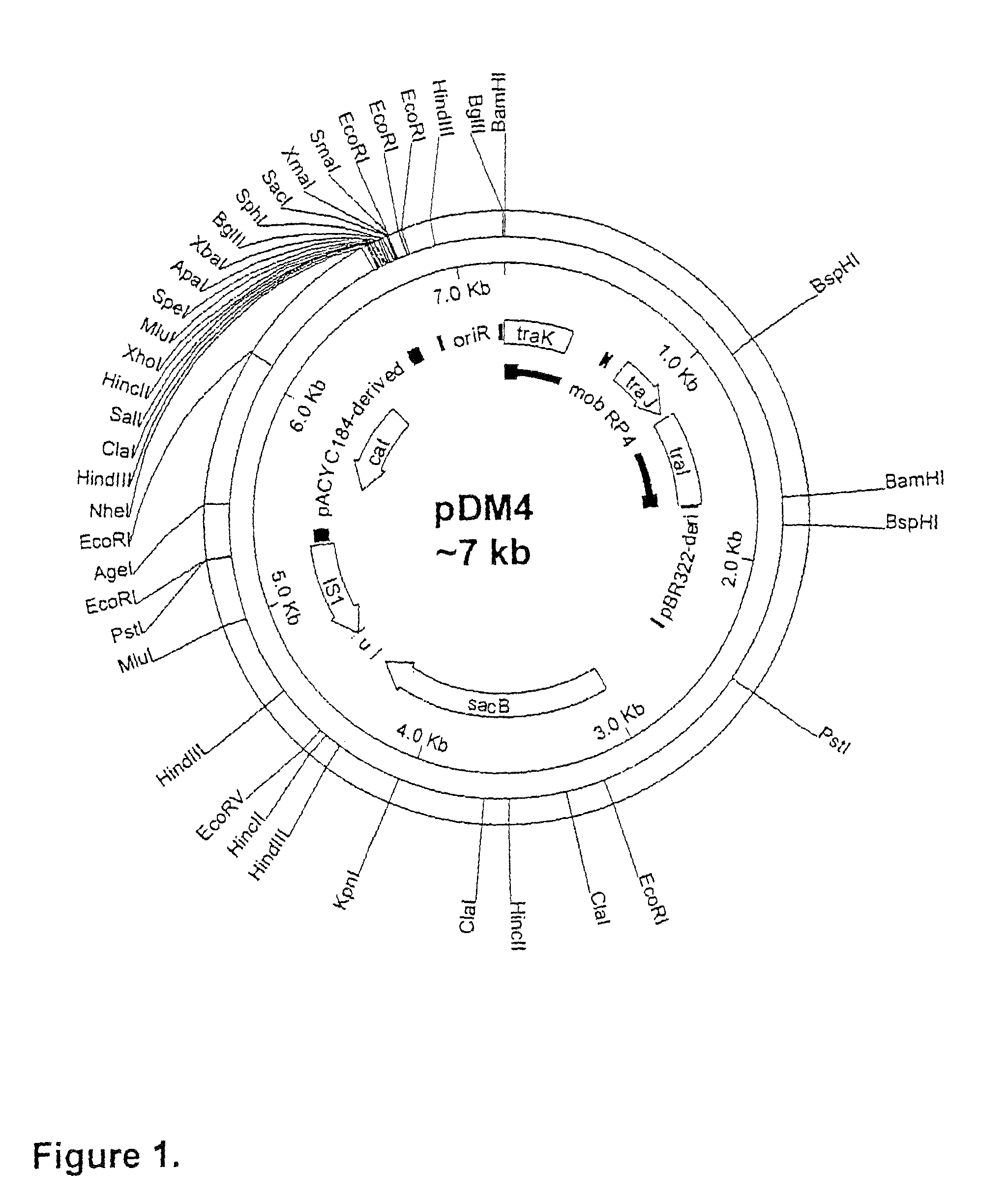
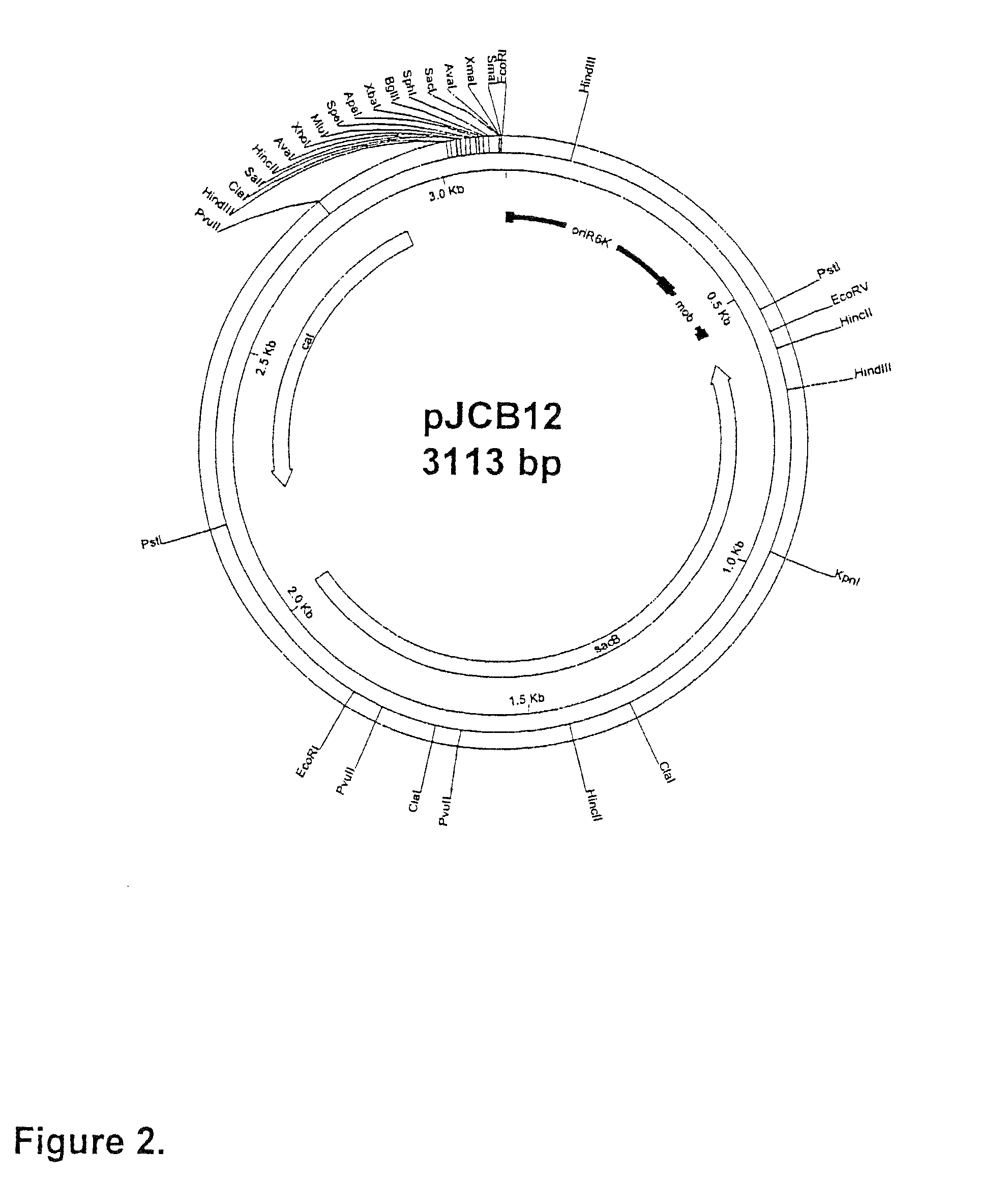
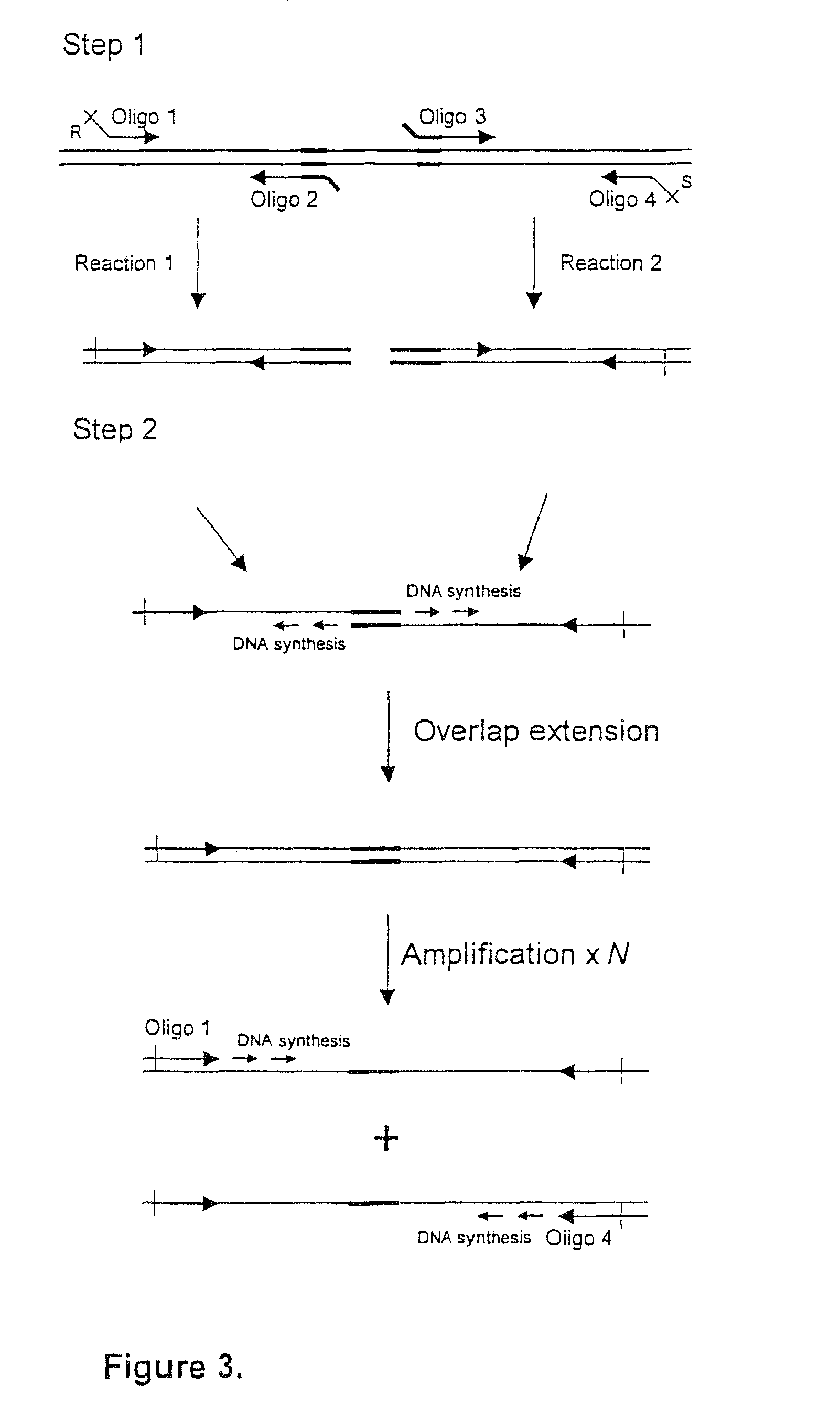
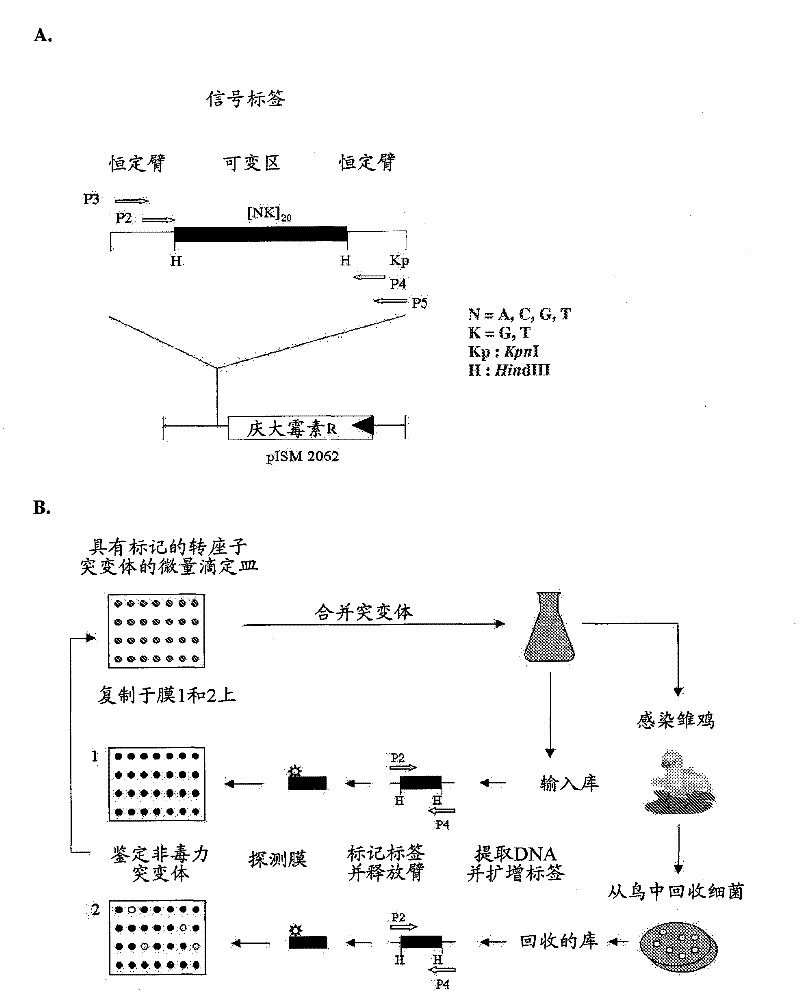
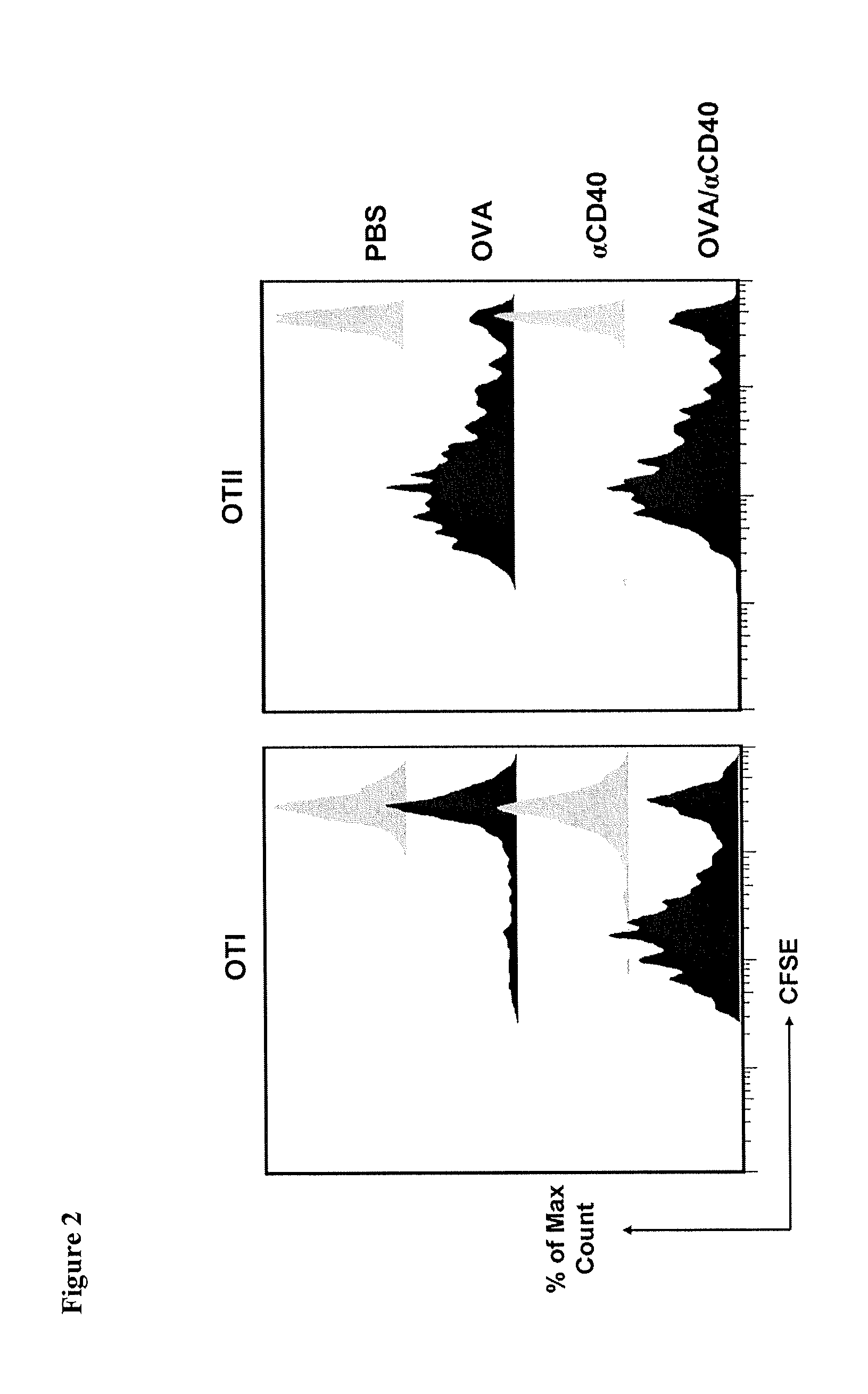
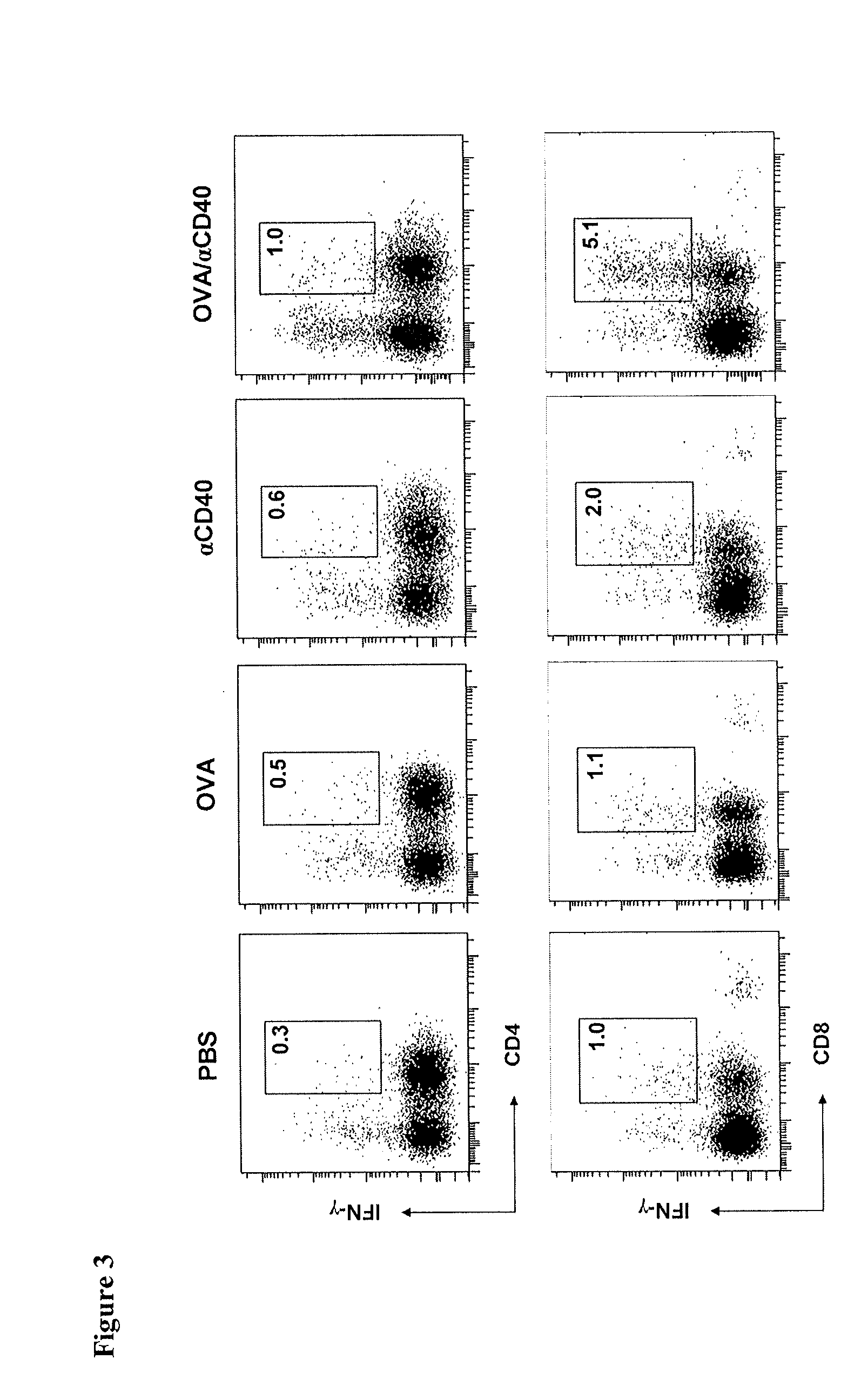
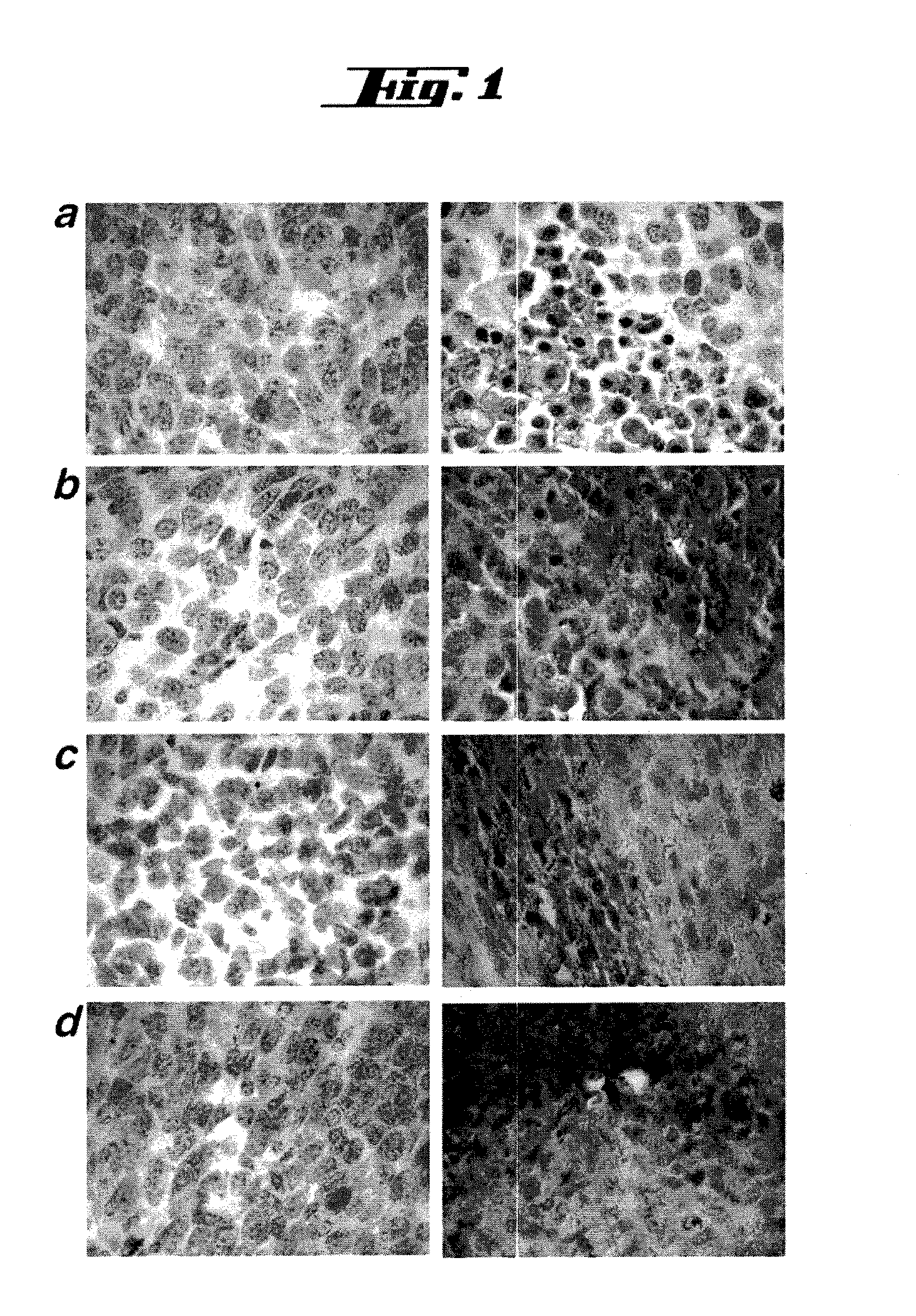
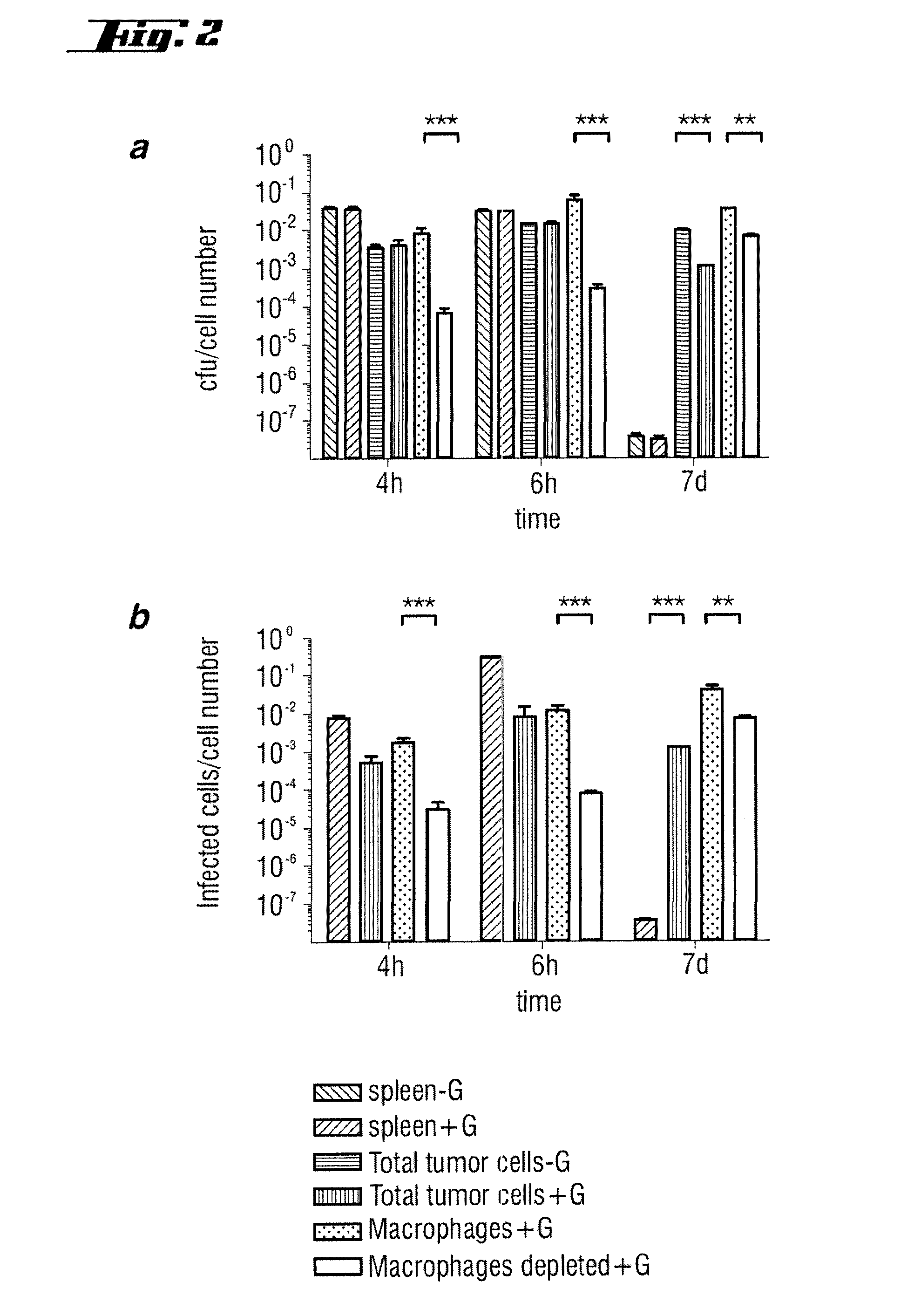
Patents
Literature
43 results about "Attenuated Bacteria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions and methods for delivery of an agent using attenuated Salmonella containing phage
The present application generally discloses delivery of an agent which can be therapeutic or prophylactic and, more particularly, the preparation and use of attenuated bacteria, such as Salmonella, containing a bacteriophage in which the genome of the bacteriophage has been modified to encode for a gene product of interest, e.g., an antigen or an anti-tumor protein. The bacteria functions as a vector for delivering the bacteriophage encoded gene product of interest to an appropriate site of action, e.g., the site of a solid tumor.
Owner:NANOTHERAPEUTICS INC
Compositions and Methods for Tumor-Targeted Delivery of Effector Molecules
InactiveUS20070298012A1Reduce Toxicity RiskReduce riskHeavy metal active ingredientsBiocideParanasal Sinus CarcinomaMelanoma
The present application discloses the preparation and use of attenuated tumor-targeted bacteria vectors for the delivery of one or more primary effector molecule(s) to the site of a solid tumor. The primary effector molecule(s) of the invention is used in the methods of the invention to treat a solid tumor cancer such as a carcinoma, melanoma, lymphoma, or sarcoma. The invention relates to the surprising discovery that effector molecules, which may be toxic when administered systemically to a host, can be delivered locally to tumors by attenuated tumor-targeted bacteria with reduced toxicity to the host. The application also discloses to the delivery of one or more optional effector molecule(s) (termed secondary effector molecules) which may be delivered by the attenuated tumor-targeted bacteria in conjunction with the primary effector molecule(s).
Owner:NANOTHERAPEUTICS INC
Compositions and methods for delivery of an agent using attenuated Salmonella containing phage
InactiveUS20040219169A1Reduce Toxicity RiskReduce riskBacterial antigen ingredientsBacteriaGene productBacteriophage
Owner:NANOTHERAPEUTICS INC
Antibody-mediated enhancement of immune response
InactiveUS20070190063A1Reduces conditionEnhance affinityBacterial antigen ingredientsDigestive systemCancerAntigen binding site
Provided are reagents and methods for administering an attenuated bacterium and a binding compound for treating a cancerous or infectious condition, where the binding compound comprises an antibody or an antigen binding site derived from an antibody.
Owner:ANZA THERAPEUTICS INC
Recombinant vaccines comprising immunogenic attenuated bacteria having RpoS positive phenotype
InactiveUS7083794B2Improve balanceImproving immunogenicityAntibacterial agentsBiocideSalmonella entericaSalmonella serotype typhi
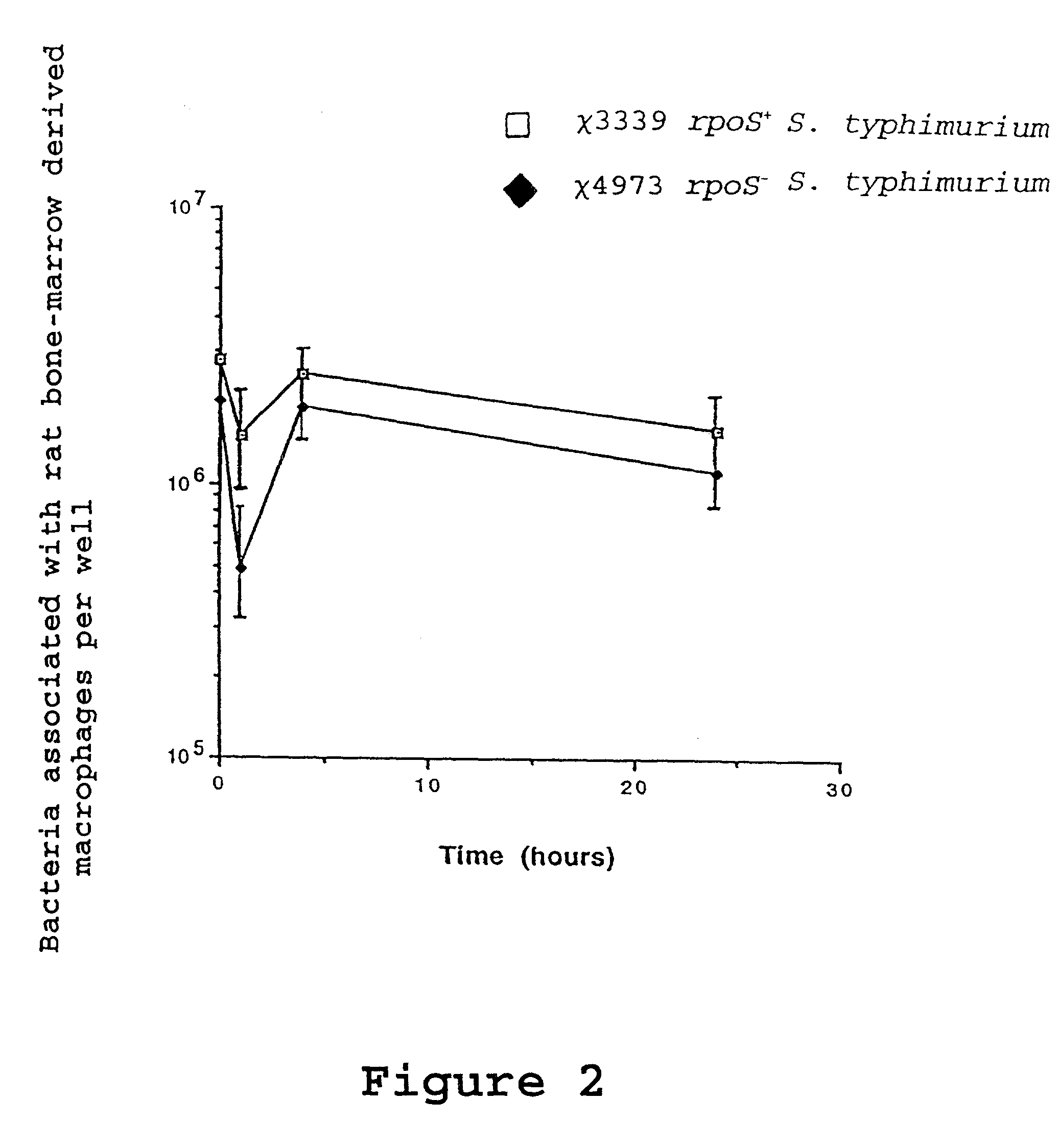
Attenuated immunogenic bacteria having an RpoS+ phenotype, in particular, Salmonella enterica serotype Typhi having an RpoS+ phenotype and methods therefor are disclosed. The Salmonella have in addition to an RpoS+ phenotype, an inactivating mutation in one or more genes which render the microbe attenuated, and a recombinant gene capable of expressing a desired protein. The Salmonella are attenuated and have high immunogenicity so that they can be used in vaccines and as delivery vehicles for genes and gene products. Also disclosed are methods for preparing the vaccine delivery vehicles.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Attenuated bacteria useful in vaccines
InactiveUS20050054075A1Improve protectionReliable and rapid isolationAntibacterial agentsBiocideBacterial strainImmunogenicity
The invention provides strains of bacteria, especially enterotoxigenic E. coli, attenuated by mutations in the genes encoding enterotoxins (LT, ST, EAST1) and optionally further attenuated by deletion of additional chromosomal genes. In addition the invention provides strains of attenuated bacteria expressing immunogenic but non-toxic variants of one or more of these enterotoxins. These bacteria are useful as a vaccine against diarrhoeal disease.
Owner:ACAMBIS RES LTD
Use of bacterium for manufacture of a vaccine
InactiveUS6682745B1Reduce decreaseImprove protectionBiocideBacterial antigen ingredientsBacteroidesMicrobiology
The present invention relates to the use of live attenuated bacteria for the manufacture of a vaccine for submucosal administration.
Owner:INTERVET INT BV
Two-step immunization procedure against Chlamydia infection
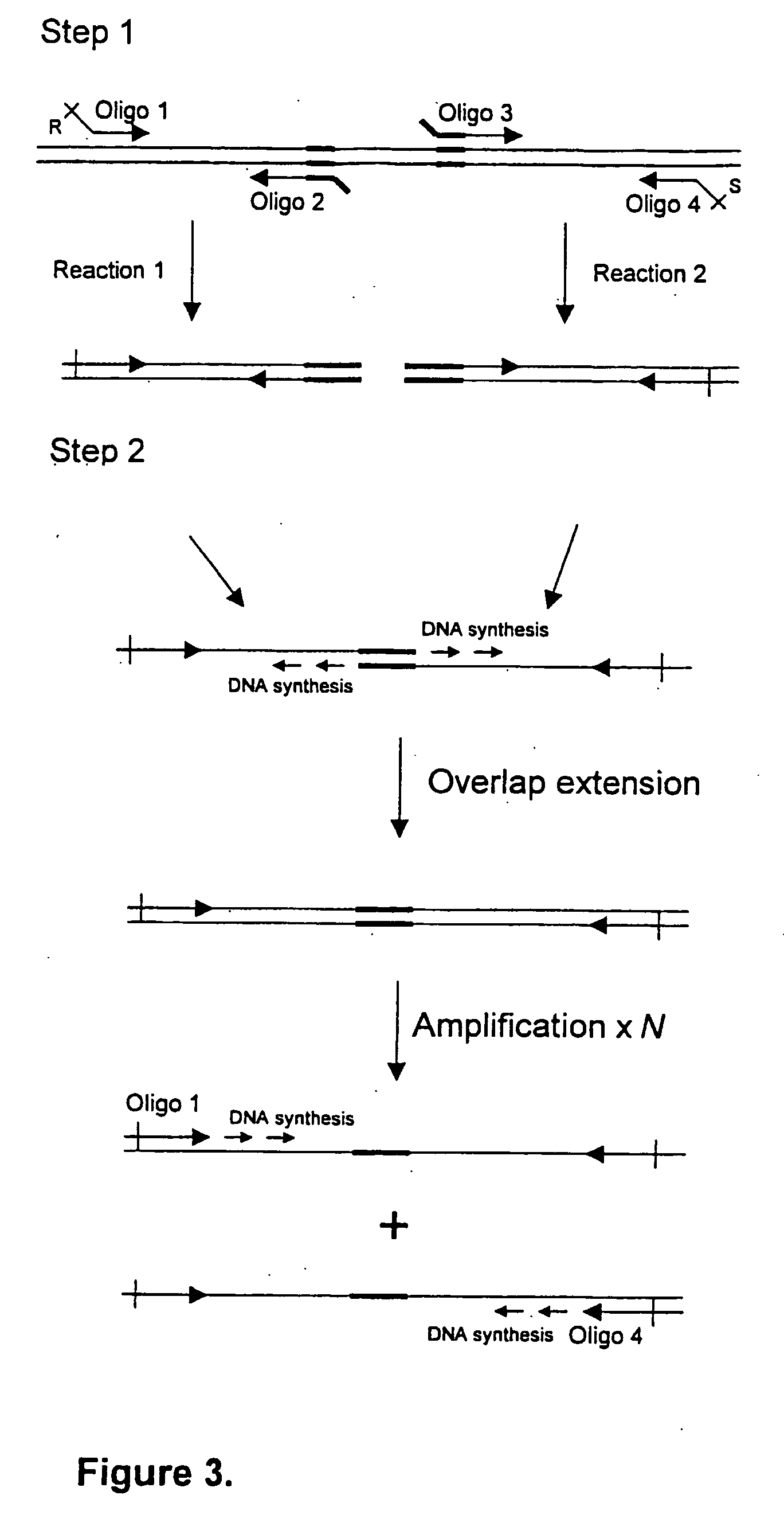
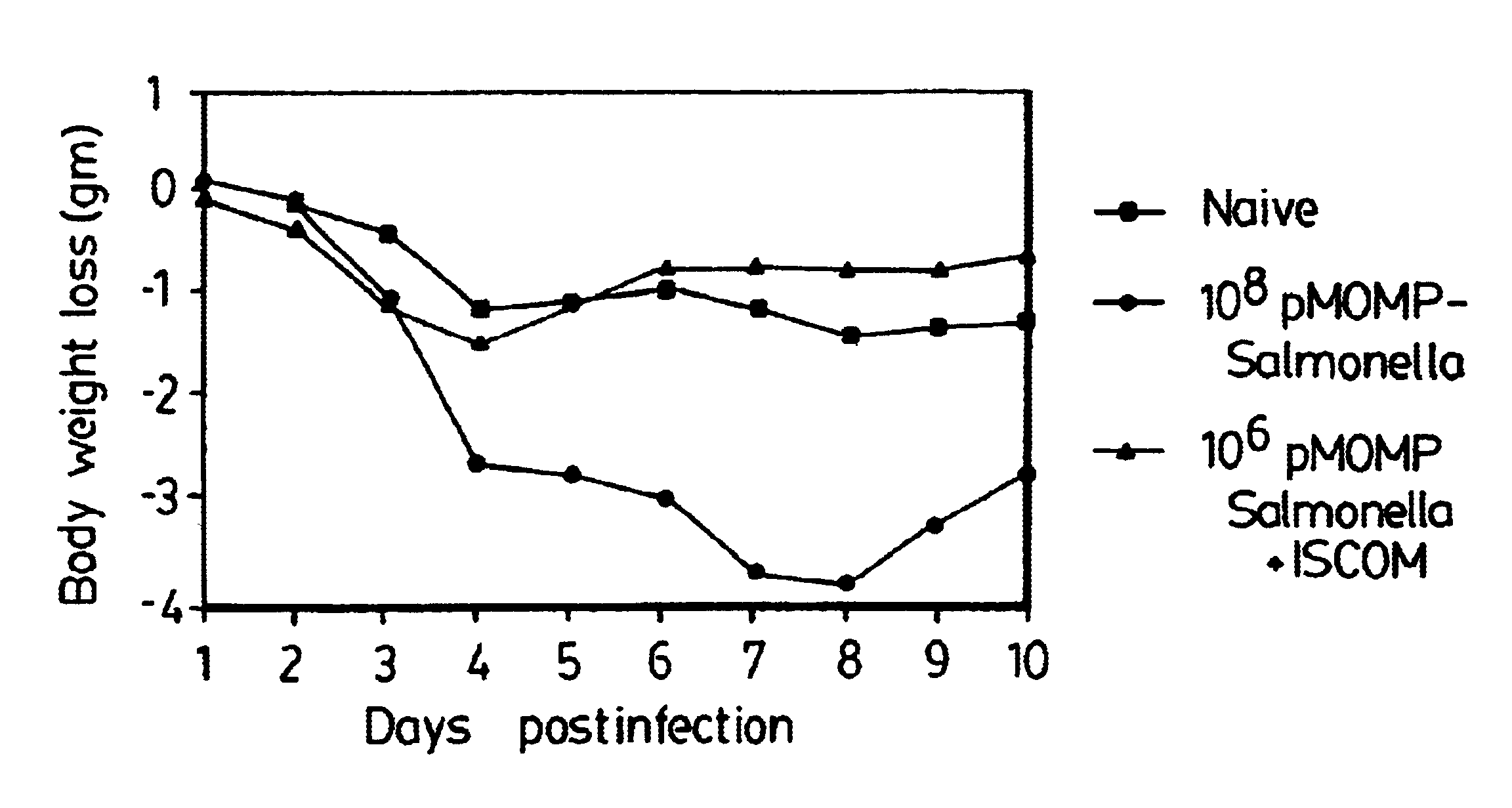
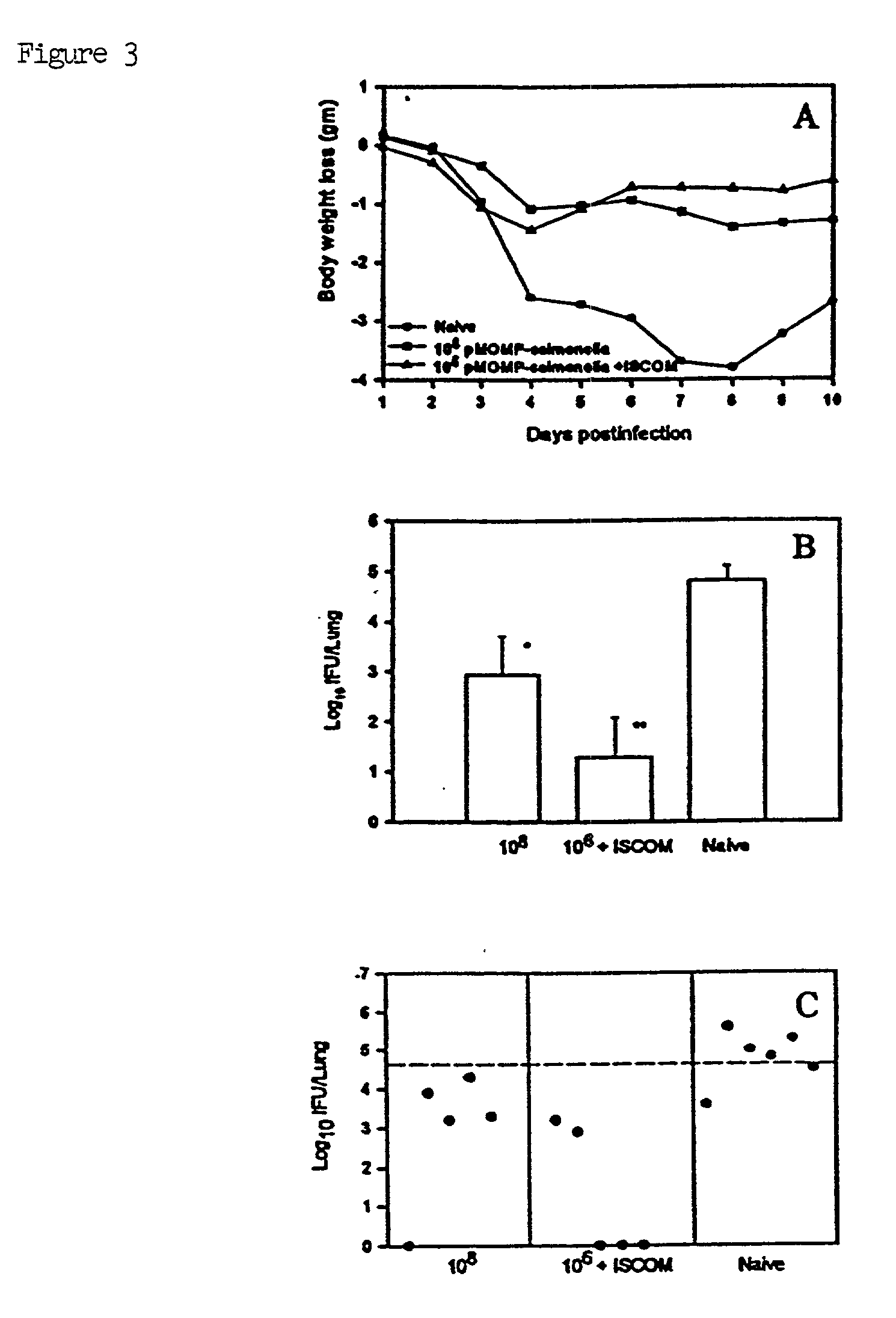
InactiveUS6676949B2Strong immune responseImproving immunogenicityAntibacterial agentsSenses disorderTwo stepChlamydia virus
A host is immunized against infection by a strain of Chlamydia by initial administration of an attenuated bacteria harbouring a nucleic acid encoding a Chlamydia protein followed by administration of a Chlamydia protein in ISCOMs. This procedure enables a high level of protection to be achieved.
Owner:AVENTIS PASTEUR LTD
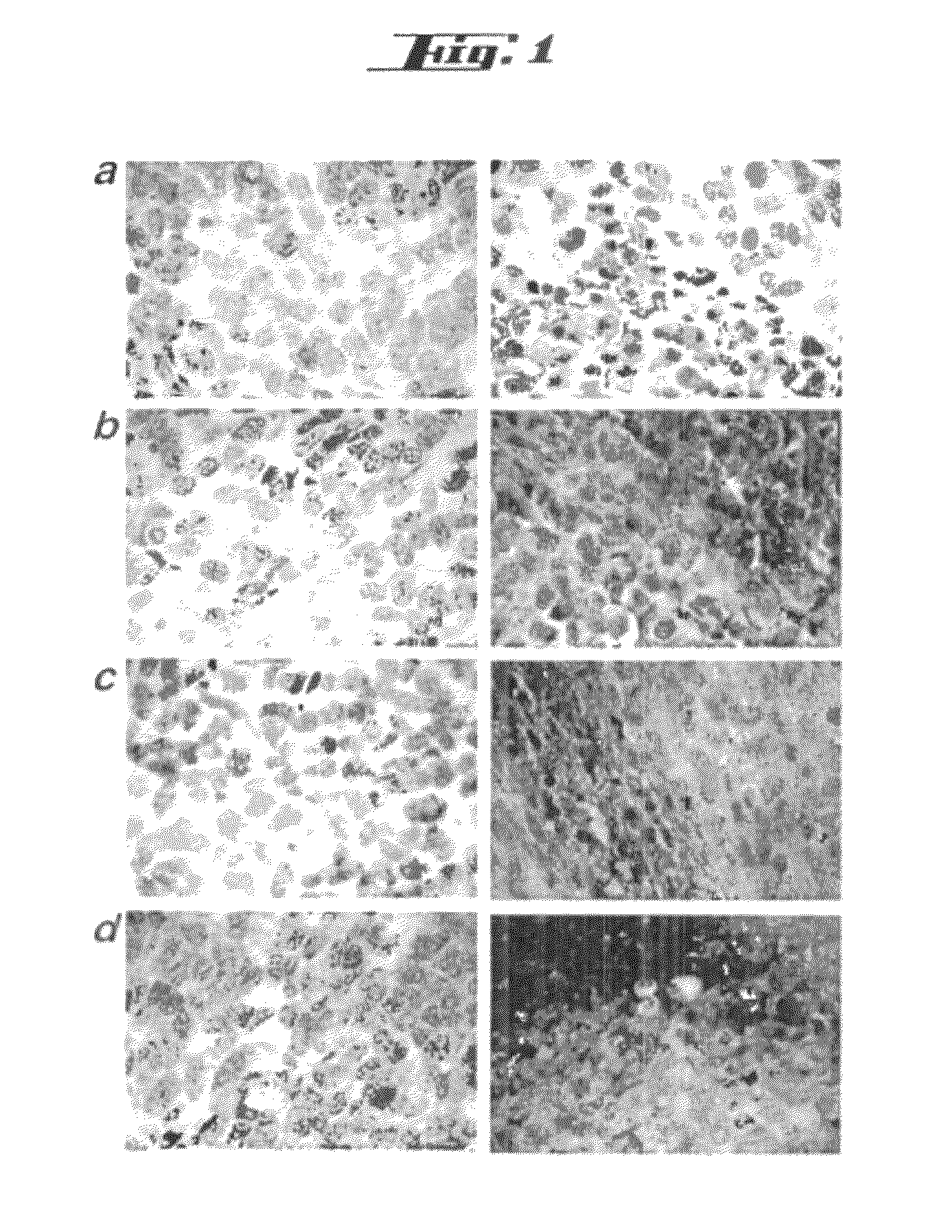
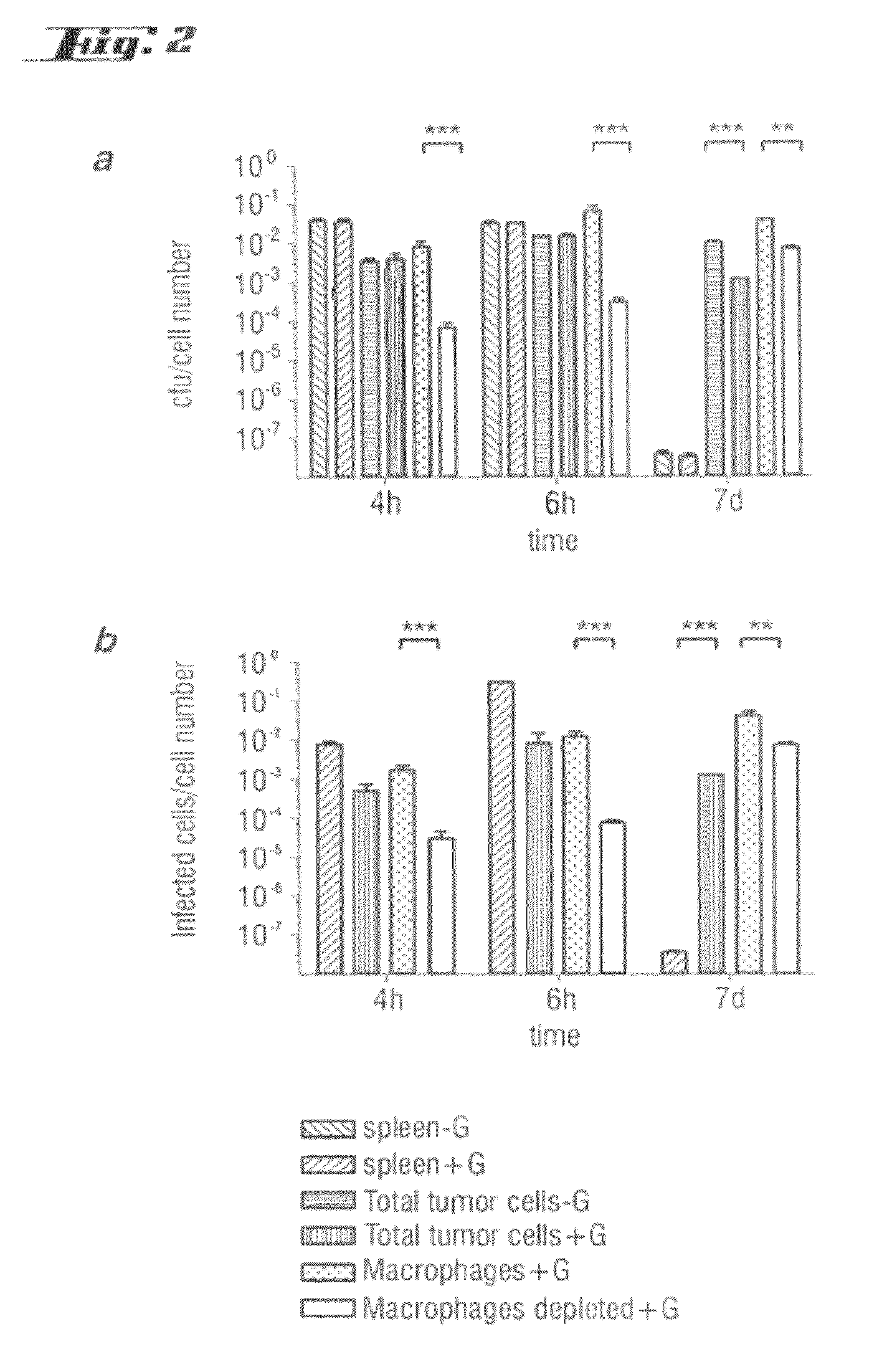
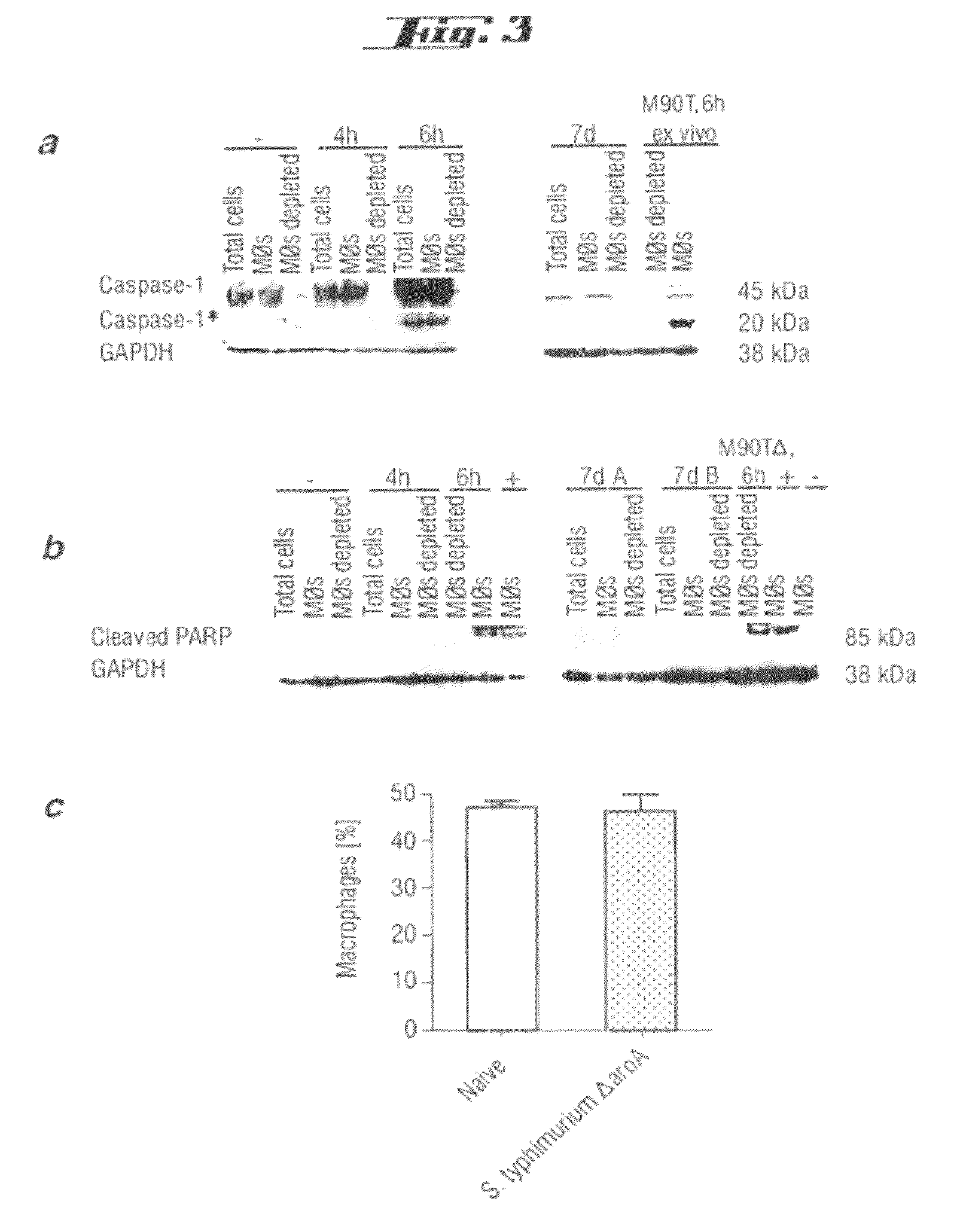
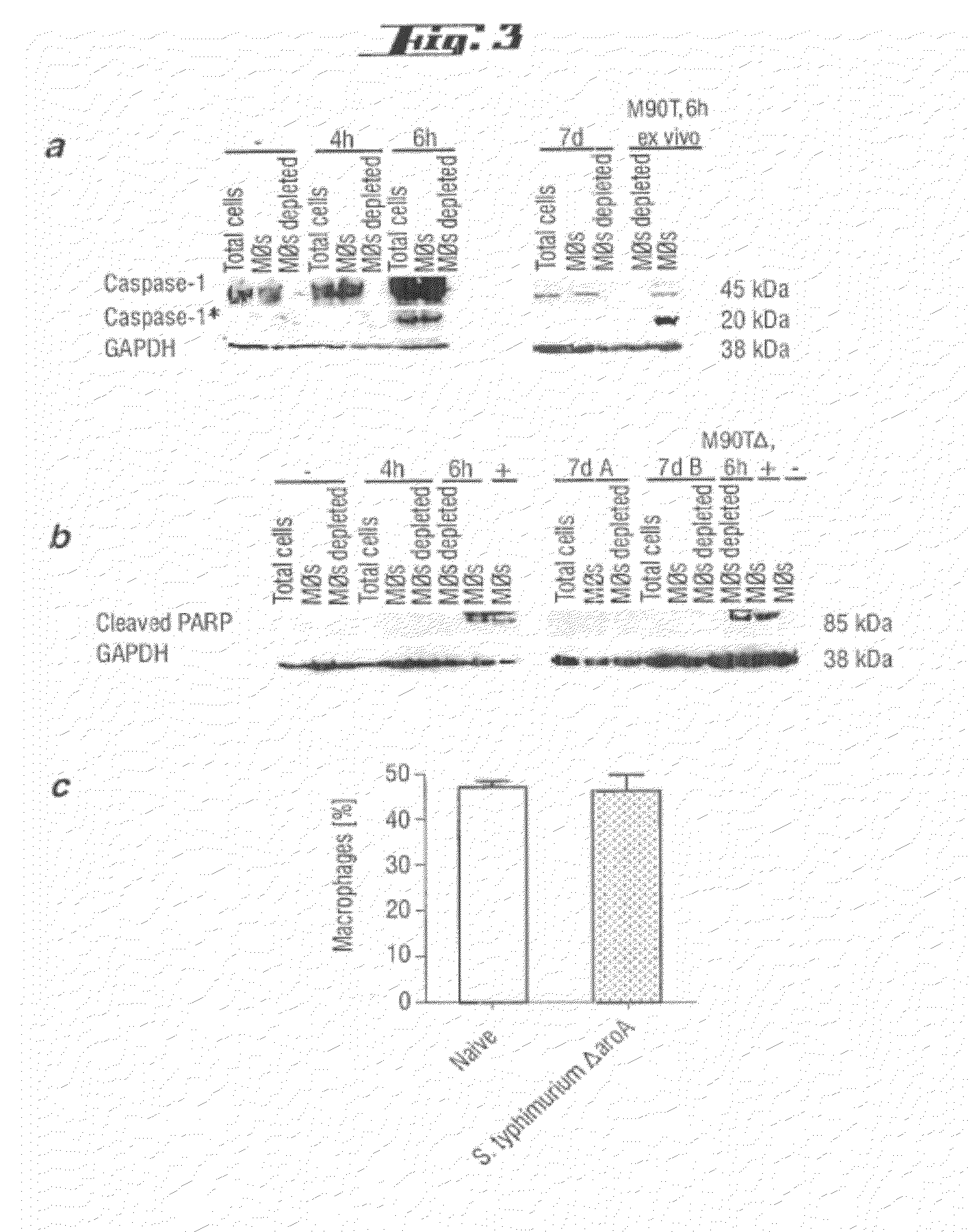
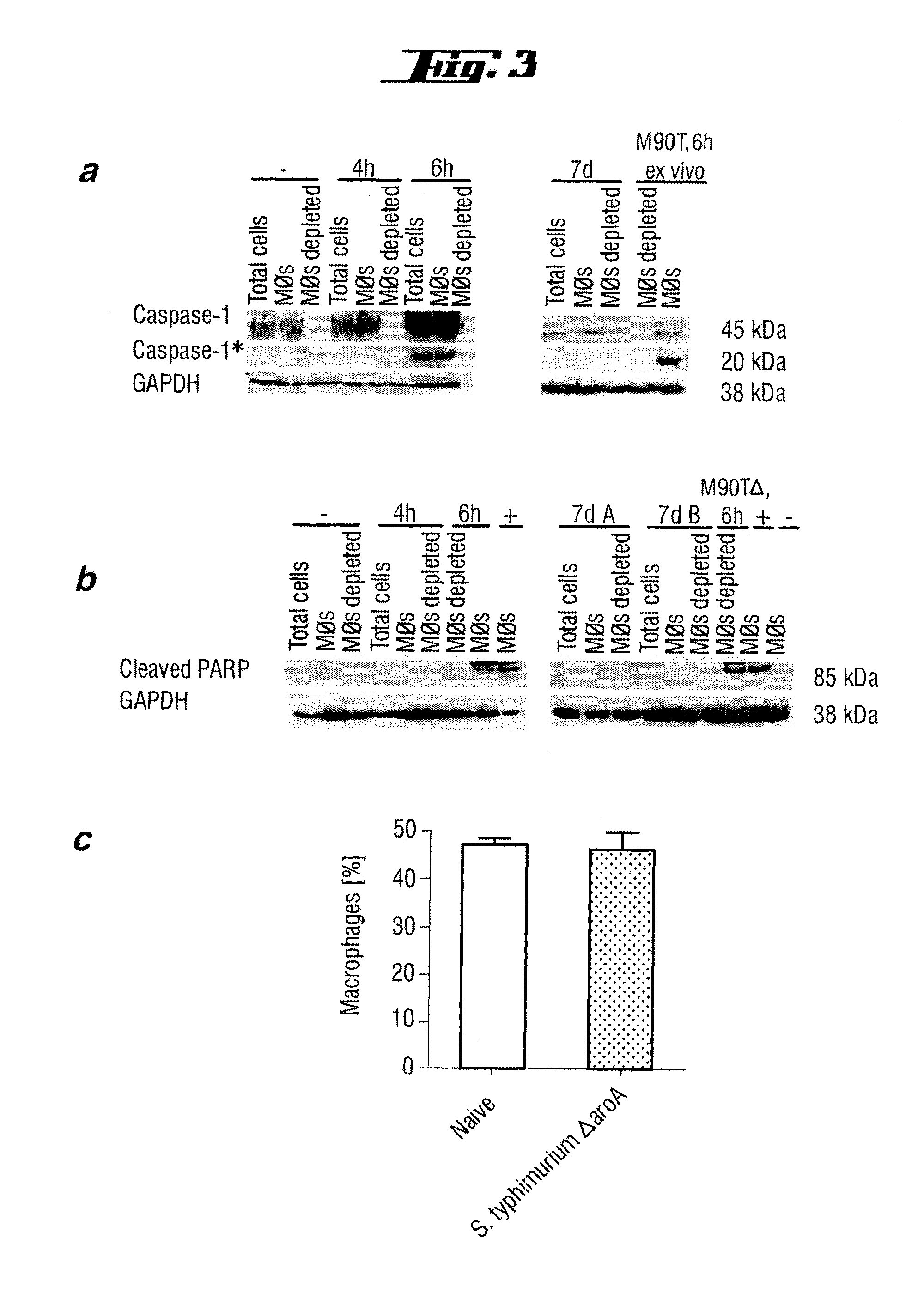
Non-pathogenic and/or attenuated bacteria capable of inducing apoptosis in macrophages, process of manufacturing and uses thereof
The invention relates to an non-pathogenic and / or attenuated bacterium which is capable of inducing apoptosis in macrophages.
Owner:AETERNA ZENTARIS GMBH
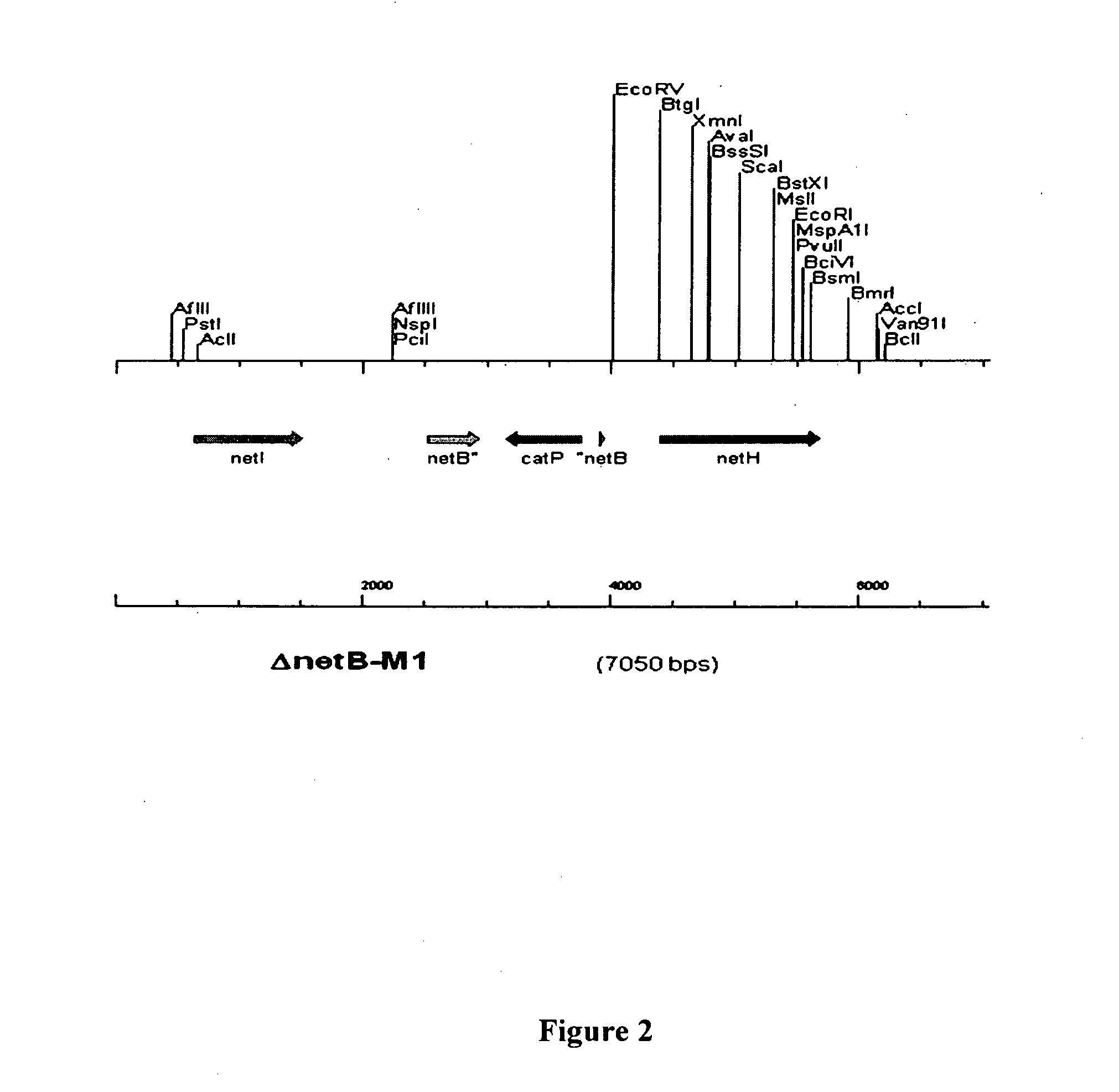
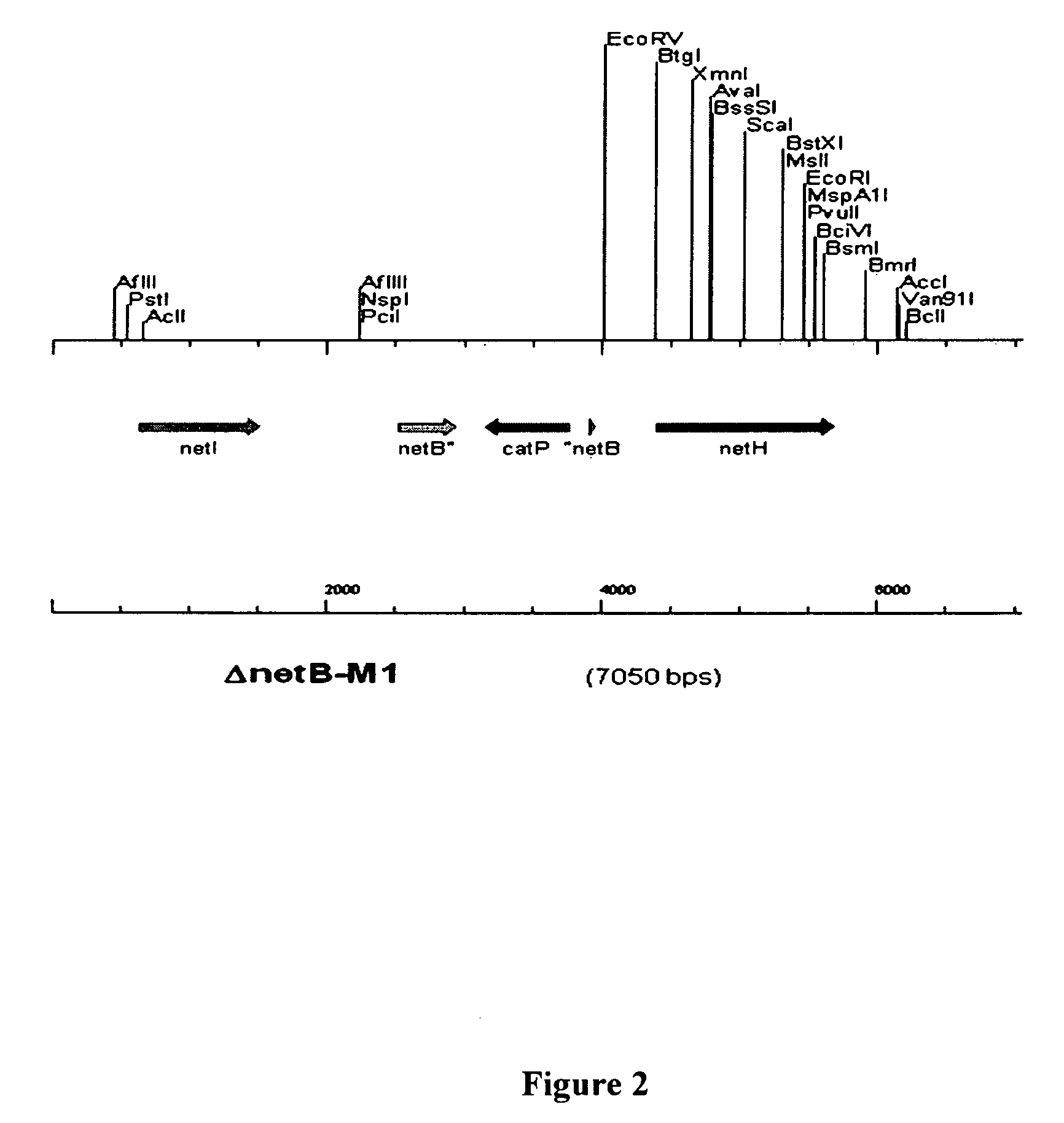
Clostridial toxin netb
ActiveUS20100291131A1Reduce infectionReduce colonizationAntibacterial agentsBacteriaDiseaseClostridial toxin
The present invention relates to a polypeptide based toxin that originates from Clostridium perfringens. The invention further relates to immunogenic compositions comprising the toxin and methods to vaccinate animals, for example chickens, such that they are less susceptible to clostridial diseases. Methods to determine whether an animal has been exposed to the toxin, polynucleotides encoding the toxin and attenuated bacteria that express a reduced or less active form of the toxin are also disclosed.
Owner:AUSTRALIAN POULTRY CRC
Virulence attenuated bacteria based protein delivery
ActiveUS20200123207A1Stably encodePolypeptide with localisation/targeting motifBacteriaMicrobiologyBacterial strain
Owner:UNIVERSITY OF BASEL
Vaccines containing attenuated bacteria
InactiveUS6905691B1Easy to foldIncidenceBacterial antigen ingredientsBacteriaMucosal Immune ResponsesBacteroides
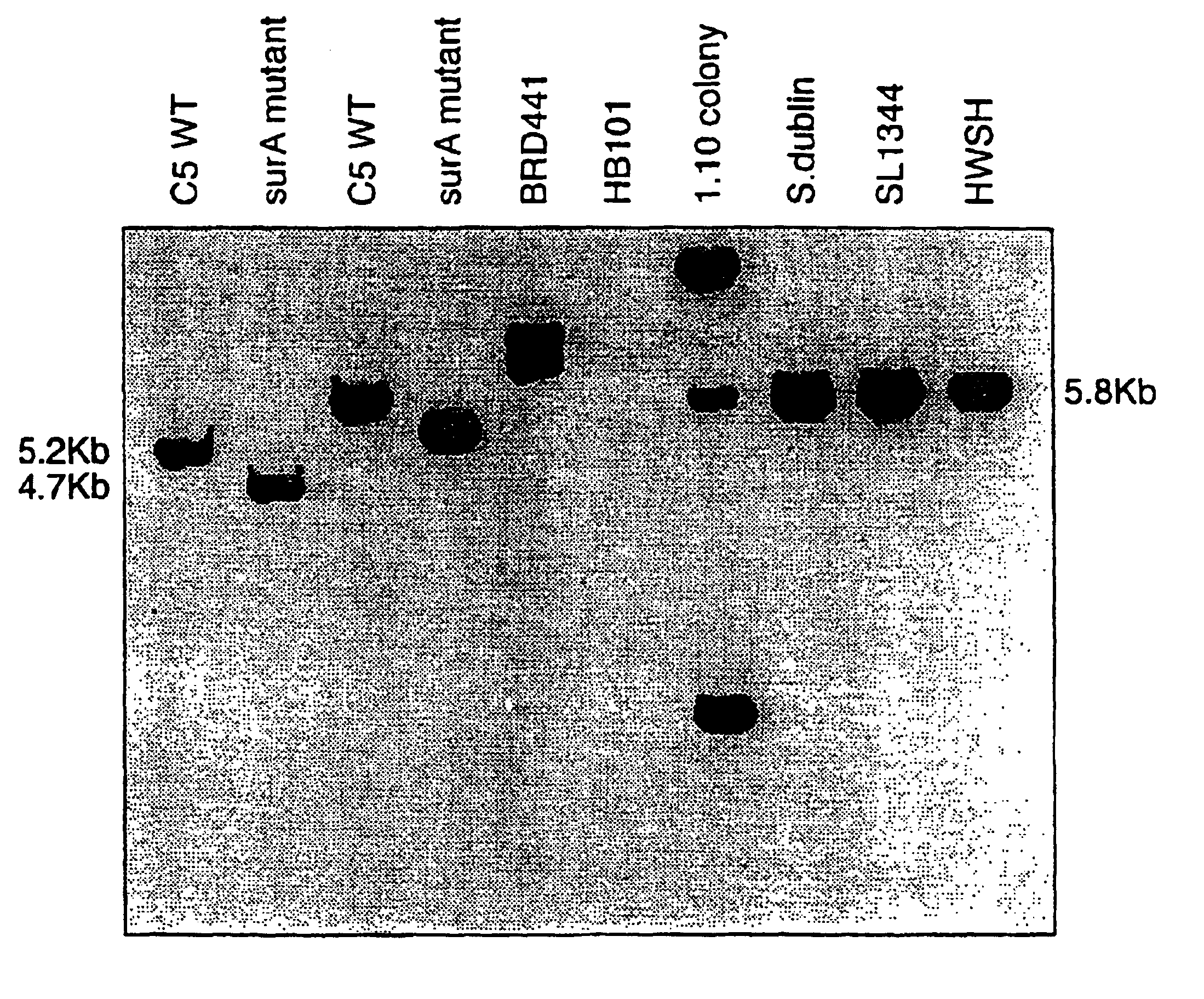
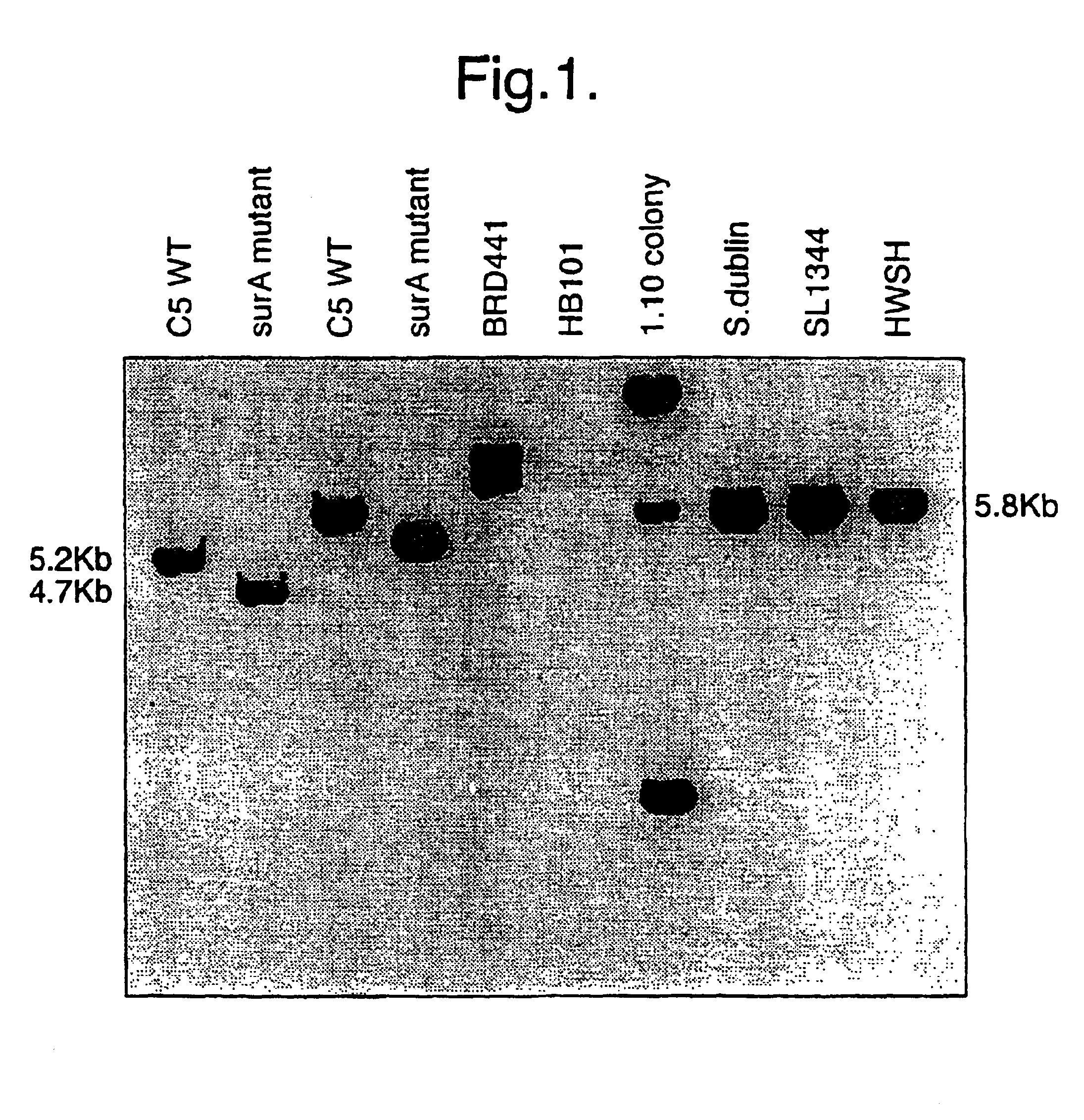
The invention relates to a vaccine comprising a bacterium attenuated by a non-reverting mutation in a gene encoding a protein which promotes folding of extracytoplasmic proteins. Such mutations were initially identified as being useful in vaccines from a bank of randomly inserted, transposon mutants in which attenuation was determined as a reduction in virulence of the organism in the mouse model of infection. Site directed mutation of the gene results in a strain which shows at least 4 logs of attenuation when delivered both orally and intravenously. Animals vaccinated with such a strain are protected against subsequent challenge with the parent wild type strain. Finally, heterologous antigens such as the non-toxic and protective, binding domain from tetanus toxin, fragment C, can be delivered via the mucosal immune system using such strains of bacteria. This results in the induction of a fully protective immune response to subsequent challenge with native tetanus toxin.
Owner:CELLTECH PHARMA EURO
Mutagenesis technique
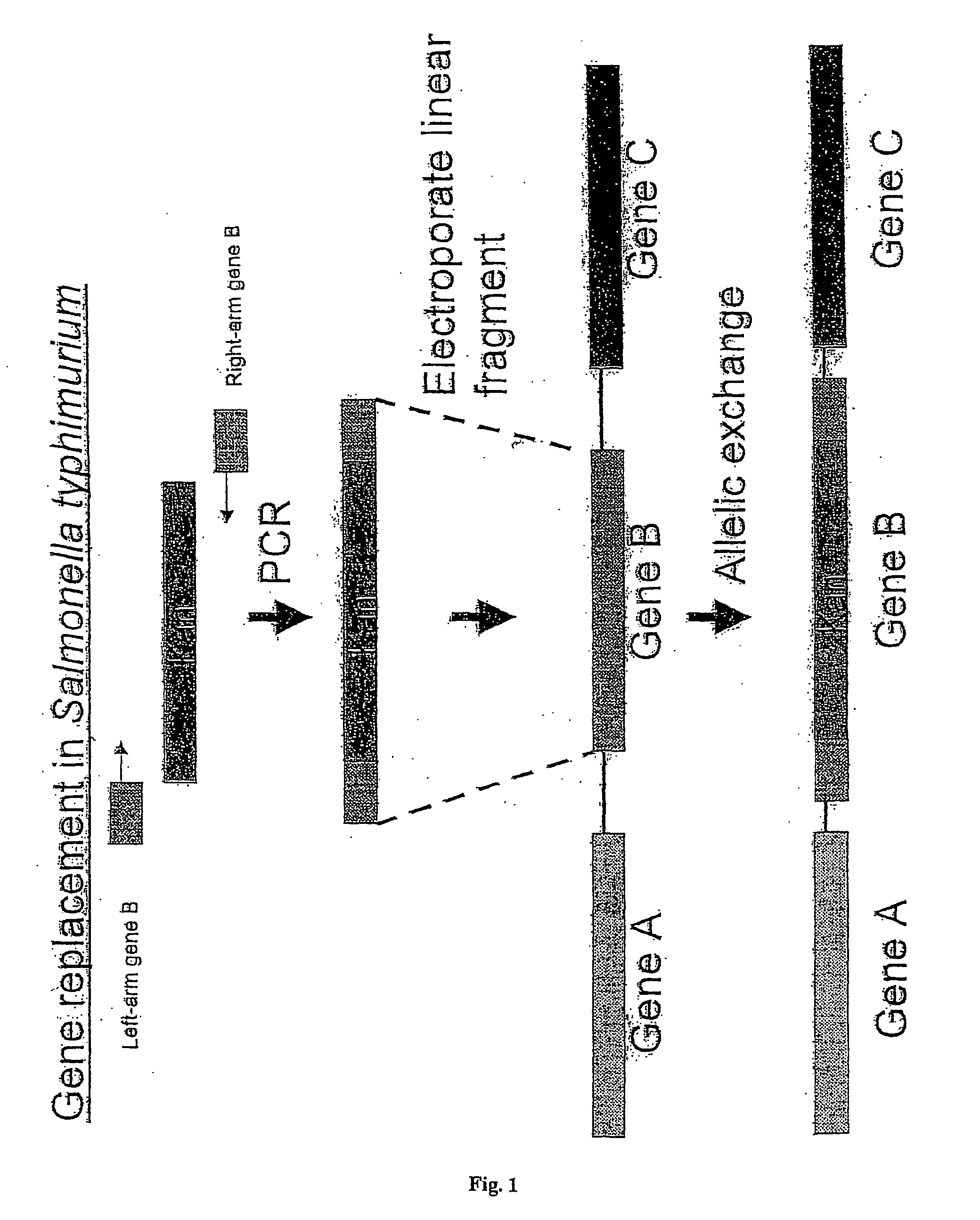
A method for replacing a target nucleotide / polynucleotide sequence of a bacterial chromosome with a different nucleotide / polynucleotide sequence, which method comprises: (a) providing a bacterium which is capable of expressing: (1) the gamma genes exo or a functional equivalent thereof and bet or a functional equivalent thereof, but not the gamma gene gam or a functional equivalent thereof; or (2) the gamma gene bet or a functional equivalent thereof and gam or a functional equivalent thereof; (b) providing a polynucleotide construct which comprises: (i) a sequence which corresponds to a first sequence flanking the left hand side of the target sequence; (ii) a second sequence corresponding to a sequence flanking the right hand side of the target sequence; and (iii) positioned between (i) and (ii), the donor sequence; and (c) introducing the polynucleotide construct into the bacterium, thereby to replace the target nucleotide sequence with the sequence different from that of the target nucleotide sequences. The method can be used in the preparation of attenuated bacteria and in the identification of essential genes.
Owner:CHARLES IAN +1
Attenuated bacteria useful in vaccines
InactiveUS7943122B2Improve protectionReliable and rapid isolationAntibacterial agentsBiocideBacteroidesDiarrhoeal disease
The invention provides strains of bacteria, especially enterotoxigenic E. coli, attenuated by mutations in the genes encoding enterotoxins (LT, ST, EAST1) and optionally further attenuated by deletion of additional chromosomal genes. In addition the invention provides strains of attenuated bacteria expressing immunogenic but non-toxic variants of one or more of these enterotoxins. These bacteria are useful as a vaccine against diarrhoeal disease.
Owner:ACAMBIS RES LTD
Live attenuated vaccines
The present invention relates to a bacterium attenuated by a mutation in at least one ABC transporter gene wherein the mutation renders the corresponding ABC transporter protein non-functional and wherein the attenuated bacterium persists in a subject.
Owner:AUSTRALIAN POULTRY CRC
Pasteurella multocida vaccine
InactiveCN101087803ABacteriaBacteria material medical ingredientsBacteroidesLive attenuated influenza vaccine
The present invention relates to live attenuated bacteria of the species Pasteurella multocida, to live attenuated vaccines comprising such live attenuated bacteria, to the use of such bacteria for the manufacture of such vaccines, to methods for the preparation of such vaccines and to diagnostic tests for the detection of such bacteria.
Owner:INTERVET INT BV
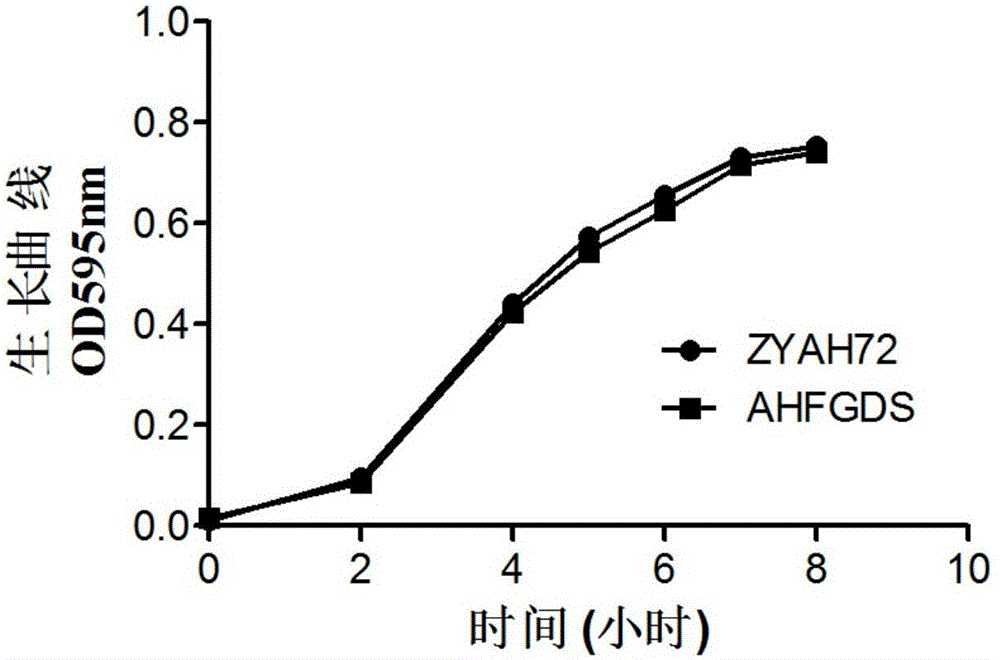
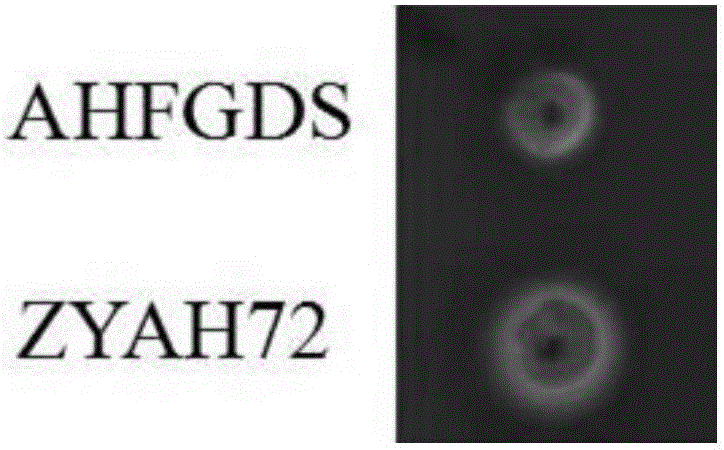
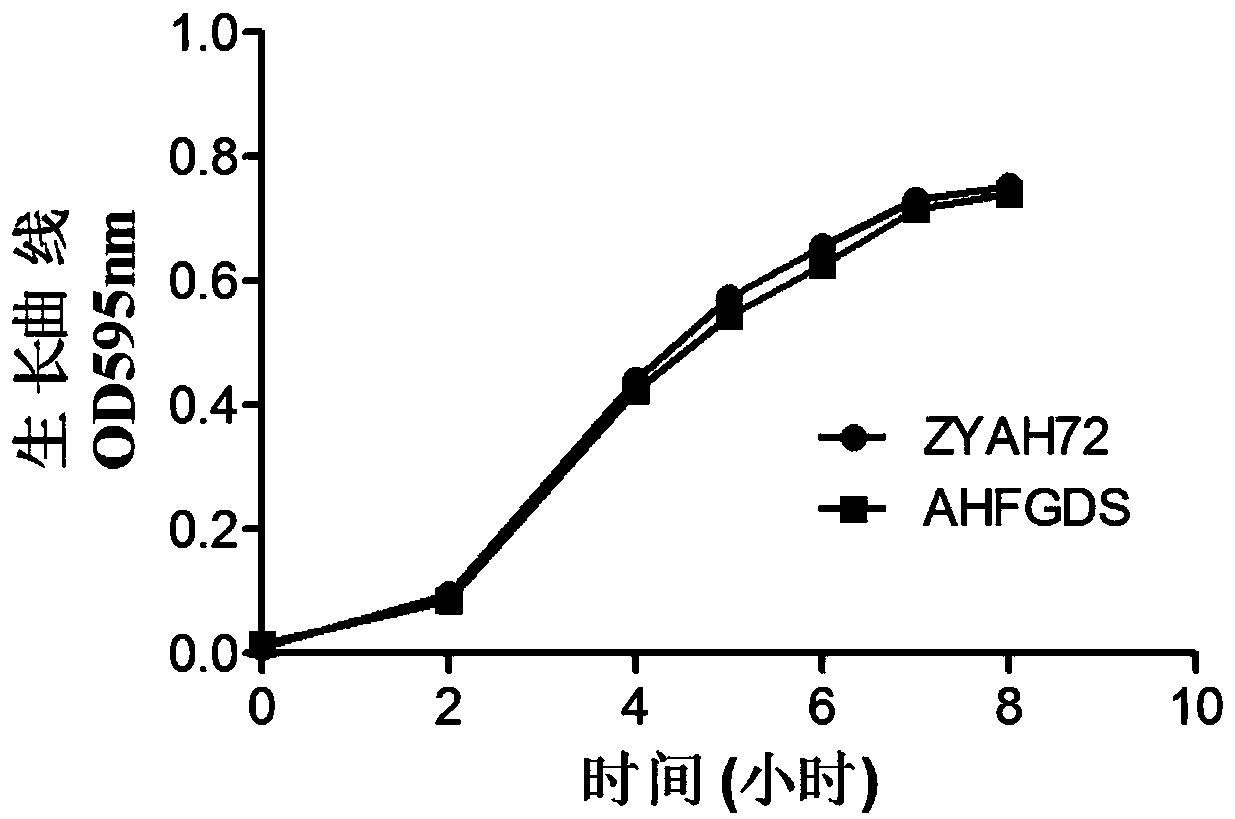
AHFGDS (aeromonas hydrophila five-gene deletion strain) attenuated bacteria without antibiotic markers and application
ActiveCN105886429ANo potential hazardGood genetic stabilityAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsAquatic animal
The invention discloses AHFGDS (aeromonas hydrophila five-gene deletion strain) attenuated bacteria without antibiotic markers and an application. Based on high-virulent strain ZYAH72, five virulence factors including aerA, alt, ahp, ast and hly are deleted with a genetic engineering method and a marker-free screening strategy, an AHFGDS with five genes deleted is obtained, and the preservation number of the strain is CCTCC (China Center for Type Culture Collection) No:M2015798. Compared with wild bacteria, the strain can still be subjected to a strong reaction with positive serum even if the pathogenicity is remarkably weakened. Experiments prove that the strain serving as an attenuated live vaccine can effectively protect susceptible aquatic animals against infection of wild AHFGDSs.
Owner:HUAZHONG AGRI UNIV
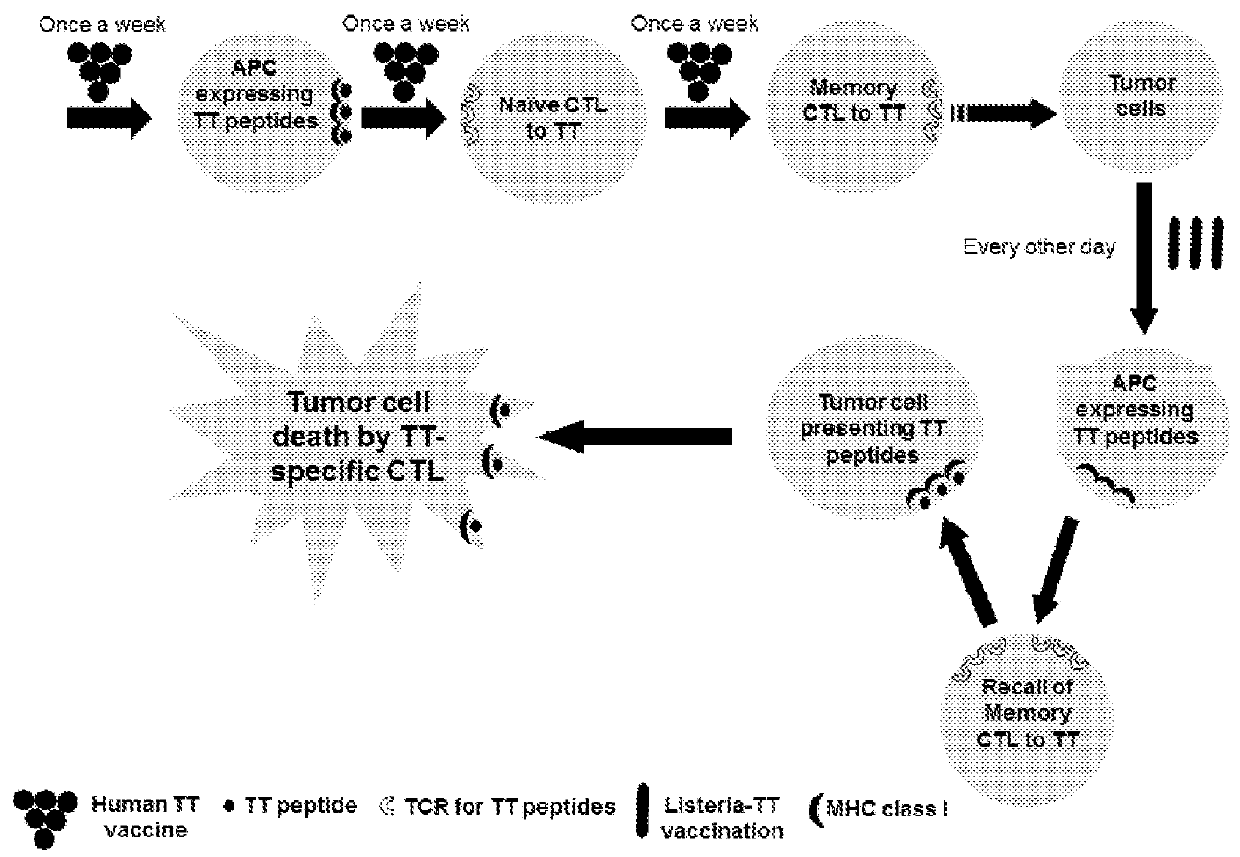
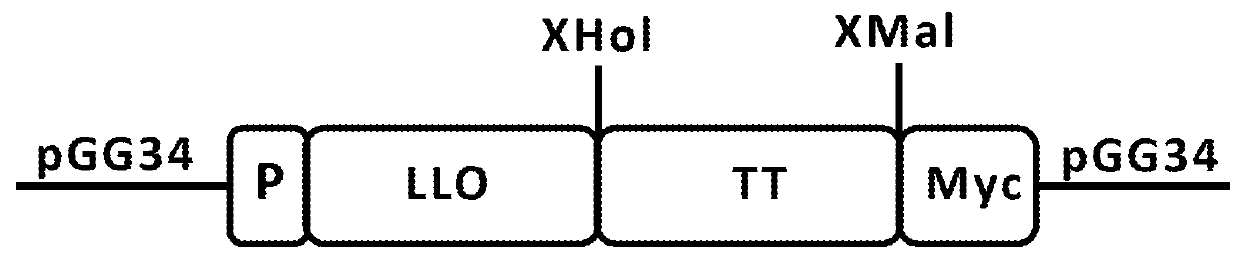
Treatment of cancer using recall antigens delivered by attenuated bacteria
ActiveUS20180104320A1Reduce and prevent metastasisSsRNA viruses negative-senseOrganic active ingredientsBacteroidesGemella
Methods, pharmaceutical compositions and vaccines comprising an attenuated bacteria that expresses a recall antigen are disclosed for treatment of cancer.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
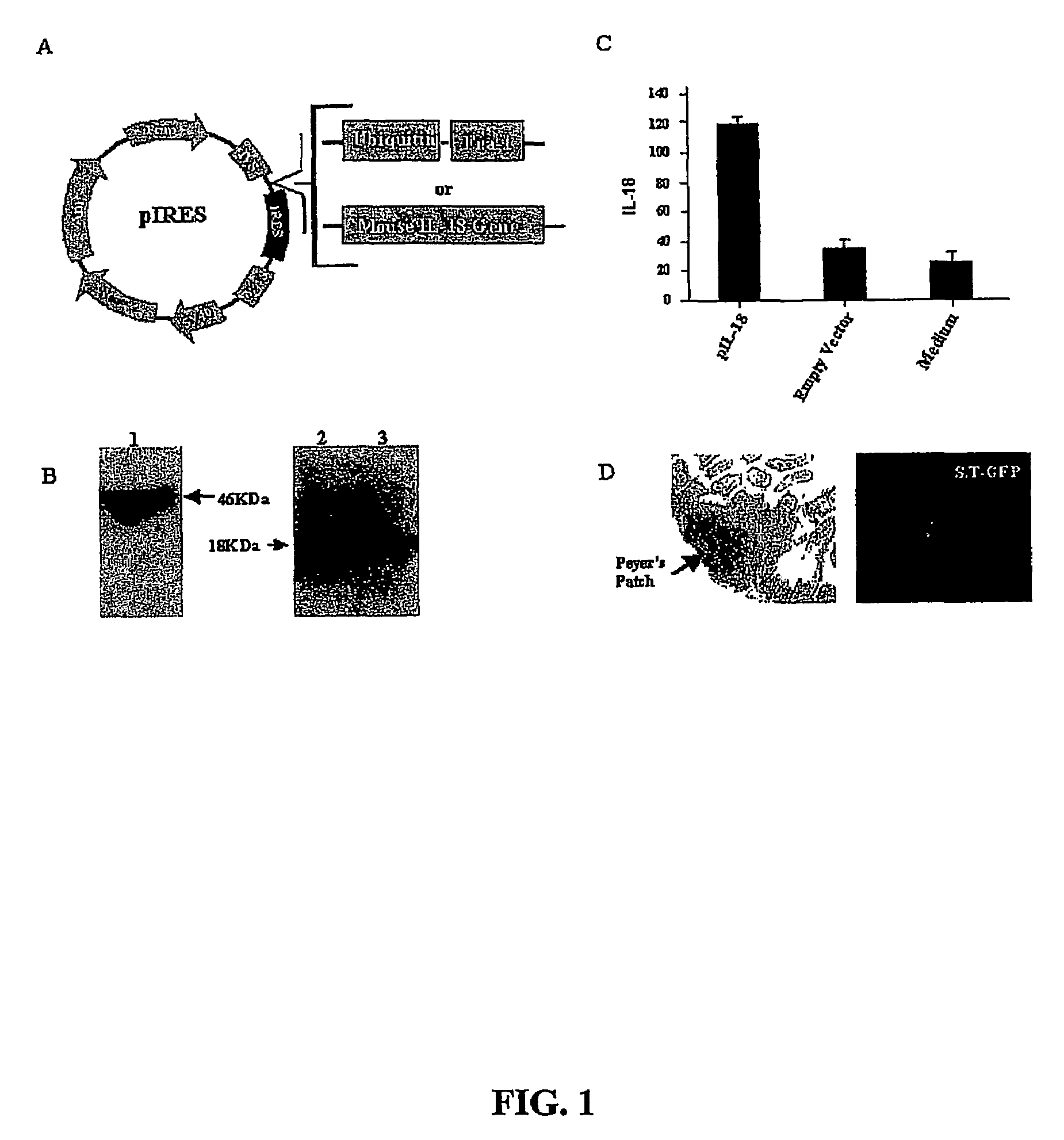
DNA vaccines against tumor growth and methods of use therof
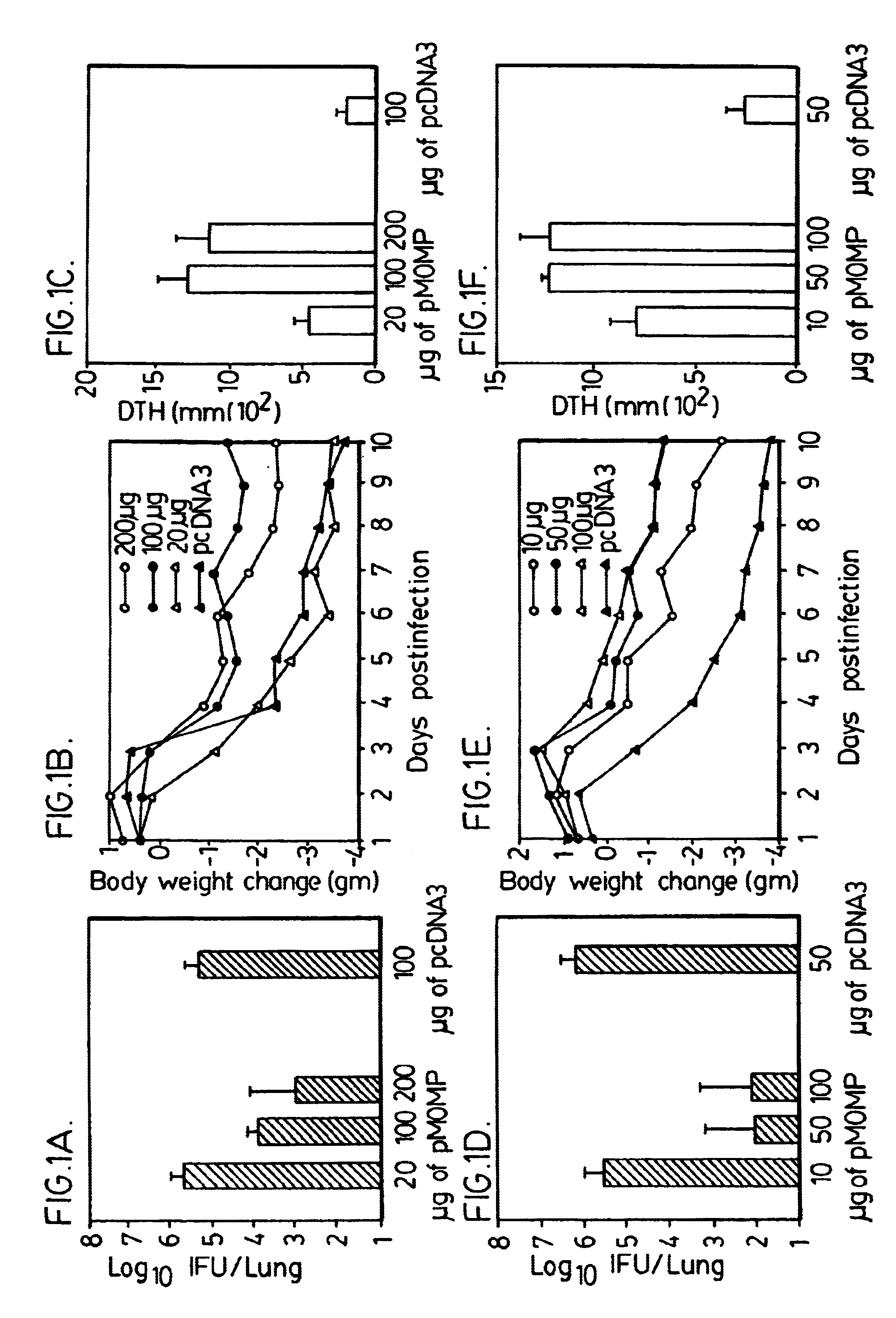
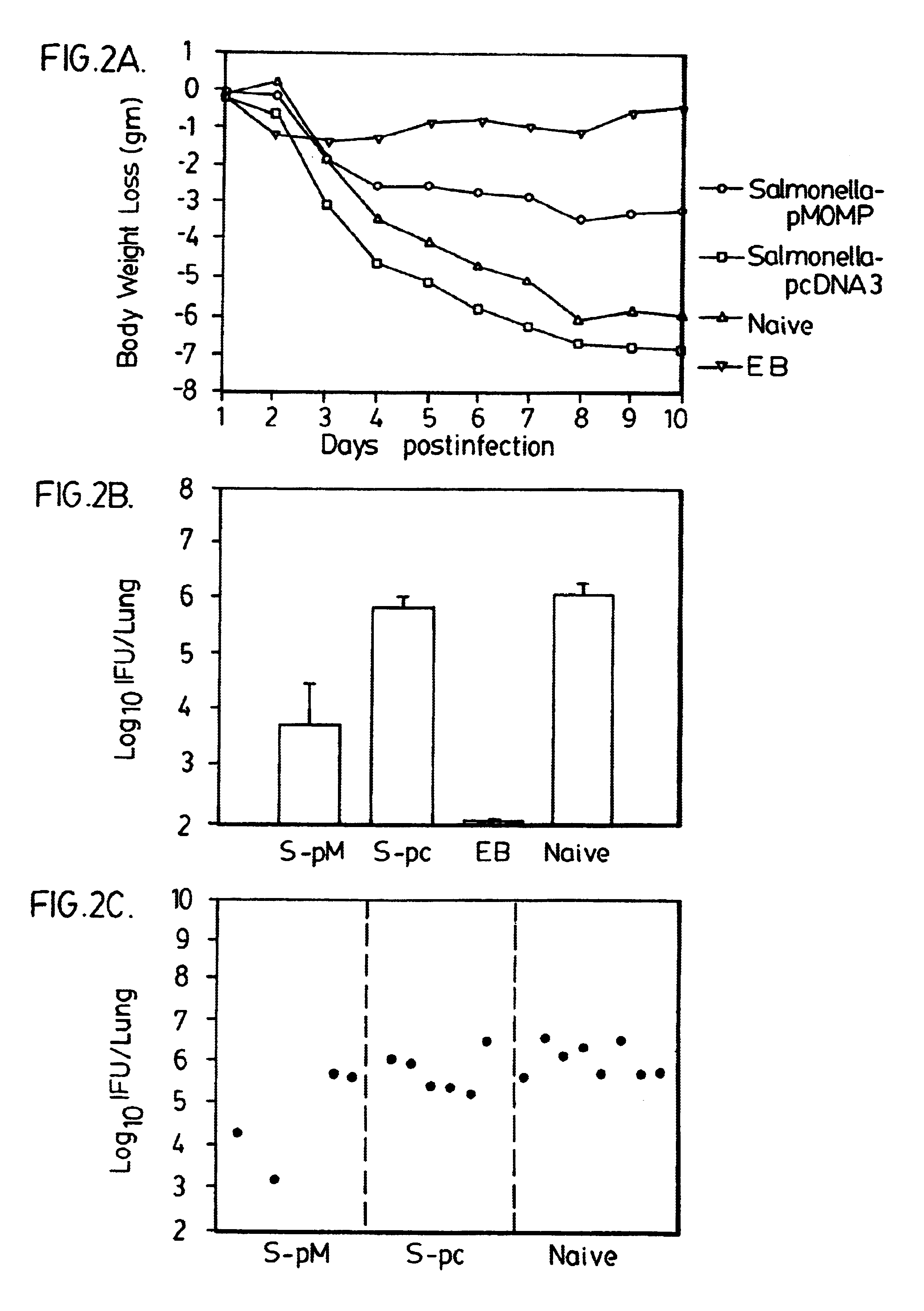
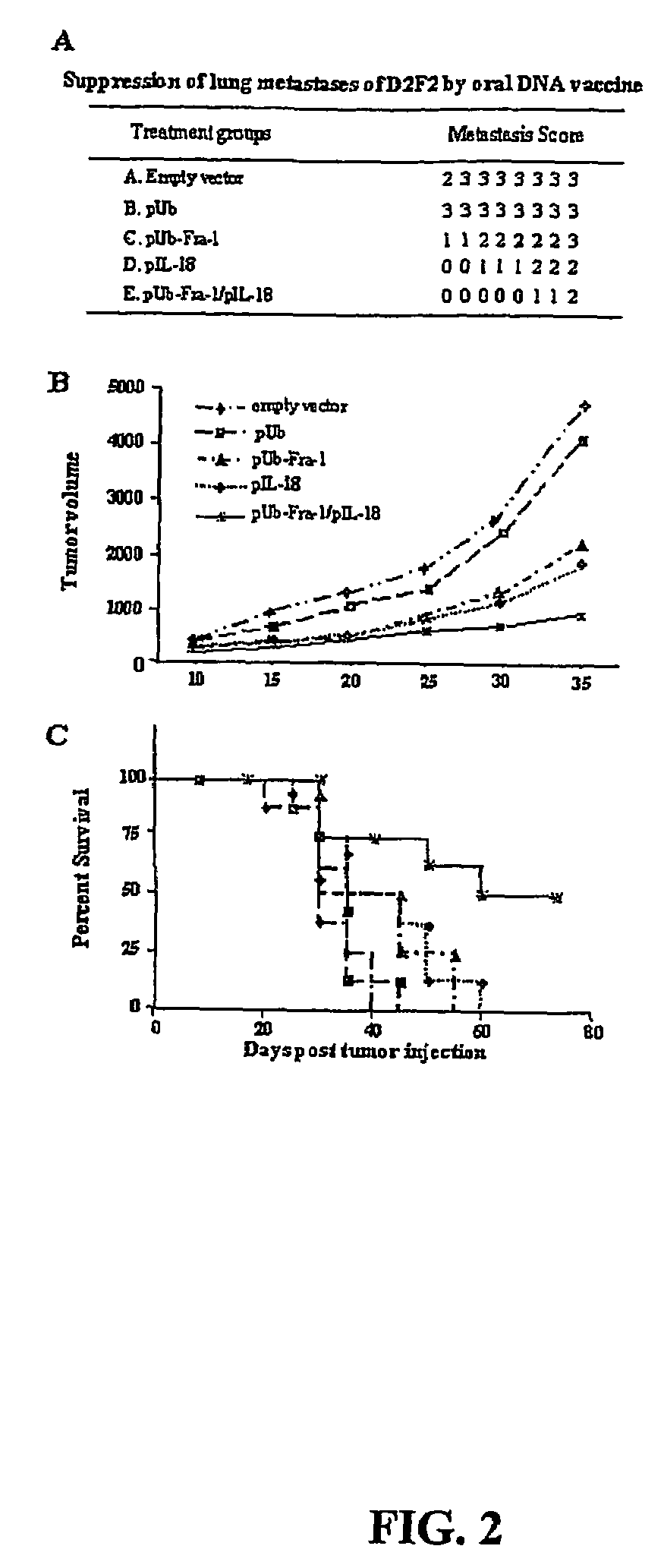
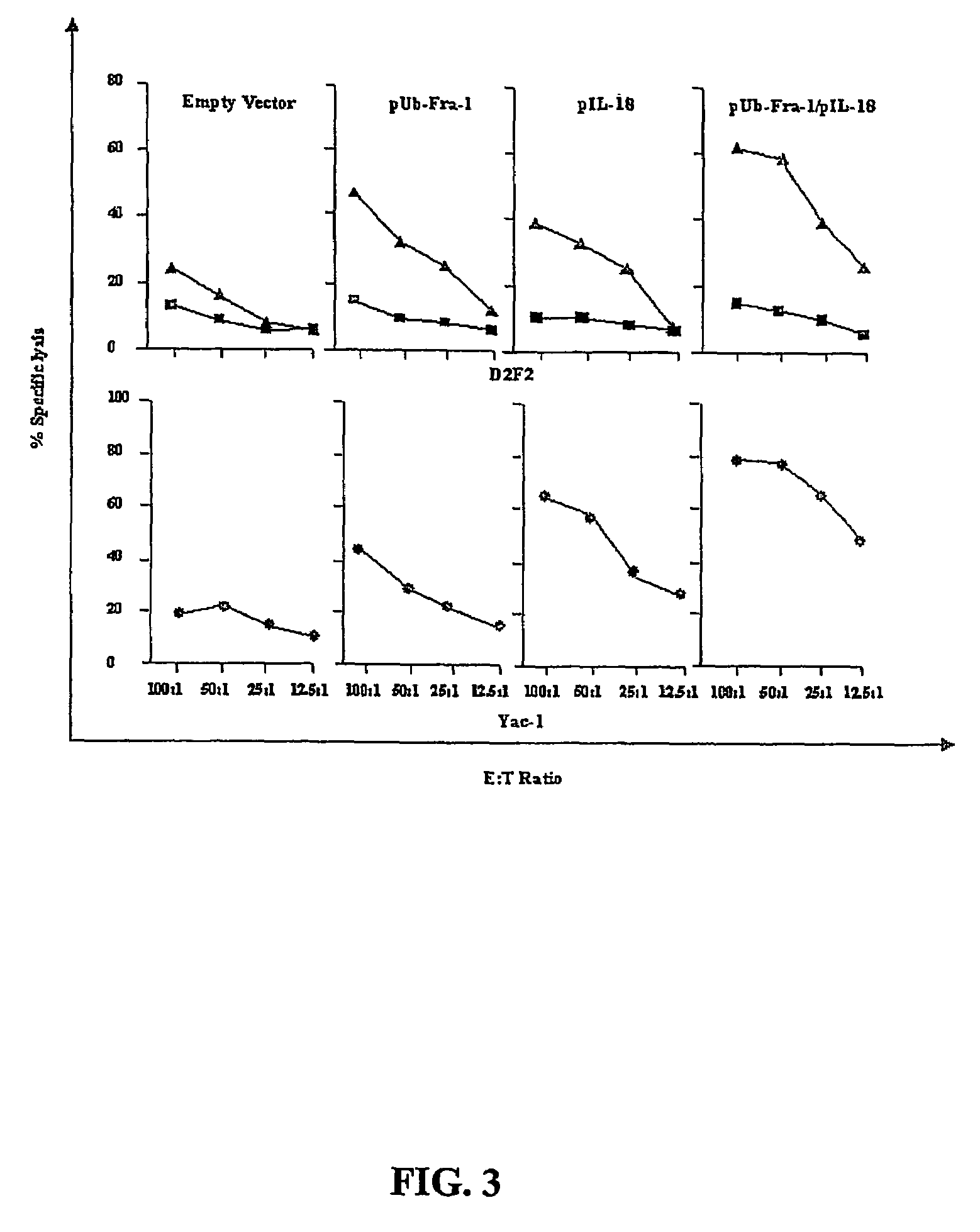
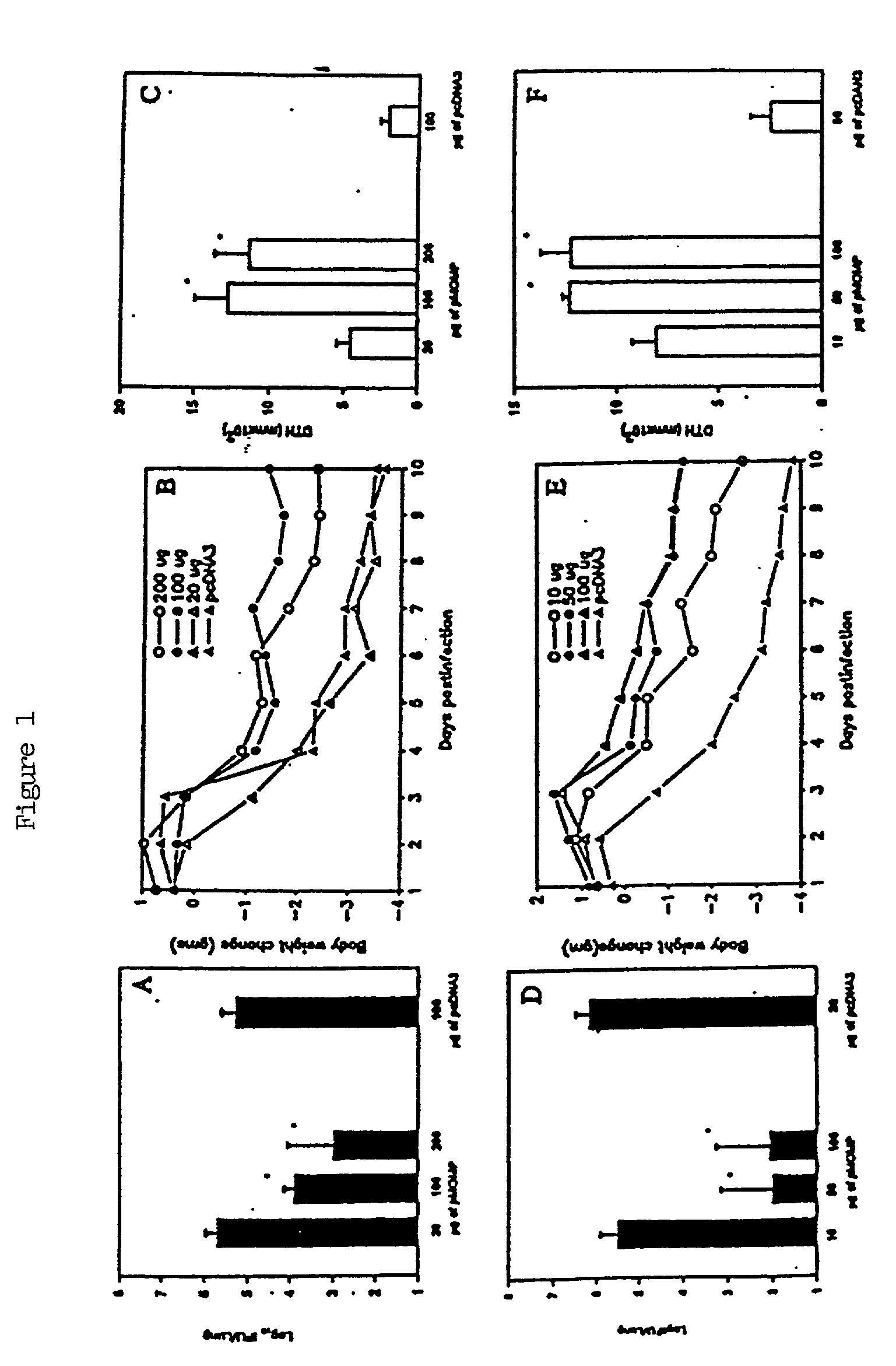
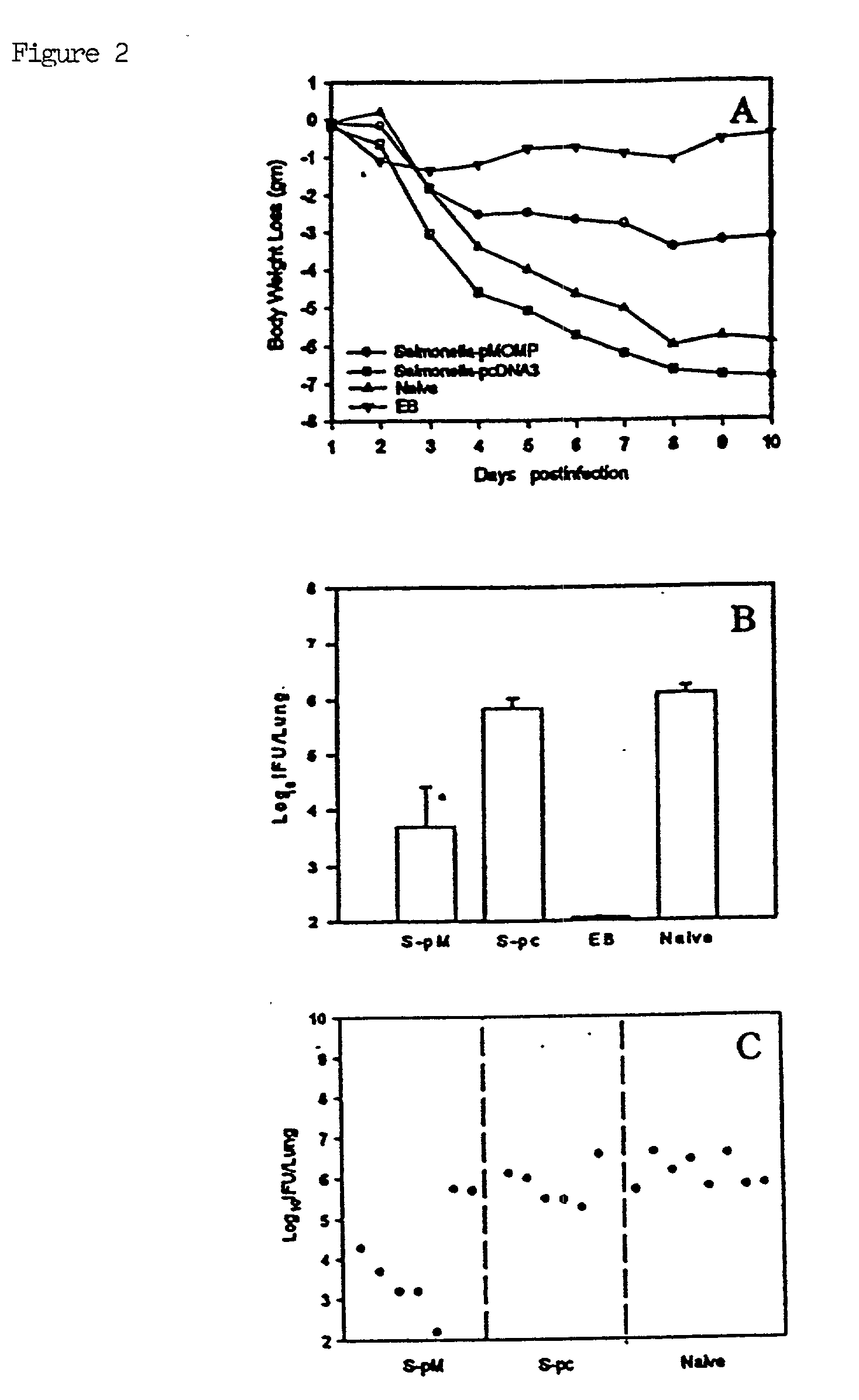
A DNA vaccine suitable for eliciting an immune response against cancer cells comprises a polynucleotide construct operably encoding an a Fra-1 protein, such as a polyubiquitinated human Fra-1 protein, and IL-18, such as human IL-18, in a pharmaceutically acceptable carrier. In a preferred embodiment, the polynucleotide construct is operably incorporated in an attenuated bacterial vector, such as an attenuated Salmonella typhimurium, particularly a doubly attenuated aroA− dam− S. typhimurium. Transformed host cells, methods of inhibiting tumor growth, of vaccinating a patient against cancer, and of delivering genetic material to a mammalian cell in vivo are also described.
Owner:THE SCRIPPS RES INST
Non-pathogenic and/or attenuated bacteria capable of inducing apoptosis in macrophages, process of manufacturing and uses thereof
The invention relates to an non-pathogenic and / or attenuated bacterium which is capable of inducing apoptosis in macrophages.
Owner:AETERNA ZENTARIS GMBH
Virulence Attenuated Bacteria For Treatment Of Malignant Solid Tumors
ActiveUS20190015497A1Bacterial antigen ingredientsBacteria material medical ingredientsBacteroidesVirulent characteristics
Owner:UNIVERSITY OF BASEL
Clostridial toxin NetB
ActiveUS8263088B2Improve stabilityImprove propertiesAntibacterial agentsBacteriaDiseaseClostridial toxin
Owner:AUSTRALIAN POULTRY CRC
Attenuation bacteria carrier tuberculosis vaccine carrying tuberculosis, preparation method and application thereof
InactiveCN101428143AEfficient expressionEffective mucosal immune responseAntibacterial agentsBacterial antigen ingredientsBacteroidesElectroporation
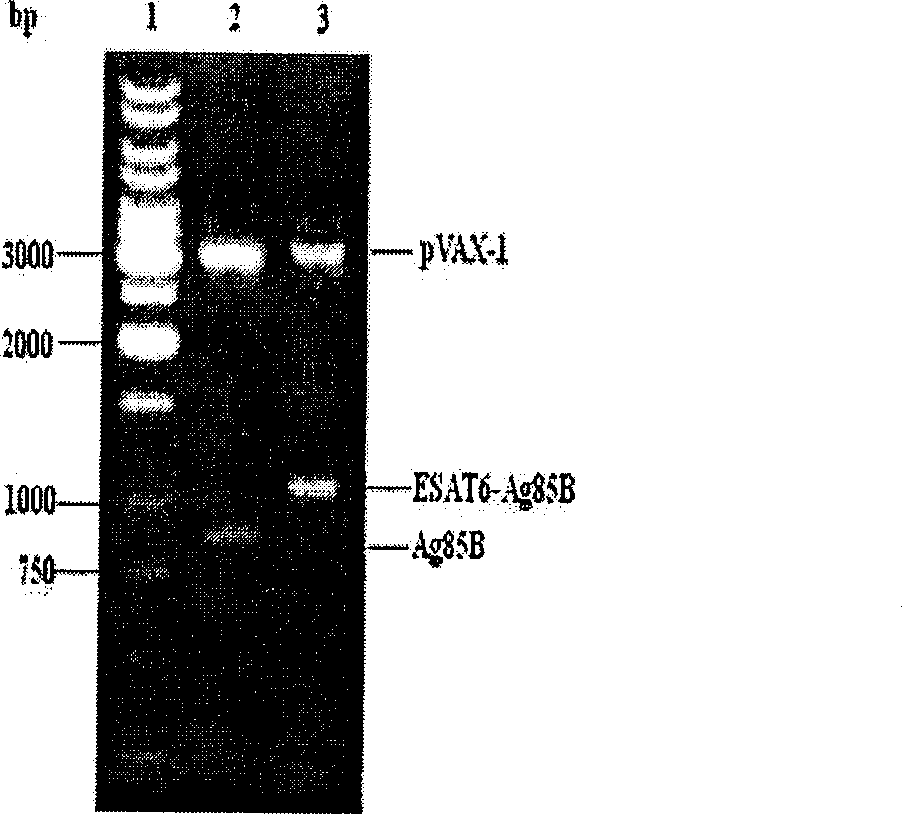
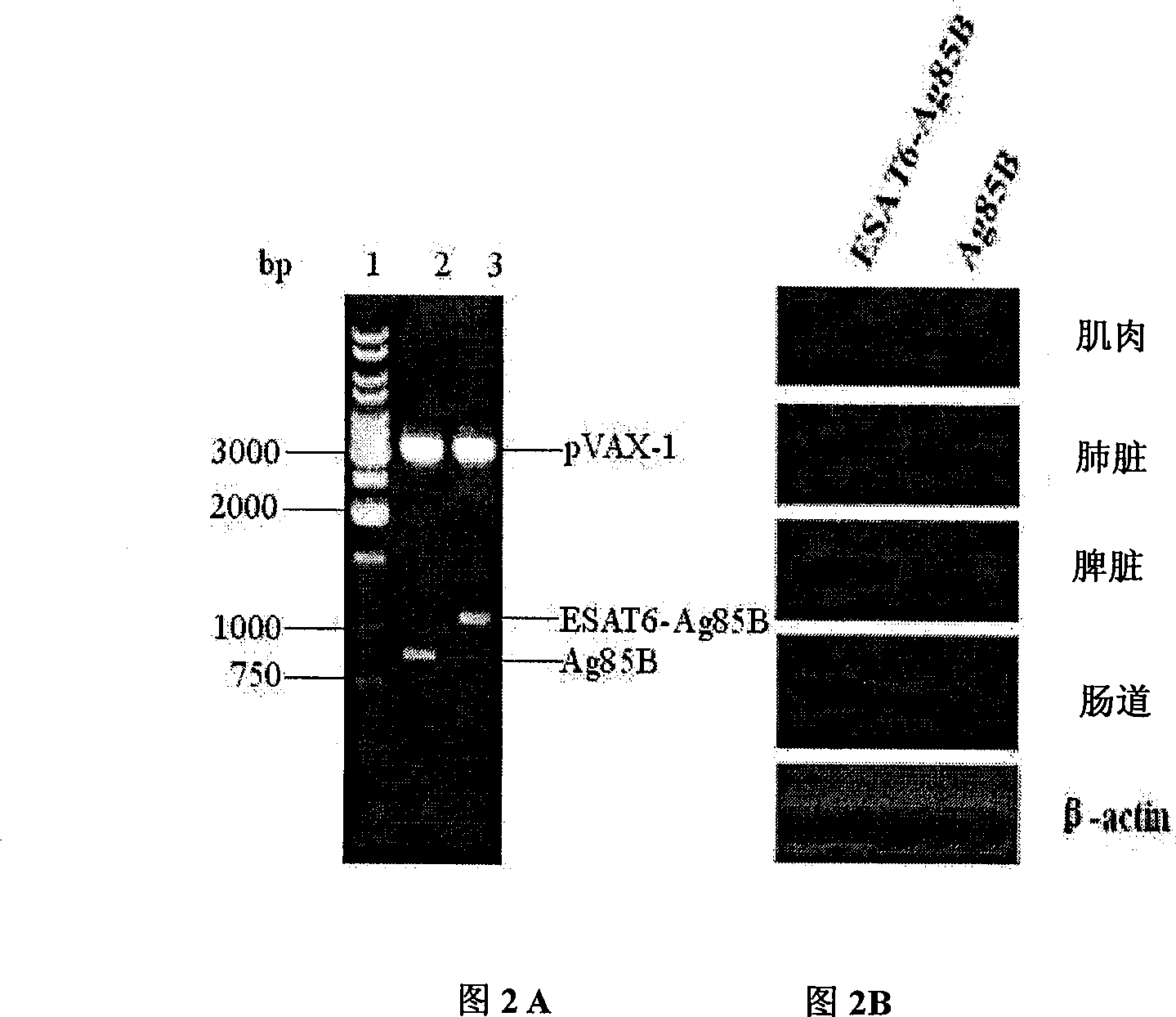
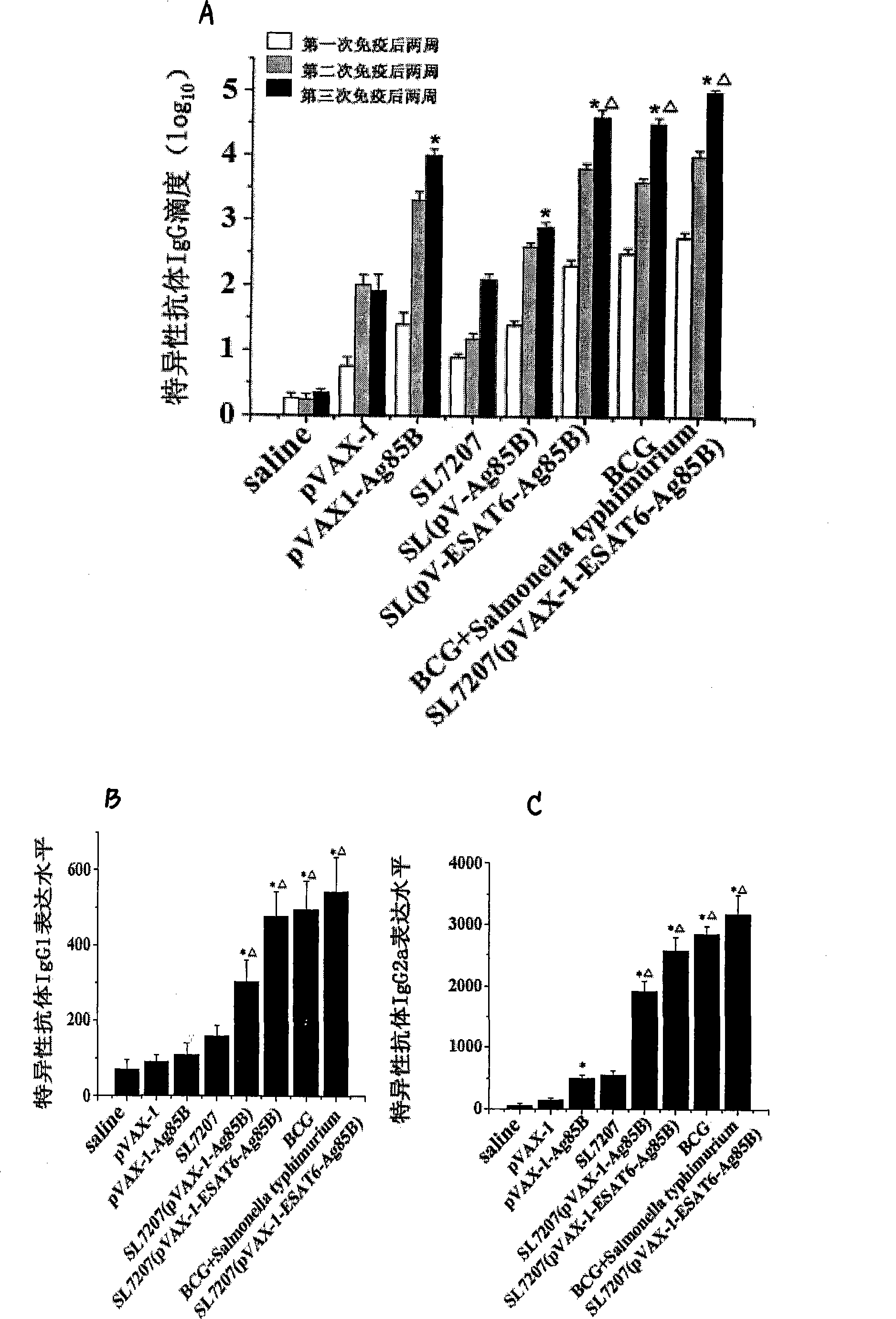
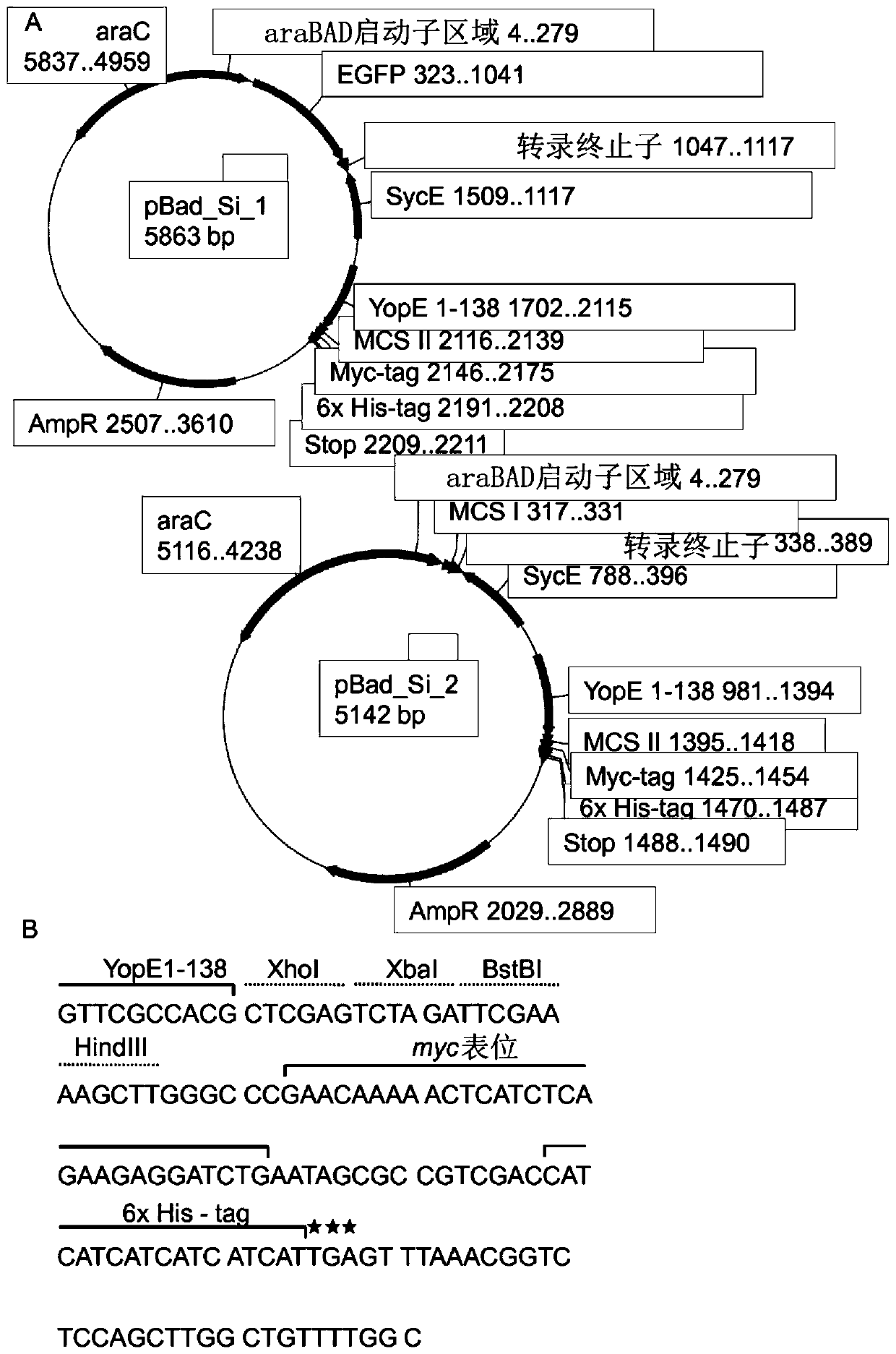
The invention discloses an attenuated bacteria vector tuberculosis vaccine carrying tuberculosis genes as well as a preparation method and application thereof. The preparation process includes the following steps: firstly, structuring a eukaryotic expression plasmid containing mycobacterium tuberculosis Ag85B and ESAT-6Agb5B genes; and secondly, using the electroporation method to convert the structure eukaryotic expression plasmid into attenuated Salmonella typhimurium which can stably express mycobacterium tuberculosis proteins. The combined utilization of Salmonella typhimurium SL7207 (pVAX-1-ESAT6-Ag85B) and BCG can achieve greater immune protective effect in resisting mycobacterium tuberculosis infection than by the BCG only; and the SIgA level of mucosa and serum produced by the Salmonella typhimurium SL7207 (pVAX-1-ESAT6-Ag85B) and BCG combined is higher than BCG and DNA vaccines. In addition, the Salmonella typhimurium SL7207 (pVAX-1-ESAT6-Ag85B) has greater immune protective ability than DNA vaccines or Salmonella typhimurium SL7207 (pVAX-1-Ag85B). The invention has the advantages of simple method, low cost, and convenient use, and can not only resist Salmonella typhosa infection, but also achieve immune protection against mycobacterium tuberculosis infection. Moreover, the stable expression of the mycobacterium tuberculosis proteins after being immunized can induce mucosal and systematic immune responses, and the invention has remarkable economic benefits.
Owner:WUHAN UNIV
Live attenuated RTX-producing bacteria of family pasteurellaceae
InactiveCN1117862CEffective immune responseBacteriaViral antigen ingredientsBacteroidesUltrasound attenuation
The present invention relates to live attenuated RTX-toxin producing bacteria of the family Pasteurellaceae, of which the attenuation is due to the fact that they produce RTX toxin in a non-activated form. The invention also relates to vaccines for the protection of mammals against infection with RTX-toxin producing bacteria of the family Pasteurellaceae, and to methods for the preparation of said live attenuated bacteria and vaccines.
Owner:INTERVET INT BV
Attenuated Mycoplasma gallisepticum strains
ActiveUS7935356B2Reduce expressionLow toxicityAntibacterial agentsBacterial antigen ingredientsBacteroidesVaccination
The present invention provides live, attenuated Mycoplasma gallisepticum bacteria that exhibit reduced expression of a protein identified as MGA_0621. In certain embodiments, the attenuated bacteria may additionally exhibit reduced expression of one or more proteins selected from the group consisting of pyruvate dehydrogenase, phosphopyruvate hydratase, 2-deoxyribose-5-phosphate aldolase, and ribosomal protein L35, relative to a wild-type M. gallisepticum bacterium. Also provided are vaccines and vaccination methods involving the use of the live, attenuated M. gallisepticum bacteria, and methods for making live attenuated M. gallisepticum bacteria. An exemplary live, attenuated strain of M. gallisepticum is provided, designated MGx+47, which was shown by proteomics analysis to exhibit significantly reduced expression of MGA_0621, and was shown to be safe and effective when administered as a vaccine against M. gallisepticum infection in chickens.
Owner:ZOETIS SERVICE LLC
Combination for use in the treatment and/or prevention of mastitis
The present invention relates a combination for use in the treatment and / or prevention of mastitis containing i) an agonistic anti-CD40 monoclonal antibody or a CD40 ligand or a vector coding for the anti-CD40 antibody or a vector coding for the CD40L; and ii) inactivated or attenuated bacteria selected from the group consisting of Staphylococcus, Streptococcus, Listeria or Escherichia.
Owner:UNIV LIEGE
Non-pathogenic and/or attenuated bacteria capable of inducing apoptosis in macrophages, process of manufacturing and uses thereof
The invention relates to an non-pathogenic and / or attenuated bacterium which is capable of inducing apoptosis in macrophages.
Owner:AETERNA ZENTARIS GMBH
Two-step immunization procedure against chlamydia infection
InactiveUS20020168382A1Strong protective immune responseProvide protectionAntibacterial agentsSenses disorderTwo stepChlamydia virus
A host is immunized against infection by a strain of Chlamydia by initial administration of an attenuated bacteria harbouring a nucleic acid encoding a Chlamydia protein followed by administration of a Chlamydia protein in ISCOMs. This procedure enables a high level of protection to be achieved.
Owner:AVENTIS PASTUER LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com