Patents
Literature
115 results about "Mycobacterium bovis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycobacterium bovis (M. bovis) is a slow-growing (16- to 20-hour generation time) aerobic bacterium and the causative agent of tuberculosis in cattle (known as bovine TB). It is related to Mycobacterium tuberculosis, the bacterium which causes tuberculosis in humans. M. bovis can jump the species barrier and cause tuberculosis-like infection in humans and other mammals.
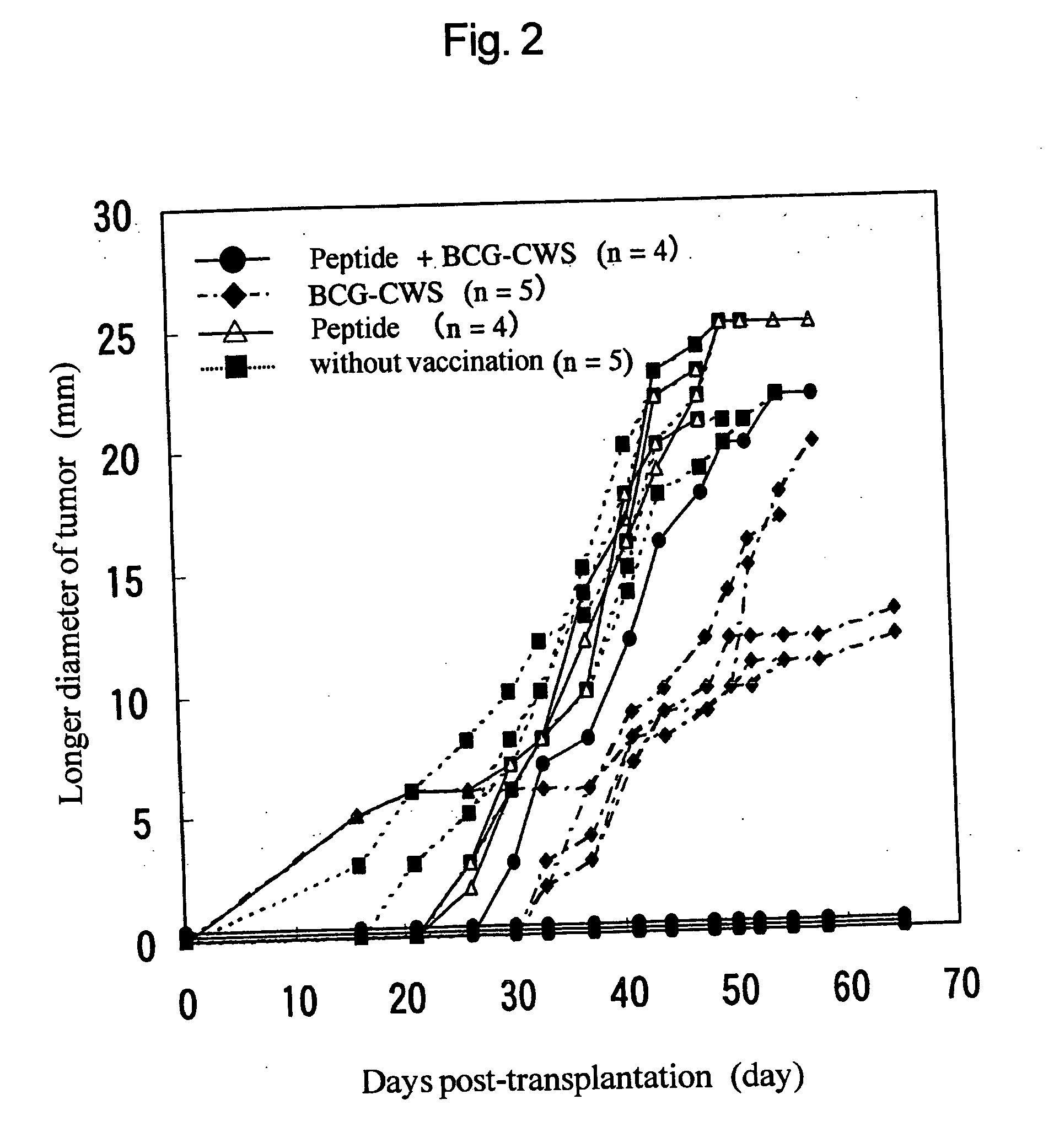
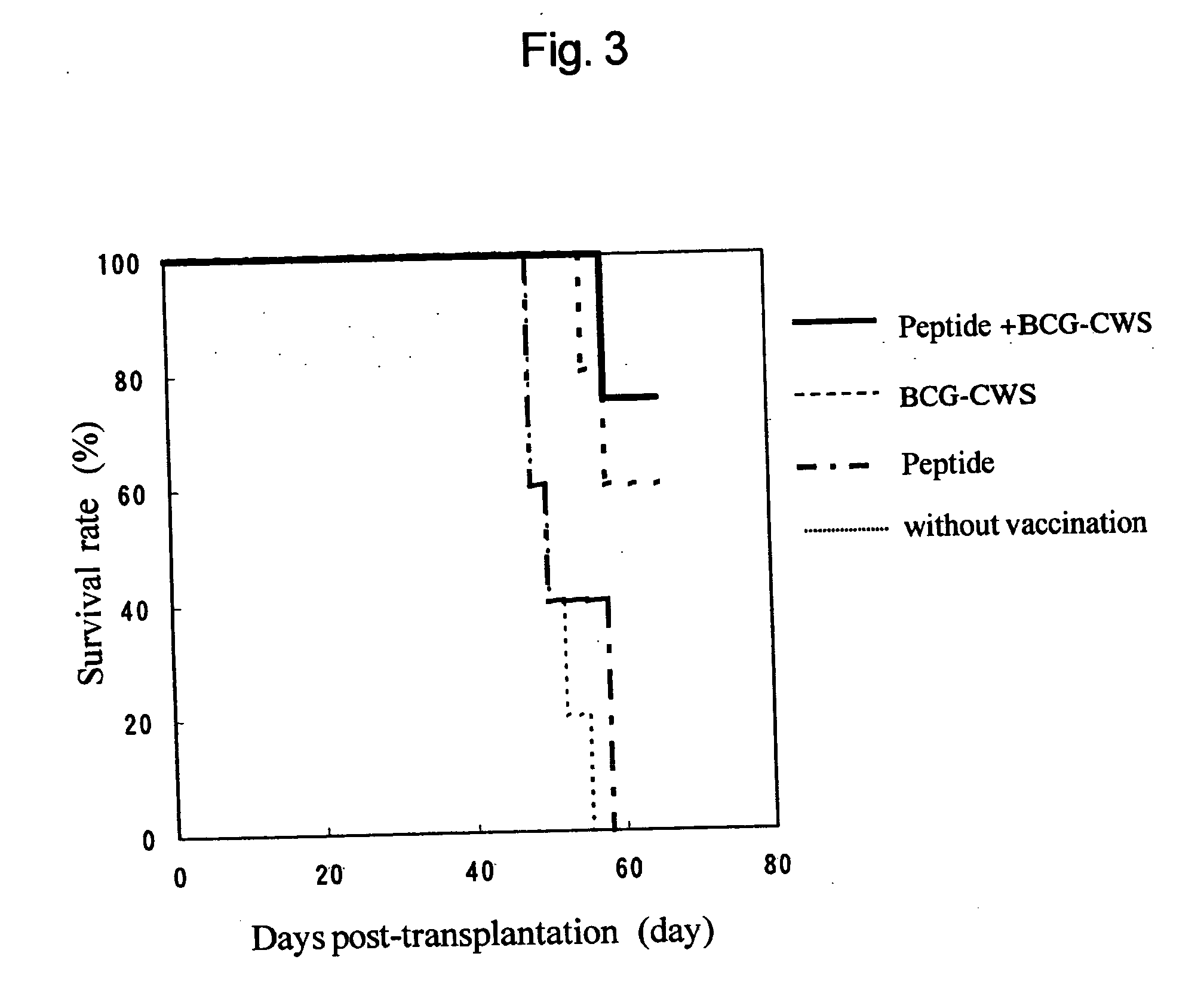
Novel method of inducing antigen-specific t cells
InactiveUS20050002951A1Efficient inductionBacterial antigen ingredientsCancer antigen ingredientsPharmaceutical drugCell Wall Skeleton
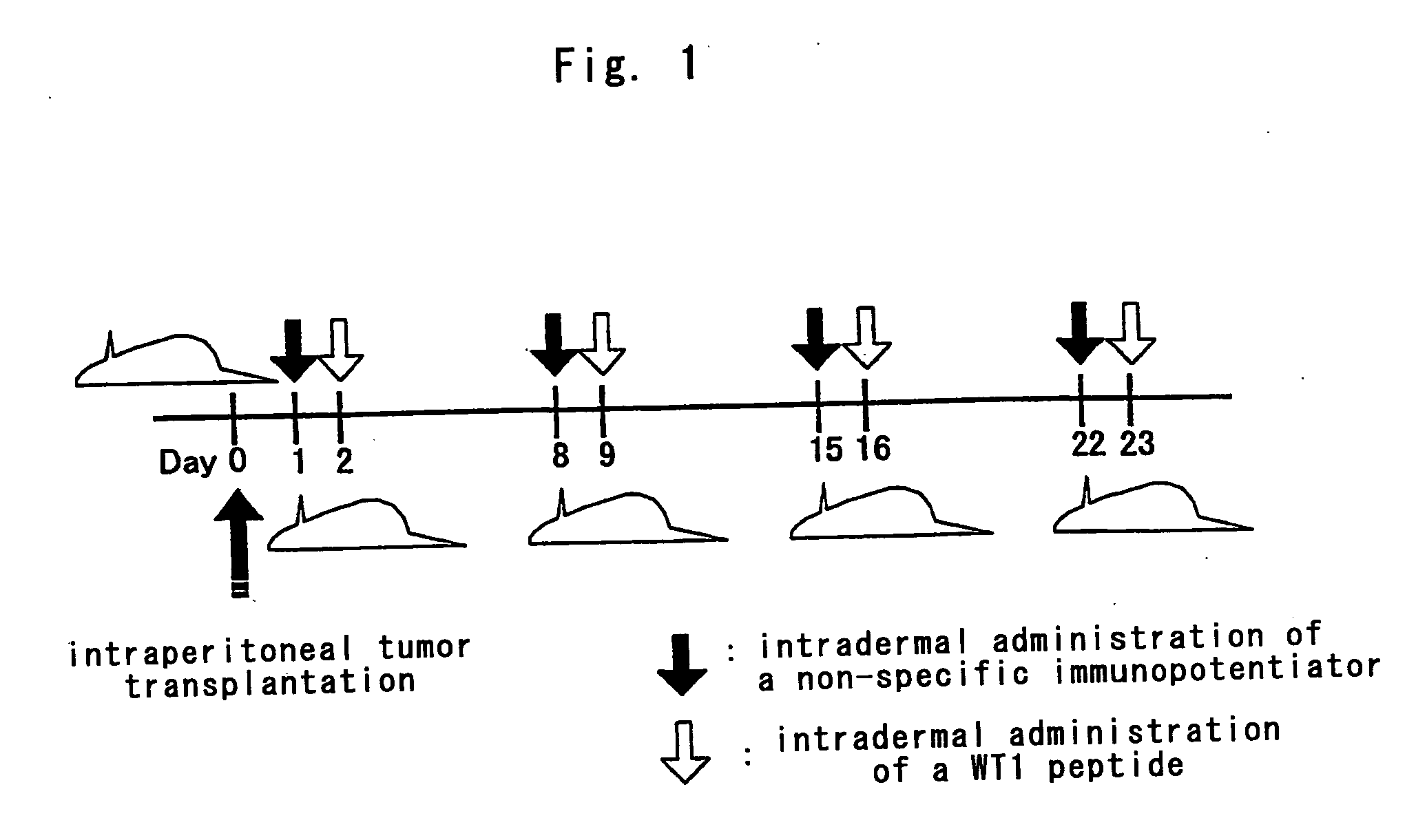
The present invention provides a novel method for inducing antigen-specific T cells. A method for inducing antigen-specific T cells in a patient comprising administering to said patient in need thereof composition (a) which comprises a therapeutically effective amount of an antigen protein or an antigen peptide as an active ingredient, and composition (b) which comprises a therapeutically effective amount of a cell wall skeleton integrant of the BCG strain of Mycobacterium bovis as an active ingredient, wherein composition (b) is administered in advance and then composition (a) is administered, and related pharmaceutical compositions are provided.
Owner:SUGIYAMA HARUO +1
Composition and method for the treatment of carcinoma
InactiveUS20070134273A1Good effectConvenient treatmentAntibacterial agentsBiocideMycobacterial antigenCompound (substance)
The present invention relates to compositions and methods useful for treating a carcinoma or viral infection in mammals, including humans. The methods and compositions typically comprise use of an immunogenic or immunomodulatory compound, and a gamma delta T cell activator, such that the composition is effective for treating a carcinoma or viral infection. In a preferred aspect of the invention, the methods comprise use of a gamma delta T cell activator and a Mycobacterium antigen, which for example is an attenuated strain of Mycobacterium bovis (Bacillus Calmette-Guerin (BCG)).
Owner:ROMAGNE FRANCOIS +1
Adjuvant combinations of liposomes and mycobacterial lipids for immunization compositions and vaccines
ActiveUS8241610B2Good auxiliary effectEnhance immune responseAntibacterial agentsBiocideLiposomeMycobacterium
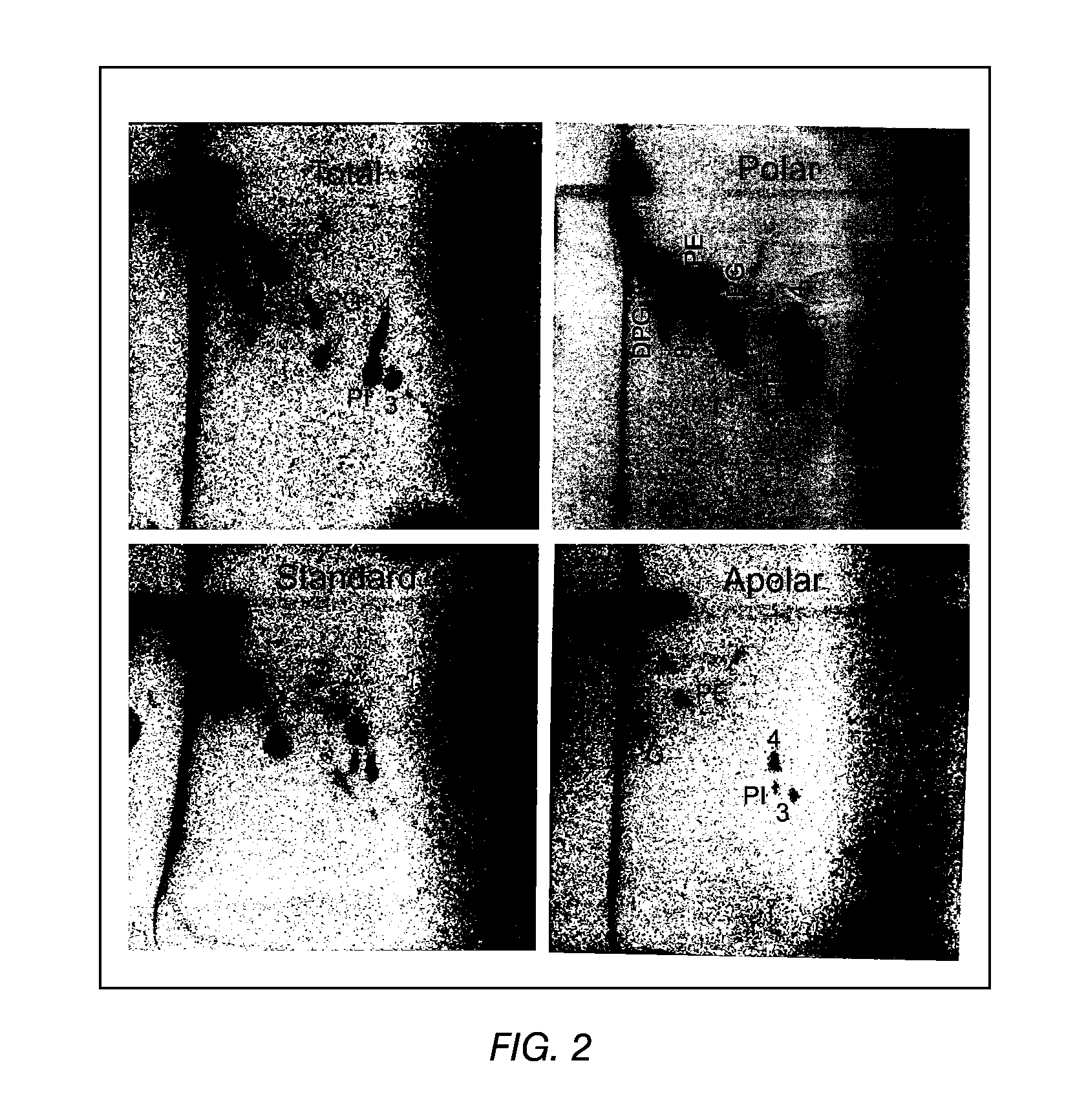
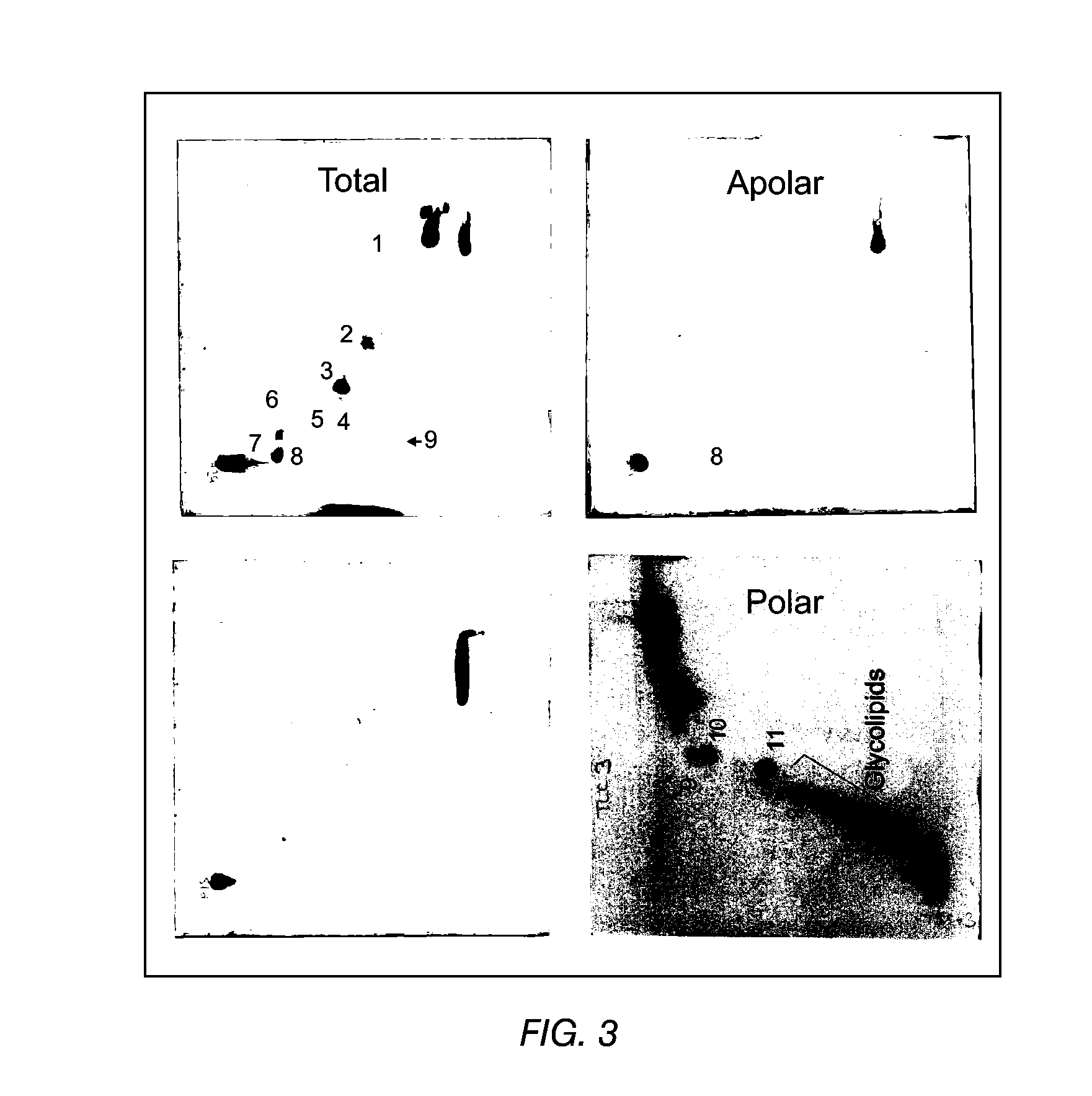
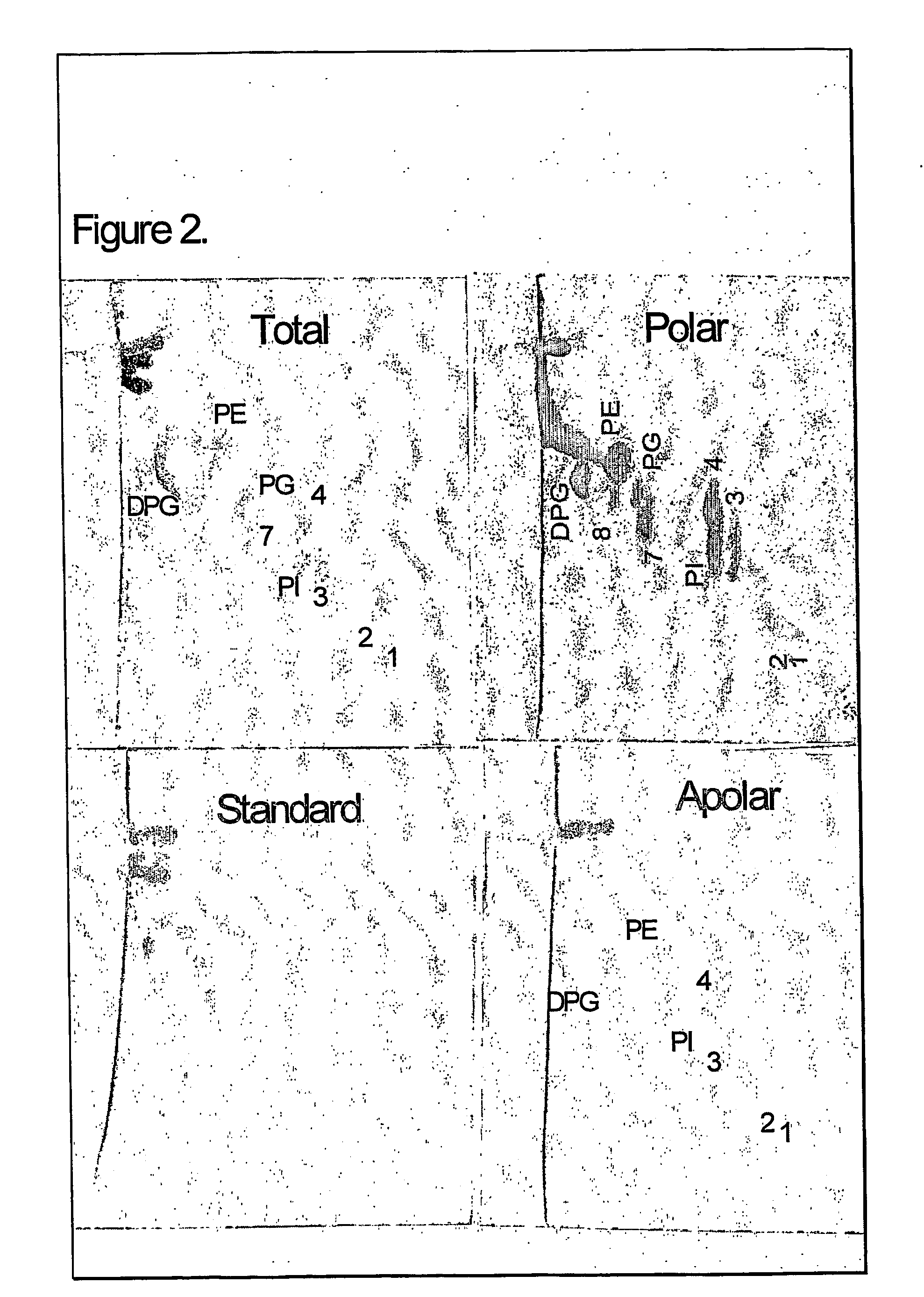
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:STATENS SERUM INST
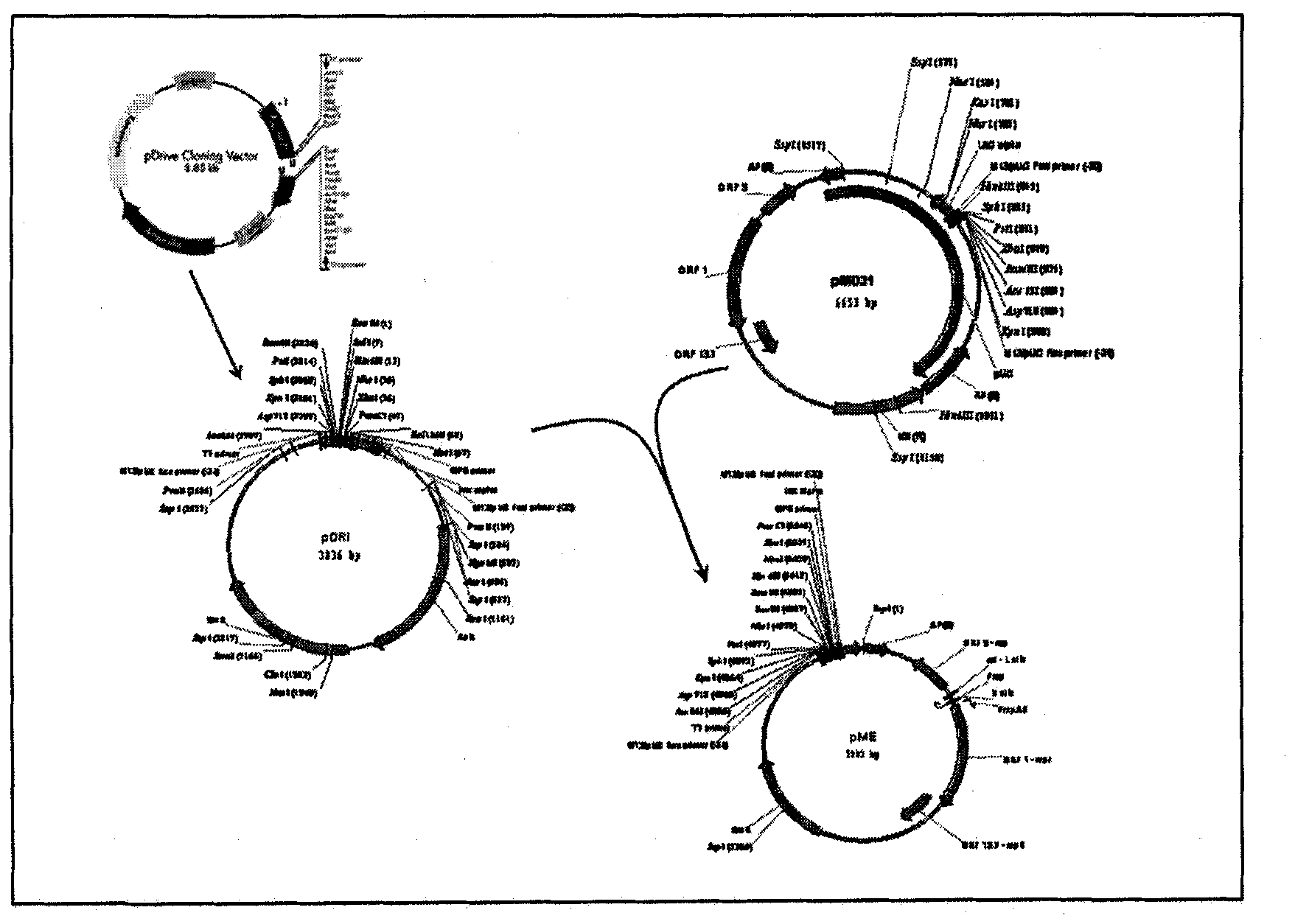
Method for isolating a polynucleotide of interest from the genome of a mycobacterium using a BAC-based DNA library application to the detection of mycobacteria
InactiveUS6183957B1Reducing potential for recombinationAvoiding lethal overexpressionSugar derivativesMicrobiological testing/measurementNucleotideGenomic DNA
The present invention relates to a method for isolating a polynucleotide of interest that is present in the genome of a first mycobacterium strain and / or is expressed by the first mycobacterium strain, where the polynucleotide of interest is also absent or altered in the genome of a second mycobacterium strain and / or is not expressed in the second mycobacterium. The method comprises: (a) contacting the genomic DNA of the first mycobacterium strain under hybridizing conditions with the DNA of a least one clone that belongs to a bacterial artificial chromosome (BAC) genomic DNA library of the second mycobacterium strain, and (b) isolating the polynucleotide of interest that does not form a hybrid with the DNA of the second mycobacterium strain. This invention further pertains to a Mycobacterium tuberculosis strain H37Rv genomic DNA library, as well as a Mycobacterium bovis BCG strain Pasteur genomic DNA library, and the recombinant BAC vectors that belong to those genomic DNA libraries. This invention also relates to a method, as well as a kit, for detecting a nucleic acid of a mycobacterium in a biological sample.
Owner:INST PASTEUR
Mycobacterial vaccine
Compositions and methods for enhancing the immunity of a subject or vaccinating a subject against mycobacterial infections are disclosed. The invention provides compositions comprising formalin inactivated cultures of a mycobacterium, such as M. bovis, and a Novasome® adjuvant, as well as methods for using such compositions.
Owner:NOVAVAX
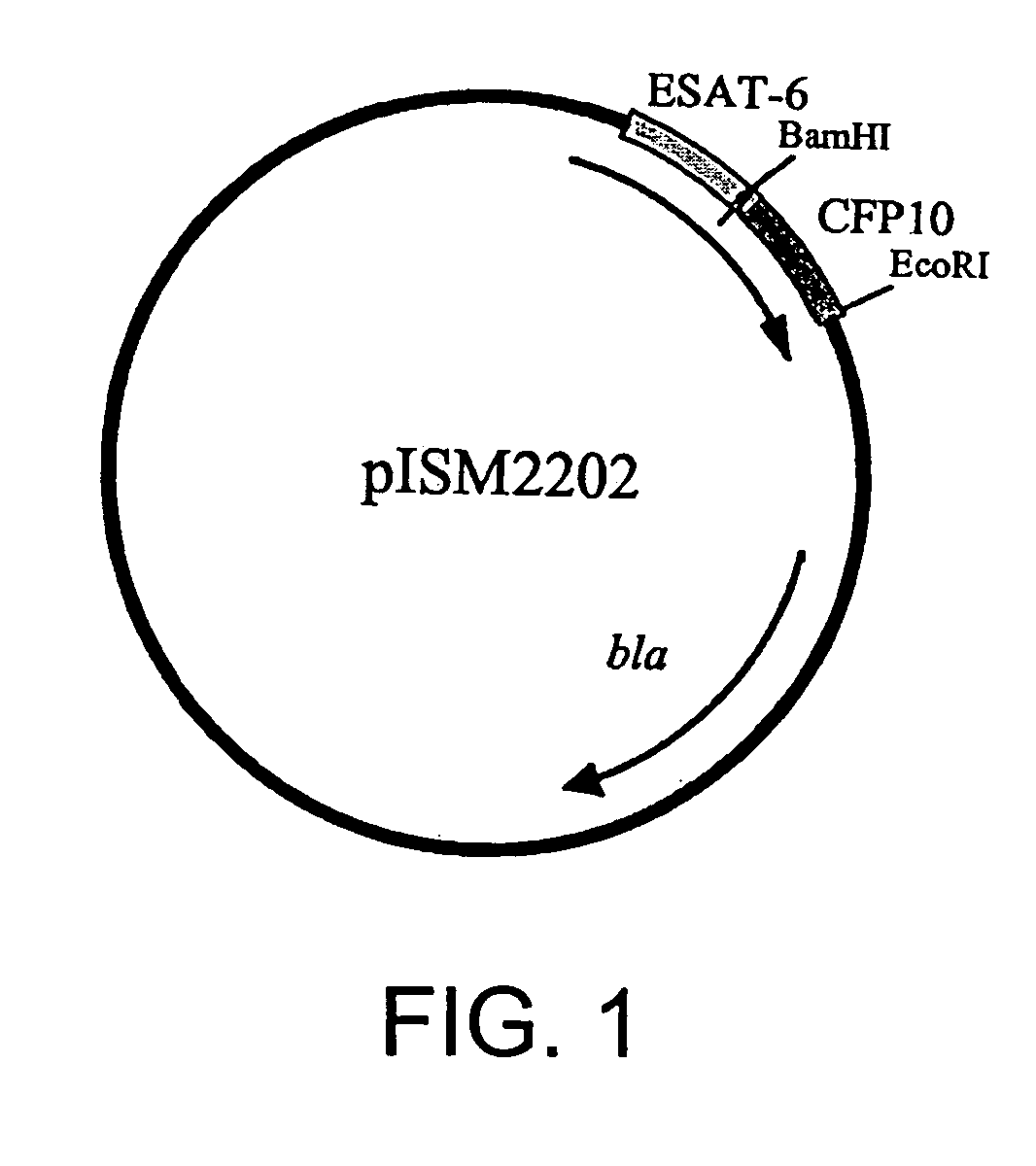
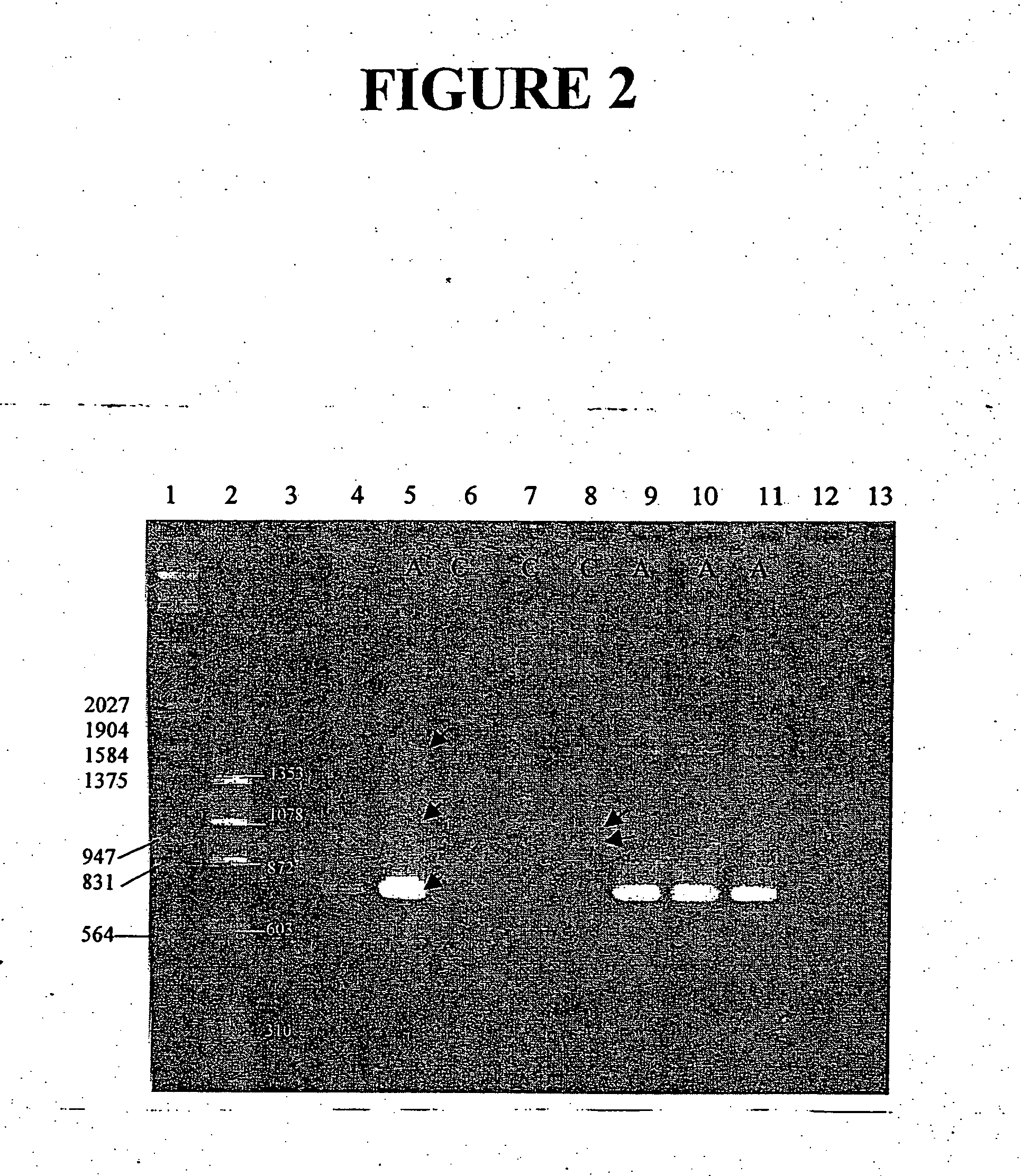
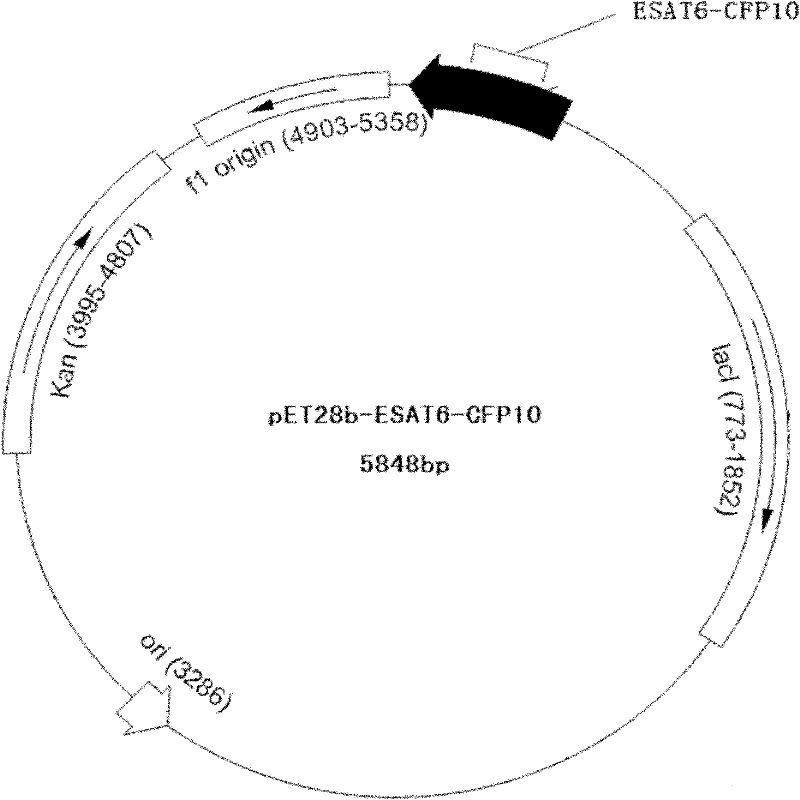
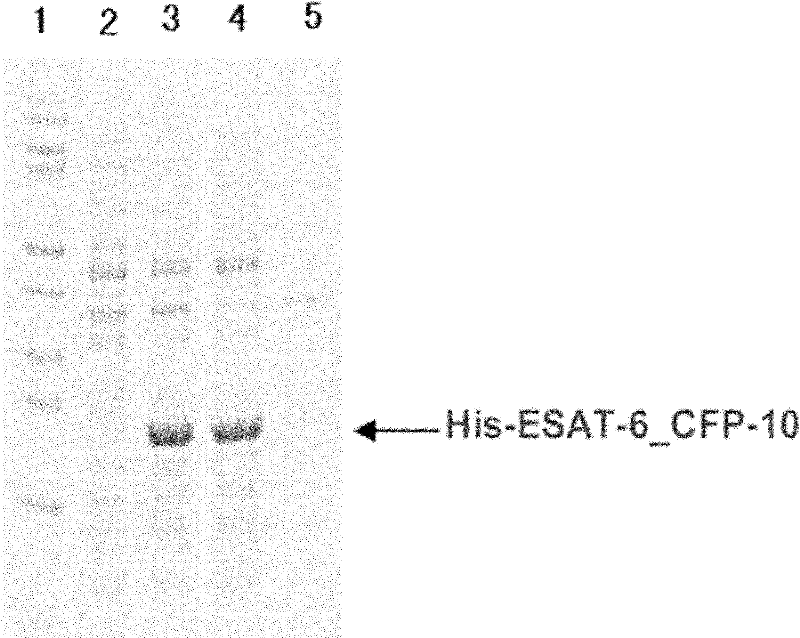
Recombinant ESAT-6:CFP-10 fusion protein useful for specific diagnosis of tuberculosis
InactiveUS20060024332A1Avoid unnecessary slaughterEasy to testBacterial antigen ingredientsAntibody mimetics/scaffoldsDiseaseNitric oxide
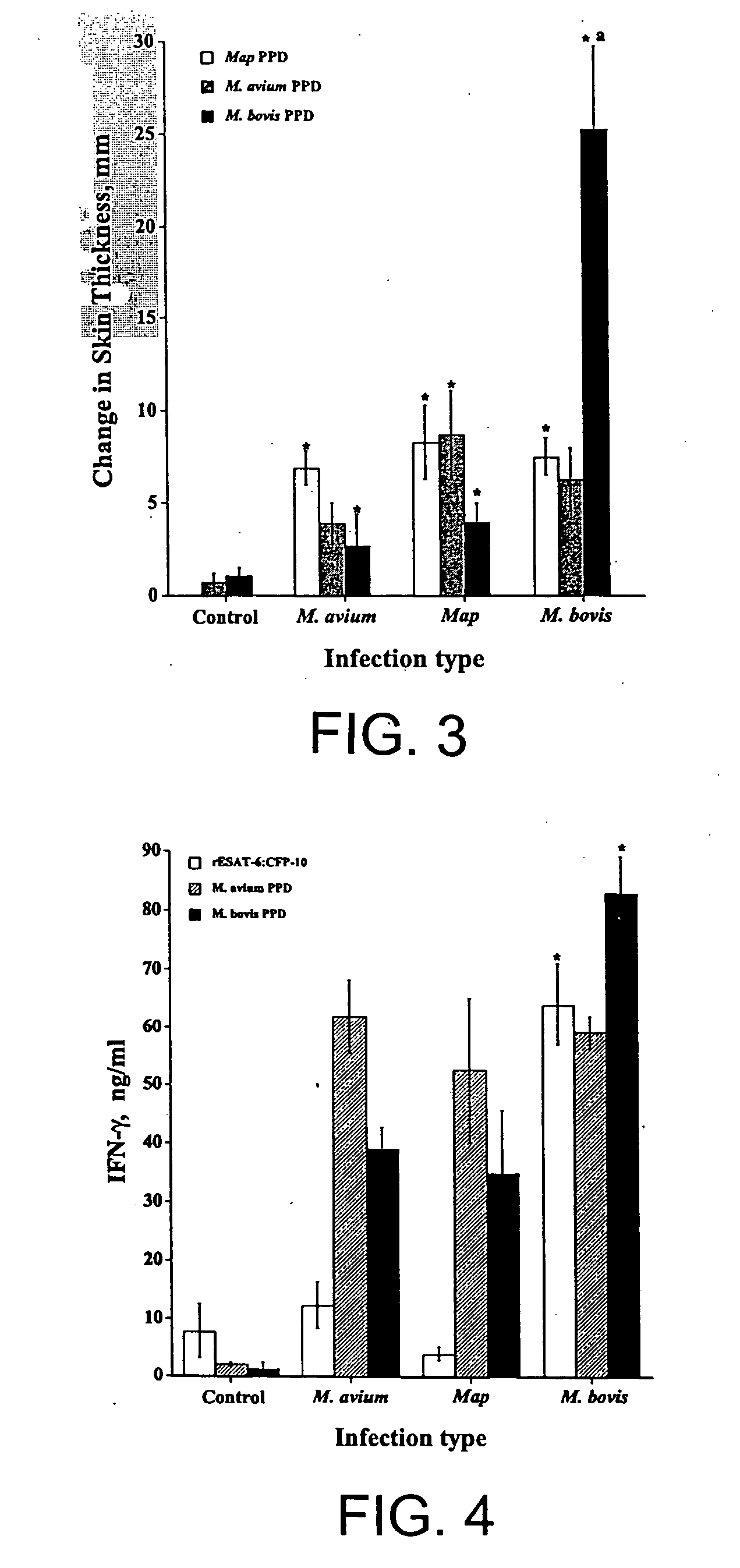
The fusion protein rESAT-6:CFP-10 is useful for differentiating infection of an animal with Mycobacterium bovis from exposure of the animal to other species of Mycobacteria, especially M. avium and M. avium subspecies paratuberculosis (Map). Cells stimulated with the fusion protein are capable of eliciting a variety of in vivo and in vitro responses (e.g. hypersensitivity skin response, IFN-γ, nitric oxide, and TNF-α) indicative of M. bovis infection. This invention will facilitate diagnosis of tuberculosis in cattle, reindeer and other susceptible animal species, thereby preventing unnecessary slaughter of uninfected animals suspected of having of the disease.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
Vaccines for Mycoplasma bovis and methods of use
The invention of novel, effective vaccines against Mycoplasma bovis for use in cattle is described. These vaccines demonstrate no undesirable side effects and protect against M. bovis related disease, such as contagious mastitis, respiratory pneumonia, joint infections, keratoconjunctivitis and middle ear infections. The novel vaccines also lessen the effect M. bovis infections on milk production, weight gain and animal health. Methods of diagnosing characterizing and treating M. bovis infections as specific biotypes are also disclosed. Vaccine compositions made in accordance with the invention may be either of the attenuated or inactivated variety. Vaccines may also include antigens from other pathogens so as to provide a protective immunogenic response to diseases other than those caused by M. bovis.
Owner:BIOMUNE
Detection kit for diagnosing tuberculosis
ActiveCN102175875ARapid diagnosisSpecific diagnosisBiological testingHybrid peptidesBiologyVaccine Immunogenicity
The invention relates to a detection kit for diagnosing tuberculosis, comprising a recombinant antigenic protein, wherein the recombinant antigenic protein is the fusion protein obtained by connecting ESAT-6 (early secreting antigen target 6KD), CFP-10 (cultufiltrate protein 10KD), and MPB64 (Mycobacterium bovis 64KD) by connecting peptides according to an arbitrary sequence, wherein the connecting peptides are short peptides which do not influence the immunogenicity of ESAT-6, CFP-10 and MPB64. Preferably, the recombinant antigenic protein in the detection kit for diagnosing tuberculosis is the antigenic protein with an amino acid sequence which is SEQ ID: No.1. When the detection kit in the invention is used for diagnosing tuberculosis, the diagnosis time is short, the diagnosis is fast, the sensitivity is strong, and the specificity is high; and the detection kit has an important significance on early diagnosis of tuberculosis.
Owner:武汉海吉力生物科技有限公司
Quick identifying method and use for new low mycobacterium and tubercle mycobacterium
InactiveCN1715422ASolve the identification problemHigh sensitivityMicrobiological testing/measurementBacteroidesMicrobiology
The quick method of identifying bovine mycobacterium and tubercle mycobacterium includes: adding bovine mycobacterium Vallee strain genome DNA to proliferate one 294 bp segment, detecting target gene from bovine mycobacterium DNA with bovine mycobacterium primer, adding tubercle mycobacterium H37vR strain genome DNA to proliferate one 294 bp segment, proliferating target genome from tubercle mycobacterium DNA with tubercle mycobacterium primer, adopting the same upstream primer and different downstream primers and adopting two gene segments of 294 bp and annealing temperature of 67 deg c. The method of present invention is used in the direct detection of bacterial cultured matter and clinical sample.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
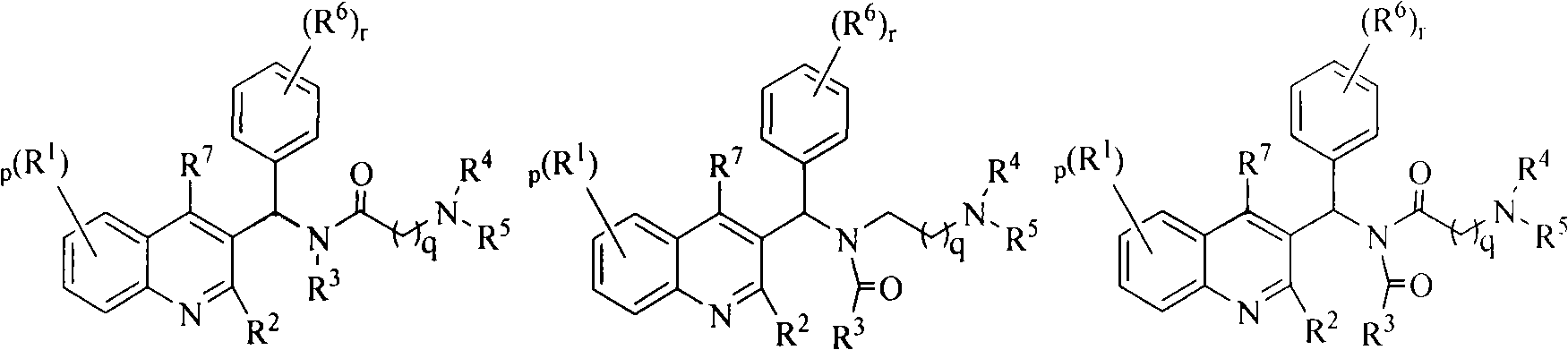
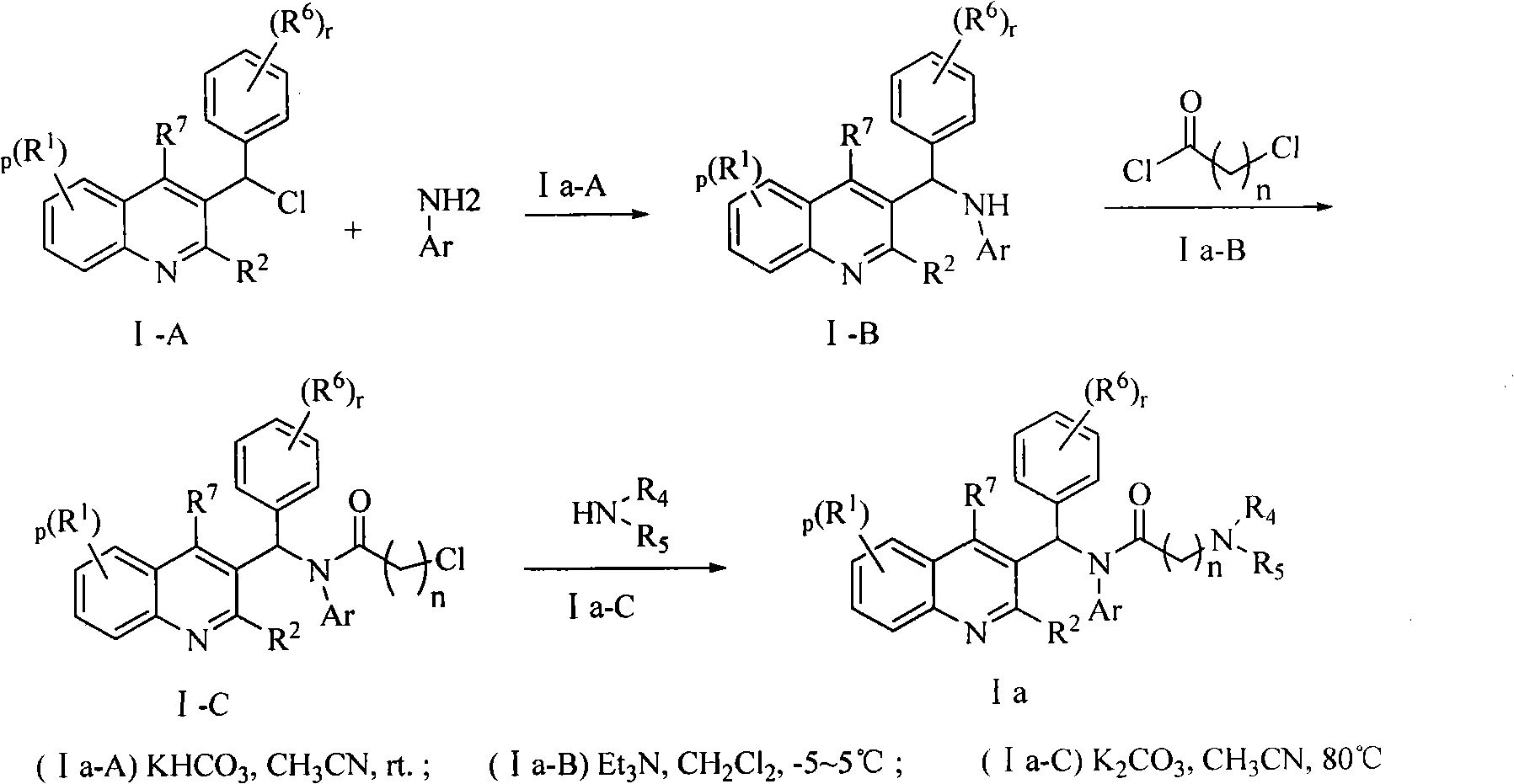
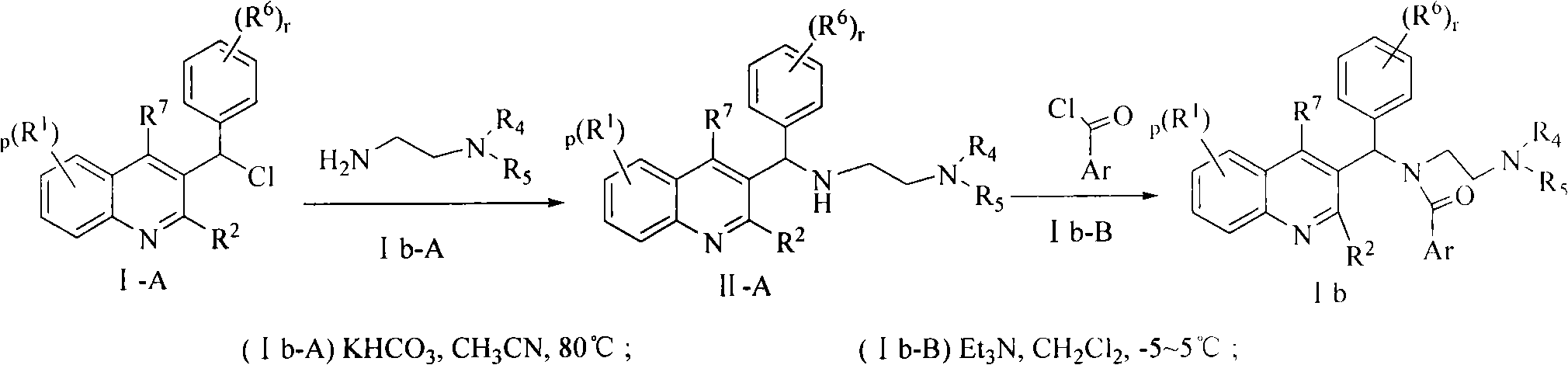
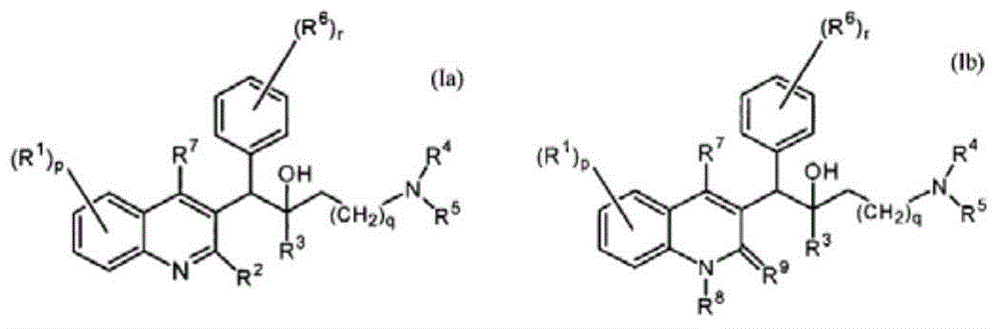
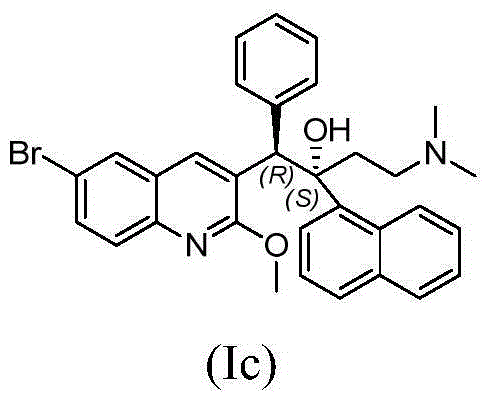
Quinoline derivatives, preparation method and applications thereof
InactiveCN101525316AGrowth inhibitionGood anti-tuberculosis activityAntibacterial agentsOrganic chemistryDiseaseHydrogen
The invention relates to new substituted quinoline derivatives presented in general formula (1a), or general formula (1b) or general formula (1c), a medical acid added salt, a stereochemical heterogeneous formula and a tautomeric form thereof. The compound can be used for curing mycobacterium diseases, in particular to diseases caused by pathogenic mycobacteria, such as mycobacterium tuberculosis, mycobacterium bovis, mycobacterium avium and mycobacterium marinum and is particularly defined as following: R1 is mutually independent bromines; p is equal to 1, R2 is an alkoxy, and R3 is a randomly substituted phenyl or naphthyl; q is equal to 1, R4 and R5 are mutually independent hydrogen and a methyl or an ethyl, and R6 is hydrogen; r is equal to 0 or 1 and R7 is hydrogen. The invention also relates to a composition of the compound, applications of the compound of the composition to medicines for curing the mycobacterium diseases, and a method for preparing the compound of the composition, which comprises a medical carrier and is used as activated ingredient of curable effective quantity.
Owner:SHENYANG PHARMA UNIVERSITY
Mycobacterial vaccine
Compositions and methods for enhancing the immunity of a subject or vaccinating a subject against mycobacterial infections are disclosed. The invention provides compositions comprising formalin inactivated cultures of a mycobacterium, such as M. bovis, and a Novasome® adjuvant, as well as methods for using such compositions.
Owner:NOVAVAX
Multiplex polymerase chain reaction (PCR) kit for identifying mycobacterium tuberculosis
InactiveCN102533959ASimple and fast operationEasy to operateMicrobiological testing/measurementSequence designAcid-fast
Owner:HUAZHONG AGRI UNIV
Immunological adjuvant with immunity vegulating agent for treating and preventing diabetic from insulin-dependent
InactiveCN1831012AReduce immune damageDoes not induce atherosclerosisPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesInsulin dependent
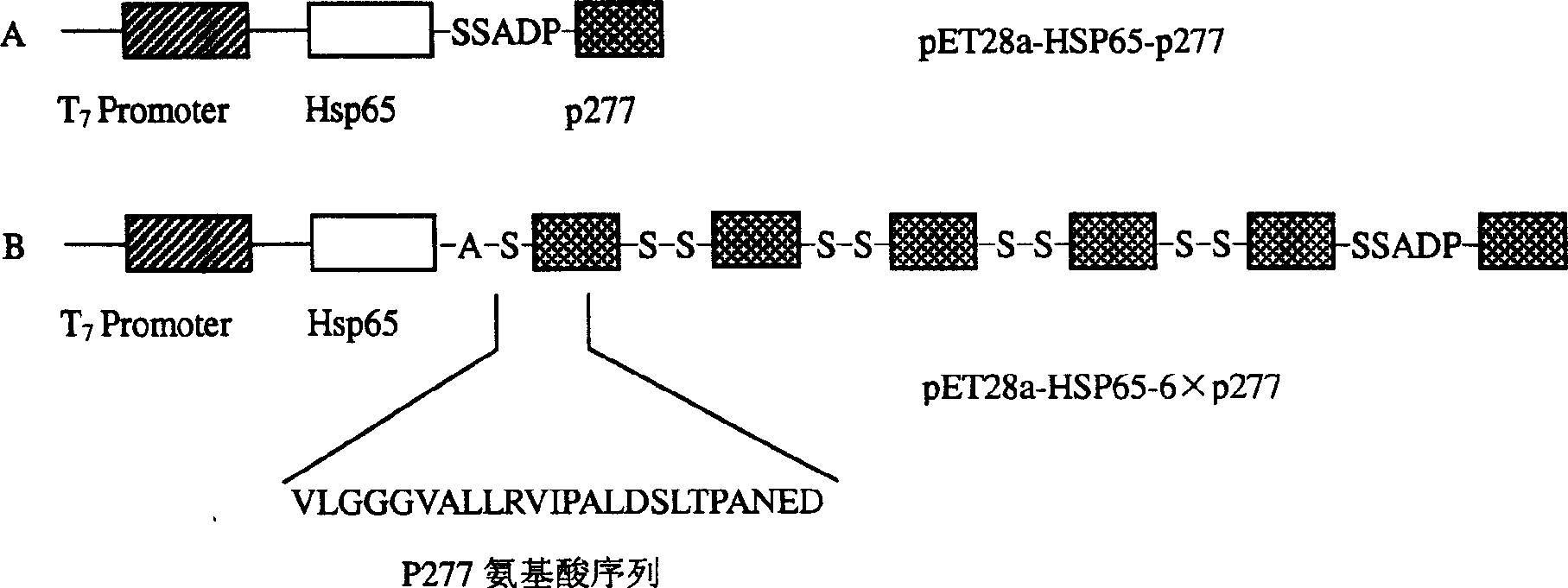
The invention provides the immunomodulator with the free adjuvant having the function of preventing and curing the insulin-dependent diabetes mellitus. Aiming at the weak immunogenicity, the antigen epitope polypeptide gene (a length of antigen epitope p277 rooted from human HSP60) is associated repeatedly six times and inserted repeatedly into the backward position of the heat shock protein HSP65 gene rooted from the mycobacterium bovis, the fusing expression between the repeat series connecting antigen polyeptide and the HSP65 is realized. The produced fusing albumen did not need the adjuvant; the antigen polyeptide don't need be coupled with the carrier chemistry to the immunity. The fusing albumen can inspire the organism to produce the high titer aiming at p277 by means of the mucous membrane immunity, so the nosogeny of NOD chmice diabetic are depressed highly. The invention can prevent the 1 type and 1.5 type diabete characterized in depending on the insulin.
Owner:CHINA PHARM UNIV
Adjuvant combinations of liposomes and mycobacteriaial lipids for immunization compositions and vaccines
InactiveUS20060286128A1High protective immune responseIncreased protective immune responseAntibacterial agentsBacterial antigen ingredientsLipid formationActive agent
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldioctadecylammonium-bromide / chloride (DDA) and a lipid extract from The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from <The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis. The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis<The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG <The present invention provides a vaccin adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from <i>Mycobacterium bovis BCG<i>. <The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from <i>Mycobacterium bovis BCG<i>. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:AGGER ELSE
Detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and method thereof
ActiveCN101533018AStrong characteristicIncreased sensitivityHybrid peptidesMaterial analysisBCG immunizationMycobacterium Infections
The invention belongs to the field of immunodetection and relates to a detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and a method thereof. The detection reagent comprises combined fusion protein rE6-M63-H70 used as a specific stimulation origin, the combined fusion protein can effectively stimulate sensitized peripheral blood lymphocyte cultured in vitro to release Gamma-interferon (IFN-Gamma) at a high level. The cow IFN-Gamma release test established by using the detection reagent rE6-M63-H70 combined fusion protein as the stimulation origin overcomes the insufficiencies of serology detection method and the IFN-Gamma release test with PPD as the stimulation origin, thus enjoying very high sensitivity and specificity and distinguishing cow pathogenic mycobacteria ( such as mycobacterium bovis) infection from non-pathogenic mycobacteria (such as mycobacterium avium or non-pathogenic mycobacteria) infection and even distinguishing the cow pathogenic mycobacteria infection from BGG immunity; therefore, the detection kit and the method of the invention can be effectively used to detect the clinical cow pathogenic mycobacteria infection.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Mycobacterium mutants for vaccines with improved protective efficacy
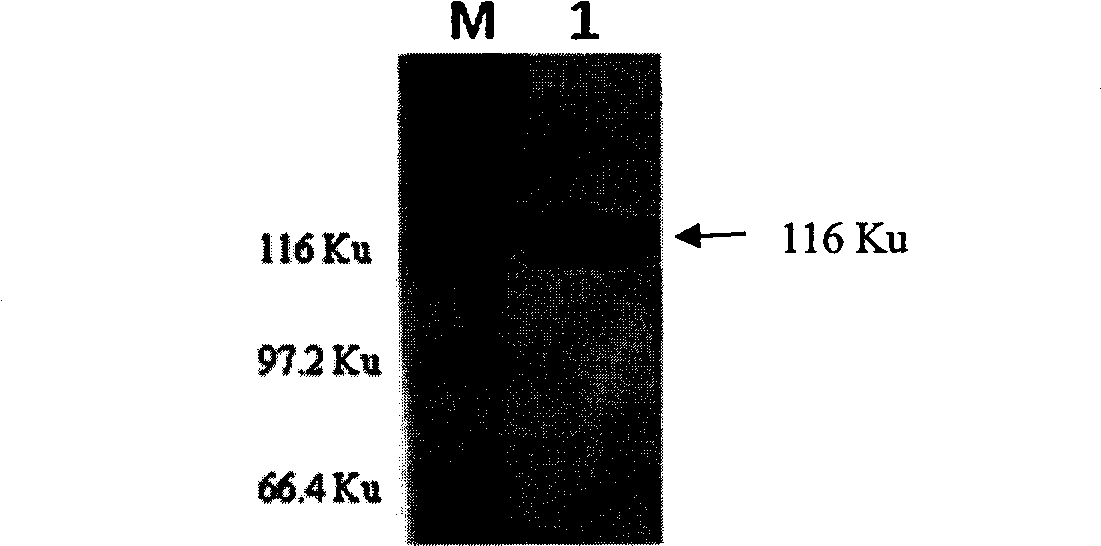
Tuberculosis (TB) is a major health problem and currently, the only licensed TB vaccine is Mycobacterium bovis Bacille Calmette-Guerin (M. bovis BCG). In the present invention, mutation of mycobacterial components reportedly involved in phagosome maturation inhibition was evaluated for vaccine purposes, as such mutations should result in better vaccine antigen processing and presentation. Thus, BCG mutants in genes coding for ManLAM capping a-1,2-mannosyltransferases and the PI3P phosphatase SapM were evaluated as TB vaccines in a stringent mouse model. Vaccination with both ManLAM capping mutants and the SapM mutant resulted in significantly longer survival as compared to non-vaccinated mice, whereas vaccination with the parental BCG did not. Moreover, mice vaccinated with the SapM mutant survived significantly longer than mice vaccinated with the parental BCG.; The mutant BCG strains showed unaltered phagocytosis, replication, lysosome colocalization and oxidant activity in macrophages and similarly induced autophagy in the latter. Additionally, replication and granuloma formation in mice was unaffected, indicating BCG-equivalent safety of these vaccines.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
Mycobacterium antigens
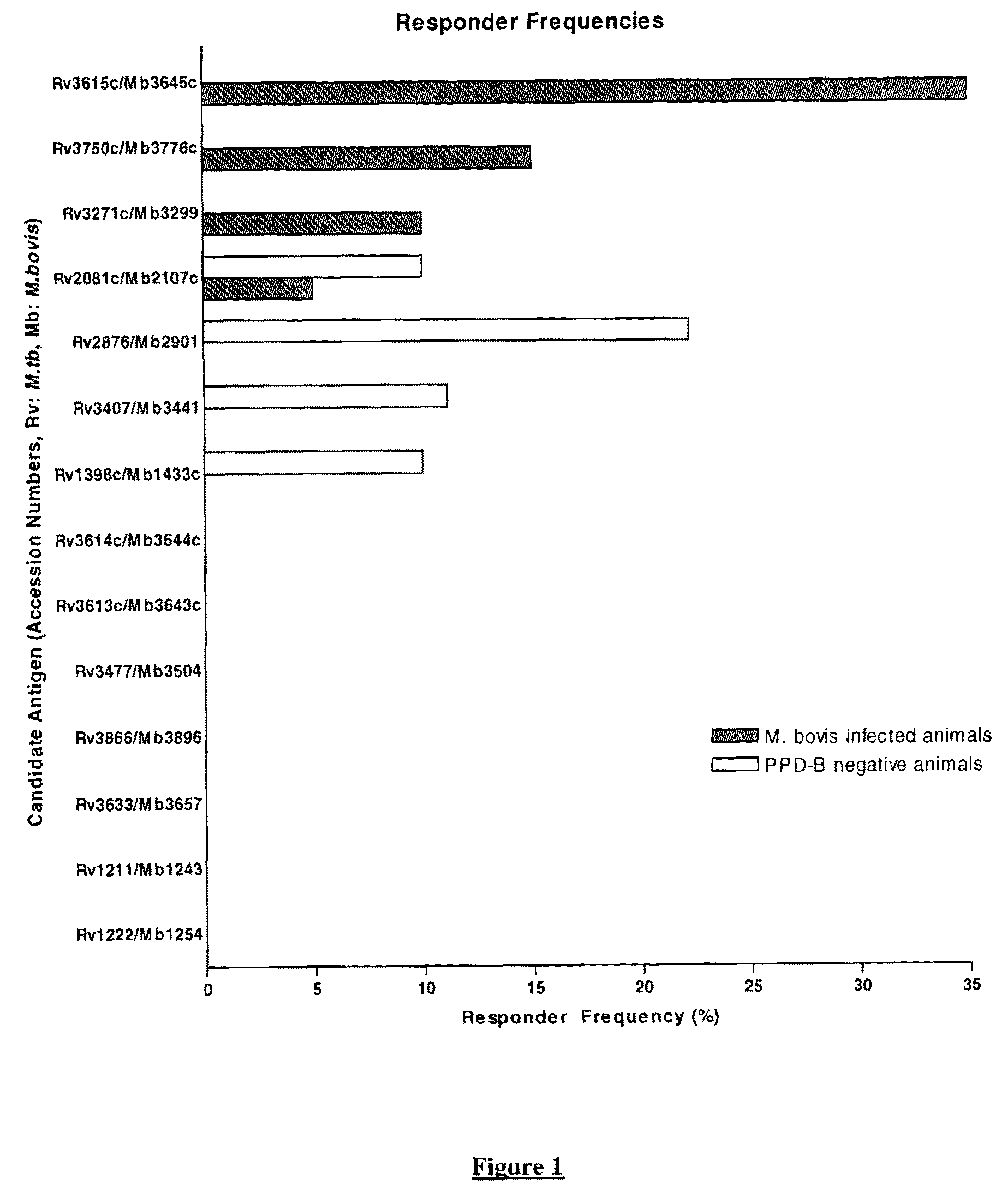
There is provided a diagnostic reagent for use in the detection of M. bovis or M. tuberculosis infection in an animal, comprising a peptide which has an epitope from Mycobacterium bovis hypothetic protein Mb3645c (SEQ ID NO: 1) or an epitope from a polypeptide having at least 76% identity with SEQ ID NO: 1.
Owner:THE UK SEC FOR ENVIRONMENT FOOD & RURAL AFFAIRS
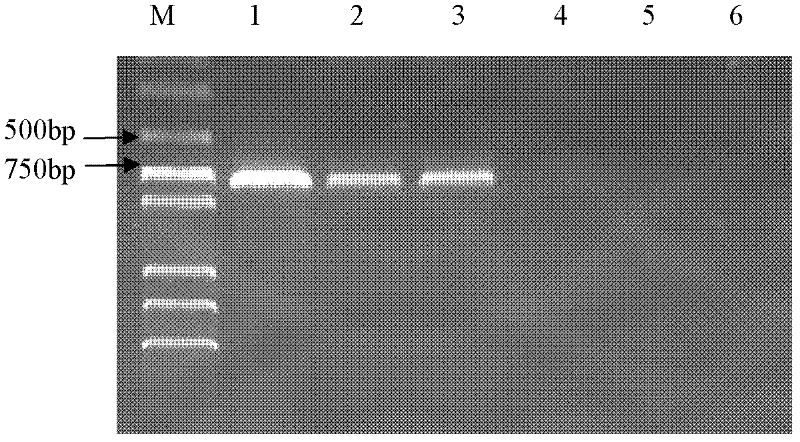
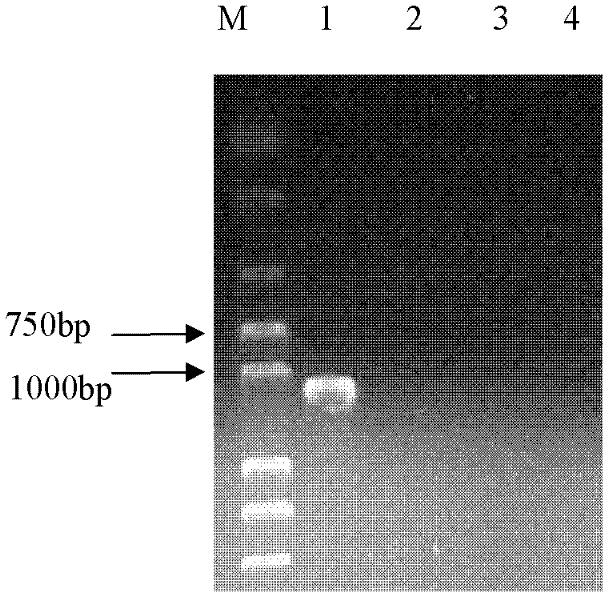
PCR(Polymerase Chain Reaction) primers and method for identifying mycobacterium bovis
InactiveCN102409102ASimple and precise identificationEasy to operateMicrobiological testing/measurementMicroorganism based processesBacteroidesNucleotide
The invention provides five pairs of primers and an identification method for PCR(Polymerase Chain Reaction) identification of mycobacterium bovis. The five pairs of primers respectively amplify aiming at a 16SrRNA conserved region of mycobacterium bacteria, a mycobacterium tuberculosis complex(MTBC)Rv0577 gene, Rv1970 of a mycobacterium tuberculosis RD7 region, a mycobacterium bovis pncA gene and an RD1 region gene to generate specific amplified fragments, and the nucleotide sequences of the specific amplified fragments are as shown in SEQ ID No.1-5. According to the identification method provided by the invention, the total DNA of a sample is taken as a template, PCR amplification is respectively performed by the five pairs of primers, and the result is judged according to the size of an amplified band. The primers provided by the invention can specifically identify mycobacteria, MTBC, mycobacterium tuberculosis, mycobacterium bovis and mycobacterium bovis BCG(Bacillus Calmette-Guerin), and the detection method has good sensitivity and simplicity of method and operation, and can realize quick large-flux detection of mycobacterium bovis.
Owner:CHINA AGRI UNIV
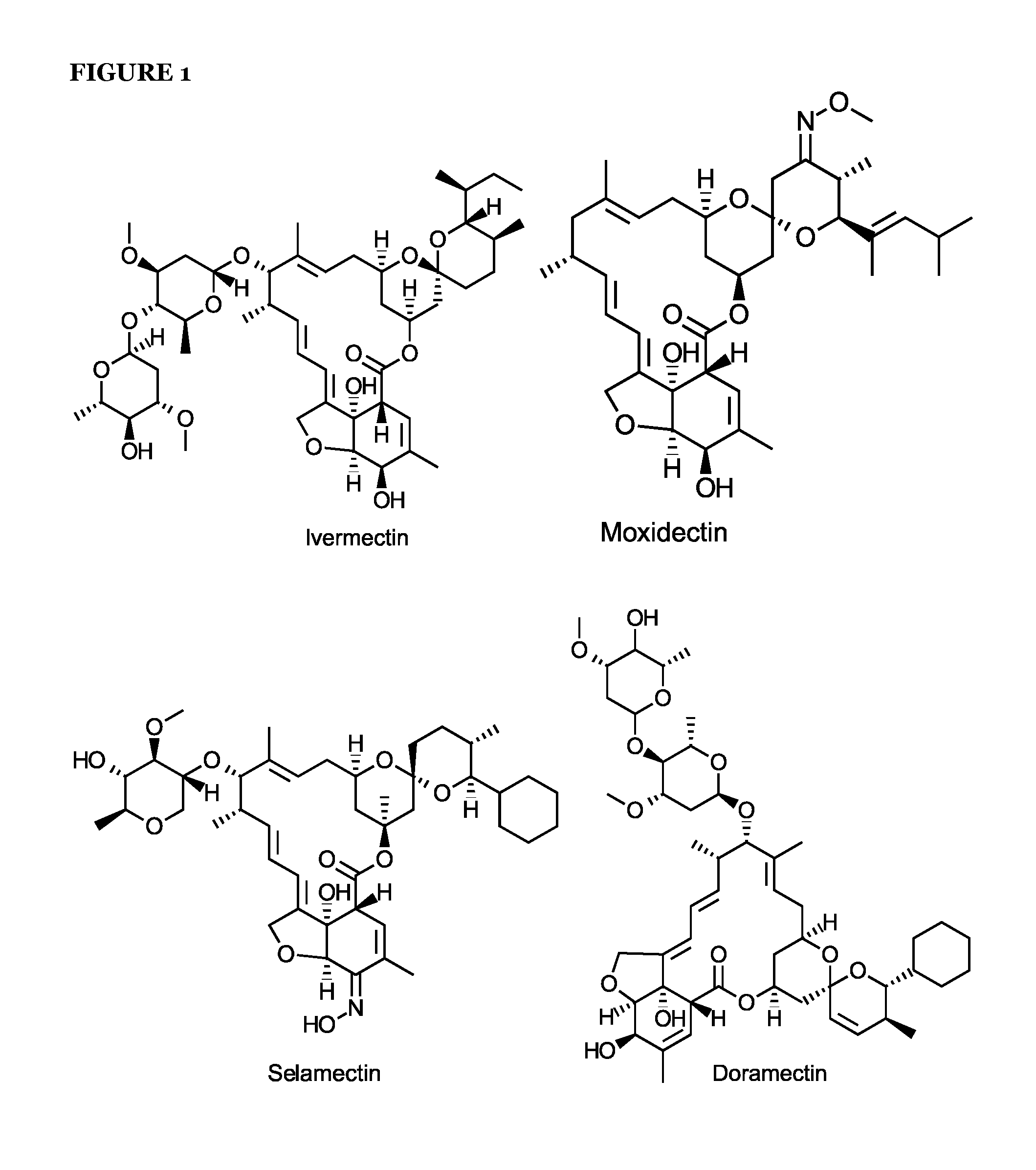
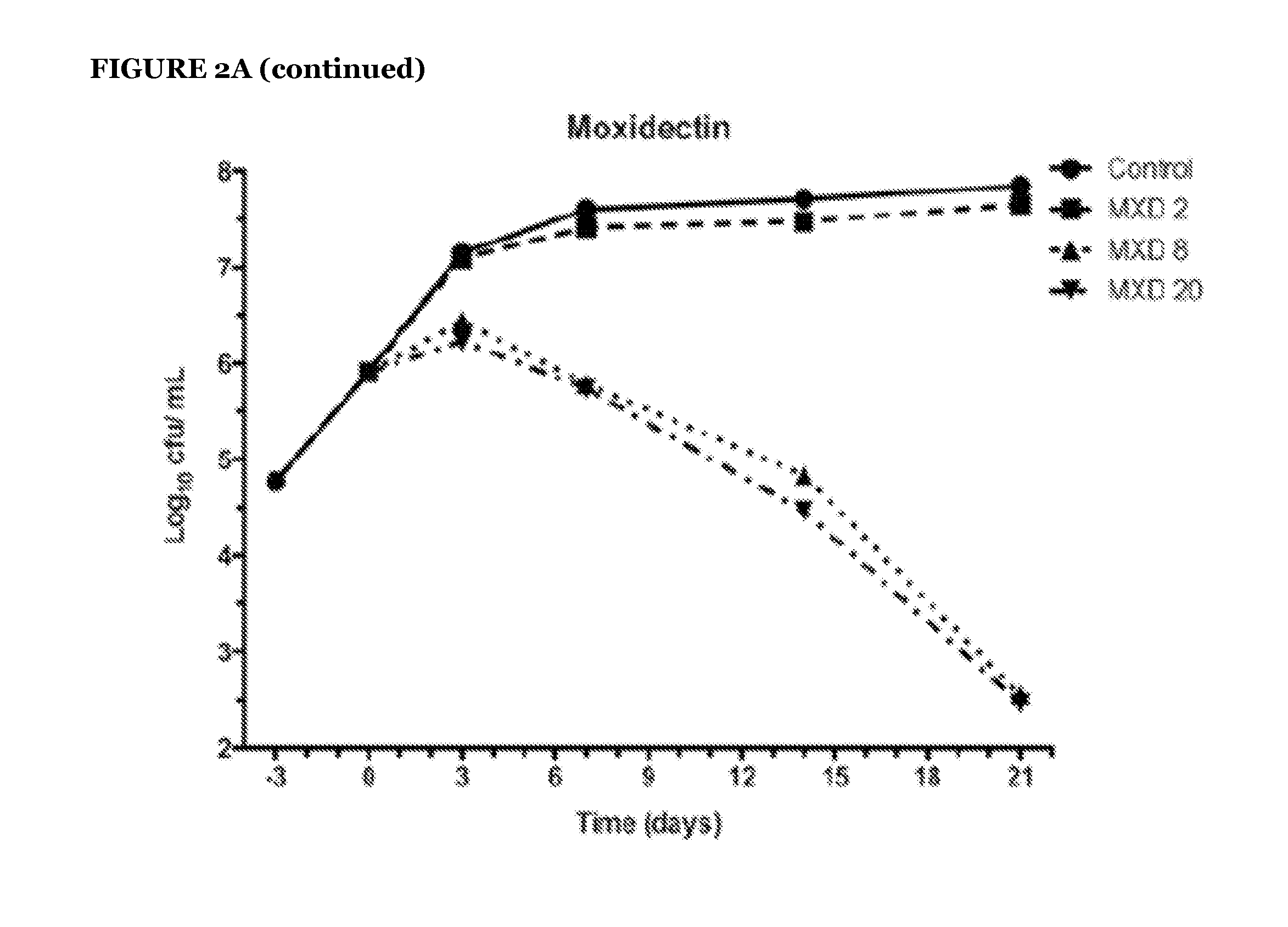
Avermectins and Milbemycins as Anti-Mycobacterial Agents
ActiveUS20140315842A1BiocideCarbohydrate active ingredientsMycobacterium ulceransMycobacterium Infections
The present invention relates to methods of treating a mycobacterial infection. In particular, this invention relates to methods, uses and compounds for use in treating mycobacterial infections, including tuberculosis. For example, the compounds may be an avermectin or a milbemycin. The avermectin or milbemycin may be selected from, but not limited to, one or more of ivermectin, moxidectin or selamectin. Exemplary microbial infections that may be treated include, but are not limited to, infections caused by Mycobacterium tuberculosis, Mycobacterium bovis, other mycobacteria of the tuberculosis complex, and non-tuberculous mycobacteria, including Mycobacterium ulcerans.
Owner:THOMPSON CHARLES J +2
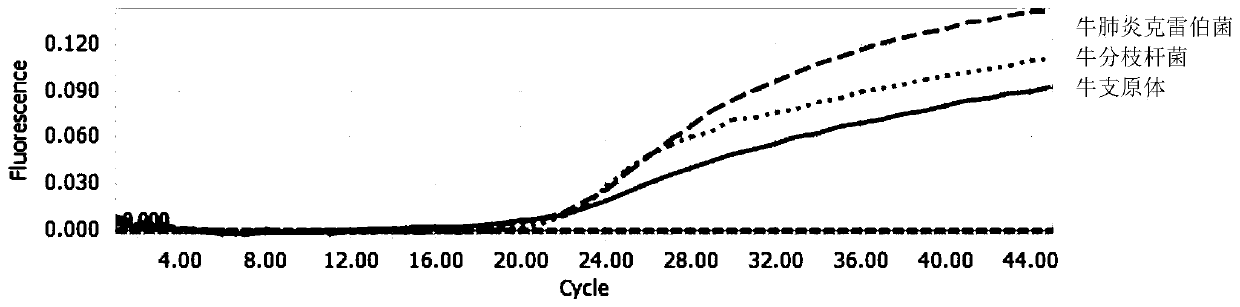
Multi-fluorescent quantitative PCR reagent kit capable of synchronously detecting three bovine respiratory pathogens, and multi-fluorescent quantitative PCR detection method capable of synchronously detecting three bovine respiratory pathogens
InactiveCN110016512AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseaseBovine respiratory disease
The invention belongs to the technical field of livestock disease detection, and discloses a multi-fluorescent quantitative PCR reagent kit capable of synchronously detecting three bovine respiratorypathogens as well as a multi-fluorescent quantitative PCR detection method capable of synchronously detecting the three bovine respiratory pathogens, wherein the three bovine respiratory pathogens arerespectively mycobacterium bovis, mycoplasma bovis and Klebsiella pneumoniae. According to the multi-fluorescent quantitative PCR detection method capable of synchronously detecting the three bovinerespiratory pathogens, 3 groups of specific primers and probes are designed and synthesized on basis of a specific sequence 229bp of the mycobacterium bovis, a uvrC gene of the mycoplasma bovis and aKhe gene of the Klebsiella pneumoniae, thereby establishing the multi-fluorescent quantitative PCR detection method capable of synchronously detecting the mycobacterium bovis, the mycoplasma bovis andthe Klebsiella pneumoniae which are associated with respiratory diseases in cattle. The multi-fluorescent quantitative PCR detection method is capable of synchronously detecting the 3 bacteria; and sensitivity, repeatability and specificity tests have proven that the detection method is high in sensitivity, strong in specificity and relatively good in repeatability.
Owner:CHINA AGRI UNIV
Modified bcg strains with reduced or eliminated activity of lsr2 and pharmaceutical composition comprising same
The invention discloses a live modified Mycobacterium bovis�BCG strain in which the lsr2 gene is inactivated or its expression is reduced and a pharmaceutical composition comprising the same for the treatment or prophylaxis of a mammal against challenge by mycobacteria or against cancer. The invention further discloses a method for the treatment or prophylaxis of a mammal against challenge by Mycobacterium tuberculosis or Mycobacterium bovis or against cancer by administering to the mammal the live modified Mycobacterium bovis�BCG strain or the pharmaceutical composition of the present invention.
Owner:CHENGDU YONGAN PHARMA CO LTD +1
Tuberculosis vaccines including recombinant bcg strains overexpressing phop, and/or phop regulon protein(s)
A live recombinant Mycobacterium bovis-BCG strain comprising a nucleic acid encoding PhoP protein and / or one or more phoP regulon proteins is provided, in which the nucleic acid is capable of being overexpressed. A vaccine or immunogenic composition comprising the live recombinant Mycobacterium bovis-BCG strain and a method for treatment or prophylaxis of a mammal against challenge by Mycobacterium tuberculosis or Mycobacterium bovis are also provided.
Owner:成都安永鼎业生物技术有限公司
Pcr-based genotyping
InactiveUS20110059437A1Improve analysisHigh sample throughputSugar derivativesMicrobiological testing/measurementInsertion sequenceBiology
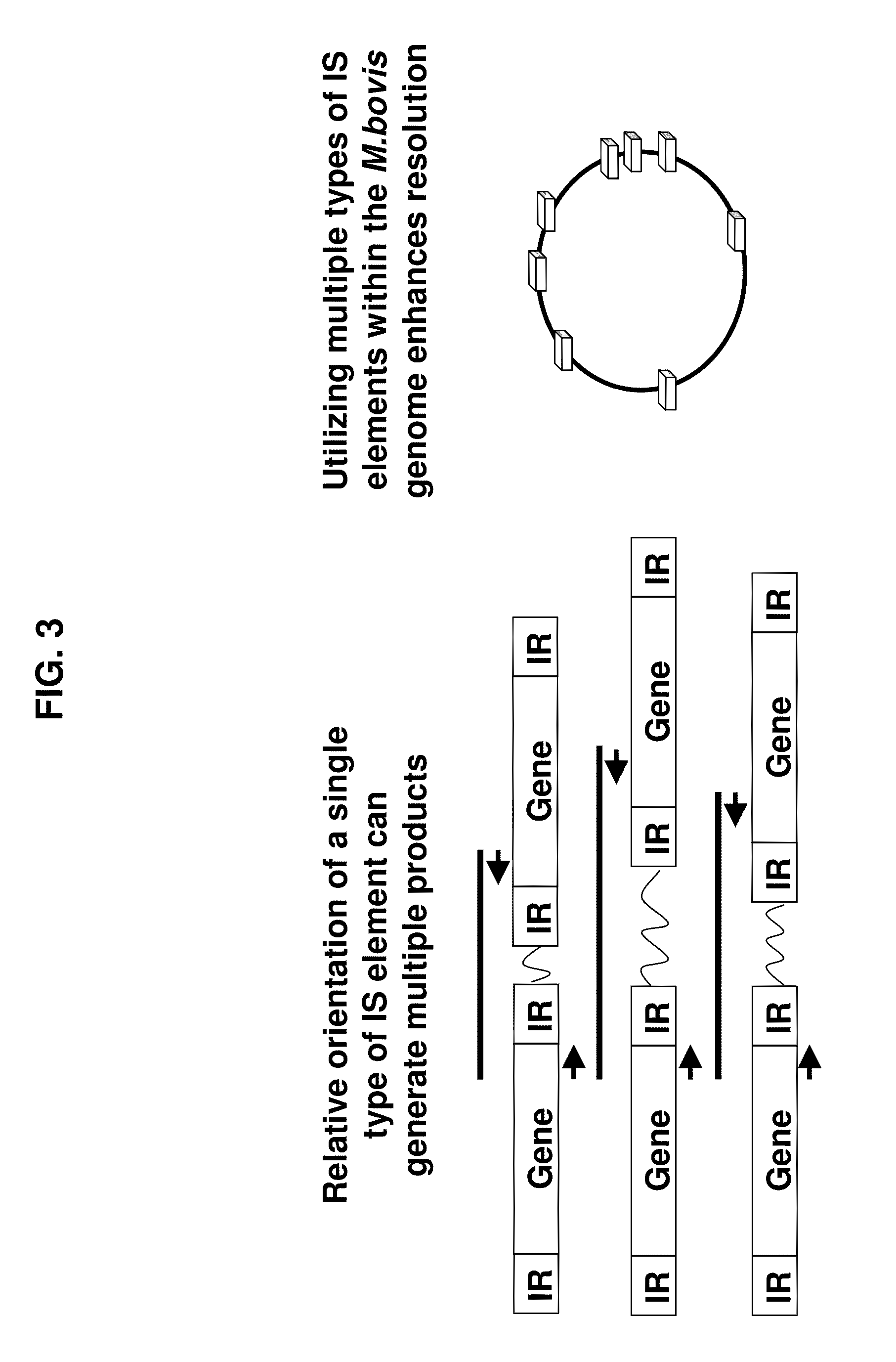
A Mycoplasma bovis PCR-based genotyping method was developed that exploits the proximity of insertion sequences (IS) within the genome by using outward facing primers that selectively amplify sequences between IS elements. The method was applied to 16 field isolates of M. bovis, originating from pneumonic lung or arthritic joints, collected from the United States (Iowa or Kansas) between 2004 and 2005. The genomic fingerprints generated 14 distinct amplification profiles consisting of 4-8 fragments ranging in size from 200-3000 bp. Three isolates presented identical patterns and were isolated from two calves (one calf with pneumonic lung and the other with both pneumonic lung and arthritic joint) from a single farm during an outbreak and probably represent multiple infections with the same genotype. To demonstrate the stability of IS markers for molecular fingerprinting, 3 of the 16 field isolates were subjected to high number passage which resulted in patterns identical to the initial isolates. The results of these studies demonstrate the method can be used for simple and rapid molecular fingerprinting and differentiating M. bovis isolates with extension to epidemiology.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
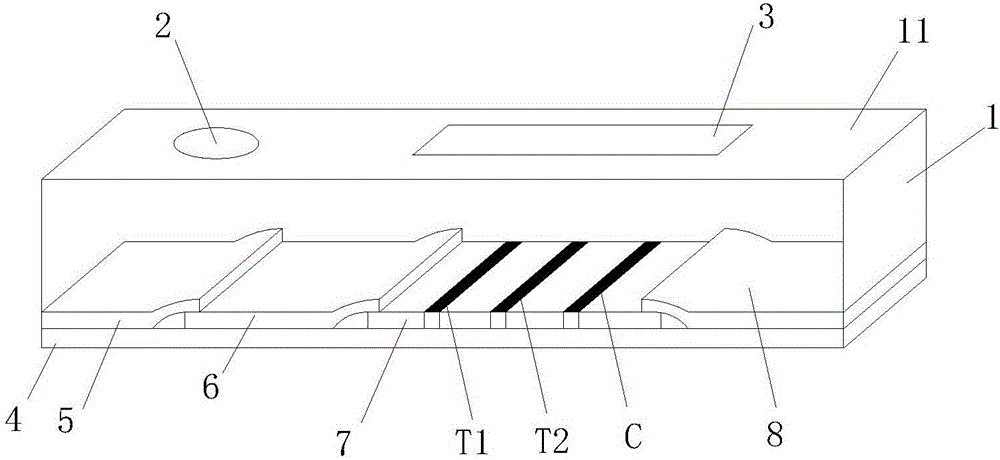
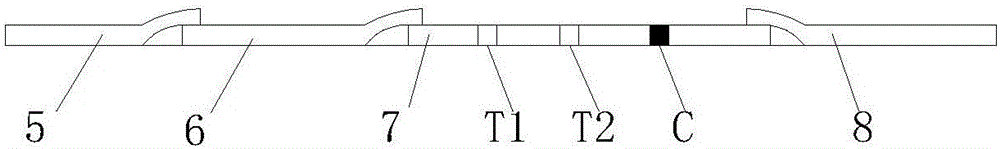
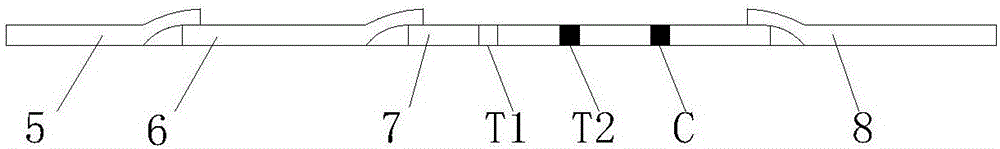
Mycobacterium bovis and brucella abortus dual detection card and preparation method thereof
InactiveCN106706906AHigh compliance rateStrong specificityMaterial analysisBrucella abortusQuality control
The invention discloses a mycobacterium bovis and brucella abortus dual detection card and a preparation method thereof and aims to provide a detection card which is capable of simultaneously detecting mycobacterium bovis and brucella abortus in a bovine serum specimen, is short in detection time, high in stability, simple in operation and intuitive and reliable in result judgment and does not need other instruments or professional personnel. According to the technical key points, the detection card comprises a shell (1), wherein a sample adding hole (2) and an observation window (3) are formed in the shell (1); and a colloidal gold test strip is arranged in the shell (1). The detection card is characterized in that the colloidal gold test strip comprises a bottom plate (4); a sample pad (5), a gold label pad (6), a coating membrane (7) and an absorbent pad (8) are sequentially connected onto the bottom plate (4); a mycobacterium bovis membrane protein MPB70-MPB83 detection line T1, a brucella abortus LPS detection line T2 and a goat anti-rabbit polyclonal antibody quality control line C are formed in the coating membrane (7); and the three lines are arranged in parallel. The detection card belongs to the technical field of biology.
Owner:深圳市绿诗源生物技术有限公司
Pyridine derivative and application thereof to mycobacterium resistance
ActiveCN105330595AGrowth inhibitionAntibacterial agentsOrganic chemistryMycobacterium marinumMycobacterium species
The invention discloses a preparation method and application of a series of novel pyridine derivatives. The derivatives can be used for the treatment of related diseases caused by mycobacterium species, especially for diseases caused by pathogenic mycobacterium, such as Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium avium and Mycobacterium marinum.
Owner:SHANGHAI JIA TAN PHARMATECH CO LTD +1
DNA cancer vaccines
InactiveUS20060189556A1Effectively attenuate and suppress growthHigh activityHeavy metal active ingredientsBiocideAbnormal tissue growthAntigen
A plurality of DNA cancer vaccines and their uses in treating cancer are disclosed. Genes encoding Mycobacterium bovis bacillus Calmette-Guérin (M. bovis BCG) recombinant antigens and genes encoding interlukin-12 heterodimer were respectively cloned into eukaryotic expression vectors to express the encoded recombinant proteins in mammalian cells in vivo whereby specific immune responses are evoked and are effective in preventing, attenuating and / or suppressing the growth of a tumor.
Owner:NAT DEFENSE MEDICAL CENT
RECOMBINANT BCG OVEREXPRESSING phoP-phoR
The present invention provides a live recombinant Mycobacterium bovis-BCG strain and a tuberculosis (TB) vaccine or immunogenic composition comprising a nucleic acid capable of overexpression, the nucleic acid encoding PhoP and PhoR proteins. A method for treatment or prophylaxis of a mammal against challenge by Mycobacterium tuberculosis or Mycobacterium bovis using the strain is also provided.
Owner:成都安永鼎业生物技术有限公司
Combination vaccines against Mycobacterium sp. and methods of using the same
The invention relates to a combination vaccine against Mycobacterium avium subspecies paratuberculosis (MAP) and M. tuberculosis and / or M. bovis for use in methods of immunizing a subject against mycobacterial infection, preventing or treating mycobacterial infection, and preventing a disease associated with mycobacterial infection.
Owner:GREENSTEIN ROBERT J
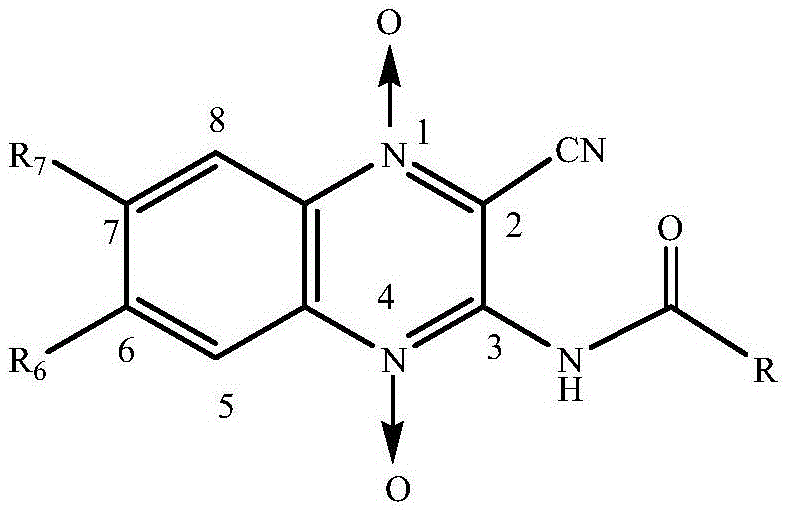
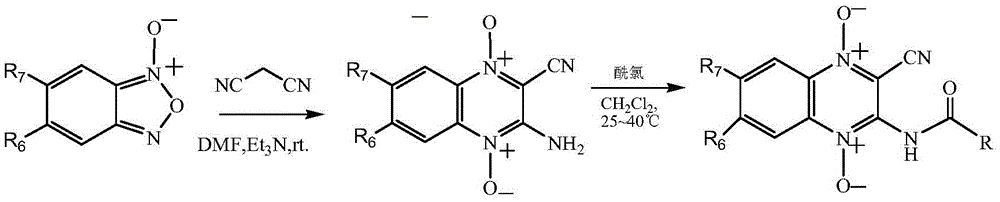
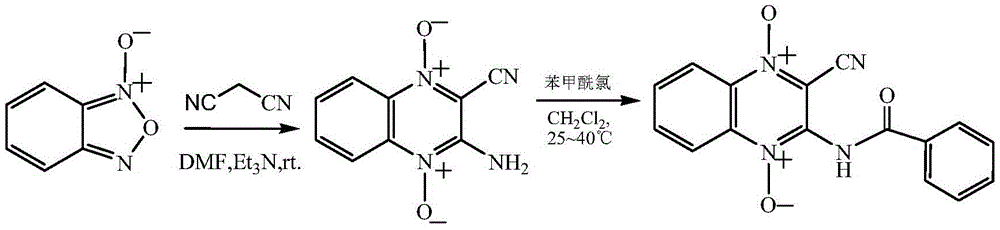
Quinoxaline-N1,N4-dioxide derivatives with antimicrobial activity
The invention belongs to the technical field of pharmaceutical chemosynthesis, and particularly relates to quinoxaline-N1,N4-dioxide derivatives with antimicrobial activity. The invention also relates to preparation of the derivatives and antimicrobial activity testing of the derivatives. The new synthetic compounds are prepared by the following steps: carrying out Beirut reaction on the raw material N-benzofuroxan oxide and malononitrile under alkaline conditions to obtain 3-amino-2-cyano-quinoxaline-N1,N4-dioxide, and reacting with appropriate acyl chloride to obtain a series of 2-cyano-amidoquinoxaline-N1,N4-dioxides. The in-vitro antibacterial activity test result indicates that the quinoxaline-N1,N4-dioxide derivatives have favorable antibacterial activity for mycobacterium hominis and mycobacterium bovis and also have antibacterial activity for Staphylococcus aureus, Streptococcus pneumoniae and Pasteurella. The invention also discloses a structural formula of the compounds used as a target antibacterial drug.
Owner:HUAZHONG AGRI UNIV
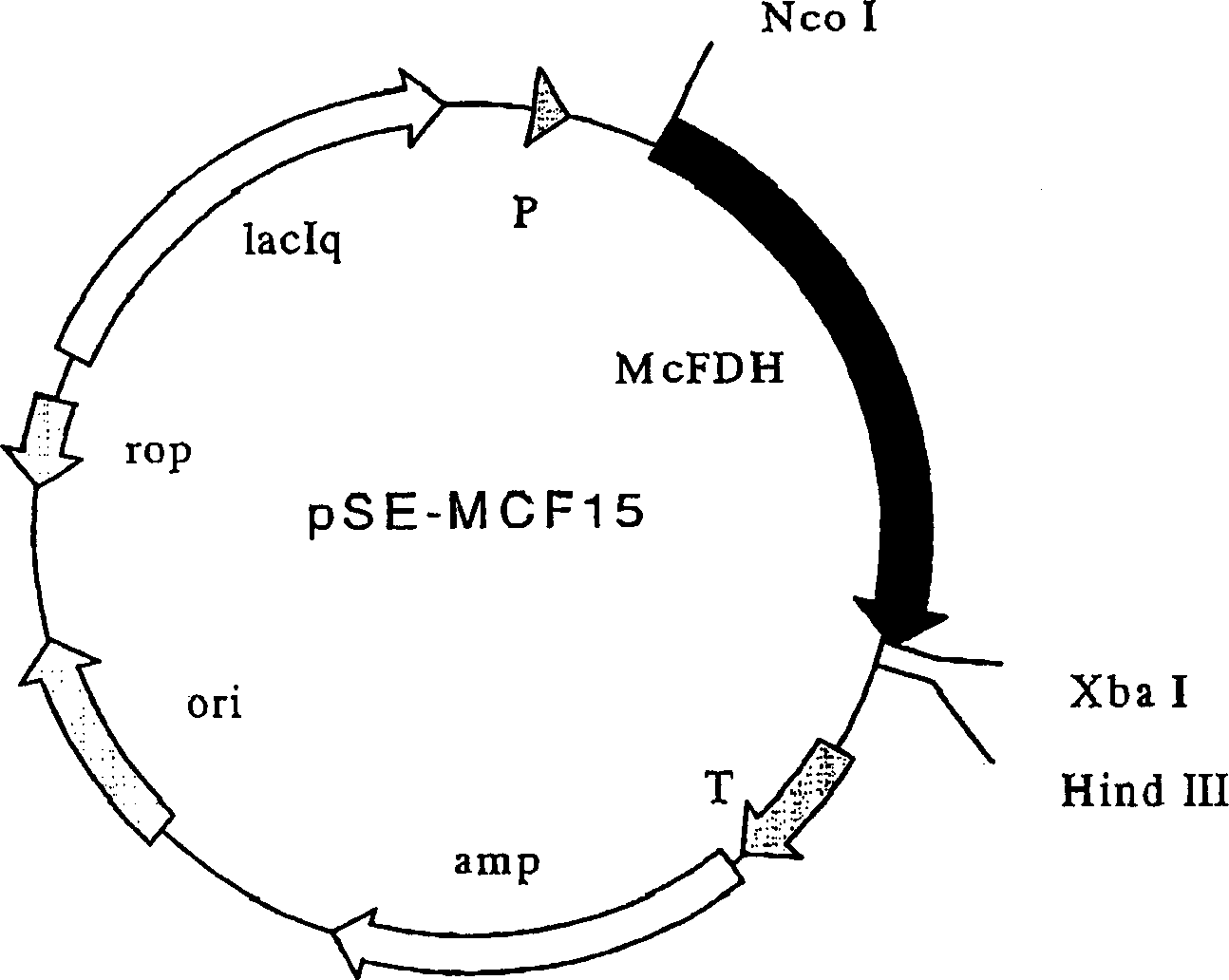
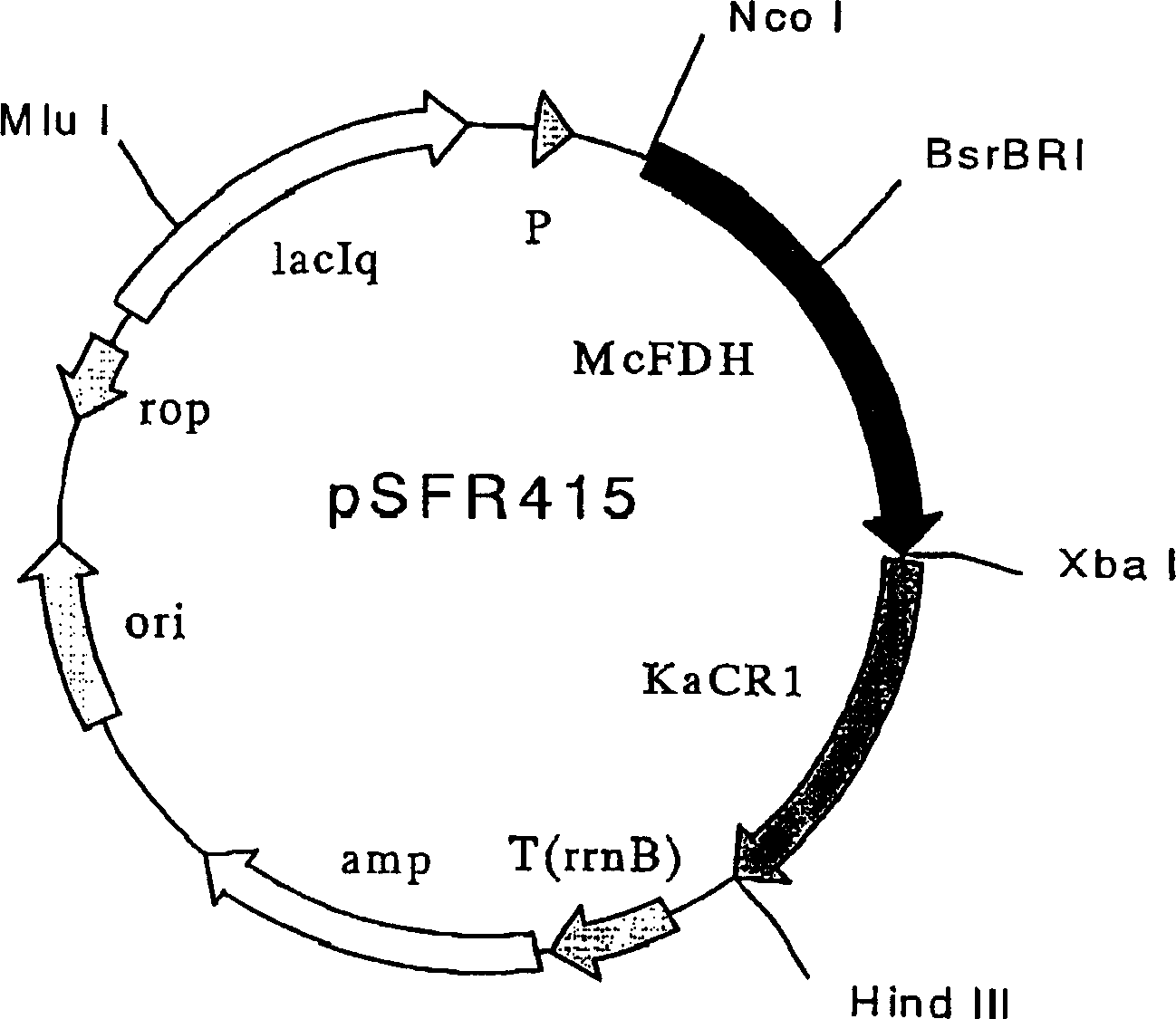
Mycobacterium vaccae formic dehydrogenase mutant and its use
The object of the present invention is to provide polypeptides that can maintain strong formate dehydrogenase activity in the presence of organic solvents, and their applications. By replacing cysteine at positions 146 and / or 256 in the amino acid sequence of formate dehydrogenase derived from Mycobacterium vaccae through site-directed mutagenesis, a formate dehydrogenase mutant polypeptide is constructed, which is resistant to organic solvents. sex. This type of polypeptide has strong formate dehydrogenase activity in the presence of organic solvents. Such mutants can use ketones as raw materials to produce alcohols, etc.
Owner:DAICEL CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com