Human anti-anthrax protective antigen pa antibody IgG and its application
A technology of protective antigen and cloned antibody, applied in application, antibody, antibacterial drug, etc., can solve the problem of no effect, and achieve the effect of good affinity, high specificity and high protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
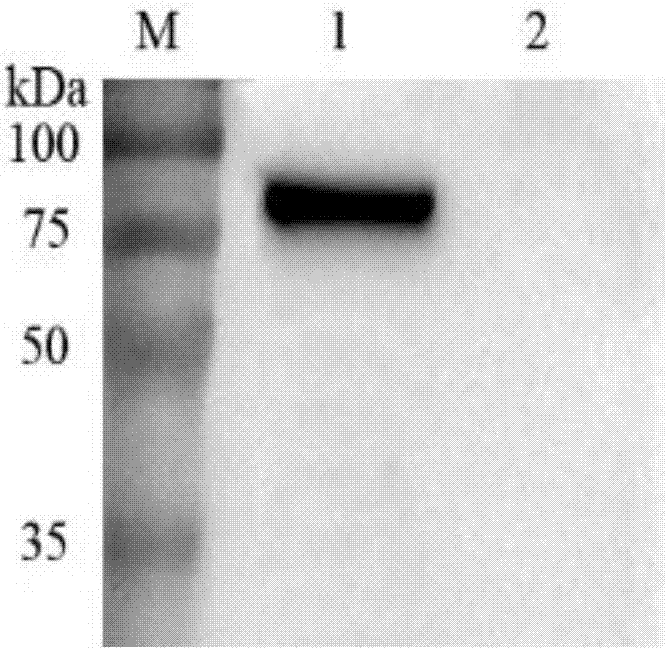
Image
Examples
Embodiment 1
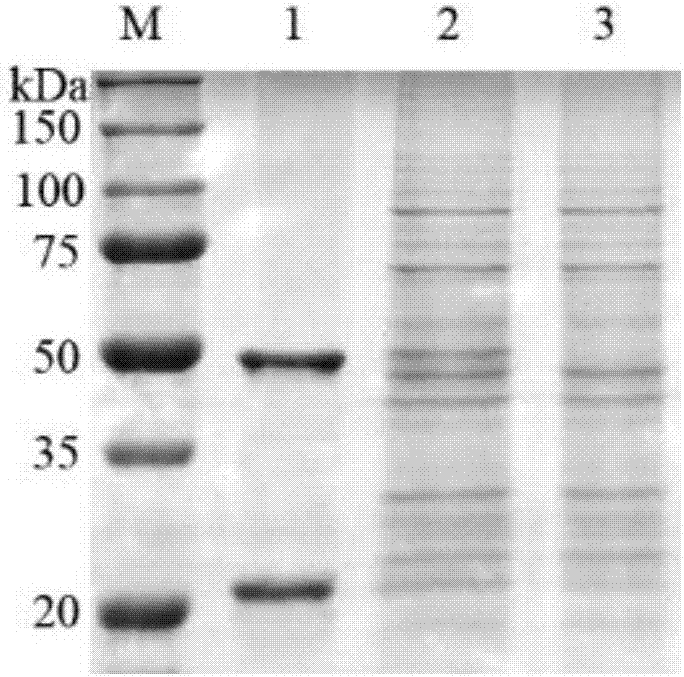
[0033] Example 1 Screening of Human Anti-PA Antibody Fab
[0034] 1) Coat the solid-phase screening ELISA plate with purified recombinant PA83 protein, 2 μg per well, wash, add blocking solution, wash, add natural phage antibody library antibody prepared in our laboratory, and wash to remove unbound phage antibody.
[0035] 2) Add trypsin to elute the specifically bound phage antibody, increase the infection value, and help superinfection with phage VCSM13.
[0036] 3) The above screening steps were repeated, and a total of five rounds of "adsorption-elution-amplification" enrichment screening were performed.
[0037] 4) Dilute the phage obtained from the last round of screening and multiplication, spread on a culture plate with 100 μg / mL of ampicillin and culture overnight, pick 60 single colonies on the cell culture plate, and culture overnight with shaking.
[0038] 5) After overnight, transfer 5 μL of bacterial solution from each well of the first plate to the second plat...
Embodiment 2
[0042] Example 2 Preparation of Human Anti-PA IgG Antibody PA21
[0043] 1) According to the variable region sequence of the obtained antibody, design primers for Infusion PCR
[0044] According to the principle of Infusion PCR, design the heavy and light chain PCR amplification primers of PA21 antibody. The primers need to include 15 bp bases on the expression vector and at least 15 bp bases inserted into the target fragment. The bases inserted into the target fragment are designed according to common primers. Principled design.
[0045] Heavy and light chain PCR amplification primers:
[0046] Primers for heavy chain amplification:
[0047] F: 5'-GGTGTCCACTCGCTAGAGGTGCAGCTGTTGGAGTCTGGGGGAG-3'
[0048] R: 5'-GCCCTTGGTGGATGCTGGGGAGACGGTGACCAGGGT-3'
[0049] Light chain amplification primers:
[0050] F: 5'-ACAGACGCTCGCTGCGAGCTCGTGATGACTCAGTCTCCAGAC-3'
[0051] R: 5'-TGCAGCCACCGTACGTTTGATCTCCAGCTTGG-3'
[0052] 2) Amplify human anti-PA IgG antibody PA21 heavy chain, ligh...
Embodiment 3
[0081] Example 3 Functional Activity Identification of Human Anti-PA IgG Antibody PA21
[0082] 1) ELISA
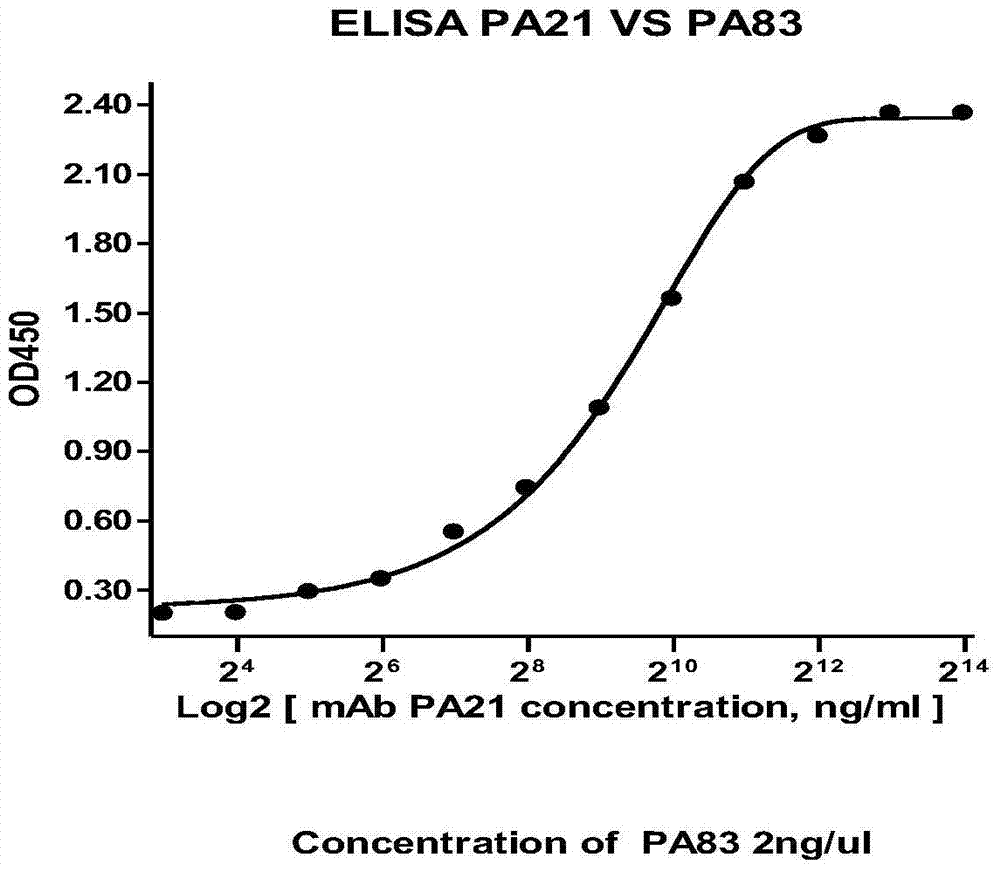
[0083] Dilute the attenuated strain PA83 protein (gifted by the Plague Department of the Chinese Center for Disease Control and Prevention) with coating solution (0.1M carbonate buffer, pH9.6) to 2 μg / mL coated ELISA 96-well plate, add 100 μL to each well, 4 overnight at ℃; PBST (PBS containing 0.5% Tween20) 5% skimmed milk-washing buffer was blocked, incubated at 37°C for 2 hours; after washing with PBST for 5 times, 100 μL of PA21 antibody (2 μg / mL initial concentration, 14 concentrations) was added to each well gradient dilution) at 37°C for 2 hours; 100 μL / well of goat anti-human secondary antibody diluted at 1:4000 was added to the well, and incubated at 37°C for 1 hour; 100 μL / well of peroxidase substrate chromogenic solution was used after 10 minutes at room temperature 2M sulfuric acid was used to stop the reaction, and the colorimetry on the machine was detected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com