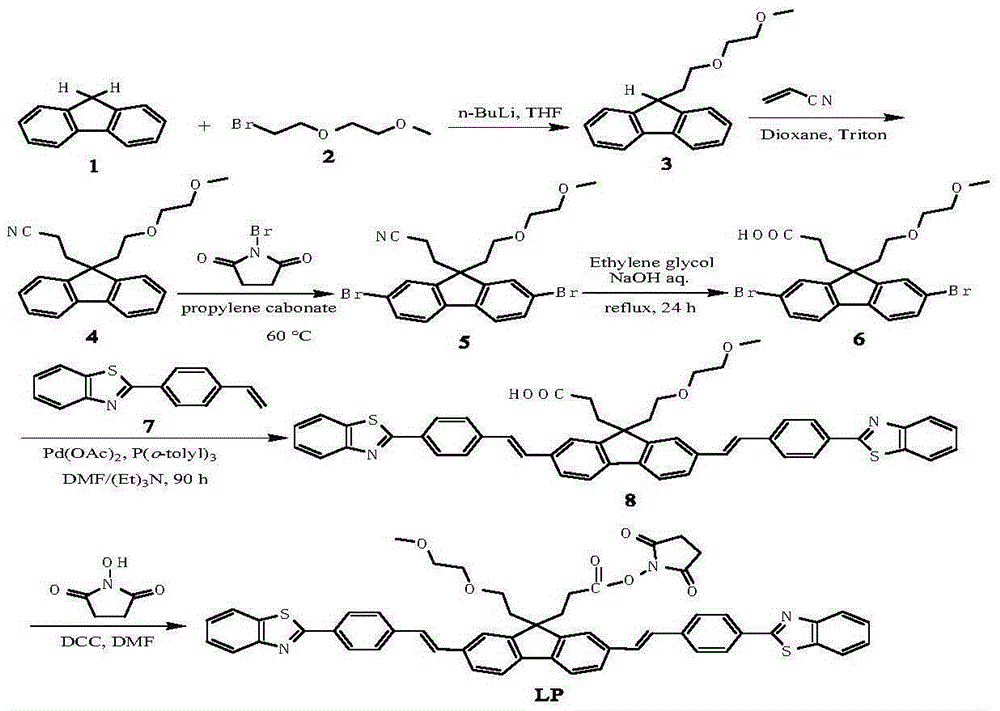
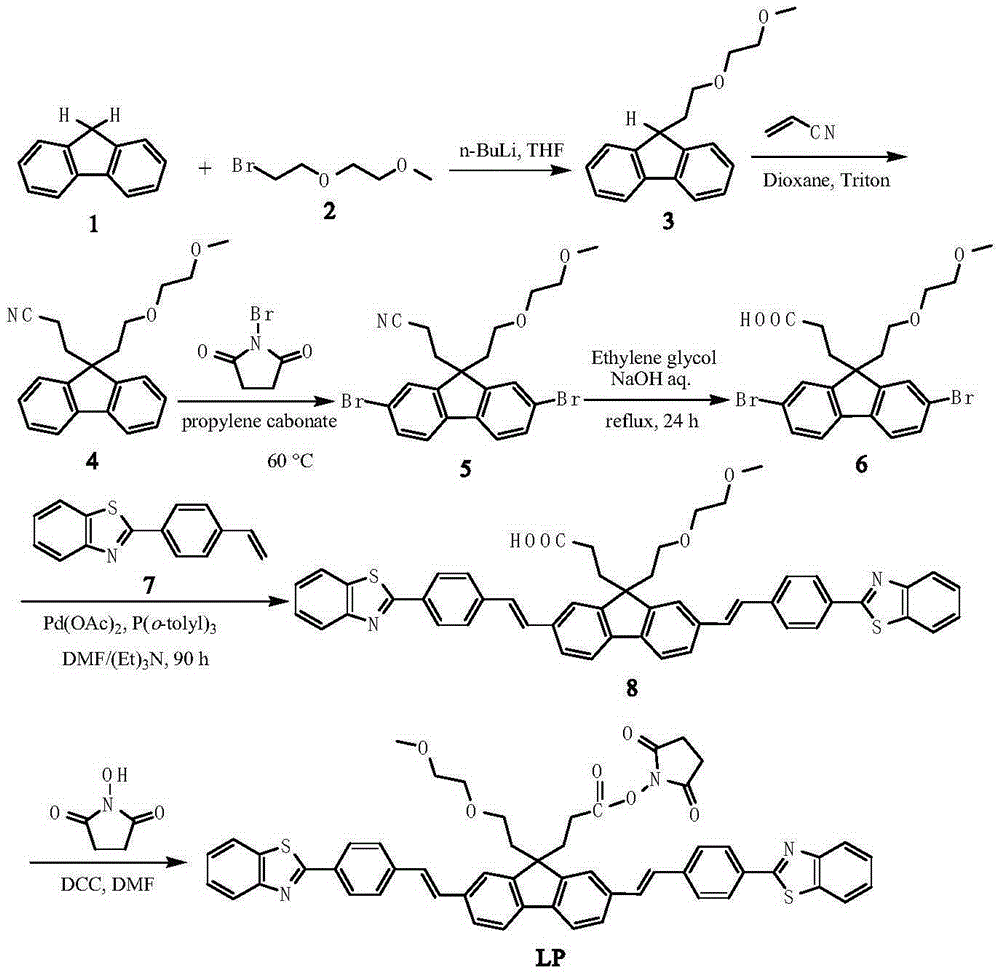
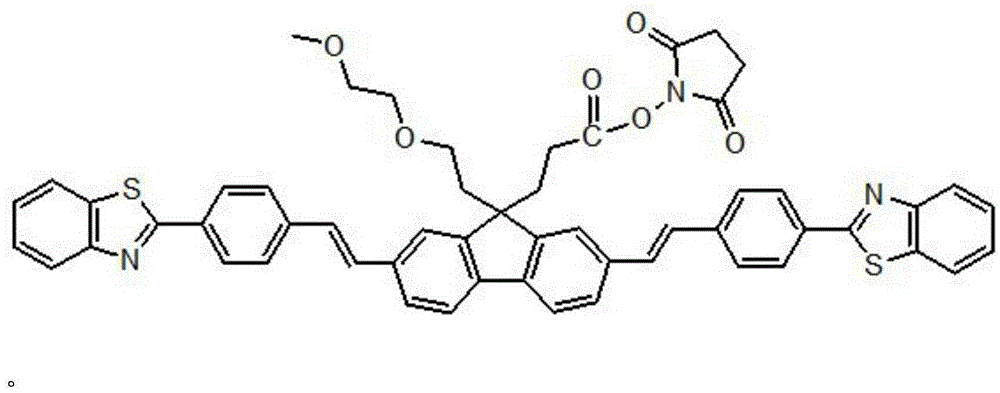
Patents
Literature
36 results about "Antigen response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions of targeted drug development
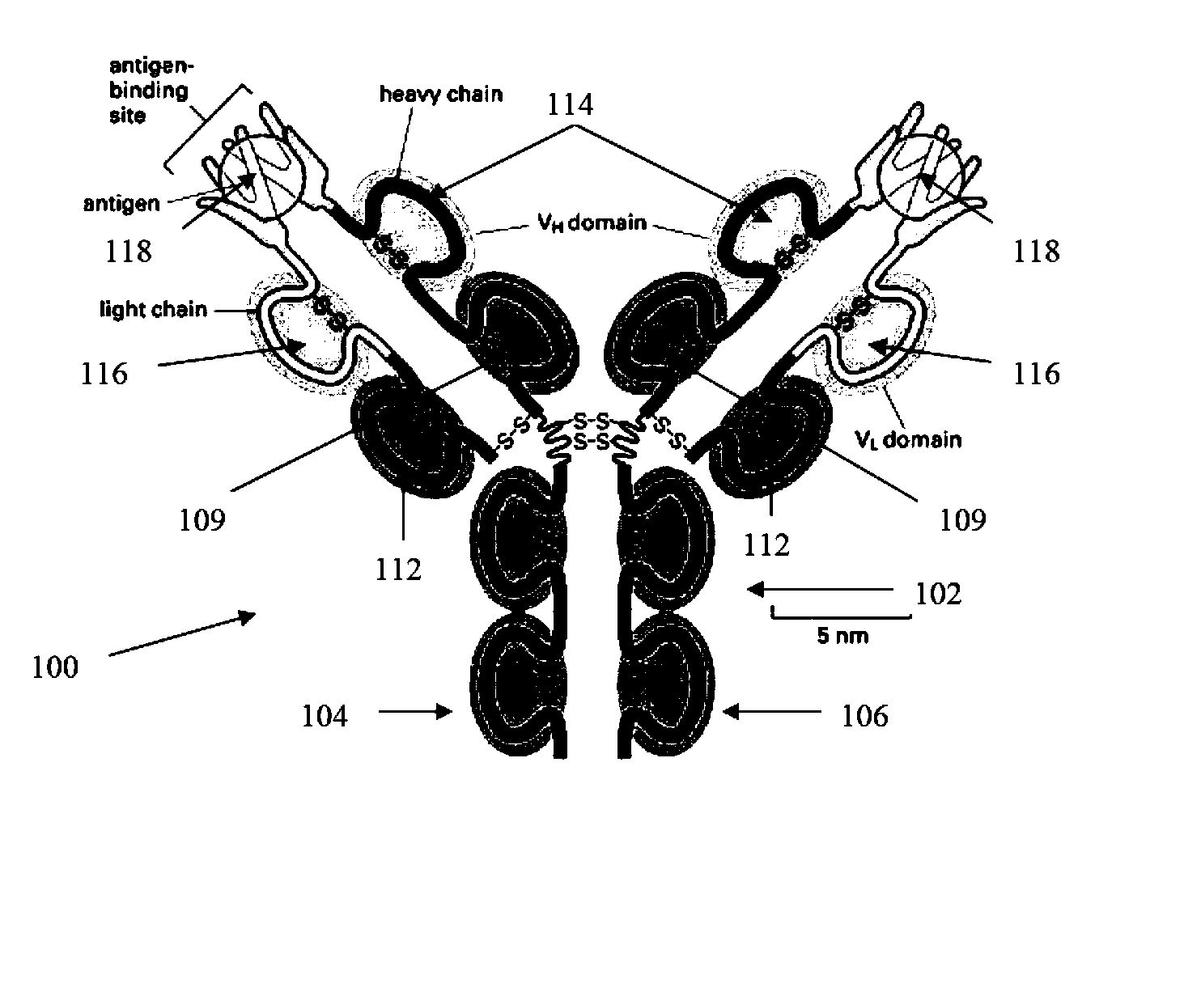
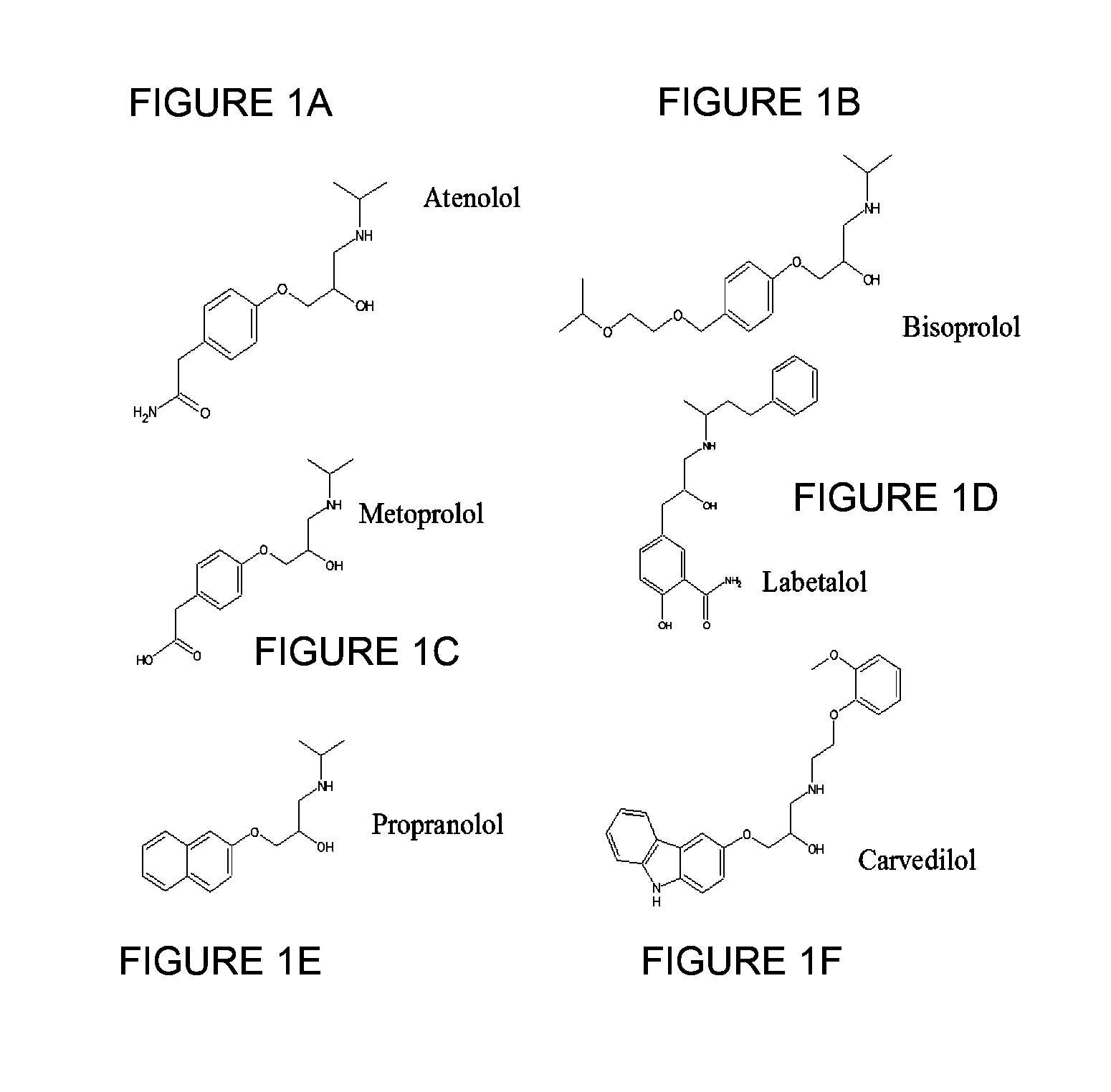
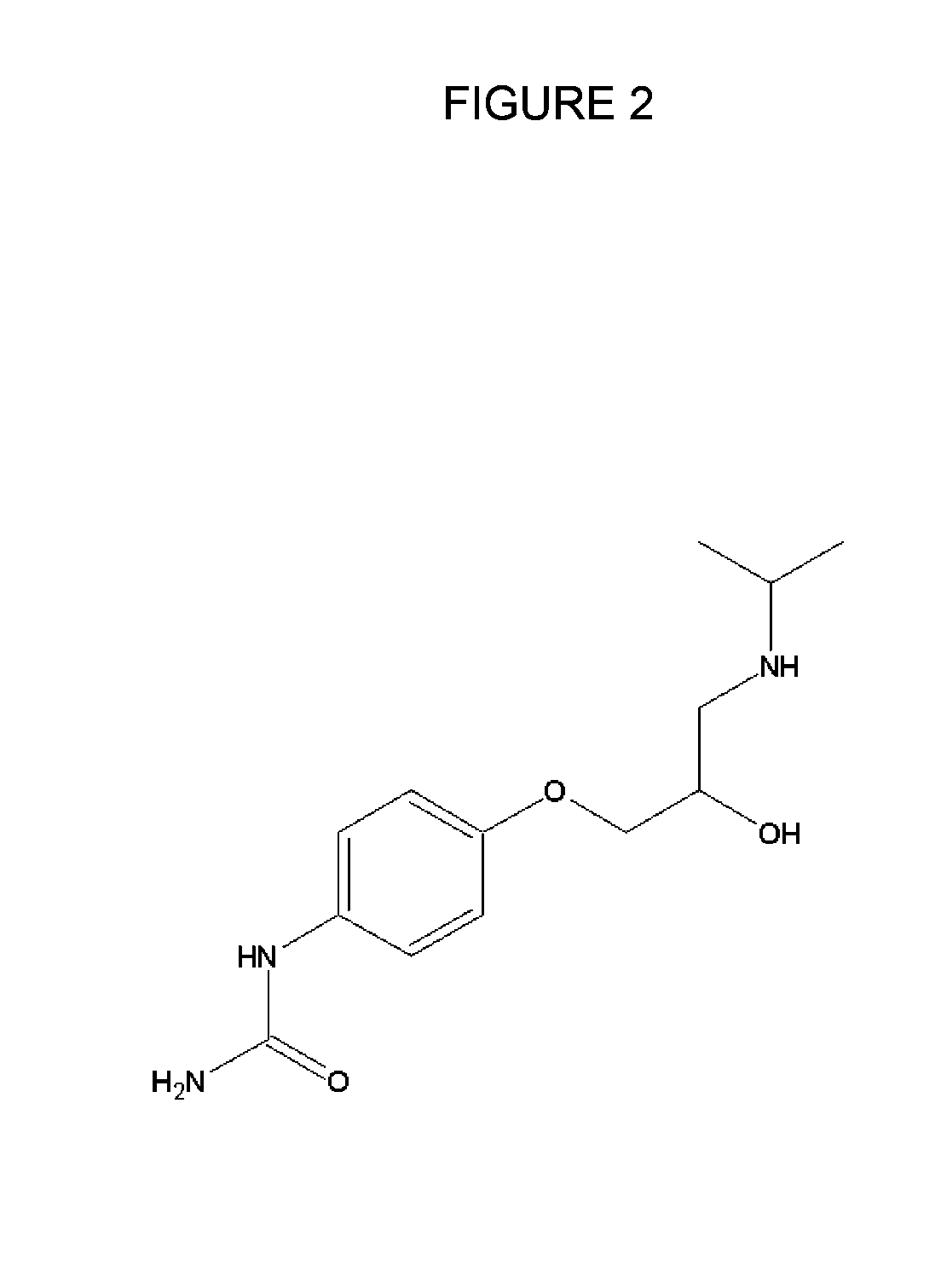
The present invention is directed to methods for developing one or more drugs for one or more targeted therapies and compositions derived therefrom. In accordance with one aspect of the present invention, combinatorial chemistry techniques for use with high throughput screening techniques for identifying small molecule affinity and / or activity interactions are avoided by instead utilizing the natural mechanisms of antigen response to effect a massively parallel screening of naturally occurring molecules against an antigen. Other aspects of the invention provide compositions derived therefrom as well as therapeutic methods of use for the compounds.
Owner:ERRICO JOSEPH PETER
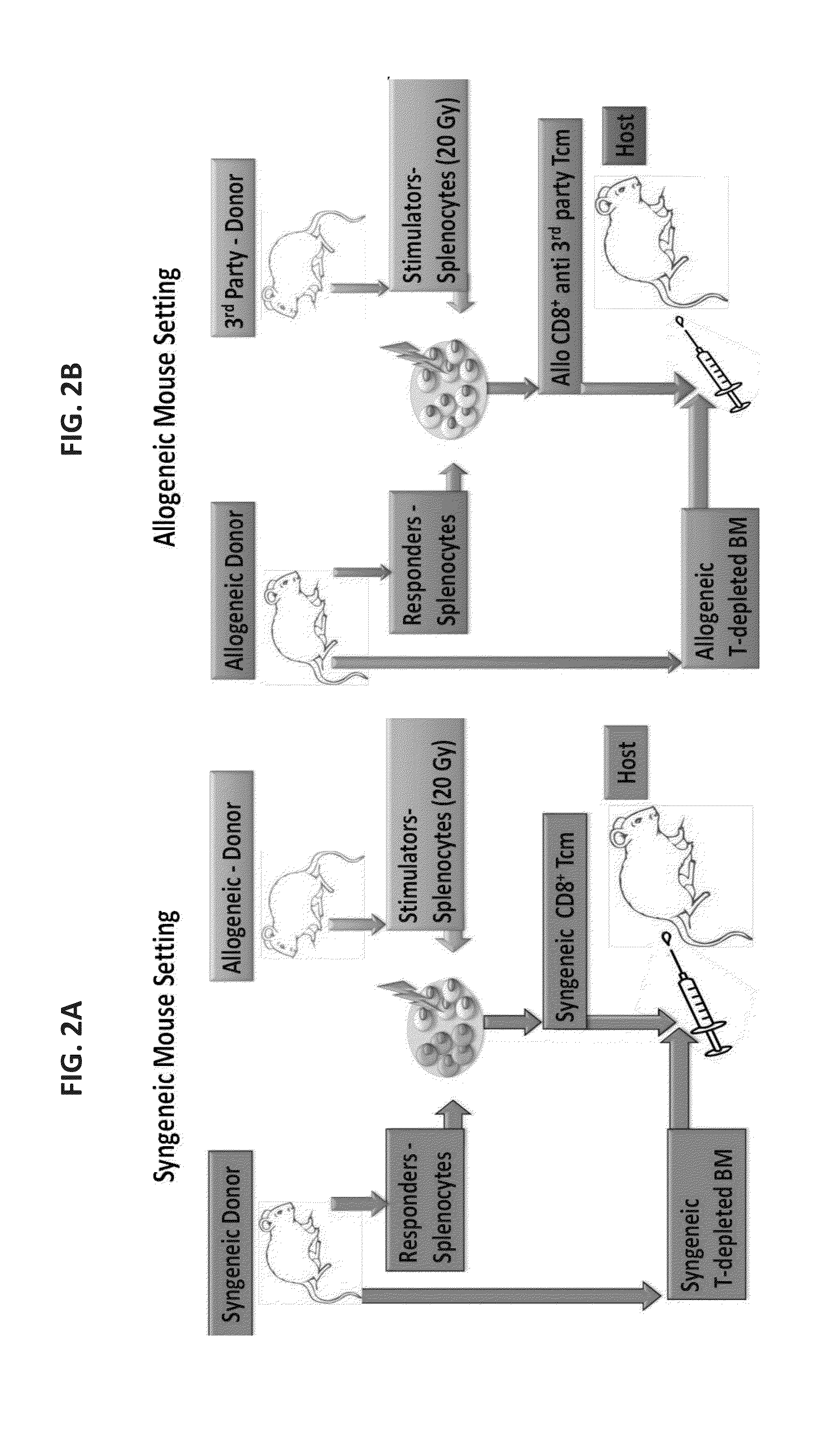
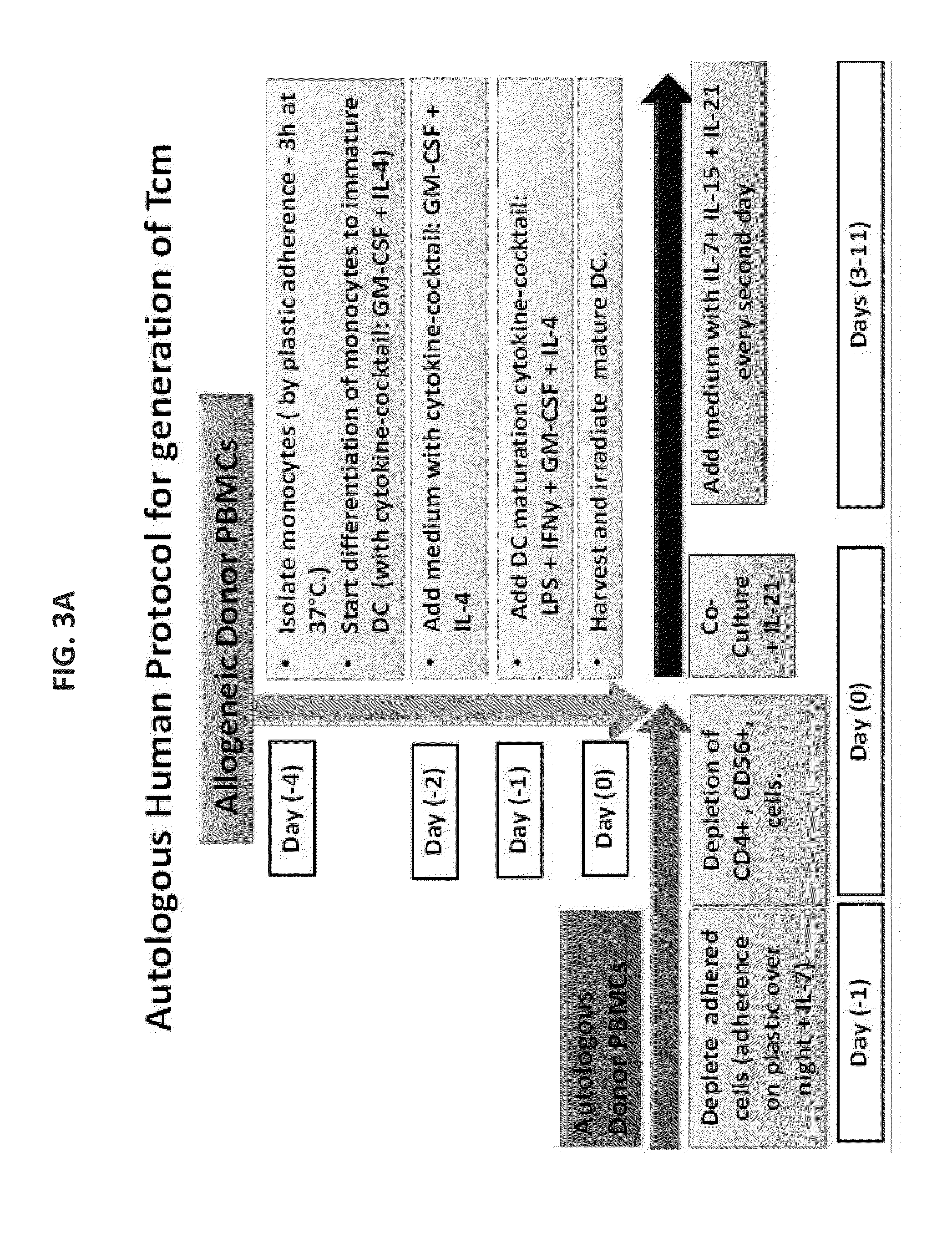
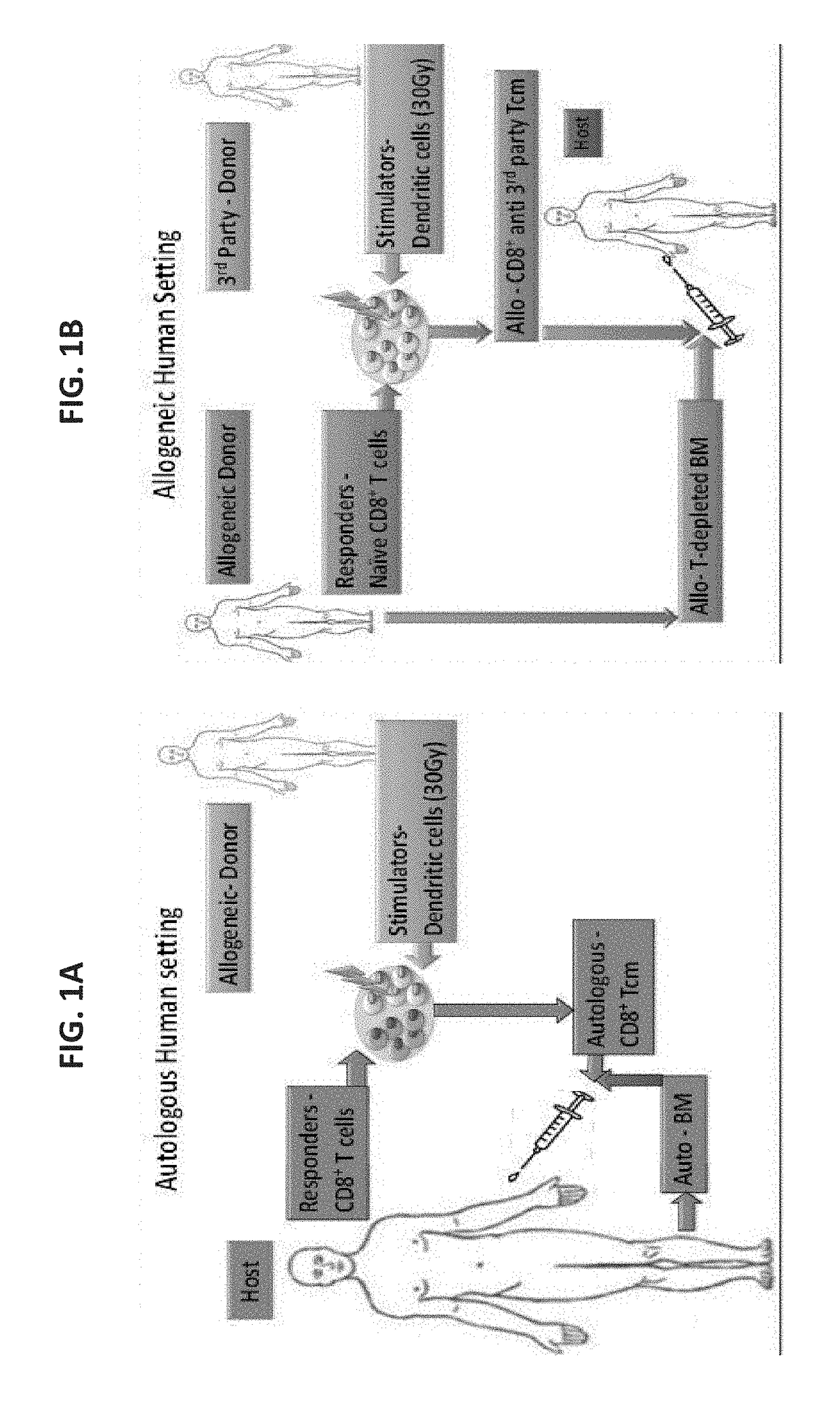
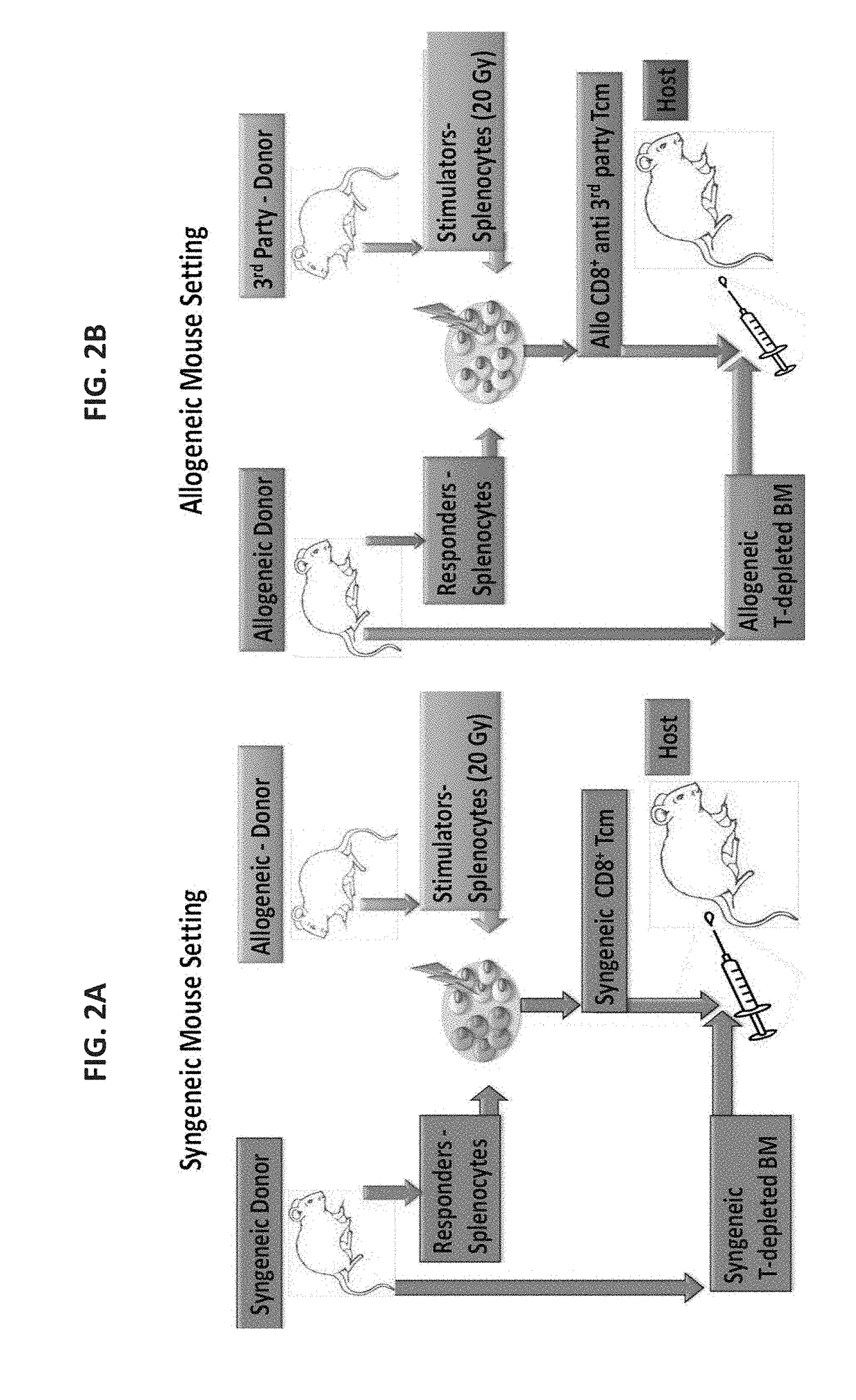
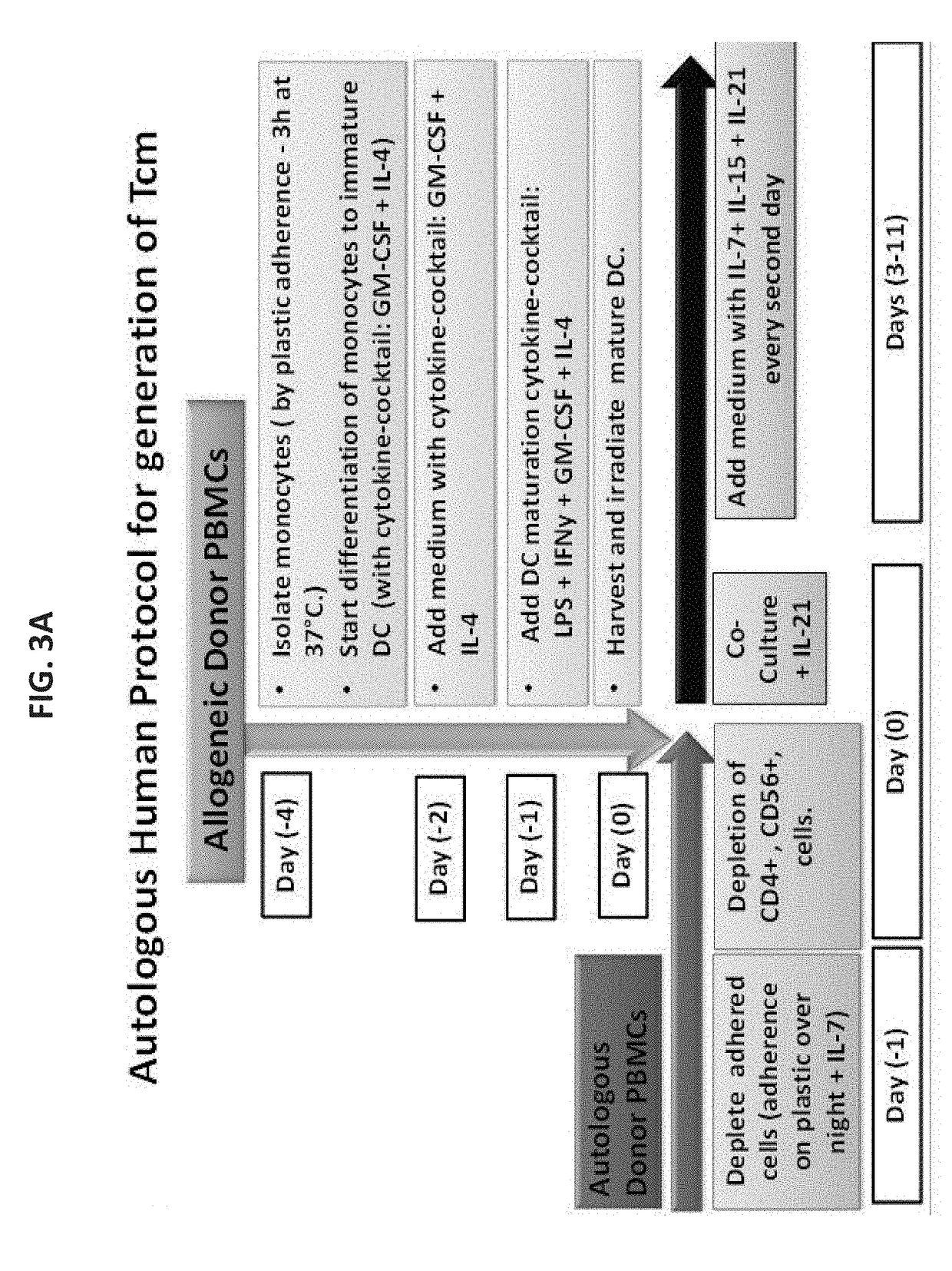
Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
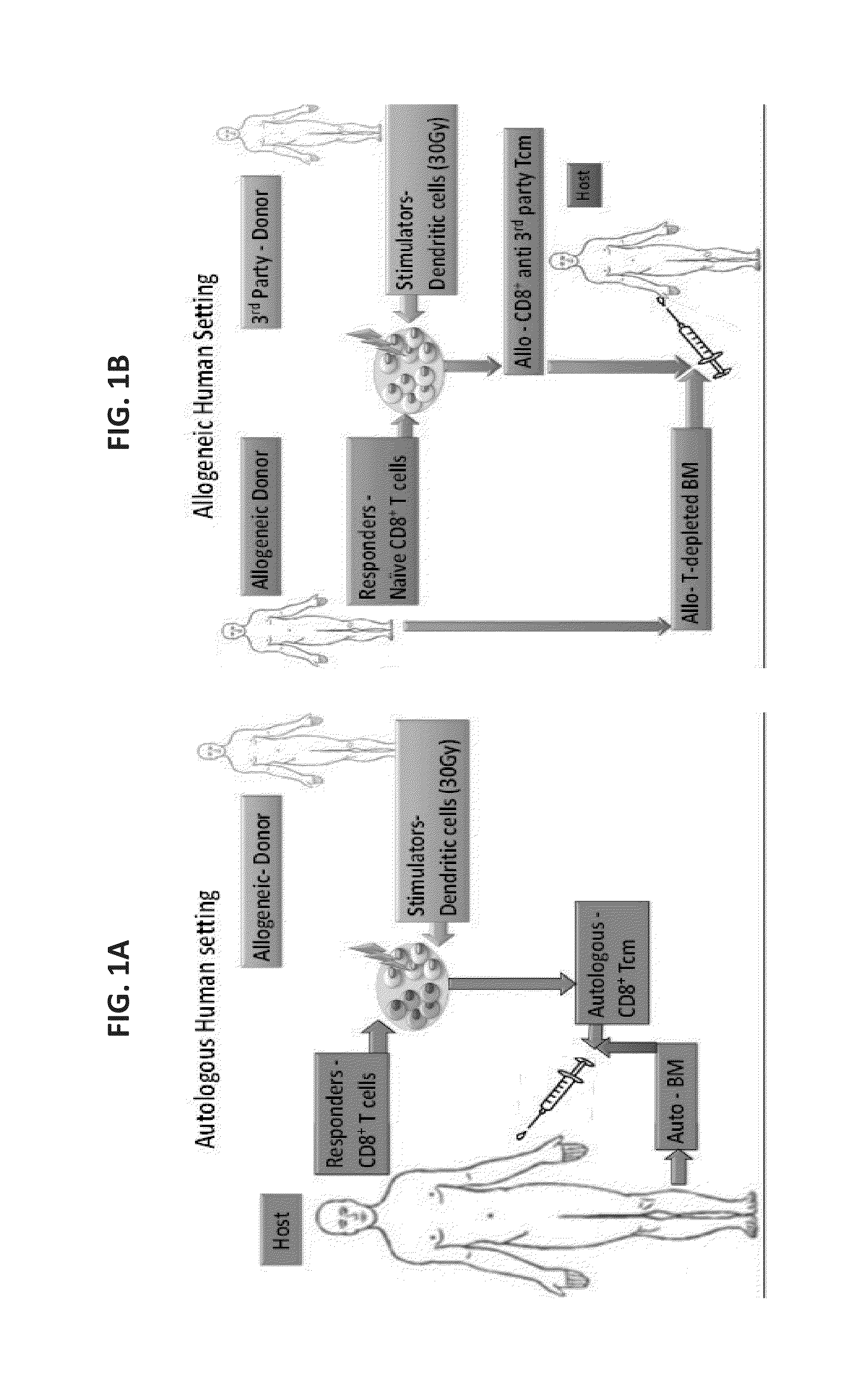
A method of generating an isolated population of cells comprising anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance-inducing cells and / or endowed with anti-disease activity, and capable of homing to the lymph nodes following transplantation is disclosed. The method comprising: (a) contacting peripheral blood mononuclear cells (PBMC) with a third party antigen or antigens in the presence of IL-21 so as to allow enrichment of antigen reactive cells; and (b) culturing the cells resulting from step (a) in the presence of IL-21, IL-15 and IL-7 in an antigen free environment so as to allow proliferation of cells comprising the central memory T-lymphocyte (Tcm) phenotype.
Owner:YEDA RES & DEV CO LTD
Antibody profiling sensitivity through increased reporter antibody layering
InactiveUS7695919B2Bioreactor/fermenter combinationsBiological substance pretreatmentsImmune complex formationImmune complex deposition
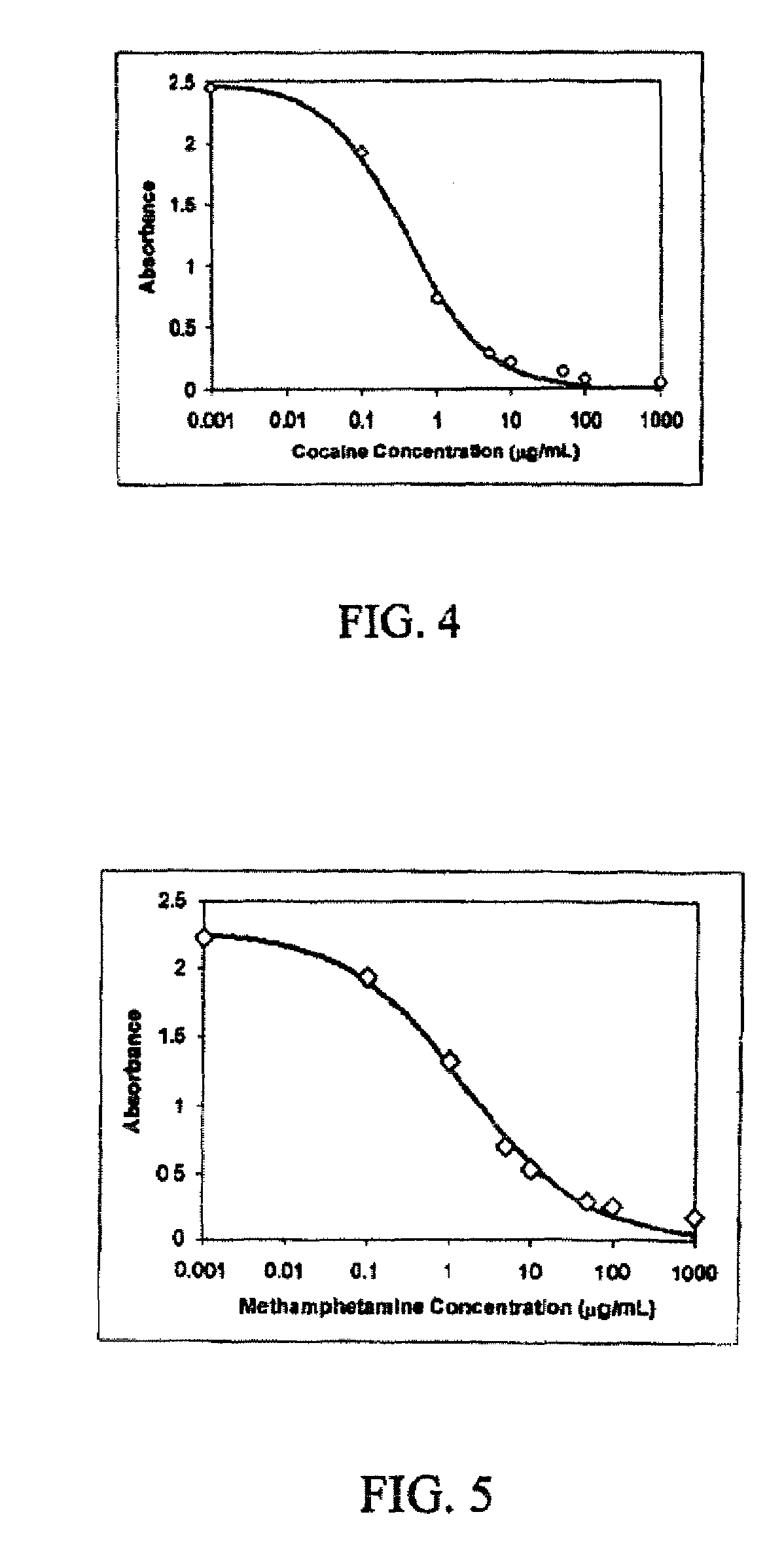
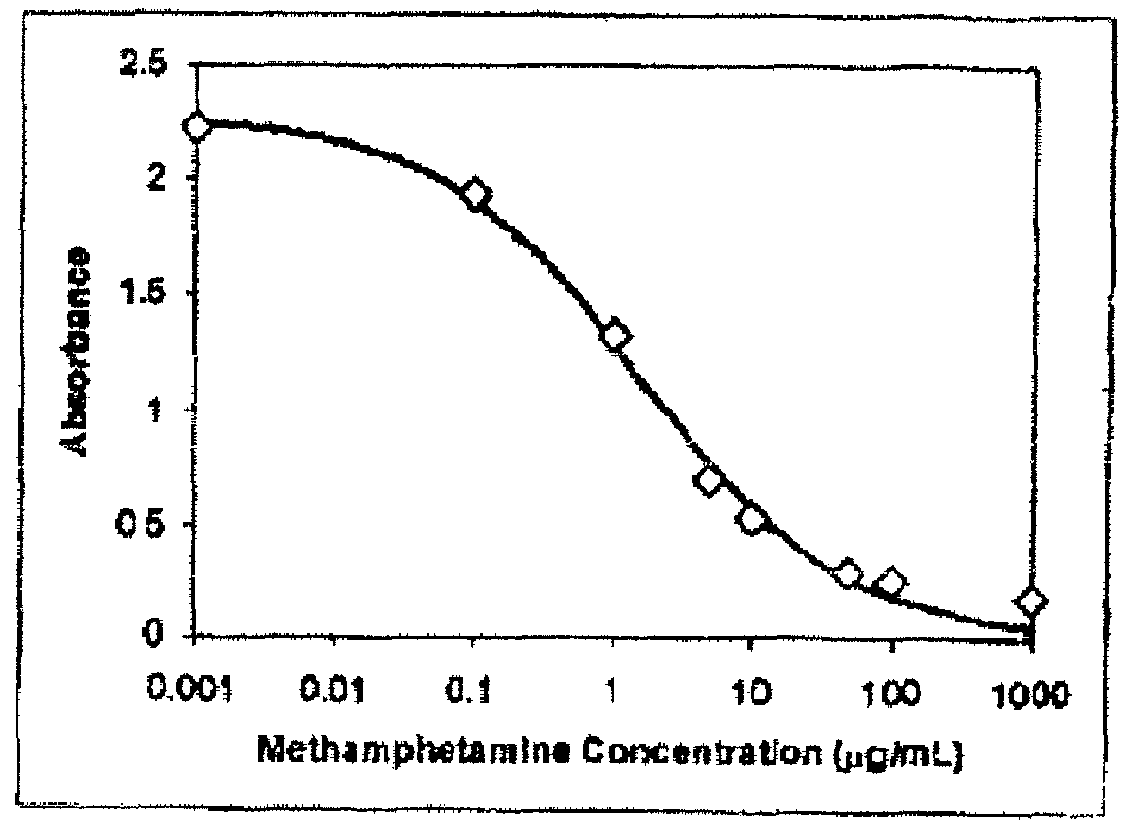
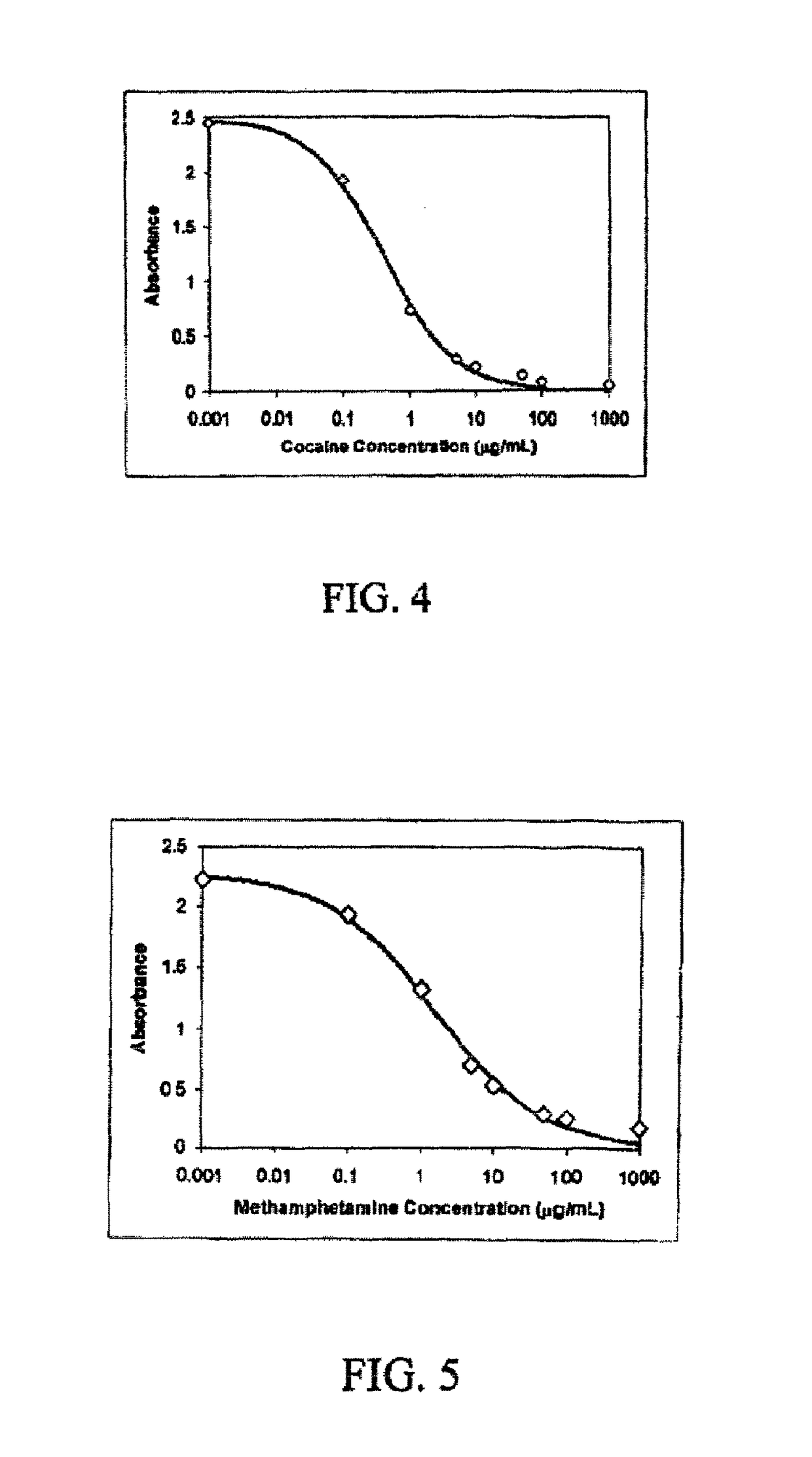
A method for analyzing a biological sample by antibody profiling for identifying forensic samples or for detecting the presence of an analyte. In an embodiment of the invention, the analyte is a drug, such as marijuana, Cocaine (crystalline tropane alkaloid), methamphetamine, methyltestosterone, or mesterolone. The method comprises attaching antigens to a surface of a solid support in a preselected pattern to form an array wherein locations of the antigens are known; contacting the array with the biological sample such that a portion of antibodies in the sample reacts with and binds to the antigens in the array to form immune complexes; washing away antibodies that do form immune complexes; and detecting the immune complexes, to form an antibody profile. Forensic samples are identified by comparing a sample from an unknown source with a sample from a known source. Further, an assay, such as a test for illegal drug use, can be coupled to a test for identity such that the results of the assay can be positively correlated to the subject's identity.
Owner:BATTELLE ENERGY ALLIANCE LLC
Antibody profiling sensitivity through increased reporter antibody layering
InactiveUSRE44031E1Analysis using chemical indicatorsLaboratory glasswaresImmune complex formationImmune complex deposition
A method for analyzing a biological sample by antibody profiling for identifying forensic samples or for detecting the presence of an analyte. In an embodiment of the invention, the analyte is a drug, such as marijuana, Cocaine (crystalline tropane alkaloid), methamphetamine, methyltestosterone, or mesterolone. The method comprises attaching antigens to a surface of a solid support in a preselected pattern to form an array wherein locations of the antigens are known; contacting the array with the biological sample such that a portion of antibodies in the sample reacts with and binds to the antigens in the array to form immune complexes; washing away antibodies that do form immune complexes; and detecting the immune complexes, to form an antibody profile. Forensic samples are identified by comparing a sample from an unknown source with a sample from a known source. Further, an assay, such as a test for illegal drug use, can be coupled to a test for identity such that the results of the assay can be positively correlated to the subject's identity.
Owner:BATTELLE ENERGY ALLIANCE LLC
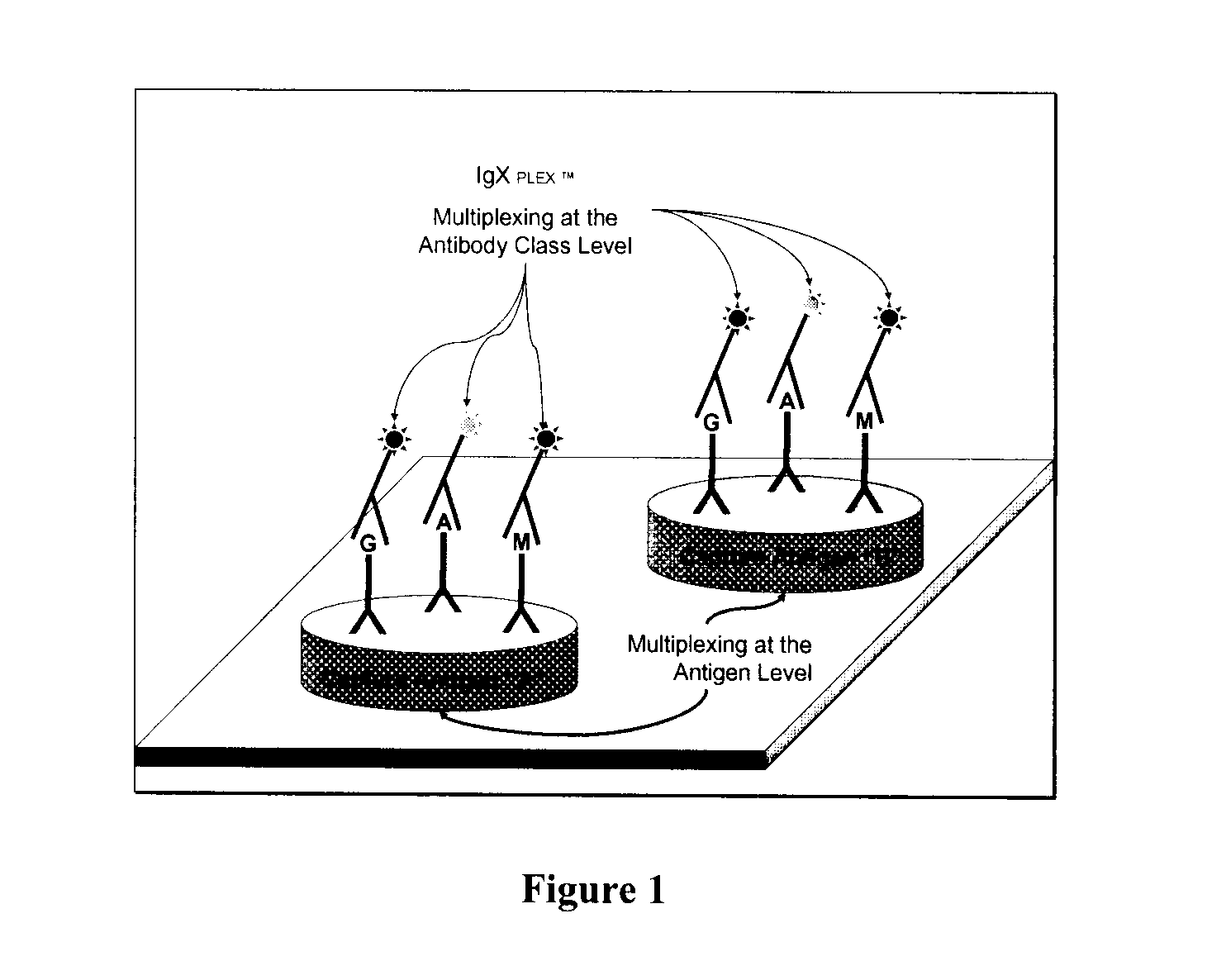
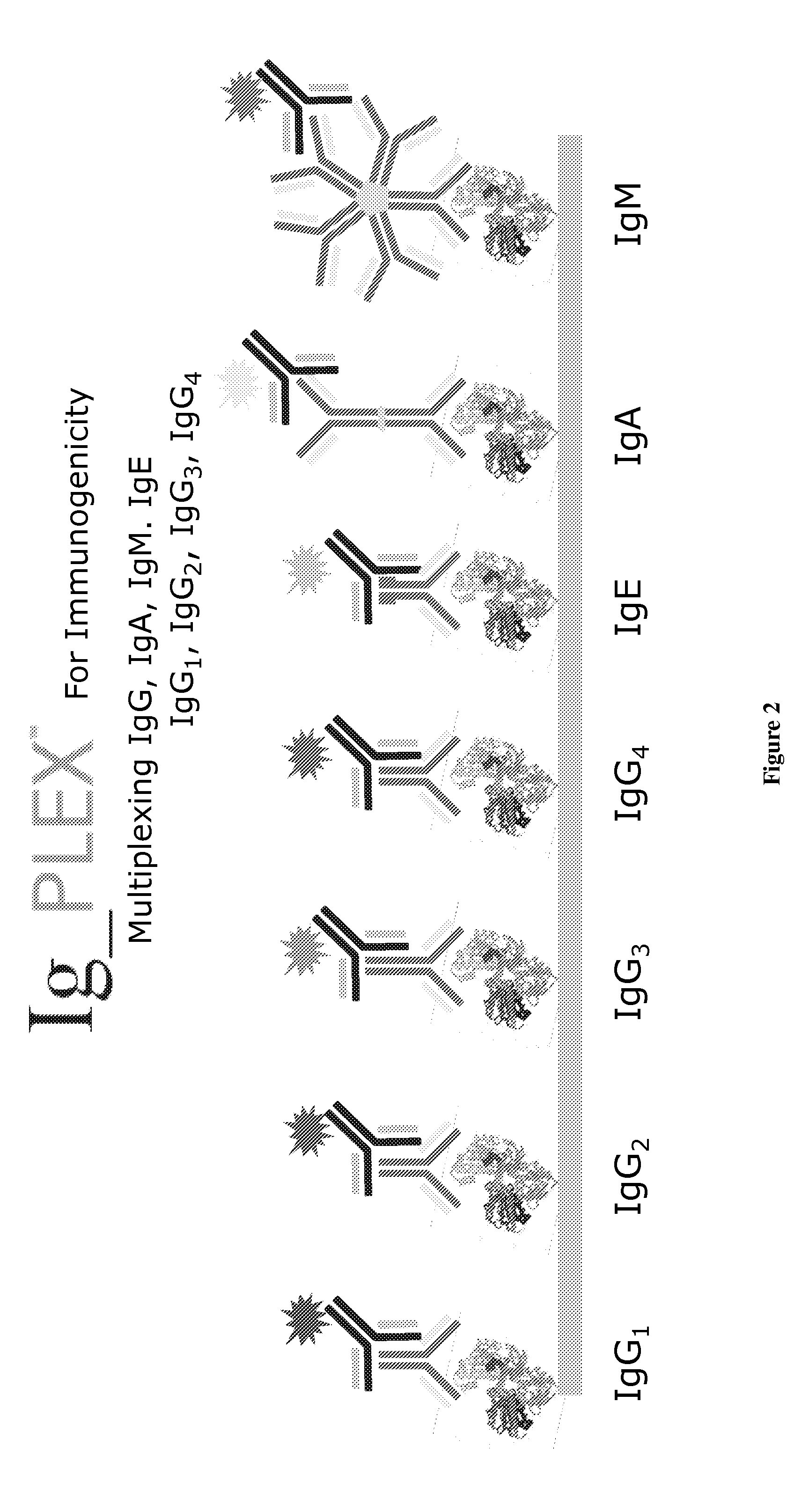
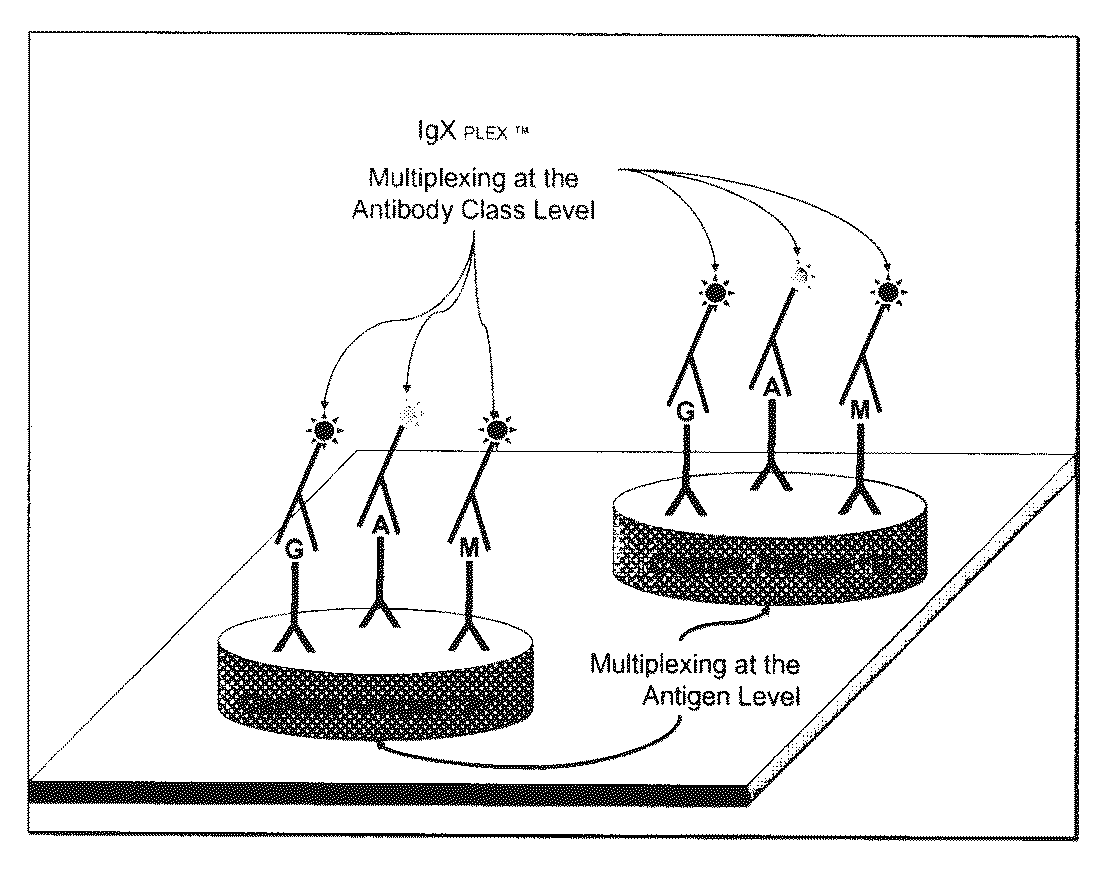
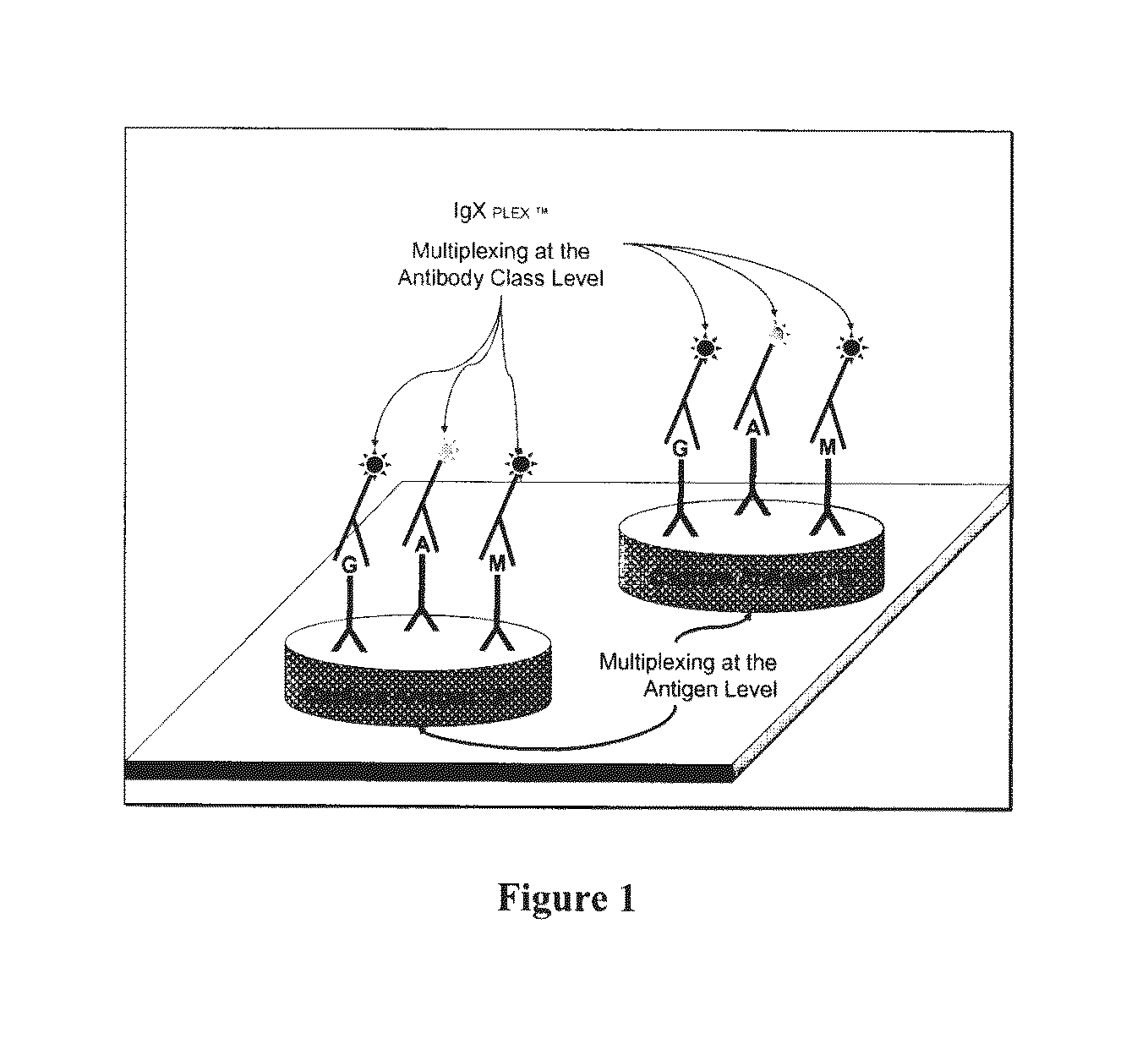
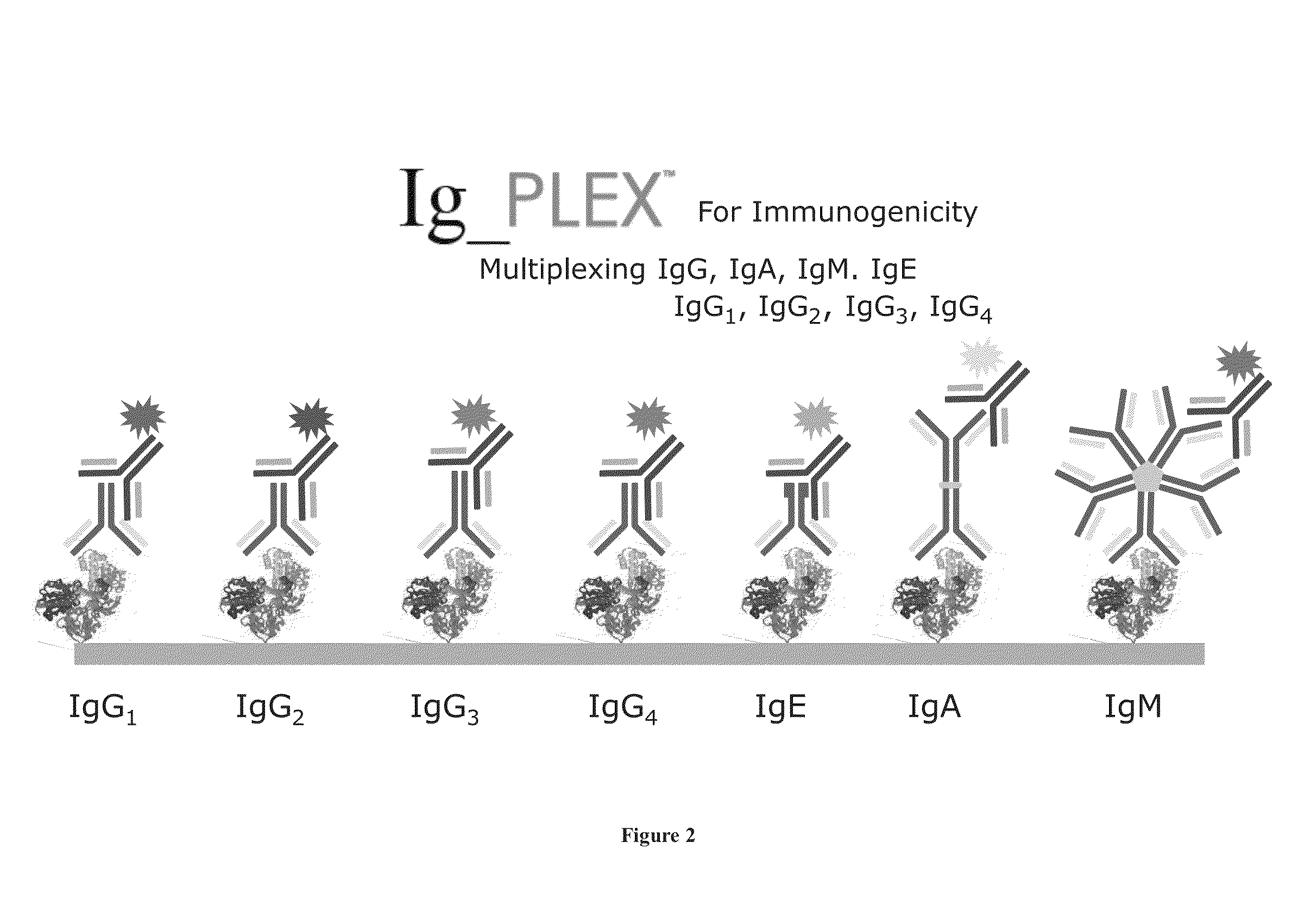
Multiplex measure of isotype antigen response
InactiveUS20130165335A1Fast and cost-effective methodLibrary screeningFluorescence/phosphorescenceImmunoglobulin isotypeTherapeutic protein
The application discloses methods for simultaneous detection and quantifying multiple target analytes, including immunoglobulin isotypes and sub-classes, single and multiple protein antibodies within a test sample in a single reaction vessel. The method uses reaction wells as on a multi-well plate, each single well comprising microarrays of: calibration spots, having a predetermined quantity of a target analyte; and capture spots, having multiple agent antibodies, including isotypes and subclasses that specifically bind the target analytes. The captured analytes and the calibration spots are detected with fluorescently labeled antibodies specific for each analyte. The application also discloses methods for detecting and quantifying biomarkers, therapeutic proteins and patient derived antibodies; the use of secondary reagents to determine immunoglobulin classes Ig G, A, M, E and sub-classes including IgG1, IgG2, IgG3, IgG4 and IgA. The intensity of each fluorescent signal allows measurement of a specific immune response to a therapeutic protein and associated analytes.
Owner:SQI DIAGNOSTICS SYST
Biotinylation bovine gamma globulin-streptavidin ELISA (enzyme linked immunosorbent assay) plate and preparation method thereof
InactiveCN102809650AExpand the scope of detectionDose-response curve is flatColor/spectral properties measurementsSolid phasesAntigen
The invention provides a biotinylation bovine gamma globulin-streptavidin ELISA (enzyme linked immunosorbent assay) plate and a preparation method thereof. The biotinylation bovine gamma globulin-streptavidin ELISA plate comprises a polystyrene micropore plate, wherein the interior of each micropore of the polystyrene micropore plate is precoated with 1.5-3.0 micrograms of biotinylated bovine gamma globulin and 2.7-3.3 micrograms of streptavidin and is sealed by a sealing solution. By utilizing the ELISA plate provided by the invention, a capture antibody cannot be directly coated and is biotinylated, a biotin-avidin system can be used for combining the catch antibody with a solid-phase material (the micropore plate) in the to-be-detected antigen reaction process, thereby achieving the purpose of solid phase adsorption and separation and overcoming the defects that the antibody utilization ratio is low caused by a traditional method of directly coating the catch antibody, the coating amount cannot be met and the reaction equilibration time is retarded.
Owner:天津中企华科生物科技发展有限公司
Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
A method of generating an isolated population of cells comprising anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance-inducing cells and / or endowed with anti-disease activity, and capable of homing to the lymph nodes following transplantation is disclosed. The method comprising: (a) contacting peripheral blood mononuclear cells (PBMC) with a third party antigen or antigens in the presence of IL-21 so as to allow enrichment of antigen reactive cells; and (b) culturing the cells resulting from step (a) in the presence of IL-21, IL-15 and IL-7 in an antigen free environment so as to allow proliferation of cells comprising the central memory T-lymphocyte (Tcm) phenotype.
Owner:YEDA RES & DEV CO LTD
Methods and compositions of targeted drug development
The present invention is directed to methods for developing one or more drugs for one or more targeted therapies and compositions derived therefrom. In accordance with one aspect of the present invention, combinatorial chemistry techniques for use with high throughput screening techniques for identifying small molecule affinity and / or activity interactions are avoided by instead utilizing the natural mechanisms of antigen response to effect a massively parallel screening of naturally occurring molecules against an antigen. Other aspects of the invention provide compositions derived thereform as well as therapeutic methods of use for the compounds.
Owner:约瑟夫・P・埃里科
Kit for detecting urine transferrin through chemiluminescence enzyme immunoassay method, and preparation method thereof
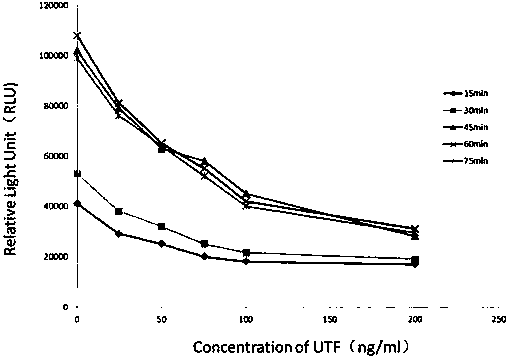
InactiveCN107643282AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingImmune complex depositionEnzyme immunoassays
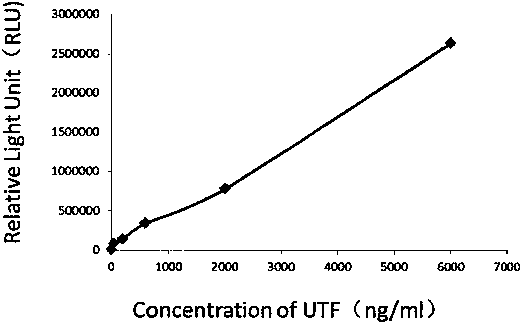
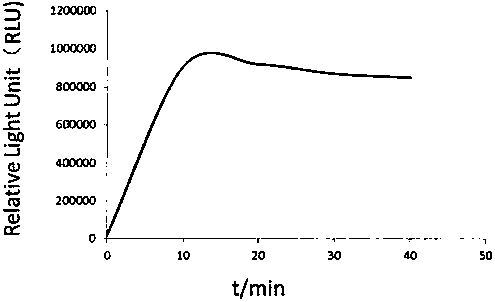
The invention relates to a kit for detecting urine transferrin through a chemiluminescence enzyme immunoassay method, and a preparation method thereof. The kit comprises an antibody-coated plate, a urine transferrin enzyme antibody, a coating buffer solution, a blocking solution, a urine transferrin standard substance, a standard substance dilution solution, a washing solution, a sample dilution solution and a chemiluminescent substrate solution. According to the present invention, the kit can detect the UTF concentration level of human based on chemiluminescence enzyme immunoassay; the chemiluminescent substrate catalytic enzyme HRP and the anti-UTF antibody are conjugated, the obtained conjugate reacts with the sample or the antigen to form the immune complexes, the chemiluminescent substrate solution is added, the relative light unit of the chemiluminescent reaction is measured, the UTF antigen content in the standard substance / the sample is proportional to the relative light unit measured by the optical system, and through standard curve fitting, the UTF content in the urine sample is determined; and the kit of the invention has advantages of high sensitivity, strong specificity, easy operation, low cost and the like.
Owner:长春市诺克因生物科技有限公司
Methods of obtaining antigen-specific T cell populations
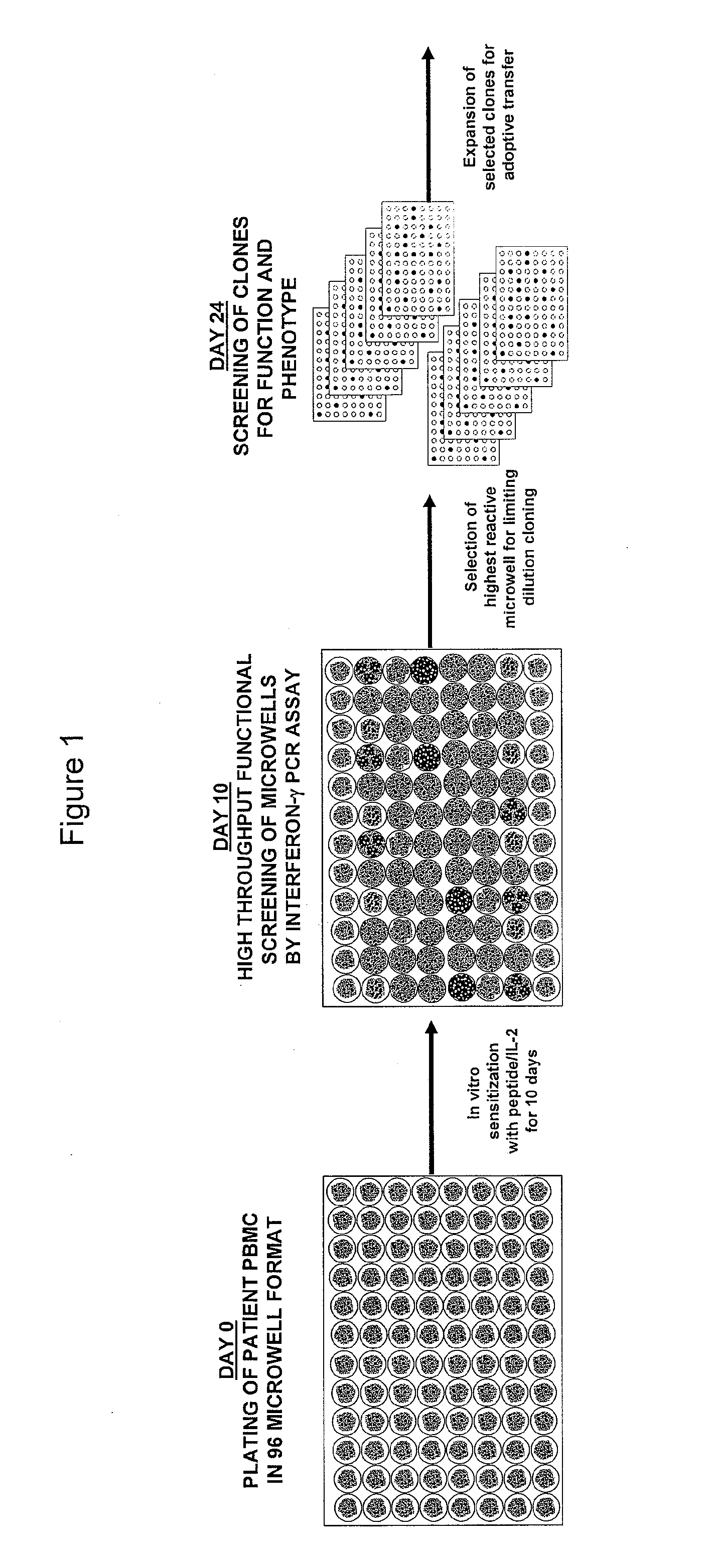
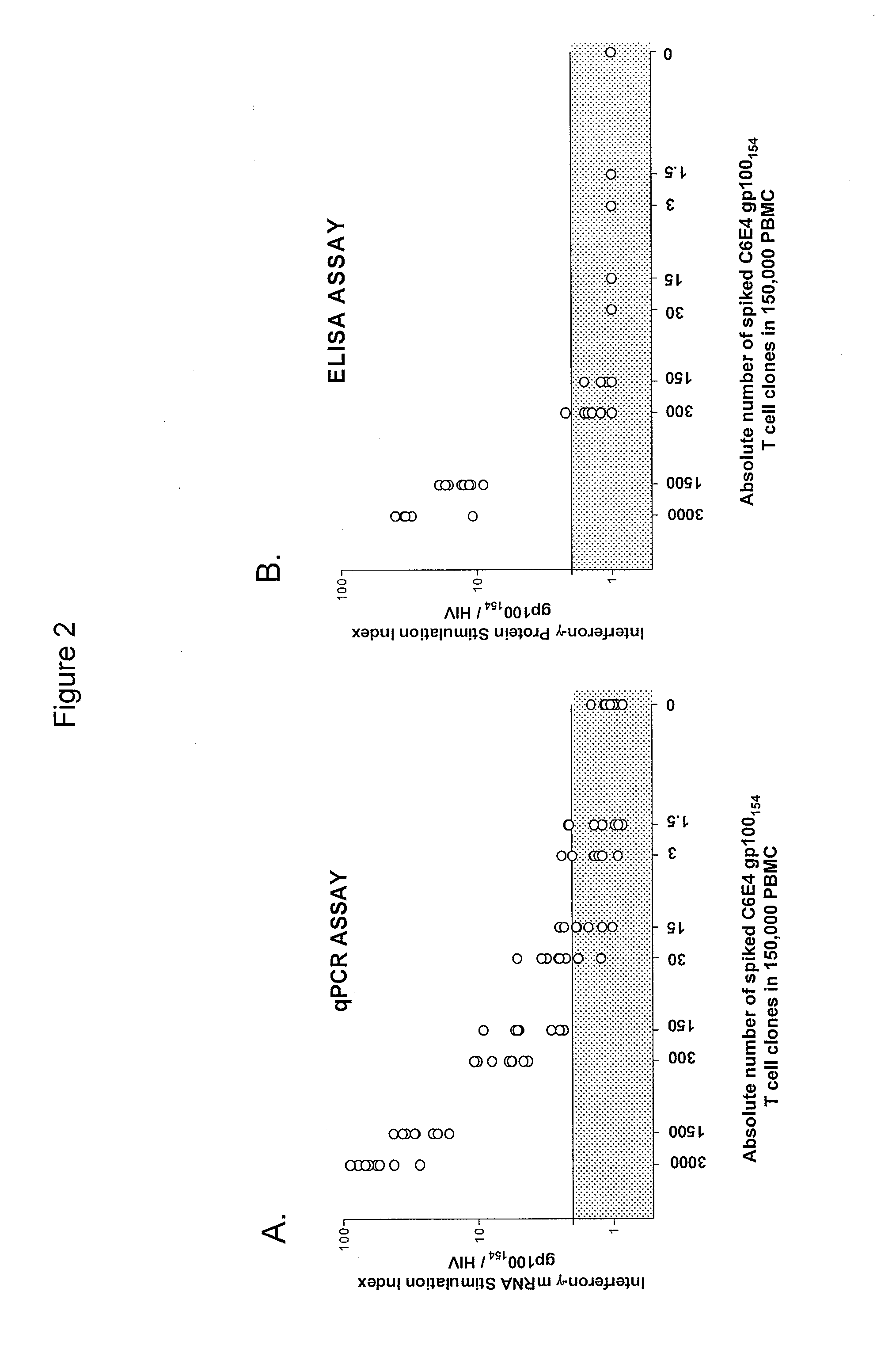
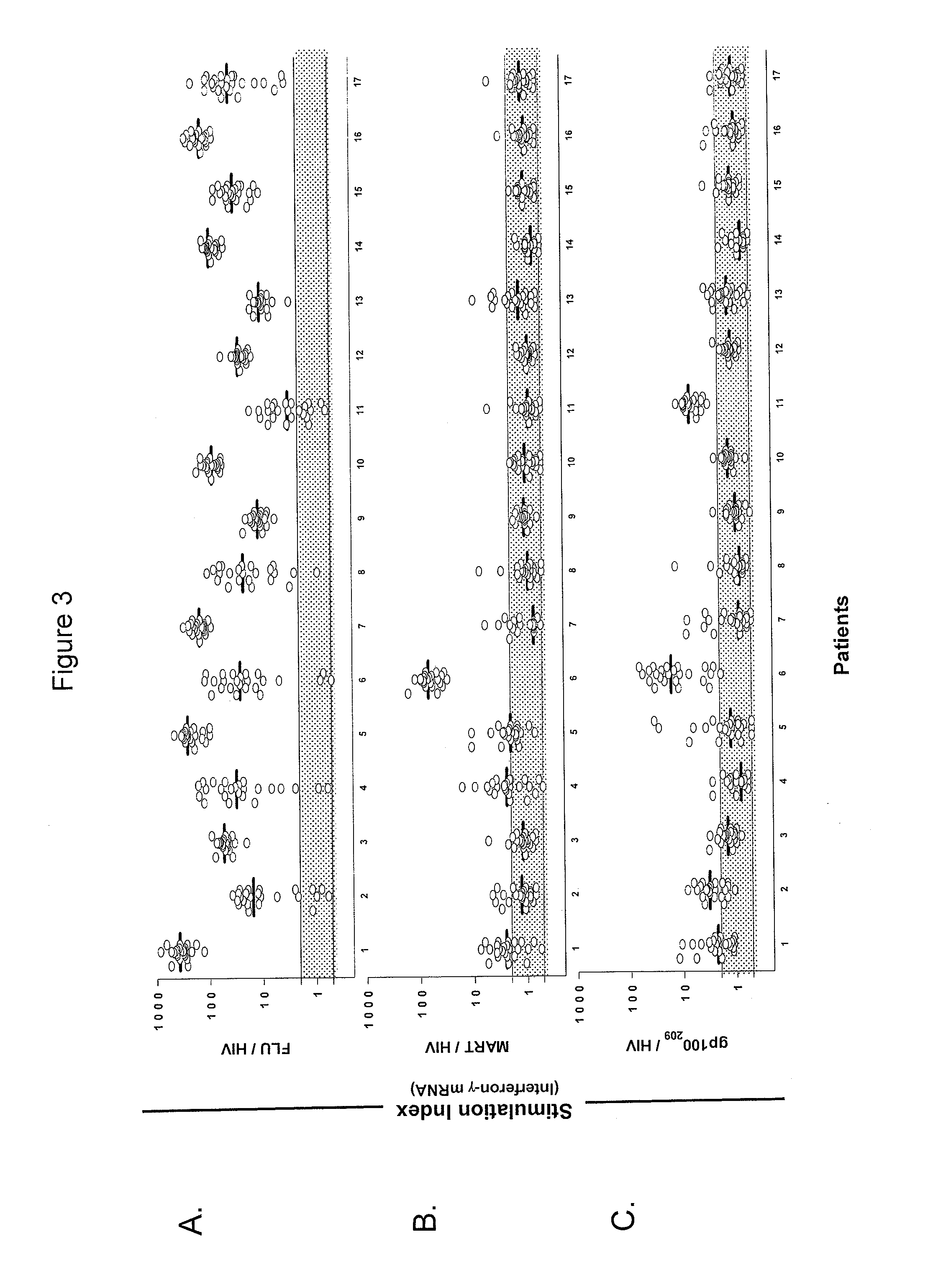
InactiveUS8759014B2Fast wayImprove throughputBiocideMicrobiological testing/measurementDiseaseSub populations
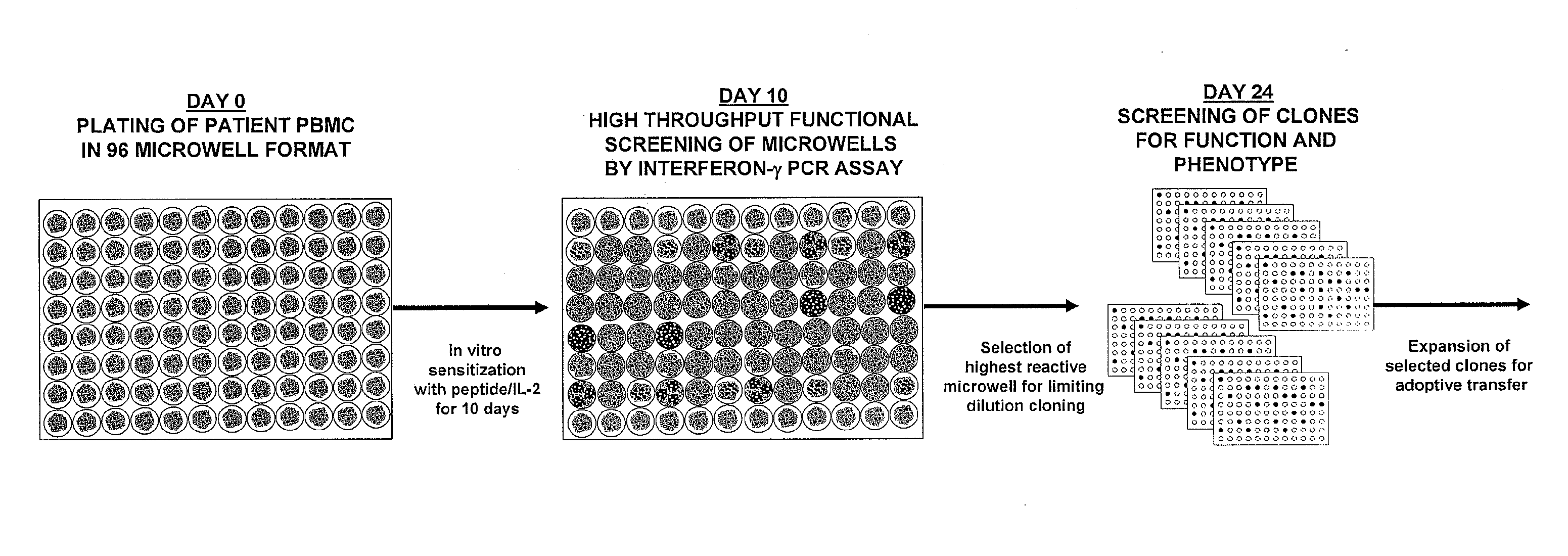
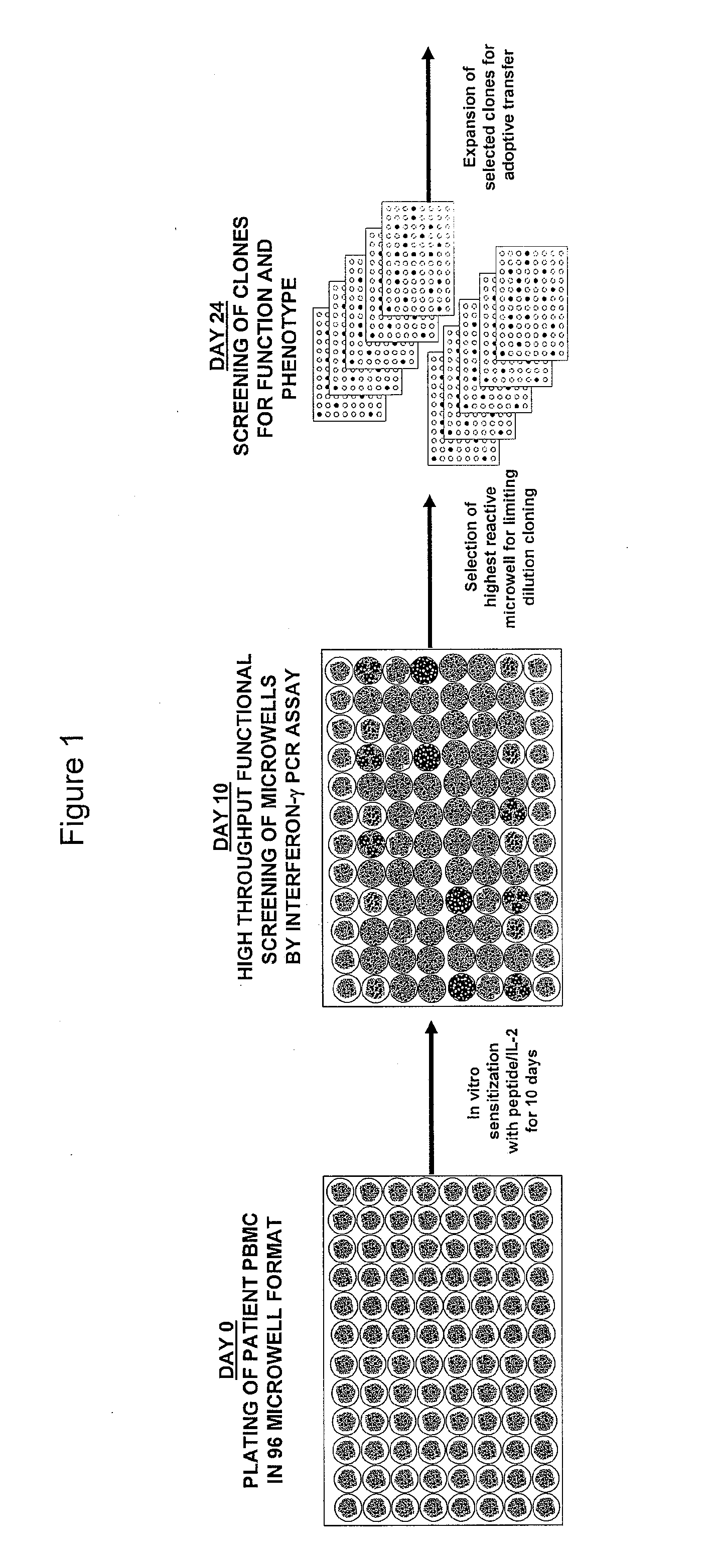
The invention provides a method of obtaining a population of antigen-specific T cells from peripheral blood of a host. An embodiment of the method of the invention comprises (i) dividing PBMCs from peripheral blood of a host into more than one sub-population; (ii) contacting the PBMCs with an antigen and IL-2; (iii) obtaining a sample of PBMCs from each sub-population; (iv) identifying an antigen-reactive sub-population by determining by high throughput quantitative PCR the expression of a factor produced by the PBMCs of each sample; (v) dividing the antigen-reactive sub-population into microcultures; (vi) identifying the antigen-reactive microculture; and (vii) expanding the microculture, thereby obtaining a population of T cells specific for the antigen. The invention also provides a population of T cells obtained by the inventive method, a pharmaceutical composition comprising the same, and a method of treating a disease in a host using the pharmaceutical composition. Related isolating and screening methods are further provided.
Owner:UNITED STATES OF AMERICA
Enzyme-linked immunologic adsorption test method for identification and diagnosis of chicken infected with avian influenza
The invention relates to the enzyme combined immune absorbing testing for diagnosing bird flu affected chicks. It expands H9N2 bird flu virus, acquiring target section to connect with the carrier to restructure the expressing particle, injecting into bacillus coli expression to get a conjugated protein which is purified to get H9N2 bird flu virus NS1 protein to be antibody wrapping enzyme reaction plate, to build the antibody level to be reacted to inspect of the serum, getting the marginal value and least sample number of the enzyme combined immune adhesive test through statistics, and finally based on the average OD value to identify the bird flu affection status of the overall poultry. Through overall diagnosis, it alleviates nonspecific reaction, provides scientific proof for timely diagnosis and prevention of bird flu.
Owner:SHANGHAI JIAO TONG UNIV
Two-photon fluorescent probe, fluorescence-labeled kit and detection method used for biopsy of intracellular carbohydrate chain antigen
ActiveCN105238388AHigh sensitivityImprove stabilityBiological testingFluorescence/phosphorescenceCarbohydrate antigenImmune complex deposition
The invention relates to the technical field of biopsy of intracellular carbohydrate chain antigen, specifically to a two-photon fluorescent probe, a fluorescence-labeled kit and a detection method used for biopsy of intracellular carbohydrate chain antigen. According to the invention, a two-photon fluorescence labeled antibody LP-IgG and a nitrocellulose-membrane-coated solid-phase antibody Ab are used; during detection, the solid-phase antibody specifically captures to-be-detected antigen through antigen reaction, so an immune complex Ab-Ag is formed; and the two-photon fluorescence labeled antibody LP-IgG is added and fully reacts with the immune complex (LP-IgG.Ab.Ag), then non-specific substances are washed and removed, and results can be detected and determined. The detection method provided by the invention overcomes the disadvantages of poor sensitivity and stability and complicated operation steps in a traditional detection method for carbohydrate antigen; meanwhile, detection samples are no longer limited to blood and peripheral blood, and any sample with carbohydrate chain antigen can be detected by using the kit prepared from the two-photon fluorescence-labeled probe LP.
Owner:ZUNYI NORMAL COLLEGE
Antibody profiling sensitivity through increased reporter antibody layering
A method for analyzing a biological sample by antibody profiling for identifying forensic samples or for detecting the presence of an analyte. In an embodiment of the invention, the analyte is a drug, such as marijuana, Cocaine (crystalline tropane alkaloid), methamphetamine, methyltestosterone, or mesterolone. The method comprises attaching antigens to a surface of a solid support in a preselected pattern to form an array wherein locations of the antigens are known; contacting the array with the biological sample such that a portion of antibodies in the sample reacts with and binds to the antigens in the array to form immune complexes; washing away antibodies that do form immune complexes; and detecting the immune complexes, to form an antibody profile. Forensic samples are identified by comparing a sample from an unknown source with a sample from a known source. Further, an assay, such as a test for illegal drug use, can be coupled to a test for identity such that the results of the assay can be positively correlated to the subject's identity.
Owner:BATTELLE ENERGY ALLIANCE LLC
Methods of obtaining antigen-specific t cell populations
InactiveUS20100322910A1High throughput screeningSensitive highBiocideMicrobiological testing/measurementDiseasePharmaceutical drug
The invention provides a method of obtaining a population of antigen-specific T cells from peripheral blood of a host. An embodiment of the method of the invention comprises (i) dividing PBMCs from peripheral blood of a host into more than one sub-population; (ii) contacting the PBMCs with an antigen and IL-2; (iii) obtaining a sample of PBMCs from each sub-population; (iv) identifying an antigen-reactive sub-population by determining by high throughput quantitative PCR the expression of a factor produced by the PBMCs of each sample; (v) dividing the antigen-reactive sub-population into microcultures; (vi) identifying the antigen-reactive microculture; and (vii) expanding the microculture, thereby obtaining a population of T cells specific for the antigen. The invention also provides a population of T cells obtained by the inventive method, a pharmaceutical composition comprising the same, and a method of treating a disease in a host using the pharmaceutical composition. Related isolating and screening methods are further provided.
Owner:UNITED STATES OF AMERICA
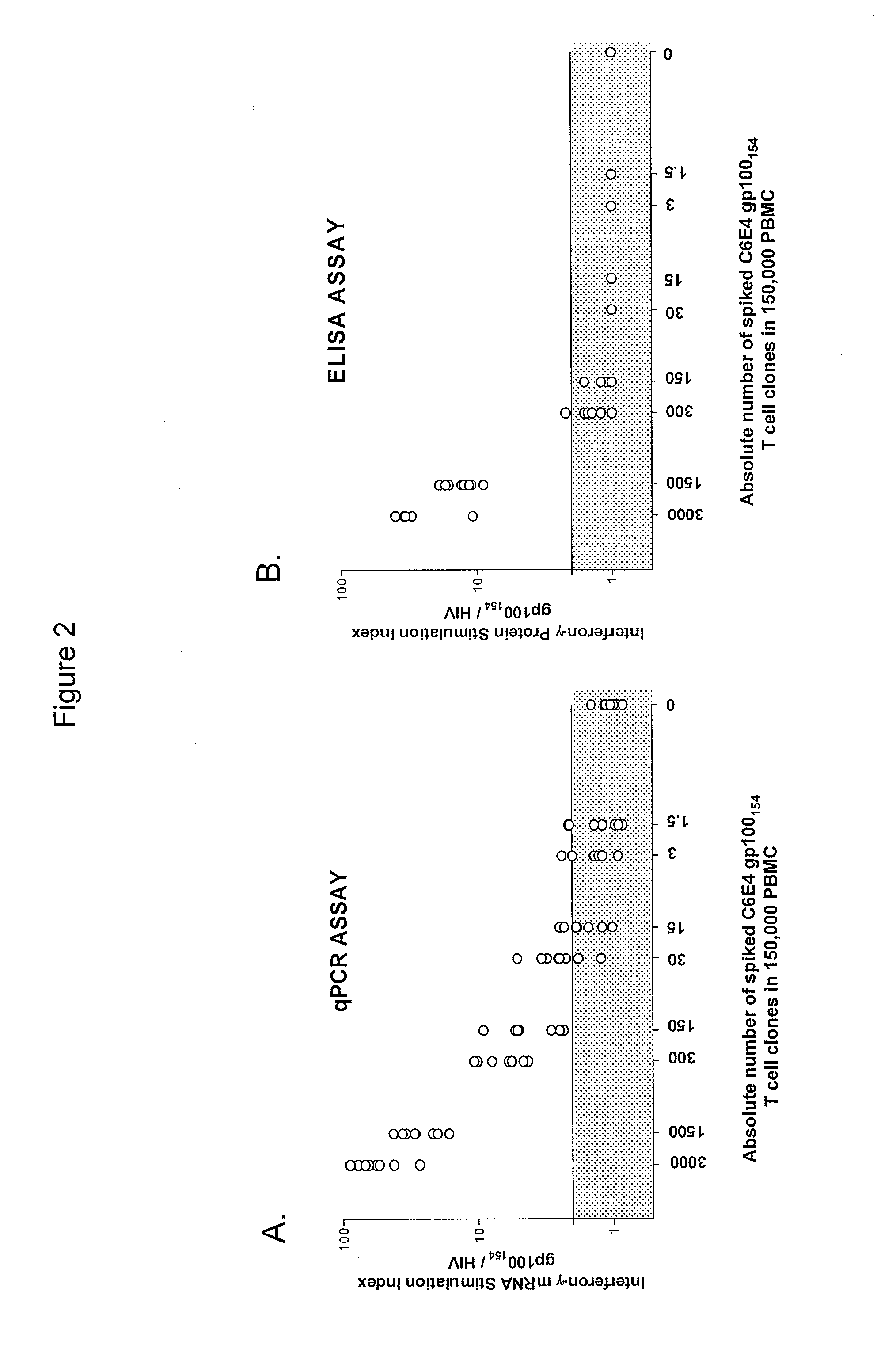
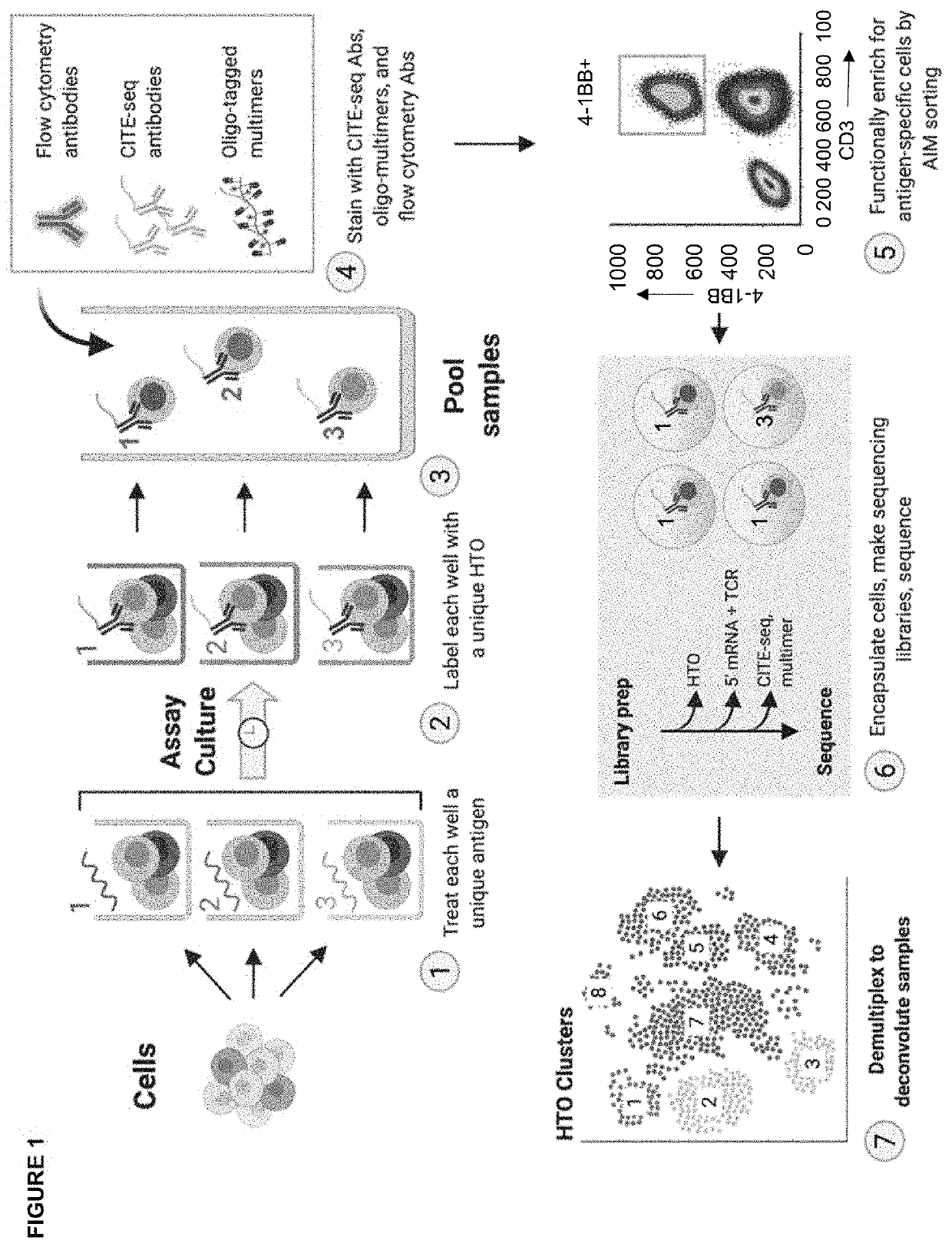
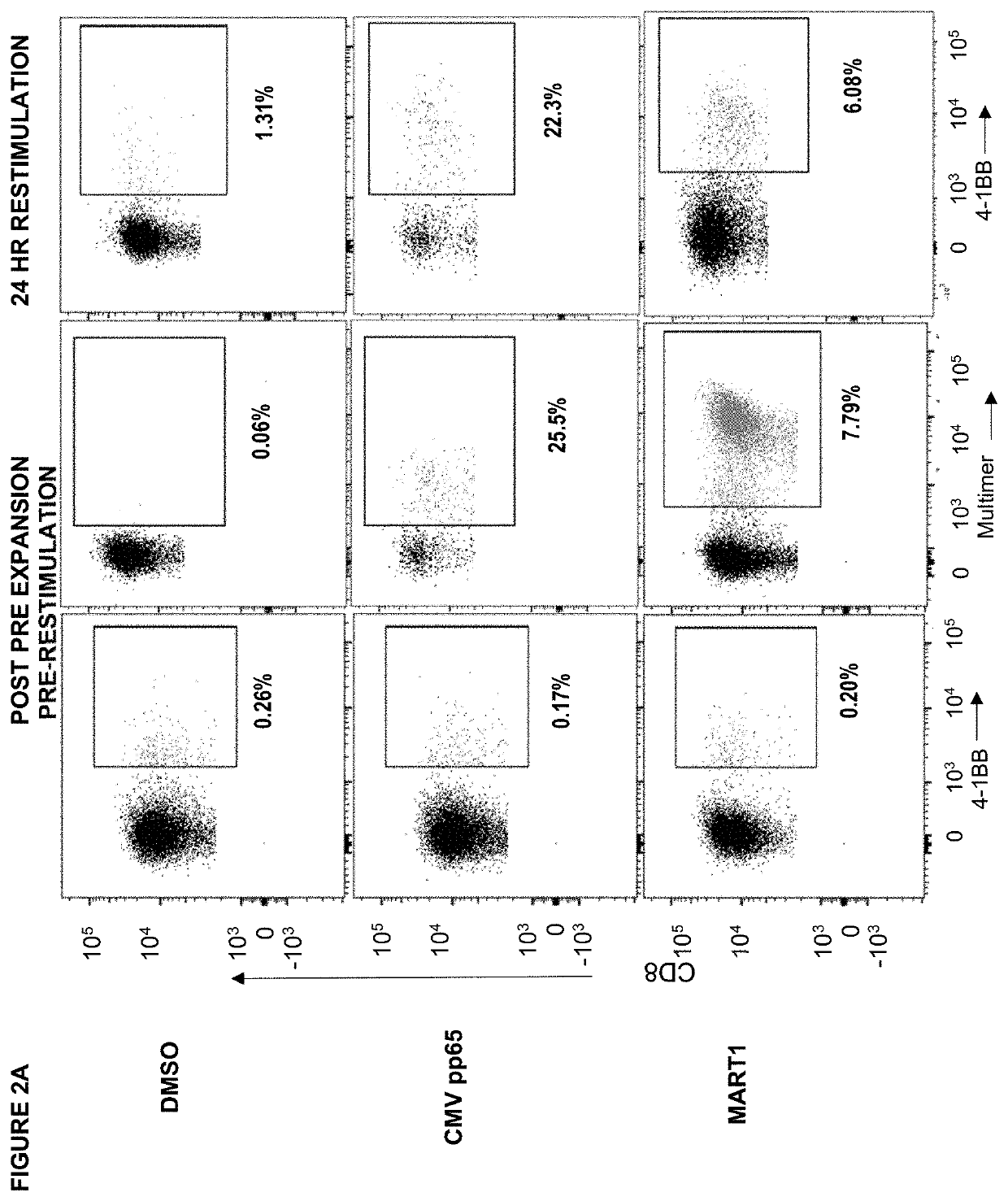
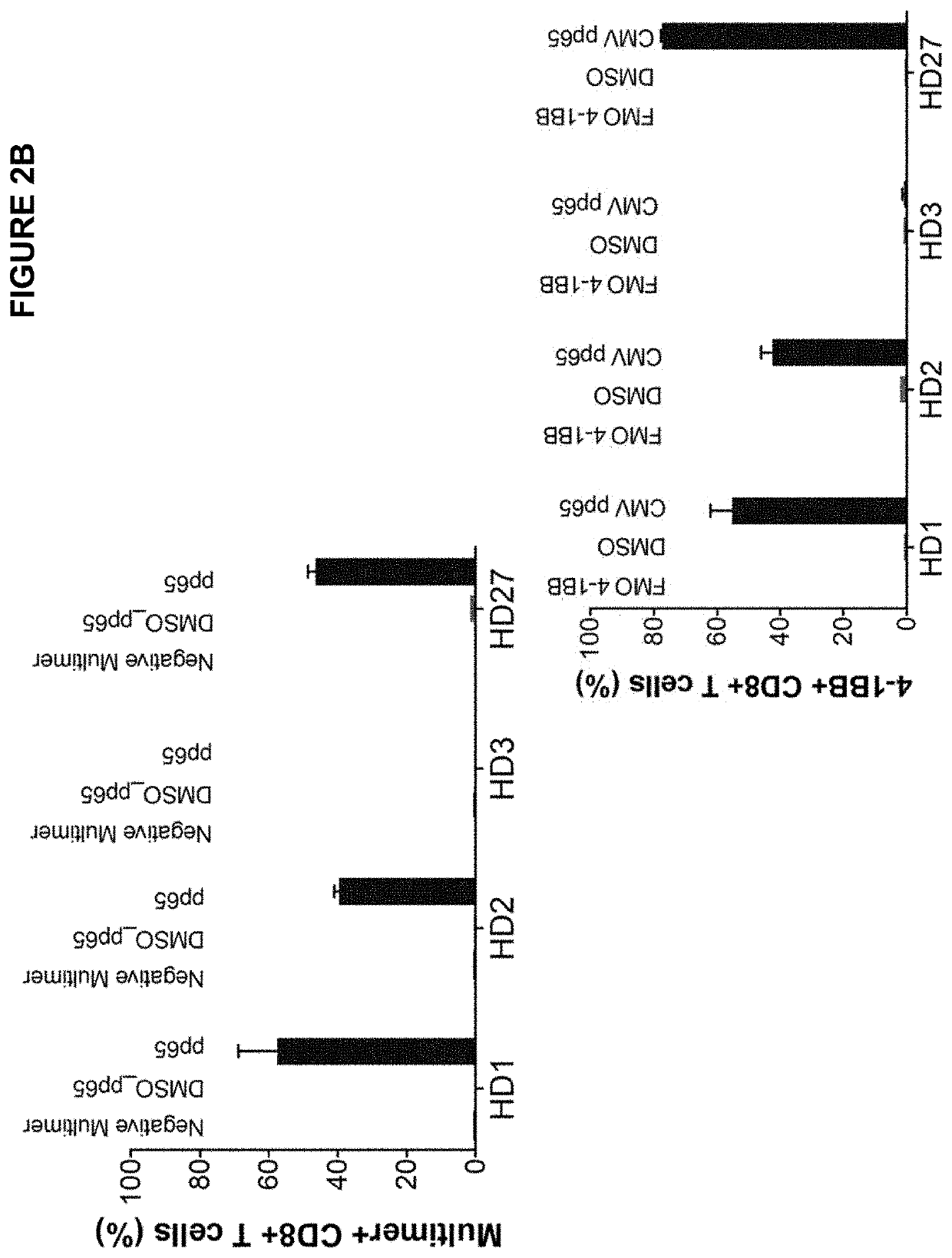
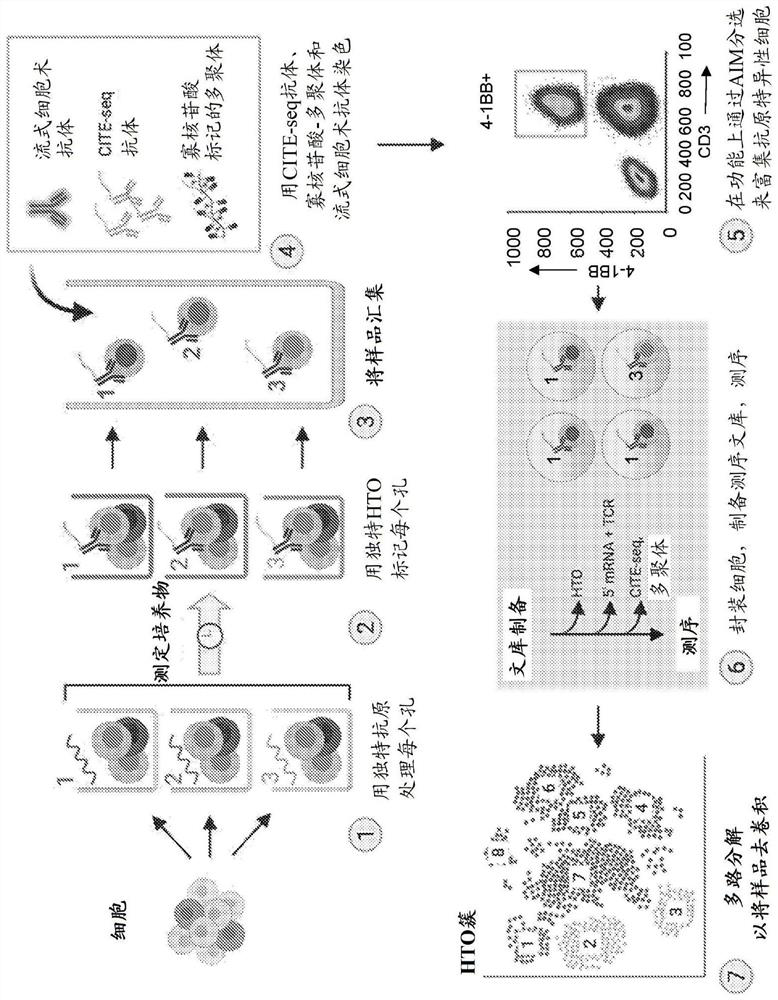
High-throughput method to screen cognate T cell and epitope reactivities in primary human cells
PendingUS20210102942A1Microbiological testing/measurementBiological material analysisAntigenOligonucleotide
Owner:REGENERON PHARM INC
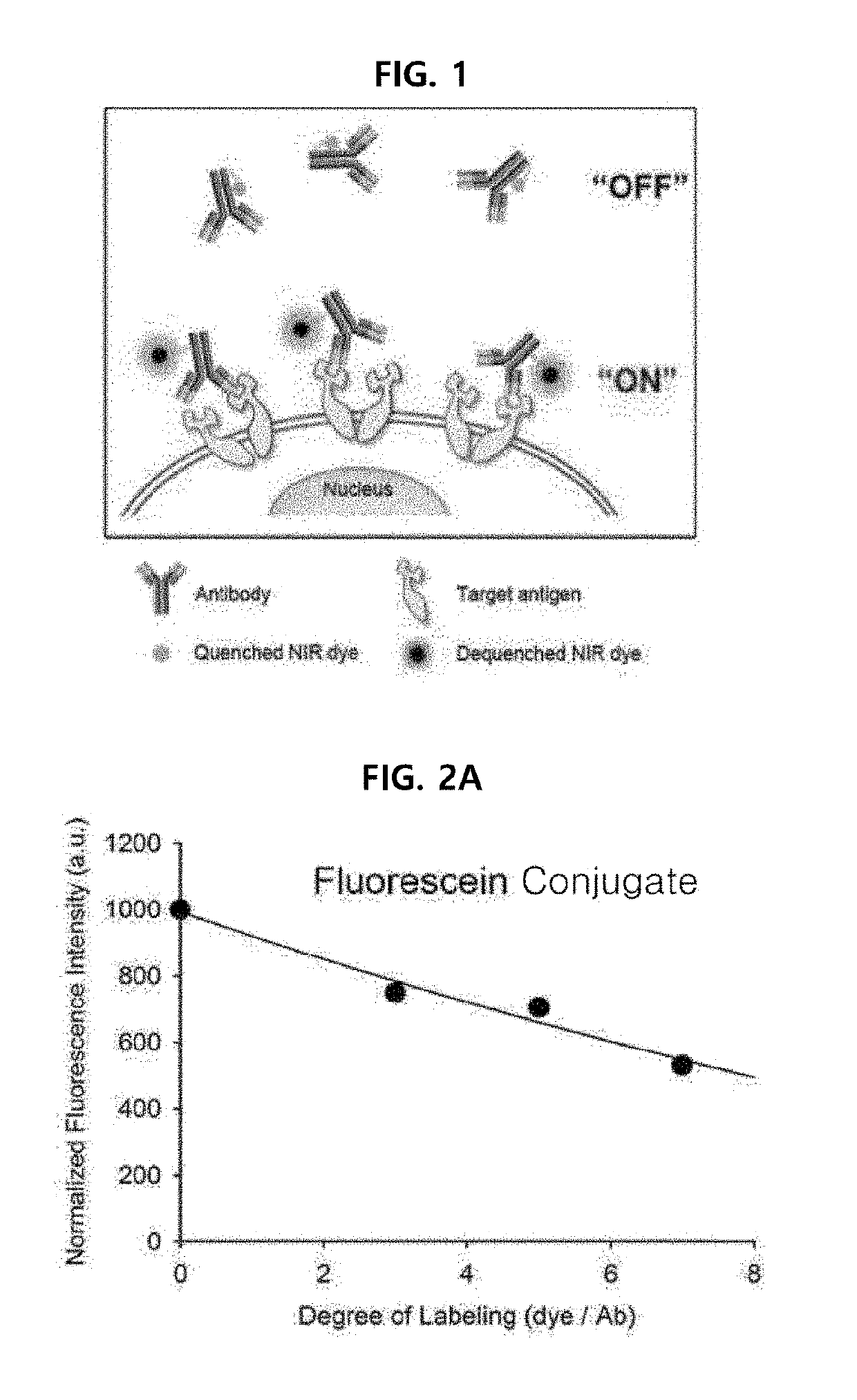
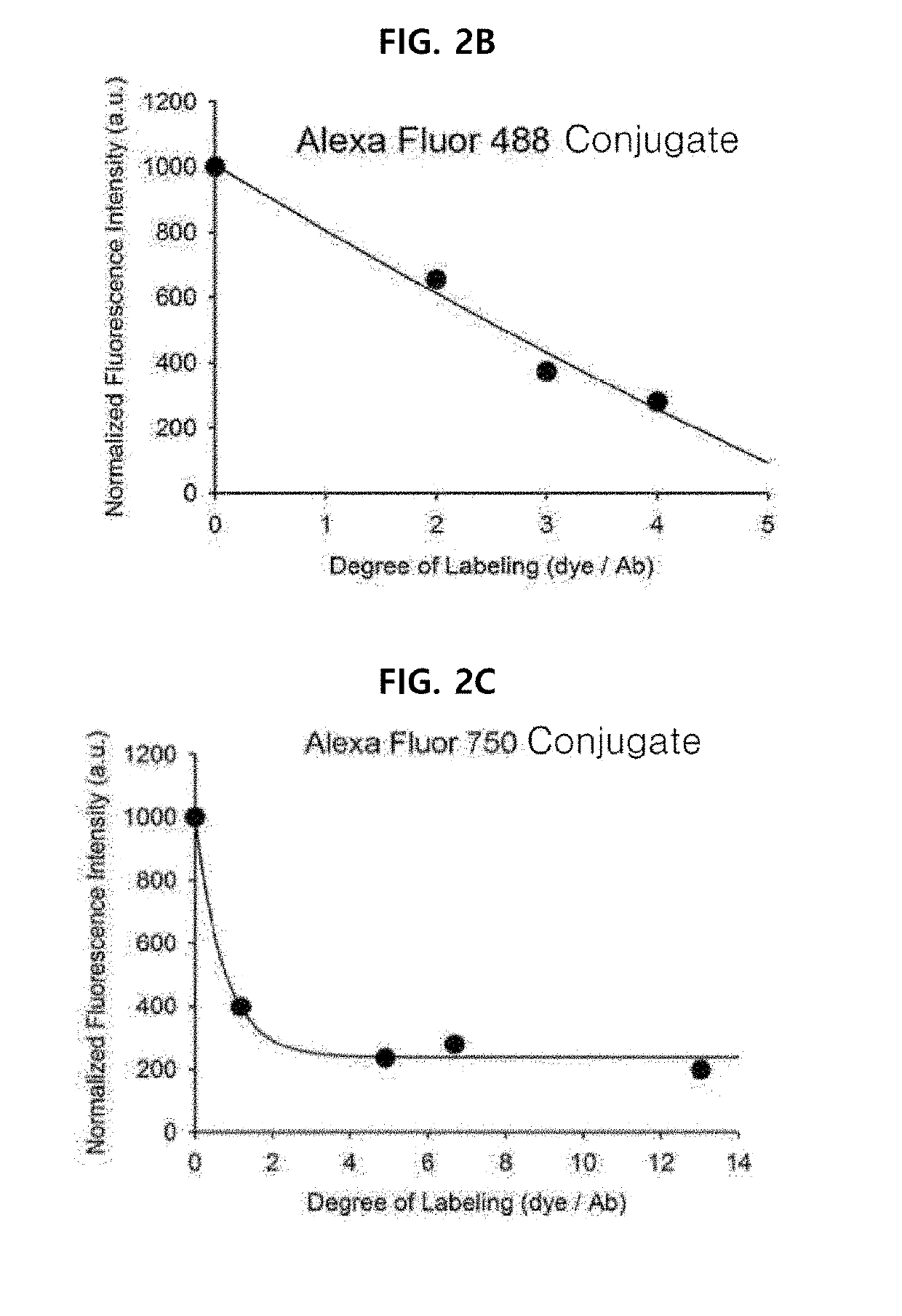
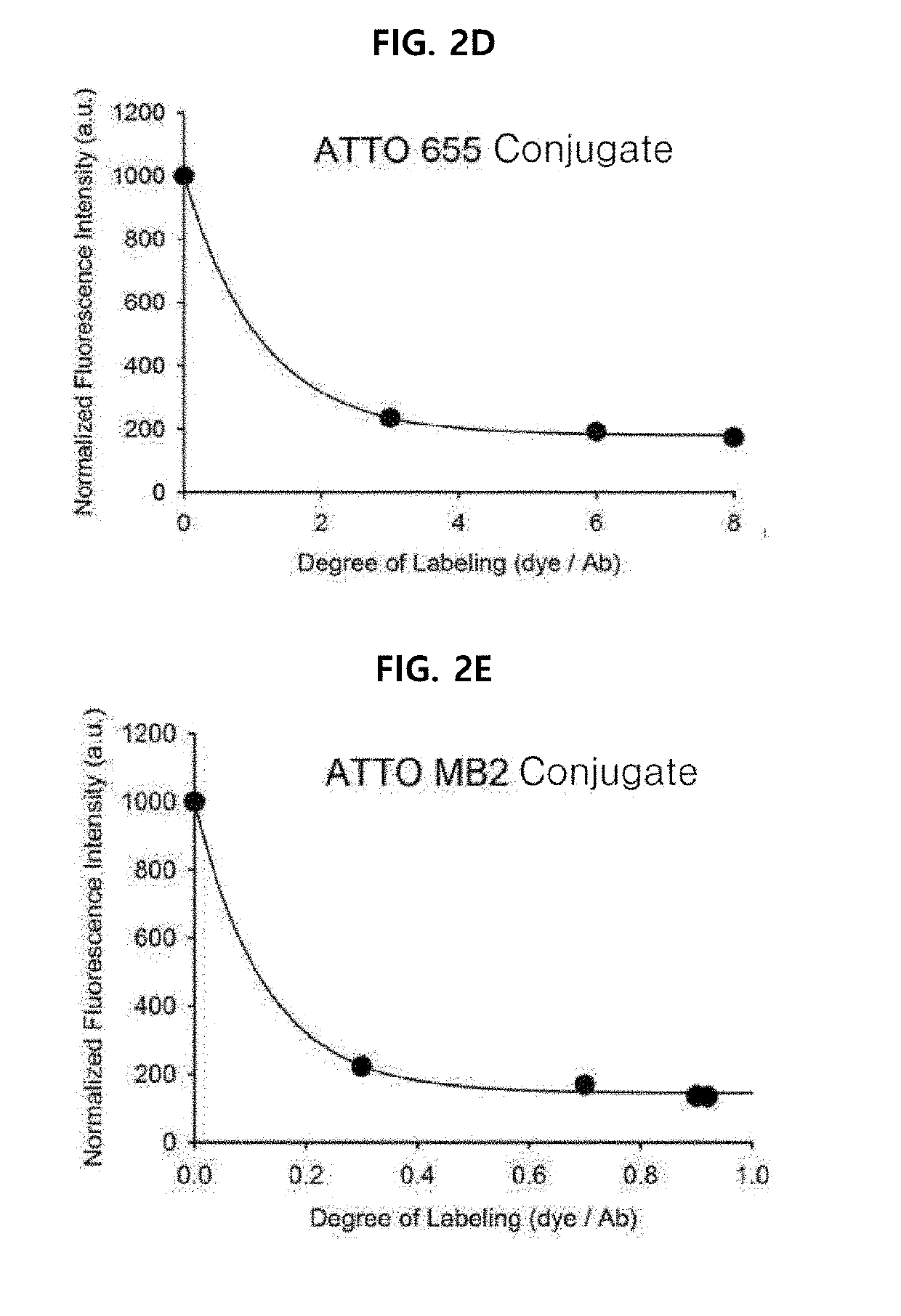
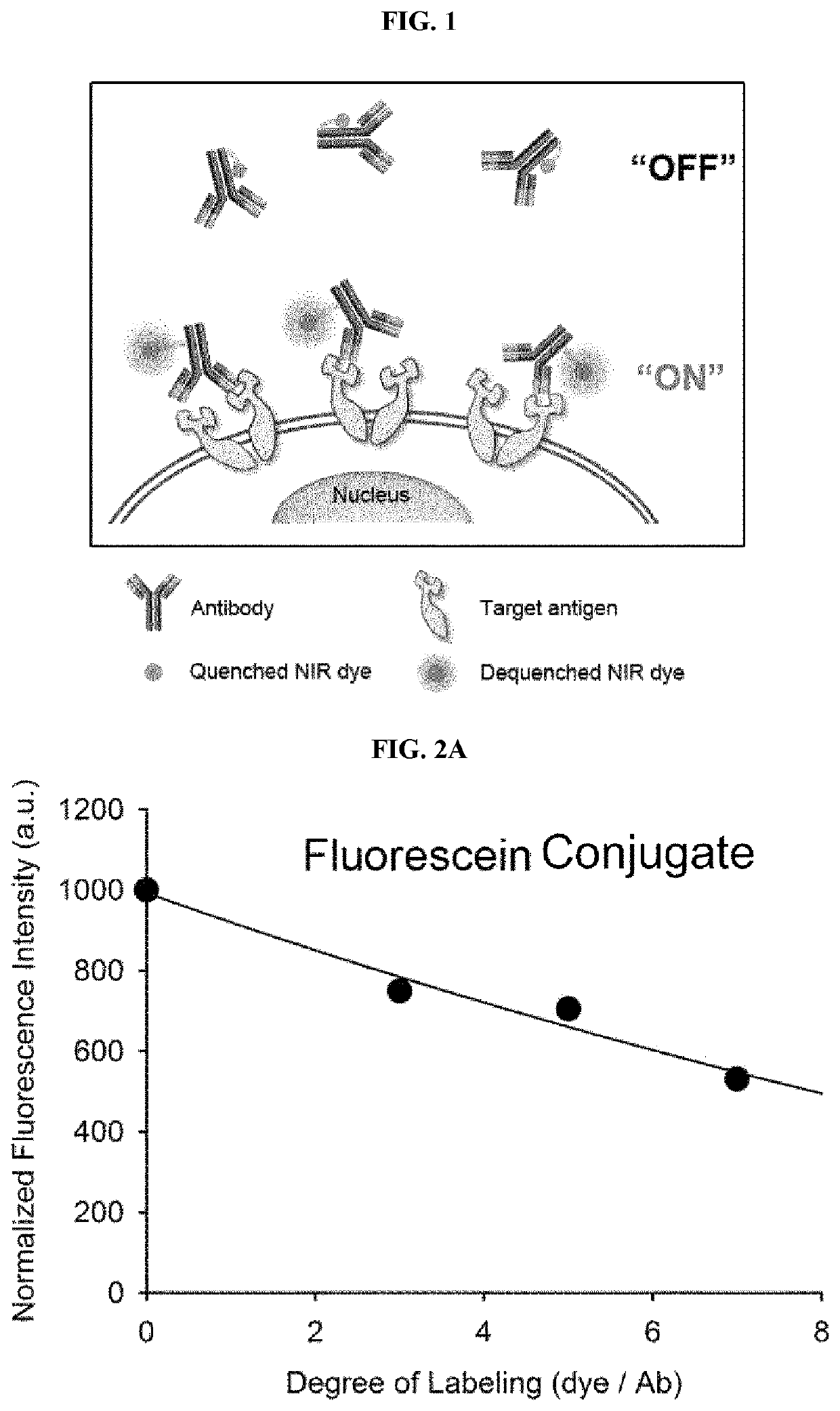
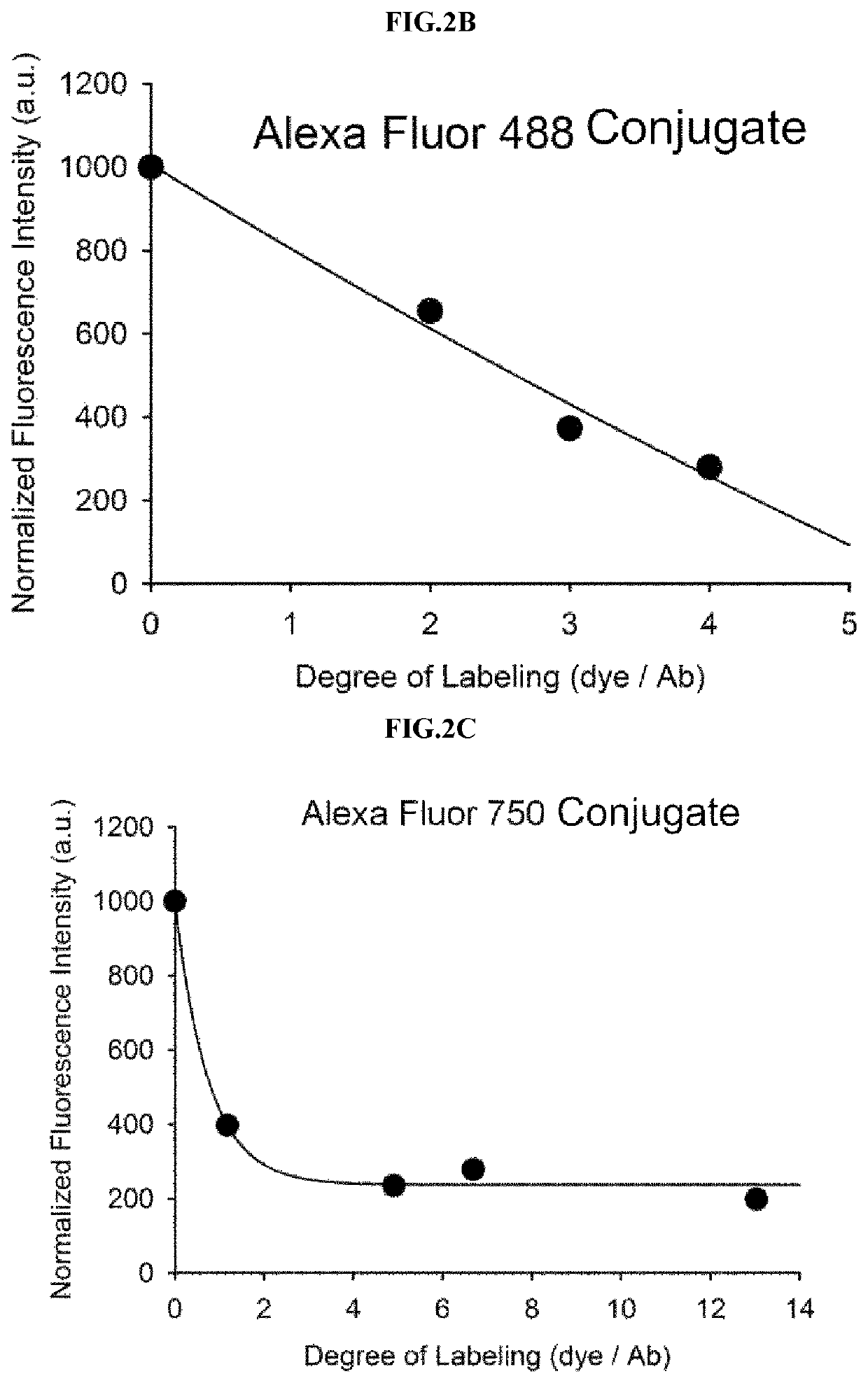
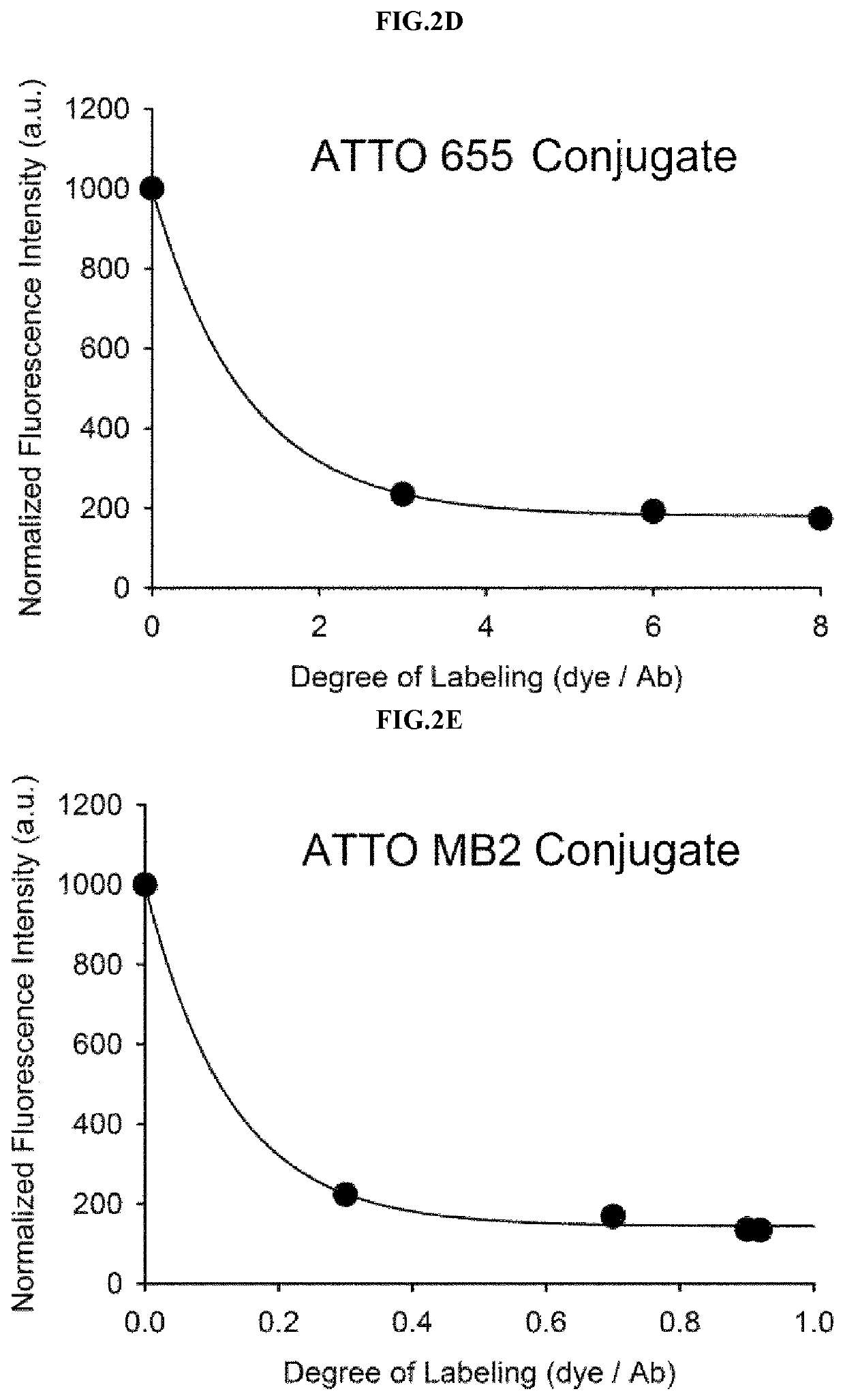
Antigen responsive antibody-fluorescent dye conjugate and method for fluorescence detection and imaging of target cell using the same
ActiveUS20190091349A1Improve accuracyImprove efficiencyImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellHigh-Throughput Screening Methods
The present invention relates to an antibody-fluorescent dye conjugate capable of cancer cell-specific fluorescence imaging diagnosis, wherein the fluorescent dye comprises a covalently labeled antibody and is structured to be quenched by interaction with an amino acid residues in the antibody, selected from the group consisting of tryptophan, tyrosine, histidine, and methionine, and to be dequenched upon binding of the antibody to an antigen present on a cell surface to emit fluorescence, whereby cells having a target antigen thereon can be imaged for diagnosis. When using the antibody-fluorescent dye conjugate according to the present invention, in vitro cell assays, high-throughput screening of cells, and cytodiagnosis based on microfluidics exhibit the effect of detecting the presence of cancer cells having a specific antigen expressed thereon at high specificity and sensitivity without a washing process, and can detect the position of primary and metastatic cancer cells at high contrast within a short time, thereby enhancing the accuracy of fluorescent image-guided surgeries and a therapeutic effect.
Owner:NAT CANCER CENT
Lactobacillus reuteri MRD01 and application thereof
ActiveCN114410502AGood hypoglycemic functionLose weightBacteriaMetabolism disorderBiotechnologyProbiotic bacterium
The invention relates to the technical field of microorganisms, in particular to lactobacillus reuteri MRD01 and application thereof. The preservation number of the lactobacillus reuteri MRD01 is GDMCC 62017, and research finds that after intervention of the lactobacillus reuteri MRD01, the fat weight of a mouse is reduced, the total weight of the mouse is reduced, and the lactobacillus reuteri MRD01 has the effects of losing weight and the like; meanwhile, the strain also has an excellent hypoglycemic function; the lactobacillus reuteri MRD01 can relieve constipation at the same time, is free of toxic and antigen reactions, can serve as probiotics, and can be prepared into medicines, functional foods or health-care foods with multiple functions of reducing body weight, losing weight and reducing blood sugar.
Owner:ANHUI MEDICAL UNIV
Method for enhancing antigen response of dendrite cells by modifying dendrite cells
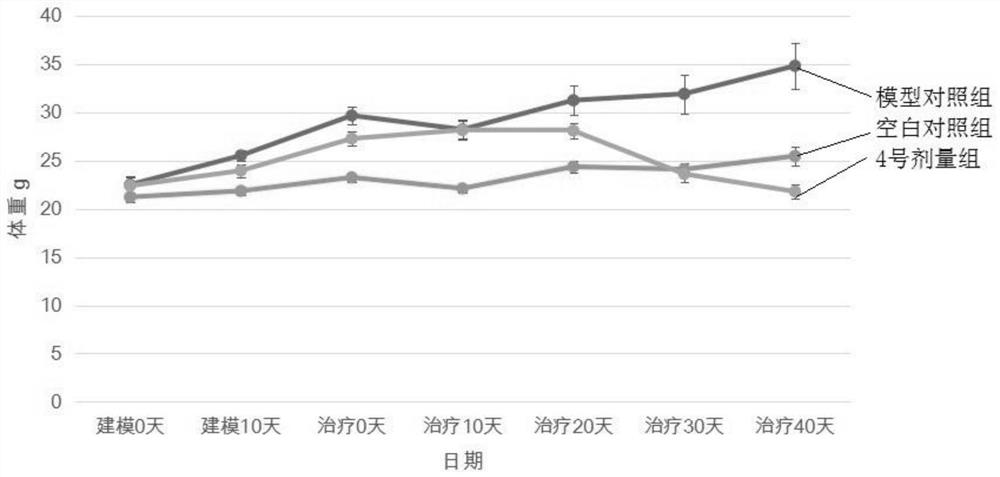
InactiveCN104630274APromote absorptionComplete understandingOther foreign material introduction processesForeign genetic material cellsAntigenAntigen response
Owner:陶英亮
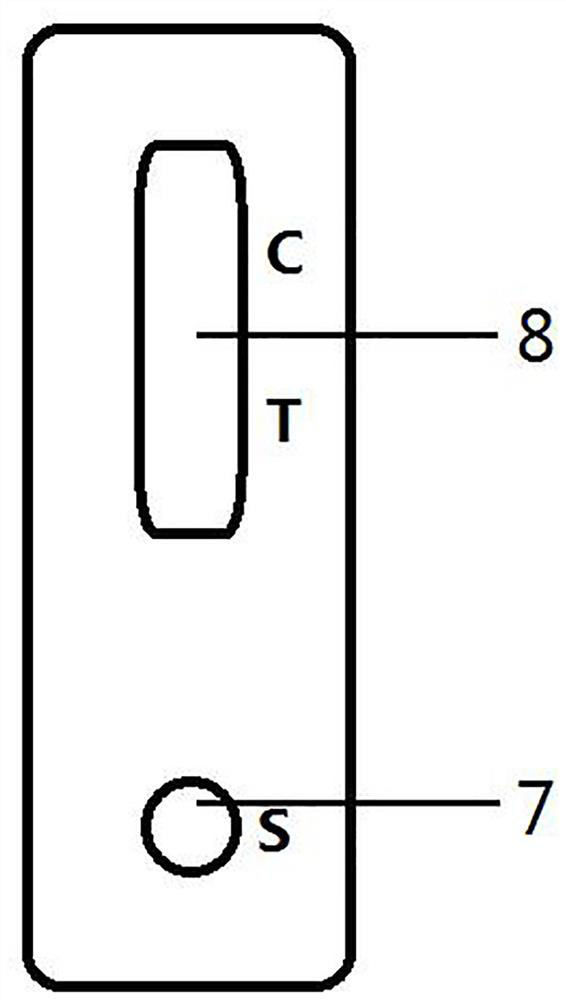
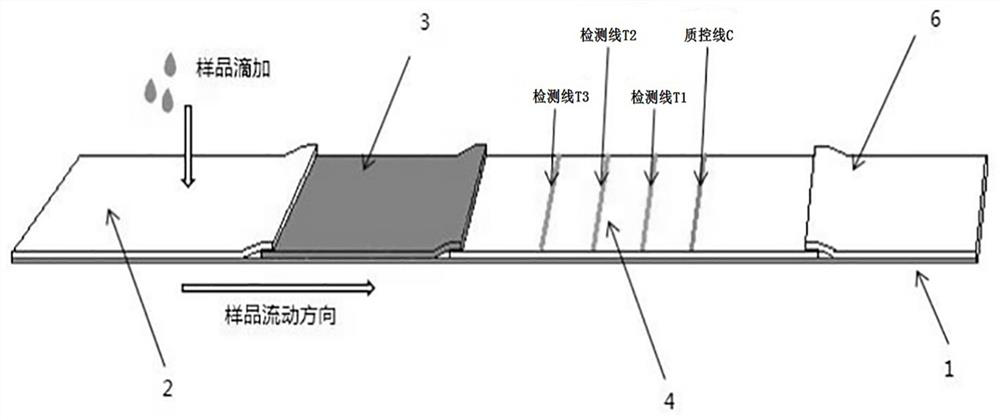
Immunochromatography test paper and preparation method and application thereof
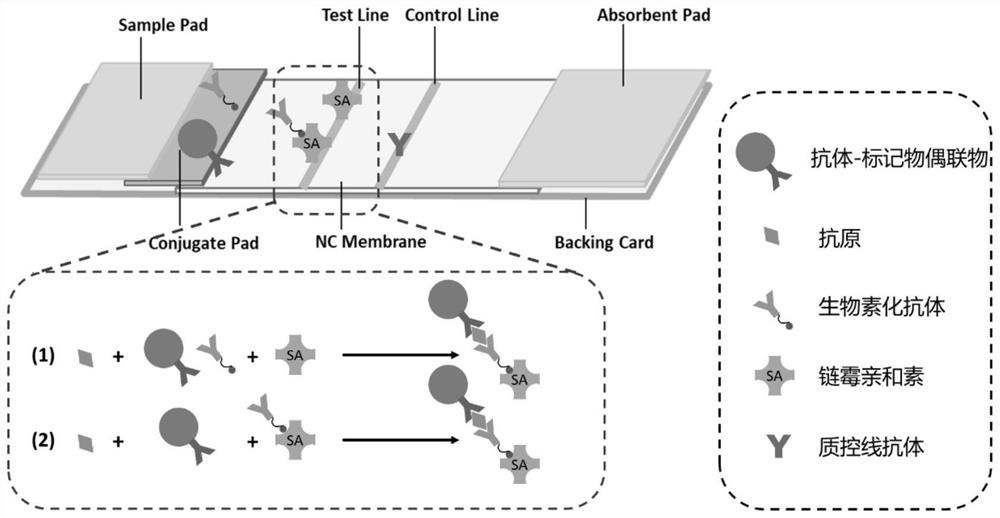
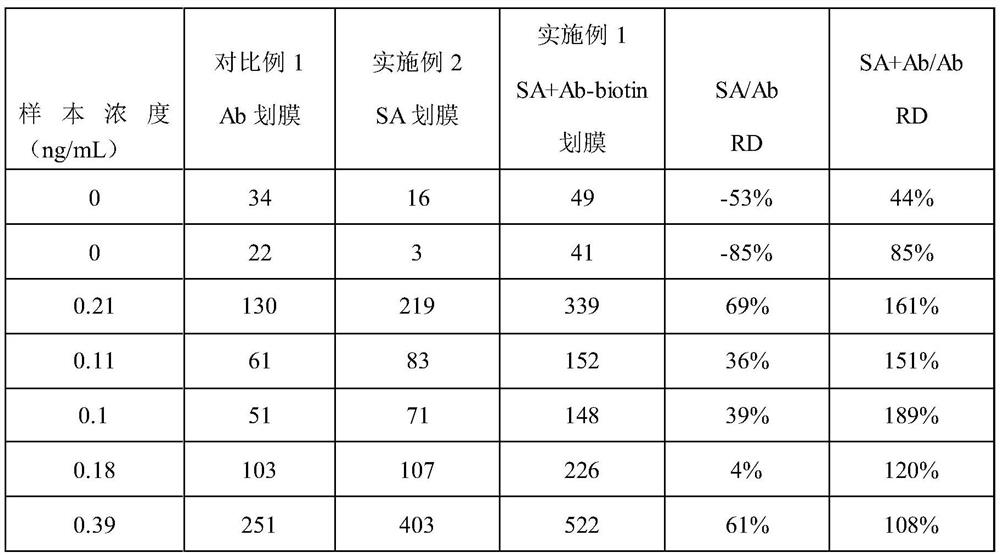
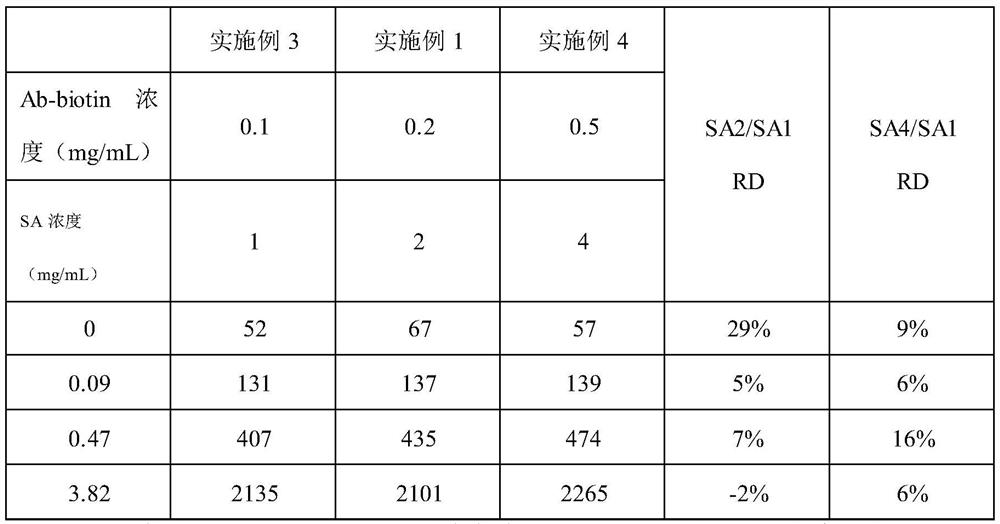
PendingCN113970635AImprove production efficiencyExtended reaction timeMaterial analysisAntigen responseAntigen-antibody reactions
The invention discloses immunochromatography test paper and a preparation method and application thereof, and relates to the technical field of immunochromatography detection.The immunochromatography test paper is characterized in that streptavidin is used for conducting detection line membrane scratching, and biotinylated antibodies are transferred to a combination pad, so that double-antibody sandwich reaction of antigens and antibodies starts from the combination pad, the reaction time is prolonged, and the reaction efficiency is improved; the biotinylated antibody can flow on the nitrocellulose membrane, and the reaction capacity with the antigen is higher than that of the antibody fixed on the detection line, so that the production efficiency of the double-antibody sandwich compound is improved, and the sensitivity is improved. Meanwhile, a capture reaction on the nitrocellulose membrane is converted from an antigen-antibody reaction to a streptavidin-biotin reaction, the reaction efficiency is improved by 3-4 orders of magnitude, and the capture efficiency of a detection line on the membrane is improved, so that the detection sensitivity is improved.
Owner:上海艾瑞德生物科技有限公司
Colorimetric sensor using polydiacetylene supramolecule
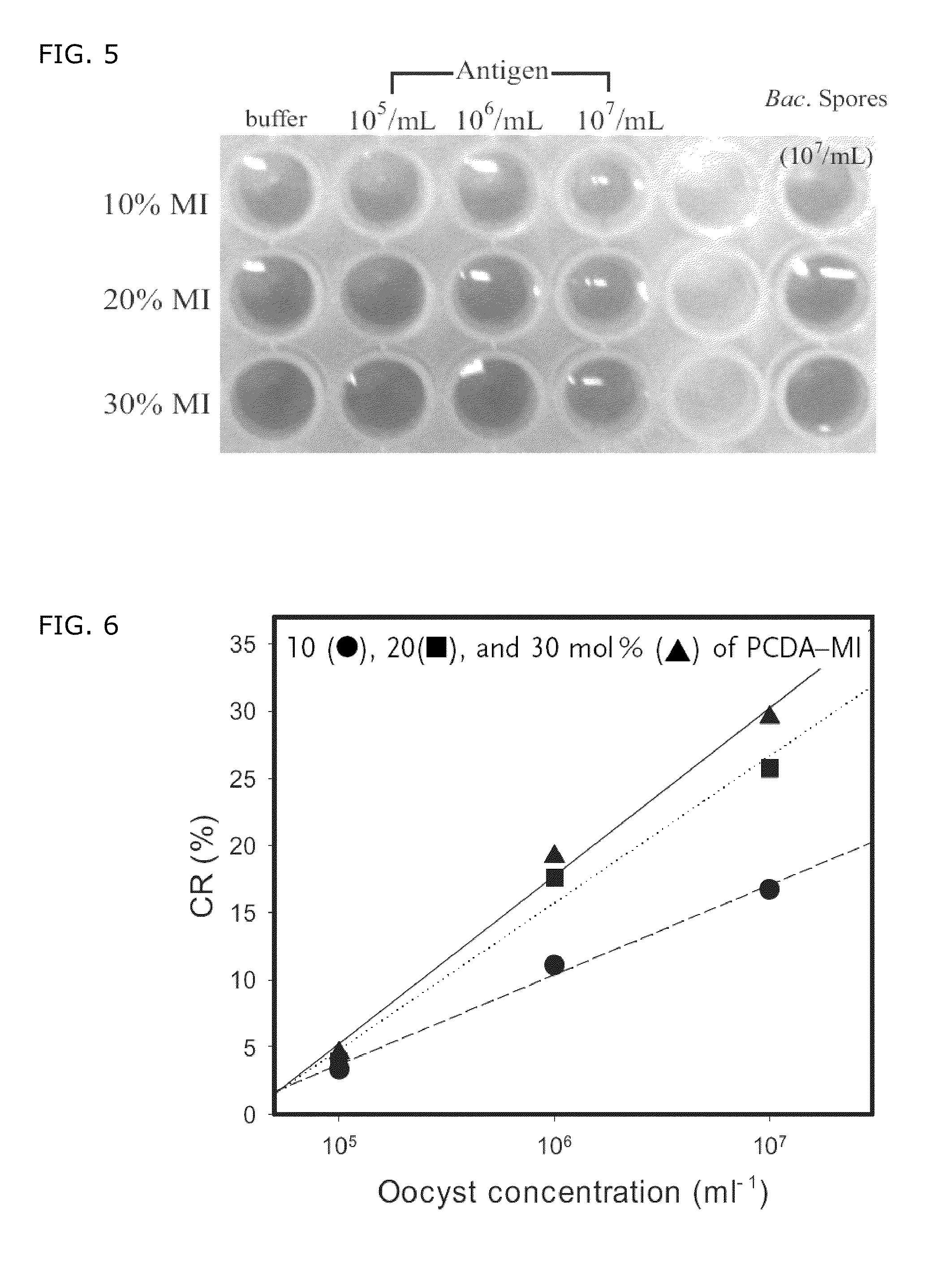
ActiveUS7776554B2Organic chemistryMicrobiological testing/measurementAntiendomysial antibodiesAntibody antigen reactions
There is provided a polydiacetylene supramolecule comprising diacetylene molecules, capable of immobilizing a receptor molecule having a thiol group. Since the polydiacetylene supramolecule has a receptor immobilized thereon having a thiol group, for example, an antibody, and thus shows color transition when reacting with a sample, an antigen can be detected through specific color transition of the polydiacetylene when employing in a receptor-ligand reaction, for example, an antibody-antigen reaction.
Owner:SUNGKYUNKWAN UNIVERSITY
Multiplex measure of isotype antigen response
InactiveUS20140031249A1Fast and cost-effective methodLibrary screeningDisease diagnosisTherapeutic proteinFluorescence
Described are methods for simultaneous detection and quantifying multiple target analytes, including immunoglobulin isotypes and sub-classes, single and multiple protein antibodies within a test sample contained in a single reaction vessel. Such methods use reaction wells as on a multi-well plate, each single well comprising microarrays of calibration spots, each having a predetermined quantity of a target analyte; and capture spots, each having multiple agent antibodies, including isotypes and subclasses that specifically bind the target analytes. The captured analytes and the calibration spots are detected with fluorescently labeled antibodies specific for each different target analyte. Calibration spots generate calibration curves for quantitative determinations of different target analytes. Also described are methods for detecting and quantifying biomarkers, therapeutic proteins and patient derived antibodies; the use of secondary reagents to determine immunoglobulin classes Ig G, A, M, E and sub-classes including IgG1, IgG2, IgG3, IgG4 and IgA. The intensity of each fluorescent signal allows measurement of a specific immune response to a therapeutic protein and associated analytes; interrogates neutralizing effects of patient antibodies on therapeutic proteins, e.g., insulin therapy.
Owner:SQI DIAGNOSTICS SYST
Preparation method and application of antigen reactive T cell based on RNA vaccine
InactiveCN113355283AShorten the production cycleLow costGenetically modified cellsMammal material medical ingredientsAntigen responseT cell
The invention discloses a preparation method and application of an antigen reactive T cell based on an RNA vaccine. The preparation method comprises the following steps of constructing an in-vitro expression vector, and carrying out plasmid linearization, in-vitro transcription and purification to obtain the RNA vaccine; obtaining a T cell and a DC cell modified by the RNA vaccine based on a collected PBMC; and carrying out multiplication culture on the DC cell modified by the RNA vaccine and the T cell to obtain the antigen reactive T cell based on the RNA vaccine. According to the preparation method and application of the antigen reactive T cell based on the RNA vaccine, pcDNA3.1 + plasmids are used for constructing the mammal in-vitro expression vector, and compared with traditional polypeptide synthesis, the preparation period is short, the cost is low, and the preparation success rate is high; an electrotransfection instrument is used for electrotransfection, and compared with a traditional transfection mode, the repeatability is good, reagent cost is avoided, and the electrotransfection rate is high; the RNA vaccine is used for electrotransfecting the DC cell, compared with other biological treatment drugs, in-vitro production and purification are easy, and the production process is high in universality and high in safety; and the DC cell and the T cell which are subjected to electrotransfection are co-incubated and amplified, and the obtained activated vaccine is high in proportion and strong in immunogenicity.
Owner:浙江格源致臻生物医药科技有限公司
A kind of tobacco leaf multi-in-one heavy metal rapid quantitative detection card and detection method
The invention belongs to the field of heavy metal detection, and relates to a tobacco leaf multi-in-one heavy metal rapid quantitative detection card and a detection method. The gold-labeled antibody formed by the combination of modified colloidal gold and an antibody is stable and reduces the time when the gold-labeled antibody reacts with the antigen. The antagonism between the antibodies can effectively improve the reaction sensitivity and reduce the amount of the antibody, and effectively overcome the immune reaction during detection; the multi-in-one heavy metal rapid quantitative detection card using the gold-labeled antibody of the present invention can simultaneously detect a variety of heavy metals, and realize the realization of a variety of heavy metals. The rapid detection and quantification greatly simplifies the operation process and improves the detection efficiency.
Owner:成都安普诺生物科技有限公司
Biosensing device for enhancing antigen and antibody combined optical detection signal
InactiveCN112730297AGood material biocompatibilityKeep aliveColor/spectral properties measurementsAntibody antigen reactionsAntigen response
The invention discloses a biosensing device for enhancing antigen and antibody binding optical detection signals, and the device comprises an adjusting box, wherein a first working box and a second working box are arranged at the top of the adjusting box; by arranging the heating piece, the material box and the metal plate, a solution pump in the material box pumps a solution for reaction into the reaction cavity, a good liquid environment is provided for reaction of antibodies and antigens, regular replacement can be achieved, the activity of liquid is kept, the metal plate is fixed to the bottom in the reaction cavity, and the metal plate is good in material biological compatibility; therefore, the good environment is provided for the antibody-antigen reaction, and the heating sheet can heat the interior of the reaction chamber so as to increase the temperature to the temperature beneficial to the enzyme reaction so as to promote the antibody-antigen reaction; the adjusting box, the second sliding block and the first sliding block are arranged, so the reflection receiving plate and the light emitter can slide at the bottom of the adjusting box at a constant speed, and constant-speed scanning is achieved.
Owner:KUNMING MEDICAL UNIVERSITY
Antigen responsive antibody-fluorescent dye conjugate and method for fluorescence detection and imaging of target cell using the same
ActiveUS11213595B2Strong specificityStrong fluorescent signalImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellTyrosine
The present invention relates to an antibody-fluorescent dye conjugate capable of cancer cell-specific fluorescence imaging diagnosis. The fluorescent dye comprises a covalently labeled antibody and is quenched by interaction with an amino acid residue such as tryptophan, tyrosine, histidine, and methionine in the antibody and upon binding of the antibody to an antigen present on a cell surface to emit fluorescence, whereby cells having a target antigen thereon can be imaged for diagnosis. When using the antibody-fluorescent dye conjugate according to the present invention during in vitro cell assays, high-throughput screening of cells, and cytodiagnosis based on microfluidics, the presence of cancer cells having a specific antigen expressed thereon can be detected at high specificity and sensitivity without a washing process, and the position of primary and metastatic cancer cells can be detected at high contrast within a short time.
Owner:NAT CANCER CENT
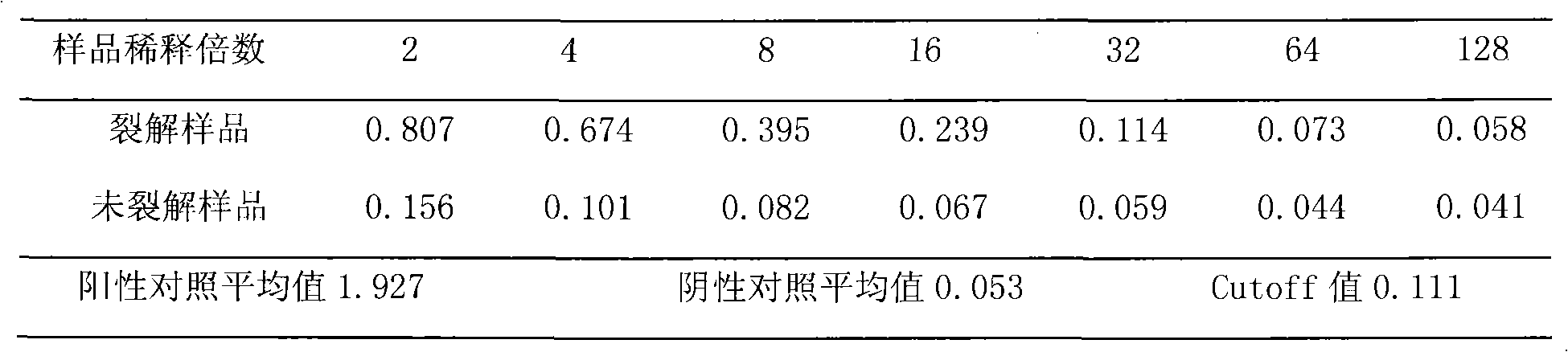
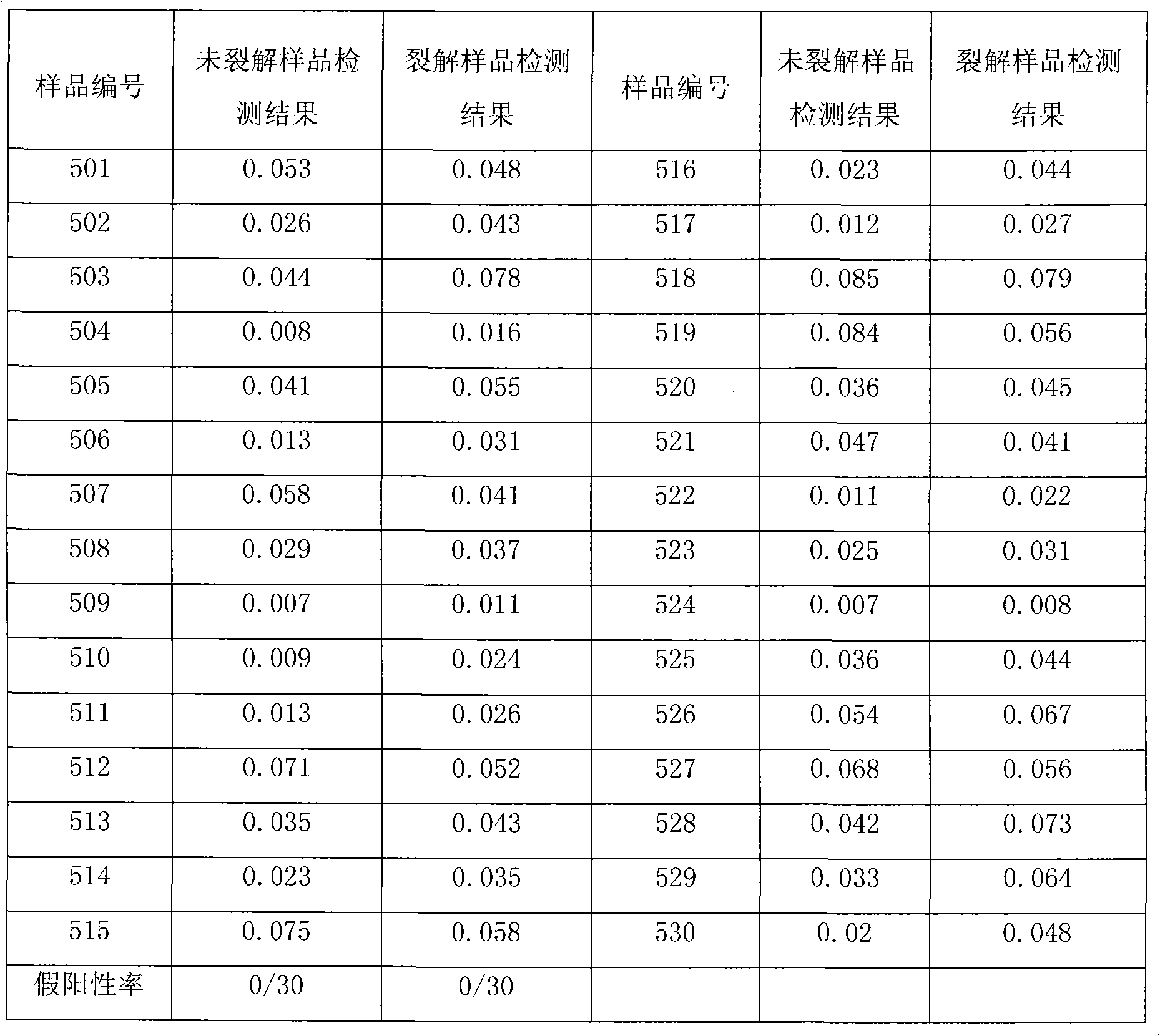
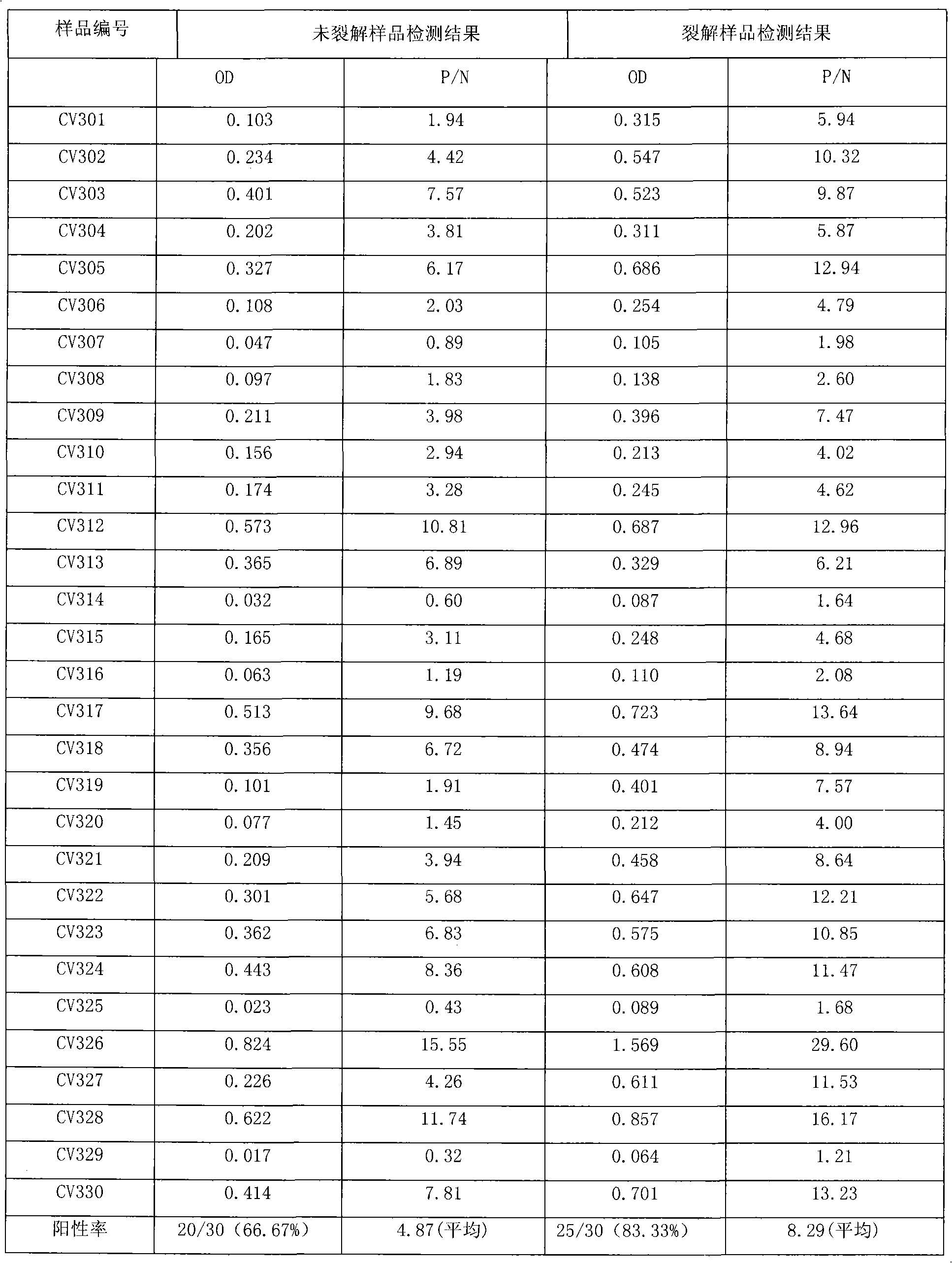
Virus disaggregating agent and method for disaggregating virus antigen-antibody complex and detecting HCV (Hepatitis C Virus) antigen
ActiveCN101975857BImprove securityPreserve antigen reactivitySerum immunoglobulinsImmunoglobulins against virusesWhole blood productHepatitis A viruses
The invention provides a virus disaggregating agent and a method for disaggregating virus antigen-antibody complex and detecting HCV (Hepatitis C Virus) antigen. The virus disaggregating agent is characterized in that each 100ml of disaggregating agent comprises 0.1-1ml of detergent, 0-20g of protein denaturant, 0.01-1ml of reducing agent, 0.1-1ml of fat solvent, and base liquid as remainder amount. The antigen-antibody complex obtained by the neutralization reaction of testing blood sample or antiserum and virus antigen is disaggregated into free components, or virus core antigen is fully exposed and the antigen reactivity of the virus core antigen is remained, thereby remarkably improving the sensitivity by comparing with the prior HCV core antigen detection method; in addition, the detection window period of the antigen is 49 days shorter than that of the anti-HCV antibody averagely, the situation appears within 1-2d after HCV-RNA appears, thereby efficiently shortening the window period of the blood serum before transformation, which is of important signification on improving the detection rate of affected person in the window period and the safety of blood transfusion and blood products. In the invention, the virus disaggregating agent is suitable for the disaggregation of common virus antigen-antibody complex including HCV and HAV (Hepatitis A Virus).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
High throughput method for screening for homologous t cells and epitope reactivity in primary human cells
PendingCN114616467AMicrobiological testing/measurementBiological material analysisAntigenOligonucleotide
An assay for autologous primary immune cells is described in which the blood cells of an individual can be functionally screened simultaneously against individual antigens of interest, such as T cell epitopes, without HLA haplotype-specific agents. The antigen reactivity is associated with individual T cells using an oligonucleotide labeled hash tracking system followed by deconvolution by single cell sequencing.
Owner:REGENERON PHARM INC
Method for identifying i(mycobacterium) species and diagnosing reagent kit therefor
InactiveCN1181345CMicrobiological testing/measurementMaterial analysisMycobacterial antigenMycobacterium species
The invention relates to a method for identifying a Mycobacterium species, comprising the steps of: a) contacting at least one immuno-cross reactive antigen component of a mycobacterial species with a sample of a body fluid of a human or animal individual; b) contacting at least one antibody, which is capable of reacting with a mycobacterial antigen, with said body fluid sample; c) detecting the presence of antigen-antibody complexes, and identifying the Mycobacterium species present in said body fluid sample.
Owner:RAPID MEDICAL DIAGNOSTICS CORP
Chemically controlled monoclonal antibody target engagement
PendingUS20220289866A1Useful in diagnosisUseful in treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigen responseTumor specific
The present disclosure relates generally to antibodies reactive with tumor-specific neoantigens, as well as neoantigen-binding fragments thereof. The present disclosure also relates to nucleic acids, expression cassettes, and expression vectors encoding the antibodies and neoantigen-binding fragments. The antibodies and neoantigen binding-fragments are useful for diagnosis and treatment of cancer.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com