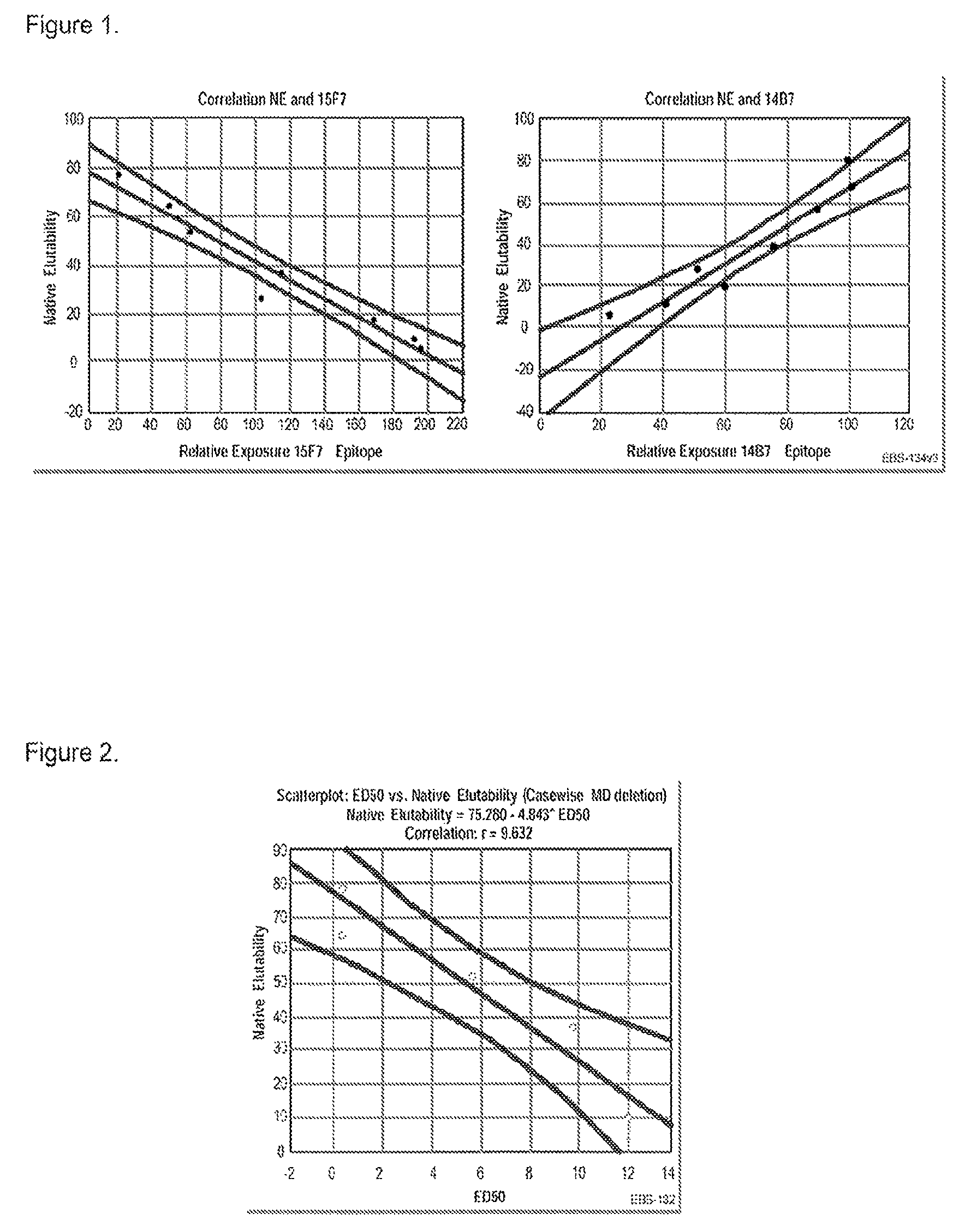
Patents
Literature
45 results about "Anthrax protective antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
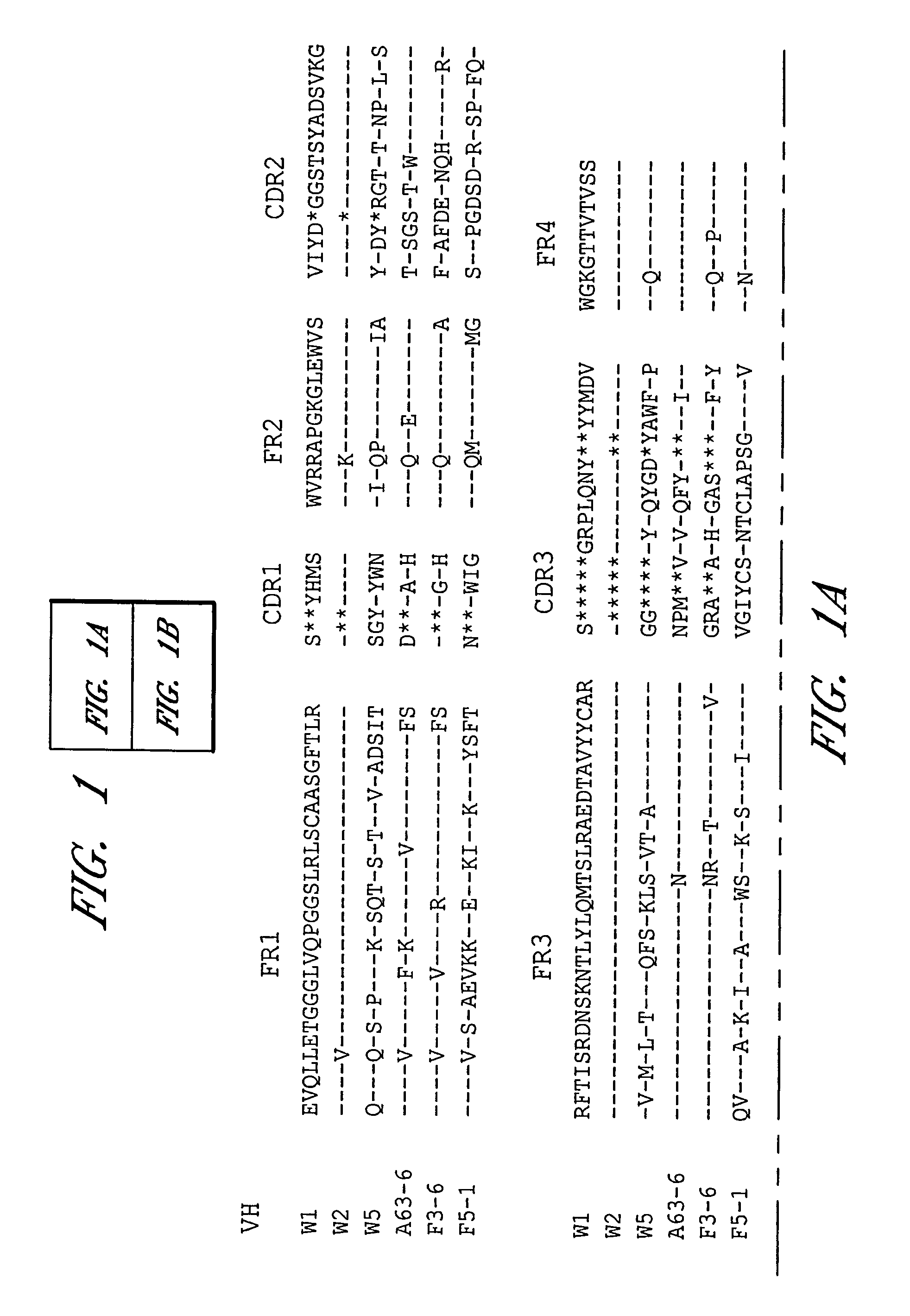
Human monoclonal antibodies against bacillus anthracis protective antigen
ActiveUS20050287149A1Neutralizing activityLess immunogenicAntibacterial agentsBacteriaV(D)J recombinationBispecific antibody
Isolated human monoclonal antibodies which bind to Anthrax protective antigen are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:MEDAREX LLC
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Human monoclonal antibodies against Bacillus anthracis protective antigen
ActiveUS7456264B2Avoid infectionNeutralizing activityAntibacterial agentsBacteriaAntigenV(D)J recombination
Isolated human monoclonal antibodies which bind to Anthrax protective antigen are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:ER SQUIBB & SONS INC
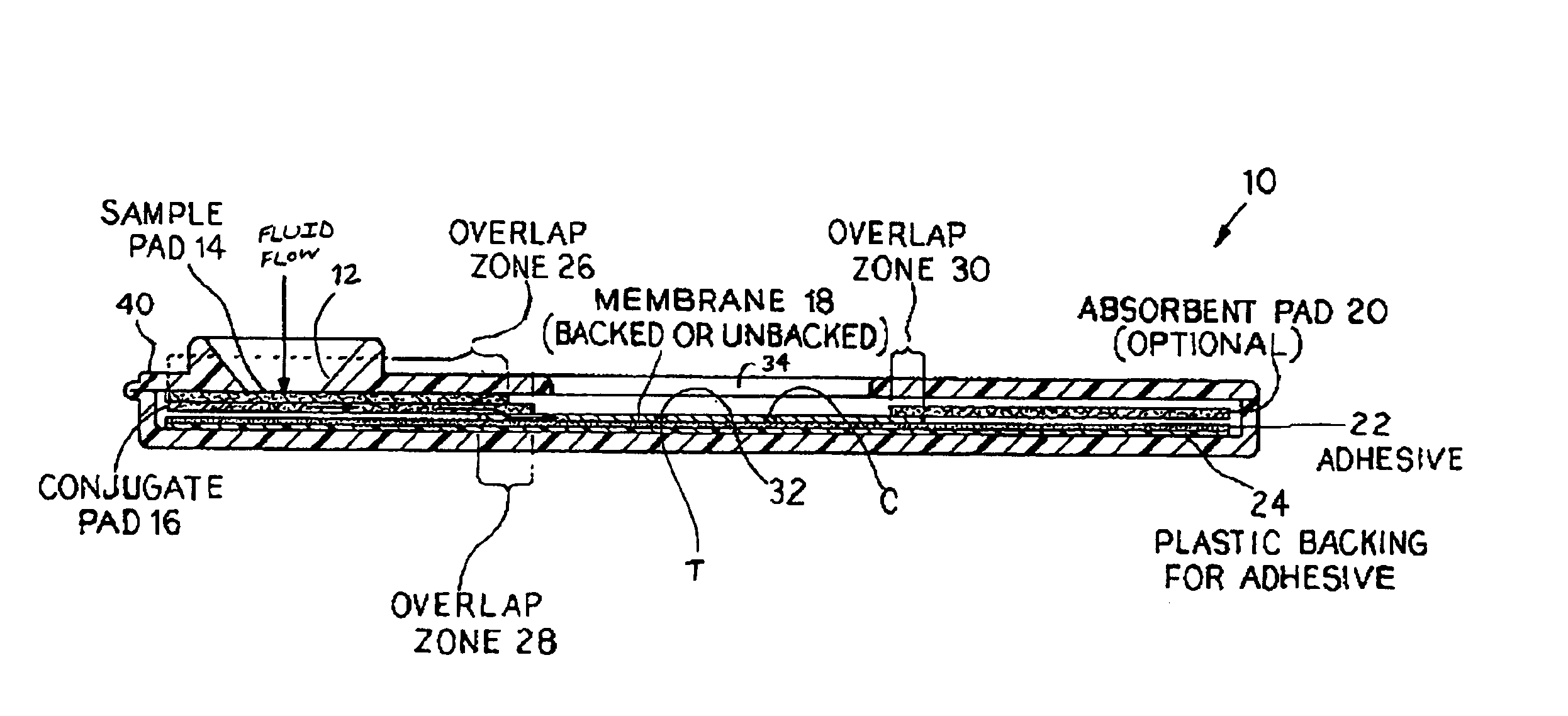
Rapid and non-invasive method to evaluate immunization status of a patient
InactiveUS6927068B2Rapid and reliable and non-invasive and safe testingRapidly evaluate immunization status of a patientBioreactor/fermenter combinationsBiological substance pretreatmentsSpecific iggAnthrax protective antigen
An assay method and kit for detecting the presence of a predesignated, target IgG antibody in a sample selected from one or more patient bodily fluids. The method comprises the following steps: (a) contacting the sample of one or more patient bodily fluids with a membrane-bound recombinant protective antigen to bind to the target IgG antibody in the sample; (b) previously, simultaneously or subsequently to step (a), binding the protective antigen (PA) with a conjugated label producing a detectable signal; and (c) detecting the signal whereby the presence of the target IgG antibody is determined in the sample by the intensity of the signal. The method can further comprise the step of evaluating immunization status of the patient from whom the sample came by comparing the signal or lack thereof with immunizations previously received by the patient. In a preferred embodiment, the recombinant protective antigen (PA) specifically binds to anthrax protective antigen-specific IgG antibodies. Preferably, the immunoassay of the present invention comprises a lateral-flow assay comprising a membrane, a conjugated label pad, and a recombinant protective antigen (PA) bound to the membrane.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Novel targets and compositions for use in decontamination, immunoprophylaxis, and post-exposure therapy against anthrax
InactiveUS20050271689A1Precise positioningEfficient methodBacterial antigen ingredientsDepsipeptidesEpitopeSpore germination
The present invention relates to the decontamination of anthrax spores, prophylaxis and treatment of anthrax infections and, more particularly, to compounds that act as specific inhibitors of B. anthracis germination / outgrowth-associated proteins, methods and means for making such inhibitors and their use as pharmaceuticals and / or vaccines. The invention also relates to the prophylaxis and treatment of anthrax infections and, more particularly, to vaccines and compositions that comprise B. anthracis antigens, epitopes, proteins, or nucleic acid molecules, including anthrax protective antigen, anthrax lethal factor, anthrax edema factor and anthrax proteins associated with spore germination and outgrowth, as well as methods and means for making such compositions and their use pharmaceuticals and / or vaccines.
Owner:ALTIMMUNE INC
High level constitutive production of anthrax protective antigen
InactiveUS20050054038A1Maximizing harvestingMinimized volumeAntibacterial agentsBacterial antigen ingredientsEscherichia coliAntigen
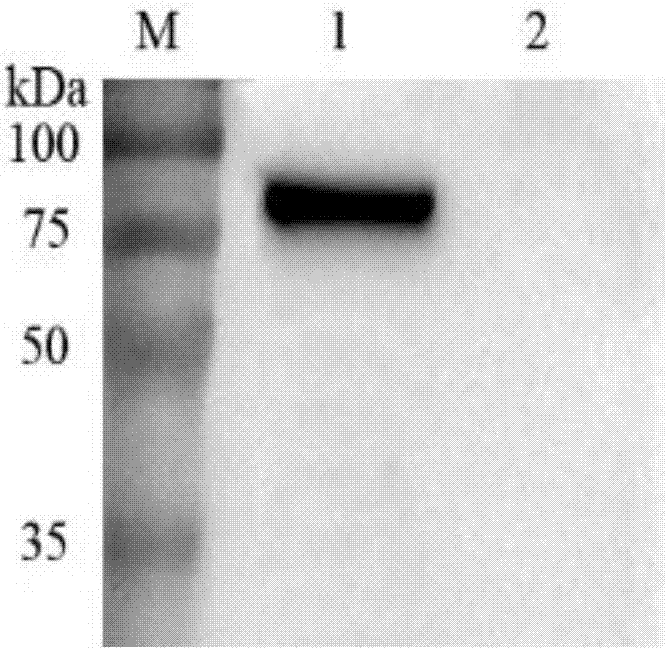
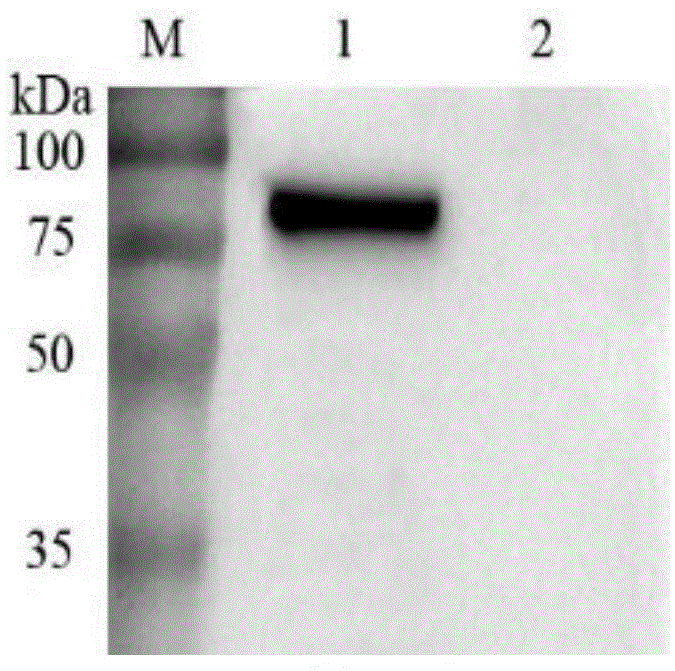
The present invention relates to a process for preparing anthrax protective antigen protein from E. coli using fed batch culture. This process creates a constitutively expressing system for rapid, efficient, cost-effective and high-level production of anthrax PA from E. coli. The steps of the process involves, transforming E. coli DH5α cells with the recombinant constitutive expression plasmid containing the PA gene to obtain recombinant DH5α cells and testing the PA expression by lysis of said cells followed by denaturing gel electrophoresis and Western Blotting technique using PA antibodies. This is followed by fermentation and harvesting of the high cell density cells. The said cells are solubilized using 6-8 Molar Urea and separated by centrifugation. The urea denatured PA is isolated from said supernatant and purified and thereafter eluted.
Owner:BHATNAGAR RAKESH
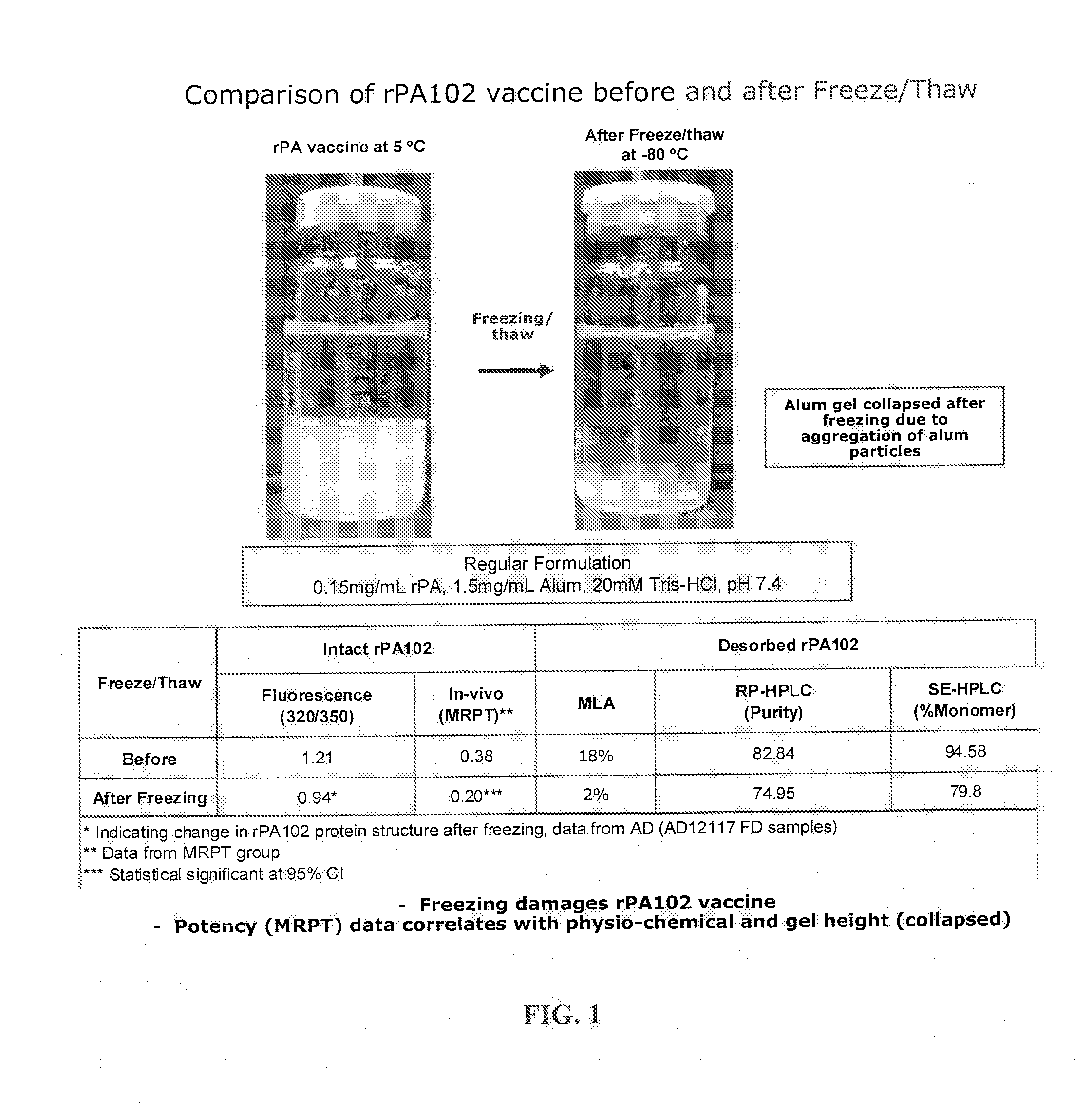
Stable anthrax vaccine formulations
ActiveUS20110229507A1Used for stabilizationImprove stabilityAntibacterial agentsBacterial antigen ingredientsAnthrax protective antigenVirology
Formulations of anthrax protective antigen are provided that are stable in storage for prolonged periods. Methods of using the formulations to prepare vaccine are also provided. Vaccines comprising the formulations are useful, for example, to protect against anthrax infection.
Owner:EMERGENT BIOSOLUTIONS
Translocation of non-natural chemical entities through anthrax protective antigen pore
Disclosed is a new approach for delivering compounds and drugs to the cytosol of living cells through the use of engineered protein transporters. The engineered protein transporters include a pore and a pore specific delivery protein, wherein a reagent such as a drug is attached to one or more of the engineered protein transporters.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Human monoclonal antibodies against bacillus anthracis protective antigen
Isolated human monoclonal antibodies that bind anthrax protective antigens are disclosed. Human antibodies can be produced in non-human transgenic animals, such as transgenic mice, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions containing human antibodies, non-human transgenic animals and hybridomas producing human antibodies, and therapeutic and diagnostic uses of human antibodies method.
Owner:ER SQUIBB & SONS INC
High level constitutive production of anthrax protective antigen
InactiveUS7329513B2Maximizing harvestingMinimized volumeAntibacterial agentsBacterial antigen ingredientsAntigenEscherichia coli
The present invention relates to a process for preparing anthrax protective antigen protein from E. coli using fed batch culture. This process creates a constitutively expressing system for rapid, efficient, cost-effective and high-level production of anthrax PA from E. coli. The steps of the process involves, transforming E. coli DH5α cells with the recombinant constitutive expression plasmid containing the PA gene to obtain recombinant DH5α cells and testing the PA expression by lysis of said cells followed by denaturing gel electrophoresis and Western Blotting technique using PA antibodies. This is followed by fermentation and harvesting of the high cell density cells. The said cells are solubilized using 6-8 Molar Urea and separated by centrifugation. The urea denatured PA is isolated from said supernatant and purified and thereafter eluted.
Owner:BHATNAGAR RAKESH
Targeting antigens to the MHC class I processing pathway with an anthrax toxin fusion protein
The present invention provides a vaccine for inducing an immune response in mammal to a specific antigen, where the vaccine comprises a unit dose of a binary toxin protective antigen and the antigen, which is bound to a binary toxin protective antigen binding protein. In one embodiment the vaccine is comprised of an anthrax protective antigen and the antigen bound to anthrax protective antigen binding protein. The present invention also provides a method of immunizing a mammal against an antigen using the vaccine, and a method of inducing antigen-presenting mammalian cells to present specific antigens via the MHC class I processing pathway.
Owner:UNITED STATES OF AMERICA
Rapid immunoassay of anthrax protective antigen in vaccine cultures and bodily fluids by fluorescence polarization
The inventive subject matter relates to a competitive method for estimating the concentration in a sample of a Bacillus anthracis protein or antibody thereof selected from the group consisting of protective antigen (PA), lethal factor (LF) and edema factor (EF). The method may employ the use of Fluorescence Polarization, FLT or FRET. The competitive methods are capable of detecting a target protein within 5 minutes within the range of 0.1 to 10.0 nM. The methods provide for the rapid detection and quantitation of bacteria, bacterial antigen or antibody in culture media or broth of growing cultures of bacteria, including B. anthracis by fluorescent methods.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Salmonella based oral vaccines for anthrax
A vaccine for the prevention of anthrax, including a live, attenuated Salmonella and at least one nucleotide sequence encoding anthrax protective antigen (PA) or a fragment thereof and a nonlethal mutated form of anthrax lethal factor (LF) or a fragment thereof. In another implementation, the vaccine is constituted for the prevention of anthrax and at least one additional pathogen, as including a live, attenuated Salmonella and at least one nucleotide sequence encoding at least a fragment of a nonlethal mutated form of anthrax lethal factor (LF) and at least one nucleotide sequence encoding at least a fragment of an antigen of an additional pathogen. Vaccines of such types can be administered to stimulate antibody response in a subject, whereby the antibody response confers immunity to the subject.
Owner:UNIV OF MARYLAND BALTIMORE
Antibody of humanized anti-anthrax protective antigen PA and application thereof
InactiveCN103789270AImprove protectionStrong specificityAntibacterial agentsImmunoglobulins against bacteriaAnthrax protective antigenBiology
The invention discloses an antibody of a humanized anti-anthrax protective antigen PA and an application thereof. According to the antibody, a heavy chain variable region has an amino acid sequence as shown in SEQ ID NO:4; a light chain variable region has an amino acid sequence as shown in SEQ ID NO:4; a heavy chain constant region has an amino acid sequence as shown in SEQ ID NO:6; and a light chain constant region has an amino acid sequence as shown in SEQ ID NO:8. The antibody can be specifically combined with the anthrax protective antigen (PA) and can be used for preventing a lethal factor LF so as to play a protective role.
Owner:中国人民解放军南京军区军事医学研究所 +1
Salmonella Based Oral Vaccines for Anthrax
A vaccine for the prevention of anthrax, including a live, attenuated Salmonella and at least one nucleotide sequence encoding anthrax protective antigen (PA) or a fragment thereof and a nonlethal mutated form of anthrax lethal factor (LF) or a fragment thereof. In another implementation, the vaccine is constituted for the prevention of anthrax and at least one additional pathogen, as including a live, attenuated Salmonella and at least one nucleotide sequence encoding at least a fragment of a nonlethal mutated form of anthrax lethal factor (LF) and at least one nucleotide sequence encoding at least a fragment of an antigen of an additional pathogen. Vaccines of such types can be administered to stimulate antibody response in a subject, whereby the antibody response confers immunity to the subject.
Owner:UNIV OF MARYLAND
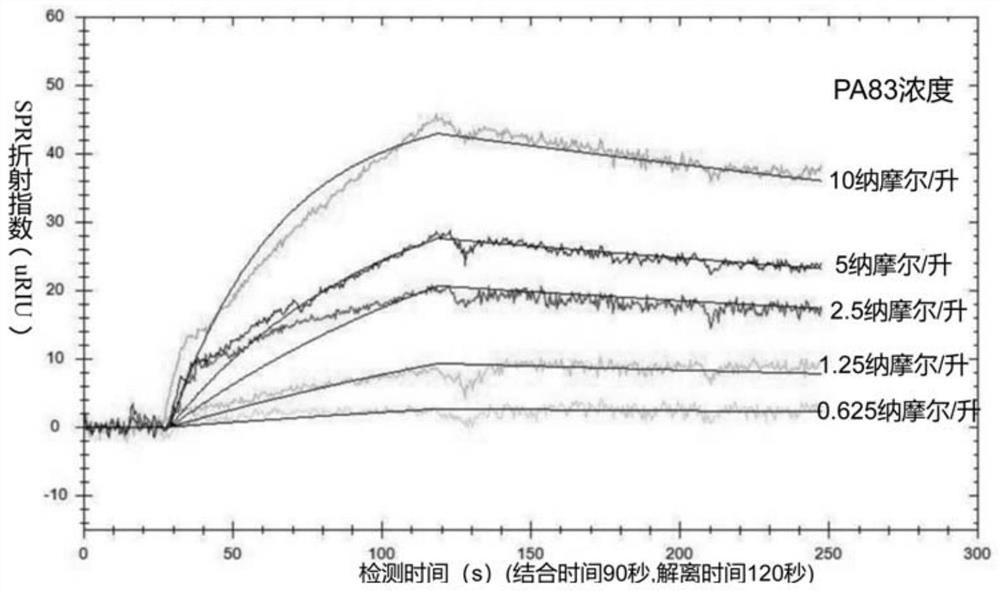
Chemically modified basic group-containing single-stranded DNA aptamer capable of specifically recognizing anthrax protective antigen PA83 and application thereof
The invention discloses a chemically modified base-containing single-stranded DNA aptamer capable of specifically recognizing anthrax protective antigen PA83 and application thereof. According to thechemically modified base-containing single-stranded DNA aptamer, the anthrax protective antigen (PA83) is taken as a target, a nucleic acid library containing chemically modified nucleotides is utilized, a high-affinity aptamer AP5 is screened out by a paramagnetic particle method SELEX, and the aptamer can specifically recognize PA83 protein in the environment of various interference proteins; the aptamer can be applied to an aptamer biosensor based on a surface plasmon resonance technology and a fluorescence polarization technology and used for detecting PA83 protein so as to detect bacillusanthracis, and the aptamer has the potential of being applied to construction of a novel bacillus anthracis protective antigen detection technology.
Owner:中国人民解放军疾病预防控制中心
Temperature Stable Vaccine Formulations
InactiveUS20150335752A1Improve effectivenessReduce the amount of solutionAntibacterial agentsPowder deliveryDiseaseFreeze and thaw
Formulations of vaccine antigen, such as anthrax protective antigen, are provided that are stable after undergoing freeze and thaw conditions. Methods of using the formulations to prepare vaccine are also provided. Vaccines comprising the formulations are useful, for example, to protect against, inhibit or alleviate a disease or infection, such as related to anthrax infection.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Stable anthrax vaccine formulations
ActiveUS8778359B2Improve stabilityImprove featuresAntibacterial agentsBacterial antigen ingredientsAnthrax protective antigenVirology
Formulations of anthrax protective antigen are provided that are stable in storage for prolonged periods. Methods of using the formulations to prepare vaccine are also provided. Vaccines comprising the formulations are useful, for example, to protect against anthrax infection.
Owner:EMERGENT BIOSOLUTIONS
Monoclonal antibodies to anthrax protective antigen
ActiveUS20100003263A1Avoid toxicityAntibacterial agentsPeptide/protein ingredientsLarge fragmentAnthrax protective antigen
The characterization and isolation of F20G75, F20G76 and F20G77, anti-PA monoclonal antibodies which also have neutralizing activities is described. The monoclonal antibodies may be used as a pharmaceutical composition for treating individuals suspected of or at risk of or having a Bacillus anthracis infection. The monoclonal antibodies bind to a specific region comprising amino acids 311-316 of PA, ASFFDI or a larger fragment comprising amino acids 301-330 of PA, SEVHGNAEVHASFFDIGSSVSAGFSNSNSS. Vaccines comprising these peptides may be used to immunize individuals against Bacillus anthracis infection.
Owner:TSANG RAYMOND +6
Translocation of non-natural chemical entities through anthrax protective antigen pore
InactiveUS20170049906A1Current is limitedTechnology is limitedOrganic active ingredientsPharmaceutical non-active ingredientsCytosolADAMTS Proteins
Disclosed is a new approach for delivering compounds and drugs to the cytosol of living cells through the use of engineered protein transporters. The engineered protein transporters include a pore and a pore specific delivery protein, wherein a reagent such as a drug is attached to one or more of the engineered protein transporters.
Owner:MASSACHUSETTS INST OF TECH +1
Method for preparing antigen effective for preventing anthrax infection
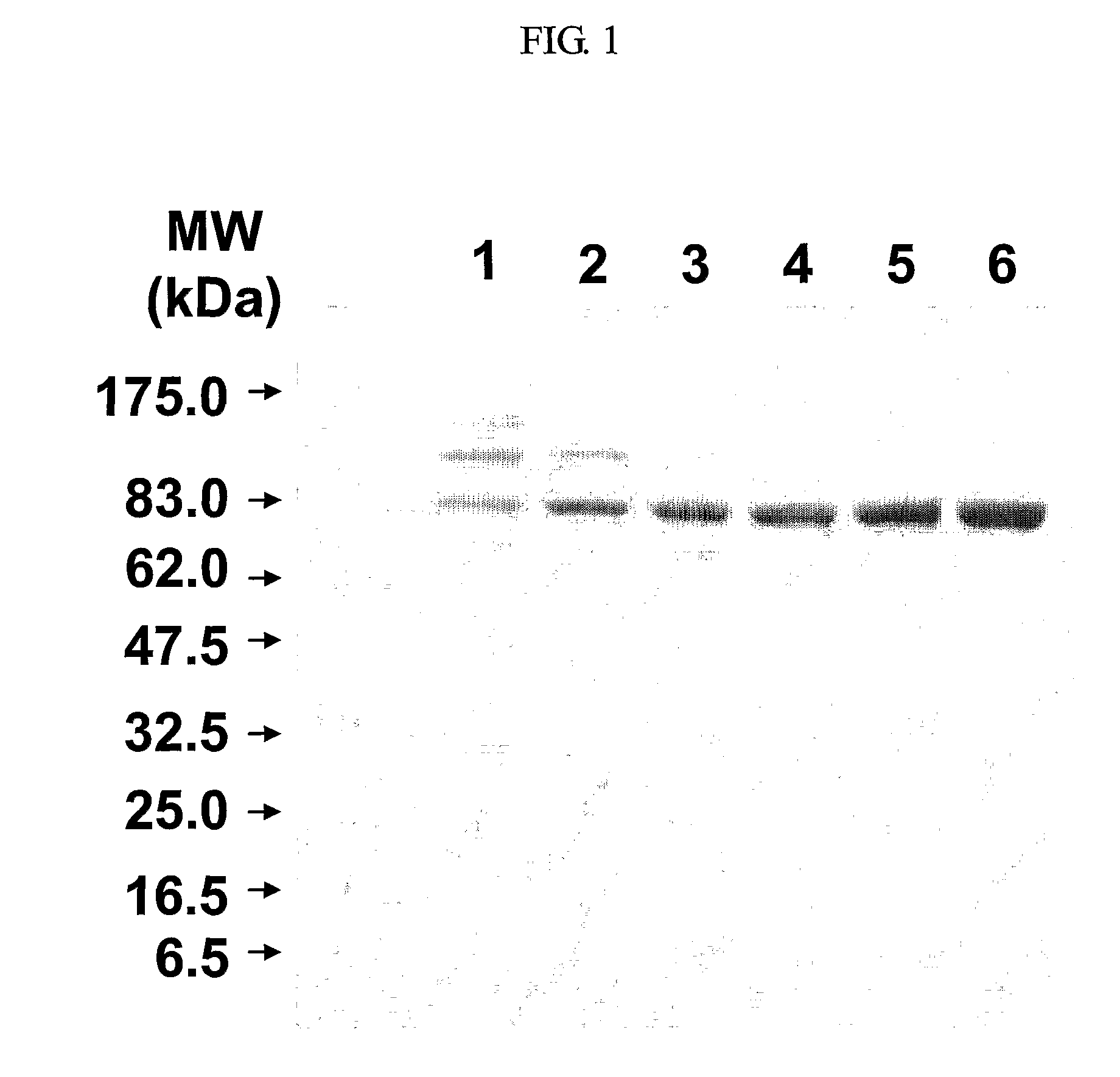
ActiveUS20110015377A1Efficient and high yieldBacteriaViral antigen ingredientsAntigenAnthrax protective antigen
The method of the present invention comprising successive column chromatography processes for the purification of an anthrax protective antigen can achieve an improved purity of the anthrax protective antigen product by effectively removing impurities (e.g., cellular residual proteins in the culture solution) without the loss of anthrax protective antigen. Therefore, the method of the present invention can be advantageously used for economically producing the anthrax protective antigen on a large scale.
Owner:KOREA CENT FOR DISEASE CONTROL & PREVENTION +1
Monoclonal antibodies to anthrax protective antigen
The characterization and isolation of F20G75, F20G76 and F20G77, anti-PA monoclonal antibodies which also have neutralizing activities is described. The monoclonal antibodies may be used as a pharmaceutical composition for treating individuals suspected of or at risk of or having a Bacillus anthracis infection. The monoclonal antibodies bind to a specific region comprising amino acids 311-316 of PA, ASFFDI or a larger fragment comprising amino acids 301-330 of PA, SEVHGNAEVHASFFDIGSSVSAGFSNSNSS. Vaccines comprising these peptides may be used to immunize individuals against Bacillus anthracis infection.
Owner:TSANG RAYMOND +6
Production of recombinant anthrax protective antigen and its special expression plasmid
ActiveCN100336908CAvoid degradationHigh purityBacteriaMicroorganism based processesEscherichia coliMetabolite
The invention provides a method and the expressed plasmid, which prepare the recombined anthrax protective antigen. The DNA sequence of the plasmid is the sequence 1 or the sequence which can cross with the sequence 1.The method gets the engineering strain by translating the E.coli using the expressed plasmid of the recombined anthrax protective antigen, so next to ferment the strain and get the metabolite. The method can get the more rPA, which have the true structure and avoid degrading by the proteinase. So we can get the 15mg / L rPA that's purity is above 95%, and the N terminal is accordance with the nature antigen.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
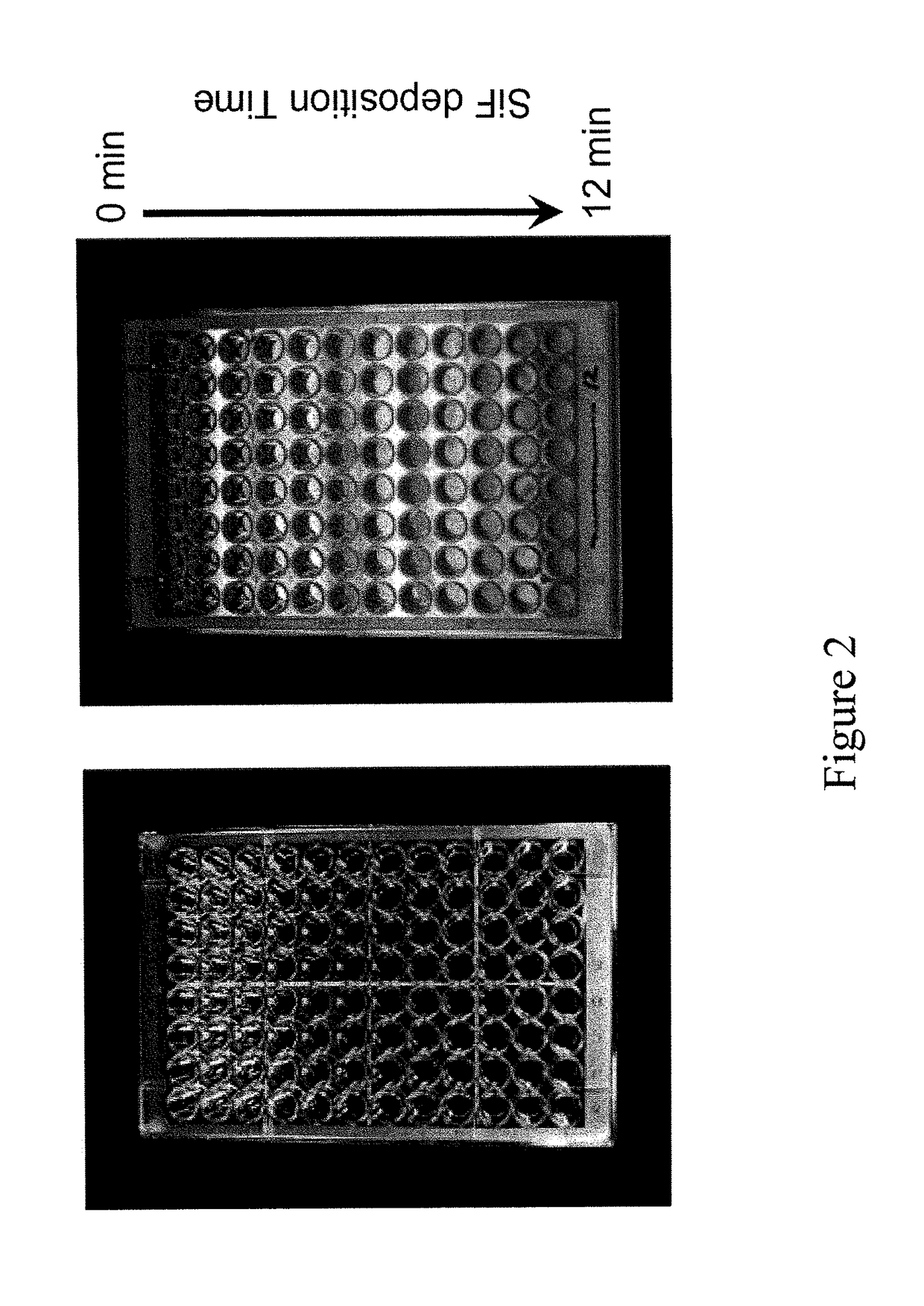
Ultra-fast pathogen toxin detection assay based on microwave-accelerated metal-enhanced fluorescence
ActiveUS10060923B2Early diagnosisCompound screeningApoptosis detectionBacterial exotoxinFluorescence
The present invention provides for a system and method to detect low levels of the anthrax protective antigen (PA) exotoxin in biological fluids, wherein the system uses a metal-enhanced fluorescence (MEF)-PA assay in combination with microwave-accelerated PA protein surface absorption. Microwave irradiation rapidly accelerates PA deposition onto the surface adjacent to deposited metallic particles and significantly speeding up the MEF-PA assay and resulting in a total assay run time of less than 40 min with an analytical sensitivity of less than 1 pg / ml PA.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Human anti-anthrax protective antigen pa antibody IgG and its application
ActiveCN104628853BInhibit assemblyAvoid infectionAntibacterial agentsImmunoglobulins against bacteriaHeavy chainAnthrax protective antigen
The present invention relates to a full-molecular human monoclonal antibody against anthrax protective antigen and its application. The amino acid sequence of the heavy chain variable region of the antibody is shown in SEQ ID NO.2, and the amino acid sequence of the light chain variable region is As shown in SEQ ID NO.4. The functional identification and in vitro cell neutralization experiments of the whole molecule human antibody showed that it can specifically bind to the anthrax protective antigen PA and effectively block the pathogenic effect of anthrax toxin. Therefore, the whole molecule human antibody of the present invention The original monoclonal antibody can be used in the diagnosis, treatment and prevention of anthrax.
Owner:中国人民解放军南京军区军事医学研究所
Temperature stable vaccine formulations
Formulations of vaccine antigen, such as anthrax protective antigen, are provided that are stable after undergoing freeze and thaw conditions. Methods of using the formulations to prepare vaccine are also provided. Vaccines comprising the formulations are useful, for example, to protect against, inhibit or alleviate a disease or infection, such as related to anthrax infection.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Monoclonal antibodies that neutralize anthrax protective antigen (PA) toxin
InactiveUS8685396B2Antibacterial agentsSugar derivativesAnthrax protective antigenHumanized antibody
Owner:UNITED STATES OF AMERICA
Human-derived antibody IgG of anti-anthrax protective antigen PA and application of human-derived antibody IgG
ActiveCN104628853AInhibit assemblyAvoid infectionAntibacterial agentsImmunoglobulins against bacteriaFunctional identificationHeavy chain
The invention relates to a total-molecular human-derived monoclonal antibody of an anti-anthrax protective antigen and application of the total-molecular human-derived monoclonal antibody. The amino acid sequence of a heavy-chain variable area of the antibody is shown in SEQ ID NO.2, and the amino acid sequence of a light-chain variable area is shown in SEQ ID NO.4. The total-molecular human-derived antibody is subjected to functional identification and an in vitro cell neutralization experiment, and results show that the total-molecular human-derived antibody can be specifically bound with the anti-anthrax protective antigen PA and has an effect of inhibiting the pathogenic effect of anthrax toxin, so the total-molecular human-derived monoclonal antibody disclosed by the invention can be applied to diagnosis, treatment and prevention of relevant anthraces.
Owner:中国人民解放军南京军区军事医学研究所
A chemically modified base-containing single-stranded DNA aptamer that specifically recognizes the anthrax protective antigen pa83 and its application
The invention discloses a chemically modified base-containing single-stranded DNA aptamer specifically recognizing anthrax protective antigen PA83 and its application. The present invention takes the anthrax protective antigen (PA83) as the target, uses the nucleic acid library containing chemically modified nucleotides, and uses the magnetic bead method SELEX to screen out the high-affinity aptamer AP5, which can be used in the environment of various interfering proteins Under this method, it can specifically recognize PA83 protein; it can be applied to aptamer biosensor based on surface plasmon resonance technology and fluorescence polarization technology to detect PA83 protein, thereby detecting Bacillus anthracis. Potential of novel detection techniques for Bacillus protective antigens.
Owner:中国人民解放军疾病预防控制中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com