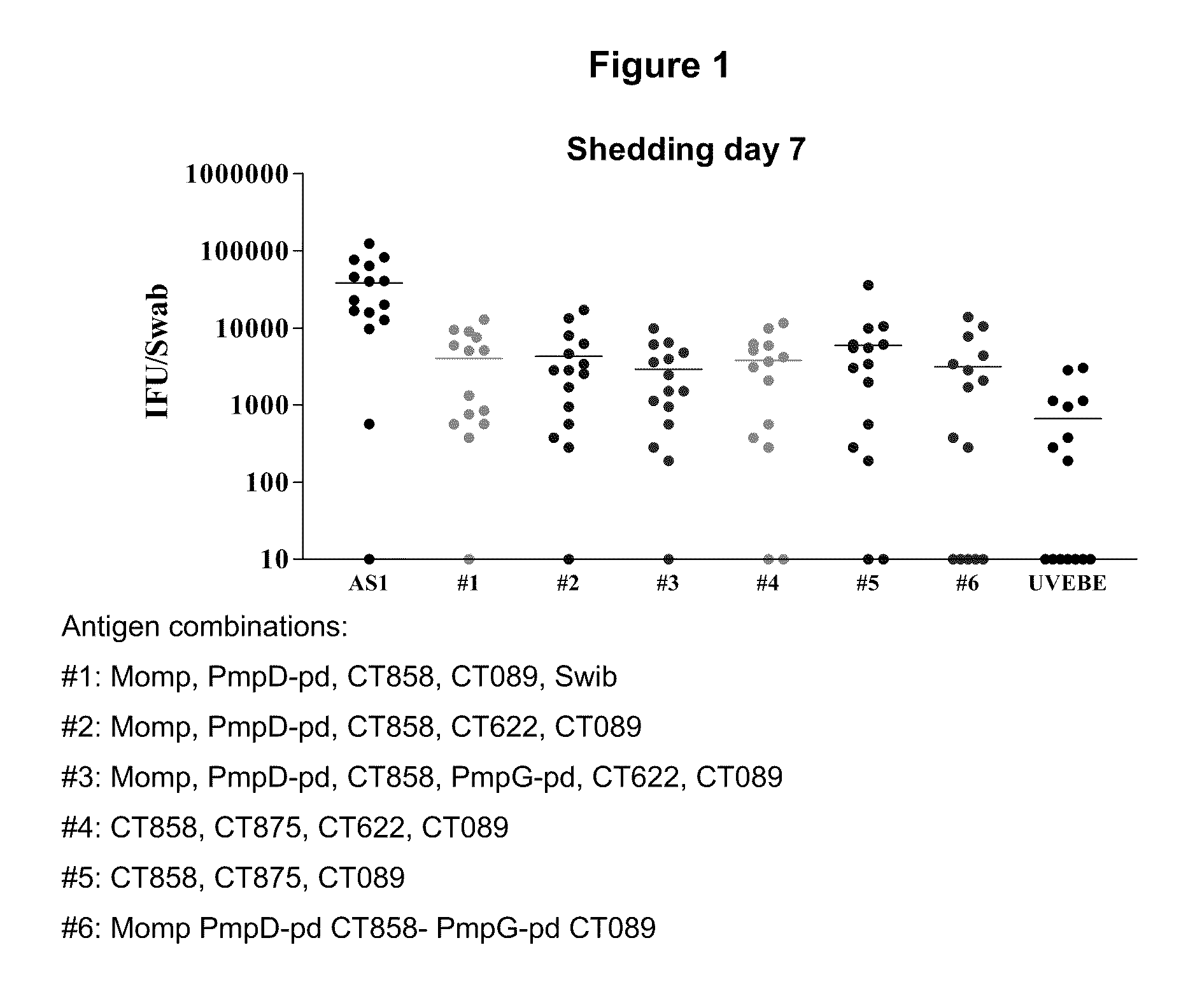
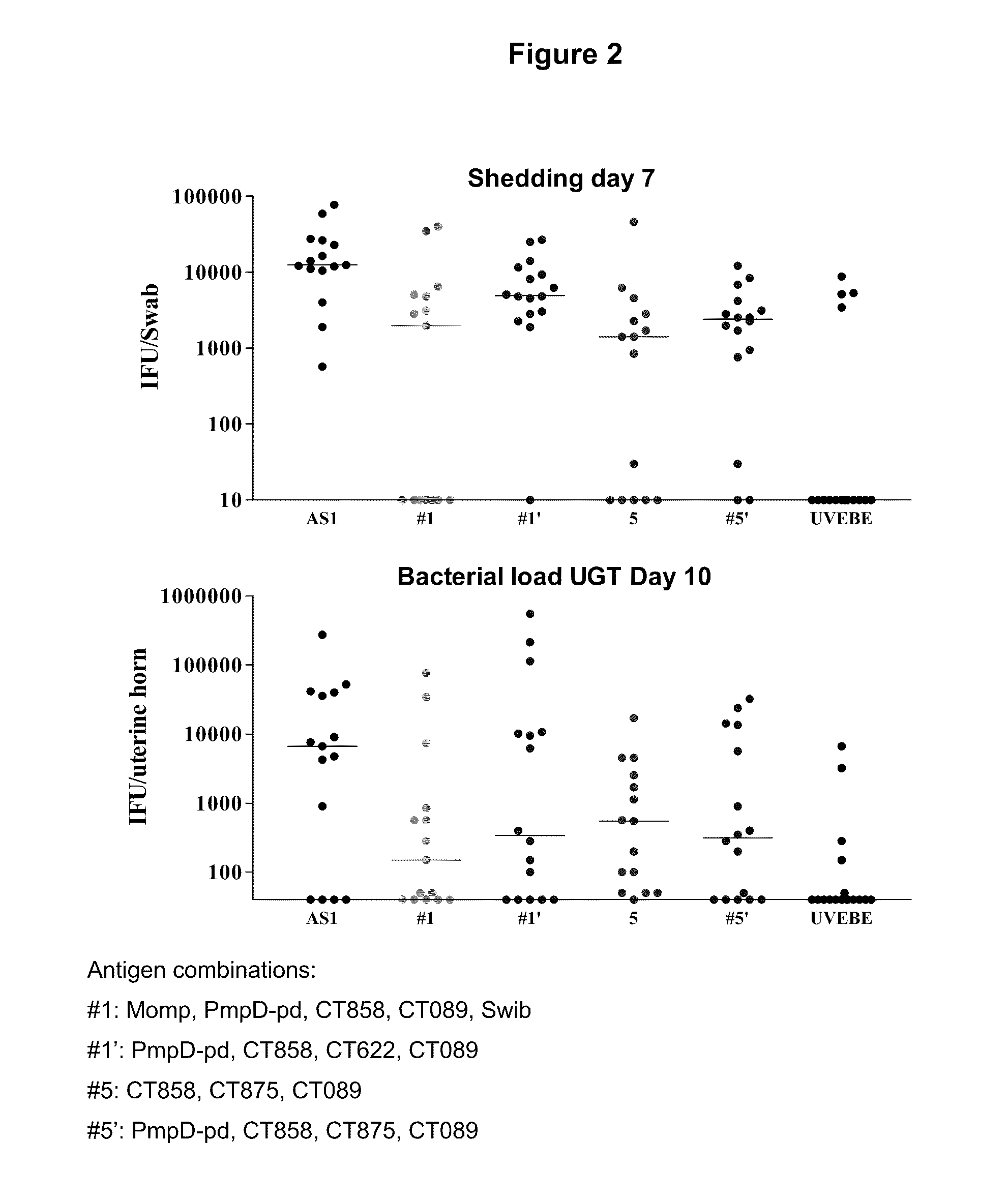
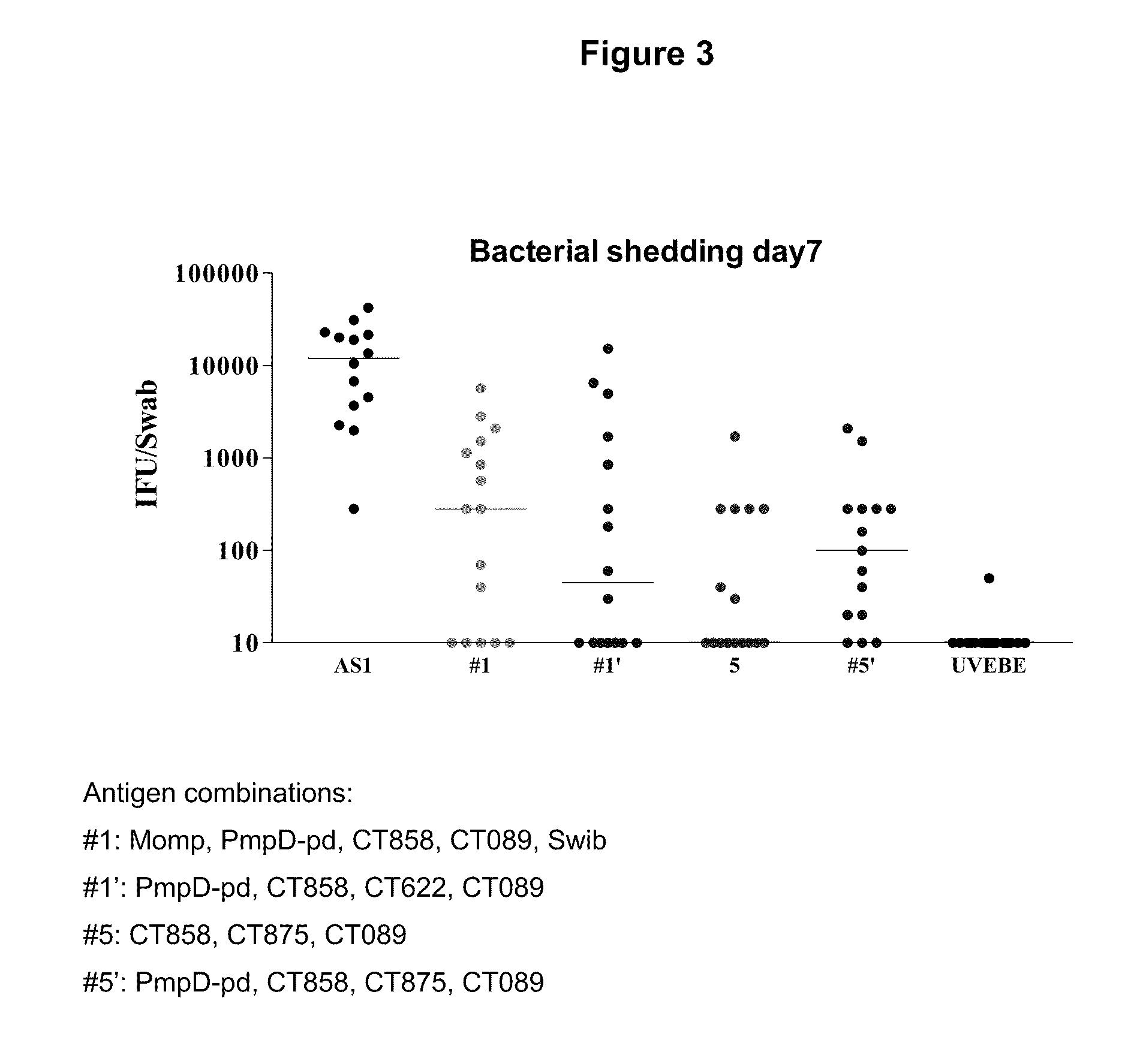
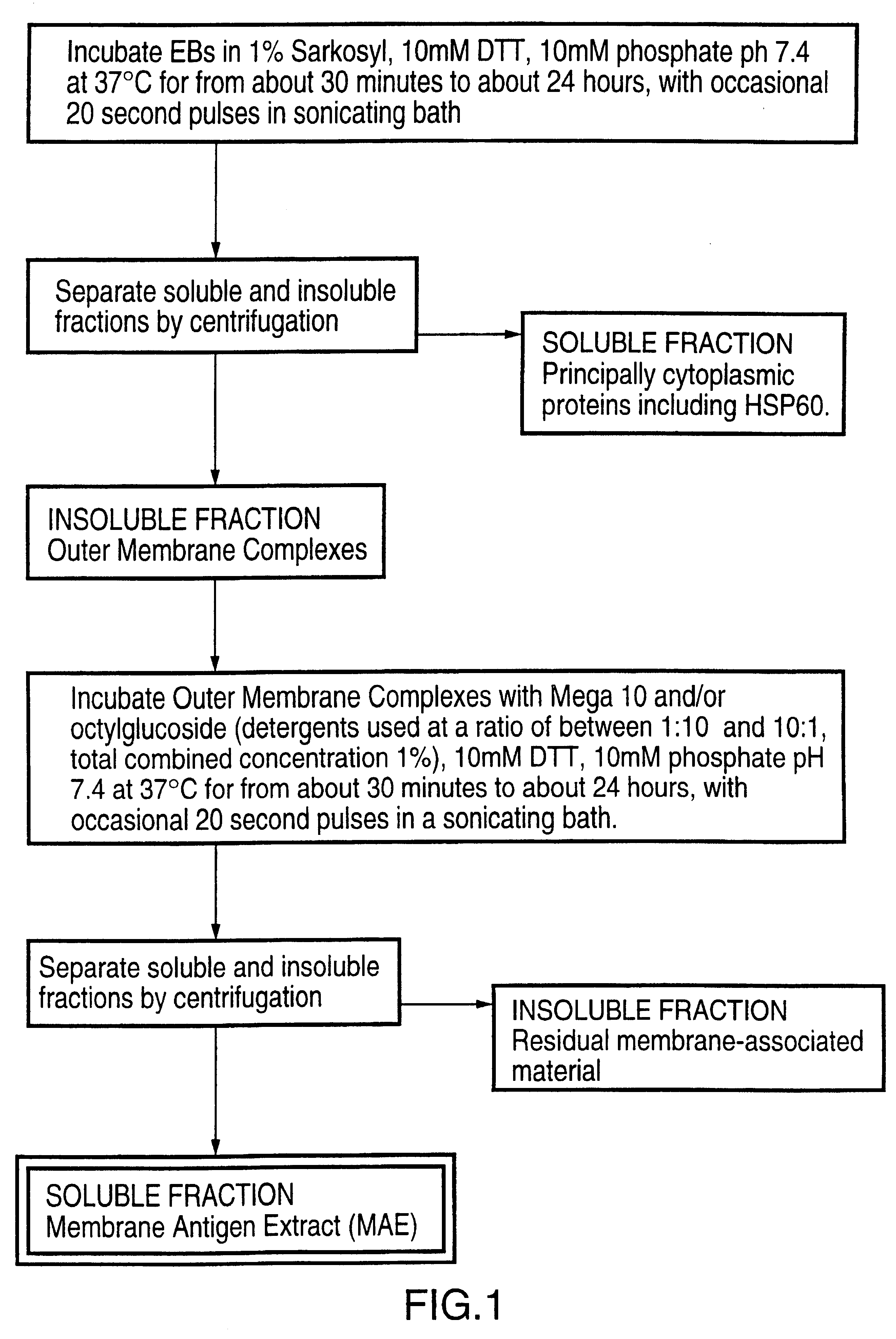
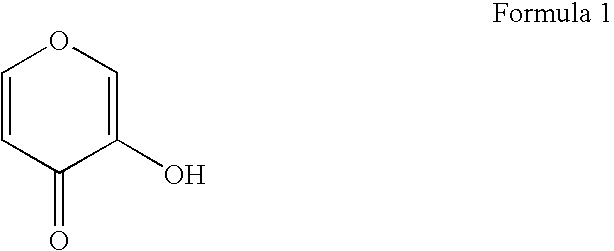Patents
Literature
42 results about "Chlamydia virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlamydia infection, often simply known as chlamydia, is a sexually transmitted infection caused by the bacterium Chlamydia trachomatis. Most people who are infected have no symptoms. When symptoms do develop this can take a few weeks following infection to occur.
Vaccines against chlamydial infection
InactiveUS20090022755A1Stimulate immune responseProvide protectionBacterial antigen ingredientsChlamydiaceae ingredientsPolynucleotideChlamydia sp
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:CORIXA CORP +1
DNA immunization against chlamydia infection
InactiveUS6696421B2Significantly more efficient in inducing protective immunityRapid recruitment of dendritic cellsBiocideGenetic material ingredientsNucleotidePhylum Chlamydiae
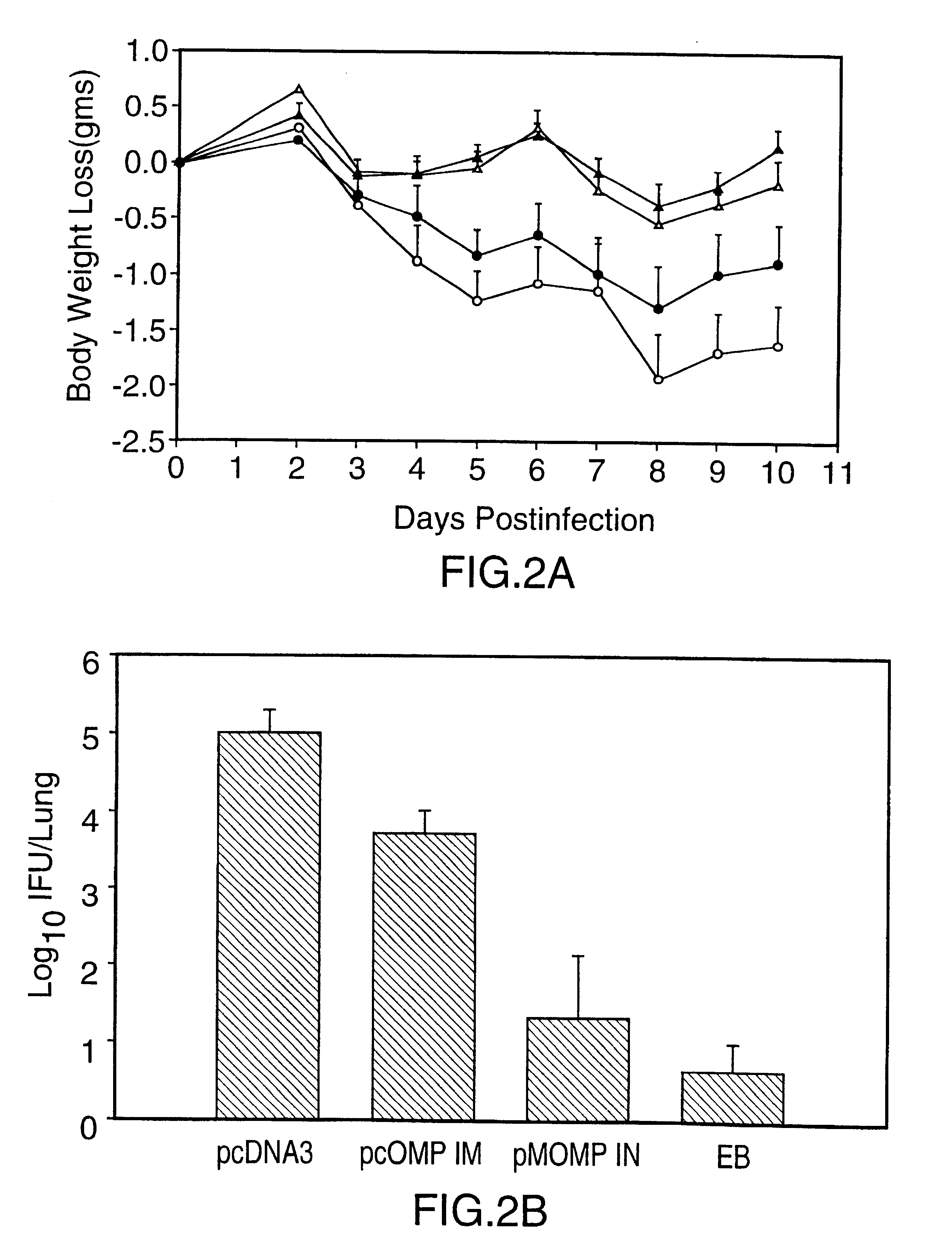
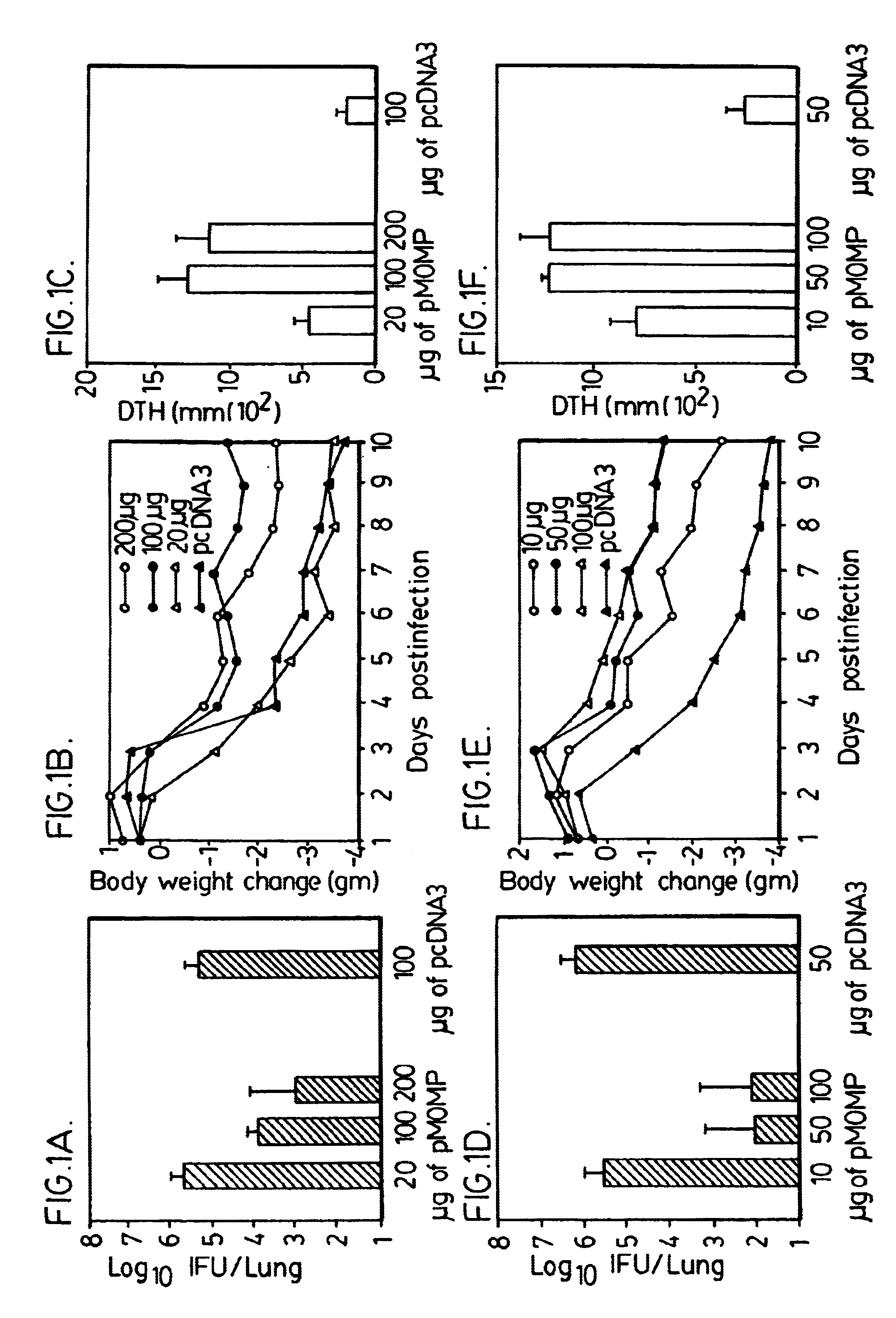
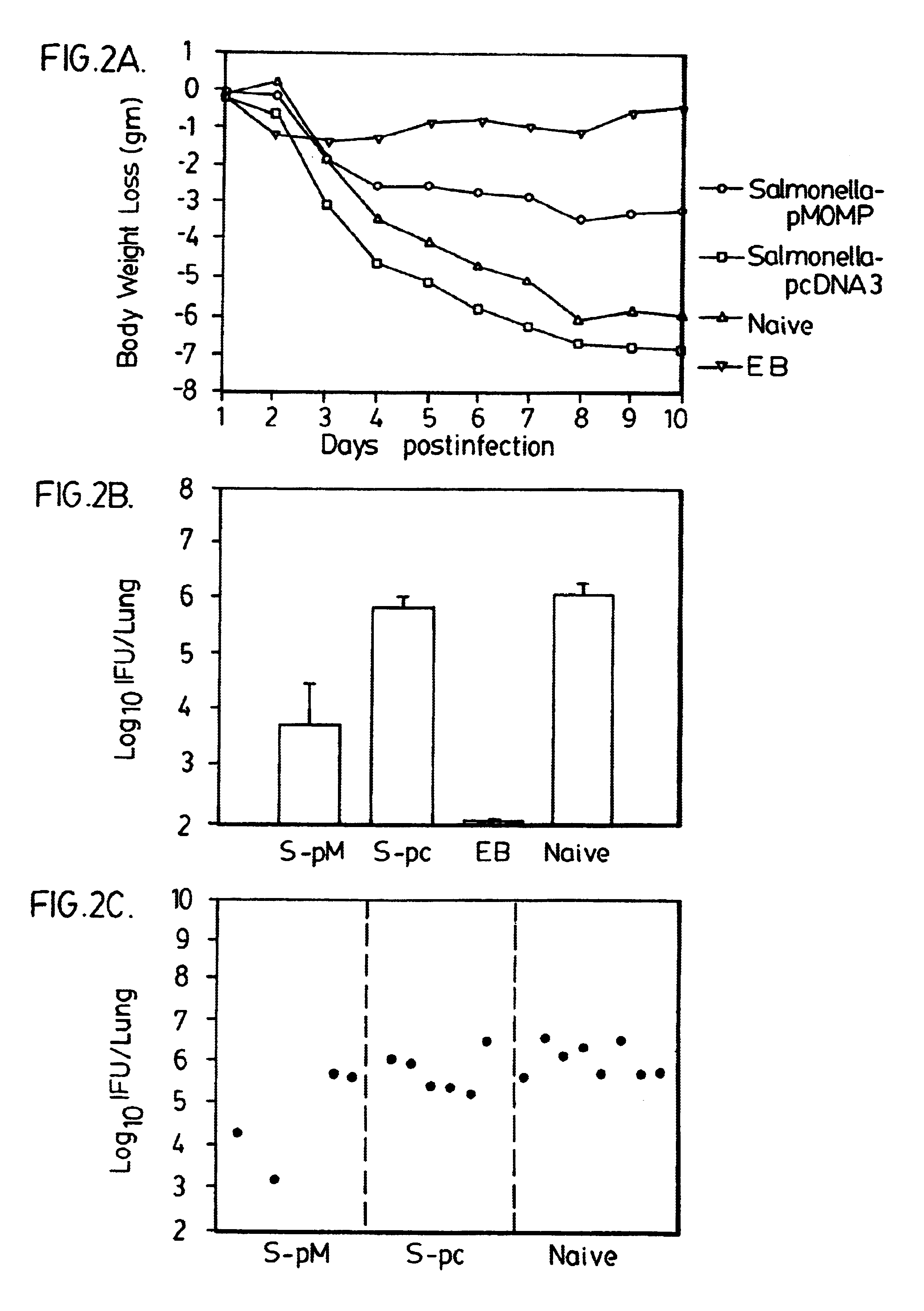
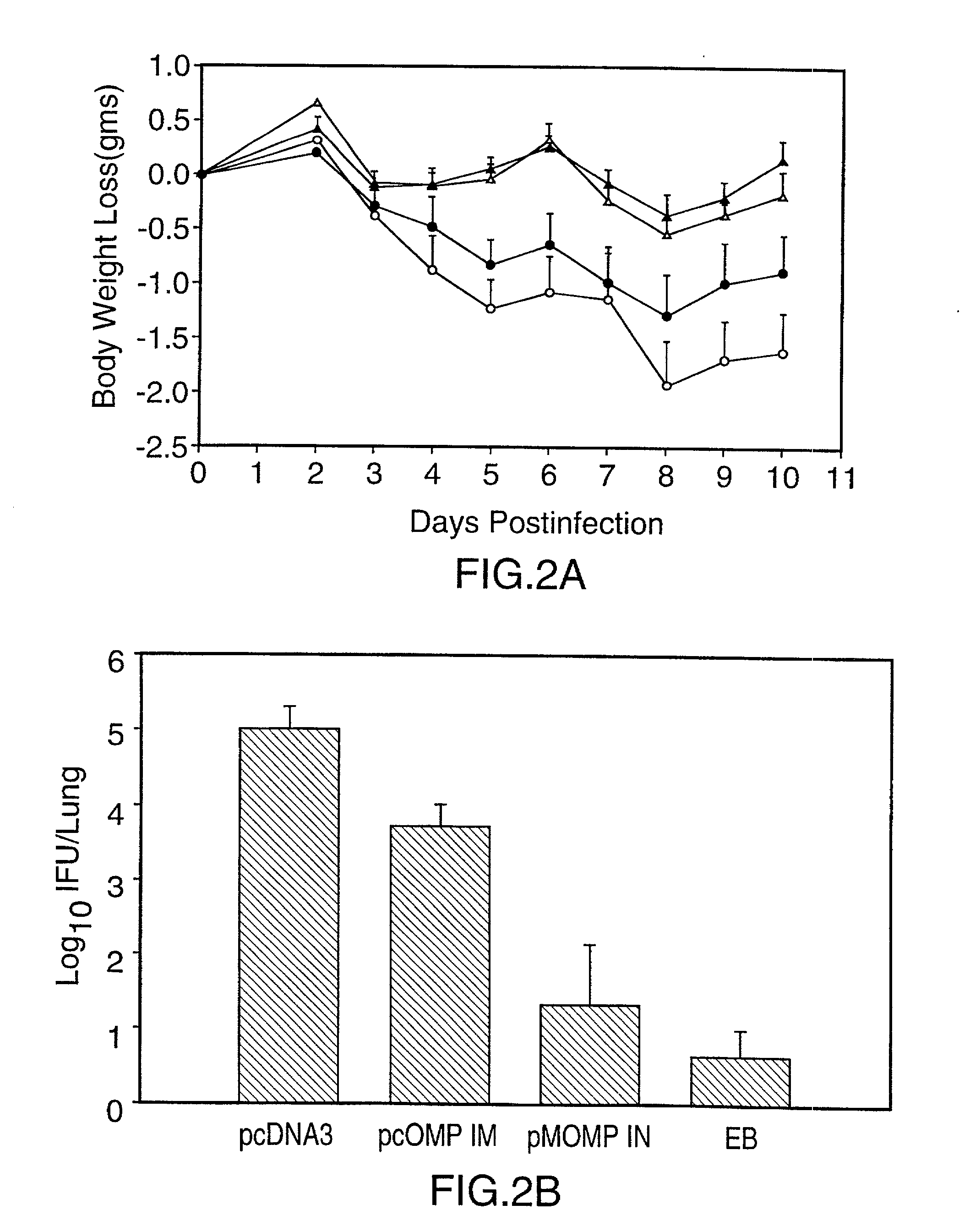
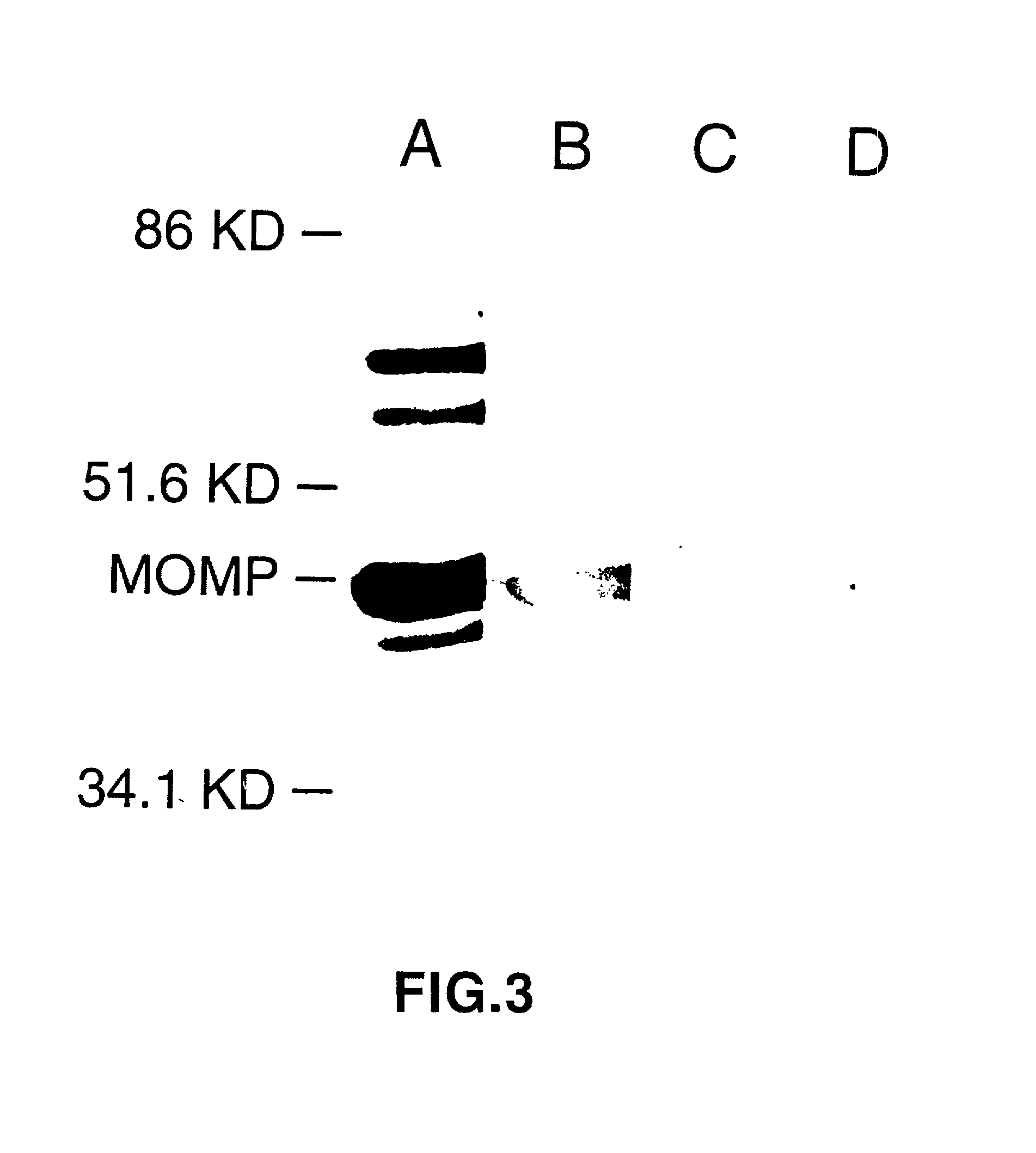
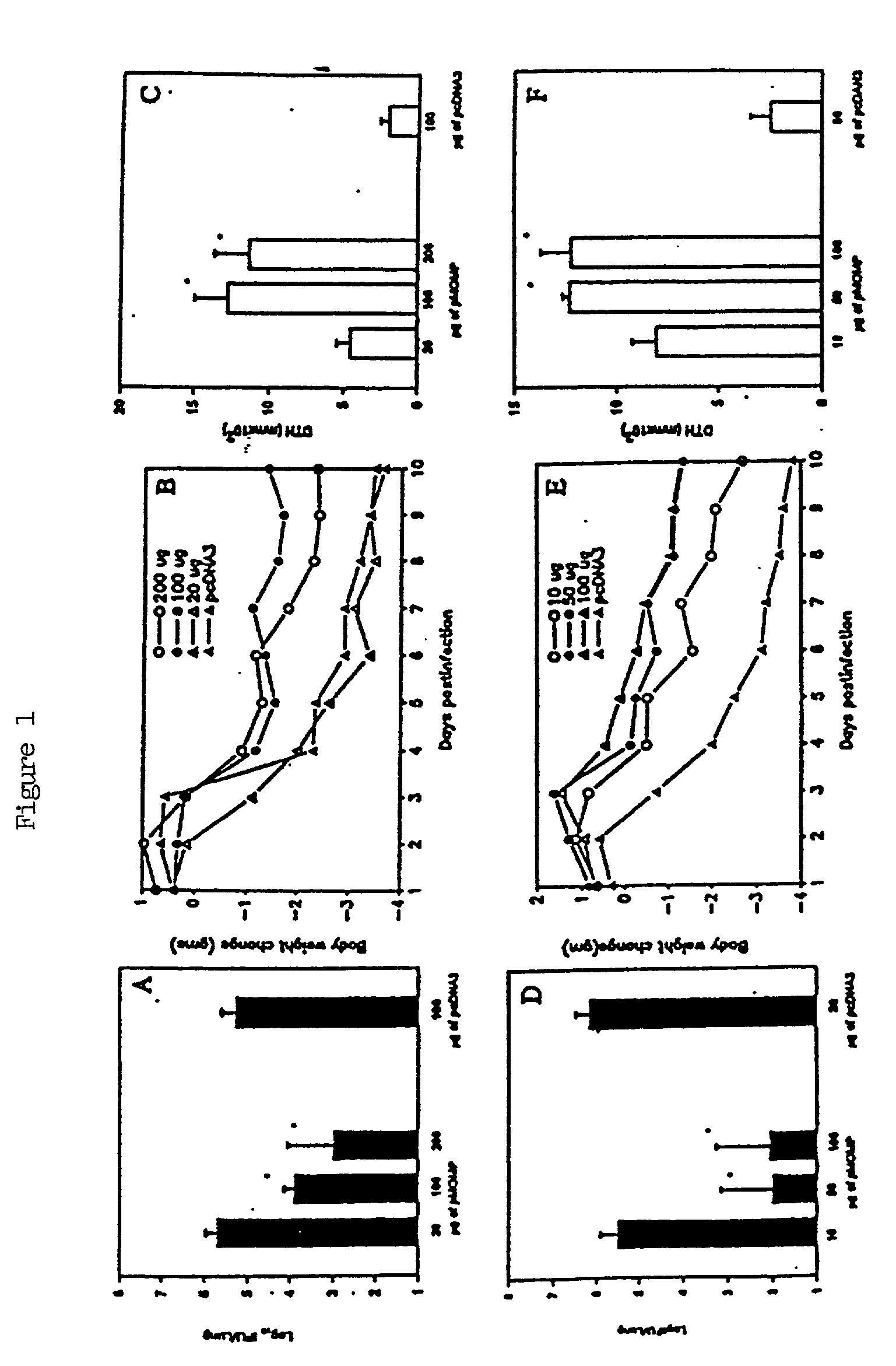
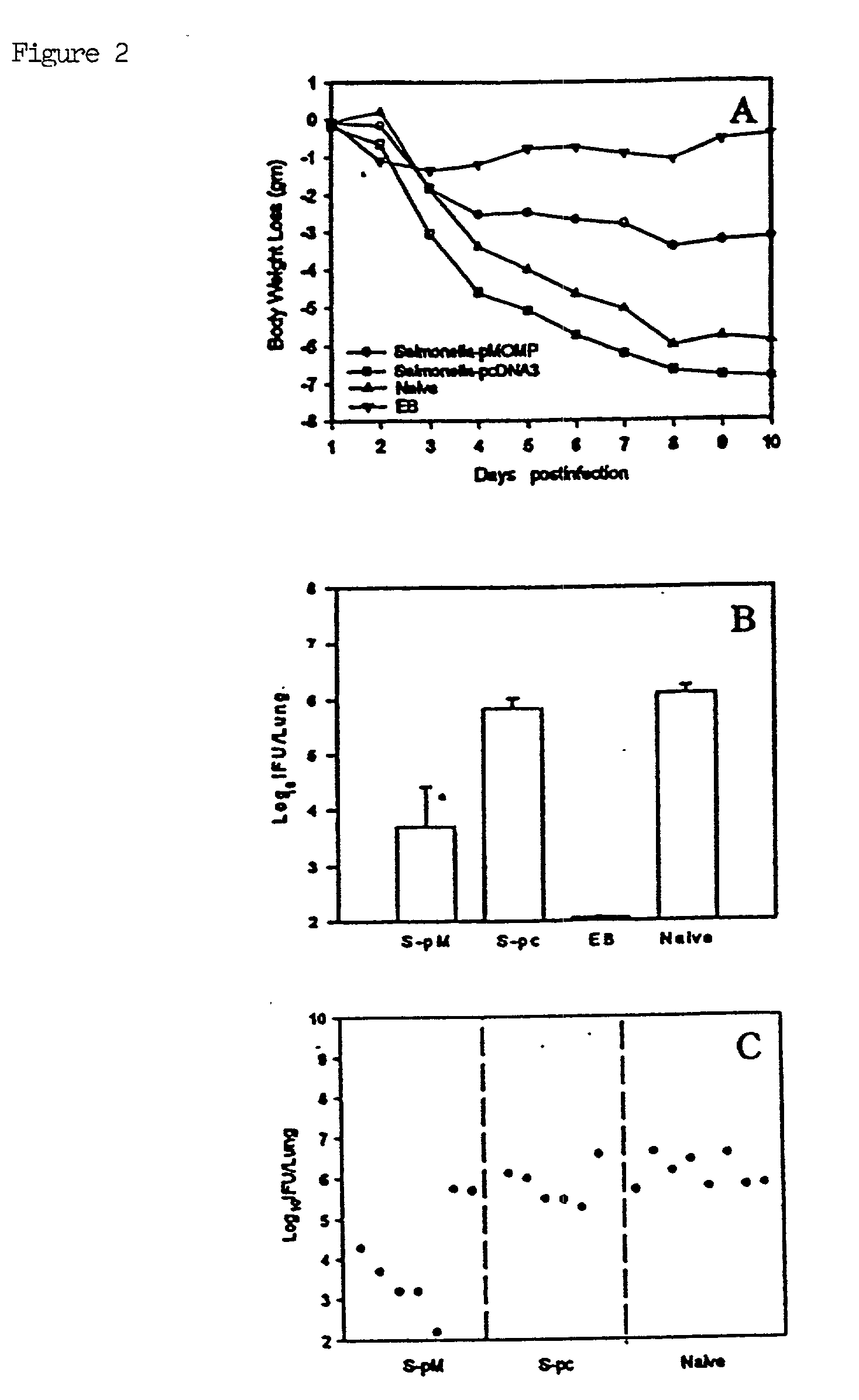
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Vaccines Against Chlamydia Infection
InactiveUS20100310593A1Improve isolationEasy to purifyAntibacterial agentsBacteriaPolynucleotideChlamydia virus
The present invention is directed to providing a vaccine to enhance the immune response of an animal in need of protection against a Chlamydia infection. The present invention is also directed toward an isolated nucleic acid encoding a polypeptide comprising at least 70% identity to any one of SEQ ID NOS: 2, 11, 13, 19, or 21, wherein the polypeptide is soluble in the absence of denaturing agents. In some aspects of the invention, the polynucleotide is codon-optimized. In some embodiments, the present invention is related to the polypeptide encoded by the polynucleotide of the invention. Administration of polypeptides of the present invention can be used as a method to treat or prevent a Chlamydia infection in an animal in need thereof.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
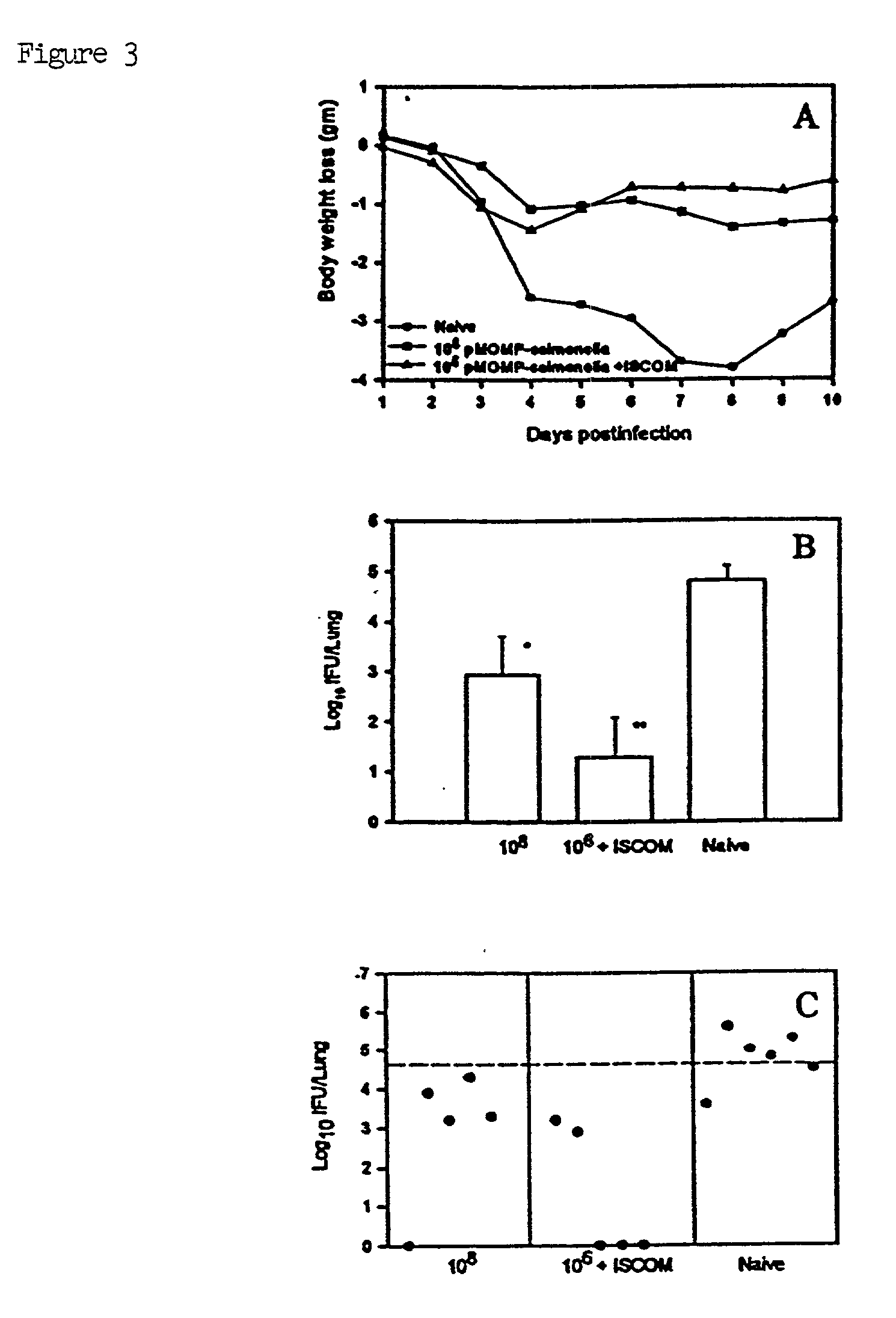
Two-step immunization procedure against Chlamydia infection
InactiveUS6676949B2Strong immune responseImproving immunogenicityAntibacterial agentsSenses disorderTwo stepChlamydia virus
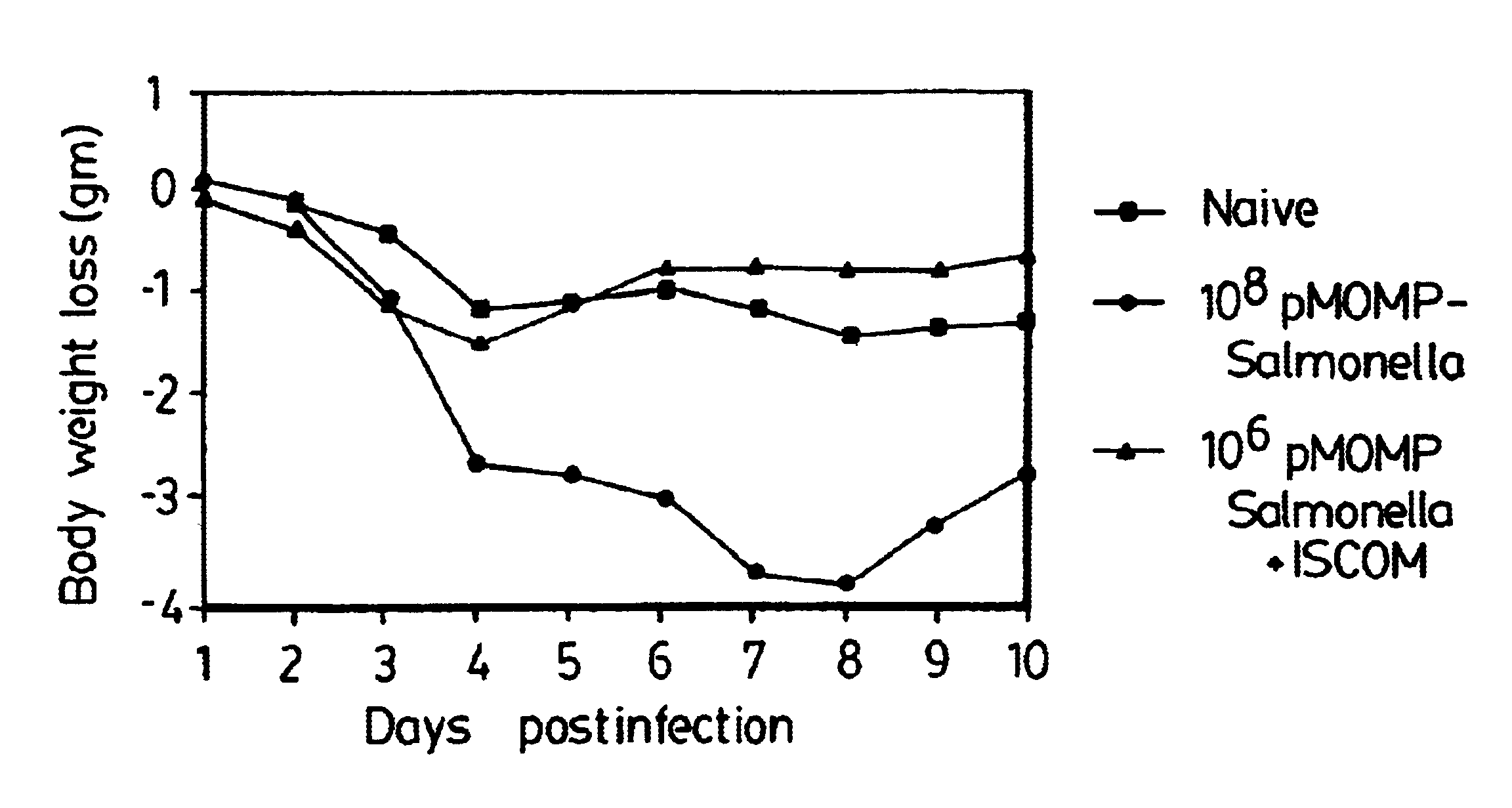
A host is immunized against infection by a strain of Chlamydia by initial administration of an attenuated bacteria harbouring a nucleic acid encoding a Chlamydia protein followed by administration of a Chlamydia protein in ISCOMs. This procedure enables a high level of protection to be achieved.
Owner:AVENTIS PASTEUR LTD
Chlamydial vaccines and methods of preparation thereof
InactiveUS6464979B1Strong immune responseImproving immunogenicityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseImmunostimulating Complexes
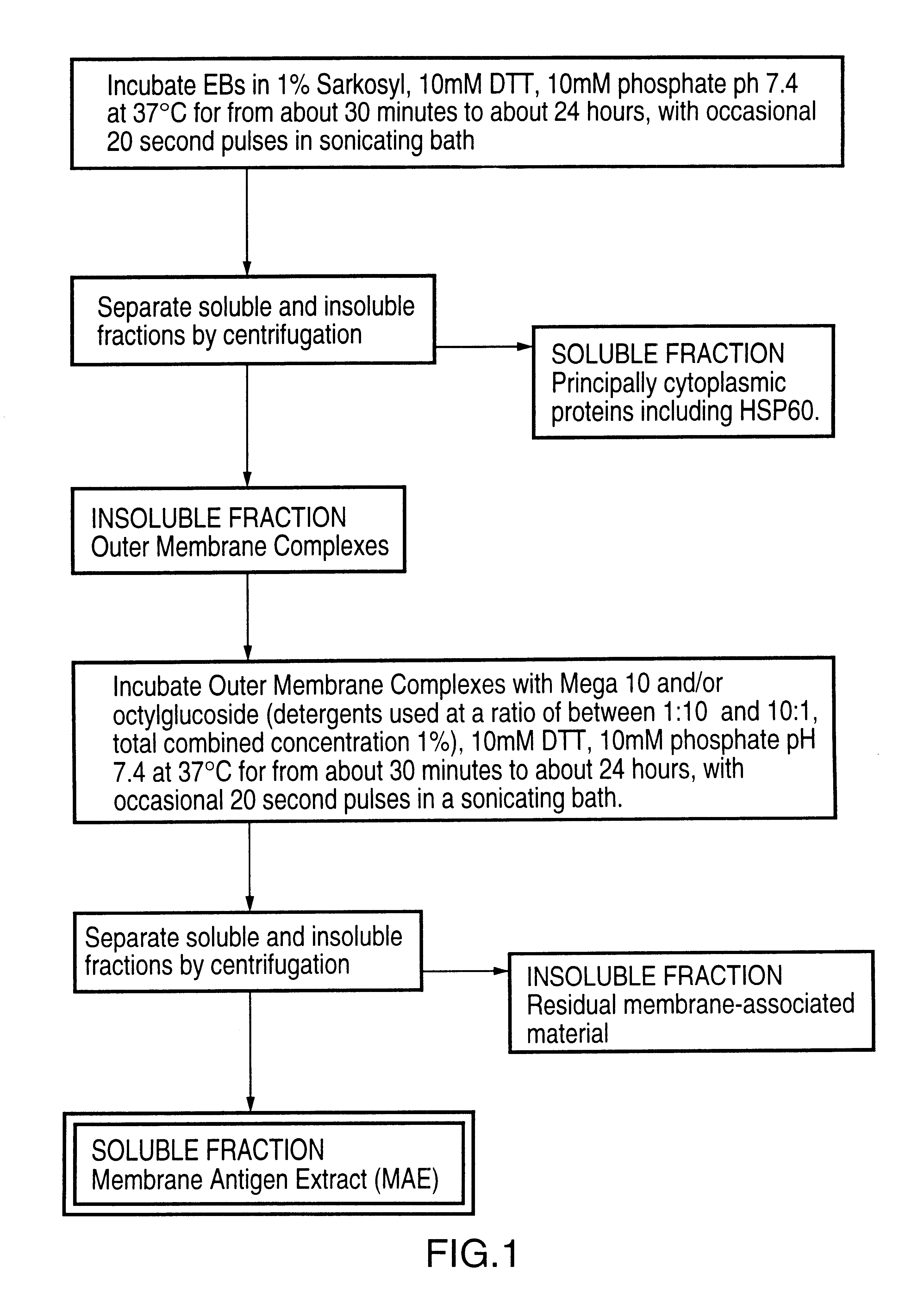
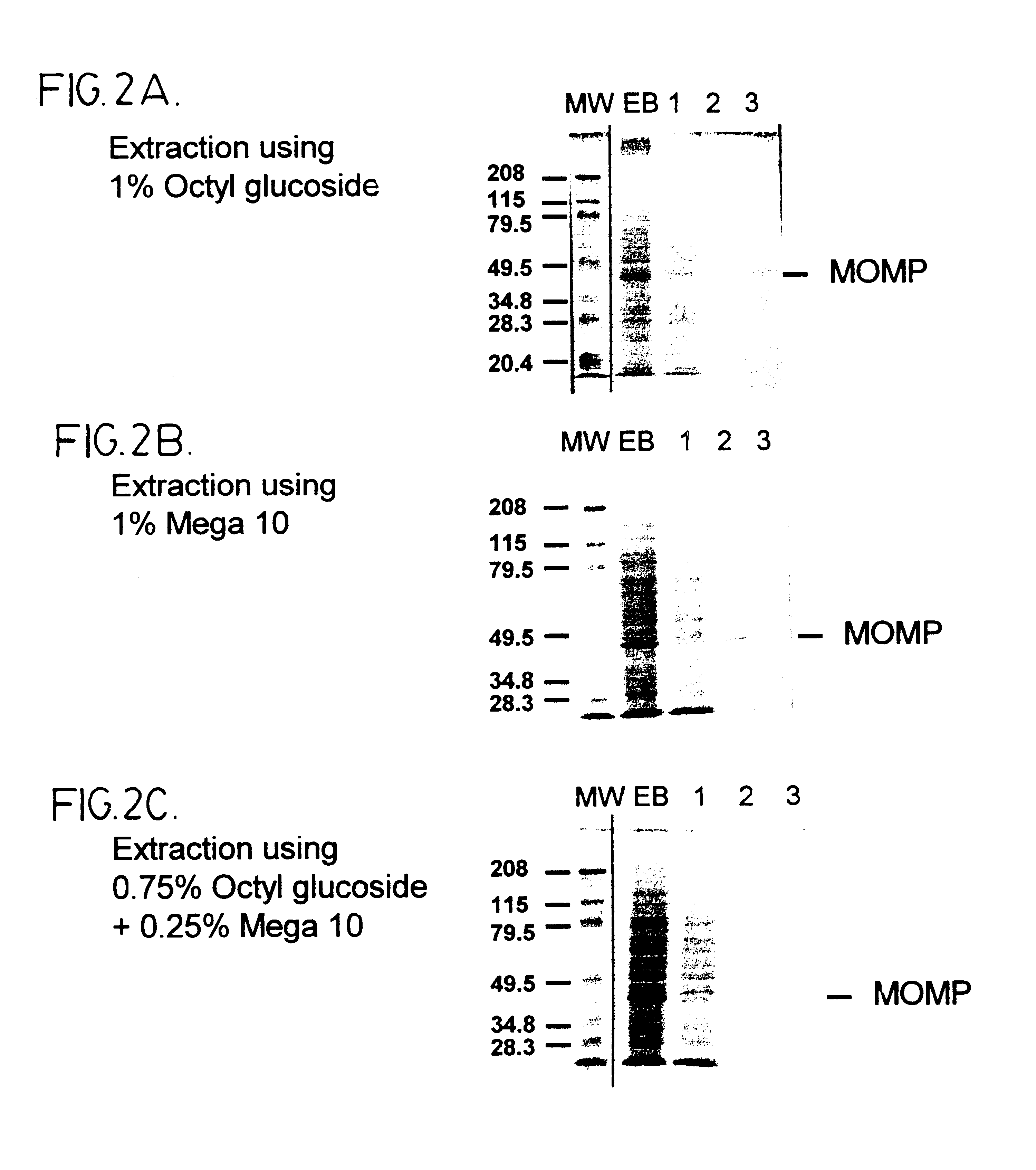
Immunogenic compositions including vaccines are described that comprise an outer membrane antigen extract of a strain of Chlamydia and are effective in protection against disease caused by Chlamydia infection. The immunogenic compositions may comprise the major outer membrane protein (MOMP) of Chlamydia which may be in a homooligomeric form or complexed with at least one other antigen of Chlamydia. The immunogenic composition may include an immunostimulating complex (ISCOM) and the outer membrane antigen may be incorporated therein. The immunogenic compositions have utility as chlamydial vaccines and in diagnostic applications.
Owner:AVENTIS PASTEUR LTD
Immunization Against Chlamydia Infection
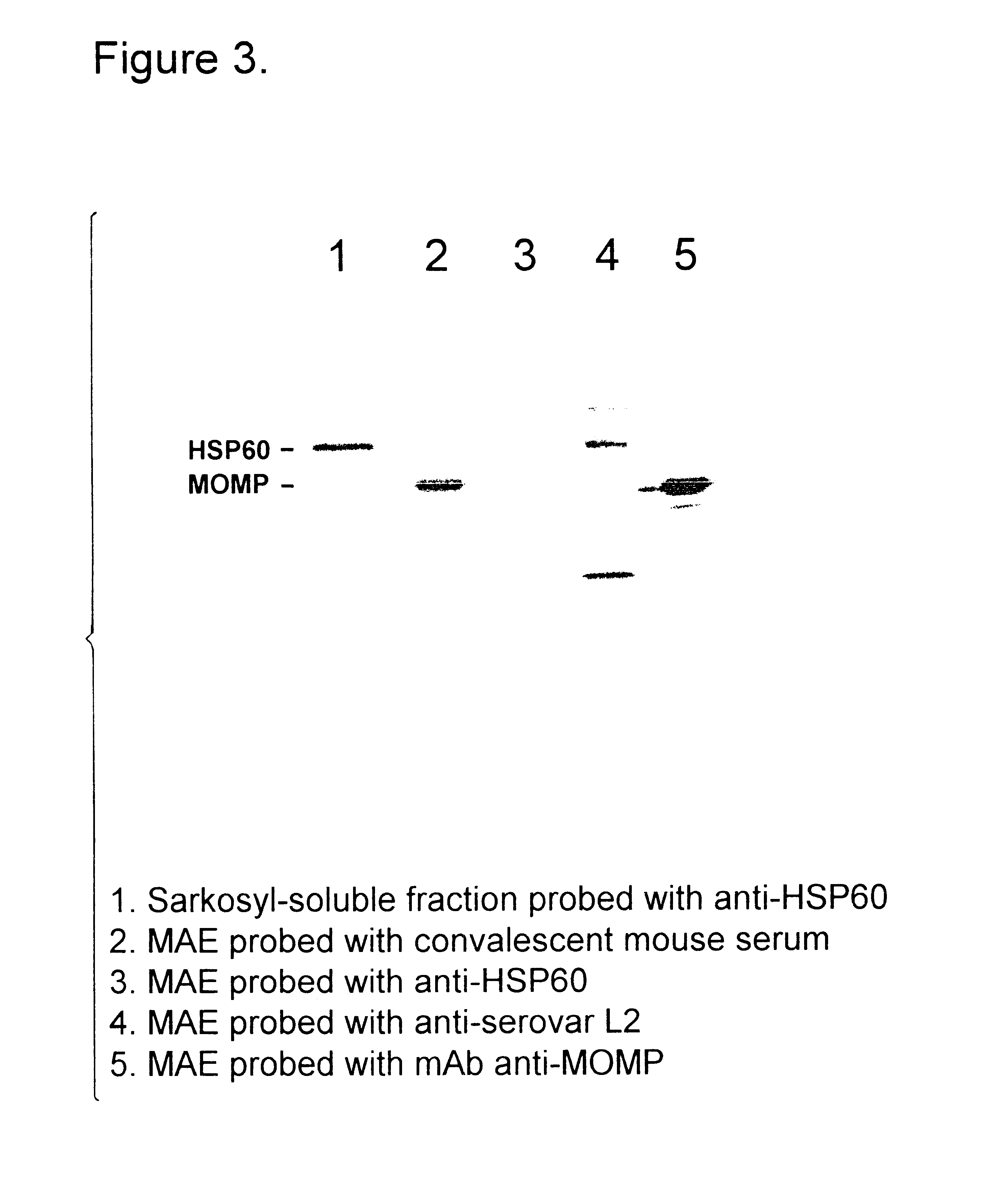
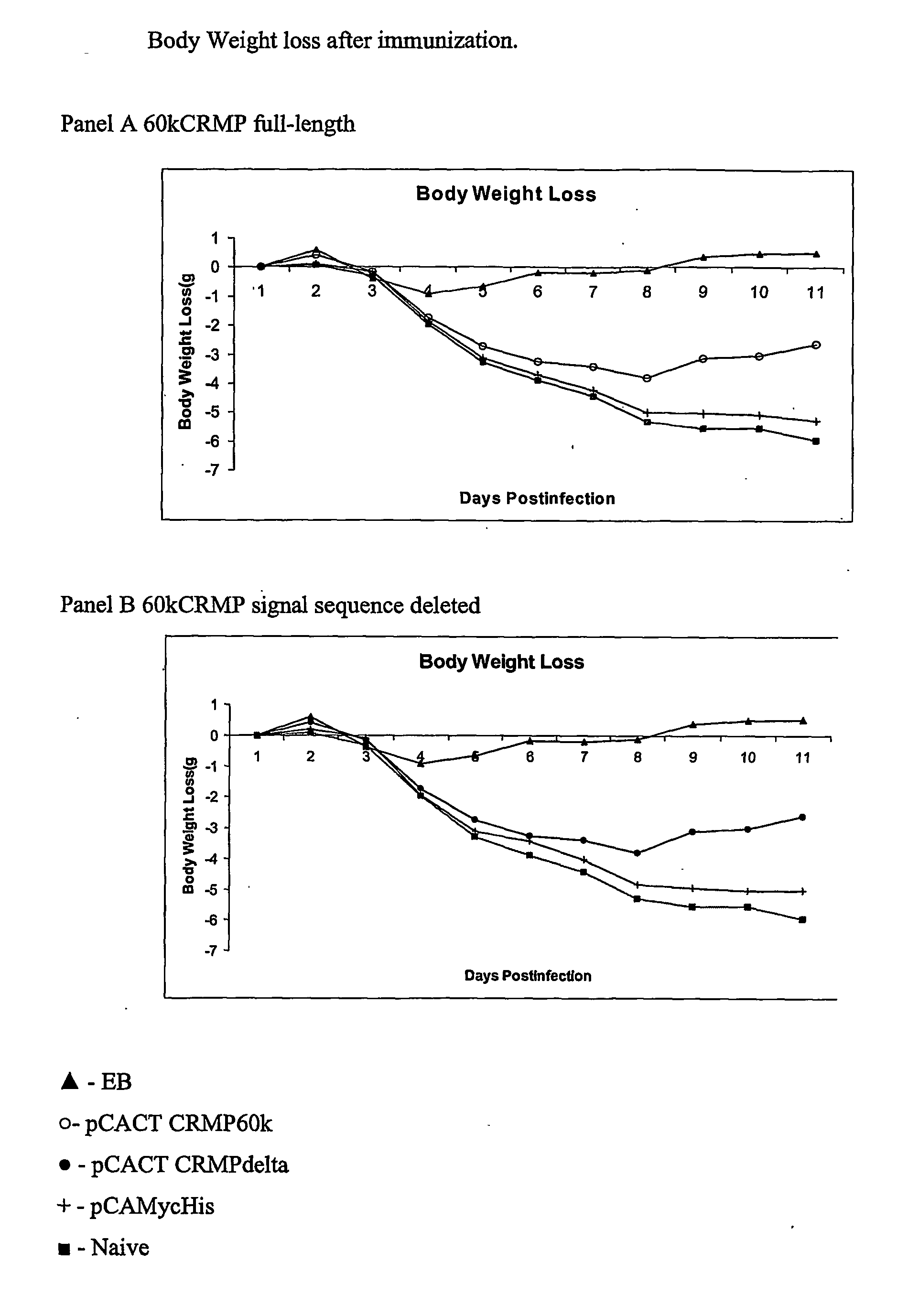
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. trachomatis. The method employs a vector containing a nucleotide sequence encoding a polypeptide of a strain of Chlamydia operably linked to a promoter to effect expression of the gene product in the host. The polypeptides are derived from the Chalmydia gene 60kCRMP gene including truncated forms of the gene. The invention further provides recombinant 60kCRMP protein useful for protecting against disease caused by infection with Chlamydia.
Owner:BRUNHAM ROBERT +3
Vaccines against chlamydial infection
InactiveUS20110300206A1Stimulate immune responseImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsNucleotideADAMTS Proteins
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Chlamydial vaccines and immunogenic compositions containing an outer membrane antigen and methods of preparation thereof
InactiveUS6635746B1Strong immune responseImproving immunogenicityPeptide/protein ingredientsPeptide preparation methodsDiseaseImmunostimulating Complexes
Immunogenic compositions including vaccines are described that comprise an outer membrane antigen extract of a strain of Chlamydia and are effective in protection against disease caused by Chlamydia infection The immunogenic compositions may comprise the major outer membrane protein (MOMP) of Chlamydia which may be in a homooligomeric form or complexed with at least one other antigen of Chlamydia. The immunogenic composition may include an immunostimulating complex (ISCOM) and the outer membrane antigen may be incorporated therein. The immunogenic compositions have utility as chlamydial vaccines and in diagnostic applications.
Owner:AVENTIS PASTEUR LTD
Photosensitizers and MRI enhancers
InactiveCN101237883AEnergy modified materialsIn-vivo testing preparationsDiabetic retinopathyProstate cancer
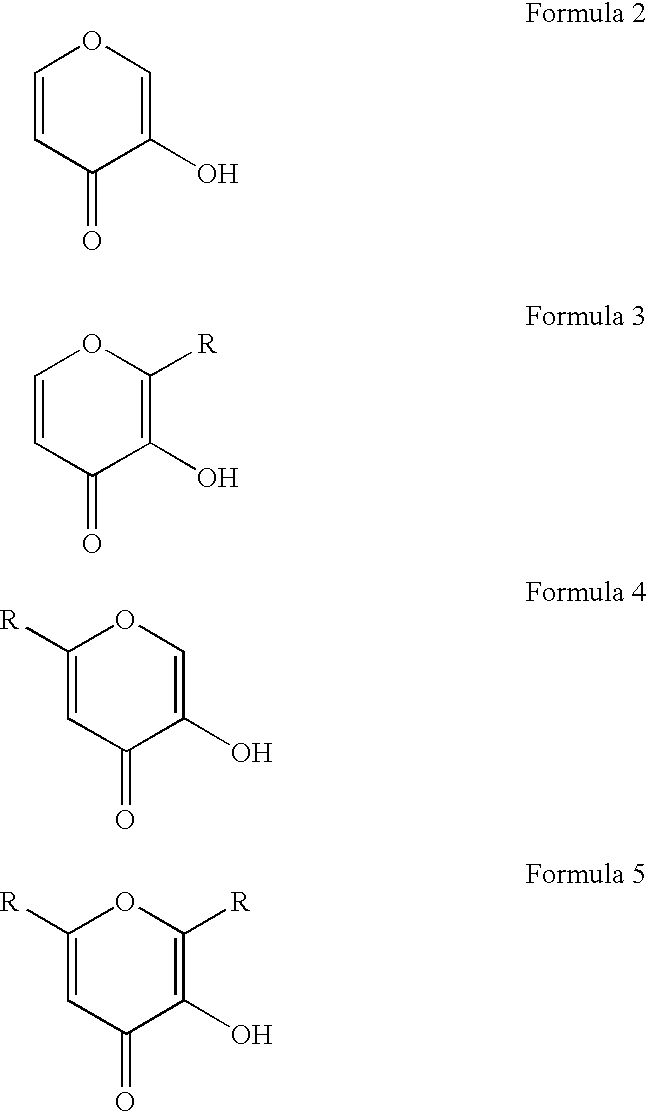
The present invention relates to the use of a compound of formula 3 or a salt thereof to prepare a medicament or phototherapeutic agent for treating the following diseases, including: acne; AIDS; viral hepatitis; diabetic retinopathy; SARS virus infection; coronavirus Arterial stenosis; carotid artery stenosis; intermittent claudication; Asian (chicken) avian influenza virus infection; cervical dysplasia or various cancers, including: blood cancer, cervical cancer, nasopharyngeal cancer, tracheal cancer, laryngeal cancer, bronchial cancer , bronchiolar cancer, bladder cancer, esophageal cancer, stomach cancer, rectal cancer, colon cancer, prostate cancer, hollow organ cancer, bile duct cancer, urinary tract cancer, kidney cancer, uterine cancer, vaginal cancer and other gynecological adnexal cancer. The present invention also relates to methods of treating the above diseases. The present invention further relates to the use of the compound of formula 3 or a salt thereof to prepare a photodiagnostic agent for detecting the above-mentioned diseases and the following diseases, including: atherosclerosis, multiple sclerosis, diabetes, arthritis, rheumatism Arthritis, fungal infections, viral infections, chlamydial infections, bacterial infections, or parasitic diseases, HIV viral infections, hepatitis, herpes simplex, shingles, psoriasis, cardiovascular disease, and skin diseases. The present invention also relates to methods for detecting the above diseases using photodiagnostic agents. The present invention further relates to a method for low-temperature sterilization of surgical devices or other devices, including the steps of: providing a compound of Chemical Formula 3 or a salt thereof on the device; and subjecting the device to radiation treatment or sonication treatment. The invention further relates to a compound of formula 3 or a salt thereof linked or attached to a magnetic element. This compound acts as an MRI enhancer. The invention also relates to the use of such MRI enhancers for performing MRI scans.
Owner:PHOTO DIAGNOSTIC DEVICES PDD
Chlamydia vaccines
Vaccine preparations are provided for the prevention of Chlamydia infections comprising a major outer membrane protein from chlamydia and a mucosal adjuvant such as a combination of QS21 and 3D-MPL, or chlorea Toxin or Heat labile enterotoxin. Such preparations provide protection from Chlamydia induced fertility.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
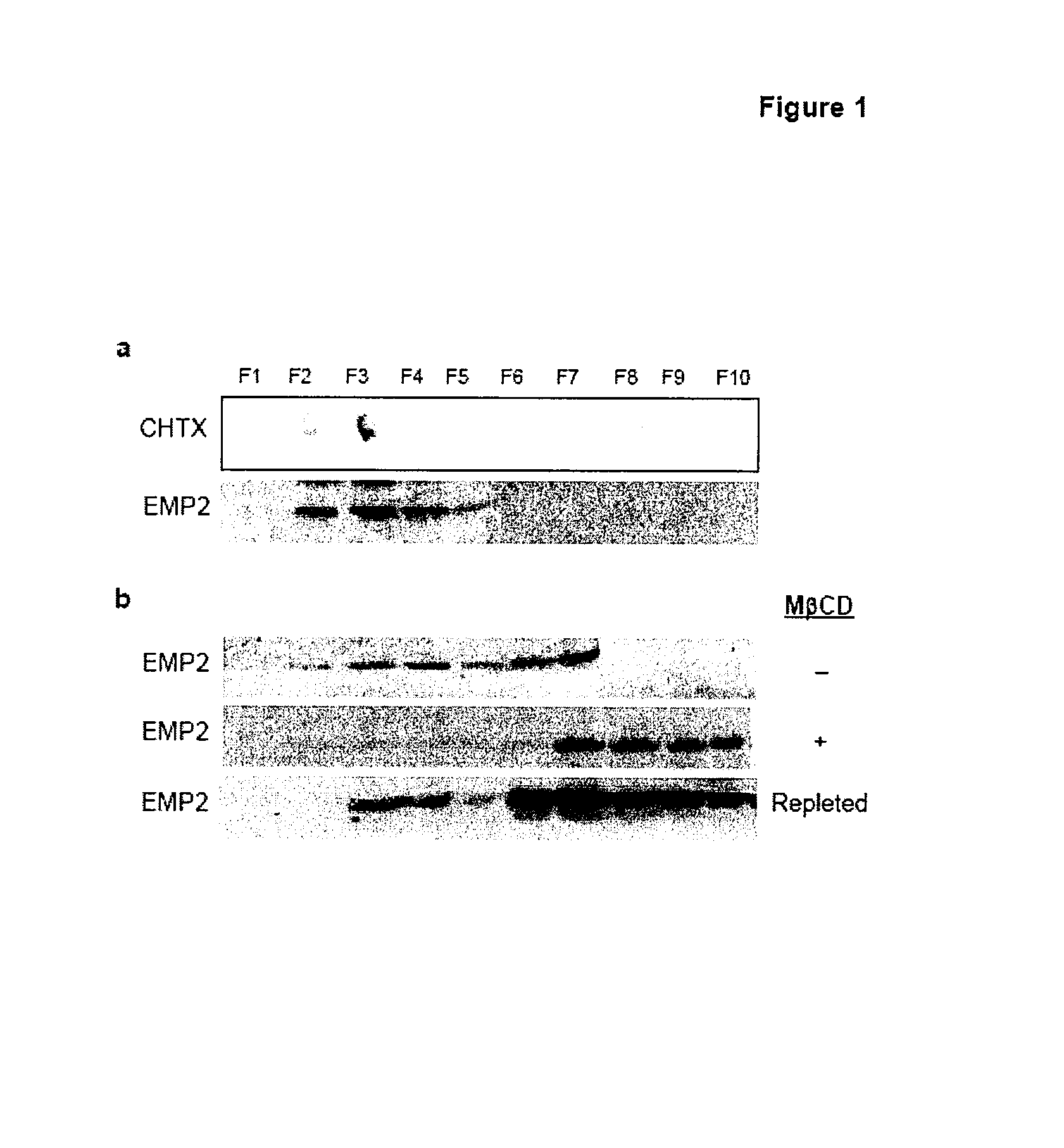
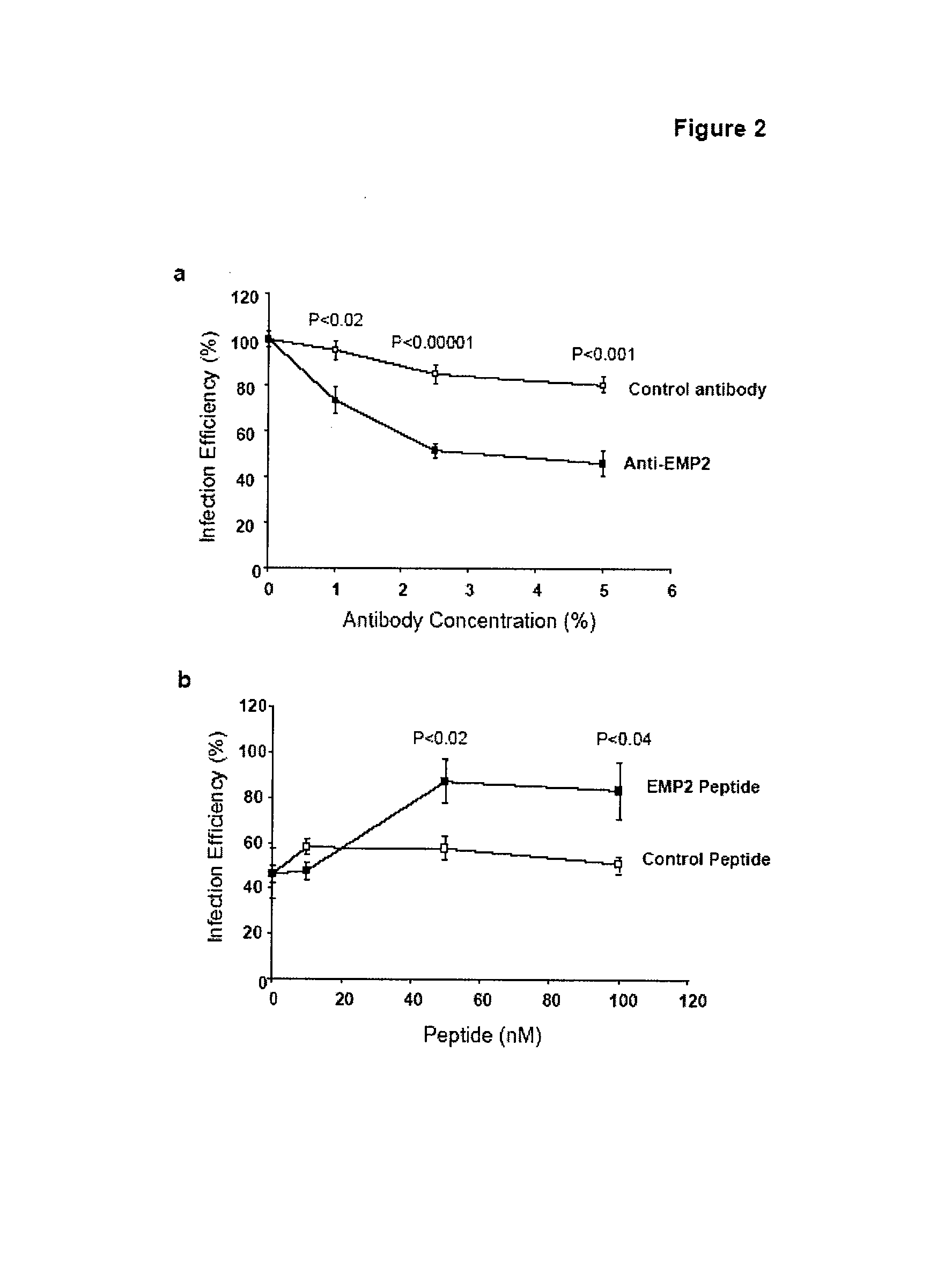
Prevention of chlamydia infection using a protective antibody
InactiveUS20080181889A1InhibitionBiocideOrganic active ingredientsChlamydia virusCHLAMYDIAL INFECTIONS
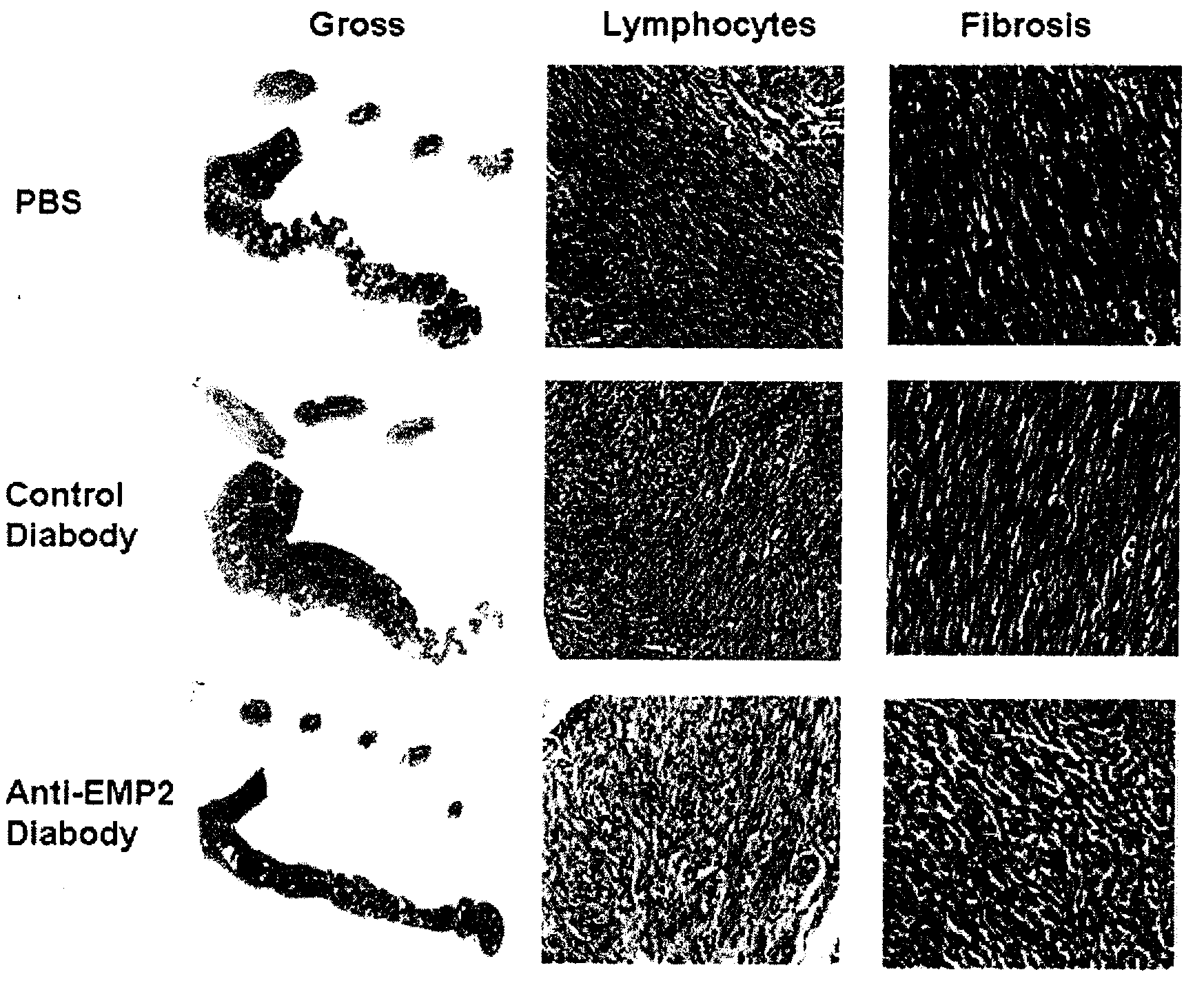
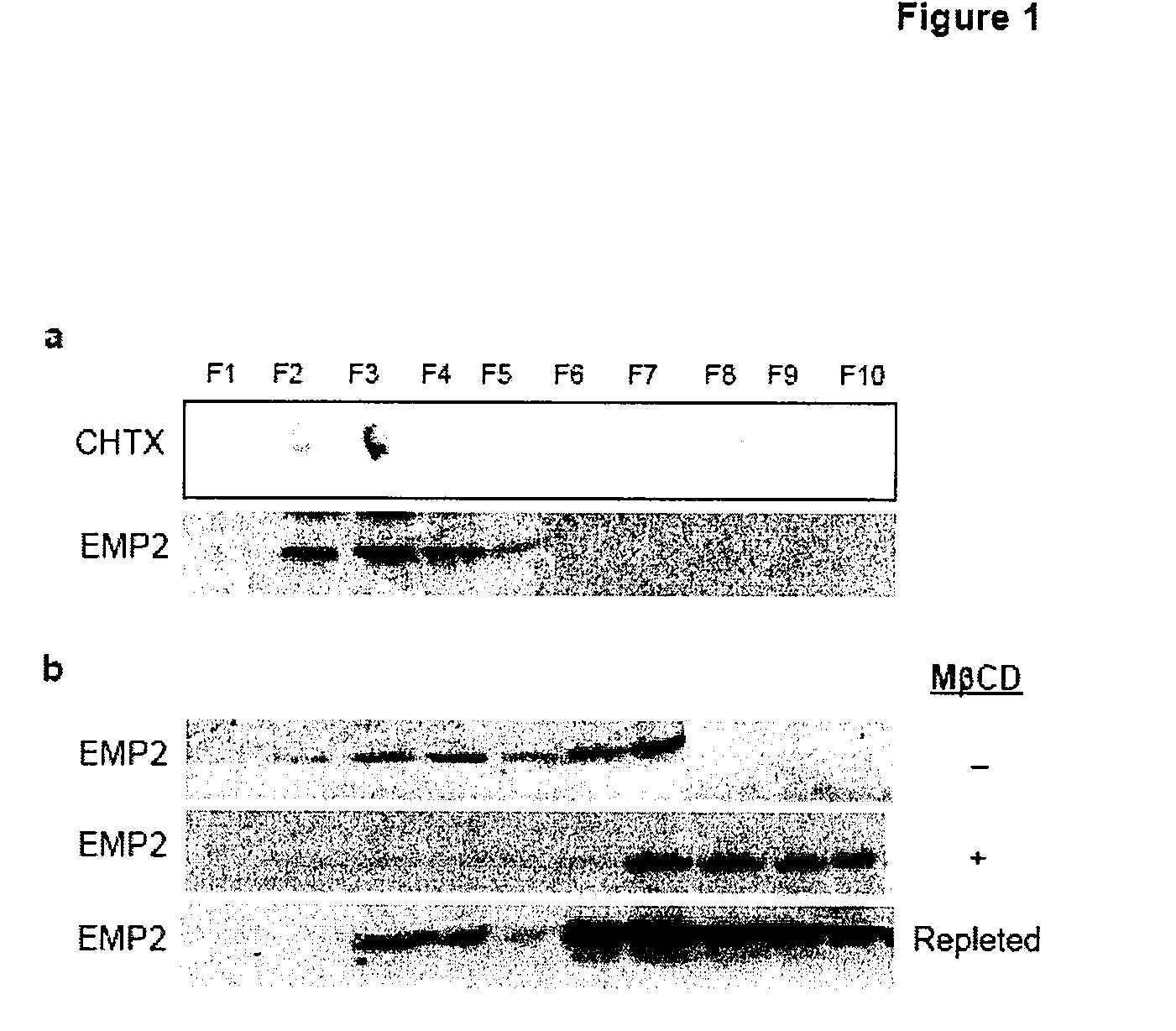
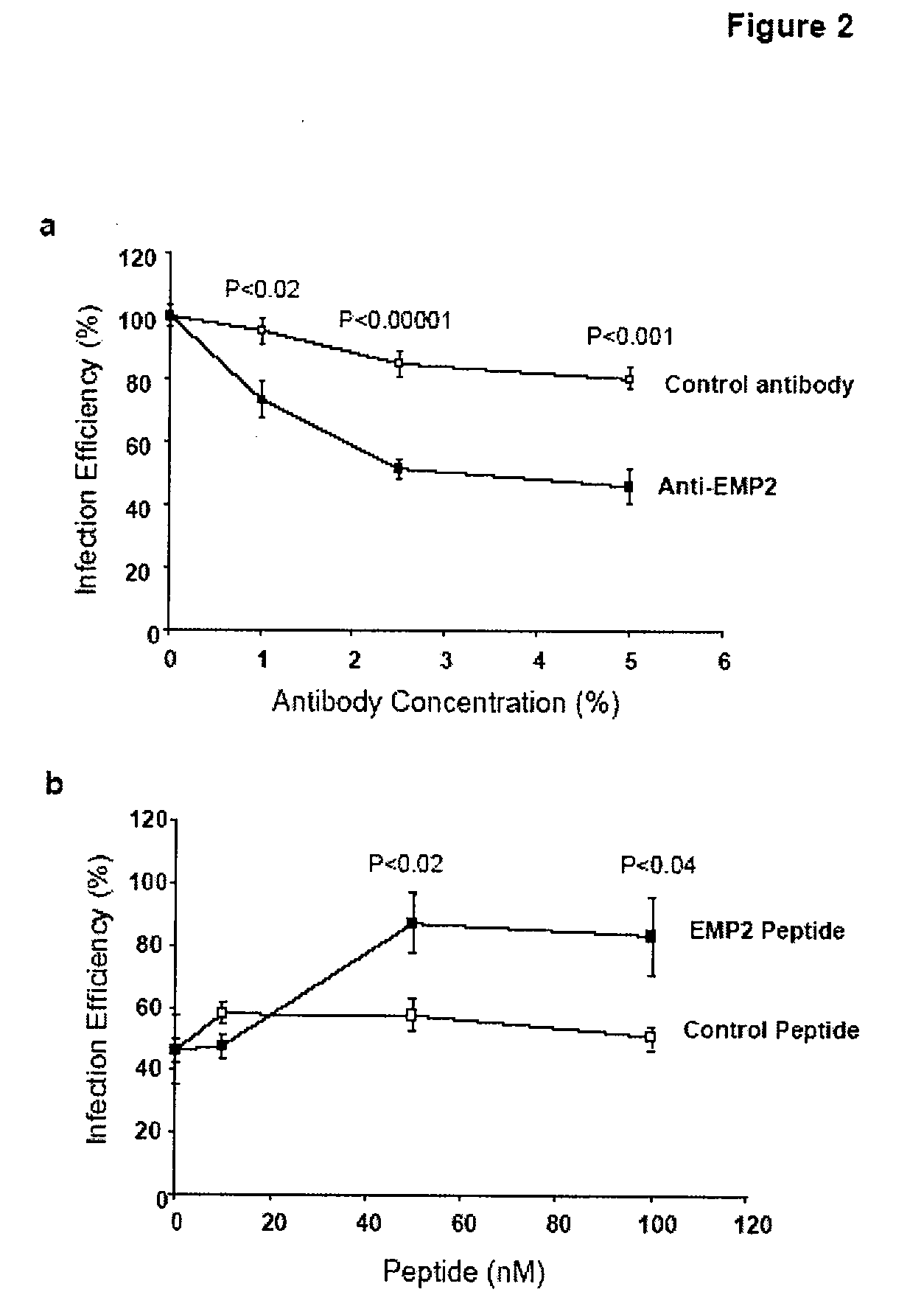
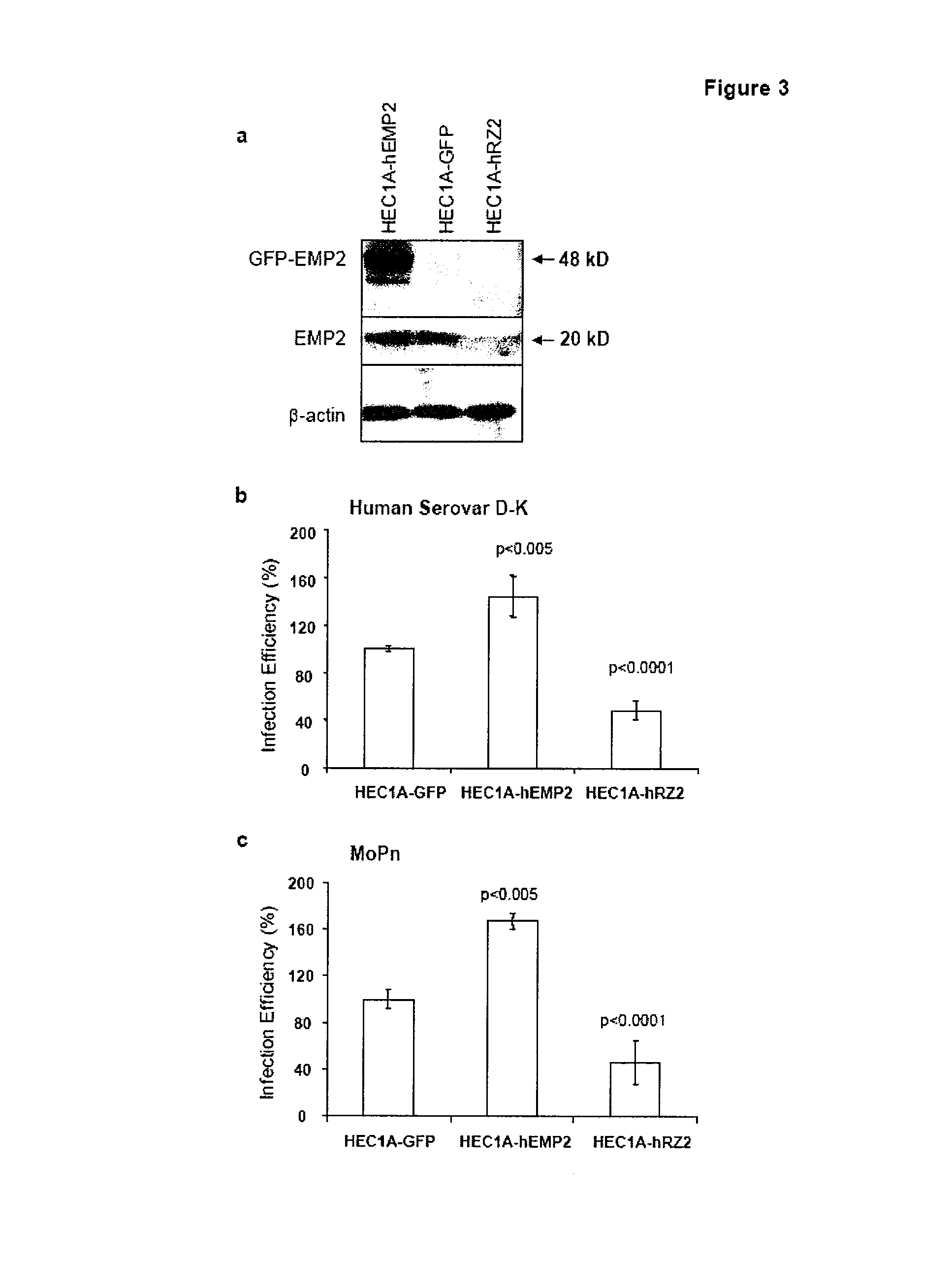
The present invention provides methods and compositions useful in the treatment or prevention of Chlamydia infections. The methods and compositions inhibit the entry of Chlamydia into a host cell expressing EMP2 by interfering with the interaction between the Chlamydia and EMP2. The compositions include EMP2 nucleic acids and polypeptides as well as anti-EMP2 antibodies.
Owner:RGT UNIV OF CALIFORNIA
Method of intracellular infectious agent detection in sperm cells
InactiveCN104303059AHigh sensitivityMicrobiological testing/measurementDisease diagnosisMicroorganismImmunofluorescence
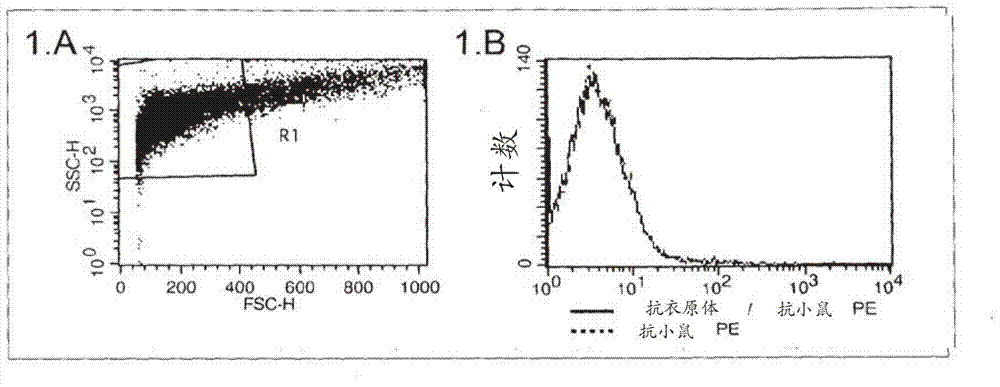
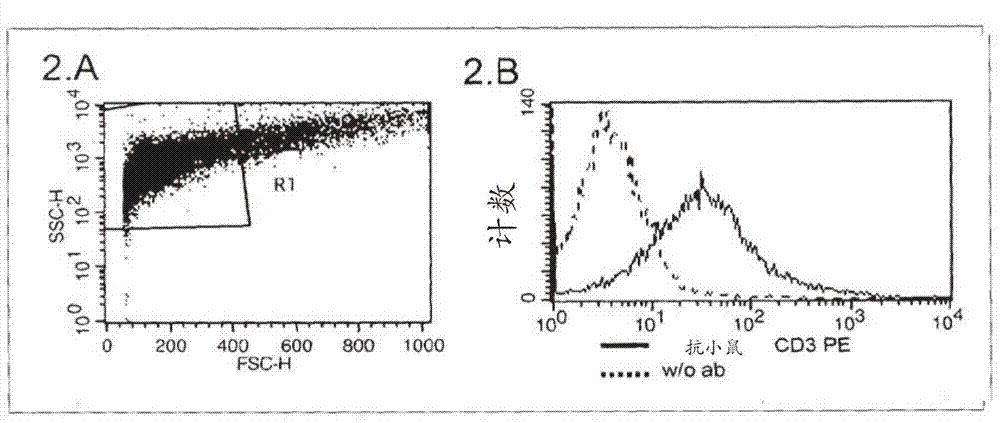
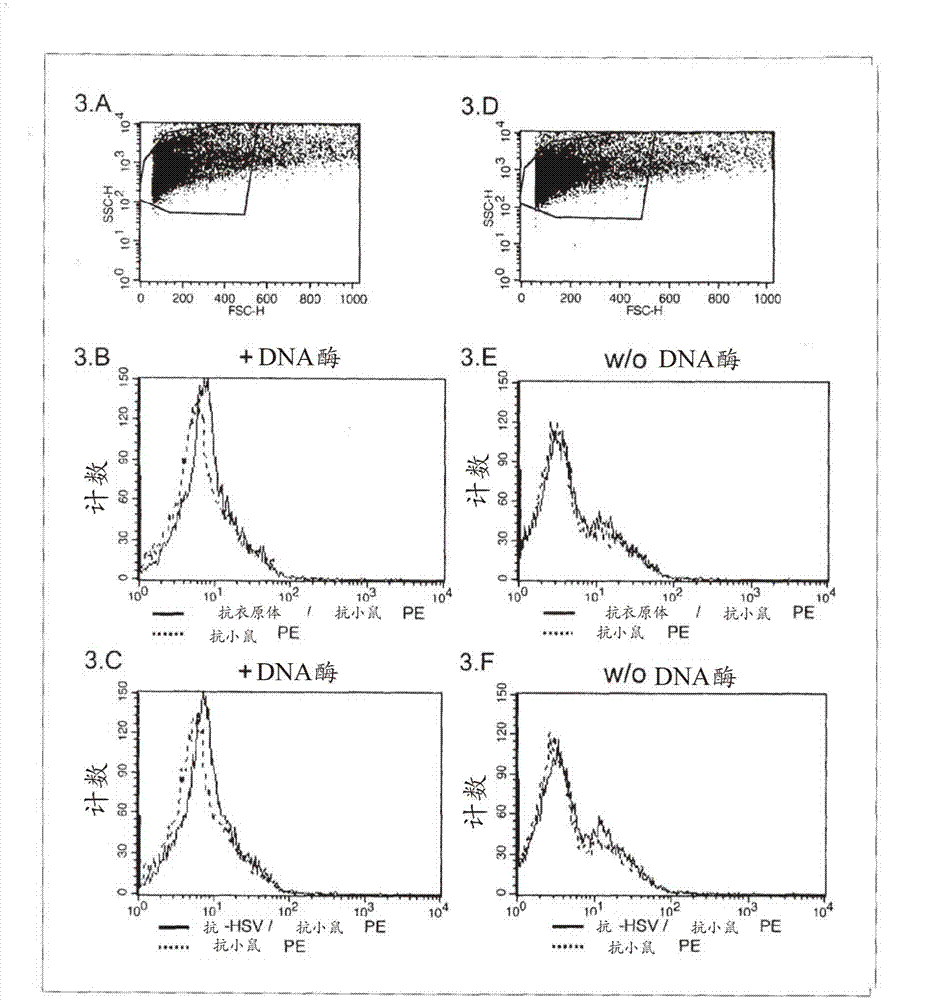
The present invention describes a method for investigating the presence of chlamydia, viruses and other infectious agents in spermatozoa, with the use of immunofluorescence in combination with flow cytometry. This method is used for the intracellular detection of microorgaqnisms within sperm cells through the use of a DNA easing procedure, as well as for the detection of microorganisms adherent to the surface of spermatozoa.
Owner:瓦西利奥斯·齐列瓦可斯 +1
Gallium complexes of 3-hydroxy-4-pyrones to treat infection by intracellular prokaryotes and DNA viruses
InactiveUS20060222628A1Guaranteed accuracyAntibacterial agentsHeavy metal active ingredientsHerpesvirus infectionHerpesvirus papio HVP
Methods are provided for treating or preventing infections by obligate intracellular prokaryotes, including mycoplasma, rickettsia and chlamydia, and DNA viruses, including herpes viruses, papillomaviruses, adenoviruses and hepatitis B virus. The methods involve the administration of 3:1 complexes of 3-hydroxy-4-pyrones with gallium, e.g., gallium maltolate. Therapies incorporating gallium maltolate in combination with agents used against obligate intracellular prokaryote and DNA virus pathogens are also provided, as are multi-combination therapies designed to treat co-infection by an obligate intracellular prokaryote or DNA virus in an immunocompromised individual. These multi-combination therapies rely on the ability of gallium maltolate to complement antiviral medication regimes against both HIV and other pathogens such as herpesvirus infections, including Kaposi sarcoma, CMV retinitis and blindness, and lymphomas, in patients immunocompromised by HIV infection.
Owner:BERNSTEIN LAWRENCE R
DNA immunization against chlamydia infection
InactiveUS6632663B1Low backgroundImproving immunogenicityTransferasesDepsipeptidesNucleotidePlasmid Vector
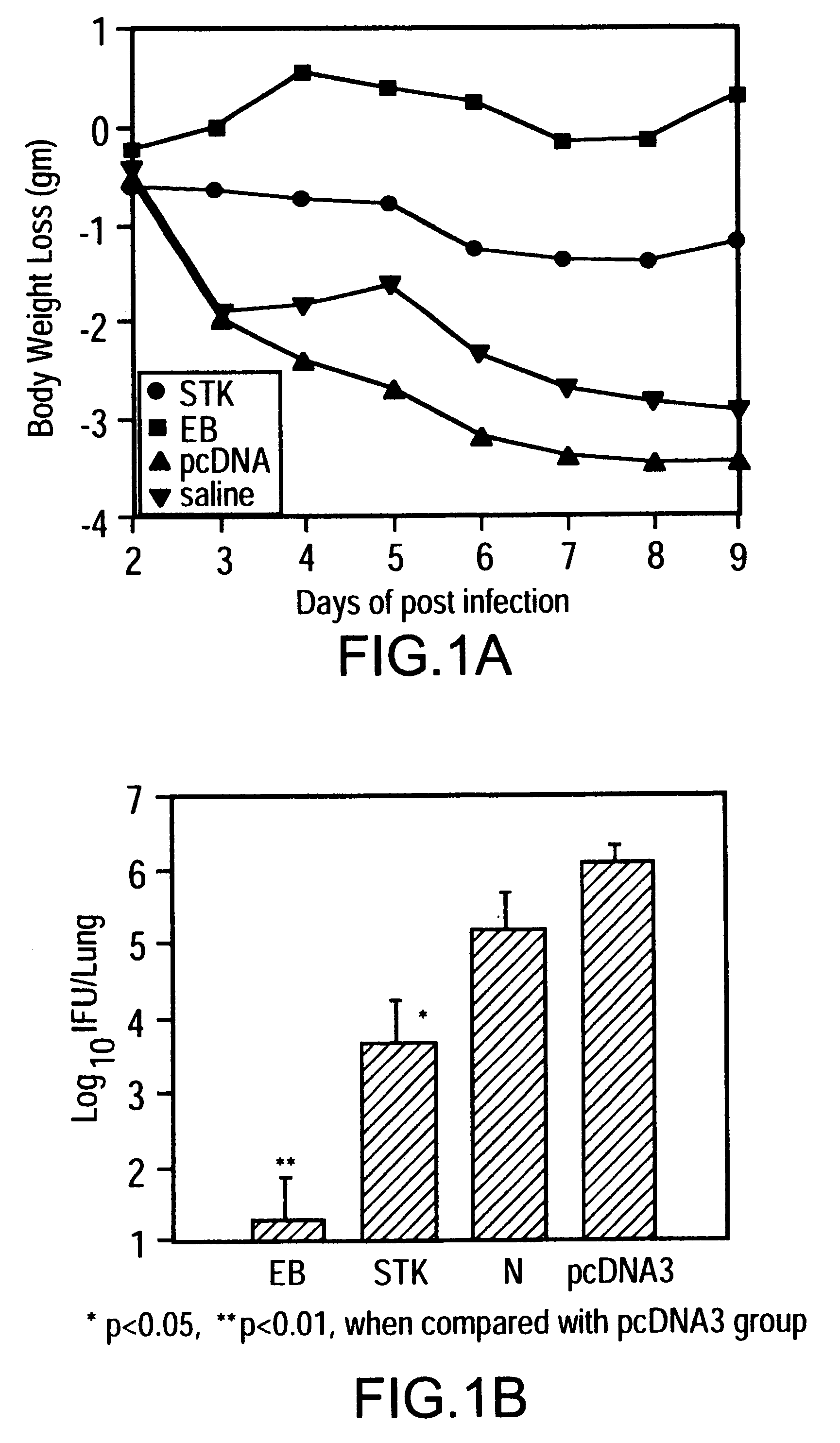
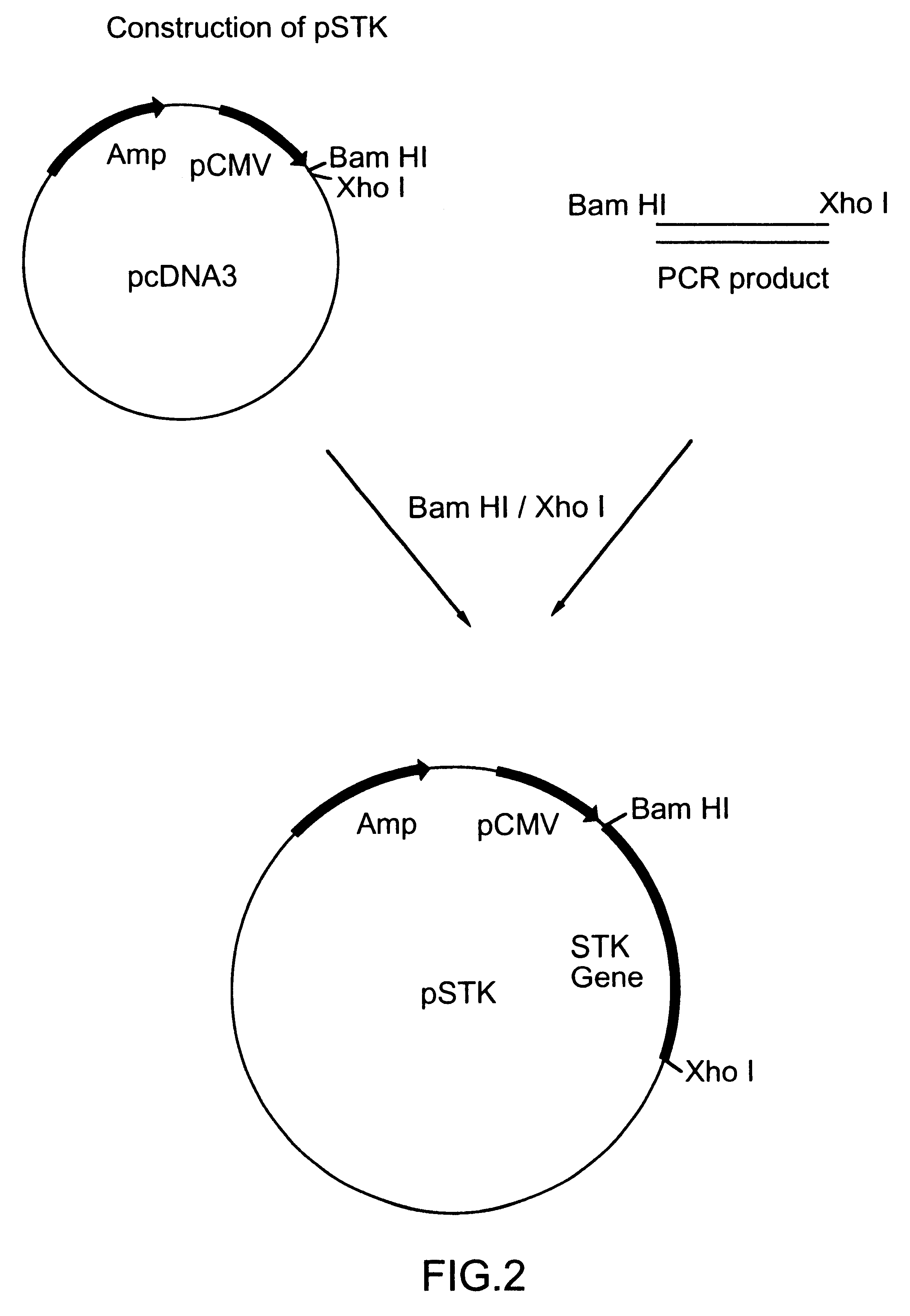
Nucleic acid, including DNA, immunization is used to generate a protective immune response in a host, including humans, to a serine-threonine kinase (STK) of a strain of Chlamydia. A non-replicating vector, including a plasmid vector, contains a nucleotide sequence encoding a STK or a fragment of the STK that generates antibodies that specifically react with STK and a promoter sequence operatively coupled co the first nucleotide sequence for expression of the STK in the host. The non-replicating vector may be formulated with a pharmaceutically-acceptable carrier for in vivo administration to the host.< / PTEXT>
Owner:UNIVERSITY OF MANITOBA
Antiseptic Chinese medicine composition and its prepn process
The antiseptic Chinese medicine composition is orally taken preparation comprising the active components extracted from skullcap root, phellodendron bark, flavescent sophora root, honeysuckle, pulsatillae root and oldenlandia and medicinal supplementary material. The Chinese medicine composition is developed based on Chinese medicine theory and prepared through modern extraction and analysis process, and has controllable effective components, stable quality and determined curative effect. The Chinese medicine composition is especially suitable for in preparing medicine for treating diseases of reproductive system and urinary system caused by mycoplasma and chlamydia infection.
Owner:上海海天医药科技开发有限公司
Vaccines against chlamydial infection
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Externally appiled traditional Chinese medicine preparation for treating venereal diseases, mycoplasma infection and Chlamydia infection
InactiveCN101991753AGood treatment effectImprove toleranceHydroxy compound active ingredientsAntiinfectivesDiseaseTolerability
The invention discloses an externally applied traditional Chinese medicine preparation for treating venereal diseases, mycoplasma infection and Chlamydia infection, which comprises the following raw material medicaments in parts by weight: cnidium, lightyellow sophora root, atractylis, smilax glabra, coptis, tribulus terrestris, borneol and radix notoginseng. The preparation includes a powdery preparation and a suppository preparation which are directly applied to an affected part. The adopted medicaments work together to achieve the efficacy of killing insects and detoxicating, converging bleeding, removing fever and eliminating cold, changing rotten matter into muscles, and dispersing pathogenic wind and relieving itching, are directly used on the affected part, and have good therapeutic effects on mycoplasma infection, Chlamydia infection, gonorrhoea and various venereal diseases. Through clinical application, the external traditional Chinese medicine preparation has the advantages of quick action, obvious therapeutic effect, good durability, low cost and no any toxic or side effect.
Owner:吴昊
Feline vaccine compositions and method for preventing chlamydia infections or diseases using the same
InactiveUS20030170251A1Increase loadAvoid infectionChlamydiaceae ingredientsSnake antigen ingredientsAdjuvantActive component
This invention provides a feline vaccine composition comprising an immunogenically active component having inactivated mammalian chlamydial cells or antigens derived therefrom, in combination with an effective amount of an immunogencally suitable adjuvant; and a veterinary pharmaceutically acceptable carrier or diluent. The vaccine composition is useful to prevent chlamydia, e.g. C. psittaci, infections or diseases in felines, and may also be combined with other vaccine compositions or therapy. A process for producing C. psittaci suitable for use in the production of safe and effective chlamydia vaccines, and a method for preventing chlamydia infections or diseases in felines, are also provided.
Owner:AMERICAN HOME PRODUCTS CORPORATION
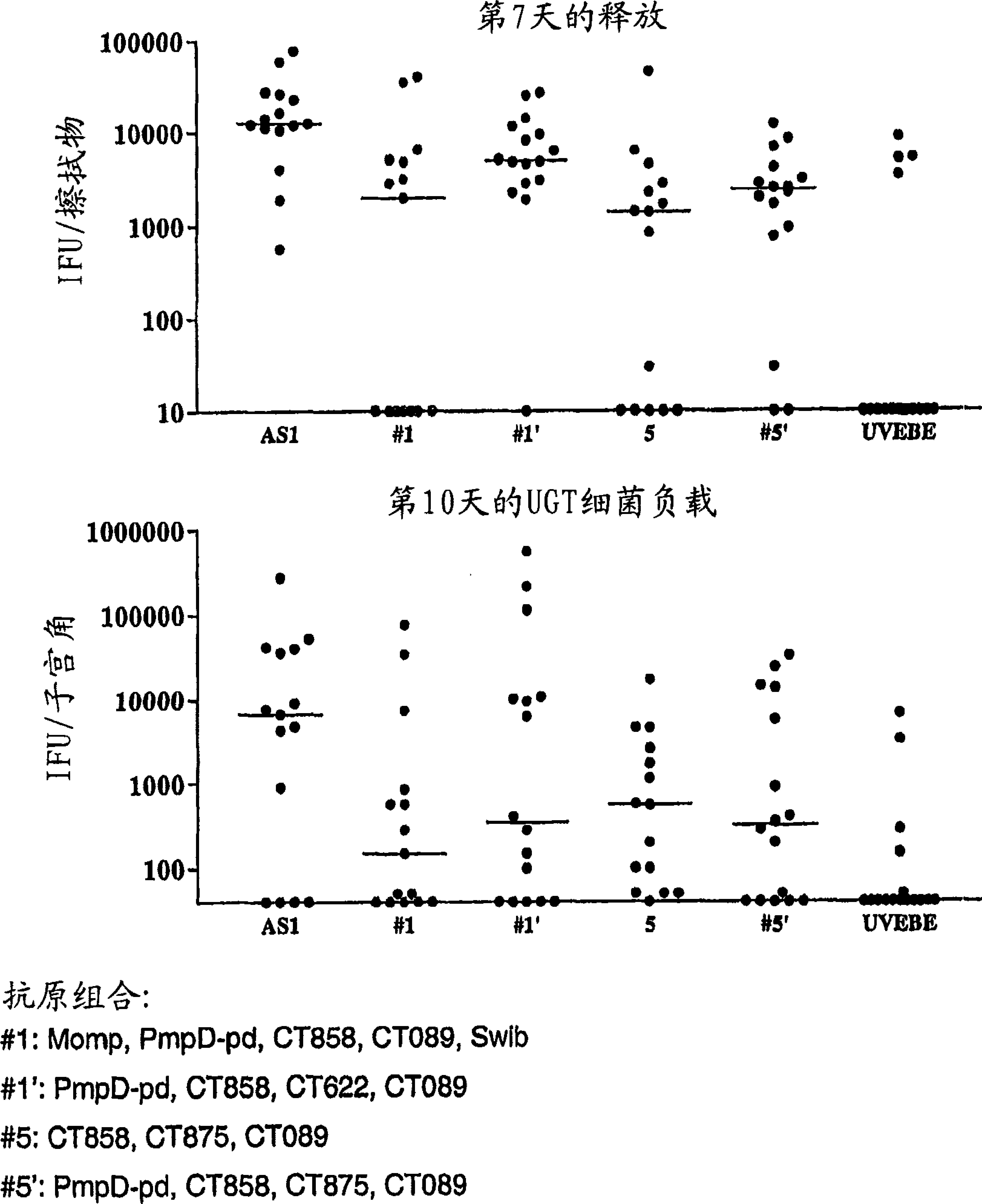
Chlamydia antigen compositions and uses thereof
InactiveCN104812406ANitro compound active ingredientsChlamydiaceae ingredientsAntigenChlamydia virus
Owner:THE UNIV OF BRITISH COLUMBIA
DNA immunization against chlamydia infection
InactiveUS20020110542A1Effectively induces protective immunityGood curative effectBiocideChlamydiaceae ingredientsNucleotideDna immunization
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The nonreplicating vector may be formulated with a pharmaceutically acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Two-step immunization procedure against chlamydia infection
InactiveUS20020168382A1Strong protective immune responseProvide protectionAntibacterial agentsSenses disorderTwo stepChlamydia virus
A host is immunized against infection by a strain of Chlamydia by initial administration of an attenuated bacteria harbouring a nucleic acid encoding a Chlamydia protein followed by administration of a Chlamydia protein in ISCOMs. This procedure enables a high level of protection to be achieved.
Owner:AVENTIS PASTUER LTD
Compounds and methods for treatment and diagnosis of chlamydial infection
The present invention discloses compounds and methods for the diagnosis and treatment of Chlamydia infection. Compounds provided by the invention include polypeptides comprising at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding these polypeptides. The present invention also provides pharmaceutical compositions and vaccines comprising these polypeptides or DNA sequences, as well as antibodies against these polypeptides. Diagnostic kits comprising these polypeptide or DNA sequences and suitable detection agents can be used to detect Chlamydia infection in patients and biological samples.
Owner:CORIXA CORP
Immunochromatographic assay test paper for diagnosing psittacosis chlamydia infection and preparation method thereof
InactiveCN1866015BEasy to operateStrong specificityMaterial analysis by observing effect on chemical indicatorBiological testingStaphylococcus aureusChlamydia psittaci infections
The disclosed immune chromatography test paper to detect chlamydia psittaci comprises: in series, a sample pad, a gold marked pad contained colloid gold probe marked by staphylococcus aureus protein A or IgG, a fiber film with separated detection line made of main out film protein of chlamydia psittaci and quality-control line as antibody fit to combine with former protein A or IgG, and the absorbent pad. Compared with IHA method, this invention has strong specificity and well sensitivity, and more fit to clinic.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Immunization Against Chlamydia Infection
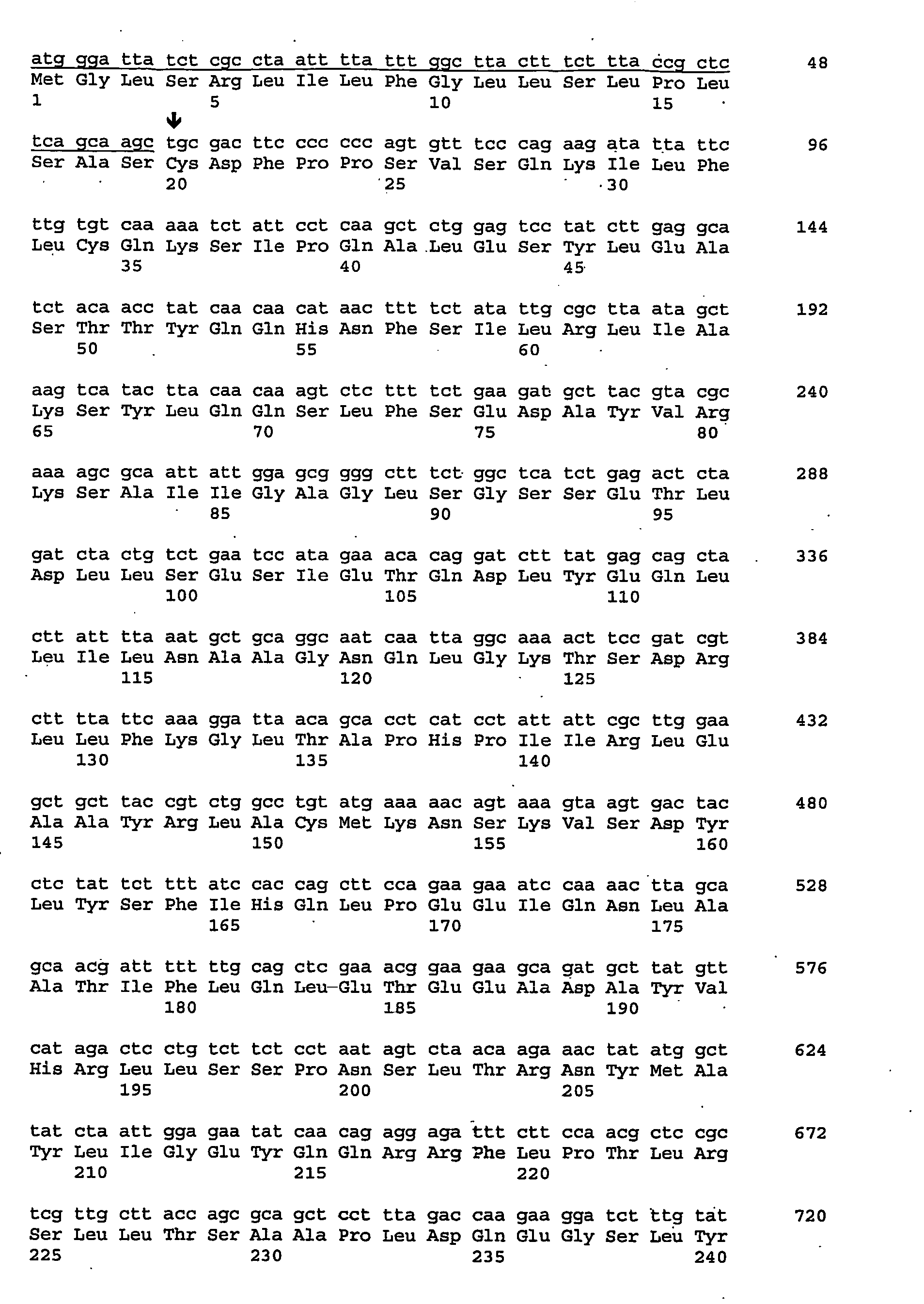
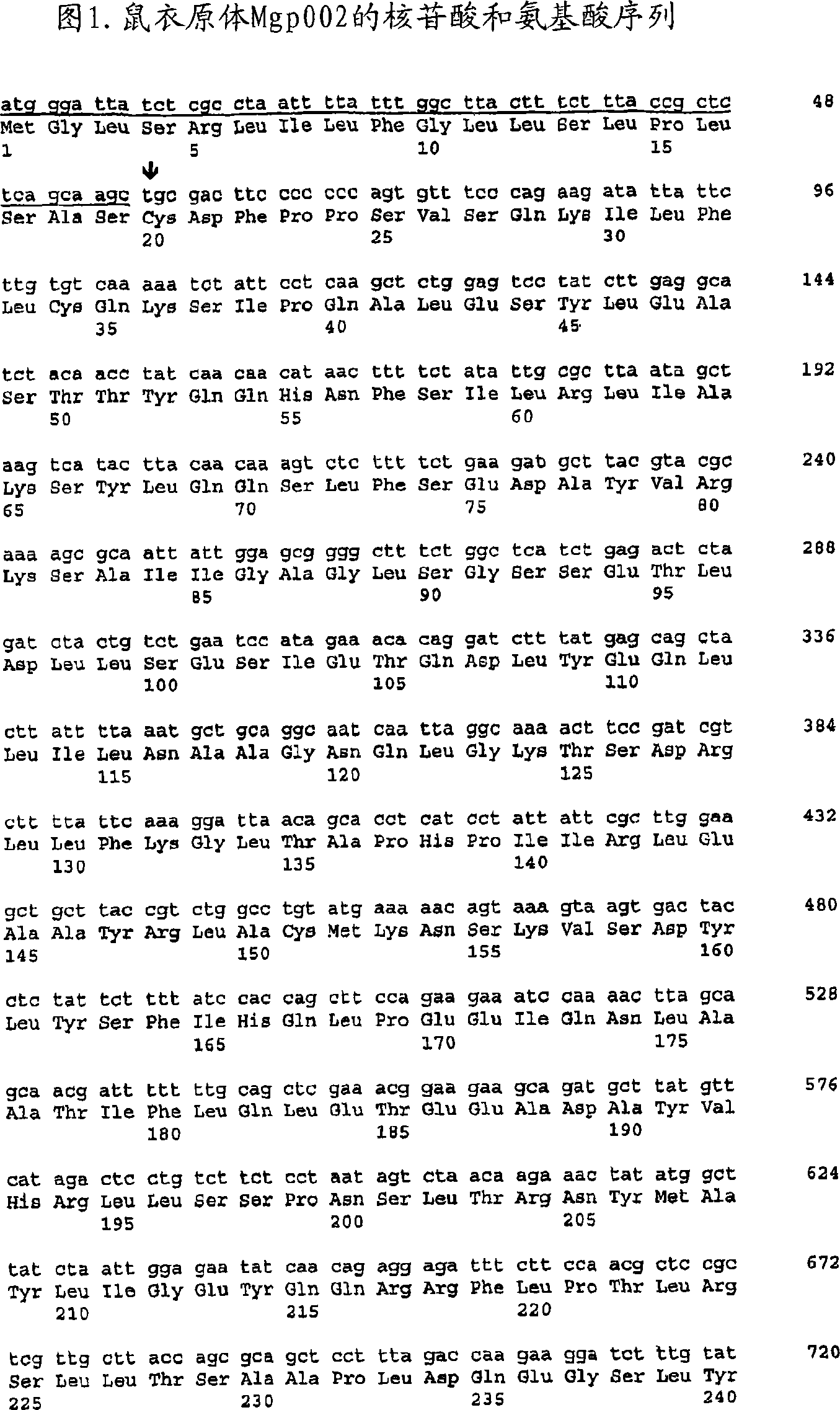
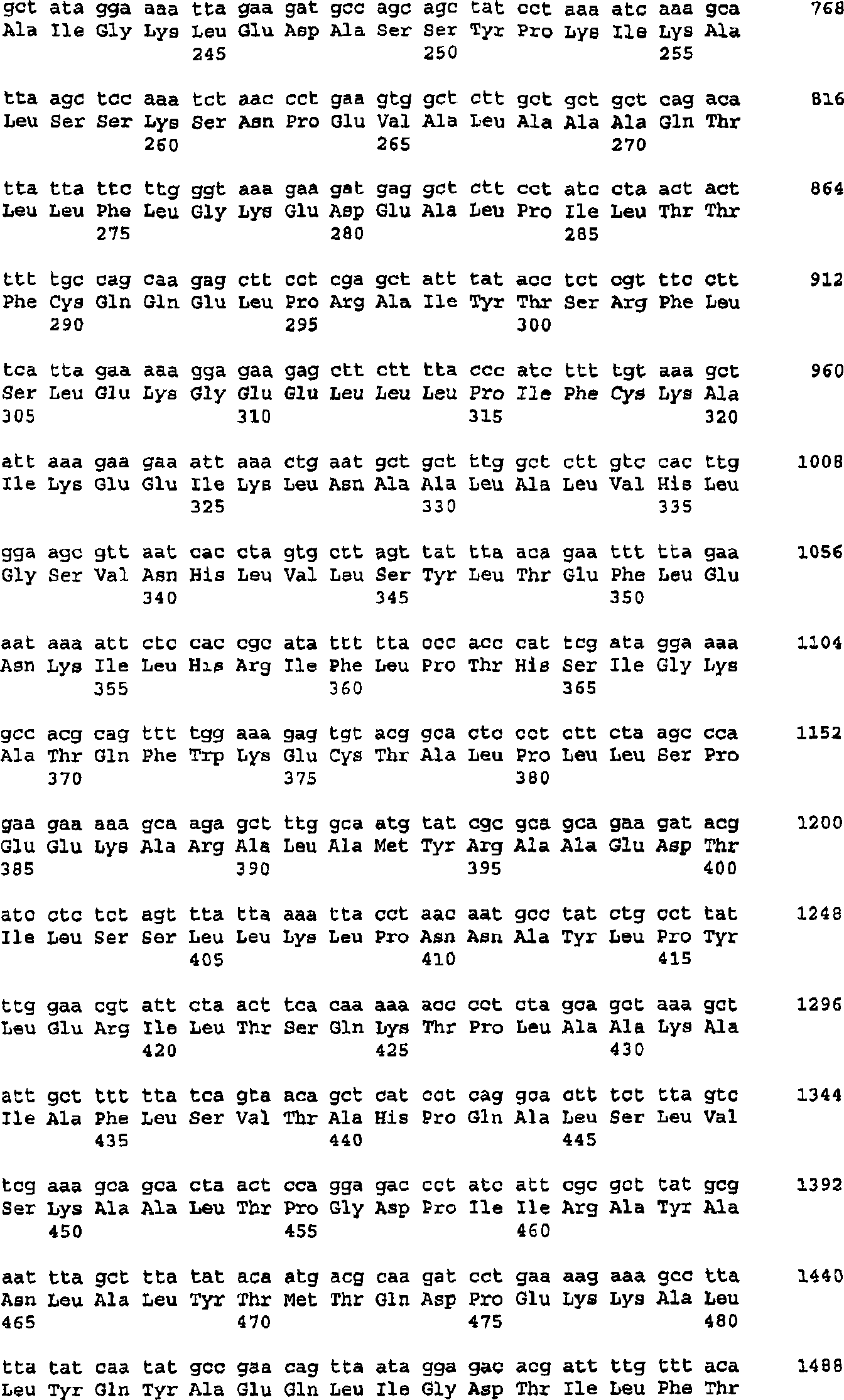
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. trachomatis. The method employs a vector containing a nucleotide sequence encoding a Mgp002 polypeptide of a strain of Chlamydia operably linked to a promoter to effect expression of the gene product in the host. Truncated forms of the full-length Mgp002 gene are useful immunogens for protecting against disease caused by infection with Chlamydia. The invention further provides recombinant Mgp002 protein useful for protecting against disease caused by infection with Chlamydia.
Owner:BE INTPROP
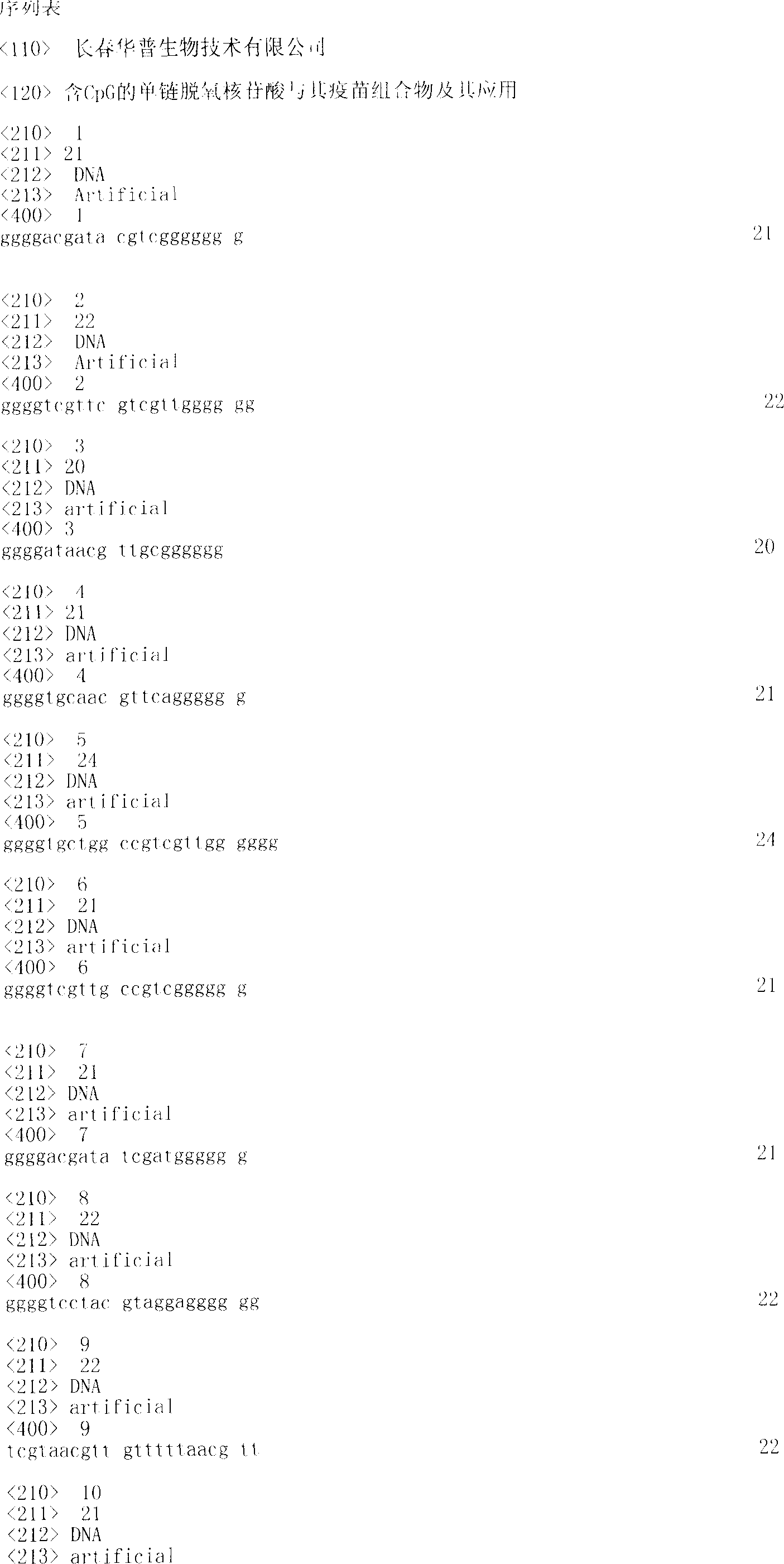
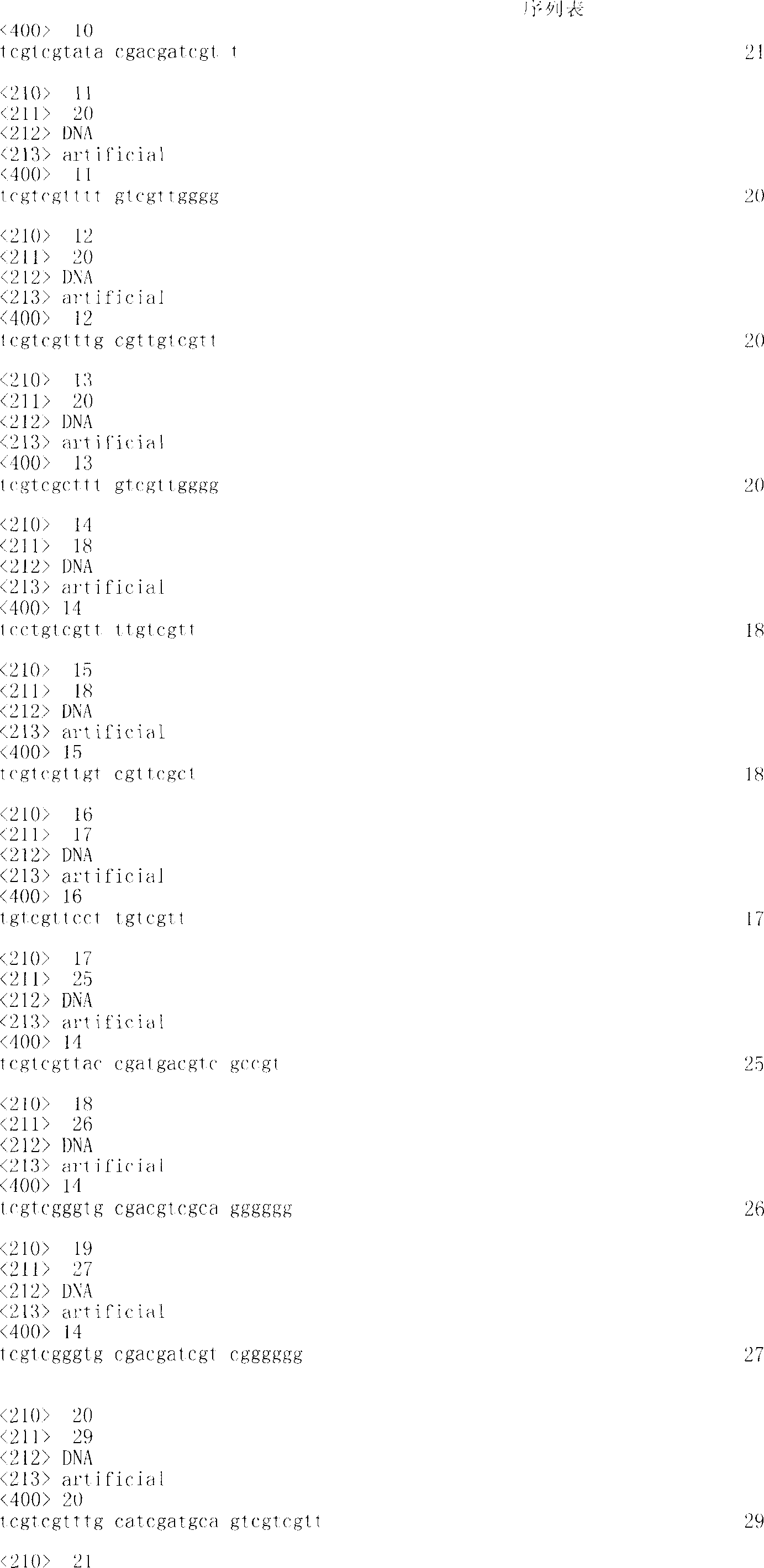
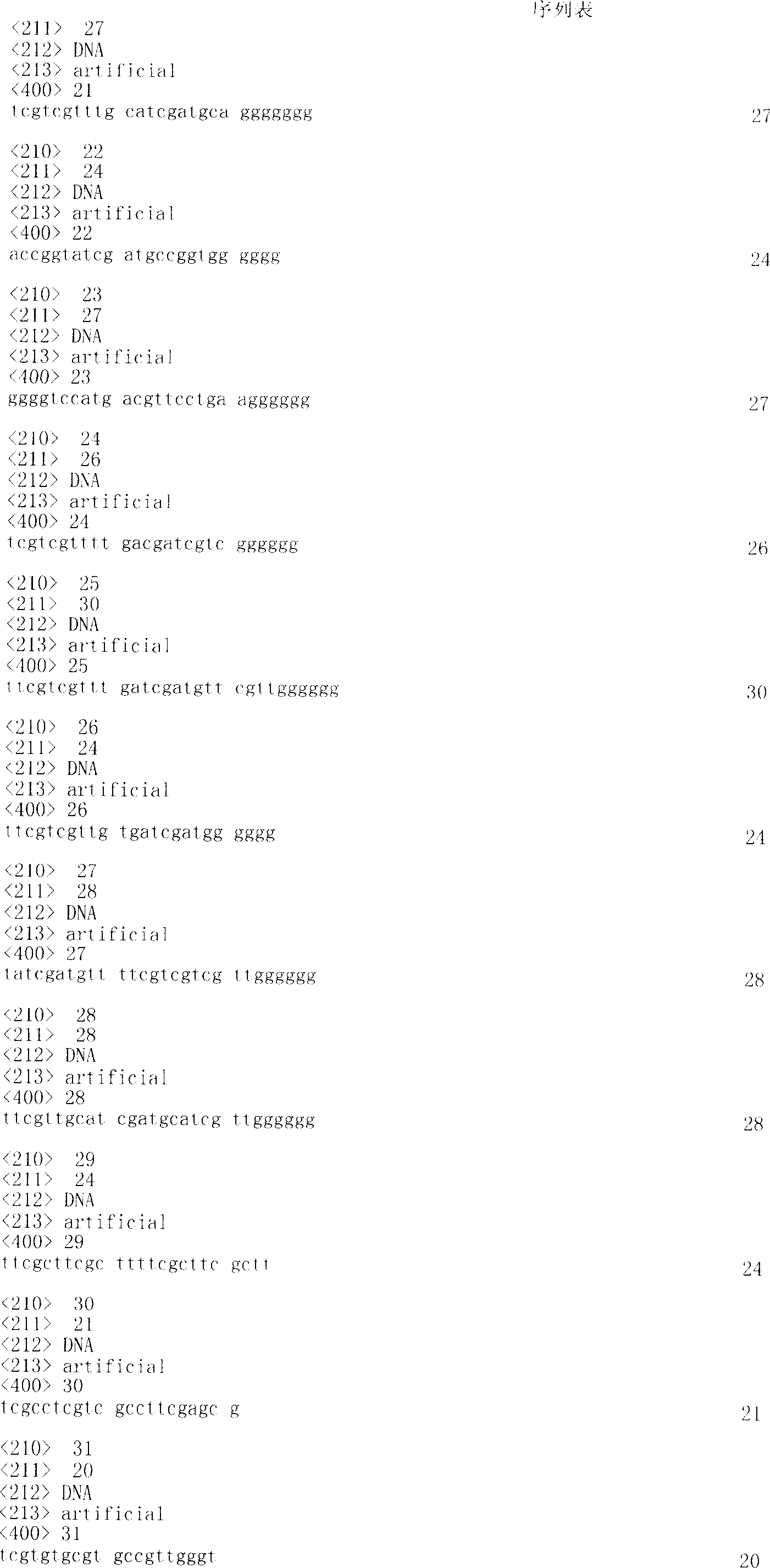
CpG-containing single-stranded deoxynucleotide, its vaccine composition and their application
The present invention relates to one kind of CpG-containing single-stranded deoxynucleotide capable of strengthening the immunostimulation of antigen or antigen composite, the vaccine composition of the single-stranded deoxynucleotide and antigen or antigen composite and its preparation process. The CpG-containing single-stranded deoxynucleotide can strengthen the specific antitumor, antiviral and chlamydia infection resisting activity of heat shock protein-antigen peptide fusion protein induced antigen peptide. In addition, the present invention also relates to the application of the CpG-containing single-stranded deoxynucleotide in preparing vaccine composition and the method of strengthening the immunostimulation. The vaccine composition may be used as treating medicine for viral infection, bacterial infectio, parasite infection, allergic reaction, cancer and relevant diseases.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
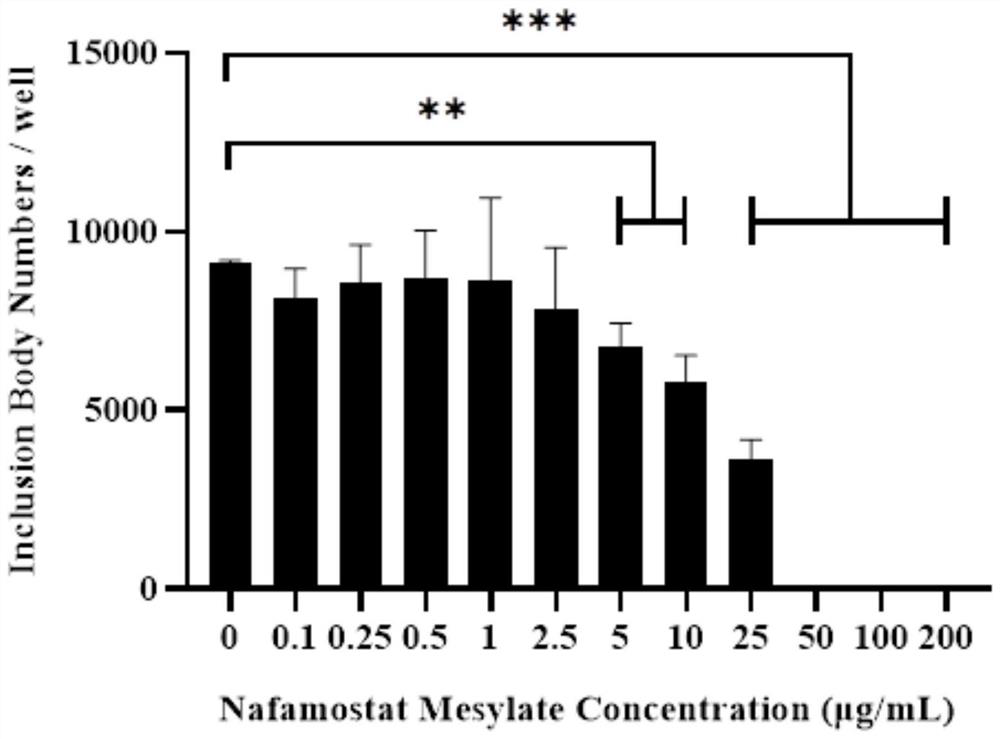
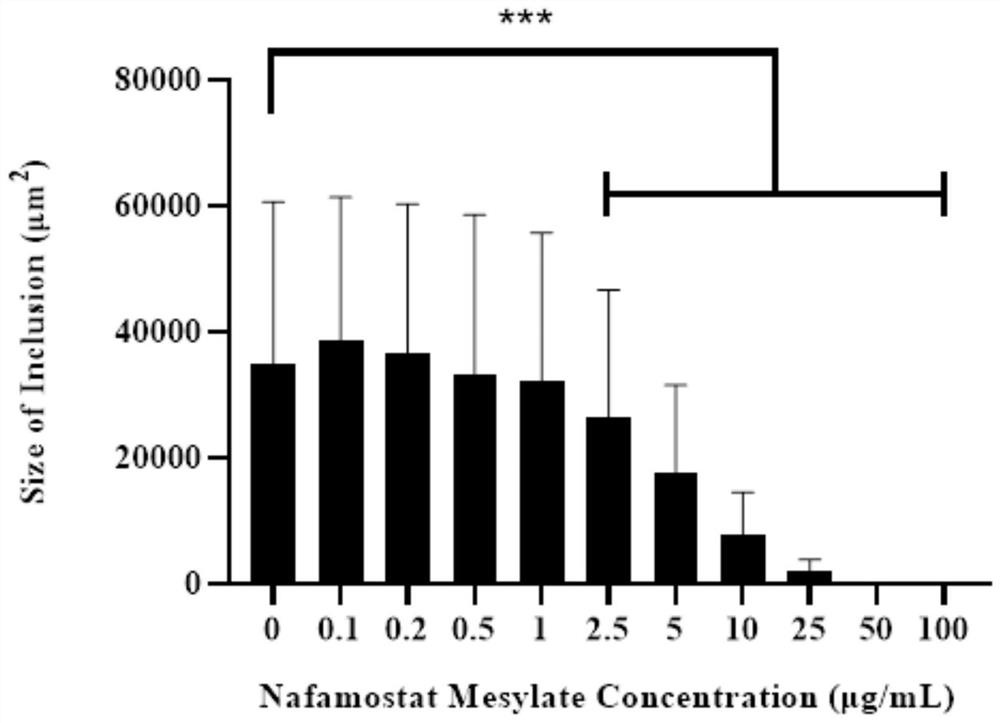
Application method of nafamostat mesylate to treatment of chlamydia infection of genital tract
InactiveCN111821292ADelay drug resistanceIncrease concentrationAntibacterial agentsOrganic active ingredientsTubal ObstructionsDisease
The invention relates to the technical field of biomedicine, in particular to an application method of nafamostat mesylate to treatment of chlamydia infection of a genital tract. The application method comprises the following steps of: A, selecting experimental materials; B, developing an experimental method; C, observing experimental results; and D, drawing experimental conclusions. The inventionprovides a new application of the nafamostat mesylate to treatment of chlamydia infection of the genital tract and particularly relates to an application of the nafamostat mesylate to preparation ofdrugs for treating chlamydia infection of the genital tract, and an application of the nafamostat mesylate to preparation of drugs for preventing tubal obstructions, hydrosalpinx and tubal infertilitycaused by chlamydia infection of the genital tract. Through in vitro cell experiments, animal experiments and other aspects, it is proved that the nafamostat mesylate can effectively treat chlamydiainfection of the genital tract to prevent and treat a tubal infertility disease.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Prevention of chlamydia infection using SIRNA
The present invention provides methods and compositions useful in the treatment or prevention of Chlamydia infections. The methods and compositions inhibit the entry of Chlamydia into a host cell expressing EMP2 by interfering with the interaction between the Chlamydia and EMP2. The compositions include EMP2 nucleic acids and polypeptides as well as anti-EMP2 antibodies.
Owner:RGT UNIV OF CALIFORNIA
Immunization against chlamydia infection
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. trachomatis. The method employs a vector containing a nucleotide sequence encoding a Mgp002 polypeptide of a strain of Chlamydia operably linked to a promoter to effect expression of the gene product in the host. Truncated forms of the full-length Mgp002 gene are useful immunogens for protecting against disease caused by infection with Chlamydia. The invention further provides recombinant Mgp002 protein useful for protecting against disease caused by infection with Chlamydia.
Owner:圣诺菲·帕斯图尔公司
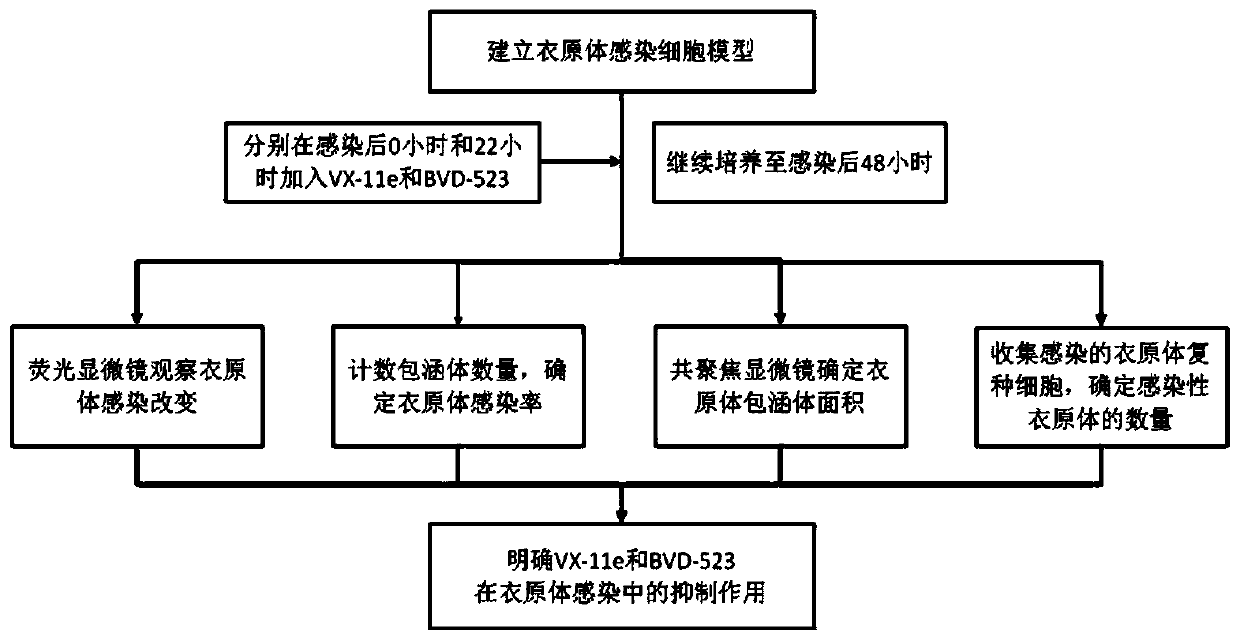
Application of ERK signal pathway small molecule inhibitor in inhibiting chlamydia infection
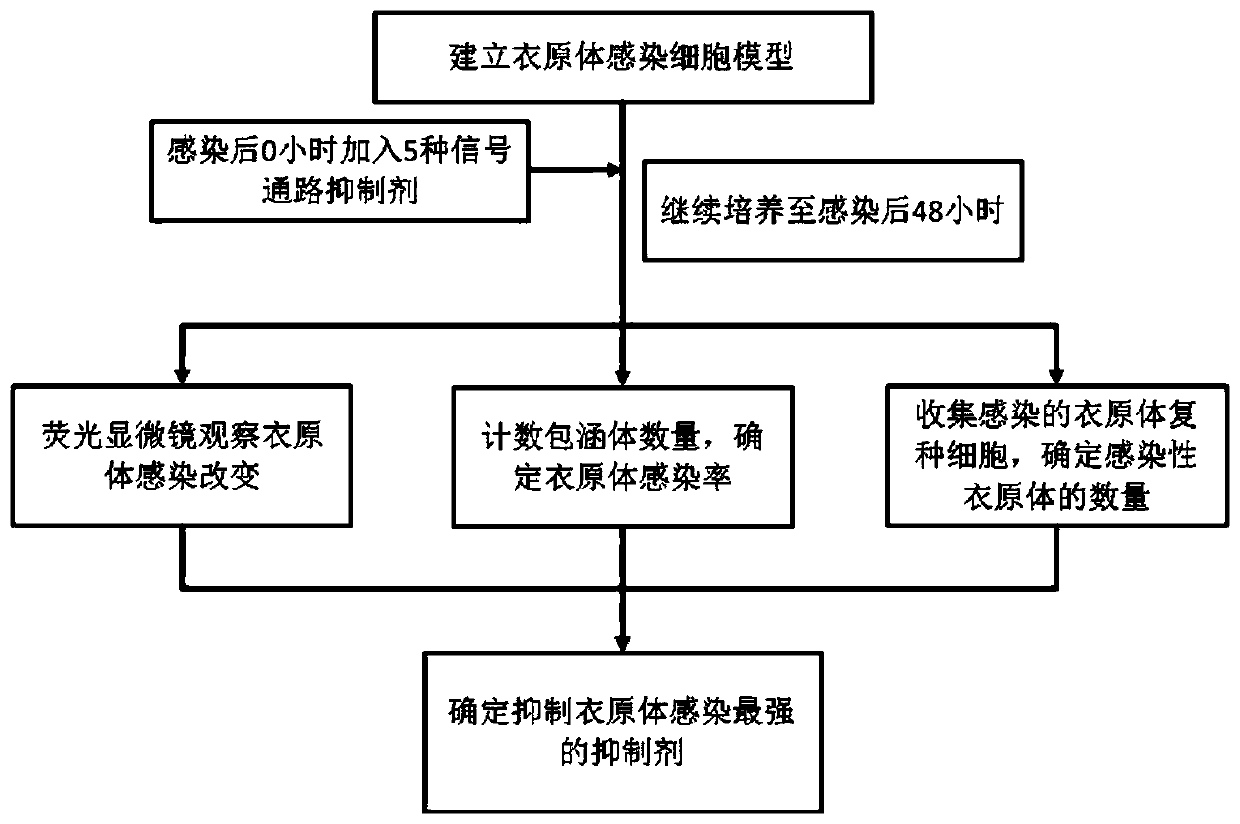
ActiveCN109745562AAvoid infectionStrong anti-infection effectOrganic active ingredientsAntiinfectivesSignalling pathwaysCHLAMYDIAL INFECTIONS
The invention discloses an application of small molecule inhibitors VX-11e and BVD-523 in inhibiting chlamydia infection. The research found that the signal pathway inhibitors VX-11e and BVD-523 can obviously inhibit the infection of the chlamydia trachomatis, and have significant difference compared with the reported MEK inhibitor U0126; according to the application of small molecule inhibitors VX-11e and BVD-523 in inhibiting chlamydia infection, the small molecule inhibitors VX-11e and BVD-523 are applied to chlamydia infection for the first time, and are expected to be new drugs for chlamydia targeting host therapy; the research also found that inhibitors VX-11e and BVD-523 had synergistic effect with azithromycin. After chlamydia infection, VX-11e and BVD-523 could promote the anti-infection effect of azithromycin, which was of great value in the treatment of chlamydia infection. The application of small molecule inhibitors VX-11e and BVD-523 in inhibiting chlamydia infection hasimportant significance for the development of new drugs for chlamydia trachomatis infection and the search of new targets for auxiliary host therapy.
Owner:DERMATOLOGY HOSPITAL SOUTHERN MEDICAL UNIV (GUANGDONG PROVINCIAL DERMATOLOGY HOSPITAL GUANGDONG PROVINCIAL CENT FOR STI & SKIN DISEASES CONTROL & PREVENTION RES CENT FOR LEPROSY CONTROL & PREVENTION CHINA)
Pigeon chlamydia PCR diagnosis kit and detection method thereof
InactiveCN107937576AAddressing the diagnosis of infectionQuick checkMicrobiological testing/measurementPositive controlDistilled water
The invention aims to provide a pigeon chlamydia PCR diagnosis kit which comprises a lysate, an amplification reaction mixture, a negative control and a positive control, wherein a component in the lysate is a DNAiso Reagent; the amplification reaction mixture contains sterilized tri-distilled water, PCR Buffer, dNTP, a CPS-F upstream primer, a CPS-R downstream primer and rTaq DNA polymerase; thenegative control is sterilized tri-distilled water; the positive control is a pigeon Chlamydia positive plasmid. The pigeon chlamydia PCR diagnosis kit provided by the invention is high in sensitivity, good in specificity, high in stability and intuitive in results; a detection method of the pigeon chlamydia PCR diagnosis kit is easy to operate, convenient and quick, and the detection time can begreatly shortened. Therefore, a powerful technical means is provided for clinical control on pigeon chlamydia infection.
Owner:XIANYANG VOCATIONAL TECHN COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com