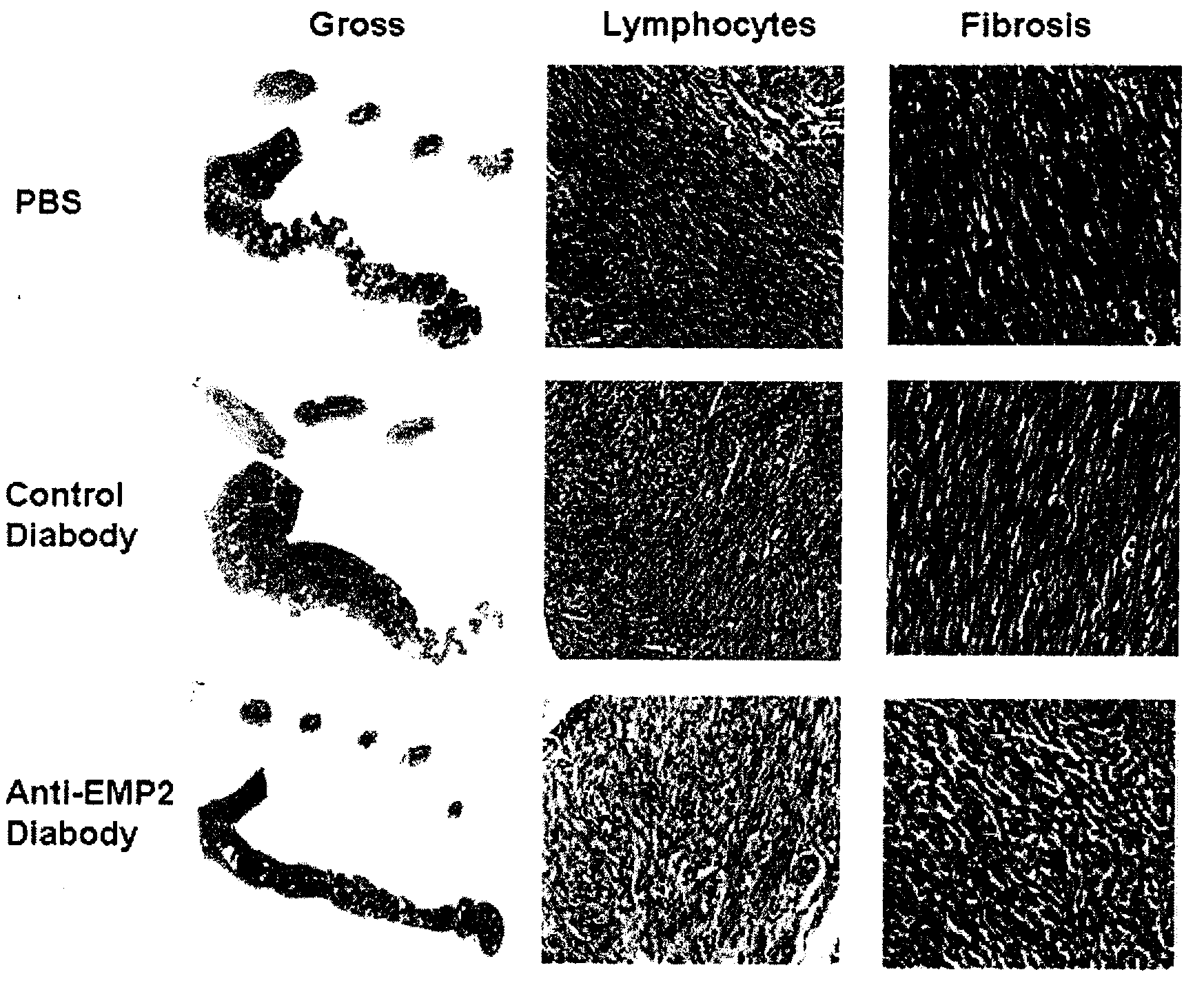
Prevention of chlamydia infection using a protective antibody
a technology of chlamydia infection and protective antibody, which is applied in the field of new anti-chlamydia antibodies, can solve the problems of serious pneumonia, chlamydia infections are particularly serious health threats to newborns, and the risk of tubal obstruction and infertility in the pid, so as to inhibit the ability of chlamydia to bind and inhibit the binding of emp2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods for Studying Chlamydial Infectivity and EMP2 Chlamydial Inhibitors
[0138]A. Endometrial cell lines and Chlamydia strains. The human endometrial adenocarcinoma cell line HEC1A (HTB112, ATCC, Manassas, Va.) was cultured in McCoy 5a media (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal calf serum (Hyclone, Logan, Utah) at 37° C. in a humidified 5% CO2 and passaged every 7 days. EMP2-modulated HEC1A sublines were stable transfectants with expression plasmids for GFP, a human EMP2-GFP fusion protein, or a human EMP2-specific ribozyme (HEC1A-GFP, HEC1A-hEMP2, and HEC1A-hRV2). EMP2 protein expression levels relative to HEC1A-GFP were 1.0, 8.7, and 0.2, respectively.
[0139]An 8-strain mix of human C. trachomatis (serovars D, E, F, and K) and C. muridarum were purified, aliquoted, and stored at −80° C. until ready for use (see, Caldwell, H. D., et al., Infect Immun 31, 1161-76 (1981). All Chlamydia samples were made in Eagle MEM (Invitrogen) with 10% fetal calf serum (Atlant...
example 2
Localization of EMP2 to Lipid Rafts
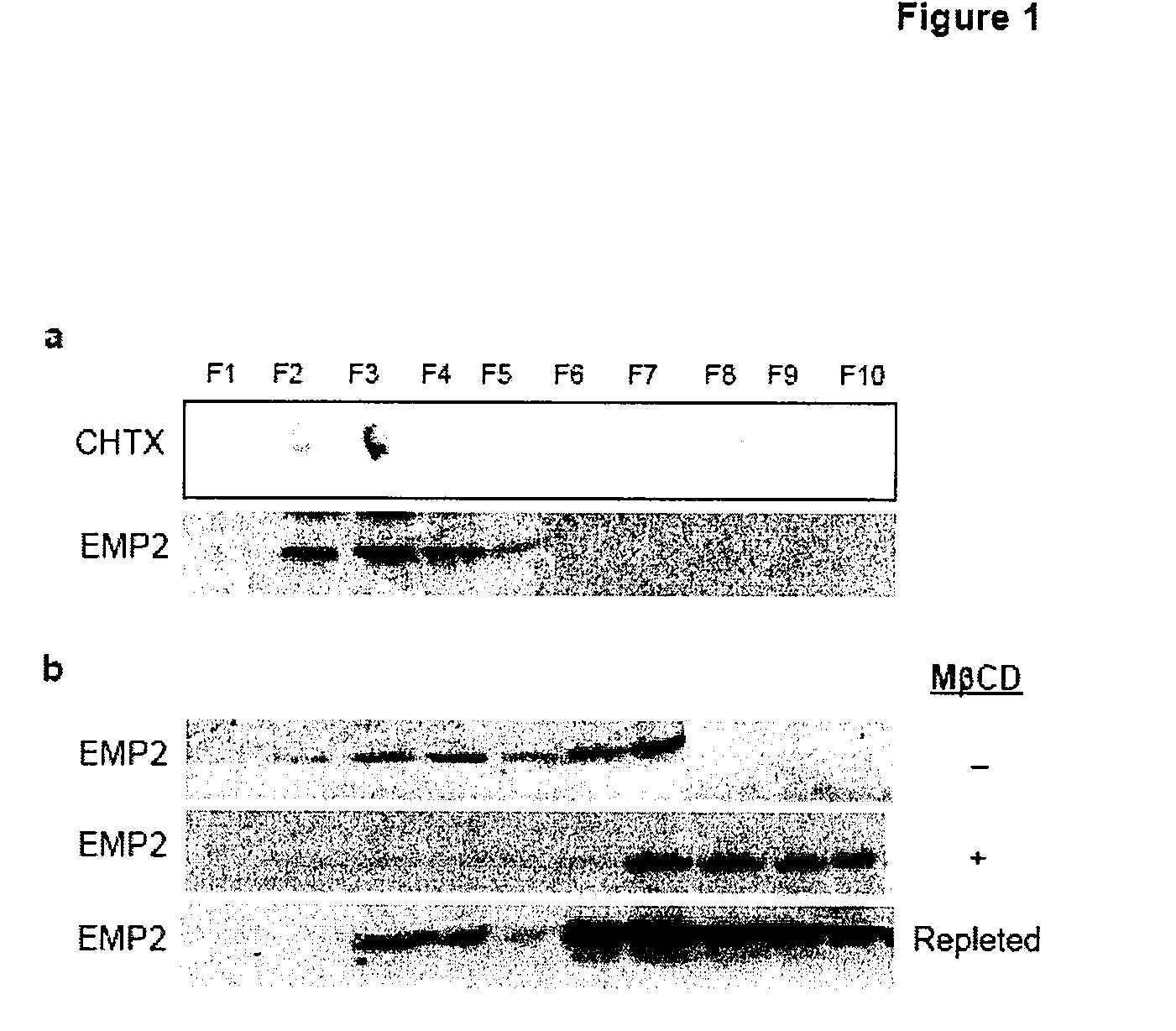
[0147]To assess whether EMP2 is localized to lipid raft domains in endometrial cells (a Chlamydial host target), EMP2 was evaluated in the HEC1A human endometrial cancer cell line by lipid raft fractionation with Brij 58 and Triton X-100. In HEC1A cells, EMP2 localized to the light, detergent-resistant gradient fractions coinciding with GM1 ganglioside, a lipid raft component (FIG. 1a,b). To confirm the localization of EMP2 to lipid rafts, lysates were prepared in 1% Triton X-100 in the presence or absence of methyl-β-cyclodextrins (MβCD) that selectively deplete cholesterol from cellular membranes and causes loss of protein localization into lipid rafts. In 1% Triton X-100, EMP2 localized to both light, detergent-resistant fractions 3-4 as well as dense fractions 6-7 (FIG. 1c). When cells were incubated for 60 minutes with MβCD in serum free conditions, EMP2 expression completely shifted to soluble, dense fractions in the presence of 1% Triton X-1...
example 3
Effect of Anti-EMP2 Antibody on Chlamydia Infectivity
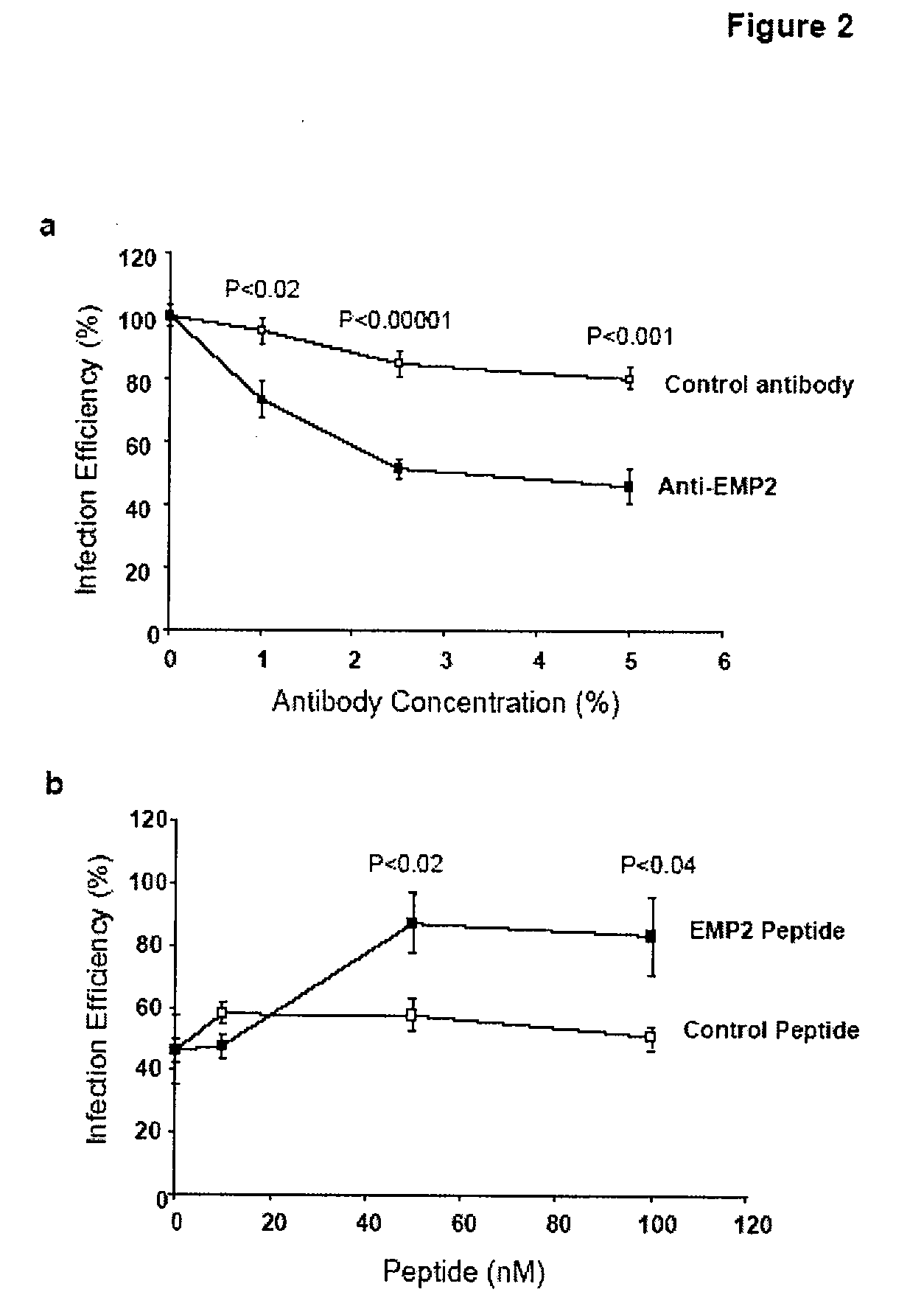
[0148]The lipid raft localization of EMP2 in endometrial cells and its control of lipid raft trafficking by integrins, caveolins, and glycosylphosphatidyl inositol-linked proteins (18, 20, 21), raised the possibility that EMP2 might directly or indirectly affect Chlamydial infectivity. To begin testing this hypothesis, anti-EMP2 antibody (specific for the 2nd extracellular loop of EMP2) was added to HEC1A cell cultures, then incubated with C. trachomatis, and infection was measured (Chlamydial inclusions, expressed as “infection efficiency”, % inclusions relative to HEC1A cells without antibody treatment) (FIG. 2a). Anti-EMP2 antibody produced a dose-dependent inhibition of infection efficiency (reaching less than 50% of HEC1A cells without antibody), at levels that were highly significant compared to control antibody. To determine if the observed inhibition was due to EMP2 specificity, anti-EMP2 was pre-incubated with the relevan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com