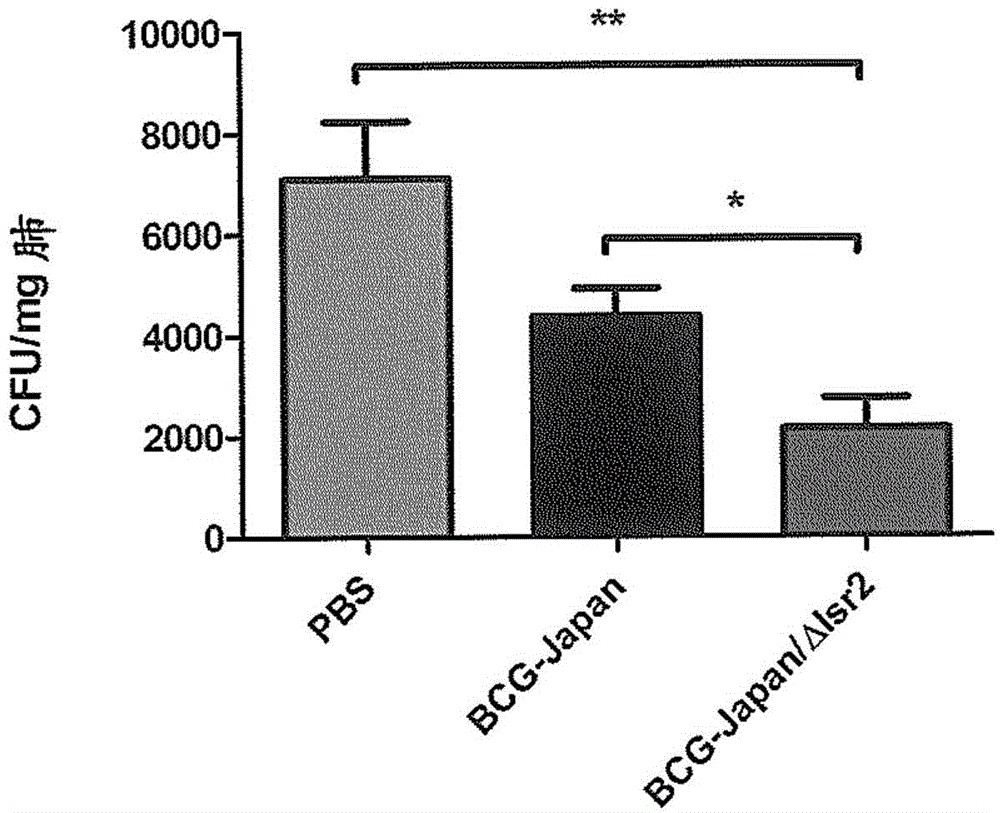
Modified bcg strains with reduced or eliminated activity of lsr2 and pharmaceutical composition comprising same
A composition and strain technology, applied in the field of tuberculosis vaccine, can solve the problem of insufficient immunogenicity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation of live recombinant vaccines is known to those skilled in the art. Typically, such vaccines are prepared as injectables, either as liquid solutions or suspensions; solid forms suitable for solution, or suspension, in liquid prior to injection may also be prepared. The preparation can also be emulsification, or encapsulation of the protein into liposomes. The live immunogenic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. Suitable excipients are, for example, water, saline, dextrose, glycerol, ethanol, etc. or combinations thereof. Furthermore, if desired, the vaccine may contain minor amounts of auxiliary substances, such as wetting or emulsifying agents, pH buffers and / or adjuvants which increase the efficacy of the vaccine. Examples of potentially effective adjuvants include, but are not limited to: aluminum hydroxide, N-acetyl-muramoyl-L-threonyl-D-isoglutamine (thr-MDP), ...
Embodiment 1
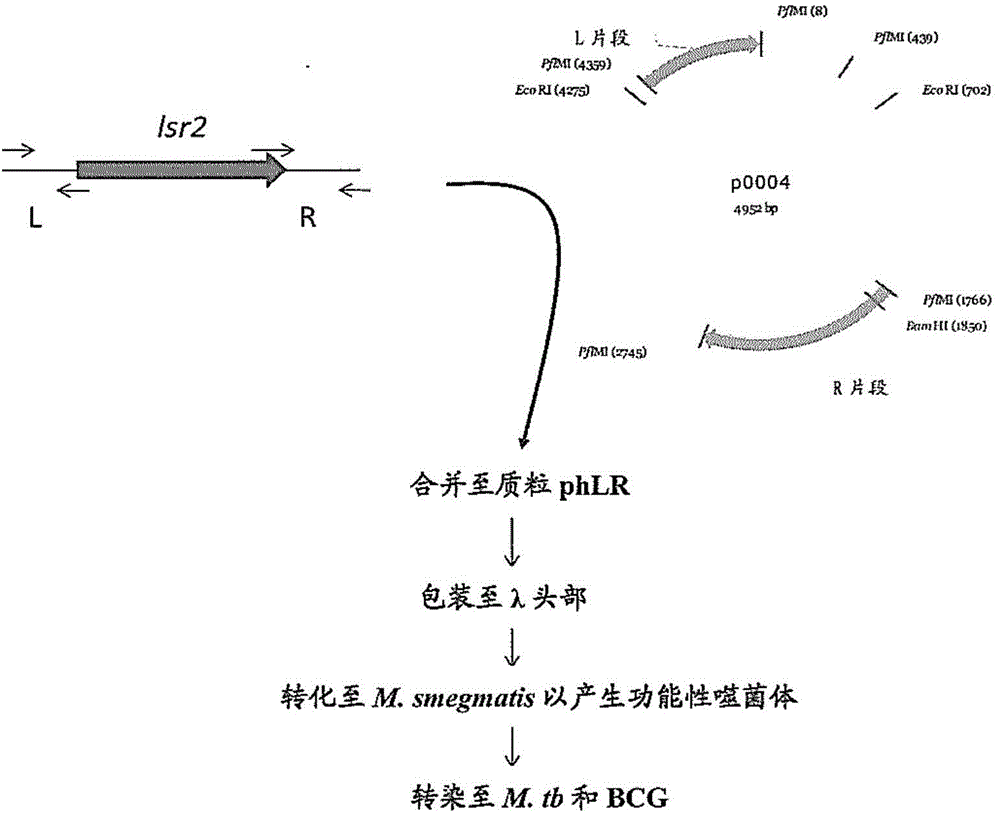
[0056] Embodiment 1: Construction of the lsr2 deletion mutant of M.tb and BCG
[0057] M.tb H37Rv (a laboratory virulent strain of M.tb purchased from ATCC, ATCC No. 25618) and BCG-Japan (30) (a gift from Marcel Behr) were generated by using a temperature-sensitive transducing phage system (26) The lsr2 deletion mutant, the main step in figure 2 shown in . DNA manipulations were performed essentially as described by Sambrook et al. (Sambrook, J., E.F. Fritsch and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.). Plasmid p0004 is a Hyg R - A counter-selectable suicide vector for the sacB cassette (31). An upstream left fragment (L-fragment) and a downstream right fragment (R-fragment) flanking the lsr2 gene were generated by two primer pairs. Using primer pair L-forward SEQ ID NO: 3 (CGGCTT CCATAAATTGG GCAGCTGGATCACCTGCTGGCGCAC) and L-reverse SEQ ID NO:4 (CGGCTT CCATTTTCTTGG CATTTGGCTACCGGCGCCCAG...
Embodiment 2
[0058] Example 2: Confirmation of the deletion of the lsr2 gene from M.tb H37Rv and BCG-Japan
[0059] Three clones of each strain (M.tb H37Rv and BCG-Japan) emerging after 4 weeks of the above experiments were randomly picked and grown in 20 ml of 7H9 medium containing 10% ADC for 4 weeks at 37°C. To isolate chromosomal DNA, 10 mL of each culture was centrifuged at 2,000×g for 20 minutes, and the pellet was washed with 1 ml of GTE solution (25 mM Tris-HCl pH 8.0, 10 mM EDTA, 50 mM glucose) and resuspended in 450 μl of GTE solution. 50 μl of lysozyme solution (10 mg / ml in Tris pH 8.5) was added, mixed gently, and incubated overnight at 37°C. Then 100 μl of 10% SDS and 50 μl of 10 mg / ml proteinase K (Sigma) were added, mixed gently, and incubated at 55° C. for 40 minutes. Then 200 μl of 5M NaCl and 160 μl of CTAB were added, mixed gently, and incubated at 65 °C for 10 min. An equal volume (≈1 ml) of chloroform:isoamyl alcohol (24:1) was added to the tube, and the aqueous phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com