Patents
Literature
149 results about "Protective antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protective antibody. An antibody produced in response to an infectious disease. antibody. specialized serum proteins produced by B lymphocytes in response to an immense number of different antigens (>10 7) to which an animal may be exposed.
Novel proteosome-liposaccharide vaccine adjuvant
InactiveUS20030044425A1Increase secretionUniform processSsRNA viruses negative-senseBiocideImmunotherapeutic agentCytokine
An adjuvant complex composed of bacterial outer membrane protein proteosomes complexed to bacterial liposaccharide is prepared to contain the component parts under a variety of conditions. The complex can be formulated with antigenic material to form immunogenic compositions, vaccines and immunotherapeutics. An induced immune response includes protective antibodies and / or type 1 cytokines is shown for a variety of protocols.
Owner:ID BIOMEDICAL CORP LAVAL
Porcine circovirus and Helicobacter combination vaccines and methods of use
InactiveUS20060029617A1Viral antigen ingredientsMicrobiological testing/measurementDiseasePorcine Circoviruses
The present invention is based on the discovery of novel species of the genus Helicobacter that are associated with gastro-esophageal ulceration in pigs. In particular, a novel species, H. cerdo, has been used as a source of antigenic material for the development of vaccine for the treatment of the gastro-esophageal disorders. Most advantageously, the novel Helicobacter and the porcine circoviruses associated with PMWS in pigs are useful for providing combination vaccines whereby immunogens derived from both types of pathogens may be codelivered to the target animal to stimulate the generation of protective antibodies and immunity. The invention, therefore, provides vaccines that are useful for the tratment of gastro-esophageal ulceration and PMWS in porcines. The present invention includes, therefore, multivalent immunogenic compositions and vaccines, multivaccine kits, and combined immunization or vaccination methods which make it possible to use such combined immunization or vaccination programmes.
Owner:MERIAL LTD
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Water-in-oil-in-water adjuvant vaccine and preparation method thereof
ActiveCN103223164APromote absorptionGenerate fastAntiinfectivesAntibody medical ingredientsEngineeringImmunity response
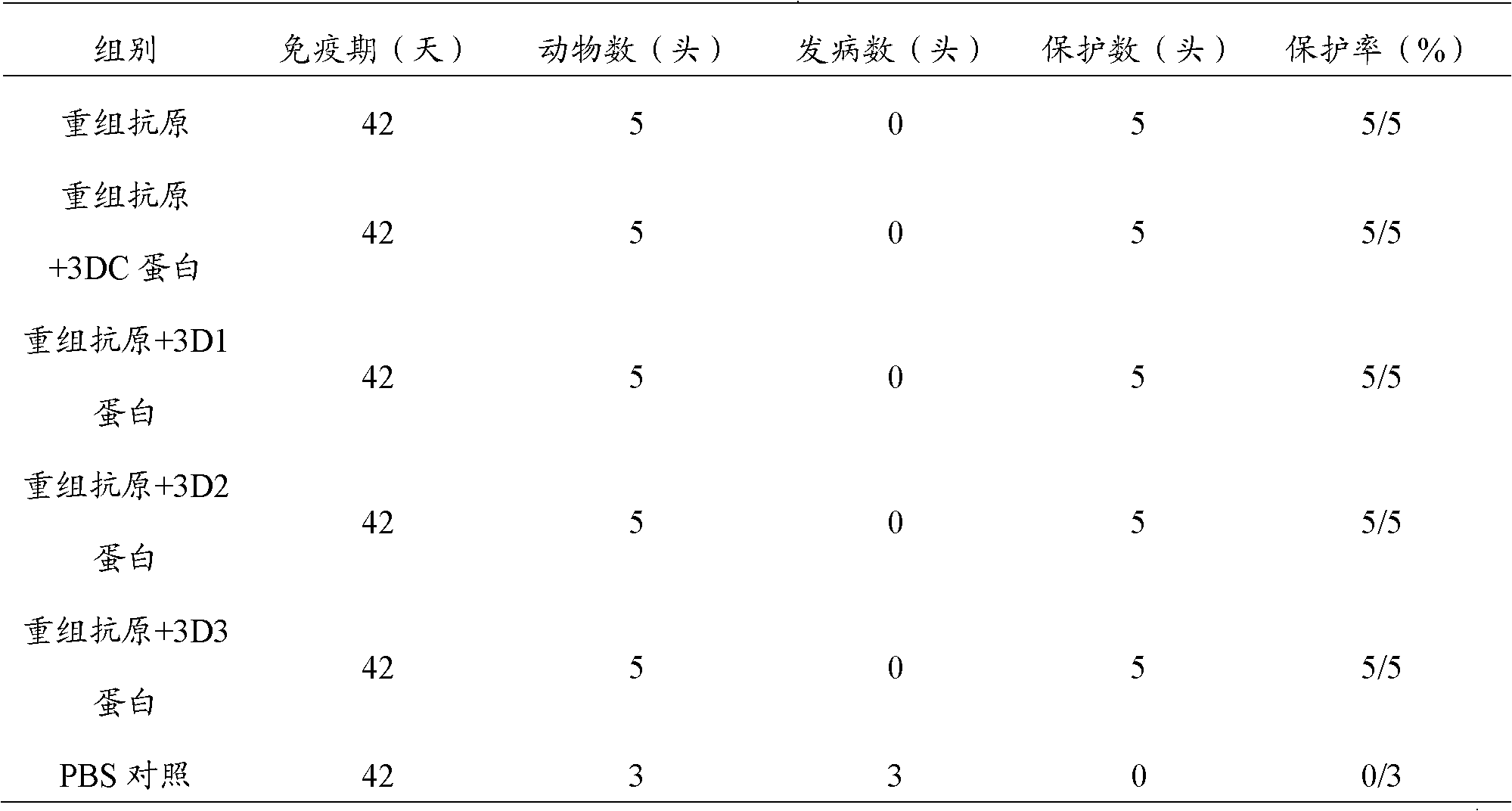
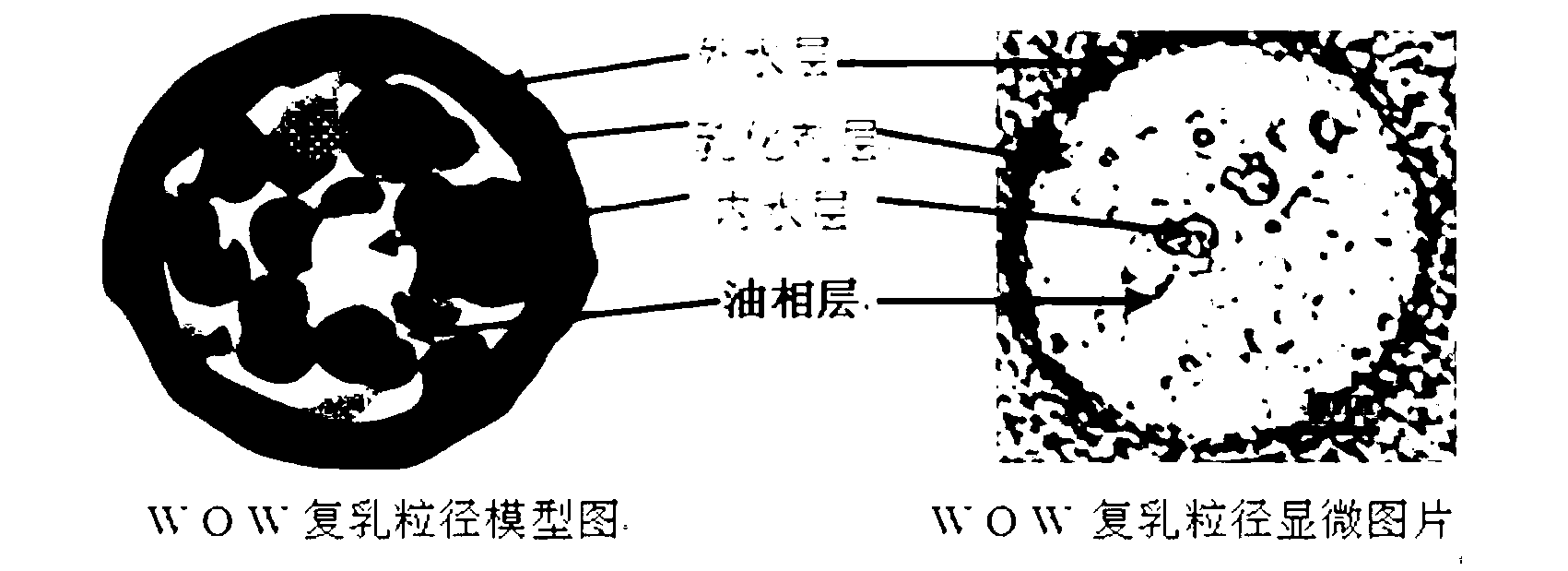
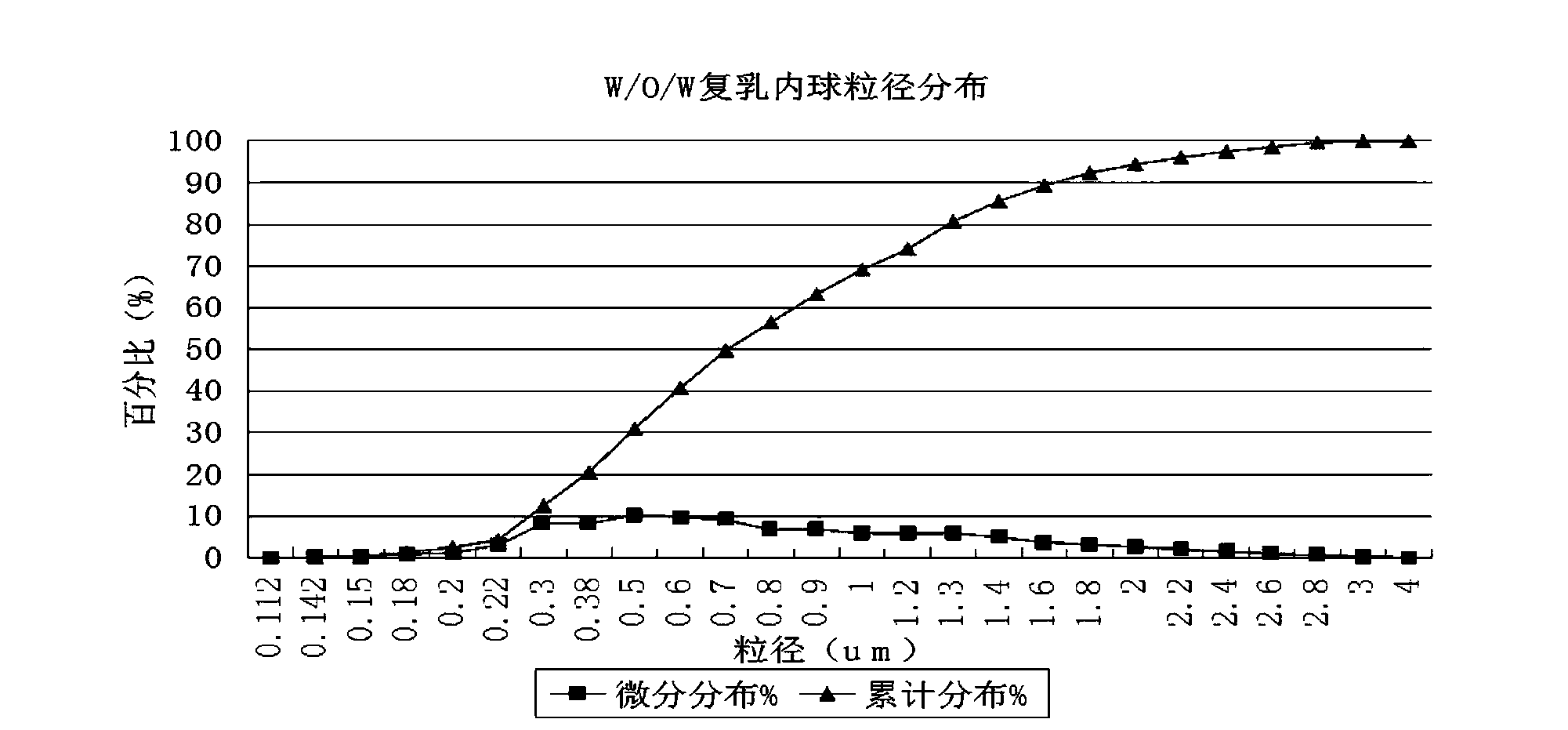
The invention discloses a water-in-oil-in-water adjuvant vaccine and a preparation method thereof. The adjuvant vaccine is composed of an internal water phase, a middle oil phase used for cladding the internal water phase, and an external water phase for cladding the middle oil phase. The external water phase comprises: a composite surfactant, a hydrophilic surfactant and mormal saline. The middle oil phase consists of: a lipophilic surfactant, a stabilizer and mineral oil. The internal water phase comprises: an immunopotentiator solution, the hydrophilic surfactant, and an inactivated antigen solution. The W / O / W (water-in-oil-in-water) type adjuvant vaccine provided in the invention can induce an immunoreaction earlier than traditional W / O (water-in-oil) type adjuvant vaccines, and can make experimental animals obtain earlier protective antibodies. The vaccine has good absorption, and causes a small inflammatory response at an inoculation site. The adjuvant vaccine disclosed in the invention has better stability than existing W / O / W type vaccines, and the antibody generation speed is fast.
Owner:SOUTH CHINA AGRI UNIV +1
Genotype VII Newcastle disease virus marker vaccine strain and application thereof
ActiveCN104988124AHigh growth titerHigh biological propertiesViral antigen ingredientsMicroorganism based processesViral MarkersChick embryos
The invention discloses a genotype VII Newcastle disease virus marker vaccine strain and an application thereof, and belongs to the field of genotype VII Newcastle disease virus marker vaccine strain rescue and application. A built Newcastle disease virus reverse genetic operating platform is utilized for enabling NP protein of a G7 strain to miss 18 amino acids and conducting mutation on F-protein cleavage loci, and the highly-weak virulence and high-virus titer genotype VII Newcastle disease virus marker vaccine strain MG7-NPdelta18+Fmut is rescued through screening. The microbial preservation serial number is CCTCC NO: V201505. The marker vaccine strain has the biological characteristics of high growth titer and low virulence in chick embryos and is genetically stable. The immune protection test result shows that the marker vaccine strain is good in immunogenicity, capable of inducing high-level protective antibodies, and capable of completely protecting immunized chicken, can be used for preventing and controlling a currently-popular genotype VII Newcastle disease virus and lays the foundation of identifying vaccine immunity and wild virus infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Cross-protective pathogen protection, methods and compositions thereof
ActiveUS20130129747A1Inhibition of activationSure easySsRNA viruses negative-senseViral antigen ingredientsImmunogenicityInfluenza a
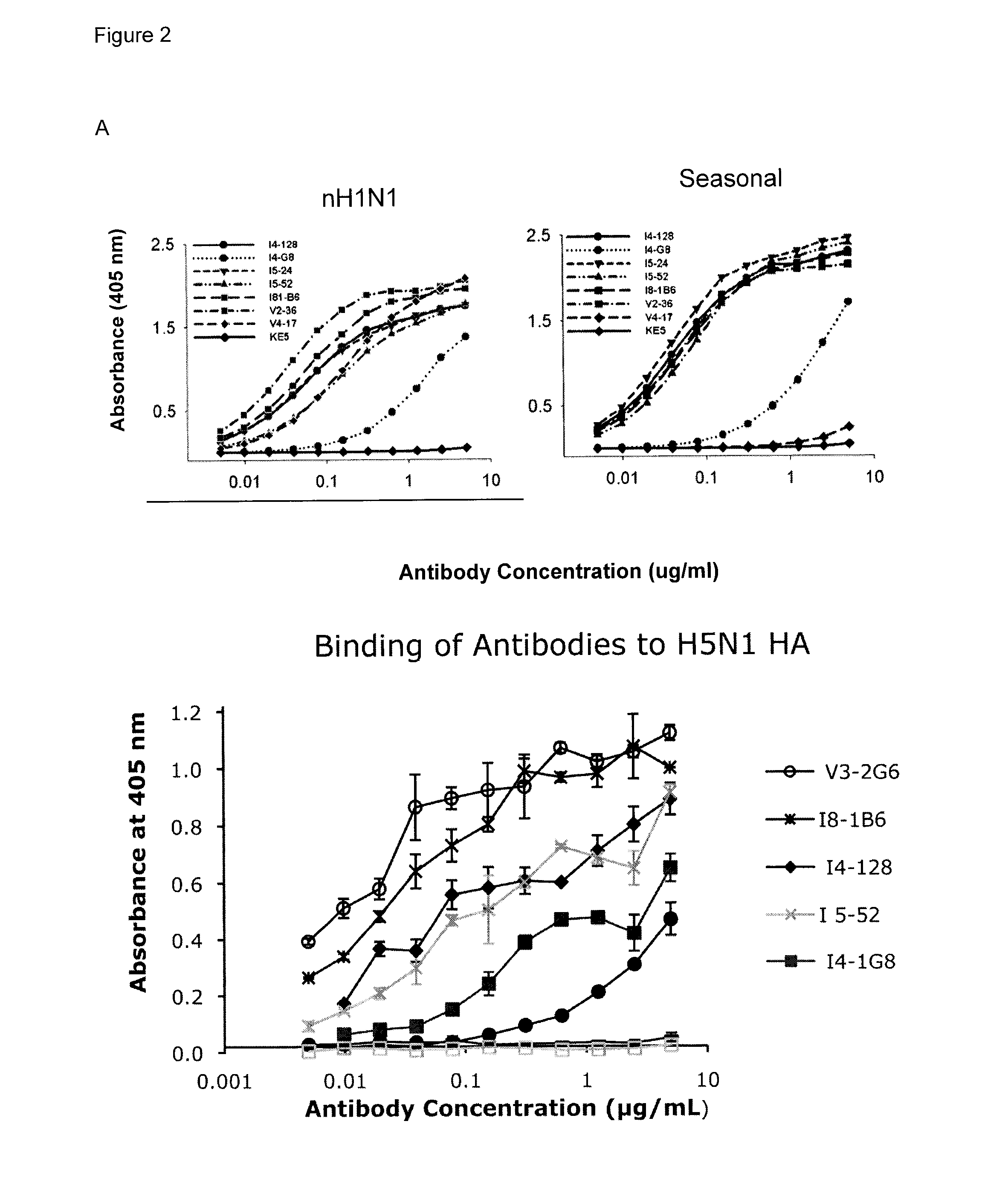
The present disclosure provides a method of inducing a cross-protective immune response in a subject against a pathogen, such as influenza, comprising administering a first unique pathogen antigen to the subject; and administering a second unique pathogen antigen 3-52 weeks after a); wherein the second unique pathogen antigen and the first unique pathogen antigen are immunologically distinct but share conserved sites that are not normally immunogenic for antibodies. Also disclosed herein are assays for detecting cross-protective antibodies, methods of generating novel cross-protective antibodies. Further provided are novel antibodies against influenza.
Owner:SCHRADER SABARIAH
Transformed bacteria producing CS6 antigens as vaccines
Disclosed herein are antigens that stimulate protective antibodies against enterotoxigenic Escherichia coli. Also disclosed herein are proteins encoded by cssA and cssB genes as well as constructs containing the genes and methods of using thereof.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Novel compositions and uses therefor
ActiveUS20040241177A1Increase ratingsMore responsiveViral antigen ingredientsGenetic material ingredientsLong latencyProtection sex
The invention is directed to the use of (i) a first antigen corresponding to a target antigen of interest, together with (ii) a second antigen, corresponding to a modified form of the target antigen, whose rate of intracellular proteolytic degradation is increased, enhanced or otherwise elevated relative to the first antigen, in compositions and methods for inducing both humoral and cellular immunity in an individual. The ability to provide compositions, which are capable of inducing both host-protective antibody and cell-mediated immune responses, facilitates the generation of immunogenic compositions capable of combating, inter alia, conditions that have long latency periods and, therefore, benefit from the dual approach of prophylaxis and therapy in one delivery.
Owner:JINGANG MEDICINE AUSTRALIA PTY LTD
Protective antigen of Epstein Barr Virus
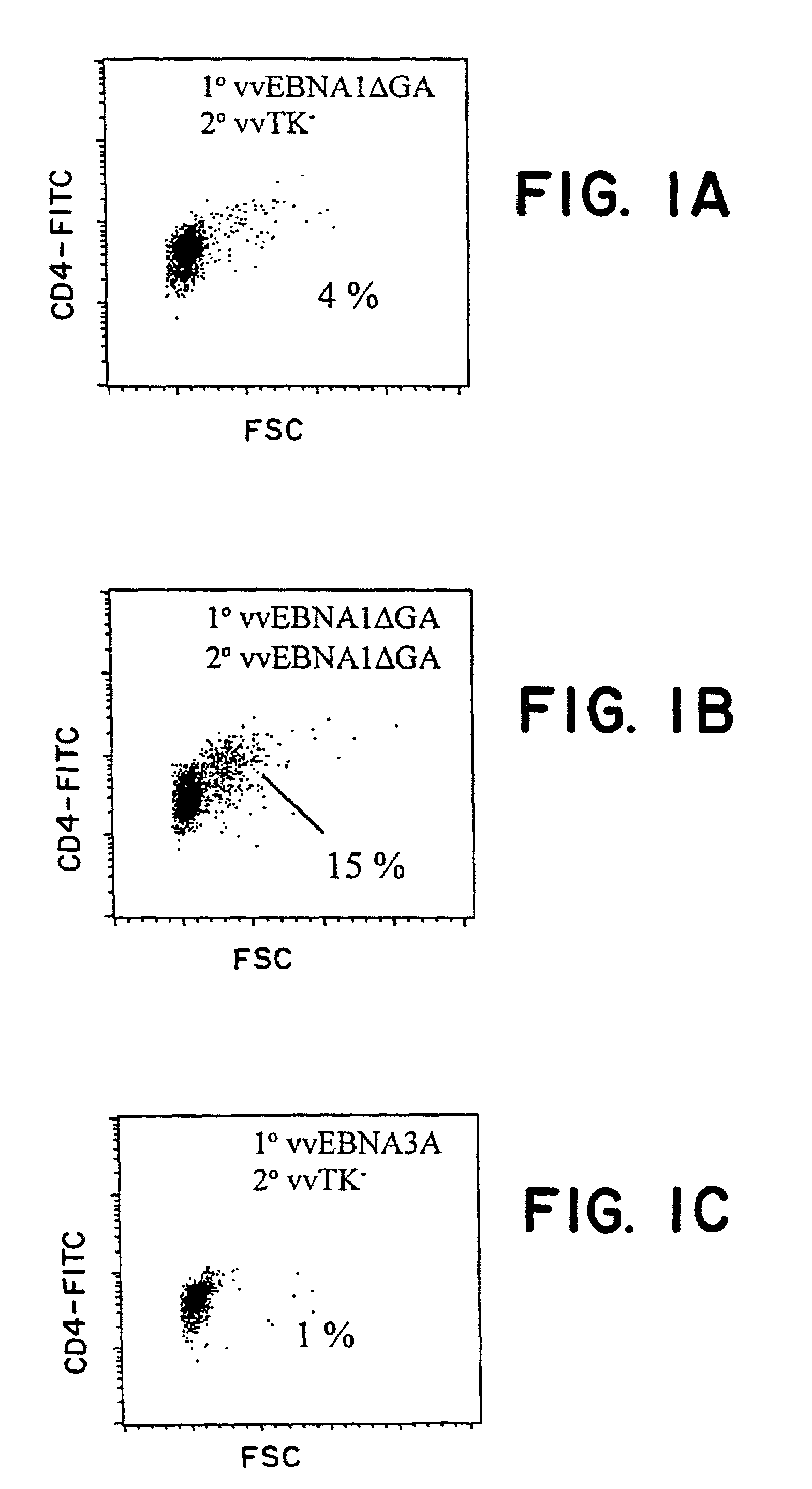
The present invention relates to the identification of a subunit vaccine to prevent or treat infection of Epstein Barr Virus. In particular, EBNA-1 was identified as a vaccine antigen. In a specific embodiment, a purified protein corresponding to EBNA-1 elicited a strong CD4+ T cell response. The responsive CD4+ T cell are primarily TH1 in function. EBNA-1 is an attractive candidate for a protective vaccine against EBV, and for immunotherapy of EBV infection and neoplasms, particularly with dendritic cells charged with EBNA-1.
Owner:THE ROCKEFELLER UNIV
High-level expression of tetanus toxin receptor binding domain Hc in Escherichia coli and application
The invention relates to a method for a tetanus toxin receptor binding domain Hc to be subjected to high-level soluble expression in Escherichia coli through nucleotide sequence optimization. According to the sequencing result of a domestic C.Tetani virulent strain CMCC64008, the tetanus toxin receptor binding domain Hc sequence is analyzed and optimized, the optimized sequence is SEQ ID No.1 and the coded protein sequence is SEQ ID No.2. The synthesized Hc gene is linked into an expression vector pET32a(+) after undergoing double enzyme digestion, the recombinant Hc is subjected to high soluble expression in Escherichia coli and the target protein accounts for about 46% of the total protein in the supernatant undergoing bacteriociasis. After QFF column purification, phenyl hydrophobic column purification and SP column purification, the purity of the target protein can be more than 95% and the yield thereof is more than 300mg / L. The recombinant protein prepared by the method of the invention has good immunogenicity, can induce the mice to produce high-titre protective antibodies and can resist attack of high-dose lethal toxins. The method has extensive application prospect in large-scale high-level preparation of the tetanus toxin recombinant subunit vaccine Hc.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Respiratory syncytial virus vaccines expressing protective antigens from promoter-proximal genes
InactiveUS6923971B2Altered immunogenicitySmall sizeSsRNA viruses negative-senseSugar derivativesProtective antigenGene Position
Recombinant respiratory syncytial virus (RSV) having the position of genes shifted within the genome or antigenome of the recombinant virus are constructed by insertion, deletion or rearrangement of genes or genome segments within the recombinant genome or antigenome and are useful for eliciting an anti-RSV immune response. Shifting the position of genes in this manner provides for a selected increase or decrease in expression of the gene. In one embodiment, expression of RSV glycoproteins is upregulated by shifting one or more glycoprotein-encoding genes to a more promoter-proximal position. Genes of interest for manipulation to create gene position-shifted RSV include any of the NS1, NS2, N, P, M, SH, M2(ORF1), M2(ORF2), L, F or G genes or a genome segment that may be part of a gene or extragenic. Additional mutations and nucleotide modifications are provided within gene position-shifted RSV to yield desired phenotypic and structural effects.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE +1
RPA optimization
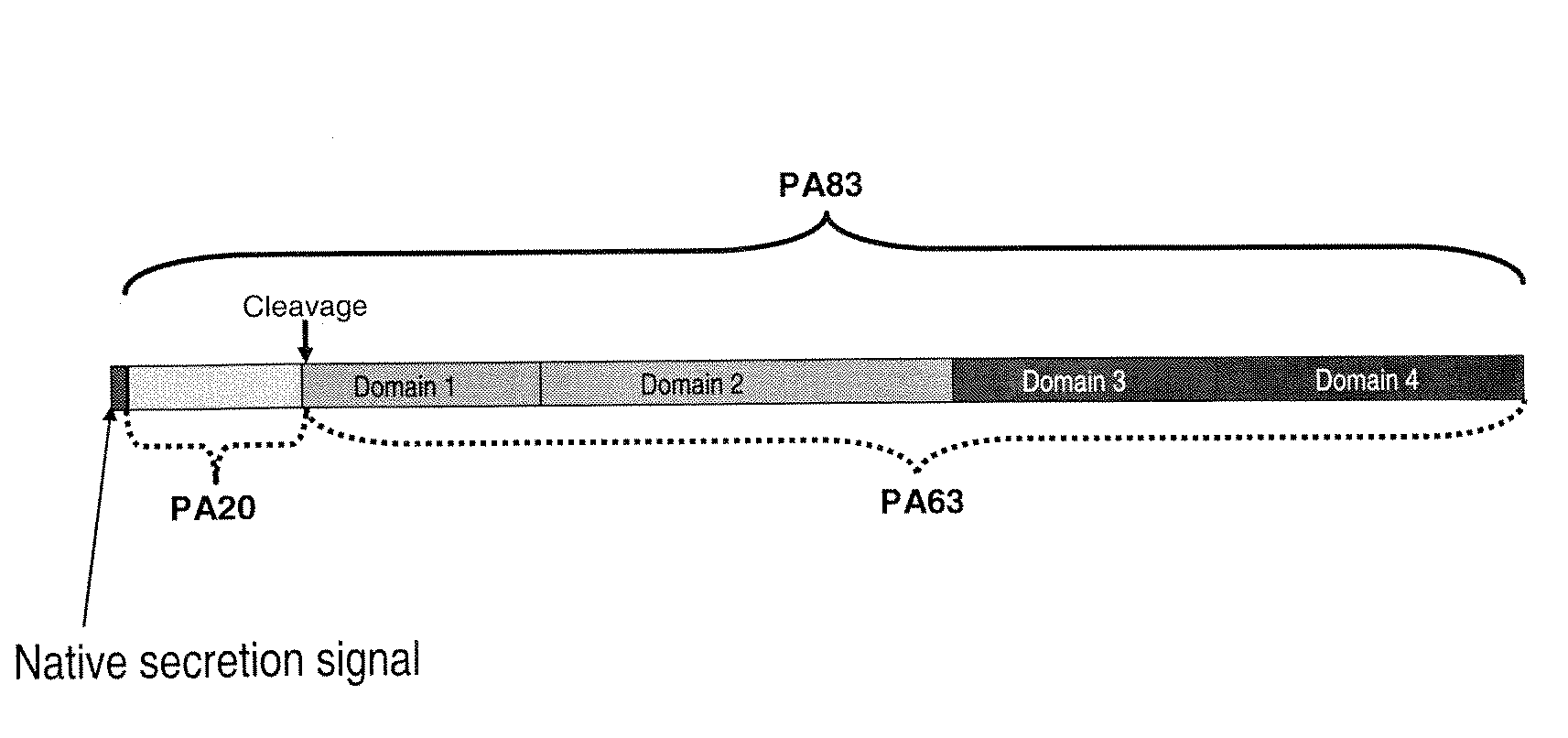
An optimized synthetic polynucleotide encoding a Bacillus anthracis protective antigen and an anthrax vaccine based on the encoded protective antigen. Furthermore, heterologous expression in a host Pseudomonas fluorescens bacteria of an optimized polynucleotide sequence encoding a Bacillus anthracis protective antigen.
Owner:PELICAN TECH HLDG INC
Preparation method of active microbial agent of forage anti-diarrhea yeast
InactiveCN103652320AThe overall impact is smallAvoid damageAnimal feeding stuffAnimal ForagingMicrobial agent
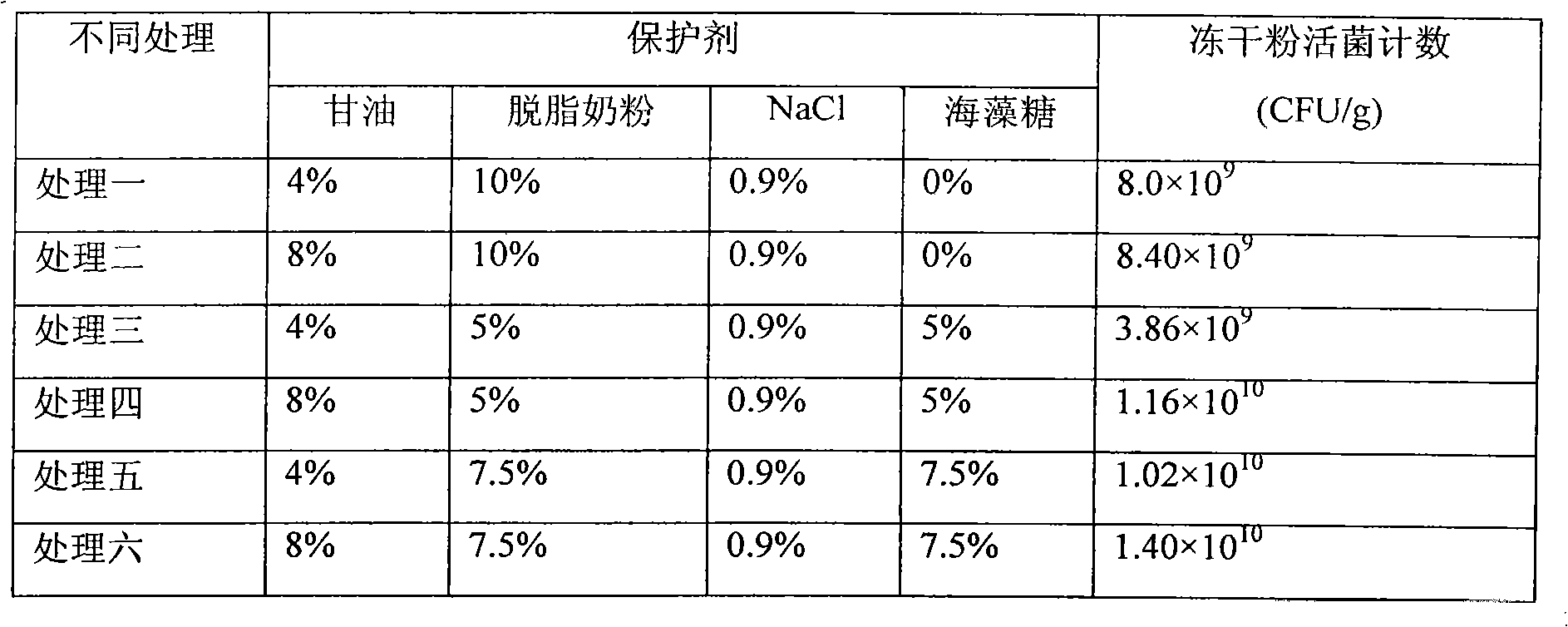
The invention relates to a preparation method of a high-density fermented and freeze-dried active microbial agent of a forage anti-diarrhea yeast, and belongs to the field of an agriculture biotechnology. The method comprises the following steps: inoculating an original starting strain (Saccharomyces boulardii, ATCC MYA-796) into yeast leaching peptone powder glucose liquid culture medium after activating; cultivating at 30 DEG C for 12-16 hours at 200-250rpm as a seed solution; inoculating the seed solution by 10% of inoculation amount, cultivating at 30 DEG C for 12 hours at 800rpm in liquid-expanded yeast leaching peptone powder glucose liquid culture medium, and then feeding 25% glucose for 6 hours, wherein the feeding amount is 20ml / h / L of fermentation liquor; centrifugally collecting thalli and adding protective agents (8% of glycerinum, 7.5% of skim milk powder, 7.5% of mycose and 0.9% of sodium chloride), wherein the mixing ratio of the protective agents to the thalli is 2:1; stewing and balancing for an hour after evenly mixing; pre-cooling at -80 DEG C for 12 hours, and then freeze-drying the thalli in a vacuum freezer dryer; weighing freeze-dried powder to count viable bacteria, wherein the viable bacteria count achieves 1.4*10<10> CFU / g.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Stable lyophilized preparation of recombinant human anti-CD20 monoclonal antibody
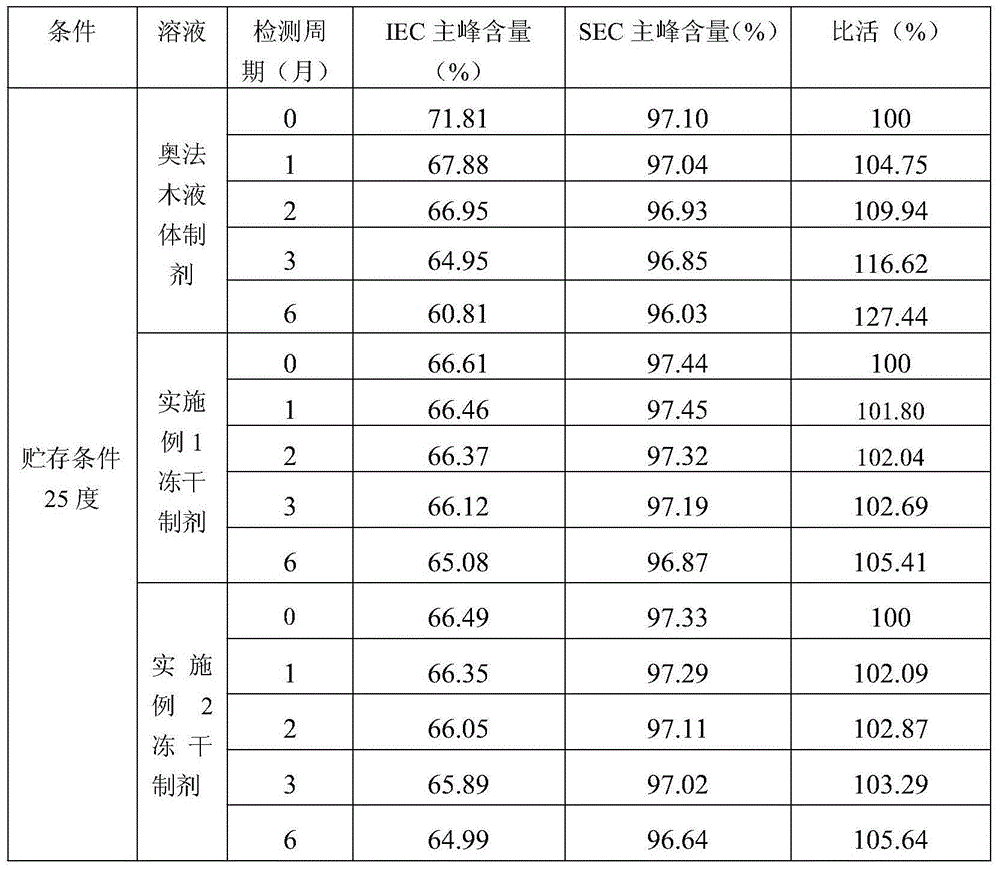
InactiveCN105708811AReduce degradationReduce aggregationPowder deliveryAntibody ingredientsProtective antigenProtective antibody
The invention relates to a stable lyophilized preparation of a recombinant human anti-CD20 monoclonal antibody. The lyophilized preparation is composed of the recombinant human anti-CD20 monoclonal antibody, a buffer system, a protective agent, an excipient and the like. The lyophilized preparation provided by the invention has a loose, full and complete appearance, has stability significantly superior to that of liquid preparations, and is suitable for long-term storage.
Owner:HAISCO PHARMA GRP INC
Proteosome-liposaccharide vaccine adjuvant
InactiveUS7524509B2Provide immunityAdvantageously producedSsRNA viruses negative-senseBacterial antigen ingredientsImmunotherapeutic agentProtection sex
An adjuvant complex composed of bacterial outer membrane protein proteosomes complexed to bacterial liposaccharide is prepared to contain the component parts under a variety of conditions. The complex can be formulated with antigenic material to form immunogenic compositions, vaccines and immunotherapeutics. An induced immune response includes protective antibodies and / or type 1 cytokines is shown for a variety of protocols.
Owner:ID BIOMEDICAL CORP LAVAL
Swine fever virus subunit vaccine and preparation method and purpose thereof
The invention belongs to the technical field of biological medicine, and concretely relates to a swine fever virus subunit vaccine and a preparation method and a purpose thereof. The invention provides a kluyveromyces marxianus yeast recombination strain used for preparing the swine fever virus subunit vaccine. The recombination strain is constructed by the following steps: swine fever virus envelope protein E2 is intercepted, through codon optimization, a coding sequence of the swine fever virus mE2 protein is obtained, and then is cloned to a kluyveromyces marxianus yeast expression vector,and the kluyveromyces marxianus yeast host strain is transformed. The invention also provides the method for preparing the swine fever virus subunit vaccine, which comprises the following steps: the kluyveromyces marxianus yeast host strain is subjected to recombination expression by using mE2, steps of culture, centrifugation, cell disruption, and separating purifying are carried out to obtain the swine fever virus mE2 protein antigen, and the purified antigen and an adjuvant are subjected to complex formulation to prepare the swine fever virus subunit vaccine. The injection immunotherapy ofswine fever virus mE2 protein recombination subunit vaccine can obtain a protective IgG antibody, and the subunit vaccine can reduce and prevent the swine fever virus infection-related disease.
Owner:FUDAN UNIV
Antigen of hybrid M protein and carrier for group A streptococcal vaccine
Recombinant hybrid streptococcal M protein antigens are provided which elicit protective antibodies against Group A streptococci and prevent rheumatic fever. Recombinant hybrid genes which encode the antigen are provided. Vaccine compositions and methods of administering the compositions are provided to elicit immunity against Group A streptococci.
Owner:UNIV OF TENNESSEE RES FOUND
Rhodococcus ruber fermentation method and application thereof as adjuvant in animal vaccines
ActiveCN109666609AUnusual humoral immunomodulatory effectsImprove securityBacteriaMicroorganism based processesAdjuvantAnimals vaccines
The invention relates to a special rhodococcus ruber (CGMCC NO. 17012) fermentation method, a preparation process of adjuvants with different dosage forms and an application as an adjuvant in animalvaccines. Rhodococcus ruber strains are separated from a farm and obtained through single colony cloning purification and identification, the invention provides a special fermentation process where the strain inactivation product is used as an animal vaccine adjuvant, and provides a preparation process of different dosage forms of adjuvants containing the strain and the product. Animal experimentsprove that when the adjuvant product is used for univalent or multivalent animal vaccines, particularly Newcastle disease inactivated vaccine, avian influenza inactivated vaccine and swine fever livevaccine, definite non-specific immune enhancement effect can be provided, specifically, the antibody peak value level induced by the animal vaccine is obviously improved, the time for producing the protective antibody level is advanced, the antibody maintenance period is prolonged, and the immune effect of the animal vaccines is enhanced.
Owner:刘春郁
Cross-protective pathogen protection, methods and compositions thereof
ActiveUS9707288B2Inhibition of activationSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityInfluenza a
The present disclosure provides a method of inducing a cross-protective immune response in a subject against a pathogen, such as influenza, comprising administering a first unique pathogen antigen to the subject; and administering a second unique pathogen antigen 3-52 weeks after a); wherein the second unique pathogen antigen and the first unique pathogen antigen are immunologically distinct but share conserved sites that are not normally immunogenic for antibodies. Also disclosed herein are assays for detecting cross-protective antibodies, methods of generating novel cross-protective antibodies. Further provided are novel antibodies against influenza.
Owner:SCHRADER SABARIAH
Bovine A-type foot-and-mouth disease multi-epitope vaccine, and preparation method and application thereof
The invention discloses a bovine A-type foot-and-mouth disease multi-epitope recombinant vaccine, and a preparation method and an application thereof, and belongs to the field of veterinary vaccine research. The preparation method comprises the following steps: carrying out reasonable serial connection on the dominant antigen epitopes of bovine A-type foot-and-mouth disease virus representative strains once pandemic in China by adopting an antigenized antibody strategy and a brand new reverse vaccinology technology and strategy, coupling with a bovine IgG immunostimulatory fragment (IgG heavy chain constant region), cloning into a prokaryotic expression vector to construct a recombinant expression vector, transforming Escherichia coli cells to express a recombinant antigen, purifying by adopting Ni-NAT column chromatography, quantifying by a Bio-Rad protein quantification kit, and preparing the vaccine individually or combining with a recombinant foot-and-mouth disease virus 3D protein. A result of animal immune experiments shows that the recombinant protein or combined vaccine can stimulate the body to produce a protective antibody with high titer and also can protect immune animals against a virus attack, so the bovine A-type foot-and-mouth disease multi-epitope recombinant vaccine has a good application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation method of aluminum-containing adjuvant hepatitis B vaccine
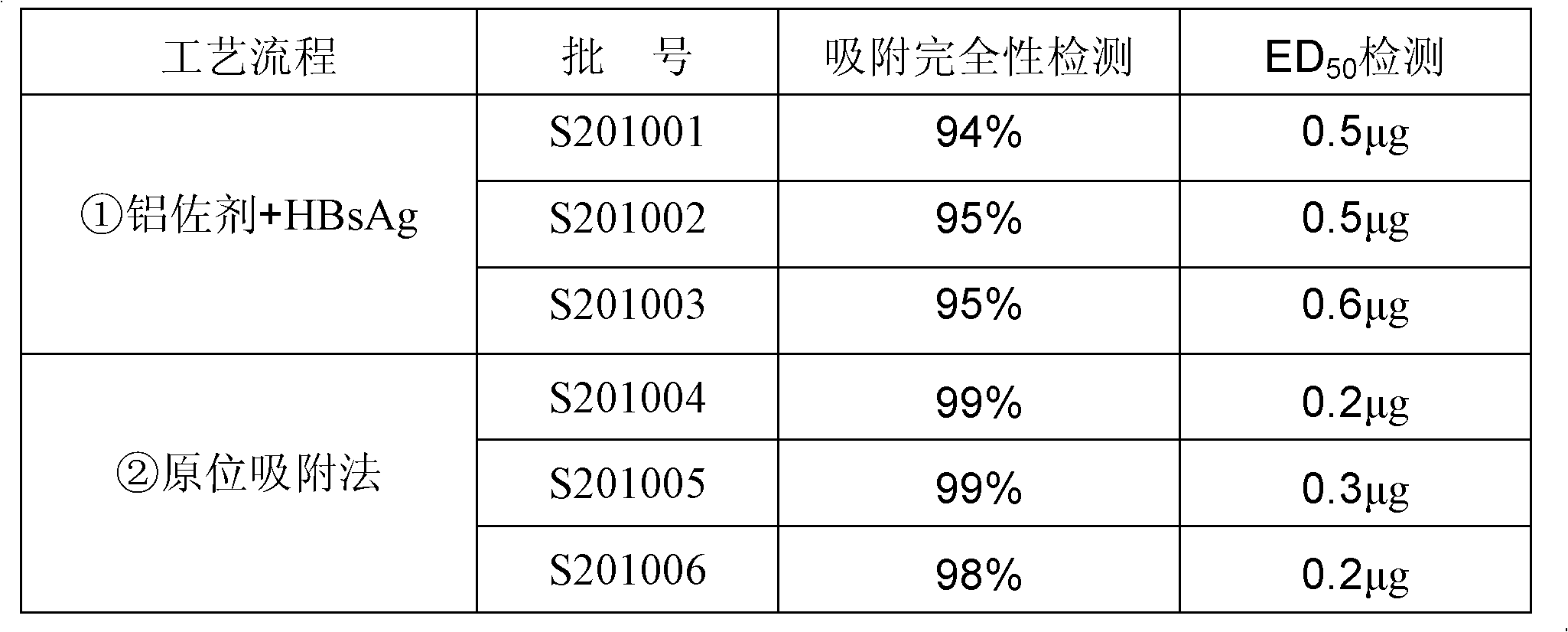
ActiveCN102198270AIncrease inoculum volumeHigh positive conversion rateViral antigen ingredientsDigestive systemAdjuvantIn situ adsorption
The invention discloses a preparation method of an aluminum-containing adjuvant hepatitis B vaccine, belonging to the biotechnology field. The preparation method is characterized in that an aluminum adjuvant Al(OH)3 is produced by an on-line reaction, i.e. after a phosphate buffer solution (PBS), a KAl(SO4)2 solution and a hepatitis B surface antigen stock solution are mixed, an NaOH solution is added to the mixed solution, an Al(OH)3 adjuvant is continuously produced, and simultaneously, hepatitis B surface antigens are continuously coated and adsorbed; and the process is called 'in-situ adsorption'. In the invention, the Al(OH)3 adjuvant is produced by an in-situ reaction to greatly improve the adsorption rate of the hepatitis B surface antigens, thereby improving the immunogenicity of the antigens, being capable of more effectively causing organisms to generate an immune response, and producing more protective antibodies. The practice proves that the aluminum adjuvant hepatitis B vaccine produced by the method disclosed by the invention has the advantages of small inoculation amount, few adverse responses, high antibody positive conversion rate and the like, and can induce high-level antibody response after being immunized. Simultaneously, the processing steps are also simplified, and the production cost is greatly lowered.
Owner:DALIAN HISSEN BIO-PHARM CO LTD
EV71 subunit vaccine of mixed adjuvant and preparation method thereof
InactiveCN105535963AHigh School and ValenceGood immunogenic responseSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliImmunogenicity
The invention discloses an EV71 subunit vaccine of a mixed adjuvant and a preparation method thereof. According to the vaccine, C4 sub-type EV71 virus VP1 protein is used as an antigen, and aluminum hydroxide and monophosphoryl lipid A are used as a mixed adjuvant. VP1 protein which is expressed and purified by escherichia coli and does not have any label is used as an antigen; the prepared protein is combined with an aluminum adjuvant under a denaturing condition, is subjected to detergence determination, and is mixed with an MPLA adjuvant to immune mice; and the prepared polyvalent antibody can be used for specifically neutralizing the EV71 virus to generate a neutral protective antibody having a titer nearly 1:128. Compared with an EV71 inactivated vaccine in current clinical tests, the human enterovirus 71 type subunit vaccine has the characteristics of low cost, simple operation, high safety, easy large-scale production and the like in the production process. The vaccine can generate relatively good immunogenicity in a human body, has a relatively high titer of the neutralizing antibody, and is an alternative vaccine with potential clinical application value.
Owner:BEIJING KYNING BIOSCI
Bovine A-type foot-and-mouth disease broad-spectrum multi-epitope vaccine, and preparation method and application thereof
ActiveCN104119441AResist attackGenetic material ingredientsAntiviralsAntigen epitopeEscherichia coli
The invention discloses a bovine A-type foot-and-mouth disease broad-spectrum multi-epitope recombinant vaccine, and a preparation method and an application thereof, and belongs to the field of veterinary vaccine research. The preparation method comprises the following steps: carrying out reasonable serial connection on the major antigen epitopes of pandemic in China and bovine A-type foot-and-mouth disease virus representative strains recently pandemic in countries bordering China by adopting a brand new reverse vaccinology idea and strategy, coupling with a bovine IgG heavy chain constant region, cloning into a prokaryotic expression vector to construct a recombinant expression vector, transforming Escherichia coli cells to express a recombinant antigen, purifying by adopting Ni-NAT column chromatography, quantifying by a Bio-Rad protein quantification kit, and preparing the vaccine individually or combining with a recombinant foot-and-mouth disease virus 3D protein. A result of animal immune experiments shows that the multi-epitope antigen or 3D protein combined vaccine can stimulate the body to produce a protective antibody with high titer and also can protect immune animals against a virus attack, so the bovine A-type foot-and-mouth disease broad-spectrum multi-epitope recombinant vaccine has a good application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Plasmodium falciparum antigens inducing protective antibodies
InactiveUS6472519B1Easy accessConvenient researchBacteriaSugar derivativesProtective antigenMonocyte
A molecule, protein or peptide characterized in that it is recognized by cytophilic antibodies from individuals who are immune to infection by Plasmodia, and recognized by non-cytophilic antibodies from individuals who are vulnerable to infection by Plasmodiae. Said antibodies are capable of blocking the erythrocytic phase of the parasite by co-operating with accessory cells such as monocytes.
Owner:INST PASTEUR
Antibodies against protective antigen
ActiveUS7906119B1Efficient killingIncreased activationAntibacterial agentsAntibody ingredientsProtective antigenAnthrax toxin
The present invention relates to antibodies and related molecules that specifically bind to protective antigen of Bacillus anthracis (PA). Such antibodies have uses, for example, in the prevention and treatment of anthrax and anthrax toxin poisoning. The invention also relates to nucleic acid molecules encoding anti-PA antibodies, vectors and host cells containing these nucleic acids, and methods for producing the same.
Owner:EMERGENT MFG OPERATIONS BALTIMORE LLC
Antibodies against protective antigen and methods of use for passive immunization and treatment of anthrax
InactiveUS20080063647A1Avoid toxicityReduce severityAntibacterial agentsImmunoglobulins against bacteriaProtective antigenMammal
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Production of hygrophilous monospermous bacterium main-protective antigen univalent and multivalent vitelline antibody and use in aquatic animal
InactiveCN101074260AIncrease productionLow costAntibacterial agentsEgg immunoglobulinsDiseaseProtective antigen
Production of various Aeromonas hydrophila protective antigen monovalent and multivalent vitelline antibody and its usage in prevention of aquatic animal diseases are disclosed. It utilizes minor-protective antibody to make immune irritant composite egged hen and produce vitelline antibody product. It's safe, efficient, fast, has no harm and residue. It has more output, better immune-system development and functions of nutrients-added, disease-prevention and health-care.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +4
Preparation and application of vaccine immunity effect evaluation protein chip
InactiveCN102175874AEasy replantingEvaluation of Convenience EffectMaterial analysis by observing effect on chemical indicatorBiological testingProtective antigenProtein chip
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Application of using streptococcus suis type-2 hy0245 gene encoded proteins as protective antigens
InactiveCN102443053AImproving immunogenicityGood immune protectionAntibacterial agentsBacterial antigen ingredientsEscherichia coliProtective antigen
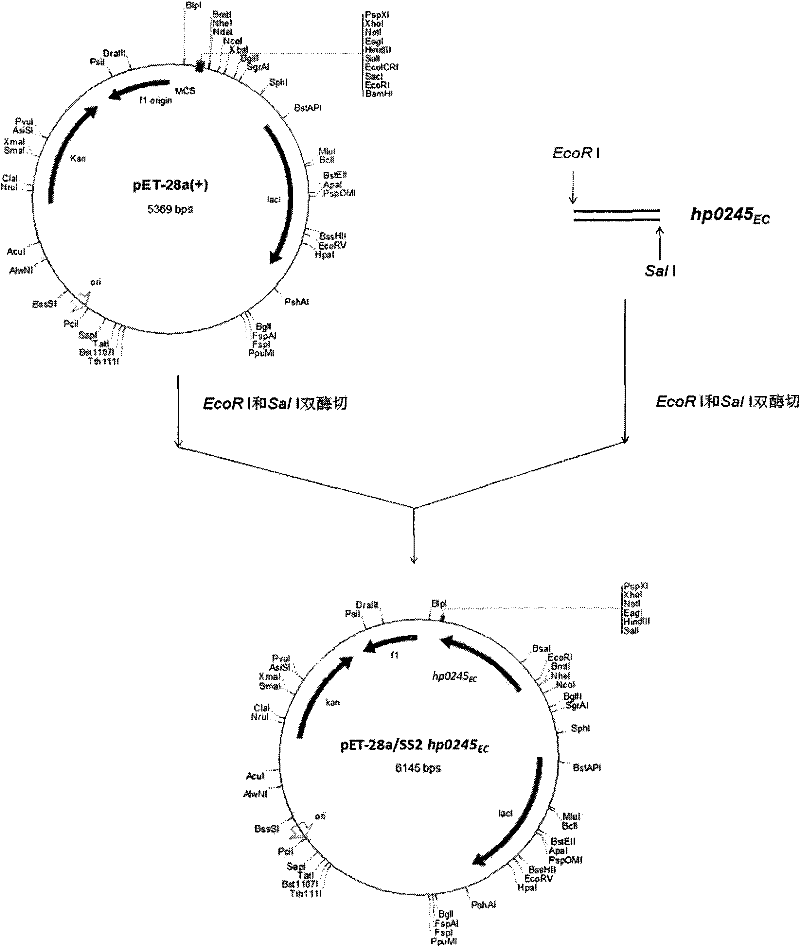
The invention belongs to the technical fields of veterinary microbiology and zoonotic diseases, and in particular relates to application of immunogenic protein genes of streptococcus suis type-2 for separating, cloning and expressing exocellular peptides and recombinant proteins of the encoded proteins in vaccine and diagnosis. In the invention, a new immunogenic protein gene hp0245 is separated from SC-19 bacterial strains of streptococcus suis type-2 virulent strains, wherein DNA (hp0245EC) for encoding the exocellular peptides has nucleotide sequences represented in the sequence table SEQ ID No:1 and is encoded with 261 amino acids. The recombinant protein HP0245EC remains the immunogenic feature of the primitive protein, can provide effective immunological protection to the SC-19 bacterial strain of the streptococcus suis type-2 infected in mice and has potential application value of vaccine. The invention further comprises a composition and preparation method for a streptococcus suis type-2 HP0245-ELISA diagnostic reagent kit, and a preparation of hp0245EC for cloning hp0245EC escherichia coli; the recombinant escherichia coli has been preserved in the China Center for Type Culture Collection (CCTCC) and the preservation number is CCTCC No: M2010258.
Owner:HUAZHONG AGRI UNIV
Bovini Asia 1/O type foot-and-mouth disease bivalent multi-epitope vaccine and preparation method and application thereof
ActiveCN103897065ARealize prevention and controlOvercome limitationsGenetic material ingredientsAntiviralsIgG.heavy chainImmunogenicity
The invention discloses a bovini Asia 1 / O type foot-and-mouth disease bivalent multi-epitope vaccine and a preparation method and an application thereof and belongs to the field of veterinary biological vaccines. The bovini Asia 1 / O type foot and mouth disease virus compound multi-epitope recombinant antigen is obtained by coupling dominant epitope of epidemic bovini O and Asia 1 type foot-and-mouth disease virus representative strains connected in series in China with a bovini IgG heavy chain constant region. Immune efficacy experiments verify that the bovini Asia 1 / O type foot-and-mouth disease bivalent multi-epitope vaccine prepared by the invention has good immunogenicity, and high level protective antibodies can be generated by the body induced by immune guinea pig or immune bovine. The inoculated vaccine is safe and harmless to immune animals. The vaccine is a novel vaccine which is abroad in prospect, supports matter storage and technical support to prevention and control of bovini Asia 1 / O type foot-and-mouth disease in China, and is of great importance.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com