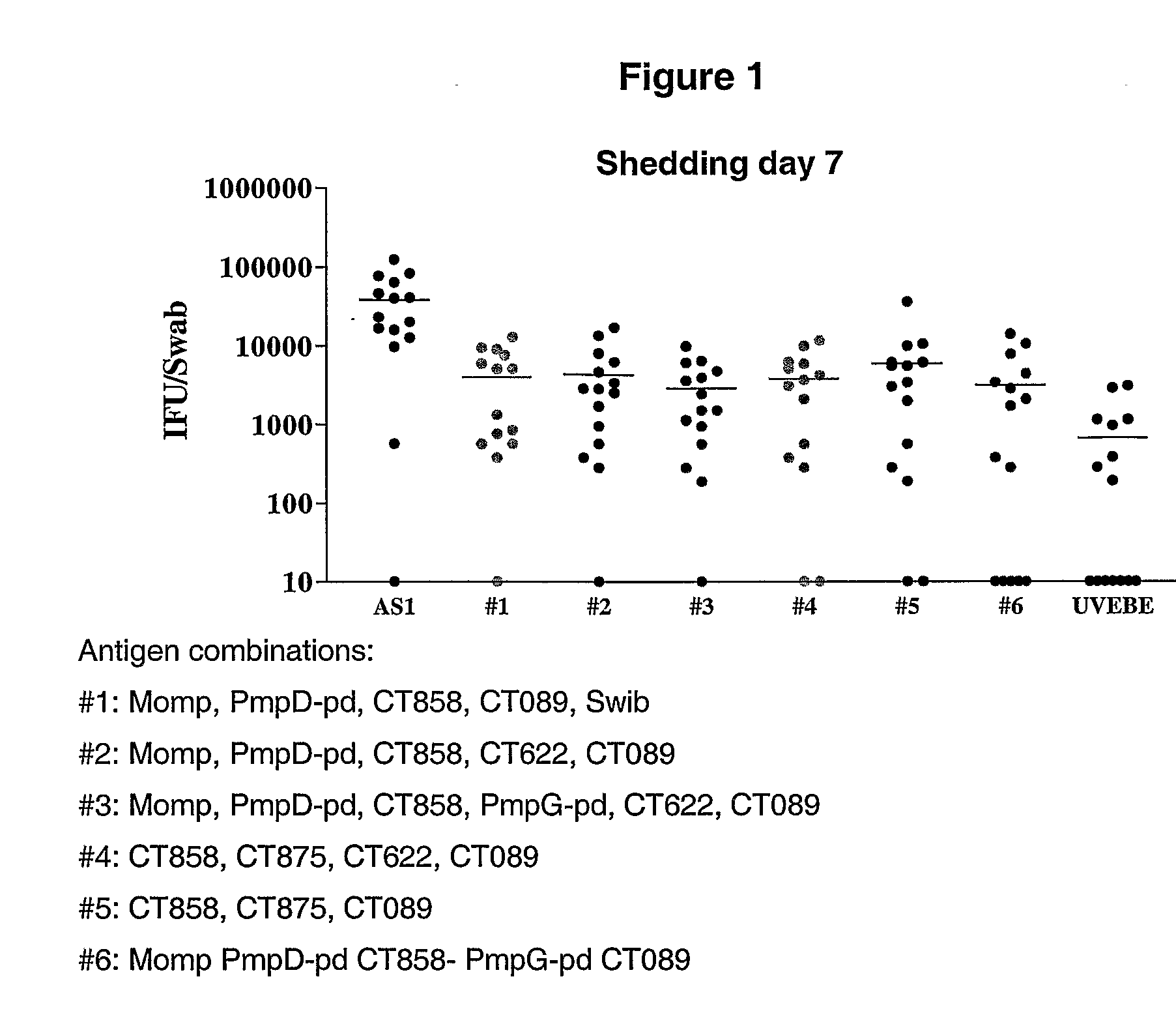
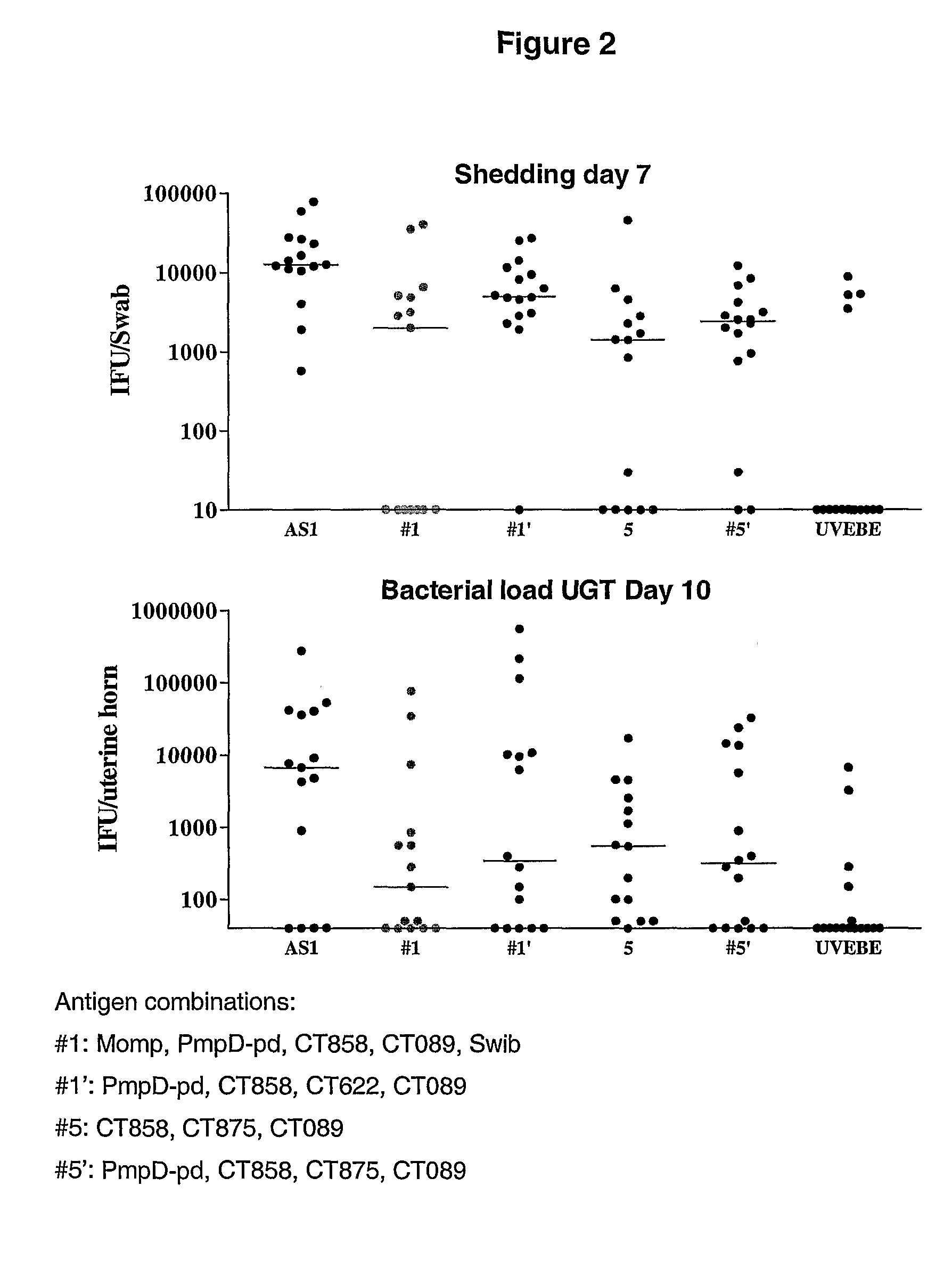
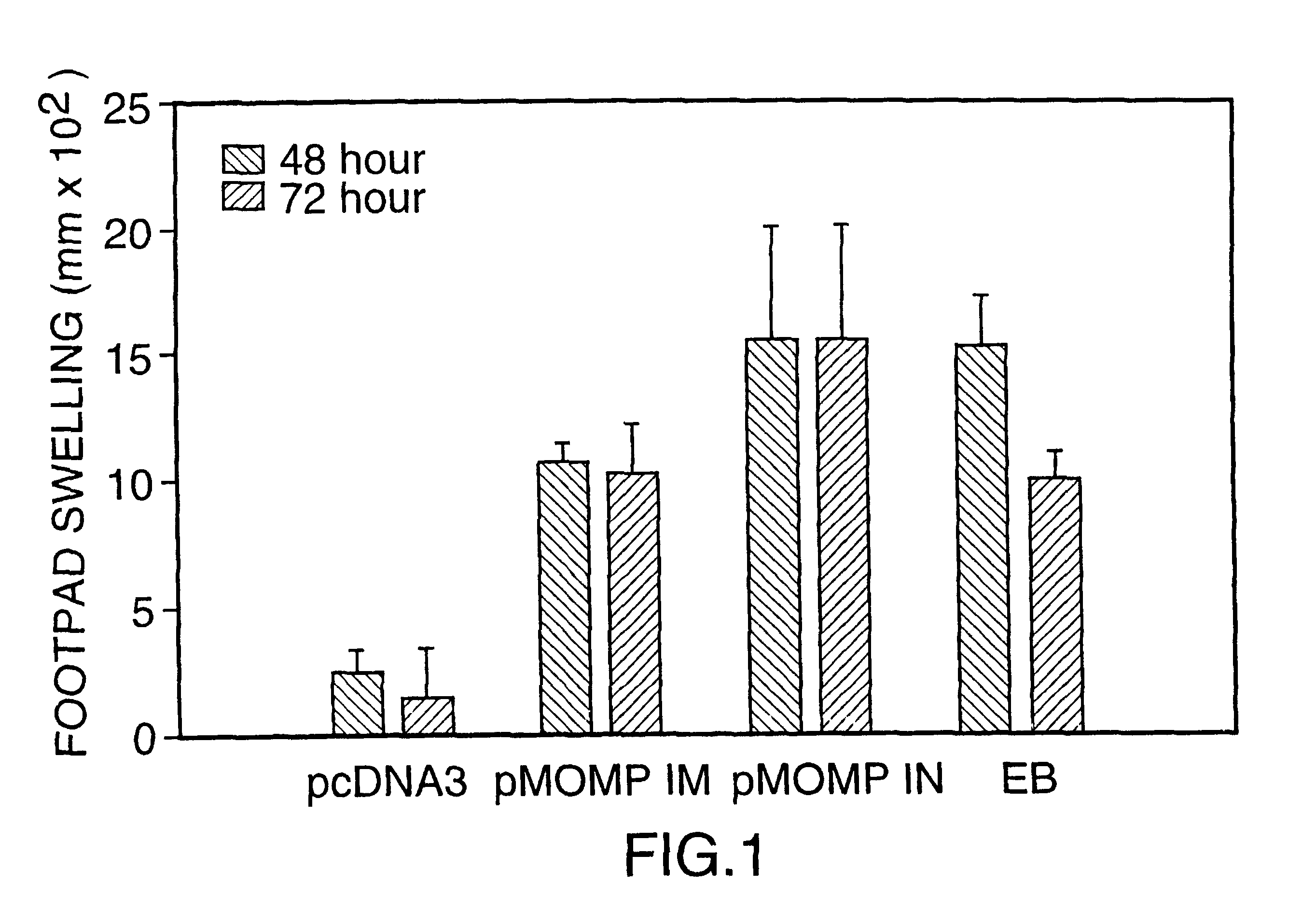
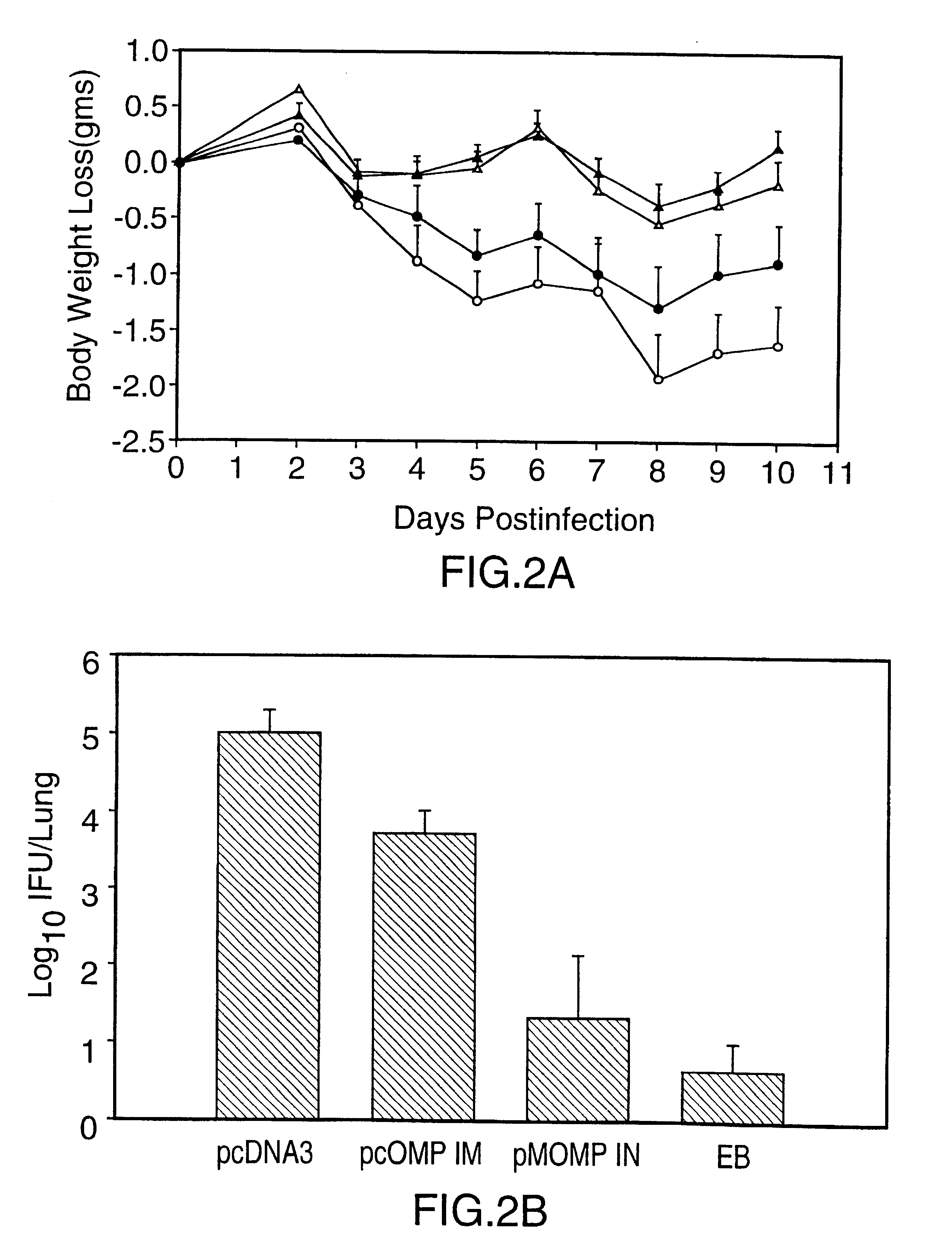
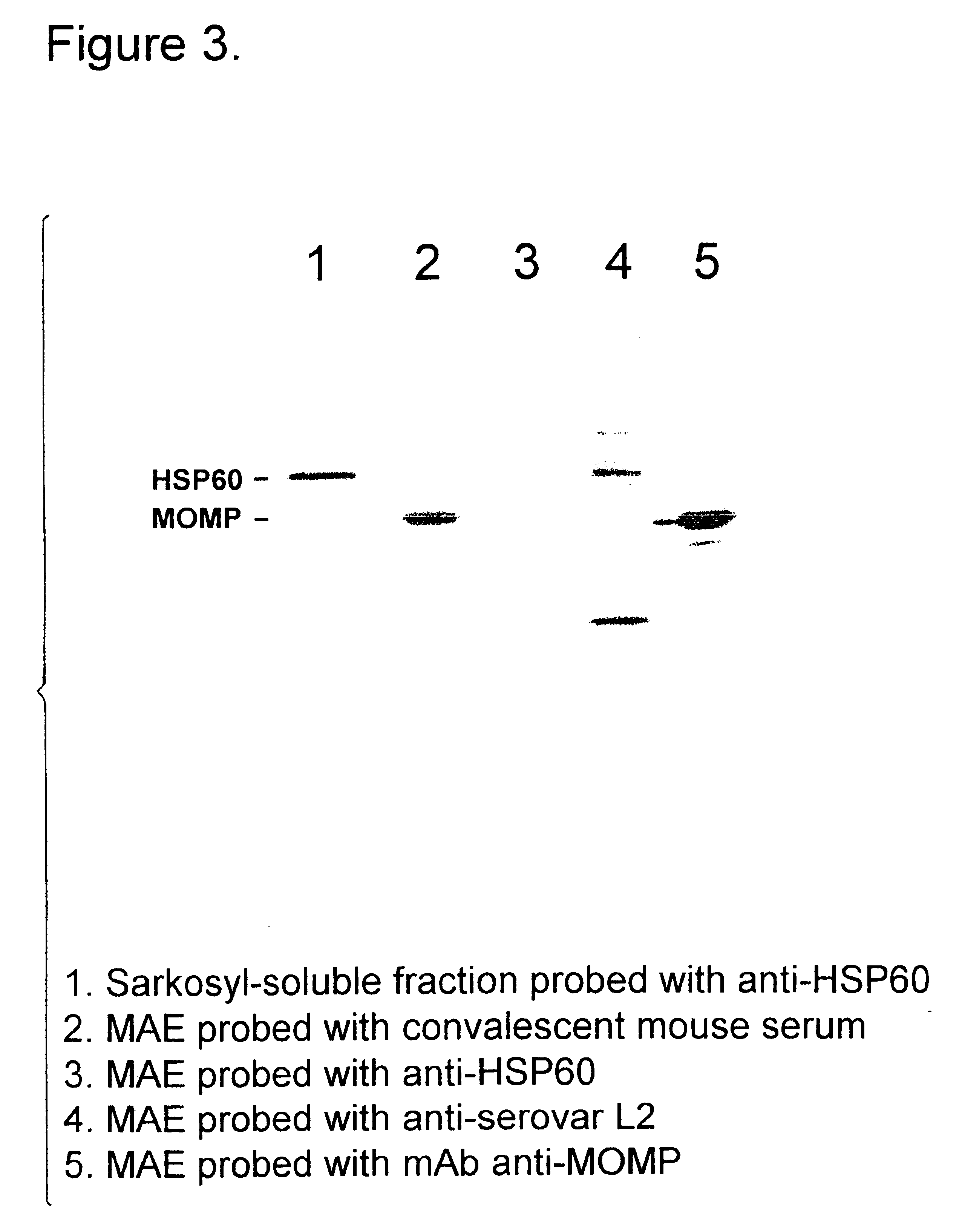
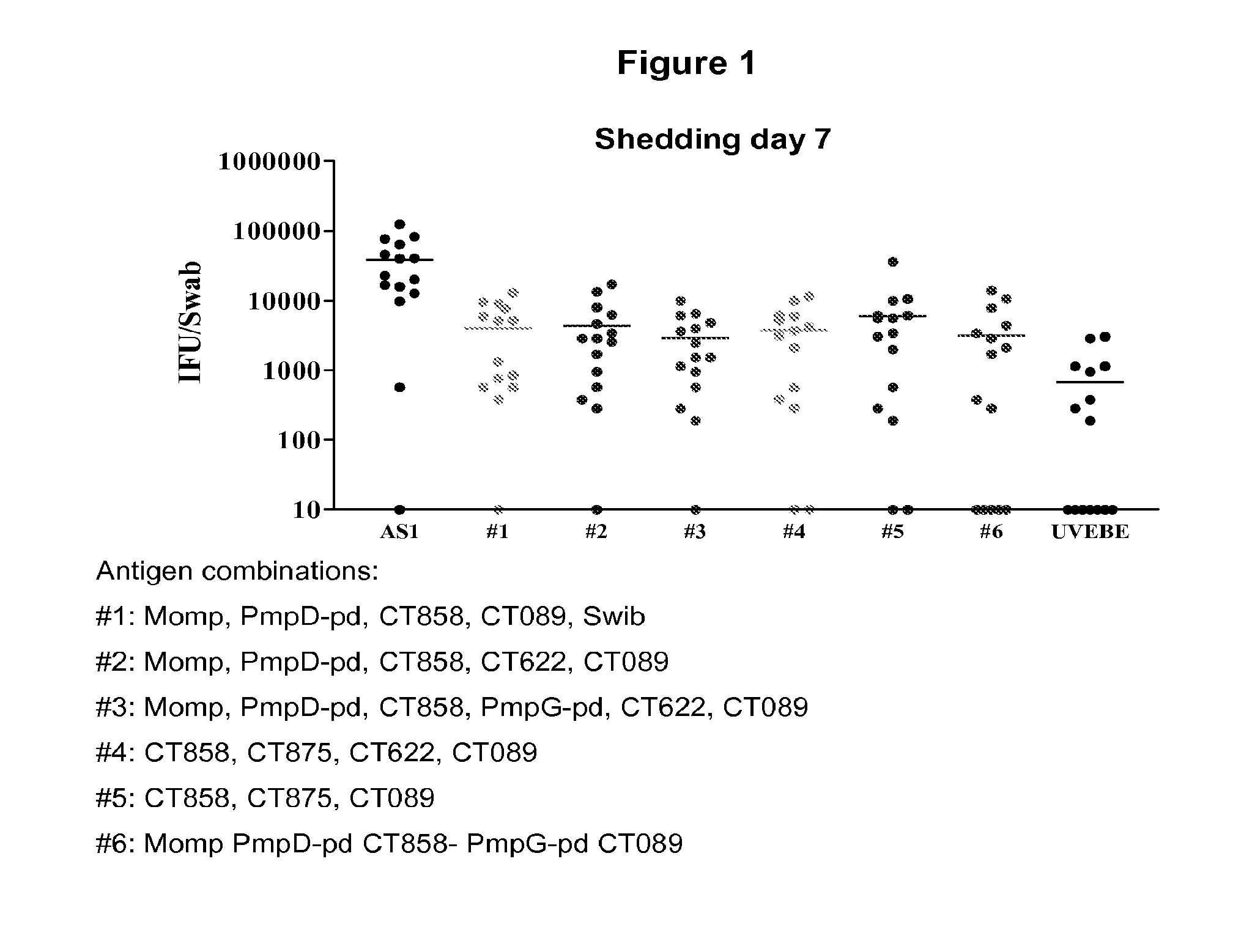
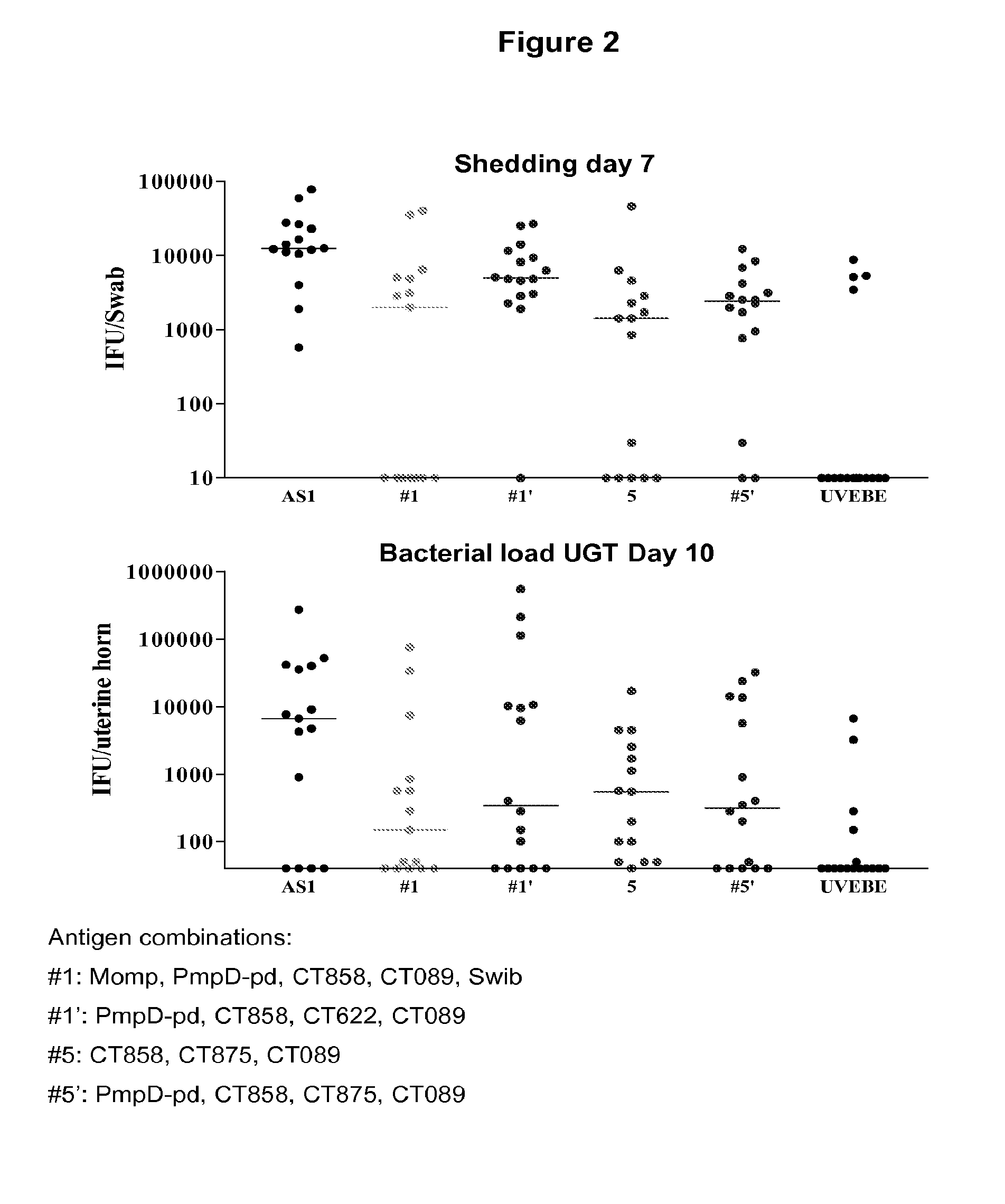
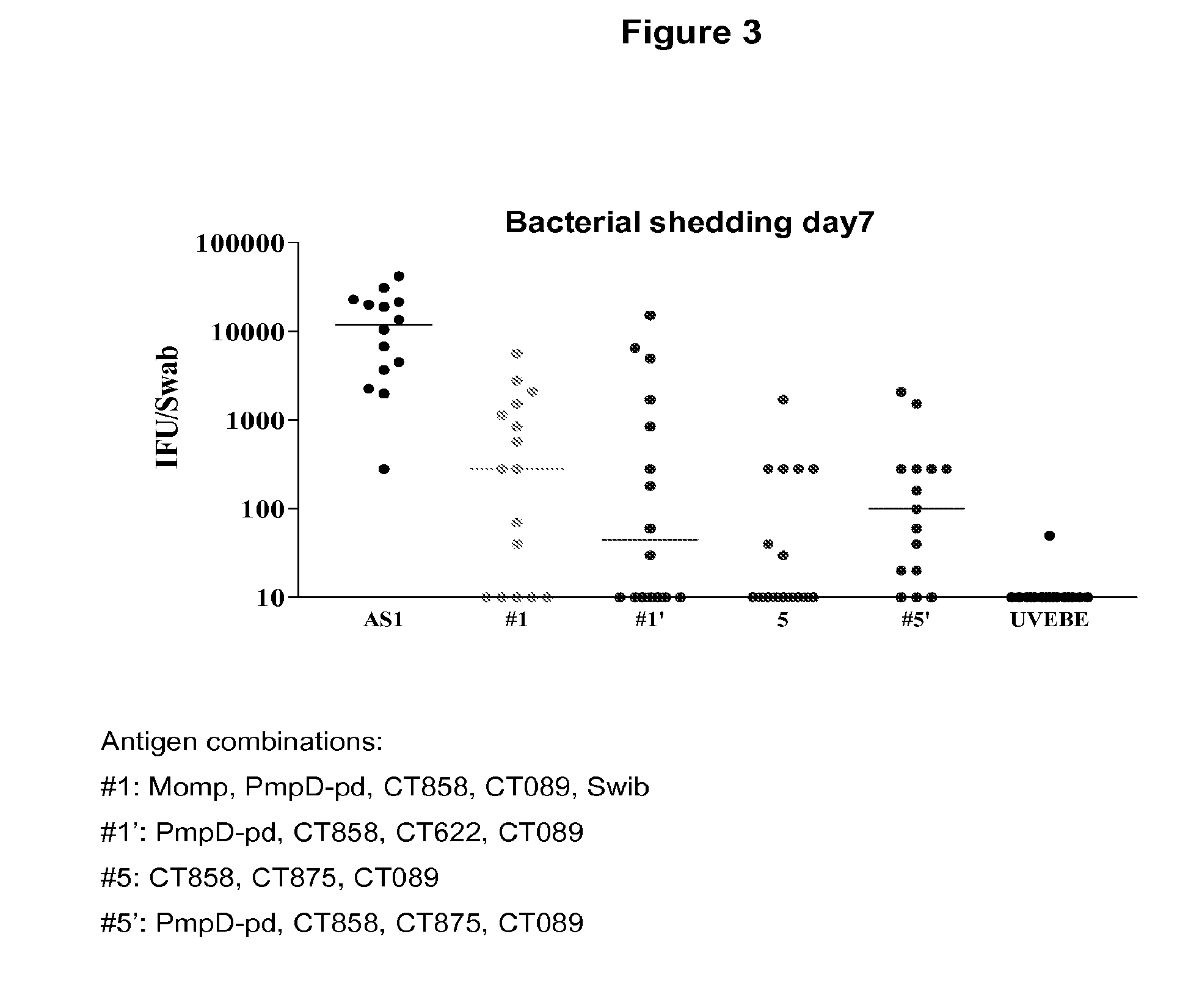
Patents
Literature
75 results about "CHLAMYDIAL INFECTIONS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlamydia infection (from the Greek, χλαμύδος meaning "cloak") is a common sexually transmitted infection (STI) in humans. It is caused by the bacterium Chlamydia trachomatis.
Compounds and methods for treatment and diagnosis of chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
Methods and compositions for immunization against chlamydial infection and disease
ActiveUS20090098165A1Reduce morbidityReduce the possibilityAntibacterial agentsBacterial antigen ingredientsCHLAMYDIAL INFECTIONSDisease cause
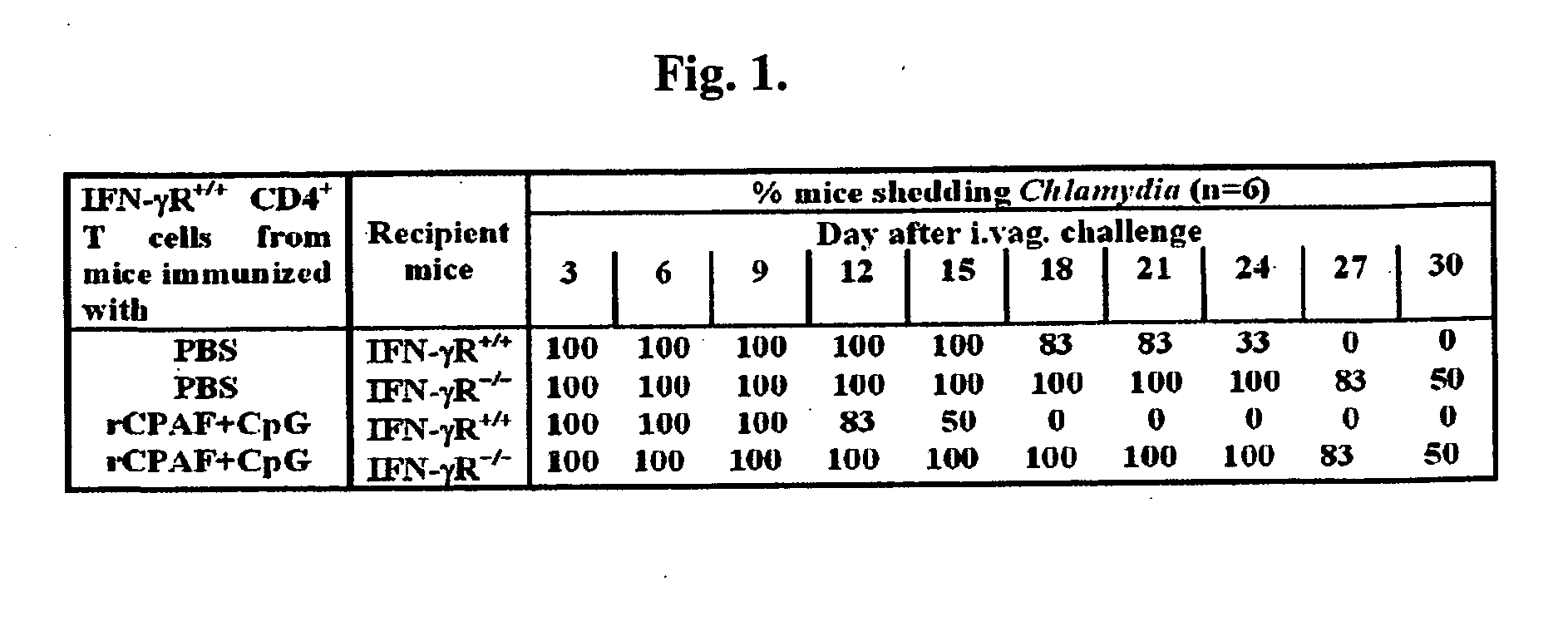
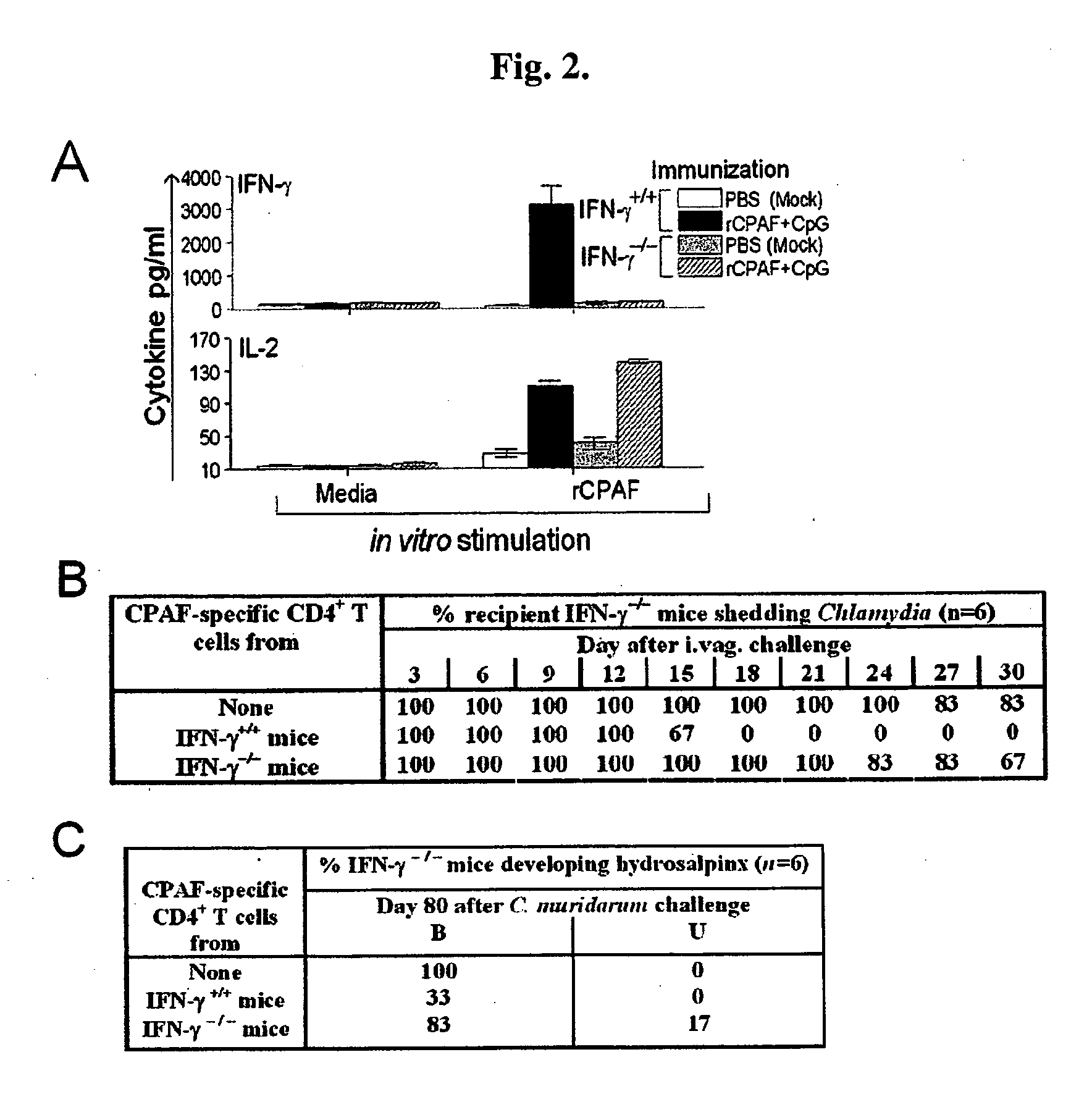
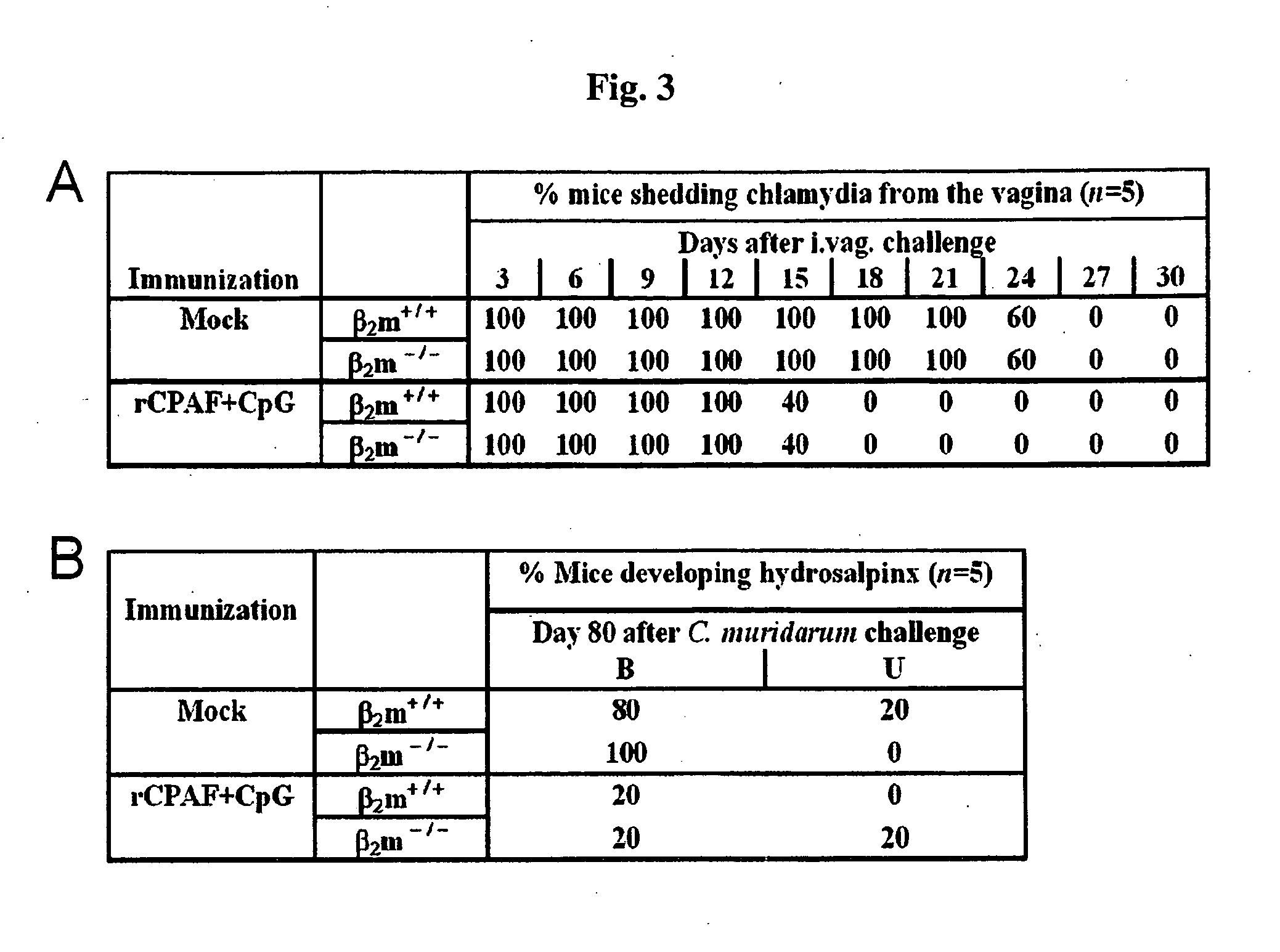
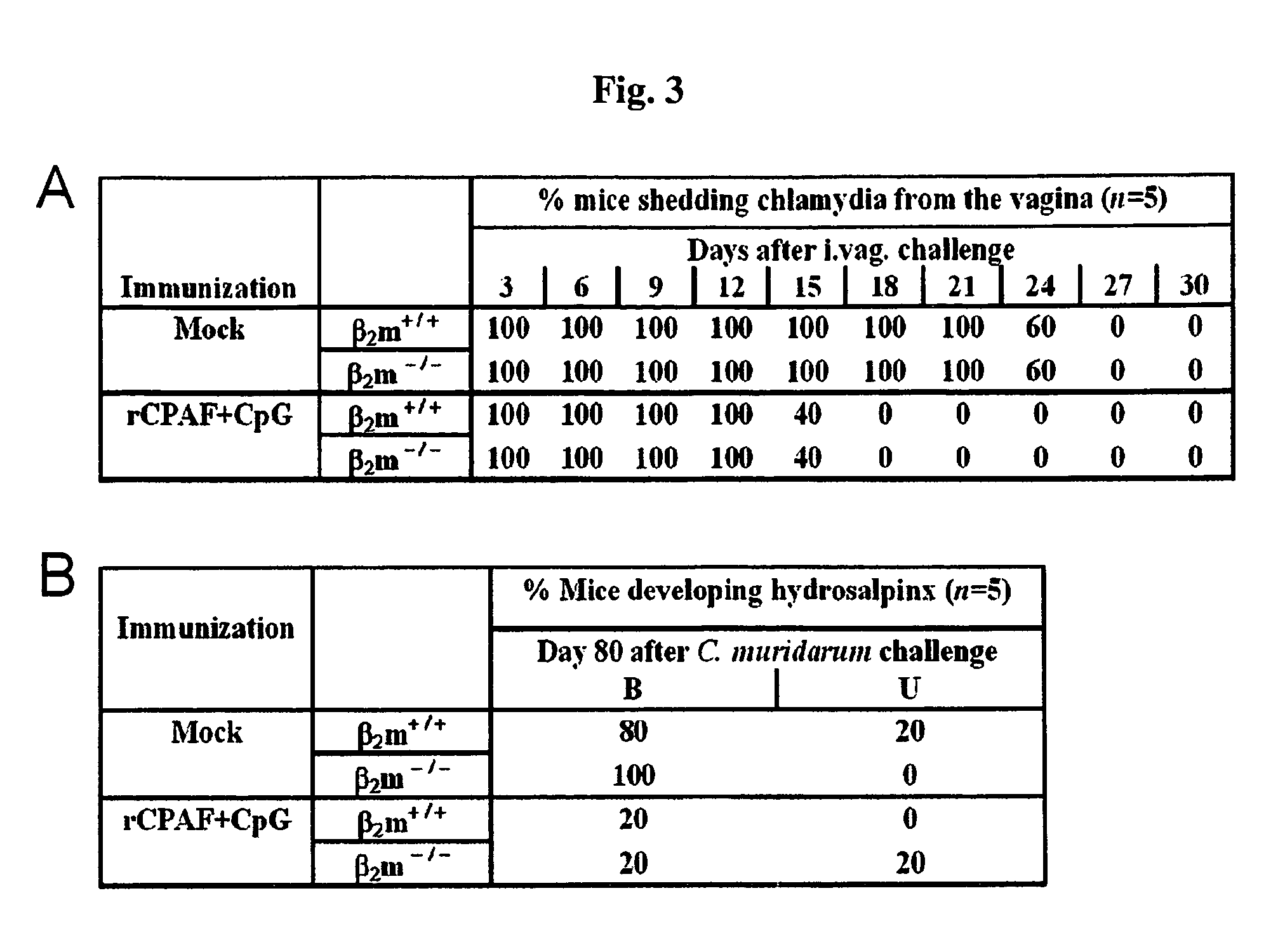
The present invention provides Chlamydia proteins and compositions and methods of use in the treatment / prevention of chlamydial infection in a subject, for eliciting an immune response in a subject and for reducing the likelihood of infertility and reducing the incidence and / or degree of hydrosalpinx due to Chlamydia infection in a subject.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Vaccines against chlamydial infection
InactiveUS20090022755A1Stimulate immune responseProvide protectionBacterial antigen ingredientsChlamydiaceae ingredientsPolynucleotideChlamydia sp
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:CORIXA CORP +1
Compounds and methods for treatment and diagnosis of Chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
Compounds and methods for treatment and diagnosis of chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
DNA immunization against chlamydia infection
InactiveUS6696421B2Significantly more efficient in inducing protective immunityRapid recruitment of dendritic cellsBiocideGenetic material ingredientsNucleotidePhylum Chlamydiae
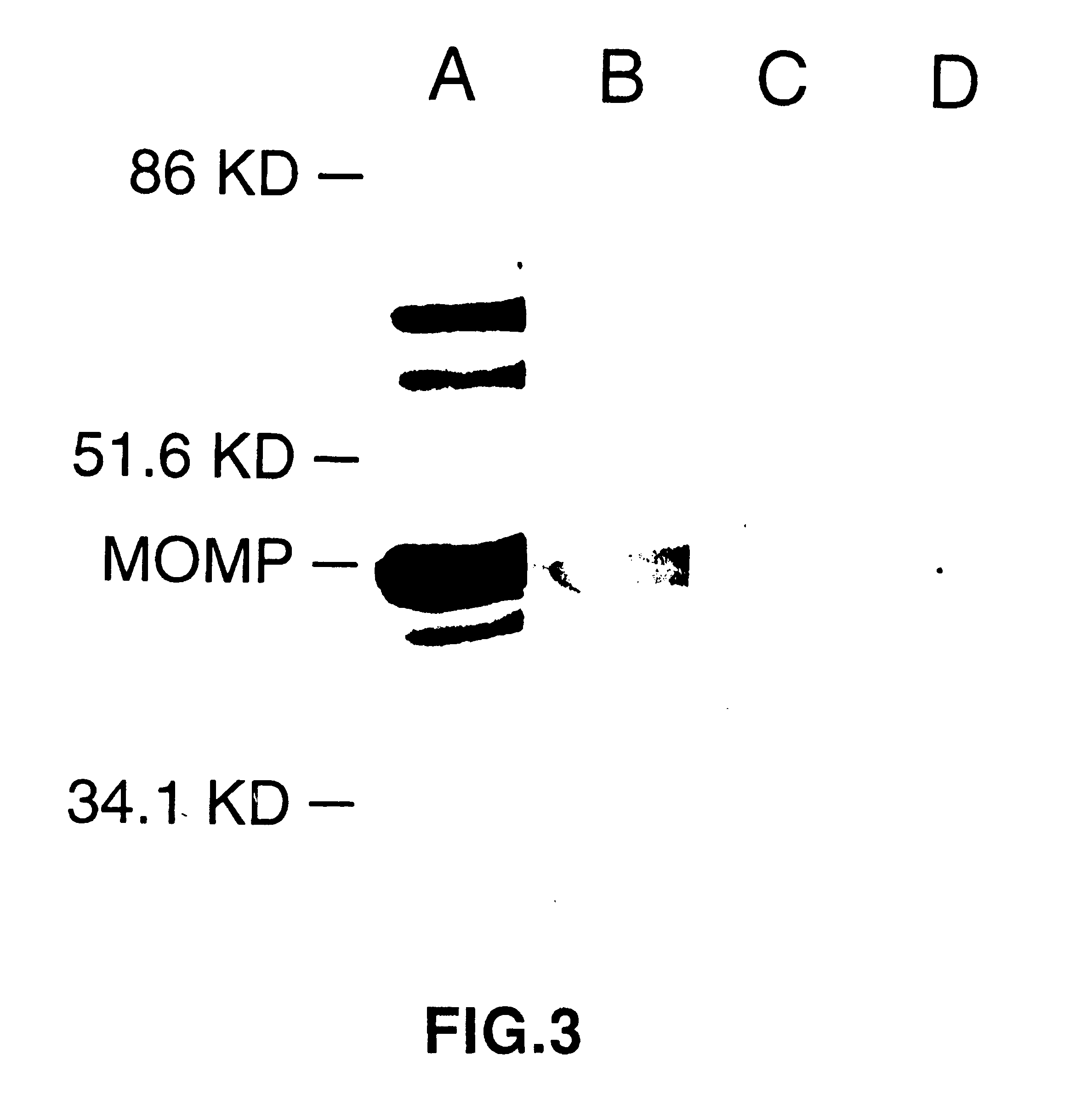
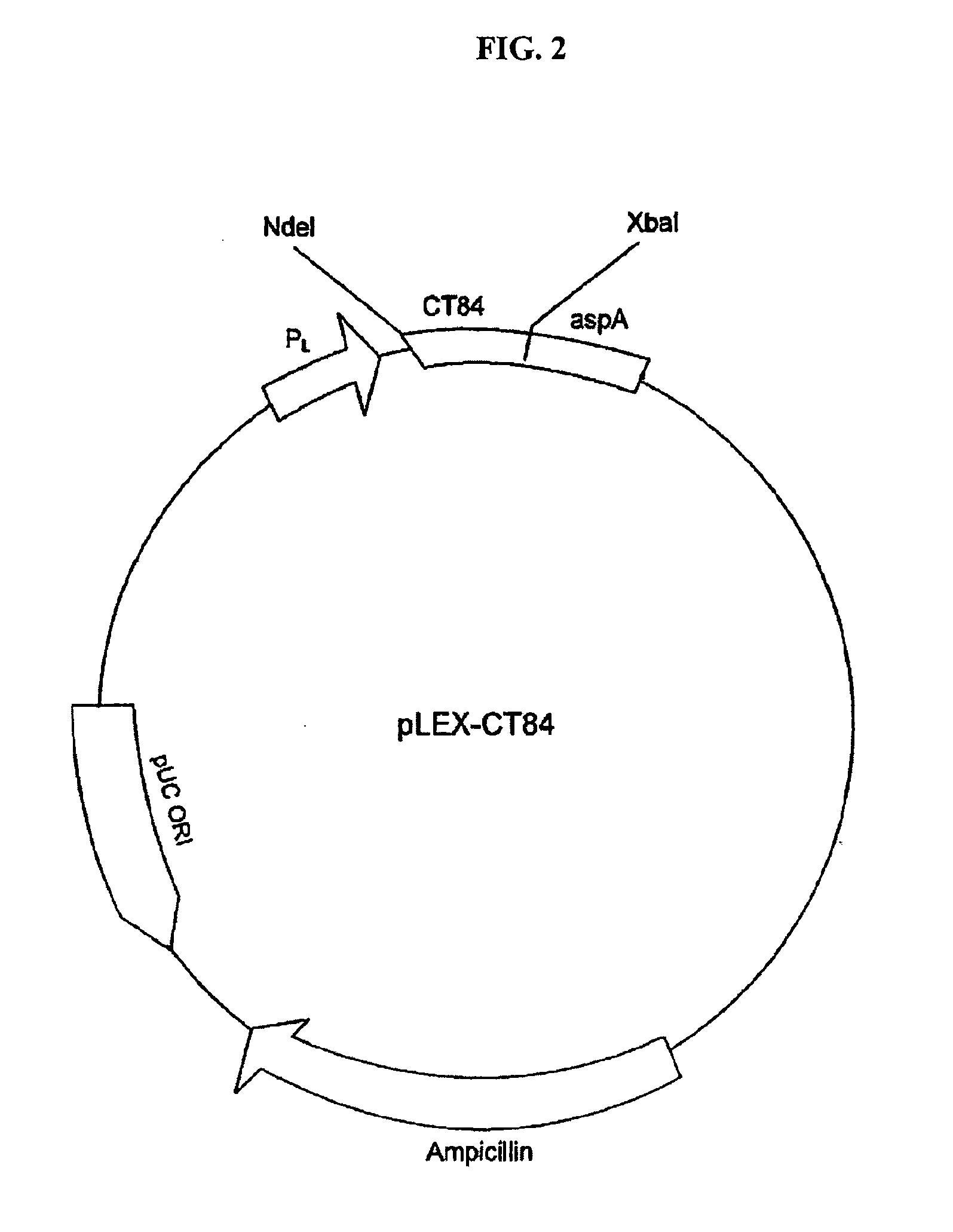
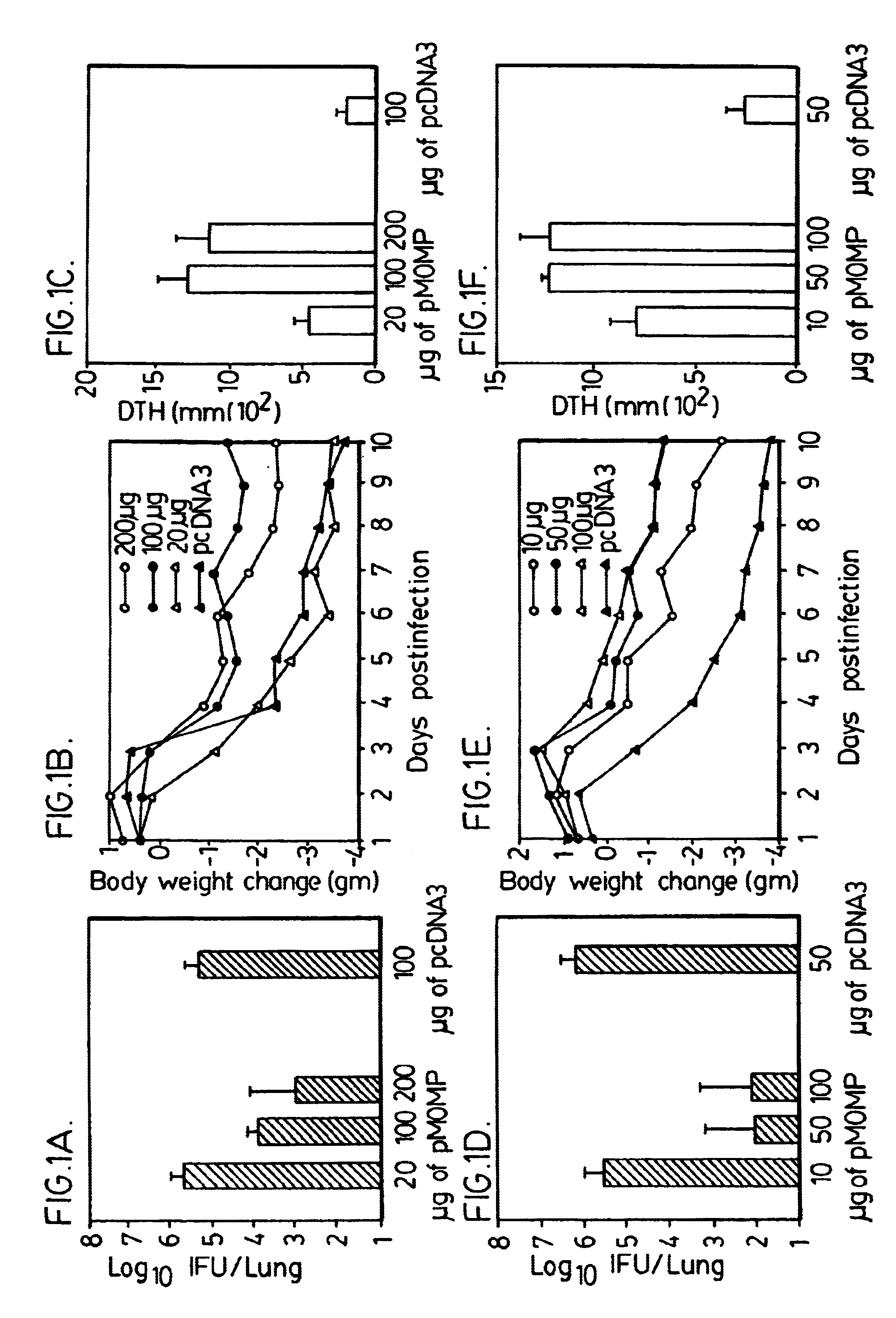
Nucleic acid, including DNA, immunization to generate a protective immune response in a host, including humans, to a major outer membrane protein of a strain of Chlamydia, preferably contains a nucleotide sequence encoding a MOMP or a MOMP fragment that generates antibodies that specifically react with MOMP and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The non-replicating vector may be formulated with a pharmaceutically acceptable carrier for in vivo administration to the host.
Owner:UNIVERSITY OF MANITOBA
Immunogenic compositions for protection against Chlamydial infection
InactiveUS20050065106A1Improve efficacySugar derivativesChlamydiaceae ingredientsNucleotideProtection sex
Owner:MURDIN ANDREW +1
Vaccines Against Chlamydial Infection
InactiveUS20100172927A1Improving immunogenicityAntibacterial agentsBacterial antigen ingredientsPolynucleotideImmunogenicity
Owner:CORIXA CORP +1
Vaccines Against Chlamydia Infection
InactiveUS20100310593A1Improve isolationEasy to purifyAntibacterial agentsBacteriaPolynucleotideChlamydia virus
The present invention is directed to providing a vaccine to enhance the immune response of an animal in need of protection against a Chlamydia infection. The present invention is also directed toward an isolated nucleic acid encoding a polypeptide comprising at least 70% identity to any one of SEQ ID NOS: 2, 11, 13, 19, or 21, wherein the polypeptide is soluble in the absence of denaturing agents. In some aspects of the invention, the polynucleotide is codon-optimized. In some embodiments, the present invention is related to the polypeptide encoded by the polynucleotide of the invention. Administration of polypeptides of the present invention can be used as a method to treat or prevent a Chlamydia infection in an animal in need thereof.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Two-step immunization procedure against Chlamydia infection
InactiveUS6676949B2Strong immune responseImproving immunogenicityAntibacterial agentsSenses disorderTwo stepChlamydia virus
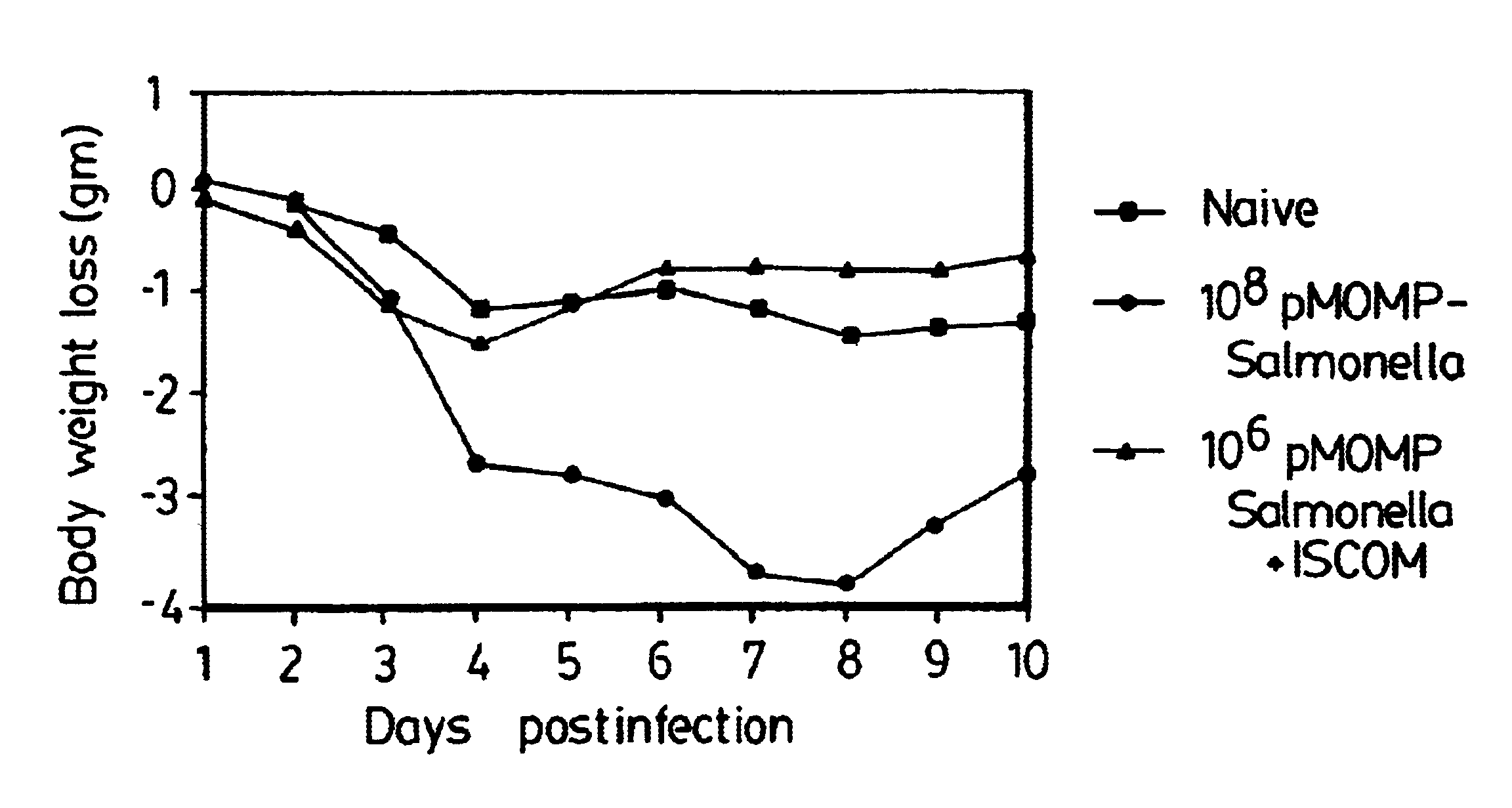
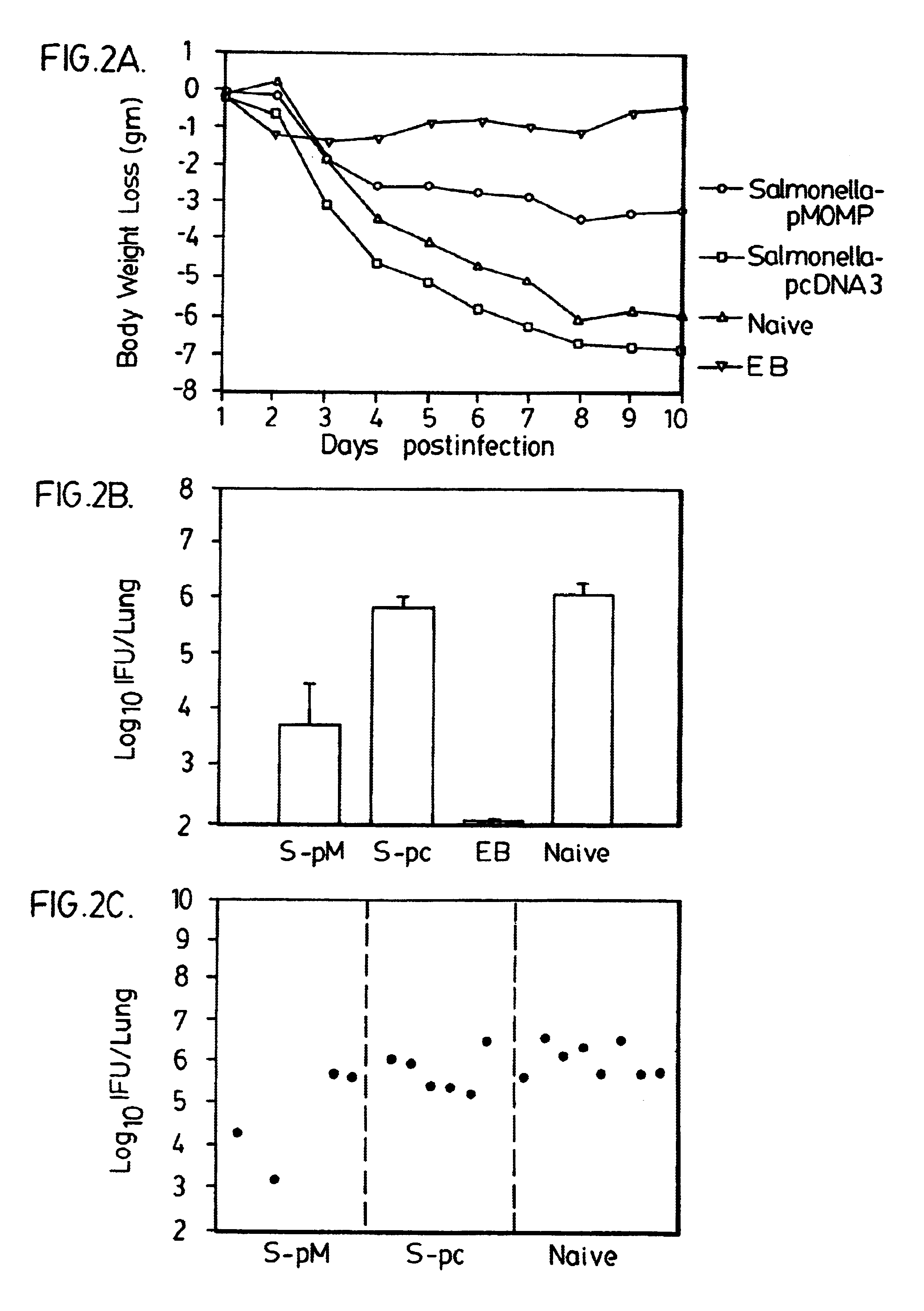
A host is immunized against infection by a strain of Chlamydia by initial administration of an attenuated bacteria harbouring a nucleic acid encoding a Chlamydia protein followed by administration of a Chlamydia protein in ISCOMs. This procedure enables a high level of protection to be achieved.
Owner:AVENTIS PASTEUR LTD
Chlamydial vaccines and methods of preparation thereof
InactiveUS6464979B1Strong immune responseImproving immunogenicityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseImmunostimulating Complexes
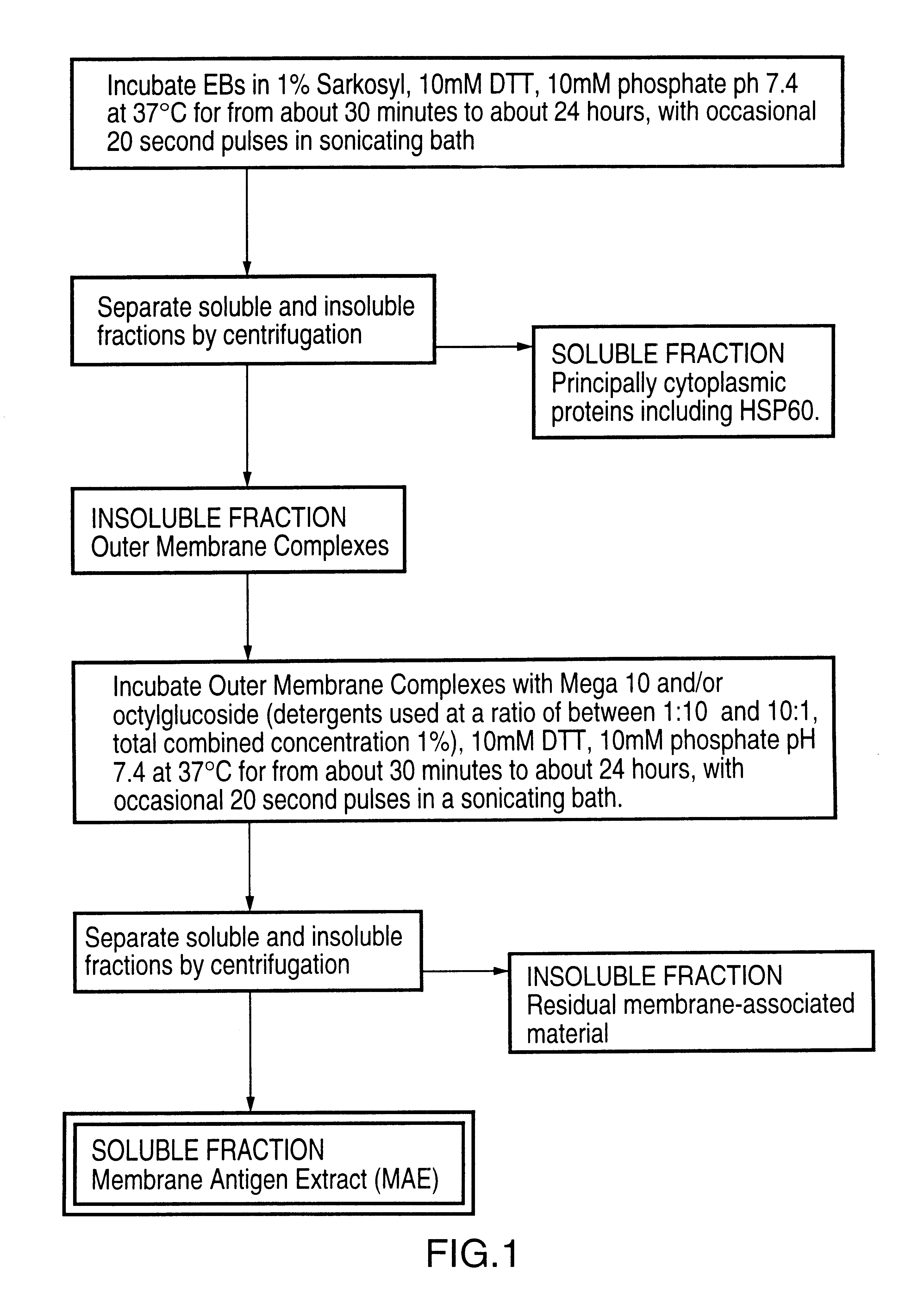
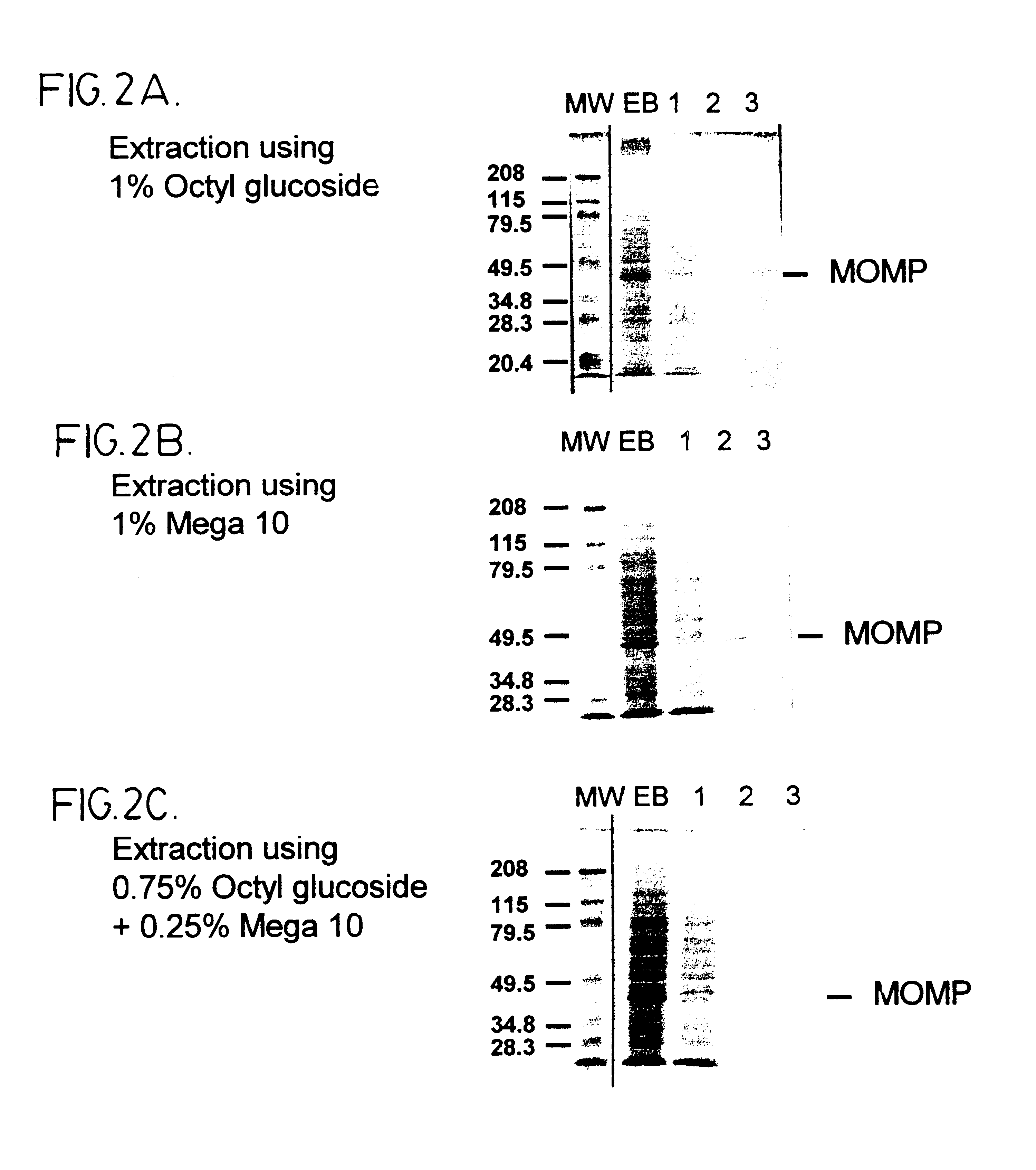
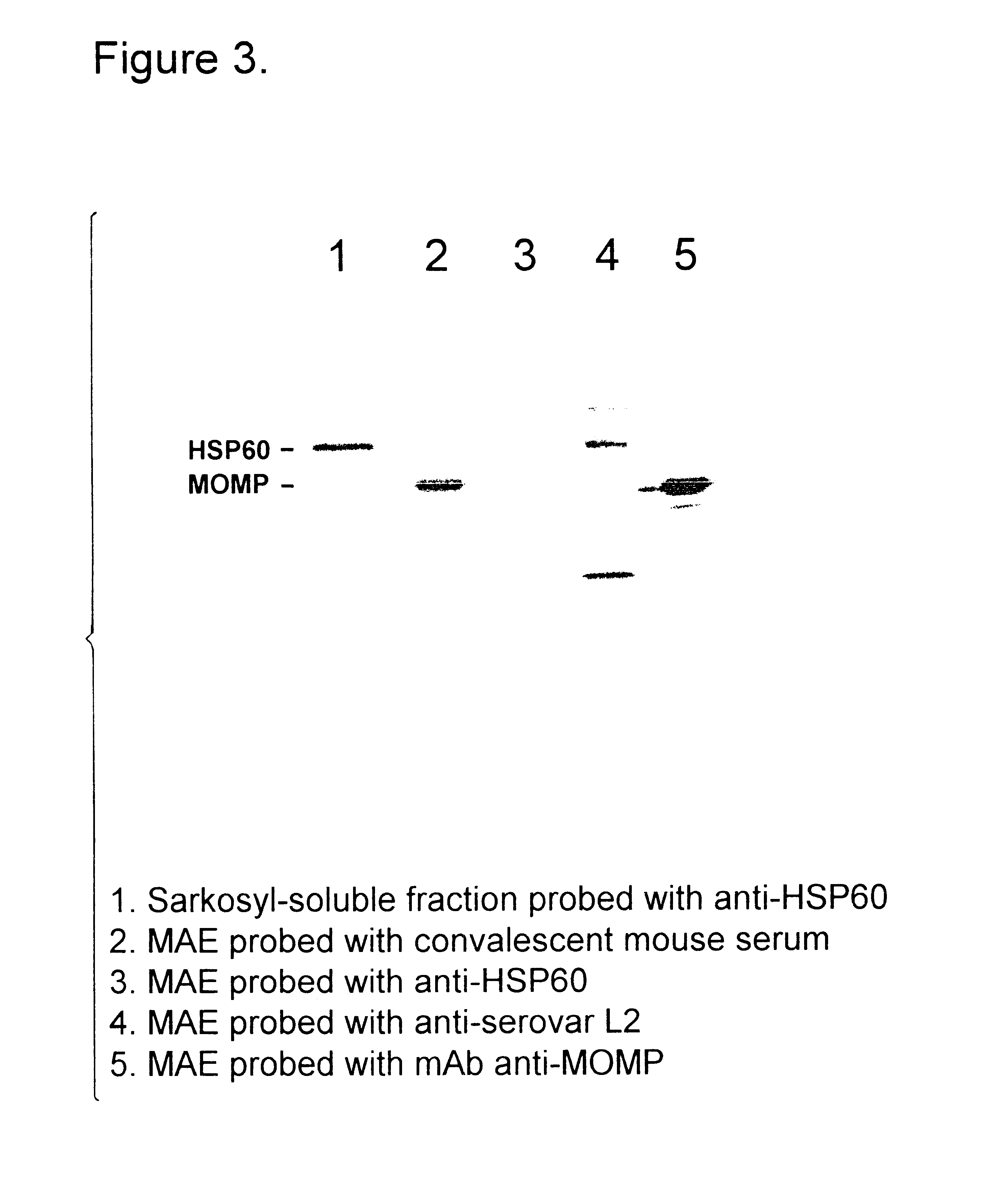
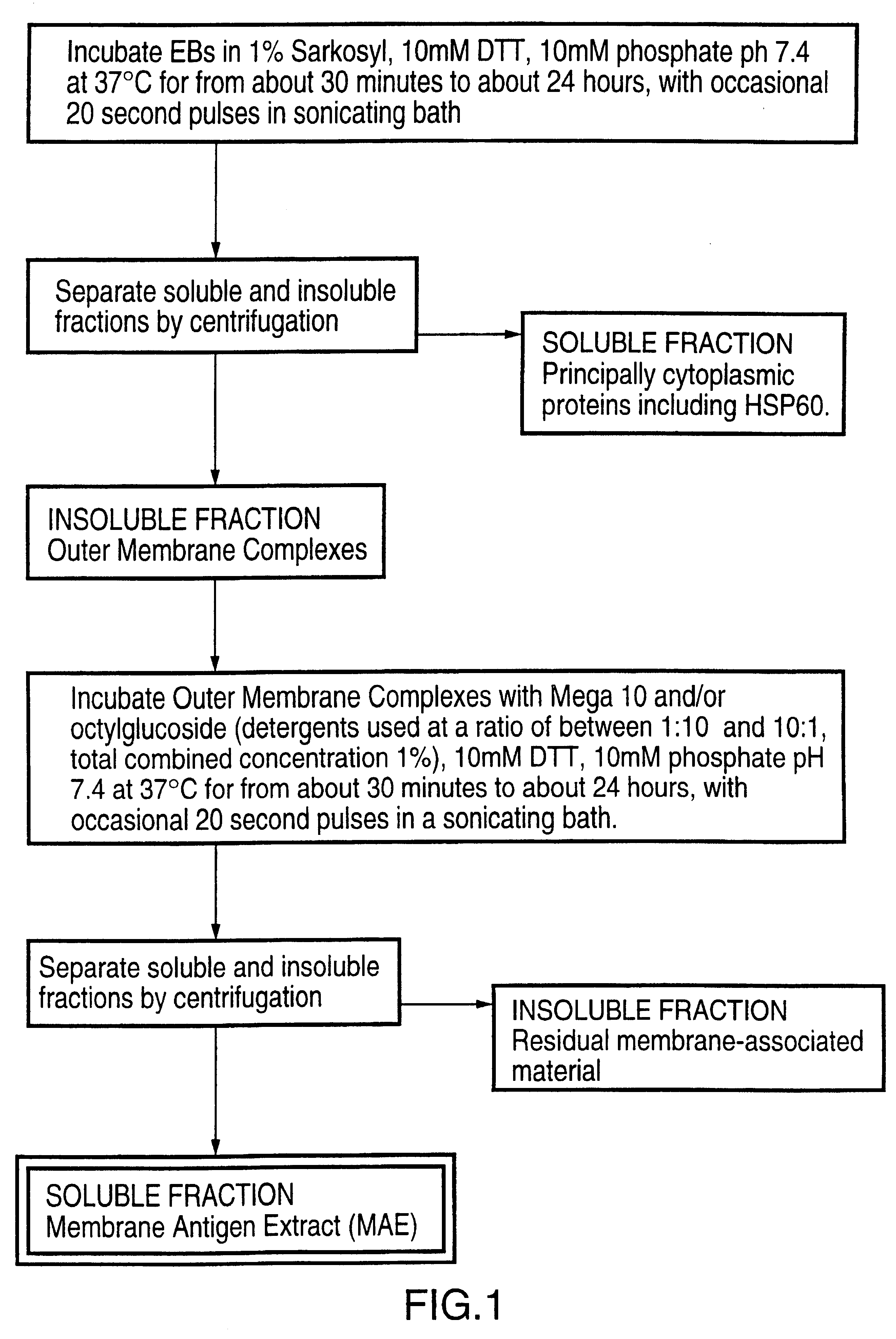
Immunogenic compositions including vaccines are described that comprise an outer membrane antigen extract of a strain of Chlamydia and are effective in protection against disease caused by Chlamydia infection. The immunogenic compositions may comprise the major outer membrane protein (MOMP) of Chlamydia which may be in a homooligomeric form or complexed with at least one other antigen of Chlamydia. The immunogenic composition may include an immunostimulating complex (ISCOM) and the outer membrane antigen may be incorporated therein. The immunogenic compositions have utility as chlamydial vaccines and in diagnostic applications.
Owner:AVENTIS PASTEUR LTD
Immunization Against Chlamydia Infection
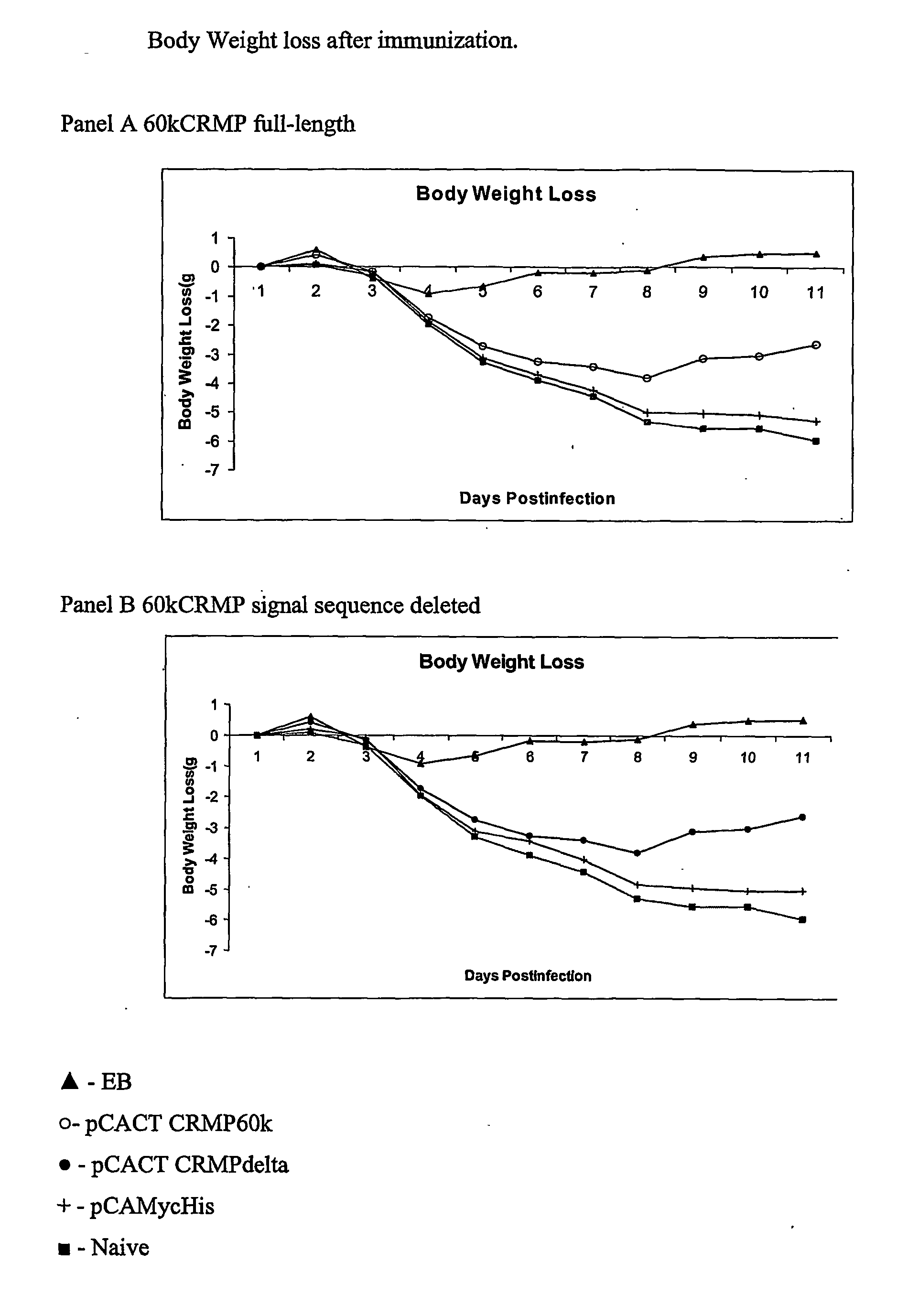
The present invention provides nucleic acids, proteins and vectors for a method of nucleic acid, including DNA, immunization of a host, including humans, against disease caused by infection by a strain of Chlamydia, specifically C. trachomatis. The method employs a vector containing a nucleotide sequence encoding a polypeptide of a strain of Chlamydia operably linked to a promoter to effect expression of the gene product in the host. The polypeptides are derived from the Chalmydia gene 60kCRMP gene including truncated forms of the gene. The invention further provides recombinant 60kCRMP protein useful for protecting against disease caused by infection with Chlamydia.
Owner:BRUNHAM ROBERT +3
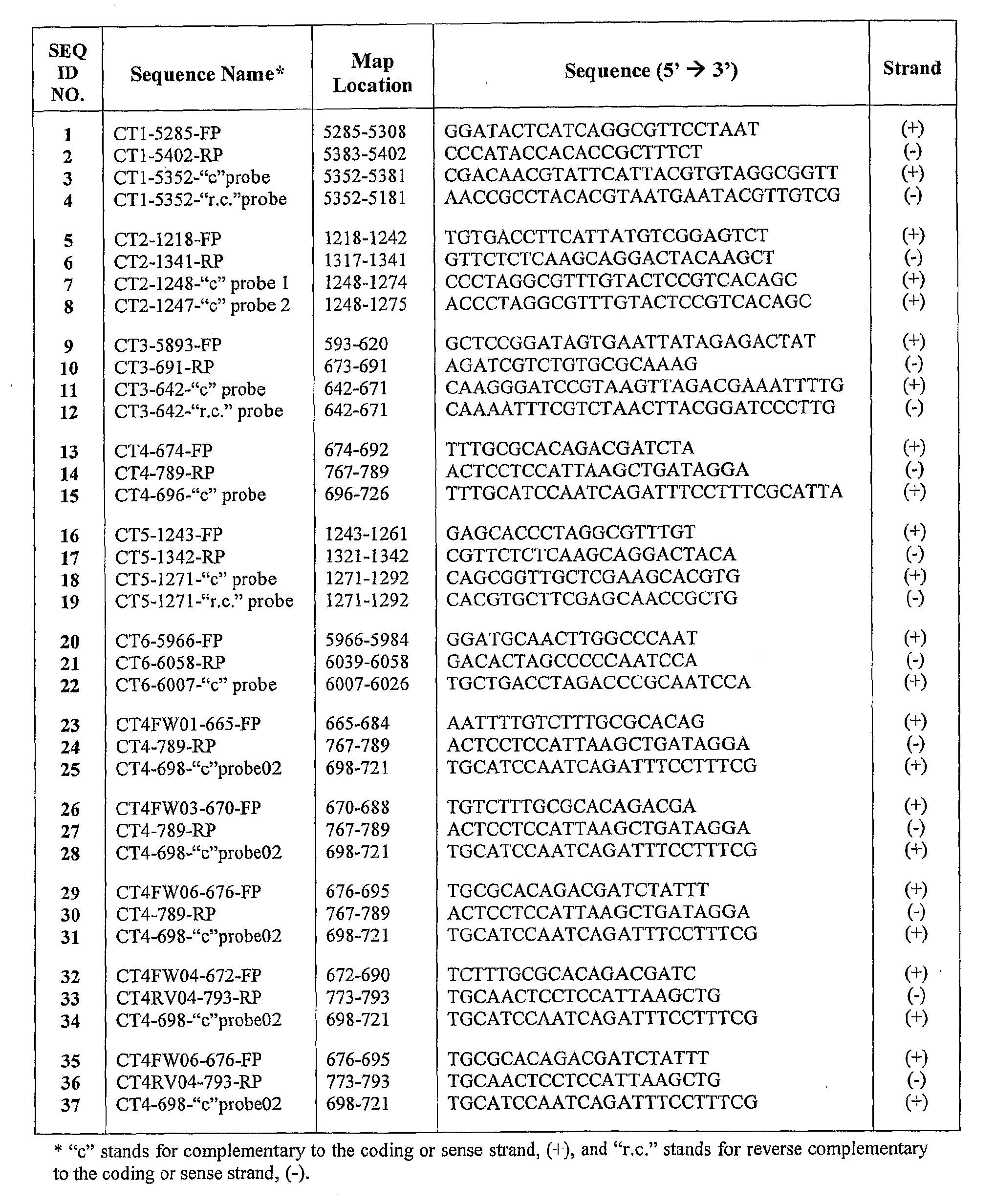
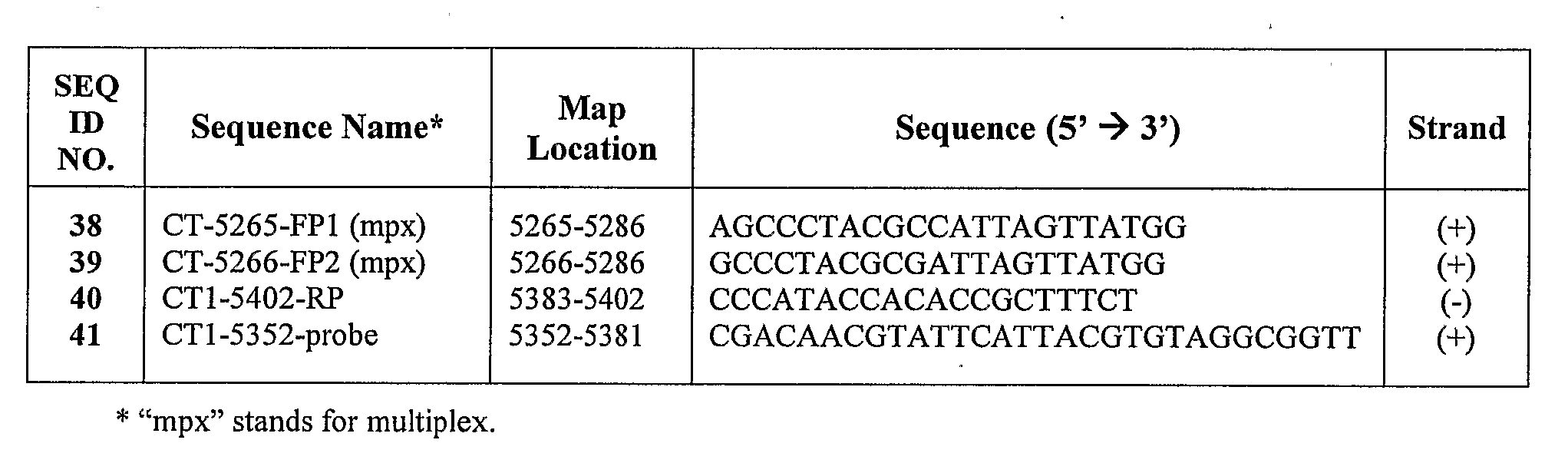
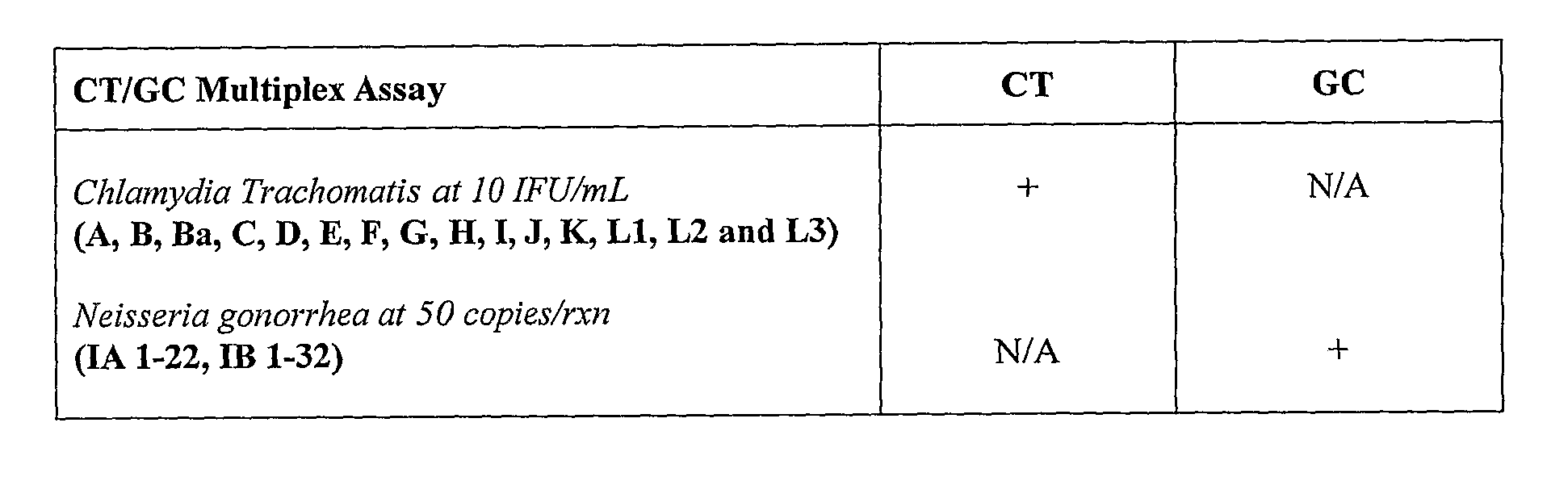
Chlamydia trachomatis specific oligonucleotide sequences
The present invention relates to oligonucleotide sequences for amplification primers and detection probes and to their use in nucleic acid amplification methods for the selective and specific detection of Chlamydia trachomatis in biological samples. The invention also provides oligonucleotide primer sets and primer / probe sets in the form of kits for the detection and diagnosis of chlamydial infection. The inventive oligonucleotide primers and probes can also be used in combination with other specific oligonucleotide primers and probes for the simultaneous detection ofChlamydia trachomatis and other target organisms, such as Neisseria gonorrhea.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Compounds and methods for treatment and diagnosis of chlamydial infection
Compounds and methods for the diagnosis and treatment of Chlamydial infection are disclosed. The compounds provided include polypeptides that contain at least one antigenic portion of a Chlamydia antigen and DNA sequences encoding such polypeptides. Pharmaceutical compositions and vaccines comprising such polypeptides or DNA sequences are also provided, together with antibodies directed against such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Chlamydial infection in patients and in biological samples.
Owner:CORIXA CORP
Methods and compositions for immunization against chlamydial infection and disease
ActiveUS7892567B2Reduce the possibilityReduce morbidityAntibacterial agentsBacterial antigen ingredientsCHLAMYDIAL INFECTIONSDisease cause
The present invention provides Chlamydia proteins and compositions and methods of use in the treatment / prevention of chlamydial infection in a subject, for eliciting an immune response in a subject and for reducing the likelihood of infertility and reducing the incidence and / or degree of hydrosalpinx due to Chlamydia infection in a subject.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Vaccines against chlamydial infection
InactiveUS20110300206A1Stimulate immune responseImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsNucleotideADAMTS Proteins
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Immunogenic compositions for protection against chlamydial infection
InactiveUS6811783B1Improving immunogenicitySlow and sustained releaseBacterial antigen ingredientsSugar derivativesNucleotideProtection sex
A protective immune response against Chlamydial infection is achieved by in vivo administration of an immunogenic composition comprising two vectors and a pharmaceutically-acceptable carrier therefor. One of the vectors comprises a first nucleotide sequence encoding a major outer membrane protein (MOMP) of a strain of Chlamydia, preferably C. pneumoniae, and a promoter sequence operatively coupled to the first nucleotide sequence for expression of the MOMP in the host. The other of the vectors comprises a second nucleotide sequence encoding a 76 kDa protein of a strain of Chlamydia, preferably C. pneumoniae, and a promoter sequence operatively coupled to the second nucleotide sequence for expression of the 76 kDa protein in the host. The protection efficiency which is achieved by the immunization procedure is enhanced over that attained with the individual vectors alone.
Owner:AVENTIS PASTUER LTD
Chlamydial vaccines and immunogenic compositions containing an outer membrane antigen and methods of preparation thereof
InactiveUS6635746B1Strong immune responseImproving immunogenicityPeptide/protein ingredientsPeptide preparation methodsDiseaseImmunostimulating Complexes
Immunogenic compositions including vaccines are described that comprise an outer membrane antigen extract of a strain of Chlamydia and are effective in protection against disease caused by Chlamydia infection The immunogenic compositions may comprise the major outer membrane protein (MOMP) of Chlamydia which may be in a homooligomeric form or complexed with at least one other antigen of Chlamydia. The immunogenic composition may include an immunostimulating complex (ISCOM) and the outer membrane antigen may be incorporated therein. The immunogenic compositions have utility as chlamydial vaccines and in diagnostic applications.
Owner:AVENTIS PASTEUR LTD
Vaccines against chlamydial infection
InactiveUS20140056967A1Stimulate immune responseProvide protectionBacterial antigen ingredientsAntibody mimetics/scaffoldsNucleotidePolynucleotide
The present invention relates to compositions comprising proteins or polynucleotides of Chlamydia sp., in particular combinations of proteins or polynucleotides encoding them, and methods for the use of the proteins or polynucleotides in the treatment, prevention and diagnosis of Chlamydia infection.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Methods and Compositions for Chlamydial Antigens for Diagnosis and Treatment of Chlamydial Infection and Disease
ActiveUS20110256094A1Reduce the possibilityReduce morbidityAntibacterial agentsBacterial antigen ingredientsAdjuvantTreatment chlamydia
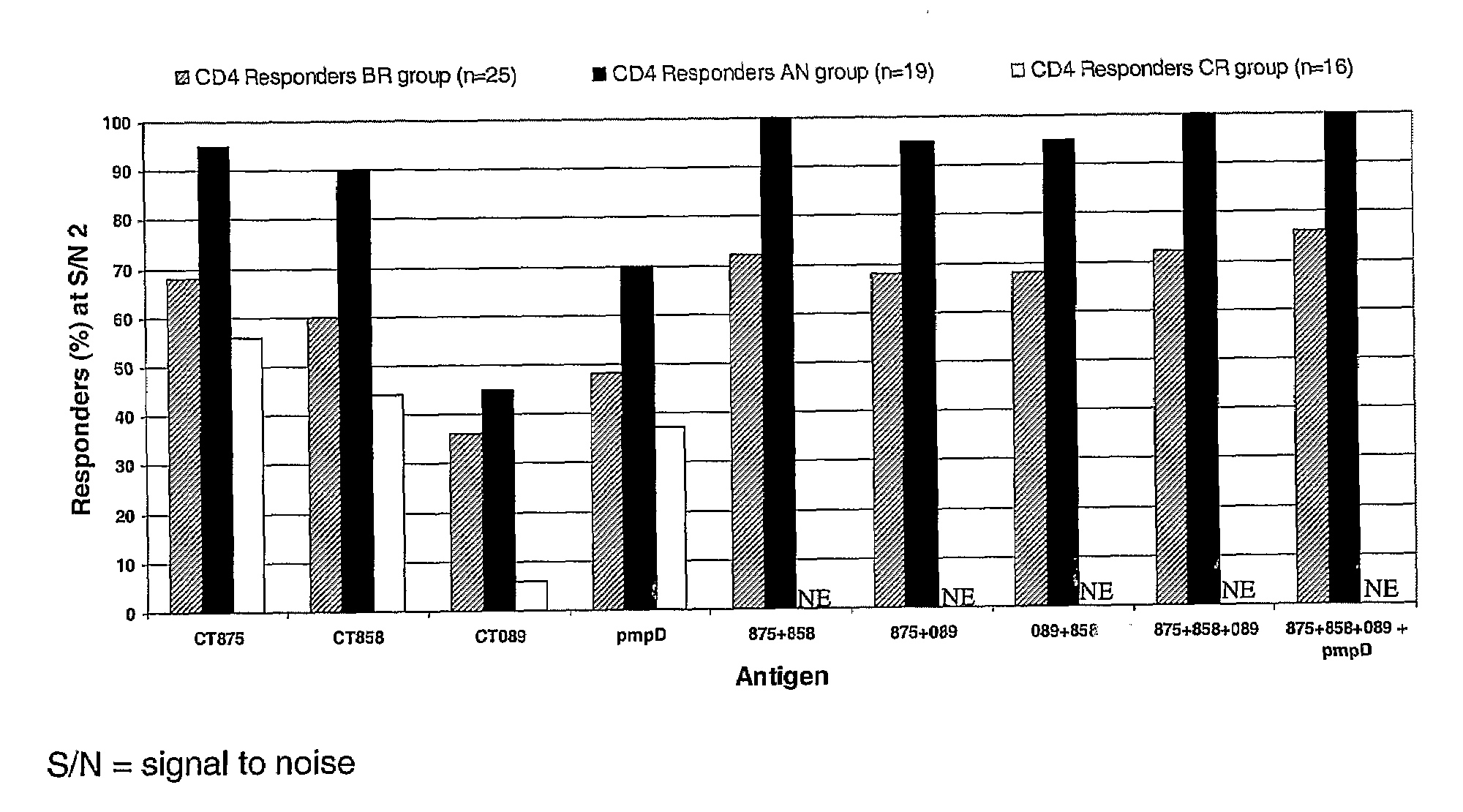
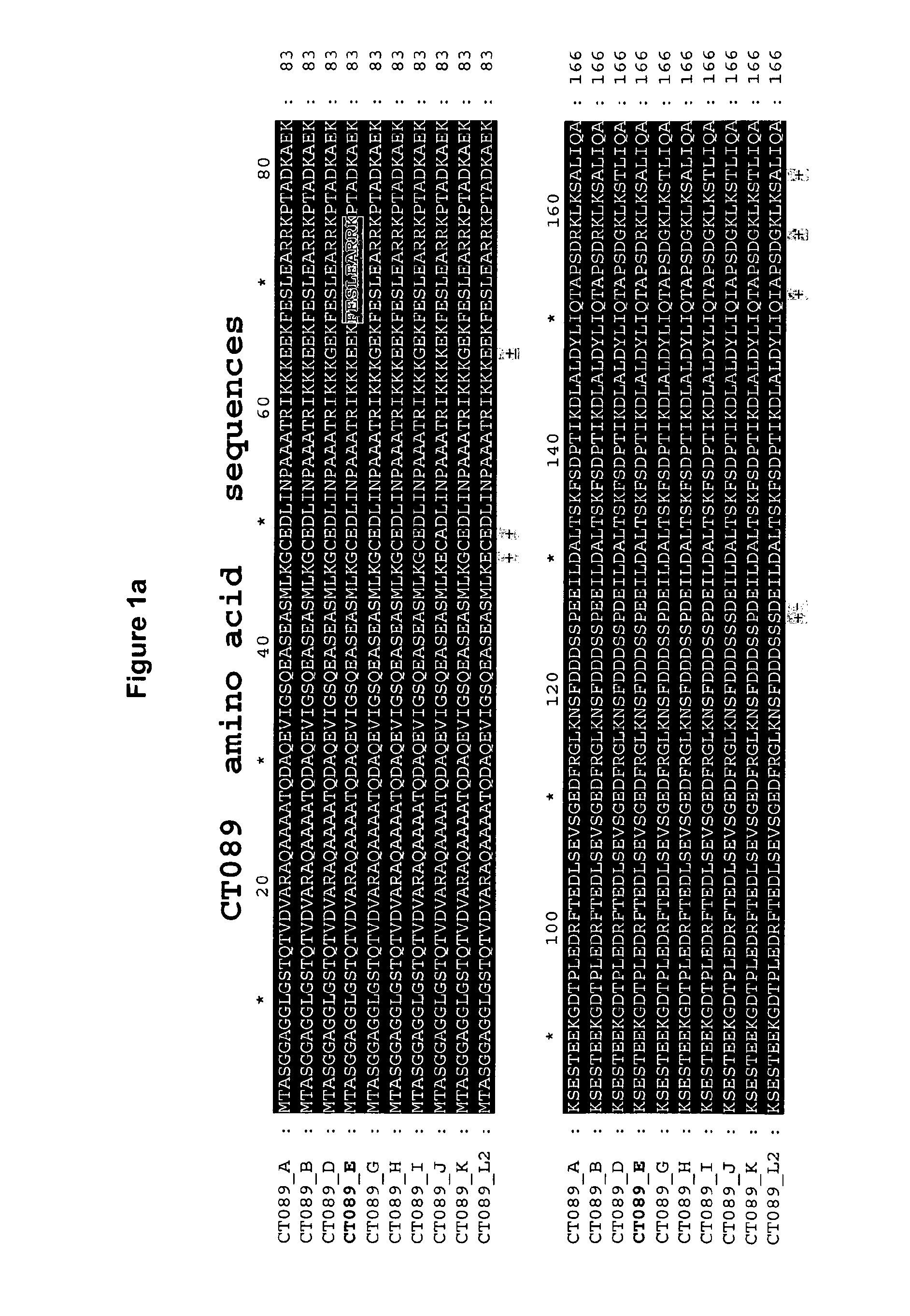
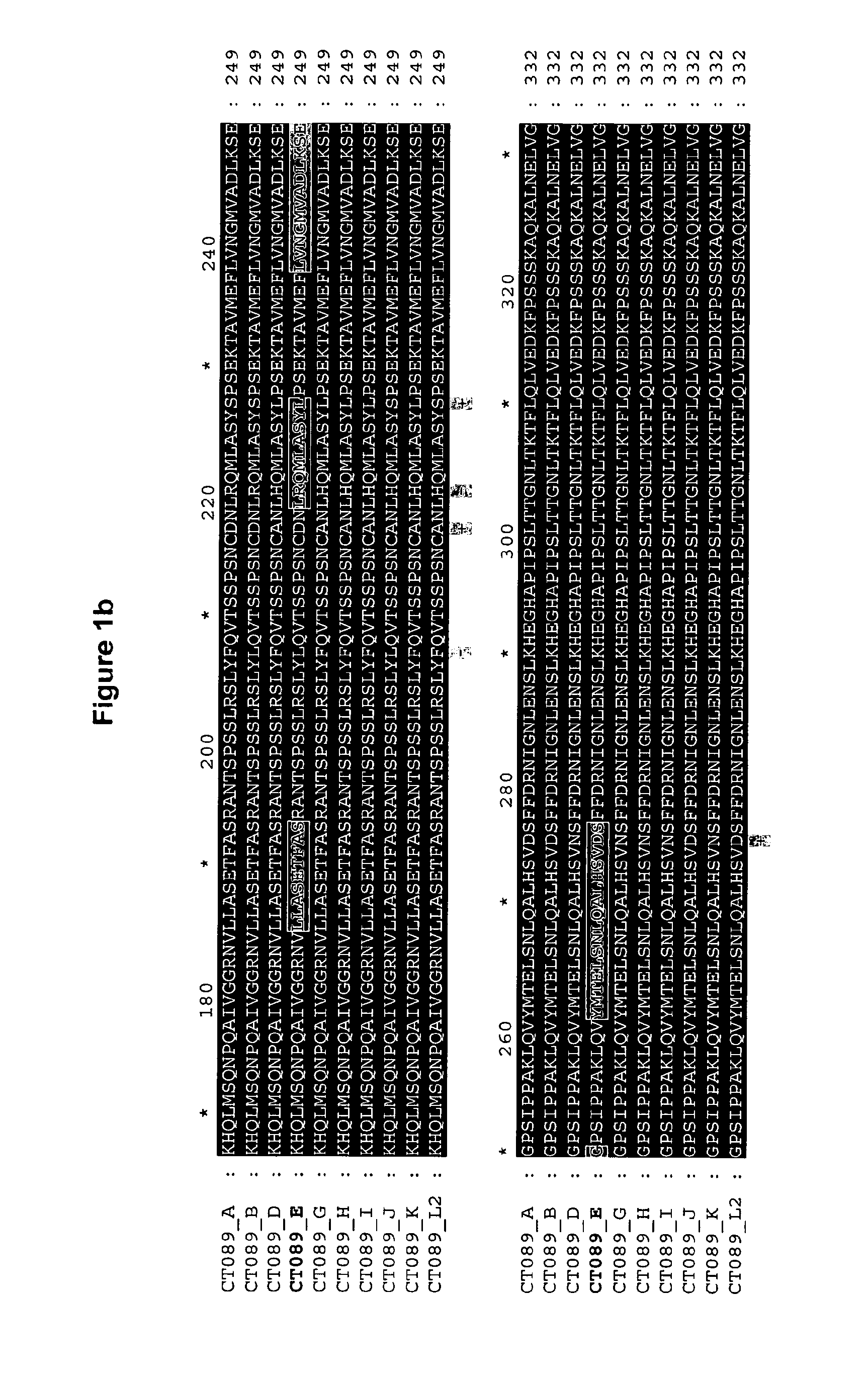
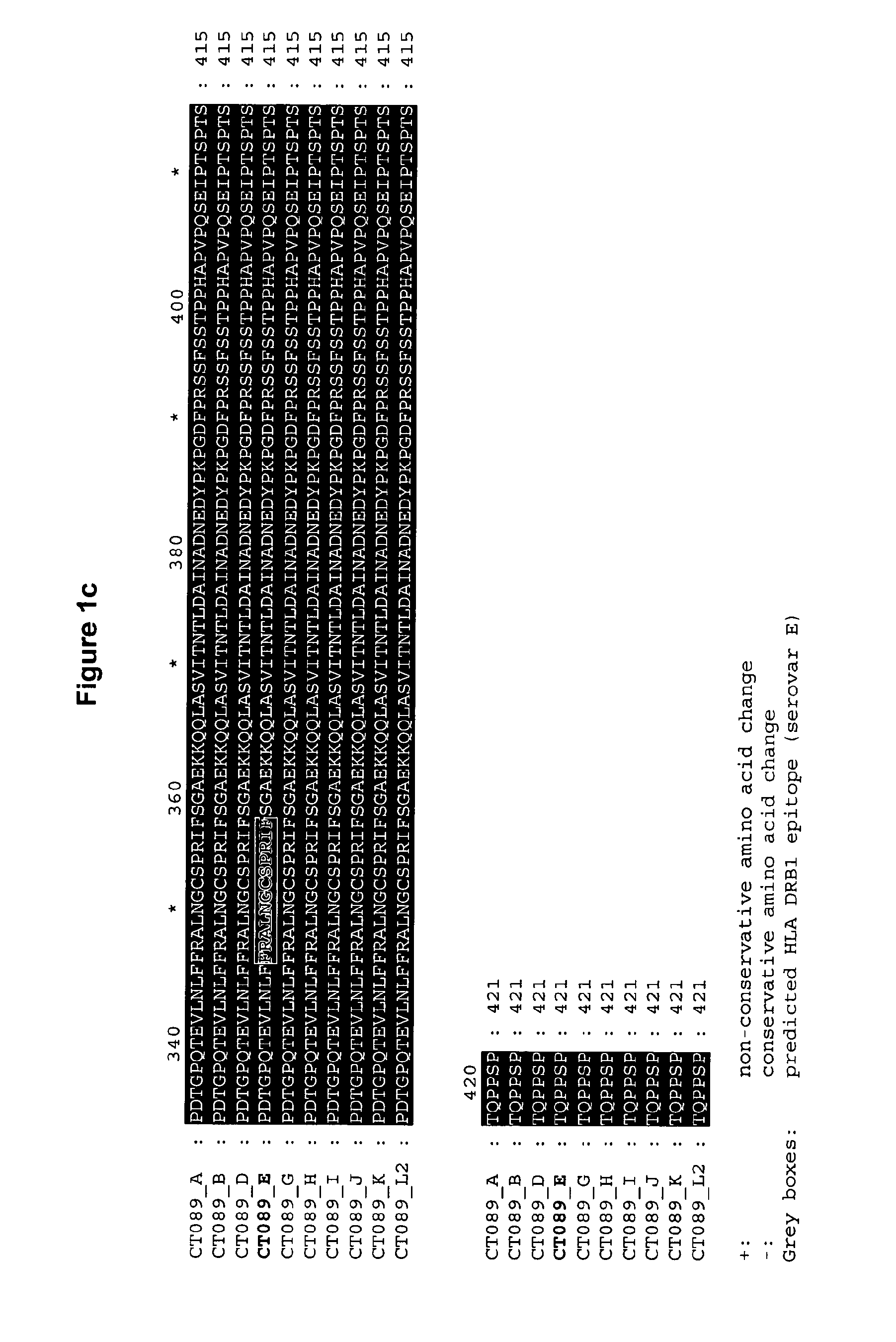
Disclosed are isolated Chlamydia trachomatis proteins, methods of fusion protein and associated antibody production, and methods of using isolated proteins and antibodies in diagnosis and detection. Also disclosed are compositions comprising isolated proteins, wherein the compositions can further comprising pharmaceutically acceptable carriers, an adjuvant and / or an immunostimulant, and methods using the pharmaceutical compositions for treating or preventing an infection by Chlamydia in a subject. The compositions may also comprise a protein or immunogenic fragment of a pathogenic organism other than Chlamydia trachomatis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Photosensitizers and MRI enhancers
InactiveCN101237883AEnergy modified materialsIn-vivo testing preparationsDiabetic retinopathyProstate cancer
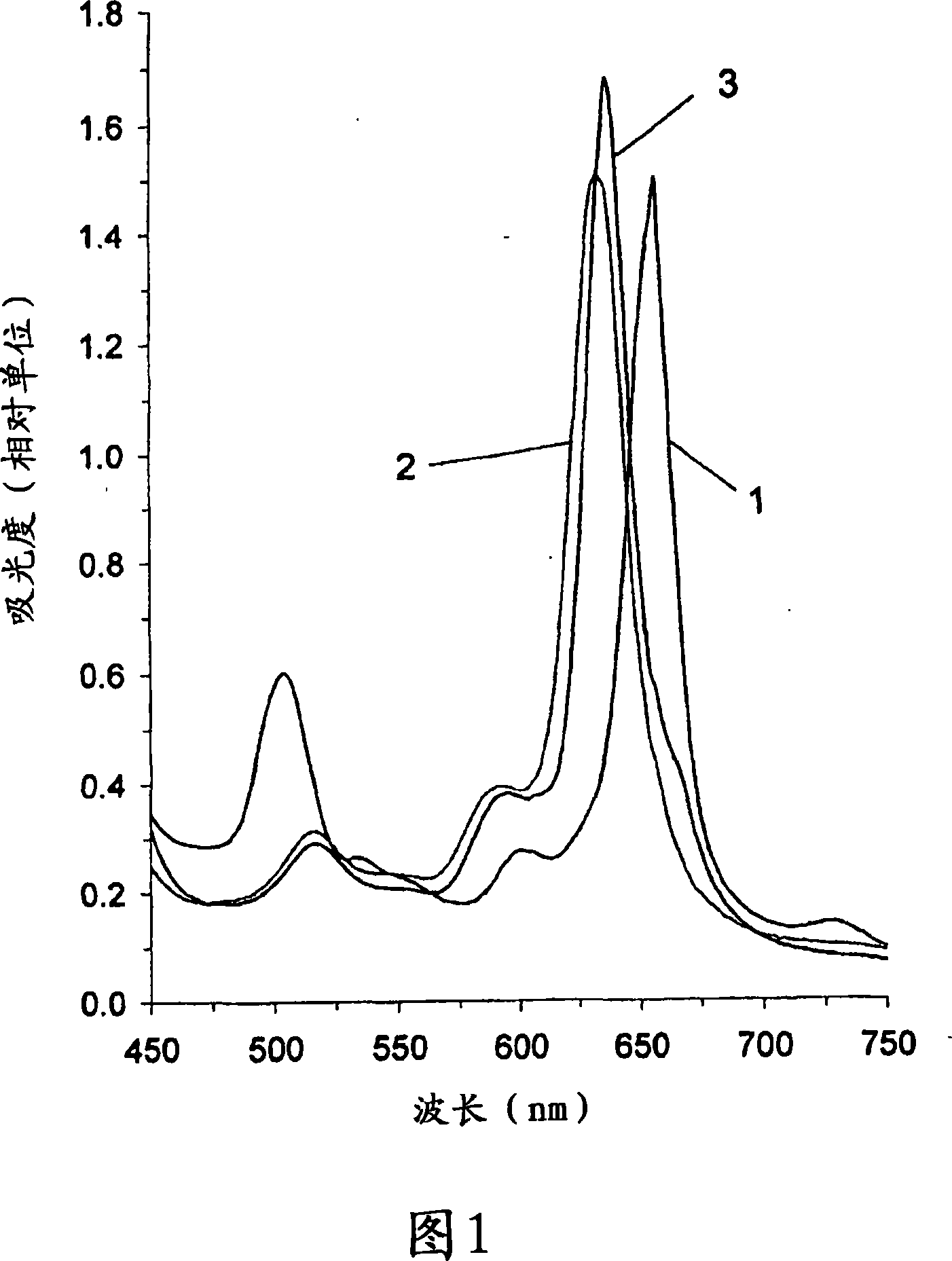
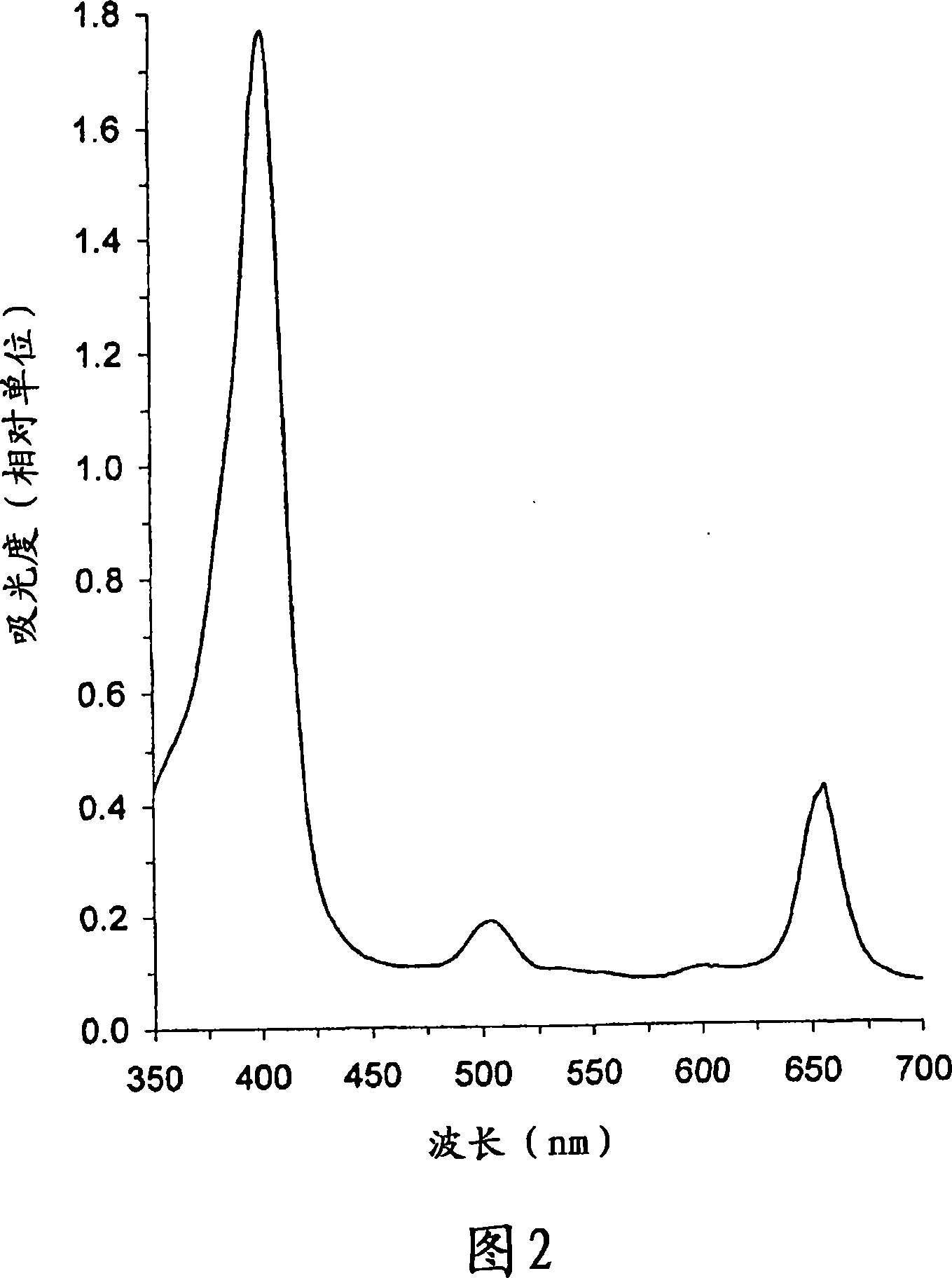
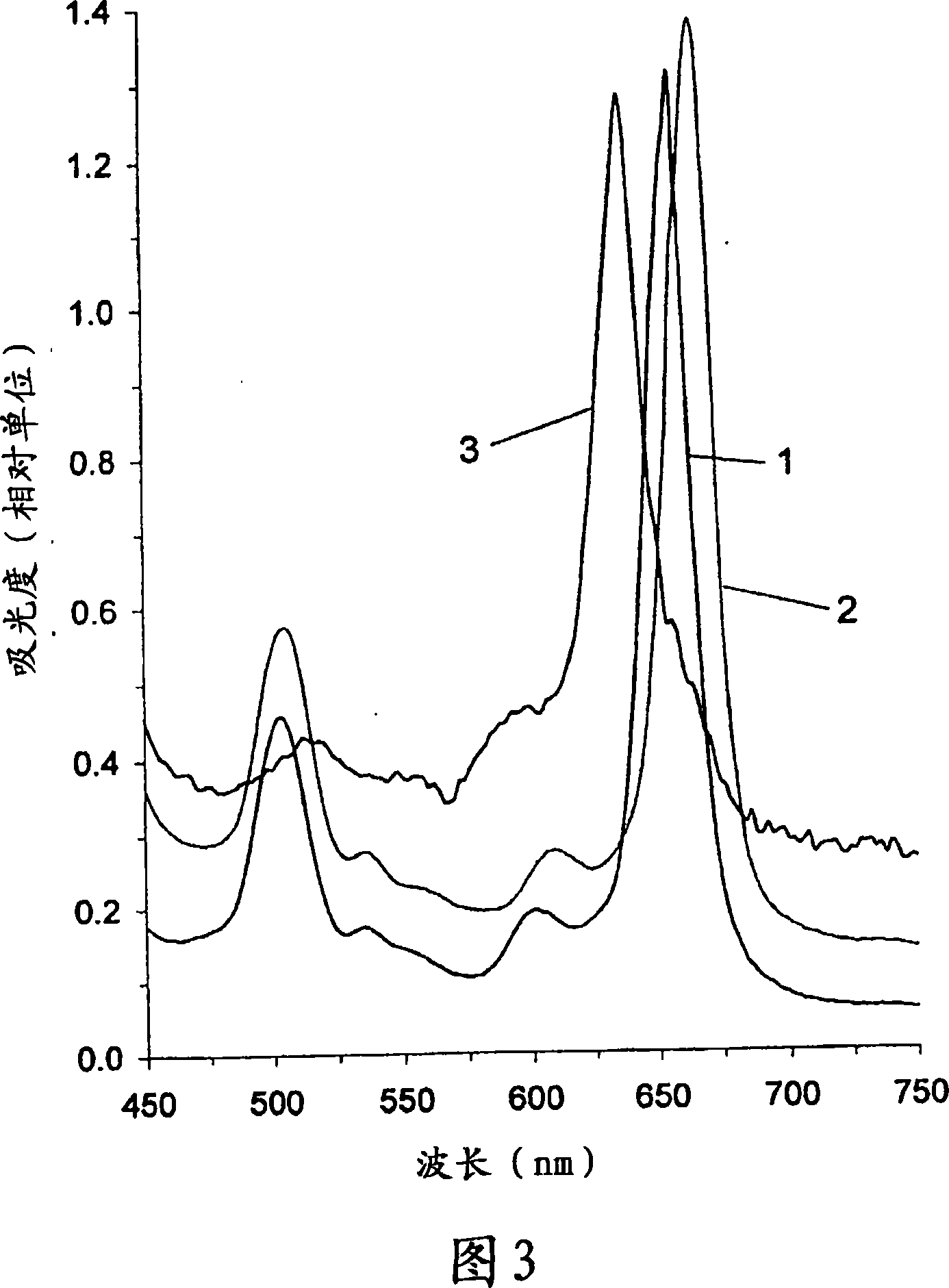
The present invention relates to the use of a compound of formula 3 or a salt thereof to prepare a medicament or phototherapeutic agent for treating the following diseases, including: acne; AIDS; viral hepatitis; diabetic retinopathy; SARS virus infection; coronavirus Arterial stenosis; carotid artery stenosis; intermittent claudication; Asian (chicken) avian influenza virus infection; cervical dysplasia or various cancers, including: blood cancer, cervical cancer, nasopharyngeal cancer, tracheal cancer, laryngeal cancer, bronchial cancer , bronchiolar cancer, bladder cancer, esophageal cancer, stomach cancer, rectal cancer, colon cancer, prostate cancer, hollow organ cancer, bile duct cancer, urinary tract cancer, kidney cancer, uterine cancer, vaginal cancer and other gynecological adnexal cancer. The present invention also relates to methods of treating the above diseases. The present invention further relates to the use of the compound of formula 3 or a salt thereof to prepare a photodiagnostic agent for detecting the above-mentioned diseases and the following diseases, including: atherosclerosis, multiple sclerosis, diabetes, arthritis, rheumatism Arthritis, fungal infections, viral infections, chlamydial infections, bacterial infections, or parasitic diseases, HIV viral infections, hepatitis, herpes simplex, shingles, psoriasis, cardiovascular disease, and skin diseases. The present invention also relates to methods for detecting the above diseases using photodiagnostic agents. The present invention further relates to a method for low-temperature sterilization of surgical devices or other devices, including the steps of: providing a compound of Chemical Formula 3 or a salt thereof on the device; and subjecting the device to radiation treatment or sonication treatment. The invention further relates to a compound of formula 3 or a salt thereof linked or attached to a magnetic element. This compound acts as an MRI enhancer. The invention also relates to the use of such MRI enhancers for performing MRI scans.
Owner:PHOTO DIAGNOSTIC DEVICES PDD
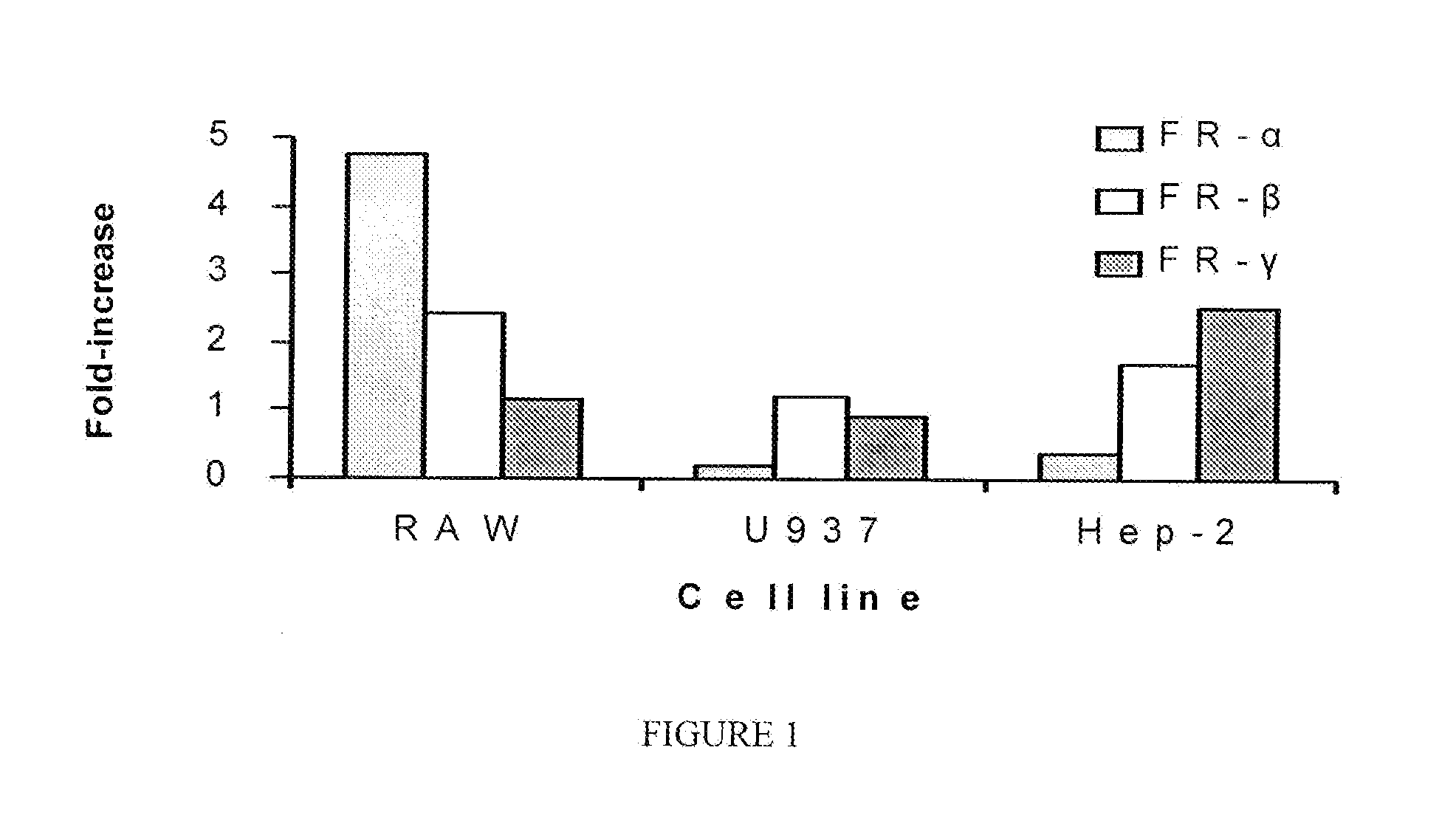
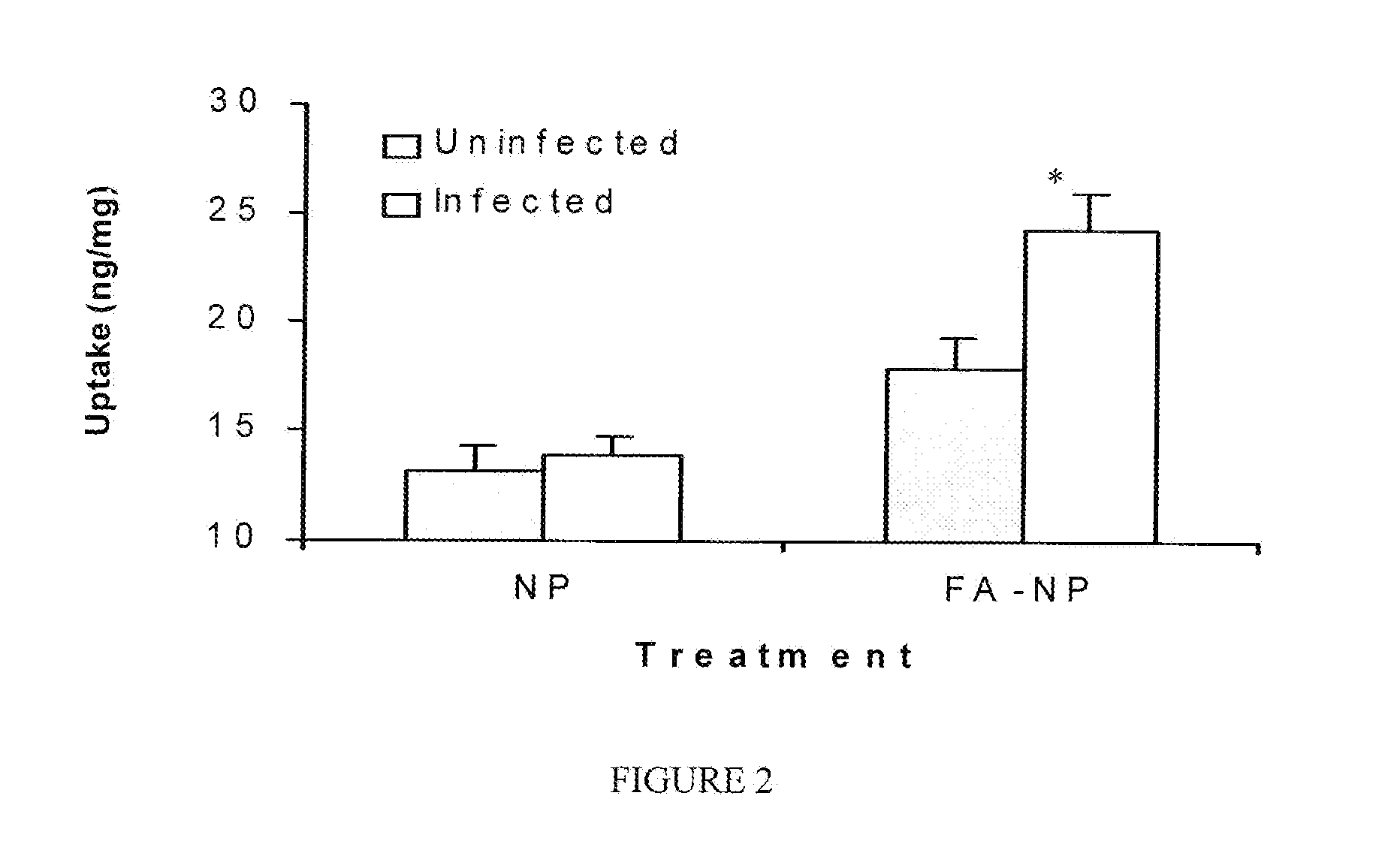
Nanoparticles for imaging and treating chlamydial infection
Compositions of nanoparticles and targeting moieties for imaging and treating Chlamydial infection are provided, including nanoparticles conjugated to folic acid and comprising at least one antibiotic effective against Chlamydia.
Owner:WAYNE STATE UNIV
Methods and Compositions for Chlamydial Antigens for Diagnosis and Treatment of Chlamydial Infection and Disease
InactiveUS20130045181A1Reducing likelihood of infertilityReduce the possibilityAntibacterial agentsChlamydiaceae ingredientsAntigenDisease
The present invention provides Chlamydia proteins and methods of use in treatment and immunization protocols as well as in diagnostic and detection assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Vaccines against chlamydial infection
InactiveUS8541007B2Stimulate immune responseImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsNucleotidePolynucleotide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Methods and Compositions for Treatment of Chlamydial Infection and Related Diseases and Disorders
The present invention provides compositions and methods of use in the treatment / prevention of chlamydial infection and / or diseases and disorders associated with chlamydial infection in a subject.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Chlamydial antigens as reagents for diagnosis and treatment of chlamydial infection and disease
ActiveUS20100119549A1Avoid developmentAvoid infectionAntibacterial agentsBacterial antigen ingredientsAntigenTreatment chlamydia
The present invention provides Chlamydia proteins and methods of use in diagnostic and detection assays as well as in treatment and immunization protocols.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chlamydia vaccines
Vaccine preparations are provided for the prevention of Chlamydia infections comprising a major outer membrane protein from chlamydia and a mucosal adjuvant such as a combination of QS21 and 3D-MPL, or chlorea Toxin or Heat labile enterotoxin. Such preparations provide protection from Chlamydia induced fertility.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Chlamydia Trachomatis Specific Oligonucleotide Sequences
InactiveUS20100248220A1Rapid and selective and specific detectionQuick checkSugar derivativesMicrobiological testing/measurementSpecific detectionOligonucleotide Primer
The present invention relates to oligonucleotide sequences for amplification primers and detection probes and to their use in nucleic acid amplification methods for the selective and specific detection of Chlamydia trachomatis in biological samples. The invention also provides oligonucleotide primer sets and primer / probe sets in the form of kits for the detection and diagnosis of chlamydial infection. The inventive oligonucleotide primers and probes can also be used in combination with other specific oligonucleotide primers and probes for the simultaneous detection of Chlamydia trachomatis and other target organisms, such as Neisseria gonorrhea.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
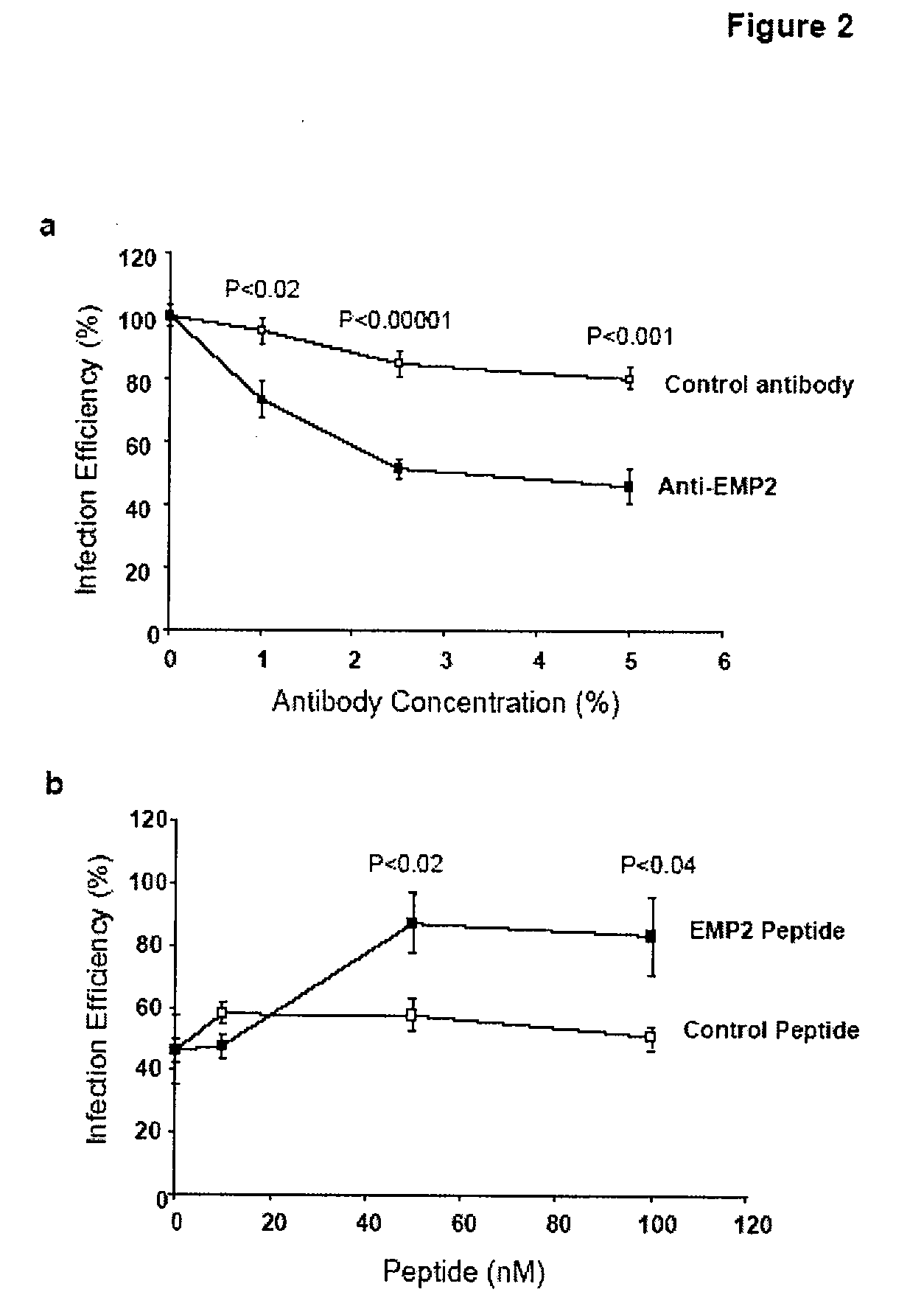
Prevention of chlamydia infection using a protective antibody
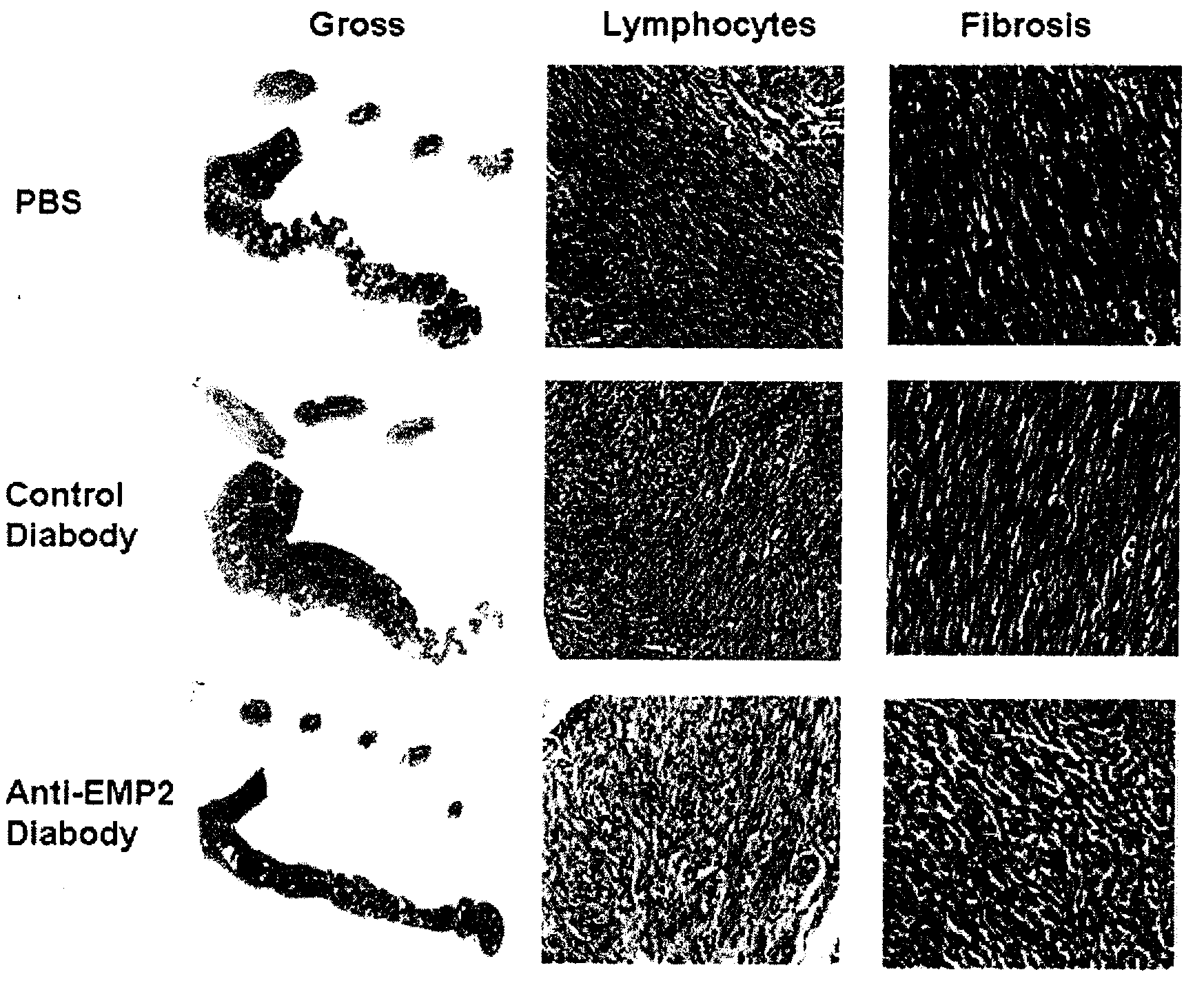
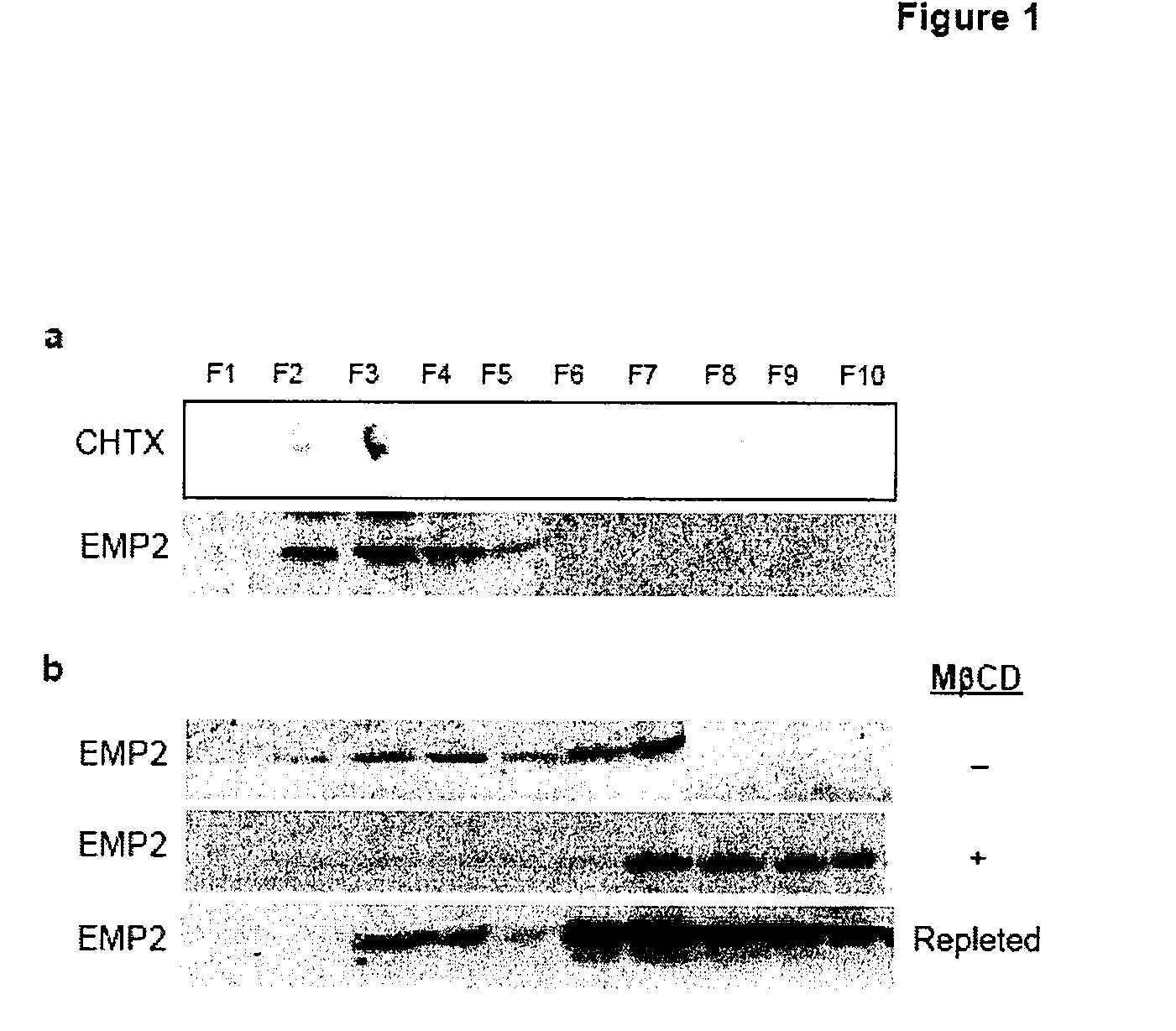
InactiveUS20080181889A1InhibitionBiocideOrganic active ingredientsChlamydia virusCHLAMYDIAL INFECTIONS
The present invention provides methods and compositions useful in the treatment or prevention of Chlamydia infections. The methods and compositions inhibit the entry of Chlamydia into a host cell expressing EMP2 by interfering with the interaction between the Chlamydia and EMP2. The compositions include EMP2 nucleic acids and polypeptides as well as anti-EMP2 antibodies.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com