Tuberculosis vaccines including recombinant bcg strains overexpressing phop, and/or phop regulon protein(s)
A technology of overexpression and bacterial strains, applied in the field of recombinant BCG strains, can solve the problems of loss of immune protection and weakened potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation of recombinant live vaccines is known to those skilled in the art. Typically the vaccines are prepared as injectables, either as liquid solutions or suspensions; solid forms suitable for solution in, or suspension in, liquid prior to injection can also be prepared. The formulation can also be emulsified, or the protein encapsulated in liposomes. The live immunogenic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. Suitable excipients are, for example, water, saline, dextrose, glycerol, ethanol, etc., and combinations thereof. In addition, if desired, the vaccine may contain minor amounts of auxiliary substances, such as wetting or emulsifying agents, pH buffering agents and / or adjuvants which enhance the efficacy of the vaccine. Examples of potentially effective adjuvants include, but are not limited to: aluminum hydroxide, N-acetylmuramoyl-L-threonyl-D-isoglutamine (thr-MDP), ...
Embodiment 1
[0074] Example 1. Construction of BCG strains overexpressing the transcription regulator protein phoP
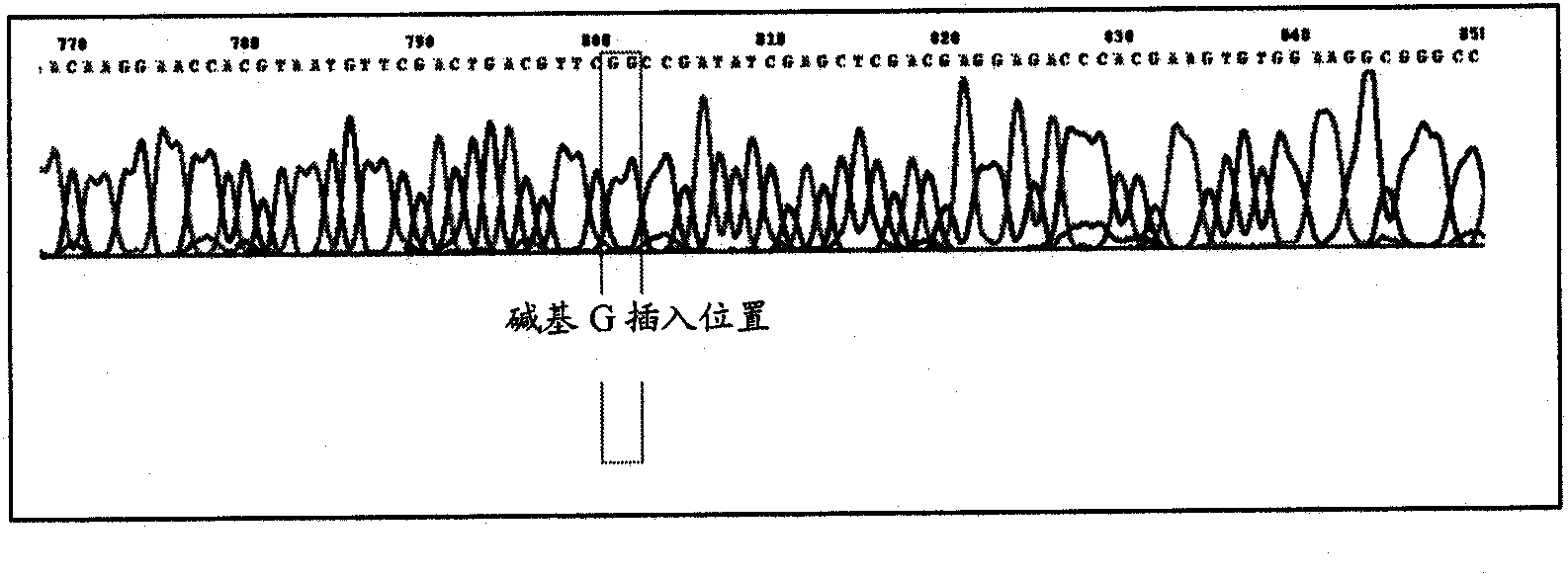
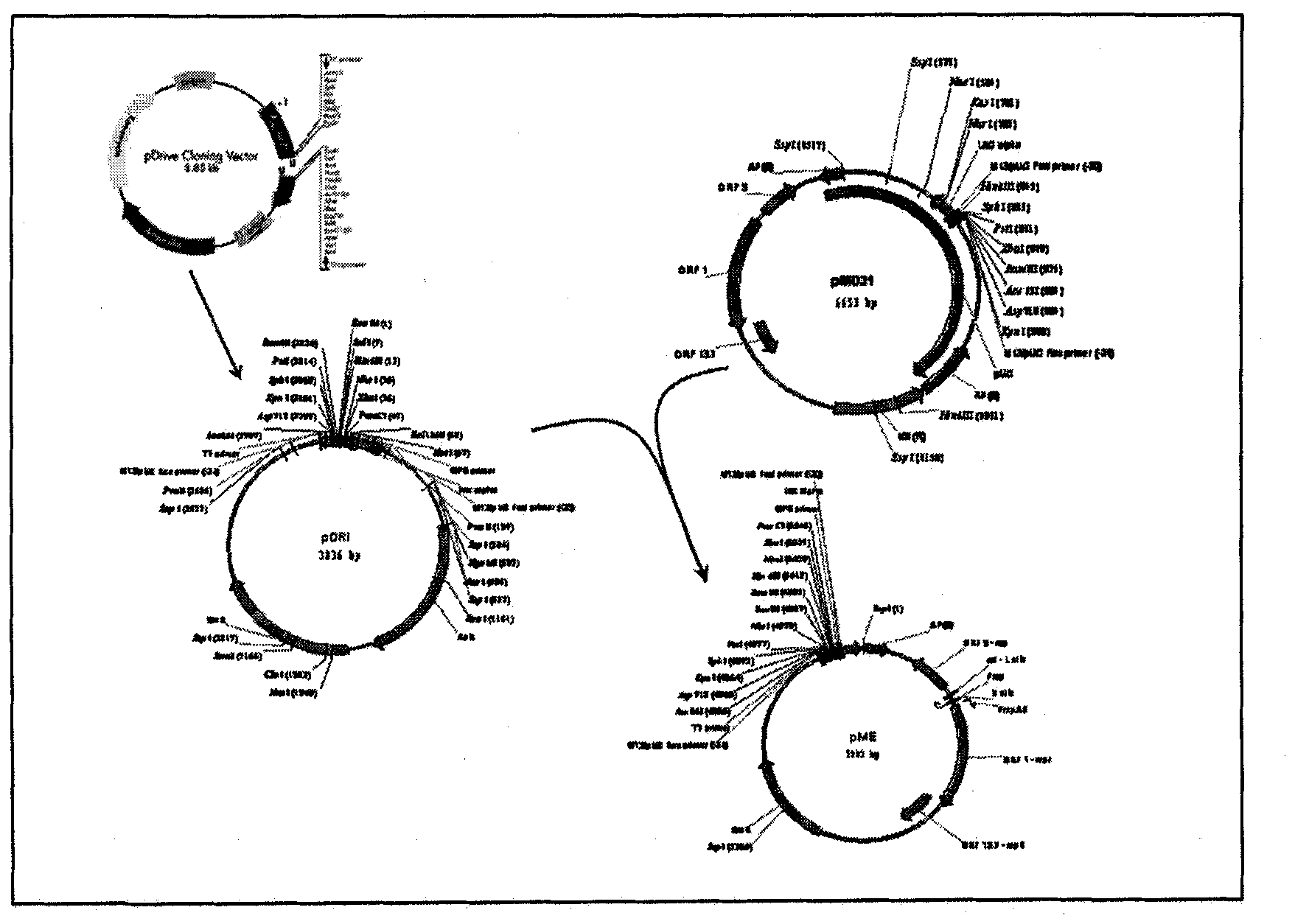
[0075] A kanamycin-resistant shuttle vector containing the T7 promoter was obtained by cutting the pDrive cloning vector (obtained from Qiagen) with EcoRI and generating pDRI by self-ligation. pDRI was digested with SspI and the 1903 base pair fragment product was isolated. The pMD31 shuttle vector (Wu et al., 1993, Molecular Microbiology, 7, 407-417) was digested with SspI and a fragment of 3379 base pairs was isolated. The ligation of these two SspI-generated fragments produces pME (5282 bp), which contains the original T7 promoter of pDRIVE (see image 3 ).
[0076] Forward primers (5'-AAAAA) containing KpnI and pstI restriction sites (underlined parts) respectively GGTACC GCTTGTTTGGCCATGTCAAC-3') and reverse primer (5'-AAAAA CTGCAG GCTGCCGATCCGATTAACTAC-3'), the wild-type M. tuberculosis phoP gene was amplified from the M. tuberculosis H37Rv strain (ATCC 25618). T...
Embodiment 2
[0078] Example 2. Determination of Induction of Recombinant BCG-Prague Genes Overexpressing phoP
[0079] To determine the effect of phoP overexpression on BCG gene expression, microarray analysis was performed to compare the transcriptional profiles of BCG-Prague / pME:phoP and wild-type BCG-Prague harboring pME empty vector. The strain was cultured at 37°C in 7H9 liquid medium supplemented with 10% ADC (Difco) and 0.05% Tween 80 until OD 600 Values reach 0.4 to 0.5. To isolate total RNA, cells were pelleted and transferred to 2 ml screw cap tubes containing 1 ml RNA protect Bacterial Reagent (Qiagen) and incubated for 5 min at room temperature. The cells were pelleted again, and 400 μl of lysate (20 mM NaCH 3 COOH, 0.5% SDS, 1 mM EDTA, pH 4) and 1 ml phenol / chloroform (pH 4.5, Sigma) were resuspended. Cells were disrupted by beating with glass beads in three 30 sec-pulses. Cells were then incubated at 65°C for 4 minutes, followed by 4°C for 5 minutes and centrifuged at 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com