragment BCMA scFv of anti-BCMA antibody, CD8a hinge region and transmembrane region, CD28 intracellular signal domain and CD3 Zeta intracellular signal domain; the CD3 Zeta intracellular signal domain is wild CD3 Zetaintracellular signal domain or mutant CD3 Zeta mut intracellular signal domain; the BCMA-based chimeric antigen receptor can enhance anti-apoptotic capability of T-cells and enhance CAR T-cell and antigen bonding and signal conduction; T-cells with the BCMA-based chimeric antigen receptor show good tumor targeting performance in in-vivo experiments, tumors diminish significantly after two weeks of dosage, and the T-cells have excellent therapeutic effect in vivo.
Owner:李斯文 +1
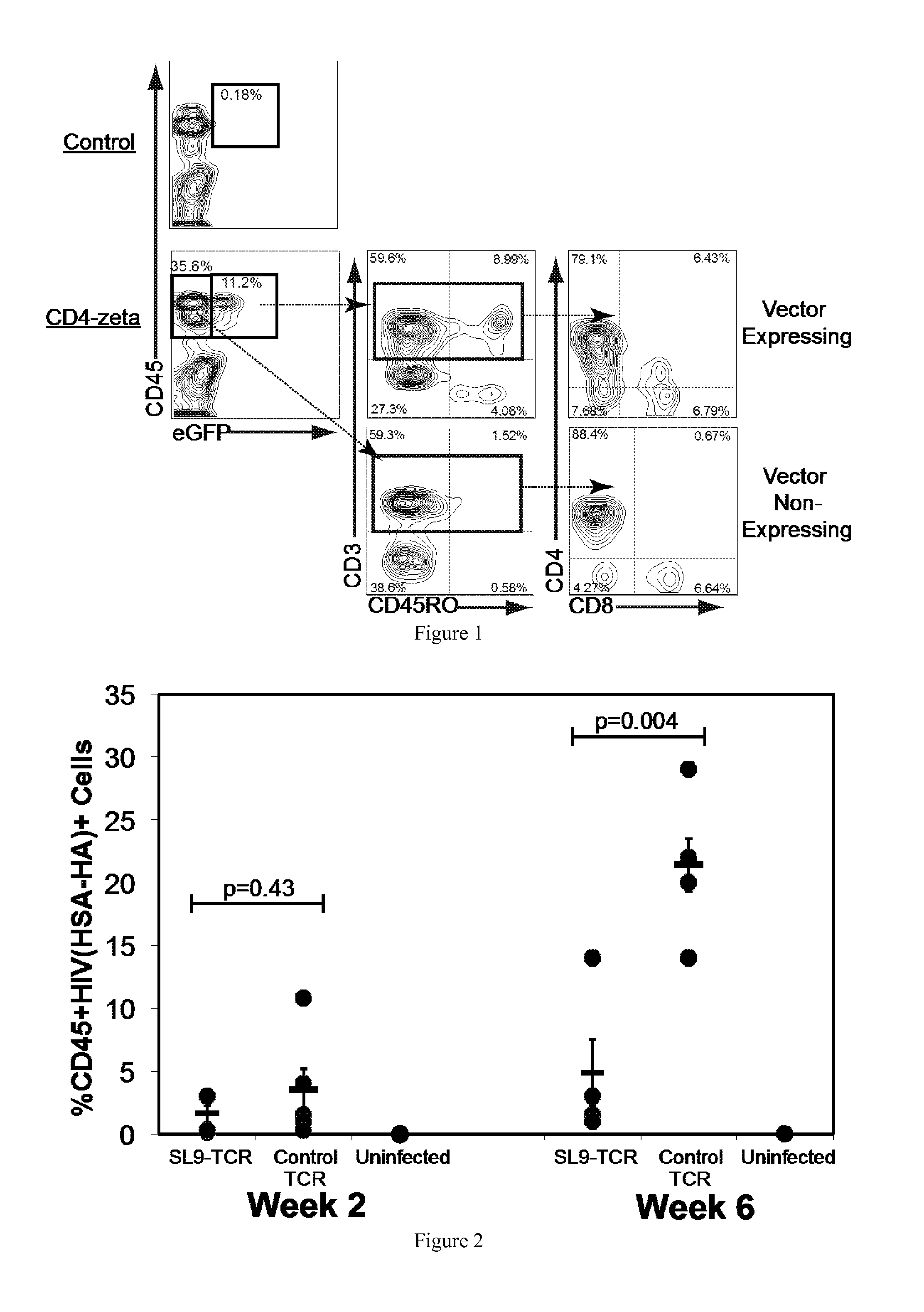
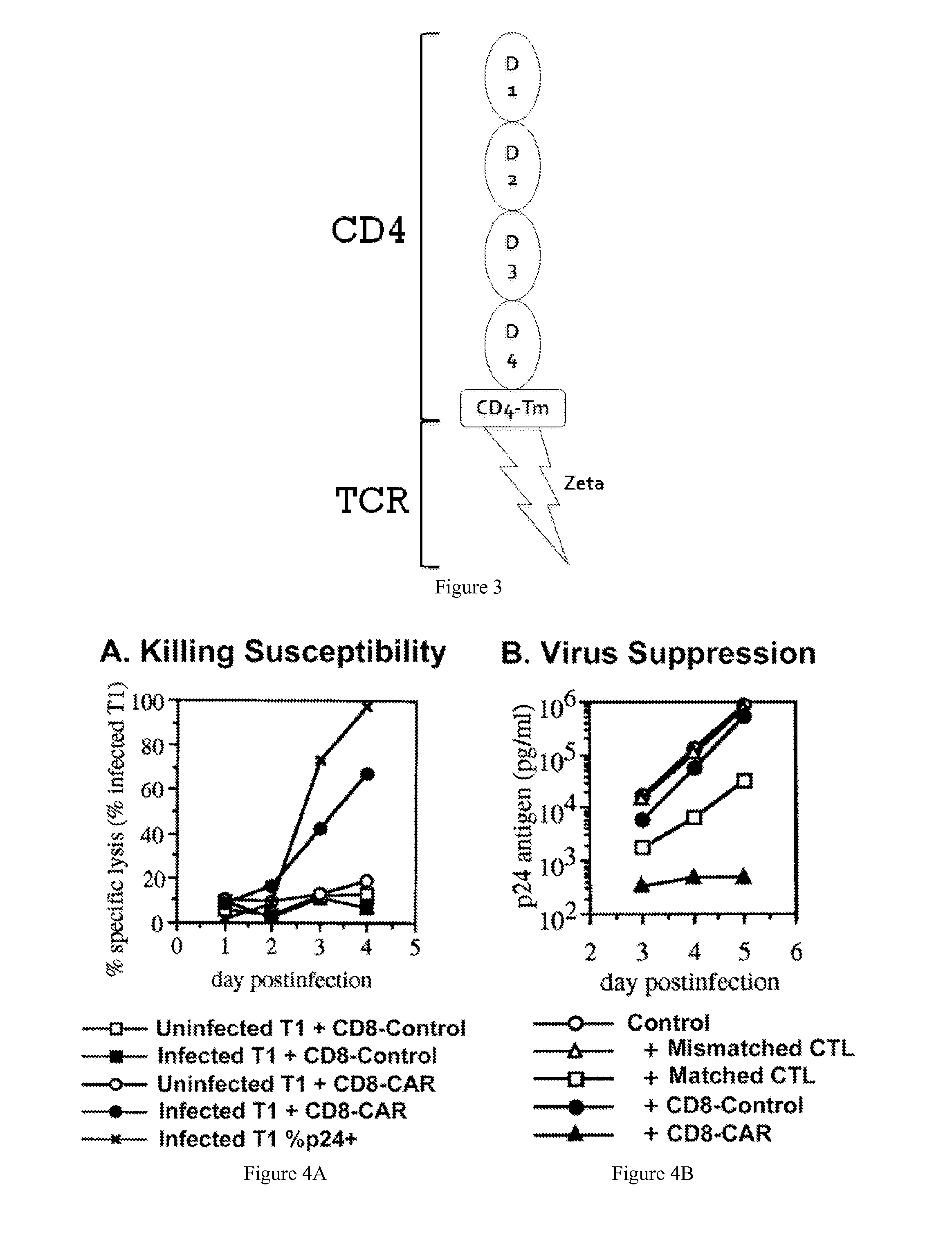
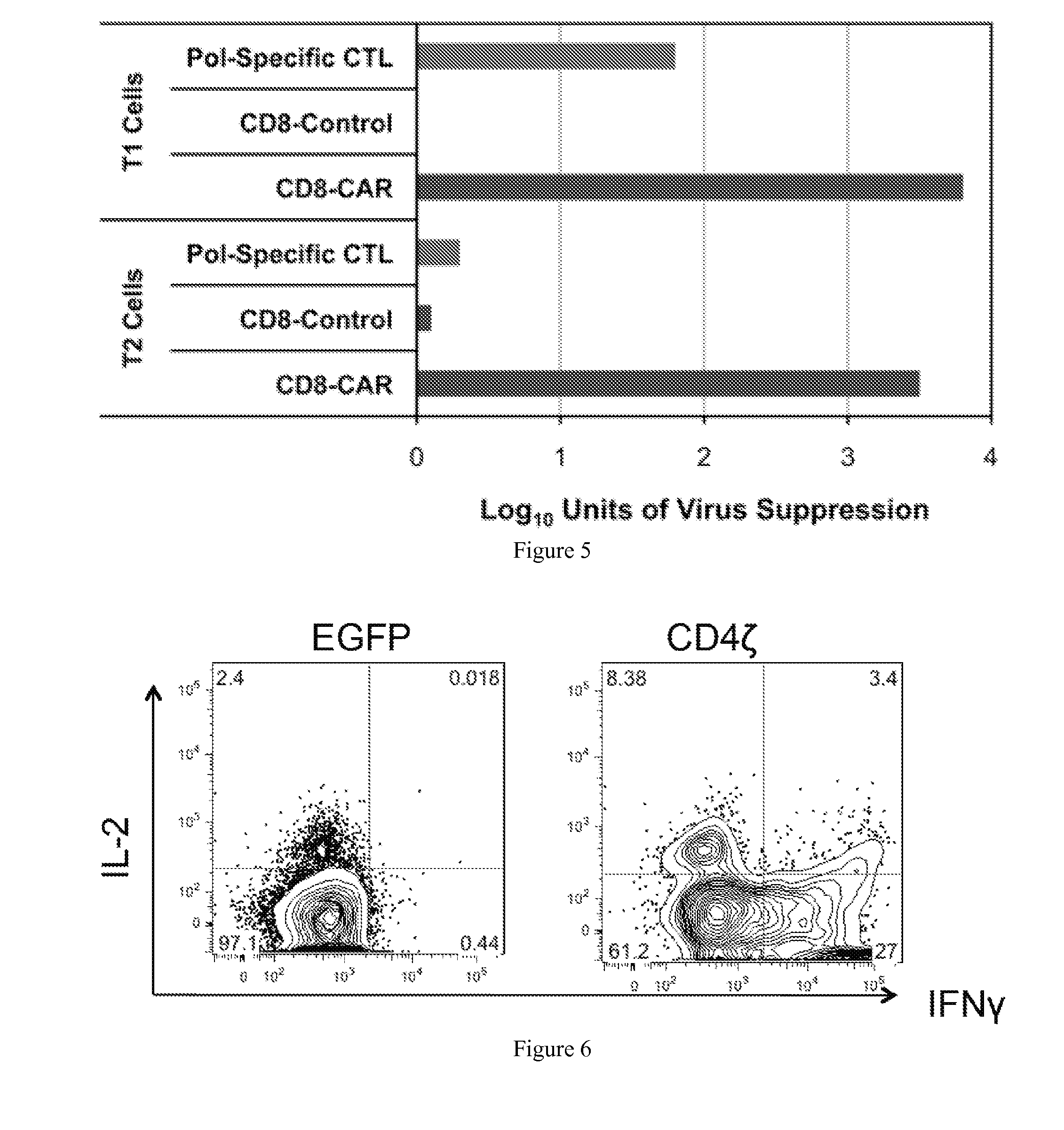
Engineering Antiviral T Cell Immunity through Stem Cells and Chimeric Antigen Receptors
The HIV-specific cytotoxic T lymphocyte (CTL) response is a critical component in controlling HIV replication and is an important part of the ultimate failure to eradicate the virus. Disclosed herein are methods for genetically enhancing the HIV-specific CTL response to allow long-term viral suppression or viral clearance. Human hematopoietic stem cells (HSCs) were genetically modified such that they differentiate into mature CTLs that will kill HIV infected cells. As disclosed herein, the functional effector cells are not human leukocyte antigen (HLA)-restricted. As disclosed herein, stem cells are transduced with non HLA-restricted chimeric antigen receptors (CARs) that allow the recognition of HIV or HIV infected cells when expressed by a CTL.
Owner:RGT UNIV OF CALIFORNIA
Chimeric antigen receptor
ActiveUS9175308B2High expressionHigh cytotoxic activityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenTumor necrosis factor receptor
Provided are a chimeric antigen receptor comprising an extracellular domain capable of binding to an antigen, a transmembrane domain and at least one intracellular domain, the chimeric antigen receptor being characterized in that an intracellular domain of a glucocorticoid-induced tumor necrosis factor receptor (GITR) is contained as the intracellular domain; a nucleic acid encoding the chimeric antigen receptor; a cell expressing the chimeric antigen receptor; and a method for producing the cell.
Owner:MIE UNIVERSITY
Novel chimeric antigen receptors
InactiveUS20170313759A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenNucleotide
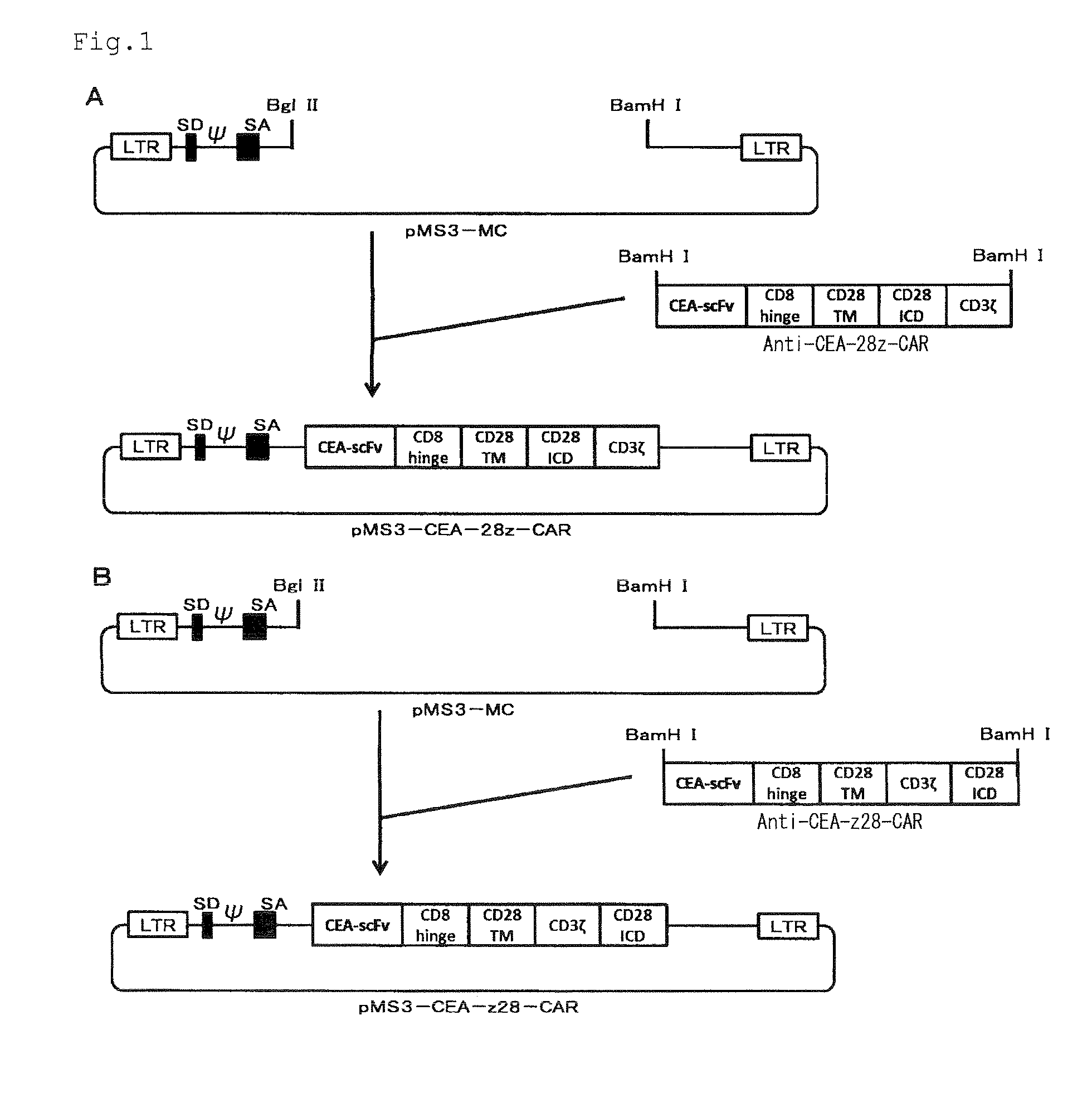
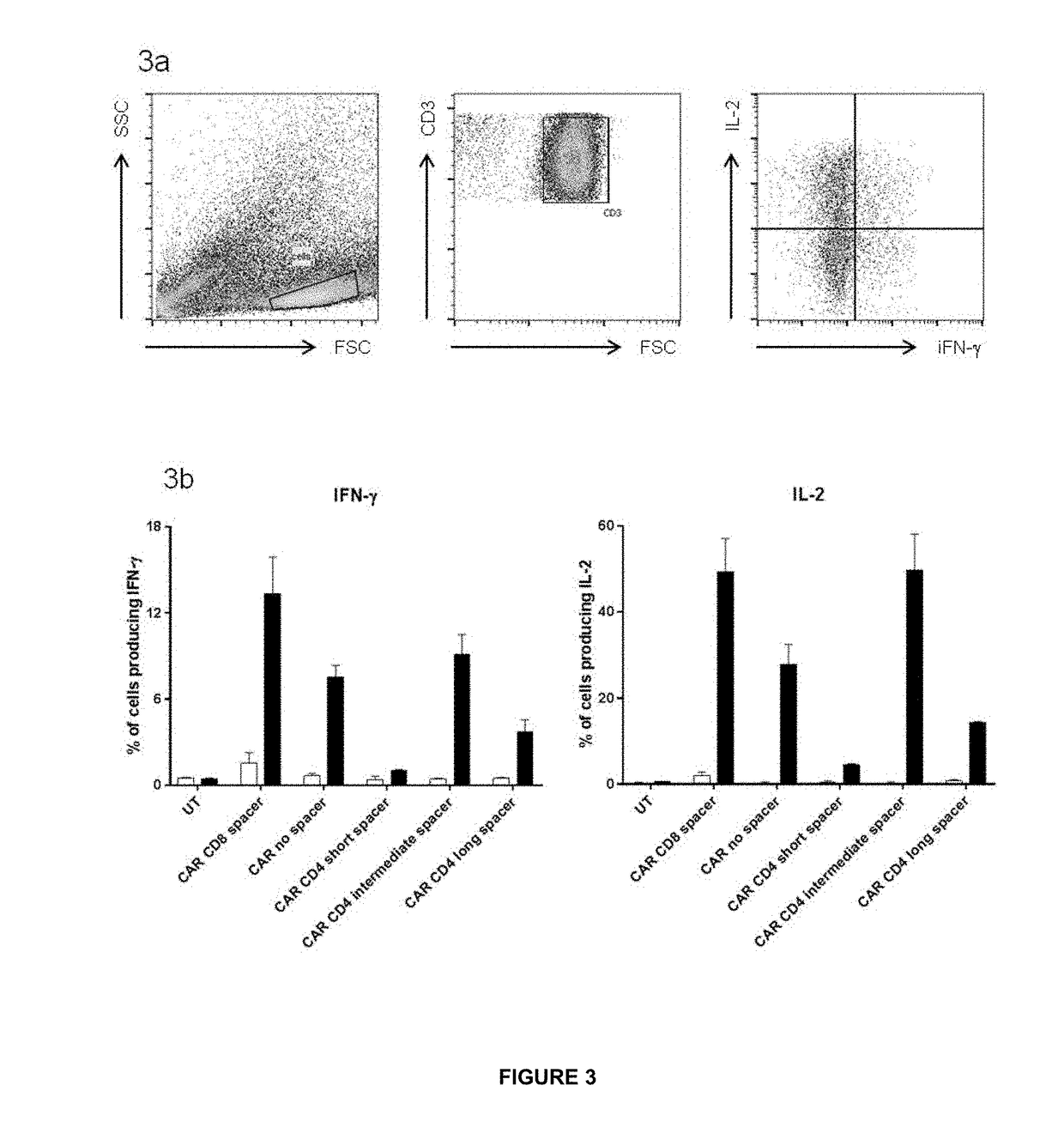
The invention relates to chimeric antigen receptor (CAR) scaffolds comprising: a target binding domain; a spacer region; a transmembrane domain; and an intracellular effector domain, wherein the spacer region comprises at least one, or multiples of, domains 2, 3 or 4 or a combination thereof of a CD4 molecule. The invention also relates to polynucleotides and expression vectors encoding said CAR scaffold and immunomodulatory cells comprising said CAR scaffold. The invention also relates to methods of engineering an immunomodulatory cell to comprise said CAR scaffold
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
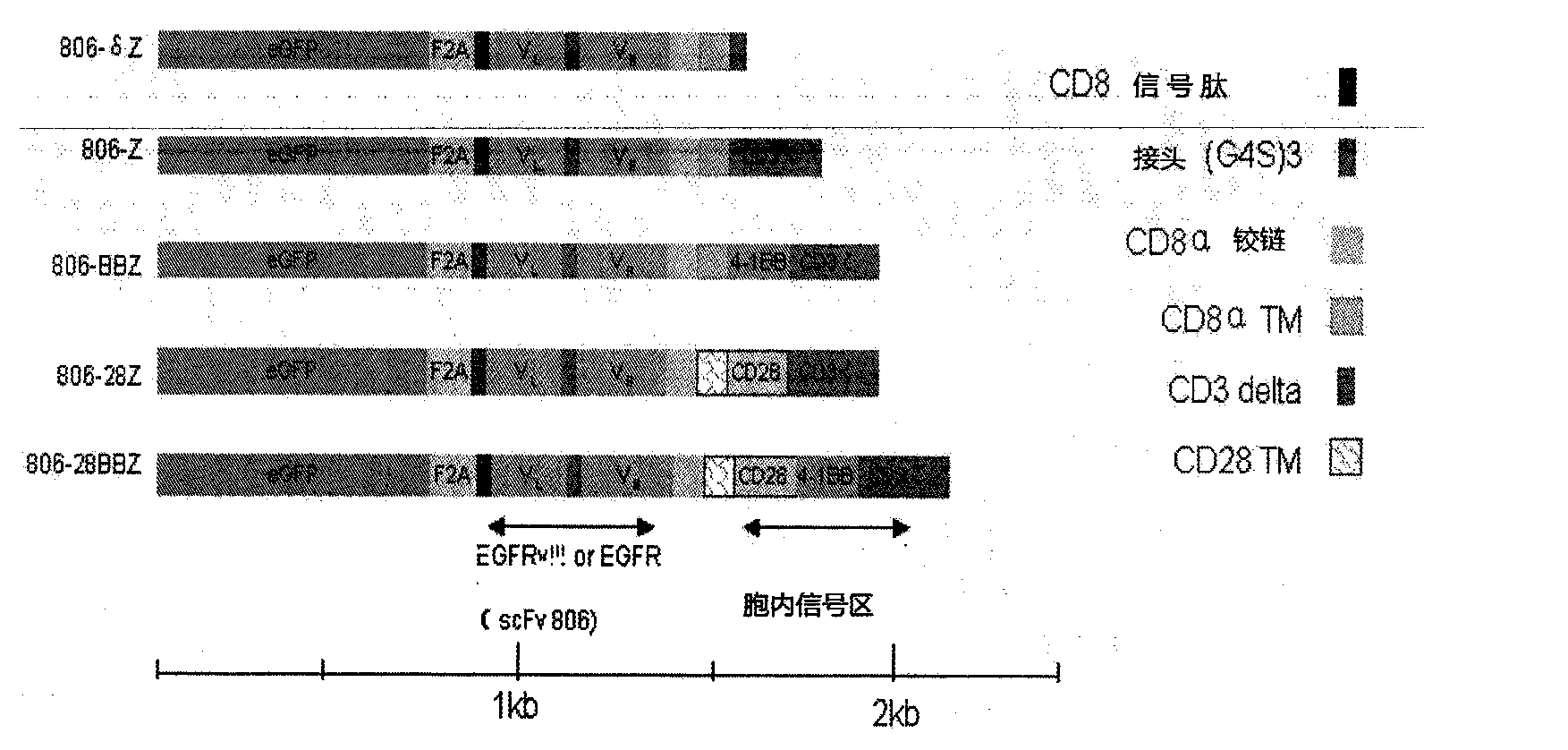
Nucleic acid for coding chimeric antigen receptor protein and T lymphocyte for expression of chimeric antigen receptor protein
InactiveCN104087607AHigh transduction efficiencyEffective immunotherapyAntibody mimetics/scaffoldsBlood/immune system cellsAntigen receptorEpitope
The invention discloses a nucleic acid for coding a chimeric antigen receptor protein expressed on the surface of the human T lymphocyte. The chimeric antigen receptor protein comprises an extracellular binding domain, a transmembrane domain and an intracellular signal domain, and the extracellular binding domain, the transmembrane domain and the intracellular signal domain are orderly connected. The extracellular binding domain comprises a single-chain antibody scFv (epidermal growth factor receptor EGFR) for specific recognition of 287th-302th amino acid epitopes of EGFR.
Owner:CRAGE MEDICAL CO LTD
Method for in situ inhibition of regulatory t cells
ActiveUS20170067022A1Reducing FoxP transcriptional activityHydrolasesAntibody mimetics/scaffoldsAntigenInfected cell
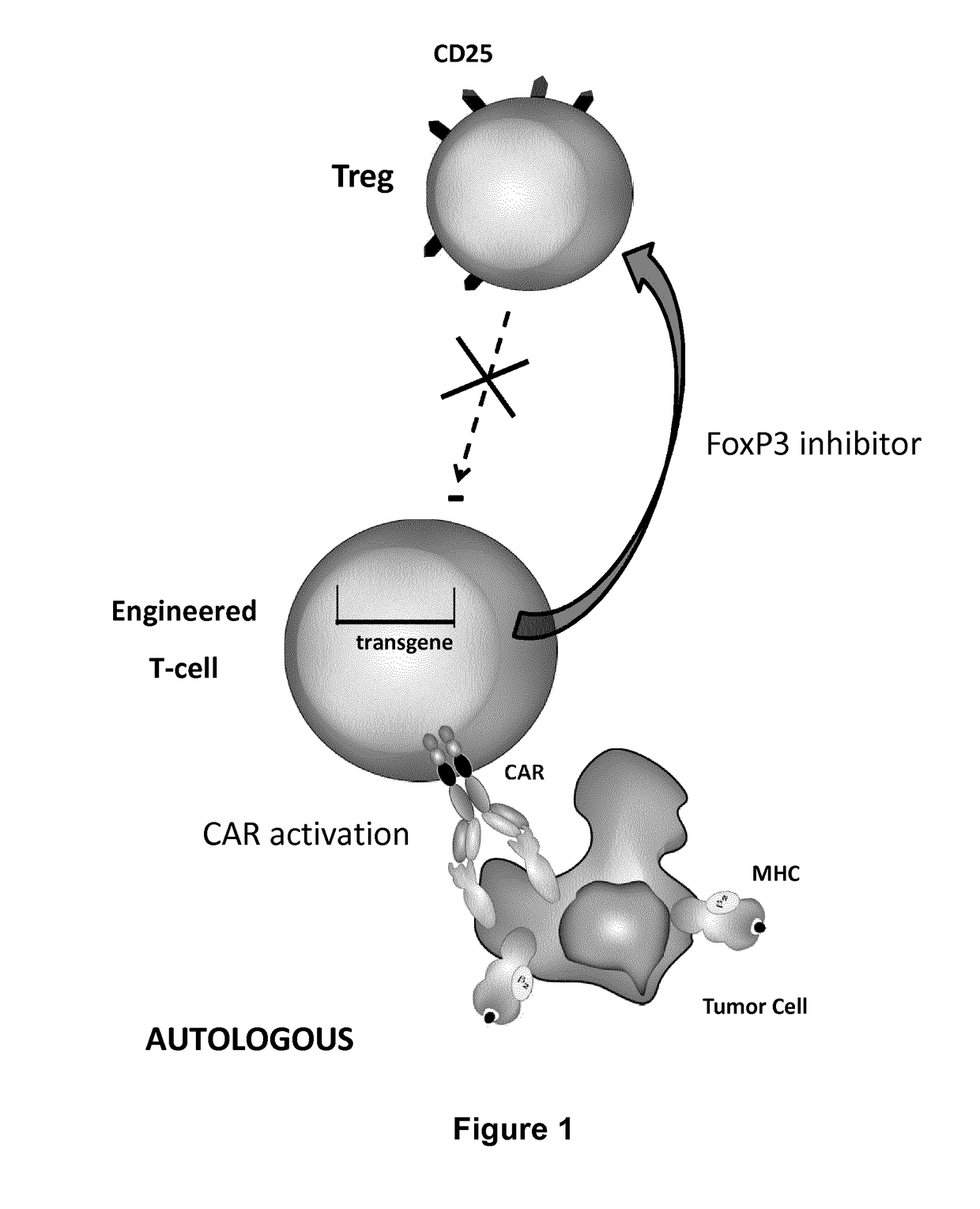
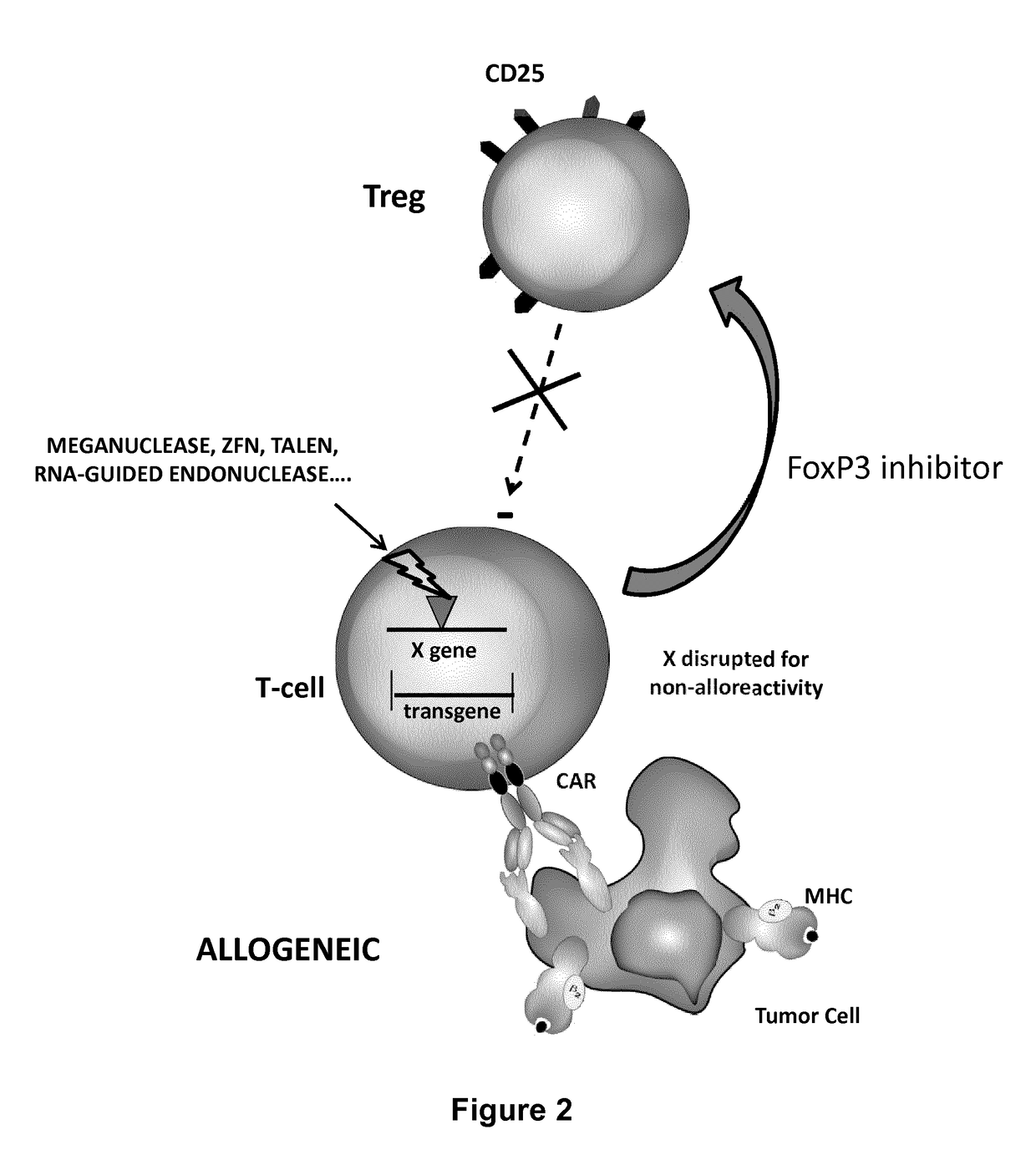
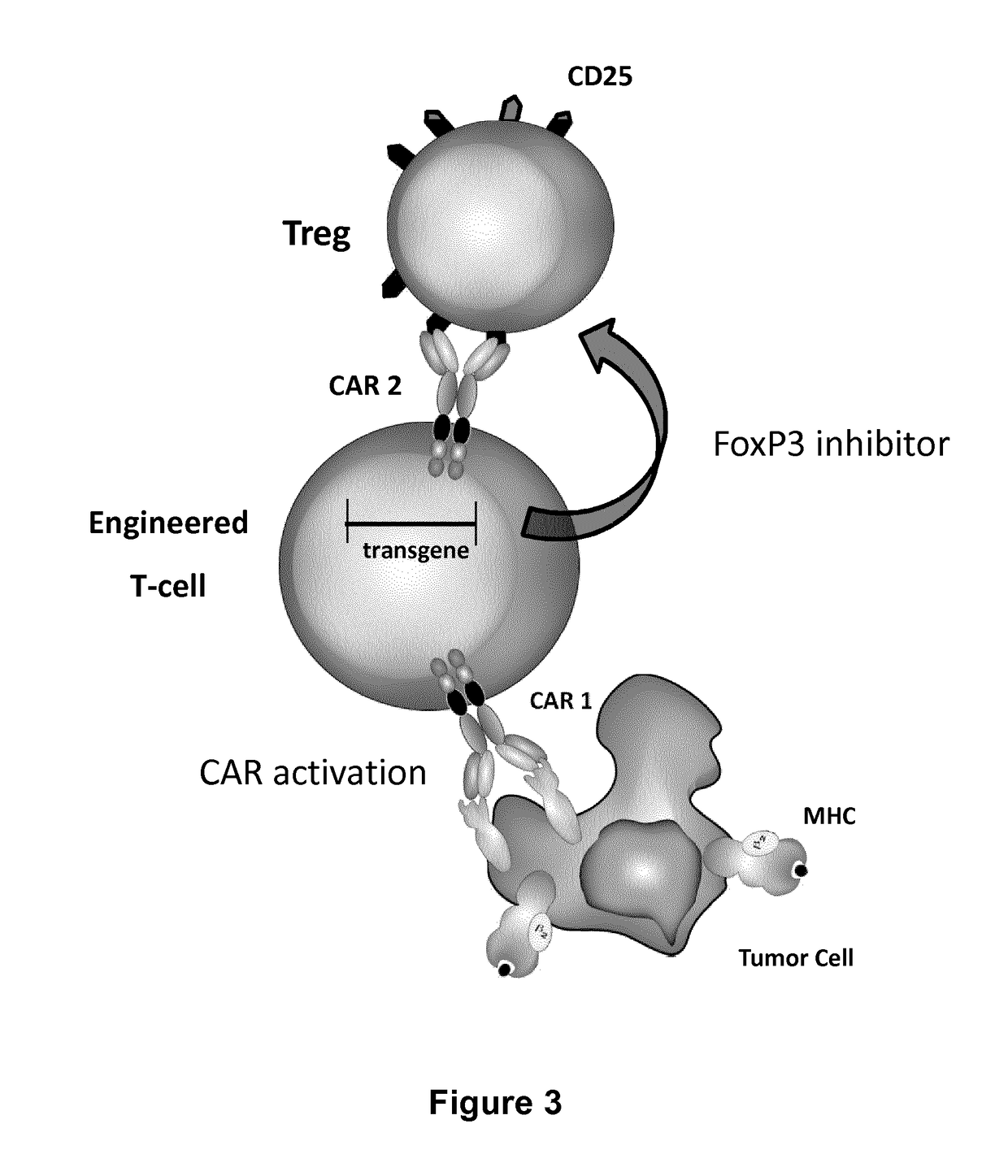
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are designed to express both a Chimeric Antigen Receptor (CAR) directed against at least one antigen expressed at the surface of a malignant or infected cell, and a secreted inhibitor of regulatory T-cells (Treg). Preferably, such secreted inhibitor is a peptide inhibitor of forkhead / winged helix transcription factor 3 (FoxP3), a specific factor involved into the differentiation of T-cells into regulatory T-cells. The engineered T-cells of the invention direct their immune activity towards specific malignant or infected cells, while at the same time will prevent neighbouring regulatory T-cells from modulating the immune response. The invention opens the way to standard and affordable adoptive immunotherapy strategies, especially for treating or preventing cancer, and bacterial or viral infections.
Owner:CELLECTIS SA
Reversing the effects of the tumor microenvironment using chimeric cytokine receptors
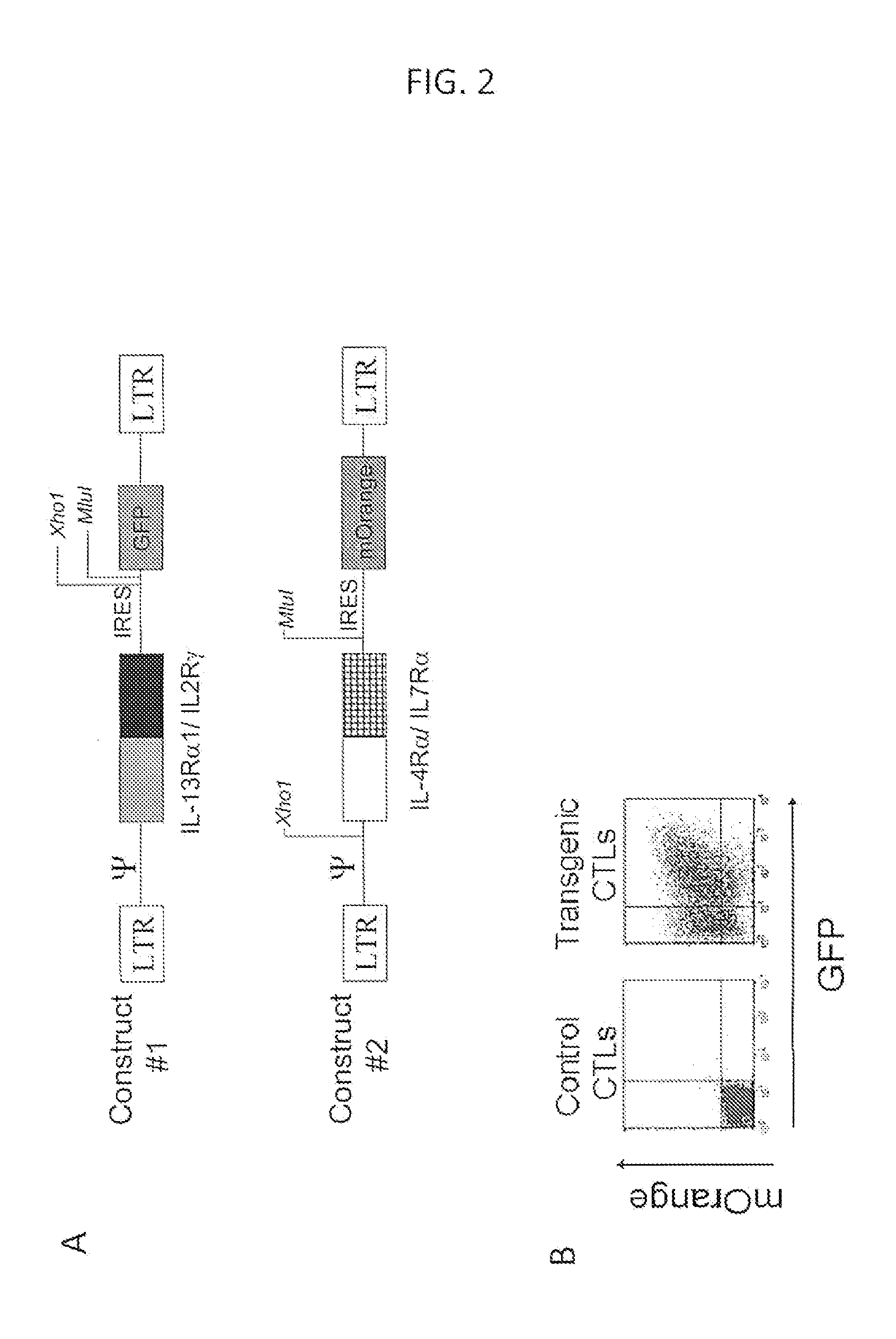
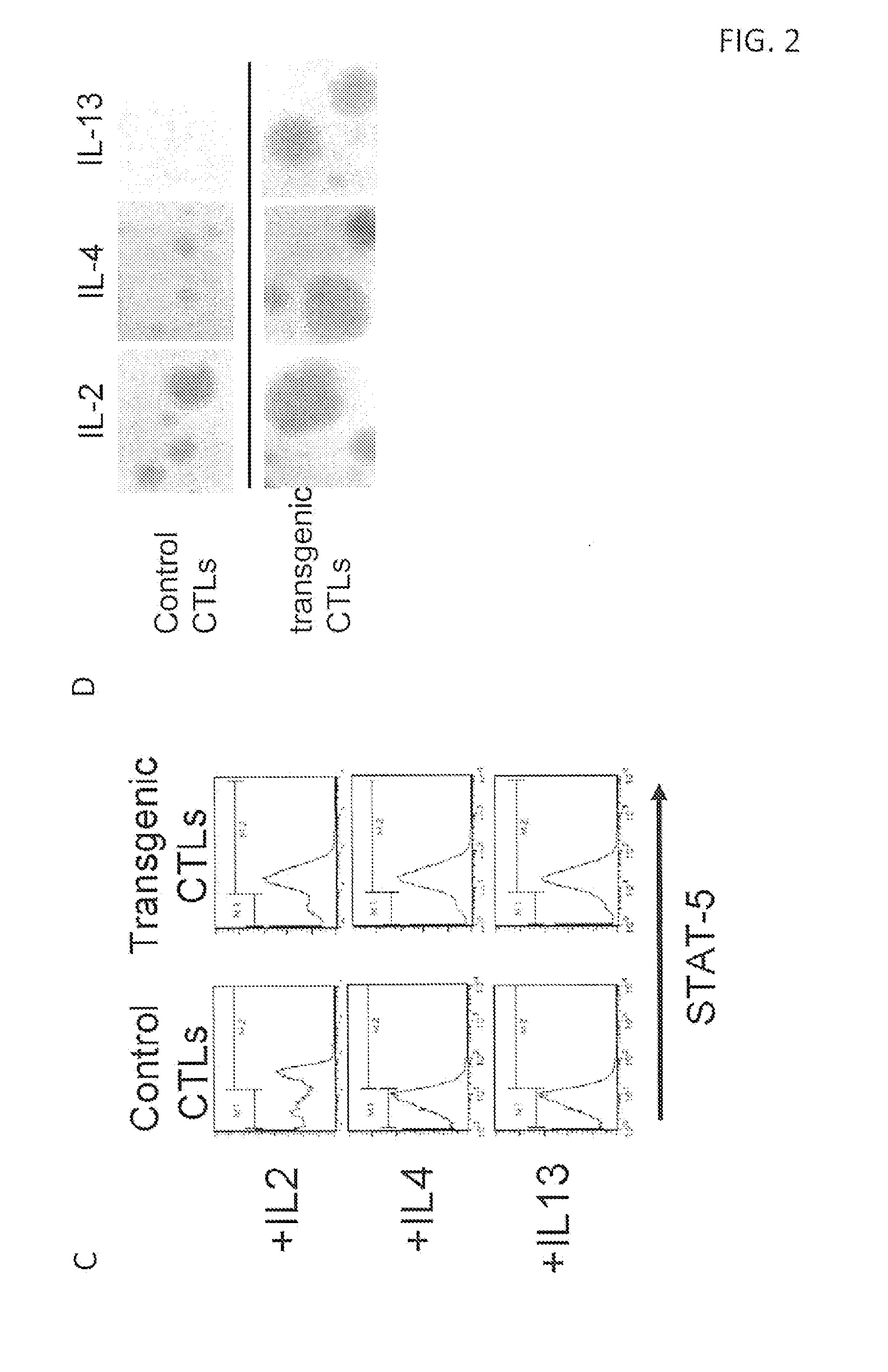
ActiveUS20140050709A1High activityIncrease expansionBiocideGenetic material ingredientsAntigen receptorCytokine milieu
Disclosed are compositions and methods related to rendering ineffective Th1 T cells resistant to the inhibitory cytokine milieu present in a cancer microenvironment. Tumor-specific T cells are modified to employ a chimeric receptor that binds inhibitory / suppressive cytokines and converts their intracellular consequences to a Th1 immunostimulatory / activating signal. The T cells employ a chimeric antigen receptor having exodomains for IL10, IL13 and / or IL4 fused with the signal transducing endodomains for IL2 and / or IL7.
Owner:BAYLOR COLLEGE OF MEDICINE
BCMA (B-cell maturation antigen) chimeric antigen receptor on basis of single-domain antibodies and application of BCMA chimeric antigen receptor
ActiveCN109134665AGood treatment effectLethalAntibody mimetics/scaffoldsMammal material medical ingredientsHeavy chainB-Cell Maturation Antigen
The invention relates to a chimeric antigen receptor (CAR) and application thereof. The CAR comprises BCMA (B-cell maturation antigen) binding structural domains, transmembrane structural domains, oneor a plurality of co-simulation structural domains and intracellular signal transduction structural domains. The BCMA binding structural domains comprise heavy chain complementary determining regionsHCDR 1-3, and amino acid sequences of the HCDR 1-3 are sequentially shown as SEQ ID NO:1-3.
Owner:SHANGHAI SIMNOVA BIOTECHNOLOGY CO LTD
Use of the cd2 signaling domain in second-generation chimeric antigen receptors
InactiveCN104136458AAvoid deathPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell expressing a CAR having an antigen binding domain, a transmembrane domain, a CD2 signaling domain, and a CD3 zeta signaling domain. The invention also includes incorporating CD2 into the CAR to alter the cytokine production of CAR-T cells in both negative and positive directions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com