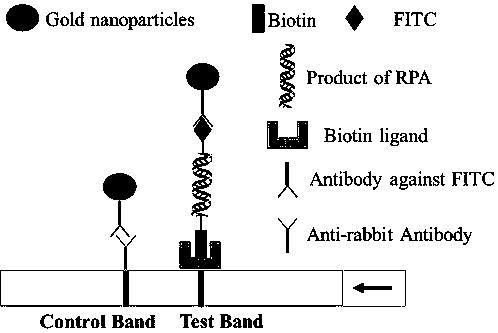
Patents
Literature
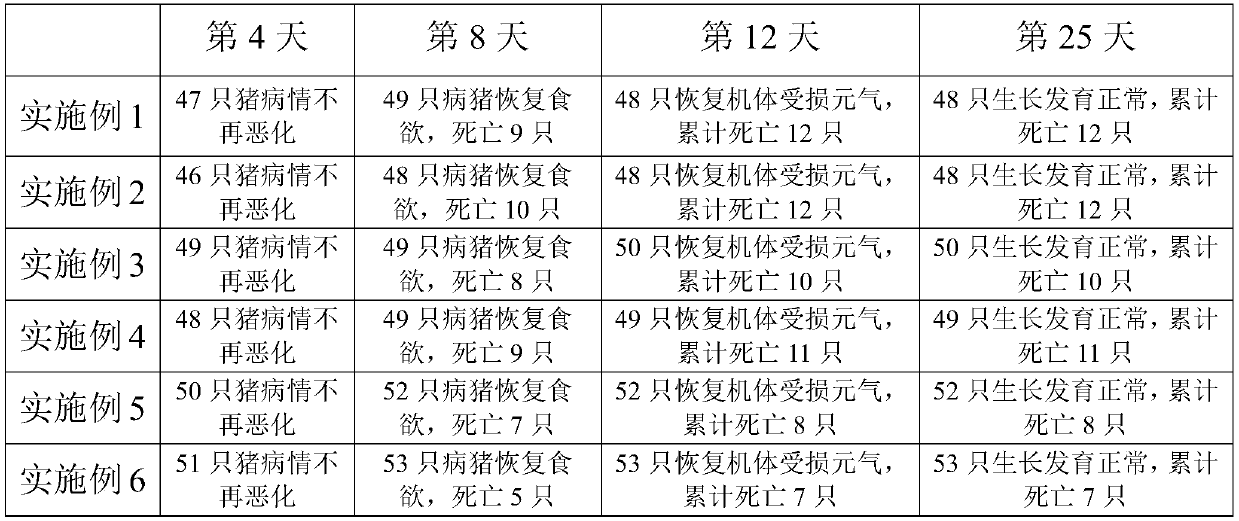
37 results about "Swine virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
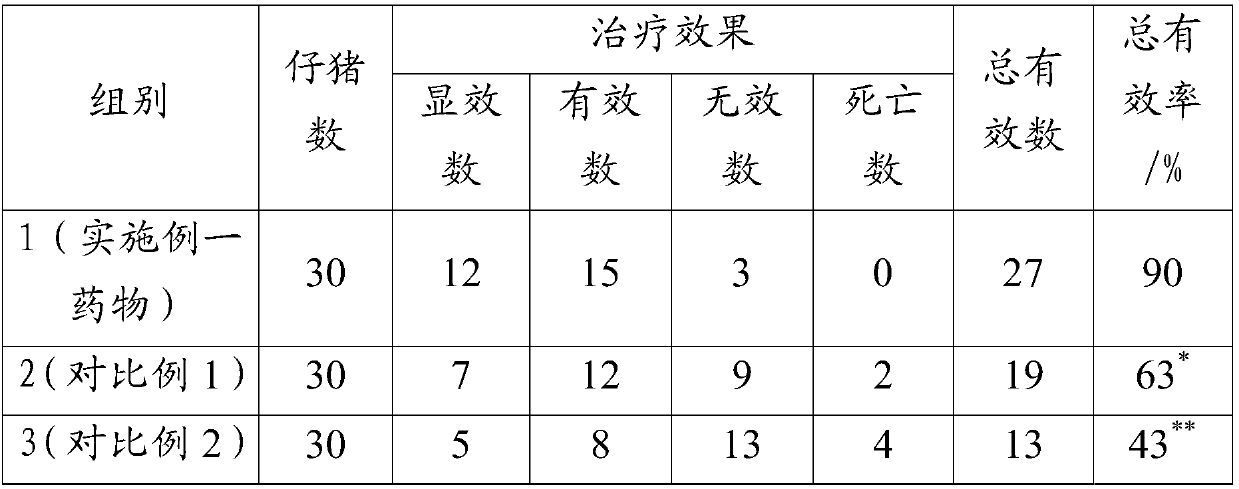
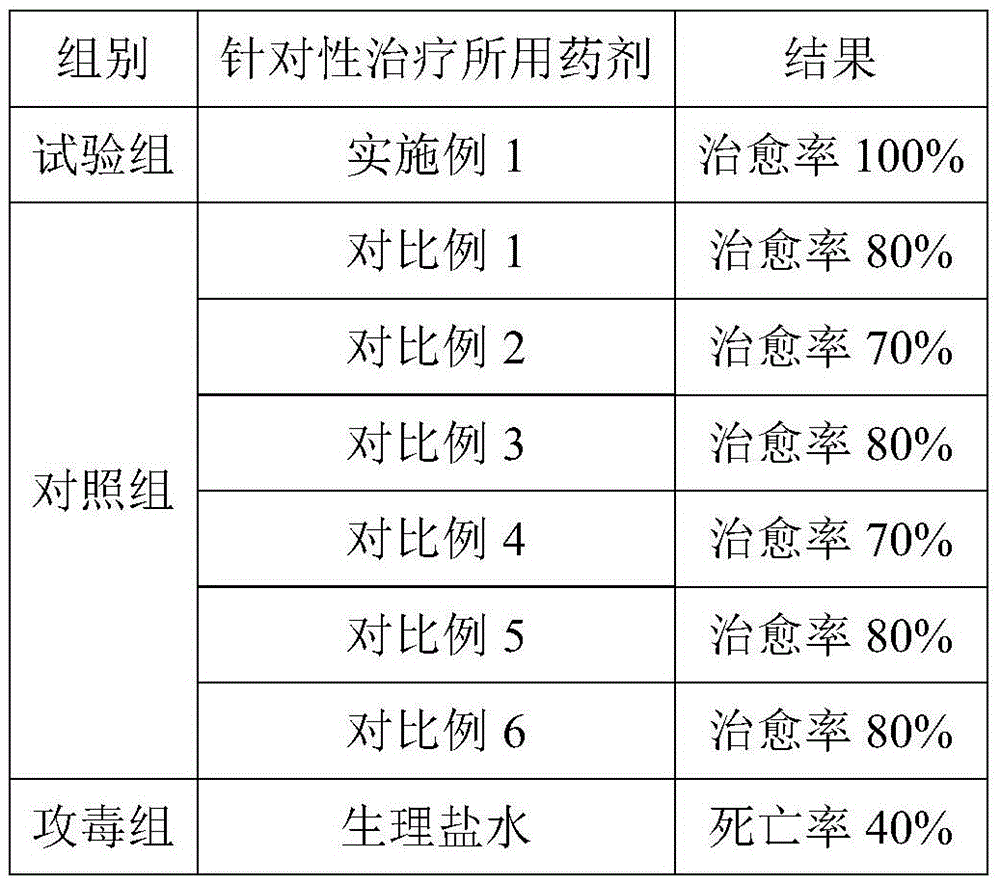
Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease
InactiveCN101695342APrevention and treatment of asthmaPrevention and treatment of contagious pleuropneumoniaFood processingAnimal feeding stuffMedicinal herbsBaical Skullcap Root
The invention provides a Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease. The swine feed additive consists of 37 Chinese medicinal herbs, namely, gypsum, rehmannia root, rhinoceros horn, golden thread, cape jasmine fruit, tree peony bark, baical skullcap root, red paeony root, figwort root, common anemarrhena rhizome, forsythia suspensa , platycodon root, liquorice root, common lophatherum herb, amur corktree bark, honeysuckle flower, Chinese pulsatilla root, indigowoad root, heartleaf houttuynia herb, astragalus, szechwon tangshan root, hawkthorn fruit, medicated leaven, barley sprout, radish seed, chicken's gizzard -membrane, Chinese thorowax root, common andrographis herb, philippine violet herb, tuber fleeceflower root, massa medicata fermentata fujianensis, cyrtomium rhizome, tung leaf, tangerine peel, white paeony root, pine needle and indigowoad leaf through scientific compatibility. The feed additive is added into swine feed in the proportion; under the condition of not using any vaccine, the feed additive can effectively prevent and cure severe mixed flu symptoms, infection and other syndromes caused by swine respiratory disease, asthma, contagious pleuropneumonia, swine virus mixed flu, high swine fever, porcine circovirus, swine fever, flu, pseudorabies, salmonellosis, bacillosis, streptococcus, erysipelas, paratyphoid, eperythrozoon, toxoplasm and other multi-pathogeny and provides genuine green food for the market.
Owner:孟祥合
RPA (recombinase polymerase amplification) primer and detection kit for rapidly detecting African swine fever viruses
PendingCN109797246AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesQuarantinePorcine reproductive and respiratory syndrome virus
The invention discloses an RPA (recombinase polymerase amplification) primer and a detection kit for rapidly detecting African swine fever viruses. Target genes can be effectively amplified, specificity is 100%, detection sensitivity is 102 copy / reaction, and sensitivity is equivalent to that of fluorescent quantitative PCR (polymerase chain reaction). Cross reaction between the RPA amplificationprimer and one of classical swine fever viruses, vesicular exanthema swine viruses I, porcine reproductive and respiratory syndrome viruses, porcine circoviruses and the like is omitted. A RPA isothermal amplification system is rapidness in reaction and wide in temperature range, effective amplification of the target genes can be achieved at the temperature of 38-46 DEG C, and the detection kit can rapidly, efficiently and sensitively detect the African swine fever viruses and has the advantages that the kit is simple to operate, high in specificity, safe and free from pollution, reaction results are easily observed and the like. Effective technical means are provided for on-site rapidness detecting and screening of infection nucleic acid of the African swine fever viruses, and the RPA amplification primer has great significance for control of infection spreading of the African swine fever viruses in China and inspection and quarantine in infected areas and entry and exit ports.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Porcine circovirus type 2 antigen capture ELISA kit
The invention discloses a porcine circovirus type 2 antigen capture ELISA kit. The kit internally comprises an enzyme labeled monoclonal antibody which is secreted by a hybridoma cell strain with the preservation number of CGMCC NO.10205. The invention also discloses a capture ELISA method for rapidly detecting the porcine circovirus type 2 and established by utilizing the monoclonal antibody. A porcine circovirus type 2 polyclonal antibody and a porcine circovirus type 2 cap protein monoclonal antibody respectively act as a capture antibody and a detecting antibody. The method can be used for detecting multiple gene type PCV2 viruses; the detection sensibility is 400 TICD50 / ml; the porcine circovirus type 2 has no crossed reaction with other swine viruses, and the toxic value determination method coincidence rate is 88%. The result shows that the method has the advantages of operation simplicity, good specificity, high sensitiveness, short time consumption and the like, can be used for estimating the toxic value of viruses, and can conveniently and rapidly control the quality of PCV2 inactivated vaccine semi-finished products.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Monoclonal antibody specially bound to TGEV (transmissible gastroenteritis of swine virus), pharmaceutical composition, kit and applications of monoclonal antibody, pharmaceutical composition and kit
ActiveCN109232736ABiological material analysisImmunoglobulins against virusesAstrovirus gastroenteritisMurine monoclonal antibody
The invention provides a monoclonal antibody specially bound to TGEV (transmissible gastroenteritis of swine virus) and a kit prepared from the monoclonal antibody. The TGEV can be determined rapidlyand accurately, and strains can be widely determined. Pharmaceutical composition prepared from the antibody can be used for broad-spectrum prevention and treatment of different TGEVs.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Pig viral infectious disease gene recombined live vaccine using canine II type adenovirus as carrier and preparation process thereof
InactiveCN1827172AGood genetic stabilityEasy to storePowder deliveryGenetic material ingredientsAntigenVp4 gene
This invention supplies a series of production techniques of gene recombination live vaccine of swine virus contagion with canine ó� adenovirus as carrier and finished goods. The viral live vectors vaccine takes swine important virus zymad protective antigens gene as object gene, which are chosen from HCV-E1, E2 gene, FMDV-VP1íóVP2íóVP3íóVP4 gene, TGEV-SíóNíóM gene, PEDV-SíóNíóM geneú¼SIV-HAíóNA geneú¼RV-GíóN gene, etc. The produced vaccines contain recombined swine influenza virus HA gene adenovirus carrier live vaccine, swine plague virus E2 gene adenovirus carrier live vaccine and recombination swine AsiaI foot-and-mouth disease virus VPI gene adenovirus carrier live vaccine. Recombination virus has good inheritance stability, and vaccine immunization can induct pig develop differential antiviral neutralization antibody. It has good immune protection effect and has no toxic side effect. The goods are facilitating for preserve and transportú”it has long storage life and simple technics, and it fits for commercial manufacture.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Anti-African swine fever virus p54 protein monoclonal antibody and preparation method and application thereof
ActiveCN112552396AStrong specificityHigh sensitivityImmunoglobulins against virusesFermentationBALB/cSwine plague
The invention relates to an anti-African swine fever virus p54 protein monoclonal antibody and a preparation method and application thereof. According to the invention, polypeptide capable of simulating conservative p54 protein NTD is designed and synthesized, and the polypeptide is coupled with BSA to serve as immunogen, an anti-ASFVp54 protein monoclonal antibody is prepared by immunizing a BALB / c mouse through an immunological method, the obtained antibody can specifically identify and bind to an N-terminal region of p54 protein, and the recognition region (aa 1-29) is different from the existing commercial monoclonal antibody. The invention provides gene sequences of a heavy chain variable region and a light chain variable region of the antibody. On this basis, the monoclonal antibodyof the invention can be obtained by adopting a conventional genetic engineering method or a protein engineering method. The monoclonal antibody is high in specificity and high in sensitivity, capableof specifically reacting with the ASFV HLJ / 18 virus strain, without reaction with CSFV, PCV2, PRRSV, PEDV, PRV and other swine viruses, thus laying a foundation for the research of ASFV etiology and pathogenesis and the clinical detection research of ASFV pathogens.
Owner:河南中泽生物工程有限公司
Preventive and therapeutic use of polypeptides from African Swine virus as vaccines
InactiveUS7396531B2Effective in prevention and treatment and diagnosisPeptide/protein ingredientsViral antigen ingredientsMedicineImmunodeficiency
The present invention relates to the use of selected polypeptides from African Swine virus for the prevention and therapy of African Swine infections as well as other infections, including immune deficiencies in mammals and humans.
Owner:RATH MATTHIAS +1
Porcine interferon alpha and application thereof
ActiveCN106939042AHigh potencyGood antiviral effectPeptide/protein ingredientsAntiviralsEscherichia coliInclusion bodies
The invention provides a porcine interferon alpha and an application thereof. The porcine interferon alpha is synthesized through selecting high-expression codon design according to the codon bias of the of a Escherichia coli prokaryotic expression system, the amino acid sequence of the porcine interferon alpha is represented by SEQ ID NO. 1, and the nucleotide sequence for encoding the porcine interferon alpha is represented by SEQ ID NO. 2. A method for preparing the porcine interferon alpha has the advantages of simplicity, low cost, easiness in industrial production, high expression level of the porcine interferon alpha, thorough denaturation in the inclusion body treatment process, and high protein renaturation rate porcine. The porcine interferon alpha prepared in the invention has high titer, can inhibit 100TCID50 vesicular stomatitis virus, has a specific activity reaching up to 10<8>, has good bioactive functions, can be used to prepare medicines for preventing and treating the swine virus infection and enhancing the immune function, and has a broad market application prospect.
Owner:SOUTH CHINA AGRI UNIV +1
Swine virus vaccines that are liquid stable
ActiveUS9855336B2Overcome deficienciesPrevent diseaseSsRNA viruses negative-senseSsRNA viruses positive-senseAnimal subjectSwine virus
The present invention is drawn to liquid stable swine vaccines that comprise a live porcine virus. The invention is also drawn to the manufacture of such vaccines and methods of vaccinating animal subjects with these vaccines.
Owner:INTERVET INC
Swine virus vaccines that are liquid stable
ActiveUS20170014513A1Overcome deficienciesPrevent diseaseSsRNA viruses negative-senseSsRNA viruses positive-senseViral VaccineAnimal subject
The present invention is drawn to liquid stable swine vaccines that comprise a live porcine virus. The invention is also drawn to the manufacture of such vaccines and methods of vaccinating animal subjects with these vaccines.
Owner:INTERVET INC
Dual fluorescent microsphere immunological detection method for pseudorabies virus gE and gB IgG antibodies
ActiveCN109307772AGood repeatabilityStrong specificityBiological material analysisBiological testingWild typeDifferential diagnosis
The invention discloses a dual fluorescent microsphere immunological detection method for pseudorabies virus gE and gB IgG antibodies. The detection method is based on a liquid protein chip technology-based carboxylated fluorescent microsphere group for detecting pseudorabies virus antibodies; the group comprises carboxylated fluorescent microspheres coupled with gE truncated proteins and gB truncated proteins, respectively; and the amino acid sequences of gE and gB truncated proteins are shown in SEQ ID NOs: 2 and 4, respectively. The dual fluorescent microsphere immunological detection method for simultaneously detecting PRV gE and gB IgG antibodies is good in reproducibility, high in sensitivity and good in specificity, and has no cross-reaction with other common porcine virus-positiveserums. The method can be used for the rapid differential diagnosis of pseudorabies virus wild-type infected pigs and vaccine-vaccinated pigs and the detection of protective antibodies, thereby providing an important method for the monitoring of swine virus diseases, having a great application value and being worthy of large-scale promotion.
Owner:SOUTH CHINA AGRI UNIV
PR-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit and detection method of swine transmissible gastroenteritis virus
InactiveCN103740860AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlSwine Transmissible Gastroenteritis
The invention discloses a PR-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit of swine transmissible gastroenteritis virus, which comprises an RT reaction solution, a positive control, a negative control, DEPC (diethylpyrocarbonate) water, 2*Taq PCR Master Mix and a primer mixed solution. Compared with an existing detection technology, the PR-PCR detection kit of the swine transmissible gastroenteritis virus has the advantages of high sensitivity, good specificity, simple and practical and the like, and can be used for rapidly detecting transmissible gastroenteritis of swine virus in various clinical samples on a large scale.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD +1
Recombinant bacillus subtilis for expressing S protein of transmissible gastroenteritis of swine virus
InactiveCN105886523ABacteriaMicroorganism based processesSwine Transmissible GastroenteritisSpecific immunity
The invention relates to recombinant bacillus subtilis for expressing S protein of transmissible gastroenteritis of swine virus (TGEV), and belongs to the fields of biotechnology and genetic engineering. According to the recombinant bacillus subtilis disclosed by the invention, a TGEV S protein recombinant bacillus subtilis integrated expression plasmid pIncotGSR is successfully constructed, and the expression plasmid is successfully transformed into bacillus subtilis WB800 through electric shock. Based upon Western-blot verification, the TGEV S protein can be successfully expressed in the recombinant bacillus subtilis. An effective TGEV specific immune response level can effectively generate in the body of a one-month-old piglet through stimulation of oral immunization. The recombinant bacillus subtilis is expected to be developed as a genetic engineering oral vaccine for preventing transmissible gastroenteritis of swine.
Owner:NANJING AGRICULTURAL UNIVERSITY
Medicine composition capable of preventing Africa swine fever, extractive thereof, injection and application
InactiveCN110101783AInhibit entryFunction increasePharmaceutical delivery mechanismAntiviralsHarrisonia perforataCallicarpa nudiflora
The invention provides a medicine composition capable of preventing Africa swine fever, an extractive thereof, an injection and application. The medicine composition is prepared from, by weight, 10-30parts of lucid ganoderma, 4-12 parts of Drosera burmanii, 4-12 parts of Harrisonia perforata, 30-50 parts of areca nuts, 4-12 parts of Callicarpa nudiflora, 4-12 parts of epigeal srephaia roots and 4-12 parts of common nepenthes. The medicine composition has the efficient prevention effect on Africa swine fever, can be widely applied to preventing Africa swine virus, and solves the major problemof the pork industry.
Owner:HAINAN JINZHU AGRI DEV CO LTD
A kind of kit and method for detecting porcine boca virus
InactiveCN102286640AThe result is easy to judgeEasy to makeMicrobiological testing/measurementFluorescence/phosphorescencePositive controlFluorescence
The invention belongs to the field of virus diagnosis and detection, solves the technical problems of complicated and long time for detecting porcine boca virus in the prior art, and provides a kit for detecting porcine boca virus, which contains a fluorescent PCR reaction solution, The fluorescent PCR reaction solution contains specific primers, and the sequence of the upstream primer is shown in SEQ ID NO: 1, and the sequence of the downstream primer is shown in SEQ ID NO: 2; it also contains a TaqMan specific probe for porcine boca virus, The sequence of the probe is shown in SEQ ID NO: 3, and it also contains the DNA positive control of the Boca virus plasmid. The present invention also provides a method for detecting porcine Boca virus by using the above-mentioned kit. The kit of the invention can quickly and accurately detect porcine boca virus, has simple operation, short time consumption, high sensitivity and strong specificity, and can qualitatively or quantitatively detect porcine boca virus in various types of specimens.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Primer and probe for detecting porcine circovirus type 2 (PCV2), fluorescence quantitative PCR kit, method and application
InactiveCN107974516AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceLinear relationship
The invention discloses a primer and a probe for detecting porcine circovirus type 2 (PCV2), a fluorescent quantitative PCR kit, a method and application, wherein the primer has the following nucleotide sequences: the upstream primer is 5'CTACTGCTGTGAGTACCTTGTTGGA 3', the downstream primer is 5'CAGGGTGCTGCTCTGCAA 3'; the sequence of the probe is as follows: 5'FAM-AGCGGGAGTCTGGT-MGB 3'. The invention have the beneficial effects that by adopting the kit disclosed by the invention, PCV2 can be quickly and accurately detected, and the kit has a good linear relationship in the template range of 5.0*10<1> to 5.0*10<9>copies*muL<-1>, has the sensitivity which is 100 times that of conventional PCR, no cross reaction with other swine viruses, and a repeatability test variation coefficient which isless than 1.5%; compared with the conventional PCR method, the method is higher in positive detection rate. In summary, the method has strong specificity, high sensitivity and good repeatability and provides a rapid and accurate detection means for laboratory diagnosis and epidemiological investigation of PCV2.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Application of SVA 3C protein in promotion of porcine virus replication
InactiveCN111690669AIncrease productionSolve outputSsRNA viruses positive-senseViral antigen ingredientsVector vaccineGenetic engineering
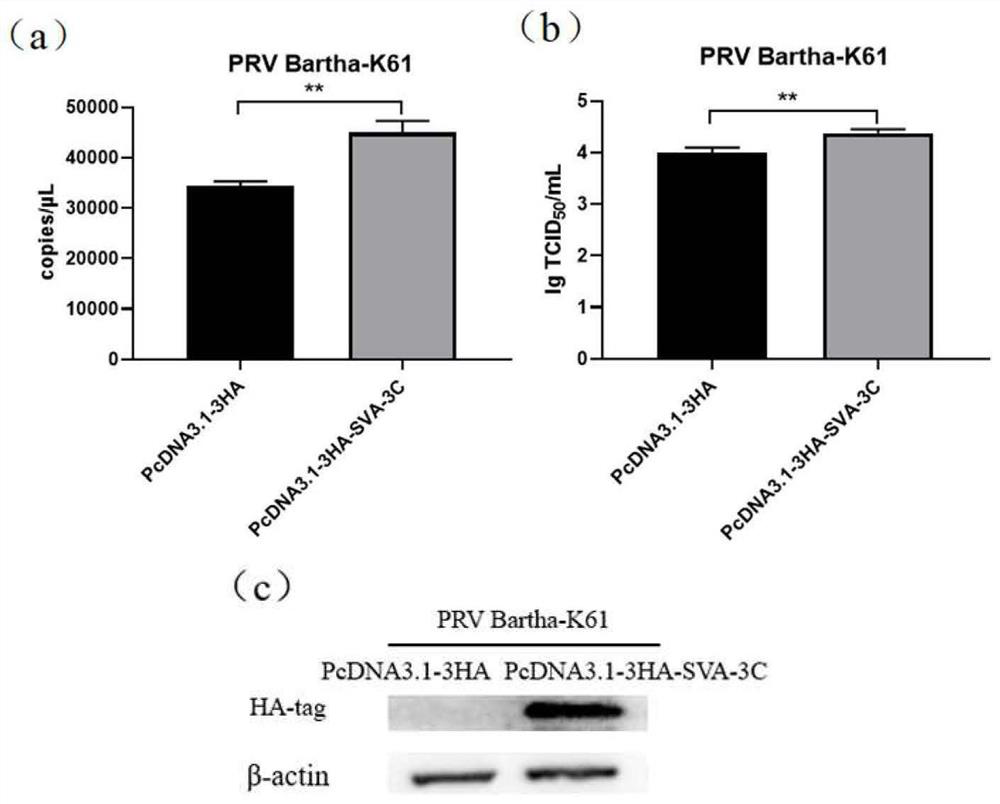
The invention belongs to the technical field of biology, and experimental research finds for the first time that a protein encoded by an SVA 3C gene can be used as a virus replication enhancer to significantly promote replication of swine viruses including attenuated vaccine strains and inactivated vaccine strains. Therefore, the problems of low virus yield and low vaccine immunogenicity in production and practice at present are well solved. The invention not only provides a new direction for revealing a virus mixed infection mechanism, but also can apply the SVA 3C protein to improve the yield of viruses in production practice, and is more beneficial to optimizing the construction strategies of related genetic engineering live vaccines and live virus vaccine vectors and improving the immune activity of the vector vaccines.
Owner:SOUTH CHINA AGRI UNIV
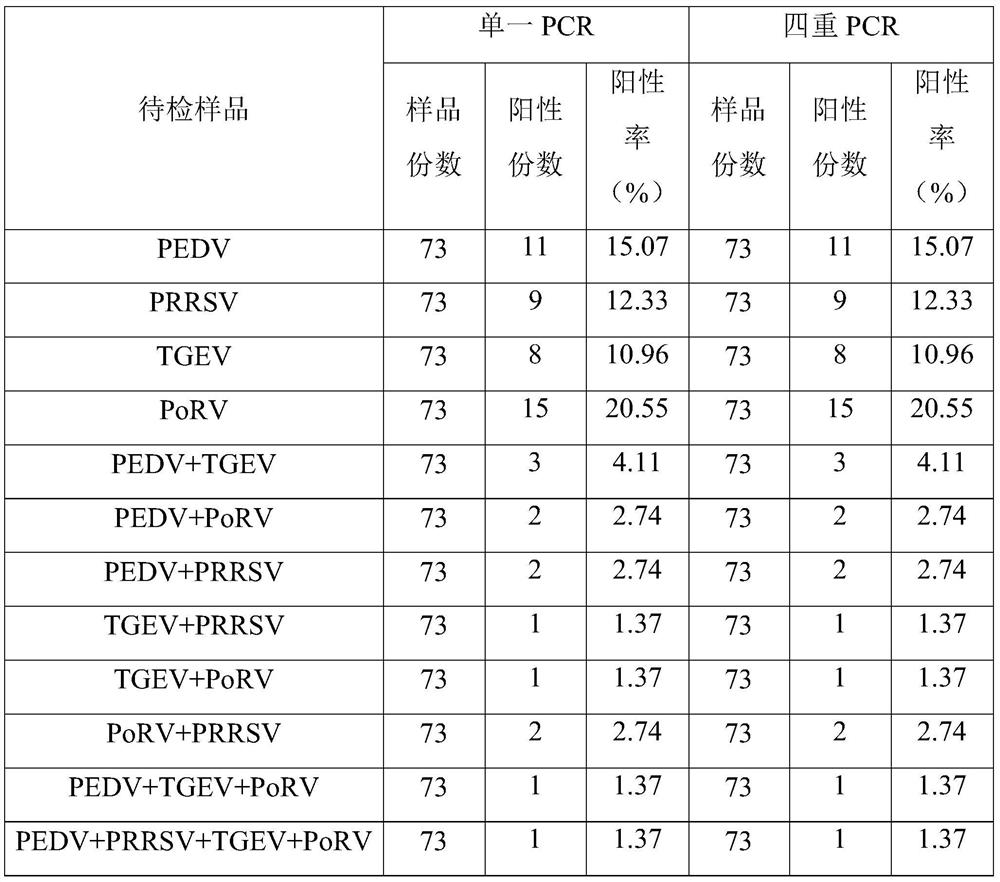
Primer and kit for simultaneously detecting PEDV, PRRSV, TGEV and PoRV and use method thereof
PendingCN111647692AHigh sensitivityRapid identificationMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVPorcine rotavirus
The invention relates to the technical field of virus detection, and particularly discloses a primer and kit for simultaneously detecting PEDV, PRRSV, TGEV and PoRV and a use method thereof. The PCR detection primer provided by the invention can be used for simultaneously detecting four kinds of viruses including the PEDV, the PRRSV, the TGEV and the PoRV, has no cross amplification reaction withswine fever virus, swine Japanese encephalitis virus, swine Bocavirus, porcine circovirus 2, swine delta circovirus, porcine pseudorabies virus and other swine viruses, and has relatively strong specificity; and the primer is high in sensitivity, the lowest detection concentrations of the primer to the PEDV, the PRRSV, the TGEV and the PoRV are 9.12x10<5> pg / [mu]L, 2.48x10<3> pg / [mu]L, 7.55x10<5>pg / [mu]L and 8.23x10<5> pg / [mu]L respectively, and the detection accuracy is 100%.
Owner:河北三狮生物科技有限公司
Method for screening CTL epitopes from autonomously constructed SLA-2-HB01-pCDH/sT2 cell line
PendingCN114181895AImproved delivery efficiencyConsistent delivery efficiencySkeletal/connective tissue cellsBiological testingCtl epitopeT lymphocyte
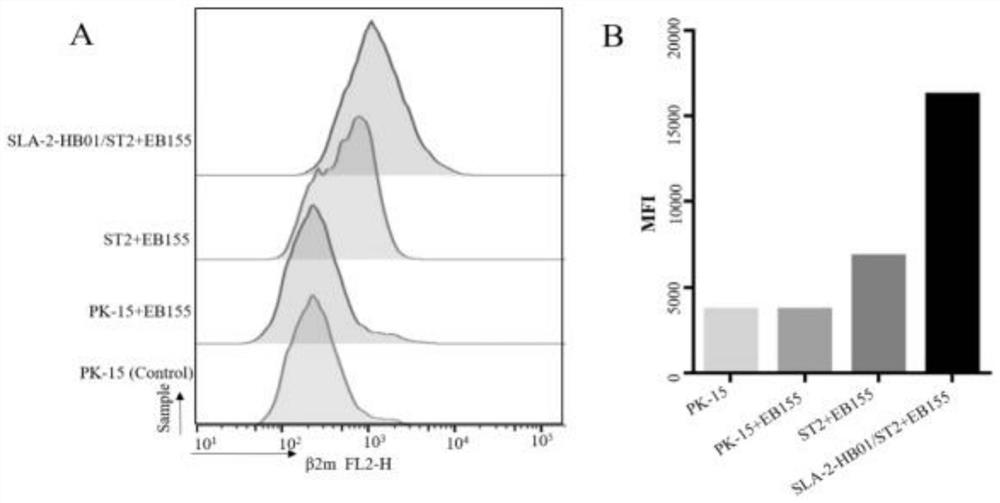
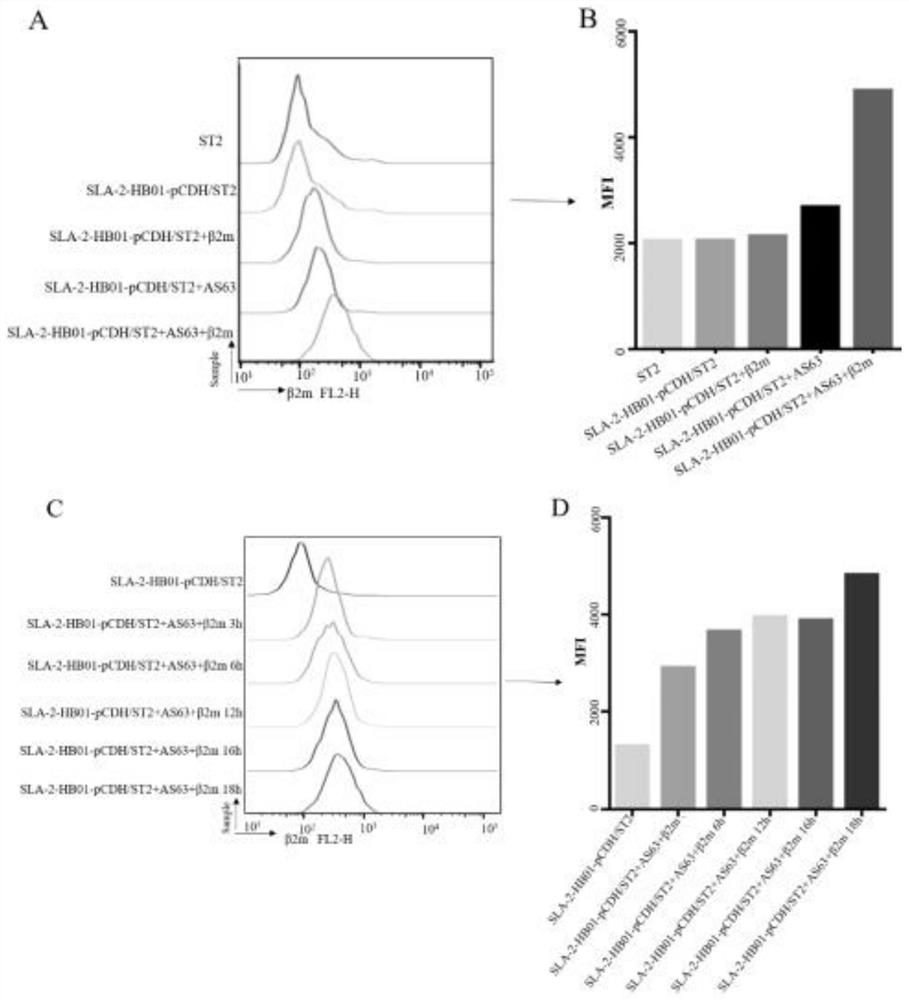
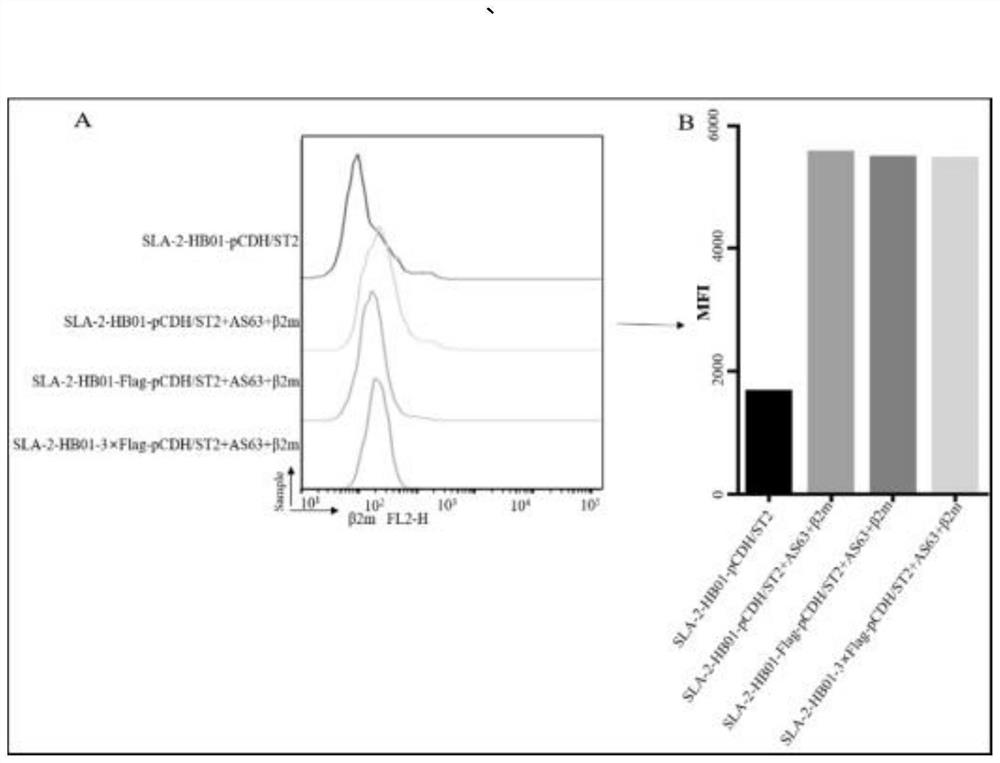
The invention belongs to the field of biological medicines, and particularly relates to a method for screening CTL (cytotoxic T lymphocyte) epitopes by an independently constructed SLA-2-HB01-pCDH / sT2 cell line. According to the invention, an exogenous SLA-2-HB01 gene is transfected to an sT2 cell, and a stable SLA-2 gene expression model is established. The method comprises the following steps: loading EB155 and other positive epitope peptides on the surfaces of cells, detecting an SLA-2-peptide-beta2m complex expressed on the surfaces of the cells through FACs, and judging that the SLA-2-HB01-pCDH / sT2 has the function of presenting exogenous polypeptide epitopes. In order to promote the formation of cell surface complexes, the addition of the beta2m is beneficial to the improvement of the presentation efficiency of the antigen polypeptide. In the result of the invention, As63, EB155 and Hu62 peptides can be presented by an SLA-2-HB01-pCDH / sT2 cell line, As63 is presented in the SLA-2-HB01-pCDH / sT2, SLA-2-HB01-Flag-pCDH / sT2 and SLA-2-HB01-3 * Flag-pCDH / in the sT2 cell line, and the presentation efficiency is basically consistent, which indicates that a series of sT2 for expressing the SLA-2-HB01 gene constructed by the invention has the function of presenting the antigen, and the SLA-2-HB01-3 * Flag-pCDH / sT2 can be used for expressing the SLA-2-HB01 gene. Therefore, the SLA-2 heavy chain molecule can be used for screening polypeptide epitopes of the swine viruses on the cellular level, and it is further proved that the antigen presentation function of a cell line is not affected when a Flag tag is added to the C-terminal of the SLA-2 heavy chain molecule.
Owner:DALIAN UNIV
Preparation method for lyophilized agent of swine interferon
InactiveCN105380911AReduce Toxic DamageLow cytotoxicityPowder deliveryPeptide/protein ingredientsSerum igeMedicine
The invention discloses a preparation method for a lyophilized agent of swine interferon, belonging to the field of animal vaccines. The preparation method for the lyophilized agent of swine interferon comprises the following six steps: acquiring fresh blood of a healthy pig under aseptic conditions, centrifuging the blood at 4 DEG C for 20 min, sucking up leucocytes at a middle layer, washing the leucocytes with NH4Cl with a mass fraction of 0.85% twice, then carrying out centrifugation, collecting precipitated leucocytes, removing NH4Cl, adding the leucocytes into an Eagle's MEM mixed nutrient solution containing 5% of swine serum according to a volume ratio of the leucocytes to the nutrient solution of 1: 15, carrying out suspension and diluting the swine leucocytes until the number of the swine leucocytes is in a range of 10 * 10<7> / ml to 25 * 10<7> / ml so as to prepare a swine leucocyte suspension liquid; etc. The preparation method is capable of preparing the long-acting swine virus interferon preparation which is injected once every 10 days and has good effect on swine viral diseases.
Owner:宋宏婷
ST IRF3/IRF7 KO cell line and construction method and application thereof
ActiveCN113717943AReduce poison priceInhibition of growth replicationCompound screeningApoptosis detectionWestern blotViral Vaccine
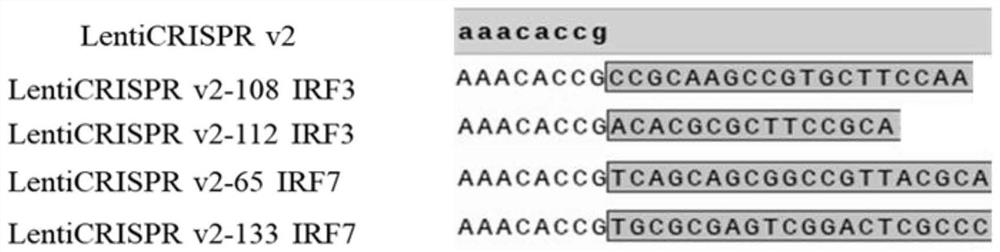
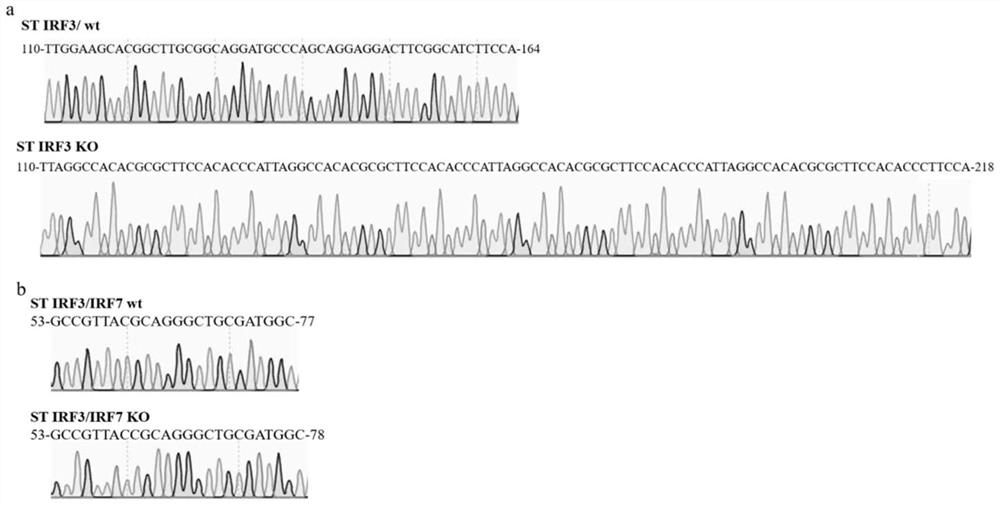
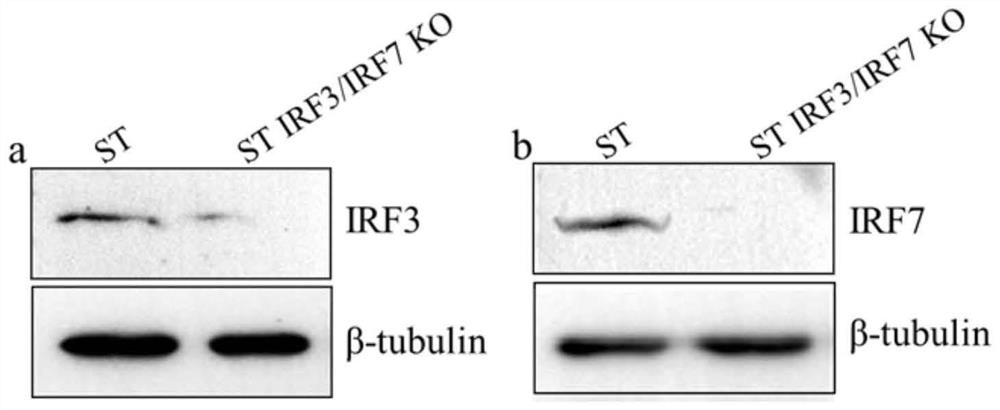
The invention belongs to the field of molecular biology, and relates to an ST IRF3 / IRF7KO cell line and a construction method and application thereof. Specific sgRNA is designed for exons of porcine IRF3 and IRF7 gene sequences and inserted into a vector lentiCRISPR v2, and lentiviral vectors lentiCRISPR v2-IRF3 and lentiCRISPR v2-IRF7 are obtained. After HEK293T cells are transfected by the recombinant plasmids, lentivirus infected ST cells are collected. Successfully infected cells are screened out through antibiotics, sequencing is carried out, and Western Blot verification is carried out. The ST IRF3 / IRF7KO cell line disclosed by the invention can be applied to basic and application research of swine viruses, and a valuable new material is provided for selection of cell lines in production of viral vaccines.
Owner:YANGZHOU UNIV
Composition and preparation method thereof, and traditional Chinese medicine preparation and application thereof
InactiveCN107753596AEasy to prepareLow costAntibacterial agentsDigestive systemMedicineWolfiporia extensa
The invention relates to a traditional Chinese medicine composition. The traditional Chinese medicine composition comprises 200 to 2000 parts of euphorbiae humifusae herb, 10 to 200 parts of catechu,10 to 100 parts of dark plum, 10 to 200 parts of scorched hawthorn fruit, 20 to 400 parts of Poria cocos, 10 to 200 parts of white peony roots and 10 to 100 parts of licorice roots. The invention alsorelates to a preparation method for the traditional Chinese medicine composition. The invention further relates to a traditional Chinese medicine preparation and application of the same as a drug fortreating pathogenic diarrhea, nonpathogenic diarrhea, damp-heat type diarrhea or spleen-deficiency type diarrhea caused by swine viruses and bacteria.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
A grinding processing device for porcine virus samples
ActiveCN109107650BAchieve secondary grindingSimple structureGrain treatmentsStructural engineeringGrinding wheel
Owner:SHANGHAI JIAOTONG UNIV
Application of a kind of n-acetyl-l-cysteine in preparing medicine for treating or preventing swine virus infection
ActiveCN104398498BImprove securityWill not result inOrganic active ingredientsAntiviralsSide effectMortality rate
The invention discloses an application of N-acetyl-L-cysteine in preparing a drug for treating or preventing porcine virus infection. The application comprises the steps: A, preparing N-acetyl-L-cysteine, absolute ethyl alcohol, tween-80, lactose, soluble starch and bran into a granule, oral liquid or powder, B, adding the granule, the oral liquid or the powder into feed or drinking water, or C, independently administering N-acetyl-L-cysteine, D, continuously using N-acetyl-L-cysteine during a whole feeding period as preventive addition and long-term usage in the feed, E, feeding the powder and the granule directly for treatment, or F, mixing the powder and the granule with daily ration, adding into the feed in the morning, at noon and in the evening every day, and continuously using the powder and the granule. N-acetyl-L-cysteine has high safety, no toxic or side effects, and antiviral activity, and can effectively prevent and treat the porcine circovirus type 2 and porcine epidemic diarrhea virus infection, reduce a mortality rate of infected pigs, and effectively increase a survival rate and productivity of the pigs.
Owner:HUBEI HAOHUA BIOTECH
A kind of porcine getavirus strain, vaccine composition and its preparation method and application
ActiveCN106636012BImproving immunogenicityReduce yieldSsRNA viruses positive-senseViral antigen ingredientsMicroorganismAdjuvant
The invention discloses a newly separated pig Getah virus HNJZ-S1 strain. The microbial preservation number of the pig Getah virus HNJZ-S1 strain is CGMCC NO 12550; the pig Getah virus is separated from a naturally infected pig group for the first time in China, and has good immunogenicity. The invention also discloses a vaccine composition which is prepared from the pig Getah virus HNJZ-S1 strain, and a preparation method thereof. The vaccine composition contains inactivated pig Getah virus strain HNJZ-S1 strain and a pharmaceutically acceptable adjuvant; the preparation method for the vaccine composition comprises the steps of virus culturing, virus liquid inactivating, aqueous phase preparing, oil phase preparing emulsifying and the like. The vaccine composition has good immunogenicity, and can produce high immunity after immunization; the attacking protecting rate is high; after inoculation, the abortion rate and the stillbirth rate of sows are obviously reduced; the incidence of piglets is remarkably reduced; the popularizing and spreading of the pig Getah virus can be effectively prevented; the vaccine composition has a broad application prospect.
Owner:HENAN AGRICULTURAL UNIVERSITY
Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease
InactiveCN101695342BPrevention and treatment of asthmaPrevention and treatment of contagious pleuropneumoniaFood processingAnimal feeding stuffMedicinal herbsHouttuynia
The invention provides a Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease. The swine feed additive consists of 37 Chinese medicinal herbs, namely, gypsum, rehmannia root, rhinoceros horn, golden thread, cape jasmine fruit, tree peony bark, baical skullcap root, red paeony root, figwort root, common anemarrhena rhizome, forsythia suspensa , platycodon root, liquorice root, common lophatherum herb, amur corktree bark, honeysuckle flower, Chinese pulsatilla root, indigowoad root, heartleaf houttuynia herb, astragalus, szechwon tangshan root, hawkthorn fruit, medicated leaven, barley sprout, radish seed, chicken's gizzard -membrane, Chinese thorowax root, common andrographis herb, philippine violet herb, tuber fleeceflower root, massa medicata fermentata fujianensis, cyrtomium rhizome, tung leaf, tangerine peel, white paeony root, pine needle and indigowoad leaf through scientific compatibility. The feed additive is added into swine feed in the proportion; under the condition of not using any vaccine, the feed additive can effectively prevent and cure severe mixed flu symptoms, infection and other syndromes caused by swine respiratory disease, asthma, contagious pleuropneumonia, swine virus mixed flu, high swine fever, porcine circovirus, swine fever, flu, pseudorabies, salmonellosis, bacillosis, streptococcus, erysipelas, paratyphoid,eperythrozoon, toxoplasm and other multi-pathogeny and provides genuine green food for the market.
Owner:孟祥合
A double fluorescent microsphere immunological detection method for pseudorabies virus ge and gb IgG antibodies
ActiveCN109307772BGood repeatabilityStrong specificityBiological material analysisBiological testingImmunological testingDisease
The invention discloses a double fluorescent microsphere immunological detection method for pseudorabies virus gE and gB IgG antibodies. The detection method is based on a group of carboxylated fluorescent microsphere combinations based on liquid-phase protein chip technology for detecting porcine pseudorabies virus antibodies , which includes carboxylated fluorescent microspheres respectively coupled with gE truncated protein and gB truncated protein, and the amino acid sequences of gE and gB truncated protein are respectively shown in SEQ ID NO: 2 and 4. The dual fluorescent microsphere immunological detection method for simultaneous detection of PRV gE and gB IgG antibodies constructed by the invention has good repeatability, high sensitivity and good specificity, and has no cross-reaction with other common porcine viral disease positive sera. The establishment of this method can be used for the rapid differential diagnosis of wild pseudorabies virus-infected pigs and vaccine-immunized pigs and the detection of protective antibodies. It provides an important method for the monitoring of swine viral diseases and has great application value. Widespread promotion.
Owner:SOUTH CHINA AGRI UNIV
A kind of anti-porcine virus disease mixed freeze-dried powder and preparation method thereof
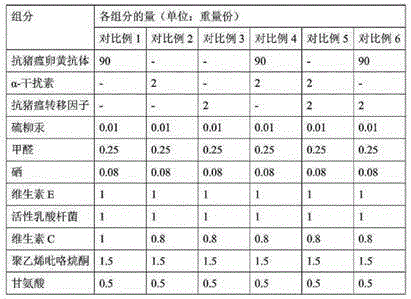
InactiveCN104208674BImprove biological activityGood control effectPowder deliveryPeptide/protein ingredientsYolkAnti virus
The invention discloses a mixed freeze-dried powder for resisting swine viral diseases and a preparation method thereof. The mixed freeze-dried powder comprises the following components in parts by weight: 90-95 parts of anti-swine virus disease yolk antibody, anti-swine virus disease 1-2 parts of transfer factor, 1-2 parts of α-interferon, 1-2 parts of antioxidant, 0.01-0.02 part of preservative, 0.2-0.3 part of inactivator, 0.05-0.1 part of selenium, 1-2 parts of polyvinylpyrrolidone 0.5-1 part of glycine, 0.5-1 part of active lactobacillus. The anti-porcine viral disease mixed freeze-dried powder of the present invention is compounded with anti-porcine viral disease egg yolk antibody, anti-porcine viral disease transfer factor and immune enhancer, supplemented with various auxiliary materials, and each component cooperates and coordinates It can significantly improve the effect of preventing and treating porcine viral diseases, and has the advantages of strong pertinence, long storage time at room temperature, high antibody titer, and good biological activity.
Owner:ZHENGZHOU HOUYI PHARMA
A kind of primer combination, probe combination and its application in detecting porcine virus, detection reagent, kit and detection method
ActiveCN112342319BEnable high-throughput screeningReduce dosageMicrobiological testing/measurementMicroorganism based processesDiseaseViral infection
Owner:北京市动物疫病预防控制中心
RT-CPA detection primer and kit for swine transmissible gastroenteritis virus
ActiveCN114196784AApplicable to on-site rapid detectionReduce testing costsMicrobiological testing/measurementAgainst vector-borne diseasesDiseaseSwine Transmissible Gastroenteritis
The invention provides an RT-CPA detection primer and kit for swine transmissible gastroenteritis virus, and belongs to the technical field of swine virus disease virus detection, the RT-CPA detection primer comprises SD-CPF, SD-CPR, SD-F, SD-R, SD-DF and SD-DR; and the nucleotide sequences are shown as SEQ ID No.1-6 in sequence. The primer provided by the invention is good in specificity and can effectively detect the swine transmissible gastroenteritis virus; the method is rapid and efficient, the detection time is about 90 min, and the method is very suitable for on-site rapid detection of TGEV; the method does not depend on expensive instruments and equipment and professional detection personnel, and the detection cost is low; the detection result is simple, objective and visual to judge; the sensitivity is high, and 10 copies / mu L of TGEV can be detected.
Owner:GANSU ANIMAL HUSBANDRY & VETERINARY MEDICINE INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com