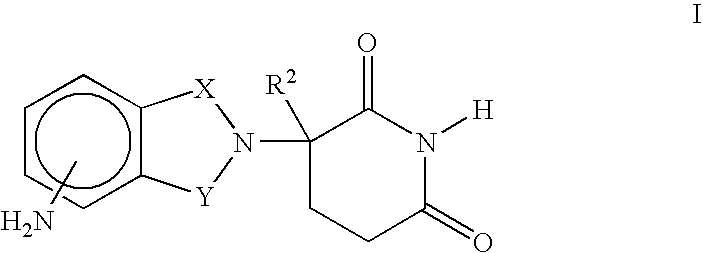
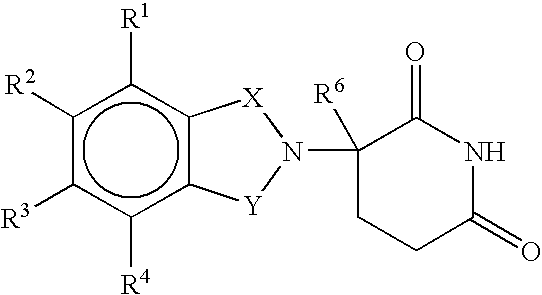
Patents
Literature
7823results about "Bacterial antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
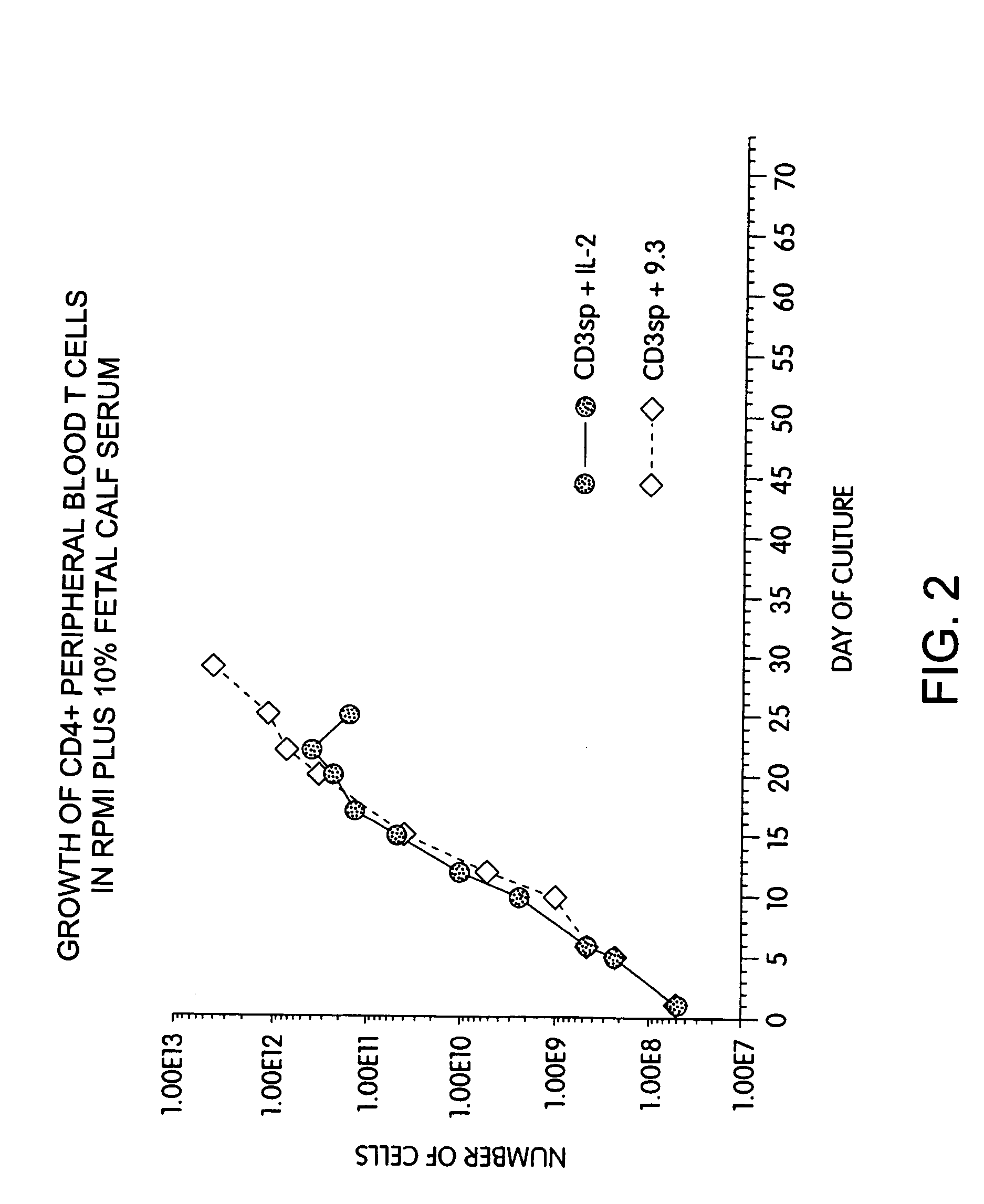
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
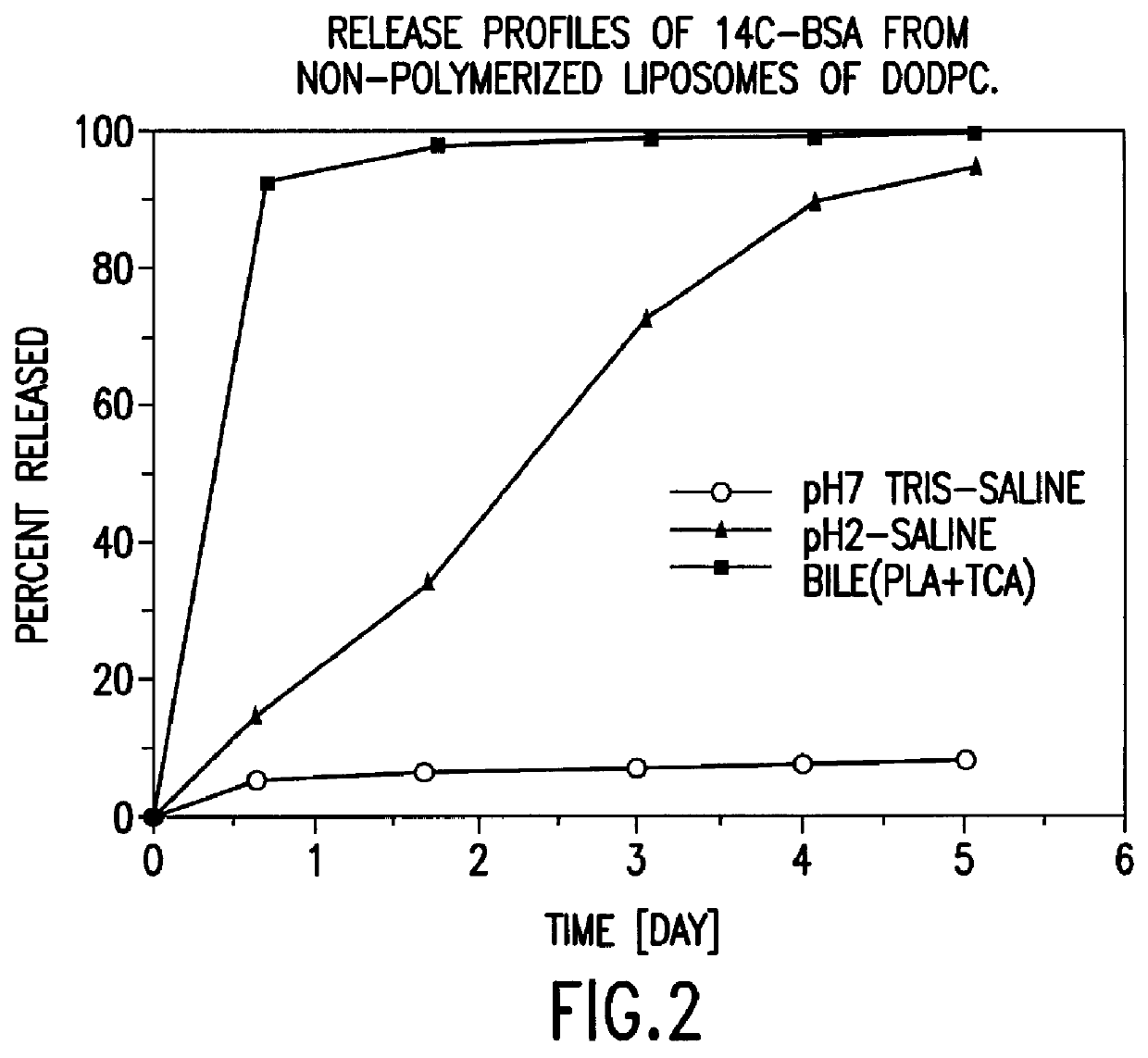
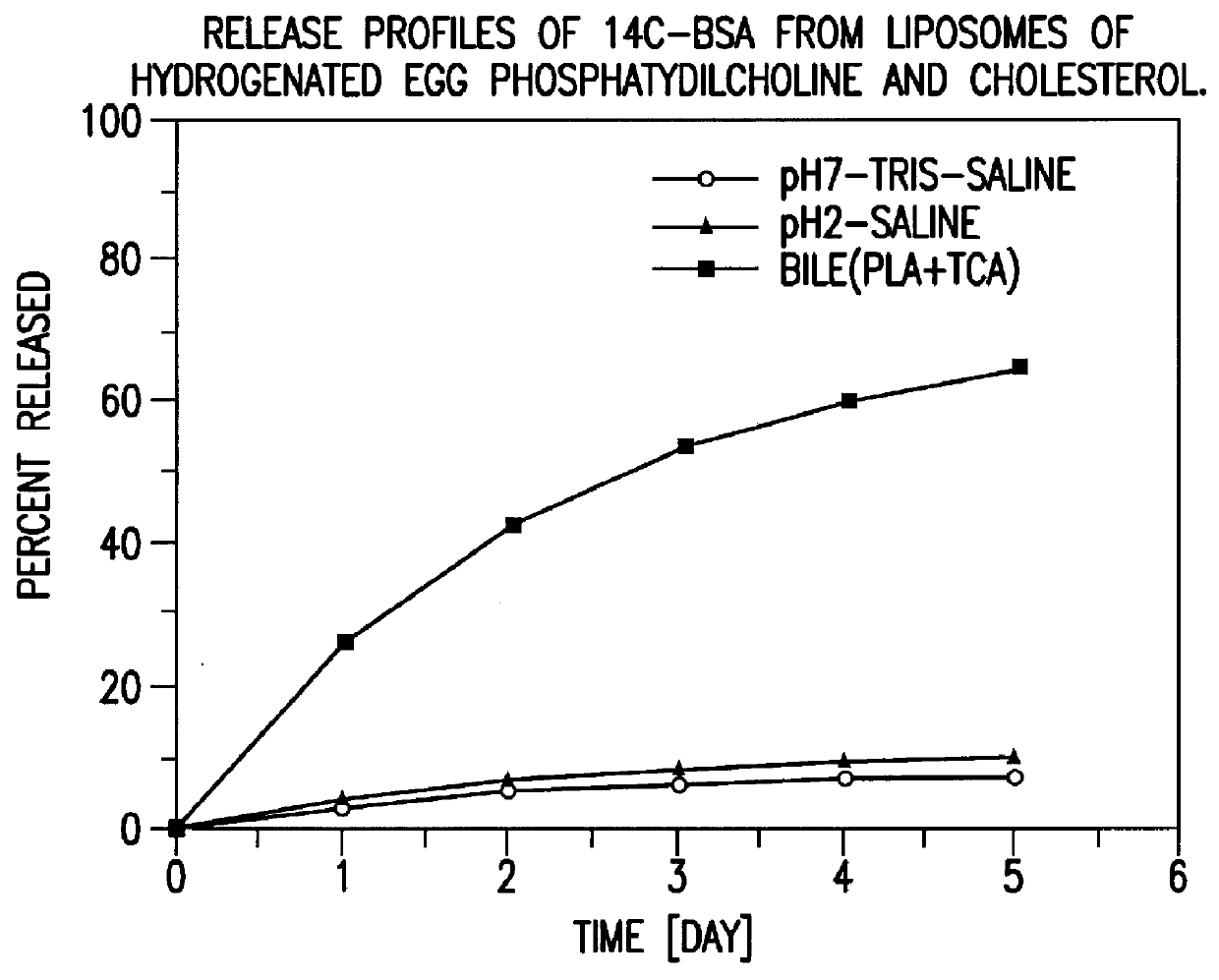
Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery
InactiveUS6060082ALessContainment leakBacterial antigen ingredientsPeptide/protein ingredientsCell typeLiposome
The present invention relates to targeted polymerized liposomes for oral and / or mucosal delivery of vaccines, allergens and therapeutics. In particular, the present invention relates to polymerized liposomes which have been modified on their surface to contain a molecule or ligand which targets the polymerized liposome to a specific site or cell type. More particularly, the invention relates to the use of polymerized liposomes modified to contain a carbohydrate or lectin on their surface.
Owner:MASSACHUSETTS INST OF TECH
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Receptor specific transepithelial transport of therapeutics
InactiveUS6485726B1Effective strategyImprove abilitiesBacterial antigen ingredientsPeptide/protein ingredientsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
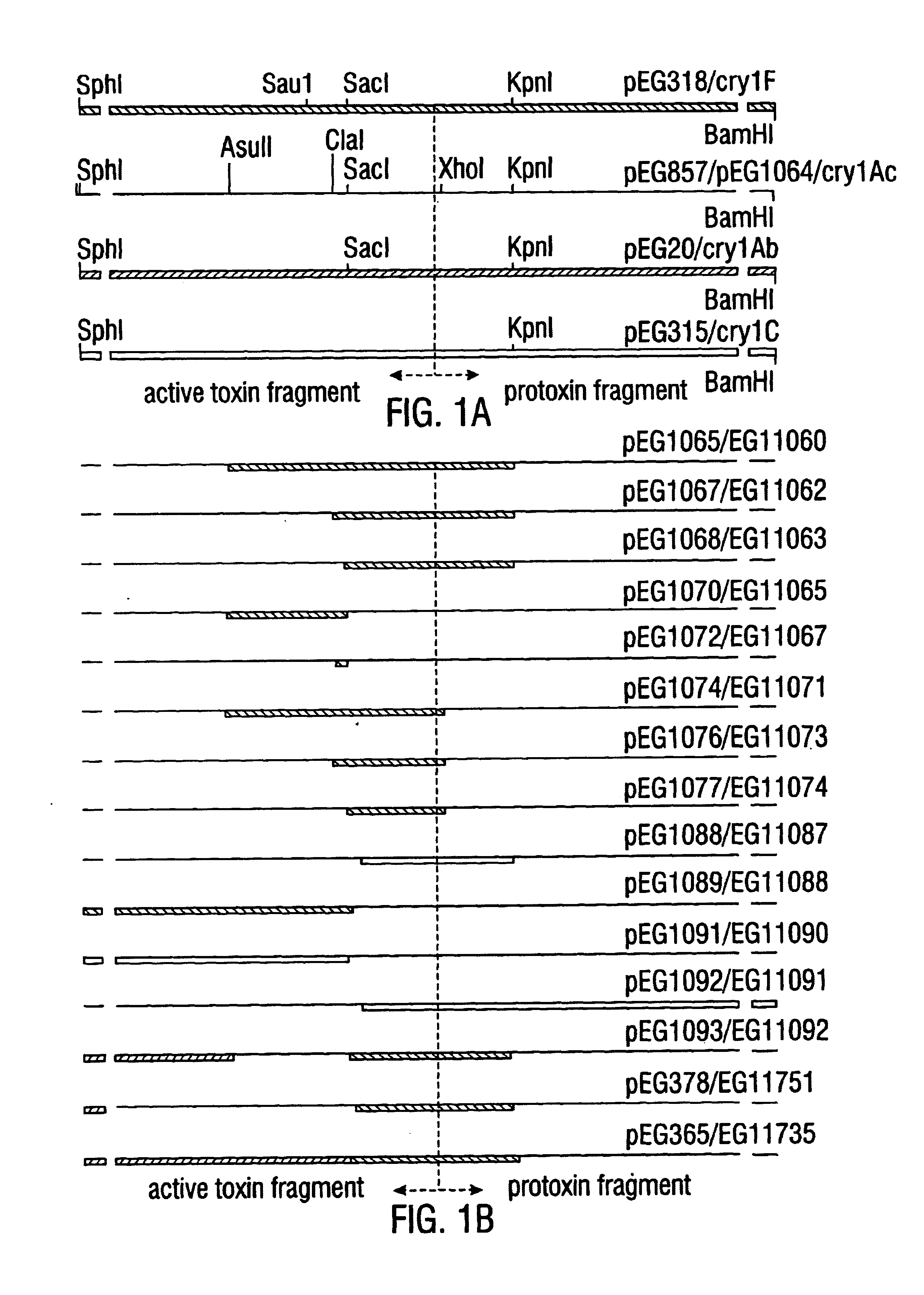
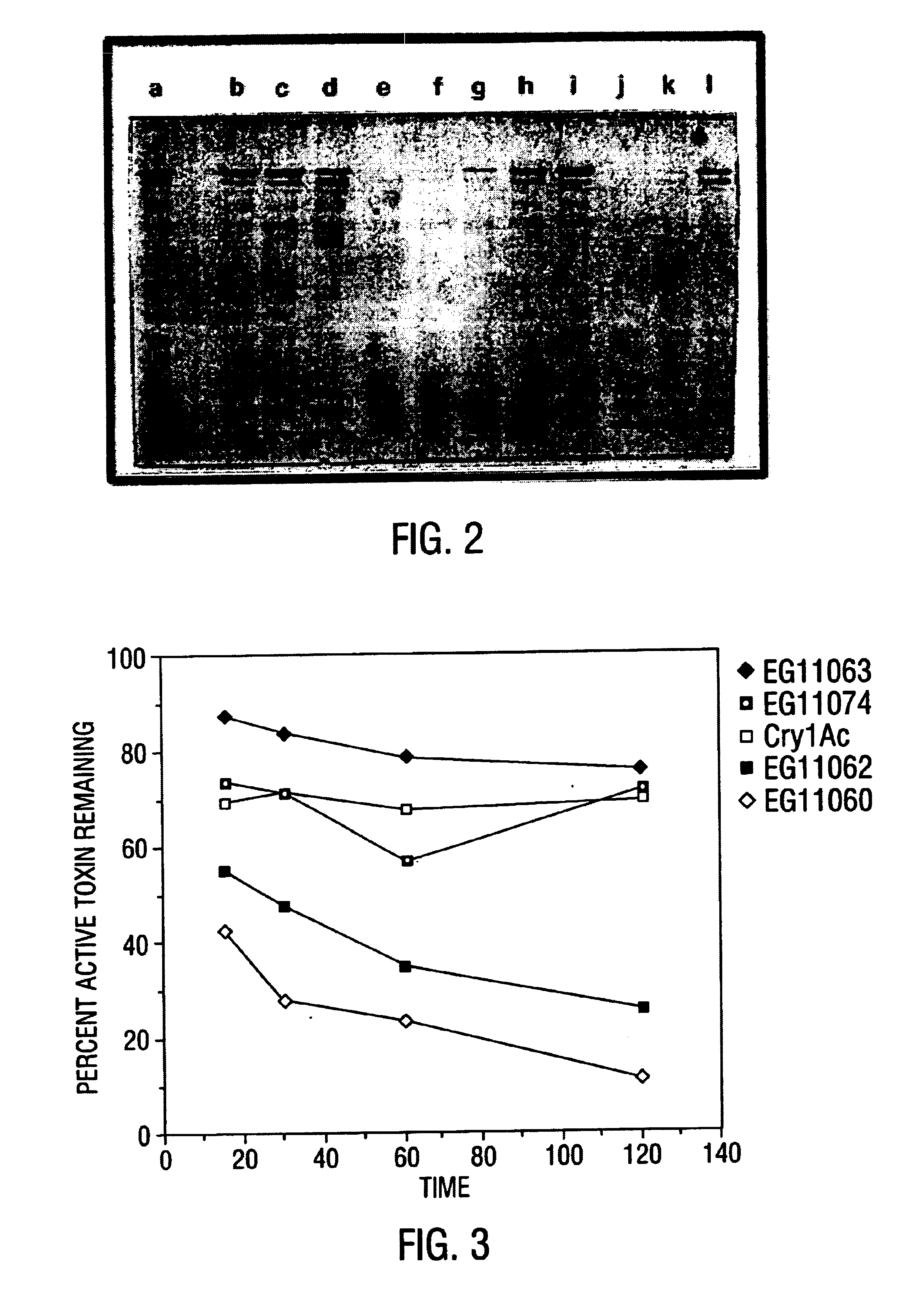
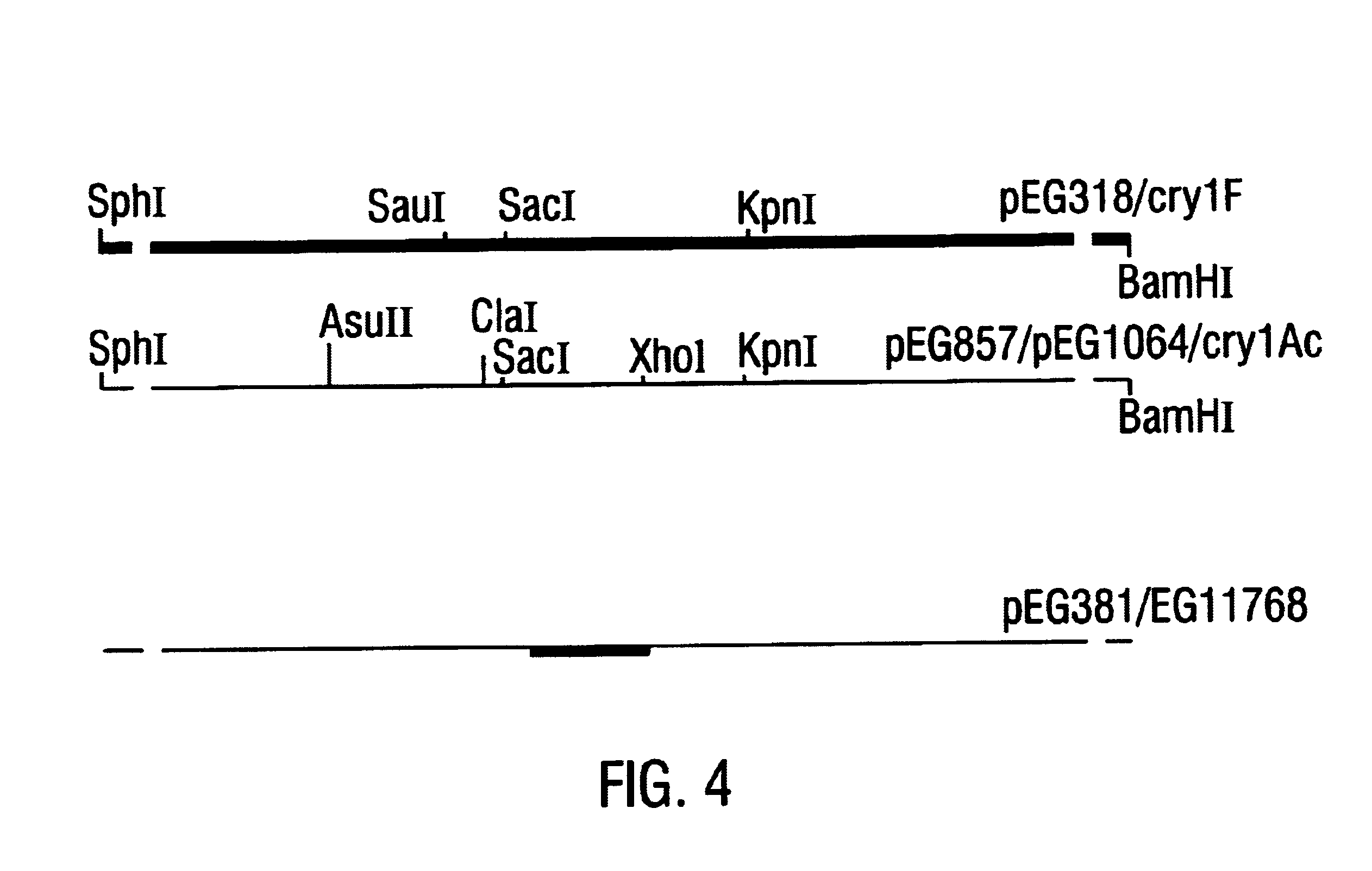
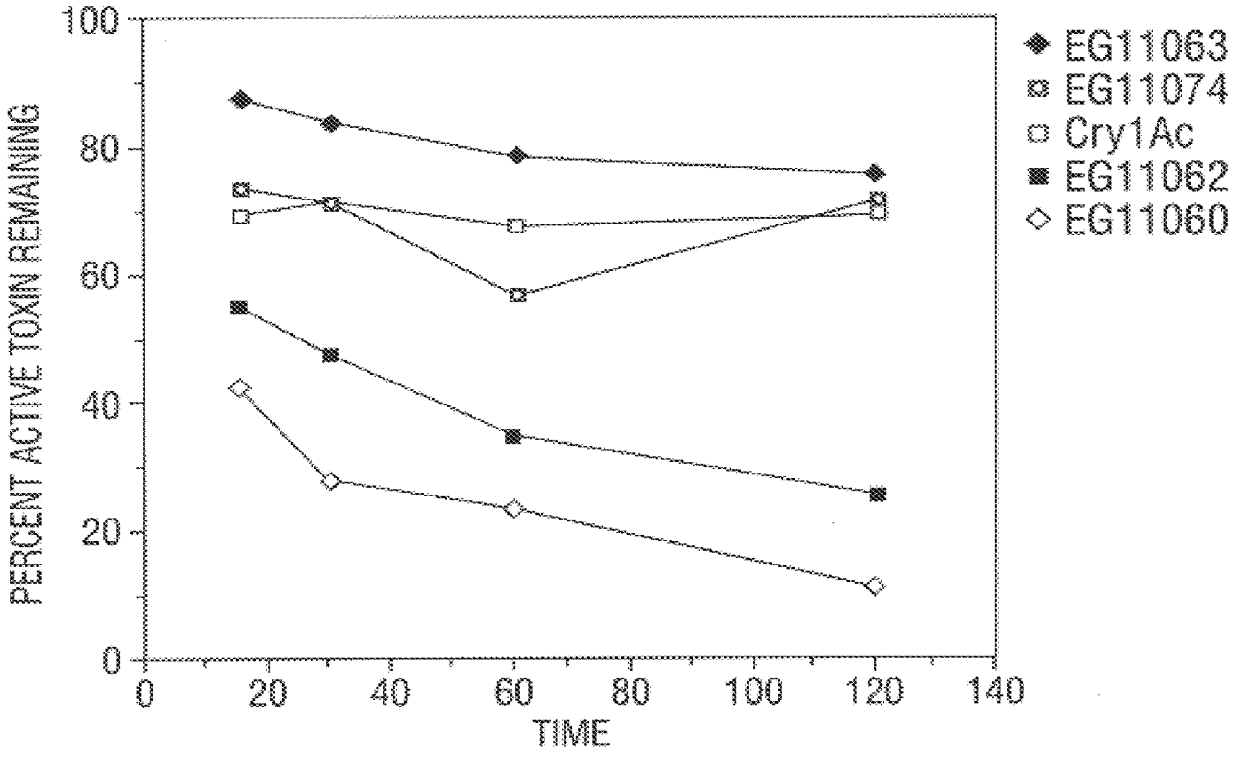
Broad-spectrum delta-endotoxins
InactiveUS6713063B1Improved insecticidal activity and broader host-range activityImproving immunogenicityBiocidePeptide/protein ingredientsAureobasidium sp.Toxin
Disclosed are novel synthetically-modified B. thuringiensis chimeric crystal proteins having improved insecticidal activity and broader insect host range against coleopteran, dipteran and lepidopteran insects. Also disclosed are the nucleic acid segments encoding these novel peptides. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and transgenic plants expressing the modified endotoxin are also aspects of the invention.
Owner:MONSANTO TECH LLC
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
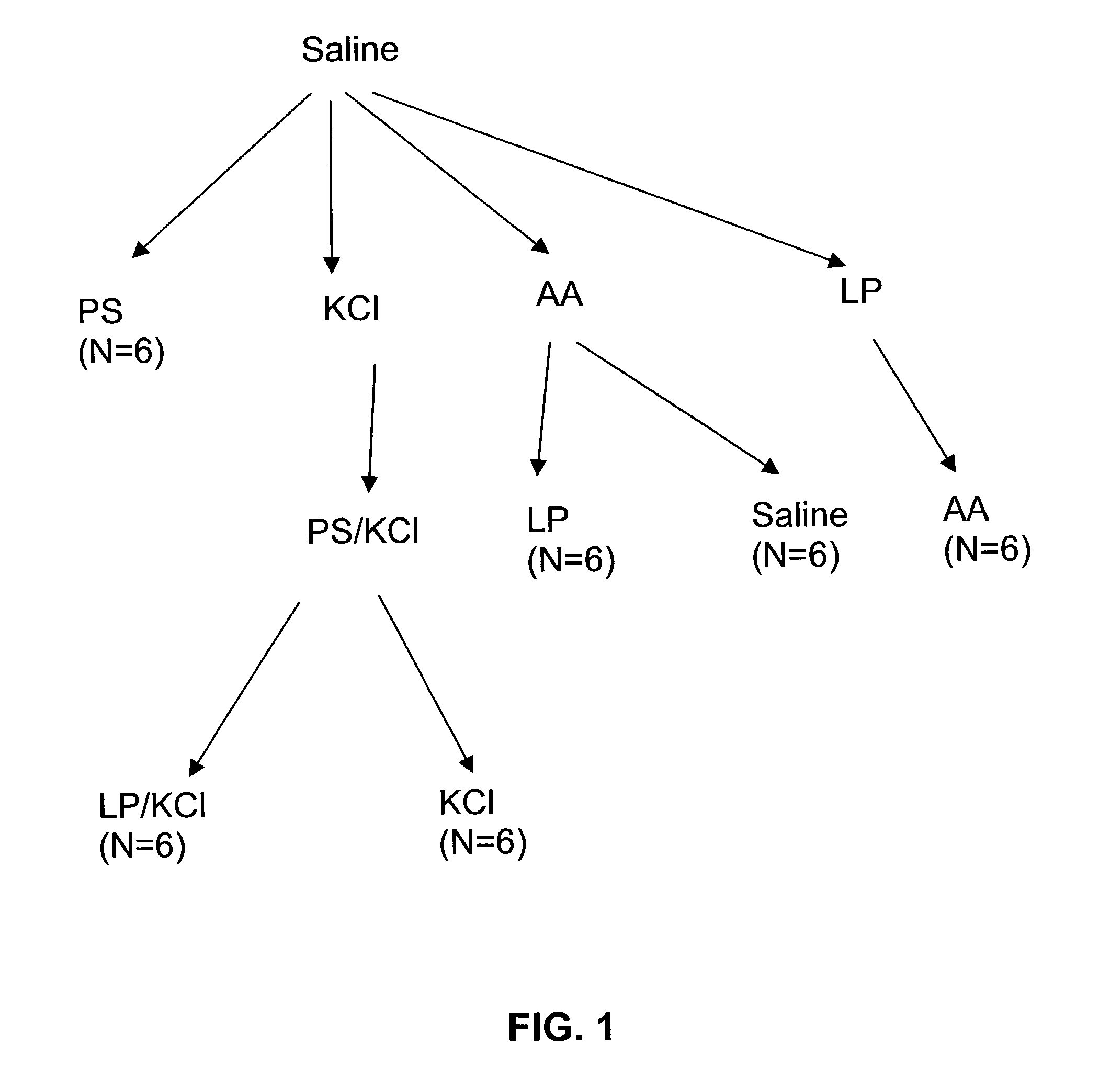
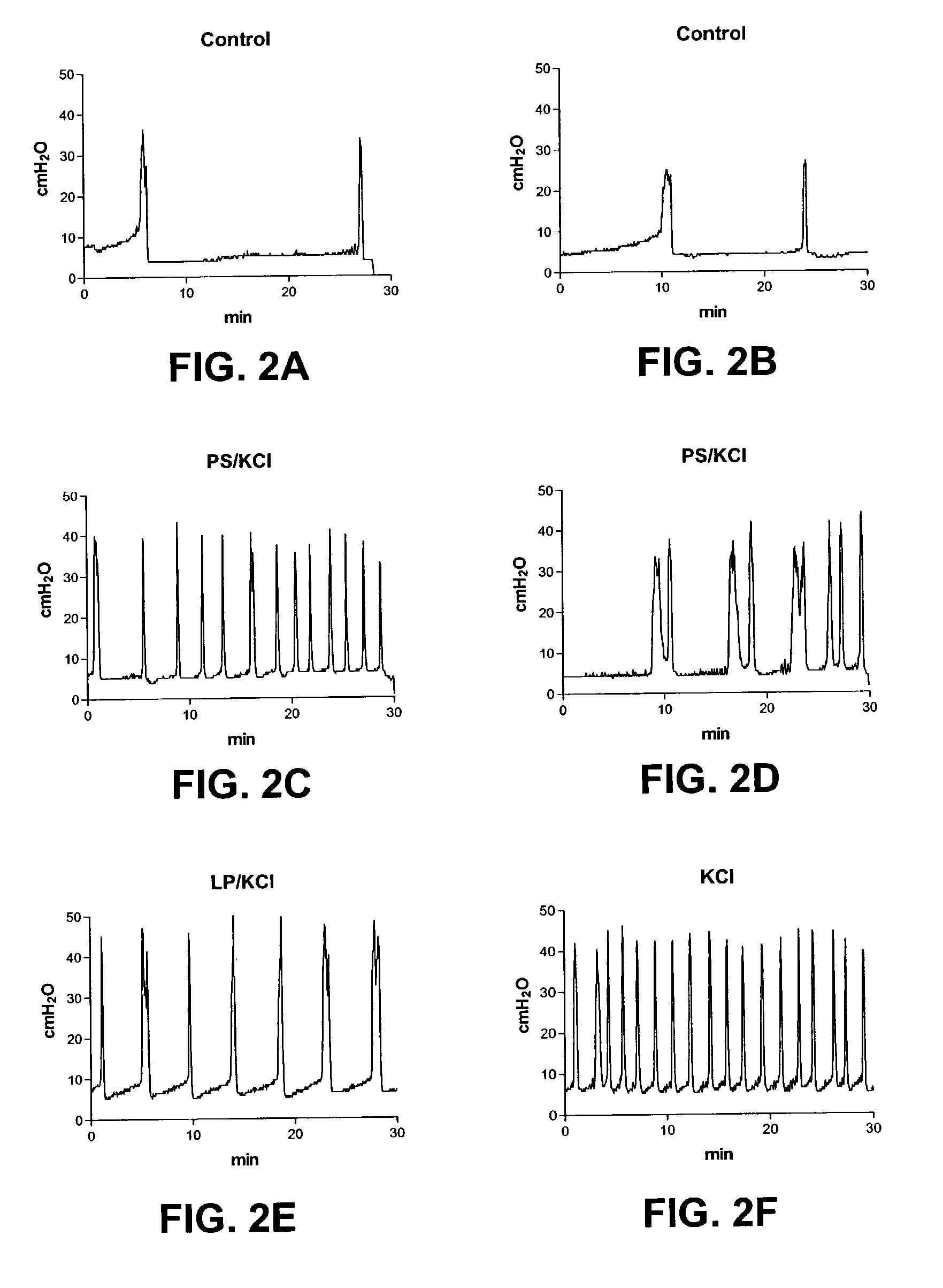
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
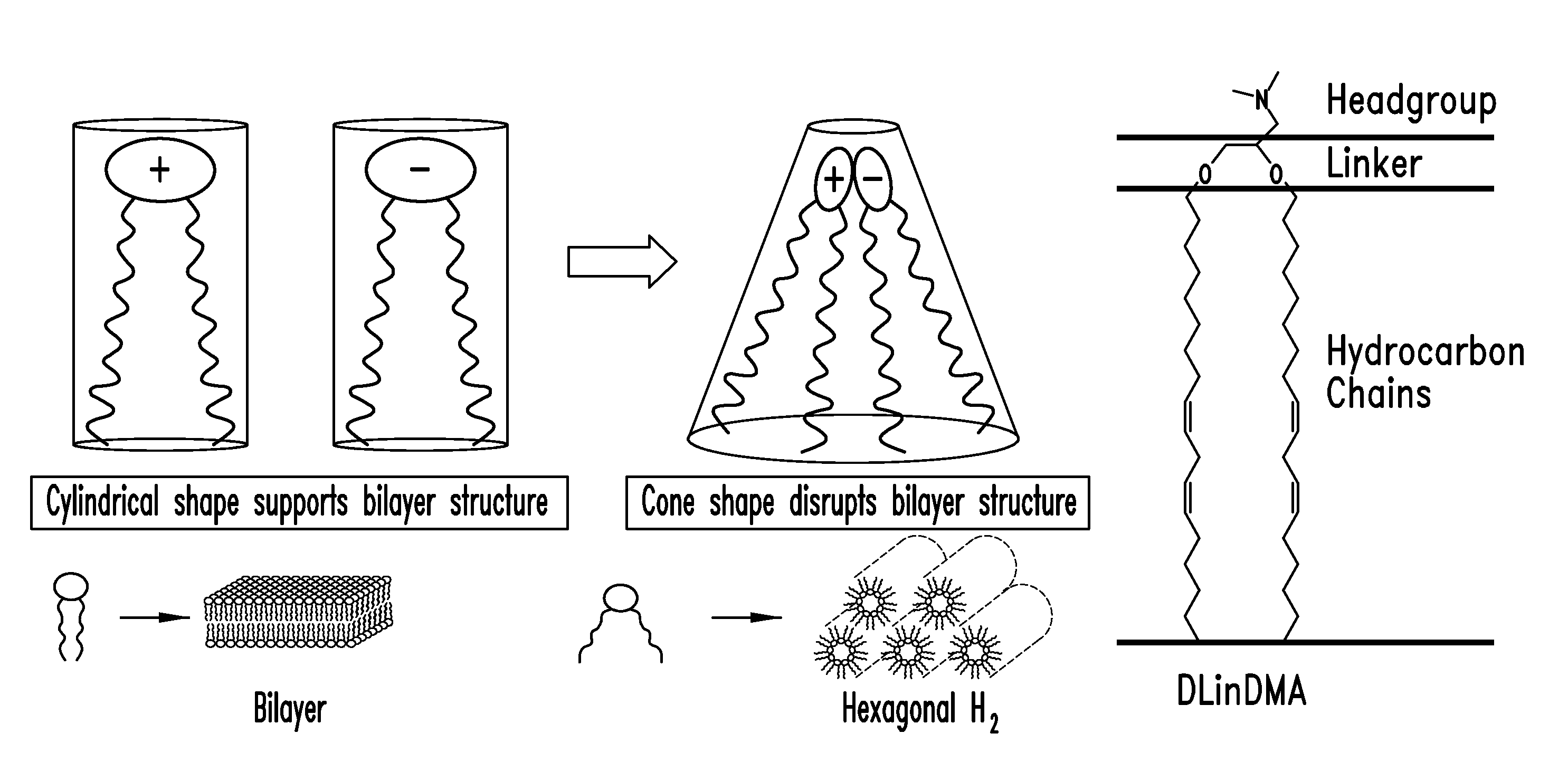
Amino lipids and methods for the delivery of nucleic acids
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Broad-spectrum delta -endotoxins
InactiveUS6110464AImprove insecticidal effectBroad-range specificityNanotechBacteriaAureobasidium sp.Toxin
Disclosed are novel synthetically-modified B. thuringiensis chimeric crystal proteins having improved insecticidal activity against coleopteran, dipteran and lepidopteran insects. Also disclosed are the nucleic acid segments encoding these novel peptides. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and transgenic plants expressing the modified endotoxin are also aspects of the invention.
Owner:MONSANTO TECH LLC
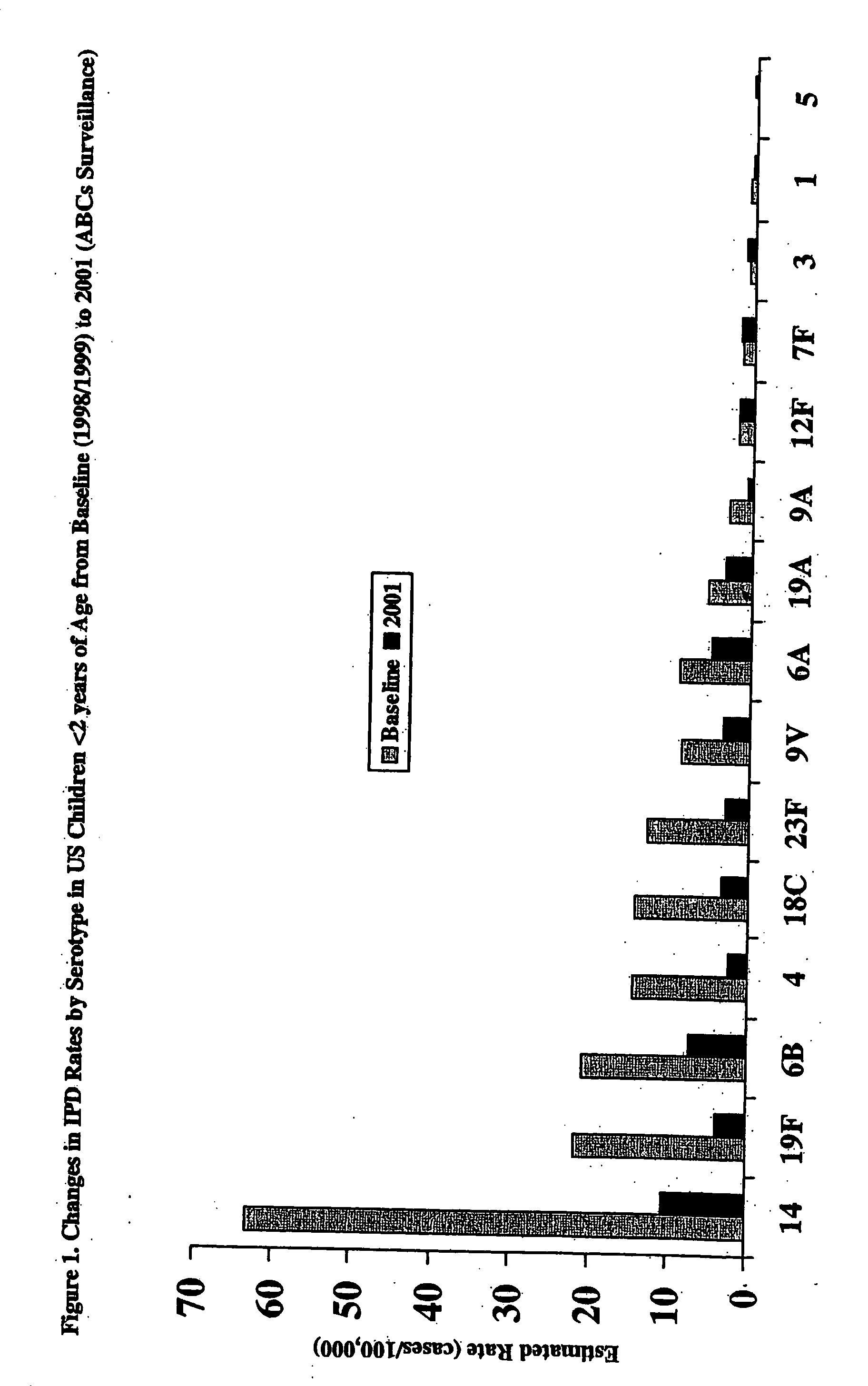
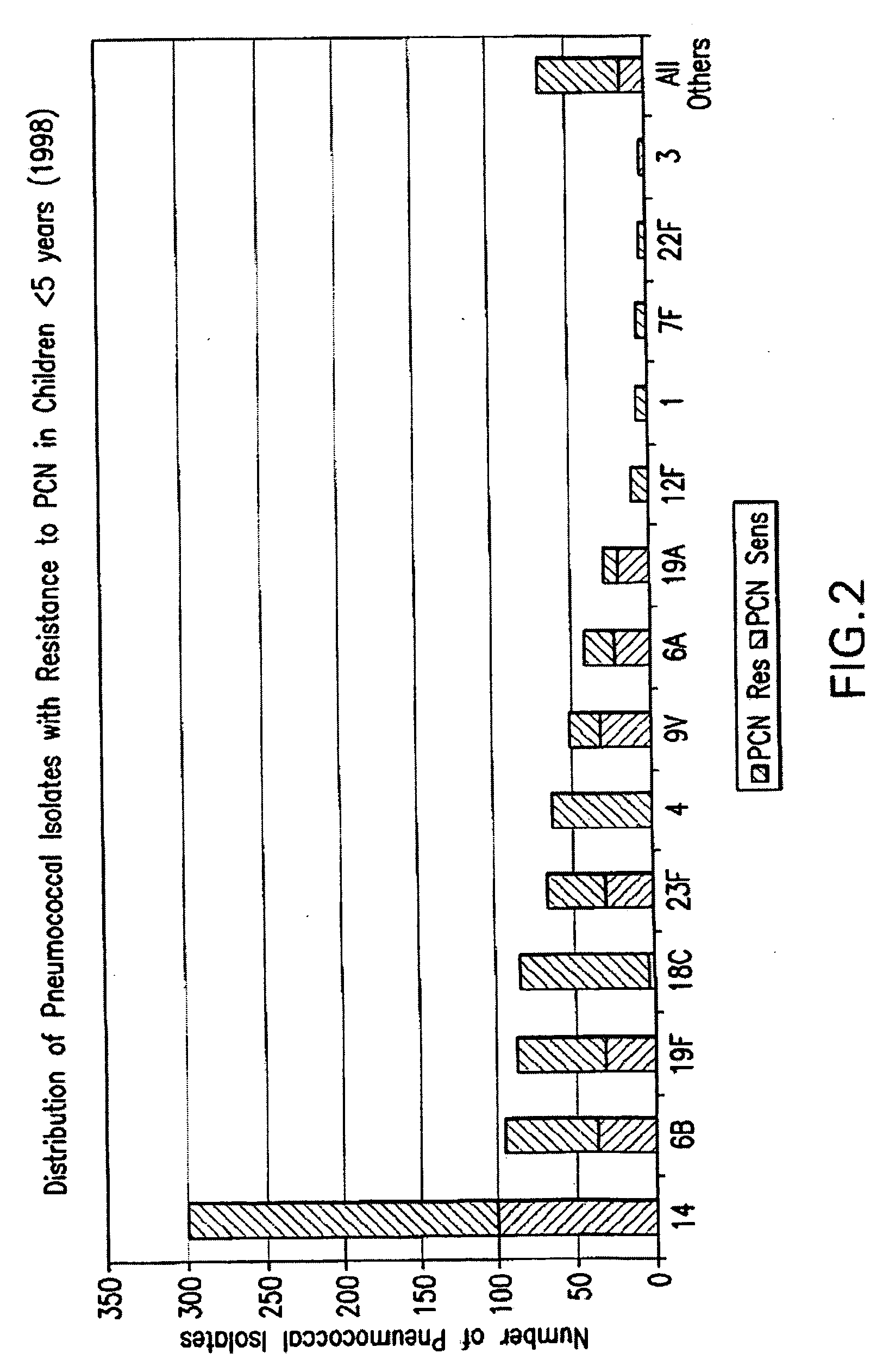
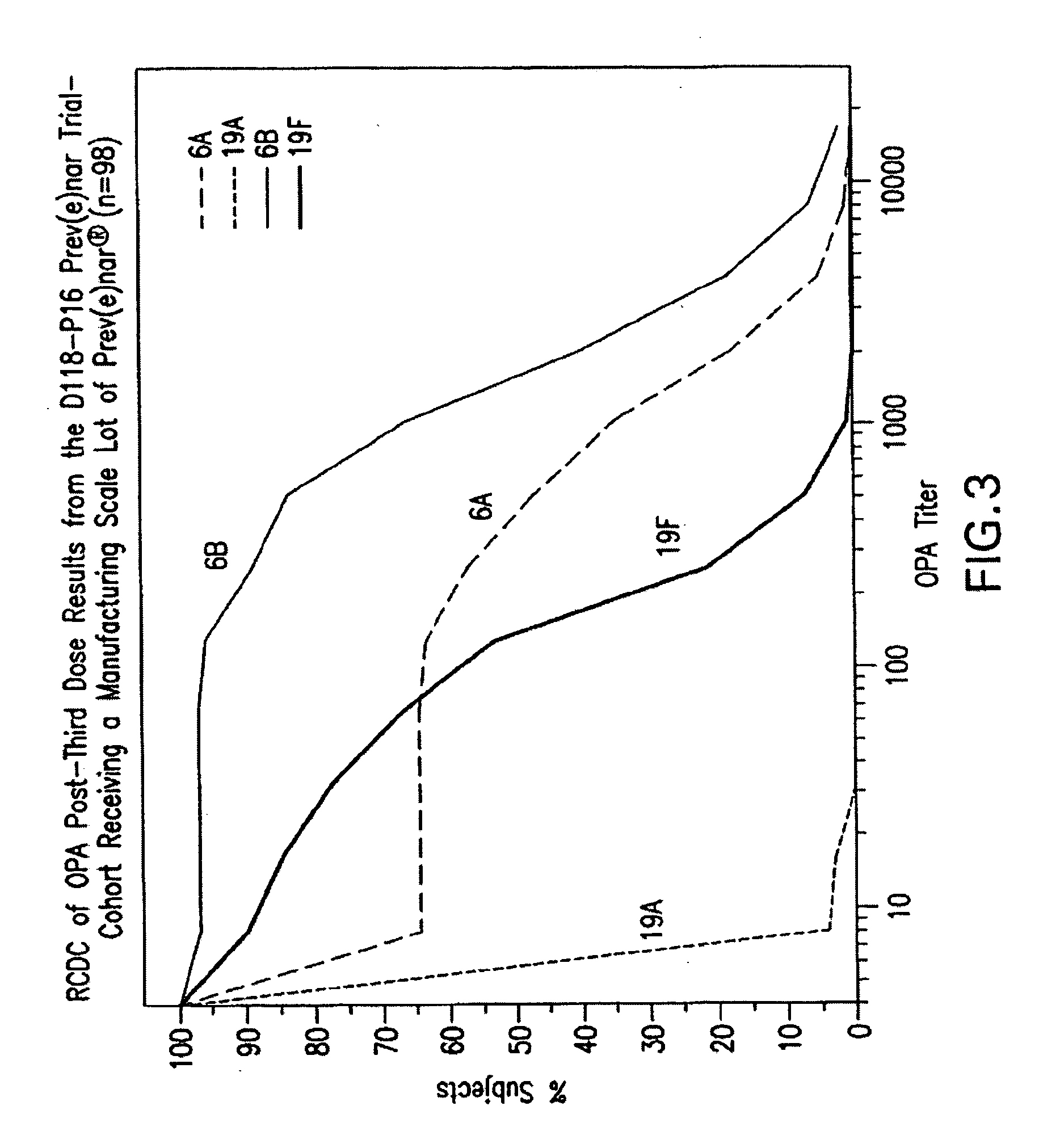
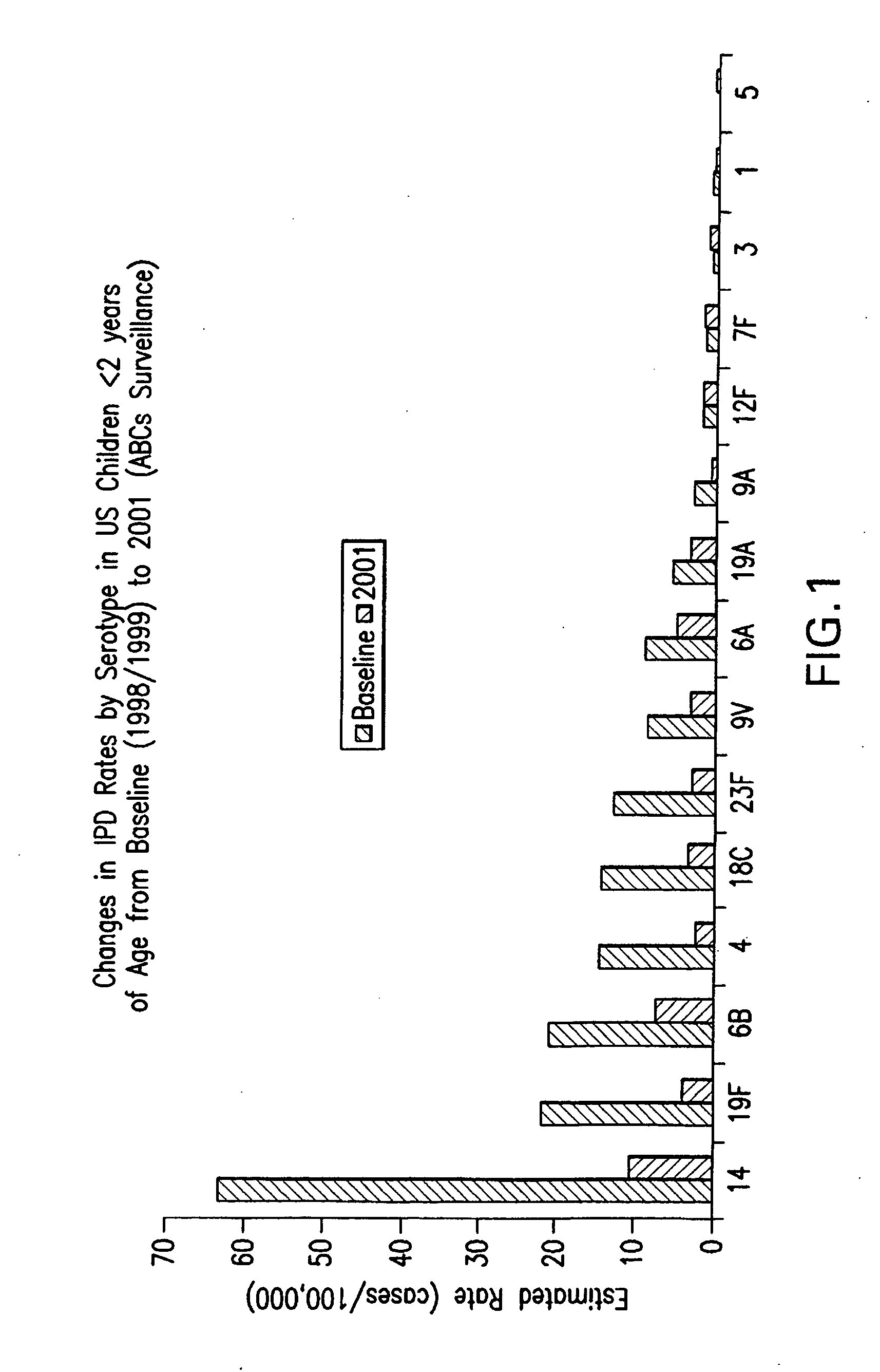
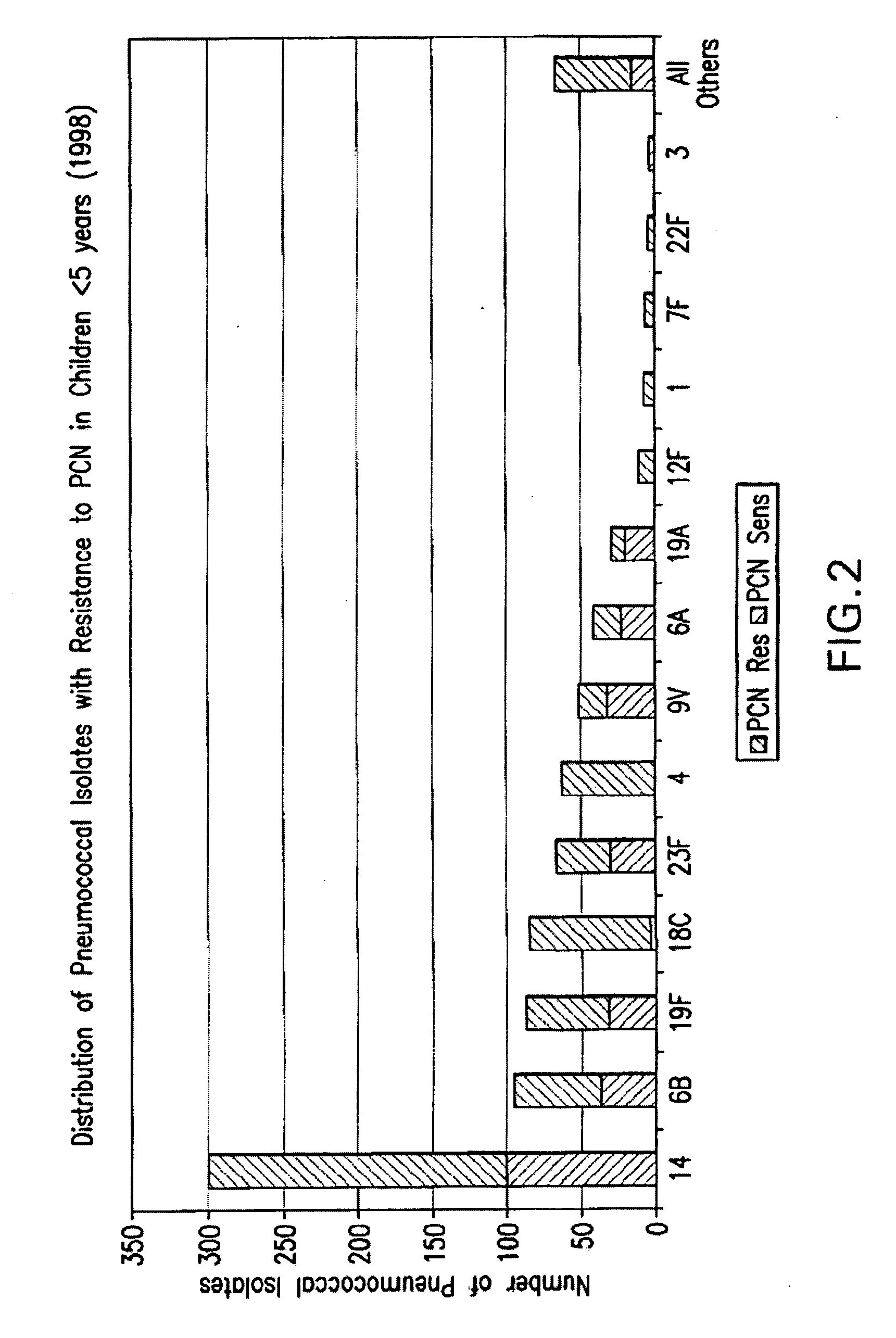
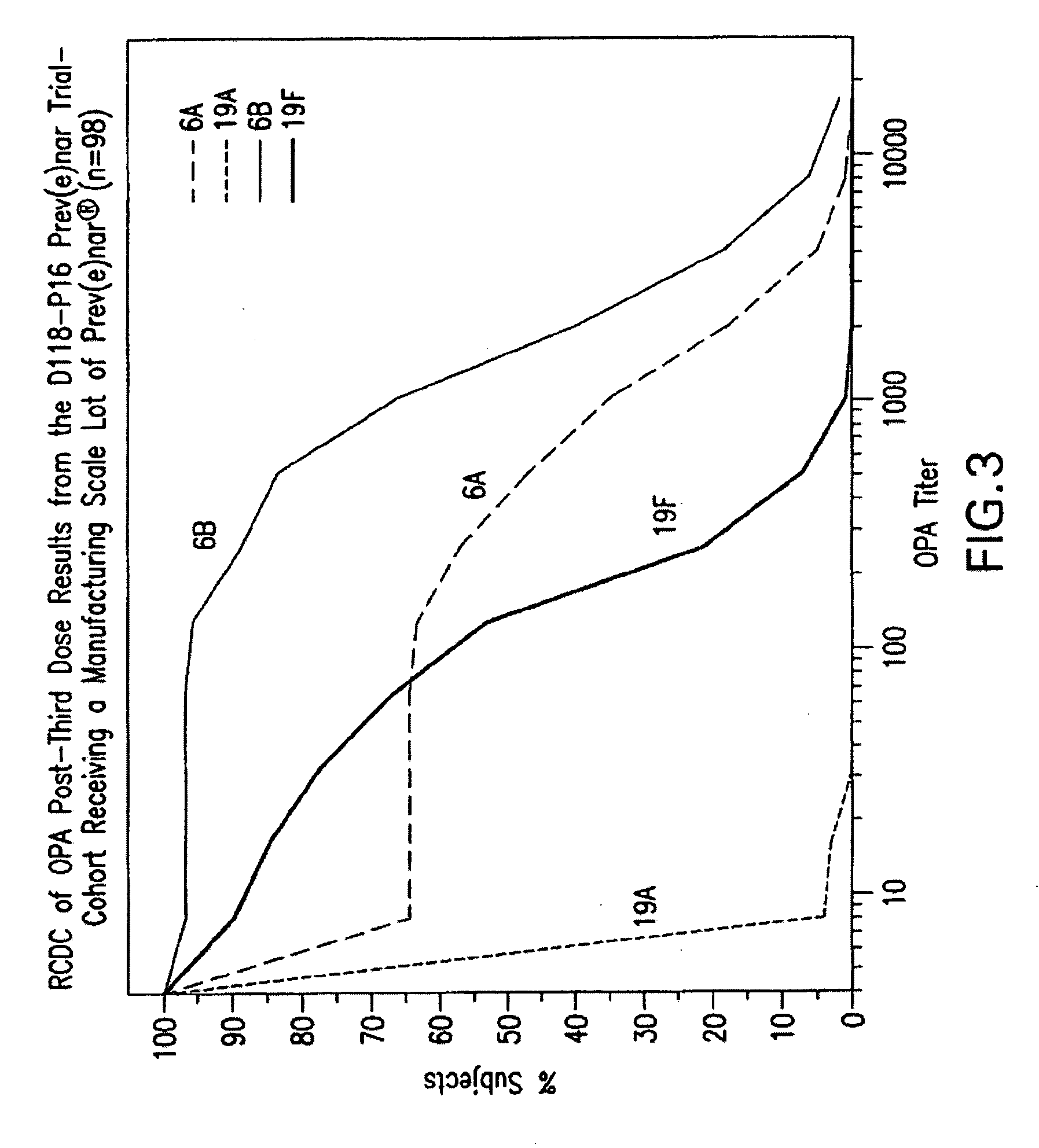
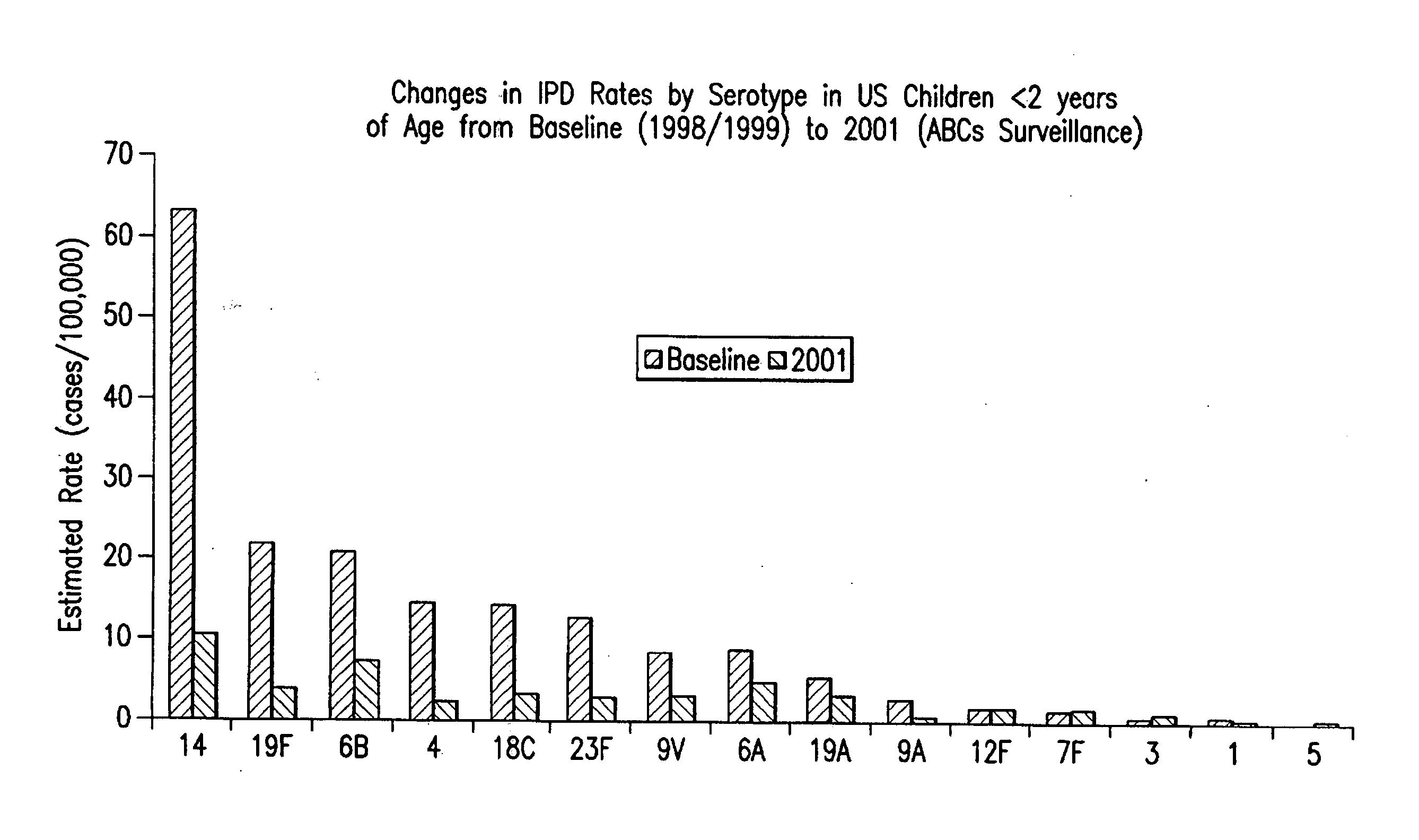
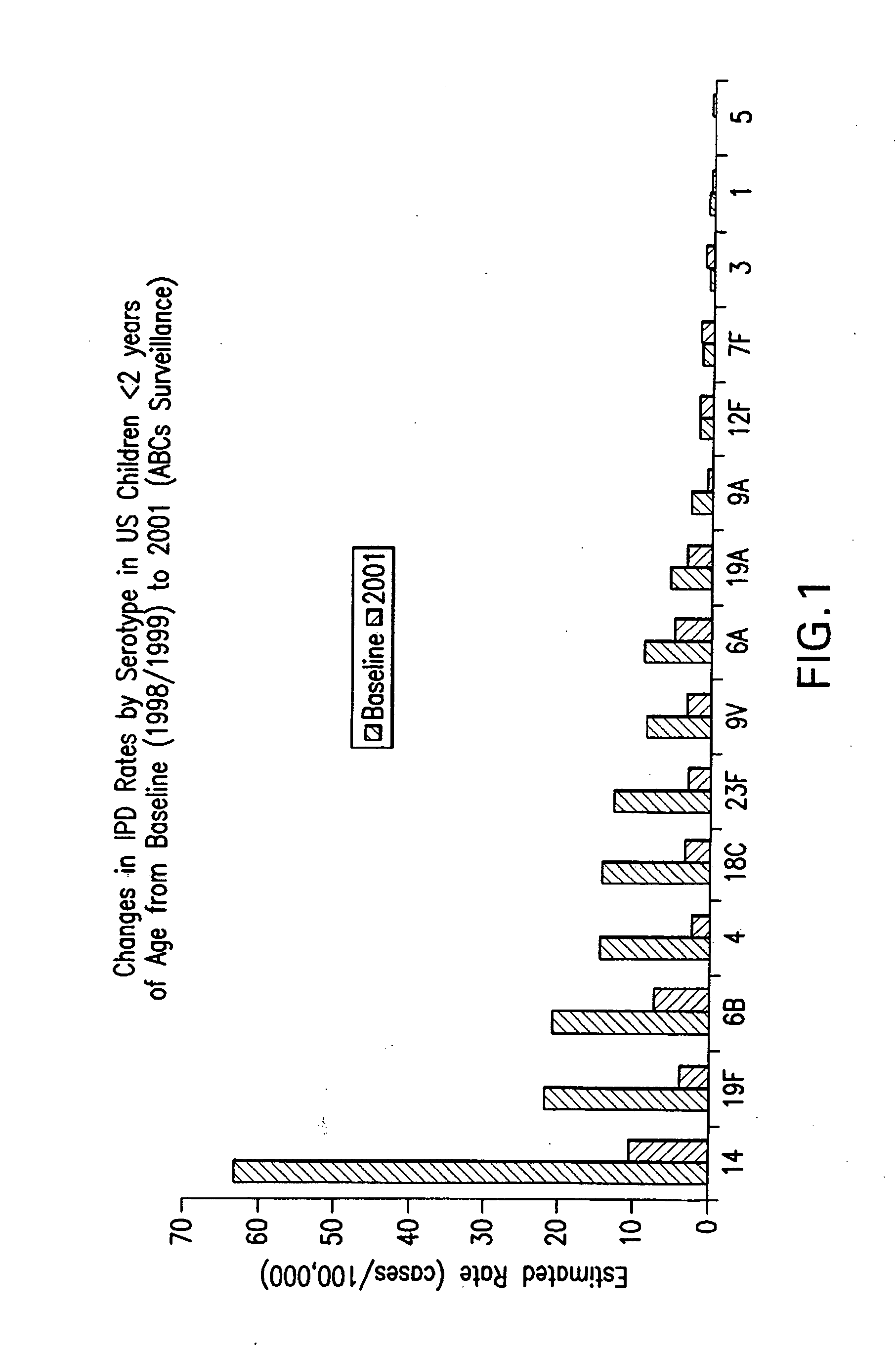
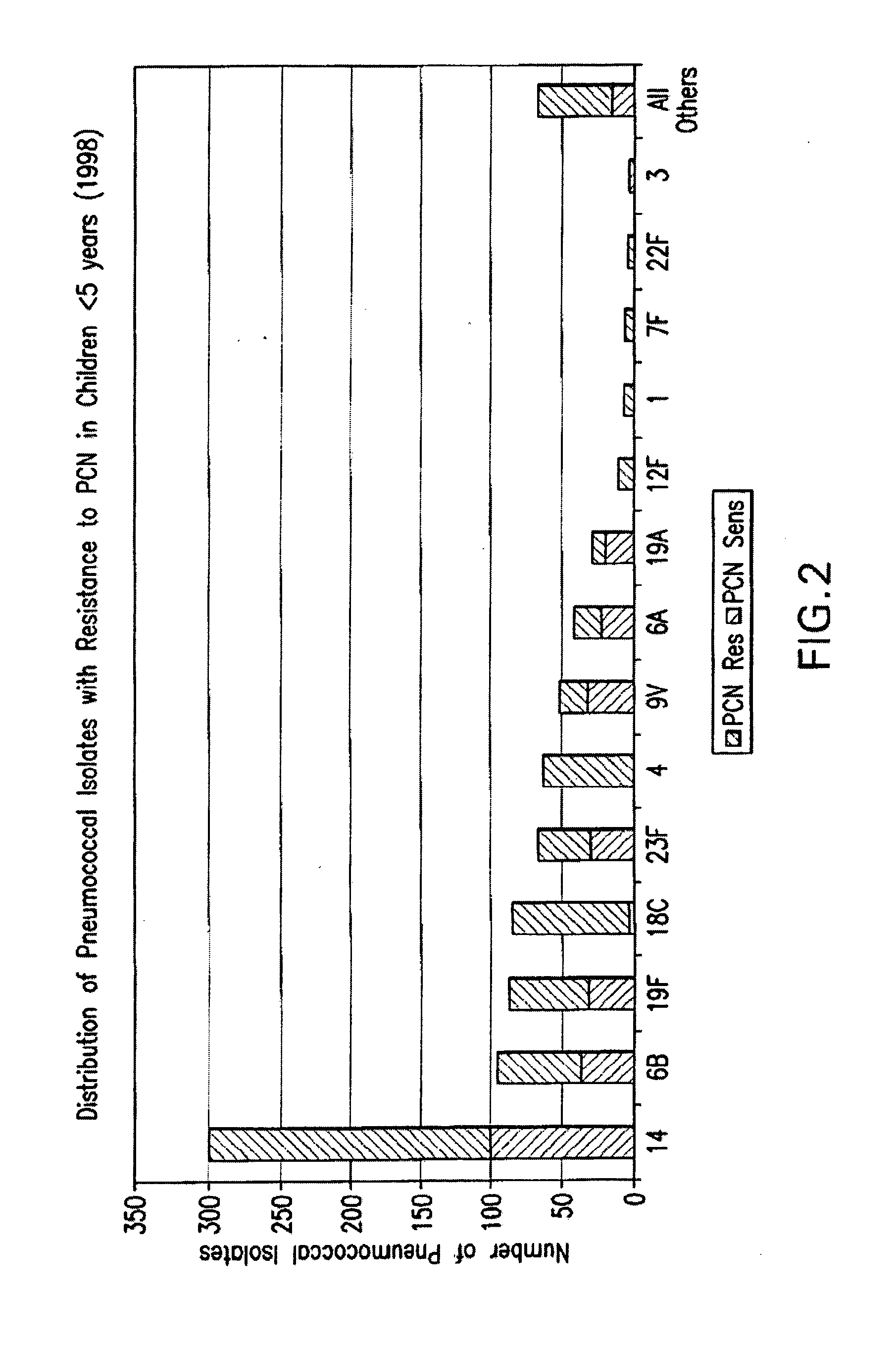
Multivalent pneumococcal polysaccharide-protein conjugate composition
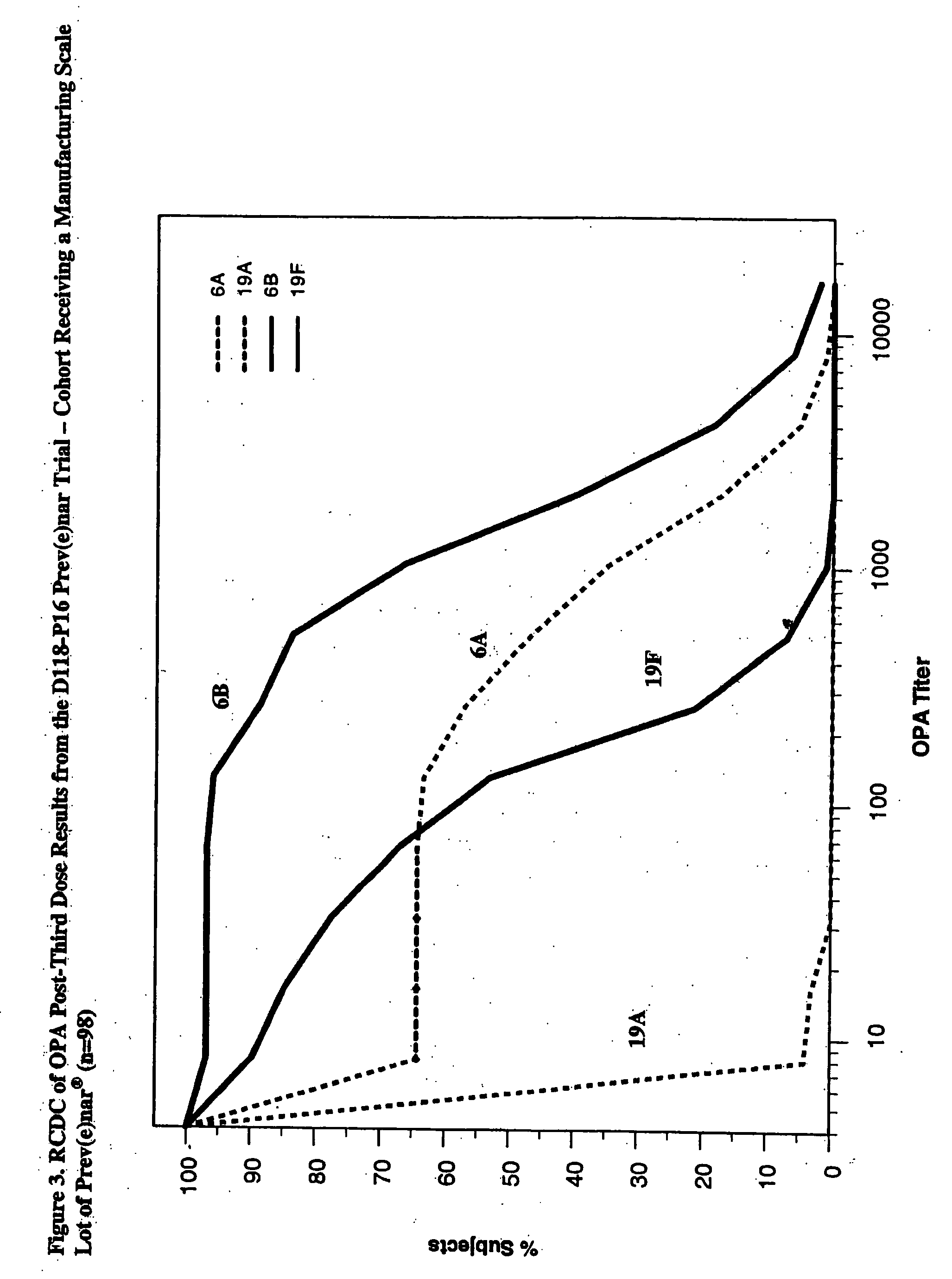
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
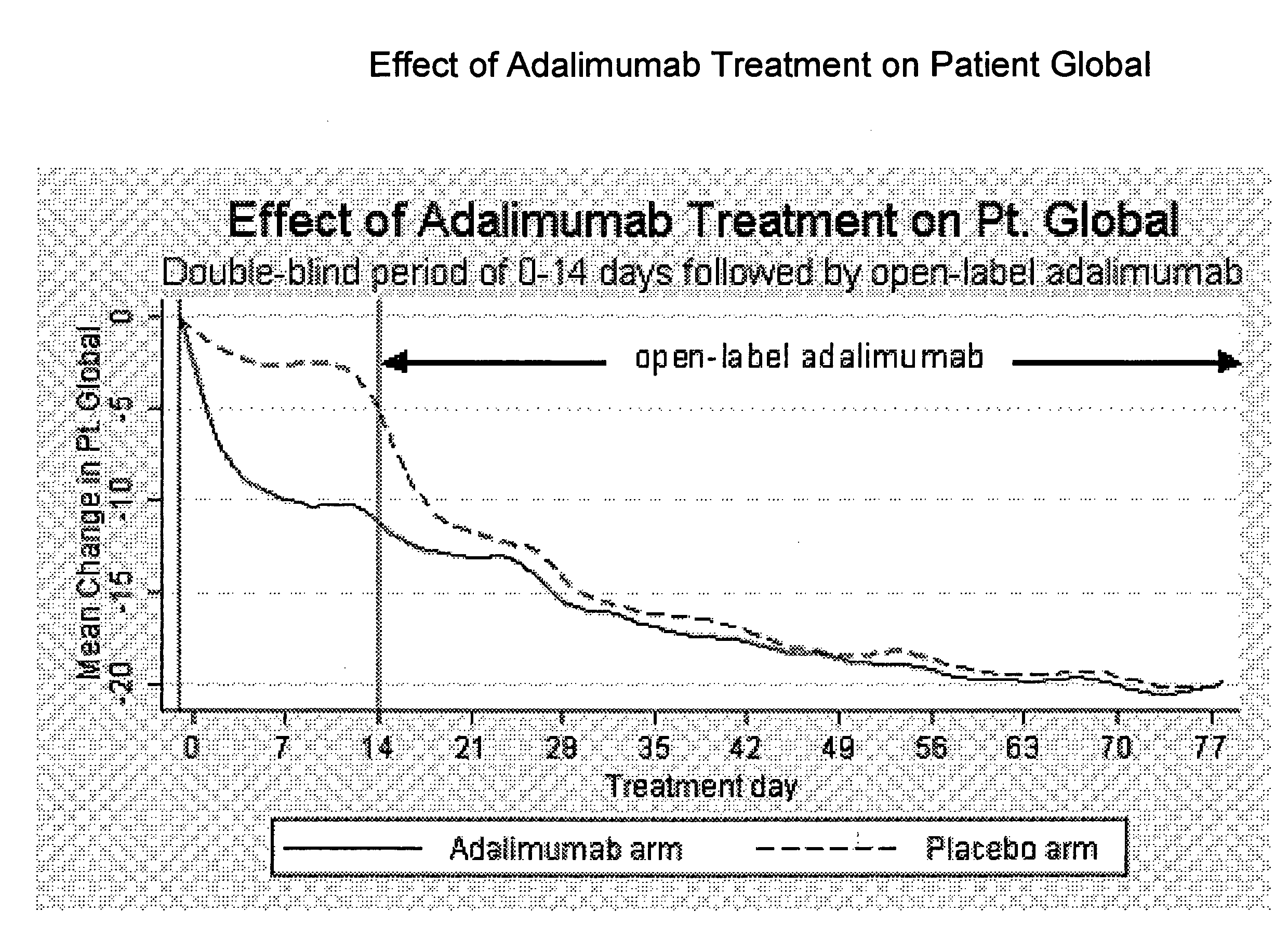
Uses and compositions for treatment of rheumatoid arthritis
ActiveUS20080166348A1Relieve symptomsInhibit progressSsRNA viruses negative-senseBiocideAntigen bindingRheumatoid arthritis
The invention provides methods, uses and compositions for the treatment of rheumatoid arthritis. The invention describes methods and uses for treating rheumatoid arthritis wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of rheumatoid arthritis in a subject.
Owner:ABBVIE BIOTECHNOLOGY LTD
Pancreatic cancer associated antigen, antibody thereto, and diagnostic and treatment methods
The present invention is directed to an antigen found on the surface of rat and human pancreatic cancer cells and provides antibodies of high specificity and selectivity to this antigen as well as hybridomas secreting the subject antibodies. Methods for both the diagnosis and treatment of pancreatic cancer are also provided. This tissue marker of pancreatic adenocarcinoma, an approximately 43.5 kD surface membrane protein designated PaCa-Ag1, is completely unexpressed in normal pancreas but abundantly expressed in pancreatic carcinoma cells. Moreover, a soluble form of PaCa-Ag1 exists, having a molecular weight about 36 to about 38 kD, that is readily identified in sera and other body fluids of pancreatic cancer patients, using a subject antibody.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
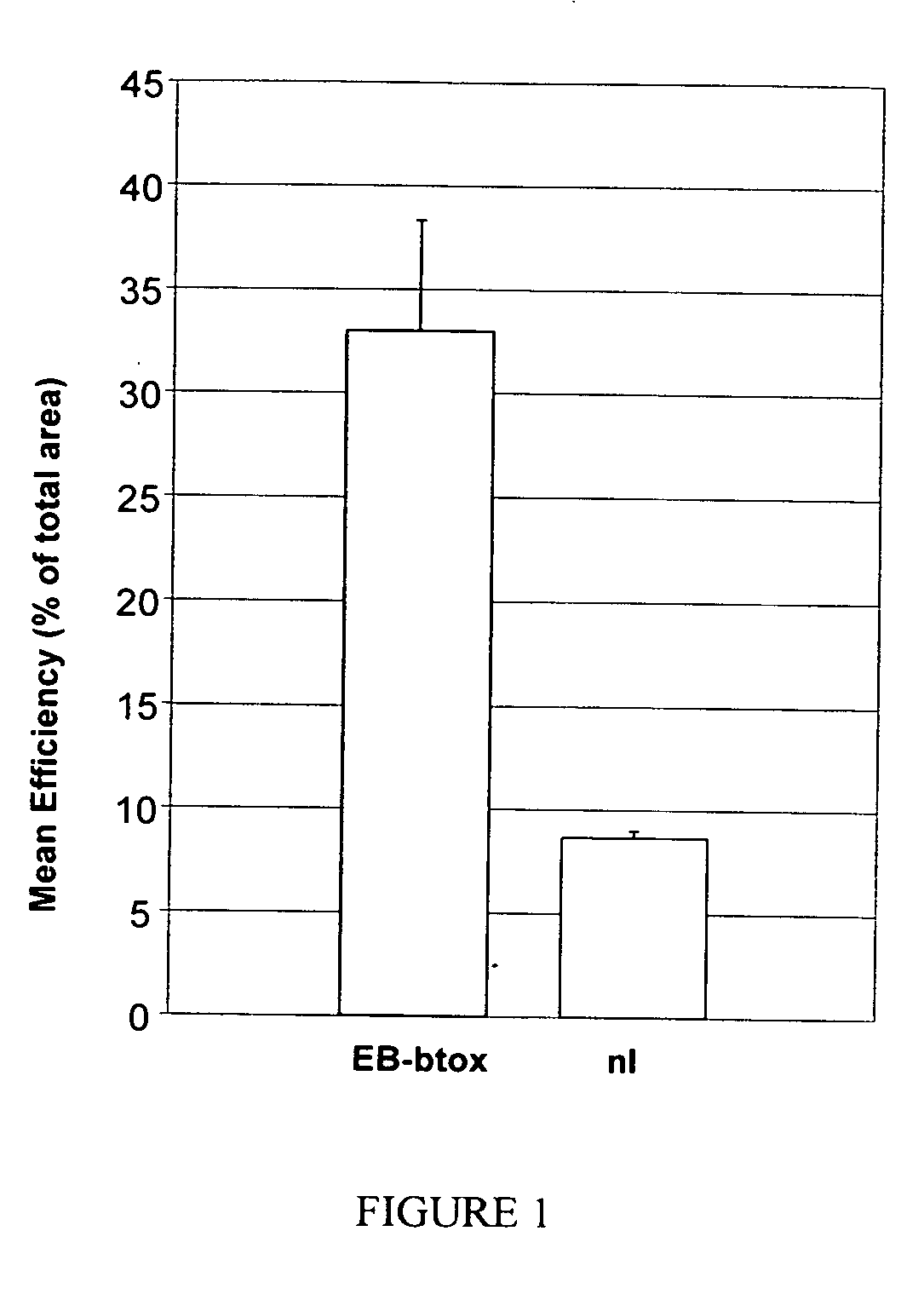
Compositions and methods for topical application and transdermal delivery of botulinum toxins
ActiveUS20050196414A1Reduce hypersecretionReduce sweatingCosmetic preparationsSenses disorderEpitheliumMedicine
A composition for topical application of a botulinum toxin (including botulinum toxin derivatives) comprises a botulinum toxin and a carrier comprising a polymeric backbone comprising a long-chain polypeptide or nonpeptidyl polymer having attached positively charged branching or “efficiency” groups. The invention also relates to methods for reducing muscle paralysis and other conditions that may be treated with a botulinum toxin, particularly paralysis of subcutaneous, and most particularly, facial, muscles, by topically applying an effective amount of the botulinum toxin and carrier, in conjunction, to the subject's skin or epithelium. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
Treatment of renal hypertension or carotid sinus syndrome with adventitial pharmaceutical sympathetic denervation or neuromodulation
ActiveUS20110104061A1Improve concentrationOrganic active ingredientsBacterial antigen ingredientsRenal HypertensionsCvd risk
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict medication regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
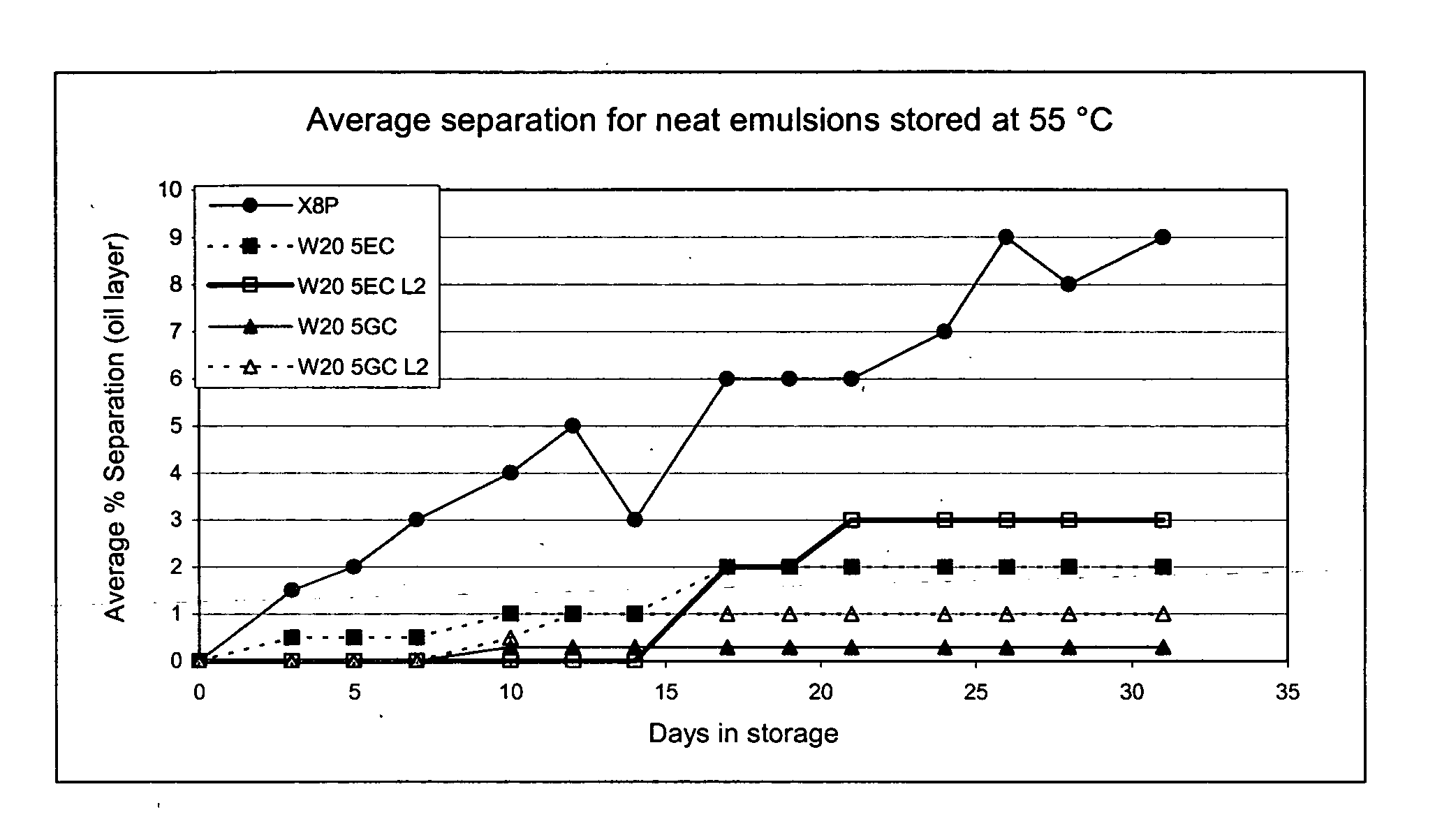
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
InactiveUS20060251684A1Reduce infectivityReduce morbiditySsRNA viruses negative-senseAntibacterial agentsPathogenic microorganismOrganic solvent
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Use of neurotoxin therapy for treatment of urologic and related disorders
The present invention relates to methods for treating neurological-urological conditions. This is accomplished by administration of at least one neurotoxin.
Owner:ALLERGAN INC
Therapeutic and prophylactic methods using heat shock proteins
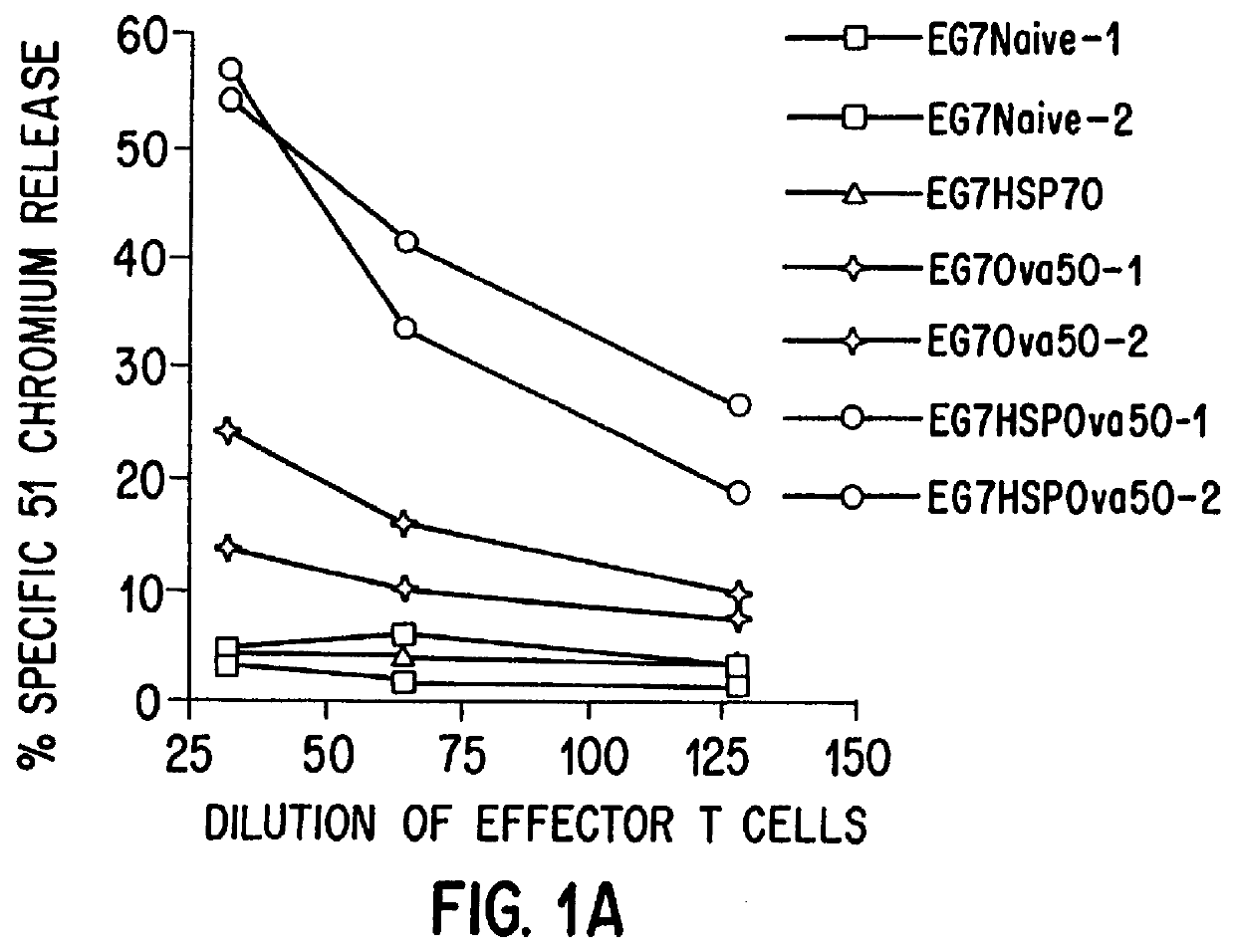
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
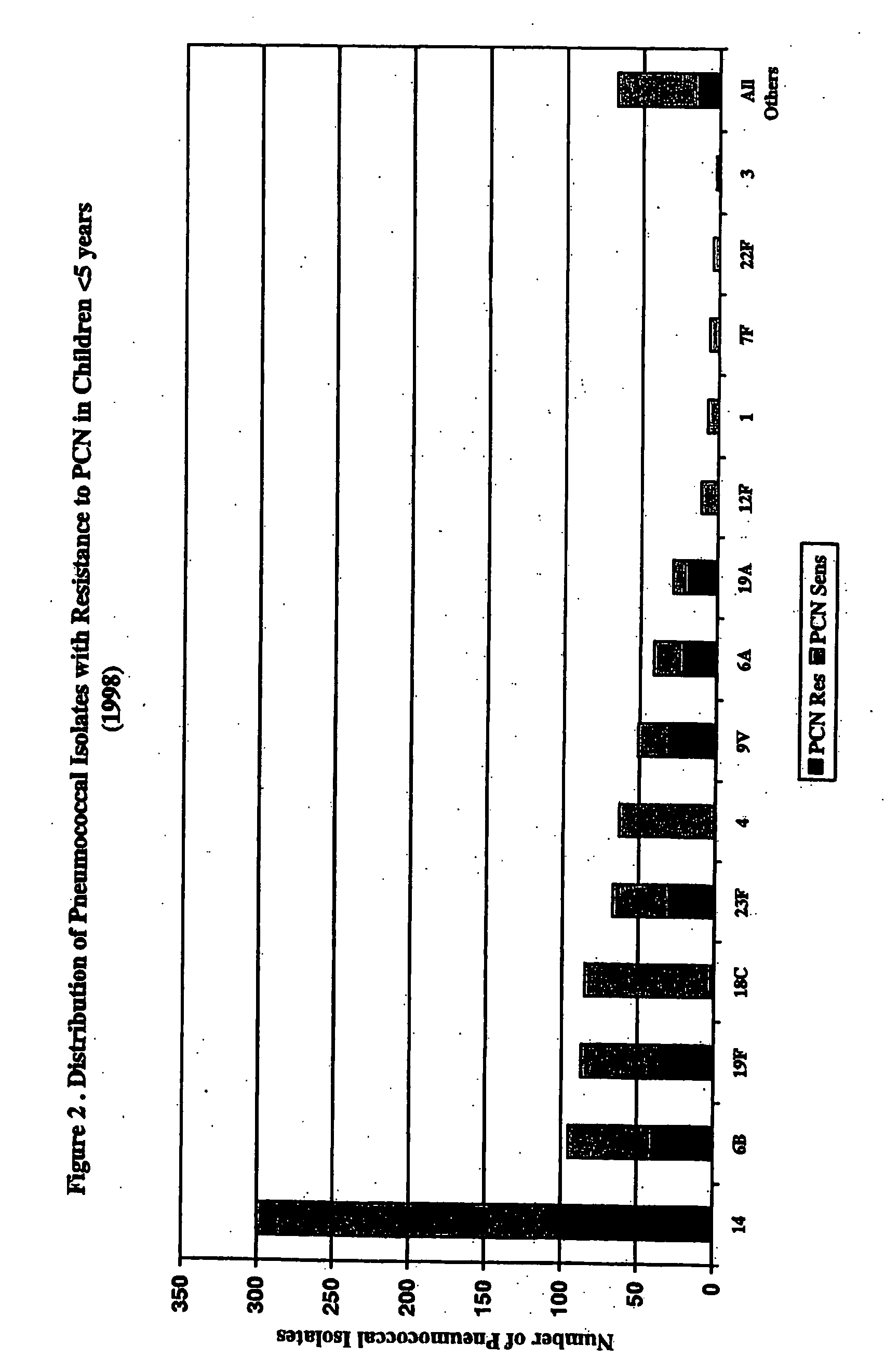
Separation of contaminants from Streptococcus pneumoniae polysaccharide by pH manipulation
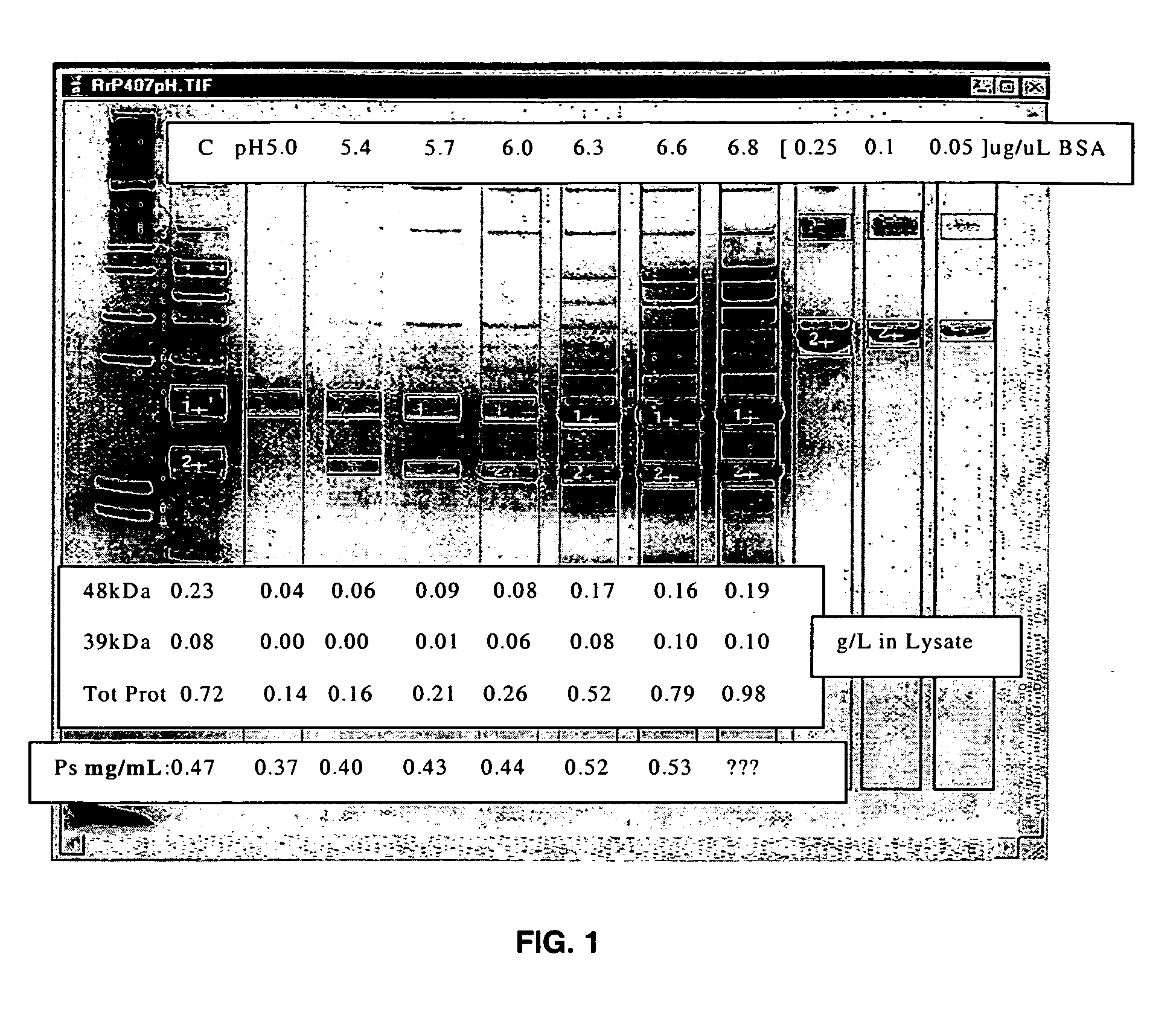
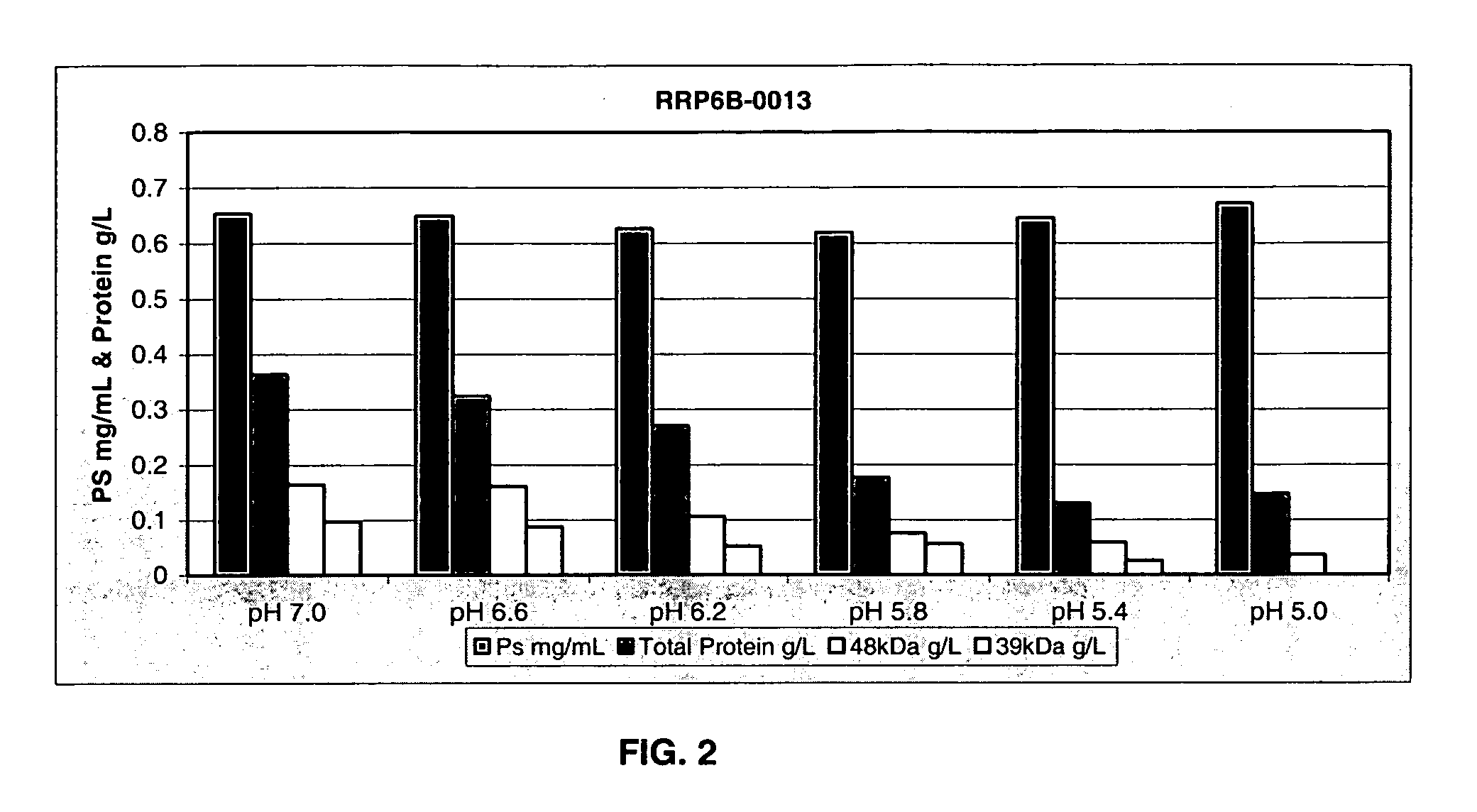
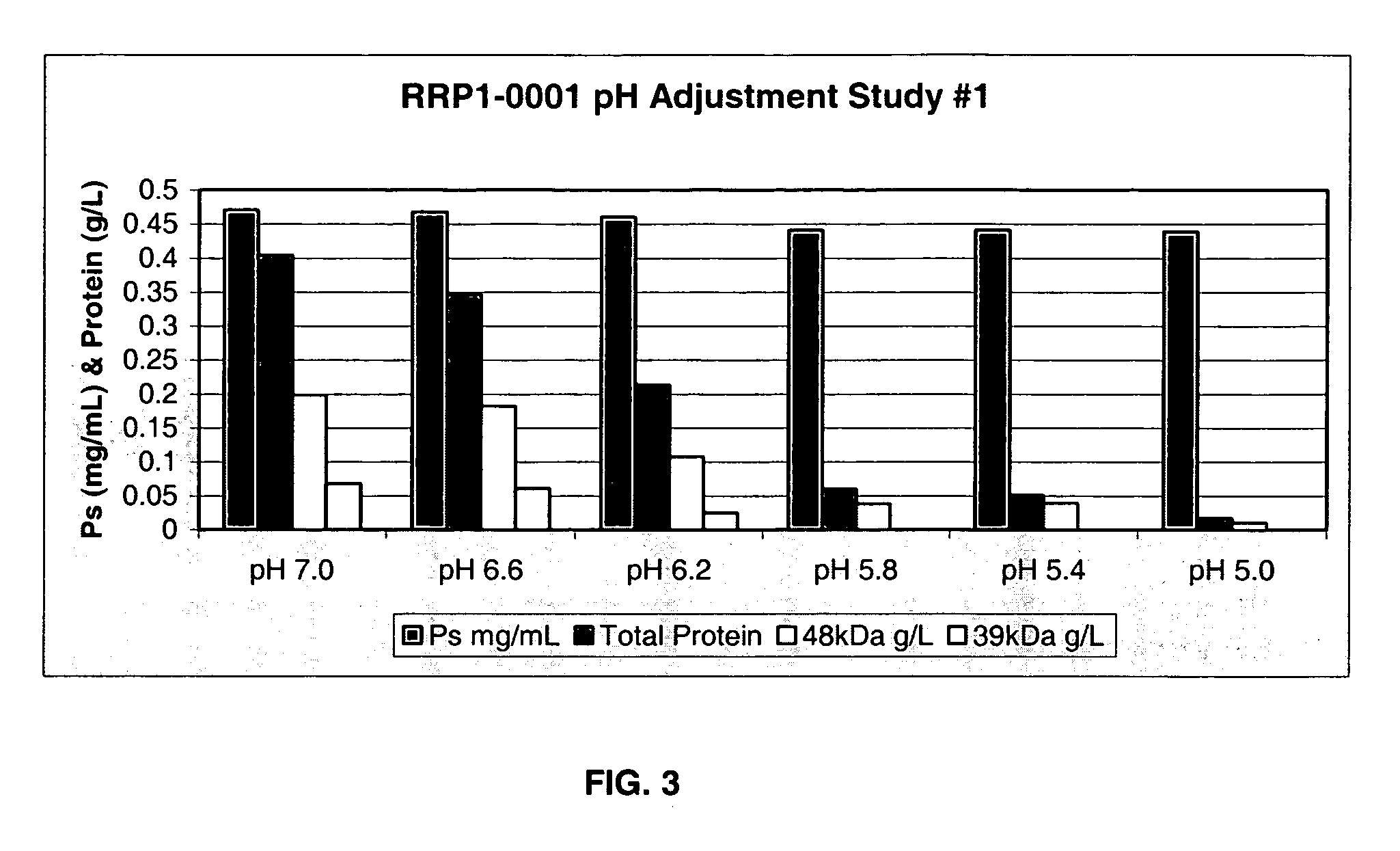
ActiveUS20060228381A1Soluble protein is effectively reducedBacterial antigen ingredientsSugar derivativesStreptococcus pneumoniaeLysis
A process for reducing the protein content and preserving the capsular polysaccharide content in a complex cellular Streptococcus pneumoniae lysate broth prior to purification is described. Utilizing pH reduction after cellular lysis has resulted in a purified polysaccharide that consistently meets the protein specification, and higher recovery yields of polysaccharide during the purification process.
Owner:WYETH LLC
Compositions and methods for the delivery of nucleic acids
InactiveUS20110117125A1Reduce particle aggregationReduce selection requirementsAntibacterial agentsOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:THE UNIV OF BRITISH COLUMBIA +2
Methods for treating sinus headache
InactiveUS6838434B2Effective treatmentReduce and inhibit and eliminateAntibacterial agentsBiocideToxin typesSurgery
A sinus headache can be treated by administration of a botulinum toxin to a patient. The botulinum toxin can be botulinum toxin type A and the botulinum toxin can be administered to or to the vicinity of a sinus membrane of a patient with a sinus headache.
Owner:ALLERGAN INC
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of skin diseases or disorders
Methods of treating, preventing, correcting and / or managing skin diseases or disorders characterized by overgrowths of the epidermis, keratoses, scleroderma, cutaneous vasculitis, acne or wrinkles are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent. Specific second active ingredients are capable of affecting or inhibiting cell growth or proliferation, removing or improving acne scars, or reducing or correcting wrinkle lines. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Multi-actuating trigger anchor delivery system
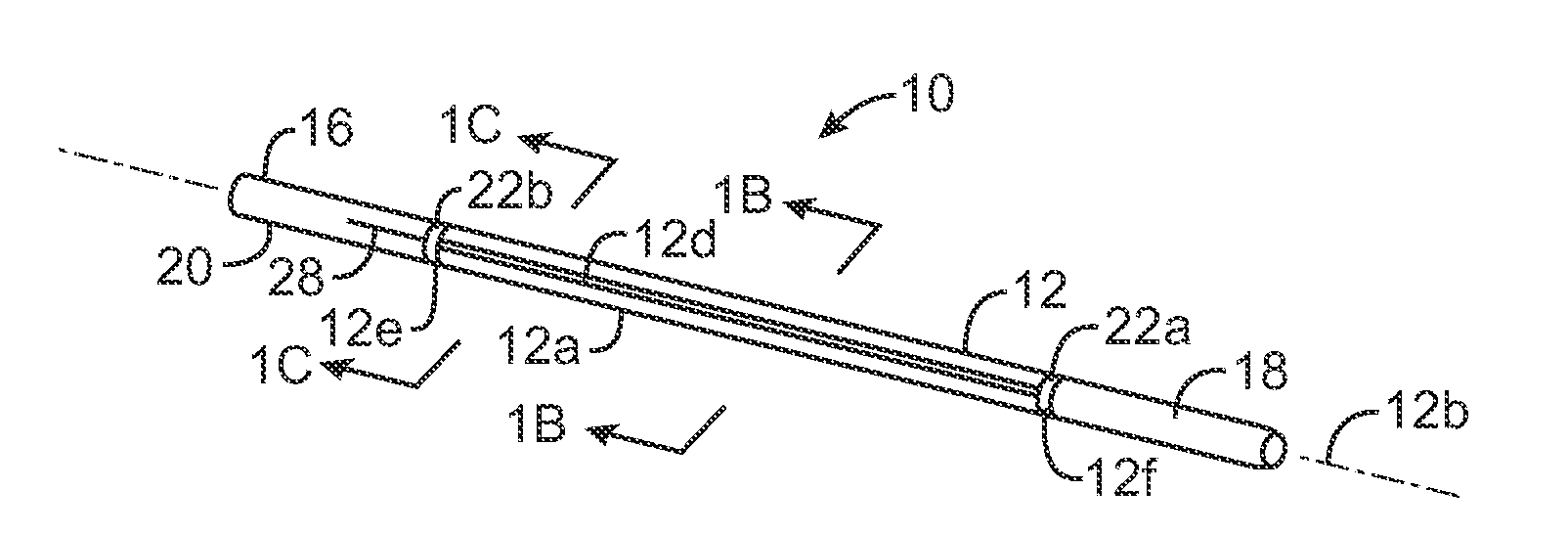
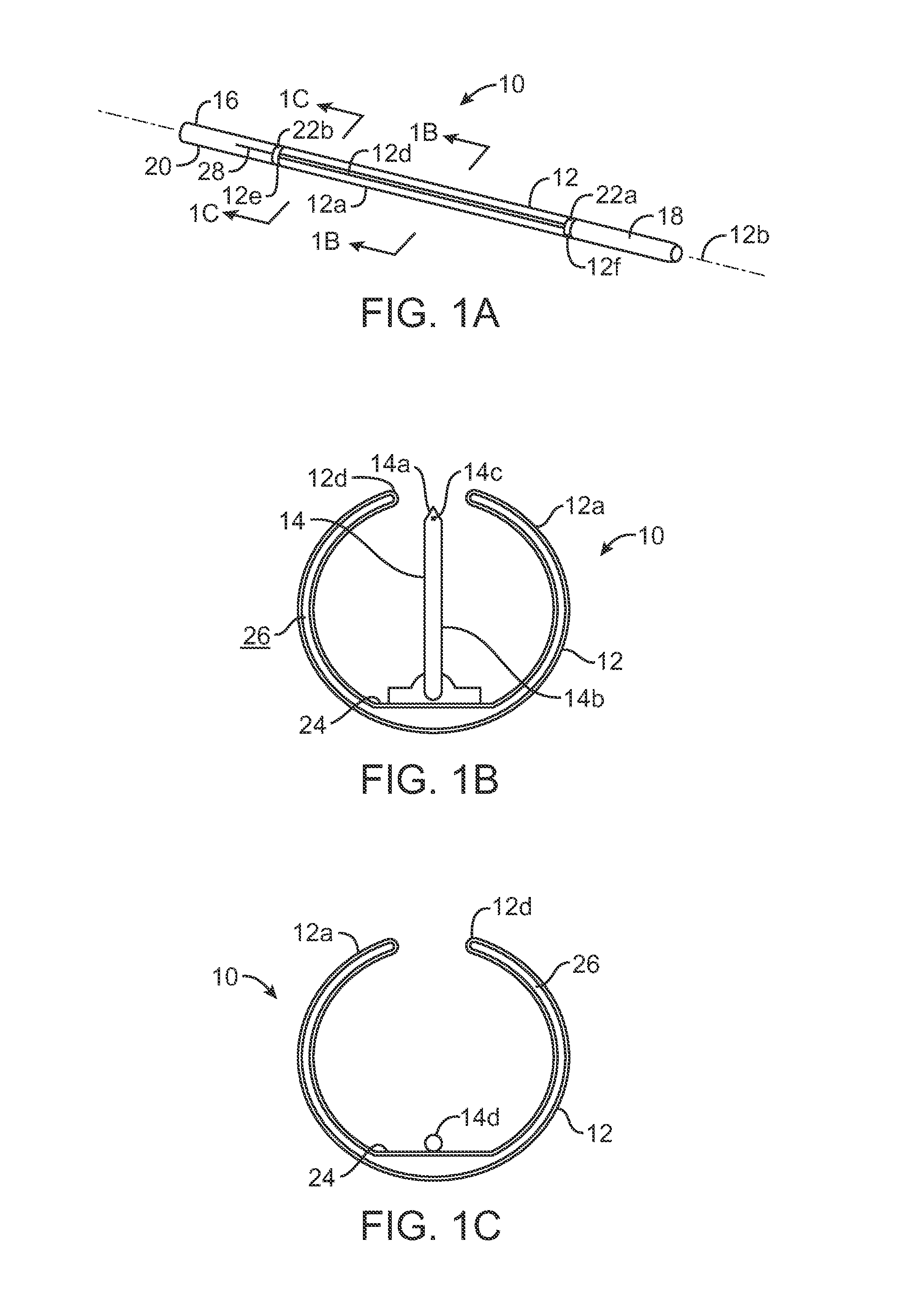
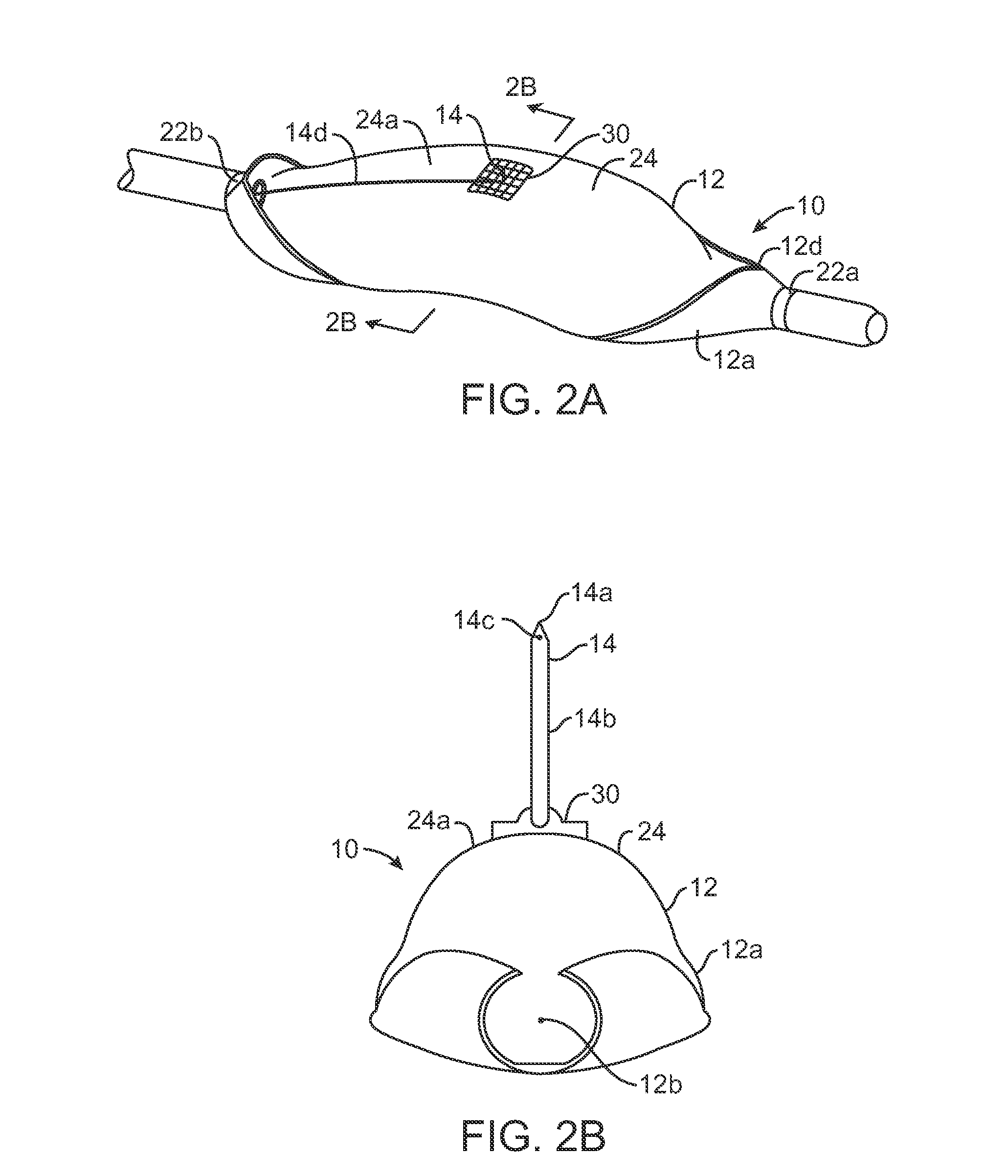
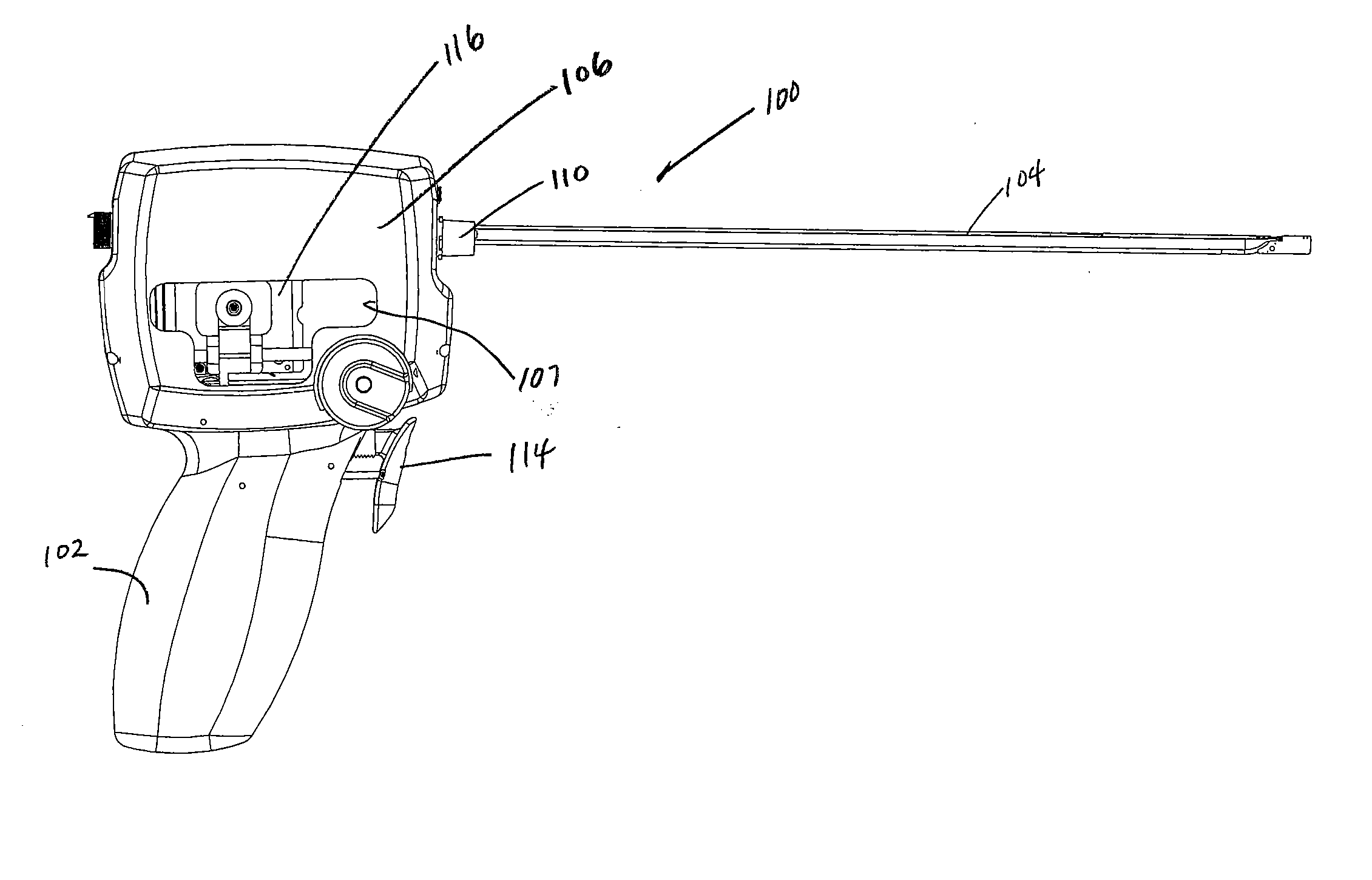
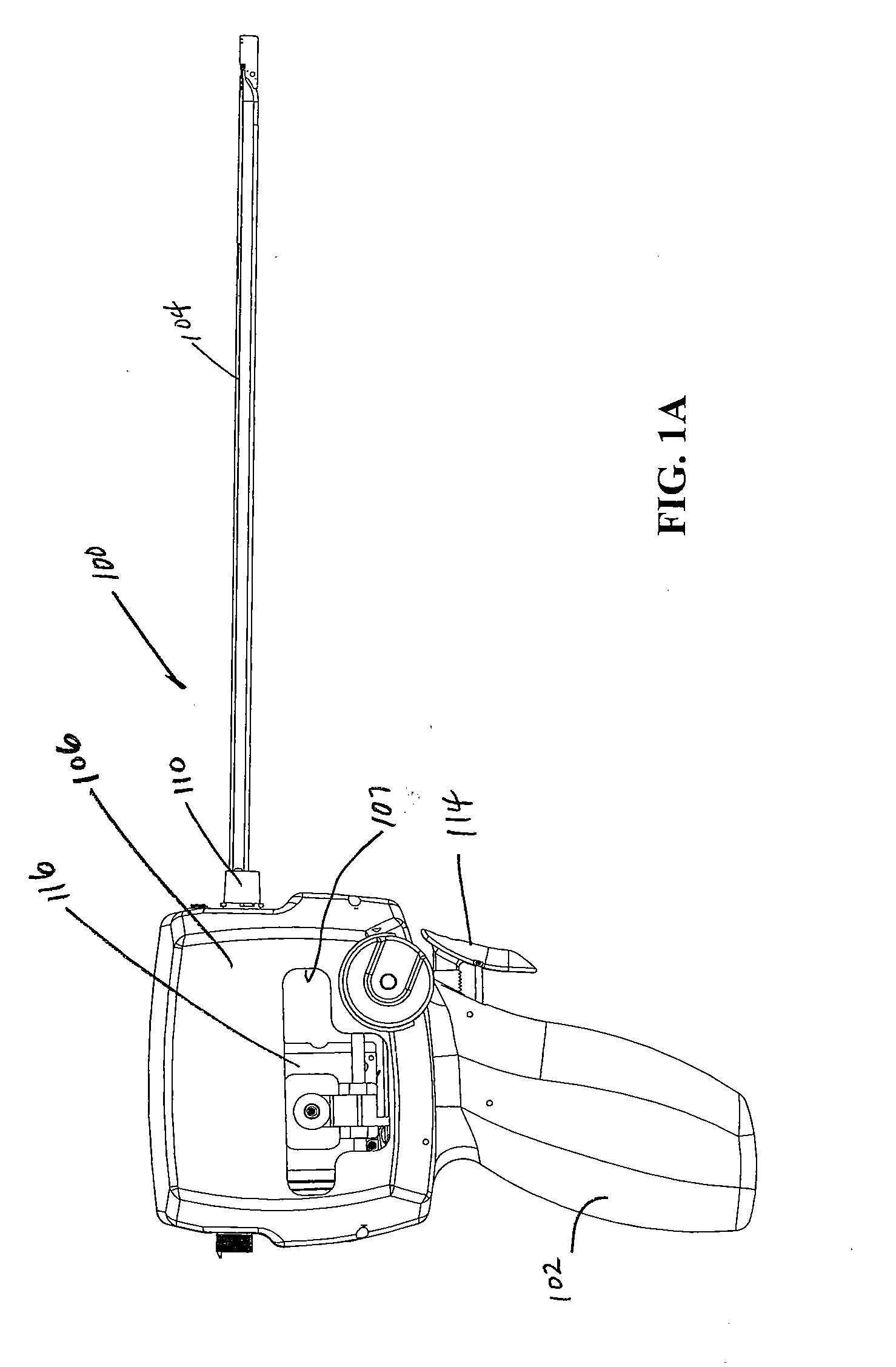
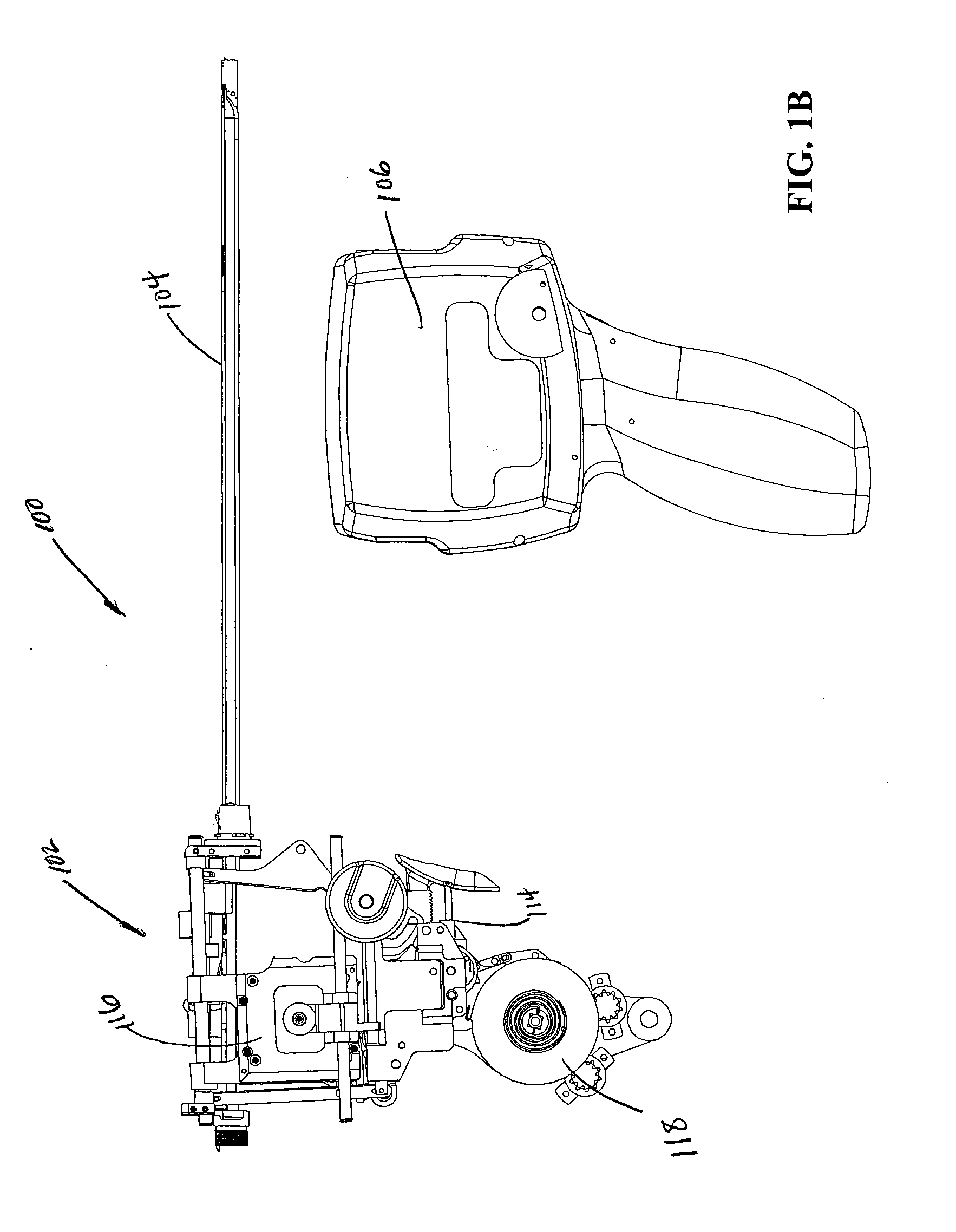
ActiveUS20080033458A1Promote healingMinimize infection risk riskSuture equipmentsBacterial antigen ingredientsBiomedical engineeringDelivery system
A single trigger system and associated method for manipulating tissues and anatomical or other structures in medical applications for the purpose of treating diseases or disorders or other purposes. In one aspect, the system includes a delivery device configured to deploy and implant anchor devices for such purposes.
Owner:TELEFLEX LIFE SCI LTD
Popular searches
Antibody mimetics/scaffolds Genetic material ingredients Dead animal preservation Immunoglobulins against cell receptors/antigens/surface-determinants Mammal material medical ingredients Blood/immune system cells Animal repellants Bacteriophages Cell receptors/surface-antigens/surface-determinants Artificial cell constructs
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com