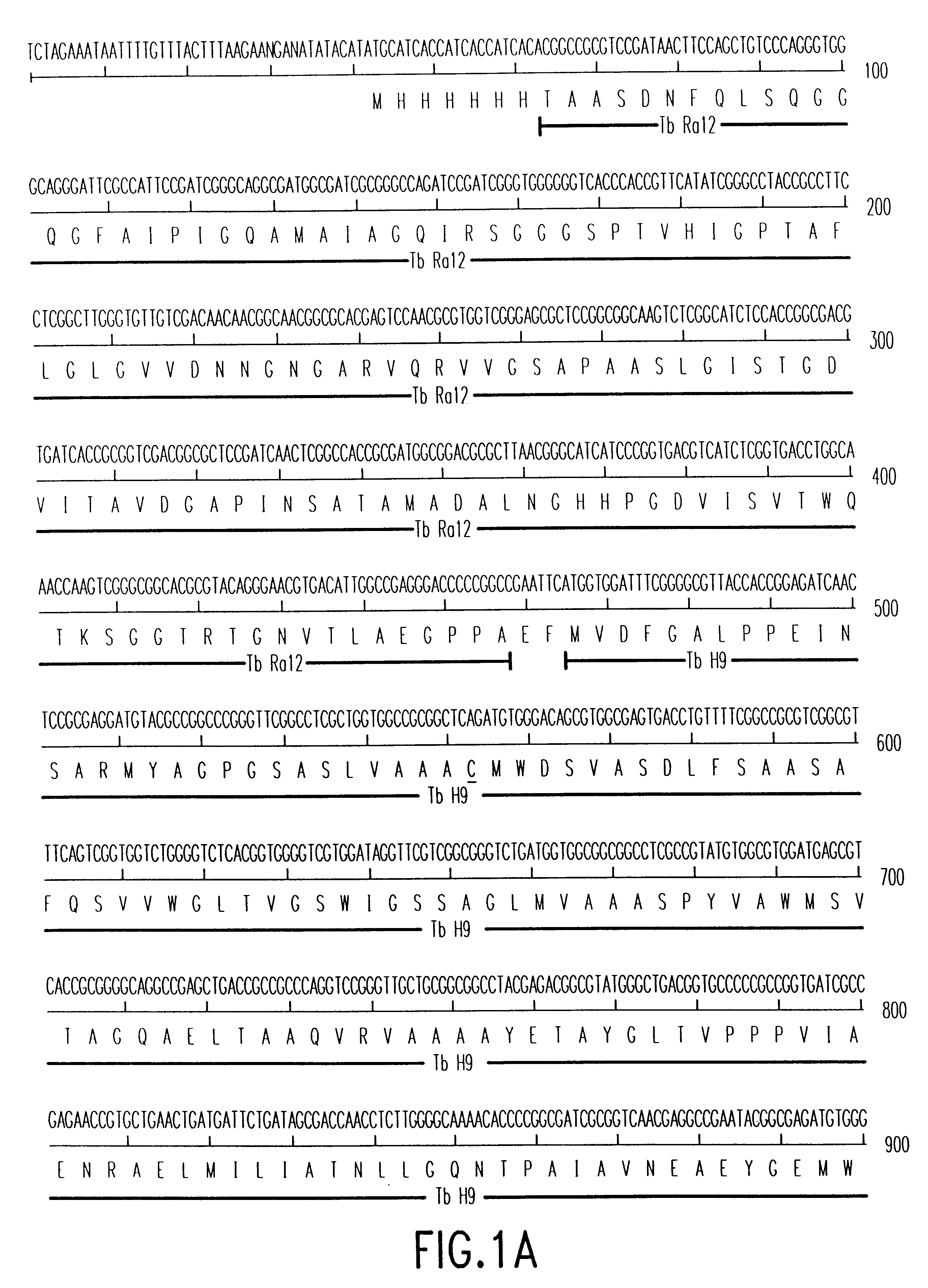
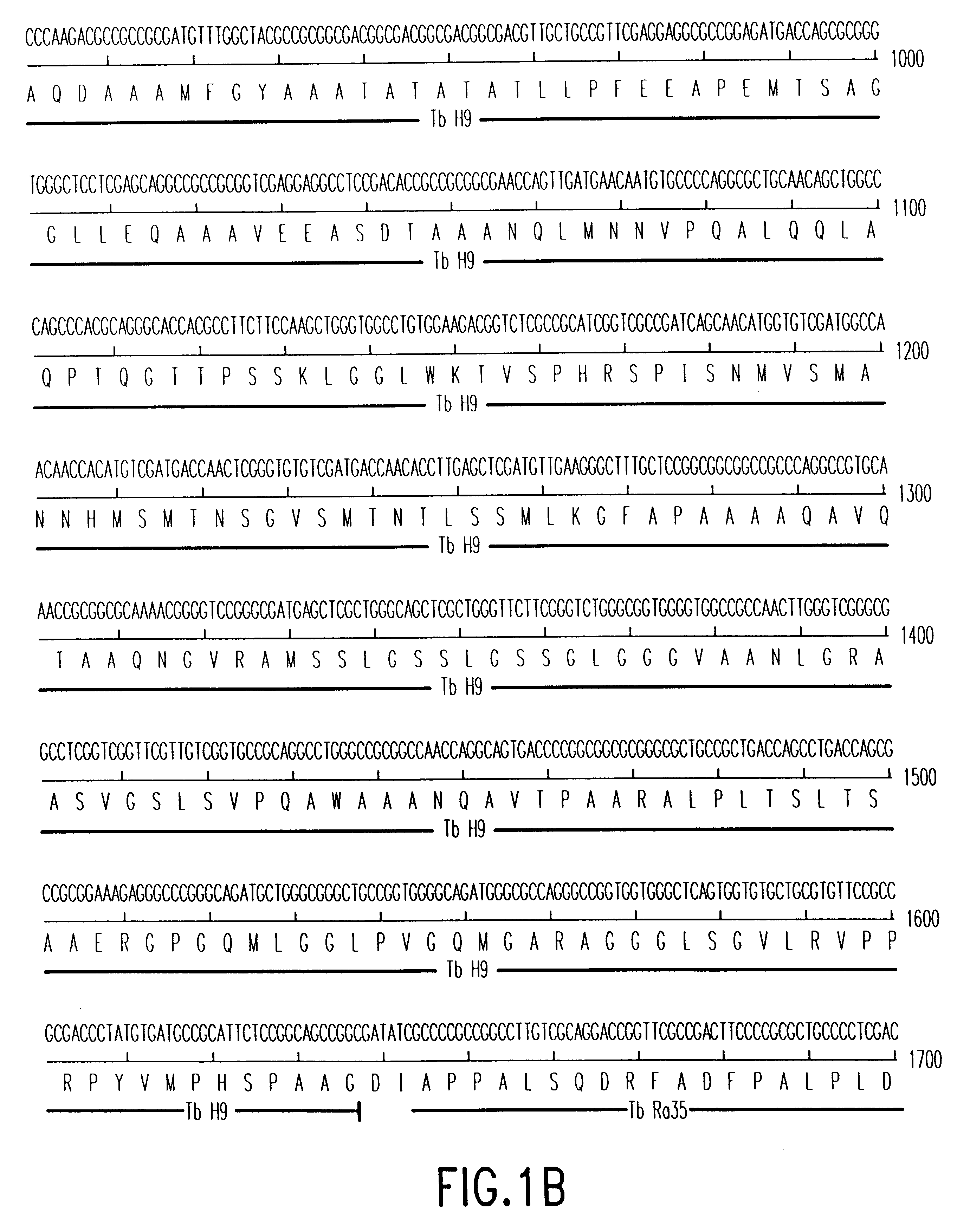
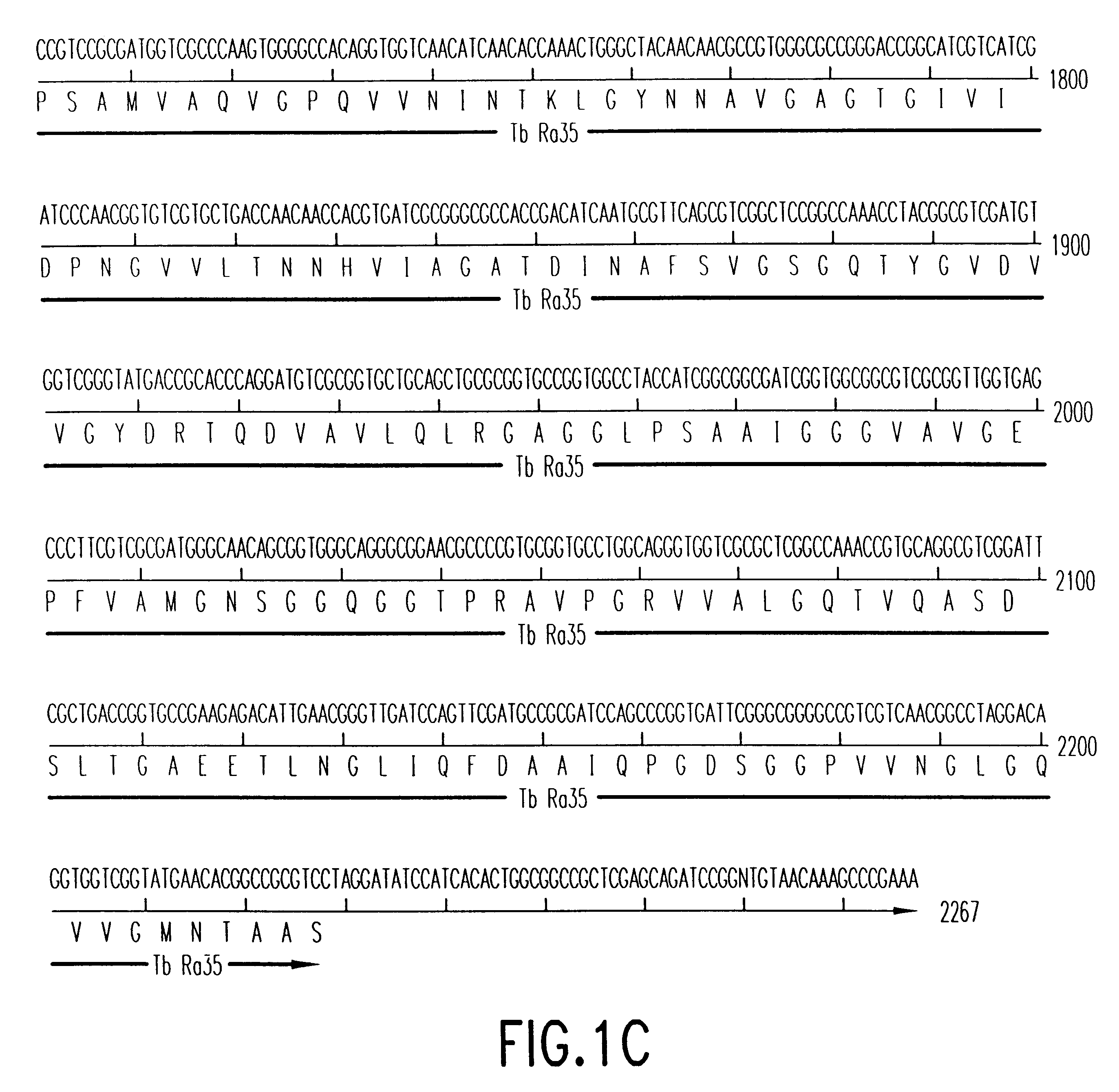
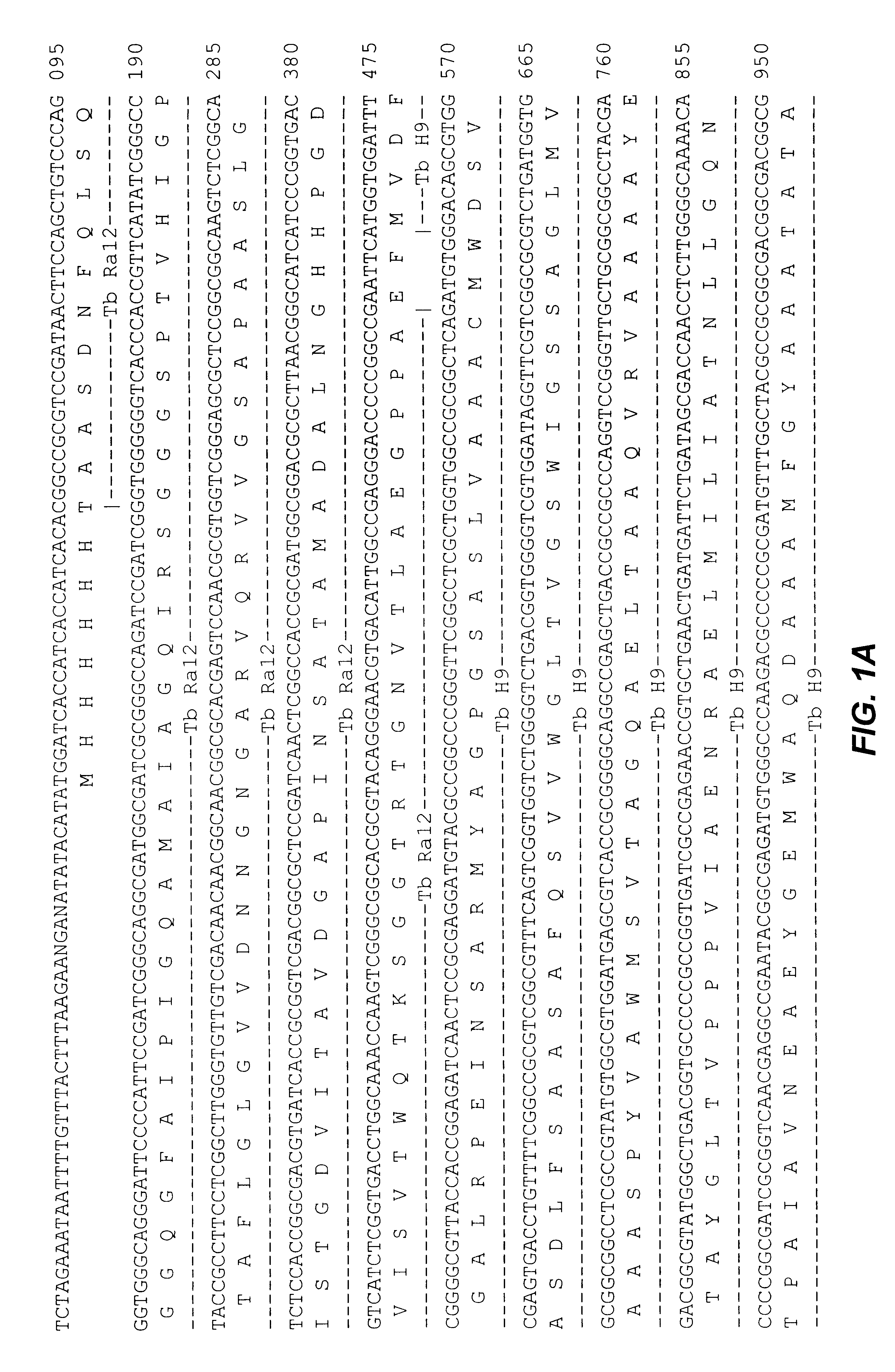
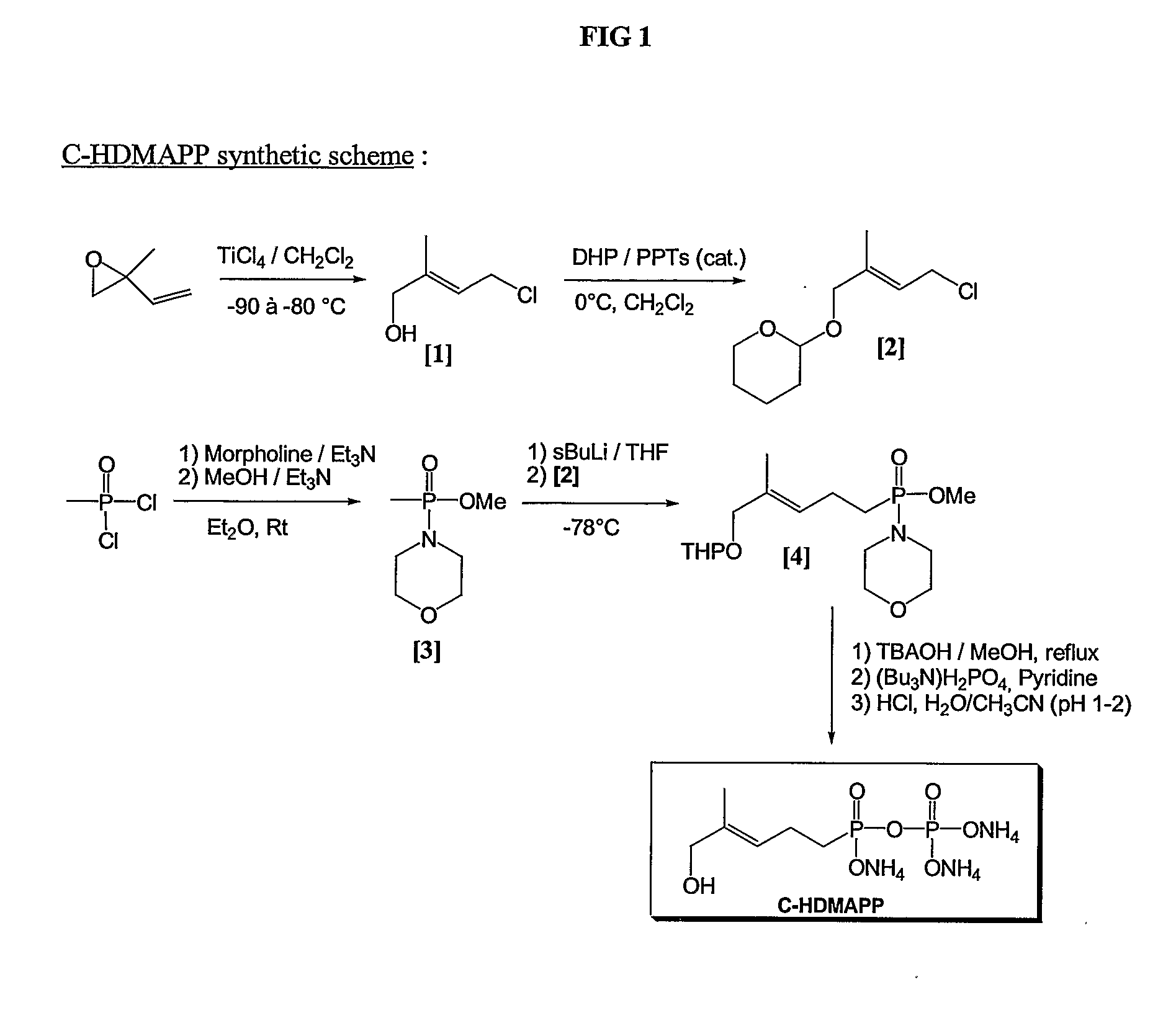
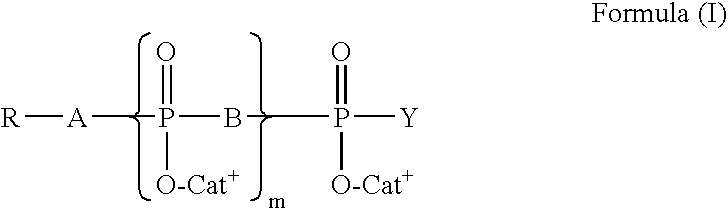
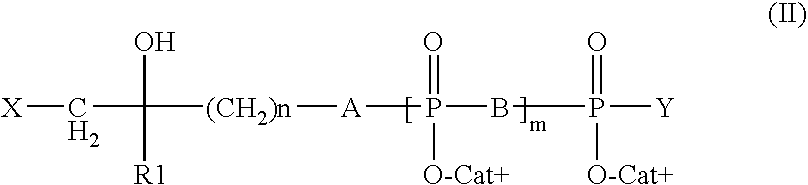
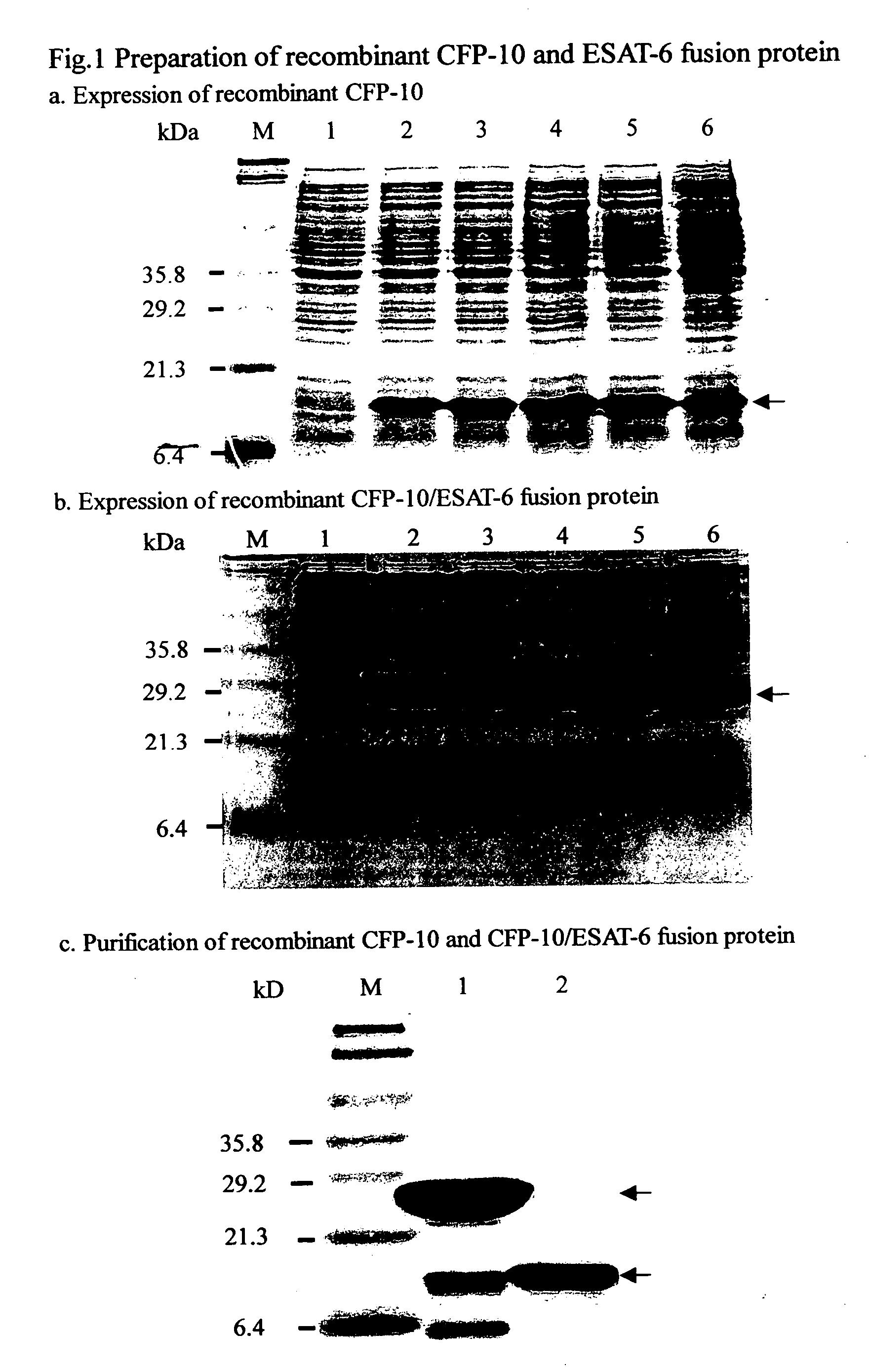
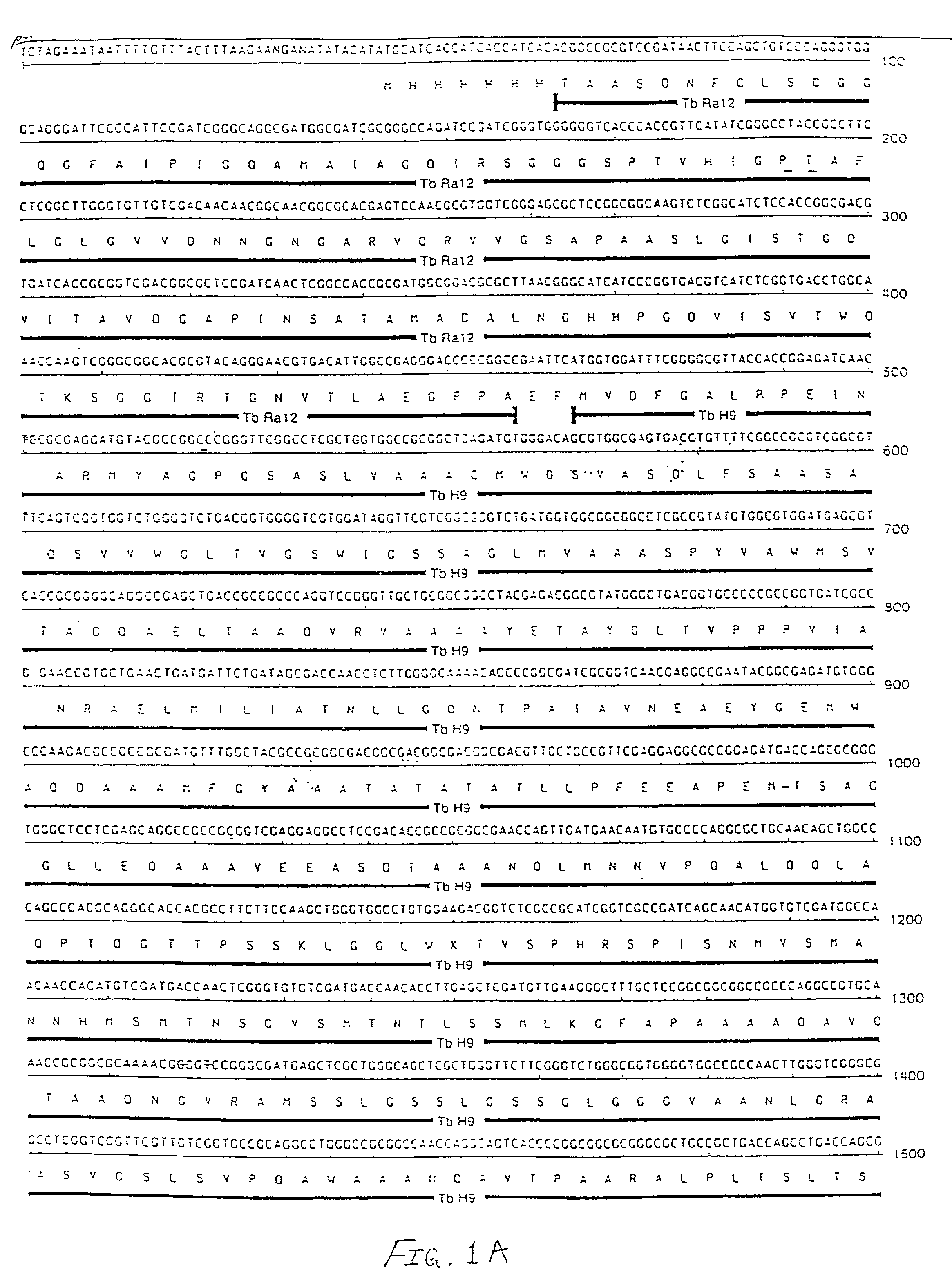
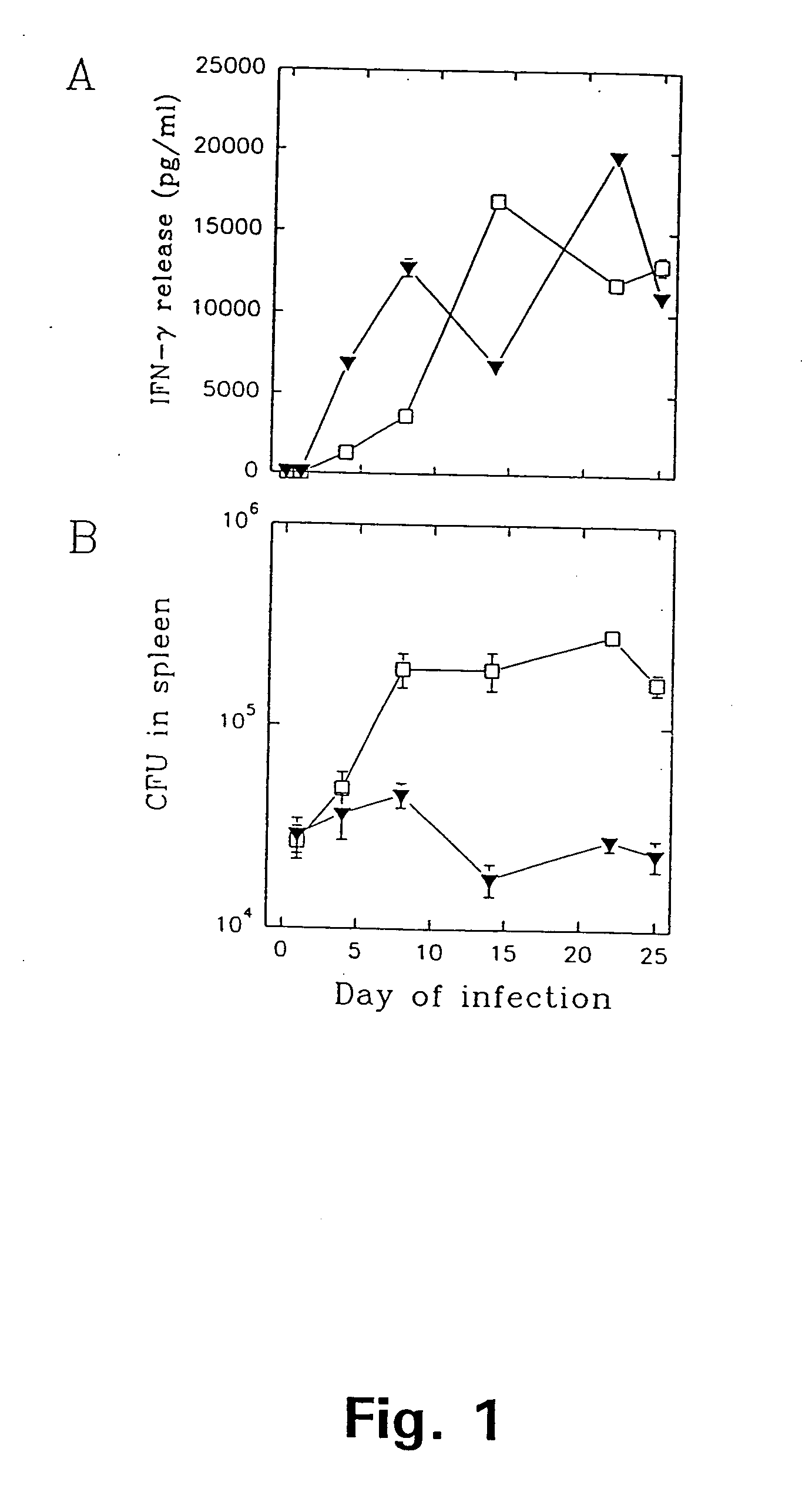
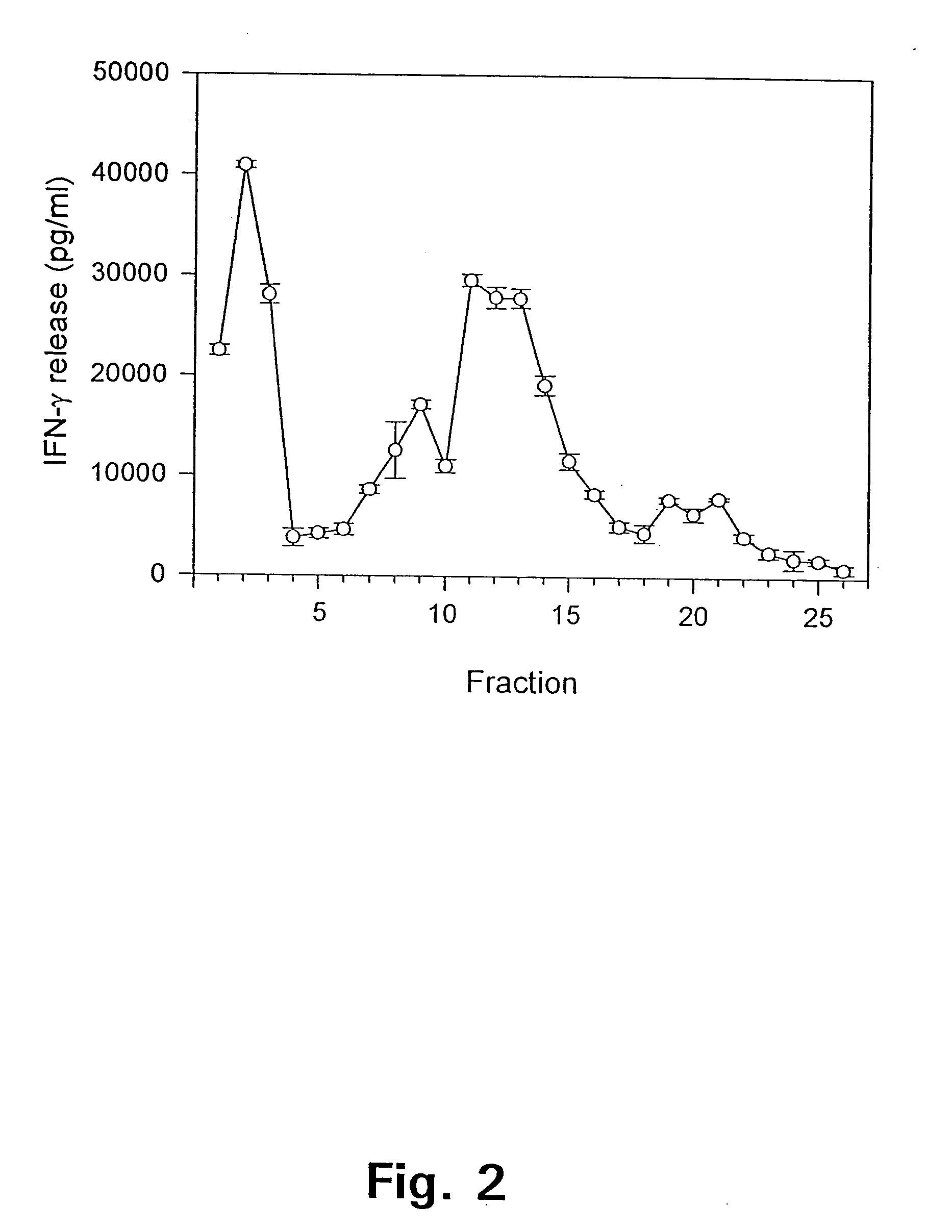
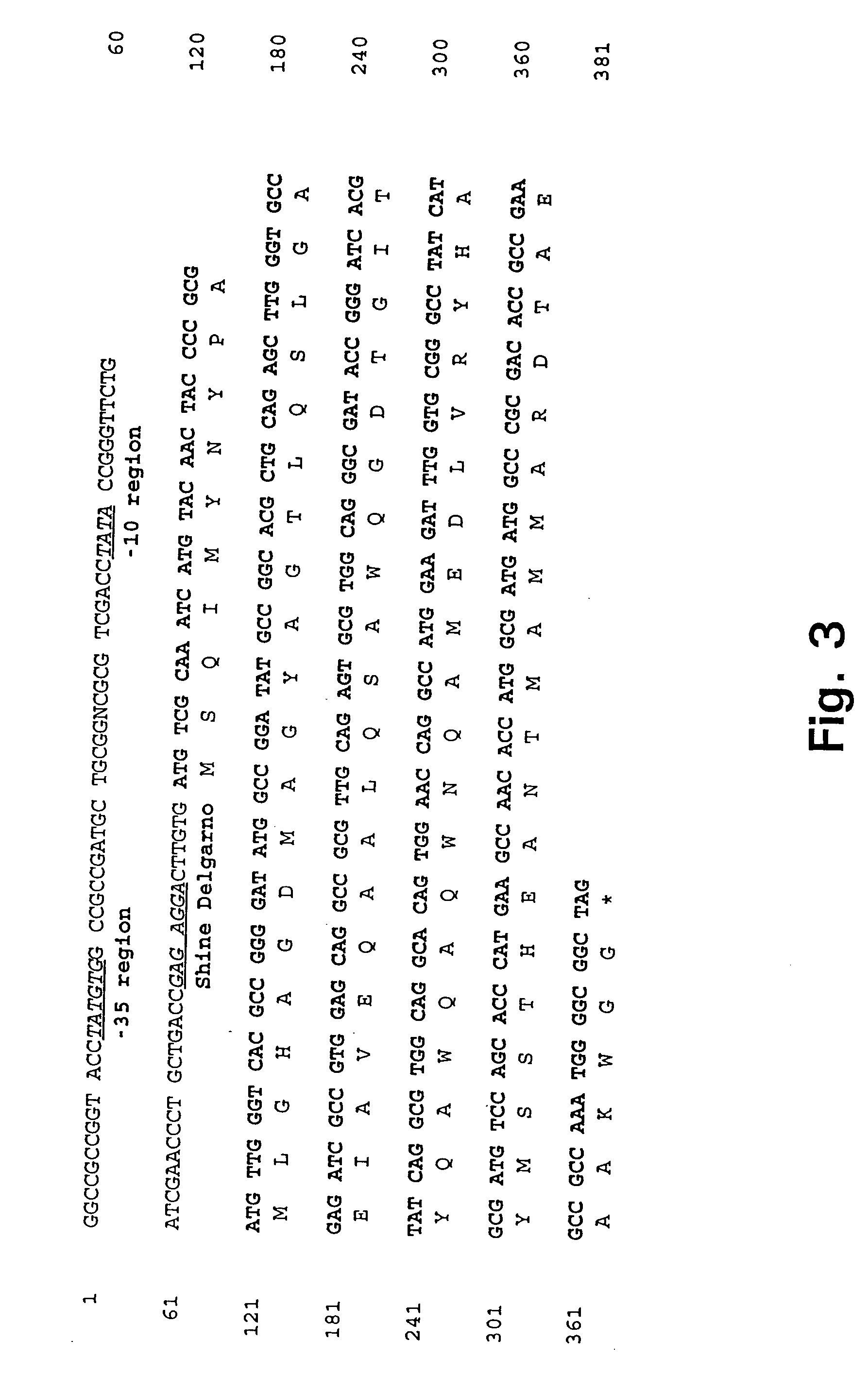
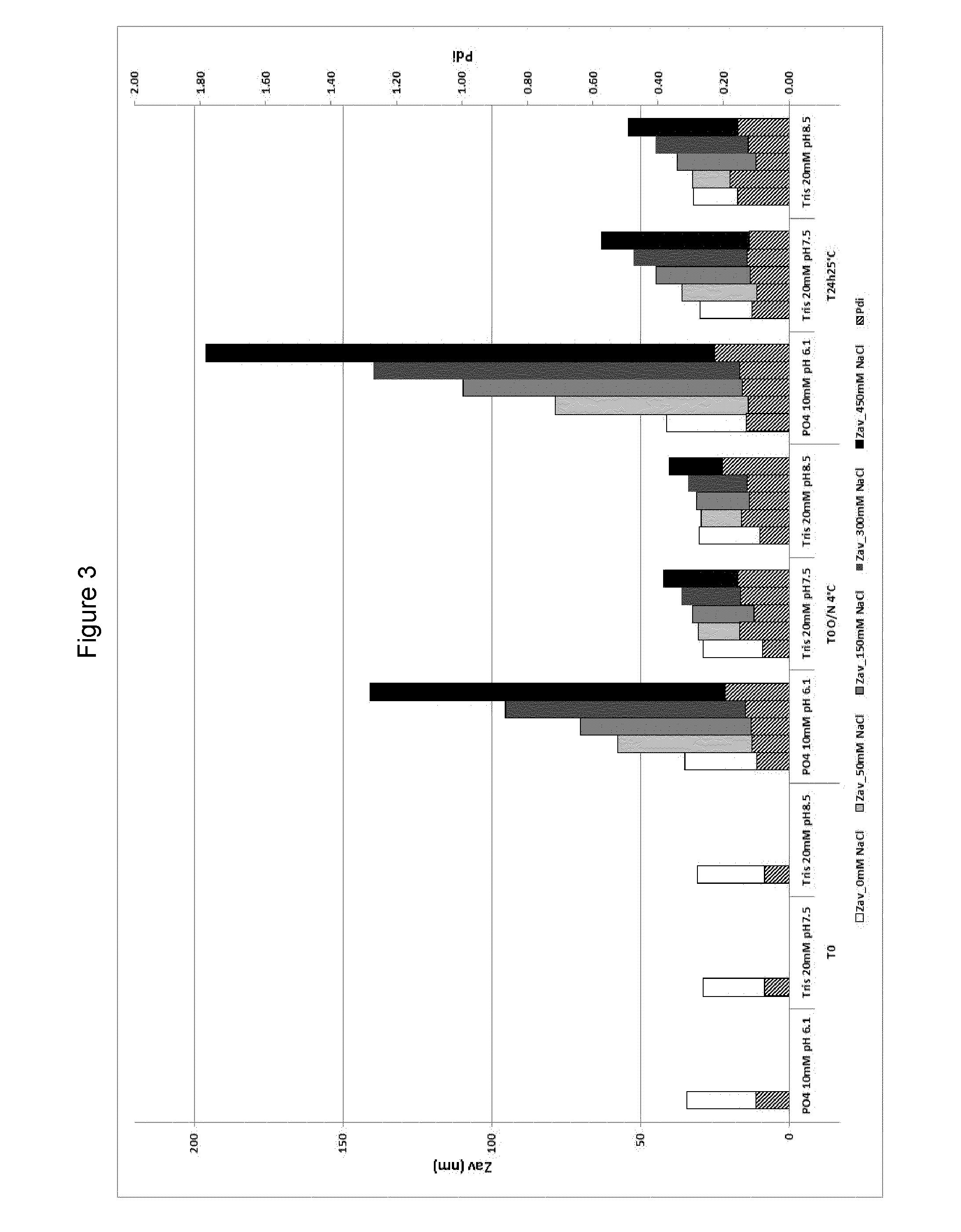
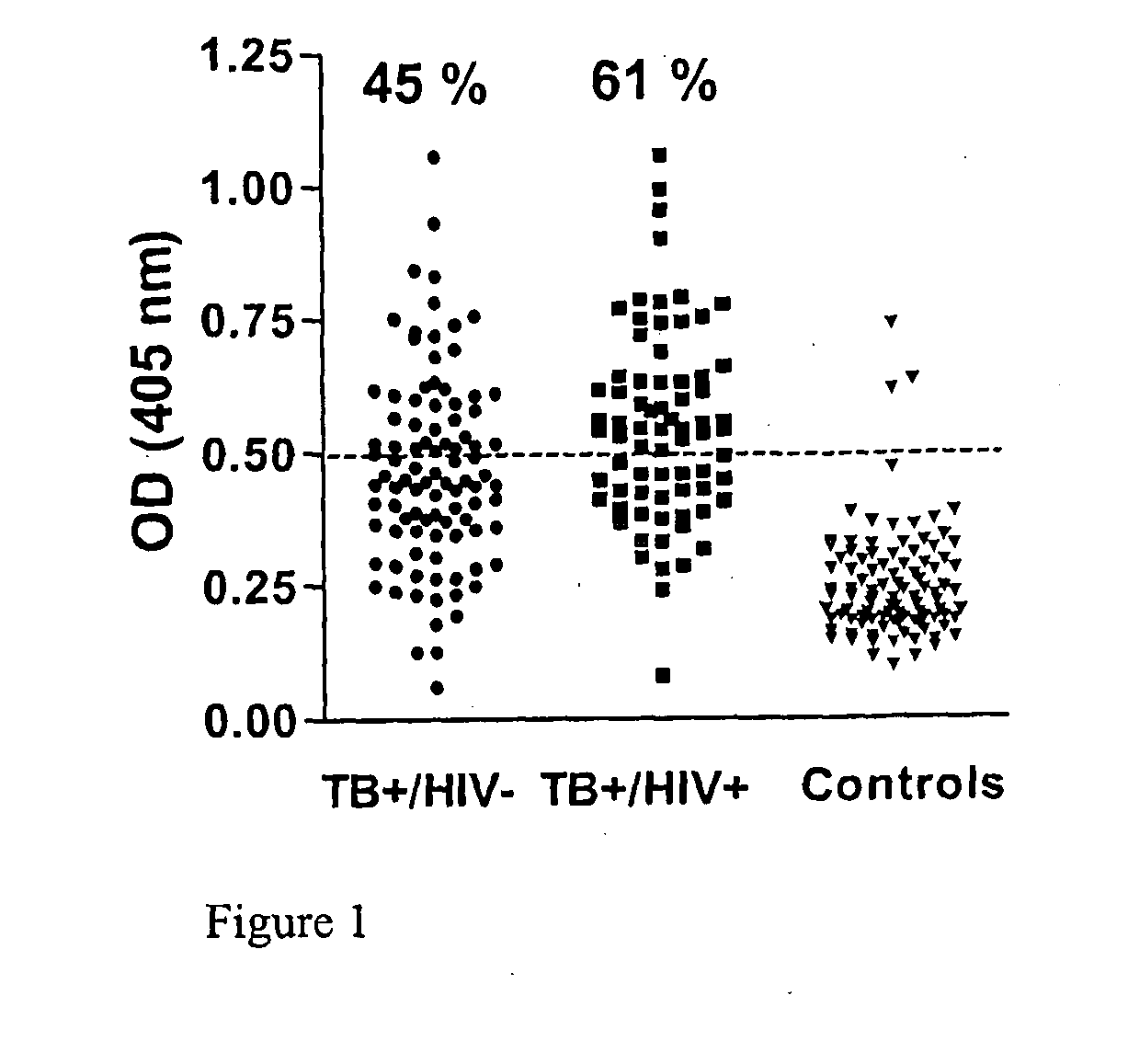
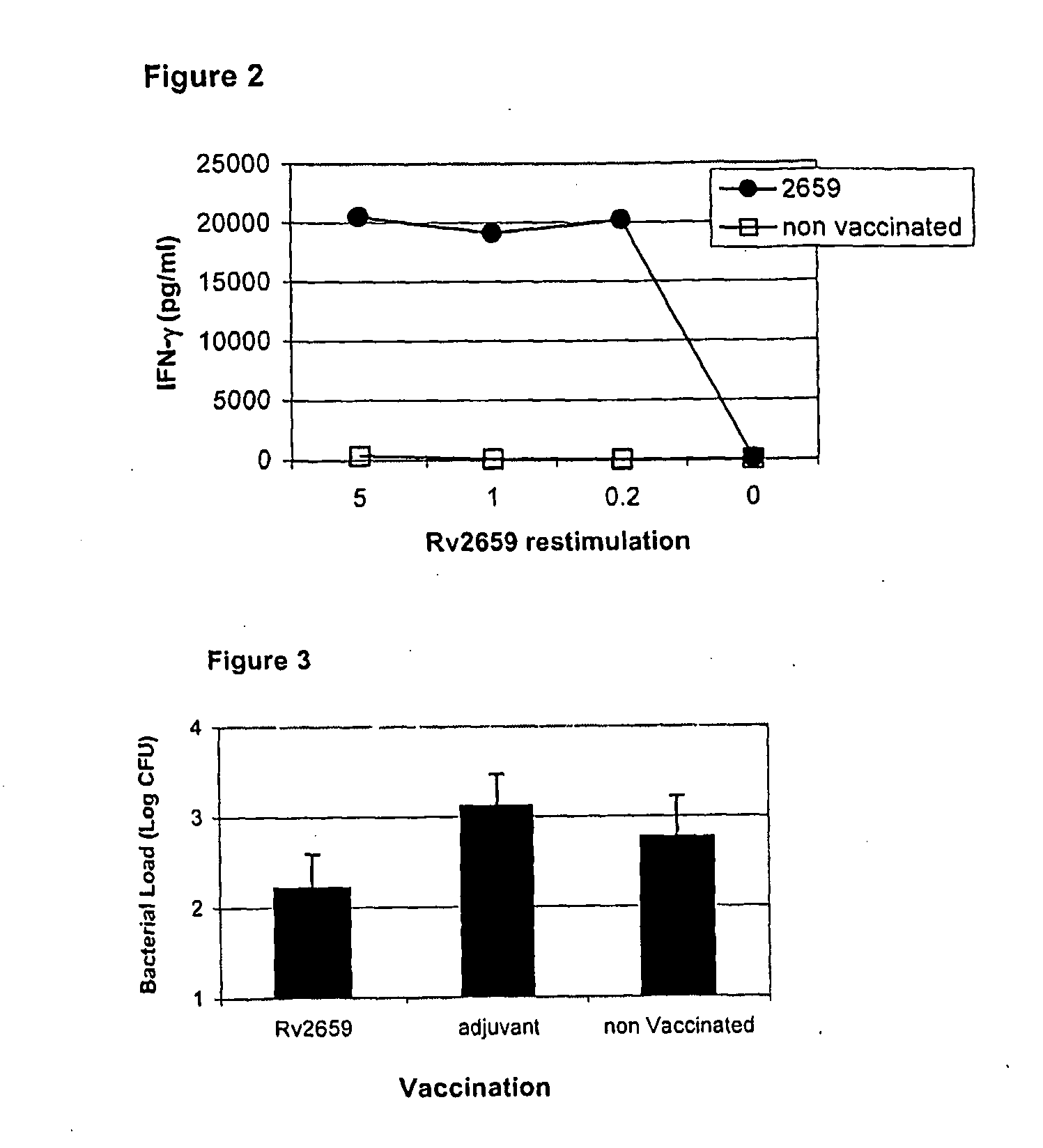
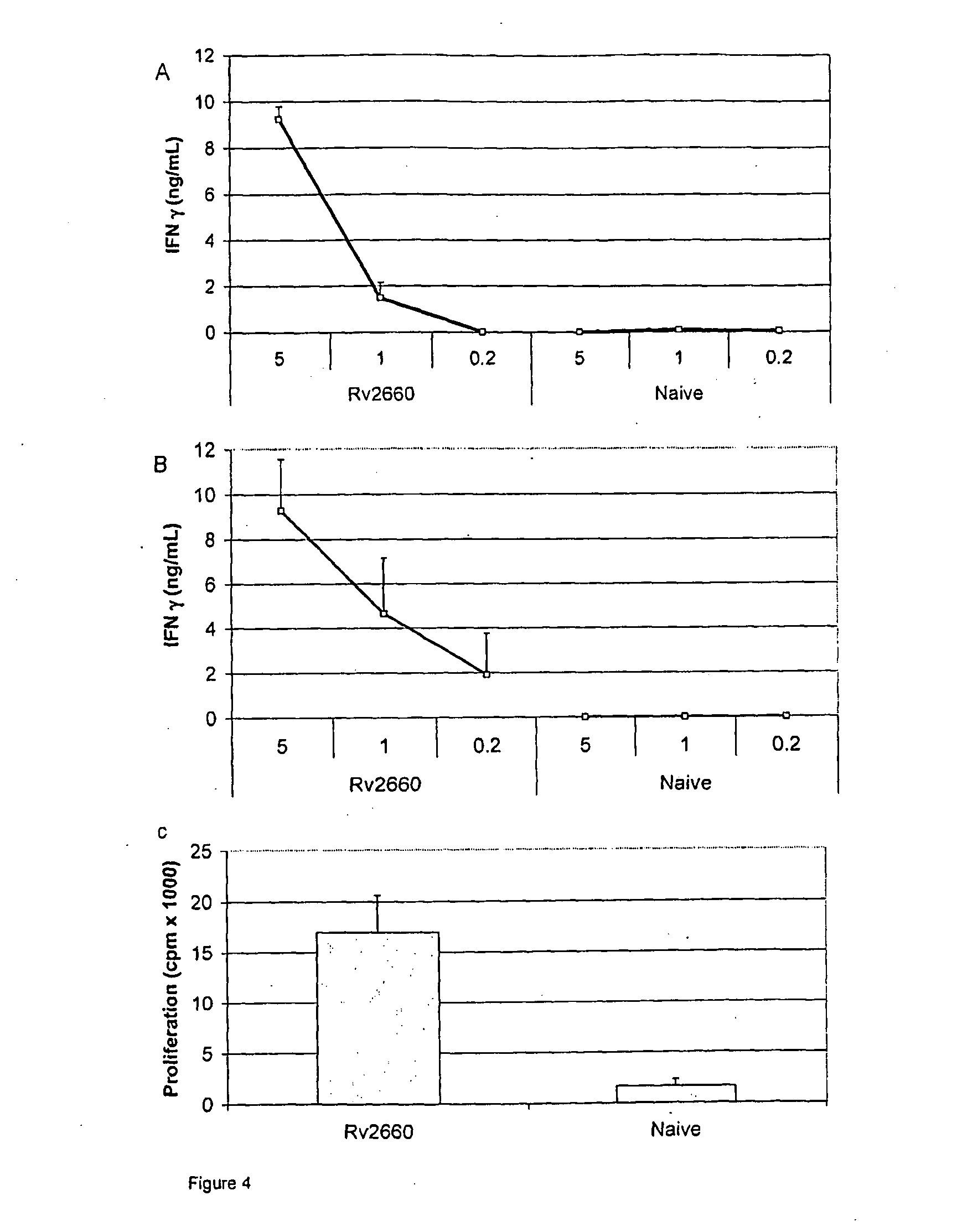
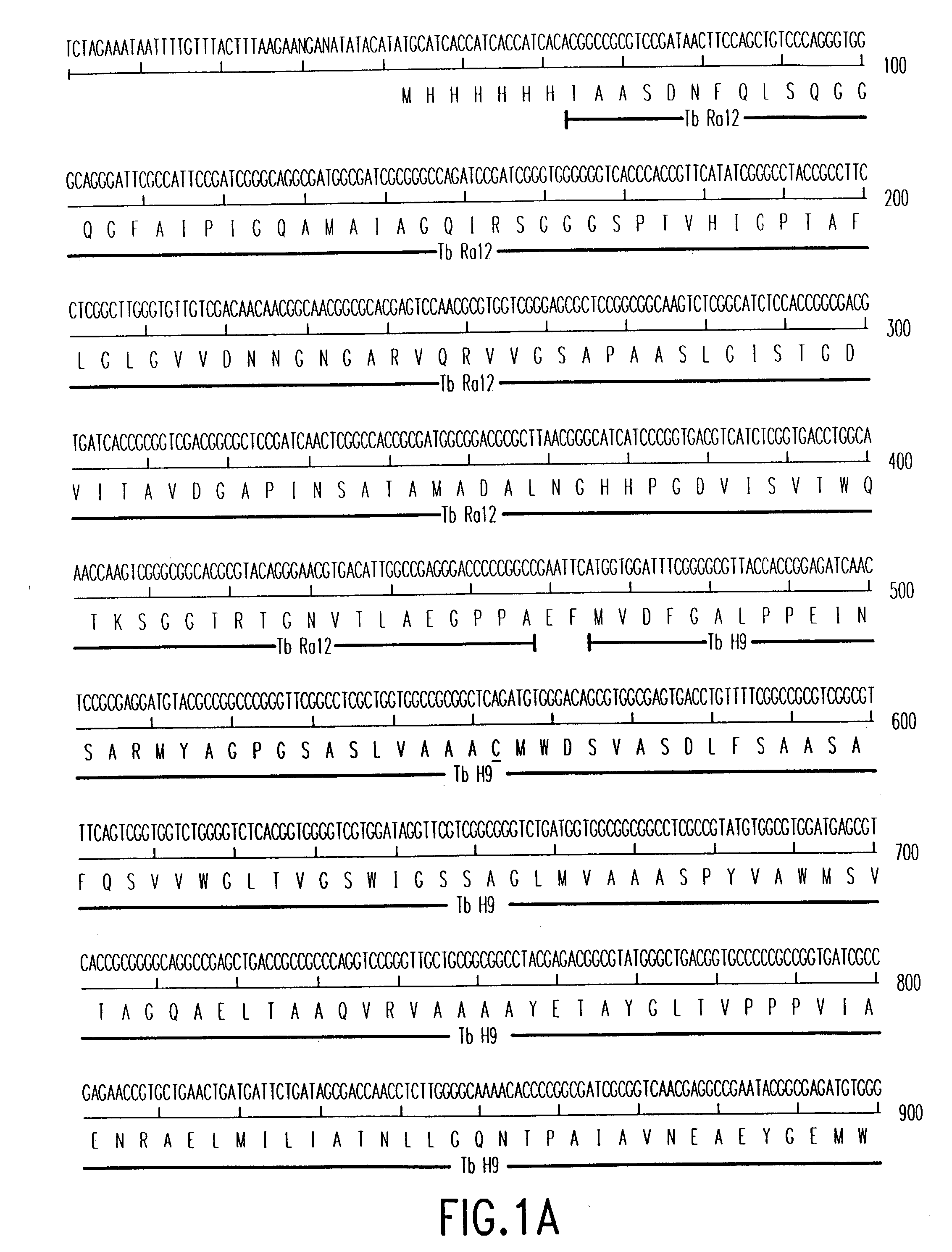
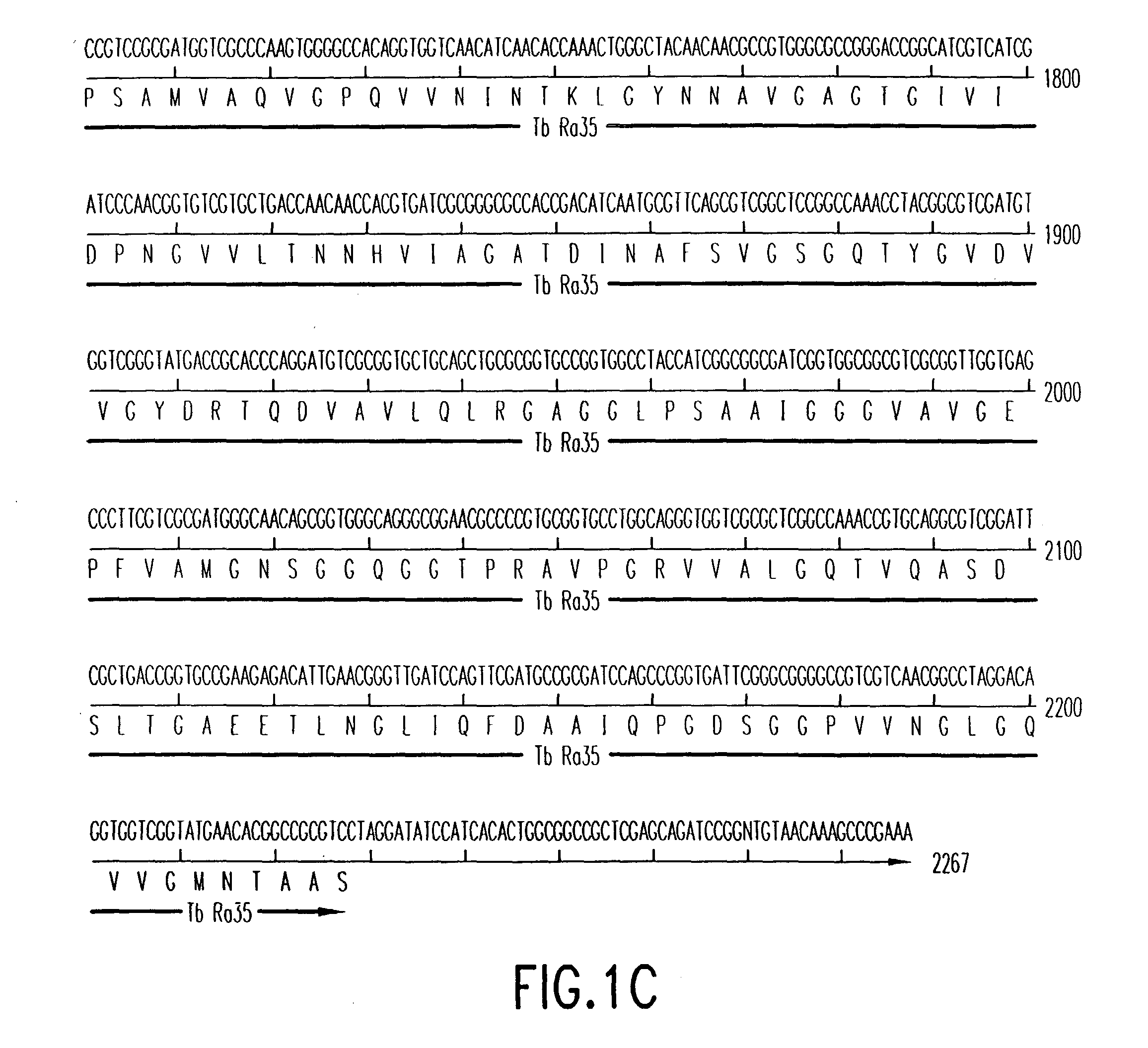
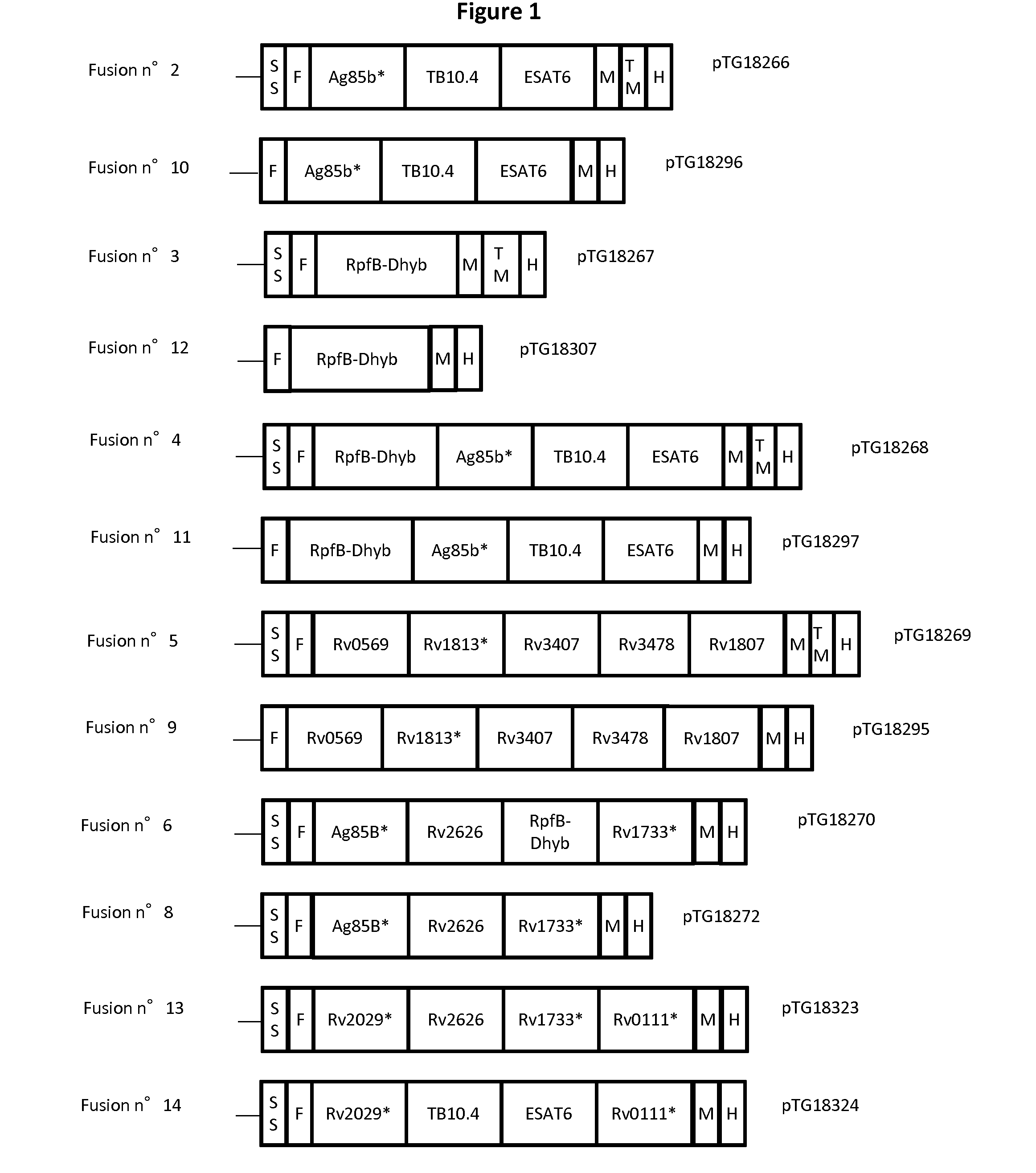
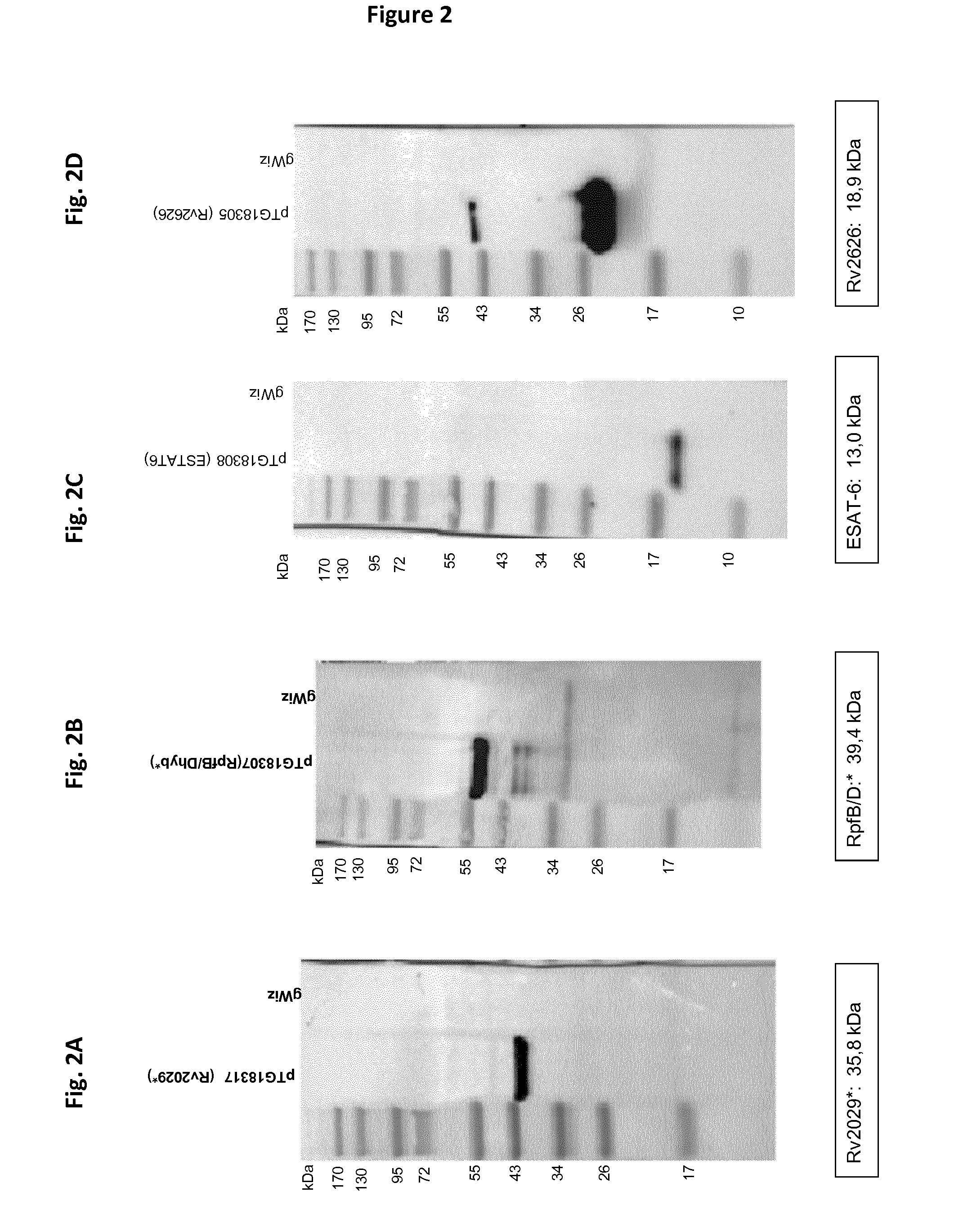
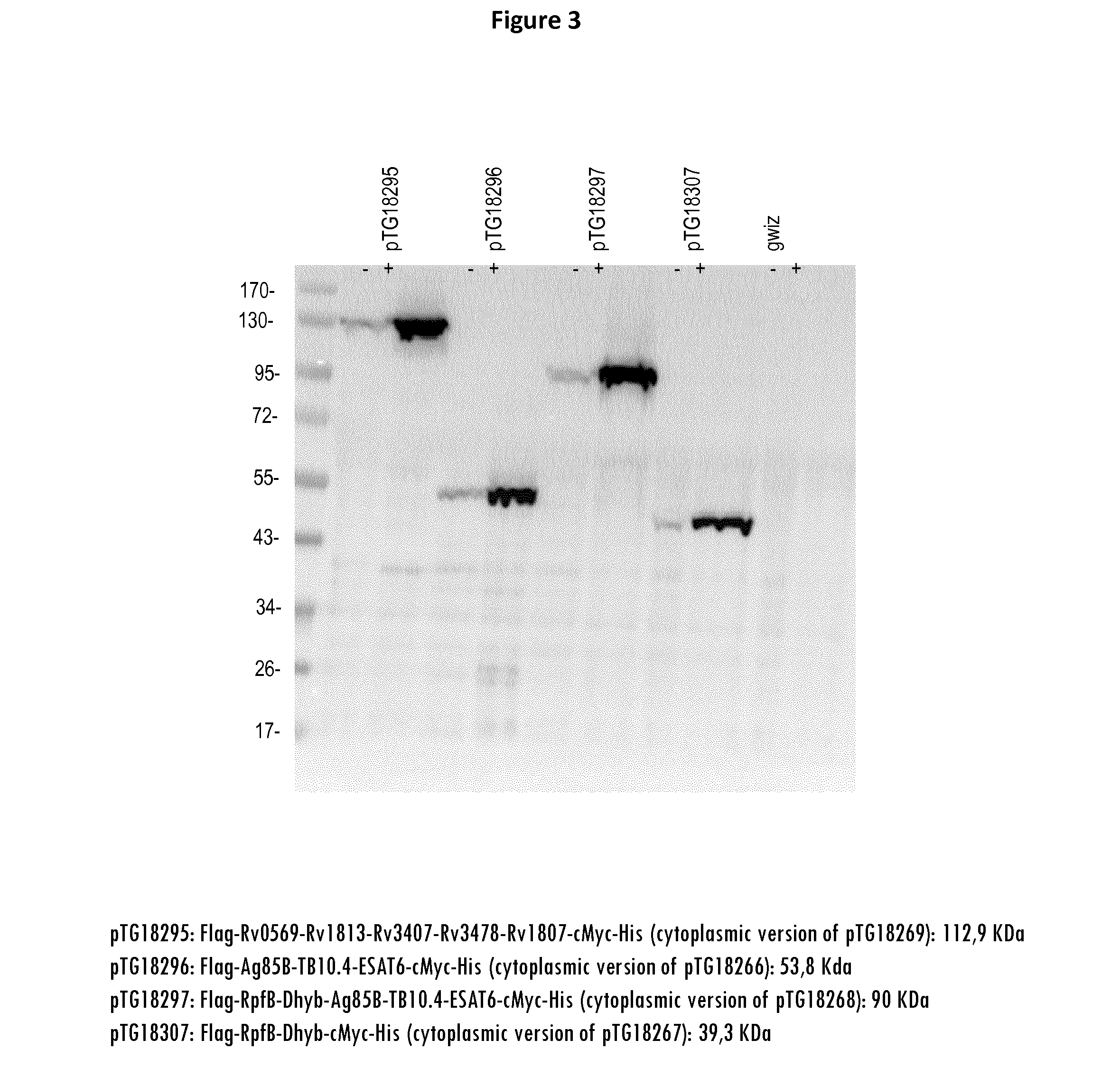
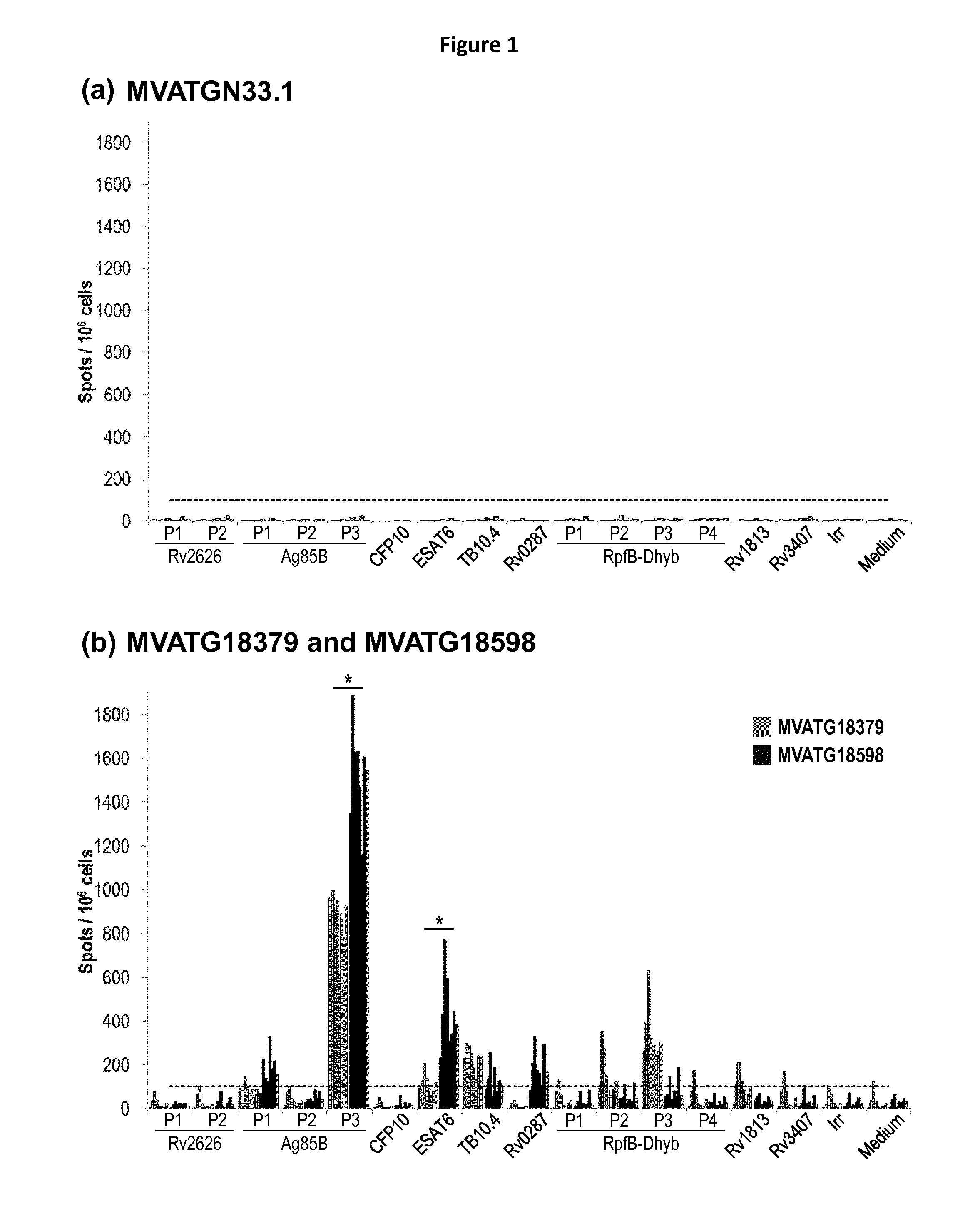
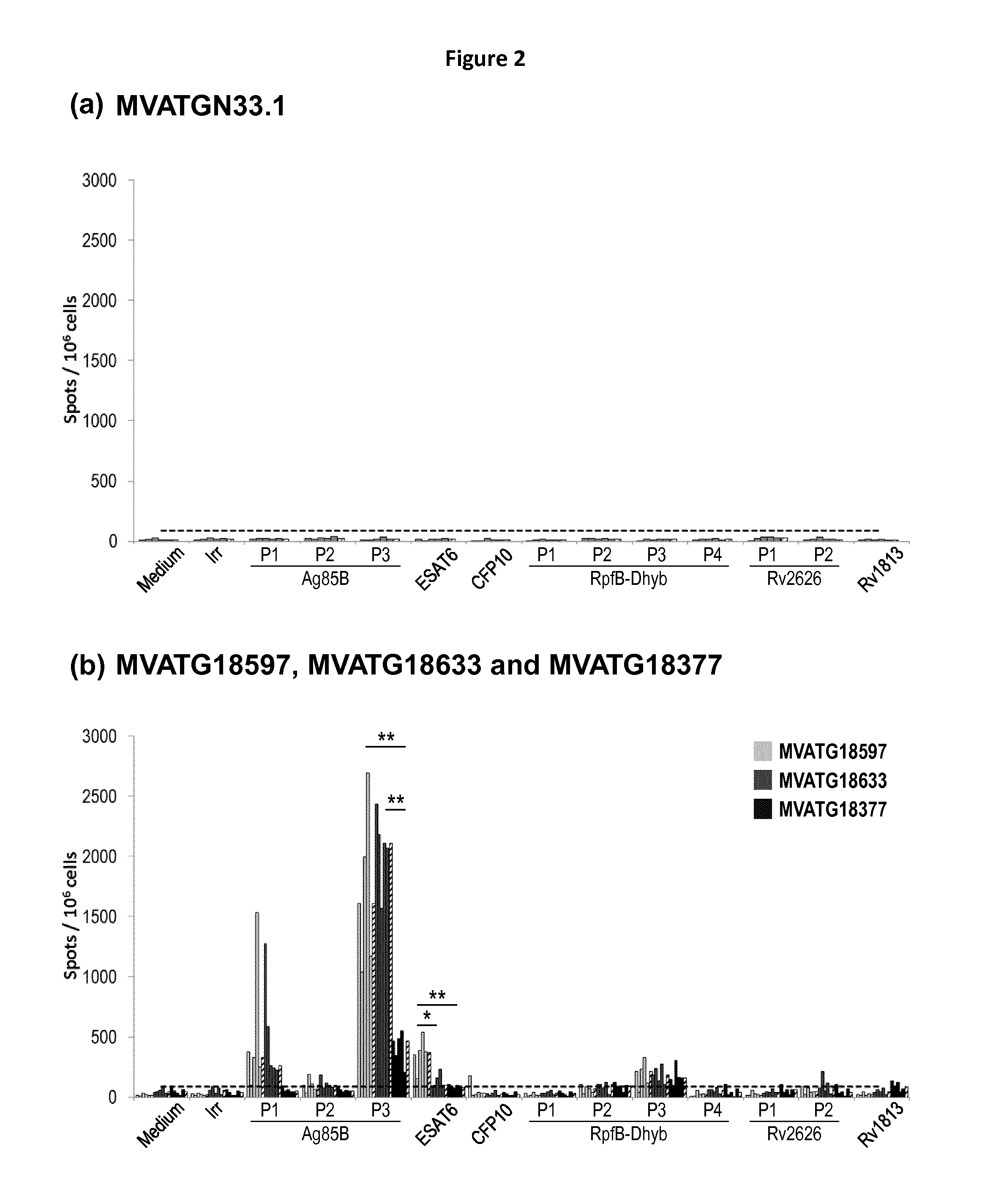
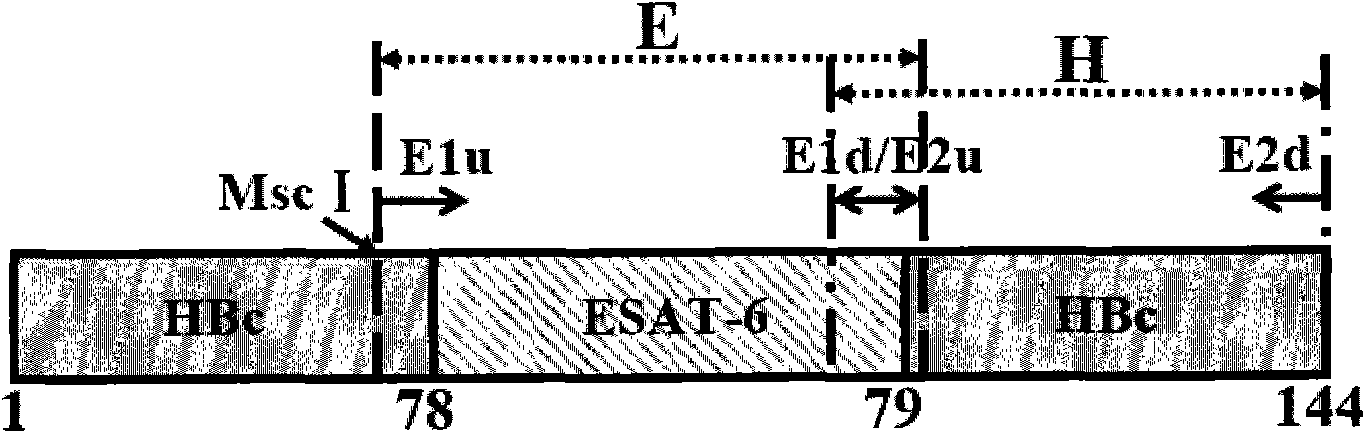
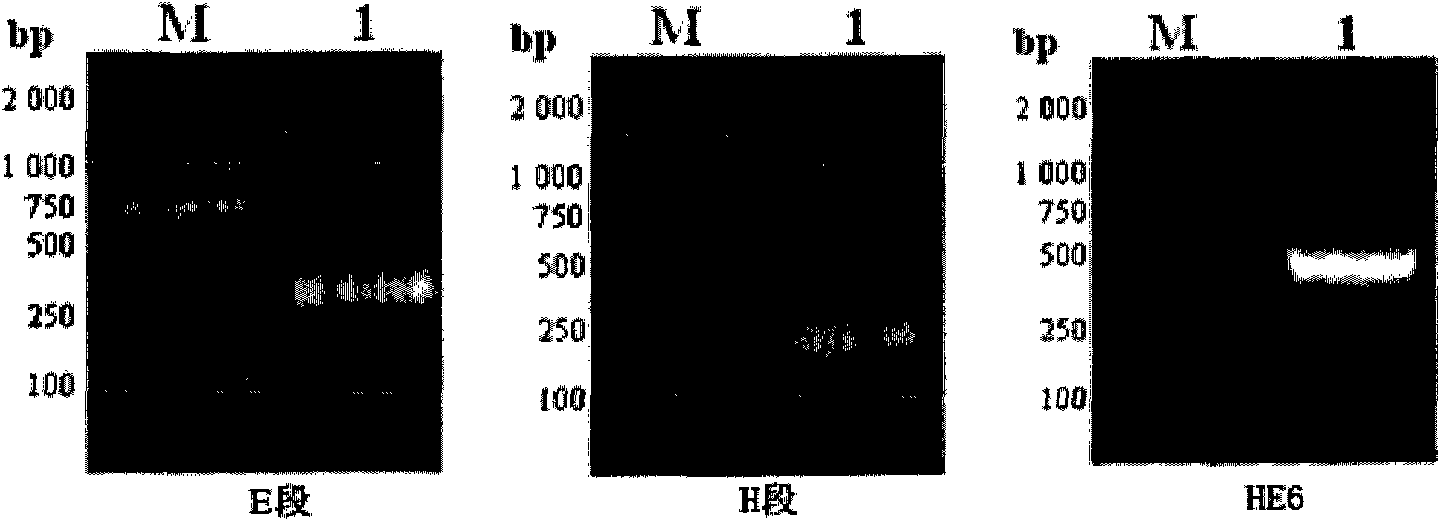
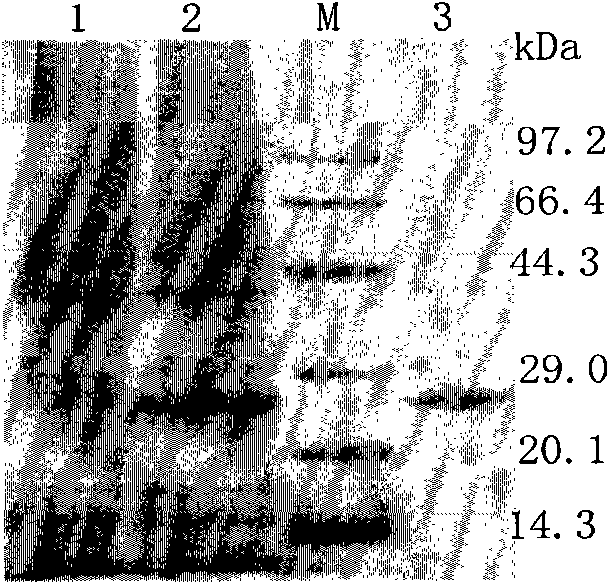
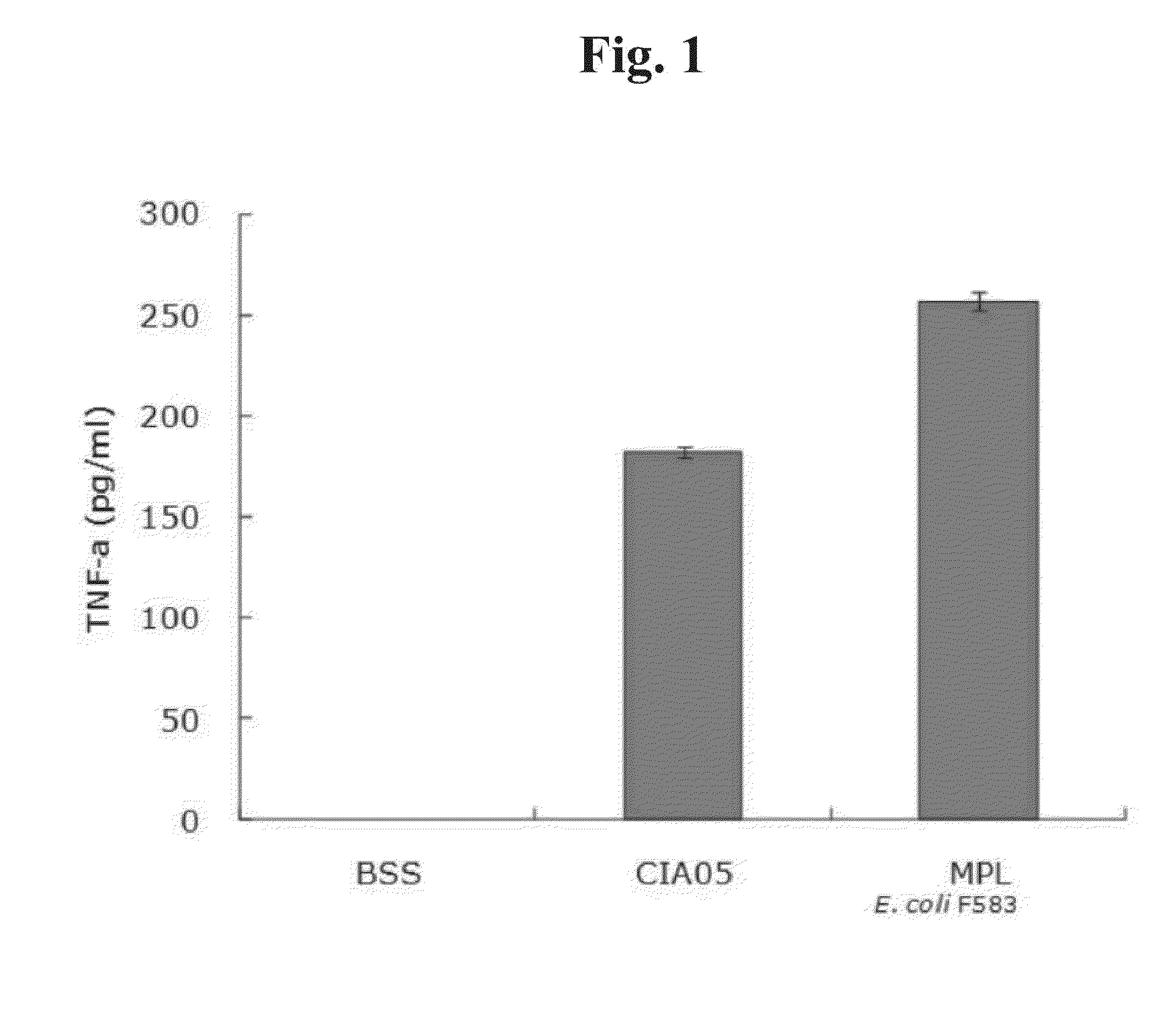
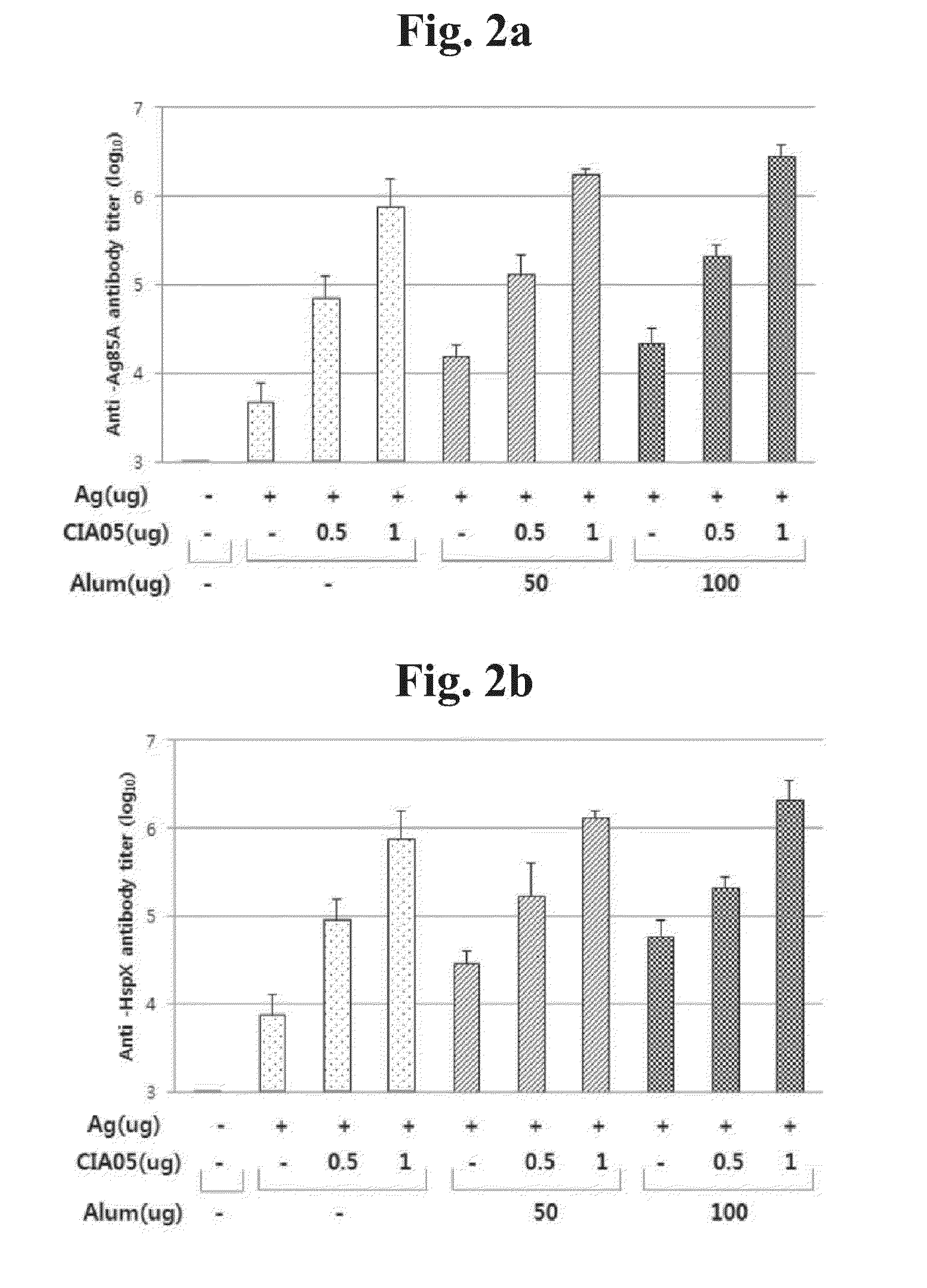
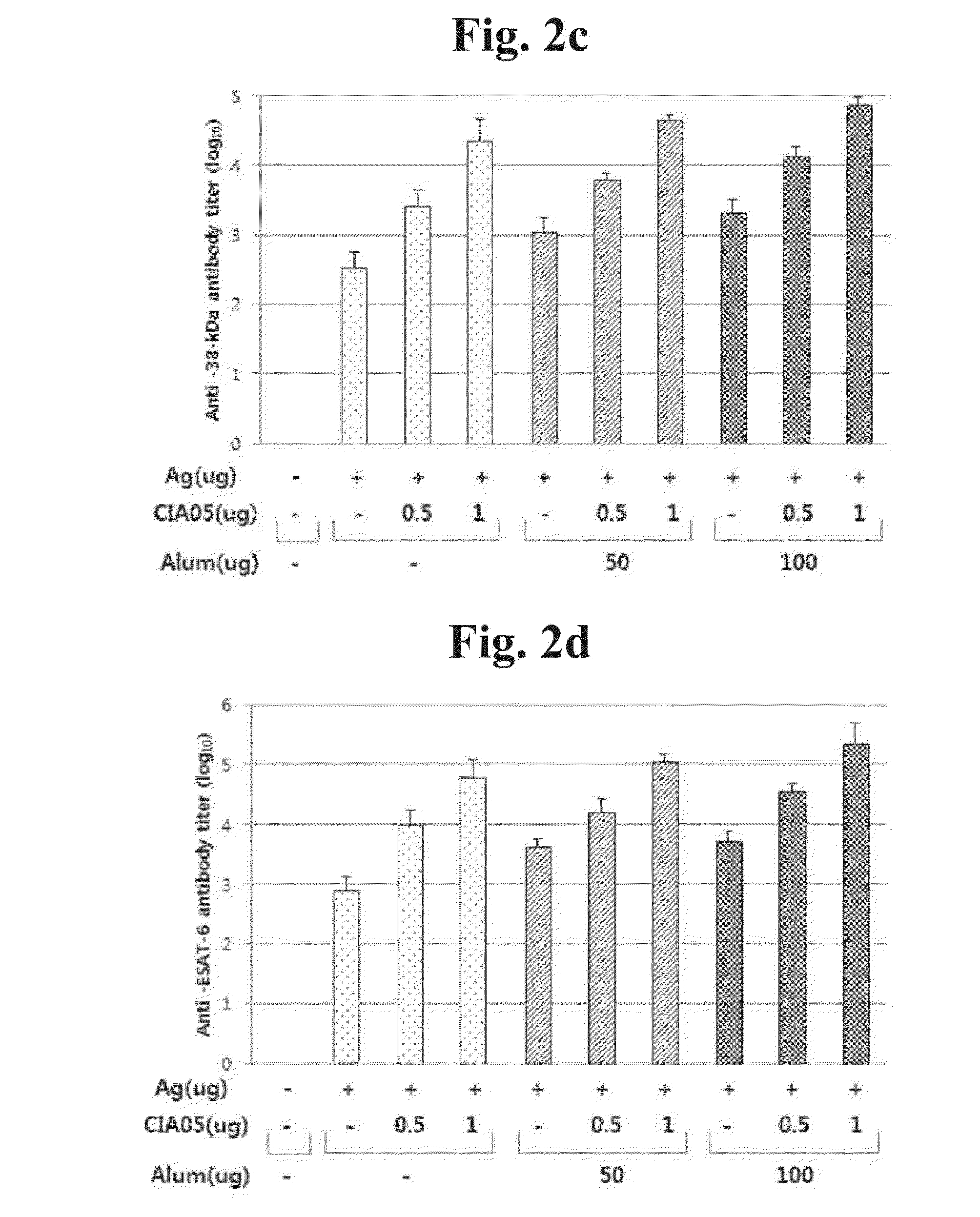
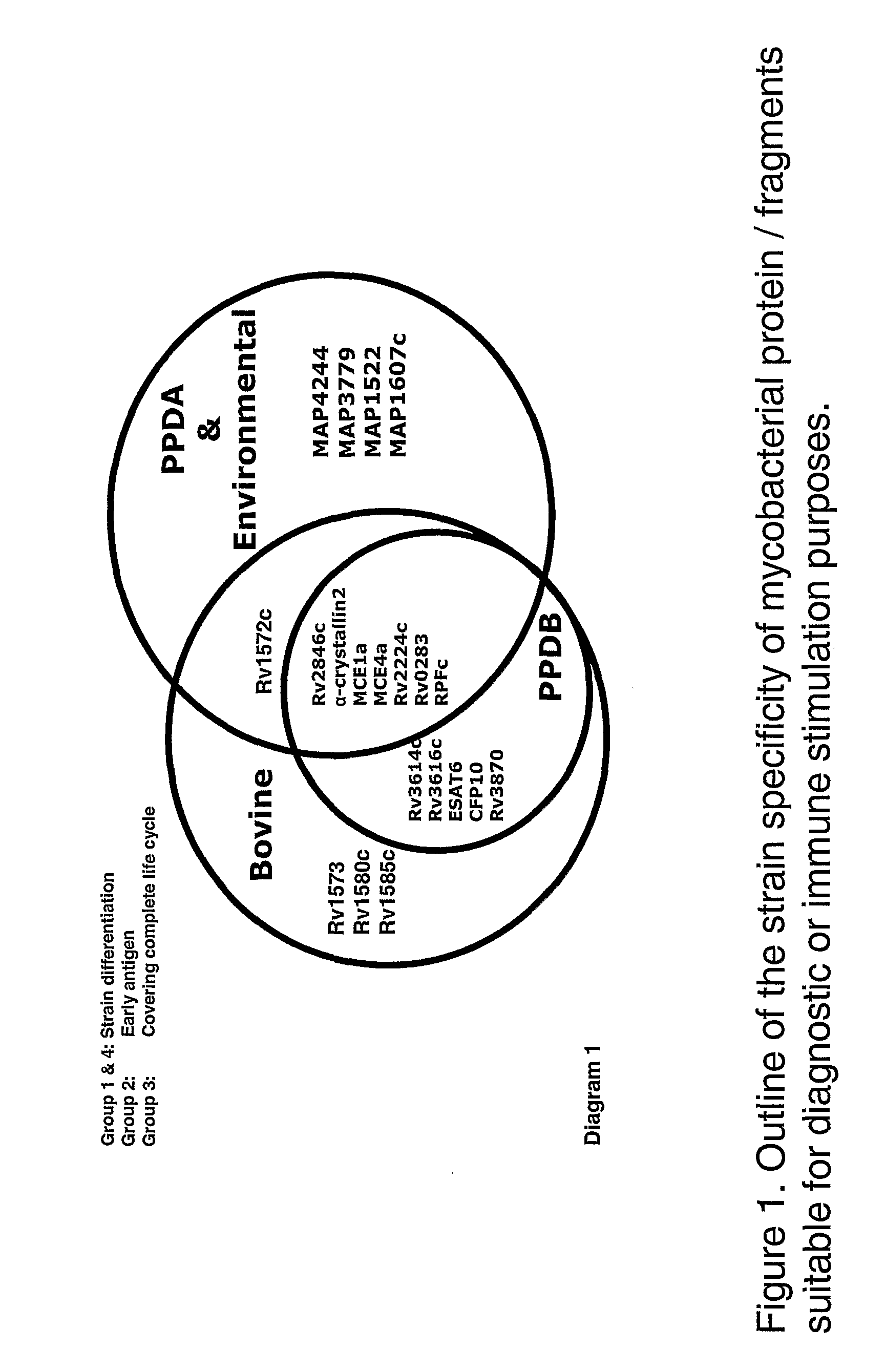
Patents
Literature
89 results about "Mycobacterial antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
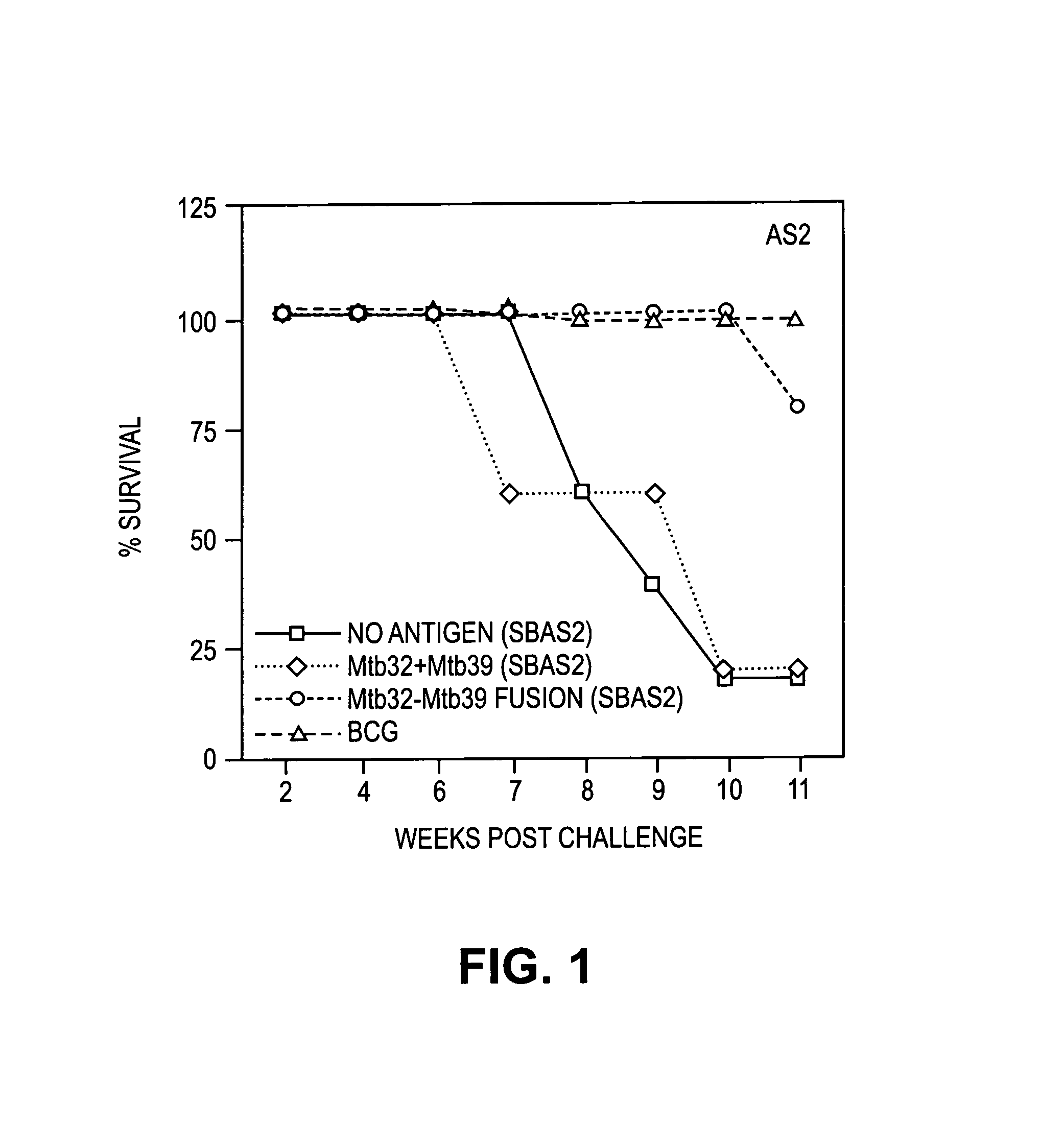
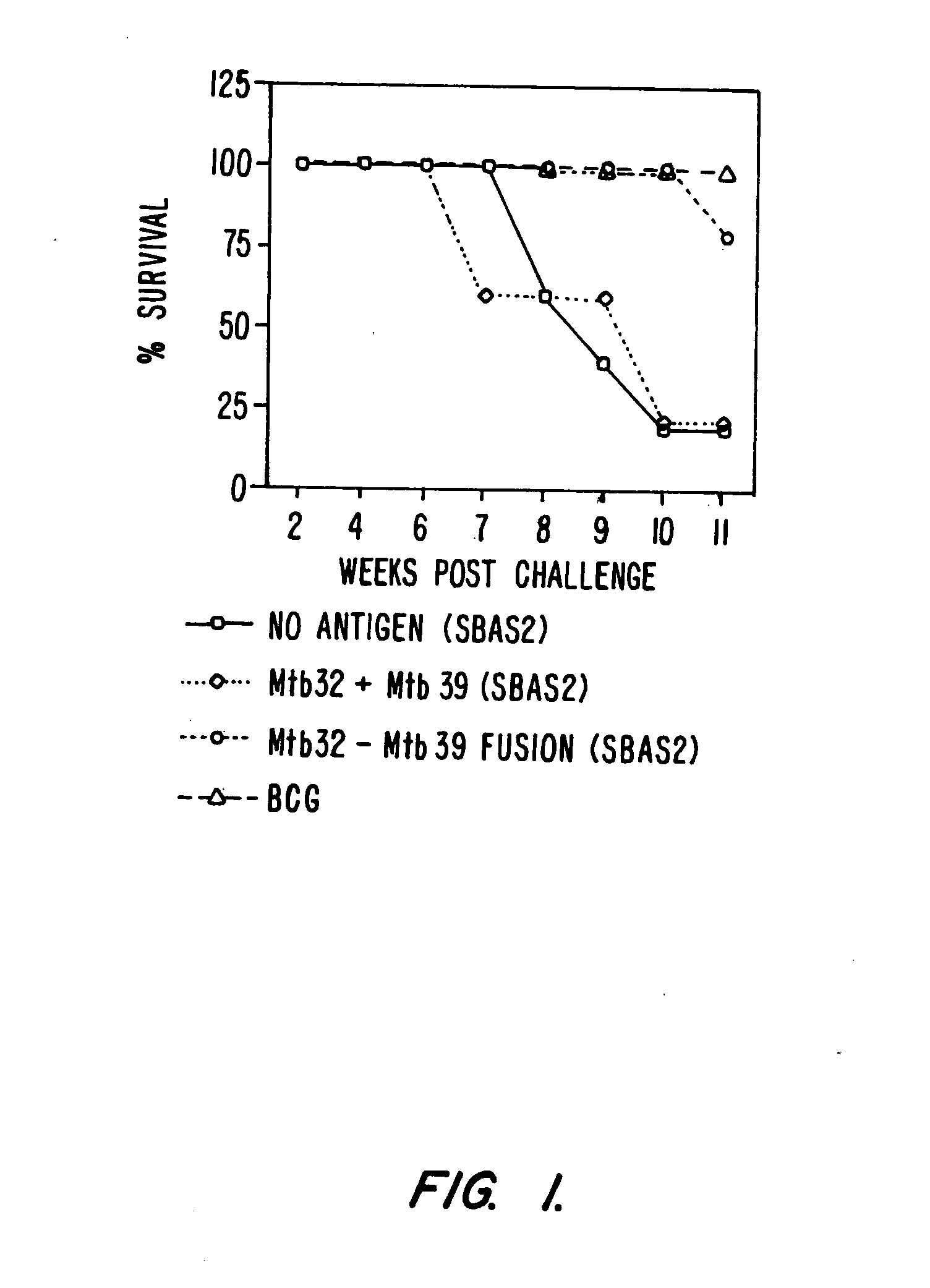
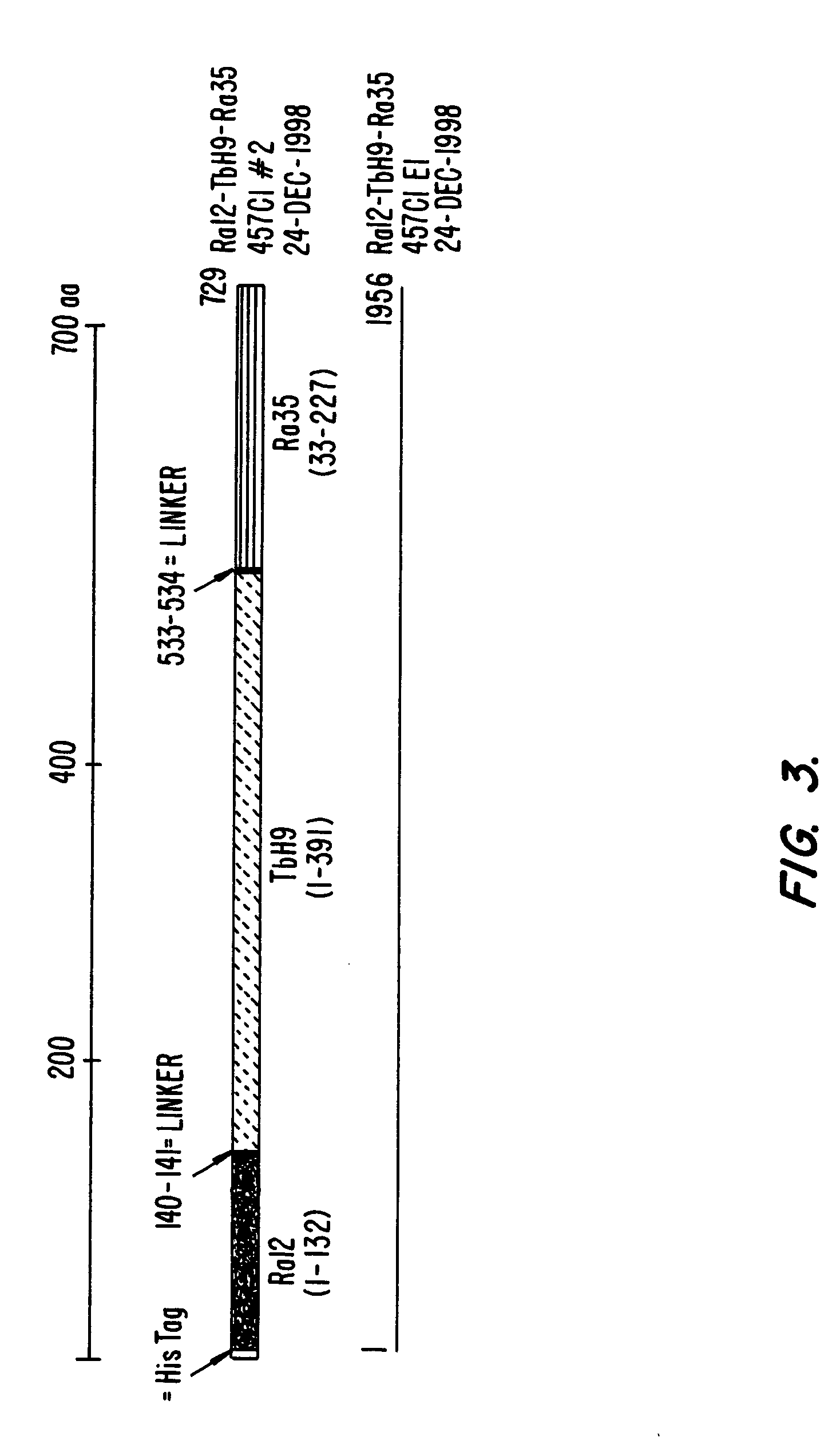
Fusion proteins of Mycobacterium tuberculosis antigens and their uses
InactiveUS6627198B2Improving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenMycobacterial antigen
The present invention relates to fusion proteins containing at least two Mycobacterium tuberculosis antigens. In particular, it relates to bi-fusion proteins which contain two individual M. tuberculosis antigens, tri-fusion proteins which contain three M. tuberculosis antigens, tetra-fusion proteins which contain four M. tuberculosis antigens, and penta-fusion proteins which contain five M. tuberculosis antigens, and methods for their use in the diagnosis, treatment and prevention of tuberculosis infection.
Owner:CORIXA CORP
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS6544522B1Improving immunogenicityAntibacterial agentsPeptide/protein ingredientsAntigenMycobacterial antigen
The present invention relates to fusion proteins of Mycobacterium tuberclosis antigens. In particular, it relates to two fusion proteins, each of which contains three individual M. tuberculosis antigens, and a fusion protein of two M. tuberculosis antigens, their coding sequences, and methods for their use in the treatment and prevention of tuberculosis.
Owner:CORIXA CORP
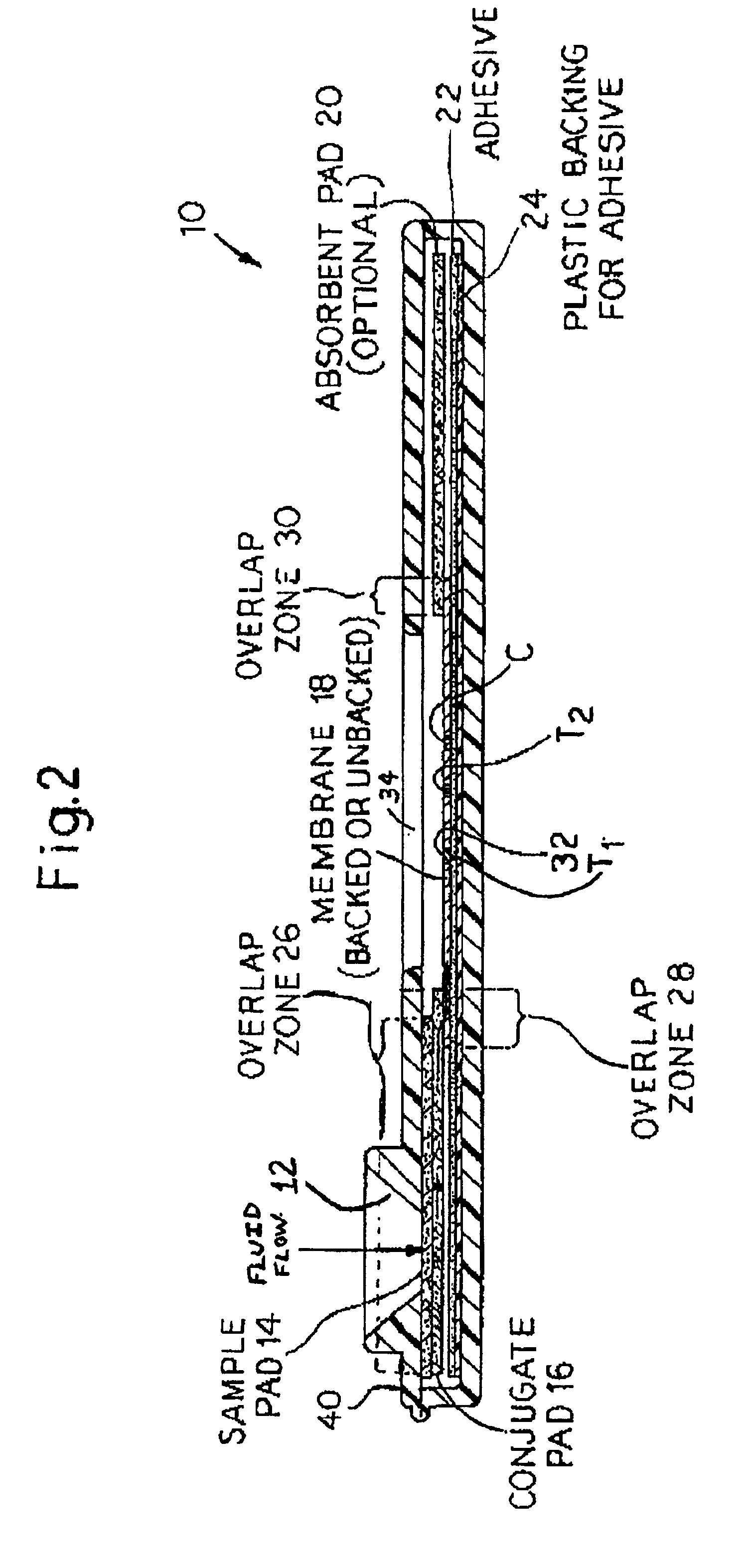
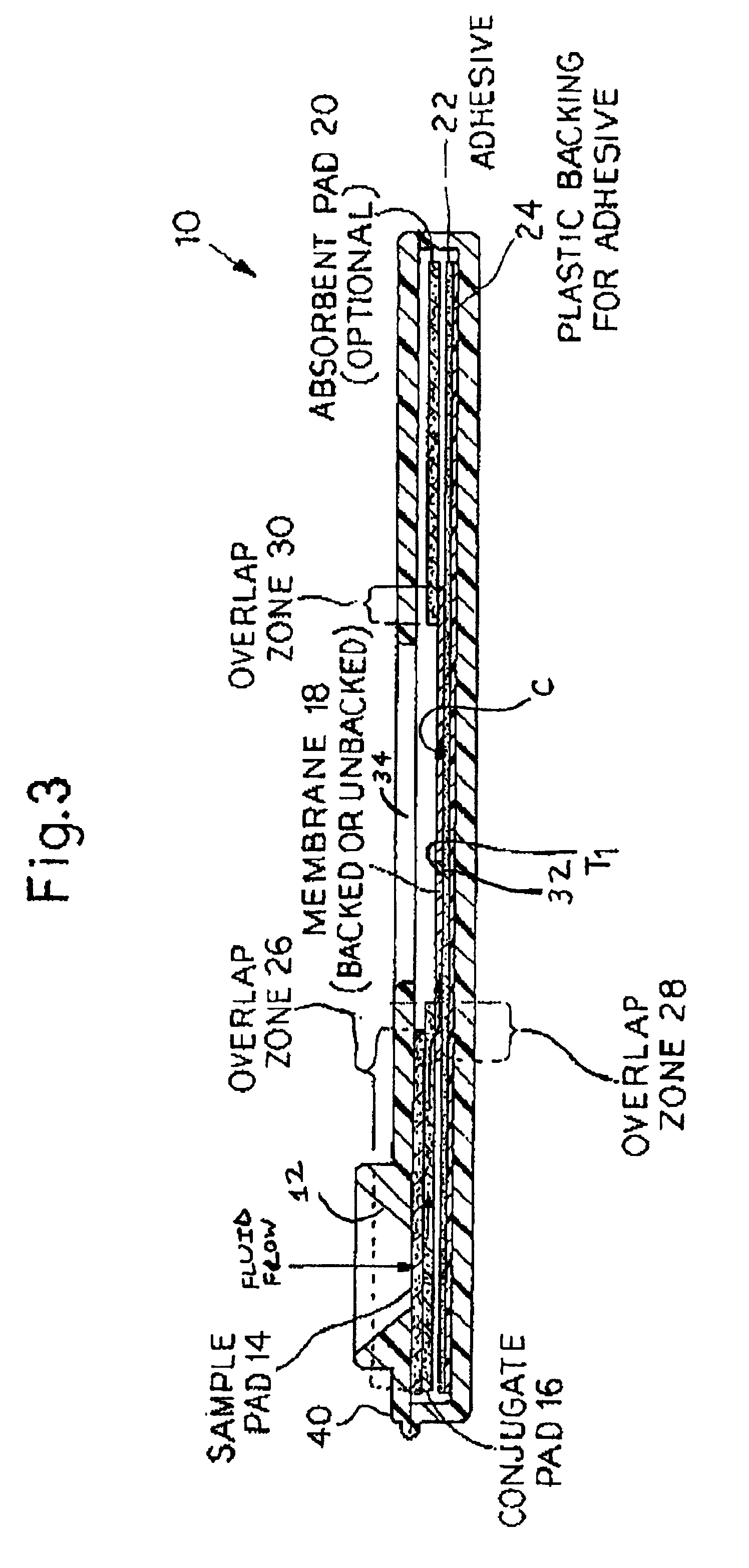
Rapid lateral flow assay for determining exposure to Mycobacterium tuberculosis and other mycobacteria
InactiveUS6841159B2Auxiliary diagnosisAuxiliary judgmentBacterial antigen ingredientsMicrobiological testing/measurementMycobacterial antigenImmunization status
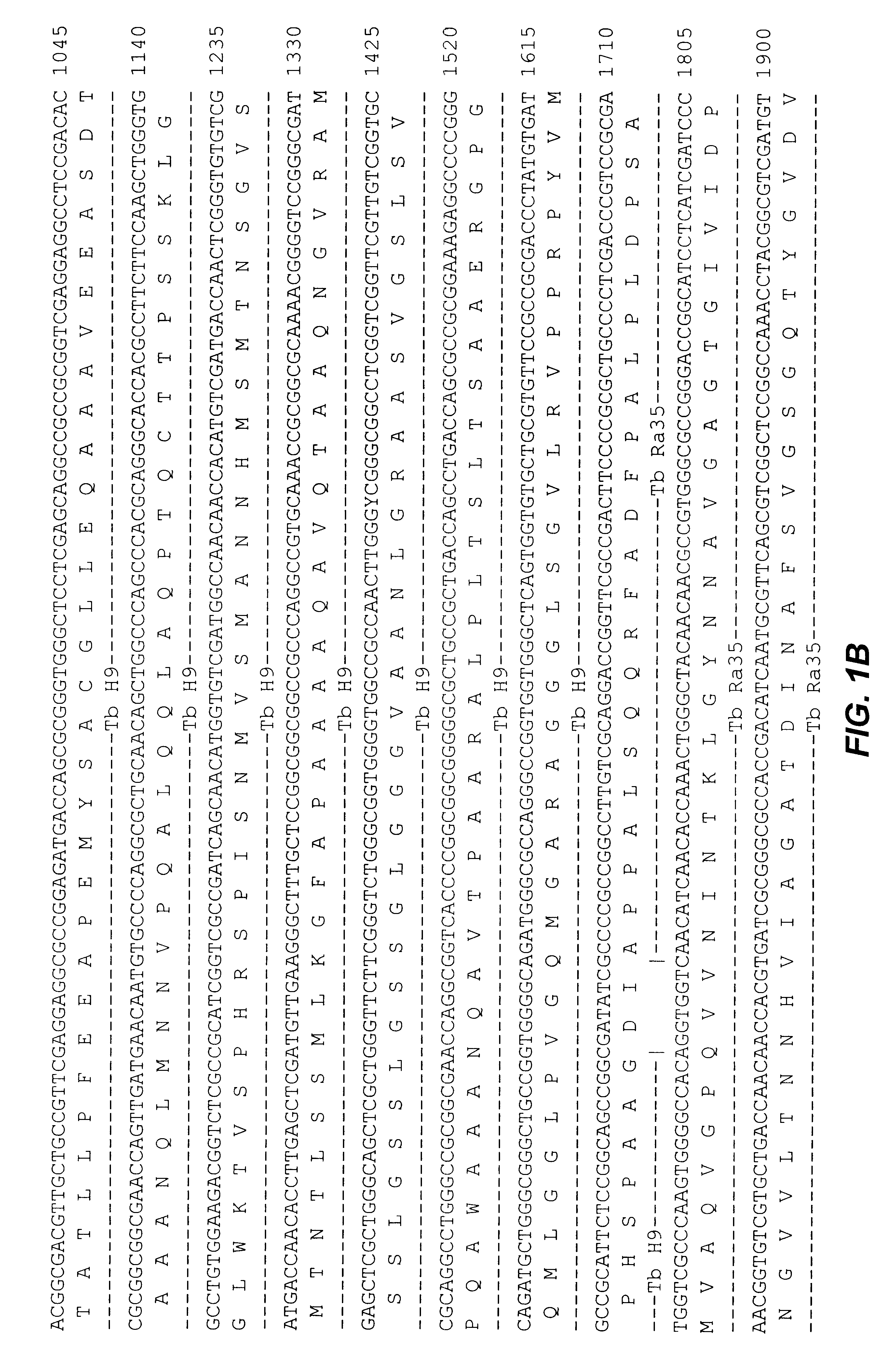
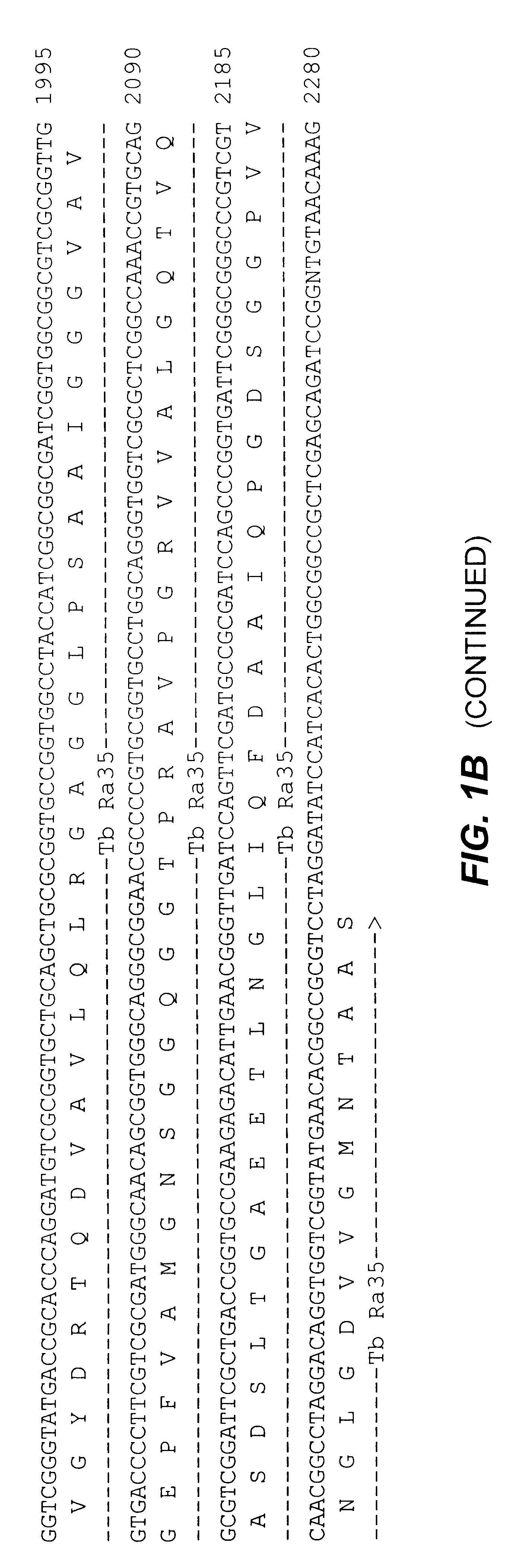
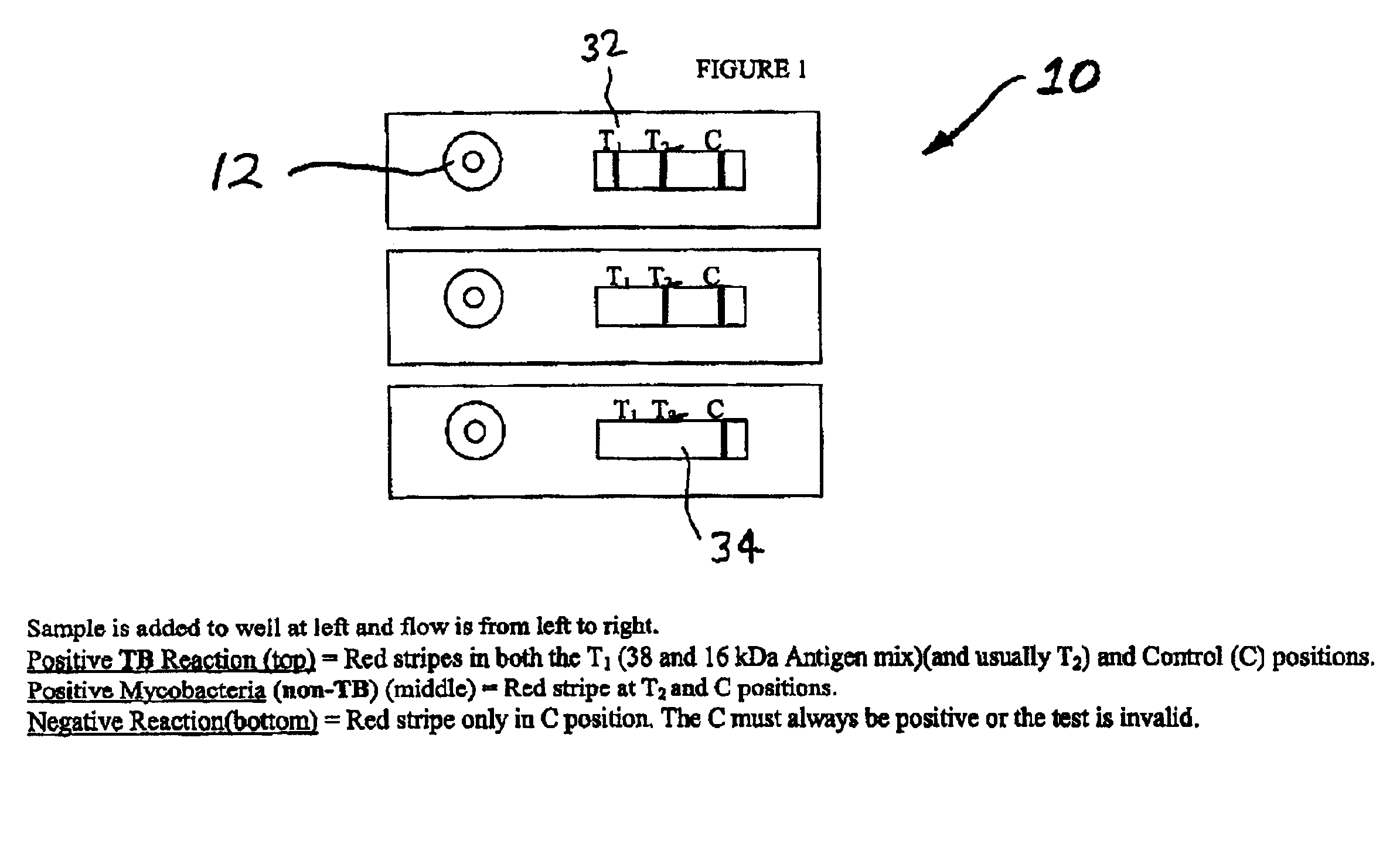
An assay method and kit is disclosed for detecting the presence of at least one predesignated, target antibody to a mycobacterium in a sample selected from one or more patient bodily fluids. The method comprises the following steps: (a) contacting the sample of one or more patient bodily fluids with at least one mycobacterium antigen on a lateral-flow assay membrane to bind to the target antibody in the sample; (b) previously, simultaneously or subsequently to step (a), binding the at least one mycobacterium antigen with a conjugated label producing a detectable signal; and (c) detecting the signal whereby the presence of the target antibody is determined in the sample by the intensity or presence of the signal. The method can further comprise the step of evaluating immunization status of the patient from whom the sample came by comparing the signal or lack thereof with immunizations previously received by the patient and in comparison to a known standard control. In a preferred embodiment, the mycobacterium antigen specifically binds to Mycobacterium tuberculosis specific antibodies. Preferably, the immunoassay of the present invention comprises a lateral-flow assay comprising a membrane, a conjugated label pad, and at least one mycobacterium antigen bound to the membrane. In a preferred embodiment, the at least one mycobacterium antigen is selected from the group consisting of 38 kDa and 16 kDa antigens.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +1
Fusion proteins of Mycobacterium tuberculosis
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Composition and method for the treatment of carcinoma
InactiveUS20070134273A1Good effectConvenient treatmentAntibacterial agentsBiocideMycobacterial antigenCompound (substance)
The present invention relates to compositions and methods useful for treating a carcinoma or viral infection in mammals, including humans. The methods and compositions typically comprise use of an immunogenic or immunomodulatory compound, and a gamma delta T cell activator, such that the composition is effective for treating a carcinoma or viral infection. In a preferred aspect of the invention, the methods comprise use of a gamma delta T cell activator and a Mycobacterium antigen, which for example is an attenuated strain of Mycobacterium bovis (Bacillus Calmette-Guerin (BCG)).
Owner:ROMAGNE FRANCOIS +1
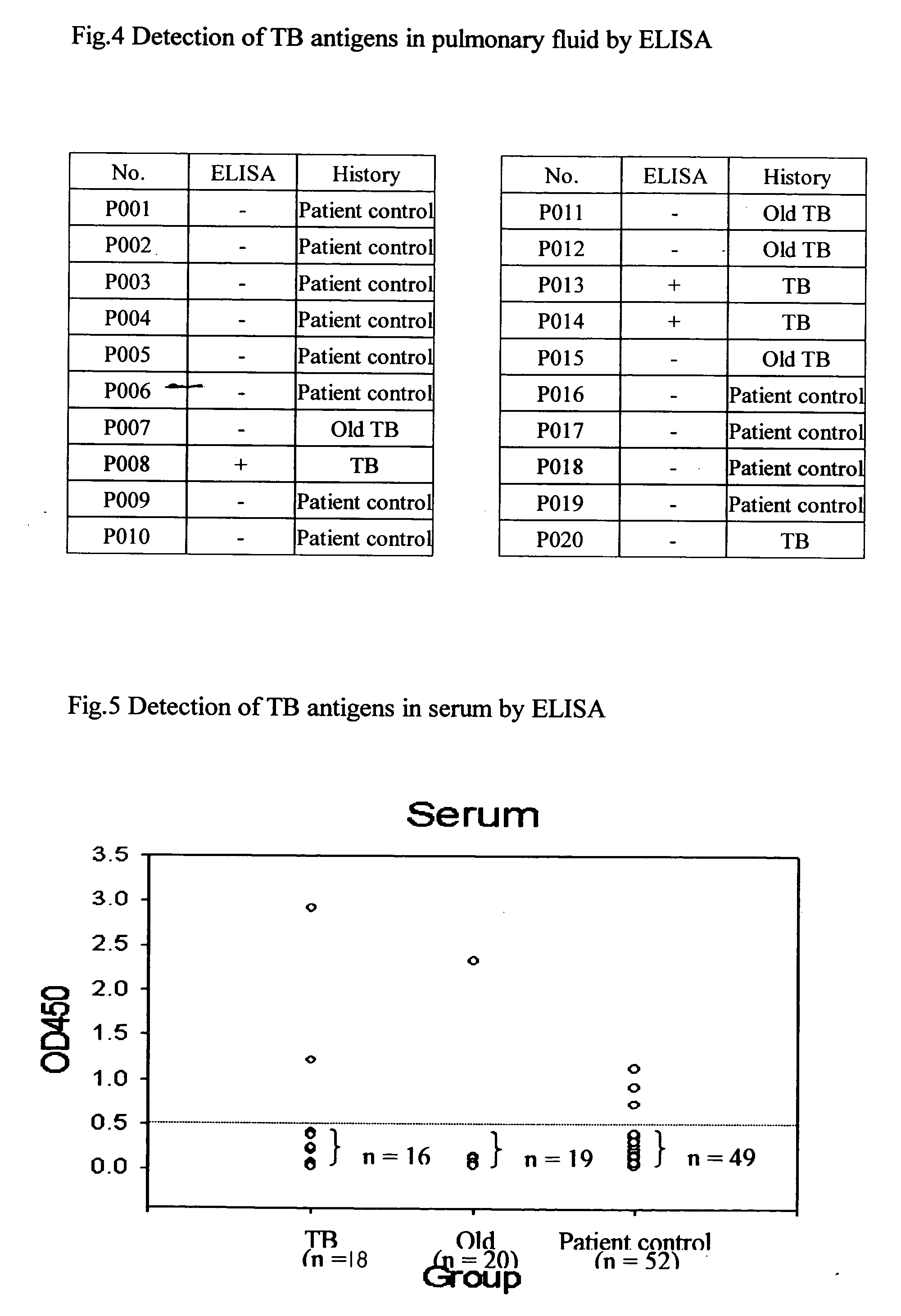
Method for detection of Mycobacterium tuberculosis antigens in biological fluids
A method for detection of mycobacterium tuberculosis antigens in biological fluids provides immunoassay methods, diagnostic kits, and an immunochromatoraphic assay device for detection of Mycobacterium tuberculosis antigens in biological specimens, preferably body fluids and tissues. The preferred body fluids are blood, serum, plasma, urine, pulmonary fluid, sputum, cerebrospinal fluid, and the preferred tissue is the lung biopsy specimen. The immunoassays require two primary antibodies against RD1, RD2, or RD3 of Mycobacterium tuberculosis. At least one of the primary antibodies is attached to a solid carrier. Optional, a second antibody against an animal species producing one of the primary antibodies can be added. Either the other primary antibody or the secondary antibody is labeled with a detection agent, which can be an enzymatic marker, a fluorescent or luminescent agent, a radio active label or a color particle. The biological specimens may be used directly, concentrated or diluted for the immunoassays.
Owner:CHANG GUNG UNIVERSITY
Fusion proteins of Mycobacterium tuberculosis
InactiveUS20060193876A1Antibacterial agentsBacterial antigen ingredientsMycobacterial antigenSerology
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
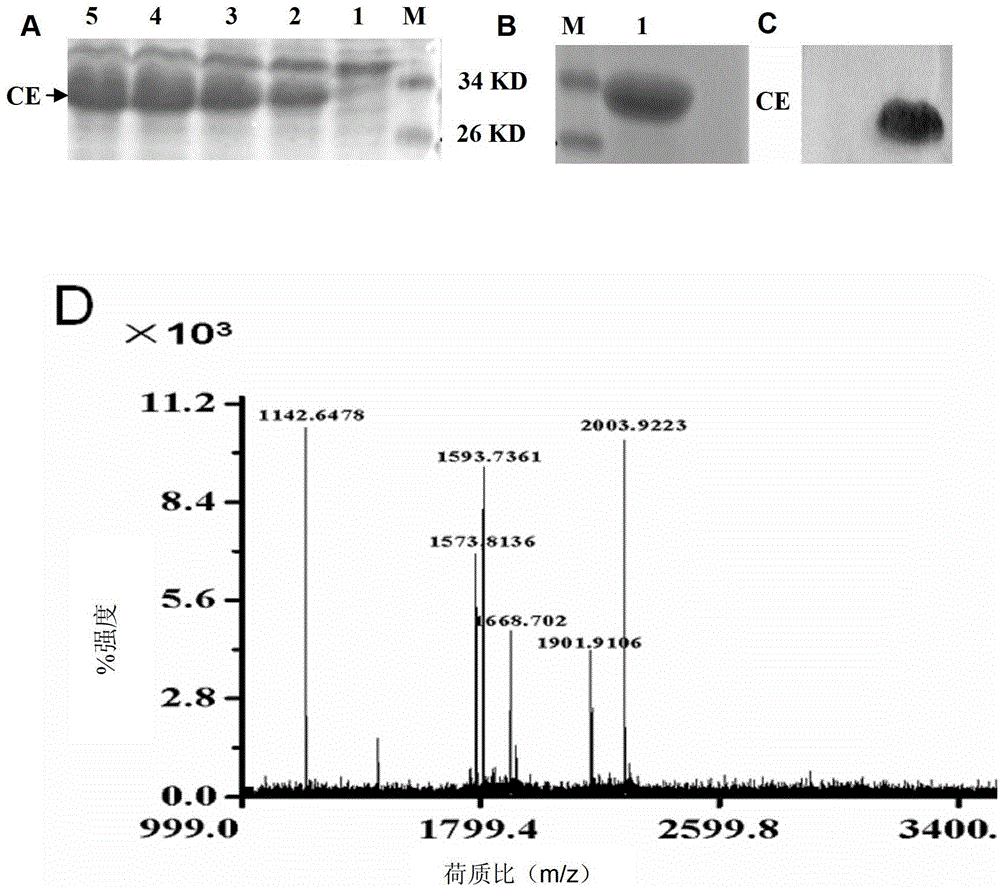
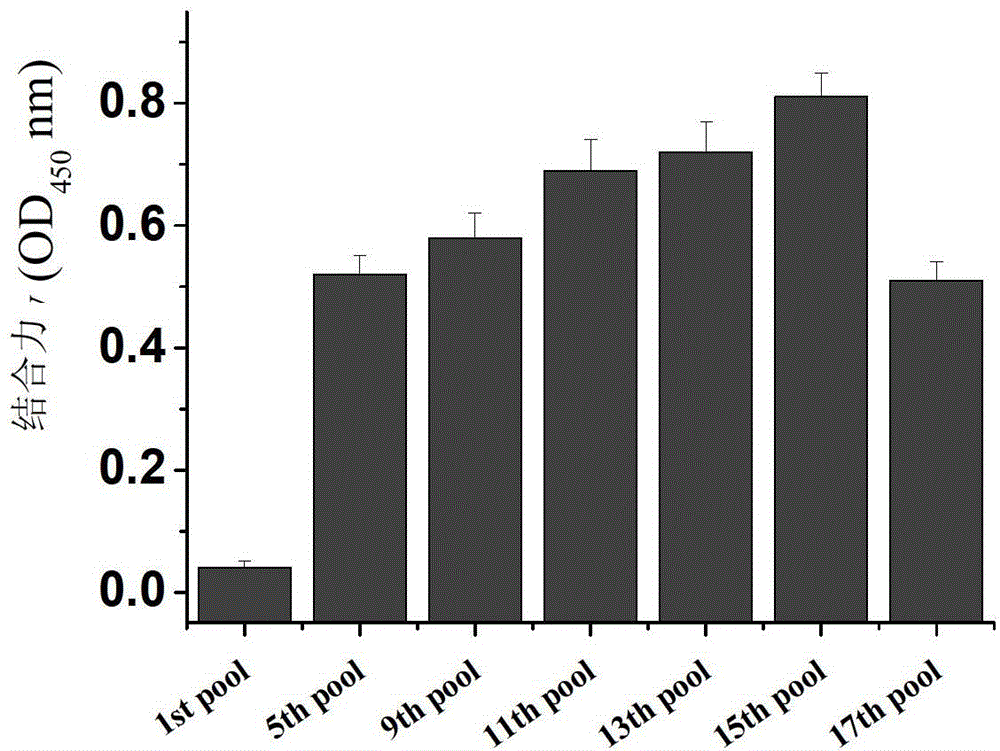
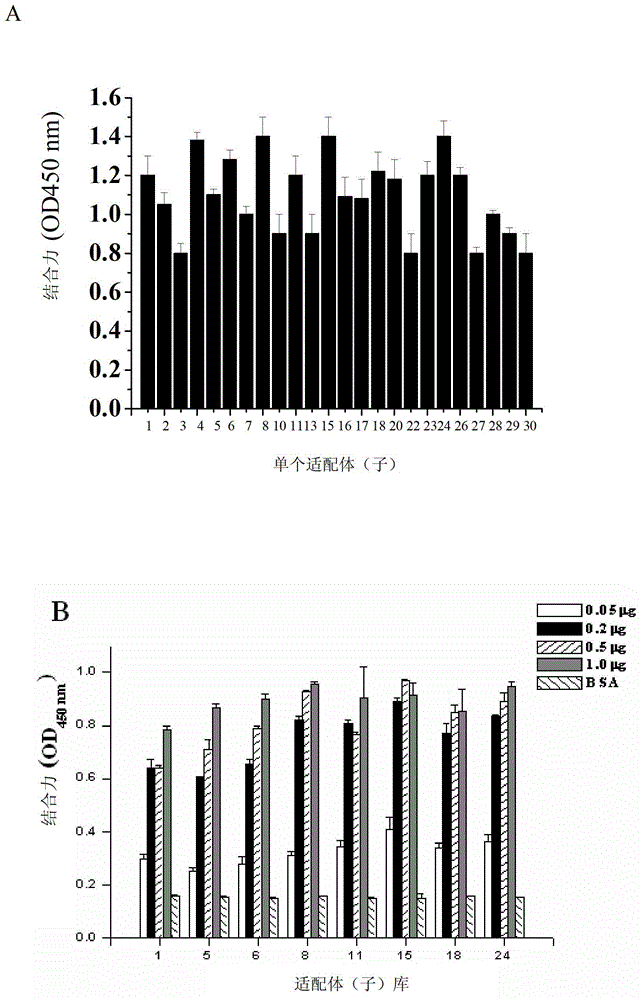
Nucleic acid aptamer capable of being specifically bound to tubercle bacillus antigen and application thereof
Owner:武汉顺可达生物科技有限公司
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS20020009459A1Improving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenMycobacterial antigen
The present invention relates to fusion proteins containing at least two Mycobacterium tuberculosis antigens. In particular, it relates to bi-fusion proteins which contain two individual M. tuberculosis antigens, tri-fusion proteins which contain three M. tuberculosis antigens, tetra-fusion proteins which contain four M. tuberculosis antigens, and penta-fusion proteins which contain five M. tuberculosis antigens, and methods for their use in the diagnosis, treatment and prevention of tuberculosis infection.
Owner:CORIXA CORP
TB diagnostic based on antigens from M. tuberculosis
The present invention is based on the identification and characterization of a number of M. tuberculosis derived novel proteins and protein fragments (SEQ ID NOs: 2, 4, 6, 8, 10, 12, 14, 16, 17-23, 42, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72-86, 88, 90, 92, 94, 141, 143, 145, 147, 149, 151, 153, and 168-171). The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as vaccines and skin test reagents containing the polypeptides. Another part of the invention is based on the surprising discovery that fusions between ESAT-6 and MPT59 are superior immunogens compared to each of the unfused proteins, respectively.
Owner:STATENS SERUM INST
Assay for detecting tuberculosis in nonhuman primates
ActiveUS20060057621A1Easy to use and sensitiveBacterial antigen ingredientsMicrobiological testing/measurementMycobacterial antigenIgm antibody
Systems, methods, and compositions for a diagnostic method for detecting TB in non-human primates that is easy to use, sensitive, and specific. The method utilizes recombinant mycobacterial antigens, such as polyfusion proteins. The method utilizes an antigen-antibody-antigen arrangement to detect TB infection in nonhuman primates. The method can detect IgM antibodies to TB, in addition to IgG antibodies, providing ability to detect TB earlier in nonhuman primate TB infection as compared to conventional TB tests.
Owner:CHEMBIO DIAGNOSTIC SYST +1
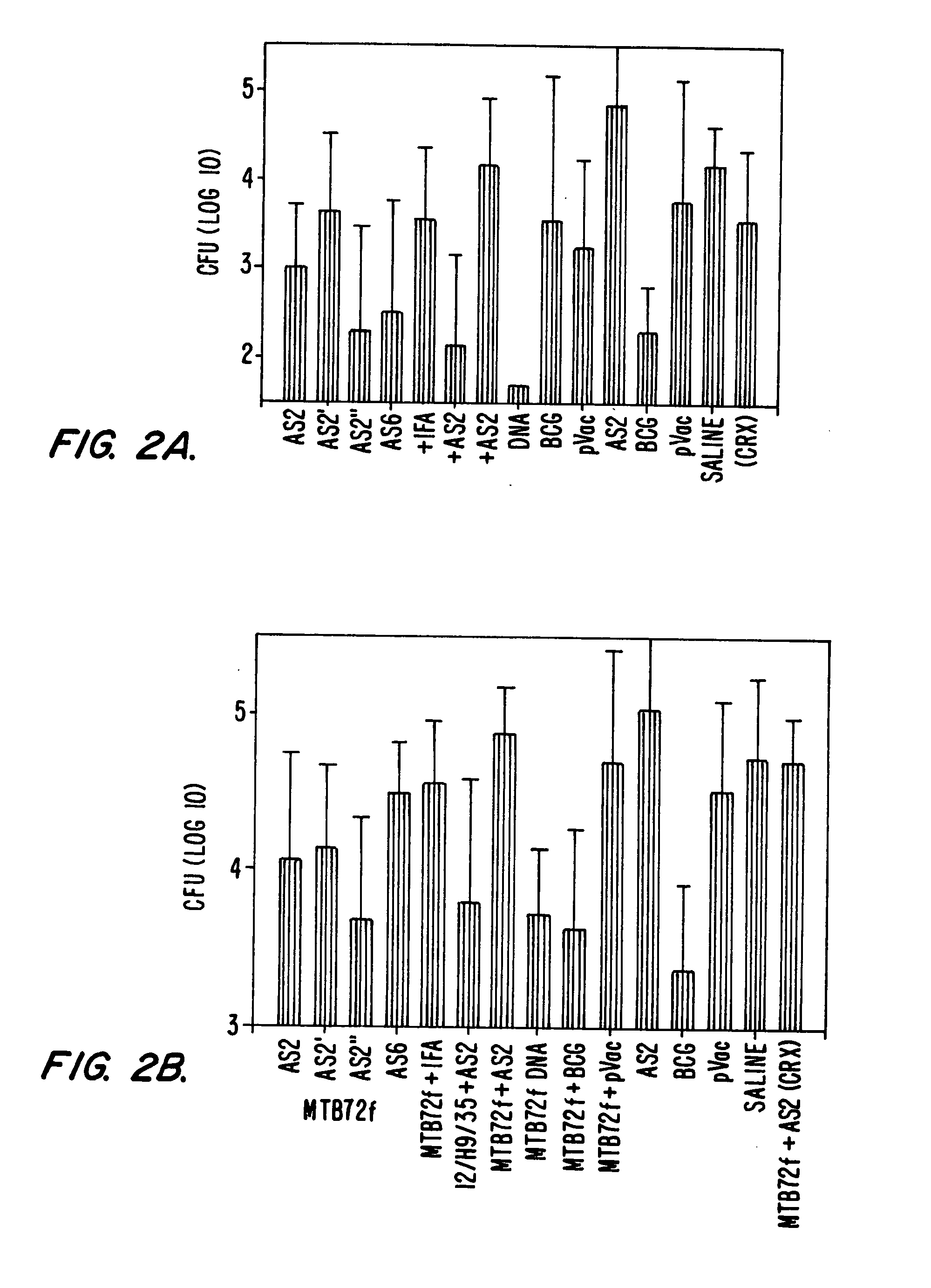
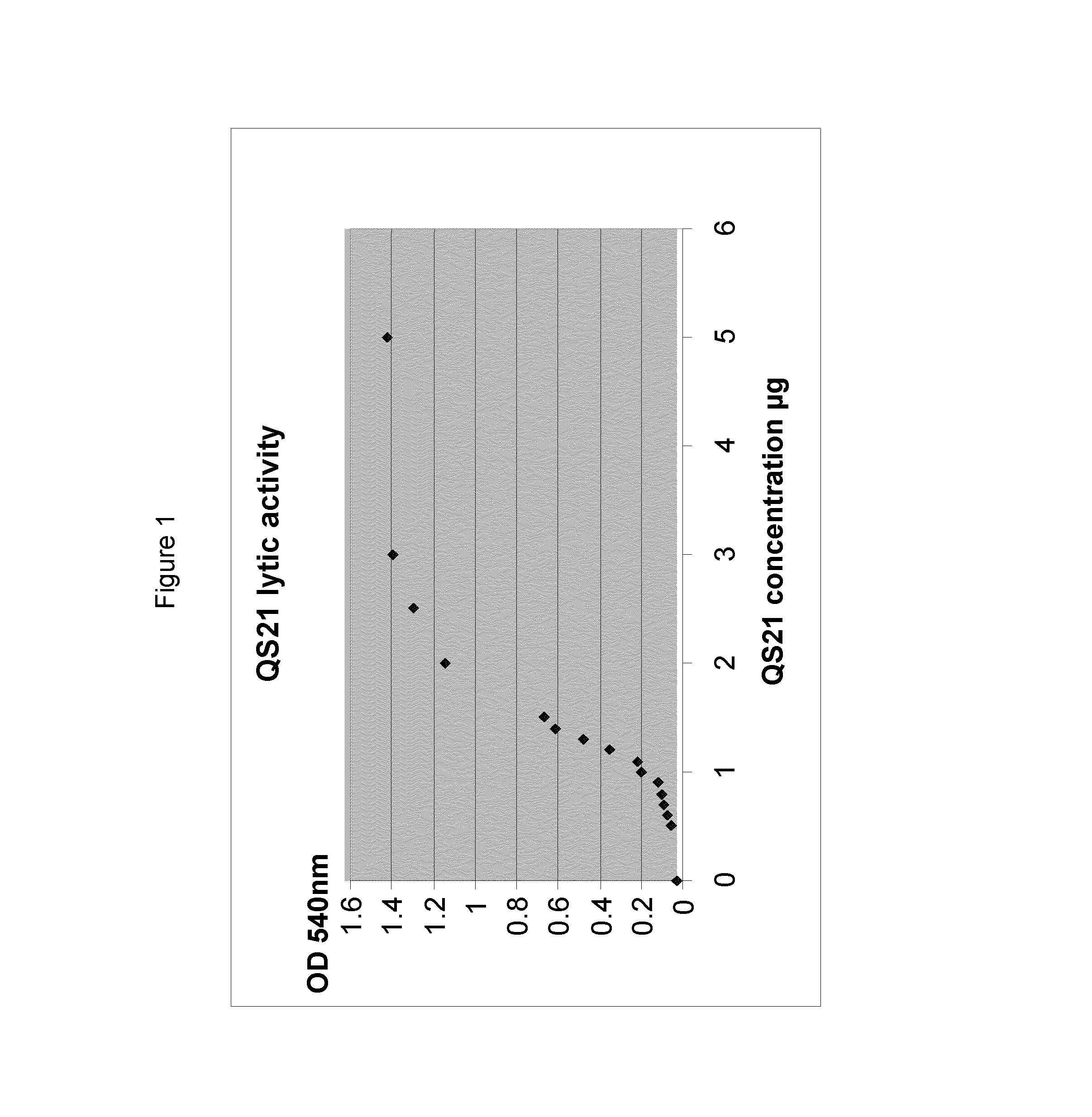
Mycobacterium antigenic composition
ActiveUS20130280289A1Improve stabilityComposition is stableAntibacterial agentsBacterial antigen ingredientsMycobacterial antigenImmunogenicity
Immunogenic compositions comprising an M72 related antigen, wherein the conductivity of the composition is 13 mS / cm or lower, or the concentration of salts of the composition is 130 mM or lower, and their use in medicine, are provided.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
TB diagnostic based on antigens from M. tuberculosis
The present invention is based on the identification and characterization of a number of novel M. tuberculosis derived proteins and protein fragments. The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as diagnostic reagents containing the polypeptides.
Owner:STATENS SERUM INST
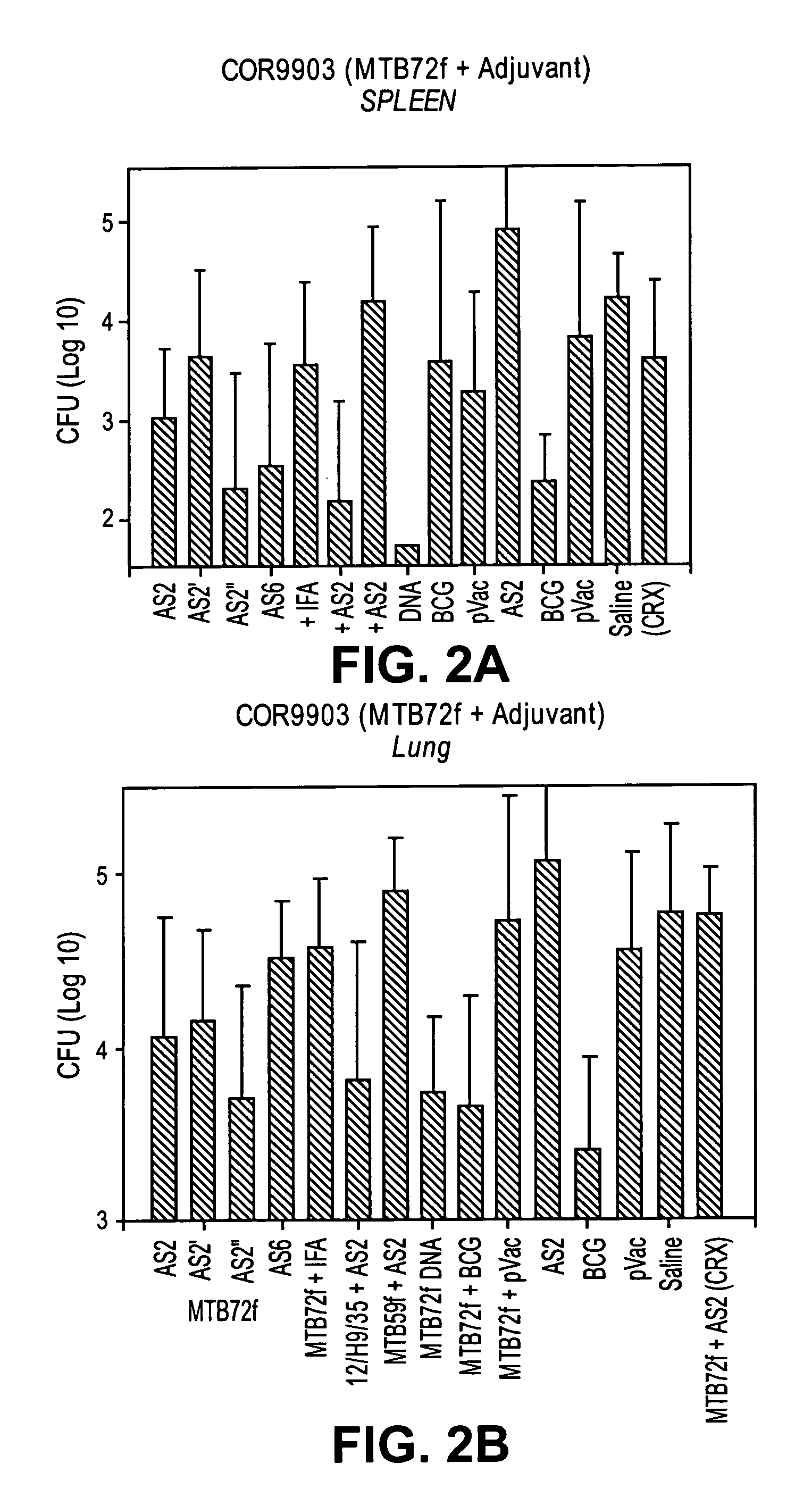
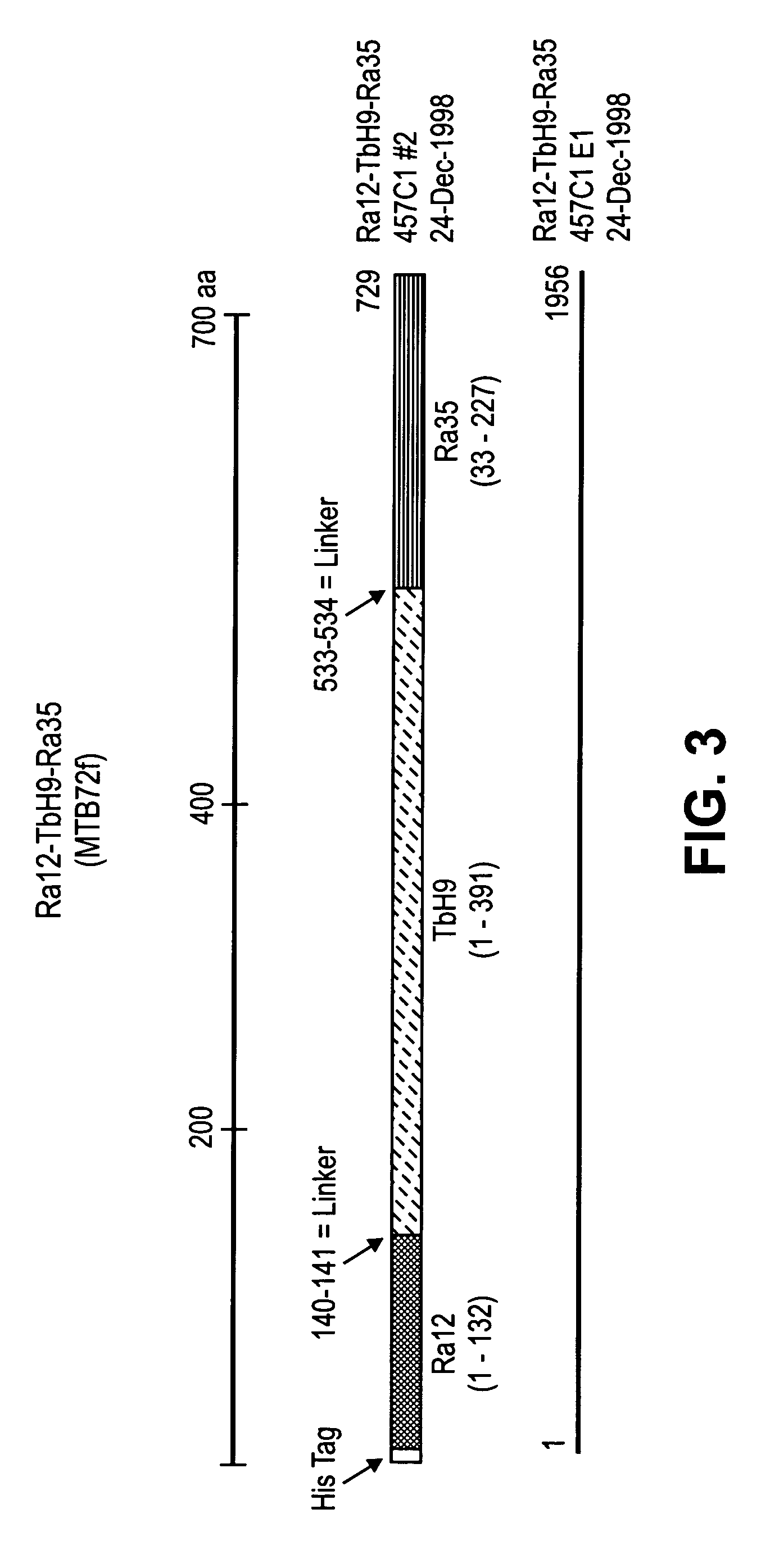
Tuberculosis vaccines comprising antigens expressed during the latent infection phase
ActiveUS20090186048A1Reduce bacterial loadProlong survival timeAntibacterial agentsBacterial antigen ingredientsMycobacterial antigenNucleic acid sequencing
The invention is related to an immunogenic composition, vaccine or pharmaceutical composition for preventing, boosting or treating infection caused by a species of the tuberculosis complex (M. tuberculosis, M. bovis, M. africanum, M. microti). The immunogenic composition, vaccine or pharmaceutical composition comprise a fusion polypeptide, which comprises one or more starvation antigens from M. tuberculosis, the units of the fusion polypeptide being M. tuberculosis antigens. Further, the invention is related to the use of a vaccine comprising a fusion polypeptide sequence or nucleic acid sequence of the invention given at the same time as BCG, either mixed with BCG or administered separately at different sites or routes for preparing said immunogenic composition, vaccine, or pharmaceutical composition.
Owner:SUN MICROSYSTEMS INC +1
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS20030147911A1Improving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenMycobacterial antigen
The present invention relates to fusion proteins containing at least two Mycobacterium tuberculosis antigens. In particular, it relates to bi-fusion proteins which contain two individual M. tuberculosis antigens, tri-fusion proteins which contain three M. tuberculosis antigens, tetra-fusion proteins which contain four M. tuberculosis antigens, and penta-fusion proteins which contain five M. tuberculosis antigens, and methods for their use in the diagnosis, treatment and prevention of tuberculosis infection.
Owner:CORIXA CORP
Mycobacterial Antigen Vaccine
InactiveUS20150165014A1Improve propertiesSure easyAntibacterial agentsBacteriaMycobacterial antigenMycobacterium Infections
The present invention relates generally to immunogenic combinations comprising at least five antigens of a Mycobacterium species as well as fusion thereof and nucleic acid molecules encoding such combined antigens and fusion. The present invention also relates to nucleic acid molecules, vectors, host cells and compositions comprising or encoding said combinations of mycobacterial antigens and fusion polypeptides as well as to methods for recombinantly producing them. The present invention also relates to methods of using said combinations of mycobacterial antigens, fusion polypeptides, vectors, host cells, compositions particularly for inducing or stimulating an immune response against a Mycobacterium infection or any disease caused by or associated with a Mycobacterium infection. The present invention also concerns antibodies directed to such mycobacterial antigens and fusion polypeptides that can be used in the diagnosis of a Mycobacterium infection and method of detection as well as kits of reagent comprising said combinations of mycobacterial antigens, fusion polypeptides, vectors, host cells, compositions or antibodies.
Owner:TRANSGENE SA
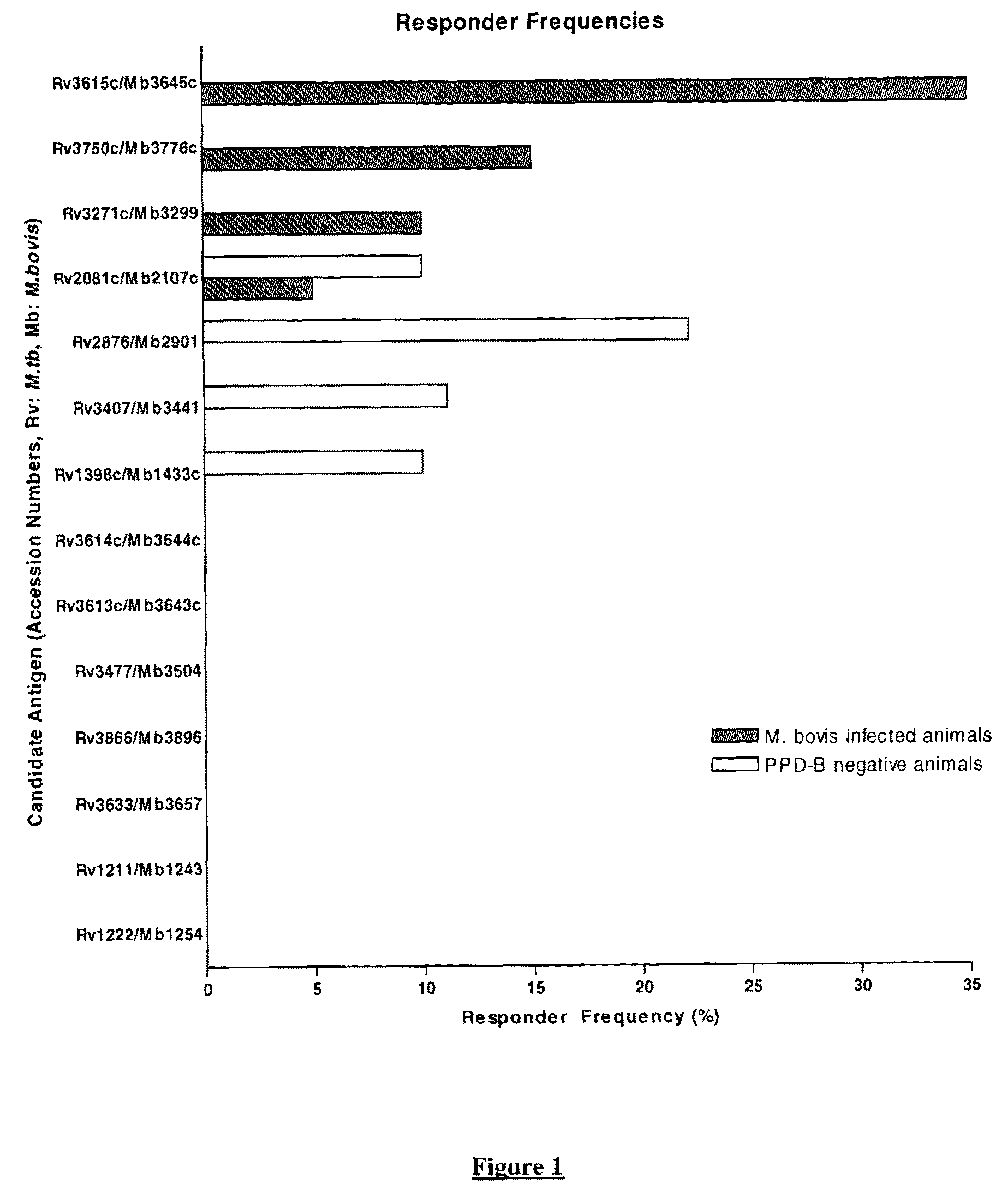
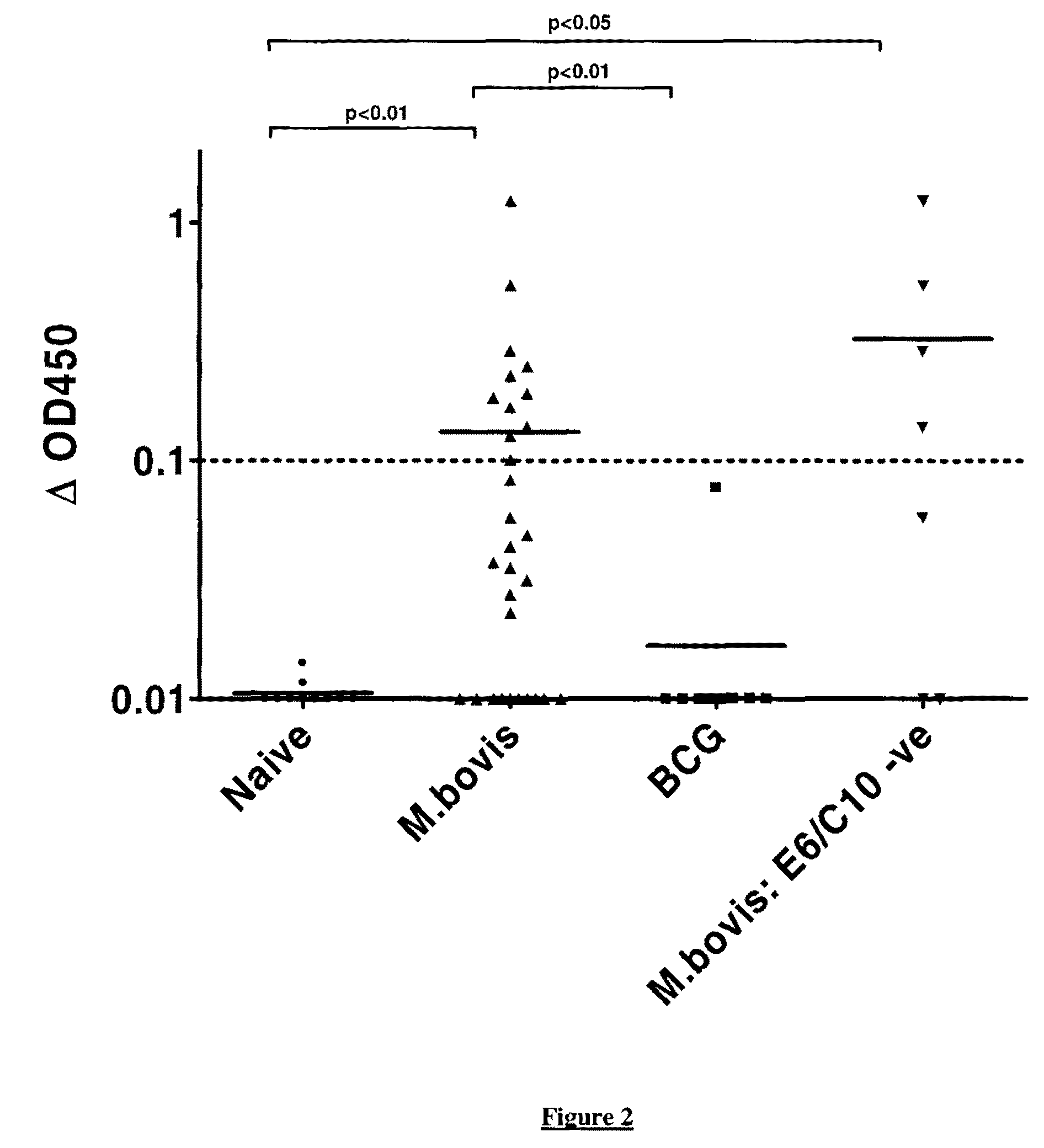
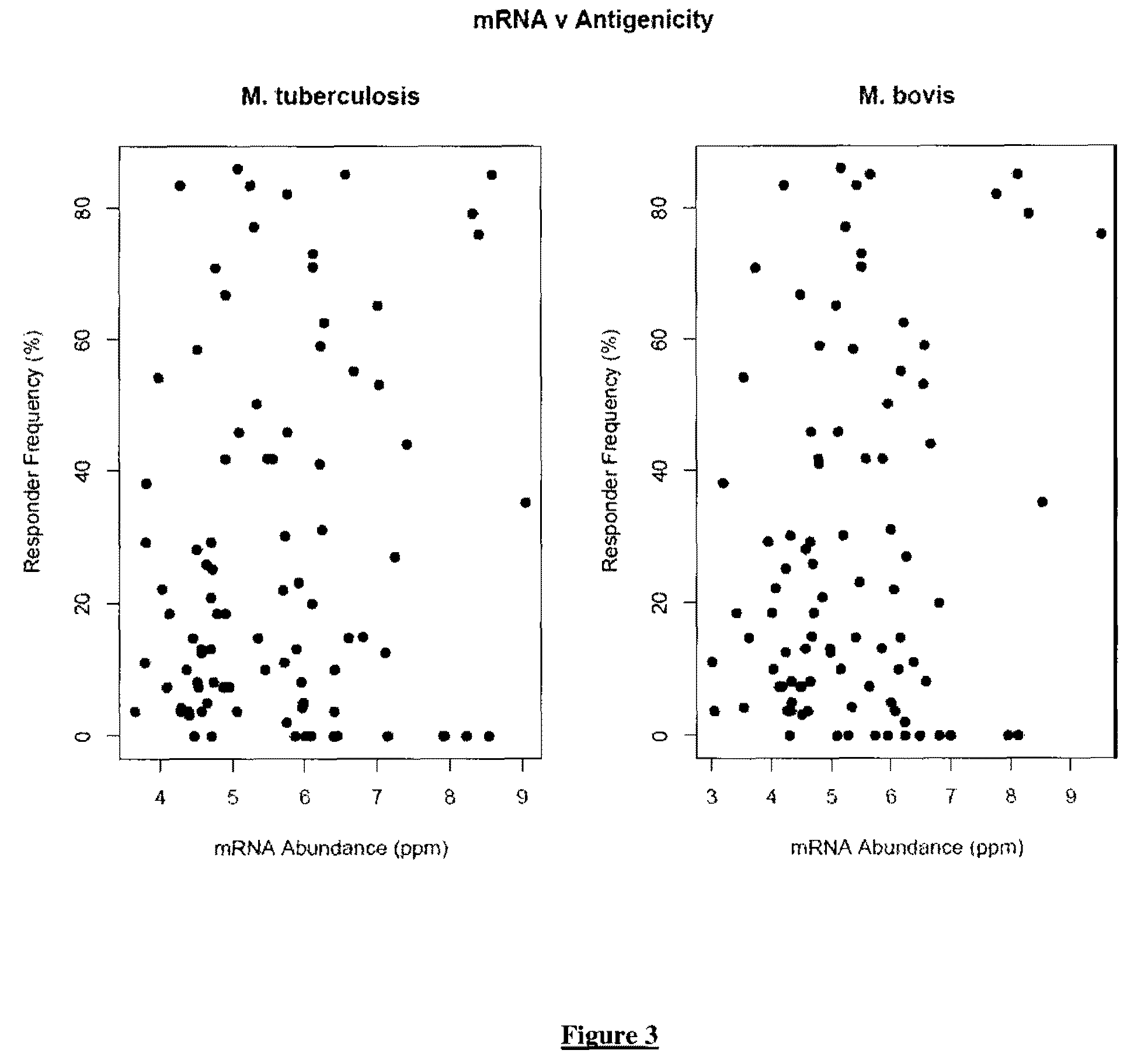
Mycobacterium antigens
There is provided a diagnostic reagent for use in the detection of M. bovis or M. tuberculosis infection in an animal, comprising a peptide which has an epitope from Mycobacterium bovis hypothetic protein Mb3645c (SEQ ID NO: 1) or an epitope from a polypeptide having at least 76% identity with SEQ ID NO: 1.
Owner:THE UK SEC FOR ENVIRONMENT FOOD & RURAL AFFAIRS
Fusion proteins of Mycobacterium tuberculosis
InactiveUS20070184074A1Improve the level ofAntibacterial agentsBacteriaMycobacterial antigenSerology
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Stress protein compositions and methods for prevention and treatment of cancer and infectious disease
InactiveUS7378096B2Good curative effectIncrease pressureAntibacterial agentsBiocideCancer preventionMycobacterial antigen
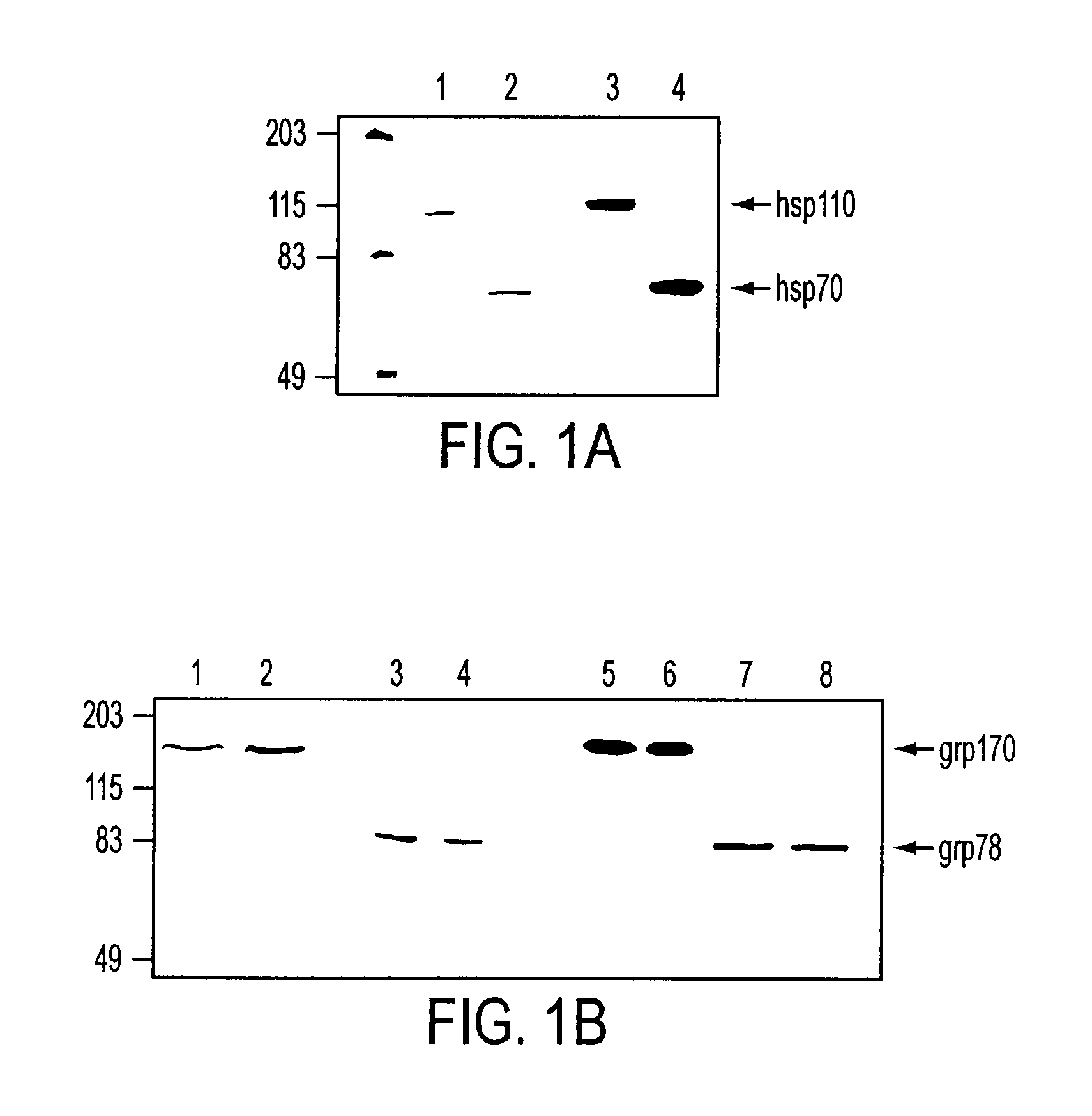
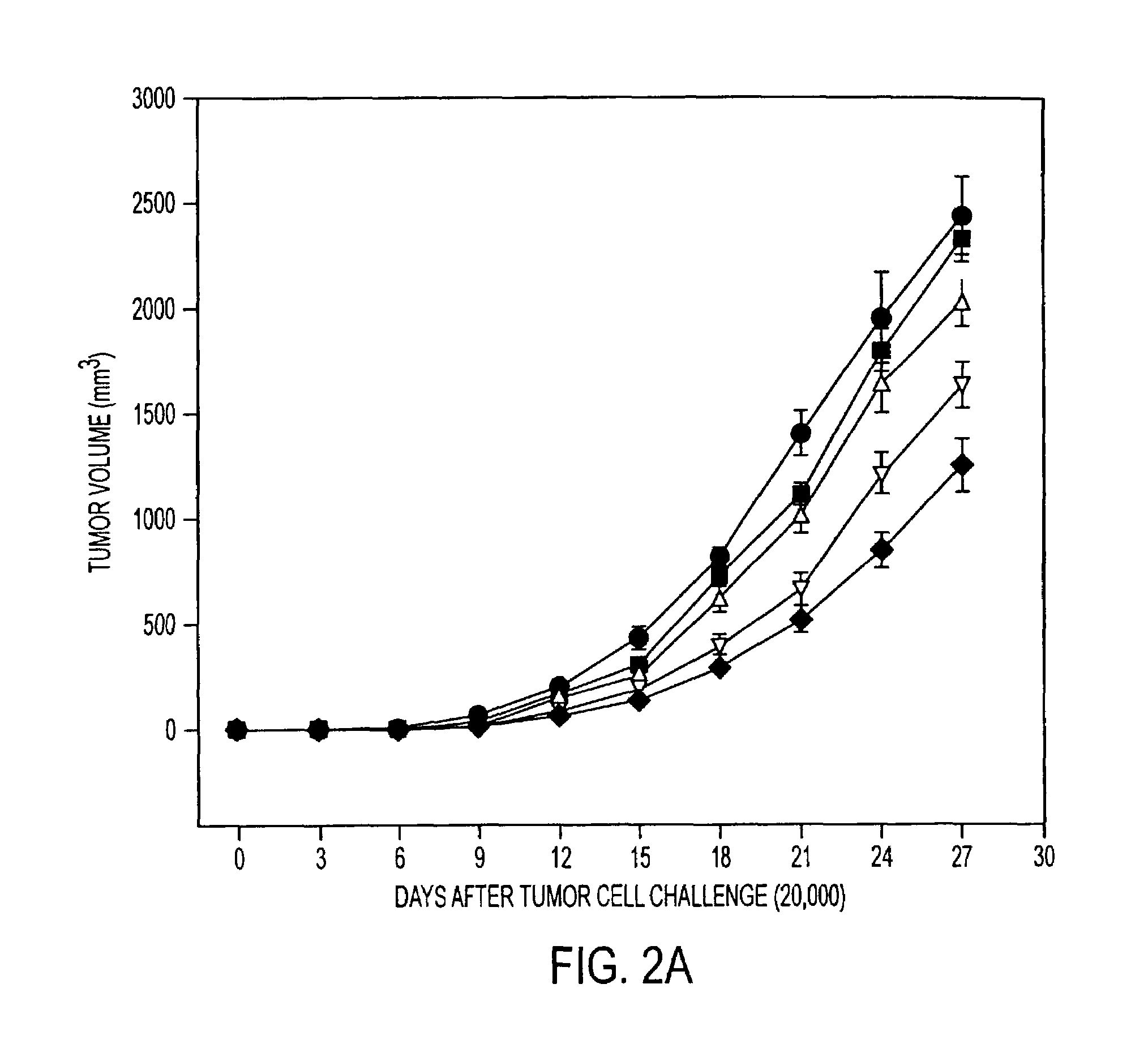
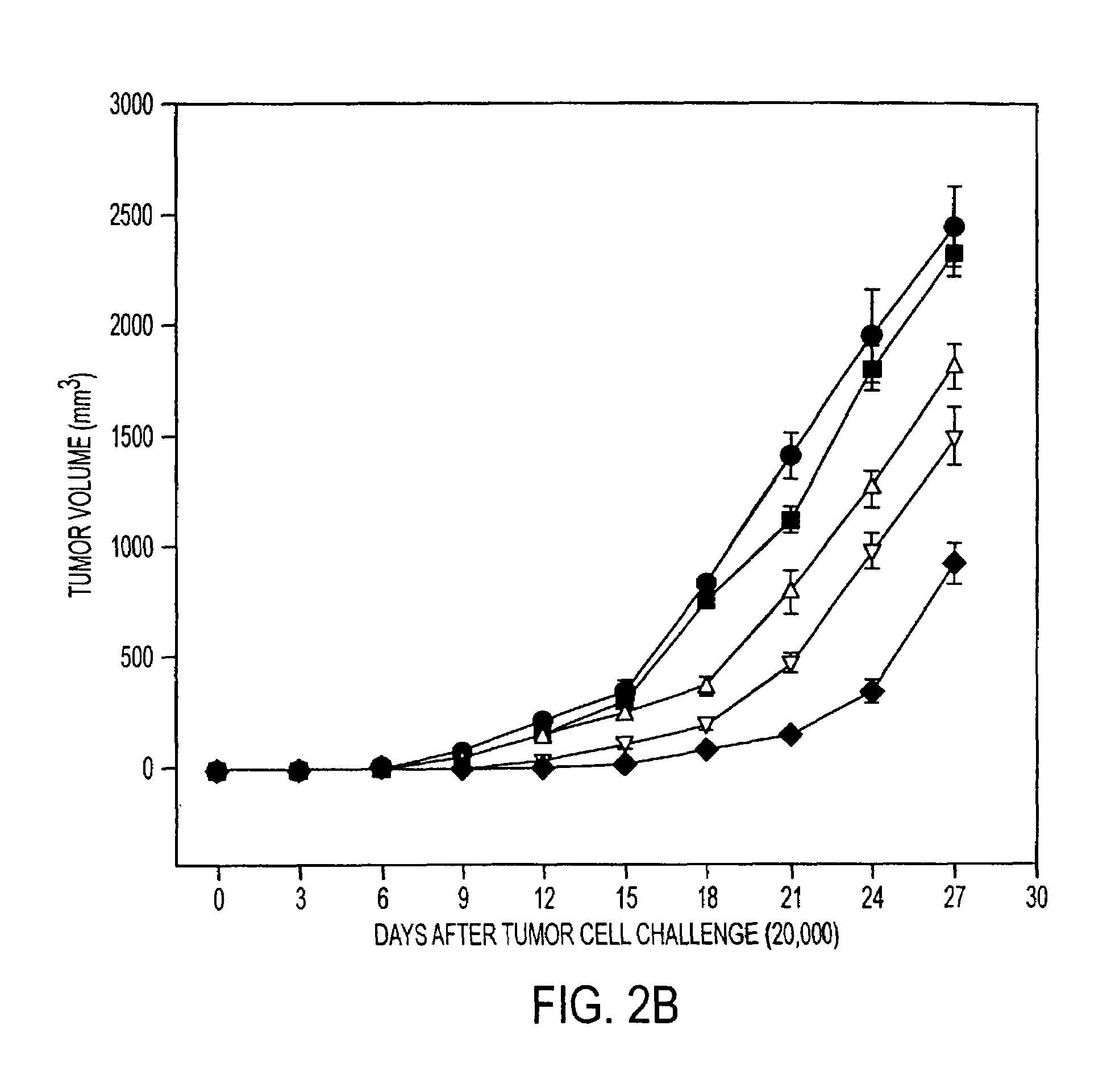
Pharmaceutical compositions comprising a stress protein complex and related molecules encoding or cells presenting such a complex are provided. The stress protein complex comprises an hsp110 or grp170 polypeptide complexed with an immunogenic polypeptide. The immunogenic polypeptide of the stress protein complex can be associated with a cancer or an infectious disease. Preferred immunogenic polypeptides include gp100, her2 / neu ECD-PD, ICD and M. tuberculosis antigens. The pharmaceutical compositions of the invention can be used for the treatment or prevention of cancer or infectious disease.
Owner:HEALTH RES INC
Applications of Mycobacterium tuberculosis antigen protein Rv0446c and T-cell epitope peptide thereof
ActiveCN106248934AReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsAntigenStimulant
The present invention relates to applications of Mycobacterium tuberculosis antigen protein Rv0446c and a T-cell epitope peptide thereof in preparation of tuberculosis detection reagents, vaccines and medicines, wherein the amino acid sequences of the antigen protein Rv0446c and the T-cell epitope peptide thereof are respectively represented by SEQ ID NO:1-5. According to the present invention, the Mycobacterium tuberculosis antigen protein Rv0446c and the T-cell epitope peptide thereof are used as the stimulants for the specific T cell and B cell immune response caused by Mycobacterium tuberculosis infection, and the false positive caused by the impure antigen can be reduced compared with the use of the complete antigen in the prior art; and the detection reagents prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be widely used for assisted diagnosis of tuberculosis, epidemiological surveillance and other related fields, and the tuberculosis vaccines and the anti-tuberculosis drugs prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be used for prevention and treatment of tuberculosis.
Owner:ICDC CHINA CDC
Mycobacterium tuberculosis ESAT6 antigen protein serial recombinant expression method and application in tuberculosis detection thereof
The invention discloses mycobacterium tuberculosis ESAT6 serial recombinant fusion protein and application thereof. The invention provides a mycobacterium tuberculosis ESAT6 codon optimization method, a serial recombinant fusion method and a method for preparing the fusion protein, and also provides a method for manufacturing and applying the recombinant fusion protein in a whole-blood IFN-gamma tuberculosis diagnosis kit and a TCELL-SPOT tuberculosis infection diagnosis kit. The fusion protein has the advantages of high specificity, high sensitivity and the like, and the requirements of the tuberculosis (TB) infection clinical diagnosis are well met.
Owner:郑州博赛生物技术股份有限公司
Mycobacterium tuberculosis combined antigen for diagnosing pulmonary tuberculosis
ActiveCN104678097AHigh diagnostic efficiencyHigh sensitivityDepsipeptidesDisease diagnosisOrganismAntibody level
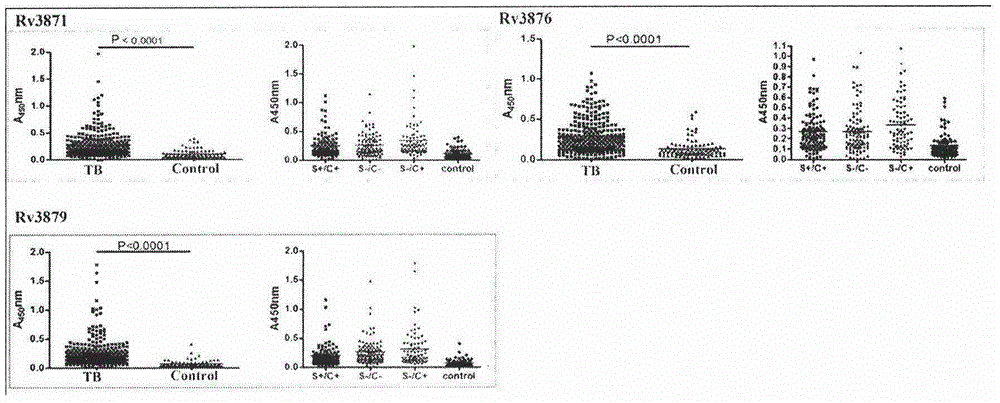
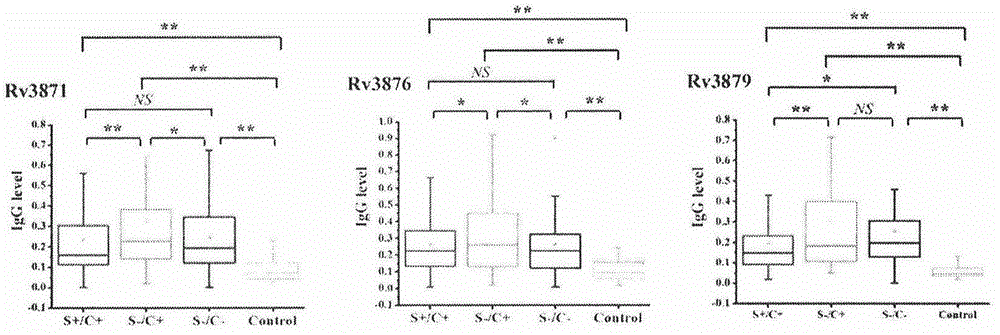
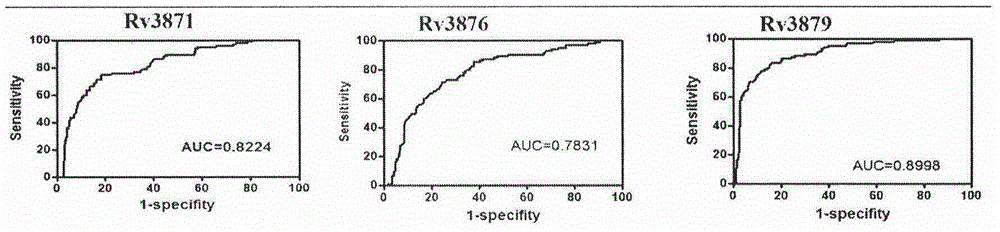
The invention provides a combined antigen comprising three mycobacterium tuberculosis antigens and an application of the combined antigen to preparation of a reagent for diagnosing active tuberculosis. The combined antigen comprises Rv3871, Rv3876 and Rv3879. Corresponding antibody detection methods are established by the aid of the three antigens and used for detecting corresponding antibody levels in biological samples, and the three detected antibody levels are particularly higher in sputum smear negative / sputum culture positive tuberculosis patients. A pulmonary tuberculosis diagnosing method is established by the aid of the combined antigen, and a positive sample is defined in such a manner that antibody levels corresponding to two or more antigens reach a positive judgment value. Verification of a lot of clinical samples indicates that the diagnosing method has the high sensitivity and the high specificity for sputum smear positive / sputum culture positive, sputum smear negative / sputum culture positive and sputum smear negative / sputum culture negative tuberculosis patients.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Novel adenovirus
There is provided adenoviral vectors encoding a mycobacterial antigen derived from a chimp adenovirus, and to related aspects.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multi-T cell epitope tuberculosis gene vaccine with HSP65 as epitope scaffold
InactiveCN104127883AEfficient removalImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsTreating tuberculosisTGE VACCINE
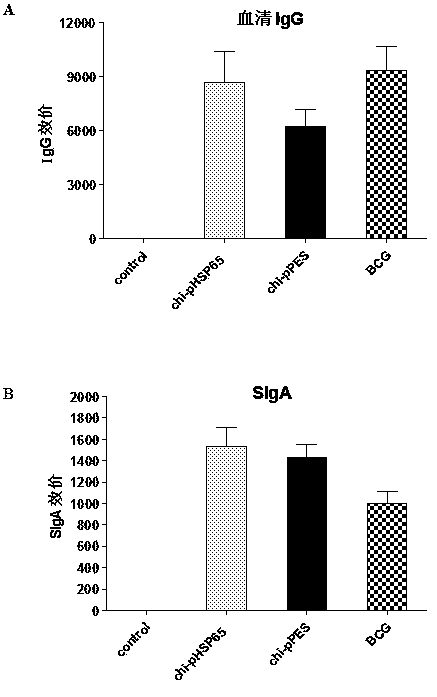
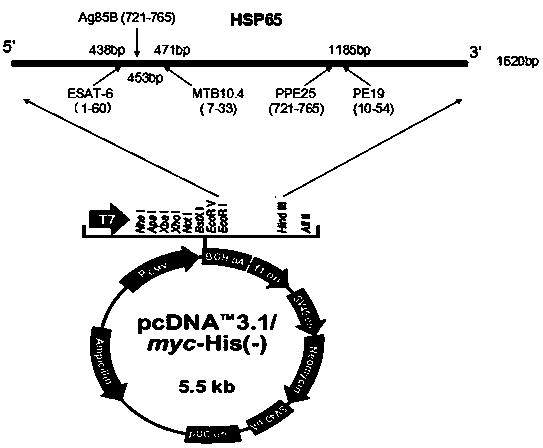
The invention discloses a multi-T cell epitope tuberculosis gene vaccine with HSP65 as an epitope scaffold. The full-length gene of HSP65 protein in which 5 T cell epitope polypeptide genes originated from mycobacterium tuberculosis antigen are embedded is inserted into a vector. A preparation method for the vaccine comprises a step of inserting the 5 T cell epitope genes originated from mycobacterium tuberculosis antigen into the HSP65 full-length gene, wherein the 5 T cell epitope genes comprise EAST-61-60, Ag85B721-765, MTB10.47-33, PPE25721-765 and PE1910-54. It is proved that the tuberculosis gene vaccine can induce response of serum IgG and lung SIgA to the HSP65 vector and induce cellular immunologic response of specific strong secretion of high-level IFN gamma to multiple tuberculosis antigen epitopes through intranasal immunization of mice with a nanometer granular compound formed by the gene vaccine and deacetylated chitosan, so the tuberculosis gene vaccine is a potential vaccine for prevention and treatment of tuberculosis.
Owner:SUZHOU UNIV
Fusion of Heterooligomeric Mycobacterial Antigens
ActiveUS20160331823A1Avoid impairmentSure easyAntibacterial agentsBacterial antigen ingredientsMycobacterial antigenImmunogenicity
The present invention relates generally to novel immunogenic combinations comprising or encoding at least two heterooligomeric mycobacterial antigens and preferably a fusion polypeptide comprising said two heterooligomeric mycobacterial antigens, where the mycobacterial antigens are selected from the group of Esx, PE and PPE antigens of a Mycobacterium species, particularly a Mycobacterium of the tuberculosis complex such as Mycobacterium tuberculosis (Mtb). The present invention also relates to vectors, host cells and compositions comprising or encoding said immunogenic combination as well as to methods for expressing and producing it. The present invention also relates to methods of using said immunogenic combination, fusion polypeptide, vector, host cell, composition particularly for inducing or stimulating an immune response with the goal of providing a protective response against a Mycobacterium infection or any disease caused by or associated with a Mycobacterium infection.
Owner:TRANSGENE SA
Recombinant fusion proteins of hepatitis B core proteins and tuberculosis antigen or antigen fragments and application thereof
ActiveCN101891825AEvaluate immunogenicityAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterial antigen
The invention relates to one or more types of recombinant fusion proteins formed by hepatitis B core proteins and mycobacterium tuberculosis antigens or antigen fragments. The tuberculosis antigens or antigen fragments are inserted in the same or different permission sites of hepatitis B core proteins in a mode of single antigen or antigen fragment or various types of antigens or antigen fragments which are combined together. The recombinant fusion proteins may comprise a plurality of cofactors. In addition, the invention also relates to the type of the fusion of the tuberculosis antigen or antigen fragments and cofactors and hepatitis B core proteins, nucleic acid and amino acid sequences of coded corresponding recombinant fusion proteins, a method for preparing the recombinant fusion proteins and application of the recombinant fusion proteins in the preparation of medicaments and vaccines for preventing and / or treating tuberculosis.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Vaccine for inducing an improved immune reaction
ActiveUS20130273101A1Efficient preparationGood effectAntibacterial agentsSsRNA viruses negative-senseHepatitis B virusNeisseria meningitidis
The present invention relates to a pharmaceutical vaccine composition comprising: (a) a pathogen-derived antigen selected from the group consisting of Mycobacterium tuberculosis antigen, Bacillus anthracis antigen, HAV (hepatitis A virus) antigen, HBV (hepatitis B virus) antigen, HCV (hepatitis C virus) antigen, HIV (human immunodeficiency virus) antigen, influenza virus antigen, HSV (herpes simplex virus) antigen, Hib (Haemophilus influenzae type b) antigen, Neisseria meningitidis antigen, Corynebacterium diphtheriae antigen, Bordetella pertussis antigen, Clostridium tetani antigen and Varicella virus antigen; (b) a deacylated non-toxic LOS (lipooligosaccharide); and (c) a pharmaceutically acceptable carrier.
Owner:EYEGENE INC
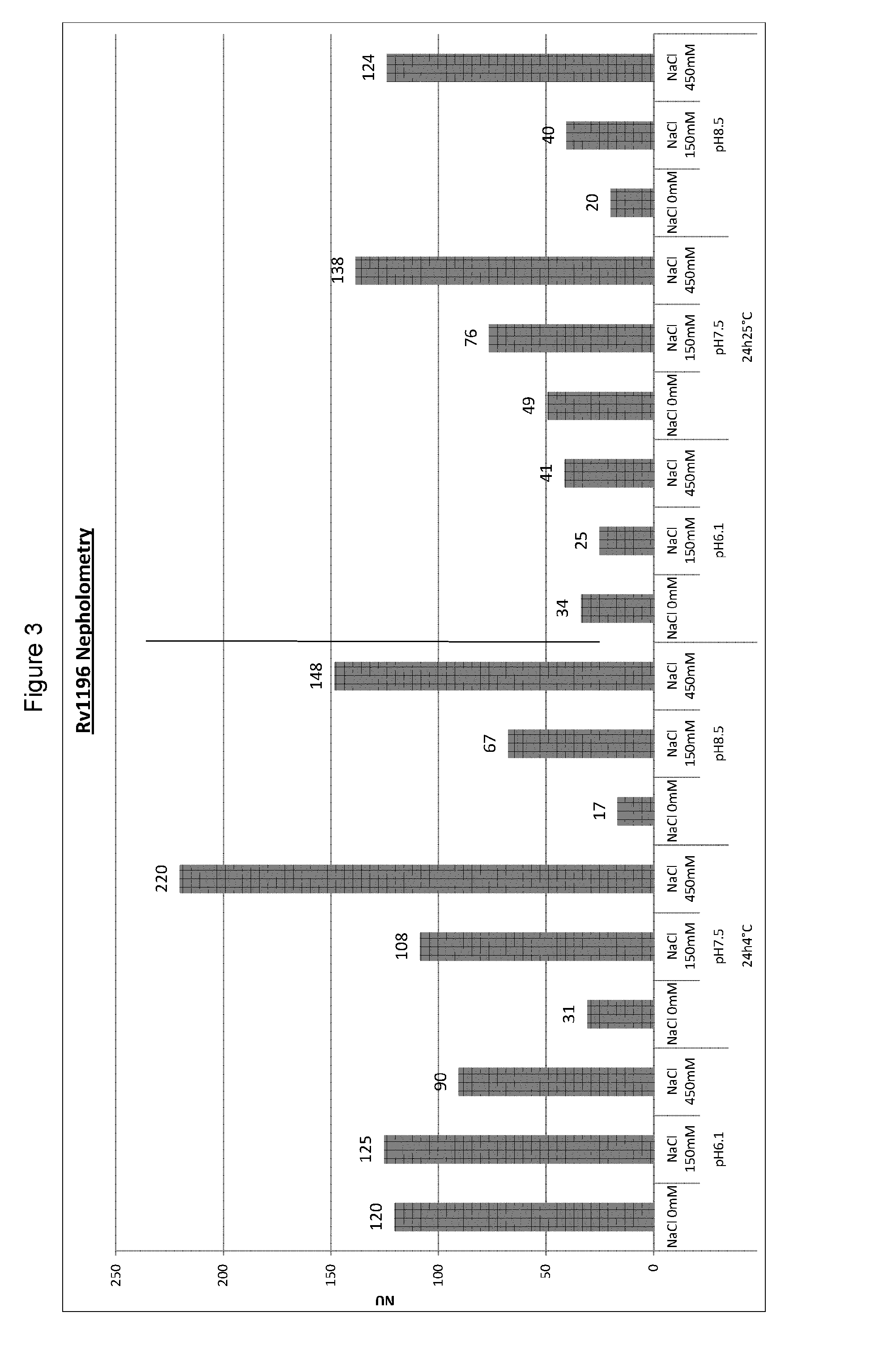
Mycobacterium antigenic composition
ActiveUS20130287809A1Composition is stableImprove stabilityAntibacterial agentsBacterial antigen ingredientsMycobacterial antigenImmunogenicity
Immunogenic compositions comprising an Rv1196 related antigen, wherein the conductivity of the composition is 13 mS / cm or lower, or the concentration of salts of the composition is 130 mM or lower, and their use in medicine, are provided.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Assays for diagnosis of tuberculosis and uses thereof
InactiveUS20090053258A1Strong immune responseFacilitate sensitiveBacterial antigen ingredientsAnalysis using chemical indicatorsMycobacterial antigenImmunogenicity
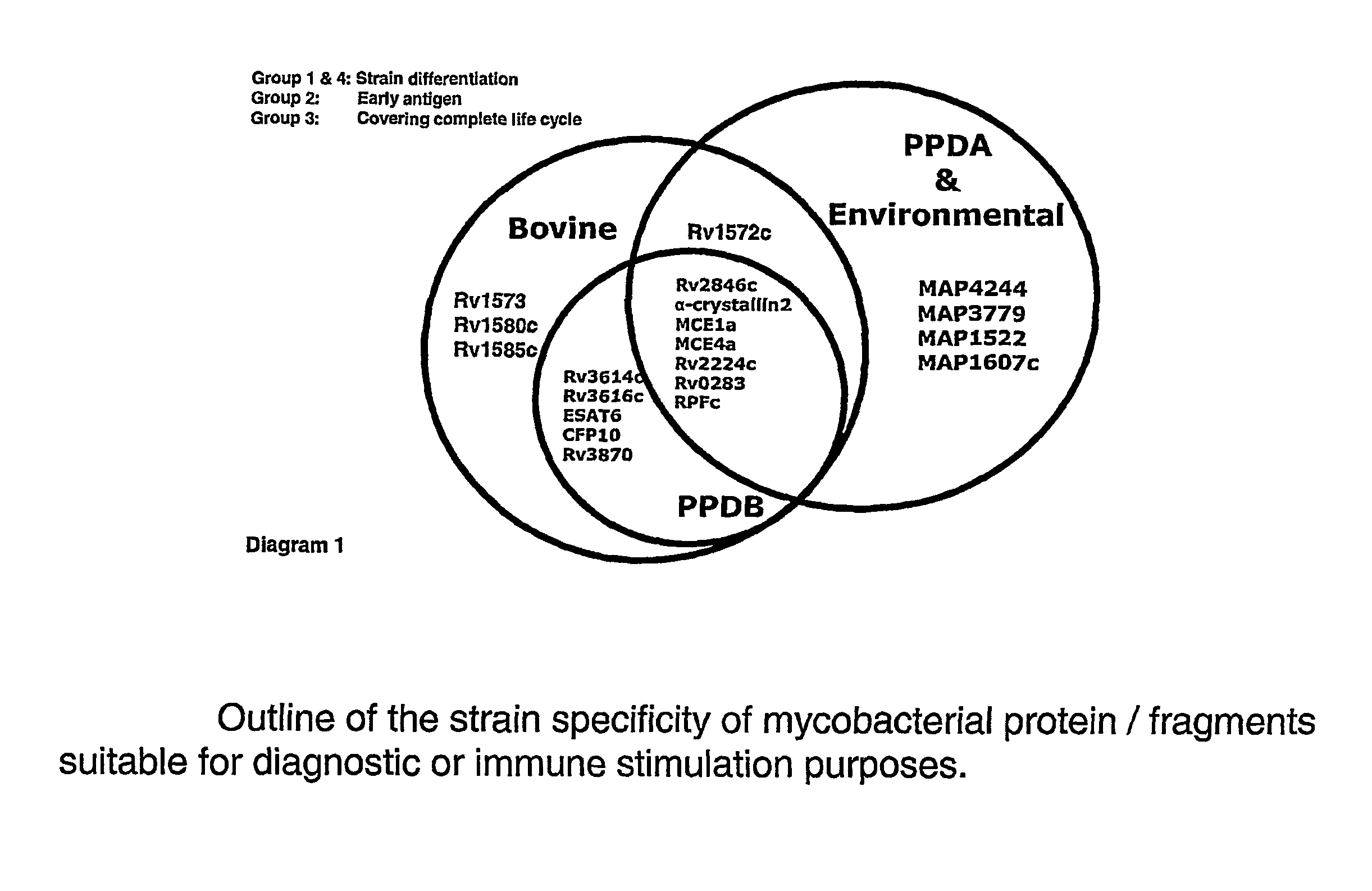
The invention relates to the identification of mycobacterial antigens which are highly immunogenic and which may be used in assays and methods for the diagnosis of tuberculosis and the discrimination between infected animals and animals previously exposed to vaccines.
Owner:FUSION ANTIBODIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com