Preparation and application of vaccine immunity effect evaluation protein chip
A technology of protein chips and vaccines, which is applied in the field of preparation of protein chips for vaccine immune effect evaluation, can solve the problems of time-consuming detection methods, large-scale and effective evaluation of vaccine immune effect antibody levels, and reduced evaluation efficiency, so as to promote research and development Application, high-throughput detection, and efficient evaluation of tool effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Treatment of Partial Disease Pathogen Protective Antigens
[0036] 1. Materials
[0037] Group A meningococcal polysaccharide vaccine was purchased from Beijing Tiantan Biological Products Co., Ltd.; Bacillus anthracis lysate was donated by Professor Wang Xiliang; live attenuated polio vaccine sugar pills were purchased from the Institute of Medical Biology, Chinese Academy of Medical Sciences; Live viruses (Types I, II, III) were obtained from the 302 Hospital of the People’s Liberation Army; Adsorbed Diphtheria-Tetanus Vaccine was purchased from Shanghai Institute of Biological Products; MMR combined live attenuated vaccine was purchased from Beijing Tiantan Biological Products Co., Ltd.; The glass sheet base was purchased from CEL Associates; Cy3-labeled goat anti-human IgG was purchased from sigma.
[0038] 2. Methods and Results
[0039] 1. Comparison of the effects of different methods for treating group A meningococcal polysaccharide vaccine
[0040]...
Embodiment 2
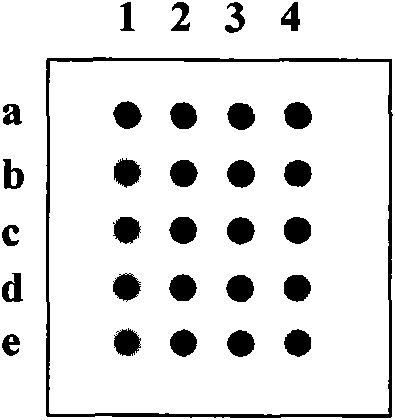
[0059] Example 2 National immunization program disease protective antibody detection protein chip probe lattice design and chip preparation
[0060] 1. Materials
[0061] HBsAg was expressed and preserved in our laboratory; measles virus H protein, rubella virus E1 protein, mumps virus NP protein, hepatitis A virus vp1 and vp3 proteins, Japanese encephalitis virus E protein, Mycobacterium tuberculosis Ag85B antigen were purchased from abcam company; IgG, BSA, pertussis Bacillus FHA, diphtheria toxin, and tetanus toxin were purchased from Sigma; live attenuated polio vaccine sugar pills were purchased from the Institute of Medical Biology, Chinese Academy of Medical Sciences; group A meningococcal polysaccharide vaccine was purchased from Beijing Tiantan Biological Products Co., Ltd. The NP protein of hemorrhagic fever virus was donated by Professor Liang Mifang; the Bacillus anthracis PA protein was purified by our laboratory; the Leptospira antigen was donated by Professor Du...
Embodiment 3
[0072] Example 3 Protein Chip Detection of Protective Antibodies in Serum Samples
[0073] 1. Materials
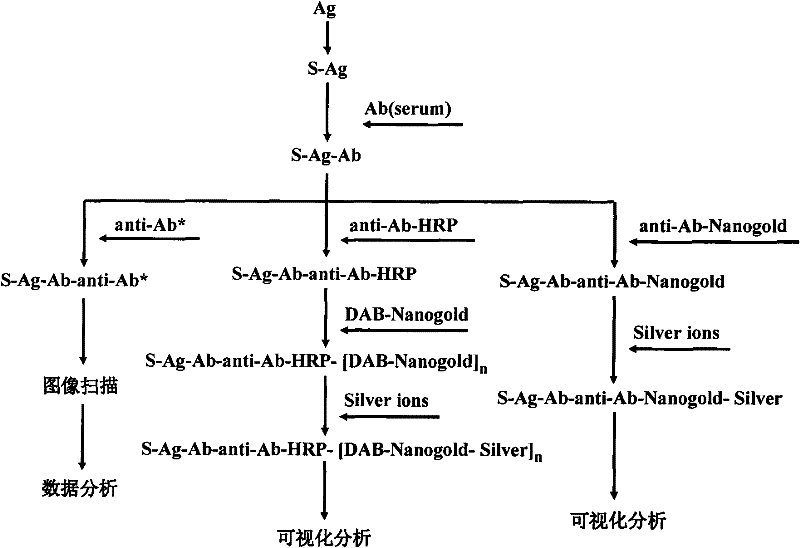
[0074] Cy3-labeled goat anti-human IgG, nano-gold-labeled goat anti-human IgG, and HRP-labeled goat anti-human IgG were purchased from Sigma; nano-gold composite substrate (Nanogold-DAB), silver enhancement reagents A and B were prepared and stored in our laboratory.
[0075] 2. Methods and Results
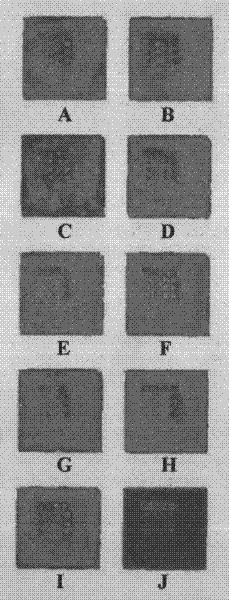
[0076] Take the protein chip prepared in the above example and equilibrate to room temperature, dilute the serum sample 1:5 with sample diluent 0.01mol / L PBS (25% FCS, pH=7.4), add 10 microliters to each reaction zone, The chip was incubated at 37°C for 30 minutes. Wash the chip with washing solution PBST (0.01 mol / L, 0.05% Tween-20, pH=7.4) gently for 3 times, 20 seconds each time, and prepare for color development after drying.
[0077] 1. Fluorescent color development
[0078] Cy3-labeled goat anti-human IgG was diluted 1:400, 10 microliters were added to each reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com