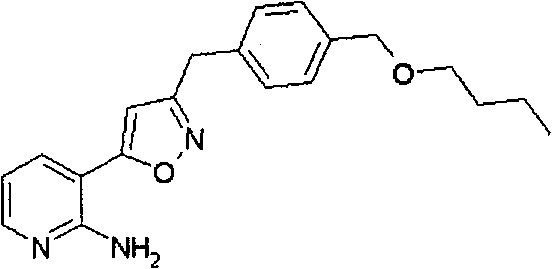
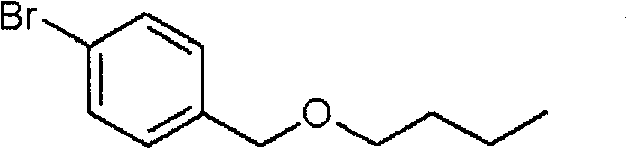
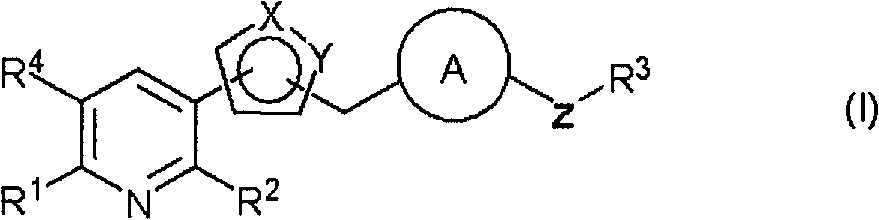
Heterocycles substituted pyridine derivatives and antifungal agent containing thereof
A technology of pyridine and pyridine ring, which is applied in antifungal agents, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of undisclosed antifungal effects and unrecorded compounds, and achieve excellent metabolic stability and safety excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0174] [Production method 1-1] Preparation method of compound (1a)
[0175]
[0176] [In the formula, ring A, R 1 , R 2 , R 3 and Z represent the same meanings as defined above. 〕
[0177] Compound (1b) may be used as it is from a commercial product, or may be prepared from a commercial product by a known method. In addition, it can also be prepared by the methods described in the preparation examples in the examples or [Production method 1-2-1] and the like.
[0178] Compound (1c) can be produced from commercially available products by known methods. Furthermore, it can also be prepared by the method described in the preparation example in an Example or [production method 1-3-1] etc.
[0179] [step 1]
[0180] This step is a step of reacting compound (1b) and compound (1c) in the presence of a base to obtain compound (1a). The solvent used for this reaction is not particularly limited as long as it can dissolve the starting material to a certain extent and does no...
Embodiment 1
[0728] [Example 1] 3-(3-(4-Benzyloxy-benzyl)-isoxazol-5-yl)-pyridin-2-ylamine
[0729]
[0730]At 0°C, add the mixture of 4-benzyloxy-phenyl-acetohydroximoyl chloride (acetohydroximoyl chloride) (1.2g, 4.4mmol) and tetrahydrofuran (34mL) described in Preparation Example 1-1-3 to prepare 3-Ethynyl-pyridin-2-ylamine (260 mg, 2.2 mmol) and triethylamine (3.0 mL, 22 mmol) described in Example 1-2-3 were stirred at room temperature for 1 hour. At room temperature, water was added to the reaction mixture, followed by extraction with ethyl acetate-tetrahydrofuran (2:1). The organic layer was washed with saturated brine, and the solvent was distilled off under reduced pressure. The residue was purified by NH silica gel column chromatography (ethyl acetate:heptane=1:3) to obtain the title compound (240 mg, 15%).
[0731] 1 H-NMR spectrum (CDCl 3 )δ (ppm): 4.00 (2H, s), 5.05 (2H, s), 5.41 (2H, s), 6.24 (1H, s), 6.71 (1H, dd, J=4.9, 7.6Hz), 6.93- 6.97(2H, m), 7.18-7.22(2H, m), 7....
preparation example 1-1-1
[0733] [Preparation Example 1-1-1] 1-Benzyloxy-4-((E)-2-nitro-vinyl)-benzene
[0734]
[0735] At 0°C, nitromethane (330 μL, 6.1 mmol) and sodium methoxide (28% in methanol, 1.0 mL, 4.9 mmol), and stirred at room temperature for 10 minutes. The reaction mixture was cooled to 0°C, and 5N aqueous hydrochloric acid solution (20 mL) was added at the same temperature. The reaction mixture was stirred at room temperature for 15 minutes. The precipitated solid was obtained by filtration to obtain the title compound (1.2 g, 100%).
[0736] 1 H-NMR spectrum (DMSO-d 6 )δ (ppm): 5.20 (2H, s), 7.10-7.14 (2H, m), 7.32-7.48 (5H, m), 7.82-7.85 (2H, m), 8.12 (2H, dd, J = 13.5, 18.2Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com