Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
A-CD28-CD3, hcd19scfv-cd8 technology, applied in the field of tumor cell immunotherapy, can solve the problem that the immune system cannot clear the tumor well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Determination of hCD19scFv-CD8α-CD28-CD3ζ gene sequence
[0031] 1.1 The human CD8α signal peptide gene, human CD8α hinge region gene, human CD28 transmembrane region and intracellular region gene, and human CD3ζ intracellular region gene sequence. The anti-CD19 single-chain antibody (anti-CD19scFv) gene sequence comes from a patent (patent number: ZL200610015650.9), and its codons are optimized to ensure that it is more suitable for human cell expression without changing the encoded amino acid sequence.
[0032] For the sequence information of each gene, see SEQUENCE LISTING (SEQ ID NO.1-8 in the sequence listing).
[0033] 1.2 The above gene sequences were sequentially linked according to the human CD8α signal peptide gene, anti-CD19 scFv, human CD8α hinge region gene, human CD28 transmembrane region and intracellular region gene, and human CD3ζ intracellular region gene sequence, and introduced Kozac sequence, and introduce different restriction sites at...
Embodiment 2
[0034] Example 2: Construction of hCD19scFv-CD8α-CD28-CD3ζ expression plasmid
[0035] 2.1 Whole gene synthesis:
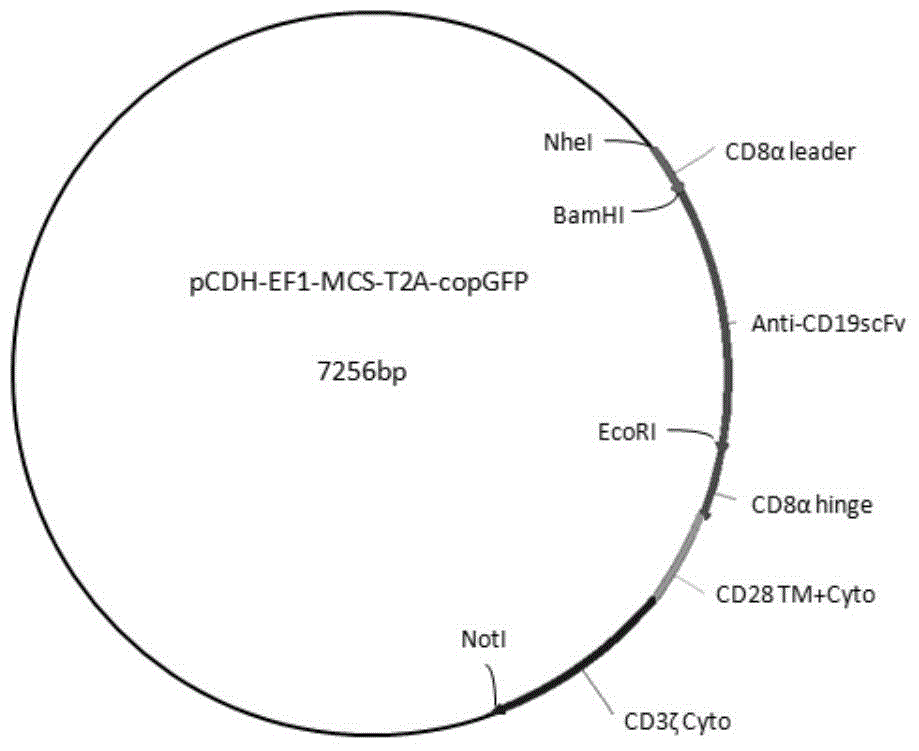
[0036] The complete hCD19-CAR sequence optimized by whole gene synthesis was cloned into the pCDH-EF1-MCS-T2A-copGFP lentiviral expression vector (pCDH-empty vector) to obtain the anti-human CD19-CAR expression plasmid (pCDH-CAR plasmid) and the DH5α bacterial fluid containing the plasmid. Plasmid information see figure 1 .
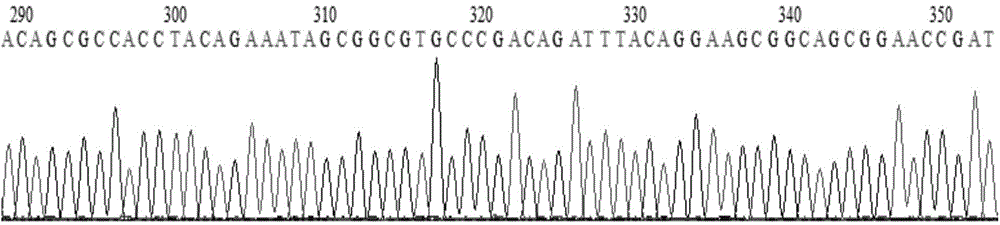
[0037] 2.2 Sequencing of recombinant plasmids:
[0038]The recombinant plasmid was sent to Shanghai Yingjun Biotechnology Co., Ltd. for sequencing, and the sequencing results were compared with the fitted hCD19-CAR sequence to confirm that the sequence was correct. The sequencing primers are the universal sequencing primers on the pCDH-EF1-MCS-T2A-copGFP vector:
[0039] Sense: 5'ctccacgctttgcctgaccctgctt 3'
[0040] Anti-sense: 5'ggtgatgcggcactcgatctccatg 3'
[0041] For some sequencing results, see figure 2 .
Embodiment 3
[0042] Example 3: Large-scale extraction of target plasmids and packaging plasmids
[0043] The strains of pCDH-CAR plasmid, pCDH-empty vector plasmid, and psPAX2 and pMD2.G packaging plasmids were cultured in LB culture medium in large quantities, and a large number of plasmids were extracted by alkaline lysis method (Beijing Tiangen Biochemical Technology Co., Ltd. endotoxin-free plasmid extraction kit) for transfection.
[0044] 1) Add the bacterial solution to 150-200ml LB culture medium containing Amp at a ratio of 1:1000, and shake at 200rpm in a constant temperature incubator at 37°C for 12-16h;
[0045] 2) Take a small amount of bacterial liquid to test A 600 value, take it out after reaching about 0.4;
[0046] 3) Add 2.5ml of balance solution BL to the adsorption column CP5, centrifuge at 10,000rpm for 2min, discard the waste liquid in the collection tube, and put the adsorption column back into the collection tube for later use.
[0047] 4) Centrifuge the bacteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com