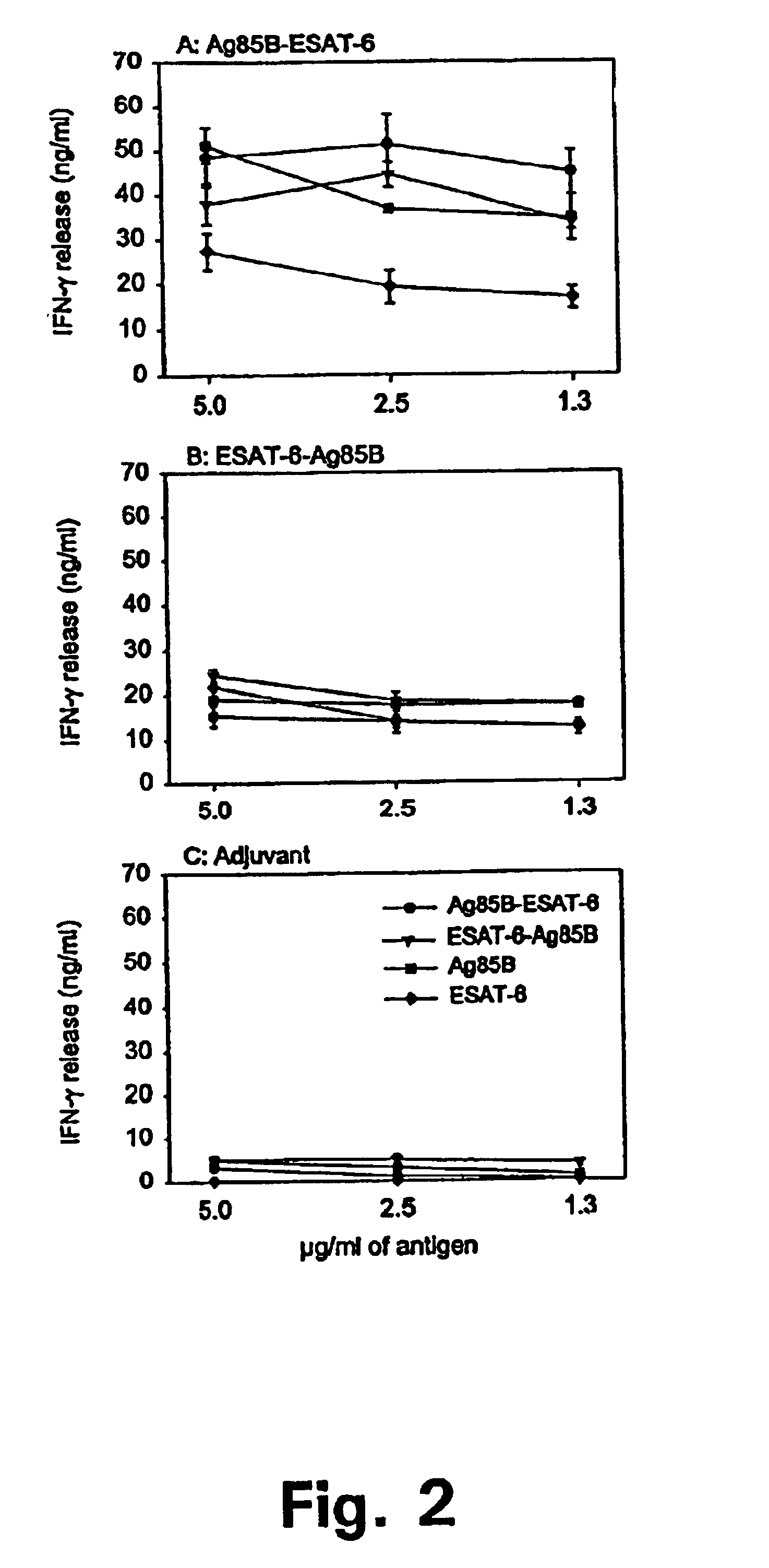
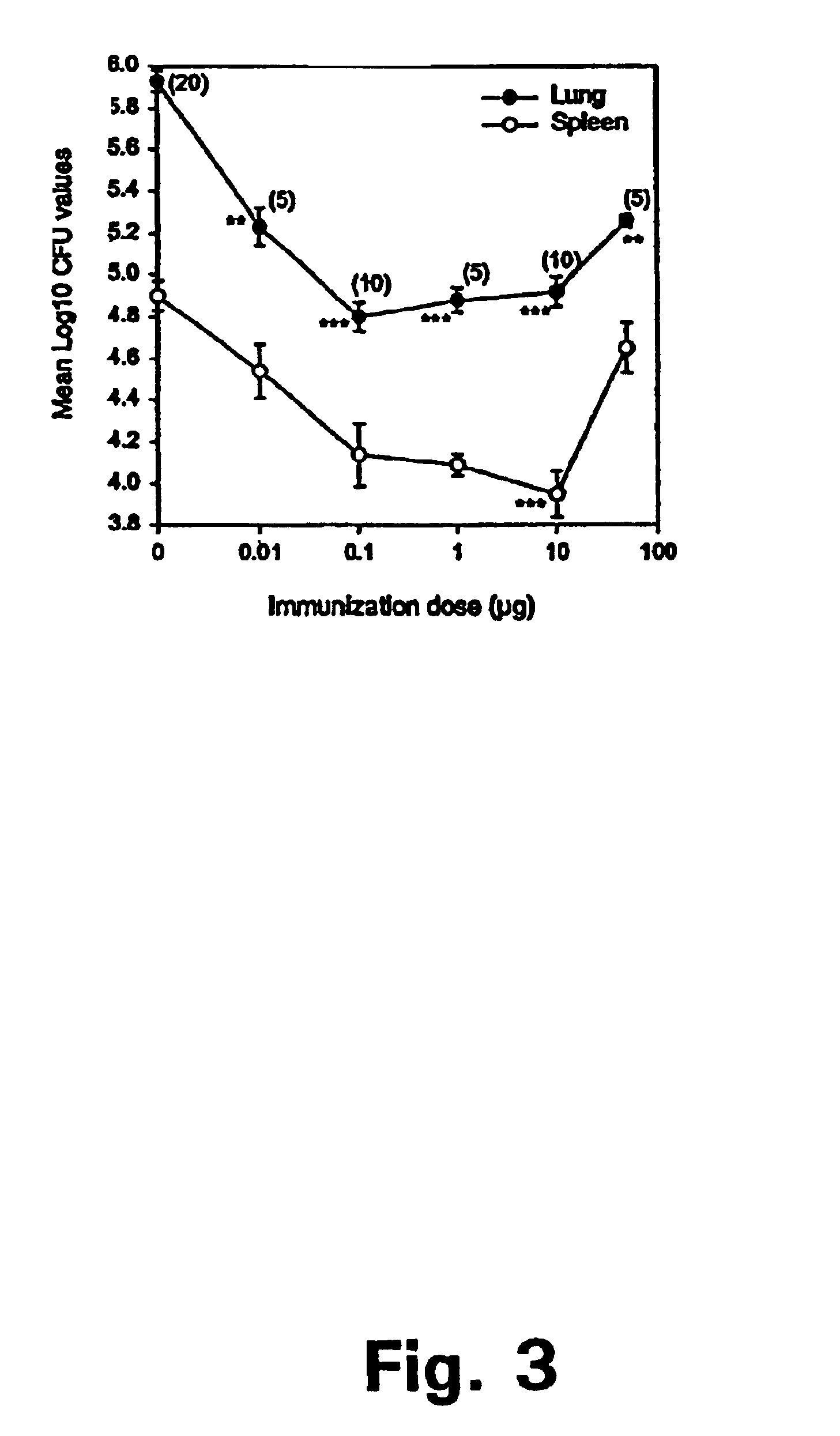
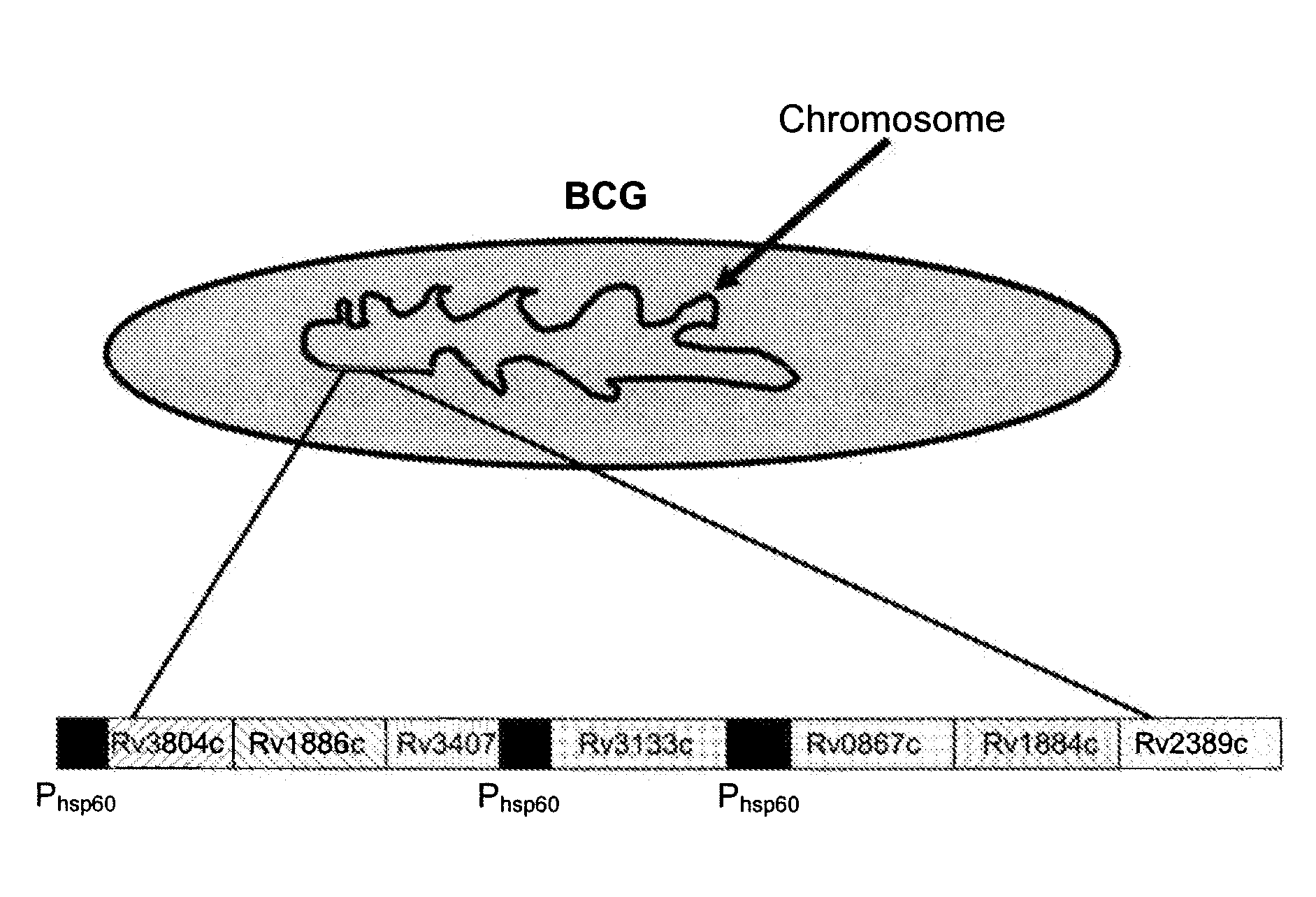
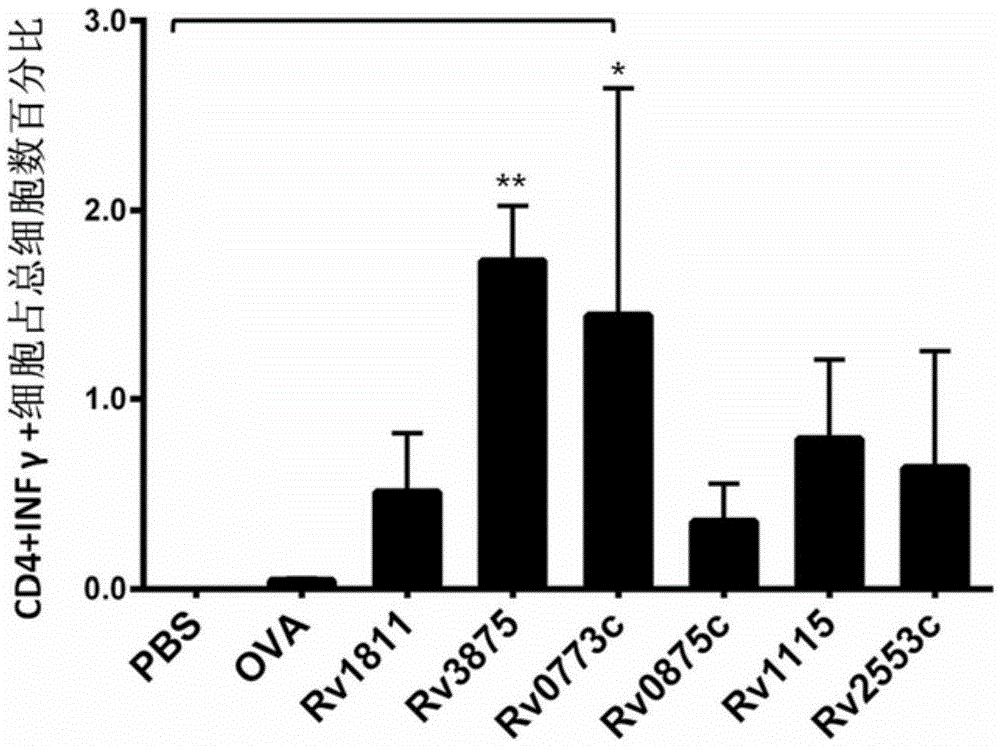
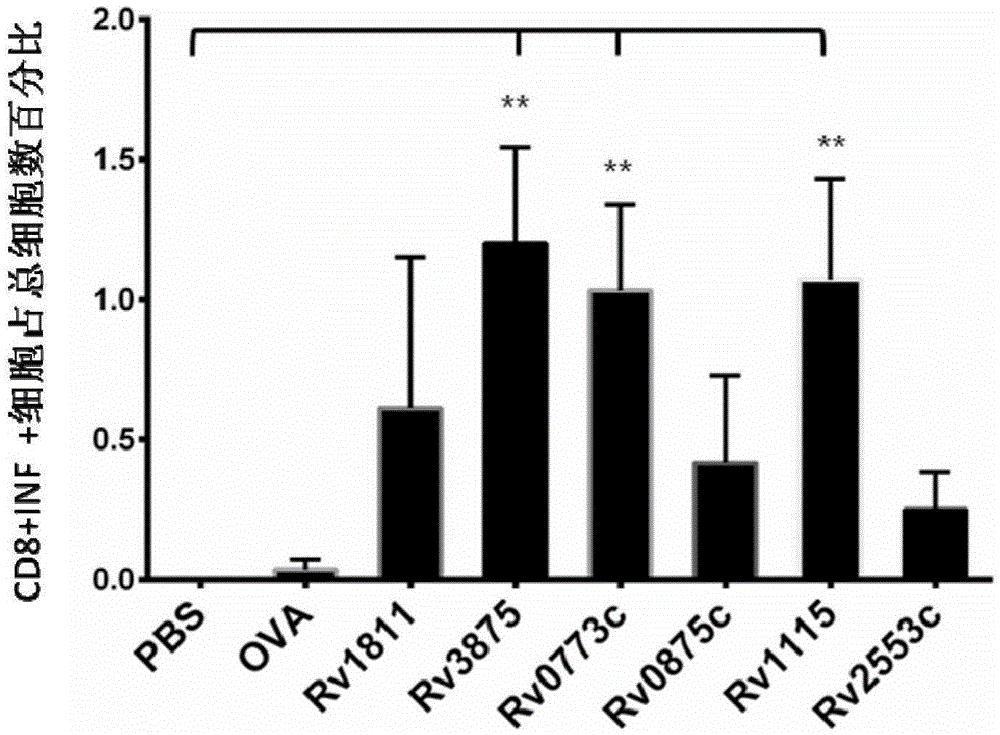
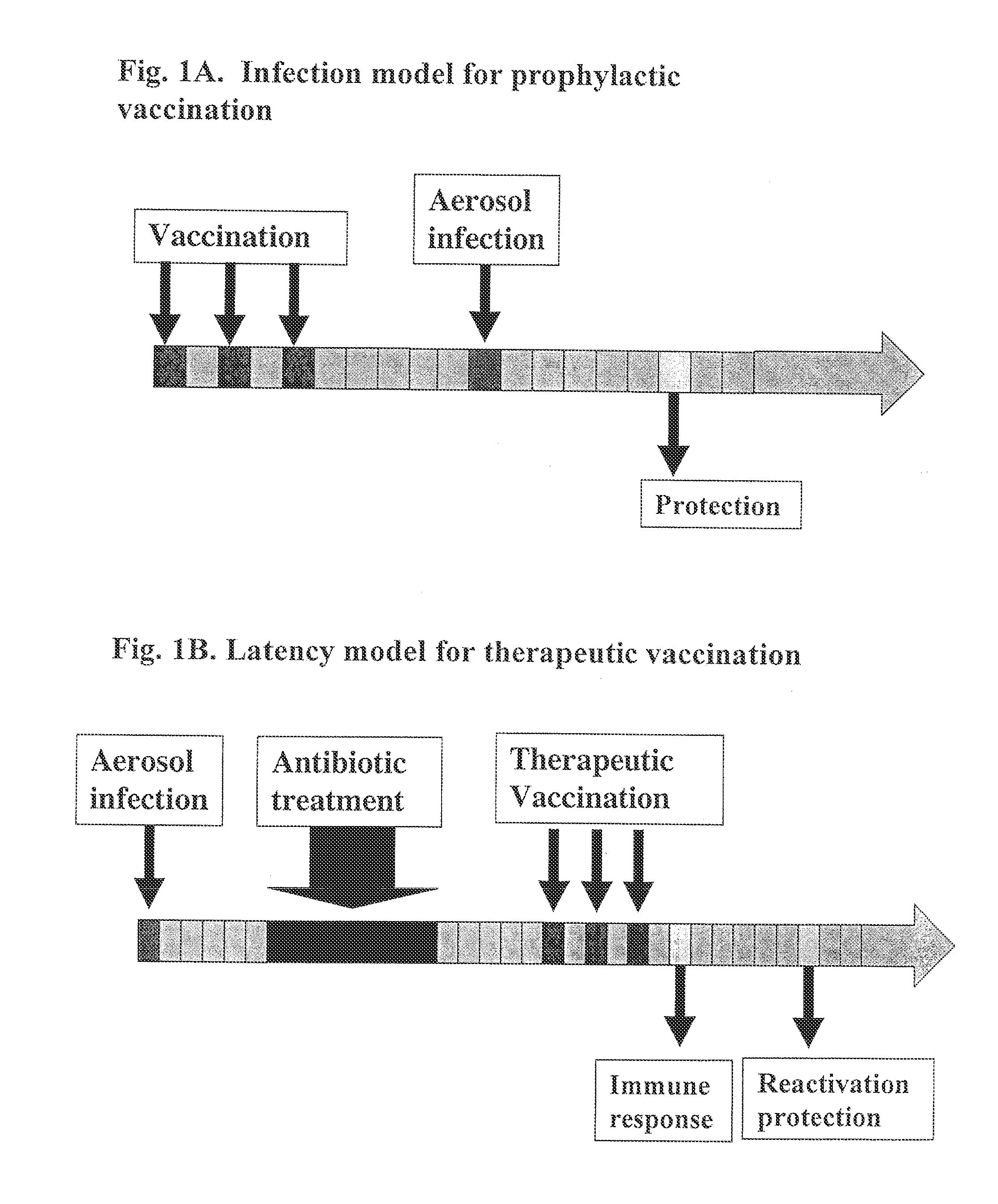
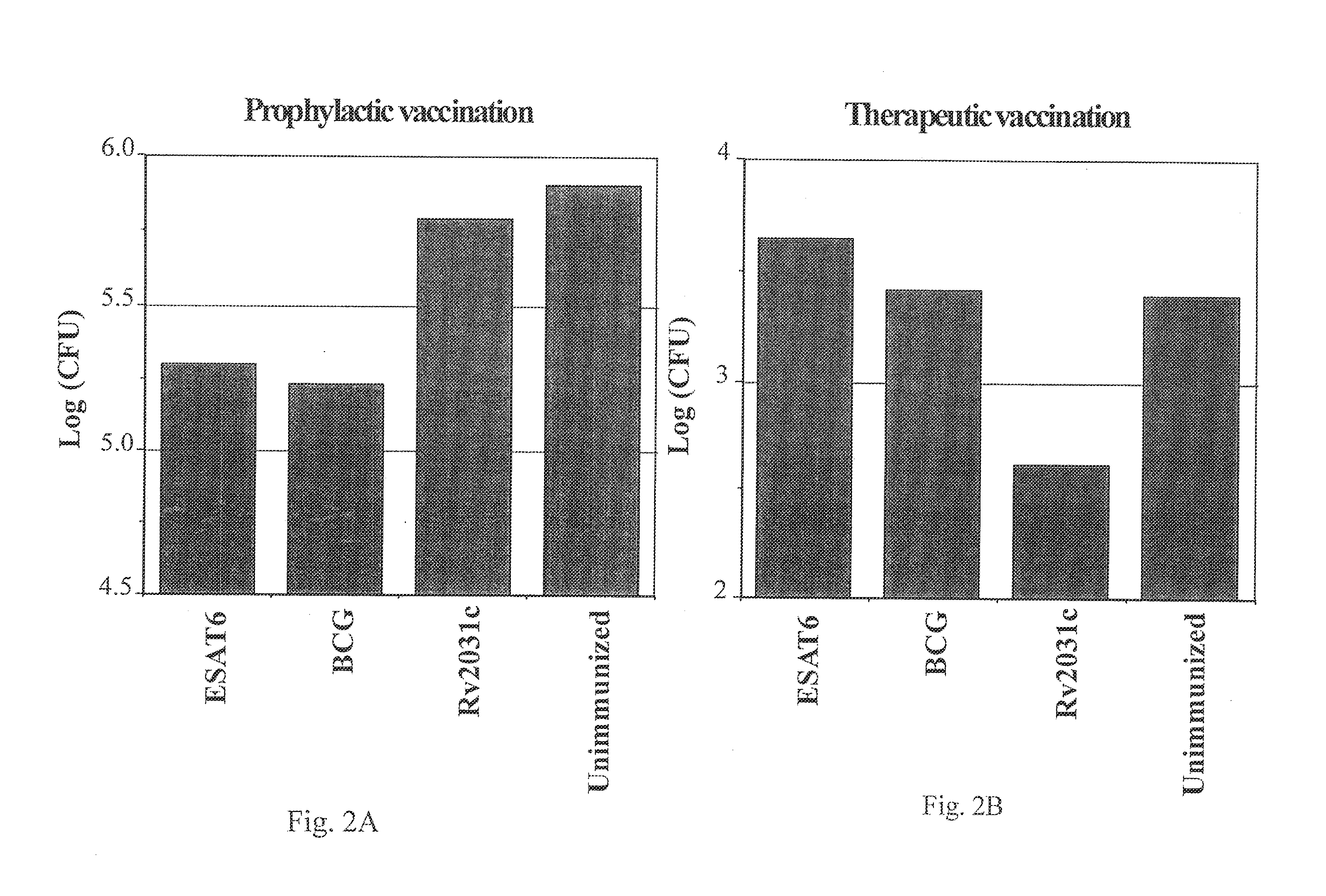
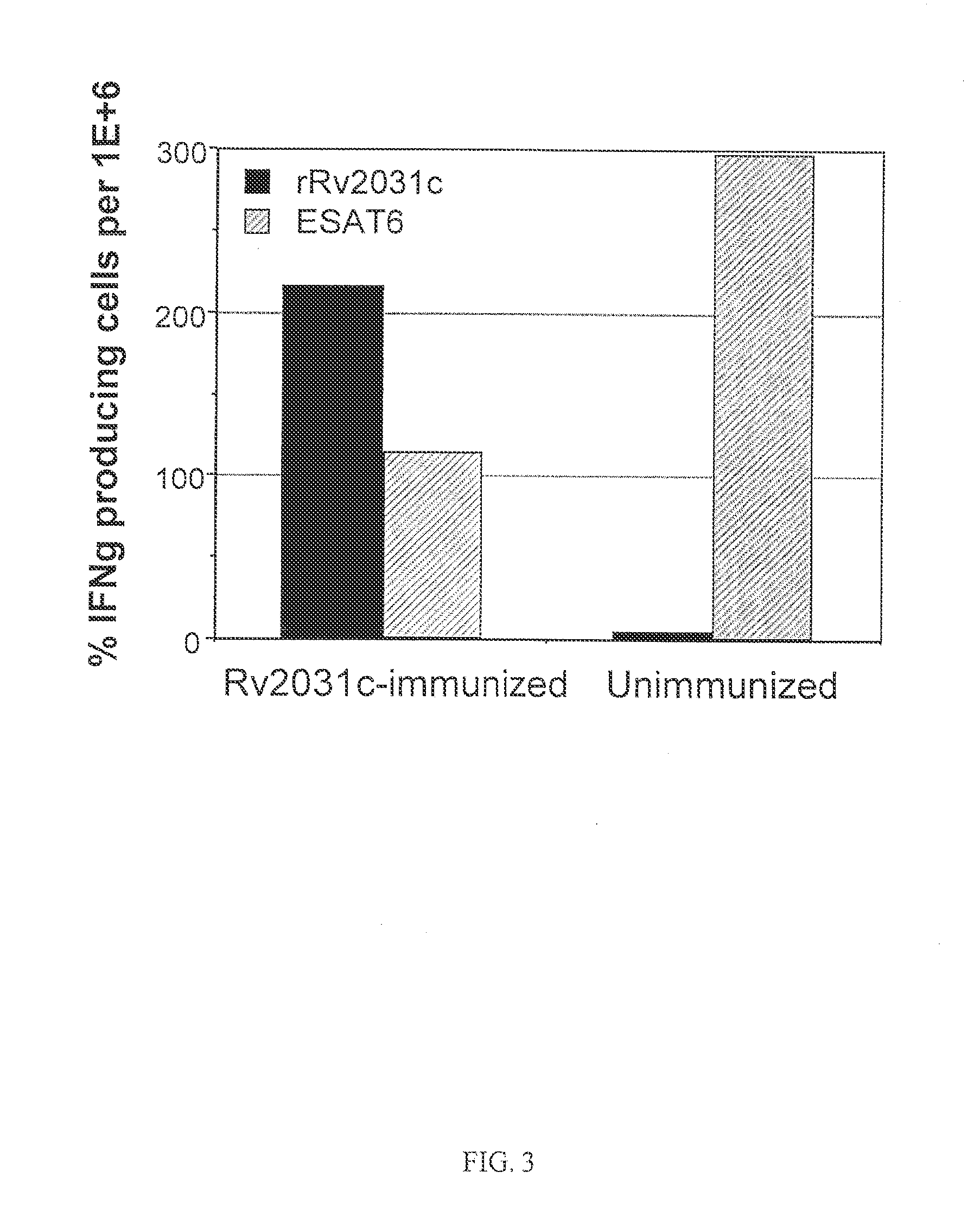
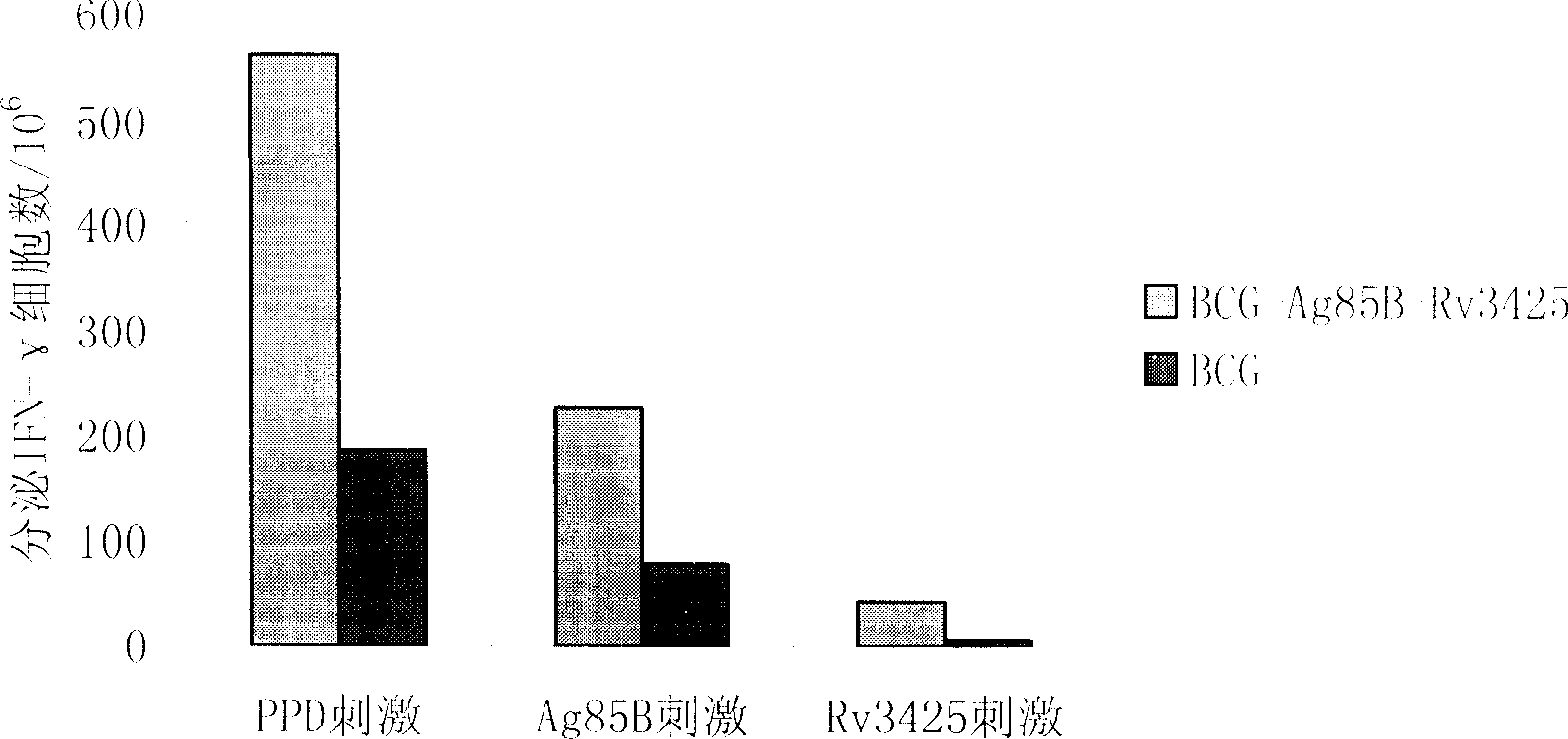Patents
Literature
148 results about "Tuberculosis vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tuberculosis (TB) vaccines are vaccinations intended for the prevention of tuberculosis. Immunotherapy as a defence against TB was first proposed in 1890 by Robert Koch. Today, the only effective tuberculosis vaccine in common use is bacilli Calmette-Guérin (BCG), first used in 1921. About three out of every 10,000 people who get the vaccine experience side effects, which are usually minor except in severely immuno-depressed individuals. While BCG immunization provides fairly effective protection for infants and young children, (including defence against TB meningitis and miliary TB), its efficacy in adults is variable, ranging from 0% to 80%. Several variables have been considered as responsible for the varying outcomes. Demand for TB immunotherapy advancement exists because the disease has become increasingly drug-resistant.
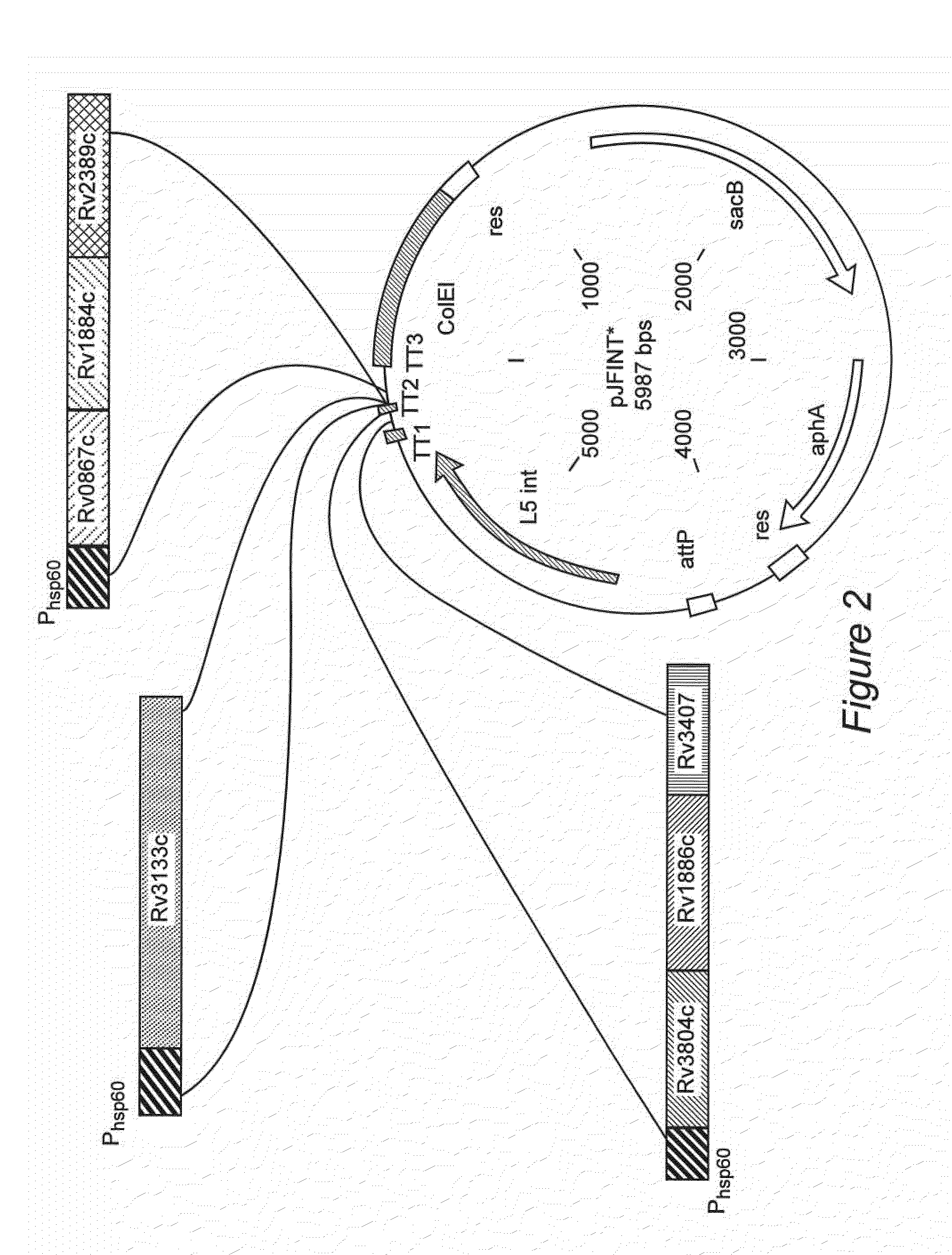
Hybrids of M. tuberculosis antigens
InactiveUS7037510B2Improving immunogenicityImprove propertiesBacteriaPeptide/protein ingredientsImmunological memoryImmunodominant Antigens
The present invention discloses fusion proteins of the immunodominant antigens ESAT-6 and Ag85B from Mycobacterium tuberculosis or homologues thereof, and a tuberculosis vaccine based on the fusion proteins, which vaccine induces efficient immunological memory.
Owner:STATENS SERUM INST
Novel recombinant bcg tuberculosis vaccine designed to elicit immune responses to mycobacterium tuberculosis in all physiological stages of infection and disease
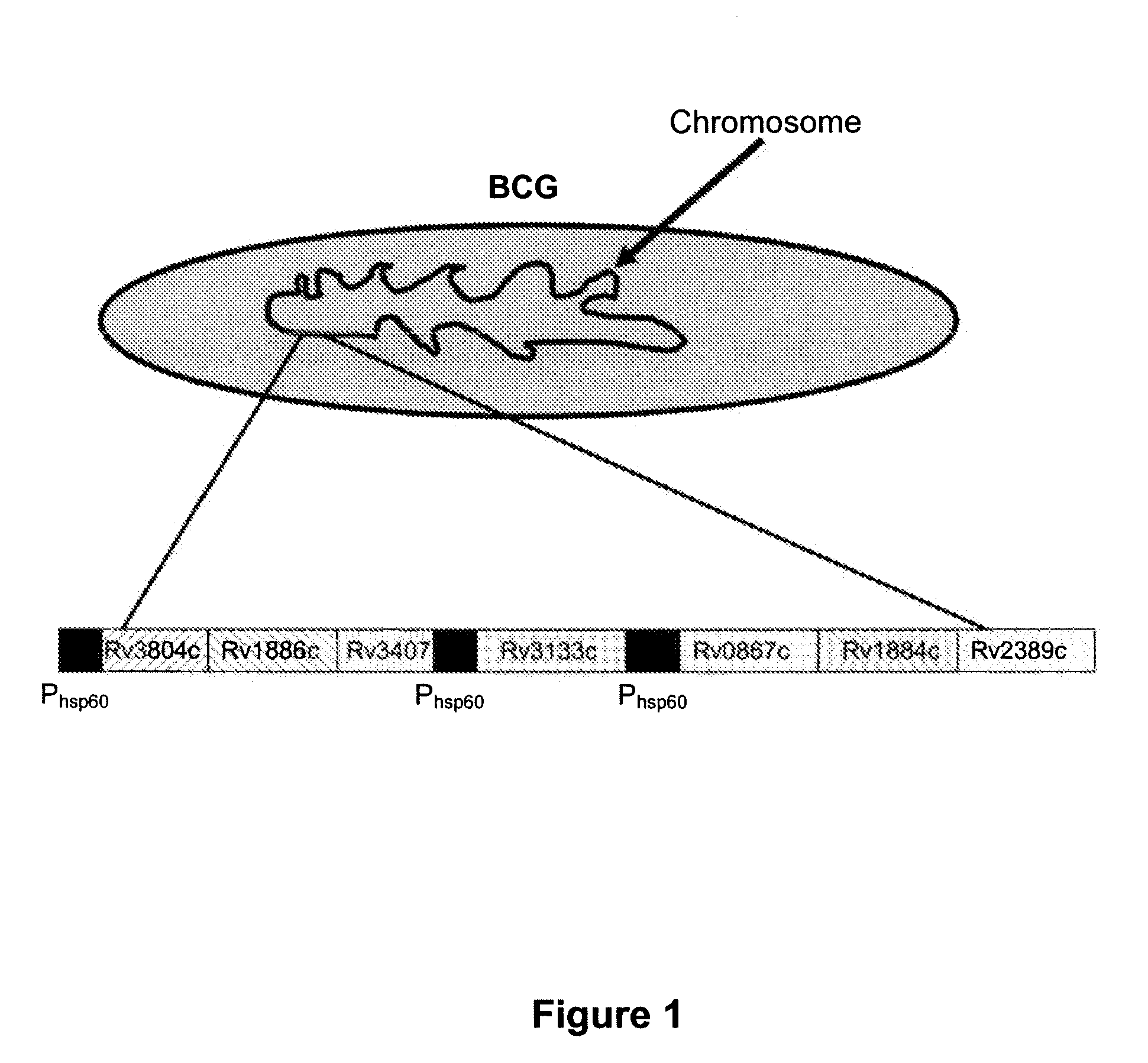
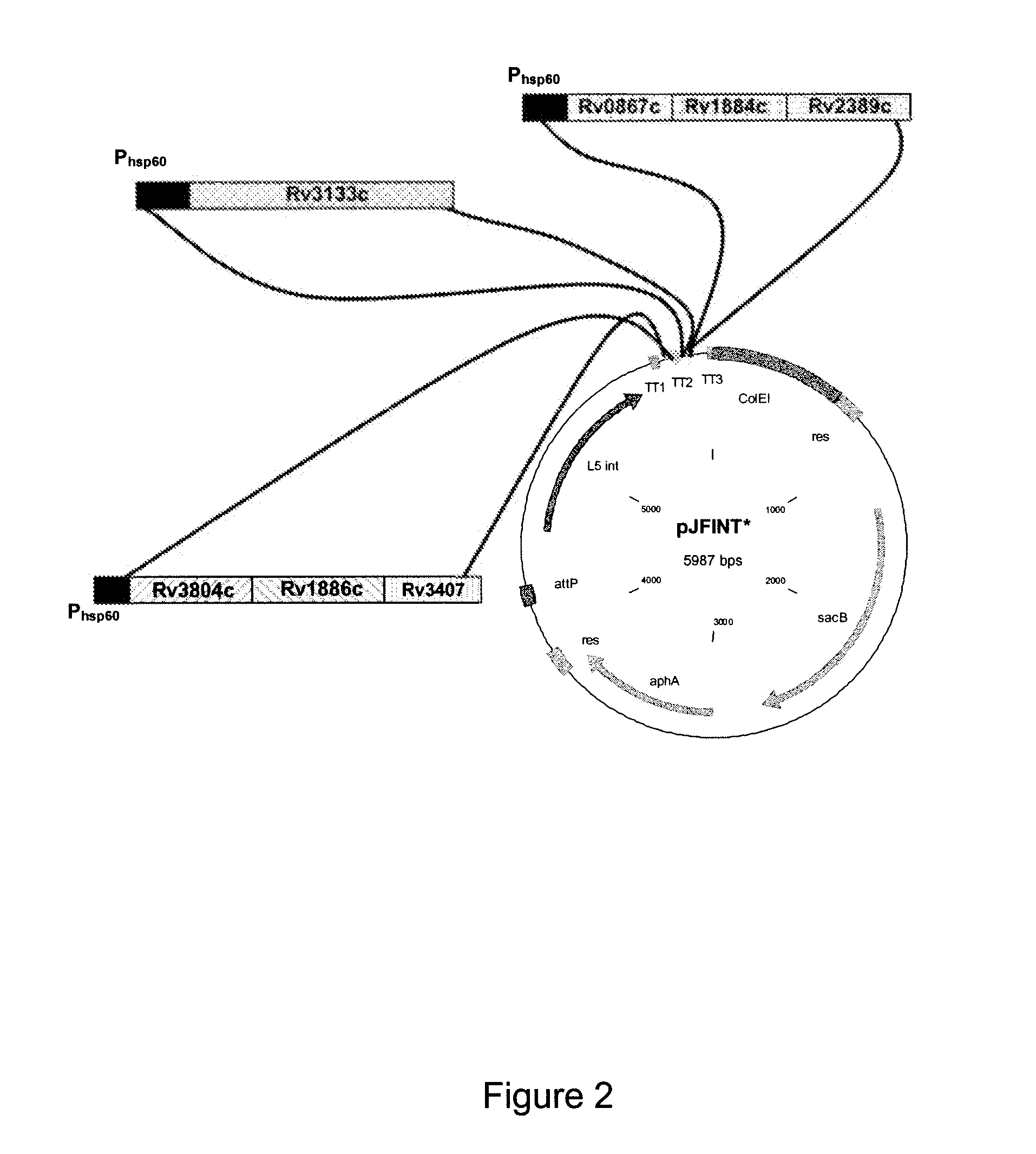
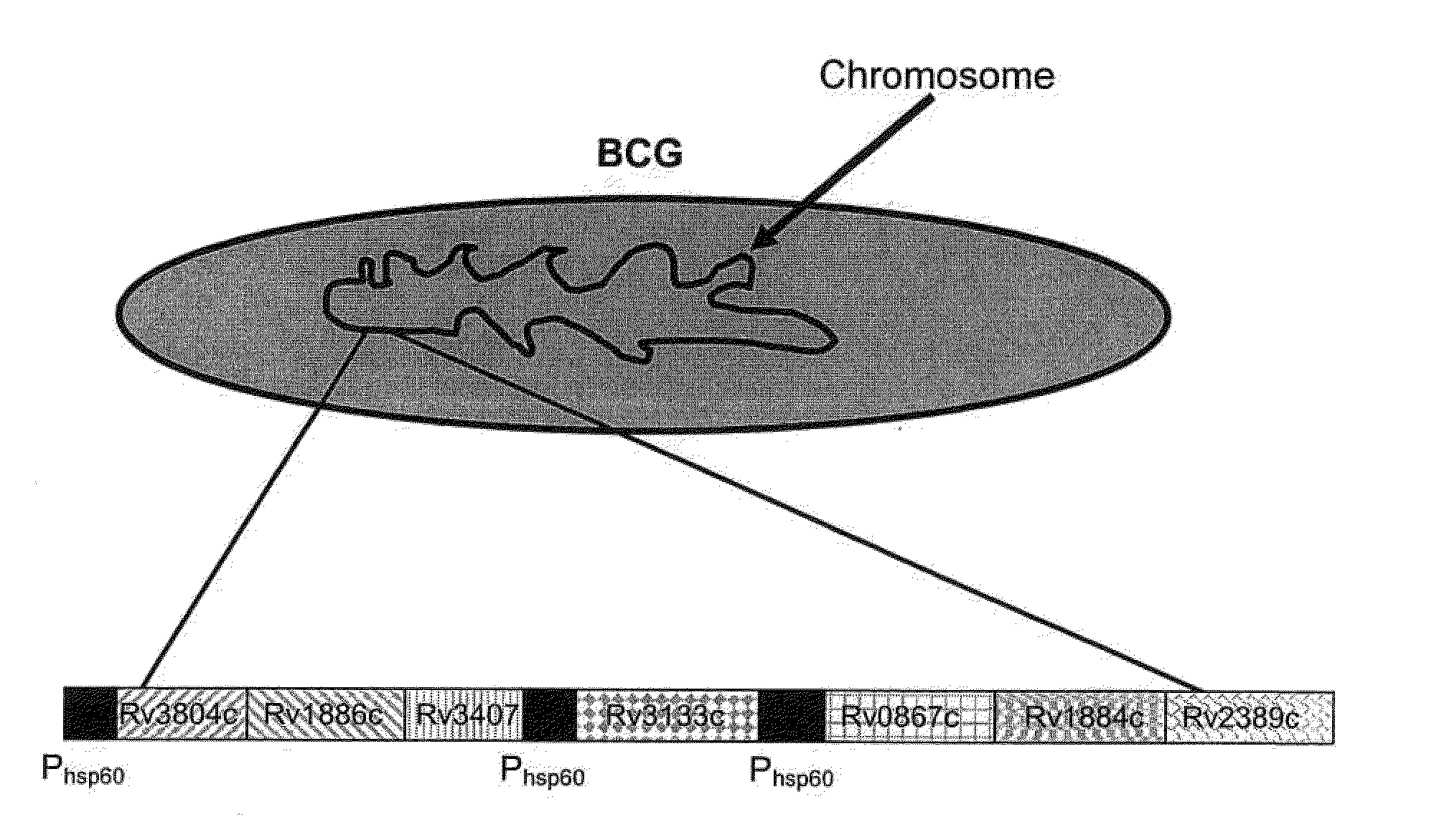
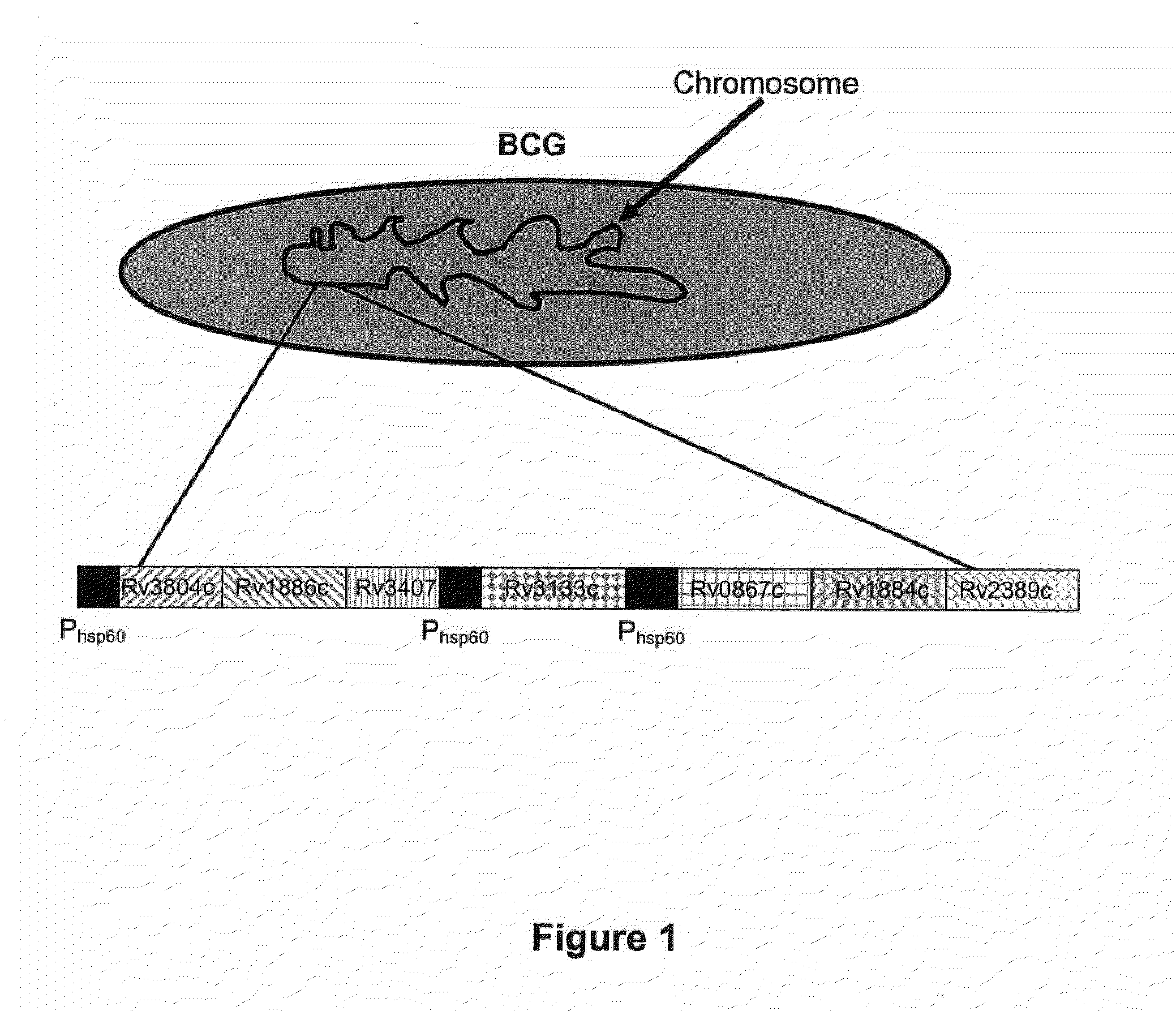
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Novel Recombinant BCG Tuberculosis Vaccine Designed to Elicit Immune Responses to Mycobacterium Tuberculosis in all Physiological Stages of Infection and Disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
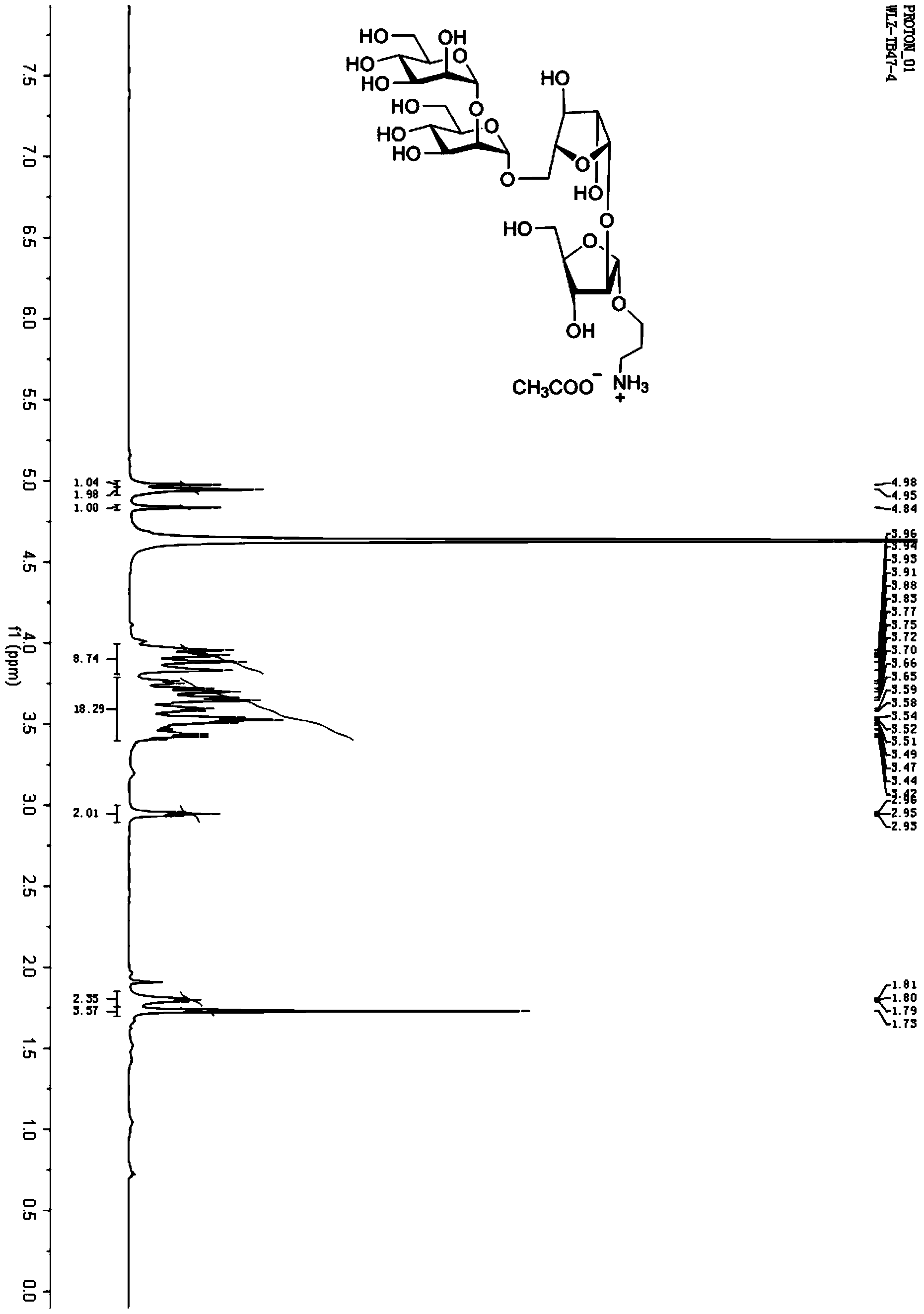
Mycobacterium tuberculosis LAM oligosaccharide conjugate as well as preparation method and application thereof
ActiveCN104004085AGood immune protectionAvoid drug resistanceAntibacterial agentsBacterial antigen ingredientsVaccination against tuberculosisChemical structure
The invention relates to a mycobacterium tuberculosis LAM oligosaccharide conjugate as well as a preparation method and application thereof. A structural general formula of the mycobacterium tuberculosis LAM oligosaccharide conjugate is described in the specification. The invention also relates to application of the mycobacterium tuberculosis LAM oligosaccharide conjugate in preparation of a tuberculosis vaccine. In the mycobacterium tuberculosis LAM oligosaccharide conjugate, a chemical structure of oligosaccharide is definite and single, is not a mixture and can be synthesized by adopting a chemical method, the problem that immune protection force of a Bacilli Calmette Guerin vaccine is small can be solved, better immune effect can be produced to the crowd with low immunity, and the problem that bacterial drug resistance is produced as antibiotics are greatly used, can be solved.
Owner:SHANDONG UNIV
Epitope screening method capable of exciting anti-mycobacterium tuberculosis protective immunological reaction of body and uses
ActiveCN101289496AHelp predictHelp determineAntibacterial agentsPeptide preparation methodsScreening methodPeptide vaccine
The invention relates to a selection method for epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof, in particular to the molecule mimic peptide of the epitope with vaccine development prospect from the mycobacterium tuberculosis and the coding DNA thereof. The selection method of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof provides T lymphocyte epitope contained in one important gene Ag85B in the research of mycobacterium tuberculosis and the method of deducting or selecting the epitope, which is beneficial to further developing novel multivalent and poly epitope tuberculosis vaccine and prevent and control the happening and development of tuberculosis; the method makes a foundation of the future development of synthesizing peptide vaccine epitope vaccine and dna vaccine by using epitope and provides molecule mimic peptide of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the peptide has the amino acid sequence of FVRSSNLKFQDAYNA(SEQ ID NO:1).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Antigen epitope for exciting human anti-tubercle bacillus protective immunoreaction and its use
InactiveCN1858059AHelp preventAids in healingAntibacterial agentsBacterial antigen ingredientsMolecular ImmunologyBCG vaccine
The present invention relates to molecular immunology technology, and aims at screening out antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, researching protective immunoreaction mechanism against tuberculosis, and further developing new type of concatenate polyepitope tuberculosis vaccine. The present invention provides one kind of antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, and the peptide contains the amino acid sequence selected from SEQ ID Nos. 2, 5, 10, 12, 14 and 15. The present invention also provides the screening process and use of the peptide. The present invention may be used in preventing and controlling tuberculosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Recombinant BCG tuberculosis vaccine designed to elicit immune responses to Mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Preparation of novel tuberculosis vaccine and use thereof
ActiveCN101376891AImprove immunityEnhance immune responseAntibacterial agentsBacterial antigen ingredientsAntigenTherapeutic effect
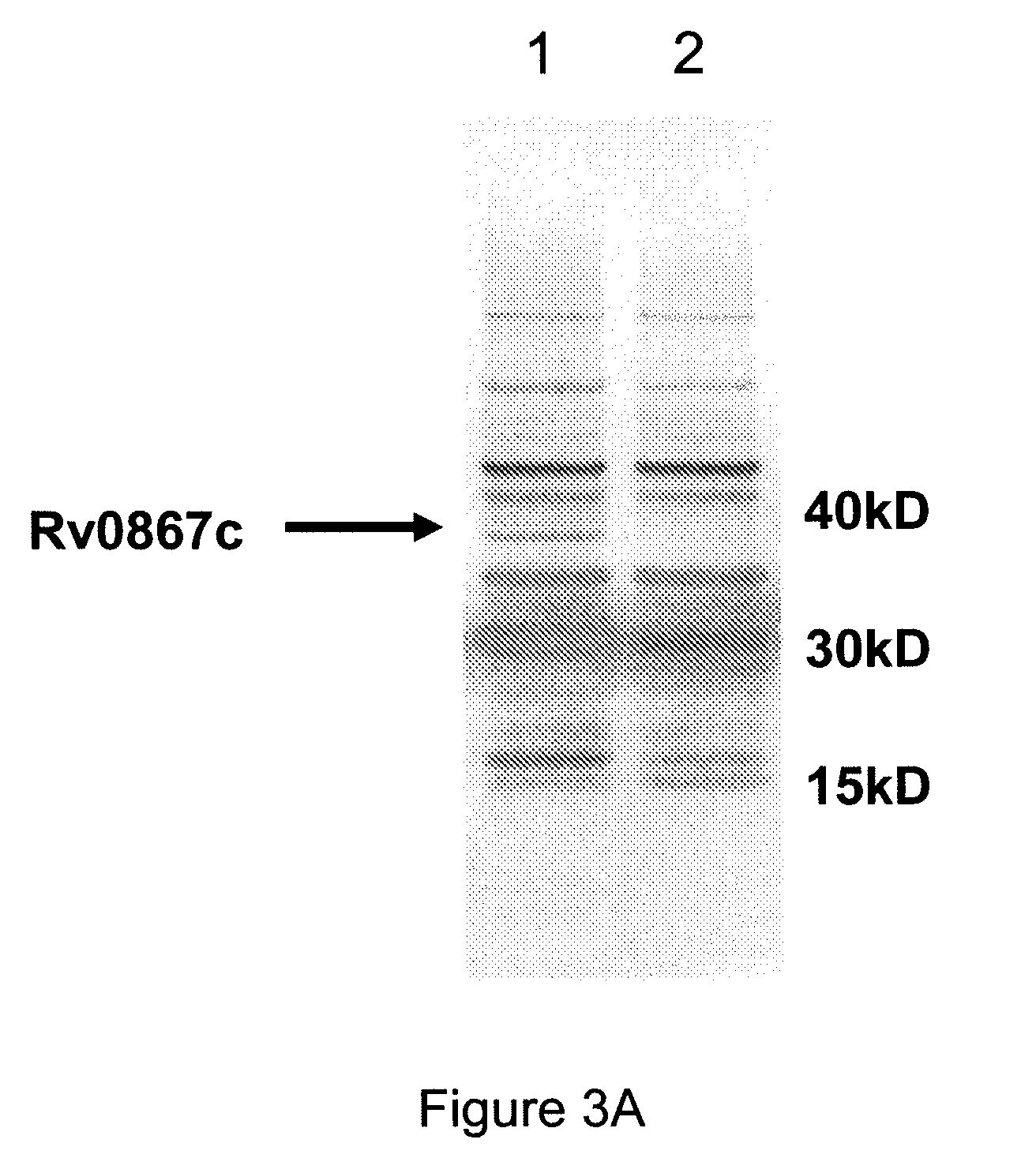
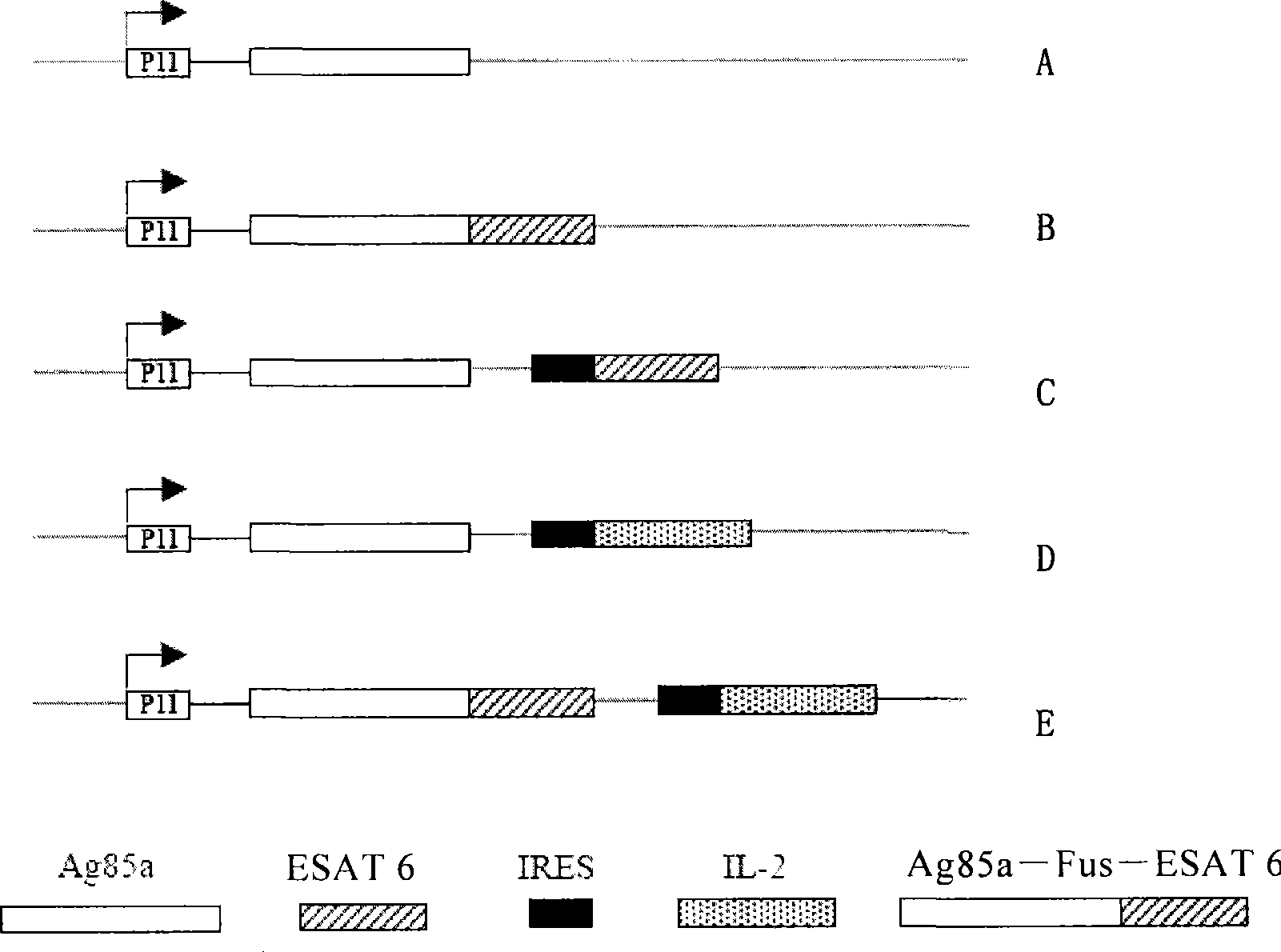
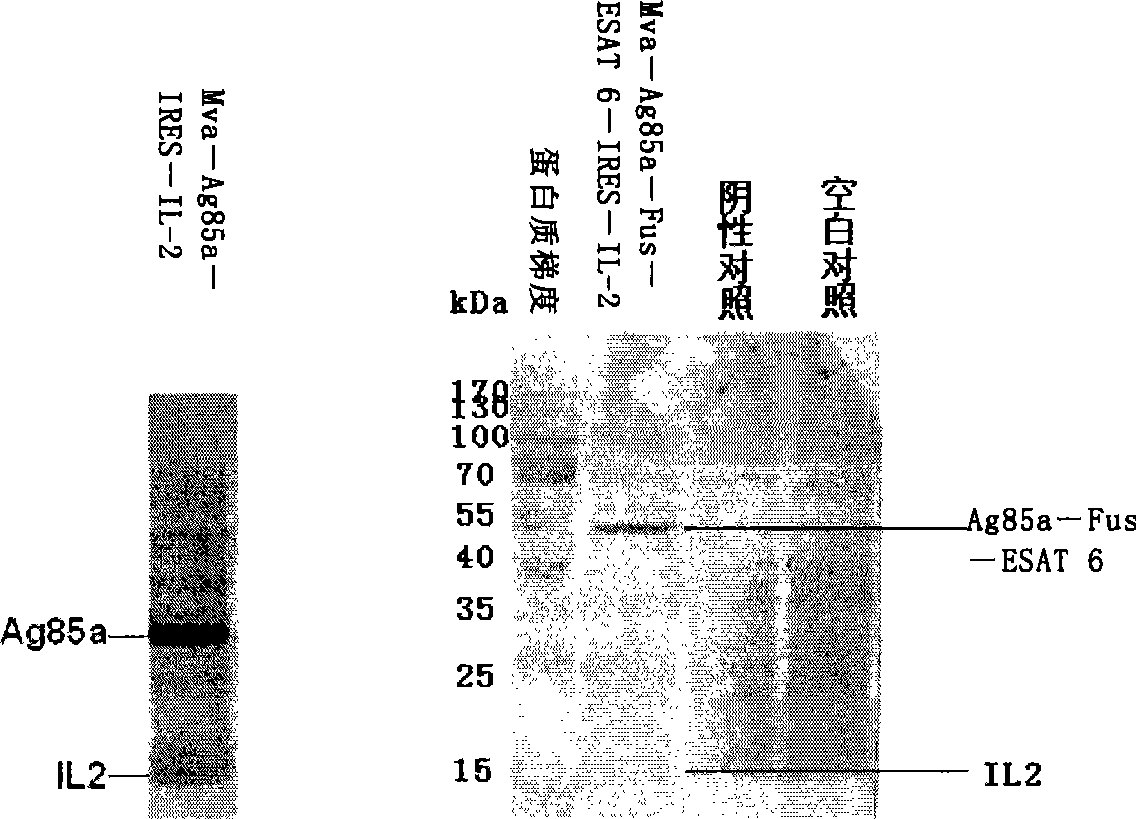
The invention relates to a recombinant virus carrier which can co-express tubercle bacillus Ag85a, ESAT6 and / or Ag85a / ESAT6 fusion protein as well as any immune auxiliary factor; an expression cassette containing at least one type of tubercle bacillus Ag85a coding sequence, ESAT6 coding sequence and Ag85a / ESAT6 fusion protein coding sequence and the any immune auxiliary factor coding sequence is inserted into a gene group DNA of the recombinant virus carrier. The invention also relates to a host cell containing the virus carrier, a pharmaceutical composition and a preparation method thereof. The recombinant virus carrier can co-express a plurality of antigens of m.tuberculosis and / or cell factors, and has improved prevention and / or curing effect to tuberculosis.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein and application thereof
The invention discloses a tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein, namely, nonapeptide. The amino acid sequence of the nonapeptide is as follows: P5: YLGGTTGPV, or P6: YIVGFCLLV, or P7: TLTWLFAFV, or P8: GLVAGLSAV, or P9: ALGMLIAGL, or P10: MLIAGLPCL, or P11: LLCAIFAEV, or P12: RLWPTVGCL. Accordingto the invention, the HLA-A*0201 restrictive CTL epitope of a tuberculosis medicament resistance related protein antigen is predicted and analyzed by applying SYFPEITHI, BIMAS and NetCTL1.2 databasesand using an immunoinformatics mean according to a primary structure of the antigen so that the epitope peptide is obtained by virtue of selection, and the identified nonapeptide is not reported in documents. The epitope peptide is identified through an in-vitro enzyme linked immunospot (ELISPOT) experiment; and according to the result, a theoretical basis is provided for developing tuberculosis vaccine based on the medicament resistance related protein antigen and more information is provided for designing a tuberculosis polyepitope peptide vaccine based on mixed T cell epitope.
Owner:ZHENGZHOU UNIV
Tuberculosis vaccine and diagnostics based on the Mycobacterium tuberculosis sat-6 gene family
InactiveUS7867502B1Improve featuresFacilitate export of the polypeptideAntibacterial agentsBacteriaGene productMycobacterium
This invention relates to a polypeptide fragment which comprises an amino acid sequence encoded by a member of the esat-6 gene. A member of the esat-6 gene family is defined as gene encoding a small protein and that two such genes are arranged next to each other on the genome and that at least one of the gene products has an amino acid sequence identity to either Rv3874, Rv3875, or Rv0288 of at least 15%.
Owner:STATENS SERUM INST
Tuberculosis vaccine and method of using same
InactiveUS20100112007A1Reduce deliveryAntibacterial agentsPowder deliveryGamma irradiationMycobacterium
Provided is a pharmaceutical composition that includes one or more inactivated Mycobacterium spp., which are preferably inactivated using gamma irradiation, and which is than formulated for mucosal or pulmonary delivery to a subject. The pharmaceutical compositions are useful for preventing or treating mycobacterium-associated infections in a subject, including a human subject.
Owner:MICO BIO
Tuberculosis vaccines comprising antigens expressed during the latent infection phase
ActiveUS20090186048A1Reduce bacterial loadProlong survival timeAntibacterial agentsBacterial antigen ingredientsMycobacterial antigenNucleic acid sequencing
The invention is related to an immunogenic composition, vaccine or pharmaceutical composition for preventing, boosting or treating infection caused by a species of the tuberculosis complex (M. tuberculosis, M. bovis, M. africanum, M. microti). The immunogenic composition, vaccine or pharmaceutical composition comprise a fusion polypeptide, which comprises one or more starvation antigens from M. tuberculosis, the units of the fusion polypeptide being M. tuberculosis antigens. Further, the invention is related to the use of a vaccine comprising a fusion polypeptide sequence or nucleic acid sequence of the invention given at the same time as BCG, either mixed with BCG or administered separately at different sites or routes for preparing said immunogenic composition, vaccine, or pharmaceutical composition.
Owner:SUN MICROSYSTEMS INC +1
Tuberculosis vaccine
Owner:UNIV DE ZARAGOZA
Improved recombinant bacillus calmetter Guerin (BCG)
InactiveCN101721693AHigh expressionSolving the problem of insufficient immune stimulationAntibacterial agentsBacterial antigen ingredientsEscherichia coliShuttle plasmid
The invention discloses an improved recombinant bacillus calmetter Guerin (BCG), belonging to the technical field of new medicaments. The immune protecting effect of a unique anti-tuberculosis vaccine BCG for tuberculosis is not exact and the tuberculosis morbidity gradually rises over the past 10 years. Therefore, the development of a more effective anti-tuberculosis vaccine is important. Gene sequences of mycobacterium tuberculosis ESAT6 and Ag85A are inserted into shuttle plasmids of colon bacillus-mycobacterium tuberculosis for forming recombinant plasmids; and the recombinant plasmids are converted into the BCG to form a recombinant anti-tuberculosis vaccine. Proved by research, the recombinant BCG is used for expressing introduced foreign genes and is a novel anti-tuberculosis vaccine.
Owner:SICHUAN UNIV
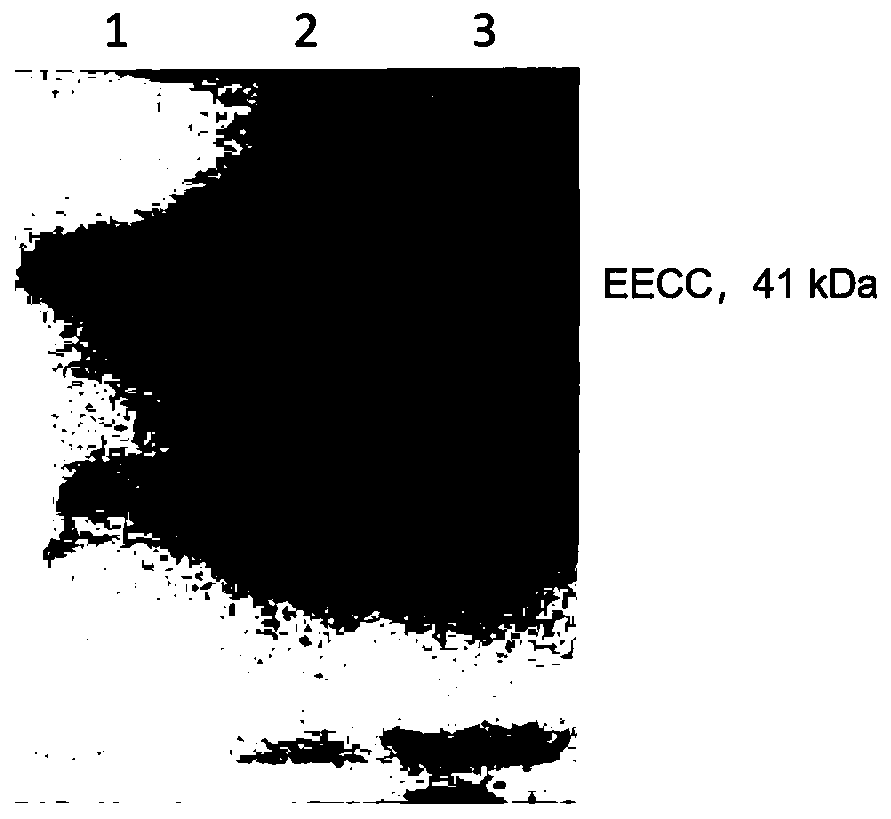
Mycobacterium tuberculosis recombinant fusion protein EECC as well as preparation method and application thereof
ActiveCN110423279ALower doseEffectively differentiate infectionAntibacterial agentsBacterial antigen ingredientsTGE VACCINECytokine
The invention relates to the biotechnology field, in particular to mycobacterium tuberculosis recombinant fusion protein EECC as well as a preparation method and application thereof. The mycobacteriumtuberculosis recombinant fusion protein EECC is EAST6-EAST6-CFP10-CFP10, with an amino acid sequence shown in SEQ ID NO.1 or SEQ ID NO.2. The recombinant fusion protein EECC has excellent antigenicity, has higher sensitivity while guaranteeing higher specificity during diagnosis of tuberculosis, can effectively reduce the use dosage, reduce the detection cost and effectively distinguish and diagnose viable bacterium infection of tubercle bacillus, dead bacterium sensitization and BCG inoculation, can be applied to diagnosis of the tuberculosis, preparation of tuberculosis vaccines and detection of antigen specific cell factors and has better promotion and application values.
Owner:扩增生物科技(北京)有限公司
Recombinant bacillus Calmette-Guerin vaccine and its preparation method
InactiveCN1737153AIncrease secretionGood effectBacterial antigen ingredientsVector-based foreign material introductionEscherichia coliTuberculosis mycobacterium
The invention relates to a recombinant bacillus Calmette-Guerin vaccine and its preparation method, wherein tuberculosis mycobacterium ag85b, esat-6 and IFN-gamma gene sequences are inserted into colibacillus-tuberculosis mycobacterium shuttle plasmids to form recombinant plasmid, and are transformed into BCG to form recombinant tuberculosis vaccine. The recombinant vaccine can be used for prevention and treatment of tuberculosis with better immunological effects than BCG.
Owner:FUDAN UNIV
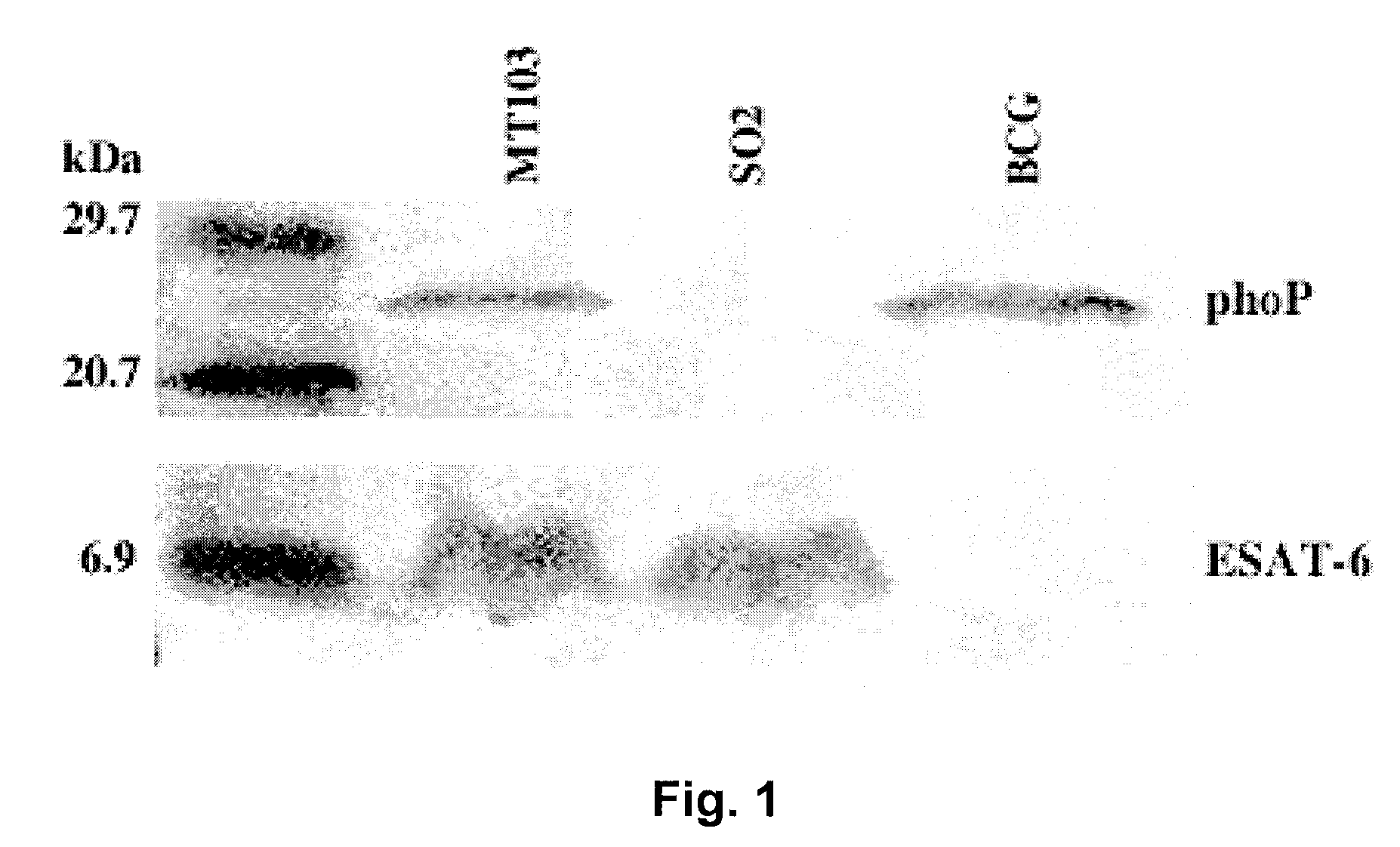
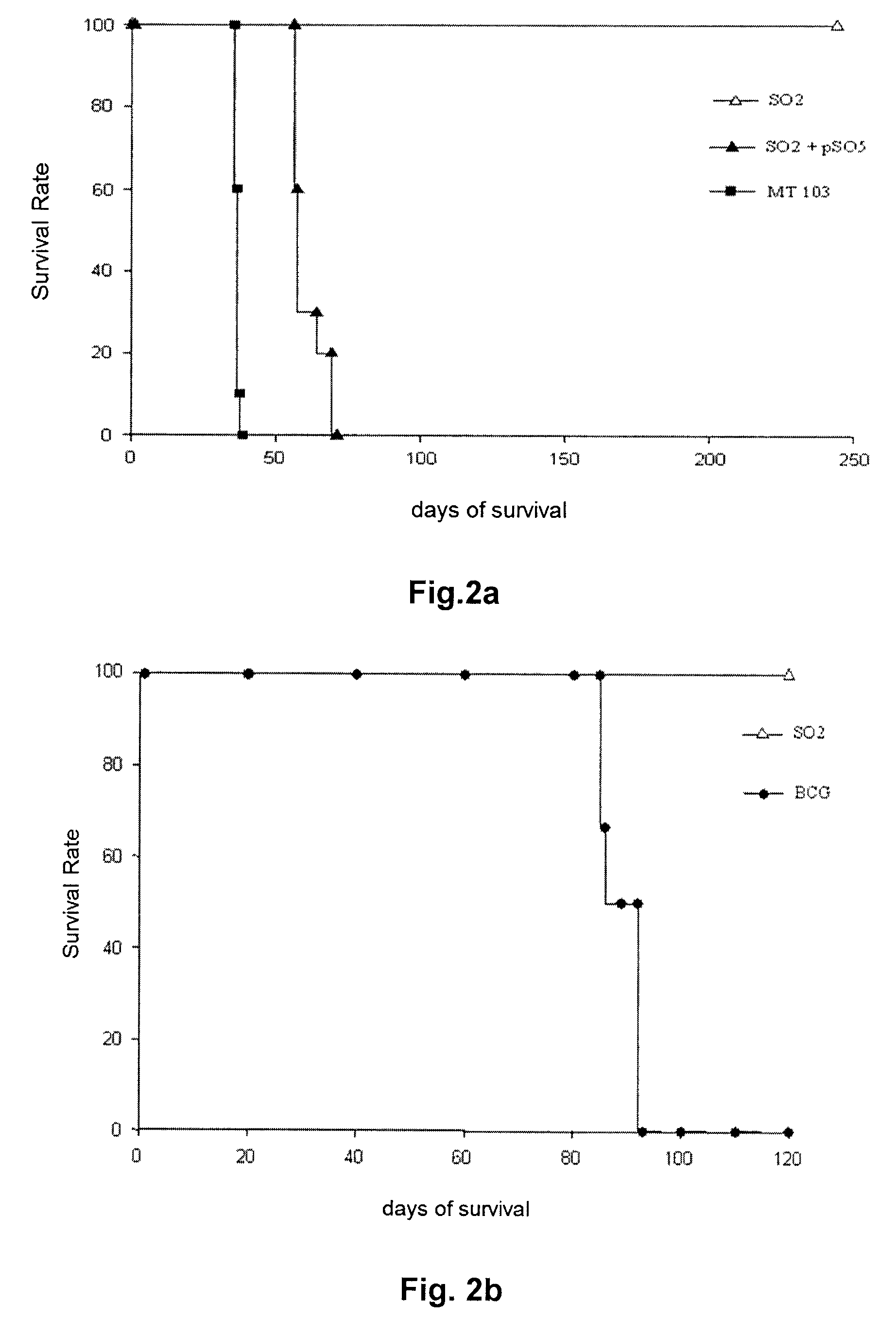
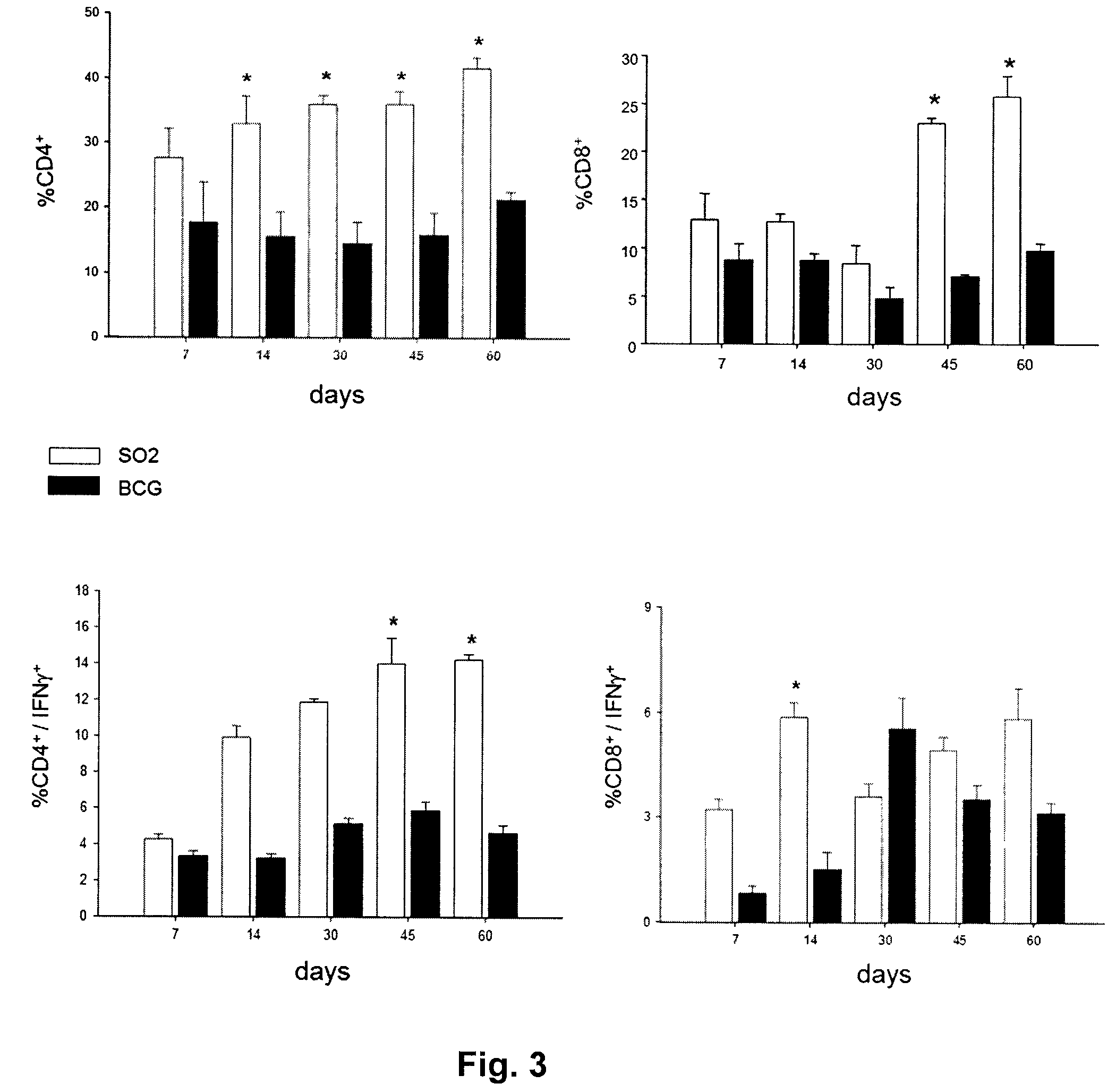
Tuberculosis vaccines including recombinant bcg strains overexpressing phop, and/or phop regulon protein(s)
A live recombinant Mycobacterium bovis-BCG strain comprising a nucleic acid encoding PhoP protein and / or one or more phoP regulon proteins is provided, in which the nucleic acid is capable of being overexpressed. A vaccine or immunogenic composition comprising the live recombinant Mycobacterium bovis-BCG strain and a method for treatment or prophylaxis of a mammal against challenge by Mycobacterium tuberculosis or Mycobacterium bovis are also provided.
Owner:成都安永鼎业生物技术有限公司
Prophylactic tuberculosis vaccine
The invention relates to the use of an immunotherapeutic agent containing cell wall fragments of a virulent strain of Mycobacterium tuberculosis-complex (MTB-C) for the preparation of a drug for the prophylactic treatment of tuberculosis, in which said agent can be obtained using a method comprising the following steps: cultivate the virulent MTB-C strain over a period equal to or greater than three weeks; and, subsequently, homogenate the cell culture in the presence of a nonionic surfactant.
Owner:ARCHIVEL FARMA SL
Novel recombinant vaccine used for preventing tuberculosis
InactiveCN101850112AStable expressionImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to the construction of a human GM-CSF gene and Mycobacterium tuberculosis ESAT6 gene chimerically expressed GMCSF-ESAT6 protein recombinant Bacillus Calmette Guerin (BCG) vaccine and immunogenicity research thereof, namely the sequence of the human GM-CFS gene and the sequence of the Mycobacterium tuberculosis ESAT6 gene are inserted into the sequence of the same escherichiacoli-Mycobacterium tuberculosis shuttle plasmid pMV361 by gene engineering technology so as to construct a recombinant shuttle plasmid rpMV361GMCSF-ESAT6; and a vector is introduced into a BCG vaccine by an electroporation method so as to construct a recombinant BCG vaccine rBCG:GMCSF-ESAT6. The recombinant BCG vaccine can express the human GM-CSF and Mycobacterium tuberculosis ESAT6 gene chimeric protein GMCSF-ESAT6 stably, and has an immunogenicity superior to that of the conventional BCG vaccine. The invention provides a process for preparing the recombinant BCG vaccine, researches the immunity thereof, and belongs to the field of gene engineering and the field of tuberculosis vaccine. The novel recombinant vaccine prevents the generation and the propagration of tuberculosis more effectively.
Owner:SICHUAN UNIV
Applications of Mycobacterium tuberculosis antigen protein Rv0446c and T-cell epitope peptide thereof
ActiveCN106248934AReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsAntigenStimulant
The present invention relates to applications of Mycobacterium tuberculosis antigen protein Rv0446c and a T-cell epitope peptide thereof in preparation of tuberculosis detection reagents, vaccines and medicines, wherein the amino acid sequences of the antigen protein Rv0446c and the T-cell epitope peptide thereof are respectively represented by SEQ ID NO:1-5. According to the present invention, the Mycobacterium tuberculosis antigen protein Rv0446c and the T-cell epitope peptide thereof are used as the stimulants for the specific T cell and B cell immune response caused by Mycobacterium tuberculosis infection, and the false positive caused by the impure antigen can be reduced compared with the use of the complete antigen in the prior art; and the detection reagents prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be widely used for assisted diagnosis of tuberculosis, epidemiological surveillance and other related fields, and the tuberculosis vaccines and the anti-tuberculosis drugs prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be used for prevention and treatment of tuberculosis.
Owner:ICDC CHINA CDC
Tuberculosis immunodiagnosis molecular marker and vaccine use thereof
ActiveCN105388300AHigh strengthAntibacterial agentsBacterial antigen ingredientsParatuberculosisTuberculosis immunization
The invention belongs to the fields of immunology and cytobiology, relates to a tuberculosis immunodiagnosis molecular marker and vaccine use thereof, and in particular relates to use of any one or more of proteins, selected from amino acid sequences shown as SEQ ID NO: 1-4, in preparing a tuberculosis diagnostic agent; preferably, the tuberculosis is phthisis. The protein shown by any sequence in SEQ ID NO: 1-4 can serve as a tuberculosis (such as phthisis) immunodiagnosis molecular marker, and has good coincidence rate and reaction intensity; besides, the tuberculosis immunodiagnosis molecular marker has the potential for being applied to preparation of a tuberculosis vaccine.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Therapeutic tb vaccine
InactiveUS20110020384A1Prevents and reduces establishmentImmunityAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsEngineering
Therapeutic vaccines comprising polypeptides expressed during the latent stage of mycobacteria infection are provided, as are multiphase vaccines, and methods for treating and preventing tuberculosis.
Owner:STATENS SERUM INST
Mycobacterium tuberculosis antigen protein Rv0865 and application of B cell epitope peptide thereof
ActiveCN106226520AReduce false negativesHigh detection sensitivityAntibacterial agentsBacterial antigen ingredientsT cellTreating tuberculosis
The invention relates to a mycobacterium tuberculosis antigen protein Rv0865 and an application of a B cell epitope peptide thereof in preparing a tuberculosis detection reagent, a vaccine and a drug. The amino acid sequences of the antigen protein Rv0865 and the B cell epitope peptide thereof are respectively shown as SEQ ID NO: 1-5. According to the invention, the mycobacterium tuberculosis antigen protein Rv0865 and the B cell epitope peptide thereof are utilized as stimulants for specific T cell and B cell immune response caused by mycobacterium tuberculosis infection. Compared with the traditional condition adopting complete antigen, the false positive caused by impure antigen can be reduced. The detection reagent prepared by adopting the protein Rv0865 and the epitope peptide thereof can be widely applied to the field of phthisic auxiliary diagnosis and epidemic disease monitoring. The vaccine and the drug prepared by adopting the protein Rv0865 and the epitope peptide thereof can be used for preventing and treating tuberculosis.
Owner:ICDC CHINA CDC
Antituberculous CTL (Cytotoxic T Lymphocyte) epitope peptide with drug-resistant related efflux protein source for tuberculosis and application of epitope peptide
The invention discloses an antituberculous CTL (Cytotoxic T Lymphocyte) epitope peptide with a drug-resistant related efflux protein source for tuberculosis. The antituberculous CTL epitope peptide is nonapeptide, wherein the amino acid sequence of the nonapeptide is P9: ALGML IAGL. Predicative analysis is carried out on HLA-A*0210 restrictive CTL epitope of drug-resistant related protein antigen for tuberculosis by adopting immune-informatics means and SYFPEITH1, BIMAS and NetCTL1.2 databases according to the primary structure of the antigen, so that the epitope peptide is obtained by screening; and the identified nonapeptide has not been reported in any document. An in-virto ELISPOT (Enzyme-Linked Immunospot Assay) is adopted to identify the epitope peptide; and the identification result provides a theoretical basis for developing tuberculosis vaccine based on drug-resistant related protein antigen and provides more information for designing multi-epitope peptide vaccine for tuberculosi based on the mixed T cell epitope.
Owner:ZHENGZHOU UNIV
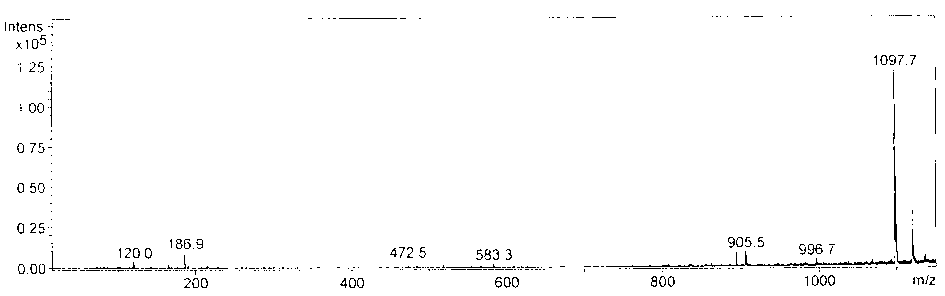
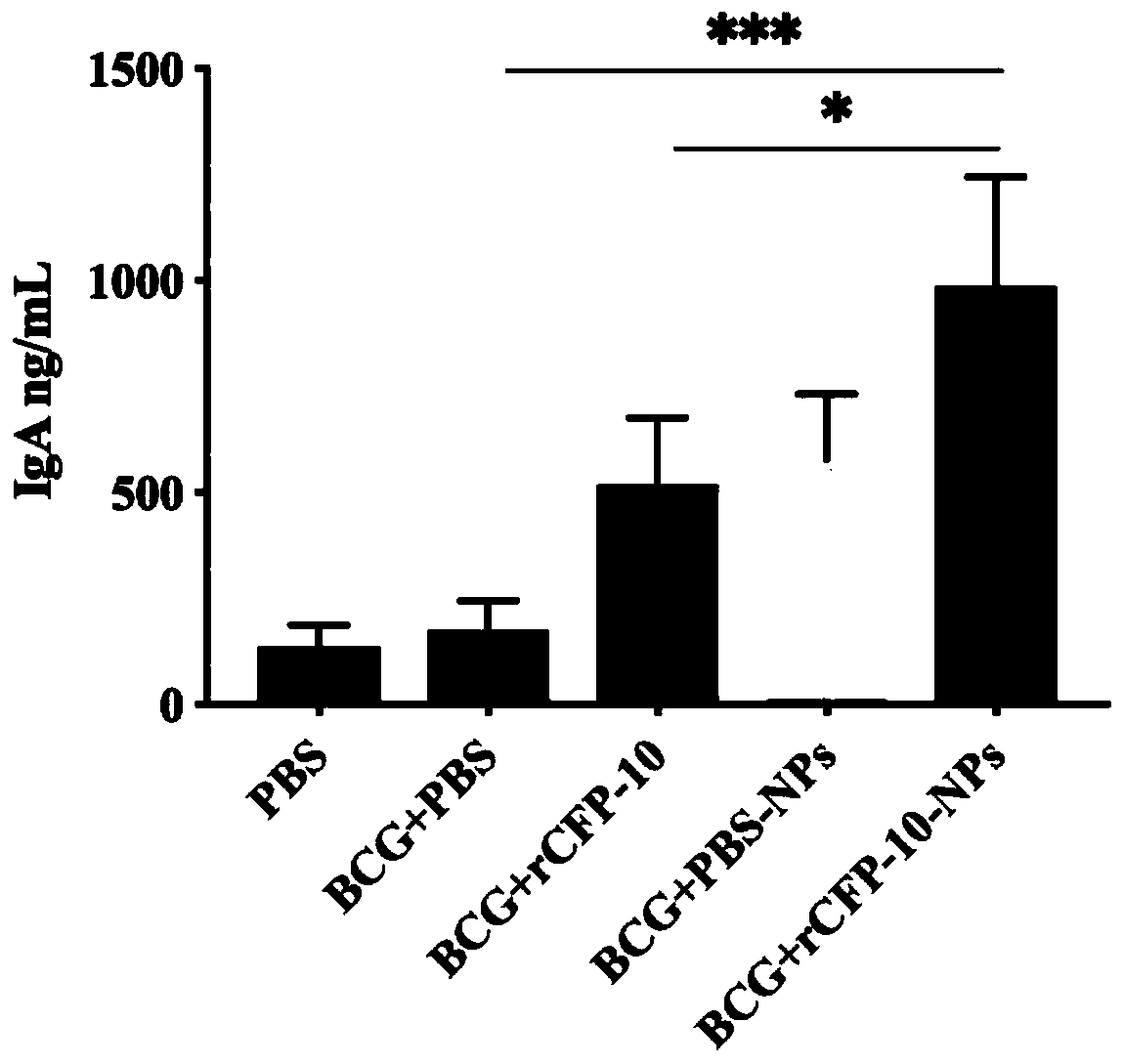
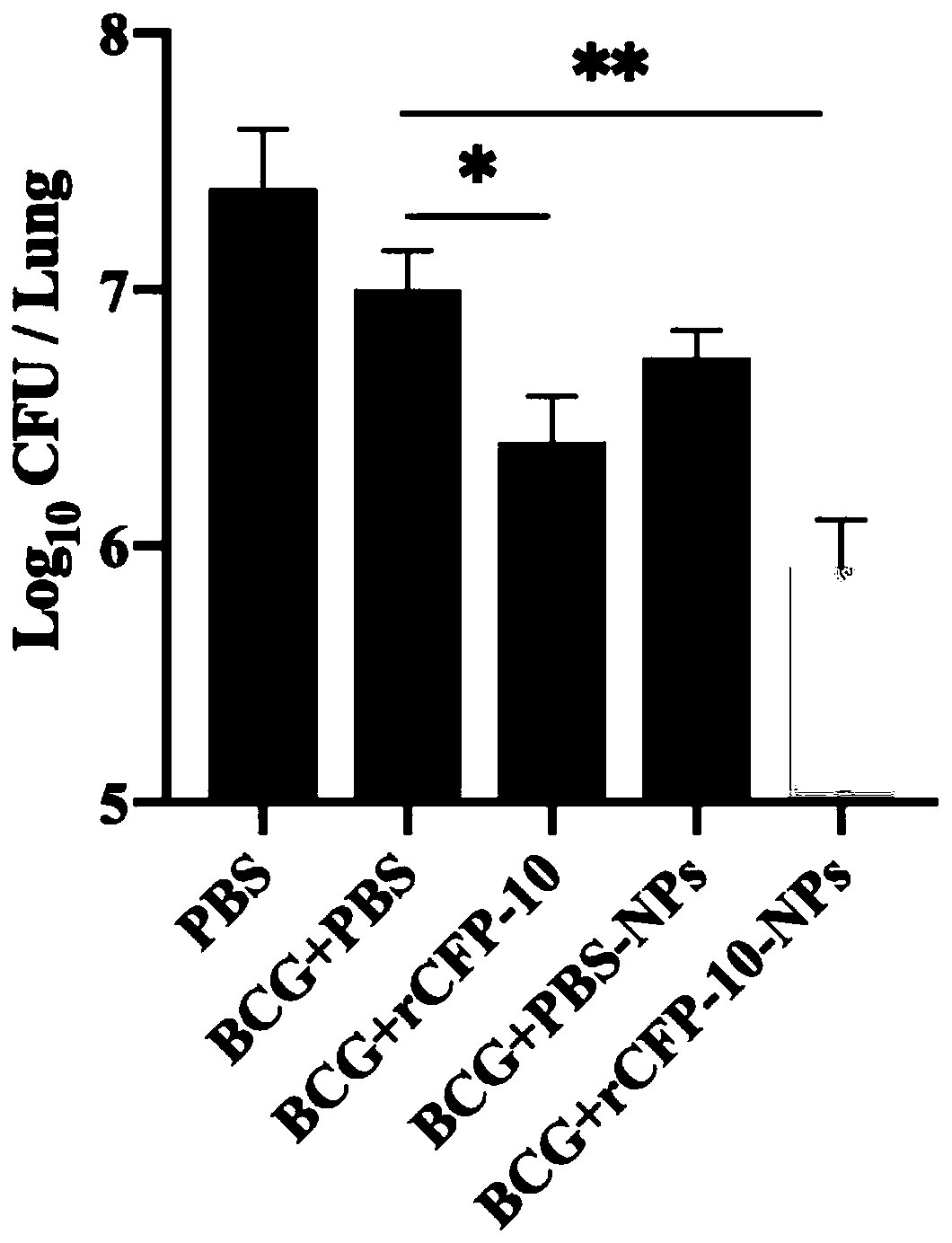
Preparation and application of recombinant protein CFP-10 nanoparticles
ActiveCN110354098AImprove immunityReduce bacterial loadAntibacterial agentsBacterial antigen ingredientsBCG immunizationEmulsion
The invention provides a preparation method of recombinant protein CFP-10 nanoparticles and the application thereof in preparing tuberculosis prevention vaccines. The preparation method includes the steps of (1) configuration of a polylactic acid-glycolic acid copolymer PLGA solution; (2) configuration of a recombinant protein CFP-10 solution; (3) preparation of primary emulsion; (4) preparation of compound emulsion; (5) formation of the nanoparticles. The recombinant protein CFP-10 nanoparticles prepared by the preparation method are used for enhancing the immunity of BCG-immunized mice through intranasal drip, and it is confirmed that the recombinant protein CFP 10 nanoparticles have the effects of improving mucosal immunity level and promoting BCG immunity.
Owner:CHINA AGRI UNIV
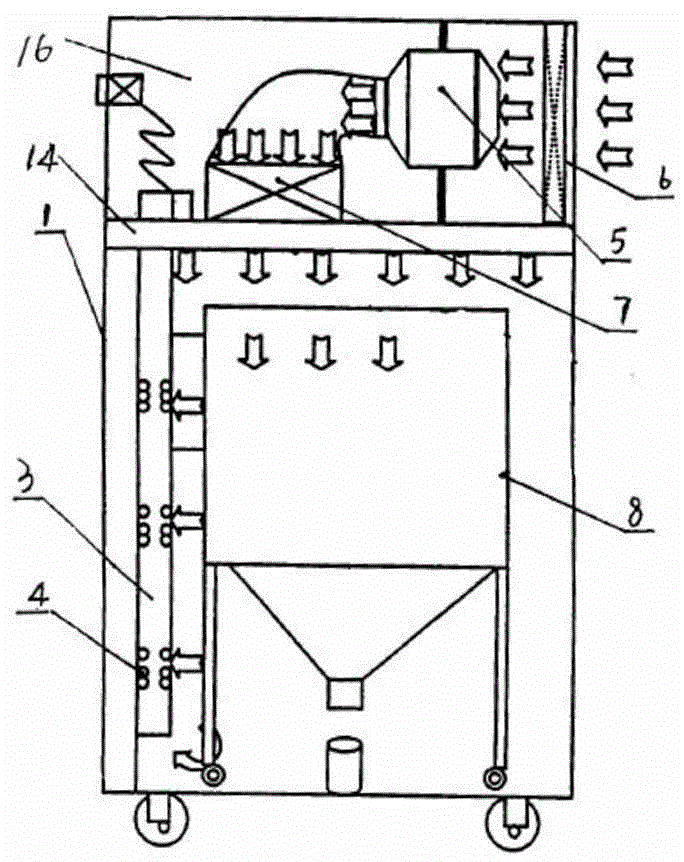
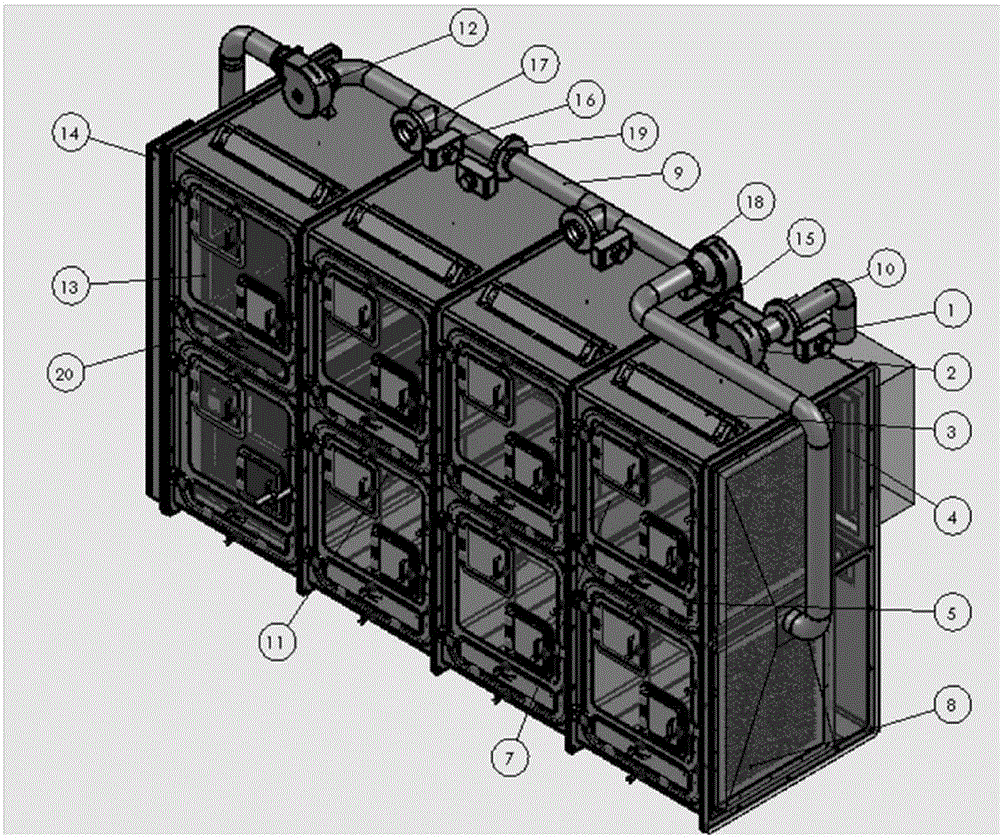
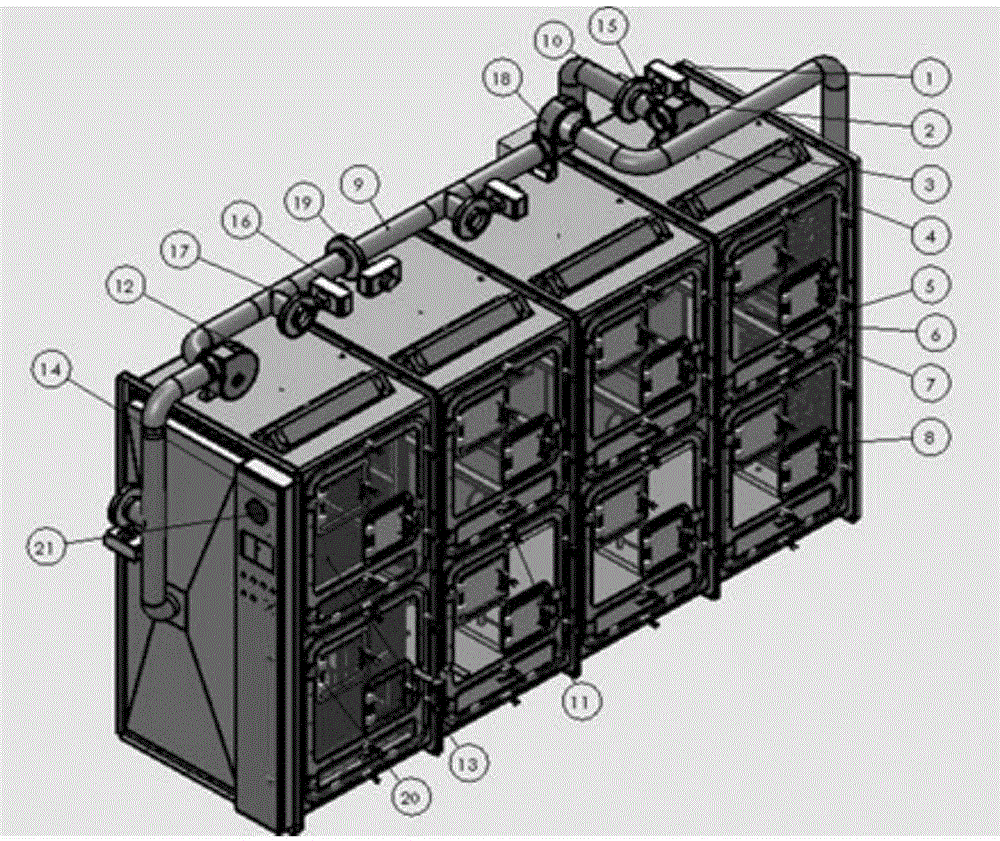
Method and device for establishment of Mycobacterium tuberculosis naturally infected non-human primate model
InactiveCN104127444ABacteria material medical ingredientsAnimal housingLatent tuberculosisMycobacterium w
The invention provides a method for establishment of a Mycobacterium tuberculosis naturally infected non-human primate model. According to the invention, a healthy non-human primate and a non-human primate artificially infected with tuberculosis and suffering cough are raised in an air flow self-circulation combined type negative pressure isolator so as to infect the healthy non-human primate with tuberculosis. Establishment of the natural infection model helps to reveal conditional factors (like HIV) activating latent tuberculosis infection and related mechanisms. The non-human primate model with more clinical significance is provided for research, development and evaluation of anti-tuberculosis vaccines and drugs.
Owner:WUHAN UNIV
Recombinant Ag85B-Rv3425 bacillus Calmette-Guerin vaccine
This invention is associated with the gene engineering field and tuberculosis vaccines field. In recent decades, the increase of tuberculosis drug resistant strain and the coinfection of mycobacterium tuberculosis and HIV lead the increase of incidence of tuberculosis, and made it the second killer of human after HIV. Recently, the only tuberculosis vaccines used globally is BCG. This invention inserts the most important protective antigen gene of mycobacterium tuberculosis and missed mycobacterium tuberculosis gene in BCG to the multi-cloning coliform - mycobacterium tuberculosis shuttle-plasmid multiple cloning sites to form recombined plasmid and transforms it to the BCG to form recombined tuberculosis vaccines. The result of experiment shows that, compared with BCG vaccine, the protective antigen can express more efficient, the original missing Rv3425 antigen also get expressed, and the induced IFN- gamma expression also increased significently. This indicates that the recombined tuberculosis vaccines can induce higher cell immunity level than BCG.
Owner:FUDAN UNIV
Antituberculosis vaccine as well as preparation method and application thereof
ActiveCN108743931APreserve antigenic propertiesLow immunogenicityAntibacterial agentsOrganic active ingredientsAntigenSpecific immunity
The invention belongs to the field of tuberculosis vaccines, and particularly relates to an antituberculosis vaccine as well as a preparation method and application thereof. Aiming at the problems that an existing vaccine has a poor effect on adults or is unsuitable for hypoimmunity patients and the like, the invention provides the preparation method of the antituberculosis vaccine. The preparation method comprises the following steps: firstly, obtaining a mycobacterium monocell thallus; secondly, interruptedly and circularly irradiating the monocell thallus by adopting rays at low dose to obtain a mycobacterium vaccine, wherein the rays are X-rays, gamma rays or rays generated by an isotope radioactive source Co60; the radiation dose rate lies in that radiation is performed at the rate of10 to 20Gy / min for 8 to 10 times at an interval of 5 to 10 minutes each radiation of 20 minutes. According to the antituberculosis vaccine disclosed by the invention, all antigen characteristics of the thallus are completely retained, stronger specific immune response can be excited more quickly, and effective durable immunity is obtained. The prepared vaccine has the advantages of low toxicity,quick onset and higher safety, and can be used for preventing and treating tituberculosis of immunocompromised patients.
Owner:WEST VAC BIOPHARMA CO LTD
Recombinant BCG tuberculosis vaccine designed to elicit immune responses to mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Mycobacterium tuberculosis EEC fusion protein, and preparation method and application thereof
ActiveCN110684116AEffectively differentiate infectionEffectively distinguish dead bacteria sensitizedAntibacterial agentsBacterial antigen ingredientsPolynucleotideTGE VACCINE
The invention discloses a mycobacterium tuberculosis EEC fusion protein. The amino acid sequence of the fusion protein is SEQ ID NO.8 in a sequence table; polynucleotide for encoding the polypeptide is shown as SEQ ID NO.7 in the sequence table; and the fusion protein contains a carrier of the polynucleotide and host cells. The invention further relates to preparation of the fusion protein, and effects of the fusion protein in tuberculosis auxiliary diagnosis, tubercle bacillus infection screening and tuberculosis vaccine development.
Owner:成都可恩生物科技有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com