Medicine composition capable of preventing Africa swine fever, extractive thereof, injection and application
A technology of African swine fever and composition, applied in the field of pharmaceutical composition for preventing and treating African swine fever, to achieve the effect of improving meat color, widely used, and reducing drip loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
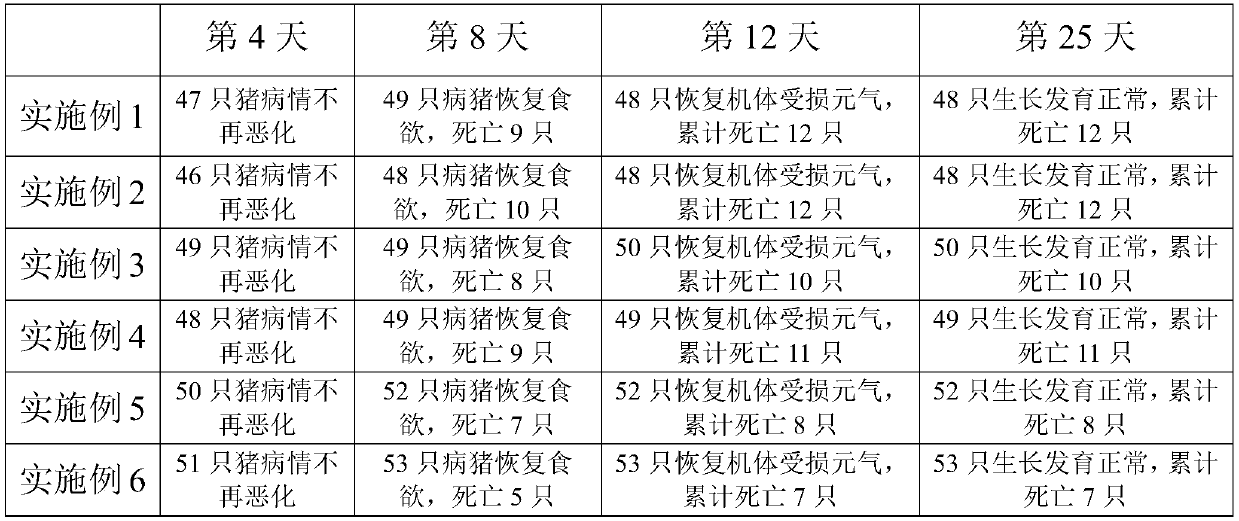
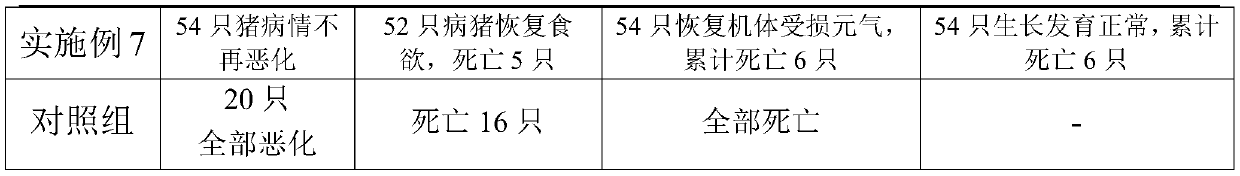
[0036] A pharmaceutical composition for preventing and treating African swine fever, the pharmaceutical composition comprising the following raw materials in parts by weight: 10 parts of ganoderma lucidum, 4 parts of jindiluo, 12 parts of acanthus, 50 parts of betel nut, 4 parts of pachyderm, There are 4 parts of ground tolerance and 12 parts of pitcher plant. The above-mentioned raw materials are prepared according to conventional methods (such as direct water extraction or alcohol extraction) to obtain extracts, and the extracts are prepared into injections according to conventional methods, and each 1 ml of injections contains 2.0 g of the equivalent raw medicinal materials.
Embodiment 2
[0038] A pharmaceutical composition for preventing and treating African swine fever, the pharmaceutical composition comprising the following raw materials in parts by weight: 30 parts of ganoderma lucidum, 12 parts of jindiluo, 4 parts of acanthus, 30 parts of betel nut, 12 parts of pachyderm, There are 12 parts of ground cannon and 4 parts of nepenthes. The above-mentioned raw materials are prepared according to conventional methods (such as direct water extraction or alcohol extraction) to obtain extracts, and the extracts are prepared into injections according to conventional methods, and each 1 ml of injections contains 2.0 g of the equivalent raw medicinal materials.
Embodiment 3
[0040] A pharmaceutical composition for preventing and treating African swine fever, the pharmaceutical composition comprising the following raw materials in parts by weight: 18 parts of ganoderma lucidum, 6 parts of jindiluo, 10 parts of acanthus, 44 parts of betel nut, 10 parts of pachyderm, 6 parts of ground cannon and 6 parts of pitcher plant. Extract the above raw materials according to conventional methods (such as direct water extraction or alcohol extraction), and prepare the extracts into injections according to conventional methods, and each 1ml of injection is equivalent to 2.0g of the original medicinal material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com