Pig viral infectious disease gene recombined live vaccine using canine II type adenovirus as carrier and preparation process thereof
A production process and gene recombination technology, applied in genetic engineering, gene therapy, antiviral agents, etc., to achieve the effects of long shelf life, good immune protection effect, and good genetic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
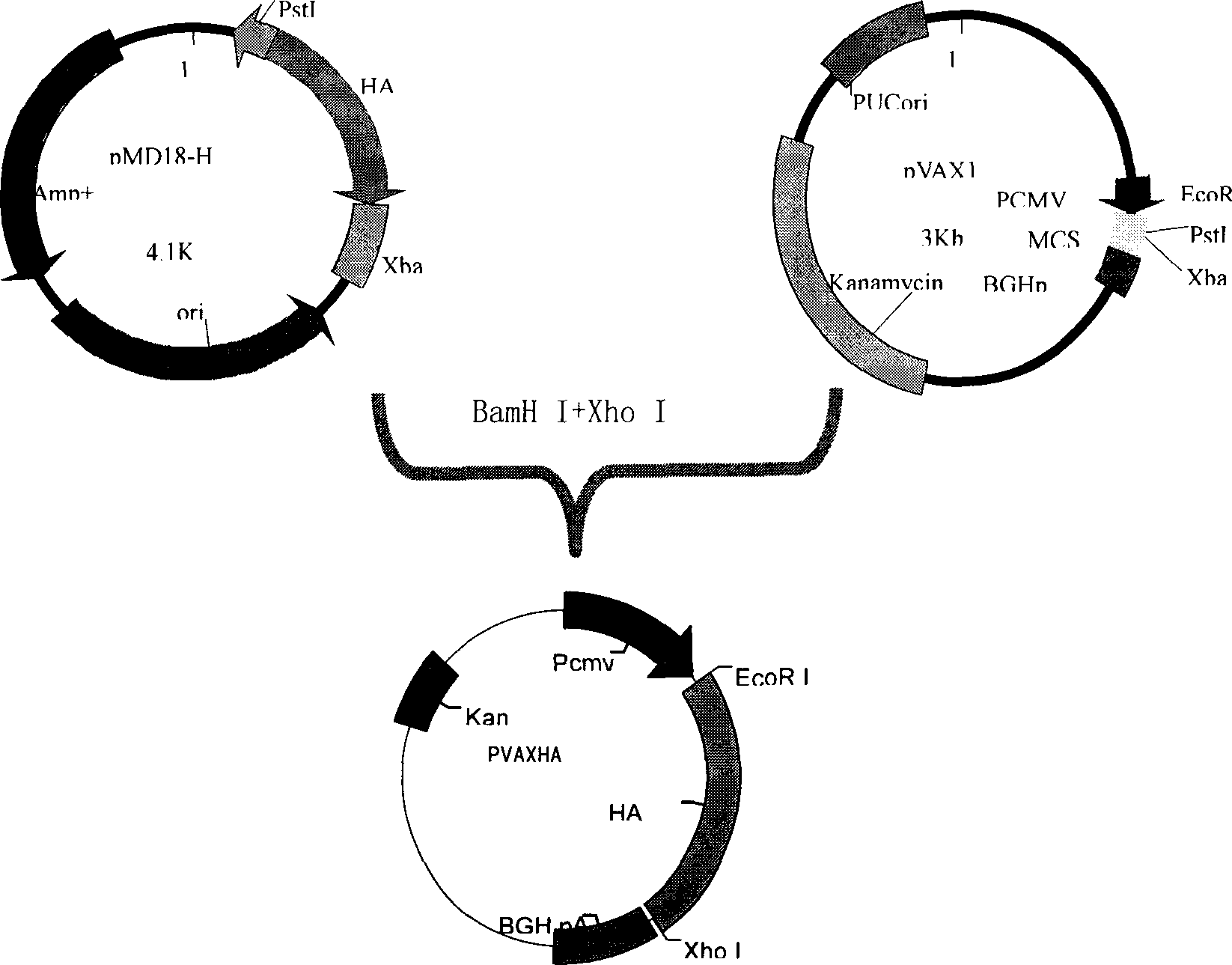
[0037]The preparation of embodiment 1 recombinant swine influenza virus HA gene adenovirus vector live vaccine
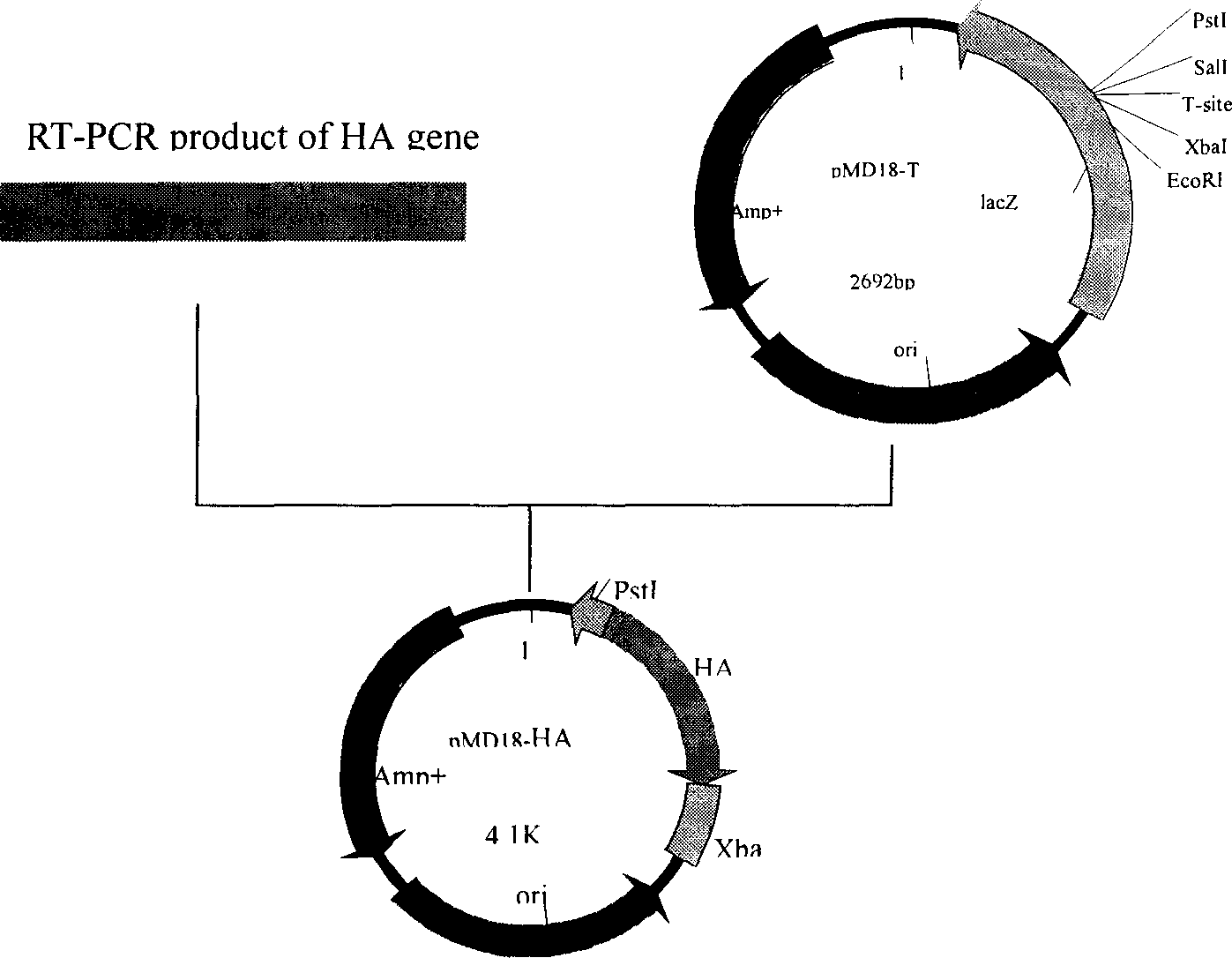
[0038] According to the process route shown in Figure 1, the recombinant adenovirus live vector vaccine was prepared. Under the electron microscope, the recombinant virus had the general characteristics of adenovirus. After infecting MDCK cells, specific cytopathic changes could occur. See attached drawing 4. Specific steps are as follows:
[0039] 1. The rescue plasmid pPoly2-UD containing the paired ends of the CAV-2 genome was obtained by PCR method, which was linearized with BstB I+Hind III enzymes and then homologously recombined with the CAV-2 genomic DNA to obtain the CAV-2 genome-wide cloning plasmid pPoly2 -CAV / 2.
[0040] PCR primers:
[0041] P 1 : 5′ end GCG-TGC-CTA-ACT-ACA-AAC-TCA-AT
[0042] 5' end G-CCT-GCT-GCT-TGA-TTT-TGT-GAT-C
[0043] P 2 : 5′ end A-CTC-ATA-GAA-GTA-GGC-AGC-TCC-G
[0044] 5' end CAT-CAT-CAA-TAA-TAT-ACA-GGA-CAA-AG
[0...
Embodiment 2
[0065] Example 2 Preparation of recombinant classical swine fever virus E2 gene adenovirus vector live vaccine
[0066] According to attached image 3 The process route shown is to prepare the recombinant adenovirus live vector vaccine. The recombinant virus has the general characteristics of adenovirus under the electron microscope. After infecting MDCK cells, specific cytopathic changes may occur. See attached drawing 4. Specific steps are as follows:
[0067] 1. The rescue plasmid pPoly2-UD containing the paired ends of the CAV-2 genome was obtained by PCR method, which was digested with PstI+Hind III and linearized, and then homologously recombined with the CAV-2 genomic DNA to obtain the CAV-2 whole genome cloning plasmid pPoly2- CAV / 2.
[0068] PCR primers:
[0069] P 1 : 5′ end GCG-TGC-CTA-ACT-ACA-AAC-TCA-AT
[0070] 5' end G-CCT-GCT-GCT-TGA-TTT-TGT-GAT-C
[0071] P 2 : 5′ end A-CTC-ATA-GAA-GTA-GGC-AGC-TCC-G
[0072] 5' end CAT-CAT-CAA-TAA-TAT-ACA-GGA-CAA-...
Embodiment 3
[0092] Embodiment 3 Preparation of recombinant porcine AsiaI type foot-and-mouth disease virus VPI gene adenovirus vector live vaccine
[0093] According to attached image 3 The process route shown is to prepare the recombinant adenovirus live vector vaccine. The recombinant virus has the general characteristics of adenovirus under the electron microscope. After infecting MDCK cells, specific cytopathic changes may occur. See attached drawing 4. Specific steps are as follows:
[0094] 1. Obtain the CAV-2 whole genome cloning plasmid pPoly2-CAV2 by the same method as described in Examples 1, 2, and 3.
[0095] 2. The PCR method obtains VPI from the AsiaI type foot-and-mouth disease virus PI whole gene, and the PCR primers are:
[0096] Upstream primer: 5'-CCGGAATTCGCCATGGCTCGCCGGCAGACTACCAC-3'
[0097] Downstream primer: 5'-CCGCTCGAGTTATAGCCTGTTTCTCGGGTGCAA-3' Restriction sites EcoRI and XhoI were added to the upstream and downstream primers respectively.
[0098] 3. Diges...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com